-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
DNA Demethylation and USF Regulate the Meiosis-Specific Expression of the Mouse
Miwi, a member of the Argonaute family, is required for initiating spermiogenesis; however, the mechanisms that regulate the expression of the Miwi gene remain unknown. By mutation analysis and transgenic models, we identified a 303 bp proximal promoter region of the mouse Miwi gene, which controls specific expression from midpachytene spermatocytes to round spermatids during meiosis. We characterized the binding sites of transcription factors NF-Y (Nuclear Factor Y) and USF (Upstream Stimulatory Factor) within the core promoter and found that both factors specifically bind to and activate the Miwi promoter. Methylation profiling of three CpG islands within the proximal promoter reveals a markedly inverse correlation between the methylation status of the CpG islands and germ cell type–specific expression of Miwi. CpG methylation at the USF–binding site within the E2 box in the promoter inhibits the binding of USF. Transgenic Miwi-EGFP and endogenous Miwi reveal a subcellular co-localization pattern in the germ cells of the Miwi-EGFP transgenic mouse. Furthermore, the DNA methylation profile of the Miwi promoter–driven transgene is consistent with that of the endogenous Miwi promoter, indicating that Miwi transgene is epigenetically modified through methylation in vivo to ensure its spatio-temporal expression. Our findings suggest that USF controls Miwi expression from midpachytene spermatocytes to round spermatids through methylation-mediated regulation. This work identifies an epigenetic regulation mechanism for the spatio-temporal expression of mouse Miwi during spermatogenesis.
Published in the journal: . PLoS Genet 8(5): e32767. doi:10.1371/journal.pgen.1002716
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002716Summary
Miwi, a member of the Argonaute family, is required for initiating spermiogenesis; however, the mechanisms that regulate the expression of the Miwi gene remain unknown. By mutation analysis and transgenic models, we identified a 303 bp proximal promoter region of the mouse Miwi gene, which controls specific expression from midpachytene spermatocytes to round spermatids during meiosis. We characterized the binding sites of transcription factors NF-Y (Nuclear Factor Y) and USF (Upstream Stimulatory Factor) within the core promoter and found that both factors specifically bind to and activate the Miwi promoter. Methylation profiling of three CpG islands within the proximal promoter reveals a markedly inverse correlation between the methylation status of the CpG islands and germ cell type–specific expression of Miwi. CpG methylation at the USF–binding site within the E2 box in the promoter inhibits the binding of USF. Transgenic Miwi-EGFP and endogenous Miwi reveal a subcellular co-localization pattern in the germ cells of the Miwi-EGFP transgenic mouse. Furthermore, the DNA methylation profile of the Miwi promoter–driven transgene is consistent with that of the endogenous Miwi promoter, indicating that Miwi transgene is epigenetically modified through methylation in vivo to ensure its spatio-temporal expression. Our findings suggest that USF controls Miwi expression from midpachytene spermatocytes to round spermatids through methylation-mediated regulation. This work identifies an epigenetic regulation mechanism for the spatio-temporal expression of mouse Miwi during spermatogenesis.
Introduction
Spermatogenesis is a complex process consisting of two types of cell division, mitosis and meiosis. Meiosis is initiated in B spermatogonia, eventually leading to the production of spermatozoa. Spermatozoa generated in the testis enter the epididymis and undergo maturation processes necessary for them to gain motility and become capable of fertilization. During spermatogenesis, differential gene expression is orderly and accurately regulated at the transcriptional level to ensure differentiation of germ cells [1]. Male germ cells in the adult mouse exhibit a highly distinct methylation pattern, and both de novo methylation and demethylation occur during spermatogenesis [2], [3]. DNA methylation of postmigratory germ cell-specific genes is also observed in both premigratory germ cells and somatic cells [4]. These methylation modifications in germ cells are important for accurate spermatogenesis. Another salient feature of gene expression regulation during spermatogenesis is translational delay or repression [5], [6], [7]. After transcription, some mRNAs are assembled in ribonucleoprotein particles, which contain RNA-binding proteins, export proteins and processing factors. Transcripts are then transported through nuclear pore complexes into the cytoplasm, where they join in formation of the chromatoid body (CB) for both storage and processing after meiosis [8]. Evidence is accumulating that microRNAs (miRNAs) can also reduce translation [9]. miRNAs likely mediate translational repression by decreasing the rate of translation initiation.
Miwi, a PIWI subfamily member of Argonaute protein family, is characterized by conserved PAZ and Piwi domains for RNA-binding, and required for initiating spermiogenesis [10]. In mice, there are three Piwi members (Miwi, Mili/Piwil2 and Miwi2/Piwil4). Miwi interacts directly with piwi-interacting RNAs (piRNAs) [11], [12], [13], and is required for the expression of not only piRNAs but also a subset of miRNAs [14]. Miwi is a cytoplasmic protein expressed specifically in the testis from midpachytene spermatocytes to round spermatids [10], [15]. Particularly, Miwi is present throughout the cytoplasm in spermatocytes, and gradually concentrates in the CB in round spermatids [14], [16]. A complex formation between Miwi, Mili and Tdrd1 is critical for the integrated subcellular localizations of these proteins [17]. The Piwi interactome has revealed that arginine methylated Miwi can interact with multiple tudor domain containing proteins [18], [19]. Tudor proteins recognize methyl-arginine of Piwi proteins, driving the localization of Piwi proteins to the CB [19]. It has been suggested that Tudor proteins regulate biological functions of Piwi proteins via specific association with methylation modifications of the Piwi arginine [20]. Despite the wealth of information, expression regulation mechanisms of the gene Miwi remain poorly understood.
In this study, we have identified the functional promoter of the mouse Miwi. Miwi-EGFP transgenic mice reveal that a 303 bp core promoter of the mouse Miwi gene directs specific expression during meiosis. Notably, transcription factors NF-Y (Nuclear Factor-Y) and USFs (Upstream Stimulatory Factors) bind to and activate the promoter of Miwi. Methylation analysis of the CpG islands in the Miwi promoter reveals that CpG methylation at the USF-binding site within the E2 box inhibits the binding of USFs. In addition, both transgenic Miwi-EGFP and endogenous Miwi reveal a co-localization pattern in Miwi-EGFP transgenic mouse testis. Furthermore, the promoter region of Miwi-EGFP transgene in transgenic mice is epigenetically modified in vivo as that of the endogenous Miwi. These results suggest an epigenetic regulation mechanism for the spatio-temporal expression of the mouse Miwi during spermatogenesis.
Results
Identification of the mouse Miwi promoter
To identify the promoter region and regulatory elements of mouse Miwi, a series of deletions of the potential promoter were introduced upstream of the luciferase gene based on the prediction of CpG islands. Luciferase activity was determined in both GC-1 and COS7 cells. Promoter analysis has revealed that the 5′ flanking sequence from −106 to −92 is important for its transcriptional activity (Figure 1a, 1b). Because the Miwi gene lacks a TATA box, we identified putative transcription elements using the TFSEARCH software (http://www.cbrc.jp/research/db/TFSEARCH.html). A CCAAT box (−97∼−93) and two E boxes (−107∼−102, −82∼−77) were observed within the core promoter region from −106 to −92, which are putative binding sites of transcription factors NF-Y and USF respectively. To functionally determine the importance of these elements, site-directed mutagenesis was performed using wild type pGL3-5M4 construct as a template. The mutated CCAAT box or E2 box showed obvious decreases in promoter activity, as compared to the wild type pGL3-5M4 construct, especially the E2 mutant, where very little transcription was observed (Figure 1c). Meanwhile, mutations for E1 box had no effect on transcription activity. The results indicate that both the CCAAT box and the E2 boxes are important for Miwi promoter activity.
Fig. 1. Deletion and point mutation analysis of the Miwi promoter. 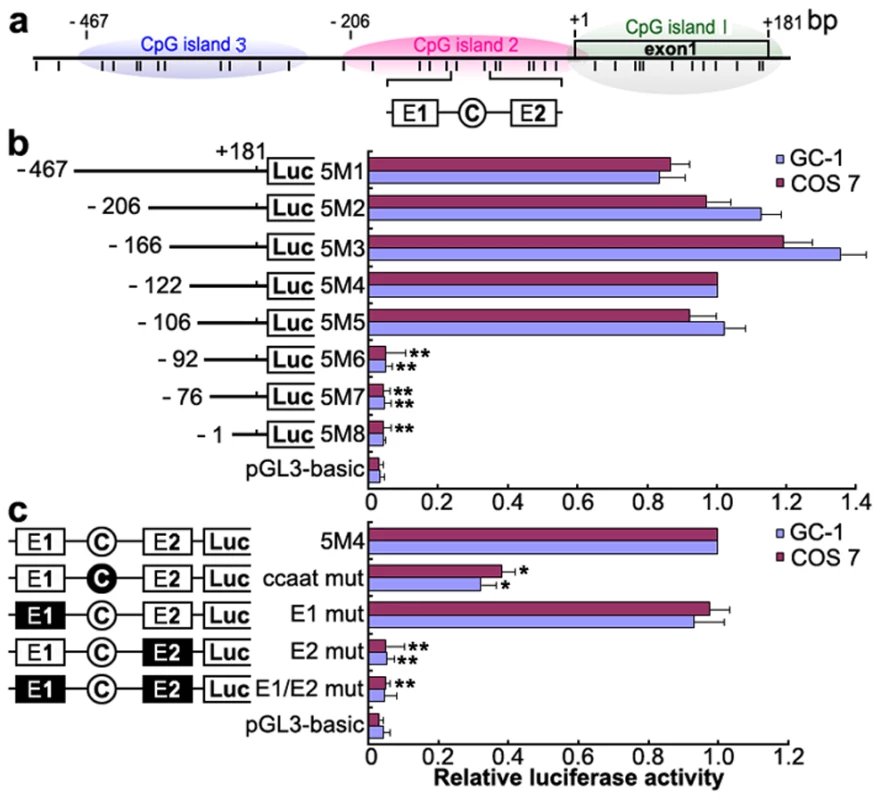
a), Schematic diagram of the CpG islands and E1, E2 and CCAAT boxes in the Miwi promoter. The short vertical bars represent the CpG dinucleotides. b), Promoter activities of a series of deleted constructs determined by luciferase assay. Left panel, schematic representation of the mutants linked with luciferase gene in pGL3 vector. The nucleotides are numbered from the potential transcriptional start site that was assigned +1. Right panel, the relative activities to the 5M4 construct of a series of mutant constructs determined by luciferase assays. c), Point mutation analysis in the E1, E2 and CCAAT boxes of the Miwi promoter by luciferase assay. The 5M4 construct of the promoter (303 bp) was used for these point mutation analyses by linkage to the luciferase gene. The relative activities to the 5M4 construct of the mutant constructs were determined by luciferase assays. The open box and open circle indicate the intact binding site of E1 and E2 or CCAAT box respectively. The filled box or circle indicates the corresponding mutations. Non modified pGL3 was used as a negative control. The firefly luciferase activity was normalized to the Renilla luciferase activity, and the data is shown as the fold increase/decrease over the luciferase activity of pGL3-5M4. The results are the mean ± S.D. of three independent experiments. *, P<0.05; **, P<0.001 compared to the 5M4 wild-type construct. Transcription factors NF-Y and USF bind to the Miwi promoter both in vitro and in vivo
To determine binding of transcription factors NF-Y and USF to the CCAAT and E2 boxes of the promoter respectively, electrophoretic mobility shift assays were carried out using nuclear extracts prepared from mouse testis and double stranded oligonucleotides. Incubation of a CCAAT probe spanning −102/−80 with nuclear extract gave rise to the formation of a DNA–protein complex. The addition of an excessive amount of unlabelled oligo DNA, but not the mutated CCAAT box, could compete with this binding (Figure 2a). Furthermore, addition of anti-NFYa, anti-NFYb or anti-NFYc antibody to the binding reaction caused the disappearance of the specific DNA/protein complex, and the appearance of the super-shift band (Figure 2a). These results show that the CCAAT box located from −97/−93 of the Miwi promoter region is capable of binding with transcription factors NF-Ya, NF-Yb and NF-Yc in vitro.
Fig. 2. Electrophoretic mobility shift and ChIP assays of NF-Y and USF binding to the Miwi promoter. 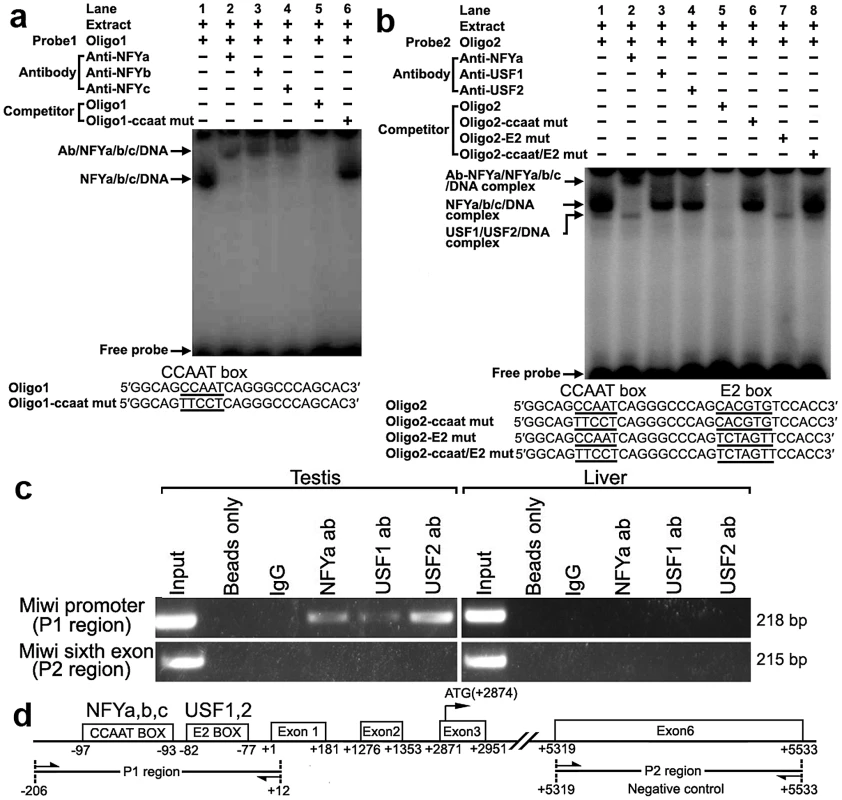
a), NF-Y binding to Miwi promoter. The oligo1 corresponding to −102/−80 was γ-32P-ATP labeled and incubated with 5 µg of nuclear extract of mouse testis in the absence or presence of 50-fold excess of various competitor DNA oligos (mutant or non-labeled oligo1) or antibodies (NF-Ya, NF-Yb and NF-Yc), as indicated on the top of the gel image. The specific DNA/protein complex and the super-shift bands are indicated by arrows. The DNA/protein complex formation cannot be affected by adding competitor mutated DNA (lane 6). The sequences of oligo1 and corresponding mutant are show under the panel. b), USF binding to Miwi promoter. The oligo2 corresponding to −102/−71 was γ-32P-ATP labeled and incubated with 5 µg of nuclear extract of mouse testis in the absence or presence of 50-fold excess of various competitor DNA oligos (mutants or non-labeled oligo2) or antibodies (NF-Ya,USF1/2) as indicated. The specific DNA/protein complex and the super-shift band are indicated by arrows. The sequence of oligo2 and corresponding mutants are indicated under the panel. Two distinct migrating complexes were detected (lane 1). Supershift complex with anti-NFYa showed in lane 2. The addition of anti-USF1 or anti-USF2 antibody to the binding reaction caused the disappearance of the DNA/protein complex (lane 3,4). The DNA/protein complex formation can not be affected by adding mutated DNA competitor (lane 6,7,8). c), ChIP assay of NF-Y and USF binding to the Miwi promoter in testis. The interaction of NF-Y and USF in vivo with the Miwi promoter was determined by chromatin immunoprecipitation analysis. Mouse testis and livers were chopped into small pieces in 1% formaldehyde to cross-link endogenous proteins and DNA. Samples of sonicated chromatin were immunoprecipitated with anti-NFYA, anti-USF1, anti-USF2, no antibody (beads only) or preimmuno IgG (control) respectively. DNA isolated from immunoprecipitated material was amplified by PCR with primers to amplify the 218-bp mouse Miwi promoter sequence corresponding to the −206 to +12 region. Primers for an unrelated part of the sixth exon of Miwi were used as negative controls. The amplified PCR fragments were analyzed on 2% agarose gel. d), The relative positions of the primers in the ChIP assay. With use of nuclear extracts prepared from mouse testis and oligo spanning −102/−71 bearing the CCAAT and E2 boxes, two specific DNA/protein complexes were observed (Figure 2b). Both of the complexes could be competed by the addition of a 50-fold molar excess of unlabelled oligo DNA, but not by the mutated CCAAT box or E2 box, or mutant of both sites. Furthermore, the addition of anti-NF-Ya antibody to the binding reaction generated a super-shift band, and the addition of anti-USF1 or anti-USF2 antibody caused the disappearance of the DNA/protein complex (Figure 2b). These results indicate that both transcription factors USF1 and USF2 bind to the E2 box located from −82/−77 of the Miwi promoter region in vitro.
To determine whether NF-Y and USF bind to the mouse Miwi promoter in vivo, we performed chromatin immunoprecipitation (ChIP) analysis. As shown in Figure 2c, a 218 bp DNA fragment was amplified from the precipitates of anti-NF-Ya, anti-USF1 and anti-USF2 in testis, but it was not amplified from liver (control tissue)(Figure 2c). The amplified fragments were confirmed by sequencing. To further rule out the possibility that the Miwi promoter region precipitated by anti-NF-Y, anti-USF1 and anti-USF2 is due to non-specific binding of these antibodies, an additional PCR amplification of a distinct genomic region (exon 6) was performed on all of the precipitated chromatin DNAs. No band from the precipitate by anti-NFY, anti-USF1 or anti-USF2 antibody was observed. These results indicate that NF-Y, USF1 and USF2 specifically bind to the Miwi promoter region in vivo.
NF-Y and USF activate the Miwi promoter
To further investigate the role of transcription factors NF-Y and USF in activation of the Miwi promoter, both GC-1 and COS7 cells were co-transfected with luciferase reporter driven by Miwi promoter or the mutant for CCAAT or E2 sites with expression plasmid for NF-Ya/b/c or USF1/2 respectively. The luciferase activity increased when co-transfected with NF-Ya/b/c or USF1/2 (Figure 3a, 3d). However, the activities in the mutants for CCAAT or E2 sites were not upregulated (Figure 3b, 3e). Replacing the NF-Ya/b/c or USF1/2 expression plasmid with relevant dominant-negative constructs (NF-YAm29 and A-USF) resulted in a clear reduction of luciferase activity in a dosage-dependent manner (Figure 3c, 3f). NF-YAm29 has a three amino acid substitutions in the C-terminal region of the NF-Ya, which impairs DNA binding activity and acts as a dominant-negative mutant by sequestering the NF-Yb/Yc subunits into a defective complex [21]. A-USF contains the USF heterodimerization domain but lacks the USF-specific region, which is required for transcriptional activation [22], [23]. The results suggest that NF-Y and USF activate the Miwi promoter.
Fig. 3. Over-expression of NF-Y or USF increases Miwi-luciferase activity. 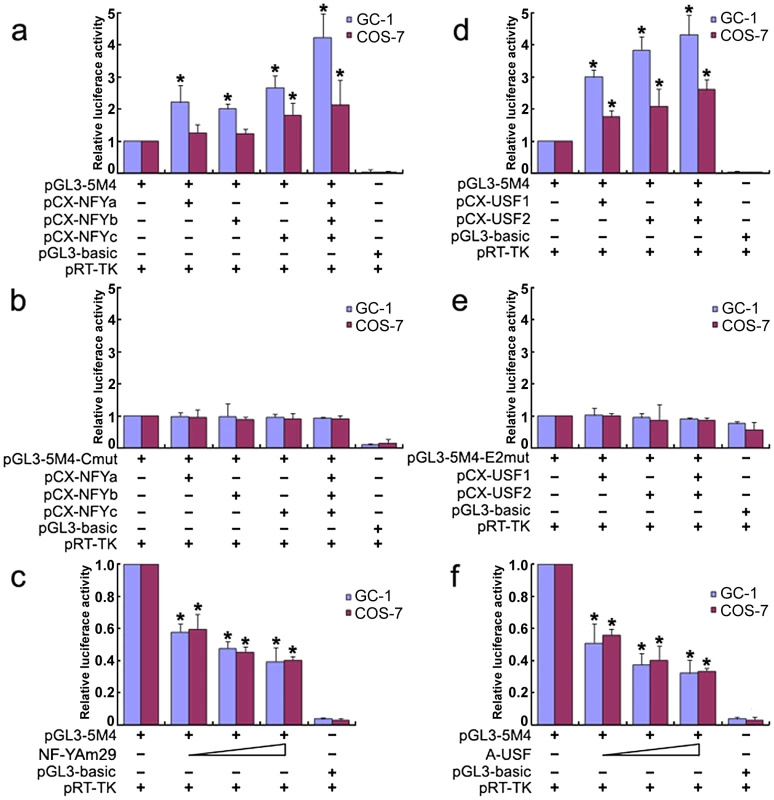
a), Over-expression of NF-Y upregulates Miwi-luciferase activity. GC-1 or COS7 cells were transfected with 0.2 µg pGL3-5M4 and 0.2 µg NF-Y expression plasmids (pCX-NFYa, pCX-NFYb, pCX-NFYc) as indicated. b), The mutated CCAAT box construct pGL3-5M4-Cmut (0.2 µg) contransfected with NF-Y expression plasmids (pCX-NFYa, pCX-NFYb, pCX-NFYc) as indicated, does not increase the Miwi-luciferase activity. c), The dominant negative NF-Ya expression plasmid NF-YAM29 (0.1 µg, 0.2 µg, 0.3 µg) was cotransfected with 0.2 µg pGL3-5M4, which inhibits Miwi-luciferase activity. d), Over-expression of USF1/2 upregulates Miwi-luciferase activity. pGL3-5M4 (0.2 µg) was cotransfected with 0.2 µg expression plasmids (pCX-USF1 and/or pCX-USF2) into GC-1 or COS7 cells. e), E2 box mutant pGL3-5M4-E2mut (0.2 µg) was cotransfected with 0.2 µg USF1 and/or USF2 expression plasmids (pCX-USF1 and/or pCX-USF2), indicating no increase in luciferase activity. f), The dominant negative USF expression plasmid A-USF (0.1 µg, 0.2 µg, 0.3 µg) was cotransfected with 0.2 µg pGL3-5M4, which inhibits Miwi-luciferase activity. pGL3-basic was employed as a negative control. pRT-TK served as an inner control for transfection efficiency. The relative luciferase activities to the 5M4 construct were determined by luciferase assay. Cells were harvested 24 hours post-transfection, and luciferase activity was measured and normalized to Renilla luciferase activity. All transfection experiments were repeated at least three times and the data are shown as the fold increase or decrease over the luciferase activity of pGL3-5M4. Mean ± S.D. are shown. *, P<0.05, as compared to the 5M4 wild-type construct. Methylation profile of the CpG islands in the Miwi promoter
We detected three CpG islands that span positions −466 to −240, −215 to +17, and −7 to +199 around the 2 kb promoter region using the MethPrimer program (Figure 4a). To determine the methylation status of the three CpGs islands, we performed bisulfite sequencing analysis using DNA isolated from spermatogonia, pachytene spermatocytes, round spermatids, epididymal spermatozoa, Sertoli cells and liver tissue. The CpG islands from bisulfite-treated DNA were amplified and five randomly selected clones were subjected to sequencing. Methylation analysis of the three CpG islands in the Miwi promoter revealed a differential methylation profile of CpGs among these cell types (Figure 4b). The CpG islands were always hypermethylated in the spermatogonia, Sertoli cells and liver tissue where Miwi was not expressed, while the CpG islands were unmethylated in the pachytene spermatocytes and round spermatids where Miwi was expressed. The CpG islands were also unmethylated in the epididymal spermatozoa, where Miwi was not expressed.
Fig. 4. The cytosine methylation profile of the CpG islands in the Miwi promoter, and methylation at the E2 box inhibits USF binding. 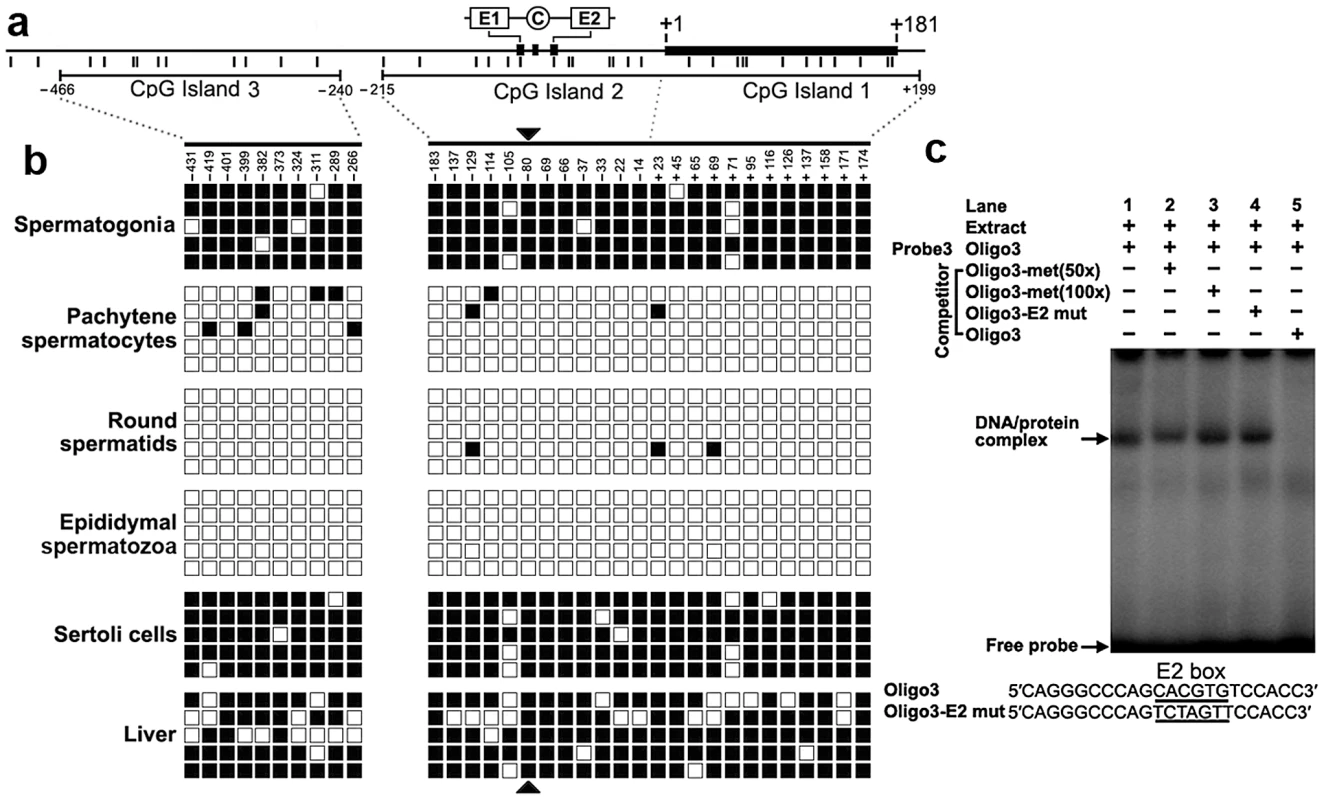
a), Schematic depiction of the CpG islands and CpG dinucleotides (short verticals) spanning the promoter. The relative positions of the E1, E2 and CCAAT box are indicated. b), Methylation status of each CpG dinucleotide in three CpG islands in various cell types of testis and liver. The spermatogonia, pachytene spermatocytes, round spermatids, epididymal spermatozoa and Sertoli cells were isolated from adult testis by flow cytometry. Bisulfite-treated genomic DNA was amplified and cloned into T-easy vector. Five independent clones from each sample were sequenced. Each row represents the sequence of an individual clone, whereas, each column depicts the position of each CpG site relative to the potential transcription start site. Open squares indicate unmethylated CpG sites; filled squares indicate methylated CpG sites; black triangles represent CpG site in the E2 box. c), The CpG methylation at the E2 box inhibits USF binding. The oligo3 corresponding to −92/−71 was γ-32P-ATP labeled and incubated with 5 µg of nuclear extract prepared from mouse testis in the absence or presence of various competitor DNA, as indicated on the top of the gel image. Nuclear extracts were incubated with labeled oligo3 in the absence (lane 1) or in the presence of various DNA competitors: 50-fold or 100-fold excess methylated oligo3 (lanes 2,3), mutant oligo3 (lane 4) or non-labeled oligo3 (lane 5). The specific DNA/protein complexes were indicated by arrows. The sequences of oligo3 and mutant competitor are shown under the panel. CpG methylation at the USF–binding site within the E2 box inhibits the binding of USF
From the methylation profile of CpG islands in the Miwi promoter, we noted an inverse association between the methylation status of the CpG dinucleotide (−80) within the E2 box and the Miwi expression pattern. To examine the importance of CpG methylation within the E2 box for Miwi expression, we performed EMSAs using a methylated double-stranded oligonucleotide, in which the cytosine residue in the CpG dinucleotide was methylated with M.SssI (CpG Methyltransferase) in vitro. Incubation of nuclear extract from mouse testis with a wild-type oligo probe resulted in the formation of a protein/DNA complex. This complex could be competed with an excessive amount of unlabelled oligo DNA. However, the addition of 50-excess or 100-excess of unlabelled methylated oligo DNA or E2 mutant did not affect the complex formation (Figure 4c). Our previous ChIP experiment in testis and liver showed that USF1/2 could bind to the E2 box only in testis (Figure 2), which is consistent with the methylation status at the CpG site within the E2 box. These results suggest that CpG methylation at the USF-binding site within the E2 box inhibits the binding of USF.
A 303 bp core promoter of the mouse Miwi directs specific expression during meiosis in transgenic mice
To examine the functional role of the core promoter of Miwi during spermatogenesis, we have made Miwi-drived EGFP transgenic mice. The Miwi promoter sequence spanning from −122 to +181 was cloned into the pEGFP-1 vector. A 1.3 kb fragment digested with both HindIII and AflII was purified for the production of transgenic mice (Figure 5a). Western blot analysis revealed that Miwi-drived EGFP was specifically expressed in testis of transgenic founders (Figure 5b). To determine whether transgenic Miwi-EGFP and endogenous Miwi are co-expressed in the seminiferous tubules of transgenic mouse testis, we first examined the subcellular localization by immunofluorescence and confocal microscopy. Transgenic Miwi-EGFP and endogenous Miwi were nearly co-localized in the cytoplasm of spermatocytes and round spermatids, particularly in round spermatids, both Miwi and EGFP concentrate in the chromatoid body (Figure 6). The cell types expressing EGFP in the seminiferous tubules of transgenic mice were further confirmed by immunochemical localization analysis in both transgenic and wild mice (Figure 7). These results suggest that EGFP transgene expression, driven by the 303 bp Miwi promoter, is germline-specific during spermatogenesis. Other promoters (e.g. Dazl and H1t) have also been observed to drive reporter expression in a germ-cell specific manner [24], [25].
Fig. 5. Miwi-EGFP transgenic construct and expression in transgenic mice. 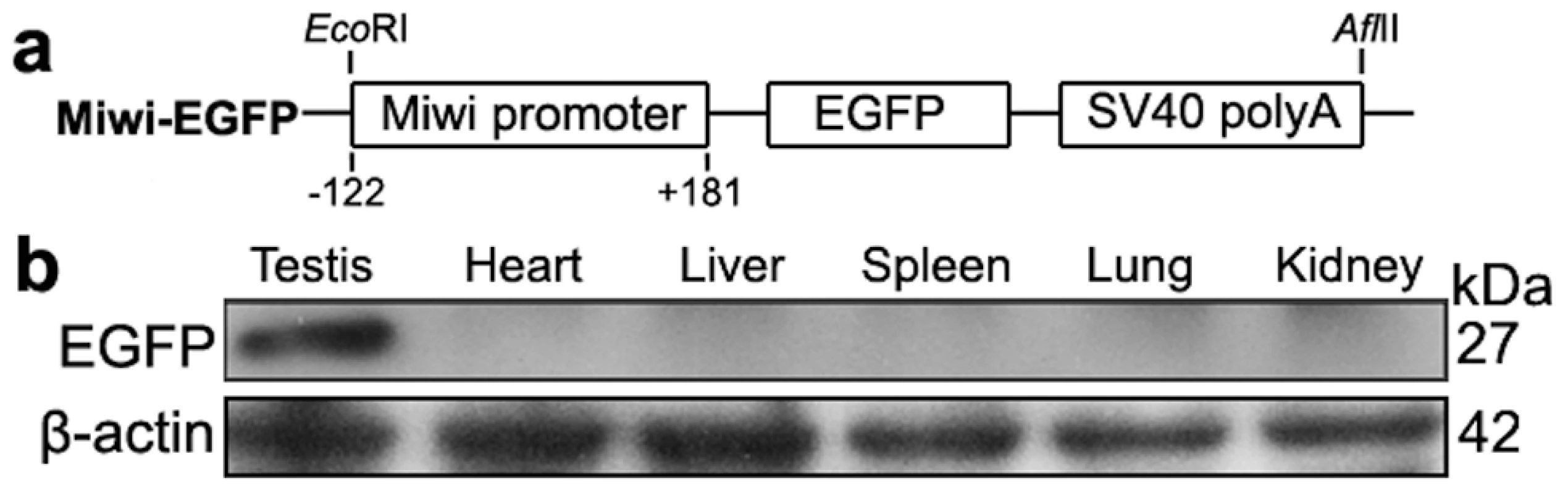
a), Schematic depiction of transgenic Miwi-EGFP construct, which contains a region of the Miwi promoter from −122∼+181 cloned into a pEGFP-1 vector. The EcoRI-AflII fragment was used for microinjection. b), Western blot analysis of the EGFP protein in the adult testis, heart, liver, spleen, lung and kidney in Miwi-EGFP transgenic mouse. The EGFP expression was only detected in the testis. β-actin served as an internal control. Fig. 6. Co-localization of transgenic Miwi-EGFP and endogenous Miwi in transgenic mouse. 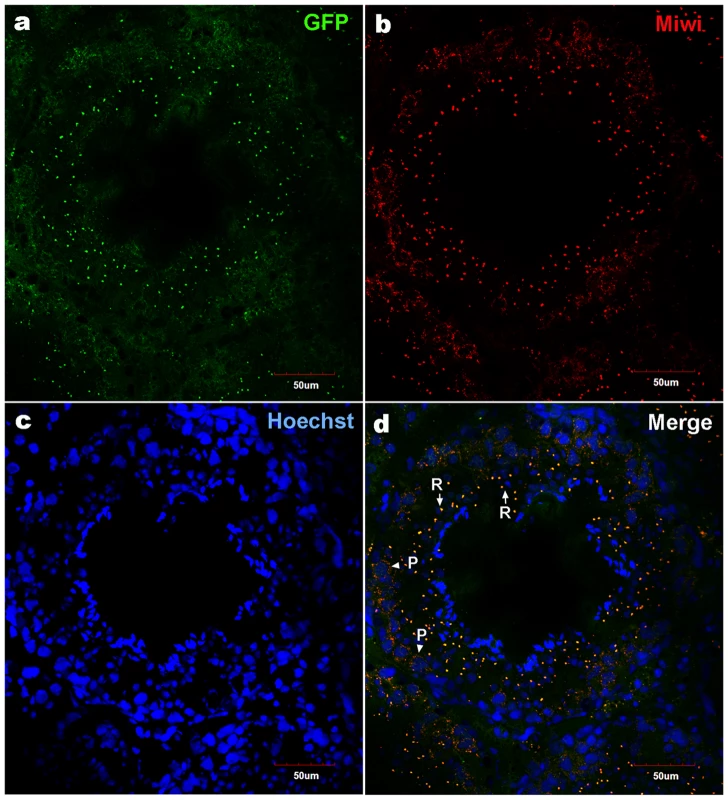
Transgenic Miwi-EGFP and endogenous Miwi proteins in the Miwi-EGFP transgenic mouse testis were examined by indirect immunofluorescence and confocal microscopy using anti-GFP and anti-Miwi antibodies. Transgenic Miwi-EGFP (a) and endogenous Miwi (b) are nearly co-expressed in the cytoplasm of spermatocytes (P, arrowheads in panel d) and round spermatids (R, arrows in panel d), especially in round spermatids, both Miwi and GFP concentrate in the chromatoid body. Nuclei were stained using Hoechst33258 (blue). Green, transgenic EGFP; Red, endogenous Miwi; Merged image was showed in panel d. Negative controls were showed in Figure S1A. Fig. 7. Cell types of Miwi-EGFP expression in transgenic mouse testis. 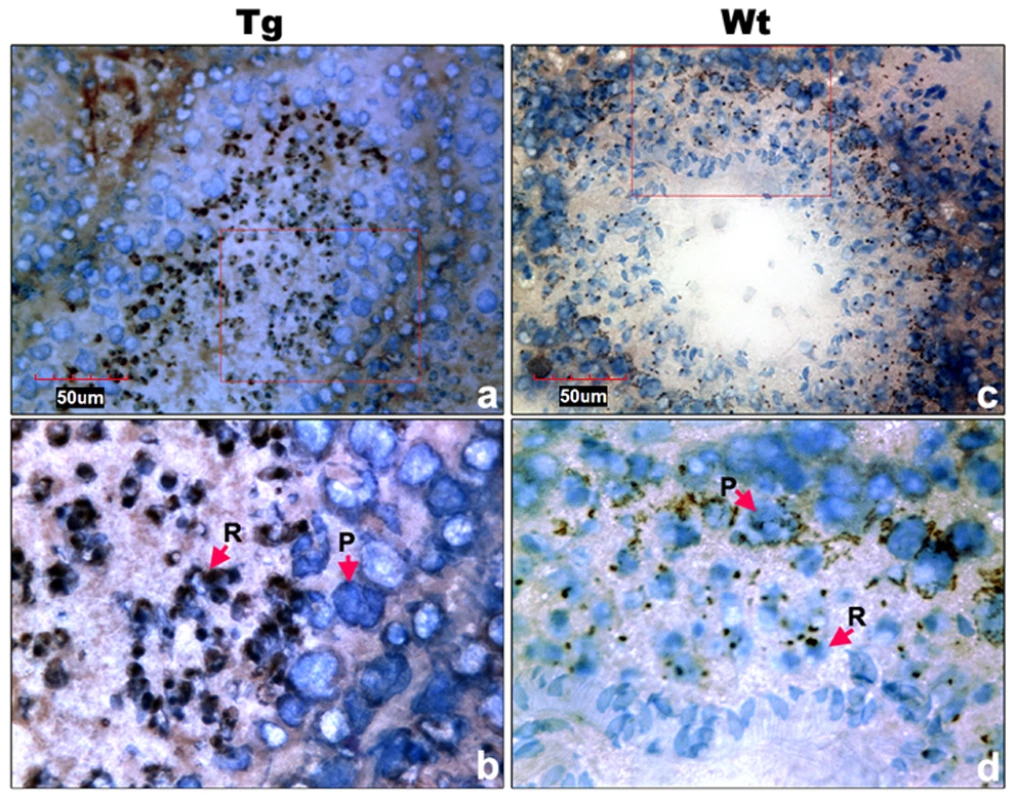
a), Immunocytochemical localization of EGFP in transgenic mouse testis using anti-EGFP antibody. c), Endogenous Miwi expression was detected in the wild-type testis using anti-Miwi antibody. Signals were detected with horseradish peroxidase-conjugated secondary antibody (brown). Bottom panels (b,d) are enlarged images (100×) from the squares in their respective upper panels (a,c) (40×). Negative controls were showed in Figure S1B. Nuclei were re-dyed with hematoxylin (blue). P, pachytene spermatocytes; R, round spermatids. The Miwi-EGFP transgene promoter is epigenetically modified through methylation in vivo in transgenic mice
To verify whether the DNA methylation profile of the Miwi promoter driven transgene matches that of the endogenous Miwi promoter, we performed flow cytometric analysis of male germ cells from the testis of three Miwi-EGFP heterozygous transgenic mice. Around 10% of cells were EGFP-positive in the transgenic mice (Figure 8a, 8b), while male germ cells from wild-type mice did not contain EGFP-positive cells (Figure 8c, 8d). To compare the methylation status of both EGFP-positive and negative cells in the transgenic mice testis, both types of cells were separated and collected from three heterozygous transgenic Miwi-EGFP mice. We then performed bisulfate sequencing analysis using DNA isolated from both EGFP-positive and negative cells. Sixty randomly selected clones were subjected to sequencing. Biased methylation status was observed between EGFP-positive cells and negative cells from the testes of Miwi-EGFP transgenic mice. Methylation frequency for EGFP-negative cells was 90–100%, however the frequency for EGFP-positive cells was lower than 30%. Further, in the CpG positions −105 and −80 (key USF binding site), the methylation frequency for EGFP-negative cells was 93% and 96% respectively, while the CpG dinucleotides in both positions of the EGFP-positive cells were un-methylated (Figure 8e, 8f). These results show that the DNA methylation profile of the Miwi promoter driven transgene is completely consistent with that of the endogenous Miwi promoter and further demonstrate that USF and demethylation of its binding site regulate male germline-specific expression of Miwi gene.
Fig. 8. Methylation profile of the Miwi promoter–driven transgene in Miwi-EGFP transgenic mice. 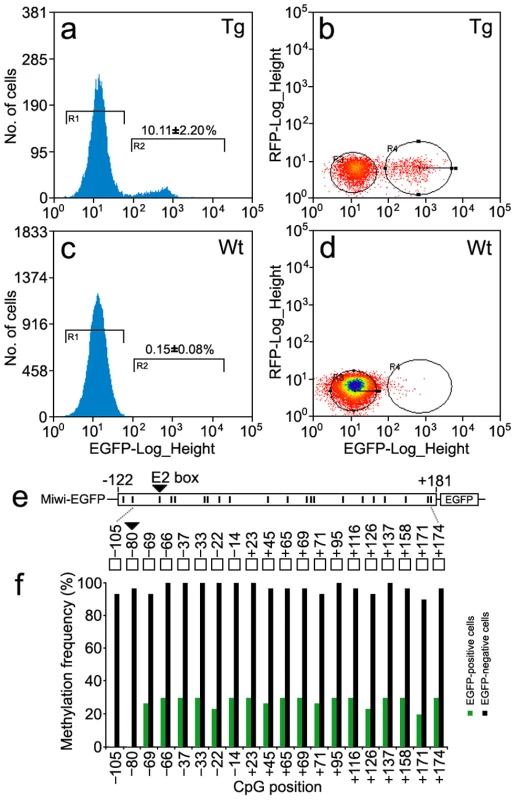
a,c), Flow cytometric analysis of male germ cells from heterozygous Miwi-EGFP transgenic mice (a) and wild type (c). Values on the y-axis represent the number of cells, and values on the x-axis represent EGFP fluorescence intensity. The number in the R2 gate represents the percentage of the cells with EGFP fluorescence. The data is representative of three independent experiments. b,d) Flow cytometric separation and collection of EGFP-positive male germ cells from Miwi-EGFP transgenic mice (b) and wild type mice were used as a control (d). Values on the x-axis represent EGFP fluorescence intensity, and values on the y-axis represent red fluorescence intensity. The cell population in the R4 gate shows clear EGFP fluorescence in panel b. e). The positions of 20 CpG dinucleotides within the promoter region of the Miwi-EGFP transgene. f). Comparison of methylation frequencies along the CpG positions between EGFP-positive cells and EGFP-negative cells from the Miwi-EGFP transgenic mouse testis. The CpG dinucleotides in the E2 box (−80) were hyper-methylated (96%) in the EGFP negative cells, however, the CpG in the same position were completely un-methylated in the EGFP-positive cells. Discussion
To produce functional sperm, male germ stem cells gradually lose their stem cell potential and initiate differentiation to become highly specialized spermatozoa. During the differentiation process, accurate spatio-temporal expression regulation of a variety of genes is important to germ cell fate. The current study reveals a clear methylation regulatory mechanism of the Miwi gene during spermatogenesis; CpG methylation inhibits the binding of the USF, thereby repressing Miwi expression. When demethylation occurs, the transcription factor USF binds to and activates the Miwi promoter. Importantly, methylation-mediated regulation of Miwi by transcription factor USF occurs in a small region of the Miwi core promoter, which controls germline-specific expression of Miwi in testis. Thus, we present an epigenetic regulation mechanism for the spatio-temporal expression of mouse Miwi, which is driven by transcription factor USF during spermatogenesis.
The present study has contributed to our understanding of how ubiquitous protein USF regulates testis-specific expression of the Miwi gene in a methylation-dependent manner. Both USF1 and USF2 are ubiquitous proteins characterized by highly conserved C-terminal basic helix-loop-helix and leucine zipper domains responsible for their dimerization and DNA binding activities [23], [26]. A number of genes have an E-box in their promoter, and the consensus sequence CANNTG, is known as the binding site for USFs to regulate transcription [27], [28]. For example, Mitf activates transcription through binding to the E-box element on the regulatory sequences of three germ genes (dazl, dnd and vasa) in medaka [29]. Although expressed ubiquitously, USFs appear to be involved in regulation of developmental and tissue-specific expression of other target genes such as surfactant protein A, steroidogenic factor 1, chipmunk hibernation-specific HP-27, transcription factor homeobox B4, carboxyl ester lipase and Prolyl-4-hydroxylase (I) [30], [31], [32], [33], [34], [35]. Our results show that USF is required for the specific expression of the Miwi gene. This USF regulation is methylation-dependent, which ensures developmental and cell type-specific expression of Miwi during meiosis of male germ cells.
The methylation of genomic DNA at CpG dinucleotides is a major epigenetic modification of the genome [36] and contributes to general transcriptional suppression, either by blocking the binding of transcription factors to their CpG-containing binding sites [31] or by recruiting methyl-CpG-binding proteins, which in turn recruit chromatin-remodeling machinery to induce the formation of a repressive chromatin structure [37]. Promoter methylation is associated with down-regulation of gene expression during spermatogenesis [38], [39], [40]. Miwi is a gene expressed specifically in the testis from midpachytene spermatocytes to round spermatids. To address how Miwi gene is regulated during spermatogenesis, we used bisulfite genomic sequencing to profile CpG methylation within the proximal promoter in cells where the gene is transcribed or silenced. We indeed observed an obvious correlation between Miwi gene expression and CpG methylation status in concert with differentiation of germ cell types. Importantly, we observed a key binding site for transcription factor USF, which plays a vital role in regulation of Miwi expression in a demethylation dependent manner. A selective demethylation and a consequent remethylation late in germ cell development have also been observed in several other testis-specific genes such as ALF and Pgk2 [41], [42], [43]. Taken together, these data support that methylation modification is a key regulation mechanism for spermatogenesis.
In mammals, both DNA methylation and demethylation events occur during germ cell development and differentiation. Molecular mechanisms of regulating these processes remain largely unknown. There are five methyltransferases (DNMTs), DNMT1, DNMT2, DNMT3a, DNMT3b and DNMT3L [44], and three of them DNMT1, DNMT3a, and DNMT3b are involved in methylation in vivo. It has been proposed that DNMT3a and DNMT3b may contribute differentially to the establishment and/or maintenance of methylation patterns in male germ cells [45], [46]. Understanding of mammalian DNA demethylation represents a great challenge. There are probably two kinds of molecular mechanisms of DNA demethylation in mammals [47], passive demethylation occuring by blocking methylation of newly synthesized DNA during DNA replication, and active demethylation which is demethylation process is initiated by the same enzymes that establish the methylation, DNMT3A and DNMT3B [48], [49]. The question remains, as to how the demethylation of such an important E2 cis-element is regulated during spermatogenesis. Further study on the mechanism will provide new insight into mammalian spermatogenesis.
Materials and Methods
All animal work has been conducted according to relevant national and international guidelines. Detailed Materials and Methods are described in Text S1.
In silico sequence analysis
Transcription factor binding sites were predicted in the genomic DNA sequence of mouse Miwi using the TFsearch program (http://www.cbrc.jp/research/db/TFSEARCH.html) with a threshold score of 85 and Vertebrate Matrix. CpG islands in Miwi promoter were predicted by MethPrimer (http://www.urogene.org/methprimer/index1.html) with observed/expected ratio >0.6 and percent C+G>50%.
Cell culture, transient transfection, and dual-luciferase reporter assay
GC-1 (a mouse germ-cell line at a stage between a type B spermatogonia and primary spermatocyte, has the capacity to differentiate into spermatids within the seminiferous tubules.) and COS7 (African Green Monkey SV40-transformed kidney fibroblast cell line) cells (for comparison, both germ-line and non germ-line cells were chosen) were maintained in high glucose Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, and plated in 48-well plates and transfected using 2 µl Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) in each well. Plasmid DNAs were prepared in a dam - and dcm - E.coli strain (SCS110). Each deletion construct (pGL3-5M1, pGL3-5M2, pGL3-5M3, pGL3-5M4, pGL3-5M5, pGL3-5M6, pGL3-5M7 and pGL3-5M8) was transfected at 0.4 µg along with 10 ng/well pRT-TK (an internal control). For co-transfection luciferase assays, 0.2 µg of Miwi promoter construct pGL3-5M4 or mutated constructs, and 0.2 µg transcription factor expression vectors or corresponding dominant negative plasmid were co-transfected into cells. Empty vector pCX was added to equalize the final DNA content among each well. Luciferase activities were measured 24 h after transfection using the dual-luciferase reporter assay system (Promega, Madison, WI, USA) and a Modulus Single Tube Multimode Reader (Turner Biosystems, Sunnyvale, CA, USA) based on the manufacturer's protocol. Assays were performed in triplicates and expressed as means ± S.D.
Isolation of spermatogenic cells by fluorescence-activated cell sorting (FACS)
Spermatogonia, pachytene spermatocytes, round spermatids, and Sertoli cells were isolated using the method described by Mays-Hoopes et al. [50]. Briefly, after the tunica albunigea was removed, the seminiferous tubules of adult testis of KunMing mice were treated with collagenase and washed to release the Leydig cells as well as interstitial cells. 0.25% trypsin treatment for 20 min at 37°C was followed by pipetting to release the cells. After fixed by the high citrate fixative method, the cells were ready for fluorescence-activated cell sorting.
For separation and collection of the EGFP-positive and negative cells from the testis of Miwi-EGFP transgenic mice, the seminiferous tubules were treated with trypsin and pipetted to release the cells. The cells were then analyzed on a MoFlo-XDP High-performance cell sorter (Beckman Coulter, Inc., Fullerton, CA, USA). Excitation of GFP was at 488 nm. Sorted cell populations were collected by centrifugation for 3 min at 3000 g and resuspended in PBS.
Bisulfite–PCR methylation analysis
Sodium bisulfite treatment of genomic DNA was performed as follows. Briefly, 2 µg of genomic DNA was denatured with 2 M NaOH for 15 min, then treated with 3 M sodium bisulfate (pH 5.0) (Sigma-Aldrich, St.Louis, MO, USA) and 10 mM hydroquinone at 50°C for 18 h. DNA was purified using a Wizar DNA Clean-up kit (Sigma-Aldrich, St Louis, MO, USA), and NaOH was added to 0.3M. Bisulfite treated DNA, which was then precipitated with ethanol and resuspended in 20 µl sterile distilled water.
Electrophoretic mobility shift assays (EMSA)
Oligonucleotides corresponding to the CCAAT and E boxes of the Miwi promoter were synthesized and annealed into double strands. Radiolabeled probes were generated by incubation of 250 ng annealed oligonucleotides with 20 µCi [γ-32P] dATP in the presence of T4 Polynucleotide Kinase (Promega, Madison, WI, USA) for 1 h at 37°C, and were subsequently separated from free nucleotide using G-50 column purification (Amersham Biosciences, Uppsala, Sweden). Testis nuclear extract was then incubated at room temperature for 30 min with a 100,000 dpm radiolabeled probe and 1 µg poly (dI-dC) in 10 mmol/l Tris-HCl, pH 7.5, 50 mmol/l NaCl, 1 mmol/l dithiothreitol, 1 mmol/l EDTA, and 5% glycerol. For supershift experiments, binding reactions were subsequently incubated with 5 µg of antibody for 30 min at room temperature. For competition experiments, the unlabeled competitor oligos (in 50-fold molar excess) were added together with the probe at the start of the incubation. Samples were resolved on 5% polyacrylamide gels in 0.5% TBE running buffer at 10 V/cm for 2 h. The dried gel was exposed to a phosphorimager cassette and scanned with typhoon 9200 instrument (GE-Healthcare, Amersham bioscience, Uppsala, Sweden).
Chromatin immunoprecipitation (ChIP)
Mouse testis and livers were chopped into small pieces with a scalpel in cold phosphate-buffered saline (PBS) and cross-linked in 1% formaldehyde-PBS for 15 min with constant shaking. The tissue was rinsed in cold PBS and homogenized with a Dounce homogenizer in 1 ml cold cell lysis buffer (10 mM Tris-Cl, pH 8.0, 10 mM NaCl, 3 mM MgCl2, 0.5% NP-40) supplemented with protease inhibitors (Roche Diagnostics Ltd, Mannheim, Germany). Cells were incubated at 4°C for 5 min to allow the release of nuclei. Nuclei were sedimented by centrifugation at 13,000 g for 5 min. After lysis in the buffer (1% sodium dodecyl sulfate [SDS], 5 mM EDTA, 50 mM Tris-Cl, pH 8.1), sonication was performed with a Sonic Dismembrator model 100 sonicator (Fisher Scientific, Inc., Pittsburgh, PA, USA). After centrifugation, the supernatant chromatin was immunoprecipitated with no antibody (beads only), preimmuno IgG (IgG), anti-NF-Ya (Abcam Inc., Cambridge, CA, USA), anti-USF1 (Santa Cruz Biotech, CA, USA) or anti-USF2 (Santa Cruz), together with Protein G PLUS-Agarose (Santa Cruz) respectively. DNA isolated from the immunoprecipitated complex was amplified by PCR with primers flanking the CCAAT/E box binding sites or control primers. The PCR products were cloned into T-easy vector (Promega, Madison, WI, USA) and sequenced.
Production of transgenic mice
A 303 bp fragment of mouse Miwi promoter ranging from −122 to +181 was amplified from genomic DNA of the KunMing mouse using primers designed with an EcoRI recognition site at the 5′ end of the forward primer and a BamHI recognition site on the 5′ end of the reverse primer. PCR fragments were subcloned into EcoRI/BamHI-digested pEGFP-1 vector. A 1.3 kb fragment digested with both HindIII and AflII was separated by agarose gel electrophoresis, dissolved in buffer of 5 mM Tris and 0.1 mM EDTA at 1 ng/µl. The purified DNA was microinjected into pronuclei of fertilized eggs from the KunMing mice on an inverted microscope (Leica DM IRB) (Leica Microscopy Systems Ltd, Wetzlar, Germany) equipped with a Leica manually operated micromanipulator (Leica) attached to a micro-injection system (Narishige, Tokyo, Japan). The injected eggs were transferred into the oviduct of day 1 pseudopregnant recipients. Transgenic animals were identified by PCR screening of genomic DNA isolated from tail with a pair of primers in the transgenic fragment and GFP checking of testis sections under fluorescent microscopy (Leica). Two independent transgenic lines have been analyzed in this study. Homozygotes were identified by crossing experiments and 100% presence of transgene in offsprings.
Immunofluorescence and immunocytochemistry
Mouse testes were embedded in OCT medium (Tissue Tek, Miles, Elkhart, IN, USA) and cut into a series of 6 µm sections with a cryostat (Leica, Bensheim, Germany). To determine immunocytochemical localization, the sections were fixed with methanol for 20 min at −20°C and then permeabilized with 0.1% Triton X-100 in PBS for 30 min. Then sections were treated with 5% BSA for 20 min at room temperature and incubated with anti-Miwi (G82) (Cell Signaling Technology Inc., Danvers, MA, USA) or anti-GFP (D5.1) (Cell Signaling Technology). Then SABC and DAB were used for color visualization according to the manufacturer's instructions (Boster Company, China). For immunofluorescence analysis, after sections were fixed with methanol for 20 min at −20°C, permeabilized with 0.1% Triton X-100 in PBS for 30 min, and treated with 5% BSA for 20 min at room temperature, the sections were incubated at 4°C overnight in 1∶50 anti-Miwi (Cell Signaling Technology). After washing 3 times with PBS, 1∶100 dilution of the corresponding DylightTM 594-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) was applied at 37°C for 1 hour. The sections were washed in PBS for 5 times. After blocked again at room temperature for 20 min in 5% BSA, the sections were incubated at 4°C overnight in 1∶50 anti-GFP (Abcam). The corresponding FITC-conjugated secondary antibody (1∶100 dilution)(Proteintech Group, Chicago, IL, USA) were then incubated at 37°C for 1 hour. The nuclei were counterstained with Hoechst33258. Images were taken by confocal fluorescence microscopy (FV1000, Olympus, Tokyo, Japan).
Supporting Information
Zdroje
1. TanakaHBabaT 2005 Gene expression in spermiogenesis. Cell Mol Life Sci 62 344 354
2. OakesCCLa SalleSSmiragliaDJRobaireBTraslerJM 2007 A unique configuration of genome-wide DNA methylation patterns in the testis. Proc Natl Acad Sci U S A 104 228 233
3. OakesCCLa SalleSSmiragliaDJRobaireBTraslerJM 2007 Developmental acquisition of genome-wide DNA methylation occurs prior to meiosis in male germ cells. Dev Biol 307 368 379
4. MaatoukDMKellamLDMannMRLeiHLiE 2006 DNA methylation is a primary mechanism for silencing postmigratory primordial germ cell genes in both germ cell and somatic cell lineages. Development 133 3411 3418
5. FajardoMAHaugenHSCleggCHBraunRE 1997 Separate elements in the 3′ untranslated region of the mouse protamine 1 mRNA regulate translational repression and activation during murine spermatogenesis. Dev Biol 191 42 52
6. GiorginiFDaviesHGBraunRE 2002 Translational repression by MSY4 inhibits spermatid differentiation in mice. Development 129 3669 3679
7. YangJMedvedevSReddiPPSchultzRMHechtNB 2005 The DNA/RNA-binding protein MSY2 marks specific transcripts for cytoplasmic storage in mouse male germ cells. Proc Natl Acad Sci U S A 102 1513 1518
8. ZhaoWZhouFZhouXHouYHeY 2010 Mago, a vertebrate homolog of Drosophila Mago nashi protein, is a component of the chromatoid body in the cytoplasm of the postmeiotic spermatid. J Exp Zool B Mol Dev Evol 314 232 241
9. Valencia-SanchezMALiuJHannonGJParkerR 2006 Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev 20 515 524
10. DengWLinH 2002 miwi, a murine homolog of piwi, encodes a cytoplasmic protein essential for spermatogenesis. Dev Cell 2 819 830
11. GirardASachidanandamRHannonGJCarmellMA 2006 A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature 442 199 202
12. GrivnaSTBeyretEWangZLinH 2006 A novel class of small RNAs in mouse spermatogenic cells. Genes Dev 20 1709 1714
13. WatanabeTTakedaATsukiyamaTMiseKOkunoT 2006 Identification and characterization of two novel classes of small RNAs in the mouse germline: retrotransposon-derived siRNAs in oocytes and germline small RNAs in testes. Genes Dev 20 1732 1743
14. GrivnaSTPyhtilaBLinH 2006 MIWI associates with translational machinery and PIWI-interacting RNAs (piRNAs) in regulating spermatogenesis. Proc Natl Acad Sci U S A 103 13415 13420
15. Kuramochi-MiyagawaSKimuraTYomogidaKKuroiwaATadokoroY 2001 Two mouse piwi-related genes: miwi and mili. Mech Dev 108 121 133
16. KotajaNBhattacharyyaSNJaskiewiczLKimminsSParvinenM 2006 The chromatoid body of male germ cells: similarity with processing bodies and presence of Dicer and microRNA pathway components. Proc Natl Acad Sci U S A 103 2647 2652
17. KojimaKKuramochi-MiyagawaSChumaSTanakaTNakatsujiN 2009 Associations between PIWI proteins and TDRD1/MTR-1 are critical for integrated subcellular localization in murine male germ cells. Genes Cells 14 1155 1165
18. ChenCJinJJamesDAAdams-CioabaMAParkJG 2009 Mouse Piwi interactome identifies binding mechanism of Tdrkh Tudor domain to arginine methylated Miwi. Proc Natl Acad Sci U S A 106 20336 20341
19. VaginVVWohlschlegelJQuJJonssonZHuangX 2009 Proteomic analysis of murine Piwi proteins reveals a role for arginine methylation in specifying interaction with Tudor family members. Genes Dev 23 1749 1762
20. SiomiMCMannenTSiomiH 2010 How does the royal family of Tudor rule the PIWI-interacting RNA pathway? Genes Dev 24 636 646
21. MantovaniRLiXYPessaraUHooft van HuisjduijnenRBenoistC 1994 Dominant negative analogs of NF-YA. J Biol Chem 269 20340 20346
22. QyangYLuoXLuTIsmailPMKrylovD 1999 Cell-type-dependent activity of the ubiquitous transcription factor USF in cellular proliferation and transcriptional activation. Mol Cell Biol 19 1508 1517
23. LuoXSawadogoM 1996 Functional domains of the transcription factor USF2: atypical nuclear localization signals and context-dependent transcriptional activation domains. Mol Cell Biol 16 1367 1375
24. NicholasCRXuEYBananiSFHammerREHamraFK 2009 Characterization of a Dazl-GFP germ cell-specific reporter. Genesis 47 74 84
25. BartellJGDavisTKremerEJDeweyMJKistlerWS 1996 Expression of the rat testis-specific histone H1t gene in transgenic mice. One kilobase of 5′-flanking sequence mediates correct expression of a lacZ fusion gene. J Biol Chem 271 4046 4054
26. GregorPDSawadogoMRoederRG 1990 The adenovirus major late transcription factor USF is a member of the helix-loop-helix group of regulatory proteins and binds to DNA as a dimer. Genes Dev 4 1730 1740
27. JiangBMendelsonCR 2003 USF1 and USF2 mediate inhibition of human trophoblast differentiation and CYP19 gene expression by Mash-2 and hypoxia. Mol Cell Biol 23 6117 6128
28. JiangBMendelsonCR 2005 O2 enhancement of human trophoblast differentiation and hCYP19 (aromatase) gene expression are mediated by proteasomal degradation of USF1 and USF2. Mol Cell Biol 25 8824 8833
29. ZhaoHLiMPurwantiYILiuRChenT 2012 Mitf is a transcriptional activator of medaka germ genes in culture. Biochimie 94 759 767
30. GaoEWangYAlcornJLMendelsonCR 1997 The basic helix-loop-helix-zipper transcription factor USF1 regulates expression of the surfactant protein-A gene. J Biol Chem 272 23398 23406
31. FujiiGNakamuraYTsukamotoDItoMShibaT 2006 CpG methylation at the USF-binding site is important for the liver-specific transcription of the chipmunk HP-27 gene. Biochem J 395 203 209
32. ChenLShenYHWangXWangJGanY 2006 Human prolyl-4-hydroxylase alpha(I) transcription is mediated by upstream stimulatory factors. J Biol Chem 281 10849 10855
33. HarrisANMellonPL 1998 The basic helix-loop-helix, leucine zipper transcription factor, USF (upstream stimulatory factor), is a key regulator of SF-1 (steroidogenic factor-1) gene expression in pituitary gonadotrope and steroidogenic cells. Mol Endocrinol 12 714 726
34. BengtssonSHMadeyski-BengtsonKNilssonJBjursellG 2002 Transcriptional regulation of the human carboxyl ester lipase gene in THP-1 monocytes: an E-box required for activation binds upstream stimulatory factors 1 and 2. Biochem J 365 481 488
35. ZhuJGiannolaDMZhangYRiveraAJEmersonSG 2003 NF-Y cooperates with USF1/2 to induce the hematopoietic expression of HOXB4. Blood 102 2420 2427
36. GruenbaumYSteinRCedarHRazinA 1981 Methylation of CpG sequences in eukaryotic DNA. FEBS Lett 124 67 71
37. OrdwayJMCurranT 2002 Methylation matters: modeling a manageable genome. Cell Growth Differ 13 149 162
38. NishinoKHattoriNTanakaSShiotaK 2004 DNA methylation-mediated control of Sry gene expression in mouse gonadal development. J Biol Chem 279 22306 22313
39. ChoiYCChaeCB 1991 DNA hypomethylation and germ cell-specific expression of testis-specific H2B histone gene. J Biol Chem 266 20504 20511
40. IannelloRCGouldJAYoungJCGiudiceAMedcalfR 2000 Methylation-dependent silencing of the testis-specific Pdha-2 basal promoter occurs through selective targeting of an activating transcription factor/cAMP-responsive element-binding site. J Biol Chem 275 19603 19608
41. XieWHanSKhanMDeJongJ 2002 Regulation of ALF gene expression in somatic and male germ line tissues involves partial and site-specific patterns of methylation. J Biol Chem 277 17765 17774
42. McCarreyJRGeyerCBYoshiokaH 2005 Epigenetic regulation of testis-specific gene expression. Ann N Y Acad Sci 1061 226 242
43. ArielMCedarHMcCarreyJ 1994 Developmental changes in methylation of spermatogenesis-specific genes include reprogramming in the epididymis. Nat Genet 7 59 63
44. GollMGBestorTH 2005 Eukaryotic cytosine methyltransferases. Annual review of biochemistry 74 481 514
45. La SalleSTraslerJM 2006 Dynamic expression of DNMT3a and DNMT3b isoforms during male germ cell development in the mouse. Dev Biol 296 71 82
46. KatoYKanedaMHataKKumakiKHisanoM 2007 Role of the Dnmt3 family in de novo methylation of imprinted and repetitive sequences during male germ cell development in the mouse. Hum Mol Genet 16 2272 2280
47. OoiSKBestorTH 2008 The colorful history of active DNA demethylation. Cell 133 1145 1148
48. KangaspeskaSStrideBMetivierRPolycarpou-SchwarzMIbbersonD 2008 Transient cyclical methylation of promoter DNA. Nature 452 112 115
49. MetivierRGallaisRTiffocheCLe PeronCJurkowskaRZ 2008 Cyclical DNA methylation of a transcriptionally active promoter. Nature 452 45 50
50. Mays-HoopesLLBolenJRiggsADSinger-SamJ 1995 Preparation of spermatogonia, spermatocytes, and round spermatids for analysis of gene expression using fluorescence-activated cell sorting. Biol Reprod 53 1003 1011
Štítky
Genetika Reprodukční medicína
Článek Functional Centromeres Determine the Activation Time of Pericentric Origins of DNA Replication inČlánek Dynamic Deposition of Histone Variant H3.3 Accompanies Developmental Remodeling of the TranscriptomeČlánek Integrin α PAT-2/CDC-42 Signaling Is Required for Muscle-Mediated Clearance of Apoptotic Cells inČlánek Prdm5 Regulates Collagen Gene Transcription by Association with RNA Polymerase II in Developing BoneČlánek Acquisition Order of Ras and p53 Gene Alterations Defines Distinct Adrenocortical Tumor Phenotypes
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 5- Akutní intermitentní porfyrie
- Souvislost haplotypu M2 genu pro annexin A5 s opakovanými reprodukčními ztrátami
- Vztah užívání alkoholu a mužské fertility
- Doporučení pro diagnostiku a léčbu akutních jaterních porfyrií
- Doc. Miloš Kubánek: Nemocní se srdeční amyloidózou jsou často skryti a sledováni pod jinými diagnózami
-
Všechny články tohoto čísla
- Slowing Replication in Preparation for Reduction
- Chromosome Pairing: A Hidden Treasure No More
- Loss of Imprinting Differentially Affects REM/NREM Sleep and Cognition in Mice
- Six Novel Susceptibility Loci for Early-Onset Androgenetic Alopecia and Their Unexpected Association with Common Diseases
- Regulation by the Noncoding RNA
- UDP-Galactose 4′-Epimerase Activities toward UDP-Gal and UDP-GalNAc Play Different Roles in the Development of
- Deletion of PTH Rescues Skeletal Abnormalities and High Osteopontin Levels in Mice
- Karyotypic Determinants of Chromosome Instability in Aneuploid Budding Yeast
- Genome-Wide Copy Number Analysis Uncovers a New HSCR Gene:
- MicroRNA-277 Modulates the Neurodegeneration Caused by Fragile X Premutation rCGG Repeats
- Functional Centromeres Determine the Activation Time of Pericentric Origins of DNA Replication in
- Dynamic Deposition of Histone Variant H3.3 Accompanies Developmental Remodeling of the Transcriptome
- Scientist Citizen: An Interview with Bruce Alberts
- YY1 Regulates Melanocyte Development and Function by Cooperating with MITF
- Congenital Heart Disease–Causing Gata4 Mutation Displays Functional Deficits
- Recombination Drives Vertebrate Genome Contraction
- KATNAL1 Regulation of Sertoli Cell Microtubule Dynamics Is Essential for Spermiogenesis and Male Fertility
- Re-Patterning Sleep Architecture in through Gustatory Perception and Nutritional Quality
- Using Whole-Genome Sequence Data to Predict Quantitative Trait Phenotypes in
- Genome-Wide Analysis of GLD-1–Mediated mRNA Regulation Suggests a Role in mRNA Storage
- Meiotic Chromosome Pairing Is Promoted by Telomere-Led Chromosome Movements Independent of Bouquet Formation
- LINT, a Novel dL(3)mbt-Containing Complex, Represses Malignant Brain Tumour Signature Genes
- The H3K27 Demethylase UTX-1 Is Essential for Normal Development, Independent of Its Enzymatic Activity
- Suppresses Senescence Programs and Thereby Accelerates and Maintains Mutant -Induced Lung Tumorigenesis
- Genome-Wide Association of Pericardial Fat Identifies a Unique Locus for Ectopic Fat
- An Essential Role for Katanin p80 and Microtubule Severing in Male Gamete Production
- Identification of Genes That Promote or Antagonize Somatic Homolog Pairing Using a High-Throughput FISH–Based Screen
- Principles of Carbon Catabolite Repression in the Rice Blast Fungus: Tps1, Nmr1-3, and a MATE–Family Pump Regulate Glucose Metabolism during Infection
- Integrin α PAT-2/CDC-42 Signaling Is Required for Muscle-Mediated Clearance of Apoptotic Cells in
- Histone H3 Localizes to the Centromeric DNA in Budding Yeast
- Collapse of Telomere Homeostasis in Hematopoietic Cells Caused by Heterozygous Mutations in Telomerase Genes
- Hypersensitive to Red and Blue 1 and Its Modification by Protein Phosphatase 7 Are Implicated in the Control of Arabidopsis Stomatal Aperture
- Extent, Causes, and Consequences of Small RNA Expression Variation in Human Adipose Tissue
- TBC-8, a Putative RAB-2 GAP, Regulates Dense Core Vesicle Maturation in
- Regulating Repression: Roles for the Sir4 N-Terminus in Linker DNA Protection and Stabilization of Epigenetic States
- Common Genetic Determinants of Intraocular Pressure and Primary Open-Angle Glaucoma
- Prdm5 Regulates Collagen Gene Transcription by Association with RNA Polymerase II in Developing Bone
- Fitness Landscape Transformation through a Single Amino Acid Change in the Rho Terminator
- Repeated, Selection-Driven Genome Reduction of Accessory Genes in Experimental Populations
- Allelic Variation and Differential Expression of the mSIN3A Histone Deacetylase Complex Gene Promote Mammary Tumor Growth and Metastasis
- DNA Demethylation and USF Regulate the Meiosis-Specific Expression of the Mouse
- Knowledge-Driven Analysis Identifies a Gene–Gene Interaction Affecting High-Density Lipoprotein Cholesterol Levels in Multi-Ethnic Populations
- A Duplication CNV That Conveys Traits Reciprocal to Metabolic Syndrome and Protects against Diet-Induced Obesity in Mice and Men
- EMT Inducers Catalyze Malignant Transformation of Mammary Epithelial Cells and Drive Tumorigenesis towards Claudin-Low Tumors in Transgenic Mice
- Inactivation of a Novel FGF23 Regulator, FAM20C, Leads to Hypophosphatemic Rickets in Mice
- Genome-Wide Association for Abdominal Subcutaneous and Visceral Adipose Reveals a Novel Locus for Visceral Fat in Women
- Stratifying Type 2 Diabetes Cases by BMI Identifies Genetic Risk Variants in and Enrichment for Risk Variants in Lean Compared to Obese Cases
- New Insight into the History of Domesticated Apple: Secondary Contribution of the European Wild Apple to the Genome of Cultivated Varieties
- Activated Cdc42 Kinase Has an Anti-Apoptotic Function
- The Region Is Critical for Birth Defects and Electrocardiographic Dysfunctions Observed in a Down Syndrome Mouse Model
- COP9 Signalosome Integrity Plays Major Roles for Hyphal Growth, Conidial Development, and Circadian Function
- Bmps and Id2a Act Upstream of Twist1 To Restrict Ectomesenchyme Potential of the Cranial Neural Crest
- Psip1/Ledgf p52 Binds Methylated Histone H3K36 and Splicing Factors and Contributes to the Regulation of Alternative Splicing
- The Number of X Chromosomes Causes Sex Differences in Adiposity in Mice
- Target Gene Analysis by Microarrays and Chromatin Immunoprecipitation Identifies HEY Proteins as Highly Redundant bHLH Repressors
- Acquisition Order of Ras and p53 Gene Alterations Defines Distinct Adrenocortical Tumor Phenotypes
- ELK1 Uses Different DNA Binding Modes to Regulate Functionally Distinct Classes of Target Genes
- Histone H1 Depletion Impairs Embryonic Stem Cell Differentiation
- IDN2 and Its Paralogs Form a Complex Required for RNA–Directed DNA Methylation
- Separation of DNA Replication from the Assembly of Break-Competent Meiotic Chromosomes
- Genomic Hypomethylation in the Human Germline Associates with Selective Structural Mutability in the Human Genome
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Inactivation of a Novel FGF23 Regulator, FAM20C, Leads to Hypophosphatemic Rickets in Mice
- Genome-Wide Association of Pericardial Fat Identifies a Unique Locus for Ectopic Fat
- Slowing Replication in Preparation for Reduction
- An Essential Role for Katanin p80 and Microtubule Severing in Male Gamete Production
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



