-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaEvolution after Introduction of a Novel Metabolic Pathway Consistently Leads to Restoration of Wild-Type Physiology
Organisms cope with physiological stressors through acclimatizing mechanisms in the short-term and adaptive mechanisms over evolutionary timescales. During adaptation to an environmental or genetic perturbation, beneficial mutations can generate numerous physiological changes: some will be novel with respect to prior physiological states, while others might either restore acclimatizing responses to a wild-type state, reinforce them further, or leave them unchanged. We examined the interplay of acclimatizing and adaptive responses at the level of global gene expression in Methylobacterium extorquens AM1 engineered with a novel central metabolism. Replacing central metabolism with a distinct, foreign pathway resulted in much slower growth than wild-type. After 600 generations of adaptation, however, eight replicate populations founded from this engineered ancestor had improved up to 2.5-fold. A comparison of global gene expression in wild-type, engineered, and all eight evolved strains revealed that the vast majority of changes during physiological adaptation effectively restored acclimatizing processes to wild-type expression states. On average, 93% of expression perturbations from the engineered strain were restored, with 70% of these occurring in perfect parallel across all eight replicate populations. Novel changes were common but typically restricted to one or a few lineages, and reinforcing changes were quite rare. Despite this, cases in which expression was novel or reinforced in parallel were enriched for loci harboring beneficial mutations. One case of parallel, reinforced changes was the pntAB transhydrogenase that uses NADH to reduce NADP+ to NADPH. We show that PntAB activity was highly correlated with the restoration of NAD(H) and NADP(H) pools perturbed in the engineered strain to wild-type levels, and with improved growth. These results suggest that much of the evolved response to genetic perturbation was a consequence rather than a cause of adaptation and that physiology avoided “reinventing the wheel” by restoring acclimatizing processes to the pre-stressed state.
Published in the journal: . PLoS Genet 9(4): e32767. doi:10.1371/journal.pgen.1003427
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003427Summary
Organisms cope with physiological stressors through acclimatizing mechanisms in the short-term and adaptive mechanisms over evolutionary timescales. During adaptation to an environmental or genetic perturbation, beneficial mutations can generate numerous physiological changes: some will be novel with respect to prior physiological states, while others might either restore acclimatizing responses to a wild-type state, reinforce them further, or leave them unchanged. We examined the interplay of acclimatizing and adaptive responses at the level of global gene expression in Methylobacterium extorquens AM1 engineered with a novel central metabolism. Replacing central metabolism with a distinct, foreign pathway resulted in much slower growth than wild-type. After 600 generations of adaptation, however, eight replicate populations founded from this engineered ancestor had improved up to 2.5-fold. A comparison of global gene expression in wild-type, engineered, and all eight evolved strains revealed that the vast majority of changes during physiological adaptation effectively restored acclimatizing processes to wild-type expression states. On average, 93% of expression perturbations from the engineered strain were restored, with 70% of these occurring in perfect parallel across all eight replicate populations. Novel changes were common but typically restricted to one or a few lineages, and reinforcing changes were quite rare. Despite this, cases in which expression was novel or reinforced in parallel were enriched for loci harboring beneficial mutations. One case of parallel, reinforced changes was the pntAB transhydrogenase that uses NADH to reduce NADP+ to NADPH. We show that PntAB activity was highly correlated with the restoration of NAD(H) and NADP(H) pools perturbed in the engineered strain to wild-type levels, and with improved growth. These results suggest that much of the evolved response to genetic perturbation was a consequence rather than a cause of adaptation and that physiology avoided “reinventing the wheel” by restoring acclimatizing processes to the pre-stressed state.
Introduction
Physiological stressors affect organisms across individual and evolutionary timescales: they invoke in individuals processes that work to restore homeostasis, and become over evolutionary timescales the selective pressures that drive adaptation in populations. How organisms generate innate and evolved responses to stressors – often termed physiological acclimation (or phenotypic plasticity) and adaptation, respectively – is a driving question today in many different fields of science, from the origins of drug resistance to the effects of global climate change. A common goal in many of these areas is to move from case-by-case studies towards a predictive understanding of how organisms will adapt to future stressors. However, whereas acclimatizing responses are generally “prewired” and relatively uniform between individuals of a population, the paths and outcomes of adaptation can be many and varied. Even under a simplified scenario of consistent selective pressures across replicate populations, evolution is not deterministic. There are many potential explanations for this variability - such as the randomness of mutations, escaping drift, epistasis, and clonal interference - all of which can give rise to multiple and sometimes quite disparate evolutionary outcomes [1]–[7]; yet, in other instances, adaptation is remarkably parallel between independently-evolved lineages, even down to the genetic level [8]–[10].
In replicate populations of laboratory-evolved organisms, parallelism is commonly interpreted as a sign of selection in either genetic [8] or phenotypic [11] data. Most studies determine the basis and parallelism of adaptation by comparing ancestral versus evolved states. However, in cases of adaptation to an environmental or genetic perturbation, there exists a third “wild-type” state that existed prior to the exposure to stressors that is often ignored. Exposure to genetic or environmental stressors invokes numerous processes that shift organisms from a wild-type to a perturbed physiological state, and it is this perturbed physiological state that is optimized over evolutionary timescales by natural selection. Thus, during experimental evolution all evolved strains share an initial set of acclimatizing responses that could be resolved differently by natural selection across replicate lineages. Given only a comparison of the ancestral (perturbed) and evolved states, it would be unclear how much of parallel adaptation represents convergent evolution of truly novel physiology, versus a wholesale restoration of cellular function to the pre-perturbed state. This has the potential to greatly conflate which physiological changes are likely causes versus consequences of improved fitness, and falsely identify highly parallel instances of adaptive evolution. To our knowledge, only one other study [12] has explicitly addressed the extent to which organisms adopt novel versus restored physiological states during adaptation to an environmental stressor, versus a genetic alteration.
By including data on the wild-type state prior to an environmental or genetic perturbation, it becomes possible to distinguish which evolved changes were truly novel versus simply altering the acclimatized state. This allows physiological changes to be categorized into four patterns (Figure 1A): restored, unrestored, or reinforced refer to whether acclimatizing responses were reversed, left unchanged, or enhanced through evolution, whereas novel changes did not manifest during initial acclimation, but appeared only later during evolution. Importantly, these classifications can be applied to various levels of physiological processes – from alterations in gene expression, to protein activity, metabolite concentrations and flux, and even higher-order properties such as growth rate or fitness – and could conceivably differ between levels. Ultimately, this framework provides a “direction” to orient the interpretation of physiological changes that occurred during adaptation, revealing the level and degree to which adaptation either restores prior cellular states or finds novel solutions to improve growth or fitness. We hypothesized that physiological changes that are simply restorative would occur commonly, and would frequently arise in parallel between replicate evolved lineages. By sorting out these restorative changes, the novel and reinforcing changes that remain should more clearly reflect the physiological bases of adaptation. Particularly when these novel or reinforcing changes occur in parallel, they may identify loci in which the causative, beneficial mutations occurred.
Fig. 1. Acclimation and adaptation in an experimentally engineered and evolved bacterium. 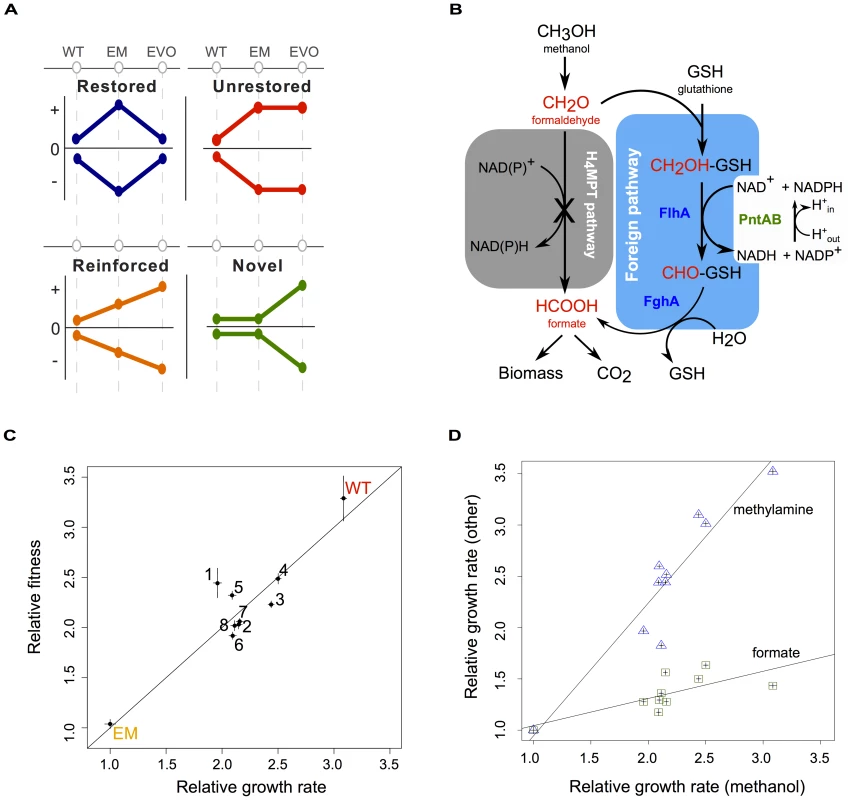
A) Combinations of acclimatizing and adaptive responses can be classified into four basic patterns based on wild-type (WT), perturbed (here, the engineered Methylobacterium strain, or “EM”), and evolved (EVO) physiological states. Physiological processes that were perturbed but return to a WT-like state are restored (blue); other processes that remain in a perturbed state are unrestored (red); those that are augmented from acclimation to adaptation are reinforced (orange); and still others are novel with respect to WT and EM states (green). B) Central one-carbon (C1) metabolism of WT and EM strains. In EM, the native pathway of formaldehyde oxidation (grey box) has been disabled and replaced by a foreign plasmid expressing two genes – flhA and fghA, from Paracoccus denitrificans – whose protein products co-opt endogenous glutathione to generate a functionally analogous, yet non-homologous substitute for C1 metabolism (blue box). This replacement results in the requirement for PntAB transhydrogenase to generate NADPH. C) EM was evolved in eight replicate cultures on methanol for over 600 generations. Isolates from each of the evolved populations (F1–F8) showed marked increases in growth rate and fitness relative to their EM ancestor. Line indicates y = x. D) Growth rates relative to EM on methanol are plotted for WT and the evolved isolates against two other C1 compounds: methylamine and formate. Lines show linear regression with an r2 of 0.94 and 0.73 for methylamine and formate, respectively, calculated in a Pearson correlation. As a model system in which to examine the interaction between acclimation and adaptation to perturbations, we employed a combination of metabolic engineering plus experimental evolution to study physiological and evolutionary responses to a novel, sub-optimal central metabolism in Methylobacterium extorquens AM1. As a facultative methylotroph, M. extorquens AM1 is capable of utilizing one-carbon (C1) compounds like methanol as a sole source of carbon and energy, as well as other multi-carbon compounds like succinate [13]. Its metabolism of C1 compounds is a complex process that requires over 100 different genes [14], many of which were acquired via horizontal gene transfer [15], [16]. C1 substrates such as methanol or methylamine are oxidized first to formaldehyde, and in wild-type (WT), this toxic intermediate is then oxidized to formate using a pathway linked to tetrahydromethanopterin (H4MPT), an analog of folate [15], [17] (Figure 1B). From formate, C1 units can be further oxidized into CO2 for the production of NADH, or assimilated into biomass [18], [19]. To create an engineered Methylobacterium (EM) strain, the native H4MPT-based pathway of formaldehyde oxidation was disabled and replaced by a functionally analogous, yet non-homologous C1 pathway. Two genetic changes were required to make EM: (1) the deletion of the mptG locus, which encodes the enzyme that drives the first committed step in H4MPT biosynthesis and is necessary for growth or survival in the presence of methanol [17], and (2) the introduction of an expression plasmid with two genes – flhA and fghA, both from Paracoccus dentrificans – that drive the oxidation of formaldehyde to formate using glutathione (GSH) as a C1 carrier [20]. The introduction of the engineered GSH-dependent pathway restores the ability of the ΔmptG strain to grow on methanol, however this EM strain is approximately 3-times slower growing than WT. Furthermore, the EM strain exhibits morphological abnormalities that arose from overexpression of the foreign GSH pathway [20].
Eight replicate populations (F1–F8) were founded from an EM ancestor and propagated on methanol for over 600 generations in batch culture to study adaptation to a novel metabolic module. Adaptation in the F populations was substantial, rapid, and largely methanol-specific [20]. The cellular abnormalities that emerged as a consequence of introducing the foreign pathway were also eliminated, representing an example of a restored (morphological) change. Several beneficial mutations have been identified in these evolved lines, including four from an isolate from the population with the highest fitness gains (F4). Notably, all four of these beneficial mutations altered gene expression. The targets and apparent physiological pressures acting upon these beneficial mutations are as follows. (1) Increased pntAB expression: switching from the native to the engineered pathway of formaldehyde oxidation eliminated the cell's only direct source of NADPH production, and a transhydrogenase encoded by pntAB can overcome this limitation by reducing NADP+ to NADPH using NADH [21]. (2) Increased gshA expression, which encodes an enzyme in GSH biosynthesis: GSH is needed to react with formaldehyde in the engineered pathway, and its recruitment into central metabolism might dilute GSH away from its native functions to protect against oxidative stress [22]. (3) Increased icuAB, which encodes a cobalt transporter: this mutation allowed cells to overcome metal limitation in the medium [10]. And (4), decreased expression of the introduced GSH pathway (i.e. flhA and fghA) [20], [23]: Foreign genes and plasmids introduced through engineering or natural gene transfers are often sub-optimal in terms of their sequence, expression, or activity for their new host and function [23]–[25]. Correspondingly, mutations that decreased expression of flhA and fghA balanced the benefits of formaldehyde oxidation with the costs of gene expression, and these occurred in all eight evolved populations through a variety of genetic mechanisms [23]. While these mutations to pntAB, gshA, icuAB, and the foreign pathway are known to have improved fitness in one or more lineages, many other changes in cellular physiology were also altered as a consequence. It is unclear whether these mutations produced novel or restorative physiological states, nor the extent to which these changes occurred in parallel across replicate populations.
Here, we sought to examine the extent to which evolution creates truly novel physiological states in the F lines, versus simply restoring acclimatizing processes towards WT-like levels. To this end, we used DNA microarrays to analyze changes in global gene expression from WT, to EM, to each of the eight F strains, and classified significant changes into patterns of restored, unrestored, reinforced, and novel gene expression as in Figure 1A. Without knowledge of acclimatizing processes, the substantial transcriptional changes observed in the evolved lineages would have been perceived as novel; however, our analysis revealed an overwhelming trend towards restoring gene expression to the WT state. Furthermore, whereas over 300 genes restored expression in parallel across all eight replicates, novel or reinforced changes tended to be unique to one or a few populations. Rare examples of parallelism amongst the novel or reinforced changes were particularly enriched for the loci with known beneficial mutations described above, or other probable candidates. One example of a highly parallel and beneficial reinforced change – PntAB transhydrogenase – translated into a restorative change in physiology, as it appeared to return NAD(P)(H) pools perturbed in EM back toward WT levels. Thus, incorporating information from physiological acclimation to a genetic or environmental perturbation can “orient” the interpretation of evolutionary adaptation, thereby distinguishing restorations from novelty, and greatly enriching for physiological changes that were causes, and not just consequences of increased fitness.
Results
Growth and fitness gains in the evolved strains were substantial and focused largely on formaldehyde oxidation
To interpret gene expression and other physiological data, we first characterized the growth rate and competitive fitness of WT, EM, and isolates of each F population after 600 generations of evolution. Relative growth rates measured using a high-throughput, automated robotic system [26]–[28] indicated the evolved isolates were now 1.95 to 2.5 times that of their EM ancestor on methanol, while WT was 3 times as fast (Figure 1C). Improvements in the F isolates were similar to the gains previously measured at the population level [20], suggesting that our isolates were representative of their respective evolved populations. Furthermore, these improvements in growth rate correlated well with increases in relative fitness, as determined by head-to-head competition [29], confirming that selection focused largely on exponential growth (Figure 1C).
Given that the sole difference between the slow EM strain and WT was the replacement of the formaldehyde oxidation pathway, we hypothesized that adaptation in the F lines would largely focus upon this stage of methanol metabolism. To test this hypothesis, we determined the specific growth rate of strains on two additional C1 substrates: methylamine, and formate. Growth on methylamine is nearly identical to growth on methanol, except that formaldehyde enters C1 metabolism by way of methylamine dehydrogenase [30]; while, in contrast, growth on formate skips the steps of formaldehyde oxidation altogether (Figure 1B). Relative to EM, the improvement of strains on methylamine was nearly comparable to their respective gains on methanol, while there were much smaller gains on formate (Figure 1D). The large difference between improvement in the selective environment (with methanol) versus formate, or succinate [20], contrasts with the generic improvements across substrates that was observed after adaptation of WT on methanol [29]. Overall, these data suggest that selection in the evolved lineages was focused predominantly focused on the formaldehyde oxidation pathway of C1 metabolism required for both methanol and methylamine growth.
Evolved changes in global gene expression were mostly restorative and highly parallel across populations
To investigate large-scale changes in physiology arising due to the replacement (acclimation) and subsequent evolution (adaptation) of the formaldehyde oxidation pathway, we used DNA microarrays to examine differences in global gene expression. We identified 878 genes that were differentially expressed relative to EM: 455 of which arose as acclimatizing responses to metabolic engineering, while the remaining 423 genes appeared only in the evolved isolates. Patterns of restored, unrestored, reinforced, or novel gene expression were categorized by following the fate of EM perturbations (if present) into each of the evolved lineages. Due either to experimental noise or an intermediate reversal in gene expression, a significant number of genes fell in-between our criteria for restored and unrestored, and were thus classified as a fifth group of “partially restored” changes. We present changes in gene expression in two ways (Figure 2A). First is a scatter plot that depicts both the changes that occurred during acclimation to the introduced pathway (WT vs. EM, x axis), versus those that occurred during adaptive evolution (EVO vs. EM, y axis). Second, we present a histogram (grey box) that compiles these data solely in terms of the changes that occurred during adaptation.
Fig. 2. Microarray analysis of changes in gene expression in WT, EM, and each of the evolved strains. 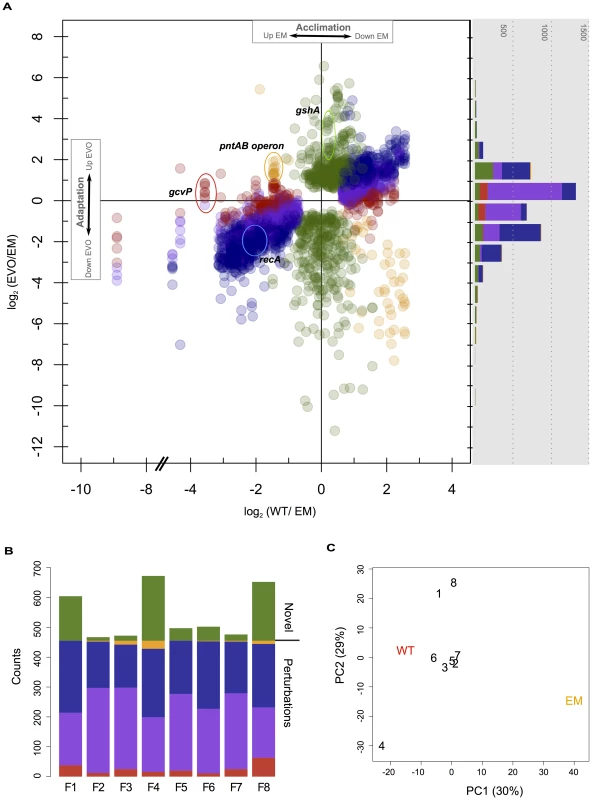
A) Genes with significant differences in expression in WT or the F isolates relative to EM. Each gene has a single value for the log2 difference in expression of WT relative to EM, and up to eight different values for each evolved strain. Changes in expression were categorized and colored as in Figure 1A as restored (blue), unrestored (red), reinforced (orange), or novel (green), as well as a fifth class of “partially restored” expression (purple). The histogram (right) bins each of these observations only considering adaptation, and thus just the differences between EM and the evolved strains. B) Instances of restored, unrestored, partially restored, reinforced, or novel expression across each of the eight evolved strains. C) Principal component analysis of all differentially expressed genes from physiological acclimation and adaptation. The majority of gene expression changes that occurred in the evolved strains were not novel, but restored perturbations to a WT-like state. Genes whose expression was fully or partially restored greatly outnumber the other categories. This is apparent by the large number of restored and partially restored genes in the scatter plot (Figure 2A), as well as by tabulating the number of genes satisfying each category across independent evolved isolates (Figure 2B, blue and purple). The next most numerous category was novel changes, followed by unrestored and then reinforced. As an additional method to explore similarity between transcriptional profiles, we used principal component analysis (PCA). Including all significant expression changes, PC1 clearly separats EM from WT and the evolved isolates. In contrast, PC2 distinguishes three evolved isolates from the remainder: separating F4 from F1 and F8 (Figure 2C). This highlights that, despite the great degree of parallelism in restorative gene expression, the transcriptomes of a few F lines appear to be quite distinct. Considering just those genes that are perturbed in EM (i.e., acclimatizing responses, with no novel changes), all evolved isolates cleanly fall between EM and WT, while F4, F1, and F8 remain quite distinct (Figure S1A). Considering just novel changes, only F4 and the pair of F1 and F8 are distinct from the rest (Figure S1B).
Given that most of the 455 genes with perturbed expression in EM were restored during adaptation, we hypothesized that this class of changes may be particularly likely to occur in parallel across the F lines. For each gene with significant expression changes, we tabulated how many instances of each class occurred across the F lines (Figure 3). Only about 10% (46/455) of perturbed genes satisfied the strict criterion for restoration in all eight populations (solid blue). By grouping strictly restored genes with cases of partial restoration, 72% (328/455) perturbed genes were restored across all eight populations (dashed-blue), and 98% (444/455) moved toward WT in at least four populations. In contrast, partially restored changes had little affect when combined with fully unrestored genes. This tremendous degree of parallelism was not observed for novel expression changes. Over 70% (330/483) of these occurred in just one strain, and of those that occurred in two populations, 81% (83/102) of these were specific to the F1 and F8 isolates that exhibited a particularly distinct transcriptional pattern.
Fig. 3. Parallelism of gene expression changes across categories. 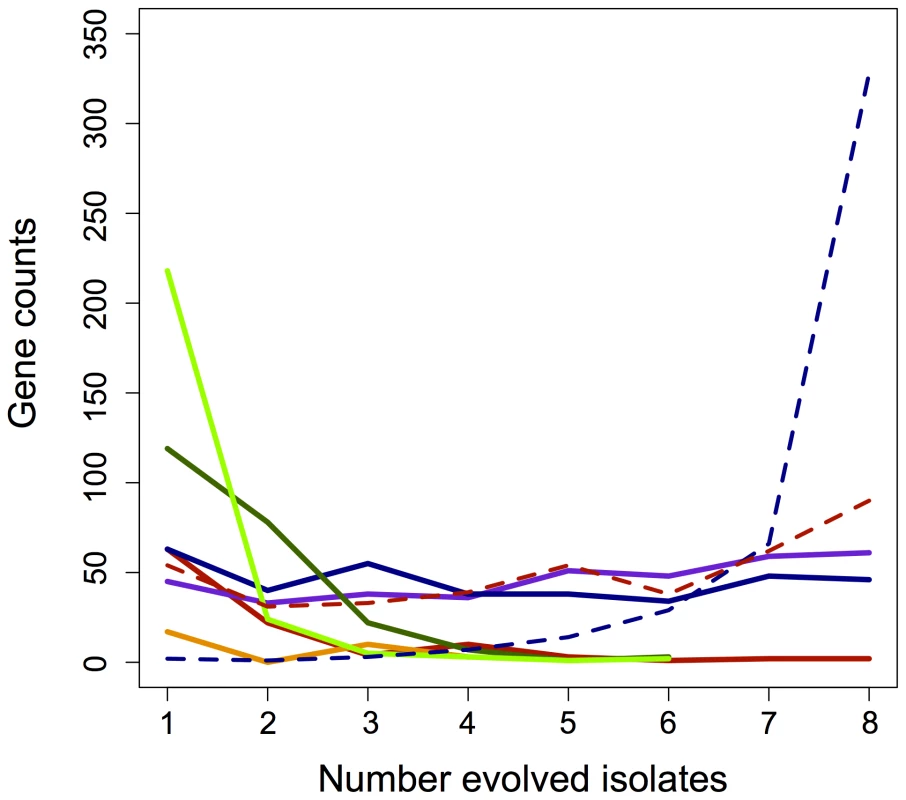
Instances of fully (blue) and partially (purple) restored, unrestored (red), reinforced (orange), or novel (green) changes in gene expression that occurred in parallel across the evolved lines. Novel is separated here into both increases (light green) and decreases (dark green) in expression for that gene. Dashed lines represent the parallelism of partially restored genes when combined with either fully restored (dashed blue) or fully unrestored (dashed red) changes. Gene expression that was restored or unrestored in perfect parallel highlights the major acclimatizing responses of EM to perturbations invoked by metabolic engineering. The 46 genes that were restored across all lineages function in heat shock and stress responses (including recA), C1 metabolism (components of methanol dehydrogenase), chemotaxis response regulators, and various genes putatively of phage origin. Conversely, only two genes – a glycine riboswitch (META1_misc_RNA_19) and a conserved hypothetical protein (META2_0338) were never restored in any lineage. In the adaptation of the F lines, we hypothesized that a greater number of restored genes would indicate a closer return to the wild-type state, and thus faster growth. This could occur either directly through mutations to pathways controlling the expression of perturbed genes, or secondarily as other physiological processes are restored (e.g., increase in growth rate or decrease in stress). Contrary to this hypothesis, however, growth rate did not correlate with either increased instances of restored or partially restored gene expression, or with a decrease in unrestored expression (Figure S2A–S2C). Furthermore, none of the four aforementioned loci known to have experienced beneficial mutations fell into this class. This suggests that the overall extent to which expression of evolved isolates returned to the WT-like state is not a good indicator of growth improvement.
Instances of novel gene expression were unique to one or a few evolved strains
Many if not most evolution experiments focus only on changes that are novel with respect to their ancestor. Provided with only knowledge of the EM state and the commonly-used threshold of 2-fold differential expression, our analysis would have wrongly classified many instances of fully or partially restored and reinforced expression as being novel in the evolved lineages (see histogram in Figure 2A). However, by incorporating WT physiology, we were able to identify 423 genes whose expression is wholly novel in at least one evolved lineage. The number of genes with novel expression varied between the F strains from only 12 in F2, to 217 in F4 (Figure 2B). Most instances of novel expression were unique to one or a few evolved lineages, with the exception of a few loci. One might therefore expect that an increased number of novel changes would be correlated with higher fitness, however no correlation was found between growth rate and increased instances of novel gene expression (Figure S2D). In fact, the F1 and F8 strains share a large number of uniquely derived changes in gene expression - with functions in DNA transcription and translation, DNA synthesis, and a number of C1-related genes – yet are amongst the least improved lineages. In F4, many novel down-regulated genes (Figure 2A) are in fact instances of gene loss from a previously identified deletion on the M. extorquens AM1 megaplasmid [20], [31] that has been shown to be beneficial and recurring across experiments [32]. While individual cases of novel gene expression are no doubt important to growth and fitness gains in the F isolates, we found that these are in general less frequent than restorative changes, mostly restricted to one or a few strains, and on the whole a poor indicator of improvements gained in the F lines.
Rare instances of reinforced gene expression highlight important links between acclimatizing and adaptive responses
The rarest, and perhaps most interesting class of gene expression changes were those that were reinforced, in which the acclimatizing response of EM to metabolic engineering was augmented through the evolutionary process. We identified only 30 genes with reinforced expression in at least one evolved isolate (7% of perturbed genes), which include the increased expression of the pntAB operon, the up-regulation of two genes with putative functions in cobalamin biosynthesis, the down-regulation of genes with predicted functions in fatty acid metabolism, and other genes with poorly-annotated functions that were down-regulated. Most genes with reinforced expression were unique to one or a few F strains, and remained unrestored or were restored in the other isolates (Figure 3). Unlike the above tests, instances of reinforced expression were strongly correlated with improvements in growth rate (Figure S2E; R2 = 0.87, p = 0.005), however the sample size of reinforced changes is small. As described above, pntAB was known to contain a beneficial mutation in its promoter in F4 [20], and these data now show that increased expression at this locus was not novel, but rather a response that arose first in the acclimation of EM and was reinforced through evolution.
We hypothesized that highly parallel instances of novel or reinforcing changes in gene expression might be enriched for loci with beneficial mutations. Although 306 genes showed parallel changes in expression across at least six populations, and 453 genes were either novel or reinforcing, only 5 instances were observed that satisfied both criteria, and they were all novel. We identifed those loci with known beneficial mutations (gshA and icuAB); one other gene with parallel increases (META1_0936, a putative type I secretion membrane fusion protein); and two genes with parallel decreases (META1_2657, a putative soxC sulfite oxidase; and META2_1007, a putative beta-lactamase). Regarding the parallelism of reinforcing changes, the three loci composing the pntAB operon were increased in 8/8 lineages relative to EM, but only significantly so in 4/8 lineages. This suggests that beneficial mutations may be particularly common in expression changes that are both parallel and buck the trend of restoration to the WT-like state.
Divergent mechanisms lead to parallel increases in the expression and activity of PntAB transhydrogenase
PntAB transhydrogenase functions in redox homeostasis, and thus it was intriguing to find that perturbed pntAB expression was not restored but actually increased further away from WT levels. We hypothesized that the consequence of increased pntAB could actually be restorative to the levels of pyridine nucleotides NAD(H) and NADP(H), despite its enhanced expression. We first examined the evolved isolates for mutations in the pntAB locus beyond that known for F4, and only one other strain – F3 – had a similar mutation in the upstream region (Figure 4A). Next, we investigated whether increased expression of pntAB equated to increased enzyme function. Transhydrogenase activity measured in WT, EM, and each of the evolved isolates closely mirrored changes in the expression of pntAB measured in the microarray analysis (Figure 4B). Our data suggest that, outside of F3 and F4, increased transhydrogenase in the F strains occurs either as a consequence of outside physiological changes (e.g., via allostery) or through trans-acting factors that drive increased expression in these lineages. We further examined the relationship between transhydrogenase levels and growth rate, and found a highly correlated positive relationship amongst the evolved F isolates (Figure 4C).
Fig. 4. Multiple evolved mechanisms reinforce increased pntAB expression and transhydrogenase activity. 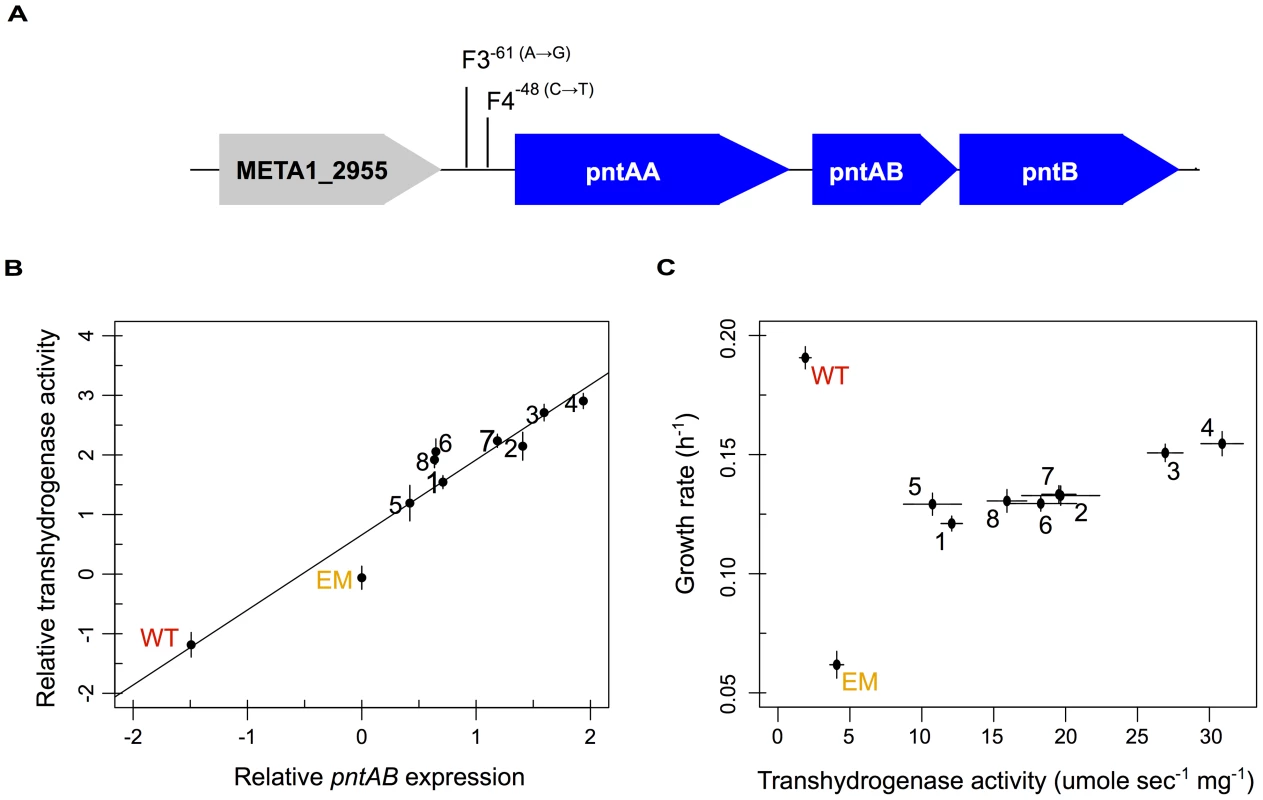
A) Known (F4) and candidate (F3) mutations that increase pntAB expression. B) Relative increases in pntAB expression were highly correlated with increased overall transhydrogenase activity (Pearson correlation, r2 = 0.96 with p = 1.27×10−5). C) Plot of increasing transhydrogenase activity with increasing growth rate. WT is able to grow well in the absence of transhydrogenase; however, enzyme activity is significantly increased from WT to EM, and reinforced even further from EM to each of the evolved lineages (p<0.05, Welch two-sample t-test). A significant positive relationship exists between transhydrogenase activity and growth rate for the evolved isolates (Pearson correlation, r2 = 0.86 with p<0.01). The reinforcement of PntAB restores perturbations in NAD(P)(H) to wild-type
To determine whether the effect of increased transhydrogenase activity in the evolved strains was to restore the redox balance of pyridine nucleotides, we examined strain differences in the ratios of NADPH/NADP+ and NADH/NAD+. Interpreting changes in the steady-state concentrations of metabolites such as NADPH is complicated by the fact that these values represent a balance between production (such as by transhydrogenase) and consumption via biosynthesis. As there is relatively little degeneracy in the network of biosynthetic reactions, the rate of NADPH use should be nearly directly proportional to growth rate, such that mutations that increase the cell's capacity to grow can actually decrease the steady-state concentration of currency metabolites. Indeed, data from a variety of other organisms, such as Escherichia coli [33] and Lactococcus lactis [34] grown at different rates in chemostats have confirmed this intuition.
Consistent with the above expectations, the slow-growing EM strain possessed a much higher ratio of both NADPH/NADP+ and NADH/NAD+ than WT. Including the evolved isolates, the ratios (or redox state) of reduced to oxidized NADP(H) and NAD(H) were both highly negatively correlated with growth rate, such that faster-growing strains possess substantially lower ratios for each (Figure 5A and 5B, respectively). Variation in levels of NADPH/NADP+ also correlated well with changes in pntAB expression: strains with significant increases in pntAB (n = 4 strains) showed significantly lower NADPH/NADP+ ratios than those with marginal increases (p<0.05, Welch two-sample t-test); however, the same was not true for NADH/NAD+ ratios. Even amongst strains with significantly increased pntAB expression, those with cis-acting mutations (F3 and F4) were significantly faster and had lower NADPH/NADP+ ratios than strains with significant increases apparently driven in trans (F2 and F7). Importantly, almost all strains statistically significantly restore the redox states of NADP(H) and NAD(H) towards WT-like levels. Overall, these data suggest that the reinforcement of transhydrogenase activity increased the rate of NADPH production and drove the restoration of pyridine nucleotide metabolism back toward a WT-like state through an apparent variety of adaptive mechanisms.
Fig. 5. The redox states of pyridine nucleotides are perturbed in EM but restored through evolution. 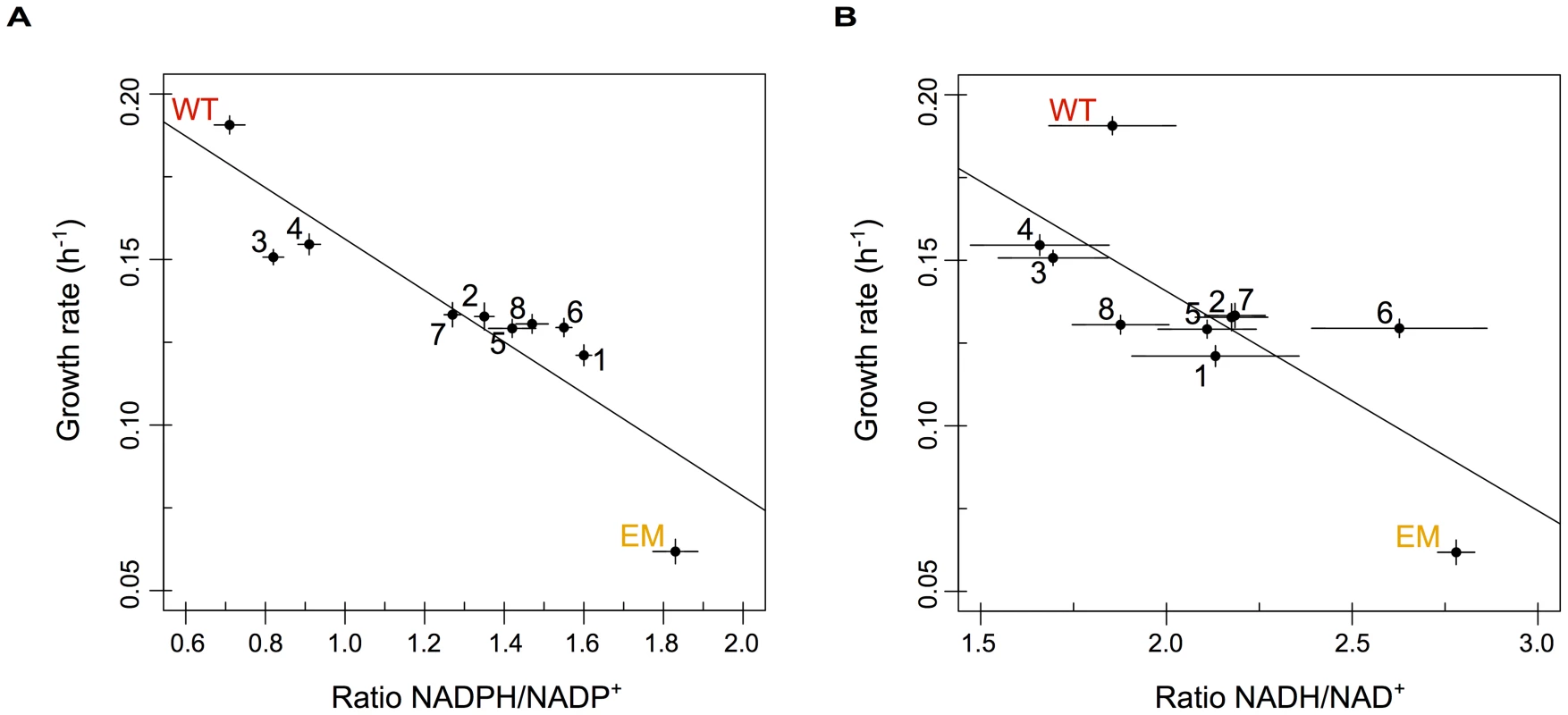
The relative ratios of NADPH/NADP+ (A) and NADH/NAD+ (B) plotted against growth rate for WT, EM, and each of the evolved strains. The redox states of NADP(H) and NAD(H) were perturbed in EM but returned toward WT-like values in almost all of the evolved lineages. Ratios were highly correlated with growth rate for both NADP(H) (r2 = −0.88 with p = 6.9×10−4) and NAD(H) (r2 = −0.76 with p = 0.011) in a Pearson correlation. Discussion
Organisms are constantly pressured by ever-changing and potentially disruptive cellular and environmental conditions. Large-scale changes in physiology can occur due to ecological or environmental transitions, or upon sudden changes in genomic composition due to mutation, horizontal gene transfer, or genetic engineering in the laboratory. When a perturbed, sub-optimal physiology persists over multiple generations, transient acclimatizing responses begin to overlap with responses from evolutionary adaptation. Conceptually, processes of physiological acclimation and adaptation are intimately linked: as beneficial mutations should revert many acclimatizing processes from a perturbed to a baseline physiological state. In practice, the interplay between acclimatizing and adaptive responses to perturbations has often been ignored, leading to the scenario where large-scale, parallel restoration of physiology to a pre-stress state will appear as novel. We argue that a proper interpretation of evolved physiological states is only possible given knowledge of the initial acclimation to a new environment or genomic composition.
Our work sought to determine the extent to which cells adopt novel versus restorative physiological states by examining acclimatizing and adaptive responses to a novel central metabolism. We utilized a strain of M. extorquens AM1 (EM) that was metabolically engineered to utilize a foreign, GSH-based central pathway to oxidize formaldehyde during growth on C1 compounds, and was subsequently propagated in eight replicate F populations for over 600 generations of evolution to optimize growth using the engineered pathway. The physiology of the EM ancestor was perturbed in many ways: it was three-fold slower; adopted an elongated, curved or branched cell morphology [20], and exhibited a unique density-threshold for growth on methanol [35]. Here we document two additional levels of physiological perturbation: microarray analyses revealed 455 genes with altered expression from WT to EM, as well as perturbations in the central redox cofactors, NAD(H) and NADP(H). By orienting our analyses based upon the initial acclimation from WT to EM, we categorized evolved changes as restored, unrestored, reinforced, or novel as in Figure 1A. Given the particularly interesting connection between acclimatizing and adaptive processes in reinforcement, we further examined the systems-level consequences of enhanced PntAB activity.
The major pattern seen for evolved changes in physiology was an overwhelming trend to return to a wild-type state. Our work highlights a few general trends to be explored in other systems. First, the majority of gene expression differences distinguishing the ancestor and the evolved isolates were not novel, but instead restorative. Most restored genes were not themselves targets of beneficial mutations, but altered in response to other changes such things as NAD(P)(H) levels, or indirectly, improved growth rate (increased methanol dehydrogenase), or reduced stress (decreased recA and heat shock proteins). So much of gene expression was restorative that it outweighed instances of novel expression in all evolved strains. Similarly, PCA analysis confirmed that expression in the evolved isolates was more like WT than their common EM ancestor. One interesting future direction would be to examine the temporal component of adaptation, studying the degree to which physiology is restored as populations acquire sequential beneficial mutations. Second, the restoration of WT physiology occurred highly in parallel. Indeed, the vast majority of genes were restored, at least partially so, in all eight lineages. This is perhaps intuitive as a shared set of acclimatizing processes from EM were simply “turned off” in the case of stress-related responses, or “turned up” in the case of growth related genes, in the evolved lines. Without specific knowledge of these acclimatizing processes, however, most of these restorative changes would be wrongly classified as novel (Figure 2A, histogram). Third, some acclimatizing processes were left unrestored because physiological adaptation cannot, or has not yet, addressed these perturbations. These may represent fundamental and perhaps inescapable differences separating WT and EM physiologies. And finally, changes that are both highly parallel and either novel or reinforced are potentially enriched for loci targeted by beneficial mutations, and thus causal changes during adaptation. Increases in expression in gshA (6/8 novel), icuAB (6/8 novel), and pntAB (4/8 reinforced) are all outliers when comparing the parallelism of changes in each category across genes (Figure 3). In fact, by filtering out (highly parallel) restorative changes, we find only 19 genes (out of 878) that are novel or reinforced changes in half or more of the evolved strains. Including the parallel, beneficial decreases in the expression and/or activity of the foreign pathway that occurred in 8/8 strains [23], parallel changes in gene expression that are not restorative appear to be particularly enriched for beneficial mutations that drove adaptation.
Looking closer, we did find variation in how the various F lines adapted to an engineered C1 metabolism. Novel expression of genes very rarely occurred in more than one strain, and where observed, it was nearly always to the F1 and F8 strains. These isolates consistently showed different transcriptional profiles than the other F isolates, not only amongst novel genes, but also in the number, types, and degree to which genes are restored. Interestingly, both F1 and F8 are also amongst the slowest growing of the F strains, suggesting perhaps the presence of a multi-peaked fitness landscape in which these strains have found a local optimum. While it appears that the F populations restored many genes in parallel, and share at least a few common molecular and physiological mechanisms, additional work is needed to understand the full extent to which these strains found parallel versus divergent paths to optimize growth using an engineered central metabolism.
Reinforcing changes to physiology, while rare in our system, are an important link between processes of physiological acclimation and adaptation. We focused on one particular instance of reinforcement – the up-regulation of pntAB transhydrogenase – to investigate both the genetic basis for enhancing expression beyond acclimation and to uncover its physiological consequences. Normally, pntAB is expressed during multi-C and not C1 growth [36], however in EM, the only direct source of NAD(P)(H) production was lost with the deletion of the native pathway of formaldehyde oxidation. This perturbation might invoke increased pntAB to maintain NAD(P)(H) homeostasis during growth on methanol. Supporting this hypothesis, the deletion of pntAB was found to be neutral for C1 growth in WT but lethal in the EM strain (H.-H. Chou, data not shown), and the mutation in the F4 lineage that drives increased pntAB expression provides a 10% selective benefit in the ancestral background [20]. These results demonstrate the irreplaceable role of pntAB as an acclimatizing response in EM, and the benefit of reinforcing this function even further through adaptation.
While the increased expression and activity of PntAB transhydrogenase was reinforcing, this translated into a restorative effect upon metabolism. All eight F strains increased transhydrogenase activity significantly, despite significant increases in expression for only half of these. Upon sequencing the genomic neighborhood of pntAB, we identified only two strains – F4 and F3 – that possess known or candidate mutations to drive increased expression. As for the physiological consequence of increased transhydrogenase activity, all evolved strains tend to restore NAD(P)(H) metabolism, and strains with greater increases to pntAB – particularly the pair with mutations in the upstream region – have levels of NAD(H) and NADP(H) that are the closest to WT. The reinforcement of pntAB expression and transhydrogenase activity, as well as the restoration of NAD(P)(H) levels, are both well correlated with increased growth rate in the F populations. By increasing activity during acclimation, and reinforcing this response further during adaptation, transhydrogenase activity appears to have been critical in maintaining and improving growth in the EM strain.
Information on acclimatizing and adaptive responses in the engineering and evolution of EM allowed us to develop a framework in which to examine the true nature of evolved physiological change. We defined four basic patterns to describe not only novel changes to physiology, but also changes that restore, disregard, or reinforce the initial acclimatizing responses to perturbations (Figure 1A). To our knowledge, this linkage between immediate physiological acclimation and subsequent adaptation has explicitly been explored only once before [12]. This paper described a large number of “compensatory” changes in gene expression that effectively restored the wild-type (glucose-grown) state during the acclimation and experimental evolution of E. coli to sub-optimal carbon sources. The commonalities between adaptation to a poor environment versus a novel, suboptimal metabolic pathway are remarkable: whereas their study showed that 87% of genes were restored after adaptation to a poor substrate, we found that on average that 93% of genes were restored after adaptation to the foreign pathway; their change was environmental, ours genetic. Furthermore, reinforcing changes are reminiscent of the fixation of traits via genetic accommodation or assimilation [37]–[39], in that both processes stem from exposure to genetic or environmental stressors to reveal beneficial phenotypes that are “assimilated” and possibly reinforced by positive selection. However, in genetic assimilation, stress-induced phenotypes arise from cryptic genetic variation in populations [40] while, at least for the reinforced up-regulation of pntAB expression, the initial response required no standing genetic variation at all. In fact, the initial acclimatizing response of EM to increase pntAB was merely a generic response to NADPH shortage typically experienced during growth on multi-carbon substrates such as succinate [36], that was co-opted for methanol growth in EM, and further increased and optimized by selection during adaption of the F lines.
Overall, our results suggest that much of evolutionary adaptation effectively relieves processes of physiological acclimation. Rather than “reinvent the wheel” of C1 metabolism, a few causal mutations in the adaptation of the F populations propagated through physiology to restore WT homeostasis. In fact, more changes in gene expression occurred as a result of acclimation to genetic engineering (n = 455) than novel changes seen in any of the isolates after 600 generations of experimental evolution (12 to 217). Beneficial mutations were enriched toward novel and reinforcing changes that occurred in parallel. By distinguishing acclimatizing versus adaptive processes, a more accurate depiction on the nature and parallelism of physiological evolution is revealed.
Materials and Methods
Growth conditions
All growth was performed using a modified “Hypho” minimal medium as in [20]. One liter of Hypho was prepared from 799 mL of deionized water, 100 mL phosphate salts (25.3 g of K2HPO4 plus 22.5 g NaH2PO4 in 1 L deionized water), 100 mL sulfate salts (5 g of (NH4)2SO4 plus 0.98 g MgSO4 in 1 L deionized water), and 1 mL of modified, high-iron “Vishniac” trace metal solution [10], [20]. All solutions were autoclaved separately and combined under sterile conditions, and the final medium was stored in the dark. Carbon substrates added just prior to inoculation consisted of: 20 mM methanol, 3.5 mM sodium succinate, 15 mM methylamine hydrochloride, or 20 mM sodium formate. Growth experiments were initiated by inoculating 10 µL of freezer stock into 9.6 mL Hypho in a 50 mL Erlenmeyer flask containing 10 mM methanol and 1.75 mM succinate plus 50 µg/mL kanamycin. Flasks were grown at 225 rpm in a 30°C shaker-incubator until reaching stationary phase (2–4 days). A second acclimation cycle was accomplished by transferring 150 µL of saturated culture into 9.45 mL fresh medium with 0.5× kanamycin plus the carbon substrate to be tested; then transferred again into the same conditions for experimental (measured) growth. A 1∶64 dilution of cultures with the given substrate concentrations allowed for six doublings per growth cycle.
All physiological assays (e.g., microarray analyses, enzyme assays, metabolite concentrations) were performed using cells that had reached half-maximal density following transfer from acclimation cultures also grown on methanol. This protocol results in eleven doublings of growth in a consistent environment while ensuring cells were still growing exponentially at the time of harvest. This gave the maximal possible time to approach steady-state physiology while staying within the constraints of the selective conditions. Furthermore, since it was previously found that the EM ancestor exhibits a unique cell-density threshold for growth [35], it would not have been possible to have diluted the cultures much more than the 1/64 used here.
Specific growth rates were determined in 48-well plates using a high-throughput, robotic system that automates measurements of optical density (i.e., OD600) in growing cultures at timed intervals [27]. This system consists of a plate-shaking tower, a plate reader, a robotic arm, and de-lidding station that transfer cultures between growth and measurements, all of which is scheduled with an open software manager program [26]. Strains for growth measurements were inoculated first into flasks, transferred to plates with for an acclimation phase, and transferred once more for measurement during the third cycle. All growth was performed in 640 µL total medium and were transferred in a 1/64 dilution (10 µL culture into 630 µL medium). To limit clumping and reduce noise in OD600 measurements in growing cultures, 0.1 mg/mL of prepared cellulase enzyme (Sigma-Aldrich, St. Louis, MO) was added to the growth medium (SMC, unpublished). The specific growth rate was calculated from the log-linear phase of growth for at least triplicate cultures of each strain using an open software analysis package [28].
Strain construction and evolution
Strains and plasmids relevant to this study are listed in Table S1 and were generated previously, unless otherwise noted. The ancestral strains for the F populations were described previously [20]. Briefly, they derive from two WT M. extorquens AM1 strains - one that is naturally pink (CM501), and another that is white (CM502) due to a neutral mutation in carotenoid biosynthesis [41] – to limit contamination between cultures. The EM strain was constructed in two steps: 1) the H4MPT-dependent pathway was disabled by deleting the mptG locus (encoding β-ribofuranosylaminobenzene 5′-phosphate synthase), the product of which drives the first committed step in the H4MPT biosynthesis [42]; and 2) the introduction of a GSH-dependent formaldehyde oxidation pathway on the plasmid pCM410 – which expresses the genes flhA (encoding S-hydroxymethyl-GSH dehydrogenase) and fghA (encoding S-formyl-GSH hydrolase) from Paracoccus denitrificans –into the ΔmptG backgrounds, generating completed pink (CM701) and white (CM702) EM strains [20].
Eight replicate populations were founded from either the pink (odd populations; CM701) or white (even populations; CM702) EM strains and evolved for over 600 generations in 9.6 mL Hypho medium plus 15 mM methanol in batch culture with transfers of 1/64 of the volume every four days for the first 300 generations, and every two days thereafter. These evolved “F” populations (F1-8) were streaked at generation 600 onto Hypho agar plates to isolate colonies for further characterization. In addition to the previously characterized isolate from the F4 population, CM1145 [20], we chose for this study the second of three random isolates from each of the other F populations for further investigation (Table S1).
Other strains relevant to this study were as follows. Fluorescence-based fitness assays required an EM reference strain (CM1232) that had been generated by replacing the katA locus with mCherry driven by a constitutive Ptac promoter [20]. To standardize the use of kanamycin in all cultures, we used a WT strain in which the kan resistance marker was inserted into katA (CM611) [29].
Competition assays of relative fitness
The relative fitness of WT and evolved strains was assessed in a head-to-head competition of co-cultures with a fluorescently-labeled reference as in [29]. Briefly, fully-grown cultures of WT and each evolved isolate were mixed in roughly equal optical densities with an EM strain expressing mCherry (CM1232). A sample of this mixture (T0) was diluted with Hypho plus 8% DMSO and stored at −80°C in 96-well plates; the rest was diluted 1∶64 into 640 µL of Hypho methanol medium in a 48-well plate and incubated with shaking at 30°C for 4 days, after which samples of the co-culture after competition (T1) were frozen for later analysis using flow cytometry. Because of the 4-day growth cycle, this amortizes fitness over all growth phases (i.e., lag, exponential, and stationary).
The ratio of labeled to unlabeled cells before and after co-culture growths was measured using a BD LSR Fortessa flow cytometer with an HTS attachment for 96-well plates (BD Biosciences, San Jose, CA). Recently it was found that the forward scatter (FSC) and side scatter (SSC) settings used in earlier work [20] systematically underestimated fitness increases relative to EM because of the cells' larger size. Here we set both scatter measurements set to 300 V to accommodate small bacterial cell sizes [23], and the flow-rate was adjusted to the lowest setting to produce reliable measurements of labeled and unlabeled events in dilute co-cultures. The ratio of nonfluorescent to fluorescent cells before (R0, from T0) and after (R1, from T1) competition were used to calculate the fitness (W) of strains relative to the EM reference (CM1232) using the following formula, assuming a 64-fold expansion of cells from six doublings per growth cycle:
Microarray analyses
Triplicate cultures of strains were grown to half-maximal OD600 in 15 mM methanol before harvesting and total RNA extraction using the RNeasy kit (Qiagen, Valencia, CA). Genomic DNA was removed using the TURBO DNA-free kit (Ambion, Austin, TX) and the RNA samples were concentrated using Amicon Ultra centrifugal filters (Millipore, Billerica, MA). Microarray analyses for all (n = 30) samples were performed by MOgene, Inc (St. Louis, MO) using one-color cDNA labeling and hybridization. The array probes and platform were designed previously [43] to include 60-mer oligonucleotides that provide two or more probes for confirmed and predicted ORFs in the Methylobacterium genome [31]. Raw and normalized expression data are available from the Gene Expression Omnibus, accession GSE42116. Pre-processing, normalization, and analysis of expression data was performed using the limma package [44], [45] with Bioconductor [46] and R [47]. Differentially expressed genes were identified by the proportion of differentially expressed probes in a limma contrast given: 1) at least two-thirds probes significant at p<0.05 in the moderated t-statistic, 2) at least one-half of probes significant at p<0.01, and 3) all significant probes with uniform changes either up or down. Probes that met these criteria were averaged in each strain to estimate the log2 difference in expression relative to EM. Genes differentially expressed in both EVO:EM and EVO:WT contrasts, and in the same direction, were classified as novel. Expression perturbations from acclimation were identified in a WT:EM contrast and further partitioned given information from EVO:EM and EVO:WT contrasts to define patterns of: restored expression, given an EVO:EM change back in the direction of WT expression; unrestored expression, given no EVO:EM difference but a significant EVO:WT difference; and reinforced expression, having an EVO:EM difference in the same direction (up or down) as the change from WT to EM (Figure 1A). Partially restored genes showed no EVO:EM difference and were not significant in a EVO:WT contrast. Principal component analysis was used to cluster and contrast the expression profile of WT, EM, and evolved strains, and was calculated using the prcomp function in R with scaling to account for large variance of expression changes between genes.
Transhydrogenase enzyme activity
Cultures for the determination of TH activity and NAD(P)(H) ratios (below) were grown to half-maximal OD600 on methanol, spiked with another 15 mM methanol, and allowed to return to mid-exponential growth for approximately 16 hours to increase yield. Cultures for transhydrogenase activity measurements were pelleted and washed with 50 mM Tris-HCl (pH 7.5) before storage at −80°C. Upon thawing, cells were re-suspended in 2 mL Tris buffer and lysed by bead beating (MP Biomedicals, Solon, OH). Cell extracts were centrifuged for less than 15 s to collect the beads. The supernatant was removed and combined with a reaction mix consisting of: 20 µL of 40 mM MgCl2 (10×), 20 µL 5 mM NADPH (10×), 20 µL 10 mM 3-acetylpyridine adenine dinucleotide (10×), plus Tris buffer to equal 200 µL, total, in a 96-well plate. The increase in absorbance at 375 nm was measured immediately after addition of the reaction mix and the slope of the linear regression was used to calculate transhydrogenase activity (µmole of 3-acetylpyridine adenine dinucleotide reduced sec−1 mg−1) as follows: TH activity (µmole sec−1 mg−1) = slope (sec−1)×1/exctinction coefficient (0.0051 mol cm L−1)×1/path length (0.42 cm−1)×reaction volume (0.2 mL)×1/cell protein (mg)×1000 (conversion to µmole L−1).
Measurement of relative NAD(H) and NADP(H) concentrations
Cell extracts for the measurement of pyridine nucleotide concentrations were prepared as follows. Metabolism in mid-exponential cells was quenched using vacuum-filtration and rapid immersion into hot extraction solutions. Oxidized pyridine nucleotides (NAD+ and NADP+) were selectively preserved in an acidic extraction solution consisting of 100 mM HCl plus 500 mM NaCl; reduced species (NADH and NADPH) were extracted using a basic solution of 100 mM NaOH plus 500 mM NaCl. For both acidic and basic extractions, 750 µL of culture was vacuum-filtered onto 0.45 µm nylon membranes (Millipore, Billerica, MA), immediately immersed into the appropriate extraction solution, briefly vortexed, and heated to 95°C for 5 m. Extracts were again briefly vortexed, centrifuged at maximum speed for 30 s, and the supernatant removed, flash frozen, and stored at −80°C for later use. Three biological replicates stemming from separate inoculations were extracted for each strain.
Pyridine nucleotides in cell extracts were quantified using enzymatic cycling [48] with alcohol dehydrogenase (ADH) or glucose-6-phosphate dehydrogenase (G6PDH) to measure NAD(H) and NADP(H), respectively. Each assay was performed using 20 µL of either acidic extraction solutions for oxidized species, basic solutions for reduced, or a serial dilution of (reduced) standards. For NAD(H), to 20 µL of cell extract or standard was added 180 µL of master solution consisting of: 20 µL 1 M bicine (pH 8.0) plus 40 mM EDTA (10×), 20 µL of 16.6 mM phenazine ethosulfate (10×), 20 µL of 4.2 mM thiazolyl blue tetrazolium bromide (10×), 20 µL of 100% ethanol, 2 µL of ADH (Sigma-Aldrich, St. Louis, MO) at 0.1 U/µL, and 98 µL water. The same mixes were used for the determination of NADP(H) with G6PDH, except that ethanol and ADH were replaced by 20 µL of 50 mM glucose-6-phosphate (10×) and 2 µL of G6PDH at 0.1 U/µL. Assays were conducted in 96-well plate format and measured in a Safire2 spectrophotometer (Tecan, Morrisville, NC) at 30°C by following the increase in absorbance at 550 nm over time.
Supporting Information
Zdroje
1. CooperTF, RozenDE, LenskiRE (2003) Parallel changes in gene expression after 20,000 generations of evolution in Escherichia coli. P Natl Acad Sci Usa 100 : 1072–1077 doi:10.1073/pnas.0334340100.
2. CooperTF, RemoldSK, LenskiRE, SchneiderD (2008) Expression profiles reveal parallel evolution of epistatic interactions involving the CRP regulon in Escherichia coli. PLoS Genet 4: e35 doi:10.1371/journal.pgen.0040035.
3. GreshamD, DesaiMM, TuckerCM, JenqHT, PaiDA, et al. (2008) The repertoire and dynamics of evolutionary adaptations to controlled nutrient-limited environments in yeast. PLoS Genet 4: e1000303 doi:10.1371/journal.pgen.1000303.
4. McDonaldMJ, GehrigSM, MeintjesPL, ZhangX-X, RaineyPB (2009) Adaptive divergence in experimental populations of Pseudomonas fluorescens. IV. Genetic constraints guide evolutionary trajectories in a parallel adaptive radiation. Genetics 183 : 1041–1053 doi:10.1534/genetics.109.107110.
5. KvitekDJ, SherlockG (2011) Reciprocal sign epistasis between frequently experimentally evolved adaptive mutations causes a rugged fitness landscape. PLoS Genet 7: e1002056 doi:10.1371/journal.pgen.1002056.
6. ToprakE, VeresA, MichelJ-B, ChaitR, HartlDL, et al. (2012) Evolutionary paths to antibiotic resistance under dynamically sustained drug selection. Nat Genet 44 : 101–105 doi:10.1038/ng.1034.
7. MaharjanR, SeetoS, Notley-McRobbL, FerenciT (2006) Clonal adaptive radiation in a constant environment. Science 313 : 514–517 doi:10.1126/science.1129865.
8. WoodsR, SchneiderD, WinkworthCL, RileyMA, LenskiRE (2006) Tests of parallel molecular evolution in a long-term experiment with Escherichia coli. P Natl Acad Sci Usa 103 : 9107–9112 doi:10.1073/pnas.0602917103.
9. WoodTE, BurkeJM, RiesebergLH (2005) Parallel genotypic adaptation: when evolution repeats itself. Genetica 123 : 157–170.
10. ChouH-H, BerthetJ, MarxCJ (2009) Fast growth increases the selective advantage of a mutation arising recurrently during evolution under metal limitation. PLoS Genet 5: e1000652 doi:10.1371/journal.pgen.1000652.
11. CooperVS, LenskiRE (2000) The population genetics of ecological specialization in evolving Escherichia coli populations. Nature 407 : 736–739 doi:10.1038/35037572.
12. FongSS, JoyceAR, PalssonBØ (2005) Parallel adaptive evolution cultures of Escherichia coli lead to convergent growth phenotypes with different gene expression states. Genome Res 15 : 1365–1372 doi:10.1101/gr.3832305.
13. PeelD, QuayleJ (1961) Microbial growth on C1 compounds. I. Isolation and characterization of Pseudomonas AM 1. Biochem J 81 : 465–469.
14. ChistoserdovaL, ChenS-W, LapidusA, LidstromME (2003) Methylotrophy in Methylobacterium extorquens AM1 from a genomic point of view. J Bacteriol 185 : 2980–2987.
15. ChistoserdovaL, VorholtJA, ThauerRK, LidstromME (1998) C1 transfer enzymes and coenzymes linking methylotrophic bacteria and methanogenic Archaea. Science 281 : 99–102.
16. VorholtJA, ChistoserdovaL, StolyarSM, ThauerRK, LidstromME (1999) Distribution of tetrahydromethanopterin-dependent enzymes in methylotrophic bacteria and phylogeny of methenyl tetrahydromethanopterin cyclohydrolases. J Bacteriol 181 : 5750–5757.
17. MarxCJ, ChistoserdovaL, LidstromME (2003) Formaldehyde-detoxifying role of the tetrahydromethanopterin-linked pathway in Methylobacterium extorquens AM1. J Bacteriol 185 : 7160–7168.
18. MarxCJ, Van DienSJ, LidstromME (2005) Flux analysis uncovers key role of functional redundancy in formaldehyde metabolism. PLoS Biol 3: e16 doi:10.1371/journal.pbio.0030016.
19. CrowtherGJ, KosályG, LidstromME (2008) Formate as the main branch point for methylotrophic metabolism in Methylobacterium extorquens AM1. J Bacteriol 190 : 5057–5062 doi:10.1128/JB.00228-08.
20. ChouH-H, ChiuH-C, DelaneyNF, SegrèD, MarxCJ (2011) Diminishing returns epistasis among beneficial mutations decelerates adaptation. Science 332 : 1190–1192 doi:10.1126/science.1203799.
21. SauerU, CanonacoF, HeriS, PerrenoudA, FischerE (2004) The soluble and membrane-bound transhydrogenases UdhA and PntAB have divergent functions in NADPH metabolism of Escherichia coli. J Biol Chem 279 : 6613–6619 doi:10.1074/jbc.M311657200.
22. MasipL, VeeravalliK, GeorgiouG (2006) The many faces of glutathione in bacteria. Antioxid Redox Signal 8 : 753–762 doi:10.1089/ars.2006.8.753.
23. Chou H-H, Marx CJ (2012) Optimization of gene expression through divergent mutational paths. Cell Reports: 1–15. doi:10.1016/j.celrep.2011.12.003.
24. ParkC, ZhangJ (2012) High expression hampers horizontal gene transfer. Genome Biol Evol 4 : 523–532 doi:10.1093/gbe/evs030.
25. DekelE, AlonU (2005) Optimality and evolutionary tuning of the expression level of a protein. Nature 436 : 588–592 doi:10.1038/nature03842.
26. DelaneyNF, Rojas EcheniqueJI, MarxCJ (2012) Clarity: an open-source manager for laboratory automation. J Lab Autom doi:10.1177/2211068212460237.
27. Delaney NF, Kaczmarek ME, Ward LM, Swanson PK, Lee MC, et al.. (n.d.) Development of an optimized medium, strain and high-throughput culturing methods for Methylobacterium extorquens. Submitted.
28. DelaneyNF, KaczmarekME, MarxCJ (n.d.) Evaluating sources of bias when estimating microbial growth rates in microtiter plates and development of the open-source program Curve Fitter. Submitted
29. LeeM-C, ChouH-H, MarxCJ (2009) Asymmetric, bimodal trade-offs during adaptation of Methylobacterium to distinct growth substrates. Evolution 63 : 2816–2830 doi:10.1111/j.1558-5646.2009.00757.x.
30. EadyRR, LargePJ (1968) Purification and properties of an amine dehydrogenase from Pseudomonas AM1 and its role in growth on methylamine. Biochem J 106 : 245–255.
31. VuilleumierS, ChistoserdovaL, LeeM-C, BringelF, LajusA, et al. (2009) Methylobacterium genome sequences: a reference blueprint to investigate microbial metabolism of C1 compounds from natural and industrial sources. PLoS ONE 4: e5584 doi:10.1371/journal.pone.0005584.t004.
32. LeeM-C, MarxCJ (2012) Repeated, selection-driven genome reduction of accessory genes in experimental populations. PLoS Genet 8: e1002651 doi:10.1371/journal.pgen.1002651.
33. KayserA, WeberJ, HechtV, RinasU (2005) Metabolic flux analysis of Escherichia coli in glucose-limited continuous culture. I. Growth-rate-dependent metabolic efficiency at steady state. Microbiology (Reading, Engl) 151 : 693–706 doi:10.1099/mic.0.27481-0.
34. LahtveeP-J, AdambergK, ArikeL, NahkuR, AllerK, et al. (2011) Multi-omics approach to study the growth efficiency and amino acid metabolism in Lactococcus lactis at various specific growth rates. Microb Cell Fact 10 : 12 doi:10.1186/1475-2859-10-12.
35. MarxCJ (2012) Recovering from a bad start: rapid adaptation and tradeoffs to growth below a threshold density. BMC Evol Biol 12 : 109 doi:10.1186/1471-2148-12-109.
36. SkovranE, CrowtherGJ, GuoX, YangS, LidstromME (2010) A systems biology approach uncovers cellular strategies used by Methylobacterium extorquens AM1 during the switch from multi - to single-carbon growth. PLoS ONE 5: e14091 doi:10.1371/journal.pone.0014091.
37. WaddingtonCH (1953) Genetic assimilation of an acquired character. Evolution 118–126.
38. West-Eberhard MJ (2003) Developmental plasticity and evolution. Oxford, UK: Oxford University Press. 816 p.
39. BraendleC, FlattT (2006) A role for genetic accommodation in evolution? Bioessays 28 : 868–873 doi:10.1002/bies.20456.
40. GibsonG, DworkinI (2004) Uncovering cryptic genetic variation. Nat Rev Genet 5 : 681–U11 doi:10.1038/nrg1426.
41. MarxCJ (2008) Development of a broad-host-range sacB-based vector for unmarked allelic exchange. BMC Res Notes 1 : 1 doi:10.1186/1756-0500-1-1.
42. ScottJW, RascheME (2002) Purification, overproduction, and partial characterization of beta-RFAP synthase, a key enzyme in the methanopterin biosynthesis pathway. J Bacteriol 184 : 4442–4448.
43. OkuboY, SkovranE, GuoX, SivamD, LidstromME (2007) Implementation of microarrays for Methylobacterium extorquens AM1. OMICS 11 : 325–340 doi:10.1089/omi.2007.0027.
44. SmythGK (2004) Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3: Article3 doi:10.2202/1544-6115.1027.
45. RitchieME, SilverJ, OshlackA, HolmesM, DiyagamaD, et al. (2007) A comparison of background correction methods for two-colour microarrays. Bioinformatics 23 : 2700–2707 doi:10.1093/bioinformatics/btm412.
46. GentlemanRC, CareyVJ, BatesDM, BolstadB, DettlingM, et al. (2004) Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5: R80 doi:10.1186/gb-2004-5-10-r80.
47. R Core Team (2012) R: A Language and Environment for Statistical Computing. Vienna, Austria.
48. ThompsonLR, ZengQ, KellyL, HuangKH, SingerAU, et al. (2011) Phage auxiliary metabolic genes and the redirection of cyanobacterial host carbon metabolism. P Natl Acad Sci Usa 108: E757–E764 doi:10.1073/pnas.1102164108/-/DCSupplemental.
Štítky
Genetika Reprodukční medicína
Článek The G4 GenomeČlánek Mondo/ChREBP-Mlx-Regulated Transcriptional Network Is Essential for Dietary Sugar Tolerance inČlánek RpoS Plays a Central Role in the SOS Induction by Sub-Lethal Aminoglycoside Concentrations inČlánek Tissue Homeostasis in the Wing Disc of : Immediate Response to Massive Damage during DevelopmentČlánek Disruption of TTDA Results in Complete Nucleotide Excision Repair Deficiency and Embryonic LethalityČlánek DJ-1 Decreases Neural Sensitivity to Stress by Negatively Regulating Daxx-Like Protein through dFOXO
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 4- Akutní intermitentní porfyrie
- Příjem alkoholu a menstruační cyklus
- Souvislost haplotypu M2 genu pro annexin A5 s opakovanými reprodukčními ztrátami
- Hysteroskopická resekce děložního septa zlepšuje šanci na graviditu žen s jinak nevysvětlenou infertilitou
- Transfer zmraženého embrya zlepšuje výsledky IVF
-
Všechny články tohoto čísla
- Epigenetic Upregulation of lncRNAs at 13q14.3 in Leukemia Is Linked to the Downregulation of a Gene Cluster That Targets NF-kB
- A Big Catch for Germ Cell Tumour Research
- The Quest for the Identification of Genetic Variants in Unexplained Cardiac Arrest and Idiopathic Ventricular Fibrillation
- A Nonsynonymous Polymorphism in as a Risk Factor for Human Unexplained Cardiac Arrest with Documented Ventricular Fibrillation
- The Hourglass and the Early Conservation Models—Co-Existing Patterns of Developmental Constraints in Vertebrates
- Smaug/SAMD4A Restores Translational Activity of CUGBP1 and Suppresses CUG-Induced Myopathy
- Balancing Selection on a Regulatory Region Exhibiting Ancient Variation That Predates Human–Neandertal Divergence
- The G4 Genome
- Extensive Natural Epigenetic Variation at a Originated Gene
- Mouse Oocyte Methylomes at Base Resolution Reveal Genome-Wide Accumulation of Non-CpG Methylation and Role of DNA Methyltransferases
- The Environment Affects Epistatic Interactions to Alter the Topology of an Empirical Fitness Landscape
- TIP48/Reptin and H2A.Z Requirement for Initiating Chromatin Remodeling in Estrogen-Activated Transcription
- Aconitase Causes Iron Toxicity in Mutants
- Tbx2 Terminates Shh/Fgf Signaling in the Developing Mouse Limb Bud by Direct Repression of
- Mondo/ChREBP-Mlx-Regulated Transcriptional Network Is Essential for Dietary Sugar Tolerance in
- Sex-Differential Selection and the Evolution of X Inactivation Strategies
- Identification of a Tissue-Selective Heat Shock Response Regulatory Network
- Phosphorylation-Coupled Proteolysis of the Transcription Factor MYC2 Is Important for Jasmonate-Signaled Plant Immunity
- RpoS Plays a Central Role in the SOS Induction by Sub-Lethal Aminoglycoside Concentrations in
- Six Homeoproteins Directly Activate Expression in the Gene Regulatory Networks That Control Early Myogenesis
- Rtt109 Prevents Hyper-Amplification of Ribosomal RNA Genes through Histone Modification in Budding Yeast
- ATP-Dependent Chromatin Remodeling by Cockayne Syndrome Protein B and NAP1-Like Histone Chaperones Is Required for Efficient Transcription-Coupled DNA Repair
- Iron-Responsive miR-485-3p Regulates Cellular Iron Homeostasis by Targeting Ferroportin
- Mutations in Predispose Zebrafish and Humans to Seminomas
- Cytotoxic Chromosomal Targeting by CRISPR/Cas Systems Can Reshape Bacterial Genomes and Expel or Remodel Pathogenicity Islands
- Tissue Homeostasis in the Wing Disc of : Immediate Response to Massive Damage during Development
- All SNPs Are Not Created Equal: Genome-Wide Association Studies Reveal a Consistent Pattern of Enrichment among Functionally Annotated SNPs
- Functional 358Ala Allele Impairs Classical IL-6 Receptor Signaling and Influences Risk of Diverse Inflammatory Diseases
- The Tissue-Specific RNA Binding Protein T-STAR Controls Regional Splicing Patterns of Pre-mRNAs in the Brain
- Neutral Genomic Microevolution of a Recently Emerged Pathogen, Serovar Agona
- Genetic Requirements for Signaling from an Autoactive Plant NB-LRR Intracellular Innate Immune Receptor
- SNF5 Is an Essential Executor of Epigenetic Regulation during Differentiation
- Dialects of the DNA Uptake Sequence in
- Reference-Free Population Genomics from Next-Generation Transcriptome Data and the Vertebrate–Invertebrate Gap
- Senataxin Plays an Essential Role with DNA Damage Response Proteins in Meiotic Recombination and Gene Silencing
- High-Resolution Mapping of Spontaneous Mitotic Recombination Hotspots on the 1.1 Mb Arm of Yeast Chromosome IV
- Rod Monochromacy and the Coevolution of Cetacean Retinal Opsins
- Evolution after Introduction of a Novel Metabolic Pathway Consistently Leads to Restoration of Wild-Type Physiology
- Disruption of TTDA Results in Complete Nucleotide Excision Repair Deficiency and Embryonic Lethality
- Insulators Target Active Genes to Transcription Factories and Polycomb-Repressed Genes to Polycomb Bodies
- Signatures of Diversifying Selection in European Pig Breeds
- The Chromosomal Passenger Protein Birc5b Organizes Microfilaments and Germ Plasm in the Zebrafish Embryo
- The Histone Demethylase Jarid1b Ensures Faithful Mouse Development by Protecting Developmental Genes from Aberrant H3K4me3
- Regulates Synaptic Development and Endocytosis by Suppressing Filamentous Actin Assembly
- Sensory Neuron-Derived Eph Regulates Glomerular Arbors and Modulatory Function of a Central Serotonergic Neuron
- Analysis of Rare, Exonic Variation amongst Subjects with Autism Spectrum Disorders and Population Controls
- Scavenger Receptors Mediate the Role of SUMO and Ftz-f1 in Steroidogenesis
- DNA Double-Strand Breaks Coupled with PARP1 and HNRNPA2B1 Binding Sites Flank Coordinately Expressed Domains in Human Chromosomes
- High-Resolution Mapping of H1 Linker Histone Variants in Embryonic Stem Cells
- Comparative Genomics of and the Bacterial Species Concept
- Genetic and Biochemical Assays Reveal a Key Role for Replication Restart Proteins in Group II Intron Retrohoming
- Genome-Wide Association Studies Identify Two Novel Mutations Responsible for an Atypical Hyperprolificacy Phenotype in Sheep
- The Genetic Correlation between Height and IQ: Shared Genes or Assortative Mating?
- Comprehensive Assignment of Roles for Typhimurium Genes in Intestinal Colonization of Food-Producing Animals
- An Essential Role for Zygotic Expression in the Pre-Cellular Drosophila Embryo
- The Genome Organization of Reflects Its Lifestyle
- Coordinated Cell Type–Specific Epigenetic Remodeling in Prefrontal Cortex Begins before Birth and Continues into Early Adulthood
- Improved Detection of Common Variants Associated with Schizophrenia and Bipolar Disorder Using Pleiotropy-Informed Conditional False Discovery Rate
- Site-Specific Phosphorylation of the DNA Damage Response Mediator Rad9 by Cyclin-Dependent Kinases Regulates Activation of Checkpoint Kinase 1
- Npc1 Acting in Neurons and Glia Is Essential for the Formation and Maintenance of CNS Myelin
- Identification of , a Retrotransposon-Derived Imprinted Gene, as a Novel Driver of Hepatocarcinogenesis
- Aag DNA Glycosylase Promotes Alkylation-Induced Tissue Damage Mediated by Parp1
- DJ-1 Decreases Neural Sensitivity to Stress by Negatively Regulating Daxx-Like Protein through dFOXO
- Asynchronous Replication, Mono-Allelic Expression, and Long Range -Effects of
- Differential Association of the Conserved SUMO Ligase Zip3 with Meiotic Double-Strand Break Sites Reveals Regional Variations in the Outcome of Meiotic Recombination
- Focusing In on the Complex Genetics of Myopia
- Continent-Wide Decoupling of Y-Chromosomal Genetic Variation from Language and Geography in Native South Americans
- Breakpoint Analysis of Transcriptional and Genomic Profiles Uncovers Novel Gene Fusions Spanning Multiple Human Cancer Types
- Intrinsic Epigenetic Regulation of the D4Z4 Macrosatellite Repeat in a Transgenic Mouse Model for FSHD
- Bisphenol A Exposure Disrupts Genomic Imprinting in the Mouse
- Genetic and Genomic Architecture of the Evolution of Resistance to Antifungal Drug Combinations
- Transposable Elements Are Major Contributors to the Origin, Diversification, and Regulation of Vertebrate Long Noncoding RNAs
- Functional Dissection of the Condensin Subunit Cap-G Reveals Its Exclusive Association with Condensin I
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The G4 Genome
- Neutral Genomic Microevolution of a Recently Emerged Pathogen, Serovar Agona
- The Histone Demethylase Jarid1b Ensures Faithful Mouse Development by Protecting Developmental Genes from Aberrant H3K4me3
- The Tissue-Specific RNA Binding Protein T-STAR Controls Regional Splicing Patterns of Pre-mRNAs in the Brain
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání




