-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Evolutionary Tuning of Protein Expression Levels of a Positively Autoregulated Two-Component System
Cellular adaptation relies on the development of proper regulatory schemes for accurate control of gene expression levels in response to environmental cues. Over - or under-expression can lead to diminished cell fitness due to increased costs or insufficient benefits. Positive autoregulation is a common regulatory scheme that controls protein expression levels and gives rise to essential features in diverse signaling systems, yet its roles in cell fitness are less understood. It remains largely unknown how much protein expression is ‘appropriate’ for optimal cell fitness under specific extracellular conditions and how the dynamic environment shapes the regulatory scheme to reach appropriate expression levels. Here, we investigate the correlation of cell fitness and output response with protein expression levels of the E. coli PhoB/PhoR two-component system (TCS). In response to phosphate (Pi)-depletion, the PhoB/PhoR system activates genes involved in phosphorus assimilation as well as genes encoding themselves, similarly to many other positively autoregulated TCSs. We developed a bacteria competition assay in continuous cultures and discovered that different Pi conditions have conflicting requirements of protein expression levels for optimal cell fitness. Pi-replete conditions favored cells with low levels of PhoB/PhoR while Pi-deplete conditions selected for cells with high levels of PhoB/PhoR. These two levels matched PhoB/PhoR concentrations achieved via positive autoregulation in wild-type cells under Pi-replete and -deplete conditions, respectively. The fitness optimum correlates with the wild-type expression level, above which the phosphorylation output saturates, thus further increase in expression presumably provides no additional benefits. Laboratory evolution experiments further indicate that cells with non-ideal protein levels can evolve toward the optimal levels with diverse mutational strategies. Our results suggest that the natural protein expression levels and feedback regulatory schemes of TCSs are evolved to match the phosphorylation output of the system, which is determined by intrinsic activities of TCS proteins.
Published in the journal: . PLoS Genet 9(10): e32767. doi:10.1371/journal.pgen.1003927
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003927Summary
Cellular adaptation relies on the development of proper regulatory schemes for accurate control of gene expression levels in response to environmental cues. Over - or under-expression can lead to diminished cell fitness due to increased costs or insufficient benefits. Positive autoregulation is a common regulatory scheme that controls protein expression levels and gives rise to essential features in diverse signaling systems, yet its roles in cell fitness are less understood. It remains largely unknown how much protein expression is ‘appropriate’ for optimal cell fitness under specific extracellular conditions and how the dynamic environment shapes the regulatory scheme to reach appropriate expression levels. Here, we investigate the correlation of cell fitness and output response with protein expression levels of the E. coli PhoB/PhoR two-component system (TCS). In response to phosphate (Pi)-depletion, the PhoB/PhoR system activates genes involved in phosphorus assimilation as well as genes encoding themselves, similarly to many other positively autoregulated TCSs. We developed a bacteria competition assay in continuous cultures and discovered that different Pi conditions have conflicting requirements of protein expression levels for optimal cell fitness. Pi-replete conditions favored cells with low levels of PhoB/PhoR while Pi-deplete conditions selected for cells with high levels of PhoB/PhoR. These two levels matched PhoB/PhoR concentrations achieved via positive autoregulation in wild-type cells under Pi-replete and -deplete conditions, respectively. The fitness optimum correlates with the wild-type expression level, above which the phosphorylation output saturates, thus further increase in expression presumably provides no additional benefits. Laboratory evolution experiments further indicate that cells with non-ideal protein levels can evolve toward the optimal levels with diverse mutational strategies. Our results suggest that the natural protein expression levels and feedback regulatory schemes of TCSs are evolved to match the phosphorylation output of the system, which is determined by intrinsic activities of TCS proteins.
Introduction
Cells constantly face challenges from a wide variety of environmental perturbations that require evolution of appropriate mechanisms for adaptive responses. Cellular adaptation is often through modulation of gene expression that benefits cells under specific conditions. However, expressing proteins using cellular resources carries a fitness cost. Hence, evolutionary adaptation relies on development of proper signaling and gene regulatory schemes to produce appropriate amounts of proteins under particular environmental conditions, balancing cost and benefit to maximize fitness. Bacteria use the two-component system (TCS) as one of the major signal transduction schemes to respond to environmental cues. A sensor histidine kinase (HK), whose autokinase, phosphotransferase and/or phosphatase activities can be tuned by input signals, adjusts the phosphorylation level of its cognate response regulator (RR), ultimately determining output responses, mostly via transcriptional regulation [1]–[3]. Naturally, not only the physico-chemical properties but also the quantities of TCS proteins can influence the output, and thus could be subject to evolutionary optimization. Adaptation to various environments requires appropriate expression levels of TCS-regulated genes as well as genes encoding TCS proteins themselves to provide fitness advantages. How different environments shape the fitness profile and select particular TCS quantities remains largely unknown.
In many cases, the quantities of HK and RR are autoregulated. In E. coli, nearly half of the 30 RR transcription factors auto-activate expression of operons encoding themselves [4]. Genetic mechanisms and regulatory features of this feedback control have been explored [4]–[8] but the potential fitness benefit of TCS autoregulation in environmental adaptation are less examined experimentally. Positive feedback can lead to an ultra-sensitive switch-like response, an increase of regulatory capacity, a delay of response time, and promotion of a bistable system that can yield all-or-none output [4], [5], [9]. The ability to switch between two discrete “ON” and “OFF” states or to defer responses after multiple cell division cycles could be beneficial to some differentiation and developmental processes [10]–[12]. Elimination of the positive feedback has been shown to affect regulatory and temporal precision of these developmental processes [12], [13]. However, a binary or greatly delayed response is not likely preferred by all signaling systems; rather, a continuous well-defined output in relation to input signals allows cells to make accurate and prompt adjustments. It has been suggested that most TCSs tend to be monostable and the TCS autoregulatory architecture is distinct from conventional positive feedback loops due to the negative phosphatase activities of bifunctional HKs [6], [7], [14]. Constitutively expressed TCSs often complement the loss of autoregulated systems without causing apparent differences in steady-state outputs under laboratory conditions [7], [15]–[19]. This raises the question of what evolutionary advantages autoregulation brings over constitutive expression in these systems and demands a comprehensive mapping of cell fitness at different TCS expression levels to understand the evolution of TCS autoregulation.
Fitness benefits of expressing TCS regulators rely upon the concerted transcription of ensemble of TCS-regulated output genes. The fitness landscape may well be correlated with TCS concentration-dependent output profiles. Intuitively, selection should produce TCSs that in environments of stimulation will have optimal concentrations of TCS proteins to elicit sufficient output to offset the cost of TCS expression. Autoregulation reflects the need for different optimal TCS levels in different environments. To examine the dependence of fitness on TCS expression levels and output responses, the autoregulated E. coli PhoB/PhoR system was used as a model system because the relation between its output phosphorylation profiles and protein levels has been well defined [19]. The PhoB/PhoR system activates genes responsible for assimilation of phosphorus in response to limitation of environmental phosphate (Pi) concentrations [20], [21]. Phosphorylated RR, PhoB, also binds to the pho box at its own promoter and stimulates the expression of PhoB and PhoR (Figure 1A). A ∼20 fold increase of PhoB concentration has been observed upon Pi-depletion. Replacing the autoregulatory phoB promoter with IPTG-inducible promoters allowed the characterization of phosphorylation profiles at different PhoB/PhoR levels [19].
Fig. 1. Autoregulation of PhoB/PhoR amplifies the graded output response. 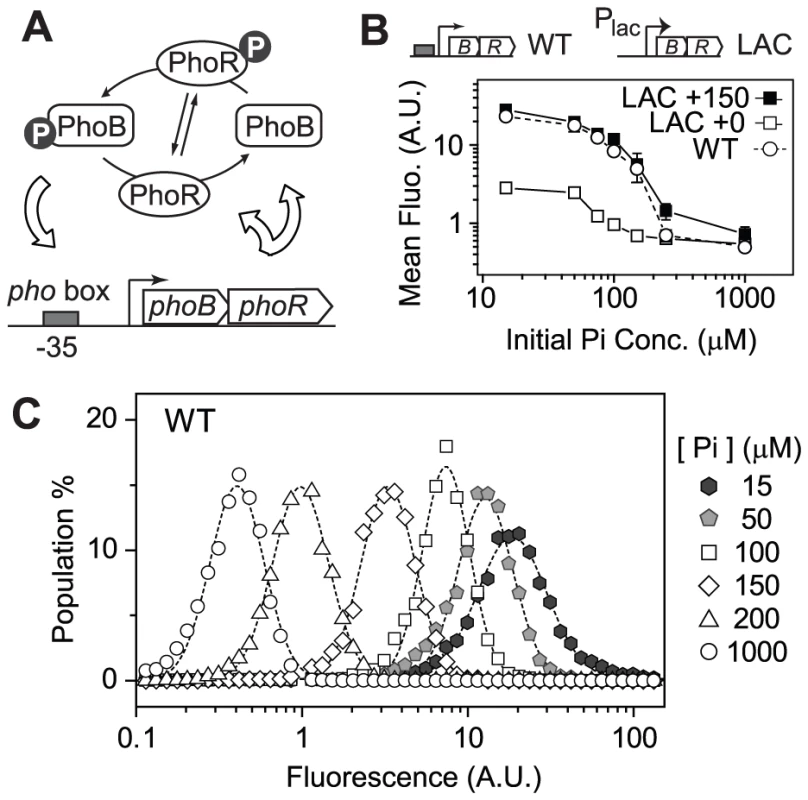
(A) Positive autoregulation of the PhoB/PhoR system. (B) PhoB-regulated transcription output of autoregulated (WT) and non-autoregulated (LAC) strains. RU1465 (WT) and RU1653 (LAC) carrying a phoA-yfp reporter were grown in MOPs medium with indicated initial Pi concentrations. Depletion of Pi led to activation of the phoA-yfp reporter and mean fluorescence of ∼20000 cells was determined. IPTG concentrations of 0 and 150 µM were used to induce PhoB expression in the LAC strain to achieve two different constant levels, corresponding to basal and autoregulated WT PhoB concentrations, respectively (Table S1). Error bars are SDs of three independent experiments and unseen error bars are smaller than symbols. (C) Population distribution of single-cell output responses of RU1465 (WT) at indicated initial Pi concentrations. Dashed lines represent lognormal fit of cell population distribution. Here we report comparison of output responses of individual cells for the autoregulated and constitutively expressed PhoB/PhoR system to examine whether bistability exists for the autoregulated WT system and how autoregulation contributes to cell fitness. The correlation of output to PhoB/PhoR levels prompted a thorough examination of cell fitness at different constitutive PhoB/PhoR levels in the absence and presence of stimuli. A competition assay in continuous cultures was developed for revealing the dependence of cell fitness on phosphorylation output and PhoB/PhoR expression levels. Under Pi-replete conditions high constitutive expression of PhoB/PhoR caused a decrease in fitness. In contrast, under Pi-deplete conditions, cell fitness peaked at an expression level close to the wild-type (WT) PhoB/PhoR concentration, at which the phosphorylation output starts to plateau. Further increase of expression apparently no longer offers sufficient advantages to overcome the cost of protein expression. To investigate whether bacteria can tune their expression levels to optimality through evolution, laboratory evolution experiments were performed for cells with unfavorable amounts of proteins under specific Pi conditions. These conditions led to evolution of diverse mutants that all shifted protein expression toward optimal levels to have greater fitness. Our results demonstrate that expression levels of TCS proteins are evolutionarily optimized and the autoregulatory scheme of the PhoB/PhoR system allows cells to adapt to both Pi - replete and -deplete conditions by expressing different optimal levels of TCS proteins to balance the cost and benefit.
Results
Graded Response for the Autoregulated PhoB/PhoR System
To examine output responses of single cells, the gene encoding yellow fluorescent protein (yfp) was fused to the promoter of the PhoB-regulated gene phoA and placed into the chromosome. The resulting strain still carries the original copy of phoA encoding an alkaline phosphatase (AP) and showed identical AP response curves to Pi concentrations as the WT strain (Figure S1A). YFP fluorescence followed a similar increasing trend as AP activities when the starting Pi concentration in the medium decreased (Figure 1B). Once the phoBR operon was placed behind a non-autoregulatory lac promoter in the LAC strain, mean fluorescence output clearly depended on expression levels of PhoB and PhoR (Figure 1B). In the absence of IPTG, PhoB concentration of the LAC strain is at a low level similar to the uninduced WT level observed under Pi-replete conditions (Table S1) and fluorescence output is correspondingly limited. The presence of 150 µM IPTG resulted in a level of PhoB comparable to the autoregulated WT level under Pi-deplete conditions and the output response curves were also comparable. It appears that positive autoregulation allows auto-amplification of PhoB and PhoR levels, leading to amplification of output responses. Analyses of single-cell fluorescence indicated that all strains, constitutive or autoregulated, displayed a graded dependence of output on Pi concentrations (Figure 1C and Figure S1). Bistability, or bimodal distribution of responses, was not observed under experimental conditions. At the timescale of the experiments, no significant delay of response time was observed for the autoregulated PhoB/PhoR system because the level of phosphorylated PhoB (PhoB∼P) was shown to increase with comparable response time in either the autoregulated WT strain or the constitutive LAC strain [19].
Dependence of Cell Fitness on TCS Expression Levels
The observed concentration-dependent output differences may have a direct consequence on cell fitness, which is often assessed by comparing cell growth rates under various conditions. Despite different constitutive levels of PhoB achieved in the LAC, TRC or KON strains whose phoBR promoter was replaced by IPTG-inducible (LAC and TRC) or constitutive (KON) promoters (Figure 2A and Table S1), cells displayed similar growth curves in MOPs medium, indistinguishable from the autoregulated WT strain (Figure 2B). As reported previously [22], [23], Pi-depletion causes a halt of the logarithmic growth and cells enter into a Pi-limited stationary phase. There is minimal difference in optical densities within the Pi-limited growth phase for these strains. Constitutive expression of the PhoB/PhoR TCS proteins also did not result in significant growth defects for the logarithmic growth phase and all shared similar doubling times (Figure 2C). Therefore, it appears that either the autoregulated WT strain does not confer fitness advantages over constitutive strains or the fitness costs of constitutively high expression are too small to be manifested under experimental conditions.
Fig. 2. Growth rates in batch cultures are similar for the autoregulated WT and constitutive strains. 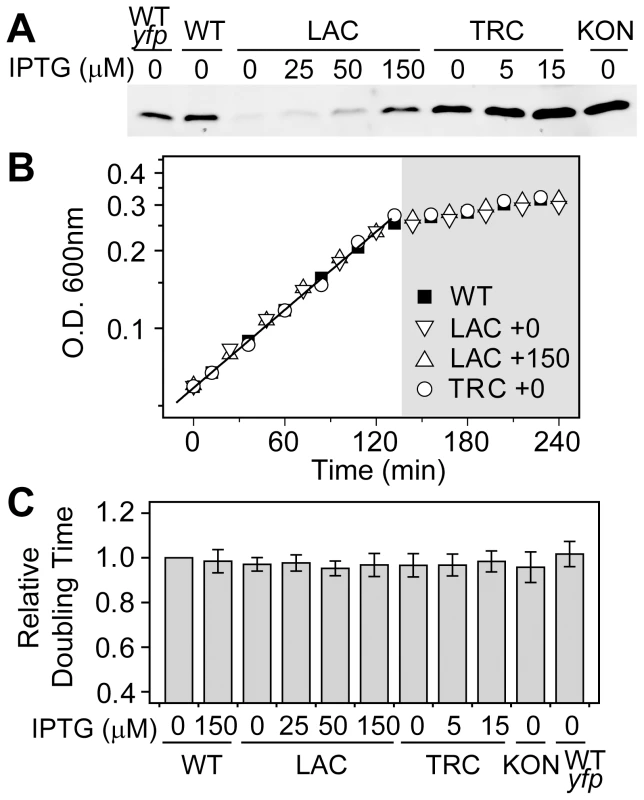
(A) Western blot analyses of PhoB expression. Wild type strains BW25113 (WT) and RU1622 (WT-yfp), IPTG-inducible strains RU1616 (LAC) and RU1618 (TRC), and the constitutive strain RU1617 (KON) were grown to Pi-depletion under different IPTG concentrations and assayed for PhoB expression. Growth curves (B) and doubling times (C) of strains with different PhoB levels. Indicated strains were grown in MOPS media with an initial Pi concentration of 50 µM. Depletion of Pi accompanies the change of the growth rate and cells quickly enter into the stationary phase (shaded). Numbers after the “+” sign indicate IPTG concentrations. Relative doubling times were determined by fitting the exponential growth curves with an exponential function and compared to the doubling time of the WT strain. Results are shown as the mean and SD of at least four independent experiments. Batch cultures in MOPs medium only allowed approximately 2 h of logarithmic growth before reaching the stationary phase, which may not be adequate to reveal any growth differences. To examine potential fitness costs and benefits, bacteria competition was assayed in continuous cultures to allow prolonged constant growth. Because bacteria growth in continuous cultures is significantly different from that in batch cultures and bacteria consumed different amount of Pi in batch and continuous cultures to reach different O.D., AP activities were used to define the activating Pi-deplete and inactive Pi-replete conditions in continuous cultures (Figure S2). All strains were competed against a yfp-carrying WT strain, WT-yfp, which allows determination of the population distribution by cellular fluorescence. Starting from a 50∶50 mixture, the population of the non-fluorescent WT strain remained constant under both Pi-deplete and -replete conditions (Figure 3A), suggesting an equal fitness for WT and WT-yfp under both experimental conditions. In contrast, the constitutive LAC strain that expresses PhoB at a low level displayed a fitness dependence on Pi concentrations. Under the Pi-replete condition, LAC is equally fit as WT-yfp, while a gradual decrease in population was observed under the Pi-deplete condition, presumably due to its limited output response. After 24 h, the population of the LAC strain decreased to less than 1% of the total bacteria population.
Fig. 3. PhoB expression levels correlate with cell fitness in continuous competition assays. 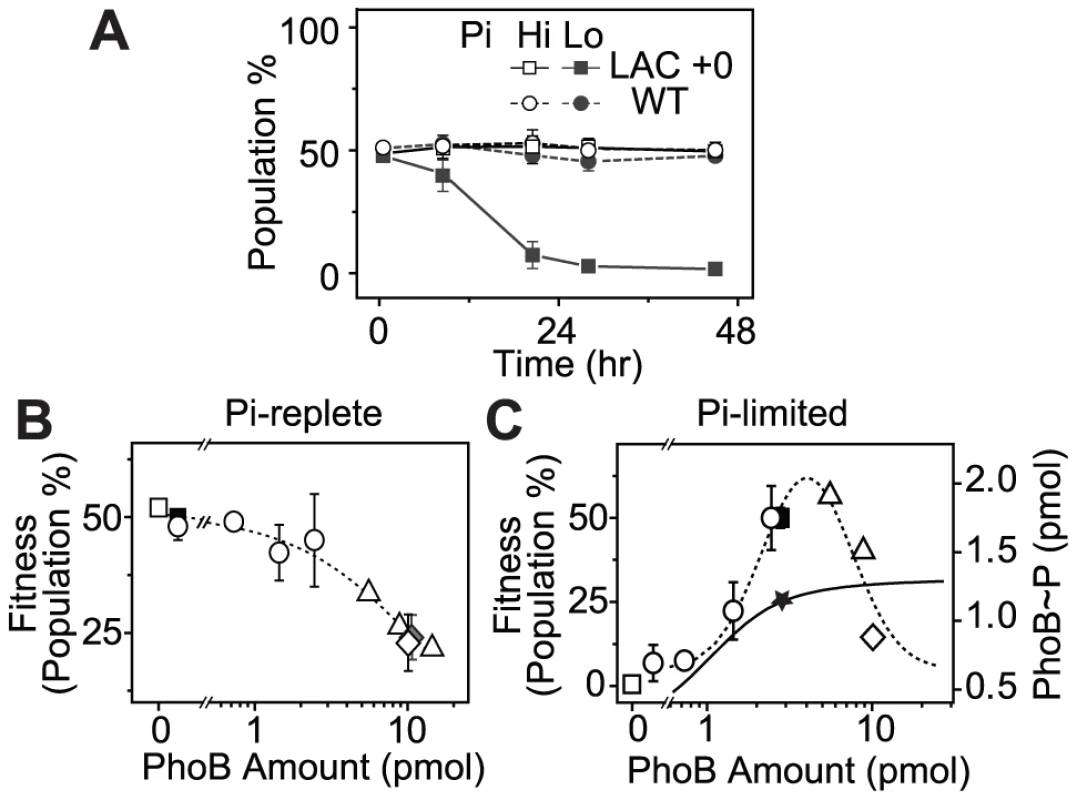
(A) Non-fluorescent bacteria population in continuous cultures when competed against WT-yfp (RU1622). A 50∶50 mixture of WT-yfp with indicated strains were grown in continuous cultures. Pi-replete and -deplete conditions were achieved with different Pi concentrations, 300 µM (Hi) and 12 µM (Lo), in the fresh medium supplied through the chemostat inlet. Bacteria population at 24 h was used to evaluate cell fitness under Pi-replete (B) and -deplete (C) conditions. Different PhoB levels were achieved with different IPTG concentrations using the following strains: BW25113 (WT, solid square), RU1631 (ΔphoB, open square), RU1616 (LAC, open circle), RU1617 (KON, open diamond), RU1618 (TRC, open triangle) and RU1619 (KON phoBD53A, solid diamond). PhoB amount was determined previously from 0.3 OD600*ml of cells [19]. A value of 3 pmol in these cells is estimated to correspond to a PhoB concentration of ∼10 µM. Dotted lines represent linear and lognormal trend lines under respective conditions. The solid line represents the saturating dependence of output PhoB∼P levels on total amount of PhoB measured previously [19]. The star marks the PhoB∼P level at the autoregulated WT concentration of PhoB. Error bars are SDs and the number of independent experiments is documented in Table S1. Bacteria populations after 24 h of competition against WT-yfp were thus used to evaluate cell fitness at a range of PhoB expression levels (Figure 3B, 3C and Table S1). Strains with no or low PhoB expression, such as the phoB deletion or the WT strain, had high fitness under Pi-replete conditions. Increasing PhoB/PhoR concentrations reduced cell fitness (Figure 3B). Because high levels of PhoB/PhoR can promote a modest increase of expression of PhoB-activated genes in the absence of stimuli [19], fitness reduction can be attributed to cost of protein production as well as activities of proteins encoded by these PhoB-activated genes, which are tailored for Pi-depleted environments and detrimental under Pi-replete conditions [20], [24], [25]. However, the elevated basal activity of PhoB at high expression levels is dependent on the conserved D53 residue in PhoB [19] yet the D53A mutant showed similar fitness to the constitutive KON strain without the mutation, arguing against the protein activities as a main cost of fitness.
When the system is stimulated under Pi-deplete conditions, cell fitness followed a different pattern of dependence on TCS expression levels (Figure 3C). Cells with no or low PhoB expression were unable to compete with WT-yfp that expressed PhoB at a high level through autoregulation. For the same LAC strain, increasing IPTG concentrations raised PhoB levels as well as the output responses. Correspondingly, the fitness of bacteria increased until the PhoB concentration became comparable to the WT level. Further increase of PhoB levels eventually led to diminishing fitness of cells. As discovered previously [19], the output of the system, indicated by the concentration of phosphorylated PhoB (PhoB∼P) shown on the right axis of Figure 3C, increased along with PhoB/PhoR levels and started to saturate around the WT concentration of PhoB (solid line, Figure 3C). Thereafter, increase of PhoB concentration does not enhance the beneficial output further but still carries great costs of protein production under Pi-depleted conditions, reducing the overall cell fitness. It appears that the WT level of PhoB has been optimized to provide close to maximal fitness under Pi-deplete conditions.
Evolutionary Tuning of PhoB/PhoR Levels under Pi-Deplete Conditions
The optimal PhoB level of WT is likely a result of adaptive evolution to balance costs and benefits. For a given E. coli strain with non-optimal expression of PhoB and reduced fitness, such as the LAC strain in the absence of IPTG (LAC0), do bacteria actually evolve toward the optimal expression level? We tested this by following laboratory evolution of bacteria in Pi-limited continuous cultures. At the start of the continuous culture, the LAC strain in the absence of IPTG displayed an extremely low level of output in AP activity as well as minimal PhoB levels (Figure 4). Only after 48 h of growth, approximately ∼18 generations, AP activity rose to a level comparable to that of the WT strain and the PhoB expression level reached the optimal level observed for the WT strain. This adaptation is clearly dependent on stimuli because PhoB concentration and AP activity remained constantly low when Pi was replete. On the other hand, when 150 µM IPTG is present in continuous cultures (LAC150), LAC displayed an optimal PhoB level, high output and high fitness that are all comparable to WT, thus no further increase of PhoB level or AP activity were observed.
Fig. 4. Cells expressing low levels of PhoB adapt to Pi-deplete environments by increasing PhoB expression. 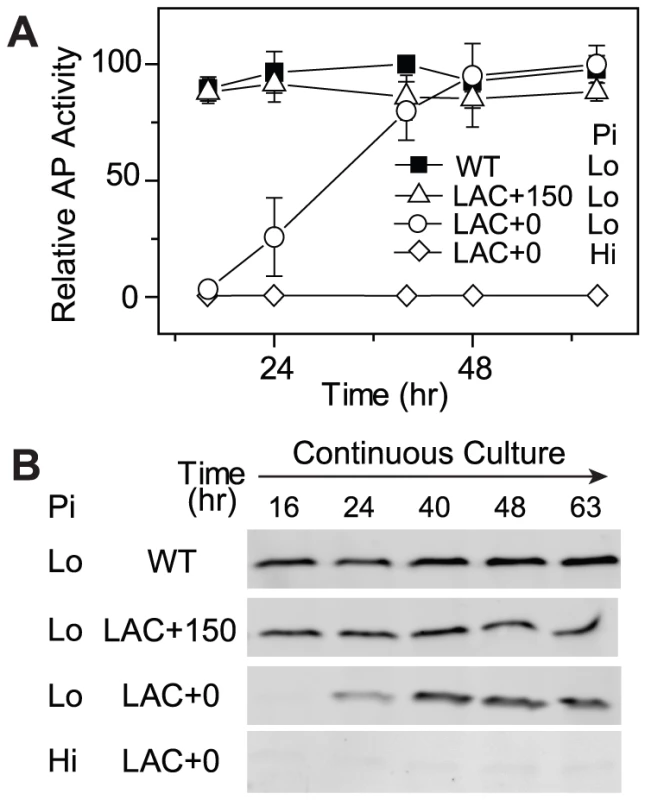
Time-dependent AP activities (A) and PhoB levels (B) are shown for continuous cultures with indicated strains, BW25113 (WT) and RU1616 (LAC). Pi concentrations of 300 µM (Hi) and 12 µM (Lo) in the inlet fresh medium were used for Pi-replete and -deplete environments, respectively. Numbers after the “+” sign indicate IPTG concentrations. 0.3 OD600*ml of cells collected from the chemostat outlet flow were used for analyses of AP activities and PhoB expression. Error bars are SDs of three independent experiments and unseen error bars are smaller than symbols. To investigate mechanisms behind the observed adaptation in the LAC0 continuous culture, individual colonies were isolated to compare their PhoB expression levels and output responses to the original LAC strain in batch cultures (Figure 5). Not surprisingly, as bacteria in LAC150 cultures can manage adequate output responses with sufficient levels of PhoB, colonies isolated from LAC150 cultures showed similar outputs in AP activity to the original LAC strain, implying the absence of mutations in the PhoB/PhoR pathway. In contrast, all six colonies isolated from LAC0 cultures showed elevated AP activities even in the absence of IPTG while the low PhoB concentration of the original LAC strain under the same condition only gave limited output (Figure 5A). Increased AP activities are not the result of stimuli-independent constitutive activation of phoA expression, and all colonies maintained low AP activities under Pi-replete conditions. Among six LAC0 colonies, colony 4 and 6 showed slightly lower AP activities while other colonies had high AP activities, similar to WT, even in the absence of IPTG. Correspondingly, colonies 4 and 6 showed sub-optimal levels of PhoB while other LAC0 colonies expressed phoB at the optimal WT level in the absence of IPTG (Figure 5B). The lac promoters before phoB in colonies 1, 2, 3 and 5 appeared to be no longer regulated by IPTG and the regulation in colonies 4 and 6 was weakened with a high basal expression (Figure 5C). The adaptation observed in LAC0 cultures occurred via altering promoter regulation that shifted the PhoB concentration toward the optimal level.
Fig. 5. Adapted LAC0 cells carry promoter regulation mutations for optimal expression of PhoB. 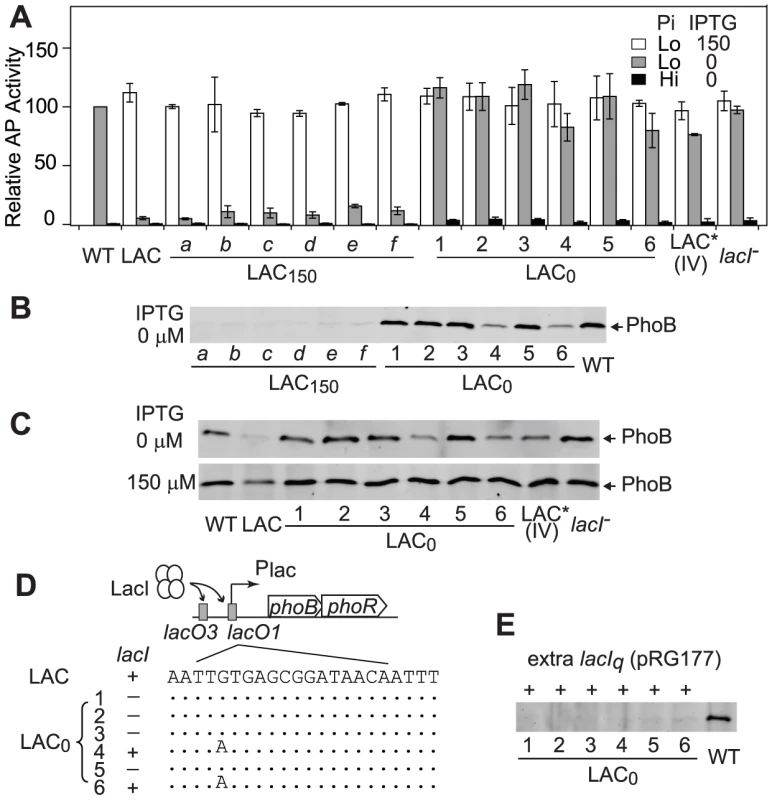
AP activities (A) and PhoB levels (B) of individual colonies isolated from LAC0 and LAC150 cultures. Indicated strains were grown in batch cultures with 50 µM (Lo) or 2 mM Pi (Hi) for 3 h. PhoB levels are shown for cells grown in Pi-replete medium (2 mM) except for WT. Error bars are SDs of at least four independent experiments. (C) IPTG-induced PhoB expression of indicated strains under Pi-deplete conditions. (D) Schematic representation of adaptive mutations. In the LAC strain, LacI binds to lacO regions at the lac promoter before phoBR. The presence or absence of lacI is indicated by “+” or “−” symbols. Sequences of the lacO1 site from adaptive mutants are shown with dots representing identical sequences. (E) Repression of PhoB expression by additional copies of lacI. Indicated colonies were transformed with the lacIq-containing plasmid pRG177 and probed for PhoB expression in the absence of IPTG. The lac promoter is repressed by LacI through binding to lacO regions of the promoter. Therefore the lac promoter, lacI and phoB were sequenced to map the adaptive mutations. Consistent with the fact that PhoB expression and AP activity profiles of LAC150 colonies were similar to those of the original LAC strain, no mutations were found in these regions for colonies of the LAC150 culture. In contrast, colonies 4 and 6 isolated from the evolved LAC0 culture carry a G to A mutation in the lacO1 region at the promoter of phoBR while lacI appeared to be missing in other LAC0 colonies (Figure 5D). No mutations were identified in the coding sequences of phoB for any colonies. Re-introduction of the same G to A mutation into the lac promoter (LAC* IV) resulted in identical AP activity profiles and PhoB expression levels observed for LAC0 colonies 4 and 6 while deletion of lacI (lacI−) in the LAC strain gave the same phenotype as other LAC0 colonies (Figure 5A and 5C). Further, introduction of an additional plasmid-encoded lacIq into LAC0 colonies re-established repression of the lac promoter and suppressed expression of phoB (Figure 5E). Combined, the above analyses indicate that LAC0 cells evolved to express phoB at or close to the optimal level by mutating different promoter regulatory elements that allowed them to generate sufficient output responses to survive under Pi-deplete conditions.
In LAC0 cultures, PhoB concentration is extremely limited at the initial stage and cells faced a great challenge of Pi-depletion that drove the evolution. An intermediate concentration (40 µM) of IPTG in continuous cultures induced the PhoB concentration to a moderate and sub-optimal level in LAC40 cultures, yet a Pi-deplete environment still prompted evolution of LAC cells, although at a slower pace (Figure 6). Increase of PhoB levels was observed after 48 h of continuous growth in LAC40 cultures while only 24 h were required for the LAC0 culture to show the first sign of adaptation (Figure 6A). Correspondingly, for individual colonies isolated from 24 h growth samples, all LAC40 colonies displayed a low PhoB level in the absence of IPTG similar to that of the original LAC strain while one adaptive mutant with high PhoB expression started to emerge in LAC0 cultures (Figure 6C and S3). At 48 h, the majority of LAC0 colonies had already adapted with high levels of PhoB comparable to the optimal WT level. At the same time, adaptation was observed in less than half of the LAC40 colonies. Further growth until 86 h increased the population of adapted cells in LAC40 cultures. Interestingly, most of the adapted LAC40 colonies shared a similar phenotype with intermediate uninduced PhoB levels, different from the high optimal PhoB level seen in adapted LAC0 cells. Sequencing results revealed that all colonies with high PhoB levels lost functional LacI through deletion, frame-shifting insertion or early termination of lacI. In contrast, most of the LAC40 colonies with intermediate PhoB levels had an unaltered lacI but carried diverse mutations in the LacI repressing site lacO1 (Figure 6D). Such mutations relaxed LacI repression and 40 µM IPTG was sufficient to induce the PhoB level to the optimal WT level in these mutants (Figure 6E). Despite different initial conditions and diverse genotypes in adapted cells, evolution in LAC0 and LAC40 cultures both led to the optimal PhoB concentration that confers maximal fitness to cells in Pi-deplete environments.
Fig. 6. Intermediate PhoB levels lead to a slower adaptation pace and distinct adaptive genotypes. 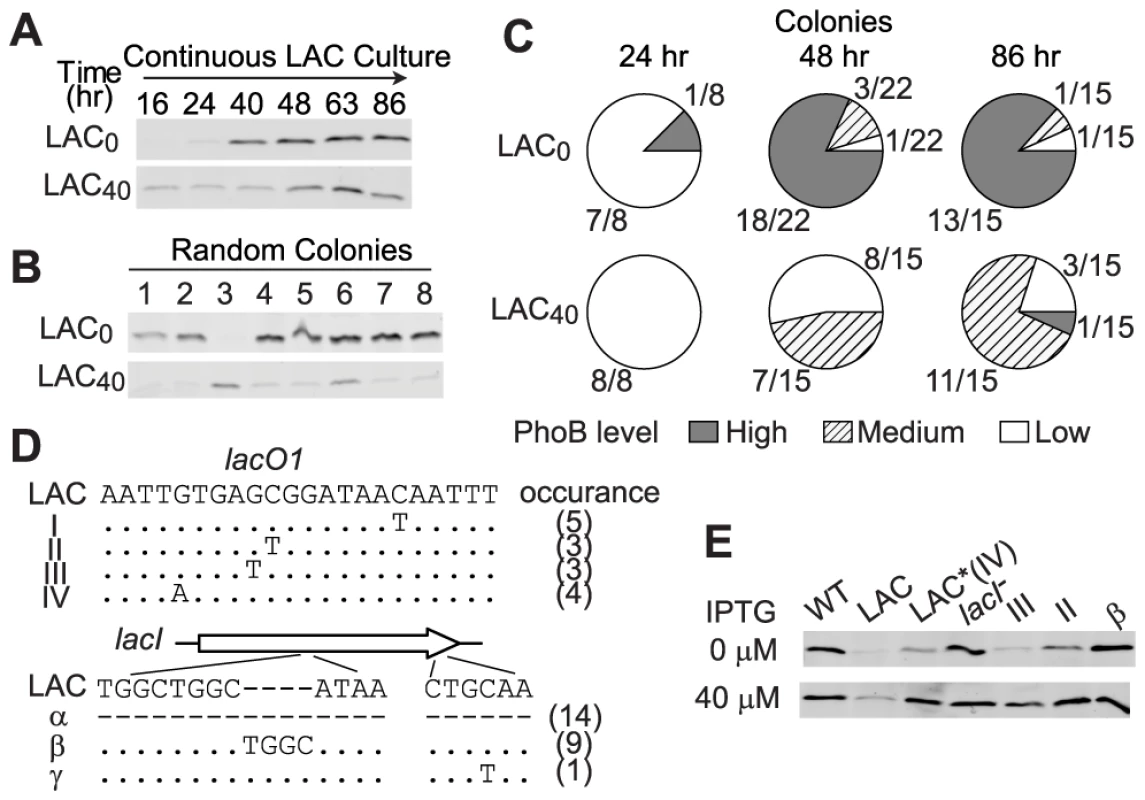
(A) PhoB levels of the indicated adapting cultures. IPTG concentrations of 0 µM and 40 µM were included in the medium to give low and intermediate initial PhoB levels, both of which were lower than the optimal concentration. (B) PhoB expression in batch cultures from random colonies isolated from above cultures. No IPTG was added to batch cultures and PhoB levels were used for phenotype classification of individual colonies. (C) Population of individual colonies with different PhoB expression phenotypes. Colonies isolated from adapting cultures were classified into three groups by PhoB expression levels (Figure S3). High level corresponds to the autoregulated WT PhoB concentration seen under Pi-deplete conditions and low level is comparable to the basal expression of the original LAC strain. Colonies with intermediate levels substantially different from the above two levels were classified as the medium PhoB expression phenotype. (D) Mapping of adaptive mutations. Colonies with elevated PhoB levels were selected for sequencing of the lac promoter and lacI. Mutations in lacO1 and lacI as well as numbers of colonies carrying the particular mutation are indicated. Dots represent identical nucleotides and dashes represent absence of nucleotides. For genotype α, PCR with multiple lacI-specific primers failed to yield any products, suggesting the loss of the entire lacI, possibly due to deletion. (E) IPTG-induced PhoB levels of selected colonies with different genotypes. Adaptation under Pi-Replete Conditions
As discussed above, a high concentration of PhoB does not provide benefits under Pi-replete conditions and the cost of protein production reduced cell fitness. Therefore, a laboratory evolution experiment similar to LAC0 and LAC40 continuous cultures was performed to examine whether a strain with a high PhoB level would adapt to Pi-replete environments by reducing PhoB expression, thus increasing its fitness. An IPTG concentration of 15 µM induced a high level of PhoB in the TRC strain and continuous growth in the Pi-replete culture resulted in a gradual decrease of PhoB levels (Figure 7A). Analyses of individual colonies revealed that adapted cells with reduced or abolished PhoB expression already emerged after 48 h of growth (Figure 7B). After 86 h, approximately 30 generations, the majority of colonies isolated from the culture no longer expressed significant amounts of PhoB. Again, different mutational strategies were discovered among these adapted cells that yielded similar phenotypes with reduced PhoB expression (Figure 7C).
Fig. 7. Cells overexpressing PhoB adapt to Pi-replete environments by abolishing PhoB expression. 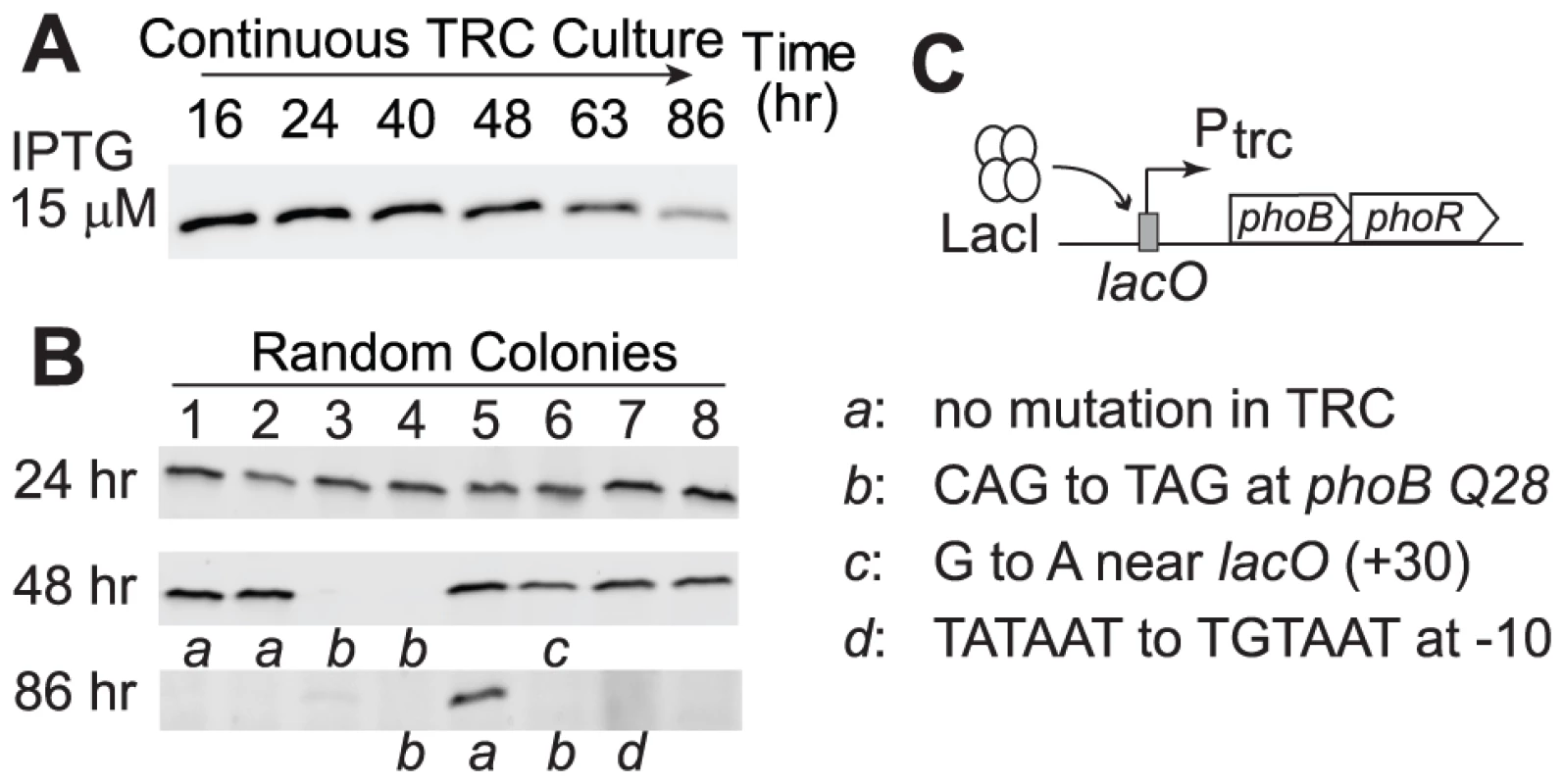
(A) PhoB levels of the adapting culture under the Pi-replete condition. RU1618 (TRC) was induced with 15 µM IPTG to achieve overexpression of PhoB and allowed for adaptation in continuous cultures. (B) PhoB expression in batch cultures from random colonies isolated at different times. Lower case letters indicate genotypes identified in selected colonies that were subjected to sequencing analyses. (C) List of mutations for adapted cells with altered PhoB expression. Discussion
Although expression of a significant number of genes is suggested to be non-optimal or even maladaptive under laboratory conditions [26], responsive gene regulation is generally considered to be an evolutionary strategy that allows cells to adjust the levels of beneficial proteins as needed under specific environmental conditions. Costs of gratuitous gene expression and benefits of induced protein production have been characterized for many adaptive genes, particularly those involved in antibiotic resistance or utilization of specific metabolites [27]–[30]. It has been shown that cells can rapidly evolve to different optimal protein levels with balanced fitness costs and benefits under different environments [27], [31]. For the PhoB/PhoR TCS regulators, cost of PhoB/PhoR expression appears to be minimized under Pi-replete conditions via low expression. Under Pi-deplete conditions, benefits derived from the output responses of regulated gene products offset the expression cost and a different optimal PhoB/PhoR concentration has been selected to reach a cost-benefit balance. Autoregulation of PhoB/PhoR expression serves as a mechanism for cells to achieve respective optimal concentration and fitness under these conditions.
Cost of PhoB/PhoR Expression
Production of unneeded proteins often carries a fitness cost [32]. One of the most extensively studied examples is the E. coli lac operon that encodes genes for utilization of lactose. In the absence of lactose, gratuitous induction of the lac operon reduces the growth rate and this reduction reflects the deleterious activity of the LacY permease as well as the cost of producing unneeded proteins with cellular resources [27], [28], [30]. Similarly, the fitness cost of high TCS expression can arise from protein production cost and potential detrimental protein activities of RR-regulated genes. It has been shown that constitutive activation of PhoB-regulated genes, particularly the pst operon encoding a Pi-transport system, hampers growth under Pi-replete conditions [24], [25]. However, high levels of PhoB/PhoR do not cause significant phosphorylation of PhoB in the absence of stimuli and only a minor basal activation of PhoB-regulated genes were observed at high PhoB levels [19]. Mutation of the conserved D53 residue almost completely abolished the basal activation [19] yet high expression of the mutant gave similar fitness reduction as high expression of intact PhoB/PhoR proteins. Thus detrimental activities of PhoB-regulated genes do not appear to be a major contributor to fitness reduction under the tested Pi-replete conditions although it cannot be excluded that some fitness cost may still originate from residual phosphorylation-independent basal activation of PhoB-regulated genes.
Under Pi-replete conditions, WT E. coli cells produce approximately 0.13 pmol of PhoB per 0.3 OD*ml of cells (Table S1), corresponding to a concentration of ∼0.5 µM or 200–300 molecules per cell. Apparently, maintaining such a low level of this 26-kDa protein and an even lower level of PhoR does not have much fitness cost. Increasing expression 50–100 fold in the constitutive TRC strain results in a PhoB concentration of only 25–50 µM. It has been reported that full induction of the lac operon yields ∼50 µM LacZ molecules (116 kDa) [33] and causes a 4.5% reduction in batch culture growth rates [27]. Therefore, it is not surprising that a fitness cost of PhoB overexpression was not revealed above the observed 5% data variance of growth rates in batch cultures. In contrast, a gradual fitness reduction caused by PhoB production was apparent in continuous cultures. Because production of useless proteins is an inefficient use of limited resources in nitrogen-limited chemostat cultures, fitness differences likely were magnified. Cost of protein production is clearly dependent on growth conditions and cells tend to reduce the cost with various mechanisms [32], [34], including complete abolishment of expression as observed in our adaptation experiments.
Cost-Benefit Balance and Evolutionary Optimization of PhoB/PhoR Levels
Pi-depletion leads to an ∼20 fold increase of PhoB level in WT cells. The cost of producing ∼6000 PhoB molecules per cell is compensated by the beneficial function of PhoB/PhoR proteins to provide a close-to-peak fitness. Fitness benefits arise from PhoB∼P-dependent expression of regulated genes, such as the pst operon encoding the Pi transporter system and the outermembrane porin gene phoE. Although the exact fitness contribution of individual PhoB-regulated genes is difficult to track, the overall fitness landscape correlates well with the output PhoB phosphorylation profile. Peak fitness occurs at a PhoB level close to where PhoB∼P starts to saturate, a point determined by the specific balance of PhoR kinase and phosphatase activities [19]. The constitutive strain in laboratory evolution experiments and the autoregulated WT strain all evolved to express PhoB close to this optimal level for maximal fitness. Above this level, high PhoB levels presumably increase cost without providing further benefits because of the saturation of phosphorylation. RR phosphorylation saturation is not unique to the PhoB/PhoR system but rather a result of the intrinsic HK-RR phosphorylation cycle determined by TCS protein activities [7], [19], [35], [36]. A similar output saturation profile has also been revealed for the autoregulated E. coli PhoQ/PhoP system and the induced WT PhoP level is again close to the beginning of saturation [7]. It remains to be investigated whether the expression levels of PhoQ/PhoP and other TCSs are similarly optimized to the phosphorylation output profile.
The laboratory evolution experiments indicated that bacteria with non-ideal TCS expression could increase their fitness through mutations that produced optimal levels of TCS proteins for the specific environment. The majority of mutations were at the promoter of phoBR or the regulatory gene of the promoter, suggesting the expression of TCS as a convenient and efficient evolutionary target for environmental adaptation. As the lac promoter of phoBR in the LAC strain is under negative regulation by LacI, any loss-of-function mutants of lacI can result in higher expression of phoBR and this may contribute to the rapid evolution pace observed in continuous cultures.
For bacteria with different initial sub-optimal fitness, such as the LAC0 and LAC40 cultures, adaptation occurred at different rates yet produced similar optimal PhoB expression levels with distinct genotypes. In the absence of IPTG, null lacI results in an unrepressed optimal PhoB level that gives adapted LAC cells higher fitness than lacO mutants whose PhoB levels are below the optimal level, thus lacI null mutants predominate in LAC0 cultures. In continuous cultures with 40 µM IPTG, both lacI deletion and lacO mutations were able to give optimal expression of TCS proteins. All isolated lacO mutants carry G:C-to-T:A substitutions and constitute a majority of evolved cells in LAC40 cultures. The dominance of lacO mutants may reflect a higher mutation frequency for single nucleotide substitution than for frame-shifting insertion or gene deletion observed in lacI null mutants. Indeed, nutrient-limited continuous cultures have been known to cause mutations in a mismatch repair gene mutY, which greatly increases the frequency of G:C-to-T:A transversions [37]. Despite diverse types of mutations observed in the evolved population, adapted bacteria cells all converged to similar phenotypes in TCS expression to match the demand of the environment.
Role of Autoregulation in Expressing Optimal PhoB/PhoR Levels
Responsive gene regulation is generally a favored mechanism for adaptation to variable environments with conflicting demands of protein expression. In natural habitats where E. coli cells face Pi-rich conditions, such as intestinal lumen, and Pi-limited conditions, such as aquatic environments, Pi-responsive autoregulation of the PhoB/PhoR regulators appears to be an evolutionary consequence that achieves optimal PhoB/PhoR levels under both environments. Under constant environments in continuous cultures, the autoregulated WT strain does not confer apparent fitness advantages over the constitutive strains as long as phoB expression matches the WT concentration. However, fixed constitutive expression can only achieve optimal fitness in one particular environment whereas autoregulation gives cells maximal fitness in variable habitats.
Positive autoregulation allows elevated output responses and higher fitness benefits under stimulated conditions. It is this output-amplifying feature of positive autoregulation that enables cells to reduce protein production cost in the absence of stimuli without sacrificing the capacity of output responses. Other features commonly associated with positive autoregulation, such as bistability and response delay, were not observed for the WT PhoB/PhoR system. For strains with non-native genetic background, all-or-none responses have been documented when PhoR is absent and PhoB is cross-phosphorylated by overexpressed non-cognate HKs [38]. However, such cross-phosphorylation is suppressed by the phosphatase activity of the cognate HK and may not be physiologically relevant [19], [39]. Phosphatase-defective or monofunctional HKs are known to promote bistability [7], [40], thus bimodal responses in these non-native cross-talk systems may arise from the lack of negative phosphatase activity in non-cognate HKs [41]. Positive autoregulation has been shown advantageous for the Salmonella PhoQ/PhoP system to promote virulence in mice [42]. The fitness gain over the constitutive strain was suggested to be associated with a transient surge in RR phosphorylation observed for the autoregulated strain even though the activation surge was later attributed to intrinsic negative feedback of biochemical activities in bifunctional HKs [8], [43]. It is possible that part of the fitness advantages for the autoregulated PhoQ/PhoP system may come from lowered costs in unstimulated environments, similarly to fitness profiles observed for the PhoB/PhoR system. Balancing costs and benefits by positive autoregulation may be a recurring scheme in TCSs to select proper TCS protein quantities and biochemical activities.
Materials and Methods
Strains and Growth Conditions
The strains and plasmids used in this study are listed in Table S2. λ red recombination [44] was used to make chromosomal gene disruption or alteration in strain BW25113 or derivatives of BW25113 similarly to the constitutive strains, RU1616 (LAC), RU1617 (KON) and RU1618 (TRC), in which the WT autoregulated phoB promoter was replaced with constitutive (KON) or IPTG-inducible (LAC and TRC) promoters [19]. Strains with the chromosomal reporter phoA-yfp or YFP marker were created using the reported recombination strategies [45]. Briefly, the plasmid pRG261 containing PphoA-yfp reporter or pRG278 containing Ptet-yfp was integrated into the chromosome of indicated strains at the HK022 or lamda phage attachment sites to generate RU1465 (WT, phoA-yfp), RU1653 (LAC, phoA-yfp) and RU1622 (WT-yfp), respectively. Details of strain and plasmid construction were described in Text S1.
Bacteria batch cultures were grown in MOPs minimal media [46] with 0.4% glucose, containing either 2 mM (Pi-replete) or 50 µM (Pi-deplete) KH2PO4. Continuous cultures were grown at 37°C in a home-built chemostat modified from the design described in [47]. Fresh feed medium was supplied to a 50-ml glass vessel through silicone tubing with a peristaltic pump at a flow rate of 6 ml/h while bacteria culture flowed out through an outflow tube at the same rate. The total culture volume is 24 ml, set by the depth of the outflow tube in the chemostat vessel. Thus the dilution rate was 0.25 h−1, corresponding to a generation time of ∼2.8 h. The feed medium was identical to the MOPs medium used in batch cultures except that the concentration of NH4Cl was limited at 250 µM instead of 5 mM in batch cultures. Optical densities (600 nm) of the nitrogen-limited chemostat were ∼0.09, corresponding to ∼2.2×109 cells in the chemostat culture. Pi concentrations of 300 µM and 12 µM were included in feed media for Pi-replete and -deplete conditions, respectively.
YFP Reporter, AP Activity and Protein Level Measurements
As described previously [19], to assay bacterial responses to phosphate concentrations, cells from fresh Pi-replete MOPs cultures were inoculated in MOPs medium containing 2 mM (Pi-replete), 50 µM (Pi-deplete) or indicated concentrations of KH2PO4 with a starting OD600 at 0.04 followed by 3 h growth. For YFP reporter assays, fluorescence of individual cells was measured with a Beckman Coulter FC500 flow cytometer. Mean fluorescence of the whole population was calculated from the fitted lognormal distribution. For AP activities and protein level determination, bacteria pellets equivalent to 0.3 OD600*ml from batch or continuous cultures were collected and assayed as described before [19]. AP activities were determined by monitoring the rate of absorbance change at 420 nm using a microplate reader (Varioskan, ThermoFisher) following addition of 7 mM p-nitrophenylphosphate. Values of absorbance changing rates were multiplied by 100000 to represent absolute AP activities while they are compared to AP activity of the WT strain for relative AP activities. PhoB levels from sample lysates were determined by western blot. Blots probed with anti-PhoB primary antisera and Cy5-conjugated secondary antibodies were visualized by fluorescence imaging with a FluorChem Q (Alpha Innotech). Selected blots were simultaneously probed with the anti-Sigma70 and Cy3-conjugated secondary antibodies to confirm equal loading of samples.
Cell Fitness Assays
Cell fitness was evaluated with growth rates in batch cultures and competition assays in continuous cultures. Indicated strains were induced with indicated IPTG concentrations in MOPs media for 2–3 h to achieve different PhoB/PhoR levels. Cells from these fresh MOPs cultures were inoculated in 96-well plates and grown at 37°C with a starting OD600 of ∼0.03 under respective IPTG conditions. The exponential growth rates before Pi-depletion were determined by fitting the data with a single exponential function.
Competition assays were performed in continuous cultures with the indicated non-fluorescent strains and WT-yfp. Cells from fresh MOPs cultures were mixed at a 1∶1 ratio and inoculated into the chemostat with a starting OD600 of ∼0.04. Indicated IPTG concentrations were included in the feed medium to achieve different PhoB levels. After 24 h of growth, cells collected from the outlet stream were analyzed by microscope imaging or flow cytometry to determine the population of individual strains. Bacteria cells were immobilized on 1% agarose pads made with MOPs medium as described [48]. Microscope images were obtained using an Olympus IX70 microscope (Olympus) with a 100× NA PlanApo 1.3 objective, 100 W mercury lamp and the HiQ fluorescein filter set (Chroma). Phase-contrast images were taken to determine the total cell numbers and fluorescent images were used to count the population of fluorescent WT-yfp cells. Cell numbers were counted using ImageJ software (NIH) and bacteria population was determined from at least 1000 cells from a total of 6–10 images. In selected samples, cell populations measured by microscope imaging were confirmed by flow cytometry.
Adaptation Experiments in Continuous Cultures
Indicated bacteria strains were grown in continuous cultures for ∼4 days. Pi concentrations of 300 µM (Pi-replete) and 12 µM (Pi-deplete) were included in feed media together with indicated concentrations of IPTG to yield non-optimal PhoB expression levels under different Pi conditions. Cells were collected from the outlet stream at indicated time intervals. Collected cultures were pelleted and 0.3 OD600*ml of cells were stored at −80°C for later examination of AP activities and PhoB levels. Diluted cultures from the chemostat were streaked on LB plates for colony isolation. Single colonies were randomly chosen to grow in MOPS batch cultures and characterized for their phenotypes in PhoB expression and AP activities. To investigate the genotype of individual clones, colony PCR was performed to amplify the chromosomal DNA regions corresponding to the lacI gene, phoB and its promoter. Sequences of these regions were determined and compared to the WT sequence. For some colonies, primers specific to lacI yielded no PCR products and further use of additional primers corresponding to different coding, upstream and downstream regions of lacI still did not give PCR products, suggesting the loss of lacI.
Supporting Information
Zdroje
1. StockAM, RobinsonVL, GoudreauPN (2000) Two-component signal transduction. Annu Rev Biochem 69 : 183–215.
2. CapraEJ, LaubMT (2012) Evolution of two-component signal transduction systems. Annu Rev Microbiol 66 : 325–347.
3. GalperinMY (2010) Diversity of structure and function of response regulator output domains. Curr Opin Microbiol 13 : 150–159.
4. HermsenR, EricksonDW, HwaT (2011) Speed, sensitivity, and bistability in auto-activating signaling circuits. PLoS Comput Biol 7: e1002265.
5. MitrophanovAY, GroismanEA (2008) Positive feedback in cellular control systems. Bioessays 30 : 542–555.
6. GoulianM (2010) Two-component signaling circuit structure and properties. Curr Opin Microbiol 13 : 184–189.
7. MiyashiroT, GoulianM (2008) High stimulus unmasks positive feedback in an autoregulated bacterial signaling circuit. Proc Natl Acad Sci USA 105 : 17457–17462.
8. RayJC, IgoshinOA (2010) Adaptable functionality of transcriptional feedback in bacterial two-component systems. PLoS Comput Biol 6: e1000676.
9. IgoshinOA, BrodyMS, PriceCW, SavageauMA (2007) Distinctive topologies of partner-switching signaling networks correlate with their physiological roles. J Mol Biol 369 : 1333–1352.
10. FerrellJEJr (2002) Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Curr Opin Cell Biol 14 : 140–148.
11. SmitsWK, KuipersOP, VeeningJW (2006) Phenotypic variation in bacteria: the role of feedback regulation. Nat Rev Microbiol 4 : 259–271.
12. LevineJH, FontesME, DworkinJ, ElowitzMB (2012) Pulsed feedback defers cellular differentiation. PLoS Biol 10: e1001252.
13. WilliamsCL, CotterPA (2007) Autoregulation is essential for precise temporal and steady-state regulation by the Bordetella BvgAS phosphorelay. J Bacteriol 189 : 1974–1982.
14. TiwariA, RayJC, NarulaJ, IgoshinOA (2011) Bistable responses in bacterial genetic networks: designs and dynamical consequences. Math Biosci 231 : 76–89.
15. MukhopadhyayA, GaoR, LynnDG (2004) Integrating input from multiple signals: the VirA/VirG two-component system of Agrobacterium tumefaciens. Chembiochem 5 : 1535–1542.
16. GaoR, LynnDG (2005) Environmental pH sensing: resolving the VirA/VirG two-component system inputs for Agrobacterium pathogenesis. J Bacteriol 187 : 2182–2189.
17. ClarkeMB, SperandioV (2005) Transcriptional autoregulation by quorum sensing Escherichia coli regulators B and C (QseBC) in enterohaemorrhagic E. coli (EHEC). Mol Microbiol 58 : 441–455.
18. PerezJC, GroismanEA (2007) Acid pH activation of the PmrA/PmrB two-component regulatory system of Salmonella enterica. Mol Microbiol 63 : 283–293.
19. GaoR, StockAM (2013) Probing kinase and phosphatase activities of two-component systems in vivo with concentration-dependent phosphorylation profiling. Proc Natl Acad Sci USA 110 : 672–677.
20. Wanner BL (1996) Phosphorus assimilation and control of the phosphate regulon. In: Neidhardt FC, Curtiss III R, Ingraham JL, Lin ECC, Low KB, Jr et al.., editors. Escherichia coli and Salmonella. Washington, D.C.: American Society for Microbiology Press. pp. 1357–1381.
21. HsiehYJ, WannerBL (2010) Global regulation by the seven-component Pi signaling system. Curr Opin Microbiol 13 : 198–203.
22. GentryDR, HernandezVJ, NguyenLH, JensenDB, CashelM (1993) Synthesis of the stationary-phase sigma factor σs is positively regulated by ppGpp. J Bacteriol 175 : 7982–7989.
23. TaschnerNP, YagilE, SpiraB (2004) A differential effect of σs on the expression of the PHO regulon genes of Escherichia coli. Microbiology 150 : 2985–2992.
24. SteedPM, WannerBL (1993) Use of the rep technique for allele replacement to constuct mutants with deletions of the pstSCAB-phoU operon: evidence of a new role for the PhoU protein in the phosphate regulon. J Bacteriol 175 : 6797–6809.
25. HaldimannA, DanielsLL, WannerBL (1998) Use of new methods for construction of tightly regulated arabinose and rhamnose promoter fusions in studies of the Escherichia coli phosphate regulon. J Bacteriol 180 : 1277–1286.
26. PriceMN, DeutschbauerAM, SkerkerJM, WetmoreKM, RuthsT, et al. (2013) Indirect and suboptimal control of gene expression is widespread in bacteria. Mol Syst Biol 9 : 660.
27. DekelE, AlonU (2005) Optimality and evolutionary tuning of the expression level of a protein. Nature 436 : 588–592.
28. StoebelDM, DeanAM, DykhuizenDE (2008) The cost of expression of Escherichia coli lac operon proteins is in the process, not in the products. Genetics 178 : 1653–1660.
29. FoucaultML, DepardieuF, CourvalinP, Grillot-CourvalinC (2010) Inducible expression eliminates the fitness cost of vancomycin resistance in enterococci. Proc Natl Acad Sci USA 107 : 16964–16969.
30. EamesM, KortemmeT (2012) Cost-benefit tradeoffs in engineered lac operons. Science 336 : 911–915.
31. BabuMM, AravindL (2006) Adaptive evolution by optimizing expression levels in different environments. Trends Microbiol 14 : 11–14.
32. ShachraiI, ZaslaverA, AlonU, DekelE (2010) Cost of unneeded proteins in E. coli is reduced after several generations in exponential growth. Mol Cell 38 : 758–767.
33. KaliskyT, DekelE, AlonU (2007) Cost-benefit theory and optimal design of gene regulation functions. Phys Biol 4 : 229–245.
34. ChouHH, MarxCJ (2012) Optimization of gene expression through divergent mutational paths. Cell Rep 1 : 133–140.
35. BatchelorE, GoulianM (2003) Robustness and the cycle of phosphorylation and dephosphorylation in a two-component regulatory system. Proc Natl Acad Sci USA 100 : 691–696.
36. ShinarG, MiloR, MartinezMR, AlonU (2007) Input output robustness in simple bacterial signaling systems. Proc Natl Acad Sci USA 104 : 19931–19935.
37. Notley-McRobbL, PintoR, SeetoS, FerenciT (2002) Regulation of mutY and nature of mutator mutations in Escherichia coli populations under nutrient limitation. J Bacteriol 184 : 739–745.
38. ZhouL, GregoriG, BlackmanJM, RobinsonJP, WannerBL (2005) Stochastic activation of the response regulator PhoB by noncognate histidine kinases. Journal of Integrative Bioinformatics 2 : 11.
39. LaubMT, GoulianM (2007) Specificity in two-component signal transduction pathways. Annu Rev Genet 41 : 121–145.
40. IgoshinOA, AlvesR, SavageauMA (2008) Hysteretic and graded responses in bacterial two-component signal transduction. Mol Microbiol 68 : 1196–1215.
41. SiryapornA, PerchukBS, LaubMT, GoulianM (2010) Evolving a robust signal transduction pathway from weak cross-talk. Mol Syst Biol 6 : 452.
42. ShinD, LeeEJ, HuangH, GroismanEA (2006) A positive feedback loop promotes transcription surge that jump-starts Salmonella virulence circuit. Science 314 : 1607–1609.
43. YeoWS, ZwirI, HuangHV, ShinD, KatoA, et al. (2012) Intrinsic negative feedback governs activation surge in two-component regulatory systems. Mol Cell 45 : 409–421.
44. DatsenkoKA, WannerBL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97 : 6640–6645.
45. HaldimannA, WannerBL (2001) Conditional-replication, integration, excision, and retrieval plasmid-host systems for gene structure-function studies of bacteria. J Bacteriol 183 : 6384–6393.
46. NeidhardtFC, BlochPL, SmithDF (1974) Culture medium for enterobacteria. J Bacteriol 119 : 736–747.
47. ChaoL, LevinBR, StewartFM (1977) A Complex Community in a Simple Habitat: An Experimental Study with Bacteria and Phage. Ecology 58 : 369–378.
48. MiyashiroT, GoulianM (2007) Single-cell analysis of gene expression by fluorescence microscopy. Methods Enzymol 423 : 458–475.
Štítky
Genetika Reprodukční medicína
Článek Defending Sperm FunctionČlánek How to Choose the Right MateČlánek Conserved Translatome Remodeling in Nematode Species Executing a Shared Developmental TransitionČlánek Genome-Wide and Cell-Specific Epigenetic Analysis Challenges the Role of Polycomb in SpermatogenesisČlánek The Integrator Complex Subunit 6 (Ints6) Confines the Dorsal Organizer in Vertebrate EmbryogenesisČlánek Multiple bHLH Proteins form Heterodimers to Mediate CRY2-Dependent Regulation of Flowering-Time inČlánek Playing the Field: Sox10 Recruits Different Partners to Drive Central and Peripheral MyelinationČlánek A Minimal Nitrogen Fixation Gene Cluster from sp. WLY78 Enables Expression of Active Nitrogenase in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 10- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Intrauterinní inseminace a její úspěšnost
- Délka menstruačního cyklu jako marker ženské plodnosti
-
Všechny články tohoto čísla
- Defending Sperm Function
- How to Choose the Right Mate
- A Mutation in the Gene in Labrador Retrievers with Hereditary Nasal Parakeratosis (HNPK) Provides Insights into the Epigenetics of Keratinocyte Differentiation
- Conserved Translatome Remodeling in Nematode Species Executing a Shared Developmental Transition
- A Novel Actin mRNA Splice Variant Regulates ACTG1 Expression
- Tracking Proliferative History in Lymphocyte Development with Cre-Mediated Sister Chromatid Recombination
- Correlated Occurrence and Bypass of Frame-Shifting Insertion-Deletions (InDels) to Give Functional Proteins
- Chimeric Protein Complexes in Hybrid Species Generate Novel Phenotypes
- Loss of miR-10a Activates and Collaborates with Activated Wnt Signaling in Inducing Intestinal Neoplasia in Female Mice
- Both Rare and Copy Number Variants Are Prevalent in Agenesis of the Corpus Callosum but Not in Cerebellar Hypoplasia or Polymicrogyria
- Reverse PCA, a Systematic Approach for Identifying Genes Important for the Physical Interaction between Protein Pairs
- Partial Deletion of Chromosome 8 β-defensin Cluster Confers Sperm Dysfunction and Infertility in Male Mice
- Genome-Wide and Cell-Specific Epigenetic Analysis Challenges the Role of Polycomb in Spermatogenesis
- Coordinate Regulation of Mature Dopaminergic Axon Morphology by Macroautophagy and the PTEN Signaling Pathway
- Cooperation between RUNX1-ETO9a and Novel Transcriptional Partner KLF6 in Upregulation of in Acute Myeloid Leukemia
- Mobility of the Native Conjugative Plasmid pLS20 Is Regulated by Intercellular Signaling
- FliZ Is a Global Regulatory Protein Affecting the Expression of Flagellar and Virulence Genes in Individual Bacterial Cells
- Specific Tandem Repeats Are Sufficient for Paramutation-Induced Trans-Generational Silencing
- Condensin II Subunit dCAP-D3 Restricts Retrotransposon Mobilization in Somatic Cells
- Dominant Mutations in Identify the Mlh1-Pms1 Endonuclease Active Site and an Exonuclease 1-Independent Mismatch Repair Pathway
- The Insulator Homie Promotes Expression and Protects the Adjacent Gene from Repression by Polycomb Spreading
- Human Intellectual Disability Genes Form Conserved Functional Modules in
- Coordination of Cell Proliferation and Cell Fate Determination by CES-1 Snail
- ORFs in Drosophila Are Important to Organismal Fitness and Evolved Rapidly from Previously Non-coding Sequences
- Different Roles of Eukaryotic MutS and MutL Complexes in Repair of Small Insertion and Deletion Loops in Yeast
- The Spore Differentiation Pathway in the Enteric Pathogen
- Acceleration of the Glycolytic Flux by Steroid Receptor Coactivator-2 Is Essential for Endometrial Decidualization
- The Human Nuclear Poly(A)-Binding Protein Promotes RNA Hyperadenylation and Decay
- Genome Wide Analysis Reveals Zic3 Interaction with Distal Regulatory Elements of Stage Specific Developmental Genes in Zebrafish
- Xbp1 Directs Global Repression of Budding Yeast Transcription during the Transition to Quiescence and Is Important for the Longevity and Reversibility of the Quiescent State
- The Integrator Complex Subunit 6 (Ints6) Confines the Dorsal Organizer in Vertebrate Embryogenesis
- Incorporating Motif Analysis into Gene Co-expression Networks Reveals Novel Modular Expression Pattern and New Signaling Pathways
- The Bacterial Response Regulator ArcA Uses a Diverse Binding Site Architecture to Regulate Carbon Oxidation Globally
- Direct Monitoring of the Strand Passage Reaction of DNA Topoisomerase II Triggers Checkpoint Activation
- Multiple bHLH Proteins form Heterodimers to Mediate CRY2-Dependent Regulation of Flowering-Time in
- A Reversible Histone H3 Acetylation Cooperates with Mismatch Repair and Replicative Polymerases in Maintaining Genome Stability
- ALS-Associated Mutations Result in Compromised Alternative Splicing and Autoregulation
- Robust Demographic Inference from Genomic and SNP Data
- Preferential Binding to Elk-1 by SLE-Associated Risk Allele Upregulates Expression
- Rad52 Sumoylation Prevents the Toxicity of Unproductive Rad51 Filaments Independently of the Anti-Recombinase Srs2
- The Serum Resistome of a Globally Disseminated Multidrug Resistant Uropathogenic Clone
- Identification of 526 Conserved Metazoan Genetic Innovations Exposes a New Role for Cofactor E-like in Neuronal Microtubule Homeostasis
- SUMO Localizes to the Central Element of Synaptonemal Complex and Is Required for the Full Synapsis of Meiotic Chromosomes in Budding Yeast
- Integrated Enrichment Analysis of Variants and Pathways in Genome-Wide Association Studies Indicates Central Role for IL-2 Signaling Genes in Type 1 Diabetes, and Cytokine Signaling Genes in Crohn's Disease
- Genome-Wide High-Resolution Mapping of UV-Induced Mitotic Recombination Events in
- Genome-Wide Analysis of Cell Type-Specific Gene Transcription during Spore Formation in
- Playing the Field: Sox10 Recruits Different Partners to Drive Central and Peripheral Myelination
- Two Portable Recombination Enhancers Direct Donor Choice in Fission Yeast Heterochromatin
- Mining the Human Phenome Using Allelic Scores That Index Biological Intermediates
- Yeast Tdh3 (Glyceraldehyde 3-Phosphate Dehydrogenase) Is a Sir2-Interacting Factor That Regulates Transcriptional Silencing and rDNA Recombination
- A Minimal Nitrogen Fixation Gene Cluster from sp. WLY78 Enables Expression of Active Nitrogenase in
- A Review of Bacteria-Animal Lateral Gene Transfer May Inform Our Understanding of Diseases like Cancer
- High Throughput Sequencing Reveals Alterations in the Recombination Signatures with Diminishing Spo11 Activity
- Partitioning the Heritability of Tourette Syndrome and Obsessive Compulsive Disorder Reveals Differences in Genetic Architecture
- Eleven Candidate Susceptibility Genes for Common Familial Colorectal Cancer
- A GDF5 Point Mutation Strikes Twice - Causing BDA1 and SYNS2
- Systematic Unraveling of the Unsolved Pathway of Nicotine Degradation in
- Natural Genetic Variation of Integrin Alpha L () Modulates Ischemic Brain Injury in Stroke
- Evolutionary Tuning of Protein Expression Levels of a Positively Autoregulated Two-Component System
- Evolutionary Change within a Bipotential Switch Shaped the Sperm/Oocyte Decision in Hermaphroditic Nematodes
- Limiting of the Innate Immune Response by SF3A-Dependent Control of MyD88 Alternative mRNA Splicing
- Multiple Signaling Pathways Coordinate to Induce a Threshold Response in a Chordate Embryo
- Distinct Regulatory Mechanisms Act to Establish and Maintain Pax3 Expression in the Developing Neural Tube
- Genome Wide Analysis of Narcolepsy in China Implicates Novel Immune Loci and Reveals Changes in Association Prior to Versus After the 2009 H1N1 Influenza Pandemic
- Mismatch Repair Genes and Modify CAG Instability in Huntington's Disease Mice: Genome-Wide and Candidate Approaches
- The Histone H3 K27 Methyltransferase KMT6 Regulates Development and Expression of Secondary Metabolite Gene Clusters
- Hsp70-Hsp40 Chaperone Complex Functions in Controlling Polarized Growth by Repressing Hsf1-Driven Heat Stress-Associated Transcription
- Function and Evolution of DNA Methylation in
- Stimulation of mTORC1 with L-leucine Rescues Defects Associated with Roberts Syndrome
- Transcription Termination and Chimeric RNA Formation Controlled by FPA
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dominant Mutations in Identify the Mlh1-Pms1 Endonuclease Active Site and an Exonuclease 1-Independent Mismatch Repair Pathway
- Eleven Candidate Susceptibility Genes for Common Familial Colorectal Cancer
- The Histone H3 K27 Methyltransferase KMT6 Regulates Development and Expression of Secondary Metabolite Gene Clusters
- A Mutation in the Gene in Labrador Retrievers with Hereditary Nasal Parakeratosis (HNPK) Provides Insights into the Epigenetics of Keratinocyte Differentiation
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



