-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The AmAZI1ng Roles of Centriolar Satellites during Development
article has not abstract
Published in the journal: . PLoS Genet 9(12): e32767. doi:10.1371/journal.pgen.1004070
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1004070Summary
article has not abstract
The precise trafficking and spatial organization of signaling molecules within cells is critical for many fundamental cellular processes. Two interconnected microtubule-based organelles, the centrosome and primary cilium, have been making headlines recently due to their role as central “hubs” for coordinating such signaling events. The centrosome is the major microtubule-nucleating center in animal cells, which polarizes microtubule arrays and thereby directs microtubule-based trafficking toward itself and its associated structure, the primary cilium. [1]. The primary cilium is a tiny hair-like sensory organelle that is templated by one of two centrioles, core elements of the centrosome, and protrudes above the apical surface of almost every cell in the human body (Figure 1). Together, the centrosome and cilium mediate the initiation and transmission of extracellular signals to the interior of the cell, thus controlling many aspects of cell physiology [2], [3]. Defects in the structure and/or function of these organelles result in human disease conditions termed “ciliopathies,” a heterogeneous group of disorders with phenotypes including cystic kidneys; digit, bone, and brain anomalies; infertility; and even cancer [4], [5].
Fig. 1. Schematic of centriolar satellites undergoing microtubule-dependent movement towards the centrosome and cilium. 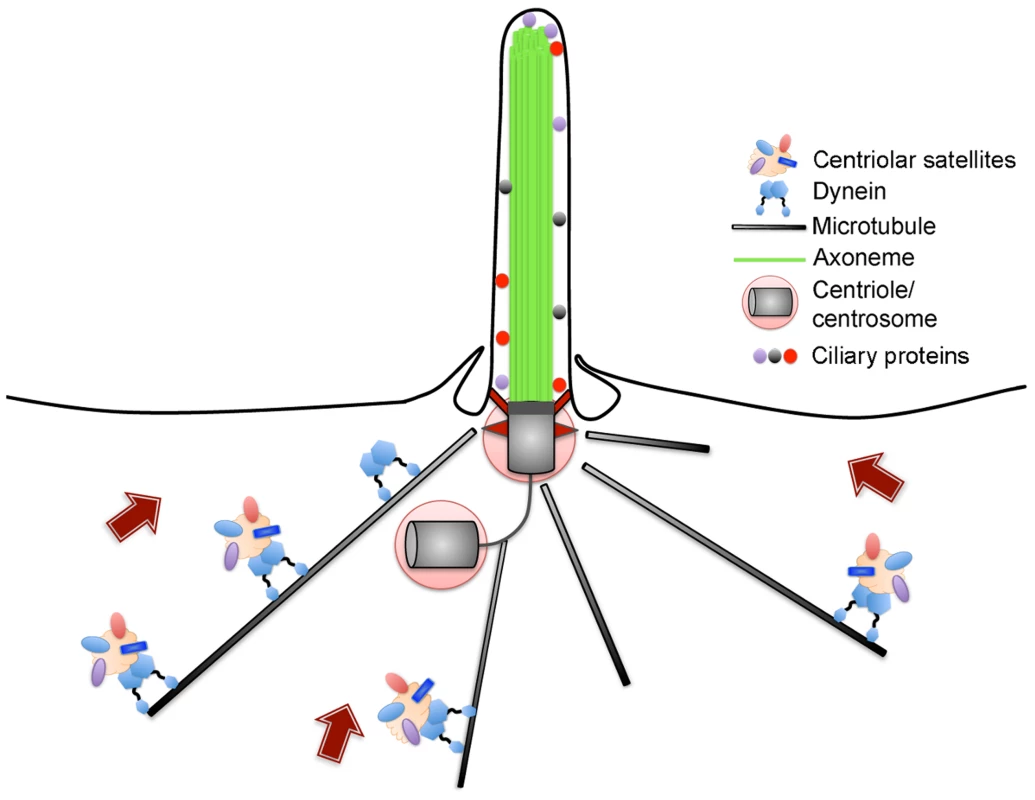
Satellites are composed of a number of proteins, including Azi1, which are typically found in a region surrounding the centrosome. Satellites mediate the trafficking of some proteins destined for the cilium but can also inhibit the movement of other proteins to the centrosome-cilium complex. Human diseases associated with centrosome-cilium aberrations generally result from defects in the proper assembly of the centrosome or cilium, or the trafficking of proteins to these structures. It is now well established that movement of proteins to and from the centrosome-cilium complex is facilitated by centriolar satellites, non-membranous 70–100 nm cytoplasmic granules that concentrate around the centrosome in animal cells (Figure 1) [6]. Centriolar satellites are generally defined by PCM1, a large scaffolding protein reported to function in dynein-dependent, microtubule-based trafficking of centrosomal and ciliary proteins [7], [8]. Although the proteome of centriolar satellites remains unknown, a number of proteins have now been shown to associate with PCM1, many of which are mutated in patients with ciliopathies [9]–[14]. However, the functional significance of centriolar satellites in mammalian development and ciliogenesis in vivo remains unclear. In this issue, Hall and colleagues [15] address the role and requirement of Azi1, a conserved satellite protein, in regulating cilium function during development.
Azi1 was originally identified as a centrosomal protein [16] and localized to centriolar satellites [17]. Hall et al. spatially refined the localization pattern of mouse Azi1, and discovered that Azi1 is fairly dynamic and undergoes microtubule-based trafficking to and from the centrosome. They noted higher levels of Azi1 around centrioles and surrounding centriolar satellites of ciliated cells compared to non-ciliated cells, and identified a further pool of Azi1 at the transition zone, an area at the base of cilia involved in regulating protein entry into the ciliary space [18]. Together, these results show a redistribution of Azi1 upon initiation of ciliogenesis, hinting at a functional role in regulating ciliary protein trafficking. Indeed, Azi1 was previously shown to be essential for ciliogenesis in zebrafish and flies [19], [20], and a siRNA screen in human cells also identified Azi1 as necessary for cilium formation [21]. Similarly, Hall et al. find that knockdown of Azi1 in mouse cells using siRNA, which they refer to as “acute” loss of the protein, results in a significant proportion of cells that lack primary cilia. Given the high conservation of Azi1 among ciliated organisms, the loss-of-function phenotypes from various model organisms, as well as the siRNA results, it was safe to predict that Azi1 would have a central role in mammalian cilia biology in vivo. Thus, the authors generated mouse mutants null for Azi1 to test the role of this protein (and centriolar satellites) in mammalian development.
Remarkably, Azi1 null mice are viable, and display none of the gross abnormalities commonly associated with either primary or motile cilia dysfunction. Examination of primary mouse embryonic fibroblasts (MEFs) isolated from Azi1 null mice showed that formation and compartmentalization of primary cilia appear normal, with normal distribution of ciliary membrane and transition zone proteins. Similarly, there were no gross defects with regard to centrosome duplication or centriolar satellite composition and distribution in cells lacking Azi1. Ultrastructure analysis of motile multiciliated tracheal epithelia also failed to detect any defects in centrioles/basal bodies and cilia. Collectively, and in stark contrast with the acute Azi1 loss in human and mouse cells, primary and motile cilia structure and function appear grossly normal in Azi1 null animals in vivo, as well as in Azi1 null MEFs grown in vitro.
The difference in phenotypes observed following acute knockdown of Azi1 versus permanent deletion of the gene (referred to as “chronic” loss by the authors) is suggestive of a compensation mechanism that, in essence, rescues the defects caused by loss of Azi1. Intriguingly, while such compensation was seen in most tissues, Azi1 null male mice were infertile, exhibiting post-meiotic defects in spermatogenesis. Sperm cells lacked flagella and any remaining axonemes were truncated and immotile, likely due to mistrafficking of proteins required for flagellar assembly. This indicates that Azi1 (and by extension centriolar satellites) plays a critical role in regulating trafficking of proteins in the highly specialized sperm cells that, unlike other ciliated cell types, cannot compensate for loss of Azi1. This tissue-specific phenotype is reminiscent of human mutations in a number of genes required for centrosome assembly and duplication, where the predominant phenotype appears to be microcephaly [22]–[26]. These latter findings suggest that, during development, most tissues can compensate and overcome mutations in centrosomal proteins, except for neural progenitor cells, which appear most sensitive to these perturbations. The findings by Hall et al. similarly indicate that sperm cells may be especially sensitive to mutations in centriolar satellite genes.
The functional compensation reported by Hall et al. highlights the complexity and challenge in understanding cilia assembly and function in the context of a developing organism. The basis of the compensation, at this point, is unclear, as it cannot be simply attributed to genetic redundancy, but a mechanistic understanding of the process will be critical if more cases like Azi1 are discovered in the future. Interestingly, the Azi1 null MEFs isolated by Hall et al. are shown to be “active” in compensating for the loss of Azi1; it will be very useful to test whether the compensation can be reversed (or switched on/off) by inducibly expressing exogenous Azi1 in these cells. One interesting strategy would be to utilize the Azi1 null MEFs as an effective in vitro system to understand how functional compensation works in this case, or with centriolar satellites in general. Moreover, this study also highlights the importance of functional follow-up studies of siRNA-based data and cautions against direct extrapolation of transient loss-of-function phenotypes to the genetic in vivo phenotype. On the other hand, transient loss-of-function studies may expose roles of genes that would otherwise be overlooked due to compensation in long-term mutagenesis studies in vivo. As such, it will be important to combine both approaches in future studies.
Zdroje
1. NiggEA, StearnsT (2011) The centrosome cycle: Centriole biogenesis, duplication and inherent asymmetries. Nat Cell Biol 13 : 1154–1160.
2. NachuryMV, SeeleyES, JinH (2010) Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier? Annu Rev Cell Dev Biol 26 : 59–87.
3. VincensiniL, BlisnickT, BastinP (2011) 1001 model organisms to study cilia and flagella. Biol Cell 103 : 109–130.
4. Bettencourt-DiasM, HildebrandtF, PellmanD, WoodsG, GodinhoSA (2011) Centrosomes and cilia in human disease. Trends Genet 27 : 307–315.
5. NiggEA, RaffJW (2009) Centrioles, centrosomes, and cilia in health and disease. Cell 139 : 663–678.
6. BarenzF, MayiloD, GrussOJ (2011) Centriolar satellites: busy orbits around the centrosome. Eur J Cell Biol 90 : 983–989.
7. BalczonR, BaoL, ZimmerWE (1994) PCM-1, A 228-kD centrosome autoantigen with a distinct cell cycle distribution. J Cell Biol 124 : 783–793.
8. KuboA, SasakiH, Yuba-KuboA, TsukitaS, ShiinaN (1999) Centriolar satellites: molecular characterization, ATP-dependent movement toward centrioles and possible involvement in ciliogenesis. J Cell Biol 147 : 969–980.
9. DammermannA, MerdesA (2002) Assembly of centrosomal proteins and microtubule organization depends on PCM-1. J Cell Biol 159 : 255–266.
10. KimJ, KrishnaswamiSR, GleesonJG (2008) CEP290 interacts with the centriolar satellite component PCM-1 and is required for Rab8 localization to the primary cilium. Hum Mol Genet 17 : 3796–3805.
11. KimJC, BadanoJL, SiboldS, EsmailMA, HillJ, et al. (2004) The Bardet-Biedl protein BBS4 targets cargo to the pericentriolar region and is required for microtubule anchoring and cell cycle progression. Nat Genet 36 : 462–470.
12. KodaniA, TonthatV, WuB, SutterlinC (2010) Par6 alpha interacts with the dynactin subunit p150 Glued and is a critical regulator of centrosomal protein recruitment. Mol Biol Cell 21 : 3376–3385.
13. LopesCA, ProsserSL, RomioL, HirstRA, O'CallaghanC, et al. (2011) Centriolar satellites are assembly points for proteins implicated in human ciliopathies, including oral-facial-digital syndrome 1. J Cell Sci 124 : 600–612.
14. StoweTR, WilkinsonCJ, IqbalA, StearnsT (2012) The centriolar satellite proteins Cep72 and Cep290 interact and are required for recruitment of BBS proteins to the cilium. Mol Biol Cell 23 : 3322–3335.
15. HallEA, KeighrenM, FordMJ, DaveyT, JarmanAP, et al. (2013) Acute versus chronic loss of mammalian Azi1/Cep131 results in distinct ciliary phenotypes. PLoS Genet 9: e1003928 doi:10.1371/journal.pgen.1003928
16. AndersenJS, WilkinsonCJ, MayorT, MortensenP, NiggEA, et al. (2003) Proteomic characterization of the human centrosome by protein correlation profiling. Nature 426 : 570–574.
17. StaplesCJ, MyersKN, BeveridgeRD, PatilAA, LeeAJ, et al. (2012) The centriolar satellite protein Cep131 is important for genome stability. J Cell Sci 125 : 4770–4779.
18. CzarneckiPG, ShahJV (2012) The ciliary transition zone: from morphology and molecules to medicine. Trends Cell Biol 22 : 201–210.
19. WilkinsonCJ, CarlM, HarrisWA (2009) Cep70 and Cep131 contribute to ciliogenesis in zebrafish embryos. BMC Cell Biol 10 : 17.
20. MaL, JarmanAP (2011) Dilatory is a Drosophila protein related to AZI1 (CEP131) that is located at the ciliary base and required for cilium formation. J Cell Sci 124 : 2622–2630.
21. GraserS, StierhofYD, LavoieSB, GassnerOS, LamlaS, et al. (2007) Cep164, a novel centriole appendage protein required for primary cilium formation. J Cell Biol 179 : 321–330.
22. RauchA, ThielCT, SchindlerD, WickU, CrowYJ, et al. (2008) Mutations in the pericentrin (PCNT) gene cause primordial dwarfism. Science 319 : 816–819.
23. KumarA, GirimajiSC, DuvvariMR, BlantonSH (2009) Mutations in STIL, encoding a pericentriolar and centrosomal protein, cause primary microcephaly. Am J Hum Genet 84 : 286–290.
24. GuernseyDL, JiangH, HussinJ, ArnoldM, BouyakdanK, et al. (2010) Mutations in centrosomal protein CEP152 in primary microcephaly families linked to MCPH4. Am J Hum Genet 87 : 40–51.
25. HussainMS, BaigSM, NeumannS, NurnbergG, FarooqM, et al. (2012) A truncating mutation of CEP135 causes primary microcephaly and disturbed centrosomal function. Am J Hum Genet 90 : 871–878.
26. MarthiensV, RujanoMA, PennetierC, TessierS, Paul-GilloteauxP, et al. (2013) Centrosome amplification causes microcephaly. Nat Cell Biol 15 : 731–740.
Štítky
Genetika Reprodukční medicína
Článek Interaction between and during Mammalian Jaw Patterning and in the Pathogenesis of SyngnathiaČlánek Clustering of Tissue-Specific Sub-TADs Accompanies the Regulation of Genes in Developing LimbsČlánek Transcription Factor Occupancy Can Mediate Active Turnover of DNA Methylation at Regulatory RegionsČlánek Tay Bridge Is a Negative Regulator of EGFR Signalling and Interacts with Erk and Mkp3 in the Wing
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 12- Akutní intermitentní porfyrie
- Souvislost haplotypu M2 genu pro annexin A5 s opakovanými reprodukčními ztrátami
- Vztah užívání alkoholu a mužské fertility
- Doc. Miloš Kubánek: Nemocní se srdeční amyloidózou jsou často skryti a sledováni pod jinými diagnózami
- Hysteroskopická resekce děložního septa zlepšuje šanci na graviditu žen s jinak nevysvětlenou infertilitou
-
Všechny články tohoto čísla
- Stressing the Importance of CHOP in Liver Cancer
- The AmAZI1ng Roles of Centriolar Satellites during Development
- Flies Get a Head Start on Meiosis
- Recommendations from Jane Gitschier's Bookshelf
- And Baby Makes Three: Genomic Imprinting in Plant Embryos
- Bugs in Transition: The Dynamic World of in Insects
- Defining the Role of ATP Hydrolysis in Mitotic Segregation of Bacterial Plasmids
- Synaptonemal Complex Components Promote Centromere Pairing in Pre-meiotic Germ Cells
- Cohesinopathies of a Feather Flock Together
- Genetic Recombination Is Targeted towards Gene Promoter Regions in Dogs
- Parathyroid-Specific Deletion of Unravels a Novel Calcineurin-Dependent FGF23 Signaling Pathway That Regulates PTH Secretion
- MAN1B1 Deficiency: An Unexpected CDG-II
- Phosphate Flow between Hybrid Histidine Kinases CheA and CheS Controls Cyst Formation
- Basolateral Mg Extrusion via CNNM4 Mediates Transcellular Mg Transport across Epithelia: A Mouse Model
- Truncation of Unsilences Paternal and Ameliorates Behavioral Defects in the Angelman Syndrome Mouse Model
- Autozygome Sequencing Expands the Horizon of Human Knockout Research and Provides Novel Insights into Human Phenotypic Variation
- Huntington's Disease Induced Cardiac Amyloidosis Is Reversed by Modulating Protein Folding and Oxidative Stress Pathways in the Heart
- Low Frequency Variants, Collapsed Based on Biological Knowledge, Uncover Complexity of Population Stratification in 1000 Genomes Project Data
- Targeted Ablation of and in Retinal Progenitor Cells Mimics Leber Congenital Amaurosis
- Genomic Imprinting in the Embryo Is Partly Regulated by PRC2
- Binary Cell Fate Decisions and Fate Transformation in the Larval Eye
- The Stress-Regulated Transcription Factor CHOP Promotes Hepatic Inflammatory Gene Expression, Fibrosis, and Oncogenesis
- A Global RNAi Screen Identifies a Key Role of Ceramide Phosphoethanolamine for Glial Ensheathment of Axons
- Functional Analysis of the Interdependence between DNA Uptake Sequence and Its Cognate ComP Receptor during Natural Transformation in Species
- Cross-Modulation of Homeostatic Responses to Temperature, Oxygen and Carbon Dioxide in
- Alcohol-Induced Histone Acetylation Reveals a Gene Network Involved in Alcohol Tolerance
- Molecular Characterization of Host-Specific Biofilm Formation in a Vertebrate Gut Symbiont
- CRIS—A Novel cAMP-Binding Protein Controlling Spermiogenesis and the Development of Flagellar Bending
- Dual Regulation of the Mitotic Exit Network (MEN) by PP2A-Cdc55 Phosphatase
- Expanding the Marine Virosphere Using Metagenomics
- Detection of Slipped-DNAs at the Trinucleotide Repeats of the Myotonic Dystrophy Type I Disease Locus in Patient Tissues
- Interaction between and during Mammalian Jaw Patterning and in the Pathogenesis of Syngnathia
- Mutations in the UQCC1-Interacting Protein, UQCC2, Cause Human Complex III Deficiency Associated with Perturbed Cytochrome Protein Expression
- Reactivation of Chromosomally Integrated Human Herpesvirus-6 by Telomeric Circle Formation
- Anoxia-Reoxygenation Regulates Mitochondrial Dynamics through the Hypoxia Response Pathway, SKN-1/Nrf, and Stomatin-Like Protein STL-1/SLP-2
- The Midline Protein Regulates Axon Guidance by Blocking the Reiteration of Neuroblast Rows within the Drosophila Ventral Nerve Cord
- Tomato Yield Heterosis Is Triggered by a Dosage Sensitivity of the Florigen Pathway That Fine-Tunes Shoot Architecture
- Selection on Plant Male Function Genes Identifies Candidates for Reproductive Isolation of Yellow Monkeyflowers
- Role of Tomato Lipoxygenase D in Wound-Induced Jasmonate Biosynthesis and Plant Immunity to Insect Herbivores
- Meiotic Cohesin SMC1β Provides Prophase I Centromeric Cohesion and Is Required for Multiple Synapsis-Associated Functions
- Identification of Sphingolipid Metabolites That Induce Obesity via Misregulation of Appetite, Caloric Intake and Fat Storage in
- Genome-Wide Screen Reveals Replication Pathway for Quasi-Palindrome Fragility Dependent on Homologous Recombination
- Histone Methylation Restrains the Expression of Subtype-Specific Genes during Terminal Neuronal Differentiation in
- A Novel Intergenic ETnII-β Insertion Mutation Causes Multiple Malformations in Mice
- The NuRD Chromatin-Remodeling Enzyme CHD4 Promotes Embryonic Vascular Integrity by Transcriptionally Regulating Extracellular Matrix Proteolysis
- A Domesticated Transposase Interacts with Heterochromatin and Catalyzes Reproducible DNA Elimination in
- Acute Versus Chronic Loss of Mammalian Results in Distinct Ciliary Phenotypes
- MBD3 Localizes at Promoters, Gene Bodies and Enhancers of Active Genes
- Positive and Negative Regulation of Gli Activity by Kif7 in the Zebrafish Embryo
- A Hereditary Spastic Paraplegia Mouse Model Supports a Role of ZFYVE26/SPASTIZIN for the Endolysosomal System
- The CCR4-NOT Complex Mediates Deadenylation and Degradation of Stem Cell mRNAs and Promotes Planarian Stem Cell Differentiation
- Reconstructing Native American Migrations from Whole-Genome and Whole-Exome Data
- Contributions of Protein-Coding and Regulatory Change to Adaptive Molecular Evolution in Murid Rodents
- Comprehensive Analysis of Transcriptome Variation Uncovers Known and Novel Driver Events in T-Cell Acute Lymphoblastic Leukemia
- A -Acting Protein Effect Causes Severe Eye Malformation in the Mouse
- Clustering of Tissue-Specific Sub-TADs Accompanies the Regulation of Genes in Developing Limbs
- Germline Progenitors Escape the Widespread Phenomenon of Homolog Pairing during Development
- Transcription Factor Occupancy Can Mediate Active Turnover of DNA Methylation at Regulatory Regions
- Somatic mtDNA Mutation Spectra in the Aging Human Putamen
- ESCRT-I Mediates FLS2 Endosomal Sorting and Plant Immunity
- Ethylene Promotes Hypocotyl Growth and HY5 Degradation by Enhancing the Movement of COP1 to the Nucleus in the Light
- The PAF Complex and Prf1/Rtf1 Delineate Distinct Cdk9-Dependent Pathways Regulating Transcription Elongation in Fission Yeast
- Dual Regulation of Gene Expression Mediated by Extended MAPK Activation and Salicylic Acid Contributes to Robust Innate Immunity in
- Quantifying Missing Heritability at Known GWAS Loci
- Smc5/6-Mms21 Prevents and Eliminates Inappropriate Recombination Intermediates in Meiosis
- Smc5/6 Coordinates Formation and Resolution of Joint Molecules with Chromosome Morphology to Ensure Meiotic Divisions
- Tay Bridge Is a Negative Regulator of EGFR Signalling and Interacts with Erk and Mkp3 in the Wing
- Meiotic Crossover Control by Concerted Action of Rad51-Dmc1 in Homolog Template Bias and Robust Homeostatic Regulation
- Active Transport and Diffusion Barriers Restrict Joubert Syndrome-Associated ARL13B/ARL-13 to an Inv-like Ciliary Membrane Subdomain
- An Regulatory Circuit Modulates /Wnt Signaling and Determines the Size of the Midbrain Dopaminergic Progenitor Pool
- Variants Induce Differential Protection to Viruses in : A Phenotypic and Phylogenomic Analysis
- Base Pairing Interaction between 5′- and 3′-UTRs Controls mRNA Translation in
- Evidence That Masking of Synapsis Imperfections Counterbalances Quality Control to Promote Efficient Meiosis
- Insulin/IGF-Regulated Size Scaling of Neuroendocrine Cells Expressing the bHLH Transcription Factor in
- Sumoylated NHR-25/NR5A Regulates Cell Fate during Vulval Development
- TATN-1 Mutations Reveal a Novel Role for Tyrosine as a Metabolic Signal That Influences Developmental Decisions and Longevity in
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The NuRD Chromatin-Remodeling Enzyme CHD4 Promotes Embryonic Vascular Integrity by Transcriptionally Regulating Extracellular Matrix Proteolysis
- Mutations in the UQCC1-Interacting Protein, UQCC2, Cause Human Complex III Deficiency Associated with Perturbed Cytochrome Protein Expression
- The Midline Protein Regulates Axon Guidance by Blocking the Reiteration of Neuroblast Rows within the Drosophila Ventral Nerve Cord
- Tomato Yield Heterosis Is Triggered by a Dosage Sensitivity of the Florigen Pathway That Fine-Tunes Shoot Architecture
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



