-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaGenome-Wide DNA Methylation Analysis of Systemic Lupus Erythematosus Reveals Persistent Hypomethylation of Interferon Genes and Compositional Changes to CD4+ T-cell Populations
Systemic lupus erythematosus (SLE) is an autoimmune disease with known genetic, epigenetic, and environmental risk factors. To assess the role of DNA methylation in SLE, we collected CD4+ T-cells, CD19+ B-cells, and CD14+ monocytes from 49 SLE patients and 58 controls, and performed genome-wide DNA methylation analysis with Illumina Methylation450 microarrays. We identified 166 CpGs in B-cells, 97 CpGs in monocytes, and 1,033 CpGs in T-cells with highly significant changes in DNA methylation levels (p<1×10−8) among SLE patients. Common to all three cell-types were widespread and severe hypomethylation events near genes involved in interferon signaling (type I). These interferon-related changes were apparent in patients collected during active and quiescent stages of the disease, suggesting that epigenetically-mediated hypersensitivity to interferon persists beyond acute stages of the disease and is independent of circulating interferon levels. This interferon hypersensitivity was apparent in memory, naïve and regulatory T-cells, suggesting that this epigenetic state in lupus patients is established in progenitor cell populations. We also identified a widespread, but lower amplitude shift in methylation in CD4+ T-cells (>16,000 CpGs at FDR<1%) near genes involved in cell division and MAPK signaling. These cell type-specific effects are consistent with disease-specific changes in the composition of the CD4+ population and suggest that shifts in the proportion of CD4+ subtypes can be monitored at CpGs with subtype-specific DNA methylation patterns.
Published in the journal: . PLoS Genet 9(8): e32767. doi:10.1371/journal.pgen.1003678
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003678Summary
Systemic lupus erythematosus (SLE) is an autoimmune disease with known genetic, epigenetic, and environmental risk factors. To assess the role of DNA methylation in SLE, we collected CD4+ T-cells, CD19+ B-cells, and CD14+ monocytes from 49 SLE patients and 58 controls, and performed genome-wide DNA methylation analysis with Illumina Methylation450 microarrays. We identified 166 CpGs in B-cells, 97 CpGs in monocytes, and 1,033 CpGs in T-cells with highly significant changes in DNA methylation levels (p<1×10−8) among SLE patients. Common to all three cell-types were widespread and severe hypomethylation events near genes involved in interferon signaling (type I). These interferon-related changes were apparent in patients collected during active and quiescent stages of the disease, suggesting that epigenetically-mediated hypersensitivity to interferon persists beyond acute stages of the disease and is independent of circulating interferon levels. This interferon hypersensitivity was apparent in memory, naïve and regulatory T-cells, suggesting that this epigenetic state in lupus patients is established in progenitor cell populations. We also identified a widespread, but lower amplitude shift in methylation in CD4+ T-cells (>16,000 CpGs at FDR<1%) near genes involved in cell division and MAPK signaling. These cell type-specific effects are consistent with disease-specific changes in the composition of the CD4+ population and suggest that shifts in the proportion of CD4+ subtypes can be monitored at CpGs with subtype-specific DNA methylation patterns.
Introduction
Systemic lupus erythematosus (SLE) is a complex autoimmune disease characterized by an impaired clearance of apoptotic cells, the production of auto-antibodies against nuclear antigens, and the deposition of immune complexes that lead to tissue damage in multiple organs. SLE patients suffer from chronic dermatological, musculoskeletal, renal, and cardiovascular problems, and like many autoimmune diseases, these symptoms typically worsen during periods of active disease, called flares, and improve during quiescent phases of the disease. SLE predominantly affects females (∼90% of cases), and is more prevalent in individuals of African descent [1].
SLE is known to have a strong genetic basis, with high sibling risk ratios (λs>8) and higher concordance among monozygotic twins compared to dizygotic twins or full siblings [2]–[4]. Recent genetic studies, including genome-wide association studies, have identified multiple common genetic risk factors, the strongest of which are in the MHC region of chromosome 6, but also include ITGAM, IRF5, STAT4, and at least twenty other genes [5]–[10]. While a few rare variants of strong effect have been identified, the currently favored hypothesis is one of complex etiology involving multiple genetic and environmental risk factors.
Given the complex nature of SLE etiology, epigenetic analyses are likely to provide new insights into the disease, as chromatin structure and DNA methylation patterns are influenced both by the inherited DNA sequence and by environmental exposures. In fact, the importance of DNA methylation in lupus has been appreciated for over 20 years. T-cells from patients with SLE have reduced expression of DNA methyltransferases [11], and DNA methylation inhibitors like 5-azacytidine can induce T-cell autoreactivity and lupus symptoms in mice [12]. Furthermore, drug-induced lupus is associated with reduced DNA methylation and aberrant expression of DNA methyltransferases [13].
A few recent studies have been published on genome-wide analyses of DNA methylation patterns in SLE. These include studies of a few thousand CpGs in CD4+ T-cells from discordant monozygotic twins [14], and either buffy coat DNA or sorted CD4+ T-cells from unrelated individuals [15], [16]. Here, we report the most comprehensive study to date of SLE epigenetics, where we have analyzed >460,000 CpGs, covering >95% of known genes, in CD4+ T-cells, CD19+ B-cells and CD14+ monocytes. Our results uncover a profound hypomethylation of genes regulated by interferon (type I) that is present in patients during and after flares, suggesting that this epigenetic state persists beyond stages when circulating interferon levels are at their highest. Our results also suggest a compositional remodeling of the CD4+ T-cell population in SLE patients that can be observed in DNA methylation patterns.
Results
To search for epigenetic risk factors for SLE, we performed genome-wide DNA methylation analysis of 49 patients with SLE and 58 control individuals with no known autoimmune disease. The patients were all seen at the UAB Rheumatology Clinic and diagnosed according to the revised ACR criteria (see Materials & Methods). Tables S1 and S2 describe the gender, age, and ethnic makeup of our initial SLE and control samples. Approximately 15 ml of peripheral blood was collected from each of subject, and the blood sample was split into aliquots for isolation of specific cell-types by positive selection with antigen-specific magnetic beads. We collected CD4+ T-cells and CD19+ B-cells from all individuals, and CD14+ monocytes from approximately half of our subjects (27 SLE patients and 27 controls). DNA from each cell type was analyzed with the Illumina Methylation450 array platform to assess DNA methylation genome-wide. After extensive quality filtering, batch normalization, and chemistry correction, we performed linear regression analysis at each CpG, in each cell type independently, to test for differences in DNA methylation levels between patients and controls. Our regression models also included covariates for age, gender and ethnicity at autosomal CpGs (See Materials & Methods). On the X-chromosome, we limited our analysis to females due to the inherent gender differences in methylation due to X-inactivation. These association tests identified highly significant methylation differences (p < 1×10−8) at autosomal CpGs in all three cell-types, including 1,033 CpGs in T-cells, 166 CpGs in B-cells, and 97 CpGs in monocytes, where our smaller sample size provides us reduced power. At this p-value significance threshold, our FDR is less than 0.0005% in T-cells, 0.003% in B-cells, and 0.012% in monocytes. Table S3 lists these highly significant CpGs. At sites with the strongest disease association, the mean difference in methylation between SLE patients and controls after covariate corrections was as high as 40%, and p-values were observed below 1×10−20 in T - and B-cells, and 1×10−12 in monocytes. These large shifts in methylation were almost entirely composed of hypomethylation events where SLE patients showed lower methylation than controls.
Many of the CpGs that showed the strongest effects were clustered within 5 kb of the same genes, such that 622 genes in T-cells, 95 genes in B-cells, and 27 genes in monocytes were strongly associated with lupus in our study. 50 genes were highly significant in both T - and B-cells, and 19 genes were highly significant across all 3 cell-types. This shared gene list expands to 60 genes if we identify those where at least one CpG is highly significant (p<1×10−8) in at least one of the cell types, and there is at least one moderately significant CpG in the other cell types. We defined moderate significance at CpGs where the false discovery rate was <1%, corresponding to p-values of less than 3.6×10−4, 3×10−5, and 4×10−6, in T-cells, B-cells, and monocytes, respectively. This list of 60 shared genes and the significant CpGs within them are shown in Table S4. Although these 60 genes contained SLE-related methylation changes in all three cell-types, the effects were not always observed at the same CpG, and there were numerous examples of cell type-specific effects, even within these shared-effect genes. For example, IRF7 contains both common and cell type-specific methylation changes in SLE patients (Figure 1A).
Fig. 1. Common and cell type-specific DNA methylation changes in SLE. 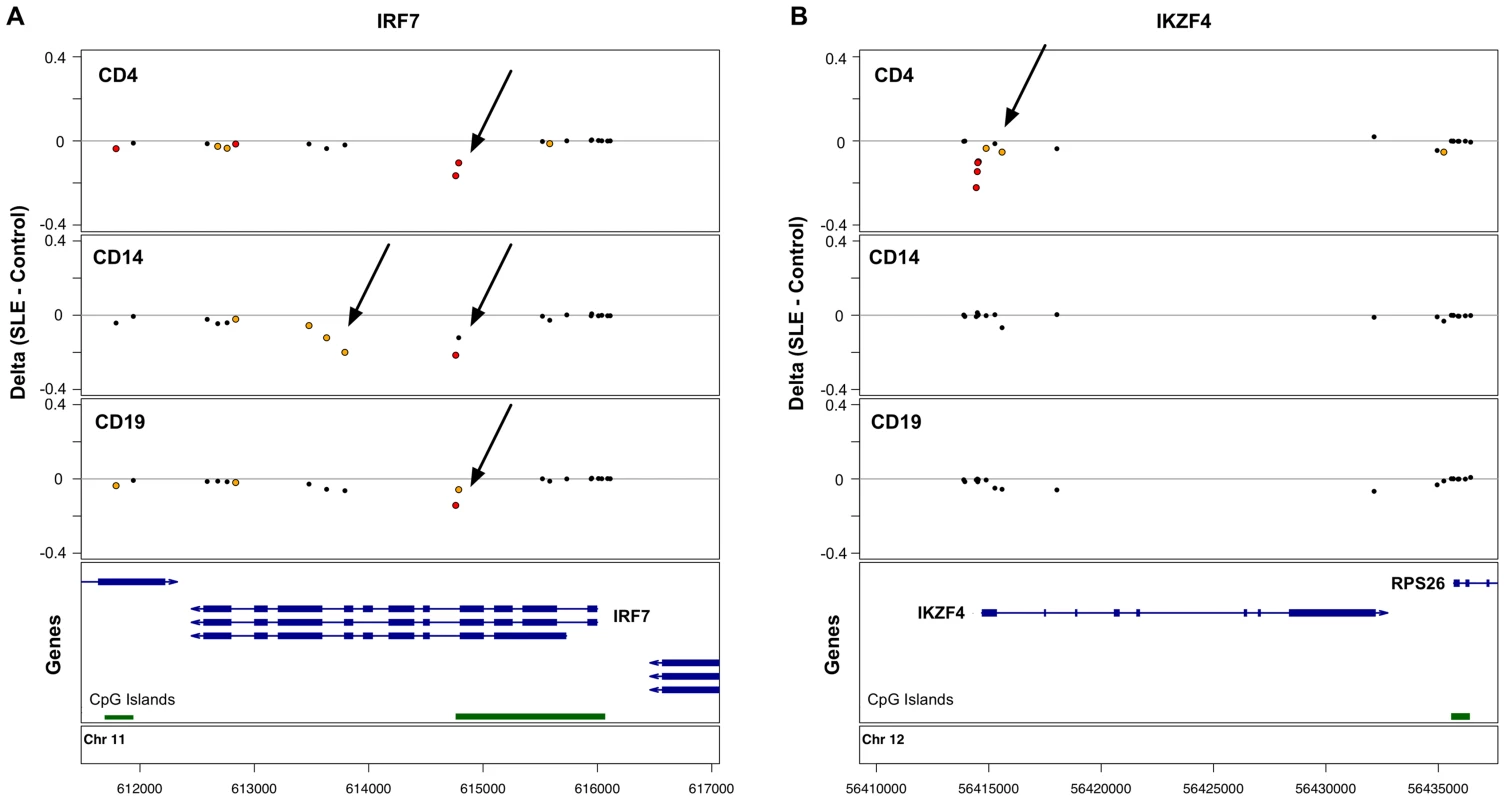
Differences in mean methylation between SLE and controls are plotted for each cell type at each probe near two genes. A. The IRF7 gene shows hypomethylation across all three cell-types at a CpG island, plus monocyte-specific hypomethylation further into the gene body. B. The IKZF4 gene shows T-cell-specific hypomethylation at the 5′ end of the gene. Red dots indicate p<1×10−8. Yellow dots indicate FDR<1%. In addition to the genes with SLE-related methylation differences in all three cell-types, there were 446 genes in T-cells and 7 genes in B-cells with highly significant effects in that cell-type alone. Figure 1B illustrates one such example at IKZF4, where only T-cells show hypomethylation in SLE patients at multiple CpGs near the transcription start site. These cell type-specific genes are listed in Table S5.
In addition to the CpGs with strong, highly significant effects, QQ plots of the p-values in each cell type (Figure 2A) suggested that T-cells had many more mild disease associations than either B-cells or monocytes. We found that >16,000 CpGs were significant at an FDR less than 1% in T-cells, while 1,403 and 199 were significant at this threshold in B-cells and monocytes, respectively. In addition, the QQ plot for T-cells displayed an unusual inflation between p-values of 1×10−5 and 1×10−11, indicating a bi-phasic p-value distribution and suggesting that two overlapping phenomenon were occurring in this cell-type. Most of these CpGs displayed milder shifts in methylation between patients and controls, typically less than 10%. Furthermore, when we plotted the log ratio of hypomethylated to hypermethylated CpGs across bins of the p-value distribution (Figure 2B), we found that T-cells contained a unique shift toward hypermethylation at these lesser effect CpGs. This observation supported the hypothesis that two independent phenomena were occurring in T-cells. These CpGs in this secondary phase of p-values also had a unique distribution of mean methylation levels, both in SLE cases and controls. While the CpGs represented on the Illumina Methylation450 array have a bimodal distribution of methylation, with most CpGs carrying less than 20% or greater than 80% methylation (Figure S1A), the SLE-associated CpGs in T-cells were heavily enriched for intermediate methylation levels. As seen in Figure S1B, the majority of these CpGs had mean methylation levels between 20% and 80%, both in SLE patients and controls. This may indicate that these CpGs are either sites of dynamic regulation with fluctuating methylation levels that average to an intermediate level, or that they are sites with methylation levels that are specific to subpopulations of CD4+ T-cells, and that the mixed population of CD4+ subsets gives rise to an observation of intermediate methylation levels. A third possibility is that these CpGs are maintained in all cells at an intermediate methylation level, similar to an imprinted locus where only one allele is methylated. However, we found no enrichment for imprinted genes near these CpGs (data not shown).
Fig. 2. SLE QQ-Plots and the ratio of hyper- and hypomethylation events. 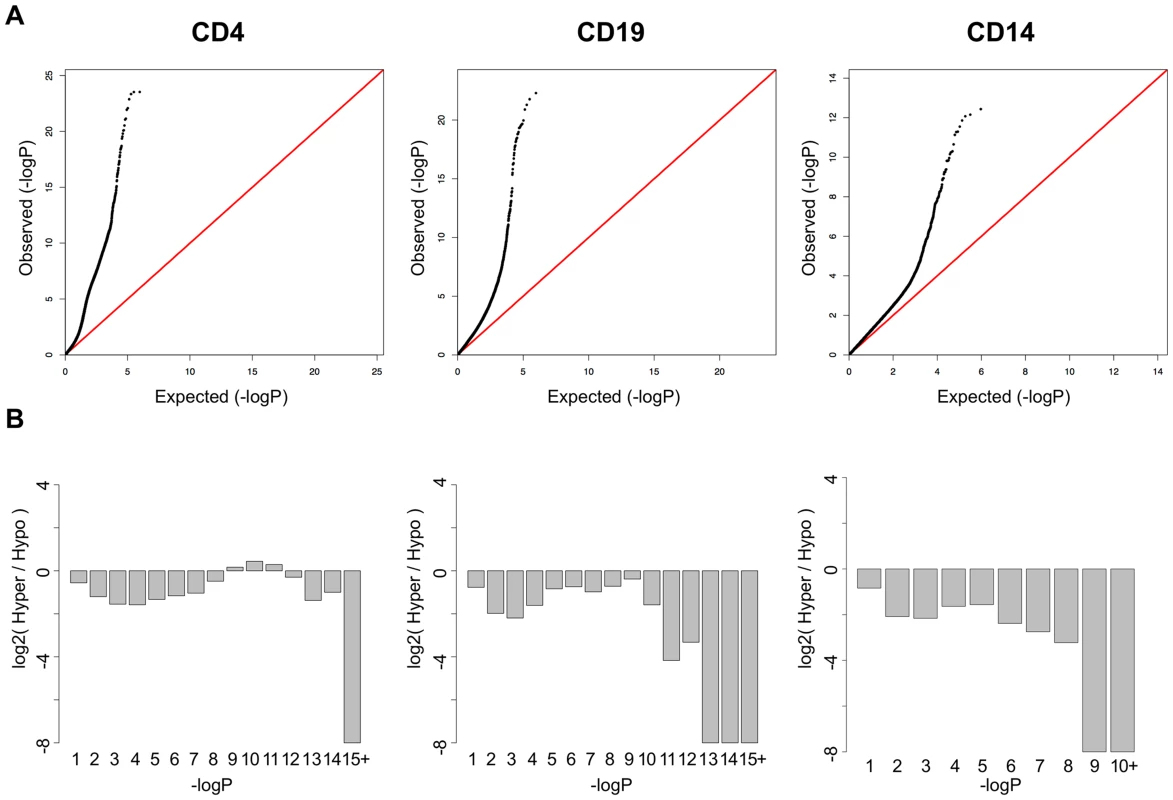
A. QQ-Plots of the p-values from the SLE association analysis for each cell-type, with a unique inflation in CD4+ T-cells. B. Log2 ratios of hyper-to-hypomethylated CpGs within bins of the p-value distribution for each cell type, showing a unique enrichment for hypermethylation among the significant CpGs in CD4+ T-cells. Functional Analysis
To identify common functional characteristics of genes with aberrant DNA methylation in SLE patients, we performed DAVID Panther GO term analysis and Ingenuity Pathway Analysis (IPA) on the genes that were proximal to each of the most significant (top 100) CpGs in each cell type. Both analyses clearly identified interferon signaling as a common feature of the genes showing the most significant changes in methylation among SLE patients. Table 1 and Table S7 list the results of these analyses, including the top Panther GO terms and IPA canonical pathways. IPA also indicated the type-I interferon IFNA2 (interferon alpha 2) as a common upstream regulator, so we suspect that type-I interferon pathways are the targets of the epigenetic changes in lupus. However, IL-29 (IFNL1) was also significant as a potential upstream regulator of these genes, so it is possible that both type-I and type-III interferons are contributing to the epigenetic patterns we observed.
Tab. 1. Functional analysis of significant CpGs in three cell types. 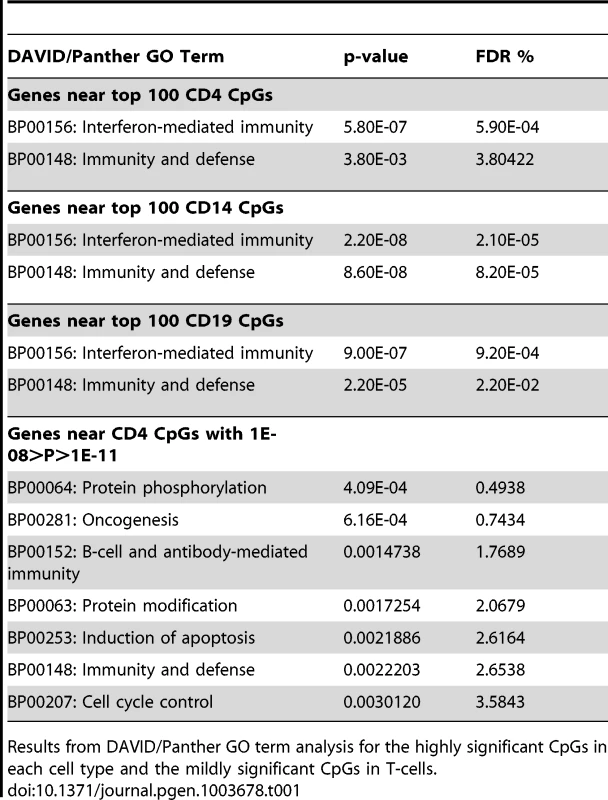
Results from DAVID/Panther GO term analysis for the highly significant CpGs in each cell type and the mildly significant CpGs in T-cells. As Panther and IPA use different gene annotations, their lists of interferon-regulated genes are not identical. Furthermore, many of the putative interferon-inducible genes (IFI44, IFITM1, etc.) are not always properly annotated with interferon GO terms. When we combined the gene lists from each software package with type-I interferon annotations and included these “IFI” genes, we found that at least half of the top 50 most significant CpGs in each cell type were proximal to genes involved in interferon signaling (50% in T-cells, 60% in B-cells, and 54% in monocytes). This represents more than 125 fold enrichment over the ∼0.4% of autosomal CpGs represented on the Methylation450 array that are adjacent to interferon type-I genes (Fisher's exact test p<5×10−46). Remarkably, of the 63 CpGs in T-cells, 58 CpGs in B-cells, and 23 CpGs in monocytes that had highly significant changes in methylation (p < 1×10−8) near an interferon type-I regulated gene, only 1 CpG in B-cells, located at the 3′ end of STAT3, was hypermethylated in SLE patients. Every other highly significant methylation change near an interferon gene was a hypomethylation effect. This widespread hypomethylation suggests that the primary methylation defect in SLE is a hyper-sensitization of interferon signaling pathways, and this is consistent with gene expression studies that have shown an overexpression of interferon-regulated genes in SLE patients, particularly during flares of the disease [17]–[19].
In addition to the most significant CpGs, we also performed a separate functional analysis of genes in the second phase of the T-cell p-value distribution (limited to p-values between 1×10−8 and 1×10−11), where we suspect a secondary phenomenon. Both Panther and IPA analyses indicated that these genes were enriched for functions associated with cell division and cancer. IPA specifically identified the p38 mitogen-activated protein kinase pathway as a common feature of these genes, a pathway that has been linked to autoimmune diseases, including SLE [20]. This functional difference between the two phases of the p-value distribution, in addition to the enrichment for hypermethylation effects and intermediate mean methylation levels, is further evidence that two independent phenomena were present in T-cells.
Disease Activity
Previous reports of increased expression of interferon-regulated genes in SLE patients have indicated that this effect is primarily observed during active phases of the disease, while those patients in quiescent phases have normal levels of expression. This observation coincides well with reports that circulating interferon levels correlate with disease activity [21]. We compared the DNA methylation levels between our active and quiescent SLE patients to identify activity-dependent methylation in these patients that might coincide with this gene expression effect. We performed regression analysis in a case-case comparison of flare versus quiescent SLE patients. As seen in the QQ plot from these association tests (Figure 3A), we found no significant differences between these groups. Regression analyses of methylation versus SLEDAI scores as continuous values were also negative (data not shown). Even the strong hypomethylation at interferon-regulated genes was similar in active and quiescent patients (Figure 3B), with no statistically significant difference between the disease groups in any cell type. These results indicate that the methylation changes in SLE persist beyond flares and may be maintained for many months after interferon levels normalize. It also indicates that SLE patients in quiescent stages remain poised for interferon response at an epigenetic level, with a significant number of immune cells carrying this phenotype.
Fig. 3. Disease activity QQ-Plots and the persistence of hypomethylation in quiescent patients. 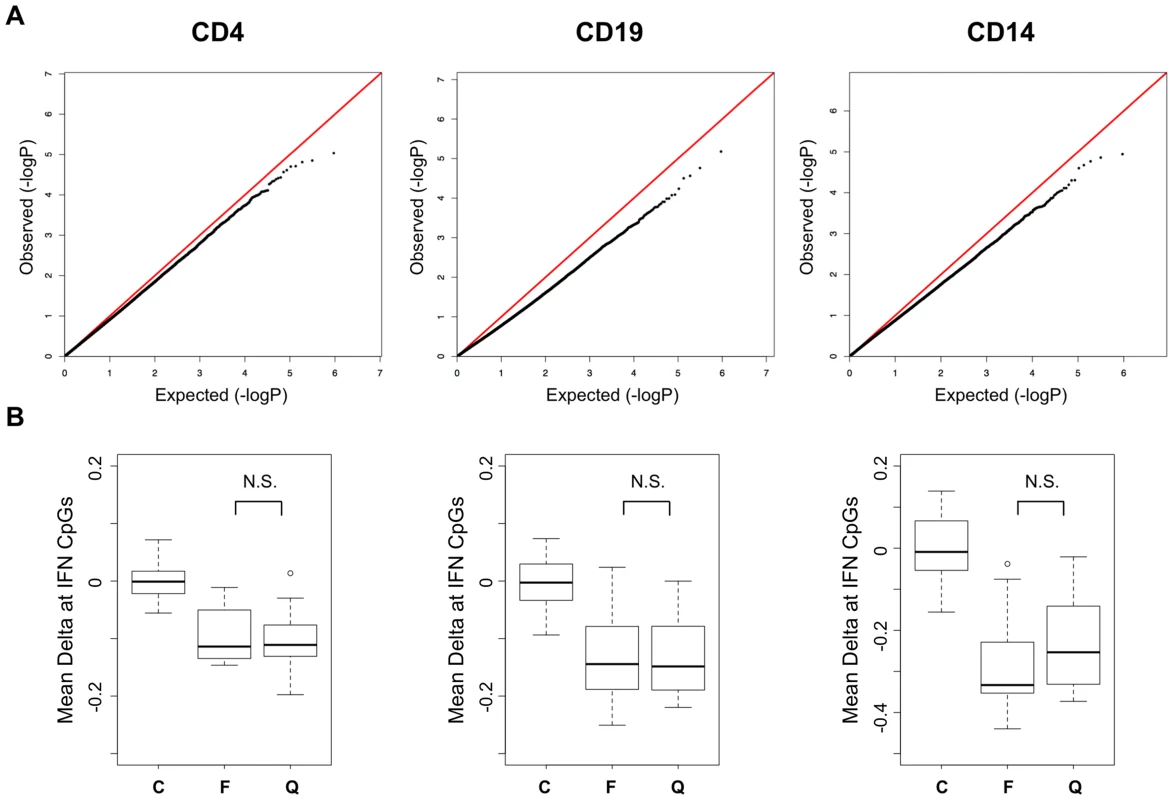
A. QQ-Plots of the p-values from the flare versus quiescent association analysis for each cell type, illustrating the lack of activity-dependent DNA methylation. B. Boxplots of the methylation difference between each individual and the mean of all controls at CpGs in IFN-regulated genes among those that were highly significant in the SLE-control tests. The groups are labeled C, Control, F, SLE collected during a flare, and Q, SLE collected during quiescence. CD4+ T-cell Subsets
One possible explanation for the persistent hypomethylation of interferon-regulated genes could be the endurance of memory cells that carry this epigenetic state since the last flare. Furthermore, some of the methylation changes we observed might be specific to T-cell subtypes, rather than a general feature of the CD4+ pool. To examine these possibilities, we collected CD4+ T-cells from an independent cohort of 26 SLE patients and 18 controls, and further sorted a fraction of these into CD45RA+RO − naïve, CD45RA-RO+ memory, and CD25+CD127 − regulatory T-cells. To ensure that this independent validation set recapitulated the results from our initial cohort, we re-tested for SLE-related methylation changes in CD4+ T-cells using our regression model at 1,031 CpGs that were highly significant in the initial cohort (2 of the original 1,033 failed QC in the validation set). Despite the smaller size of the validation set, 76.8% of the CpGs were significant at p<0.01 in these validation tests (see Figure 4A, black line, and 4B, gray bar). Furthermore, a comparison of the direction and amplitude of the changes in methylation observed in the SLE patients' T-cells indicated a very high correlation with the initial cohort (R2 = 0.92, see Figure 5A). These tests strongly validate our initial findings in an independent cohort.
Fig. 4. Comparison of the SLE-control p-values in the sorted T-cells from the validation cohort. 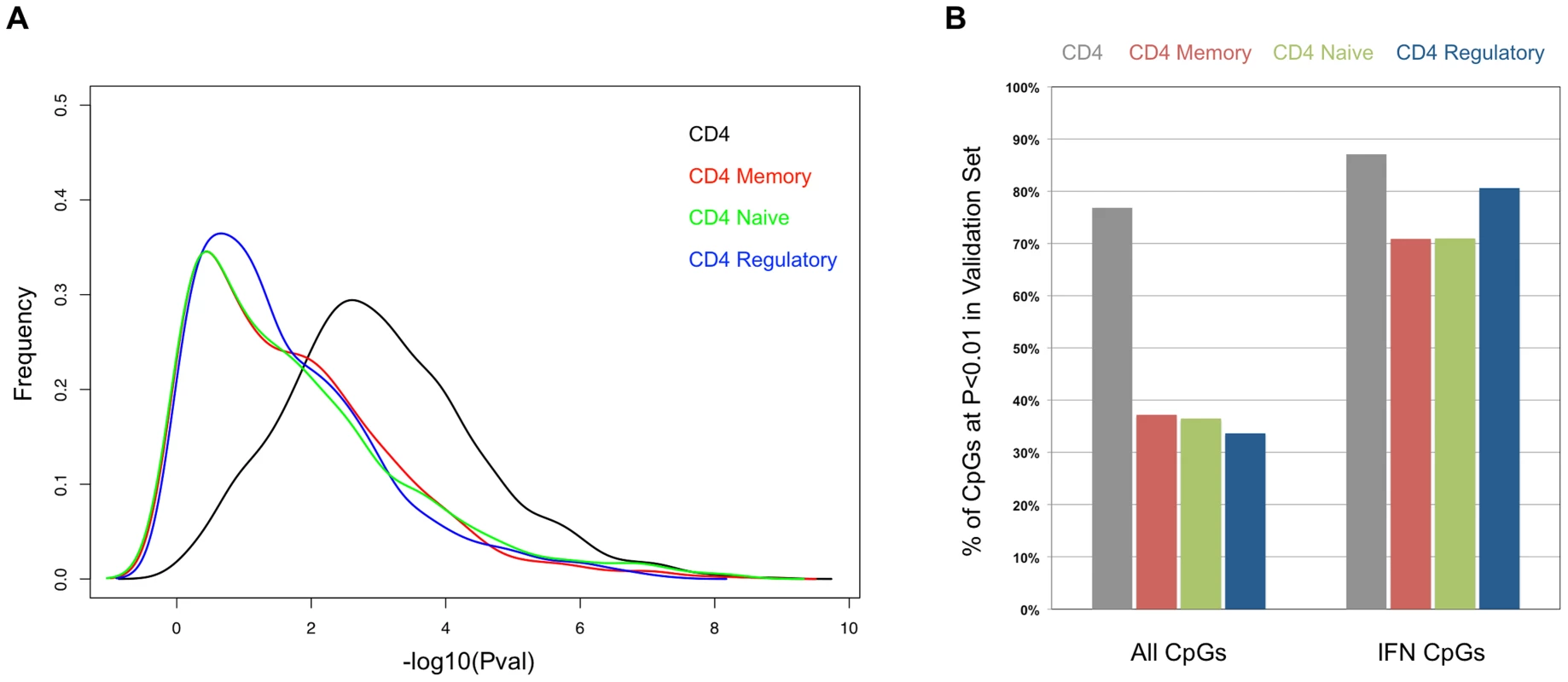
(A) Distribution of the −log10 of the p-values from association tests in the sorted T-cells cells from the validation cohort at CpGs that were highly significant (p<1×10−8) in the initial cohort. (B) Percentage of CpGs that reported p<0.01 in the sorted T-cells cells from the validation cohort. Values in the left set of bars are from all 1,031 CpGs tested. Values in the right set of bars are from the subset of 62 CpGs near interferon-regulated genes. Fig. 5. Comparison of the SLE-control methylation differences in sorted T-cell populations. 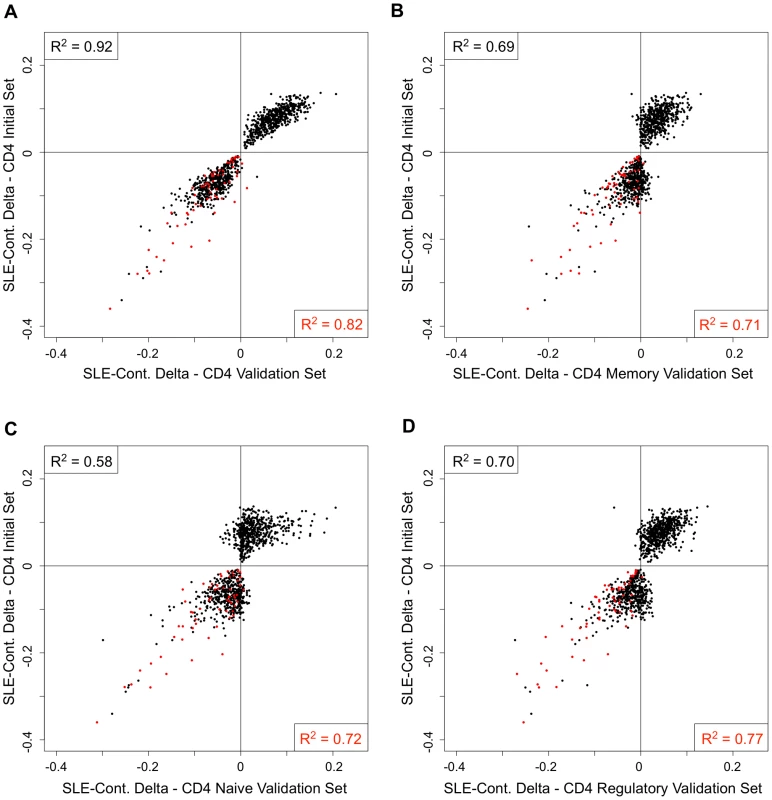
Each scatter plot represents 1,031 CpGs that had p<1×10−8 in CD4+ T-cells in our SLE-control association tests. The Y-axis for all plots is the mean SLE-control methylation delta at these CpGs in the initial cohort. The X-axis for each plot is the mean SLE-control methylation delta at the same CpGs in our validation cohort, using (A) total CD4+, (B) CD4+Memory, (C) CD4+Naïve, or (D) CD4+Regulatory cells. The red dots represent CpGs near IFN-regulated genes and the squared correlation coefficients (R2) represent the values for all plotted CpGs (upper left) or IFN CpGs only (lower right). We next tested for SLE-related methylation changes in the sorted T-cell subsets from the same individuals from our validation cohort. If any of the observed methylation changes were specific to memory, naïve, or regulatory T-cells, the enrichment of these cell types should reveal a stronger effect than is seen in the CD4+ pool as a whole. However, when we ran our regression tests on the same 1,031 CpGs in the sorted subsets, the distribution of p-values indicated much weaker effects than those seen in the CD4+ pool (Figure 4A). The number of CpGs that validated at p<0.01 was less than 38% in all three sorted subtypes, or approximately half of that observed in the CD4+ pool from the same individuals (Figure 4B). Furthermore, the correlations in direction and amplitude of the SLE-related methylation changes were weaker in the sorted CD4+ subtypes, where the R2 dropped below 0.70 for each sorted cell type (Figure 5 B–D).
When we limited our analysis to only those significant CpGs near interferon-regulated genes, the trend was dramatically different. The number of these CpGs that validated at p<0.01 was similar in the sorted subtypes (71% in naïve and memory, 81% in regulatory) compared to the CD4+ pool as a whole (87%) (Figure 4B). Furthermore, the direction and amplitude correlations with the initial CD4+ results were stronger at the interferon CpGs than at the non-interferon CpGs (Figure 5 B–D, red dots), but no stronger than the correlations observed in the CD4+ population as a whole. This suggests that the methylation changes we observed at interferon-regulated genes in CD4+ T-cells are intrinsic to memory, naïve, and regulatory T-cells, but not specific to any one population. So, it is unlikely that the persistence of these changes during quiescent stages of SLE can be explained simply by the endurance of memory cells. Furthermore, since the milder changes in methylation at non-interferon loci that were observed in the CD4+ T-cells, appear to be absent or greatly diminished in the sorted subtypes, the observed differences in methylation are not likely to be intrinsic to memory, naïve or regulatory T-cells. Thus, the most likely explanation for the widespread, moderate changes at thousands of CpGs in the CD4+ T-cells is a change in the composition of the CD4+ pool. Changes in the proportions of CD4+ subtypes in SLE patients would generate disease associations at any loci that had cell type-specific methylation patterns, and as we have observed, these loci would likely have intermediate mean methylation levels due to the mixture of these cell types in the CD4+ population. For example, a 10% methylation difference between SLE patients and controls could be due to a 50% difference in methylation within a CD4+ subtype that makes up 20% of CD4+ cells. Conversely, the same 10% methylation difference could be generated if that same subtype dropped in number among SLE patients to alter the composition of the CD4+ population. Any CpG with a subtype-specific methylation pattern would show this trend. Our data on sorted CD4+ subsets is consistent with the latter, as we observe a reduction, rather than an enrichment of the SLE-control methylation differences, as we purify CD4+ subtypes.
X-chromosome
The analysis of X-chromosome methylation is hampered by the inherent differences in methylation between males and females, so our disease association tests on this chromosome were limited to females, in which we have the largest sample size. For this reason, it is difficult to compare test statistics to those at the autosomal CpGs. Nonetheless, we ran regression tests at 11,122 X-chromosome CpGs to compare female SLE patients to females controls. Only in T-cells did we observe moderately significant associations (FDR<1%), although none were genome-wide significant (p<1×10−8). Table S6 lists the 43 significant X-chromosome CpGs in T-cells. These include TLR7 and FOXP3, both of which have been previously linked to SLE.
Discussion
We have performed a comprehensive analysis of DNA methylation changes in SLE in two lymphoid cell-types (T - and B-cells), and one myeloid cell-type (monocytes). Our analysis has identified a strong hypomethylation of loci involved in type-I interferon signaling, which indicates that SLE patients are hypersensitive to interferon. While this is not entirely surprising, given that interferon-related gene expression changes have been documented in active SLE patients, we have also discovered that the hypomethylation is observed in both active and quiescent patients. This is remarkable because circulating interferon and the expression of the genes it induces, are known to increase during flares of the disease, but return to normal during quiescent periods. So, the epigenetic hypersensitivity at the DNA methylation level appears to be independent of interferon levels and is maintained in the immune system beyond active stages of the disease. Exactly when these epigenetic changes occur is not clear. Studies have demonstrated mildly elevated IFN-α in unaffected relatives of SLE patients, suggesting that there is a genetic basis of higher IFN levels [22]. So it is feasible that SLE patients had higher baseline IFN prior to disease onset, and that chronic exposure could have induced long-lasting epigenetic hypersensitivity. In any case, the persistence of the hypomethylation in patients during quiescence is important, as it may help explain the chronic nature of the disease and the potential for recurrent flares in SLE patients. Our data suggest that these patients are poised for elevated interferon responses, but until some event triggers IFN-α production, the responsive genes remain near normal expression levels.
We have also observed the hypomethylation of interferon genes in sorted subpopulations of CD4+ T-cells, including memory, naïve and regulatory T-cells. Given that this appears to be a universal effect, and is apparent in lymphoid and myeloid lineages, the most likely explanation is that a multi-potent progenitor population carries this epigenetic state and produces lineages that are programmed to respond to interferon. Future studies of DNA methylation in early progenitor populations from SLE patients will be needed to establish the responsible cells, and to define the events that might induce this epigenetic state in progenitor cells.
In addition to the primary interferon effect, we have identified widespread moderate changes in methylation in T-cells that are best explained by SLE-related compositional changes to the CD4+ population, rather than intrinsic methylation changes in any CD4+ subtype. We did not observe an enrichment of these effects in sorted memory, naïve or regulatory T-cells, although we cannot rule out a role for subtypes such as Th1, Th2, or Th17, as we did not sort along these lines. This is not to suggest that methylation effects are absent from CD4+ subtypes, but rather that the widespread, moderate changes we can observe in the CD4+ population cannot be explained solely by intrinsic methylation changes in memory, naïve or regulatory T-cells. Further sub-fractionation of the CD4+ cells will be required to establish which subtypes are responsible for these subtle changes in SLE patients, either because they carry subtype-specific methylation patterns and are changing in number, or because they carry intrinsic methylation differences in SLE patients. Some studies have indicated that regulatory T-cells are reduced in number in SLE patients. While this may be one contributor to the compositional effect, our quantification of memory, naïve and regulatory T-cells is insufficient to explain the entirety of the methylation changes we observe in CD4+ cells. Our functional analysis of the genes affected by these methylation changes, indicate that they are involved in immune cell signaling and cell division. All of these might be interpreted as part of the T-cell activation process, and perhaps the compositional changes occurring in the CD4+ population are due to increases in the number of activated T-cells that cut across traditional definitions of the CD4+ subsets. A complete characterization of the genome-wide DNA methylation profiles in the CD4+ milieu will be required to understand how different epigenetic states correlate with classic cell type definitions.
Finally, while our study was not designed to detect methylation patterns that were induced by medications or might be predictive of a patient's response to medications, this is clearly an area of great interest. The fact that we observe similar methylation patterns in quiescent and active SLE patients, who typically increase their medication levels during a flare, suggests that these medications do not induce a large epigenetic effect. Nonetheless, studies that examine the epigenetic impact of anti-inflammatories, as well as the epigenetic states that modulate their efficacy, may have an impact on the clinical management of SLE.
Materials and Methods
Ethics Statement
All patient samples were collected with consent at UAB under compliance with the Institutional Review Board.
Patient Samples
Patients were recruited through the UAB outpatient Rheumatology clinic. Diagnosis was performed according to revised ACR criteria [23]–[25] and disease activity and SLEDAI scores were collected from each patient, along with gender, age and ethnicity information. Disease activity (flare versus quiescent) was defined by a recent increase in SLEDAI without using a specific SLEDAI threshold. However, all patients considered to be active had a SLEDAI > = 4 (mean = 8.5), and all of our quiescent patients had a SLEDAI < = 6 (mean = 1.5).
Cell and DNA Isolation
CD4+ T-cells, CD19+ B-cells and CD14+ monocytes were isolated from ∼5 ml each of freshly collected peripheral blood. All three cell types were isolated in parallel using positive selection by antigen-specific Dynabeads (Invitrogen), according to the manufacturer's standard protocol. The cells captured on the beads were lysed and DNA was extracted with QIAGEN DNAeasy kits. Purity of separated populations was verified to be above 95%.
For experiments with CD4+ subsets, CD4+ cells were isolated using positive selection (Invitrogen) followed by sorting of subsets by flow cytometry (FACSAriaII, BD Biosciences). Very pure populations (95–100%) of memory T cells (CD45RO+RA−), naïve T cells (CD45RA+RO−), and T regulatory cells (CD25+CD127−) were collected using anti-CD4-Alexa488, anti-CD45RO-APC, anti-CD45RA-PE, anti-CD25-PerCP-Cy5.5 and anti-CD127-Pacific Blue antibodies (Biolegend, Inc). Cells were then lysed and DNA extracted with QIAGEN DNAeasy kits.
Methylation450 Assays, Data QC and Batch Normalization
500 ng of each DNA sample was treated with sodium bisulfite (Zymo EZ DNA) prior to standard Illumina amplification, hybridization, and imaging steps. To limit confounding from batch effects, we distributed SLE cases and controls equally among the 12 slots on each array. The samples were also grouped on the arrays by cell type. The resulting intensity files were analyzed with Illumina's GenomeStudio, which generated beta scores (proportion of total signal from the methylation-specific probe or color channel) and “detection p-values” (probability that the total intensity for a given probe falls within the background signal intensity). Beta scores were generated without background subtraction or Illlumina normalization options. Those beta scores with an associated detection p-value greater than 0.01 were removed and samples with more than 1.5% missing data points across ∼470,000 autosomal CpGs were eliminated from further analysis. Furthermore, any CpG probes where more than 10% of samples failed to yield adequate intensity were removed.
The filtered beta scores were then subjected to non-parametric batch normalization with the ComBat package for R software (http://http://www.bu.edu/jlab/wp-assets/ComBat/Abstract.html). To parallelize this process on our computational cluster, normalization was performed on non-overlapping subsets of no more than 20,000 CpGs per job (randomly selected), and each array of 12 samples was used as a “batch”. We also separately normalized probes from the Infinium I and II chemistries, as their beta score distributions are slightly different. For example, the 131,715 autosomal Infinium I CpGs were split into 6 randomly chosen sets of 20,000 CpGs each, plus one set of 11,715 CpGs, and each set was batch normalized in parallel. Figure S3A shows QQ-plots of explicit tests for batch effects at each CpG, before and after ComBat normalization. These tests were linear regression tests for batch ID, with disease, age, gender, and ethnicity as covariates. In addition, we compared our subsetting approach of 20,000 CpGs to similar ComBat runs with larger numbers of CpGs, but the efficacy of batch correction was virtually identical, while greatly reducing the computational time for normalization. Furthermore, as indicated in Figure S3B, our batch normalization process did not introduce any systematic bias into our data, as our disease-specific regression results applied before and after ComBat were highly similar. Data from the X chromosome was normalized separately for males and females due to the gender-specific effect of X-inactivation on the beta score distribution. After batch normalization, we further adjusted the beta scores for probes that utilized the Infinium II chemistry to better match the Infinium I chemistry using the equation β′ = 0.001514 + 0.3323* β + 0.7411* β 2. This equation was derived from fitting a second order polynomial to the observed pairs of beta scores across all pairs of probes located <50 bp apart, where one probe was Infinium I and one was Infinium II. At this proximity, within-chemistry correlations are extremely high (R>0.99) due to locally correlated methylation patterns, and the non-linear relationship between the two chemistries is easily estimated. Figure S4 illustrates the improved scaling of the two chemistries after our corrections have been applied.
Our dataset was further reduced by eliminating any CpGs where the probe sequence either mapped to a location in the genome that was different that the location found in Illumina's annotation file, or where the probe could potentially map to more than one locus. The list of these problematic CpGs was generated by re-aligning all probes (with unconverted Cs) to the human reference genome with BLAT. We also maintained a list of probes where known SNPs would fall within the probe sequence or at the CpG itself, but did not explicitly filter out these probes. There was no apparent enrichment for CpG probes that overlapped a SNP in dbSNP 135 among our most significant results.
Data Analysis
To perform genome-wide association testing, we ran linear regression models at each CpG (lm package in R) to test for associations between DNA methylation levels and SLE disease state (case/control comparison) or flare status (case/case comparison). Since DNA methylation is influenced by age, gender, and ethnicity, we included these as covariates in our models.
For analysis of the X-chromosome CpGs, females were analyzed separately so gender correction was unnecessary. The p-values and beta coefficients for the disease term in our regression models were used to establish the significance of the association at each CpG, and to estimate the post-correction differences in methylation between cases and controls, respectively. FDR correction was performed on the p-values using R (p.adjust function). We also selected 20,000 CpGs at random to perform permutation tests that randomized the disease state variable to estimate empirical p-values (lmp package). After 108 permutations, the permutation-based p-value was compared to the regression estimate, and both p-values were highly correlated. Figure S2 displays the genome-wide QQ plot for CD4+ cells, with the permuted p-values for 20,000 random CpGs overlayed in green. The biphasic trend in the QQ plot was recapitulated with permutation-based p-values.
We performed two types of analyses (Ingenuity and DAVID) to identify gene annotation terms that were enriched among our most significant associations. Our annotation of interferon-regulated genes was expanded to include the “IFI” gene symbols, which have been termed “interferon-inducible transcripts”, but have not all been given GO terms that reflect this functionality.
Supporting Information
Zdroje
1. HirakiLT, FeldmanCH, LiuJ, AlarcónGS, FischerMA, et al. (2012) Prevalence, incidence, and demographics of systemic lupus erythematosus and lupus nephritis from 2000 to 2004 among children in the US medicaid beneficiary population. Arthritis Rheum 64 : 2669–2676 doi:10.1002/art.34472
2. Alarcón-SegoviaD, Alarcón-RiquelmeME, CardielMH, CaeiroF, MassardoL, et al. (2005) Familial aggregation of systemic lupus erythematosus, rheumatoid arthritis, and other autoimmune diseases in 1,177 lupus patients from the GLADEL cohort. Arthritis Rheum 52 : 1138–1147 doi:10.1002/art.20999
3. DeapenD, EscalanteA, WeinribL, HorwitzD, BachmanB, et al. (1992) A revised estimate of twin concordance in systemic lupus erythematosus. Arthritis Rheum 35 : 311–318.
4. LawrenceJS, MartinsCL, DrakeGL (1987) A family survey of lupus erythematosus. 1. Heritability. J Rheumatol 14 : 913–921.
5. GatevaV, SandlingJK, HomG, TaylorKE, ChungSA, et al. (2009) A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1 and IL10 as risk loci for systemic lupus erythematosus. Nat Genet 41 : 1228–1233 doi:10.1038/ng.468
6. GrahamRR, HomG, OrtmannW, BehrensTW (2009) Review of recent genome-wide association scans in lupus. Journal of Internal Medicine 265 : 680–688 doi:10.1111/j.1365-2796.2009.02096.x
7. HanJW, ZhengHF, CuiY, SunLD, YeDQ, et al. (2009) Genome-wide association study in a Chinese Han population identifies nine new susceptibility loci for systemic lupus erythematosus. Nature Genetics 41 : 1234–1237.
8. SawalhaAH, WebbR, HanS, KellyJA, KaufmanKM, et al. (2008) Common variants within MECP2 confer risk of systemic lupus erythematosus. PLoS ONE 3: e1727 doi:10.1371/journal.pone.0001727
9. YangW, ShenN, YeD-Q, LiuQ, ZhangY, et al. (2010) Genome-wide association study in Asian populations identifies variants in ETS1 and WDFY4 associated with systemic lupus erythematosus. PLoS Genet 6: e1000841 doi:10.1371/journal.pgen.1000841
10. ZhouX-JX, LuX-LX, NathSKS, LvJ-CJ, ZhuS-NS, et al. (2012) Gene-gene interaction of BLK, TNFSF4, TRAF1, TNFAIP3, and REL in systemic lupus erythematosus. Arthritis Rheum 64 : 222–231 doi:10.1002/art.33318
11. RichardsonBB, ScheinbartLL, StrahlerJJ, GrossLL, HanashSS, et al. (1990) Evidence for impaired T cell DNA methylation in systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum 33 : 1665–1673 doi:10.1002/art.1780331109
12. QuddusJ, JohnsonKJ, GavalchinJ (1993) Treating activated CD4+ T cells with either of two distinct DNA methyltransferase inhibitors, 5-azacytidine or procainamide, is sufficient to cause a lupus-like disease in syngeneic mice. Journal of Clinical Investigation 92 : 38–53.
13. CornacchiaE, GolbusJ, MaybaumJ, StrahlerJ, HanashS, et al. (1988) Hydralazine and procainamide inhibit T cell DNA methylation and induce autoreactivity. J Immunol 140 : 2197–2200.
14. JavierreBM, FernandezAF, RichterJ, Al-ShahrourF, Martin-SuberoJI, et al. (2010) Changes in the pattern of DNA methylation associate with twin discordance in systemic lupus erythematosus. Genome Res 20 : 170–179 doi:10.1101/gr.100289.109
15. JeffriesMA, DozmorovM, TangY, MerrillJT, WrenJD, et al. (2011) Genome-wide DNA methylation patterns in CD4+ T cells from patients with systemic lupus erythematosus. Epigenetics 6 : 593–601.
16. LinS-Y, HsiehS-C, LinY-C, LeeC-N, TsaiM-H, et al. (2012) A whole genome methylation analysis of systemic lupus erythematosus: hypomethylation of the IL10 and IL1R2 promoters is associated with disease activity. Genes Immun 13 : 214–220 doi:10.1038/gene.2011.74
17. BaechlerEC, BatliwallaFM, KarypisG, GaffneyPM, OrtmannWA, et al. (2003) Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci USA 100 : 2610–2615 doi:10.1073/pnas.0337679100
18. KirouKA, LeeC, GeorgeS, LoucaK, PapagiannisIG, et al. (2004) Coordinate overexpression of interferon-?-induced genes in systemic lupus erythematosus. Arthritis Rheum 50 : 3958–3967 doi:10.1002/art.20798
19. KirouKA, LeeC, GeorgeS, LoucaK, PetersonMGE, et al. (2005) Activation of the interferon-α pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum 52 : 1491–1503 doi:10.1002/art.21031
20. DengCC, KaplanMJM, YangJJ, RayDD, ZhangZZ, et al. (2001) Decreased Ras-mitogen-activated protein kinase signaling may cause DNA hypomethylation in T lymphocytes from lupus patients. Arthritis Rheum 44 : 397–407 doi:;10.1002/1529-0131(200102)44 : 2<397::AID-ANR59>3.0.CO;2-N
21. Becker-MerokA, Ostli-EilerstenG, LesterS, NossentJ (2012) Circulating interferon-α2 levels are increased in the majority of patients with systemic lupus erythematosus and are associated with disease activity and multiple cytokine activation. Lupus 22 : 155–63 doi:10.1177/0961203312468964
22. NiewoldTB, HuaJ, LehmanTJA, HarleyJB, CrowMK (2007) High serum IFN-α activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun 8 : 492–502 doi:10.1038/sj.gene.6364408
23. HochbergMC (1997) Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40 : 1725–1725 doi:;10.1002/1529-0131(199709)40 : 9<1725::AID-ART29>3.0.CO;2-Y
24. PetriMM, OrbaiA-MA, AlarcónGSG, GordonCC, MerrillJTJ, et al. (2012) Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 64 : 2677–2686 doi:10.1002/art.34473
25. TanEME, CohenASA, FriesJFJ, MasiATA, McShaneDJD, et al. (1982) The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 25 : 1271–1277 doi:10.1002/art.1780251101
Štítky
Genetika Reprodukční medicína
Článek Re-Ranking Sequencing Variants in the Post-GWAS Era for Accurate Causal Variant IdentificationČlánek Bypass of 8-oxodGČlánek Integrated Model of and Inherited Genetic Variants Yields Greater Power to Identify Risk GenesČlánek Comparative Genomic and Functional Analysis of 100 Strains and Their Comparison with Strain GGČlánek A Nuclear Calcium-Sensing Pathway Is Critical for Gene Regulation and Salt Stress Tolerance inČlánek Computational Identification of Diverse Mechanisms Underlying Transcription Factor-DNA OccupancyČlánek Reversible and Rapid Transfer-RNA Deactivation as a Mechanism of Translational Repression in StressČlánek Genome-Wide Association of Body Fat Distribution in African Ancestry Populations Suggests New Loci
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 8- Souvislost haplotypu M2 genu pro annexin A5 s opakovanými reprodukčními ztrátami
- Intrauterinní inseminace a její úspěšnost
- Pánevní endometrióza spojená s volnou tekutinou v peritoneální dutině snižuje úspěšnost otěhotnění po intrauterinní inseminaci
- Mateřský haplotyp KIR ovlivňuje porodnost živých dětí po transferu dvou embryí v rámci fertilizace in vitro u pacientek s opakujícími se samovolnými potraty nebo poruchami implantace
- Akutní intermitentní porfyrie
-
Všechny články tohoto čísla
- Genome-Wide DNA Methylation Analysis of Systemic Lupus Erythematosus Reveals Persistent Hypomethylation of Interferon Genes and Compositional Changes to CD4+ T-cell Populations
- Re-Ranking Sequencing Variants in the Post-GWAS Era for Accurate Causal Variant Identification
- Histone Variant HTZ1 Shows Extensive Epistasis with, but Does Not Increase Robustness to, New Mutations
- Past Visits Present: TCF/LEFs Partner with ATFs for β-Catenin–Independent Activity
- Functional Characterisation of Alpha-Galactosidase A Mutations as a Basis for a New Classification System in Fabry Disease
- A Flexible Approach for the Analysis of Rare Variants Allowing for a Mixture of Effects on Binary or Quantitative Traits
- Masculinization of Gene Expression Is Associated with Exaggeration of Male Sexual Dimorphism
- Genic Intolerance to Functional Variation and the Interpretation of Personal Genomes
- Endogenous Stress Caused by Faulty Oxidation Reactions Fosters Evolution of 2,4-Dinitrotoluene-Degrading Bacteria
- Transposon Domestication versus Mutualism in Ciliate Genome Rearrangements
- Comparative Anatomy of Chromosomal Domains with Imprinted and Non-Imprinted Allele-Specific DNA Methylation
- An Essential Function for the ATR-Activation-Domain (AAD) of TopBP1 in Mouse Development and Cellular Senescence
- Depletion of Retinoic Acid Receptors Initiates a Novel Positive Feedback Mechanism that Promotes Teratogenic Increases in Retinoic Acid
- Bypass of 8-oxodG
- Calpain-6 Deficiency Promotes Skeletal Muscle Development and Regeneration
- ATM Release at Resected Double-Strand Breaks Provides Heterochromatin Reconstitution to Facilitate Homologous Recombination
- Generation of Tandem Direct Duplications by Reversed-Ends Transposition of Maize Elements
- Loss of a Conserved tRNA Anticodon Modification Perturbs Cellular Signaling
- Integrated Model of and Inherited Genetic Variants Yields Greater Power to Identify Risk Genes
- High-Throughput Genetic and Gene Expression Analysis of the RNAPII-CTD Reveals Unexpected Connections to SRB10/CDK8
- Dynamic Rewiring of the Retinal Determination Network Switches Its Function from Selector to Differentiation
- β-Catenin-Independent Activation of TCF1/LEF1 in Human Hematopoietic Tumor Cells through Interaction with ATF2 Transcription Factors
- Genetic Mapping of Specific Interactions between Mosquitoes and Dengue Viruses
- A Highly Redundant Gene Network Controls Assembly of the Outer Spore Wall in
- Origin and Functional Diversification of an Amphibian Defense Peptide Arsenal
- Myc-Driven Overgrowth Requires Unfolded Protein Response-Mediated Induction of Autophagy and Antioxidant Responses in
- Integrative Modeling of eQTLs and Cis-Regulatory Elements Suggests Mechanisms Underlying Cell Type Specificity of eQTLs
- Species and Population Level Molecular Profiling Reveals Cryptic Recombination and Emergent Asymmetry in the Dimorphic Mating Locus of
- Ras-Induced Changes in H3K27me3 Occur after Those in Transcriptional Activity
- Characterization of the p53 Cistrome – DNA Binding Cooperativity Dissects p53's Tumor Suppressor Functions
- Global Analysis of Fission Yeast Mating Genes Reveals New Autophagy Factors
- Deficiency Suppresses Intestinal Tumorigenesis
- Introns Regulate Gene Expression in in a Pab2p Dependent Pathway
- Meiotic Recombination Initiation in and around Retrotransposable Elements in
- Comparative Oncogenomic Analysis of Copy Number Alterations in Human and Zebrafish Tumors Enables Cancer Driver Discovery
- Comparative Genomic and Functional Analysis of 100 Strains and Their Comparison with Strain GG
- A Model-Based Analysis of GC-Biased Gene Conversion in the Human and Chimpanzee Genomes
- Masculinization of the X Chromosome in the Pea Aphid
- The Architecture of a Prototypical Bacterial Signaling Circuit Enables a Single Point Mutation to Confer Novel Network Properties
- Distinct SUMO Ligases Cooperate with Esc2 and Slx5 to Suppress Duplication-Mediated Genome Rearrangements
- The Yeast Environmental Stress Response Regulates Mutagenesis Induced by Proteotoxic Stress
- Mediator Directs Co-transcriptional Heterochromatin Assembly by RNA Interference-Dependent and -Independent Pathways
- The Genome of and the Basis of Host-Microsporidian Interactions
- Regulation of Sister Chromosome Cohesion by the Replication Fork Tracking Protein SeqA
- Neuronal Reprograming of Protein Homeostasis by Calcium-Dependent Regulation of the Heat Shock Response
- A Nuclear Calcium-Sensing Pathway Is Critical for Gene Regulation and Salt Stress Tolerance in
- Cross-Species Array Comparative Genomic Hybridization Identifies Novel Oncogenic Events in Zebrafish and Human Embryonal Rhabdomyosarcoma
- : A Mouse Strain with an Ift140 Mutation That Results in a Skeletal Ciliopathy Modelling Jeune Syndrome
- The Relative Contribution of Proximal 5′ Flanking Sequence and Microsatellite Variation on Brain Vasopressin 1a Receptor () Gene Expression and Behavior
- Combining Quantitative Genetic Footprinting and Trait Enrichment Analysis to Identify Fitness Determinants of a Bacterial Pathogen
- The Innocence Project at Twenty: An Interview with Barry Scheck
- Computational Identification of Diverse Mechanisms Underlying Transcription Factor-DNA Occupancy
- GUESS-ing Polygenic Associations with Multiple Phenotypes Using a GPU-Based Evolutionary Stochastic Search Algorithm
- H2A.Z Acidic Patch Couples Chromatin Dynamics to Regulation of Gene Expression Programs during ESC Differentiation
- Identification of DSB-1, a Protein Required for Initiation of Meiotic Recombination in , Illuminates a Crossover Assurance Checkpoint
- Binding of TFIIIC to SINE Elements Controls the Relocation of Activity-Dependent Neuronal Genes to Transcription Factories
- Global Analysis of the Sporulation Pathway of
- Genetic Circuits that Govern Bisexual and Unisexual Reproduction in
- Deletion of microRNA-80 Activates Dietary Restriction to Extend Healthspan and Lifespan
- Fifty Years On: GWAS Confirms the Role of a Rare Variant in Lung Disease
- The Enhancer Landscape during Early Neocortical Development Reveals Patterns of Dense Regulation and Co-option
- Gene Expression Regulation by Upstream Open Reading Frames and Human Disease
- Sociogenomics of Cooperation and Conflict during Colony Founding in the Fire Ant
- The Intronic Long Noncoding RNA Recruits PRC2 to the Promoter, Reducing the Expression of and Increasing Cell Proliferation
- The , p.E318G Variant Increases the Risk of Alzheimer's Disease in -ε4 Carriers
- The Wilms Tumor Gene, , Is Critical for Mouse Spermatogenesis via Regulation of Sertoli Cell Polarity and Is Associated with Non-Obstructive Azoospermia in Humans
- Reversible and Rapid Transfer-RNA Deactivation as a Mechanism of Translational Repression in Stress
- QTL Analysis of High Thermotolerance with Superior and Downgraded Parental Yeast Strains Reveals New Minor QTLs and Converges on Novel Causative Alleles Involved in RNA Processing
- Genome Wide Association Identifies Novel Loci Involved in Fungal Communication
- Chromatin Sampling—An Emerging Perspective on Targeting Polycomb Repressor Proteins
- A Recessive Founder Mutation in Regulator of Telomere Elongation Helicase 1, , Underlies Severe Immunodeficiency and Features of Hoyeraal Hreidarsson Syndrome
- Genome-Wide Association of Body Fat Distribution in African Ancestry Populations Suggests New Loci
- Causal and Synthetic Associations of Variants in the Gene Cluster with Alpha1-antitrypsin Serum Levels
- Hard Selective Sweep and Ectopic Gene Conversion in a Gene Cluster Affording Environmental Adaptation
- Brittle Culm1, a COBRA-Like Protein, Functions in Cellulose Assembly through Binding Cellulose Microfibrils
- Chromosomal Copy Number Variation, Selection and Uneven Rates of Recombination Reveal Cryptic Genome Diversity Linked to Pathogenicity
- The Ribosomal Protein Rpl22 Controls Ribosome Composition by Directly Repressing Expression of Its Own Paralog, Rpl22l1
- Ras1 Acts through Duplicated Cdc42 and Rac Proteins to Regulate Morphogenesis and Pathogenesis in the Human Fungal Pathogen
- The DSB-2 Protein Reveals a Regulatory Network that Controls Competence for Meiotic DSB Formation and Promotes Crossover Assurance
- Recurrent Modification of a Conserved -Regulatory Element Underlies Fruit Fly Pigmentation Diversity
- Associations of Mitochondrial Haplogroups B4 and E with Biliary Atresia and Differential Susceptibility to Hydrophobic Bile Acid
- The Conditional Nature of Genetic Interactions: The Consequences of Wild-Type Backgrounds on Mutational Interactions in a Genome-Wide Modifier Screen
- A Critical Function of Mad2l2 in Primordial Germ Cell Development of Mice
- A Role for CF1A 3′ End Processing Complex in Promoter-Associated Transcription
- Vitellogenin Underwent Subfunctionalization to Acquire Caste and Behavioral Specific Expression in the Harvester Ant
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Chromosomal Copy Number Variation, Selection and Uneven Rates of Recombination Reveal Cryptic Genome Diversity Linked to Pathogenicity
- Genome-Wide DNA Methylation Analysis of Systemic Lupus Erythematosus Reveals Persistent Hypomethylation of Interferon Genes and Compositional Changes to CD4+ T-cell Populations
- Associations of Mitochondrial Haplogroups B4 and E with Biliary Atresia and Differential Susceptibility to Hydrophobic Bile Acid
- A Role for CF1A 3′ End Processing Complex in Promoter-Associated Transcription
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání




