-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaBacterial Regulon Evolution: Distinct Responses and Roles for the Identical OmpR Proteins of Typhimurium and in the Acid Stress Response
Salmonella Typhimurium is closely related to Escherichia coli and they possess identical OmpR DNA binding proteins. S. Typhimurium uses OmpR to control the expression of genes involved in adaptation to acid rather than osmotic stress. OmpR expression increases in response to acid stress in S. Typhimurium but not in E. coli due to structural differences in the ompR regulatory region. S. Typhimurium OmpR controls many genes, few of which are in E. coli. Many OmpR-regulated S. Typhimurium-specific targets have been acquired by horizontal gene transfer and contribute to pathogenesis. During infection, S. Typhimurium adapts to the macrophage vacuole, an acidic niche where S. Typhimurium DNA becomes relaxed. DNA relaxation accompanies acid stress in S. Typhimurium but not E. coli and enhances OmpR binding to DNA. Drug-induced DNA relaxation mimics the effect of acid stress on OmpR binding to DNA. Thus acid-sensitive OmpR activity in S. Typhimurium allows OmpR to control many S. Typhimurium-specific genes through a mechanism that depends on changes to DNA topology. We propose that this allosteric role for DNA, combined with a weak requirement on the part of OmpR for binding site sequence specificity, accommodates flexibility in regulon membership and facilitates bacterial evolution.
Published in the journal: . PLoS Genet 10(3): e32767. doi:10.1371/journal.pgen.1004215
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004215Summary
Salmonella Typhimurium is closely related to Escherichia coli and they possess identical OmpR DNA binding proteins. S. Typhimurium uses OmpR to control the expression of genes involved in adaptation to acid rather than osmotic stress. OmpR expression increases in response to acid stress in S. Typhimurium but not in E. coli due to structural differences in the ompR regulatory region. S. Typhimurium OmpR controls many genes, few of which are in E. coli. Many OmpR-regulated S. Typhimurium-specific targets have been acquired by horizontal gene transfer and contribute to pathogenesis. During infection, S. Typhimurium adapts to the macrophage vacuole, an acidic niche where S. Typhimurium DNA becomes relaxed. DNA relaxation accompanies acid stress in S. Typhimurium but not E. coli and enhances OmpR binding to DNA. Drug-induced DNA relaxation mimics the effect of acid stress on OmpR binding to DNA. Thus acid-sensitive OmpR activity in S. Typhimurium allows OmpR to control many S. Typhimurium-specific genes through a mechanism that depends on changes to DNA topology. We propose that this allosteric role for DNA, combined with a weak requirement on the part of OmpR for binding site sequence specificity, accommodates flexibility in regulon membership and facilitates bacterial evolution.
Introduction
The relationship between a given regulatory protein and its target genes is subject to evolutionary change, allowing genes to join or to leave a given regulon over time. Evidence for this regulatory flexibility comes from comparisons of orthologous regulators and their regulons from related bacterial species. Four types of variation have been described so far: (i) changes in the presence/absence and the distribution of binding sites for a common regulatory protein at orthologous targets (ii) genes acquired via lateral transfer coming under the control of a regulator already established in the cell (iii) architectural adjustment of a promoter under the control of a regulator to add or subtract regulatory influence and (iv) changes that affect the DNA binding protein itself [1]. The OmpR DNA binding protein of Gram-negative bacteria has the potential to govern collectives of genes whose membership is subject to change. This is because OmpR demonstrates only moderate specificity for its DNA targets [2], allowing new binding sites to arise de novo in relatively few mutagenic steps. Furthermore, OmpR has the potential to respond to more than one environmental signal, giving it the potential to participate in regulon evolution from the standpoint of regulatory signal reception. Its activity is under allosteric control through phosphorylation by the EnvZ sensor kinase and the OmpR/EnvZ regulatory cascade is an important component of the osmotic stress response system of E. coli. However, OmpR is also sensitive to allosteric effects acting through its DNA target: it binds best to DNA that has adopted a relaxed topology, both in vivo and in vitro [3] and DNA topology can be modulated by a variety of environmental stressors [4]–[8].
The OmpR/EnvZ two-component system consists of a sensor kinase (EnvZ) located in the cytoplasmic membrane and a response regulator DNA binding protein (OmpR) located in the cytoplasm [9], [10]. The EnvZ protein has two trans-membrane helices, a periplasmic domain and a cytoplasmic domain that undergoes auto-phosphorylation at His-243 [11]. Environmental signal transduction involves phosphorylation of OmpR on Asp-55 by EnvZ [12], [13]. In E. coli, this regulatory system has been shown to transmit an osmotic stress signal and among the OmpR targets are the ompF and ompC genes that encode major porin proteins located in the outer membrane [14]–[16]. Recent evidence shows EnvZ senses changes in osmolarity through its cytoplasmic domain [17].
Mammalian infection by S. Typhimurium and E. coli exposes the bacteria to acid stress as they transit the stomach. S. Typhimurium must also endure acidification within the Salmonella-containing vacuole of the host macrophage [18], [19]. In addition to a reduction in pH, the harsh vacuolar environment also exposes the bacterium to Mg2+ starvation and toxic defensin peptides [20]. Both species employ general resistance mechanisms that allow them to tolerate external pH values outside the preferred cytoplasmic range. Despite being very closely related at the genetic level, E. coli is much more acid-tolerant than S. Typhimurium. It can survive pH 2 for several hours whereas S. Typhimurium dies rapidly under these conditions [21], [22].
In S. Typhimurium, OmpR plays an important role in infection and ompR knockout mutants are attenuated for virulence in mice [23]. OmpR regulates the transcription of a number of horizontally acquired genes that are not present in E. coli and are key to S. Typhimurium virulence [24], [25]. These include the hilC and hilD regulatory genes in the SPI-1 pathogenicity island [3], a 40-kb genetic element that encodes a type III secretion system and effector proteins required for invasion of mammalian cells [26], [27]. OmpR also regulates the transcription of the ssrA and ssrB genes of the 40-kb SPI-2 pathogenicity island [3], [28], [29]. These genes encode a two-component system consisting of the sensor kinase (SsrA) and cognate DNA binding protein (SsrB) that regulates the expression of a type III secretion/effector protein system required for intracellular survival and replication of S. Typhimurium inside the acidified vacoular compartment [30].
Adaptation to the vacuole involves a complicated process of transcription regulation and this process is modulated by DNA relaxation [31]. Recently, DNA relaxation was shown to enhance the binding of the OmpR protein at specific target genes in SPI-1 (hilC, hilD), SPI-2 (ssrA) and the core genome (ompR) in vivo and in vitro [3]. Previous work has shown that DNA relaxation occurs in S. Typhimurium when the bacterium is exposed to acid stress in vitro [4] and that transcription of the ompR gene is stimulated by DNA relaxation [3], [32], [33]. These observations led us to hypothesize that exposure of S. Typhimurium to low pH might result in elevated levels of active OmpR protein and enhanced binding to its targets throughout the genome. We were also interested to learn if a similar mechanism is used by the closely related bacterium E. coli to regulate its OmpR regulon, not least because the two OmpR proteins in these bacterial species have identical amino acid sequences [34, this study]. Our findings show that OmpR expression is regulated differently in S. Typhimurium and E. coli and that there are very large differences in the composition of the OmpR regulons in these bacterial species. We find that OmpR shows little DNA sequence specificity in its binding sites and propose that this makes OmpR particularly useful for bringing horizontally acquired genes into an established regulatory circuit encoded by the core genome.
Results and Discussion
ompR gene expression is differentially sensitive to pH in S. Typhimurium and E. coli
Because the pH-responsiveness of the S. Typhimurium ompR promoter does not fit with the E. coli osmosensing paradigm, the expression of the orthologous ompR envZ operons of S. Typhimurium and E. coli were compared for pH sensitivity. We used quantitative PCR to measure ompR transcript levels in both S. Typhimurium (SL1344) and E. coli (CSH50) after 90 min at pH 7 or pH 4.5. As expected, ompR transcript levels in S. Typhimurium were ∼2.7-fold higher at pH 4.5 than at pH 7 (Figure 1A: S. Typhimurium). Conversely, the mean level of ompR gene expression in E. coli was equal at pH 4.5 and at pH 7 (Figure 1A; E. coli). OmpR protein levels were measured by western blotting in S. Typhimurium strain SL1344 and E. coli strain CSH50 that each expressed OmpR with a 3xFLAG epitope fused to its C-terminal domain; the fusion genes were specially engineered to preserve expression and function of the overlapping envZ genes. OmpR protein was expressed to a higher level in S. Typhimurium at pH 4.5 than at pH 7 whereas its level of expression was constant at pH 4.5 and pH 7 in E. coli (Figure 1B). Thus, both ompR mRNA and OmpR protein levels were elevated in S. Typhimurium in response to acid pH but neither responded to acid pH in E. coli. We sought to discover the molecular mechanism responsible for this difference.
Fig. 1. The promoter region is responsible for the pH sensitivity of ompR in S. Typhimurium. 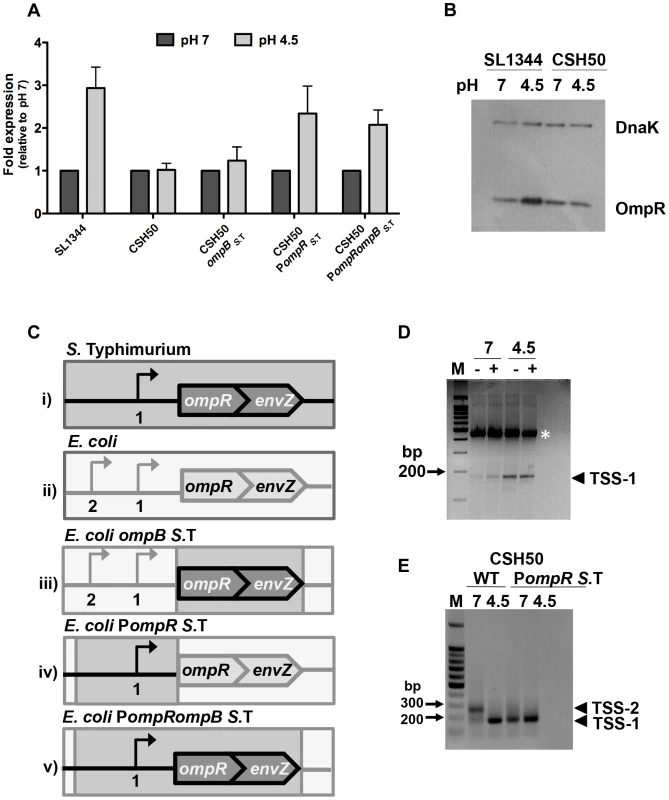
(A) Quantitative PCR measurements of ompR transcript levels in S. Typhimurium (SL1344) and E. coli (CSH50) and constructs with exchanged ompR regulatory regions (see C) at pH 7 and pH 4.5. Mean (N≥3) values are reported and the error bars represent the standard deviation of the mean. (B) OmpR protein levels in S. Typhimurium (SL1344 ompR::3xFLAG) and E. coli (CSH50 ompR::3xFLAG) at pH 7 and pH 4.5. Anti-FLAG antibody was used to detect the FLAG epitope and DnaK was used as a loading control. (C) Diagrams illustrating the constructs (i–v) used in the study. Bent arrows denote transcription start sites (TSSs) The wild type ompB locus in S. Typhimurium (i). The wild type ompB locus in E. coli (ii). In E. coli ompBS. T. the native E. coli ompR and envZ genes are replaced by the corresponding ompR/envZ genes from S. Typhimurium (dark grey). The native E. coli ompR promoter remains (light grey) (iii). In E. coli PompR S.T. the ompR promoter in E. coli is replaced by the ompR promoter from S. Typhimurium (dark grey). The native E. coli ompB locus is retained (light grey) (iv). In E. coli PompR ompBS. T. both the ompR promoter and ompR/envZ genes in E. coli are replaced by the ompR promoter and ompR/envZ genes from S. Typhimurium (iv). (D) Gel electrophoresis of 5′ RACE-amplified ompR cDNA ends analysed on a 3% agarose gel. RNA was extracted after 90 min at pH 7 or pH 4.5. Samples contained either tobacco acid pyrophosphatase (TAP)-treated (+) or untreated (−) RNA, generating 5′ monophosphate for ligation of the RNA-linker (A4; see Table S2) [87]. Ligation of the linker was more efficient in the TAP treated sample. RNA was reverse-transcribed into cDNA and PCR was performed on each cDNA sample using primers RACE_ompR and JVO-0367; see Table S2. Arrowhead denotes the PCR product found to be TSS-1. M, 100-bp ladder. The white asterisk denotes a non-specific PCR product that was sequenced and identified as a 23S ribosomal RNA product using BLAST (Basic Local Alignment Search Tool). (E) Gel electrophoresis of 5′ RACE-amplified ompR cDNA ends analysed on a 1% agarose gel. RNA was extracted from CSH50 and CSH50 PompRS.T after 90 min at pH 7 or pH 4.5. Arrowheads TSS-1 and TSS-2 denote the PCR products. M, 100-bp ladder. The locations of the transcription start sites are shown in Figure S1. pH sensitivity resides in the ompB promoter region
The ompR and envZ genes constitute the bicistronic ompB operon (Figure 1C) [15]. Transcription of ompB is driven from the promoter region upstream of ompR. An acid-inducible promoter and associated transcription start site (TSS-1) has been identified in this region in S. Typhimurium (Figure S1) [33]. Following acid shock, the ompR gene is positively autoregulated through a mechanism that requires the sensor kinase EnvZ [33]. We reasoned that species-specific differences in one or more of these factors (ompB promoter region, EnvZ protein structure and function) might account for the distinct expression patterns of the ompR genes in S. Typhimurium and E. coli during acid stress.
The nucleotide sequences of the regulatory regions of ompB in S. Typhimurium and E. coli are 88% identical (Figure S1). In addition to differences in nucleotide sequence, the species also differ in the location of ompR transcription start sites that have been characterised (Figure S1). The amino acid sequences of the OmpR proteins in the two species are completely identical while those of the EnvZ sensor kinases show a 5% difference in amino acid sequence. Thus the ompB regulatory region and the envZ gene were analysed as potential contributors to differential expression of ompR. This was done by making a series of transgenic strains in which the regulatory regions and the ompR envZ open reading frames from S. Typhimurium were exchanged individually and then together for their E. coli counterparts (Figure 1C). Quantitative PCR was used to measure ompR transcript levels after 90 min growth at pH 7 or pH 4.5.
E. coli ompBS.T has had the open reading frames of its own copies of the ompR and envZ genes replaced by the corresponding genes from S. Typhimurium; it retains the E. coli ompB promoter region. Transcription of ompR in this strain was similar at both pH 4.5 and 7, i.e. it retained the previously observed E. coli pattern of ompR expression (Figure 1A; E. coli ompBS.T) implicating the promoter region in conferring pH sensitivity. E. coli PompRS. T. has had the promoter region of its ompB operon replaced with the equivalent region from S. Typhimurium; it retains its E. coli ompR and envZ open reading frames. In this strain the ompR gene showed enhanced expression at pH 4.5 (Figure 1A; E. coli PompRS. T.), showing that it has acquired the S. Typhimurium pattern of ompR gene expression in response to acid stress. In E. coli PompR S. T. ompBS. T. the entire S. Typhimurium ompB locus and its regulatory region has replaced the equivalent region of the E. coli genome. This strain expresses ompR in the acid-inducible pattern that is characteristic of S. Typhimurium (Figure 1A; E. coli PompRS. T. ompB S. T.). Transcription of the envZ gene, which is 3′ to ompR in the bicistronic ompB operon, showed a similar pH response to that of ompR (Figure S2A). EnvZ protein levels did not show a marked response to acid pH, either in SL1344 itself or when the S. Typhimurium ompB operon and its promoter were transferred to the E. coli chromosome (Figure S2B) indicating that while envZ transcription shares the pH sensitivity of ompR, its posttranscriptional response to pH is distinct.
These data showed that the S. Typhimurium ompR promoter region determines the low-pH sensitivity that is a characteristic of the S. Typhimurium ompR gene. Further, pH sensitivity can be conferred upon E. coli even in the presence of endogenous E. coli EnvZ, which is tuned to osmotic sensing. Thus the 5% difference in amino acid sequence between the E. coli and S. Typhimurium EnvZ proteins is not the basis of the differential sensitivity of E. coli and S. Typhimurium ompR expression to pH.
The ompR regulatory region has pH-sensitive transcription start sites in both species
Previous studies have mapped ompR transcription start sites in both S. Typhimurium and E. coli [33], [35]–[38]. In this study we focussed on ompR acid-inducible TSSs, examining the possibility that S. Typhimurium and E. coli each utilises a unique ompR TSS profile at different pH values thus explaining the pH sensitivity of ompR in S. Typhimurium. The transcription architectures of the E. coli and S. Typhimurium ompR genes were compared at pH 4.5 and pH 7 using 5′ RACE (Figure 1D, E). The previously described acid-inducible transcription start site (TSS-1) in S. Typhimurium was confirmed; the transcript initiating at this position was detectable at both pH values but was more abundant at acidic pH (Figure 1D). In E. coli strain CSH50 two transcripts were detected at pH 7, with one being much more abundant than the other; this pattern of predominance was reversed at pH 4.5 (Figure 1E). The predominant transcript at pH 7 (henceforth called TSS-2) mapped to the same location as that identified in E. coli by Tsui et al. [36]. The second and previously unidentified E. coli transcript (TSS-1) was present at pH 7 and pH 4.5. We mapped TSS-1 in E. coli to a location that was identical to that of TSS-1 in S. Typhimurium. Therefore, although overall ompR transcript levels in E. coli appear to be pH insensitive (Figure 1A) there is a clear shift in TSS utilisation and the output of TSS-1-originating transcription in both species under acid conditions (Figure 1D, E). We next mapped TSSs in the E. coli derivative harbouring the S. Typhimurium ompR regulatory region (Figure 1E; E. coli PompRS. T.). We found that the S. Typhimurium ompR promoter maintained its native transcriptional start site profile in an E. coli background and that only a single transcript from TSS-1 was detected at both pH values. Thus, in response to changes in pH, a re-prioritising of TSS usage occurs in E. coli. This results in a distinct species-specific TSS profile at ompR and directly influences the level of OmpR protein produced in either species (Figure 1B). We next wished to investigate what effect this differential response to pH may have on the OmpR regulon in both organisms.
Genome-wide binding of OmpR in S. Typhimurium and E. coli
We investigated the impact of acid pH on genome-wide binding of OmpR to assess the physiological consequences of differential ompR gene regulation in each species. Chromatin immunoprecipitation-on-chip (ChIP-on-chip) was used to ascertain the in vivo distribution of OmpR on the chromosomes of S. Typhimurium and E. coli at pH 4.5 and pH 7 using S. Typhimurium strain SL1344 ompR::3xFLAG and E. coli strain CSH50 ompR::3xFLAG, respectively. We have previously shown the OmpR protein with a FLAG tag at the C-terminus is functional [3]. To generate a ‘snapshot’ of OmpR binding, DNA-protein interactions were cross-linked after 90 min at pH 4.5 or pH 7 and OmpR-protein-DNA complexes were immunoprecipitated using an anti-FLAG antibody. As a control for possible noise produced by non-specific immunoprecipitation during ChIP-chip, we performed a control ‘mock’ ChIP-chip experiments under identical culture conditions. We used normal mouse IgG antibody during the immunoprecipitation step, the log2 ratio was then subtracted from the experimental log2 ratio. The 100% identity of the amino acid sequences of the OmpR proteins in the two species allowed direct comparisons to be made of OmpR binding patterns in these two organisms. In considering our data we kept in mind the fact that binding of OmpR to a particular gene was not in itself evidence that OmpR regulates the expression of that gene.
OmpR binding was increased at low pH throughout the chromosome of S. Typhimurium as indicated by a larger number of DNA binding peaks in the pH 4.5 samples compared to the corresponding samples from E. coli (Figure 2A, B). The ChIPOTle ChIP data analysis program was used to identify high-confidence OmpR binding regions in each species. In S. Typhimurium, ChIPOTle identified 85 significantly enriched OmpR binding regions at pH 7 and this increased to 225 peaks at pH 4.5 (Figure 2C). Of these OmpR-bound regions, 61 were detected in both pH 7 and pH 4.5 conditions (Figure 2C; pH insensitive). In E. coli, ChIPOTle analysis identified 67 OmpR binding regions at pH 7, and 49 binding regions at pH 4.5; 28 OmpR-targets were bound at both pH 7 and pH 4.5 (Figure 2C; pH insensitive). The results showed that OmpR bound more targets in S. Typhimurium at acidic pH compared to neutral pH (Figure 2C) whereas in E. coli there was a small decrease in the number of targets bound by OmpR at acidic pH. Perhaps the greater abundance of OmpR at pH 4.5 in S. Typhimurium compared to E. coli (Figure 1C) accounts, at least in part, for these differences in protein binding patterns. We detected binding of OmpR to previously identified OmpR-regulated genes, validating our experimental approach. These genes were: ompF, ompC [34], [37]–[42], tppB [43], [44], csgD [45]–[47], ompR [32], [33], flhD [48], ssrA [3], [28], [29], hilC (3] omrA [49], omrB [49], hyaB2 [49] and hyaA2 [50].
Fig. 2. Genome-wide distribution of OmpR in E. coli and S. Typhimurium. 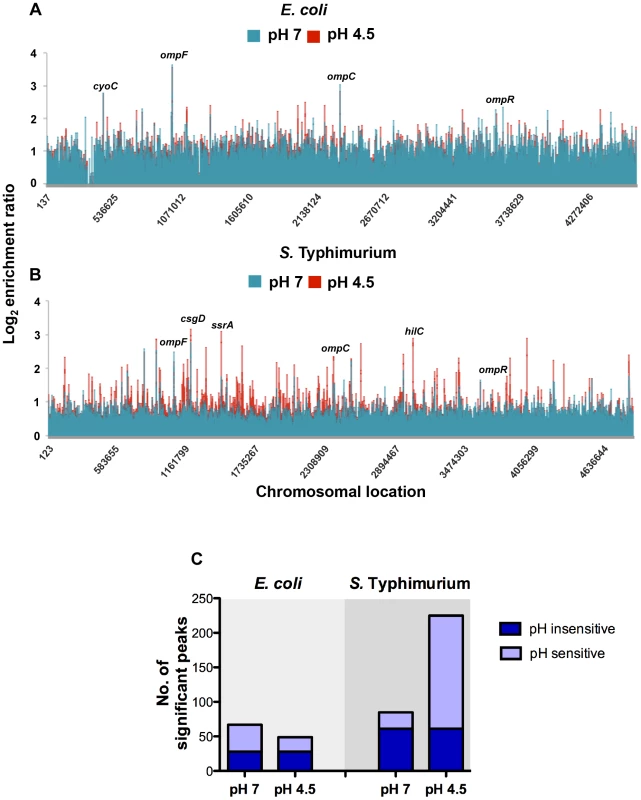
Results from genome-wide analysis of OmpR binding in E. coli (A) and S. Typhimurium (B) at pH 7 (blue) and pH 4.5 (red). The log2 enrichment ratio (ChIP/input) is plotted on the y-axis and the locations of the probes are shown on the x-axis. (C) Overlap between OmpR binding sites at pH 7 and pH 4.5 in E. coli and S. Typhimurium. The histogram illustrates the number of significant OmpR binding peaks bound at pH 7 and pH 4.5 in E. coli and S. Typhimurium. The number of targets occupied at pH 7 and pH 4.5 is shown in dark blue (pH sensitive). The number of pH-specific targets occupied at pH 7 or pH 4.5 is shown in light blue (pH insensitive). In addition to previously characterised OmpR targets, we found OmpR bound at genes associated with pH homeostasis in both organisms. In E. coli OmpR bound the cadBA operon (Figure S5C) that encodes an acid stress response system responsible for consuming intracellular protons by decarboxylation of a specific amino acid substrate [51]. In S. Typhimurium OmpR was bound at the atpI gene encoding a component of the F1Fo ATP synthase. This enzyme promotes ATP-dependent extrusion of protons and ATP synthesis under acidic conditions [52]. We also identified OmpR binding at additional ATPases (e.g. STM1635, STM0723 and ybiT) and to cation transporters (e.g. ybaL, STM0765 and STM3116). OmpR binding to acid stress genes in both species indicates that OmpR performs a shared function of regulating pH homeostasis. Surprisingly, we found that overall there was only a small number of common OmpR targets between these organisms.
The S. Typhimurium and E. coli OmpR regulons have limited overlap
Only 15 OmpR targets were found to be in common between both organisms (Table S3), indicating significant divergence in the E. coli and S. Typhimurium OmpR regulons. In both species, OmpR binds its own promoter, as well as the well characterised E. coli targets ompF and ompC. In the context of differences in the OmpR regulons of E. coli and Salmonella, it is interesting to note that ompC expression is differentially affected by osmolarity in E. coli and S. Typhi [34]. Although OmpR had very few common target genes in S. Typhimurium and E. coli, those it did bind shared the common theme of contributing to cell envelope composition. These include the small RNA gene rseX which downregulates the expression of RNAs encoding the outer membrane porins OmpC and OmpA [53]. Other cross-species OmpR targets included factors involved in biofilm formation, motility, chemotaxis and genes involved in fimbrial production such as csgD, the regulator of curli synthesis and assembly [45], [46]. OmpR bound the flhDC operon that encodes the master flagellar regulator in both species. OmpR has been shown to repress expression of flhDC in E. coli [54], [48]. We noted OmpR binding was elevated at the lrhA-alaA intergenic region. lrhA encodes the LysR-like protein LrhA which is a global regulator of flagellar, motility and chemotaxis genes [55] including flhDC. LrhA also regulates expression of type 1 fimbriae that promote attachment to host cells and biofilm formation on abiotic surfaces [56]. OmpR binding was also increased at the ycb operon (an orthologue of bcfA in S. Typhimurium) that encodes cryptic fimbriae for attachment to abiotic surfaces [57].
Interestingly, the pattern of OmpR binding was not conserved at all members within the shared regulon. For example OmpR binding at pH 4.5 was increased at micF in S. Typhimurium but not in E. coli (Figure S3A). OmpR binding was similar and pH insensitive at the ompF gene in both species (Figure S2, B). OmpR binding at rseX was more abundant at pH 4.5 in E. coli but not in S. Typhimurium (Figure S3C) suggesting that although total OmpR protein levels in E. coli are insensitive to pH OmpR levels at target genes are influenced by pH. These results suggest that common gene targets may differ in the detail of their OmpR mediated regulation between S. Typhimurium and E. coli. The core OmpR regulon includes genes encoding surface expressed appendages such as outer membrane porins, curli fibres, motility factors and fimbriae. These targets are consistent with a generalized role for OmpR in the regulation of cell surface composition in both organisms. The retention of these targets in both species is not surprising because the bacterial envelope is the first barrier against acidic pH and OmpR is known to regulate expression of the outer membrane porins in response to shifts in external pH such that expression of the smaller-channel porin OmpC is favoured over OmpF [58]. Following from this any species-specific OmpR targets absorbed into the OmpR regulon may represent examples of regulon evolution enabling niche adaptation.
S. Typhimurium-specific OmpR targets and DNA topology
During infection orally-ingested S. Typhimurium experience acidic pH in both the host stomach and within the acidified-Salmonella-containing vacuole of the host macrophage [18]–[20]. Acidic pH is both a threat to S. Typhimurium's survival and an environmental cue directing S. Typhimurium to upregulate expression of virulence genes [59]. We found OmpR targeted some of these virulence genes located within the horizontally acquired pathogenicity islands -1 and -2 (SPI-1 and SPI-2) and that binding there was pH sensitive. SPI-1 and -2 are required for infection and enable S. Typhimurium to invade host epithelium and survival inside host macrophage respectively [24]. Crucially, these islands are absent from E. coli [25], thus these OmpR targets were acquired by the OmpR regulon after lateral DNA transfer. In keeping with findings from previous studies we found OmpR binding to be increased within SPI-1 and SPI-2 [3], [28], [29]. In addition we found OmpR bound at SPI effector genes located outside these islands providing further evidence of regulon evolution (discussed below).
SPI-1 expression is triggered by multiple environmental signals and is regulated by the SPI-1-encoded HilC and HilD proteins. Together these activate expression of HilA and in turn this activates expression of SPI-1 structural genes encoding a type III secretion system (T3SS) [60], [61]. Expression of the SPI-1 genes also requires core-genome-encoded regulators such as OmpR, Fis and H-NS [62]–[65]. OmpR acts as a positive regulator of hilC and a negative regulator of hilD expression [3]. Our ChIP analysis revealed OmpR binding to be significantly increased upstream of the hilC regulatory gene (Figure 3A, inset) and the level of this binding was higher still in acidic conditions. In addition, OmpR binding was elevated in the prgH-hilD intergenic region and within the prgHIJK operon which encodes structural components of the SPI-1 needle complex that delivers bacterial effectors to host cell [66].
Fig. 3. The effect of pH and DNA relaxation on OmpR binding across Salmonella pathogenicity islands 1 and 2 (SPI-1 and SPI-2). 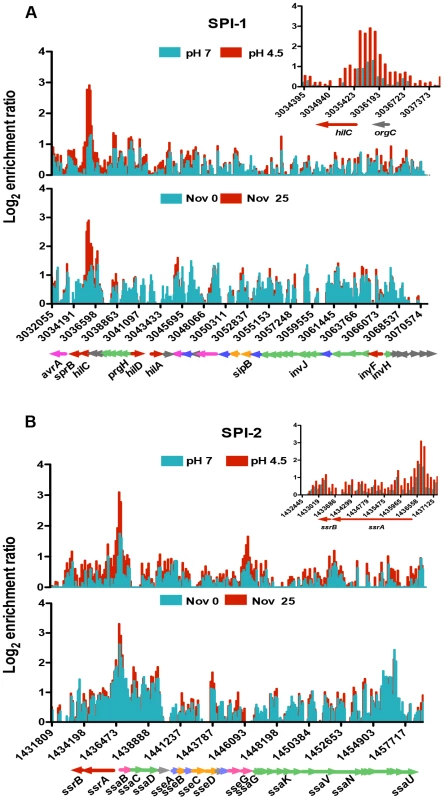
(A) Log2 enrichment ratio (ChIP/input) is plotted on the y-axis and the locations of the probes are shown on the x-axis for SPI-1. Coloured arrows below the x-axis show the locations of SPI-1 genes determined using Jbrowse [38]. The inset shows the OmpR binding pattern at the hilC regulatory gene of SPI-1 in more detail. (B) Log2 enrichment ratio (ChIP/input) is plotted on the y-axis and location of the probes are shown on the x-axis for SPI-2. Genes colour is based on their function regulatory genes are red, effector genes are pink, structural genes are green, translocon genes are orange, chaperone genes are blue and genes of unknown function are grey. The inset shows the OmpR binding pattern at the ssrA ssrB regulatory genes of SPI-2 in more detail. In (A) and (B), the top histogram shows OmpR binding at pH 7 (blue) and pH 4.5 (red) and the bottom histogram shows OmpR binding without (blue) and with (red) novobiocin treatment (25 µg ml−1). Genes are coloured to indicate their function: regulatory genes are red, effector genes are pink, structural genes are green, translocon genes are orange, chaperone genes are blue and genes of unknown function are grey. SPI-2 gene expression is dependent on the SsrA/SsrB two-component system; where the SsrB response regulator activates SPI-2 structural genes encoding a T3SS [67], [68]. OmpR binds to the ssrA promoter and positively regulates its transcription [3], [28], [29]. We found that within SPI-2, OmpR was most significantly enriched in the intergenic region of the divergently transcribed ssrA and ssaB genes. OmpR binding there increased at low pH (Figure 3B, inset) coinciding with the 6-fold OmpR-dependent increase in ssrA transcript levels detected at pH 4.5 (Figure S4). OmpR also bound the sseA promoter that controls expression of the genes within the effector/chaperone operon of SPI-2. Binding was also detected within the coding sequence of sseG and ssaV the latter of which forms an essential structural component of the T3SS. [69]. The effect of OmpR binding within the coding sequences of these genes is unknown.
We found OmpR bound at both SPI-1 and -2 secreted effector protein genes located outside these pathogenicity islands. These included the SPI-1 effector genes sopE2, sopA and gtgE and the SPI-2 effector genes sseK2 gogB, pipB2, sopD2 and gtgE [20]. Thus, the relationship between OmpR and the pathogenicity islands is complex and OmpR is likely to regulate virulence in S. Typhimurium at levels beyond its influence at the SPI master regulators ssrA/ssrB, hilC and hilD. In keeping with this possibility, we detected elevated OmpR binding in the laterally-acquired pathogenicity island SPI-4 (Figure S5A) that encodes a type 1 secretion system for transporting the SiiE large adhesin to the cell surface (Figure S5B) [70]. This non-fimbrial adhesin is required for attachment and invasion of polarized epithelial cells during the intestinal phase of infection [71]. OmpR binds directly to SPI-4 within the coding region of the siiABCDE genes; an effect on gene expression here would provide a further example of the expansion and evolution of the OmpR regulon to control pathogenicity gene expression.
Whilst we observed an increase in OmpR protein levels in S. Typhimurium at low pH we also considered the possibility that DNA topology influenced OmpR binding to its chromosomal targets. Two lines of evidence supported the hypothesis that OmpR binding to its DNA targets in acid-stressed cells is modulated by the topology of the target DNA. First, acid treatment of S. Typhimurium results in relaxation of plasmid DNA supercoiling [4]. Second, we found that OmpR binding to the hilC, hilD, ompR, and ssrA promoters was enhanced by relaxation of DNA supercoiling [3].
We investigated the possibility that global relaxation of DNA supercoiling caused by acidic pH contributes to the dramatic increase in OmpR regulon size at pH 4.5 in S. Typhimurium. First we measured DNA supercoiling in S. Typhimurium after 90 min at pH 7 or pH 4.5 and found that reporter plasmid DNA became more relaxed at acid pH (Figure 4) in agreement with previous findings [4]. Interestingly, we found DNA in E. coli became more supercoiled at low pH, this is quite clear in the plasmid dimers (Figure 4). This is important as we have previously shown DNA in both species can be artificially relaxed using the drug novobiocin [72]. Despite this, we find acidic pH relaxes DNA topology in S. Typhimurium but not in E. coli.
Fig. 4. The effect of pH on DNA supercoiling in Salmonella Typhimurium strain SL1344 (top) and E. coli strain CSH50 (bottom). 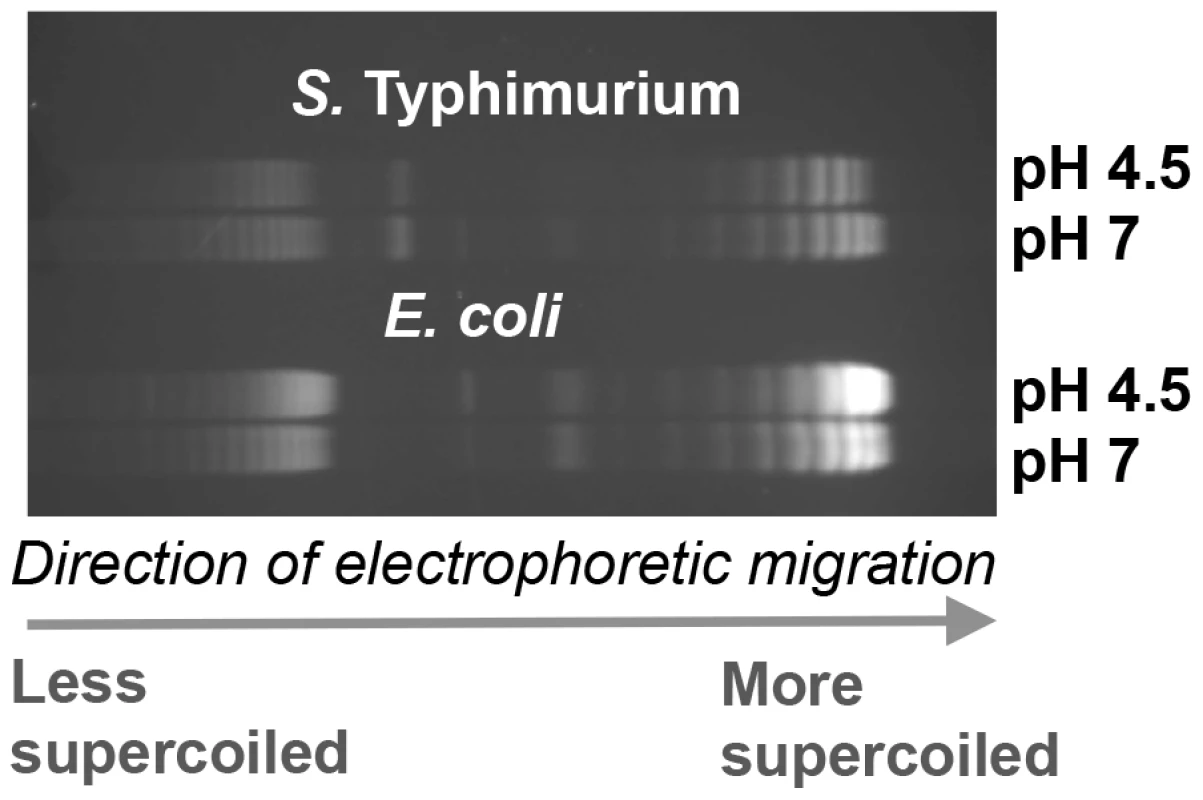
Electrophoretic mobility of plasmid pUC18 topoisomers in agarose gel containing chloroquine at 2.5 µg ml−1. At this concentration of chloroquine more supercoiled topoisomers run further in the gel. Cultures were pelleted after 90 min at pH 7 or pH 4.5 E-minimal medium and plasmids were extracted immediately. Gel image is representative of three independent experiments. We mapped OmpR binding using ChIP-on-chip in S. Typhimurium cells treated with a sub-inhibitory concentration of the DNA-gyrase-inhibiting drug novobiocin during exponential growth. DNA becomes relaxed in these cells because novobiocin blocks access to the ATP binding pocket of the GyrB subunit of DNA gyrase, preventing that copy of the gyrase enzyme from performing a further round of negative DNA supercoiling [73]. Strikingly, the OmpR ChIP-on-chip profiles at acidic pH and those obtained after novobiocin treatment showed similar increases in OmpR binding at several loci (Figure 3A, B); OmpR binding was focused at the hilC promoter in SPI-1 and at the ssrA promoter within SPI-2. Thus, acidic pH and relaxation of DNA supercoiling due to DNA gyrase inhibition created very similar OmpR binding profiles across the major pathogenicity islands of S. Typhimurium.
OmpR and the PhoQ/PhoP regulon of S. Typhimurium
At low pH, OmpR binding was elevated at several genes that belong to the PhoQ/PhoP two-component system regulon (Table S4). In S. Typhimurium, the PhoP DNA binding protein regulates genes involved in virulence, magnesium transport, intramacrophage survival and resistance to antimicrobial peptides, with PhoP activity being dependent on phosphorylation by the membrane-associated sensor kinase PhoQ [74], [75]. Our ChIP-chip data showed increased binding of OmpR at the promoter region of mgtC, a PhoP-regulated gene that encodes an inner membrane protein involved in intramacrophage survival (Figure 5A) [76]. Electrophoretic mobility shift analysis (EMSA) confirmed that OmpR binds specifically to the mgtC promoter (Figure 5B). A DNA probe encompassing the ompC promoter which is a well-characterized OmpR target was included as a positive control. A DNA probe encompassing a portion of the kanamycin resistance gene which contains no known OmpR binding sites was included as a negative control. OmpR bound the ompC promoter and did not bind the kanamycin gene as expected (Figure 5B). Using qRT-PCR we found that mgtC expression was induced at pH 4.5 and required OmpR (Figure 5C). Thus, OmpR is an important activator of mgtC expression.
Fig. 5. OmpR regulates PhoP-regulated genes. 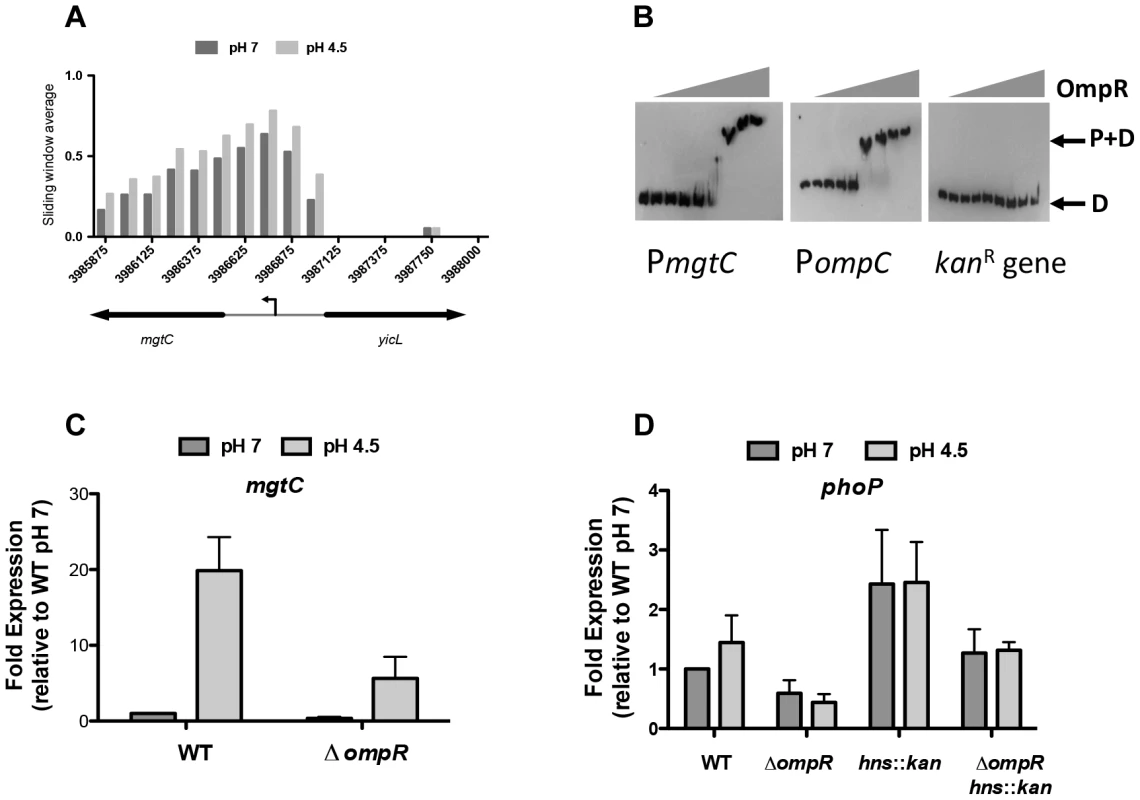
(A) OmpR binding at mgtC at pH 7 and pH 4.5. Sliding window average of log2 enrichment as calculated by ChIPOTle [88] is shown on the y-axis. (B) EMSA analysis showing OmpR binding to the mgtC promoter as well as the ompC promoter (positive control) and the kanR (kanamycin resistance gene; negative control). D, free DNA probe; P+D, protein + DNA complex. OmpR concentrations used were 0, 0.02, 0.04,.078,.16, 0.31, 0.63, 1.25, 2.5 µM. A representative gel image is shown from three independent replicates (C) Quantitative PCR measurements of mgtC transcript levels at pH 7 and pH 4.5 in the wild type strain and an ompR knockout mutant. (D) Quantitative PCR measurements of phoP transcript levels at pH 7 and pH 4.5 in the wild type strain and an ompR knockout mutant and in the ompR hns::kan double mutant. N≥3; standard deviations from the mean are shown as error bars. OmpR binding was also elevated at low pH at the PhoP-activated genes pagK and pagO and at the PhoP-repressed operon prgHIJK, as indicated earlier in the discussion of SPI-1. OmpR also targeted the PhoP-dependent mig14 gene that encodes a protein mediating resistance to the cathelicidin-related antimicrobial peptide CRAMP [77] (Table S4.).
In addition to overlap with the PhoP regulon, OmpR was found to bind and regulate the phoP promoter. Genetic evidence indicated that OmpR was required for fine-tuning phoP expression both in the presence and absence of H-NS (Figure 5D). However, ChIP data indicated that the level of OmpR binding at phoP was not significantly above that seen in the mock immunoprecipitated control. A potential OmpR binding site that was identified bioinformatically at the phoP promoter was mutated by site-directed mutagenesis (Figure S6). The result was a mild reduction in the ability of OmpR to bind here; approximately 50% of the wild-type PphoP DNA shifted at 2 µM OmpR whereas >2 µM OmpR protein was required to shift ∼50% of the mutant OmpR-I- PphoP. These results suggest that OmpR positively regulates phoP via a mechanism that remains to be elucidated.
The PhoQ/PhoP and EnvZ/OmpR two-component systems are found throughout the Enterobacteriaceae and the results presented here demonstrate an overlap between their regulons in S. Typhimurium. The genes listed in Table S4 are absent from E. coli, with the exception of slyB and phoP. The slyB and phoP genes are PhoP-regulated in E. coli, yet we did not detect OmpR binding at these genes. This observation indicates that the integration of the OmpR and PhoP regulatory circuits may have occurred after divergence of S. Typhimurium and E. coli. Many of the Salmonella genes in Table S4 are associated with virulence and their expression is governed by PhoP either directly or indirectly, this may enable these shared targets respond to multiple signals sensed by either the OmpR/EnvZ and PhoP/PhoQ two-component systems.
OmpR binding site motif analysis
OmpR demonstrates only moderate sequence specificity for binding sites in DNA [2]; consequently, the biochemically-characterized OmpR binding sites in Regulon DB do not reveal a good consensus for the binding site [78]. Using MEME we conducted an unbiased motif search of the DNA sequences from our ChIP-chip datasets that were bound by OmpR [79]. We tested for the presence of any sequence motif that may be enriched in DNA bound by OmpR, but no significant motif was detected in the E. coli or S. Typhimurium ChIP datasets. We next conducted a biased search using a position weight matrix built from characterized E. coli and S. Typhimurium OmpR binding sites (listed in Table S5) that contain elements of the GTnTCA motif to which OmpR binds with high affinity [2]. Searches were performed using the RSAT matrix-scan program [80], and putative OmpR sites were identified in all our ChIP-chip datasets (listed in Table S6). To control for random matches to the weight matrix, we analysed in parallel replicate datasets composed of random DNA sequence generated by the RSAT random-sequence program and matched to the size of the experimental ChIP datasets. In all cases, the OmpR-bound sequences from the ChIP-chip datasets contained significantly more putative OmpR sites than the random DNA sequences (Figure 6) consistent with the presence of specific OmpR binding sites in the experimental ChIP datasets. Perhaps it is unsurprising that no OmpR logo was identified in our ChIP datasets by unbiased motif searching given the low DNA sequence-specificity displayed by the OmpR protein for binding. This may also explain the observation of numerous weak peaks of OmpR binding detected throughout the genomes of both S. Typhimurium and E. coli (Figure 2A, B). The lack of an OmpR binding site motif and genome-wide binding may allow OmpR to act as a global regulator of transcription and to incorporate newly-acquired genes into its regulon, as observed with SPI-1 and SPI-2. OmpR binding sites that contain the GTnTCA motif may represent a subclass of high-specificity OmpR targets that nucleate formation of higher-order nucleoprotein structures.
Fig. 6. The number of high-scoring OmpR sites identified within the ChIP datasets. 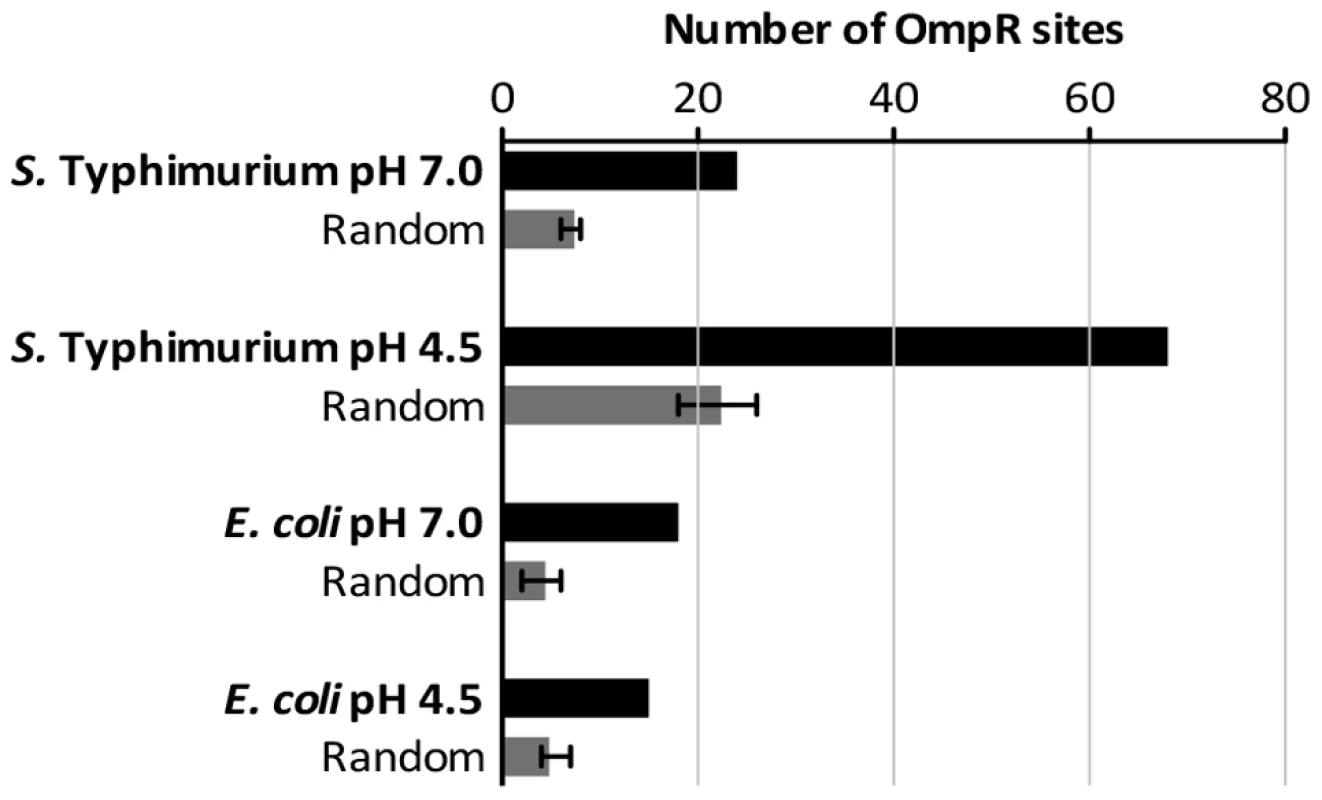
Sequences of the binding sites are listed in Table S6. Random DNA datasets were generated as described in Materials and Methods. Perspective
The OmpR protein in S. Typhimurium binds to a much larger number of targets than does its identical orthologue in E. coli. It bound to the same gene in the two species in only 15 cases; these genes make up the core OmpR regulon and all contribute in some fashion to the composition of the cell surface. We found members of the species-specific regulons included genes involved in pathogenesis in S. Typhimurium and stress-management in E. coli (Figure 7). Although OmpR levels did not change in acid-treated E. coli, OmpR did appear to assume a pH-specific role in that species. For example, OmpR targeted the genes of the lysine/cadaverine decarboxylase system that is a part of the acid stress response system [51]. Such a pH-specific role for OmpR in E. coli is in agreement with recent findings that identified OmpR as a regulator of the acid stress response in E. coli [81].
Fig. 7. The OmpR regulons of S. Typhimurium and E. coli in acid pH. 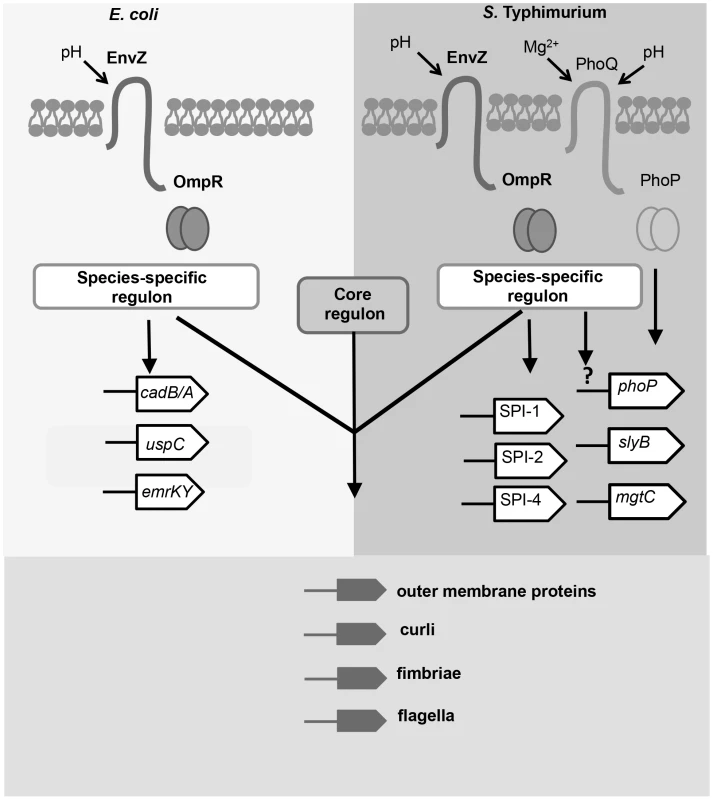
Members of the OmpR regulon in has evolved since the divergence of these closely-related species. OmpR (pairs of circles) binds to a species-specific regulon in Salmonella and E. coli; examples of these targets are shown. In E. coli, OmpR binds to genes involved in acid resistance e.g. cad operon [13] and general stress resistance e.g. the uspC [14] and emrK [15] genes. In S. Typhimurium, OmpR binds to pathogenicity islands SPI-1, -2, and -4, and to genes regulated by PhoP. OmpR positively regulates phoP expression by an unknown mechanism (denoted by the question mark). The genes identified as the core OmpR regulon (conserved targets; bound by OmpR in both species) encode surface-associated organelles and proteins. Modulation of the cell-surface composition may be an important function of the core OmpR regulon in response to acidic stress. The large OmpR regulon in S. Typhimurium includes many genes that have been acquired by horizontal gene transfer. In many cases, the same genes are targets of H-NS-mediated transcription silencing and OmpR is known to be an effective antagonist of H-NS silencing when accompanied by DNA relaxation [3]. OmpR and H-NS share a weak requirement for specific sequences for DNA binding, although in both cases high-affinity consensus sequences have been described [41], [64], [82]. Thus, these proteins are especially suitable for imposing dual control at promoters that have at least the DNA structural features if not the specific sites that these proteins require for binding. In the case of OmpR, relaxed DNA is a better target for binding than is supercoiled DNA, as has been shown in vitro and in vivo [3; this work]. In this context it is interesting to note that the mgtC gene is a target for acid-pH-dependent positive regulation by OmpR (Figure 5) and it encodes a protein that inhibits the activity of the F1Fo ATP synthase [75]. DNA gyrase requires ATP to supercoil DNA negatively so a reduction in ATP results in a general relaxation of cellular DNA [7], [8], [73], [83]. These observations suggest that a regulatory circuit exists in which the production of ATP is down-regulated in S. Typhimurium in low-pH growth conditions, resulting in up-regulation of OmpR expression and a concomitant enhancement of OmpR binding to its genomic targets, which is reinforced by the negative impact of MgtC on ATP synthesis. The operation of such a circuit is consistent with empirical data showing that DNA in S. Typhimurium becomes relaxed when the bacterium is in the low-pH environment of the macrophage vacuole [31].
Our findings demonstrate an allosteric role for DNA topology in the operation of the OmpR regulon in S. Typhimurium. OmpR interacts with DNA via both a helix-turn-helix (H-T-H) motif and a winged helix [2]. The H-T-H motif interacts with the major groove in DNA while the wing contacts the minor groove [2]. A+T-rich DNA sequences have a narrow minor groove whose width is adjustable by changes in DNA supercoiling [84]. This structural variation provides a mechanism for modulating the interaction of OmpR with a given DNA target that is additional to any allosteric control that operates at the level of the protein. Our finding that OmpR binds to many A+T-rich genes that have been acquired by HGT in S. Typhimurium is particularly interesting given that its interactions with those targets shows the greatest sensitivity to DNA topological change. The H-NS nucleoid-associated protein targets these genes too and silences their transcription, but H-NS binding seems to less affected by relaxation of the DNA target than is OmpR [3]. These two proteins seem to be particularly well matched for the purpose of imposing environmentally-responsive dual control on A+T-rich genes: H-NS binds and silences them while OmpR binds and antagonises H-NS-mediated repression but only when both the OmpR protein and its DNA target are appropriately primed by allosteric control. Newly acquired genes that meet the structural requirements for this type of dual control may be expected to make good candidates for membership of the OmpR regulon, contributing to the evolution of the bacterium.
Materials and Methods
Strains and growth media
Salmonella Typhimurium serovar Typhimurium strain SL1344, Escherichia coli K-12 strain CSH50 and isogenic derivatives used in this study are listed in Table S1. Cells were grown in E-minimal medium [85] supplemented with L-histidine (0.5 mM), L-proline (100 µg ml−1) and thiamine (1 µg ml−1) or LB broth at 37°C and 200 rpm. Antibiotics were used at the following final concentrations; chloramphenicol 20 µg ml−1; kanamycin 50 µg ml−1; carbenicillin 100 µg ml−1.
Culture conditions
To measure the effect of pH on ompR expression and OmpR binding cells were grown overnight in LB broth. The culture optical density (OD600) was equalized to 0.15 units. Cells were collected by centrifugation and washed with 1 ml EG-minimal medium pH 7. This was repeated twice more to remove residual nutrients from the LB broth. Cells were grown in 40 ml EG-minimal medium (pH 7) in a 250 ml flask to an OD600 ∼0.5–0.6 at 37°C and 200 rpm. The culture was then split into two 20 ml volumes and collected by centrifugation at room temperature. One cell pellet was resuspended in 1 ml EG-minimal medium at pH 7, the second pellet was resuspended in 1 ml EG-minimal medium at pH 4.5. Each suspension was added to a final volume of 20 ml pre-warmed EG-minimal medium of the same pH in a 250 ml flask and kept at 37°C and 200 rpm for 90 min before harvesting for analysis. To measure the effect of novobiocin treatment cells were treated as previously described [3]. Novobiocin was used at a final concentration of 25 µg ml−1 and cells treated for 40 min before harvesting for ChIP analysis.
Cloning and mutant construction
Detailed information on cloning and mutant construction can be found in S1 text.
Western blotting
Cells were normalized to 0.2 OD600 units and western blot analysis was performed as previously described [3]. Anti-FLAG (F3165, Sigma-Aldrich) was used at 1 in 10,000 and Anti-DnaK (1.0 mg ml−1, Enzo life sciences) was used at 1 in 200,000. Goat anti-mouse IgG horseradish peroxidase conjugate (Millipore) was used at 1 in 10,000. Autoradiography was used to visualize chemiluminescent emissions derived from horseradish peroxidase oxidation of luminol, blots were developed using Hyperfilm MP (Amersham Biosciences) film.
Electrophoretic mobility shift assays
These were performed using purified OmpR (D55E) protein as previously described [3] with the following exceptions; biotinylated primers were used to PCR amplify DNA probes (mgtC; PmgtC_F_EM and PmgtC_R_EM, ompC; PompC_EM_F and PompC_EM_R, kan gene; kan_bio_F and kan_bio_R, phoP; Bio_PphoP_F and Bio_PphoP_R listed in Table S2). After running the gels as described previously the gels were transferred to a Biodyne B membrane (Pall) for 1 h at 30 V in 0.5 X TBE buffer. Blots were UV cross-linked (GS GeneLinker, Bio-Rad) and developed using the Chemiluminescent nucleic acid detection module (Thermoscientific).
RNA extraction, cDNA synthesis and quantitative PCR
Total RNA was extracted from SL1344 and CSH50 cells normalized to 2.0 OD600 units. Cultures were prepared as previously described [38]. RNA was then extracted using the SV Total RNA Isolation System (Promega) according to manufacturers instructions. 50 µl of RNA was DNase treated using the Turbo-DNA-free kit (Ambion). RNA was analyzed on a 1% TAE agarose gel. 1 µg of RNA was synthesized to cDNA using the GoScript Reverse Transcription System (Promega) according to manufacturers instructions. 100 µl nuclease-free H2O was added to each cDNA pool. Quantitative PCR was performed using oligonucleotides listed in Table S2. Primers specific for ompR in SL1344 were ompR_RT_S.e_F and ompR_RT_S.e_R, ompR in CSH50; ompR_RT_E.c_F and ompR_RT_E.c_R, ssrA in SL134; ssrA_RT_F and ssrA_RT_R, mgtC in SL1344 mgtC_RT_F and mgtC_RT_R, phoP in SL134; phoP_S.e_RT_F and phoP S.e_RT_R. PCR reactions were performed as previously described [3] Primers specific for the gmk gene were used as an internal control as previously described [86], SL1344 specific gmk primers are listed gmk_F_S.e and gmk_R_S.e and CSH50 specific gmk primers are listed gmk_F_E.c and gmk_R_E.c in Table S2.
5′RACE
This was performed as previously described [87] using RNA extracted from SL1344 and CSH50 after 90 min in EG-minimal medium pH 7 or pH 4.5. PCR was then performed on cDNA samples using adapter-specific (JVO-0367; see Table S2) and ompR specific primer (RACE_ompR; see Table S2). The oligonucleotide RACE_ompR is complementary to a region of conserved nucleotide sequence in both species. PCR products were cloned into the linearized vector pJET (Fermentas) and transformed into E. coli strain XL-1 and at least 5 clones were DNA sequenced.
Motif analysis
Unbiased motif finding was conducted using MEME 4.9, and the following parameters were tested: motifs could range in size from 10 to 50 bp, each DNA sequence could contain multiple or no motif sites, and motifs could be palindromic or non-palindromic. The RSAT matrix-scan program was run with default settings except that the background model was based on the genome subset of non-coding upstream DNA from E. coli K12 or S. Typhimurium LT2, depending on the ChIP dataset being analyzed. The RSAT random-sequence program used the nucleotide hexamer frequencies in non-coding upstream sequences from E. coli K12 or S. Typhimurium LT2 to generate random sequence datasets of sizes matching the experimental datasets.
Plasmid topoisomer gel electrophoresis
The high-copy plasmid pUC18 was used to report DNA supercoiling levels in this study [7]. Cultures were harvested after 90 min at pH 7 and pH 4.5 and plasmid DNA was extracted using the Promega PureYield plasmid miniprep system. Plasmid DNA was ran on an agarose gel containing 2.5 µg ml−1 chloroquine with 2X Tris Borate EDTA (TBE) used as gel and running buffer as previously described [3].
Chromatin immunoprecipitation and microarray analysis
ChIP was performed as previously described [64] using the strains SL1344 ompR::3xFLAG and CSH50 ompR::3xFLAG. Anti-FLAG antibody (Sigma-Aldrich, cat no. F3165) was used to immunoprecipitated the OmpR-FLAG tagged protein. Normal mouse IgG (Millipore) was used as a control for non-specific (‘mock’) immunoprecipitation. Fluorescent labelling of DNA was performed as previously described [64]. Samples were prepared for hybridisation as follows: 50 µl 2X Hi-RPM Hybridisation buffer (Agilent), 25 µl ChIP or ‘mock’ DNA, 5 µl input DNA, 10 µl 10X GE blocking Agent (Agilent) and 10 µl nuclease-free H2O. Samples were added to a microfuge tube, vortex-mixed briefly and collected by short centrifugation spin. This was then added to the appropriate species-specific (SL1344 or MG1655) microarray slide (Oxford Gene Technology; 4×44 K identical arrays). Arrays were sealed with the appropriate gasket slide, loaded into the Agilent Microarray Hybridisation Chamber Kit (G2534A) and hybridised for 24 h at 65°C in a hybridization oven (Agilent). Slides were washed according to instructions provided by Oxford Gene Technology and scanned on Agilent High-Resolution C scanner at 635 and 532 nm. The median intensities for both channels were acquired by Agilent Feature Extraction Software version 10.5.1.1. This software calculates the background fluorescence for each spot. These values were used to calculate the background subtracted ChIP/input ratio. The data was median-normalised by calculating the median fluorescence for each channel (Cy3 or Cy5) and using a scaling factor to ensure the median of the data set was equal to 1. Three independent ChIP-on-chip replicates were performed for SL1344 and CSH50 experiments in EG-minimal medium at pH 7 and pH 4.5. Two independent ChIP-on-chip replicates were performed for SL1344 experiments without novobiocin and with novobiocin at 25 µg ml−1. Two independent control ChIP-on-chip replicates were performed using ‘mock’ antibody in SL1344 and CSH50 backgrounds. The median ChIP/input ratio and the mock/input ratio were calculated. The median log2 ratio for each independent biological replicate was calculated. To correct for non-specifically enriched peaks, the mock median log2 ratio was subtracted from each ChIP median log2 ratio. The log2 ratios were used in the ChIPOTle programme [88] to define peaks of enrichment as described previously [64] a P-value of P = 0.001 was assigned. The complete ChIP-on-chip datasets have been submitted to the Gene Expression Omnibus (GEO) database (accession number GSE49914).
Supporting Information
Zdroje
1. PerezJC, GroismanEA (2009) Evolution of transcriptional regulatory circuits in bacteria. Cell 138 : 233–244.
2. RheeJE, ShengW, MorganLK, NoletR, LiaoX, et al. (2008) Amino acids important for DNA recognition by the response regulator OmpR. J Biol Chem 13 : 8664–8677.
3. CameronADS, DormanCJ (2012) A fundamental regulatory mechanism operating through OmpR and DNA topology controls expression of Salmonella Pathogenicity islands SPI-1 and SPI-2. PLoS Genet 3: e1002615.
4. KaremK, FosterJW (1993) The influence of DNA topology on the environmental regulation of a pH-regulated locus in Salmonella Typhimurium. Mol Microbiol 10 : 75–86.
5. GoldsteinE, DrlicaK (1984) Regulation of bacterial DNA supercoiling: plasmid linking numbers vary with growth temperature. Proc Natl Acad Sci USA 81 : 4046–4050.
6. DormanCJ, BarrGC, Ní BhriainN, HigginsCF (1988) DNA supercoiling and the anaerobic and growth phase regulation of tonB gene expression. J Bacteriol 170 : 2816–2826.
7. HsiehLS, BurgerRM, DrlicaK (1991) Bacterial DNA supercoiling and [ATP]/[ADP]. Changes associated with a transition to anaerobic growth. J Mol Biol 219 : 443–450.
8. HsiehLS, Rouvière-YanivJ, DrlicaK (1991) Bacterial DNA supercoiling and [ATP]/[ADP] ratio: changes associated with salt shock. J Bacteriol 173 : 3914–3917.
9. MizunoT, MizushimaS (1990) Signal transduction and gene regulation through the phosphorylation of two regulatory components: the molecular basis for the osmotic regulation of the porin genes. Mol Microbiol 7 : 1077–1082.
10. Pratt L, Silhavy TJ, (1995) In: Hoch J, Silhavy TJ editors. Two-component signal transduction. Washington, DC: American Society for Microbiology Press. pp 105–107.
11. RobertsDL, BennettDW, ForstSA (1994) Identification of the site of phosphorylation on the osmosensor, EnvZ, of Escherichia coli. J Biol Chem 269 : 8728–8733.
12. Martinez-HackeretE, StockAM (1997) The DNA binding domain of OmpR: crystal structures of a winged helix transcription factor. Structure 5 : 109–124.
13. DelgadoJ, ForstS, HarlockerS, InouyeM (1993) Identification of a phosphorylation site and functional analysis of conserved aspartic acid residues of OmpR, a transcriptional activator for ompF and ompC in Escherichia coli.. Mol Microbiol 5 : 1037–1047.
14. AlphenWV, LugtenbergB (1977) Influence of osmolarity of the growth medium on the outer membrane protein pattern of Escherichia coli. J Bacteriol 131 : 623–630.
15. SarmaV, ReevesP (1977) Genetic locus (ompB) affecting a major outer-membrane protein in Escherichia coli K-12. J Bacteriol 132 : 23–27.
16. SlauchJM, GarrettS, JacksonDE, SilhavyTJ (1988) EnvZ functions through OmpR to control porin gene expression in Escherichia coli K-12. J Bacteriol 170 : 439–441.
17. WangLC, MorganLK, GodakumburaP, KenneyLJ, AnandGS (2012) The inner membrane histidine kinase EnvZ senses osmolality via helix-coil transitions in the cytoplasm. EMBO J 11 : 2648–59.
18. Alpuche-ArandaCM, SwansonJA, LoomisWP, MillerSI (1992) Salmonella Typhimurium activates virulence gene transcription within acidified macrophage phagosomes. Proc Natl Acad Sci USA 89 : 10079–10083.
19. RathmanM, SjaastadMD, FalkowS (1996) Acidification of phagosomes containing Salmonella Typhimurium in murine macrophages. Infect Immun 64 : 2765–2773.
20. HaragaA, OhlsonMB, MillerSI (2008) Salmonellae interplay with host cells. Nat Rev Microbiol 1 : 53–66.
21. SmallP, BlankenhornD, WeltyD, ZinserE, SlonczewskiJL (1994) Acid and base resistance in Escherichia coli and Shigella flexneri: Role of rpoS and growth pH. J Bacteriol 176 : 1729–1737.
22. LinJ, LeeIS, FreyJ, SlonczewskiJL, FosterJW (1995) Comparative analysis of extreme acid survival in Salmonella Typhimurium, Shigella flexneri and Escherichia coli. J Bacteriol 177 : 4097–4104.
23. DormanCJ, ChatfieldS, HigginsCF, HaywardC, DouganG (1989) Characterization of porin and ompR mutants of a virulent strain of Salmonella Typhimurium: ompR mutants are attenuated in vivo. Infect Immun 57 : 2136–2140.
24. OchmanH, SonciniFC, SolomonF, GroismanEA (1996) Identification of a pathogenicity island required for Salmonella survival in host cells. Proc Natl Acad Sci USA 15 : 7800–7804.
25. OchmanH, GroismanEA (1996) Distribution of pathogenicity islands in Salmonella spp. Infect Immun 12 : 5410–5412.
26. SheaJE, HenselM, GleesonC, HoldenDW (1996) Identification of a virulence locus encoding a second type III secretion system in Salmonella Typhimurium. Proc Natl Acad Sci USA 93 : 2593–2597.
27. RhenM, DormanCJ (2005) Hierarchical gene regulators adapt Salmonella Typhimurium to its host milieus. Int J Med Microbiol 294 : 487–502.
28. LeeAK, DetweilerCS, FalkowS (2000) OmpR regulates the two-component system SsrA-SsrB in Salmonella pathogenicity island 2. J Bacteriol 182 : 771–781.
29. FengX, OropezaR, KenneyLJ (2003) Dual regulation by phospho-OmpR of ssrA/B gene expression in Salmonella pathogenicity island 2. Mol Microbiol 1 : 231–247.
30. FassE, GroismanEA (2009) Control of Salmonella pathogenicity island-2 gene expression. Curr Opin Microbiol 12 : 199–204.
31. Ó CróinínT, CarrollRK, KellyA, DormanCJ (2006) Roles for DNA supercoiling and the Fis protein in modulation expression of virulence genes during intracellular growth of Salmonella enterica serovar Typhimurium. Mol Microbiol 62 : 869–882.
32. BangIS, KimBH, FosterJW, ParkYK (2000) OmpR regulates the stationary-phase acid tolerance response of Salmonella enterica serovar Typhimurium. J Bacteriol 182 : 2245–2252.
33. BangIS, AudiaJP, ParkYK, FosterJW (2002) Autoinduction of the ompR response regulator by acid shock and control of the Salmonella typhimurium acid tolerance response. Mol Microbiol 44 : 1235–1250.
34. Martinez-FloresI, CanoR, BustamanteVH, CalvaE, PuenteJL (1999) The ompB operon partially determines differential expression of OmpC in Salmonella Typhi and Escherichia coli. J Bacteriol 2 : 556–562.
35. LiljestromP, LaamanenI, PalvaET (1988) Structure and expression of the ompB operon, the regulatory locus for the outer membrane porin regulon in Salmonella Typhimurium LT-2. J Mol Biol 201 : 663–673.
36. TsuiP, HuangL, FreundlichM (1991) Integration host factor binds specifically to multiple sites in the ompB promoter of Escherichia coli and inhibits transcription. J Bacteriol 173 : 5800–5807.
37. HuangKJ, SchieberlJL, IgoMM (1994) A distant upstream site involved in the negative regulation of the Escherichia coli ompF gene. J Bacteriol 5 : 1309–1315.
38. KrogerC, DillonSC, CameronADS, PapenfortK, SivasankaranSK, et al. (2012) The transcriptional landscape and small RNAs of Salmonella enterica serovar Typhimurium. Proc Natl Acad Sci USA 20: E1277–1286.
39. MattisonK, OropezaR, ByersN, KenneyLJ (2002) A phosphorylation site mutant of OmpR reveals different binding conformations at ompF and ompC. J Mol Biol 4 : 497–511.
40. ForstS, KalveI, DurskiW (1995) Molecular analysis of OmpR binding sequences involved in the regulation of ompF in Escherichia coli. FEMS Microbiol Lett 2 : 147–15.
41. HarlockerSL, BergstromL, InouyeM (1995) Tandem binding of six OmpR proteins to the ompF upstream regulatory sequence of Escherichia coli. J Biol Chem 45 : 26849–26856.
42. YoshidaT, QinL, EggerLA, InouyeM (2006) Transcription regulation of ompF and ompC by a single transcription factor, OmpR. J Biol Chem 25 : 17114–17123.
43. GibsonMM, EllisEM, Graeme-CookKA, HigginsCF (1987) OmpR and EnvZ are pleiotropic regulatory proteins: positive regulation of the tripeptide permease (tppB) of Salmonella Typhimurium. Mol Gen Genet 1 : 120–129.
44. GohEB, SiinoDF, IgoMM (2004) The Escherichia coli tppB (ydgR) gene represents a new class of OmpR-regulated genes. J Bacteriol 12 : 4019–4024.
45. RomlingU, BianZ, HammarM, SierraltaWD, NormarkS (1998) Curli fibers are highly conserved between Salmonella Typhimurium and Escherichia coli with respect to operon structure and regulation. J Bacteriol 3 : 722–731.
46. GerstelU, ParkC, RomlingU (2003) Complex regulation of csgD promoter activity by global regulatory proteins. Mol Microbiol 3 : 639–654.
47. Prigent-CombaretC, BrombacherE, VidalO, AmbertA, LejeuneP, et al. (2001) Complex regulatory network controls initial adhesion and biofilm formation in Escherichia coli via regulation of the csgD gene. J Bacteriol 24 : 7213–7223.
48. ShinS, ParkC (1995) Modulation of flagellar expression in Escherichia coli by acetyl phosphate and the osmoregulator OmpR. J Bacteriol 16 : 4696–4702.
49. GuillierM, GottesmanS (2006) Remodelling of the Escherichia coli outer membrane by two small regulatory RNAs. Mol Microbiol 1 : 231–247.
50. PerkinsTT, KingsleyRA, FookesMC, GardnerPP, JamesKD, et al. (2009) A strand-specific RNA-Seq analysis of the transcriptome of the typhoid bacillus Salmonella Typhi. PLoS Genet 7: e1000569.
51. NeelyMN, OlsonER (1996) Kinetics of expression of the Escherichia coli cad operon as a function of pH and lysine. J Bacteriol 178 : 5522–5528.
52. KobayashiH, SuzukiT, UnemotoT (1986) Streptococcal cytoplasmic pH is regulated by changes in amount and activity of a proton-translocating ATPase. J Biol Chem 261 : 627–630.
53. DouchinV, BohnC, BoulocP (2006) Down-regulation of porins by a small RNA bypasses the essentiality of the regulated intramembrane proteolysis protease RseP in Escherichia coli. J Biol Chem 18 : 12253–12259.
54. ClaretL, HughesC (2002) Interaction of the atypical prokaryotic transcription activator FlhD2C2 with early promoters of the flagellar gene hierarchy. J Mol Biol 2 : 185–199.
55. LehnenD, BlumerC, PolenT, WackwitzB, WendischVF, et al. (2002) LrhA as a new transcriptional key regulator of flagella, motility and chemotaxis genes in Escherichia coli. Mol Microbiol 2 : 521–532.
56. BlumerC, KleefeldA, LehnenD, HeintzM, Dobrindt, etal (2005) Regulation of type 1 fimbriae synthesis and biofilm formation by the transcriptional regulator LrhA of Escherichia coli. Microbiology 10 : 3287–3298.
57. KoreaCG, BadouralyR, PrevostMC, GhigoJM, BeloinC (2010) Escherichia coli K-12 possesses multiple cryptic but functional chaperone-usher fimbriae with distinct surface specificities. Environ microbiol 7 : 1957–1977.
58. ThomasAD, BoothIR (1992) The regulation of expression of the porin gene ompC by acid pH. J Gen Microbiol 9 : 1829–35.
59. YuXJ, McGourtyK, LiuM, UnsworthKE, HoldenDW (2010) pH sensing by intracellular Salmonella induces effector translocation. Science 5981 : 1040–3.
60. BajajV, HwangC, LeeCA (1995) hilA is a novel ompR/toxR family member that activates the expression of Salmonella Typhimurium invasion genes. Mol Microbiol 4 : 715–727.
61. EllermeierCD, EllermeierJR, SlauchJM (2005) HilD, HilC and RtsA constitute a feed forward loop that controls expression of the SPI1 type three secretion system regulator hilA in Salmonella enterica serovar Typhimurium. Mol Microbiol 3 : 691–705.
62. KellyA, GoldbergMD, CarrollRK, DaninoV, HintonJC, et al. (2004) A global role for Fis in the transcriptional control of metabolism and type III secretion in Salmonella enterica serovar Typhimurium. Microbiology 7 : 2037–2053.
63. LucchiniS, RowleyG, GoldbergMD, HurdD, HarrisonM, et al. (2006) H-NS mediates the silencing of laterally acquired genes in bacteria. PLoS Pathog 8: e81.
64. DillonSC, CameronAD, HokampK, LucchiniS, HintonJC, et al. (2010) Genome-wide analysis of the H-NS and Sfh regulatory networks in Salmonella Typhimurium identifies a plasmid-encoded transcription silencing mechanism. Mol Microbiol 5 : 1250–1265.
65. OsborneSE, CoombesBK (2011) Transcriptional priming of Salmonella pathogenicity Island-2 precedes cellular invasion. PloS One 6: e21648.
66. KuboriT, MatsushimaY, NakamuraD, UralilJ, Lara-TejeroM, et al. (1998) Supramolecular structure of the Salmonella Typhimurium type III protein secretion system. Science 5363 : 602–605.
67. FengX, WalthersD, OropezaR, KenneyLJ (2004) The response regulator SsrB activates transcription and binds to a region overlapping OmpR binding sites at Salmonella pathogenicity island 2. Mol Microbiol 54 : 823–835.
68. WalthersD, CarrollRK, NavarreWW, LibbySJ, FangFC, et al. (2007) The response regulator SsrB activates expression of diverse Salmonella pathogenicity island 2 promoters and counters silencing by the nucleoid-associated protein H-NS. Mol Microbiol 2 : 477–493.
69. Tomljenovic-BerubeAM, MulderDT, WhitesideMD, BrinkmanFS, CoombesBK (2010) Identification of the regulatory logic controlling Salmonella pathoadaptation by the SsrA-SsrB two-component system. PLoS Genet 3: e1000875.
70. GerlachRG, JäckelD, StecherB, WagnerC, LupasA, et al. (2007) Salmonella Pathogenicity Island 4 encodes a giant non-fimbrial adhesin and the cognate type 1 secretion system. Cell Microbiol 9 : 1834–1850.
71. WagnerC, PolkeM, GerlachRG, LinkeD, StierhofYD, et al. (2011) Functional dissection of SiiE, a giant non-fimbrial adhesin of Salmonella Typhimurium. Cell Microbiol 13 : 1286–1301.
72. CameronAD, StoebelDM, DormanCJ (2011) DNA supercoiling is differentially regulated by environmental factors and FIS in Escherichia coli and Salmonella Typhimurium. Mol Microbiol 80 : 85–101.
73. GellertM, O'DeaMH, ItohT, TomizawaJ (1976) Novobiocin and coumermycin inhibit DNA supercoiling catalyzed by DNA gyrase. Proc Natl Acad Sci USA 12 : 4474–4478.
74. GroismanEA (2001) The pleiotropic two-component regulatory system PhoP-PhoQ. J Bacteriol 6 : 1835–1842.
75. LeeE-J, PontesMH, GroismanEA (2013) A bacterial virulence protein promotes pathogenicity by inhibiting the bacterium's own F1Fo ATP synthase. Cell 154 : 146–156.
76. Blanc-PotardAB, GroismanEA (1997) The Salmonella selC locus contains a pathogenicity island mediating intramacrophage survival. EMBO J 17 : 5376–5385.
77. BrodskyIE, ErnstRK, MillerSI, FalkowS (2002) mig-14 is a Salmonella gene that plays a role in bacterial resistance to antimicrobial peptides. J Bacteriol 184 : 3203–3213.
78. Salgado H, Peralta-Gil M, Gama-Castro S, Santos-Zavaleta A, Muñiz-Rascado L, et al. (2013) RegulonDB v8.0: omics data sets, evolutionary conservation, regulatory phrases, cross-validated gold standards and more. Nucleic Acids Res 41 (Database issue): D203–213.
79. Bailey TL, Boden M, Buske FA, Frith M, Grant CE, et al. (2009) MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res 37 (Web Server issue): W202–208.
80. TuratsinzeJV, Thomas-ChollierM, DefranceM (2008) van HeldenJ (2008) Using RSAT to scan genome sequences for transcription factor binding sites and cis-regulatory modules. Nature protocols 10 : 1578–1588.
81. StinconeA, DaudiN, RathmanAS, AntczakP, HendersonI, et al. (2011) A systems biology approach sheds new light on Escherichia coli acid resistance. Nucleic Acids Res 39 : 7512–7528.
82. LangB, BlotN, BouffartiguesE, BuckleM, GeertzM, et al. (2007) High-affinity DNA binding sites for H-NS provide a molecular basis for selective silencing within proteobacterial genomes. Nucleic Acids Res 35 : 6330–6337.
83. WesterhoffHV, O'DeaMH, MaxwellA, GellertM (1988) DNA supercoiling by DNA gyrase. A static head analysis. Cell Biophys. 12 : 157–181.
84. RohsR, WestSM, SosinskyA, Liu P MannRS, et al. (2009) The role of DNA shape in protein-DNA recognition. Nature. 461 : 1248–53.
85. VogelHJ, BonnerDM (1956) Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem 1 : 97–106.
86. MullerC, BangIS, VelayudhanJ, KarlinseyJ, PapenfortK, et al. (2009) Acid stress activation of the sigma(E) stress response in Salmonella enterica serovar Typhimurium. Mol Microbiol 71 : 1228–1238.
87. UrbanJH, VogelJ (2009) A green fluorescent protein (GFP)-based plasmid system to study post-transcriptional control of gene expression in vivo. Methods Mol Biol 540 : 301–319.
88. BuckMJ, NobelAB, LiebJD (2005) ChIPOTle: a user-friendly tool for the analysis of ChIP-chip data. Genome Biol 6: R97.
Štítky
Genetika Reprodukční medicína
Článek Sex Chromosome Turnover Contributes to Genomic Divergence between Incipient Stickleback SpeciesČlánek Final Pre-40S Maturation Depends on the Functional Integrity of the 60S Subunit Ribosomal Protein L3
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 3- Akutní intermitentní porfyrie
- Souvislost haplotypu M2 genu pro annexin A5 s opakovanými reprodukčními ztrátami
- Příjem alkoholu a menstruační cyklus
- Doporučení pro diagnostiku a léčbu akutních jaterních porfyrií
- Hysteroskopická resekce děložního septa zlepšuje šanci na graviditu žen s jinak nevysvětlenou infertilitou
-
Všechny články tohoto čísla
- , a Gene That Influences the Anterior Chamber Depth, Is Associated with Primary Angle Closure Glaucoma
- Genomic View of Bipolar Disorder Revealed by Whole Genome Sequencing in a Genetic Isolate
- The Rate of Nonallelic Homologous Recombination in Males Is Highly Variable, Correlated between Monozygotic Twins and Independent of Age
- Genetic Determinants Influencing Human Serum Metabolome among African Americans
- Heterozygous and Inherited Mutations in the Smooth Muscle Actin () Gene Underlie Megacystis-Microcolon-Intestinal Hypoperistalsis Syndrome
- Genome-Wide Meta-Analysis of Homocysteine and Methionine Metabolism Identifies Five One Carbon Metabolism Loci and a Novel Association of with Ischemic Stroke
- Cancer Evolution Is Associated with Pervasive Positive Selection on Globally Expressed Genes
- Genetic Diversity in the Interference Selection Limit
- Integrating Multiple Genomic Data to Predict Disease-Causing Nonsynonymous Single Nucleotide Variants in Exome Sequencing Studies
- An Evolutionary Analysis of Antigen Processing and Presentation across Different Timescales Reveals Pervasive Selection
- Cleavage Factor I Links Transcription Termination to DNA Damage Response and Genome Integrity Maintenance in
- DNA Dynamics during Early Double-Strand Break Processing Revealed by Non-Intrusive Imaging of Living Cells
- Genetic Basis of Metabolome Variation in Yeast
- Modeling 3D Facial Shape from DNA
- Dysregulated Estrogen Receptor Signaling in the Hypothalamic-Pituitary-Ovarian Axis Leads to Ovarian Epithelial Tumorigenesis in Mice
- Photoactivated UVR8-COP1 Module Determines Photomorphogenic UV-B Signaling Output in
- Local Evolution of Seed Flotation in Arabidopsis
- Chromatin Targeting Signals, Nucleosome Positioning Mechanism and Non-Coding RNA-Mediated Regulation of the Chromatin Remodeling Complex NoRC
- Nucleosome Acidic Patch Promotes RNF168- and RING1B/BMI1-Dependent H2AX and H2A Ubiquitination and DNA Damage Signaling
- The -Induced Arabidopsis Transcription Factor Attenuates ABA Signaling and Renders Seedlings Sugar Insensitive when Present in the Nucleus
- Changes in Colorectal Carcinoma Genomes under Anti-EGFR Therapy Identified by Whole-Genome Plasma DNA Sequencing
- Selection of Orphan Rhs Toxin Expression in Evolved Serovar Typhimurium
- FAK Acts as a Suppressor of RTK-MAP Kinase Signalling in Epithelia and Human Cancer Cells
- Asymmetry in Family History Implicates Nonstandard Genetic Mechanisms: Application to the Genetics of Breast Cancer
- Co-translational Localization of an LTR-Retrotransposon RNA to the Endoplasmic Reticulum Nucleates Virus-Like Particle Assembly Sites
- Sex Chromosome Turnover Contributes to Genomic Divergence between Incipient Stickleback Species
- DWARF TILLER1, a WUSCHEL-Related Homeobox Transcription Factor, Is Required for Tiller Growth in Rice
- Functional Organization of a Multimodular Bacterial Chemosensory Apparatus
- Genome-Wide Analysis of SREBP1 Activity around the Clock Reveals Its Combined Dependency on Nutrient and Circadian Signals
- The Yeast Sks1p Kinase Signaling Network Regulates Pseudohyphal Growth and Glucose Response
- An Interspecific Fungal Hybrid Reveals Cross-Kingdom Rules for Allopolyploid Gene Expression Patterns
- Temperate Phages Acquire DNA from Defective Prophages by Relaxed Homologous Recombination: The Role of Rad52-Like Recombinases
- Dying Cells Protect Survivors from Radiation-Induced Cell Death in
- Determinants beyond Both Complementarity and Cleavage Govern MicroR159 Efficacy in
- The bHLH Factors Extramacrochaetae and Daughterless Control Cell Cycle in Imaginal Discs through the Transcriptional Regulation of the Phosphatase
- The First Steps of Adaptation of to the Gut Are Dominated by Soft Sweeps
- Bacterial Regulon Evolution: Distinct Responses and Roles for the Identical OmpR Proteins of Typhimurium and in the Acid Stress Response
- Final Pre-40S Maturation Depends on the Functional Integrity of the 60S Subunit Ribosomal Protein L3
- Mitogen-Activated Protein Kinase (MAPK) Pathway Regulates Branching by Remodeling Epithelial Cell Adhesion
- CDP-Diacylglycerol Synthetase Coordinates Cell Growth and Fat Storage through Phosphatidylinositol Metabolism and the Insulin Pathway
- Coronary Heart Disease-Associated Variation in Disrupts a miR-224 Binding Site and miRNA-Mediated Regulation
- TBX3 Regulates Splicing : A Novel Molecular Mechanism for Ulnar-Mammary Syndrome
- Identification of Interphase Functions for the NIMA Kinase Involving Microtubules and the ESCRT Pathway
- Is a Cancer-Specific Fusion Gene Recurrent in High-Grade Serous Ovarian Carcinoma
- LILRB2 Interaction with HLA Class I Correlates with Control of HIV-1 Infection
- Worldwide Patterns of Ancestry, Divergence, and Admixture in Domesticated Cattle
- Parent-of-Origin Effects Implicate Epigenetic Regulation of Experimental Autoimmune Encephalomyelitis and Identify Imprinted as a Novel Risk Gene
- The Phospholipid Flippase dATP8B Is Required for Odorant Receptor Function
- Noise Genetics: Inferring Protein Function by Correlating Phenotype with Protein Levels and Localization in Individual Human Cells
- DUF1220 Dosage Is Linearly Associated with Increasing Severity of the Three Primary Symptoms of Autism
- Sugar and Chromosome Stability: Clastogenic Effects of Sugars in Vitamin B6-Deficient Cells
- Pheromone-Sensing Neurons Expressing the Ion Channel Subunit Stimulate Male Courtship and Female Receptivity
- Gene-Based Sequencing Identifies Lipid-Influencing Variants with Ethnicity-Specific Effects in African Americans
- Telomere Shortening Unrelated to Smoking, Body Weight, Physical Activity, and Alcohol Intake: 4,576 General Population Individuals with Repeat Measurements 10 Years Apart
- A Combination of Activation and Repression by a Colinear Hox Code Controls Forelimb-Restricted Expression of and Reveals Hox Protein Specificity
- An ER Complex of ODR-4 and ODR-8/Ufm1 Specific Protease 2 Promotes GPCR Maturation by a Ufm1-Independent Mechanism
- Epigenetic Control of Effector Gene Expression in the Plant Pathogenic Fungus
- Genetic Dissection of Photoreceptor Subtype Specification by the Zinc Finger Proteins Elbow and No ocelli
- Validation and Genotyping of Multiple Human Polymorphic Inversions Mediated by Inverted Repeats Reveals a High Degree of Recurrence
- CYP6 P450 Enzymes and Duplication Produce Extreme and Multiple Insecticide Resistance in the Malaria Mosquito
- GC-Rich DNA Elements Enable Replication Origin Activity in the Methylotrophic Yeast
- An Epigenetic Signature in Peripheral Blood Associated with the Haplotype on 17q21.31, a Risk Factor for Neurodegenerative Tauopathy
- Lsd1 Restricts the Number of Germline Stem Cells by Regulating Multiple Targets in Escort Cells
- RBPJ, the Major Transcriptional Effector of Notch Signaling, Remains Associated with Chromatin throughout Mitosis, Suggesting a Role in Mitotic Bookmarking
- The Membrane-Associated Transcription Factor NAC089 Controls ER-Stress-Induced Programmed Cell Death in Plants
- The Functional Consequences of Variation in Transcription Factor Binding
- Comparative Genomic Analysis of N-Fixing and Non-N-Fixing spp.: Organization, Evolution and Expression of the Nitrogen Fixation Genes
- An Insulin-to-Insulin Regulatory Network Orchestrates Phenotypic Specificity in Development and Physiology
- Suicidal Autointegration of and Transposons in Eukaryotic Cells
- A Multi-Trait, Meta-analysis for Detecting Pleiotropic Polymorphisms for Stature, Fatness and Reproduction in Beef Cattle
- Genome-Wide DNA Methylation Analysis Predicts an Epigenetic Switch for GATA Factor Expression in Endometriosis
- Genome-Wide DNA Methylation Analysis of Human Pancreatic Islets from Type 2 Diabetic and Non-Diabetic Donors Identifies Candidate Genes That Influence Insulin Secretion
- The and Hybrid Incompatibility Genes Suppress a Broad Range of Heterochromatic Repeats
- The Kil Peptide of Bacteriophage λ Blocks Cytokinesis via ZipA-Dependent Inhibition of FtsZ Assembly
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Worldwide Patterns of Ancestry, Divergence, and Admixture in Domesticated Cattle
- Genome-Wide DNA Methylation Analysis of Human Pancreatic Islets from Type 2 Diabetic and Non-Diabetic Donors Identifies Candidate Genes That Influence Insulin Secretion
- Genetic Dissection of Photoreceptor Subtype Specification by the Zinc Finger Proteins Elbow and No ocelli
- GC-Rich DNA Elements Enable Replication Origin Activity in the Methylotrophic Yeast
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



