-
Články
- Vzdělávání
- Časopisy
Top články
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Genomic Tools for Evolution and Conservation in the Chimpanzee: Is a Genetically Distinct Population
In spite of its evolutionary significance and conservation importance, the population structure of the common chimpanzee, Pan troglodytes, is still poorly understood. An issue of particular controversy is whether the proposed fourth subspecies of chimpanzee, Pan troglodytes ellioti, from parts of Nigeria and Cameroon, is genetically distinct. Although modern high-throughput SNP genotyping has had a major impact on our understanding of human population structure and demographic history, its application to ecological, demographic, or conservation questions in non-human species has been extremely limited. Here we apply these tools to chimpanzee population structure, using ∼700 autosomal SNPs derived from chimpanzee genomic data and a further ∼100 SNPs from targeted re-sequencing. We demonstrate conclusively the existence of P. t. ellioti as a genetically distinct subgroup. We show that there is clear differentiation between the verus, troglodytes, and ellioti populations at the SNP and haplotype level, on a scale that is greater than that separating continental human populations. Further, we show that only a small set of SNPs (10–20) is needed to successfully assign individuals to these populations. Tellingly, use of only mitochondrial DNA variation to classify individuals is erroneous in 4 of 54 cases, reinforcing the dangers of basing demographic inference on a single locus and implying that the demographic history of the species is more complicated than that suggested analyses based solely on mtDNA. In this study we demonstrate the feasibility of developing economical and robust tests of individual chimpanzee origin as well as in-depth studies of population structure. These findings have important implications for conservation strategies and our understanding of the evolution of chimpanzees. They also act as a proof-of-principle for the use of cheap high-throughput genomic methods for ecological questions.
Published in the journal: . PLoS Genet 8(3): e32767. doi:10.1371/journal.pgen.1002504
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002504Summary
In spite of its evolutionary significance and conservation importance, the population structure of the common chimpanzee, Pan troglodytes, is still poorly understood. An issue of particular controversy is whether the proposed fourth subspecies of chimpanzee, Pan troglodytes ellioti, from parts of Nigeria and Cameroon, is genetically distinct. Although modern high-throughput SNP genotyping has had a major impact on our understanding of human population structure and demographic history, its application to ecological, demographic, or conservation questions in non-human species has been extremely limited. Here we apply these tools to chimpanzee population structure, using ∼700 autosomal SNPs derived from chimpanzee genomic data and a further ∼100 SNPs from targeted re-sequencing. We demonstrate conclusively the existence of P. t. ellioti as a genetically distinct subgroup. We show that there is clear differentiation between the verus, troglodytes, and ellioti populations at the SNP and haplotype level, on a scale that is greater than that separating continental human populations. Further, we show that only a small set of SNPs (10–20) is needed to successfully assign individuals to these populations. Tellingly, use of only mitochondrial DNA variation to classify individuals is erroneous in 4 of 54 cases, reinforcing the dangers of basing demographic inference on a single locus and implying that the demographic history of the species is more complicated than that suggested analyses based solely on mtDNA. In this study we demonstrate the feasibility of developing economical and robust tests of individual chimpanzee origin as well as in-depth studies of population structure. These findings have important implications for conservation strategies and our understanding of the evolution of chimpanzees. They also act as a proof-of-principle for the use of cheap high-throughput genomic methods for ecological questions.
Introduction
The history and population structure of the common chimpanzee, Pan troglodytes, are incompletely understood. Traditionally, three subspecies have been described: the western chimpanzee (P. t. verus), central chimpanzee (P. t. troglodytes) and eastern chimpanzee (P. t. schweinfurthii). Analysis of mitochondrial DNA (mtDNA) variation led to the proposal of a fourth, “Nigerian” chimpanzee subspecies (P. t. vellerosus, since renamed P. t. ellioti [1]) as a sister taxon to P. t. verus occurring in an area of Nigeria and Cameroon east of the Niger river and north of the Sanaga river (Figure 1) [2], [3]. This new subspecies has been recognized by many taxonomists and conservation biologists [4], [5]. Subsequent analyses of autosomal microsatellite data, in one case based on few loci [6], and in another including few individuals designated a priori as P. t. ellioti [7], found little evidence to distinguish P. t. ellioti from P. t. troglodytes, which is distributed south of the Sanaga river (Figure 1). Very recently however a microsatellite-based study of 94 individuals with 27 loci [8] has established that up to five groups of common chimpanzees, including P. t. ellioti, can be distinguished genetically. In this study we provide a complementary analysis using very different data and analytical methodology that allows a direct comparison with human data.
Fig. 1. Map of the geographic distribution of four populations of common chimpanzee. 
After [3], Figure 6b. Colours show the ranges of each population (yellow - P. t. troglodytes, red - P. t. ellioti, blue - P. t. verus, green - P. t. schweinfurthii) with major rivers indicated. The Sanaga River in Cameroon has been proposed to form the boundary between the ranges of P. t. ellioti and P. t. troglodytes. For most animals, the definition of a subspecies as “a collection of populations occupying a distinct breeding range and diagnosably distinct from other populations” [9] would be uncontroversial. However, our close evolutionary relationship with chimpanzees, and the parallels that can be drawn between chimpanzees and humans, makes this terminology increasingly uncomfortable, and in some cases controversial, and so we prefer to avoid it. Whatever term is used, modern genetic methods clearly have the potential to make the assessment of distinctiveness more objective and precise than in the past and it should now be possible to confirm or refine earlier judgments that were based on other criteria or limited data.
The development of modern high-throughput SNP genotyping technologies has revolutionized many aspects of human genetics, including our understanding of the history and demography of human populations [10]–[13]. To date, the impact of such methods in non-human species has been limited (e.g. [14], [15]). Here we apply these technologies to chimpanzees, and show that they can clearly resolve the genetic distinctness of P. t. ellioti, and that, for conservation purposes, small subsets of SNPs can be used to distinguish previously recognized populations. Our major source of SNPs was those arising from sequencing reads of a single individual (“Clint”) from the chimpanzee genome project [16]. A notable finding is that, in spite of the severe ascertainment biases inherent in this SNP discovery (largely a single individual, from only one of the populations), analyses based on the resulting SNPs remain powerful, suggesting that the same may be true in other species for which there have been genome projects. We also demonstrate the potential benefits of haplotype-based analyses in combination with genomic SNP data in defining and quantifying population relationships.
Results
To address the question of whether Pan t. ellioti is genetically distinct from other populations, we obtained DNA samples from Cameroonian chimpanzees which we analysed along with samples from captive Western (verus) and troglodytes chimpanzees. Eastern chimpanzees (P. t. schweinfurthii), with their distinct geographical distribution, were not sampled in the current study. We sequenced 12 autosomal fragments of ∼1 kb and genotyped 691 SNPs from 22 autosomal regions of 40–80 kb [17] in order to resolve genome-wide relationships, and compared the results with inference from the mitochondrial HV-I locus.
We applied a number of different methods to the analysis of these data to assess the relationships and genetic clustering amongst the sampled individuals. The first set of methods (principal components and Structure) were based on the marginal data at each genotyped SNP. We then calculated FST from the DNA sequence data, and finally applied recently developed methods which exploited information on the joint distribution of SNP alleles within haplotypes.
Using the first two principal components of the data from all 818 SNPs, 52 of the 54 chimpanzees studied clustered into three distinct, non-overlapping groups (Figure 2a). These clusters are consistent with three genetically distinct populations represented amongst the study chimpanzees: captive Western (P. t. verus) chimpanzees form one cluster while Cameroonian chimpanzees are divided into two genetically distinct clusters, one of which we infer to correspond to P. t. ellioti, whose existence had been the subject of uncertainty. We note that two individuals in the P. t. ellioti cluster had previously been designated P. t. troglodytes based on mtDNA sequence, a point to which we return below. Two individuals (C024, C025) with P. t. troglodytes-like mtDNA lie between the presumptive P. t. verus and P. t. troglodytes clusters, and records have subsequently revealed that these are indeed first-generation hybrids produced in captivity.
Fig. 2. Clustering of chimpanzees based on principal components. 
(a) Clustering of chimpanzees based on principal components using data from 818 SNPs. Plots of the first two principal components of data from 818 SNPs show that chimpanzees in this study form three genetically distinct groups. Two chimpanzees (C127, C541) have P. t. troglodytes-like mtDNA but group with P. t. ellioti at autosomal loci. Two chimpanzees (C024, C025) known to be hybrids between P. t. troglodytes and P. t. verus lie between these populations on the PCA plot. (b) Clustering of chimpanzees based on principal components using population-informative SNPs. Plots of the first two principal components of data from just 10 selected SNPs (Table S4) reveal the same three groups as the full dataset. Plotted positions are shown with jitter to separate individuals with the same genotypes at the subset of SNPs. Plotting characters show the inferred population of origin of each chimpanzee: (triangles - P. t. troglodytes, squares - P. t. ellioti, circles - P. t. verus, ‘+’ - hybrids). A similar conclusion comes from a different perspective when the software Structure [18], [19] is used to estimate the proportion of each individual's genome that comes from each of several ancestral populations. With k = 3 presumptive populations, the same three groups were recovered cleanly with little estimated admixture except for the two hybrids (Figure 3), and where there was evidence for co-ancestry, it was detected between the ellioti and troglodytes groups, rather than involving verus chimpanzees. This suggests more recent interaction between P. t. ellioti and P. t. troglodytes than either has had with P. t. verus, although an effect of SNP ascertainment could not be ruled out. We note that the model underlying Structure assumes no linkage disequilibrium between loci, whereas our data do exhibit such correlations because of the clustering of SNPs. The expected effect of this in the Structure model is an over-estimation of precision, rather than bias [19], but nonetheless our Structure analysis should be interpreted with some caution.
Fig. 3. Structure estimates of ancestry in three populations. 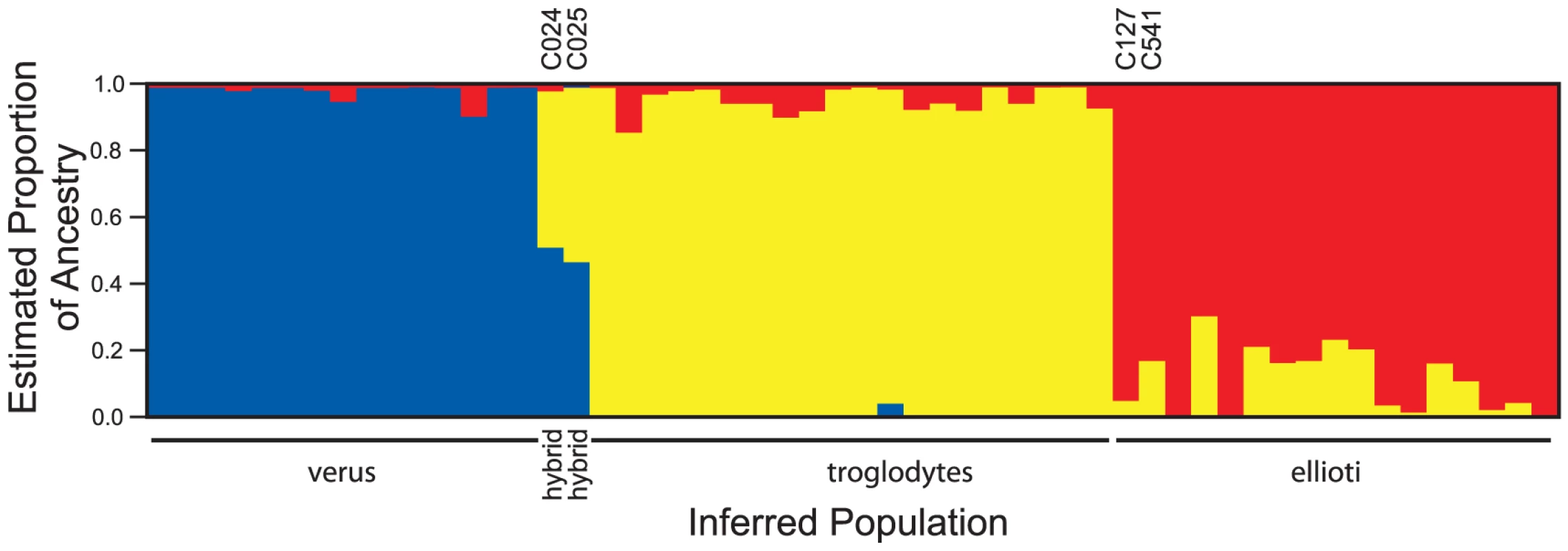
For each sampled individual the figure shows the estimated proportion of ancestry from Structure's three putative ancestral populations, with P. t. troglodytes in yellow, P. t. ellioti in red and P. t. verus in blue. Structure reveals the same pattern of group memberships as PCA, and additionally suggests that P. t. troglodytes and P. t. ellioti individuals may share more DNA from the other group than either shares with P. t. verus (blue). The two known hybrid individuals (C024, C025, with ancestry estimated at close to 50% in each of P. t. troglodytes and P. t. verus) and two P. t. ellioti chimpanzees with P. t. troglodytes-like mtDNA (C127, C541) are labelled. Next, we calculated pairwise FST, a commonly-used measure of the proportion of total genetic variation occurring between populations. Potential confounding effects from SNP ascertainment complicate interpretation of FST values calculated from the genotype data, so we restricted these analyses to our re-sequencing data alone (104 of 818 SNPs, also eliminating 3 sequenced loci showing evidence of positive selection) [20]. Consistent with Structure's view of relative amounts of co-ancestry, FST between P. t. ellioti and P. t. troglodytes (0.134, 95% CI 0.105–0.162) is slightly lower than, but cannot be formally distinguished from, that between P. t. troglodytes and P. t. verus (0.177, 95% CI 0.129–0.225) or between P. t. ellioti and P. t. verus (0.190, 95% CI 0.145–0.235). The troglodytes – verus figure in our data is lower than the 0.29 for Central vs. Western chimpanzees previously estimated from re-sequencing data [21], presumably due to sampling differences (either of loci or individuals) between the two studies.
When genetic data is collected from tightly linked variable sites, exploiting patterns of non-random association (i.e. linkage disequilibrium) can increase power to identify population structure over single-SNP analyses [22], [23]. Informally, haplotype-based approaches have many of the advantages in terms of discriminatory power of other multi-allelic systems such as microsatellites, but in addition, our understanding of the evolutionary mechanisms involved means that there is a natural sense of the evolutionary distance between haplotypes. Sensible haplotype-based analyses can thus be more powerful than SNP-based approaches in using considerably more genetic information in comparing individuals, and in our context can thus be informative about differentiation at timescales shorter than those over which drift can be detected in SNP frequency differences. Additionally, haplotype-based analyses may be less susceptible to biases in SNP discovery [22]. Conversely, while haplotype-based methods can increase power to detect population structure, statistical methodology to fit explicit models of isolation, migration and fluctuating population size [24] to such data is so far lacking.
We analysed similarities in patterns of haplotype variation among individuals for the 691 clustered autosomal SNPs using a so-called copying model applied to estimated haplotypes from each individual [25], [26]. In effect, for each small chromosomal segment in one of the haplotypes of a particular individual, the approach looks amongst the haplotypes of the other sampled individuals to find the one with which it is most closely related, in the sense of most recently sharing a common ancestor. This is done under a model in which shared ancestry is likely to be the same for chromosomal segments which are very near to each other (in terms of genetic distance). The primary results of such an analysis are estimates of the most recent shared ancestry across each locus in each haplotype. For a particular chimpanzee, these can be aggregated to calculate the estimated proportion of the sampled regions for which it is most closely related to each of the other chimpanzees. These estimates are shown in Figure 4a. The figure provides a visual summary of the patterns of most-recently-shared ancestry within and between the three population groups. In a randomly mating population, the haplotypes in a particular individual will share similarities with many others across the sample, while in the presence of population structure haplotypes will tend to be more similar to those of other individuals within the same population than to those in other populations. Figure 4b (see also Table 1) provides a higher-level summary which aggregates information across populations to show, for each chimpanzee, the proportion of its sampled regions for which the most closely related haplotype comes from each of the three populations. Strikingly, Figure 4a and 4b show that across most of the sampled regions in each individual, the most closely related haplotype comes from the same population; in other words that the three populations are genetically quite distinct. This effect is most marked for the P. t. verus individuals, for whom the most closely related haplotype is virtually always in the same population. Haplotypes of P. t. ellioti and P. t. troglodytes chimpanzees respectively are typically most similar to those of other individuals within the same population, but occasionally to those of individuals from the other (P. t. troglodytes and P. t. ellioti respectively) population. The two previously noted hybrid individuals are clearly identified, and in addition it emerges that two of the P. t. ellioti chimpanzees had a higher level of shared ancestry than the other chimpanzees. The qualitative conclusions from the haplotype-based analysis thus mimic those from principal components and Structure, although reassuringly they explicitly model the correlations between nearby SNPs, in contrast to Structure.
Fig. 4. Haplotype-based analyses of population relationships. 
(a) (chimpanzee) and (c) (human): heat maps show the estimates from a copying model of the proportion of sampled genetic material of each individual (X axis) inferred to be closest to that in each other individual in the sample (Y axis). Human data was sampled from HapMap data for the three continental populations: Europe (CEU), Africa (YRI) and East Asia (Han Chinese, CHB) using an ascertainment scheme designed to match properties of SNPs in the chimpanzee data. Chimpanzees have less estimated copying from outside their own population than do humans. Individuals are labeled by their inferred (chimpanzee) or known (human) population of origin, or as hybrids. (b) and (d): summaries of estimated copying (ancestry) proportions by population, for each individual. (b) Chimpanzees: P. t. troglodytes in yellow, P. t. ellioti in red and P. t. verus in blue. P. t. troglodytes and P. t. ellioti appear to be less differentiated from other populations than is P. t. verus. (d) Human Continental populations: CEU Europe in yellow, YOR Africa in red and CHB East Asia in blue. Human individuals have higher proportions of ancestry from other populations than do chimpanzees. Tab. 1. Estimates of the proportion of the sampled genomic regions for which the most closely related haplotype comes from each study population, for chimpanzees and humans. 
Parentheses show the empirical central 95% region of the distribution of values for the 100 re-samples of the human data. By applying the haplotype-based copying model to human data, we can compare quantitatively the extent of differentiation between the three chimpanzee groups with that between various human populations. Importantly, such analyses can allow for ascertainment effects. We show the copy model results for human data from the Phase II HapMap (Frazer et al. 2007) in Figure 4c and 4d, comparing sampled individuals of European (CEPH), African (Yoruba, YRI) and East-Asian (Han Chinese, CHB) descent in an analysis in which SNPs in the human data were re-ascertained to match characteristics of the chimpanzee data (see “Data Analysis”). The average within - vs. between-population copying frequencies, that is, frequencies for the most-closely-related-haplotype, in these analyses are summarized in Table 1. Levels of between-population similarity among the chimpanzee populations are lower than among the HapMap populations, suggesting that the chimpanzee populations are more distinct than even continental human populations. To test the robustness of this conclusion to choice of comparison data, we re-sampled Phase II HapMap individuals, genomic regions and ascertained SNPs, 100 times. Only three times was the level of within-population copying of a pair of human populations greater than that between any chimp population (estimated within-population copying in each of Africa and East-Asia was greater than the estimated within-population copying in P. t. troglodytes for 3 of 100 re-samples). In Figures S1 and S2, we colour fragments of chromosomes according to their assigned population of origin under the copying model, illustrating that the probabilities with which individual chimpanzee chromosome segments are assigned to specific populations are also higher than for human data.
An equivalent analysis of the HapMap III African populations [27] showed that these African human populations are considerably less structured than the chimpanzee populations (Figures S3 and S4), as might be expected given the observation above that the chimpanzee populations are more differentiated even than continental human populations. Note that our comparisons with the human population samples are based on similar amounts of data as in our chimpanzee samples. With larger SNP datasets, the power to separate the human populations increases.
We note that while it is theoretically possible to use the lengths of copied fragments in the copying model to estimate the timescale over which differentiation has occurred, our data is not well-suited to this because the shortness of the assayed regions means that relatively few breakpoints are observed, providing little information about the times of events in the history of chimpanzee populations.
Discussion
We have applied a number of different analytical methods to an extensive set of SNP data from 54 chimpanzees. All of the methods point clearly to the existence of three distinct population groups, corresponding to three of the previously-described “subspecies” of chimpanzee P. t. verus, P. t. troglodytes, and P. t. ellioti, with the latter two groups sharing somewhat more similarity with each other than either does with P. t. verus. P. t. troglodytes and P. t. verus are two securely defined populations estimated to have diverged 0.4–0.6 million years ago [7], [8], [28]–[30]. Our analyses show P. t. ellioti to be clearly distinct from P. t. troglodytes with both groups equally distinct from P. t. verus, so that whatever terminology (“population” or “subspecies”) is applied to verus and troglodytes should equally be applied to ellioti.
By way of comparison, we have shown that these three chimpanzee populations are more differentiated than even continental human populations, and also that in spite of the relatively close geographic proximity of the groups, particularly troglodytes and ellioti, the chimpanzee populations are considerably more distinct than the African populations sampled in HapMap III, suggesting rather differing demographic histories for the two sister species.
In order to compare population comparisons based on the copying model with those based on more traditional FST approaches, we also calculated pairwise FST values for each of the 100 resamples of individuals and SNPs in our analyses of the three continental population samples. The results are summarized in Table 2. We note that while the average values of pairwise FST across the 100 samples show the same pattern as copying proportions in the copying model, the sample-to-sample variation is larger. For example, the FST intervals for the central 95% of resamples for Europe-East Asia overlap those of Africa-Europe and Africa-East Asia, and for example for five of the 100 resamples the pairwise FST between Africa and Europe was actually smaller than that between Europe and East-Asia. In contrast, for the copying model analysis the 95% intervals for the proportion that Europe and East Asia copy from each other do not overlap with the 95% intervals for either copying from Africa, and the proportion that Europe copied from Africa was lower than the proportion Europe copied from East Asia in each of the 100 re-samples. This accurately reflects the fact that on average East Asia and Europe share more recent ancestry with each other than with Africa.
Tab. 2. Pairwise FST values for human samples. 
Parentheses show the empirical central 95% region of the distribution of values for the 100 re-samples of the human data. One weakness of our study (and some others) is that we do not have definitive information on the geographic origin of all of the chimpanzees we have studied. All our analyses point to two very distinct population groups for the chimpanzees originating from eastern Nigeria and Cameroon. In the light of other genetic evidence for distinctiveness of individuals sampled from either side of the Sanaga River [3], [8], our assignment of one of our sampled groups as troglodytes and one as ellioti seems reasonable. Whilst our data alone could not rule out two distinct populations, one or both of which extends across the Sanaga River, this seems a priori unlikely – the river provides a natural barrier between the distinct populations, whereas if both were to exist on the same side of the river there seems no reason for their reproductive isolation—and at variance to other available evidence. Notwithstanding our lack of complete geographical information on sampled chimpanzees, the clear separation between all three populations, relative to the similarities within the populations, seems hard to reconcile with the suggestion that chimpanzee genetic variation is distributed more or less continuously across the species range (cf [21]).
The initial genetic description of P. t. ellioti was based on mtDNA sequence analysis [2], [3], which places most chimpanzees from parts of Nigeria and Cameroon north of the Sanaga river in a group sharing a common ancestor with P. t. verus, to the exclusion of P. t. troglodytes, a description made more robust by a recent analysis of complete mitochondrial genomes [31], [32]. We compared the classification based on mtDNA with our genome-wide analysis and found that it classified 50 of 52 non-hybrid individuals correctly. Chimpanzees C127 and C541 had troglodytes-like mtDNA but ellioti autosomal SNP genotypes. (The two known hybrid chimpanzees C024 and C025 had troglodytes-like mtDNA but were detectably intermediate in autosomal genotype). Thus the two systems generally agree, but, not surprisingly, single-locus mtDNA data is less reliable for classification than genome-wide data.
The mtDNA-based picture of demographic relationships suggests that P. t. verus and P. t. ellioti are sister taxa [3], [31]. Our data suggests this to be misleading, in two different respects. Firstly, as noted above, two individuals who are clearly P. t. ellioti, on the basis of extensive autosomal data, have mtDNA which clusters with P. t. troglodytes. Thus, mtDNA from ellioti individuals does not fall into a single clade on a mtDNA tree. If mtDNA is used both to classify individuals and to estimate trees for the resulting groups, there is always a danger, as seems to have occurred in this instance, that misclassification of individuals will lead to a simpler-looking tree than is actually the case. Secondly, the suggestion from the mtDNA data that (many, but as noted above, not all) ellioti individuals have mtDNA types which are closer to verus than to troglodytes individuals is strikingly different from the results of our analyses based on many independent autosomal loci, which places P. t. ellioti clearly closer to P. t. troglodytes than to P. t. verus. It is interesting to note that a study of morphological variation agreed with the picture obtained from autosomal loci [4]. Taken together, the mtDNA and autosomal results are difficult to reconcile with a simple demographic scenario based on population splitting, and suggest a more complex demographic history for the three populations we have studied, possibly including sex-biased gene flow.
For many conservation applications, it would be desirable to be able to assign or classify individuals to populations based on a small number of loci. We developed and applied a method for choosing subsets of SNPs for classification based on their contribution to assignment probabilities (see Methods). To avoid over-fitting, we divided our data set in two. A training dataset comprising half the samples from each population (27 of the 52 non-hybrid individuals) was used to select informative SNPs for classification, with the other half of the individuals forming a test dataset in which the ability of the chosen SNPs to accurately classify individuals to populations was measured.
For our data, we could essentially reproduce the discrimination obtained with the complete dataset of 818 SNPs with as few as 8 carefully selected SNPs in distinct regions of the genome (Figure 2b). While there is still some danger of over-fitting from our relatively small sample sizes, we conclude that a small, well-chosen panel of probably 10–20 SNPs, assayed via either a set of PCR-based single-locus assays or a single multiplex SNP assay for forensic and conservation work, would be capable of analysing and classifying limited DNA samples at low cost. The exact size of panel used would depend on the requirement to identify individuals of mixed ancestry. This is particularly encouraging considering the extreme ascertainment bias inherent in our genotyped SNPs: for the chimpanzee, dbSNP at the time of our SNP selection reflected the composition of the chimpanzee draft genome, in which ∼91% of sequence traces came from a single P. t. verus individual (‘Clint’), a further 4% from four other verus, and less than 5% from three P. t. troglodytes [16]. Notwithstanding this bias, 12 of our SNPs have an estimated allele frequency difference of >0.5 between ellioti and pooled troglodytes and verus chimpanzees. Our study thus confirms the utility of genomic resources even when ascertainment is sub-optimal.
The confirmation of P. t. ellioti as a genetically distinct population of chimpanzee strongly supports efforts to treat this population as a separate management unit for conservation [33] This is of particular importance since while all chimpanzees are considered to be endangered [34], P. t. ellioti, with an estimated 6,500 individuals remaining, is the least numerous population.
In conclusion, using genomic resources we have assembled the largest SNP-based dataset for investigating chimpanzee population structure. It resolves an outstanding controversy in clearly establishing the fourth putative subspecies, Pan troglodytes ellioti, as a genetically distinct group. More generally, our results confirm the utility of high throughput SNP typing for evolutionary genetic and conservation analysis. However, we recognize that a full appraisal of chimpanzee population structure would require denser sampling from all four populations in addition potentially to comparative studies across primates that go beyond great apes and humans.
Materials and Methods
Chimpanzee samples
Blood samples were obtained from 35 wild-born orphaned chimpanzees of unknown geographic origin within Cameroon. Genomic DNA, extracted using standard procedures, was amplified (GenomiPhi, GE Healthcare) before genotyping. DNA samples were also obtained from 15 P. t. verus (from Sierra Leone) and 4 putative P. t. troglodytes (unknown geographic origin) chimpanzees held at the Biomedical Primate Research Centre in the Netherlands (Table S1). For chimpanzees in the Netherlands, all blood sampling was done in accordance with a protocol that was approved by the Institutional Animal Care and User Committee (IACUC) of the Biomedical Primate Research Center (BPRC). For chimpanzees in Cameroon, blood samples were taken from orphaned individuals for haematological analysis as part of veterinary health screens.
Re-sequencing
Mitochondrial HV-I fragments of 534 bp and fragments of ∼1 kbp from the genes CCR5, SDF, CXCR4, CX3CR1, RANTES, CCR2, SEC22L3, ZNF445, PTPN23, CCRL2, MC1R and HBB (Table S2, Table S3) were amplified by PCR and sequenced directly. PCR products with heterozygous indels were cloned and 10 clones were sequenced for each sample. For pairwise Fst analyses, 3 loci with evidence for directional selection (CCR5, CXCR4 and CX3CR1; 23 SNPs, MacFie et al. 2009) were removed from the analysis.
SNP genotyping
A panel of 768 SNPs was designed for the GoldenGate Genotyping Assay (Illumina, San Diego), using polymorphism information from the Chimpanzee Genome Project [16] via dbSNP v26 [http://www.ncbi.nlm.nih.gov/projects/SNP/]. The SNPs, arranged in 22 clusters of size 40–80 kbp on several autosomes, were screened using BLAST to ensure unique context. The panel has also been used to assess recombination rates in the 22 regions, orthologous to recombination hotspots in humans [17]. Across 54 samples, 58 SNPs failed visual inspection, 14 gave at least one no-call and 5 SNPs departed strongly from Hardy-Weinberg equilibrium within a population (as initially labelled), leaving 691 SNPs for analysis.
Data analysis
Population structure was assessed by pairwise FST in Arlequin (with 95% CIs estimated by jackknifing) [35], PCA and SNP selection for assignment in the R Package [36], and with Structure [18], [19], using the admixture model of ancestry, with correlated allele frequencies, run with a ‘burn-in’ of 100,000 iterations followed by a further 1,000,000 iterations. This model is not strictly applicable to data from sites in linkage disequilibrium, so this analysis is indicative only. SNPs were chosen for classification as follows: for each SNP a sample was assigned to the population in which its genotype was most probable, the 818 SNPs were ranked by their ability to classify the training samples and the best SNPs, from distinct loci, were chosen (Table S4).
For the haplotype-based analysis, we inferred haplotypes and population-scaled recombination rates between adjacent SNPs using PHASEv2.1.1 [25], [37]–[39] with ten times the default number of MCMC iterations. We then applied the Li and Stephens (2003) copying model to the inferred “best-guess” haplotypes as described in [40] but fixing the PHASE recombination rate estimates, inferring the expected number of haplotype segments that each chimp copies from every other chimp via 100 iterations of an Expectation-Maximization (EM) algorithm and precluding copying from the other haplotype within the same individual. Figures S1, S2, and S3 are based on 100 samples from the model using the converged E-M values.
For comparisons with human data, we matched features of the chimpanzee dataset by randomly selecting 18 individuals per population using HapMap Phase 2 Release 21 or HapMap Phase 3 Release 2 consensus haplotypes. For each analysis, we then randomly selected 22 autosomal genomic regions, randomly selecting SNPs to match the SNP density and minor allele frequency distribution (in bins of (0.0,0.1], (0.1,0.2], (0.2,0.3], (0.3,0.4], (0.4,0.5]) for the respective 22 chimp regions. We ran the copying model using fixed genetic map estimates (build 35 estimates for HapMap2 populations and build 36 estimates for HapMap3 populations) scaled by an effective population size value of 30000, the value that maximized the expected log-likelihood over a fixed grid of (10K,20K,30K,40K,60K,300K,25000K), though we note that results were similar for all scaling factors we considered. Ascertaining SNPs on a single randomly selected HapMap Phase2 CEPH individual or HapMap Phase3 Luhya (Kenya) individual not included in the sample gave similar results to those presented. Pairwise FST for each re-sample was calculated using the approach described in [41].
Supporting Information
Zdroje
1. OatesJFGrovesCPJenkinsPD 2009 The type locality of Pan troglodytes vellerosus (Gray, 1862), and implications for the nomenclature of West African chimpanzees. Primates 50 78 80
2. GonderMKOatesJFDisotellTRForstnerMRMoralesJC 1997 A new west African chimpanzee subspecies? Nature 388 337
3. GonderMKDisotellTROatesJF 2006 New genetic evidence on the evolution of chimpanzee populations and implications for taxonomy. Int J Primatol 27 1103 1127
4. GrovesCP 2001 Primate Taxonomy Washington DC Smithsonian Institution Press 350
5. GrubbPButynskiTOatesJFBearderSDisotellT 2003 Assessment of the diversity of African primates. Int J Primatol 24 1301 1357
6. GonderMKDisotellTR 2006 Contrasting phylogeographic histories of chimpanzees in Nigeria and Cameroon: A multilocus analysis. LehmanSFleagleJ Primate Biogeography New York Springer 129 161
7. BecquetCPattersonNStoneACPrzeworskiMReichD 2007 Genetic structure of chimpanzee populations. PLoS Genet 3 e66 doi:10.1371/journal.pgen.0030066
8. GonderMKLocatelliSGhobrialLMitchellMWKujawskiJT 2011 Evidence from Cameroon reveals differences in the genetic structure and histories of chimpanzee populations. Proc Natl Acad Sci U S A 108 4766 4771
9. PattenMAUnittPSheldonF 2002 Diagnosability versus mean differences of sage sparrow subspecies. The Auk 119 26 35
10. International HapMap Consortium 2005 A haplotype map of the human genome. Nature 437 1299 1320
11. KeinanAMullikinJCPattersonNReichD 2007 Measurement of the human allele frequency spectrum demonstrates greater genetic drift in East Asians than in Europeans. Nat Genet 39 1251 1255
12. LiJZAbsherDMTangHSouthwickAMCastoAM 2008 Worldwide human relationships inferred from genome-wide patterns of variation. Science 319 1100 1104
13. HennBMGignouxCRJobinMGrankaJMMacphersonJM 2011 Hunter-gatherer genomic diversity suggests a southern African origin for modern humans. Proc Natl Acad Sci U S A 108 5154 5162
14. KarlssonEKBaranowskaIWadeCMSalmon HillbertzNHCZodyMC 2007 Efficient mapping of Mendelian traits in dogs through genome-wide association. Nat Genet 39 1321 1328
15. WillingEVABentzenPvan OosterhoutCHoffmannMCableJ 2010 Genome wide single nucleotide polymorphisms reveal population history and adaptive divergence in wild guppies. Mol Ecol 19 968 984
16. Chimpanzee Sequencing and Analysis Consortium 2005 Initial sequence of the chimpanzee genome and comparison with the human genome. Nature 437 69 87
17. MyersSBowdenRTumianABontropREFreemanC 2010 Drive against hotspot motifs in primates implicates the PRDM9 gene in meiotic recombination. Science 327 876 879
18. PritchardJKStephensMDonnellyP 2000 Inference of population structure using multilocus genotype data. Genetics 155 945 959
19. FalushDStephensMPritchardJK 2003 Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics 164 1567 1587
20. MacfieTSNerrienetEde GrootNGBontropREMundyNI 2009 Patterns of diversity in HIV-related loci among subspecies of chimpanzee: concordance at CCR5 and differences at CXCR4 and CX3CR1. Mol Biol Evol 26 719 727
21. FischerAPollackJThalmannONickelBPaaboS 2006 Demographic history and genetic differentiation in apes. Curr Biol 16 1133 1138
22. ConradDFJakobssonMCoopGWenXWallJD 2006 A worldwide survey of haplotype variation and linkage disequilibrium in the human genome. Nat Genet 38 1251 1260
23. HellenthalGAutonAFalushD 2008 Inferring human colonization history using a copying model. PLoS Genet 4 e1000078 doi:10.1371/journal.pgen.1000078
24. PinhoCHeyJ 2010 Divergence with gene flow: Models and data. Annu Rev Ecol Evol Syst 41 215 230
25. LiNStephensM 2003 Modeling linkage disequilibrium and identifying recombination hotspots using single-nucleotide polymorphism data. Genetics 165 2213 2233
26. LawsonDJHellenthalGMyersSFalushD 2012 Inference of population structure using dense haplotype data. PLoS Genet 8 e1002453 doi:10.1371/journal.pgen.1002453
27. AltshulerDMGibbsRAPeltonenLSchaffnerSFYuF 2010 Integrating common and rare genetic variation in diverse human populations. Nature 467 52 58
28. CaswellJLMallickSRichterDJNeubauerJSchirmerC 2008 Analysis of chimpanzee history based on genome sequence alignments. PLoS Genet 4 e1000057 doi:10.1371/journal.pgen.1000057
29. HeyJ 2010 The divergence of chimpanzee species and subspecies as revealed in multipopulation isolation-with-migration analyses. Mol Biol Evol 27 921 933
30. WegmannDExcoffierL 2010 Bayesian inference of the demographic history of chimpanzees. Mol Biol Evol 27 1425
31. BjorkALiuWWertheimJOHahnBHWorobeyM 2011 Evolutionary history of chimpanzees inferred from complete mitochondrial genomes. Mol Biol Evol 28 615 623
32. StoneACBattistuzziFUKubatkoLSPerryGHJrTrudeauE 2010 More reliable estimates of divergence times in Pan using complete mtDNA sequences and accounting for population structure. Philos Trans R Soc Lond B Biol Sci 365 3277 3288
33. KormosRBoeschC 2003 Regional action plan for the conservation of chimpanzees in West Africa Washington DC Conservation International
34. OatesJFTutinCEGHumleTWilsonMLBaillieJEM 2008 Pan troglodytes. IUCN 2008 2008 IUCN Red List of Threatened Species
35. ExcoffierLLavalGSchneiderS 2005 Arlequin ver. 3.0: An integrated software package for population genetics data analysis. Evolutionary Bioinformatics Online 1 47 50
36. R Development Core Team 2007 R: A language and environment for statistical computing Vienna R Foundation for Statistical Computing, Vienna, Austria
37. StephensMSmithNJDonnellyP 2001 A new statistical method for haplotype reconstruction from population data. Am J Hu Genet 68 978 989
38. StephensMScheetP 2005 Accounting for decay of linkage disequilibrium in haplotype inference and missing-data imputation. Am J Hum Genet 76 449 462
39. CrawfordDCBhangaleTLiNHellenthalGRiederMJ 2004 Evidence for substantial fine-scale variation in recombination rates across the human genome. Nat Genet 36 700 706
40. HellenthalGAutonAFalushD 2008 Inferring human colonization history using a copying model. PLoS Genet 4 e1000078 doi:10.1371/journal.pgen.1000078
41. HudsonRRSlatkinMMaddisonWP 1992 Estimation of levels of gene flow from DNA sequence data. Genetics 132 583
Štítky
Genetika Reprodukční medicína
Článek Physiological Notch Signaling Maintains Bone Homeostasis via RBPjk and Hey Upstream of NFATc1Článek Intronic -Regulatory Modules Mediate Tissue-Specific and Microbial Control of / TranscriptionČlánek Probing the Informational and Regulatory Plasticity of a Transcription Factor DNA–Binding DomainČlánek Repression of Germline RNAi Pathways in Somatic Cells by Retinoblastoma Pathway Chromatin ComplexesČlánek An Alu Element–Associated Hypermethylation Variant of the Gene Is Associated with Childhood ObesityČlánek Three Essential Ribonucleases—RNase Y, J1, and III—Control the Abundance of a Majority of mRNAs
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 3- Akutní intermitentní porfyrie
- Souvislost haplotypu M2 genu pro annexin A5 s opakovanými reprodukčními ztrátami
- Růst a vývoj dětí narozených pomocí IVF
- Srdeční frekvence embrya může být faktorem užitečným v předpovídání výsledku IVF
- Vztah užívání alkoholu a mužské fertility
-
Všechny články tohoto čísla
- Comprehensive Research Synopsis and Systematic Meta-Analyses in Parkinson's Disease Genetics: The PDGene Database
- Genomic Analysis of the Hydrocarbon-Producing, Cellulolytic, Endophytic Fungus
- Networks of Neuronal Genes Affected by Common and Rare Variants in Autism Spectrum Disorders
- Akirin Links Twist-Regulated Transcription with the Brahma Chromatin Remodeling Complex during Embryogenesis
- Too Much Cleavage of Cyclin E Promotes Breast Tumorigenesis
- Imprinted Genes … and the Number Is?
- Genetic Architecture of Highly Complex Chemical Resistance Traits across Four Yeast Strains
- Exploring the Complexity of the HIV-1 Fitness Landscape
- MNS1 Is Essential for Spermiogenesis and Motile Ciliary Functions in Mice
- A Fundamental Regulatory Mechanism Operating through OmpR and DNA Topology Controls Expression of Pathogenicity Islands SPI-1 and SPI-2
- Evidence for Positive Selection on a Number of MicroRNA Regulatory Interactions during Recent Human Evolution
- Variation in Modifies Risk of Neonatal Intestinal Obstruction in Cystic Fibrosis
- PIF4–Mediated Activation of Expression Integrates Temperature into the Auxin Pathway in Regulating Hypocotyl Growth
- Critical Evaluation of Imprinted Gene Expression by RNA–Seq: A New Perspective
- A Meta-Analysis and Genome-Wide Association Study of Platelet Count and Mean Platelet Volume in African Americans
- Mouse Genetics Suggests Cell-Context Dependency for Myc-Regulated Metabolic Enzymes during Tumorigenesis
- Transcriptional Control in Cardiac Progenitors: Tbx1 Interacts with the BAF Chromatin Remodeling Complex and Regulates
- Synthetic Lethality of Cohesins with PARPs and Replication Fork Mediators
- APOBEC3G-Induced Hypermutation of Human Immunodeficiency Virus Type-1 Is Typically a Discrete “All or Nothing” Phenomenon
- Interpreting Meta-Analyses of Genome-Wide Association Studies
- Error-Prone ZW Pairing and No Evidence for Meiotic Sex Chromosome Inactivation in the Chicken Germ Line
- -Dependent Chemosensory Functions Contribute to Courtship Behavior in
- Diverse Forms of Splicing Are Part of an Evolving Autoregulatory Circuit
- Phenotypic Plasticity of the Drosophila Transcriptome
- Physiological Notch Signaling Maintains Bone Homeostasis via RBPjk and Hey Upstream of NFATc1
- Precocious Metamorphosis in the Juvenile Hormone–Deficient Mutant of the Silkworm,
- Igf1r Signaling Is Indispensable for Preimplantation Development and Is Activated via a Novel Function of E-cadherin
- Accurate Prediction of Inducible Transcription Factor Binding Intensities In Vivo
- Mitochondrial Oxidative Stress Alters a Pathway in Strongly Resembling That of Bile Acid Biosynthesis and Secretion in Vertebrates
- Mammalian Neurogenesis Requires Treacle-Plk1 for Precise Control of Spindle Orientation, Mitotic Progression, and Maintenance of Neural Progenitor Cells
- Tcf7 Is an Important Regulator of the Switch of Self-Renewal and Differentiation in a Multipotential Hematopoietic Cell Line
- REST–Mediated Recruitment of Polycomb Repressor Complexes in Mammalian Cells
- Intronic -Regulatory Modules Mediate Tissue-Specific and Microbial Control of / Transcription
- Age-Dependent Brain Gene Expression and Copy Number Anomalies in Autism Suggest Distinct Pathological Processes at Young Versus Mature Ages
- A Genome-Wide Association Study Identifies Variants Underlying the Shade Avoidance Response
- -by- Regulatory Divergence Causes the Asymmetric Lethal Effects of an Ancestral Hybrid Incompatibility Gene
- Genome-Wide Association and Functional Follow-Up Reveals New Loci for Kidney Function
- A Natural System of Chromosome Transfer in
- Cell Size and the Initiation of DNA Replication in Bacteria
- Probing the Informational and Regulatory Plasticity of a Transcription Factor DNA–Binding Domain
- Repression of Germline RNAi Pathways in Somatic Cells by Retinoblastoma Pathway Chromatin Complexes
- Temporal Transcriptional Profiling of Somatic and Germ Cells Reveals Biased Lineage Priming of Sexual Fate in the Fetal Mouse Gonad
- Rapid Analysis of Genome Rearrangements by Multiplex Ligation–Dependent Probe Amplification
- Metabolic Profiling of a Mapping Population Exposes New Insights in the Regulation of Seed Metabolism and Seed, Fruit, and Plant Relations
- The Atypical Calpains: Evolutionary Analyses and Roles in Cellular Degeneration
- The Silkworm Coming of Age—Early
- Development of a Panel of Genome-Wide Ancestry Informative Markers to Study Admixture Throughout the Americas
- Balanced Codon Usage Optimizes Eukaryotic Translational Efficiency
- The Min System and Nucleoid Occlusion Are Not Required for Identifying the Division Site in but Ensure Its Efficient Utilization
- Neurobeachin, a Regulator of Synaptic Protein Targeting, Is Associated with Body Fat Mass and Feeding Behavior in Mice and Body-Mass Index in Humans
- Statistical Analysis of Readthrough Levels for Nonsense Mutations in Mammalian Cells Reveals a Major Determinant of Response to Gentamicin
- Gene Reactivation by 5-Aza-2′-Deoxycytidine–Induced Demethylation Requires SRCAP–Mediated H2A.Z Insertion to Establish Nucleosome Depleted Regions
- The miR-35-41 Family of MicroRNAs Regulates RNAi Sensitivity in
- Genetic Basis of Hidden Phenotypic Variation Revealed by Increased Translational Readthrough in Yeast
- An Alu Element–Associated Hypermethylation Variant of the Gene Is Associated with Childhood Obesity
- Modelling Human Regulatory Variation in Mouse: Finding the Function in Genome-Wide Association Studies and Whole-Genome Sequencing
- Novel Loci for Adiponectin Levels and Their Influence on Type 2 Diabetes and Metabolic Traits: A Multi-Ethnic Meta-Analysis of 45,891 Individuals
- Polycomb-Like 3 Promotes Polycomb Repressive Complex 2 Binding to CpG Islands and Embryonic Stem Cell Self-Renewal
- Insulin/IGF-1 and Hypoxia Signaling Act in Concert to Regulate Iron Homeostasis in
- EMF1 and PRC2 Cooperate to Repress Key Regulators of Arabidopsis Development
- Three Essential Ribonucleases—RNase Y, J1, and III—Control the Abundance of a Majority of mRNAs
- Contrasted Patterns of Molecular Evolution in Dominant and Recessive Self-Incompatibility Haplotypes in
- A Machine Learning Approach for Identifying Novel Cell Type–Specific Transcriptional Regulators of Myogenesis
- Genomic Tools for Evolution and Conservation in the Chimpanzee: Is a Genetically Distinct Population
- Nos2 Inactivation Promotes the Development of Medulloblastoma in Mice by Deregulation of Gap43–Dependent Granule Cell Precursor Migration
- Intracranial Aneurysm Risk Locus 5q23.2 Is Associated with Elevated Systolic Blood Pressure
- Heritability and Genetic Correlations Explained by Common SNPs for Metabolic Syndrome Traits
- A Genome-Wide Association Study of Nephrolithiasis in the Japanese Population Identifies Novel Susceptible Loci at 5q35.3, 7p14.3, and 13q14.1
- DNA Damage in Nijmegen Breakage Syndrome Cells Leads to PARP Hyperactivation and Increased Oxidative Stress
- DNA Resection at Chromosome Breaks Promotes Genome Stability by Constraining Non-Allelic Homologous Recombination
- Genetic Analysis of Floral Symmetry in Van Gogh's Sunflowers Reveals Independent Recruitment of Genes in the Asteraceae
- A Splice Site Variant in the Bovine Gene Compromises Growth and Regulation of the Inflammatory Response
- Promoter Nucleosome Organization Shapes the Evolution of Gene Expression
- The Nucleoside Diphosphate Kinase Gene Acts as Quantitative Trait Locus Promoting Non-Mendelian Inheritance
- The Ciliogenic Transcription Factor RFX3 Regulates Early Midline Distribution of Guidepost Neurons Required for Corpus Callosum Development
- Phosphorylation of the RNA–Binding Protein HOW by MAPK/ERK Enhances Its Dimerization and Activity
- A Genome-Wide Scan of Ashkenazi Jewish Crohn's Disease Suggests Novel Susceptibility Loci
- Parkinson's Disease–Associated Kinase PINK1 Regulates Miro Protein Level and Axonal Transport of Mitochondria
- LMW-E/CDK2 Deregulates Acinar Morphogenesis, Induces Tumorigenesis, and Associates with the Activated b-Raf-ERK1/2-mTOR Pathway in Breast Cancer Patients
- Mapping the Hsp90 Genetic Interaction Network in Reveals Environmental Contingency and Rewired Circuitry
- Autoregulation of the Noncoding RNA Gene
- The Human Pancreatic Islet Transcriptome: Expression of Candidate Genes for Type 1 Diabetes and the Impact of Pro-Inflammatory Cytokines
- Spo0A∼P Imposes a Temporal Gate for the Bimodal Expression of Competence in
- Antagonistic Regulation of Apoptosis and Differentiation by the Cut Transcription Factor Represents a Tumor-Suppressing Mechanism in
- A Downstream CpG Island Controls Transcript Initiation and Elongation and the Methylation State of the Imprinted Macro ncRNA Promoter
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- PIF4–Mediated Activation of Expression Integrates Temperature into the Auxin Pathway in Regulating Hypocotyl Growth
- Metabolic Profiling of a Mapping Population Exposes New Insights in the Regulation of Seed Metabolism and Seed, Fruit, and Plant Relations
- A Splice Site Variant in the Bovine Gene Compromises Growth and Regulation of the Inflammatory Response
- Comprehensive Research Synopsis and Systematic Meta-Analyses in Parkinson's Disease Genetics: The PDGene Database
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



