-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Genetic and Biochemical Assays Reveal a Key Role for Replication Restart Proteins in Group II Intron Retrohoming
Mobile group II introns retrohome by an RNP-based mechanism in which the intron RNA reverse splices into a DNA site and is reverse transcribed by the associated intron-encoded protein. The resulting intron cDNA is then integrated into the genome by cellular mechanisms that have remained unclear. Here, we used an Escherichia coli genetic screen and Taqman qPCR assay that mitigate indirect effects to identify host factors that function in retrohoming. We then analyzed mutants identified in these and previous genetic screens by using a new biochemical assay that combines group II intron RNPs with cellular extracts to reconstitute the complete retrohoming reaction in vitro. The genetic and biochemical analyses indicate a retrohoming pathway involving degradation of the intron RNA template by a host RNase H and second-strand DNA synthesis by the host replicative DNA polymerase. Our results reveal ATP-dependent steps in both cDNA and second-strand synthesis and a surprising role for replication restart proteins in initiating second-strand synthesis in the absence of DNA replication. We also find an unsuspected requirement for host factors in initiating reverse transcription and a new RNA degradation pathway that suppresses retrohoming. Key features of the retrohoming mechanism may be used by human LINEs and other non-LTR-retrotransposons, which are related evolutionarily to mobile group II introns. Our findings highlight a new role for replication restart proteins, which function not only to repair DNA damage caused by mobile element insertion, but have also been co-opted to become an integral part of the group II intron retrohoming mechanism.
Published in the journal: . PLoS Genet 9(4): e32767. doi:10.1371/journal.pgen.1003469
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003469Summary
Mobile group II introns retrohome by an RNP-based mechanism in which the intron RNA reverse splices into a DNA site and is reverse transcribed by the associated intron-encoded protein. The resulting intron cDNA is then integrated into the genome by cellular mechanisms that have remained unclear. Here, we used an Escherichia coli genetic screen and Taqman qPCR assay that mitigate indirect effects to identify host factors that function in retrohoming. We then analyzed mutants identified in these and previous genetic screens by using a new biochemical assay that combines group II intron RNPs with cellular extracts to reconstitute the complete retrohoming reaction in vitro. The genetic and biochemical analyses indicate a retrohoming pathway involving degradation of the intron RNA template by a host RNase H and second-strand DNA synthesis by the host replicative DNA polymerase. Our results reveal ATP-dependent steps in both cDNA and second-strand synthesis and a surprising role for replication restart proteins in initiating second-strand synthesis in the absence of DNA replication. We also find an unsuspected requirement for host factors in initiating reverse transcription and a new RNA degradation pathway that suppresses retrohoming. Key features of the retrohoming mechanism may be used by human LINEs and other non-LTR-retrotransposons, which are related evolutionarily to mobile group II introns. Our findings highlight a new role for replication restart proteins, which function not only to repair DNA damage caused by mobile element insertion, but have also been co-opted to become an integral part of the group II intron retrohoming mechanism.
Introduction
Mobile group II introns are non-long-terminal-repeat (non-LTR) retroelements that are commonly found in prokaryotes and in organellar genomes of eukaryotes and are thought to be evolutionary ancestors of splicesomal introns and retrotransposons in higher organisms [1]. They consist of an autocatalytic intron RNA (“ribozyme”) and an intron-encoded protein (IEP), which has reverse transcriptase (RT) activity. These two components function together in a ribonucleoprotein complex (RNP) to promote intron mobility by a mechanism in which the excised intron lariat RNA uses its ribozyme activity to reverse splice directly into a DNA site and is then reverse transcribed by the IEP, yielding an intron cDNA that is integrated into the genome by host enzymes [2]–[5]. By using this mechanism, group II introns insert at high frequency into specific DNA target sites in a process called “retrohoming” and at low frequency into ectopic sites that resemble the normal homing site in a process called “retrotransposition” or “ectopic retrohoming” [6]. These processes enabled the dispersal of group II introns to a wide variety of bacteria and some archaea and likely into eukaryotic nuclear genomes, where ancestral group II introns are thought to have evolved into both spliceosomal introns and non-LTR-retrotransposons [3], [7], [8].
Although the early reverse splicing and reverse transcription steps catalyzed by group II intron RNPs are common to retrohoming pathways in all organisms, the late host-mediated steps of second-strand DNA synthesis and cDNA integration can occur in different ways. In Saccharomyces cerevisiae mitochondria, where retrohoming was studied initially, cDNA integration occurs largely by a recombination mechanism in which the nascent intron cDNA initiated at the recipient allele invades an intron-containing allele for completion of intron DNA synthesis before switching back to the recipient DNA in the upstream exon [9], [10]. In bacteria, however, the fully reverse spliced intron RNA is reverse transcribed to yield a full-length intron cDNA that is integrated directly into the recipient DNA by a RecA-independent mechanism hypothesized to involve host DNA repair enzymes [5], [11], [12]. Recently, non-lariat, linear forms of the Lactococcus lactis Ll.LtrB intron RNA were found to retrohome in Drosophila melanogaster by using host non-homologous end-joining enzymes for cDNA integration [13]. However, host factors that function in late steps in the retrohoming of group II intron lariat RNAs, the major retrohoming pathway used in nature, have not been identified conclusively in any organism, and consequently, the mechanisms used for these steps have remained poorly understood.
Figure 1 diagrams the major steps elucidated thus far in the retrohoming pathway of the L. lactis Ll.LtrB intron, which has been studied extensively as a model system for group II intron lariat RNA retrohoming in bacteria. The Ll.LtrB intron was discovered in a relaxase gene (ltrB) in a conjugative element, where its splicing is required to produce functional relaxase for conjugation [14], [15]. Its IEP, denoted LtrA protein, is multifunctional, with RT, RNA splicing (“maturase”), DNA binding, and DNA endonuclease activities [16], [17]. Transcription of the ltrB gene yields a precursor RNA, which contains the intron flanked by the 5′ and 3′ ltrB exons (E1 and E2, respectively). The IEP, which is translated from within the intron, binds to the intron in the unspliced precursor RNA and promotes its splicing by stabilizing the catalytically active RNA structure [18]. Splicing occurs via two sequential RNA-catalyzed transesterification reactions that yield ligated ltrB exons and an excised intron lariat RNA to which the IEP remains tightly bound in an RNP. RNPs then initiate retrohoming by recognizing a DNA target sequence (corresponding to the ligated ltrB E1–E2 DNA sequence), using both the IEP and base pairing of the intron RNA [19]–[21]. After DNA target site recognition, the intron RNA fully reverse splices into the top strand of the DNA, leading to insertion of the intron RNA between the two DNA exons, while the IEP cleaves the bottom strand 9 nts downstream of the intron-insertion site and uses the 3′ end of the cleaved DNA strand for target DNA-primed reverse transcription (TPRT) of the inserted intron RNA [16], [17]. Finally, the resulting intron cDNA is integrated into the recipient DNA by host factors in late steps that minimally include the degradation or displacement of the intron RNA template strand, second (top)-strand DNA synthesis, resection of DNA overhangs, and ligation to seal DNA nicks [12].
Fig. 1. Retrohoming pathway of Ll.LtrB intron lariat RNA in bacteria. 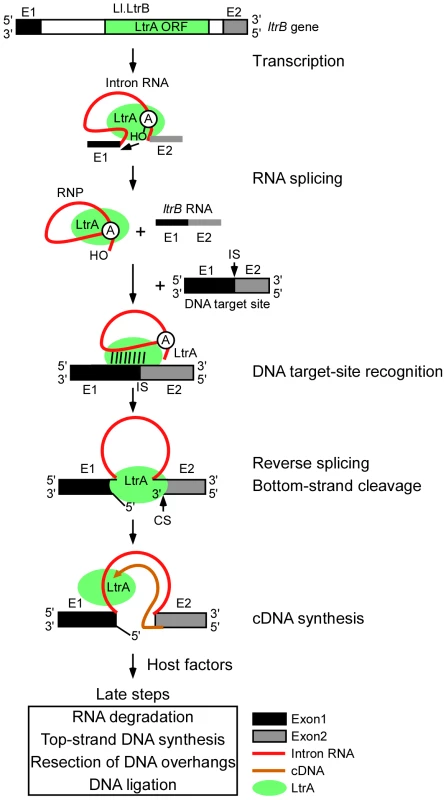
The Ll.LtrB intron, found in a relaxase gene (ltrB) in an L. lactis conjugative element, encodes a multi-functional RT (LtrA protein) with RT, RNA splicing, DNA-binding, and DNA endonuclease activities. Transcription of the ltrB gene yields a precursor RNA containing the intron flanked by 5′ and 3′ exons (E1 and E2, respectively). LtrA is translated from within the intron using its own Shine-Dalgarno sequence and then binds to the intron in the precursor RNA to promote formation of the catalytically active RNA structure for RNA splicing. RNA splicing occurs via two sequential RNA-catalyzed transesterification reactions that are initiated by nucleophilic attack of the 2′ OH of a branch point A-residue near the 3′ end of the intron at the 5′-splice site and results in ligated ltrB exons and an excised intron lariat RNA with a 2′-5′ phosphodiester linkage. After splicing, LtrA remains tightly bound to the excised intron lariat RNA in an RNP. RNPs initiate retrohoming by recognizing a DNA target site (the ligated ltrB E1–E2 sequence), using both the IEP and base pairing of the intron RNA. The intron RNA then inserts via reversal of the two transesterification reactions used for RNA splicing (referred to as “full reverse splicing”) into the intron-insertion site (IS) at the ligated-exon junction in the top strand of the DNA target site. LtrA uses its DNA endonuclease activity to cleave the bottom strand at a site (CS) between positions +9 and +10 of E2 and uses the 3′ DNA end at the cleavage site as a primer for reverse transcription of the inserted intron RNA. The resulting intron cDNA is then integrated into the genome by host enzymes in late steps that minimally include degradation of the intron RNA template strand, second (top)-strand DNA synthesis, resection of DNA overhangs, and sealing of DNA strand nicks [12]. In addition to its native host, the Ll.LtrB intron splices and retrohomes efficiently in a wide variety of other bacteria, including Escherichia coli, where it has been studied by using the facile genetic and biochemical methods available for that organism [5]. By screening E. coli mutants using two different plasmid-based retrohoming assays, we in collaboration with the Belfort laboratory previously identified candidate host factors that potentially function in the late steps in retrohoming, including RNase H1 and the 5′→3′ exonuclease activity of Pol I, both of which could contribute to degrading the intron RNA template strand; the host replicative polymerase Pol III, which may function in second-strand DNA synthesis; and DNA ligase A, which presumably seals strand nicks [12]. Decreased retrohoming frequencies were also found in mutants deficient in host exo - and endonucleases activities [RecJ, DnaQ (MutD), and SbcD], which could function to resect overhangs or resolve intermediates, and increased retrohoming frequencies were found for mutants deficient in RNases I and E and exonuclease III, which in wild-type strains may suppress retrohoming by degrading the intron RNA or nascent cDNA [12].
More recently, Coros et al. [22], [23] extended this work by screening an E. coli transposon-insertion library for mutants defective in group II intron retrohoming into chromosomal sites, using a donor plasmid to express an Ll.LtrB-ΔORF intron carrying a kanR marker. This screen identified additional host factors potentially involved in retrohoming, including polynucleotide phosphorylase (PNPase), the DNA helicase Rep, and MnmE (TrmE), which functions in tRNA modification, with additional host proteins (CyaA, SpoT, and AtpA) acting by affecting accessibility of chromosomal target sites or energy metabolism, and RNase E acting to impede retrohoming by degrading the intron RNA.
Although the genetic screens described above identified host genes in which mutations decrease retrohoming efficiency, it remains possible that some or many of these mutations affect retrohoming indirectly. Such indirect effects could result from mutations that impair the propagation or expression of the intron-donor plasmid, decrease the intracellular levels or activity of group II intron RNPs, or impede the accessibility of group II intron RNPs to DNA target sites. Additionally, all previous genetic screens relied upon the expression of an antibiotic-resistance marker to identify retrohoming events and are thus vulnerable to false positives arising from mutations that affect the expression of antibiotic-resistance (e.g., by affecting the expression of the antibiotic-resistance gene or cellular permeability to the antibiotic). In some cases, studies of “indirect” effects revealed by the genetic screens have provided rich information about host responses and the regulation of group II intron mobility [22], [23]. However, further insight into the retrohoming mechanism requires the identification of host factors that function directly in this process.
Here we used an E. coli genetic screen and Taqman qPCR assay that mitigate indirect effects to identify candidate host factors for Ll.LtrB retrohoming, and we tested their function in retrohoming by using a newly developed biochemical assay that combines group II intron RNPs with cellular extracts to reconstitute the complete retrohoming reaction in vitro. By using these multiple approaches, we confirmed some previously identified host factors but not others. Additionally, we found that replication restart enzymes play a key role in intron retrohoming by initiating second-strand DNA synthesis. Our findings indicate a novel mechanism for the major pathway of group II intron lariat retrohoming in bacteria, with features that may be shared by human LINE elements and other non-LTR retrotransposons.
Results
Genetic and Taqman qPCR screens to identify host proteins that function in retrohoming
To identify host factors that function in retrohoming of the Ll.LtrB group II intron, we used two complementary approaches that mitigate weaknesses of previous genetic screens. First, we used a plasmid-based group II intron retrohoming assay that controls for indirect effects to screen an E. coli mariner transposon-insertion library for mutants that have decreased or increased retrohoming efficiency. This screen used the intron-donor plasmid pALG3 and recipient plasmid pBRR3-ltrB (Figure 2A) and was done in E. coli host strain HMS174(DE3), which is RecA− and encodes an isopropyl β-D-1 thiogalactopyranoside (IPTG)-inducible T7 RNA polymerase for donor-plasmid transcription. The donor plasmid pALG3, which was newly constructed for this screen, uses a T7lac promoter to synthesize a precursor RNA, which contains an Ll.LtrB-ΔORF intron (i.e., an intron deleted for the LtrA ORF) flanked by short 5′ and 3′ exons, with the 3′ exon linked in frame to an ORF encoding GFP. The LtrA protein, which functions stoichiometrically to splice and mobilize the Ll.LtrB intron, is expressed from a position downstream of the GFP ORF, where it is co-transcribed with the intron RNA and translated using its own Shine-Dalgarno sequence. Because the expression of the Ll.LtrB-ΔORF intron and its splicing to produce RNPs are linked to GFP expression, mutants with decreased group II intron RNP production as a result of defects in donor plasmid replication, LtrA protein expression, or the expression and splicing of the Ll.LtrB intron RNA, are identified readily by decreased GFP fluorescence after IPTG induction.
Fig. 2. Genetic and Taqman qPCR assays used to identify E. coli mutants deficient in retrohoming. 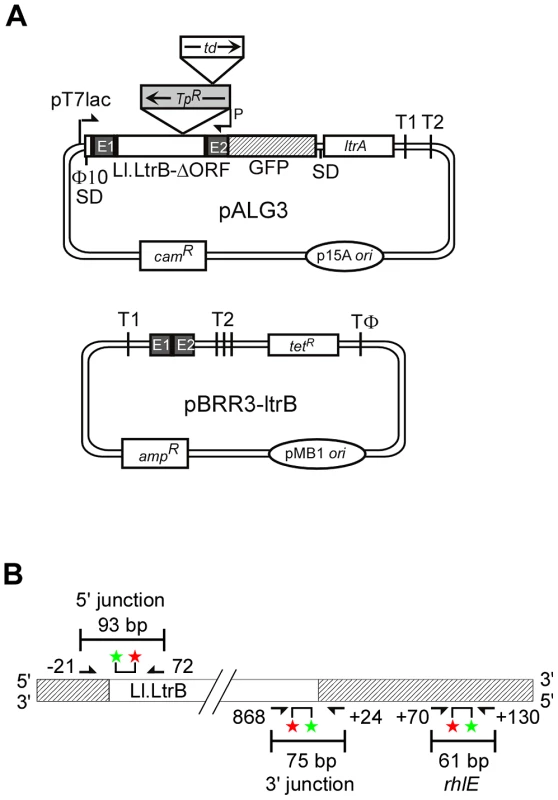
(A) Genetic assay. The CamR intron-donor plasmid pALG3 uses a T7lac promoter and phage Φ10 Shine-Dalgarno (SD) sequence to express an ltrB/GFP fusion cassette. This cassette consists of a 0.9-kb Ll.LtrB-ΔORF intron and flanking 5′- and 3′-exons (E1 and E2, respectively) [19], with the intron carrying a trimethoprim-resistance retrotransposition-activated genetic marker (TpR-RAM), and E2 linked in-frame to an ORF encoding GFP. The LtrA ORF preceded by its own Shine-Dalgarno sequence is co-transcribed from a position downstream of the GFP ORF. The AmpR recipient plasmid contains a 45-bp Ll.LtrB target site (ligated E1–E2 sequence) upstream of a promoterless tetR gene. T1 and T2 are E. coli rrnB transcription terminators, and TΦ is a phage T7 transcription terminator. (B) Taqman qPCR assay. The assay quantifies 5′- and 3′-intron integration junctions resulting from retrohoming of a retargeted Ll.LtrB-ΔORF intron into a site in the rhlE gene in the E. coli chromosome. Retrohoming events are quantified by Taqman qPCR, which utilizes the 5′→3′ exonuclease activity of Taq DNA polymerase to cleave a fluorescently labeled DNA probe that base pairs to an internal region of a PCR amplicon. Digestion of the probe by Taq DNA polymerase releases the FAM label (red star) free of the MGB quencher (green star), resulting in a quantifiable fluorescence signal for each amplification event. The numbers of 5′- and 3′-intron integration junctions relative to the number of rhlE targets were determined by quantifying the fluorescence signals in three separate PCRs relative to standard curves generated from serial dilutions of a reference plasmid (Materials and Methods). Primers for these PCRs are depicted by arrows with numbers indicating the positions of the 5′ nucleotide of the upstream primer and 3′ nucleotide of the downstream primer relative to the intron-integration junction. To enable direct selection for retrohoming events, the Ll.LtrB intron in pALG3 carries a trimethoprim-resistance retrotransposition-activated genetic marker (TpR-RAM), which consists of a small trimethoprim-resistance gene inserted in the orientation opposite group II intron transcription, but interrupted by an efficiently self-splicing group I intron, the phage T4 td intron, in the forward orientation [24]. During retrohoming via an RNA intermediate, the td intron is spliced, thereby reconstituting the TpR marker and enabling its expression after the intron retrohomes into the DNA target site. By using this combination of TpR-RAM and GFP markers, mutants with transposon insertions that inhibit retrohoming without affecting Ll.LtrB expression or splicing are identified as TpS and GFP+.
The screen was done in 96-well plates under calibrated selective conditions in which the cell density for log phase cells provides a measure of retrohoming efficiency (Figure S1; Materials and Methods). Screening of 9,200 colonies by two rounds of 96-well plate assays identified 61 transposon insertions that reproducibly gave a >4-fold decrease in retrohoming efficiency compared to a wild-type control and had no defect in RNP production, as judged by a fluorescence-activated cell sorter (FACS) assay of GFP synthesis (Table S1). After mapping of transposon-insertion sites by thermal-asymmetric-interlaced (TAIL) PCR [25], we identified 67 candidate protein-encoding genes, whose disruption or altered expression due to the proximity of the transposon insertion results in decreased retrohoming efficiency. Six of these candidate genes were sites of multiple transposon insertions, and 12 were genes with nucleic acid-related functions found downstream of transposon-insertion sites within operons. An additional transposon insertion (C0719) that decreased retrohoming efficiency mapped to a site predicted to encode a small non-coding RNA (sRNA) (Table S1). All the candidates were confirmed by Southern hybridization to contain a single mariner-transposon insertion at the indicated genomic location (data not shown).
To complement the transposon-insertion screen, our second approach was to screen individual candidate strains for efficient integration into a chromosomal target site directly by using a Taqman qPCR assay to quantify both the 5′ - and 3′-intron-integration junctions, thereby eliminating false positives that arise from mutations affecting expression of a drug-resistance phenotype (Figure 2B). Mobile group II introns can be retargeted to retrohome into different chromosomal DNA sites simply by modifying the intron RNA sequences that base pair to the DNA target sequence (see Introduction), a gene targeting technology known as “targetron” [19], [21], [26]. The Taqman qPCR assay uses an Ll.LtrB-ΔORF intron that was retargeted in this way to retrohome efficiently into a site in the rhlE gene, which encodes a non-essential DEAD-box protein whose disruption has no effect on cellular growth rate [21], [27]. The intron was expressed from the broad-host range donor plasmid pBL1, which has a different DNA replication origin than pALG3 and employs an m-toluic acid-inducible promoter; the latter functions independently of host factors and is activated by a freely permeable inducer (m-toluic acid) that does not require cellular transporters to enter the cell [28]. Additionally, the screen was carried out in mutant strains from the Keio collection in which deleted genes are replaced with a kanR marker, thereby mitigating polarity effects on downstream genes in operons [29]. The Keio strains were supplemented by temperature-sensitive mutants to test the contribution of essential genes.
We used the Taqman qPCR assay to test all 68 candidate host factors identified in our initial transposon-insertion screen, as well as 30 additional candidate proteins that act on nucleic acids, including all 21 such candidates identified in previous mutant screens [12], [22], [30]. Table 1 shows results of the Taqman qPCR assay for notable mutants, and Tables S1 and Table S2 show complete results for the mariner-transposon screen and Taqman qPCR assay, respectively. Among the 68 candidates identified in the initial transposon library screen (Table S1), only ten (dnaC, dnaT, gyrB, mdoB, paoD, rpoH, rpoN, tonB, ydcM, and yjjB) had statistically significant decreases in retrohoming efficiency in the Taqman qPCR assay, and only four (dnaC, dnaT, gyrB, and rpoH) had substantial decreases (10–67% of wild type retrohoming efficiency; Table 1 and Table S2). This poor correlation highlights the difficulty of distinguishing direct and indirect effects and the necessity of using multiple approaches to identify host factors that function in retrohoming.
Tab. 1. Taqman qPCR assays of retrohoming in notable E. coli mutants. 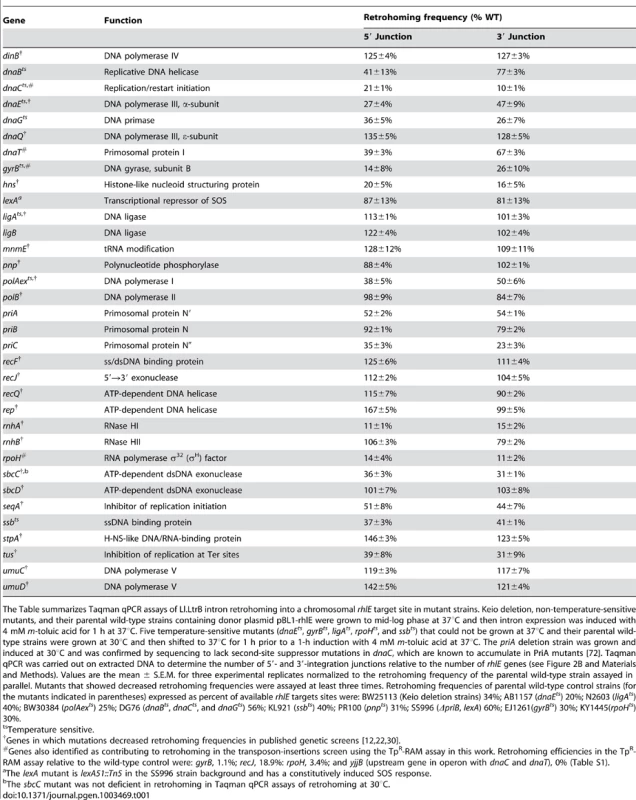
The Table summarizes Taqman qPCR assays of Ll.LtrB intron retrohoming into a chromosomal rhlE target site in mutant strains. Keio deletion, non-temperature-sensitive mutants, and their parental wild-type strains containing donor plasmid pBL1-rhlE were grown to mid-log phase at 37°C and then intron expression was induced with 4 mM m-toluic acid for 1 h at 37°C. Five temperature-sensitive mutants (dnaEts, gyrBts, ligAts, rpoHts, and ssbts) that could not be grown at 37°C and their parental wild-type strains were grown at 30°C and then shifted to 37°C for 1 h prior to a 1-h induction with 4 mM m-toluic acid at 37°C. The priA deletion strain was grown and induced at 30°C and was confirmed by sequencing to lack second-site suppressor mutations in dnaC, which are known to accumulate in PriA mutants [72]. Taqman qPCR was carried out on extracted DNA to determine the number of 5′- and 3′-integration junctions relative to the number of rhlE genes (see Figure 2B and Materials and Methods). Values are the mean ± S.E.M. for three experimental replicates normalized to the retrohoming frequency of the parental wild-type strain assayed in parallel. Mutants that showed decreased retrohoming frequencies were assayed at least three times. Retrohoming frequencies of parental wild-type control strains (for the mutants indicated in parentheses) expressed as percent of available rhlE targets sites were: BW25113 (Keio deletion strains) 34%; AB1157 (dnaEts) 20%; N2603 (ligAts) 40%; BW30384 (polAexts) 25%; DG76 (dnaBts, dnaCts, and dnaGts) 56%; KL921 (ssbts) 40%; PR100 (pnpts) 31%; SS996 (ΔpriB, lexA) 60%; EJ1261(gyrBts) 30%; KY1445(rpoHts) 30%. Candidate host factors that function in retrohoming
Among the candidates identified as potential retrohoming factors in previous screens [12], [22], [30], the Taqman qPCR assay confirmed significant reductions in retrohoming efficiency in the Keio deletions of rnhA (RNase H1, the major cellular RNase H [31]); seqA (initiation of chromosomal DNA replication); sbcC (ATP-dependent exonuclease); hns (histone-like nucleoid structuring protein); and tus (DNA replication termination site-binding protein); as well as at restrictive temperatures in the temperature-sensitive mutants polAexts, which is defective in the 5′→3′ exonuclease but not the DNA polymerase activity of Pol I [32]; and dnaEts in the catalytic (α) subunit of the host replicative DNA polymerase Pol III [33]. Also in agreement with previous results, we found no strong decrease in retrohoming efficiency in a Keio deletion of rnhB (RNase H2 [34]).
In contrast to results of genetic assays, the Taqman qPCR assays found no decrease in retrohoming efficiency for Keio deletions of recJ (single-stranded DNA exonuclease [35]); dnaQ (Pol III ε subunit, which has the proofreading exonuclease activity [36]); rep and recQ (DNA helicases [37], [38]); recF (RecA-dependent recombination); stpA (H-NS-like DNA - and RNA-binding protein with RNA chaperone activity [39]); mnmE (trmE; tRNA modification); ligA and ligB (DNA ligases [40], [41]); and pnp (polynucleotide phosphorylase [42]). Additionally, the Taqman qPCR assay found no decrease in retrohoming efficiency for Keio deletions of the genes encoding DNA repair polymerases polB (Pol II [43]), dinB (Pol IV [44]), and umuC or D (Pol V [45]), whereas polB and dinB deletions in a different strain showed moderate decreases in previous genetic assays [12].
The new candidates that were identified in the transposon-insertion screen (Table S1) and confirmed to have substantial decreases in retrohoming efficiency in the Taqman qPCR assay (10–67% wild type; Table 1) were: DnaC and DnaT, which function in replication restart (identified as genes downstream of the transposon insertion in the yjjB operon [46], [47]); GyrB (DNA gyrase subunit B); and RpoH (RNA polymerase σ32 factor).
For several mutants in which inhibition of retrohoming was found in genetic assays but not in the Taqman qPCR assay, we subsequently found significant effects on top - or bottom-strand DNA synthesis in biochemical assays below (e.g., dinB, dnaQ, ligA, recJ, pnp, polB, and stpA). The disagreement between the genetic and Taqman qPCR assays for these mutants may reflect: (i) that qPCR monitors only short DNA regions at the intron-integration junctions; (ii) the longer time of the Taqman qPCR assay, which may give alternative enzymes a greater chance to act; or (iii) the different genetic backgrounds of the strains used in the two assays. The results again emphasize the need to use multiple assays to identify retrohoming factors, with biochemical support for a genetic assay in our view providing the most definitive identification.
Mutants deficient in group II intron RNP synthesis
The transposon-library screen in E. coli HMS174(DE3) identified eight retrohoming-deficient mutants that are TpS/GFP−, potentially indicating a defect in the production of Ll.LtrB RNPs (Table S3). Although we hoped that such mutants would identify host factors required for Ll.LtrB intron splicing, all eight of these mutants have transposon insertions that likely affect donor plasmid transcription (four in the lacUV5 promoter of the λDE3 prophage, one in the T7 RNA polymerase gene, and three in genes encoding membrane transporters that could affect IPTG induction or trimethoprim uptake: ugpA (glycerol-3-phosphate uptake transporter subunit); xylF (xylose transporter subunit); and yjbB (a putative transporter). Consistent with an effect on transcription, all eight mutants showed decreased GFP fluorescence when counter-screened with the control plasmid pALE, which lacks the Ll.LtrB intron and contains the ligated ltrB exons fused directly to GFP (Figure S2, Figure S3, and Table S3). A direct screen of the transposon library for splicing-defective mutants using plasmid pALG2, in which an Ll.LtrB-ΔORF intron lacking the TpR-RAM marker is linked to GFP expression [48], also identified only mutants that are defective in GFP expression with both the reporter construct containing the intron and the control reporter construct lacking the intron [T7 RNA polymerase of the λDE3 prophage; malE (maltose ABC transporter subunit); kdsD (arabinose-5-phosphate isomerase); dppD (dipeptide ABC transporter subunit); clcA (H+/Cl− exchange transporter); yfeN (conserved outer membrane protein); pgi (phosphoglucose isomerase; and yjbE (predicted protein)] (Table S4). These findings suggest either that splicing of the Ll.LtrB intron in vivo requires only the LtrA protein as it does in vitro [17] or that host-encoded splicing factors are essential proteins that are not readily identified in a transposon screen.
Mutants with increased retrohoming efficiencies
The TpR-RAM screen also identified five transposon insertions that give increased retrohoming efficiencies and potentially encode host factors that function to suppress retrohoming. Surprisingly, all five of these transposon-insertions mapped to three closely linked genes, rnlA (RNase LS), yfjK (DExH/D-box protein), and yfjL (protein of unknown function), which are part of a cryptic prophage (CP4-57) in E. coli K12 (Figure S4, Table S5; [49], [50]). In addition to higher group II intron retrohoming efficiencies indicated by high levels of TpR, all five disruptants showed increased levels of GFP fluorescence both with the intron-donor plasmid pALG3, in which splicing of the Ll.LtrB-ΔORF intron is required for GFP expression, and with the control plasmid pALE, which has ligated ltrB exons fused directly to GFP (Figure S4). Given the identity of the affected genes, these findings suggest that the increased retrohoming efficiencies and GFP fluorescence result from decreased rates of RNA degradation, leading to elevated levels of group II intron RNPs and GFP mRNA. The degradosome, which functions in mRNA turnover in E. coli, is a multiprotein complex consisting of RNase E (an endoribonuclease), PNPase (an exoribonuclease), RhlB (a DEAD-box RNA helicase), and enolase [51]. The close linkage of the three genes potentially involved in intron RNA degradation in our screen suggests that they may function together in a previously unknown RNA degradation pathway, possibly a second degradosome.
Replication restart proteins play a role in group II intron retrohoming
The decreased retrohoming efficiency resulting from a transposon insertion in the yjjB operon containing dnaC and dnaT in the TpR-RAM screen (see above; Table S1) focused our attention on replication restart proteins as attractive candidates for playing a role in the late steps of retrohoming. We therefore carried out systematic Taqman qPCR assays of replication restart mutants and found significant reductions in retrohoming in Keio deletions of priA, priC, and dnaT, and in a temperature-sensitive mutant of dnaB (Table 1). PriA and PriC are key proteins that independently recognize stalled or collapsed replication forks in the three major E. coli replication restart pathways (denoted the PriA-PriB, PriA-PriC, and PriC-Rep pathways; [47], [52]), while DnaT interacts with PriA and PriC to load the replicative DNA helicase DnaB [53], [54]. We also found decreased retrohoming efficiencies in temperature-sensitive mutants of several essential genes that function in replication restart, including those encoding DnaC, which interacts with DnaB prior to loading [55]; DnaG, the DNA primase [56]; and the single-stranded DNA binding protein Ssb, which has been shown to promote the formation of the primosome at the chromosomal replication origin (oriC) and interacts with PriA to stimulate the loading of DnaB during replication restart [57], [58]. However, deletion of the genes encoding PriB, an auxiliary component of the PriA-dependent pathway, and Rep, which functions in conjunction with PriC [59], showed only small (1–21%) reductions in retrohoming efficiency.
We also carried out Taqman qPCR assays of retrohoming in a different set of replication restart mutants in the genetic background of E. coli SS996, a recA+ strain containing a GFP reporter for SOS induction. The results indicated that the decreased retrohoming efficiencies in the affected replication restart mutants are not allele specific and are larger than can be accounted for by the proportion of cells undergoing the SOS response (Figure S5; different alleles tested for all genes except dnaE and dnaB). Additionally, the mutant strain SS4610 (lexA51::Tn5), which has a lexA null allele and is constitutively induced for the SOS response in nearly 100% of cells [60], showed only minimally decreased retrohoming frequencies in the Taqman qPCR assay (81–87% wild type; Figure S5 and Table 1). Collectively, the above findings indicate that the decreased retrohoming efficiency in the replication restart mutants is not a secondary effect of cell cycle arrest during SOS induction and indicate a requirement for replication restart proteins in group II intron retrohoming.
An E. coli extract assay that reconstitutes retrohoming in vitro
To further test the function of individual host proteins, we developed a biochemical assay in which host factors function together with group II intron RNPs to reconstitute the complete retrohoming reaction in vitro. This assay uses an E. coli S12 extract similar to those used for in vitro transcription and translation [61], [62]. Figure 3 shows experiments in which 5′ top - or bottom-strand labeled, 73-bp DNA oligonucleotide substrates containing the Ll.LtrB target site were incubated with group II intron RNPs in the presence of the extract, dNTPs (dATP, dCTP, dGTP, and dTTP), ATP, and an ATP-regenerating system (phosphoenolpyruvate+pyruvate kinase) at 37°C. The products were then analyzed in a denaturing polyacrylamide gel before and after digestion with RNases A+H to degrade the reverse spliced intron RNA leaving only 5′-labeled DNA products.
Fig. 3. E. coli extract assay for bottom-strand (cDNA) and top-strand DNA synthesis. 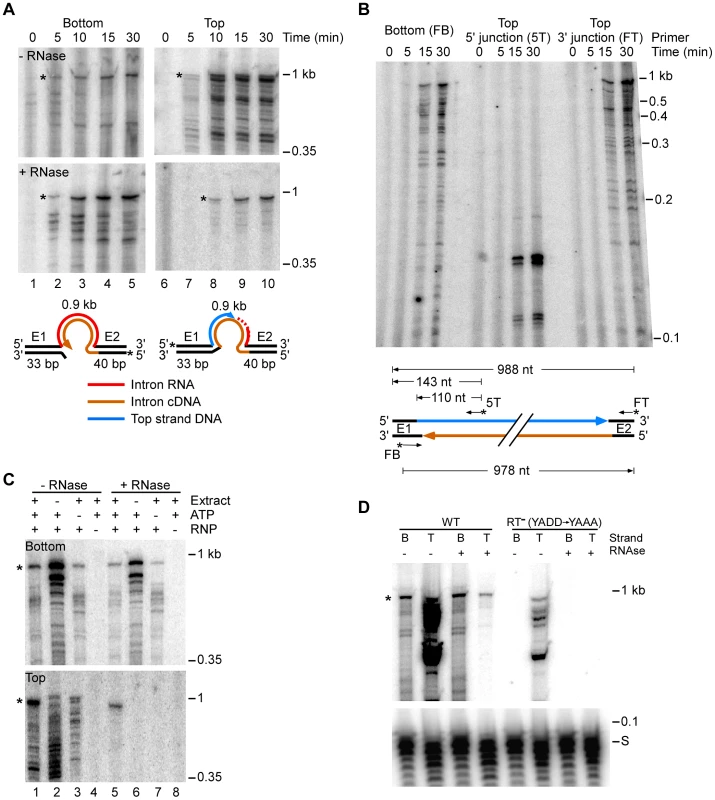
(A) Time courses. Group II intron RNPs and labeled DNA substrates (73 bp) containing the Ll.LtrB-insertion site (ligated E1–E2 sequence) were incubated with E. coli HMS174(DE3) extract in the presence of 1 mM dNTPs, 1.5 mM ATP, and an ATP-regenerating system (phosphoenolpyruvate+pyruvate kinase) at 37°C. The DNA substrates were labeled at the 5′ end of either the top (T) or bottom (B) strands to separately assay top- and bottom-strand DNA synthesis. After terminating portions of the reaction at the indicated times, samples were split into halves, which were incubated without or with RNases A+H, and the products were analyzed in a denaturing 6% polyacrylamide gel, which was dried and scanned with a PhosphorImager. RNase-sensitive top-strand products contain the reverse-spliced intron RNA. Schematics below the gels depict bottom- and top-strand synthesis on the DNA substrates (intron and exons not drawn to scale; star indicates 5′ 32P-label). (B) Primer extension analysis. DNA products synthesized in a time course were digested with RNase A+H, purified in a 1% agarose gel (0.85–1.2 kb gel slice), and analyzed by primer extension using 5′ -labeled primers to detect bottom-strand cDNAs (primer FB); the top-strand 5′-intron-integration junction (primer 5T); and top-strand DNAs (primer FT). Major products are diagrammed below the gel. (C) Requirements for bottom- and top-strand DNA synthesis. Reactions with the indicated components were incubated at 37°C for 30 min and then processed and analyzed in a denaturing 6% polyacrylamide gel, as described above. (D) Bottom- and top-strand products obtained with RNPs containing wild-type LtrA protein or an RT-deficient mutant LtrA (RT−; YADD motif changed to YAAA). For simplicity, the bottom part of the gel with the labeled DNA substrate (S) is shown only for panel D. Asterisks indicate gels bands of the size expected for full-length bottom- and top-strand products. Time courses with the 5′-labeled bottom-strand substrate, which monitors cDNA synthesis, showed that an RNase-resistant band of the size expected for full-length bottom strand (988 nt) appeared after 5 min and accumulated for up to 30 min, along with a series of smaller bands (Figure 3A). These smaller bands are likely incomplete cDNAs rather than degradation products, as controls showed that exogenous 32P-labeled ssDNA and dsDNA corresponding to the retrohoming products were not degraded when incubated in the extracts under the same conditions (Figure S6).
Time courses with the 5′-labeled top-strand substrate, which monitors second-strand DNA synthesis, showed that an RNase-resistant band of the size expected for full-length top strand appeared later (10 min) and continued to accumulate during the reaction (Figure 3A, bottom right panel). We confirmed by primer extension that both the 5′ - and 3′-junctions in the newly synthesized top strand DNA are continuous (Figure 3B).
Figure 3C shows that the appearance of labeled top - and bottom-strand products is dependent upon the addition of RNPs (lanes 4 and 8) and that top-strand DNA synthesis is completely dependent upon the presence of extract (lane 6). ATP increased the levels of reverse splicing and cDNA synthesis and was required for top-strand DNA synthesis in the extracts (cf., lane 1 and 5 with lanes 3 and 7), indicating that energy-dependent processes, perhaps involving DNA or RNA helicases, are involved at both stages of the reaction. Further, RNPs containing a mutant LtrA protein that lacks RT activity (RT−; YADD→YAAA) carried out reverse splicing, but showed no detectable cDNA synthesis, indicating that bottom-strand synthesis is dependent upon the group II intron RT activity and is not done by a host polymerase in the extracts (Figure 3D).
Biochemical analysis of retrohoming-deficient mutants
Finally, the E. coli extract assay enabled us to directly analyze bottom - and top-strand DNA synthesis in retrohoming-deficient mutants identified in genetic screens. In these experiments, extracts from Keio deletion or temperature-sensitive mutants were compared with those from their parental wild-type strains. In most cases, extracts were prepared from cells grown continuously at 37°C, which is a semi-permissive temperature for most of the temperature-sensitive mutants. The priA deletion strain was grown at 30°C to avoid accumulation of suppressor mutations, and five temperature-sensitive mutants (dnaEts, gyrBts, ligAts, rpoHts, and ssbts) that could not grow at 37°C along with their parental wild-type strains were grown at 30°C and then shifted to 37°C for 2 h before preparing the extracts. For all strains, the extract assays were done at 37°C for relatively short times (15 min) to remain within the linear range and minimize dephosphorylation of the labeled DNA substrate for several mutants whose extracts appear to have elevated phosphatase activity (DnaQ, DnaT, PriA, and DnaB). Table 2 and Table S6 summarize quantitation of the assays, and representative assays for notable mutants are shown in Figure 4 and for the remaining mutants in Figure S7. All values shown in Table 2 and Table S6 were reproducible to within <30% in replicate experiments.
Fig. 4. Assays of top- and bottom-strand DNA synthesis with extracts from E. coli mutants. 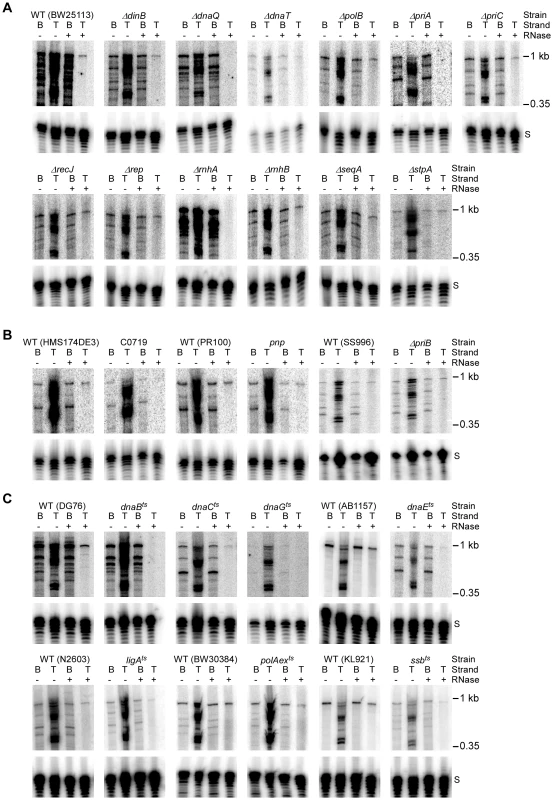
DNA substrate labeled with 32P at the 5′-end of the top (T) or bottom (B) strand were incubated with group II intron RNPs for 15 min at 37°C in reaction medium containing extracts from: (A) Keio deletion mutants and their parental wild-type strain BW25113. (B) Mutant strain C0719, which contains a mariner-transposon at the site of a predicted sRNA, a pnp mutant, a priB deletion (non-Keio), and their parental wild-type strains; and (C) temperature-sensitive mutants and their parental wild-type strains. After phenol-CIA extraction and proteinase K digestion, samples were split into halves, which were incubated without or with RNase A+H at 37°C for 30 min. The products were analyzed in a denaturing 6% polyacrylamide gel, which was dried and scanned with a PhosphorImager. Extracts were confirmed to contain equal amounts of protein by SDS-polyacrylamide gels stained with Coomassie blue (not shown). The amount of radiolabel in the indicated product band or bands was normalized for the amount of substrate (S) in each lane and expressed as a percent of that in the parental wild-type strain (Table 2). At least two assays were done for each mutant and were reproducible to within <30%. Tab. 2. E. coli extract assays of retrohoming in wild-type and mutant strains. 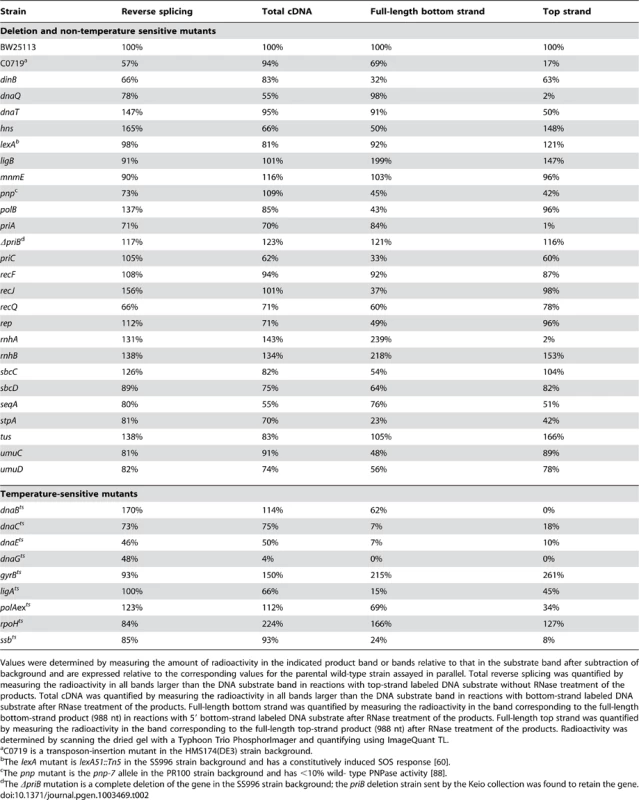
Values were determined by measuring the amount of radioactivity in the indicated product band or bands relative to that in the substrate band after subtraction of background and are expressed relative to the corresponding values for the parental wild-type strain assayed in parallel. Total reverse splicing was quantified by measuring the radioactivity in all bands larger than the DNA substrate band in reactions with top-strand labeled DNA substrate without RNase treatment of the products. Total cDNA was quantified by measuring the radioactivity in all bands larger than the DNA substrate band in reactions with bottom-strand labeled DNA substrate after RNase treatment of the products. Full-length bottom strand was quantified by measuring the radioactivity in the band corresponding to the full-length bottom-strand product (988 nt) in reactions with 5′ bottom-strand labeled DNA substrate after RNase treatment of the products. Full-length top strand was quantified by measuring the radioactivity in the band corresponding to the full-length top-strand product (988 nt) after RNase treatment of the products. Radioactivity was determined by scanning the dried gel with a Typhoon Trio PhosphorImager and quantifying using ImageQuant TL. First, the biochemical assays confirmed the function of several candidate retrohoming factors that were expected to be required for top-strand DNA synthesis, including RNase H1 (rnhA), the 5′→3′ exonuclease activity of Pol I (polAexts), and the replicative polymerase Pol III (dnaQ, dnaEts). Extracts from Keio deletions or mutants having temperature-sensitive defects in these activities showed high levels of reverse splicing and cDNA synthesis, but strongly decreased top-strand DNA synthesis (rnhA and dnaQ, 2% wild type; polAexts, 34% wild type; and dnaEts, 10% wild type; Figure 4A, Figure 4C, and Table 2). The ligAts mutant also showed a substantial decrease in top-strand DNA synthesis in the extract assays (45% wild type; Figure 4C), consistent with a role for DNA ligase A in sealing nicks. The incomplete inhibition of top-strand DNA synthesis in the temperature-sensitive mutants may reflect residual activity at the semi-permissive temperature used in the experiment. The Keio deletion of the second E. coli RNase H gene, rnhB, which had no effect on retrohoming in vivo, did not inhibit top-strand synthesis in the in vitro assays (153% wild type; Figure 4A). However, extracts from both the rnhA and rnhB deletions showed elevated levels of full-length intron cDNAs (239 and 218% wild type, respectively), possibly reflecting that RNase H2 makes some contribution to degrading the intron RNA template in vitro.
Among the replication restart mutants, we found substantial decreases in top-strand synthesis in extracts from Keio deletions priA (1% wild type), priC (60% wild type), and dnaT (50% wild type), as well as the temperature-sensitive mutants of dnaB, which encodes the replicative DNA helicase (0%), and dnaC, which interacts with DnaB prior to loading (18% wild type) (Figure 4A, Table 2). The greater effect of the Keio priA deletion in vitro than in vivo may reflect that the short time of the in vitro assays (15 min) favors intermediates with short gaps that are recognized by PriA, while the longer time of the in vivo assays (1 h) favors intermediates with longer gaps that are recognized by PriC [47]. In agreement with Taqman qPCR assays, we found no effect for the deletion of the genes encoding PriB, an accessory protein that facilitates PriA-DnaT complex formation (116% wild type; Figure 4B) [63], nor the DNA helicase Rep (96% wild type; Figure 4A), which ordinarily functions in conjunction with PriC [59].
We also found severe defects in top-strand DNA synthesis in the extracts from temperature-sensitive mutants of two essential proteins that function in replication restart pathways, the single-stranded DNA-binding protein Ssb (ssbts; 8% wild type) [57], [58] and the DNA primase (dnaGts, 0%; [56]) (Figure 4C). Surprisingly, the DNA primase mutant (dnaGts) was also strongly inhibited in bottom-strand cDNA synthesis (<1% wild type; Table 2; see Discussion). Importantly, the LexA SS4610 (lexA51::Tn5) mutant, which has a constitutively induced SOS response [60], showed no significant decrease in top - or bottom-strand DNA synthesis in the extract assays (92 and 121% wild type, respectively; Table 2, Figure S7), consistent with the minimal effect of this mutation on retrohoming in vivo (Table 1 and Figure S5).
Only four other protein mutants showed significantly decreased retrohoming in the extract assays (<70% wild-type top-strand synthesis): the Keio deletions of dinB (Pol IV; 63% wild type), seqA (51% wild type), and stpA (42% wild type; Figure 4A); and a pnp mutant that retains <10% of the wild-type PNPase activity (42% wild type; Figure 4B). Three of these mutants along with the Keio deletion of polB also showed substantially decreased synthesis of full-length bottom strands (dinB, 32% wild type; pnp, 45% wild type; polB, 43% wild type; and stpA, 23% wild type; Table 2). The effect of the DNA repair polymerase mutations is consistent with a possible role in helping to initiate at or traverse the intron RNA/DNA junctions in retrohoming intermediates [12] and/or in initiating replication restart before being replaced by Pol III [64]. PNPase, a component of the E. coli RNA degradosome, has multiple activities that could affect retrohoming, including 3′→5′ exoribonuclease, 3′-terminal oligonucleotide polymerase, high affinity binding to ssRNA and ssDNA, and lower affinity binding to dsDNA [65], [66]. StpA, an H-NS-like DNA - and RNA-binding protein that has RNA chaperone activity, could affect retrohoming by acting on either the target DNA or intron RNA [39], [67], [68]. How SeqA, a negative regulator of the initiation of chromosome replication [69], might contribute to retrohoming is unclear.
The RecJ deletion (single-stranded DNA exonuclease) is noteworthy for showing substantially decreased synthesis of full-length bottom strands (37% wild type; Figure 4A; Table 2) with no decrease in top-strand synthesis. RecJ mutants were identified as retrohoming-deficient both in initial genetic assays [12] and in the transposon screen in this work (Table 1), although not in the Taqman qPCR assays (see above). The effect of the recJ deletion on bottom-strand synthesis in the extract assays is consistent with its previously suggested function in resecting the 5′ overhang of the bottom-strand resulting from the staggered double-strand break made by group II intron RNPs [12].
Notably, we also found strong inhibition of top strand synthesis with extracts from the transposon-insertion mutant C0719, which is at the site of a predicted sRNA (17% wild type; Figure 4B and Table 2). Both reverse splicing and full-length cDNA synthesis were also decreased in this strain (57 and 69% wild type, respectively). How an sRNA might affect retrohoming warrants further investigation.
None of the other mutants tested strongly inhibited retrohoming in the in vitro assays (<70% wild type top-strand DNA synthesis; Figure 4, Table 2, Figure S7, and Table S6). A number of these mutants showed decreased retrohoming efficiencies in in vivo assays and may affect retrohoming indirectly by affecting chromosome structure, DNA replication, DNA target site accessibility, or energy production, e.g., gyrBts (DNA gyrase subunit B); hns (histone-like nucleoid structuring protein); rpoHts and rpoN (RNA polymerase sigma factors); tonB (membrane protein involved in energy production); and tus (DNA termination site binding protein). Notably, the gyrBts mutant showed decreased retrohoming efficiencies in vivo, but elevated levels of both top - and bottom-strand synthesis in vitro (261 and 215% wild type, respectively), possibly reflecting that DNA gyrase impedes retrohoming in wild-type extracts in vitro by unwinding some proportion of the dsDNA oligonucleotide substrate.
Discussion
Here, we used genetic and biochemical approaches to identify E. coli host factors that function in group II intron retrohoming. First, we used a plasmid-based genetic assay that controls for indirect effects to screen a transposon-insertion library for mutants in which the Ll.LtrB group II intron shows decreased or increased retrohoming efficiency. We then used a Taqman qPCR assay to quantify retrohoming into a chromosomal site in Keio deletions or temperature-sensitive mutants of the candidates identified in this and previous screens. Finally, we compared retrohoming activity in wild-type and candidate mutant strains by using a new biochemical assay that combines Ll.LtrB RNPs with E. coli extracts to reconstitute the complete retrohoming reaction in vitro. Although the initial transposon screen remained vulnerable to false positives, it yielded a manageable group of candidates, whose function in retrohoming was verified by the more direct Taqman qPCR and/or biochemical assays.
Considered together, our results suggest a model for retrohoming of Ll.LtrB intron lariat RNAs in E. coli shown in Figure 5. In initial previously characterized steps, Ll.LtrB RNPs recognize the double-stranded DNA target site and the intron RNA reverse splices into one DNA strand, while the IEP cuts the opposite DNA strand and uses the cleaved strand as a primer for reverse transcription of the reverse-spliced intron RNA [5], [16]. The major host RNase H, RNase H1 encoded by rnhA, degrades the intron RNA template strand during or after cDNA synthesis, leaving residual RNA fragments that could serve as primers for top-strand DNA synthesis. Crucially, after synthesis of a full-length intron cDNA, either the group II intron RT or host DNA polymerase extends bottom-strand synthesis into the 5′ exon, yielding a branched intermediate that is recognized by the replication restart proteins PriA or PriC, which act preferentially on intermediates with short or long gaps between the branch and the 3′ end of the nascent strand [47]. These replication restart proteins then initiate a replisome-loading cascade leading to top-strand DNA synthesis by the host replicative polymerase, Pol III. We find that the 5′→3′ exonuclease activity of Pol I is required for second-strand synthesis during retrohoming, presumably to degrade RNA primers attached to newly synthesized DNA, and Pol I DNA polymerase activity could additionally contribute by helping to fill gaps, both functions of Pol I in host cell DNA replication [70], [71]. Surprisingly, although bottom-strand cDNA synthesis in the extracts is completely dependent upon the RT activity of the LtrA protein, it was nevertheless strongly inhibited in the DNA primase mutant dnaGts, suggesting a previously unsuspected contribution of host factors to initiating cDNA synthesis (see below). The genetic screens also revealed a new putative E. coli RNA degradation pathway that impedes retrohoming and whose disruption leads to increased retrohoming efficiencies.
Fig. 5. Model for function of host factors in group II intron retrohoming in E. coli. 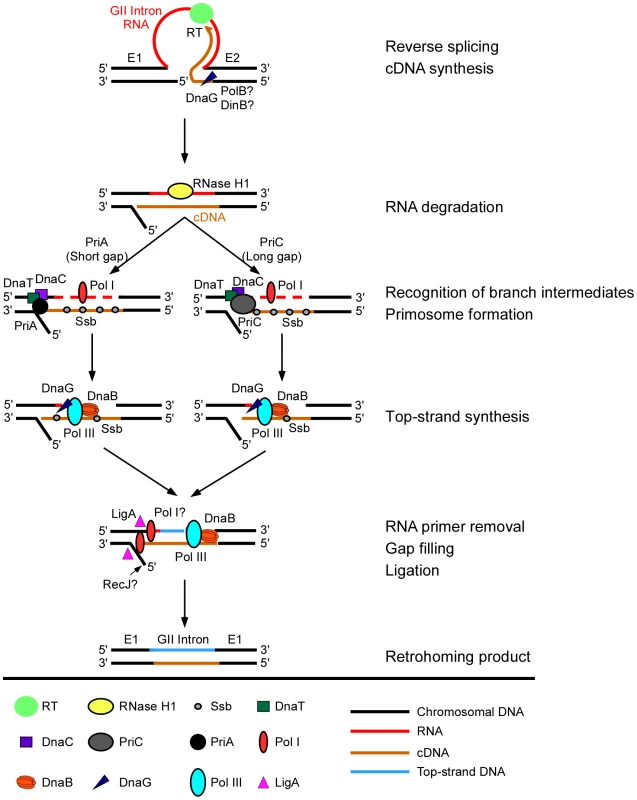
In initial steps, the group II intron lariat RNA reverse splices into the top strand of the DNA target site, while the intron-encoded RT cuts the bottom DNA strand and uses the 3′ end of the cleaved strand as a primer for target DNA-primed reverse transcription of the intron RNA. During or after cDNA synthesis, a host RNase H (RNase H1) degrades the intron RNA template strand. Extension of the intron cDNA into the 5′ exon displaces the bottom-DNA strand resulting in a branched intermediate that is recognized by the replication restart proteins PriA or PriC, with PriA preferentially recognizing intermediates with short gaps in the bottom strand and PriC preferentially recognizing intermediates with long gaps in the bottom strand. PriA and PriC then initiate a replisome loading cascade involving the sequential recruitment of the replicative helicase DnaB, the primase DnaG, and the replicative polymerase Pol III for second-strand DNA synthesis. Ssb stabilizes single-stranded DNA in gapped regions and interacts with PriA to stimulate the loading of DnaB. The 5′→3′ exonuclease activity of Pol I contributes to the removal of residual RNA primers and its DNA polymerase activity may contribute to filling in gaps, and a host DNA ligase (LigA) seals nicks in the top and bottom strands. Although bottom-strand synthesis is completely dependent on group II RT activity (Figure 3D), biochemical assays show that it is strongly inhibited in a DNA primase (DnaG) mutant and moderately inhibited in repair DNA polymerase DinB and PolB mutants, suggesting a previously unsuspected role for host factors in initiating bottom-strand (cDNA) synthesis. Deletion of RecJ moderately inhibits synthesis of full-length bottom strands in extracts, consistent with a role in resection of the 5′-overhang resulting from the staggered cleavage of the DNA substrate by group II intron RNPs [12]. Importantly, our results confirm that the Ll.LtrB group II intron relies on host DNA polymerases for second-strand DNA synthesis, with a major role for the host replicative polymerase Pol III. The involvement of Pol III in second-strand DNA synthesis was postulated previously based on two findings: (i) that LtrA has low DNA-dependent DNA polymerase activity on artificial substrates in vitro [12], [13], and (ii) that DnaQ and DnaEts mutants are deficient in retrohoming in plasmid-based genetic assays [12]. Here, biochemical assays with cell extracts show that second-strand synthesis is completely dependent upon host DNA polymerases and is strongly inhibited in extracts from DnaQ and DnaEts mutants (2 and 10% wild-type activity, respectively). Notably, although on-going DNA replication may contribute to retrohoming in vivo, we observed the synthesis of a complete second-strand DNA in vitro in the absence of DNA replication.
A major function for RNase H1 in retrohoming is indicated by the findings that a Keio deletion and other mutations in the rnhA gene strongly inhibit retrohoming in genetic, Taqman qPCR, and biochemical assays in this work, and in two different genetic assays used in previous work [12]. By contrast, mutations in the rnhB gene encoding RNase H2 do not significantly inhibit retrohoming or top-strand synthesis in these assays (this work and [12]). Biochemical analysis using extracts from the Keio rnhA deletion strain show that deficiency of RNase H1 results in the accumulation of an intermediate containing the reverse spliced intron RNA, as expected, and that inability to degrade the intron RNA template strand strongly inhibits top-strand DNA synthesis. Extracts from the Keio rnhB deletion also showed some accumulation of the reverse-spliced intermediate, but no deficiency in top-strand synthesis.
A major finding is that replication restart proteins function in retrohoming and are required for second-strand DNA synthesis. We hypothesize that these proteins recognize the branched intermediate formed after RNase H degradation of the intron RNA template strand and extension of intron cDNA synthesis into the 5′ exon and then initiate replisome loading and second-strand DNA synthesis by Pol III by mechanisms similar or identical to those ordinarily used for replication restart at stalled or collapsed replication forks. The replication restart components found here to function in retrohoming by both in vivo and in vitro assays include PriA and PriC, the host proteins that initiate replication restart by recognizing stalled or collapsed replication forks [47]; the accessory proteins DnaC and DnaT [72]; and the replicative helicase DnaB [54]. Our biochemical assays with mutant extracts show directly that all these components are required for second-strand DNA synthesis. During replication restart, PriA recognizes a branched intermediate in which the 3′ OH of the nascent leading strand is close to the replication fork (no gap or a gap of <3 nts), while PriC recognizes an intermediate with a larger gap (>7 nts) [47]. During retrohoming, longer or shorter gaps in the branched intermediate could result from more or less resection of a stalled nascent bottom strand after dissociation of the RT prior to reinitiation of DNA synthesis by a host DNA polymerase. Although the top strand of the retrohoming intermediate contains annealed RNA fragments that result from RNase H digestion and may thus resemble a nascent lagging strand, the location of this strand relative to the branch differs from that at a replication fork, and it is unclear how or if it might also contribute to recognition by PriA or PriC.
The genetic and biochemical assays also indicate a major role in retrohoming for two other essential proteins that function in conjunction with replication restart machinery, the single-stranded DNA binding protein Ssb and the primase DnaG, with mutations in these proteins inhibiting both retrohoming in vivo and top-strand DNA synthesis in vitro. Ssb binds ssDNA regions after unwinding by Rep or PriA and has been shown to physically interact with PriA to stimulate the loading of DnaB at stalled forks [47], [58]. DnaG synthesizes short RNA primers, which are used for initiation of DNA synthesis by Pol III, and triggers the release of DnaC from DnaB [73]. The very stringent requirement for the helicase DnaB in the in vitro retrohoming reaction with a small DNA oligonucleotide substrate could reflect that in addition to DNA unwinding, it is needed to recruit the primase DnaG.
In contrast to other replication restart components, we found no contribution to group II intron retrohoming for PriB, an accessory protein in the PriA-PriB pathway, or Rep, which ordinarily functions together with PriC on the stalled fork by unwinding the dsDNA, especially when the 5′ end of the newly synthesized lagging strand is close to the fork [59], [74]. The dispensability of these factors presumably reflects that PriA can function independently of PriB in the PriA-PriC pathway and that PriC can load DnaB on stalled forks independently of either PriA or Rep [47], [59], [74]. Although the lack of requirement for Rep in vitro could also reflect that the biochemical assay uses a small DNA oligonucleotide substrate, it is nevertheless consistent with the lack of requirement for Rep in our in vivo assays (Table 1; in agreement with [12] but not [22]). An intriguing possibility is that a replisome assembled by replication restart proteins at the site of a group II intron insertion initiates a new round of host DNA replication from this location, thereby rapidly fixing the group II intron insertion into the genome.
The genetic and biochemical assays are consistent with the previously suggested role for RecJ, a 5′→3′ DNA exonuclease, which may resect the 5′ overhang on the bottom strand resulting from the staggered double-strand break made by the group II intron RNP [12]. A surprising finding, however, was that bottom-strand DNA synthesis in extracts requires not only the group II intron RT, but is also strongly inhibited in extracts from DnaG primase mutants and moderately inhibited in extracts from DinB and PolB DNA repair polymerase mutants. The DnaG mutant extracts showed strongly decreased synthesis of even short cDNAs (Figure 4C), suggesting that DnaG may be needed for initiation of TPRT, possibly by functioning in conjunction with DNA repair polymerases to copy the 5′-top-strand DNA overhang before the group II intron RT is engaged to copy the reverse-spliced intron RNA. As LtrA can by itself efficiently initiate TPRT directly from the bottom-strand cleavage site in in vitro reactions with purified RNPs [16], [75], we speculate that host proteins, such as Ssb or RecA, may block initiation by the foreign group II intron RT in extracts, whereas host DNA repair enzymes have mechanisms for overcoming such blocks. Either or both mechanisms for initiation of TPRT could be employed in vivo.
We also identified a number of host proteins in which mutations inhibit retrohoming in vivo, but not in vitro. A number of these proteins act on chromosomal DNA or in transcription (e.g., GyrB, Hns, RpoH, SbcC, Tus) and could impact group II intron retrohoming in vivo by affecting chromosome structure, DNA replication, or target site accessibility. Also affecting retrohoming in vivo but not in our in vitro assay is MnmE, which functions in tRNA modification and may affect the activity or intracellular levels of group II intron RNPs (see also [22], [30]). The failure to observe a decrease in retrohoming activity in some mutant extracts could also be due to replacement of the required activity by other host enzymes.
In addition to mutants that decrease retrohoming efficiency, our transposon-library screen also identified host genes whose disruption leads to increased retrohoming efficiencies. Although we hoped such mutants would identify a variety of host defense factors that function in different ways, all five transposon-insertions that increased retrohoming efficiency in our screen mapped to three closely linked genes associated with a cryptic prophage: rnlA, which encodes RNase LS; yfjK, which encodes a DExH/D-box helicase; and yfjL, which encodes a protein of unknown function. The identity of these genes and the finding that their disruption also leads to elevated GFP expression from a control reporter construct that lacks the Ll.LtrB intron suggest that they suppress retrohoming by degrading group II intron RNAs. Previous studies showed that mutations in RNase E, an essential protein, increase retrohoming frequencies by inhibiting group II intron RNA degradation [12], [22]. Together, these findings indicate that cellular RNases function as a major host defense mechanism for suppressing retrohoming. Additionally, our findings identify a new putative RNA degradation pathway in E. coli K12 that may have been acquired from another bacteria via insertion of a temperate phage and may constitute a second degradosome. The suppression of group II intron mobility by intron RNA degradation in bacteria may be analogous to the suppression of mobility of LINE-1 elements by sequestration into stress granules in human cells [76], [77].
Given our findings for Ll.LtrB retrohoming, we anticipate that replication restart proteins may also function in alternate group II intron retromobility pathways in which a nascent strand at a DNA replication fork rather than a cleaved DNA strand is used to prime reverse transcription of the intron RNA [78]–[80]. In these pathways, which are used by group II introns whose IEPs lack DNA endonuclease activity, reverse splicing is thought to result in the insertion of a group II intron RNP into a DNA target site ahead of a replication fork, with the RT positioned to use either a nascent leading or lagging strand as a primer for reverse transcription, depending upon the strand into which the intron inserted [80]. The stalling of the replication fork when it encounters the inserted group II intron RNP may lead first to dissociation of the replisome, enabling the group II intron RT to access the nascent DNA strand for the priming of cDNA synthesis, and then contribute to its re-recruitment via replication restart proteins for second-strand synthesis and continuation of host DNA replication. We note that yeast mtDNA group II introns primarily use a recombination mechanism rather than replication restart for cDNA integration (see Introduction), and the extent to which the replication restart, DNA recombination, or other pathways are used for the retromobility of different group II introns in different bacteria remains to be elucidated.
Key features of the Ll.LtrB intron retrohoming mechanism delineated here may be relevant to the propagation of LINES and other non-LTR-retrotransposons in eukaryotic nuclear genomes. Non-LTR-retrotransposons, which are thought to be evolutionary descendants of mobile group II introns, encode closely related RTs and use an analogous TPRT mechanism for cDNA synthesis [1], [7]. Like mobile group II introns, most non-LTR-retrotransposons do not encode RNase H and presumably rely on a cellular enzyme to degrade the RNA template strand after cDNA synthesis [7]. The mechanism used for second-strand DNA synthesis by non-LTR-retrotransposons is unknown, but given that non-LTR-retrotransposons carry out reverse transcription in the nucleus could well involve the use of a host DNA polymerase and replication restart proteins as found here for group II introns. The use of replication restart for second-strand synthesis by LINE-1 elements is consistent with their ability to retrotranspose in non-dividing cells [81]. In contrast to non-LTR-retroelements, retroviruses and LTR-containing retrotransposons carry out reverse transcription in the cytosol and rely on RTs that have acquired an RNase H domain and efficient DNA-dependent DNA polymerase activity to synthesize a pre-integration complex containing dsDNA that must then enter the nucleus for integration into the genome [7]. These evolutionary advances, which enable LTR-containing retroelements to carry out major steps of their replication pathway in the cytosol, may contribute to their greater propensity to be transferred horizontally between species and evolve into infectious viruses.
Finally, our results have implications for replication restart pathways. In E. coli, replication restart occurs on stalled or collapsed DNA replication forks and is thus dependent upon on-going DNA synthesis. Surprisingly, our extract assays indicate that replication restart components can synthesize a complete second-strand DNA without on-going DNA replication. These findings resemble recent results for bacteriophage Mu where PriA was found to be required for filling in 5-bp gaps at each end of the Mu insertion in the absence of DNA replication [82]. Thus, replication restart proteins may play a more general role both in the repair of DNA damage and propagation of mobile elements than was thought previously, including as an integral part of the group II intron retrohoming mechanism.
Materials and Methods
E. coli strains and growth conditions
E. coli HMS174(DE3) (Novagen) was used for the transposon library screen and retrohoming assays and DH5α was used for cloning. The construction of the mariner transposon library in HMS174(DE3) was described previously [83]. E. coli Keio deletions and their parental wild-type strain BW25113 were obtained from the National BioResource Project (National Institute of Genetics, Japan). Wild-type SS996 and mutant strains in this genetic background were obtained from Dr. Steven Sandler (University of Massachusetts) [84]. A complete listing of strains and genotypes is given in Table S7.
Cells were grown in Luria-Bertani (LB), Mueller-Hinton (MH) [85], or 2xYT medium [86], as specified for individual experiments. Antibiotics were added at the following concentrations: ampicillin, 100 µg/ml; chloramphenicol, 25 µg/ml; kanamycin, 40 µg/ml; rifampicin, 50 µg/ml; trimethoprim (Tp), 10 µg/ml. Thymine was added at 2 µg/ml.
Recombinant plasmids
The intron-donor plasmids pALG2 (Figure S2A; [48]) and pALG3 (Figure 2A) have the vector backbone of pACYC184 with a camR marker and use a T7lac promoter to express an ltrB/GFP fusion cassette, followed by the LtrA ORF. The ltrB/GFP fusion cassette consists of the Shine-Dalgarno sequence and the first eight codons of the phage T7 Φ10 gene linked in-frame to a segment of the L. lactis ltrB gene [58-bp exon 1 (E1), 915-bp Ll.LtrB-ΔORF intron, and 38-bp exon 2 (E2)], with E2 linked to codons 2 to 238 of the GFP ORF. The GFP ORF is derived from pGFPuv (Clontech), which has the amino acid substitutions F64L and S65T to improve performance in FACS assays [87]. The LtrA ORF is cloned downstream of the ltrB/GFP fusion cassette and has its own Shine-Dalgarno sequence. pALG3 additionally contains a trimethoprim-resistance retrotransposition-activated marker (TpR-RAM [24]) inserted at the MluI site in DIV of the Ll.LtrB-ΔORF intron, enabling selection for retrohoming events.
The intron-recipient plasmid pBRR3-ltrB (Figure 2A) is a derivative of pBR322 with an ampR marker [26]. It contains a 45-bp wild-type ltrB target site cloned upstream of a promoterless tetR gene, enabling detection of mobility events either by using the TpR-RAM marker, as in the present work, or by integration of an intron carrying a phage T7 promoter for reporter gene activation [26].
The control plasmid pALE is a derivative of pALG2 that lacks the Ll.LtrB-ΔORF intron and has the ligated ltrB exon sequences linked directly to GFP (Figure S2B; [48]).
pBL1Cap is a broad host range intron-donor plasmid that uses an m-toluic acid-inducible promoter to express the Ll.LtrB-ΔORF intron and flanking exons. It was derived from the broad host range intron-donor plasmid pBL1 [28] by replacing the tetR marker with a camR marker (1.5-kb NheI/PshAI fragment of pACD3 blunt ended and cloned in place of tetR between the FspI sites of the pBL1).
pBL1-rhlE is a derivative of pBL1Cap that expresses an Ll.LtrB-ΔORF intron that was retargeted to insert into a site in the antisense strand of the rhlE gene [21].
Transposon-library screen and TpR-RAM selection assay
The intron-donor plasmid pALG3 and recipient plasmid pBRR3-ltrB were co-transformed into HMS174(DE3) containing random mariner-transposon insertions [83], and the cells were plated on LB medium containing ampicillin and chloramphenicol to select for the markers on the plasmids. Individual colonies were resuspended in 500 µl of LB medium containing the same antibiotics in 96-deep-well plates and incubated overnight at 37°C. A portion (20 µl) of the overnight culture was transferred into 500 µl of fresh LB medium containing ampicillin and chloramphenicol in new 96-deep-well plates, incubated with shaking at 37°C for 2 h, and then induced with 0.5 mM IPTG for 3 h at 30°C. After induction, 105 cells from each sample were transferred to another set of 96-deep-well plates containing 600 µl of MH medium or MH medium with thymine and trimethoprim. The plates were then incubated overnight at 30°C, and the O.D.595 of individual wells was read by a DTX880 multimode plate reader (Beckman Coulter). DNA was isolated by using a Bacterial Genomic DNA Prep kit (Qiagen) and transposon-insertion sites were mapped by Thermal-Asymmetric-Interlaced (TAIL) PCR [25]. Southern hybridizations to confirm that each mutant contained a single transposon insertion at the identified site were as described [83].
FACS analysis of GFP expression
E. coli strains carrying plasmids with ltrB/GFP fusions cassettes were grown to log phase (O.D.595 = 0.2–0.4) and induced for 3 h at 30°C with 0.1 mM IPTG (pALG2 or pALE) or 0.5 mM IPTG (pALG3). Wild-type and mutant SS996 (PsulA-GFP) strains were grown at 30°C to O.D.595 = 0.2–0.3 and induced with 4 mM m-toluic acid for 1 h at 37°C. Cells were collected by centrifugation, resuspended in phosphate-buffered saline (140 mM NaCl, 2.7 mM KCl, 9 mM Na2HPO4, 1.6 mM KH2PO4, pH 7.4), and analyzed by using a FACS Caliber (Becton Dickinson), with filter FL1 set at 530±30 nm. FACS data were analyzed with the CELLQuest Pro program (Becton Dickinson).
Taqman qPCR assay of retrohoming
Retrohoming frequencies in Keio deletion and temperature-sensitive mutant strains were determined by using a retargeted Ll.LtrB-ΔORF intron (rhlE481a) that inserts at a site in the chromosomal rhlE gene [21] and quantifying the 5′ - and 3′-integration junctions relative to the number of rhlE genes by Taqman qPCR. For this assay, E. coli strains were transformed with pBL1-rhlE and grown overnight at 37°C in LB medium containing chloramphenicol. The overnight culture was subcultured into fresh medium and incubated at 37°C until O.D.595 = 0.2–0.3. Then, triplicate 3-ml cultures were induced with 4 mM m-toluic acid for 1 h at 37°C. The priA Keio deletion strain was grown and induced at 30°C to avoid the accumulation of suppressor mutations. Five temperature-sensitive mutants (dnaEts, gyrBts, ligAts, rpoHts, and ssbts) that could not be grown at 37°C were grown at 30°C and then shifted to 37°C for 1 h followed by a 1-h induction with m-toluic acid. After induction, cells were lysed immediately and DNA was extracted by using a DNeasy Blood and Tissue Kit (Qiagen).
Taqman qPCR was carried out on 10 ng of total DNA in 384-well plates using a Universal qPCR Master Mix kit (Applied Biosystems), with the following primer/probe sets: (i) 5′-integration junction. P5-forward 5′-GGTGCAAACCAGTCACAGTAATG; reverse 5′-GTCAGCTTCATCGAGGACGAG; Taqman probe 5′-CAAGGCGGTACCTCC; (ii) 3′-integration junction. P3-forward 5′-ATAAAGCCCATGTCGAGCATG; reverse 5′-TGTAAGATAACACAGAAAACAGCCAA; Taqman probe 5′-TGCGCCCAGATAGGGTGTTAAGTCAAGTAGT; (iii) rhlE gene. rhlE-forward 5′-CAGCAACGTCCCGGGG; reverse 5′-ACGCAGTTTCATCATCTGCG; Taqman probe 5′-CCACCAGCACATCAACGCCGC. Taqman qPCR primers and probes with a 5′ FAM (6-carboxyfluorescein) label and 3′ MGB (dihydrocyclopyrroloindole tripeptide major groove binder) quencher were obtained from Applied Biosystems. Standard curves were generated by serial ten-fold dilutions of a TOPO-2.1 vector carrying a cloned DNA fragment containing the Ll.LtrB-ΔORF intron integrated within the rhlE gene. All primer/probe sets had >90% amplification efficiency over the concentration range of the standard curve. Retrohoming frequencies were calculated as the numbers of 5′ - and 3′-integration junctions relative to the number of rhlE genes after subtraction of background signal without m-toluic acid induction and are the mean ± standard error of the mean (S.E.M.) for triplicate 1-h inductions.
E. coli extract assays
E. coli S12 extracts were prepared by a modification of procedures used to prepare extracts for in vitro transcription and translation [61], [62]. Cells were grown at 37°C in 200 ml of 2xYT medium at 200 reciprocations per min to O.D.595 = 0.8–1.0, harvested by centrifugation, and washed three times with 20 ml of buffer A [10 mM Tris-acetate, pH 8.2, 14 mM magnesium acetate, 60 mM potassium glutamate, 1 mM dithiothreitol (DTT), and 0.05% (v/v) 2-mercaptoethanol (2-ME)]. The five temperature-sensitive strains (dnaEts, gyrBts, ligAts, rpoHts, and ssbts) that could not be grown at 37°C were instead grown to O.D.595 = 0.4–0.6 at 30°C, then shifted to 37°C and incubated for an additional 2 h. The priA deletion strain was grown at 30°C. After growth, the cells were resuspended in 1.27 ml of buffer B (buffer A without 2-ME) per g wet weight and disrupted by using a BeadBeater [3.24 g of 0.1-mm glass beads (BioSpec Products) per g wet weight; 3 cycles of 1 min at 4°C followed by 1-min incubation on ice]. The crude lysate was centrifuged twice (12,000× g for 10 min at 4°C), and the supernatant was removed and pre-incubated at 37°C for 30 min to complete endogenous reactions and release required components. Extracts prepared without this pre-incubation step showed no detectable top-strand DNA synthesis in retrohoming assays. The extracts were divided into 50-µl aliquots and stored at −80°C. Cell extracts from different strains were checked by Coomassie blue staining of SDS-polyacrylamide gels to confirm that they contained equal amounts of total protein.
The DNA substrates used for extract assays consisted of the top-strand oligonucleotide (5′ - GCAACCCACGTCGATCGTGAACACATCCATAACCATATCATTTTTAATTCTACGAATCTTTATACTGGCAAAC) and the bottom-strand oligonucleotide (5′ - GTTTGCCAGTATAAAGATTCGTAGAATTAAAAATGATATGGTTATGGATGTGTTCACGATCGACGTGGGTTGC). The strands were annealed by mixing 1 µM of each strand in RNase-free water, heating to 100°C for 5 min, and slowly cooling to room temperature. DNA oligonucleotides were 5′-labeled with 32P using T4 polynucleotide kinase (New England Biolabs), according to the manufacturer's protocol.
Assays were carried out in 20 µl of reaction mixture containing 50 nM DNA substrate, 3 µl of in vitro reconstituted Ll.LtrB RNPs (5–10 µg based on O.D.260; RNPs prepared as described in ref. [13]), 6 µl of S12 extract, 20 µM carrier DNA oligonucleotide (5′-GTGATGTCTGAAAAGAACGGGAAG) as protection against DNase activity, 56.4 mM Tris-acetate buffer, pH 7.5, 100 mM potassium acetate, 35.9 mM ammonium acetate, 24 mM magnesium acetate, 1.5 mM ATP, 1 mM each of dATP, dCTP, dGTP, and dTTP (collectively denoted dNTPs), 500 µM of CTP, GTP, and UTP, 5 mM phosphoenolpyruvate, 50 µg/ml pyruvate kinase, 2 units/µl RNaseOUT (Invitrogen), and 1% (v/v) protease inhibitor cocktail [made by dissolving a mini EDTA-free tablet (Roche) in 1 ml of RNase-free water]. For time-course experiments, the reactions were scaled up to 100 µl, and a 20-µl portion was withdrawn at each time point. Reactions were initiated by adding labeled DNA substrate, incubated at 37°C for times specified for individual experiments, and terminated by extraction with phenol-chloroform-isoamyl alcohol (phenol-CIA; 25∶24∶1 by volume). After digestion with proteinase K (2 µg/µl, 30 min at 37°C) and re-extraction with phenol-CIA, nucleic acids were ethanol precipitated and dissolved in 20 µl of nuclease-free water. For RNase treatment, 0.4 units RNase H (Invitrogen) and 0.1 µg RNase A (Roche) were added, and the sample was incubated for 30 min at 37°C before the ethanol-precipitation step. Samples were analyzed in a denaturing 6% polyacrylamide gel, which was dried and scanned with a PhosphorImager.
For primer-extension analysis, DNA products were separated in a 1% low-melting point agarose gel (Fermentas). A gel slice containing DNAs of 0.85–1.2 kb was heated to 70°C for 10 min and then digested with agarase (Fermentas) for 30 min at 42°C. The released DNAs were ethanol precipitated in the presence of glycogen carrier (Fermentas), washed with 70% ethanol, and dissolved in 10 µl of RNase-free water. For primer extension, a 1-µl portion of the DNA was incubated with 10 µM labeled primer in a 10-µl PCR reaction (95°C, 5 min; then 10–20 cycles of 95°C, 15 sec, 58°C, 30 sec, slow ramp to 72°C, 60 sec; 72°C, 4 min) in an ABI 9700 PCR apparatus. Primers were FB, 5′-TCGATCGTGAACACATCCATAAC; 5T, 5′-TGCTCTGTTCCCGTATCAGCT; and FT, 5′-GTTTGCCAGTATAAAGATTCGTAGAA. Samples were analyzed in a denaturing 6% polyacrylamide gel, which was dried and scanned with a PhosphorImager.
Supporting Information
Zdroje
1. LambowitzAM, ZimmerlyS (2011) Group II introns: mobile ribozymes that invade DNA. Cold Spring Harb Perspect Biol 3: a003616 doi:10.1101/cshperspect.a003616.
2. ZimmerlyS, GuoH, EskesR, YangJ, PerlmanPS, et al. (1995) A group II intron RNA is a catalytic component of a DNA endonuclease involved in intron mobility. Cell 83 : 529–538.
3. ZimmerlyS, GuoH, PerlmanPS, LambowitzAM (1995) Group II intron mobility occurs by target DNA-primed reverse transcription. Cell 82 : 545–554.
4. YangJ, ZimmerlyS, PerlmanPS, LambowitzAM (1996) Efficient integration of an intron RNA into double-stranded DNA by reverse splicing. Nature 381 : 332–335.
5. CousineauB, SmithD, Lawrence-CavanaghS, MuellerJE, YangJ, et al. (1998) Retrohoming of a bacterial group II intron: mobility via complete reverse splicing, independent of homologous DNA recombination. Cell 94 : 451–462.
6. Belfort M, Derbyshire V, Parker MM, Cousineau B, Lambowitz AM (2002) Mobile introns: pathways and proteins. In: Craig N, Craigie R, Gellert M, Lambowitz AM, editors. Mobile DNA II. Washington DC: ASM Press. pp. 761–783.
7. Eickbush TH, Malik HS (2002) Evolution of retrotransposons. In: Craig N, Craigie R, Gellert M, Lambowitz AM, editors. Mobile DNA II. Washinton DC: ASM Press. pp. 1111–1114.
8. KooninEV (2009) Intron-dominated genomes of early ancestors of eukaryotes. J Hered 100 : 618–623.
9. EskesR, YangJ, LambowitzAM, PerlmanPS (1997) Mobility of yeast mitochondrial group II introns: engineering a new site specificity and retrohoming via full reverse splicing. Cell 88 : 865–874.
10. EskesR, LiuL, MaH, ChaoMY, DicksonL, et al. (2000) Multiple homing pathways used by yeast mitochondrial group II introns. Mol Cell Biol 20 : 8432–8446.
11. MillsDA, ManiasDA, McKayLL, DunnyGM (1997) Homing of a group II intron from Lactococcus lactis subsp. lactis ML3. J Bacteriol 179 : 6107–6111.
12. SmithD, ZhongJ, MatsuuraM, LambowitzAM, BelfortM (2005) Recruitment of host functions suggests a repair pathway for late steps in group II intron retrohoming. Genes Dev 19 : 2477–2487.
13. WhiteTB, LambowitzAM (2012) The retrohoming of linear group II intron RNAs in Drosophila melanogaster occurs by both DNA ligase 4-dependent and -independent mechanisms. PLoS Genet 8: e1002534 doi:10.1371/journal.pgen.1002534.
14. MillsDA, McKayLL, DunnyGM (1996) Splicing of a group II intron involved in the conjugative transfer of pRS01 in Lactococci. J Bacteriol 178 : 3531–3538.
15. ShearmanC, GodonJJ, GassonM (1996) Splicing of a group II intron in a functional transfer gene of Lactococcus lactis. Mol Microbiol 21 : 45–53.
16. MatsuuraM, SaldanhaR, MaH, WankH, YangJ, et al. (1997) A bacterial group II intron encoding reverse transcriptase, maturase, and DNA endonuclease activities: biochemical demonstration of maturase activity and insertion of new genetic information within the intron. Genes Dev 11 : 2910–2924.
17. SaldanhaR, ChenB, WankH, MatsuuraM, EdwardsJ, et al. (1999) RNA and protein catalysis in group II intron splicing and mobility reactions using purified components. Biochemistry 38 : 9069–9083.
18. MatsuuraM, NoahJW, LambowitzAM (2001) Mechanism of maturase-promoted group II intron splicing. EMBO J 20 : 7259–7270.
19. GuoH, KarbergM, LongM, JonesJPIII, SullengerB, et al. (2000) Group II introns designed to insert into therapeutically relevant DNA target sites in human cells. Science 289 : 452–457.
20. MohrG, SmithD, BelfortM, LambowitzAM (2000) Rules for DNA target-site recognition by a lactococcal group II intron enable retargeting of the intron to specific DNA sequences. Genes Dev 14 : 559–573.
21. PerutkaJ, WangW, GoerlitzD, LambowitzAM (2004) Use of computer-designed group II introns to disrupt Escherichia coli DExH/D-box protein and DNA helicase genes. J Mol Biol 336 : 421–439.
22. CorosCJ, PiazzaCL, ChalamcharlaVR, BelfortM (2008) A mutant screen reveals RNase E as a silencer of group II intron retromobility in Escherichia coli. RNA 14 : 2634–2644.
23. CorosCJ, PiazzaCL, ChalamcharlaVR, SmithD, BelfortM (2009) Global regulators orchestrate group II intron retromobility. Mol Cell 34 : 250–256.
24. ZhongJ, KarbergM, LambowitzAM (2003) Targeted and random bacterial gene disruption using a group II intron (targetron) vector containing a retrotransposition-activated selectable marker. Nucleic Acids Res 31 : 1656–1664.
25. LiuYG, WhittierRF (1995) Thermal asymmetric interlaced PCR: automatable amplification and sequencing of insert end fragments from P1 and YAC clones for chromosome walking. Genomics 25 : 674–681.
26. KarbergM, GuoH, ZhongJ, CoonR, PerutkaJ, et al. (2001) Group II introns as controllable gene targeting vectors for genetic manipulation of bacteria. Nat Biotechnol 19 : 1162–1167.
27. OhmoriH (1994) Structural analysis of the rhlE gene of Escherichia coli. Jpn J Genet 69 : 1–12.
28. YaoJ, LambowitzAM (2007) Gene targeting in gram-negative bacteria by use of a mobile group II intron (“targetron”) expressed from a broad-host-range vector. Appl Environ Microbiol 73 : 2735–2743.
29. BabaT, AraT, HasegawaM, TakaiY, OkumuraY, et al. (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2 : 2006.0008 doi:10.1038/msb4100050.
30. BeauregardA, ChalamcharlaVR, PiazzaCL, BelfortM, CorosCJ (2006) Bipolar localization of the group II intron Ll.LtrB is maintained in Escherichia coli deficient in nucleoid condensation, chromosome partitioning and DNA replication. Mol Microbiol 62 : 709–722.
31. KanayaS, CrouchRJ (1983) DNA sequence of the gene coding for Escherichia coli ribonuclease H. J Biol Chem 258 : 1276–1281.
32. UyemuraD, EichlerDC, LehmanIR (1976) Biochemical characterization of mutant forms of DNA polymerase I from Escherichia coli. II. The PolAex1 mutation. J Biol Chem 251 : 4085–4089.
33. MakiH, KornbergA (1985) The polymerase subunit of DNA polymerase III of Escherichia coli. II. Purification of the a subunit, devoid of nuclease activities. J Biol Chem 260 : 12987–12992.
34. RydbergB, GameJ (2002) Excision of misincorporated ribonucleotides in DNA by RNase H (type 2) and FEN-1 in cell-free extracts. Proc Natl Acad Sci U S A 99 : 16654–16659.
35. LovettST, ClarkAJ (1984) Genetic analysis of the recJ gene of Escherichia coli K-12. J Bacteriol 157 : 190–196.
36. DiFrancescoR, BhatnagarSK, BrownA, BessmanMJ (1984) The interaction of DNA polymerase III and the product of the Escherichia coli mutator gene, mutD. J Biol Chem 259 : 5567–5573.
37. TakahashiS, HoursC, ChuA, DenhardtDT (1979) The rep mutation. VI. Purification and properties of the Escherichia coli rep protein, DNA helicase III. Can J Biochem 57 : 855–866.
38. UmezuK, NakayamaK, NakayamaH (1990) Escherichia coli RecQ protein is a DNA helicase. Proc Natl Acad Sci U S A 87 : 5363–5367.
39. CusickME, BelfortM (1998) Domain structure and RNA annealing activity of the Escherichia coli regulatory protein StpA. Mol Microbiol 28 : 847–857.
40. OliveraBM, LehmanIR (1967) Linkage of polynucleotides through phosphodiester bonds by an enzyme from Escherichia coli. Proc Natl Acad Sci U S A 57 : 1426–1433.
41. SriskandaV, ShumanS (2001) A second NAD+-dependent DNA ligase (LigB) in Escherichia coli. Nucleic Acids Res 29 : 4930–4934.
42. KinscherfTG, ApirionD (1975) Polynucleotide phosphorylase can participate in decay of mRNA in Escherichia coli in the absence of ribonuclease II. Mol Gen Genet 139 : 357–362.
43. KornbergT, GefterML (1971) Purification and DNA synthesis in cell-free extracts: properties of DNA polymerase II. Proc Natl Acad Sci U S A 68 : 761–764.
44. WagnerJ, GruzP, KimS-R, YamadaM, MatsuiK, et al. (1999) The dinB gene encodes a novel E. coli DNA polymerase, DNA Pol IV, involved in mutagenesis. Mol Cell 4 : 281–286.
45. ShinagawaH, KatoT, IseT, MakinoK, NakataA (1983) Cloning and characterization of the umu operon responsible for inducible mutagenesis in Escherichia coli. Gene 23 : 167–174.
46. MasaiH, AraiK (1988) Operon structure of dnaT and dnaC genes essential for normal and stable DNA replication of Escherichia coli chromosome. J Biol Chem 263 : 15083–15093.
47. HellerRC, MariansKJ (2005) The disposition of nascent strands at stalled replication forks dictates the pathway of replisome loading during restart. Mol Cell 17 : 733–743.
48. CuiX, MatsuuraM, WangQ, MaH, LambowitzAM (2004) A group II intron-encoded maturase functions preferentially in cis and requires both the reverse transcriptase and X domains to promote RNA splicing. J Mol Biol 340 : 211–231.
49. OtsukaY, YonesakiT (2005) A novel endoribonuclease, RNase LS, in Escherichia coli. Genetics 169 : 13–20.
50. WangX, KimY, WoodTK (2009) Control and benefits of CP4-57 prophage excision in Escherichia coli biofilms. ISME J 3 : 1164–1179.
51. CarpousisAJ (2007) The RNA degradosome of Escherichia coli: an mRNA-degrading machine assembled on RNase E. Annu Rev Genet 61 : 71–87.
52. LiuJ, XuL, SandlerSJ, MariansKJ (1999) Replication fork assembly at recombination intermediates is required for bacterial growth. Proc Natl Acad Sci U S A 96 : 3552–3555.
53. UedaK, McMackenR, KornbergA (1978) DnaB protein of Escherichia coli. Purification and role in the replication of φX174 DNA. J Biol Chem 253 : 261–269.
54. AllenGCJr, KornbergA (1993) Assembly of the primosome of DNA replication in Escherichia coli. J Biol Chem 268 : 19204–19209.
55. WahleE, LaskenRS, KornbergA (1989) The dnaB-dnaC replication protein complex of Escherichia coli. I. Formation and properties. J Biol Chem 264 : 2463–2468.
56. RowenL, KornbergA (1978) Primase, the dnaG protein of Escherichia coli. An enzyme which starts DNA chains. J Biol Chem 253 : 758–764.
57. MeyerRR, LainePS (1990) The single-stranded DNA-binding protein of Escherichia coli. Microbiol Mol Biol Rev 54 : 342–380.
58. CadmanCJ, McGlynnP (2004) PriA helicase and SSB interact physically and functionally. Nucleic Acids Res 32 : 6378–6387.
59. SandlerSJ (2000) Multiple genetic pathways for restarting DNA replication forks in Escherichia coli K-12. Genetics 155 : 487–497.
60. McCoolJD, LongE, PetrosinoJF, SandlerHA, RosenbergSM, et al. (2004) Measurement of SOS expression in individual Escherichia coli K-12 cells using fluorescence microscopy. Mol Microbiol 53 : 1343–1357.
61. YamaneT, IkedaY, NagasakaT, NakanoH (2005) Enhanced cell-free protein synthesis using a S30 extract from Escherichia coli grown rapidly at 42°C in an amino acid enriched medium. Biotechnol Prog 21 : 608–613.
62. KimT-W, KeumJ-W, OhI-S, ChoiC-Y, ParkC-G, et al. (2006) Simple procedures for the construction of a robust and cost-effective cell-free protein synthesis system. J Biotechnol 126 : 554–561.
63. LiuJ, NurseP, MariansKJ (1996) The ordered assembly of the φX174-type primosome. J Biol Chem 271 : 15656–15661.
64. RangarajanS, WoodgateR, GoodmanMF (1999) A phenotype for enigmatic DNA polymerase II: A pivotal role for pol II in replication restart in UV-irradiated Escherichia coli. Proc Natl Acad Sci U S A 96 : 9224–9229.
65. SoreqH, LittauerUZ (1977) Purification and characterization of polynucleotide phosphorylase from Escherichia coli. Probe for the analysis of 3′ sequences of RNA. J Biol Chem 252 : 6885–6888.
66. Bermúdez-CruzRM, García-MenaJ, MontañezC (2002) Polynucleotide phosphorylase binds to ssRNA with same affinity as to ssDNA. Biochimie 84 : 321–328.
67. WolfT, JanzenW, BlumC, SchnetzK (2006) Differential dependence of StpA on H-NS in autoregulation of stpA and in regulation of bgl. J Bacteriol 188 : 6728–6738.
68. GrossbergerR, MayerO, WaldsichC, SemradK, UrschitzS, et al. (2005) Influence of RNA structural stability on the RNA chaperone activity of the Escherichia coli protein StpA. Nucleic Acids Res 33 : 2280–2289.
69. LuM, CampbellJL, BoyeE, KlecknerN (1994) SeqA: a negative modulator of replication initiation in E. coli. Cell 77 : 413–426.
70. OkazakiR, ArisawaM, SuginoA (1971) Slow joining of newly replicated DNA chains in DNA polymerase I-deficient Escherichia coli mutants. Proc Natl Acad Sci U S A 68 : 2954–2957.
71. KonradEB, LehmanIR (1974) A conditional lethal mutant of Escherichia coli K12 defective in the 5′→3′ exonuclease associated with DNA polymerase I. Proc Natl Acad Sci U S A 71 : 2048–2051.
72. SandlerSJ, MariansKJ, ZavitzKH, CoutuJ, ParentMA, et al. (1999) dnaC mutations suppress defects in DNA replication - and recombination-associated functions in priB and priC double mutants in Escherichia coli K-12. Mol Microbiol 34 : 91–101.
73. Makowska-GrzyskaM, KaguniJM (2010) Primase directs the release of DnaC from DnaB. Mol Cell 37 : 90–101.
74. HellerRC, MariansKJ (2005) Unwinding of the nascent lagging strand by Rep and PriA enables the direct restart of stalled replication forks. J Biol Chem 280 : 34143–34151.
75. MastroianniM, WatanabeK, WhiteTB, ZhuangF, VernonJ, et al. (2008) Group II intron-based gene targeting reactions in eukaryotes. PLoS ONE 3: e3121 doi:10.1371/journal.pone.0003121.
76. GoodierJL, ZhangL, VetterMR, KazazianHH (2007) LINE-1 ORF1 protein localizes in stress granules with other RNA-binding proteins, including components of RNA interference RNA-induced silencing complex. Mol Cell Biol 27 : 6469–6483.
77. DoucetAJ, HulmeAE, SahinovicE, KulpaDA, MoldovanJB, et al. (2010) Characterization of LINE-1 ribonucleoprotein particles. PLoS Genet 6: e1001150 doi:10.1371/journal.pgen.1001150.
78. IchiyanagiK, BeauregardA, LawrenceS, SmithD, CousineauB, et al. (2002) Retrotransposition of the Ll.LtrB group II intron proceeds predominantly via reverse splicing into DNA targets. Mol Microbiol 46 : 1259–1272.
79. Martínez-AbarcaF, Barrientos-DuránA, Fernández-LópezM, ToroN (2004) The RmInt1 group II intron has two different retrohoming pathways for mobility using predominantly the nascent lagging strand at DNA replication forks for priming. Nucleic Acids Res 32 : 2880–2888.
80. ZhongJ, LambowitzAM (2003) Group II intron mobility using nascent strands at DNA replication forks to prime reverse transcription. EMBO J 22 : 4555–4565.
81. KuboS, SelemeMdC, SoiferHS, PerezJLG, MoranJV, et al. (2006) L1 retrotransposition in nondividing and primary human somatic cells. Proc Natl Acad Sci U S A 103 : 8036–8041.
82. JangS, SandlerSJ, HarsheyRM (2012) Mu insertions are repaired by the double-strand break repair pathway of Escherichia coli. PLoS Genet 8: e1002642 doi:10.1371/journal.pgen.1002642.
83. ZhaoJ, NiuW, YaoJ, MohrS, MarcotteEM, et al. (2008) Group II intron protein localization and insertion sites are affected by polyphosphate. PLoS Biol 6: e150 doi:10.1371/journal.pbio.0060150.
84. LongJE, MassoniSC, SandlerSJ (2010) RecA4142 causes SOS constitutive expression by loading onto reversed replication forks in Escherichia coli K-12. J Bacteriol 192 : 2575–2582.
85. MuellerHJ, HintonJ (1941) A protein-free medium for primary isolation of the Gonococcus and Meningococcus. Proc Soc Exp Biol Med 48 : 330–333.
86. Davis LG, Dibner MD, Battey JF (1986) Basic methods in molecular biology. New York: Elsovier.
87. CrameriA, WhitehornEA, TateE, StemmerWPC (1996) Improved green fluorescent protein by molecular evolution using DNA shuffling. Nat Biotechnol 14 : 315–319.
88. ReinerAM (1969) Isolation and mapping of polynucleotide phosphorylase mutants of Escherichia coli. J Bacteriol 97 : 1431–1436.
Štítky
Genetika Reprodukční medicína
Článek The G4 GenomeČlánek Mondo/ChREBP-Mlx-Regulated Transcriptional Network Is Essential for Dietary Sugar Tolerance inČlánek RpoS Plays a Central Role in the SOS Induction by Sub-Lethal Aminoglycoside Concentrations inČlánek Tissue Homeostasis in the Wing Disc of : Immediate Response to Massive Damage during DevelopmentČlánek Disruption of TTDA Results in Complete Nucleotide Excision Repair Deficiency and Embryonic LethalityČlánek DJ-1 Decreases Neural Sensitivity to Stress by Negatively Regulating Daxx-Like Protein through dFOXO
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 4- Akutní intermitentní porfyrie
- Souvislost haplotypu M2 genu pro annexin A5 s opakovanými reprodukčními ztrátami
- Vztah užívání alkoholu a mužské fertility
- Doporučení pro diagnostiku a léčbu akutních jaterních porfyrií
- Doc. Miloš Kubánek: Nemocní se srdeční amyloidózou jsou často skryti a sledováni pod jinými diagnózami
-
Všechny články tohoto čísla
- Epigenetic Upregulation of lncRNAs at 13q14.3 in Leukemia Is Linked to the Downregulation of a Gene Cluster That Targets NF-kB
- A Big Catch for Germ Cell Tumour Research
- The Quest for the Identification of Genetic Variants in Unexplained Cardiac Arrest and Idiopathic Ventricular Fibrillation
- A Nonsynonymous Polymorphism in as a Risk Factor for Human Unexplained Cardiac Arrest with Documented Ventricular Fibrillation
- The Hourglass and the Early Conservation Models—Co-Existing Patterns of Developmental Constraints in Vertebrates
- Smaug/SAMD4A Restores Translational Activity of CUGBP1 and Suppresses CUG-Induced Myopathy
- Balancing Selection on a Regulatory Region Exhibiting Ancient Variation That Predates Human–Neandertal Divergence
- The G4 Genome
- Extensive Natural Epigenetic Variation at a Originated Gene
- Mouse Oocyte Methylomes at Base Resolution Reveal Genome-Wide Accumulation of Non-CpG Methylation and Role of DNA Methyltransferases
- The Environment Affects Epistatic Interactions to Alter the Topology of an Empirical Fitness Landscape
- TIP48/Reptin and H2A.Z Requirement for Initiating Chromatin Remodeling in Estrogen-Activated Transcription
- Aconitase Causes Iron Toxicity in Mutants
- Tbx2 Terminates Shh/Fgf Signaling in the Developing Mouse Limb Bud by Direct Repression of
- Mondo/ChREBP-Mlx-Regulated Transcriptional Network Is Essential for Dietary Sugar Tolerance in
- Sex-Differential Selection and the Evolution of X Inactivation Strategies
- Identification of a Tissue-Selective Heat Shock Response Regulatory Network
- Phosphorylation-Coupled Proteolysis of the Transcription Factor MYC2 Is Important for Jasmonate-Signaled Plant Immunity
- RpoS Plays a Central Role in the SOS Induction by Sub-Lethal Aminoglycoside Concentrations in
- Six Homeoproteins Directly Activate Expression in the Gene Regulatory Networks That Control Early Myogenesis
- Rtt109 Prevents Hyper-Amplification of Ribosomal RNA Genes through Histone Modification in Budding Yeast
- ATP-Dependent Chromatin Remodeling by Cockayne Syndrome Protein B and NAP1-Like Histone Chaperones Is Required for Efficient Transcription-Coupled DNA Repair
- Iron-Responsive miR-485-3p Regulates Cellular Iron Homeostasis by Targeting Ferroportin
- Mutations in Predispose Zebrafish and Humans to Seminomas
- Cytotoxic Chromosomal Targeting by CRISPR/Cas Systems Can Reshape Bacterial Genomes and Expel or Remodel Pathogenicity Islands
- Tissue Homeostasis in the Wing Disc of : Immediate Response to Massive Damage during Development
- All SNPs Are Not Created Equal: Genome-Wide Association Studies Reveal a Consistent Pattern of Enrichment among Functionally Annotated SNPs
- Functional 358Ala Allele Impairs Classical IL-6 Receptor Signaling and Influences Risk of Diverse Inflammatory Diseases
- The Tissue-Specific RNA Binding Protein T-STAR Controls Regional Splicing Patterns of Pre-mRNAs in the Brain
- Neutral Genomic Microevolution of a Recently Emerged Pathogen, Serovar Agona
- Genetic Requirements for Signaling from an Autoactive Plant NB-LRR Intracellular Innate Immune Receptor
- SNF5 Is an Essential Executor of Epigenetic Regulation during Differentiation
- Dialects of the DNA Uptake Sequence in
- Reference-Free Population Genomics from Next-Generation Transcriptome Data and the Vertebrate–Invertebrate Gap
- Senataxin Plays an Essential Role with DNA Damage Response Proteins in Meiotic Recombination and Gene Silencing
- High-Resolution Mapping of Spontaneous Mitotic Recombination Hotspots on the 1.1 Mb Arm of Yeast Chromosome IV
- Rod Monochromacy and the Coevolution of Cetacean Retinal Opsins
- Evolution after Introduction of a Novel Metabolic Pathway Consistently Leads to Restoration of Wild-Type Physiology
- Disruption of TTDA Results in Complete Nucleotide Excision Repair Deficiency and Embryonic Lethality
- Insulators Target Active Genes to Transcription Factories and Polycomb-Repressed Genes to Polycomb Bodies
- Signatures of Diversifying Selection in European Pig Breeds
- The Chromosomal Passenger Protein Birc5b Organizes Microfilaments and Germ Plasm in the Zebrafish Embryo
- The Histone Demethylase Jarid1b Ensures Faithful Mouse Development by Protecting Developmental Genes from Aberrant H3K4me3
- Regulates Synaptic Development and Endocytosis by Suppressing Filamentous Actin Assembly
- Sensory Neuron-Derived Eph Regulates Glomerular Arbors and Modulatory Function of a Central Serotonergic Neuron
- Analysis of Rare, Exonic Variation amongst Subjects with Autism Spectrum Disorders and Population Controls
- Scavenger Receptors Mediate the Role of SUMO and Ftz-f1 in Steroidogenesis
- DNA Double-Strand Breaks Coupled with PARP1 and HNRNPA2B1 Binding Sites Flank Coordinately Expressed Domains in Human Chromosomes
- High-Resolution Mapping of H1 Linker Histone Variants in Embryonic Stem Cells
- Comparative Genomics of and the Bacterial Species Concept
- Genetic and Biochemical Assays Reveal a Key Role for Replication Restart Proteins in Group II Intron Retrohoming
- Genome-Wide Association Studies Identify Two Novel Mutations Responsible for an Atypical Hyperprolificacy Phenotype in Sheep
- The Genetic Correlation between Height and IQ: Shared Genes or Assortative Mating?
- Comprehensive Assignment of Roles for Typhimurium Genes in Intestinal Colonization of Food-Producing Animals
- An Essential Role for Zygotic Expression in the Pre-Cellular Drosophila Embryo
- The Genome Organization of Reflects Its Lifestyle
- Coordinated Cell Type–Specific Epigenetic Remodeling in Prefrontal Cortex Begins before Birth and Continues into Early Adulthood
- Improved Detection of Common Variants Associated with Schizophrenia and Bipolar Disorder Using Pleiotropy-Informed Conditional False Discovery Rate
- Site-Specific Phosphorylation of the DNA Damage Response Mediator Rad9 by Cyclin-Dependent Kinases Regulates Activation of Checkpoint Kinase 1
- Npc1 Acting in Neurons and Glia Is Essential for the Formation and Maintenance of CNS Myelin
- Identification of , a Retrotransposon-Derived Imprinted Gene, as a Novel Driver of Hepatocarcinogenesis
- Aag DNA Glycosylase Promotes Alkylation-Induced Tissue Damage Mediated by Parp1
- DJ-1 Decreases Neural Sensitivity to Stress by Negatively Regulating Daxx-Like Protein through dFOXO
- Asynchronous Replication, Mono-Allelic Expression, and Long Range -Effects of
- Differential Association of the Conserved SUMO Ligase Zip3 with Meiotic Double-Strand Break Sites Reveals Regional Variations in the Outcome of Meiotic Recombination
- Focusing In on the Complex Genetics of Myopia
- Continent-Wide Decoupling of Y-Chromosomal Genetic Variation from Language and Geography in Native South Americans
- Breakpoint Analysis of Transcriptional and Genomic Profiles Uncovers Novel Gene Fusions Spanning Multiple Human Cancer Types
- Intrinsic Epigenetic Regulation of the D4Z4 Macrosatellite Repeat in a Transgenic Mouse Model for FSHD
- Bisphenol A Exposure Disrupts Genomic Imprinting in the Mouse
- Genetic and Genomic Architecture of the Evolution of Resistance to Antifungal Drug Combinations
- Transposable Elements Are Major Contributors to the Origin, Diversification, and Regulation of Vertebrate Long Noncoding RNAs
- Functional Dissection of the Condensin Subunit Cap-G Reveals Its Exclusive Association with Condensin I
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The G4 Genome
- Neutral Genomic Microevolution of a Recently Emerged Pathogen, Serovar Agona
- The Histone Demethylase Jarid1b Ensures Faithful Mouse Development by Protecting Developmental Genes from Aberrant H3K4me3
- The Tissue-Specific RNA Binding Protein T-STAR Controls Regional Splicing Patterns of Pre-mRNAs in the Brain
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



