-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaWidespread Dysregulation of Peptide Hormone Release in Mice Lacking Adaptor Protein AP-3
The regulated secretion of peptide hormones, neural peptides and many growth factors depends on their sorting into large dense core vesicles (LDCVs) capable of regulated exocytosis. LDCVs form at the trans-Golgi network, but the mechanisms that sort proteins to this regulated secretory pathway and the cytosolic machinery that produces LDCVs remain poorly understood. Recently, we used an RNAi screen to identify a role for heterotetrameric adaptor protein AP-3 in regulated secretion and in particular, LDCV formation. Indeed, mocha mice lacking AP-3 have a severe neurological and behavioral phenotype, but this has been attributed to a role for AP-3 in the endolysosomal rather than biosynthetic pathway. We therefore used mocha mice to determine whether loss of AP-3 also dysregulates peptide release in vivo. We find that adrenal chromaffin cells from mocha animals show increased constitutive exocytosis of both soluble cargo and LDCV membrane proteins, reducing the response to stimulation. We also observe increased basal release of both insulin and glucagon from pancreatic islet cells of mocha mice, suggesting a global disturbance in the release of peptide hormones. AP-3 exists as both ubiquitous and neuronal isoforms, but the analysis of mice lacking each of these isoforms individually and together shows that loss of both is required to reproduce the effect of the mocha mutation on the regulated pathway. In addition, we show that loss of the related adaptor protein AP-1 has a similar effect on regulated secretion but exacerbates the effect of AP-3 RNAi, suggesting distinct roles for the two adaptors in the regulated secretory pathway.
Published in the journal: . PLoS Genet 9(9): e32767. doi:10.1371/journal.pgen.1003812
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003812Summary
The regulated secretion of peptide hormones, neural peptides and many growth factors depends on their sorting into large dense core vesicles (LDCVs) capable of regulated exocytosis. LDCVs form at the trans-Golgi network, but the mechanisms that sort proteins to this regulated secretory pathway and the cytosolic machinery that produces LDCVs remain poorly understood. Recently, we used an RNAi screen to identify a role for heterotetrameric adaptor protein AP-3 in regulated secretion and in particular, LDCV formation. Indeed, mocha mice lacking AP-3 have a severe neurological and behavioral phenotype, but this has been attributed to a role for AP-3 in the endolysosomal rather than biosynthetic pathway. We therefore used mocha mice to determine whether loss of AP-3 also dysregulates peptide release in vivo. We find that adrenal chromaffin cells from mocha animals show increased constitutive exocytosis of both soluble cargo and LDCV membrane proteins, reducing the response to stimulation. We also observe increased basal release of both insulin and glucagon from pancreatic islet cells of mocha mice, suggesting a global disturbance in the release of peptide hormones. AP-3 exists as both ubiquitous and neuronal isoforms, but the analysis of mice lacking each of these isoforms individually and together shows that loss of both is required to reproduce the effect of the mocha mutation on the regulated pathway. In addition, we show that loss of the related adaptor protein AP-1 has a similar effect on regulated secretion but exacerbates the effect of AP-3 RNAi, suggesting distinct roles for the two adaptors in the regulated secretory pathway.
Introduction
In contrast to most proteins which undergo immediate and unregulated secretion after biosynthesis, proteins destined for regulated release require sorting into LDCVs, but the mechanisms responsible for sorting to LDCVs and indeed LDCV formation remain poorly understood. LDCVs bud from the trans-Golgi network (TGN) [1], [2], [3], and previous work has suggested that lumenal interactions such as the aggregation of granulogenic proteins drive their formation [4], [5]. Indeed, sorting to LDCVs has been suggested to occur by default, with proteins destined for other organelles removed during the well-established process of LDCV maturation [6], [7]. However, direct analysis of budding from the TGN has demonstrated the sorting of regulated from constitutive cargo at this early step, before maturation [3]. In addition, LDCV membrane proteins such as carboxypeptidase E and sortilin have been proposed to serve as the receptors for soluble cargo [8], [9]. In contrast to these lumenal and membrane interactions, the cytosolic machinery involved in sorting to LDCVs and LDCV formation has remained poorly understood.
Several membrane proteins contain cytosolic sequences that direct them to LDCVs. For example, the neuronal vesicular monoamine transporter VMAT2, which fills neurosecretory vesicles with monoamine transmitter, depends on a conserved, C-terminal, cytoplasmic dileucine-like motif for sorting to LDCVs [10], [11], [12], and the LDCV membrane protein IA-2β (phogrin) relies on a remarkably similar sequence [13]. Since the requirement for a cytoplasmic motif suggested an interaction with cytosolic sorting machinery, we recently used VMAT as a reporter to screen by RNAi in Drosophila S2 cells for proteins involved in biogenesis of the regulated secretory pathway, identifying multiple subunits of the heterotetrameric adaptor protein AP-3 [14]. Loss of AP-3 results in mis-sorting of VMAT in both S2 and mammalian neuroendocrine PC12 cells, dysregulated secretion, a reduction in the number and alteration in the morphology of LDCVs [14]. Indeed, AP-3 RNAi disrupts sorting at the TGN and impairs the concentration of membrane proteins such as synaptotagmin that are required for regulated release [14]. However, most work in mammalian cells has focused on the role of AP-3 within the endolysosomal pathway, in trafficking from early endosome to lysosome.
Consistent with a role in the endolysosomal pathway, mocha mice (Mus musculus) lacking AP-3 show defects in lysosome-related organelles (LROs) such as melanosomes, platelet granules and synaptic vesicles [15], [16]. In addition to the abnormal coat color and a bleeding diathesis, however, the animals exhibit perinatal lethality, hyperactivity, head tilt, seizures and reduced fertility [17], [18], [19], and it has remained unclear whether a defect in the endolysosomal pathway can fully account for the severe phenotype. Importantly, previous work in S. cerevisiae has indicated a primary role for AP-3 in the biosynthetic pathway [20], [21], [22]. We have thus now used mocha mice to investigate the physiological role of mammalian AP-3 in regulated protein secretion.
Results
The mocha Mutation Dysregulates Release by Adrenal Chromaffin Cells
To determine whether the loss of AP-3 in vivo affects regulated secretion, we cultured adrenal chromaffin cells from control and AP-3-deficient mocha mice, measuring the release of endogenous secretogranin II (SgII) in response to the nicotinic agonist DMPP [23]. Western blotting of the medium indicated that DMPP stimulates SgII secretion from control cells, but SgII was undetectable in the medium of mocha cells (Figure S1). However, the substantial reduction in cellular SgII content of mocha adrenal glands [14] and of cultured mocha adrenal chromaffin cells (Figure S1) made it difficult to determine whether the cells simply do not contain and release enough SgII to detect, or actually exhibit a defect in regulated release.
To assess regulated exocytosis by chromaffin granules, we used total internal reflection fluorescence (TIRF) microscopy to image neuropeptide and LDCV membrane protein reporters fused to the superecliptic pHluorin [24], [25]. The pHluorin is a modified form of green fluorescent protein (GFP) with increased sensitivity to protons that is quenched at the low internal pH of LDCVs and therefore increases in fluorescence with exposure to the higher external pH on exocytosis. Since neuropeptide Y (NPY)-pHluorin has been shown to undergo regulated exocytosis [26], we used lentiviral transduction to express this fusion protein and monitored individual exocytotic events at the plasma membrane of living chromaffin cells. In the absence of stimulation, control cells showed very few spontaneous fusion events over 90 s of imaging, but AP-3-deficient mocha cells exhibited substantially more (Figure 1A–1B). Both control and mocha cells showed a clear increase in exocytosis in response to stimulation by DMPP, but the extent of stimulation relative to baseline reveals an ∼70% reduction in mocha cells compared to controls (Figure 1B). To extend these findings to an LDCV membrane protein, we transduced chromaffin cells with a virus encoding VMAT2-pHluorin, with the lumenal location of pHluorin enabling detection of release events [27], [28]. Similar to NPY-pHluorin, VMAT2-pHluorin also showed a clear increase in basal, unstimulated exocytosis in mocha relative to control cells, and this again resulted in an ∼75% reduction in stimulated release (Figure 1C). The loss of AP-3 thus dysregulates the release of LDCVs as monitored using either soluble or membrane cargo.
Fig. 1. mocha chromaffin cells display dysregulated release of NPY and VMAT2. 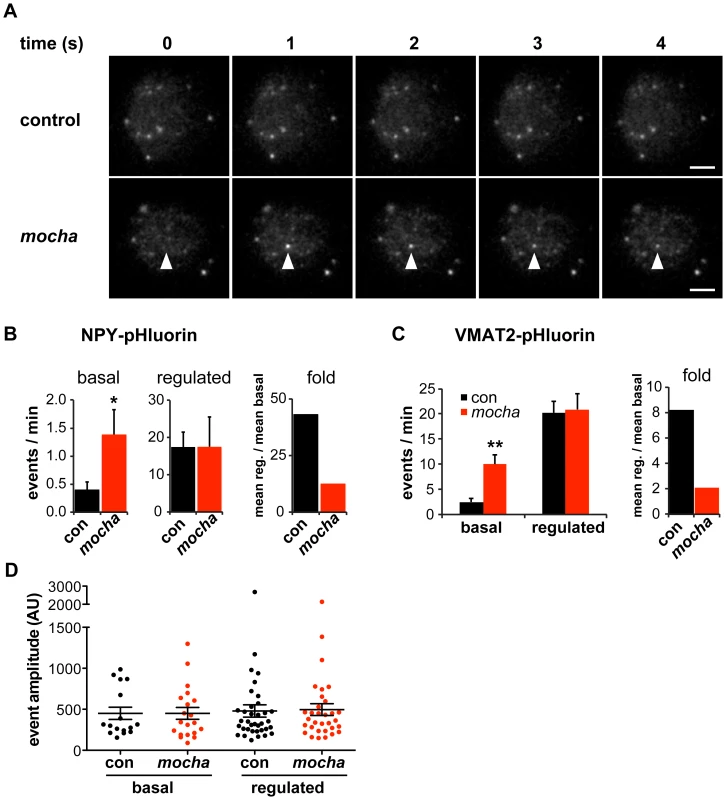
Chromaffin cells were transduced with lentivirus encoding either NPY- or VMAT2-pHluorin, then imaged live 4–7 days later by TIRF microscopy. Basal exocytosis was measured in Tyrode's solution, and release stimulated in Tyrode's containing 5 µM DMPP. (A) Representative images acquired during the unstimulated phase show a typical basal exocytotic event in a mocha cell. Scale bars indicate 5 µm. (B) Quantification of individual exocytotic events shows increased basal NPY-pHluorin exocytosis in mocha cells, and a similar number of events relative to control cells after stimulation. *p<0.04 relative to control; for basal, n = 38 for control and n = 38 for mocha; for stimulated, n = 22 for control and n = 22 for mocha. Normalizing mean regulated events to mean basal events shows that mocha cells have an ∼70% reduction in secretion fold change in response to stimulation relative to control cells. (C) Quantification of exocytotic events as above shows increased basal VMAT2-pHluorin exocytosis in mocha cells. **p<0.0003 relative to control; n = 18 for control and n = 16 for mocha. Analysis of the secretion fold change shows that mocha cells have an ∼75% reduction relative to control. (D) Analysis of the amplitude of exocytotic events monitored using NPY-pHluorin reveals that basal and regulated events of both control and mocha cells are comparable in size. The bars indicate mean values, and error bars the s.e.m. The role of AP-3 in defining LDCV membrane protein composition has suggested that loss of the adaptor results in mixing of constitutive and regulated secretory pathways [14]. The resulting constitutive secretion (measured biochemically in the case of endogenous SgII [14]) could thus reflect either the spontaneous release of constitutive secretory vesicles which contain mis-sorted LDCV cargo but no dense core, or the dysregulated release of LDCVs. To distinguish between these possibilities, we again took advantage of TIRF microscopy. Release from constitutive vesicles without a dense core should yield events with reduced amplitude relative to controls, whereas dysregulated LDCV fusion should yield events with a size similar to controls. Analyzing individual exocytotic events, we observed that the basal as well as stimulated events observed in mocha cells have an amplitude similar to those observed in controls (Figure 1D). The heightened basal secretion observed in mocha cells thus apparently results from dysregulated exocytosis of LDCVs rather than the fusion of constitutive secretory vesicles without a dense core.
Dysregulated Peptide Hormone Release by Pancreatic Islet Cells from mocha Mice
The increased basal exocytosis of NPY and VMAT2 from mocha chromaffin cells is consistent with earlier experiments using RNAi in PC12 cells [14], but does the loss of AP-3 also dysregulate release from other neuroendocrine tissues? Pancreatic β cells store insulin in LDCVs morphologically and biochemically similar to chromaffin granules [29], [30]. To assess the regulated release of insulin in vivo, we measured baseline serum insulin levels while fasting and stimulated levels 15–20 minutes after intraperitoneal injection of glucose (Figure 2A). Before glucose administration, we observed a slight reduction in the serum insulin levels of mocha mice relative to controls, but this did not reach significance. After glucose administration, the control animals showed a clear increase in serum insulin but the mocha mice did not (Figure 2A), suggesting a failure of regulated release.
Fig. 2. mocha mice show dysregulated secretion of insulin and glucagon. 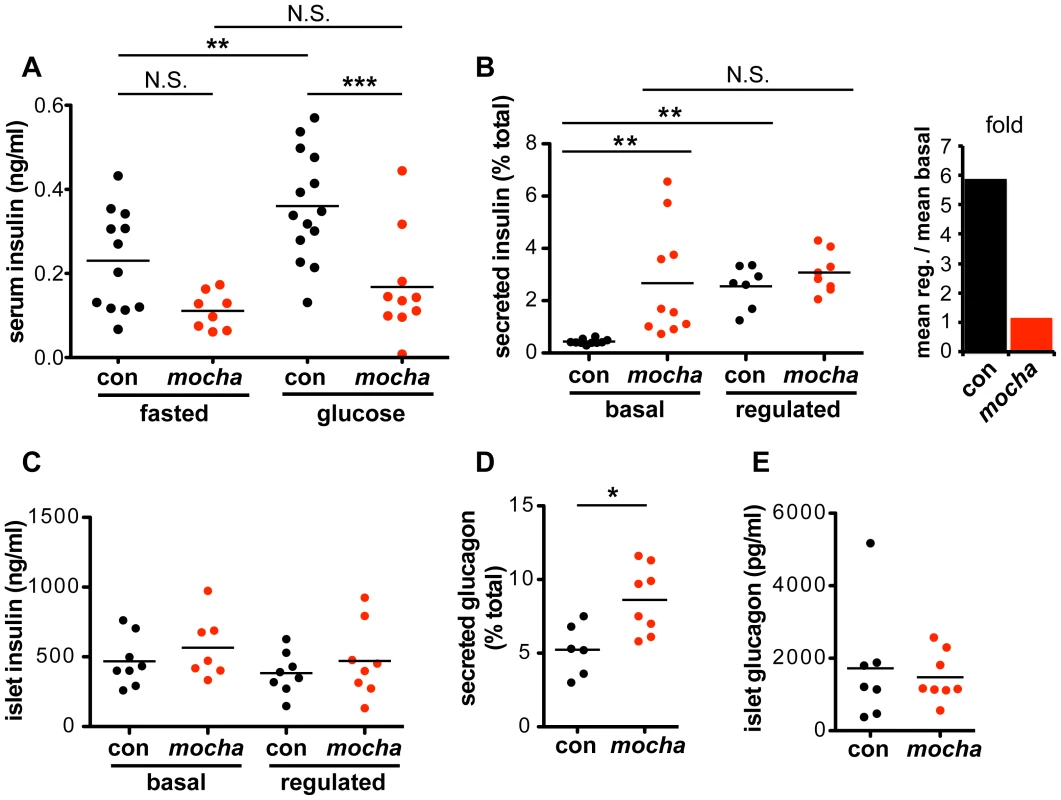
(A) Serum insulin levels were determined after an overnight fast (baseline) and 15–20 min after i.p. injection of glucose (2 mg/g body weight). In contrast to the controls, mocha insulin levels do not significantly increase after glucose injection. The data derive from two independent experiments; n = 14 control and n = 10 mocha mice. **p<0.01; ***p<0.001 by Newman-Keuls post-hoc test. Pancreatic islets were isolated acutely and bathed in either 2.8 mM (basal) or 28 mM (regulated) glucose for 1 h at 37°C. (B) mocha islets display markedly increased basal insulin secretion compared to controls. In contrast, control and mocha islets secrete comparable amounts of insulin in response to high glucose. For control islets, this represents an ∼6-fold increase in secretion in response to glucose elevation. For mocha islets, insulin secretion is not significantly increased in response to glucose elevation. **p<0.01 by Dunnett's post-hoc test, n = 7–10. (C) After collecting secreted insulin, the islets were pelleted and lysed by sonication to extract cellular insulin. Cellular insulin levels are similar between all groups. (D) Basal glucagon secretion measured in 28 mM glucose is significantly increased by the mocha mutation. (E) Cellular glucagon levels do not differ between mocha and control mice. *p<0.02, n = 6–8. The bars represent mean values. To assess the consequences of dysregulated insulin release for carbohydrate metabolism, we also measured blood glucose. Surprisingly, we observed no clear difference in fasting blood glucose levels between control and mocha mice (Figure S2A). After glucose administration, mocha animals show an increase in glycemia but less than controls (Figure S2A), a surprising effect in light of the lower serum insulin levels which would have been expected to impair glucose tolerance (Figure 2A). The effects of the mocha mutation on blood glucose levels thus do not correlate with the effects on insulin. However, blood glucose levels reflect the combined action of multiple circulating hormones, many of which may be affected by the loss of AP-3. Indeed, the dysregulated release of other peptide hormones may indirectly affect the release of insulin.
To examine insulin release independent of systemic effects, we isolated pancreatic islets and acutely incubated them in medium containing either low or high concentrations of glucose. Figure 2B shows that in contrast to the clear stimulation of insulin release by high concentrations of glucose in control islet cells, mocha cells exhibit increased basal release with little if any stimulation by high glucose. As opposed to the reduced content of SgII in mocha chromaffin cells, cellular insulin levels show no difference between mocha and control islets (Figure 2C), indicating that the change in basal secretion is not secondary to altered expression of the hormone. In the case of insulin, the mocha mutation thus dramatically and selectively impairs regulated secretion.
Since the effects of the mocha mutation on release of other peptide hormones may complicate the observations in vivo, we also examined glucagon, a peptide released from pancreatic α cells that opposes the action of insulin: glucagon raises blood glucose levels in response to starvation. Although we observed no effect of the mocha mutation on fasting serum glucagon (Figure S2B), baseline glucagon secretion was increased in acutely isolated islets (Figure 2D). In addition, the glucagon content of mocha islet cells did not differ from controls (Figure 2E), and the morphology of the islets in situ appears unchanged (Figure S2C). Thus, mocha mice show dysregulated release of two peptide hormones with opposing actions, suggesting a global effect on peptide hormone release that makes it difficult to predict the net consequences for glucose homeostasis in vivo.
Loss of Both Ubiquitous and Neural AP-3 Isoforms Is Required to Recapitulate the LDCV Phenotype of mocha Mice
The heterotetrameric AP-3 complex is known to exist in two isoforms, one expressed by all tissues, and another expressed more specifically by neurons and endocrine tissue including the adrenal gland and pancreatic islets [16], [31], [32]. In metazoan cells, the ubiquitous isoform contributes to trafficking from early endosomes to the lysosome through a pathway that does not involve multivesicular bodies [15], [33]. In contrast, the neural isoform has been implicated in the formation of synaptic vesicles from an endosomal intermediate [34], [35], suggesting that this isoform may also contribute to the formation of LDCVs. To test this possibility, we used pearl mice lacking the ubiquitous isoform of the β3 subunit (β3A) and β3B knockouts lacking the neural isoform of β3. Since the δ subunit of AP-3 exists as only a single isoform, and the loss of one subunit usually destabilizes the complex [17], [36], we first stained cells in culture for δ to assess the effect of the mutations on the complex as a whole. We were surprised to observe no effect of losing either β3 isoform on the levels of immunoreactive δ in chromaffin cells (Figure 3A), particularly considering the abundance of the ubiquitous β3A subunit in most tissues. However, we did observe reduced expression of δ by β3A-deficient, non-chromaffin cells in the culture (Figure 3B), presumably because they do not express the neural isoform and therefore cannot exhibit redundancy. Consistent with the relative abundance of adrenal cortical cells [37] and of the ubiquitous AP-3 isoform, western analysis of adrenal homogenates showed low levels of AP-3 δ in β3A-deficient pearl animals (Figure 3C) but normal levels in β3B knockouts (Figure 3D).
Fig. 3. Concomitant loss of both β3A and β3B is required to reduce adrenal SgII. 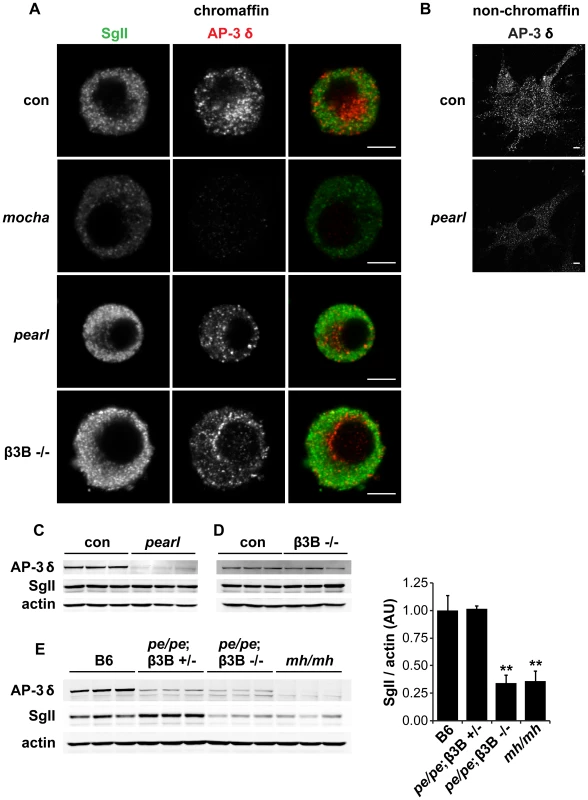
(A) Effect of individual AP-3 subunit mutations on SgII and AP-3 δ levels by immunofluorescence. Adrenal chromaffin cells from mocha (δ), pearl (β3A), β3B−/−, and control mice were stained with a rabbit polyclonal antibody to SgII and a mouse monoclonal antibody to AP-3 δ, followed by an anti-rabbit antibody conjugated to Alexa Fluor 488 and an anti-mouse antibody conjugated to Alexa Fluor 594. Representative confocal micrographs show the expected reduction in SgII and absence of AP-3 δ staining in mocha chromaffin cells, but unchanged SgII and AP-3 δ in both pearl and β3B−/− cells. (B) Non-chromaffin, fibroblast-like pearl cells present in the culture show a clear reduction in AP-3 δ. Scale bars indicate 5 µm. (C) The adrenal glands of pearl mice show a clear reduction in overall AP-3 levels, but unchanged SgII. (D) Adrenal glands of β3B−/− mice show no change in overall AP-3 or SgII levels. (E) Adrenal glands of double mutant pe/pe; β3B−/− mice show a clear reduction in SgII levels relative to age-matched C57BL/6 controls and pe/pe; β3B+/− controls. mocha adrenals show a reduction in SgII similar to that in pe/pe; β3B−/− mice. **p<0.001 relative to control by Dunnett's post-hoc test, n = 3. The data show mean values, and error bars indicate s.e.m. To determine whether the loss of β3A or β3B influences the formation of LDCVs, we then examined the effects on SgII. We were surprised to observe that in contrast to mocha cells which showed the reduction previously reported [14], both β3 mutants had normal levels of SgII by immunofluorescence (Figure 3A). By western analysis of adrenal extracts, both β3A-deficient pearl and β3B knockouts also contained normal levels of immunoreactive SgII (Figure 3C–3D). However, loss of both isoforms in the adrenal gland of double mutant mice produced a reduction in SgII comparable to that observed in mocha mice (Figure 3E). With regard to the cellular content of SgII, the two isoforms thus exhibit redundancy.
Reduced Granin Content Reflects Reduced Gene Expression but Not Increased Degradation
The reduced expression of SgII in mice lacking AP-3 might reflect increased basal secretion or an entirely distinct process. LDCV contents have indeed been shown to undergo transcriptional regulation through a variety of mechanisms [30], [38]. We therefore measured adrenal SgII and chromogranin A (CgA) transcripts from control and mocha adrenals by quantitative reverse transcription (qRT)-PCR. Both SgII and CgA mRNA were substantially reduced (by ∼50%) in mocha mice, although not to the same extent as the protein [14] (Figure 4A). PC12 cells showed a similar reduction in SgII mRNA after AP-3 RNAi (Figure 4B).
Fig. 4. Loss of AP-3 reduces granin mRNA but does not increase its degradation. 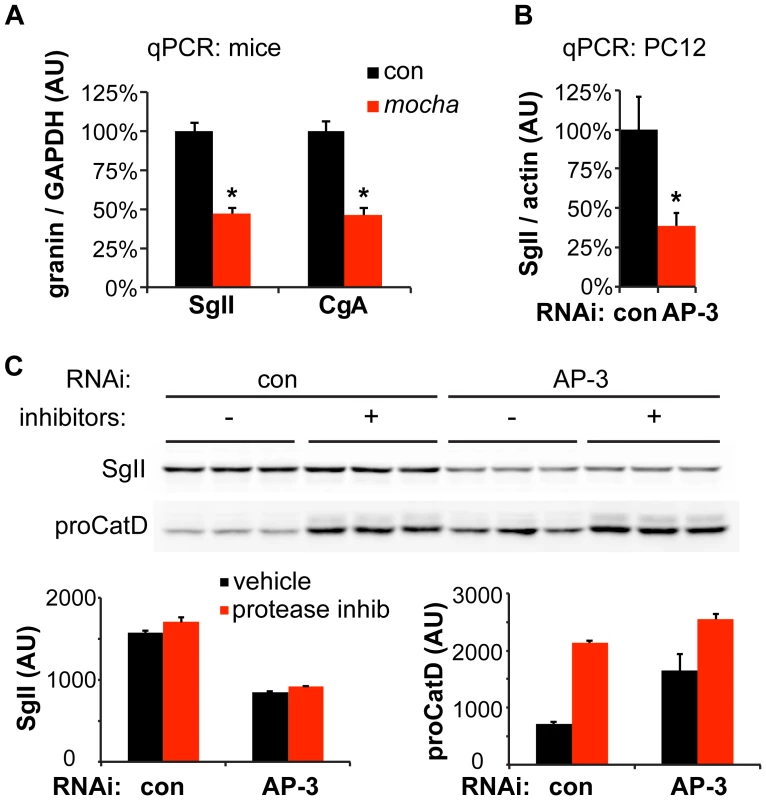
(A) Quantitative RT-PCR (qPCR) analysis of adrenal gland transcripts from mocha mice shows an ∼50% reduction in SgII and CgA expression relative to the adrenals of littermate controls. *p<1×10−4, n = 6–7 adrenals per genotype. (B) qPCR analysis of PC12 cells transfected with AP-3 δ siRNA shows an ∼60% reduction in SgII expression relative to cells transfected with control siRNA. *p<0.02, n = 5–6 transfections. (C) Incubation of PC12 cells with lysosomal protease inhibitors for ∼24 h has no effect on cellular SgII levels in cells transfected with either AP-3 δ or control siRNA. In contrast, the protease inhibitors markedly elevate the levels of the lysosomal hydrolase precursor procathepsin D. The data show mean values, and error bars indicate s.e.m. Since AP-3 influences trafficking within the endolysosomal pathway, loss of the adaptor may also influence SgII levels through increased degradation in the lysosome. To test this possibility, we inhibited lysosomal proteases after AP-3 knockdown in PC12 cells, but did not observe any increase in the levels of SgII (Figure 4C). On the other hand, the level of lysosomal hydrolase precursor procathepsin D dramatically increased in response to the inhibition of lysosomal degradation, indicating the effectiveness of the inhibitors (Figure 4C). The reduction in cellular SgII observed with loss of AP-3 thus reflects reduced expression as well as increased constitutive release, but not increased degradation.
Non-redundant Roles for AP-3 and AP-1 in the Regulated Secretory Pathway
AP-3 resembles AP-1 in terms of sequence, the ability to recognize similar trafficking motifs and subcellular location at endosomes and the Golgi apparatus [39], [40], [41]. In addition, AP-1 associates with immature LDCVs and promotes their maturation through the clathrin-dependent removal of proteins destined for other organelles [6], [42]. In mouse pituitary AtT-20 cells, maturation indeed contributes to regulated release by removing the inhibitory protein synaptotagmin 4 [43]. In PC12 and pancreatic islet cells, however, immature LDCVs can undergo regulated release [44], [45]. To determine whether silencing of AP-1 impairs regulated release from PC12 cells, we initially targeted AP-1 β-adaptin since this is the only mammalian AP-1 subunit without multiple isoforms and hence with reduced potential for redundancy. Despite highly efficient knockdown of the β1 subunit by RNAi, μ1A levels were not reduced (data not shown), raising the possibility that the β2 subunit (of AP-2) stabilized the complex by replacing β1 [46], [47]. We therefore targeted the γ subunit of AP-1, in particular the γ1 isoform which appears to show little redundancy with γ2 [48]. siRNA transfection reduced endogenous γ1 protein by ∼80% (Figure 5A). It also reduced the stimulated secretion of SgII and the intracellular accumulation of SgII, very similar to AP-3 δ RNAi (Figure 5B,C). However, normalizing to the reduced cellular stores of SgII revealed more of an increase in basal SgII secretion than a reduction in stimulated release with AP-1 knockdown, in contrast to AP-3 RNAi (Figure 5D). AP-1 RNAi thus still reduces the extent of stimulation (stimulated/basal release) from 19-fold for control to 10-fold for AP-1 RNAi (p<0.005). AP-1 knockdown also potentiates the effect of AP-3 RNAi on stimulated secretion even after normalization to the reduced intracellular stores of SgII (Figure 5D). AP-1 thus contributes to regulated secretion, and its role appears independent at least in part from that of AP-3.
Fig. 5. AP-1 knockdown potentiates the effect of AP-3 knockdown on regulated secretion. 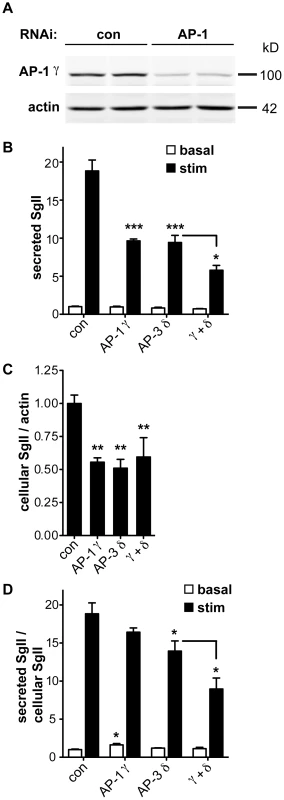
(A) PC12 cells were transfected with pooled siRNA directed against AP-1 γ or pooled nontargeting siRNA as control. Knockdown was assessed by quantitative fluorescent immunoblotting for AP-1 γ with actin as loading control. (B) PC12 cells were transfected with control, AP-1 γ, AP-3 δ or both siRNAs, washed, and incubated for 30 min in Tyrode's solution containing 2.5 mM (basal) or 50 mM (stimulated) K+. Secreted SgII was measured by quantitative fluorescent immunoblotting and normalized to basal secretion from cells treated with control siRNA. AP-1 γ RNAi alone reduces the depolarization-induced secretion of SgII very similar to AP-3 δ RNAi, and combined AP-1 γ/AP-3 δ RNAi potentiates the decrease in regulated secretion. (C) Cells were lysed and intracellular SgII measured as above, normalizing to actin. AP-1 γ and AP-3 δ RNAi both reduce cellular SgII, but the combined knockdown does not show an additional effect. (D) Secreted SgII was normalized to cellular SgII levels for each RNAi condition, and expressed relative to basal secretion from cells treated with control siRNA. Normalizing to cellular SgII shows a significant increase in basal secretion of SgII with AP-1 γ RNAi whereas AP-3 δ RNAi significantly reduces depolarization-induced secretion. Combined AP-1 γ/AP-3 δ RNAi potentiates the reduction in stimulated release. *p<0.05; **p<0.01; ***p<0.001 (Newman-Keuls post-hoc test; n = 4 transfections). The data show mean values, and error bars indicate s.e.m. Discussion
The results show that mocha mice have a major defect in the regulated secretion of peptide hormones. mocha animals exhibit hyperactivity, poor fertility, seizures and premature lethality [18], [19], but this has generally been attributed to a role for AP-3 in the endolysosomal pathway and the formation of LROs [15], [34]. We now find that mocha animals exhibit dysregulated exocytosis of adrenal chromaffin granules and both insulin - and glucagon-containing granules from pancreatic islet cells. All of the mocha cells examined show increased constitutive release relative to their cellular stores. In addition, they all show a reduced effect of stimulation, with a virtually complete loss of regulation in pancreatic β cells. Consistent with these findings in mocha mice, AP-3 RNAi increases constitutive and reduces stimulated LDCV release in PC12 cells [14]. Considering the parallel effects of AP-3 deficiency on LDCV behavior in chromaffin and pancreatic islet as well as PC12 cells, we infer that the dysregulated release observed in mocha mice reflects a common disturbance in the formation of LDCVs. The dysregulation of release by multiple neuroendocrine populations further suggests that a global defect in regulated protein secretion contributes to the phenotype of mocha mice, although the specific peptides contributing to individual features of the phenotype such as hyperactivity and seizures remain unknown.
In previous work, we found that the loss of AP-3 reduces the amount of SgII stored in PC12 cells and the adrenal gland [14]. This reflects the increased baseline exocytosis of LDCVs, but we now find that multiple granin mRNAs down-regulate as well, indicating unanticipated transcriptional effects of AP-3 deficiency. Indeed, the transcriptional down-regulation of certain LDCV cargo may account for the apparently different effects of AP-3 deficiency on different reporters and in different cells. The down-regulation of SgII mRNA in PC12 cells presumably makes it difficult to detect an increase in the absolute amount of SgII released constitutively, but transfection into PC12 cells of pHluorin-based reporters, which are not subject to this down-regulation, reveal the increased basal secretion [14]. We observe a similar increase in basal exocytosis of VMAT2 - and NPY-pHluorin expressed in AP-3-deficient chromaffin cells using a lentivirus. In the case of pancreatic islets from mocha mice, cellular levels of insulin do not fall, presumably enabling us to detect the increase in basal insulin release.
Similar to AP-3 deficiency, loss of the major LDCV protein IA-2 reduces expression of multiple LDCV cargo [49], [50], suggesting that AP-3 may sort IA-2 to LDCVs. In the absence of AP-3, decreased LDCV IA-2 may thus result in reduced granin gene expression. Although AP-3 has a role in the endolysosomal pathway, we also find that the reduced granin content does not reflect increased degradation. The role for AP-3 in regulated secretion thus appears distinct from its well-established role in trafficking to the lysosome.
The analysis of isoform-specific knockouts indicates redundancy between the ubiquitous and neural isoforms of AP-3 with regard to LDCV formation. Using SgII as a reporter for a defect in the regulated secretory pathway, we find that only the loss of both ubiquitous β3A and neural β3B causes a reduction in adrenal SgII levels. However, the loss of these isoform-specific subunits has differential effects on other trafficking phenomena. In neurons, loss of β3B mimics the effect of the full mocha mutation, with reduced presynaptic expression of proteins such as the zinc transporter ZnT3 and the chloride carrier ClC-3 [35]. Loss of β3A, on the other hand, increases presynaptic expression of these proteins [35]. The redundancy of neural and ubiquitous AP-3 forms in LDCV formation thus contrasts with the opposing roles of the two isoforms in delivery of specific proteins to the nerve terminal.
How does AP-3 promote regulated secretion? In PC12 cells, the loss of AP-3 reduces the number of LDCVs and changes their morphology [14]. They appear less dense by gradient fractionation and larger by electron microscopy (EM). Consistent with this, previous work in mocha mice has shown enlarged chromaffin granules by amperometry and EM [31]. In addition, we found that AP-3 deficiency affects the membrane proteins required for regulated exocytosis: the calcium sensor synaptotagmin 1 shifts from LDCVs to lighter membranes [14]. An assay for budding from the TGN further shows that AP-3 deficiency impairs LDCV formation [14]. Despite the importance of AP-3 for LDCV formation, however, its role may be indirect, and previous work has indeed localized AP-3 primarily to endosomes [33], [41].
Several observations have suggested a role for AP-3 at the Golgi complex. In yeast, AP-3 contributes to a direct pathway from the Golgi to the vacuole [20], [22], [51]. Although this has been considered specific to yeast, work in mammalian cells has more recently supported a role for AP-3 in delivery of membrane proteins from the biosynthetic pathway to the lysosome [52], [53]. Biochemical studies have further demonstrated the specific binding of AP-3 to membranes derived from the Golgi or to artificial membranes containing the Golgi lipid phosphatidylinositol-4-phosphate (PI4P) [54], [55]. Ultrastructural studies with immunogold have also demonstrated a small pool of AP-3 at the Golgi complex [33], [41]. However, it remains possible that the role of AP-3 in LDCV formation is indirect, helping to recycle critical LDCV membrane proteins to the Golgi, or adding these proteins to LDCVs during their maturation [56].
Despite the complete loss of AP-3 and increased basal secretion, adrenal chromaffin cells from mocha mice still show residual stimulated release, raising the possibility that another system also contributes to LDCV formation. Interestingly, previous work has implicated the related adaptor AP-1 in the formation of secretory granules by other cell types, such as glue granules of the Drosophila exocrine salivary gland, and the Weibel-Palade bodies of mammalian endothelial cells [57], [58]. AP-1 also has a clear role in LDCV maturation [6], [42], but immature LDCVs can undergo release from PC12 cells [45]. We were therefore surprised to find that loss of AP-1 impairs regulated release by PC12 cells. It remains possible that maturation promotes regulated secretion even if it is not absolutely required. Alternatively, AP-1 may promote regulated release independent of LDCV maturation.
We also find that the loss of AP-1 exacerbates the dysregulation of release by AP-3 RNAi, suggesting that the two adaptors act through distinct mechanisms. If AP-1 promotes regulated secretion through its role in LDCV maturation, it may indeed act to remove proteins that interfere with regulated release, a process that occurs in AtT-20 cells [43]. We speculate that a proofreading role for AP-1 may become even more important in the absence of AP-3 to concentrate the membrane proteins required for regulated secretion.
Materials and Methods
Ethics Statement
All procedures involving animals were approved by the UCSF Institutional Animal Care and Use Committee.
Antibodies
The rabbit SgII antibody was obtained from Meridian Life Science, the mouse actin monoclonal antibody from Millipore, the mouse δ SA4 monoclonal antibody from the Developmental Studies Hybridoma Bank, the goat cathepsin D antibody from Santa Cruz, the mouse HA.11 monoclonal antibody from Covance, the mouse adaptin γ monoclonal antibody from BD Transduction Laboratories, the mouse insulin monoclonal antibody from Sigma, the guinea pig glucagon antibody from Linco, the mouse glucagon monoclonal antibody from Sigma and the rabbit somatostatin antibody from Thermo Scientific.
siRNAs
Silencer Select rat Ap3d1 (sense, 5′-CAUGGAUCAUGACCAAGAA-3′) and corresponding non-targeting control siRNAs were from Ambion. ON-TARGETplus rat Ap1g1 (sense, 5′-CAUAAAUAUUCUUGGUCGA-3′, 5′-GUGUGGAGAUGCACGCUUA-3′, 5′-UGUAACAGUGAUAACGAUA-3′, 5′-GGACUGGAAUUCACGGCAA-3′) and corresponding non-targeting pooled control siRNAs were from Dharmacon.
Molecular Biology
The sequences of NPY-pHluorin (a generous gift of R. Holz, U. Michigan) and VMAT2 - pHluorin were amplified by PCR to add 5′ BamHI and 3′ EcoRI sites, then subcloned into the FUGW lentiviral expression vector, replacing the EGFP coding sequence.
Cell Culture and Lentivirus Production
PC12 cells were maintained in DMEH-21 medium supplemented with 10% horse serum (HS) and 5% cosmic calf serum (CCS; HyClone) in 5% CO2 at 37°C. siRNA transfection was performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. HEK293T cells were maintained in DMEH-21 medium with 10% fetal bovine serum (FBS) in 5% CO2 at 37°C. Lentivirus was produced by transfecting HEK293T cells with FUGW, psPAX2 and pVSVG and Fugene HD (Roche) according to the manufacturer's instructions [59].
Chromaffin Cell Isolation and Culture
Mouse adrenal chromaffin cells were isolated and cultured as previously described [60]. Briefly, adrenal glands were dissected and placed in cold Ca++-, Mg++-free (CMF) Hank's balanced salt solution (HBSS). The surrounding fat and cortex were removed and the medullae transferred to tubes containing 300 U/ml Collagenase I (Worthington) in CMF-HBSS. Medullae were dissociated by shaking for 40 min at 37°C. Collagenase solution was then replaced by CMF-HBSS containing 200 µg/ml DNAse I (Sigma) and 1% heat-inactivated FBS (Gibco), the tissue triturated first with a P200 pipette tip, then with a 23 gauge needle. The cells were pelleted at 300 g for 8 min at room temperature and resuspended in pre-warmed culture medium. Cells were maintained in DMEH-21 medium supplemented with 10% FBS and antibiotics. For lentiviral transduction, freshly isolated chromaffin cells were plated in viral supernatant, and fresh medium was added the following morning.
Total Internal Reflection Fluorescence (TIRF) Microscopy
For TIRF microscopy, control or mocha chromaffin cells were plated onto glass chambers coated with poly-L-lysine, immediately transduced with lentivirus encoding either NPY - or VMAT2-pHluorin and imaged live 4–7 days later. Images were typically collected for 40–50 ms at 10 Hz and room temperature using an inverted TIRF microscope (TE2000E; Nikon) with 100× Plan Apo 1.49 NA oil objective, a 1.5× tube lens and an electron-multiplying charge-coupled device camera (QuantEM; Photometrics). Basal exocytosis was measured in Tyrode's solution containing (in mM, 119 NaCl, 25 HEPES-NaOH, pH 7.4, 30 glucose, 2.5 KCl, 2 CaCl2, 2 MgCl2) over 90 s, and release stimulated for 60 s in Tyrode's containing 5 µM 1,1-Dimethyl-4-phenylpiperazinium (DMPP; Sigma). Individual exocytotic events were quantified manually using NIS-Elements software (Nikon). The amplitude of individual exocytotic events was measured by placing 2×2 pixel ROIs manually over the center of events, and the mean ROI intensity prior to an event subtracted from the maximum event intensity.
Glucose Tolerance Tests and Serum Insulin Measurements
Glucose tolerance was assessed and serum insulin levels measured using 5–15 week-old mocha mice and age-matched controls. Mice were fasted overnight (∼16 h), weighed the following morning, and blood samples collected for baseline glucose and insulin levels. Mice were then injected intraperitoneally with glucose at 2 mg/g body weight and blood samples collected from the tail vein at the time points indicated. Blood glucose levels were measured using the FreeStyle glucometer (Abbott). To measure serum insulin, the blood was allowed to clot, sedimented at 2000 g and 4°C for 20 min, and insulin levels determined using the Ultra Sensitive Mouse Insulin ELISA kit (Crystal Chem).
Pancreatic Islet Isolation and Insulin Secretion
Islets were isolated as previously described from 9–16 week-old mocha mice and age-matched controls [61]. Briefly, islets were purified on a Ficoll gradient and allowed to recover for 1 h at 37°C. Five islets were aliquoted into tubes containing HEPES-buffered RPMI medium supplemented with either 2.8 mM glucose (basal) or 28 mM glucose (regulated) and incubated for 1 h at 37°C. The islets were then sedimented and the supernatants collected to measure insulin secretion. The pellets were resuspended and sonicated in 2 mM acetic acid, 0.25% RIA-grade BSA to extract intracellular insulin. Finally, the nuclei were lysed by additional sonication in 67 mM ammonium hydroxide, 0.2% Triton X-100. Secreted and cellular insulin were quantified by ELISA (Mercodia) according to the manufacturer's instructions, and islet DNA quantified to confirm that the amount of islets per tube was similar between conditions. Glucagon was measured by ELISA (R&D Systems).
Immunofluorescence
Chromaffin cells were fixed by adding an equal volume of 4% formaldehyde in CMF-PBS to the culture medium and incubating for 20 min at room temperature. Cells were blocked and permeabilized in CMF-PBS containing 2% BSA, 1% fish skin gelatin and 0.02% saponin. Primary antibodies were diluted in blocking solution at 1∶500 (SgII) and 1∶100 (δ SA4). The following secondary antibodies conjugated to Alexa Fluor dyes (Invitrogen) were used at 1∶1000 in blocking solution: goat anti-rabbit IgG 488 and goat anti-mouse IgG 594. Images were acquired using a Zeiss LSM 510 confocal microscope and 100× oil objective.
Adrenal Gland Granin Content
pearl and mocha mice were obtained from the Jackson Laboratory, and mocha animals were backcrossed to C57BL/6 to remove grizzled and Pde6brd1 alleles. Ap3b2 KO mice were obtained from V. Faundez (Emory) and S. Voglmaier (UCSF). Double mutant pe/pe; Ap3b2−/ − mice were generated by crossing pe/pe; Ap3b2+/ − males to pe/+; Ap3b2+/ − females. Adrenal glands from 3–6 week-old mice were homogenized in 150 mM NaCl, 50 mM Tris-HCl, pH 8.0, 1% NP-40, 0.5% sodium deoxycholate, and Complete protease inhibitors (Roche) with 1 mM EGTA and 1 mM PMSF. After sedimentation at 14,000 g to remove nuclei and cell debris, 20–40 µg protein was separated by electrophoresis through polyacrylamide, transferred to nitrocellulose, and the membranes immunoblotted for AP-3δ and SgII, with actin as loading control and the appropriate secondary antibodies conjugated to IRDye800 (Rockland Immunochemicals). The immunoreactivity was quantified by imaging with an Odyssey system (LI-COR Biosciences) and ImageJ (National Institutes of Health), and the signals normalized to actin. For western analysis of the SgII secreted from chromaffin cells, Tyrode's solution was collected, sedimented at 300 g for 3 min at 4°C, and the supernatant mixed with SDS-PAGE sample buffer before electrophoresis through polyacrylamide. Chromaffin cells were directly lysed by the addition of sample buffer. In this case, SgII and actin were detected using ECL plus (GE Healthcare).
qPCR
Total RNA was isolated from PC12 cells and mouse adrenal glands using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. To improve the yield of adrenal RNA, ultrapure glycogen (Invitrogen) was added to the TRIzol as carrier. In addition, adrenal RNA was treated with RNAse-free DNAse I (NEB) to remove contaminating genomic DNA. cDNA was synthesized using oligo(dT) or gene-specific primers and a Transcriptor First Strand cDNA Synthesis kit (Roche). qPCR was performed with SYBR Green (Applied Biosystems) on a Stratagene Mx4000 machine. The following primers were used: rat SgII fwd: 5′-ACAATATAAGACAGAGGAAAATTTT-3′, rev: 5′-TGGATAAGAAGCAGAACTG-3′; rat β-actin fwd: 5′-CCGTGAAAAGATGACCCAGATC-3′, rev: 5′-CAGGGACAACACAGCCTG-3′; mouse CgA fwd: 5′-CCAACCGCAGAGCAGAG-3′, rev: 5′-AGCTGGTGGGCCACCTT-3′; mouse SgII fwd: 5′-AAGTGCTGGAGTACCTCAACC-3′, rev: 5′-TTACATGTTTTCCATGGCCCG-3′; mouse GAPDH fwd: 5′-ATGGTGAAGGTCGGTGTGAAC-3′, rev: 5′-TCCACTTTGCCACTGCAAATG-3′.
Lysosomal Inhibition
Two days after the second siRNA transfection, PC12 cells were incubated for ∼24 h in complete medium supplemented with vehicle or a cocktail of lysosomal protease inhibitors (Sigma) including (in µM) 10 antipain, 10 leupeptin and 5 pepstatin A. Cells were washed on ice with cold PBS and lysed by the addition of 50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1% Triton X-100, and Complete protease inhibitors (Roche) plus 10 mM EDTA and 1 mM PMSF. Samples were analyzed by quantitative fluorescent immunoblotting.
Secretion Assays
Cells were transfected with siRNA (100 nM) on days 1 and 3 after plating, washed 2 days later and incubated in Tyrode's solution containing 2.5 mM K+ (basal) or 50 mM K+ (stimulated) for 30 min at 37°C. The supernatants were collected, cell lysates prepared as described above, and the samples analyzed by quantitative fluorescent immunoblotting.
Statistical Analysis
Statistical analysis was performed using the Student's two-tailed t-test unless otherwise indicated.
Supporting Information
Zdroje
1. OrciL, RavazzolaM, AmherdtM, PerreletA, PowellSK, et al. (1987) The trans-most cisternae of the Golgi complex: a compartment for sorting of secretory and plasma membrane proteins. Cell 51 : 1039–1051.
2. SossinWS, FisherJM, SchellerRH (1990) Sorting within the regulated secretory pathway occurs in the trans-Golgi network. J Cell Biol 110 : 1–12.
3. ToozeSA, HuttnerWB (1990) Cell-free protein sorting to the regulated and constitutive secretory pathways. Cell 60 : 837–847.
4. KimT, Tao-ChengJH, EidenLE, LohYP (2001) Chromogranin A, an “on/off” switch controlling dense-core granule biogenesis. Cell 106 : 499–509.
5. TurkewitzAP (2004) Out with a bang! Tetrahymena as a model system to study secretory granule biogenesis. Traffic 5 : 63–68.
6. KlumpermanJ, KuliawatR, GriffithJM, GeuzeHJ, ArvanP (1998) Mannose 6-phosphate receptors are sorted from immature secretory granules via adaptor protein AP-1. clathrin and syntaxin 6-positive vesicles. J Cell Biol 141 : 359–371.
7. MorvanJ, ToozeSA (2008) Discovery and progress in our understanding of the regulated secretory pathway in neuroendocrine cells. Histochem Cell Biol 129 : 243–252.
8. ChenZY, IeraciA, TengH, DallH, MengCX, et al. (2005) Sortilin controls intracellular sorting of brain-derived neurotrophic factor to the regulated secretory pathway. J Neurosci 25 : 6156–6166.
9. CoolDR, NormantE, ShenF, ChenHC, PannellL, et al. (1997) Carboxypeptidase E is a regulated secretory pathway sorting receptor: genetic obliteration leads to endocrine disorders in Cpe(fat) mice. Cell 88 : 73–83.
10. KrantzDE, WaitesC, OorschotV, LiuY, WilsonRI, et al. (2000) A phosphorylation site in the vesicular acetylcholine transporter regulates sorting to secretory vesicles. J Cell Biol 149 : 379–395.
11. LiH, WaitesCL, StaalRG, DobryyY, ParkJ, et al. (2005) Sorting of vesicular monoamine transporter 2 to the regulated secretory pathway confers the somatodendritic exocytosis of monoamines. Neuron 48 : 619–633.
12. LiuY, SchweitzerES, NirenbergMJ, PickelVM, EvansCJ, et al. (1994) Preferential localization of a vesicular monoamine transporter to dense core vesicles in PC12 cells. J Cell Biol 127 : 1419–1433.
13. ToriiS, SaitoN, KawanoA, ZhaoS, IzumiT, et al. (2005) Cytoplasmic transport signal is involved in phogrin targeting and localization to secretory granules. Traffic 6 : 1213–1224.
14. AsensioCS, SirkisDW, EdwardsRH (2010) RNAi screen identifies a role for adaptor protein AP-3 in sorting to the regulated secretory pathway. J Cell Biol 191 : 1173–1187.
15. Dell'AngelicaEC (2009) AP-3-dependent trafficking and disease: the first decade. Curr Opin Cell Biol 21 : 552–559.
16. Newell-LitwaK, SeongE, BurmeisterM, FaundezV (2007) Neuronal and non-neuronal functions of the AP-3 sorting machinery. J Cell Sci 120 : 531–541.
17. KanthetiP, QiaoX, DiazME, PedenAA, MeyerGE, et al. (1998) Mutation in AP-3 delta in the mocha mouse links endosomal transport to storage deficiency in platelets, microsomes and synaptic vesicles. Neuron 21 : 111–122.
18. LanePW, DeolMS (1974) Mocha, a new coat color and behavior mutation on chromosome 10 of the mouse. J Heredity 65 : 362–364.
19. NoebelsJL, SidmanRL (1989) Persistent hypersynchronization of neocortical neurons in the mocha mutant of mouse. J Neurogenet 6 : 53–56.
20. CowlesCR, OdorizziG, PayneGS, EmrSD (1997) The AP-3 adaptor complex is essential for cargo-selective transport to the yeast vacuole. Cell 91 : 109–118.
21. NickersonDP, BrettCL, MerzAJ (2009) Vps-C complexes: gatekeepers of endolysosomal traffic. Curr Opin Cell Biol 21 : 543–551.
22. SteppJD, HuangK, LemmonSK (1997) The yeast adaptor protein complex, AP-3, is essential for the efficient delivery of alkaline phosphatase by the alternate pathway to the vacuole. J Cell Biol 139 : 1761–1774.
23. ChungSH, JobertyG, GelinoEA, MacaraIG, HolzRW (1999) Comparison of the effects on secretion in chromaffin and PC12 cells of Rab3 family members and mutants. Evidence that inhibitory effects are independent of direct interaction with Rabphilin3. J Biol Chem 274 : 18113–18120.
24. MiesenböckG, De AngelisDA, RothmanJE (1998) Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature 394 : 192–195.
25. SankaranarayananS, De AngelisD, RothmanJE, RyanTA (2000) The use of pHluorins for optical measurements of presynaptic activity. Biophys J 79 : 2199–2208.
26. KogelT, RudolfR, HodnelandE, HellwigA, KuznetsovSA, et al. (2010) Distinct roles of myosin Va in membrane remodeling and exocytosis of secretory granules. Traffic 11 : 637–650.
27. AnantharamA, OnoaB, EdwardsRH, HolzRW, AxelrodD (2010) Localized topological changes of the plasma membrane upon exocytosis visualized by polarized TIRFM. J Cell Biol 188 : 415–428.
28. OnoaB, LiH, Gagnon-BartschJA, EliasLA, EdwardsRH (2010) Vesicular monoamine and glutamate transporters select distinct synaptic vesicle recycling pathways. J Neurosci 30 : 7917–7927.
29. ArvanP, HalbanPA (2004) Sorting ourselves out: seeking consensus on trafficking in the beta-cell. Traffic 5 : 53–61.
30. SuckaleJ, SolimenaM (2010) The insulin secretory granule as a signaling hub. Trends in endocrinology and metabolism: TEM 21 : 599–609.
31. GrabnerCP, PriceSD, LysakowskiA, CahillAL, FoxAP (2006) Regulation of large dense-core vesicle volume and neurotransmitter content mediated by adaptor protein 3. Proc Natl Acad Sci U S A 103 : 10035–10040.
32. SuckowAT, CraigeB, FaundezV, CainWJ, ChesslerSD (2010) An AP-3-dependent mechanism drives synaptic-like microvesicle biogenesis in pancreatic islet beta-cells. Am J Physiol Endocrin metab 299: E23–32.
33. PedenAA, OorschotV, HesserBA, AustinCD, SchellerRH, et al. (2004) Localization of the AP-3 adaptor complex defines a novel endosomal exit site for lysosomal membrane proteins. J Cell Biol 164 : 1065–1076.
34. FaundezV, HorngJT, KellyRB (1998) A function for the AP3 coat complex in synaptic vesicle formation from endosomes. Cell 93 : 423–432.
35. SeongE, WainerBH, HughesED, SaundersTL, BurmeisterM, et al. (2005) Genetic analysis of the neuronal and ubiquitous AP-3 adaptor complexes reveals divergent functions in brain. Mol Biol Cell 16 : 128–140.
36. PedenAA, RudgeRE, LuiWW, RobinsonMS (2002) Assembly and function of AP-3 complexes in cells expressing mutant subunits. J Cell Biol 156 : 327–336.
37. BielohubyM, HerbachN, WankeR, Maser-GluthC, BeuschleinF, et al. (2007) Growth analysis of the mouse adrenal gland from weaning to adulthood: time - and gender-dependent alterations of cell size and number in the cortical compartment. Am J Physiol Endocrin metab 293: E139–146.
38. OhnedaK, EeH, GermanM (2000) Regulation of insulin gene transcription. Sem Cell Dev Biol 11 : 227–233.
39. JanvierK, KatoY, BoehmM, RoseJR, MartinaJA, et al. (2003) Recognition of dileucine-based sorting signals from HIV-1 Nef and LIMP-II by the AP-1 gamma-sigma1 and AP-3 delta-sigma3 hemicomplexes. J Cell Biol 163 : 1281–1290.
40. MatteraR, BoehmM, ChaudhuriR, PrabhuY, BonifacinoJS (2011) Conservation and diversification of dileucine signal recognition by adaptor protein (AP) complex variants. J Biol Chem 286 : 2022–2030.
41. TheosAC, TenzaD, MartinaJA, HurbainI, PedenAA, et al. (2005) Functions of adaptor protein (AP)-3 and AP-1 in tyrosinase sorting from endosomes to melanosomes. Mol Biol Cell 16 : 5356–5372.
42. DittieAS, HajibagheriN, ToozeSA (1996) The AP-1 adaptor complex binds to immature secretory granules from PC12 cells and is regulated by ADP-ribosylation factor. J Cell Biol 132 : 523–536.
43. EatonBA, HaugwitzM, LauD, MooreHP (2000) Biogenesis of regulated exocytotic carriers in neuroendocrine cells. J Neurosci 20 : 7334–7344.
44. MolineteM, DupuisS, BrodskyFM, HalbanPA (2001) Role of clathrin in the regulated secretory pathway of pancreatic beta-cells. J Cell Sci 114 : 3059–3066.
45. ToozeSA, FlatmarkT, ToozeJ, HuttnerWB (1991) Characterization of the immature secretory granule, an intermediate in granule biogenesis. J Cell Biol 115 : 1491–1503.
46. PageLJ, RobinsonMS (1995) Targeting signals and subunit interactions in coated vesicle adaptor complexes. J Cell Biol 131 : 619–630.
47. AguilarRC, OhnoH, RocheKW, BonifacinoJS (1997) Functional domain mapping of the clathrin-associated adaptor medium chains mu1 and mu2. J Biol Chem 272 : 27160–27166.
48. ZizioliD, MeyerC, GuhdeG, SaftigP, von FiguraK, et al. (1999) Early embryonic death of mice deficient in gamma-adaptin. J Biol Chem 274 : 5385–5390.
49. MziautH, TrajkovskiM, KerstingS, EhningerA, AltkrugerA, et al. (2006) Synergy of glucose and growth hormone signalling in islet cells through ICA512 and STAT5. Nat Cell Biol 8 : 435–445.
50. TrajkovskiM, MziautH, AltkrugerA, OuwendijkJ, KnochKP, et al. (2004) Nuclear translocation of an ICA512 cytosolic fragment couples granule exocytosis and insulin expression in {beta}-cells. J Cell Biol 167 : 1063–1074.
51. RehlingP, DarsowT, KatzmannDJ, EmrSD (1999) Formation of AP-3 transport intermediates requires Vps41 function. Nat Cell Biol 1 : 346–353.
52. ChapuyB, TikkanenR, MuhlhausenC, WenzelD, von FiguraK, et al. (2008) AP-1 and AP-3 Mediate Sorting of Melanosomal and Lysosomal Membrane Proteins into Distinct Post-Golgi Trafficking Pathways. Traffic 9 : 1157–1172.
53. ScheuberA, RudgeR, DanglotL, RaposoG, BinzT, et al. (2006) Loss of AP-3 function affects spontaneous and evoked release at hippocampal mossy fiber synapses. Proc Natl Acad Sci U S A 103 : 16562–16567.
54. BaustT, AniteiM, CzupallaC, ParshynaI, BourelL, et al. (2008) Protein networks supporting AP-3 function in targeting lysosomal membrane proteins. Mol Biol Cell 19 : 1942–1951.
55. DrakeMT, ZhuY, KornfeldS (2000) The assembly of AP-3 adaptor complex-containing clathrin-coated vesicles on synthetic liposomes. Mol Biol Cell 11 : 3723–3736.
56. Harrison-LavoieKJ, MichauxG, HewlettL, KaurJ, HannahMJ, et al. (2006) P-selectin and CD63 use different mechanisms for delivery to Weibel-Palade bodies. Traffic 7 : 647–662.
57. BurgessJ, JaureguiM, TanJ, RollinsJ, LalletS, et al. (2011) AP-1 and clathrin are essential for secretory granule biogenesis in Drosophila. Mol Biol Cell 22 : 2094–2105.
58. Lui-RobertsWW, CollinsonLM, HewlettLJ, MichauxG, CutlerDF (2005) An AP-1/clathrin coat plays a novel and essential role in forming the Weibel-Palade bodies of endothelial cells. J Cell Biol 170 : 627–636.
59. LoisC, HongEJ, PeaseS, BrownEJ, BaltimoreD (2002) Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science 295 : 868–872.
60. Kolski-AndreacoA, CaiH, CurrleDS, ChandyKG, ChowRH (2007) Mouse adrenal chromaffin cell isolation. Journal of visualized experiments:JoVE 129.
61. SzotGL, KoudriaP, BluestoneJA (2007) Murine pancreatic islet isolation. Journal of visualized experiments: JoVE 255.
Štítky
Genetika Reprodukční medicína
Článek Rapid Intrahost Evolution of Human Cytomegalovirus Is Shaped by Demography and Positive SelectionČlánek Common Variants in Left/Right Asymmetry Genes and Pathways Are Associated with Relative Hand SkillČlánek Manipulating or Superseding Host Recombination Functions: A Dilemma That Shapes Phage EvolvabilityČlánek Maternal Depletion of Piwi, a Component of the RNAi System, Impacts Heterochromatin Formation inČlánek Hsp104 Suppresses Polyglutamine-Induced Degeneration Post Onset in a Drosophila MJD/SCA3 ModelČlánek Cooperative Interaction between Phosphorylation Sites on PERIOD Maintains Circadian Period inČlánek VAPB/ALS8 MSP Ligands Regulate Striated Muscle Energy Metabolism Critical for Adult Survival inČlánek Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2AČlánek A Link between ORC-Origin Binding Mechanisms and Origin Activation Time Revealed in Budding YeastČlánek Genotype-Environment Interactions Reveal Causal Pathways That Mediate Genetic Effects on PhenotypeČlánek Chromatin-Specific Regulation of Mammalian rDNA Transcription by Clustered TTF-I Binding SitesČlánek Meiotic Recombination in Arabidopsis Is Catalysed by DMC1, with RAD51 Playing a Supporting Role
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 9- Souvislost haplotypu M2 genu pro annexin A5 s opakovanými reprodukčními ztrátami
- Akutní intermitentní porfyrie
- Příjem alkoholu a menstruační cyklus
- Hysteroskopická resekce děložního septa zlepšuje šanci na graviditu žen s jinak nevysvětlenou infertilitou
- Transfer zmraženého embrya zlepšuje výsledky IVF
-
Všechny články tohoto čísla
- The Pathway Gene Functions together with the -Dependent Isoprenoid Biosynthetic Pathway to Orchestrate Germ Cell Migration
- Take Off, Landing, and Fly Anesthesia
- Nucleosome Assembly Proteins Get SET to Defeat the Guardian of Chromosome Cohesion
- Whole-Exome Sequencing Reveals a Rapid Change in the Frequency of Rare Functional Variants in a Founding Population of Humans
- Evidence Is Evidence: An Interview with Mary-Claire King
- Rapid Intrahost Evolution of Human Cytomegalovirus Is Shaped by Demography and Positive Selection
- Convergent Transcription Induces Dynamic DNA Methylation at Loci
- Environmental Stresses Disrupt Telomere Length Homeostasis
- Ultra-Sensitive Sequencing Reveals an Age-Related Increase in Somatic Mitochondrial Mutations That Are Inconsistent with Oxidative Damage
- Common Variants in Left/Right Asymmetry Genes and Pathways Are Associated with Relative Hand Skill
- Genetic and Anatomical Basis of the Barrier Separating Wakefulness and Anesthetic-Induced Unresponsiveness
- The Locus, Exclusive to the Ambulacrarians, Encodes a Chromatin Insulator Binding Protein in the Sea Urchin Embryo
- Binding of NF-κB to Nucleosomes: Effect of Translational Positioning, Nucleosome Remodeling and Linker Histone H1
- Manipulating or Superseding Host Recombination Functions: A Dilemma That Shapes Phage Evolvability
- Dynamics of DNA Methylation in Recent Human and Great Ape Evolution
- Functional Dissection of Regulatory Models Using Gene Expression Data of Deletion Mutants
- PAQR-2 Regulates Fatty Acid Desaturation during Cold Adaptation in
- N-alpha-terminal Acetylation of Histone H4 Regulates Arginine Methylation and Ribosomal DNA Silencing
- A Genome-Wide Systematic Analysis Reveals Different and Predictive Proliferation Expression Signatures of Cancerous vs. Non-Cancerous Cells
- Maternal Depletion of Piwi, a Component of the RNAi System, Impacts Heterochromatin Formation in
- miR-1/133a Clusters Cooperatively Specify the Cardiomyogenic Lineage by Adjustment of Myocardin Levels during Embryonic Heart Development
- Hsp104 Suppresses Polyglutamine-Induced Degeneration Post Onset in a Drosophila MJD/SCA3 Model
- Genome-Wide Analysis of Genes and Their Association with Natural Variation in Drought Tolerance at Seedling Stage of L
- Deep Resequencing of GWAS Loci Identifies Rare Variants in , and That Are Associated with Ulcerative Colitis
- Cooperative Interaction between Phosphorylation Sites on PERIOD Maintains Circadian Period in
- VAPB/ALS8 MSP Ligands Regulate Striated Muscle Energy Metabolism Critical for Adult Survival in
- Analysis of Genes Reveals Redundant and Independent Functions in the Inner Ear
- Predicting the Risk of Rheumatoid Arthritis and Its Age of Onset through Modelling Genetic Risk Variants with Smoking
- Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2A
- A Shift to Organismal Stress Resistance in Programmed Cell Death Mutants
- Fragile Site Instability in Causes Loss of Heterozygosity by Mitotic Crossovers and Break-Induced Replication
- Tracking of Chromosome and Replisome Dynamics in Reveals a Novel Chromosome Arrangement
- The Condition-Dependent Transcriptional Landscape of
- Ago1 Interacts with RNA Polymerase II and Binds to the Promoters of Actively Transcribed Genes in Human Cancer Cells
- Nebula/DSCR1 Upregulation Delays Neurodegeneration and Protects against APP-Induced Axonal Transport Defects by Restoring Calcineurin and GSK-3β Signaling
- System-Wide Analysis Reveals a Complex Network of Tumor-Fibroblast Interactions Involved in Tumorigenicity
- Meta-Analysis of Genome-Wide Association Studies Identifies Six New Loci for Serum Calcium Concentrations
- and Are Required for Cellularization and Differentiation during Female Gametogenesis in
- Growth factor independent-1 Maintains Notch1-Dependent Transcriptional Programming of Lymphoid Precursors
- Whole Genome Sequencing Identifies a Deletion in Protein Phosphatase 2A That Affects Its Stability and Localization in
- An Alteration in ELMOD3, an Arl2 GTPase-Activating Protein, Is Associated with Hearing Impairment in Humans
- Genomic Identification of Founding Haplotypes Reveals the History of the Selfing Species
- Plasticity Regulators Modulate Specific Root Traits in Discrete Nitrogen Environments
- The IDD14, IDD15, and IDD16 Cooperatively Regulate Lateral Organ Morphogenesis and Gravitropism by Promoting Auxin Biosynthesis and Transport
- Stochastic Loss of Silencing of the Imprinted Allele, in a Mouse Model and Humans with Prader-Willi Syndrome, Has Functional Consequences
- The Prefoldin Complex Regulates Chromatin Dynamics during Transcription Elongation
- PKA Controls Calcium Influx into Motor Neurons during a Rhythmic Behavior
- A Pre-mRNA-Splicing Factor Is Required for RNA-Directed DNA Methylation in
- Cell-Type Specific Features of Circular RNA Expression
- The Uve1 Endonuclease Is Regulated by the White Collar Complex to Protect from UV Damage
- An Atypical Kinase under Balancing Selection Confers Broad-Spectrum Disease Resistance in Arabidopsis
- Genome-Wide Mutation Avalanches Induced in Diploid Yeast Cells by a Base Analog or an APOBEC Deaminase
- Extensive Divergence of Transcription Factor Binding in Embryos with Highly Conserved Gene Expression
- Bi-modal Distribution of the Second Messenger c-di-GMP Controls Cell Fate and Asymmetry during the Cell Cycle
- Cell Interactions and Patterned Intercalations Shape and Link Epithelial Tubes in
- A Link between ORC-Origin Binding Mechanisms and Origin Activation Time Revealed in Budding Yeast
- The Genome and Development-Dependent Transcriptomes of : A Window into Fungal Evolution
- SKN-1/Nrf, A New Unfolded Protein Response Factor?
- The Highly Prolific Phenotype of Lacaune Sheep Is Associated with an Ectopic Expression of the Gene within the Ovary
- Fusion of Large-Scale Genomic Knowledge and Frequency Data Computationally Prioritizes Variants in Epilepsy
- IL-17 Attenuates Degradation of ARE-mRNAs by Changing the Cooperation between AU-Binding Proteins and microRNA16
- An Enhancer Element Harboring Variants Associated with Systemic Lupus Erythematosus Engages the Promoter to Influence A20 Expression
- Genome Analysis of a Transmissible Lineage of Reveals Pathoadaptive Mutations and Distinct Evolutionary Paths of Hypermutators
- Type I-E CRISPR-Cas Systems Discriminate Target from Non-Target DNA through Base Pairing-Independent PAM Recognition
- Divergent Transcriptional Regulatory Logic at the Intersection of Tissue Growth and Developmental Patterning
- MEIOB Targets Single-Strand DNA and Is Necessary for Meiotic Recombination
- Transmission of Hypervirulence Traits via Sexual Reproduction within and between Lineages of the Human Fungal Pathogen
- Integration of the Unfolded Protein and Oxidative Stress Responses through SKN-1/Nrf
- Guanine Holes Are Prominent Targets for Mutation in Cancer and Inherited Disease
- Regulation of the Boundaries of Accessible Chromatin
- Natural Genetic Transformation Generates a Population of Merodiploids in
- Ablating Adult Neurogenesis in the Rat Has No Effect on Spatial Processing: Evidence from a Novel Pharmacogenetic Model
- Genotype-Environment Interactions Reveal Causal Pathways That Mediate Genetic Effects on Phenotype
- The Molecular Mechanism of a -Regulatory Adaptation in Yeast
- Phenotypic and Genetic Consequences of Protein Damage
- Recent Acquisition of by Baka Pygmies
- Fatty Acid Taste Signals through the PLC Pathway in Sugar-Sensing Neurons
- A Critical Role for PDGFRα Signaling in Medial Nasal Process Development
- Chromatin-Specific Regulation of Mammalian rDNA Transcription by Clustered TTF-I Binding Sites
- Meiotic Recombination in Arabidopsis Is Catalysed by DMC1, with RAD51 Playing a Supporting Role
- dTULP, the Homolog of Tubby, Regulates Transient Receptor Potential Channel Localization in Cilia
- Widespread Dysregulation of Peptide Hormone Release in Mice Lacking Adaptor Protein AP-3
- , a Direct Transcriptional Target, Modulates T-Box Factor Activity in Orofacial Clefting
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Genome-Wide Systematic Analysis Reveals Different and Predictive Proliferation Expression Signatures of Cancerous vs. Non-Cancerous Cells
- Recent Acquisition of by Baka Pygmies
- The Condition-Dependent Transcriptional Landscape of
- Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2A
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



