-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
A Critical Role for PDGFRα Signaling in Medial Nasal Process Development
The primitive face is composed of neural crest cell (NCC) derived prominences. The medial nasal processes (MNP) give rise to the upper lip and vomeronasal organ, and are essential for normal craniofacial development, but the mechanism of MNP development remains largely unknown. PDGFRα signaling is known to be critical for NCC development and craniofacial morphogenesis. In this study, we show that PDGFRα is required for MNP development by maintaining the migration of progenitor neural crest cells (NCCs) and the proliferation of MNP cells. Further investigations reveal that PI3K/Akt and Rac1 signaling mediate PDGFRα function during MNP development. We thus establish PDGFRα as a novel regulator of MNP development and elucidate the roles of its downstream signaling pathways at cellular and molecular levels.
Published in the journal: . PLoS Genet 9(9): e32767. doi:10.1371/journal.pgen.1003851
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003851Summary
The primitive face is composed of neural crest cell (NCC) derived prominences. The medial nasal processes (MNP) give rise to the upper lip and vomeronasal organ, and are essential for normal craniofacial development, but the mechanism of MNP development remains largely unknown. PDGFRα signaling is known to be critical for NCC development and craniofacial morphogenesis. In this study, we show that PDGFRα is required for MNP development by maintaining the migration of progenitor neural crest cells (NCCs) and the proliferation of MNP cells. Further investigations reveal that PI3K/Akt and Rac1 signaling mediate PDGFRα function during MNP development. We thus establish PDGFRα as a novel regulator of MNP development and elucidate the roles of its downstream signaling pathways at cellular and molecular levels.
Introduction
Neural crest cells (NCCs) are a transient and multipotent cell population unique to vertebrates. During development, NCCs give rise to a broad variety of cell types, which contribute to the formation of the peripheral nervous system, cardiac outflow tract, pigment cells, and the majority of craniofacial bones and cartilages [1]–[4]. Alterations of cranial NCC (cNCC) development often lead to craniofacial malformations, one of the most prevalent birth defects [5]. These facts underscore the significance of understanding the mechanisms regulating NCCs during craniofacial morphogenesis.
At the onset of craniofacial development, the facial primordium is composed of five prominences surrounding the stomodeum: the frontonasal prominence (FNP) at the rostral region, two paired maxillary processes in the middle, and a pair of mandibular processes at the caudal end [6], [7]. These primordia are populated predominantly by NCC derived cells, surrounding a mesodermal core and covered by the overlying ectoderm. The ectoderm then thickens and invaginates to form two bilateral nasal placodes, dividing the FNP into the medial nasal process (MNP) and a pair of lateral nasal processes (LNP). The MNP and bilateral maxillary processes contribute together to form the upper lip [8]. In mammals, the MNP further develops into the philtrum and the nasal septum, which later forms the nasal cartilage and bone [9]. Disruption of the MNP usually causes a variety of craniofacial defects, ranging from mild hypoplasia of the nasal bones to complete midfacial clefting. A number of genes regulate maxilla and mandible development, but it remains largely unknown how MNP development is controlled at the molecular and cellular level. Vital dye labeling studies reveal that the NCCs giving rise to different facial prominences share distinct origins along the rostral-caudal axis: NCCs from the diencephalon and anterior mesencephalon give rise to the MNP and LNP, while those originating from the posterior mesencephalon and hindbrain give rise to the maxilla and mandible [10], [11]. These results suggest that the MNP and other prominences may be regulated through different mechanisms.
Multiple genetic factors have been implicated in cranial NCC (cNCC) development. Among these, growth factor signaling pathways are essential for induction, proliferation, survival and migration [12]–[14]. BMP, FGF and Wnt signaling together mediate induction of cNCCs from neural ectoderm [13], [15]. cNCC proliferation and survival are under the control of BMP, FGF and TGFβ signaling, and migration of the cNCCs at the caudal level is regulated by BMP, Wnt, Semaphorin and Ephrin signaling [13]. Growth factors act via binding and activation of their cell surface receptors, which in turn engage multiple intracellular signaling pathways. It remains to be elucidated how these intracellular signaling pathways mediate the receptors' function, especially in developmental contexts.
Platelet Derived Growth Factor (PDGF) signaling plays essential roles in development and disease [16]–[19]. In mammals, PDGF signaling can be activated by four PDGF ligands (A, B, C and D) operating through two receptor tyrosine kinases, PDGFRα and β [17], [20]. Activation of PDGFRs leads to phosphorylation of intracellular tyrosines and docking of intracellular effectors, which in turn engage downstream signaling cascades including the MAPK, PI3K, PLCγ, STAT and Src pathways. Previous studies from our laboratory and others have shown that PDGFRα and its downstream signaling pathways are crucial for cardiac and cranial NCCs [21]–[24]. PDGFA/PDGFRα signaling has also been implicated in cell migration in zebrafish palatogenesis [25]. However, the precise mechanisms by which PDGFRα regulates cNCCs and MNP development still remain to be elucidated. To this end, we have carried out a detailed study of PDGFRα NCC conditional knockout embryos. Our work reveals novel roles of PDGFRα in regulating NCC migration, and for PDGFRα engaged PI3K signaling in MNP neural crest cell proliferation. Moreover, we show that Rac1 and PI3K signaling mediate these processes under the control of PDGFRα.
Results
Loss of PDGFRα leads to facial clefting due to loss of MNP derived structures
To understand how PDGFRα and its downstream signaling pathways regulate NCC development and craniofacial morphogenesis, we generated NCC-specific PDGFRα conditional knockout (cKO) embryos by intercrossing PDGFRαfl/fl and Wnt1Cre transgenic mice [21], [26]. Morphological differences first became visible at E11.5, as the medial nasal processes (MNP) of cKO littermates remained separated by an obvious gap relative to the control embryos (Fig. 1A, B). By E13.5, cKO embryos lacked the philtrum and the primary palate (Fig. 1C, D), both of which are derived from the MNP. This observation was confirmed by histological analysis that showed the absence of the primary palate and philtrum in the cKO embryos (Fig. 1E, F). At E18.5, skeletal preparations showed that PDGFRαfl/fl; Wnt1 Cre embryos exhibited a significant cleft (6 out of 6) and shortening of nasal cartilage (88±4.8% relative to control) and premaxilla (81.2±2.0% relative to control) (Fig. 1G, H). Skeletal analysis of cKO mutants also revealed malformation of the neural crest derived basisphenoid, alisphenoid and pterygoid bones (Fig. 1G, H) [1], [27], [28]. Other cNCC derived structures, such as the mandible, developed normally in the cKO mutants. No cKO embryos survived past birth.
Fig. 1. Tissue specific inactivation of PDGFRα from NCCs causes facial clefting and absence of MNP derived structures. 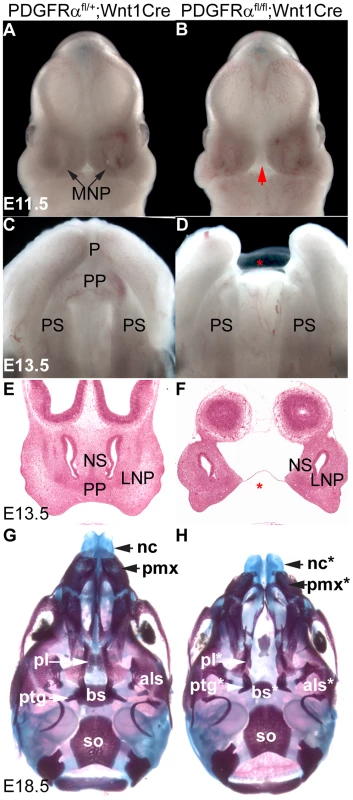
(A, B) Frontal view of PDGFRαfl/+; Wnt1Cre (A) and PDGFRαfl/fl; Wnt1Cre embryo (B) at E11.5. An obvious gap was observed between MNPs in PDGFRαfl/fl; Wnt1Cre embryo (arrow in B). (C, D) Ventral view of PDGFRαfl/+; Wnt1Cre (C) and PDGFRαfl/fl; Wnt1Cre embryo (D) at E13.5. The PDGFRαfl/fl; Wnt1Cre embryo shows cleft lip and lacks of philtrum and primary palate (asterisk in D). The mandible has been removed for visualization. (E, F) Histological coronal sections of PDGFRαfl/+; Wnt1Cre (E) and PDGFRαfl/fl; Wnt1Cre embryo (F) at E13.5. PDGFRαfl/fl; Wnt1Cre embryo shows a cleft of the nasal septum and lacks the primary palate (asterisk in F). (G, H) Ventral view of skeletal preparation of PDGFRαfl/+; Wnt1Cre (G) and PDGFRαfl/fl; Wnt1Cre embryo (H) at E18.5. Note the shortening and clefting of the nasal cartilage in the PDGFRαfl/fl; Wnt1Cre embryo. Asterisks refer to defective bones or cartilages. Als, alisphenoid; bs, basisphenoid; LNP, lateral nasal process; MNP, medial nasal process; nc, nasal cartilage; NS, nasal septum; P, philtrum; pl, palatine; pmx, premaxilla; PP, primary palate; PS, palatal shelf; ptg, pterygoid. To further understand how PDGFRα signals during MNP development, we analyzed the expression of PDGFRα and its ligands. Using PDGFRαGFP/+ knockin reporter mice [29], we observed broad GFP expression in the facial structures at E10.5 (Fig. 2A). Coronal sections of PDGFRαGFP/+ embryos at different stages revealed that PDGFRα is exclusively expressed in mesenchymal cells of the future facial structures, which are derived predominantly from cNCCs [1]. At E10.5, PDGFRα appears to be expressed equally in both the MNP and the LNP (Fig. 2B); however by E11.5, PDGFRα expression is reduced in the LNP relative to the MNP mesenchyme (Fig. 2C). PDGFA and PDGFC encode two endogenous ligands that bind specifically to PDGFRα, and inactivation of the two genes together recapitulates the PDGFRα null mutant phenotype [30]. In situ hybridization showed that these genes exhibit overlapping expression in the MNP: PDGFA is expressed in the MNP and LNP epithelium and PDGFC is expressed in both the epithelium and mesenchyme (Fig. 2D–G). The expression patterns of PDGFRα and its ligands indicate paracrine and possibly autocrine PDGFRα signaling during MNP development.
Fig. 2. PDGFRα and its ligands PDGFA and PDGFC are expressed during midface development. 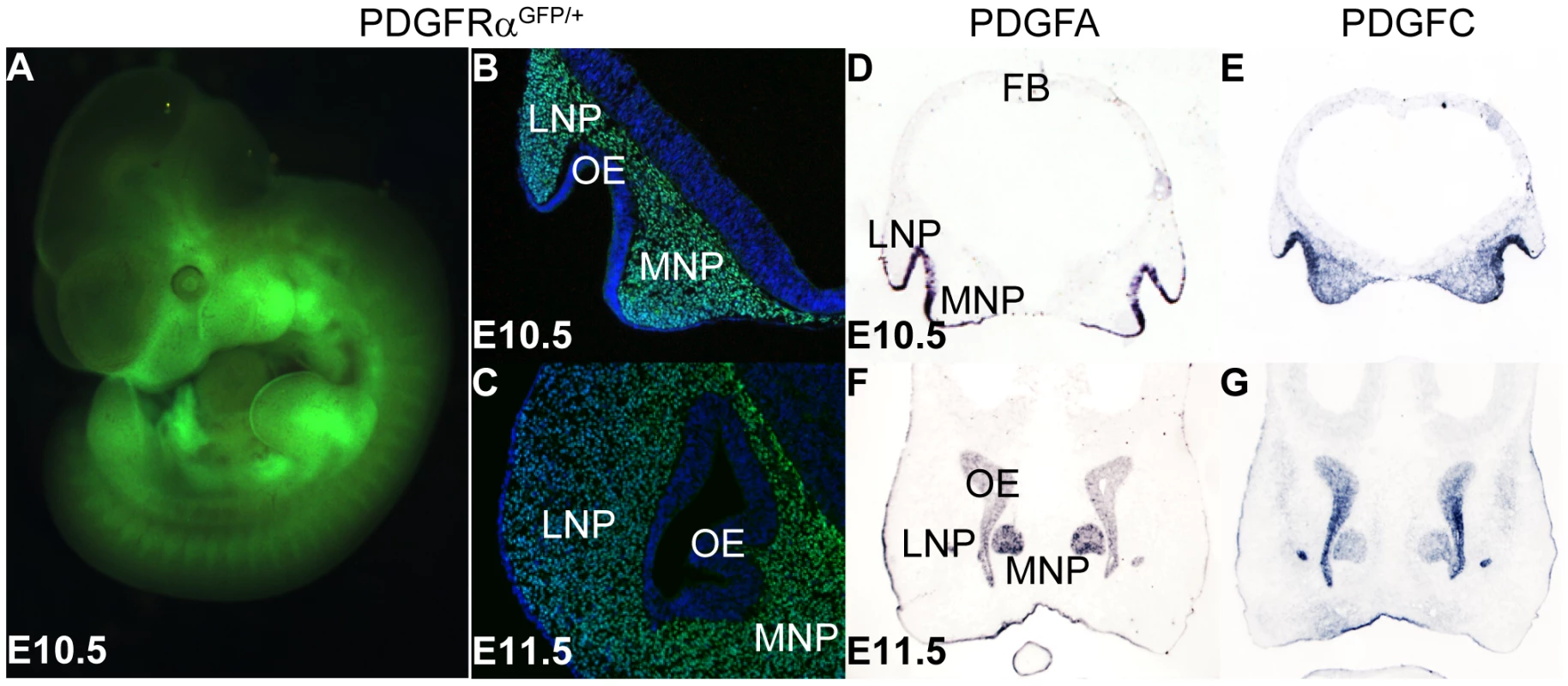
(A) Whole mount view of PDGFRα expression, represented by H2B-GFP in the E10.5 PDGFRαGFP/+ knockin mouse embryo. (B, C) Coronal section shows PDGFRα expression in the mesenchyme of MNP and LNP at E10.5 and E11.5. E11.5 PDGFRαGFP/+ embryo shows high PDGFRα expression in the MNP mesenchyme, and a moderate level in the LNP. (D–G) In situ hybridization results show PDGFA and PDGFC are expressed in MNP and LNP at E10.5 and E11.5. At both stages, PDGFA is expressed in the epithelium of LNP and MNP, and the olfactory epithelium (D, F). PDGFC expression is detected in the epithelium, as well as in the LNP and MNP mesenchyme (E, G). LNP: Lateral Nasal Process; MNP: Medial Nasal Process; OE: Olfactory Epithelium. PDGFRα regulates cell proliferation in the FNP and MNP through PI3K signaling
To examine the role of PDGFRα in the MNP, we first analyzed cell proliferation and apoptosis in developing embryos. BrdU labeling revealed that in control embryos (n = 9), 40±3% of MNP mesenchymal cells and 35±2% of LNP mesenchymal cells were proliferating at E11.5. In cKO embryos, MNP mesenchymal cell proliferation was decreased by 30% relative to littermate controls, while LNP mesenchymal cell division was maintained a comparable level (Fig. 3A, B and E). No ectopic apoptosis was identified in cKO MNP cells (data not shown). Since the MNP and LNP are derived from the FNP during embryogenesis, we further traced these defects to the FNP. We found that at E9.5, cKO FNP mesenchymal cell proliferation was significantly downregulated by 19% (Fig. 3C–E), and no ectopic cell apoptosis was identified (data not shown). We also assayed expression of genes critical for MNP development in E10.5 embryos. Among them, Six1 and Alx4 expression remained unaltered in cKO and control embryos (Fig. S1), while Alx3 expression was downregulated in the cKO MNP mesenchyme, but not in the mandible (Fig. 3F, G). Because Alx3 is essential for MNP cell survival and no ectopic apoptosis was found in the cKO MNP (data not shown) [31], the reduction of Alx3 mRNA might be caused by a decrease in cell number in the cKO MNP. A similar change in gene expression has been observed in Pax6 mutant embryos, which showed a migration defect of MNP progenitor cells [32]. Together, these data suggest that PDGFRα regulates cell proliferation in the FNP and specifically in the MNP.
Fig. 3. PDGFRα is essential to maintain cell proliferation and gene expression of MNP. 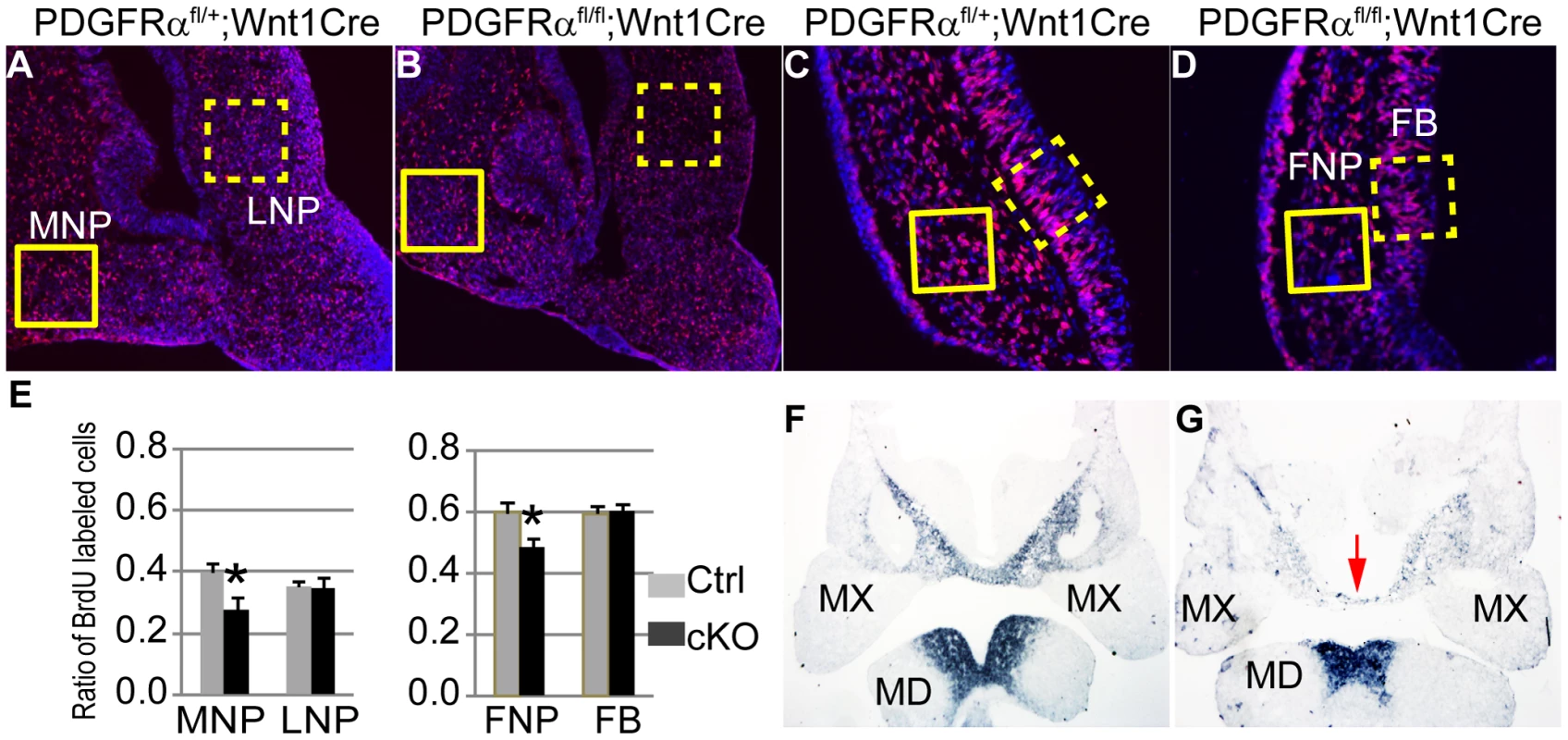
(A–D) Coronal sections of BrdU (red) labeled PDGFRαfl/+; Wnt1Cre (A, C) and PDGFRαfl/fl; Wnt1Cre (B, D) embryos at E11.5 and E9.5. Nuclei are counterstained with DAPI (blue). (E) The cell proliferation rate is significantly decreased in the cKO MNP at E11.5 (PDGFRαfl/fl; Wnt1Cre), as compared to the Control (Ctrl; PDGFRαfl/+; Wnt1Cre). Control and cKO embryos exhibit comparable cell proliferation rates in the LNP. cKO embryos show decreased rate of cell proliferation in the FNP at E9.5, but a comparable rate in the FB relative to the control embryos. N = 9; asterisk, p<0.01. (F, G) Alx3 expression in PDGFRαfl/+; Wnt1Cre and PDGFRαfl/fl; Wnt1Cre embryos at E10.5. In the control embryo (F), Alx3 is expressed in the mesenchyme of medial nasal process and mandible at E10.5. Arrow points to the MNP. In the cKO embryo (G), Alx3 mRNA expression level is decreased specifically in the MNP mesenchyme, and remains comparable to the control in the mandible. FB, forebrain; FNP, frontonasal prominence; MD, mandible; MNP, medial nasal process; MX, maxilla; LNP, lateral nasal process. PDGFRα regulates cell fates and behaviors through a number of downstream signaling pathways. Previous studies in our laboratory showed that PI3K signaling is the major effector of PDGFRα in craniofacial development. Loss of PDGFRα-mediated PI3K signaling alone caused cleft palate with incomplete penetrance, but inactivation of PDGFRα together with PDGFRβ-mediated PI3K signaling caused facial clefting similar to PDGFRα null mutant embryos [24]. To examine the role of PI3K signaling in MNP development, we first analyzed the phenotype of PDGFRαPI3K/PI3K mice. PDGFRαPI3K/PI3K embryos exhibited a shortened nasal septum at E13.5 (Fig. 4A, B) and shortened nasal bones at E18.5 (Fig. 4C, D). The mutant nasal cartilage was 10% shorter than the heterozygous control and the premaxilla was 14% shorter than the control (n = 5, p<0.05). As with the cKO MNP, BrdU labeling results revealed that a decrease in cell proliferation in the MNP of PDGFRαPI3K/PI3K embryos at E11.5 (Fig. 4E–G). To further substantiate these results, we extended these studies to Mouse Embryonic Palatal Mesenchymal cells (MEPMs). Although MNP cells would be the ideal material at this point, we were not able to maintain MNP cells in culture beyond passage 1. Similar to MNP mesenchymal cells, MEPMs originate from cNCCs, exhibit stable PDGFRα expression and response to PDGFA stimulation (Fig. S2) and are thus a robust tool to study PDGFRα function. PDGFA treatment significantly increased cell proliferation in WT MEPMs, but failed to do so in PDGFRαPI3K/PI3K MEPMs (Fig. S3). Conversely, PDGFRαPI3K/PI3K MEPMs exhibited a small decrease in cell proliferation (data not shown). Together these results indicate that PI3K signaling is essential for PDGFRα-regulated neural crest derived cell proliferation.
Fig. 4. PI3K signaling mediates PDGFRα regulation on cell proliferation during MNP development. 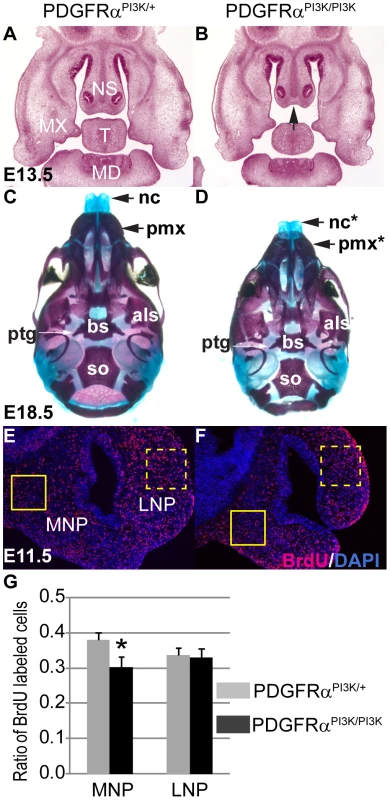
(A, B) Histological coronal sections of embryos at E13.5. PDGFRαPI3K/PI3K embryo (B) exhibits a shortened nasal septum (arrow) and hypoplastic mandible and tongue, as compared to the PDGFRαPI3K/+ control (A). (C, D) PDGFRαPI3K/PI3K E18.5 embryos develop hypoplastic nasal cartilages and shorter premaxilla bones than control (C). Asterisks refer to defective bones or cartilages. (E, F) At E11.5, BrdU labeling results show a decreased proliferation rate in PDGFRαPI3K/PI3K MNP mesenchymal cells (F) as compared to the control (E). (G) The ratio of proliferating cells is significantly decreased in PDGFRαPI3K/PI3K MNP mesenchyme when compared to that of PDGFRαPI3K/+. The LNP cell proliferation rate in PDGFRαPI3K/PI3K is not affected. N = 9; asterisk, p<0.01. Als, alisphenoid; bs, basisphenoid; FB, forebrain; MD, mandible; MNP, medial nasal process; MX, maxilla; nc, nasal cartilage; NS, nasal septum; pmx, premaxilla; ptg, pterygoid; so, supraoccipital bone; T, tongue. Neural crest cells fail to populate the frontonasal area efficiently
Lineage studies have revealed that MNP cells are predominantly derived from NCCs [1], [2]. To be able to trace NCCs in the conditional mutants, we introduced the R26R Cre reporter allele [33] into the PDGFRαfl/fl; Wnt1Cre background. CKO and control embryos were age matched by counting the number of somites. Lineage tracing showed that at E8.5 LacZ reporter expression was comparable in cKO and control embryos (Fig. 5A–D, cKO n = 7, ctrl n = 11), indicating that PDGFRα is not critical for neural crest specification. This observation was confirmed by quantifying the expression of neural crest marker genes Sox10 and Ap2α in E8.5 embryos using RT q-PCR (Fig. 5E, n = 3). By E9.5 however, R26R expression was attenuated in the cKO FNP as compared to the control (Fig. 5F–I, cKO n = 4, ctrl n = 15). Consistent with the above observations, Sox10 expression was also disrupted in E9.5 cKO embryos (Fig. 5J, K). At E10.5, cKO embryos showed fewer NCCs in pharyngeal arches III to VI (Fig. 5L, M, cKO n = 5, ctrl n = 7) and abnormal bifurcation of the NCCs streams migrating to these pharyngeal arches was observed in some embryos (Fig. S4A, B). Skeletal elements derived from these structures including the hyoid bone, the stapes, and the styloid process were severely deformed or missing in cKO embryos at E18.5 (Fig. S4C–H, n = 6). The cell lineage tracing results, along with the altered gene expression signature and defects in skeletal development indicate that PDGFRα is essential for NCCs to migrate in normal numbers and populate craniofacial regions.
Fig. 5. PDGFRα is required for proper population and migration of cranial neural crest cells. 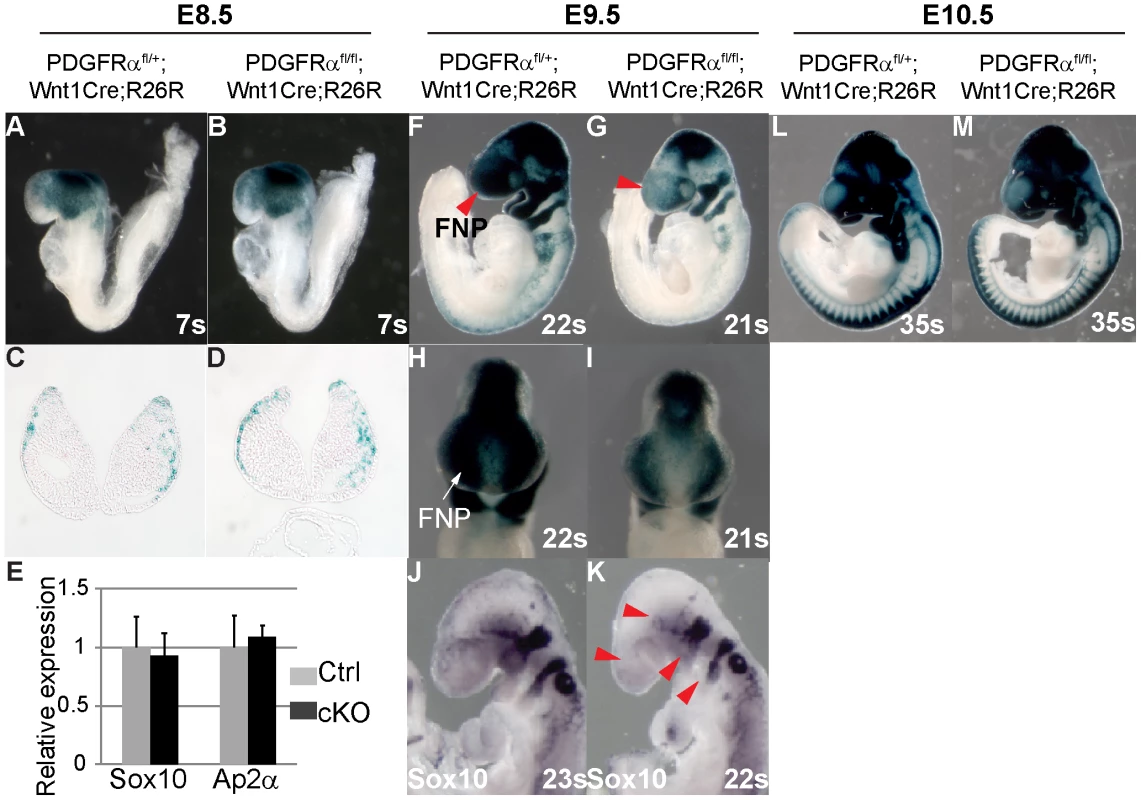
Embryos carrying the ROSA26R Cre reporter were examined by X-gal staining to highlight neural crest derived tissues. (A, B) Whole mount of E8.5 embryos. PDGFRαfl/+; Wnt1Cre (Ctrl) (A) and PDGFRαfl/fl; Wnt1Cre (cKO) (B) show comparable neural crest derived tissues. (C, D) Coronal sections of embryos through head mesenchyme. (E) qRT-PCR quantification of Sox10 and Ap2α mRNA expression of control and cKO embryos at E8.5. N = 3; asterisk, p>0.05. (F, G) CKO embryos (G) at E9.5 show fewer neural crest derived cells than control (F) in multiple tissues including frontal nasal process (FNP) (arrow in G) and pharyngeal arches. (H, I) Frontal view of stained embryos further confirms a neural crest populating defect in cKO FNP. (J, K) In situ hybridization of Sox10 reveals disrupted NCC formation in FNP, trigeminal ganglia, and pharyngeal arches in E9.5 cKO embryo (arrowheads in K). (L, M) Whole mount of E10.5 embryos. FNP, frontonasal prominence. Defective migration and morphology of cKO NCCs
To analyze the function of PDGFRα in NCC migration, explant cultures were established from the cranial neural tube (anterior to the first pharyngeal arch) of cKO and control embryos. To uncouple potential cell migration defects from alterations in cell proliferation, explants were plated on fibronectin in the presence of mitomycin C. The explant cultures exhibited a significant decrease in emigration of cKO NCC cells (Fig. 6A–D, n = 3). Moreover morphometric analysis indicated that primary cKO NCC cells were significantly smaller (33.1%) than control cells (n = 50), and exhibited fewer lamellipodia (Fig. 6E, F), which are required for cell migration. The cKO NCCs also exhibited an increased nuclear-cytoplasmic ratio (12.7% in cKO cells and 4.3% in wild type cells), as well as fewer focal adhesions (34.5 per cKO cell and 89.1 per wild type cell, n = 50; Fig. 6 G).
Fig. 6. Altered NCC morphology in PDGFRα mutants. 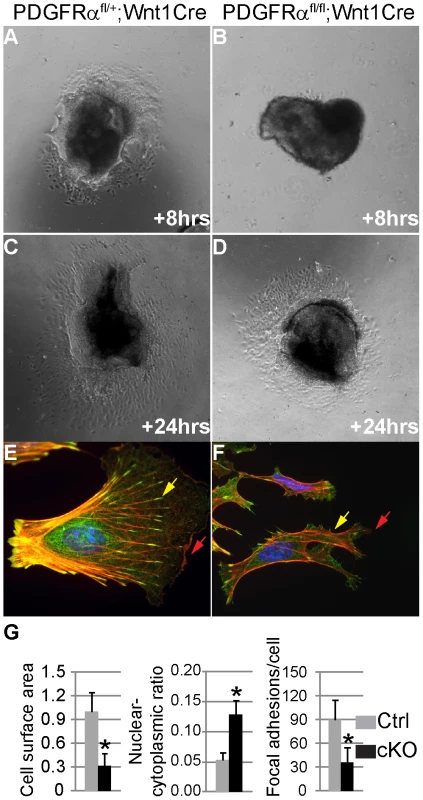
(A–D) Explant cultures of NCC. After 8 and 24 hour growth in 0.5% FBS and 10 µg/ml mitomycin C, cells emigrating from cKO (PDGFRαfl/fl; Wnt1Cre) neural crest explants (B, D) cover a smaller area than those from control (PDGFRαfl/+; Wnt1Cre) (A, C). (E, F) Immunostaining of emigrating NCCs of explants culture. F-actin was stained with Rhodamine-phalloidin (red), vinculin was stained with anti-vinculin antibody (green) and nuclei were stained with DAPI (blue). (E) A typical migrating NCC from control explants. (F) cKO NCCs show aberrant morphology, malformed lamellipodia (red arrows) and focal adhesions (yellow arrows). (G) Morphometric analysis of NCCs from control and cKO explants culture using Image J software. CKO NCCs exhibit smaller cell size, increased nuclear-cytoplasmic ratio and fewer focal adhesions (n = 50; asterisk, p<0.01). These results suggest that PDGFRα is essential for neural crest cell motility, possibly by regulating cytoskeletal architecture. Alternatively, PDGFA/PDGFRα might also regulate NCC migration by regulating cell guidance [25], [34]. To distinguish between these possibilities, we carried out further experiments in primary MEPMs at passage 1. PDGFA acted as a chemo-attractant of primary MEPMs in transwell assays (Fig. 7A). In addition, PDGFA treatment sped up the wound healing rate of MEPMs (Fig. 7B). These data indicate that activation of PDGFRα plays dual roles in neural crest derived cell migration, by stimulating chemotaxis and by regulating cell motility.
Fig. 7. PDGFRα controls chemotaxis and cell motility. 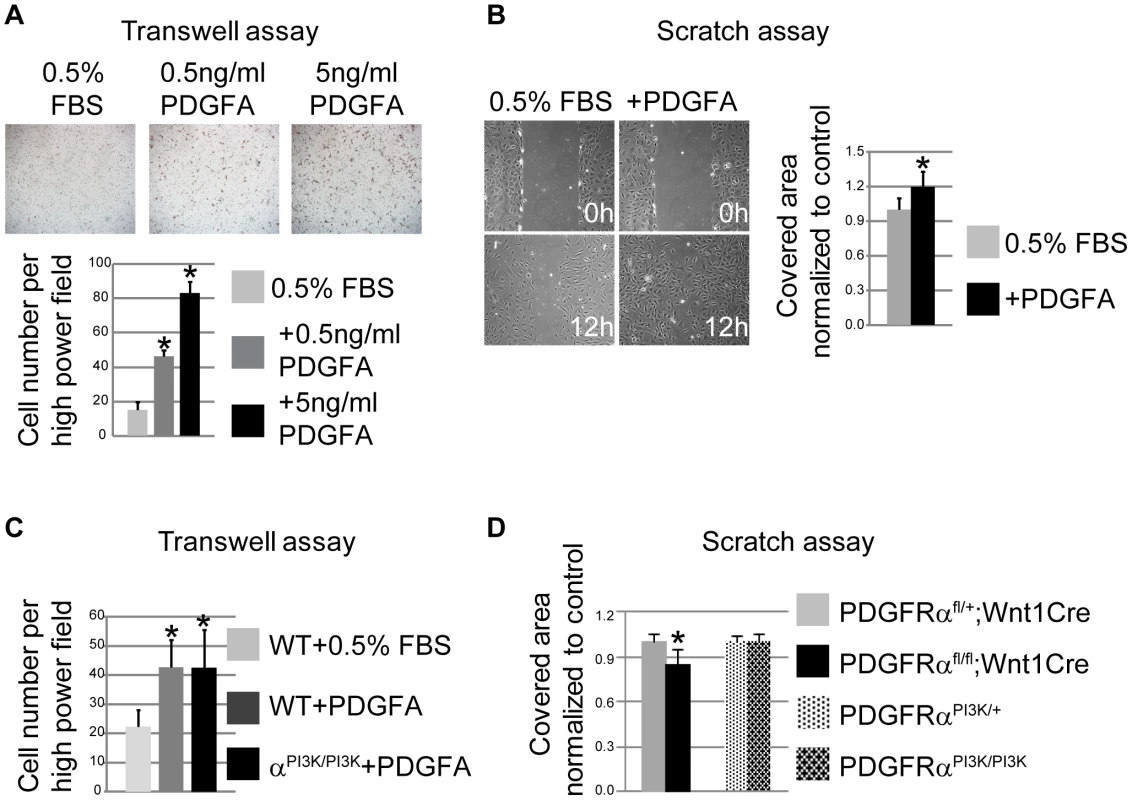
(A) PDGFA (0.5 ng/ml and 5 ng/ml) stimulates chemotaxis of MEPMs in transwell assays. N = 9; asterisk, p<0.01. (B) PDGFA (30 ng/ml) accelerates wound healing in MEPMs in scratch assays. N = 9; asterisk, p<0.01. (C) PDGFRαPI3K/PI3K MEPMs migrate towards PDGFA at a comparable level to PDGFRαPI3K/+ MEPMs in transwell assays. N = 9; asterisk, p<0.01. (D) The speed of wound healing in scratch assays is significantly affected in cKO MEPMs, but not in PDGFRαPI3K/PI3K MEPMs. N = 9; asterisk, p<0.01. To investigate if PI3K signaling plays a role in NCC migration, we generated PDGFRPI3K/PI3K; Wnt1Cre; R26R+/− embryos. Lineage tracing showed no obvious cNCC migration defects in PDGFRαPI3K/PI3K; Wnt1Cre; R26R+/− embryos (Fig. S5, n = 6). Transwell assays revealed that PDGFRαPI3K/PI3K MEPMs respond and migrate towards a source of PDGFA at a level comparable to wild type cells (Fig. 7C). In addition, the wound healing speed of PDGFRαPI3K/PI3K MEPMs remains comparable to that of heterozygous cells in a scratch assay (Fig. 7D). In summary, these results indicate that PI3K signaling engaged by PDGFRα is not essential for cell migration, in contrast to its role in regulating proliferation of neural crest derived cells during development.
Rac1 signaling mediates PDGFRα regulation of proliferation and migration of MEPMs
The abnormal morphology of cKO NCCs indicates other signaling might be essential to regulate the cytoskeleton downstream of PDGFRα. Rho GTPases constitute a group of major regulators of cell migration that mediate actin reorganization, and lamellipodia and filopodia formation [35], [36]. PDGF and PI3K/Akt signaling have been shown to phosphorylate guanine nucleotide-exchange factors (GEFs), which in turn prompt the formation of GTP-bound, active small GTPases such as RhoA, Cdc42 and Rac1 [37]–[40]. Inactivation of Rac1 in NCCs caused facial clefting, strikingly resembling the PDGFRα cKO phenotype [41], [42], suggesting Rac1 to be a potential mediator of PDGFRα in NCC development. Rac1 activity was attenuated in lysates of E10.5 cKO MNP cells (Fig. 8A), and we observed a reduction in the expression of phosphorylated cofilin 1, an actin depolymerization enzyme required for cNCC development (Fig. 8 B) [43]. PDGFA stimulation facilitates phosphorylation of cofilin in MEPMs (Fig. 8C), indicating that PDGFRα can regulate Rac1 activity in neural crest derived cells. Consistent with an important role for Rac1 in mediating PDGF driven functions, treatment of MEPMs with the Rac1 specific inhibitor NSC 23766 blocked PDGFA stimulated proliferation and wound healing (Fig. 8D, E). Further examination revealed that inactivation of Rac1 affected lamellipodia formation at the leading edge of migrating MEPMs, reminiscent of the phenotype of PDGFRα deficient MEPMs (Fig. 8F–K). Inhibition of Rac1 activity in MEPMs also led to smaller size (31% of untreated cells, n = 50), fewer focal adhesion complexes (22.1 per treated cell vs. 83.2 per untreated cell, n = 50), and increased nuclear-cytoplasmic ratio (9% in treated cells vs. 5% in untreated MEPMs, n = 50) (Fig. 8L). Taken together, these results indicate a prominent role for Rac1 in the regulation of PDGF-induced cell migration and proliferation.
Fig. 8. Rac1 mediates PDGFRα regulation of proliferation and migration in MNP cells and MEPMs. 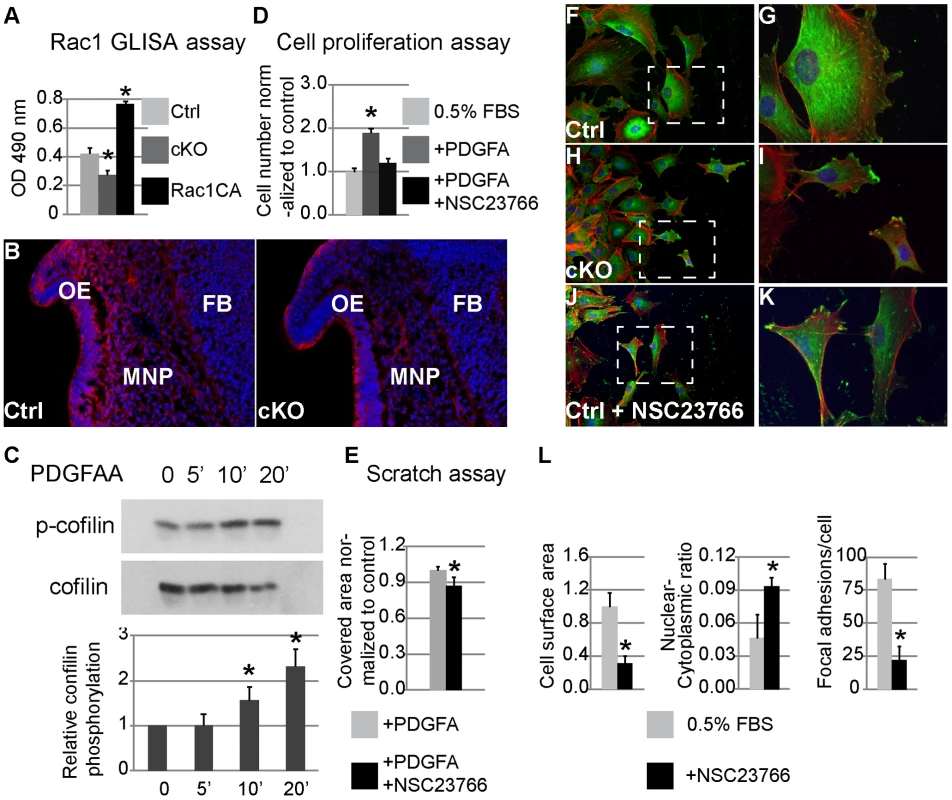
(A) Rac1 G-LISA assay. Rac1 activity is decreased in cKO MNP lysate; recombinant constitutively active (CA) Rac1 is included as a control. (B) Cofilin phosphorylation as seen by immunostaining (red) is attenuated primarily in cKO MNP mesenchymal cells (DAPI blue) (n = 3; asterisk, p<0.05). (C) Western blot analysis shows elevated cofilin phosphorylation in MEPMs following exposure to PDGFAA for 5 and 10 min. N = 3; asterisk, p<0.05. (D) Inhibition of Rac1 activity abrogates PDGFA stimulation of cell proliferation in MEPMs. N = 3; asterisk, p<0.05. (E) Inhibition of Rac1 activity antagonizes the effect of PDGFA on wound healing in MEPMs. N = 9; asterisk, p<0.01. (F–K) Immunostaining of MEPMs at the leading edge of scratch assay. Expression of Vinculin (green), F-actin (red) and nuclei (blue) was examined. G, I and K represent magnification of areas outlined by the dashed lines in F, H and J, respectively. (L) Compared to untreated wild type cells (F, G), inhibition of Rac1 leads to smaller cells, abnormal elongation and disrupted lamellipodia formation (J, K), similar to PDGFRαfl/fl; Wnt1Cre MEPMs (H, I). N = 50; asterisk, p<0.01. Discussion
Facial clefting is a rare birth defect and its etiology remains poorly understood. In this work, we show that the facial clefting phenotype of PDGFRα mutants is not associated with a defect in NCC specification but rather a subsequent defect in the medial nasal process (MNP), a facial primordium derived from the frontonasal prominence (FNP). We further show that this defect is associated with alterations in both cell proliferation and cell migration, and that PI3K and Rac1 signaling are essential to maintain a normal level of cell proliferation. Last, we provide evidence that Rac1 regulates cell migration at the level of cell motility as well as chemotaxis under the regulation of PDGFRα.
A previous study from our laboratory had shown that the facial clefting observed in PDGFRα mutants was of neural crest origin, using chimeric analysis and conditional mutagenesis with the Wnt1Cre driver [21]. Although global defects in cell proliferation and migration were not documented, chimeric analysis identified a role for PDGFRα in development of the pharyngeal arches, which we now show by lineage tracing are deficient in cNCCs by E10.5 in cKO embryos. In this work, we have considerably refined the analysis by examining specific rather than overall craniofacial subregions. We were thus able to document disruption of cell migration in the FNP by cell lineage analysis at E9.5, and of cell proliferation in the MNP of cKO embryos by E10.5. Therefore, both the previous study and the present work identify a crucial role for PDGFRα in NCC development.
We found that PDGFRα exhibits a strong expression pattern in the MNP mesenchyme at different stages. PDGFA is expressed in the MNP epithelium, and PDGFC is expressed in both the MNP epithelium and the mesenchyme, consistent with paracrine or autocrine PDGFRα signaling during craniofacial development. Prior genetic evidence from our lab, using point mutations in the PI3K binding sites in the PDGFRs, has implicated PI3K as the key signaling pathway regulating craniofacial development [24]. We also found that that PI3K signaling regulates p44/42 MAPK (data not shown). Strikingly, mice carrying a neural crest-specific deletion of Erk1/Erk2 display facial clefting [23]. p44/42 MAPK can also be engaged by other pathways than PI3K, and by other RTKs that are critical for craniofacial development including Fgfrs, Eph receptors, EGFR, Ror or Ryk. Although these RTKs share some similar intracellular domains and engage overlapping signaling pathways, their impact on craniofacial development might reflect tissue-restricted expression patterns of receptors and ligands, as well as engagement of unique combinations of downstream signaling cascades. It will be important to understand if the phenotypic differences associated with different RTKs are due to dosage variation of PI3K signaling, involvement of other unique signaling pathways, or a combination of both.
Neural crest cells form through delamination of cells at the lateral plate border of the neural tube that undergo an epithelial to mesenchymal transition. There is extensive evidence that cell-cell contacts through intricate lamellipodial and filipodial extensions play critical roles in regulating how cells exit the neural tube and migrate to their proper destination (for a review, see [13]). Small GTPases, including Rho, Rac and Cdc42 are well known to regulate such cell behaviors, but also cell proliferation and gene transcription under the regulation of multiple RTKs [44]. Recent gene targeting studies showed that inactivation of Rac1 or Cdc42, or overexpression of a dominant negative Rho kinase in mouse NCCs causes a severe facial clefting phenotype, which strikingly resembles PDGFRα homozygous mutant embryos [21], [22], [41], [42], [45]. In particular, Rac1 deficient NCCs exhibit decreased proliferation, abnormal cell morphology, as well as disrupted lamellipodia formation [42], very similar to the defects we have observed in PDGFRα deficient NCCs and MEPMs. The notion that PDGFRα might be a major regulator of Rac1 activity is further supported by our present work and other studies that show that PDGFA stimulates Rac1-GTP levels in a variety of biological settings [46]–[48]. It has been suggested that EGFR could be the major effector of the Rac1 mutant phenotype [41], as EGF has been used as an agonist of Rac1 activity in a variety of in vitro studies. However EGFR null mutant mice exhibit a much milder craniofacial phenotype with only a cleft palate. In addition, gene expression data showed that EGFR and Rac1 only partially overlap in ectodermal cells during early stages of craniofacial morphogenesis (Emage, www.emouseatlas.org), whereas PDGFRα shows a broad expression pattern similar to Rac1 in cNCCs at E10.5. Functionally, PDGFRα deficient NCCs exhibit abnormal morphology and defective formation of lamellipodia and focal adhesions. These lines of evidence point to a major role for Rac1 in mediating PDGFRα functions in NCC development and craniofacial morphogenesis.
Materials and Methods
Ethics statement
The Mount Sinai School of Medicine Institutional Animal Care and Use Committee (IACUC) approved all animal work and procedures used in this study. The Mount Sinai animal facility is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC).
Mice and genotyping
PDGFRαfl/fl, PDGFRαGFP/+, PDGFRαPI3K/+, Wnt1Cre and R26R mice have been described previously [21], [24], [26], [29], [33]. PDGFRαfl/fl, PDGFRαPI3K/+ and Wnt1Cre mice were maintained on a 129S4 co-isogenic genetic background, and PDGFRαGFP/+ and ROSA26R mice were kept on a C57BL/6J co-isogenic background. Mice and embryos used in lineage tracing studies were maintained on a mixed genetic background.
Histology, skeletal analysis, immunostaining, in situ hybridization and X-gal staining
For histology, staged embryos were dissected in ice-cold PBS, fixed in Bouin's fixative, dehydrated through a graded series of ethanol washes, and embedded in paraffin. Sections were cut at 10 µm for hematoxylin and eosin staining. Skeletal analysis was performed on E18.5 embryos as described [22]. Craniofacial morphometry was performed as described in Fig. S6. For immunostaining, embryos were fixed in 4% PFA overnight at 4°C, dehydrated in 30% sucrose/PBS and embedded in OCT. Cryosections were prepared at a thickness of 10 µm. Immunostaining was performed according to standard protocols using antibodies to PDGFRα (1∶60; Santa Cruz), vinculin (1∶200, Sigma), BrdU (1∶500, DSHB), Cleaved-Caspase 3 (1∶200, Cell Signaling Technology) and rhodamine phalloidin (0.2 µM, Biotium). For in situ hybridization, embryos were dissected in ice-cold PBS, fixed in 4% paraformaldehyde (PFA), dehydrated through graded ethanol washes, and embedded in paraffin. Coronal sections were cut at 10 µm and in situ hybridization was performed as described [49]. X-gal staining was performed as described [33].
Cell proliferation analysis
To measure cell proliferation rates in vivo, BrdU was injected intraperitoneally into pregnant females at a dosage of 50 µg per gram of body weight. Embryos were dissected after 1 hour, fixed in 4% PFA and processed for cryosections and immunostaining using standard protocols. BrdU labeled cells were counted in a random area in the defined mesenchyme at comparable levels in mutant and control samples. Three continuous sections were counted from each of triplicate samples. The result of BrdU labeling was presented as percentage of BrdU-positive cells against total nuclei labeled by DAPI. Student's t-test was used to determine statistical significance.
Neural crest explant cultures
Neural tubes anterior to the first pharyngeal arch were dissected from E8.5 embryos. Following two brief washes in ice-cold PBS, heads were sagitally split into two equal halves. The tissue was incubated in 0.5% trypsin/2.5% pancreatin in PBS for 5 minutes on ice, and then in DMEM with 10% FBS for 10 minutes to stop the reaction. The head mesenchyme was carefully dissected and isolated with fine-tipped Dumont #5 tweezers, and the neural tube was transferred to fibronectin-coated cover slips in a 6 well plate. For NCC emigration assays, neural tubes were cultured in DMEM/F12 with 10% FBS for 6 hours. The explants were then treated with low serum media (DMEM/F12 containing 0.5% FBS) with 10 µg/ml mitomycin C for two hours. The culture medium was replaced with low serum media and maintained for 24 hours. Results were recorded at 8 hours and 24 hours respectively. The explants were then removed and the emigrating cells were subjected to immunostaining.
Cell culture, scratch assay and transwell assay
Primary Mouse Embryonic Palatal Mesenchymal cells (MEPMs) were isolated as described [50]. For scratch assays, MEPMs at passage 1 were seeded on fibronectin-coated cover slips in 6 well plates at a density of 100,000 cells per well. After reaching 70–80% confluency, cells were starved for 24 hours in DMEM containing 0.5% FBS. In some experiments, serum starved cells were pretreated with 30 µM Rac1 inhibitor NSC23766 (R&D Systems) for 3 hrs before stimulation with 30 ng/ml PDGFA. The scratch was mechanically created using a sterile P200 pipette tip and washed twice with starving medium to remove cell debris. The wound area was then photographed at marked positions (3 different fields per well). Cells were allowed to migrate for 12 hours at 37°C before the same fields were recorded. All experiments were performed in triplicate. Scratch results were measured with Image J software (NIH, Bethesda, USA) and analyzed using the extension package MiToBo [51].
For transwell assays, cell culture inserts for a 24-well plate (Fisher) with a pore size of 8 µm were coated with fibronectin. P1 MEPMs were trypsinized, washed, and suspended in serum free medium at a concentration of 5×106 cells/ml. 300 µl of cell suspension was added to the insert chambers immersed in 500 µl medium with 10% FBS, 0.5% FBS, or 0.5% FBS with PDGFAA. After incubation at 37°C and 5% CO2 for 3.5 hours, the inserts were fixed in 3.7% formaldehyde and stained in Mayer's hematoxylin solution. Filters of the inserts were then isolated with a scalpel and mounted. The numbers of cells on the bottom were counted. Data were recorded from 9 high power fields from three independent experiments.
Western blot and Rac1 activity analysis
Western blot analysis was carried out as described previously [24], using primary antibodies from Abcam: anti-cofilin (phospho-S3) and from Cell Signaling Technology: anti-cofilin, anti-p44/42 MAPK, anti-phospho p44/42 MAPK, anti-Akt, anti-phospho-Akt (Ser473). Chemical inhibitors for MAPK (U0126, Promega), PI3K/Akt (LY294002, Stemgent) and Rac1 (NSC23766, R&D systems) were used in lysate generation for western blot analysis and the Rac1 activity assay, following the manufacturers' instruction. Rac1 activity analysis was performed using the colorimetric based G-LISA Rac1 Activation Assay Biochem Kit (Cytoskeleton Inc.).
Supporting Information
Zdroje
1. ChaiY, JiangX, ItoY, et al. (2000) Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development 127 : 1671–1679.
2. JiangX, IsekiS, MaxsonRE, SucovHM, Morriss-KayGM (2002) Tissue origins and interactions in the mammalian skull vault. Developmental Biology 241 : 106–116.
3. CorderoDR, BrugmannS, ChuY, BajpaiR, JameM, et al. (2011) Cranial neural crest cells on the move: their roles in craniofacial development. American Journal of Medical Genetics Part A 155A: 270–279.
4. GammillLS, Bronner-FraserM (2003) Neural crest specification: migrating into genomics. Nature Reviews Neuroscience 4 : 795–805.
5. WehbyGL, CassellCH (2010) The impact of orofacial clefts on quality of life and healthcare use and costs. Oral Diseases 16 : 3–10.
6. DixonMJ, MarazitaML, BeatyTH, MurrayJC (2011) Cleft lip and palate: understanding genetic and environmental influences. Nature Reviews Genetics 12 : 167–178.
7. HelmsJA, CorderoD, TapadiaMD (2005) New insights into craniofacial morphogenesis. Development 132 : 851–861.
8. JiangR, BushJO, LidralAC (2006) Development of the upper lip: morphogenetic and molecular mechanisms. Dev Dyn 235 : 1152–1166.
9. SantagatiF, RijliFM (2003) Cranial neural crest and the building of the vertebrate head. Nature Reviews Neuroscience 4 : 806–818.
10. Osumi-YamashitaN, NinomiyaY, EtoK, DoiH (1994) The contribution of both forebrain and midbrain crest cells to the mesenchyme in the frontonasal mass of mouse embryos. Developmental Biology 164 : 409–419.
11. SerbedzijaGN, Bronner-FraserM, FraserSE (1992) Vital dye analysis of cranial neural crest cell migration in the mouse embryo. Development 116 : 297–307.
12. JonesNC, TrainorPA (2005) Role of morphogens in neural crest cell determination. Journal of Neurobiology 64 : 388–404.
13. KulesaPM, BaileyCM, Kasemeier-KulesaJC, McLennanR (2010) Cranial neural crest migration: New rules for an old road. Developmental Biology 344 : 543–554.
14. MinouxM, RijliFM (2010) Molecular mechanisms of cranial neural crest cell migration and patterning in craniofacial development. Development 137 : 2605–2621.
15. NietoMA (2001) The early steps of neural crest development. Mechanisms of Development 105 : 27–35.
16. AndraeJ, GalliniR, BetsholtzC (2008) Role of platelet-derived growth factors in physiology and medicine. Genes and Development 22 : 1276–1312.
17. HochR, SorianoP (2003) Roles of PDGF in animal development. Development 130 : 4769–4784.
18. TallquistM, KazlauskasA (2004) PDGF signaling in cells and mice. Cytokine & Growth Factor Reviews 15 : 205–213.
19. SmithCL, TallquistMD (2010) PDGF function in diverse neural crest cell populations. Cell Adh Migr 4 : 561–566.
20. HeldinCH, WestermarkB (1999) Mechanism of Action and In Vivo Role of Platelet-Derived Growth Factor. Physiological reviews 79 : 1283–1316.
21. TallquistMD, SorianoP (2003) Cell autonomous requirement for PDGFR in populations of cranial and cardiac neural crest cells. Development 130 : 507.
22. SorianoP (1997) The PDGF alpha receptor is required for neural crest cell development and for normal patterning of the somites. Development 124 : 2691–2700.
23. NewbernJ, ZhongJ, WickramasingheRS, LiX, WuY, et al. (2008) Mouse and human phenotypes indicate a critical conserved role for ERK2 signaling in neural crest development. Proceedings of the National Academy of Sciences of the United States of America 105 : 17115–17120.
24. KlinghofferRA, HamiltonTG, HochR, SorianoP (2002) An Allelic Series at the PDGFalphaR Locus Indicates Unequal Contributions of Distinct Signaling Pathways During Development. Developmental Cell 2 : 103–113.
25. EberhartJK, HeX, SwartzME, YanYL, SongH, et al. (2008) MicroRNA Mirn140 modulates Pdgf signaling during palatogenesis. Nature Genetics 40 : 290–298.
26. DanielianPS, MuccinoD, RowitchDH, MichaelSK, McMahonAP (1998) Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Current Biology 8 : 1323–S1322, 1323-1326, S1321-S1322.
27. NodenDM, TrainorPA (2005) Relations and interactions between cranial mesoderm and neural crest populations. Journal of Anatomy 207 : 575–601.
28. NodenDM, SchneiderRA (2006) Neural crest cells and the community of plan for craniofacial development: historical debates and current perspectives. Advances in Experimental Medicine and Biology 589 : 1–23.
29. HamiltonTG, KlinghofferRA, CorrinPD, SorianoP (2003) Evolutionary Divergence of Platelet-Derived Growth Factor Alpha Receptor Signaling Mechanisms. Molecular and Cellular Biology 23 : 4013–4025.
30. DingH, WuX, BostromH, KimI, WongN, et al. (2004) A specific requirement for PDGF-C in palate formation and PDGFR-alpha signaling. Nat Genet 36 : 1111–1116.
31. BeverdamA, BrouwerA, ReijnenM, KorvingJ, MeijlinkF (2001) Severe nasal clefting and abnormal embryonic apoptosis in Alx3/Alx4 double mutant mice. Development 128 : 3975.
32. CompagnucciC, FishJL, SchwarkM, TarabykinV, DepewMJ (2011) Pax6 regulates craniofacial form through its control of an essential cephalic ectodermal patterning center. Genesis 49 : 307–325.
33. SorianoP (1999) Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature Genetics 21 : 70.
34. KawakamiM, UmedaM, NakagataN, TakeoT, YamamuraK (2011) Novel migrating mouse neural crest cell assay system utilizing P0-Cre/EGFP fluorescent time-lapse imaging. BMC Developmental Biology 11 : 68.
35. RidleyAJ (2001) Rho GTPases and cell migration. Journal of Cell Science 114 : 2713–2722.
36. Etienne-MannevilleS, HallA (2002) Rho GTPases in cell biology. Nature 420 : 629–635.
37. KashishianA, KazlauskasA, CooperJA (1992) Phosphorylation sites in the PDGF receptor with different specificities for binding GAP and PI3 kinase in vivo. EMBO Journal 11 : 1373–1382.
38. RidleyAJ, HallA (1992) The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70 : 389–399.
39. HawkinsPT, EguinoaA, QiuRG, StokoeD, CookeFT, et al. (1995) PDGF stimulates an increase in GTP-Rac via activation of phosphoinositide 3-kinase. Current Biology 5 : 393–403.
40. ZubiaurM, SanchoJ, TerhorstC, FallerDV (1995) A small GTP-binding protein, Rho, associates with the platelet-derived growth factor type-beta receptor upon ligand binding. Journal of Biological Chemistry 270 : 17221–17228.
41. FuchsS, HerzogD, SumaraG, Büchmann-MøllerS, CivenniG, et al. (2009) Stage-Specific Control of Neural Crest Stem Cell Proliferation by the Small Rho GTPases Cdc42 and Rac1. Cell Stem Cell 4 : 236–247.
42. ThomasPS, KimJ, NunezS, GlogauerM, KaartinenV (2010) Neural crest cell-specific deletion of Rac1 results in defective cell-matrix interactions and severe craniofacial and cardiovascular malformations. Developmental Biology 340 : 613–625.
43. GurniakCB, PerlasE, WitkeW (2005) The actin depolymerizing factor n-cofilin is essential for neural tube morphogenesis and neural crest cell migration. Developmental Biology 278 : 231–241.
44. HallA (2005) Rho GTPases and the control of cell behaviour. Biochemical Society Transactions 33 : 891–895.
45. PhillipsHM, PapoutsiT, SoenenH, Ybot-GonzalezP, HendersonDJ, et al. (2012) Neural crest cell survival is dependent on Rho kinase and is required for development of the mid face in mouse embryos. PLoS ONE 7: e37685.
46. FengH, HuB, LiuKW, LiY, LuX, et al. (2011) Activation of Rac1 by Src-dependent phosphorylation of Dock180(Y1811) mediates PDGFRalpha-stimulated glioma tumorigenesis in mice and humans. Journal of Clinical Investigation 121 : 4670–4684.
47. PickettEA, OlsenGS, TallquistMD (2008) Disruption of PDGFRalpha-initiated PI3K activation and migration of somite derivatives leads to spina bifida. Development 135 : 589–598.
48. FengH, LiuKW, GuoP, ZhangP, ChengT, et al. (2012) Dynamin 2 mediates PDGFRalpha-SHP-2-promoted glioblastoma growth and invasion. Oncogene 31 : 2691–2702.
49. St. AmandTR, ZhangY, SeminaEV, ZhaoX, HuYP, et al. (2000) Antagonistic signals between BMP4 and FGF8 define the expression of Pitx1 and Pitx2 in mouse tooth-forming anlage. Developmental biology 217 : 323–332.
50. BushJO, SorianoP (2010) Ephrin-B1 forward signaling regulates craniofacial morphogenesis by controlling cell proliferation across Eph-ephrin boundaries. Genes and Development 24 : 2068–2080.
51. Glaß M, Möller B, Zirkel A, Wächter K, Hüttelmaier S, et al.. (2011) Scratch Assay Analysis with Topology-Preserving Level Sets and Texture Measures. In: Vitrià J, Sanches J, Hernández M, editors. Pattern Recognition and Image Analysis: Springer Berlin Heidelberg. pp. 100–108.
Štítky
Genetika Reprodukční medicína
Článek Rapid Intrahost Evolution of Human Cytomegalovirus Is Shaped by Demography and Positive SelectionČlánek Common Variants in Left/Right Asymmetry Genes and Pathways Are Associated with Relative Hand SkillČlánek Manipulating or Superseding Host Recombination Functions: A Dilemma That Shapes Phage EvolvabilityČlánek Maternal Depletion of Piwi, a Component of the RNAi System, Impacts Heterochromatin Formation inČlánek Hsp104 Suppresses Polyglutamine-Induced Degeneration Post Onset in a Drosophila MJD/SCA3 ModelČlánek Cooperative Interaction between Phosphorylation Sites on PERIOD Maintains Circadian Period inČlánek VAPB/ALS8 MSP Ligands Regulate Striated Muscle Energy Metabolism Critical for Adult Survival inČlánek Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2AČlánek A Link between ORC-Origin Binding Mechanisms and Origin Activation Time Revealed in Budding YeastČlánek Genotype-Environment Interactions Reveal Causal Pathways That Mediate Genetic Effects on PhenotypeČlánek Chromatin-Specific Regulation of Mammalian rDNA Transcription by Clustered TTF-I Binding SitesČlánek Meiotic Recombination in Arabidopsis Is Catalysed by DMC1, with RAD51 Playing a Supporting Role
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 9- Akutní intermitentní porfyrie
- Souvislost haplotypu M2 genu pro annexin A5 s opakovanými reprodukčními ztrátami
- Vztah užívání alkoholu a mužské fertility
- Doc. Miloš Kubánek: Nemocní se srdeční amyloidózou jsou často skryti a sledováni pod jinými diagnózami
- Hysteroskopická resekce děložního septa zlepšuje šanci na graviditu žen s jinak nevysvětlenou infertilitou
-
Všechny články tohoto čísla
- The Pathway Gene Functions together with the -Dependent Isoprenoid Biosynthetic Pathway to Orchestrate Germ Cell Migration
- Take Off, Landing, and Fly Anesthesia
- Nucleosome Assembly Proteins Get SET to Defeat the Guardian of Chromosome Cohesion
- Whole-Exome Sequencing Reveals a Rapid Change in the Frequency of Rare Functional Variants in a Founding Population of Humans
- Evidence Is Evidence: An Interview with Mary-Claire King
- Rapid Intrahost Evolution of Human Cytomegalovirus Is Shaped by Demography and Positive Selection
- Convergent Transcription Induces Dynamic DNA Methylation at Loci
- Environmental Stresses Disrupt Telomere Length Homeostasis
- Ultra-Sensitive Sequencing Reveals an Age-Related Increase in Somatic Mitochondrial Mutations That Are Inconsistent with Oxidative Damage
- Common Variants in Left/Right Asymmetry Genes and Pathways Are Associated with Relative Hand Skill
- Genetic and Anatomical Basis of the Barrier Separating Wakefulness and Anesthetic-Induced Unresponsiveness
- The Locus, Exclusive to the Ambulacrarians, Encodes a Chromatin Insulator Binding Protein in the Sea Urchin Embryo
- Binding of NF-κB to Nucleosomes: Effect of Translational Positioning, Nucleosome Remodeling and Linker Histone H1
- Manipulating or Superseding Host Recombination Functions: A Dilemma That Shapes Phage Evolvability
- Dynamics of DNA Methylation in Recent Human and Great Ape Evolution
- Functional Dissection of Regulatory Models Using Gene Expression Data of Deletion Mutants
- PAQR-2 Regulates Fatty Acid Desaturation during Cold Adaptation in
- N-alpha-terminal Acetylation of Histone H4 Regulates Arginine Methylation and Ribosomal DNA Silencing
- A Genome-Wide Systematic Analysis Reveals Different and Predictive Proliferation Expression Signatures of Cancerous vs. Non-Cancerous Cells
- Maternal Depletion of Piwi, a Component of the RNAi System, Impacts Heterochromatin Formation in
- miR-1/133a Clusters Cooperatively Specify the Cardiomyogenic Lineage by Adjustment of Myocardin Levels during Embryonic Heart Development
- Hsp104 Suppresses Polyglutamine-Induced Degeneration Post Onset in a Drosophila MJD/SCA3 Model
- Genome-Wide Analysis of Genes and Their Association with Natural Variation in Drought Tolerance at Seedling Stage of L
- Deep Resequencing of GWAS Loci Identifies Rare Variants in , and That Are Associated with Ulcerative Colitis
- Cooperative Interaction between Phosphorylation Sites on PERIOD Maintains Circadian Period in
- VAPB/ALS8 MSP Ligands Regulate Striated Muscle Energy Metabolism Critical for Adult Survival in
- Analysis of Genes Reveals Redundant and Independent Functions in the Inner Ear
- Predicting the Risk of Rheumatoid Arthritis and Its Age of Onset through Modelling Genetic Risk Variants with Smoking
- Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2A
- A Shift to Organismal Stress Resistance in Programmed Cell Death Mutants
- Fragile Site Instability in Causes Loss of Heterozygosity by Mitotic Crossovers and Break-Induced Replication
- Tracking of Chromosome and Replisome Dynamics in Reveals a Novel Chromosome Arrangement
- The Condition-Dependent Transcriptional Landscape of
- Ago1 Interacts with RNA Polymerase II and Binds to the Promoters of Actively Transcribed Genes in Human Cancer Cells
- Nebula/DSCR1 Upregulation Delays Neurodegeneration and Protects against APP-Induced Axonal Transport Defects by Restoring Calcineurin and GSK-3β Signaling
- System-Wide Analysis Reveals a Complex Network of Tumor-Fibroblast Interactions Involved in Tumorigenicity
- Meta-Analysis of Genome-Wide Association Studies Identifies Six New Loci for Serum Calcium Concentrations
- and Are Required for Cellularization and Differentiation during Female Gametogenesis in
- Growth factor independent-1 Maintains Notch1-Dependent Transcriptional Programming of Lymphoid Precursors
- Whole Genome Sequencing Identifies a Deletion in Protein Phosphatase 2A That Affects Its Stability and Localization in
- An Alteration in ELMOD3, an Arl2 GTPase-Activating Protein, Is Associated with Hearing Impairment in Humans
- Genomic Identification of Founding Haplotypes Reveals the History of the Selfing Species
- Plasticity Regulators Modulate Specific Root Traits in Discrete Nitrogen Environments
- The IDD14, IDD15, and IDD16 Cooperatively Regulate Lateral Organ Morphogenesis and Gravitropism by Promoting Auxin Biosynthesis and Transport
- Stochastic Loss of Silencing of the Imprinted Allele, in a Mouse Model and Humans with Prader-Willi Syndrome, Has Functional Consequences
- The Prefoldin Complex Regulates Chromatin Dynamics during Transcription Elongation
- PKA Controls Calcium Influx into Motor Neurons during a Rhythmic Behavior
- A Pre-mRNA-Splicing Factor Is Required for RNA-Directed DNA Methylation in
- Cell-Type Specific Features of Circular RNA Expression
- The Uve1 Endonuclease Is Regulated by the White Collar Complex to Protect from UV Damage
- An Atypical Kinase under Balancing Selection Confers Broad-Spectrum Disease Resistance in Arabidopsis
- Genome-Wide Mutation Avalanches Induced in Diploid Yeast Cells by a Base Analog or an APOBEC Deaminase
- Extensive Divergence of Transcription Factor Binding in Embryos with Highly Conserved Gene Expression
- Bi-modal Distribution of the Second Messenger c-di-GMP Controls Cell Fate and Asymmetry during the Cell Cycle
- Cell Interactions and Patterned Intercalations Shape and Link Epithelial Tubes in
- A Link between ORC-Origin Binding Mechanisms and Origin Activation Time Revealed in Budding Yeast
- The Genome and Development-Dependent Transcriptomes of : A Window into Fungal Evolution
- SKN-1/Nrf, A New Unfolded Protein Response Factor?
- The Highly Prolific Phenotype of Lacaune Sheep Is Associated with an Ectopic Expression of the Gene within the Ovary
- Fusion of Large-Scale Genomic Knowledge and Frequency Data Computationally Prioritizes Variants in Epilepsy
- IL-17 Attenuates Degradation of ARE-mRNAs by Changing the Cooperation between AU-Binding Proteins and microRNA16
- An Enhancer Element Harboring Variants Associated with Systemic Lupus Erythematosus Engages the Promoter to Influence A20 Expression
- Genome Analysis of a Transmissible Lineage of Reveals Pathoadaptive Mutations and Distinct Evolutionary Paths of Hypermutators
- Type I-E CRISPR-Cas Systems Discriminate Target from Non-Target DNA through Base Pairing-Independent PAM Recognition
- Divergent Transcriptional Regulatory Logic at the Intersection of Tissue Growth and Developmental Patterning
- MEIOB Targets Single-Strand DNA and Is Necessary for Meiotic Recombination
- Transmission of Hypervirulence Traits via Sexual Reproduction within and between Lineages of the Human Fungal Pathogen
- Integration of the Unfolded Protein and Oxidative Stress Responses through SKN-1/Nrf
- Guanine Holes Are Prominent Targets for Mutation in Cancer and Inherited Disease
- Regulation of the Boundaries of Accessible Chromatin
- Natural Genetic Transformation Generates a Population of Merodiploids in
- Ablating Adult Neurogenesis in the Rat Has No Effect on Spatial Processing: Evidence from a Novel Pharmacogenetic Model
- Genotype-Environment Interactions Reveal Causal Pathways That Mediate Genetic Effects on Phenotype
- The Molecular Mechanism of a -Regulatory Adaptation in Yeast
- Phenotypic and Genetic Consequences of Protein Damage
- Recent Acquisition of by Baka Pygmies
- Fatty Acid Taste Signals through the PLC Pathway in Sugar-Sensing Neurons
- A Critical Role for PDGFRα Signaling in Medial Nasal Process Development
- Chromatin-Specific Regulation of Mammalian rDNA Transcription by Clustered TTF-I Binding Sites
- Meiotic Recombination in Arabidopsis Is Catalysed by DMC1, with RAD51 Playing a Supporting Role
- dTULP, the Homolog of Tubby, Regulates Transient Receptor Potential Channel Localization in Cilia
- Widespread Dysregulation of Peptide Hormone Release in Mice Lacking Adaptor Protein AP-3
- , a Direct Transcriptional Target, Modulates T-Box Factor Activity in Orofacial Clefting
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Genome-Wide Systematic Analysis Reveals Different and Predictive Proliferation Expression Signatures of Cancerous vs. Non-Cancerous Cells
- Recent Acquisition of by Baka Pygmies
- The Condition-Dependent Transcriptional Landscape of
- Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2A
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



