-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe , p.E318G Variant Increases the Risk of Alzheimer's Disease in -ε4 Carriers
The primary constituents of plaques (Aβ42/Aβ40) and neurofibrillary tangles (tau and phosphorylated forms of tau [ptau]) are the current leading diagnostic and prognostic cerebrospinal fluid (CSF) biomarkers for AD. In this study, we performed deep sequencing of APP, PSEN1, PSEN2, GRN, APOE and MAPT genes in individuals with extreme CSF Aβ42, tau, or ptau levels. One known pathogenic mutation (PSEN1 p.A426P), four high-risk variants for AD (APOE p.L46P, MAPT p.A152T, PSEN2 p.R62H and p.R71W) and nine novel variants were identified. Surprisingly, a coding variant in PSEN1, p.E318G (rs17125721-G) exhibited a significant association with high CSF tau (p = 9.2×10−4) and ptau (p = 1.8×10−3) levels. The association of the p.E318G variant with Aβ deposition was observed in APOE-ε4 allele carriers. Furthermore, we found that in a large case-control series (n = 5,161) individuals who are APOE-ε4 carriers and carry the p.E318G variant are at a risk of developing AD (OR = 10.7, 95% CI = 4.7–24.6) that is similar to APOE-ε4 homozygous (OR = 9.9, 95% CI = 7.2.9–13.6), and double the risk for APOE-ε4 carriers that do not carry p.E318G (OR = 3.9, 95% CI = 3.4–4.4). The p.E318G variant is present in 5.3% (n = 30) of the families from a large clinical series of LOAD families (n = 565) and exhibited a higher frequency in familial LOAD (MAF = 2.5%) than in sporadic LOAD (MAF = 1.6%) (p = 0.02). Additionally, we found that in the presence of at least one APOE-ε4 allele, p.E318G is associated with more Aβ plaques and faster cognitive decline. We demonstrate that the effect of PSEN1, p.E318G on AD susceptibility is largely dependent on an interaction with APOE-ε4 and mediated by an increased burden of Aβ deposition.
Published in the journal: . PLoS Genet 9(8): e32767. doi:10.1371/journal.pgen.1003685
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003685Summary
The primary constituents of plaques (Aβ42/Aβ40) and neurofibrillary tangles (tau and phosphorylated forms of tau [ptau]) are the current leading diagnostic and prognostic cerebrospinal fluid (CSF) biomarkers for AD. In this study, we performed deep sequencing of APP, PSEN1, PSEN2, GRN, APOE and MAPT genes in individuals with extreme CSF Aβ42, tau, or ptau levels. One known pathogenic mutation (PSEN1 p.A426P), four high-risk variants for AD (APOE p.L46P, MAPT p.A152T, PSEN2 p.R62H and p.R71W) and nine novel variants were identified. Surprisingly, a coding variant in PSEN1, p.E318G (rs17125721-G) exhibited a significant association with high CSF tau (p = 9.2×10−4) and ptau (p = 1.8×10−3) levels. The association of the p.E318G variant with Aβ deposition was observed in APOE-ε4 allele carriers. Furthermore, we found that in a large case-control series (n = 5,161) individuals who are APOE-ε4 carriers and carry the p.E318G variant are at a risk of developing AD (OR = 10.7, 95% CI = 4.7–24.6) that is similar to APOE-ε4 homozygous (OR = 9.9, 95% CI = 7.2.9–13.6), and double the risk for APOE-ε4 carriers that do not carry p.E318G (OR = 3.9, 95% CI = 3.4–4.4). The p.E318G variant is present in 5.3% (n = 30) of the families from a large clinical series of LOAD families (n = 565) and exhibited a higher frequency in familial LOAD (MAF = 2.5%) than in sporadic LOAD (MAF = 1.6%) (p = 0.02). Additionally, we found that in the presence of at least one APOE-ε4 allele, p.E318G is associated with more Aβ plaques and faster cognitive decline. We demonstrate that the effect of PSEN1, p.E318G on AD susceptibility is largely dependent on an interaction with APOE-ε4 and mediated by an increased burden of Aβ deposition.
Introduction
Dementias are complex, polygenic and genetically heterogeneous disorders [1]. The most common form of dementia is Alzheimer's disease (AD), which affects more than 5.3 million people in the US [2]. Late-onset AD (LOAD) is the most common form of dementia. However, the current model of AD pathogenesis is based on the genetic findings in rare and phenotypically extreme AD cases [3]. LOAD heritability varies from 58% to 79% [4] and, despite the tremendous progress in AD genetics in the last twenty years, the total proportion of phenotypic variance explained by all the combined variants (including APOE genotype and genome wide association studies [GWAS] signals) is estimated to be 23% [5], which suggests a large proportion of the heritability of AD still remains unexplained. Three important factors may account for the missing heritability in AD; first, the clinical heterogeneity of AD remains a significant confounding variable in case-control studies [6], second, much of the unexplained variance of complex phenotypes may be attributed to low frequency or rare alleles [7] and third, gene by gene or gene by environment interactions [8]. Quantitative intermediate phenotypes have helped to overcome some of these obstacles in complex diseases [9], [10]. Endophenotype-oriented approaches have greater statistical power, less clinical heterogeneity and offer important insights into the mechanisms by which genetic variants modulate the disease phenotype [6], [9], .
The primary constituents of plaques (Aβ42/Aβ40) and neurofibrillary tangles (tau and phosphorylated forms of tau [ptau]) are the current leading diagnostic and prognostic cerebrospinal fluid (CSF) biomarkers for AD [12]. Recently, it was shown that CSF biomarker abnormalities typically precede clinical AD symptoms by decades and reflect the timing and magnitude of pathophysiological changes [13]. These findings suggest that a better understanding of the genetic contribution to the variance in these CSF biomarkers can provide important information about susceptibility to AD. In fact, the two most important known risk factors for AD, APOE genotype and age account for 13% and 14% of the variance in CSF Aβ42 and tau levels, respectively [14]. Likewise, pathogenic mutations in the most important causal genes for familial AD, amyloid-beta precursor protein (APP), and presenilin 1 and 2 (PSEN1, PSEN2) alter CSF Aβ42 levels [13], [15], [16]. Additionally, some genetic variants initially discovered by their association with CSF biomarkers have recently been proven to be modifiers of risk, age at onset (AAO) or rate of AD progression [17], [18], [19]. Likewise, it was recently described that carriers of PSEN1 mutations exhibit very low CSF Aβ42, and high tau or ptau levels [13], [20], [21], [22]. Similar CSF biomarker level profiles have been described in sporadic AD cases [23]. However, the genetic variants responsible for CSF changes in sporadic AD have not been found yet. Together, these results suggest that CSF biomarker levels as quantitative traits are useful tools in uncovering genetic variants that are closely related to the physiopathological mechanisms underlying AD.
Rare or low frequency coding and non-coding variants have been predicted to be enriched in functional alleles and to exhibit strong effect size [7], [10]. Recently, a rare (minor allele frequency [MAF] = 0.02) coding variant in TREM2 gene p.P47H was found to confer a high risk for AD (Odd ratios from 2 to 5) [24], [25], [26]. Two recent studies analyzed the association of genetic variants of APP, PSEN1, PSEN2, MAPT, and GRN on risk for AD [27], [28]. One study was focused on common variants in sporadic AD [27] while the other focused on the identification of very rare coding variants in familial LOAD [28]. However, the impact of low-frequency coding variants of APP, PSEN1, PSEN2, GRN and MAPT on sporadic LOAD has not been well studied. Identification of low frequency variants associated with disease remains challenging because standard case-controls design requires very large sample sizes. To overcome this problem we have used quantitative phenotypes. Previously, we identified a pathogenic mutation in a family with LOAD within the PSEN1 gene by selecting the top and bottom 5% from the distributions of Aβ40, Aβ42, and Aβ42/40 ratio [29] In the present study, we sequenced individuals with extremes levels of CSF-based biomarkers in order to identify variants in APOE, APP, PSEN1, PSEN2, GRN and MAPT genes associated with the CSF biomarker levels. This approach allowed us to identify known pathogenic variants, AD risk factors and identify a low frequency variant that increases risk for AD in a gene-gene interaction mode.
Results
Rare variants found by targeted-pooled-DNA and Next Generation sequencing
We hypothesized that the coding variants found in individuals at the extremes of the phenotypic distribution of CSF biomarker levels are more likely to have a functional impact on CSF biomarker levels. In order to identify rare or low frequency variants that affect the CSF levels of Aβ42, tau and ptau levels, we used a two-stage extreme phenotype sequencing design (Figure S1). A 10-fold difference between the lowest and highest raw values in Aβ42, tau and ptau CSF levels in each series was found among individuals in these studies. The individuals were selected regardless of their clinical status (based on the clinical dementia rating [CDR]) (Table 1). We combined both series (WU-ADRC [n = 475] and ADNI [n = 259]) by normalizing the CSF Aβ42, tau and ptau levels and adjusting for covariates [17], [18]. We selected 212 individuals from the top and bottom 15% for each phenotype (Table 1). The 212 samples were divided in two pools (Pool 1 and 2, respectively); targeted and pooled-sample sequencing was performed. All the validated variants were genotyped in the total CSF sample and tested for association with each CSF biomarker. Linear regression (assuming an additive genetic effect) was utilized for each variant by adjusting for significant covariates (age, gender, CDR and site [WU-ADRC or ADNI]) (Table S1 in Text S1) [17], [18].
Tab. 1. Summary of sample characteristics. 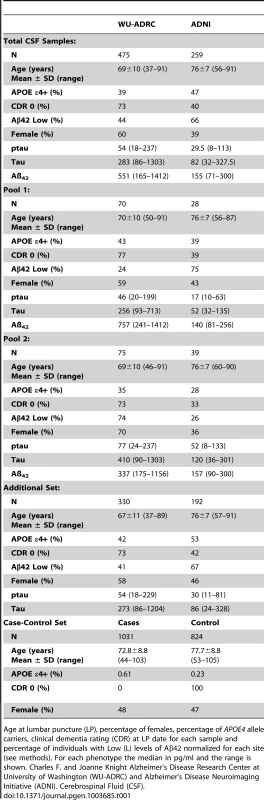
Age at lumbar puncture (LP), percentage of females, percentage of APOE4 allele carriers, clinical dementia rating (CDR) at LP date for each sample and percentage of individuals with Low (L) levels of Aβ42 normalized for each site (see methods). For each phenotype the median in pg/ml and the range is shown. Charles F. and Joanne Knight Alzheimer's Disease Research Center at University of Washington (WU-ADRC) and Alzheimer's Disease Neuroimaging Initiative (ADNI). Cerebrospinal Fluid (CSF). A greater than 30-fold coverage per allele at all positions within the 62 amplicons designed to cover the protein coding regions of the APP, APOE, PSEN1, PSEN2, MAPT and GRN were obtained (Table S2 in Text S1). After adjusting for the sensitivity and specificity parameters of the base-calling algorithm (SPLINTER) using negative and positive controls, a total of 396 and 369 variants were called and perfectly annotated in the targeted genomic regions of Pool 1 and 2, respectively. 73% of these variants were intronic, 8% were missense, 5% were coding-synonymous, 1% were at splicing sites, 12% were located at the untranslated regions (UTR) and 2% were called to be near-gene (Table S2b in Text S1) We focused on missense and splicing-affecting variants with a predicted minor allele frequency (MAF) below 5% (by SPLINTER) in each pool.
A total of 27 rare or low frequency non-synonymous variants were validated by direct genotyping in the discovery samples (both pools). 33% of these variants identified (9/27) were novel. Seven of nine (77%) are located in highly conserved nucleotide (GERP>4) and 88% (8/9) are predicted to be damaging for the respective protein (SIFT and polyphen2 algorithms) [30]. As expected, 48% of these variants are singletons (9/27) or doubletons (4/27) (Table 2).
Tab. 2. Summary of the rare variants found in the extreme values of CSF biomarker levels. 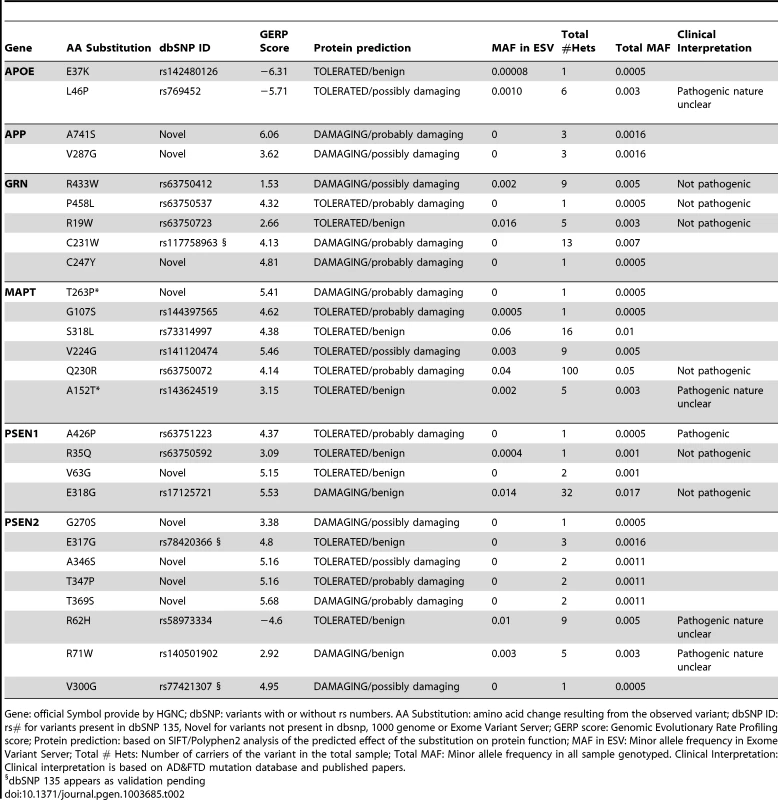
Gene: official Symbol provide by HGNC; dbSNP: variants with or without rs numbers. AA Substitution: amino acid change resulting from the observed variant; dbSNP ID: rs# for variants present in dbSNP 135, Novel for variants not present in dbsnp, 1000 genome or Exome Variant Server; GERP score: Genomic Evolutionary Rate Profiling score; Protein prediction: based on SIFT/Polyphen2 analysis of the predicted effect of the substitution on protein function; MAF in ESV: Minor allele frequency in Exome Variant Server; Total # Hets: Number of carriers of the variant in the total sample; Total MAF: Minor allele frequency in all sample genotyped. Clinical Interpretation: Clinical interpretation is based on AD&FTD mutation database and published papers. Among the 18 previously reported variants; we found one known pathogenic mutation PSEN1 p.A426P. PSEN1 p.A426P (rs63751223) was reported in a five members of a family with autosomal dominant AD [31].We also found four high-risk variants for LOAD (APOE, p.L46P; MAPT, p.A152T; PSEN2, p.R62H and p.R71W) [28], [32], [33], six variants that were previously reported in families with AD or frontotemporal dementia (FTD), but classified as non-pathogenic (GRN, p.R433W, p.P458L, p.R19W; MAPT, p.Q230R; PSEN1, p.R35Q and p.E318G) [34], and seven variants that have been recently reported in public databases with no clear role in human disease to date (APOE, p.E37K; GRN, p.C231W; MAPT, p.G107S, p.S318L, p.V224G; PSEN2, p.E317G and p.V300G) (A detailed description of each variant can be found in the supporting material in Text S1).
These results highlight the relative enrichment of rare and low frequency variants in six genes involved in AD and FTD among individuals at the extremes of the CSF biomarker distribution [29].
Association with CSF biomarker levels
Next, we tested whether any of the variants identified by an endophenotype-based approach could improve our understanding of both the genetic architecture and pathophysiology of LOAD [17], [18]. We ran a linear regression analysis for single SNP using CSF biomarkers as quantitative traits, but we failed to find significant association with CSF tau, ptau or Aβ42 levels for most of the identified variants, even after we collapsed all of the potentially damaging variants in each gene and analyzed the dataset for carriers vs. non-carriers of these variants (Table 3). Surprisingly, a low frequency coding variant in PSEN1, p.E318G (rs17125721) (MAF = 0.02 for Europeans Americans, Exome Variant Server EVS: http://evs.gs.washington.edu/EVS/), whose pathogenic role is currently debated [34] exhibited a statistically significant association (multiple test correction threshold, p = 7.0×10−3) with CSF tau (p = 9.2×10−4, Beta = 0.14) and ptau levels (P = 1.8×10−3, Beta = 0.12), but not with Aβ42 (p = 0.14, Beta = −0.05). Interestingly, it has been reported that the combination of Aβ42 and tau or ptau as a ratio provides the best discriminative value to date for AD cases [35], [36] and predict the conversion from non-dementia clinical status to dementia [37]. p.E318G exhibited a significant association with the ratio of ptau∶Aβ42 (p = 9.5×10−5, Beta = 0.08) and tau∶Aβ42 (p = 2.0×10−4, Beta = 0.06) (Figure 1A–C, 2A) suggesting that the association of p.E318G with CSF biomarker levels may be an association with clinical AD.
Fig. 1. Distribution of PSEN1 p.E318G mutation carriers in CSF Biomarker quartiles. 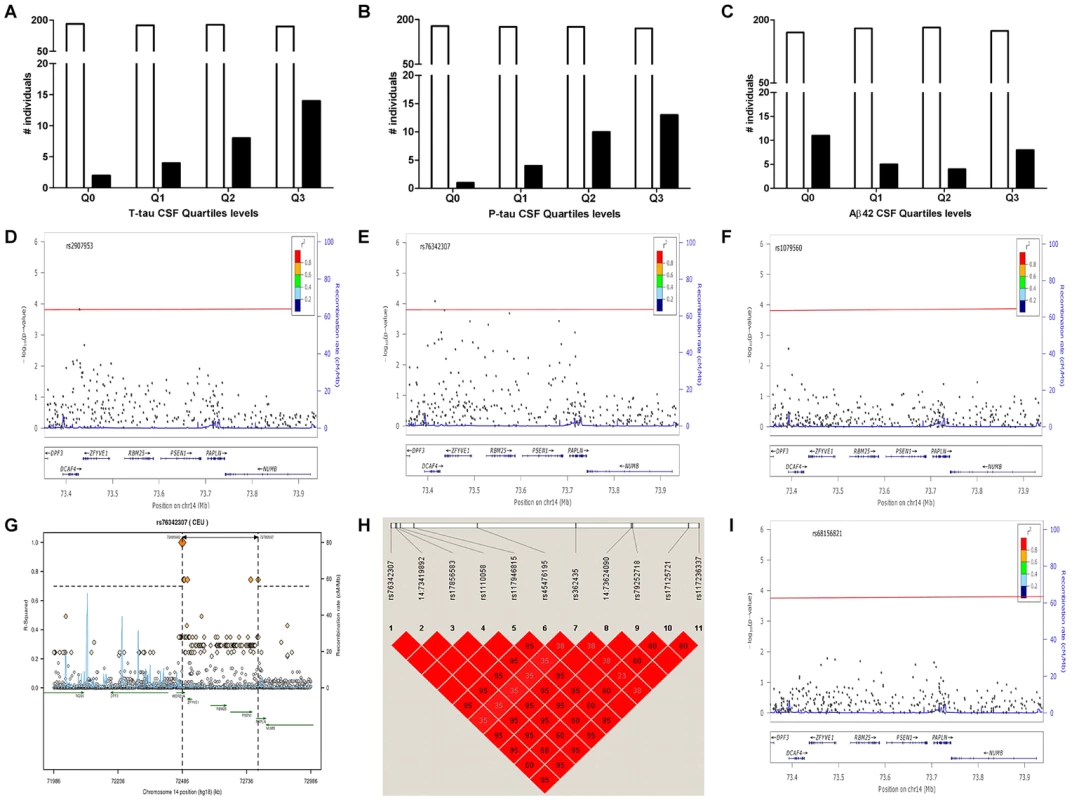
A. CSF tau, Logistic regression model p = 6.0×10−4. B. CSF pTau, Logistic regression model p = 3.0×10−4. C. CSF Aβ42, Logistic regression model p = 0.38. White bars represent the number of non carriers. Black bars represent the number of carriers D. Association of PSEN1 gene with CSF tau. E. Association of PSEN1 gene with CSF ptau. F. Association of PSEN1 gene with CSF Aβ42. Plots are showing the most significant SNP at a given locus along with the combined-analysis results for SNPs in the region surrounding it (typically ±500 kb). Symbols are colored according to the LD of the SNP with the top SNP (r2 color-based insert). The red line represents the threshold for significance. The light blue line represents the estimated recombination rate. G. LD Block for the most significant SNP associated with biomarker levels at PSEN1 genomic region: SNP rs76342307 based on the 1000 genome project for Europeans. Gene annotations are shown as dark green lines. H. LD Block for rs76342307 and rs17125721 in our own data set. I. Plot after the conditional analysis including both SNPs (rs76342307 and rs17125721) in the model. Fig. 2. Distribution of biomarker levels in PSEN1 p.E318G and APOE carriers. 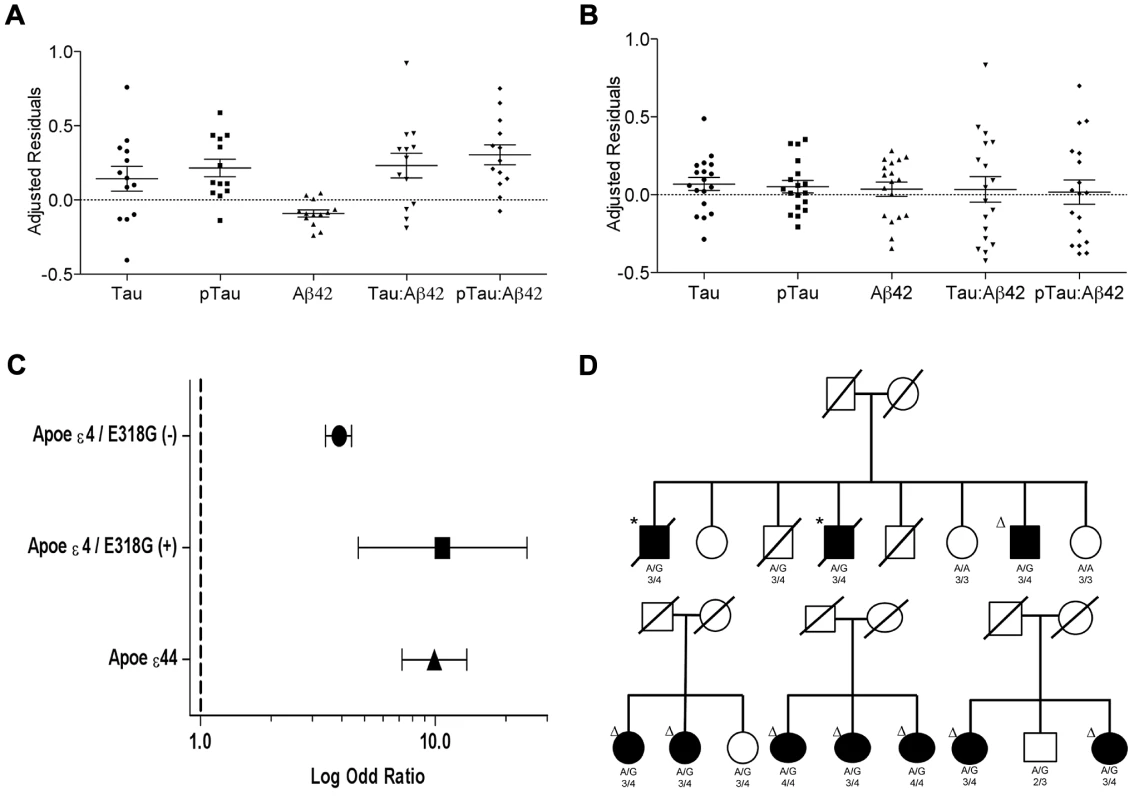
Distribution of the covariate-adjusted residuals of the CSF tau, ptau, Aβ42, Tau∶Aβ42 and ptau∶Aβ42 ratio. A. APOE ε4 positive/PSEN1 p.E318G carriers and. B. APOE ε4 negative/PSEN1 p.E318G carriers. C. Forest plot of the odd ratios of the effect of PSEN1 p.E318G in APOE ε4 heterozygous. D. Pedigrees for some of the families with p.E318G carriers illustrating the segregation analysis and the presence of APOE ε4 allele. A/G or A/A is the genotype for p.E318G variant. 2/3, 3/3, 3/4, 4/4 is the APOE genotype. * Symbol means confirmed AD by autopsy. Δ Symbol indicates probable AD diagnosed using NINCDS-ADRDA criteria. Tab. 3. Frequency of the rare variants in cases and controls and in individuals with and without Aβ deposition. 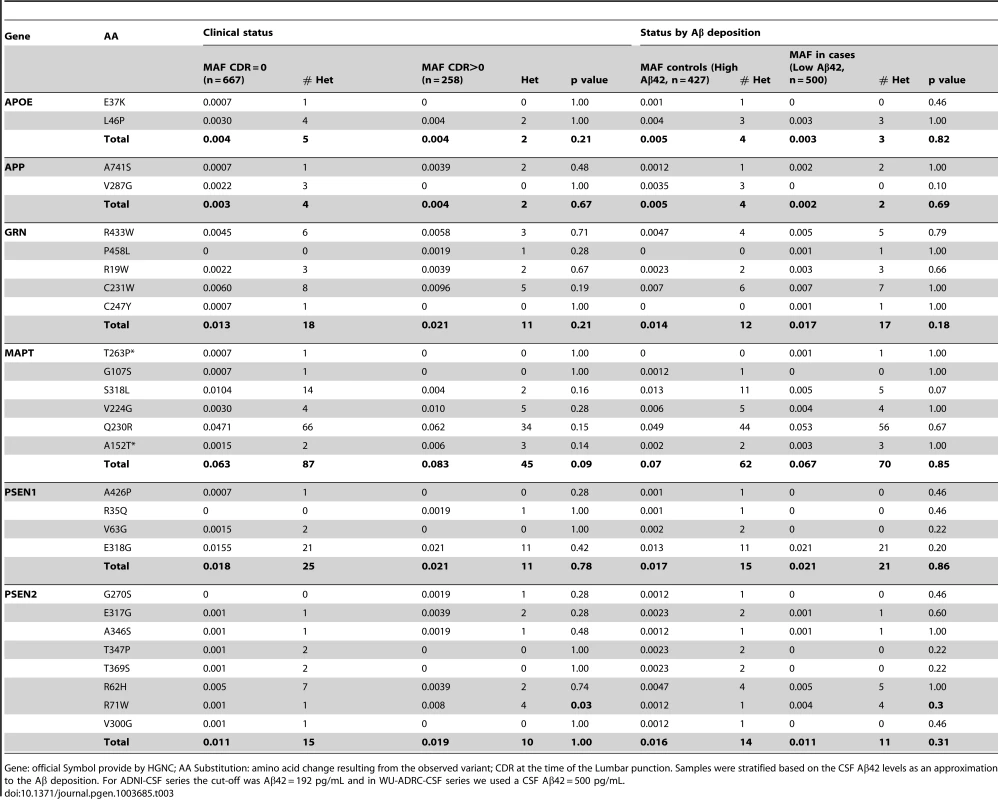
Gene: official Symbol provide by HGNC; AA Substitution: amino acid change resulting from the observed variant; CDR at the time of the Lumbar punction. Samples were stratified based on the CSF Aβ42 levels as an approximation to the Aβ deposition. For ADNI-CSF series the cut-off was Aβ42 = 192 pg/mL and in WU-ADRC-CSF series we used a CSF Aβ42 = 500 pg/mL. In order to confirm this association with CSF biomarkers and to determine whether this or any other SNP in linkage disequilibrium (LD) was driving the association, we combined genotype and imputed data from 895 individuals (WU-ADRC, n = 501, and ADNI, n = 394, this dataset constitute the same CSF series that we genotyped (Table 1) plus additional 161 individuals) to perform a dense fine mapping analysis of PSEN1 genomic region. The number of independent tests (Meff = 317) was calculated based on the number of SNPs after correcting for LD structure (r2 = 0.8) within the genomic region (250 Kb in each side) [38]. We performed linear regression assuming an additive genetic model to test the association between each SNP and CSF biomarker levels by adjusting for age, gender and the first three principal components from the population stratification analysis. We confirmed a significant association (multiple-testing threshold = 1.6×10−4) between an intronic SNP, rs76342307 (MAF = 0.016) and CSF ptau (p = 8.0×10−5, Beta = 0.14), tau (p = 8.4×10−3, Beta = 0.10), and Aβ42 levels (p = 0.02, Beta = −0.06) (Figure 1D–F) for the PSEN1 genomic region. Rs76342307 is located 0.2 Mb 3′ upstream from the PSEN1 gene. We used data from the HapMap and the 1000 Genomes Project to identify all of the SNPs in linkage disequilibrium (LD, r2>0.8) with rs76342307. Six SNPs (rs76342307, rs17856583, rs1110058, rs117946815, rs117236337 and rs2091912) were found to be in strong LD (r2 = 0.95, D′ = 1) with rs76342307 spanning 0.3 Mb (Figure 1G, H). 100% and 97% concordance rates were observed among the directly typed and imputed results for rs76342307 and rs117236337, respectively. Interestingly, rs117236337 is an intronic SNP in PSEN1 gene, which is also associated with extreme CSF tau (p = 0.02, Beta = 0.08), ptau (p = 5.7×10−4, Beta = 0.09) and Aβ42 levels (p = 0.01, Beta = −0.06). Next, we tested whether PSEN1, p.E318G was in LD with the SNPs identified by the fine mapping analysis. In fact, rs17125721 (PSEN1, p.E318G) is in moderate LD with all of them (R2 = 0.68, D′ = 1) (Figure 1H). To analyze whether the p.E318G and rs76342307 are two independent signals, we ran a conditional analysis including both SNPs (rs76342307 and rs17125721) in the model. When one of the SNPs was included in the model, the association from the other SNP disappeared, suggesting that the association in this locus is driven by a single signal (Figure 1I).
Effect of PSEN1, p.E318G on Aβ deposition is APOE ε4-dependent
We observed that in the subset of individuals with Aβ deposition (CSF Aβ42 levels lower than 500 pg/ml in WU-ADRC, and 192 pg/ml in ADNI) [35], [39], the frequency of p.E318G carriers (4.2%, 21/500) was higher than in individuals without Aβ deposition (2.5%, 11/427), although this difference did not achieve statistical significance (p = 0.18, OR = 1.6, 95%CI = 0.78–3.4) (Table 3, 4). In addition, we observed that 93% (15/16) of the individuals carrying PSEN1, p.E318G along with APOE ε4 exhibited low CSF Aβ42 levels, while only 45% (9/20) of the individuals carrying PSEN1, p.E318G but do not carry the APOE ε4 allele showed low CSF Aβ42 levels, suggesting that APOE ε4 allele is modifying the profile of Aβ deposition in PSEN1, p.E318G carriers (Table 4 and Figure 2A). APOE ε4 is strongly associated with CSF Aβ42 levels (Table 4) [14], [18], and APOE genotype has been reported to modify disease expression in individuals with mutations in PSEN1 [40] and PSEN2 [41] genes. However, previous reports have not found any significant interaction between APOE and PSEN1 p.E318G, most likely due to the low frequency of PSEN1, p.E318G and small sample sizes [42], [43], [44]. To analyze whether there was an APOE-dependent effect on this variant, we tested the association of p.E318G with CSF Aβ42 levels by stratifying it in the presence (+) or absence (−) of the APOE ε4 allele. We found that the risk of having Aβ deposition is greater for carriers of PSEN1, p.E318G and APOE ε4 together (OR = 18.3 CI = 2.0–166.8, p = 3.5×10−3) than those carrying APOE ε4 allele alone (OR = 4.5, CI = 3.4–6.0, p<1.0×10−5) (Table 4). These individuals are more likely to have a CSF biomarker profile similar consistent with AD (low CSF Aβ42, and high tau or ptau levels) (Figure 2A). p.E318G carriers who also carry APOE ε4+ allele (n = 20) exhibited significantly higher CSF tau (p = 0.04) and ptau (p = 0.01) levels and significantly lower CSF levels of Aβ42 (p = 0.02) compared to those that are p.E318G carriers but do not carry the APOE ε4 allele (Figure 2 A, B). We also found a significant interaction (p = 0.03) between APOE ε4+ and p.E318G in individuals with increased burden of Aβ deposition. Taken together, the results of the biomarker analyses suggest that PSEN1, p.E318G is associated with higher levels of neuronal loss (reflected by CSF tau and ptau levels) and with Aβ deposition (low Aβ42 CSF levels) in an APOE ε4-dependent fashion.
Tab. 4. Effect of the interaction of PSEN1 p.E318G with APOE in individuals with and without Aβ deposition. 
Samples were stratified based on the CSF Aβ42 levels as an approximation to the Aβ deposition. For ADNI-CSF series the cut-off was Aβ42 = 192 pg/mL and in WU-ADRC-CSF series we used a CSF Aβ42 = 500 pg/mL. Replication of the PSEN1, p.E318G-APOE interaction in large case-control datasets
Because the purpose of this endophenotype-based approach is to identify variants implicated in disease, we tested whether the PSEN1, p.E318G is associated with AD risk, tau/Aβ pathology or rate of cognitive decline in an APOE dependent manner.
Analyses of the association between PSEN1 p.E318G and clinical AD status in an independent AD case-control series (n = 1,855, WU series) revealed that the risk of AD is significantly higher for p.E318G/APOE ε4 carriers (OR = 9.9 CI = 2.6–37.5, p = 1.7×10−4) compared to individuals carrying APOE ε4 alone (OR = 5.1, CI = 4.1–6.3, p = 3.2×10−59) (Table 5). This finding was replicated in an independent sample from the GERAD consortium (n = 4,058). In this dataset, the association of p.E318G with AD case-control status in the presence of at least one APOE ε4 allele (OR = 10.3, 95% CI = 4.1–25.5, p = 4.1×10−8) was double the risk for AD in the presence of APOE ε4 alone (OR = 4.1, 95% CI = 3.5–4.8, p = 1.1×10−79). In the joint-analysis of these two independent series (5,161 individuals), the risk of developing AD in the p.E318G/APOE ε4 carriers (OR = 10.1, 95% CI = 4.8–20.9, p = 9.0×10−12) is two-fold the AD risk of those that carry APOE ε4 allele alone (OR = 4.4, 95% CI = 3.9–5.0, p = 6.8×10−139) (Table 5).
Tab. 5. Replication analysis of <i>PSEN1</i> p.E318G interaction with <i>APOE</i> in AD case-control status. 
In fact, we found that individuals who are APOE ε4 heterozygous and also carry the p.E318G variant are at similar AD risk (OR = 10.7, 95% CI = 4.7–24.6, p = 2.5×10−10) as APOE ε4 homozygous (OR = 9.9, 95% CI = 7.2.9–13.6, p = 5.5×10−76) and are at double the AD risk compared to APOE ε4 heterozygous that are not carrying p.E318G (OR = 3.9, 95% CI = 3.4–4.4, p = 2.8×10−106) (Table 6, Figure 2C).
Tab. 6. <i>PSEN1</i>, p.E318G modifies Alzheimer's risk in <i>APOE</i> e4 carriers. 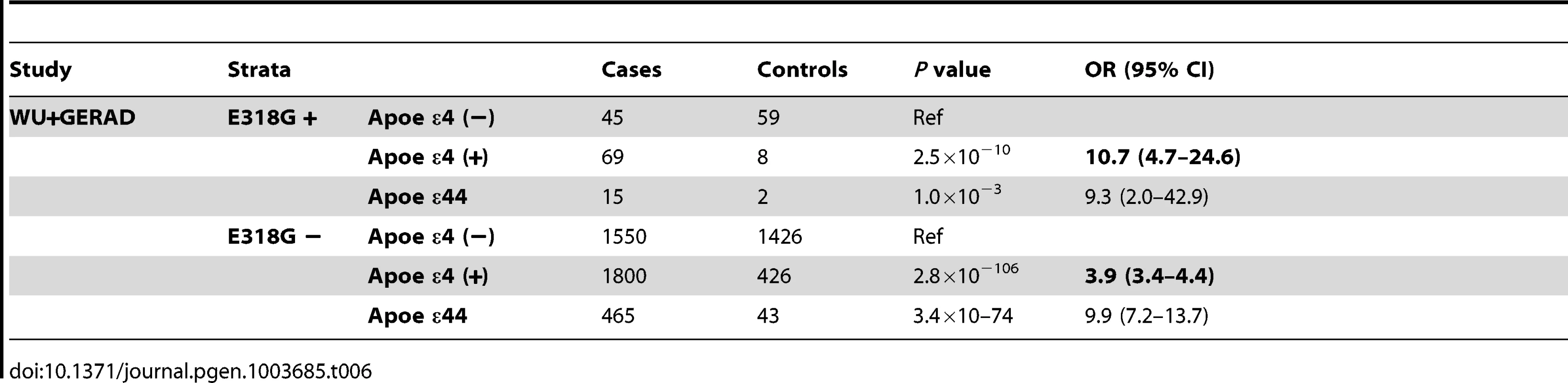
In an independent analysis leveraging two prospective cohorts, the Religious Orders Study and Rush Memory and Aging Project, we confirmed a significant interaction between APOE4 and p.E318G with burden of neuritic plaques at autopsy (n = 748; P = 0.01) but we failed to detect any significant association with neurofibrillary tangles (p = 0.47). Interestingly, the effect of APOE ε4 allele alone on neuritic plaques (n = 748, p = 4.5×10−24, Beta = 0.39) was increased by two fold the presence of p.E318G (n = 204, p = 0.08, Beta = 0.74). p.E318G has previously associated with lower cognitive performance [45]. We tested whether the interaction between APOE4 and p.E318G affect the episodic memory. We found that there is trend between interaction between APOE4 and p.E318G with episodic memory decline (p = 0.08).Furthermore, the significant effect of APOE ε4 allele on episodic memory decline (p = 1.7×10−16, Beta = −0.06) was modified by the presence of p.E318G (p = 0.14, Beta = −0.16).However, these interactions showed the predicted direction of effects for these phenotypes based on the results of the biomarker data: In the presence of at least one APOE-ε4 allele, p.E318G is associated with more Aβ plaques, faster cognitive decline and higher risk for AD.
Family based and segregation analysis
The p.E318G variant has been associated with familial AD in different populations [42], [44], [46]. However, this association has not been consistently replicated [43], [47], [48], [49]. Our previous analyses indicate that in sporadic AD cases the effect of the p.E318G variant can be detected only in presence of the APOE ε4 allele. We wanted to analyze whether the same effect is found in familial cases. We genotyped probands from 565 total LOAD families and found the presence of PSEN1 p.E318G in 30 families (MAF = 2.5%). PSEN1 p.E318G exhibited a higher frequency in individuals with familial LOAD than those with sporadic LOAD (MAF = 1.6%, n = 3,989, p = 0.02) and a group of age matched control subjects (MAF = 1.5%, n = 830, p = 0.03). Next, we tested whether the association with familial LOAD was due to the interaction of p.E318G with APOE-ε4 allele. The presence of APOE-ε4 allele in p.E318G carriers in familial AD (70%, 21/30) was higher than that in sporadic AD (65%, 84/129) but not statistically significant (p = 0.61). On the other hand, APOE-ε4/p.E318G carriers in familial AD were significantly higher (p = 4.0×10−4) than those in the control group (15%, 10/69). Therefore, the risk conferred by APOE-ε4 and p.E318G carriers in familial AD (OR = 16.4, 95% CI = 5.6–48.2, p = 5.8×10−8) compared to the control group was higher than the risk associated with sporadic AD (OR = 10.1, 95% CI = 4.8–20.9, p = 9.0×10−12). These results suggest that higher risk of the p.E318G variant in familial cases is mostly due to the high frequency of APOE ε4 allele in this population [28].
Interestingly, the p.E318G variant has been reported in multigenerational families with AD [42], [50]. However, PSEN1 p.E318G is not considered pathogenic in part due to the absence of conclusive evidence for cosegregation with AD [34], [43], [47], [48]. We observed 8 families (with more than two affected individuals carrying p.E318G) in which p.E318G segregates with disease (Figure 2D), even in the absence of APOE-ε4 allele (two families) (Figure S2). These families do not carry any other mutations in APP, PSEN1, PSEN2, GRN and MAPT genes [28]. In three additional families the cosegregation p.E318G with AD was inconclusive because only a few family members had been sampled and/or because p.E318G carriers were below the mean age of onset for AD in their respective families. Thus, using the largest sample of familial LOAD screened to date for the role of p.E318G in AD, we have demonstrated that minor allele p.E318G increases the risk of familial LOAD. Furthermore, p.E318G cosegregates with AD in 26% of all the familial LOAD carriers.
Effect on age at onset of AD
Carriers of PSEN1, p.E318G have been reported across a wide range of ages (45 to 93 yrs.) [42], [44], [46], [50]. Thus, we tested whether PSEN1, p.E318G affects AAO regardless of the APOE genotype; we found that PSEN1, p.E318G carriers have a lower AAO than non-carriers (73.9 yr. vs. 78.2 yr.; p = 0.01) (Figure S3).
Discussion
Resequencing genes in individuals from the extremes of the biomarker distribution constitutes a powerful and efficient strategy to identify functional sequence variants associated with complex traits [10]. CSF-based biomarker profiles have proven to be powerful tools in endophenotype-oriented approaches, by which we have been able to identify common genetic variants associated with the rate of progression, AAO or the risk of AD [11], [14], [17], [18], [51]. Previously, we identified a pathogenic mutation in a family with LOAD within the PSEN1 gene by selecting the top and bottom 5% from the distributions of CSF levels of Aβ40, Aβ42, and Aβ42/40 ratio [29]. Here, we have used a novel and powerful approach by using next-generation sequencing to sequence individuals with extreme phenotypes: individuals from the bottom and top 15% of Aβ42, tau, or ptau CSF levels.
Pathogenic mutations and high-risk AD variants
Previous data have suggested that mutations in APP, PSEN1, and PSEN2 genes only cause early-onset familial AD. However, this study and previous studies from our group [28], [52] indicate that pathogenic mutations in these genes can be also found in late-onset familial and sporadic AD cases. In this study, we observed a known and confirmed pathogenic mutation (PSEN1 p.A426P, rs63751223) in one individual (57 years old) without a clear family history of dementia, out of 258 individuals (CDR>0), which constitutes 0.3% of AD cases.
In a previous study, Cruchaga et al, found that 2.3% of families with multiple members affected by LOAD carried pathogenic mutations [28]. In this study, we expanded our analyses to sporadic cases, which constitute 95% of the total number of AD cases. Although we found only one case with a pathogenic mutation (0.3%), this could be an underestimate because both of the novel mutations, PSEN2: p.G270S and MAPT p.T263P were found in single cases that met biomarker criteria for AD. A novel variant in GRN, p.C247Y and a known variant in PSEN1, p.R35Q were found in demented individuals with a non-AD CSF profile suggesting another type of dementia. However, without segregation analyses, additional functional studies are required to determine the potential pathogenicity of these variants.
The classification of mutations as not pathogenic, possibly pathogenic, probably pathogenic and definitely pathogenic based on segregation analyses, amino acid conservation, effects on Aβ metabolism in in vitro studies, association studies and presence in healthy individuals has been useful in prioritizing mutations and their likelihood of affecting risk for disease [47]. However, this classification is likely to miss variants with a smaller but real effect (OR>2.0) on risk for sporadic AD. The variant GRN, p.P458L is classified as non-pathogenic [34] due to fact that it was reported in an ALS/FTD patient and in 25 out of 492 controls (MAF = 2.5%) [53]. However, this variant is not reported in the EVS server (6,515 exomes) (EVS-v.0.0.18, (February 8, 2013) or in our control population of 824 samples (Table 2). Here, this variant was found in an individual with early onset dementia and with typical biomarker criteria for AD. PSEN2, p.R71W has been classified as non-pathogenic because it was reported in controls and EOAD cases [34]. However, in a previous study the frequency of the p.R71W variant in AD cases was significantly higher than in controls (n = 3,152, p = 9.0×10−4 OR = 6.45; 95%CI = 1.95–21.39) and carriers have a significantly earlier age at onset than affected non-carriers (p.R71W: 70.2 vs. 76.7, p = 5.0×10−4), suggesting that this variant could be a modifier of LOAD risk [28]. Here, we found the same trend, PSEN2 p.R71W was also found to be present more frequently in clinical cases than in controls (p = 0.03, OR = 10.3, 95%CI = 1.1–96.2). However, it did not reach statistical significance in individuals with Aβ deposition (p = 0.27, OR = 3.4, 95%CI = 0.38–30.7).
PSEN1 p.E318G increases the risk of AD in APOE ε4 allele carriers
The PSEN1, p.E318G variant has been considered to be a non-pathogenic variant, because it has been found in non-demented individuals [43], [48], [49] and the absence of conclusive evidence for cosegregation with AD [43]. However, it has been suggested that phenocopies, potential presymptomatic individuals, reduced penetrance and gene by gene interactions complicate the interpretation of the p.E318G variant in familial and sporadic LOAD [42], [44]. This is the first study to systematically screen the presence of PSEN1 p.E318G in a large (n = 565) clinical series of well-characterized families densely affected by LOAD with no mutations in APP, PSEN2, GRN or MAPT genes. PSEN1 p.E318G was found in 5.3% and cosegregated with the disease in 1.4% of all families. We also found that PSEN1 p.E318G exhibited a higher frequency in familial LOAD than in sporadic LOAD (p = 0.025), supporting earlier findings that the p.E318G variant has higher frequencies among AD cases with a family history of AD in different populations [42], [44], [46]. Additionally, our analyses indicate that PSEN1 p.E318G carriers have an average age at onset that is 4.3 years earlier than that in non-carriers (73.9 yr. vs. 78.2 yr). Putative pathogenic variants in genes that cause late-onset rather than early-onset dementia could have a less severe effect on protein function due to genetic or environmental modifiers [28]. Our CSF biomarker analyses suggested that PSEN1 p.E318G was associated with higher levels of neuronal loss (reflected by high CSF tau and ptau levels) and with Aβ deposition (low Aβ42 CSF levels) in an APOE ε4-dependent fashion. Furthermore, in the largest AD case-control series (n = 5,161) analyzed for the interaction between PSEN1 p.E318G and APOE ε4 allele to date, we found that the presence of p.E318G and APOE ε4 doubles the risk for AD (OR = 10.3, 95% CI = 4.1–25.5) compared to the risk with the presence of APOE ε4 alone (OR = 4.1, 95% CI = 3.5–4.8). There are several reports of variants that modify the risk of AD in APOE ε4 carriers such as α-1-antichymotrypsin (ACT) gene (APOE ε4/ACT, [OR = 6.4, non 95% CI reported]) [54], Cholesteryl ester transfer protein (CETP) gene (APOE ε4/CETP [–629] C allele [OR 7.12, non 95% CI reported]) [55], GRB-associated binding protein 2 (GAB2) gene (APOE ε4/rs2373115 genotype GG [OR = 2.36, 95% CI 1.55–3.58]) [56], CUG triplet repeat, and RNA binding protein 2 (CUGBP2) gene (APOE ε4/ε4/rs62209 [OR = 1.75, 95% CI 1.27–2.41]) [57]. However, all these variants have a modest effect increasing the risk due to APOE ε4 allele. Here, we provided evidence of a low frequency variant in PSEN1 gene with a significant effect on the AD risk in APOE ε4 carriers (OR = 10.7, 95% CI = 4.7–24.6) comparable only to the effect of a second APOE ε4 allele (OR = 9.9, 95% CI = 7.2.9–13.6). Moreover, we also found that in the presence of at least one APOE ε4 allele, p.E318G is associated with more Aβ plaques and faster cognitive decline, as recently reported for a low frequency variant in complement receptor 1 (CR1) [58] In addition, p.E318G has previously associated with lower cognitive performance, which support our findings of cognitive decline [45]. The interaction of the p.E318G with APOE ε4 allele was replicated in four different datasets: the CSF dataset (discovery set), WU_ADRC case-control dataset, GERAD1 and the Religious Orders Study and Rush Memory and Aging Project, indicating that this association and interaction is not a type I error, but a real association. All these results together support the role of PSEN1 p.E318G as one of the most important modifiers of the risk of LOAD reported to date.
Functional studies, especially concerning the effect on Aβ metabolism in vitro, have further questioned the pathogenicity of the p.E318G variant. One study showed no alteration in the production of Aβ42 induced by p.E318G [43]. However, a recent study using skin fibroblasts from individuals with the p.E318G variation showed an increase in the production of Aβ40, a decrease in Aβ42 and a subsequent significant reduction in the Aβ42/Aβ40 ratio compare to non-carriers [42], along with a lack of an inhibitory effect of the exon 9 loop in the presence of the p.E318G variant reported by an independent study [59]. It has been proposed that the activation of γ-secretase results from a cleavage-induced conformational change that relieves the inhibitory effect of the intact exon 9 loop, which is mediated by occupying the substrate-binding site on the immature enzyme before it is cleaved [59]. It was reported that p.E318G abolishes the inhibitory effect of the intact exon 9 loop, which favors the production of Aβ40 [59]. It was also reported that p.E318G affects the processing of PSEN1 by reducing the amount of N-terminal fragment that is generated after cleavage [60], and augments levels of neuronal cell death after overexpression [61]. We suggest that another approach to test the impact of pathogenic mutations on Aβ metabolism is to examine the effect on the CSF biomarker levels. Most of the published data about CSF biomarkers reveal that PSEN1 gene mutation carriers display a typical AD biomarker signature with low CSF levels of Aβ42 and high CSF tau levels [13], [20]. There is no published data on the levels of CSF biomarkers for PSEN1, p.E318G carriers. Here, for the first time we demonstrate that PSEN1, p.E318G/APOE ε4 carriers have a CSF biomarker profile similar to AD cases.
In summary, these results highlight the relative enrichment of low frequency variants in six genes involved in AD and FTD that are at the extremes of the distribution of CSF biomarker levels [29]. We provide evidence that the PSEN1, p.E318G variant increases the risk for AD in APOE ε4 heterozygous, equivalent to that of APOE ε4 homozygous. We also found that p.E318G increases the risk of familial LOAD and cosegregates with AD in 26% of all the familial LOAD carriers. All these findings have important implications for genetic counseling since PSEN1, p.E318G is currently considered a non-pathogenic variant [50].
By using CSF biomarker levels as a quantitative trait, we were able to identify a low frequency variant associated with AD risk, PSEN1, p.E318G. This association is mediated by a SNP-by-SNP interaction, which has not been found using the standard case-control design [43], [48], [49]. Together, these results indicate that there are potentially many more low frequency variants associated with complex disease, and that the association results from complex interactions. We were able to identify the association of PSEN1, p.E318G with risk for AD and its interaction with the APOE ε4 allele because both genes are known to be associated with AD. However, the identification of such an association and interactions in a genome-wide approach remains still challenging and requires novel, powerful approaches.
We believe that this endophenotype-based approach is a good alternative to case-control studies and can allow us to gain a better understanding of both the genetic architecture and pathophysiology of LOAD [17], [18]. In terms of genetics and factors that may explain some of the missing hereditability of complex diseases, these results are important because they are a clear example of low frequency variants that are associated with disease and how such associations are due to epistatic gene by gene interactions.
Materials and Methods
Ethics statement
The Institutional Review Board (IRB) at the Washington University School of Medicine in Saint Louis approved the study. Prior to their participation, a written informed consent was reviewed and obtained from family members. The Human Research Protection Office (HRPO) approval number for our ADRC Genetics Core family studies is 93-0006.
Samples
Two CSF series were used for this study. A total sample of 475 individuals enrolled in longitudinal studies at the Alzheimer's disease Research Center at Washington University School of Medicine (ADRC) and 259 participants of the Alzheimer's disease Neuroimaging Initiative (ADNI) were used in this study. A subset of 145 participants from ADRC and 67 from ADNI were included in the discovery series (two DNA pools). CSF samples were from individuals of European descent. In the WU-ADRC-CSF series: 60% of sample is female, ranging from 37–91 years of age. 73% of the sample has a clinical dementia rating (CDR) of 0 (cognitively normal) and 39% of the individuals carry at least one APOE ε4 allele. In the ADNI-CSF series: 44% of sample is female, ranging from 56–91 years of age. 60% of the sample has a CDR higher than 0 (demented) and 47% are APOE ε4 allele positive. Table 1 summarizes the demographic data for the CSF series. Covariate-adjusted residuals of CSF Aβ42, tau and p-tau were used to define the pools (see statistical analysis, Table S3 in Text S1). 114 individuals in the bottom 15% of CSF Aβ42 levels or individuals in the top 15% of CSF tau or p-tau levels were included in a pool. The second pool consisted of 98 individuals in the top 15% of CSF Aβ42 or individuals in the bottom 15% of tau and p-tau181 levels (Table 1).
The Religious Orders Study (ROS) and the Rush Memory and Aging Project (MAP) recruit participants without known dementia who agree to annual clinical evaluations and sign an Anatomic Gift Act donating their brains at death. The full cohort with genotype data included 1,708 subjects (817 ROS and 891 MAP). The mean age at enrollment was 78.5 years and 69.1% were female. At the last evaluation, 24.9% met clinical diagnostic criteria for AD and 21.8% had mild cognitive impairment. The summary measure of global cognitive performance was based on annual assessments of 17 neuropsychiatric tests. A nested autopsy cohort consisted of 651 deceased subjects (376 ROS and 275 MAP); mean age at death was 81.5 years and 37.6% were male. Proximate to death, 40.9% of subjects included in the autopsy cohort met clinical diagnostic criteria for AD. Bielschowsky silver stain was used to visualize neurofibrillary tangles in tissue sections from the midfrontal, middle temporal, inferior parietal, and entorhinal cortices, and the hippocampal CA1 sector. A quantitative composite score for neurofibrillary tangle pathologic burden was created by dividing the raw counts in each region by the standard deviation of the region specific counts, and then averaging the scaled counts over the 5 brain regions to create a single standardized summary measure. Additional details of the ROS and MAP cohorts as well as the cognitive and pathologic phenotypes are described in prior publications [58], [62]. Follow-up series included 1,031 sporadic AD cases, 824 unrelated elderly cognitively normal controls and a single case from NIA-LOAD families (n = 595) [28]. All these samples are independent of the CSF samples. Cases received a diagnosis of dementia of the Alzheimer's type (DAT), using criteria equivalent to the National Institute of Neurological and Communication Disorders and Stroke-Alzheimer's Disease and Related Disorders Association for probable AD [63], [64]. Controls received the same assessment as the cases but were non-demented. All individuals were of European descent and written consent was obtained from all participants.
DNA from ROS and MAP subjects was extracted from whole blood, lymphocytes or frozen post-mortem brain tissue and genotyped on the Affymetrix Genechip 6.0 platform, as previously described [58]. Following standard QC procedures, imputation was performed using MACH software (version 1.0.16a) and HapMap release 22 CEU (build 36) as a reference.
Statistical and association analyses
Association of Aβ42, tau and p-tau181 with genetic variants was analyzed as previously reported [14], [17], [18]. Briefly, Aβ42, tau and p-tau181 values were log transformed to approximate a normal distribution. Because the CSF biomarker levels were measured using different platforms (Innotest plate ELISA vs. AlzBia3 bead-based ELISA, respectively) we were not able to combine the raw data. For the combined analyses we standardized the mean of the log transformed values from each dataset to zero. A stepwise discriminant analysis identified CDR, APOE genotype, gender and age as significant covariates in both series (Table S1b in Text S1) [17], [18]. No significant differences in the transformed and standardized CSF values for different series were found (Table S1b in Text S1).
CSF biomarker levels were used as a quantitative trait for most analyses. It has been shown that CSF Aβ42 is an accurate predictor of brain amyloid burden regardless of clinical diagnosis [39]. Therefore, the Aβ plaque deposition was assumed using the biomarker levels as a dichotomous variable (low and high CSF Aβ42). Levels of CSF biomarkers were as follows: for the ADNI-CSF series the cut-off was Aβ42<192 pg/mL [35]. In the WU-ADRC-CSF series, we used CSF Aβ42<500 pg/mL as the cut-off [39].
We used Plink (http://pngu.mgh.harvard.edu/~purcell/plink/) to analyze the association of variants (individually or collapsed by gene) with CSF biomarker levels. Age, gender and site were included as covariates. In order to determine whether the association of variants with CSF biomarker levels was driven by case-control status we included clinical dementia rating (CDR) or CSF Aβ42 levels as a covariate in the model or stratified the data by case control status. We also performed analyses including APOE genotype as a covariate. Association with AAO was carried out using the Kaplan-Meier method and tested for significant differences, using a log-rank test [17].
Fisher's exact test was used to compare the frequency of each variant and collapse by gene in the case control series defined by CDR or CSF Aβ42 levels (Table 3). All variants were included in the model independent of their pathogenicity.
Analyses of SNP effects on global cognitive decline in ROS and MAP were performed as in prior publications [62]. Briefly, we first fit linear mixed effects models using the global cognitive summary measure in order to characterize individual paths of change, adjusted for age, sex, years of education, and their interactions with time. At least two longitudinal measures of cognition were required for inclusion in these analyses, for which data on 1,593 subjects was available. We then used these person-specific, residual cognitive decline slopes as the outcome variable in our linear regression models, with each SNP of interest coded additively relative to the minor allele, and further adjusted for study membership (ROS vs. MAP) and the first 3 principal components from population structure analysis. For analyses of neurofibrillary tangle burden, linear regression was used to relate SNPs to the pathologic summary measure, adjusting for age at death, study membership, and 3 principal components. Because the data were skewed, square root of the scaled neurofibrillary tangle burden summary score was used in analyses.
Pooled sequencing analysis
Pooled-DNA sequencing was performed, as previously described by Druley TE et al. [28], [52], [65], [66]. Briefly, equimolar amounts of individual DNA samples were pooled together after being measured using Quant-iT PicoGreen reagent. Two different pools with 100 ng of DNA from 114 and 98 individuals were made. The coding exons and flanking regions (a minimum of 50 bp each side) were individually PCR amplified using specific primers and Pfu Ultra high-fidelity polymerase (Stratagene). An average of 20 diploid genomes (approximately 0.14 ng DNA) per individual were used as input into a total of 62 PCR reactions that covered 46,319 bases from the 6 genes. PCR products were cleaned using QIAquick PCR purification kits, quantified using Quant-iT PicoGreen reagent and ligated in equimolar amounts using T4 Ligase and T4 Polynucleotide Kinase. After ligation, concatenated PCR products were randomly sheared by sonication and prepared for sequencing on an Illumina Genome Analyzer IIx (GAIIx) according to the manufacturer's specifications. pCMV6-XL5 amplicon (1908 base pairs) was included in the reaction as a negative control. As positive controls, ten different constructs (p53 gene) with synthetically engineered mutations at a relative frequency of one mutated copy per 250 normal copies was amplified and pooled with the pcr products. Six DNA samples heterozygous for previously known mutants in GRN, PSEN1, MAPT genes were also included.
Single reads (36 bp) were aligned to the human genome reference assembly build 36.1 (hg18) using SPLINTER [67]. SPLINTER uses the positive control to estimate sensitivity and specificity for variant calling. The wild type: mutant ratio in the positive control is similar to the relative frequency expected for a single mutation in one pool (1 chromosome mutated in 125 samples = 1/250). SPLINTER uses the negative control (first 900 bp) to model the errors across the 36-bp Illumina reads and to create an error model from each sequencing run of the machine. Based on the error model SPLINTER calculates a p-value for the probability that a predicted variant is a true positive. A p-value at which all mutants in the positive controls were identified was defined as the cut-off value for the best sensitivity and specificity. All mutants included as part of the amplified positive control vector were found upon achieving >30-fold coverage at mutated sites (sensitivity = 100%) and only ∼80 sites in the 1908 bp negative control vector were predicted to be polymorphic (specificity = ∼95%). The variants with a p-value below this cut-off value were considered for follow-up confirmation. All rare missense or splice site variants (with an estimated allelic frequency less than 5%) were then validated by Sequenom and KASPar genotyping in each individual included in the pools [28], [52], [66]. The validated SNPs were then genotyped in all members of the WU-ADRC-CSF and ADNI-CSF series. Common variants (>5%) and synonymous variants were not followed up.
An average coverage of 30X-fold per allele per pool is the minimum coverage necessary to get an optimal positive predictive value for the SNP-calling algorithm [67]. The necessary number of lanes to obtain a minimum of 30-fold coverage per base and sample were run (Table S2 in Text S1).
The WU-ADRC samples were genotyped with the Illumina 610 or OmniExpress. The ADNI samples were genotyped with the Illumina 610 chip. Prior to association analysis, all samples and genotypes underwent stringent quality control (QC). Genotype data were cleaned applying a minimum call rate for SNPs and individuals (98%) and minimum minor allele frequencies (0.02). SNPs not in Hardy-Weinberg equilibrium (P<1×10−6) were excluded. The QC cleaning steps were applied for each genotyping array separately. We tested for unanticipated duplicates and cryptic relatedness using pairwise genome-wide estimates of proportion identity-by-descent. When a pair of identical samples or a pair of samples with cryptic relatedness was identified, the sample from the WU-ADRC or samples with a higher number of SNPs passing QC were prioritized. Eigenstrat was used to calculate principal component factors for each sample and confirm the ethnicity of the samples [68]. The 1000 Genome Project data (June 2011 release) and Beagle software were used to impute up to 6 million SNPs. SNPs with a Beagle R2 of 0.3 or lower, a minor allele frequency (MAF) lower than 0.05, out of Hardy-Weinberg equilibrium (p<1×10-6), a call rate lower than 95% or a Gprobs score lower than 0.90 were removed. A total of 5,815,690 SNPs passed the QC process.
We used PLINK to select the list of SNPs in the gene region (approximately 250 kb of flanking sequence each side) from the imputed data. These SNPs were pruned with an r2 cutoff of 0.8.. The simple method [38] was used to calculate the number of informative SNPs within the genomic region for each gene. This measure was then used in a Bonferroni adjustment to estimate the significance threshold. Significant SNPs that were imputed or have a MAF<10% were directly genotyped in all the samples to confirm the association.
Bioinformatics
The AD&FTD mutation database [34] was used to identify sequence variants previously found in other studies of early onset familial dementia and to determine whether or not they are considered to be disease-causative variants. The sequencing data from the 1,000 Genome Project and the Exome Variant Server data base (http://evs.gs.washington.edu/EVS/) were used to estimate the frequency of novel and rare (minor allele frequency less than 5%) missense, nonsense and splice site variants in samples unselected for studies of AD. Conservation was determined by using the GERP score, which calculates the conservation of each nucleotide in multi-species alignment. A site was called conserved when the GERP score was greater than or equal to 4 [69], [70].
ADNI material and methods
Data used in the preparation of this article were obtained from the ADNI database (www.loni.ucla.edu\ADNI). The ADNI was launched in 2003 by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, the Food and Drug Administration, private pharmaceutical companies and non-profit organizations, as a $60 million, 5-year public-private partnership. The Principal Investigator of this initiative is Michael W. Weiner, M.D. ADNI is the result of efforts of many co-investigators from a broad range of academic institutions and private corporations, and subjects have been recruited from over 50 sites across the U.S. and Canada. The initial goal of ADNI was to recruit 800 adults, ages 55 to 90, to participate in the research -approximately 200 cognitively normal older individuals to be followed for 3 years, 400 people with MCI to be followed for 3 years, and 200 people with early AD to be followed for 2 years.” For up-to-date information see www.adni-info.org.
GERAD data information
Data used in the preparation of this article were obtained from the Genetic and Environmental Risk for Alzheimer's disease (GERAD1) Consortium [71]. The GERAD1 sample comprised up to 3941 AD cases and 7848 controls. A subset of this sample has been used in this study, comprising 3333 cases and 1225 elderly screened controls genotyped at the Sanger Institute on the Illumina 610-quad chip. These samples were recruited by the Medical Research Council (MRC) Genetic Resource for AD (Cardiff University; Kings College London; Cambridge University; Trinity College Dublin), the Alzheimer's Research Trust (ART) Collaboration (University of Nottingham; University of Manchester; University of Southampton; University of Bristol; Queen's University Belfast; the Oxford Project to Investigate Memory and Ageing (OPTIMA), Oxford University); Washington University, St Louis, United States; MRC PRION Unit, University College London; London and the South East Region AD project (LASER-AD), University College London; Competence Network of Dementia (CND) and Department of Psychiatry, University of Bonn, Germany and the National Institute of Mental Health (NIMH) AD Genetics Initiative. All AD cases met criteria for either probable (NINCDS-ADRDA, DSM-IV) or definite (CERAD) AD. All elderly controls were screened for dementia using the MMSE or ADAS-cog, were determined to be free from dementia at neuropathological examination or had a Braak score of 2.5 or lower.
Supporting Information
Zdroje
1. HoltzmanDM, MorrisJC, GoateAM (2011) Alzheimer's disease: the challenge of the second century. Sci Transl Med 3 : 77sr71.
2. 2009 Alzheimer's disease facts and figures. Alzheimers Dement 5 : 234–270.
3. GoateA, Chartier-HarlinMC, MullanM, BrownJ, CrawfordF, et al. (1991) Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature 349 : 704–706.
4. GatzM, MortimerJA, FratiglioniL, JohanssonB, BergS, et al. (2006) Potentially modifiable risk factors for dementia in identical twins. Alzheimers Dement 2 : 110–117.
5. SoHC, GuiAH, ChernySS, ShamPC (2011) Evaluating the heritability explained by known susceptibility variants: a survey of ten complex diseases. Genet Epidemiol 35 : 310–317.
6. ShulmanJM, ChibnikLB, AubinC, SchneiderJA, BennettDA, et al. (2010) Intermediate phenotypes identify divergent pathways to Alzheimer's disease. PLoS One 5: e11244.
7. TennessenJA, BighamAW, O'ConnorTD, FuW, KennyEE, et al. (2012) Evolution and functional impact of rare coding variation from deep sequencing of human exomes. Science 337 : 64–69.
8. LiuY, MaxwellS, FengT, ZhuX, ElstonRC, et al. (2012) Gene, pathway and network frameworks to identify epistatic interactions of single nucleotide polymorphisms derived from GWAS data. BMC Syst Biol 6 Suppl 3: S15.
9. NolanDK, SuttonB, HaynesC, JohnsonJ, SebekJ, et al. (2012) Fine mapping of a linkage peak with integration of lipid traits identifies novel coronary artery disease genes on chromosome 5. BMC Genet 13 : 12.
10. CohenJC, KissRS, PertsemlidisA, MarcelYL, McPhersonR, et al. (2004) Multiple rare alleles contribute to low plasma levels of HDL cholesterol. Science 305 : 869–872.
11. KauweJS, CruchagaC, BertelsenS, MayoK, LatuW, et al. (2010) Validating predicted biological effects of Alzheimer's disease associated SNPs using CSF biomarker levels. J Alzheimers Dis 21 : 833–842.
12. HoltzmanDM (2011) CSF biomarkers for Alzheimer's disease: current utility and potential future use. Neurobiol Aging 32 Suppl 1: S4–9.
13. BatemanRJ, XiongC, BenzingerTL, FaganAM, GoateA, et al. (2012) Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med 367 : 795–804.
14. CruchagaC, KauweJS, NowotnyP, BalesK, PickeringEH, et al. (2012) Cerebrospinal fluid APOE levels: an endophenotype for genetic studies for Alzheimer's disease. Hum Mol Genet 21 : 4558–4571.
15. ScheunerD, EckmanC, JensenM, SongX, CitronM, et al. (1996) Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nat Med 2 : 864–870.
16. ForteaJ, LladoA, BoschB, AntonellA, OlivaR, et al. (2011) Cerebrospinal fluid biomarkers in Alzheimer's disease families with PSEN1 mutations. Neurodegener Dis 8 : 202–207.
17. CruchagaC, KauweJS, MayoK, SpiegelN, BertelsenS, et al. (2010) SNPs associated with cerebrospinal fluid phospho-tau levels influence rate of decline in Alzheimer's disease. PLoS Genet 6: e1001101.
18. KauweJS, CruchagaC, MayoK, FenoglioC, BertelsenS, et al. (2008) Variation in MAPT is associated with cerebrospinal fluid tau levels in the presence of amyloid-beta deposition. Proc Natl Acad Sci U S A 105 : 8050–8054.
19. KauweJS, WangJ, MayoK, MorrisJC, FaganAM, et al. (2009) Alzheimer's disease risk variants show association with cerebrospinal fluid amyloid beta. Neurogenetics 10 : 13–17.
20. WallonD, RousseauS, Rovelet-LecruxA, Quillard-MuraineM, Guyant-MarechalL, et al. (2012) The French series of autosomal dominant early onset Alzheimer's disease cases: mutation spectrum and cerebrospinal fluid biomarkers. J Alzheimers Dis 30 : 847–856.
21. BalasaM, Vidal-PineiroD, LladoA, AntonellA, BoschB, et al. (2012) PSEN1 mutation carriers present lower cerebrospinal fluid amyoid-beta42 levels than sporadic early-onset Alzheimer's disease patients but no differences in neuronal injury biomarkers. J Alzheimers Dis 30 : 605–616.
22. RingmanJM, GylysKH, MedinaLD, FoxM, KepeV, et al. (2011) Biochemical, neuropathological, and neuroimaging characteristics of early-onset Alzheimer's disease due to a novel PSEN1 mutation. Neurosci Lett 487 : 287–292.
23. JackCRJr, KnopmanDS, JagustWJ, ShawLM, AisenPS, et al. (2010) Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol 9 : 119–128.
24. GuerreiroR, WojtasA, BrasJ, CarrasquilloM, RogaevaE, et al. (2013) TREM2 variants in Alzheimer's disease. N Engl J Med 368 : 117–127.
25. JonssonT, StefanssonH, SteinbergS, JonsdottirI, JonssonPV, et al. (2013) Variant of TREM2 associated with the risk of Alzheimer's disease. N Engl J Med 368 : 107–116.
26. BenitezBA, CooperB, PastorP, JinSC, LorenzoE, et al. (2013) TREM2 is associated with the risk of Alzheimer's disease in Spanish population. Neurobiol Aging 34 : 1711 e1715–1717.
27. GerrishA, RussoG, RichardsA, MoskvinaV, IvanovD, et al. (2012) The role of variation at AbetaPP, PSEN1, PSEN2, and MAPT in late onset Alzheimer's disease. J Alzheimers Dis 28 : 377–387.
28. CruchagaC, HallerG, ChakravertyS, MayoK, VallaniaFL, et al. (2012) Rare variants in APP, PSEN1 and PSEN2 increase risk for AD in late-onset Alzheimer's disease families. PLoS One 7: e31039.
29. KauweJS, JacquartS, ChakravertyS, WangJ, MayoK, et al. (2007) Extreme cerebrospinal fluid amyloid beta levels identify family with late-onset Alzheimer's disease presenilin 1 mutation. Ann Neurol 61 : 446–453.
30. AdzhubeiIA, SchmidtS, PeshkinL, RamenskyVE, GerasimovaA, et al. (2010) A method and server for predicting damaging missense mutations. Nat Methods 7 : 248–249.
31. PoorkajP, SharmaV, AndersonL, NemensE, AlonsoME, et al. (1998) Missense mutations in the chromosome 14 familial Alzheimer's disease presenilin 1 gene. Hum Mutat 11 : 216–221.
32. ScacchiR, GambinaG, FerrariG, CorboRM (2003) Screening of two mutations at exon 3 of the apolipoprotein E gene (sites 28 and 42) in a sample of patients with sporadic late-onset Alzheimer's disease. Neurobiol Aging 24 : 339–343.
33. CoppolaG, ChinnathambiS, LeeJJ, DombroskiBA, BakerMC, et al. (2012) Evidence for a role of the rare p.A152T variant in MAPT in increasing the risk for FTD-spectrum and Alzheimer's diseases. Hum Mol Genet 21 : 3500–3512.
34. CrutsM, TheunsJ, Van BroeckhovenC (2012) Locus-specific mutation databases for neurodegenerative brain diseases. Hum Mutat 33 : 1340–1344.
35. ShawLM, VandersticheleH, Knapik-CzajkaM, ClarkCM, AisenPS, et al. (2009) Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol 65 : 403–413.
36. WelgeV, FiegeO, LewczukP, MollenhauerB, EsselmannH, et al. (2009) Combined CSF tau, p-tau181 and amyloid-beta 38/40/42 for diagnosing Alzheimer's disease. J Neural Transm 116 : 203–212.
37. FaganAM, RoeCM, XiongC, MintunMA, MorrisJC, et al. (2007) Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol 64 : 343–349.
38. GaoX, BeckerLC, BeckerDM, StarmerJD, ProvinceMA (2010) Avoiding the high Bonferroni penalty in genome-wide association studies. Genet Epidemiol 34 : 100–105.
39. FaganAM, HeadD, ShahAR, MarcusD, MintunM, et al. (2009) Decreased cerebrospinal fluid Abeta(42) correlates with brain atrophy in cognitively normal elderly. Ann Neurol 65 : 176–183.
40. PastorP, RoeCM, VillegasA, BedoyaG, ChakravertyS, et al. (2003) Apolipoprotein Eepsilon4 modifies Alzheimer's disease onset in an E280A PS1 kindred. Ann Neurol 54 : 163–169.
41. WijsmanEM, DawEW, YuX, SteinbartEJ, NochlinD, et al. (2005) APOE and other loci affect age-at-onset in Alzheimer's disease families with PS2 mutation. Am J Med Genet B Neuropsychiatr Genet 132B: 14–20.
42. AlbaniD, RoiterI, ArtusoV, BatelliS, PratoF, et al. (2007) Presenilin-1 mutation E318G and familial Alzheimer's disease in the Italian population. Neurobiol Aging 28 : 1682–1688.
43. DermautB, CrutsM, SlooterAJ, Van GestelS, De JongheC, et al. (1999) The Glu318Gly substitution in presenilin 1 is not causally related to Alzheimer disease. Am J Hum Genet 64 : 290–292.
44. TaddeiK, FisherC, LawsSM, MartinsG, PatonA, et al. (2002) Association between presenilin-1 Glu318Gly mutation and familial Alzheimer's disease in the Australian population. Mol Psychiatry 7 : 776–781.
45. LawsSM, ClarnetteRM, TaddeiK, MartinsG, PatonA, et al. (2002) Association between the presenilin-1 mutation Glu318Gly and complaints of memory impairment. Neurobiol Aging 23 : 55–58.
46. HelisalmiS, HiltunenM, MannermaaA, KoivistoAM, LehtovirtaM, et al. (2000) Is the presenilin-1 E318G missense mutation a risk factor for Alzheimer's disease? Neurosci Lett 278 : 65–68.
47. GuerreiroRJ, BaqueroM, BlesaR, BoadaM, BrasJM, et al. (2010) Genetic screening of Alzheimer's disease genes in Iberian and African samples yields novel mutations in presenilins and APP. Neurobiol Aging 31 : 725–731.
48. MattilaKM, ForsellC, PirttilaT, RinneJO, LehtimakiT, et al. (1998) The Glu318Gly mutation of the presenilin-1 gene does not necessarily cause Alzheimer's disease. Ann Neurol 44 : 965–967.
49. AldudoJ, BullidoMJ, FrankA, ValdiviesoF (1998) Missense mutation E318G of the presenilin-1 gene appears to be a nonpathogenic polymorphism. Ann Neurol 44 : 985–986.
50. GoldmanJS, JohnsonJK, McElligottK, SuchowerskyO, MillerBL, et al. (2005) Presenilin 1 Glu318Gly polymorphism: interpret with caution. Arch Neurol 62 : 1624–1627.
51. KauweJS, CruchagaC, KarchCM, SadlerB, LeeM, et al. (2011) Fine mapping of genetic variants in BIN1, CLU, CR1 and PICALM for association with cerebrospinal fluid biomarkers for Alzheimer's disease. PLoS One 6: e15918.
52. JinSC, PastorP, CooperB, CervantesS, BenitezBA, et al. (2012) Pooled-DNA sequencing identifies novel causative variants in PSEN1, GRN and MAPT in a clinical early-onset and familial Alzheimer's disease Ibero-American cohort. Alzheimers Res Ther 4 : 34.
53. SchymickJC, YangY, AndersenPM, VonsattelJP, GreenwayM, et al. (2007) Progranulin mutations and amyotrophic lateral sclerosis or amyotrophic lateral sclerosis-frontotemporal dementia phenotypes. J Neurol Neurosurg Psychiatry 78 : 754–756.
54. KambohMI, SangheraDK, FerrellRE, DeKoskyST (1995) APOE*4-associated Alzheimer's disease risk is modified by alpha 1-antichymotrypsin polymorphism. Nat Genet 10 : 486–488.
55. RodriguezE, MateoI, InfanteJ, LlorcaJ, BercianoJ, et al. (2006) Cholesteryl ester transfer protein (CETP) polymorphism modifies the Alzheimer's disease risk associated with APOE epsilon4 allele. J Neurol 253 : 181–185.
56. ReimanEM, WebsterJA, MyersAJ, HardyJ, DunckleyT, et al. (2007) GAB2 alleles modify Alzheimer's risk in APOE epsilon4 carriers. Neuron 54 : 713–720.
57. WijsmanEM, PankratzND, ChoiY, RothsteinJH, FaberKM, et al. (2011) Genome-wide association of familial late-onset Alzheimer's disease replicates BIN1 and CLU and nominates CUGBP2 in interaction with APOE. PLoS Genet 7: e1001308.
58. KeenanBT, ShulmanJM, ChibnikLB, RajT, TranD, et al. (2012) A coding variant in CR1 interacts with APOE-epsilon4 to influence cognitive decline. Hum Mol Genet 21 : 2377–2388.
59. KnappenbergerKS, TianG, YeX, Sobotka-BrinerC, GhanekarSV, et al. (2004) Mechanism of gamma-secretase cleavage activation: is gamma-secretase regulated through autoinhibition involving the presenilin-1 exon 9 loop? Biochemistry 43 : 6208–6218.
60. MurayamaO, MurayamaM, HondaT, SunX, NihonmatsuN, et al. (1999) Twenty-nine missense mutations linked with familial Alzheimer's disease alter the processing of presenilin 1. Prog Neuropsychopharmacol Biol Psychiatry 23 : 905–913.
61. HashimotoY, TsukamotoE, NiikuraT, YamagishiY, IshizakaM, et al. (2004) Amino - and carboxyl-terminal mutants of presenilin 1 cause neuronal cell death through distinct toxic mechanisms: Study of 27 different presenilin 1 mutants. J Neurosci Res 75 : 417–428.
62. De JagerPL, ShulmanJM, ChibnikLB, KeenanBT, RajT, et al. (2012) A genome-wide scan for common variants affecting the rate of age-related cognitive decline. Neurobiol Aging 33 : 1017 e1011–1015.
63. McKhannG, DrachmanD, FolsteinM, KatzmanR, PriceD, et al. (1984) Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 34 : 939–944.
64. BergL, McKeelDWJr, MillerJP, StorandtM, RubinEH, et al. (1998) Clinicopathologic studies in cognitively healthy aging and Alzheimer's disease: relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Arch Neurol 55 : 326–335.
65. DruleyTE, VallaniaFL, WegnerDJ, VarleyKE, KnowlesOL, et al. (2009) Quantification of rare allelic variants from pooled genomic DNA. Nat Methods 6 : 263–265.
66. HallerG, DruleyT, VallaniaFL, MitraRD, LiP, et al. (2012) Rare missense variants in CHRNB4 are associated with reduced risk of nicotine dependence. Hum Mol Genet 21 : 647–655.
67. VallaniaFL, DruleyTE, RamosE, WangJ, BoreckiI, et al. (2010) High-throughput discovery of rare insertions and deletions in large cohorts. Genome Res 20 : 1711–1718.
68. PriceAL, PattersonNJ, PlengeRM, WeinblattME, ShadickNA, et al. (2006) Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 38 : 904–909.
69. CooperGM, GoodeDL, NgSB, SidowA, BamshadMJ, et al. (2010) Single-nucleotide evolutionary constraint scores highlight disease-causing mutations. Nat Methods 7 : 250–251.
70. CooperGM, StoneEA, AsimenosG, GreenED, BatzoglouS, et al. (2005) Distribution and intensity of constraint in mammalian genomic sequence. Genome Res 15 : 901–913.
71. HaroldD, AbrahamR, HollingworthP, SimsR, GerrishA, et al. (2009) Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer's disease. Nat Genet 41 : 1088–1093.
Štítky
Genetika Reprodukční medicína
Článek Re-Ranking Sequencing Variants in the Post-GWAS Era for Accurate Causal Variant IdentificationČlánek Bypass of 8-oxodGČlánek Integrated Model of and Inherited Genetic Variants Yields Greater Power to Identify Risk GenesČlánek Comparative Genomic and Functional Analysis of 100 Strains and Their Comparison with Strain GGČlánek A Nuclear Calcium-Sensing Pathway Is Critical for Gene Regulation and Salt Stress Tolerance inČlánek Computational Identification of Diverse Mechanisms Underlying Transcription Factor-DNA OccupancyČlánek Reversible and Rapid Transfer-RNA Deactivation as a Mechanism of Translational Repression in StressČlánek Genome-Wide Association of Body Fat Distribution in African Ancestry Populations Suggests New Loci
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 8- Příjem alkoholu a menstruační cyklus
- Mateřský haplotyp KIR ovlivňuje porodnost živých dětí po transferu dvou embryí v rámci fertilizace in vitro u pacientek s opakujícími se samovolnými potraty nebo poruchami implantace
- Intrauterinní inseminace a její úspěšnost
- Růst a vývoj dětí narozených pomocí IVF
- Akutní intermitentní porfyrie
-
Všechny články tohoto čísla
- Genome-Wide DNA Methylation Analysis of Systemic Lupus Erythematosus Reveals Persistent Hypomethylation of Interferon Genes and Compositional Changes to CD4+ T-cell Populations
- Re-Ranking Sequencing Variants in the Post-GWAS Era for Accurate Causal Variant Identification
- Histone Variant HTZ1 Shows Extensive Epistasis with, but Does Not Increase Robustness to, New Mutations
- Past Visits Present: TCF/LEFs Partner with ATFs for β-Catenin–Independent Activity
- Functional Characterisation of Alpha-Galactosidase A Mutations as a Basis for a New Classification System in Fabry Disease
- A Flexible Approach for the Analysis of Rare Variants Allowing for a Mixture of Effects on Binary or Quantitative Traits
- Masculinization of Gene Expression Is Associated with Exaggeration of Male Sexual Dimorphism
- Genic Intolerance to Functional Variation and the Interpretation of Personal Genomes
- Endogenous Stress Caused by Faulty Oxidation Reactions Fosters Evolution of 2,4-Dinitrotoluene-Degrading Bacteria
- Transposon Domestication versus Mutualism in Ciliate Genome Rearrangements
- Comparative Anatomy of Chromosomal Domains with Imprinted and Non-Imprinted Allele-Specific DNA Methylation
- An Essential Function for the ATR-Activation-Domain (AAD) of TopBP1 in Mouse Development and Cellular Senescence
- Depletion of Retinoic Acid Receptors Initiates a Novel Positive Feedback Mechanism that Promotes Teratogenic Increases in Retinoic Acid
- Bypass of 8-oxodG
- Calpain-6 Deficiency Promotes Skeletal Muscle Development and Regeneration
- ATM Release at Resected Double-Strand Breaks Provides Heterochromatin Reconstitution to Facilitate Homologous Recombination
- Generation of Tandem Direct Duplications by Reversed-Ends Transposition of Maize Elements
- Loss of a Conserved tRNA Anticodon Modification Perturbs Cellular Signaling
- Integrated Model of and Inherited Genetic Variants Yields Greater Power to Identify Risk Genes
- High-Throughput Genetic and Gene Expression Analysis of the RNAPII-CTD Reveals Unexpected Connections to SRB10/CDK8
- Dynamic Rewiring of the Retinal Determination Network Switches Its Function from Selector to Differentiation
- β-Catenin-Independent Activation of TCF1/LEF1 in Human Hematopoietic Tumor Cells through Interaction with ATF2 Transcription Factors
- Genetic Mapping of Specific Interactions between Mosquitoes and Dengue Viruses
- A Highly Redundant Gene Network Controls Assembly of the Outer Spore Wall in
- Origin and Functional Diversification of an Amphibian Defense Peptide Arsenal
- Myc-Driven Overgrowth Requires Unfolded Protein Response-Mediated Induction of Autophagy and Antioxidant Responses in
- Integrative Modeling of eQTLs and Cis-Regulatory Elements Suggests Mechanisms Underlying Cell Type Specificity of eQTLs
- Species and Population Level Molecular Profiling Reveals Cryptic Recombination and Emergent Asymmetry in the Dimorphic Mating Locus of
- Ras-Induced Changes in H3K27me3 Occur after Those in Transcriptional Activity
- Characterization of the p53 Cistrome – DNA Binding Cooperativity Dissects p53's Tumor Suppressor Functions
- Global Analysis of Fission Yeast Mating Genes Reveals New Autophagy Factors
- Deficiency Suppresses Intestinal Tumorigenesis
- Introns Regulate Gene Expression in in a Pab2p Dependent Pathway
- Meiotic Recombination Initiation in and around Retrotransposable Elements in
- Comparative Oncogenomic Analysis of Copy Number Alterations in Human and Zebrafish Tumors Enables Cancer Driver Discovery
- Comparative Genomic and Functional Analysis of 100 Strains and Their Comparison with Strain GG
- A Model-Based Analysis of GC-Biased Gene Conversion in the Human and Chimpanzee Genomes
- Masculinization of the X Chromosome in the Pea Aphid
- The Architecture of a Prototypical Bacterial Signaling Circuit Enables a Single Point Mutation to Confer Novel Network Properties
- Distinct SUMO Ligases Cooperate with Esc2 and Slx5 to Suppress Duplication-Mediated Genome Rearrangements
- The Yeast Environmental Stress Response Regulates Mutagenesis Induced by Proteotoxic Stress
- Mediator Directs Co-transcriptional Heterochromatin Assembly by RNA Interference-Dependent and -Independent Pathways
- The Genome of and the Basis of Host-Microsporidian Interactions
- Regulation of Sister Chromosome Cohesion by the Replication Fork Tracking Protein SeqA
- Neuronal Reprograming of Protein Homeostasis by Calcium-Dependent Regulation of the Heat Shock Response
- A Nuclear Calcium-Sensing Pathway Is Critical for Gene Regulation and Salt Stress Tolerance in
- Cross-Species Array Comparative Genomic Hybridization Identifies Novel Oncogenic Events in Zebrafish and Human Embryonal Rhabdomyosarcoma
- : A Mouse Strain with an Ift140 Mutation That Results in a Skeletal Ciliopathy Modelling Jeune Syndrome
- The Relative Contribution of Proximal 5′ Flanking Sequence and Microsatellite Variation on Brain Vasopressin 1a Receptor () Gene Expression and Behavior
- Combining Quantitative Genetic Footprinting and Trait Enrichment Analysis to Identify Fitness Determinants of a Bacterial Pathogen
- The Innocence Project at Twenty: An Interview with Barry Scheck
- Computational Identification of Diverse Mechanisms Underlying Transcription Factor-DNA Occupancy
- GUESS-ing Polygenic Associations with Multiple Phenotypes Using a GPU-Based Evolutionary Stochastic Search Algorithm
- H2A.Z Acidic Patch Couples Chromatin Dynamics to Regulation of Gene Expression Programs during ESC Differentiation
- Identification of DSB-1, a Protein Required for Initiation of Meiotic Recombination in , Illuminates a Crossover Assurance Checkpoint
- Binding of TFIIIC to SINE Elements Controls the Relocation of Activity-Dependent Neuronal Genes to Transcription Factories
- Global Analysis of the Sporulation Pathway of
- Genetic Circuits that Govern Bisexual and Unisexual Reproduction in
- Deletion of microRNA-80 Activates Dietary Restriction to Extend Healthspan and Lifespan
- Fifty Years On: GWAS Confirms the Role of a Rare Variant in Lung Disease
- The Enhancer Landscape during Early Neocortical Development Reveals Patterns of Dense Regulation and Co-option
- Gene Expression Regulation by Upstream Open Reading Frames and Human Disease
- Sociogenomics of Cooperation and Conflict during Colony Founding in the Fire Ant
- The Intronic Long Noncoding RNA Recruits PRC2 to the Promoter, Reducing the Expression of and Increasing Cell Proliferation
- The , p.E318G Variant Increases the Risk of Alzheimer's Disease in -ε4 Carriers
- The Wilms Tumor Gene, , Is Critical for Mouse Spermatogenesis via Regulation of Sertoli Cell Polarity and Is Associated with Non-Obstructive Azoospermia in Humans
- Reversible and Rapid Transfer-RNA Deactivation as a Mechanism of Translational Repression in Stress
- QTL Analysis of High Thermotolerance with Superior and Downgraded Parental Yeast Strains Reveals New Minor QTLs and Converges on Novel Causative Alleles Involved in RNA Processing
- Genome Wide Association Identifies Novel Loci Involved in Fungal Communication
- Chromatin Sampling—An Emerging Perspective on Targeting Polycomb Repressor Proteins
- A Recessive Founder Mutation in Regulator of Telomere Elongation Helicase 1, , Underlies Severe Immunodeficiency and Features of Hoyeraal Hreidarsson Syndrome
- Genome-Wide Association of Body Fat Distribution in African Ancestry Populations Suggests New Loci
- Causal and Synthetic Associations of Variants in the Gene Cluster with Alpha1-antitrypsin Serum Levels
- Hard Selective Sweep and Ectopic Gene Conversion in a Gene Cluster Affording Environmental Adaptation
- Brittle Culm1, a COBRA-Like Protein, Functions in Cellulose Assembly through Binding Cellulose Microfibrils
- Chromosomal Copy Number Variation, Selection and Uneven Rates of Recombination Reveal Cryptic Genome Diversity Linked to Pathogenicity
- The Ribosomal Protein Rpl22 Controls Ribosome Composition by Directly Repressing Expression of Its Own Paralog, Rpl22l1
- Ras1 Acts through Duplicated Cdc42 and Rac Proteins to Regulate Morphogenesis and Pathogenesis in the Human Fungal Pathogen
- The DSB-2 Protein Reveals a Regulatory Network that Controls Competence for Meiotic DSB Formation and Promotes Crossover Assurance
- Recurrent Modification of a Conserved -Regulatory Element Underlies Fruit Fly Pigmentation Diversity
- Associations of Mitochondrial Haplogroups B4 and E with Biliary Atresia and Differential Susceptibility to Hydrophobic Bile Acid
- The Conditional Nature of Genetic Interactions: The Consequences of Wild-Type Backgrounds on Mutational Interactions in a Genome-Wide Modifier Screen
- A Critical Function of Mad2l2 in Primordial Germ Cell Development of Mice
- A Role for CF1A 3′ End Processing Complex in Promoter-Associated Transcription
- Vitellogenin Underwent Subfunctionalization to Acquire Caste and Behavioral Specific Expression in the Harvester Ant
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Chromosomal Copy Number Variation, Selection and Uneven Rates of Recombination Reveal Cryptic Genome Diversity Linked to Pathogenicity
- Genome-Wide DNA Methylation Analysis of Systemic Lupus Erythematosus Reveals Persistent Hypomethylation of Interferon Genes and Compositional Changes to CD4+ T-cell Populations
- Associations of Mitochondrial Haplogroups B4 and E with Biliary Atresia and Differential Susceptibility to Hydrophobic Bile Acid
- A Role for CF1A 3′ End Processing Complex in Promoter-Associated Transcription
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Revma Focus: Spondyloartritidy
nový kurz
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



