-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Genetic Requirements for Signaling from an Autoactive Plant NB-LRR Intracellular Innate Immune Receptor
Plants react to pathogen attack via recognition of, and response to, pathogen-specific molecules at the cell surface and inside the cell. Pathogen effectors (virulence factors) are monitored by intracellular nucleotide-binding leucine-rich repeat (NB-LRR) sensor proteins in plants and mammals. Here, we study the genetic requirements for defense responses of an autoactive mutant of ADR1-L2, an Arabidopsis coiled-coil (CC)-NB-LRR protein. ADR1-L2 functions upstream of salicylic acid (SA) accumulation in several defense contexts, and it can act in this context as a “helper” to transduce specific microbial activation signals from “sensor” NB-LRRs. This helper activity does not require an intact P-loop. ADR1-L2 and another of two closely related members of this small NB-LRR family are also required for propagation of unregulated runaway cell death (rcd) in an lsd1 mutant. We demonstrate here that, in this particular context, ADR1-L2 function is P-loop dependent. We generated an autoactive missense mutation, ADR1-L2D484V, in a small homology motif termed MHD. Expression of ADR1-L2D848V leads to dwarfed plants that exhibit increased disease resistance and constitutively high SA levels. The morphological phenotype also requires an intact P-loop, suggesting that these ADR1-L2D484V phenotypes reflect canonical activation of this NB-LRR protein. We used ADR1-L2D484V to define genetic requirements for signaling. Signaling from ADR1-L2D484V does not require NADPH oxidase and is negatively regulated by EDS1 and AtMC1. Transcriptional regulation of ADR1-L2D484V is correlated with its phenotypic outputs; these outputs are both SA–dependent and –independent. The genetic requirements for ADR1-L2D484V activity resemble those that regulate an SA–gradient-dependent signal amplification of defense and cell death signaling initially observed in the absence of LSD1. Importantly, ADR1-L2D484V autoactivation signaling is controlled by both EDS1 and SA in separable, but linked pathways. These data allows us to propose a genetic model that provides insight into an SA–dependent feedback regulation loop, which, surprisingly, includes ADR1-L2.
Published in the journal: . PLoS Genet 9(4): e32767. doi:10.1371/journal.pgen.1003465
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003465Summary
Plants react to pathogen attack via recognition of, and response to, pathogen-specific molecules at the cell surface and inside the cell. Pathogen effectors (virulence factors) are monitored by intracellular nucleotide-binding leucine-rich repeat (NB-LRR) sensor proteins in plants and mammals. Here, we study the genetic requirements for defense responses of an autoactive mutant of ADR1-L2, an Arabidopsis coiled-coil (CC)-NB-LRR protein. ADR1-L2 functions upstream of salicylic acid (SA) accumulation in several defense contexts, and it can act in this context as a “helper” to transduce specific microbial activation signals from “sensor” NB-LRRs. This helper activity does not require an intact P-loop. ADR1-L2 and another of two closely related members of this small NB-LRR family are also required for propagation of unregulated runaway cell death (rcd) in an lsd1 mutant. We demonstrate here that, in this particular context, ADR1-L2 function is P-loop dependent. We generated an autoactive missense mutation, ADR1-L2D484V, in a small homology motif termed MHD. Expression of ADR1-L2D848V leads to dwarfed plants that exhibit increased disease resistance and constitutively high SA levels. The morphological phenotype also requires an intact P-loop, suggesting that these ADR1-L2D484V phenotypes reflect canonical activation of this NB-LRR protein. We used ADR1-L2D484V to define genetic requirements for signaling. Signaling from ADR1-L2D484V does not require NADPH oxidase and is negatively regulated by EDS1 and AtMC1. Transcriptional regulation of ADR1-L2D484V is correlated with its phenotypic outputs; these outputs are both SA–dependent and –independent. The genetic requirements for ADR1-L2D484V activity resemble those that regulate an SA–gradient-dependent signal amplification of defense and cell death signaling initially observed in the absence of LSD1. Importantly, ADR1-L2D484V autoactivation signaling is controlled by both EDS1 and SA in separable, but linked pathways. These data allows us to propose a genetic model that provides insight into an SA–dependent feedback regulation loop, which, surprisingly, includes ADR1-L2.
Introduction
Plants encounter a wide variety of pathogens. To defend against infection, plants rely on their organ surfaces as pre-formed barriers to infection. Plants have also evolved an active, two-layered immune system [1]. The first branch utilizes transmembrane receptors (PRRs, or pattern recognition receptors) which detect microbe-associated molecular patterns (MAMPs) of various pathogens [2]. MAMP detection elicits a rapid, relatively low-amplitude host transcriptional response resulting in MAMP-triggered immunity (MTI) which is sufficient to halt growth of many microbes [1], [3]. Successful pathogens can suppress or delay MTI via delivery of effector molecules into host cells. Effectors are typically virulence proteins [4]. Gram-negative bacterial pathogens deliver effectors via injection into the plant cell by the Type III Secretion System (TTSS). Plants respond to effectors with the second tier of recognition, which is dependent on highly polymorphic intracellular disease resistance (R) proteins of the NB-LRR family. NB-LRRs are specifically activated by the presence and/or action of effectors to trigger robust defense responses termed Effector-Triggered Immunity (ETI), which can include localized hypersensitive cell death [1].
NB-LRR proteins are members of the signal transduction ATPases with numerous domains (STAND) superfamily, which also includes animal innate immune sensors of the nucleotide-binding domain and leucine-rich repeat-containing (NLR) class [5], [6]. STAND proteins are ATPases that function as molecular switches: in the “off” position they bind ADP, and in the “on” position they bind ATP, activating nucleotide hydrolysis and triggering downstream defense responses. This model is proposed for plant NB-LRRs, though there is very little experimental data pertinent to it [7]. Two essential, conserved homology regions necessary for proper plant NB-LRR activity are the P-loop (Walker-A) and the thus far plant-specific ‘MHD motif’ located in the ARC2 sub-domain of the extended NB-ARC domain. Mutations in the P-loop typically lead to loss of function [8], [9]. Conversely, mutation of the Asp (D) in the MHD motif often leads to autoactivity of the NB-LRR protein [10]–[15], resulting in either lethality or a severely dwarfed morphology. These pleiotropic phenotypes are thought to be the consequence of ectopic accumulation of SA, a key defense hormone whose synthesis from chorismate is controlled by the isochorismate synthase gene (ICS1/SID2) [16], and consequent defense activation [11], [13], [15]. Additionally, several NB-LRRs, in both plants and animals, work in pairs: in these cases, one can function as an effector-specific ‘sensor’, and the other as a ‘helper’ protein. This may allow or drive the formation of higher-order protein complexes necessary for properly regulated defense activation [17]–[20].
ADR1-L2 (Activated Disease Resistance 1-like 2) is one of a small family of NB-LRR proteins that includes ADR1 and ADR1-L1 [21]. We recently demonstrated that ADR1-L2 functions downstream of the production of reactive oxygen intermediates (ROI), and upstream of SA accumulation, in basal defense (defined as the response that limits the growth and proliferation of genetically virulent pathogens). ADR1-L2 also functions in MAMP-triggered SA accumulation, and as a ‘helper’ protein during some, but not all ETI responses driven by effector-mediated activation of specific sensor NB-LRR proteins [22].
Surprisingly, none of the ADR1-L2 functions above required an intact P-loop [22]. In addition to these ‘non-canonical’ activities, we suggested that ADR1-L2 might have as yet undefined P-loop dependent, ‘canonical’ functions that, in the absence of the specific effector required for activation, are difficult to define. ADR1-L2 would not be the first NB-LRR protein to have multiple, independent functions. The mouse NLR protein NLRC4 has two separate functions as a ‘helper’ protein in the recognition of both the MAMP flagellin and PrgJ, a component of the Salmonella TTSS. These activities are downstream of the activation of two different sensor NLRs: NAIP5 is necessary for flagellin perception, and NAIP2 is required for PrgJ recognition [17], [20]. Importantly, NLRC4 ‘helper’ activity is also P-loop independent [17], [20].
Canonical, effector-driven NB-LRR activation typically leads to an NADPH oxidase-dependent ROI burst [23]. The adr1 family triple mutant (adr1 adr1-L1 adr1-L2) exhibited normal ROI production after successful pathogen recognition [22]. Thus, the ADR1-L2 helper function noted above is downstream or independent of this oxidative burst. However, adr1 triple mutants failed to accumulate wild-type levels of SA in this context [22]. Another protein that functions downstream of the effector-driven oxidative burst and both regulates and responds to SA accumulation is Lesion Simulating Disease resistance 1 (LSD1) [23], [24]. Loss of LSD1 leads to improper regulation of runaway cell death, or rcd [24] that eventually engulfs the affected leaf. The Arabidopsis NADPH oxidase AtRbohD, which is required for effector-driven oxidative burst, is not required for lsd1-mediated cell death [23]. On the other hand, lsd1 rcd is both induced by, and requires, SA [24], [25]. lsd1 rcd is also regulated by Enhanced Disease Susceptibility 1 (EDS1) and a type I metacaspase, AtMC1; eds1 lsd1 and atmc1 lsd1 plants do not exhibit rcd [26], [27]. EDS1 is a defense response regulator, required for both basal defense and Toll/interleukin-1 (TIR)-NB-LRR mediated ETI [28]. EDS1 and SA act in a regulatory feedback loop, with SA up-regulating EDS1 expression and EDS1 functioning as a potentiator of SA-mediated signaling [29], [30]. AtMC1 is a positive regulator of ETI-mediated cell death [27].
To define the genetic requirements of putative canonical functions of ADR1-L2 in the absence of an effector known to activate it, we created an autoactive MHD mutant, ADR1-L2D484V. This allele displayed the dwarfed morphology that is the hallmark of MHD mutants [11], [13], [15]. We demonstrate that this autoactivity is P-loop dependent, downstream of AtRbohD-mediated ROI production, partially dependent on SA synthesis, and negatively regulated by EDS1 and AtMC1. We then present and validate a model for the interaction of EDS1, LSD1, and ADR1-L2, showing that these proteins function in both SA-dependent and SA-independent feedback regulatory loops that are interconnected.
Results
Members of the ADR1 family of NB-LRRs are required for runaway cell death in lsd1
ADR1-L2 is a CC-NB-LRR that is a positive regulator of lsd1 rcd [22]. It is part of a small family of NB-LRRs that includes ADR1 and ADR1-L1 [21], [22]. We generated adr1 lsd1-2 and adr1-L1 lsd1-2 double mutants and sprayed them with the SA analog benzothiadiazole (BTH) [31] to test whether adr1 and adr1-L1 also suppress the initiation and propagation of lsd1 rcd. Col-0 wild-type plants were unaffected by BTH treatment, whereas lsd1-2 plants sprayed with BTH showed typical rcd [24]. As reported, the adr1-L2 lsd1-2 double mutants fully suppressed lsd1 rcd [22]. adr1-L1 also fully suppressed lsd1-2 rcd, while adr1 had only a slight effect (Figure 1A, 1B). We quantified this phenotype by monitoring cellular ion leakage via changes in media conductivity, an established proxy for membrane damage associated with cell death [32]. Col-0 plants did not exhibit significant changes in media conductivity, but lsd1-2 plants showed increasing conductivity, with the highest reading at 92 hours post-BTH treatment. adr1-L1 lsd1-2 and adr1-L2 lsd1-2 both exhibited complete ion leakage suppression, while adr1 lsd1-2 exhibited a marginal effect (Figure 1C). Thus, ADR1-L1 and ADR1-L2 are each required for lsd1 rcd.
Fig. 1. A family of CC-NB-LRR proteins is required for lsd1 runaway cell death. 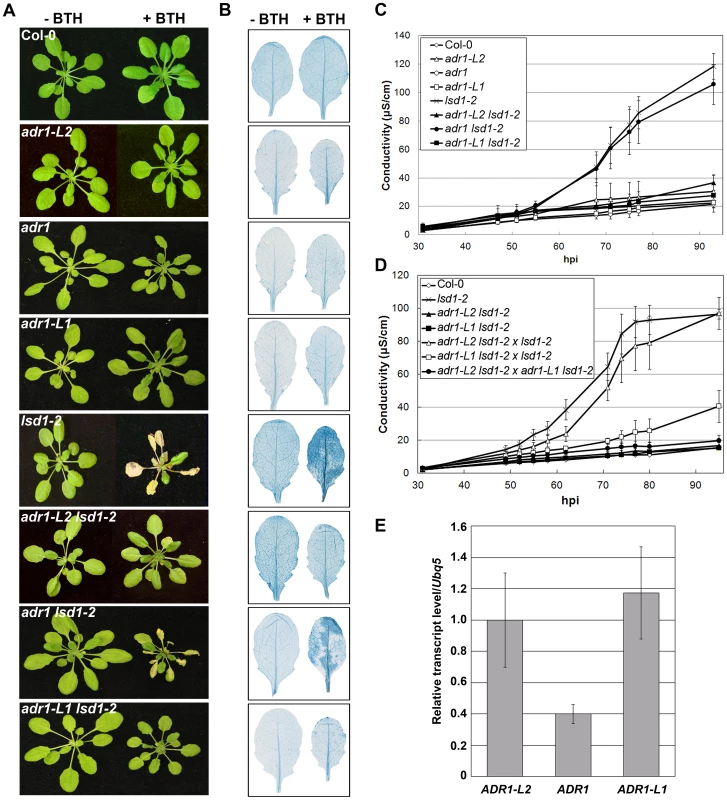
(A) Four-week-old plants were sprayed with 300 µM BTH or water. Pictures of plants were taken 5 days post-inoculation (dpi). (B) Leaves from plants in (A) were stained with trypan blue to visualize cell death. Leaves on the left are water-treated controls, leaves on the right are sprayed with 300 µM BTH. (C) Ion leakage measurements from (A), 5 days post-BTH treatment. Values are means ±2× SE (n = 5). (D) Ion leakage measurements for NANC. adr1-L1 lsd1-2×lsd1-2, adr1-L2 lsd1-2×lsd1-2, adr1-L1 lsd1-2×adr1-L2 lsd1-2 represent F1 plants of the indicated crosses, and are thus lsd1 homozygous and heterozygous for the indicated adr mutations. (E) Quantitative real time PCR for the transcript amounts of the three members of the ADR family in wild-type Col-0 plants, normalized to UBQ5. We noted that adr1-L1 and adr1-L2 exhibited non-allelic non-complementation (NANC), a rare genetic condition where plants that are heterozygous at both loci phenotypically resemble either homozygous single mutant. Thus, plants homozygous for lsd1-2 and heterozygous for both ADR1-L1 and ADR1-L2 exhibited full suppression of lsd1 rcd (Figure 1D). We also found that adr1-L2 was fully recessive, whereas adr1-L1 appeared to be semi-dominant (Figure 1D). NANC frequently indicates that the two genes act closely together or that the two proteins physically interact or are a part of the same protein complex, and that their overall dose is important for their shared function [33]. Because all three ADR1 proteins share significant amino acid identity, we speculated that lowering of the overall ADR1 dose might be sufficient to suppress lsd1 rcd. Thus, the weak adr1 rcd suppression phenotype might simply reflect low expression of ADR1 relative to ADR1-L1 and ADR1-L2. Quantitative RT-PCR analysis of gene specific mRNA levels confirmed that ADR1 is expressed at lower levels than ADR1-L1 and ADR1-L2 under our growth conditions, consistent with this model (Figure 1E).
ADR1-L2 is required at the specific site undergoing cell death
ADR1-L2 is a positive regulator of lsd1-mediated cell death. This could be due either to (i) a requirement for ADR1-L2 activation in cells destined to die, followed by its continued activation in neighboring cells, as the SA-dependent signal for rcd spreads in the absence of LSD1 [23], [34]; or (ii) a requirement for ADR1-L2 activation in cells initially triggered to die, with this activation contributing to the spread of an ADR1-L2-independent cell death signal beyond the primary cell death site. To distinguish between these two hypotheses, we generated an estradiol-driven (Est) conditional expression system, which induces local target gene expression [35]. adr1-L2 lsd1-2 plants expressing an estradiol-induced, HA epitope-tagged ADR1-L2 transgene were constructed (Materials and Methods). Expression of ADR1-L2 was activated by local application of estradiol on only part of a leaf, thus creating an artificial chimera containing both adr1-L2 lsd1-2 and ADR1-L2 lsd1-2 sectors (Figure 2A). ADR1-L2 expression was limited to the area of estradiol application as measured via Western blot (Figure 2B). BTH treatment was then used to induce lsd1-mediated rcd. We observed that cell death was limited to the zone of estradiol treatment and did not expand into the adr1-L2 lsd1-2 sector (Figure 2C). This result supports our first hypothesis: ADR1-L2 expression is continuously required in cells undergoing lsd1-mediated rcd.
Fig. 2. ADR1-L2 is required at the site undergoing cell death. 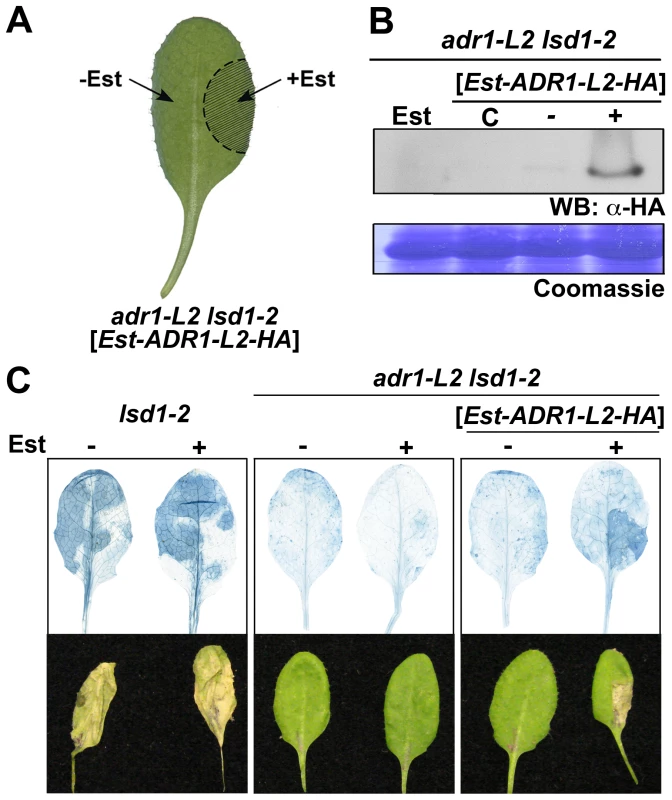
(A) Schematic of the chimera. adr1-L2 lsd1-2 expressing an estradiol inducible C-terminal HA-tagged ADR1-L2 were infiltrated in the indicated area with 20 µM estradiol, making that portion of the leaf ADR1-L2 lsd1-2. (B) Western blot to confirm expression of ADR1-L2 was limited to the estradiol-induced area. Estradiol + and − leaf areas were cored and protein was extracted from these cores. Protein extracts were run on SDS-Page gels and immunoblotted with anti-HA antibody. Coomassie stained blot confirms equal loading control (bottom). C, samples from un-infiltrated leaves; +, estradiol-infiltrated plant tissue; −, un-infiltrated tissue from the same leaf. In all samples, the entire leaf was treated with 300 µM BTH. (C) Trypan blue staining (top) of representative leaves (bottom) to show cell death in lsd1 control and tissue chimera plants. Leaves from four-week-old plants were treated as indicated in (A). Plants were sprayed with BTH 16 hours after estradiol treatment, and leaves were stained with trypan blue 5 days after BTH treatment. The requirement for ADR1-L2 in lsd1 rcd is P-loop dependent
We previously noted that ADR1-L2 is required for SA accumulation following effector and MAMP recognition, and that this does not require an intact P-loop motif [22]. However, these results do not preclude additional, canonical P-loop-dependent functions for ADR1-L2. Thus, we tested whether or not the positive regulatory function of ADR1-L2 in lsd1 rcd is P-loop dependent. We generated adr1-L2 lsd1-2 plants expressing ADR1-L2AAA, a mutated allele of ADR1-L2 which carries alanine (A) substitutions in the three consecutive conserved residues within the P-loop motif which are essential for nucleotide binding [22]. Interestingly, ADR1-L2AAA fails to complement for lsd1 rcd following BTH treatment (Figure 3A), even though this construct retains wild type BTH-induced ADR1-L2 protein accumulation (Figure 3B). Despite repeated attempts, we could not recover adr1-L2 plants over-expressing ADR1-L2, presumably due to lethality of ectopic over-expression as noted for other sensor NB-LRR proteins (data not shown). Together these results suggest that ADR1-L2 activation in lsd1 rcd proceeds in a canonical, P-loop dependent manner.
Fig. 3. The requirement for ADR1-L2 in lsd1 rcd is P-loop dependent. 
(A) Four-week-old plants of the indicated genotypes were sprayed with BTH or water. ADR1-L2 and AAA indicate adr1-L2 lsd1 plants expressing C-terminally HA-tagged wild-type ADR1-L2 or the mutated P-loop allele ADR1-L2AAA, respectively. In both transgenics expression is driven by the native ADR1-L2 promoter. Pictures of plants were taken 5 dpi. (B) Protein from the indicated genotypes was extracted before or after BTH treatment, run on a denaturing gel, and probed with anti-HA antibody. Ponceau-stained blot shows relative loading. An autoactive version of ADR1-L2 exhibits P-loop-dependent, ectopically activated immune responses
Mutations of the aspartic acid (D) in the conserved MHD motif in plant NB-LRRs typically lead to autoactivity [10]–[14]. Mechanistically, this is thought to reflect either a preference for ATP binding or a lack of ATPase activity, either of which would favor the “on” state, according to current models of NB-LRR activation [7], [19]. Thus, a similar mutation in the MHD motif of ADR1-L2 should result in a permanent ‘on’ state, resulting in ectopic autoactivity. In the cases where it has been examined, NB-LRR autoactivity via MHD mutation has been shown to require an intact P-loop [10]–[14]. Thus, given the P-loop dependent function of ADR1-L2 in lsd1 rcd, we speculated that ADR1-L2 activity in additional defense contexts might also require an intact P-loop.
We generated adr1-L2 plants expressing ADR1-L2 with a Val (V) for Asp (D) substitution at amino acid 484 (Figure 4A; hereafter ADR1-L2D484V). As expected, ADR1-L2D484V transgenics exhibited a dwarfed, cpr (Constitutive PR expression)-like phenotype [36] with short hypocotyls, pointed leaves (Figure 4B), and a bushy appearance after bolting. In contrast, adr1-L2 plants expressing wild-type ADR1-L2 appeared morphologically similar to wild-type Col-0 plants (Figure 4B). Both transgenes were expressed from the native ADR1-L2 promoter, with C-terminal HA epitope tags (Figure 4C). We note that the majority of ADR1-L2D484V transgenic lines accumulated higher protein levels than those expressing the wild-type ADR1-L2 allele. We selected ADR1-L2 and ADR1-L2D484V lines expressing similar levels of protein to show that the cpr-like phenotype is not simply a result of higher protein levels in the autoactive mutant (Figure 4C); the differences in morphology persist. Additional ADR1-L2D484V lines expressing less ADR1-L2D484V protein were also recovered; these did not exhibit strong cpr-like phenotypes, suggesting that there is a threshold amount of ADR1-L2D484V required for the associated phenotypes (data not shown).
Fig. 4. ADR1-L2D484V ectopically activates basal defense. 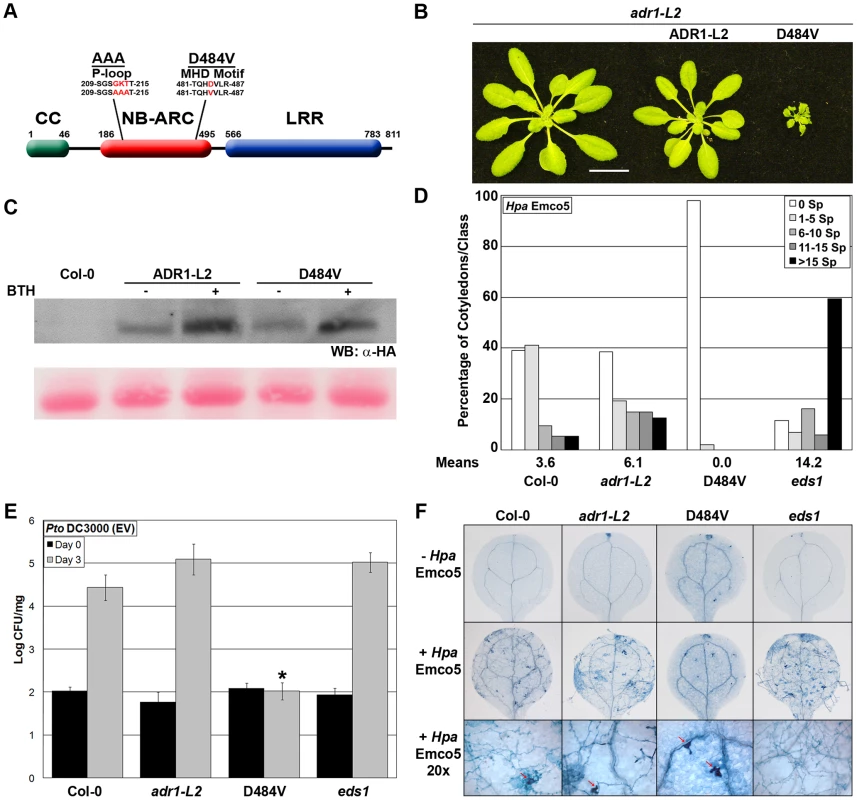
(A) Schematic representation of ADR1-L2 showing the P-loop and MHD mutations used in this study. (B) Morphology of five-week-old adr1-L2, and adr1-L2 complemented with pADR1-L2::ADR1-L2-HA or pADR1-L2::ADR1-L2D484V-HA, showing relative size. White bar is 2 cm. (C) Western blot of HA-tagged protein from the indicated genotypes before and after BTH application. Protein was extracted from plants, run on a denaturing gel and probed with anti-HA antibody. Ponceau-stained blot shows relative loading. (D) Ten-day-old seedlings were inoculated with 5×104 sporangia/mL Hpa Emco5 via spray inoculation. Sporangiophores per cotyledon were counted 4 dpi, with an average of 80 cotyledons per genotype counted. Sporangiophore counts were classified into: no sporulation (0 sporangiophores/cotyledon), light sporulation (1–5), medium sporulation (6–10), heavy sporulation (11–15), or very heavy sporulation (>15). Means of sporangiophore per cotyledon are listed below the graph. (E) Twenty-day-old seedlings were dip-inoculated with Pto DC3000(EV). Bacterial growth was assayed at 0 and 3 dpi. Values are mean cfu/mg ±2× SE, n = 4. Asterisk indicates significant difference (Post Hoc test, p<0.0001). (F) Trypan blue stained leaves from (D) and magnified sites (20×). Leaves were collected and stained 4 dpi. Red arrows indicate HR sites. The ADR1 family members work additively to limit pathogen growth, with adr1 triple mutant plants exhibiting increased susceptibility to virulent pathogens [22]. We therefore tested the ability of autoactive ADR1-L2D484V to confer enhanced basal defense against otherwise virulent pathogens. ADR1-L2D484V plants displayed increased resistance to both Hyaloperonospora arabidopsidis (Hpa) Emco5 and Pseudomonas syringae pv tomato (Pto) DC3000 (Figure 4D, 4E). Trypan blue staining of cotyledons after inoculation with Hpa Emco5 revealed predominantly free hyphal growth in the wild-type Col-0 control and adr1-L2, which was enhanced in the fully susceptible control, eds1 (Figure 4F). ADR1-L2D484V plants, on the other hand, exhibited only localized hypersensitive cell death (HR) as well as a basal level of cell death (Figure 4F, top row) not seen in the other genotypes. Thus, ADR1-L2D484V constitutively triggers downstream signaling and increased immune function.
We examined the dependence of the ADR1-L2D484V cpr-like phenotype on the P-loop. The triple missense P-loop dead mutation, ADR1-L2AAA [22], and the autoactive ADR1-L2D484V mutation were combined in cis (Figure 4A) and transformed into adr1-L2 plants. ADR1-L2AAA D484V plants did not exhibit the cpr-like phenotype (Figure 5A) despite the fact that they expressed levels of ADR1-L2AAA D484V protein that are similar to ADR1-L2D484V levels sufficient to cause the dwarfed phenotype (Figure 5B). Thus, an intact P-loop domain is required for ADR1-L2D484V autoactivity. We infer that ADR1-L2D484V is an activated version of this NB-LRR which can be used to study the canonical, P-loop dependent functions of ADR1-L2.
Fig. 5. An intact P-loop catalytic domain is required for the ADR1-L2D484V morphological phenotype. 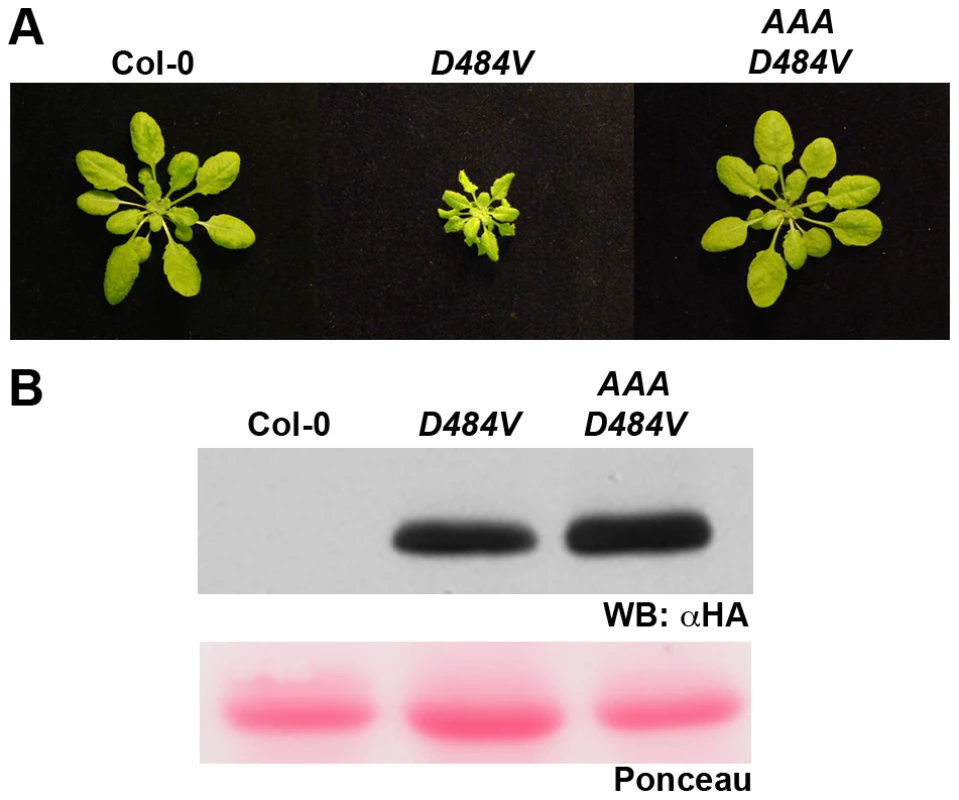
(A) Pictures of five-week-old Col-0, ADR1-L2D484V, and ADR1-L2AAA D484V plants show relative morphology. (B) Western blot of Col-0 and HA-tagged ADR1-L2D484V and ADR1-L2AAA D484V protein from plants in (A). Relative loading indicated by Ponceau stained blot. ADR1-L2D484V autoactivity is regulated by lsd1 suppressors
ADR1-L2 was identified as a positive regulator of lsd1 rcd ([34], above). LSD1 and ADR1-L2 both function downstream of the NADPH oxidase-dependent ROI burst driven by NB-LRR sensor activation, but upstream of SA accumulation [22], [25], [26]. Additionally, ADR1-L2 is locally required for lsd1-mediated rcd and its function in this context is P-loop dependent (Figure 2, Figure 5). Thus, we hypothesized that genetic components known to regulate lsd1 rcd might also be required for ADR1-L2D484V activity. We generated double mutants between ADR1-L2D484V and the lsd1 suppressors sid2, eds1, and atmc1 to define genetic interactions required for the ADR1-L2D484V phenotypes. We also generated ADR1-L2D484V atrbohD double mutants to define whether an oxidative burst is required for the ADR1-L2D484V phenotypes. We examined these double mutants for ADR1-L2D484V protein accumulation, alterations in the ADR1-L2D484V cpr-like morphology, enhanced resistance to the virulent Hpa isolate Emco5, and steady-state SA levels.
AtRbohD is generally required for effector-driven, NB-LRR-dependent superoxide production, but not for lsd1 rcd [23]. In fact, lsd1-2 atrbohD plants exhibit increased rcd compared to lsd1-2 single mutants, a phenotype that depends on SA accumulation [25]. This result suggests that the NADPH oxidase can down-regulate the spread of cell death as SA-dependent signals emanate from an infection site [23]. atrbohD ADR1-L2D484V plants morphologically resembled the ADR1-L2D484V parent (Figure 6A, Figure S1) and expressed a similar level of ADR1-L2D484V protein (Figure 6B). Like the ADR1-L2D484V parent, atrbohD ADR1-L2D484V plants were significantly more resistant to Hpa Emco5 (Figure 6C), and had extremely high steady-state levels of SA (Figure 6D). We conclude that ADR1-L2D484V autoactivity, unlike effector-driven NB-LRR activation, is downstream, or independent, of AtRbohD.
Fig. 6. lsd1 suppressors are regulators of ADR1-L2D484V autoactivity. 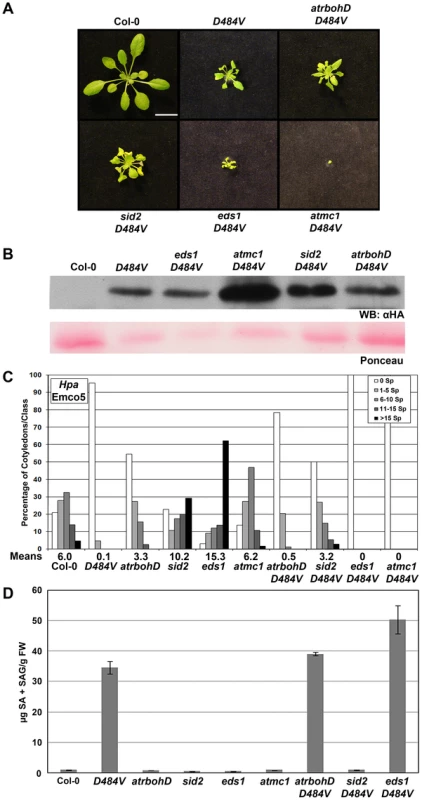
(A) Pictures of five-week-old Col-0, ADR1-L2D484V, atrbohD ADR1-L2D484V, sid2-1 ADR1-L2D484V, eds1-2 ADR1-L2D484V, or atmc1-1 ADR1-L2D484V plants, showing morphological differences between the genotypes. White bar is 2 cm. (B) Western blots of HA-tagged ADR1-L2D484V proteins from plants in (A). Ponceau staining shows relative loading. (C) Ten-day-old seedlings of the indicated genotypes were inoculated with 5×104 sporangia/mL Hpa Emco5. At 4 dpi, sporangiophores were counted and classified as in Figure 4. Means per cotyledon are listed below the graph. (D) Steady-state total SA levels were measured from leaves of the indicated genotypes. Values are average µg of total SA from 4 replicates, ±2× SE. SA is required for lsd1 rcd [25] and mediates basal defense in plants [37]. Additionally, SA levels are reduced in adr1-family triple mutant plants, corresponding to diminished basal defense and an increase in disease susceptibility [22]. Thus, it seemed likely that the increased basal defense in ADR1-L2D484V plants could be due to the massive increase in SA observed in this line (Figure 6D). We tested this hypothesis using the sid2 mutant, which is unable to synthesize SA due to a mutation in the biosynthetic isochorismate synthase gene, ICS1 [16]. sid2 ADR1-L2D484V plants morphologically resembled the ADR1-L2D484V parent (Figure 6A, Figure S1) and accumulated similar amounts of ADR1-L2D484V protein (Figure 6B). sid2 ADR1-L2D484V plants exhibited enhanced basal defense to Hpa Emco5, though not to the same extent as ADR1-L2D484V (Figure 6C). As expected, sid2 ADR1-L2D484V plants did not accumulate SA (Figure 6D). These observations indicate that the defense cpr-like phenotypes of ADR1-L2D484V consist of both SA-dependent and SA-independent components, whereas the cpr-like growth phenotype is SA-independent.
EDS1 is required for lsd1-mediated rcd [26] and is an essential regulator of both basal defense against virulent pathogens [38], [39] and TIR-NB-LRR dependent ETI [40]–[42]. Exogenous SA rescues eds1 basal defense phenotypes, suggesting that EDS1 acts upstream of ICS1, at least for the phenotypes assayed [42], [43]. Importantly, eds1 ADR1-L2D484V plants were significantly more dwarfed than ADR1-L2D484V (Figure 6A, Figure S1), though these two lines expressed similar levels of ADR1-L2D484V protein (Figure 6B). eds1 ADR1-L2D484V double mutants were completely resistant to Hpa Emco5 (Figure 6C), and had steady-state SA levels that were higher than the ADR1-L2D484V single mutant (Figure 6D). These surprising results demonstrate that EDS1 is a negative regulator of the SA-accumulation observed in ADR1-L2D484V.
AtMC1 is a metacaspase required for lsd1 rcd; AtMC1 also contributes significantly to ETI-dependent HR [27]. atmc1 ADR1-L2D484V plants were extremely dwarfed (Figure 6A, Figure S1). However, these plants were not sterile; they produced small amounts of seed and had a very long life cycle compared to wild-type Col-0 or ADR1-L2D484V plants (data not shown). They also accumulated more ADR1-L2D484V protein than the ADR1-L2D484V parent (Figure 6B). Cotyledons of the atmc1 ADR1-L2D484V plants were similar in size to those of ADR1-L2D484V plants, and we were thus able to perform Hpa infection assays. We determined that atmc1 ADR1-L2D484V cotyledons are completely resistant to Hpa Emco5 (Figure 6C). Due to the extremely small size of the atmc1 ADR1-L2D484V double mutant, we were unable to perform SA analysis on this line. Collectively, these data indicate that AtMC1 negatively regulates ADR1-L2D484V protein accumulation, and likely subsequent SA accumulation leading to a hyper-cpr phenotype.
lsd1 ADR1-L2D484V is lethal, and this lethality requires EDS1
ADR1-L2 is required for lsd1-mediated rcd [22]. We therefore examined whether ADR1-L2D484V affects the lsd1 phenotype. We crossed lsd1-2 and ADR1-L2D484V plants, and in the F3 generation homozygous ADR1-L2D484V plants were selected via Basta resistance markers on the transgene (Materials and Methods). ADR1-L2D484V homozygotes were genotyped for lsd1-2; none were lsd1-2 homozygous (Table S1). Additionally, we carried lsd1-2 homozygous, ADR1-L2D484V heterozygous plants forward an additional generation, and again used the Basta resistance marker to identify homozygous ADR1-L2D484V plants. None were recovered. Next, we attempted to transform lsd1-2 mutant plants with the same ADR1-L2D484V construct used in the adr1-L2 transformation. No lines were recovered that expressed detectable levels of ADR1-L2D484V protein, and no plants that were recovered displayed the dwarfed phenotype (data not shown). We concluded that lsd1-2 ADR1-L2D484V is lethal.
We therefore looked for genetic determinants required for lsd1 ADR1-L2D484V lethality. As stated above, eds1 and atmc1 are both suppressors of lsd1 rcd. We therefore crossed atmc1 lsd1-2 or eds1 lsd1-2 plants, which express wild-type growth, to ADR1-L2D484V. atmc1 lsd1-2 ADR1-L2D484V plants could not be recovered (data not shown), indicating that AtMC1 is not required for lethality of lsd1-2 ADR1-L2D484V. However, we did recover eds1 lsd1-2 ADR1-L2D484V plants. These plants surprisingly exhibited wild-type morphology (Figure 7A), resembling eds1 lsd1 [26]. The suppression of the ADR1-L2D484V cpr-like phenotype is likely due to a much lower level of steady state ADR1-L2D484V accumulation in the eds1 lsd1-2 ADR1-L2D484V plants compared to parental plants (Figure 7B). Despite examining many eds1 lsd1-2 ADR1-L2D484V plants from 4 independent progenies, no plant with ADR1-L2D484V parental expression levels was recovered. Additionally, eds1 lsd1-2 ADR1-L2D484V plants did not accumulate the high levels of SA observed in ADR1-L2D484V (Figure 7C).
Fig. 7. eds1 lsd1 ADR1-L2D484V plants lose ectopic activation phenotypes. 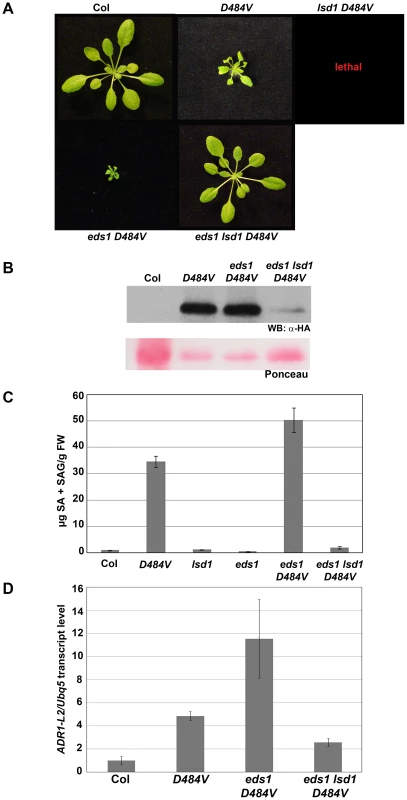
(A) Pictures of five-week-old plants of the indicated genotypes show suppression of the eds1-2 ADR1-L2D484V phenotype in an lsd1-2 background. (B) Western blot of HA-tagged ADR1-L2D484V protein from the indicated genotypes. Ponceau stain shows relative loading. (C) Total SA amounts (mean ±2× SE) were measured from plants of the indicated genotypes. Values are average µg of total SA from 4 replicates. Error bar represents ±2× SE. Controls here are from same experiment as data shown in Figure 6D. (D) Quantitative real time PCR for the transcript amounts of ADR1-L2 in the indicated genotypes. Error bars represent ±2× SE. In light of the surprising result that eds1 lsd1-2 ADR1-L2D484V plants are essentially wild-type, we re-confirmed the genotypes and phenotypes of eds1 ADR1-L2D484V and eds1 lsd1-2 ADR1-L2D484V. For this, we used a line that was homozygous for eds1 and ADR1-L2D484V but heterozygous for LSD1 and expressed the wild-type morphology. In the next generation, both dwarfed and wild-type size plants were identified (Figure S2A). These plants were genotyped for LSD1, and all dwarfed plants were found to be LSD1 homozygotes (Figure S2B, 20 of 70 plants were LSD1 homozygotes). Wild-type size plants were either LSD1/lsd1 heterozygotes (34 of 70 plants) or lsd1 mutants (16 of 70 plants), suggesting that the dominant wild-type phenotype in this context is the result of LSD1 haploinsufficiency. We therefore conclude that the difference in the phenotypes between eds1 lsd1-2 ADR1-L2D484V (wild-type) and both eds1 ADR1-L2D484V (nearly lethal) and lsd1 ADR1-L2D484V (lethal) is genuine. Further, in the presence of autoactive ADR1-L2D484V, the combined absence of EDS1 and the loss, or reduction, of LSD1 leads to down-regulation of ADR1-L2D484V protein accumulation and restoration of wild-type morphology.
We addressed whether the lowered accumulation of ADR1-L2D484V protein in eds1 lsd1-2 ADR1-L2D484V was due to transcriptional regulation. We performed quantitative RT-PCR, and discovered that the ADR1-L2D484V transcript levels in eds1 lsd1-2 ADR1-L2D484V plants were lower than in ADR1-L2D484V (Figure 7D), generally consistent with the diminution of ADR1-L2D484V protein in eds1 lsd1-2 ADR1-L2D484V (Figure 7B). LSD1 and EDS1 are known to work together in an SA regulatory feedback loop [26]. Given that eds1 lsd1-2 ADR1-L2D484V plants are morphologically normal, express lower levels of SA than ADR1-L2D484V, and accumulate lower levels of ADR1-L2 transcript and protein than ADR1-L2D484V (Figure 7), and that ADR1-L2 accumulation is up-regulated by BTH application (Figure 4C), we speculate that this loop also regulates ADR1-L2 expression. In support of this hypothesis, we also noted that ADR1-L2D484V transcript accumulated to significantly higher levels than the endogenous ADR1-L2 transcript in wild-type Col-0 plants (Figure 7D), indicating that plants expressing the activated ADR1-L2 allele constitutively up-regulate ADR1-L2 transcription.
ADR1-L2D484V autoactivity is synergistically regulated by EDS1 function and SA accumulation
The phenotypic suppression of lsd1 lethality and of eds1 ADR1-L2D484V morphological defects in eds1 lsd1 ADR1-L2D484V plants suggests that ADR1-L2D484V autoactivity signals via two parallel pathways leading to SA accumulation, one EDS1 - and one LSD1-dependent. These converge through mutual negative regulation exerted by EDS1 on the LSD1-dependent pathway and vice versa. LSD1 dampens an SA regulatory feed-forward loop that requires EDS1 [26]. EDS1 dampens an LSD1-dependent SA-accumulation (Figure 7). Thus it is plausible that eds1 lsd1 ADR1-L2D484V resembles a wild-type plant because the SA levels cannot be feed-forward amplified. To test this hypothesis, we generated sid2 eds1 ADR1-L2D484V plants by crossing sid2 ADR1-L2D484V to eds1 ADR1-L2D484V. Similar to eds1 lsd1 ADR1-L2D484V, these plants exhibited complete suppression of the nearly lethal eds1 ADR1-L2D484V phenotype (Figure 8A). Additionally, the steady state accumulation of the transgene was lowered compared to either parental line (Figure 8B). We noted that the reduced protein accumulation was not caused by transgene silencing, as F2 progeny from sid2 ADR1-L2D484V×eds1 ADR1-L2D484V segregated the SID2 eds1 ADR1-L2D484V morphological phenotype (Figure 8A). Quantitative RT-PCR on ADR1-L2 transcript suggested that, similar to eds1 lsd1 ADR1-L2D484V, the reduced transgene accumulation is transcriptional (Figure 8C). As noted above, an additional hallmark of ADR1-L2D484V autoactivity is enhanced immune function. We thus tested whether the enhanced basal defense response of ADR1-L2D484V is affected in the eds1 sid2 mutant background. Strikingly, sid2 eds1 ADR1-L2D484V plants were extremely susceptible to Hpa Emco5, more so than either single sid2 or eds1 mutants (Figure 8D). A model consistent with these observations and previous publications is presented in Figure 9 and discussed below.
Fig. 8. ADR1-L2D484V autoactivity signaling requires both SA and EDS1. 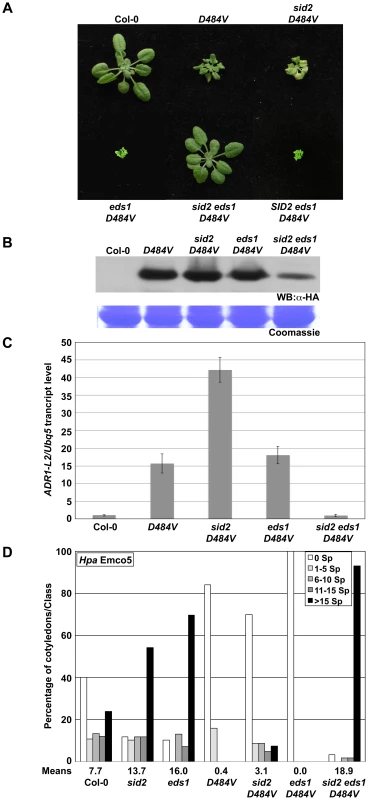
A) Pictures of five-week-old plants representative of the indicated genotypes. SID2 eds1 ADR1-L2D484V is a segregating F2 derived from the eds1 ADR1-L2D484V×sid2 ADR1-L2D484V cross. (B) Western blot of HA-tagged ADR1-L2D484V protein from plants in (A). Coomassie stain shows relative loading. (C) Quantitative real-time PCR for the transcript amounts of ADR1-L2 in the indicated genotypes. Error bar represents ±2×SE. (D) Ten-day-old seedlings were inoculated with 5×104 sporangia/mL Hpa Emco5. At 4 dpi, sporangiophores were counted and classified as in Figure 4. Means per cotyledon are listed below the graph. Fig. 9. A model for the regulation of ADR1-L2D484V activity. 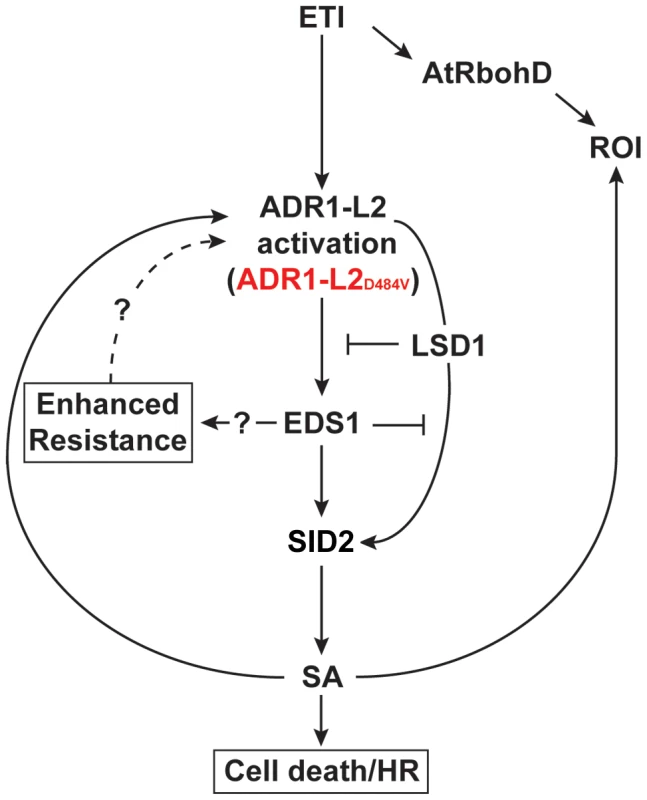
ETI activates both an AtRbohD-dependent ROI burst and SID2-dependent SA accumulation via ADR1-L2. Activated ADR1-L2 initiates cell death and disease resistance via SA-dependent and -independent pathways. EDS1 functions downstream of activated ADR1-L2 as a positive regulator of both SA accumulation and of the SA-independent pathway. ADR1-L2 also triggers SA via a pathway that is controlled by LSD1 and antagonized by EDS1. Therefore, the spread of this SA accumulation is spatially down-regulated through a combined action of EDS1 and LSD1. Due to its position in these feedback loops, SA functions both up- and down-stream of ADR1-L2. RAR1 is dispensable for accumulation of ADR1-L2
The autoactive phenotypes of ADR1-L2D484V plants require ADR1-L2D484V protein accumulation above a threshold. This indicates that the expression level of wild-type ADR1-L2 may also be under exquisite control. The co-chaperone RAR1, while not necessary for the function of all NB-LRRs, is required for the steady state accumulation of all NB-LRRs tested to date [44]–[47]. We thus crossed adr1-L2 pADR1-L2:ADR1-L2-HA to rar1-21 [46]. Plants genotyped as homozygous rar1-21 and homozygous RAR1 exhibited similar levels of ADR1-L2-HA protein (Figure S3A), indicating that RAR1 is not required for ADR1-L2 accumulation. The rar1 genotype was confirmed by Western blot for RAR1 protein (Figure S3B). ADR1-L2 expression can be up-regulated with BTH [22]. We therefore also tested whether RAR1 is required for the high levels of ADR1-L2 accumulating after BTH treatment. BTH induced ADR1-L2 protein in rar-21 ADR1-L2-HA plants accumulated to levels at least as high as those in RAR1 ADR1-L2-HA plants (Figure S3A). Therefore, RAR1 is dispensable for both steady-state ADR1-L2 accumulation, in contrast to other assayed NB-LRR proteins [44]–[47], and for its BTH-induced up-regulation.
Discussion
We recently demonstrated that the plant NB-LRR immune receptor ADR1-L2 can have non-canonical, P-loop independent ‘helper’ functions in plant defense [22]. Here, we sought first to define canonical, P-loop dependent function(s) for ADR1-L2, and then to understand the genetic requirements for these functions. We demonstrated that wild-type ADR1-L2 is required locally at the site of BTH-driven cell death activation in the lsd1 cell death control mutant. This activity requires an intact P-loop and is thus canonical. In this context, ADR1-L2 genetically interacts with ADR1-L1 to control runaway cell death, as shown by NANC, further suggesting that members of the ADR1 family function together in cell death signaling. ADR1-L2 does not require RAR1 for either its steady state accumulation, nor for its induced accumulation following BTH treatment. This is the first report of either steady state or inducible NB-LRR accumulation that is not RAR1-dependent. This result may differentiate ‘helper’ NB-LRRs from ‘sensor’ NB-LRRs. We propose that levels of the former might be dictated by the signaling partners with which they function in specific stoichiometries, while the latter, acting as effector-sensors, are threshold-regulated by the NB-LRR co-chaperone complex [48].
Given the canonical P-loop-dependent function of ADR1-L2 as a positive regulator of lsd1 cell death, we inferred that ADR1-L2, like other NB-LRRs studied to date, retains the ability to undergo a nucleotide-dependent conformational switch to regulate its activation. Thus, we sought a context in which we could analyze canonical ADR1-L2 P-loop dependent functions, despite the absence of an effector to trigger it. We created an autoactive allele, ADR1-L2D484V. ADR1-L2D484V plants exhibit the dwarfed morphology and constitutively active defense responses observed in other autoactive NB-LRR mutants. We showed that this autoactivity requires an intact P-loop. We then used this allele as a proxy for canonical activation of ADR1-L2 in a series of epistasis experiments. We present a model consistent with our new findings and previous genetic analyses [22], [23], [25], [26], [30] (Figure 9).
Canonical, P-loop dependent, ‘sensor’ NB-LRR functions typically drive both the AtrbohD NADPH oxidase-dependent oxidative burst following effector perception and SID2-dependent SA accumulation [23]. By contrast, ADR1-L2D484V autoactivity is downstream, or independent, of AtrbohD, yet still drives SID2-dependent SA accumulation. This is consistent with the previously defined, P-loop-independent ‘helper’ activity of ADR1-L2 [22].
Plants expressing ADR1-L2D484V exhibited increased disease resistance and very high steady-state levels of SA. sid2 ADR1-L2D484V plants expressed, as expected, very low levels of SA, but these plants did not completely revert to wild-type morphology, and they maintained an increased level of enhanced disease resistance. Thus, there must be SA-independent regulation of activated ADR1-L2. Redundant functions of EDS1 and SA in plant defense mediated by ‘sensor’ NB-LRR functions have been reported [30]. In that work, sid2 or eds1 mutants were insufficient to disrupt CC-NB-LRR-mediated disease resistance, while combined loss of both gene products led to loss of resistance [30]. Our results support this model, since the constitutive activation of ADR1-L2D484V results in both SA-dependent and SA-independent phenotypes (Figure 9). Given these data, as well as the fact that eds1 lsd1 ADR1-L2D484V phenocopies sid2 eds1 ADR1-L2D484V we conclude that the SA-independent pathway we describe here requires EDS1 (Figure 9, left).
One of our most surprising observations is the phenotypic rescue of both the lethal lsd1 ADR1-L2D484V phenotype and the nearly lethal eds1 ADR1-L2D484V phenotype in eds1 lsd1 ADR1-L2D484V plants. It is important to recall that either adr1-L2 or eds1 suppresses lsd1 rcd [22], [26]. Recall also that the P-loop independent function of ADR1-L2 as a ‘helper’ is downstream of AtRbohD, but upstream of SA accumulation [22]. This is in agreement with the autoactive ADR1-L2D484V phenotype, which bypasses AtRbohD but still drives enhanced SA levels. Notably, loss of LSD1 in the eds1 ADR1-L2D484V context functionally resembles loss of SID2. Since SID2-dependent SA accumulation is regulated by LSD1, we conclude that both SA and EDS1 are required for ADR1-L2D484V autoactivity. Loss of either genetic component destroys the fine-tuned equilibrium between EDS1-dependent and SA-dependent processes in this autoactivity.
P-loop-dependent activation of ADR1-L2 results in SID2-dependent SA accumulation via two separate pathways (Figure 9). In the first pathway, ADR1-L2D484V constitutively signals to EDS1, which in turn positively regulates SID2, increasing SA levels. ADR1-L2D484V also triggers additional SA production in a parallel pathway that requires LSD1 and is antagonized by EDS1. In support of our model, SA regulates EDS1 transcription [29], which in turn regulates SID2 [49]. Once activated, ADR1-L2 causes cell death, which drives more AtRbohD-dependent ROI [34] and SA accumulation in surrounding cells [34], [50]. In both pathways, SA is part of a feedback loop that further potentiates the P-loop dependent activity of ADR1-L2, as indicated by the fact that ADR1-L2 is BTH inducible. Thus, ADR1-L2 is also both upstream and downstream of SA accumulation (Figure 9).
Our data are consistent with ADR1-L2 transcriptional regulation by both SA-dependent and -independent pathways (Figure 9). In an otherwise wild-type plant expressing activated ADR1-L2, the antagonism between EDS1 and LSD1 maintains SA production below toxic levels. In an lsd1 plant, the level of SA surpasses this level via ectopic ADR1-L2 activation and consequent SA production. This increased SA in turn drives higher ADR1-L2 expression, and the cycle repeats. This is exacerbated, and lethal, in lsd1 ADR1-L2D484V. eds1 and sid2 suppress lsd1 because feed forward regulation of the SA accumulation cycle is blocked. The surprising eds1 lsd1 ADR1-L2D484V and sid2 eds1 ADR1-L2D484V phenotypes are consistent with the low level of SA in these lines being insufficient to up-regulate ADR1-L2 expression. Thus, even though there is chronic signaling feeding the cycle in ADR1-L2D484V, the EDS1-dependent, SA-independent pathway is interrupted in eds1 lsd1 ADR1-L2D484V and sid2 eds1 ADR1-L2D484V. How LSD1 and EDS1 negatively regulate each other has yet to be determined, although our data suggest that LSD1 might regulate EDS1 function through transcriptional control, as EDS1 transcription levels are increased in an lsd1 mutant (Figure S4). In support of this hypothesis, a role for LSD1 as a cytosolic retention factor for the AtbZIP10 transcription factor [51] may provide a mechanism for LSD1 control of EDS1 expression.
Our model (Figure 9) supports a scenario in which in wild-type, P-loop dependent NB-LRR activation leads to local increased levels of SA via an AtRbohD-dependent ROI burst and SID2-dependent SA accumulation. The spread of this SA accumulation is spatially down-regulated through a combined action of EDS1 and LSD1 at increasing distance from the infection site. As stated above, our model also implies that SA functions both up - and down-stream of ADR1-L2. This may readily reconciled with our previous finding that ADR1-L2 helper function is required for SA accumulation and cell death, since ARD1-L2 is SA-up-regulated [22].
Overall, we present a general approach to characterize canonical, P-loop dependent functions of NB-LRR proteins in the absence of a specific effector. We applied this to a recently characterized ‘helper’ NB-LRR protein, ADR1-L2. We identified genetic components that regulate its P-loop-dependent, canonical functions, and found that they, in turn, are regulated by suppressors of the lsd1 rcd phenotype. Our work suggests that the genetic requirements for ‘helper’ NB-LRR function may differ from the effector-driven activation of canonical ‘sensor’ NB-LRRs. Given that ADR1-L2, unlike other NB-LRRs, is required for lsd1 rcd, we note that our results may be mainly relevant to the dissection of the functions of ADR1-L2 and its paralogues, rather than being broadly applicable to understanding of ‘sensor’ NB-LRRs. Nevertheless, in agreement with previous reports on ‘sensor’ NB-LRR function [30], we conclude that the P-loop-dependent autoactivity of ADR1-L2 relies on signaling pathways that differ in their requirement for SA accumulation, but which are both regulated by EDS1. Thus, though the requirements for ‘sensor’ and ‘helper’ NB-LRR functions may be separable, they could still share some overlapping features.
A significant challenge remains to address the sub-cellular localization of these regulatory circuits [52]. Resting state NB-LRRs are localized to diverse sub-cellular compartments, and dynamic re-localization may accompany effector-driven activation of some [19]. Defining any dynamics of protein localization associated with the differential ADR1-L2 canonical and non-canonical functions will be ultimately important for understanding the genetic network that we describe.
Materials and Methods
Plant lines and pathogen strains
All Arabidopsis lines are in the Columbia (Col-0) ecotype. adr1-1 [22], adr1-L1-1 [22], adr1-L2-4 [22], eds1-2 [49], sid2-1, atrbohD [23], lsd1-2 [24], atmc1 [27], and rar1-21 [46] are described elsewhere; primers used to genotype these lines are in Table S2. For generation of adr1-L2 plants expressing ADR1-L2-HA, ADR1-L2D484V-HA, and ADR1-L2AAA D484V-HA lines, the C-terminal HA-tagged coding sequence of wild-type ADR1-L2 or the mutated alleles were fused to its native promoter (500 bp) and cloned in the pBAR (Basta resistant) Gateway vector [53]. For generation of adr1-L2 lsd1-2 plants expressing an estradiol inducible ADR1-L2-HA, the coding sequence of ADR1-L2 was cloned into a modified pMDC7 (hygromicin resistant) Gateway vector carrying a C-terminal HA tag. Arabidopsis transgenics were generated using Agrobacterium (GV3101)-mediated floral dip transformation [54]. Basta selection of transgenic plants was performed by spraying 10-day-old seedlings. Plants were grown under short day conditions (9 hrs light, 21°C; 15 hrs dark, 18°C).
Immunoblot analysis
Leaves from 4-week-old plants were harvested and total proteins were extracted by grinding frozen tissue in a buffer containing 20 mM Tris-HCl (pH 7.0), 150 mM NaCl, 1 mM EDTA (pH 8.0), 1% Triton X-100, 0.1% SDS, 10 mM DTT, and plant protein protease inhibitor cocktail (Sigma-Aldrich). Samples were centrifuged at 14,000 rpm for 15 min at 4°C to pellet debris. Proteins were separated on 7.5% (ADR1-HA) or 12% (RAR1) SDS-PAGE gels and were transferred to polyvinylidene difluoride membrane. Western blots were performed using standard methods. Anti-HA (Santa Cruz Biotechnology) antibody was used at a 1∶3000 dilution; anti-RAR1 (custom anti-RAR1 polyclonal antibody was made against the full length RAR1 with C-terminus GST tag by Cocalico Biologicals, Inc.) was used at a 1∶2000 dilution. Signals were detected by enhanced chemiluminescence using ECL Plus (Amersham Biosciences). For BTH induction experiments (300 µM), plants were collected 24 hpi.
SA measurement
SA and SAG measurements were performed as described [55]. Briefly, 100 mg of leaves were collected from 4-week-old plants and frozen in liquid nitrogen. Samples were ground and tissue was homogenized in 200 µl 0.1M acetate buffer pH 5.6. Samples were centrifuged for 15 min at 16,000 g at 4°C. 100 µl of supernatant was transferred to a new tube for free SA measurement, and 10 µl was incubated with 1 µl 0.5 U/µl β-glucosidase for 90 min at 37°C for total SA measurement. After incubation, plant extracts were diluted 5-fold with 44 µl acetate buffer for free SA measurement. 60 µl of LB, 5 µl of plant extract (treated or not with β-glucosidase), and 50 µl of Acinetobacter sp. ADPWH-lux (OD = 0.4) were added to each well of a black 96-well plate (BD Falcon). The plate was incubated at 37°C for 60 min and luminescence was read with Spectra Max L (Molecular Devices) microplate reader. For the standard curve, 1 µl of a known amount of SA (Sigma; from 0 to 1000 µg/ml) was diluted 10-fold in sid2-1 plant extract, and 5 µl of each standard (undiluted for free SA measurement, or 5-fold diluted for total SA) was added to the wells of the plate containing 60 µl of LB and 50 µl of Acinetobacter. SA standards were read in parallel with the experimental samples. For BTH induction experiments (300 µM), plants were collected 24 hpi.
Pathogen strains and growth quantification
Ten-day-old seedlings were spray-inoculated with 50,000 spores/ml of Hyaloperonospora arabidopsidis isolate Emco5. Pots were covered with a lid to increase humidity during inoculation and pathogen growth. Sporangiophores were counted at 4 dpi as described [56]. Pto DC3000(EV) was resuspended in 10 mM MgCl2 to a final concentration of 2.5×105 cfu/ml (OD600 = 0.0005). Twenty-day-old seedlings were dipped in the bacterial solution and growth was assessed as described [57].
Cell death assays
4-week-old plants were sprayed with 300 µM BTH, or 10-day-old plants were inoculated with Hpa Emco5 as described above. Leaves were harvested and stained with lactophenol Trypan Blue (TB) to visualize dead cells as described [58]. For the conductivity measurements, 4-week-old plants were sprayed with 300 µM BTH. Plants were harvested and 4 leaf discs (7 mm) were cored and then floated in water for 30 min. These leaf discs were transferred to tubes containing 6 ml distilled water. Conductivity of the solution (μSiemens/cm) was determined with an Orion Conductivity Meter at the indicated time points [59].
Creation of an artificial chimera
The central portion of the right halves of leaves from 4-week-old transgenic adr1-L2 lsd1-2 plants expressing an estradiol inducible allele of ADR1-L2 were hand-infiltrated with Est (20 µM) using a needleless syringe. 300 µM BTH was sprayed on the whole plant 24 h post-Est application. 20 µM Est was then hand-infiltrated on the same portion of the leaves 2 dpi to ensure expression of ADR1-L2. Leaves were collected 5 dpi from the first Est infiltration.
Quantitative RT–PCR
Leaves from 4-week-old plants were collected, frozen into liquid nitrogen and ground into powder with a mortar and pestle. RNA was extracted using TRIzol (Invitrogen), DNased (Ambion Turbo DNase), and cleaned up with Qiagen RNeasy Mini kit. Reverse transcription was performed (Ambion RETROscript) using 1 µg total RNA, and cDNA was analyzed with SYBR green (Applied Biosystem) using an Applied Biosystems ViiA7. Primers used are listed in Table S2.
Selection of segregating plants
Pots of sibling plants fixed for eds1 and segregating lsd1-2 (LSD1 heterzygotes) were Basta sprayed to check for segregation of ADR1-L2D484V. Those found to be eds1 ADR1-L2D484V were transplanted individually into pots, monitored for size, and genotyped for the T-DNA insertion of the lsd1-2 mutation.
Supporting Information
Zdroje
1. JonesJD, DanglJL (2006) The plant immune system. Nature 444 : 323–329.
2. SegonzacC, ZipfelC (2011) Activation of plant pattern-recognition receptors by bacteria. Current opinion in microbiology 14 : 54–61.
3. BollerT, FelixG (2009) A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol 60 : 379–406.
4. DoddsPN, RathjenJP (2010) Plant immunity: towards an integrated view of plant-pathogen interactions. Nat Rev Genet 11 : 539–548.
5. LeipeDD, KooninEV, AravindL (2004) STAND, a class of P-loop NTPases including animal and plant regulators of programmed cell death: multiple, complex domain architectures, unusual phyletic patterns, and evolution by horizontal gene transfer. Journal of molecular biology 343 : 1–28.
6. LukasikE, TakkenFL (2009) STANDing strong, resistance proteins instigators of plant defence. Current opinion in plant biology 12 : 427–436.
7. TakkenFL, AlbrechtM, TamelingWI (2006) Resistance proteins: molecular switches of plant defence. Current opinion in plant biology 9 : 383–390.
8. TamelingWI, ElzingaSD, DarminPS, VossenJH, TakkenFL, et al. (2002) The tomato R gene products I-2 and MI-1 are functional ATP binding proteins with ATPase activity. The Plant cell 14 : 2929–2939.
9. HansonPI, WhiteheartSW (2005) AAA+ proteins: have engine, will work. Nat Rev Mol Cell Biol 6 : 519–529.
10. WilliamsSJ, SornarajP, deCourcy-IrelandE, MenzRI, KobeB, et al. (2011) An autoactive mutant of the M flax rust resistance protein has a preference for binding ATP, whereas wild-type M protein binds ADP. Molecular plant-microbe interactions : MPMI 24 : 897–906.
11. GaoZ, ChungEH, EitasTK, DanglJL (2011) Plant intracellular innate immune receptor Resistance to Pseudomonas syringae pv. maculicola 1 (RPM1) is activated at, and functions on, the plasma membrane. Proceedings of the National Academy of Sciences of the United States of America 108 : 7619–7624.
12. TamelingWI, VossenJH, AlbrechtM, LengauerT, BerdenJA, et al. (2006) Mutations in the NB-ARC domain of I-2 that impair ATP hydrolysis cause autoactivation. Plant physiology 140 : 1233–1245.
13. HowlesP, LawrenceG, FinneganJ, McFaddenH, AyliffeM, et al. (2005) Autoactive alleles of the flax L6 rust resistance gene induce non-race-specific rust resistance associated with the hypersensitive response. Mol Plant Microbe Interact 18 : 570–582.
14. BendahmaneA, FarnhamG, MoffettP, BaulcombeDC (2002) Constitutive gain-of-function mutants in a nucleotide binding site-leucine rich repeat protein encoded at the Rx locus of potato. The Plant journal : for cell and molecular biology 32 : 195–204.
15. ZhangZ, WuY, GaoM, ZhangJ, KongQ, et al. (2012) Disruption of PAMP-induced MAP kinase cascade by a Pseudomonas syringae effector activates plant immunity mediated by the NB-LRR protein SUMM2. Cell Host Microbe 11 : 253–263.
16. WildermuthMC, DewdneyJ, WuG, AusubelFM (2001) Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414 : 562–565.
17. ZhaoY, YangJ, ShiJ, GongYN, LuQ, et al. (2011) The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477 : 596–600.
18. EitasTK, DanglJL (2010) NB-LRR proteins: pairs, pieces, perception, partners, and pathways. Current opinion in plant biology 13 : 472–477.
19. BonardiV, CherkisK, NishimuraMT, DanglJL (2012) A new eye on NLR proteins: focused on clarity or diffused by complexity? Current opinion in immunology 24 : 41–50.
20. KofoedEM, VanceRE (2011) Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature 477 : 592–595.
21. ChiniA, LoakeGJ (2005) Motifs specific for the ADR1 NBS-LRR protein family in Arabidopsis are conserved among NBS-LRR sequences from both dicotyledonous and monocotyledonous plants. Planta 221 : 597–601.
22. BonardiV, TangS, StallmannA, RobertsM, CherkisK, et al. (2011) Expanded functions for a family of plant intracellular immune receptors beyond specific recognition of pathogen effectors. Proceedings of the National Academy of Sciences of the United States of America 108 : 16463–16468.
23. TorresMA, JonesJD, DanglJL (2005) Pathogen-induced, NADPH oxidase-derived reactive oxygen intermediates suppress spread of cell death in Arabidopsis thaliana. Nature genetics 37 : 1130–1134.
24. DietrichRA, DelaneyTP, UknesSJ, WardER, RyalsJA, et al. (1994) Arabidopsis mutants simulating disease resistance response. Cell 77 : 565–577.
25. AvivDH, RusterucciC, HoltBF3rd, DietrichRA, ParkerJE, et al. (2002) Runaway cell death, but not basal disease resistance, in lsd1 is SA - and NIM1/NPR1-dependent. The Plant journal : for cell and molecular biology 29 : 381–391.
26. RusterucciC, AvivDH, HoltBF3rd, DanglJL, ParkerJE (2001) The disease resistance signaling components EDS1 and PAD4 are essential regulators of the cell death pathway controlled by LSD1 in Arabidopsis. The Plant cell 13 : 2211–2224.
27. CollNS, VercammenD, SmidlerA, CloverC, Van BreusegemF, et al. (2010) Arabidopsis type I metacaspases control cell death. Science 330 : 1393–1397.
28. WiermerM, FeysBJ, ParkerJE (2005) Plant immunity: the EDS1 regulatory node. Curr Opin Plant Biol 8 : 383–389.
29. FalkA, FeysBJ, FrostLN, JonesJD, DanielsMJ, et al. (1999) EDS1, an essential component of R gene-mediated disease resistance in Arabidopsis has homology to eukaryotic lipases. Proceedings of the National Academy of Sciences of the United States of America 96 : 3292–3297.
30. VenugopalSC, JeongRD, MandalMK, ZhuS, Chandra-ShekaraAC, et al. (2009) Enhanced disease susceptibility 1 and salicylic acid act redundantly to regulate resistance gene-mediated signaling. PLoS Genet 5: e1000545 doi:10.1371/journal.pgen.1000545.
31. GorlachJ, VolrathS, Knauf-BeiterG, HengyG, BeckhoveU, et al. (1996) Benzothiadiazole, a novel class of inducers of systemic acquired resistance, activates gene expression and disease resistance in wheat. Plant Cell 8 : 629–643.
32. DellagiA, BrissetMN, PaulinJP, ExpertD (1998) Dual role of desferrioxamine in Erwinia amylovora pathogenicity. Mol Plant Microbe Interact 11 : 734–742.
33. StearnsT, BotsteinD (1988) Unlinked noncomplementation: isolation of new conditional-lethal mutations in each of the tubulin genes of Saccharomyces cerevisiae. Genetics 119 : 249–260.
34. JabsT, DietrichRA, DanglJL (1996) Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science 273 : 1853–1856.
35. BrandL, HorlerM, NueschE, VassalliS, BarrellP, et al. (2006) A versatile and reliable two-component system for tissue-specific gene induction in Arabidopsis. Plant Physiol 141 : 1194–1204.
36. BowlingSA, GuoA, CaoH, GordonAS, KlessigDF, et al. (1994) A mutation in Arabidopsis that leads to constitutive expression of systemic acquired resistance. Plant Cell 6 : 1845–1857.
37. LoakeG, GrantM (2007) Salicylic acid in plant defence–the players and protagonists. Curr Opin Plant Biol 10 : 466–472.
38. AartsN, MetzM, HolubE, StaskawiczBJ, DanielsMJ, et al. (1998) Different requirements for EDS1 and NDR1 by disease resistance genes define at least two R gene-mediated signaling pathways in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America 95 : 10306–10311.
39. FeysBJ, WiermerM, BhatRA, MoisanLJ, Medina-EscobarN, et al. (2005) Arabidopsis SENESCENCE-ASSOCIATED GENE101 stabilizes and signals within an ENHANCED DISEASE SUSCEPTIBILITY1 complex in plant innate immunity. Plant Cell 17 : 2601–2613.
40. ZhangY, GoritschnigS, DongX, LiX (2003) A gain-of-function mutation in a plant disease resistance gene leads to constitutive activation of downstream signal transduction pathways in suppressor of npr1-1, constitutive 1. Plant Cell 15 : 2636–2646.
41. WirthmuellerL, ZhangY, JonesJD, ParkerJE (2007) Nuclear accumulation of the Arabidopsis immune receptor RPS4 is necessary for triggering EDS1-dependent defense. Curr Biol 17 : 2023–2029.
42. FeysBJ, MoisanLJ, NewmanMA, ParkerJE (2001) Direct interaction between the Arabidopsis disease resistance signaling proteins, EDS1 and PAD4. EMBO J 20 : 5400–5411.
43. ParkerJE, HolubEB, FrostLN, FalkA, GunnND, et al. (1996) Characterization of eds1, a mutation in Arabidopsis suppressing resistance to Peronospora parasitica specified by several different RPP genes. Plant Cell 8 : 2033–2046.
44. HoltBF3rd, BelkhadirY, DanglJL (2005) Antagonistic control of disease resistance protein stability in the plant immune system. Science 309 : 929–932.
45. BelkhadirY, NimchukZ, HubertDA, MackeyD, DanglJL (2004) Arabidopsis RIN4 negatively regulates disease resistance mediated by RPS2 and RPM1 downstream or independent of the NDR1 signal modulator and is not required for the virulence functions of bacterial type III effectors AvrRpt2 or AvrRpm1. The Plant cell 16 : 2822–2835.
46. TorneroP, MerrittP, SadanandomA, ShirasuK, InnesRW, et al. (2002) RAR1 and NDR1 contribute quantitatively to disease resistance in Arabidopsis, and their relative contributions are dependent on the R gene assayed. The Plant cell 14 : 1005–1015.
47. BieriS, MauchS, ShenQH, PeartJ, DevotoA, et al. (2004) RAR1 positively controls steady state levels of barley MLA resistance proteins and enables sufficient MLA6 accumulation for effective resistance. The Plant cell 16 : 3480–3495.
48. ShirasuK (2009) The HSP90-SGT1 chaperone complex for NLR immune sensors. Annu Rev Plant Biol 60 : 139–164.
49. BartschM, GobbatoE, BednarekP, DebeyS, SchultzeJL, et al. (2006) Salicylic acid-independent ENHANCED DISEASE SUSCEPTIBILITY1 signaling in Arabidopsis immunity and cell death is regulated by the monooxygenase FMO1 and the Nudix hydrolase NUDT7. Plant Cell 18 : 1038–1051.
50. EnyediAJ, YalpaniN, SilvermanP, RaskinI (1992) Localization, conjugation, and function of salicylic acid in tobacco during the hypersensitive reaction to tobacco mosaic virus. Proc Natl Acad Sci U S A 89 : 2480–2484.
51. KaminakaH, NakeC, EppleP, DittgenJ, SchutzeK, et al. (2006) bZIP10-LSD1 antagonism modulates basal defense and cell death in Arabidopsis following infection. The EMBO journal 25 : 4400–4411.
52. BonardiV, DanglJL (2012) How complex are intracellular immune receptor signaling complexes? Front Plant Sci 3 : 237.
53. NakagawaT, KuroseT, HinoT, TanakaK, KawamukaiM, et al. (2007) Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J Biosci Bioeng 104 : 34–41.
54. CloughSJ, BentAF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 : 735–743.
55. DefraiaCT, SchmelzEA, MouZ (2008) A rapid biosensor-based method for quantification of free and glucose-conjugated salicylic acid. Plant Methods 4 : 28.
56. HoltBF3rd, BoyesDC, EllerstromM, SiefersN, WiigA, et al. (2002) An evolutionarily conserved mediator of plant disease resistance gene function is required for normal Arabidopsis development. Developmental cell 2 : 807–817.
57. TorneroP, DanglJL (2001) A high-throughput method for quantifying growth of phytopathogenic bacteria in Arabidopsis thaliana. The Plant journal : for cell and molecular biology 28 : 475–481.
58. KochE, SlusarenkoA (1990) Arabidopsis is susceptible to infection by a downy mildew fungus. Plant Cell 2 : 437–445.
59. EppleP, MackAA, MorrisVR, DanglJL (2003) Antagonistic control of oxidative stress-induced cell death in Arabidopsis by two related, plant-specific zinc finger proteins. Proceedings of the National Academy of Sciences of the United States of America 100 : 6831–6836.
Štítky
Genetika Reprodukční medicína
Článek The G4 GenomeČlánek Mondo/ChREBP-Mlx-Regulated Transcriptional Network Is Essential for Dietary Sugar Tolerance inČlánek RpoS Plays a Central Role in the SOS Induction by Sub-Lethal Aminoglycoside Concentrations inČlánek Tissue Homeostasis in the Wing Disc of : Immediate Response to Massive Damage during DevelopmentČlánek Disruption of TTDA Results in Complete Nucleotide Excision Repair Deficiency and Embryonic LethalityČlánek DJ-1 Decreases Neural Sensitivity to Stress by Negatively Regulating Daxx-Like Protein through dFOXO
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 4- Akutní intermitentní porfyrie
- Souvislost haplotypu M2 genu pro annexin A5 s opakovanými reprodukčními ztrátami
- Vztah užívání alkoholu a mužské fertility
- Doporučení pro diagnostiku a léčbu akutních jaterních porfyrií
- Doc. Miloš Kubánek: Nemocní se srdeční amyloidózou jsou často skryti a sledováni pod jinými diagnózami
-
Všechny články tohoto čísla
- Epigenetic Upregulation of lncRNAs at 13q14.3 in Leukemia Is Linked to the Downregulation of a Gene Cluster That Targets NF-kB
- A Big Catch for Germ Cell Tumour Research
- The Quest for the Identification of Genetic Variants in Unexplained Cardiac Arrest and Idiopathic Ventricular Fibrillation
- A Nonsynonymous Polymorphism in as a Risk Factor for Human Unexplained Cardiac Arrest with Documented Ventricular Fibrillation
- The Hourglass and the Early Conservation Models—Co-Existing Patterns of Developmental Constraints in Vertebrates
- Smaug/SAMD4A Restores Translational Activity of CUGBP1 and Suppresses CUG-Induced Myopathy
- Balancing Selection on a Regulatory Region Exhibiting Ancient Variation That Predates Human–Neandertal Divergence
- The G4 Genome
- Extensive Natural Epigenetic Variation at a Originated Gene
- Mouse Oocyte Methylomes at Base Resolution Reveal Genome-Wide Accumulation of Non-CpG Methylation and Role of DNA Methyltransferases
- The Environment Affects Epistatic Interactions to Alter the Topology of an Empirical Fitness Landscape
- TIP48/Reptin and H2A.Z Requirement for Initiating Chromatin Remodeling in Estrogen-Activated Transcription
- Aconitase Causes Iron Toxicity in Mutants
- Tbx2 Terminates Shh/Fgf Signaling in the Developing Mouse Limb Bud by Direct Repression of
- Mondo/ChREBP-Mlx-Regulated Transcriptional Network Is Essential for Dietary Sugar Tolerance in
- Sex-Differential Selection and the Evolution of X Inactivation Strategies
- Identification of a Tissue-Selective Heat Shock Response Regulatory Network
- Phosphorylation-Coupled Proteolysis of the Transcription Factor MYC2 Is Important for Jasmonate-Signaled Plant Immunity
- RpoS Plays a Central Role in the SOS Induction by Sub-Lethal Aminoglycoside Concentrations in
- Six Homeoproteins Directly Activate Expression in the Gene Regulatory Networks That Control Early Myogenesis
- Rtt109 Prevents Hyper-Amplification of Ribosomal RNA Genes through Histone Modification in Budding Yeast
- ATP-Dependent Chromatin Remodeling by Cockayne Syndrome Protein B and NAP1-Like Histone Chaperones Is Required for Efficient Transcription-Coupled DNA Repair
- Iron-Responsive miR-485-3p Regulates Cellular Iron Homeostasis by Targeting Ferroportin
- Mutations in Predispose Zebrafish and Humans to Seminomas
- Cytotoxic Chromosomal Targeting by CRISPR/Cas Systems Can Reshape Bacterial Genomes and Expel or Remodel Pathogenicity Islands
- Tissue Homeostasis in the Wing Disc of : Immediate Response to Massive Damage during Development
- All SNPs Are Not Created Equal: Genome-Wide Association Studies Reveal a Consistent Pattern of Enrichment among Functionally Annotated SNPs
- Functional 358Ala Allele Impairs Classical IL-6 Receptor Signaling and Influences Risk of Diverse Inflammatory Diseases
- The Tissue-Specific RNA Binding Protein T-STAR Controls Regional Splicing Patterns of Pre-mRNAs in the Brain
- Neutral Genomic Microevolution of a Recently Emerged Pathogen, Serovar Agona
- Genetic Requirements for Signaling from an Autoactive Plant NB-LRR Intracellular Innate Immune Receptor
- SNF5 Is an Essential Executor of Epigenetic Regulation during Differentiation
- Dialects of the DNA Uptake Sequence in
- Reference-Free Population Genomics from Next-Generation Transcriptome Data and the Vertebrate–Invertebrate Gap
- Senataxin Plays an Essential Role with DNA Damage Response Proteins in Meiotic Recombination and Gene Silencing
- High-Resolution Mapping of Spontaneous Mitotic Recombination Hotspots on the 1.1 Mb Arm of Yeast Chromosome IV
- Rod Monochromacy and the Coevolution of Cetacean Retinal Opsins
- Evolution after Introduction of a Novel Metabolic Pathway Consistently Leads to Restoration of Wild-Type Physiology
- Disruption of TTDA Results in Complete Nucleotide Excision Repair Deficiency and Embryonic Lethality
- Insulators Target Active Genes to Transcription Factories and Polycomb-Repressed Genes to Polycomb Bodies
- Signatures of Diversifying Selection in European Pig Breeds
- The Chromosomal Passenger Protein Birc5b Organizes Microfilaments and Germ Plasm in the Zebrafish Embryo
- The Histone Demethylase Jarid1b Ensures Faithful Mouse Development by Protecting Developmental Genes from Aberrant H3K4me3
- Regulates Synaptic Development and Endocytosis by Suppressing Filamentous Actin Assembly
- Sensory Neuron-Derived Eph Regulates Glomerular Arbors and Modulatory Function of a Central Serotonergic Neuron
- Analysis of Rare, Exonic Variation amongst Subjects with Autism Spectrum Disorders and Population Controls
- Scavenger Receptors Mediate the Role of SUMO and Ftz-f1 in Steroidogenesis
- DNA Double-Strand Breaks Coupled with PARP1 and HNRNPA2B1 Binding Sites Flank Coordinately Expressed Domains in Human Chromosomes
- High-Resolution Mapping of H1 Linker Histone Variants in Embryonic Stem Cells
- Comparative Genomics of and the Bacterial Species Concept
- Genetic and Biochemical Assays Reveal a Key Role for Replication Restart Proteins in Group II Intron Retrohoming
- Genome-Wide Association Studies Identify Two Novel Mutations Responsible for an Atypical Hyperprolificacy Phenotype in Sheep
- The Genetic Correlation between Height and IQ: Shared Genes or Assortative Mating?
- Comprehensive Assignment of Roles for Typhimurium Genes in Intestinal Colonization of Food-Producing Animals
- An Essential Role for Zygotic Expression in the Pre-Cellular Drosophila Embryo
- The Genome Organization of Reflects Its Lifestyle
- Coordinated Cell Type–Specific Epigenetic Remodeling in Prefrontal Cortex Begins before Birth and Continues into Early Adulthood
- Improved Detection of Common Variants Associated with Schizophrenia and Bipolar Disorder Using Pleiotropy-Informed Conditional False Discovery Rate
- Site-Specific Phosphorylation of the DNA Damage Response Mediator Rad9 by Cyclin-Dependent Kinases Regulates Activation of Checkpoint Kinase 1
- Npc1 Acting in Neurons and Glia Is Essential for the Formation and Maintenance of CNS Myelin
- Identification of , a Retrotransposon-Derived Imprinted Gene, as a Novel Driver of Hepatocarcinogenesis
- Aag DNA Glycosylase Promotes Alkylation-Induced Tissue Damage Mediated by Parp1
- DJ-1 Decreases Neural Sensitivity to Stress by Negatively Regulating Daxx-Like Protein through dFOXO
- Asynchronous Replication, Mono-Allelic Expression, and Long Range -Effects of
- Differential Association of the Conserved SUMO Ligase Zip3 with Meiotic Double-Strand Break Sites Reveals Regional Variations in the Outcome of Meiotic Recombination
- Focusing In on the Complex Genetics of Myopia
- Continent-Wide Decoupling of Y-Chromosomal Genetic Variation from Language and Geography in Native South Americans
- Breakpoint Analysis of Transcriptional and Genomic Profiles Uncovers Novel Gene Fusions Spanning Multiple Human Cancer Types
- Intrinsic Epigenetic Regulation of the D4Z4 Macrosatellite Repeat in a Transgenic Mouse Model for FSHD
- Bisphenol A Exposure Disrupts Genomic Imprinting in the Mouse
- Genetic and Genomic Architecture of the Evolution of Resistance to Antifungal Drug Combinations
- Transposable Elements Are Major Contributors to the Origin, Diversification, and Regulation of Vertebrate Long Noncoding RNAs
- Functional Dissection of the Condensin Subunit Cap-G Reveals Its Exclusive Association with Condensin I
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The G4 Genome
- Neutral Genomic Microevolution of a Recently Emerged Pathogen, Serovar Agona
- The Histone Demethylase Jarid1b Ensures Faithful Mouse Development by Protecting Developmental Genes from Aberrant H3K4me3
- The Tissue-Specific RNA Binding Protein T-STAR Controls Regional Splicing Patterns of Pre-mRNAs in the Brain
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



