-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaDysregulated Estrogen Receptor Signaling in the Hypothalamic-Pituitary-Ovarian Axis Leads to Ovarian Epithelial Tumorigenesis in Mice
Ovarian cancer is currently the most lethal gynecological cancer in the United States. Multiple epidemiological studies indicate that women who take hormone replacement therapy, estrogen or estrogen with progesterone, peri - or postmenopause will have an increased chance of developing ovarian cancer. Unfortunately, the five-year survival rate after diagnosis is very low indicating that better tools are needed to diagnose and treat ovarian cancer. The models that would allow investigation of this disease are severely limited. In this article we introduce a mouse model that develops epithelial ovarian tumors, and by employing inhibitors of estrogen synthesis, we show that ovarian tumorigenesis in this model is dependent on estrogen production within the ovarian tumor. These studies suggest that estrogen may play a role in promoting ovarian tumor growth.
Published in the journal: . PLoS Genet 10(3): e32767. doi:10.1371/journal.pgen.1004230
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004230Summary
Ovarian cancer is currently the most lethal gynecological cancer in the United States. Multiple epidemiological studies indicate that women who take hormone replacement therapy, estrogen or estrogen with progesterone, peri - or postmenopause will have an increased chance of developing ovarian cancer. Unfortunately, the five-year survival rate after diagnosis is very low indicating that better tools are needed to diagnose and treat ovarian cancer. The models that would allow investigation of this disease are severely limited. In this article we introduce a mouse model that develops epithelial ovarian tumors, and by employing inhibitors of estrogen synthesis, we show that ovarian tumorigenesis in this model is dependent on estrogen production within the ovarian tumor. These studies suggest that estrogen may play a role in promoting ovarian tumor growth.
Introduction
Ovarian cancer is the most lethal malignancy of the female reproductive system and the fifth leading cause of cancer-related death among women [1]. Approximately 90% of malignant ovarian tumors are derived from either the ovarian surface epithelium (OSE) or fallopian tube epithelium (FTE) [2]. Due to the absence of specific symptoms and the lack of strategies for early detection of ovarian cancer, the majority (70%) of women with this disease are diagnosed at a late stage when the cancer has spread beyond the confines of the ovary [1]. Despite its clinical significance, the etiology of ovarian cancer is poorly understood, mainly due to the lack of an appropriate experimental model for studying the onset and progression of this disease.
Multiple theories regarding the etiology of ovarian cancer have been proposed, but the precise molecular defects underlying the development of this disease remain elusive [3]. The “gonadotropin hypothesis” proposes that high gonadotropin levels can have a stimulatory effect on OSE cells, promoting their neoplastic transformation [4], [5]. It was reported that the addition of gonadotropins to rodents in which ovarian cancer was induced upon treatment with the chemical carcinogen, 7,12-dimethylbenz(a)anthracene (DMBA) led to increased lesion severity, suggesting that gonadotropins play a role in tumor progression [6]. In humans, epidemiologic evidence, indirectly supporting this hypothesis, includes the well-documented protective effects of oral contraceptives and multiparity, which suppress gonadotropin secretion by the pituitary gland [5], [7]. The majority of women with epithelial ovarian cancer present the disease at a postmenopausal stage where circulating follicle stimulating hormone (FSH) and lutenizing hormone (LH) levels are elevated, indicating a causal relationship between chronically elevated gonadotropin levels and ovarian cancer development [5], [8].
Besides gonadotropins, epidemiological studies have reported altered ovarian cancer risk associated with the use of steroid hormones to ease menopausal symptoms. Estrogen (E) is a well-known mitogenic factor associated with the genesis of many cancers. It has been reported previously that the risk of developing ovarian cancer increases in women who use hormone replacement therapy (HRT) for more than five years or use E-only regimens [9]–[13]. While most of these studies comprise a small number of subjects and fail to control for all of the factors that may influence cancer risk, in patients with ovarian cancers, intratumoral production of E via in situ aromatization has been suggested to promote growth of breast, endometrial and ovarian cancer cells [14]. However, only few animal models have been used to investigate the role of E in ovarian tumorigenesis. Bai et al reported the effects of prolonged E exposure on the morphology of rabbit ovaries and found an increase in both OSE cell proliferation and the number of papillae covering the ovarian surface, but no ovarian tumors [15]. In a recent study, Laviotte et al conditionally activated an oncogene, SV40 TAg, in OSE cells and treated the mice with exogenous E [16]. These investigators reported that E treatment resulted in an earlier onset of ovarian tumors and a significantly decreased survival time [16]. While the results from this animal model underscore the importance of E in the progression of ovarian cancer, it is clear that new animal models independent of specifically directed single oncogenic mutations are needed for assessment of the role of E signaling in ovarian epithelial tumorigenesis.
In this study, we present a novel transgenic mouse model of ovarian tumorigenesis. In this model, termed ERαd/d, the estrogen receptor alpha (ERα) gene is dysregulated in the hypothalamic-pituitary-ovarian axis. Conditional deletion of this gene in the anterior pituitary, but not in the hypothalamus and the ovary, led to elevated circulating LH. Hyperstimulation by LH resulted in luteinization of the ovarian stromal cells, expression of P450 aromatase in these cells, and increased E synthesis in the ovarian microenvironment. Our study suggests that E critically controls ovarian tumor growth, presumably by stimulating the proliferation of OSE cells to drive epithelial tumorigenesis. The ERαd/d mouse, therefore, provides a useful model to study the mechanisms by which dysregulated E signaling promotes the initiation and progression of ovarian epithelial tumors.
Results
ERα conditional knockout mice (ERαd/d) were generated by crossing progesterone receptor cre recombinase (PR-Cre) knock-in mice with ERα floxed (ERαf/f) mice [17], [18]. By five months of age, the ERαd/d mice developed palpable ovarian tumors with 100% penetrance. In contrast, the ERαf/f and the global ERα knockout mice did not develop any tumor (Fig. 1A). The ovarian tumors of ERαd/d mice grew progressively with age and became as large as 11 mm in size with an average weight of 300 mg by eight months of age (Fig. S1A). Because of this large tumor burden, 80% of the ERαd/d mice die by 68 weeks of age (Fig. S1C). Histological analyses of the ERαd/d ovaries showed cystic hemorrhagic follicles at 3 months of age. By 6 months, there was evidence of neoplastic epithelial cells migrating into the ovarian stroma, and by 11 months, extensive cellular proliferation occurred, resulting in the formation of a large tumor mass (Fig. S1B). Immunohistochemical analysis of ovaries of ERαf/f mice at 6 months of age, using cell proliferation markers, revealed that the follicular granulosa cells were proliferative but the OSE cells were quiescent (Fig. 1B, panel a,c). In sharp contrast, both OSE and the tumor cells within ERαd/d ovaries exhibited pronounced proliferative activity (panels b, d; proliferative cells indicated by arrow) (Fig. 1B).
Fig. 1. The ERαd/d mice form proliferative ovarian tumors. 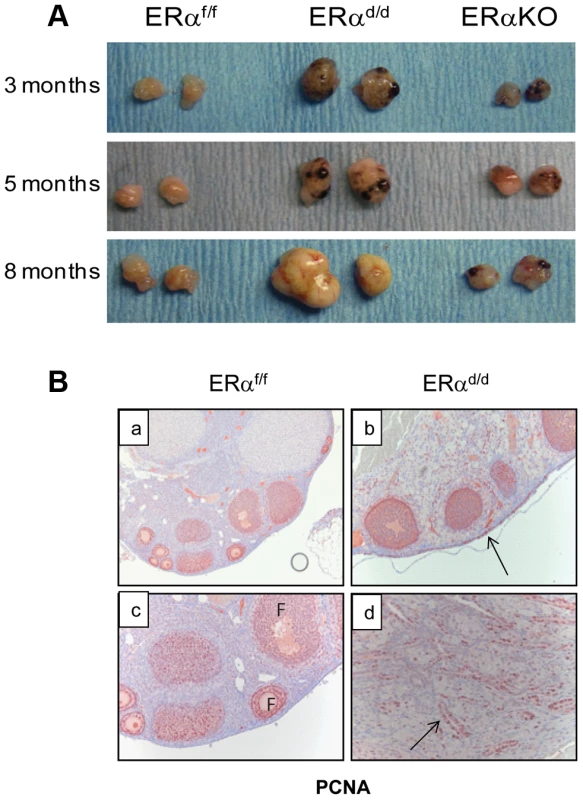
(A) Gross morphology of ERαd/d, ERαf/f, and ERα global KO mouse ovaries at 3, 5, and 8 months of age. (B) Immunohistochemistry of ERαf/f ovary (panels a, c) and ERαd/d ovaries (panels b, d) with tumors at 6 months of age using anti-PCNA antibody. Red staining indicates proliferating PCNA positive cells. Arrows point to hyperproliferative OSE (b) and tumor cells (d) in ERαd/d ovarian tumors. F indicates follicle. We next assessed the expression of ERα in the key tissues of the hypothalamic-pituitary-ovarian (HPO) axis. As shown in Fig. 2A, ERα expression was detected near the third ventricle of the hypothalamus in ERαf/f mice, and this expression remained intact in ERαd/d mice. Widespread expression of ERα was also observed in the anterior pituitary of ERαf/f mice. However, the pituitary expression of ERα was absent in ERαd/d mice. The ERα expression was evident in OSE of ERαf/f mice and remained intact in ERαd/d OSE (panels e,f). In addition theca cell expression of ERα also remained intact in the ERαd/d ovaries (panel h). Most notably, ERα was present in the tumor cells of ERαd/d ovaries (inset j, Fig. 2A). The Cre-mediated excision of the floxed ERα gene in the anterior pituitary is consistent with earlier reports indicating high levels of progesterone receptor (PR) expression in this tissue. The lack of Cre-mediated excision of the ERα gene in the hypothalamus and OSE, on the other hand, is presumably due to relatively low levels of PR expression in these tissues [18], [19].
Fig. 2. ERα localization in the tissues of HPO axis. 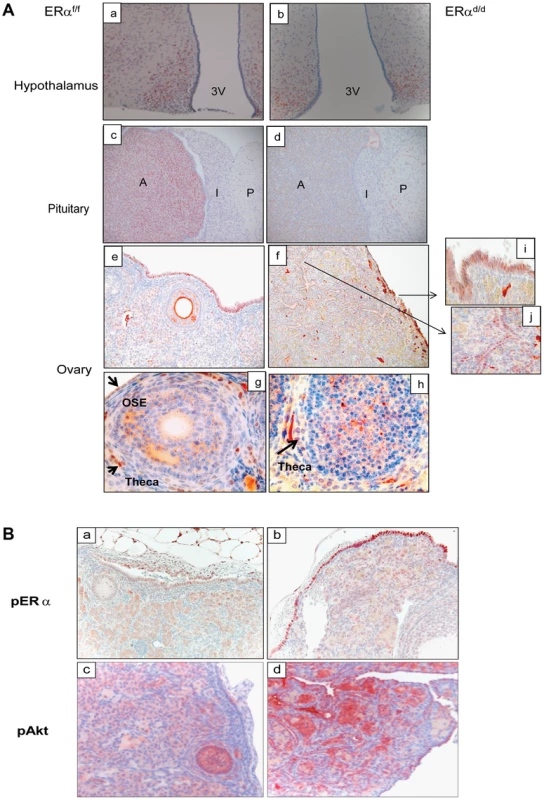
(A) Histological sections of ERαf/f (a) and ERαd/d (b) hypothalami, ERαf/f (c) and ERαd/d (d) pituitaries, ERαf/f ovary (e, g) and ERαd/d ovarian tumor (f, h) from adult mice at 6 months of age stained with anti-ERα. Inserts i and j indicate higher magnification depicting ERα positive cells (red staining) in the OSC and ovarian tumor cells. 3 V indicates the third ventricle. A indicates the anterior lobe, I indicates the intermediate lobe, and P indicates the posterior lobe of the pituitaries. Arrows point to OSE cells or theca cells expressing ERα. (B) Ovarian sections obtained from ERαf/f (left pictures) and ERαd/d (right pictures) mice were subjected to immunohistochemistry using antibodies against phospho-ERα (S118) (panels a, b) and phospho-Akt (S473) (panels c,d). Due to selective ablation of pituitary ERα expression, the ERαd/d mice are likely to experience a loss of negative-feedback regulation by E at the level of pituitary. Consistent with this prediction, the serum level of LH was significantly elevated in ERαd/d mice (Table 1). Hyperstimulation of ovarian cells by LH resulted in increased steroidogenesis, leading to high circulating levels of progesterone, testosterone and E in ERαd/d mice (Table 1). In contrast, the level of FSH was not statistically different between ERαd/d and ERαf/f mice. According to previous reports, the levels of LH, progesterone, testosterone, and E are also elevated in ERαKO mice [19], [20]. Consistent with the ERαKO mouse phenotype, ERαd/d mice are infertile. Adult mice fail to ovulate due to chronic high levels of LH. Due to the lack of ERα expression in uterine epithelial and stromal cells, the ERαd/d uteri are unable to receive an implanting embryo. Furthermore, uterine tumors are not found in the ERαd/d mice, presumably because the major uterine cell types do not express ERα. However, in contrast to the ERαKO mice, which lack ERα in all cells, including the ovarian cells, ERα was intact in OSE of ERαd/d mice. This raised the possibility that elevated systemic E levels contribute to tumor initiation by stimulating ER signaling in OSE of ERαd/d mice but fails to do so in OSE of ERαKO mice.
Tab. 1. Serum hormone measurements of ERαf/f and ERαd/d mice at six months of age. 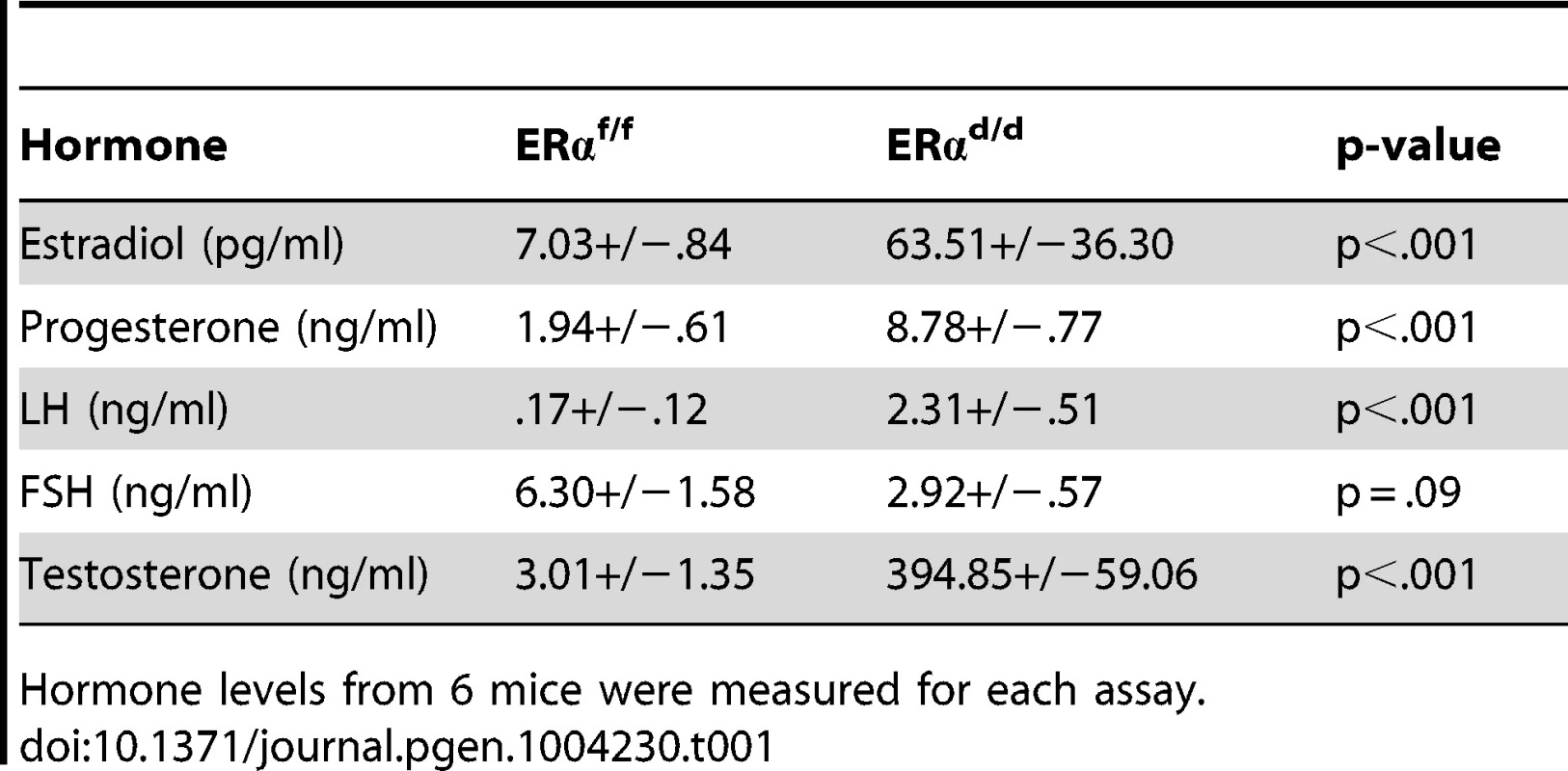
Hormone levels from 6 mice were measured for each assay. In agreement with this view, we observed marked up-regulation of a transcriptionally active form of ERα, phosphorylated at serine 118, in OSE of ERαd/d mice (Fig. 2B, b). We also examined the status of the phosphoinositide 3-kinase (PI3K)/AKT pathway, which is reported to be activated in response to E treatment of ovarian cancer cell lines [21]–[23]. We noted that the level of AKT phosphorylated at Ser 473 (p-AKT) is elevated in the OSE and tumor cells of ERαd/d ovaries, while p-AKT level is maintained at a low level in ERαf/f ovaries (Fig. 2B, c,d). It is likely that the increased level of phosphorylated AKT is linked to the elevated E signaling in ERαd/d ovaries.
To further characterize the nature of the ovarian tumor in ERαd/d mice, we performed immunohistochemical analyses using epithelial and granulosa cell biomarkers. Anti-mullerian hormone (AMH) is a well-known marker for normal granulosa cells and granulosa cell tumors [24]. While both ERαf/f and ERαd/d ovaries expressed AMH exclusively in the granulosa cells of follicles, ERαd/d ovaries did not express AMH in the tumor cells, indicating that these tumors are not of granulosa cell origin (Fig. 3A). Analysis using anti-cytokeratin 8 (CK8) antibody revealed that ERαf/f mice express this epithelial marker exclusively in a single layer of OSE at 3, 6, and 11 months of age (Fig. 3B, panels a,c,e). In contrast, the OSE of ERαd/d mice at 3 months of age exhibited multiple layers of cytokeratin-positive cells (panel b). At 6 months of age, we observed pronounced cytokeratin 8 expression within the ovaries of ERαd/d mice, indicating the presence of epithelial cells within the tumor mass (panel d). By 11 months of age, widespread cytokeratin 8 immunostaining was observed within the ovarian tumor, highlighting its remarkable epithelial component (Fig. 3B, panel f).
Fig. 3. Development of ovarian tumors in ERαd/d mice. 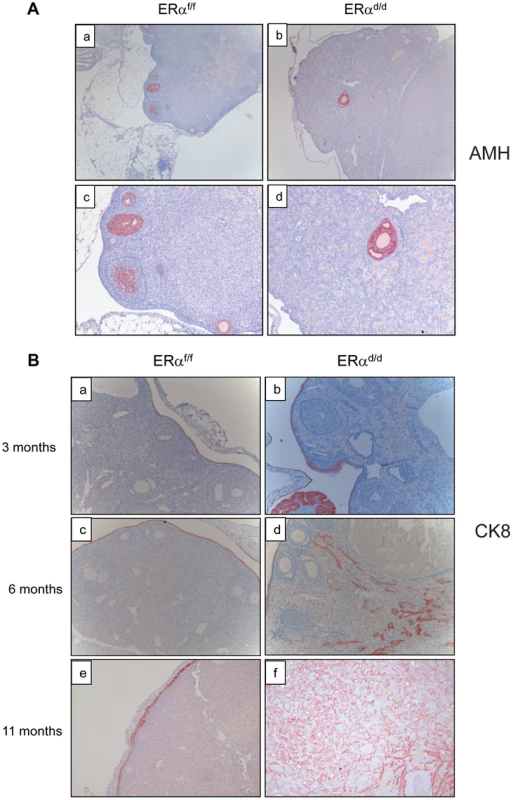
(A) Ovarian sections from ERαf/f (a, c) and ERαd/d (b, d) mice at 11 months of age were subjected to immunohistochemistry using an antibody against AMH. Red staining indicates AMH positive granulosa cells. (B) Ovarian sections from ERαf/f mice at 3, 6, and 11 months of age (left panels a, c, e respectively) and ERαd/d mice at 3, 6, and 11 months of age (right panels b, d, f respectively) were subjected to immunohistochemistry using anti-cytokeratin 8 antibody. Red staining indicates cytokeratin 8 positive epithelial cells. Current literature suggests that the human ovarian epithelial tumors are derived from either OSE or FTE [2], [25]. Although these epithelia are derived from a common embryologic precursor, OSE is thought to retain mesothelial characteristics, while FTE is terminally differentiated [25]–[27]. Recent studies on the serous subtype of ovarian cancer have suggested that either OSE differentiates to resemble FTE or the cancer originates in the fallopian tube and spreads to the ovary [27]. To investigate further the origin of epithelial ovarian tumor cells in ERαd/d mice, we removed the oviducts of these mice prior to tumor formation. Interestingly, removal of the oviducts from pre-pubertal ERαd/d mice did not prevent the onset of ovarian tumor growth in these animals, indicating that the tumor cells originate from the OSE rather than the oviductal epithelium (Fig. 4A). We also examined the epithelia of ERαf/f and ERαd/d ovaries by monitoring the expression of biomarkers specific for either OSE or FTE. As shown in Fig. 4B, we detected prominent expression of calretinin, a mesothelial marker [2], [28], in OSE of ERαf/f ovaries but not in OSE of ERαd/d ovaries. We also noted marked up regulation of tubal-specific makers, including PAX8, WT1, and Ber-EP4 in the ovaries of ERαd/d mice, while the ovaries of ERαf/f mice lacked their expression. Since PAX8, WT1, and Ber-EP4 are normally expressed in FTE and are present in serous epithelial ovarian tumors [2], [28]–[30], it is likely that the OSE cells of ERαd/d ovaries have undergone differentiation to resemble FTE. Furthermore, it has been reported previously that PAX8 is expressed in serous, endometrioid, and mucinous ovarian cancer while expression of WT1 is restricted to the serous subtype of ovarian cancer [29]. Currently there are no available biomarkers that can differentiate between high and low-grade serous ovarian carcinoma. It is clear that the ERαd/d tumors do not grow aggressively.
Fig. 4. OSE is the site of origin for ovarian tumorigenesis in ERαd/d mice. 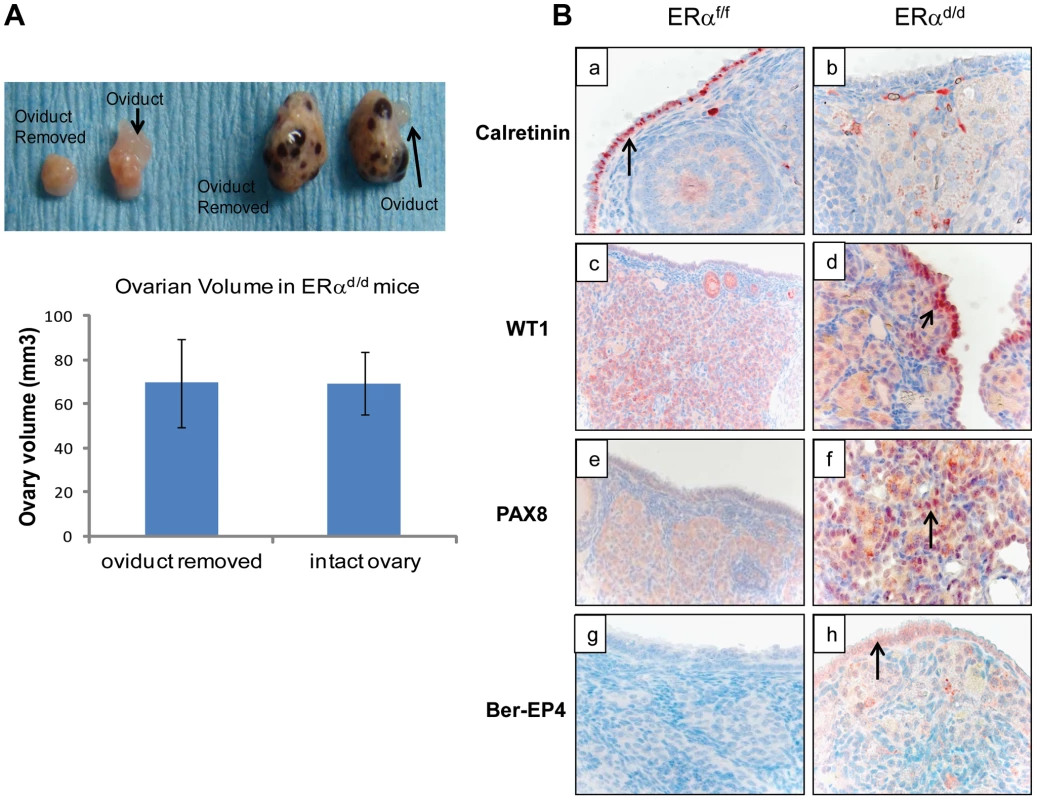
(A) Ovarian tumor growth in ERαd/d mice after surgical removal of oviducts. The right oviduct was surgically removed from ERαf/f and ERαd/d mice at four weeks of age, leaving the left oviduct intact as an internal control. Upper panel, Gross morphology of ERαf/f and ERαd/d ovaries at 5 months after oviduct removal surgery. Lower panel, The graph depicts ovarian volume comparing ERαd/d tumors with and without the oviduct. Two-tailed t-test was performed for statistical analysis: n = 5; p = 0.99. (B) Immunohistochemistry of ERαf/f (left panel) and ERαd/d (right panel) ovaries from mice at 6 months of age using calretinin (a, b); WT1 (c, d); PAX8 (e, f); and Ber-EP4 (g, h) antibodies. Red staining indicates immunostaining. To investigate the molecular pathways underlying ovarian tumorigenesis in ERαd/d mice, we next performed gene expression profiling, using RNA isolated from the ovaries of ERαf/f and ERαd/d mice. We identified more than 2500 genes that were differentially expressed in the tumor tissue compared to the normal ovaries. The GEO accession number for the microarray data is GSE39402. When we compared the differentially regulated genes to three different datasets of differential gene expression profiles of human serous adenocarcinoma versus control human ovaries that exist in the Oncomine database, we noted that a large number of genes, which are differentially expressed in human serous ovarian cancer specimens, are also present in ERαd/d ovarian tumors (Fig. S2A). Remarkably, the identity of genes expressed in ERαd/d ovaries and human serous ovarian cancer ranged from 25–40%. Prominent among these genes were those encoding platelet derived growth factor receptor alpha (PDGFRα), vascular cell adhesion molecule (VCAM), clusterin, intercellular adhesion molecule 1 (ICAM-1), and serine/threonine phosphatase 1 (Wip1), which are overexpressed in human serous ovarian cancer [31]–[36]. We observed that the levels of PDGFRα, VCAM, ICAM1, and clusterin were markedly elevated in the ovaries of ERαd/d mice compared to those of ERαf/f mice (Fig. S2B). Collectively, the presence of these cancer biomarkers in ERαd/d ovarian tumors underscored the importance of this model in deciphering the pathways involved in genesis and progression of epithelial ovarian tumorigenesis.
Although the elevated systemic levels of E in ERαd/d mice likely contribute to the initiation of ovarian tumors by stimulating ERα signaling in OSE, we considered the possibility that, as the follicles are depleted with tumor progression, intratumoral E biosynthesis becomes a major regulator of tumorigenesis. Studies in postmenopausal women reported significantly increased expression and activity of P450 aromatase in serous ovarian carcinomas, but not in benign adenomas, supporting the view that intratumoral E derived from in situ aromatization could function as an autocrine growth regulator for cancer cells [14], [37]. Previous studies have also reported elevated aromatase activity in tumors and ovarian cancer cell lines [38]–[41]. We observed that ovarian tumors of ERαd/d mice do indeed express high levels of P450 aromatase mRNA (Fig. S3A). To localize aromatase expression we digested ERαd/d ovarian tumors into single-cell suspension, plated both fibroblast stromal and epithelial cells, and completed immunocytochemistry co-localizing both aromatase and a marker indicating the cell type. We observed that ovarian tumor cells isolated from ERαd/d mice express high levels of P450 aromatase protein in luteinized stromal cells of the tumor, suggesting that these cells acquired the ability to synthesize E (Figs. S3B). Furthermore, the activated form of ERα, phosphorylated at Ser-118, is abundantly expressed in the OSC, while aromatase is expressed in ovarian stroma of ERαd/d mice as early as 3 months (Fig. S3C). We postulated that the epithelial ERα signaling remains elevated in response to this locally produced E in ERαd/d ovarian tumors, supporting increased ERα-dependent gene expression, abnormal cell proliferation, and tumorigenesis.
To examine whether E plays a critical role in ovarian tumor progression in ERαd/d mice, we chronically treated these mice at 3 months of age with letrozole, a specific inhibitor of P450 aromatase, by implanting silastic capsules containing this drug. Following three months of letrozole treatment, ovarian tumors of 6-month old ERαd/d mice displayed a remarkable reduction, up to 60%, in tumor volume when compared to sham-treated ERαd/d mice (Fig. 5A). It is important to note that while ERαd/d mice treated with letrozole exhibited significantly lower levels of serum E compared to sham-treated ERαd/d mice, their serum LH levels were not altered in response to this treatment (Fig. 5A). Interestingly, ERαd/d mice treated with the letrozole exhibited significantly lower levels of ovarian expression of PDGFRα and VCAM transcripts compared to sham-treated ERαd/d mice (Fig. 5B). Similarly, the levels of Wip1 mRNA and protein were markedly decreased in ovarian tumors of ERαd/d mice upon letrozole treatment (Fig. 5, B and C). Taken together, these results confirmed that elevated E signaling in the ovarian tumors of ERαd/d mice leads to dysregulated expression of a subset of genes with known links to ovarian epithelial cancer.
Fig. 5. ERαd/d ovarian tumor growth is inhibited by P450 aromatase inhibitor. 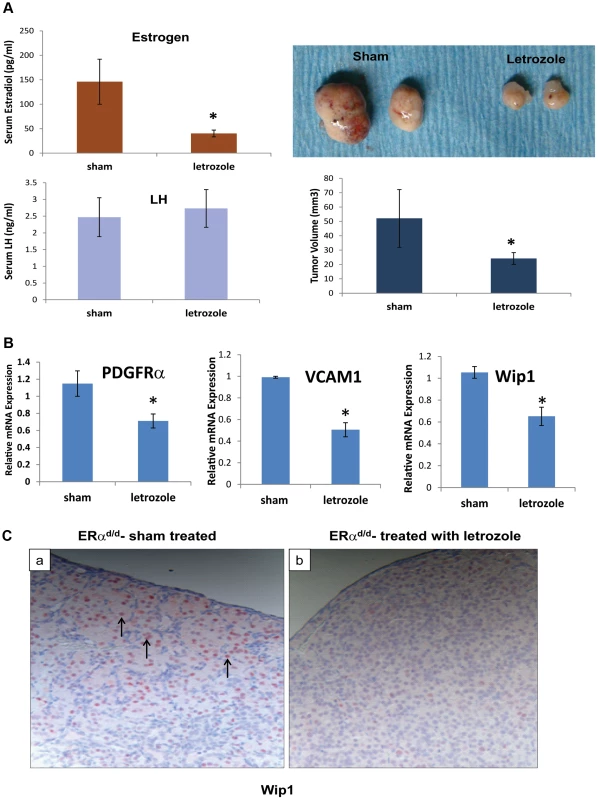
(A) Ovaries with tumors from ERαd/d mice treated with letrozole, a P450 aromatase inhibitor, filled silastic capsules or sham empty silastic capsules for 3 months are analyzed by gross morphology and tumor volume. Serum estradiol and LH is assayed by radioimmunoassay. (B) Real-time quantitative PCR was employed to measure mRNA levels of genes associated with ovarian carcinoma, PDGFRα, VCAM, and Wip1, in ovaries with tumors of ERαd/d mice treated with letrozole-containing or sham empty silastic capsules. (C) Wip1 protein localization in ovaries with tumors of ERαd/d mice either treated with sham control (a) or treated with letrozole (b). Arrow points to nuclear expression of Wip1. * indicates p<.05. Each treatment group had n = 8 samples. Discussion
Genetically engineered mouse models are considered to be among the most powerful and promising tools presently available for studying the biology of various forms of cancer and for developing therapeutics. Although the creation of mouse models of ovarian cancer has lagged behind models for many other neoplastic diseases, significant advances have been made in the last decade. Orsulic et al have shown that p53-deficient ovarian cells engineered to overexpress multiple oncogenes, c-myc, Kras, and Akt, develop ovarian tumors when injected in mice [42]. Similar mouse models for ovarian epithelial tumors were developed via inactivation of various tumor suppressors, such as Pten, APC, p53 and Rb, through intrabursal administration of adenoviral vectors [43], [44]. Conditional inactivation of multiple genes, such as Pten and Kras or PTEN and Dicer, by expression of Cre recombinase driven by the Amhr2 promoter also led to ovarian cancer in mice [45], [46]. These mouse models have provided compelling evidence that OSE or FTE can be transformed by altering the expression of a variety of oncogenic factors or tumor suppressors. Some of these models display tumor histotypes similar to ovarian cancer subtypes seen in women. However, it is clear that these models typically require multiple genetic changes and are limited by very rapid tumor onset, which limits their usefulness for studying early modulators of ovarian tumorigenesis. In the present study, we report the development of a unique animal model, in which initiation of ovarian tumorigenesis is independent of any oncogenic insult but dependent on elevated E signaling in the ovary. Since the onset and progression of tumorigenesis is relatively slow in ERαd/d mice, this model is potentially useful in providing insights into the factors involved in the initiation and early phases of ovarian epithelial tumorigenesis.
In ERαd/d mice, ERα is conditionally ablated in the pituitary but retained in the hypothalamus and ovary. The loss of negative-feedback regulation by E in the HPO axis led to elevated production of LH by the pituitary. Interestingly, high levels of gonadotropins in women in early postmenopause have been postulated to play a role in the development of epithelial ovarian neoplasms [4], [5]. Consistent with this notion, it has been found that women with polycystic ovary syndrome, which is accompanied by high LH levels, have a greater risk of developing ovarian cancer [47]. Further supporting a role of gonadotropins in ovarian cancer development, gonadotropin levels in cysts and peritoneal fluid from ovarian cancer patients have been shown to be elevated [48]. However, not all studies have led to similar findings and it is clear that elevated gonadotropin levels alone do not cause ovarian cancer. In fact, our studies using the ERαd/d model suggested that hyperstimulation of ovarian cells by LH results in increased steroidogenesis, leading to high levels of circulating E as well as locally produced E in the ovarian tissue. High levels of testosterone coupled with increased expression of aromatase in the ovarian tissue would lead to increased synthesis of local E. We propose that this elevated E is an important factor in epithelial ovarian tumorigenesis as it stimulates signaling in the OSE, promoting its proliferation and phenotypic transformation. These results are supported by epidemiological and clinical studies, which indicate that postmenopausal women with elevated gonadotropin levels and receiving E replacement therapy exhibit an increased incidence of ovarian tumors [9]–[13]. Consistent with a role of E in the genesis of ovarian tumors, recent reports point to the clinical use of anti-estrogen drugs in stabilization of ovarian cancers [49], [50].
Although many previous studies indicated that epithelial ovarian cancer arises from OSE, recent studies have revealed that the fimbriae of the fallopian tube is a possible site of origin of this malignancy, particularly high-grade serous carcinoma [51]. The common embryologic precursor of OSE and FTE is the coelomic epithelium, which gives rise to the epithelial linings of the fallopian tube and the ovary [27]. Unlike FTE, OSE retains mesothelial characteristics and is not terminally differentiated. It has been proposed that either OSE terminally differentiates to resemble FTE, or the cancer originates in fallopian tube and then spreads to the ovary. In support of the latter hypothesis, a recent study showed that conditional deletion of Pten and Dicer, using the Amhr2-Cre, led to tumor development in the fallopian tube, which subsequently metastasized to the ovary [46]. Our studies, on the other hand, appear to indicate that ovarian tumorigenesis in ERαd/d mice is associated with differentiation of OSE to FTE. We observed prominent expression of FTE marker proteins, such as PAX8, WT1, and Ber-EP4, which are not normally expressed in OSE, in the ovaries of ERαd/d mice. Furthermore, removal of oviducts from ERαd/d mice did not prevent the onset of ovarian tumorigenesis, indicating that FTE is not the precursor tissue for tumorigenesis in ERαd/d mice.
Interestingly, we did not observe any intraperitoneal metastatic spread of the ovarian tumor in ERαd/d mice. This could be partly due to the fact that the majority of the mutant mice died by 10 months of age due to the enlarged tumor, making it difficult to follow the progression of tumorigenesis beyond this point. The absence of overt malignancy in our model is not entirely surprising as several recent studies indicate that multiple genetic changes are necessary for metastatic transformation. It is conceivable that additional mutation(s) in tumor suppressor genes, such as p53, is required to drive the tumorigenic pathways in ERαd/d ovaries to rapidly progressing ovarian carcinoma, which will culminate in metastasis. Indeed, recent studies, utilizing genomic sequencing data from human high-grade serous ovarian cancer specimens, have shown that these cancers exhibit genomic instability and harbor genetic mutations in p53, Rb, BRCA1, and/or BRCA2 loci [52]–[55].
The ovarian tumors in ERαd/d mice are composed of cells of both epithelial and stromal origins. These tumors appear to be distinct from the tubular or tubulostromal adenomas, which are reported to occur spontaneously in a number of mutant mouse strains, including the WXWX mice [56], [57]. The adenomas, composed of numerous tube-like structures plus abundant large luteinized stromal cells, arise due to a defect in primordial germ cell proliferation and rapid loss of oocytes at birth, resulting in destruction of graafian follicles [58], [59]. They also arise when mice are irradiated and there is a rapid loss of oocytes after radiation exposure [60]. However, these adenomas are not lethal and administration of E prevents rather than promotes their development [61]. Furthermore, in contrast to these mutant mouse strains, the ERαd/d mice exhibit normal number of oocytes at 3 months of age, which then start to decline when ovarian epithelial and stromal cells expand and form the tumor mass at 6 months. Therefore, the initial stages of tumorigenesis in ERαd/d mice are independent of the oocyte loss. Most importantly, the growth of the ovarian tumors exhibited by the ERαd/d mice is inhibited by letrozole, indicating that these tumors, unlike adenomas, are E-dependent. The ovarian tumors in the ERαd/d mice are presumably dependent on pituitary LH production, which help luteinize the stromal cells. However, the local production of E by these tumors and the resulting estrogenic effects on ovarian surface epithelial expansion and transformation appear to be the two key features that distinguish these tumors from the endocrinologically inactive tubular adenomas or tubulostromal adenomas.
Although the ovarian neoplasm in ERαd/d mice did not show signs of overt malignancy, there was nevertheless clear evidence of tumorigenic transformation. Particularly striking is the finding that a large number of genes, associated with human serous ovarian cancer, are also expressed in ERαd/d ovarian tumors. Specifically, these tumors exhibit dysregulated expression of PDGFRα, VCAM, and Wip1, which were previously reported to be involved in human ovarian cancer. PDGFRα, a cell surface tyrosine kinase receptor for members of the platelet-derived growth factor family, is over-expressed in human serous ovarian tumors and is targeted in clinical trials to treat ovarian cancers [32], [33]. VCAM, a vascular cell adhesion molecule, is found in the blood circulation of cancer patients and has recently been proposed as a marker to detect early stages of ovarian cancer [34], [35]. Wip1, a p53-inducible phosphatase and an oncogene, is of particular interest. Under normal conditions, it restores cellular homeostasis following DNA-damage by cooperating with p53 to induce G2/M cell cycle arrest, thereby allowing ample time for repair of the damaged DNA [62]. However, amplification of Wip1 leads to sustained inhibition of DNA damage response and tumor suppressors, and consequently, its overexpression has been implicated in a variety of human malignancies, including ovarian carcinoma [37], [63]. Recent studies have revealed that Wip1 is regulated by ERα [64]. Consistent with this finding, administration of letrozole to ERαd/d mice, which decreased the ovarian tumor size, also markedly reduced the expression of Wip1 along with PDGFRα, and VCAM. These results are consistent with our hypothesis that accentuated E signaling in the ovarian tissue promotes aberrant expression of genes that participate in tumorigenesis.
In summary, we describe a unique mouse model that allows us to identify hormonal effectors, particularly elevated E signaling, which play an important role in the development of ovarian epithelial tumorigenesis. In the future, the ERαd/d model will serve as a valuable tool for exploring the involvement of E-dependent signaling pathways in the onset and progression of this deadly disease.
Materials and Methods
Animals
Mice (C57BL/6; Jackson Laboratory) were maintained in the designated animal care facility at the University of Illinois College of Veterinary Medicine according to the institutional guidelines for the care and use of laboratory animals. We crossed mice harboring ‘floxed’ ERα gene (Esr1tm1.2Mma), termed ERαf/f, with PR-Cre mice expressing Cre recombinase under the control of progesterone receptor promoter (Pgrtm2(cre)Lyd) to develop mice of genotype Esr1tm1.2Mma/Esr1tm1.2Mma Pgrtm2(cre)Lyd/Pgr+, which we termed ERαd/d. The PR-Cre knock-in mice expression of cre recombinase in pituitary, uterus, oviduct, mammary gland, and corpora lutea of the ovary have been described previously [18]. It has been used extensively to ablate “floxed” genes in tissues expressing PR [17], [18].
Immunohistochemistry (IHC)
Paraffin-embedded ovarian tissue sectioned at 4 µm, mounted on slides and subjected to immunohistochemistry as described previously [65]. Sections were incubated at 4°C with polyclonal antibodies against PCNA (Santa Cruz sc-56), cytokeratin 8 (Developmental Studies Hybridoma Bank, TROMA I), ERα (Novacastra Laboratories), p-Akt1/2/3 serine 473 (Santa Cruz SC-33437), AMH (Santa Cruz Biotechnology SC-6886), WT1 (Santa Cruz Biotechnology), PAX8 (Proteintech group 10336-1-AP), calretinin (Invitrogen 18-0291), Ber-EP4 (Dako), aromatase (Abcam ab35604), vimentin (Sigma Aldrich V5255). Biotinylated secondary antibodies were used followed by incubation with horseradish peroxidase-conjugated streptavidin (Invitrogen). Sections were stained in AEC Solution.
Real-time PCR analysis
Total RNA was isolated from ovaries by standard Trizol-based protocols and converted to cDNA. The cDNA was amplified by real-time PCR to quantify gene expression using gene-specific primers and SYBR Green (Applied Biosystems). As a loading control, the expression level of RPLP0 (36B4), which encodes a ribosomal protein, was determined. For each treatment, the mean threshold cycle (CT) and standard deviation were calculated from CT values obtained individually from 3 to 4 replicates of that sample. Each sample was subjected to three independent real-time PCR trials. The fold change was derived from the mean CT values. Primer sequences recognizing each gene are located in Table S1.
Measurement of serum hormones
Hormones were measured by radioimmunoassay (RIA) at the Ligand Core facility, University of Virginia, Charlottesville. Statistical significance was determined on SAS program using the Tukey procedure to control for comparison-wise error rate. Significance cutoff value of p<.05 was determined to be statistically significant.
Isolation of cells from ovaries with tumors
Ovarian tumors were removed from mice and digested with either 6 g/liter dispase (Invitrogen) and 25 g/liter pancreatin (Sigma Aldrich), or 0.5 g/liter collagenase (Sigma Aldrich) in Hank's balanced salt solution (HBSS). After incubation for 1 h at 37°C, the tubes were vortexed for 10–12 s until the supernatant became turbid with dispersed cells. The contents were then passed through an 80-µm gauze filter (Millipore). Cells were re-suspended in Dulbecco's modified Eagle's F12 medium (DMEM-F12; with 100 unit/liter penicillin, 0.1 g/liter streptomycin, 1.25 mg/liter fungizone) containing 10% heat-inactivated fetal calf serum. Cell culture was continued for 48 h after addition of fresh medium.
Immunocytochemistry
Ovarian tumor cells were fixed with 10% formalin solution for 10 m. Cells were treated with 25% Triton X-100 (Sigma Aldrich) in PBS for 10 m and exposed to a blocking serum for 1 h. Cells were treated with primary antibodies and incubated at 4°C and exposed to cy3 or cy5-conjugated secondary antibodies.
Silastic capsule implant
Silastic capsules were made by filling silastic laboratory tubing with 0.8 mg of ground Novartis Femara tablets (letrozole) and sealing with medical adhesive silicone type A (Dow Corning). For surgery, mice were first treated with analgesic 1 h prior to surgery and then anesthetized. A small dorsal incision was made just below the neck, and the silastic capsule was inserted underneath the skin. The incision was held together with wound clips until healed. After 3 months of exposure to either empty silastic capsules (sham control) or silastic capsules containing letrozole, mice were euthanized and ovarian tumors were fixed or frozen for analysis.
Statistical analysis
Statistical analysis was performed by ANOVA or two-tailed student's ttest. Values of P<0.05 were considered significant.
Supporting Information
Zdroje
1. SiegelR, NaishadhamD, JemalA (2012) Cancer statistics. CA Cancer J Clin 62 (1) 10–29.
2. AuerspergN (2011) The origin of ovarian carcinomas: a unifying hypothesis. Int J Gynecol Pathol 30 (1) 12–21.
3. BastRC, HennessyB, MillsGB (2009) The biology of ovarian cancer: new opportunities for translation. Nature Reviews Cancer 9 : 415–428.
4. StadelBV (1975) Letter: the etiology and prevention of ovarian cancer. Am J Obstet Gynecol 123 : 772–774.
5. Mertens-WalkerI, BaxterRC, MarshDJ (2012) Gonadotropin signaling in epithelial ovarian cancer. Cancer Letters 324 : 152–159.
6. StewartSL, QuerecTD, OchmanAR, GruverBN, BaoR, et al. (2004) Characterization of a carcinogenesis rat model of ovarian preneoplasia and neoplasia. Cancer Res 64 : 8177–8183.
7. RimanT, PerssonS, NilssonS (1998) Hormonal aspects of epithelial ovarian cancer: review of epidemiological evidence. Clin Endocrinol 49 : 695–707.
8. ChakravartiS, CollinsWP, ForecastJD, NewtonJR, OramDH, et al. (1976) Hormonal profiles after the menopause. Br Med J 2 : 784–787.
9. LaceyJVJr, MinkPJ, LubinJH, ShermanME, TroisiR, et al. (2002) Menopausal hormone replacement therapy and risk of ovarian cancer. JAMA 288 : 334–341.
10. GludE, KjaerSK, ThomsenBL, HøgdallC, ChristensenL, et al. (2004) Hormone therapy and the impact of estrogen intake on the risk of ovarian cancer. Arch Intern Med 164 : 2253–2259.
11. BeralV (2007) Million Women Study Collaborators (2007) BullD, GreenJ, ReevesG (2007) Ovarian cancer and hormone replacement therapy in the Million Women Study. Lancet 369 : 1703–1710.
12. RossingMA, Cushing-HaugenKL, WicklundKG, DohertyJA, WeissNS (2007) Menopausal hormone therapy and risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev 16 : 2548–2556.
13. MørchLS, LøkkegaardE, AndreasenAH, Krüger-KjaerS, LidegaardO (2009) Hormone therapy and ovarian cancer. JAMA 302 : 298–305.
14. SasanoH, HaradaN (1998) Intratumoral aromatase in human breast, endometrial, and ovarian malignancies. Endocr Rev 19 : 593–607.
15. BaiW, Oliveros-SaundersB, WangQ, Acevedo-DuncanME, NicosiaSV (2000) Estrogen stimulation of ovarian surface epithelial cell proliferation. In Vitro Cell Dev Biol Anim 36 : 657–66.
16. LavioletteLA, GarsonK, MacdonaldEA, SentermanMK, CourvilleK, et al. (2010) 17beta-estradiol accelerates tumor onset and decreases survival in a transgenic mouse model of ovarian cancer. Endocrinology 151 : 929–38.
17. DupontS, KrustA, GansmullerA, DierichA, ChambonP, et al. (2000) Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development 127 : 4277–4291.
18. SoyalSM, MukherjeeA, LeeKY, LiJ, LiH, et al. (2005) Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis 41 : 58–66.
19. HewittSC, KorachKS (2000) Progesterone action and responses in the alphaERKO mouse. Steroids 65 : 551–7.
20. CouseJF, YatesMM, WalkerVR, KorachKS (2003) Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) Null mice reveals hypergonadism and endocrine sex reversal in females lacking ERalpha but not ERbeta. Mol Endocrinol 17 : 1039–53.
21. AltomareDA, WangHQ, SkeleKL, De RienzoA, Klein-SzantoAJ, et al. (2004) AKT and mTOR phosphorylation is frequently detected in ovarian cancer and can be targeted to disrupt ovarian tumor cell growth. Oncogene 23 : 5853–5857.
22. VivancoI, SawyersCL (2002) The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer 2 : 489–501.
23. KimuraA, OhmichiM, KawagoeJ, KyoS, MabuchiS, et al. (2004) Induction of hTERT expression and phosphorylation by estrogen via Akt cascade in human ovarian cancer cell lines. Oncogene 23 : 4505–15.
24. ReyR, SabourinJC, VenaraM, LongWQ, JaubertF, et al. (2000) Anti-Mullerian hormone is a specific marker of sertoli - and granulosa-cell origin in gonadal tumors. Hum Pathol 31 : 1202–1208.
25. AuerspergN, WongAS, ChoiKC, KangSK, LeungPC (2001) Ovarian surface epithelium: biology, endocrinology and pathology. Endocr Rev 22 : 255–288.
26. AuerspergN, WooMM, GilksCB (2008) The origin of ovarian carcinomas: a developmental view. Gynecol Oncol 110 : 452–454.
27. KingSJ, BurdetteJE (2011) Evaluating the progenitor cells of ovarian cancer: analysis of current animal models. BMB reports 44 : 435–445.
28. KurmanRJ, ShihLM (2010) The origin and pathogenesis of epithelial ovarian cancer-a proposed unifying theory. Am J Surg Pathol 34 : 433–443.
29. NonakaD, ChiribogaL, SoslowRA (2008) Expression of pax8 as a useful marker in distinguishing ovarian carcinomas from mammary carcinomas. Am J Surg Pathol 32 : 1566–1571.
30. TongGX, DevarajK, Hamele-BenaD, YuWM, TurkA, et al. (2011) Pax8: a marker for carcinoma of Mullerian origin in serous effusions. Diagn Cytopathol 39 : 567–574.
31. WilczynskiSP, ChenYY, ChenW, HowellSB, ShivelyJE, et al. (2005) Expression and mutational analysis of tyrosine kinase receptors c-kit, PDGFRalpha, and PDGFRbeta in ovarian cancers. Hum Pathol 36 : 242–9.
32. GiavazziR, NicolettiMI, ChiriviRG, HemingwayI, BernasconiS, et al. (1994) Soluble intercellular adhesion molecule 1 (ICAM-1) is released into the serum and ascites of human ovarian carcinoma patients and in nude mice bearing tumor xenogtafts. Eur J Cancer 30A: 1865–70.
33. BanksRE, GearingAJ, HemingwayIK, NorfolkDR, PerrenTJ, et al. (1993) Circulating intercellular adhesion molecule-1 (ICAM-1), E-selectin and vascular cell adhesion molecule-1 (VCAM-1) in human malignancies. Br J Cancer 68 : 122–4.
34. YurkovetskyZ, SkatesS, LomakinA, NolenB, PulsipherT, et al. (2010) Development of a multimarker assay for early detection of ovarian cancer. J Clin Oncol 28 : 2159–66.
35. GunawardanaCG, KukC, SmithCR, BatruchI, SoosaipillaiA, et al. (2009) Comprehensive analysis of conditioned media from ovarian cancer cell lines identifies novel candidate markers of epithelial ovarian cancer. J Proteome Res 8 : 4705–13.
36. TanDS, LambrosMB, RayterS, NatrajanR, VatchevaR, et al. (2009) PPM1D (Wip1) is a potential therapeutic target in ovarian clear cell carcinomas. Clin Cancer Res 15 : 2269–2280.
37. MacLuskyNJ, VoitR, LazoJS, SchwartzPE, MerinoMJ, et al. (1987) Aromatase activity in human ovarian cancer. Steroids 50 : 423–433.
38. ThompsonMA, AdelsonMD, KaufmanLM, MarshallLD, CobleDA (1988) Aromatization of testosterone by epithelial tumor cells cultured from patients with ovarian carcinoma. Cancer Res 48 : 6491–6497.
39. ZimniskiSJ, GarolaRE, FendlK, PetersonCM (1989) Endocrine characterization of a human ovarian carcinoma (BG-1) established in nude mice. Steroids 54 : 593–606.
40. CunataS, HoffmannbP, PujolP (2004) Estrogens and epithelial ovarian cancer. Gynecologic Oncology 94 (1) 25–32.
41. GoodmanMT, LurieG, ThompsonPJ, McDuffieKE, CarneyME (2008) Association of two common single-nucleotide polymorphisms in the CYP19A1 locus and ovarian cancer risk. Endocr Relat Cancer 15 (4) 1055–60.
42. OrsulicS, LiY, SoslowRA, Vitale-CrossLA, GutkindJS, et al. (2002) Induction of ovarian cancer by defined multiple genetic changes in a mouse model system. Cancer Cell 1 : 53–62.
43. Flesken-NikitinA, ChoiKC, EngJP, ShmidtEN, NikitinAY (2003) Induction of carcinogenesis by concurrent inactivation of p53 and Rb1 in the mouse ovarian surface epithelium. Cancer Res 63 : 3459–3463.
44. WuR, Hendrix-LucasN, KuickR, ZhaiY, SchwartzDR, et al. (2007) Mouse model of human ovarian endometrioid adenocarcinoma based on somatic defects in the Wnt/beta-catenin and PI3K/Pten signaling pathways. Cancer Cell 11 : 321–33.
45. FanHY, LiuZ, PaquetM, WangJ, LydonJP, et al. (2009) Cell type specific targeted mutation of Kras and Pten document proliferation arrest in granulosa cells versus oncogenic insult in ovarian surface epithelial cells. Cancer Res 69 : 6463–6472.
46. KimJ, CoffeyDM, CreightonCJ, YuZ, HawkinsSM, et al. (2012) High-grade serous ovarian cancer arises from fallopian tube in a mouse model. Proc Natl Acad Sci U S A 109 : 3921–6.
47. SchildkrautJM, SchwinglPJ, BastosE, EvanoffA, HughesC (1996) Epithelial ovarian cancer risk among women with polycystic ovary syndrome. Obstet Gynecol 88 : 554–559.
48. HalperinR, HadasE, LangerR, BukovskyI, SchneiderD (1999) Peritoneal fluid gonadotropins and ovarian hormones in patients with ovarian cancer. Int J Gynecol Cancer 9 : 502–507.
49. KothariR, ArgentaP, FowlerJ, CarterJ, ShimpW (2010) Antiestrogen therapy in recurrent ovarian cancer resulting in 28 months of stable disease: a case report and review of the literature. Arch Oncol 18 : 32–35.
50. ArgentaPA, ThomasSG, JudsonPL, DownsLSJr, GellerMA, et al. (2009) A phase II study of fulvestrant in the treatment of multiply-recurrent epithelial ovarian cancer. Gynecol Oncol 113 : 205–9.
51. JarboeE, FolkinsA, NucciMR, KindelbergerD, DrapkinR, et al. (2008) Serous carcinogenesis in the fallopian tube: a descriptive classification. Int J Gynecol Pathol 27 : 1–9.
52. Cancer Genome Atlas Research Network (2011) Integrated genomic analyses of ovarian carcinoma. Nature 474 : 609–615.
53. MullanyLK, RichardsJS (2012) Minireview: Animal models and mechanisms of ovarian cancer development. Endocrinology 153 : 1585–1592.
54. HashiguchiY, TsudaH, YamamotoK, InoueT, IshikoO, et al. (2001) Combined analysis of p53 and RB pathways in epithelial ovarian cancer. Hum Pathol 32 : 988–96.
55. RischHA, McLaughlinJR, ColeDE, RosenB, BradleyL, et al. (2001) Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am J Hum Genet 68 : 700–710.
56. RehmS, DierksenD, DeerbergF (1984) Spontaneous ovarian tumors in Han:NMRI mice: histological classification, incidence and influence of food restriction. J Natl Cancer Inst 72 : 1383–96.
57. AllisonRH, MorganKT (1987) Ovarian neoplasms in F344 rats and B6C3F1 mice. Environ Helath Perspect 73 : 91–106.
58. CapenCharles C (2004) Mechanisms of hormone-mediated carcinogenesis of the ovary. Toxicologic Pathology 32 (Suppl.2) 1–5.
59. MurphyED (1972) Hyperplastic and early neoplastic changes in the ovaries of mice after genic deletion of germ cells. J Natl Cancer Inst 48 : 1283–95.
60. GardnerWU (1950) Ovarian and lymphoid tumors in female mice subsequent to Roentgen-ray irradiation and hormone treatment. Proc Soc Exp Biol Med 75 : 434–6.
61. CapenCC, BeamerWG, TennentBJ, StitzelKA (1995) Mechanisms of hormone-mediated carcinogenesis of the ovary in mice. Mutation Research 333 : 143–51.
62. LuX, NannengaB, DonehowerLA (2005) PPM1D dephosphorylates Chk1 and p53 and abrogates cell cycle checkpoints. Genes Dev 19 : 1162–74.
63. BulavinDV, DemidovON, SaitoS, KauraniemiP, PhillipsC, et al. (2002) Amplification of PPM1D in human tumors abrogates p53 tumor-suppressor activity. Nat Genet 31 : 210–215.
64. HanHS, YuE, SongJY, ParkJY, JangSJ, et al. (2009) The estrogen receptor alpha pathway induces oncogenic Wip1 phosphatase gene expression. Mol Cancer Res 7 : 713–23.
65. LiQ, CheonYP, et al. (2004) “A novel pathway involving progesterone receptor, 12/15-lipoxygenase-derived eicosanoids, and peroxisome proliferator-activated receptor gamma regulates implantation in mice.”. J Biol Chem 279 (12) 11570–11581.
Štítky
Genetika Reprodukční medicína
Článek Sex Chromosome Turnover Contributes to Genomic Divergence between Incipient Stickleback SpeciesČlánek Final Pre-40S Maturation Depends on the Functional Integrity of the 60S Subunit Ribosomal Protein L3
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 3- Akutní intermitentní porfyrie
- Souvislost haplotypu M2 genu pro annexin A5 s opakovanými reprodukčními ztrátami
- Příjem alkoholu a menstruační cyklus
- Doporučení pro diagnostiku a léčbu akutních jaterních porfyrií
- Hysteroskopická resekce děložního septa zlepšuje šanci na graviditu žen s jinak nevysvětlenou infertilitou
-
Všechny články tohoto čísla
- , a Gene That Influences the Anterior Chamber Depth, Is Associated with Primary Angle Closure Glaucoma
- Genomic View of Bipolar Disorder Revealed by Whole Genome Sequencing in a Genetic Isolate
- The Rate of Nonallelic Homologous Recombination in Males Is Highly Variable, Correlated between Monozygotic Twins and Independent of Age
- Genetic Determinants Influencing Human Serum Metabolome among African Americans
- Heterozygous and Inherited Mutations in the Smooth Muscle Actin () Gene Underlie Megacystis-Microcolon-Intestinal Hypoperistalsis Syndrome
- Genome-Wide Meta-Analysis of Homocysteine and Methionine Metabolism Identifies Five One Carbon Metabolism Loci and a Novel Association of with Ischemic Stroke
- Cancer Evolution Is Associated with Pervasive Positive Selection on Globally Expressed Genes
- Genetic Diversity in the Interference Selection Limit
- Integrating Multiple Genomic Data to Predict Disease-Causing Nonsynonymous Single Nucleotide Variants in Exome Sequencing Studies
- An Evolutionary Analysis of Antigen Processing and Presentation across Different Timescales Reveals Pervasive Selection
- Cleavage Factor I Links Transcription Termination to DNA Damage Response and Genome Integrity Maintenance in
- DNA Dynamics during Early Double-Strand Break Processing Revealed by Non-Intrusive Imaging of Living Cells
- Genetic Basis of Metabolome Variation in Yeast
- Modeling 3D Facial Shape from DNA
- Dysregulated Estrogen Receptor Signaling in the Hypothalamic-Pituitary-Ovarian Axis Leads to Ovarian Epithelial Tumorigenesis in Mice
- Photoactivated UVR8-COP1 Module Determines Photomorphogenic UV-B Signaling Output in
- Local Evolution of Seed Flotation in Arabidopsis
- Chromatin Targeting Signals, Nucleosome Positioning Mechanism and Non-Coding RNA-Mediated Regulation of the Chromatin Remodeling Complex NoRC
- Nucleosome Acidic Patch Promotes RNF168- and RING1B/BMI1-Dependent H2AX and H2A Ubiquitination and DNA Damage Signaling
- The -Induced Arabidopsis Transcription Factor Attenuates ABA Signaling and Renders Seedlings Sugar Insensitive when Present in the Nucleus
- Changes in Colorectal Carcinoma Genomes under Anti-EGFR Therapy Identified by Whole-Genome Plasma DNA Sequencing
- Selection of Orphan Rhs Toxin Expression in Evolved Serovar Typhimurium
- FAK Acts as a Suppressor of RTK-MAP Kinase Signalling in Epithelia and Human Cancer Cells
- Asymmetry in Family History Implicates Nonstandard Genetic Mechanisms: Application to the Genetics of Breast Cancer
- Co-translational Localization of an LTR-Retrotransposon RNA to the Endoplasmic Reticulum Nucleates Virus-Like Particle Assembly Sites
- Sex Chromosome Turnover Contributes to Genomic Divergence between Incipient Stickleback Species
- DWARF TILLER1, a WUSCHEL-Related Homeobox Transcription Factor, Is Required for Tiller Growth in Rice
- Functional Organization of a Multimodular Bacterial Chemosensory Apparatus
- Genome-Wide Analysis of SREBP1 Activity around the Clock Reveals Its Combined Dependency on Nutrient and Circadian Signals
- The Yeast Sks1p Kinase Signaling Network Regulates Pseudohyphal Growth and Glucose Response
- An Interspecific Fungal Hybrid Reveals Cross-Kingdom Rules for Allopolyploid Gene Expression Patterns
- Temperate Phages Acquire DNA from Defective Prophages by Relaxed Homologous Recombination: The Role of Rad52-Like Recombinases
- Dying Cells Protect Survivors from Radiation-Induced Cell Death in
- Determinants beyond Both Complementarity and Cleavage Govern MicroR159 Efficacy in
- The bHLH Factors Extramacrochaetae and Daughterless Control Cell Cycle in Imaginal Discs through the Transcriptional Regulation of the Phosphatase
- The First Steps of Adaptation of to the Gut Are Dominated by Soft Sweeps
- Bacterial Regulon Evolution: Distinct Responses and Roles for the Identical OmpR Proteins of Typhimurium and in the Acid Stress Response
- Final Pre-40S Maturation Depends on the Functional Integrity of the 60S Subunit Ribosomal Protein L3
- Mitogen-Activated Protein Kinase (MAPK) Pathway Regulates Branching by Remodeling Epithelial Cell Adhesion
- CDP-Diacylglycerol Synthetase Coordinates Cell Growth and Fat Storage through Phosphatidylinositol Metabolism and the Insulin Pathway
- Coronary Heart Disease-Associated Variation in Disrupts a miR-224 Binding Site and miRNA-Mediated Regulation
- TBX3 Regulates Splicing : A Novel Molecular Mechanism for Ulnar-Mammary Syndrome
- Identification of Interphase Functions for the NIMA Kinase Involving Microtubules and the ESCRT Pathway
- Is a Cancer-Specific Fusion Gene Recurrent in High-Grade Serous Ovarian Carcinoma
- LILRB2 Interaction with HLA Class I Correlates with Control of HIV-1 Infection
- Worldwide Patterns of Ancestry, Divergence, and Admixture in Domesticated Cattle
- Parent-of-Origin Effects Implicate Epigenetic Regulation of Experimental Autoimmune Encephalomyelitis and Identify Imprinted as a Novel Risk Gene
- The Phospholipid Flippase dATP8B Is Required for Odorant Receptor Function
- Noise Genetics: Inferring Protein Function by Correlating Phenotype with Protein Levels and Localization in Individual Human Cells
- DUF1220 Dosage Is Linearly Associated with Increasing Severity of the Three Primary Symptoms of Autism
- Sugar and Chromosome Stability: Clastogenic Effects of Sugars in Vitamin B6-Deficient Cells
- Pheromone-Sensing Neurons Expressing the Ion Channel Subunit Stimulate Male Courtship and Female Receptivity
- Gene-Based Sequencing Identifies Lipid-Influencing Variants with Ethnicity-Specific Effects in African Americans
- Telomere Shortening Unrelated to Smoking, Body Weight, Physical Activity, and Alcohol Intake: 4,576 General Population Individuals with Repeat Measurements 10 Years Apart
- A Combination of Activation and Repression by a Colinear Hox Code Controls Forelimb-Restricted Expression of and Reveals Hox Protein Specificity
- An ER Complex of ODR-4 and ODR-8/Ufm1 Specific Protease 2 Promotes GPCR Maturation by a Ufm1-Independent Mechanism
- Epigenetic Control of Effector Gene Expression in the Plant Pathogenic Fungus
- Genetic Dissection of Photoreceptor Subtype Specification by the Zinc Finger Proteins Elbow and No ocelli
- Validation and Genotyping of Multiple Human Polymorphic Inversions Mediated by Inverted Repeats Reveals a High Degree of Recurrence
- CYP6 P450 Enzymes and Duplication Produce Extreme and Multiple Insecticide Resistance in the Malaria Mosquito
- GC-Rich DNA Elements Enable Replication Origin Activity in the Methylotrophic Yeast
- An Epigenetic Signature in Peripheral Blood Associated with the Haplotype on 17q21.31, a Risk Factor for Neurodegenerative Tauopathy
- Lsd1 Restricts the Number of Germline Stem Cells by Regulating Multiple Targets in Escort Cells
- RBPJ, the Major Transcriptional Effector of Notch Signaling, Remains Associated with Chromatin throughout Mitosis, Suggesting a Role in Mitotic Bookmarking
- The Membrane-Associated Transcription Factor NAC089 Controls ER-Stress-Induced Programmed Cell Death in Plants
- The Functional Consequences of Variation in Transcription Factor Binding
- Comparative Genomic Analysis of N-Fixing and Non-N-Fixing spp.: Organization, Evolution and Expression of the Nitrogen Fixation Genes
- An Insulin-to-Insulin Regulatory Network Orchestrates Phenotypic Specificity in Development and Physiology
- Suicidal Autointegration of and Transposons in Eukaryotic Cells
- A Multi-Trait, Meta-analysis for Detecting Pleiotropic Polymorphisms for Stature, Fatness and Reproduction in Beef Cattle
- Genome-Wide DNA Methylation Analysis Predicts an Epigenetic Switch for GATA Factor Expression in Endometriosis
- Genome-Wide DNA Methylation Analysis of Human Pancreatic Islets from Type 2 Diabetic and Non-Diabetic Donors Identifies Candidate Genes That Influence Insulin Secretion
- The and Hybrid Incompatibility Genes Suppress a Broad Range of Heterochromatic Repeats
- The Kil Peptide of Bacteriophage λ Blocks Cytokinesis via ZipA-Dependent Inhibition of FtsZ Assembly
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Worldwide Patterns of Ancestry, Divergence, and Admixture in Domesticated Cattle
- Genome-Wide DNA Methylation Analysis of Human Pancreatic Islets from Type 2 Diabetic and Non-Diabetic Donors Identifies Candidate Genes That Influence Insulin Secretion
- Genetic Dissection of Photoreceptor Subtype Specification by the Zinc Finger Proteins Elbow and No ocelli
- GC-Rich DNA Elements Enable Replication Origin Activity in the Methylotrophic Yeast
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



