-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaMitosis in Neurons: Roughex and APC/C Maintain Cell Cycle Exit to Prevent Cytokinetic and Axonal Defects in Photoreceptor Neurons
The mechanisms of cell cycle exit by neurons remain poorly understood. Through genetic and developmental analysis of Drosophila eye development, we found that the cyclin-dependent kinase-inhibitor Roughex maintains G1 cell cycle exit during differentiation of the R8 class of photoreceptor neurons. The roughex mutant neurons re-enter the mitotic cell cycle and progress without executing cytokinesis, unlike non-neuronal cells in the roughex mutant that perform complete cell divisions. After mitosis, the binucleated R8 neurons usually transport one daughter nucleus away from the cell body into the developing axon towards the brain in a kinesin-dependent manner resembling anterograde axonal trafficking. Similar cell cycle and photoreceptor neuron defects occurred in mutants for components of the Anaphase Promoting Complex/Cyclosome. These findings indicate a neuron-specific defect in cytokinesis and demonstrate a critical role for mitotic cyclin downregulation both to maintain cell cycle exit during neuronal differentiation and to prevent axonal defects following failed cytokinesis.
Published in the journal: . PLoS Genet 8(11): e32767. doi:10.1371/journal.pgen.1003049
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003049Summary
The mechanisms of cell cycle exit by neurons remain poorly understood. Through genetic and developmental analysis of Drosophila eye development, we found that the cyclin-dependent kinase-inhibitor Roughex maintains G1 cell cycle exit during differentiation of the R8 class of photoreceptor neurons. The roughex mutant neurons re-enter the mitotic cell cycle and progress without executing cytokinesis, unlike non-neuronal cells in the roughex mutant that perform complete cell divisions. After mitosis, the binucleated R8 neurons usually transport one daughter nucleus away from the cell body into the developing axon towards the brain in a kinesin-dependent manner resembling anterograde axonal trafficking. Similar cell cycle and photoreceptor neuron defects occurred in mutants for components of the Anaphase Promoting Complex/Cyclosome. These findings indicate a neuron-specific defect in cytokinesis and demonstrate a critical role for mitotic cyclin downregulation both to maintain cell cycle exit during neuronal differentiation and to prevent axonal defects following failed cytokinesis.
Introduction
The near universality of neuronal cell cycle exit implies robust barriers to division. These could include additional neuron-specific and redundant barriers to cell cycle entry and progression, and surveillance mechanisms to eliminate neurons that evade them. Even in tumors, to date the only candidate for a dividing neuron is the horizontal interneuron suggested to be the cell of origin for retinoblastoma [1].
It is often speculated that the cell cycle might be lethal for neurons, or that inability to duplicate axons may prevent neuronal division. In the case of horizontal cells that give rise to retinoblastoma in the mouse retina, mutations at three Rb-family loci are required for transformation [2]. Such genotypes cause other types of retinal neuron to die [2]. In Drosophila, in which rbf1 is the only positively-acting Rb family gene, differentiating retinal neurons only re-enter the cell cycle if both Rbf1 and the p21/27-like gene dacapo are removed, and the contribution of mitotic rbf dap neurons to the differentiated nervous system has not been determined [3]. There are reports of cell cycle entry by a subset of neurons in the normal mammalian CNS, including retinal ganglion cells, but it is thought that such cell cycles are usually not completed [4]. It has been suggested that unscheduled cell cycle re-entry contributes to neurological diseases [5]–[9]. On the other hand, substantial evidence links multiple neurodegenerative conditions to defects in axonal trafficking [10]–[13]. Overall, much remains to be learned concerning cell cycle status and mechanisms in neurons, and its relationship to disease.
In this paper, we describe cell cycle re-entry in differentiating photoreceptor neurons lacking the cyclin dependent kinase inhibitor, roughex, or certain components of the Anaphase Promoting Complex/Cyclosome (APC/C), and the cellular consequences of such deregulated neuronal cell cycles in the differentiating Drosophila eye. The Drosophila adult eye is derived from the eye imaginal disc, which during the last larval stage is transformed from a sheet of undifferentiated, proliferating cells to highly organized neuroepithelium (Figure 1) [14], [15]. The recruitment and specification of cells begins at the posterior of the organ and progresses anteriorly as diffusible signals from more differentiated cells induce undifferentiated cells to exit the cell cycle in G1 [16]. These G1 arrested cells change shape to form a transient depression called the morphogenetic furrow that approximately separates the progenitor cell population from the region where retinal patterning and differentiation has begun (Figure 1) [15]. An array of R8 photoreceptor neuron precursor cells is selected from clusters of cells called intermediate groups that express the transcription factors Atonal and Senseless [17]–[20]. Cell interactions refine the expression of Atonal and Senseless to individual cells in each intermediate group that are specified as R8 photoreceptor precursors, the first neuron of each unit eye or ommatidium [21], [22]. R8 cells then coordinate the recruitment of some neighboring cells to the ommatidium while the remaining cells undergo an additional round of cell division, called the Second Mitotic Wave, to generate more progenitors required to form a complete unit eye, or ommatidium (Figure 1) [23], [15], [24], [16]. Soon after being specified, photoreceptor neurons form axons that grow below the basal surface of the disc and project to the optic lobe [23], [25], [26].
Fig. 1. Mitotic R8 cells re-enter the cell cycle in rux mutants. 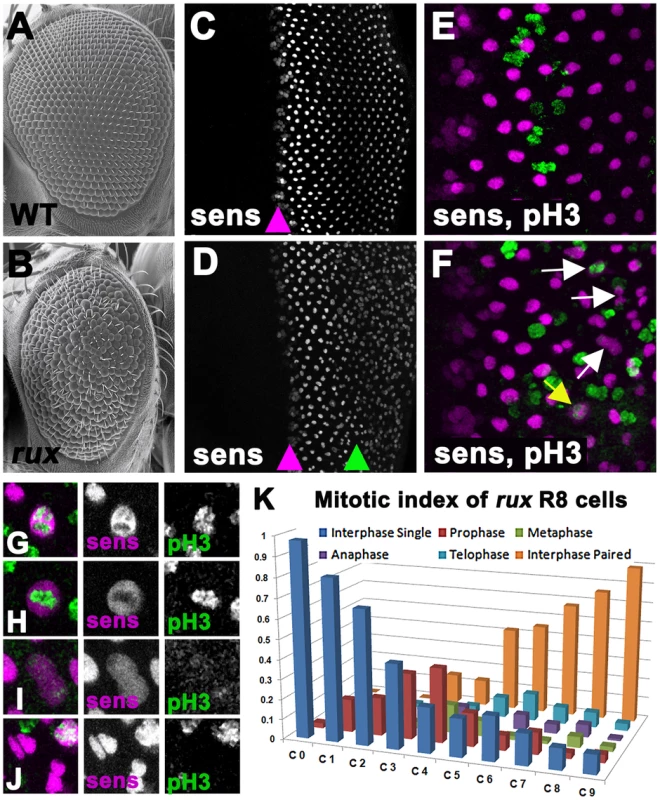
Scanning electron micrographs of the wild type (A) and rux (B) adult eyes. In both wild type (C) and rux (D) third instar eye discs, normal Sens staining appears in intermediate group cells (purple arrowheads) before becoming isolated to single R8 cells. In rux R8 nuclei become disorganized after their number increases (D, green arrowhead). Mitotic figures labeled with H3p (green) are seen only in non-neuronal cells during the Second Mitotic Wave of wild type eye discs (E), but in rux discs (F) mitotic R8 cells and paired R8 nuclei are also seen (yellow and white arrows). G–K. Mitotic R8 cells from a rux eye disc in prophase (G), metaphase (H), anaphase (I), and as closely paired nuclei (J). K. Mitotic index of R8 cells showing the fraction of mitotic R8 cells in each of the first 10 columns (n = 5 eye discs and 620 R8 cells). See also Figure S1. The roughex (rux) locus encodes a cyclin dependent kinase inhibitor that suppresses Cyclin A/CDK1 activity and regulates the cell cycle in several tissues [27]–[32]. Cyclin A is required for entry into mitosis in Drosophila whereas Cyclin E/CDK2 is required for entry into S-phase [33], [34]. Cyclin A is able to drive S phase entry if it is stabilized in G1, however, bypassing the requirement for Cyclin E [29], [35], [36]. Adult rux mutants exhibit deformed eyes composed of disorganized eye units containing irregular, reduced numbers of photoreceptors [28]. In rux mutant eye discs, G1 arrest is less effective both anterior and posterior to the morphogenetic furrow and generalized mitotic activity replaces the orderly second mitotic wave. The ectopic mitosis was previously thought to be limited to non-neuronal progenitor cells, and the fact that multiple R8 precursors were seen was attributed to an effect of proliferation on R8 specification [28]. Here, we report instead that cell cycle re-entry during R8 differentiation leads to the appearance of multiple R8 cells. The R8 neurons complete mitosis without undergoing cytokinesis, and their syncytial daughter nuclei become involved in axonal trafficking, demonstrating an unexpected link between the two main proposed mechanisms of neurodegeneration.
Results
Rux activity is required in R8 cells
Genetic mosaic analysis was used to assess the requirement for rux in distinct ommatidial cells. Adult rux mutant eyes have a reduced number of ommatidia that often lack a variable number of cells(Figure 1A, 1B) [28]. Mosaic eyes containing clones of the null mutation rux8 were sectioned, and how often ommatidia developed normally despite containing rux null cells was determined for each cell type (Table 1). Strikingly, less than 1% of the mosaic, normal ommatidia contained mutant R8 cells. In addition, the rux mutation was tolerated in other photoreceptor cells less often than the 50% recovery that would be expected were it entirely neutral, consistent with a lesser requirement in cells other than R8. These findings indicate that the rux gene is directly required in R8 cells, and challenge the conclusion that R8 development is affected in rux as an indirect consequence of mitotic defects affecting other cells. It was unexpected that a cell cycle regulator would be required in R8 precursor cells, a post-mitotic neuronal cell type.
Tab. 1. Analysis of adult retina mosaic for rux8. 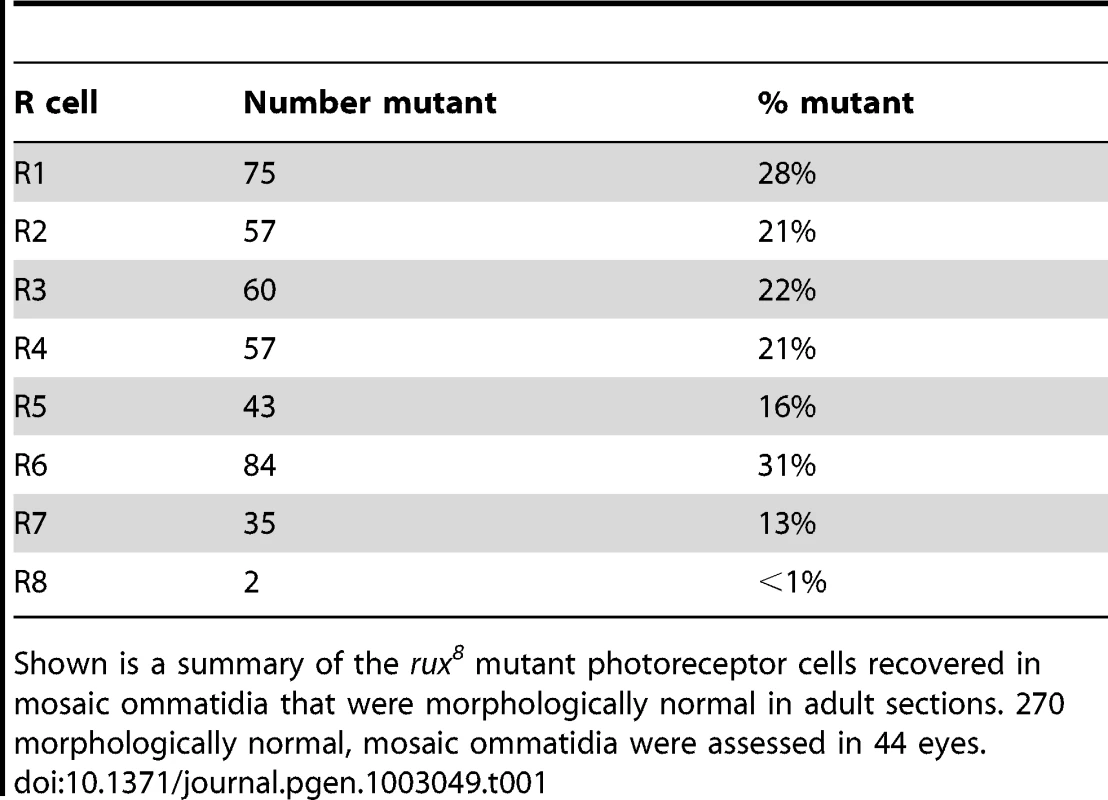
Shown is a summary of the rux8 mutant photoreceptor cells recovered in mosaic ommatidia that were morphologically normal in adult sections. 270 morphologically normal, mosaic ommatidia were assessed in 44 eyes. Rux is required to maintain of cell cycle exit in R8 cells
The presence of extra R8 cells in rux mutants was observed previously using antibodies against the R8-specific apical membrane protein Boss and the pattern of expression of the R8-specific lac-Z reporter BB02 [28]. Thus R8 development must be abnormal by the time these markers are expressed, about 12 hours after specification. We re-examined R8 development using an antibody against the Senseless protein, which is expressed from the onset of R8 specification [37]. Senseless expression in wild type animals is normally seen in intermediate groups within the furrow and becomes isolated to a single R8 cell in the next column, column 0, thereafter being maintained in R8 cells for the remainder of eye development (Figure 1C). Contrary to the previous model, we found that intermediate group formation and the selection of a single R8 cell occurred almost normally in rux mutants, and that multiple R8 nuclei were not apparent until further posterior in the eye disc (Figure 1D). Furthermore, it was evident that these were associated with R8 cell mitosis.
Starting at column 3, Senseless labeling transiently expanded through the R8 cells, an appearance previously seen in mitotic photoreceptor neurons where the nuclear membrane breaks down [3]. Consistent with this, mitotic chromatin labeled by an antibody against Histone 3 phosphorylated on Ser 10 (H3p) [38] was seen in R8 cells from column 3–8 (Figure 1E, 1F), progressing through prophase, metaphase and anaphase morphologies, followed by Sens labeling of pairs of sens-positive R8 nuclei (Figure 1F–1J). The regular array of R8 nuclei also became less organized as their numbers increased. Figure 1K catalogs the mitotic stages of R8 nuclei in rux. They entered prophase between columns 0 and 3. Some quickly progressed through mitosis, since paired R8 nuclei in interphase were first seen in column 3, but R8 cells in anaphase and telophase were commonest between columns 7 and 10, and pairs of R8 nuclei accumulated most rapidly after column 6. Most or all R8 nuclei were paired by column 10. Taken together with the mosaic analysis, these data suggest defects in R8 number and patterning do not reflect defects in R8 specification but are a consequence of cell-cycle re-entry by R8 cells that have undergone fate specification.
Previous studies established that rux prevents division of non-neuronal cells by inhibiting CycA [30], [39]. The effect of reducing cycA gene dose on R8 cell cycles was examined to confirm CycA dependence. Consistent with this expectation, the number of and rate of R8 mitosis was significantly reduced in rux8;cycA/+ eye discs (Figure S1).
We also examined the mitotic status of other photoreceptor cell types. Based on quantifying the co-labeling of H3p with the pan-neuronal marker Elav, we estimate that only about 20% of photoreceptor cells other than R8 re-entered the cell cycle (data not shown).
R8 cell mitosis in rux is insensitive to p21
In an attempt to separate pre - and post-specification effects of rux we expressed human p21 using the GMR-p21 transgenic line [38]. This cyclin dependent kinase inhibitor (CDI) strongly inhibits G1 cyclin activity of CDK2 and CDK4 complexes, but is considerably less effective at inhibiting CDK1 [40]–[43]. Cell cycle progression was monitored by labeling for mitotic H3p and for Cyclin B, which accumulates in cells between S-phase entry and mitotic metaphase [44], [34](Figure 2A, 2B). GMR-p21 completely blocks the second mitotic wave in wild type, without affecting progenitor cell proliferation anterior to the morphogenetic furrow [38](Figure 2C, 2D). In rux mutant eye discs, G1 arrest ahead of the furrow was less effective and ended prematurely, with high levels of Cyclin B accumulating in many cells from the earliest stages of R8 specification, and widespread mitosis of R8 and undifferentiated cells from column 2 onwards (Figure 2E, 2F) [28]. GMR-p21 had no discernible effect on any aspect of the cell cycle activity in rux mutants (Figure 2G, 2H). This supports the notion that ectopic cell cycles are independent of CycE/Cdk2, although it could also be explained if rux mutant cells enter the cell cycle before the GMR-p21 transgene is expressed.
Fig. 2. Mitotic activity in rux mutants is not affected by p21. 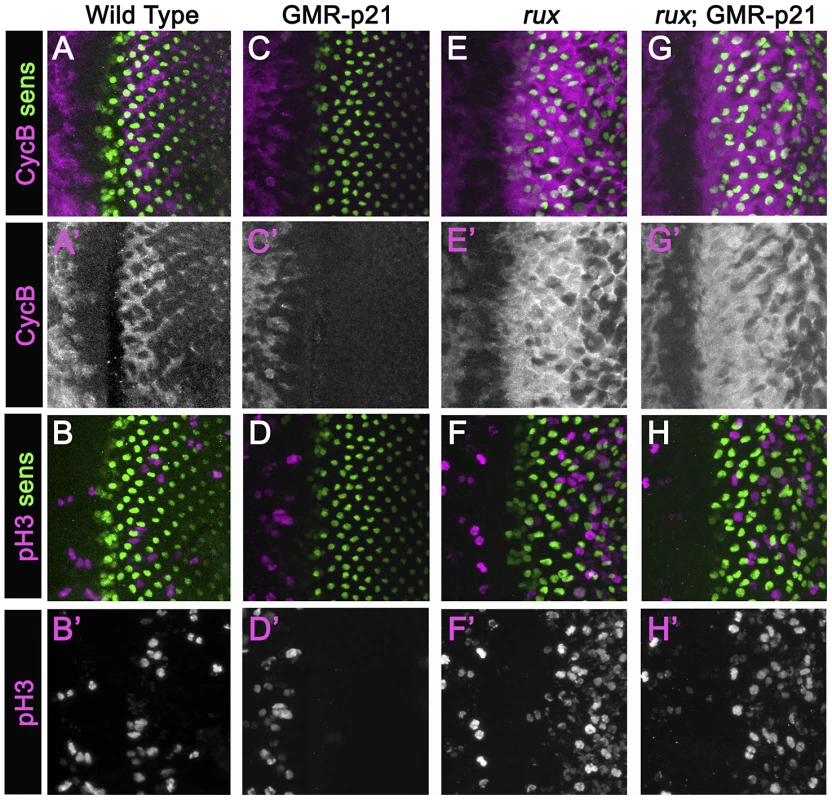
Eye discs from different genotypes labeled with Sens (green) and Cyclin B (A,C,G,E) or H3p (B,D,F,H) in magenta. (A)(B) Wild type eye discs exhibit the pattern of cells entering the SMW. (C)(D) When p21 is expressed this blocks the Second Mitotic Wave. (E)(F) In rux, arrest ahead of the morphogenetic furrow is incomplete and truncated, and there is much more mitotic activity posterior to the furrow. (G,H). rux; GMR-p21 eye discs are identical to rux (compare panels E,F). Mitotic R8 cells do not complete cytokinesis
To characterize the effects of cell cycle re-entry on R8 cells in more detail the G109-68-Gal4 driver was used to express CD8:GFP, a fusion protein effective for labeling neurons [45]. In eye discs, G109-68 expression is almost entirely restricted to R8 cells (Figure 3A) [46]. In confocal micrographs of otherwise wild type eye discs, R8 nuclei ascend apically within the eye disc epithelial layer following specification and then by column 3 drop somewhat as additional photoreceptors are recruited to each ommatidium, but remain in the apical half of the disc epithelium [23](Figure 3A). In rux mutant eye discs, R8 morphology resembled normal until column 3 (Figure 3B). By column 6, most rux R8 cells contained two separated nuclei within which nuclear proteins such as Sens and Elav appeared confined. Horizontal pairs of nuclei occupied an apico-basal location similar to that of R8 cells from wild type in 38% of such cells (Figure 3C). In most, however, one R8 nucleus at the normal apical position was accompanied by a second progressively more basal (Figure 3B). In columns 15–20, 36.5% of these basal R8 nuclei were found among the axons under the eye disc epithelium, which are normally devoid of nuclei (Figure 3D,3E). These observations suggested R8 cells remained binucleate in rux, with one nucleus often moving into the developing axon.
Fig. 3. Mitotic R8 cells remain bi-nucleated without completing cytokinesis. 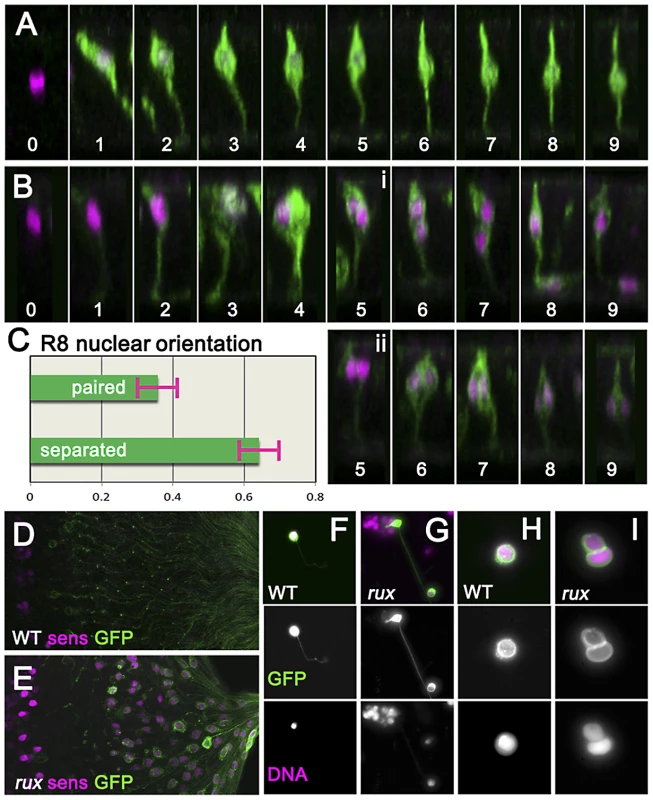
(A) Fixed eye discs stained with anti-Sens (magenta). Z-slices of differentiating R8 cells from wild type expressing GFP (green; ommatidial column denoted by numbers). The apical surface is at the top, anterior is to the left. Nuclei rise apically soon after specification, dropping progressively to a somewhat more basal location from column 3 onwards. (B) Z projections of differentiating R8 cells from rux. 109-68Gal4>GFP expression is often weaker in rux. Around column 3–4, many R8 nuclei are mitotic. Posterior to column 6, most R8 cells retain one nucleus in a normal location while the second drops basally and into the developing axon (i), but in some R8 cells both nuclei remain together in the cell body (ii). (C) quantification of nuclear behavior. (D) the basal third of wild type discs lack R8 nuclei (magenta) near the axons (green). (E) In rux, R8 nuclei accumulate in the basal axons. (F) Trypsin treated, dissociated, R8 cells exclusively exhibit a single nucleus as labeled for DNA (magenta). (G) In partially dissociated rux mutants, R8 cells containing a second nucleus in the axon can be seen. The figure shows an R8 cell whose cell body remains within an ommatidial cluster dissociated from the rest of the eye disc. (H). After extensive dissociation, wt R8 cells become round without axons. (I) 35% of rux 35% cells appear binucleated. See also Video S1. In order to determine directly whether rux R8 cells divide, living eye discs were digested with trypsin [47]. Samples of dissociated cells were generated from late third instar and white pre-pupal eye discs whose R8 cells were marked with 109-68>CD8:GFP. No axonal nuclei were ever seen in samples from wild type discs (Figure 3F). When partially dissociated, rux eye discs yielded isolated ommatidia containing GFP labeled R8 cells clearly appearing to contain axonal nuclei (Figure 3G). When completely dissociated, single R8 cells rapidly lost their neuronal morphology and rounded up (this process could be observed and recorded under the microscope and often resulted in nuclear fusion: Video S1). Under conditions where all cells were dissociated, all GFP-labelled R8 cells from wild type preparations were mononculeate (Figure 3H). With rux mutant discs, ∼35% of GFP positive cells were binucleated (Figure 3I). Thus, many or all R8 cells from rux mutant eye discs had not undergone cytokinesis.
Mitotic defects in R8 neurons
To explore how cell division fails in R8 neurons, mitotic figures were examined in neuronal and non-neuronal cells from fixed eye discs from wild type and rux mutant discs. Mitotic spindles were characterized using antibodies against α-tubulin. Dividing undifferentiated cells in the SMW from wild type exhibited characteristic prophase, metaphase, anaphase, and telophase morphologies that have been described in other cells, although SMW cells were smaller and embedded in a more complex tissue than S2 cells or embryonic cleavage nuclei [48]–[50](Figure 4Ai–iv). During cytokinesis the nuclear material was entirely separated in each of the daughter cells, H3p diminished, and midbody remnants of the central spindle were observed (Figure 4Av and data not shown).
Fig. 4. rux mutant R8 nuclei separate but exhibit late mitotic defects. 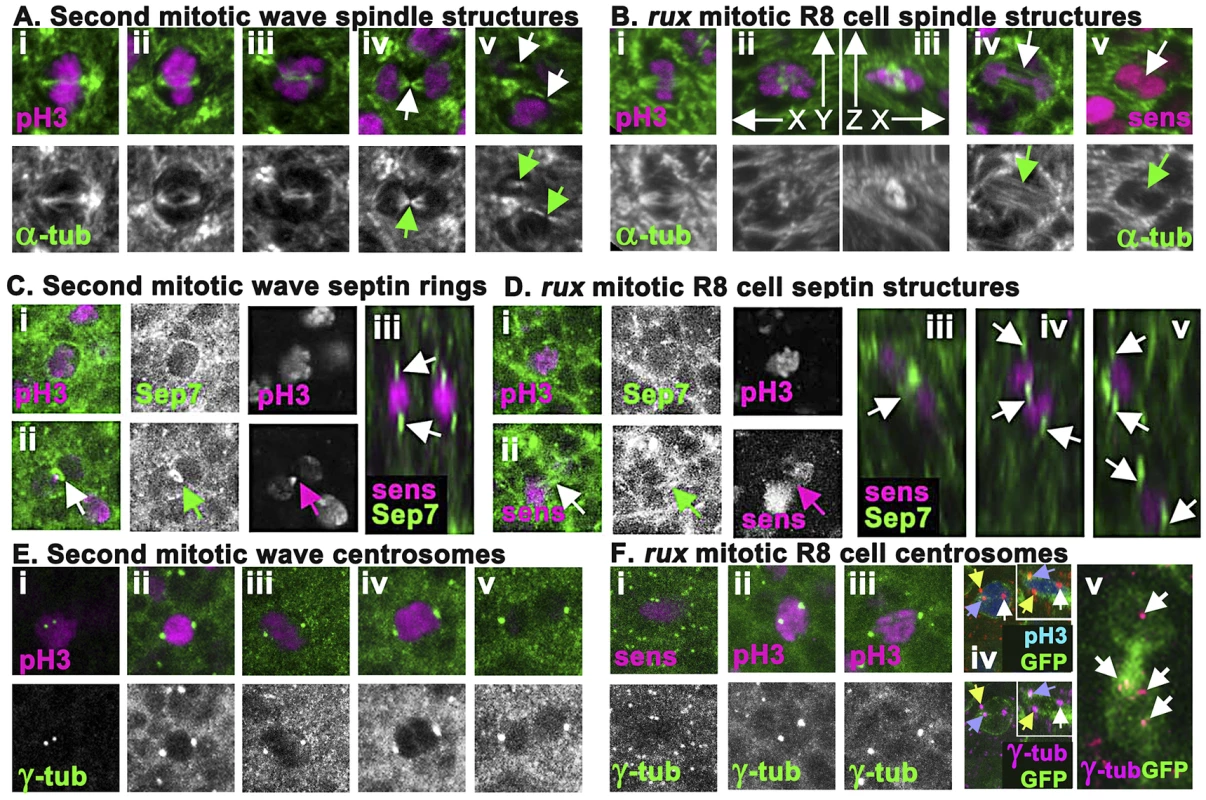
(A) Wild type second mitotic wave cells labeled with α-tubulin (green): prophase (i) metaphase (ii) early anaphase (iii), the central spindle (arrow) of a late anaphase cell (iv), and the midbodies indicative of telophase/cytokinesis (arrows) (v). (B) α-tubulin staining (green) of mitotic R8 cells from rux eye discs: a metaphase R8 cell with a spindle parallel to the epithelial surface (i), confocal section through a metaphase R8 cell with a vertically oriented spindle (ii) and a rotated reconstruction of the same cell with apical uppermost (iii). (iv) an R8 cell in anaphase in column 3. Arrow highlights absence of a midbody. (v) an R8 cell in anaphase in column 5. Arrow highlights no apparent central spindle. (C) Septin-7 (green) and H3p or Sens labeling of WT second mitotic waves cells in prophase (i) and telophase (ii). Arrow indicates contractile ring. (iii). An x-y reconstruction of the eye disc showing focal concentrations of Septin-7 apical and basal to photoreceptor nuclei. (D) Septin-7 staining of mitotic R8 cells in prophase (i). Although Septin7 sometmes seems elevated between telophase pronuclei (arrow), no contractile ring is appears (ii). (iii–v) x-y reconstructions of rux eye discs showing Septin-7 foci near photoreceptor nuclei, as in wild type. (E) Υ tubulin (green) and H3p (magenta). Prophase cell with recently duplicated centrosomes (i), prometaphase cell (ii), metaphase cell (iii), anaphase cell (iv) and telophase cell (v). Note the background of many tiny foci of Υ tubulin labeling in addition to the centrosomes, thought to represent the location of centrosomes at the apex of G1 cells [56]. (F) Υ tubulin and Sens or H3p (magenta) in R8 cells from rux eye discs: a prophase (i), prometaphase (ii), metaphase (iii). The background of small Υ tubulin foci typical of G1 interphase cells in wild type is replaced in rux by apical centrosomes of many dividing cells (i). These are less apparent in panels (ii) and (iii) because R8 nuclei are typically more basally located at these stages. (iv) A subset of mitotic R8 cells appear to contain extra centrosomes. (v) x-z reconstruction confirms that all three centrosomes share one R8 cell. (vi) A binucleated R8 cell in column 7containing multiple Υ tubulin structures (the Υ-tubulin signal has been gain amplified to reveal these weakly-stained structures). See also Figure S2. The spindle morphology of non-neuronal cells dividing in rux mutant eye discs could not be distinguished from those of wild type, except that the number of such divisions was greatly increased in rux (Figure S2). Mitotic R8 neurons in rux exhibited relatively normal spindles through metaphase (Figure 4Bi–iii), but about 20% (n = 50) had spindles oriented perpendicular to the epithelial surface (Figure 4Bii–iii), not parallel as typical of epithelial cells [51], [52]. By anaphase and telophase, however, most R8 cells lacked central spindles (compare Figure 4Biv with Figure 4Aiv). Although R8 pronuclei separated, the midbodies typical of cytokinesis were rarely present (compare Figure 4Av with Figure 4Bv). It may be noteworthy that R8 spindles appeared more normal closer to the morphogenetic furrow. Normally, mitotic cells lose their basal attachments and rise apically in the eye disc so the cells divide within the epithelial plane above the level of most other cells in the disc [14]. In rux, mitotic nuclei in R8 cells within the first few columns posterior to the morphogenetic furrow were near the apical surface, but after column 8 H3p labeled chromosome condensed in mitotic R8 cells often remained at their original location more than 5 microns from the apical disc surface (data not shown).
To monitor contractile ring dynamics, we stained third instar eye discs with antibodies against the Drosophila homolog of Septin 7 (aka Peanut), which labels contractile ring structures from membrane ingression to the completion of cytokinesis [53]. In undifferentiated cells dividing in the Second Mitotic Wave of wild type eye discs, Septin7 surrounded mitotic cells until anaphase (Figure 4Ci), when it began concentrating to a band around the central spindle that occupied <1 µ between nearly separated cells by the end of cytokinesis (Figure 4Cii). In wildtype, Septin 7 was also evident in differentiating, postmitotic photoreceptor neurons, concentrated above and below the nuclei (Figure 4Ciii).
Septin7 labeling of undifferentiated cells dividing in rux mutant eye discs was indistinguishable from wild type (Figure S2). In mitotic R8 cells, Septin7 surrounded the apical cortex (Figure 4Di), but never concentrated into tight bands between separating pronuclei, irrespective of the division axis (Figure 4Dii). After division, Septin 7 concentrated in juxtanuclear foci in the binucleated R8 cells (Figure 4Diii–v). These findings corroborate the conclusion from α-tubulin labeling that mitotic R8 neurons are abnormal by telophase and lack structures typical of cytokinesis.
To characterize the centrosomes we stained Υ-tubulin with antibody GTU-88. Υ-tubulin is associated with pericentric material surrounding centromeres where it acts to nucleate and anchor microtubules to orient and regulate the spindle [54], [55]. This antibody also weakly detailed spindles, and post-mitotic eye disc cells in wild type exhibit a weak apical spot of Υ-tubulin thought to represent the location of the centrosome in G1 cells [56] (Figure 4E). In wild type eye discs, second mitotic wave cells exhibited duplicated centrosomes around column 2; these increased in size and separated to define opposite poles of the mitotic spindle during mitotic prophase, metaphase and anaphase (Figure 4Ei–iv). The centrosomes separated into daughter cells with cytokinesis (Figure 4Ev), then Υ-tubulin labeling faded.
In rux discs, centrosome dynamics of non-neuronal cells resembled that of wild type (Figure S2). The centrosomes of mitotic R8 neurons mostly followed a similar mitotic program (Figure 4Fi–iii), with the exception that about 20% appeared to have one or more extra centrosomes based on Υ-tubulin labeling (Figure 4Fiv). Extra centrosomes might result from the ability of Cyclin A/CDK1 to promote centrosome nucleation [57]. They were not associated with multipolar spindles (data not shown). In rux R8 neurons after column 7 the weaker Υ-tubulin labeling typical of G1 cells was usually observed just apical to each nucleus, and some cells contained multiple such puncta (Figure 4Fv).
Kinesin Heavy Chain mediates movement of R8 nuclei
A striking feature of the rux mutant phenotype was the behavior of R8 nuclei after mitosis. In the majority of R8 cells, one nucleus retained a normal position in the cell body while the other traveled basally and into the developing axon (Figure 3B). Accordingly, the number of basal nuclei progressively increased in the posterior region of rux eye discs (Figure 3E), and R8 nuclei were even found in the optic stalk, the conduit for retinal axons to the optic lobe (Figure 5A, 5B).
Fig. 5. Kinesin and Dynein regulate nuclear position in post mitotic rux R8 cells. 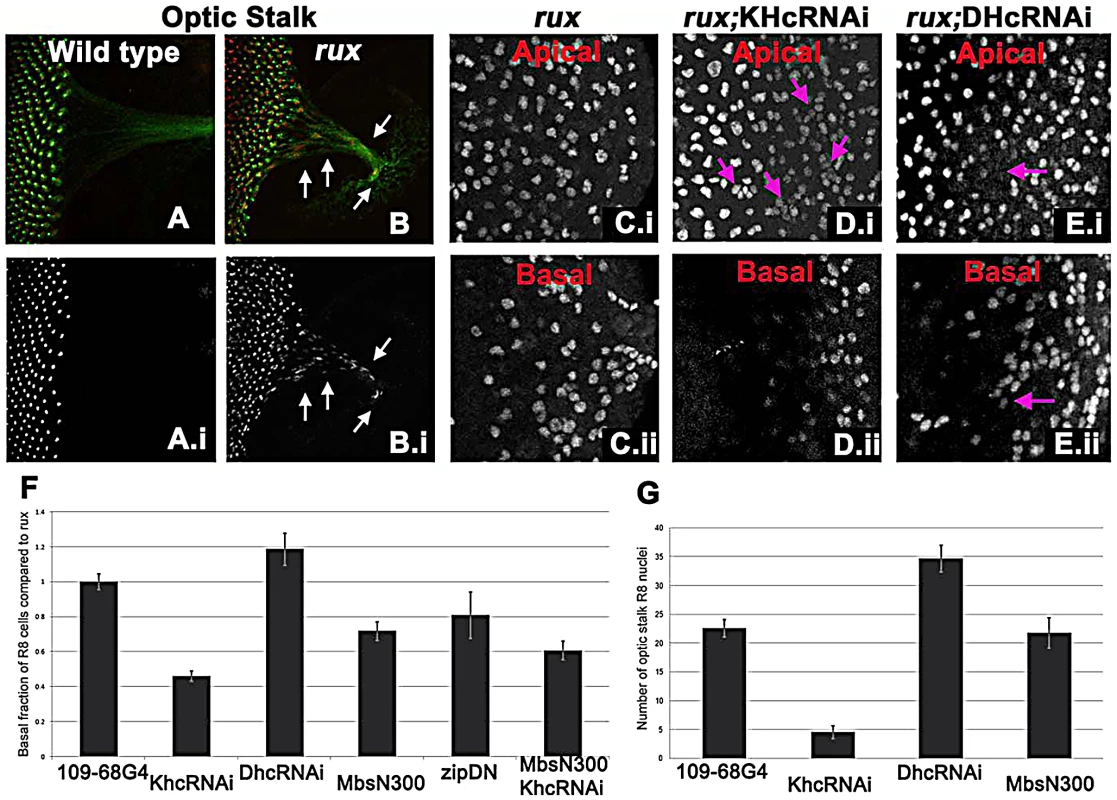
(A–B) Wild type (A) and rux (B) eye discs and optic stalks labeled with anti-Sens (red) and anti-Futsch (mAb22C10; green). Arrows showing R8 cells in the optic stalk in rux. (C) R8 cells from the apical (i) and basal (ii) portions of rux eye discs expressing GFP. (D) Coexpressing dsRNA for KHC retains R8 nuclei apically (i) while fewer drop basally (ii). Arrows indicate clusters of R8 nuclei apically. (E) Coexpressing dsRNA for DHC leads to apical regions lacking R8 nuclei (arrow in (i) while R8 nuclei accumulate basally (arrow in ii). (F). Results are quantified as the percentage of R8 nuclei observed in the bottom third of the eye disc (in all cases n>2000, from at least 5 different eye discs) and have been normalized to rux alone (35%). bars indicate standard errors. (G) The average number of R8 nuclei identified in the optic stalk of rux mutants, and discs expressing inhibitors of molecular motors under the control of G109-68-Gal4 (n>5 in all cases, bars indicate standard error). To determine if a motor acts on R8 nuclei, kinesin, dynein, and myosin function were inhibited in rux mutant R8 neurons. To investigate the role of kinesin, a plus-end microtubule motor, dsRNA against Kinesin heavy chain (Khc) was expressed in R8 cells using 109-68 Gal4. This had no effect on the morphology of photoreceptors in the wild type background (data not shown). In rux, we quantified the proportion of R8 nuclei found in the basal one-third of the eye disc posterior to column 10, including the axons that run along the base of the epithelium (Figure 5C). Khc dsRNA reduced the number of basal R8 nuclei in rux by more than half (Figure 5D, 5F). The total number of R8 nuclei was unaffected, resulting in more R8 nuclei clustered at the apical surface (Figure 5D). Knocking down Khc also reduced the number of R8 nuclei that migrate into the optic stalk by over 80% (Figure 5G). Similar results were obtained with two independent dsRNA constructs (data not shown).
Dynein knockdown in R8 cells had no effect on R8 nuclear position in the wild type (data not shown). In rux larvae, dsRNAi against Dhc increased the basal R8 nuclei by nearly 20% (Figure 5E, 5F). As some apical disc regions lacked R8 nuclei (Figure 5E) it is likely that both daughter nuclei became basal in some R8 cells. The number of R8 nuclei in the optic stalk increased (Figure 5G), suggesting an increased rate of movement.
We also inhibited Myosin, the primary source of mechanical force in the contractile ring, by expressing MbsN300, a constitutively active form of the myosin binding subunit (Mbs) a central component of myosin phosphatase [58]. MbsN300 expression in R8 cells reduced basal R8 nuclei in rux by almost 30% (Figure 5F). A similar result was obtained after expressing dominant-negative Myosin II (zipDN), but there was no additional effect of doubling the MbsN300 transgene copy number, or coexpressing MbsN300 with Khc dsRNA (Figure 5F and data not shown).
Taken together, these results suggest that the nuclei in rux R8 neurons are moved into and through axons by kinesin, acting in opposition to dynein. Myosin II may be contribute to all movement, and affect the location of nuclei in rux R8 cells indirectly.
The Anaphase Promoting Complex/Cyclosome also maintains cell cycle exit of R8 cells
Cyclins A and B activities are kept low in most G1 cells by the Anaphase Promoting Complex/Cyclosome (APC/C), a ubiquitin ligase complex that normally remains active until the G1-S transition [59]. Viable hypomorphic mutations of APC/C subunits Cdh1 and APC1 cause rough eye phenotypes [60]–[62]. Mitotic neurons had not previously been described in cdh1 mutations. Very similar to the situation for rux, mutations in cdh1 (also known as retina aberrant in pattern: rap) increase cycling of undifferentiated progenitors, and yet mosaic analysis shows that the adult eye defects in rap mutants are caused by a requirement in R8 neurons [60], [61]. We report that in eye discs mutant for the cdh1G0418 allele, many R8 cells labeled with the mitotic marker H3p (Figure 6A, 6B). Pairs of apparent postmitotic R8 nuclei were abundant. R8 nuclei moved basally in the eye disc and into axons and the optic stalk (Figure 6C,D) and R8 cells were binucleated (Figure 6E). Thus it appears that just as for rux, mutations in cdh1 affect not only undifferentiated progenitor cells, but also return the R8 neurons to the cell cycle, which is responsible for the major patterning defects. Like rux and rap, the shtd3 mutant allele of Apc1 exhibits cell cycle re-entry by R8 cells [62]. We find that in shtd3 mutant eye discs, post-mitotic nuclei are also transported basally in the R8 cells and into the axons of the optic stalk (Figure 6F, 6G), and binucleated R8 cells were seen (Figure 6H). Taken together these data indicate that rux, cdh1 and apc1 are all required to prevent R8 neurons from re-entering the cell cycle, and that cell cycle entry always leads to a similar phenotype of acytokinetic mitosis and nuclear mislocalization in these three mutants.
Fig. 6. APC1 and Cdh1 maintain cell cycle exit. 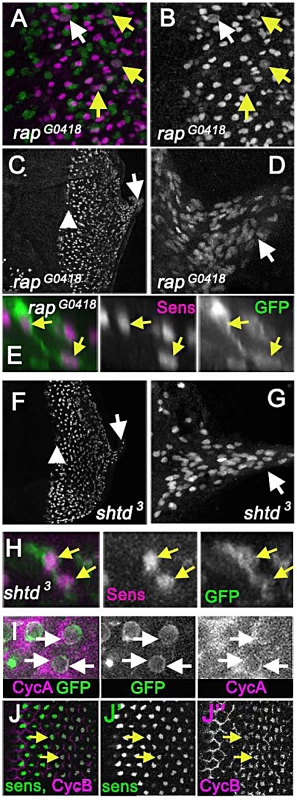
(A) Eye disc carrying the rapG0418 mutation in the cdh1 locus labelled for Senseless (magenta) and mitotic figures (anti-H3p in green) showing mitotic R8 cells (arrows). R8 neurons appeared to enter the cell cycle earlier than in rux, so that nearly 10% of intermediate groups contained a mitotic cell and 20% of R8 cells in column 0 labeled with H3p (white arrow). (B) Sens channel reveals mitotic R8 nuclei (arrows). (C) R8 cells become disorganized soon after the morphogenetic furrow (arrow head). R8 nuclei can be seen in the optic stalk (arrow). (D) R8 nuclei labeled with sens in the optic stalk of a rapG0418 eye disc. (E) A binucleated rapG0418 mutant R8 cell expressing GFP (green) and labeled for Sens (magenta). (F) Senseless staining of an eye discs carrying the shtd3 mutation in the apc1 locus. R8 cells become disorganized soon after the morphogenetic furrow (arrow head). R8 nuclei can be seen in the optic stalk (arrow). (G) Enlargement of shtd3 optic stalk. (H) A binucleated rapG0418 mutant R8 cell expressing GFP (green) and labeled for Sens (magenta). (I) Cytoplasmic Cyclin A (magenta) is observed around the R8 nuclei in columns 0 and 1 (arrows), in addition to the labeling of unspecified cells in the second mitotic wave observed previously [28]. R8 cells labeled by 109-68>GFP (green). (J) Cyclin B staining (magenta) co-localizes with senseless (green) staining in wild type eye discs as beginning in column 4, and in all R8 cells by column 6 (arrows). Cyclin B appears nuclear in R8 cells. Multiple cell cycle proteins are expressed in wild-type R8 neurons
Cyclin A could sometimes be detected in R8 cells in columns 0 and 1 close to the furrow, the stage where R8 cells enter S phase in rux mutants (Figure 6I), highlighting the importance of mechanisms to prevent inappropriate cell cycle entry. When eye discs were labeled with antibodies for cdk1 and cdk2, these proteins were seen to be ubiquitous in both neurons and progenitor cells (data not shown). It is already known that Cyclin E accumulates in post-mitotic photoreceptor neuron precursors [3]. Unexpectedly, Cyclin B antibody clearly labels wild type post-mitotic R8 cells after column 5 in the wild type (Figure 6J). Neuronal expression of all these proteins, thought to be specific for the cell cycle, is surprising and emphasizes the importance of post-translational suppression of the cell cycle to these neurons.
Discussion
Here we show that the rux mutation produces a defective Drosophila eye because of cell cycle re-entry by differentiating photoreceptor neurons of the R8 class. The rux gene encodes a CDI that affects the stability and function of Cyclin A [30], [39]. Cyclin A is normally required in G2/M in Drosophila, but if present in G1 can obviate the requirement for Cyclin E/Cdk2 activity to initiate S phase [29], [35]. Although rux predominantly affects the eye and male germline, and does not appear to be conserved in vertebrates [63], failure to maintain R8 cell cycle arrest was also seen in hypomorphic mutant alleles of two components of the Anaphase Promoting Complex, APC1 and Cdh1, which are conserved and function similarly in most cell types [59]. APC/C is active in G1 cells until inactivated by Cyclin E/Cdk2 at the G1/S transition, and so also renders Cyclin A unstable in G1 [64]–[66]. These findings support a critical role for eliminating Cyclin A after the terminal mitosis to ensure cell cycle exit by differentiating R8 photoreceptor neurons and by other classes of retinal photoreceptor neuron, which is achieved by APC/C and rux together.
The role of APC/C in maintaining cell cycle exit has recently been highlighted in terminally differentiated epidermal cells and non-neuronal cells of the retina [61], [67]. Rux maintains cell cycle exit by non-neuronal cells in the eye also [28]. Mitotic R8 neurons differed from non-neuronal cells of the same genotypes by performing a nuclear mitosis without cytokinesis. There was no evidence that Cyclin A itself prevents cytokinesis, because none of the non-neuronal cells driven into ectopic cell divisions in the rux mutant exhibited abnormalities in cell division (Figure S1), and because previous studies have found that stabilizing Cyclin A does not perturb cytokinesis unless the stabilized forms of Cyclin A are over-expressed [36]. We think that failure to execute cytokinesis likely represents an independent barrier to cell division in these neurons, downstream of the G1 exit that is sensitive to Cyclin A.
Perhaps surprisingly, R8 neurons assembled mitotic spindles that appeared to segregate chromosomes relatively normally. Although other abnormalities were noted, the main findings were the absence of proper contractile ring structures and the rarity and disorganization of central spindles and midbodies typical of anaphase and telophase, which might be related. In tissue culture studies of mitosis, treatments that delay cytokinesis at this stage often abort cell division with subsequent development of polyploid cells, similar to the binuclear R8 cells [68]–[70]. We surmise that the differentiation program of neuronal R8 cells precludes normal spindle development and contractile ring formation. This could be related to axon formation, which begins at about the same time R8 cells enter mitosis in the rux mutants [23]. Acytokinetic mitosis has also been reported, however, after inactivation of Rb in post-mitotic inner ear hair cells, a cell type that is excitable but lacks an axon [71]. Another feature of mitotic R8 neurons is that they remain anchored to the basal surface of the eye imaginal disc during their abnormal cell cycle (see Figure 3), unlike the normal divisions of unspecified cells that lose their basal footing during cytokinesis [72].
A striking feature of most mitotic R8 cells is the migration of a daughter nucleus into the developing photoreceptor cell axon. We considered the possibility that the cells remain in an extended telophase, transporting one nucleus into the axon as if to the furthest end of the cell in preparation for cytokinesis, but rule this mechanism out for two reasons. In dividing cells the pronuclei are separated by dynein pulling microtubules towards spindle poles, reeling them in towards the centrosome [73]. If such a mechanism were moving nuclei in R8 cells, then knocking down dynein heavy chain would suppress nuclear movement. In contrast to this expectation, we observed more rapid nuclear movement when dynein was knocked down, and in some cells both daughter nuclei were found basally. In addition, centrosomes were generally observed apical to R8 cell nuclei at this stage (Figure 4Biv–v), inconsistent with a role moving nuclei in a telophase-like process. We think it unlikely that Rux protein plays a direct role in locating the nucleus, both because one nucleus is always unaffected in rux mutant R8 cells, and because rux mutations are not dominant modifiers of Glued, a dynactin mutant that affects the position of photoreceptor nuclei ([74] and data not shown) By contrast, the nuclear phenotype was suppressed by reducing kinesin heavy chain expression, suggesting that anterograde transport was the transport mechanism.
Anterograde transport of photoreceptor nuclei has been reported in both Drosophila and zebrafish when the dynactin complex is inhibited [74]–[77]. It has been proposed that Dynein and the dynactin complex maintain the position of the nucleus in the photoreceptor cell body, opposing the tendency of kinesins to move the nucleus into the axons [76]. Our observations suggest a more complicated model, since the two nuclei within each cell usually behave differently from one another. We suggest that there may be a single dynactin-dependent berth or niche in the cell body that a second nucleus cannot often share.
Although it is often suggested that cell cycle entry is lethal for neurons, polyploid neurons exist, and It has been suggested that mammalian neurons that re-enter the cell cycle can remain viable by arresting in G2 [4]. In one model, eventual breakdown of the arrest and subsequent entry into M phase is hypothesized as a cause of chronic neuronal death in Alzheimer's Disease [4], [9]. Apparently binucleated neurons resembling mitotic R8 cells in rux have been reported in hippocampus from Alzheimer's Disease patients, although rarely [78]. Our findings demonstrate an unexpected connection between failure to exit the cell cycle and axonal trafficking. Defects in axonal trafficking have been implicated in many types of neurodegeneration [10]–[12]. Like rux, other mutations in which photoreceptor nuclei are relocated to axons also result in retinal degeneration [74]–[77]. It will now be necessary to determine why mitotic neurons do not form cleavage structures, whether abnormal cytokinesis and nuclear trafficking occur in mitotic neurons of other types, and if such secondary defects of mitotic neurons contribute to neurodegeneration.
Materials and Methods
Drosophila husbandry and strains
All experiments were conducted at 25 C.
The following fly strains were used in the experiments described above:
y1 cv1 rux8/FM7i, p{ActGFPJMR3} [28]; y1 w*; P{GawB}sca109-68 (Chien et al., 1996); y1 w*; P{w+mC = UAS-mCD8::GFP.L}LL5 [45]; w; UAS-MbsN300 [58]; y1 v1; P{y+t7.7 v+t1.8 = TRiP.JF01939}attP2 (KhcRNAi) [79];b1 pr1 Khc8/Cyo [80]; w1118; y1 w*; P{Sep2-GFP.SG}3 [81]; w67c23 P{lacW}rapG0418/FM7a [82]y1 w1118 shtd3/FM7i, P{ActGFP}JMR3 [62]; P{GD12258}v28053 and w1118; P{GD12278}v44338 [37; VDRC]; cycAC8LR1.
Immunohistochemistry
Antibody labeling was conducted as previously described [83]. Antibodies: Guinea pig-anti Sensless [20]; mAb22C10; mouse anti-PNUT (mAb4C9H4) [53]; mouse anti-Cyclin B (mAbF2F4) and mouse anti-Cyclin A (mAbA12) [33]; rabbit anti-phospho-Histone 3 (Upstate Laboratories); rat-anti GFP (Nacalia, USA); mouse-anti-tubulin-α - Ab-2 (Thermo Scientific); mouse anti-Υ-tubulin, Clone GTU-88 (Sigma Aldrich). Secondary antibodies were conjugated to Cy2, Cy3, or Cy5 (Jackson ImmunoResearch Laboratories). Confocal microscopy was conducted using BioRad 2000, Leica SP2 or Leica SP5 instruments. Single channels were collected and analyzed separately in NIH ImageJ1.6 software, and figures were arranged in Photoshop.
Cell dissociation assays
Eye imaginal discs were dissected and separated from antennal discs in PBS buffer and transferred to 4 ml Trypsin EDTA (Sigma) in PBS, containing Draq5 (diluted 1∶500; Bio Status Limited) in batches of 10–20 and dissociated as previously described [47]. Partial dissociated samples were imaged for 30–45 minutes after 90 minutes of gentle nutation at room temperature, and discs were fully dissociated after 150 minutes of gentle nutation. Epi-fluorescence imaging was carried out using on DeltaVision Core, with an mCherry/GFP filter set.
Supporting Information
Zdroje
1. AjiokaI, DyerMA (2008) A new model of tumor susceptibility following tumor suppressor gene inactivation. Cell Cycle 7 : 735–740.
2. AjiokaI, MartinsRA, BayazitovIT, DonovanS, JohnsonDA, et al. (2007) Differentiated horizontal interneurons clonally expand to form metastatic retinoblastoma in mice. Cell 131 : 378–390.
3. FirthLC, BakerNE (2005) Extracellular signals responsible for spatially regulated proliferation in the differentiating Drosophila eye. Developmental Cell 8 : 541–551.
4. Lopez-SanchezN, Ovejero-BenitoMC, BorregueroL, FradeJM (2011) Control of neuronal ploidy during vertebrate development. Results Probl Cell Differ 53 : 547–563.
5. HeintzN (1993) Cell death and the cell cycle: a relationship between transformation and neurodegeneration? Trends in Biochemical Sciences 18 : 157–159.
6. ZhuX, RainaAK, PerryG, SmithMA (2004) Alzheimer's disease: the two-hit hypothesis. Lancet Neurol 3 : 219–226.
7. HerrupK, YangY (2007) Cell cycle regulation in the postmitotic neuron: oxymoron or new biology? Nature Reviews Neuroscience 8 : 368–378.
8. HoglingerGU, BreunigJJ, DepboyluC, RouauxC, MichelPP, et al. (2007) The pRb/E2F cell-cycle pathway mediates cell death in Parkinson's disease. Proceedings of the National Academy of Sciences of the United States of America 104 : 3585–3590.
9. MohC, KubiakJZ, BajicVP, ZhuX, SmithMA, et al. (2011) Cell cycle deregulation in the neurons of Alzheimer's disease. Results Probl Cell Differ 53 : 565–576.
10. DuncanJE, GoldsteinLS (2006) The genetics of axonal transport and axonal transport disorders. PLoS Genet 2: e124 doi:10.1371/journal.pgen.0020124.
11. IkedaY, DickKA, WeatherspoonMR, GincelD, ArmbrustKR, et al. (2006) Spectrin mutations cause spinocerebellar ataxia type 5. Nature Genetics 38 : 184–190.
12. StokinGB, GoldsteinLS (2006) Axonal transport and Alzheimer's disease. Annual Review of Biochemistry 75 : 607–627.
13. LorenzoDN, LiMG, MischeSE, ArmbrustKR, RanumLP, et al. (2010) Spectrin mutations that cause spinocerebellar ataxia type 5 impair axonal transport and induce neurodegeneration in Drosophila. The Journal of Cell Biology 189 : 143–158.
14. ReadyDF, HansonTE, BenzerS (1976) Development of the Drosophila retina, a neurocrystalline lattice. Developmental Biology 53 : 217–240.
15. Wolff T, Ready DF (1993) Pattern formation in the Drosophila retina. In: M. Bate AMA, editor. The Development of Drosophila melanogaster. Cold Spring Harbor, NY Cold Spring Harbor Laboratory Press. pp. 1277–1325.
16. BakerNE (2007) Patterning signals and proliferation in Drosophila imaginal discs. Current Opinion in Genetics & Development 17 : 287–293.
17. MlodzikM, BakerNE, RubinGM (1990) Isolation and expression of scabrous, a gene regulating neurogenesis in Drosophila. Genes & Development 4 : 1848–1861.
18. JarmanAP, GrellEH, AckermanL, JanLY, JanYN (1994) Atonal is the proneural gene for Drosophila photoreceptors. Nature 369 : 398–400.
19. JarmanAP, SunY, JanLY, JanYN (1995) Role of the proneural gene, atonal, in formation of Drosophila chordotonal organs and photoreceptors. Development 121 : 2019–2030.
20. NoloR, AbbottLA, BellenHJ (2000) Senseless, a Zn finger transcription factor, is necessary and sufficient for sensory organ development in Drosophila. Cell 102 : 349–362.
21. BakerNE (2002) Notch and the patterning of ommatidial founder cells in the developing Drosophila eye. Results and Problems in Cell Differentiation 35–58.
22. FrankfortBJ, MardonG (2002) R8 development in the Drosophila eye: a paradigm for neural selection and differentiation. Development 129 : 1295–1306.
23. TomlinsonA, ReadyDF (1987) Neuronal differentiation in Drosophila ommatidium. Developmental Biology 120 : 366–376.
24. FreemanM (1997) Cell determination strategies in the Drosophila eye. Development 124 : 261–270.
25. Gaul U, editor (2002) The establishment of retinal connectivity Heidelberg, Berlin: Springer Verlag. 205–218 p.
26. TaylerTD, GarrityPA (2003) Axon targeting in the Drosophila visual system. Current Opinion in Neurobiology 13 : 90–95.
27. GonczyP, ThomasBJ, DiNardoS (1994) roughex is a dose-dependent regulator of the second meiotic division during Drosophila spermatogenesis. Cell 77 : 1015–1025.
28. ThomasBJ, GunningDA, ChoJ, ZipurskyL (1994) Cell cycle progression in the developing Drosophila eye: roughex encodes a novel protein required for the establishment of G1. Cell 77 : 1003–1014.
29. DongX, ZavitzKH, ThomasBJ, LinM, CampbellS, et al. (1997) Control of G1 in the developing Drosophila eye: rca1 regulates Cyclin A. Genes & Development 11 : 94–105.
30. ThomasBJ, ZavitzKH, DongX, LaneME, WeigmannK, et al. (1997) roughex down-regulates G2 cyclins in G1. Genes & Development 11 : 1289–1298.
31. FoleyE, O'FarrellPH, SprengerF (1999) Rux is a cyclin-dependent kinase inhibitor (CKI) specific for mitotic cyclin-Cdk complexes. Current Biology : CB 9 : 1392–1402.
32. FoleyE, SprengerF (2001) The cyclin-dependent kinase inhibitor Roughex is involved in mitotic exit in Drosophila. Current Biology : CB 11 : 151–160.
33. KnoblichJA, LehnerCF (1993) Synergistic action of Drosophila cyclins A and B during the G2-M transition. The EMBO Journal 12 : 65–74.
34. KnoblichJA, SauerK, JonesL, RichardsonH, SaintR, et al. (1994) Cyclin E controls S phase progression and its down-regulation during Drosophila embryogenesis is required for the arrest of cell proliferation. Cell 77 : 107–120.
35. SprengerF, YakubovichN, O'FarrellPH (1997) S-phase function of Drosophila cyclin A and its downregulation in G1 phase. Current Biology : CB 7 : 488–499.
36. JacobsHW, KeidelE, LehnerCF (2001) A complex degradation signal in Cyclin A required for G1 arrest, and a C-terminal region for mitosis. The EMBO Journal 20 : 2376–2386.
37. DietzlG, ChenD, SchnorrerF, SuKC, BarinovaY, et al. (2007) A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448 : 151–156.
38. de NooijJC, HariharanIK (1995) Uncoupling cell fate determination from patterned cell division in the Drosophila eye. Science 270 : 983–985.
39. AvedisovSN, KrasnoselskayaI, MortinM, ThomasBJ (2000) Roughex mediates G(1) arrest through a physical association with cyclin A. Mol Cell Biol 20 : 8220–8229.
40. HarperJW, AdamiGR, WeiN, KeyomarsiK, ElledgeSJ (1993) The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 75 : 805–816.
41. HarperJW, ElledgeSJ, KeyomarsiK, DynlachtB, TsaiLH, et al. (1995) Inhibition of cyclin-dependent kinases by p21. Molecular Biology of the Cell 6 : 387–400.
42. RussoAA, JeffreyPD, PattenAK, MassagueJ, PavletichNP (1996) Crystal structure of the p27Kip1 cyclin-dependent-kinase inhibitor bound to the cyclin A-Cdk2 complex. Nature 382 : 325–331.
43. WangY, FisherJC, MathewR, OuL, OtienoS, et al. (2011) Intrinsic disorder mediates the diverse regulatory functions of the Cdk inhibitor p21. Nature Chemical Biology 7 : 214–221.
44. EvansT, RosenthalET, YoungblomJ, DistelD, HuntT (1983) Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell 33 : 389–396.
45. LeeT, LuoL (1999) Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22 : 451–461.
46. WhiteNM, JarmanAP (2000) Drosophila atonal controls photoreceptor R8-specific properties and modulates both receptor tyrosine kinase and Hedgehog signalling. Development 127 : 1681–1689.
47. de la CruzAF, EdgarBA (2008) Flow cytometric analysis of Drosophila cells. Methods in Molecular Biology 420 : 373–389.
48. Foe V, Odell GM, Edgar B (1993) Mitosis and morphegenesis in the Drosophila embryo. Point and counterpoint. In: M. Bate AMA, editor. The Development of Drosophila melanogaster. Cold Spring Harbor, NY Cold Spring Harbor Laboratory Press. pp. 1277–1325.
49. KarsentiE, VernosI (2001) The mitotic spindle: a self-made machine. Science 294 : 543–547.
50. GoshimaG (2010) Assessment of mitotic spindle phenotypes in Drosophila S2 cells. Methods in Cell Biology 97 : 259–275.
51. LuB, RoegiersF, JanLY, JanYN (2001) Adherens junctions inhibit asymmetric division in the Drosophila epithelium. Nature 409 : 522–525.
52. den ElzenN, ButteryCV, MaddugodaMP, RenG, YapAS (2009) Cadherin adhesion receptors orient the mitotic spindle during symmetric cell division in mammalian epithelia. Molecular Biology of the Cell 20 : 3740–3750.
53. NeufeldTP, RubinGM (1994) The Drosophila peanut gene is required for cytokinesis and encodes a protein similar to yeast putative bud neck filament proteins. Cell 77 : 371–379.
54. StearnsT, EvansL, KirschnerM (1991) Gamma-tubulin is a highly conserved component of the centrosome. Cell 65 : 825–836.
55. RaffJW, KelloggDR, AlbertsBM (1993) Drosophila gamma-tubulin is part of a complex containing two previously identified centrosomal MAPs. The Journal of Cell Biology 121 : 823–835.
56. BastoR, LauJ, VinogradovaT, GardiolA, WoodsCG, et al. (2006) Flies without centrioles. Cell 125 : 1375–1386.
57. BuendiaB, DraettaG, KarsentiE (1992) Regulation of the microtubule nucleating activity of centrosomes in Xenopus egg extracts: role of cyclin A-associated protein kinase. The Journal of Cell Biology 116 : 1431–1442.
58. LeeA, TreismanJE (2004) Excessive Myosin activity in mbs mutants causes photoreceptor movement out of the Drosophila eye disc epithelium. Molecular Biology of the Cell 15 : 3285–3295.
59. AcquavivaC, PinesJ (2006) The anaphase-promoting complex/cyclosome: APC/C. Journal of Cell Science 119 : 2401–2404.
60. KarpilowJ, KolodkinA, BorkT, VenkateshT (1989) Neuronal development in the Drosophila compound eye: rap gene function is required in photoreceptor cell R8 for ommatidial pattern formation. Genes & Development 3 : 1834–1844.
61. PimentelAC, VenkateshTR (2005) rap gene encodes Fizzy-related protein (Fzr) and regulates cell proliferation and pattern formation in the developing Drosophila eye-antennal disc. Developmental Biology 285 : 436–446.
62. Tanaka-MatakatsuM, ThomasBJ, DuW (2007) Mutation of the Apc1 homologue shattered disrupts normal eye development by disrupting G1 cell cycle arrest and progression through mitosis. Developmental Biology 309 : 222–235.
63. AvedisovSN, RogozinIB, KooninEV, ThomasBJ (2001) Rapid evolution of a cyclin A inhibitor gene, roughex, in Drosophila. Molecular Biology and Evolution 18 : 2110–2118.
64. LukasC, SorensenCS, KramerE, Santoni-RugiuE, LindenegC, et al. (1999) Accumulation of cyclin B1 requires E2F and cyclin-A-dependent rearrangement of the anaphase-promoting complex. Nature 401 : 815–818.
65. BlancoMA, Sanchez-DiazA, de PradaJM, MorenoS (2000) APC(ste9/srw1) promotes degradation of mitotic cyclins in G(1) and is inhibited by cdc2 phosphorylation. The EMBO Journal 19 : 3945–3955.
66. BakerNE, YuSY (2001) The EGF receptor defines domains of cell cycle progression and survival to regulate cell number in the developing Drosophila eye. Cell 104 : 699–708.
67. ButtittaLA, KatzaroffAJ, EdgarBA (2010) A robust cell cycle control mechanism limits E2F-induced proliferation of terminally differentiated cells in vivo. The Journal of Cell Biology 189 : 981–996.
68. KosakoH, YoshidaT, MatsumuraF, IshizakiT, NarumiyaS, InagakiM (2000) Rho-kinase/ROCK is involved in cytokinesis through the phosphorylation of myosin light chain and not ezrin/radixin/moesin proteins at the cleavage furrow. Oncogene 19 : 6059–6064.
69. SommaMP, FasuloB, CenciG, CundariE, GattiM (2002) Molecular dissection of cytokinesis by RNA interference in Drosophila cultured cells. Molecular Biology of the Cell 13 : 2448–2460.
70. ShiQ, KingRW (2005) Chromosome nondisjunction yields tetraploid rather than aneuploid cells in human cell lines. Nature 437 : 1038–1042.
71. WeberT, CorbettMK, ChowLM, ValentineMB, BakerSJ, et al. (2008) Rapid cell-cycle reentry and cell death after acute inactivation of the retinoblastoma gene product in postnatal cochlear hair cells. Proceedings of the National Academy of Sciences of the United States of America 105 : 781–785.
72. TomlinsonA (1985) The cellular dynamics of pattern formation in the eye of Drosophila. Journal of Embryology and Experimental Morphology 89 : 313–331.
73. SharpDJ, RogersGC, ScholeyJM (2000) Cytoplasmic dynein is required for poleward chromosome movement during mitosis in Drosophila embryos. Nature Cell Biology 2 : 922–930.
74. FanSS, ReadyDF (1997) Glued participates in distinct microtubule-based activities in Drosophila eye development. Development 124 : 1497–1507.
75. AllenMJ, ShanX, CaruccioP, FroggettSJ, MoffatKG, et al. (1999) Targeted expression of truncated glued disrupts giant fiber synapse formation in Drosophila. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience 19 : 9374–9384.
76. WhitedJL, CassellA, BrouilletteM, GarrityPA (2004) Dynactin is required to maintain nuclear position within postmitotic Drosophila photoreceptor neurons. Development 131 : 4677–4686.
77. TsujikawaM, OmoriY, BiyanwilaJ, MalickiJ (2007) Mechanism of positioning the cell nucleus in vertebrate photoreceptors. Proceedings of the National Academy of Sciences of the United States of America 104 : 14819–14824.
78. ZhuX, SiedlakSL, WangY, PerryG, CastellaniRJ, et al. (2008) Neuronal binucleation in Alzheimer disease hippocampus. Neuropathol Appl Neurobiol 34 : 457–465.
79. Perkins LA, Shim HS, Perrimon N. Initial TRiP stock collection.
80. SaxtonWM, HicksJ, GoldsteinLS, RaffEC (1991) Kinesin heavy chain is essential for viability and neuromuscular functions in Drosophila, but mutants show no defects in mitosis. Cell 64 : 1093–1102.
81. Hales K. (2008) P{Sep2-GFP.SG}3.
82. BierE, VaessinH, ShepherdS, LeeK, McCallK, et al. (1989) Searching for pattern and mutation in the Drosophila genome with a P-lacZ vector. Genes & Development 3 : 1273–1287.
83. FirthLC, LiW, ZhangH, BakerNE (2006) Analyses of RAS regulation of eye development in Drosophila melanogaster. Methods in Enzymology 407 : 711–721.
Štítky
Genetika Reprodukční medicína
Článek The Covariate's DilemmaČlánek Plant Vascular Cell Division Is Maintained by an Interaction between PXY and Ethylene SignallingČlánek Lessons from Model Organisms: Phenotypic Robustness and Missing Heritability in Complex Disease
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 11- Příjem alkoholu a menstruační cyklus
- Mateřský haplotyp KIR ovlivňuje porodnost živých dětí po transferu dvou embryí v rámci fertilizace in vitro u pacientek s opakujícími se samovolnými potraty nebo poruchami implantace
- Intrauterinní inseminace a její úspěšnost
- Růst a vývoj dětí narozených pomocí IVF
- Akutní intermitentní porfyrie
-
Všechny články tohoto čísla
- Inference of Population Splits and Mixtures from Genome-Wide Allele Frequency Data
- The Covariate's Dilemma
- Plant Vascular Cell Division Is Maintained by an Interaction between PXY and Ethylene Signalling
- Plan B for Stimulating Stem Cell Division
- Discovering Thiamine Transporters as Targets of Chloroquine Using a Novel Functional Genomics Strategy
- Is a Modifier of Mutations in Retinitis Pigmentosa with Incomplete Penetrance
- Evolutionarily Ancient Association of the FoxJ1 Transcription Factor with the Motile Ciliogenic Program
- Genome Instability Caused by a Germline Mutation in the Human DNA Repair Gene
- Transcription Factor Oct1 Is a Somatic and Cancer Stem Cell Determinant
- Controls of Nucleosome Positioning in the Human Genome
- Disruption of Causes Defective Meiotic Recombination in Male Mice
- A Novel Human-Infection-Derived Bacterium Provides Insights into the Evolutionary Origins of Mutualistic Insect–Bacterial Symbioses
- Trps1 and Its Target Gene Regulate Epithelial Proliferation in the Developing Hair Follicle and Are Associated with Hypertrichosis
- Zcchc11 Uridylates Mature miRNAs to Enhance Neonatal IGF-1 Expression, Growth, and Survival
- Population-Based Resequencing of in 10,330 Individuals: Spectrum of Genetic Variation, Phenotype, and Comparison with Extreme Phenotype Approach
- HP1a Recruitment to Promoters Is Independent of H3K9 Methylation in
- Transcription Elongation and Tissue-Specific Somatic CAG Instability
- A Germline Polymorphism of DNA Polymerase Beta Induces Genomic Instability and Cellular Transformation
- Interallelic and Intergenic Incompatibilities of the () Gene in Mouse Hybrid Sterility
- Comparison of Mitochondrial Mutation Spectra in Ageing Human Colonic Epithelium and Disease: Absence of Evidence for Purifying Selection in Somatic Mitochondrial DNA Point Mutations
- Mutations in the Transcription Elongation Factor SPT5 Disrupt a Reporter for Dosage Compensation in Drosophila
- Evolution of Minimal Specificity and Promiscuity in Steroid Hormone Receptors
- Blockade of Pachytene piRNA Biogenesis Reveals a Novel Requirement for Maintaining Post-Meiotic Germline Genome Integrity
- RHOA Is a Modulator of the Cholesterol-Lowering Effects of Statin
- MIG-10 Functions with ABI-1 to Mediate the UNC-6 and SLT-1 Axon Guidance Signaling Pathways
- Loss of the DNA Methyltransferase MET1 Induces H3K9 Hypermethylation at PcG Target Genes and Redistribution of H3K27 Trimethylation to Transposons in
- Genome-Wide Association Studies Reveal a Simple Genetic Basis of Resistance to Naturally Coevolving Viruses in
- The Principal Genetic Determinants for Nasopharyngeal Carcinoma in China Involve the Class I Antigen Recognition Groove
- Molecular, Physiological, and Motor Performance Defects in DMSXL Mice Carrying >1,000 CTG Repeats from the Human DM1 Locus
- Genomic Study of RNA Polymerase II and III SNAP-Bound Promoters Reveals a Gene Transcribed by Both Enzymes and a Broad Use of Common Activators
- Long Telomeres Produced by Telomerase-Resistant Recombination Are Established from a Single Source and Are Subject to Extreme Sequence Scrambling
- The Yeast SR-Like Protein Npl3 Links Chromatin Modification to mRNA Processing
- Deubiquitylation Machinery Is Required for Embryonic Polarity in
- dJun and Vri/dNFIL3 Are Major Regulators of Cardiac Aging in Drosophila
- CtIP Is Required to Initiate Replication-Dependent Interstrand Crosslink Repair
- Notch-Mediated Suppression of TSC2 Expression Regulates Cell Differentiation in the Intestinal Stem Cell Lineage
- A Combination of H2A.Z and H4 Acetylation Recruits Brd2 to Chromatin during Transcriptional Activation
- Network Analysis of a -Mouse Model of Autosomal Dominant Polycystic Kidney Disease Identifies HNF4α as a Disease Modifier
- Mitosis in Neurons: Roughex and APC/C Maintain Cell Cycle Exit to Prevent Cytokinetic and Axonal Defects in Photoreceptor Neurons
- CELF4 Regulates Translation and Local Abundance of a Vast Set of mRNAs, Including Genes Associated with Regulation of Synaptic Function
- Mechanisms Employed by to Prevent Ribonucleotide Incorporation into Genomic DNA by Pol V
- The Genomes of the Fungal Plant Pathogens and Reveal Adaptation to Different Hosts and Lifestyles But Also Signatures of Common Ancestry
- A Genome-Scale RNA–Interference Screen Identifies RRAS Signaling as a Pathologic Feature of Huntington's Disease
- Lessons from Model Organisms: Phenotypic Robustness and Missing Heritability in Complex Disease
- Population Genomic Scan for Candidate Signatures of Balancing Selection to Guide Antigen Characterization in Malaria Parasites
- Tissue-Specific Regulation of Chromatin Insulator Function
- Disruption of Mouse Cenpj, a Regulator of Centriole Biogenesis, Phenocopies Seckel Syndrome
- Genome, Functional Gene Annotation, and Nuclear Transformation of the Heterokont Oleaginous Alga CCMP1779
- Antagonistic Gene Activities Determine the Formation of Pattern Elements along the Mediolateral Axis of the Fruit
- Lung eQTLs to Help Reveal the Molecular Underpinnings of Asthma
- Identification of the First ATRIP–Deficient Patient and Novel Mutations in ATR Define a Clinical Spectrum for ATR–ATRIP Seckel Syndrome
- Cooperativity of , , and in Malignant Breast Cancer Evolution
- Loss of Prohibitin Membrane Scaffolds Impairs Mitochondrial Architecture and Leads to Tau Hyperphosphorylation and Neurodegeneration
- Microhomology Directs Diverse DNA Break Repair Pathways and Chromosomal Translocations
- MicroRNA–Mediated Repression of the Seed Maturation Program during Vegetative Development in
- Selective Pressure Causes an RNA Virus to Trade Reproductive Fitness for Increased Structural and Thermal Stability of a Viral Enzyme
- The Tumor Suppressor Gene Retinoblastoma-1 Is Required for Retinotectal Development and Visual Function in Zebrafish
- Regions of Homozygosity in the Porcine Genome: Consequence of Demography and the Recombination Landscape
- Histone Methyltransferases MES-4 and MET-1 Promote Meiotic Checkpoint Activation in
- Polyadenylation-Dependent Control of Long Noncoding RNA Expression by the Poly(A)-Binding Protein Nuclear 1
- A Unified Method for Detecting Secondary Trait Associations with Rare Variants: Application to Sequence Data
- Genetic and Biochemical Dissection of a HisKA Domain Identifies Residues Required Exclusively for Kinase and Phosphatase Activities
- Informed Conditioning on Clinical Covariates Increases Power in Case-Control Association Studies
- Biochemical Diversification through Foreign Gene Expression in Bdelloid Rotifers
- Genomic Variation and Its Impact on Gene Expression in
- Spastic Paraplegia Mutation N256S in the Neuronal Microtubule Motor KIF5A Disrupts Axonal Transport in a HSP Model
- Lamin B1 Polymorphism Influences Morphology of the Nuclear Envelope, Cell Cycle Progression, and Risk of Neural Tube Defects in Mice
- A Targeted Glycan-Related Gene Screen Reveals Heparan Sulfate Proteoglycan Sulfation Regulates WNT and BMP Trans-Synaptic Signaling
- Dopaminergic D2-Like Receptors Delimit Recurrent Cholinergic-Mediated Motor Programs during a Goal-Oriented Behavior
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Mechanisms Employed by to Prevent Ribonucleotide Incorporation into Genomic DNA by Pol V
- Inference of Population Splits and Mixtures from Genome-Wide Allele Frequency Data
- Zcchc11 Uridylates Mature miRNAs to Enhance Neonatal IGF-1 Expression, Growth, and Survival
- Histone Methyltransferases MES-4 and MET-1 Promote Meiotic Checkpoint Activation in
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Revma Focus: Spondyloartritidy
nový kurz
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



