-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamadTULP, the Homolog of Tubby, Regulates Transient Receptor Potential Channel Localization in Cilia
Mechanically gated ion channels convert sound into an electrical signal for the sense of hearing. In Drosophila melanogaster, several transient receptor potential (TRP) channels have been implicated to be involved in this process. TRPN (NompC) and TRPV (Inactive) channels are localized in the distal and proximal ciliary zones of auditory receptor neurons, respectively. This segregated ciliary localization suggests distinct roles in auditory transduction. However, the regulation of this localization is not fully understood. Here we show that the Drosophila Tubby homolog, King tubby (hereafter called dTULP) regulates ciliary localization of TRPs. dTULP-deficient flies show uncoordinated movement and complete loss of sound-evoked action potentials. Inactive and NompC are mislocalized in the cilia of auditory receptor neurons in the dTulp mutants, indicating that dTULP is required for proper cilia membrane protein localization. This is the first demonstration that dTULP regulates TRP channel localization in cilia, and suggests that dTULP is a protein that regulates ciliary neurosensory functions.
Published in the journal: . PLoS Genet 9(9): e32767. doi:10.1371/journal.pgen.1003814
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003814Summary
Mechanically gated ion channels convert sound into an electrical signal for the sense of hearing. In Drosophila melanogaster, several transient receptor potential (TRP) channels have been implicated to be involved in this process. TRPN (NompC) and TRPV (Inactive) channels are localized in the distal and proximal ciliary zones of auditory receptor neurons, respectively. This segregated ciliary localization suggests distinct roles in auditory transduction. However, the regulation of this localization is not fully understood. Here we show that the Drosophila Tubby homolog, King tubby (hereafter called dTULP) regulates ciliary localization of TRPs. dTULP-deficient flies show uncoordinated movement and complete loss of sound-evoked action potentials. Inactive and NompC are mislocalized in the cilia of auditory receptor neurons in the dTulp mutants, indicating that dTULP is required for proper cilia membrane protein localization. This is the first demonstration that dTULP regulates TRP channel localization in cilia, and suggests that dTULP is a protein that regulates ciliary neurosensory functions.
Introduction
The auditory system allows animals to communicate and obtain information about their environment. The hearing organs transform sound into an electrical signal through a process called mechanotransduction, the conversion of a mechanical force impinging on a cell into an intracellular signal [1]. Although the recent discovery of several molecules involved in mechanotransduction allows interpretation of the biophysical properties of the mechanotransduction process for hearing [2], many additional molecular players in auditory development and function are waiting to be unveiled.
Drosophila melanogaster has been suggested as a model organism to study the fundamental process of hearing [3], [4]. Hearing in the fly is necessary for the detection of courtship songs [5]–[7]. Male-generated courtship song causes females to reduce locomotion and enhances female receptivity, whereas it causes males to chase each other [8]. The ability to hear courtship songs is ascribed to Johnston's organ (JO) in the second antennal segment. Near-field sounds rotate the sound receiver; the third antennal segment and the arista and this rotation of the antennal receiver transmits mechanical forces to the JO in the second antennal segment, which is connected to the third antennal segment by a thin stalk [9]. Each JO sensilla consists of two or three chordotonal neurons and several supporting cells. The outer dendritic segments of the JO neurons are compartmentalized cilia which are directly connected to the antennal sound receiver via extracellular caps. The distortion of the junction between the second and third segment stretches the cilia and stimulates the JO neurons.
Several transient receptor potential (TRP) channels have been shown to be required for Drosophila hearing transduction and amplification [4], [10]–[14]. Mutation in nompC, the Drosophila TRPN channel, resulted in substantial reduction of sound-evoked potentials [4]. Reports showing that NompC and TRP-4 (the C. elegans ortholog of NompC) are bona fide mechanotransduction channels support the idea that NompC is the Drosophila hearing transducer [15], [16]. Two Drosophila TRPV channel, inactive (iav) and nanchung (nan), mutants showed complete loss of sound-evoked action potentials [11]. However, they have not been considered to be the hearing transduction complex per se; rather they are thought to be required to amplify the electric signal generated by the hearing transduction complex, since Iav and Nan reside in the proximal cilia which are distant from the distal cilia where NompC is localized and mechanical force is directly transmitted [17], [18]. A recent study which employed a new method to measure subthreshold signals from the JO neurons suggested the opposite possibility that the TRPV (Iav and Nan) complex is the hearing transduction complex modulated by TRPN (NompC) [14]. Although the exact roles of each TRP in Drosophila hearing are still controversial, it is clear that TRPN and TRPV have essential and distinct roles in Drosophila hearing.
Several attempts have been made to identify molecular players regulating the function of the ciliated mechanoreceptor neurons. Gene expression profiling identified chordotonal organ-enriched genes from campaniform mechanoreceptors, developing embryo chordotonal neurons, and the second antennal segment [19]–[21]. Alternatively, chordotonal neuron-specific genes were identified by searching for regulatory factor X (RFX)-binding sites, because ciliogenesis of the chordotonal neurons mainly depends on the RFX transcription factor [22]. However, so far only a limited number of genes involved in TRP channel localization in the JO neuron cilia have been identified and characterized, including axonemal components and intraflagellar transports (IFTs) [17], [23]. IFTs are indispensable for the formation and maintenance of cilia as well as for the transport of proteins along the microtubules in and out of the cilia [24]–[26]. Therefore, mutation of many of the characterized genes results in not only delocalization of the TRPs but also profound structural abnormality in cilia, rendering it difficult to delineate the gene functions specific to TRP localization.
Tubby is the founding member of Tubby-like proteins (TULPs) [27]. Loss-of-function of the Tubby gene exhibits adult-onset obesity, retinal degeneration, and hearing loss in mice. The Drosophila genome encodes one Tubby homolog called King tubby (hereafter designated dTULP), which shares approximately 43% amino acid identity with mouse Tubby (Figure S1A) [28]. At the embryonic stage, dTULP is expressed in various types of neuronal cells including the chordotonal neurons. Although previous expression analyses and bioinformatic approaches detected dTulp in the chordotonal organs, its presence did not attract much interest because of its distribution in various neuronal cell types [22].
In this study, we aimed to investigate the novel molecular function of dTULP in Drosophila hearing. dTULP is localized to the well-defined ciliary structure of Drosophila auditory organs. Loss of dTULP has no effect on the ciliary structure of the JO neurons, but NompC and Iav localization in cilia was severely altered. These data demonstrate a new role of dTULP as a regulator of TRP localization in the hearing organs.
Results
Generation of dTulp mutants
To test whether dTULP plays a role in Drosophila hearing, we generated two dTulp mutant alleles by ends-out homologous recombination [29]. The first allele was dTulp1, which harbours a deleted C-terminal containing the conserved “tubby domain” (residues 220 to 460; Figure 1A). The second allele, dTulpG, was generated by replacing an N-terminal portion of the dTULP coding region (residues 18 to 261; Figure S1B) with GAL4 coding sequences at the site corresponding to the initiation codon of the short splicing variant of dTulp. Genomic PCR analyses showed that the dTulp genomic locus was deleted in dTulp1 and dTulpG flies (Figure 1B and Figure S1B). We raised antibodies to dTULP, which recognized a 51 kDa protein as predicted in wild-type fly extracts on a Western blot, and confirmed that dTULP was not detected in dTulp1 and dTulpG fly extracts (Figure 1C). Both alleles are homozygous viable and fertile.
Fig. 1. Hearing loss in the dTulp mutant. 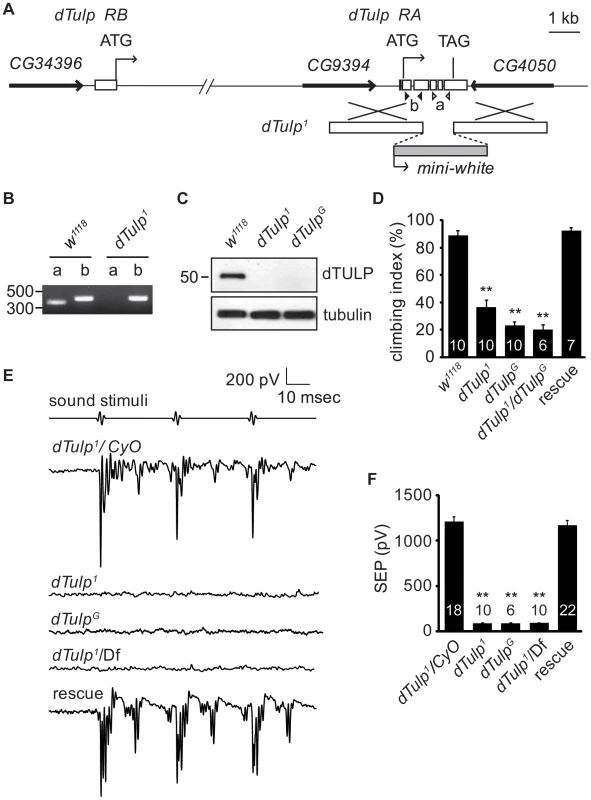
(A) Physical map of the dTulp locus and the targeting schemes used to generate the dTulp1 allele by homologous recombination. The boxes indicate dTulp exons. Open (a) and solid triangles (b) indicate the locations of primers used for genomic PCR to confirm dTulp deletion. (B) Confirmation of the deletion of the dTulp locus using genomic PCR. (C) Western blot analyses using extracts from wild-type, dTulp1, and dTulpG flies. (D) dTULP-deficient flies exhibit climbing defects. A single insertion of the genomic rescue construct rescues the dTulp1climbing defect. The number of tests for each genotype is indicated inside the bars. **p<0.01 compared with wild type (w1118) using one-way ANOVA with post-hoc analysis (Tukey). (E) Representative traces of the sound-evoked potentials recorded from the antennal nerve of the indicated genotypes. Df indicates Df(2R)BSC462. The hearing defect was rescued by introduction of a genomic DNA fragment. (F) Quantification of the sound-evoked potentials. Genotypes are indicated. The number of flies used for quantification for each genotype is indicated. **p<0.01 compared to dTulp1/CyO. p values were calculated using ANOVA with post-hoc Tukey assay. All error bars indicate SEM. Hearing impairment in dTulp mutant flies
Since both dTulp1and dTulpG mutant alleles showed postural problems and uncoordinated movement, we performed a climbing assay. Flies were banged down to the bottom of a vertical tube and the percentage of the flies climbing above half of the height of the vertical tube within 10 seconds was recorded as the climbing index. dTulp1, dTulpG, and transheterozygote flies exhibited a decreased climbing index compared to control flies (Figure 1D). Introduction of a P[acman] clone containing the dTULP coding region (CH321-59C17) in the dTulp1 mutant background rescued this phenotype [30]. These data suggested that dTulp mutants may have functional defects in the JO neurons [13].
To check for hearing defects in dTulp mutant flies, we recorded extracellular sound-evoked potentials in wild-type and dTULP-deficient flies. Sound-evoked potentials were completely abolished in dTulp1, dTulpG, and dTulp1 in trans with a deletion that completely removed dTulp, Df(2R)BSC462. Genomic rescue using the P[acman] clone produced sound-evoked potentials similar to those in the wild-type, suggesting that the hearing defect was specifically due to dTulp ablation (Figure 1E and 1F).
Expression of dTULP in the chordotonal neurons
To test whether dTULP is expressed in the JO neurons, we first attempted to take advantage of the GAL4/UAS system using the dTulpG allele. However, the GAL4 reporter inserted in dTulpG was not expressed. This may be caused by inserting GAL4 at the site corresponding to the initiation codon of the short splicing variant of dTulp rather than the long splicing variant.
Therefore, we performed immunohistochemistry with dTULP antibodies. We found that dTULP was expressed in the cilia as well as the cell body of the chordotonal neurons (Figure 2A, left). We did not detect dTULP immunoreactivity in the JO neurons in dTulp1flies, indicating that the immunosignal is specific for dTULP (Figure 2A, right). To further characterize the ciliary localization of dTULP, we compared the localization of dTULP with that of Iav and NompC. The subcellular localization of Iav and NompC are in the proximal and distal cilia, respectively, in a mutually exclusive manner (Figure 2B) [11], [17], [18], [31]. dTULP staining extended from the proximal to distal cilia with a much weaker signal observed in the distal portion (Figure 2C and 2D). The mouse Tubby protein has been reported to shuttle from the plasma membrane to the nucleus upon Gq-coupled G protein-coupled receptor (GPCR) activation [32]. dTULP was also detected in the cell body as well as the nucleus in the JO neurons (Figure 2A and Figure S7B). We also found that dTULP was expressed in other types of sensory neurons with cilia (Figure S2).
Fig. 2. Ciliary localization of dTULP in Johnston's organ. 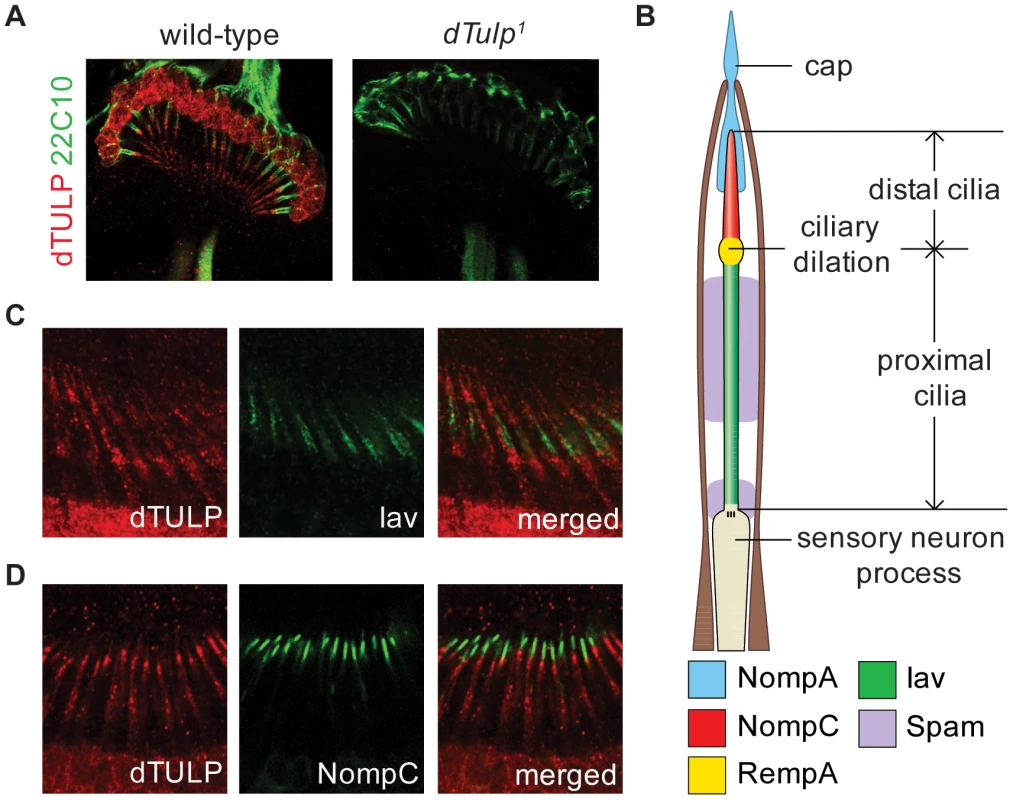
(A) Expression of dTULP in the chordotonal neurons of the Johnston's organ in wild type (left) and dTulp1 (right). 22C10 is a monoclonal antibody that stains all chordotonal neurons except for the cilia. (B) Schematic diagram of the scolopidium in the Johnston's organ. For simplicity, only one sensory neuron process is depicted. The localization of molecular components is marked with shaded colors. (C–D) dTULP localization was analyzed in comparison with two ciliary proteins, Iav and NompC. (C) Immunostaining of dTULP and Iav–GFP (anti-GFP) on the antennae of Iav-GFP flies. (D) Immunostaining of dTULP and NompC-GFP (anti-GFP) on the antennae of F>UAS-NompC:GFP flies. Normal scolopidia structure in dTulp mutants
To examine whether the dTulp mutants have developmental defects in the JO neuron structure, we observed the expression of a membrane-targeted GFP (UAS-mCD8:GFP) driven by the pan-neuronal promoter (elav-GAL4) in the JO neurons. We found no gross structural abnormalities in dTulp1flies (Figure 3A). Electron microscopy of the JO revealed that most dTulp mutants had normal ciliary ultrastructure (Figure 3B). Approximately 9.3% of chordotonal scolopidia appeared abnormal in terms of cilia number or cap-cilia connections (Figure S3). In addition, we did not observe any discernible changes in the expression of the dendritic cap protein NompA, which transmits mechanical stimuli to the distal segment of chordotonal neurons in dTulp mutants (Figure 3C) [33]. These observations suggest that structural changes in the JO cannot account for severe hearing impairment in dTulp mutants.
Fig. 3. Normal scolopidia structure in the dTulp mutant. 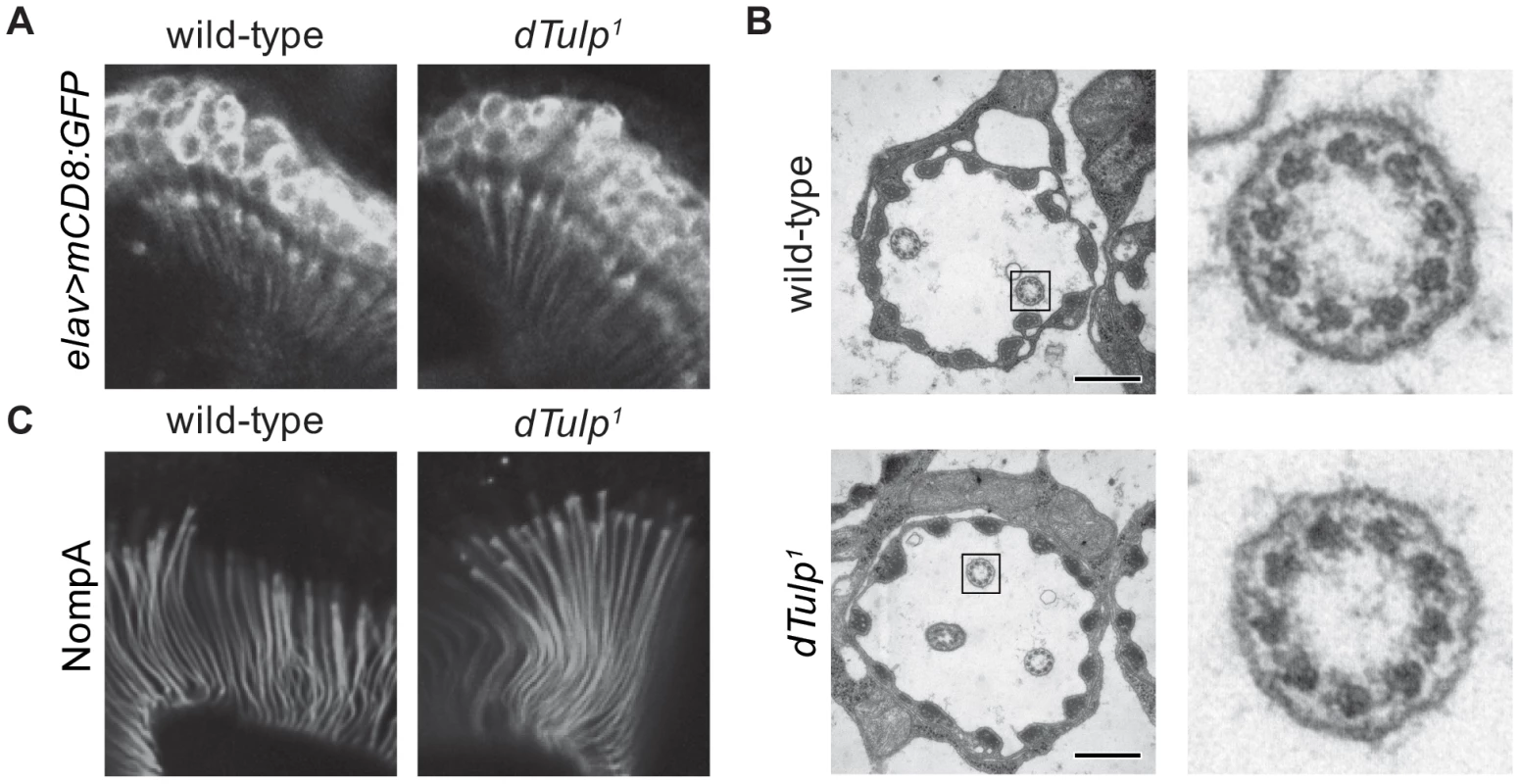
(A) Confocal images of the second antennal segment expressing mCD8:GFP. Genotypes of animals are dTulp1/+;elav-GAL4/UAS-mCD8:GFP (left) and dTulp1/dTulp1;elav-GAL4/UAS-mCD8:GFP (right). (B) Transmission electron microscopic examination of wild-type and dTulp1mutant antennal scolopidium. Scale bars represent 0.2 µm. The black boxes mark the inset fields shown at higher magnification on the right. (C) Confocal images of second segment antennae from pupae expressing GFP-tagged NompA. Genotypes of animals are dTulp1/+;NompA-GFP/+ (left) and dTulp1/dTulp1;NompA-GFP/+ (right). Requirement of dTULP for proper ciliary localization of Iav, NompC, and Spam
Mutations of trps, including iav and nompC, cause hearing defects in Drosophila [4], [11]. To investigate the possibility that dTULP controls the expression of TRPs and other genes which are indispensable for Drosophila hearing, we performed quantitative PCR analysis of such genes and no significant differences in expression levels were present between wild-type and dTulp1 antennae (Figure S4). This suggested that dTULP plays other roles in Drosophila chordotonal neurons rather than as a transcription factor that controls transcription of known hearing related genes, although we cannot exclude the possibility that dTULP regulates the expression of hearing related genes we did not survey.
Next we examined the ciliary localization of Iav and NompC in the dTulp mutants. Surprisingly, Iav was not localized to the proximal cilia in dTulp1 flies (Figure 4A). Furthermore, NompC, characteristically localized to the distal cilia (Figure 2B), was redistributed toward the proximal cilia (Figure 4B). Spacemaker (Spam) is an extracellular protein which protects cells from massive osmotic stress [34]. Localization of Spam was also altered in dTulp mutants from its two typical locations: the luminal space adjacent to the cilia dilation and the scolopidium base (Figure 4C) [35]. Introduction of the dTulp+ transgene rescued the localization of Iav, NompC, and Spam (Figure 4).
Fig. 4. Mislocalization of Iav, NompC and Spam in the dTulp mutant. 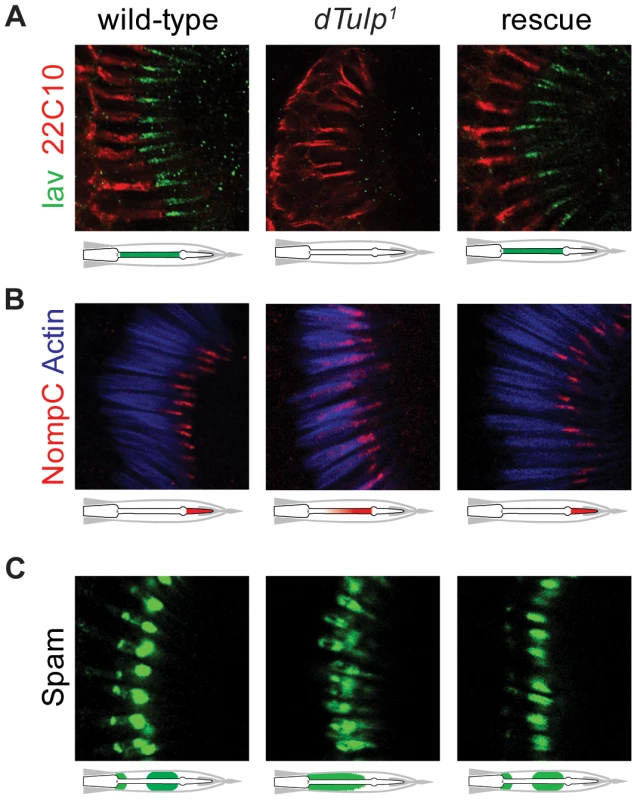
(A–C) Confocal imaging of the second antennal segment from wild-type, dTulp1, and genomic rescue flies. The localization of Iav-GFP and NompC are depicted by schematic diagrams. (A) Immunostaining of Iav-GFP (anti-GFP) counterstained with 22C10 which stains neuronal cells except for the outer segments, i.e. cilia. (B) Immunostaining of NompC counterstained with phalloidin that specifically stains actin-rich scolopales. (C) Immunostaining of Spam detected with the monoclonal antibody 21A6. IFTs are involved in the localization of Iav, NompC, and Spam [17], [23]. Because IFT mutants show similar phenotypes to the dTulp mutant, we investigated the localization of IFT proteins in dTULP-deficient flies. Ciliary localization of the two IFTs, NompB (the ortholog of human IFT-B, IFT88) and RempA (the ortholog of human IFT-A, IFT140), was unaffected in dTulp1 mutants (Figure S5).
To further address the functional relationship between dTULP and IFTs, we examined distribution of dTULP in three IFT (nompB, rempA, and oseg1) mutants and a retrograde motor dynein heavy chain (beethoven) mutant. Although the rempA, oseg1, and beethoven mutants show different degrees of defective cilia structure, dTULP is localized to the deteriorated cilia of each mutant, suggesting that rempA, Oseg1, and beethoven are not required for the transport of dTULP into the cilia (Figure S6A–S6C). Since the nompB mutant does not develop cilia structure, dTULP was present in the inner segment at a high level (Figure S6D) [36]. However, it is possible that other IFTs may play a role for dTULP ciliary localization even though the IFTs we examined are not involved in ciliary localization of dTULP.
Requirement of both IFT - and phosphoinositide-binding domains of dTULP for the proper cilia localization of TRPs
Mammalian Tubby have two distinct domains: nuclear localization signal (NLS) and phosphoinositide (PIP)-binding domain. An NLS, which allows Tubby to translocate into the nucleus, resides in the N-terminal region of Tubby [32]. Recently, a short stretch of amino acids including the NLS in TULP3, a mammalian member of the Tubby-like protein family, has been reported as an IFT-A binding domain [37]. A PIP-binding domain in the C-terminal tubby domain allows Tubby to be localized under the inner leaflet of the plasma membrane through binding to specific phosphoinositides. These domains are also conserved in dTULP (Figure 5A).
Fig. 5. Functional domain mapping of dTULP for ciliary localization of Iav and NompC. 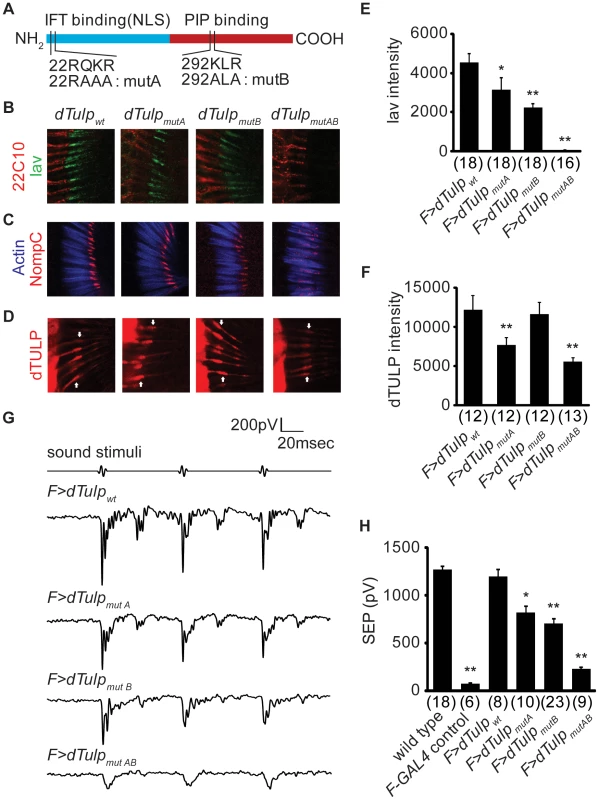
(A) Schematic diagram showing different domains of dTULP as well as dTULP mutant forms (mutA, mutB, and mutAB). Location and identity of each mutation are marked. IFT, intraflagellar transport; NLS, nuclear localization signal; PIP, phosphoinositide. (B–D) Confocal imaging of the second antennal segment in the dTulp knockout flies expressing dTULP wild-type (dTULPwt), dTULPmutA, dTULPmutB, and dTULPmutAB. (B) Confocal imaging of Iav-GFP counterstained with 22C10 which stains neuronal cells except for the cilia located in the outer segment. (C) Immunostaining of NompC counterstained with phalloidin that specifically stains actin-rich scolopales. (D) Immunostaining of dTULP. Arrows indicate the junction between inner and outer segment. (E–F) Quantification of Iav-GFP and dTULP expression levels in the proximal cilia. The number of images analyzed is shown in parentheses. (E) Quantification of Iav-GFP expression level in the proximal cilia. *p<0.05 and **p<0.01 compared to dTULPwt-expressing dTulp mutant. (F) Quantification of dTULP expression level in the proximal cilia. **p<0.01 compared to dTULPwt-expressing dTulp mutant. (G) Representative traces of sound-evoked potentials recorded from the antennal nerve of dTULPwt, dTULPmutA, dTULPmutB, and dTULPmutAB-expressing dTulp1 flies. (H) Quantification of sound-evoked potentials of indicated genotypes. Genotypes of animal are dTulp1/CyO, dTulp1,F-GAL4/dTulp1, dTulp1,F-GAL4/dTulp1;UAS-dTulpwt/+, dTulp1,F-GAL4/dTulp1;UAS-dTulpmutA/+, dTulp1,F-GAL4/dTulp1;UAS-dTulpmutB/+, and dTulp1,F-GAL4/dTulp1;UAS-dTulpmutAB/+. *p<0.05 and **p<0.01 compared to dTulp1/CyO. The number of flies used for quantification of each genotype is indicated in parentheses. All p values were calculated using ANOVA with post-hoc Tukey assay. All error bars represent SEM. In order to investigate the mechanism by which dTULP regulates the ciliary localization of Iav and NompC, we introduced mutations into the putative NLS/IFT-binding (dTULPmutA), PIP-binding domain (dTULPmutB), or both domains (dTULPmutAB) of dTulp cDNA and generated UAS-wild-type dTulp (UAS-dTulpwt), UAS-dTulpmutA, UAS-dTulpmutB, and UAS-dTulpmutAB transgenic flies, respectively. To eliminate positional effects, all transgenes were integrated into the same loci using site-specific recombination with an attP landing site on the third chromosome [38].
To test the effect of each mutation on the subcellular localization of dTULP, we examined the subcellular localization of dTULPwt, dTULPmutA, and dTULPmutB in Drosophila salivary glands. dTULPwt was detected mainly in the plasma membrane and nucleus (Figure S7A). Mutations in the NLS/IFT-binding domain or PIP-binding domain of dTULP resulted in significant exclusion from the nucleus or accumulation in the nucleus, respectively, which suggested that the NLS/IFT-binding and PIP-binding properties of mouse Tubby are conserved in dTULP in Drosophila salivary glands (Figure S7A). However, the localization of dTULPwt, dTULPmutA, and dTULPmutB in the JO neurons in terms of the cell body and nuclear distribution was virtually the same (Figure S7B). These data suggested that dTULP is not shuttled between the plasma membrane and the nucleus in the JO neurons and these domains may have other functions in the JO neurons rather than controlling the translocation of dTULP from the plasma membrane to the nucleus.
To evaluate the functional consequences of each mutation, we expressed dTULPwt, dTULPmutA, dTULPmutB, or dTULPmutAB in the JO neurons of dTulp1 flies. The expression of dTULPwt in the dTulp mutant background restored the distribution and the expression level of Iav and NompC similar to those of wild type (Figure 5B and 5C). The expression of dTULPmutA or dTULPmutB rescued the Iav trafficking defect of the dTulp mutant, but the expression levels of Iav in the proximal cilia in dTULPmutA - or dTULPmutB-expressing flies were reduced compared to those of dTULPwt-expressing flies (Figure 5B and 5E). NompC localization to the distal cilia in dTULPmutA - or dTULPmutB-expressing flies was similar to that in dTULPwt-expressing flies (Figure 5C). dTULPmutAB could not rescue the Iav or NompC localization defects of the dTulp mutant. This difference was not due to the expression levels of the mutant dTulp transgene since the expression levels of mutant forms of dTULP were similar to those of wild-type dTULP (Figure S8).
Next, we examined whether the different degrees of rescue of Iav and NompC localization was due to differential ciliary trafficking of variant forms of dTULP. The ciliary expression level of dTULPmutB was similar to that of dTULPwt, whereas the ciliary expression levels of dTULPmutA and dTULPmutAB were reduced compared with those of dTULPwt (Figure 5D and 5F). These data suggested that the putative NLS/IFT-binding domain of dTULP has a regulatory function to control the trafficking of dTULP into the cilia.
Consistent with immunohistochemical analyses, dTULPwt fully rescued the hearing defect of the dTulp mutant. dTULPmutA and dTULPmutB restored a partial function and dTULPmutAB had no such activity (Figure 5G and 5H).
Discussion
In the current study, we demonstrate that dTULP is a cilia trafficking regulator in the Drosophila hearing system. Mutation of dTulp results in hearing loss due to the mislocalization of two TRP channels, Iav and NompC, which are ciliary membrane proteins. In addition, Spam, whose localization is dependent on the IFT machinery, is also mislocalized in dTulp mutants.
How does dTULP regulate the ciliary distribution of TRPs in the JO neurons? Several studies have shown that mutations in IFT machinery or cilia components result in mislocalization of Iav, NompC, and Spam, along with abnormal axonemal structure [17], [23]. It is notable that, in contrast to IFT or cilia component mutants, ciliogenesis and maintenance appear normal in dTULP-missing flies. Furthermore, the altered distribution of Iav, NompC, and Spam in dTulp mutants was not due to the mislocalization of IFT proteins, since the localization of two IFTs (NompB and RempA) was normal in dTulp mutants (Figure S6). These data suggest that dTULP acts downstream of the IFTs to regulate TRP localization.
Even though the mutation of dTulp affected the trafficking of both Iav and NompC, the compartmentalized ciliary localization of Iav and NompC is differentially regulated by dTULP. An individual mutation in either the putative IFT - or PIP-binding domain reduced Iav expression levels in cilia, whereas NompC localization was not altered until both domains were mutated. Even after the double mutations in both domains of dTULP, NompC is still situated inside the cilia, but in abnormal locations. These findings demonstrate that ciliary entry of NompC is not dependent on dTULP while the distal ciliary localization of NompC is dependent on dTULP. One possibility is that dTULP allows NompC to disengage from the IFT complex at the distal cilia so that NompC is enriched in the distal cilia through the mechanism that required both IFT - and PIP-binding domains. It is also possible that the distal ciliary localization of NompC is regulated by an unidentified factor(s) whose ciliary localization is dTULP-dependent as is Iav.
Both the putative IFT - and PIP-binding domains play important roles in the proper Iav distribution in cilia, but they appear to have different roles. Even though the IFT - or PIP-binding mutant forms of dTULP could only partially rescue the ciliary levels of Iav to the similar extent, the mutation of the IFT-binding domain reduced the ciliary levels of dTULP while disruption of the PIP-binding domain had no effect on the ciliary levels of dTULP. These findings suggest that two domains play distinct roles in the regulation of the ciliary localization of Iav. The IFT-binding domain is the motif required for the ciliary entry for dTULP, and the PIP-binding domain is not related to dTULP ciliary entry itself, rather it affects recruitment of Iav-containing preciliary vesicles to dTULP. By these two linked steps, Iav localization to cilia would be facilitated by dTULP.
In mammals, IFT-A directs the ciliary localization of TULP3 through physical interaction between TULP3 and the IFT-A core complex (WDR19, IFT122, and IFT140), and in turn, promotes trafficking of GPCR to the cilia. Indeed, the depletion of individual IFT-A core complex components affects the ciliary localization of TULP3, which results in the inhibition of GPCR trafficking to the cilia [37]. It appears that dTULP and TULP3 have the similar molecular mechanisms to regulate ciliary membrane proteins. However, unlike TULP3, dTULP ciliary access is not dependent on IFT-A. dTULP ciliary trafficking was not affected by the mutation of Oseg1 (an ortholog of human IFT-A, IFT122) or rempA (an ortholog of human IFT-A, IFT140). Furthermore, the presence of dTULP in cilia did not determine the normal localization of Iav. For example, in the rempA mutant, even when dTULP was localized to the cilia (Figure S6B), Iav was not found in cilia [23]. Taken together, dTULP facilitates the relay of preciliary vesicles to the IFT complex at the base of cilia rather than moving together with ciliary membrane proteins into the cilia as an adaptor between IFT and cargo. dTULP may have other additional roles in cilia, which needs to be explored in the future. Based on our finding that dTULP but not Iav could be found in cilia of IFT mutants, it is also possible that recruitment of Iav-containing preciliary vesicles requires dTULP and additional unknown factors, whose function is altered in IFT mutants. Thus, Iav-containing preciliary vesicles may not be able to form stable interactions with dTULP and IFTs.
After the cloning of the Tubby gene two decades ago, one promising hypothesis has been that Tubby is a transcription factor, since Tubby translocates to the nucleus upon GPCR activation and the N-terminal region of Tubby has transactivation potentials [32], [39]. However, candidate target genes for Tubby have not been identified. Tubby is thought to have additional functions including vesicular trafficking, insulin signaling, endocytosis, or phagocytosis [40]–[43]. It is still not clear how these molecular functions lead to the in vivo phenotypes observed in the tubby mouse. Meanwhile, several studies have hinted at possible connections between the phenotypes of tubby mutant mice and ciliary dysfunction. Tubby mice phenotypes comprise syndromic manifestations that are commonly observed in ciliopathies such as Bardet-Biedle syndrome [44] and Usher syndrome [45], [46]. Recently, GPCR trafficking into neuronal cilia was reported to be misregulated in tubby mice [47]. Mutation of Tulp1, a member of the TULPs, in human and mice, exhibits retinal degeneration due to the mislocalization of rhodopsin [48]. TULP3 represses Hedgehog signalling, which is a crucial signalling cascade in cilia, via the regulation of the ciliary localization of GPCRs [49]. Our current study provides additional supports for the idea that TULPs play an important role in ciliary signalling and that the tubby mouse syndrome might be due to the ciliary defects.
In contrast to mammalian cells, only specialized cell types have the ciliary structure in Drosophila, and the expression of dTULP is not restricted to organs with the ciliary structure, which suggested that dTULP may have other roles not related to the ciliary function [28]. For example, dTULP mediates rhodopsin endocytosis in Drosophila photoreceptor cells which do not have cilium in contrast to its mammalian counterpart [50].
In summary, we demonstrate an intriguing role of dTULP in governing the ciliary localization of TRP proteins. This is the first in vivo evidence showing that dTULP may have important roles in the maintenance of ciliary functions by regulating the localization of ciliary proteins, thereby maintaining sensory functions.
Materials and Methods
Fly stocks
All fly stocks were maintained in regular laboratory conditions (conventional cornmeal agar molasses medium, 12-h light/12-h dark cycle at 25°C, 60% humidity). Iav-GFP and NompA-GFP were reported previously [13], [33]. RempA-YFP and NompB-GFP were from M. Kernan. Y. Jan and M. Noll provided UAS-NompC:GFP and Poxn-GAL4, respectively. Df(2R)BSC462, elav-GAL4, UAS-mCD8:GFP, AB1-GAL4, F-GAL4, and Orco-GAL4 were from the Bloomington Stock Center (Bloomington, IN).
Generation of dTulp mutant flies
We employed ends-out homologous recombination to generate dTulp mutant alleles. To make the dTulp1 allele, 3 kb genomic DNA at the 5′ and 3′ ends of the tubby domain (220 to 460 residues) coding sequence was PCR amplified from w1118 and subcloned into the pw35 vector. The primer sequences for the 5′ homologous arm of the pw35 vector are 5′-AAAGCGGCCGCCACCGGTGACATCCTCATGTTC-3′ and 5′-AAAGCGGCCGCGTTGCATCACGAACTGGTCGATATTG-3′. The primer sequences for the 3′ homologous arm of the pw35 vector are 5′-TGAGCTGGCTGGGATCCTCGGGTTGG-3′ and 5′-GTGGATCCTTCCTGGTTGGCATCACGTTGAC-3′. To generate the dTulpG allele, we used the pw35GAL4loxP vector in which GAL4 and white are flanked by loxP sequences so the cassette can be removed by introducing Cre recombinase. We subcloned the 3 kb of genomic DNA from each of the 5′ and 3′ ends of the dTULP coding region (18 to 261 residues) into the pw35GAL4loxP vector. The primer sequences for the 5′ homologous arm of the pw35GAL4loxP vector are5′-ACAGATCTCACCGTCGCCTGGCTCAGTGCCC-3′ and 5′-GTGGTACCCAGCTGGCGCTGCAAAGCAGTTAAATC-3′. The primer sequences for the 3′ homologous arm of the pw35GAL4loxP vector are5′-AAAGCGGCCGCGTGGGTTATTGATAGTGATCCTCTA-3′ and 5′-AACCGCGGCGTACAGAATACTCCCTGTTCATGTCT-3′. We generated transgenic flies by germ line transformation (BestGene Inc., Chino Hills, CA) and screened for the targeted alleles as described previously [51]. Targeted alleles were subjected to outcross for five generations into a w1118 genetic background.
Molecular biology and generation of transgenic flies
We amplified dTulp cDNAs from cDNA clones (RE38560) with PCR and subcloned the fragments into the pUASTattB vector. These constructs were subjected to further modification. We generated the dTulpmutA and dTulpmutB mutant constructs using site-directed mutagenesis to change the sequence encoding R23QKR to L23AAA, and K292LR to A292LA, respectively. The dTulpmutAB construct was generated by introducing the mutation corresponding to dTulpmutB into the dTulpmutA construct. To generate genomic rescue transgenic flies, we obtained the BAC clones CH321-59C17 from the BACPAC Resource Center (Oakland, CA) and used these as genomic rescue constructs. Transgenic flies were generated using PhiC31 integrase-mediated transgenesis on the third chromosome to minimize position effect (Bloomington stock number 24749).
Electrophysiology
Sound-evoked potentials were recorded as described by Eberl et al [4]. Briefly, the fly's antennal sound receivers were stimulated by computer-generated pulse songs. Neuronal responses were detected using a recording electrode inserted in the junction between the first and second antennal segment and a reference electrode was inserted in the dorsal head cuticle. The signals were subtracted with a DAM50 differential amplifier (World Precision Instruments, Sarasota, FL) and digitized using Superscope 3.0 software (GW Instruments, Somerville, MA). Each trace represents the average responses to 10 stimuli.
Immunohistochemistry
For whole-mount staining, antennae were dissected at the pupa stage and the labellum and legs were prepared at the adult stage. Salivary glands were dissected from third instar larvae. For antenna sections, fly heads were embedded in OCT medium and 14 µm frozen cryostat sections were collected. Dissected tissues and sections were fixed for 15 min with 4% paraformaldehyde in 1× PBS containing 0.2% TritonX-100 (PBS-T) and washed three times with PBS-T. The fixed samples were blocked for 30 min with 5% heat-inactivated goat serum in PBS-T and incubated overnight at 4°C in primary antibodies diluted in the same blocking solution. The tissues were washed three times for 10 min with PBS-T and incubated for 1 h at room temperature in secondary antibodies diluted 1∶500 in blocking solution. Following three washes with PBS-T, the samples were mounted with Vectashield (Vector Laboratories, Burlingame, CA) and examined using a Zeiss LSM710 confocal microscope (Jena, Germany).
To quantify Iav-GFP and dTULP expression levels in cilia, all samples were prepared at the same time and all confocal images were obtained under the same conditions. The pixel intensity of each protein was measured using Zen Software (Jena, Germany). Iav-GFP intensity was measured without immunostaining.
Antibodies
Rabbit dTULP antibodies were raised by injecting animals with a purified His-tagged dTULP fusion protein (residue 95–339), followed by affinity purification. The primary antibodies were used in immunohistochemistry at the following dilutions: rabbit anti-dTULP, 1∶400; 22C10, 1∶200 (Hybridoma Bank, University of Iowa); 21A6, 1∶200 (Hybridoma Bank); rabbit anti-Orco, 1∶1,000 (gift from L. Vosshall); rabbit anti-NompC, 1∶20; rabbit anti-GFP, 1∶1,000 (Molecular Probes, Eugene, OR); mouse anti-GFP, 1∶500 (Molecular Probes). The secondary antibodies used were Alexa 488-, Alexa 568-, and Alexa 633-conjugated anti-mouse or anti-rabbit IgG (Molecular Probes; 1∶500). DNA and actin were visualized by DAPI and Alexa Fluor 633 Phalloidin (Molecular Probes) staining, respectively.
Western blot
Fly head or antennae lysates from each genotype were subjected to electrophoresis on SDS-polyacrylamide gels and transferred onto polyvinylidene fluoride membranes. The membranes were blocked for 1 h with 5% nonfat milk plus 0.1% Tween-20. Membrane-bound proteins were analyzed by immunoblotting with primary antibodies against dTULP (1∶1,000) and tubulin (Hybridoma Bank, 1∶2,000).
Transmission electron microscopy
Fly heads were dissected and fixed in 2% paraformaldehyde, 2.5% glutaraldehyde, 0.1 M cacodylate, and 2 mM CaCl2, pH 7.4. The tissue was embedded in LR white resin. Thin sections were cut, mounted on formvar-coated single slot nickel grids, counterstained with uranyl acetate and lead citrate, and examined on a Hitachi H-7500 electron microscope (Hitachi, Tokyo, Japan).
Real-time PCR
Total RNA was extracted from adult antennae using Trizol reagent (Invitrogen, Carlsbad, CA). cDNA was generated from 0.5 µg of RNA from each genotype using the SuperScript III First Strand Synthesis System (Invitrogen). Quantitative PCR was performed using an ABI7500 real-time PCR machine (Applied Biosystems, Foster City, CA) and the ABI SYBR green system. Transcript levels were normalized to rp49 as an internal control and the ΔCT (CT = threshold cycle) method was used to calculate the relative amount of mRNAs.The primers used for qRT-PCR are listed in Table S1.
Behavioural test (bang test)
Fifteen 3 - to 6-day-old flies were placed in an empty fly food vial. The climbing index is the fraction of flies that climb halfway up the vials in 10 s after being tapped down to the bottom of the tube. We performed each experiment twice and used the average of the two trials to calculate the climbing index.
Statistical analyses
Data shown are the mean ± SEM. To compare two sets of data, unpaired Student's t-tests were used. ANOVA with the Tukey post-hoc test was used to compare multiple sets of data. Asterisks indicate statistical significance.
Supporting Information
Zdroje
1. HudspethAJ (1992) Hair-bundle mechanics and a model for mechanoelectrical transduction by hair cells. Soc Gen Physiol Ser 47 : 357–370.
2. GillespiePG, MullerU (2009) Mechanotransduction by hair cells: models, molecules, and mechanisms. Cell 139 : 33–44.
3. LuQ, SenthilanPR, EffertzT, NadrowskiB, GopfertMC (2009) Using Drosophila for studying fundamental processes in hearing. Integr Comp Biol 49 : 674–680.
4. EberlDF, HardyRW, KernanMJ (2000) Genetically similar transduction mechanisms for touch and hearing in Drosophila. J Neurosci 20 : 5981–5988.
5. GreenspanRJ, FerveurJF (2000) Courtship in Drosophila. Annu Rev Genet 34 : 205–232.
6. HallJC (1994) The mating of a fly. Science 264 : 1702–1714.
7. Von SchilcherF (1976) The behavior of cacophony, a courtship song mutant in Drosophila melanogaster. Behav Biol 17 : 187–196.
8. EberlDF, DuykGM, PerrimonN (1997) A genetic screen for mutations that disrupt an auditory response in Drosophila melanogaster. Proc Natl Acad Sci U S A 94 : 14837–14842.
9. GopfertMC, RobertD (2001) Biomechanics. Turning the key on Drosophila audition. Nature 411 : 908.
10. EffertzT, WiekR, GopfertMC (2011) NompC TRP channel is essential for Drosophila sound receptor function. Curr Biol 21 : 592–597.
11. GongZ, SonW, ChungYD, KimJ, ShinDW, et al. (2004) Two interdependent TRPV channel subunits, inactive and Nanchung, mediate hearing in Drosophila. J Neurosci 24 : 9059–9066.
12. GopfertMC, AlbertJT, NadrowskiB, KamikouchiA (2006) Specification of auditory sensitivity by Drosophila TRP channels. Nat Neurosci 9 : 999–1000.
13. KimJ, ChungYD, ParkDY, ChoiS, ShinDW, et al. (2003) A TRPV family ion channel required for hearing in Drosophila. Nature 424 : 81–84.
14. LehnertBP, BakerAE, GaudryQ, ChiangAS, WilsonRI (2013) Distinct roles of TRP channels in auditory transduction and amplification in Drosophila. Neuron 77 : 115–128.
15. KangL, GaoJ, SchaferWR, XieZ, XuXZ (2010) C. elegans TRP family protein TRP-4 is a pore-forming subunit of a native mechanotransduction channel. Neuron 67 : 381–391.
16. YanZ, ZhangW, HeY, GorczycaD, XiangY, et al. (2013) Drosophila NOMPC is a mechanotransduction channel subunit for gentle-touch sensation. Nature 493 : 221–225.
17. LeeJ, MoonS, ChaY, ChungYD (2010) Drosophila TRPN( = NOMPC) channel localizes to the distal end of mechanosensory cilia. PLoS One 5: e11012.
18. LiangX, MadridJ, SalehHS, HowardJ (2011) NOMPC, a member of the TRP channel family, localizes to the tubular body and distal cilium of Drosophila campaniform and chordotonal receptor cells. Cytoskeleton (Hoboken) 68 : 1–7.
19. BechstedtS, AlbertJT, KreilDP, Muller-ReichertT, GopfertMC, et al. (2010) A doublecortin containing microtubule-associated protein is implicated in mechanotransduction in Drosophila sensory cilia. Nat Commun 1 : 11.
20. CacheroS, SimpsonTI, Zur LagePI, MaL, NewtonFG, et al. (2011) The gene regulatory cascade linking proneural specification with differentiation in Drosophila sensory neurons. PLoS Biol 9: e1000568.
21. SenthilanPR, PiepenbrockD, OvezmyradovG, NadrowskiB, BechstedtS, et al. (2012) Drosophila auditory organ genes and genetic hearing defects. Cell 150 : 1042–1054.
22. LaurenconA, DubruilleR, EfimenkoE, GrenierG, BissettR, et al. (2007) Identification of novel regulatory factor X (RFX) target genes by comparative genomics in Drosophila species. Genome Biol 8: R195.
23. LeeE, Sivan-LoukianovaE, EberlDF, KernanMJ (2008) An IFT-A protein is required to delimit functionally distinct zones in mechanosensory cilia. Curr Biol 18 : 1899–1906.
24. ColeDG, DienerDR, HimelblauAL, BeechPL, FusterJC, et al. (1998) Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J Cell Biol 141 : 993–1008.
25. PazourGJ, WilkersonCG, WitmanGB (1998) A dynein light chain is essential for the retrograde particle movement of intraflagellar transport (IFT). J Cell Biol 141 : 979–992.
26. ScholeyJM, AndersonKV (2006) Intraflagellar transport and cilium-based signaling. Cell 125 : 439–442.
27. NorthMA, NaggertJK, YanY, Noben-TrauthK, NishinaPM (1997) Molecular characterization of TUB, TULP1, and TULP2, members of the novel tubby gene family and their possible relation to ocular diseases. Proc Natl Acad Sci U S A 94 : 3128–3133.
28. RonshaugenM, McGinnisN, InglisD, ChouD, ZhaoJ, et al. (2002) Structure and expression patterns of Drosophila TULP and TUSP, members of the tubby-like gene family. Mech Dev 117 : 209–215.
29. GongWJ, GolicKG (2003) Ends-out, or replacement, gene targeting in Drosophila. Proc Natl Acad Sci U S A 100 : 2556–2561.
30. VenkenKJ, HeY, HoskinsRA, BellenHJ (2006) P[acman]: a BAC transgenic platform for targeted insertion of large DNA fragments in D. melanogaster. Science 314 : 1747–1751.
31. ChengLE, SongW, LoogerLL, JanLY, JanYN (2010) The role of the TRP channel NompC in Drosophila larval and adult locomotion. Neuron 67 : 373–380.
32. SantagataS, BoggonTJ, BairdCL, GomezCA, ZhaoJ, et al. (2001) G-protein signaling through tubby proteins. Science 292 : 2041–2050.
33. ChungYD, ZhuJ, HanY, KernanMJ (2001) nompA encodes a PNS-specific, ZP domain protein required to connect mechanosensory dendrites to sensory structures. Neuron 29 : 415–428.
34. CookB, HardyRW, McConnaugheyWB, ZukerCS (2008) Preserving cell shape under environmental stress. Nature 452 : 361–364.
35. HusainN, PellikkaM, HongH, KlimentovaT, ChoeKM, et al. (2006) The agrin/perlecan-related protein eyes shut is essential for epithelial lumen formation in the Drosophila retina. Dev Cell 11 : 483–493.
36. HanYG, KwokBH, KernanMJ (2003) Intraflagellar transport is required in Drosophila to differentiate sensory cilia but not sperm. Curr Biol 13 : 1679–1686.
37. MukhopadhyayS, WenX, ChihB, NelsonCD, LaneWS, et al. (2010) TULP3 bridges the IFT-A complex and membrane phosphoinositides to promote trafficking of G protein-coupled receptors into primary cilia. Genes Dev 24 : 2180–2193.
38. BischofJ, MaedaRK, HedigerM, KarchF, BaslerK (2007) An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc Natl Acad Sci U S A 104 : 3312–3317.
39. BoggonTJ, ShanWS, SantagataS, MyersSC, ShapiroL (1999) Implication of tubby proteins as transcription factors by structure-based functional analysis. Science 286 : 2119–2125.
40. CaberoyNB, ZhouY, LiW (2010) Tubby and tubby-like protein 1 are new MerTK ligands for phagocytosis. EMBO J 29 : 3898–3910.
41. KapellerR, MoriartyA, StraussA, StubdalH, TheriaultK, et al. (1999) Tyrosine phosphorylation of tub and its association with Src homology 2 domain-containing proteins implicate tub in intracellular signaling by insulin. J Biol Chem 274 : 24980–24986.
42. MukhopadhyayA, DeplanckeB, WalhoutAJ, TissenbaumHA (2005) C. elegans tubby regulates life span and fat storage by two independent mechanisms. Cell Metab 2 : 35–42.
43. MukhopadhyayA, PanX, LambrightDG, TissenbaumHA (2007) An endocytic pathway as a target of tubby for regulation of fat storage. EMBO Rep 8 : 931–938.
44. GreenJS, ParfreyPS, HarnettJD, FaridNR, CramerBC, et al. (1989) The cardinal manifestations of Bardet-Biedl syndrome, a form of Laurence-Moon-Biedl syndrome. N Engl J Med 321 : 1002–1009.
45. VernonM (1969) Usher's syndrome–deafness and progressive blindness. Clinical cases, prevention, theory and literature survey. J Chronic Dis 22 : 133–151.
46. ArdenGB, FoxB (1979) Increased incidence of abnormal nasal cilia in patients with retinitis pigmentosa. Nature 279 : 534–536.
47. SunX, HaleyJ, BulgakovolegOV, CaiX, McGinnisJ, et al. (2012) Tubby is required for trafficking g protein-coupled receptors to neuronal cilia. Cilia 1 : 21.
48. HagstromSA, AdamianM, ScimecaM, PawlykBS, YueG, et al. (2001) A role for the Tubby-like protein 1 in rhodopsin transport. Invest Ophthalmol Vis Sci 42 : 1955–1962.
49. MukhopadhyayS, WenX, RattiN, LoktevA, RangellL, et al. (2013) The Ciliary G-Protein-Coupled Receptor Gpr161 Negatively Regulates the Sonic Hedgehog Pathway via cAMP Signaling. Cell 152 : 210–223.
50. ChenSF, TsaiYC, FanSS (2012) Drosophila king tubby (ktub) mediates light-induced rhodopsin endocytosis and retinal degeneration. J Biomed Sci 19 : 101.
51. MoonSJ, LeeY, JiaoY, MontellC (2009) A Drosophila gustatory receptor essential for aversive taste and inhibiting male-to-male courtship. Curr Biol 19 : 1623–1627.
Štítky
Genetika Reprodukční medicína
Článek Rapid Intrahost Evolution of Human Cytomegalovirus Is Shaped by Demography and Positive SelectionČlánek Common Variants in Left/Right Asymmetry Genes and Pathways Are Associated with Relative Hand SkillČlánek Manipulating or Superseding Host Recombination Functions: A Dilemma That Shapes Phage EvolvabilityČlánek Maternal Depletion of Piwi, a Component of the RNAi System, Impacts Heterochromatin Formation inČlánek Hsp104 Suppresses Polyglutamine-Induced Degeneration Post Onset in a Drosophila MJD/SCA3 ModelČlánek Cooperative Interaction between Phosphorylation Sites on PERIOD Maintains Circadian Period inČlánek VAPB/ALS8 MSP Ligands Regulate Striated Muscle Energy Metabolism Critical for Adult Survival inČlánek Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2AČlánek A Link between ORC-Origin Binding Mechanisms and Origin Activation Time Revealed in Budding YeastČlánek Genotype-Environment Interactions Reveal Causal Pathways That Mediate Genetic Effects on PhenotypeČlánek Chromatin-Specific Regulation of Mammalian rDNA Transcription by Clustered TTF-I Binding Sites
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 9- Souvislost haplotypu M2 genu pro annexin A5 s opakovanými reprodukčními ztrátami
- Akutní intermitentní porfyrie
- Příjem alkoholu a menstruační cyklus
- Hysteroskopická resekce děložního septa zlepšuje šanci na graviditu žen s jinak nevysvětlenou infertilitou
- Transfer zmraženého embrya zlepšuje výsledky IVF
-
Všechny články tohoto čísla
- The Pathway Gene Functions together with the -Dependent Isoprenoid Biosynthetic Pathway to Orchestrate Germ Cell Migration
- Take Off, Landing, and Fly Anesthesia
- Nucleosome Assembly Proteins Get SET to Defeat the Guardian of Chromosome Cohesion
- Whole-Exome Sequencing Reveals a Rapid Change in the Frequency of Rare Functional Variants in a Founding Population of Humans
- Evidence Is Evidence: An Interview with Mary-Claire King
- Rapid Intrahost Evolution of Human Cytomegalovirus Is Shaped by Demography and Positive Selection
- Convergent Transcription Induces Dynamic DNA Methylation at Loci
- Environmental Stresses Disrupt Telomere Length Homeostasis
- Ultra-Sensitive Sequencing Reveals an Age-Related Increase in Somatic Mitochondrial Mutations That Are Inconsistent with Oxidative Damage
- Common Variants in Left/Right Asymmetry Genes and Pathways Are Associated with Relative Hand Skill
- Genetic and Anatomical Basis of the Barrier Separating Wakefulness and Anesthetic-Induced Unresponsiveness
- The Locus, Exclusive to the Ambulacrarians, Encodes a Chromatin Insulator Binding Protein in the Sea Urchin Embryo
- Binding of NF-κB to Nucleosomes: Effect of Translational Positioning, Nucleosome Remodeling and Linker Histone H1
- Manipulating or Superseding Host Recombination Functions: A Dilemma That Shapes Phage Evolvability
- Dynamics of DNA Methylation in Recent Human and Great Ape Evolution
- Functional Dissection of Regulatory Models Using Gene Expression Data of Deletion Mutants
- PAQR-2 Regulates Fatty Acid Desaturation during Cold Adaptation in
- N-alpha-terminal Acetylation of Histone H4 Regulates Arginine Methylation and Ribosomal DNA Silencing
- A Genome-Wide Systematic Analysis Reveals Different and Predictive Proliferation Expression Signatures of Cancerous vs. Non-Cancerous Cells
- Maternal Depletion of Piwi, a Component of the RNAi System, Impacts Heterochromatin Formation in
- miR-1/133a Clusters Cooperatively Specify the Cardiomyogenic Lineage by Adjustment of Myocardin Levels during Embryonic Heart Development
- Hsp104 Suppresses Polyglutamine-Induced Degeneration Post Onset in a Drosophila MJD/SCA3 Model
- Genome-Wide Analysis of Genes and Their Association with Natural Variation in Drought Tolerance at Seedling Stage of L
- Deep Resequencing of GWAS Loci Identifies Rare Variants in , and That Are Associated with Ulcerative Colitis
- Cooperative Interaction between Phosphorylation Sites on PERIOD Maintains Circadian Period in
- VAPB/ALS8 MSP Ligands Regulate Striated Muscle Energy Metabolism Critical for Adult Survival in
- Analysis of Genes Reveals Redundant and Independent Functions in the Inner Ear
- Predicting the Risk of Rheumatoid Arthritis and Its Age of Onset through Modelling Genetic Risk Variants with Smoking
- Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2A
- A Shift to Organismal Stress Resistance in Programmed Cell Death Mutants
- Fragile Site Instability in Causes Loss of Heterozygosity by Mitotic Crossovers and Break-Induced Replication
- Tracking of Chromosome and Replisome Dynamics in Reveals a Novel Chromosome Arrangement
- The Condition-Dependent Transcriptional Landscape of
- Ago1 Interacts with RNA Polymerase II and Binds to the Promoters of Actively Transcribed Genes in Human Cancer Cells
- Nebula/DSCR1 Upregulation Delays Neurodegeneration and Protects against APP-Induced Axonal Transport Defects by Restoring Calcineurin and GSK-3β Signaling
- System-Wide Analysis Reveals a Complex Network of Tumor-Fibroblast Interactions Involved in Tumorigenicity
- Meta-Analysis of Genome-Wide Association Studies Identifies Six New Loci for Serum Calcium Concentrations
- and Are Required for Cellularization and Differentiation during Female Gametogenesis in
- Growth factor independent-1 Maintains Notch1-Dependent Transcriptional Programming of Lymphoid Precursors
- Whole Genome Sequencing Identifies a Deletion in Protein Phosphatase 2A That Affects Its Stability and Localization in
- An Alteration in ELMOD3, an Arl2 GTPase-Activating Protein, Is Associated with Hearing Impairment in Humans
- Genomic Identification of Founding Haplotypes Reveals the History of the Selfing Species
- Plasticity Regulators Modulate Specific Root Traits in Discrete Nitrogen Environments
- The IDD14, IDD15, and IDD16 Cooperatively Regulate Lateral Organ Morphogenesis and Gravitropism by Promoting Auxin Biosynthesis and Transport
- Stochastic Loss of Silencing of the Imprinted Allele, in a Mouse Model and Humans with Prader-Willi Syndrome, Has Functional Consequences
- The Prefoldin Complex Regulates Chromatin Dynamics during Transcription Elongation
- PKA Controls Calcium Influx into Motor Neurons during a Rhythmic Behavior
- A Pre-mRNA-Splicing Factor Is Required for RNA-Directed DNA Methylation in
- Cell-Type Specific Features of Circular RNA Expression
- The Uve1 Endonuclease Is Regulated by the White Collar Complex to Protect from UV Damage
- An Atypical Kinase under Balancing Selection Confers Broad-Spectrum Disease Resistance in Arabidopsis
- Genome-Wide Mutation Avalanches Induced in Diploid Yeast Cells by a Base Analog or an APOBEC Deaminase
- Extensive Divergence of Transcription Factor Binding in Embryos with Highly Conserved Gene Expression
- Bi-modal Distribution of the Second Messenger c-di-GMP Controls Cell Fate and Asymmetry during the Cell Cycle
- Cell Interactions and Patterned Intercalations Shape and Link Epithelial Tubes in
- A Link between ORC-Origin Binding Mechanisms and Origin Activation Time Revealed in Budding Yeast
- The Genome and Development-Dependent Transcriptomes of : A Window into Fungal Evolution
- SKN-1/Nrf, A New Unfolded Protein Response Factor?
- The Highly Prolific Phenotype of Lacaune Sheep Is Associated with an Ectopic Expression of the Gene within the Ovary
- Fusion of Large-Scale Genomic Knowledge and Frequency Data Computationally Prioritizes Variants in Epilepsy
- IL-17 Attenuates Degradation of ARE-mRNAs by Changing the Cooperation between AU-Binding Proteins and microRNA16
- An Enhancer Element Harboring Variants Associated with Systemic Lupus Erythematosus Engages the Promoter to Influence A20 Expression
- Genome Analysis of a Transmissible Lineage of Reveals Pathoadaptive Mutations and Distinct Evolutionary Paths of Hypermutators
- Type I-E CRISPR-Cas Systems Discriminate Target from Non-Target DNA through Base Pairing-Independent PAM Recognition
- Divergent Transcriptional Regulatory Logic at the Intersection of Tissue Growth and Developmental Patterning
- MEIOB Targets Single-Strand DNA and Is Necessary for Meiotic Recombination
- Transmission of Hypervirulence Traits via Sexual Reproduction within and between Lineages of the Human Fungal Pathogen
- Integration of the Unfolded Protein and Oxidative Stress Responses through SKN-1/Nrf
- Guanine Holes Are Prominent Targets for Mutation in Cancer and Inherited Disease
- Regulation of the Boundaries of Accessible Chromatin
- Natural Genetic Transformation Generates a Population of Merodiploids in
- Ablating Adult Neurogenesis in the Rat Has No Effect on Spatial Processing: Evidence from a Novel Pharmacogenetic Model
- Genotype-Environment Interactions Reveal Causal Pathways That Mediate Genetic Effects on Phenotype
- The Molecular Mechanism of a -Regulatory Adaptation in Yeast
- Phenotypic and Genetic Consequences of Protein Damage
- Recent Acquisition of by Baka Pygmies
- Fatty Acid Taste Signals through the PLC Pathway in Sugar-Sensing Neurons
- A Critical Role for PDGFRα Signaling in Medial Nasal Process Development
- Chromatin-Specific Regulation of Mammalian rDNA Transcription by Clustered TTF-I Binding Sites
- Meiotic Recombination in Arabidopsis Is Catalysed by DMC1, with RAD51 Playing a Supporting Role
- dTULP, the Homolog of Tubby, Regulates Transient Receptor Potential Channel Localization in Cilia
- Widespread Dysregulation of Peptide Hormone Release in Mice Lacking Adaptor Protein AP-3
- , a Direct Transcriptional Target, Modulates T-Box Factor Activity in Orofacial Clefting
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Genome-Wide Systematic Analysis Reveals Different and Predictive Proliferation Expression Signatures of Cancerous vs. Non-Cancerous Cells
- Recent Acquisition of by Baka Pygmies
- The Condition-Dependent Transcriptional Landscape of
- Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2A
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



