-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaMyopathic Lamin Mutations Cause Reductive Stress and Activate the Nrf2/Keap-1 Pathway
Mutations in the human LMNA gene cause muscular dystrophy that is often accompanied by heart disease. The LMNA gene makes proteins that form a network on the inner side of the nuclear envelope, a structure that reinforces the cell nucleus. How mutations in the LMNA gene cause muscle disease is not well understood. Our studies provide evidence that LMNA mutations activate an intracellular signaling pathway and alter the redox homeostasis of muscle tissue. Thus, our results suggest that blocking the signaling pathway and maintaining the oxidative state of the diseased muscle are potential therapies for muscular dystrophy patients with LMNA mutations.
Published in the journal: . PLoS Genet 11(5): e32767. doi:10.1371/journal.pgen.1005231
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005231Summary
Mutations in the human LMNA gene cause muscular dystrophy that is often accompanied by heart disease. The LMNA gene makes proteins that form a network on the inner side of the nuclear envelope, a structure that reinforces the cell nucleus. How mutations in the LMNA gene cause muscle disease is not well understood. Our studies provide evidence that LMNA mutations activate an intracellular signaling pathway and alter the redox homeostasis of muscle tissue. Thus, our results suggest that blocking the signaling pathway and maintaining the oxidative state of the diseased muscle are potential therapies for muscular dystrophy patients with LMNA mutations.
Introduction
The human LMNA gene exemplifies the rich source of genetic variation that exists in the human genome. Over 283 sequence variants and 460 disease-causing mutations have been identified to date. These mutations cause at least 13 distinct clinical diseases, called laminopathies, which have mainly tissue-restricted phenotypes, despite the fact that A-type lamins are expressed in nearly all cells [1]. For any given disease, mutations are scattered throughout the LMNA gene [2]. Furthermore, neighboring missense mutations can give rise to dramatically different disease phenotypes. These findings suggest that defined protein domains do not have tissue-specific functions.
The LMNA gene encodes alternatively spliced mRNAs for lamin A and C that have a common domain structure [3]. The N-terminal region of lamins forms a globular domain, the central region forms a coiled coil domain, and the carboxy terminus contains an Ig-fold domain [4]. Lamins dimerize through the rod domain and form filaments via head-to-tail interactions of the dimers. Lateral interactions between lamin filaments are thought to generate higher order structures that form the network that underlies the inner membrane of the nuclear envelope. This network provides structural stability to the nucleus, serves as a scaffold for inner nuclear envelope proteins, and organizes the genome through contacts made with chromatin [5].
The mechanisms by which mutant lamins cause disease remain incompletely understood. It has been proposed that mutant lamins cause nuclear fragility, leading to nuclear deformation and breakage under mechanical stress [6]. This idea provides an explanation for the tissue-restricted phenotypes associated with muscular dystrophy and cardiomyopathy. However, sensitivity to mechanical stress does not explain why mutant lamins cause other diseases, such as lipodystrophy. For tissues that do not experience mechanical stress, mutant lamins are proposed to dysregulate gene expression [7]. While evidence exists for both the mechanical stress and gene expression models, it is also possible that lamins are required for adult stem cell homeostasis [8].
To gain novel insights into mechanisms by which mutant lamins cause disease, we previously developed a Drosophila model of lamin associated muscular dystrophy [9]. Mutations identified in patients are modeled into Drosophila Lamin C. Tissue-specific expression achieved by the Gal4/UAS system provides a means of expressing the mutant lamins in desired tissues [10]. Expression of the mutant lamins in larval body wall muscle causes larval locomotion defects and pupal death [9].
Here, we report in-depth structural and functional analyses of the mutant lamins identified in muscular dystrophy patients. Structural studies, which included NMR analysis, showed that the pathogenic mutations perturb the tertiary structure of the lamin Ig-fold domain. These structural perturbations are associated with cytoplasmic lamin aggregation, activation of the Nrf2/Keap-1 pathway, and reductive stress, yet have minimal effects on nuclear stiffness. These data lead to a novel hypothesis suggesting that cytoplasmic aggregation of nuclear envelope proteins causes Nrf2 target gene activation. Our findings provide new potential avenues for therapy involving protein metabolism and redox homeostasis.
Results
Mutant lamins alter the tertiary structure of the Ig-fold domain
To identify mechanisms by which LMNA mutations cause muscle disease, we performed an in-depth structural analysis on four mutations identified in patients with skeletal muscular dystrophy. Each patient possessed a single nucleotide substitution in LMNA that caused an amino acid substitution in the Ig-fold domain of A-type lamins. These amino acid substitutions (G449V, N456I, L489P and W514R) were dispersed throughout the Ig-fold domain and map to loop regions, making their effect on protein structure challenging to predict (S1 Fig). To analyze the effects of these amino acid substitutions on Ig-fold structure, sequences encoding the wild type and mutant human A-type lamin Ig-fold domain were cloned into an expression vector, expressed and purified from E. coli (S2A Fig). The peptides were analyzed by circular dichroism (CD) and NMR. The wild type A-type lamin Ig-fold domain contains eight anti-parallel and one parallel beta strands that form a beta barrel structure [11] (S1 Fig). The CD spectra for the wild type and three of the mutant Ig-fold domains showed similar peak intensity at 220 nm (S2B Fig) demonstrating that the beta-sheet content of the wild type and mutant Ig-fold domains was comparable.
Given the absence of obvious changes in beta sheet content between the wild type and mutant Ig-fold domains, we examined whether the amino acid substitutions altered the tertiary structure of the Ig-fold domain. Changes in tertiary structure often affect the thermal stability of a protein. We determined the T1/2 for denaturation of the wild type Ig-fold to be 55°C (S2C Fig), which was slightly lower than the published value of 62°C [11]. This variation might be accounted for by slight differences in the size of the domain in the expression constructs. Our construct included amino acid residues 435–552, whereas the published construct included amino acid residues 411–553 [11]. The T1/2 values for denaturation of G449V, N456I and W514R were reduced to 40, 35 and 35°C, respectively (S2C Fig). Thus, all three of the mutants analyzed had significantly lowered the thermal stability of the Ig-fold compared to that of the wild type Ig-fold domain.
In the absence of changes in secondary structure, a lower T1/2 value for thermal stability for the mutant proteins suggested structural perturbations (i.e. altered positioning of amino acids) within the Ig-fold domain tertiary structure. The 15N/1H Heteronuclear Single Quantum Coherence (HSQC) NMR spectrum of the wild type Ig-fold domain of our construct showed well dispersed cross peaks, similar to those reported, indicating that the wild type protein is well folded and has similar tertiary structures as reported previously [11] (S3A Fig). However, subtle differences are clearly observed between the two 15N/1H HSQC spectra, due to differences in the expression constructs used (see above). To determine whether the amino acid substitutions caused changes in the tertiary structure of the Ig-fold domain, we assigned the backbone amide cross peaks in the 15N/1H HSQC spectrum of the wild type Ig-fold (S3B Fig) and compared it to that generated from the 15N/1H HSQC spectrum of each mutant (Fig 1A). All mutants showed significant changes in the 15N/1H HSQC spectrum. In all cases, perturbations were observed in the loop in which the amino acid substitution occurred. In addition, G449V and W514R showed chemical shift perturbations throughout the beta sheets of the Ig-fold domain, indicating that these two mutations caused large structural changes. Among the four mutants tested, N456I exhibited a spectrum most similar to that of the wild type Ig-fold, while the L489P mutant showed a mixed population of folded and unfolded protein. Analysis of the chemical shift perturbation data revealed two clusters of commonly perturbed residues, one shared between G449V and W514R (Fig 1B) and other shared between N456I and L489P (Fig 1C). These commonly perturbed residues are on opposite sides of the Ig-fold barrel (Fig 1C). Taken together, our analysis identified two surfaces on the Ig-fold that are critical for the function of A-type lamins in muscle.
Fig. 1. The LMNA mutations cause perturbations of the A-type lamin Ig-fold tertiary structure. 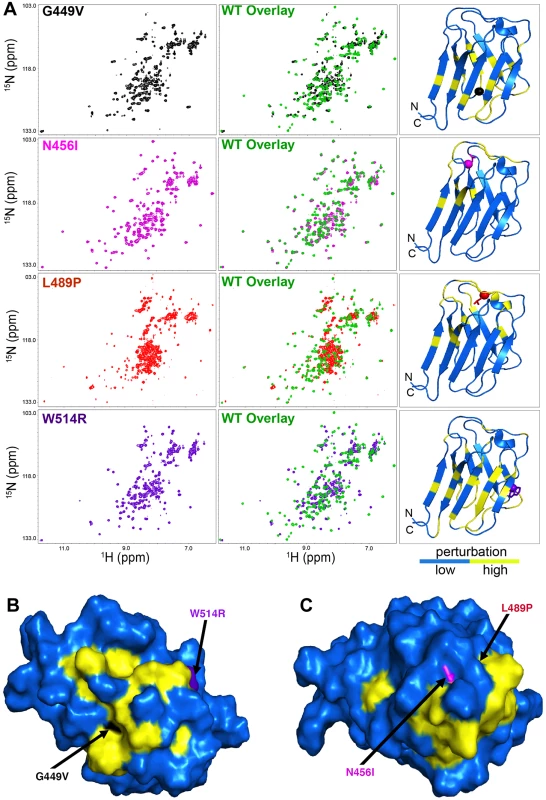
(A) The 15N/1H HSQC NMR spectrum of each mutant Ig-fold (left column) was superimposed onto that of the wild type Ig-fold in green (middle column) PDB 1IVT was used to generate a ribbon plot of the wild type Ig-fold showing the perturbed amino acids (yellow, Δδppm ≥ 0.15 ppm) and the unperturbed backbone atoms (blue, Δδppm ≤ 0.15 ppm) backbone (right column). Perturbation was determined by calculating the chemical shift difference for each backbone amide cross peak using the equation Δδppm = ([Δδ(1Hppm)]2 + [0.1 • Δδ(15Nppm)]2) 1/2. Since the 15N/1H HSQC spectrum of the wild type was assigned (S3B Fig) and the spectra of the mutants were not assigned, the Δδppm was calculated by comparing each backbone amide cross peak in the wild type spectrum (assigned) to all cross peaks present in the mutant spectrum. The smallest Δδppm from this calculation was taken as the chemical shift perturbation value for that residue. In the ribbon plots, the mutated residues are shown in stick mode with their C atoms indicated by spheres. (B and C) Surface display of the Ig-fold domain showing common residues perturbed by G449V and W514R (B) and N456I and L489P (C). Amino acids not perturbed are shown in blue. Amino acid residues altered by the LMNA mutations are colored by black (G449V), purple (W514R), magenta (N456I) and red (L489P) and indicated by an arrow. Note that L489P is buried and not visible from the surface. Mutant lamins have minimal dominant effects on nuclear stiffness
Lamins A and C are contributors to nuclear stiffness [12,13]. Given the structural perturbations of the mutant Ig-fold domains, we tested whether full-length lamins possessing the amino acid substitutions within the Ig-fold altered nuclear deformation in response to mechanical stress. To accomplish this, we made use of a Drosophila model [9]. Drosophila Lamin C shows conservation of amino acid sequence and domain organization with human lamin A/C, including the amino acid residues under investigation [9]. Note that the amino acid numbering for the substituted residues is different between the human and Drosophila Ig-fold (S1 Fig) due to differences in the size of the domain between the two species. Importantly, the carboxyl sequence of Drosophila Lamin C is predicted to form an Ig-fold structure that is highly similar to that of human lamin A (S4 Fig). In addition, the spatial and temporal expression pattern of Drosophila Lamin C is conserved with that of human lamin A/C [14]. Mutations identified in human LMNA were modeled into the Drosophila Lamin C gene, transgenic Drosophila were generated and the Gal4/UAS system [10] was used to express wild type and mutant lamins in Drosophila larval body wall muscle at levels comparable to endogenous Lamin C [9].
Expression of wild type Drosophila Lamin C in the larval body wall muscle caused no obvious phenotypes and did not affect viability [9]. In contrast, muscle-specific expression of mutant Lamin C caused larval locomotion defects and semi-lethality at the pupal stage [9]. Approximately 30% of the larval body wall muscles showed abnormally shaped and spaced nuclei, disorganization of the actin cytoskeleton, and cytoplasmic aggregation of nuclear envelope proteins including mutant Lamin C (Fig 2A) and nuclear pore proteins as shown previously [9]. None of these cellular phenotypes were observed in the wild type Lamin C control where lamin localization was confined to the nucleus (Fig 2A).
Fig. 2. Mutant lamins mislocalize in Drosophila larval body wall muscles and have minimal dominant effects on nuclear stiffness. 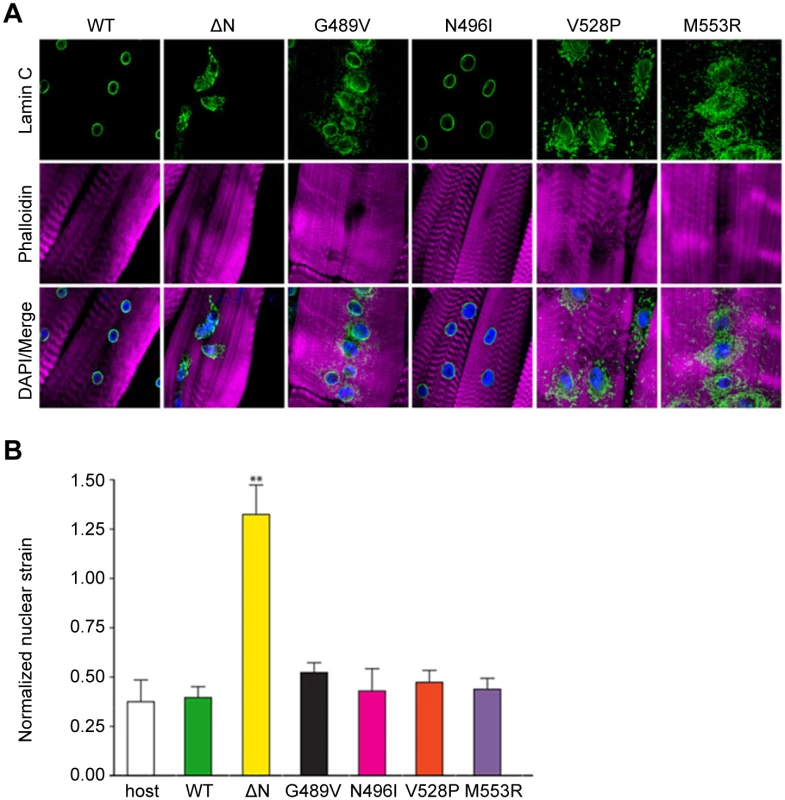
(A) Immunohistochemical staining of Drosophila body wall muscles from transgenic larvae expressing full length wild type (WT) and mutant Lamin C. Phalloidin staining is represented by magenta, Lamin C by green, and DAPI by blue. (B) Normalized nuclear strain values for nuclei in Drosophila larval body wall muscle. Eleven nuclei from three different larvae were analyzed per genotype. Error bars represent standard error of the mean. Increased normalized nuclear strain values correspond to decreased nuclear stiffness. The ** indicates p ≤ 0.01 compared to the value obtained for muscles expressing wild type Lamin C. To examine the effect of mutant lamins on nuclear stability in muscle tissue, larval body wall muscle fillets were hand-dissected from transgenic Drosophila larvae and attached to a flexible silicone membrane for nuclear strain measurements [13]. Expression of wild type Lamin C in an otherwise wild type background did not alter nuclear stiffness relative to that of a non-transgenic stock (Fig 2B). In contrast, expression of Lamin C in which the N-terminal head domain had been deleted (ΔN) showed larger nuclear deformation (Fig 2A) corresponding to decreased nuclear stiffness (Fig 2B). These results are consistent with the dominant negative effects observed for headless lamin A in the cultured cells [13]. No statistical difference in nuclear tension was observed in myonuclei expressing wild type and mutant Lamin C. Thus, these show that the mutant lamins do not have dominant effects on the nuclear tension in Drosophila muscle fibers.
Mutant lamins alter muscle gene expression
In the absence of dominant changes in nuclear stiffness, we reasoned that mutant lamins might exert their pathogenic effects by changing muscle gene expression [15–17]. To minimize indirect effects on gene expression, we exploited the Drosophila model to capture changes in muscle gene expression 24–48 hours following induction of mutant Lamin C expression. Total RNA was isolated from larval body wall muscles and used for Affymetrix gene expression profiling. The analysis was performed on transgenic larvae expressing wild type, ΔN and G489V full-length versions of Lamin C. Lamin C ΔN was selected because it is known to have dominant negative effects on lamin assembly. Lamin C G489V was selected because it caused the greatest percent lethality (95%) [9]. Using the Partek software suite and a two-fold cut off with a p value of 0.05 or greater, 28 genes showed changes in expression between muscle expressing wild type Lamin C and ΔN (Fig 3A and S1 Table). A total of 87 genes showed changes in expression between wild type Lamin C and the G489V mutant (Fig 3A and S2 Table), with 21 genes overlapping with those altered by the ΔN mutant (Fig 3A and Table 1). The majority of these genes were up-regulated in response to the mutant lamins, consistent with a repressive role of wild type lamins in gene expression [15,16]. The relatively small number of genes that changed expression is consistent with the idea that ‘first responder’ genes were captured by the analysis.
Fig. 3. Mutant lamins cause changes in Drosophila muscle gene expression and reductive stress. 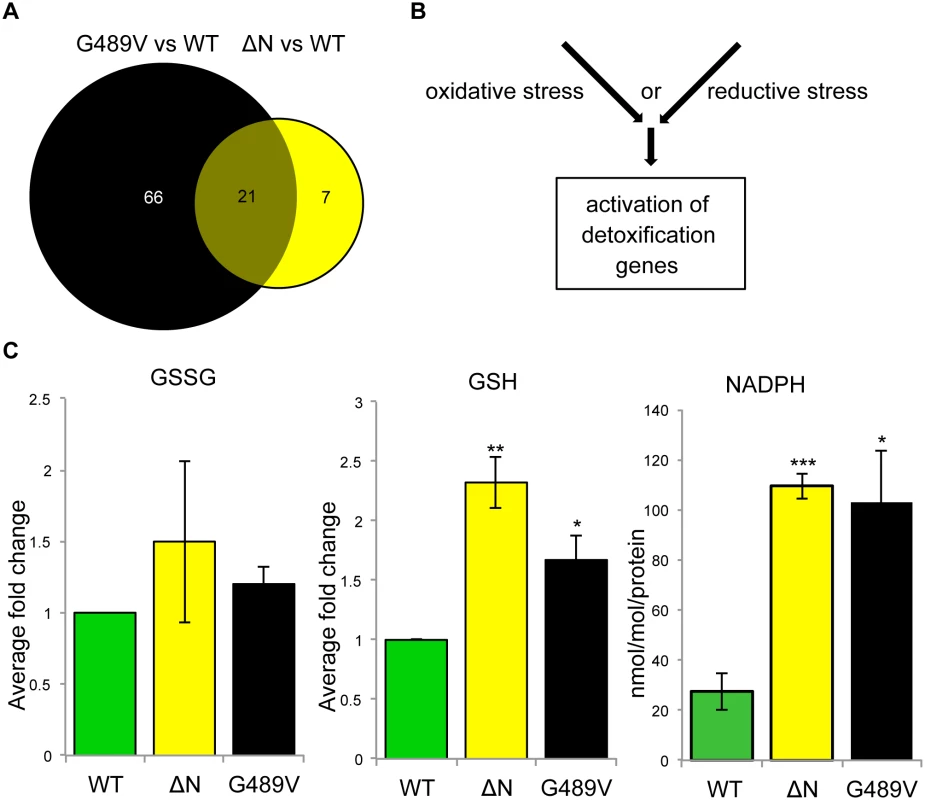
(A) Venn diagram of the changes in gene expression caused by ΔN and G489V versus wild type Lamin C. (B) Alternative pathways known to cause activation of cellular detoxification genes. (C) Quantitation of oxidized glutathione (GSSG), reduced glutathione (GSH) and NADPH in Drosophila body wall muscles of larvae expressing wild type and mutant Lamin C. Analysis was performed on three independent biological samples. Error bars represent standard error of the mean. Statistical significance is indicated by * for p ≤ 0.05, ** for p ≤ 0.01, and *** for p ≤ 0.001 when compared to values obtained for muscles expressing wild type Lamin C. Tab. 1. Genes misregulated by Lamin C ΔN and G489V. 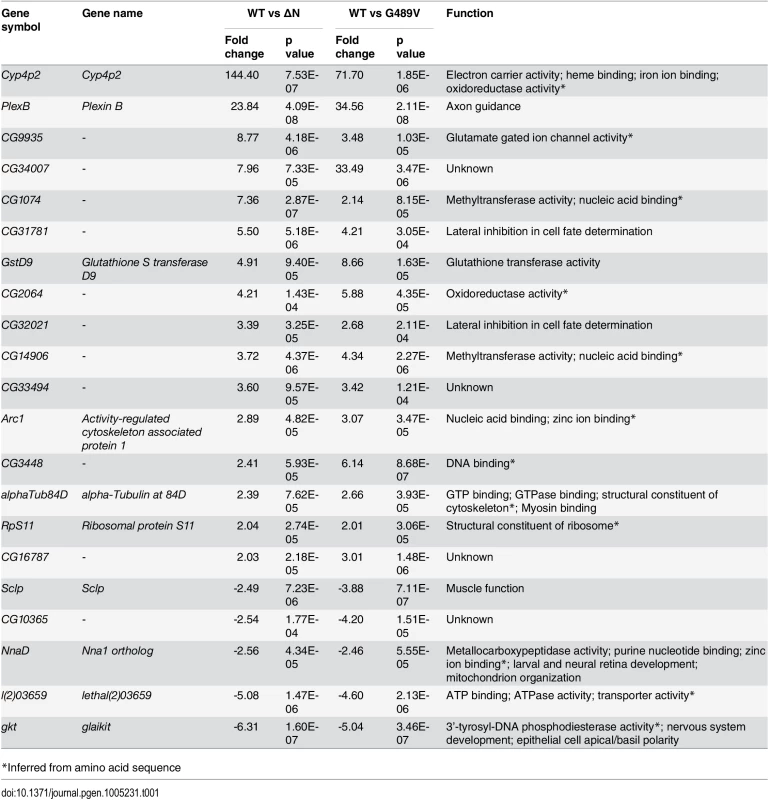
*Inferred from amino acid sequence Using Partek and Flybase gene annotations, we discovered that cellular detoxification genes, such as glutathione S transferase (Gst) genes, were enriched among those that changed expression (Table 2). These genes are typically activated in response to oxidative stress [18,19]. Other genes, including those involved in neuromuscular junction function (Table 2, S1 and S2 Tables) might be activated to compensate for deterioration of the muscles at the neuromuscular junction. Thus, the gene expression analysis provided insights on the initial stages of pathogenesis.
Tab. 2. Categories of mis-regulated genes differentially expressed in wild type Lamin C vs G489V. 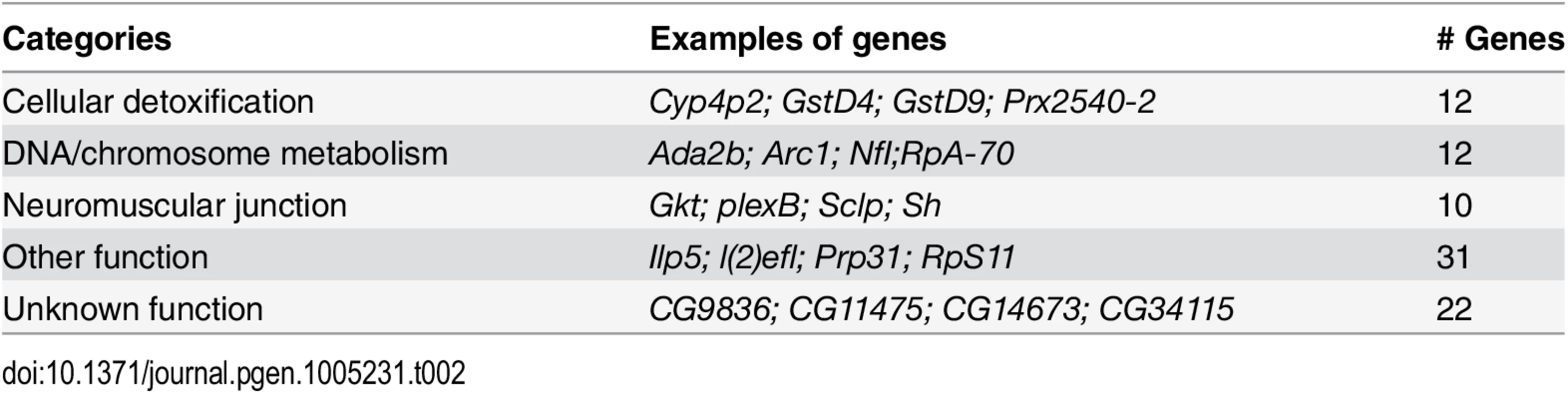
Mutant lamins cause reductive stress
Cellular anti-oxidant genes are typically activated in response to a redox imbalance. Measurements of the levels of oxidized (GSSG) and reduced (GSH) glutathione were determined in extracts from body wall muscles from larvae expressing wild type, ΔN and G489V Lamin C transgene. This revealed similar levels of GSSG among all genotypes (Fig 3C, left panel). In contrast, GSH levels were elevated in muscle expressing the mutant Lamin C relative to wild type (Fig 3C, middle panel). Elevated levels of GSH and NADPH are hallmarks of a condition known as ‘reductive stress’ [20]. We found that NADPH levels were also elevated in the muscle of the larvae expressing ΔN and G489V, relative to wild type Lamin C (Fig 3C, right panel). These findings demonstrate that mutant lamins cause reductive stress in muscle.
To identify the source of the reductive stress, we measured the activity of NADPH-producing enzymes in larval body wall muscle. We discovered that the activity of glucose-6-phosphate dehydrogenase (G6PDH) and 6-phosphogluconate dehydrogenase (6PGH) were similar between muscles expressing wild type and mutant Lamin C (S5 Fig, left and middle panel). In contrast, the activity of isocitrate dehydrogenase (IDH) was elevated in muscles expressing mutant lamins, compared to that of wild type (S5 Fig, right panel). Thus, the elevated IDH activity provides a potential explanation for the increased levels of NADPH.
Mutant lamins activate the Nrf2/Keap-1 pathway
Genes involved in cellular detoxification, such as the Gst genes, are typically activated by the conserved Nuclear factor erythroid 2-related factor 2 (Nrf2)/Kelch-like ECH associated protein 1 (Keap-1) signaling pathway [21,22]. Under normal conditions, the antioxidant transcription factor Nrf2 is sequestered in the cytoplasm by Keap-1. Under conditions of oxidative stress, cysteine residues within Keap-1 are oxidized, causing Nrf2 to no longer associate and translocate into the nucleus, where it activates target genes possessing anti-oxidant response elements (AREs) [21,22]. However, an alternative mechanism for Nrf2 target gene activation has been described for conditions of reductive stress [23] (Fig 3B). This mechanism relies on the competition between Nrf2 and p62/SQSTM1, an autophagy cargo acceptor, for the binding of Keap-1. Increased levels of p62/SQSTM1 sequester Keap-1, allowing Nrf2 to translocate into the nucleus and activate target genes.
Our finding that cellular detoxification genes were upregulated in response to mutant lamins suggested that the Nrf2/Keap-1 pathway was activated. To determine if this was the case, we performed immunohistochemistry on larval body wall muscle expressing wild type and mutant Lamin C with antibodies to Cap-and-collar C (CncC), the Drosophila homologue of Nrf2 [24]. In muscles expressing wild type Lamin C, we observed little to no staining, consistent with the fact that Nrf2/Keap-1 is rapidly turned over under normal conditions (Fig 4A) [25]. In contrast, we observed enhanced nuclear staining in muscles expressing each of the mutant Lamin C, relative to controls (Fig 4A). Thus, mutant lamins cause nuclear accumulation of CncC (Nrf2), which is consistent with activation of the Nrf2/Keap-1 pathway and expression of many CncC target genes.
Fig. 4. Mutant lamins cause enrichment of CncC/Nrf2 in myonuclei. 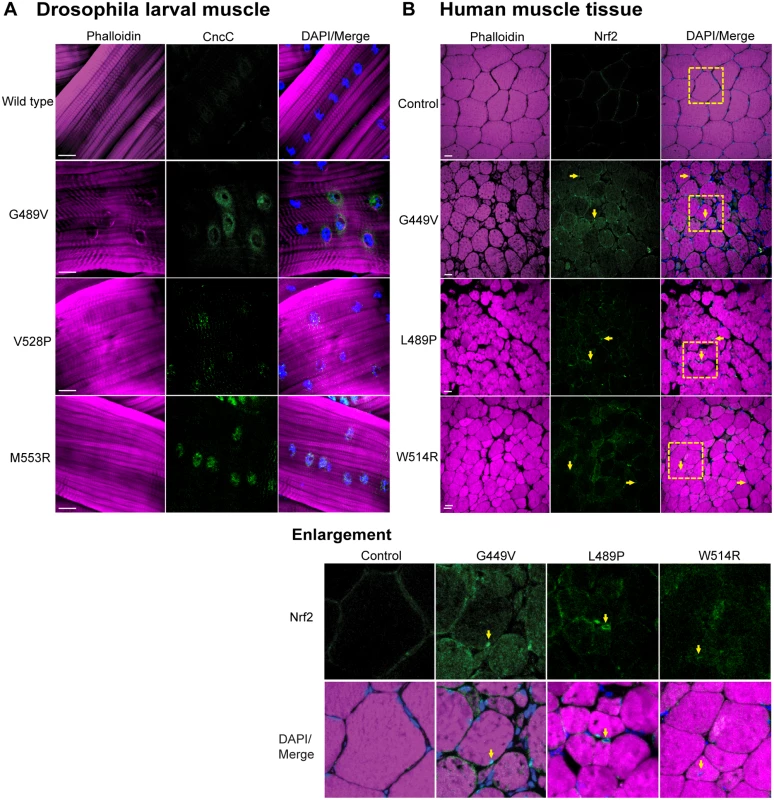
(A) Drosophila larval body wall muscles from transgenic larvae stained with phalloidin (magenta), DAPI (blue) and CncC (green). Increased intensity of staining was observed for all mutants, relative to wild type. (B) Human muscle biopsy tissues were stained with phalloidin (magenta), DAPI (blue) and antibodies to Nrf2 (green). Arrows indicate myonuclei enriched for staining with Nrf2 antibodies. Boxed areas are those shown enlarged below. To determine if the Nrf2/Keap-1 pathway is active in the human disease state, we stained muscle biopsy tissues from patients (possessing the mutations that were modeled in Drosophila) with antibodies to human Nrf2 [24]. In control muscle tissue, little to no staining was observed with the Nrf2 antibody (Fig 4B). In contrast, enhanced staining within the myonuclei of the patient muscle biopsy tissue was apparent (Fig 4B). Thus, expression of mutant lamins correlates with nuclear translocation of Nrf2 in both Drosophila and diseased human muscle.
In mammalian systems Nrf2 and p62/SQSTM1 are co-regulated [21]. Given our findings of reductive stress and Nrf2 myonuclei enrichment, we hypothesized that levels of the autophagy cargo protein p62/SQSTM1 would be elevated in the muscles expressing mutant lamins relative to controls. To test this hypothesis, we stained Drosophila larval body wall muscles with antibodies to Drosophila p62/Ref(2)P, a homologue of human p62/SQSTM1 [26]. Muscles expressing wild type Lamin C showed hardly any staining for p62/Ref(2)P (Fig 5A). In contrast, muscles expressing mutant lamins showed increased cytoplasmic foci of staining (Fig 5A). The elevated levels of p62/Ref(2)P were validated by western analysis of protein extract from larval body wall muscles (S6 Fig). Thus, mutant lamins cause elevated levels of cytoplasmic p62/Ref(2)P, which is consistent with the increased cytoplasmic aggregation of mutant lamins (Fig 2A).
Fig. 5. Mutant lamins cause increased levels of p62/SQSTM1 in Drosophila muscles and human muscle biopsy tissues. 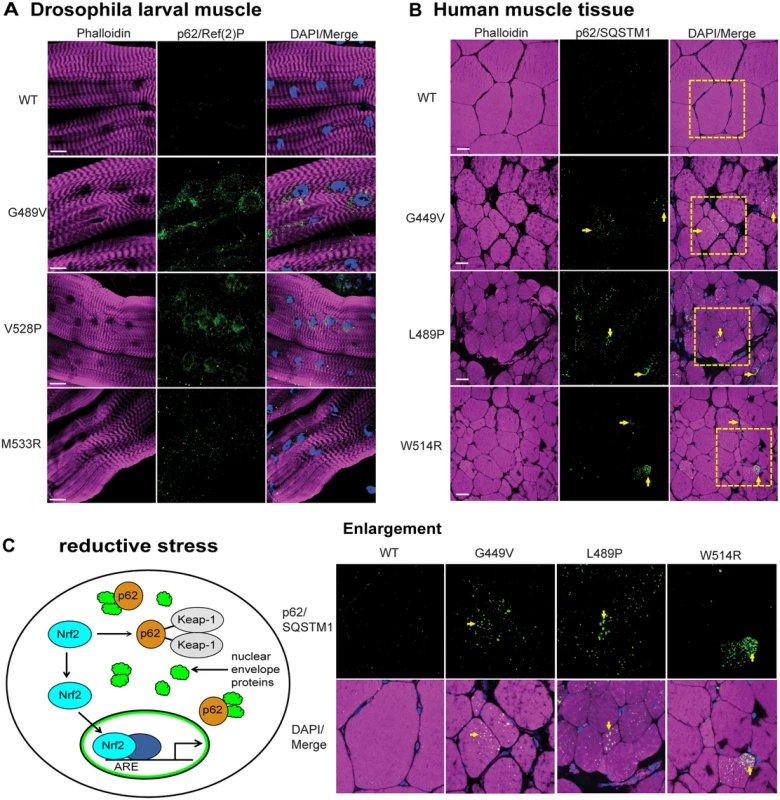
(A) Drosophila larval body wall muscles from transgenic larvae were stained with phalloidin (magenta), DAPI (blue) and antibodies to p62/Ref(2)P (green). Increased intensity of staining and the number of cytoplasmic foci were greater in muscle expressing mutant lamins compared to wild type. (B) Human muscle biopsy tissues were stained with phalloidin (magenta), DAPI (blue) and antibodies to p62/SQSTM1 (green). Arrows indicate muscle fibers with enhanced staining with anti-p62/SQSTM1 antibodies. Boxed areas are those shown enlarged below. (C) Unified model to explain the activation of cellular detoxification genes due to mutant lamins. Mutant lamins have altered tertiary structures that promote cytoplasmic aggregation. In addition, other nuclear envelope proteins such as LEM domain proteins and nuclear pore proteins accumulate in the cytoplasm via unknown mechanisms [9] (green cloud structures). These aggregates cause elevated levels of the autophagy adaptor protein p62/SQSTM1 (orange circles), which in turn binds Keap-1 (grey oval). This competitive binding allows Nrf2 (light blue oval) to translocate into the nucleus, bind to a partner protein (dark blue oval), and activate target genes possessing anti-oxidant response elements (AREs). To determine if the elevated levels of p62/Ref(2)P also occur in the human disease state, we stained muscle biopsy samples from the patients (possessing the mutations that were modeled in Drosophila) with antibodies to human p62/SQSTM1 [26]. Scoring muscle fibers positive for p62/SQSTM1 if they had ten or more visible foci containing p62/SQSTM1, showed that the control patient muscle tissue had low levels of p62/SQSTM1; only 5/200 muscle fibers were positive (Fig 5B). In contrast, 48–62/200 fibers were scored positive in the patient samples. Furthermore, the diameter of the p62/SQSTM foci in the patient tissues was larger than those in the controls (Fig 5B). These findings strongly suggest that the Nrf2/Keap-1 pathway activation in both Drosophila and human muscle occurs through an alternative mechanism that is triggered by elevated levels of p62/SQSTM1.
Discussion
Our structural studies of the lamin Ig-fold demonstrated that single amino acid substitutions in the loop regions perturb the tertiary structure, leaving the secondary structure of the folded domain largely intact (S2 Fig). These data were consistent with single-molecule force spectroscopy showing that the lamin Ig-fold possessing an R453W substitution required less force to unfold than the wild type Ig-fold domain [27]. Our structural data are consistent with in silico modeling in which amino acid substitutions in the Ig-fold that cause muscular dystrophy were predicted to alter the structure, more so than those that cause lipodystrophy or progeria [28]. Our NMR analysis of the mutant Ig-fold domains identified surfaces on opposite sides of the Ig-fold barrel that are critical for muscle function. This finding predicts that substitution of other amino acids that comprise these surfaces might result in muscular dystrophy. Consistent with this prediction, amino acid substitutions in eight of the 21 amino acids that make up these surfaces cause muscular dystrophy (Leiden muscular dystrophy database http://www.dmd.nl).
It is interesting to note that the largest structural perturbations were observed for the G449V and W514R mutants, which correspond to the most severe patient phenotypes. The corresponding amino acid substitutions in Drosophila Lamin C caused the greatest percentage of lethality [9]. The N456I mutant showed the least structural perturbations in the Ig-fold domain (Fig 1), though the relative severity of symptoms in this patient was not assertained [29]. Consistent with the structural data, the corresponding amino acid substitution in Drosophila Lamin C gave the least percentage of lethality [9]. Thus, our investigations showed an obvious correlation between the severity of the Ig-fold structural perturbations and phenotypic severity.
The structural perturbations within the Ig-fold might generate novel interaction surfaces that promote lamin aggregation (Figs 1 and 2A). Both nuclear and cytoplasmic aggregation of mutant lamins have been reported [30,31], however, they are not commonly observed in human muscle biopsy tissue or tissue from a laminopathy mouse model [9,32]. Cytoplasmic aggregation was observed for a truncated form of A-type lamin that causes Hutchinson-Gilford progeria syndrome [30]. Lamin aggregation is supported by X-ray crystallography studies of a R482W substitition in the A-type lamin Ig-fold domain that causes lipodystrophy [33]. The R482W Ig-fold domain possesses unique interaction surfaces not present in the wild type Ig-fold that form a unique platform for tetramerization.
The structural perturbations in the Ig-fold domain are likely to affect many functions of the mutant lamins. The lamin Ig-fold domain interacts with many partners to build the network that underlies the inner membrane of the nuclear envelope [5]. Mutant lamins can be inappropriately incorporated into the lamin network and function as dominant negatives [15,34,35]. This is the case for the headless lamin, which has dominant effects on nuclear shape and stiffness in both Drosophila muscle tissue and MEFs (Fig 2) [13]. In contrast, the Ig-fold substitutions do not cause major dominant effects on stiffness, however, there might be undetected alterations in the lamina and/or nuclear organization. Changes in nuclear organization could explain the misregulation of gene expression that we observed in muscles expressing mutant lamins (S1 and S2 Tables). In addition, the amino acid substitutions within the lamin Ig-fold domain might disrupt posttranslational modifications that occur on lamins, similar to what has been shown for familial partial lipodystrophy LMNA mutations that disrupt SUMOylation of Lamin A [36]. Changes in posttranslational modifications have the potential to alter interaction with partner proteins and/or affect aggregation properties.
Cytoplasmic protein aggregation has been linked to reductive stress [37,38]. Here, we show that cytoplasmic lamin aggregation correlates with elevated levels of both GSH and NADPH, hallmarks of reductive stress [20] (Fig 3). Elevated levels of isocitrate dehydrogenase enzyme activity (S5 Fig) contribute to the additional NADPH. In a similar manner, dominant negative forms of alphaB-crystallin (CryAB) result in cytoplasmic CryAB misfolding/aggregation and reductive stress in the mouse heart, ultimately leading to dilated cardiomyopathy [39]. These findings suggest that reductive stress might contribute to the dilated cardiomyopathy in cases of lamin associated muscular dystrophy. Interestingly, mutations in the human CRYAB gene cause disease phenotypes that are strikingly similar to those observed for lamin associated muscular dystrophy, including skeletal muscle weakness and dilated cardiomyopathy in cases of lamin-associated muscular dystrophy. It is worthwhile to note that CryAB functions as a chaperone to prevent aggregation of intermediate filament proteins such as desmin, suggesting a common link between intermediate filament aggregation and reductive stress.
An imbalance in redox homeostasis can provide an environment that promotes protein misfolding and aggregation. The redox state influences aggregation of lamins; aggregation has been observed under both oxidative and reductive conditions [33,40]. In fact, the formation of the novel tetramer generated by the R482W mutant Ig-fold domain (see above) required a reductive environment [33]. Reductive stress has also been observed in healthy individuals predisposed to Alzheimer disease, a disease of protein aggregation [41]. Alzheimer disease is typically accompanied by oxidative stress, however, lymphocytes from patients carrying an ApoE4 allele that predisposes them to Alzheimer disease show reductive stress. It is hypothesized that continual activation of antioxidant defense systems, such as Nrf2/Keap-1 signaling, becomes exhausted over time, particularly later in life, resulting in the inability to properly defend against oxidative stress. Our redox analysis in Drosophila muscle occurred 24–48 hours post expression of the mutant lamins. Our findings suggest reductive stress at the onset of pathology that could resolve into oxidative stress later in disease progression [42].
Typically lamins are thought to regulate gene expression from inside the nucleus, by interacting with transcription factors and organizing the genome [5,43]. Our data support a novel model in which genes are misregulated as a consequence of mutant lamin aggregation in the cytoplasm. Cytoplasmic lamin aggregates have been found in high molecular weight complexes in cases of liver injury [44]. Such complexes contain nuclear pore proteins, signaling mediators, transcription factors and ribosomal proteins, which are thought to disrupt the normal cellular physiology. Lamin aggregation might also serve a cytoprotective function by facilitating the coalescence of mutant lamin so that the contractile apparatus can properly function. A similar mechanism exists in Huntington’s disease, where sequestration of mutant huntingtin in inclusion bodies correlates with better neuron health [45].
Collectively, our findings continue to support this Drosophila model of laminopathies, as many of the phenotypes discovered here in Drosophila have been validated in human muscle biopsies (Figs 4B and 5B) [9]. It is now possible to use this rapid genetic model to (1) determine if mutations in other domains of lamin produce similar phenotypes and (2) if lamin mutations have similar effects in other tissues, such as the heart. Our data suggest that cytoplasmic lamin aggregation contributes to muscle pathology. Consistent with this idea, increased rates of autophagy suppress phenotypes caused by mutant A-type lamin in cultured cells and mouse models [32,46]. Furthermore, electron microscopy of skeletal muscle biopsies from patients with LMNA mutations showed large perinuclear autophagosomes [47], similar to the localization of lamin aggregates and p62 foci in the Drosophila muscle (Figs 2A and 5A). Thus, the regulation of autophagy, a process that removes both damaged organelles and proteins, might be central to the development of therapies. The Drosophila model will allow for genetic dissection of both the autophagy and reductive stress pathways to identify the key factors responsible for the muscle pathogenesis and its suppression.
Materials and Methods
Protein expression
LMNA mutations identified in patients were introduced into a wild type copy of the human LMNA gene in a pCR2.1 vector via site-directed mutagenesis (Quick Change, Stratagene). The sequences of the PCR primers used to make these mutations are listed in S3 Table. DNA fragments encoding amino acids 435 through 552 of human lamin A were amplified using primers containing BamH1 and HindIII sites. The resulting PCR products were cloned into the pQE-30 Xa vector (Qiagen) and the constructs was expressed in M15[pREP4] E. coli cells. Expression was induced by IPTG overnight. Expression of wild type Ig-fold, G449V and W514R yielded protein that was purified by nickel column chromatography followed by Superdex-75 size exclusion chromatography. Expression of N456I and L489P yielded proteins that resided within inclusion bodies. Subsequent purification required denaturing conditions in 8 M urea during purification nickel and Superdex-200 size exclusion chromatography. Material from the monomeric peak eluted from the Superdex-200 column was dialyzed overnight to eliminate the urea and then re-purified on the Superdex-75 size exclusion column. Approximately 30 mg of wild type Ig-fold domain and approximately 8 mg of each mutant were purified per liter of cell culture, as determined spectrophotometrically (Nanodrop, Thermo Scientific).
Biophysical analyses
Circular dichroism (CD) data was collected using 1 μM protein in 20 mM phosphate buffer with 100 mM NaCl and 0.1 mM DTT using a Jasco J815 CD Spectrophotometer. The spectral scan was performed between 190 nm and 280 nm. For T1/2 determination, melting curves were monitored under tryptophan absorbance at 230 nm; samples were heated at the rate of 2°C per minute. All CD experiments were performed in triplicate with independently prepared protein samples. Nuclear magnetic resonance (NMR) spectra were recorded at 20°C on a Bruker 500 or 800 MHz NMR spectrometer (NMR Core Facility, University of Iowa). NMR data were processed using NMRPipe [48] and analyzed using Sparky [49] and/or NMRView [50]. For the wild type Ig-fold domain, 1H, 15N, and 13C resonances of the backbone were assigned using the triple resonance experiments [HNCA, HN(CO)CA, HNCACB, HN(CO)CACB, HNCO, HN(CA)CO, and C(CO)NH-TOCSY] with a 500 uM 15N/13C-labeled sample. All NMR experiments were conducted in a NMR buffer containing 20 mM sodium phosphate (pH 7.0), 100 mM NaCl, 2 mM DTT, 1 mM EDTA and 0.1 mM sodium azide.
Drosophila stocks
Drosophila stocks were cultured on standard corn meal media at 25°C [51]. Stocks with the wild type and mutant lamin transgenes were previously described [9,15,52]. All lamins were expressed using the Gal4/UAS system and the C57 muscle-specific Gal4 driver stock [10].
Western analysis
Western analysis of protein extract from MEFs was according to published procedures [13]. Western analysis using Drosophila muscle was performed by extracting protein from 10 muscle fillets hand-dissected from third instar larvae in 2X Laemmli grinding buffer (125 mM Tris HCL, pH 6.8, 20% glucerol, 4% SDS, 0.005% bromophenol blue) plus 10 mM DL-Dithiothreitol. Anti-Drosophila p62 (1 : 8,000; kind gift of G. Juhász) and anti-Drosophila tubulin (1 : 300,000, Sigma) were used as primary antibodies and detected with anti-rabbit-HRP (1 : 400, Sigma) and anti-mouse-HRP (1 : 400, Sigma).
Nuclear strain assays
Nuclear strain analysis of modified and unmodified MEFs was performed as previously described [53]. Nuclear strain analysis of Drosophila muscle was previously described [13].
Gene expression analyses
Total RNA was isolated from hand-dissected body wall muscle from 40 third instar larvae per sample. RNA was purified using Trizol (Ambion) followed by RNAeasy (Qiagen). The RNA was used to generate labeled cRNA and hybridized to Drosophila 2.0 GeneChip arrays (Affymetrix) (DNA Core Facility, University of Iowa). Triplicate biological samples were analyzed for each genotype. The microarray data were analyzed using the Partek Genomic Suite [54]. Differentially expressed genes were identified using analysis of variance (ANOVA) with a two-fold change in expression and a P value of 0.05 or higher used as a cut off.
Immunohistochemistry
Immunohistochemistry of Drosophila larval body wall muscles and human muscle biopsy tissues were performed as previously described [9]. Drosophila larval body wall muscles were stained with affinity purified anti-CncC antibodies (1 : 100; gift from H. Deng and T. Kerppola) [24], anti-p62/Ref(2)P (1 : 3,000, kind gift of G. Juhász) [55] that was detected with Alexa Fluor 488 goat anti-rabbit (1 : 400 dilution; Invitrogen). Filamentous actin was detected with Texas Red Phalloidin (1 : 400, Invitrogen). Human muscle biopsy cryosections were obtained from the Iowa Wellstone Muscular Dystrophy Cooperative Research Center and stained according to published procedures [9] with human p62/SQSTM1 (1 : 3,000, Sigma) and Nrf2 (1 : 300, Santa Cruz Biotech), followed by Alexa Fluor 488 goat anti-rabbit (1 : 400, Invitrogen). Filamentous actin was detected with Texas Red Phalloidin (1 : 400, Invitrogen).
Glutathione and NADPH measurements
Drosophila larval body wall muscles were hand-dissected from 15 larvae, placed in 200 μl of 5% 5-sulfosalicylic acid and quantitation of reduced glutathione (GSH) and oxidized glutathione disulfide (GSSG) was performed as published [56]. GSSG was determined by adding a 1 : 1 mixture of 2-vinylpyridine and ethanol to the samples and incubating for two hours before assaying as described previously [57]. Enzymatic rates were compared to standard curves obtained from control samples. GSH and GSSG amounts were normalized to the protein content of the insoluble pellet from the 5-sulfosalicylic acid treatment, dissolved in 2.5% SDS in 0.1N bicarbonate, using the BCA Protein Assay Kit (Thermo Scientific). For measurements of NADPH, larval body wall muscles were hand-dissected from 15 larvae and immediately frozen in liquid nitrogen. Muscle samples were thawed in 170 μl of buffer [100 mM Tris HCl, 10 mM EDTA, 0.05% Triton X (v/v), pH 7.6] and sonicated four times for 30 seconds each. Assays were performed according to previous published procedures [58]. Absorbance was read at 310nm using a DU670 Spectrophotometer (Beckman) and enzyme activity was expressed as micromoles of NADPH per milligram of total protein.
Enzymatic activity measurements
For measurements of the NADPH-producing enzymes, larval body wall muscles were hand-dissected from 15 larvae and immediately frozen in liquid nitrogen. For assays of G6PD and 6PGD, diethylenetriaminepentaacetic acid (DETAPAC) was added to pellet and the mixture sonicated using a Sonics Vibra-cell sonicator with a cup horn at 20% amplitude. Enzymatic assays were performed according to previous published procedures [59] using 0.1 M Tris HCl-MgCl2, 2mM NADP with either 0.034 grams of glucose-6-phosphate or 0.041 grams 6-phosphogluconic acid, pH 8.0. Absorbance was read at 340 nm using a DU670 Spectrophotometer (Beckman) and enzyme activity was reported as milliunits per milligram of protein. IDH activity was measured according to published procedures [60] using 100mM Tris-HCl, 0.10 mM NADP, 0.84 mM MgSO4, and1.37 mM isocitrate (pH 8.6). Absorbance was measured at 340 nm, every 9 sec, over 3 minutes at 25°C using a DU670 Spectrophotometer (Beckman) and enzyme activity was expressed as micromoles of NADPH produced per minute per microgram soluble protein X 10,000.
Supporting Information
Zdroje
1. Worman HJ (2012) Nuclear lamins and laminopathies. The Journal of Pathology 226 : 316–325. doi: 10.1002/path.2999 21953297
2. Worman HJ, Bonne G (2007) "Laminopathies": a wide spectrum of human diseases. Exp Cell Res 313 : 2121–2133. 17467691
3. Worman HJ, Fong LG, Muchir A, Young SG (2009) Laminopathies and the long strange trip from basic cell biology to therapy. J Clin Invest 119 : 1825–1836. doi: 10.1172/JCI37679 19587457
4. Herrmann H, Bar H, Kreplak L, Strelkov SV, Aebi U (2007) Intermediate filaments: from cell architecture to nanomechanics. Nat Rev Mol Cell Biol 8 : 562–573. 17551517
5. Wilson KL, Berk JM (2010) The nuclear envelope at a glance. J Cell Science 123 : 1973–1978. doi: 10.1242/jcs.019042 20519579
6. Davidson PM, Lammerding J (2014) Broken nuclei—lamins, nuclear mechanics, and disease. Trends Cell Biol 24 : 247–256. doi: 10.1016/j.tcb.2013.11.004 24309562
7. Worman HJ, Courvalin JC (2004) How do mutations in lamins A and C cause disease? The Journal of Clinical Investigation 113 : 349–351. 14755330
8. Gesson K, Vidak S, Foisner R (2014) Lamina-associated polypeptide (LAP)2alpha and nucleoplasmic lamins in adult stem cell regulation and disease. Semin Cell Dev Biol 29 : 116–124. doi: 10.1016/j.semcdb.2013.12.009 24374133
9. Dialynas G, Flannery KM, Zirbel LN, Nagy PL, Mathews KD, Moore SA, et al. (2012) LMNA variants cause cytoplasmic distribution of nuclear pore proteins in Drosophila and human muscle. Hum Mol Genet 21 : 1544–1556. doi: 10.1093/hmg/ddr592 22186027
10. Duffy JB (2002) GAL4 system in Drosophila: a fly geneticist's Swiss army knife. Genesis 34 : 1–15. 12324939
11. Krimm I, Ostlund C, Gilquin B, Couprie J, Hossenlopp P, Mornon JP, et al. (2002) The Ig-like structure of the C-terminal domain of lamin A/C, mutated in muscular dystrophies, cardiomyopathy, and partial lipodystrophy. Structure 10 : 811–823. 12057196
12. Lammerding J, Fong LG, Ji JY, Reue K, Stewart CL, Young SG, et al. (2006) Lamins A and C but not lamin B1 regulate nuclear mechanics. J Biol Chem 281 : 25768–25780. 16825190
13. Zwerger M, Jaalouk DE, Lombardi ML, Isermann P, Mauermann M, Dialynas G, et al. (2013) Myopathic lamin mutations impair nuclear stability in cells and tissue and disrupt nucleo-cytoskeletal coupling. Hum Mol Genet 22 : 2335–2349. doi: 10.1093/hmg/ddt079 23427149
14. Riemer D, Stuurman N, Berrios M, Hunter C, Fisher PA, Weber K, et al. (1995) Expression of Drosophila Lamin-C Is Developmentally-Regulated—Analogies with Vertebrate a-Type Lamins. J Cell Science 108 : 3189–3198. 7593280
15. Dialynas G, Speese S, Budnik V, Geyer PK, Wallrath LL (2010) The role of Drosophila Lamin C in muscle function and gene expression. Development 137 : 3067–3077. doi: 10.1242/dev.048231 20702563
16. Kind J, van Steensel B (2010) Genome-nuclear lamina interactions and gene regulation. Curr Opin Cell Biol 22 : 320–325. doi: 10.1016/j.ceb.2010.04.002 20444586
17. Mattout A, Pike BL, Towbin BD, Bank EM, Gonzalez-Sandoval A, Stadler MB, et al. (2011) An EDMD mutation in C. elegans lamin blocks muscle-specific gene relocation and compromises muscle integrity. Curr Biol: CB 21 : 1603–1614. doi: 10.1016/j.cub.2011.08.030 21962710
18. Saisawang C, Wongsantichon J, Ketterman AJ (2012) A preliminary characterization of the cytosolic glutathione transferase proteome from Drosophila melanogaster. Biochem J 442 : 181–190. doi: 10.1042/BJ20111747 22082028
19. Zhou S, Campbell TG, Stone EA, Mackay TF, Anholt RR (2012) Phenotypic plasticity of the Drosophila transcriptome. PLoS Genet 8: e1002593. doi: 10.1371/journal.pgen.1002593 22479193
20. Brewer AC, Mustafi SB, Murray TV, Rajasekaran NS, Benjamin IJ (2013) Reductive stress linked to small HSPs, G6PD, and Nrf2 pathways in heart disease. Antioxid Redox Signal 18 : 1114–1127. doi: 10.1089/ars.2012.4914 22938199
21. Jain A, Lamark T, Sjottem E, Larsen KB, Awuh JA, Overvatn A, et al. (2010) p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. Journal Biol Chem 285 : 22576–22591. doi: 10.1074/jbc.M110.118976 20452972
22. Nezis IP, Simonsen A, Sagona AP, Finley K, Gaumer S, Contamine D, et al. (2008) Ref(2)P, the Drosophila melanogaster homologue of mammalian p62, is required for the formation of protein aggregates in adult brain. J Cell Biol 180 : 1065–1071. doi: 10.1083/jcb.200711108 18347073
23. Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, et al. (2010) The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol 12 : 213–223. doi: 10.1038/ncb2021 20173742
24. Deng H, Kerppola TK (2013) Regulation of Drosophila metamorphosis by xenobiotic response regulators. PLoS Genet 9: e1003263. doi: 10.1371/journal.pgen.1003263 23408904
25. Hayes JD, Dinkova-Kostova AT (2014) The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci 39 : 199–218. doi: 10.1016/j.tibs.2014.02.002 24647116
26. Bartlett BJ, Isakson P, Lewerenz J, Sanchez H, Kotzebue RW, Cumming RC, et al. (2011) p62, Ref(2)P and ubiquitinated proteins are conserved markers of neuronal aging, aggregate formation and progressive autophagic defects. Autophagy 7 : 572–583. 21325881
27. Bera M, Kotamarthi HC, Dutta S, Ray A, Ghosh S, Bhattacharyya D, et al. (2014) Characterization of Unfolding Mechanism of Human Lamin A Ig Fold by Single-Molecule Force Spectroscopy-Implications in EDMD. Biochemistry 53 : 7242–7258.
28. Scharner J, Lu HC, Fraternali F, Ellis JA, Zammit PS (2014) Mapping disease-related missense mutations in the immunoglobulin-like fold domain of lamin A/C reveals novel genotype-phenotype associations for laminopathies. Proteins 82 : 904–915. doi: 10.1002/prot.24465 24375749
29. Brown CA, Lanning RW, McKinney KQ, Salvino AR, Cherniske E, Crowe CA, et al. (2001) Novel and recurrent mutations in lamin A/C in patients with Emery-Dreifuss muscular dystrophy. Am J Med Genet 102 : 359–367. 11503164
30. Cao K, Blair CD, Faddah DA, Kieckhaefer JE, Olive M, Erdos MR, et al. (2011) Progerin and telomere dysfunction collaborate to trigger cellular senescence in normal human fibroblasts. J Clin Invest 121 : 2833–2844. doi: 10.1172/JCI43578 21670498
31. Ostlund C, Bonne G, Schwartz K, Worman HJ (2001) Properties of lamin A mutants found in Emery-Dreifuss muscular dystrophy, cardiomyopathy and Dunnigan-type partial lipodystrophy. Journal of cell science 114 : 4435–4445. 11792809
32. Choi JC, Muchir A, Wu W, Iwata S, Homma S, Morrow JP, et al. (2012) Temsirolimus activates autophagy and ameliorates cardiomyopathy caused by lamin A/C gene mutation. Science translational medicine 4 : 144ra102. doi: 10.1126/scitranslmed.3003875 22837537
33. Magracheva E, Kozlov S, Stewart CL, Wlodawer A, Zdanov A (2009) Structure of the lamin A/C R482W mutant responsible for dominant familial partial lipodystrophy (FPLD). Acta crystallographica Section F, Structural biology and crystallization communications 65 : 665–670. doi: 10.1107/S1744309109020302 19574635
34. Burke B, Stewart CL (2006) The laminopathies: the functional architecture of the nucleus and its contribution to disease. Annu Rev Genomics Hum Genet 7 : 369–405. 16824021
35. Gotzmann J, Foisner R (2006) A-type lamin complexes and regenerative potential: a step towards understanding laminopathic diseases? Histochem Cell Biol 125 : 33–41. 16142451
36. Simon DN, Domaradzki T, Hofmann WA, Wilson KL (2013) Lamin A tail modification by SUMO1 is disrupted by familial partial lipodystrophy-causing mutations. Mol Biol Cell 24 : 342–350. doi: 10.1091/mbc.E12-07-0527 23243001
37. Christians ES, Benjamin IJ (2012) Proteostasis and REDOX state in the heart. Am J Physiol Heart Circ Physiol 302: H24–37. doi: 10.1152/ajpheart.00903.2011 22003057
38. Christians ES, Mustafi SB, Benjamin IJ (2014) Chaperones and cardiac misfolding protein diseases. Curr Protein Pept Sci 15 : 189–204. 24694370
39. Zhang X, Min X, Li C, Benjamin IJ, Qian B, Ding Z, et al. (2010) Involvement of reductive stress in the cardiomyopathy in transgenic mice with cardiac-specific overexpression of heat shock protein 27. Hypertension 55 : 1412–1417. doi: 10.1161/HYPERTENSIONAHA.109.147066 20439823
40. Verstraeten VL, Caputo S, van Steensel MA, Duband-Goulet I, Zinn-Justin S, Kamps M, et al. (2009) The R439C mutation in LMNA causes lamin oligomerization and susceptibility to oxidative stress. J Cell Mol Med 13 : 959–971. doi: 10.1111/j.1582-4934.2009.00690.x 19220582
41. Badia MC, Giraldo E, Dasi F, Alonso D, Lainez JM, Lloret A, et al. (2013) Reductive stress in young healthy individuals at risk of Alzheimer disease. Free Radic Biol Med 63 : 274–279. doi: 10.1016/j.freeradbiomed.2013.05.003 23665394
42. Teodoro JS, Rolo AP, Palmeira CM (2013) The NAD ratio redox paradox: why does too much reductive power cause oxidative stress? Toxicol Mech Methods 23 : 297–302. doi: 10.3109/15376516.2012.759305 23256455
43. Wilson KL, Foisner R (2010) Lamin-binding Proteins. Cold Spring Harbor perspectives in biology 2: a000554. doi: 10.1101/cshperspect.a000554 20452940
44. Singla A, Griggs NW, Kwan R, Snider NT, Maitra D, Ernst SA, et al. (2013) Lamin aggregation is an early sensor of porphyria-induced liver injury. J Cell Sci 126 : 3105–3112. doi: 10.1242/jcs.123026 23641075
45. Arrasate M, Mitra S, Schweitzer ES, Segal MR, Finkbeiner S (2004) Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature 431 : 805–810. 15483602
46. Ramos FJ, Chen SC, Garelick MG, Dai DF, Liao CY, Schreiber KH, et al. (2012) Rapamycin reverses elevated mTORC1 signaling in lamin A/C-deficient mice, rescues cardiac and skeletal muscle function, and extends survival. Sci Transl Med 4 : 144ra103. doi: 10.1126/scitranslmed.3003802 22837538
47. Park YE, Hayashi YK, Bonne G, Arimura T, Noguchi S, Nonaka I, et al. (2009) Autophagic degradation of nuclear components in mammalian cells. Autophagy 5 : 795–804. 19550147
48. Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6 : 277–293. 8520220
49. Lee W, Westler WM, Bahrami A, Eghbalnia HR, Markley JL (2009) PINE-SPARKY: graphical interface for evaluating automated probabilistic peak assignments in protein NMR spectroscopy. Bioinformatics 25 : 2085–2087. doi: 10.1093/bioinformatics/btp345 19497931
50. Johnson BA (2004) Using NMRView to visualize and analyze the NMR spectra of macromolecules. Methods Mol Biol 278 : 313–352. 15318002
51. Shaffer CD, Wuller JM, Elgin SC (1994) Raising large quantities of Drosophila for biochemical experiments. Method Cell Biol 44 : 99–108. 7707979
52. Schulze SR, Curio-Penny B, Li Y, Imani RA, Rydberg L, Geyer PK, et al. (2005) Molecular genetic analysis of the nested Drosophila melanogaster Lamin C gene. Genetics 171 : 185–196. 15965247
53. Lammerding J, Lee RT (2009) Mechanical properties of interphase nuclei probed by cellular strain application. Method Mol Biol 464 : 13–26. doi: 10.1007/978-1-60327-461-6_2 18951177
54. Downey T (2006) Analysis of a multifactor microarray study using Partek genomics solution. Method Enzymol 411 : 256–270. 16939794
55. Pircs K, Nagy P, Varga A, Venkei Z, Erdi B, Hegedus K, et al. (2012) Advantages and limitations of different p62-based assays for estimating autophagic activity in Drosophila. PLoS One 7: e44214. doi: 10.1371/journal.pone.0044214 22952930
56. Tietze F (1969) Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem 27 : 502–522. 4388022
57. Griffith OW (1980) Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem 106 : 207–212. 7416462
58. Zhang Z, Yu J, Stanton RC (2000) A method for determination of pyridine nucleotides using a single extract. Anal Biochem 285 : 163–167. 10998277
59. Glock GE, Mc LP (1953) Further studies on the properties and assay of glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase of rat liver. Biochem J 55 : 400–408. 13105646
60. Rzezniczak TZ, Merritt TJ (2012) Interactions of NADP-reducing enzymes across varying environmental conditions: a model of biological complexity. G3 (Bethesda) 2 : 1613–1623. doi: 10.1534/g3.112.003715 23275884
Štítky
Genetika Reprodukční medicína
Článek Minor Type IV Collagen α5 Chain Promotes Cancer Progression through Discoidin Domain Receptor-1Článek A Unique Virulence Gene Occupies a Principal Position in Immune Evasion by the Malaria ParasiteČlánek The Centrosomal Linker and Microtubules Provide Dual Levels of Spatial Coordination of CentrosomesČlánek Dissecting the Function and Assembly of Acentriolar Microtubule Organizing Centers in Cells In VivoČlánek MicroRNA-Dependent Transcriptional Silencing of Transposable Elements in Drosophila Follicle CellsČlánek PARP2 Is the Predominant Poly(ADP-Ribose) Polymerase in Arabidopsis DNA Damage and Immune ResponsesČlánek Cooperative Action of Cdk1/cyclin B and SIRT1 Is Required for Mitotic Repression of rRNA SynthesisČlánek The Developmental Intestinal Regulator ELT-2 Controls p38-Dependent Immune Responses in Adult .Článek Feeding and Fasting Signals Converge on the LKB1-SIK3 Pathway to Regulate Lipid Metabolism in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 5- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- IVF a rakovina prsu – zvyšují hormony riziko vzniku rakoviny?
-
Všechny články tohoto čísla
- Genomic Heritability: What Is It?
- Triglyceride-Increasing Alleles Associated with Protection against Type-2 Diabetes
- Epistasis Is a Major Determinant of the Additive Genetic Variance in
- Genetic Regulation of Bone Metabolism in the Chicken: Similarities and Differences to Mammalian Systems
- Minor Type IV Collagen α5 Chain Promotes Cancer Progression through Discoidin Domain Receptor-1
- The Philosophical Approach: An Interview with Ford Doolittle
- Downregulation of the Host Gene by miR-92 Is Essential for Neuroblast Self-Renewal in
- A Unique Virulence Gene Occupies a Principal Position in Immune Evasion by the Malaria Parasite
- Y Fuse? Sex Chromosome Fusions in Fishes and Reptiles
- Regulation of Active DNA Demethylation by a Methyl-CpG-Binding Domain Protein in
- Overlapping Patterns of Rapid Evolution in the Nucleic Acid Sensors cGAS and OAS1 Suggest a Common Mechanism of Pathogen Antagonism and Escape
- Autoselection of Cytoplasmic Yeast Virus Like Elements Encoding Toxin/Antitoxin Systems Involves a Nuclear Barrier for Immunity Gene Expression
- Genetic Architecture of Abdominal Pigmentation in
- Whole Genome DNA Binding Analysis of the Bacterial Replication Initiator and Transcription Factor DnaA
- The Centrosomal Linker and Microtubules Provide Dual Levels of Spatial Coordination of Centrosomes
- Parp3 Negatively Regulates Immunoglobulin Class Switch Recombination
- Burden Analysis of Rare Microdeletions Suggests a Strong Impact of Neurodevelopmental Genes in Genetic Generalised Epilepsies
- Cell Cycle Control by the Master Regulator CtrA in
- Myopathic Lamin Mutations Cause Reductive Stress and Activate the Nrf2/Keap-1 Pathway
- Monoallelic Loss of the Imprinted Gene Promotes Tumor Formation in Irradiated Mice
- Phylum-Level Conservation of Regulatory Information in Nematodes despite Extensive Non-coding Sequence Divergence
- Clustering and Negative Feedback by Endocytosis in Planar Cell Polarity Signaling Is Modulated by Ubiquitinylation of Prickle
- Dissecting the Function and Assembly of Acentriolar Microtubule Organizing Centers in Cells In Vivo
- MicroRNA-Dependent Transcriptional Silencing of Transposable Elements in Drosophila Follicle Cells
- β-Catenin Signaling Biases Multipotent Lingual Epithelial Progenitors to Differentiate and Acquire Specific Taste Cell Fates
- A Simple Auxin Transcriptional Response System Regulates Multiple Morphogenetic Processes in the Liverwort
- Parallel Gene Expression Differences between Low and High Latitude Populations of and .
- The Nutrient-Responsive Hormone CCHamide-2 Controls Growth by Regulating Insulin-like Peptides in the Brain of
- Characterization of TCF21 Downstream Target Regions Identifies a Transcriptional Network Linking Multiple Independent Coronary Artery Disease Loci
- PARP2 Is the Predominant Poly(ADP-Ribose) Polymerase in Arabidopsis DNA Damage and Immune Responses
- Drosophila Spaghetti and Doubletime Link the Circadian Clock and Light to Caspases, Apoptosis and Tauopathy
- Coronary Artery Disease Associated Transcription Factor TCF21 Regulates Smooth Muscle Precursor Cells That Contribute to the Fibrous Cap
- Rescue of DNA-PK Signaling and T-Cell Differentiation by Targeted Genome Editing in a Deficient iPSC Disease Model
- Disruption of Transcriptional Coactivator Sub1 Leads to Genome-Wide Re-distribution of Clustered Mutations Induced by APOBEC in Active Yeast Genes
- Yeast Killer Elements Hold Their Hosts Hostage
- Keeping in Shape the Dogma of Mitochondrial DNA Maternal Inheritance
- Extreme-Depth Re-sequencing of Mitochondrial DNA Finds No Evidence of Paternal Transmission in Humans
- Trading Places—Switching Frataxin Function by a Single Amino Acid Substitution within the [Fe-S] Cluster Assembly Scaffold
- Natural Variation Identifies , a Universal Gene Required for Cell Proliferation and Growth at High Temperatures in
- Mutations in Gene Are Associated with Predisposition to Breast Cancer
- The Whole of a Scientific Career: An Interview with Oliver Smithies
- Cell Specific eQTL Analysis without Sorting Cells
- Cooperative Action of Cdk1/cyclin B and SIRT1 Is Required for Mitotic Repression of rRNA Synthesis
- Systemic Regulation of RAS/MAPK Signaling by the Serotonin Metabolite 5-HIAA
- Reprogramming LCLs to iPSCs Results in Recovery of Donor-Specific Gene Expression Signature
- The Developmental Intestinal Regulator ELT-2 Controls p38-Dependent Immune Responses in Adult .
- Genetic Mechanism of Human Neutrophil Antigen 2 Deficiency and Expression Variations
- Feeding and Fasting Signals Converge on the LKB1-SIK3 Pathway to Regulate Lipid Metabolism in
- Early Lineage Priming by Trisomy of Leads to Myeloproliferation in a Down Syndrome Model
- Turning into a Frataxin-Dependent Organism
- Accounting for Experimental Noise Reveals That mRNA Levels, Amplified by Post-Transcriptional Processes, Largely Determine Steady-State Protein Levels in Yeast
- Biological Significance of Photoreceptor Photocycle Length: VIVID Photocycle Governs the Dynamic VIVID-White Collar Complex Pool Mediating Photo-adaptation and Response to Changes in Light Intensity
- CTXφ Replication Depends on the Histone-Like HU Protein and the UvrD Helicase
- Disruption of miR-29 Leads to Aberrant Differentiation of Smooth Muscle Cells Selectively Associated with Distal Lung Vasculature
- Notch Is Required in Adult Sensory Neurons for Morphological and Functional Plasticity of the Olfactory Circuit
- Post-transcriptional Regulation of Keratinocyte Progenitor Cell Expansion, Differentiation and Hair Follicle Regression by
- PERK Limits Lifespan by Promoting Intestinal Stem Cell Proliferation in Response to ER Stress
- Casein Kinase 1 and Phosphorylation of Cohesin Subunit Rec11 (SA3) Promote Meiotic Recombination through Linear Element Formation
- Fibroblast Growth Factor 9 Regulation by MicroRNAs Controls Lung Development and Links Loss to the Pathogenesis of Pleuropulmonary Blastoma
- Auxin-Mediated Transcriptional System with a Minimal Set of Components Is Critical for Morphogenesis through the Life Cycle in
- The Broad-Spectrum Antiviral Protein ZAP Restricts Human Retrotransposition
- The 4E-BP Caf20p Mediates Both eIF4E-Dependent and Independent Repression of Translation
- Turning into a Frataxin-Independent Organism
- Promotion of Bone Morphogenetic Protein Signaling by Tetraspanins and Glycosphingolipids
- Essential Role of the ESX-5 Secretion System in Outer Membrane Permeability of Pathogenic Mycobacteria
- The Zinc-Finger Antiviral Protein ZAP Inhibits LINE and Alu Retrotransposition
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Drosophila Spaghetti and Doubletime Link the Circadian Clock and Light to Caspases, Apoptosis and Tauopathy
- Autoselection of Cytoplasmic Yeast Virus Like Elements Encoding Toxin/Antitoxin Systems Involves a Nuclear Barrier for Immunity Gene Expression
- Parp3 Negatively Regulates Immunoglobulin Class Switch Recombination
- PERK Limits Lifespan by Promoting Intestinal Stem Cell Proliferation in Response to ER Stress
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



