-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
A Big Catch for Germ Cell Tumour Research
article has not abstract
Published in the journal: . PLoS Genet 9(4): e32767. doi:10.1371/journal.pgen.1003481
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1003481Summary
article has not abstract
Testicular germ cell tumour (TGCT) is the most common cancer in young men, and the incidence of TGCT is rising worldwide for unknown reasons [1], [2]. Treatments for TGCT are overall quite effective, but at the cost of significant toxicity [3], creating a powerful incentive for the development of more specific, molecularly guided therapies. TGCTs are generally thought to arise from a pluripotent fetal or embryonic germ cell [4]. Reflecting this pluripotency, these tumours can present in a wide range of histologic forms. Seminomas are TGCTs that retain features of pluripotent, primitive germ cells. In contrast, non-seminoma TGCTs exhibit differentiation into forms resembling somatic tissues (teratomas) or extraembryonic structures such as yolk sac (yolk sac tumour) or placenta (choriocarcinoma) (Figure 1). Family members of TGCT patients have a markedly increased risk of developing TGCT, strongly implicating an underlying genetic basis. Recent genome-wide association studies of TGCT have identified SNPs near ATF7IP, BAK1, DMRT1, KITLG, SPRY4, and TERT-CLPTM1L that increase TGCT risk [5]–[7]; however, the mechanisms associating most of these loci with tumourigenesis remain unclear.
Fig. 1. Animal models of TGCT and correlation to human histologic subtypes. 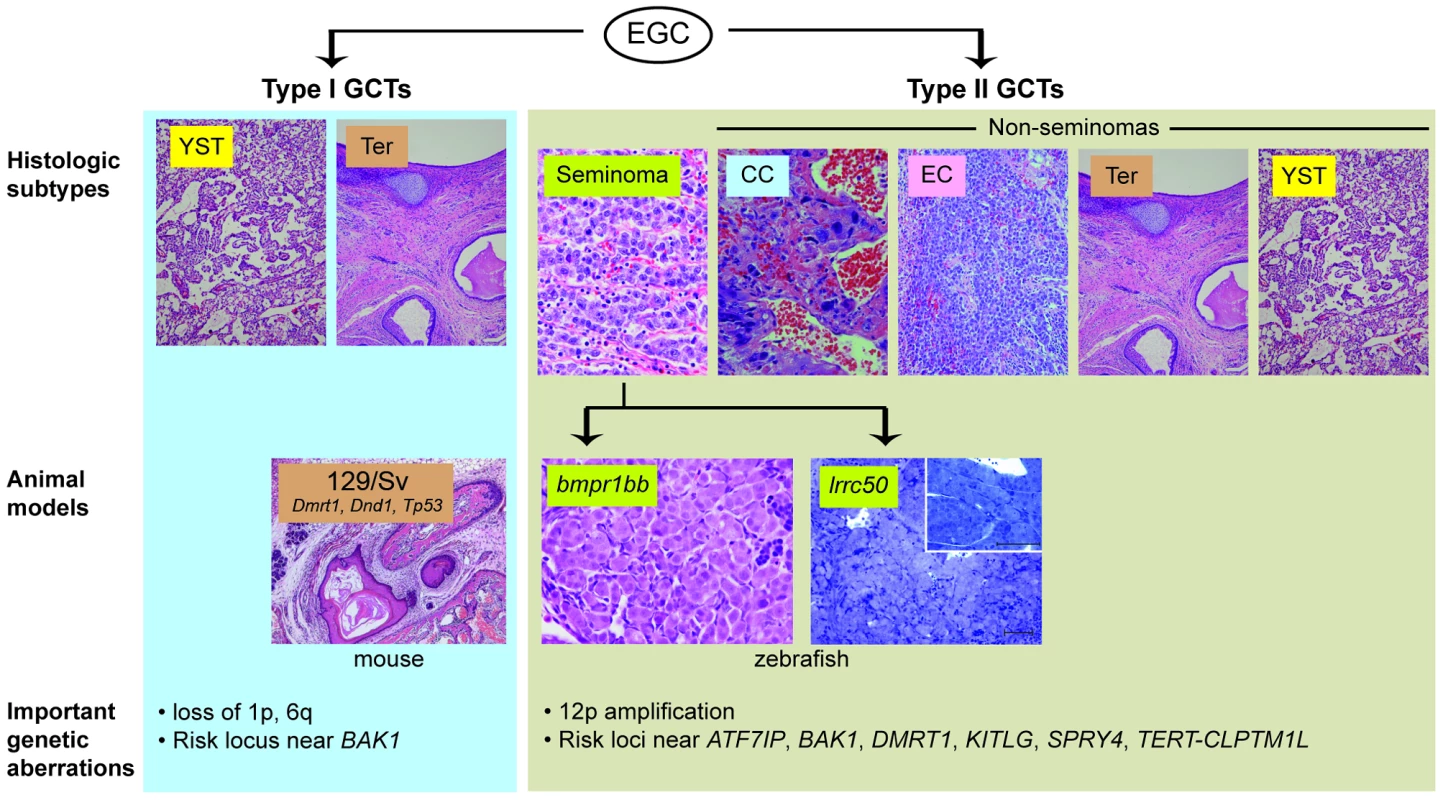
TGCTs appear to arise from embryonic germ cells (EGC) in infants and young children (“type I”) or in adolescents and young adults (“type II”) [4]. (For clarity, the presumed carcinoma in situ precursor cell for type II tumours is omitted). These are further sub-divided by histologic subtype: yolk sac tumour (YST), teratoma (Ter), seminoma, choriocarcinoma (CC), embryonal carcinoma (EC). Current animal models of TGCT correlate to type I teratomas (129/Sv mice) and type II seminomas (bmpr1bb or lrrc50 fish). Important genetic aberrations in human tumours are listed below. Mouse teratoma photo courtesy of Dr. David Zarkower. Researchers seeking to identify such mechanisms have been hindered by the limited TGCT animal models available thus far. Two heritable TGCT models have been previously described: one in mouse and one in zebrafish. The mouse model arose through the observation by Leroy Stevens in the late 1950s that testicular teratomas arise spontaneously at low frequency during embryonic development in mice of the 129/Sv strain [8]. This discovery, which ultimately led to the experimental derivation of embryonic stem cells [9], [10], has also proved to be a useful model of teratoma formation. A number of genes have been identified as modifier loci that increase teratoma incidence in the 129/Sv background, including Tp53, Dmrt1, and Dnd1, an RNA-binding protein that is central to germ cell maintenance [11]–[13]. Forward genetic screening led to the discovery of a second in vivo TGCT model. Zebrafish carrying nonsense mutations in alk6b/bmpr1bb, an ortholog of the human bone morphogenetic protein (BMP) receptor BMPR1B, develop TGCTs resembling human seminomas [14], [15]. This finding illuminated the importance of BMP signaling in germ cell development and implicated disruption of BMP signaling in human germ cell tumourigenesis [16]. These two models have provided insight into the roles of pluripotency and differentiation pathways in TGCT development; however, their direct correlation to human tumourigenesis has been limited, as genes such as DND1 and BMPR1B have not been found to be mutated in human TGCTs [14], [17].
In this issue of PLOS Genetics, Basten and coworkers describe a new zebrafish TGCT model with a direct connection to human TGCT mutations [18]. Zebrafish with homozygous mutations in the ciliary protein lrrc50 were previously described to have kidney cysts homologous to human polycystic kidney disease [19]. In this paper, the authors report that male lrrc50 heterozygotes develop testicular tumours late in life with near complete penetrance. Morphologically, these tumours, similar to those arising in bmpr1bb-deficient zebrafish, contain sheets of uniform, undifferentiated germ cells, resembling human seminoma. Loss of heterozygosity at the lrrc50 locus was found in some tumours, consistent with a role of lrrc50 as a tumour suppressor. The authors then conducted a mutational analysis of LRRC50 in a collection of human seminoma samples and identified different mutations in two pedigrees with family history of seminomas, as well as heterozygosity for a different germline LRRC50 mutation in five of 38 patients with sporadic seminomas. LRRC50 is thus the first gene specifically linked to seminoma predisposition in humans. The mutations were found to be functional nulls through their inability to complement lrrc50 knockdown in zebrafish embryos, an elegant example of the utility of the fish system for both gene discovery (forward genetics) and functional genomics (reverse genetics).
lrrc50 has heretofore been characterized solely as a ciliary motor protein, and its connection to GCT suppression is intriguing. Cilia have not previously been thought to be present in spermatogonia, but the authors show that normal spermatogonia do indeed have a cilium and that LRRC50 colocalized with the axoneme in spermatogonial stem cells. In addition, the authors provide evidence that its role may not be solely structural by showing that its expression is cell cycle–regulated and that it localizes with condensed chromosomes. The development of both renal cysts in homozygotes and seminomas in heterozygotes may implicate an underlying role for lrrc50 in early genitourinary development. Furthermore, the primary cilium has emerged as a signaling center for Hedgehog, Wnt, and other developmental pathways [20], and this, along with the TGCT phenotype of bmpr1bb mutants, raises the interesting possibility that interplay of these developmental signaling pathways is central to TGCT tumour suppression. Follow-up mechanistic studies will be critical to testing these hypotheses.
This paper provides another example of the power of zebrafish forward genetic screens for discovery of genes with novel roles in cancer and other diseases, and is a welcome addition to the list of animal models of TGCT. Some caveats apply when comparing analogous tumours arising in animals separated by a large evolutionary distance; for example, the fish seminomas are benign compared to human seminomas, and may well arise at a different stage of germ cell development. More significantly, neither the fish nor the mouse models currently reflect the biology of human non-seminomatous GCTs, such as embryonal carcinoma, choriocarcinoma, or yolk sac tumour. Clinically the non-seminomas are more likely to be metastatic and resistant to standard treatments, meaning that new models of these GCT subtypes are urgently needed to provide insight into tumour biology, as well as platforms for testing new therapeutic strategies for these cancers.
Zdroje
1. VisfeldtJ, JorgensenN, MullerJ, MollerH, SkakkebaekNE (1994) Testicular germ cell tumours of childhood in Denmark, 1943–1989: incidence and evaluation of histology using immunohistochemical techniques. J Pathol 174 : 39–47.
2. WuXC, ChenVW, SteeleB, RoffersS, KlotzJB, et al. (2003) Cancer incidence in adolescents and young adults in the United States, 1992–1997. J Adolesc Health 32 : 405–415.
3. Frazier AL, Amatruda JF (2009) Germ cell tumors. In: Fisher DE, Nathan D, Look AT, editors. Nathan and Oski's textbook of pediatric hematology-oncology. London: Elsevier.
4. OosterhuisJW, LooijengaLH (2005) Testicular germ-cell tumours in a broader perspective. Nat Rev Cancer 5 : 210–222.
5. RapleyEA, TurnbullC, Al OlamaAA, DermitzakisET, LingerR, et al. (2009) A genome-wide association study of testicular germ cell tumor. Nat Genet 41 : 807–810.
6. TurnbullC, RapleyEA, SealS, PernetD, RenwickA, et al. (2010) Variants near DMRT1, TERT and ATF7IP are associated with testicular germ cell cancer. Nat Genet 42 : 604–607.
7. PoynterJN, HootenAJ, FrazierAL, RossJA (2012) Associations between variants in KITLG, SPRY4, BAK1, and DMRT1 and pediatric germ cell tumors. Genes Chromosomes Cancer 51 : 266–271.
8. StevensLC, HummelKP (1957) A description of spontaneous congenital testicular teratomas in strain 129 mice. J Natl Cancer Inst 18 : 719–747.
9. MintzB, IllmenseeK (1975) Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc Natl Acad Sci U S A 72 : 3585–3589.
10. StevensLC (1970) The development of transplantable teratocarcinomas from intratesticular grafts of pre - and postimplantation mouse embryos. Dev Biol 21 : 364–382.
11. KrentzAD, MurphyMW, KimS, CookMS, CapelB, et al. (2009) The DM domain protein DMRT1 is a dose-sensitive regulator of fetal germ cell proliferation and pluripotency. Proc Natl Acad Sci U S A 106 : 22323–22328.
12. LamMY, NadeauJH (2003) Genetic control of susceptibility to spontaneous testicular germ cell tumors in mice. APMIS 111 : 184–190; discussion 191.
13. YoungrenKK, CoveneyD, PengX, BhattacharyaC, SchmidtLS, et al. (2005) The Ter mutation in the dead end gene causes germ cell loss and testicular germ cell tumours. Nature 435 : 360–364.
14. NeumannJC, ChandlerGL, DamoulisVA, FustinoNJ, LillardK, et al. (2011) Mutation in the type IB bone morphogenetic protein receptor Alk6b impairs germ-cell differentiation and causes germ-cell tumors in zebrafish. Proc Natl Acad Sci U S A 108 : 13153–13158.
15. NeumannJC, DoveyJS, ChandlerGL, CarbajalL, AmatrudaJF (2009) Identification of a heritable model of testicular germ cell tumor in the zebrafish. Zebrafish 6 : 319–327.
16. FustinoN, RakhejaD, AteekCS, NeumannJC, AmatrudaJF (2011) Bone morphogenetic protein signalling activity distinguishes histological subsets of paediatric germ cell tumours. Int J Androl 34: e218–233.
17. LingerR, DudakiaD, HuddartR, TuckerK, FriedlanderM, et al. (2008) Analysis of the DND1 gene in men with sporadic and familial testicular germ cell tumors. Genes Chromosomes Cancer 47 : 247–252.
18. BastenSG, DavisEE, GillisAJM, van RooijenE, StoopH, et al. (2013) Mutations in LRRC50 predispose zebrafish and humans to seminomas. PLoS Genet 9: e1003384 doi:10.1371/journal. pgen.1003384.
19. van RooijenE, GilesRH, VoestEE, van RooijenC, Schulte-MerkerS, et al. (2008) LRRC50, a conserved ciliary protein implicated in polycystic kidney disease. J Am Soc Nephrol 19 : 1128–1138.
20. VelandIR, AwanA, PedersenLB, YoderBK, ChristensenST (2009) Primary cilia and signaling pathways in mammalian development, health and disease. Nephron Physiol 111: p39–53.
Štítky
Genetika Reprodukční medicína
Článek The G4 GenomeČlánek Mondo/ChREBP-Mlx-Regulated Transcriptional Network Is Essential for Dietary Sugar Tolerance inČlánek RpoS Plays a Central Role in the SOS Induction by Sub-Lethal Aminoglycoside Concentrations inČlánek Tissue Homeostasis in the Wing Disc of : Immediate Response to Massive Damage during DevelopmentČlánek Disruption of TTDA Results in Complete Nucleotide Excision Repair Deficiency and Embryonic LethalityČlánek DJ-1 Decreases Neural Sensitivity to Stress by Negatively Regulating Daxx-Like Protein through dFOXO
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 4- Akutní intermitentní porfyrie
- Souvislost haplotypu M2 genu pro annexin A5 s opakovanými reprodukčními ztrátami
- Vztah užívání alkoholu a mužské fertility
- Doc. Miloš Kubánek: Nemocní se srdeční amyloidózou jsou často skryti a sledováni pod jinými diagnózami
- Hysteroskopická resekce děložního septa zlepšuje šanci na graviditu žen s jinak nevysvětlenou infertilitou
-
Všechny články tohoto čísla
- Epigenetic Upregulation of lncRNAs at 13q14.3 in Leukemia Is Linked to the Downregulation of a Gene Cluster That Targets NF-kB
- A Big Catch for Germ Cell Tumour Research
- The Quest for the Identification of Genetic Variants in Unexplained Cardiac Arrest and Idiopathic Ventricular Fibrillation
- A Nonsynonymous Polymorphism in as a Risk Factor for Human Unexplained Cardiac Arrest with Documented Ventricular Fibrillation
- The Hourglass and the Early Conservation Models—Co-Existing Patterns of Developmental Constraints in Vertebrates
- Smaug/SAMD4A Restores Translational Activity of CUGBP1 and Suppresses CUG-Induced Myopathy
- Balancing Selection on a Regulatory Region Exhibiting Ancient Variation That Predates Human–Neandertal Divergence
- The G4 Genome
- Extensive Natural Epigenetic Variation at a Originated Gene
- Mouse Oocyte Methylomes at Base Resolution Reveal Genome-Wide Accumulation of Non-CpG Methylation and Role of DNA Methyltransferases
- The Environment Affects Epistatic Interactions to Alter the Topology of an Empirical Fitness Landscape
- TIP48/Reptin and H2A.Z Requirement for Initiating Chromatin Remodeling in Estrogen-Activated Transcription
- Aconitase Causes Iron Toxicity in Mutants
- Tbx2 Terminates Shh/Fgf Signaling in the Developing Mouse Limb Bud by Direct Repression of
- Mondo/ChREBP-Mlx-Regulated Transcriptional Network Is Essential for Dietary Sugar Tolerance in
- Sex-Differential Selection and the Evolution of X Inactivation Strategies
- Identification of a Tissue-Selective Heat Shock Response Regulatory Network
- Phosphorylation-Coupled Proteolysis of the Transcription Factor MYC2 Is Important for Jasmonate-Signaled Plant Immunity
- RpoS Plays a Central Role in the SOS Induction by Sub-Lethal Aminoglycoside Concentrations in
- Six Homeoproteins Directly Activate Expression in the Gene Regulatory Networks That Control Early Myogenesis
- Rtt109 Prevents Hyper-Amplification of Ribosomal RNA Genes through Histone Modification in Budding Yeast
- ATP-Dependent Chromatin Remodeling by Cockayne Syndrome Protein B and NAP1-Like Histone Chaperones Is Required for Efficient Transcription-Coupled DNA Repair
- Iron-Responsive miR-485-3p Regulates Cellular Iron Homeostasis by Targeting Ferroportin
- Mutations in Predispose Zebrafish and Humans to Seminomas
- Cytotoxic Chromosomal Targeting by CRISPR/Cas Systems Can Reshape Bacterial Genomes and Expel or Remodel Pathogenicity Islands
- Tissue Homeostasis in the Wing Disc of : Immediate Response to Massive Damage during Development
- All SNPs Are Not Created Equal: Genome-Wide Association Studies Reveal a Consistent Pattern of Enrichment among Functionally Annotated SNPs
- Functional 358Ala Allele Impairs Classical IL-6 Receptor Signaling and Influences Risk of Diverse Inflammatory Diseases
- The Tissue-Specific RNA Binding Protein T-STAR Controls Regional Splicing Patterns of Pre-mRNAs in the Brain
- Neutral Genomic Microevolution of a Recently Emerged Pathogen, Serovar Agona
- Genetic Requirements for Signaling from an Autoactive Plant NB-LRR Intracellular Innate Immune Receptor
- SNF5 Is an Essential Executor of Epigenetic Regulation during Differentiation
- Dialects of the DNA Uptake Sequence in
- Reference-Free Population Genomics from Next-Generation Transcriptome Data and the Vertebrate–Invertebrate Gap
- Senataxin Plays an Essential Role with DNA Damage Response Proteins in Meiotic Recombination and Gene Silencing
- High-Resolution Mapping of Spontaneous Mitotic Recombination Hotspots on the 1.1 Mb Arm of Yeast Chromosome IV
- Rod Monochromacy and the Coevolution of Cetacean Retinal Opsins
- Evolution after Introduction of a Novel Metabolic Pathway Consistently Leads to Restoration of Wild-Type Physiology
- Disruption of TTDA Results in Complete Nucleotide Excision Repair Deficiency and Embryonic Lethality
- Insulators Target Active Genes to Transcription Factories and Polycomb-Repressed Genes to Polycomb Bodies
- Signatures of Diversifying Selection in European Pig Breeds
- The Chromosomal Passenger Protein Birc5b Organizes Microfilaments and Germ Plasm in the Zebrafish Embryo
- The Histone Demethylase Jarid1b Ensures Faithful Mouse Development by Protecting Developmental Genes from Aberrant H3K4me3
- Regulates Synaptic Development and Endocytosis by Suppressing Filamentous Actin Assembly
- Sensory Neuron-Derived Eph Regulates Glomerular Arbors and Modulatory Function of a Central Serotonergic Neuron
- Analysis of Rare, Exonic Variation amongst Subjects with Autism Spectrum Disorders and Population Controls
- Scavenger Receptors Mediate the Role of SUMO and Ftz-f1 in Steroidogenesis
- DNA Double-Strand Breaks Coupled with PARP1 and HNRNPA2B1 Binding Sites Flank Coordinately Expressed Domains in Human Chromosomes
- High-Resolution Mapping of H1 Linker Histone Variants in Embryonic Stem Cells
- Comparative Genomics of and the Bacterial Species Concept
- Genetic and Biochemical Assays Reveal a Key Role for Replication Restart Proteins in Group II Intron Retrohoming
- Genome-Wide Association Studies Identify Two Novel Mutations Responsible for an Atypical Hyperprolificacy Phenotype in Sheep
- The Genetic Correlation between Height and IQ: Shared Genes or Assortative Mating?
- Comprehensive Assignment of Roles for Typhimurium Genes in Intestinal Colonization of Food-Producing Animals
- An Essential Role for Zygotic Expression in the Pre-Cellular Drosophila Embryo
- The Genome Organization of Reflects Its Lifestyle
- Coordinated Cell Type–Specific Epigenetic Remodeling in Prefrontal Cortex Begins before Birth and Continues into Early Adulthood
- Improved Detection of Common Variants Associated with Schizophrenia and Bipolar Disorder Using Pleiotropy-Informed Conditional False Discovery Rate
- Site-Specific Phosphorylation of the DNA Damage Response Mediator Rad9 by Cyclin-Dependent Kinases Regulates Activation of Checkpoint Kinase 1
- Npc1 Acting in Neurons and Glia Is Essential for the Formation and Maintenance of CNS Myelin
- Identification of , a Retrotransposon-Derived Imprinted Gene, as a Novel Driver of Hepatocarcinogenesis
- Aag DNA Glycosylase Promotes Alkylation-Induced Tissue Damage Mediated by Parp1
- DJ-1 Decreases Neural Sensitivity to Stress by Negatively Regulating Daxx-Like Protein through dFOXO
- Asynchronous Replication, Mono-Allelic Expression, and Long Range -Effects of
- Differential Association of the Conserved SUMO Ligase Zip3 with Meiotic Double-Strand Break Sites Reveals Regional Variations in the Outcome of Meiotic Recombination
- Focusing In on the Complex Genetics of Myopia
- Continent-Wide Decoupling of Y-Chromosomal Genetic Variation from Language and Geography in Native South Americans
- Breakpoint Analysis of Transcriptional and Genomic Profiles Uncovers Novel Gene Fusions Spanning Multiple Human Cancer Types
- Intrinsic Epigenetic Regulation of the D4Z4 Macrosatellite Repeat in a Transgenic Mouse Model for FSHD
- Bisphenol A Exposure Disrupts Genomic Imprinting in the Mouse
- Genetic and Genomic Architecture of the Evolution of Resistance to Antifungal Drug Combinations
- Transposable Elements Are Major Contributors to the Origin, Diversification, and Regulation of Vertebrate Long Noncoding RNAs
- Functional Dissection of the Condensin Subunit Cap-G Reveals Its Exclusive Association with Condensin I
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The G4 Genome
- Neutral Genomic Microevolution of a Recently Emerged Pathogen, Serovar Agona
- The Histone Demethylase Jarid1b Ensures Faithful Mouse Development by Protecting Developmental Genes from Aberrant H3K4me3
- The Tissue-Specific RNA Binding Protein T-STAR Controls Regional Splicing Patterns of Pre-mRNAs in the Brain
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



