-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Casein Kinase 1 and Phosphorylation of Cohesin Subunit Rec11 (SA3) Promote Meiotic Recombination through Linear Element Formation
The formation of haploid gametes (sex cells, such as eggs and sperm) from diploid precursor cells involves two nuclear divisions but one round of chromosomal replication. In the unique first meiotic division, centromeres of sister chromatids remain connected and homologous chromosomes (homologs) segregate from each other. In most species proper homolog segregation requires that crossover recombination occur between homologs to impart tension between homologs as they move apart. A protein kinase (casein kinase 1) has long been known to regulate proper sister centromere connections by phosphorylating Rec8, a meiosis-specific sister chromatid cohesin subunit. We report here that in fission yeast this kinase has a second critical role—to mediate phosphorylation of another meiosis-specific cohesin subunit Rec11. Phosphorylation of Rec11 enhances loading of two meiosis-specific components of linear elements, which are related to the synaptonemal complex and help pair homologs. These linear element proteins lead to high-level DNA breakage and crossovers between homologs. Thus, casein kinase regulates two crucial but separate events in meiosis. The mammalian functional homolog of Rec11, called STAG3, is also phosphorylated during meiosis and appears to be required for fertility in human females. These observations suggest wide-spread conservation of the roles of casein kinase 1 and Rec11 in ensuring proper meiotic chromosome segregation and sexual reproduction.
Published in the journal: . PLoS Genet 11(5): e32767. doi:10.1371/journal.pgen.1005225
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005225Summary
The formation of haploid gametes (sex cells, such as eggs and sperm) from diploid precursor cells involves two nuclear divisions but one round of chromosomal replication. In the unique first meiotic division, centromeres of sister chromatids remain connected and homologous chromosomes (homologs) segregate from each other. In most species proper homolog segregation requires that crossover recombination occur between homologs to impart tension between homologs as they move apart. A protein kinase (casein kinase 1) has long been known to regulate proper sister centromere connections by phosphorylating Rec8, a meiosis-specific sister chromatid cohesin subunit. We report here that in fission yeast this kinase has a second critical role—to mediate phosphorylation of another meiosis-specific cohesin subunit Rec11. Phosphorylation of Rec11 enhances loading of two meiosis-specific components of linear elements, which are related to the synaptonemal complex and help pair homologs. These linear element proteins lead to high-level DNA breakage and crossovers between homologs. Thus, casein kinase regulates two crucial but separate events in meiosis. The mammalian functional homolog of Rec11, called STAG3, is also phosphorylated during meiosis and appears to be required for fertility in human females. These observations suggest wide-spread conservation of the roles of casein kinase 1 and Rec11 in ensuring proper meiotic chromosome segregation and sexual reproduction.
Introduction
The two specialized nuclear divisions during meiosis convert a diploid precursor cell into one or more haploid cells (gametes). Uniquely in the first meiotic division, centromeres of homologous chromosomes (homologs) segregate from each other, whereas centromeres of sister chromatids segregate from each other only in the second meiotic division, as in mitotic divisions. Proper chromosome segregation is essential for the formation of gametes with viable chromosome complements and requires two chromosomal events special to meiosis—crossing-over between homologs, and spatially and temporally regulated cohesion between sister chromatids. For homologs to segregate properly from each other in the first meiotic division, they must pair and the sister centromeres must remain connected and move as a unit to one pole of the cell; in most species pairing is accompanied by physical exchange of DNA between homologs to form crossovers that provide the interhomolog tension required for their proper segregation. For sister centromeres to segregate properly from each other in the second meiotic division, pericentric cohesion must be established in the first division but be removed only during the second division. This is accomplished by protection of a meiosis-specific cohesin subunit only at and near the centromere before and during the first division and its degradation specifically just before the second division.
Although the outlines of these two events are known [1], how they are regulated and coordinated remains unclear. Previous data and our new results reported here show that casein kinase 1 plays an essential role in both of these events, by mediating the phosphorylation of two separate subunits of cohesin. Our observations are in fission yeast, but the wide-spread conservation of these subunits suggests that our conclusions apply broadly to eukaryotes.
Meiotic cohesin is a large protein complex composed of Smc1 and Smc3, which are common to both the mitotic and meiotic forms, and the meiosis-specific Rec8 and Rec11 subunits [2–5]. (Some species, including budding yeast and Tetrahymena, lack a clear Rec11 homolog and retain the mitotic form, Scc3, in meiosis [6,7].) This complex forms a ring that connects sister chromatids from the time of replication to the time of chromatid segregation [8,9]. To allow segregation, Rec8 is phosphorylated and then cleaved by a protease called separase [10]. During meiosis, Rec8 cleavage occurs in two steps: along the chromosome arms during the first meiotic division (MI) and in the pericentric region during the second meiotic division (MII) [11]. In MI pericentric Rec8 is protected by shugoshin (Sgo1), which recruits PP2A protein phosphatase and thereby prevents Rec8 cleavage. During MII, cohesin is no longer protected from separase, which then cleaves pericentric Rec8 to allow sister centromere segregation [12]. In the absence of Rec8, chromosome segregation is like that in mitosis: sister chromatids, rather than homologs, segregate at MI [13]. In the absence of Rec11, chromosome segregation is similar to that in a recombination-deficient mutant: sister centromeres remain connected until MII, when they segregate, but aberrant arm cohesion and a paucity of crossovers reduce proper homolog segregation at MI [5].
Rec8 and Rec11 are also required for formation of crossovers, which result from the repair of DNA double-strand breaks (DSBs) programmed to occur during meiosis [14,15]. DSBs are made by the highly conserved topoisomerase-like protein Spo11 (named Rec12 in fission yeast) [16]. To be active, Rec12 requires six essential partner proteins, which likely function as a large complex similar to that of the Spo11 complex of budding yeast [17]. In a proposed pathway, Rec8 and Rec11 cohesin subunits are loaded onto chromosomes during S phase [14,18]. Their loading allows loading of the linear element (LinE) complex, related to the synaptonemal complex of other species; LinEs contain Rec10, Rec25, Rec27, and Mug20 [14,19–21]. In accord with this pathway, LinEs are rare or absent in each of the six corresponding mutants [14,19–22]. LinE protein loading activates the Rec12 complex to make DSBs [14]. Although Rec10 is required for DSB-formation and recombination throughout the genome, the other three LinE proteins, like Rec8 and Rec11, are required more strongly in some chromosomal intervals than in others [14].
Cleavage of Rec8 to allow proper chromosome segregation requires phosphorylation of Rec8 by one or more protein kinases, including casein kinase 1 orthologs in both budding and fission yeasts [23]. In fission yeast two casein kinase 1 paralogs, Hhp1 and Hhp2 (collectively called Hhp here), function redundantly to phosphorylate Rec8 [12,23]. In their absence Rec8 cleavage is delayed and persistent sister-chromatid cohesion along chromosome arms often prevents chromosome segregation, leading to many fewer viable gametes (spores) than in wild-type cells. Because of the close connection between meiotic chromosome segregation and recombination, exemplified by the role of Rec8 in both processes [13,15], we examined meiotic recombination in Hhp-deficient mutants. We found that Hhp is indeed required for recombination but that the substrate for this process is, unexpectedly, the meiosis-specific cohesin subunit Rec11, not Rec8. Our findings indicate that Hhp regulates chromosome segregation and recombination separately, by regulating Rec8 cleavage and by activating Rec11 to promote DSB-formation and recombination. We discuss parallels in the roles of meiotic cohesin subunits common to fission yeast and mammals.
Results
Casein kinase 1 (hhp) null mutants are meiotic recombination-deficient
To test for a possible role of casein kinase 1 homologs Hhp1 and Hhp2 in meiotic recombination of the fission yeast Schizosaccharomyces pombe, we measured recombination in hhp1Δ hhp2Δ double deletion mutants. Recombination was reduced by factors ranging from about 5 to 170, depending on the interval measured (Table 1). Both intergenic recombination (crossing over) and intragenic recombination (gene conversion) were reduced in the double mutant but only slightly in each single mutant, indicating that Hhp1 and Hhp2 have redundant roles in recombination, as previously reported for chromosome segregation [12]. Similar differential reductions, depending on the interval measured, are observed in cohesin - and LinE-deficient mutants [19,24,25], leading us to suspect that the Hhp substrate required for recombination is a cohesin subunit or LinE protein. Our results below bear out this suspicion.
Tab. 1. Meiotic recombination depends on redundant Hhp1 and Hhp2 kinases. 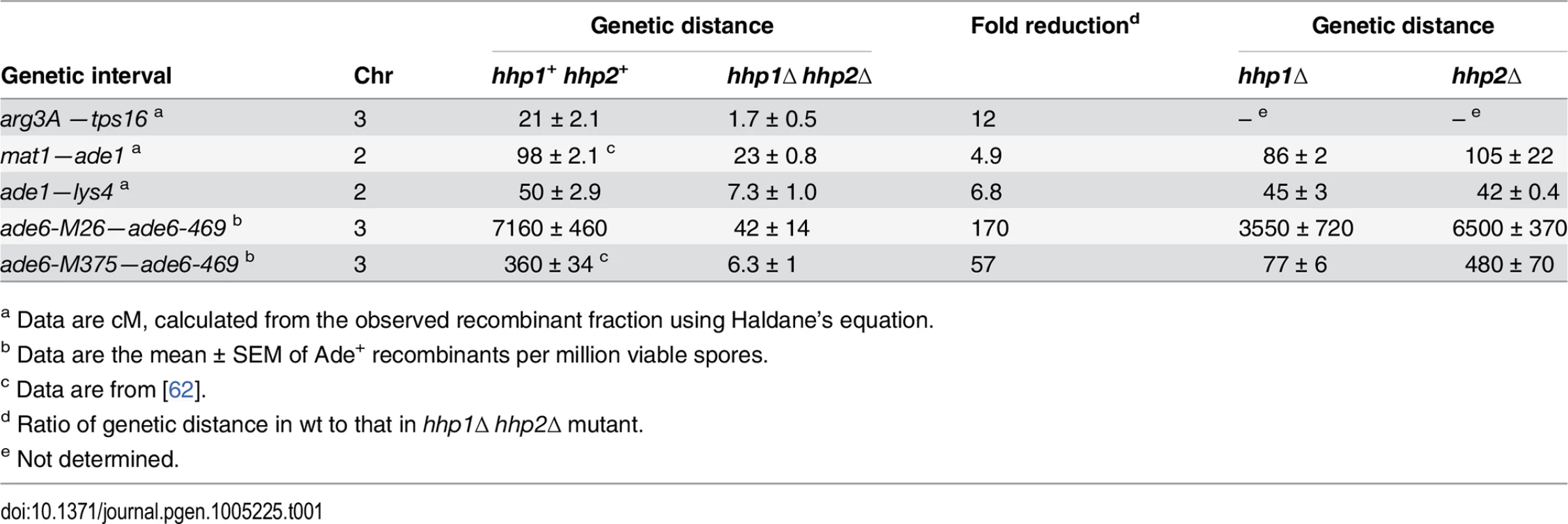
a Data are cM, calculated from the observed recombinant fraction using Haldane’s equation. An ATP analog-sensitive hhp mutant is DSB - and recombination-deficient even without analog
Because mitotic growth and viable spore yield are severely impaired in hhp1Δ hhp2Δ mutants [26,27], we used an Hhp1 mutant (hhp1-as encoding the Met84 → Gly alteration) sensitive to 1-NM-PP1, an analog of the purine moiety of ATP, by alteration of its ATP-binding site [28,29]. In conjunction with hhp2Δ we could thereby allow Hhp function (in the absence of analog) or block Hhp function (in its presence). As expected from the results above, recombination was strongly reduced in the presence of the analog: we observed ~15 - and 100-fold reductions in the two intervals tested (Table 2), comparable to the reductions seen in hhp1Δ hhp2Δ. Recombination was also reduced to essentially the same extent in the absence of the analog, a condition that allowed much higher viable spore yield and nearly wild-type chromosome segregation (Table 2 and S1 Table). This fortuitous result, presumably a reflection of hhp1-as allowing adequate phosphorylation of some substrates but not others, allowed us to conduct experiments under conditions allowing nearly wild-type mitotic growth and high viable spore yield. We discuss later the putative separation of functions of hhp1-as.
Tab. 2. Even without ATP-analog, hhp1-as hhp2∆ mutant has strongly reduced meiotic recombination but only weakly reduced viable spore yield. 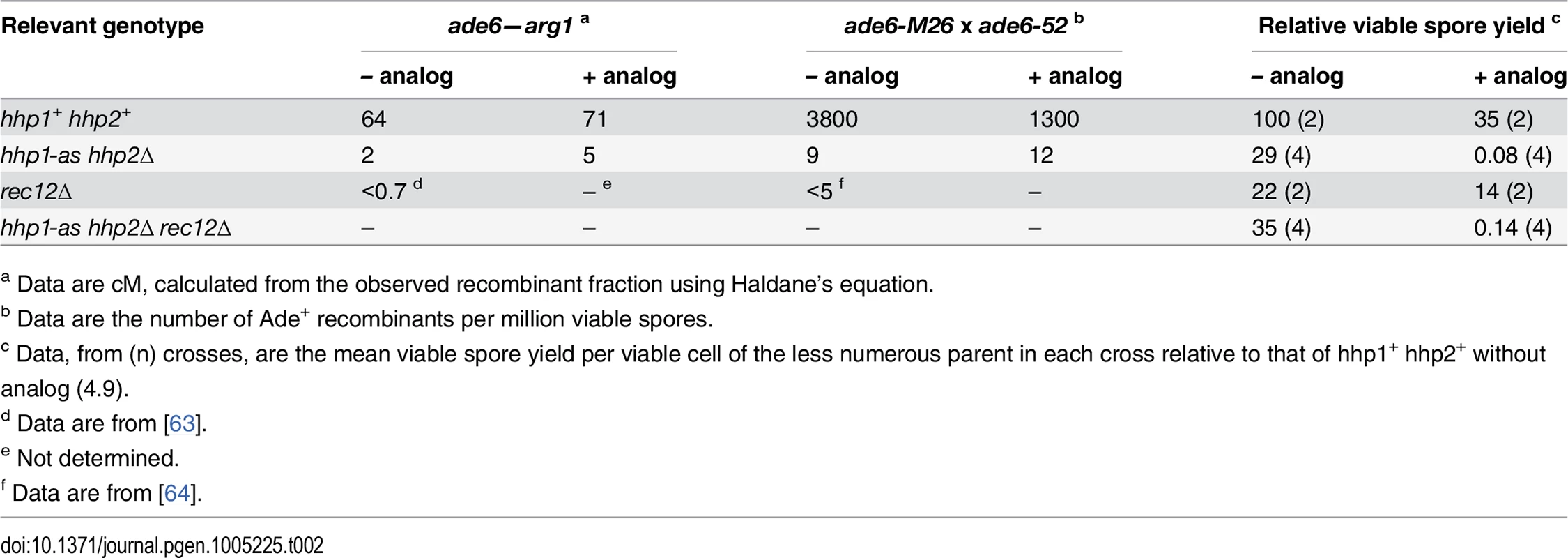
a Data are cM, calculated from the observed recombinant fraction using Haldane’s equation. When we coupled the hhp1-as hhp2Δ mutations with rec12Δ, we obtained results indicating that, in the absence of analog, recombination but not Rec8 cleavage was defective in the hhp1-as hhp2Δ mutant. Because S. pombe has only three chromosomes and has a mechanism that enhances proper segregation of non-recombinant homologs [30], rec12Δ mutants form ~25% as many viable spores as wild type (Table 2) [31]. This yield was not further reduced by the hhp1-as hhp2Δ mutations in the absence of analog, but it was greatly reduced in its presence, as expected from failure of Rec8 to be cleaved under this condition [12,32]. In the absence of analog, the failure of rec12Δ to further reduce the viable spore yield of hhp1-as hhp2Δ indicates that without analog hhp1-as hhp2Δ blocks recombination but not chromosome segregation. We infer that one or more recombination-promoting protein(s) is not properly phosphorylated by Hhp1-as in the absence of the analog and that in the presence of the analog Rec8 is also hypo-phosphorylated.
Meiotic recombination requires both formation and repair of DSBs. hhp1Δ hhp2Δ mutants have DNA repair defects in vegetative cells [26]. To determine if meiotic DSB repair is blocked in the hhp1-as hhp2Δ mutant in the absence of the analog, we artificially introduced DSBs with the I-SceI homing endonuclease, controlled by the meiosis-specific rec12 promoter [33]. DSBs were introduced in the ade6 gene at the site of the ade6-3061 mutation, which can recombine with another mutation ade6-52 to generate Ade+ recombinants. In the absence of analog the frequency of recombinants was indistinguishable in wild type and in hhp1-as hhp2Δ, indicating that under this condition Hhp is not required for DSB repair (S2 Table). rec8Δ, however, reduced the recombinant frequency by a factor of ~3, suggesting that Rec8 is required for DSB repair of I-SceI DSBs, as it is at some chromosomal sites in budding yeast [6]. We infer that during meiosis Hhp is required for DSB formation, but we found no evidence for its having a role in DSB repair under this condition. This conclusion is consistent with the hhp mutant having high viable spore yield but low recombination-proficiency in the absence of analog (Table 2).
To directly test for a role of Hhp in DSB formation, we assayed DSBs by Southern blot hybridizations of DNA extracted from hhp1-as hhp2Δ mutants induced for meiosis in the absence of analog; DNA from wild type and rec8Δ was analyzed for comparison (Fig 1 and S1 Fig). In wild type there were six prominent, meiosis-dependent DSB hotspots on the 501 kb NotI restriction fragment J, as seen before [34,35]. DSBs were barely detectable at these sites in hhp1-as hhp2Δ, as is the case in rec8Δ and rec11Δ [14,18]. DSBs were also barely detectable in hhp1-as hhp2Δ at the strong DSB hotspot ade6-3049, as is the case in rec8Δ and rec11Δ [14,18]. In contrast, DSBs were detectable, though at reduced levels, at some hotspots on the 1500 kb NotI restriction fragment C in hhp1-as hhp2Δ and in rec8Δ and rec11Δ (Fig 1B) [14,18]. These results show that Hhp is required for most meiotic DSB formation, but residual DSBs with a spatial pattern similar to that in rec8Δ and rec11Δ, which are indistinguishable [14,18], remain in the absence of Hhp function.
Fig. 1. Meiotic DNA breakage in hhp, rec11, and rec8 mutants. 
Strains with the indicated mutations were induced for meiosis in the absence of an ATP analog. At the indicated times, DNA was extracted and analyzed by Southern blot hybridization. All time points for each mutant were run on the same gel, one gel for the two NotI fragments and another for the ade6-3049 fragment (S1 Fig). Data below each lane with meiotically induced DNA are the percent of total DNA in the bands labeled a—f after subtraction of the intensity in the corresponding 0 hr lane. These data reflect DNA breakage at DSB hotspots. DSBs at the ade6-3049 hotspot are spread over about 1 kb, indicated by the bar (f) on the right. See also S1 Fig. (A) The 501 kb NotI fragment J on chromosome 1 was analyzed with a probe at its left end [60]. (B) The 1.5 Mb NotI fragment C on chromosome 2 was analyzed with a probe near its left end [18]. (C) The 6.6 kb AflII fragment containing ade6 on chromosome 3 was analyzed with a probe at its right end [61]. Rec8 phosphorylation-deficient mutants are not recombination-deficient
Because the residual patterns of DSB formation and recombination in hhp1-as hhp2Δ resemble those in rec8Δ (Fig 1) [14,18] and because Rec8 is an Hhp substrate [12,23], we tested the hypothesis that the Hhp substrate required for DSB formation is Rec8. One of the Hhp-dependent phosphorylation sites on Rec8 (S412) is critical for cleavage of Rec8 to allow chromosome segregation [12]. The non-phosphorylatable mutant rec8-S412A was, however, as recombination-proficient as wild type (S3 Table) and had DSB patterns on NotI fragments J and D similar to those of wild type. Similar recombination results were obtained with six additional rec8 mutants lacking seven, 12, 13, 17, or 18 phosphorylation sites (S3 Table). In S. cerevisiae, Rec8 phosphorylation is also not essential for meiotic recombination, although crossing-over is about one-half as frequent and delayed relative to wild type [36]. These results suggest that Rec8 is not, under the conditions used here, the major substrate of Hhp required for DSB formation and recombination, although minor effects cannot be ruled out.
Rec11 is phosphorylated in an Hhp-dependent manner
To search for additional Hhp substrates during meiosis, we immunoprecipitated TAP-tagged versions of Hhp1 and Hhp2 and analyzed the precipitated proteins by mass spectrometry (S2 Fig, S4 Table and S5 Table). In meiotically induced cells, but not in mitotically growing cells, we found that Hhp1 co-immunoprecipitated with Hhp2-TAP and, conversely, Hhp2 with Hhp1-TAP. We confirmed this interaction in meiotic extracts by standard co-immunoprecipitation and Western blotting (S3 Fig). Physical interaction between Hhp1 and Hhp2 was previously observed in checkpoint-activated mitotic cells [37]. Importantly, we found the cohesin subunits Rec8, Psm1, and Psm3 (orthologs of Smc1 and Smc3 in other species) in precipitates of Hhp1 and Hhp2 from meiotic cells (S4 Table). To test the possibility that other cohesin subunits interact with Hhp, we analyzed Rec11-TAP precipitates, in which we found known cohesin subunits and Hhp2, suggesting that cohesin and Hhp physically interact. Interestingly, we did not detect interaction between Hhp and cohesin in extracts from mitotically growing cells, suggesting that this interaction is stronger during meiosis or is meiosis-specific (S4 Table).
To test directly for phosphorylation of Rec11, we determined the mobility of Rec11-TAP by gel electrophoresis (Fig 2). The mobility of Rec11 from wild-type cells was considerably increased by phosphatase treatment (Fig 2A, lanes 1 and 2 vs. lanes 3 and 4), indicating that Rec11 indeed was phosphorylated during meiosis. In the presence of analog the mobility of Rec11 from the hhp1-as hhp2Δ mutant induced into meiosis was greater than that from wild-type cells (lane 5 vs. lane 1); its mobility was further increased by phosphatase treatment (lanes 7 and 8 vs. lanes 5 and 6). As expected, the mobility of Rec11 from wild type (hhp+) was the same with or without analog (lane 1 vs. lane 2), demonstrating that the increased mobility of Rec11 from the mutant was due to inhibition of Hhp and not to an off-target effect. In the absence of analog the mobility of Rec11 from the hhp1 mutant was also greater than that from wild type (Fig 2B, lanes 1 and 4 vs. lane 2) and was further increased by adding analog (Fig 2B, lane 2 vs. lane 3), as also evident in Fig 2A (lane 5 vs. lane 6).
Fig. 2. Rec11 is phosphorylated during meiosis, at least partially dependent on Hhp. 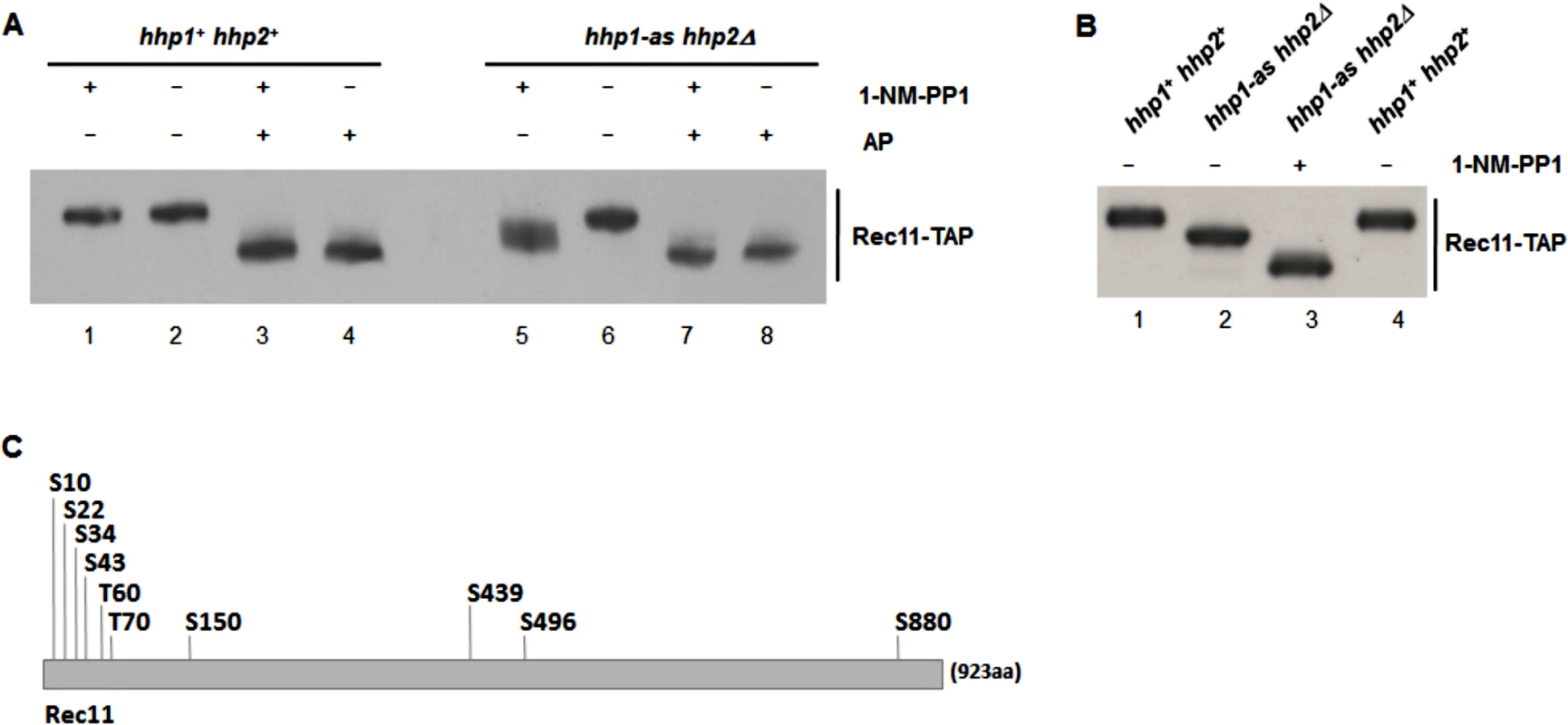
(A and B) pat1-114 rec11-TAP cells (hhp1+ hhp2+ or hhp1-as hhp2Δ, as indicated) were induced for meiosis in medium containing (+) or lacking (–) 30 μM 1-NM-PP1, an ATP analog. Extracted proteins were treated with alkaline phosphatase (AP) (+) or not (–) and separated by gel electrophoresis. Rec11-TAP protein was detected by western blotting using peroxidase anti-peroxidase antibody. (C) Positions of phosphorylation on Rec11-TAP isolated from meiotically induced pat1-114 cells were determined by mass spectrometry; S, serine; T, threonine. Their codons were mutated to create rec11-10A (encoding alanine at each position) and rec11-10D (encoding aspartate at each position). These results indicate that Rec11 phosphorylation in the hhp1-as hhp2Δ mutant is reduced even in the absence of the inhibitor, consistent with the hhp1-as hhp2Δ mutant having a recombination-deficient phenotype in the absence of inhibitor (Table 2). They also indicate that Rec11 phosphorylation is reduced even more, but not completely, in the presence of analog. This outcome is consistent with viable spore yield being somewhat reduced in the absence of analog but reduced much more in the presence of analog (Table 2). Residual Rec11 phosphorylation in the presence of analog (Fig 2A, lane 5 vs. lane 7) may depend on residual Hhp1-as function or on another protein kinase.
Rec11 phosphorylation-site mutants are recombination - and DSB-deficient but segregation-proficient
To determine the nature of Rec11 phosphorylation during meiosis, we analyzed the immunoprecipitates of Rec11-TAP for phosphopeptides via mass spectrometry in two independent experiments. We found that Rec11 is phosphorylated on eight serine (S10, S22, S34, S43, S150, S439, S496 and S880) and two threonine (T60 and T70) residues during meiosis (Fig 2C).
To test the potential functional significance of Rec11 phosphorylation, we generated rec11 mutants encoding alanine at these ten phosphorylation sites (allele rec11-10A) or the phosphomimetic aspartate at those positions (rec11-10D) (Fig 2C). The recombination-proficiency of the rec11-10A mutant was lower than that of wild type by factors of ~5–10, whereas that of the rec11-10D mutant was near that of wild type (Tables 3). These data indicate that phosphorylation of Rec11 at one or more of these sites is important for recombination. [Since in recombination assays the phosphomimetic aspartate is less deleterious than alanine (Table 3), we presume that the deficiency in the rec11-10A mutant results from reduced phosphorylation, although other structural deficiencies of Rec11 cannot be excluded. The abundance of both Rec11 mutant proteins during meiosis was similar to that of wild-type Rec11 (S4 Fig), indicating that the mutations do not significantly alter the stability of Rec11.] Because the recombination-deficiency of the rec11-10A mutants was not as great as that of the rec11Δ null mutant or of the hhp1-as hhp2Δ mutant in the absence of analog, Rec11 presumably has additional phosphorylation sites important for its promotion of recombination; these may be the residual sites phosphorylated in hhp1-as hhp2Δ (Fig 2). Alternatively, Rec11 may have phosphorylation-independent functions important for recombination, still present in the rec11-10A mutants but lacking in rec11Δ.
Tab. 3. Recombination defects in hhp1-as hhp2∆ and rec11 phosphorylation-site mutants resemble those in rec8∆ and rec11∆ mutants, and phosphomimetic rec11 mutation partially suppresses hhp1-as hhp2∆. 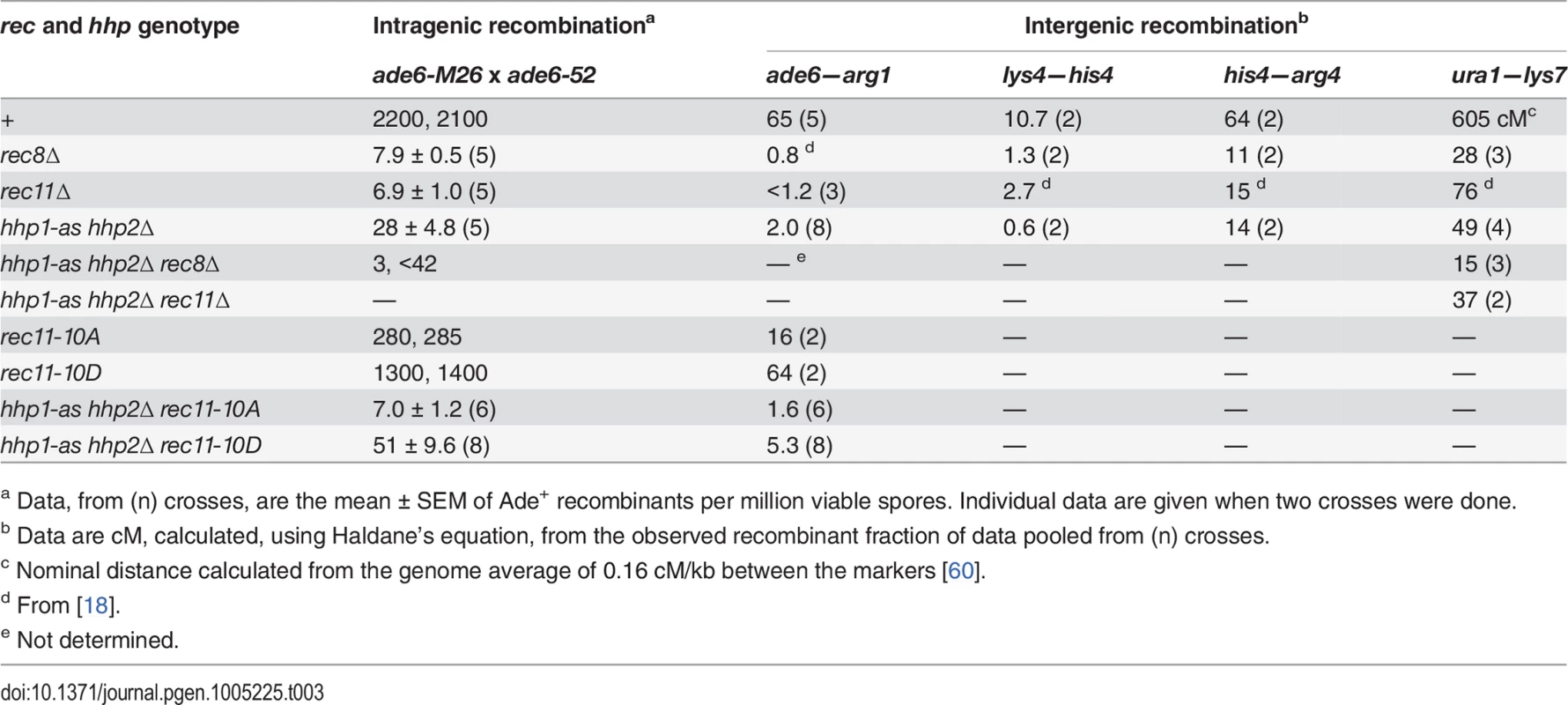
a Data, from (n) crosses, are the mean ± SEM of Ade+ recombinants per million viable spores. Individual data are given when two crosses were done. If phosphorylation of Rec11 depends on Hhp and is important for recombination, the rec11 phosphomimetic mutation, rec11-10D, might suppress the loss of Hhp function in the hhp1-as hhp2Δ mutant. Indeed, rec11-10D partially suppressed hhp1-as hhp2Δ for both gene conversion (ade6 intragenic recombination) and crossing-over (ade6—arg1 intergenic recombination). The rec11-10D mutation slightly raised the Ade+ recombinant frequency in the hhp1-as hhp2Δ mutant from 28 to 51 (per million viable spores; p = 0.05 by one-tailed t-test) and the ade6—arg1 crossover distance from 2.0 to 5.3 (cM; p < 0.001 by Fisher’s exact test) (Table 3). Suppression may be only partial owing to sites of phosphorylation other than those mutated in the rec11-10D mutant or to the phosphomimetic mutations being only partially effective, as is frequently observed [38,39].
As expected from the greater recombination-proficiency of rec11-10D than of rec11-10A, DSBs were more abundant in rec11-10D than in rec11-10A (Fig 1, middle two pairs of lanes in each panel). Only at one DSB hotspot, denoted “c” on NotI fragment C (Fig 1B, middle panel), were DSBs close to wild-type levels in rec11-10A; DSBs at this hotspot were also most abundant in hhp1-as hhp2Δ, rec8Δ, and rec11Δ (Fig 1) [14,18], showing that DSB formation at this hotspot is largely independent of these factors. At other hotspots, DSBs were slightly reduced in rec11-10D relative to wild type, but they were reduced more in rec11-10A though in most cases not to the level in hhp1-as hhp2Δ, rec8Δ, or rec11Δ (as noted above, DSB levels are indistinguishable in rec8Δ and rec11Δ [14,18]). These data are in accord with the recombination data in Table 3 —the rec11-10A mutation reduces both DSB formation and recombination more than the rec11-10D mutation but not as much as the hhp1-as hhp2Δ, rec8Δ, and rec11Δ mutations.
Because Hhp is required for cohesin removal and proper meiotic divisions [12,40], we tested whether Rec11 phosphorylation is required for these steps of meiosis. Although Rec8 phosphorylation is essential for its cleavage and removal and thus for segregation of chromosomes during meiosis I [12,40], meiotic divisions occurred normally in Rec11 phosphorylation-site mutants 10A and 10D, and no defect in Rec8 removal at the onset of anaphase I was observed (S5 Fig). Mutating one of the two separase cleavage sites on Rec8 (Rec8-RD1) leads to only a minor defect in chromosome segregation during meiosis [41]. We did not observe any further impairment of meiotic chromosome segregation when we analyzed cells expressing both Rec11-10A and Rec8-RD1 (S6 Fig). These results suggest that the Rec11 phosphorylation sites identified in our study are required for meiotic recombination but not for segregation of chromosomes during meiotic divisions.
Hhp promotes chromosomal loading of LinE proteins Rec10 and Rec27
Rec8 and Rec11 appear to act before the LinE proteins, because the formation of LinEs or nuclear LinE protein foci largely depends on Rec8 and Rec11, but Rec8 focus-formation does not depend on LinE proteins [22]; Rec11 focus-formation has not, to our knowledge, been similarly tested. We therefore tested the dependence on Hhp of focus-formation by LinE proteins, using fluorescence microscopy of Rec10-GFP and Rec27-GFP, both of which are functional [14,19,20]. The number of foci formed by each protein and the fraction of cells with visible foci were significantly reduced in hhp1-as hhp2Δ in the absence of analog (p < 0.01 by Fisher’s exact test) as well as in rec11-10A (p < 0.01) (Fig 3 and S7 Fig). Comparison of either time point for wt (3 and 3.5 hr) with either time point for hhp1-as hhp2∆ (4 and 4.5 hr) or for rec11-10A (3 and 3.5 hr) shows that the mutants have fewer foci and cells with foci. [We used later time points for the hhp1-as hhp2∆ mutant because meiosis is delayed in the mutant (S8 Fig).] The residual levels were similar to those reported in rec8Δ and rec11Δ mutants, which behave similarly in other recombination-related assays [19,20]. In contrast, Rec11-GFP appeared to localize to the nucleus to nearly the same extent in wild type and in hhp1-as hhp2Δ (S9 Fig) but not in rec8∆ (S10 Fig) as noted previously [42]. Rec11-GFP did not consistently form distinct foci, making quantification difficult. Nevertheless, the frequency of nuclei with Rec11-GFP fluorescence appeared to be similar in wild type and in hhp1-as hhp2Δ. These results show that the loading of LinE proteins depends on Hhp and likely on the phosphorylation of Rec11, which itself depends on Hhp (Fig 2).
Fig. 3. hhp and rec11-10A mutations reduce the number and intensity of foci of linear element proteins. 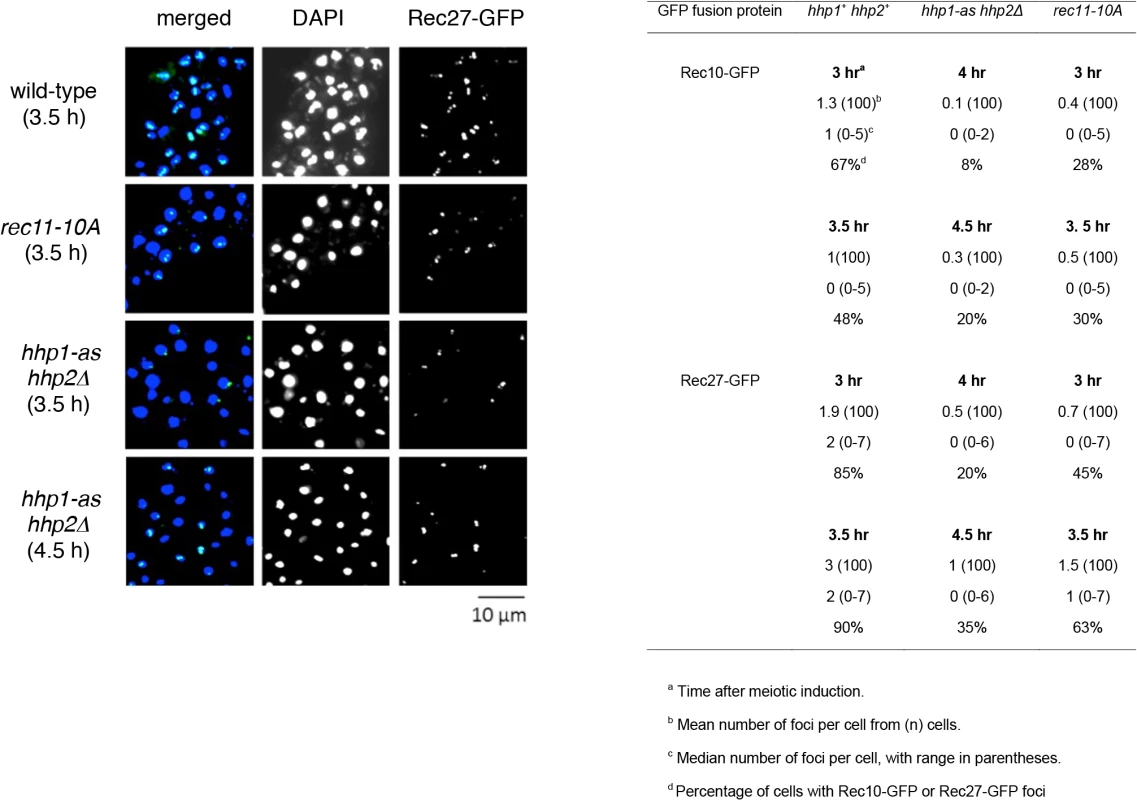
Strains with the indicated mutations were induced for meiosis in the absence of an ATP analog. At the indicated times, cells were fixed, stained with DAPI, and examined by fluorescence microscopy for foci of the indicated GFP fusion protein (green) and DNA (blue). Representative cells with Rec27-GFP are shown, and quantification is given in the table below. Data for the hhp1-as hhp2Δ mutant were taken 1 hr later than for hhp+ because replication is delayed by about 1 hr in the hhp1-as hhp2Δ mutant (S8 Fig). See also S6 Fig. Discussion
Our results reveal that casein kinase 1 homologs in fission yeast, Hhp1 and Hhp2 (Hhp), have, in addition to their known substrate Rec8 [12,40], a second substrate that must be phosphorylated by Hhp during meiosis to promote DSB formation and recombination. We infer that the second substrate is Rec11, since inactivating Rec11 phosphorylation sites (by Ser or Thr → Ala changes) reduced recombination more in wild type cells than in Hhp-deficient cells (Table 3). Furthermore, phosphomimetic alterations (Ser or Thr → Asp) in Rec11 left the cells recombination-proficient, though not quite to the wild-type level, and partially suppressed the recombination-deficiency of Hhp-deficient cells (Table 3). Rec8 mutants lacking Hhp-dependent phosphorylation sites are deficient for cohesin cleavage [12,40] but are recombination-proficient (S3 Table). Conversely, Rec11 mutants lacking phosphorylation sites are deficient for recombination and DSB formation (Table 3; Fig 1 and S1 Fig) but are segregation-proficient (S5 Fig and S6 Fig). Therefore, our data combined with the cited published data show that Hhp phosphorylates Rec8 to regulate cohesin cleavage for proper chromosome segregation and mediates phosphorylation of Rec11 to activate DSB formation for recombination. A recent independent report drew the same conclusion [43]. These two actions of Hhp are separable, since lack of Rec8 phosphorylation leaves recombination (Rec11 action) intact (S3 Table) and reduction of Rec11 phosphorylation leaves chromosome segregation (Rec8 action) intact (S5 Fig and S6 Fig). Below, we discuss the implications of these findings for the mechanism of meiotic recombination and chromosome segregation, and their co-ordination. The conservation of Rec8 and Rec11 in most species suggests that these two separate roles of the cohesin subunits regulate meiotic chromosome dynamics in widely divergent species, including humans.
Separable functions of Hhp during meiosis
We were surprised that the hhp1-as (M84G) ATP-analog-sensitive mutant had a dramatic, differential phenotype even in the absence of added analog (Tables 2 and 3; Figs 1, 2, and 3)—it strongly reduced recombination but had much less effect on viable spore yield or chromosome segregation (Tables 2 and 3). We infer that this mutation differentially alters the substrate specificity or activity of Hhp1 such that in the absence of analog the mutant Hhp1 adequately phosphorylates Rec8 but not Rec11 (at least not completely) (Tables 2 and 3; Figs 1, 2, and 3). This fortuitous result greatly aided our experiments because hhp1Δ hhp2Δ mutants grow poorly and have very low viable spore yields [26–28], whereas the hhp1-as mutants grow like wild type and have quite high viable spore yield in the absence of analog (Table 2). There are precedents for such differential inactivation of protein kinases by ATP-binding site mutations. For example, mutation of the “gatekeeper” residue in the ATP-binding site can reduce kinase activity even without analog present [29]. In addition, differential regulation of cellular events can arise from differential threshold levels for kinase activity [44,45].
During meiosis, Hhp plays major roles both in the timely removal of cohesin from chromosome arms at the onset of anaphase I, via phosphorylation of Rec8, and in DSB formation and recombination, via phosphorylation of Rec11. Since both of these processes are meiosis-specific, it is not surprising that we found physical interaction between Hhp and cohesin only in meiotic cells (S4 Table and S3 Fig); this association may aid the coordination of Rec8 cleavage and recombination. The abundance of hhp1 and hhp2 transcripts is greatly increased during meiosis [46], a feature consistent with Hhp playing especially important roles during meiosis. Hhp directly phosphorylates Rec8 [12,23], and it may directly phosphorylate Rec11 or activate another protein kinase that does; our data do not distinguish these possibilities, but it was recently reported that Hhp1 and Hhp2 can directly phosphorylate Rec11 [43]. Hhp clearly regulates separately two events essential for the formation of viable gametes and species propagation.
A role for Hhp in the regional specificity of meiotic recombination
We found that the Hhp mutants (hhp2Δ coupled with hhp1-as or hhp1Δ) reduced recombination more in some intervals than in others (Tables 1, 2, and 3, and S3 Table), much like the region-dependent reductions by rec8 and rec11 mutations, including deletions [18,25]. Furthermore, hhp1-as abolished meiotic DSBs at some hotspots but not at others (Fig 1 and S1 Fig). The residual DSB patterns are reminiscent of those of cohesin and certain LinE mutants [14,18]. These observations lead us to propose that Rec11 phosphorylation is required for the loading of the putative Rec25-Rec27-Mug20 complex and Rec10 at DSB hotspots [14]. This proposal is consistent with the reduction, but not elimination, of Rec27-GFP foci in the hhp1-as hhp2Δ mutant (Fig 3 and S7 Fig). Rec11 phosphorylation seems not to be required for Rec11 to localize to the nucleus and possibly to load onto chromosomes, since Rec11-GFP nuclear localization was similar in wild type and in the hhp1-as hhp2Δ mutant (S9 Fig).
Our results, coupled with previous data [14], suggest the following scheme for formation of meiotic DSBs and crossovers (Fig 4). Rec8 is loaded onto chromosomes during S phase. Rec11 is concurrently or subsequently loaded in a Rec8-dependent manner (S10 Fig) [42] and then phosphorylated by Hhp, which allows the preferential loading of Rec25-Rec27-Mug20 at DSB hotspots. Rec10 is loaded at low levels independently of any of these proteins and, in their absence, activates the Rec12 complex to form DSBs at low level across the genome and at a few DSB hotspots. The Rec25-Rec27-Mug20 complex, whose loading at high levels at DSB hotspots depends on Rec8 and phosphorylated Rec11, along with Rec10 strongly activates the Rec12 complex to form DSBs at the hundreds of DSB hotspots dependent upon these proteins [14]. In this view, the main role of Hhp is to promote high level DSB formation at hotspots, which collectively account for about 70% of all DSBs and about half of all crossovers across the genome [47].
Fig. 4. Scheme for dual action of casein kinase 1 (Hhp) on cohesin subunits to regulate meiotic chromosome segregation via Rec8 phosphorylation and to promote linear element formation, meiotic DSB formation, and recombination via Rec11 phosphorylation. 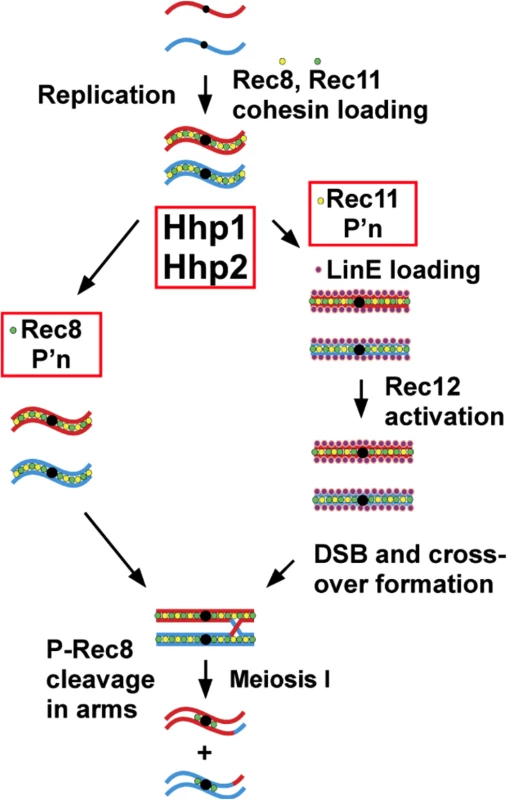
Cohesin subunits Rec8 and Rec11 are loaded onto chromosomes prior to premeiotic replication, during which sister chromatid cohesion is established. Before, during, or after loading they are phosphorylated by casein kinase homologs Hhp1 and Hhp2. Right: Phosphorylation (P’n) of Rec11 leads to loading of the linear element (LinE) proteins Rec10, Rec25, Rec27, and Mug20, which in turn activate Rec12 (Spo11 homolog) and its partner proteins for DSB formation; DSBs lead to crossovers, which allow segregation of homologous centromeres, not sister centromeres, at the first meiotic division (MI). Left: Phosphorylation of Rec8 (generating P-Rec8) allows its cleavage, first in the arms, which allows segregation of homologous centromeres at MI; later, cleavage of Rec8 in the centromeres allows segregation of sister centromeres at MII. Rec8 is also needed for loading of Rec11 (S10 Fig) [42] and is thus indirectly required for DSB formation and recombination [14,18]. The precise timing of Rec8 and Rec11 phosphorylation is unknown. Rec8 and Rec11 (STAG3 functional homolog) and their phosphorylation may play similar roles in mammalian gametogenesis (see Discussion). Conservation of meiosis-specific cohesin subunits and their roles
The proteins discussed here are widely conserved across all eukaryotic phyla, including humans. To our knowledge, casein kinase homologs are present, often as multiple paralogs, in all eukaryotes examined, and all except apparently the protist Tetrahymena thermophila have meiosis-specific Rec8 cohesin subunits [7]. In only rare cases, such as the budding yeast Saccharomyces cerevisiae and T. thermophila, is there no identified meiosis-specific homolog of the Rec11 cohesin subunit [6,7,48,49]. Vertebrates harbor three Rec11-like STAG (stromal antigen) proteins, STAG1, STAG2, and STAG3. STAG3 is meiosis-specific, is required for meiotic sister chromatid cohesion and chromosome axis formation, and is closely related to the Rec11 protein studied here [50–52]. Murine STAG3 is phosphorylated during meiosis, and this modification appears to be required for meiotic progression [53]. STAG3-deficient mice, both male and female, are sterile and display severe meiosis I defects [54,55]. A STAG3 frameshift mutation is apparently the cause of premature ovarian failure in humans [56]. Thus, our observations on Rec11 phosphorylation and its role in meiotic chromosome behavior are likely to pertain to meiosis and fertility in most species, including humans.
Materials and Methods
S. pombe strains, mutant constructions, and genetic methods
Strains were constructed by standard meiotic crosses [57]; genotypes of strains and the figures and tables in which each was used are in S6 Table. Mutations were introduced into cloned genes using the QuikChangeII kit (Agilent Technologies), which were inserted into the genome by transformation to antibiotic-resistance [28]. Transformants were confirmed by PCR-based analysis and, in some cases, by nucleotide sequencing.
Meiotic crosses and analysis of random spore colonies were conducted as described [57]. The ATP analog 1-NM-PP1 (Toronto Research Chemicals) was added to the sporulation agar (SPA) at 50 μM; required amino acid, purine, and pyrimidine supplements were added at 100 μg/ml. Ade+ recombinant frequencies were determined by differential plating on yeast extract agar (YEA) with and without guanine (200 μg/ml). To determine intergenic recombinant frequencies, spore suspensions were plated on YEA; colonies were transferred with toothpicks to YEA supplemented with adenine (100 μg/ml), incubated overnight, and replicated to appropriate media to determine phenotypes. Recombinant frequencies were converted to genetic distance (cM) using Haldane’s equation [x = -½ ln(1-2R), where x is the distance in Morgans (M) and R is the recombinant frequency].
To prepare large meiotic cultures, cells were grown to log phase at 25° C in supplemented liquid minimal medium (EMM2), washed in H2O, resuspended in supplemented EMM2 without a nitrogen source, and incubated for 18–19 hr, at which time NH4Cl was added to 5 mg/ml and the temperature raised to 34° C to inactivate the Pat1-114 temperature-sensitive protein kinase [58]. Cells were harvested at appropriate times after induction and analyzed for DNA content by flow cytometry (to determine the time of replication) and for other features as described below.
DNA and protein analysis
To determine DSBs, meiotic cells were washed, concentrated by centrifugation, and embedded in agarose plugs, which were sequentially treated with lytic enzymes, proteinase K, RNase, and appropriate restriction enzymes [58]. The digested DNA was separated by gel electrophoresis and analyzed by Southern blot hybridization.
To analyze phosphorylation and electrophoretic mobility of Rec11, cells expressing Rec11-TAP were arrested in G1 by nitrogen starvation, and meiosis was induced by shifting the culture to 34°C. Four hr later, cells from 20 mL of culture were concentrated by centrifugation, suspended in IPP150 buffer (50 mM Tris-HCl pH 8.0, 150 mM NaCl, 10% glycerol, 0.1% NP-40), and homogenized using glass beads (0.4–0.6 mm diameter). Extracted proteins were immunoprecipitated with IgG Sepharose 6 Fast Flow beads (GE Healthcare), treated with alkaline phosphatase (Thermo Scientific) as indicated and separated by electrophoresis through 5% polyacrylamide gels containing SDS (0.1%). Proteins were transferred to a PVDF membrane (Millipore), and Rec11-TAP and Hhp2-TAP were detected using rabbit antiperoxidase antibody linked to peroxidase (Dako; 1 : 30,000 dilution) in 0.1% PBS-T (8 gm NaCl, 0.2 gm KCl, 1.44 gm Na2HPO4, 0.24 gm KH2PO4, 1 mL Tween-20 per L). Hhp1-PK9 was detected using mouse-anti-PK (V5) antibody (Serotec; 1 : 2000 dilution) and goat anti-mouse IgG-HRP secondary antibody (Santa Cruz Biotechnology; 1 : 5000 dilution) in 0.1% PBS-T.
Affinity purification of proteins
Cells from six-liter (mitotic) or fifteen-liter (meiotic) cultures of strains expressing TAP-tagged proteins were collected by centrifugation; meiotic cultures were harvested at 2.5–3.5 hr after induction of meiosis. Yeast cell powder was made from frozen pellets using a SamplePrep 6870 Freezer Mill (SPEX, Inc.). Proteins were extracted using IPP150 buffer containing complete protease and phosphatase inhibitors (Roche) and 1 mM PMSF (Sigma). All washing steps were performed in Poly-Prep columns (Bio-Rad) by gravity flow. IgG Sepharose™ 6 Fast Flow beads (500 μl; GE Healthcare) were washed with IPP150 buffer, mixed with protein extract, and rotated for 2 hr at 4°C. Beads were washed with IPP150 buffer and then with TEV cleavage buffer (TCB: 10 mM Tris-HCl pH 8.0, 150 mM NaCl, 10% glycerol, 0.1% NP-40, 0.5 mM EDTA, 1 mM DTT). Protein cleavage was performed in 2 ml of TCB buffer supplemented with 400 units of AcTEV protease (Life Technologies) for 2 hr at 16°C. The eluate (2 ml) was supplemented with 6 μl of 1 M CaCl2 and mixed with 6 ml of calmodulin binding buffer 1 (CBB1 : 10 mM Tris-HCl pH 8.0, 150 mM NaCl, 10% glycerol, 0.1% NP-40, 1 mM imidazole, 1 mM Mg(OAc)2, 2 mM CaCl2, 10 mM β-mercaptoethanol). Calmodulin Sepharose 4B beads (150 μl; GE Healthcare) were washed with CBB1 buffer, added to a mixture of eluate and CBB1 buffer, and incubated for 2 hr at 4°C. The beads were washed with CBB1 and calmodulin binding buffer 2 (CBB2 : 10 mM Tris-HCl pH 8.0, 150 mM NaCl, 1 mM Mg(OAc)2, 2 mM CaCl2, 1 mM β-mercaptoethanol). The proteins were step-eluted using one bed volume of elution buffer (EB: 10 mM Tris-HCl pH 8.0, 150 mM NaCl, 1 mM Mg(OAc)2, 2 mM EGTA, 1 mM β-mercaptoethanol). Eluted proteins were separated by SDS-PAGE and silver stained. Eluates from peak fractions were analyzed by LC-MS/MS as described in S1 Text and by Cipak et al. [59].
Fluorescence microscopy
Haploid pat1-114 cells were arrested by nitrogen starvation for 16 hr and released into meiosis at 34°C by inactivation of Pat1 and addition of nitrogen. Cells were harvested at the indicated time points after meiotic induction, fixed with 70% ethanol (Fig 3 and S7 Fig) or with 99.8% methanol (S9 Fig and S10 Fig), and stained with DAPI; nuclei were counted in 100 cells at each time point. The Rec10-GFP protein was visualized in unfixed cells (S7 Fig) as follows. Cells from 100 μl of culture were collected by centrifugation, washed once with water, and spread on a cover slip coated with poly-L-lysine. Slides with a drop of mounting medium containing DAPI were covered with the cover slips, and the cells were analyzed the next day. All images were from a single focal plane; out-of-focus cells were not scored. Images for Fig 3 and S7 Fig were obtained with a Zeiss Axio Imager.Z2 microscope equipped with a Plan Apochromat 63x/1.4 oil-immersion lens and an AxioCamMRm camera; images were analysed with ZEN 2011 software. Images for S9 Fig and S10 Fig were obtained with a Zeiss Apotome microscope equipped with a 100X /1.4 oil-immersion lens and were analyzed using Axiovision software. All inductions and localization analyses were performed at least twice. Haploid strains were analysed because diploid hhp1-as hhp2∆ cells grow poorly even without analog and produce grossly abnormal asci.
Supporting Information
Zdroje
1. Phadnis N, Hyppa RW, Smith GR (2011) New and old ways to control meiotic recombination. Trends Genet 27 : 411–421. doi: 10.1016/j.tig.2011.06.007 21782271
2. Li YF, Numata M, Wahls WP, Smith GR (1997) Region-specific meiotic recombination in S. pombe: the rec11 gene. Molecular Microbiology 23 : 869–878. 9076725
3. Lin Y, Larson KL, Dorer R, Smith GR (1992) Meiotically induced rec7 and rec8 genes from Schizosaccharomyces pombe. Genetics 132 : 75–85. 1339382
4. Nasmyth K, Haering CH (2005) The structure and function of SMC and kleisin complexes. Annu Rev Biochem 74 : 595–648. 15952899
5. Kitajima TS, Yokobayashi S, Yamamoto M, Watanabe Y (2003) Distinct cohesin complexes organize meiotic chromosome domains. Science 300 : 1152–1155. 12750522
6. Klein F, Mahr P, Galova M, Buonomo SBC, Michaelis C, et al. (1999) A central role for cohesins in sister chromatid cohesion, formation of axial elements, and recombination during yeast meiosis. Cell 98 : 91–103. 10412984
7. Howard-Till RA, Lukaszewicz A, Novatchkova M, Loidl J (2013) A single cohesin complex performs mitotic and meiotic functions in the protist tetrahymena. PLoS Genet 9: e1003418. doi: 10.1371/journal.pgen.1003418 23555314
8. Uhlmann F, Nasmyth K (1998) Cohesion between sister chromatids must be established during DNA replication. Curr Biol 8 : 1095–1101. 9778527
9. Gruber S, Haering CH, Nasmyth K (2003) Chromosomal cohesin forms a ring. Cell 112 : 765–777. 12654244
10. Buonomo SB, Clyne RK, Fuchs J, Loidl J, Uhlmann F, et al. (2000) Disjunction of homologous chromosomes in meiosis I depends on proteolytic cleavage of the meiotic cohesin Rec8 by separin. Cell 103 : 387–398. 11081626
11. Brar GA, Kiburz BM, Zhang Y, Kim JE, White F, et al. (2006) Rec8 phosphorylation and recombination promote the step-wise loss of cohesins in meiosis. Nature 441 : 532–536. 16672979
12. Ishiguro T, Tanaka K, Sakuno T, Watanabe Y (2010) Shugoshin-PP2A counteracts casein-kinase-1-dependent cleavage of Rec8 by separase. Nat Cell Biol 12 : 500–506. doi: 10.1038/ncb2052 20383139
13. Watanabe Y, Nurse P (1999) Cohesin Rec8 is required for reductional chromosome segregation at meiosis. Nature 400 : 461–464. 10440376
14. Fowler KR, Gutiérrez-Velasco S, Martín-Castellanos C, Smith GR (2013) Protein determinants of meiotic DNA break hotspots. Molecular Cell 49 : 983–996. doi: 10.1016/j.molcel.2013.01.008 23395004
15. Ponticelli AS, Smith GR (1989) Meiotic recombination-deficient mutants of Schizosaccharomyces pombe. Genetics 123 : 45–54. 2806887
16. Keeney S, Giroux CN, Kleckner N (1997) Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell 88 : 375–384. 9039264
17. Cromie GA, Smith GR (2008) Meiotic recombination in Schizosaccharomyces pombe: A paradigm for genetic and molecular analysis. In: Egel R, Lankenau D-H, editors. Recombination and meiosis: Models, means, and evolution. Berlin: Springer-Verlag. pp. 195–230.
18. Ellermeier C, Smith GR (2005) Cohesins are required for meiotic DNA breakage and recombination in Schizosaccharomyces pombe. Proceedings of the National Academy of Sciences of the United States of America 102 : 10952–10957. 16043696
19. Davis L, Rozalén AE, Moreno S, Smith GR, Martin-Castellanos C (2008) Rec25 and Rec27, novel components of meiotic linear elements, link cohesin to DNA breakage and recombination in fission yeast. Current Biology 18 : 849–854. doi: 10.1016/j.cub.2008.05.025 18514516
20. Lorenz A, Wells JL, Pryce DW, Novatchkova FE, Eisenhaber F, et al. (2004) S. pombe meiotic linear elements contain proteins related to synaptonemal complex components. J Cell Sci 117 : 3343–3351. 15226405
21. Estreicher A, Lorenz A, Loidl J (2012) Mug20, a novel protein associated with linear elements in fission yeast meiosis. Current Genetics 58 : 119–127. doi: 10.1007/s00294-012-0369-3 22362333
22. Molnar M, Doll E, Yamamoto A, Hiraoka Y, Kohli J (2003) Linear element formation and their role in meiotic sister chromatid cohesion and chromosome pairing. J Cell Sci 116 : 1719–1731. 12665553
23. Rumpf C, Cipak L, Dudas A, Benko Z, Pozgajova M, et al. (2010) Casein kinase 1 is required for efficient removal of Rec8 during meiosis I. Cell Cycle 9 : 2657–2662. doi: 10.4161/cc.9.13.12146 20581463
24. Krawchuk MD, DeVeaux LC, Wahls WP (1999) Meiotic chromosome dynamics dependent upon the rec8+, rec10+ and rec11+ genes of the fission yeast Schizosaccharomyces pombe. Genetics 153 : 57–68. 10471700
25. DeVeaux LC, Smith GR (1994) Region-specific activators of meiotic recombination in Schizosaccharomyces pombe. Genes Development 8 : 203–210. 8299939
26. Dhillon N, Hoekstra MF (1994) Characterization of two protein kinases from Schizosaccharomyces pombe involved in the regulation of DNA repair. EMBO Journal 13 : 2777–2788. 8026462
27. Kearney PH, Ebert M, Kuret J (1994) Molecular cloning and sequence analysis of two novel fission yeast casein kinase-1 isoforms. Biochemical and Biophysical Research Communications 203 : 231–236. 8074660
28. Gregan J, Zhang C, Rumpf C, Cipak L, Li Z, et al. (2007) Construction of conditional analog-sensitive kinase alleles in the fission yeast Schizosaccharomyces pombe. Nat Protoc 2 : 2996–3000. 18007635
29. Bishop AC, Ubersax JA, Petsch DT, Matheos DP, Gray NS, et al. (2000) A chemical switch for inhibitor-sensitive alleles of any protein kinase. Nature 407 : 395–401. 11014197
30. Davis L, Smith GR (2005) Dynein promotes achiasmate segregation in Schizosaccharomyces pombe. Genetics 170 : 581–590. 15802518
31. Davis L, Smith GR (2003) Non-random homolog segregation at meiosis I in Schizosaccharomyces pombe mutants lacking recombination. Genetics 163 : 857–874. 12663528
32. Rumpf C, Cipak L, Dudas A, Benko Z, Pozgajova M, et al. (2010) Casein kinase 1 is required for efficient removal of Rec8 during meiosis I. Cell Cycle 9 : 2657–2662. doi: 10.4161/cc.9.13.12146 20581463
33. Farah JA, Cromie GA, Smith GR (2009) Ctp1 and Exonuclease 1, alternative nucleases regulated by the MRN complex, are required for efficient meiotic DNA repair and recombination. Proceedings of the National Academy of Sciences of the United States of America 106 : 9356–9361. doi: 10.1073/pnas.0902793106 19470480
34. Cromie GA, Hyppa RW, Cam HP, Farah JA, Grewal SI, et al. (2007) A discrete class of intergenic DNA dictates meiotic DNA break hotspots in fission yeast. PLoS Genet 3: e141. 17722984
35. Young JA, Schreckhise RW, Steiner WW, Smith GR (2002) Meiotic recombination remote from prominent DNA break sites in S. pombe. Mol Cell 9 : 253–263. 11864600
36. Brar GA, Hochwagen A, Ee LS, Amon A (2009) The multiple roles of cohesin in meiotic chromosome morphogenesis and pairing. Mol Biol Cell 20 : 1030–1047. doi: 10.1091/mbc.E08-06-0637 19073884
37. Johnson AE, Chen JS, Gould KL (2013) CK1 is required for a mitotic checkpoint that delays cytokinesis. Current biology 23 : 1920–1926. doi: 10.1016/j.cub.2013.07.077 24055157
38. Niu H, Wan L, Busygina V, Kwon Y, Allen JA, et al. (2009) Regulation of meiotic recombination via Mek1-mediated Rad54 phosphorylation. Mol Cell 36 : 393–404. doi: 10.1016/j.molcel.2009.09.029 19917248
39. Tsukahara T, Tanno Y, Watanabe Y (2010) Phosphorylation of the CPC by Cdk1 promotes chromosome bi-orientation. Nature 467 : 719–723. doi: 10.1038/nature09390 20739936
40. Rumpf C, Cipak L, Dudas A, Benko Z, Pozgajova M, et al. (2010) Casein kinase 1 is required for efficient removal of Rec8 during meiosis I. Cell Cycle 9 2657–62. doi: 10.4161/cc.9.13.12146 20581463.
41. Kitajima TS, Miyazaki Y, Yamamoto M, Watanabe Y (2003) Rec8 cleavage by separase is required for meiotic nuclear divisions in fission yeast. Embo J 22 : 5643–5653. 14532136
42. Ding DQ, Sakurai N, Katou Y, Itoh T, Shirahige K, et al. (2006) Meiotic cohesins modulate chromosome compaction during meiotic prophase in fission yeast. J Cell Biol 174 : 499–508. 16893973
43. Sakuno T, Watanabe Y (2015) Phosphorylation of cohesin Rec11/SA3 by casein kinase 1 promotes homologous recombination by assembling the meiotic chromosome axis. Dev Cell 32 : 220–230. doi: 10.1016/j.devcel.2014.11.033 25579976
44. Stern B, Nurse P (1996) A quantitative model for the cdc2 control of S phase and mitosis in fission yeast. Trends Genet 12 : 345–350. 8855663
45. Henderson KA, Kee K, Maleki S, Santini PA, Keeney S (2006) Cyclin-dependent kinase directly regulates initiation of meiotic recombination. Cell 125 : 1321–1332. 16814718
46. Mata J, Lyne R, Burns G, Bahler J (2002) The transcriptional program of meiosis and sporulation in fission yeast. Nat Genet 32 : 143–147. 12161753
47. Fowler KR, Sasaki M, Milman N, Keeney S, Smith GR (2014) Evolutionarily diverse determinants of meiotic DNA break and recombination landscapes across the genome. Genome Research 24 1650–1664. doi: 10.1101/gr.172122.114 25024163
48. Wood AJ, Severson AF, Meyer BJ (2010) Condensin and cohesin complexity: the expanding repertoire of functions. Nat Rev Genet 11 : 391–404. doi: 10.1038/nrg2794 20442714
49. Petronczki M, Siomos MF, Nasmyth K (2003) Un menage a quatre: the molecular biology of chromosome segregation in meiosis. Cell 112 : 423–440. 12600308
50. Winters T, McNicoll F, Jessberger R (2014) Meiotic cohesin STAG3 is required for chromosome axis formation and sister chromatid cohesion. EMBO J 33 : 1256–1270. doi: 10.1002/embj.201387330 24797474
51. Fukuda T, Fukuda N, Agostinho A, Hernandez-Hernandez A, Kouznetsova A, et al. (2014) STAG3-mediated stabilization of REC8 cohesin complexes promotes chromosome synapsis during meiosis. EMBO J 33 : 1243–1255. doi: 10.1002/embj.201387329 24797475
52. Prieto I, Suja JA, Pezzi N, Kremer L, Martinez AC, et al. (2001) Mammalian STAG3 is a cohesin specific to sister chromatid arms in meiosis I. Nature Cell Biology 3 : 761–766. 11483963
53. Fukuda T, Pratto F, Schimenti JC, Turner JM, Camerini-Otero RD, et al. (2012) Phosphorylation of chromosome core components may serve as axis marks for the status of chromosomal events during mammalian meiosis. PLoS Genet 8: e1002485. doi: 10.1371/journal.pgen.1002485 22346761
54. Hopkins J, Hwang G, Jacob J, Sapp N, Bedigian R, et al. (2014) Meiosis-specific cohesin component, Stag3 is essential for maintaining centromere chromatid cohesion, and required for DNA repair and synapsis between homologous chromosomes. PLoS Genet 10: e1004413. doi: 10.1371/journal.pgen.1004413 24992337
55. Llano E, Gomez HL, Garcia-Tunon I, Sanchez-Martin M, Caburet S, et al. (2014) STAG3 is a strong candidate gene for male infertility. Hum Mol Genet 23 : 3421–3431. doi: 10.1093/hmg/ddu051 24608227
56. Caburet S, Arboleda VA, Llano E, Overbeek PA, Barbero JL, et al. (2014) Mutant cohesin in premature ovarian failure. N Engl J Med 370 : 943–949. doi: 10.1056/NEJMoa1309635 24597867
57. Smith GR (2009) Genetic analysis of meiotic recombination in Schizosaccharomyces pombe. In: Keeney S, editor. Meiosis. Totowa, NJ: Humana Press. pp. 65–76.
58. Hyppa RW, Smith GR (2009) Using Schizosaccharomyces pombe meiosis to analyze DNA recombination intermediates. In: Keeney S, editor. Meiosis. Totowa, NJ: Humana Press. pp. 235–252.
59. Cipak L, Spirek M, Novatchkova M, Chen Z, Rumpf C, et al. (2009) An improved strategy for tandem affinity purification-tagging of Schizosaccharomyces pombe genes. Proteomics 9 : 4825–4828. doi: 10.1002/pmic.200800948 19750511
60. Young JA, Schreckhise RW, Steiner WW, Smith GR (2002) Meiotic recombination remote from prominent DNA break sites in S. pombe. Molecular Cell 9 : 253–263. 11864600
61. Steiner WW, Schreckhise RW, Smith GR (2002) Meiotic DNA breaks at the S. pombe recombination hotspot M26. Molecular Cell 9 : 847–855 11983175
62. Grishchuk AL, Kohli J (2003) Five RecA-like proteins of Schizosaccharomyces pombe are involved in meiotic recombination. Genetics 165 : 1031–1043. 14668362
63. Farah JA, Cromie G, Davis L, Steiner WW, Smith GR (2005) Activation of an alternative, Rec12 (Spo11)-independent pathway of fission yeast meiotic recombination in the absence of a DNA flap endonuclease. Genetics 171 : 1499–1511. 16118186
64. Lin Y, Smith GR (1994) Transient meiosis-induced expression of the rec6 and rec12 genes of Schizosaccharomyces pombe. Genetics 136 : 769–779. 8005432
Štítky
Genetika Reprodukční medicína
Článek Minor Type IV Collagen α5 Chain Promotes Cancer Progression through Discoidin Domain Receptor-1Článek A Unique Virulence Gene Occupies a Principal Position in Immune Evasion by the Malaria ParasiteČlánek The Centrosomal Linker and Microtubules Provide Dual Levels of Spatial Coordination of CentrosomesČlánek Dissecting the Function and Assembly of Acentriolar Microtubule Organizing Centers in Cells In VivoČlánek MicroRNA-Dependent Transcriptional Silencing of Transposable Elements in Drosophila Follicle CellsČlánek PARP2 Is the Predominant Poly(ADP-Ribose) Polymerase in Arabidopsis DNA Damage and Immune ResponsesČlánek Cooperative Action of Cdk1/cyclin B and SIRT1 Is Required for Mitotic Repression of rRNA SynthesisČlánek The Developmental Intestinal Regulator ELT-2 Controls p38-Dependent Immune Responses in Adult .Článek Feeding and Fasting Signals Converge on the LKB1-SIK3 Pathway to Regulate Lipid Metabolism in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 5- Růst a vývoj dětí narozených pomocí IVF
- Délka menstruačního cyklu jako marker ženské plodnosti
- Vztah užívání alkoholu a mužské fertility
- Šanci na úspěšný průběh těhotenství snižují nevhodné hladiny progesteronu vznikající při umělém oplodnění
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Genomic Heritability: What Is It?
- Triglyceride-Increasing Alleles Associated with Protection against Type-2 Diabetes
- Epistasis Is a Major Determinant of the Additive Genetic Variance in
- Genetic Regulation of Bone Metabolism in the Chicken: Similarities and Differences to Mammalian Systems
- Minor Type IV Collagen α5 Chain Promotes Cancer Progression through Discoidin Domain Receptor-1
- The Philosophical Approach: An Interview with Ford Doolittle
- Downregulation of the Host Gene by miR-92 Is Essential for Neuroblast Self-Renewal in
- A Unique Virulence Gene Occupies a Principal Position in Immune Evasion by the Malaria Parasite
- Y Fuse? Sex Chromosome Fusions in Fishes and Reptiles
- Regulation of Active DNA Demethylation by a Methyl-CpG-Binding Domain Protein in
- Overlapping Patterns of Rapid Evolution in the Nucleic Acid Sensors cGAS and OAS1 Suggest a Common Mechanism of Pathogen Antagonism and Escape
- Autoselection of Cytoplasmic Yeast Virus Like Elements Encoding Toxin/Antitoxin Systems Involves a Nuclear Barrier for Immunity Gene Expression
- Genetic Architecture of Abdominal Pigmentation in
- Whole Genome DNA Binding Analysis of the Bacterial Replication Initiator and Transcription Factor DnaA
- The Centrosomal Linker and Microtubules Provide Dual Levels of Spatial Coordination of Centrosomes
- Parp3 Negatively Regulates Immunoglobulin Class Switch Recombination
- Burden Analysis of Rare Microdeletions Suggests a Strong Impact of Neurodevelopmental Genes in Genetic Generalised Epilepsies
- Cell Cycle Control by the Master Regulator CtrA in
- Myopathic Lamin Mutations Cause Reductive Stress and Activate the Nrf2/Keap-1 Pathway
- Monoallelic Loss of the Imprinted Gene Promotes Tumor Formation in Irradiated Mice
- Phylum-Level Conservation of Regulatory Information in Nematodes despite Extensive Non-coding Sequence Divergence
- Clustering and Negative Feedback by Endocytosis in Planar Cell Polarity Signaling Is Modulated by Ubiquitinylation of Prickle
- Dissecting the Function and Assembly of Acentriolar Microtubule Organizing Centers in Cells In Vivo
- MicroRNA-Dependent Transcriptional Silencing of Transposable Elements in Drosophila Follicle Cells
- β-Catenin Signaling Biases Multipotent Lingual Epithelial Progenitors to Differentiate and Acquire Specific Taste Cell Fates
- A Simple Auxin Transcriptional Response System Regulates Multiple Morphogenetic Processes in the Liverwort
- Parallel Gene Expression Differences between Low and High Latitude Populations of and .
- The Nutrient-Responsive Hormone CCHamide-2 Controls Growth by Regulating Insulin-like Peptides in the Brain of
- Characterization of TCF21 Downstream Target Regions Identifies a Transcriptional Network Linking Multiple Independent Coronary Artery Disease Loci
- PARP2 Is the Predominant Poly(ADP-Ribose) Polymerase in Arabidopsis DNA Damage and Immune Responses
- Drosophila Spaghetti and Doubletime Link the Circadian Clock and Light to Caspases, Apoptosis and Tauopathy
- Coronary Artery Disease Associated Transcription Factor TCF21 Regulates Smooth Muscle Precursor Cells That Contribute to the Fibrous Cap
- Rescue of DNA-PK Signaling and T-Cell Differentiation by Targeted Genome Editing in a Deficient iPSC Disease Model
- Disruption of Transcriptional Coactivator Sub1 Leads to Genome-Wide Re-distribution of Clustered Mutations Induced by APOBEC in Active Yeast Genes
- Yeast Killer Elements Hold Their Hosts Hostage
- Keeping in Shape the Dogma of Mitochondrial DNA Maternal Inheritance
- Extreme-Depth Re-sequencing of Mitochondrial DNA Finds No Evidence of Paternal Transmission in Humans
- Trading Places—Switching Frataxin Function by a Single Amino Acid Substitution within the [Fe-S] Cluster Assembly Scaffold
- Natural Variation Identifies , a Universal Gene Required for Cell Proliferation and Growth at High Temperatures in
- Mutations in Gene Are Associated with Predisposition to Breast Cancer
- The Whole of a Scientific Career: An Interview with Oliver Smithies
- Cell Specific eQTL Analysis without Sorting Cells
- Cooperative Action of Cdk1/cyclin B and SIRT1 Is Required for Mitotic Repression of rRNA Synthesis
- Systemic Regulation of RAS/MAPK Signaling by the Serotonin Metabolite 5-HIAA
- Reprogramming LCLs to iPSCs Results in Recovery of Donor-Specific Gene Expression Signature
- The Developmental Intestinal Regulator ELT-2 Controls p38-Dependent Immune Responses in Adult .
- Genetic Mechanism of Human Neutrophil Antigen 2 Deficiency and Expression Variations
- Feeding and Fasting Signals Converge on the LKB1-SIK3 Pathway to Regulate Lipid Metabolism in
- Early Lineage Priming by Trisomy of Leads to Myeloproliferation in a Down Syndrome Model
- Turning into a Frataxin-Dependent Organism
- Accounting for Experimental Noise Reveals That mRNA Levels, Amplified by Post-Transcriptional Processes, Largely Determine Steady-State Protein Levels in Yeast
- Biological Significance of Photoreceptor Photocycle Length: VIVID Photocycle Governs the Dynamic VIVID-White Collar Complex Pool Mediating Photo-adaptation and Response to Changes in Light Intensity
- CTXφ Replication Depends on the Histone-Like HU Protein and the UvrD Helicase
- Disruption of miR-29 Leads to Aberrant Differentiation of Smooth Muscle Cells Selectively Associated with Distal Lung Vasculature
- Notch Is Required in Adult Sensory Neurons for Morphological and Functional Plasticity of the Olfactory Circuit
- Post-transcriptional Regulation of Keratinocyte Progenitor Cell Expansion, Differentiation and Hair Follicle Regression by
- PERK Limits Lifespan by Promoting Intestinal Stem Cell Proliferation in Response to ER Stress
- Casein Kinase 1 and Phosphorylation of Cohesin Subunit Rec11 (SA3) Promote Meiotic Recombination through Linear Element Formation
- Fibroblast Growth Factor 9 Regulation by MicroRNAs Controls Lung Development and Links Loss to the Pathogenesis of Pleuropulmonary Blastoma
- Auxin-Mediated Transcriptional System with a Minimal Set of Components Is Critical for Morphogenesis through the Life Cycle in
- The Broad-Spectrum Antiviral Protein ZAP Restricts Human Retrotransposition
- The 4E-BP Caf20p Mediates Both eIF4E-Dependent and Independent Repression of Translation
- Turning into a Frataxin-Independent Organism
- Promotion of Bone Morphogenetic Protein Signaling by Tetraspanins and Glycosphingolipids
- Essential Role of the ESX-5 Secretion System in Outer Membrane Permeability of Pathogenic Mycobacteria
- The Zinc-Finger Antiviral Protein ZAP Inhibits LINE and Alu Retrotransposition
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Drosophila Spaghetti and Doubletime Link the Circadian Clock and Light to Caspases, Apoptosis and Tauopathy
- Autoselection of Cytoplasmic Yeast Virus Like Elements Encoding Toxin/Antitoxin Systems Involves a Nuclear Barrier for Immunity Gene Expression
- Parp3 Negatively Regulates Immunoglobulin Class Switch Recombination
- PERK Limits Lifespan by Promoting Intestinal Stem Cell Proliferation in Response to ER Stress
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



