-
Články
- Vzdělávání
- Časopisy
Top články
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Gene Pathways That Delay Reproductive Senescence
Female reproductive senescence is one hallmark of human aging, and as the germline ages, there is increased incidence of chromosome non-dysjunction and DNA damage. Delayed childbearing is a general feature in modern society, resulting in high risk of infertility, miscarriage and birth defects. Thus, understanding the molecular mechanisms regulating reproductive senescence and its association with somatic senescence is increasingly relevant to human health, and will shed light on the prolongation of reproductive longevity and the improvement of post-reproductive health. Here we conducted a genomic screen in Caenorhabditis elegans, searching for genetic regulators of reproductive senescence. We identified 32 gene inactivations that extend reproductive lifespan. Functional characterization of these genes has revealed their interactions with insulin/IGF-1 and TGF-β signaling pathways, their effects in different genders on the regulation of reproductive longevity, and their implications in the control of healthy life expectancy. Many of these genes are conserved between worms and humans. Our studies thus provide new insights into the molecular control of reproductive aging and the mechanistic link between reproductive senescence and organism longevity.
Published in the journal: . PLoS Genet 10(12): e32767. doi:10.1371/journal.pgen.1004752
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004752Summary
Female reproductive senescence is one hallmark of human aging, and as the germline ages, there is increased incidence of chromosome non-dysjunction and DNA damage. Delayed childbearing is a general feature in modern society, resulting in high risk of infertility, miscarriage and birth defects. Thus, understanding the molecular mechanisms regulating reproductive senescence and its association with somatic senescence is increasingly relevant to human health, and will shed light on the prolongation of reproductive longevity and the improvement of post-reproductive health. Here we conducted a genomic screen in Caenorhabditis elegans, searching for genetic regulators of reproductive senescence. We identified 32 gene inactivations that extend reproductive lifespan. Functional characterization of these genes has revealed their interactions with insulin/IGF-1 and TGF-β signaling pathways, their effects in different genders on the regulation of reproductive longevity, and their implications in the control of healthy life expectancy. Many of these genes are conserved between worms and humans. Our studies thus provide new insights into the molecular control of reproductive aging and the mechanistic link between reproductive senescence and organism longevity.
Introduction
Age-associated reproductive decline is one of the first aging phenotypes to manifest in humans. As women age, they experience both a decline in fertility and an increased risk of miscarriage and birth defects [1], [2], and completely cease reproduction (reproductive senesce) after menopause. The mean age of natural menopause is 50–51 years in the western world, with a variation from 40–61 years [3]. Studies of menopausal onset demonstrated an association between mothers and daughters and also between sister pairs, suggesting the involvement of genetic factors in regulating reproductive senescence [4], [5], [6]. The genetic contributions have been estimated to range from 30 to 85% [4], [5], [6]. However, the exact genes and signaling pathways that regulate the onset and progression of reproductive senescence remain largely unknown.
C. elegans has been proven an effective model for studying reproduction and aging processes using various genetic approaches, genome-scale screens and live microscopy imaging. Although evolutionarily distant, C. elegans and humans share many conserved molecular pathways. C. elegans also undergoes reproductive senescence and ceases reproducing progeny after one third of their lifespan [7]. Like the increase in chromosome nondisjunction as human females age, aging C. elegans exhibits increased chromosome nondisjunction during aging [8], suggesting conserved consequences of germline aging. Several endocrine factors were shown to influence the onset of reproductive senescence in C. elegans, including mutations in the daf-2/insulin/IGF-1 receptor gene, and the sma-2/TGF-β receptor linked Smad transcription factor gene [7], [8], [9]. Inactivating these genes significantly increases reproductive lifespan, and improves the quality of oocytes in later age [7], [8], [9]. Importantly, these genetic factors are also implicated in the regulation of mammalian reproductive lifespan and oocyte quality maintenance [10], [11].
Reproductive aging closely interacts with the decline of somatic health. The positive association between late reproduction and longevity has been observed in various invertebrate and vertebrate animals models and in human population studies. In Drosophila melanogaster, long-lived strains can be generated after more than 25 generations of artificial selection of late progeny produced by aged females, which show extended mean lifespan exceeding the maximum lifespan of the original parental strains [12], [13], [14], [15]. In wild chimpanzees, age-associated fertility declines are well correlated with decrease in survival probability [16]. In women, the age at natural menopause is inversely correlated with the rate of all-cause mortality during aging, and females who reproduced their last offspring at advanced age (>50 years) are far more likely to survive to 100 years old [17], [18]. Despite these association studies, the molecular mechanisms underlying the intersection between reproductive aging and somatic aging remain unclear.
Here, we report a comprehensive functional genomic screen to search for genes regulating reproductive senescence in C. elegans, and identified 32 gene inactivations that extend reproductive lifespan. Genetic epistasis analysis revealed interactions of some of these genes with insulin/IGF-1 and TGF-β endocrine signaling pathways. We also characterized their requirement and sufficiency in either hermaphrodites or males to promote reproductive longevity. Further demographic analysis showed that 22 of these gene inactivations reduce the mortality rate at young adulthood with or without increasing total life expectancy. Together these new reproductive aging regulators provide an entry point to further characterize the molecular control of reproductive aging, and reveal important mechanistic insights into the intersection between reproductive senescence and organism longevity.
Results
Genome-wide RNAi screen for clones extending reproductive lifespan
Wild type C. elegans hermaphrodites produce progeny for 7 days at 20°C or for 3 days at 26°C, before they undergo reproductive senescence and cease reproduction. To identify genetic regulators of reproductive aging, we designed a genome-wide RNAi screen to identify those gene inactivations that postpone reproductive senescence in C. elegans hermaphrodites. To facilitate high-throughput screening, we used a temperature-sensitive embryonic lethal mutation in a collagen gene, sqt-3(e2117), to destroy all progeny produced during the normal phase of C. elegans reproduction so that we could observe only those viable progeny produced after normal reproductive senescence. In the RNAi screen (Figure S1A), C. elegans genes were inactivated one gene per well by feeding an Escherichia coli strain expressing an approximately one kilobase C. elegans segment of double stranded RNA to the sqt-3(e2117) mutant animals starting at the first larval stage (L1). The animals were raised at 15°C during larval development, and shifted to the 26°C non-permissive temperature at the fourth larval stage (L4). At this non-permissive temperature, the eggs that are laid during the normal reproductive period are killed by the temperature-sensitive sqt-3 aberrant collagen mutation. After the normally 3-day reproductive period at 26°C, the adults were shifted back to 15°C on the fourth day, and screened for the presence of live progeny in the well three days later. As expected, we detected no live progeny from the control animals that were fed with bacteria expressing no dsRNA or “non-scoring” dsRNAs (Figure S1B). Reduction of daf-2 activity by mutation delays the onset of reproductive senescence and extends healthy reproductive lifespan [7]. As a positive control, we scored about 15 live progeny generated per well by the normally reproductively senescent adults fed with daf-2 RNAi bacteria (Figure S1B). This proves the principle of the genomic screening strategy to systemically explore the regulatory mechanisms of reproductive senescence. By screening 18,413 such dsRNAs, we could survey 94% of C. elegans genome for gene inactivations that like daf-2 gene inactivation delay reproductive senescence.
Out of 18,413 gene inactivations screened, 58 candidates were identified that extend reproductive longevity of sqt-3 mutant animals (Figure S1C and Table S1). These gene inactivations were further examined in wild type and in an RNAi hypersensitive strain, nre-1(hd20)lin-15b(hd126) where RNAi potency is enhanced, especially in neurons [19]. We found that 32 gene inactivations prolong reproductive lifespan by more than 25% in the nre-1(hd20)lin-15b(hd126) background (Figure 1A and Table S2), and increase late reproduction of progeny after the first 3 days of adulthood (Figure S2). Twenty-six of the gene inactivations extend reproductive lifespan nearly equivalently in the enhanced neuronal RNAi background and in wild type; the 6 genes that show phenotypes only in the enhanced neuronal RNAi strain background may act in neurons because wild type C. elegans generally does not silence neuronal gene functions by RNAi (Figure 1B and Table S3).
Fig. 1. Gene inactivations extending reproductive lifespan. 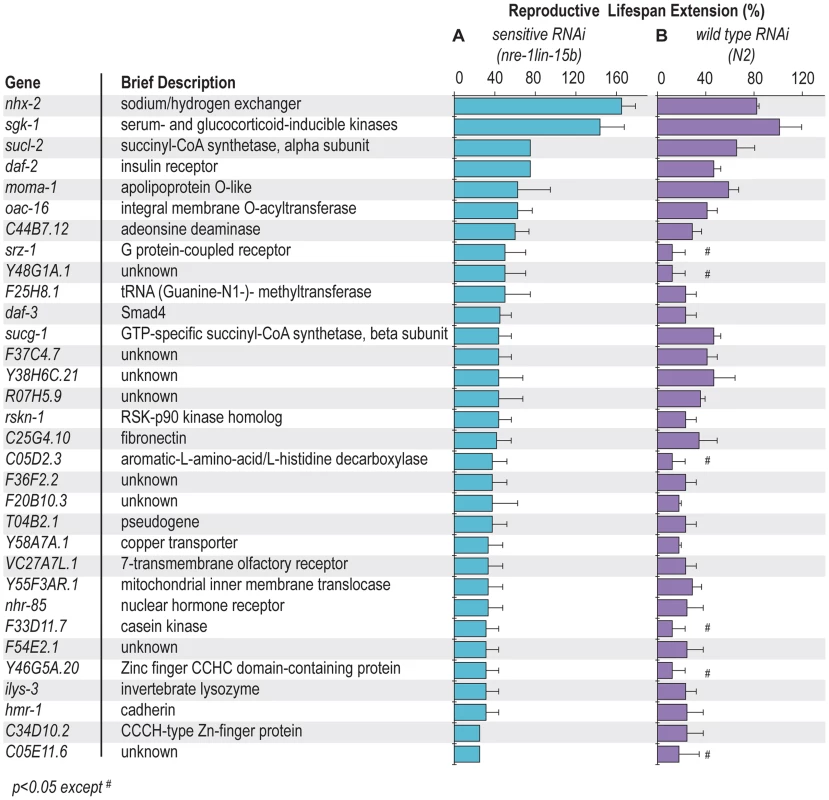
32 gene inactivations extend reproductive lifespan more than 25% in the RNAi hypersensitive strain, nre-1(hd20)lin-15b(hd126) (A). 26 of those gene inactivations also significantly increase reproductive lifespan of wild type (N2) (B). The other six gene inactivations promote reproductive longevity only in the nre-1(hd20)lin-15b(hd126) strains. They may act in neurons or their RNAi inactivations are only effective in the RNAi hypersensitive background. The average of three independent experiments is shown, p<0.05 except #. One manner by which gene inactivations can appear to increase reproductive longevity is if that gene inactivation slows down the developmental process. That is, if development of the animal or its germline during larval stages is progressing at a decreased rate, the onset of reproduction as well as its cessation may be delayed. On the other hand, if time to initial reproduction is normal, but the cessation of reproduction is delayed, the gene is more likely to regulate reproductive senescence. To distinguish these two possibilities, we examined the time needed to develop from the L1 stage to the onset of reproduction in adulthood for each of the gene inactivations. Nearly all of the reproductive senescence candidate gene inactivations have a normal developmental rate (Figure S3), suggesting that metabolic rate or other gross features of developmental control are not affected by these gene inactivations. Three gene inactivations prolong the developmental time to adulthood, but to a much lesser extent than their effects on the reproductive lifespan extension (Figure S3). Therefore, most of the identified genes indeed affect the program of reproductive senescence, and their effects on reproductive longevity are not simply the result of a developmental delay.
Genetic classification of genetic regulators of reproductive longevity
Functional annotation analysis classified these newly identified genes into different categories, such as signaling transduction, gene expression/translation control, metabolic maintenance, ion transport and innate immunity defense (Figure 2A). To further understand the regulatory mechanisms of these genes, we examined their interactions with the known reproductive longevity pathways regulated by insulin/IGF-1 and TGF-β signaling [7], [8], [9]. Mutations in the insulin/IGF-1 receptor, daf-2(e1370), cause increased reproductive lifespan, which is fully suppressed by a mutation in the negatively regulated FoxO transcription factor, daf-16(mgDf47) [7]. To analyze the functions of the identified genes in insulin/IGF-1 signaling, we inactivated the newly identified reproductive senescence genes in the sqt-3(e2117) strains carrying either daf-2(e1370) or daf-16(mgDf47) mutation, performed the similar temperature switching procedure as shown above, and examined whether reproductive senescence could be delayed. As shown in Figure 2B, ten gene inactivations delay reproductive senescence in the background with either daf-2 or daf-16 mutation, suggesting that these genes regulate reproductive senescence independently from insulin/IGF-1 signaling. Among the other 22 genes, nine of them act independently of daf-16, but their inactivations have no additive effects with the daf-2 mutation; the effects of four genes are dependent on daf-16, but are additive to daf-2; and the other nine gene inactivations fail to delay reproductive senescence in either mutant background. These 22 genes are likely acting in insulin/IGF-1 signaling, but at different positions in the pathway.
Fig. 2. Functional classification of reproductive longevity regulatory genes. 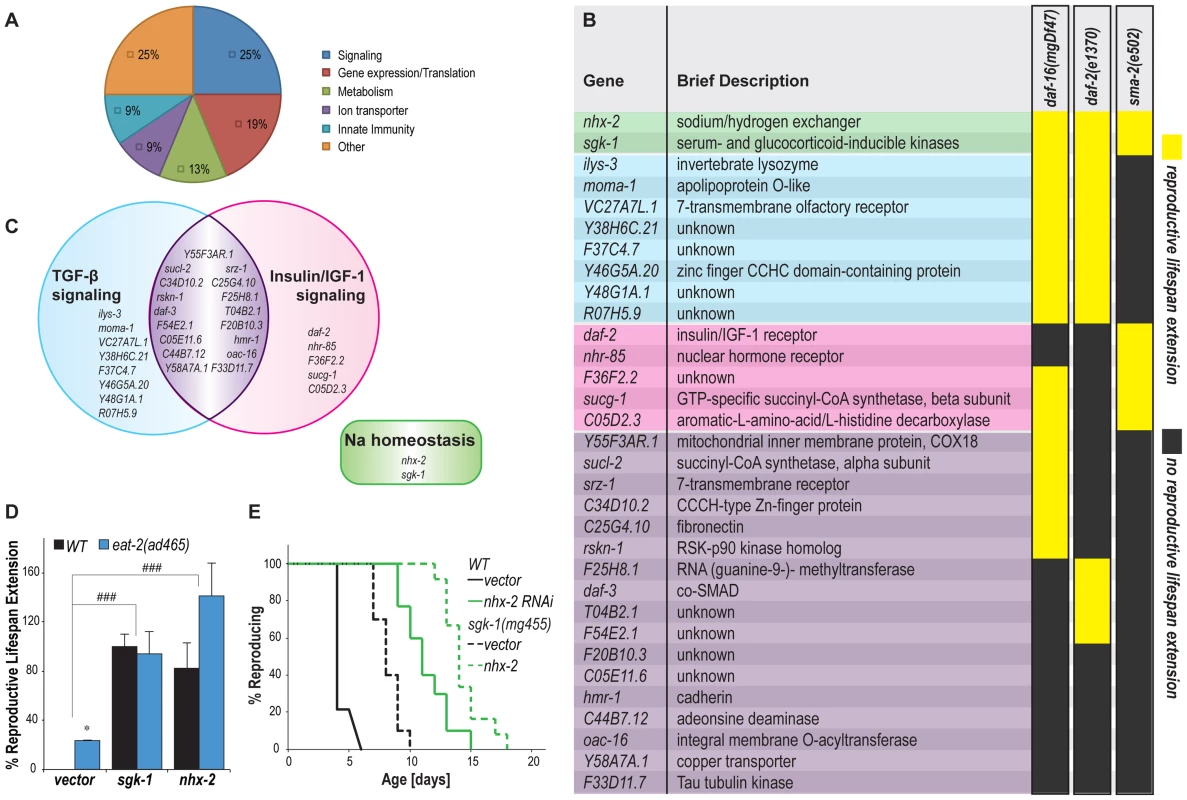
(A) Identified reproductive longevity regulatory genes are placed into different groups based on their functional annotation using DAVID bioinformatics analysis. (B) The genetic interaction between the identified genes and insulin/IGF-1 and TGF-β signaling pathways. The identified genes were inactivated in daf-2(e1370);sqt-3(e2117), daf-16(mgDf47);sqt-3(e2117) or sma-2(e502);sqt-3(e2117) mutants and examined for their effects on the onset of reproductive senescence. Yellow highlights gene inactivations that delay reproductive senescence in the mutants. Dark gray shows gene inactivations that fail to affect reproductive senescence of the mutants. All the experiments were performed at least twice independently. (C) nhx-2 and sgk-1, two regulators of sodium reabsorption, modulate reproductive senescence independently of either insulin/IGF-1 or TGF-β signaling. 17 of the identified genes interact with both pathways. Eight and five genes function specifically in the TGF-β and the insulin/IGF-1 signaling pathway, respectively. (D) nhx-2 and sgk-1 regulate reproductive lifespan additively with caloric restriction. sgk-1 and nhx-2 were inactivated in the eat-2(ad465) mutant, a genetic model of caloric restriction in C. elegans. Compared to wild type (N2), the eat-2(ad465) mutant reproduces 24% longer, * p<0.05. RNAi inactivation of either sgk-1 or nhx-2 further enhances the reproductive lifespan extension in the eat-2(ad465) mutant, ### p<0.001. The average of three independent experiments is shown. (E) Inactivation of nhx-2 and sgk-1 are additive on reproductive lifespan extension. The sgk-1(mg455) null mutation and the nhx-2 RNAi inactivation prolong reproductive lifespan by 85% and 154%, respectively. The nhx-2 RNAi inactivation further extends the reproductive lifespan of the sgk-1 mutant by 75%. p<0.0001 in all cases. TGF-β signaling also regulates C. elegans reproductive aging; mutations in sma-2(e502), which encodes the TGF-β-receptor-regulated Smad transcription factor, increase reproductive lifespan more than two-fold [8], [9]. We found that five of the identified gene inactivations interacting with insulin/IGF-1 signaling can further extend the reproductive longevity of the sma-2 mutant, as is also true for the daf-2 gene inactivation (Figure 2B and 2C, highlighted with pink). On the other hand, eight other gene inactivations delay reproductive senescence of the daf-2 and daf-16 mutants, but fail to do so in the sma-2 mutant (Figure 2B and 2C, highlighted with blue). These data suggest that insulin/IGF-1 and TGF-β signaling modulate the process of reproductive aging in parallel, which is consistent with previous findings [8]. However, our results also show that these two pathways converge on a large group of common downstream targets, considering 17 of the candidate genes interact with both pathways (Figure 2B and 2C, highlighted with purple).
The two most potent effectors, nhx-2 and sgk-1, however act independently of either pathway (Figure 2B and 2C, highlighted with green). In addition to insulin/IGF-1 and TGF-β signaling, caloric restriction using eat-2 mutants also prolongs reproductive lifespan in C. elegans [7]. We found that RNAi inactivation of either nhx-2 or sgk-1 further enhances reproductive lifespan extension in the eat-2(ad465) mutant (Figure 2D). Because the eat-2 mutation causes caloric restriction via a disability in the actual ingestion of bacteria, it is unclear if these enhancing gene inactivations act via a regulatory mechanism on the caloric restriction pathway or in parallel to that pathway. An sgk-1(mg455) null mutation and nhx-2 RNAi inactivation exert additive effects on reproductive lifespan extension (Figure 2E), suggesting their parallel functions in regulating reproductive aging. The mammalian homologs of these two genes are the sodium/hydrogen exchanger and the serum-and glucocorticoid-inducible kinase respectively, which both regulate sodium reabsorption [20], [21]. Our results suggest that sodium homeostasis may play a crucial role in the regulation of reproductive senescence.
Somatic regulation of reproductive longevity
Reproductive activities are under the control of complex interaction between germline and somatic tissues. To test whether the candidate genes function in the germline or in somatic tissues to regulate reproductive longevity, we inactivated the 32 reproductive senescence genes in rrf-1(pk1417) mutant animals, for which RNAi is predominantly effective only in the germline with a minor effect in the intestine [22], [23]. We found that only 10 gene inactivations prolong the reproductive lifespan of the rrf-1 mutant animals (Figure 3 and Table sS4). Among them, the effect of nhx-2/sodium/hydrogen exchanger, moma-1/apolipoprotein O homologue or daf-2/insulin-like receptor in the rrf-1 background is much weaker than that in wild type (Figure 3), suggesting that their functions in somatic tissues are also involved in the regulation of reproductive longevity. Moreover, the other 22 gene inactivations failed to increase reproductive lifespan in the rrf-1 mutant; those genes likely regulate reproductive longevity systemically via their effects in somatic tissues. Together these studies indicate the significance of somatic functions in the regulation of reproductive senescence.
Fig. 3. Germline genetic inactivations that prolong reproductive lifespan. 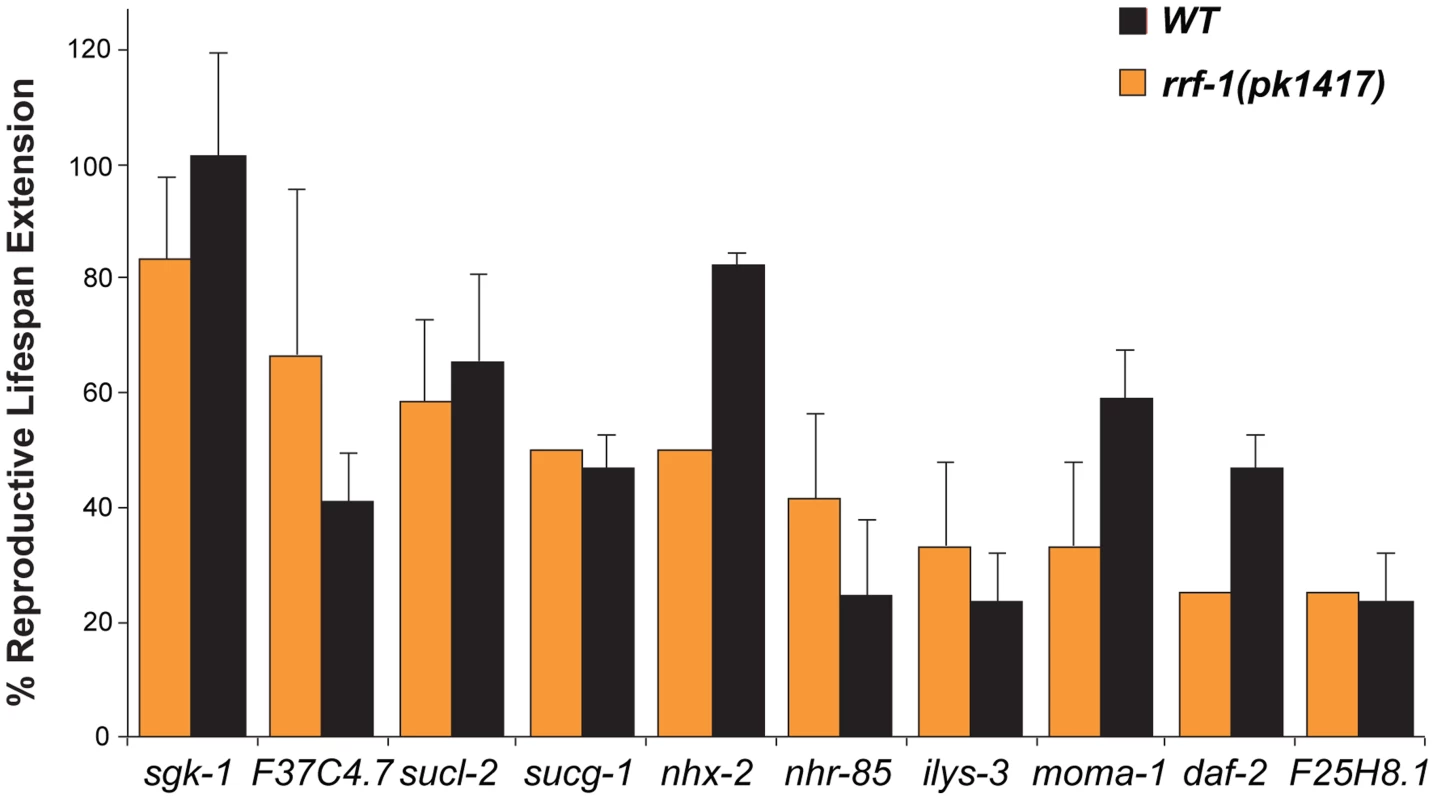
In the rrf-1(pk1417) mutant, RNAi predominantly operates in the germline. Ten of the identified genes increase reproductive lifespan when inactivated in the rrf-1 mutants. The extension levels are comparable to that in wild type (N2), except for daf-2, nhx-2 and moma-1. The average of three independent experiments is shown, p<0.05. Reproductive lifespan extending effects on mated animals
Self-fertilizing C. elegans hermaphrodites generate a limited number of sperm by virtue of a pulse of spermatogenesis before a longer run of oogenesis, which constrains their reproductive capacities. However when mated to males which produce far more sperm over a longer period, hermaphrodites use male sperm preferentially, produce double the number of progeny, and reproduce for a much longer time period [7]. To test whether the newly identified genes exert effects on the reproductive longevity of mated animals, we first inactivated these genes in hermaphrodites by RNAi and then crossed those animals with males feeding with control bacteria expressing no dsRNAs. Under this condition, sperm from the males are not affected by RNAi inactivation. We found that 19 genes when inactivated only in mated hermaphrodites are sufficient to promote reproductive longevity (Figure 4A and Table S5), suggesting that these gene inactivations predominantly delay age-associated oocyte senescence.
Fig. 4. Reproductive longevity extending effects in mated animals. 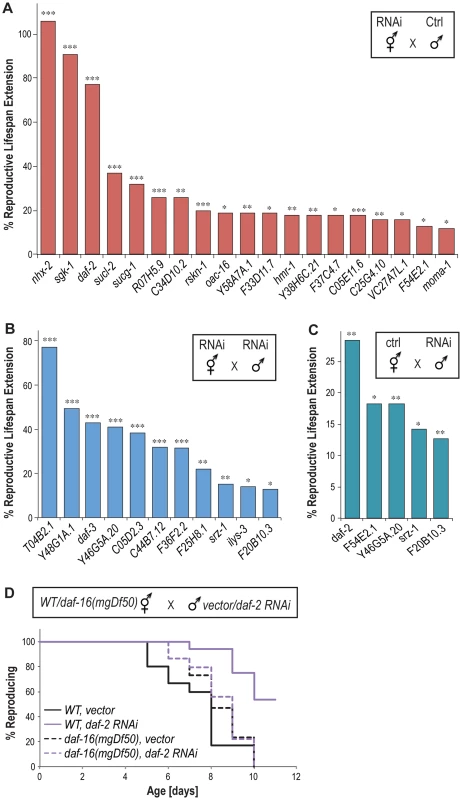
(A) 19 gene inactivations only in hermaphrodites but not males extend the reproductive lifespan of mated hermaphrodites. (B) 11 genes extend reproductive lifespan when inactivated in both hermaphrodites and males. (C) Five gene inactivations in males alone are sufficient to increase the reproductive lifespan of mated hermaphrodites. * p<0.05, ** p<0.01, ***p<0.005. (D) daf-2 RNAi inactivation only in males prolongs reproductive lifespan of mated wild type hermaphrodites by 37% (p<0.001), but fails to do so in the mated daf-16(mgDf50) mutant hermaphrodites (p = 0.79). For the other 13 candidate genes, their inactivations in hermaphrodites alone are not sufficient to enhance reproductive longevity after mating. However, when they are also inactivated in males, 11 of them increase the reproductive lifespan of the mated hermaphrodites (Figure 4B and Table S5), suggesting the requirement of their functions in males to modulate the process of reproductive senescence. To test whether these 11 genes affect sperm production, we examined the effects of their inactivations on brood size in self-fertilizing hermaphrodites where the total number of progeny is determined by sperm quantity. We found that none of these gene inactivations increase total brood size (Figure S4). Therefore, the effect of these genes on reproductive lifespan extension is not simply a result of increased sperm quantity.
We also found five genes whose inactivation only in males are sufficient to prolong the reproductive lifespan of hermaphrodites after mating, including daf-2, F54E2.1, Y46G5A.20, srz-1 and F20B10.3 (Figure 4C). Inactivation of srz-1 and F20B10.3 in males alone prolongs reproductive lifespan by 14% and 13%, respectively (Figure 4C), which are comparable to the 15% and 13% extension caused by their inactivation in both hermaphrodites and males (Figure 4B). On the other hand, male-only inactivation of Y46G5A.20 results in 18% reproductive lifespan extension (Figure 4C), which is significantly reduced compared to the 42% extension when Y46G5A.20 is inactivated in both hermaphrodites and males (Figure 4B). For daf-2 and F54E2.1, their inactivation in either hermaphrodites or males alone is sufficient to prolong reproductive lifespan (Figure 4A and 4C). Interestingly, when mated with the daf-16(mgDf50) mutant hermaphrodites, the male-only daf-2 inactivation failed to prolong reproductive lifespan (Figure 4D). Together, these studies suggest that the effects of the gene inactivations on reproductive longevity occur not only in self-fertilizing hermaphrodites, but also in mated animals. Furthermore, sperm and seminal fluid transferred by mating may induce a humoral response that regulates the process of reproductive senescence in hermaphrodites systemically, and insulin/IGF-1 signaling is involved in this humoral response.
Effects of reproductive aging regulators on somatic aging-related parameters
To characterize the interaction between reproductive aging and somatic aging, we inactivated the 32 genes in the nre-1(hd20)lin-15b(hd126) RNAi hypersensitive strain, and examined their effects on organismal lifespan and on age-specific patterns of mortality. In the fitted mortality rate curve under the Gompertz-Makeham model (Figure 5A), the slope of the curve defines the demographic rate of aging (RoA) showing the rate of increase in mortality with age; whereas the intercept with the y-axis defines initial mortality rate (IMR) or “frailty” that represents the mortality rate at the defined time zero, in this case young adulthood and is related to the baseline mortality [24].
Fig. 5. Reproductive longevity regulatory genes modulate somatic longevity. 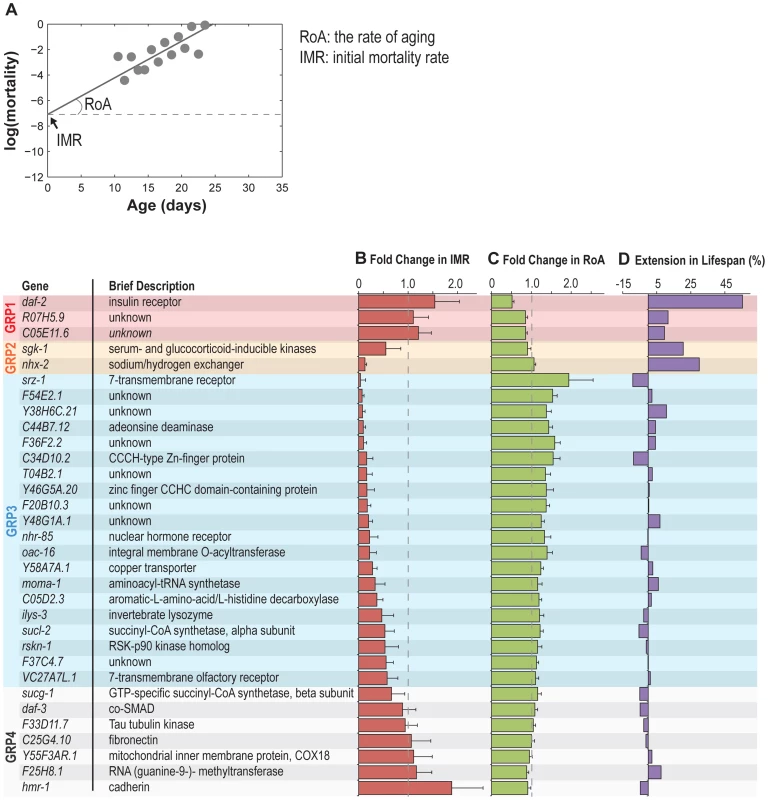
(A) The mortality rate curve of the control animals fed with bacteria expressing no dsRNA. The slope of the curve defines the demographic rate of aging (RoA), and the intercept with the y-axis shows initial mortality rate (IMR). (B–D) The effects of the reproductive senescence genes on somatic aging-related parameters, including IMR (B), RoA (C), and mean lifespan (D). Three gene inactivations including daf-2, R07H5.9 and C05E11.6 (Group 1), reduce RoA without affecting IMR and increase mean lifespan significantly. Inactivation of sgk-1 or nhx-2 (Group 2), two regulators of sodium reabsorption, reduces IMR with no effect on RoA and extends mean lifespan. There are also 20 other gene inactivations (Group 3) that reduce IMR, but increase RoA and do not alter mean lifespan. Six gene inactivations have no effect on somatic longevity (Group 4). For IMR and RoA, the average with standard deviation of three independent experiments is shown; for mean lifespan extension, three independent experiments were combined for analysis. We found that the majority (25) of the identified reproductive senescence genes affect somatic mortality trajectories (Figure 5B–D). Based on their effects on different aging-related parameters, these genes can be classified into three groups. First, gene inactivations of three genes, including daf-2, R07H5.9 and C05E11.6 decrease RoA without affecting IMR (frailty) (Figure 5B–D, Figure 6A–C). As a result, the median and maximum lifespan (defined here as age at 1% estimated survival) are both significantly increased upon their inactivations (Figure 6A–C). Secondly, gene inactivation of the two sodium homeostasis regulatory genes nhx-2 and sgk-1, reduce IMR but not RoA (Figure 5B–D, Figure 6D and 6E). Their inactivations also lead to median and maximum lifespan extension (Figure 6D and 6E), which is consistent with previous findings [25], [26]. The third group includes 20 gene inactivations that decrease IMR, but increase RoA (Figure 5B–D). As a result, these genes have little effect on median lifespan and cause no change in maximum lifespan (Figure 5B–D, one example shown in Figure 6F). Together, these results suggest that most of the gene inactivations that delay reproductive senescence decrease the baseline probability of death and consequently postpone the age-associated rise in mortality.
Fig. 6. Gene inactivations that promote somatic longevity. 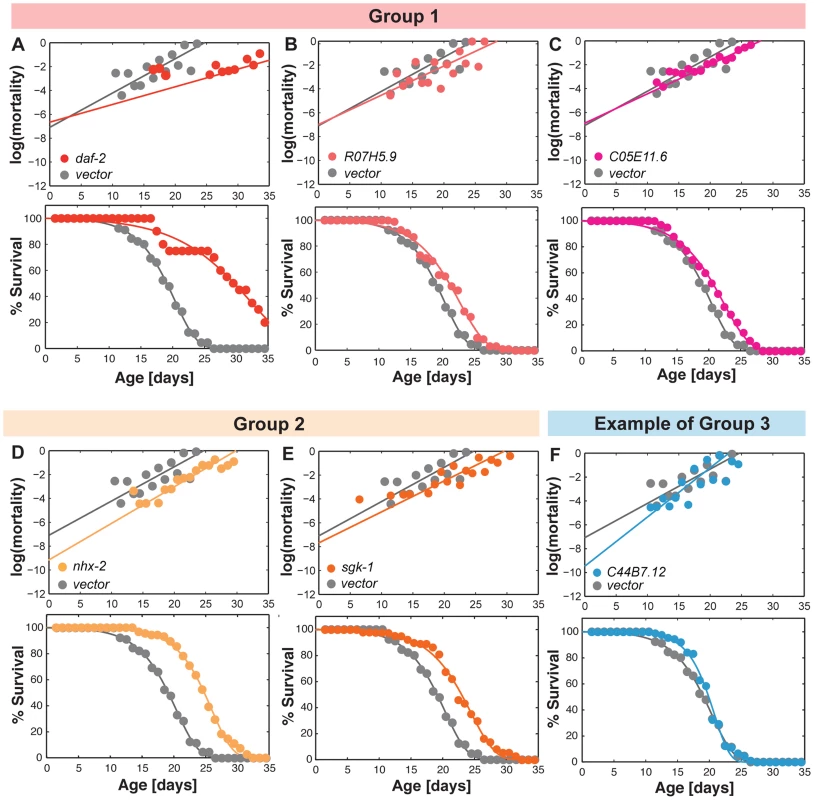
(A–C) RNAi inactivation of daf-2, R07H5.9 and C05E11.6 decrease RoA by 48%, 15% and 14%, respectively, without a significant effect on IMR. The mean lifespan is significantly increased by 55%, 11% and 10%, respectively (p<0.001). (D and E) Inactivation of nhx-2 and sgk-1 reduce IMR by 87% and 45%, respectively, but does not significantly affect RoA. The mean lifespan is increased by 30% and 21%, respectively (p<0.001). (F) C44B7.12 inactivation reduces IMR by 91%, but increases RoA by 44%. The mean lifespan is not significantly affected. This is one example to represent 20 genes in the Group 3. Discussion
Our genome scale screen for gene inactivations that delay reproductive senescence identified 32 genes that normally function to mediate reproductive senescence. Characterization of synergies and epistasis of the reproductive lifespan extension induced by these gene inactivations in strains also carrying mutations in insulin/IGF-1 and TGF-β signaling pathways places them into distinct classes. Insulin/IGF-1 and TGF-β signaling are two independent mechanisms to modulate reproductive senescence in C. elegans, which are also implicated in regulating mammalian reproductive span and oocyte quality maintenance [10], [11]. Most of our newly identified genes interact with either or both of these two signaling pathways.
sgk-1 and nhx-2 are the two most potent effectors identified from the screen and regulate reproductive senescence through a mechanism independent of insulin/IGF-1 signaling, TGF-β signaling or caloric restriction. The mammalian homologues of sgk-1, the serum and glucocorticoid activated kinase and nhx-2, a sodium/hydrogen exchanger both modulate sodium reabsorption [20], [21]. Thus our data suggest an involvement of sodium homeostasis in the regulation of reproductive senescence in C. elegans. Up-regulation of mammalian SGK-1 has been implicated in reproductive failure in women [27]. Therefore, although worms and humans have very distinct reproductive strategies, they may share some common genetic regulatory factors. Twenty-one of the newly identified C. elegans genes from the screens are well conserved in human (Table S6), and may provide new insights into the age-associated reproductive senescence in human.
Reproductive success requires proper functions of both oocyte and sperm, and their coordination. In several species including C. elegans, sperm can signal to oocyte and to the somatic gonad, and sperm-derived signals are responsible for promoting both oocyte maturation and ovulation [28]. Males can also influence hermaphrodite lifespan in C. elegans [29], [30], through secreting diffusible compounds [29]. We found 19 gene inactivations in hermaphrodites only that can promote reproductive longevity. In these cases, mated hermaphrodites use the sperm from the control males not undergoing gene inactivation. Thus the extension of reproductive lifespan under these conditions is caused by the gene inactivations that regulate oocyte activities directly or indirectly. There are 11 genes that require inactivation in both hermaphrodites and males to prolong reproductive lifespan, suggesting the significance of sperm and/or seminal fluid in regulating reproductive aging. Among those 11 genes, only ilys-3 and F25H8.1 are sufficient to prolong reproductive lifespan when inactivated in the rrf-1 mutants, which may exert cell-autonomous effects on the activity of sperm and/or seminal fluid. However, the other nine genes are likely to function in somatic tissues to affect sperm and seminal fluid activity cell-nonautonomously. On the other hand, we also found that five gene inactivations in the male alone, including daf-2, F54E2.1, Y46G5A.20, srz-1 and F20B10.3, are sufficient to prolong the reproductive lifespan of hermaphrodites carrying non-gene inactivated oocyte. These data suggest that sperm produce humoral signals to actively modulate the process of reproductive senescence, and these five genes are likely involved in the sperm humoral signaling. These humoral signals are likely to be secreted steroids and/or peptides; while it is also possible that the sperm or seminal fluid from the males undergoing RNAi treatment may bring gene-inactivating siRNAs into the hermaphrodite. We expect that future characterization of the regulatory mechanisms of those genes will provide insights into the humoral communication between males and females.
Reproductive success is the currency of evolution. To achieve this goal, somatic physiology must be well maintained to support reproductive activities. Thus it is expected that the onset of reproductive senescence would affect somatic maintenance and organism aging. Our data support this, and further reveal that delaying reproductive senescence may have predominant effects on healthy lifespan rather than total lifespan. In our demographic analysis, 3 gene inactivations reduce the mortality rate increase with age (RoA), while 22 gene inactivations decrease the baseline mortality (IMR). IMR measures the initial vulnerability to physiological damage, and its decrease is related to a delay in the onset of chronological aging, or an increase in the initial quality of the organism [24], [31]. Thus, these 22 gene inactivations likely increase healthy life expectancy without necessarily slowing the aging process. Further characterization of these genes may help us find new ways to improve healthy life expectancy, and understand the crucial link between late reproduction and organism longevity during evolution.
Materials and Methods
Strains
N2 Bristol was used as the wild-type strain. Other C. elegans mutant alleles: sqt-3(e2117)V, nre-1(hd20)lin-15b(hd126)X, daf-2(e1370)III, daf-16(mgDf47)I, sma-2(e502)III, and rrf-1(pk1417)I, eat-2(ad465)II, daf-16(mgDf50)I.
Genome-wide RNAi reproductive longevity screens
We carried out a large-scale RNAi screen using the sqt-3(e2117) strain. RNAi bacteria were cultured 12 h in LB with 50 µg/ml ampicillin and seeded onto 24-well RNAi agar plates containing 5 mM isopropylthiogalactoside (IPTG). The plates were allowed to dry in a laminar flow hood and incubated at room temperature overnight to induce dsRNA expression. Approximately 20∼30 synchronized L1 larvae were placed onto agar plate wells where each E. coli strain expressing a distinct C. elegans dsRNA corresponding to one gene, and the worms were allowed to develop at 15°C till L4 and shifted to 26°C. After 4 days, the worms were shifted back to 15°C and scored for progeny production after 2∼3 more days of incubation. Worms feeding on bacteria carrying the empty vector (L4440) were used as control. We routinely screened ∼2000–3000 RNAi clones in one experiment. RNAi wells in which live progeny were observed were scored as positives. These “positive” RNAi clones were retested at least three more times using similar high-throughput screening strategy described above. RNAi clones that were scored as positive in all the rescreening tests were deemed “primary positives”. Primary positives were retested three times in RNAi self-fertilizing reproductive lifespan assays (see below) using the nre-1(hd20)lin-15b(hd126) or wild type N2 strain. The RNAi clones that were scored positive three times in the reproductive lifespan assays define the final list of enhanced reproductive longevity gene inactivations.
Genetic interaction analysis with daf-2, daf-16 and sma-2
daf-2(e1370);sqt-3(e2117), daf-16(mgDf47));sqt-3(e2117) and sma-2(e502);sqt-3(e2117) double mutants were generated. Approximately 20∼30 synchronized L1 larvae were placed onto agar plate wells where each E. coli strain expressing a distinct C. elegans dsRNA corresponding to one gene, and the worms were allowed to develop at 15°C till L4 and shifted to 26°C. For daf-2(e1370);sqt-3(e2117), the worms were shifted back to 15°C after 9 days and scored for progeny production after 2∼3 more days of incubation. For daf-16(mgDf47));sqt-3(e2117) and sma-2(e502);sqt-3(e2117), the worms were kept at 26°C for 4 days and 9 days, respectively. Worms feeding on bacteria carrying the empty vector (L4440) were used as control, in which no progeny was detected after temperature shifting back to 15°C.
RNAi self-fertilizing reproductive lifespan assay
RNAi bacteria were prepared as described above in 60 mm RNAi agar plates. Approximately 20∼30 synchronized L1 larvae were placed onto the RNAi containing plates and allowed to develop at 20°C until the L4 stage. For each genotype and RNAi treatment, three plates were prepared. At the L4 stage, 10 larvae from each plate were hand - picked and transferred to a fresh RNAi agar plate containing the same RNAi bacteria. The animals then were kept at 20°C and were transferred to fresh RNAi plates every day. After transferring, the old plates were kept at 20°C and scored for the number of progeny two days later. Reproductive lifespan is defined as the first day of reproduction (reproductive lifespan = 1) to when no progeny were scored. The reproductive lifespan of RNAi-treatment groups are compared with that of the control groups (bacteria carrying the empty vector) using a student T-test.
RNAi mating reproductive lifespan assay
RNAi bacteria were prepared as described above in 35 mm RNAi agar plates. Synchronized L1 larvae (nre-1(hd20)lin-15b(hd126) or daf-16(mgDf50)) were placed onto the RNAi containing plates and allowed to develop at 20°C. Next, L4 hermaphrodites were mated to young males (nre-1(hd20)lin-15b(hd126) at a 1∶2 ratio for 48 hours before being transferred to individual RNAi containing plates. Successful mating was determined by the production of male progeny each day. The individual animal was transferred to fresh plates daily until no progeny scored for at least two days. For each individual, the last day of live progeny production was recorded as the day of reproduction cessation. For each experiment, at least 10 individual hermaphrodites were included. Statistical analyses were performed using SPSS software (http://www-01.ibm.com/software/analytics/spss/). Each RNAi-treatment population is compared with that of the population treated with control RNAi (bacteria carrying the empty vector) using a log rank test.
RNAi lifespan assay
RNAi bacteria were prepared as described above in 60 mm RNAi agar plates. Approximately 30∼40 synchronized L1 larvae (nre-1(hd20)lin-15b(hd126)) were placed onto RNAi-containing agar plates, allowed to develop at 20°C. The animals then were kept at 20°C and transferred to fresh RNAi plates every two days or every seven days during or past the reproductive period respectively. For each RNAi treatment, 3 plates (around 100 worms) were prepared and scored every day by gentle prodding with a platinum wire to test for viability. Lifespan is defined as the first day of adulthood (adult lifespan = 1) to when they were scored as dead. The same experiment was performed three times independently.
Demographic analysis
We generated lifetables from Nx, dx, and cx, the number entering, dying in, or censored in each age interval, respectively. Probability of death (qx) was estimated as qx = dx/Nx, and force of mortality as mx = −ln(1−qx). The Gompertz-Makeham model for age-specific mortality M(x) = M0⋅exp(G⋅x)+M∞, with initial mortality M0, rate of aging G, age x, and age-independent mortality M∞ can be shown to give the survival proportion S(x) = exp[(M0/G)(1−exp(G⋅x)−M∞⋅x]. We performed non-linear-least-squares fits to S(x) using a Trust-Region Reflective Newton algorithm implemented by the MATLAB fit() function (The MathWorks, Natick, MA). Kaplan-Meier estimates of the cumulative distribution (survival) function were computing using the MATLAB (The Mathworks, Natick, MA) function ecdf(). We also performed a log-rank test to compare each RNAi gene inactivation to it's associated vector control within the same experiment, using a Bonferroni step-down multiple testing p-value adjustment.
Progeny production analysis
10 synchronized L4 hermaphrodite larvae (nre-1(hd20)lin-15b(hd126)) were transferred to fresh RNAi plates every day and the number of progeny was counted daily until reproduction cessation. The total number of progeny per hermaphrodite was calculated. The experiments were performed three times at 20°C. The RNAi-treatment groups are compared with the control groups (bacteria carrying the empty vector) using a student T-test.
Developmental time measurement
10 synchronized L1 larvae (nre-1(hd20)lin-15b(hd126)) were allowed to develop at 20°C. After becoming L4, the animals were examined every half hour to note the transition to adulthood (oocytes in the germline). If any, the adult individual would be removed from the population. The time from L1 to adulthood when the animal was removed was recorded for each individual. The RNAi-treatment groups are compared with the control groups (bacteria carrying the empty vector) using a student T-test.
Supporting Information
Zdroje
1. BroekmansFJ, KnauffEA, te VeldeER, MacklonNS, FauserBC (2007) Female reproductive ageing: current knowledge and future trends. Trends Endocrinol Metab 18 : 58–65.
2. te VeldeER, PearsonPL (2002) The variability of female reproductive ageing. Hum Reprod Update 8 : 141–154.
3. TreloarAE (1981) Menstrual cyclicity and the pre-menopause. Maturitas 3 : 249–264.
4. de BruinJP, BovenhuisH, van NoordPA, PearsonPL, van ArendonkJA, et al. (2001) The role of genetic factors in age at natural menopause. Hum Reprod 16 : 2014–2018.
5. MurabitoJM, YangQ, FoxC, WilsonPW, CupplesLA (2005) Heritability of age at natural menopause in the Framingham Heart Study. J Clin Endocrinol Metab 90 : 3427–3430.
6. van AsseltKM, KokHS, PearsonPL, DubasJS, PeetersPH, et al. (2004) Heritability of menopausal age in mothers and daughters. Fertil Steril 82 : 1348–1351.
7. HughesSE, EvasonK, XiongC, KornfeldK (2007) Genetic and pharmacological factors that influence reproductive aging in nematodes. PLoS Genet 3: e25.
8. LuoS, KleemannGA, AshrafJM, ShawWM, MurphyCT (2010) TGF-beta and insulin signaling regulate reproductive aging via oocyte and germline quality maintenance. Cell 143 : 299–312.
9. LuoS, ShawWM, AshrafJ, MurphyCT (2009) TGF-beta Sma/Mab signaling mutations uncouple reproductive aging from somatic aging. PLoS Genet 5: e1000789.
10. HamataniT, FalcoG, CarterMG, AkutsuH, StaggCA, et al. (2004) Age-associated alteration of gene expression patterns in mouse oocytes. Hum Mol Genet 13 : 2263–2278.
11. PelosiE, OmariS, MichelM, DingJ, AmanoT, et al. (2013) Constitutively active Foxo3 in oocytes preserves ovarian reserve in mice. Nat Commun 4 : 1843.
12. PartridgeL, ProwseN, PignatelliP (1999) Another set of responses and correlated responses to selection on age at reproduction in Drosophila melanogaster. Proc Biol Sci 266 : 255–261.
13. RoseMR (1984) Laboratory evolution of postponed senescence in Drosophila melanogaster. Evolution 38 : 1004–1010.
14. LuckinbillLS, ArkingR, ClareMJ, CiroccoWC, BuckSA (1984) Selection for delayed senescence in Drosophila melanogaster. Evolution 38 : 996–1003.
15. PartridgeL, FowlerK (1992) Direct and correlated response to selection on age at reproduction in Drosophila melanogaster. Evolution 46 : 76–91.
16. Emery ThompsonM, JonesJH, PuseyAE, Brewer-MarsdenS, GoodallJ, et al. (2007) Aging and fertility patterns in wild chimpanzees provide insights into the evolution of menopause. Curr Biol 17 : 2150–2156.
17. JacobsenBK, HeuchI, KvaleG (2003) Age at natural menopause and all-cause mortality: a 37-year follow-up of 19,731 Norwegian women. Am J Epidemiol 157 : 923–929.
18. PerlsTT, AlpertL, FrettsRC (1997) Middle-aged mothers live longer. Nature 389 : 133.
19. SchmitzC, KingeP, HutterH (2007) Axon guidance genes identified in a large-scale RNAi screen using the RNAi-hypersensitive Caenorhabditis elegans strain nre-1(hd20) lin-15b(hd126). Proc Natl Acad Sci U S A 104 : 834–839.
20. LoffingJ, FloresSY, StaubO (2006) Sgk kinases and their role in epithelial transport. Annu Rev Physiol 68 : 461–490.
21. OrlowskiJ, GrinsteinS (1997) Na+/H+ exchangers of mammalian cells. J Biol Chem 272 : 22373–22376.
22. SmardonA, SpoerkeJM, StaceySC, KleinME, MackinN, et al. (2000) EGO-1 is related to RNA-directed RNA polymerase and functions in germ-line development and RNA interference in C. elegans. Curr Biol 10 : 169–178.
23. KumstaC, HansenM (2012) C. elegans rrf-1 mutations maintain RNAi efficiency in the soma in addition to the germline. PLoS One 7: e35428.
24. PletcherSD, KhazaeliAA, CurtsingerJW (2000) Why do life spans differ? Partitioning mean longevity differences in terms of age-specific mortality parameters. J Gerontol A Biol Sci Med Sci 55: B381–389.
25. HertweckM, GobelC, BaumeisterR (2004) C. elegans SGK-1 is the critical component in the Akt/PKB kinase complex to control stress response and life span. Dev Cell 6 : 577–588.
26. NehrkeK (2003) A reduction in intestinal cell pHi due to loss of the Caenorhabditis elegans Na+/H+ exchanger NHX-2 increases life span. J Biol Chem 278 : 44657–44666.
27. SalkerMS, ChristianM, SteelJH, NautiyalJ, LaveryS, et al. (2011) Deregulation of the serum - and glucocorticoid-inducible kinase SGK1 in the endometrium causes reproductive failure. Nat Med 17 : 1509–1513.
28. KuwabaraPE (2003) The multifaceted C. elegans major sperm protein: an ephrin signaling antagonist in oocyte maturation. Genes Dev 17 : 155–161.
29. MauresTJ, BoothLN, BenayounBA, IzrayelitY, SchroederFC, et al. (2014) Males shorten the life span of C. elegans hermaphrodites via secreted compounds. Science 343 : 541–544.
30. ShiC, MurphyCT (2014) Mating induces shrinking and death in Caenorhabditis mothers. Science 343 : 536–540.
31. Sacher GA (1977) Life table modification and life prolongation. In: Finch CE, Hayflick L, editors. Handbook of the Biology of Aging. New York: Van Nostrand Reinhold Co. pp. 582–638.
Štítky
Genetika Reprodukční medicína
Článek Large-scale Metabolomic Profiling Identifies Novel Biomarkers for Incident Coronary Heart DiseaseČlánek Notch Signaling Mediates the Age-Associated Decrease in Adhesion of Germline Stem Cells to the NicheČlánek Phosphorylation of Mitochondrial Polyubiquitin by PINK1 Promotes Parkin Mitochondrial TetheringČlánek Natural Variation Is Associated With Genome-Wide Methylation Changes and Temperature SeasonalityČlánek Overlapping and Non-overlapping Functions of Condensins I and II in Neural Stem Cell DivisionsČlánek Unisexual Reproduction Drives Meiotic Recombination and Phenotypic and Karyotypic Plasticity inČlánek Tetraspanin (TSP-17) Protects Dopaminergic Neurons against 6-OHDA-Induced Neurodegeneration inČlánek ABA-Mediated ROS in Mitochondria Regulate Root Meristem Activity by Controlling Expression inČlánek Mutations in Global Regulators Lead to Metabolic Selection during Adaptation to Complex EnvironmentsČlánek The Evolution of Sex Ratio Distorter Suppression Affects a 25 cM Genomic Region in the Butterfly
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 12- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Srdeční frekvence embrya může být faktorem užitečným v předpovídání výsledku IVF
- Prenatální expozice ftalátům a anogenitální vzdálenost u novorozenců
- Vztah užívání alkoholu a mužské fertility
-
Všechny články tohoto čísla
- Stratification by Smoking Status Reveals an Association of Genotype with Body Mass Index in Never Smokers
- Genome Wide Meta-analysis Highlights the Role of Genetic Variation in in the Regulation of Circulating Serum Chemerin
- Occupancy of Mitochondrial Single-Stranded DNA Binding Protein Supports the Strand Displacement Mode of DNA Replication
- Distinct Genealogies for Plasmids and Chromosome
- Large-scale Metabolomic Profiling Identifies Novel Biomarkers for Incident Coronary Heart Disease
- Non-coding RNAs Prevent the Binding of the MSL-complex to Heterochromatic Regions
- Plasmid Flux in ST131 Sublineages, Analyzed by Plasmid Constellation Network (PLACNET), a New Method for Plasmid Reconstruction from Whole Genome Sequences
- Epigenome-Guided Analysis of the Transcriptome of Plaque Macrophages during Atherosclerosis Regression Reveals Activation of the Wnt Signaling Pathway
- The Inventiveness of Nature: An Interview with Werner Arber
- Mediation Analysis Demonstrates That -eQTLs Are Often Explained by -Mediation: A Genome-Wide Analysis among 1,800 South Asians
- Generation of Antigenic Diversity in by Structured Rearrangement of Genes During Mitosis
- A Massively Parallel Pipeline to Clone DNA Variants and Examine Molecular Phenotypes of Human Disease Mutations
- Genetic Analysis of the Cardiac Methylome at Single Nucleotide Resolution in a Model of Human Cardiovascular Disease
- Genetic Analysis of Circadian Responses to Low Frequency Electromagnetic Fields in
- The Dissection of Meiotic Chromosome Movement in Mice Using an Electroporation Technique
- Altered Chromatin Occupancy of Master Regulators Underlies Evolutionary Divergence in the Transcriptional Landscape of Erythroid Differentiation
- Syd/JIP3 and JNK Signaling Are Required for Myonuclear Positioning and Muscle Function
- Notch Signaling Mediates the Age-Associated Decrease in Adhesion of Germline Stem Cells to the Niche
- Mutation of Leads to Blurred Tonotopic Organization of Central Auditory Circuits in Mice
- The IKAROS Interaction with a Complex Including Chromatin Remodeling and Transcription Elongation Activities Is Required for Hematopoiesis
- RAN-Binding Protein 9 is Involved in Alternative Splicing and is Critical for Male Germ Cell Development and Male Fertility
- Enhanced Longevity by Ibuprofen, Conserved in Multiple Species, Occurs in Yeast through Inhibition of Tryptophan Import
- Phosphorylation of Mitochondrial Polyubiquitin by PINK1 Promotes Parkin Mitochondrial Tethering
- Recurrent Loss of Specific Introns during Angiosperm Evolution
- Natural Variation Is Associated With Genome-Wide Methylation Changes and Temperature Seasonality
- SEEDSTICK is a Master Regulator of Development and Metabolism in the Arabidopsis Seed Coat
- Overlapping and Non-overlapping Functions of Condensins I and II in Neural Stem Cell Divisions
- Unisexual Reproduction Drives Meiotic Recombination and Phenotypic and Karyotypic Plasticity in
- Tetraspanin (TSP-17) Protects Dopaminergic Neurons against 6-OHDA-Induced Neurodegeneration in
- ABA-Mediated ROS in Mitochondria Regulate Root Meristem Activity by Controlling Expression in
- Mutations in Global Regulators Lead to Metabolic Selection during Adaptation to Complex Environments
- Global Analysis of Photosynthesis Transcriptional Regulatory Networks
- Mucolipin Co-deficiency Causes Accelerated Endolysosomal Vacuolation of Enterocytes and Failure-to-Thrive from Birth to Weaning
- Controlling Pre-leukemic Thymocyte Self-Renewal
- How Malaria Parasites Avoid Running Out of Ammo
- Echoes of the Past: Hereditarianism and
- Deep Reads: Strands in the History of Molecular Genetics
- Keep on Laying Eggs Mama, RNAi My Reproductive Aging Blues Away
- Analysis of a Plant Complex Resistance Gene Locus Underlying Immune-Related Hybrid Incompatibility and Its Occurrence in Nature
- Epistatic Adaptive Evolution of Human Color Vision
- Increased and Imbalanced dNTP Pools Symmetrically Promote Both Leading and Lagging Strand Replication Infidelity
- Genetic Basis of Haloperidol Resistance in Is Complex and Dose Dependent
- Genome-Wide Analysis of DNA Methylation Dynamics during Early Human Development
- Interaction between Conjugative and Retrotransposable Elements in Horizontal Gene Transfer
- The Evolution of Sex Ratio Distorter Suppression Affects a 25 cM Genomic Region in the Butterfly
- is Required for Adult Maintenance of Dopaminergic Neurons in the Ventral Substantia Nigra
- PRL1, an RNA-Binding Protein, Positively Regulates the Accumulation of miRNAs and siRNAs in Arabidopsis
- Genetic Control of Contagious Asexuality in the Pea Aphid
- Early Mesozoic Coexistence of Amniotes and Hepadnaviridae
- Local and Systemic Regulation of Plant Root System Architecture and Symbiotic Nodulation by a Receptor-Like Kinase
- Gene Pathways That Delay Reproductive Senescence
- The Evolution of Fungal Metabolic Pathways
- Maf1 Is a Novel Target of PTEN and PI3K Signaling That Negatively Regulates Oncogenesis and Lipid Metabolism
- Formation of Linear Amplicons with Inverted Duplications in Requires the MRE11 Nuclease
- Identification of Rare Causal Variants in Sequence-Based Studies: Methods and Applications to , a Gene Involved in Cohen Syndrome and Autism
- Rrp12 and the Exportin Crm1 Participate in Late Assembly Events in the Nucleolus during 40S Ribosomal Subunit Biogenesis
- The Mutations in the ATP-Binding Groove of the Rad3/XPD Helicase Lead to -Cockayne Syndrome-Like Phenotypes
- Topoisomerase I Plays a Critical Role in Suppressing Genome Instability at a Highly Transcribed G-Quadruplex-Forming Sequence
- A Cbx8-Containing Polycomb Complex Facilitates the Transition to Gene Activation during ES Cell Differentiation
- Transcriptional Frameshifting Rescues Type VI Secretion by the Production of Two Length Variants from the Prematurely Interrupted Gene
- Association Mapping across Numerous Traits Reveals Patterns of Functional Variation in Maize
- Genome-Wide Analysis of -Regulated and Phased Small RNAs Underscores the Importance of the ta-siRNA Pathway to Maize Development
- Dissemination of Cephalosporin Resistance Genes between Strains from Farm Animals and Humans by Specific Plasmid Lineages
- The Tau Tubulin Kinases TTBK1/2 Promote Accumulation of Pathological TDP-43
- Germline Signals Deploy NHR-49 to Modulate Fatty-Acid β-Oxidation and Desaturation in Somatic Tissues of
- Microevolution of in Macrophages Restores Filamentation in a Nonfilamentous Mutant
- Vangl2-Regulated Polarisation of Second Heart Field-Derived Cells Is Required for Outflow Tract Lengthening during Cardiac Development
- Chondrocytes Transdifferentiate into Osteoblasts in Endochondral Bone during Development, Postnatal Growth and Fracture Healing in Mice
- A ABC Transporter Regulates Lifespan
- RA and FGF Signalling Are Required in the Zebrafish Otic Vesicle to Pattern and Maintain Ventral Otic Identities
- , and Reprogram Thymocytes into Self-Renewing Cells
- The miR9863 Family Regulates Distinct Alleles in Barley to Attenuate NLR Receptor-Triggered Disease Resistance and Cell-Death Signaling
- Detection of Pleiotropy through a Phenome-Wide Association Study (PheWAS) of Epidemiologic Data as Part of the Environmental Architecture for Genes Linked to Environment (EAGLE) Study
- Extensive Copy-Number Variation of Young Genes across Stickleback Populations
- The and Genetic Modules Interact to Regulate Ciliogenesis and Ciliary Microtubule Patterning in
- Analysis of the Genome, Transcriptome and Secretome Provides Insight into Its Pioneer Colonization Strategies of Wood
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Tetraspanin (TSP-17) Protects Dopaminergic Neurons against 6-OHDA-Induced Neurodegeneration in
- Maf1 Is a Novel Target of PTEN and PI3K Signaling That Negatively Regulates Oncogenesis and Lipid Metabolism
- The IKAROS Interaction with a Complex Including Chromatin Remodeling and Transcription Elongation Activities Is Required for Hematopoiesis
- Echoes of the Past: Hereditarianism and
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



