-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Extreme-Depth Re-sequencing of Mitochondrial DNA Finds No Evidence of Paternal Transmission in Humans
Emerging evidence raises the possibility that human mitochondrial DNA (mtDNA) is not strictly maternally inherited, but it has not been technically possible to test this hypothesis directly. We identified trios with discordant mtDNA haplotypes, parent-offspring trios were validated using polymorphic microsatellites, and then used extreme-high depth mtDNA re-sequencing to look for paternally transmitted mtDNA. Despite having up to ~1.2 million-fold coverage of mtDNA, we find no evidence that paternal mtDNA haplotypes are transmitted to offspring in humans. Our findings exclude a simple dilution mechanism for uniparental transmission of mtDNA present in all healthy individuals.
Published in the journal: . PLoS Genet 11(5): e32767. doi:10.1371/journal.pgen.1005040
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005040Summary
Emerging evidence raises the possibility that human mitochondrial DNA (mtDNA) is not strictly maternally inherited, but it has not been technically possible to test this hypothesis directly. We identified trios with discordant mtDNA haplotypes, parent-offspring trios were validated using polymorphic microsatellites, and then used extreme-high depth mtDNA re-sequencing to look for paternally transmitted mtDNA. Despite having up to ~1.2 million-fold coverage of mtDNA, we find no evidence that paternal mtDNA haplotypes are transmitted to offspring in humans. Our findings exclude a simple dilution mechanism for uniparental transmission of mtDNA present in all healthy individuals.
Introduction
In eukaryotes, cytoplasmic genes are generally inherited from the mother. The mechanisms responsible for this appear to differ between organisms. The elimination of mitochondrial DNA (mtDNA) during spermatogenesis prevents paternal transmission in Drosophila melanogaster [1]. Conversely, in the Japanese medaka fish Oryzias latipes, sperm mtDNA is lost after fertilization [2]. In cows and humans, sperm mtDNA is eliminated from two or four cell embryos [3,4], and sperm loss may also occur throughout embryogenesis in mice [5].
Although sperm mitochondria are tagged with ubiquitin and actively destroyed through P62 and LC3-mediated autophagy [6,7], there is no direct evidence showing the destruction of sperm mitochondrial DNA (mtDNA) [8], and there are several examples where paternal mtDNA has escaped this process. Extensive paternal transmission of mtDNA has been observed in the marine mussel (Mytillus sp) [9], and occasionally in the fruit fly (Drosophila melanogaster)[10], Lepidopteran insects and the honey bee (Apis mellifera) [11]. For the most part, the “leakage” of paternal mtDNA during transmission in mammals has only been observed in atypical situations such as inter-strain breeding in mice (Mus musculus) [12], or following in vitro embryo manipulation in cattle (Bos taurus) [13]. However, the description of a paternally-derived 2 base pair pathogenic deletion in the mtDNA MTDN2 in a 28-year-old man with a mitochondrial myopathy [14], the persistence of human sperm-derived mtDNA when introduced into somatic cells [15] and in abnormal fertilised human embryos [16], coupled with evidence of paternally transmitted mtDNA in healthy sheep (Ovis aries) [17], and the great tit (Parus major) in its natural habitat [18], questions the accepted dogma of exclusive maternal transmission.
In humans, the debate hinges on the analysis of mtDNA sequences at the population level. By studying partial or complete mtDNA sequences from individuals across the globe, some have argued that the co-occurrence of phylogenetically unrelated genetic variants indicates inter-molecular recombination between paternal and maternal mitochondrial genomes [19], but others have argued that the high mtDNA mutation rate confounds this analysis through the generation of homoplasy [20], which can reach ~20%. Recent experimental data showed no evidence of the active removal of sperm mtDNA from developing mammalian embryos [8], pointing towards a passive dilution process based on differences in the amount of mtDNA between human gametes. Here we test this hypothesis directly.
Results
First we determined the proportion of paternal haplotypes transmitted to the offspring assuming no preferential destruction of sperm-derived mtDNA. We measured the amount of mtDNA in healthy human sperm and pre-fertilization oocytes on the same assay plate. This gave a mean ratio of 1 : 15,860, in keeping with previous reports [8,21–23]. Based on these observations, the 95% prediction interval for the proportion of paternal haplotypes at fertilization is 10–5 to 1.8x10-4.
Next we developed an extreme-depth mtDNA re-sequencing assay to detect very low levels of paternal haplotypes. Given previous work showing a background level of ~1% heteroplasmy for single variants using deep mtDNA re-sequencing [24], we set out to identify trios where the father and the child had two or more variant differences within a <200bp stretch of mtDNA, thus allowing the detection of extremely rare paternal haplotypes at a much lower heteroplasmy level captured within the same sequencing read. Sanger sequencing of the mtDNA from 99 mother-father-child trios from the Avon Longitudinal Survey of Parents and Children identified four different trios with >2 discordant alleles (subsequently referred to as discordant variants, S1 & S2 Tables, Fig 1A & S1 Fig). Parent-offspring trios were confirmed with >99.9% accuracy using 16 short microsatellites in all members of each trio. Next we designed PCR amplicons spanning the four discordant regions. Each amplicon was >2Kb to eliminate nuclear pseudogene amplification, and BLAST analysis predicted exclusive annealing to mtDNA (Fig 1A), which was confirmed by the failure to generate a template from rho-0 cells devoid of mtDNA. All four amplicons were amplified in all four trios using ultra-high fidelity polymerase (PrimeSTAR GXL, TaKaRa Bio Europe, fidelity = 1 error/~16,230 base pairs), allowing each trio to act as a control for the others. Extreme high-depth re-sequencing (Illumina MiSeq, 250bp paired-end reads) yielded stable coverage spanning the region of interest in the offspring (Fig 1B).
Fig. 1. Extreme deep sequencing in trios with discordant paternal and maternal mitochondrial DNA. 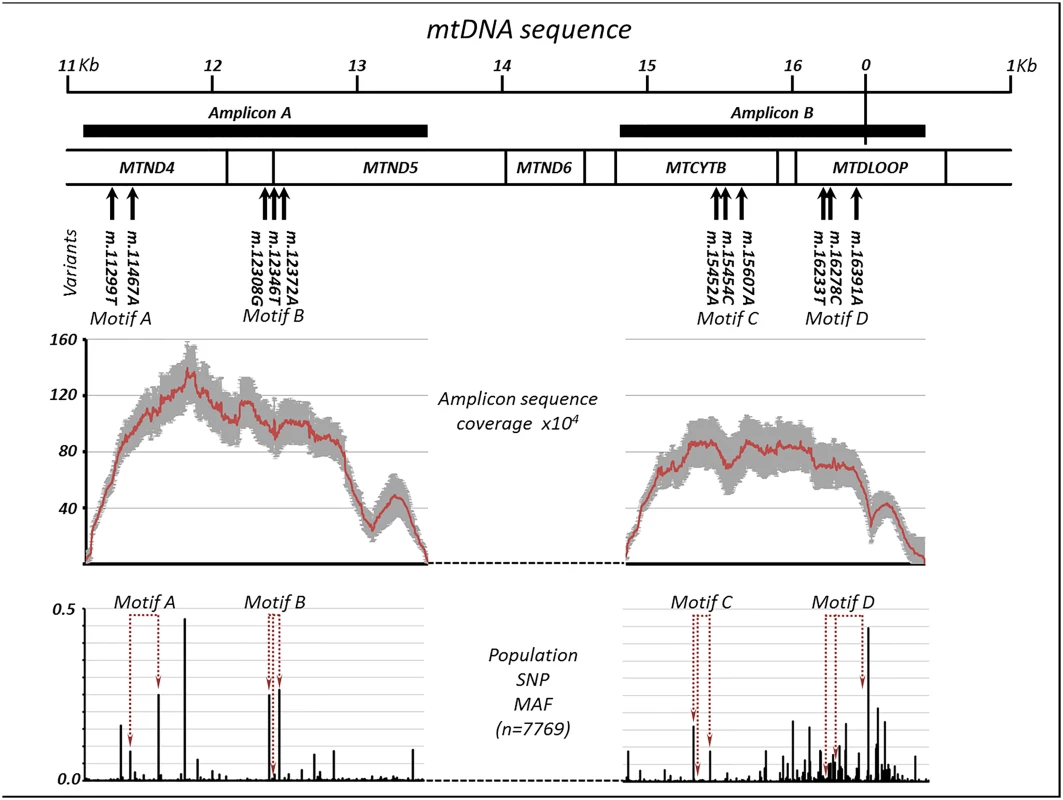
(a) Position of the discordant haplotypes on the mitochondrial genome. Thick horizontal bars show the position of the PCR amplicons. (b) Average sequencing depth +/- 95% confidence intervals for the two amplicons in all four trios. (c) Population variant allele frequencies for the two amplicons, indicating position of motif variants. We then compared the frequency of minor alleles and haplotypes both within and between the four trios. The frequency of isolated single variants was similar to that observed previously at lower depth at ~ 0.5% [24]. As expected, the number of minor haplotypes containing two or three variants was substantially less (Table 1). Overall, the frequency of minor haplotypes containing two variants approximated the square root of the single variant frequency, and the frequency of the three-allele haplotypes was approximately equal to the cube root of the single variant frequency. These observations were in keeping with a random background mutation frequency affecting single base-pairs of ~0.5% [24]. The observed alleles contributing to the ultra-rare haplotypes did not correspond to commonly co-occurring population variants, making external sample contamination unlikely (Fig 1D and S2 Table). The frequency of unexpected maternal or paternal haplotypes was greater in other members of the same family trio than in other trios. Given that all of the trios were analysed in the laboratory simultaneously, these rare shared haplotypes probably arose through very low-level contamination at the time the original samples were taken, in the order of <10–5 molecules (Table 1). We incorporated these observations in a significance test of our findings, and determined whether we had adequate statistical power to detect paternally inherited mtDNA.
Tab. 1. Frequency of rare haplotypes in trios with discordant paternal and maternal mitochondrial DNA. 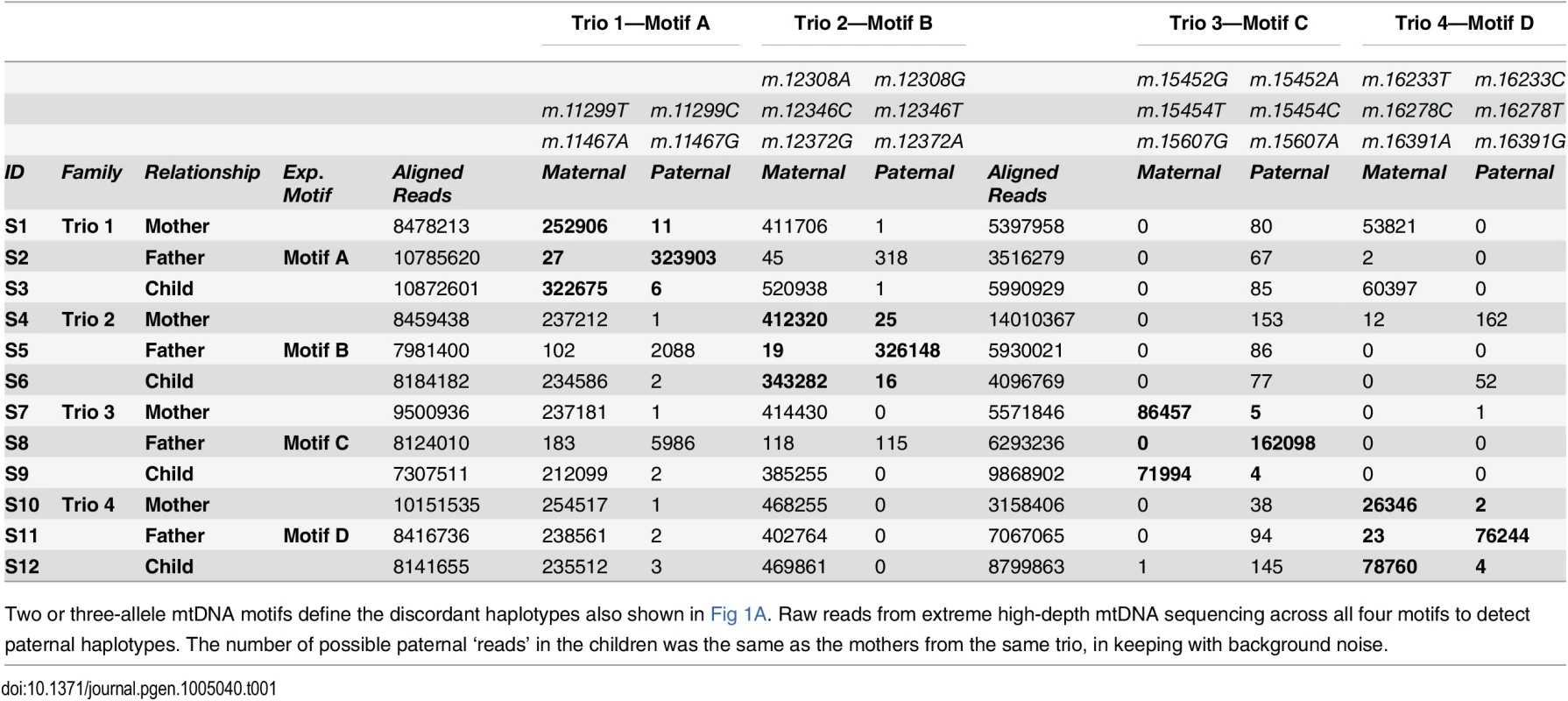
Two or three-allele mtDNA motifs define the discordant haplotypes also shown in Fig 1A. Raw reads from extreme high-depth mtDNA sequencing across all four motifs to detect paternal haplotypes. The number of possible paternal ‘reads’ in the children was the same as the mothers from the same trio, in keeping with background noise. The power to detect paternally inherited mtDNA was estimated by determining the difference between mismatched haplotype frequencies in mothers and children. These are simply modelled by Poisson distributions with a without paternal contributions, and the difference is described by a Skellam distribution [25]. The power was investigated for a range of theoretical paternal mtDNA contributions, and the observed background heteroplasmy levels in mothers (Fig 2). For the observed background heteroplasmy levels in the mothers (4.3 x 10–5, 6.0 x 10–5, 5.7 x 10–5, and 7.6 x 10–5, Table 1), sequencing at >300,000-fold depth in the relevant offspring had >80% power to detect the predicted level of transmitted paternal mtDNA.
Fig. 2. Power to detect paternally transmitted mtDNA in the children studied here based on the observed ultra-deep mtDNA sequence data. 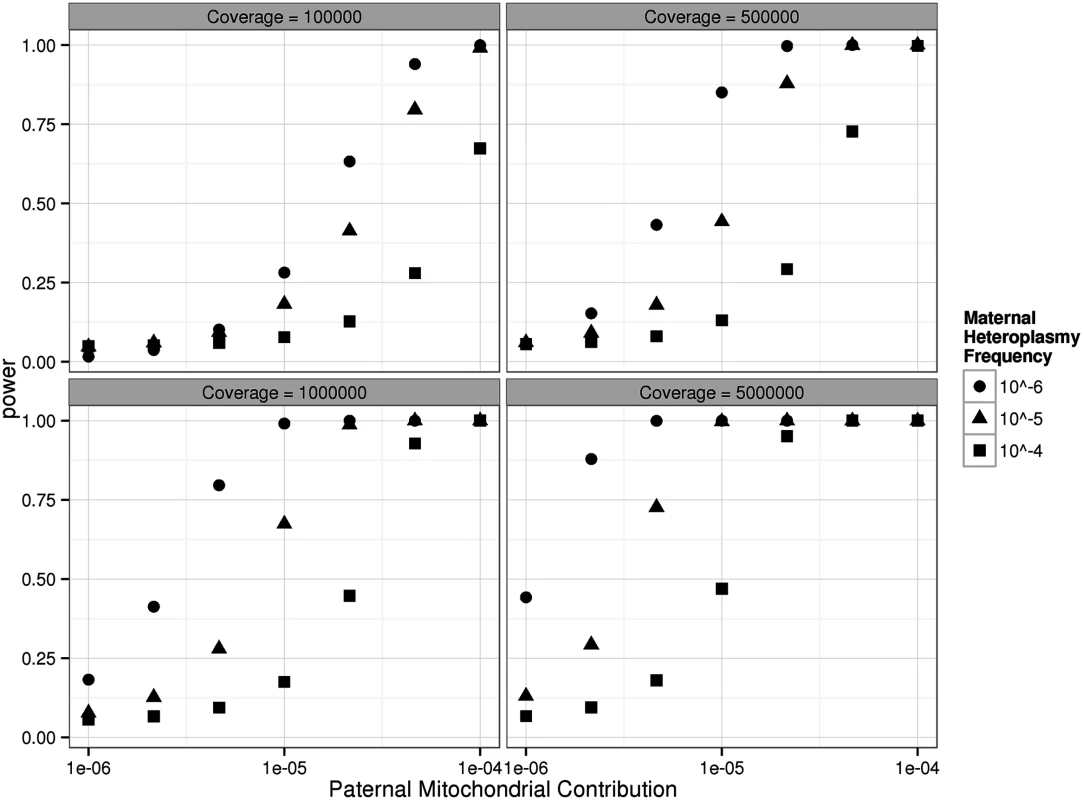
Calculations are based on the comparison of two Poisson distributions as described by Skellam [25], assuming the same mtDNA fold coverage in mothers and offspring (see text for a full description of the methods). Each graph shows the power to detect a paternal contribution to the mtDNA in the offspring of the trios studied here based for different degrees of ultra-deep sequencing coverage, and for different background levels of mtDNA heteroplasmy seen in the mothers, which in this study were 4.3 x 10–5, 6.0 x 10–5, 5.7 x 10–5, and 7.6 x 10–5. A bootstrap test was used to determine whether the observed discordant haplotypes in the children were consistent with the predicted paternal contribution, incorporating the 95% confidence intervals for proportion of sperm haplotypes in the oocytes (10–5 to 1.8x10-4). A paternal contribution was not compatible for trios A and B (p = 0.004, p = 0.016), but there was insufficient evidence to reject the other two trios (p = 0.21, p = 0.12) that had a lower total coverage for the full range of possible transmitted levels of sperm mtDNA. The power calculation suggests that the uncertainty in two trios likely reflects the confidence interval for our estimate of sperm haplotypes in the fertilized oocyte, and not directly reflect the likelihood of any paternal transmission of mtDNA.
The full data set and relevant code is available from the authors and at: https://www.staff.ncl.ac.uk/i.j.wilson/PaternalTransmission
Discussion
When taken together these findings indicate that the extremely rare variant haplotypes seen in the offspring are highly unlikely to have arisen through the passive ‘leakage’ of paternal mtDNA within the trio. Lower levels of paternal mtDNA transmission are also unlikely because we did not see common mtDNA population haplotypes in any of the individuals studied (Fig 1D), which would be expected if there were frequent paternal leakage in the human population. The lack of common population haplotypes at extreme high depth also shows that our experimental approach was not subject to significant cross-contamination. Finally, we show that there is little to be gained by sequencing at >5000-fold if single variants are of interest, because the ‘noise’ level will prevent further resolution of low-level heteroplasmy.
The ‘background noise’ level that we observed has several potential origins. First, the near exponential decrease in frequency of single variants, two variant, and three variant haplotypes is consistent with a random background mutation frequency introduced by DNA replication errors, either within the biological system or through PCR amplification. We did not observe patterns of nucleotide changes that would imply a direct insult to the DNA templates (such as C>U deamination damage). Finally, even at extreme high depth (>1 million reads in some trios) we saw negligible or no haplotypes observed in other trios. Given that the trios were analysed together, this means that our laboratory procedures were robust with negligible cross contamination. It is therefore likely that the rare paternal haplotypes seen in maternal samples (Table 1) were introduced when the samples were collected.
Our observations were made on DNA extracted from buccal swabs. It is thus theoretically possible that paternal mtDNA was originally transmitted to the offspring and subsequently lost from buccal cells before the DNA samples were acquired. The loss of mutated mtDNA has been observed in patients with mtDNA diseases, most typically m.3243A>G, probably through selection against a deleterious allele at the stem cell level [26]. However, for most inherited heteroplasmic mtDNA mutations (eg m.8993T>C), the percentage level of the mutation is the same in a wide range of different tissues [27], including buccal swabs. It is therefore likely that if there were paternal transmission, this would be detected in all tissues. Moreover, since variants we studied here are haplogroup markers and are unlikely to have significant biochemical consequences [28], it is highly unlikely that a paternal haplotype allele would be lost from a particular tissue through selection. Absolute reassurance would be provided by studying other tissues, but this approach has its own difficulties because somatic point mutations accumulate in non-dividing tissues, increasing the background ‘noise’ levels to a point that would preclude the detection of passively transmitted paternal mtDNA.
Given that human paternal mtDNA can be detected in very early embryos [4], what is the mechanism underpinning exclusive maternal inheritance of mtDNA in humans? Recent observations that ubiquitination and P63/LC3 tagging of sperm mitochondria does not lead to mitophagy in mice, the mechanism is likely to act at the level of the mitochondrial genome [8]. This is plausible, given recent observations in heteroplasmic mice where a similar mechanism was proposed for heteroplasmy segregation during inheritance [29,30], and within different tissues and organs during life [31], where selection against specific mtDNA molecules occurs at levels well below the level required to cause a biochemical defect affecting oxidative phosphorylation within the cell. A similar mechanism was proposed following the introduction of sperm mtDNA into somatic cells [15]. Although it remains to be determined how this selection process occurs at the molecular level, it is of fundamental importance in preventing the accumulation of deleterious mutations in the human population, effectively taking Muller’s ratchet [32] ‘down a gear’.
Materials and Methods
Ethics statement
Ethical approval for the study was obtained from the ALSPAC Ethics and Law Committee and the Local Research Ethics Committees. The ALSPAC study web site contains details of all the data that is available on the cohort through a fully searchable dictionary (http:www.bris.ac.uk/alspac/researchers/data-access/data-dictionary/).
Quantification of mtDNA in sperm and oocytes
Excess oocytes were collected after follicular reduction from healthy donors, lysed for 16 hours in 50mM Tris-HCl, pH 8.5, with 0.5% Tween 20 and 200ng/ml proteinase K, at 55°C, followed by heat inactivation at 95°C for 10 minutes. Three single oocytes and 43 sperm DNA samples were analysed using quantitative real-time PCR (qPCR). A multiplex qPCR assay, using probes and primers targeting a region of MT-ND1 and the nuclear housekeeping gene β-2 microglobulin (β2M), were used to measure mtDNA copy number on a CFX96 Touch™ Real-Time PCR Detection System (BioRad, USA).
Identification of mtDNA discordant trios
Buccal DNA samples were extracted in a different laboratory from 100 mother-father-child trios obtained from the Avon Longitudinal Study of Parents and Children Cohort (ALSPAC) [33,34]. Mitochondrial DNA (mtDNA) haplogroup defining single nucleotide polymorphisms (SNPs, www.phylotree.org) were determined in each mother and father by Sanger sequencing specific regions of the mitochondrial DNA genome. (Big Dye v3.1 kit and capillary electrophoresed on an ABI3130xl Genetic Analyzer, Life Technologies, Warrington, UK). Alignment and variant calling was performed using SeqScape software (v2.1.1, Applied Biosystems) reference to the GenBank sequence NC_012920.1. Four trios were identified with >2 discordant variants falling within a ~250 bp read-length (S1 Table). Parent-offspring trios were confirmed using the Promega Powerplex 16 HS system (Promega, Southampton, UK, performed by NorthGene Ltd.).
mtDNA isolation and enrichment
2x 2Kb mtDNA amplicons were designed to span the discordant regions in each trio (Fig 1A) and to avoid nuclear pseudogene co-amplification with a high-fidelity polymerase (PrimeSTAR GXL DNA Polymerase, TaKaRa Bio Europe, France). Primer sequences were: set 1 = TATCCAGTGAACCACTATCAC-F (m.11010-11030) and GGGAGGTTGAAGTGAGAGG-R (m.13453-13435); set 2 = ATTCATCGACCTCCCCACC-F (m.14797-14815) and CTGGTTAGGCTGGTGTTAGG-R (m.389-370). Initially, primer efficiency and specificity was assessed as successful after no amplification of DNA from rho0 cell lines, which contain no mtDNA. Amplified products were assessed by gel electrophoresis against DNA+ve and DNA-ve controls, and quantified using a Qubit 2.0 fluorimeter (Life Technologies, Paisley, UK). Each amplicon was individually purified using Agencourt AMPure XP beads (Beckman-Coulter, USA), pooled in equimolar concentrations and re-quantified.
Extreme depth mtDNA sequencing
Amplicons were ‘tagmented’, amplified, cleaned, normalised and pooled into a multiplex using the Illumina Nextera XT DNA sample preparation kit (Illumina, USA). Multiplex plex pools were sequenced using MiSeq Reagent Kit v3.0 (Illumina, USA) in paired-end, 250 bp reads on the same flow cell.
Bioinformatic analysis
Post-run FASTQ files were analysed using an in-house developed bioinformatic pipeline. Reads were aligned to the rCRS (NC_012920) using BWA v0.7.10 [35], invoking—mem [35]. Aligned reads were sorted and indexed using Samtools v0.1.18 [36]. Variant calling was performed in tandem using VarScan v2.3.13 [37,38], (minimum depth = 1500, supporting reads = 10 and variant threshold = 1.0%) and LoFreq v0.6.1 [39]. Concordance calling between VarScan and LoFreq was >99.5%. Concordant variants were annotated using ANNOVAR v529 [40]. In-house Perl scripts were used to extract base/read quality data and coverage data.
Potential paternal haplotypes were identified from the pool of analysed reads (*.sam files) using command line scripting, generating motif-specific counts for each trio. For example, [grep—c 'CCTCACTGCCCAAGAACTATCAAACTCCTGAGCCAACAACTTAATATGACTAGCTTACACAATAGCTTTTATAGTAAAGATACCTCTTTACGGACTCCACTTATGACTCCCTAAAGCCCATGTCGAAGCCCCCATCGCTGGGTCAATAGTACTTGCCGCAGTACTCTTG\|CAAGAGTACTGCGGCAAGTACTATTGACCCAGCGATGGGGGCTTCGACATGGGCTTTAGGGAGTCATAAGTGGAGTCCGTAAAGAGGTATCTTTACTATAAAAGCTATTGTGTAAGCTAGTCATATTAAGTTGTTGGCTCAGGAGTTTGATAGTTCTTGGGCAGTGAGG'. /*.sam] was used to identify whole reads containing m.11299C and m.11467G in forward and complement orientations (corresponding bases underlined). Counts were generated for all possible permutations of motifs, in all available samples.
Statistical analysis
The mtDNA counts for healthy human sperm had mean 77.2 (SD 53.9, n = 43) and oocytes had mean 1220000 (SD 183000, n = 3). An empirical distribution for the ratio of sperm to oocyte mtDNA levels immediately after fertilization was estimated by bootstrapping. 100,000 individual mtDNA counts were sampled from the raw sperm mtDNA counts and 105 corresponding oocyte mtDNA counts were drawn from a normal distribution with mean and standard deviation as above for the oocytes. A 95% prediction interval was then calculated from the 2.5th and 97.5th percentiles of this ratio of sperm mtDNA to oocyte count.
A hypothesis test for paternal transmission was performed by bootstrapping under the null hypothesis that the discordant haplotypes were due to paternal inheritance at the ratio predicted from the direct measurements on individual gametes. For each of the four discordant haplotypes, 105 sampled ratios were simulated in the same way as for the prediction interval, and from this 105 replicate discordant child haplotypes were generated by binomial sampling, with p equal to these ratios and n equal to the total coverage over all haplotypes for this motif and trio. Observed background noise was added to this count at a Poisson rate equal to the discordant maternal haplotype rate. The simulated maternal count was pure Poisson noise, generated at the same rate as the child noise. The bootstrapped differences in proportion were then compared to the observed data, and extreme values are evidence to reject the null hypothesis. The p-value is estimated percentile rank of the observed data in the bootstrapped distribution.
Supporting Information
Zdroje
1. DeLuca SZ, O'Farrell PH (2012) Barriers to male transmission of mitochondrial DNA in sperm development. Dev Cell 22 : 660–668. doi: 10.1016/j.devcel.2011.12.021 22421049
2. Nishimura Y, Yoshinari T, Naruse K, Yamada T, Sumi K, et al. (2006) Active digestion of sperm mitochondrial DNA in single living sperm revealed by optical tweezers. Proc Natl Acad Sci U S A 103 : 1382–1387. 16432229
3. Sutovsky P, Moreno RD, Ramalho-Santos J, Dominko T, Simerly C, et al. (1999) Ubiquitin tag for sperm mitochondria. Nature 402 : 371–372. 10586873
4. Ankel-Simons F, Cummins JM (1996) Misconceptions about mitochondria and mammalian fertilization: implications for theories on human evolution. Proc Natl Acad Sci U S A 93 : 13859–13863. 8943026
5. Shitara H, Kaneda H, Sato A, Inoue K, Ogura A, et al. (2000) Selective and continuous elimination of mitochondria microinjected into mouse eggs from spermatids, but not from liver cells, occurs throughout embryogenesis. Genetics 156 : 1277–1284. 11063701
6. Al Rawi S, Louvet-Vallee S, Djeddi A, Sachse M, Culetto E, et al. (2011) Postfertilization autophagy of sperm organelles prevents paternal mitochondrial DNA transmission. Science 334 : 1144–1147. doi: 10.1126/science.1211878 22033522
7. Sato M, Sato K (2011) Degradation of paternal mitochondria by fertilization-triggered autophagy in C. elegans embryos. Science 334 : 1141–1144. doi: 10.1126/science.1210333 21998252
8. Luo SM, Ge ZJ, Wang ZW, Jiang ZZ, Wang ZB, et al. (2013) Unique insights into maternal mitochondrial inheritance in mice. Proc Natl Acad Sci U S A 110 : 13038–13043. doi: 10.1073/pnas.1303231110 23878233
9. Cao L, Ort BS, Mizi A, Pogson G, Kenchington E, et al. (2009) The control region of maternally and paternally inherited mitochondrial genomes of three species of the sea mussel genus Mytilus. Genetics 181 : 1045–1056. doi: 10.1534/genetics.108.093229 19139146
10. Dokianakis E, Ladoukakis ED (2014) Different degree of paternal mtDNA leakage between male and female progeny in interspecific Drosophila crosses. Ecol Evol 4 : 2633–2641. doi: 10.1002/ece3.1069 25077015
11. Meusel MS, Moritz RF (1993) Transfer of paternal mitochondrial DNA during fertilization of honeybee (Apis mellifera L.) eggs. Curr Genet 24 : 539–543. 8299176
12. Gyllensten U, Wharton D, Josefsson A, Wilson AC (1991) Paternal inheritance of mitochondrial DNA in mice. Nature 352 : 255–257. 1857422
13. St John JC, Schatten G (2004) Paternal mitochondrial DNA transmission during nonhuman primate nuclear transfer. Genetics 167 : 897–905. 15238538
14. Schwartz M, Vissing J (2002) Paternal inheritance of mitochondrial DNA. N Engl J Med 347 : 576–580. 12192017
15. Manfredi G, Thyagarajan D, Papadopoulou LC, Pallotti F, Schon EA (1997) The fate of human sperm-derived mtDNA in somatic cells. American Journal of Human Genetics 61 : 953–960. 9382109
16. St John J, Sakkas D, Dimitriadi K, Barnes A, Maclin V, et al. (2000) Failure of elimination of paternal mitochondrial DNA in abnormal embryos. Lancet 355 : 200. 10675123
17. Zhao X, Li N, Guo W, Hu X, Liu Z, et al. (2004) Further evidence for paternal inheritance of mitochondrial DNA in the sheep (Ovis aries). Heredity (Edinb) 93 : 399–403. 15266295
18. Kvist L, Martens J, Nazarenko AA, Orell M (2003) Paternal leakage of mitochondrial DNA in the great tit (Parus major). Mol Biol Evol 20 : 243–247. 12598691
19. Awadalla P, Eyre-Walker A, Smith JM (1999) Linkage disequilibrium and recombination in hominid mitochondrial DNA[see comments]. Science 286 : 2524–2525. 10617471
20. Elson JL, Andrews RM, Chinnery PF, Lightowlers RN, Turnbull DM, et al. (2001) Analysis of European mtDNAs for recombination. Am J Hum Genet 68 : 145–153. 11115380
21. May-Panloup P, Chretien MF, Savagner F, Vasseur C, Jean M, et al. (2003) Increased sperm mitochondrial DNA content in male infertility. Hum Reprod 18 : 550–556. 12615823
22. Greggains GD, Lister LM, Tuppen HA, Zhang Q, Needham LH, et al. (2014) Therapeutic potential of somatic cell nuclear transfer for degenerative disease caused by mitochondrial DNA mutations. Sci Rep 4 : 3844. doi: 10.1038/srep03844 24457623
23. Craven L, Tuppen HA, Greggains GD, Harbottle SJ, Murphy JL, et al. (2010) Pronuclear transfer in human embryos to prevent transmission of mitochondrial DNA disease. Nature 465 : 82–85. doi: 10.1038/nature08958 20393463
24. Payne BA, Wilson IJ, Yu-Wai-Man P, Coxhead J, Deehan D, et al. (2013) Universal heteroplasmy of human mitochondrial DNA. Hum Mol Genet 22 : 384–390. doi: 10.1093/hmg/dds435 23077218
25. Skellam JG (1946) The frequency distribution of the difference between two Poisson variates belonging to different populations. J R Stat Soc Ser A 109 : 296. 20256640
26. Rajasimha HK, Chinnery PF, Samuels DC (2008) Selection against pathogenic mtDNA mutations in a stem cell population leads to the loss of the 3243A—>G mutation in blood. American Journal of Human Genetics 82 : 333–343. doi: 10.1016/j.ajhg.2007.10.007 18252214
27. White SL, Shanske S, McGill JJ, Mountain H, Geraghty MT, et al. (1999) Mitochondrial DNA mutations at nucleotide 8993 show a lack of tissue of age-related variation. Journal of Inherited Metabolic Diseases 22 : 899–914. 10604142
28. Amo T, Brand MD (2007) Were inefficient mitochondrial haplogroups selected during migrations of modern humans? A test using modular kinetic analysis of coupling in mitochondria from cybrid cell lines. Biochem J 404 : 345–351. 17355224
29. Freyer C, Cree LM, Mourier A, Stewart JB, Koolmeister C, et al. (2012) Variation in germline mtDNA heteroplasmy is determined prenatally but modified during subsequent transmission. Nature Genetics 44 : 1282–1285. doi: 10.1038/ng.2427 23042113
30. Fan W, Waymire KG, Narula N, Li P, Rocher C, et al. (2008) A mouse model of mitochondrial disease reveals germline selection against severe mtDNA mutations. Science 319 : 958–962. doi: 10.1126/science.1147786 18276892
31. Jokinen R, Marttinen P, Sandell HK, Manninen T, Teerenhovi H, et al. (2010) Gimap3 regulates tissue-specific mitochondrial DNA segregation. PLoS Genet 6: e1001161. doi: 10.1371/journal.pgen.1001161 20976251
32. Muller HJ (1964) The relation of recombination to mutational advance. Mutation Research 1 : 2–9.
33. Fraser A, Macdonald-Wallis C, Tilling K, Boyd A, Golding J, et al. (2013) Cohort Profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol 42 : 97–110. doi: 10.1093/ije/dys066 22507742
34. Boyd A, Golding J, Macleod J, Lawlor DA, Fraser A, et al. (2013) Cohort Profile: the 'children of the 90s'—the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol 42 : 111–127. doi: 10.1093/ije/dys064 22507743
35. Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25 : 1754–1760. doi: 10.1093/bioinformatics/btp324 19451168
36. Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, et al. (2009) The Sequence Alignment/Map format and SAMtools. Bioinformatics 25 : 2078–2079. doi: 10.1093/bioinformatics/btp352 19505943
37. Koboldt DC, Chen K, Wylie T, Larson DE, McLellan MD, et al. (2009) VarScan: variant detection in massively parallel sequencing of individual and pooled samples. Bioinformatics 25 : 2283–2285. doi: 10.1093/bioinformatics/btp373 19542151
38. Koboldt DC, Zhang Q, Larson DE, Shen D, McLellan MD, et al. (2012) VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res 22 : 568–576. doi: 10.1101/gr.129684.111 22300766
39. Wilm A, Aw PP, Bertrand D, Yeo GH, Ong SH, et al. (2012) LoFreq: a sequence-quality aware, ultra-sensitive variant caller for uncovering cell-population heterogeneity from high-throughput sequencing datasets. Nucleic Acids Res 40 : 11189–11201. doi: 10.1093/nar/gks918 23066108
40. Wang K, Li M, Hakonarson H (2010) ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 38: e164. doi: 10.1093/nar/gkq603 20601685
Štítky
Genetika Reprodukční medicína
Článek Minor Type IV Collagen α5 Chain Promotes Cancer Progression through Discoidin Domain Receptor-1Článek A Unique Virulence Gene Occupies a Principal Position in Immune Evasion by the Malaria ParasiteČlánek The Centrosomal Linker and Microtubules Provide Dual Levels of Spatial Coordination of CentrosomesČlánek Dissecting the Function and Assembly of Acentriolar Microtubule Organizing Centers in Cells In VivoČlánek MicroRNA-Dependent Transcriptional Silencing of Transposable Elements in Drosophila Follicle CellsČlánek PARP2 Is the Predominant Poly(ADP-Ribose) Polymerase in Arabidopsis DNA Damage and Immune ResponsesČlánek Cooperative Action of Cdk1/cyclin B and SIRT1 Is Required for Mitotic Repression of rRNA SynthesisČlánek The Developmental Intestinal Regulator ELT-2 Controls p38-Dependent Immune Responses in Adult .Článek Feeding and Fasting Signals Converge on the LKB1-SIK3 Pathway to Regulate Lipid Metabolism in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 5- Růst a vývoj dětí narozených pomocí IVF
- Délka menstruačního cyklu jako marker ženské plodnosti
- Vztah užívání alkoholu a mužské fertility
- Šanci na úspěšný průběh těhotenství snižují nevhodné hladiny progesteronu vznikající při umělém oplodnění
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Genomic Heritability: What Is It?
- Triglyceride-Increasing Alleles Associated with Protection against Type-2 Diabetes
- Epistasis Is a Major Determinant of the Additive Genetic Variance in
- Genetic Regulation of Bone Metabolism in the Chicken: Similarities and Differences to Mammalian Systems
- Minor Type IV Collagen α5 Chain Promotes Cancer Progression through Discoidin Domain Receptor-1
- The Philosophical Approach: An Interview with Ford Doolittle
- Downregulation of the Host Gene by miR-92 Is Essential for Neuroblast Self-Renewal in
- A Unique Virulence Gene Occupies a Principal Position in Immune Evasion by the Malaria Parasite
- Y Fuse? Sex Chromosome Fusions in Fishes and Reptiles
- Regulation of Active DNA Demethylation by a Methyl-CpG-Binding Domain Protein in
- Overlapping Patterns of Rapid Evolution in the Nucleic Acid Sensors cGAS and OAS1 Suggest a Common Mechanism of Pathogen Antagonism and Escape
- Autoselection of Cytoplasmic Yeast Virus Like Elements Encoding Toxin/Antitoxin Systems Involves a Nuclear Barrier for Immunity Gene Expression
- Genetic Architecture of Abdominal Pigmentation in
- Whole Genome DNA Binding Analysis of the Bacterial Replication Initiator and Transcription Factor DnaA
- The Centrosomal Linker and Microtubules Provide Dual Levels of Spatial Coordination of Centrosomes
- Parp3 Negatively Regulates Immunoglobulin Class Switch Recombination
- Burden Analysis of Rare Microdeletions Suggests a Strong Impact of Neurodevelopmental Genes in Genetic Generalised Epilepsies
- Cell Cycle Control by the Master Regulator CtrA in
- Myopathic Lamin Mutations Cause Reductive Stress and Activate the Nrf2/Keap-1 Pathway
- Monoallelic Loss of the Imprinted Gene Promotes Tumor Formation in Irradiated Mice
- Phylum-Level Conservation of Regulatory Information in Nematodes despite Extensive Non-coding Sequence Divergence
- Clustering and Negative Feedback by Endocytosis in Planar Cell Polarity Signaling Is Modulated by Ubiquitinylation of Prickle
- Dissecting the Function and Assembly of Acentriolar Microtubule Organizing Centers in Cells In Vivo
- MicroRNA-Dependent Transcriptional Silencing of Transposable Elements in Drosophila Follicle Cells
- β-Catenin Signaling Biases Multipotent Lingual Epithelial Progenitors to Differentiate and Acquire Specific Taste Cell Fates
- A Simple Auxin Transcriptional Response System Regulates Multiple Morphogenetic Processes in the Liverwort
- Parallel Gene Expression Differences between Low and High Latitude Populations of and .
- The Nutrient-Responsive Hormone CCHamide-2 Controls Growth by Regulating Insulin-like Peptides in the Brain of
- Characterization of TCF21 Downstream Target Regions Identifies a Transcriptional Network Linking Multiple Independent Coronary Artery Disease Loci
- PARP2 Is the Predominant Poly(ADP-Ribose) Polymerase in Arabidopsis DNA Damage and Immune Responses
- Drosophila Spaghetti and Doubletime Link the Circadian Clock and Light to Caspases, Apoptosis and Tauopathy
- Coronary Artery Disease Associated Transcription Factor TCF21 Regulates Smooth Muscle Precursor Cells That Contribute to the Fibrous Cap
- Rescue of DNA-PK Signaling and T-Cell Differentiation by Targeted Genome Editing in a Deficient iPSC Disease Model
- Disruption of Transcriptional Coactivator Sub1 Leads to Genome-Wide Re-distribution of Clustered Mutations Induced by APOBEC in Active Yeast Genes
- Yeast Killer Elements Hold Their Hosts Hostage
- Keeping in Shape the Dogma of Mitochondrial DNA Maternal Inheritance
- Extreme-Depth Re-sequencing of Mitochondrial DNA Finds No Evidence of Paternal Transmission in Humans
- Trading Places—Switching Frataxin Function by a Single Amino Acid Substitution within the [Fe-S] Cluster Assembly Scaffold
- Natural Variation Identifies , a Universal Gene Required for Cell Proliferation and Growth at High Temperatures in
- Mutations in Gene Are Associated with Predisposition to Breast Cancer
- The Whole of a Scientific Career: An Interview with Oliver Smithies
- Cell Specific eQTL Analysis without Sorting Cells
- Cooperative Action of Cdk1/cyclin B and SIRT1 Is Required for Mitotic Repression of rRNA Synthesis
- Systemic Regulation of RAS/MAPK Signaling by the Serotonin Metabolite 5-HIAA
- Reprogramming LCLs to iPSCs Results in Recovery of Donor-Specific Gene Expression Signature
- The Developmental Intestinal Regulator ELT-2 Controls p38-Dependent Immune Responses in Adult .
- Genetic Mechanism of Human Neutrophil Antigen 2 Deficiency and Expression Variations
- Feeding and Fasting Signals Converge on the LKB1-SIK3 Pathway to Regulate Lipid Metabolism in
- Early Lineage Priming by Trisomy of Leads to Myeloproliferation in a Down Syndrome Model
- Turning into a Frataxin-Dependent Organism
- Accounting for Experimental Noise Reveals That mRNA Levels, Amplified by Post-Transcriptional Processes, Largely Determine Steady-State Protein Levels in Yeast
- Biological Significance of Photoreceptor Photocycle Length: VIVID Photocycle Governs the Dynamic VIVID-White Collar Complex Pool Mediating Photo-adaptation and Response to Changes in Light Intensity
- CTXφ Replication Depends on the Histone-Like HU Protein and the UvrD Helicase
- Disruption of miR-29 Leads to Aberrant Differentiation of Smooth Muscle Cells Selectively Associated with Distal Lung Vasculature
- Notch Is Required in Adult Sensory Neurons for Morphological and Functional Plasticity of the Olfactory Circuit
- Post-transcriptional Regulation of Keratinocyte Progenitor Cell Expansion, Differentiation and Hair Follicle Regression by
- PERK Limits Lifespan by Promoting Intestinal Stem Cell Proliferation in Response to ER Stress
- Casein Kinase 1 and Phosphorylation of Cohesin Subunit Rec11 (SA3) Promote Meiotic Recombination through Linear Element Formation
- Fibroblast Growth Factor 9 Regulation by MicroRNAs Controls Lung Development and Links Loss to the Pathogenesis of Pleuropulmonary Blastoma
- Auxin-Mediated Transcriptional System with a Minimal Set of Components Is Critical for Morphogenesis through the Life Cycle in
- The Broad-Spectrum Antiviral Protein ZAP Restricts Human Retrotransposition
- The 4E-BP Caf20p Mediates Both eIF4E-Dependent and Independent Repression of Translation
- Turning into a Frataxin-Independent Organism
- Promotion of Bone Morphogenetic Protein Signaling by Tetraspanins and Glycosphingolipids
- Essential Role of the ESX-5 Secretion System in Outer Membrane Permeability of Pathogenic Mycobacteria
- The Zinc-Finger Antiviral Protein ZAP Inhibits LINE and Alu Retrotransposition
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Drosophila Spaghetti and Doubletime Link the Circadian Clock and Light to Caspases, Apoptosis and Tauopathy
- Autoselection of Cytoplasmic Yeast Virus Like Elements Encoding Toxin/Antitoxin Systems Involves a Nuclear Barrier for Immunity Gene Expression
- Parp3 Negatively Regulates Immunoglobulin Class Switch Recombination
- PERK Limits Lifespan by Promoting Intestinal Stem Cell Proliferation in Response to ER Stress
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



