-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaAn Alteration in ELMOD3, an Arl2 GTPase-Activating Protein, Is Associated with Hearing Impairment in Humans
Exome sequencing coupled with homozygosity mapping was used to identify a transition mutation (c.794T>C; p.Leu265Ser) in ELMOD3 at the DFNB88 locus that is associated with nonsyndromic deafness in a large Pakistani family, PKDF468. The affected individuals of this family exhibited pre-lingual, severe-to-profound degrees of mixed hearing loss. ELMOD3 belongs to the engulfment and cell motility (ELMO) family, which consists of six paralogs in mammals. Several members of the ELMO family have been shown to regulate a subset of GTPases within the Ras superfamily. However, ELMOD3 is a largely uncharacterized protein that has no previously known biochemical activities. We found that in rodents, within the sensory epithelia of the inner ear, ELMOD3 appears most pronounced in the stereocilia of cochlear hair cells. Fluorescently tagged ELMOD3 co-localized with the actin cytoskeleton in MDCK cells and actin-based microvilli of LLC-PK1-CL4 epithelial cells. The p.Leu265Ser mutation in the ELMO domain impaired each of these activities. Super-resolution imaging revealed instances of close association of ELMOD3 with actin at the plasma membrane of MDCK cells. Furthermore, recombinant human GST-ELMOD3 exhibited GTPase activating protein (GAP) activity against the Arl2 GTPase, which was completely abolished by the p.Leu265Ser mutation. Collectively, our data provide the first insights into the expression and biochemical properties of ELMOD3 and highlight its functional links to sound perception and actin cytoskeleton.
Published in the journal: . PLoS Genet 9(9): e32767. doi:10.1371/journal.pgen.1003774
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003774Summary
Exome sequencing coupled with homozygosity mapping was used to identify a transition mutation (c.794T>C; p.Leu265Ser) in ELMOD3 at the DFNB88 locus that is associated with nonsyndromic deafness in a large Pakistani family, PKDF468. The affected individuals of this family exhibited pre-lingual, severe-to-profound degrees of mixed hearing loss. ELMOD3 belongs to the engulfment and cell motility (ELMO) family, which consists of six paralogs in mammals. Several members of the ELMO family have been shown to regulate a subset of GTPases within the Ras superfamily. However, ELMOD3 is a largely uncharacterized protein that has no previously known biochemical activities. We found that in rodents, within the sensory epithelia of the inner ear, ELMOD3 appears most pronounced in the stereocilia of cochlear hair cells. Fluorescently tagged ELMOD3 co-localized with the actin cytoskeleton in MDCK cells and actin-based microvilli of LLC-PK1-CL4 epithelial cells. The p.Leu265Ser mutation in the ELMO domain impaired each of these activities. Super-resolution imaging revealed instances of close association of ELMOD3 with actin at the plasma membrane of MDCK cells. Furthermore, recombinant human GST-ELMOD3 exhibited GTPase activating protein (GAP) activity against the Arl2 GTPase, which was completely abolished by the p.Leu265Ser mutation. Collectively, our data provide the first insights into the expression and biochemical properties of ELMOD3 and highlight its functional links to sound perception and actin cytoskeleton.
Introduction
Many molecular components that are necessary for the development and maintenance of hearing have been discovered by identifying the genes that underlie hearing impairment in humans and mice [1]–[4]. Hearing requires the precise and efficient functioning of intricately structured mechanosensory hair cells and supporting cells in the inner ear [3]. One of the key structures in the mechanotransduction process is the hair cell stereocilium. Protruding from the apical surface of the hair cells, stereocilia are organized in three rows of decreasing height in a staircase pattern. Each stereocilium is composed of an actin core that contains cross-linked and bundled γ - and β-actin microfilaments that are uniformly polarized, with the barbed (positive) ends localized at the tip. At the tapered end of the stereocilium, the actin filaments form a rootlet that has been proposed to anchor the structure in the actin-rich meshwork of the cuticular plate [5]. Interestingly, among the identified hearing loss-associated genes, nineteen encode proteins that interact with actin [6], [7]. Numerous studies have demonstrated that actin cytoskeleton-associated proteins are involved in the development, maintenance and stabilization of the stereocilia (for review, see [6]).
Continuous depolymerization of actin filaments at the base and polymerization at the barbed end, termed treadmilling, is thought to be critical to the maintenance of the length of stereocilia [8], [9]. However, a recent study demonstrated a rapid turnover of the actin filaments only at the tip of the stereocilia, without a treadmilling process [10], emphasizing the specific role of proteins at the stereocilia tip in the regulation of actin filaments. Regardless of the precise site, it is quite clear that the proper regulation of actin dynamics is critical to the generation and maintenance of stereocilia as sensory structures.
The Rho/Rac/Cdc42 family of GTPases is well known as a regulator of actin. Rho and Rac in the inner ear are involved in the morphogenesis and growth of the otocyst [11], [12]. The depletion of Rac1 or both Rac1 and Rac3 in the murine inner ear leads to a shorter cochlear duct with an abnormal sensory epithelium. Rac may participate in cell adhesion, proliferation, and movements during otic development [11], [12]. Several studies have suggested that the activation/inhibition of Rho pathways control the actin depolymerization rate in the outer hair cells [13], [14]. Although best known for their roles in the regulation of membrane traffic, there is growing evidence that GTPases in the Arf family can also act via changes in actin. [15].
Here, we report the identification of a new deafness gene, which encodes an ELMO/CED 12 domain containing protein, ELMOD3. Our biochemical studies demonstrated that ELMOD3 possesses GAP activity against a small GTPase in the Arf family, Arl2, providing a functional link between Arf family signaling pathways and stereocilia actin-based cytoskeletal architecture. GAPs are regulators and effectors of the Ras superfamily of GTPases, which are increasingly recognized as providing specificity as well as temporal and spatial regulation to GTPase signaling [16]. Thus, we believe that the identification of ELMOD3 role in the inner ear provides new insights into signaling processes that are important to hearing in humans.
Results
Clinical findings
Family PKDF468 (Figure 1A) was recruited after obtaining Institutional Review Board approval and written informed consent. The family history revealed that the onset of hearing loss was pre-lingual, with no clear vestibular impairment among the deaf individuals. Pure-tone bone and air-conduction audiometry revealed severe-to-profound mixed (conductive and sensorineural) hearing loss in the affected individuals of family PKDF468 (Figure 1B). Individual V:2 exhibited severe-to-profound mixed hearing loss, with bone conduction thresholds for the right ear displaying a mild downward slope to the severe hearing loss range. The left ear displayed slightly better bone conduction thresholds with normal values for lower frequencies and a downward slope to severe hearing loss at higher frequencies (Figure 1B). The audiograms of individual V:5 revealed bilateral severe-to-profound mixed hearing loss, with a large conductive component in both ears. The bone conduction thresholds exhibited a mild downward slope to moderately severe hearing loss for the right ear and were slightly better on the left, for which the thresholds ranged from borderline normal to moderate hearing loss ranges (Figure 1B). The clinical evaluation revealed no clear signs of skin, renal, or retinal abnormalities.
Fig. 1. Hearing loss segregating in family PKDF468 is associated with a missense ELMOD3 allele. 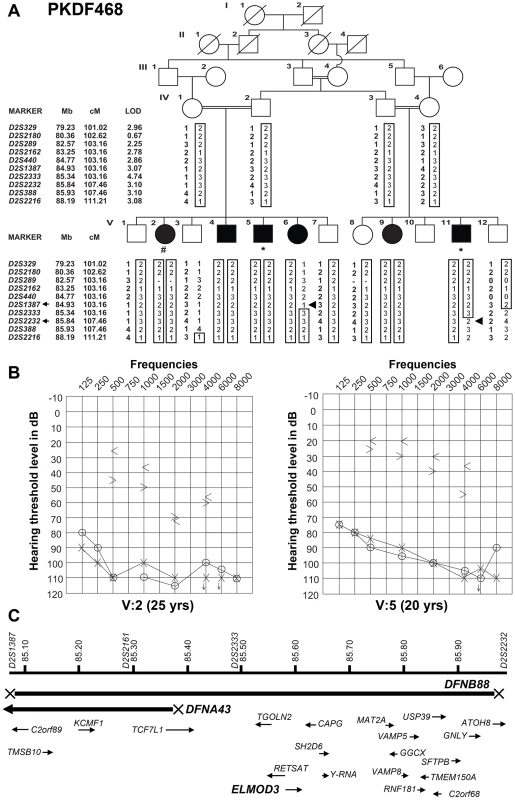
(A) Pedigree of the family in which the DFNB88 locus was mapped. The filled symbols represent affected individuals, and a double horizontal line connecting parents represents a consanguineous marriage. The alleles forming the risk haplotypes are boxed. The short tandem repeat (STR) markers, their relative map positions (Mb) according to UCSC Genome Bioinformatics Build GRCh37 (hg19), and their genetic positions (cM) based on the Marshfield genetic map are shown next to the pedigree. Haplotype analysis revealed a linkage region of 4.3 cM (0.91 Mb) that was delimited by proximal meiotic recombination in individual V:6 (arrowhead) at marker D2S1387 (103.16 cM; arrow) and distal recombination at marker D2S2232 (107.46 cM; arrow) in individual V:11 (arrowhead). (B) Pure-tone audiograms for family PKDF468 V:2 (25-year-old female) and V:5 (20-year-old male). The symbols ‘o’ and ‘x’ denote air conduction pure-tone thresholds, and the ‘<’ and ‘>’ symbols denote bone conduction thresholds in the right and the left ears, respectively. (C) The linkage interval that defines the DFNB88 locus for family PKDF468 partially overlaps with DFNA43. The relative locations and orientations of the genes and mRNAs are indicated with arrows. Individual V:2 DNA sample was used for exome sequencing, while individuals V:5 and V:11 samples were used for Sanger sequencing of coding, non-coding, and flanking sequences of the exon-intron boundaries of the known candidate genes in DFNB88 linkage interval. To determine the temporal bone malformation, we performed computed tomography (CT) scans of two affected (V:2 and V:11) along with a normal hearing sibling (V:7). CT scan of individual V:2 revealed all three semicircular and internal auditory canals were intact on both sides. The middle ear and mastoid appeared well-aerated bilaterally. Imaging of individual V:11 demonstrated a slightly narrow appearing internal auditory canal on the right side only. The mastoid air cells and middle ear cleft were well-aerated bilaterally. The external auditory canal appeared normal as well for both affected individuals.
Deafness in PKDF468 is linked with DFNB88 locus
We initially observed that deafness in family PKDF468 did not co-segregate with short tandem repeat (STR) markers for 74 of the reported recessive nonsyndromic deafness loci (data not shown). We therefore performed a genome-wide linkage analysis and observed that the deafness phenotype of family PKDF468 exhibited significant evidence of linkage to STR markers on chromosome 2p12-p11.2 (Figure 1A). Additional STRs on 2p were genotyped, and haplotype analysis revealed a 0.91 Mb linkage interval that was delimited by the markers D2S1387 and D2S2232 (Figure 1A). Under a recessive model of inheritance, with a disease allele frequency of 0.001 and full penetrance, a maximum two-point LOD [17] score of 4.74 (θ = 0) was obtained for the marker D2S2333. These results define and delimit DFNB88 [Human Genome Nomenclature Committee (HGNC) approved locus symbol], a novel recessive deafness locus on chromosome 2p11.2.
Exome sequencing revealed a mutation in ELMOD3 at the DFNB88 locus
The DFNB88 locus partially overlaps with the dominant deafness locus DFNA43 (Figure 1C) [18]. Four known candidate genes were identified within the DFNB88/DFNA43 overlapping linkage region (Figure 1C). However, Sanger sequencing of these genes did not reveal any pathogenic variants. Approximately 85% of the disease-causing mutations in Mendelian disorders reside in coding regions or in exon-intron canonical splice junctions [19]. We therefore performed exome sequencing of an affected individual from family PKDF468. The sample was enriched using the NimbleGen SeqCap EZ Exome Library v2.0 (Roche Diagnostics; San Francisco, CA), and 100 bp, paired-end sequencing was performed on the Illumina HiSeq 2000 platform (Illumina). An average of 78.94% of bases were sequenced with 20× coverage within the targeted regions. This yielded a total of 64,863 single-nucleotide variants, of which 1,928 were not found in the dbSNP133 database (Table S1). Based on the recessive mode of inheritance evident in the pedigree, we analyzed genes with homozygous changes and potential compound heterozygous changes. Additionally, we removed all of the variants that were present in six ethnically matched control samples (Table S1). No mutation segregating with hearing loss in family PKDF468 was identified in any of the known deafness-causing genes (Table S1).
We identified one homozygous transition mutation, c.794T>C (p.Leu265Ser), in ELMOD3 (Figure S1) on chromosome 2p11.3 (Figure 1C) that segregated with DFNB88-linked deafness (Tables S1 and S2). The c.794T>C change was not present near the canonical splice junctions and was not predicted to create any aberrant splice site. However, to confirm that c.794T>C did not affect splicing of ELMOD3 transcripts, we generated cDNA libraries using the total RNA extracted from the white blood cells of two affected and one normal hearing individual. Sanger sequencing of sub-cloned PCR products, amplified using primers in either exons 9 and 11 or in exons 9 and 12 (Figure S2), did not reveal any aberrant splicing product in affected individuals. Thus, the likely pathogenic affect of the c.794T>C change is substitution of a highly conserved leucine residue at amino acid position 265 of the human ELMOD3 protein with serine (Figure 2C). No carrier of c.794T>C was identified among 524 ethnically matched control chromosomes, in the 1000 Genome database or in the 6500 individuals who are listed in the NHLBI-ESP variant database (http://evs.gs.washington.edu/EVS/). Moreover, Polyphen-2 [20], SNPs3D [21], MutationTaster [22], PMut [23], and SIFT [24] predicted that the ELMOD3 mutation would be deleterious (Table S3).
Fig. 2. The transcripts and expression profiles of the genes that encode ELMOD3 in humans and mice. 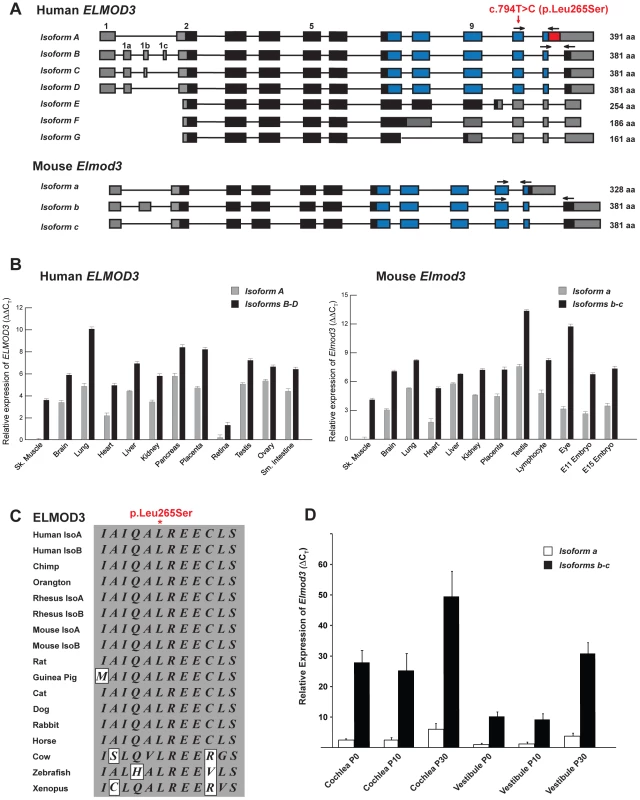
(A) Alternative splicing leads to seven isoforms of human ELMOD3. Non-coding segments, sequences encoding ELMO domain and other coding regions of exons are denoted by gray, blue and black boxes, respectively. Also depicted is the mutation that was identified in the DFNB88 family (red arrow). The numbering of the position of mutation c.794T>C (p.Leu265Ser) is based on accession number NM_032213.4. Mice have only three Elmod3 alternative transcripts. Primers used for real-time quantitative PCR analyses are represented with black arrows. (B) Real-time quantitative PCR analysis of human and mouse ELMOD3/Elmod3 isoforms A/a and B–E/b–c. (C) The leucine residue at amino acid position 265 (accession number NP_115589) is completely conserved across a wide variety of species. Identical residues are boxed in gray. (D) Real-time quantitative RT-PCR analysis of Elmod3 isoforms a and b–c in C57BL/6J mouse cochlear and vestibular tissues at three different ages (P0, P10 and P30). CT indicates the observed threshold number of PCR cycles that was required for the detection of the amplification product; the relative expression level is the calculated difference in CT between the Elmod3 and that of an internal control standard (Gapdh), which was measured in the same sample. To further confirm that the p.Leu265Ser allele of ELMOD3 is the only mutation that was associated with hearing loss at the DFNB88 locus, we sequenced the coding, non-coding, and approximately 75 bp flanking sequences of the exon-intron boundaries of all the known candidate genes present within the linkage region in two affected individuals of family PKDF468 (Figure 1C). No other potentially pathogenic mutation was identified in the affected individuals of family PKDF468. Although, ELMOD3 is located outside the reported linkage interval of DFNA43 (Figure 1C) [18], nevertheless we sequenced DNA samples of two affected individuals from the original DFNA43 family and no mutation was found.
ELMOD3/Elmod3 are expressed ubiquitously
We next examined the gene structure and expression of ELMOD3. Seven alternatively spliced isoforms of human ELMOD3 were identified (Figure 2A). Isoform A (reference sequence NM_ 032213.4) has a translation initiation codon (AUG) in exon 2, ten coding exons that encode a polypeptide of 391 residues (Figure S1B). Exons 7 to 11 encode the engulfment and cell motility (ELMO or CED12) domain, which consists of 164 amino acid residues (Figure S1B; blue box). ELMOD3 isoforms B to D include alternatively spliced exons in the 5′ untranslated region (UTR) but harbor the same coding exons and encode identical 381 residue polypeptides that differ from isoform A only at their carboxy termini (Figures 2A and S1B). The human ELMOD3 isoforms, A and B, share 87% identity, with all the differences clustered near the C-terminus. Isoforms E, F, and G do not encode the full-length ELMO domain due to alternate splicing of exons in the carboxy terminus (Figure 2A). The c.794T>C transition mutation is predicted to result in the substitution of serine for a highly conserved leucine in all of the ELMO domain-containing isoforms of ELMOD3 (Figures 2A and 2C). In comparison to the human sequence, mouse Elmod3 includes only three known alternatively spliced transcripts (Figure 2A). RT-PCR and real-time quantitative PCR analysis of multiple human and mouse tissue cDNAs (Tables S4 and S5) revealed the ubiquitous expression of isoforms A/a and B–D/b–c (Figures S3 and 2B). We also assayed the relative mRNA expression of murine Elmod3 isoforms a and b–c with real-time quantitative RT-PCR of RNA that was extracted from cochlear and vestibular inner ear tissues from postnatal day 0 (P0), P10, and P30 C57BL/6J mice (Figure 2D). The expression of Elmod3 isoform b–c was several-fold higher than isoform a, in both cochlear and vestibular tissues at all of the time points examined (Figure 2D). Therefore, we focused on the ELMOD3 isoform B for the subsequent biochemical and cellular studies. The mouse ELMOD3 protein is 70% and 80% identical to the A and B isoforms of human ELMOD3, respectively, and again the differences are greatest at the C-terminus, although single amino acid changes are scattered throughout the alignments.
ELMOD3 is localized to the mechanosensory stereocilia of hair cells in rodent organ of Corti
To characterize the cellular localization of ELMOD3, we produced a rabbit polyclonal antiserum against synthetic peptide immunogens from mouse ELMOD3 isoform b. The sensitivity and specificity of the ELMOD3 antibody was validated in immunoblot and immunofluorescence analyses, in transfected cells and mouse tissues (Figures S4 and S7). Our antibodies specifically recognized ELMOD3 isoform b but not murine ELMOD1, ELMOD2, or ELMOD3 isoform a (Figure S4). We next performed immunolocalization of ELMOD3 in the rat and mouse organ of Corti (Figures 3 and 4). In rat cochlea, ELMOD3 immunoreactivity was observed in the stereocilia, kinocilia and cuticular plate of developing hair cells (Figures 3 and S5). Before P07, ELMOD3 staining was very weak in the inner hair cells stereocilia. By P07, in auditory hair cells, patchy labeling of ELMOD3 immunostaining was detected along the length of stereocilia (Figure 3). In contrast to actin staining, ELMOD3 immunoreactivity was not uniform along the length of each stereocilium and the protein seemed to be excluded from a region near the tip (Figure 3E). ELMOD3 immunoreactivity was also found in the supporting cells, including pillar and Dieters' cells (Figure 3). Similar to that seen in the rat (Figure 3D), the stereocilia of inner hair cells in the mouse organ of Corti were more intensely labeled than those of outer hair cells (Figure 4A). In contrast to the cochlear hair cells, ELMOD3 antibody labeling was observed within the hair cell bodies in the vestibular end organs of both rat and mouse inner ear, but no prominent immunoreactivity was observed in the hair bundles (Figures 4B–4C and S6). These observations suggest a unique role for ELMOD3 in cochlear sensory cells and may reflect the functional or structural differences between cochlear and vestibular hair bundles. No specific immunoreactivity was observed when the primary antibody was omitted (data not shown) or when the antibody was pre-incubated with the ELMOD3 peptide antigen (Figure S7).
Fig. 3. ELMOD3 is expressed in sensory cells of rat organ of Corti. 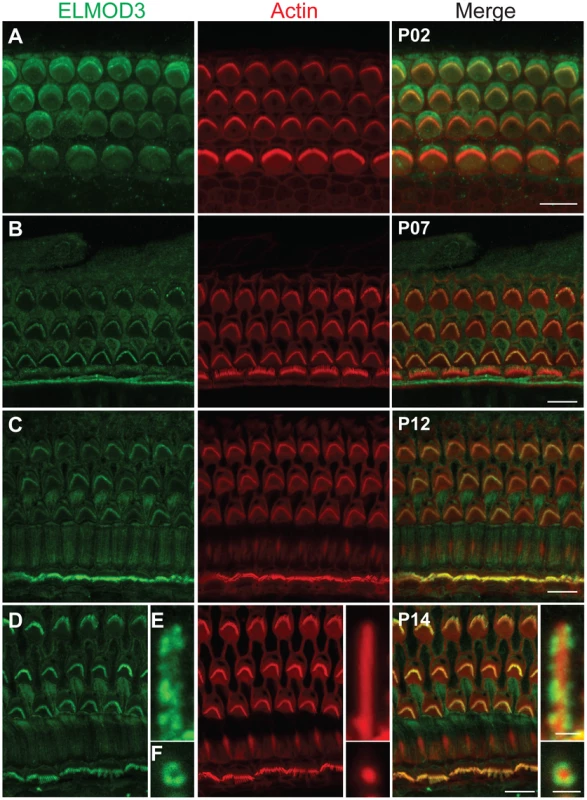
(A–F) The spatio-temporal expression pattern of ELMOD3 in the rat organ of Corti. (A) At P02, ELMOD3 immunoreactivity (green) was found in the stereocilia of outer hair cells and in the kinocilia of both outer and inner hair cells. ELMOD3 immunostaining was also concentrated at the cuticular plate level of auditory hair cells. Rhodamine-phalloidin (red) staining was used to label stereocilia and actin cytoskeleton of cochlear inner hair cells and three rows of outer hair cells. (B–D) Later in development, ELMOD3 immunostaining was detected in the stereocilia bundles and microvilli of sensory cells and also in the supporting cells bodies. Longitudinal (E) and top view (F) cross-sections of stereocilia of inner hair cells, at higher magnification, showed that ELMOD3 immunostaining is distributed in patches all along the length of the stereocilia but is absent from the very tip of each stereocilium. Scale bars in panels A, B, C and D are 10 µm. Scale bar in panels E–F is 1 µm. Images in panels A to D are projections of confocal optical sections, while images in panels E and F are single plane confocal sections. Fig. 4. Immunolocalization of ELMOD3 in the mouse organ of Corti and vestibular sensory epithelia. 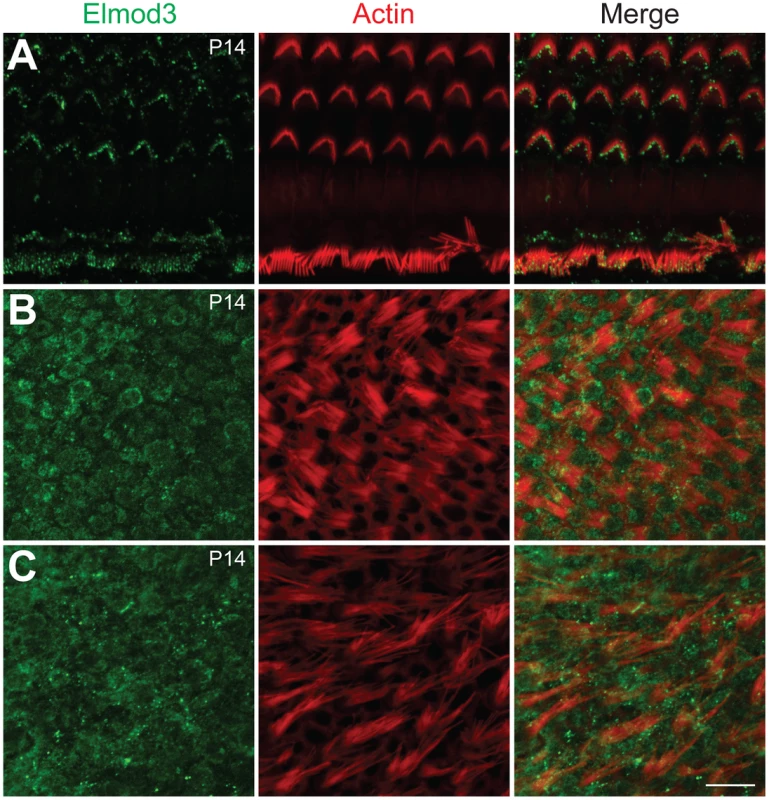
(A–C) Double staining for F-actin (red) and ELMOD3 (green) in the mouse inner ear epithelia. (A) In the organ of Corti, at P14, ELMOD3 immunoreactivity (green) was detected along the length of stereocilia of hair cells, with prominent staining in the upper half of the actin-filled structures. Staining was also observed in the microvilli on the apical surface of the hair cells. (B–C) In the vestibular end organs, ELMOD3 immunostaining was localized within the hair cell and supporting cell bodies in the saccule (B) and utricule (C) at P14, but no immunoreactivity was observed in the vestibular hair bundles. All images are projections of confocal optical sections stack. Scale bar applies to all panels and is 10 µm. p.Leu265Ser mutation affects the localization of ELMOD3 in CL4 cells and mouse inner ear explants
We examined LLC-PK1-CL4 epithelial (CL4) cells to understand the mechanism and effect of the hearing loss-associated allele of ELMOD3. CL4 cells contain actin-rich microvilli and have been used as in vitro models of stereocilia to examine F-actin and protein dynamics [25]. We transiently co-transfected GFP-ELMOD3 constructs with Espn constructs, where the latter was used to over-elongate the microvilli at the CL4 cell surface [26] (Figure 5A–5B). We observed a significant expression of GFP-ELMOD3 in the apical (microvillar) plasma membrane twenty-four hours post-transfection (Figure 5A). We also observed expression of GFP-ELMOD3 in the cytosol of transfected cells (Figure 5A). In contrast to the wild type protein, the p.Leu265Ser mutation in the ELMO domain yielded a protein that displayed either weak or no labeling in the microvilli of the transfected CL4 cells. Additionally, the protein appeared to be diffusely located throughout the cytoplasm, with a nuclear concentration in approximately half of the transfected cells (Figure 5B). Identical results were observed with tdTomato-tagged wild-type and mutant ELMOD3 constructs (data not shown).
Fig. 5. ELMOD3 accumulates at actin-based structures. 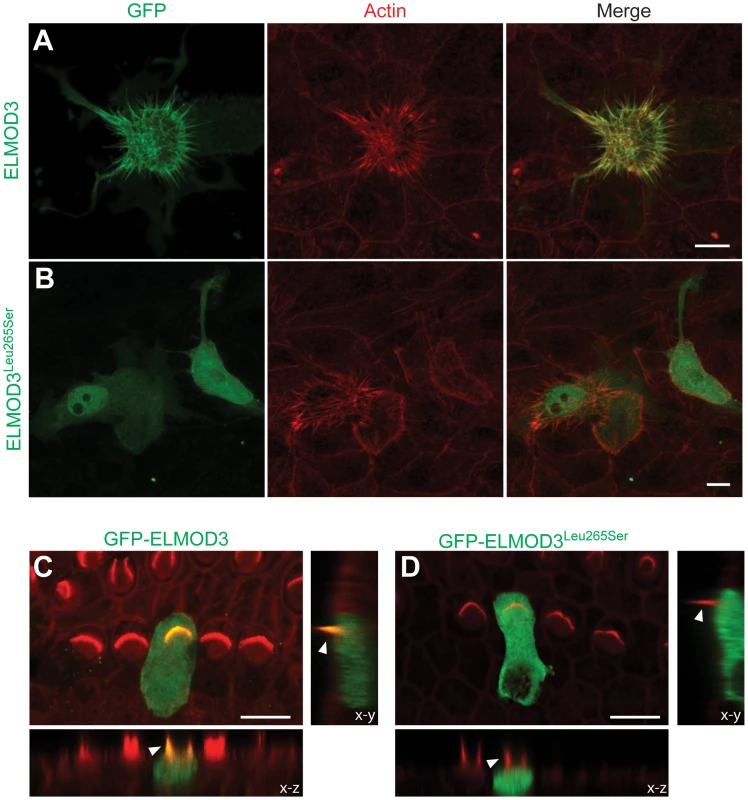
(A–B) GFP-ELMOD3 localizes on actin structures (elongated microvilli due to co-expression of espin) but the deafness-causing ELMOD3 mutant does not. (A) CL4 epithelial cells were co-transfected with GFP-ELMOD3 and un-tagged Espin expression vectors. Rhodamine phalloidin (red) was used to reveal F-actin and highlight the microvilli on the CL4 cell surface. GFP-ELMOD3 was efficiently targeted to the microvillar actin bundles and the cell membrane. (B) GFP-ELMOD3 harboring the p.Leu265Ser human deafness-associated mutation retained weak microvillar targeting and cell membrane localization; the protein remained in the cytoplasm and accumulated in the nucleus. (C–D) Representative results of the transfection of P2 C57BL/6J mouse inner ear sensory epithelial explants with expression vectors that encoded either GFP-ELMOD3 or p.Leu265Ser ELMOD3. The x–z and y–z plane projections are also presented (lower panels). (C) An inner hair cell (IHC) in the mouse organ of Corti that was transfected with GFP-ELMOD3 reveals localization along the length of stereocilia (arrowhead) and throughout the hair cell body. (D) Mouse organ of Corti hair cell that was transfected with GFP-ELMOD3 (p.Leu265Ser). No concentration and only negligible fluorescence is observed in the stereocilia (arrowhead), and mutated ELMOD3 remains in the cytosol. Scale bar applies to all panels and is 10 µm. To determine the effect of p.Leu265Ser mutation on the localization of ELMOD3 in the mouse inner ear, we performed gene gun-mediated transfection of wild-type and p.Leu265Ser mutant GFP-tagged ELMOD3 cDNA constructs in organotypic cultures of inner ear sensory epithelia of P2 C57BL/6J mice (Figure 5C–5D). Over-expressed wild-type GFP-ELMOD3 localized along the length of the stereocilia of cochlear hair cells (Figure 5C). We also observed homogeneous distribution throughout the hair cell bodies (Figure 5C). Similar to the results that were observed in CL4 cells, GFP-ELMOD3 harboring the p.Leu265Ser mutation failed to target to the stereocilia, and the protein was apparently distributed throughout the cochlear hair cell bodies (Figure 5D). Taken together, these results support our conclusion that ELMOD3 localizes to actin-based microvilli and stereocilia (Figure 5) but that a point mutation in the ELMO domain can prevent its normal localization and potentially affect its function in the stereocilia.
ELMOD3 is linked to the actin cytoskeleton
To further investigate the ELMOD3-actin association, we transfected GFP-tagged ELMOD3 into MDCK cells, which is a highly polarized cell model system (Figure S8). Forty-eight hours post-transfection, GFP-ELMOD3 accumulation was apparent at the periphery of the transfected cells near the plasma membrane (Figure S8A). The expression of GFP-ELMOD3 harboring the p.Leu265Ser mutation in MDCK cells resulted in a protein that failed to target or accumulate at the plasma membrane and instead, appeared to concentrate in the nuclei (Figure S8B). To determine whether GFP-ELMOD3 associates with the actin cytoskeleton at the plasma membrane (Figure 6A), we treated the cells with cytochalasin D (cyto-D), which is a potent inhibitor of actin polymerization, to disrupt the actin cytoskeleton [27], [28]. We hypothesized that if GFP-ELMOD3 associated with the actin cytoskeleton at the cell membrane, then treatment of the cells with cyto-D would also affect ELMOD3 localization. Indeed, we observed a significant decrease in the GFP-ELMOD3 signal at the cell membrane following disruption of the actin cytoskeleton (Figure 6B, 6D). Four hours following cyto-D treatment (i.e., the recovery period for actin re-polymerization) [27], we observed that ELMOD3 re-accumulated at the cell membrane (Figure 6C, 6D). These results suggest that the localization of ELMOD3 is dependent on the actin cytoskeleton and/or may contribute to a mechanism that supports its maintenance.
Fig. 6. ELMOD3 is linked to the F-actin cytoskeleton. 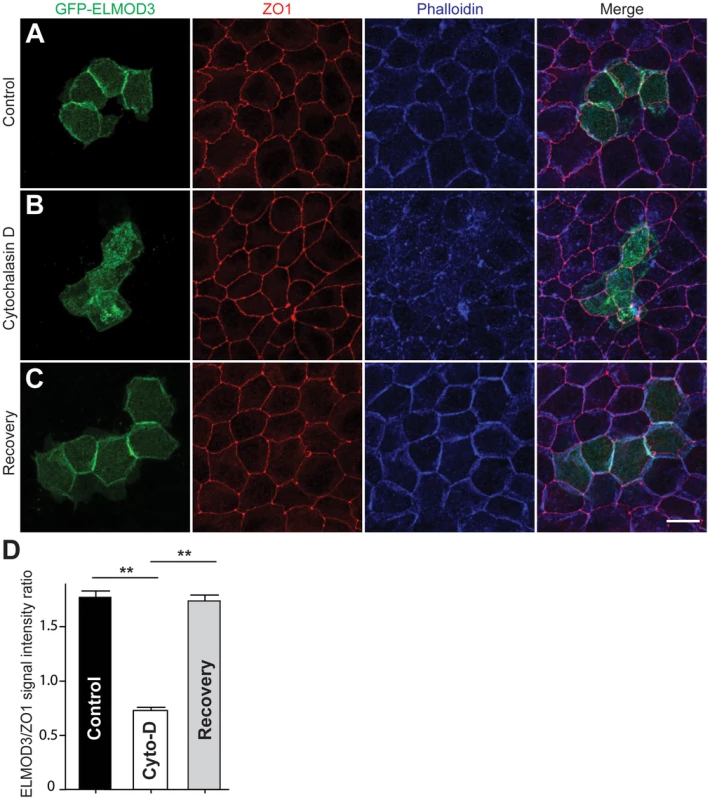
(A) In transfected MDCK cells, GFP-ELMOD3 (green) localized with F-actin at the cell membrane. Alexa647-phalloidin (blue) was used to stain F-actin, and a ZO1 antibody was used to label the tight junctions (red). (B) When the cells were treated with cytochalasin D (Cyto-D), GFP-ELMOD3 was internalized, and no or negligible localization at the cell membrane was observed. (C) Four hrs post-Cyto-D treatment, GFP-ELMOD3 re-localized with the actin cytoskeleton at the plasma membrane. Scale bar applies to all panels and is 10 µm. (D) Quantification of membranous GFP-ELMOD3 localization. The normalized ratio of GFP-ELMOD3 and ZO1 signal intensities at the cell membrane (n = 30, ** p<0.01) confirmed the internalization of ELMOD3 following Cyto-D treatment. To decipher the link between F-actin and ELMOD3, we performed a two-color stochastic optical reconstruction microscopy (STORM) imaging of EGFP-ELMOD3 transfected MDCK cells. While conventional confocal acquisitions revealed co-localization of ELMOD3 and the actin-cytoskeleton, this high resolution imaging technique allowed us to determine more precisely the relative positions of ELMOD3 and actin (Figure 7). ELMOD3 and actin were each found in close apposition to the plasma membrane and in irregularly shaped puncta (Figure 7C). Many regions of extensive overlap in staining between ELMOD3 and actin, suggest the possibility that a subset of the actin-based structures may contain ELMOD3 but that each protein is also found localized independently of the other at the plasma membrane (Figure 7C).
Fig. 7. ELMOD3 is localized at the plasma membrane. 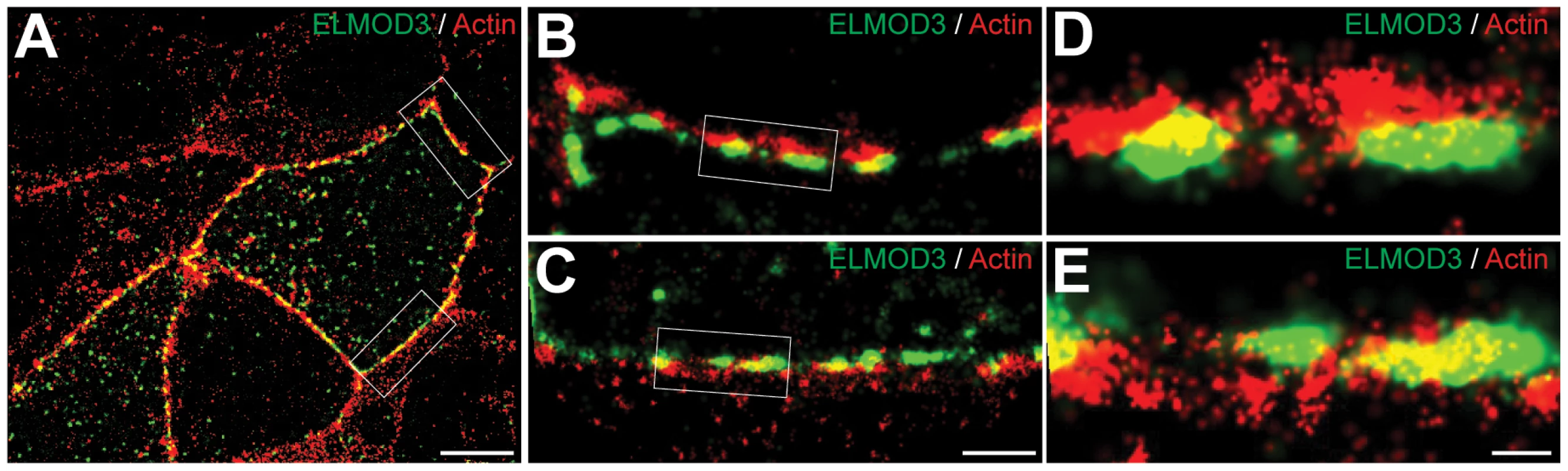
STORM imaging revealed close proximity between actin filaments and over-expressed GFP-ELMOD3 at the plasma membrane in MDCK cells. (A) A representative two-color STORM image of transfected MDCK cells is shown. Alexa647 phalloidin was used to label F-actin and a Cy3B-conjugated GFP antibody was used to detect GFP-ELMOD3. In most places the GFP-ELMOD3 (green) signal did not co-localize with actin (red) at the plasma membrane. However, at few spots we found partial overlap between ELMOD3 and actin. (B–C) Magnification of the regions inside the boxes in A. (D–E) Zoomed-in views of the regions inside the boxes in B and C, respectively. Scale bars: 5 µm for A, 1 µm for B and C, 200 nm for D and E. To test the possibility that ELMOD3 binds to actin-based structures, we performed a high-speed co-sedimentation assay that pellets actin along with its associated proteins. To obtain a source of purified ELMOD3, His6-Trigger factor-ELMOD3 (TF-ELMOD3) fusion protein was expressed in bacteria, and the recombinant protein was purified by Ni-NTA chromatography. The TF-ELMOD3 or control proteins were incubated with polymerized F-actin and subjected to high-speed centrifugation at 150,000× g for 1.5 hrs (Figure S9). TF-ELMOD3 co-sedimented, albeit weakly or incompletely, with F-actin in this assay (Figure S9). Under these conditions, the p.Leu265Ser mutation did not significantly impact the level of TF-ELMOD3 that co-sedimented with F-actin (Figure S9).
ELMOD3 exhibits GAP activity against Arl2
Human ELMOD1 and ELMOD2 each possess Arl2 GAP activity [29]. We therefore investigated whether ELMOD3 also possesses GAP activity against Arl2. Previous tests of bacterially expressed human ELMOD3 as either maltose binding proteins or trigger factor fusion proteins were negative, but the homologous preparations of ELMOD1 and ELMOD2 were found to possess very low specific activities as Arl2 GAPs, compared to the preparation purified from bovine tissues. To obtain a potentially more active preparation of human ELMOD3, we expressed ELMOD3 and the mutant p.Leu265Ser in HEK293T cells as N-terminal GST-fusion proteins to facilitate protein purification. Protein expression and purification from ∼108 HEK293T cells that expressed GST-ELMOD3 or the mutant each yielded ∼0.6 mg protein. These preparations were stable at 4°C and against freeze-thaw cycles, as judged by either GAP activity or lack of precipitation. We expressed and purified GST alone and used it as a negative control in all of our assays. We also evaluated the effect of cleavage of the GST fusion tag by TEV protease on ELMOD3 activity; no changes in activity in the Arl2 GAP assay were observed compared to un-cleaved proteins (data not shown). Thus, we believe that the presence of the GST moiety at the N-terminus does not interfere with access to the substrate or with enzymatic activity in our assay.
When we varied the amount of GST-ELMOD3 protein in the Arl2 GAP assay, we observed a dose-dependent response and evidence of saturation at higher protein concentrations (data not shown). Using the lower concentrations of GST-ELMOD3 to estimate the initial rates of GAP-dependent activity and estimating the purity of the preparation at 50% (based on visual inspection of Coomassie blue-stained gels), we obtained a specific activity of 24 pmol of GTP hydrolyzed/min/mg (Figure 8). This specific activity of GST-ELMOD3 as an Arl2 GAP is approximately 32-fold lower than that determined for GST-ELMOD1 and nearly 1000-fold lower than that of GST-ELMOD2 or bovine testes ELMOD2, which is the most active reported preparation of any Arl2 GAP [29]. Thus, in contrast to our earlier report that it is inactive, GST-ELMOD3 does exhibit Arl2 GAP activity, and we believe that its lower specific activity when expressed in bacteria likely contributed to the earlier negative findings [29]. The differences in specific activity among the three human GST-ELMOD preparations from HEK293T cells are predicted to result from differences in substrate specificity, sensitivities to co-activators (as known for Arf GAPs), or both. Thus, more studies are required to determine whether the biologically relevant substrate of ELMOD3's GAP activity in the inner ear is Arl2 or a related GTPase.
Fig. 8. ELMOD3 has Arl2 GAP activity. 
Human GST-ELMOD3 (WT), the point mutant (Leu265Ser), and GST alone (GST) were expressed and purified from HEK293T cells. The white and black bars represent the Arl2 GAP activities, with 1.5 µM or 3 µM of purified protein, respectively, assayed using 200 nM Arl2 as substrate. The activities shown are the averages of duplicate experiments. The bars indicate the range. This experiment was repeated with a different preparation, and essentially identical results were obtained. We next assessed the effects of the Leu265Ser point mutation on the Arl2 GAP activity of GST-ELMOD3. The mutant protein was expressed at the same levels in HEK293T cells and was purified in the same way, resulting in equivalent amounts of protein, indicating that the protein is equally stable in mammalian cells and in solution. However, when assayed for Arl2 GAP activity, the mutant was inactive (Figure 8). Although we observed small amounts of activity over our no protein control, this level of activity seen for the mutant was not different from that observed with GST alone (Figure 8). These activities are so low as to be at or near the lower limits of our assay. Thus, we can safely conclude that the point mutant has at least a 10-fold lower specific activity than the wild-type protein, but it might be completely inactive as an Arl2 GAP.
Discussion
Our study revealed that ELMOD3 is important for hearing in humans as a missense mutation in the gene leads to profound hearing impairment. ELMOD3 belongs to the engulfment and cell motility (ELMO) protein family, which includes six known members in mammals (ELMO1-3 and ELMOD1-3). Our ex vivo studies reveal that fluorescently tagged ELMOD3 localized with the actin-based microvilli of LLC-PK1-CL4 epithelial cells, in the stereocilia of sensory hair cells of mouse organ of Corti explants, and to a lesser extent to the actin cytoskeleton of MDCK cells, whereas the deafness-associated allele (p.Leu265Ser) was deficient in each case. Similarly, we show that human ELMOD3 possesses Arl2 GAP activity but the mutant has at least a 10-fold loss in activity.
While ELMOD3 antibody reactivity was detected in outer hair cells stereocilia at P02, more pronounced accumulation of ELMOD3 immunoreactivity was detected in rat cochlear inner hair cell stereocilia only by P12, which is when hair bundles are in the late phase of maturation. During this period, the inner hair cell stereocilia undergo a rapid elongation [30]. The observed staining suggests that ELMOD3 might be necessary for the initial development of the outer hair cell stereocilia or the organization of the bundle in a staircase pattern but may play a different role in the stereocilia of inner hair cells. Nevertheless, it is tempting to speculate that ELMOD3 may play a role in the maturation or maintenance of the cochlear stereociliary bundle. Recently, two spontaneous mutations (rda and rda2J) in mouse Elmod1 were shown to result in profound deafness and vestibular dysfunction [7], demonstrating that the function of ELMOD1 is essential for regulating the shape and maintenance of inner ear hair cell stereocilia in mice [7]. Elmod1 has been shown to be part of a large cluster of genes expressed in the developing inner ear, while Elmod3 level was below the detectable range [31]. These observations are consistent with findings from other studies, like the SHIELD database, which demonstrated that the level of Elmod3 mRNA is ∼100-fold lower than that of Elmod1 in the developing inner ear (P0–P1).
Besides stereocilia bundles, immunoreactivity for ELMOD3 was also detected along the kinocilium in developing cochlear hair cells. Recently, it has been shown that ELMO1 can act at the interface between the actin-cytoskeleton and microtubule network by interacting with ACF7 (Actin crosslinking family 7) [32]. Moreover, microtubule polymerization depends on Arl2 activity [33], and we have shown that ELMOD3 exhibits a GAP activity against Arl2. Therefore, ELMOD3 expression in the kinocilium might have a role in the assembly of the kinocilium architecture and in pathways regulating planar cell polarity.
The ELMO family proteins are functionally poorly characterized, and more information is currently available for the ELMOs than for the ELMODs, with no structural information available for any of them. So far, only one activity has been ascribed to ELMOD proteins: we previously reported that recombinant human ELMOD1 and ELMOD2 display in vitro Arl2 GAP activity, whereas ELMOD3 and bacterially expressed ELMO1-3 did not [29]. More recently, we performed additional phylogenetic and functional analyses of the ELMO domain that led us to re-examine whether ELMOD3 shares the Arl2 GAP activity of ELMOD1 and ELMOD2. Our data contrast with the earlier-published claim that ELMOD3 lacks Arl2 GAP activity; we determined that it does indeed possess this activity, albeit at a substantially lower specific activity than that of its two closest human paralogs. The large differences in specific activities observed in the Arl2 GAP assay may be due to differences in the specificities of ELMODs as GAPs for different GTPases, including the lack of one or more binding partners (e.g., one that is perhaps analogous to Dock180 binding to ELMO1 or a co-activator for activity such as has been proposed for COP-I and ArfGAP1 [34]), and/or the lack of post-translational modification.
Our in vitro experiment revealed that ELMOD3 harboring the p.Leu265Ser mutation, unlike ELMOD3, has no or few GAP activity against Arl2. The lack of Arl2 GAP activity of the mutant may suggest a reduced affinity for the Arl2 GTPase, which may play an important role in ELMOD3 localization. Elmod3 and Arl2 are expressed in developing mouse cochlear tissues and weakly in vestibular tissues (Figure S10). Even though ELMOD3 is active and has been defined as “Arl2 GAPs”, we expect it to be active against other GTPases in the Arf family as well. We therefore speculate that ELMOD3 functions as a GAP for Arl2 and perhaps other GTPases that participate in actin organization, polymerization or depolymerization in the cochlear hair bundles. If ELMOD3 is an active GAP for other GTPases, these GTPases are likely to be part of the Arf family given that GAPs are not known to cross family boundaries within the Ras superfamily. However, it is plausible that ELMOD3 functions in a signaling pathway that includes Arl2 (or an Arf family GTPase), Rac, Rho, and, ultimately, affects the actin cytoskeleton.
Future studies will address the specific roles of ELMOD3 in the development of the inner ear sensory epithelium, cytoskeletal organization, and ELMOD3-mediated signaling pathways. Revealing the interacting partners, substrate specificities for its GAP activities, as well as the means of regulation of ELMOD3 and other ELMO family proteins, will shed light on the overlapping functions of the Ras superfamily in the inner ear. These fundamental functions of this unique protein family are likely to be important in all eukaryotic cells.
Materials and Methods
Family ascertainment and phenotype analysis
Family PKDF468 was enrolled in the present study from the Punjab province of Pakistan, and written informed consent was obtained from all participating family members. The Institutional Review Boards at the Center for Excellence in Molecular Biology (Pakistan), at the National Institute on Deafness and Other Communication Disorders, and at Cincinnati Children's Hospital (USA) approved the present study. Hearing loss in the affected family members was evaluated using pure-tone audiometry, which tested frequencies that ranged from 125 Hz to 8 kHz. The family medical history stated that the onset of hearing loss was pre-lingual, and we observed no evidence of vestibular dysfunction or other balance issues using the Romberg and tandem gait tests. There were no other significant findings from the clinical exam, and the affected members had basic metabolic panel results within the normal range, indicating that they had nonsyndromic hearing loss.
Linkage analysis and whole exome sequencing
We conducted a genome-wide scan on family PKDF468 using 388 STR markers and performed linkage analysis using GeneMapper software (Applied Biosystems; Carlsbad, CA). The LOD score was calculated using a recessive model of inheritance assuming a fully penetrant disorder and a disease allele frequency of 0.001. The primers were designed with Primer3 to sequence all of the coding exons and 75 bp of the exon-intron boundaries of all of the known genes within the DFNB88 locus (Table S2). The products were amplified using either Taq polymerase (Genscript; Piscataway, NJ) or Amplitaq Gold 360 (Applied Biosystems) for the GC-rich regions. The chromatograms were read using SeqMan software (DNAStar; Madison, WI).
Exome sequencing was conducted on one affected individual from family PKDF468 and was enriched using the Nimblegen SeqCap EZ Exome v2.0 Library (Roche Diagnostics; San Francisco, CA). One hundred base pair paired-end sequencing was performed on an Illumina Hi-Seq 2000 system. The sequencing data were analyzed following the guidelines that are outlined in the Broad Institute's Genome Analysis Toolkit [35], [36]. The row data were mapped using the Burrows Wheeler Aligner [36], the variants were called using the Unified Genotyper, and the data underwent further processing and quality control [35], [36]. Low-quality reads (less than 10× coverage) were removed, and the remaining variants were filtered against the dbSNP133 database and all of the known variants in the NHLBI 6500 Exome Variant database that had a minor allele frequency (MAF) of greater than 0.05%. We also filtered out additional variants that were observed in six ethnically matched control exomes. Primers were designed, using Primer3, to screen the remaining candidate gene variants, and we performed segregation analysis by performing Sanger sequencing of the variants of all of the participating family members.
ELMOD3/Elmod3 expression analyses
Human and mouse ELMOD3/Elmod3 isoform-specific primers and TaqMan probes were designed, using Primer3 web-based program, and the transcripts were amplified from human and mouse cDNA libraries (Clontech Laboratories; Mountain View, CA).
Mouse inner ear tissues were harvested from 3 or more C57BL/6J mice at P0, P10, and P30. The cochlea and vestibular system were separated from the inner ear, and the total RNA was extracted from each tissue using TRIreagent (Life Technologies, Grand Island, NY). The RNA was reverse-transcribed into cDNA using the SMARTscribe Kit (Clontech). Real-time PCR was performed in triplicate on a StepOne Plus instrument (Applied Biosystems). The data were analyzed using the comparative Ct method, with Gapdh as the endogenously expressed reference gene. RT-PCR was performed by using LA Taq (Clontech). The products were run on a 2% agarose gel that was stained with ethidium bromide and each isoform was verified by sequencing.
To determine the in vivo effect of c.794T>C allele, if any, on the splicing of ELMOD3 transcripts, total RNA was isolated from fresh blood samples of two affected individuals (V:2 and V:11) and one normal hearing individual (V:3) by use of TRIzol reagent (Life Technologies). Oligo dT and randomly primed first strand cDNA libraries were generated using SMART 1st strand cDNA synthesis kit (Clontech). Touchdown PCR was performed with GenScript Taq (GenScript) and 1.5 mM MgCl2 at an annealing temperature of 63°C for 30 cycles using ELMOD3-specific primer pairs with a common forward primer in exon 9 and reverse primer either in exons 11 (hELMOD3_ex9-11; Table S5) or in exon 12 (hELMOD3_ex9-12; Table S5). GAPDH was used as a control and amplified under the same conditions. PCR fragments were subcloned into pCR-TOPO cloning vector (Life Technologies), and the sequences were verified.
ELMOD3 expression constructs
Human ELMOD3 B isoform, murine Elmod1, Elmod2 and Elmod3 isoforms a and b open reading frame have been amplified from commercially available human and mouse cDNA libraries (Clontech) and inserted in pEGFP-C2 vector (Clontech) to generate proteins with GFP fused to their N-termini. The construct encoding p.Leu265Ser ELMOD3 was prepared through site-directed mutagenesis (Agilent Technologies, Santa Clara, CA) using the wild-type ELMOD3 isoform B as a template. The full-length open reading frame of human ELMOD3 (isoform B) was cloned into the pCOLD-TF (Takara Bio, Inc.; Otsu Shiga, Japan) vector at the BamHI and SalI sites by PCR amplification of the cDNA using primers that inserted the appropriate restriction sites. This generated a fusion protein with a His6 tag at the N-terminus. This tag was followed by trigger factor (∼48 kDa), a thrombin cleavage site, and the ELMOD3 open reading frame. To insert the Leu265Ser mutation, we performed site-directed mutagenesis on the construct using the QuikChange Lightening Kit (Agilent Technologies). Full-length open reading frames of human ELMOD3 (Isoform B) and the Leu265Ser mutant were cloned into the pLEXm-GST vector [37] using KpnI and SphI sites that were inserted into the PCR primers, with subsequent confirmation of the correct DNA sequence. The parent vector was used to express GST alone.
Inner ear explant and gene gun
Inner ear explants were harvested from C57BL/6J mice at P2. The explants were cultured in a glass-bottom Petri dish (MatTek, Ashland, MA) that was coated with Matrigel (BD Biosciences, San Jose, CA) and were maintained in DMEM that was supplemented with 7% fetal bovine serum (FBS) (Life Technologies) for 24 hrs at 37°C with 5% CO2. The cultures were transfected using a Helios gene gun (Bio-Rad, Hercules, CA), as described elsewhere [38].
Cell culture and transfection
HEK293T, CL4 and MDCK cells were grown in DMEM that was supplemented with 10% FBS, 2 mM L-glutamine, and penicillin/streptomycin (50 U/ml) (Life Technologies) and were maintained at 37°C in 5% CO2. The cells were transfected using Fugene HD Transfection Reagent (Promega; Sunnyvale, CA), according to the manufacturer's instructions. The cells were then cultured for an additional 48 hrs prior to immunostaining. Forty-eight hrs following transfection, we added 2.5 µM Cytochalasin D (EMD Millipore, Billerica, MA) in fresh DMSO medium to the cells for two hrs to disrupt the actin cytoskeleton. The Cytochalasin D was then washed out, and the cells were grown for four additional hrs in complete medium.
Antibody
ELMOD3 antiserum was raised in rabbits against two synthetic mouse ELMOD3-specific peptides (corresponding to residues 143–156 and 346–361 of the mouse sequence [GenBank accession number GI:358679299]). The immunizations and sera collections were performed by Covance (Princeton, New Jersey). The antiserum was affinity purified (AminoLink Plus Immobilization Kit; Thermo Scientific, Rockford, IL) either using both synthetic peptides in combination or individually.
Western blot
Antibody specificity was assessed by transfections (Fugene HD Transfection Reagent; Promega) of GFP-tagged mouse ELMOD1, ELMOD2 and ELMOD3 into HEK293T cells followed by western blot analysis, as described elsewhere [39]. Inner ear and olfactory bulbs tissues were harvested from C57BL/6J mice at P30 and followed by western blot analysis, as described elsewhere [39]. Antigen competition was performed by incubating the primary antibody for 30 min at room temperature with the two immunizing peptides prior to use in Western Blot or immunofluorescence analyses.
Immunostaining
C57BL/6J mice were obtained from Jackson Laboratories (Bar Harbor, ME) and bred in house. Sprague–Dawley rats were purchased from Charles River Breeding Laboratories (Raleigh, North Carolina) and bred in house. All experiments and procedures were approved by the Institutional Animal Care and Use Committee of the Cincinnati Children's Hospital Medical Center.
The inner ears from rats and mice were fixed with 4% PFA at 4°C overnight. P12 and P14 rat cochlea were incubated for one day, at 4°C, in 0.25M EDTA. The sensory epithelia were dissected in PBS. Following permeabilization with 0.25% Triton X-100 for 45 min, the samples were incubated in blocking solution (5% normal goat serum in PBS). The samples were then incubated overnight at 4°C with primary antibody (anti-ELMOD3; anti acetylated Tubulin (Sigma-Aldrich, St Louis, MO)) in 3% NGS/PBS. This step was followed by three washes in PBS and consecutive incubation with Alexa-488 conjugated secondary antibody and Alexa-647 conjugated secondary antibody (Life Technologies) at 1∶500 dilution and with rhodamine phalloidin (Life Technologies) at 1∶200 dilution in 3% NGS/PBS for one hour. After three washes with PBS, the samples were mounted using ProLong Gold Antifade Reagent (Life Technologies).
Transfected cells and inner ear explants were washed with PBS and fixed for 20 min in 4% paraformaldehyde. Filamentous actin was detected with rhodamine phalloidin (Life Technologies) in PBS/0.1%Triton-X100 for one hour. Following subsequent washes, the coverslips were mounted using FluoroGel medium (Electron Microscopy Sciences, Hatfield, PA) for the cell monolayers or with ProLong Gold Antifade Reagent (Life Technologies) for the explant cultures.
MDCK cells were washed with PBS, fixed for 20 min in 4% paraformaldehyde and blocked with 10%NGS/PBS/0.1% Triton-X100. The cells were incubated overnight at 4°C with a primary antibody (ZO1, Life Technologies; anti-ELMOD3) in 3% NGS/PBS/0.1%Triton-X100. The cells were then washed in PBS/0.1%Triton-X100 and incubated with the Alexa-fluor 546 conjugated secondary antibody (Life Technologies) in 3% NGS/PBS/0.1% Triton-X100 for one hour at room temperature. Filamentous actin was detected with Alexa647-phalloidin (Life Technologies) in PBS/0.1%Triton-X100 for one hr. Following subsequent washes, the coverslips were placed using FluoroGel mounting medium (Electron Microscopy Sciences).
All images were acquired using a Zeiss LSM 700 scanning confocal microscope that was equipped with 63× and 100× objectives, and the analyses were performed using ImageJ software. The pixel intensity analyses were performed using ImageJ software on images that were acquired with the same microscope settings. The statistical analyses were performed using GraphPad Prism software and the ANOVA test function.
Immunofluorescence and STORM imaging
Transfected MDCK cells were washed with PBS and fixed for 15 min in 4% paraformaldehyde. Following a one hour incubation in blocking solution (10% normal goat serum in PBS with 0.1% Triton X-100), the cells were then incubated one hour with primary antibody: polyclonal chicken anti-GFP (Aves Labs Inc., Tigard, OR) custom conjugated with APEX Alexa Fluor 568 Antibody (Life Technelogies), in PBS/0.1%Triton-X100/3%NGS. Filamentous actin was labeled concomitantly with Alexa647-phalloidin (Life Technologies). After extensive washes in PBS/0.1%TritonX-100/3%NGS, the cells were fixed 15 min in 4% paraformaldehyde and stored at 4°C in PBS.
For imaging, PBS was replaced by the following imaging medium: 2-mercaptoethanol, buffer B (10% glucose, 50 mM Tris-HCl pH = 8, 10 mM NaCl) and the GLOX system (14 mg Glucose Oxidase, 50 ul Catalase, 200 ul Tris buffer) in a 1∶100∶1 volume ratio. N-STORM imaging was performed with a Nikon N-STORM super-resolution microscope system (Nikon Instruments Ltd, Melville, NY) based on an inverted microscope Nikon A1Rsi equipped with a perfect-focusing system and a 100× TIRF APO NA 1.49 oil objective. A 561 nm wavelength laser was applied for bleaching and excitation of Alexa Fluor 568 while a 647 nm wavelength laser was applied for Alexa Fluor 647. The images were acquired with an Andor Xion 897 EMCCD camera using 16 ms exposition with one frame of imaging for 5000 cycles. The analysis was performed with Nikon Elements Storm software.
Actin co-sedimentation assay
BL21 (DE3) Gold E. coli were transfected with the pCOLD-TF-ELMOD3 and pCOLD-TF-ELMOD3 Leu265Ser plasmids, and single colonies were used to inoculate cultures in LB medium with 100 µg/ml ampicillin, which were grown at 37°C with shaking. When OD600 = 0.5, the cells were moved to 15°C for 30 min without shaking. IPTG was then added to 1 mM, and the culture was grown overnight at 15°C with shaking. The cells were collected and lysed by passage through a French press in 20 mL of 20 mM HEPES (pH 7.5), 150 mM NaCl, and 5 mM imidazole with a protease inhibitor cocktail (Sigma-Aldrich). The lysates were clarified by centrifugation at 100,000× g for one hour at 4°C, and the supernatants were loaded onto a Ni-NTA column (GE Healthcare) that was pre-equilibrated in the same buffer. The column was washed with 20 mL of 20 mM HEPES (pH 7.5), 150 mM NaCl, and 55 mM imidazole prior to elution in 15 mL buffer that contained 250 mM imidazole. The protein was further purified and buffer-exchanged by gel filtration chromatography using a Superdex S200 column (24 mL) that was run in 20 mM HEPES (pH 7.5), 150 mM NaCl, and 1 mM dithiothreitol. Typical yields from this protocol were ∼10 mg/L TF-ELMOD3 that was >90% pure, as estimated by visual inspection of a Coomassie blue-stained gel. The actin binding experiment was performed with purified TF-ELMOD3 and TF-ELMOD3 Leu265Ser using an Actin Binding Protein Biochem Kit (Cytoskeleton Inc, Denver, CO), following the manufacturer's protocol and later repeated with the GST-ELMOD3 proteins purified from HEK cells.
Purification of GST-ELMOD3 from HEK293T cells
GST-ELMOD3, the point mutant, or GST alone was expressed in HEK293T cells using the pLEXm-GST vector (a kind gift from Dr. James Hurley (NIDDK)) and a modification of the method that was described in Aricescu et al [37]. Briefly, HEK293T cells (10×10 cm plates) were transfected at 90% confluency with 1 µg/mL DNA after mixing with polyethyleneimine (PEI-MAX; Polysciences, Inc.; Warrington, PA) at a 1∶3 ratio of DNA∶PEI in Opti-MEM medium (Life Technologies). The mixture was then added to cells in DMEM medium that contained 2% FBS and grown for two days. The cells were collected by centrifugation and lysed by resuspension in 1.5 ml 25 mM HEPES (pH 7.4), 100 mM NaCl, and 1% CHAPS. The solution was clarified by centrifugation for 30 min in a microfuge at a maximum speed (∼14,000× g) at 4°C. Glutathione Sepharose 4B (GE Healthcare) beads were added and incubated at 4°C for 3 hrs with mixing. The beads were then pelleted, washed twice in 25 mM HEPES (pH 7.4) and 100 mM NaCl and eluted (2×0.5 mL) in the same buffer containing 20 mM glutathione. The eluted protein was concentrated to 0.25 mL in a spin concentrator (Amicon Ultra-4; EMD Millipore). The protein concentration was determined using a Bradford assay. Typical yields of preparations from 10×10 cm plates were ∼600 µg of GST-ELMOD3 or mutant and ∼6 mg of GST. The purified proteins were quick frozen and stored at −80°C.
Arl2 GAP assays
The Arl2 GAP assay was performed as described previously by Bowzard et al [29]. Briefly, 2 µM purified recombinant Arl2, prepared as described by Clark et al [40], was pre-loaded with [γ-32P]GTP in 25 mM HEPES (pH 7.4), 2.5 mM MgCl2, 100 mM NaCl, 1 mM EDTA, 25 mM KCl, and 0.5 mM ATP in a total volume of 100 µL. The incubation was performed at 30°C for 30 min. The GAP reaction was performed in a buffer that contained 25 mM HEPES (pH 7.4), 2.5 mM MgCl2, 100 mM NaCl, 1 mM dithiothreitol, 2 mM ATP, 1 mM GTP and the pre-loaded Arl2 [γ-32P]GTP. The total 50 µL reaction was initiated by the addition of the sample that contained the Arl2 GAP and stopped after 4 min at 30°C by the addition of 750 µL of ice-cold activated charcoal (5% activated charcoal (Sigma-Aldrich, St. Louis, MO) in 50 mM Na2HPO4 (pH 7.4). The samples were clarified by centrifugation, and 400 µL was taken for counting in a liquid scintillation counter. Each GAP sample was also assayed in parallel in a tube that contained all of the above reagents except Arl2 (i.e., the same amount of [γ-32P]GTP). The resulting “blank” values were subtracted from the results that were obtained in the presence of Arl2 to determine the amount of hydrolyzed 32Pi that was dependent on Arl2 GAP activity. More detail and descriptions regarding how the specific activities were calculated from this assay can be found in the report by Bowzard et al [29].
Supporting Information
Zdroje
1. BrownSD, Hardisty-HughesRE, MburuP (2008) Quiet as a mouse: dissecting the molecular and genetic basis of hearing. Nature reviews Genetics 9 : 277–290.
2. FrolenkovGI, BelyantsevaIA, FriedmanTB, GriffithAJ (2004) Genetic insights into the morphogenesis of inner ear hair cells. Nature reviews Genetics 5 : 489–498.
3. PetitC, RichardsonGP (2009) Linking genes underlying deafness to hair-bundle development and function. Nature neuroscience 12 : 703–710.
4. RichardsonGP, de MonvelJB, PetitC (2011) How the genetics of deafness illuminates auditory physiology. Annual review of physiology 73 : 311–334.
5. KitajiriS, SakamotoT, BelyantsevaIA, GoodyearRJ, StepanyanR, et al. (2010) Actin-bundling protein TRIOBP forms resilient rootlets of hair cell stereocilia essential for hearing. Cell 141 : 786–798.
6. DrummondMC, BelyantsevaIA, FridericiKH, FriedmanTB (2011) Actin in hair cells and hearing loss. Hear Res 288 : 89–99.
7. JohnsonKR, Longo-GuessCM, GagnonLH (2012) Mutations of the mouse ELMO domain containing 1 gene (Elmod1) link small GTPase signaling to actin cytoskeleton dynamics in hair cell stereocilia. PLoS One 7: e36074.
8. RzadzinskaAK, SchneiderME, DaviesC, RiordanGP, KacharB (2004) An actin molecular treadmill and myosins maintain stereocilia functional architecture and self-renewal. J Cell Biol 164 : 887–897.
9. ManorU, KacharB (2008) Dynamic length regulation of sensory stereocilia. Semin Cell Dev Biol 19 : 502–510.
10. ZhangDS, PiazzaV, PerrinBJ, RzadzinskaAK, PoczatekJC, et al. (2012) Multi-isotope imaging mass spectrometry reveals slow protein turnover in hair-cell stereocilia. Nature 481 : 520–524.
11. Grimsley-MyersCM, SipeCW, GeleocGS, LuX (2009) The small GTPase Rac1 regulates auditory hair cell morphogenesis. J Neurosci 29 : 15859–15869.
12. Grimsley-MyersCM, SipeCW, WuDK, LuX (2012) Redundant functions of Rac GTPases in inner ear morphogenesis. Dev Biol 362 : 172–186.
13. KalinecF, ZhangM, UrrutiaR, KalinecG (2000) Rho GTPases mediate the regulation of cochlear outer hair cell motility by acetylcholine. J Biol Chem 275 : 28000–28005.
14. MatsumotoN, KitaniR, MaricleA, MuellerM, KalinecF (2010) Pivotal role of actin depolymerization in the regulation of cochlear outer hair cell motility. Biophys J 99 : 2067–2076.
15. LiCC, ChiangTC, WuTS, Pacheco-RodriguezG, MossJ, et al. (2007) ARL4D recruits cytohesin-2/ARNO to modulate actin remodeling. Mol Biol Cell 18 : 4420–4437.
16. EastM, BowzardJB, DacksJ, KahnRA (2012) ELMO domains: evolutionary and functional characterization of a novel GTPase activating protein (GAP) domain for Arf family GTPases. J Biol Chem 287 : 39538–53.
17. LindnerTH, HoffmannK (2005) easyLINKAGE: a PERL script for easy and automated two-/multi-point linkage analyses. Bioinformatics 21 : 405–407.
18. FlexE, ManginoM, MazzoliM, MartiniA, MigliosiV, et al. (2003) Mapping of a new autosomal dominant non-syndromic hearing loss locus (DFNA43) to chromosome 2p12. J Med Genet 40 : 278–281.
19. Cooper DN, Krawczak M, Antonorakis SE (1995) The nature and mechanisms of human gene mutation. In: Scriver C, Beaudet al., Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. 7th ed. New York: McGraw-Hill. pp. 259–291.
20. AdzhubeiIA, SchmidtS, PeshkinL, RamenskyVE, GerasimovaA, et al. (2010) A method and server for predicting damaging missense mutations. Nat Methods 7 : 248–249.
21. YueP, MelamudE, MoultJ (2006) SNPs3D: candidate gene and SNP selection for association studies. BMC Bioinformatics 7 : 166.
22. SchwarzJM, RodelspergerC, SchuelkeM, SeelowD (2010) MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods 7 : 575–576.
23. Ferrer-CostaC, OrozcoM, de la CruzX (2004) Sequence-based prediction of pathological mutations. Proteins 57 : 811–819.
24. KumarP, HenikoffS, NgPC (2009) Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc 4 : 1073–1081.
25. ZhengL, ZhengJ, WhitlonDS, Garcia-AnoverosJ, BartlesJR (2010) Targeting of the hair cell proteins cadherin 23, harmonin, myosin XVa, espin, and prestin in an epithelial cell model. J Neurosci 30 : 7187–7201.
26. LoomisPA, ZhengL, SekerkovaG, ChangyaleketB, MugnainiE, et al. (2003) Espin cross-links cause the elongation of microvillus-type parallel actin bundles in vivo. J Cell Biol 163 : 1045–1055.
27. CasellaJF, FlanaganMD, LinS (1981) Cytochalasin D inhibits actin polymerization and induces depolymerization of actin filaments formed during platelet shape change. Nature 293 : 302–305.
28. GoddetteDW, FriedenC (1985) The binding of cytochalasin D to monomeric actin. Biochem Biophys Res Commun 128 : 1087–1092.
29. BowzardJB, ChengD, PengJ, KahnRA (2007) ELMOD2 is an Arl2 GTPase-activating protein that also acts on Arfs. J Biol Chem 282 : 17568–17580.
30. PengAW, BelyantsevaIA, HsuPD, FriedmanTB, HellerS (2009) Twinfilin 2 regulates actin filament lengths in cochlear stereocilia. J Neurosci 29 : 15083–15088.
31. HertzanoR, ElkonR, KurimaK, MorrissonA, ChanSL, et al. (2011) Cell type-specific transcriptome analysis reveals a major role for Zeb1 and miR-200b in mouse inner ear morphogenesis. PLoS Genet 7: e1002309.
32. MargaronY, FradetN, CoteJF (2013) ELMO recruits actin cross-linking family 7 (ACF7) at the cell membrane for microtubule capture and stabilization of cellular protrusions. J Biol Chem 288 : 1184–1199.
33. ZhouC, CunninghamL, MarcusAI, LiY, KahnRA (2006) Arl2 and Arl3 regulate different microtubule-dependent processes. Mol Biol Cell 17 : 2476–2487.
34. GoldbergJ (1999) Structural and functional analysis of the ARF1-ARFGAP complex reveals a role for coatomer in GTP hydrolysis. Cell 96 : 893–902.
35. DePristoMA, BanksE, PoplinR, GarimellaKV, MaguireJR, et al. (2011) A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet 43 : 491–498.
36. LiH, DurbinR (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25 : 1754–1760.
37. AricescuAR, AssenbergR, BillRM, BussoD, ChangVT, et al. (2006) Eukaryotic expression: developments for structural proteomics. Acta crystallographica Section D, Biological crystallography 62 : 1114–1124.
38. BelyantsevaIA (2009) Helios Gene Gun-mediated transfection of the inner ear sensory epithelium. Methods Mol Biol 493 : 103–123.
39. RiazuddinS, BelyantsevaIA, GieseAP, LeeK, IndzhykulianAA, et al. (2012) Alterations of the CIB2 calcium - and integrin-binding protein cause Usher syndrome type 1J and nonsyndromic deafness DFNB48. Nat Genet 44 : 1265–1271.
40. MarthGT, YuF, IndapAR, GarimellaK, GravelS, et al. (2011) The functional spectrum of low-frequency coding variation. Genome Biol 12: R84.
Štítky
Genetika Reprodukční medicína
Článek Rapid Intrahost Evolution of Human Cytomegalovirus Is Shaped by Demography and Positive SelectionČlánek Common Variants in Left/Right Asymmetry Genes and Pathways Are Associated with Relative Hand SkillČlánek Manipulating or Superseding Host Recombination Functions: A Dilemma That Shapes Phage EvolvabilityČlánek Maternal Depletion of Piwi, a Component of the RNAi System, Impacts Heterochromatin Formation inČlánek Hsp104 Suppresses Polyglutamine-Induced Degeneration Post Onset in a Drosophila MJD/SCA3 ModelČlánek Cooperative Interaction between Phosphorylation Sites on PERIOD Maintains Circadian Period inČlánek VAPB/ALS8 MSP Ligands Regulate Striated Muscle Energy Metabolism Critical for Adult Survival inČlánek Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2AČlánek A Link between ORC-Origin Binding Mechanisms and Origin Activation Time Revealed in Budding YeastČlánek Genotype-Environment Interactions Reveal Causal Pathways That Mediate Genetic Effects on PhenotypeČlánek Chromatin-Specific Regulation of Mammalian rDNA Transcription by Clustered TTF-I Binding SitesČlánek Meiotic Recombination in Arabidopsis Is Catalysed by DMC1, with RAD51 Playing a Supporting Role
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 9- Souvislost haplotypu M2 genu pro annexin A5 s opakovanými reprodukčními ztrátami
- Akutní intermitentní porfyrie
- Příjem alkoholu a menstruační cyklus
- Hysteroskopická resekce děložního septa zlepšuje šanci na graviditu žen s jinak nevysvětlenou infertilitou
- Transfer zmraženého embrya zlepšuje výsledky IVF
-
Všechny články tohoto čísla
- The Pathway Gene Functions together with the -Dependent Isoprenoid Biosynthetic Pathway to Orchestrate Germ Cell Migration
- Take Off, Landing, and Fly Anesthesia
- Nucleosome Assembly Proteins Get SET to Defeat the Guardian of Chromosome Cohesion
- Whole-Exome Sequencing Reveals a Rapid Change in the Frequency of Rare Functional Variants in a Founding Population of Humans
- Evidence Is Evidence: An Interview with Mary-Claire King
- Rapid Intrahost Evolution of Human Cytomegalovirus Is Shaped by Demography and Positive Selection
- Convergent Transcription Induces Dynamic DNA Methylation at Loci
- Environmental Stresses Disrupt Telomere Length Homeostasis
- Ultra-Sensitive Sequencing Reveals an Age-Related Increase in Somatic Mitochondrial Mutations That Are Inconsistent with Oxidative Damage
- Common Variants in Left/Right Asymmetry Genes and Pathways Are Associated with Relative Hand Skill
- Genetic and Anatomical Basis of the Barrier Separating Wakefulness and Anesthetic-Induced Unresponsiveness
- The Locus, Exclusive to the Ambulacrarians, Encodes a Chromatin Insulator Binding Protein in the Sea Urchin Embryo
- Binding of NF-κB to Nucleosomes: Effect of Translational Positioning, Nucleosome Remodeling and Linker Histone H1
- Manipulating or Superseding Host Recombination Functions: A Dilemma That Shapes Phage Evolvability
- Dynamics of DNA Methylation in Recent Human and Great Ape Evolution
- Functional Dissection of Regulatory Models Using Gene Expression Data of Deletion Mutants
- PAQR-2 Regulates Fatty Acid Desaturation during Cold Adaptation in
- N-alpha-terminal Acetylation of Histone H4 Regulates Arginine Methylation and Ribosomal DNA Silencing
- A Genome-Wide Systematic Analysis Reveals Different and Predictive Proliferation Expression Signatures of Cancerous vs. Non-Cancerous Cells
- Maternal Depletion of Piwi, a Component of the RNAi System, Impacts Heterochromatin Formation in
- miR-1/133a Clusters Cooperatively Specify the Cardiomyogenic Lineage by Adjustment of Myocardin Levels during Embryonic Heart Development
- Hsp104 Suppresses Polyglutamine-Induced Degeneration Post Onset in a Drosophila MJD/SCA3 Model
- Genome-Wide Analysis of Genes and Their Association with Natural Variation in Drought Tolerance at Seedling Stage of L
- Deep Resequencing of GWAS Loci Identifies Rare Variants in , and That Are Associated with Ulcerative Colitis
- Cooperative Interaction between Phosphorylation Sites on PERIOD Maintains Circadian Period in
- VAPB/ALS8 MSP Ligands Regulate Striated Muscle Energy Metabolism Critical for Adult Survival in
- Analysis of Genes Reveals Redundant and Independent Functions in the Inner Ear
- Predicting the Risk of Rheumatoid Arthritis and Its Age of Onset through Modelling Genetic Risk Variants with Smoking
- Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2A
- A Shift to Organismal Stress Resistance in Programmed Cell Death Mutants
- Fragile Site Instability in Causes Loss of Heterozygosity by Mitotic Crossovers and Break-Induced Replication
- Tracking of Chromosome and Replisome Dynamics in Reveals a Novel Chromosome Arrangement
- The Condition-Dependent Transcriptional Landscape of
- Ago1 Interacts with RNA Polymerase II and Binds to the Promoters of Actively Transcribed Genes in Human Cancer Cells
- Nebula/DSCR1 Upregulation Delays Neurodegeneration and Protects against APP-Induced Axonal Transport Defects by Restoring Calcineurin and GSK-3β Signaling
- System-Wide Analysis Reveals a Complex Network of Tumor-Fibroblast Interactions Involved in Tumorigenicity
- Meta-Analysis of Genome-Wide Association Studies Identifies Six New Loci for Serum Calcium Concentrations
- and Are Required for Cellularization and Differentiation during Female Gametogenesis in
- Growth factor independent-1 Maintains Notch1-Dependent Transcriptional Programming of Lymphoid Precursors
- Whole Genome Sequencing Identifies a Deletion in Protein Phosphatase 2A That Affects Its Stability and Localization in
- An Alteration in ELMOD3, an Arl2 GTPase-Activating Protein, Is Associated with Hearing Impairment in Humans
- Genomic Identification of Founding Haplotypes Reveals the History of the Selfing Species
- Plasticity Regulators Modulate Specific Root Traits in Discrete Nitrogen Environments
- The IDD14, IDD15, and IDD16 Cooperatively Regulate Lateral Organ Morphogenesis and Gravitropism by Promoting Auxin Biosynthesis and Transport
- Stochastic Loss of Silencing of the Imprinted Allele, in a Mouse Model and Humans with Prader-Willi Syndrome, Has Functional Consequences
- The Prefoldin Complex Regulates Chromatin Dynamics during Transcription Elongation
- PKA Controls Calcium Influx into Motor Neurons during a Rhythmic Behavior
- A Pre-mRNA-Splicing Factor Is Required for RNA-Directed DNA Methylation in
- Cell-Type Specific Features of Circular RNA Expression
- The Uve1 Endonuclease Is Regulated by the White Collar Complex to Protect from UV Damage
- An Atypical Kinase under Balancing Selection Confers Broad-Spectrum Disease Resistance in Arabidopsis
- Genome-Wide Mutation Avalanches Induced in Diploid Yeast Cells by a Base Analog or an APOBEC Deaminase
- Extensive Divergence of Transcription Factor Binding in Embryos with Highly Conserved Gene Expression
- Bi-modal Distribution of the Second Messenger c-di-GMP Controls Cell Fate and Asymmetry during the Cell Cycle
- Cell Interactions and Patterned Intercalations Shape and Link Epithelial Tubes in
- A Link between ORC-Origin Binding Mechanisms and Origin Activation Time Revealed in Budding Yeast
- The Genome and Development-Dependent Transcriptomes of : A Window into Fungal Evolution
- SKN-1/Nrf, A New Unfolded Protein Response Factor?
- The Highly Prolific Phenotype of Lacaune Sheep Is Associated with an Ectopic Expression of the Gene within the Ovary
- Fusion of Large-Scale Genomic Knowledge and Frequency Data Computationally Prioritizes Variants in Epilepsy
- IL-17 Attenuates Degradation of ARE-mRNAs by Changing the Cooperation between AU-Binding Proteins and microRNA16
- An Enhancer Element Harboring Variants Associated with Systemic Lupus Erythematosus Engages the Promoter to Influence A20 Expression
- Genome Analysis of a Transmissible Lineage of Reveals Pathoadaptive Mutations and Distinct Evolutionary Paths of Hypermutators
- Type I-E CRISPR-Cas Systems Discriminate Target from Non-Target DNA through Base Pairing-Independent PAM Recognition
- Divergent Transcriptional Regulatory Logic at the Intersection of Tissue Growth and Developmental Patterning
- MEIOB Targets Single-Strand DNA and Is Necessary for Meiotic Recombination
- Transmission of Hypervirulence Traits via Sexual Reproduction within and between Lineages of the Human Fungal Pathogen
- Integration of the Unfolded Protein and Oxidative Stress Responses through SKN-1/Nrf
- Guanine Holes Are Prominent Targets for Mutation in Cancer and Inherited Disease
- Regulation of the Boundaries of Accessible Chromatin
- Natural Genetic Transformation Generates a Population of Merodiploids in
- Ablating Adult Neurogenesis in the Rat Has No Effect on Spatial Processing: Evidence from a Novel Pharmacogenetic Model
- Genotype-Environment Interactions Reveal Causal Pathways That Mediate Genetic Effects on Phenotype
- The Molecular Mechanism of a -Regulatory Adaptation in Yeast
- Phenotypic and Genetic Consequences of Protein Damage
- Recent Acquisition of by Baka Pygmies
- Fatty Acid Taste Signals through the PLC Pathway in Sugar-Sensing Neurons
- A Critical Role for PDGFRα Signaling in Medial Nasal Process Development
- Chromatin-Specific Regulation of Mammalian rDNA Transcription by Clustered TTF-I Binding Sites
- Meiotic Recombination in Arabidopsis Is Catalysed by DMC1, with RAD51 Playing a Supporting Role
- dTULP, the Homolog of Tubby, Regulates Transient Receptor Potential Channel Localization in Cilia
- Widespread Dysregulation of Peptide Hormone Release in Mice Lacking Adaptor Protein AP-3
- , a Direct Transcriptional Target, Modulates T-Box Factor Activity in Orofacial Clefting
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Genome-Wide Systematic Analysis Reveals Different and Predictive Proliferation Expression Signatures of Cancerous vs. Non-Cancerous Cells
- Recent Acquisition of by Baka Pygmies
- The Condition-Dependent Transcriptional Landscape of
- Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2A
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



