-
Články
- Vzdělávání
- Časopisy
Top články
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The Genome and Development-Dependent Transcriptomes of : A Window into Fungal Evolution
Fungi are a large group of eukaryotes found in nearly all ecosystems. More than 250 fungal genomes have already been sequenced, greatly improving our understanding of fungal evolution, physiology, and development. However, for the Pezizomycetes, an early-diverging lineage of filamentous ascomycetes, there is so far only one genome available, namely that of the black truffle, Tuber melanosporum, a mycorrhizal species with unusual subterranean fruiting bodies. To help close the sequence gap among basal filamentous ascomycetes, and to allow conclusions about the evolution of fungal development, we sequenced the genome and assayed transcriptomes during development of Pyronema confluens, a saprobic Pezizomycete with a typical apothecium as fruiting body. With a size of 50 Mb and ∼13,400 protein-coding genes, the genome is more characteristic of higher filamentous ascomycetes than the large, repeat-rich truffle genome; however, some typical features are different in the P. confluens lineage, e.g. the genomic environment of the mating type genes that is conserved in higher filamentous ascomycetes, but only partly conserved in P. confluens. On the other hand, P. confluens has a full complement of fungal photoreceptors, and expression studies indicate that light perception might be similar to distantly related ascomycetes and, thus, represent a basic feature of filamentous ascomycetes. Analysis of spliced RNA-seq sequence reads allowed the detection of natural antisense transcripts for 281 genes. The P. confluens genome contains an unusually high number of predicted orphan genes, many of which are upregulated during sexual development, consistent with the idea of rapid evolution of sex-associated genes. Comparative transcriptomics identified the transcription factor gene pro44 that is upregulated during development in P. confluens and the Sordariomycete Sordaria macrospora. The P. confluens pro44 gene (PCON_06721) was used to complement the S. macrospora pro44 deletion mutant, showing functional conservation of this developmental regulator.
Published in the journal: . PLoS Genet 9(9): e32767. doi:10.1371/journal.pgen.1003820
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003820Summary
Fungi are a large group of eukaryotes found in nearly all ecosystems. More than 250 fungal genomes have already been sequenced, greatly improving our understanding of fungal evolution, physiology, and development. However, for the Pezizomycetes, an early-diverging lineage of filamentous ascomycetes, there is so far only one genome available, namely that of the black truffle, Tuber melanosporum, a mycorrhizal species with unusual subterranean fruiting bodies. To help close the sequence gap among basal filamentous ascomycetes, and to allow conclusions about the evolution of fungal development, we sequenced the genome and assayed transcriptomes during development of Pyronema confluens, a saprobic Pezizomycete with a typical apothecium as fruiting body. With a size of 50 Mb and ∼13,400 protein-coding genes, the genome is more characteristic of higher filamentous ascomycetes than the large, repeat-rich truffle genome; however, some typical features are different in the P. confluens lineage, e.g. the genomic environment of the mating type genes that is conserved in higher filamentous ascomycetes, but only partly conserved in P. confluens. On the other hand, P. confluens has a full complement of fungal photoreceptors, and expression studies indicate that light perception might be similar to distantly related ascomycetes and, thus, represent a basic feature of filamentous ascomycetes. Analysis of spliced RNA-seq sequence reads allowed the detection of natural antisense transcripts for 281 genes. The P. confluens genome contains an unusually high number of predicted orphan genes, many of which are upregulated during sexual development, consistent with the idea of rapid evolution of sex-associated genes. Comparative transcriptomics identified the transcription factor gene pro44 that is upregulated during development in P. confluens and the Sordariomycete Sordaria macrospora. The P. confluens pro44 gene (PCON_06721) was used to complement the S. macrospora pro44 deletion mutant, showing functional conservation of this developmental regulator.
Introduction
Fungi (Eumycota) are a group of eukaryotes that are present in almost all habitats; therefore they do not only play a great role in nature, but also influence human life in many ways [1]. About 100,000 fungal species have been described, but it is estimated that the actual number might exceed 1.5 million [2]. The largest group among the Eumycota is the Ascomycota (or ascomycetes), which comprise the Saccharomycotina, Taphrinomycotina, and Pezizomycotina. The former groups contain many unicellular species (yeasts) or species that develop only few hyphae or develop hyphae only under certain conditions (dimorphic fungi), whereas the Pezizomycotina are generally filamentous fungi capable of producing highly differentiated multicellular structures, the most complex of which are fruiting bodies for the protection and dispersal of sexual spores [3], [4]. The most basal groups of Pezizomycotina are the Pezizomycetes and the Orbiliomycetes that form open fruiting bodies called apothecia with exposed meiosporangia (asci). Phylogenetically derived groups (e.g. Sordariomycetes, Eurotiomycetes and Dothideomycetes) mostly differentiate closed fruiting bodies where the asci develop within and protected by mycelial structures [3], [5]–[8].
In the last decade, genomes of many filamentous ascomycetes have been sequenced and are invaluable for the analysis of the evolution of species as well as for understanding physiological and morphological properties of fungi. In fact, fungi are among the groups of eukaryotes with the highest number of sequenced genomes to date (http://www.ncbi.nlm.nih.gov/genome/browse/), largely because they include many model organisms, species of medical, agricultural or biotechnological importance. In addition, they usually have compact genomes with short introns and relatively few repetitive regions or non-coding DNA compared to plants and animals, thus making genomic analysis less complex. However, while there are at least ten genome sequences available for each of the more derived groups (Sordariomycetes, Leotiomycetes, Eurotiomycetes and Dothideomycetes), only one Orbiliomycete and one Pezizomycete genome have been sequenced, namely those of a nematode-trapping fungus, Arthrobotrys oligospora, and the black truffle, Tuber melanosporum, respectively [9], [10]. A. oligospora (teleomorph Orbilia auricolor [11]) belongs to a group of nematode-trapping soil fungi that comprises only a few known species, which are mostly analyzed for their ability to develop specialized trapping structures, while fruiting body formation is not well studied in this group. The 40 Mb genome of A. oligospora encodes ∼11,500 protein-coding genes, similar to the size and coding capacity of other ascomycete genomes [10]. In contrast, the 125 Mb genome of T. melanosporum is much larger than those of other sequenced ascomycetes, but contains fewer protein-coding genes. This genome expansion is mostly due to a large number of transposable elements that make up 58% of the truffle genome [9]. Truffles are symbiotic fungi that form mycorrhizal interactions with plant roots; and it has been noted that a biotrophic life-style, either as symbiont or pathogen, is often correlated with an increase in genome size, e.g. caused by repetitive sequences, in many fungi [12]. Furthermore, truffles have a highly specialized fruiting body that is adapted to growth within the soil, in contrast to fruiting bodies of almost all other filamentous fungi, which develop above ground. Thus, even though the truffle genome is of great interest for both ecological and economic reasons, it is difficult to distinguish between features that are ancestral with respect to the filamentous ascomycete lineage, specifically with respect to fruiting body formation, versus features that are adaptations to the truffle-specific life style, i.e. adaptations to mycorrhizal symbiosis or to below-ground fruiting body development. Consequently, the genome sequence of another member of the Pezizomycetes with fruiting body development that is more typical of filamentous ascomycetes will be of great value for evolutionary and morphogenetic analyses. To fill this gap, we sequenced and analyzed the genome and development-dependent transcriptomes of the Pezizomycete Pyronema confluens.
P. confluens was established as a model organism for the analysis of cell biology and fruiting body development in filamentous ascomycetes during the first half of the 20th century. It was instrumental in the elucidation of the dikaryotic phase during sexual development of higher ascomycetes [13]–[17]. In the last decade, P. confluens was used in comparative studies of gene expression during sexual development of ascomycetes [6], [18], [19]. It is a soil-living saprobe found in forests in temperate climates. In nature, its fruiting bodies (apothecia) usually appear on the ground after forest fires [20]. Under laboratory conditions, P. confluens has a short life cycle where typical apothecia containing eight-spored asci are formed within six days (Figure 1). This is rare among members of the Pezizomycetes, many of which do not easily reproduce sexually in the laboratory. Also, P. confluens is homothallic (self-fertile), therefore no crossing partner of different mating type is needed for the fungus to complete its sexual cycle [17]. In addition, P. confluens can also be used to study the effects of light on fruiting body formation, because in contrast to many other filamentous ascomycetes, fruiting body development in this fungus is strictly light-dependent [6], [21]. A previous analysis based on sequence data from 15 proteins showed that the P. confluens lineage is positioned at the base of the filamentous ascomycetes in a phylogenetic tree [6]. Phylogenomic analysis based on the genome data from this study and including sequences from T. melanosporum and A. oligospora confirms this basal position with the Pezizomycetes as sister group to the Orbiliomycetes (Figure 2).
Fig. 1. Life cycle of P. confluens under continuous illumination and laboratory conditions. 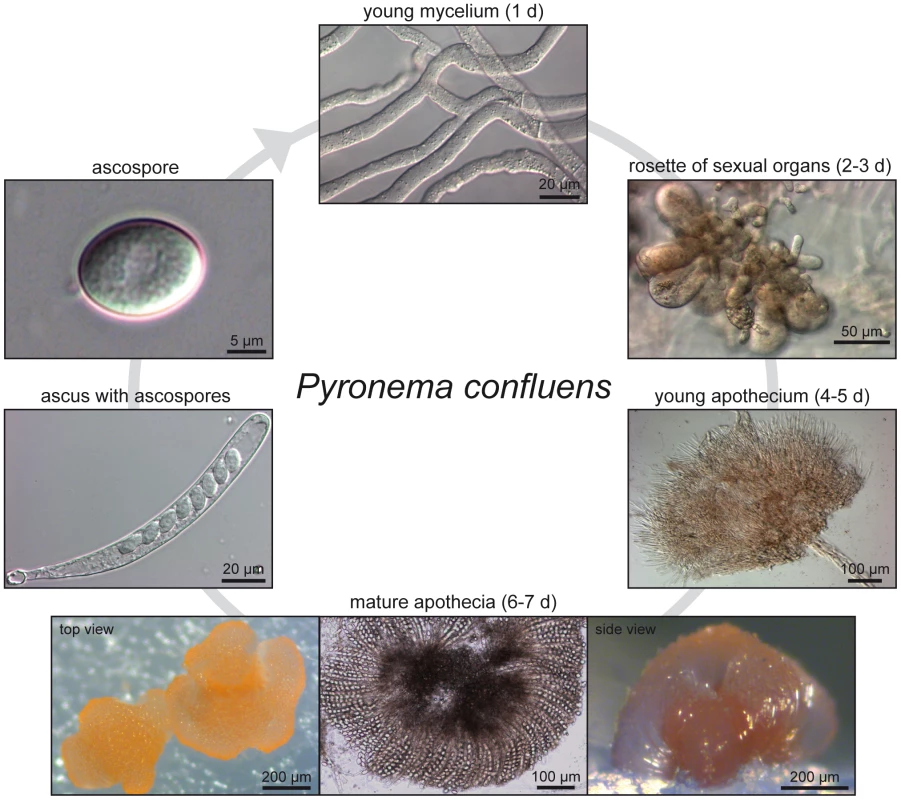
Non-pigmented, regularly septated mycelium germinates from haploid ascospores after a few hours. First orange structures of sexual organs can be observed after two days, they consists of ascogonia (enlarged, cytoplasm-rich female structures) and antheridia (male organs). Cytoplasmic fusion and transfer of nuclei are realized by a trichogyne that grows from an ascogonium towards an antheridium; the formation of dikaryotic ascogenous hyphae begins after plasmogamy and is followed by karyogamy and meiosis (not shown). After the fourth day of incubation, young pigmented apothecia can be observed; these contain many thin paraphyses, but no mature asci yet. By the sixth to seventh day, apothecia are mature and contain numerous asci (shown in top and side view to the left and right, the middle panel shows a flattened apothecium to visualize the ascus rosette). Each ascus contains eight hyaline ascospores. With the exception of the ascogenous hyphae in which karyogamy occurs leading to a diploid nucleus (which directly undergoes meiosis to yield haploid ascospores), all hyphae, both in vegetative or sexual structures, contain haploid nuclei. Fig. 2. Species tree of 18 fungal species based on phylome reconstruction. 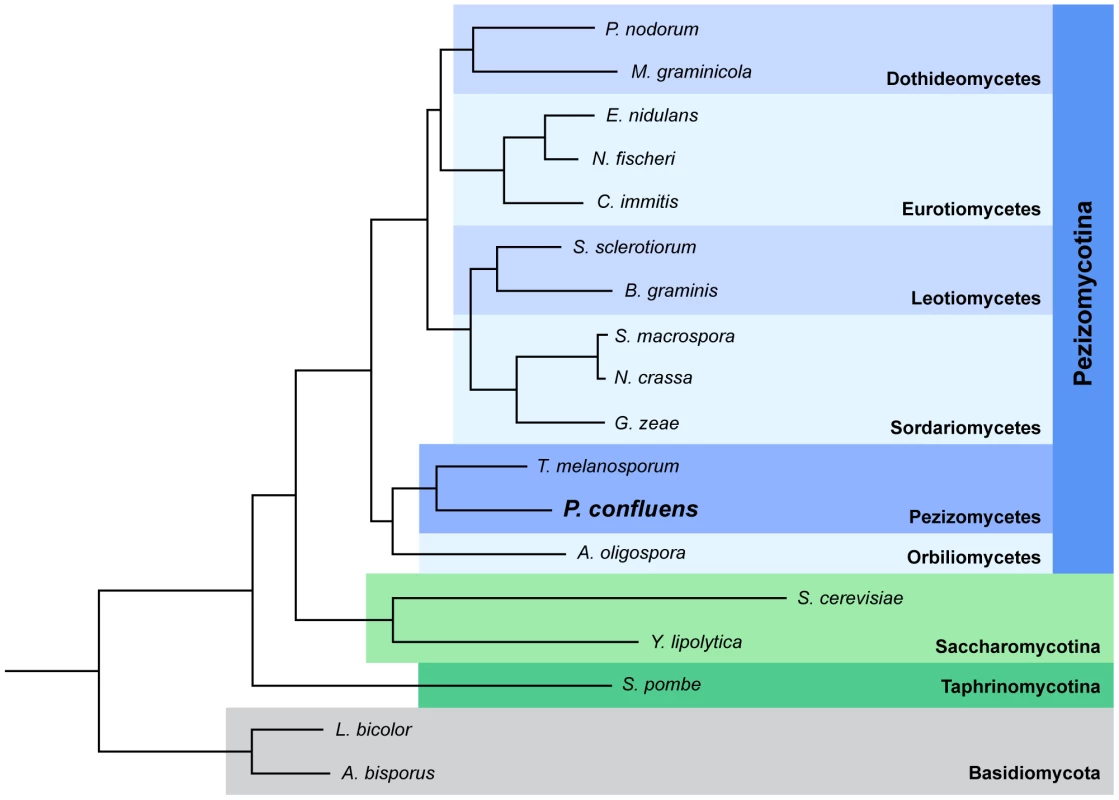
The species tree was built using PhyML [191] based on 426 single-copy, widespread genes (see Materials and Methods for details). A bootstrap of 100 repetitions was also reconstructed, bootstrap values for all branches were 100%. A species tree constructed with the super-tree reconstruction program DupTree [195] based on all 6,949 trees reconstructed in the phylome had the same topology. The divergence time of the Pyronema and Tuber lineages was estimated at 260 or 413 Mya using r8s [49] calibrated with divergence times of 723.86 Mya and 1147.78 Mya, respectively, for Schizosaccharomyces pombe and the remaining ascomycetes (see Materials and Methods). In two previous small pilot studies, P. confluens was used for comparative expression analyses to identify genes with evolutionary conserved expression patterns during fruiting body development in ascomycetes [6], [19]. These studies already indicated that gene expression patterns during development might be conserved even over large evolutionary distances. Here, we sequenced the genome and development-dependent transcriptomes of P. confluens with the following objectives: (i) To work towards closing the sequence gap among basal filamentous ascomycetes, and, thus, to learn more about the evolution of fungal genomes. (ii) To use the genome and transcriptome data to study the biology of a basal filamentous ascomycete in comparison with more derived species, with a focus on sexual development.
Results/Discussion
Sequencing and Assembly of the P. confluens Genome and Development-Dependent Transcriptomes
The genome of the P. confluens strain CBS100304 was sequenced with a combination of Roche/454 and Illumina/Solexa sequencing similar to what was described previously for Sordaria macrospora [22]. A summary of the sequence reads that were used for the P. confluens assembly is given in Table S1. The final assembly consists of 1,588 scaffolds (1,898 contigs) with a total size of 50 Mb, a scaffold N50 of 135 kb and a GC content of 47.8% (Table 1). To estimate the genome size independently of the assembly, k-mer analyses based on the Illumina/Solexa reads were performed using an algorithm described for the potato genome [23]. The analysis resulted in one clear peak, as one would expect for haploid genome (Figure S1). Based on the analysis for different k-mer lengths (31 and 41), a total genome size of ∼50.1 Mb was predicted which is close to the total length of the assembly.
Tab. 1. Main features of the P. confluens genome. 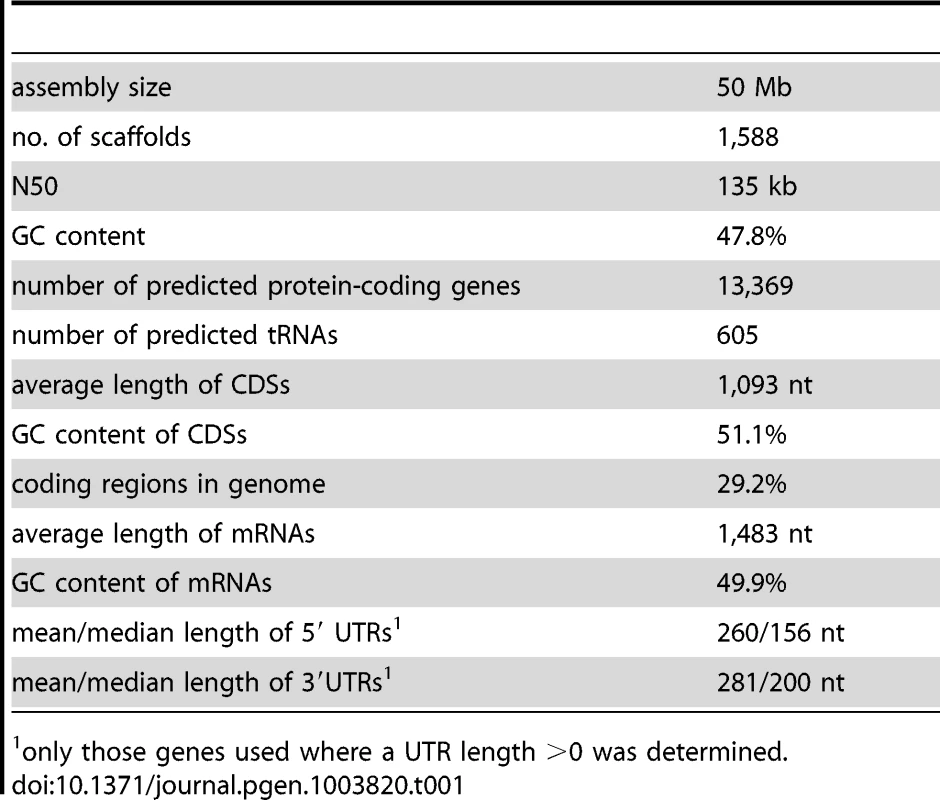
only those genes used where a UTR length >0 was determined. For transcriptomics, we performed RNA-seq for three different conditions: sexual development (sex), long-term culturing in the dark (DD), and a mixture of different vegetative tissues (vegmix), in two biological replicates per condition (Table S1). For each condition, RNA from different time points was pooled to represent a high number of genes that are expressed during the corresponding condition (see Materials and Methods for details). RNA for sex samples was extracted from mycelia grown in minimal medium in surface culture in constant light. Only under these conditions is P. confluens able to develop fruiting bodies, whereas growth in darkness, submerged, or in complete medium prevents sexual development. We used RNA from 3d, 4d, and 5d old mycelia to cover the initial stages of sexual development up to the development of young fruiting bodies (Figure 1). The DD samples comprised RNAs from mycelia grown in minimal medium in submerged culture in constant darkness, which prevents fruiting body formation. The vegmix samples also contained only RNAs from mycelia that could not develop fruiting bodies, but from a mixture of growth conditions different from the DD condition. We argue that the use of different mycelia sharing the common denominator of “no fruiting bodies” would allow us to focus on genes that are differentially expressed during fruiting body morphogenesis by comparing the three different conditions. Thus, genes that are differentially regulated in the comparisons sex/DD and sex/vegmix, but not DD/vegmix are candidates for genes that are regulated in a sexual development-dependent manner.
RNAs from different growth conditions were also used to allow a high read coverage of as many genes as possible for annotation purposes as described previously for Sordaria macrospora [24]. Therefore, gene model predictions were performed ab initio as well as evidence-based on the RNA-seq data (details in Materials and Methods). The output from different gene prediction pipelines was merged using MAKER [25]. Gene model predictions were scanned for consistency, and ∼10% of the predicted gene annotations were corrected manually to improve the exon/intron structure. To address the question whether the assembly and annotation cover the complete gene space of P. confluens, we performed a BLASTP search with a eukaryotic core gene set as previously described [26]. All of the 248 single-copy core genes were present in the P. confluens predicted peptides, suggesting that the assembly covers the complete core gene space.
Untranslated regions (UTRs) were also modeled by the gene prediction pipeline, and were refined based on the manually curated gene set using custom-made Perl scripts as described previously [24]. For the current assembly, 13,369 protein-coding genes are predicted with an average CDS length of 1,093 and an average transcript length of 1,483 bases (Table 1, Table S2). The median length of 5′ and 3′ UTRs are 156 and 200 bases, respectively, similar to findings in T. melanosporum, Aspergillus oryzae and S. macrospora [24], [27], [28]. Furthermore, we predicted 605 tRNAs, and assembled an rDNA unit comprising the 18S, 5.8S and 28S rRNA genes as well as the internal transcribed spacers (ITS) 1 and 2. Based on this annotation, the majority of the RNA-seq reads map to exonic regions as expected (Figure S2).
Spliced sequence reads identified in RNA-seq mapping results can not only be used to improve the exon/intron structures of predicted genes, but also to address the question of natural antisense transcripts (NATs), because the consensus sequences at the 5′ and 3′ ends of introns allow strand determination even in non-strand-specific RNA-seq data. NATs can play a role in the regulation of gene expression, and were found to be pervasive in metazoans [29], [30]. To identify putative NATs in P. confluens, we extracted predicted splice sites in antisense orientation to annotated genes from the mapping results obtained with Tophat [31]. Antisense splice sites covered by at least five spliced sequence reads, and with a coverage of more than 10% of the average coverage of the predicted sense-transcript were checked manually to remove splice sites that were most likely due to annotation errors, within repeat-rich regions, or close to sequence gaps. This yielded 376 antisense splice sites in 281 genes (Table S3), indicating that natural antisense transcripts are present in P. confluens. The number of genes with NATs is most likely underestimated, because we set stringent criteria, and non-spliced antisense transcripts could not be discovered by this analysis. In T. melansporum, only 33 NATs were identified from RNA-seq data by gene modeling, but this low number most likely also is an underestimate due to the method used [27]. Few studies have addressed the presence of NATs at a genome-wide scale in filamentous fungi, and the number of NATs that were identified in the ascomycetes Aspergillus flavus, Aspergillus niger, and Magnaporthe grisea [32]–[34], and in the basidiomycetes Ustilago maydis, Coprinopsis cinerea, and Schizophyllum commune [35]–[37] is in a range similar to our findings in P. confluens. Thus, NATs appear to be present across all groups of filamentous fungi, even though they do not seem to be as pervasive as in metazoans.
When compared with the genome of its closest sequenced relative, T. melanosporum [9], the P. confluens genome is much smaller (50 Mb versus 125 Mb), but contains nearly twice the number of protein-coding genes (13,369 versus 7,496). The large size of the truffle genome is mostly due to an expansion of transposons and other repeated elements, with nearly 58% (∼71 Mb) of the genome consisting of repeats larger than 200 bp [9]. Repeat analyses based on similarity to known repeat classes as well as de novo repeat finding showed that in P. confluens, transposable elements longer than 200 bp constitute only 12% (∼6 Mb) of the genome, with low complexity regions and simple repeats constituting an additional 0.21 and 1.03%, respectively (Table S4). Very few of the repeats show a high percentage of sequence identity with the repeat consensus sequences, indicating a high degree of divergence among repeats (Figure S3). This is different from findings in T. melanosporum, Fusarium oxysporum, and Pyrenophora tritici-repentis [9], [38], [39], but similar to Nectria haematococca [40]. One reason for this finding might be that long perfect repeats (longer than the Roche/454 read lengths of 300–400 bp) were lost in the assembly process; however, this would not apply to shorter perfect repeats. Also, the k-mer analysis showed a single main peak corresponding to a genome size of 50 Mb (Figure S1), and did not show any major extra peaks, indicating that most of the genome sequence is represented in the assembly. Thus, the presence of repeats with dissimilar sequences suggests that the repeats are evolutionary rather old, and that genome defense processes are active in P. confluens. A search for genes that might be involved in chromatin modification and other silencing processes showed that P. confluens comprises gene sets similar to those in other fungi where genome defense processes were identified. Interestingly, there is even a slight expansion in some families of putative RNA interference genes (Table S5). This suggests that in its current state, the P. confluens genome is well-protected against repeat spreading, and that the high repeat content of the T. melanosporum genome is not characteristic for all members of the Pezizales. Among the genes that might be involved in genome defense is PCON_06255, a homolog of the N. crassa rid gene. In N. crassa, the cytosine DNA methyltransferase RID is essential for repeat induced point mutation (RIP) [41]. The RIP process introduces C∶G to T∶A mutations in duplicated sequences of more than 400 bp and at least 80% sequence identity during the sexual cycle, and thus is a means to control the spread of duplicated sequences including transposons [42]–[44]. The rid homolog PCON_06255 is slightly upregulated during sexual development (Table S2), consistent with a role for the gene product under these conditions; however, an analysis of the genomic DNA for the presence of regions that might have been subjected to RIP yielded only a small fraction (0.46%) of the genome (Table S6). Thus, RIP does not seem to play a major role in genome defense in P. confluens. Overall, with respect to genome size, gene number, and transposon content, the P. confluens genome is more similar to those of many higher filamentous ascomycetes than to the truffle genome, indicating that these features in truffle might be a consequence of the specialized life style.
It has been shown previously that the number of introns per gene varies greatly between different fungal lineages, with filamentous ascomycetes harboring one to two introns per kilobase [45]. However, at the time of the study, no Pezizomycete genome sequences were available, therefore we performed an analysis of intron content in P. confluens and T. melanosporum in comparison with nine other filamentous fungi representing major fungal lineages (Figure 3). The comparison included 747 genes for which orthologs could be identified in all analyzed fungi. About 7% of the analyzed P. confluens genes do not contain introns, similar to chytrids and zygomycetes, and less than in most higher ascomycetes where 10–12% of the investigated genes are intron-free. This is also much less than in hemiascomycete yeasts, e.g. S. cerevisiae, where only a minority of genes contains introns [45]. However, it is almost twice the number found in basidiomycetes, where less than 4% of the analyzed genes do not contain introns. The average number of introns per kb is 1.83 in P. confluens, which is larger than that of the higher filamentous ascomcyetes with the exception of Aspergillus nidulans, but still in the same range as found in the previous study [45]. Interestingly, T. melanosporum has 2.49 introns per kb, the highest value of all investigated ascomycetes. These data might indicate that within filamentous ascomcyetes there is a tendency towards net intron loss that is more pronounced in the evolutionary derived lineages than in the basal Pezizomycetes. However, the exceptionally high intron content of T. melanosporum might also be life style-specific or connected to the high repeat content in this fungus.
Fig. 3. Intron content of protein-coding genes from P. confluens and 10 other filamentous fungi. 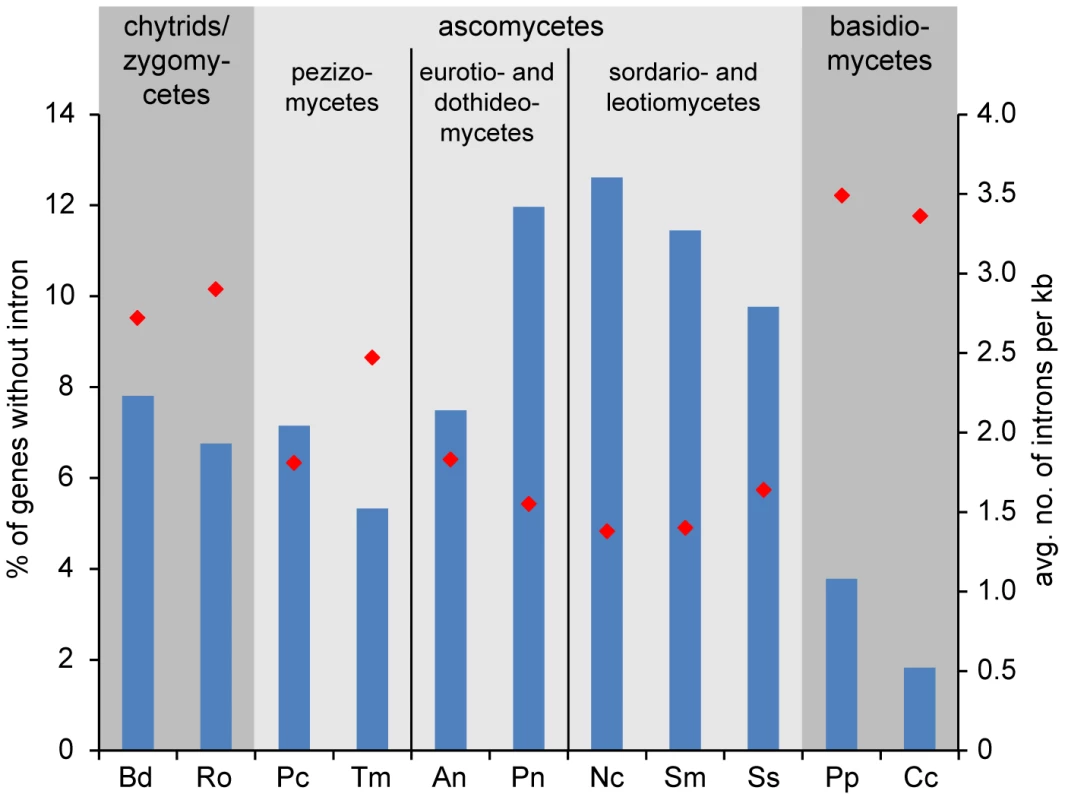
Putative orthologs to P. confluens genes were identified by reciprocal BLASTP analysis, and 747 genes with orthologs across all fungal genomes used were analyzed for intron content (analysis of CDSs only, because UTRs are not annotated in all cases). Blue bars give the percentage of genes without introns (left y-axis), red diamonds give the average number of genes per kb (right y-axis). Data from genome projects for the following species were used for the analysis: Bd, Batrachochytrium dendrobatidis (http://genome.jgi-psf.org/Batde5/Batde5.home.html); Ro, Rhizopus oryzae [207]; Pc, Pyronema confluens (this study); Tm, Tuber melanosporum [9]; An, Aspergillus nidulans [174]; Pn, Phaesphaeria nodorum [179]; Nc, Neurospora crassa [178]; Sm, Sordaria macrospora [22]; Ss, Sclerotinia sclerotiorum [182]; Pp, Postia placenta [208]; Cc, Coprinopsis cinerea [209]. The availability of transcriptome data for P. confluens also allowed us to compare overall expression levels across the genome. In a previous study with metazoans, genes could be grouped in two classes with high and low expression levels, respectively, independent of species, tissue type, or type of experiment [46]. In fungi, this has been addressed only in the filamentous ascomycete Sordaria macrospora where the situation is different, because there were up to three expression peaks depending on the conditions analyzed [24]. Interestingly, the situation in P. confluens is more similar to metazoans, with two main peaks that represent high and low expression in all three conditions investigated (for details see Text S1 and Figure S4).
Analysis of Synteny with Other Fungal Species
The closest relative of P. confluens with a sequenced genome is T. melanosporum, therefore, we used the MUMmer package [47] to determine regions of sequence similarity and possible synteny, i.e. the order of genes within the genome, between the two species. However, even though both species belong to the order Pezizales, there is little sequence similarity at nucleic acid level (data not shown), therefore we used the PROmer algorithm from the MUMmer package to compare the in silico-translated genomic sequences (Figure 4). Even at amino acid level, only ∼11% of the P. confluens genome align with the T. melanosporum genome, compared to more than 66% in the highly syntenic genomes of S. macrospora and N. crassa (Figure 4A and B). A dot plot analysis of the PROmer results also indicated a low degree of overall synteny between P. confluens and T. melanosporum (Figure S5A). In a second analyis of synteny, we identified orthologs for all P. confluens genes in the predicted proteomes of ten filamentous fungi by reciprocal BLAST analysis [48], and used the positions of orthologous proteins on sequenced chromosomes or contigs to determine synteny (Figure S5B). A dot plot for this comparison of P. confluens with T. melanosporum also shows that there is little overall synteny, in contrast to the comparison of the highly syntenic genomes of N. crassa and S. macrospora. The low degree of synteny between P. confluens and T. melanosporum might be explained by their large evolutionary distance. Estimation of divergence times of the Pyronema and Tuber lineages using r8s [49] placed their most recent common ancestor at least 260 Mya (million years ago), nearly twice the time of the estimated divergence of Tuber from its sister groups within the Tuberaceae (∼156 Mya, [50]).
Fig. 4. Synteny analysis with other fungi. 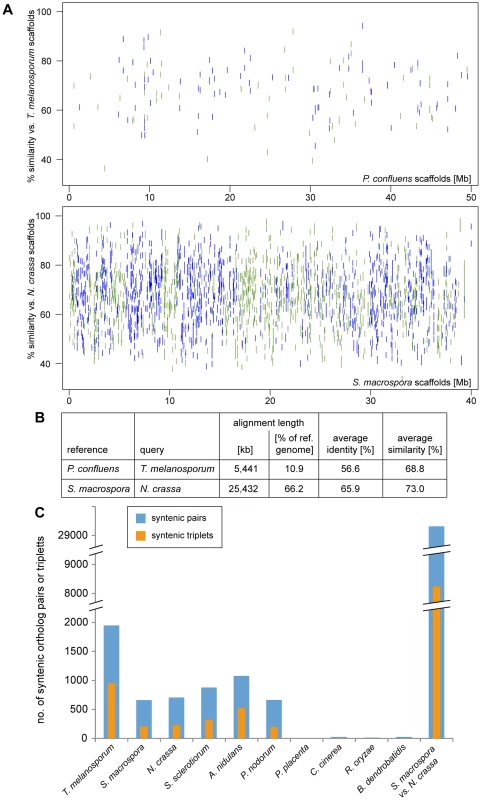
A–B. Regions of sequence identity between the in silico-translated genomic sequences of the pairs T. melanosporum/P. confluens and S. macrospora/N. crassa (for comparison) were determined with the PROmer algorithm from the MUMmer package [47]. The percent identity plot (A) was plotted with gnuplot, green indicates sequences on the forward strand, blue on the reverse strand of the reference. The PROmer analysis shows a much higher percentage of regions of similarity/identity between S. macrospora and N. crassa than between T. melanosporum and P. confluens. C. The number of pairs or triplets of orthologous genes within a 20 kb (pairs) or 40 kb (triplets) region was determined for P. confluens and 10 other fungi. For comparison, the number of syntenic pairs or triplets was also determined for S. macrospora and N. crassa (bars at the right). Note that the y-axis is interrupted in two places for better visualization. Data used for this analysis are from the genome projects as indicated in the legend of Figure 3. Nevertheless, an analysis of the number of syntenic gene pairs or gene triplets showed that although the number of syntenic pairs and triplets in P. confluens versus T. melanosporum is still lower than in N. crassa versus S. macrospora, it is much higher than in comparisons of P. confluens with other ascomycetes (Figure 4C). This indicates that many regions of microsynteny exist between P. confluens and T. melanosporum whereas overall chromosomal synteny was lost. In contrast, in comparisons of P. confluens with basidiomycete, zygomycete, or chytrid species, we found few syntenic gene pairs or triplets.
P. confluens Might Reflect an “Intermediate State” in the Evolution of the Genomic Environment of the Mating Type Loci
In filamentous ascomycetes, the master regulators of sexual reproduction are the various genes that reside at the mating type (MAT) loci [51]. They encode transcription factors that regulate the sexual cycle. Heterothallic ascomycetes have a bipolar mating type system, with isolates possessing one of two non-allelic versions (idiomorphs) of a single MAT locus, termed MAT1-1 and MAT1-2 [52]. The MAT1-1-1 and MAT1-2-1 genes encode transcription factors with a conserved alpha domain and high-mobility group (HGM)-domain, respectively [51]. Conversely, homothallic ascomycetes carry both MAT loci within a single genome, with the two loci either fused together, located within close proximity, or on separate chromosomes [53]–[57]. BLASTP searches with MAT1-1-1 proteins of different filamentous ascomycetes revealed the presence of a MAT1-1-1 gene (PCON_07491, scaffold 329) encoding a putative transcription factor with an alpha domain that is most similar to the MAT1-1-1 protein of T. melanosporum [58] (Table S7). In the majority of Sordariomycetes, two other genes are also located in the MAT1-1 locus: the MAT1-1-2 gene encoding a protein with a PPF domain harboring the three invariant residues proline (P), proline and phenylalanine (F), and the MAT1-1-3 gene encoding a protein with a high-mobility-group (HMG) domain as a DNA-binding motif [55], [59]. Homologs of these two MAT1-1-specific mating type genes could not be identified in the genome of P. confluens.
A BLAST search with MAT1-2-1 HMG domain mating type proteins identified the ORF PCON_08389 (scaffold 381) encoding a HMG domain protein as a putative MAT1-2-1 homolog. The encoded protein displayed the highest degree of identity to the mating type protein MAT1-2-1 of Gibberella indica (Table S7).
The genes APN2, encoding a putative DNA lyase, and SLA2, encoding a cytoskeleton assembly control factor, have been reported to be adjacent to MAT loci in many filamentous ascomycetes [55], [59]–[62]. The MAT1-1 locus of P. confluens is flanked by genes encoding proteins of unknown function (Figure 5, Table S7). A homolog of APN2 (PCON_08385) is located 10 kb upstream of the MAT1-2 locus. A SLA2 homolog (PCON_02178) is also present in the P. confluens genome but neither on scaffold 329 (MAT1-1) nor on scaffold 381 (MAT1-2). Recently the genes flanking the mating type locus of the Pezizomycete T. melanosporum have been identified as GSTUMT00001088001 and GSTUMT00001092001 at the left flank and at the right flank, respectively [58]. Only a homolog of GSTUMT00001088001 is conserved in P. confluens (PCON_01243, scaffold 1068) but is not located adjacent to the mating type genes. Interestingly, PCON_08391 at the right flank of MAT1-2 and PCON_07490 at the left flank of MAT1-1 encode proteins with a high degree of similarity (59.2% identity in 1005 amino acids overlap), and for both proteins the closest homolog in T. melanosporum is GSTUMT0008232001 (Figure 5, Table S7). Aspergilli also contain only one copy of this gene. Therefore, one might hypothesize that this gene was duplicated in P. confluens during a recombination event that led to the presence of both mating type loci in one genome and therefore to homothallism. Indeed the phylogenomic tree in phylomeDB shows that PCON_08391 and PCON_07490 are species-specific paralogs.
Fig. 5. Organization of the mating type loci. 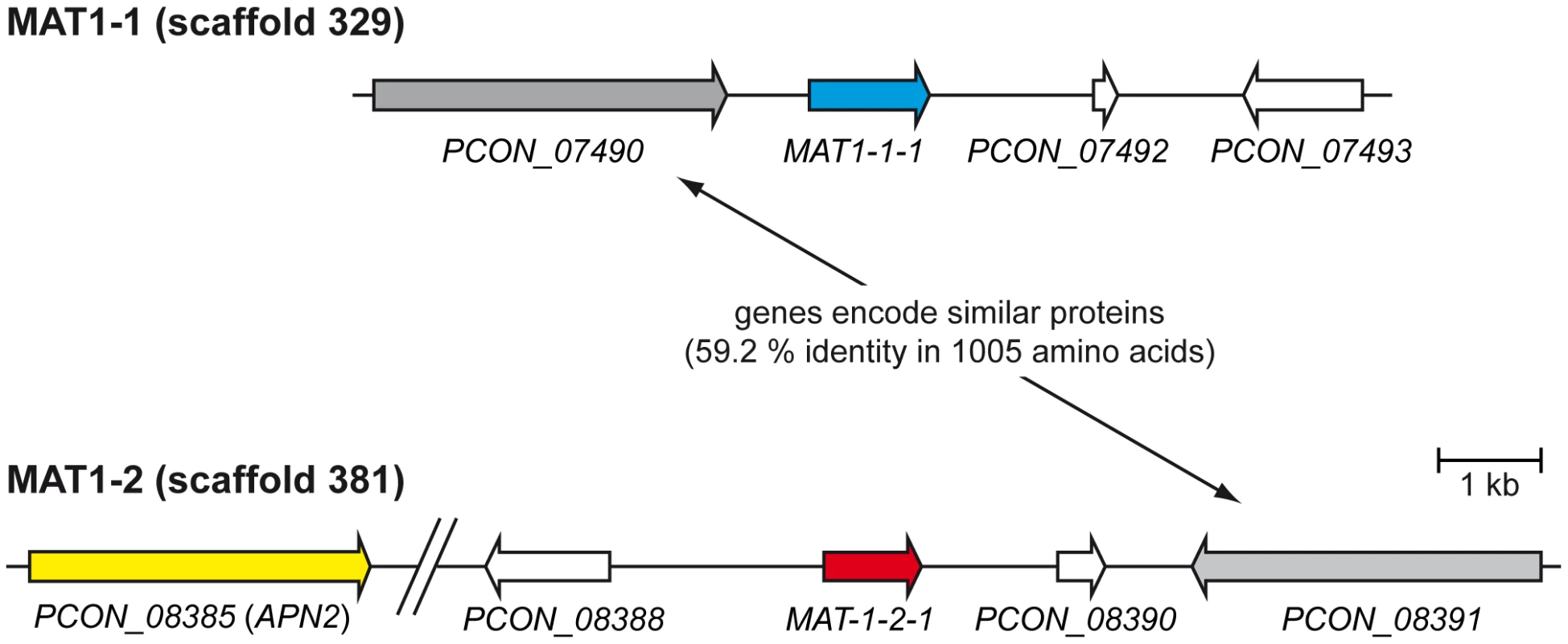
The predicted mating type genes MAT-1-1-1 and MAT-1-2-1 on scaffolds 329 and 381, respectively, are shown together with adjacent genes. The gap in scaffold 381 was introduced for better visibility and contains the predicted genes PCON_08386 and PCON_08387. In summary, the genome of the homothallic P. confluens has two putative MAT loci, typical for homothallic filamentous ascomycetes. The MAT1-1 and MAT1-2 loci encode an alpha domain and an HMG domain transcription factor, respectively. The mating type loci are not fused and not in close proximity, similar to the situation in several Eurotiomycetes [63]. Only the MAT1-2 locus is flanked by the conserved APN2 gene, whereas both MAT loci are flanked by a pair of paralogous genes not found in this location in other ascomycetes (Figure 5). In this respect, P. confluens is more similar to derived filamentous ascomycetes than to its closest sequenced relative, T. melanosporum, where the MAT loci are not flanked by either APN2 or SLA2. Thus, the P. confluens MAT loci might reflect a putative intermediate state between the relatively conserved genomic arrangement of mating type loci found in higher filamentous ascomycetes and the specific arrangements found in T. melanosporum.
We also searched for homologs to other proteins involved in sexual development and signaling, e.g. pheromone and pheromone receptor genes as well as genes involved in pheromone processing and downstream signaling. With the exception of pheromone genes, which are weakly conserved in ascomycetes, conserved genes for sexual development were found in P. confluens, too (for details see Text S2 and Table S7).
Many Orphan Genes are Upregulated during Sexual Development
A quantitative analysis of gene expression across the P. confluens genome was performed based on RNA-seq (Table S2). Of the 13,369 annotated genes, only 58 were not expressed (i.e. have no RNA-seq reads mapped to their exon sequences) in any of the conditions tested. Our analysis was focused on development-dependent gene expression with one tested condition allowing sexual development (sex), and two conditions that only allow the formation of vegetative mycelium (DD and vegmix). Genes that are regulated mostly by developmental factors should be differentially expressed in the comparisons sex/DD and sex/vegmix, but not DD/vegmix. Of the predicted 13,369 protein-coding genes, 5,565 (41%) are regulated differentially in at least one of the three comparisons with thresholds >2 or <0.5, and 3,616 genes (27%) are differentially regulated in at least one comparison with thresholds >4 and <0.25. With the less stringent thresholds, 506 (4%) genes are downregulated and 1,804 (13%) genes are upregulated in both sex/DD and sex/vegmix, but not differentially regulated in DD/vegmix; with the more stringent thresholds, the numbers are 229 (2%) and 1,460 (11%) genes.
Studies in animals and plants have shown that genes associated with sexual reproduction evolve more rapidly than genes with other functions [64], [65]. This can be observed not only for single genes, but across genes that are transcriptionally expressed in organs involved in sexual reproduction [66]. In fungi, few studies have addressed this question so far. A genome comparison of Candida and related yeast species showed that meiotic genes undergo rapid evolution [67], and similar findings were made in studies of mating type and pheromone signaling genes in filamentous ascomycetes [68], [69]. A recent EST analysis of Neurospora intermedia and comparison with other Neurospora species indicated that sex-associated genes, i.e. those genes that are preferentially expressed during sexual development, are rapidly evolving in fungi, too [70]. Here, we approached this question from a different angle by analyzing gene expression levels for P. confluens genes with different degrees of evolutionary conservation to find out if genes with different lineage-specificities are preferentially expressed under any of the conditions that we investigated (Figure 6, Table S8). First, we extended our orthology analysis as described in the previous section to include the predicted proteomes of 14 fungi from the major fungal groups (chytrids, zygomycetes, ascomycetes, basidiomycetes), adding two ascomycetous yeasts and an additional chytrid to the previous dataset (Table S8). For downstream analysis, only genes without hits (orphan genes) or with clear reciprocal BLAST hits (orthologs) were used. Genes that are members of gene families with more than one paralog where clear orthologs could not be determined in the analyzed genomes were excluded from the analysis. This left 6,706 P. confluens genes in the final analysis that were sorted in six lineage-specific groups (a–f) ranging from P. confluens orphan genes (the largest group with 5,737 genes) to genes that are conserved in all analyzed fungal genomes (Figure 6, Table S8). For these genes, we analyzed derived peptide lengths, and expression in the three conditions sex, DD, and vegmix.
Fig. 6. Lineage-specific peptide lengths and gene expression. 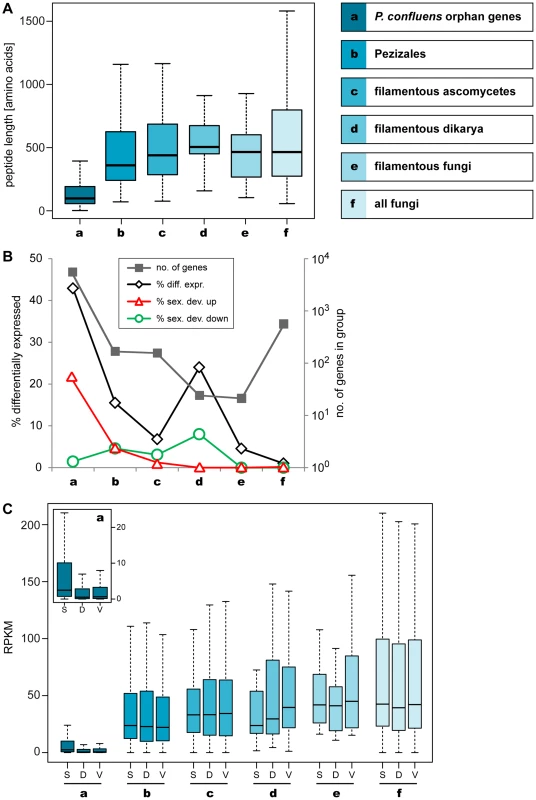
Peptide lengths and expression of P. confluens genes from groups of genes with different levels of evolutionary conservation as indicated. A. Boxplot showing the distribution of peptide lengths (outliers left out for better visibility) with the median value as a horizontal line in the box between the first and third quartiles. Peptide length of predicted P. confluens orphan genes are smaller than those for the other groups, and from groups a to d, median peptide lengths increase with increasing conservation of genes. B. The number of genes in each group are indicated on the logarithmic y-axis to the right, and percentage of genes that are differentially regulated under any condition, and up- or down-regulated during sexual development (up or down in sex/DD and sex/vegmix, data shown for stringent expression analysis) are indicated on the y-axis to the left. The orphan genes have the highest percentage of differentially expressed genes, most of which are upregulated during sexual development. In the other groups, the portion of differentially regulated genes is smaller, and the percentage of genes upregulated during sexual development is similar or smaller than that of downregulated genes. C. Overall expression levels given in RPKM (reads per kilobase per million counted reads). For each group, RPKM values were calculated for samples sexual development (S), DD (D), and vegmix (V) as mean RPKM values of the two independent experiments. The boxplot shows the distribution with the median value as a horizontal line in the box between the first and third quartiles (outliers left out for better visibility). The small inlet shows a magnification of the RPKM values for group a (orphan genes). In this group, overall expression is lower, but the distribution of RPKM values for S is significantly different from D and V samples (p<0.01 in Kolmogorov-Smirnov-test). Within the other groups, no significantly different distribution between S, V, and D can be observed. Median peptide lengths were shortest in the orphan genes (Figure 6A). This is consistent with observations from a broad range of organisms where conserved proteins are on average longer than poorly conserved proteins [71]. However, at least some of the short peptides may be artifacts of annotation problems, because no homology-based information to aid annotation was available for these genes. But while some of these genes might be due to spurious annotation, more than 40% of the orphan genes are differentially expressed in at least one condition, which might indicate some functionality (Figure 6B). Interestingly, more than 20% of the orphan genes are upregulated during sexual development (in sex/DD and sex/vegmix), while less than 2% are downregulated. This percentage of differentially expressed genes as well as genes upregulated during sexual development is much higher than in all the other groups where less than 5% of genes are upregulated during sexual development. Furthermore, the percentage of up - and downregulated genes is not much different in the other groups.
These trends were also observed when this analysis was performed with lineage-specificity groups obtained from phylogenomics analysis (Figure S6, Tables S8 and S9). This analysis allowed the differentiation between P. confluens orphan genes, Pezizales-specific genes, and genes that are specific to Pezizales and Orbiliales; and an increase in peptide lengths as well as a decrease in the percentage of genes that are upregulated during sexual development is correlated with decreasing lineage specificity (Figure S6).
The expression trends can also be seen when analyzing overall expression levels as measured by RPKM (reads per kilobase per million counted reads) values (Figure 6C, Figure S6C). Overall median expression is significantly lower for orphan genes than for the other groups, with a strong increase in Pezizales-specific genes and further slight increase in genes specific to filamentous ascomycetes. A general trend for more conserved genes to have higher expression has also been observed in other organisms [72], [73]. When looking at RPKM values in the three conditions tested, there are no significant differences between conditions within the lineage-specific groups with the exception of the orphan genes. In this group, the median expression is significantly higher in the sexual development condition. Thus, while overall expression is lower for orphan genes, this group comprises much more genes with specific expression during sexual development than the more conserved groups.
There are several hypotheses to explain this finding. One would be the above-mentioned rapid evolution of sex-associated genes leading to apparent orphan genes in its most extreme form. This should be observed especially in species where no sequence information is available for close relatives, as is the case with P. confluens. Increased evolutionary rates have generally been observed in genes with higher lineage-specificity, independent of putative function, in an analysis of seven ascomycete genomes [74]. However, there are other mechanisms that may lead to the presence of orphan genes. One is gene loss in all but one (observed) species, although this is unlikely to occur on a larger scale, i.e. for thousands of genes in a single species. Another is the de novo gene birth from previously non-coding sequences, a process that in recent years was acknowledged as probably being more common than previously thought [75], [76]. One might speculate that the high number of sex-associated orphan or less conserved genes in P. confluens indicates that sexual development allows the “testing” of novel gene-inventions. This might be feasible in filamentous fungi where sexual development is usually not the only means of propagation, and therefore novel genes that are deleterious for sexual reproduction under some circumstances might be retained by purely vegetative propagation until more compatible conditions occur. Another reason could be a more general trend for less conserved genes to be involved in group - or species-specific processes as was found in an analysis of gene expression during different stages of vegetative growth and conidiation in N. crassa [77]. Further analyses of more species and transcriptomes from different conditions will be necessary to address these questions.
Light-Dependent Development and Expression of Photoreceptor Genes
It has been shown in many fungi that light can cause developmental changes. In several ascomycetes, illumination promotes vegetative reproduction via conidiospores, while sexual reproduction is observed in darkness [78]. In contrast to this, it was noted already at the beginning of the last century that the formation of apothecia in P. confluens is light-dependent [13], [21], and this was confirmed by our studies. Both constant illumination (LL) as well as a 12 h photoperiod promote fruiting body formation, whereas in constant darkness (DD), P. confluens is sterile (Figure S7, Figure 7). This complete light-dependency of fruiting body development is uncommon in ascomycetes. It was discussed that perithecia formation in Trichoderma reseei might be activated by light [79], but it was shown later on that sexual development in this fungus can also be observed in darkness [80]. However, light-dependent fruiting body development was observed in several basidiomycetes, e.g. Schizophyllum commune and Coprinopsis cinerea, and in both species, blue light constitutes the effective part of the visible spectrum [81]–[83]. We found that this is also the case for P. confluens where blue (400–500 nm) but not green or red light allows fruiting body formation (Figure 7A). This confirms an early study from the 1920s that found wavelengths of 400–550 nm to promote sexual development in this fungus [21].
Fig. 7. Fruiting body development and gene expression under different light regimes. 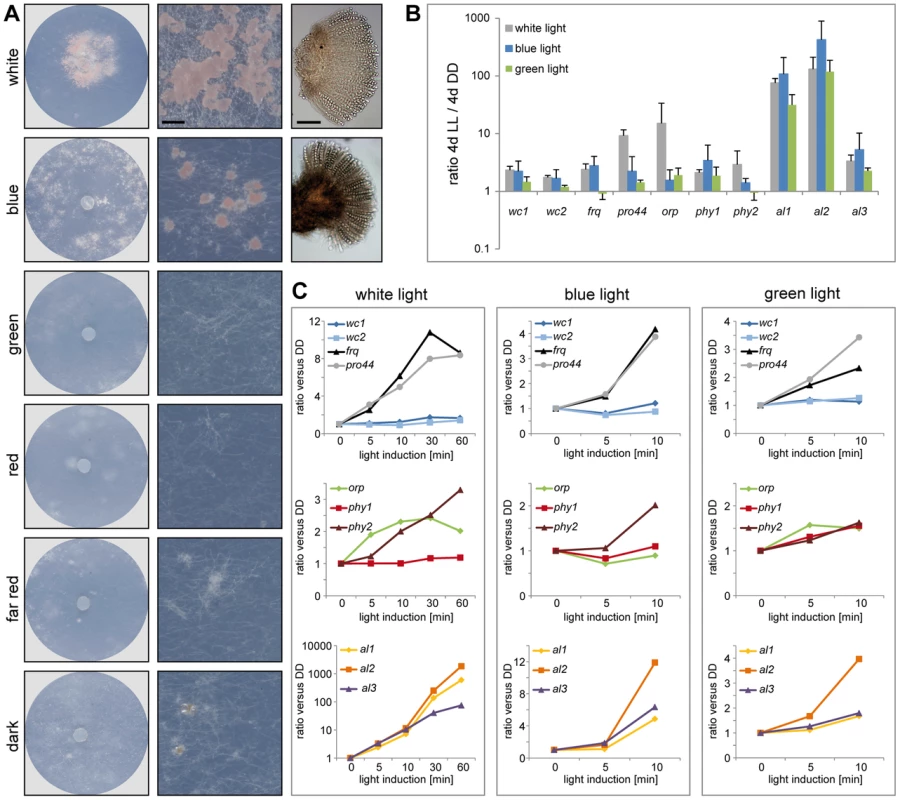
A. Fruiting body development in P. confluens is blue light-dependent. P. confluens was grown on minimal medium in constant light of different wavelengths as indicated (for filter characteristics see Figure S8). The left column shows petri dishes, the middle column sections from petri dishes (bar 500 µm), and the right column shows mature apothecia in those cases where fruiting bodies are formed (bar 100 µm). Blue light is the effective part of the visible spectrum and induces apothecia formation, similar to white light. Under other light conditions or in darkness, sometimes mycelial aggreagates are formed that can be darkly pigmented, but do not contain sexual structures (e.g. visible in section from petri dish in the dark). B and C. Expression of homologs of genes that are involved in blue light responses (wc1, wc2, frq), in light responses and/or fruiting body formation (pro44), are photoreceptors for other wavelengths (orp1, phy1, phy2) or carotenoid biosynthesis genes (al1, al2, al3) in other fungi (Table 2). Transcript levels after 4 d of illumination with white, blue, or green light compared to 4 d in darkness (DD) are shown in B, short term light induction (5–60 min after growth in darkness for 4 d) in C. P. confluens was grown in minimal liquid medium and harvested under far-red light. Transcript levels were determined by qRT-PCR from at least two independent biological replicates; ratios versus DD samples are shown, error bars in B indicate standard deviation, for standard deviations for short term light induction in C, see Table S10. Despite the fact that no phenotypic responses to wavelengths other than blue have been observed in P. confluens yet, its genome encodes putative photoreceptors that cover a range of wavelengths (Table 2), and most of these genes are expressed (Figure 7, Table S10). This includes homologs of N. crassa WC-1 and WC-2 (PCON_03119 and PCON_05086), transcription factor/photoreceptor proteins that are part of the white collar complex and mediate all blue-light responses [84]–[88]; however, no homolog was found for the VVD protein that functions in light adaptation in N. crassa and T. reseei [80], [89], [90]. Two putative phytochromes (PCON_06747 and PCON_08526) are present in the P. confluens genome, the first of which is orthologous to FphA that was shown to be the photoreceptor mediating repression of sexual development by red light in A. nidulans [91]. P. confluens also encodes an ortholog of the N. crassa opsin-related protein (PCON_01637) that lacks a conserved lysine residue for chromophore binding, but has no homolog of the rhodopsin NOP-1, a putative green-light receptor [92]–[94] (Table 2). BLAST searches in the T. melanosporum genome also failed to identify a NOP-1 homolog, suggesting that the Pezizales might lack a gene for this type of photoreceptor. A gene encoding a putative cryptochrome is present in P. confluens (PCON_04132, Table 2), but was the only putative photoreceptor gene for which no expression could be detected under the conditions tested. All other putative photoreceptor genes are expressed, and induced by white light in long-term illumination experiments (4 d LL versus 4 d DD, Figure 7B), but only moderately or not induced by short-term light pulses (from 5 to 60 min, Figure 7C). Interestingly, we also observed some induction with green light for several of the genes, which is surprising because P. confluens lacks a rhodopsin-type receptor that was hypothesized to mediate green-light responses in fungi [92]. However, the green-light filter we used has a slight transmission wavelength overlap with the blue light filter (Figure S8), therefore at least part of the green-light responses might be mediated by residual blue light. Nevertheless, P. confluens seems to have some green-light sensitivity, because the orp gene is light-induced in the short - and long-term illumination experiments, and this effect is stronger with green light than with blue light (Figure 7, B and C).
Tab. 2. P. confluens homologs of photoreceptor genes, genes involved in light signaling or light-regulated carotenoid biosynthesis genes in other fungi. 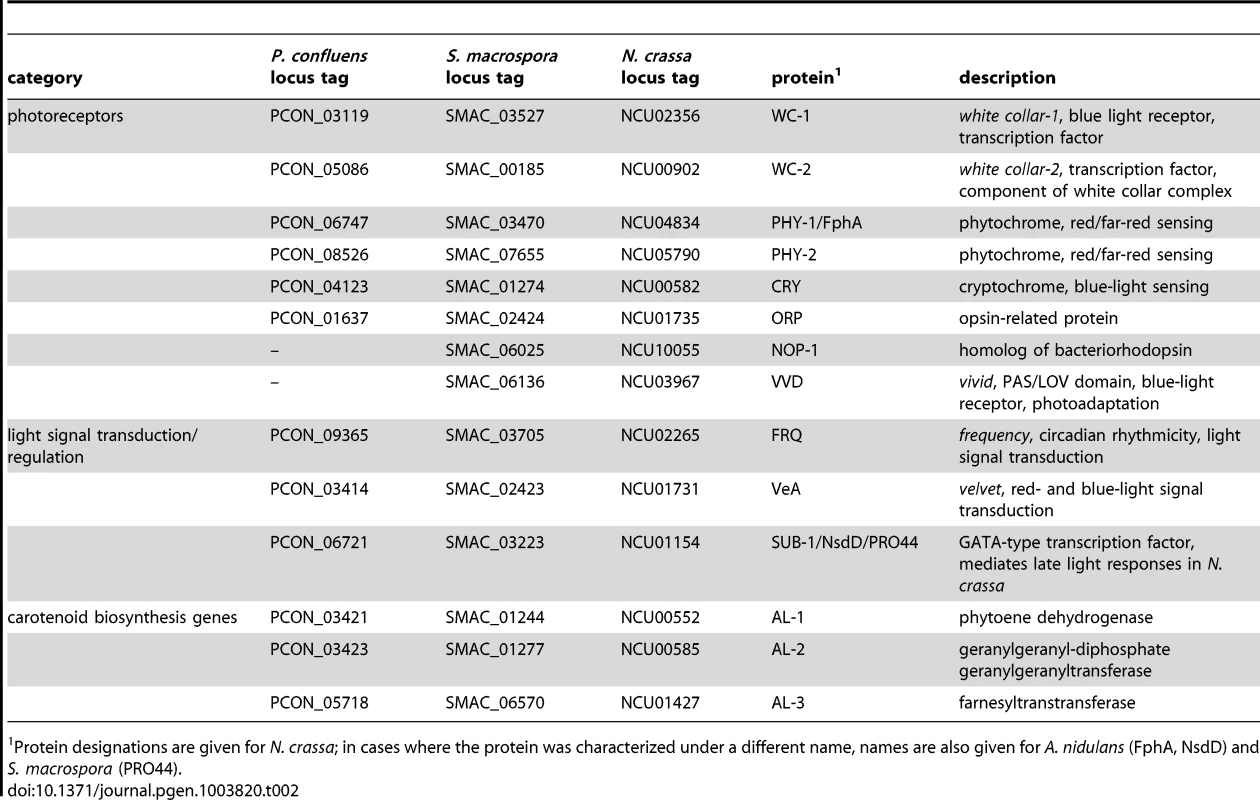
Protein designations are given for N. crassa; in cases where the protein was characterized under a different name, names are also given for A. nidulans (FphA, NsdD) and S. macrospora (PRO44). We also looked for homologs to genes that act downstream of photoreceptors in light signal transduction in other fungi. Interestingly, P. confluens contains a frequency (frq) homolog (PCON_09365), and thus is the most distant relative of N. crassa in which this gene is found. The frq gene encodes the main regulator of circadian rhythmicity in N. crassa, and is a direct target of the white collar complex, but so far frq homologs were only found in Sordariomycetes, Dothideomycetes, and Leotiomycetes [95], [96]. The identification of a frq homolog in P. confluens suggests that frq was present in the ancestor of filamentous ascomycetes and was lost several times during evolution, because the T. melanosporum genome does not contain a frq homolog (data not shown), and no homolog has been detected in the Eurotiomycetes [95], [96]. Similar to N. crassa [84], [97], the P. confluens frq is strongly induced by short light pulses, and this reaction is mainly mediated by blue light (Figure 7C). frq was also upregulated in the long-term illumination experiments (Figure 7B).
In N. crassa, an antisense transcript of frq is also upregulated by light, and is involved in light-dependent resetting of the circadian clock [98]. Analysis of antisense splice sites did not show any NATs for the P. confluens frq, although we cannot exclude the possibility of non-spliced antisense transcripts with this analysis. However, a splice site analysis of RNA-seq reads for frq indicated that there might be an alternatively spliced intron in the sense direction overlapping the predicted start codon of the open reading frame (Figure S9). In N. crassa, an intron overlapping the frq start codon is alternatively spliced resulting in two different forms of the FRQ protein [99], [100]. To determine whether a similar mechanism might occur in P. confluens, we performed RT-PCR analysis of a region covering the predicted alternatively spliced intron. Interestingly, there are indeed two different transcript, and alternative splicing of the intron is light-dependent with an increased ratio of spliced versus non-spliced transcript in the light (Figure S9). In the N. crassa frq, alternative splicing of the AUG-covering intron is temperature-dependent [99], [100], therefore it seems that similar principles might be at work in these two distantly related fungi, but with different input signals.
We also identified an ortholog for the GATA-type transcription factor NsdD/SUB-1/PRO44 (PCON_06721) that was shown to mediate late light responses in N. crassa [101] and is essential for sexual development in A. nidulans, Aspergillus fumigatus, N. crassa and S. macrospora [102]–[105]. The developmental function of this protein appears to be conserved (see later section), and expression analyses indicate that light responses, and therefore regulatory activities, might also be similar to those in higher fungi, because the P. confluens pro44 is strongly light induced after long - and short-term illumination (Figure 7).
We also searched for homologs to carotenoid biosynthesis genes that are known to be light-induced in other fungi [78], [106], [107]. P. confluens encodes homologs to the three enzymes AL-1, AL-2, and AL-3 from N. crassa [108]–[111], and the corresponding al genes (PCON_03421, PCON_03432, and PCON_05718) are strongly light-induced under short - and long-term illumination (Figure 7B, C). Under long-term illumination, blue light has an even stronger effect than white light on al gene expression. It has been shown in a previous biochemical analysis that the orange-pinkish pigments that characterize P. confluens cultures grown in white or blue light (Figure 7A) are carotenoids [112]. Thus, assuming a function of the al genes in P. confluens similar to that in N. crassa, it seems likely that these carotenoids are synthesized by the products of the al genes. Analysis of cultures grown on complete medium that does not support fruiting body formation even in the light indicates that pigment synthesis is independent of fruiting body formation, because light-grown cultures are pigmented even in the absence of apothecia formation (Figure S7).
In summary, our data suggest that light-sensing and signal transduction in P. confluens might be comparable to mechanisms in the distantly related species N. crassa, and might thus be conserved to a large degree in filamentous ascomycetes. However, output from the light-signaling pathways might be somewhat different, because fruiting body development is strictly light-dependent in P. confluens, but not in most other ascomycetes. Blue light has the strongest effect on both morphological as well as gene expression phenotypes, but our data hint at sensitivity to other wavelengths, too, especially in the green part of the visible spectrum. Similar findings were made in S. macrospora, where phenotypic changes were observed in response to green light, and in N. crassa, where gene expression was found to be modified in mutants of the putative green-light receptor NOP-1 [22], [113].
Gene Family Expansions and Contractions in P. confluens
We searched for conserved protein domains in the predicted proteins from P. confluens and seven other filamentous fungi to identify protein families that are expanded in P. confluens (Table S11). Among the expanded gene families are two that encode mostly small, extracellular proteins, namely CBM_14 and Defensin_2 domain proteins. The CBM_14 domain (Chitin binding Peritrophin-A domain) is mainly found in metazoa, and in fungi so far has been described only in the Avr4 protein from Cladosporium fulvum and, with one gene per genome, in the genomic sequences from several Aspergilli [114], [115]. BLASTP analysis in GenBank revealed that there are also some predicted CBM_14 proteins in other Eurotiales (Figure 8A), but not in other fungal groups. In contrast, there are 13 proteins with CBM_14 domain in P. confluens (Figure 8A). All of these are predicted as extracellular and have a putative cleavable N-terminal signal peptide for co-translational insertion into the ER (data not shown). They are mostly 80–140 amino acids long with the exception of PCON_04108 (351 amino acids), and contain no other recognized domains besides CBM_14. Some of the corresponding genes are clustered within the same genomic region, indicating that the genes might have arisen through duplications at certain gene loci: PCON_09939, PCON_09940, PCON_09946, and PCON_09947 lie within 20 kb of scaffold 486, and PCON_05983 and PCON_05987 lie within 9 kb on scaffold 228.
Fig. 8. The CBM_14 domain protein family is expanded in P. confluens. 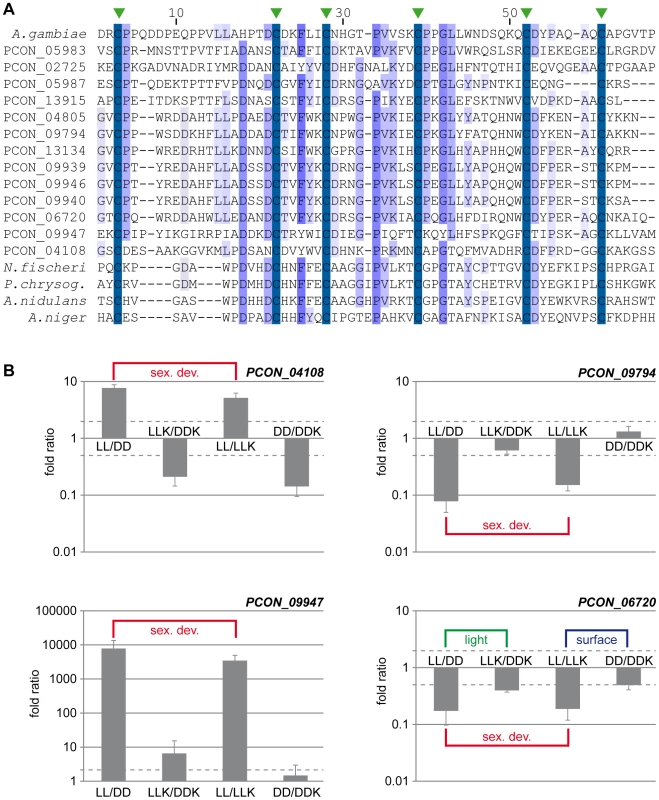
A. Multiple alignment of the CBM_14 domains of proteins from P. confluens, several Eurotiales and an insect. In addition to the P. confluens proteins, the following proteins were used for Clustal X analysis: A. gambiae, Anopheles gambiae sp|O76217.2; A. nidulans, Aspergillus nidulans ANID_00499 from the A. nidulans genome project http://www.broadinstitute.org/annotation/genome/aspergillus_group/MultiHome.html; A. niger, Aspergillus niger ref|XP_001397263.1; N. fischeri, Neosartorya fischeri ref|XP_001266454.1; P. chrysog., Penicillium chrysogenum ref|XP_002561001.1. The conserved cysteine residues are indicated by green arrowheads above the sequence. B. Quantitative real time PCR analysis of selected CBM_14 domain-encoding genes from P. confluens. Expression was analyzed for each gene in two independent biological replicates for the four conditions LL, DD, LLK and DDK (light and darkness in surface culture and submerged culture). Sexual development is only possible in condition LL. The graph shows mean and standard deviation (for better visualization, standard deviations for negative expression ratios are shown in the negative instead of the positive direction). Expression ratios were calculated to address the question if a gene is differentially regulated during sexual development (i.e. in LL/DD and in LL/LLK), or regulated by light (i.e. in LL/DD and LLK/DDK) or regulated by surface versus submerged growth (i.e. LL/LLK and DD/DDK). The RNA-seq data show that more than half of the CBM_14 domain proteins are upregulated during sexual development. Furthermore, the overall expression levels of these proteins vary greatly ranging from no sequence reads in certain conditions to >13,000 reads (normalized to kb of mRNA, Figure S10). For example, the clustered genes PCON_09939 to PCON_09947 are preferentially or only expressed during sexual development, while others are more strongly expressed under non-sexual conditions. To address the expression of some CBM_14 domain genes in more detail and distinguish between regulation by sexual development, light, and growth conditions (surface versus submerged), we performed qRT-PCR for four genes under the conditions LL, DD, LLK and DDK (light and darkness in surface culture and submerged culture, Figure 8B). These combinations distinguish if a gene is differentially regulated during sexual development (i.e. in LL/DD and in LL/LLK), or regulated by light (i.e. in LL/DD and LLK/DDK) or regulated by surface versus submerged growth (i.e. in LL/LLK and DD/DDK). PCON_04108 and PCON_09947 are upregulated during sexual development, but not consistently regulated by light or surface culture (PCON_09947 is slightly upregulated by light, but the extent of regulation is far lower than the development-dependent regulation). PCON_09794 is downregulated during sexual development, but not regulated by the other two stimuli, whereas PCON_06720 is downregulated during sexual development and (to a lesser extent) downregulated in the light and in surface cultures. Overall, the four genes have distinct expression patterns, which might indicate that they are functional in P. confluens. The Avr4 protein from the phytopathogenic C. fulvum was shown to bind chitin and protect it from hydrolysis by plant chitinases [115]. P. confluens is non-pathogenic, but one might speculate that secreted CBM_14 domain proteins might protect the fungus from microbial attacks in its soil habitat.
A second expanded gene family encoding small, secreted proteins is the Defensin_2 family (Table S11). Defensin_2 domain proteins are mostly known from arthropods where they are part of the immune system and act against bacteria [116], [117]. In fungi, only one Defensin_2 domain protein has been described in detail, namely Plectasin from Pseudoplectania nigrella, a member of the Pezizales [118], [119]. In P. confluens, the family comprises six members (PCON_01606 to PCON_01611), all of which are between 92–96 amino acids long, including predicted signal peptides of 15–23 amino acids, and are encoded by a cluster of genes within 10 kb on scaffold 1117 (Figure S11A and B). Two of the genes (PCON_01607 and PCON_01611) are pseudogenes, and interestingly the expression of the two pseudogenes is much lower than that of the other four genes under all conditions investigated by RNA-seq (Figure S11C). Both pseudogenes have one of the functional Defensin genes as closest homolog (Figure S11D), and one might speculate that they have arisen from gene duplications within the Defensin gene cluster, but lost (most of) their expression and function which was retained by their closest homologs. Interestingly, all genes including the pseudogenes are downregulated during sexual development (Figure S11C). However, a comparison of intergenic regions showed that these are not conserved, in contrast to the coding regions. On the one hand, this might indicate that the regulatory sequences responsible for development-specific regulation are too small or non-conserved to be detected in these comparisons. Another explanation could be that regulation is achieved through chromatin organization of the complete gene cluster, similar to what was described for secondary metabolism gene clusters in fungi [120], [121].
In addition to the Defensin_2 domain proteins in P. confluens and P. nigrella, searches in other sequenced fungal genomes identified Defensin_2 domain proteins only in Eurotiomycetes (Figure S8D). This phylogenetic distribution is similar to that of the CBM_14 domain proteins. One might speculate that both classes of small, secreted proteins arose from horizontal transfer events from insects into fungi; alternatively, this could be a case of gene loss or rapid evolution in the other ascomycete groups. Horizontal gene transfer has been acknowledged as an important mechanism in fungal evolution only in recent years, and the availability of genome sequences has made in-depth analyses possible [122]. A transfer event of carotenoid biosynthesis genes from fungi into insects has been shown already [123], therefore it seems possible that a transfer in the reverse direction might also occur. Interestingly, both the Defensin_2 as well as the CBM_14 domain proteins might be involved in defense mechanisms against microorganisms in arthropods. One might hypothesize that the corresponding genes were acquired by fungi through horizontal gene transfer, and were retained because they offer a selective advantage in the microorganism-rich soil habitat.
A third gene family that is expanded in P. confluens compared to T. melanosporum (but not to other ascomycetes) comprises genes with HET (heterokaryon incompatibility protein) domains. While there are only two HET-domain containing protein in T. melanosporum [124], there are 15 in P. confluens (Table S11). However, there are 11–101 predicted HET domain proteins in various species of higher filamentous ascomycetes, therefore the number in T. melanosporum might have been reduced by selective gene loss. In the Sordariomycetes N. crassa and Podospora anserina, HET domain proteins were shown to mediate heterokaryon incompatibility (HI) [125], [126]. Homologs to many known HI proteins can be found in P. confluens (Table S12); however, none of the P. confluens HET-domain proteins contains additional WD repeat, NACHT, leucine - or glycine-rich repeat domains that are found in the HET domain in HI proteins from N. crassa and P. anserina [127]. Therefore, if HI is present in P. confluens, it is unlikely to be mediated by the same proteins that regulate HI in Sordariomycetes.
A number of protein families are have fewer members or are missing in P. confluens compared to more derived ascomycete groups (Table S11). Most prominent among these are gene families involved in secondary metabolism (see next section), transporter protein families, and several gene families involved in carbohydrate metabolism. The low number of genes for some transporter families might be connected to the limited capabilities for the production (and presumably export) of secondary metabolites; whereas the reduced number of genes for certain enzymes involved carbohydrate metabolism might either be a niche-specific adaptation or indicate that the expansion of carbohydrate-activating enzymes occured only in more derived ascomycete groups. Overall, gene family contraction in P. melanosporum is much less prominent than in T. melanosporum [9].
Genes for Secondary Metabolism
In contrast to the gene families described in the previous section, genes encoding enzymes for the biosynthesis of polyketides or non-ribosomal peptides, typical secondary metabolites of filamentous ascomycetes, are underrepresented in the P. confluens genome (Figure S12A). There are seven putative non-ribosomal peptide synthase (NRPS) genes, and one polyketide synthase (PKS) gene, much fewer than in the genomes of higher filamentous ascomycetes [128]–[130]. The predicted NRPS protein PCON_02859 has the typical domain structure of siderophore NRPSs and is part of a cluster of genes homologs of which are involved in siderophore biosynthesis in other fungi [131], [132] (Figure S12B). A second putative NRPS gene (PCON_07777) is not clustered and does not have homology to NRPSs with known function. The remaining five NRPS genes all have a domain structure that is typical for alpha-aminoadipate reductase (AAR)-type NRPSs (Figure S12A), and (with the exception of PCON_04030) all have high sequence similarity to aminoadipate semialdehyde dehydrogenase, an enzyme of lysine biosynthesis that is conserved in fungi [129]. Most fungi have only one AAR-type NRPS [129], therefore the high number of corresponding genes in P. confluens is somewhat unusual. Possible explanations may be selective amplification of this specific gene family or loss of most other NRPS genes with exception of AAA-type NRPS genes. However, at least PCON_04030 might have a function other than lysine biosynthesis, because the gene is located adjacent to the single PKS gene (PCON_04029) in a gene cluster that also contains other genes encoding enzymes that might be involved in the biosynthesis of secondary metabolites (Figure S12C). The genes in this cluster might be involved in the production of a hybrid polyketide/non-ribosomal peptide. The existence of gene clusters encoding separate PKS and NRPS proteins that act in a common biosynthetic pathway was demonstrated, for example, in A. nidulans, where such a cluster is responsible for the production of Emericellamide [133].
The single predicted PKS PCON_04029 is a type I PKS. In filamentous ascomycetes, there is usually one type III PKS encoded in the genome [130], but type III PKSs are missing in P. confluens. A low number of PKS and NRPS genes was also found in T. melanosporum, and therefore seems to be typical for lower filamentous ascomycetes rather than a result of the truffle-specific life-style [9]. In summary, our analysis shows that the presence and clustering of NRPS and PKS genes is already established in P. confluens. In combination with the fact that no PKS genes and only the single AAR-type NRPS gene were found in Taphrinomycotina, Saccharomycotina, and zygomycete genomes [130], this suggests that the evolution and expansion of PKS and NRPS gene families began in a common ancestor of filamentous ascomycetes, whereas the evolution of type III PKS genes might be a later event that occurred in higher filamentous ascomycetes. However, at present it cannot be excluded that the low number of putative PKS and NRPS genes is an adaptation to specific ecological niches in both T. melanosporum and P. confluens [134], [135]; more Pezizomycete genome sequences will be needed to resolve this question.
Genes Encoding Putative Transcription Factors
The number of putative transcription factor genes (excluding general transcription factors that regulate RNA polymerase) in filamentous fungi varies from 182 in N. crassa to more than 600 to 800 in several Fusarium species [39], [136]–[138]. In truffle, 201 transcription factor genes were predicted [139], while our survey of the P. confluens genome indicated 177 putative transcription factor genes (Table 3, Table S13). Similar to other filamentous ascomycetes, the largest group comprises putative Zn2-Cys6 binuclear cluster (Zn cluster) proteins; thus, the regulatory capacity of P. confluens appears to be similar to that of other filamentous fungi. 54 of the putative transcription factor genes are differentially expressed in at least one of the comparisons that were investigated (Figure S13). Eight genes are strongly upregulated during sexual development, and among these is PCON_02619, the gene encoding the ortholog of STE12, a transcription factor that was shown to be involved in sexual development in yeast and filamentous ascomycetes [140]–[143]. Expression of PCON_02619 and five additional transcription factor genes was characterized in more detail by qRT-PCR (Figure S14). Development-dependent expression was confirmed for those genes that were predicted to be differentially regulated during sexual development by the RNA-seq analysis, showing that our sampling strategy is indeed suitable for identifying developmentally regulated genes. The STE12 ortholog PCON_02619 was confirmed as one of the most strongly upregulated transcription factors during sexual development. Interestingly, the corresponding SteA gene in truffle is downregulated in fruiting bodies [139], suggesting a functional diversification of this conserved transcription factor among Pezizales.
Tab. 3. Overview of putative transcription factor genes. 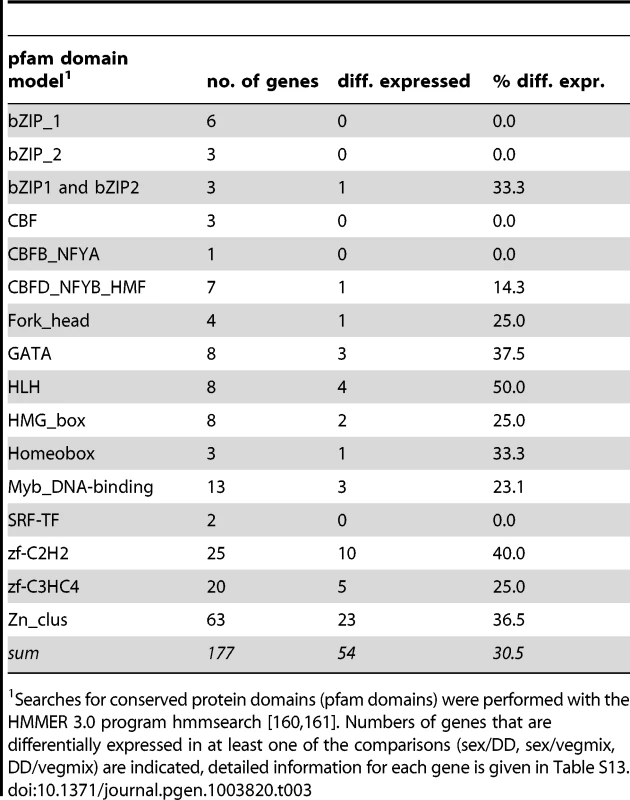
Searches for conserved protein domains (pfam domains) were performed with the HMMER 3.0 program hmmsearch [160], [161]. Numbers of genes that are differentially expressed in at least one of the comparisons (sex/DD, sex/vegmix, DD/vegmix) are indicated, detailed information for each gene is given in Table S13. Comparative Expression Analysis to Identify Genes with Conserved Expression Patterns during Sexual Development
Comparison of gene expression patterns can serve to identify core genes that are involved in biological processes, because conservation of expression is a strong indicator for functional significance [144], [145]. In previous studies, we have already demonstrated that development-dependent expression of several genes is conserved in P. confluens and other, more derived filamentous ascomycetes, and that conservation of gene expression can be used as a criterion to identify genes that play a role during sexual development in fungi [6], [18], [19]. Here, we compared the RNA-seq results from P. confluens with published data from different developmental stages of S. macrospora [24] (Table S14). A cluster analysis of RPKM values for all orthologous gene pairs showed that overall expression patterns in total vegetative or sexual mycelia from S. macrospora are more similar to those from total mycelia from P. confluens than to expression patterns from isolated young fruiting bodies (protoperithecia) of S. macrospora (Figure 9). Similar results were obtained in a cluster analysis of expression ratios where comparisons of S. macrospora protoperithecia with total mycelia cluster separately from other comparisons (Figure S15). This indicates that similar tissues/organs in different species might have more similar expression patterns than different tissues/organs from the same species; in other words, tissue/organ-specific gene expression might be conserved across fungi. While more comparative studies of specific fungal organs or cell types are needed to confirm this, this finding is similar to results from organ-specific gene expression analysis in mammals [66].
Fig. 9. Comparative transcriptome analysis with S. macrospora, and functional conservation of the transcription factor gene PCON_06721. 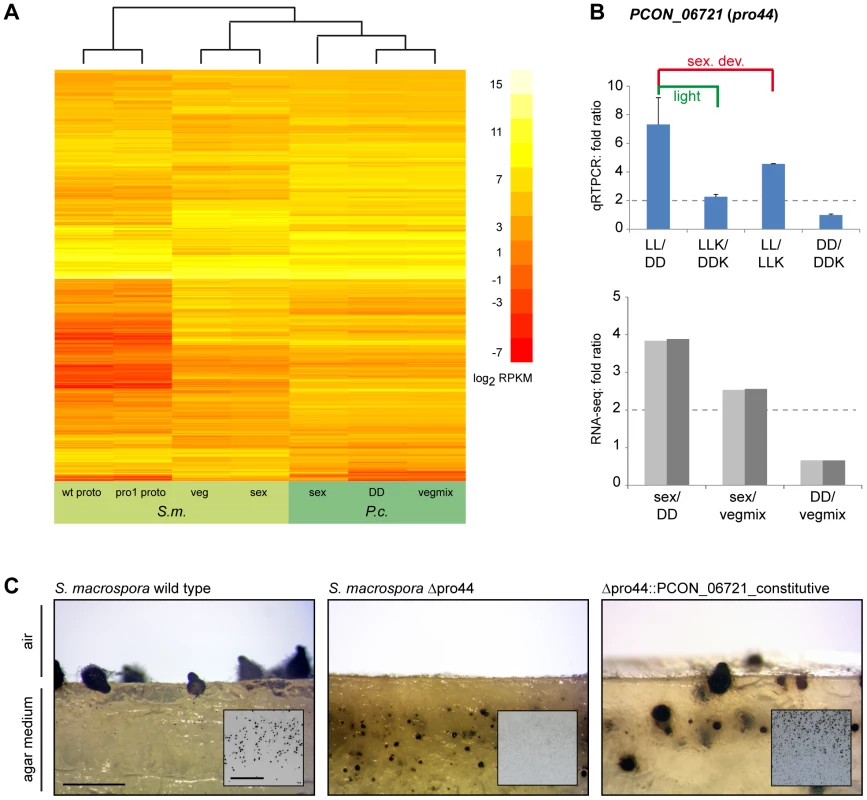
A. Comparative analysis of RPKM values for all orthologous genes for P. confluens and S. macrospora (with the exception of genes without read counts in one or more conditions). B. Quantitative real time PCR analysis of the predicted transcription factor gene PCON_06721 in P. confluens. Expression was analyzed in two independent biological replicates for the four conditions LL, DD, LLK and DDK (light and darkness in surface culture and submerged culture). Sexual development is only possible in condition LL. Expression ratios and standard deviations were calculated to address the question if the gene is differentially regulated during sexual development (i.e. in LL/DD and in LL/LLK), or regulated by light (i.e. in LL/DD and LLK/DDK) or regulated by surface versus submerged growth (i.e. LL/LLK and DD/DDK). PCON_06721 is upregulated during sexual development, but also slightly upregulated by light. RNA-seq results are given for comparison. C. Complementation of the Sordaria macrospora mutant Δpro44 with the P. confluens ortholog PCON_06721. The mutant was transformed with plasmid pFA50 that carries the PCON_06721 ORF under control of the respective gpd and trpC promoter and terminator sequences of Aspergillus nidulans. The figure shows a side view (longitudinal section) of the region comprising the agar/air interface from cultures of the wild type, the sterile mutant Δpro44 and a complemented transformant (Δpro44::PCON_06721_constitutive). The small inserts show photographs of Petri dish sections. The S. macrospora wild type forms mature perithecia at the agar/air interface, whereas the mutant only forms protoperithecia that are submerged in the agar. Complemented transformants produce mature perithecia at the agar/air interface like the wild type. Strains were grown on corn meal agar; photographs were taken after 8 d; scale bar indicates 1 mm, and 1 cm in the inserted photographs. Among the genes that were upregulated during sexual development in both species are the predicted transcription factor gene PCON_06721 and its S. macrospora ortholog pro44. The S. macrospora gene was shown previously to be essential for fruiting body development, similar to the corresponding homologous genes in A. nidulans, N. crassa, and A. fumigatus [102]–[105]. In N. crassa, the homologous sub-1 gene was also shown to be light-regulated at the level of transcription, and to be involved in light-regulation of downstream genes [101]. PCON_06721 (P. confluens pro44) is also light-induced (see section about light-dependent regulation and Figure 7), and further qRT-PCR analysis of PCON_06721 showed that it is upregulated by both light and sexual development, indicating that PCON_06721 might be involved in sexual development and development-independent light reactions (Figure 9B). PCON_06721 and its homologs in other fungi encode GATA-type transcription factors. Whereas the C-terminal GATA domain is highly conserved in all homologous proteins, the N-terminal part of the protein is only weakly conserved (Figure S16). To address the question whether the developmental function of PCON_06721 might be conserved despite limited sequence conservation, we transformed an S. macrospora Δpro44 strain with a construct expressing the P. confluens PCON_06721 gene (Figure 9C). The S. macrospora Δpro44 is sterile and forms protoperithecia, but no mature perithecia. Transformation with PCON_06721 restored the fertility of the deletion mutant, demonstrating that the gene from the basal filamentous ascomycete P. confluens is functional in the more derived Pezizomycete S. macrospora. This indicates that this transcription factor gene is one of the core regulators of sexual development across filamentous ascomycetes.
Conclusions
Here, we have analyzed the genome and development-dependent transcriptomes of P. confluens. This is the second Pezizomycete genome to be sequenced, but the first of a Pezizomycete with a more “typical” saprobic lifestyle and apothecium when compared to the more specialized life style of the truffle T. melanosporum. Overall genomic synteny with T. melanosporum is low, but regions of microsynteny between P. confluens and truffle are more numerous than between P. confluens and other ascomycetes, indicating that the two Pezizomycetes are more closely related to each other than to other ascomycete groups; however, the level of synteny still suggests a wide evolutionary range within the Pezizomycetes. This is consistent with phylogenetic analyses based on rDNA sequences that placed Pyronema and Tuber in subgroups C and B, respectively, of the Pezizomycetes [146], [147].
The P. confluens genome has a number of characteristics that are similar to higher filamentous ascomycetes, and distinct from T. melanosporum, namely its size of 50 Mb, gene content of ∼13,400 protein-coding genes, and low repeat content. However, several typical features of higher filamentous ascomycetes are different in P. confluens, allowing conclusions about the evolution of these features in fungi. For example, the mating type genes are conserved, but in contrast to higher filamentous ascomycetes, their genomic environment is not. Also, clustered genes for secondary metabolites exist, but in much lower numbers than in other species. On the other hand, P. confluens has a full complement of fungal photoreceptors, and expression studies indicate that light-sensing and signaling might be similar to more derived species and therefore represent basic features of filamentous ascomycetes. Several families encoding predicted small secreted proteins are expanded in P. confluens and present in only few other fungal groups, making it possible that they were acquired by horizontal gene transfer. By analyzing spliced RNA-seq reads in antisense direction to annotated genes, we were able to deduce the presence of natural antisense transcripts in P. confluens; and this principle might be of interest for non-strand-specific RNA-seq experiments in other organisms.
Interestingly, among the P. confluens orphan genes, a disproportionally high number is upregulated during sexual development, consistent with a hypothesis of rapid evolution of sex-associated genes. Comparative transcriptome analysis with S. macrospora identified the transcription factor gene PCON_06721, the ortholog of S. macrospora pro44, as upregulated during sexual development in both species, and complementation of an S. macrospora deletion mutant with the P. confluens gene demonstrated the conserved function of this regulator of sexual development. In summary, the P. confluens genome helps to close a sequence gap at the base of the filamentous ascomycetes, and the genome and transcriptome data are valuable resources for the analysis of fungal evolution and sexual development.
Materials and Methods
Strains and Culture Conditions
The sequenced strain is Pyronema confluens CBS100304, obtained from the CBS (Centraalbureau voor Schimmelcultures, Utrecht, NL). The strain was grown on minimal medium as previously described [6] or on cornmeal medium [148]. Sordaria macrospora strains used in this study are the wild type (FGSC 10222) and a pro44 deletion mutant from the strain collection of the Department of General and Molecular Botany at the Ruhr-Universität Bochum (Nowrousian, Teichert and Kück, unpublished). S. macrospora was grown on cornmeal medium as previously described [148].
Light Treatment for the Analysis of Light-Dependent Development and Gene Expression
For standard cultures, white light with a spectral range from 400 to 700 nm (Osram L36W/840; 1.57 lx at culture level) was used. For wavelength-dependent development and gene expression analyses, LEE filters (Andover, UK) with different transmission characteristics were used (light intensity at the level of the cultures given in lux, for transmission data see Figure S8): far red (LEE Filter 787 marius red; 0.02 lx), red (LEE Filter 106 primary red; 0.54 lx), green (LEE Filter 139 primary green; 0.60 lx) and blue (LEE Filter 363 medium blue; 0.14 lx). For light induction experiments, samples were harvested under far-red light (Philips PF712E darkroom safe light) after 4 d continuous light (LL), or continuous darkness (DD), or DD and 5–60 min of light induction with the respective wavelengths.
DNA Preparation and Sequencing
Genomic DNA from P. confluens was prepared from mycelium grown for 3 days in minimal medium. Mycelium was frozen in liquid nitrogen, pulverized, and incubated in equal volumes of lysis buffer (0.6 M, 10 mM EDTA, 100 mM Tris-HCl pH 8.0, 1% SDS) and phenol/chloroform (1∶1) at room temperature for 10 min with constant shaking. After centrifugation, the supernatant was again treated with an equal volume phenol/chloroform (1∶1), and this step was repeated until the supernatant was clear. It was then treated with RNase A, and afterwards again with phenol/chloroform. Genomic DNA was precipitated with sodium acetate (pH 7.0) and ethanol. Roche/454 sequencing was performed with 20 µg genomic DNA at Eurofins MWG GmbH (Ebersberg, Germany) on a GS FLX system. Illumina/Solexa paired-end sequencing was performed with 5 µg genomic DNA at GATC Biotech (Konstanz, Germany) on a HiSeq 2000. An overview of obtained sequence reads is given in Table S1.
Assembly and k-mer Frequency Analysis
The 454 raw data were extracted from sff files and converted to fasta files using sff_extract.py (Jose Blanca and Bastien Chevreux, http://bioinf.comav.upv.es/sff_extract/index.html). 454 and Illumina raw data were trimmed with custom-made Perl scripts to remove reads with undetermined bases (“N”) and for trimming of low quality bases (phred score <10) from the 3′ end as described (available at http://c4-1-8.serverhosting.rub.de/public/software.html) [24], [104]. 454 reads were assembled with the Celera assembler [149]. The trimmed 454 and Illumina reads as well as the 454-based Celera assembly were used for an assembly with Velvet 1.1.04 [150] with the following parameters for velveth (k) and velvetg (all others): k 41, exp_cov 100, cov_cutoff 2, long_mult_cutoff 0, ins_length 300. Overlapping Velvet scaffolds were merged further using CAP3 [151].
The rDNA unit (scaffold 1635) was assembled separately from 454 reads. BLAST searches with rDNAs from S. macrospora and several publicly available Pezizales rDNAs against the 454 reads and the Celera assembly were used to obtain sequences with homology to rDNA. Theses reads were assembled with CAP3 [151] to obtain an rDNA unit that contains the 18S, 5.8S, and 28S rRNA genes as well as the internal transcribed spacers 1 and 2.
k-mer frequencies were analyzed based on the Illumina reads with an algorithm described for potato genome [23]. The algorithm was used to write a custom Perl program. Based on the fastq data of the Illumina reads, k-mers of 31 and 41 bases were analyzed.
RNA Preparation, qRT-PCR, and RNA-seq
For RNA extraction, P. confluens mycelia were grown as described in liquid medium (minimal medium or cornmeal medium) either in darkness (and harvested in dark red light) or in light, as surface cultures or submerged (shaking cultures) [6]. For the analysis of effects of different wavelengths on fruiting body formation and gene expression, LEE filters (Andover, UK) were used as described above. RNA was prepared with the RNeasy lipid tissue mini kit (Qiagen, Hilden, Germany) as described [6]. Reverse transcription and qRT-PCR were performed as described previously [18], [152], oligonucleotide primers are given in Table S15.
For RNA-seq analysis, 50 µg total RNA from several growth conditions were pooled to generate the sex, DD, and vegmix samples. RNA for sample sex was extracted from mycelia grown in minimal medium in surface culture in constant light. Only under these conditions is P. confluens able to develop fruiting bodies. Equal amounts of RNAs from mycelia grown for 3, 4, and 5 d were pooled to represent a high number of genes that are expressed during fruiting body development. The DD samples comprised RNAs from mycelia grown for 3, 4, and 5 d in minimal medium in submerged culture in constant darkness, which prevents fruiting body formation. The vegmix samples also contained only RNAs from mycelia that could not develop fruiting bodies, but from a mixture of growth conditions different from the DD samples: for the vegmix samples, we used mycelia grown for 3 d in complete medium (cornmeal medium) in surface culture in constant darkness, mycelia grown for 3 d submerged in minimal medium in constant light, and mycelia grown for 3 d submerged in minimal medium in constant darkness. Two independent biological replicates of each condition (sex, DD, and vegmix) were used for sequencing. cDNA and library preparation for RNA-seq as well as Illumina/Solexa paired-end sequencing was performed at GATC Biotech (Konstanz, Germany). Indexed cDNA libraries for multiplexing were prepared with the TrueSeq RNA sample preparation kit (Illumina, San Diego, CA, USA). One library was prepared for each independent replicate for each of the three conditions (sex, DD, and veg), and the resulting six libraries were pooled and sequenced in one lane on a HiSeq 2000. An overview of obtained sequence reads is given in Table S1.
Annotation and Analysis of Repeat Content
RNA-seq reads were assembled with Trinity [153], and assembled transcripts were mapped to the genome sequence with PASA [154]. The longest full length ORFs identified by PASA were used to train AUGUSTUS and SNAP, then gene models were predicted independently with AUGUSTUS, SNAP, and GeneMark-ES [155]–[158]. The resulting annotation from each of the prediction programs was used together with the RNA-seq data as input to MAKER, a program that integrates the different sources of gene evidence [25]. Detailed parameters that were used for the gene predictions are available at https://github.com/hyphaltip/fungi-gene-prediction-params/. Initial automated gene predictions were checked for consistency (e.g. presence of start/stop codons) and manually curated in about 10% of all cases. UTR predictions were refined/improved based on the RNA-seq data using custom-made Perl scripts as described previously [24]. For each of the predicted proteins, the protein with the highest sequence identity in GenBank (nr) was determined using BLASTP [48], and putative localizations of the predicted proteins were determined with WoLF PSORT [159] (Table S2). Searches for conserved protein domains (Pfam-A domains, http://pfam.sanger.ac.uk/) were performed with the HMMER 3.0 program hmmsearch [160], [161]. A chi-square test to determine which domains are over - or underrepresented in P. confluens was performed in R. tRNAs were predicted using a combination of Infernal 1.0, tRNAscan-SE, TFAM 1.0, and ARAGORN [162]–[165].
Analysis of transposable elements and other repeats was performed with RepeatMasker (A.F.A. Smit, R. Hubley, P. Green; www.repeatmasker.org) based the RepbaseUpdate library [166] and a library of de novo-identified P. confluens repeat consensus sequences that was generated by RepeatModeler (A.F.A. Smit, R. Hubley; www.repeatmasker.org/RepeatModeler.html). First, the P. confluens genome sequence was analyzed using the RepBase Update library and species-specification “fungi”. In a second step, repeats were identified de novo from the P. confluens genome using RepeatModeler, and the RepeatMasker analysis was repeated with the P. confluens-specific repeat library generated by RepeatModeler. The results of both RepeatMasker runs were combined using custom-made Perl scripts to remove redundancy and only keep non-overlapping repeat regions. Histograms of percent divergence, percent insertions, and percent deletions compared to the repeat consensus sequences were generated based on the output information from RepeatMasker.
RIP Index Calculation
Composite RIP indices were calculated with Perl script RIP_index_calculation.pl (https://github.com/hyphaltip/fungaltools/blob/master/scripts/RIP_index_calculation.pl) on DNA sequences of 500 bp in sliding windows (window step size 100 bp) based on a method used in [167]. Briefly, a RIP product index (TpA/ApT) and RIP substrate index (CpA+TpG/ApC+GpT) [168], [169] are calculated. Sequences that have been subjected to RIP have a product index of at least 1.1 and a substrate index of less than 0.9, while sequences that have not been subjected to RIP have a product index of less than 0.8 and substrate index of at least 1.1. The composite RIP index is calculated by subtracting the substrate index from the product index; positive values imply that the DNA has been subjected to RIP [167].
Analysis of Natural Antisense Transcripts (NATs)
Predicted splice sites from the junctions.bed output of Tophat [31] were analyzed with respect to strand based on the intron consensus sequences (5′ GT or GC, 3′ AG) and overlap with annotated protein-coding genes using custom-made Perl scripts based on BioPerl modules [170]. Splice sites in antisense direction to annotated genes were further filtered to include only sites covered by at least five spliced sequence reads, and with a coverage of more than 10% of the average coverage of the predicted sense-transcript. Remaining putative antisense splice sites were checked manually to remove splice sites that were most likely due to annotation errors or within repeat-rich regions or close to sequence gaps.
Synteny Analysis
Regions of sequence similarity were determined with the PROmer algorithm from the MUMmer package version 3.23 [47]. The resulting files were used as input to mummerplot, and percent identity plots and dot plots of PROmer results were visualized with gnuplot (www.gnuplot.info) based on the mummerplot output files.
An orthology-based analysis of synteny was performed by determining orthologs for all P. confluens genes in the predicted proteomes of ten filamentous fungi by reciprocal BLAST analysis [48], and using custom-made Perl scripts based on BioPerl modules [170] to determine the positions of orthologous proteins on sequenced chromosomes or contigs.
Phylogenomics Analysis and Estimation of Divergence Times
The predicted proteomes of P. confluens and the following 17 other fungal species were used for the reconstruction of the phylome: Agaricus bisporus [171], Arthrobotrys oligospora [10], Blumeria graminis [172], Coccidioides immitis [173], Emericella nidulans [174], Gibberella zeae [138], Laccaria bicolor [175], Mycosphaerella graminicola [176], Neosartorya fischeri [177], Neurospora crassa [178], Phaeosphaeria nodorum [179], Saccharomyces cerevisiae [180], Schizosaccharomyces pombe [181], Sclerotinia sclerotiorum [182], Sordaria macrospora [22], Tuber melanosporum [9], Yarrowia lipolytica [183]. 13 of the proteomes belonged to other Pezizomycotina species, S. cerevisiae and Y. lipolytica represented the Saccharomycotina and S. pombe, L. bicolor and A. bisporus served as outgroups.
The phylome, meaning the complete collection of phylogenetic trees for each gene in a genome, was reconstructed in an automated process that mimics a manual phylogenetic tree reconstruction process [184]: homology search, multiple sequence alignment and phylogenetic reconstruction. For each protein encoded in the P. confluens genome we performed a Smith-Waterman search against the proteome database formed by the genomes listed above. Results were then filtered using an e-value threshold of 1e-05 and a continuous overlapping region between the query and the result of 0.5. A maximum of 150 sequences were taken. Multiple sequence alignments were then reconstructed using three different programs: MUSCLE v3.8 [185], MAFFT v6.712b [186], and DIALIGN-TX [187]. Alignments were reconstructed in forward and reverse (i.e using the Head or Tail approach [188]). The resulting alignments were then combined using M-COFFEE [189]. A trimming step was performed using trimAl v1.3 [190] (consistency-score cutoff 0.1667, gap-score cutoff 0.9). Trees were reconstructed using PhyML [191]. First the best fitting model was selected by reconstructing neighbor joining trees as implemented in BioNJ [192] using seven different models (JTT, LG, WAG, Blosum62, MtREV, VT and Dayhoff). The two best models in terms of likelihood were used to reconstruct maximum-likelihood trees. Four rate categories were used and invariant positions were inferred from the data. Branch support was computed using an aLRT (approximate likelihood ratio test) based on a chi-square distribution. Resulting trees and alignments are stored in phylomeDB [184] (http://phylomedb.org), with the phylomeID 203.
Orthologs between P. confluens and the other species included in the phylome were based on the phylogenetic trees reconstructed in the phylome (Table S9). A species-overlap algorithm, as implemented in ETE v2 [193] was used to infer orthology and paralogy relationships. The algorithm traverses the tree and at each node it calls speciation or duplication depending on whether there are common species at both sides of the node.
Expanded protein families were detected based on the trees reconstructed in the phylome. For each tree, we used ETE v2 [193] to find nodes that exclusively contained P. confluens sequences. Only those nodes with more than five sequences were considered as expansions. Overlapping expansions were fused when they shared more than 20% of their members. Expansions were then annotated using a BLAST search against UniProt.
The species tree was build using a concatenation method. 426 single-copy, widespread genes were selected. The concatenated alignment was further trimmed using trimAl [190] (gap-score cutoff 0.5 and conservation 0.5). The final alignment contained 277,192 positions. The tree was reconstructed using PhyML [191]. LG model [194] was selected and a 4-categories GAMMA distribution was used. A bootstrap of 100 repetitions was also reconstructed. Additionally a species tree based on the super-tree reconstruction program DupTree [195] was reconstructed. All the trees reconstructed in the phylome were used as input (6,949 trees). Both species trees showed the same topology.
r8s [49] was used to estimate the divergence between P. confluens and T. melanosporum based on the species tree inferred from the concatenated alignment. Two analyses were run using different estimates of the divergence between Schizosaccharomyces pombe and the remaining acomycetes as calibration point. For each analysis the smoothing parameter was estimated using cross-validation. In the first analysis, the divergence between S. pombe and the remaning ascomycetes was put at 723.86 Mya [196] (www.timetree.org), this resulted in a divergence time between T. melanosporum and P. confluens of 260.38 Mya. If a more ancestral divergence point is selected as a calibration point (1147.78 Mya, [197]), then the divergence time between the two species of interest is 413.30 Mya.
Quantitative Analysis of Gene Expression Based on RNA-seq Data
RNA-seq reads were cleaned with custom-made Perl scripts as described [24] and mapped to the P. confluens genome sequence using Tophat [31]. Custom-made Perl scripts using modules from the BioPerl toolkit [170] were used to determine the number of reads that mapped to each annotated protein-coding gene based on the SAM files with the mapping information (output from Tophat), and quantitative analysis was done with two different methods (“classical” and with LOX [198]) as described previously [24]. We also used the two Bioconductor packages DESeq [199] and baySeq [200] in the R computing environment (version 2.12.1); however, similar to previous analyses, the number of differentially expressed genes was >10%, and under these conditions the statistical models upon which these methods are based are no longer valid [24], therefore the results were not used for further analyses (data not shown). Results from both LOX and “classical” analyses agreed well with qRT-PCR results for selected genes (see e.g. Figure S14, compare qRT-PCR results for LL/DD with RNA-seq results for sex/DD). LOX calculates expression ratios and Bayesian credible intervals and p-values for differential expression. The classical analysis consists of the calculation of expression ratios, standard deviation, and coefficient of variance from read counts normalized to the total number of read counts for the sample, and genes were sorted into five groups (0–4) according to the following criteria: genes in group 4 have ratios of ≤0.25 or ≥4 in all independent biological replicates, genes in group 3 have a mean ratio of ≤0.25 or ≥4 and a coefficient of variance <0.5, genes in group 2 have ratios of ≤0.5 or ≥2 in all independent biological replicates, genes in group 1 have a mean ratio of ≤0.5 or ≥2 and a coefficient of variance <0.5, and group 0 contains all other genes (with the exception of genes for which no ratios could be calculated due to a lack of read coverage, these were not included in the analysis). To classify genes as differentially expressed, a consensus was determined for each gene based on the results from both the classical and LOX analysis; a gene was labeled as up-regulated (1), down-regulated (−1) or not differentially expressed (0) under the conditions that were compared, when the following criteria were met: (a) (“normal” analysis) expression ratios from both classical and LOX analysis had to be >2 and <0.5, LOX Bayesian probability for differential expression = 1, and the gene had to be in groups 1–4 in the classical analysis; (b) (stringent analysis) thresholds for expression ratios were set to >4 and <0.25.
For the analysis of reads that mapped to different genomic regions (e.g., exons, introns, intergenic regions), reads were counted based on the SAM files with the mapping information using custom-made Perl scripts as described previously [24]. To determine the distribution of expression frequencies (Figure S4), the coverage for locus tags of protein-coding gene was determined as the average coverage for the bases of the predicted mRNA (normalized to coverage per kilobase per million counted bases in the sample). Curve fitting and clustering of the data by expectation-maximization was performed on the log2-transformed RNA-seq data using the R package mclust [201].
Complementation of S. macrospora Δpro44 with PCON_06721
Plasmid pRSnat_06721_OE for complementation of an S. macrospora pro44 deletion mutant was generated by homologous recombination in yeast as described [102]. It contains the PCON_06721 ORF under control of the Aspergillus nidulans gpd promoter and trpC terminator in vector pRSnat, which contains a nourseothricin resistance cassette for selection in S. macrospora [202]. The S. macrospora Δpro44 strain was transformed with pRSnat_06721_OE as described [104], [203].
Phylogenetic Analysis
Multiple alignments were created in CLUSTALX [204] and trimmed with Jalview [205], and the same alignment was used for analysis by distance-matrix (DM) or maximum parsimony (MP). Phylogenetic analyses were made with PAUP version 4.0b10 for Windows (D.L. Swofford, distributed by Sinauer Associates, copyright 2001 Smithsonian Institution). DM and MP analyses were performed using 10,000 bootstrap replicates. Consensus trees were graphically displayed with TREEVIEW [206].
Accession Numbers
The sequence and annotation data are available under the accession numbers HF935090–HF936677 (annotated scaffolds at the European Nucleotide Archive ENA, http://www.ebi.ac.uk/ena/data/view/HF935090-HF936677), CATG01000001–CATG01001898 (primary, non-annotated contigs from which scaffolds were assembled), and BioProject acc. PRJNA65573. The sequence reads that were used for the assembly of the P. confluens genome were submitted to the NCBI sequence read archive (accession number SRA059523). The RNA-seq reads and derived expression ratios were submitted to the GEO database (accession number GSE41631).
Supporting Information
Zdroje
1. Buckley M (2008) The fungal kingdom - diverse and essential roles in earth's ecosystem. Washington DC: American Academy of Microbiology.
2. HawksworthDL (2001) The magnitude of fungal diversity: the 1.5 million species estimate revisited. Mycol Res 105 : 1422–1432.
3. EbersbergerI, de Matos SimoesR, KupczokA, GubeM, KotheE, et al. (2012) A consistent phylogenetic backbone for the fungi. Mol Biol Evol 29 : 1319–1334.
4. HibbettDS, BinderM, BischoffJF, BlackwellM, CannonPF, et al. (2007) A higher-level phylogenetic classification of the Fungi. Mycol Res 111 : 509–547.
5. LiuYJ, HallBD (2004) Body plan evolution of ascomycetes, as inferred from an RNA polymerase II phylogeny. Proc Nat Acad Sci USA 101 : 4507–4512.
6. NowrousianM, KückU (2006) Comparative gene expression analysis of fruiting body development in two filamentous fungi. FEMS Microbiol Lett 257 : 328–335.
7. ZhuangWY, LiuCY (2012) What an rRNA secondary structure tells about phylogeny of fungi in ascomycota with emphasis on evolution of major types of ascus. PLoS One 7: e47546.
8. SpataforaJW, SungG-H, JohnsonD, HesseC, O'RourkeB, et al. (2006) A five-gene phylogeny of Pezizomycotina. Mycologia 98 : 1018–1028.
9. MartinF, KohlerA, MuratC, BalestriniR, CoutinhoPM, et al. (2010) Périgord black truffle genome uncovers evolutionary origins and mechanisms of symbiosis. Nature 464 : 1033–1039.
10. YangJ, WangL, JiX, FengY, LiX, et al. (2011) Genomic and proteomic analyses of the fungus Arthrobotrys oligospora provide insights into nematode-trap formation. PLoS Pathog 7: e1002179.
11. PfisterDH, LiftikME (1995) Two Arthrobotrys anamorphs from Orbilia auricolor. Mycologia 87 : 684–688.
12. RaffaeleS, KamounS (2012) Genome evolution in filamentous platn pathogens: why bigger can be better. Nat Rev Microbiol 10 : 417–430.
13. ClaussenP (1912) Zur Entwicklungsgeschichte der Ascomyceten. Pyronema confluens. Zeitschr f Bot 4 : 1–63.
14. Gwynne-VaughanHCI, WilliamsonHS (1931) Contributions to the study of Pyronema confluens. Ann Bot 45 : 355–371.
15. KerlI (1937) Über Regenerationsversuche an Fruchtkörpern und andere entwicklungsphysiologische Untersuchungen bei Pyronema confluens. Zeitschr f Bot 31 : 9–174.
16. MooreEJ, KorfRP (1963) The genus Pyronema. Bull Torrey Bot Club 90 : 33–42.
17. WilsonIM (1952) The ascogenous hyphae of Pyronema confluens. Ann Bot 56 : 321–338.
18. GesingS, SchindlerD, FränzelB, WoltersD, NowrousianM (2012) The histone chaperone ASF1 is essential for sexual development in the filamentous fungus Sordaria macrospora. Mol Microbiol 84 : 748–765.
19. GesingS, SchindlerD, NowrousianM (2012) Suppression subtractive hybridization and comparative expression analysis to identify developmentally regulated genes in filamentous fungi. J Basic Microbiol doi: 10.1002/jobm.201200223
20. SeaverFJ (1909) Studies in pyrophilous fungi - I. The occurence and cultivation of Pyronema. Mycologia 1 : 131–139.
21. RobinsonW (1926) The conditions of growth and development of Pyronema confluens, Tul. (P. omphaloides, (Bull.) Fuckel). Ann Bot 40 : 245–272.
22. NowrousianM, StajichJE, ChuM, EnghI, EspagneE, et al. (2010) De novo assembly of a 40 Mb eukaryotic genome from short sequence reads: Sordaria macrospora, a model organism for fungal morphogenesis. PLoS Genet 6: e1000891.
23. The Potato Genome Sequencing Consortium (2011) Genome sequence and analysis of the tuber crop potato. Nature 475 : 189–195.
24. TeichertI, WolffG, KückU, NowrousianM (2012) Combining laser microdissection and RNA-seq to chart the transcriptional landscape of fungal development. BMC Genomics 13 : 511.
25. CantarelBL, KorfI, RobbSMC, ParraG, RossE, et al. (2008) MAKER: An easy-to-use annotation pipeline designed for emerging model organism genomes. Genome Res 18 : 188–196.
26. ParraG, BradnamK, NingZ, KeaneT, KorfI (2009) Assessing the gene space in draft genomes. Nucl Acids Res 37 : 289–297.
27. TisserantE, Da SilvaC, KohlerA, MorinE, WinckerP, et al. (2011) Deep RNA sequencing improved the structural annotation of the Tuber melanosporum transcriptome. New Phytol 189 : 883–891.
28. WangB, GuoG, WangC, LinY, WangX, et al. (2010) Survey of the transcriptome of Aspergillus oryzae via massively parallel mRNA sequencing. Nucl Acids Res 38 : 5075–5087.
29. DjebaliS, DavisCA, MerkelA, DobinA, LassmannT, et al. (2012) Landscape of transcription in human cells. Nature 489 : 101–108.
30. DonaldsonME, SavilleBJ (2012) Natural antisense transcripts in fungi. Mol Microbiol 83 : 405–417.
31. TrapnellC, PachterL, SalzbergSL (2009) TopHat: discovering splice junctions with RNA-Seq. Bioinf 25 : 1105–1111.
32. DelmasS, PullanST, GaddipatiS, KokolskiM, MallaS, et al. (2012) Uncovering the genome-wide transcriptional responses of the filamentous fungus Aspergillus niger to lignocellulose using RNA sequencing. PLoS Genet 8: e1002875.
33. GowdaM, VenuRC, RaghupathyMB, NobutaK, LiH, et al. (2006) Deep and comparative analysis of the mycelium and appressorium transcriptomes of Magnaporthe grisea using MPSS, RL-SAGE, and oligoarray methods. BMC Genomics 7 : 310.
34. SmithCA, RobertsonD, YatesB, NielsenDM, BrownD, et al. (2008) The effect of temperature on natural antisense transcript (NAT) expression in Aspergillus flavus. Curr Genet 54 : 241–269.
35. ChengCK, AuCH, WilkeSK, StajichJE, ZolanME, et al. (2013) 5′-Serial analysis of gene expression studies reveal a transcriptomic switch during fruiting body development in Coprinopsis cinerea. BMC Genomics 14 : 195.
36. DoyleEE, DonaldsonME, MorrisonEN, SavilleBJ (2011) Ustilago maydis transcript features identified through full-length cDNA analysis. Mol Genet Genomics 286 : 143–159.
37. OhmRA, de JongJF, LugonesLG, AertsA, KotheE, et al. (2010) Genome sequence of the model mushroom Schizophyllum commune. Nat Biotech 28 : 957–963.
38. ManningVA, PandelovaI, DhillonB, WilhelmLJ, GoodwinSB, et al. (2013) Comparative genomics of a plant-pathogenic fungus, Pyrenophora tritici-repentis, reveals transduplication and the impact of repeat elements on pathogenicity and population divergence. G3 (Bethesda) 3 : 41–63.
39. MaL-J, van der DoesHC, BorkovichKA, ColemanJJ, DaboussiM-J, et al. (2010) Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature 464 : 367–373.
40. ColemanJJ, RounsleySD, Rodriguez-CarresM, KuoA, WasmannCC, et al. (2009) The genome of Nectria haematococca: Contribution of supernumerary chromosomes to gene expansion. PLoS Genet 5: e1000618.
41. FreitagM, WilliamsRL, KotheGO, SelkerEU (2002) A cytosine methyltransferase homologue is essential for repeat-induced point mutation in Neurospora crassa. Proc Nat Acad Sci USA 99 : 8802–8807.
42. SelkerEU, CambareriEB, JensenBC, HaackKR (1987) Rearrangement of duplicated DNA in specialized cells of Neurospora. Cell 51 : 741–752.
43. CambareriEB, BryanJC, SchabtachE, SelkerEU (1989) Repeat-induced point mutations. Science 244 : 1571–1575.
44. GalaganJE, SelkerEU (2004) RIP: the evolutionary cost of genome defense. Trends Genet 20 : 417–423.
45. StajichJE, DietrichFS, RoySW (2007) Comparative genomic analysis of fungal genomes reveals intron-rich ancestors. Genome Biol 8: R223.
46. HebenstreitD, FangM, GuM, CharoensawanV, van OudenaardenA, et al. (2011) RNA sequencing reveals two major classes of gene expression levels in metazoan cells. Mol Syst Biol 7 : 497.
47. KurtzS, PhillippyA, DelcherAL, SmootM, ShumwayM, et al. (2004) Versatile and open software for comparing large genomes. Genome Biol 5: R12.
48. AltschulSF, MaddenTL, SchafferAA, ZhangJ, ZhangZ, et al. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25 : 3389–3402.
49. SandersonMJ (2003) r8s: inferring absolute rates of molecular evolution and divergence times in the absence of a molecular clock. Bioinf 19 : 301–302.
50. BonitoG, SmithME, NowakM, HealyRA, GuevaraG, et al. (2013) Historical biogeography and diversification of truffles in the Tuberaceae and their newly identified southern hemisphere sister lineage. PLoS One 8: e52765.
51. TurgeonG, YoderOC (2000) Proposed nomenclature for mating type genes of filamentous ascomycetes. Fungal Genet Biol 31 : 1–5.
52. PöggelerS (2001) Mating-type genes for classical strain improvements of ascomycetes. Appl Microbiol Biotechnol 56 : 589–601.
53. PöggelerS, RischS, KückU, OsiewaczHD (1997) Mating-type genes from the homothallic fungus Sordaria macrospora are functionally expressed in a heterothallic ascomycete. Genetics 147 : 567–580.
54. PöggelerS, O'GormanCM, HoffB, KückU (2011) Molecular organization of the mating-type loci in the homothallic ascomycete Eupenicillium crustaceum. Fungal Biol 115 : 615–624.
55. Debuchy R, Berteaux-Leceleir V, Silar P (2010) Mating systems and sexual morphogenesis in ascomycetes. In: Borkovich KA, Ebbole DJ, editors. Cellular and molecular biology of filamentous fungi. Washington, DC: ASM Press. pp. 501–535.
56. PaolettiM, SeymourFA, AlcocerMJC, KaurN, CalvoAM, et al. (2007) Mating type and the genetic basis of self-fertility in the model fungus Aspergillus nidulans. Curr Biol 17 : 1384–1389.
57. RydholmC, DyerPS, LutzoniF (2007) DNA sequence characterization and molecular evolution of MAT1 and MAT2 mating-type loci of the self-compatible ascomycete mold Neosartorya fischeri. Eukaryot Cell 6 : 868–874.
58. RubiniA, BelfioriB, RiccioniC, TisserantE, ArcioniS, et al. (2011) Isolation and characterization of MAT genes in the symbiotic ascomycete Tuber melanosporum. New Phytol 183 : 710–722.
59. Debuchy R, Turgeon BG (2006) Mating-type structure, evolution, and function in Euascomycetes. In: Kües U, Fischer R, editors. The Mycota I: Growth, differentiation and sexuality. Berlin, Heidelberg: Springer-Verlag. pp. 293–323.
60. ButlerG, KennyC, FaganA, KurischkoC, GaillardinC, et al. (2004) Evolution of the MAT locus and its Ho endonuclease in yeast species. Proc Nat Acad Sci USA 101 : 1632–1637.
61. Dyer PS (2007) Sexual reproduction and significance of MAT in the Aspergilli. In: Heitman J, Kronstad JW, Taylor JW, Casselton LA, editors. Sex in fungi. Washington, DC: ASM Press. pp. 123–142.
62. HoffB, PöggelerS, KückU (2008) Eighty years after its discovery, Fleming's Penicillium strain discloses the secret of its sex. Eukaryot Cell 7 : 465–470.
63. LeeSC, NiM, LiW, ShertzC, HeitmanJ (2010) The evolution of sex: a perspective from the fungal kingdom. Microbiol Mol Biol Rev 74 : 298–340.
64. SwansonWJ, VacquierVD (2002) The rapid evolution of reproductive proteins. Nat Rev Genet 3 : 137–144.
65. ClarkNL, AagarardJE, SwansonWJ (2006) Evolution of reproductive proteins from animals and plants. Reproduction 131 : 11–22.
66. BrawandD, SoumillonM, NecsuleaA, JulienP, CsardiG, et al. (2011) The evolution of gene expression levels in mammalian organs. Nature 478 : 343–348.
67. ButlerG, RasmussenMD, LinMF, SantosMAS, SakthikumarS, et al. (2009) Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature 459 : 657–662.
68. PöggelerS (1999) Phylogenetic relationships between mating-type sequences from homothallic and heterothallic ascomycetes. Curr Genet 36 : 222–231.
69. KarlssonM, NygrenK, JohannessonH (2008) The evolution of the pheromonal signal system and its potential role for reproductive isolation in heterothallic Neurospora. Mol Biol Evol 25 : 168–178.
70. NygrenK, WallbergA, SamilsN, StajichJE, TownsendJP, et al. (2012) Analyses of expressed sequence tags in Neurospora reveal rapid evolution of genes associated with the early stages of sexual reproduction in fungi. BMC Evol Biol 12 : 229.
71. LipmanDJ, SouvorovA, KooninEV, PanchenkoAR, TatusovaTA (2002) The relationship of protein conservation and sequence length. BMC Evol Biol 2 : 20.
72. PálC, PappB, HurstLD (2001) Highly expressed genes in yeast evolve slowly. Genetics 158 : 927–931.
73. SubramanianS, KumarS (2004) Gene expression intensity shapes evolutionary rates of the proteins encoded by the vertebrate genome. Genetics 168 : 373–381.
74. CaiJJ, WooPCY, LauSKP, SmithDK, YuenKY (2006) Accelerated evoutionary rate may be responsible for the emergence of lineage-specific genes in ascomycota. J Mol Evol 63 : 1–11.
75. CarvunisA-R, RollandT, WapinskiI, CalderwoodMA, YildirimMA, et al. (2012) Proto-genes and de novo gene birth. Nature 487 : 370–374.
76. TautzD, Domazet-LošoT (2011) The evolutionary origin of orphan genes. Nat Rev Genet 12 : 692–702.
77. KasugaT, GlassNL (2008) Dissecting colony development of Neurospora crassa using mRNA profiling and comparative genomics approaches. Eukaryot Cell 7 : 1549–1564.
78. Rodriguez-RomeroJ, HedtkeM, KastnerC, MüllerS, FischerR (2010) Fungi, hidden in soil or up in the air: light makes a difference. Annu Rev Microbiol 64 : 585–610.
79. SeidlV, SeibelC, KubicekCP, SchmollM (2009) Sexual development in the industrial workhorse Trichoderma reesei. Proc Nat Acad Sci USA 106 : 13909–13914.
80. ChenCL, KuoHC, TungSY, HsuPWC, WangCL, et al. (2012) Blue light acts as a s double-edged sword in regulating sexual development in Hypocrea jecorina (Trichoderma reesei). PLoS One 7: e44969.
81. PerkinsJH (1969) Morphogenesis in Schizophyllum commune. I. Effects of white light. Plant Physiol 44 : 1706–1711.
82. KüesU (2000) Life history and developmental processes in the basidiomycete Coprinus cinereus. Microbiol Mol Biol Rev 64 : 316–353.
83. PerkinsJH, GordonSA (1969) Morphogenesis in Schizophyllum commune. II. Effects of monochromatic light. Plant Physiol 44 : 1712–1716.
84. FroehlichAC, LiuY, LorosJJ, DunlapJC (2002) White Collar-1, a circadian blue light photoreceptor, binding to the frequency promoter. Science 297 : 815–819.
85. BallarioP, VittoriosoP, MagrelliA, TaloraC, CabibboA, et al. (1996) White collar-1, a central regulator of blue-light responses in Neurospora, is a zinc finger protein. EMBO J 15 : 1650–1657.
86. CrosthwaiteSC, DunlapJC, LorosJJ (1997) Neurospora wc-1 and wc-2: Transcription, photoresponses, and the origins of circadian rhythmicity. Science 276 : 763–769.
87. LindenH, MacinoG (1997) White collar 2, a partner in blue-light signal transduction, controlling expression of light-regulated genes in Neurospora crassa. EMBO J 16 : 98–109.
88. TaloraC, FranchiL, LindenH, BallarioP, MacinoG (1999) Role of a white collar-1-white collar-2 complex in blue-light signal transduction. EMBO J 18 : 4961–4968.
89. HeintzenC, LorosJJ, DunlapJC (2001) The PAS protein VIVID defines a clock-associated feedback loop that represses light input, modulates gating, and regulates clock resetting. Cell 104 : 453–464.
90. SchwerdtfegerC, LindenH (2003) VIVID is a flavoprotein and serves as a fungal blue light photoreceptor for photoadaptation. EMBO J 22 : 4846–4855.
91. BlumensteinA, VienkenK, TaslerR, PurschwitzJ, VeithD, et al. (2005) The Aspergillus nidulans phytochrome FphA represses sexual development in red light. Curr Biol 15 : 1833–1838.
92. BieszkeJA, SpudichEN, ScottKL, BorkovichKA, SpudichJL (1999) A eukaryotic protein, NOP-1, binds retinal to form an archaeal rhodopsin-like photochemically reactive pigment. Biochemistry 38 : 14138–14145.
93. BieszkeJA, BraunEL, BeanLE, KangS, NatvigDO, et al. (1999) The nop-1 gene of Neurospora crassa encodes a seven transmembrane helix retinal-binding protein homologous to archaeal rhodopsins. Proc Nat Acad Sci USA 96 : 8034–8039.
94. BrownLS (2004) Fungal rhodopsins and opsin-related proteins: eukaryotic homologues of bacteriorhodopsin with unknown functions. Photochem Photobiol Sci 3 : 555–565.
95. DunlapJC, LorosJJ (2006) How fungi keep time: circadian system in Neurospora and other fungi. Curr Opin Microbiol 9 : 579–587.
96. SalichosL, RokasA (2010) The diversity and evolution of circadian clock proteins in fungi. Mycologia 102 : 269–278.
97. CrosthwaiteSC, LorosJJ, DunlapJC (1995) Light-Induced resetting of a circadian clock is mediated by a rapid increase in frequency transcript. Cell 81 : 1003–1012.
98. KramerC, LorosJJ, DunlapJC, CrosthwaiteSK (2003) Role for antisense RNA in regulating circadian clock function in Neurospora crassa. Nature 421 : 948–952.
99. DiernfellnerACR, SchafmeierT, MerrowMW, BrunnerM (2005) Molecular mechanism of temperature sensing by the circadian clock of Neurospora crassa. Genes Dev 19 : 1968–1973.
100. ColotHV, LorosJJ, DunlapJC (2005) Temperature-modulated alternative splicing and promoter use in the circadian clock gene frequency. Mol Biol Cell 16 : 5563–5571.
101. ChenCH, RingelbergCS, GrossRH, DunlapJC, LorosJJ (2009) Genome-wide analysis of light-inducible responses reveals hierarchical light signalling in Neurospora. EMBO J 28 : 1029–1042.
102. ColotHV, ParkG, TurnerGE, RingelbergC, CrewCM, et al. (2006) A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc Nat Acad Sci USA 103 : 10352–10357.
103. HanKH, HanKY, YuJH, ChaeKS, JahngKY, et al. (2001) The nsdD gene encodes a putative GATA-type transcription factor necessary for sexual development of Aspergillus nidulans. Mol Microbiol 41 : 299–309.
104. NowrousianM, TeichertI, MasloffS, KückU (2012) Whole-genome sequencing of Sordaria macrospora mutants identifies developmental genes. G3 (Bethesda) 2 : 261–270.
105. SzewczykE, KrappmannS (2010) Conserved regulators of mating are essential for Aspergillus fumigatus cleistothecium formation. Eukaryot Cell 9 : 774–783.
106. CorrochanoLM, GarreV (2010) Photobiology in the Zygomycota: multiple photoreceptor genes for complex responses to light. Fungal Genet Biol 47 : 893–899.
107. TischD, SchmollM (2010) Light regulation of metabolic pathways in fungi. Appl Microbiol Biotechnol 85 : 1259–1277.
108. HardingRW, TurnerRV (1981) Photoregulation of the carotenoid biosynthetic pathway in albino and white collar mutants of Neurospora crassa. Plant Physiol 68 : 745–749.
109. SchmidhauserTJ, LauterFR, RussoVE, YanofskyC (1990) Cloning, sequence, and photoregulation of al-1, a carotenoid biosynthetic gene of Neurospora crassa. Mol Cell Biol 10 : 5064–5070.
110. SchmidhauserTJ, LauterFR, SchumacherM, ZhouW, RussoVE, et al. (1994) Characterization of al-2, the phytoene synthase gene of Neurospora crassa. Cloning, sequence analysis, and photoregulation. J Biol Chem 269 : 12060–12066.
111. NelsonMA, MorelliG, CarattoliA, RomanoN, MacinoG (1989) Molecular cloning of a Neurospora crassa carotenoid biosynthetic gene (albino-3) regulated by blue light and the products of the white collar locus. Mol Cell Biol 9 : 1271–1276.
112. CarlileMJ, FriendJ (1956) Carotenoids and reproduction in Pyronema confluens. Nature 178 : 369–370.
113. OlmedoM, Ruger-HerrerosC, LuqueEM, CorrochanoLM (2010) A complex photoreceptor system mediates the regulation by light of the conidiation genes con-10 and con-6 in Neurospora crassa. Fungal Genet Biol 47 : 352–363.
114. van den BurgHA, WesterinkN, FrancoijsKJ, RothR, WoestenenkE, et al. (2003) Natural disulfide bond-disrupted mutants of AVR4 of the tomato pathogen Cladosporium fulvum are sensitive to proteolysis, circumvent Cf-4-mediated resistance, but retain their chitin binding ability. J Biol Chem 278 : 27340–27346.
115. van den BurgHA, HarrisonSJ, JoostenMHAJ, VervoortJ, de WitPJ (2006) Cladosporium fulvum Avr4 protects fungal cell walls against hydrolysis by plant chitinases accumulating during infection. Mol Plant Microbe Interact 19 : 1420–1430.
116. FujiwaraS, ImaiJ, FujiwaraM, YaeshimaT, KawashimaT, et al. (1990) A potent antibacterial protein in royal jelly. Purification and determination of the primary structure of royalisin. J Biol Chem 265 : 11333–11337.
117. BuletP, HetruC, DimarcqJL, HoffmannD (1999) Antimicrobial peptides in insects; structure and function. Dev Comp Immunol 23 : 329–344.
118. MygindPH, FischerRL, SchnorrKM, HansenMT, SonksenCP, et al. (2005) Plectasin is a peptide antibiotic with therapeutic potential from a saprophytic fungus. Nature 437 : 975–980.
119. SchneiderT, KruseT, WimmerR, WiedemannI, SassV, et al. (2010) Plectasin, a fungal defensin, targets the bacterial cell wall precursor lipid II. Science 328 : 1168–1172.
120. BokJW, ChiangY-M, SzewczykE, Reyes-DominguezY, DavidsonAD, et al. (2009) Chromatin-level regulation of biosynthetic gene clusters. Nat Chem Biol 5 : 462–464.
121. BokJW, NoordermeerD, KaleSP, KellerNP (2006) Secondary metabolic gene cluster silencing in Aspergillus nidulans. Mol Microbiol 61 : 1636–1645.
122. RichardsTA, LeonardG, SoanesDM, TalbotNJ (2011) Gene transfer into the fungi. Fungal Biol Rev 25 : 98–110.
123. MoranNA, JarvikT (2010) Lateral transfer of genes from fungi underlies carotenoid production in aphids. Science 328 : 624–627.
124. IottiM, RubiniA, TisserantE, KholerA, PaolocciF, et al. (2012) Self/nonself recognition in Tuber melanosporum is not mediated by a heterokaryon incompatibility system. Fungal Biol 116 : 261–275.
125. DeleuC, ClavéC, BégueretJ (1993) A single amino acid difference is sufficient to elicit vegetative incompatibility in the fungus Podospora anserina. Genetics 135 : 45–52.
126. SaupeSJ, KuldauGA, SmithML, GlassNL (1996) The product of the het-C heterokaryon incompatibility gene of Neurospora crassa has characteristics of a glycine-rich cell wall protein. Genetics 143 : 1589–1600.
127. GlassNL, KanekoI (2003) Fatal attraction: nonself recognition and heterokaryon incompatibility in filamentous fungi. Eukaryot Cell 2 : 1–8.
128. KrokenS, GlassNL, TaylorJW, YoderOC, TurgeonBG (2003) Phylogenomic analyis of type I polyketide synthase genes in pathogenic and saprobic ascomycetes. Proc Nat Acad Sci USA 100 : 15670–15675.
129. BushleyKE, TurgeonBG (2010) Phylogenomics reveals subfamilies of fungal nonribosomal peptide synthetases and their evolutionary relationships. BMC Evol Biol 10 : 26.
130. Teichert I, Nowrousian M (2011) Evolution of genes for secondary metabolism in fungi. In: Pöggeler S, Wöstemeyer J, editors. Evolution of fungi and fungal-like organisms, The Mycota XIV. Berlin, Heidelberg: Springer-Verlag. pp. 231–255.
131. BushleyKE, RipollDR, TurgeonBG (2008) Module evolution and substrate specificity of fungal nonribosomal peptide synthetases involved in siderophore biosynthesis. BMC Evol Biol 8 : 328.
132. HaasH, EisendleM, TurgeonBG (2008) Siderophores in fungal physiology and virulence. Annu Rev Phytopathol 46 : 149–187.
133. ChiangY-M, SzewczykE, NayakT, DavidsonAD, SanchezJF, et al. (2008) Molecular genetic mining of the Aspergillus secondary metabolome: discovery of the Emericellamide biosynthetic pathway. Chem Biol 15 : 527–532.
134. KempkenF (2011) Fungal defences against animal antagonists - lectins & more. Mol Ecol 20 : 2876–2877.
135. KempkenF, RohlfsM (2010) Fungal secondary metabolite biosynthesis - a chemical defense strategy against antagonistic animals? Fungal Ecol 3 : 107–114.
136. BorkovichKA, AlexLA, YardenO, FreitagM, TurnerGE, et al. (2004) Lessons from the genome sequence of Neurospora crassa: tracing the path from genomic blueprint to multicellular organism. Microbiol Mol Biol Rev 68 : 1–108.
137. SonH, SeoYS, MinK, ParkAR, LeeJ, et al. (2011) A phenome-based functional analysis of transcription factors in the cereal head blight fungus, Fusarium graminearum. PLoS Pathog 7: e1002310.
138. CuomoCA, GüldenerU, XuJR, TrailF, TurgeonBG, et al. (2007) The Fusarium graminearum genome reveals a link between localized polymorphism and pathogen specialization. Science 317 : 1400–1402.
139. MontaniniB, LevatiE, BolchiA, KohlerA, MorinE, et al. (2011) Genome-wide search and functional identification of transcription factors in the mycorrhizal fungus Tuber melanosporum. New Phytol 189 : 736–750.
140. JohnsonAD (1995) Molecular mechanisms of cell-type determination in budding yeast. Curr Opin Genet Dev 5 : 552–558.
141. NoltingN, PöggelerS (2006) A STE12 homologue of the homothallic ascomycete Sordaria macrospora interacts with the MADS box protein MCM1 and is required for ascosporogenesis. Mol Microbiol 62 : 853–868.
142. LiD, BobrowiczP, WilkinsonHH, EbboleDJ (2005) A mitogen-activated protein kinase pathway essential for mating and contributing to vegetative growth in Neurospora crassa. Genetics 170 : 1091–1104.
143. VallimMA, MillerKY, MillerBL (2000) Aspergillus SteA (sterile12-like) is a homeodomain-C2/H2-Zn+2 finger transcription factor required for sexual reproduction. Mol Microbiol 36 : 290–301.
144. StuartJM, SegalE, KollerD, KimSK (2003) A gene-coexpression network for global discovery of conserved genetic modules. Science 302 : 249–255.
145. RomeroIG, RuvinskyI, GiladY (2012) Comparative studies of gene expression and the evolution of gene regulation. Nat Rev Genet 13 : 505–516.
146. HansenK, PfisterDH (2006) Systematics of the Pezizomycetes - the operculate discomycetes. Mycologia 98 : 1029–1040.
147. LandvikS, EggerKN, SchumacherT (1997) Towards a subordinal classification of the Pezizales (Ascomycota): phylogenetic analyses of SSU rDNA sequences. Nord J Bot 17 : 403–418.
148. Esser K (1982) Cryptogams - Cyanobacteria, Algae, Fungi, Lichens. London: Cambridge University Press.
149. MyersEW, SuttonGG, DelcherAL, DewIM, FasuloDP, et al. (2000) A whole-genome assembly of Drosophila. Science 287 : 2196–2204.
150. ZerbinoDR, BirneyE (2008) Velvet: Algorithms for de novo short read assembly using de Bruijn graphs. Genome Res 18 : 821–829.
151. HuangX, MadanA (1999) CAP3: A DNA sequence assembly program. Genome Res 9 : 868–877.
152. NowrousianM, RingelbergC, DunlapJC, LorosJJ, KückU (2005) Cross-species microarray hybridization to identify developmentally regulated genes in the filamentous fungus Sordaria macrospora. Mol Genet Genomics 273 : 137–149.
153. GrabherrMG, HaasBJ, YassourM, LevinJZ, ThompsonDA, et al. (2011) Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotech 29 : 644–652.
154. HaasBJ, DelcherAL, MountSM, WortmanJR, SmithRKJr, et al. (2003) Improving the Arabidopsis genome annotation using maximal transcript alignment assemblies. Nucl Acids Res 31 : 5654–5666.
155. KorfI (2004) Gene finding in novel genomes. BMC Bioinformatics 5 : 59.
156. StankeM, SchöffmannO, MorgensternB, WaackS (2006) Gene prediction in eukaryotes with a generalized hidden Markov model that uses hints from external sources. BMC Bioinformatics 7 : 62.
157. StankeM, WaackS (2003) Gene prediction with a hidden Markov model and a new intron submodel. Bioinformatics 19 (suppl. 2) ii215–225.
158. BesemerJ, BorodovskyM (2005) GeneMark: web software for gene finding in prokaryotes, eukaryotes and viruses. Nucl Acids Res 33: W451–454.
159. Horton P, Park KJ, Obayashi T, Nakai K (2006) Protein subcellular localization prediction with WoLF PSORT. Proceedings of the 4th Annual Asia Pacific Bioinformatics Conference APBC06, Taipei, Taiwan: pp. 39–48.
160. FinnRD, TateJ, MistryJ, CoggillPC, SammutSJ, et al. (2008) The Pfam protein families database. Nucl Acids Res 36: D281–288.
161. EddySR (2011) Accelerated profile HMM searches. PLoS Comp Biol 7: e1002195.
162. LaslettD, CanbackB (2004) ARAGORN, a program to detect tRNA genes and tmRNA genes in nucleotide sequences. Nucl Acids Res 32 : 11–16.
163. NawrockiEP, KolbeDL, EddySR (2009) Infernal 1.0: inference of RNA alignments. Bioinformatics 25 : 1335–1337.
164. TaquistH, CuiY, ArdellDH (2007) TFAM 1.0: an online tRNA function classifier. Nucl Acids Res 35: W350–353.
165. LoweTM, EddySR (1997) tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucl Acids Res 25 : 955–964.
166. JurkaJ, KapitonovVV, PavlicekA, KlonowskiP, KohanyO, et al. (2005) Repbase Update, a database of eukyrotic repetitive elements. Cytogenet Genome Res 110 : 462–467.
167. LewisZA, HondaS, KhlafallahTK, JeffressJK, FreitagM, et al. (2009) Relics of repeat-induced point mutation direct heterochromatin formation in Neurospora crassa. Genome Res 19 : 427–437.
168. MargolinBS, Garrett-EngelePW, StevensJN, FritzDY, Garrett-EngeleC, et al. (1998) A methylated Neurospora 5S rRNA pseudogene contains a transposable element inactivated by repeat-induced point mutation. Genetics 149 : 1787–1797.
169. SelkerEU, TountasNA, CrossSH, MargolinBS, MurphyJG, et al. (2003) The methylated component of the Neurospora crassa genome. Nature 422 : 893–897.
170. StajichJE, BlockD, BoulezK, BrennerSE, ChervitzSA, et al. (2002) The Bioperl Toolkit: Perl modules for the life sciences. Genome Res 12 : 1611–1618.
171. MorinE, KohlerA, BakerAR, Foulongne-OriolM, LombardV, et al. (2012) Genome sequence of the button mushroom Agaricus bisporus reveals mechanisms governing adaptation to a humic-rich ecological niche. Proc Nat Acad Sci USA 109 : 17501–17506.
172. SpanuPD, AbbottJ, C., AmselemJ, BurgisTA, SoanesDM, et al. (2010) Genome expansion and gene loss in powdery mildew fungi reveal tradeoffs in extreme parasitism. Science 330 : 1543–1546.
173. SharptonTJ, StajichJE, RounsleySD, GardnerMJ, WortmanJR, et al. (2009) Comparative genomic analyses of the human fungal pathogens Coccidioides and their relatives. Genome Res 19 : 1722–1731.
174. GalaganJE, CalvoSE, CuomoC, MaL-J, WortmanJR, et al. (2005) Sequencing of Aspergillus nidulans and comparative analysis with A. fumigatus and A. oryzae. Nature 438 : 1105–1115.
175. MartinF, AertsA, AhrenD, BrunA, DanchinEGJ, et al. (2008) The genome of Laccaria bicolor provides insights into mycorrhizal symbiosis. Nature 452 : 88–92.
176. GoodwinSB, M'barekSB, DhillonB, WittenbergAH, CraneCF, et al. (2011) Finished genome of the fungal wheat pathogen Mycosphaerella graminicola reveals dispensome structure, chromosome plasticity, and stealth pathogenesis. PLoS Genet 7: e1002070.
177. FedorovaND, KhaldiN, JoardarVS, MaitiR, AmedeoP, et al. (2008) Genomic islands in the pathogenic filamentous fungus Aspergillus fumigatus. PLOS Genet 4: e1000046.
178. GalaganJE, CalvoSE, BorkovichKA, SelkerEU, ReadND, et al. (2003) The genome sequence of the filamentous fungus Neurospora crassa. Nature 422 : 859–868.
179. HaneJK, LoweRGT, SolomonPS, TanK-C, SchochCL, et al. (2007) Dothideomycete plant interactions illuminated by genome sequencing and EST analysis of the wheat pathogen Stagonospora nodorum. Plant Cell 19 : 3347–3368.
180. GoffeauA, BarrellBG, BusseyH, DavisRW, DujonB, et al. (1996) Life with 6000 genes. Science 274 : 546–567.
181. WoodV, GwilliamR, RajandreamMA, LyneM, LyneR, et al. (2002) The genome sequence of Schizosaccharomyces pombe. Nature 415 : 871–880.
182. AmselemJ, CuomoCA, van KanJAL, ViaudM, BenitoEP, et al. (2011) Genomic analysis of the necrotrophic fungal pathogens Sclerotinia sclerotiorum and Botrytis cinerea. PLoS Genet 7: e1002230.
183. DujonB, ShermanD, FischerG, DurrensP, CasaregolaS, et al. (2004) Genome evolution in yeasts. Nature 430 : 35–44.
184. Huerta-CepasJ, Capella-GutierrezS, PryszczLP, DenisovI, KormesD, et al. (2011) PhylomeDB v3.0: an expanding repository of genome-wide collections of trees, alignments and phylogeny-based orthology and paralogy predictions. Nucl Acids Res 39: D556–560.
185. EdgarRC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucl Acids Res 32 : 1792–1797.
186. KatohK, KumaK, TohH, MiyataT (2005) MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucl Acids Res 33 : 511–518.
187. SubramanianAR, KaufmannM, MorgensternB (2008) DIALIGN-TX: greedy and progressive approaches for segment-based multiple sequence alignment. Algorithms Mol Biol 3 : 6.
188. LandanG, GraurD (2007) Heads or tails: a simple reliability check for multiple sequence alignments. Mol Biol Evol 24 : 1380–1383.
189. WallaceIM, O'SullivanO, HigginsDG, NotredameC (2006) M-Coffee: combining multiple sequence alignment methods with T-Coffee. Nucl Acids Res 34 : 1692–1699.
190. Capella-GutierrezS, Silla-MartinezJM, GabaldónT (2009) trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinf 25 : 1972–1973.
191. GuindonS, DufayardJF, LefortV, AnisimovaM, HordijkW, et al. (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59 : 307–321.
192. GascuelO (1997) BIONJ: an improved version of the NJ algorithm based on a simple model of sequence data. Mol Biol Evol 14 : 685–695.
193. Huerta-CepasJ, DopazoJ, GabaldónT (2010) ETE: a python Environment for Tree Exploration. Bioinf 11 : 24.
194. LeSQ, GascuelO (2008) An improved general amino acid replacement matrix. Mol Biol Evol 25 : 1307–1320.
195. WeheA, BansalMS, BurleighJG, EulensteinO (2008) DupTree: a program for large-scale phylogenetic analyses using gene tree parsimony. Bioinf 24 : 1540–1541.
196. HedgesSB, DudleyJ, KumarS (2006) TimeTree: a public knowledge-base of divergence times among organisms. Bioinf 22 : 2971–2972.
197. HeckmanDS, GeiserDM, EidellBR, StaufferRL, KardosNL, et al. (2001) Molecular evidence for the early colonization of land by fungi and plants. Science 293 : 1129–1133.
198. ZhangZ, López-GiráldezF, TownsendJP (2010) LOX: inferring Level Of eXpression from diverse methods of census sequencing. Bioinf 26 : 1918–1919.
199. AndersS, HuberW (2010) Differential expression analysis for sequence count data. Genome Biol 11: R106.
200. HardcastleTJ, KellyKA (2010) baySeq: empirical Bayesian methods for identifying differential expression in sequence count data. BMC Bioinf 11 : 422.
201. FraleyC, RafteryAE (2002) Model-based clustering, discriminant analysis, and density estimation J Amer Stat Assoc. 97 : 611–631.
202. KlixV, NowrousianM, RingelbergC, LorosJJ, DunlapJC, et al. (2010) Functional characterization of MAT1-1-specific mating-type genes in the homothallic ascomycete Sordaria macrospora provides new insights into essential and non-essential sexual regulators. Eukaryot Cell 9 : 894–905.
203. NowrousianM, MasloffS, PöggelerS, KückU (1999) Cell differentiation during sexual development of the fungus Sordaria macrospora requires ATP citrate lyase activity. Mol Cell Biol 19 : 450–460.
204. ThompsonJD, GibsonTJ, PlewniakF, JeanmouginF, HigginsDG (1997) The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 24 : 4876–4882.
205. WaterhouseAM, ProcterJB, MartinDMA, ClampM, BartonGJ (2009) Jalview Version 2 - a multiple sequence alignment editor and analysis workbench. Bioinformatics 25 : 1189–1191.
206. PageR (1996) TREEVIEW: an application to display phylogenetic trees on personal computers. Appl Biosci 12 : 357–358.
207. MaL-J, IbrahimAS, SkoryC, GrabherrMG, BurgerG, et al. (2009) Genomic analysis of the basal lineage fungus Rhizopus oryzae reveals a whole-genome duplication. PLoS Genet 5: e1000549.
208. MartinezD, ChallacombeJ, MorgensternI, HibbettD, SchmollM, et al. (2009) Genome, transcriptome, and secretome analysis of wood decay fungus Postia placenta supports unique mechanisms of lignocellulose conversion. Proc Nat Acad Sci USA 106 : 1954–1959.
209. StajichJE, WilkeSK, AhrénD, AuCH, BirrenBW, et al. (2010) Insights into evolution of multicellular fungi from the assembled chromosomes of the mushroom Coprinopsis cinerea (Coprinus cinereus). Proc Nat Acad Sci USA 107 : 11889–11894.
Štítky
Genetika Reprodukční medicína
Článek Rapid Intrahost Evolution of Human Cytomegalovirus Is Shaped by Demography and Positive SelectionČlánek Common Variants in Left/Right Asymmetry Genes and Pathways Are Associated with Relative Hand SkillČlánek Manipulating or Superseding Host Recombination Functions: A Dilemma That Shapes Phage EvolvabilityČlánek Maternal Depletion of Piwi, a Component of the RNAi System, Impacts Heterochromatin Formation inČlánek Hsp104 Suppresses Polyglutamine-Induced Degeneration Post Onset in a Drosophila MJD/SCA3 ModelČlánek Cooperative Interaction between Phosphorylation Sites on PERIOD Maintains Circadian Period inČlánek VAPB/ALS8 MSP Ligands Regulate Striated Muscle Energy Metabolism Critical for Adult Survival inČlánek Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2AČlánek A Link between ORC-Origin Binding Mechanisms and Origin Activation Time Revealed in Budding YeastČlánek Genotype-Environment Interactions Reveal Causal Pathways That Mediate Genetic Effects on PhenotypeČlánek Chromatin-Specific Regulation of Mammalian rDNA Transcription by Clustered TTF-I Binding SitesČlánek Meiotic Recombination in Arabidopsis Is Catalysed by DMC1, with RAD51 Playing a Supporting Role
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 9- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Srdeční frekvence embrya může být faktorem užitečným v předpovídání výsledku IVF
- Prenatální expozice ftalátům a anogenitální vzdálenost u novorozenců
- Vztah užívání alkoholu a mužské fertility
-
Všechny články tohoto čísla
- The Pathway Gene Functions together with the -Dependent Isoprenoid Biosynthetic Pathway to Orchestrate Germ Cell Migration
- Take Off, Landing, and Fly Anesthesia
- Nucleosome Assembly Proteins Get SET to Defeat the Guardian of Chromosome Cohesion
- Whole-Exome Sequencing Reveals a Rapid Change in the Frequency of Rare Functional Variants in a Founding Population of Humans
- Evidence Is Evidence: An Interview with Mary-Claire King
- Rapid Intrahost Evolution of Human Cytomegalovirus Is Shaped by Demography and Positive Selection
- Convergent Transcription Induces Dynamic DNA Methylation at Loci
- Environmental Stresses Disrupt Telomere Length Homeostasis
- Ultra-Sensitive Sequencing Reveals an Age-Related Increase in Somatic Mitochondrial Mutations That Are Inconsistent with Oxidative Damage
- Common Variants in Left/Right Asymmetry Genes and Pathways Are Associated with Relative Hand Skill
- Genetic and Anatomical Basis of the Barrier Separating Wakefulness and Anesthetic-Induced Unresponsiveness
- The Locus, Exclusive to the Ambulacrarians, Encodes a Chromatin Insulator Binding Protein in the Sea Urchin Embryo
- Binding of NF-κB to Nucleosomes: Effect of Translational Positioning, Nucleosome Remodeling and Linker Histone H1
- Manipulating or Superseding Host Recombination Functions: A Dilemma That Shapes Phage Evolvability
- Dynamics of DNA Methylation in Recent Human and Great Ape Evolution
- Functional Dissection of Regulatory Models Using Gene Expression Data of Deletion Mutants
- PAQR-2 Regulates Fatty Acid Desaturation during Cold Adaptation in
- N-alpha-terminal Acetylation of Histone H4 Regulates Arginine Methylation and Ribosomal DNA Silencing
- A Genome-Wide Systematic Analysis Reveals Different and Predictive Proliferation Expression Signatures of Cancerous vs. Non-Cancerous Cells
- Maternal Depletion of Piwi, a Component of the RNAi System, Impacts Heterochromatin Formation in
- miR-1/133a Clusters Cooperatively Specify the Cardiomyogenic Lineage by Adjustment of Myocardin Levels during Embryonic Heart Development
- Hsp104 Suppresses Polyglutamine-Induced Degeneration Post Onset in a Drosophila MJD/SCA3 Model
- Genome-Wide Analysis of Genes and Their Association with Natural Variation in Drought Tolerance at Seedling Stage of L
- Deep Resequencing of GWAS Loci Identifies Rare Variants in , and That Are Associated with Ulcerative Colitis
- Cooperative Interaction between Phosphorylation Sites on PERIOD Maintains Circadian Period in
- VAPB/ALS8 MSP Ligands Regulate Striated Muscle Energy Metabolism Critical for Adult Survival in
- Analysis of Genes Reveals Redundant and Independent Functions in the Inner Ear
- Predicting the Risk of Rheumatoid Arthritis and Its Age of Onset through Modelling Genetic Risk Variants with Smoking
- Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2A
- A Shift to Organismal Stress Resistance in Programmed Cell Death Mutants
- Fragile Site Instability in Causes Loss of Heterozygosity by Mitotic Crossovers and Break-Induced Replication
- Tracking of Chromosome and Replisome Dynamics in Reveals a Novel Chromosome Arrangement
- The Condition-Dependent Transcriptional Landscape of
- Ago1 Interacts with RNA Polymerase II and Binds to the Promoters of Actively Transcribed Genes in Human Cancer Cells
- Nebula/DSCR1 Upregulation Delays Neurodegeneration and Protects against APP-Induced Axonal Transport Defects by Restoring Calcineurin and GSK-3β Signaling
- System-Wide Analysis Reveals a Complex Network of Tumor-Fibroblast Interactions Involved in Tumorigenicity
- Meta-Analysis of Genome-Wide Association Studies Identifies Six New Loci for Serum Calcium Concentrations
- and Are Required for Cellularization and Differentiation during Female Gametogenesis in
- Growth factor independent-1 Maintains Notch1-Dependent Transcriptional Programming of Lymphoid Precursors
- Whole Genome Sequencing Identifies a Deletion in Protein Phosphatase 2A That Affects Its Stability and Localization in
- An Alteration in ELMOD3, an Arl2 GTPase-Activating Protein, Is Associated with Hearing Impairment in Humans
- Genomic Identification of Founding Haplotypes Reveals the History of the Selfing Species
- Plasticity Regulators Modulate Specific Root Traits in Discrete Nitrogen Environments
- The IDD14, IDD15, and IDD16 Cooperatively Regulate Lateral Organ Morphogenesis and Gravitropism by Promoting Auxin Biosynthesis and Transport
- Stochastic Loss of Silencing of the Imprinted Allele, in a Mouse Model and Humans with Prader-Willi Syndrome, Has Functional Consequences
- The Prefoldin Complex Regulates Chromatin Dynamics during Transcription Elongation
- PKA Controls Calcium Influx into Motor Neurons during a Rhythmic Behavior
- A Pre-mRNA-Splicing Factor Is Required for RNA-Directed DNA Methylation in
- Cell-Type Specific Features of Circular RNA Expression
- The Uve1 Endonuclease Is Regulated by the White Collar Complex to Protect from UV Damage
- An Atypical Kinase under Balancing Selection Confers Broad-Spectrum Disease Resistance in Arabidopsis
- Genome-Wide Mutation Avalanches Induced in Diploid Yeast Cells by a Base Analog or an APOBEC Deaminase
- Extensive Divergence of Transcription Factor Binding in Embryos with Highly Conserved Gene Expression
- Bi-modal Distribution of the Second Messenger c-di-GMP Controls Cell Fate and Asymmetry during the Cell Cycle
- Cell Interactions and Patterned Intercalations Shape and Link Epithelial Tubes in
- A Link between ORC-Origin Binding Mechanisms and Origin Activation Time Revealed in Budding Yeast
- The Genome and Development-Dependent Transcriptomes of : A Window into Fungal Evolution
- SKN-1/Nrf, A New Unfolded Protein Response Factor?
- The Highly Prolific Phenotype of Lacaune Sheep Is Associated with an Ectopic Expression of the Gene within the Ovary
- Fusion of Large-Scale Genomic Knowledge and Frequency Data Computationally Prioritizes Variants in Epilepsy
- IL-17 Attenuates Degradation of ARE-mRNAs by Changing the Cooperation between AU-Binding Proteins and microRNA16
- An Enhancer Element Harboring Variants Associated with Systemic Lupus Erythematosus Engages the Promoter to Influence A20 Expression
- Genome Analysis of a Transmissible Lineage of Reveals Pathoadaptive Mutations and Distinct Evolutionary Paths of Hypermutators
- Type I-E CRISPR-Cas Systems Discriminate Target from Non-Target DNA through Base Pairing-Independent PAM Recognition
- Divergent Transcriptional Regulatory Logic at the Intersection of Tissue Growth and Developmental Patterning
- MEIOB Targets Single-Strand DNA and Is Necessary for Meiotic Recombination
- Transmission of Hypervirulence Traits via Sexual Reproduction within and between Lineages of the Human Fungal Pathogen
- Integration of the Unfolded Protein and Oxidative Stress Responses through SKN-1/Nrf
- Guanine Holes Are Prominent Targets for Mutation in Cancer and Inherited Disease
- Regulation of the Boundaries of Accessible Chromatin
- Natural Genetic Transformation Generates a Population of Merodiploids in
- Ablating Adult Neurogenesis in the Rat Has No Effect on Spatial Processing: Evidence from a Novel Pharmacogenetic Model
- Genotype-Environment Interactions Reveal Causal Pathways That Mediate Genetic Effects on Phenotype
- The Molecular Mechanism of a -Regulatory Adaptation in Yeast
- Phenotypic and Genetic Consequences of Protein Damage
- Recent Acquisition of by Baka Pygmies
- Fatty Acid Taste Signals through the PLC Pathway in Sugar-Sensing Neurons
- A Critical Role for PDGFRα Signaling in Medial Nasal Process Development
- Chromatin-Specific Regulation of Mammalian rDNA Transcription by Clustered TTF-I Binding Sites
- Meiotic Recombination in Arabidopsis Is Catalysed by DMC1, with RAD51 Playing a Supporting Role
- dTULP, the Homolog of Tubby, Regulates Transient Receptor Potential Channel Localization in Cilia
- Widespread Dysregulation of Peptide Hormone Release in Mice Lacking Adaptor Protein AP-3
- , a Direct Transcriptional Target, Modulates T-Box Factor Activity in Orofacial Clefting
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Genome-Wide Systematic Analysis Reveals Different and Predictive Proliferation Expression Signatures of Cancerous vs. Non-Cancerous Cells
- Recent Acquisition of by Baka Pygmies
- The Condition-Dependent Transcriptional Landscape of
- Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2A
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



