-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaMasculinization of Gene Expression Is Associated with Exaggeration of Male Sexual Dimorphism
Gene expression differences between the sexes account for the majority of sexually dimorphic phenotypes, and the study of sex-biased gene expression is important for understanding the genetic basis of complex sexual dimorphisms. However, it has been difficult to test the nature of this relationship due to the fact that sexual dimorphism has traditionally been conceptualized as a dichotomy between males and females, rather than an axis with individuals distributed at intermediate points. The wild turkey (Meleagris gallopavo) exhibits just this sort of continuum, with dominant and subordinate males forming a gradient in male secondary sexual characteristics. This makes it possible for the first time to test the correlation between sex-biased gene expression and sexually dimorphic phenotypes, a relationship crucial to molecular studies of sexual selection and sexual conflict. Here, we show that subordinate male transcriptomes show striking multiple concordances with their relative phenotypic sexual dimorphism. Subordinate males were clearly male rather than intersex, and when compared to dominant males, their transcriptomes were simultaneously demasculinized for male-biased genes and feminized for female-biased genes across the majority of the transcriptome. These results provide the first evidence linking sexually dimorphic transcription and sexually dimorphic phenotypes. More importantly, they indicate that evolutionary changes in sexual dimorphism can be achieved by varying the magnitude of sex-bias in expression across a large proportion of the coding content of a genome.
Published in the journal: . PLoS Genet 9(8): e32767. doi:10.1371/journal.pgen.1003697
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003697Summary
Gene expression differences between the sexes account for the majority of sexually dimorphic phenotypes, and the study of sex-biased gene expression is important for understanding the genetic basis of complex sexual dimorphisms. However, it has been difficult to test the nature of this relationship due to the fact that sexual dimorphism has traditionally been conceptualized as a dichotomy between males and females, rather than an axis with individuals distributed at intermediate points. The wild turkey (Meleagris gallopavo) exhibits just this sort of continuum, with dominant and subordinate males forming a gradient in male secondary sexual characteristics. This makes it possible for the first time to test the correlation between sex-biased gene expression and sexually dimorphic phenotypes, a relationship crucial to molecular studies of sexual selection and sexual conflict. Here, we show that subordinate male transcriptomes show striking multiple concordances with their relative phenotypic sexual dimorphism. Subordinate males were clearly male rather than intersex, and when compared to dominant males, their transcriptomes were simultaneously demasculinized for male-biased genes and feminized for female-biased genes across the majority of the transcriptome. These results provide the first evidence linking sexually dimorphic transcription and sexually dimorphic phenotypes. More importantly, they indicate that evolutionary changes in sexual dimorphism can be achieved by varying the magnitude of sex-bias in expression across a large proportion of the coding content of a genome.
Introduction
Complex sexually dimorphic phenotypes are largely the result of gene expression differences between males and females for loci that are present in both sexes [1], [2], and the study of sex-biased gene expression provides a link between sexual conflict and sexual selection acting on the phenotype with the genetic loci that underpin it. It is often assumed that genes expressed more in either sex encode sexually dimorphic phenotypes that are then subject to sex-specific selection. Studies in a range of animals have demonstrated that sex-biased gene expression is widespread across the genome [3]–[7], most evident in adults as would be expected as this is when sexual phenotypes are most manifest [8]–[10], variable among closely related species [11] and subject to rates of evolution consistent with sexual selection acting primarily on males [2]. However, despite this mounting circumstantial evidence, the relationship between gene expression and the phenotype is complex, and direct connections linking sex-biased gene expression to sexually dimorphic phenotypes have remained elusive. This relationship between sex-biased transcription and sexual dimorphism is key to studies of sexual conflict and sexual selection, which are increasingly focused on sex-specific regulation, and to the broader question of the regulatory control of complex phenotypes.
The relationship between sex-biased gene expression and sexual dimorphism has been difficult to test directly, primarily because sexual dimorphism is often envisaged as a dichotomous comparison between female and male forms. Additionally, many of the model systems for sex-biased gene expression studies lack multiple sex-specific morphs, precluding detailed tests of the association between sex-biased gene expression and dimorphic phenotypes. However, sexual dimorphism is far more complex for many species, with some individuals occupying intermediate points along an axis. The wild turkey exhibits two male phenotypes in the forms of dominant and subordinate males. The species is strongly sexually dimorphic, with dominant males showing greater body size than females, along with a constellation of sexually selected traits including iridescent plumage, a long beard, vivid coloration on the head and neck, enlargement of the caruncles, wattle and snood (Supplemental Fig. 1), and distinct mating behaviours [12]–[14].
Dominance among sibling males is established via male-male competition during the winter prior to sexual maturation [15], and at this point, many males develop the subordinate male phenotype, which includes iridescent plumage and long beards similar to dominant males, but with less vivid head and neck coloration and less developed wattles, caruncles and snoods. The length of the latter appears to be key to intra-sexual and inter-sexual selection in this species [12], [13]. Although subordinate males can mate and sire offspring [15]–[17] they rarely obtain mating opportunities. Their role is mainly to assist their dominant brothers in attracting mates, and as such has been held up as an example of Hamilton's rule of kin selection [15], [16], [18]. Importantly, subordinate males can become dominant males later in life if the dominant dies, emphasising the plastic nature of the male phenotype. Subordinate males are therefore clearly male in phenotype, but occupy an intermediate position on the continuum of sexual dimorphism.
The two male phenotypes in the wild turkey make it possible to test for the first time whether the magnitude of sexual dimorphism in the phenotype is associated with the magnitude of sex-biased expression. Male-biased genes are often assumed to encode male-specific phenotypes, while female-biased genes are thought to encode female-specific phenotypes. Within this framework, the subordinate male phenotype could be the product of reduced expression of male-biased genes (demasculinized), increased expression of female-biased genes (feminized), or a combination of both, compared to the dominant male phenotype. We therefore used the female and subordinate male and dominant male phenotypes in order to directly test for the first time whether the degree of sex-biased gene expression is correlated with sexual dimorphism, and to understand the role of demasculinization and feminization in gene expression in encoding the subordinate male form.
Results and Discussion
Our initial preliminary analysis of sex-biased expression indicated, as have previous studies [7], [19], that the gonad is the most transcriptionally dimorphic tissue (Supplemental Table 1), and therefore we focused our analysis primarily on this organ, although we also assessed the spleen using lower fold-change thresholds in order to determine whether the general patterns extend from the gonad to the soma. In the gonad, 9,872 autosomal and 364 Z-linked genes were significantly expressed. Of the autosomal genes, 2,217 were significantly male-biased (dominant male: female fold change >2, adj. p<0.05), 2,908 were significantly female-biased (female: dominant male fold change >2, adj. p<0.05), and 4,747 were unbiased. The autosomal genes show a broadly similar pattern of sequence evolution to that seen in other adult animals [2], with male-biased genes showing elevated rates of functional evolution compared to female-biased and unbiased genes (Table 1), consistent with the notion that sex-specific selection is stronger in males than females in this species.
Tab. 1. Rates of non-synonymous (d<sub>N</sub>) and synonymous (d<sub>S</sub>) substitution for autosomal sex-biased and unbiased genes. 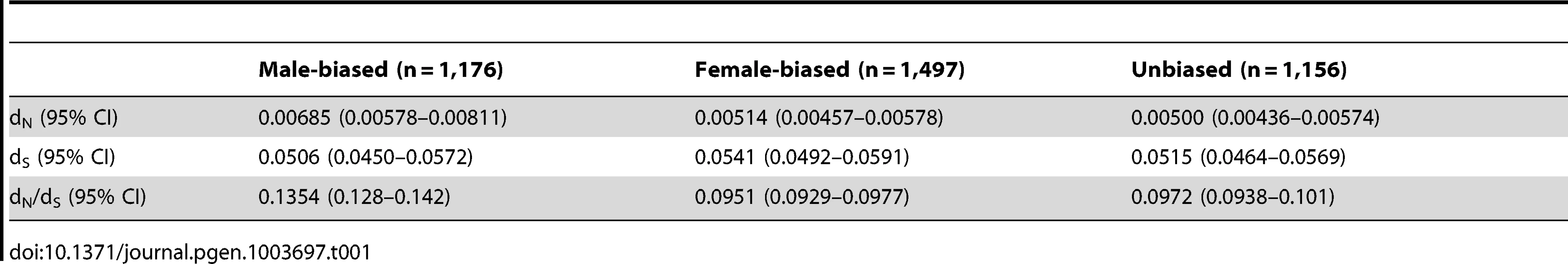
We used hierarchical clustering of expression level to visualize global transcriptomic patterns for the three morphs in the gonad. Subordinate and dominant males clustered together with high confidence for male-biased autosomal, female-biased autosomal and Z-linked genes (Fig. 1). Clustering clearly demonstrates that subordinate male transcription is on the male side of the sexual dimorphism continuum rather than intersex, however there were clear but subtle differences between the male forms in overall transcription that distinguish them. Hierarchical clustering of unbiased autosomal expression also showed the male phenotypes cluster together, with 100% bootstrap support.
Fig. 1. Heat maps and hierarchical clustering of gene expression for females, subordinate males and dominant males. 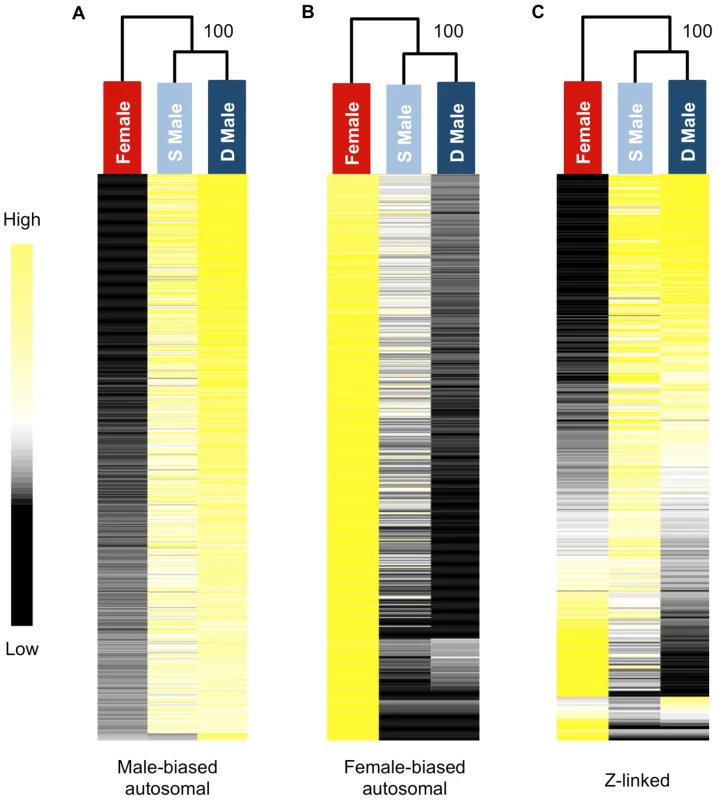
Shown is the relative expression for autosomal male-biased (n = 2,217, panel A), female-biased (n = 2,908, panel B) and Z-linked (n = 364, panel C) genes. Hierarchical gene clustering is based on Euclidean distance for average log2 expression for each gene for the three sexual morphs. The number at each node is the percentage bootstrap result from 1000 replicates. We next analysed sex-biased genes for evidence of demasculinization and/or feminization in subordinate males in order to examine how sex-biased gene expression is affected by male social dominance (Fig. 2A). Subordinate males express autosomal male-biased genes in the gonad at significantly lower levels than dominant males (Wilcoxon test, p<0.00001), suggesting that subordinate males are transcriptionally demasculinized. Just as important is the fact that subordinate males express female-biased genes at a higher level than dominant males (Wilcoxon test, p<0.00001, Fig 2A), suggesting that they are transcriptionally feminized.
Fig. 2. Average log2 expression for all sex-biased genes. 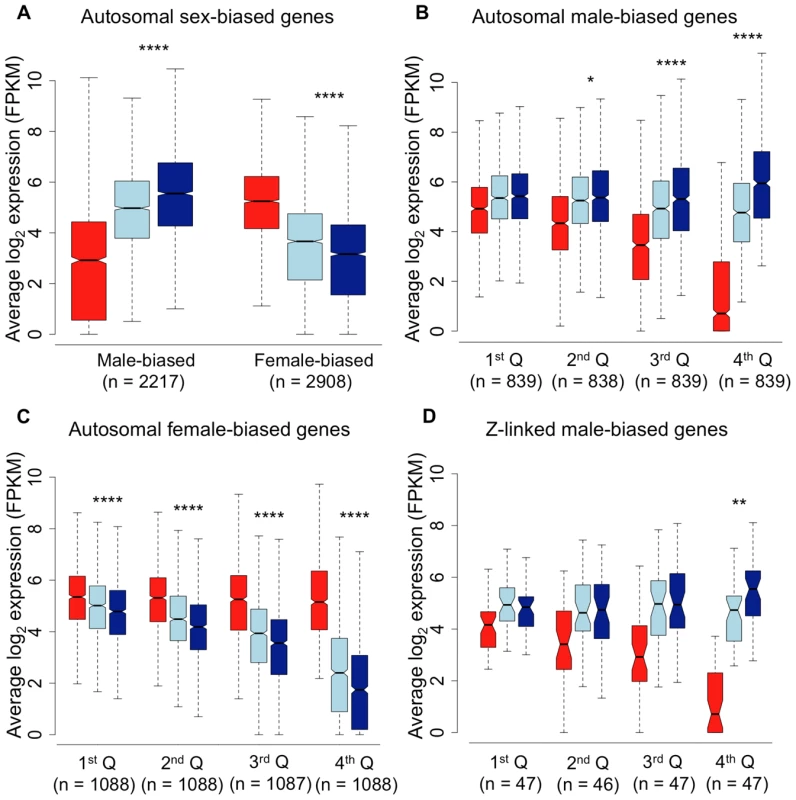
Panel A, autosomal male-biased and female-biased genes in females (red), subordinate males (light blue) and dominant males (dark blue). Panel B, autosomal male-biased genes ranked by male-bias. Panel C, autosomal female-biased genes ranked by female bias, and Panel D, Z-linked male-biased genes ranked by male-bias. Whiskers extend to the most extreme data point, excluding outliers that exceeded 1.5× the interquartile range. Significant p-values as calculated by Wilcoxon tests are indicated by asterisks above each comparison between dominant and subordinate males (* p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001). RNA-Seq data give a relative, rather than absolute measure of expression. It is therefore possible that the relative reduction in expression for male-biased genes in subordinate males could produce a false signal of a relative increase in expression for all other types of genes. In order to ascertain whether the pattern of feminization for female-biased genes was simply an artefact of relative decrease in expression for male-biased genes, we removed all reads mapping to male-biased genes in all three morphs. We remapped the remaining reads, effectively normalizing for differences in expression for male-biased genes. The resulting comparison of unbiased and female-biased genes (Supplemental Fig. 2), suggests that the feminization in transcription in subordinate males is not an artefact of demasculinization.
We assessed gene expression in the spleen, in order to determine whether the pattern we observe in the gonad extends to the soma. Because patterns of sex-bias are much reduced in somatic tissue [4], [7], we relaxed our fold-change thresholds considerably to do this. Despite the lower overall degree of sex-bias, we observed the same qualitative pattern in the spleen compared to the gonad, with subordinate males both demasculinized and feminized in overall transcription compared to dominant males. Despite the fact that the limited overall differences between males and females in transcription in the spleen limits statistical power, the pattern of demasculinization and feminization in the spleen was statistically significant (Wilcoxon test, p<0.05) in three of the four comparisons (Fig. 3). This suggests that the pattern of demasculinization and feminization is not limited to the gonad, but extends into the soma as well, although to a lesser degree.
Fig. 3. Sex-bias in the spleen of females (red), subordinate males (light blue) and dominant males (dark blue). 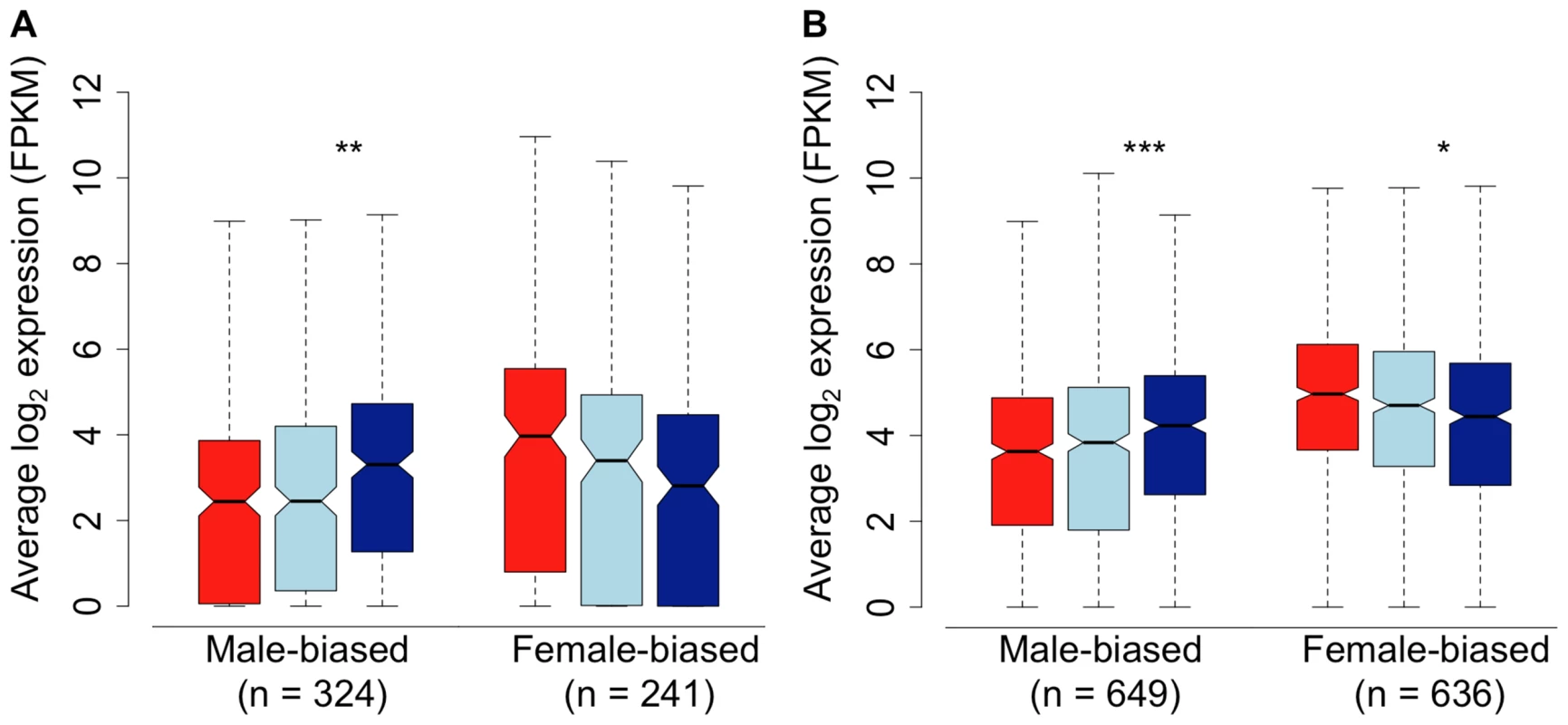
Sex-bias was defined in panel A based on a 1.5-fold change threshold between females and dominant males, with a p-value<0.05. Sex-bias in panel B is defined solely on statistical difference (p<0.05) between females and dominant males. Significant difference between dominant and subordinate males is indicated (Wilcoxon test, * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001). In order to further test whether subordinate males are intermediate, or orthogonal, to dominant males and females in overall expression, we performed factor analysis for all expressed genes in the gonad and spleen. In both tissues, subordinate males are clearly more similar to dominant males, although intermediate between dominant males and females (Supplemental Fig. 3). This is congruent with the concept that the three sexual morphs form an axis of dimorphism.
The overall pattern of demasculinization and feminization of subordinate male transcription is strongly correlated with the degree of sex-bias. Demasculinization of male-biased gene expression in subordinates is more pronounced for genes with greater male-bias in the gonad (significance for each quartile is denoted in Fig. 2B), possibly suggesting that the most extreme male-biased genes make the greatest contribution to male-specific traits. Similarly, feminization increases for genes with greater female-bias (Fig 2C), indicating feminization of the subordinate male transcriptome for female-biased genes. We lacked sufficient sex-biased genes in the spleen to do a meaningful quartile-based analysis. We calculated the overall correlation between the difference in transcription between dominant and subordinate males (log2 dominant male expression – log2 subordinate male expression) with the degree of female-bias. This analysis recovered a significant correlation for both the gonad-expressed genes (r2 = 0.307, p<0.001) and the spleen (r2 = 0.159, p<0.001), indicating that as sex-bias increases, subordinate and dominant male transcription is increasingly decoupled in both tissues.
In order to assess whether the patterns we observe are artefacts of the way in which we defined sex-bias, we further examined the gonad data, where sex-bias is most evident. Our results indicate that demasculinization and feminization of subordinate male transcriptomes is independent of how sex-bias is defined. Qualitatively similar patterns are evident when sex-bias is defined by comparing female expression to the combined dominant and subordinate male expression or to subordinate male expression alone (Supplemental Fig. 4). We also tested for the possible influence of regression toward the mean by randomizing samples, in each case picking three dominant male and three female samples to define sex-bias (greater than two-fold expression difference, adj. p<0.05), and then assessing the remaining dominant males, females and subordinate males for average expression for female-biased and male biased genes. There was no difference between female sample groups or between dominant male sample groups (Wilcoxon test, all p>0.05 after Bonferroni adjustment for multiple comparisons) in any of the 100 sample combinations. In every case, subordinate male expression was significantly different than dominant male expression (Wilcoxon test, p<0.05 after Bonferroni adjustment for multiple comparisons). We also randomized our definition of female-bias for the renormalized dataset which corrects for any artefacts of differences in male-biased expression (Supplemental Fig. 2). As with the full dataset there was no difference in female-biased genes between female sample groups or between dominant male sample groups in any of the sample combinations (Wilcoxon test, all p>0.05 after Bonferroni adjustment for multiple comparisons), and subordinate male expression was significantly different to dominant male expression in all but one of the combinations (Wilcoxon test, p<0.05 in 99 out of 100 comparisons after Bonferroni adjustment for multiple comparisons). Additionally, increasing male-bias was largely due to a reduction in expression in females rather than an increase in male expression. Similarly, increasing female-bias was primarily due to reduced male expression rather than an increase in females (Fig. 2 and Supplemental Fig. 5).
We also randomized the spleen data in order to test whether the intermediate position of subordinate males was due to regression toward the mean. There was no evidence of regression toward the mean for male - or female-biased genes between dominant male sample groups in any of the sample combinations (Wilcoxon test, all p>0.05 after Bonferroni adjustment for multiple comparisons), and subordinate male expression was significantly different to dominant male expression in all combinations (Wilcoxon test, all p<0.05 after Bonferroni adjustment for multiple comparisons). Due to the limited number of samples it was only possible to iterate on dominant males and not on female samples.
Because the Z chromosome is thought to play an important role in male sexually selected traits [20], and because incomplete dosage compensation on the avian Z chromosome results in an average male-bias of Z chromosome expression [21], we assessed Z-linked loci separately. Z-linked male-biased genes in the gonad gave the same result as seen for male-biased autosomal genes, with the most male-biased quartile showing significantly lower expression in subordinate males (Fig. 2D). However the pattern overall was not exaggerated compared to the autosomes, as might be expected if the Z chromosome represented a hotspot for genes encoding male sexually selected traits. We also regressed the magnitude of difference in expression between dominant and subordinate males against male-biased expression for all autosomal and Z-linked male-biased genes separately. The slope of each regression was 0.32 (95% CI = 0.33-0.30) and 0.35 (95% CI = 0.42-0.29) for autosomal genes and Z-linked genes respectively. The overlapping confidence intervals suggest that there is no significant difference between the two slopes, and that the Z chromosome does not play a larger role than expected in encoding the differences between dominant and subordinate males.
Although this could potentially be due to the reduced gene number of the Z chromosome, it also may suggest that the Z chromosome does not play a disproportionately large role in encoding the male sexually selected traits that differ between dominant and subordinate males, but rather its effect is in proportion to its relative size. We did not assess female-biased Z-linked genes as the lack of dosage compensation in birds means there are very few genes that fit these criteria.
Our results show that as genes become more sex-biased, the difference between subordinate and dominant male average expression increases, with subordinate males expressing the most male-biased genes at a lower level and the most female-biased genes at a higher level than their dominant counterparts. The results from male-biased and female-biased genes are the first evidence that subordinate male turkeys show both demasculinization (expressing male-biased genes less) and feminization (expression female-biased genes more) in overall expression patterns that are remarkably concordant with their phenotypic status. This pattern is most evident in the gonad, where sexual dimorphism in transcription is the greatest. However, we observe a similar pattern, although to a lesser degree, in the spleen, suggesting that the concordance between phenotypic sexual dimorphism and transcriptional dimorphism extends to the soma as well.
Given the pattern of demasculinization, we might expect reduced correlation between dominant and subordinate expression for male-biased genes on the autosomes and Z chromosome compared to other types of genes, as the erosion of intersexual correlation is one way that conflict over optimal transcription can be resolved [22]. We therefore performed Spearman rank correlations on female, dominant and subordinate male average expression for autosomal unbiased, male-biased and female-biased, as well as Z-linked, genes expressed in the gonad (Fig. 4). Male-biased autosomal genes showed a lower correlation between dominant and subordinate males (ρ = 0.881) than for female-biased (ρ = 0.970) or unbiased genes (ρ = 0.964), and this was significant (Fisher r-to-z transformation male-biased v. female-biased p<0.0001 and male-biased v. unbiased p<0.0001). Also, for Z-linked and male-biased genes, there is greater correlation between subordinate male and female expression (ρ = 0.580 for Z-linked genes, and ρ = 0.761 for autosomal male-biased genes) than is found between dominant male and female expression (ρ = 0.430 for Z-linked and ρ = 0.629 for male-biased autosomal genes, Fisher r-to-z transformation male-biased p = 0.000, Z-linked p = 0.007, unbiased p = 0.018, female-biased p = 0.472). Both these results support our prediction that subordinate and dominant males are more divergent for those genes under the greatest male-specific selection, i.e. male-biased autosomal and Z-linked genes, with subordinate male expression showing evidence of demasculinization for male-biased autosomal and Z-linked genes (which are also largely male-biased). Interestingly, the correlation between either male form with females was roughly half for Z-linked genes compared to autosomes, and this may be in part due to incomplete dosage compensation in birds [21].
Fig. 4. Expression similarity across sexual forms. 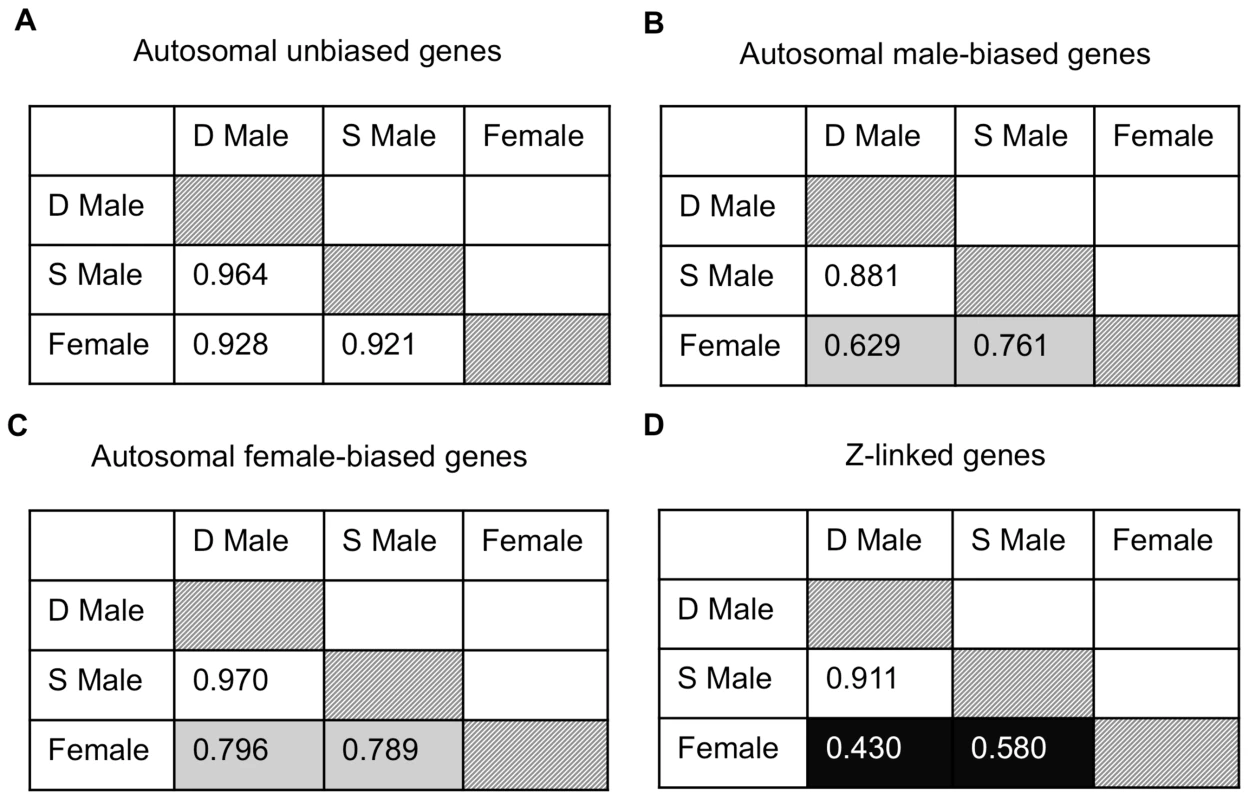
Spearman rank order (ρ) correlations for average expression for females, subordinate males and dominant males for autosomal unbiased (panel A), autosomal male-biased (panel B), autosomal female-biased (panel C), and Z-linked (panel D) genes. Correlation values are colour coded with lighter colours indicating greater correlation. Finally, we examined gene expression in the gonad within each phenotype. Of the 9,872 autosomal expressed genes, 8,918 (90.3%) were expressed to some degree in all three phenotypes, 252 were female-limited and 473 were male-limited (Fig. 5A). Of the latter, only 9 were limited to dominant males and the remainder were present in both male forms. Interestingly, females and subordinate males shared more than four times as many genes (188) as did females and dominant males (41) (Z-test p<0.00001). The same pattern was evident for Z-linked genes, although this was not statistically significant (Fig. 5B, Z-test p = 0.098). This suggests that although subordinate males share the greatest expression overlap with dominant males, they show greater similarity to females than do dominant males. There were no GO term enrichments for the genes shared between dominant males and females, and over-abundant GO terms for the genes shared between female and subordinate male turkeys and between dominant and subordinate male turkeys are listed in the supplemental materials (Supplemental Tables 2–3).
Fig. 5. Genes shared between morphs. 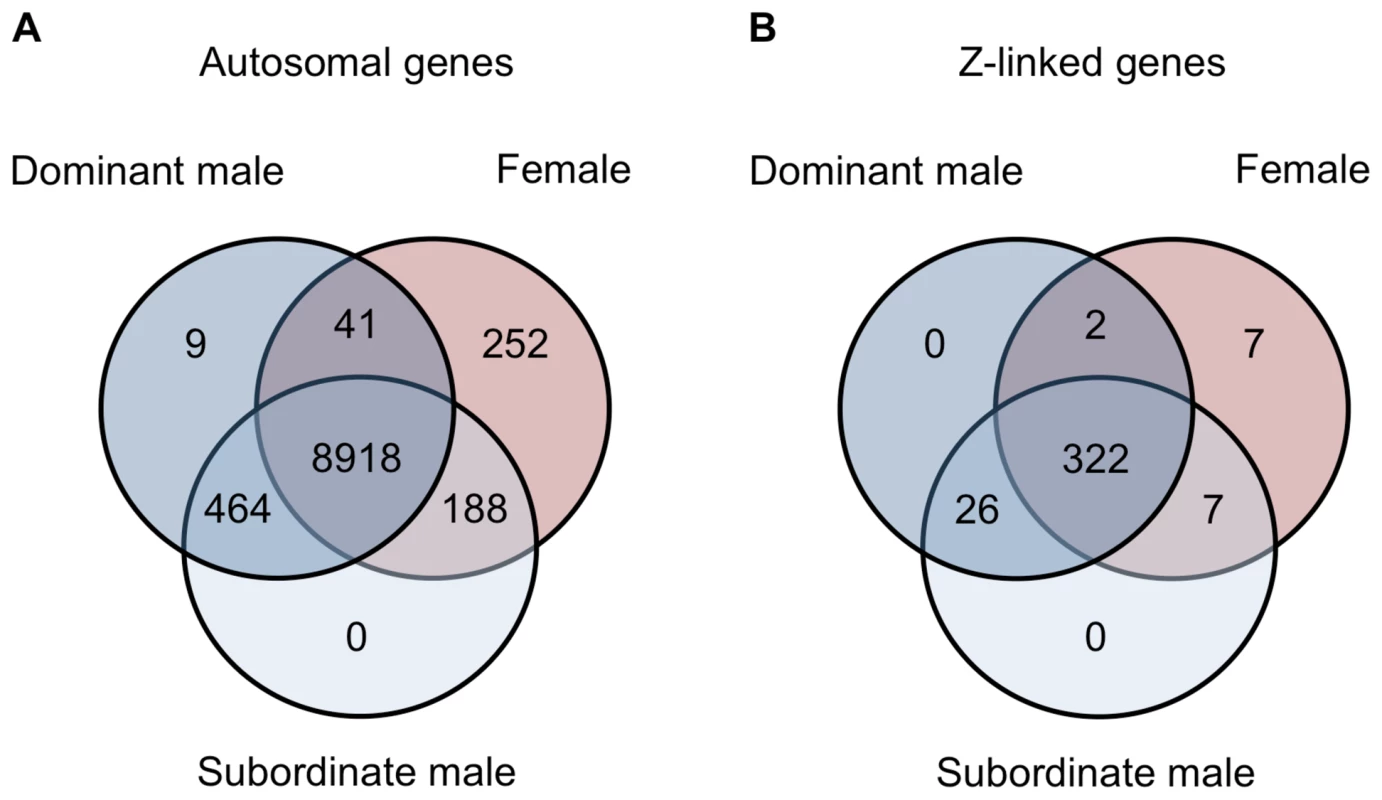
Venn diagrams for the number of autosomal (panel A) and Z-linked (panel B) genes expressed in females (red), subordinate males (light blue) and dominant males (dark blue). Although our results indicate that dominant and subordinate males differ subtly across the transcriptome, they also differ substantially for 21 genes (all autosomal) in the gonad that were significantly differentially expressed between subordinate and dominant males (fold change >2, adj. p-value<0.05, Supplemental Table 4), and there was no significant enrichment of Gene Ontology (GO) terms in this gene list. There were no genes that were statistically significant between male morphs in the spleen. The locus with the greatest expression bias toward subordinate males in the gonad, cytochrome P450, family 11, subfamily A, polypeptide 1 (CYP11A1), encodes a catalytic enzyme involved in the first and rate-limiting step in the steroid hormone biosynthesis pathway [23], [24]. Variation in CYP11A1 has been linked to serum testosterone levels [25], [26], and although this might suggest that testosterone is directly associated with the observed differences in subordinate and dominant male transcription, we found no association between male-bias and proximity to testosterone binding motifs, and the nearby presence of testosterone binding motifs does not explain the expression differences between subordinate and dominant males (Supplemental Tables 5–6). Although it has been suggested that the relative paucity of testosterone binding domains acts as a brake on the evolution of sexual dimorphism [27], our analysis suggests that sex-biased gene expression is controlled by a more complicated regulatory system.
Conclusions
Our analyses provide the first correlative support linking magnitude of sex-biased gene expression to the degree of phenotypic sexual dimorphism. Our data show a clear and strikingly direct concordance between relative expression of male sexually selected traits and transcriptional masculinization and feminization at multiple levels, in both the gonad and the soma. Synthesis and decay rates can differ for transcription and translation, which can break down the correlation between mRNA abundance and protein titer. However, in some studies, up to 70% of the variance in protein abundance is explained by mRNA levels [28]. Additionally, the broad, genome wide pattern we observe suggests that many of the differences in gene expression levels between male morphs will have functional consequences.
It is not clear whether this axis of dimorphism extends to systems with alternative male mating strategies, as observed in some fish species, where sneaker males and female mimics seek to steal fertilization events from dominant males. In these cases, males with alternative morphs likely divert effort from sexually selected somatic traits to reproductive function and sperm production [29], and so it is difficult to predict what we might expect in transcriptomic comparisons. However, our results suggest that evolutionary changes in the magnitude of sexual dimorphism, which affect a large number of species in many clades, may be achievable by changes in the magnitude of sex-biased transcription.
Materials and Methods
Two-year-old wild turkeys were obtained in the breeding season of their first reproductive year, after social dominance was established, from Vicvet Farms (Yorkshire, UK). Although the population is natural in that is has not been subject to selection for domestication traits, it is kept under controlled semi-natural conditions, allowing us to control for age, diet and many environmental influences that can potentially affect gene expression. All samples were collected under permission from institutional ethical review committees and in accordance with national guidelines. In each case, the telencephalon, spleen and left gonad were collected separately, homogenized and stored in RNAlater. RNA was prepared from the same volume of starting material with the Animal Tissue RNA Kit (Qiagen). Library and RNA-Sequence samples were prepared and barcoded by the Wellcome Trust Centre for Human Genetics, University of Oxford, using standard methods and sequenced on an Illumina HiSeq 2000 as paired-end 100 bp reads.
The resulting data was assessed for quality using FastQC (http://www.bioinformatics.bbsrc.ac.uk/projects/fastqc). Trimmomatic [30] was used to remove read pairs with residual adaptor sequence and conduct quality filtering. Reads were trimmed if the leading or trailing bases had a Phred score <4, and were also trimmed if a sliding window average Phred score over four bases was <15. Post filtering, reads where either pair was <25 bases in length were removed from subsequent analyses, leaving on average more than 26 million mappable paired-end reads per sample.
The genome of Meleagris gallopavo [31] version 2.01 (GCA_000146605.1), was obtained from Ensembl release 67 [32]. Filtered reads were mapped to the genome (excluding rRNA regions) using RSEM, version 1.1.20 [33], which leverages the short-read aligner bowtie, version 0.12.8 [34]. To remove non - and lowly-expressed genes, a minimum expression filter of four reads per million mappable reads was applied to the raw counts, as we have previously implemented for deep RNA-Seq datasets [35]–[36]. All genes expressed lower than this threshold in less than half the female, dominant male or subordinate male individuals were removed from further analysis to prevent our results being biased by the noise inherent in very lowly expressed genes. Fragments per kilobase per million mappable reads (FPKM), which corrects for variations in contig length and read depth between samples was calculated from these raw counts for each sample [37].
To explore the expression differences among the three sexual phenotypes in the gonad, we calculated average log2 expression for all females, dominant males and subordinate males for each gene, and tested for sex-bias in several ways using the R package, DESeq [38], which calculates differential expression in a pairwise fashion by negative binomial modelling and adjusts for multiple testing using the Benjamini-Hochberg method. For the gonad, we first tested for sex-bias by identifying significant expression differences (>2-fold difference, p<0.05) between females and dominant males. However, in order to verify that our results were not artefacts of how we defined sex-bias, and regression toward the mean, we also identified those genes with significant expression differences between females and subordinate males, and between females and all males. Due to the reduced level of transcriptional dimorphism in the soma, we reduced our fold-change thresholds considerably for the spleen (Supplemental Materials).
We performed hierarchical clustering using Euclidean distance with complete linkage, as implemented in Cluster 3.0 [39] and visualized in TreeView (v.1.1.6) [40]. Heat maps were separately constructed for male-biased, female-biased and unbiased autosomal genes and Z-linked genes. The reliabilities of the inferred trees were tested by bootstrap resampling (1000 replicates) using the R package, Pvclust [41].
We separated autosomal and Z-linked genes for two reasons. First, the sex chromosomes in birds show incomplete dosage compensation [21], therefore they exhibit an overall male-bias due to gene dose effects. Additionally, the unbalanced sex-specific selection acting on the sex chromosomes has been shown in chicken to masculinize Z chromosome expression [42]–[43]. These patterns mean that although the Z chromosome is interesting in its own right, it cannot be directly compared in terms of sex-bias to the autosomes. Therefore, sex-bias for autosomal genes was defined as those genes expressed two-fold higher in dominant males or females, with an adjusted p-value<0.05 (unpaired t-test, Benjamini-Hochberg correction for multiple comparisons [44]). Unbiased genes were all those not classified as either male - or female-biased. When average log2 expression values for quartiles based on sex-bias were calculated, the fold change criteria was dropped so as to include genes with a lower fold change than 2. This prevented restriction of the quartile analysis to solely the most sex-biased genes but allowed comparison to genes differentially expressed between the sexes but sex-biased to a lesser degree.
GO term enrichment analysis was performed by taking mouse Ensembl gene IDs for those genes with a 1∶1 mouse ortholog, identified via Biomart. The target list (i.e. 21 significantly differentially expressed genes between dominant and subordinate males, or genes shared between two morphs) were compared to a background list (either all expressed autosomal genes or all expressed genes) using Gorilla [45]–[46]. P-values were calculated using a hypergeometric model and corrected for multiple testing.
In order to investigate dN and dS, the turkey genome was compared to the genomes of chicken (Gallus gallus) and zebra finch (Taeniopygia guttata), obtaining 16,496, 22,194 and 18,204 peptides and corresponding cDNA sequence for each species respectively from Ensembl. Proteinortho [47], with default parameters, was used to identify single copy orthologs held in all three species. These 7,854 orthologous groups were aligned with PRANK using a guide tree obtained from Superfamily 1.75 [48]. This orthologous set was filtered with Repeatmasker (http://www.repeatmasker.org) to remove seven retrotransposons and perl scripts were used to remove two genes with in frame stop codons and 13 genes with less than 100 bp in aligned gapless length. PAML, version 4.4b [49], was used to analyse the remaining 6,839 one-to-one orthologs, utilizing the phylogeny used for PRANK above. Alignments where dS>2 were removed as this represents the point of mutational saturation in avian sequence data [50]. For those alignments that passed filtering, the number of potential nonsynonymous substitutions (NdN), the number of nonsynonymous substitutions (N), the number of potential synonymous substitutions (SdS) and the number of synonymous substitutions (S) were extracted for each orthologous group for the turkey-specific branch of the three-species phylogeny. These values were summed for each expression category in order to calculate average dN and dS for male-biased, female-biased and unbiased genes. This has the advantage of simultaneously avoiding the problem of infinitely high dN/dS values for genes lacking synonymous substitutions while weighting the data by alignment length [9].
The location of androgen transcription factor binding sites (tfbs) in the turkey genome were predicted using amniote androgen tfbs motifs [51]. The predicted tfbs locations were then compared to the start sites of all turkey genes in 2 kb, 5 kb and 10 kb upstream windows and matching hits recorded.
Supporting Information
Zdroje
1. MankJE (2009) Sex chromosomes and the evolution of sexual dimorphism: Lessons from the genome. Am Nat 173(2): 141–150.
2. EllegrenH, ParschJ (2007) The evolution of sex-biased genes and sex-biased gene expression. Nat Rev Genet 8(9): 689–698.
3. CutterAD, WardS (2005) Sexual and temporal dynamics of molecular evolution in C. elegans development. Mol Biol Evol 22 : 178–188.
4. MankJE, Hultin-RosenbergL, AxelssonE, EllegrenH (2007) Rapid evolution of female-biased, but not male-biased, genes expressed in the avian brain. Mol Biol Evol 24(12): 2698–2706.
5. RanzJM, Castillo-DavisCI, MeiklejohnCD, HartlDL (1997) Sex-dependent gene expression and the evolution of the Drosophila transcriptome. Science 300 : 1742–1745.
6. ReiniusB, SaetreP, LeonardJA, BlekhmanR, Merino-MartinezR, et al. (2008) An evolutionary conserved sexual signature in the primate brain. PLoS Genet 4(6): e1000100.
7. YangX, SchadtEE, WangS, WangH, ArnoldAP, et al. (2006) Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res 16(8): 995–1004.
8. MagnussonK, MendesAM, WindbichlerN, PapathanosP-A, NolanT, et al. (2011) Transcription regulation of sex-biased genes during ontogeny in the malaria vector Anopheles gambiae. PLoS One 6(6): e21572.
9. MankJE, NamK, BrunströmB, EllegrenH (2010) Ontogenetic complexity of sexual dimorphism and sex-specific selection. Mol Biol Evol 27(7): 1570–1578.
10. OmettoL, ShoemakerD, RossKG, KellerL (2011) Evolution of gene expression in fire ants: the effects of developmental stage, caste, and species. Mol Biol Evol 28(4): 1381–1392.
11. ZhangY, SturgillD, ParisiM, KumarS, OliverB (2007) Constraint and turnover in sex-biased gene expression in the genus Drosophila. Nature 450 : 233–238.
12. BuchholzR (1995) Female choice, parasite load and male ornamentation in Wild Turkeys. Anim Behav 50 : 929–943.
13. BuchholzR (1997) Male dominance and variation in fleshy head ornamentation in Wild Turkeys. J Avian Biol 28 : 223–230.
14. HillG, DoucetS, BuchholzR (1997) The effect of coccidial infection on iridescent plumage coloration in wild turkeys. Anim Behav 69 : 387–394.
15. WattsCR, StokesAW (1971) The social order of turkeys. Science 224 : 112–118.
16. KrakauerAH (2005) Kin selection and cooperative courtship in wild turkeys. Nature 434 : 69–72.
17. KrakauerAH (2008) Sexual selection and the genetic mating system of Wild Turkeys. Condor 110(1): 1–12.
18. HamiltonWD (1964) The genetical evolution of social behaviour. I J Theor Biol 7(1): 1–16.
19. MankJE, Hultin-RosenbergL, WebsterMT, EllegrenH (2008) The unique genomic properties of sex-biased genes: Insights from avian microarray data. BMC Genomics 9 : 148.
20. KirkpatrickM, HallDW (2004) Male-biased mutation, sex linkage, and the rate of adaptive evolution. Evolution 58(2): 437–440.
21. ItohY, ReplogleK, KimYH, WadeJ, ClaytonDF, et al. (2010) Sex bias and dosage compensation in the zebra finch versus chicken genomes: general and specialized patterns among birds. Genome Res 20(4): 512–518.
22. ConnallonT, KnowlesLL (2005) Intergenomic conflict revealed by patterns of sex-biased gene expression. Trends Genet 21(9): 495–499.
23. StoneD, HechterO (1055) Studies on ACTH action in perfused bovine adrenals: aspects of progesterone as an intermediary in corticosteroidogenesis. Arch Biochem Biophys 54 : 121.
24. HalkerstonIDK, EichhornJ, HechterOA (1961) Requirement for reduced triphosphopyridine nucleotide for cholesterol side-chain cleavage by mitochondrial fractions of bovine adrenal cortex. J Biol Chem 236 : 374.
25. GharaniN, WaterworthDM, BattyS, WhiteD, Gilling-SmithC, et al. (1997) Association of the steroid synthesis gene CYP11a with polycystic ovary syndrome and hyperandrogenism. Hum Mol Genet 6 : 397–402.
26. KatsumataN, OhtakeM, HojoT, OgawaE, HaraT, et al. (2002) Compound heterozygous mutations in the cholesterol side-chain cleavage enxyme gene (CYP11A) cause congential adrenal insufficiency in humans. J Clin Endocrinol Metab 87 : 3808–3813.
27. StewartAD, PischeddaA, RiceWR (2010) Resolving intralocus sexual conflict: genetic chanisms and time frame. J Heredity 101: S94–S99.
28. LuP, VogelC, WangR, YaoX, MarcotteEM (2007) Absolute protein expression profiling estimates the relative contribution of transcriptional and translational regulation. Nature Biotechnology 25 : 117–124.
29. MankJE, AviseJC (2006) Comparative phylogenetic analysis of male alternative reproductive tactics. Evolution 60 : 1311–1316.
30. LohseM, BolgerAM, NagelA, FernieAR, LunnJE, et al. (2012) RobiNA: a user-friendly, integrated software solution for RNA-Seq-based transcriptomics. Nucleic Acids Res 40 : 622–627.
31. DalloulRA, LongJA, ZiminAV, AslamL, BealK, et al. (2012) Multi-Platform Next-Generation Sequencing of the Domestic Turkey Meleagris gallopavo: Genome Assembly and Analysis. PLoS Biol 8(9): e1000475.
32. FlicekP, AmodeMR, BarrellD, BealK, BrentS, et al. (2012) Ensembl 2012. Nucleic Acids Res 40(D1): D84–D90.
33. LiB, DeweyC (2011) RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12(1): 323.
34. LangmeadB, TrapnellC, PopM, SalzbergS (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Gen Biol 10(3): R25.
35. HarrisonPW, MankJE, WedellN (2012) Incomplete sex chromosome dosage compensation in the Indian meal moth Plodia interpunctella, based on de novo transcriptome assembly. Genome Biol Evol 4 : 1118–1126.
36. MoghadamHK, HarrisonPW, ZacharG, SzekelyT, MankJE (2013) The plover neurotranscriptome assembly: Transcriptomic analysis in an ecological model species without a reference genome. Molecular Ecology Resources 3 : 696–705.
37. MortazaviA, WilliamsBA, McCueK, SchaefferL, WoldB (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Meth 5 : 621–628.
38. AndersS, HuberW (2010) Differential expression analysis for sequence count data. Genome Biol 11: R106.
39. de HoonMJ, ImotoS, NolanJ, MiyanoS (2004) Open source clustering software. Bioinformatics 20 : 1453–1454.
40. SaldanhaAJ (2004) Java Treeview - extensible visualization of microarray data. Bioinformatics 20 : 3246–3248.
41. SuzukiR, ShimodairaH (2006) Pvclust: An R package for assessing the uncertainty in hierarchical clustering. Bioinformatics 22 : 1540–1542.
42. WrightAE, MoghadamHK, MankJE (2012) Trade-off between selection for dosage compensation and masculinisation on the avian Z chromosome. Genetics 192 : 1433–1445.
43. MankJE, EllegrenH (2009) Sex-linkage of sexually antagonistic genes is predicted by female, but not male, effects in birds. Evolution 63(6): 1464–1472.
44. BenjaminiY, HochbergY (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc B 57(1): 289–300.
45. EdenE, NavonR, SteinfeldI, LipsonD, YakhiniZ (2009) GOrilla: A tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC Bioinformatics 10 : 48.
46. EdenE, LipsonD, YogevS, YakhiniZ (2007) Discovering motifs in ranked lists of DNA sequences. PLoS Computational Biol 3(3): e39.
47. LechnerM, FindeiβS, SteinerL, MarzM, StadlerPF, et al. (2011) Proteinortho: Detection of (Co-)orthologs in large-scale analysis. BMC Bioinformatics 12 : 124.
48. GoughJ, KarplusK, HughevR, ChothiaC (2001) Assignment of homology to genome sequences using a library of hidden markov models that represent all proteins of known structure. J Mol Biol 313(4): 903–919.
49. YangZ (2007) PAML 4: Phylogenetic analysis by maximum likelihood. Mol Biol Evol 24 : 1586–1591.
50. AxelssonE, Hultin-RosenbergL, BrandströmM, ZwahlénM, ClaytonDF, et al. (2008) Natural selection in avian protein-coding genes expressed in brain. Mol Ecol 17(12): 3008–3017.
51. SubramanianA, TamayoP, MoothaVK, MukherjeeS, EbertBL, et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 102(48): 15545–15550.
Štítky
Genetika Reprodukční medicína
Článek Re-Ranking Sequencing Variants in the Post-GWAS Era for Accurate Causal Variant IdentificationČlánek Bypass of 8-oxodGČlánek Integrated Model of and Inherited Genetic Variants Yields Greater Power to Identify Risk GenesČlánek Comparative Genomic and Functional Analysis of 100 Strains and Their Comparison with Strain GGČlánek A Nuclear Calcium-Sensing Pathway Is Critical for Gene Regulation and Salt Stress Tolerance inČlánek Computational Identification of Diverse Mechanisms Underlying Transcription Factor-DNA OccupancyČlánek Reversible and Rapid Transfer-RNA Deactivation as a Mechanism of Translational Repression in StressČlánek Genome-Wide Association of Body Fat Distribution in African Ancestry Populations Suggests New Loci
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 8- Příjem alkoholu a menstruační cyklus
- Mateřský haplotyp KIR ovlivňuje porodnost živých dětí po transferu dvou embryí v rámci fertilizace in vitro u pacientek s opakujícími se samovolnými potraty nebo poruchami implantace
- Intrauterinní inseminace a její úspěšnost
- Růst a vývoj dětí narozených pomocí IVF
- Akutní intermitentní porfyrie
-
Všechny články tohoto čísla
- Genome-Wide DNA Methylation Analysis of Systemic Lupus Erythematosus Reveals Persistent Hypomethylation of Interferon Genes and Compositional Changes to CD4+ T-cell Populations
- Re-Ranking Sequencing Variants in the Post-GWAS Era for Accurate Causal Variant Identification
- Histone Variant HTZ1 Shows Extensive Epistasis with, but Does Not Increase Robustness to, New Mutations
- Past Visits Present: TCF/LEFs Partner with ATFs for β-Catenin–Independent Activity
- Functional Characterisation of Alpha-Galactosidase A Mutations as a Basis for a New Classification System in Fabry Disease
- A Flexible Approach for the Analysis of Rare Variants Allowing for a Mixture of Effects on Binary or Quantitative Traits
- Masculinization of Gene Expression Is Associated with Exaggeration of Male Sexual Dimorphism
- Genic Intolerance to Functional Variation and the Interpretation of Personal Genomes
- Endogenous Stress Caused by Faulty Oxidation Reactions Fosters Evolution of 2,4-Dinitrotoluene-Degrading Bacteria
- Transposon Domestication versus Mutualism in Ciliate Genome Rearrangements
- Comparative Anatomy of Chromosomal Domains with Imprinted and Non-Imprinted Allele-Specific DNA Methylation
- An Essential Function for the ATR-Activation-Domain (AAD) of TopBP1 in Mouse Development and Cellular Senescence
- Depletion of Retinoic Acid Receptors Initiates a Novel Positive Feedback Mechanism that Promotes Teratogenic Increases in Retinoic Acid
- Bypass of 8-oxodG
- Calpain-6 Deficiency Promotes Skeletal Muscle Development and Regeneration
- ATM Release at Resected Double-Strand Breaks Provides Heterochromatin Reconstitution to Facilitate Homologous Recombination
- Generation of Tandem Direct Duplications by Reversed-Ends Transposition of Maize Elements
- Loss of a Conserved tRNA Anticodon Modification Perturbs Cellular Signaling
- Integrated Model of and Inherited Genetic Variants Yields Greater Power to Identify Risk Genes
- High-Throughput Genetic and Gene Expression Analysis of the RNAPII-CTD Reveals Unexpected Connections to SRB10/CDK8
- Dynamic Rewiring of the Retinal Determination Network Switches Its Function from Selector to Differentiation
- β-Catenin-Independent Activation of TCF1/LEF1 in Human Hematopoietic Tumor Cells through Interaction with ATF2 Transcription Factors
- Genetic Mapping of Specific Interactions between Mosquitoes and Dengue Viruses
- A Highly Redundant Gene Network Controls Assembly of the Outer Spore Wall in
- Origin and Functional Diversification of an Amphibian Defense Peptide Arsenal
- Myc-Driven Overgrowth Requires Unfolded Protein Response-Mediated Induction of Autophagy and Antioxidant Responses in
- Integrative Modeling of eQTLs and Cis-Regulatory Elements Suggests Mechanisms Underlying Cell Type Specificity of eQTLs
- Species and Population Level Molecular Profiling Reveals Cryptic Recombination and Emergent Asymmetry in the Dimorphic Mating Locus of
- Ras-Induced Changes in H3K27me3 Occur after Those in Transcriptional Activity
- Characterization of the p53 Cistrome – DNA Binding Cooperativity Dissects p53's Tumor Suppressor Functions
- Global Analysis of Fission Yeast Mating Genes Reveals New Autophagy Factors
- Deficiency Suppresses Intestinal Tumorigenesis
- Introns Regulate Gene Expression in in a Pab2p Dependent Pathway
- Meiotic Recombination Initiation in and around Retrotransposable Elements in
- Comparative Oncogenomic Analysis of Copy Number Alterations in Human and Zebrafish Tumors Enables Cancer Driver Discovery
- Comparative Genomic and Functional Analysis of 100 Strains and Their Comparison with Strain GG
- A Model-Based Analysis of GC-Biased Gene Conversion in the Human and Chimpanzee Genomes
- Masculinization of the X Chromosome in the Pea Aphid
- The Architecture of a Prototypical Bacterial Signaling Circuit Enables a Single Point Mutation to Confer Novel Network Properties
- Distinct SUMO Ligases Cooperate with Esc2 and Slx5 to Suppress Duplication-Mediated Genome Rearrangements
- The Yeast Environmental Stress Response Regulates Mutagenesis Induced by Proteotoxic Stress
- Mediator Directs Co-transcriptional Heterochromatin Assembly by RNA Interference-Dependent and -Independent Pathways
- The Genome of and the Basis of Host-Microsporidian Interactions
- Regulation of Sister Chromosome Cohesion by the Replication Fork Tracking Protein SeqA
- Neuronal Reprograming of Protein Homeostasis by Calcium-Dependent Regulation of the Heat Shock Response
- A Nuclear Calcium-Sensing Pathway Is Critical for Gene Regulation and Salt Stress Tolerance in
- Cross-Species Array Comparative Genomic Hybridization Identifies Novel Oncogenic Events in Zebrafish and Human Embryonal Rhabdomyosarcoma
- : A Mouse Strain with an Ift140 Mutation That Results in a Skeletal Ciliopathy Modelling Jeune Syndrome
- The Relative Contribution of Proximal 5′ Flanking Sequence and Microsatellite Variation on Brain Vasopressin 1a Receptor () Gene Expression and Behavior
- Combining Quantitative Genetic Footprinting and Trait Enrichment Analysis to Identify Fitness Determinants of a Bacterial Pathogen
- The Innocence Project at Twenty: An Interview with Barry Scheck
- Computational Identification of Diverse Mechanisms Underlying Transcription Factor-DNA Occupancy
- GUESS-ing Polygenic Associations with Multiple Phenotypes Using a GPU-Based Evolutionary Stochastic Search Algorithm
- H2A.Z Acidic Patch Couples Chromatin Dynamics to Regulation of Gene Expression Programs during ESC Differentiation
- Identification of DSB-1, a Protein Required for Initiation of Meiotic Recombination in , Illuminates a Crossover Assurance Checkpoint
- Binding of TFIIIC to SINE Elements Controls the Relocation of Activity-Dependent Neuronal Genes to Transcription Factories
- Global Analysis of the Sporulation Pathway of
- Genetic Circuits that Govern Bisexual and Unisexual Reproduction in
- Deletion of microRNA-80 Activates Dietary Restriction to Extend Healthspan and Lifespan
- Fifty Years On: GWAS Confirms the Role of a Rare Variant in Lung Disease
- The Enhancer Landscape during Early Neocortical Development Reveals Patterns of Dense Regulation and Co-option
- Gene Expression Regulation by Upstream Open Reading Frames and Human Disease
- Sociogenomics of Cooperation and Conflict during Colony Founding in the Fire Ant
- The Intronic Long Noncoding RNA Recruits PRC2 to the Promoter, Reducing the Expression of and Increasing Cell Proliferation
- The , p.E318G Variant Increases the Risk of Alzheimer's Disease in -ε4 Carriers
- The Wilms Tumor Gene, , Is Critical for Mouse Spermatogenesis via Regulation of Sertoli Cell Polarity and Is Associated with Non-Obstructive Azoospermia in Humans
- Reversible and Rapid Transfer-RNA Deactivation as a Mechanism of Translational Repression in Stress
- QTL Analysis of High Thermotolerance with Superior and Downgraded Parental Yeast Strains Reveals New Minor QTLs and Converges on Novel Causative Alleles Involved in RNA Processing
- Genome Wide Association Identifies Novel Loci Involved in Fungal Communication
- Chromatin Sampling—An Emerging Perspective on Targeting Polycomb Repressor Proteins
- A Recessive Founder Mutation in Regulator of Telomere Elongation Helicase 1, , Underlies Severe Immunodeficiency and Features of Hoyeraal Hreidarsson Syndrome
- Genome-Wide Association of Body Fat Distribution in African Ancestry Populations Suggests New Loci
- Causal and Synthetic Associations of Variants in the Gene Cluster with Alpha1-antitrypsin Serum Levels
- Hard Selective Sweep and Ectopic Gene Conversion in a Gene Cluster Affording Environmental Adaptation
- Brittle Culm1, a COBRA-Like Protein, Functions in Cellulose Assembly through Binding Cellulose Microfibrils
- Chromosomal Copy Number Variation, Selection and Uneven Rates of Recombination Reveal Cryptic Genome Diversity Linked to Pathogenicity
- The Ribosomal Protein Rpl22 Controls Ribosome Composition by Directly Repressing Expression of Its Own Paralog, Rpl22l1
- Ras1 Acts through Duplicated Cdc42 and Rac Proteins to Regulate Morphogenesis and Pathogenesis in the Human Fungal Pathogen
- The DSB-2 Protein Reveals a Regulatory Network that Controls Competence for Meiotic DSB Formation and Promotes Crossover Assurance
- Recurrent Modification of a Conserved -Regulatory Element Underlies Fruit Fly Pigmentation Diversity
- Associations of Mitochondrial Haplogroups B4 and E with Biliary Atresia and Differential Susceptibility to Hydrophobic Bile Acid
- The Conditional Nature of Genetic Interactions: The Consequences of Wild-Type Backgrounds on Mutational Interactions in a Genome-Wide Modifier Screen
- A Critical Function of Mad2l2 in Primordial Germ Cell Development of Mice
- A Role for CF1A 3′ End Processing Complex in Promoter-Associated Transcription
- Vitellogenin Underwent Subfunctionalization to Acquire Caste and Behavioral Specific Expression in the Harvester Ant
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Chromosomal Copy Number Variation, Selection and Uneven Rates of Recombination Reveal Cryptic Genome Diversity Linked to Pathogenicity
- Genome-Wide DNA Methylation Analysis of Systemic Lupus Erythematosus Reveals Persistent Hypomethylation of Interferon Genes and Compositional Changes to CD4+ T-cell Populations
- Associations of Mitochondrial Haplogroups B4 and E with Biliary Atresia and Differential Susceptibility to Hydrophobic Bile Acid
- A Role for CF1A 3′ End Processing Complex in Promoter-Associated Transcription
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Revma Focus: Spondyloartritidy
nový kurz
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



