-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaStressing the Importance of CHOP in Liver Cancer
article has not abstract
Published in the journal: . PLoS Genet 9(12): e32767. doi:10.1371/journal.pgen.1004045
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1004045Summary
article has not abstract
Hepatocellular carcinoma (HCC) is the third leading cause of cancer death and the fifth most common solid tumor worldwide [1], [2]. Liver tumorigenesis is a multistep process in which external stimuli such as chronic inflammation or cirrhosis lead to the development of clonal populations of dysplastic hepatocytes that accumulate genetic changes and evolve into malignant foci [2]. Among the most common risk factors for HCC pathogenesis include viral hepatitis, alcoholism, and obesity [1], [3].
The same insults that predispose to HCC are known to induce endoplasmic reticulum (ER) stress pathways. One such pathway, known as the unfolded protein response (UPR), is triggered by the accumulation of incompletely folded proteins in the ER lumen [4]–[6]. Stimulation of the UPR results in the activation of three transmembrane proteins that induce downstream effectors to alter gene expression and ultimately modulate ER function. One of these UPR transmembrane proteins is protein kinase RNA (PKR)-like ER kinase (PERK), which phosphorylates eIF2α, leading to a transient translational blockade. A related pathway that shares transcriptional targets with the UPR is the integrated stress response (ISR) pathway. When triggered by viral infection or amino acid starvation the ISR also initiates eIF2α-dependent signaling events [7]. Although the UPR and ISR pathways are active in distinct human tumor types and the UPR is implicated in HCC [8]–[10], their relative contribution to the pathogenesis of HCC has remained uncharacterized.
In this issue of PLOS Genetics, Rutkowski and colleagues (DeZwaan-McCabe et al., [11]) sought to determine whether the UPR pathway was induced in murine liver tumors that developed in a Sleeping Beauty (SB) transposon-induced insertional mutagenesis screen [12], [13]. The application of transposon-based approaches to cancer gene identification provides a powerful opportunity to examine the consequences of specific mutations in the context of in vivo tumor development [14]. Whole transcriptome sequencing of liver tumors generated in an SB-mediated liver tumorigenesis screen identified an induction of C/EBP Homologous Protein (CHOP), a stress-regulated transcription factor, in multiple SB-induced tumors. Upon further analysis, components of the two PERK-independent arms of the UPR pathway were not altered at the transcript level, leading the authors to further investigate the role of the ISR and CHOP in HCC.
CHOP, which has a diverse repertoire of transcriptional targets and modes of transcriptional modulation, was previously known to mediate apoptosis in response to ER stress [15]–[17]. Accordingly, several studies implicate CHOP as a putative tumor suppressor. In contrast to this, chromosomal translocations fusing CHOP to FUS/TLS and EWS have been identified in several cancers, hinting that CHOP may also play an oncogenic role in tumorigenesis in certain contexts [18], [19].
The Integrated Stress Response in HCC: Not Just CHOPped Liver
Consistent with a pro-oncogenic role for CHOP, McCabe et al. [11] hypothesized that CHOP contributes to the pathogenesis of HCC in vivo by promoting apoptosis, inflammation, fibrosis, compensatory proliferation, and development of liver tumors (Figure 1). Consistent with this hypothesis, global deletion of Chop in mice attenuated these sequelae following treatment with the chemical carcinogen diethylnitrosamine (DEN). Following administration of the hepatotoxin carbon tetrachloride in wild-type mice, the authors observed an association of CHOP-positive foci with increased fibrosis. Staining of human HCC samples with a CHOP antibody revealed CHOP-positive foci in tumors and significantly less staining in normal liver. These results suggest that activation of CHOP promotes HCC progression. Moreover, these findings provide the first link between CHOP and liver oncogenesis.
Fig. 1. The role of CHOP in HCC pathogenesis. 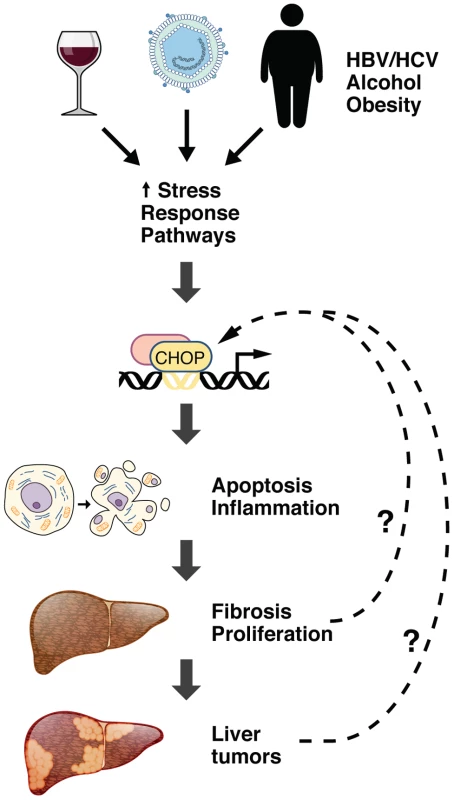
Hepatocyte injury, including toxicity from viral hepatitis, obesity, or alcoholism, promotes the induction of canonical ER stress pathways, including the UPR and ISR. This results in induction of the CHOP transcription factor, which stimulates cell death and provokes an inflammatory response. Inflammation may further stimulate fibrosis and compensatory proliferation, leading to the development of liver tumor nodules. It is also possible that stress caused by fibrosis and proliferation of dysplastic hepatocytes in HCC may induce expression of CHOP, further amplifying its effect on tumorigenesis. Gene expression profiling of liver mRNA from Chop-null and wild-type mice in the absence of hepatotoxic challenge revealed that deletion of Chop reduced the levels of basal inflammatory signaling genes. This is consistent with an important role for CHOP in promoting inflammation after liver injury. Interestingly, genes encoding ribosomal proteins were significantly increased in liver tumors derived from DEN-treated Chop-null animals relative to tumors that developed in wild-type animals. None of these genes harbored canonical CHOP binding sites, leaving the question of how this occurs unresolved. This represents the first evidence that CHOP can reduce translation by suppressing expression of ribosomal proteins. However, this is consistent with the general role of the ISR as an inhibitor of translation. Further studies are needed to fully elucidate how CHOP affects the translational machinery and the resulting effects on translational output.
The authors of this study present several lines of evidence consistent with an oncogenic role for CHOP in promoting HCC. Their findings suggest that induction of CHOP is a common feature of liver cancer caused by viral infection, alcoholism, and obesity. Recently, a novel framework has been proposed suggesting that cancer cells exhibit hallmarks of chronic stress induced by DNA damage, proteotoxic stress created by accumulation of unfolded protein aggregates, metabolic stress, and oxidative stress [20]. Additional experiments are therefore warranted to determine whether CHOP induction is a causative event that promotes liver tumorigenesis and/or a consequence of the immense cellular stress that cells are subjected to as hepatocytes acquire mutations and undergo the multistep progression to HCC. This will require the generation of inducible and tissue-specific transgenic mouse models, which are currently lacking. Temporal manipulation of CHOP expression in the liver could also tease out whether CHOP promotes the initiation of HCC, or if it enhances tumorigenesis after dysplastic liver nodules form.
Given the resistance to HCC-associated phenotypes observed in Chop-null animals and the discovery of human HCC-associated CHOP expression, this stress-responsive transcription factor may serve as a useful biomarker for liver cancer. However, several important questions remain. For example, is CHOP-mediated apoptosis of hepatocytes the major initiating event that triggers the cycle of subsequent inflammation, fibrosis, and ultimately HCC initiation? Or does hepatocyte-specific expression of CHOP indirectly stimulate inflammation, perhaps through cytokine release, initiating the inflammation-tumorigenesis sequence? The analysis of CHOP target genes that mediate these effects in HCC will shed light on these issues. Perhaps most intriguingly, the identity of the eIF2α kinase that leads to CHOP induction in liver cancer remains unknown. PERK is one candidate, and it would be useful to determine whether PERK inhibitors will blunt CHOP expression and ameliorate HCC in mouse models. Thus, further investigation of the pro - and anti-oncogenic functions of CHOP is likely to reveal important new insights into the pathogenesis of liver cancer and other tumor types.
Zdroje
1. AltekruseSF, McGlynnKA, ReichmanME (2009) Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol 27 : 1485–1491.
2. ThorgeirssonSS, GrishamJW (2002) Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet 31 : 339–346.
3. StarleyBQ, CalcagnoCJ, HarrisonSA (2010) Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology 51 : 1820–1832.
4. HardingHP, NovoaI, ZhangY, ZengH, WekR, et al. (2000) Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell 6 : 1099–1108.
5. RutkowskiDT, HegdeRS (2010) Regulation of basal cellular physiology by the homeostatic unfolded protein response. J Cell Biol 189 : 783–794.
6. WalterP, RonD (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 334 : 1081–1086.
7. HardingHP, ZhangY, ZengH, NovoaI, LuPD, et al. (2003) An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11 : 619–633.
8. Bobrovnikova-MarjonE, GrigoriadouC, PytelD, ZhangF, YeJ, et al. (2010) PERK promotes cancer cell proliferation and tumor growth by limiting oxidative DNA damage. Oncogene 29 : 3881–3895.
9. DongD, NiM, LiJ, XiongS, YeW, et al. (2008) Critical role of the stress chaperone GRP78/BiP in tumor proliferation, survival, and tumor angiogenesis in transgene-induced mammary tumor development. Cancer Res 68 : 498–505.
10. ShudaM, KondohN, ImazekiN, TanakaK, OkadaT, et al. (2003) Activation of the ATF6, XBP1 and grp78 genes in human hepatocellular carcinoma: a possible involvement of the ER stress pathway in hepatocarcinogenesis. J Hepatol 38 : 605–614.
11. DeZwaan-McCabeD, RiordanJD, ArensdorfAM, IcardiMS, DupuyAJ, et al. (2013) The stress-regulated transcription factor CHOP promotes hepatic inflammatory gene expression, fibrosis, and oncogenesis. PLoS Genet 9: e1003937 doi:10.1371/journal.pgen.1003937
12. DupuyAJ, RogersLM, KimJ, NannapaneniK, StarrTK, et al. (2009) A modified sleeping beauty transposon system that can be used to model a wide variety of human cancers in mice. Cancer Res 69 : 8150–8156.
13. RiordanJD, KengVW, TschidaBR, ScheetzTE, BellJB, et al. (2013) Identification of Rtl1, a retrotransposon-derived imprinted gene, as a novel driver of hepatocarcinogenesis. PLoS Genet 9: e1003441 doi:10.1371/journal.pgen.1003441
14. O'DonnellKA, KengVW, YorkB, ReinekeEL, SeoD, et al. (2012) A Sleeping Beauty mutagenesis screen reveals a tumor suppressor role for Ncoa2/Src-2 in liver cancer. Proc Natl Acad Sci U S A 109: E1377–1386.
15. MarciniakSJ, YunCY, OyadomariS, NovoaI, ZhangY, et al. (2004) CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev 18 : 3066–3077.
16. YamaguchiH, WangHG (2004) CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J Biol Chem 279 : 45495–45502.
17. ZinsznerH, KurodaM, WangX, BatchvarovaN, LightfootRT, et al. (1998) CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev 12 : 982–995.
18. CrozatA, AmanP, MandahlN, RonD (1993) Fusion of CHOP to a novel RNA-binding protein in human myxoid liposarcoma. Nature 363 : 640–644.
19. RabbittsTH, ForsterA, LarsonR, NathanP (1993) Fusion of the dominant negative transcription regulator CHOP with a novel gene FUS by translocation t(12;16) in malignant liposarcoma. Nat Genet 4 : 175–180.
20. LuoJ, SoliminiNL, ElledgeSJ (2009) Principles of cancer therapy: oncogene and non-oncogene addiction. Cell 136 : 823–837.
Štítky
Genetika Reprodukční medicína
Článek Interaction between and during Mammalian Jaw Patterning and in the Pathogenesis of SyngnathiaČlánek Clustering of Tissue-Specific Sub-TADs Accompanies the Regulation of Genes in Developing LimbsČlánek Transcription Factor Occupancy Can Mediate Active Turnover of DNA Methylation at Regulatory RegionsČlánek Tay Bridge Is a Negative Regulator of EGFR Signalling and Interacts with Erk and Mkp3 in the Wing
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 12- Souvislost haplotypu M2 genu pro annexin A5 s opakovanými reprodukčními ztrátami
- Akutní intermitentní porfyrie
- Příjem alkoholu a menstruační cyklus
- Hysteroskopická resekce děložního septa zlepšuje šanci na graviditu žen s jinak nevysvětlenou infertilitou
- Transfer zmraženého embrya zlepšuje výsledky IVF
-
Všechny články tohoto čísla
- Stressing the Importance of CHOP in Liver Cancer
- The AmAZI1ng Roles of Centriolar Satellites during Development
- Flies Get a Head Start on Meiosis
- Recommendations from Jane Gitschier's Bookshelf
- And Baby Makes Three: Genomic Imprinting in Plant Embryos
- Bugs in Transition: The Dynamic World of in Insects
- Defining the Role of ATP Hydrolysis in Mitotic Segregation of Bacterial Plasmids
- Synaptonemal Complex Components Promote Centromere Pairing in Pre-meiotic Germ Cells
- Cohesinopathies of a Feather Flock Together
- Genetic Recombination Is Targeted towards Gene Promoter Regions in Dogs
- Parathyroid-Specific Deletion of Unravels a Novel Calcineurin-Dependent FGF23 Signaling Pathway That Regulates PTH Secretion
- MAN1B1 Deficiency: An Unexpected CDG-II
- Phosphate Flow between Hybrid Histidine Kinases CheA and CheS Controls Cyst Formation
- Basolateral Mg Extrusion via CNNM4 Mediates Transcellular Mg Transport across Epithelia: A Mouse Model
- Truncation of Unsilences Paternal and Ameliorates Behavioral Defects in the Angelman Syndrome Mouse Model
- Autozygome Sequencing Expands the Horizon of Human Knockout Research and Provides Novel Insights into Human Phenotypic Variation
- Huntington's Disease Induced Cardiac Amyloidosis Is Reversed by Modulating Protein Folding and Oxidative Stress Pathways in the Heart
- Low Frequency Variants, Collapsed Based on Biological Knowledge, Uncover Complexity of Population Stratification in 1000 Genomes Project Data
- Targeted Ablation of and in Retinal Progenitor Cells Mimics Leber Congenital Amaurosis
- Genomic Imprinting in the Embryo Is Partly Regulated by PRC2
- Binary Cell Fate Decisions and Fate Transformation in the Larval Eye
- The Stress-Regulated Transcription Factor CHOP Promotes Hepatic Inflammatory Gene Expression, Fibrosis, and Oncogenesis
- A Global RNAi Screen Identifies a Key Role of Ceramide Phosphoethanolamine for Glial Ensheathment of Axons
- Functional Analysis of the Interdependence between DNA Uptake Sequence and Its Cognate ComP Receptor during Natural Transformation in Species
- Cross-Modulation of Homeostatic Responses to Temperature, Oxygen and Carbon Dioxide in
- Alcohol-Induced Histone Acetylation Reveals a Gene Network Involved in Alcohol Tolerance
- Molecular Characterization of Host-Specific Biofilm Formation in a Vertebrate Gut Symbiont
- CRIS—A Novel cAMP-Binding Protein Controlling Spermiogenesis and the Development of Flagellar Bending
- Dual Regulation of the Mitotic Exit Network (MEN) by PP2A-Cdc55 Phosphatase
- Expanding the Marine Virosphere Using Metagenomics
- Detection of Slipped-DNAs at the Trinucleotide Repeats of the Myotonic Dystrophy Type I Disease Locus in Patient Tissues
- Interaction between and during Mammalian Jaw Patterning and in the Pathogenesis of Syngnathia
- Mutations in the UQCC1-Interacting Protein, UQCC2, Cause Human Complex III Deficiency Associated with Perturbed Cytochrome Protein Expression
- Reactivation of Chromosomally Integrated Human Herpesvirus-6 by Telomeric Circle Formation
- Anoxia-Reoxygenation Regulates Mitochondrial Dynamics through the Hypoxia Response Pathway, SKN-1/Nrf, and Stomatin-Like Protein STL-1/SLP-2
- The Midline Protein Regulates Axon Guidance by Blocking the Reiteration of Neuroblast Rows within the Drosophila Ventral Nerve Cord
- Tomato Yield Heterosis Is Triggered by a Dosage Sensitivity of the Florigen Pathway That Fine-Tunes Shoot Architecture
- Selection on Plant Male Function Genes Identifies Candidates for Reproductive Isolation of Yellow Monkeyflowers
- Role of Tomato Lipoxygenase D in Wound-Induced Jasmonate Biosynthesis and Plant Immunity to Insect Herbivores
- Meiotic Cohesin SMC1β Provides Prophase I Centromeric Cohesion and Is Required for Multiple Synapsis-Associated Functions
- Identification of Sphingolipid Metabolites That Induce Obesity via Misregulation of Appetite, Caloric Intake and Fat Storage in
- Genome-Wide Screen Reveals Replication Pathway for Quasi-Palindrome Fragility Dependent on Homologous Recombination
- Histone Methylation Restrains the Expression of Subtype-Specific Genes during Terminal Neuronal Differentiation in
- A Novel Intergenic ETnII-β Insertion Mutation Causes Multiple Malformations in Mice
- The NuRD Chromatin-Remodeling Enzyme CHD4 Promotes Embryonic Vascular Integrity by Transcriptionally Regulating Extracellular Matrix Proteolysis
- A Domesticated Transposase Interacts with Heterochromatin and Catalyzes Reproducible DNA Elimination in
- Acute Versus Chronic Loss of Mammalian Results in Distinct Ciliary Phenotypes
- MBD3 Localizes at Promoters, Gene Bodies and Enhancers of Active Genes
- Positive and Negative Regulation of Gli Activity by Kif7 in the Zebrafish Embryo
- A Hereditary Spastic Paraplegia Mouse Model Supports a Role of ZFYVE26/SPASTIZIN for the Endolysosomal System
- The CCR4-NOT Complex Mediates Deadenylation and Degradation of Stem Cell mRNAs and Promotes Planarian Stem Cell Differentiation
- Reconstructing Native American Migrations from Whole-Genome and Whole-Exome Data
- Contributions of Protein-Coding and Regulatory Change to Adaptive Molecular Evolution in Murid Rodents
- Comprehensive Analysis of Transcriptome Variation Uncovers Known and Novel Driver Events in T-Cell Acute Lymphoblastic Leukemia
- A -Acting Protein Effect Causes Severe Eye Malformation in the Mouse
- Clustering of Tissue-Specific Sub-TADs Accompanies the Regulation of Genes in Developing Limbs
- Germline Progenitors Escape the Widespread Phenomenon of Homolog Pairing during Development
- Transcription Factor Occupancy Can Mediate Active Turnover of DNA Methylation at Regulatory Regions
- Somatic mtDNA Mutation Spectra in the Aging Human Putamen
- ESCRT-I Mediates FLS2 Endosomal Sorting and Plant Immunity
- Ethylene Promotes Hypocotyl Growth and HY5 Degradation by Enhancing the Movement of COP1 to the Nucleus in the Light
- The PAF Complex and Prf1/Rtf1 Delineate Distinct Cdk9-Dependent Pathways Regulating Transcription Elongation in Fission Yeast
- Dual Regulation of Gene Expression Mediated by Extended MAPK Activation and Salicylic Acid Contributes to Robust Innate Immunity in
- Quantifying Missing Heritability at Known GWAS Loci
- Smc5/6-Mms21 Prevents and Eliminates Inappropriate Recombination Intermediates in Meiosis
- Smc5/6 Coordinates Formation and Resolution of Joint Molecules with Chromosome Morphology to Ensure Meiotic Divisions
- Tay Bridge Is a Negative Regulator of EGFR Signalling and Interacts with Erk and Mkp3 in the Wing
- Meiotic Crossover Control by Concerted Action of Rad51-Dmc1 in Homolog Template Bias and Robust Homeostatic Regulation
- Active Transport and Diffusion Barriers Restrict Joubert Syndrome-Associated ARL13B/ARL-13 to an Inv-like Ciliary Membrane Subdomain
- An Regulatory Circuit Modulates /Wnt Signaling and Determines the Size of the Midbrain Dopaminergic Progenitor Pool
- Variants Induce Differential Protection to Viruses in : A Phenotypic and Phylogenomic Analysis
- Base Pairing Interaction between 5′- and 3′-UTRs Controls mRNA Translation in
- Evidence That Masking of Synapsis Imperfections Counterbalances Quality Control to Promote Efficient Meiosis
- Insulin/IGF-Regulated Size Scaling of Neuroendocrine Cells Expressing the bHLH Transcription Factor in
- Sumoylated NHR-25/NR5A Regulates Cell Fate during Vulval Development
- TATN-1 Mutations Reveal a Novel Role for Tyrosine as a Metabolic Signal That Influences Developmental Decisions and Longevity in
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The NuRD Chromatin-Remodeling Enzyme CHD4 Promotes Embryonic Vascular Integrity by Transcriptionally Regulating Extracellular Matrix Proteolysis
- Mutations in the UQCC1-Interacting Protein, UQCC2, Cause Human Complex III Deficiency Associated with Perturbed Cytochrome Protein Expression
- The Midline Protein Regulates Axon Guidance by Blocking the Reiteration of Neuroblast Rows within the Drosophila Ventral Nerve Cord
- Tomato Yield Heterosis Is Triggered by a Dosage Sensitivity of the Florigen Pathway That Fine-Tunes Shoot Architecture
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



