-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaDefensins and Viral Infection: Dispelling Common Misconceptions
article has not abstract
Published in the journal: . PLoS Pathog 10(7): e32767. doi:10.1371/journal.ppat.1004186
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1004186Summary
article has not abstract
Introduction
Human α - and β-defensins are cationic, amphipathic effector peptides of the innate immune system with broad antimicrobial activity [1]. α-defensins are produced by neutrophils (human neutrophil peptides [HNP] 1–4), as well as by epithelial cells in the gut and genitourinary tract (human defensins [HD] 5 and 6). β-defensins (human β-defensins [HBD] 1–4) are constitutively expressed by epithelial cells of skin and mucosal surfaces. Originally discovered due to their antibacterial activity, defensins are also active against both enveloped and non-enveloped viruses. Mechanisms important for bacterial inhibition have been historically assumed to be responsible for defensin antiviral activity; however, this assumption has not held up to experimentation. Our purpose is to address some persistent myths regarding the activity of human defensins against viruses, as well as to identify areas requiring further investigation (Figure 1).
Fig. 1. Common misconceptions of defensin antiviral activity. 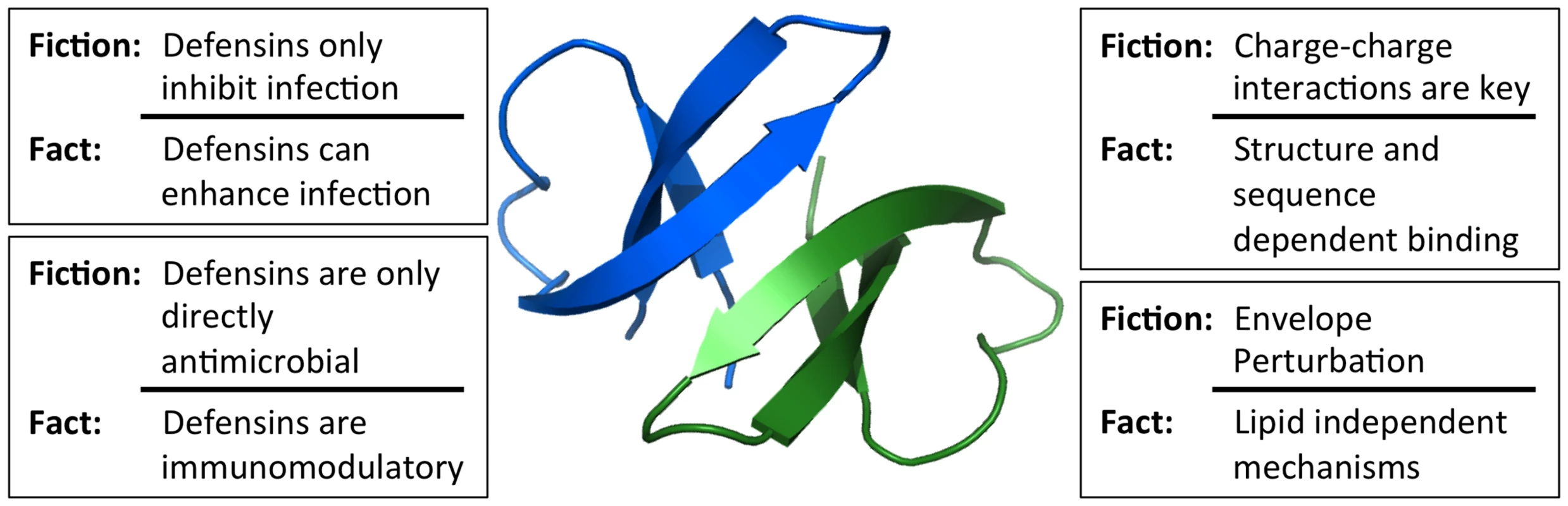
Studies of viral interactions with defensins have dispelled many misconceptions about the key properties of defensins that dictate their activity. The in vivo relevance of these mechanisms for viral pathogenesis has yet to be firmly established. Image of the HD5 dimer was generated using Pymol (PDB: 1ZMP). Misconception: Antiviral Activity Is Only Due to Defensin Positive Charge
The initial antibacterial mechanism was proposed to be largely dependent on simple charge–charge attraction to the bacterial membrane [2]. Accordingly, defensins are most potently antibacterial in hypotonic media [1], [3]. Antiviral activity, in contrast, is generally preserved at physiological salt concentrations in normal cell culture media, arguing against a dominant charge–charge component to their interaction with viruses [4]–[6]. There is additional strong evidence that charge alone does not dictate antiviral activity: linearized α-defensins that lack a disulfide-stabilized 3-D structure are nonfunctional against all viruses tested [6]. Additionally, obligate monomer forms of α-defensins are highly attenuated for binding to and/or inactivation of viral pathogens. [7], [8]. If antiviral activity were simply due to charge–charge interactions, these defensin mutants would still be active, as their charge is conserved. A second line of investigation using direct mutational analysis showed that arginine to lysine substitutions at specific residues attenuate the antiviral activity of HD5 [7]. These results, coupled with the marked preference for arginines over lysines in α-defensins, imply that other aspects of arginine residues are more important than simple charge [6], [9]. Furthermore, β-defensins are, on average, more charged than α-defensins yet largely exhibit less antiviral activity, especially against non-enveloped viruses. Thus, while charge is important for defensin function, it is not the only determining factor in antiviral activity.
Misconception: Defensins Only Work by Damaging the Viral Envelope
Lipid perturbation is a second key component of the canonical defensin antibacterial mechanism that was thought to extend to their antiviral activity. Indeed, an early study found that enveloped viruses were sensitive to neutrophil α-defensins, while the non-enveloped viruses included in this study were not [10]. Despite the pervasiveness of the notion that defensins perturb the lipid bilayer of enveloped viruses, there is scant direct evidence. In fact, the only clear data that supports this idea is the inhibition of respiratory syncytial virus (RSV) by HBD2 [11]. An inability of defensins to directly perturb viral lipid envelopes is consistent with the observation that cholesterol and other neutral lipids, which are commonly found in viral envelopes but not in bacterial membranes, attenuate the membrane lytic activity of defensins and other antimicrobial peptides [3], [12]. Thus, viral inhibition by defensins can occur by multiple mechanisms that are distinct from lipid perturbation, including effects on target cells rather than the virus particle [13]. Importantly, despite the initial study to the contrary, α-defensins have been shown to block infection by multiple non-enveloped viruses, which by definition lack a lipid target. For these viruses, mechanisms include extracellular aggregation, inhibition of viral uncoating, and blocking the viral genome from reaching the nucleus [6]. Overall, the inhibition of non-enveloped viruses and the myriad ways that defensins have been shown to inhibit viral infection do not support a role for lipid perturbation as the defining mechanism.
Misconception: Defensins Exclusively Inhibit Viral Infection
Although the majority of studies have focused on the antiviral activity of defensins, in some cases, α-defensins actually enhance human immunodeficiency virus (HIV) and human adenovirus (HAdV) infection [14], [15]. For both viruses, enhancement is not observed with linearized defensins, indicating that structure-dependent interactions are required. Treatment of HIV with HD5 or HD6 substantially increases infection, which for some strains can reach >100-fold [15]. This enhancement is sufficient to overcome the effects of entry and fusion inhibitors and acts primarily by increasing viral attachment to target cells [16]. Naturally produced HD5 from Neisseria gonorrhoeae–infected cells also enhances HIV infection, suggesting that it is likely to occur under physiological conditions in vivo [15]. We have observed a similar, albeit much more modest, HNP1 - and HD5-dependent increase in infection by certain serotypes of HAdV, which is also correlated with increased receptor-dependent and -independent attachment to cells [7], [14]. Whereas HIV is sensitive to HNPs but enhanced by HD5 and HD6, HAdV serotypes appear to be more uniformly resistant or sensitive to α-defensins in general. Given that infection by two disparate viral families is enhanced by defensins, it would not be surprising if this were true for other viruses. It also remains to be seen if enhancement occurs in vivo and whether enhancement or inhibition predominates.
Misconception: Defensin In Vitro Activity Predicts Their Role In Vivo
In addition to direct effects on viral infection, defensins also target cells and are immunomodulatory [6], [17], [18]. Thus, even defensins that are not directly antiviral could influence the activation and function of immune cells recruited to a site of viral infection, thereby impacting viral pathogenesis in vivo. These mechanisms likely explain the immunopathology of influenza virus infection in a mouse lacking β-defensin 1 [19]. They also function when defensins are used as adjuvants by altering the numbers and specific subsets of immune cells recruited in response to model antigens [20]. Whether this adjuvant effect occurs in the context of a pathogenic infection has not been shown. Moreover, the cellular receptors for many defensins are unknown, making the mechanism by which chemotaxis occurs unclear. Separate from these immunomodulatory activities, work in animal models with altered defensin expression levels has shown that the defensin repertoire impacts the composition of the commensal microbiota [21]. A logical extension of these studies is that the commensal population shaped by defensin expression could influence susceptibility to viral infection. Although these are all mechanisms by which defensins could potentially influence viral infection, there is minimal data as to their actual effect on viral pathogenesis in vivo.
Conclusions
Cell culture studies have provided the vast majority of the evidence demonstrating a direct antiviral effect of defensins under assay conditions that do not result in defensin-mediated cytotoxicity [22]. Indirect evidence for the importance of defensins in vivo comes from a limited number of association studies of defensin levels with viral disease states or progression [6], [13]. Although mouse models with altered or transgenic defensin expression exist, a clean genetic knockout has not been generated due to the complexity and the extent of the mouse defensin locus [23]. In addition, there is no animal model that completely recapitulates the human defensin repertoire. Mice, in particular, lack myeloid α-defensins. Due to the paucity of data, the most pressing issue in the field is to firmly establish in vivo relevance for the growing body of in vitro data characterizing the antiviral properties of these interesting and multifunctional peptides.
Zdroje
1. SelstedME, OuelletteAJ (2005) Mammalian defensins in the antimicrobial immune response. Nat Immunol 6 : 551–557 doi:10.1038/ni1206
2. GanzT (2003) Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol 3 : 710–720.
3. ZasloffM (2002) Antimicrobial peptides of multicellular organisms. Nature 415 : 389–395 doi:10.1038/415389a
4. SmithJG, NemerowGR (2008) Mechanism of Adenovirus Neutralization by Human α-Defensins. Cell Host Microbe 3 : 11–19 doi:10.1016/j.chom.2007.12.001
5. Quiñones-MateuME, LedermanMM, FengZ, ChakrabortyB, WeberJ, et al. (2003) Human epithelial beta-defensins 2 and 3 inhibit HIV-1 replication. AIDS 17: F39–F48 doi:10.1097/01.aids.0000096878.73209.4f
6. WilsonSS, WiensME, SmithJG (2013) Antiviral mechanisms of human defensins. J Mol Biol 425 : 4965–4980 doi:10.1016/j.jmb.2013.09.038
7. GounderAP, WiensME, WilsonSS, LuW, SmithJG (2012) Critical Determinants of Human -Defensin 5 Activity against Non-enveloped Viruses. J Biol Chem 287 : 24554–24562 doi:10.1074/jbc.M112.354068
8. PazgierM, WeiG, EricksenB, JungG, WuZ, et al. (2012) Sometimes it takes two to tango: contributions of dimerization to functions of human α-defensin HNP1 peptide. J Biol Chem 287 : 8944–8953 doi:10.1074/jbc.M111.332205
9. ZouG, de LeeuwE, LiC, PazgierM, LiC, et al. (2007) Toward understanding the cationicity of defensins. Arg and Lys versus their noncoded analogs. J Biol Chem 282 : 19653–19665 doi:10.1074/jbc.M611003200
10. DaherKA, SelstedME, LehrerRI (1986) Direct inactivation of viruses by human granulocyte defensins. Journal Virol 60 : 1068–1074.
11. KotaS, SabbahA, ChangTH, HarnackR, XiangY, et al. (2008) Role of human beta-defensin-2 during tumor necrosis factor-alpha/NF-kappaB-mediated innate antiviral response against human respiratory syncytial virus. J Biol Chem 283 : 22417–22429 doi:10.1074/jbc.M710415200
12. FujiiG, SelstedME, EisenbergD (1993) Defensins promote fusion and lysis of negatively charged membranes. Protein Sci 2 : 1301–1312 doi:10.1002/pro.5560020813
13. Shah R, Chang TL (2012) Defensins in Viral Infection. Rajasekaran K, Cary JW, Jaynes JM, Montesinos E, editors. Washington, D.C.: American Chemical Society. 35 p. doi:10.1021/bk-2012-1095.ch007
14. SmithJG, SilvestryM, LindertS, LuW, NemerowGR, et al. (2010) Insight into the mechanisms of adenovirus capsid disassembly from studies of defensin neutralization. PLoS Pathog 6: e1000959 doi:10.1371/journal.ppat.1000959
15. KlotmanME, RapistaA, TeleshovaN, MicsenyiA, JarvisGA, et al. (2008) Neisseria gonorrhoeae-induced human defensins 5 and 6 increase HIV infectivity: role in enhanced transmission. J Immunol 180 : 6176–6185.
16. RapistaA, DingJ, BenitoB, LoY-T, NeiditchMB, et al. (2011) Human defensins 5 and 6 enhance HIV-1 infectivity through promoting HIV attachment. Retrovirology 8 : 45 doi:10.1186/1742-4690-8-45
17. BowdishDME, DavidsonDJ, HancockREW (2006) Immunomodulatory properties of defensins and cathelicidins. Curr Top Microbiol Immunol 306 : 27–66.
18. LeikinaE, Delanoe-AyariH, MelikovK, ChoM-S, ChenA, et al. (2005) Carbohydrate-binding molecules inhibit viral fusion and entry by crosslinking membrane glycoproteins. Nat Immunol 6 : 995–1001 doi:10.1038/ni1248
19. RyanLK, DaiJ, YinZ, MegjugoracN, UhlhornV, et al. (2011) Modulation of human beta-defensin-1 (hBD-1) in plasmacytoid dendritic cells (PDC), monocytes, and epithelial cells by influenza virus, Herpes simplex virus, and Sendai virus and its possible role in innate immunity. J Leukoc Biol 90 : 343–356 doi:10.1189/jlb.0209079
20. LillardJW, BoyakaPN, ChertovO, OppenheimJJ, McGheeJR (1999) Mechanisms for induction of acquired host immunity by neutrophil peptide defensins. Proc Natl Acad Sci U S A 96 : 651–656 doi:10.1073/pnas.96.2.651
21. SalzmanNH, HungK, HaribhaiD, ChuH, Karlsson-SjöbergJ, et al. (2010) Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol 11 : 76–83 doi:10.1038/ni.1825
22. DingJ, TaskerC, ValereK, SihvonenT, Descalzi-MontoyaDB, et al. (2013) Anti-HIV Activity of Human Defensin 5 in Primary CD4+ T Cells under Serum-Deprived Conditions Is a Consequence of Defensin-Mediated Cytotoxicity. PLoS ONE 8: e76038 doi:10.1371/journal.pone.0076038
23. BevinsCL, SalzmanNH (2011) Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol 9 : 356–368 doi:10.1038/nrmicro2546
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Molecular and Cellular Mechanisms of KSHV Oncogenesis of Kaposi's Sarcoma Associated with HIV/AIDSČlánek The Semen Microbiome and Its Relationship with Local Immunology and Viral Load in HIV InfectionČlánek Peptidoglycan Recognition Proteins Kill Bacteria by Inducing Oxidative, Thiol, and Metal Stress
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 7- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
-
Všechny články tohoto čísla
- Bacteriophages as Vehicles for Antibiotic Resistance Genes in the Environment
- Helminth Infections, Type-2 Immune Response, and Metabolic Syndrome
- Defensins and Viral Infection: Dispelling Common Misconceptions
- Holobiont–Holobiont Interactions: Redefining Host–Parasite Interactions
- The Wide World of Ribosomally Encoded Bacterial Peptides
- Microbial Egress: A Hitchhiker's Guide to Freedom
- Molecular and Cellular Mechanisms of KSHV Oncogenesis of Kaposi's Sarcoma Associated with HIV/AIDS
- HIV-1 Capture and Transmission by Dendritic Cells: The Role of Viral Glycolipids and the Cellular Receptor Siglec-1
- Tetherin Can Restrict Cell-Free and Cell-Cell Transmission of HIV from Primary Macrophages to T Cells
- The Frustrated Host Response to Is Bypassed by MyD88-Dependent Translation of Pro-inflammatory Cytokines
- Larger Mammalian Body Size Leads to Lower Retroviral Activity
- The Semen Microbiome and Its Relationship with Local Immunology and Viral Load in HIV Infection
- Lytic Gene Expression Is Frequent in HSV-1 Latent Infection and Correlates with the Engagement of a Cell-Intrinsic Transcriptional Response
- Phase Variation of Poly-N-Acetylglucosamine Expression in
- A Screen of Mutants Reveals Important Roles for Dot/Icm Effectors and Host Autophagy in Vacuole Biogenesis
- Structure of the Trehalose-6-phosphate Phosphatase from Reveals Key Design Principles for Anthelmintic Drugs
- The Impact of Juvenile Coxsackievirus Infection on Cardiac Progenitor Cells and Postnatal Heart Development
- Vertical Transmission Selects for Reduced Virulence in a Plant Virus and for Increased Resistance in the Host
- Characterization of the Largest Effector Gene Cluster of
- Novel Drosophila Viruses Encode Host-Specific Suppressors of RNAi
- Pto Kinase Binds Two Domains of AvrPtoB and Its Proximity to the Effector E3 Ligase Determines if It Evades Degradation and Activates Plant Immunity
- Genetic Analysis of Tropism Using a Naturally Attenuated Cutaneous Strain
- Plasmacytoid Dendritic Cells Suppress HIV-1 Replication but Contribute to HIV-1 Induced Immunopathogenesis in Humanized Mice
- A Novel Mouse Model of Gastroenteritis Reveals Key Pro-inflammatory and Tissue Protective Roles for Toll-like Receptor Signaling during Infection
- Pathogenicity of Is Expressed by Regulating Metabolic Thresholds of the Host Macrophage
- BCKDH: The Missing Link in Apicomplexan Mitochondrial Metabolism Is Required for Full Virulence of and
- Independent Bottlenecks Characterize Colonization of Systemic Compartments and Gut Lymphoid Tissue by
- Peptidoglycan Recognition Proteins Kill Bacteria by Inducing Oxidative, Thiol, and Metal Stress
- G3BP1, G3BP2 and CAPRIN1 Are Required for Translation of Interferon Stimulated mRNAs and Are Targeted by a Dengue Virus Non-coding RNA
- Cytolethal Distending Toxins Require Components of the ER-Associated Degradation Pathway for Host Cell Entry
- The Machinery at Endoplasmic Reticulum-Plasma Membrane Contact Sites Contributes to Spatial Regulation of Multiple Effector Proteins
- Arabidopsis LIP5, a Positive Regulator of Multivesicular Body Biogenesis, Is a Critical Target of Pathogen-Responsive MAPK Cascade in Plant Basal Defense
- Plant Surface Cues Prime for Biotrophic Development
- Real-Time Imaging Reveals the Dynamics of Leukocyte Behaviour during Experimental Cerebral Malaria Pathogenesis
- The CD27L and CTP1L Endolysins Targeting Contain a Built-in Trigger and Release Factor
- cGMP and NHR Signaling Co-regulate Expression of Insulin-Like Peptides and Developmental Activation of Infective Larvae in
- Systemic Hematogenous Maintenance of Memory Inflation by MCMV Infection
- Strain-Specific Variation of the Decorin-Binding Adhesin DbpA Influences the Tissue Tropism of the Lyme Disease Spirochete
- Distinct Lipid A Moieties Contribute to Pathogen-Induced Site-Specific Vascular Inflammation
- Serovar Typhi Conceals the Invasion-Associated Type Three Secretion System from the Innate Immune System by Gene Regulation
- LANA Binds to Multiple Active Viral and Cellular Promoters and Associates with the H3K4Methyltransferase hSET1 Complex
- A Molecularly Cloned, Live-Attenuated Japanese Encephalitis Vaccine SA-14-2 Virus: A Conserved Single Amino Acid in the Hairpin of the Viral E Glycoprotein Determines Neurovirulence in Mice
- Illuminating Fungal Infections with Bioluminescence
- Comparative Genomics of Plant Fungal Pathogens: The - Paradigm
- Motility and Chemotaxis Mediate the Preferential Colonization of Gastric Injury Sites by
- Widespread Sequence Variations in VAMP1 across Vertebrates Suggest a Potential Selective Pressure from Botulinum Neurotoxins
- An Immunity-Triggering Effector from the Barley Smut Fungus Resides in an Ustilaginaceae-Specific Cluster Bearing Signs of Transposable Element-Assisted Evolution
- Establishment of Murine Gammaherpesvirus Latency in B Cells Is Not a Stochastic Event
- Oncogenic Herpesvirus KSHV Hijacks BMP-Smad1-Id Signaling to Promote Tumorigenesis
- Human APOBEC3 Induced Mutation of Human Immunodeficiency Virus Type-1 Contributes to Adaptation and Evolution in Natural Infection
- Innate Immune Responses and Rapid Control of Inflammation in African Green Monkeys Treated or Not with Interferon-Alpha during Primary SIVagm Infection
- Chitin-Degrading Protein CBP49 Is a Key Virulence Factor in American Foulbrood of Honey Bees
- Influenza A Virus Host Shutoff Disables Antiviral Stress-Induced Translation Arrest
- Nsp9 and Nsp10 Contribute to the Fatal Virulence of Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus Emerging in China
- Pulmonary Infection with Hypervirulent Mycobacteria Reveals a Crucial Role for the P2X7 Receptor in Aggressive Forms of Tuberculosis
- Syk Signaling in Dendritic Cells Orchestrates Innate Resistance to Systemic Fungal Infection
- A Repetitive DNA Element Regulates Expression of the Sialic Acid Binding Adhesin by a Rheostat-like Mechanism
- T-bet and Eomes Are Differentially Linked to the Exhausted Phenotype of CD8+ T Cells in HIV Infection
- Israeli Acute Paralysis Virus: Epidemiology, Pathogenesis and Implications for Honey Bee Health
- Influence of ND10 Components on Epigenetic Determinants of Early KSHV Latency Establishment
- Antibody to gp41 MPER Alters Functional Properties of HIV-1 Env without Complete Neutralization
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Molecular and Cellular Mechanisms of KSHV Oncogenesis of Kaposi's Sarcoma Associated with HIV/AIDS
- Holobiont–Holobiont Interactions: Redefining Host–Parasite Interactions
- BCKDH: The Missing Link in Apicomplexan Mitochondrial Metabolism Is Required for Full Virulence of and
- Helminth Infections, Type-2 Immune Response, and Metabolic Syndrome
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



