-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaGenome-Wide Association for Abdominal Subcutaneous and Visceral Adipose Reveals a Novel Locus for Visceral Fat in Women
Body fat distribution, particularly centralized obesity, is associated with metabolic risk above and beyond total adiposity. We performed genome-wide association of abdominal adipose depots quantified using computed tomography (CT) to uncover novel loci for body fat distribution among participants of European ancestry. Subcutaneous and visceral fat were quantified in 5,560 women and 4,997 men from 4 population-based studies. Genome-wide genotyping was performed using standard arrays and imputed to ∼2.5 million Hapmap SNPs. Each study performed a genome-wide association analysis of subcutaneous adipose tissue (SAT), visceral adipose tissue (VAT), VAT adjusted for body mass index, and VAT/SAT ratio (a metric of the propensity to store fat viscerally as compared to subcutaneously) in the overall sample and in women and men separately. A weighted z-score meta-analysis was conducted. For the VAT/SAT ratio, our most significant p-value was rs11118316 at LYPLAL1 gene (p = 3.1×10E-09), previously identified in association with waist–hip ratio. For SAT, the most significant SNP was in the FTO gene (p = 5.9×10E-08). Given the known gender differences in body fat distribution, we performed sex-specific analyses. Our most significant finding was for VAT in women, rs1659258 near THNSL2 (p = 1.6×10-08), but not men (p = 0.75). Validation of this SNP in the GIANT consortium data demonstrated a similar sex-specific pattern, with observed significance in women (p = 0.006) but not men (p = 0.24) for BMI and waist circumference (p = 0.04 [women], p = 0.49 [men]). Finally, we interrogated our data for the 14 recently published loci for body fat distribution (measured by waist–hip ratio adjusted for BMI); associations were observed at 7 of these loci. In contrast, we observed associations at only 7/32 loci previously identified in association with BMI; the majority of overlap was observed with SAT. Genome-wide association for visceral and subcutaneous fat revealed a SNP for VAT in women. More refined phenotypes for body composition and fat distribution can detect new loci not previously uncovered in large-scale GWAS of anthropometric traits.
Published in the journal: . PLoS Genet 8(5): e32767. doi:10.1371/journal.pgen.1002695
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002695Summary
Body fat distribution, particularly centralized obesity, is associated with metabolic risk above and beyond total adiposity. We performed genome-wide association of abdominal adipose depots quantified using computed tomography (CT) to uncover novel loci for body fat distribution among participants of European ancestry. Subcutaneous and visceral fat were quantified in 5,560 women and 4,997 men from 4 population-based studies. Genome-wide genotyping was performed using standard arrays and imputed to ∼2.5 million Hapmap SNPs. Each study performed a genome-wide association analysis of subcutaneous adipose tissue (SAT), visceral adipose tissue (VAT), VAT adjusted for body mass index, and VAT/SAT ratio (a metric of the propensity to store fat viscerally as compared to subcutaneously) in the overall sample and in women and men separately. A weighted z-score meta-analysis was conducted. For the VAT/SAT ratio, our most significant p-value was rs11118316 at LYPLAL1 gene (p = 3.1×10E-09), previously identified in association with waist–hip ratio. For SAT, the most significant SNP was in the FTO gene (p = 5.9×10E-08). Given the known gender differences in body fat distribution, we performed sex-specific analyses. Our most significant finding was for VAT in women, rs1659258 near THNSL2 (p = 1.6×10-08), but not men (p = 0.75). Validation of this SNP in the GIANT consortium data demonstrated a similar sex-specific pattern, with observed significance in women (p = 0.006) but not men (p = 0.24) for BMI and waist circumference (p = 0.04 [women], p = 0.49 [men]). Finally, we interrogated our data for the 14 recently published loci for body fat distribution (measured by waist–hip ratio adjusted for BMI); associations were observed at 7 of these loci. In contrast, we observed associations at only 7/32 loci previously identified in association with BMI; the majority of overlap was observed with SAT. Genome-wide association for visceral and subcutaneous fat revealed a SNP for VAT in women. More refined phenotypes for body composition and fat distribution can detect new loci not previously uncovered in large-scale GWAS of anthropometric traits.
Introduction
Obesity is an important risk factor for cardiometabolic outcomes [1]–[5]. Heterogeneity in the regional deposition of fat, particularly, visceral adipose tissue (VAT), may be more deleterious than total body obesity. Numerous epidemiologic studies have demonstrated that central obesity, measured by simple anthropometric measures including waist circumference or waist-hip-ratio (WHR), is associated with cardiovascular disease (CVD) and glucose, insulin, and lipid metabolism, independent of overall obesity as measured by body mass index (BMI) [6]–[19]. However, waist circumference is limited due to its inability to discriminate between VAT and subcutaneous adipose tissue (SAT) [20]. Computed tomography (CT) provides a more direct and precise assessment of adipose tissue compartments. In many studies, the associations between CVD risk factors and directly-measured VAT are stronger than the associations observed with other typical anthropometric measures [21]–[28].
Prior studies have shown that indices of body fat distribution, including waist circumference, VAT, and SAT are heritable [20], [29]–[31]. A recent large-scale genome-wide association study (GWAS) identified 14 loci in association with waist-hip-ratio [32], providing proof-of-principle for the concept that genetic variants are associated with body fat distribution above and beyond generalized adiposity. However, there are currently no large-scale GWAS for directly-measured VAT and SAT. Thus, the purpose of the present study was to perform GWAS for VAT and SAT in 4 large population-based cohorts. We analyzed SAT, VAT, VAT adjusted for BMI, and the VAT/SAT ratio, a metric of the propensity to store fat viscerally as compared to subcutaneously. Given the known sex differences in body fat distribution [33], we additionally performed sex-specific analyses.
Results
The characteristics of the study sample are presented in Table 1 and Table S1. Overall, 5560 women and 4997 men were available for analysis. Study participants ranged in age from their thirties to their mid seventies, and BMI ranged from 26.6 kg/m2 to 28.8 kg/m2.
Tab. 1. Study Sample Characteristics, VATGen Consortium. 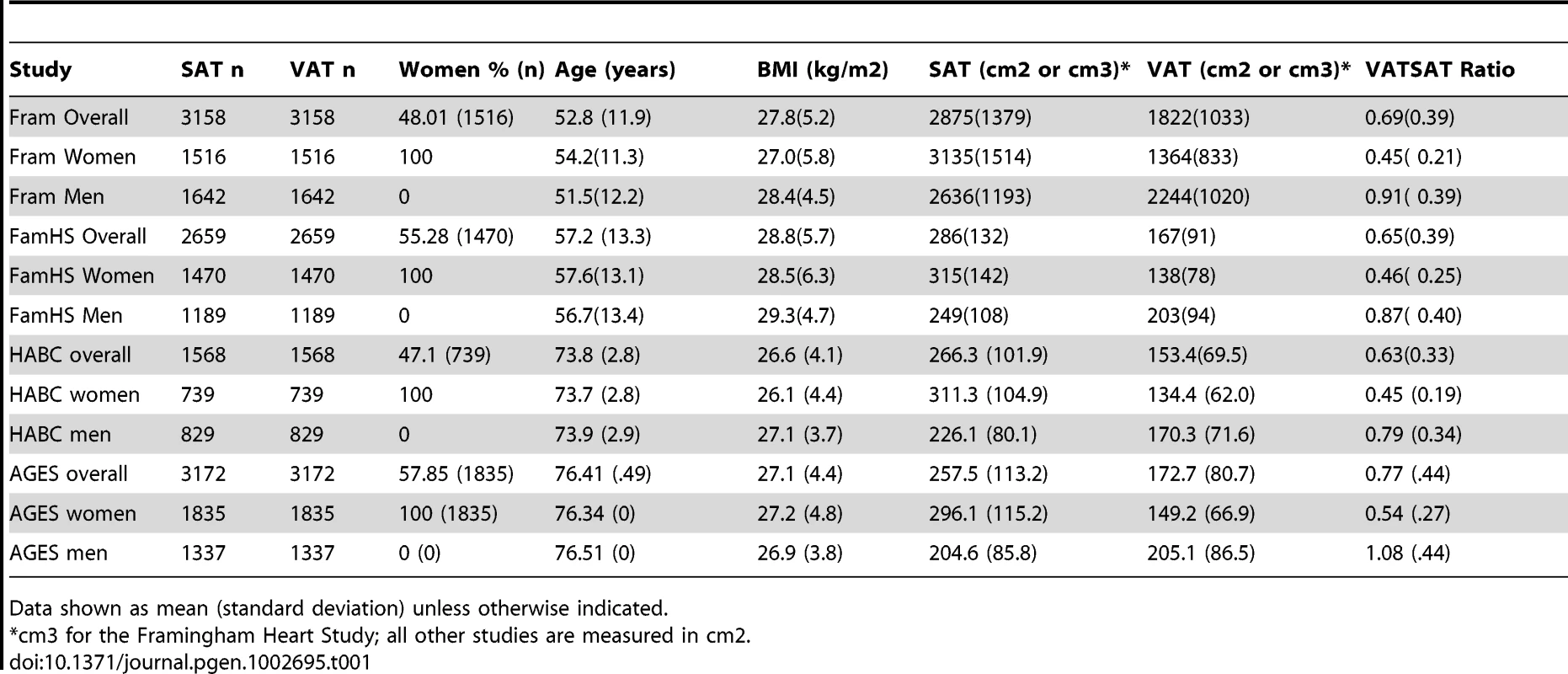
Data shown as mean (standard deviation) unless otherwise indicated. Heritability Analyses
In order to document a genetic or familial component of directly-imaged CT adipose tissue traits, we previously performed heritability analyses of VAT (h2 36%) and SAT (h2 57%) [20]. For the present analysis, we additionally calculated the heritability of VAT and SAT in the Family Heart Study and the VAT/SAT ratio in both the Family Heart Study and the Framingham Heart Study. In the Family Heart Study, the heritability of VAT and SAT was 36% and 44%, respectively. The heritability of the VAT/SAT ratio was 43%; after adjustment for either BMI or VAT, the heritability was not materially different (44% and 47%, respectively). Similarly, we found that the heritability of the VAT/SAT ratio was 55% (p<0.0001) in the Framingham Heart Study, which was essentially unchanged after adjustment for BMI (h2 55%) or VAT (64%).
Stage 1 Discovery Results
After confirming a heritable component to directly imaged CT adipose tissue traits, we proceeded with GWAS. To assess for occult population stratification, we examined q-q plots for all traits (SAT, VAT, VAT-adjusted-for-BMI, and VAT/SAT ratio in the overall sample and in women and men separately), which can be found in Figure S1. All lambda values were <1.08, with little evidence to suggest unaccounted for population stratification. Manhattan plots for these traits can be found in Figure S2, with p-values<5.0*10E08 for the VAT/SAT ratio overall and VAT in women.
Our most significant finding was for rs11118316 at LYPLAL1 for the VAT/SAT ratio (Table S2). This SNP is in low to moderate LD with rs4846567 (r2 = 0.285, D′ 0.935), which was previously identified in the GIANT consortium in association with WHR-adjusted-for-BMI [32]. It is notable that in the GIANT consortium, rs4846567 was only associated with WHR in women (p = 4.9*10E-33) but not men (p = 0.36), whereas rs11118316 was associated with both women (p = 4.5*10E-6) and men (p = 8.3*10E-5) in the present analysis. We further note that for rs4846567, the p-value is 4.4*10E-04 in women but p = 0.05 in men. Thus, it is possible that we have identified a slightly different locus with varying sex differences. Our next genome-wide significant finding was for rs1659258 at chromosome 2 for VAT in women (p = 1.58*10E-08; Table S2 and Figure 1). Imputation scores for this SNP ranged from 0.98 to 1.0. This region has not previously been identified in association with adiposity phenotypes. Table 2 shows the results for rs1659258 across the abdominal adiposity traits in our meta-analysis. We observed no association in men (p = 0.75) for VAT. In women, we observed a nominally-significant signal for SAT in women (p = 0.002), and for VAT-adjusted-for-BMI (p = 6.9*10E-05). Figure 2 shows the standardized beta coefficients in women as compared to men for all traits in each contributing study.
Fig. 1. Regional Association Plot of the Chromosome 2 region for VAT in women. 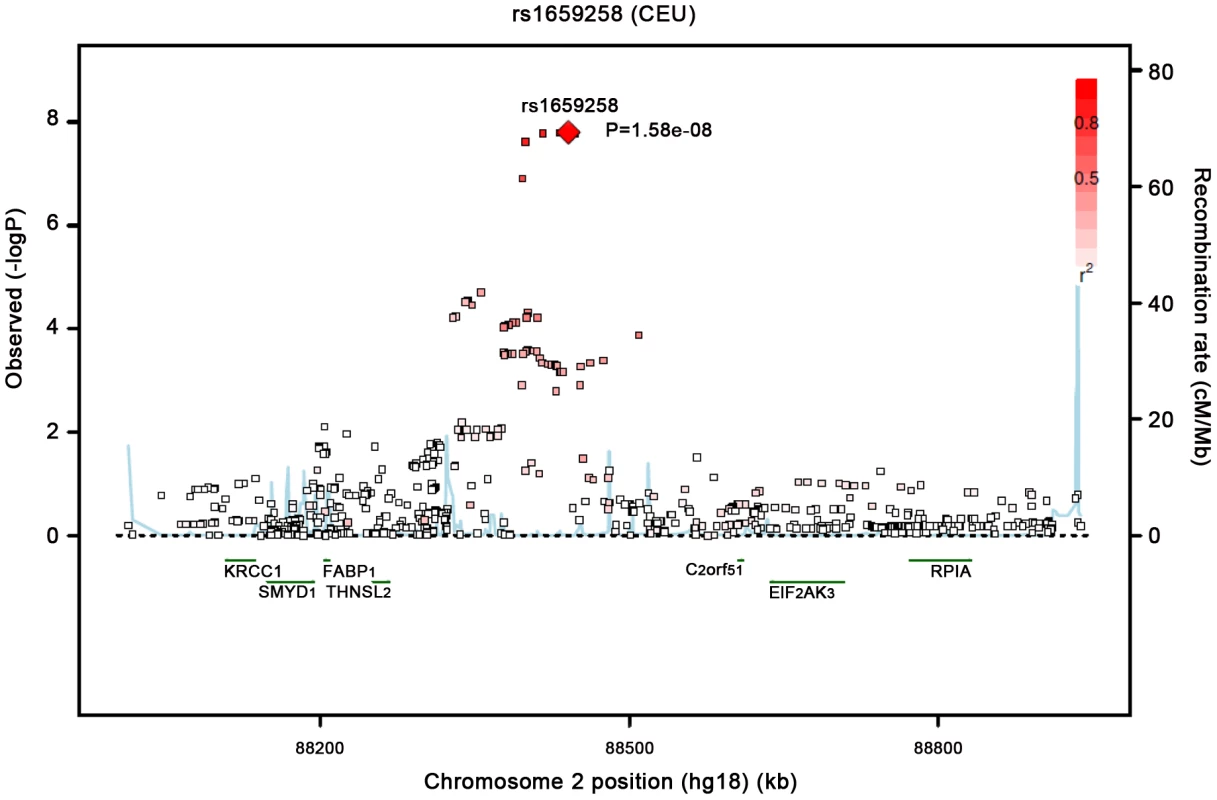
Fig. 2. Association of rs1659258 in all 4 discovery cohorts. 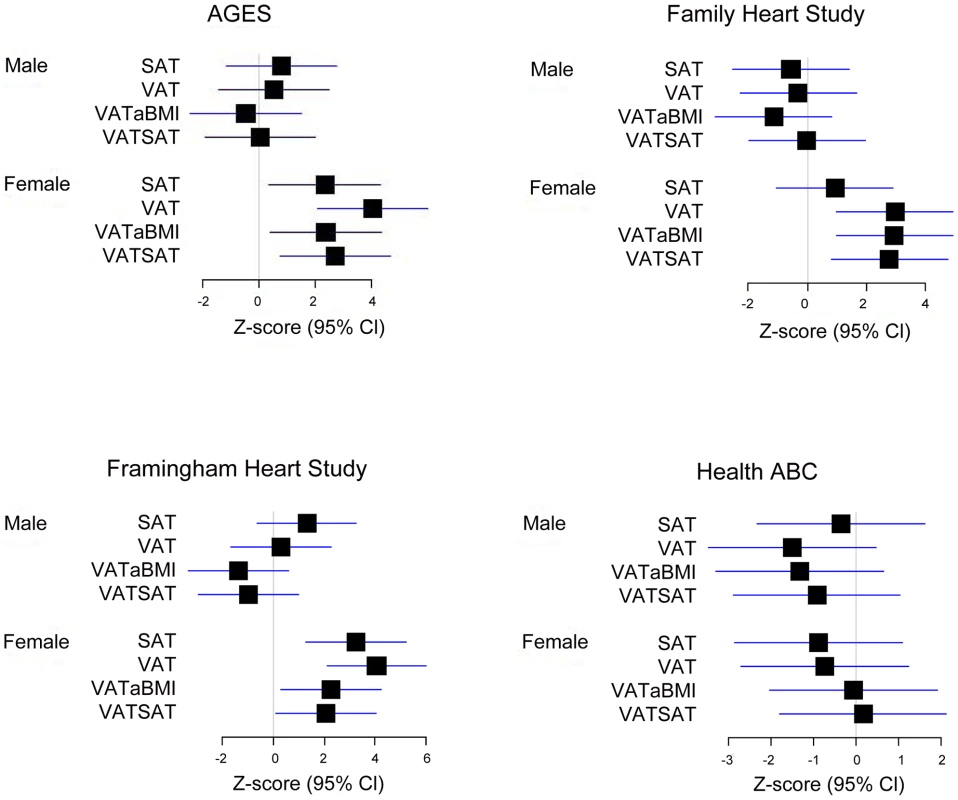
Results are shown modeled per copy of the trait-increasing A allele. Within each study, data presented represent the beta coefficient indexed to the standard error. Bars represent 95% confidence intervals. VATSAT is the VAT/SAT ratio, and VATaBMI is VAT-adjusted-for-BMI. Tab. 2. Results of rs1659258 in the VATGen meta-analysis; results modeled per copy of the trait-increasing A allele and for independent validation in the GIANT Consortium (non-overlapping studies).* 
GIANT sample sizes for women and men are as follows: BMI (58208, 49092); WC (39471, 31406). Given our finding of a stronger association in women as compared to men at rs1659258 for VAT, we formally tested a sex interaction term, and found a significant effect in the magnitude of the association between our lead SNP and VAT in women as compared to men (pinteraction = 0.0002).
All additional independent SNPs with p-values<9.9*10E-06 can be found in Table S2.
Stage 2 Validation
We focused on rs1659258 as a new locus for independent validation in the non-overlapping cohorts that are part of the GIANT consortium data. Results are displayed in Table 2, with evidence for replication at this locus in women (p = 0.006) but not men (p = 0.24) for BMI. Similar results were observed for waist circumference.
Metabolic Traits Association Studies with rs1659258
After confirming the association of rs1659258 with measures of adiposity in a sex-specific manner, we next sought to perform association studies with correlated metabolic traits. Because VAT is associated with glycemic and lipid traits [20], [34]–[37], we requested sex-specific associations of rs1659258 in the Global Lipids Genetics Consortium [38] for lipid traits and in the MAGIC consortium for traits related to glucose metabolism. Overall, we observed a nominal and direction-consistent association (i.e., same allele associated with higher VAT and lower HDL) with HDL (p = 0.019, n = 98,263), but no robust associations for LDL, triglycerides, or total cholesterol (all p>0.06), which is surprising given the phenotypic correlations between VAT and triglycerides [20]. In sex-specific analyses, we observed no association for this SNP with HDL, triglycerides, or total cholesterol for either women (all p>0.09, personal communication) or men (all p>0.73, personal communication). In MAGIC, we observed a direction-consistent and borderline statistically significant result for this SNP in women (p = 0.048, n = 43,754) but not men (p = 0.39, n = 36,514) for fasting glucose (personal communication, MAGIC consortium).
Test of Age Interaction with rs1659258
Because fat distribution can vary by age, we performed a formal age interaction test between rs1659258 and VAT in women only. Stratifying above and below age 60 years, we did not observe a significant sex interaction (p = 0.79).
Interrogation of Published Loci for WHR and BMI Loci
In order to understand whether directly-imaged adipose traits are associated with previously-identified loci in a GWAS for fat distribution using anthropometric traits, we performed an association analysis of the 14 previously-published loci for WHR-adjusted-for-BMI for all 4 of our traits in the overall sample and by sex (Table 3) in the results from the GIANT consortium [32]. Overall, we observed associations (defined as p<0.01) for 7/14 of the previously reported loci, most of which were direction-consistent (with the exception of loci associated with SAT, consistent with the fact that SAT and hip are highly correlated traits [r = 0.92 among 2656 individuals from the Family Heart Study]). We observed only one association with VAT at the NISCH-STAB1 locus.
Tab. 3. Association of SNPs from a Recently Published GWAS of Body Fat Distribution* (Heid IM et al, NG, 2010) [32]. ![Association of SNPs from a Recently Published GWAS of Body Fat Distribution<em class="ref">*</em> (Heid IM et al, NG, 2010) <em class="ref">[32]</em>.](https://www.prolekare.cz/media/cache/resolve/media_object_image_small/media/image/d57e0c77a0f86e2a689f8f594a80ef99.png)
All data modeled relative to the previously-published trait-increasing allele; the z-statistic indicates the effect direction relative to the coded allele. We additionally performed an association analysis of all of the 32 published BMI loci identified via the GIANT consortium (Table 4) [39]. Overall, we observed associations (defined as p<0.01) at 7/32 loci, the majority of which overlapped for SAT and were all direction-consistent with the exception of VAT/SAT ratio at NEGR1. We observed very few associations with VAT, with the exception of FTO and NRXN3.
Tab. 4. Association of validated SNPs for BMI (from Speliotes et al, Nature Genetics 2010) [39]. ![Association of validated SNPs for BMI (from Speliotes et al, Nature Genetics 2010) <em class="ref">[39]</em>.](https://www.prolekare.cz/media/cache/resolve/media_object_image_small/media/image/c96252631cfd6ef3a7ab7b261d4986cf.png)
All CT traits presented with the same coded allele, and all are modeled relative to the previously-published BMI trait-increasing allele. Z-statistic indicates direction relative to the coded allele. eQTL Results
In order to help identify the potential causal gene in the region surrounding our lead SNP, we first reviewed publically available databases, but did not identify any associations of rs1659258 with eQTLs (see methods for additional details). However, most of these databases consist of women and men in combined analyses; given that our GWAS finding was in women alone, we performed sex-specific eQTL analyses of rs1659258 in up to 848 patients (mean BMI 50.5 kg/m2) who underwent Roux-en-Y gastric bypass surgery who also underwent subcutaneous and visceral fat biopsy [40]. We performed eQTL testing in the 1 MB region surrounding rs1659258 (n = 31 genes). Using a corrected p-value threshold of p<0.05, only THNSL2 expression in subcutaneous fat in women was associated with our lead SNP (p = 0.03) but not men (p = 0.96). We did not observe association with expression in VAT in either women or men.
Discussion
Principal Findings
We have uncovered a new locus for VAT at THNSL2 in women that reveals a striking sexual dimorphism, where we observed significance only in women, but not men. We also observed genome-wide significance for rs11118316 at LYPLAL1 for the VAT/SAT ratio; this region was previously identified in a GWAS for WHR in the GIANT consortium, although our lead SNP is only in moderate LD with the SNP identified by GIANT. Finally, we performed targeted SNP evaluations of 14 SNPs previously identified in association with central fat distribution and identified nominal associations at 7 loci.
In the Context of the Current Literature
Prior genome-wide association studies have focused on using anthropometric measures to define body fat distribution. The GIANT consortium identified 14 loci associated with WHR adjusted for BMI [32], the majority of which demonstrated stronger associations in women as compared to men. More recently, a SNP at the IRS1 locus was identified in association with body percent fat in men, but not women [41]. We showed that the VAT/SAT ratio in men was associated with this same IRS1 SNP [41]. Taken together, these prior data suggest that additional loci may exist in association with fat distribution above and beyond those associated with generalized adiposity. In the present study, we identified a new locus in association with VAT in women, highlighting the utility of more precise phenotyping of abdominal fat distribution.
We also extend the observations of a marked sexual dimorphism that have been made using primarily anthropometric measurements to CT-imaged fat depots. It is well-known that in women as compared with men, VAT levels are relatively lower, and SAT levels are higher [42]. This gender difference underscores the need to consider women and men separately in assessing the genetic architecture of fat distribution. We also note that our most significant SNP at the LYPLAL1 locus was associated with the VAT/SAT ratio in both women and men as compared to only WHR in GIANT [32], while the lead SNP identified in GIANT demonstrated some evidence for heterogeneity by sex with the VAT/SAT ratio. Taken together, these findings highlight the potential differences in directly-imaged fat distribution traits as compared to anthropometric data.
An important consideration is whether our finding of rs1659258 at THNSL2 in association with VAT in women represents a variant that is specific to central adiposity. Indeed, our strongest result is derived from VAT itself. However, we observed associations with SAT in women, and our validation data suggests this SNP is also associated with BMI in women. Once we adjusted the VAT trait for BMI, our SNP did not completely lose its statistical significance (p = 6.9*10E-05), suggesting that there is some specificity of our finding to fat deposition in the central abdominal compartment. This result is in contrast to our prior results for a SNP in NRXN3 in association with waist circumference: upon adjustment for BMI, statistical significance was attenuated [43]. Finally, our eQTL data reveals nominal expression in subcutaneous but not visceral adipose tissue.
Potential Underlying Biology
Our lead SNP for VAT in women, rs1659258, is located in an intergenic region upstream from THNSL2 and FABP1. However, rs1659258 is not in linkage disequilibrium (LD) with any coding SNPs within 88,200-88,700 kb. In addition, the correlations between rs1659258 with coding SNPs in FOXI3, C2orf51, THNSL2, EIF2AK3, FABP1, and SMYD1 genes are low (r2<0.15). Finally, there is no evidence that 2p11-p12, where rs1659258 is located, has been previously implicated in association with copy number variation in adipose-related human disease. Nonetheless, we explored the potential biology in this region. Fatty acid binding protein is produced in the liver and is involved with fatty acid metabolism. Free fatty acid flux has previously been shown to be more strongly associated with visceral as compared to subcutaneous fat [44]. In addition, women have been shown to have a faster rate of non-oxidative free fatty acid disposal as compared to men, but without concomitant worsened metabolic risk factor profiles [45]. While FABP1 represents an exciting potential candidate gene, rs1659258 resides in a neighboring linkage disequilibrium block that does not contain any genes. THNSL2 is just downstream of FABP1 and our lead SNP demonstrates nominal gene expression to THNSL2, which is part of the threonine synthase family. A recent analysis of RNA expression in 225 healthy Pima Indian skeletal muscle biopsies showed a bimodal (ie two discrete clusters) expression of THNSL2, thought to occur due to cis-acting polymorphisms [46].
It is particularly notable that we uncovered a genome-wide significant finding with rs11118316 at LYPLAL1 for the VAT/SAT ratio overall. The VAT/SAT ratio is a metric of propensity to store visceral as compared to subcutaneous fat and has been shown to be associated with cardiometabolic risk [47]. We previously observed associations of a SNP in this gene in association with WHR-adjusted-for-BMI in the GIANT consortium [32], and LYPLAL1 has also been associated with cardiometabolic traits [48] and fatty liver [49]. LYPLAL1 encodes the lysophospholipase-like protein 1 and has been shown to be upregulated in the visceral and subcutaneous fat of obese subjects [50].
There are several potential explanations that could potentially account for our observed genetic association, particularly the marked sexual dimorphism. It has been previously shown that the familial contribution to fat distribution phenotypes is stronger in women as compared to men [33]. This concept is further strengthened by findings in mice suggesting that gonadal hormones are important in the sex-specific expression of genes related to metabolic traits [51]. In addition, known gender differences in fat distribution may also in part contribute to our findings, as women have been shown to have more subcutaneous fat but less visceral fat compared to men [20]. The relatively smaller amount of visceral fat in women may have increased our ability to detect a genetic signal. Finally, we have consistently observed stronger associations among women as compared to men with respect to metabolic risk factors in association with VAT [20]. While the reasons for this have not been fully elucidated, the stronger associations in women as compared to men is similar to what we have observed in the present analysis and in a prior GWAS of fat distribution phenotypes [32].
Strengths and Limitations
Strengths of our study include directly measured visceral and subcutaneous fat using CT imaging. Phenotyping using imaging is superior to typical anthropometric measures in the ability to partition the subcutaneous from visceral fat depots. Limitations include sample size: because of the limited number of studies with these imaging measurements and genome-wide association data, our discovery sample size was modest compared with other contemporary analyses. However, we note that performing sex-specific analyses actually enabled us to uncover a new locus, highlighting how heterogeneity can mask findings even when sample sizes are larger. Finally, the mean BMI in our gastric bypass eQTL dataset was substantially higher than the mean BMI in our discovery GWAS, which may affect generalizability of the eQTL data.
Conclusions
We have uncovered new loci for body fat distribution phenotypes, highlighting that loci exist for fat distribution that are largely independent of overall adiposity. More refined phenotypes for body composition and fat distribution can detect new loci not uncovered in large-scale GWAS of anthropometric traits.
Methods
Phenotype Definition
VAT and SAT were measured on CT following protocols established by each study as detailed in the Study-Specific Methods. We created sex-specific residuals adjusting for age, age-squared, smoking and principal components derived from genotypes denoting population stratification. The following traits were created in the overall sample and in women and men separately for each participating study: VAT, SAT, VAT-adjusted-for-BMI, and VAT/SAT ratio. VAT-adjusted-for-BMI provides insight into the relative amount of VAT controlling for the degree of generalized adiposity, whereas the VAT/SAT ratio is a metric of the propensity to deposit fat viscerally as compared to subcutaneously. The VAT/SAT ratio has been previously shown to be associated with cardiometabolic risk factors [47]. The correlation between VAT-adjusted-for-BMI and the VAT/SAT ratio in the Family Heart Study is 0.76 (p<0.0001; N = 2658).
Heritability Analyses
We created sex-and-cohort specific residuals, which were then pooled and analyzed using variance components analysis (SOLAR) [52].
Genotyping
Genotyping was conducted as specified in the Table S1 and the Study Specific Methods. We applied quality-control filters in order to exclude low-quality SNPs or samples. Based on CEU samples, each study imputed ∼2.5 million Phase 2 HapMap SNPs. We used imputed allelic dosage in the analysis. Additional details can be found in Table S1.
Primary Association Analyses and Meta-Analysis
The primary analysis was conducted in each cohort separately using regression analysis, assuming additive genetic effects and accounting for dependence among family member when appropriate. We accounted for principal components where necessary in order to prevent population stratification as well as the assumption of homogeneity within samples of European ancestry. These results were gathered together and used to conduct a fixed effects weighted Z meta-analysis (to allow for differences in phenotype scaling across the participating studies) using METAL [53]. We have previously shown that the association between single-slice VAT and SAT measurements at the L4/L5 level is highly correlated with volumetric measurements (r = 0.95–0.99) [54]. This approach weights the signed Z statistics from each study by its sample size to obtain a weighted sum, from which a p value is obtained. We applied the genomic control correction to control type I error rates. SNPs that reached a meta-analysis P value≤5×10−8 were considered to be genome-wide significant [55].
Validation Analysis
Stage 2 validation was conducted for our novel lead SNP, rs1659258, using data from studies not part of the present meta-analysis in the GIANT consortium data in sex-specific meta-analyses for BMI and waist circumference (personal communication, Heid I et al). P-values for SNPs discovered in the present effort in association with adiposity phenotypes reported by the GIANT consortium were queried. We concluded significant results when a direction-consistent p-value was at least p<0.05.
Analyses of Related Phenotypes
For each validated SNP, we obtained sex-specific association results for lipid and glycemic phenotypes from the MAGIC and GLGC consortia.
Interaction Testing
We performed formal tests of interaction of rs1659258 by sex (women as compared to men) and age (stratified above/below age 60). Briefly, each study generated the interaction regression coefficient, its standard error and its p value through regression. For the sex interaction, we included, age, age-squared, smoking (yes/no), sex, and any principal components (and study center) that were used in the original discovery analysis. We additionally added rs1659258 and the cross-product rs1659258*sex. The age interaction analysis was performed in women only. Because the AGES and Health ABC studies only included participants older than 65 years, they did not contribute to this analysis. Model covariates included age, age-squared, smoking (yes/no), and any principal components (and study center) with the addition of rs1659258 and the cross-product of rs1659258 and the dichotomized age term. We meta-analyzed the interaction terms across all studies using the weighted z-score approach.
eSNP Analysis
Using publically available datasets, we tested for the association of rs1659258 in expression SNPs (eSNP) datasets comprised of the following tissue types: lymphocytes [56], leukocytes [57], leukocytes from patients with Celiac disease [58], lymphoblastoid cell lines (LCL) from children with asthma [59], HapMap LCL from 3 populations [60], a separate study on HapMap CEU LCL [61], peripheral blood monocytes [62], [63], adipose tissue [40], [64], and blood samples [64], 2 studies on brain cortex [62], [65], three large studies of brain regions including prefrontal cortex, visual cortex and cerebellum (Emilsson, personal communication), liver [40], [66], osteoblasts [67], skin [68], and additional fibroblast, T cell and LCL samples [69]. We considered significance to be the association with gene transcript levels as originally described.
For sex-specific expression data, we queried data from patients who underwent a Roux-en-Y gastric bypass [40]. Briefly, we queried a 1 MB region surrounding our lead SNP for VAT in women and men separately. Altogether, 31 genes were included in the query. Statistical significance was considered to be a p-value<0.05 after correction for multiple testing.
Study-Specific Information: Framingham Heart Study
In 1948, the Framingham Heart Study began when the Original Cohort was enrolled [70]. Beginning in 1971, the Offspring Cohort was enrolled (5,124 participants); the methodology and design has been described. In 2002, the Third Generation cohort was enrolled (n = 4095) [71]. Participants for this study were drawn from the Framingham Heart Study Multi-detector Computed Tomography (MDCT) Study, a population-based sub-study of the community-based Framingham Heart Study Offspring and Third Generation cohorts. Participants for the current study were drawn from the MDCT sub-study. Between June 2002 to April 2005, 3529 participants (2111 Third Generation, 1418 Offspring participants) underwent MDCT assessment of coronary and aortic calcium. Inclusion in this study was weighted towards participants from larger Framingham Heart Study families and those who resided in the Greater New England area. Men had to be at least 35 years of age, women had to be at least 40 years of age and non-pregnant, and all participants had to weigh less than 350 pounds. Of the total of 3529 subjects imaged, 3394 had interpretable CT measures, 3329 of whom had both SAT and VAT measured, and 3158 participated in the present GWAS study.
We observed association with the first principal component estimated using Eigenstrat [72]; this component was included in our regression models.
Volumetric adipose tissue imaging
Subjects underwent eight-slice MDCT imaging of the chest and abdomen in a supine position as previously described (LightSpeed Ultra, General Electric, Milwaukee, WI) [73]. Briefly, twenty-five contiguous five mm thick slices (120 kVp, 400 mA, gantry rotation time 500 ms, table feed 3∶1) were acquired covering 125 mm above the level of S1.
Abdominal adipose tissue measurements
Subcutaneous and visceral adipose tissue volumes (SAT and VAT) were assessed (Aquarius 3D Workstation, TeraRecon Inc., San Mateo, CA). In order to identify pixels containing fat, an image display window width of −195 to −45 Hounsfield Units (HU) and a window center of −120 HU were used. The abdominal muscular wall separating the visceral from the subcutaneous compartment was manually traced. Inter-reader reproducibility was assessed by two independent readers measuring VAT and SAT on a subset of 100 randomly selected participants [73]. Inter-class correlations for inter-reader comparisons were 0.992 for VAT and 0.997 for SAT. Similar high correlations were noted for intra-reader comparisons.
Imputation
As a reference panel for imputation, we used Phase II CEU HapMap individuals; we imputed genotypes to nearly 2.5 million HapMap SNPs; further details are presented in Table S1. We used MACH v1.0.15/16 (http://www.sph.umich.edu/csg/abecasis/MACH/) in conjunction with 200 (101 Men and 99 Women) biologically independent individuals to establish parameter estimates and then used the parameter estimates to infer gene dosage for all study participants. We expressed imputed genotypes as allelic dosage (which is a decimal value ranging from 0–2).
Statistical analysis
We performed linear mixed effects regression modeling for SAT, VAT, and the VAT/SAT ratio to account for pedigree structure (using the R lme and kinship package).
Study-Specific Information: Family Heart Study
The Family Heart Study (FamHS) is a multicenter, population-based, family study designed to investigate the determinants of cardiovascular disease [74]. Families in the FamHS were selected at random (588 families) or ascertained for family history of CHD (656 families) using information collected in the parent studies—Framingham Heart Study (Framingham, MA, USA), the Utah Health Family Tree Study (Salt Lake City, UT, USA) or the Atherosclerosis Risk in Communities Study (Minneapolis Suburbs, MN, USA and Forsyth County, NC,USA). Between 2002 and 2003 about two-thirds of the largest families were invited to participate in a follow-up clinical examination that included measurement of the liver and abdomen with cardiac CT using standardized procedures and quality control methods developed in NHLBI's MESA and CARDIA studies [75]. Informed consent was obtained from all participants, and this project was approved by the Institutional Review Boards of all participating institutions.
CT scan–related phenotypes
Participants underwent a cardiac MDCT exam with four detectors using a standardized protocol as described previously [75]. For participants weighing 100 kg (220 lbs) or greater, the mAs were increased by 25%. The effective radiation exposure for the average participant of each coronary scan was 1.5 mSv for men and 1.9 mSv for women. Participants received two sequential scans. CT images from all study centers were sent electronically to the central CT reading center located at Wake Forest University Health Sciences, Winston Salem, NC, USA.
CT scans of the abdomen were reconstructed into 5 mm slices with the maximum 50 cm field-of-view to include the whole abdomen for body composition. Total and adipose tissues were measured volumetrically from two 5 mm contiguous slices located at the level of the lumbar disk between the 4th and 5th vertebra so as to be comparable to a single 10 mm slice used historically. Tissues with an attenuation between −190 to −30 Hounsfield units was define as adipose tissue. The MIPAV application (http://mipav.cit.nih.gov/index.php) was used by experienced analysts to segment the images based on anatomic boundaries (skin, subcutaneous fat-muscle interface and peritoneum) into the entire abdomen, abdominal wall and intra-abdominal compartments [76]. In each compartment the total volume and fat volumes were determined allowing calculation of the total abdominal adipose tissue, subcutaneous adipose tissue and visceral adipose tissue contained with the 10 mm slice located at L4–5.
Statistical analysis
We performed linear mixed effects regression modeling for SAT, VAT, and the VAT/SAT ratio, accounting for dependency among family member as a function of their kinship correlation (R kinship package).
Study-Specific Information: HABC Study
The Health ABC study is a prospective cohort study investigating the associations between body composition, weight-related health conditions, and incident functional limitation in older adults. Health ABC enrolled well-functioning, community-dwelling black (n = 1281) and white (n = 1794) men and women aged 70–79 years between April 1997 and June 1998. Participants were recruited from a random sample of white and all black Medicare eligible residents in the Pittsburgh, PA, and Memphis, TN, metropolitan areas. Participants have undergone annual exams and semi-annual phone interviews. The current study sample consists of 1559 white participants who attended the second exam in 1998–1999 with available genotyping and SAT/VAT data.
Regional fat depots were assessed from CT scans obtained in Pittsburgh on a General Electric 9800 Advantage (General Electric, Milwaukee, WI) and in Memphis on a Siemens Somatron Plus 4 (Siemens, Erlangen, Germany) or Picker PQ2000S (Marconi Medical Systems, Cleveland, OH). A single axial scan (140 kVp, 300 to 360 mAs, 10-mm thickness) was taken at the disk space between the fourth and fifth lumbar vertebrae. Images were transferred to the Reading Center at the University of Colorado Health Sciences Center on optical disc or magnetic tape. Analyses were performed on a SPARC station II (Sun Microsystems, Mountain View, CA) using IDL development software (RSI Systems, Boulder, CO). An outline was traced surrounding the abdominal cavity. The adipose tissue density range was determined with a bimodal image distribution histogram for each participant. Visceral fat was defined as the area of all adipose tissue within the abdominal cavity with exclusion of the muscle region, calculated by multiplying the number of pixels within this range by a single pixel area. Abdominal subcutaneous fat was defined as the difference in the area between the entire adipose tissue in the scan and visceral fat. To assess the reproducibility of these measurements, 5% of the data was re-read in a blinded fashion. The intra-class correlation coefficients of reliability ranged from 0.93 to 1.000.
Genotyping and imputation
Genomic DNA was extracted from buffy coat collected using PUREGENE DNA Purification Kit during the baseline exam. Genotyping was performed by the Center for Inherited Disease Research (CIDR) using the Illumina Human1M-Duo BeadChip system. Samples were excluded from the dataset for the reasons of sample failure, genotypic sex mismatch, and first-degree relative of an included individual based on genotype data. Genotyping was successful for 1,151,215 SNPs in 2,802 unrelated individuals (1663 Caucasians and 1139 African Americans). Imputation was done for the autosomes using the MACH software version 1.0.16. SNPs with minor allele frequency ≥1%, call rate ≥97% and HWE p≥10-6 were used for imputation. HapMap II phased haplotypes were used as reference panels. For EAs, genotypes were available on 914,263 high quality SNPs for imputation based on the HapMap CEPH reference panel (release 22, build 36). A total of 2,543,887 in EAs are available for analysis.
Statistical analysis
We performed linear regression modeling for SAT, VAT, and the VAT/SAT ratio. We observed association with the first principal components estimated using Eigenstrat [72]; this was accounted for in our analyses.
Study-Specific Information: Age, Gene/Environment Susceptibility–Reykjavik Study
The AGES-Reykjavik study is an ongoing study of the effects of gene-environment interactions and other risk factors on disease in old age. AGES-Reykjavik is a subset of a larger population based cohort study called the Reykjavik-study. The aim of the original study was to prospectively investigate risk factors for cardiovascular disease in the Icelandic population. The original Reykjavik-cohort was established in 1967 with a random sample of 30,795 individuals born in the years 1907–1935, and residing in Reykjavik, the capital of Iceland. A total of 18,045 individuals entered the study as participants and attended examinations. The AGES-Reykjavik sample was constructed in 2002 by randomly drawing 8,030 individuals who were still alive from the original Reykjavik-cohort (n = 11,459). A total of 5,764 individuals (58% women) entered the AGES-Reykjavik study as participants. All cohort members were European Caucasians.
The AGES-Reykjavik Study was designed to investigate aging using a multifaceted comprehensive approach. Physical, physiological and questionnaire examinations were conducted in three visits for each subject including detailed medical history, physical examination, laboratory and screening tests, and questionnaires on health-related behaviors such as alcohol consumption, smoking history, and physical activity. Pertinent to this study, these measures included anthropometric measurements and computerized tomography measures of adipose depots in the abdomen and thigh. The AGES–Reykjavik study was approved by the institutional review boards of the National Institute on Aging and the Icelandic National Bioethics Committee (VSN: 00-063), and written informed consent was obtained from all participants.
Analytic sample
Of the 5,764 individuals who agreed to participate in the AGES-Reykjavik study, 5,427 individuals attended the research center for examinations while 337 received a home visit. A total of 204 individuals did not contribute CT-data at the research center. However, among those individuals with CT, only 3,664 had genotyping at the Laboratory of Neurogenetics, Intramural Research Program, NIA, Bethesda, Maryland, and 3,219 participants passed QC criteria for genotyping. Of these, 3172 had complete genotyping and complete CT data for abdominal subcutaneous and visceral adipose depots.
Image acquisition
Images for abdominal adipose depots were acquired in a single 10 mm thick trans-axial section of the abdomen at the level of the L4–L5 vertebrae using a Siemens Somatom Sensation 4 multi-detector CT scanner (Siemens Medical Solutions, Erlangen, Germany) (standard scan setting: slice thickness: 10 mm, tube voltage; 140 kilo-voltage, tube-current-time-product; 50 milli-ampere-seconds and scan time 0.361 sec). Study participants weighing more than 110 kg (kilo-grams) underwent CT with a tube current setting that was 25% higher than the standard scan setting. The images were reconstructed into a display field of view of 350 mm to include a calibration phantom (Image Analysis, Columbia, KY, USA) which was positioned under the abdomen of each subject. VAT area was estimated from all pixels in the abdominal cavity within the range of −50 to −200 Hounsfield units. Inter-observer variability based on the re-analysis of randomly selected 365 scans from the core study population by an expert observer showed an average correlation coefficient of 0.99. Intra-observer variability based on re-analysis of 45 scans by each of the four observers resulted in an average correlation coefficient of 0.99.
Imputation
As a reference panel for imputation, we used Phase II CEU HapMap individuals; we imputed genotypes to nearly 2.5 million HapMap SNPs; further details are presented in Table S1.
Statistical analysis
We performed linear regression modeling for SAT, VAT, and the VAT/SAT ratio.
Supporting Information
Zdroje
1. RositoGAMassaroJMHoffmannURubergFLMahabadiAA 2008 Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation 117 605 613
2. DingJHsuFCHarrisTBLiuYKritchevskySB 2009 The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr 90 499 504
3. WormserDKaptogeSDiAEWoodAMPennellsL 2011 Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet 377 1085 1095
4. PischonTBoeingHHoffmannKBergmannMSchulzeMB 2008 General and abdominal adiposity and risk of death in Europe. N Engl J Med 359 2105 2120
5. RexrodeKMCareyVJHennekensCHWaltersEEColditzGA 1998 Abdominal adiposity and coronary heart disease in women. JAMA 280 1843 1848
6. RimmEBStampferMJGiovannucciEAscherioASpiegelmanD 1995 Body size and fat distribution as predictors of coronary heart disease among middle-aged and older US men. Am J Epidemiol 141 1117 1127
7. CareyVJWaltersEEColditzGASolomonCGWillettWC 1997 Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women. The Nurses' Health Study. Am J Epidemiol 145 614 619
8. CassanoPASegalMRVokonasPSWeissST 1990 Body fat distribution, blood pressure, and hypertension. A prospective cohort study of men in the normative aging study. Ann Epidemiol 1 33 48
9. CigoliniMTargherGBergamoAITonoliMAgostinoG 1996 Visceral fat accumulation and its relation to plasma hemostatic factors in healthy men. Arterioscler Thromb Vasc Biol 16 368 374
10. CoonPJRogusEMDrinkwaterDMullerDCGoldbergAP 1992 Role of body fat distribution in the decline in insulin sensitivity and glucose tolerance with age. J Clin Endocrinol Metab 75 1125 1132
11. DonahueRPAbbottRDBloomEReedDMYanoK 1987 Central obesity and coronary heart disease in men. Lancet 1 821 824
12. FolsomARKayeSASellersTAHongCPCerhanJR 1993 Body fat distribution and 5-year risk of death in older women. JAMA 269 483 487
13. KockxMLeenenRSeidellJPrincenHMKooistraT 1999 Relationship between visceral fat and PAI-1 in overweight men and women before and after weight loss. Thromb Haemost 82 1490 1496
14. LapidusLBengtssonCLarssonBPennertKRyboE 1984 Distribution of adipose tissue and risk of cardiovascular disease and death: a 12 year follow up of participants in the population study of women in Gothenburg, Sweden. Br Med J (Clin Res Ed) 289 1257 1261
15. LarssonBSvardsuddKWelinLWilhelmsenLBjorntorpP 1984 Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13 year follow up of participants in the study of men born in 1913. Br Med J (Clin Res Ed) 288 1401 1404
16. LemieuxIPascotAPrud'hommeDAlmerasNBogatyP 2001 Elevated C-reactive protein: another component of the atherothrombotic profile of abdominal obesity. Arterioscler Thromb Vasc Biol 21 961 967
17. MertensIVan derPMCorthoutsBWautersMPeifferF 2001 Visceral fat is a determinant of PAI-1 activity in diabetic and non-diabetic overweight and obese women. Horm Metab Res 33 602 607
18. SattarNTanCEHanTSForsterLLeanME 1998 Associations of indices of adiposity with atherogenic lipoprotein subfractions. Int J Obes Relat Metab Disord 22 432 439
19. ShetterlySMMarshallJABaxterJHammanRF 1993 Waist-hip ratio measurement location influences associations with measures of glucose and lipid metabolism. The San Luis Valley Diabetes Study. Ann Epidemiol 3 295 299
20. FoxCSMassaroJMHoffmannUPouKMMaurovich-HorvatP 2007 Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 116 39 48
21. BouchardCDespresJPMauriegeP 1993 Genetic and nongenetic determinants of regional fat distribution. Endocr Rev 14 72 93
22. BrochuMStarlingRDTchernofAMatthewsDEGarcia-RubiE 2000 Visceral adipose tissue is an independent correlate of glucose disposal in older obese postmenopausal women. J Clin Endocrinol Metab 85 2378 2384
23. CareyDGJenkinsABCampbellLVFreundJChisholmDJ 1996 Abdominal fat and insulin resistance in normal and overweight women: Direct measurements reveal a strong relationship in subjects at both low and high risk of NIDDM. Diabetes 45 633 638
24. DespresJP 1998 The insulin resistance-dyslipidemic syndrome of visceral obesity: effect on patients' risk. Obes Res 6 Suppl 1 8S 17S
25. LemieuxS 2001 Contribution of visceral obesity to the insulin resistance syndrome. Can J Appl Physiol 26 273 290
26. RossRFortierLHudsonR 1996 Separate associations between visceral and subcutaneous adipose tissue distribution, insulin and glucose levels in obese women. Diabetes Care 19 1404 1411
27. WagenknechtLELangefeldCDScherzingerALNorrisJMHaffnerSM 2003 Insulin sensitivity, insulin secretion, and abdominal fat: the Insulin Resistance Atherosclerosis Study (IRAS) Family Study. Diabetes 52 2490 2496
28. BoykoEJFujimotoWYLeonettiDLNewell-MorrisL 2000 Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes Care 23 465 471
29. FoxCSHeard-CostaNLWilsonPWLevyDD'AgostinoRBSr 2004 Genome-Wide Linkage to Chromosome 6 for Waist Circumference in the Framingham Heart Study. Diabetes 53 1399 1402
30. KatzmarzykPTMalinaRMPerusseLRiceTProvinceMA 2000 Familial resemblance in fatness and fat distribution. Am J Hum Biol 12 395 404
31. SellersTADrinkardCRichSSPotterJDJefferyRW 1994 Familial aggregation and heritability of waist-to-hip ratio in adult women: the Iowa Women's Health Study. Int J Obes Relat Metab Disord 18 607 613
32. HeidIMJacksonAURandallJCWinklerTWQiL 2010 Meta-analysis identifies 13 new loci associated with waist-hip ratio and reveals sexual dimorphism in the genetic basis of fat distribution. Nat Genet 42 949 960
33. ZillikensMCYazdanpanahMPardoLMRivadeneiraFAulchenkoYS 2008 Sex-specific genetic effects influence variation in body composition. Diabetologia 51 2233 2241
34. CigoliniMTargherGBergamoAITonoliMAgostinoG 1996 Visceral fat accumulation and its relation to plasma hemostatic factors in healthy men. Arterioscler Thromb Vasc Biol 16 368 374
35. CareyVJWaltersEEColditzGASolomonCGWillettWC 1997 Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women. The Nurses' Health Study. Am J Epidemiol 145 614 619
36. DespresJP 1998 The insulin resistance-dyslipidemic syndrome of visceral obesity: effect on patients' risk. Obes Res 6 Suppl 1 8S 17S
37. WagenknechtLELangefeldCDScherzingerALNorrisJMHaffnerSM 2003 Insulin sensitivity, insulin secretion, and abdominal fat: the Insulin Resistance Atherosclerosis Study (IRAS) Family Study. Diabetes 52 2490 2496
38. TeslovichTMMusunuruKSmithAVEdmondsonACStylianouIM 2010 Biological, clinical and population relevance of 95 loci for blood lipids. Nature 466 707 713
39. SpeliotesEKWillerCJBerndtSIMondaKLThorleifssonG 2010 Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet 42 937 948
40. GreenawaltDMDobrinRChudinEHatoumIJSuverC 2011 A survey of the genetics of stomach, liver, and adipose gene expression from a morbidly obese cohort. Genome Res 21 1008 1016
41. KilpelainenTOZillikensMCStancakovaAFinucaneFMRiedJS 2011 Genetic variation near IRS1 associates with reduced adiposity and an impaired metabolic profile. Nat Genet 43 753 760
42. PouKMMassaroJMHoffmannUVasanRSMaurovich-HorvatP 2007 Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation 116 1234 1241
43. Heard-CostaNLZillikensMCMondaKLJohanssonAHarrisTB 2009 NRXN3 is a novel locus for waist circumference: a genome-wide association study from the CHARGE Consortium. PLoS Genet 5 e1000539 doi:10.1371/journal.pgen.1000539
44. HouXGMoserSSarrMGThompsonGBQueFG 2009 Visceral and subcutaneous adipose tissue diacylglycerol acyltransferase activity in humans. Obesity (Silver Spring) 17 1129 1134
45. KoutsariCBasuRRizzaRANairKSKhoslaS 2011 Nonoxidative free fatty acid disposal is greater in young women than men. J Clin Endocrinol Metab 96 541 547
46. MasonCCHansonRLOssowskiVBianLBaierLJ 2011 Bimodal distribution of RNA expression levels in human skeletal muscle tissue. BMC Genomics 12 98
47. KimSChoBLeeHChoiKHwangSS 2011 Distribution of abdominal visceral and subcutaneous adipose tissue and metabolic syndrome in a Korean population. Diabetes Care 34 504 506
48. BilleDSBanasikKJustesenJMSandholtCHSandbaekA 2011 Implications of central obesity-related variants in LYPLAL1, NRXN3, MSRA, and TFAP2B on quantitative metabolic traits in adult Danes. PLoS ONE 6 e20640 doi:10.1371/journal.pone.0020640
49. SpeliotesEKYerges-ArmstrongLMWuJHernaezRKimLJ 2011 Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet 7 e1001324 doi:10.1371/journal.pgen.1001324
50. SteinbergGRKempBEWattMJ 2007 Adipocyte triglyceride lipase expression in human obesity. Am J Physiol Endocrinol Metab 293 E958 E964
51. vanNAGuhathakurtaDWangSSYehyaNHorvathS 2009 Elucidating the role of gonadal hormones in sexually dimorphic gene coexpression networks. Endocrinology 150 1235 1249
52. AlmasyLBlangeroJ 1998 Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet 62 1198 1211
53. WillerCJLiYAbecasisGR 2010 METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 26 2190 2191
54. IrlbeckTMassaroJMBambergFO'DonnellCJHoffmannU 2010 Association between single-slice measurements of visceral and abdominal subcutaneous adipose tissue with volumetric measurements: the Framingham Heart Study. Int J Obes (Lond) 34 781 787
55. Pe'erIYelenskyRAltshulerDDalyMJ 2008 Estimation of the multiple testing burden for genomewide association studies of nearly all common variants. Genet Epidemiol 32 381 385
56. GoringHHCurranJEJohnsonMPDyerTDCharlesworthJ 2007 Discovery of expression QTLs using large-scale transcriptional profiling in human lymphocytes. Nat Genet 39 1208 1216
57. IdaghdourYCzikaWShiannaKVLeeSHVisscherPM 2010 Geographical genomics of human leukocyte gene expression variation in southern Morocco. Nat Genet 42 62 67
58. HeapGATrynkaGJansenRCBruinenbergMSwertzMA 2009 Complex nature of SNP genotype effects on gene expression in primary human leucocytes. BMC Med Genomics 2 1
59. DixonALLiangLMoffattMFChenWHeathS 2007 A genome-wide association study of global gene expression. Nat Genet 39 1202 1207
60. StrangerBENicaACForrestMSDimasABirdCP 2007 Population genomics of human gene expression. Nat Genet 39 1217 1224
61. KwanTBenovoyDDiasCGurdSProvencherC 2008 Genome-wide analysis of transcript isoform variation in humans. Nat Genet 40 225 231
62. HeinzenELGeDCroninKDMaiaJMShiannaKV 2008 Tissue-specific genetic control of splicing: implications for the study of complex traits. PLoS Biol 6 e1 doi:10.1371/journal.pbio.1000001
63. ZellerTWildPSzymczakSRotivalMSchillertA 2010 Genetics and beyond—the transcriptome of human monocytes and disease susceptibility. PLoS ONE 5 e10693 doi:10.1371/journal.pone.0010693
64. EmilssonVThorleifssonGZhangBLeonardsonASZinkF 2008 Genetics of gene expression and its effect on disease. Nature 452 423 428
65. WebsterJAGibbsJRClarkeJRayMZhangW 2009 Genetic control of human brain transcript expression in Alzheimer disease. Am J Hum Genet 84 445 458
66. SchadtEEMolonyCChudinEHaoKYangX 2008 Mapping the genetic architecture of gene expression in human liver. PLoS Biol 6 e107 doi:10.1371/journal.pbio.0060107
67. GrundbergEKwanTGeBLamKCKokaV 2009 Population genomics in a disease targeted primary cell model. Genome Res 19 1942 1952
68. DingJGudjonssonJELiangLStuartPELiY 2010 Gene expression in skin and lymphoblastoid cells: Refined statistical method reveals extensive overlap in cis-eQTL signals. Am J Hum Genet 87 779 789
69. DimasASDeutschSStrangerBEMontgomerySBBorelC 2009 Common regulatory variation impacts gene expression in a cell type-dependent manner. Science 325 1246 1250
70. DawberTRMeadorsGFMooreFE 1951 Epidemiologic approaches to heart disease: the Framingham study. Am J Publich Health 41 279 286
71. SplanskyGLCoreyDYangQAtwoodLDCupplesLA 2007 The Third Generation Cohort of the National Heart, Lung, and Blood Institute's Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol 165 1328 1335
72. PriceALPattersonNJPlengeRMWeinblattMEShadickNA 2006 Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 38 904 909
73. Maurovich-HorvatPMassaroJFoxCSMoselewskiFO'DonnellCJ 2007 Comparison of anthropometric, area - and volume-based assessment of abdominal subcutaneous and visceral adipose tissue volumes using multi-detector computed tomography. Int J Obes (Lond) 31 500 506
74. HigginsMProvinceMHeissGEckfeldtJEllisonRC 1996 NHLBI Family Heart Study: objectives and design. Am J Epidemiol 143 1219 1228
75. CarrJJNelsonJCWongNDNitt-GrayMAradY 2005 Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology 234 35 43
76. YoshizumiTNakamuraTYamaneMIslamAHMenjuM 1999 Abdominal fat: standardized technique for measurement at CT. Radiology 211 283 286
Štítky
Genetika Reprodukční medicína
Článek Functional Centromeres Determine the Activation Time of Pericentric Origins of DNA Replication inČlánek Dynamic Deposition of Histone Variant H3.3 Accompanies Developmental Remodeling of the TranscriptomeČlánek Integrin α PAT-2/CDC-42 Signaling Is Required for Muscle-Mediated Clearance of Apoptotic Cells inČlánek Prdm5 Regulates Collagen Gene Transcription by Association with RNA Polymerase II in Developing BoneČlánek Acquisition Order of Ras and p53 Gene Alterations Defines Distinct Adrenocortical Tumor Phenotypes
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 5- Akutní intermitentní porfyrie
- Souvislost haplotypu M2 genu pro annexin A5 s opakovanými reprodukčními ztrátami
- Příjem alkoholu a menstruační cyklus
- Doporučení pro diagnostiku a léčbu akutních jaterních porfyrií
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- Slowing Replication in Preparation for Reduction
- Chromosome Pairing: A Hidden Treasure No More
- Loss of Imprinting Differentially Affects REM/NREM Sleep and Cognition in Mice
- Six Novel Susceptibility Loci for Early-Onset Androgenetic Alopecia and Their Unexpected Association with Common Diseases
- Regulation by the Noncoding RNA
- UDP-Galactose 4′-Epimerase Activities toward UDP-Gal and UDP-GalNAc Play Different Roles in the Development of
- Deletion of PTH Rescues Skeletal Abnormalities and High Osteopontin Levels in Mice
- Karyotypic Determinants of Chromosome Instability in Aneuploid Budding Yeast
- Genome-Wide Copy Number Analysis Uncovers a New HSCR Gene:
- MicroRNA-277 Modulates the Neurodegeneration Caused by Fragile X Premutation rCGG Repeats
- Functional Centromeres Determine the Activation Time of Pericentric Origins of DNA Replication in
- Dynamic Deposition of Histone Variant H3.3 Accompanies Developmental Remodeling of the Transcriptome
- Scientist Citizen: An Interview with Bruce Alberts
- YY1 Regulates Melanocyte Development and Function by Cooperating with MITF
- Congenital Heart Disease–Causing Gata4 Mutation Displays Functional Deficits
- Recombination Drives Vertebrate Genome Contraction
- KATNAL1 Regulation of Sertoli Cell Microtubule Dynamics Is Essential for Spermiogenesis and Male Fertility
- Re-Patterning Sleep Architecture in through Gustatory Perception and Nutritional Quality
- Using Whole-Genome Sequence Data to Predict Quantitative Trait Phenotypes in
- Genome-Wide Analysis of GLD-1–Mediated mRNA Regulation Suggests a Role in mRNA Storage
- Meiotic Chromosome Pairing Is Promoted by Telomere-Led Chromosome Movements Independent of Bouquet Formation
- LINT, a Novel dL(3)mbt-Containing Complex, Represses Malignant Brain Tumour Signature Genes
- The H3K27 Demethylase UTX-1 Is Essential for Normal Development, Independent of Its Enzymatic Activity
- Suppresses Senescence Programs and Thereby Accelerates and Maintains Mutant -Induced Lung Tumorigenesis
- Genome-Wide Association of Pericardial Fat Identifies a Unique Locus for Ectopic Fat
- An Essential Role for Katanin p80 and Microtubule Severing in Male Gamete Production
- Identification of Genes That Promote or Antagonize Somatic Homolog Pairing Using a High-Throughput FISH–Based Screen
- Principles of Carbon Catabolite Repression in the Rice Blast Fungus: Tps1, Nmr1-3, and a MATE–Family Pump Regulate Glucose Metabolism during Infection
- Integrin α PAT-2/CDC-42 Signaling Is Required for Muscle-Mediated Clearance of Apoptotic Cells in
- Histone H3 Localizes to the Centromeric DNA in Budding Yeast
- Collapse of Telomere Homeostasis in Hematopoietic Cells Caused by Heterozygous Mutations in Telomerase Genes
- Hypersensitive to Red and Blue 1 and Its Modification by Protein Phosphatase 7 Are Implicated in the Control of Arabidopsis Stomatal Aperture
- Extent, Causes, and Consequences of Small RNA Expression Variation in Human Adipose Tissue
- TBC-8, a Putative RAB-2 GAP, Regulates Dense Core Vesicle Maturation in
- Regulating Repression: Roles for the Sir4 N-Terminus in Linker DNA Protection and Stabilization of Epigenetic States
- Common Genetic Determinants of Intraocular Pressure and Primary Open-Angle Glaucoma
- Prdm5 Regulates Collagen Gene Transcription by Association with RNA Polymerase II in Developing Bone
- Fitness Landscape Transformation through a Single Amino Acid Change in the Rho Terminator
- Repeated, Selection-Driven Genome Reduction of Accessory Genes in Experimental Populations
- Allelic Variation and Differential Expression of the mSIN3A Histone Deacetylase Complex Gene Promote Mammary Tumor Growth and Metastasis
- DNA Demethylation and USF Regulate the Meiosis-Specific Expression of the Mouse
- Knowledge-Driven Analysis Identifies a Gene–Gene Interaction Affecting High-Density Lipoprotein Cholesterol Levels in Multi-Ethnic Populations
- A Duplication CNV That Conveys Traits Reciprocal to Metabolic Syndrome and Protects against Diet-Induced Obesity in Mice and Men
- EMT Inducers Catalyze Malignant Transformation of Mammary Epithelial Cells and Drive Tumorigenesis towards Claudin-Low Tumors in Transgenic Mice
- Inactivation of a Novel FGF23 Regulator, FAM20C, Leads to Hypophosphatemic Rickets in Mice
- Genome-Wide Association for Abdominal Subcutaneous and Visceral Adipose Reveals a Novel Locus for Visceral Fat in Women
- Stratifying Type 2 Diabetes Cases by BMI Identifies Genetic Risk Variants in and Enrichment for Risk Variants in Lean Compared to Obese Cases
- New Insight into the History of Domesticated Apple: Secondary Contribution of the European Wild Apple to the Genome of Cultivated Varieties
- Activated Cdc42 Kinase Has an Anti-Apoptotic Function
- The Region Is Critical for Birth Defects and Electrocardiographic Dysfunctions Observed in a Down Syndrome Mouse Model
- COP9 Signalosome Integrity Plays Major Roles for Hyphal Growth, Conidial Development, and Circadian Function
- Bmps and Id2a Act Upstream of Twist1 To Restrict Ectomesenchyme Potential of the Cranial Neural Crest
- Psip1/Ledgf p52 Binds Methylated Histone H3K36 and Splicing Factors and Contributes to the Regulation of Alternative Splicing
- The Number of X Chromosomes Causes Sex Differences in Adiposity in Mice
- Target Gene Analysis by Microarrays and Chromatin Immunoprecipitation Identifies HEY Proteins as Highly Redundant bHLH Repressors
- Acquisition Order of Ras and p53 Gene Alterations Defines Distinct Adrenocortical Tumor Phenotypes
- ELK1 Uses Different DNA Binding Modes to Regulate Functionally Distinct Classes of Target Genes
- Histone H1 Depletion Impairs Embryonic Stem Cell Differentiation
- IDN2 and Its Paralogs Form a Complex Required for RNA–Directed DNA Methylation
- Separation of DNA Replication from the Assembly of Break-Competent Meiotic Chromosomes
- Genomic Hypomethylation in the Human Germline Associates with Selective Structural Mutability in the Human Genome
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Inactivation of a Novel FGF23 Regulator, FAM20C, Leads to Hypophosphatemic Rickets in Mice
- Genome-Wide Association of Pericardial Fat Identifies a Unique Locus for Ectopic Fat
- Slowing Replication in Preparation for Reduction
- An Essential Role for Katanin p80 and Microtubule Severing in Male Gamete Production
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



