-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaA Targeted Glycan-Related Gene Screen Reveals Heparan Sulfate Proteoglycan Sulfation Regulates WNT and BMP Trans-Synaptic Signaling
A Drosophila transgenic RNAi screen targeting the glycan genome, including all N/O/GAG-glycan biosynthesis/modification enzymes and glycan-binding lectins, was conducted to discover novel glycan functions in synaptogenesis. As proof-of-product, we characterized functionally paired heparan sulfate (HS) 6-O-sulfotransferase (hs6st) and sulfatase (sulf1), which bidirectionally control HS proteoglycan (HSPG) sulfation. RNAi knockdown of hs6st and sulf1 causes opposite effects on functional synapse development, with decreased (hs6st) and increased (sulf1) neurotransmission strength confirmed in null mutants. HSPG co-receptors for WNT and BMP intercellular signaling, Dally-like Protein and Syndecan, are differentially misregulated in the synaptomatrix of these mutants. Consistently, hs6st and sulf1 nulls differentially elevate both WNT (Wingless; Wg) and BMP (Glass Bottom Boat; Gbb) ligand abundance in the synaptomatrix. Anterograde Wg signaling via Wg receptor dFrizzled2 C-terminus nuclear import and retrograde Gbb signaling via synaptic MAD phosphorylation and nuclear import are differentially activated in hs6st and sulf1 mutants. Consequently, transcriptional control of presynaptic glutamate release machinery and postsynaptic glutamate receptors is bidirectionally altered in hs6st and sulf1 mutants, explaining the bidirectional change in synaptic functional strength. Genetic correction of the altered WNT/BMP signaling restores normal synaptic development in both mutant conditions, proving that altered trans-synaptic signaling causes functional differentiation defects.
Published in the journal: . PLoS Genet 8(11): e32767. doi:10.1371/journal.pgen.1003031
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003031Summary
A Drosophila transgenic RNAi screen targeting the glycan genome, including all N/O/GAG-glycan biosynthesis/modification enzymes and glycan-binding lectins, was conducted to discover novel glycan functions in synaptogenesis. As proof-of-product, we characterized functionally paired heparan sulfate (HS) 6-O-sulfotransferase (hs6st) and sulfatase (sulf1), which bidirectionally control HS proteoglycan (HSPG) sulfation. RNAi knockdown of hs6st and sulf1 causes opposite effects on functional synapse development, with decreased (hs6st) and increased (sulf1) neurotransmission strength confirmed in null mutants. HSPG co-receptors for WNT and BMP intercellular signaling, Dally-like Protein and Syndecan, are differentially misregulated in the synaptomatrix of these mutants. Consistently, hs6st and sulf1 nulls differentially elevate both WNT (Wingless; Wg) and BMP (Glass Bottom Boat; Gbb) ligand abundance in the synaptomatrix. Anterograde Wg signaling via Wg receptor dFrizzled2 C-terminus nuclear import and retrograde Gbb signaling via synaptic MAD phosphorylation and nuclear import are differentially activated in hs6st and sulf1 mutants. Consequently, transcriptional control of presynaptic glutamate release machinery and postsynaptic glutamate receptors is bidirectionally altered in hs6st and sulf1 mutants, explaining the bidirectional change in synaptic functional strength. Genetic correction of the altered WNT/BMP signaling restores normal synaptic development in both mutant conditions, proving that altered trans-synaptic signaling causes functional differentiation defects.
Introduction
Glycans coat cell surfaces, and glycosylation decorates secreted molecules of the pericellular space and extracellular matrix (ECM) [1], [2]. It is well known that glycan modifications mediate critical functions of intercellular signaling and regulate interactions of numerous growth factors with the ECM [3], [4]. The synthesis, modification and degradation of glycoconjugates, including O/N-linked glycoproteins, glycosaminoglycan (GAG) proteoglycans and glycan-binding lectins, is controlled by a dedicated cadre of genes [5], [6]. In the nervous system, these glycan-related genes play key roles in development, including neuron fate specification, migration, formation of axon tracts and synapse maturation [7]. At synapses, glycosylated ECM molecules, membrane receptors and outer-leaflet glycolipids together form the highly specialized synaptomatrix interface [4], [8], which interacts with trans-synaptic signals to modulate synaptogenesis [9].
A prime example is the classic Agrin proteoglycan, which bears heparan sulfate (HS) chains, O/N-linked glycans and also a glycan-binding lectin domain that binds other glycoconjugates [10], [11], [12]. Reduction of GAG sulfation perturbs the Agrin signaling that drives postsynaptic acetylcholine receptor (AChR) cluster maintenance at the neuromuscular synapse [13]. Likewise, Galbeta1,4GlcNAc and Galbeta1,3GalNAc glycans inhibit Agrin signaling by suppressing muscle specific kinase (MuSK) autophosphorylation, a key step during synaptogenesis [14]. Analogous glycan-dependent mechanisms at the Drosophila neuromuscular synapse involve the secreted Mind-the-Gap (Mtg) lectin, which assembles the glycosylated synaptomatrix between presynaptic active zone and postsynaptic glutamate receptor (GluR) domains [15]. This glycan mechanism induces GluR clustering, synaptic localization of integrin ECM receptors, and shapes trans-synaptic signaling by controlling ligand/receptor abundance [16], [17], [18]. Thus, many long-term studies in vertebrate and invertebrate genetic models suggest that glycan mechanisms are a core foundation of synapse development.
In the current study, we conducted a broad transgenic RNA interference (RNAi) screen of synaptic glycan function, assaying requirements in both structural and functional development of the Drosophila neuromuscular junction (NMJ). We tested 130 genes from 8 functional categories: N-glycan, O-glycan and GAG biosynthesis; glycosyltransferases and glycan modifying/degrading enzymes; glycoprotein and proteoglycan core proteins; sugar transporters and glycan-binding lectins. We found that RNAi-knockdown of genes in all eight categories affects synaptic morphological development, with gene-specific effects on branching, bouton differentiation and synapse area. Likewise, all eight categories regulate synaptic functional development, with gene-specific effects both weakening and strengthening neurotransmission. Interestingly, only a few genes affect both structure and function, suggesting separable roles for glycans in regulating these synaptogenic pathways. The results of this genomic transgenic screen are presented as a platform from which to pursue systematic investigation of glycan mechanisms in synaptic development.
Two genes were selected for screen validation and mechanistic characterization; functionally-paired HS 6-O-endosulfatase (sulf1) and HS 6-O-sulfotransferase (hs6st). RNAi knockdown and null mutants identically alter synaptic functional development in a bidirectional manner; loss of sulf1 elevates neurotransmission strength, whereas loss of hs6st weakens it. Heparan sulfate proteoglycan (HSPG) targets Dally-like Protein (Dlp) and Syndecan (Sdc) [19], [20] are mislocalized in sulf1 and hs6st null synapses. In other developmental contexts, the sulfation state of these HSPG co-receptors strongly regulates WNT and BMP intercellular signaling [20], [21], [22]. At Drosophila synapses, WNT (Wg) is a key anterograde [23], [24] and BMP (Gbb) a key retrograde [25], [26] trans-synaptic signal. Consistently, loss of sulf1 and hs6st differentially changes synaptomatrix levels of Wg and Gbb, and downstream signaling into muscle and motor neuron nuclei, respectively. Glutamate release and receptor machinery is thereby bidirectionally altered in the two nulls. Genetic restoration of Wg/Gbb signaling to control levels restores the bidirectional changes in synaptic functional strength and pre-/post - synaptic differentiation in both sulf1 and hs6st nulls. We conclude that extracellular HSPG sulfation state in the synaptomatrix is a point of intersection between WNT/BMP trans-synaptic signaling pathways that drive functional development of the neuromuscular synapse.
Results
RNAi screen of glycan-related genes identifies multiple synaptogenesis defects
Synaptic glycans play important roles as ligands, modulators and co-receptors regulating cell-matrix and intercellular communication [3], [27], [28]. Differential glycan distribution on pre - and postsynaptic surfaces, and in the cleft, of numerous protein classes, strongly suggests that glycan mechanisms mediate synaptic structural and functional development [29], [30], [31]. To test the genomic scope of this requirement, we used confocal imaging and electrophysiological recording at the well-characterized Drosophila glutamatergic neuromuscular junction (NMJ) [32], [33], [34] to screen the Vienna Drosophila RNAi Center (VDRC) library of glycan-related genes [35]. We induced UAS-RNAi knockdown using the ubiquitous UH1-GAL4 driver [15], [36]. We assayed morphological defects by co-labeling for pre - and postsynaptic markers, and assayed functional defects with two-electrode voltage clamp (TEVC) recording of neurotransmission strength. A summary of the screen results is shown in Figure 1. Full numerical results of the screen are shown in Table S1.
Fig. 1. Glycan-related gene RNAi screen for synapse structure/function defects. 
Transgenic RNAi screen interrogating effects of glycan-related gene knockdown on the morphology and function of the Drosophila neuromuscular junction (NMJ) synapse. All VDRC UAS-RNAi lines were crossed to the UH1-GAL4 driver line. Target genes are indicated by Drosophila genome CG annotation number and categorized by function. Confocal imaging of co-labeled pre- and postsynaptic markers was used to quantify NMJ architecture, including branch number, bouton number and synaptic area. TEVC electrophysiology was used to quantify evoked excitatory junctional current (EJC) amplitudes. The magnitude of fold changes compared to control (w1118×UH1-GAL4) is shown on a color scale (see legend below the two columns). Statistical significance was calculated using one-way ANOVA analysis, and displayed as p<0.05 (*), p<0.01 (**). Candidate glycan-related genes were identified and classified into eight functional categories using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database [37] (Figure 1). Additional genes were added to the screen based on ortholog identification using the Information Hyperlinked over Proteins (iHOP) database [38]. The candidate gene list was expanded and verified using Flybase [39]. From this list, genes were cross-referenced with available VDRC UAS-RNAi transgenic lines to generate a final candidate list containing 130 genes within eight functionally-defined categories (Figure 1): N-glycan, O-glycan and glycosaminoglycan (GAG) biosynthesis; glycan core proteins (HSPG core proteins/glycoproteins); sugar transporters; glycosyltranferases; glycan modification genes (modification and degradation of glycans); and glycan-binding lectins. On genetic knockdown, 103 lines were viable until the wandering 3rd instar, whereas 27 lines showed developmental lethality at embryonic and early larval stages of development. From the 103 genetic lines characterized by confocal microscopy and TEVC electrophysiology in the 3rd instar (Figure 1), 21 exhibited pupal stage developmental lethality. Interestingly, >50% of pupal lethal lines displayed statistically significant defects in NMJ synaptic morphology and function.
For all 103 larval-viable lines, synapse morphology and function was quantified at the wandering 3rd instar NMJ (Figure 1; Table S1). Each UAS-RNAi line driven by UH1-GAL4 in the w1118 background was compared to the genetic control of w1118 crossed to UH1-GAL4 (UH1-GAL4×w1118) [35]. All morphological and functional assays were done blind to genotype, with values reported as fold-change compared to genetic control, as well as statistical significance calculated using one-way ANOVA analyses (see color scheme; P<0.05 (*), P<0.01 (**); Figure 1). The data represents ≥6 NMJs from ≥3 animals from every genotype. Synapse morphology was imaged by co-labeling with presynaptic marker anti-horse radish peroxidase (HRP) and postsynaptic marker anti-Discs Large (DLG). A synaptic bouton was defined as a varicosity of ≥2 µm in minimum diameter labeled by both HRP and DLG, and a synaptic branch was defined as a process containing at least two boutons [40]. NMJ branch number was the least affected morphological parameter, with only 2 of 103 genes showing a statistically significant change (Figure 1). Many more genes were involved in bouton development. All 27 genes showing a statistically significant change compared to genetic control exhibited elevated bouton numbers (Figure 1), suggesting that glycan mechanisms primarily limit morphological growth. Synapse area was determined by outlining the terminal area labeled by DLG using the thresholding function in ImageJ. The majority of gene knockdown conditions showed a decrease in NMJ area compared to control (Figure 1). 7 RNAi lines exhibited a statistically significant decrease in area, whereas only 2 lines exhibited a statistically significant increase in synaptic area. All raw values of measured morphological parameters are included in Table S1.
To assay functional differentiation, the motor nerve was stimulated with a suction electrode while the evoked excitatory junctional current (EJC) was recorded in the muscle (Figure 1) [41]. Nerve stimulation was applied at 4 V for 0.5 ms at a frequency of 0.2 Hz, with the muscle clamped at −60 mV. EJC amplitudes were calculated from recorded traces in the ubiquitously-driven RNAi lines (w1118 background) compared to the w1118; UH1-GAL4/+ control. Recordings were obtained from ≥3 independent trials for each RNAi knockdown condition. All electrophysiological screening was done blind to genotype, with values reported as fold-change and statistical significance calculated by one-way ANOVA analyses (see color scheme; P<0.05 (*), P<0.01 (**); Figure 1). Genes from all eight glycan classes were identified to produce changes in neurotransmission strength upon genetic knockdown. For the 103 larval-viable lines tested, 26 lines showed a trend towards increased transmission strength, and 12 were statistically elevated compared to genetic control (Figure 1). 4 gene knockdowns showed a trend towards decreased transmission strength, of which only 1 line reached statistical significance. 73 of the 103 lines tested showed no change in functional strength (Figure 1). Interestingly, only 6 RNAi lines showed statistically significant effects on both NMJ morphology parameters and EJC amplitude: CG1597, CG6657, CG7480, CG4451, CG6725 and CG11874 (Figure 1). This suggests that glycan effects on synapse morphological and functional development are largely separable. All raw values of EJC measurements are included in Table S1.
To validate results, a secondary screen was conducted using independent RNAi lines obtained from the VDRC and Harvard TRiP collections (Table S2). Of the 44 genes that showed morphological and functional defects in the primary screen, 33 were retested using independent RNAi lines, with the others lacking available secondary lines from any source. Using the same screen of morphological and functional characterization, we determined that ∼80% of retested secondary lines showed the reported structural (bouton number) and functional (EJC) phenotypes consistent with primary screen (Table S2). These primary and secondary RNAi screen results now represent a resource for the systematic characterization of glycan mechanisms underlying synaptic structural and functional development. Screen results were further studied by comparing synaptogenesis phenotypes of RNAi knockdown with defined genetic nulls for two genes, CG6725 and CG4451, from the glycosaminoglycan biosynthesis class (Figure 1). The RNAi screen of functional strength as measured by EJC amplitudes indicated opposite effects for these two lines, with CG6725 (RNAi-sulf1) knockdown exhibiting an increase in transmission strength and CG4451 (RNAi-hs6st) knockdown producing a decrease (Figure 1). Along with our goal to identify interesting glycan-related genes involved in synapse development, we show here characterization of null alleles of two genes obtained from screen results and define the associated mechanisms driving the bidirectional regulation of synaptic functional development.
Synaptogenesis is bidirectionally regulated by paired sulf1 and hs6st genes
The RNAi screen identified two functionally-paired genes, sulf1 (CG6725) and hs6st (CG4451), with similar effects on morphological development but opposite effects on synaptic functional differentiation (Figure 1). Our goal was to use these genes as a test case from the completed glycan screen, by assaying phenotypes in recently characterized null mutants of both genes [42], [43]. The gene products Sulfated (Sulf1), an HS 6-endosulfatase, and Hs6st, an HS 6-O-sulfotransferase, drive opposing changes in sulfation state of the same C6 carbon of the repeated glucosamine unit in GAG modified heparan sulfate proteoglycans [43], [44]. Viable null mutants are available for both genes, e.g. sulf1 (sulf1Δ1) and hs6st (hs6std770) [42], [43], but requirements have never been assayed in the nervous system or neuromusculature. We therefore first compared phenotypes of RNAi knockdown and null alleles at the NMJ synapse by confocal imaging of synaptic morphogenesis and TEVC recording of synaptic functional neurotransmission.
Using double-labeling for HRP (presynaptic) and DLG (postsynaptic), NMJ structural parameters including bouton number, branch number and synaptic area were quantified in sulf1 and hs6st null alleles. The mutant results closely recapitulated the RNAi knockdown findings from the screen (Table S1). To consistently compare RNAi and null mutant conditions, both animal groups were simultaneously reared and processed to visualize the NMJ (Figure S1). Structural quantification showed an increased bouton number with RNAi-mediated sulf1 knockdown (sulf1-RNAi×UH1-GAL4; 36.4±1.6, n = 10) and hs6st knockdown (hs6st-RNAi×UH1-GAL4; 35.1±1.96, n = 10) compared to the transgenic control (w1118×UH1-GAL4; 21.9±1.84, p<0.001, n = 10; Figure S1A, S1B). Consistently, increased bouton number was observed in both sulf1 (31.9±1.37, n = 10) and hs6st (36.25±2.58, n = 8) null mutants compared to genetic control (w1118, 19.3±1.69, p<0.001, n = 10; Figure S1C, S1D). In contrast, no significant change in branch number was exhibited with sulf1 knockdown (3.22±0.28, p>0.05, n = 9) or hs6st knockdown (3.22±0.22, p>0.05, n = 9) compared to control (w1118×UH1-GAL4; 2.64±0.06, n = 11). Similarly, no significant change was observed in the synaptic branch number in sulf1 (2.8±0.33, p = 0.27, n = 10,) and hs6st (3.63±0.38, p = 0.115, n = 10) nulls compared to control (w1118; 3.4±0.46, n = 8). Further, there was no significant difference in synaptic area in sulf1 (138.16±5.82, p>0.05, n = 10,) and hs6st (138.48±13.38, p>0.05, n = 8,) mutants compared to the control (w1118; 118.04±8,38, n = 10), however a slight increase in synaptic area was observed in sulf1 knockdown (178.68±10.64, p<0.05, n = 9), while no change was observed for hs6st knockdown (164±8.47, p>0.05, n = 10) as compared to control (w1118×UH1-GAL4; 134.57±11.95, n = 10). Based on these imaging studies, we conclude morphological differences in synaptic architecture observed in both sulf1 and hs6st null allele conditions are consistent with both RNAi knockdown conditions.
Functional development was next tested with electrophysiological recording to compare RNAi and null mutant phenotypes (Figure 2). Representative TEVC records are shown as an average of 10 consecutive nerve stimulus responses in 1.0 mM extracellular Ca2+ for each transgenic genotype in Figure 2A; sulf1 knockdown (UH1-GAL4×sulf1-RNAi), hs6st knockdown (UH1-GAL4×hs6st-RNAi) and genetic control (UH1-GAL4×w1118). There was a striking ∼80% difference in EJC amplitude between sulf1 and hs6st knockdown conditions, with sulf1 elevated by ∼30% and hs6st reduced by ∼30% compared to control. Quantification of EJC amplitudes showed both knockdown conditions to be highly significantly different from control and each other (control, 286.22±8.56 nA; sulf1-RNAi, 365.01±9.502 nA, p<0.001; hs6st-RNAi, 199.19±11.84 nA, p<0.001; sulf1-RNAi vs. hs6st-RNAi, p<0.001; Figure 2B). These opposite effects on neurotransmission strength were confirmed in characterized null alleles for both genes [42], [43]. Representative traces from sulf1Δ1 and hs6std770 null mutants compared to w1118 control are shown in Figure 2C. Quantification of EJC amplitudes showed null mutants to be highly significantly different from control and each other (w1118, 256.14±7.38 nA; sulf1Δ1, 372.86±18.49 nA, n = 11, p<0.001; hs6st, 209.66±13.44 nA, n = 14, p<0.01; sulf1Δ1 vs. hs6st, p<0.001; Figure 2D). These results were confirmed in an independent sulf1 null allele (sulf1ΔP1), which shows comparable elevation compared to control (w1118, 244.91±9.04 nA; sulf1ΔP1, 282.28±13.59, p<0.05, n = 22), as well as the hs6st null (hs6std770) over deficiency (Df(3R)ED6027), which shows comparable depression compared to control (w1118, 256.14±7.38 nA; hs6st/Df(3R)ED6027, 224.06±7.65 nA, p<0.05, n = 18). These results reveal a critical role for sulf1 and hs6st genes in synaptic functional development.
Fig. 2. Loss of sulf1/hs6st causes opposite effects on transmission strength. 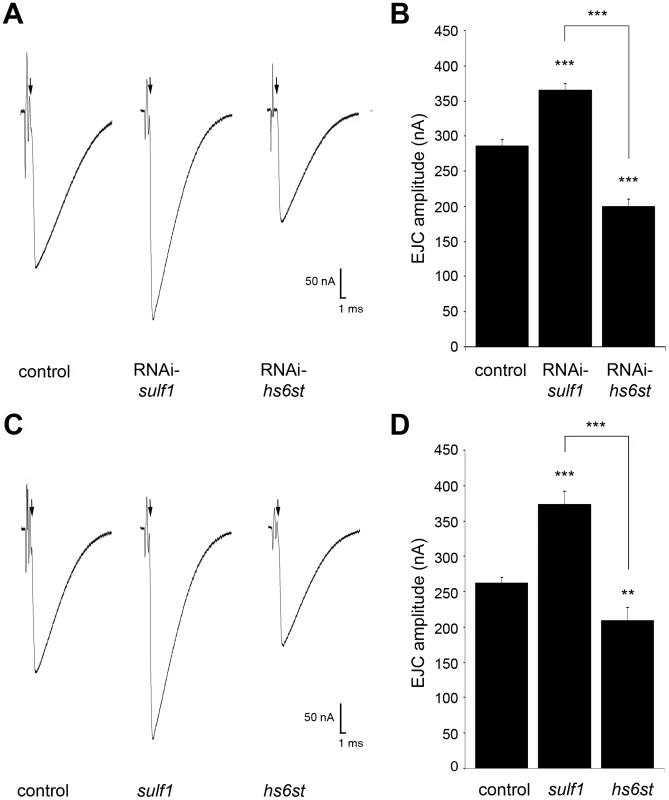
(A) Representative excitatory junctional current (EJC) traces from control (w1118×UH1-GAL4), sulf1 RNAi (UH1-GAL4×UAS-CG6725) and hs6st RNAi (UH1-GAL4×UAS-CG4451). The nerve was stimulated (arrows) in 1.0 mM external Ca2+, with TEVC records (−60 mV holding potential) from muscle 6 in segment A3. Each trace averaged from 10 consecutive recordings. (B) Quantified mean EJC amplitudes (nA) for the three genotypes shown in panel A. (C) Representative traces from control (w1118), sulf1Δ1 and hs6std770 null alleles under the same conditions described in panel A. (D) Quantified mean EJC amplitudes (nA) for the three genotypes shown in panel C. Sample sizes are at least 11 animals per indicated genotype. Statistically significant differences calculated using student's t-test, ** p<0.01, *** p<0.001. Error bars indicate S.E.M. Given the functionally-paired nature of sulf1 and hs6st activities on 6-O-S modification, and the epistatic function of hs6st to sulf1, we predicted that knocking both genes down would produce a phenotype similar to knockdown of hs6st alone. Consistently, hs6st and sulf1 double knockdown produced EJC amplitudes significantly lower than control (w1118×hs6st-RNAi; sulf1-RNAi (control), 225.17±6.28 nA, n = 12; hs6st-RNAi, sulf1-RNAi×UH1-GAL4, 198.22±9.77 nA, n = 15, p<0.05; Figure S2). Cell-specific knockdown in neural (elav-GAL4), muscle (24B-GAL4) and glia (repo-GAL4) also support the observed opposite effects in neurotransmission strength. With sulf1 knockdown in muscle, EJC amplitude was significantly elevated compared to control (w1118×sulf1-RNAi (control), 199.97±21.86 nA; 24B-GAL4×sulf1-RNAi (knockdown), 222.88±25.78 nA, p<0.01, n = 10), but no change occurred with neural knockdown (elav-GAL4×sulf1-RNAi, 196.09±25.08 nA, p = 0.72, n = 10) or glial knockdown (repo-GAL4×sulf1-RNAi, 208.40±32.45 nA, p = 0.53, n = 7). Moreover, only neural knockdown of hs6st caused a decrease in EJC amplitude (w1118×hs6st-RNAi (control), 211.496±22.142 nA, elav-GAL4×hs6st-RNAi (knockdown), 184.68±28.97 nA, p<0.05, n = 16), while no change occurred with muscle knockdown (24B-GAL4×hs6st-RNAi, 209.92±24.74 nA, p = 0.88, n = 9) or glial knockdown (repo-GAL4×hs6st-RNAi, 216.38±37.80 nA, p = 0.32, n = 7). We conclude that HSPG sulfation state strongly modulates NMJ functional development, with contributions from both motor neuron and muscle, but not glia. The clear next step was to test for differences in the localization and abundance of synaptic HSPG targets known to regulate NMJ synaptogenesis.
HSPG abundance at the synaptic interface is dependent on sulf1 and hs6st
Both GPI-anchored HSPG glypican Dally-like (Dlp) and transmembrane HSPG Syndecan (Sdc) are clearly expressed at the Drosophila NMJ (Figure S3), where they are known to regulate synaptogenesis [45]. We detect no enrichment of the secreted HSPG perelcan (Trol) at the NMJ, although it is abundantly expressed in the motor nerve leading up to the synaptic terminal and present in lower levels throughout the muscle (Figure S4). We therefore hypothesized that membrane-associated Dlp and Sdc HSPGs are targeted by sulf1 and hs6st activity to regulate their synaptic distribution and/or function. To test this hypothesis, we assayed both Dlp and Sdc under non-permeabilized, detergent-free conditions to examine their cell surface expression at the NMJ synaptic interface of sulf1 and hs6st null mutants compared to control. These data are summarized in Figure 3.
Fig. 3. Synaptic HSPG co-receptor abundance is modified by 6-O-S sulfation. 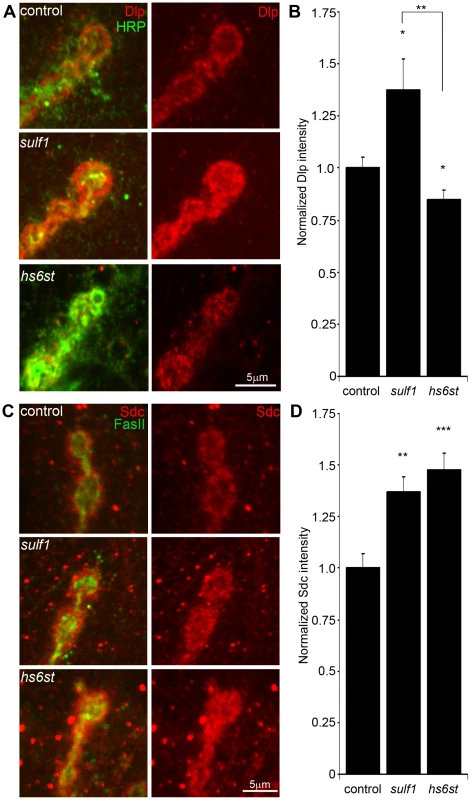
(A) Representative NMJ synaptic boutons imaged from control (w1118), sulf1 and hs6st nulls, probed with presynaptic neural marker anti-HRP (green) and Dally-like (Dlp; red). Right: Dlp distribution without the HRP signal is shown for clarity. (B) Quantification of mean fluorescent intensity levels of anti-Dlp labeling normalized to the HRP co-label at the muscle 6 NMJ, normalized to genetic control. (C) Boutons labeled with neural marker anti-Fasciclin II (FasII, green) and anti-Syndecan (Sdc, red). Right: Sdc distribution is shown alone for clarity. (D) Quantification of the mean fluorescent intensity levels of anti-Sdc labeling at the muscle 6 NMJ, normalized to genetic control. Sample sizes are at least 12 independent NMJs of at least 7 animals per indicated genotypes. Statistically significant differences calculated using student's t-test, * p<0.01, ** p<0.01, ***p<0.001. Error bars indicate S.E.M. In the genetic background control (w1118), Dlp shows a punctate expression pattern strongly concentrated in a halo-like array around the anti-HRP labeled presynaptic membrane (Figure 3A, top; Figure S3). In sulf1 mutants there was a clear and consistent increase in Dlp abundance, with more numerous and intense punctae at the synaptic interface surrounding NMJ boutons, while at hs6st mutant synapses there was an opposing decrease in Dlp abundance (Figure 3A). This bidirectional and differential effect on Dlp abundance was quantified as fluorescence intensity normalized to the internal HRP labeling control. There was a significant Dlp increase in sulf1 compared to control (∼40% elevated over control; p<0.05; n = 11), and a significant Dlp decrease in the hs6st null synapse (∼15% reduced compared to control; p<0.05; n = 11; Figure 3B). Importantly, the difference between sulf1 and hs6st nulls was very highly significant (p<0.001). In comparison, cell surface Sdc labeling also showed a dense halo-like localization around NMJ synaptic boutons labeled with cell adhesion marker Fasciclin II (FasII; Figure 3C; Figure S3). Synaptic Sdc labeling intensity was consistently greater in both sulf1 and hs6st nulls compared to control (Figure 3C). Quantification of fluorescence intensity normalized to HRP revealed that Sdc abundance was greatly increased in sulf1 null synapses compared to control (∼35% elevated over control; p<0.01; n = 17) and, to a greater degree, also in hs6st nulls (∼50% elevated over control; p<0.001; n = 12; Figure 3D). Thus, both Dlp and Sdc HSPGs are strongly altered in sulf1 and hs6st null NMJ synapses, with Dlp bidirectionally misregulated and Sdc differentially elevated in the two mutant conditions.
HSPGs act as co-receptors for WNT and BMP intercellular signaling ligands in many developmental contexts, acting to modulate extracellular ligand abundance and downstream signaling [46], [47]. Drosophila WNT Wingless (Wg) distribution and signaling is known to be modulated by Dlp, which retains Wg at the cell surface in a mechanism that is enhanced by HS GAG chains [48]. Specifically, Wg ligand abundance and signaling activity along the dorso-ventral axis of the developing Drosophila wing disc is elevated in sulf1 mutants [22]. Likewise, BMP ligands in other cellular contexts are closely regulated by HSPG co-receptors [20]. Specifically, Dlp has been suggested to similarly regulate Drosophila BMP Glass Bottom Boat (Gbb) [20]. We therefore hypothesized that altered HSPG co-receptors Dlp and/or Sdc in sulf1 and hs6st null synapses regulate Wg and Gbb abundance to drive differentially altered trans-synaptic signaling across the synaptic cleft.
HSPG sulfation regulates abundance of WNT/BMP trans-synaptic ligands
Classical WNT and BMP morphogens act locally at synapses to fine tune synaptogenesis [49], [50]. At the Drosophila NMJ, the WNT Wg is well-characterized as an anterograde trans-synaptic signal modulating synaptogenesis [23], [24], [51]. Similarly, the BMP Gbb is well-characterized as a retrograde signal driving synaptic development [25], [26], [52]. A third trans-synaptic signaling pathway, presynaptically-secreted Jelly Belly (Jeb) to postsynaptic Alk receptor [17], has no known interaction with HSPGs and therefore would not be expected to be affected in sulf1 and hs6st nulls, providing a comparison for specificity. To test the hypothesis that the observed alterations of HSPG co-receptor abundance will drive specific changes in WNT and BMP intercellular pathways, we labeled NMJ synapses with antibodies under non-permeablized conditions to reveal extracellular trans-synaptic signaling ligands (Figure S5), and compared protein abundance and distribution in controls, sulf1 and hs6st null mutants. The data are summarized in Figure 4.
Fig. 4. Synaptic WNT and BMP ligand abundance is modified by 6-O-S sulfation. 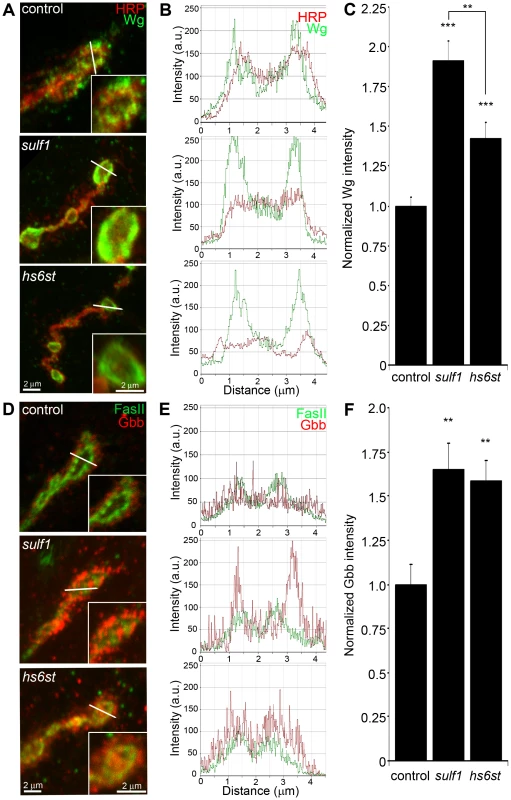
Images show muscle 6 NMJ in segment A3 probed in non-detergent conditions, so that only extracellular protein distributions are detected. The white lines indicate cross-section planes for spatial measurements. Insets indicate single synaptic boutons at higher magnification. (A) Representative NMJ boutons from control (w1118), sulf1 and hs6st null genotypes, labeled for presynaptic anti-horseradish peroxidase (HRP, red) and anti-wingless (Wg, green). (B) Extracellular distribution of Wg across the diameter of a synaptic bouton. The Y-axis indicates intensity and the X-axis shows distance in microns. The HRP intensity profile is indicated in red; Wg intensity is shown in green. (C) Quantification of Wg mean intensity levels normalized to the HRP co-label, and to genetic control. Sample sizes are at least 15 animals per indicated genotypes. (D) Representative synaptic boutons labeled with presynaptic anti-Fasciclin II (FasII; green) and anti-Glass Bottom Boat (Gbb; red). (E) Gbb distribution across the diameter of a synaptic bouton. Y-axis indicates intensity and the X-axis shows distance in microns. FasII intensity profile is indicated in green; Gbb intensity is shown in red. (F) Quantification of Gbb mean intensity levels normalized to genetic control. Sample sizes are at least 11 independent NMJs of at least 7 animals per indicated genotypes. Statistically significant differences calculated using student's t-test and Mann-Whitney test for non-parametric data, ** p<0.01, *** p<0.001. Error bars indicate S.E.M. NMJ synapses were first labeled with Wg antibody (green) together with anti-HRP (red) to label the presynaptic membrane (Figure 4A). In control animals (w1118), external Wg localized at large type Ib synaptic boutons in a dynamic pattern of punctuate distribution at the synaptic interface between motor neuron and muscle (Figure 4A, top; Figure S5). In sulf1 and hs6st mutants, Wg was consistently elevated and concentrated uniformly in the extracellular domain adjacent to, and overlapping with, the anti-HRP-labeled presynaptic membrane (Figure 4A, middle and bottom). The elevated Wg levels in mutants were clearly observed at the level of individual synaptic boutons, as shown in the magnified insets in Figure 4A. To examine changes in Wg spatial distribution, cross-sectional planes were examined in single confocal line scans through the diameter of individual synaptic boutons (Figure 4A, white lines). Representative distribution plots for membrane-marker HRP (red) and external Wg (green) are shown in Figure 4B. In all genotypes, extracellular Wg was closely associated with the HRP-labeled presynaptic membrane, but both sulf1 and hs6st nulls displayed a consistent increase in Wg label intensity and broadening of the spatial domain occupied by the secreted Wg ligand (Figure 4B, middle and bottom). To quantify changes in extracellular Wg abundance, the mean fluorescent signal intensity was normalized to the internal HRP co-label, and then normalized to analogous control intensity ratios. In sulf1Δ1 nulls, there was very highly significant elevation of Wg compared to control (∼90% increased; p<0.001; n = 16; Figure 4C). A similar increase was observed in the independent sulf1ΔP1 null (p<0.001; n = 11). The hs6st null displayed a smaller significant increase in Wg abundance (∼40% increased; p<0.001; n = 15; Figure 4C), which was again recapitulated in hs6st null over deficiency (Df(3R)ED6027) condition. Importantly, Wg abundance is differentially elevated in sulf1 vs. hs6st mutants (p<0.01, Figure 4C).
To test whether the sulf1/hs6st mechanism might coordinately regulate multiple trans-synaptic signals, we next assayed the BMP Gbb, a muscle-derived retrograde signal [25]. A barrier to previous Gbb analyses has been the absence of an anti-Gbb antibody. We therefore generated a specific anti-Gbb antibody for this study (see Methods). As above, labeling was done under non-permeabilized conditions to reveal only the extracellular Gbb, together with labeling for HRP or the cell adhesion molecule marker FasII to reveal the presynaptic membrane (Figure S5). In the control (w1118), extracellular Gbb concentrated in a ring of punctate domains around boutons (Figure 4D, top). Gbb was similarly punctate in sulf1 and hs6st nulls, but consistently more extensive and denser (Figure 4D, middle and bottom; see magnified insets). To examine Gbb spatial distribution, cross-sectional planes of confocal line scans were made through individual synaptic boutons (Figure 4D, white lines). Representative plots for FasII (green) and Gbb (red) show extracellular Gbb closely associated with the FasII-labeled presynaptic membrane in all genotypes (Figure 4E). However, sulf1 and hs6st nulls consistently displayed increased Gbb intensity and broadened expression compared to the control. Upon quantifying signal intensity of Gbb normalized to HRP co-label, sulf1Δ1 exhibited a significantly higher Gbb abundance than control (65% increased; p<0.01; n = 12; Figure 4F). The independent sulf1ΔP1 null allele showed a similar increase (p<0.001; n = 12). The hs6st null also showed Gbb elevation compared to control (59% increased; p<0.01; n = 11; Figure 4E), which was confirmed in hs6st null over deficiency (Df(3R)ED6027; p<0.05; n = 23).
To test further whether extracellular Wg and Gbb abundance was sensitive to the sulfation state of GAGs, a biochemical approach was next used to determine effects on Wg and Gbb trans-synaptic signals (Figure S6). Specifically, NMJs were acutely exposed to heparin, the most sulfated form of GAG [53], and then synaptic Wg and Gbb abundance was measured by immunolabeling as above. We found that both trans-synaptic signals were rapidly altered by heparin incubation in a dose-dependent manner. Specifically, incubation with increasing concentrations of heparin caused a reciprocal decrease in Wg labeling intensity in the NMJ synaptic domain (Figure S6A, S6C), with a significant decrease first detected with 0.315 mg/ml heparin incubation (∼50% less than control, p<0.01, n = 4). Interestingly, increasing heparin concentrations caused a parallel increase in Gbb abundance in the NMJ synaptic domain (Figure S6B, S6C) in a dose-dependent manner, with significant increases again first detected at 0.315 mg/ml heparin (∼25% greater than control, p<0.05) and rising further at 0.625 mg/ml heparin (∼40% greater than control, p<0.001). These results indicate that HSPG sulfation state does indeed affect trans-synaptic signal abundance, supporting the observed alterations in Wg and Gbb abundance in mutants of heparan sulfate modifying genes, sulf1 and hs6st.
To examine effects on other trans-synaptic signaling pathways in the sulf1 and hs6st mutant synapses, we also assayed for changes in Jeb [17] and FGF [17] signaling. In both control and mutants, extracellular Jeb labeling was tightly associated with NMJ type Ib boutons and, like other trans-synaptic ligands, occupied an extracellular domain closely associated with the presynaptic membrane (Figure S7A). However, in stark contrast to Wg and Gbb ligands in the same extracellular synaptomatrix domain, no change was observed in Jeb abundance or spatial distribution in sulf1 null (p = 0.99, n = 10) or hs6st null (p = 0.36, n = 8) compared to control (w1118) NMJ synapses (Figure S7B). FGF signaling is also well established to be affected by HSPGs [54], and one pioneering study has investigated roles for FGF signaling at the Drosophila NMJ [55]. The probe used in the previous study was an antibody against the FGF receptor Heartless (Htl) [56]. Using this antibody, we confirmed that the Htl receptor beautifully localizes to NMJ boutons to mediate FGF signaling (Figure S8A). However, Htl receptor synaptic abundance and distribution was very similar for the sulf1 (p = 0.89, n = 9) and hs6st (p = 0.69, n = 7) mutants compared to control (w1118) (Figure S8B). Unfortunately, no antibody probes are available for Drosophila FGF ligands, so these signals have not yet been queried. Together, these results show that both WNT (Wg) and BMP (Gbb) ligand abundance is coordinately upregulated by the sulf1 and hs6st mechanism at the NMJ synapse, but that a spatially overlapping signaling ligand (Jeb) and at least FGF receptor expression are unaffected. These results strongly predict that Wg and Gbb trans-synaptic signaling controlled by sulf1 and hs6st activity regulates synaptic functional development.
Trans-synaptic WNT/BMP signaling is regulated by HSPG sulfation
Wg and Gbb serve as anterograde and retrograde trans-synaptic signals, respectively, activating cognate receptors to initiate downstream signaling cascades and nuclear import pathways in muscles and motor neurons, respectively [24], [26], [50], [51]. The anterograde Wg signal drives dFrizzled-2 (dFz2) receptor internalization in the postsynaptic domain followed by cleavage of the receptor C-terminus, which then enters the muscle nuclei [57]. The muscle-derived retrograde Gbb signal activates presynaptic receptors to drive phosphorylation of the Mothers Against Decapentaplegic (Mad) transcription factor, and then P-Mad enters the motor neuron nuclei to regulate transcription [25], [26], [58]. Given the differential change in both HSPG co-receptor and Wg/Gbb ligand abundance in sulf1 vs. hs6st mutants, we hypothesized that these signaling pathways would be differentially affected during synaptogenesis. We therefore quantitatively assayed the paired muscle and motor neuron nuclear import pathways to determine whether and how trans-synaptic signaling may be modulated by sulf1 and hs6st at the NMJ synapse.
Characterized antibodies specifically recognizing the N - and C-termini of the Wg dFz2 receptor allow measurements of the receptor at the NMJ synapse (dFz2N; Figure S9) and the cleaved fragment (dFz2C; Figure 5) imported into muscle nuclei [57], [59]. We first assayed dFz2 receptor abundance at the NMJ with the N-terminal specific antibody. The dFz2 receptor is closely associated with the synaptic cell membrane marker FasII and occupies a domain that envelopes all type Ib boutons (Figure S9A). In hs6st nulls, the dFz2 receptor domain was spatially extended as compared to controls, however sulf1 alleles showed no detectable change in the receptor. Likewise, fluorescence intensity measurements showed no significant difference between control and sulf1 nulls, but hs6st null synapses displayed a ∼25% increase in dFz2 receptor abundance, a very significant elevation (p<0.01, n = 12; Figure S9B) in synaptic dFz2 abundance. Thus, importantly (see Discussion), significantly more dFz2 receptors occur in the hs6st null compared to sulf1 null synapse.
Fig. 5. Loss of sulf1 and hs6st causes opposite effects on WNT signaling. 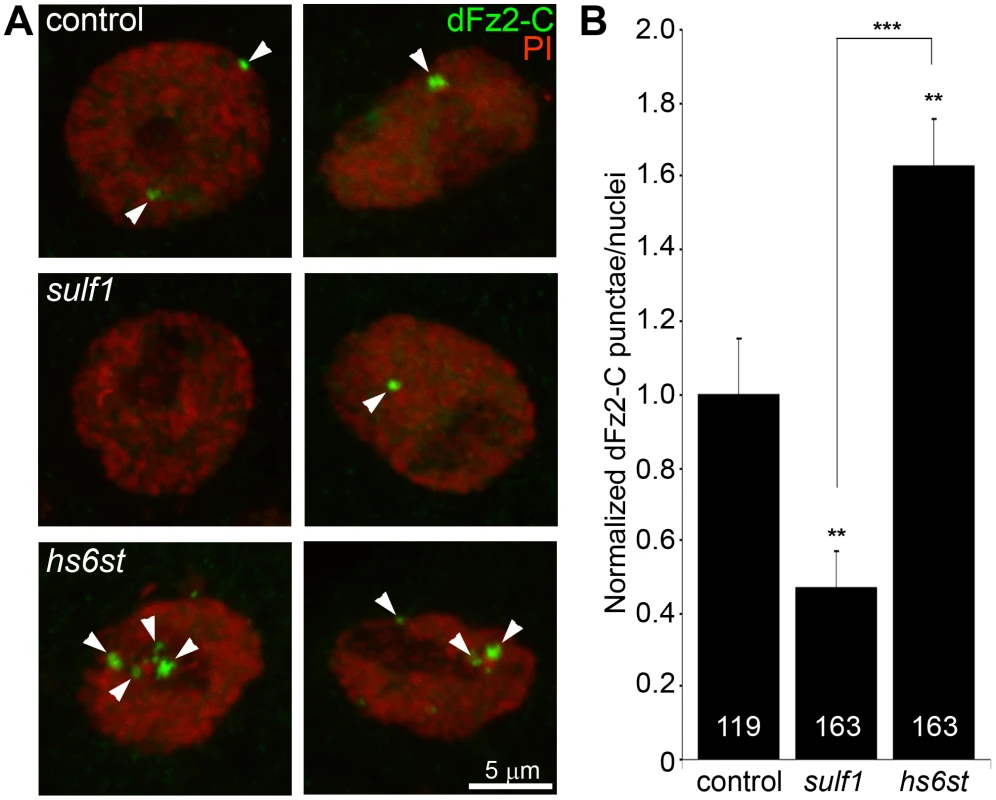
(A) Representative images of muscle nuclei from control (w1118), sulf1 and hs6st nulls, labeled with nuclear marker propidium iodide (PI, red) and for the C-terminus of the Wingless receptor Frizzled 2 (dFz2-C, green). Arrows indicate punctate dFz2-C nuclear labeling. Nuclei shown from muscle 6 in segment A3. (B) Quantification of the number of dFz2-C punctae per nuclei, normalized to genetic control. The total number of nuclei analyzed is indicated in each column; 119 for control (w1118) and 163 nuclei each for sulf1 and hs6st null mutants. Sample sizes are ≥9 animals per indicated genotypes. Statistically significant differences calculated using student's t-test; ** p<0.01 *** p<0.001. Error bars indicate S.E.M. To assay downstream signal transduction, the cleaved Fz2C fragment imported into muscle nuclei was quantified using the established method of counting dFz2C-positive punctae in nuclei proximal to the NMJ (Figure 5) [59]. In genetic control (w1118), most muscle nuclei contained a small number (1–3) of detectable dFz2C punctae, but some nuclei contained more and others were devoid of detectable dFz2C (Figure 5A, top). More than 100 muscle nuclei were quantified in >7 different animals to determine the control level of dFz2C nuclear import. In sulf1 and hs6st mutants, there was a clear and consistent bidirectional difference in the number and size of dFz2C punctae in muscle nuclei (Figure 5A, middle and bottom). Null sulf1 nuclei showed a highly significant decrease in number of dFz2C punctae per nuclei (>50% decreased; p<0.01; n = 163; Figure 5B). In contrast, hs6st nulls had an opposing highly significant increase in dFz2C punctae per nuclei (>60% increased; p<0.01; n = 163; Figure 5B). The difference between sulf1 and hs6st null mutants was very highly significant (p<0.001), with a differential change in signaling paralleling the bidirectional change in synaptic functional differentiation (Figure 2).
A characterized antibody specifically recognizing phosphorylated Mad (P-Mad) allowed independent measurements of Gbb signaling in the presynaptic terminal and P-Mad import into the motor neuron nuclei as a transcriptional regulator (Figure 6) [25], [60]. To assay this transduction pathway, P-Mad fluorescent intensity normalized to FasII was first assayed in presynaptic boutons [61], [62]. In the genetic control (w1118), P-Mad labeling was bounded by the synaptic cell adhesion molecule marker FasII, with P-Mad localized in numerous punctate domains (Figure 6A, arrows). In sulf1 and hs6st nulls, both the intensity and size of P-Mad positive punctae were obviously and consistently greater than in controls (Figure 6A, middle and bottom). In fluorescence intensity quantification, sulf1 null synapses displayed a significant increase in synaptic P-Mad (45% increased; p<0.05; n = 10; Figure 6C). An increase in P-Mad was also observed in the hs6st null boutons (42% greater than control; p<0.01; n = 15; Figure 6C). The motor neuron nuclei at the ventral nerve cord (VNC) midline accumulate P-Mad transcription factor downstream of Gbb signaling at the NMJ [25], [61], [62]. In genetic control (w1118), P-Mad nuclear labeling was consistently detected in these motor neuron nuclei (Figure 6B, arrows). A similar P-Mad distribution was observed in motor neuron nuclei of sulf1 and hs6st nulls, but the intensity of P-mad expression was clearly and consistently elevated in both mutants compared to control (Figure 6B, middle and bottom). In fluorescence intensity quantification, sulf1 null neuronal nuclei displayed a very significant increase in P-Mad accumulation (15% increased; p<0.01; n = 14; Figure 6D), paralleling increased P-Mad signaling at the NMJ (Figure 6C). Likewise, hs6st null motoneuron nuclei exhibited a smaller but still significant elevation in P-Mad accumulation (9% elevated over control; p<0.05; n = 21; Figure 6D), again paralleling the observed P-Mad signaling change at the NMJ (Figure 6C). We conclude that both anterograde WNT (Wg) and retrograde BMP (Gbb) trans-synaptic signaling in muscle and motor neuron nuclei, respectively, is differentially regulated by the sulf1 and hs6st HSPG sulfation mechanism.
Fig. 6. Loss of sulf1 and hs6st causes differential effects on BMP signaling. 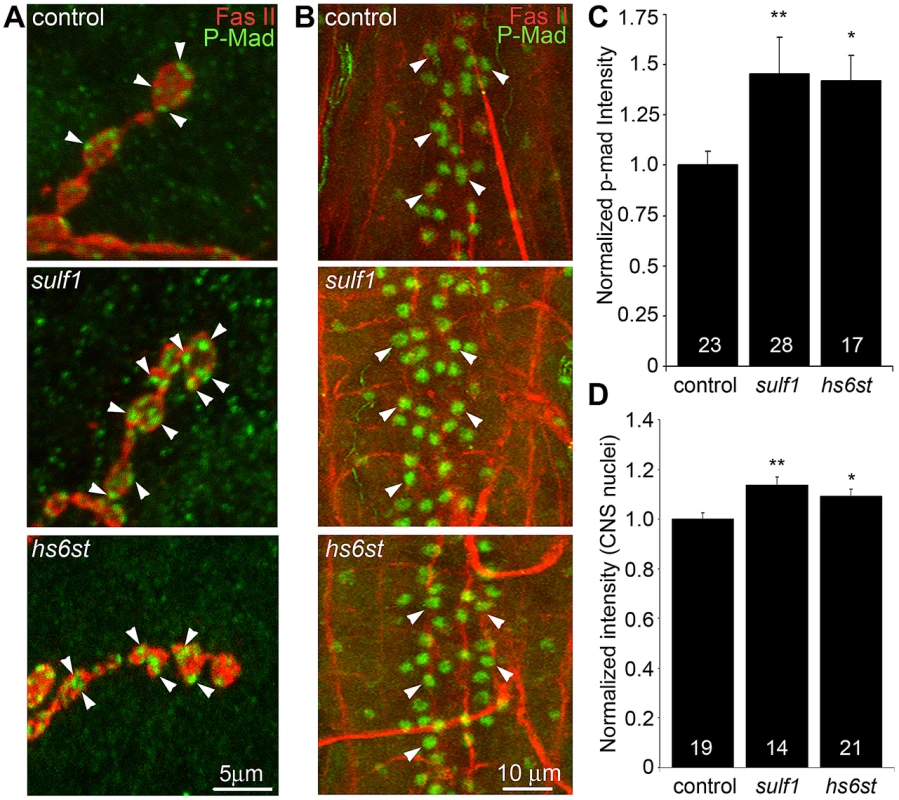
(A) Representative NMJ synaptic boutons on muscle 6 in segment A3 from control (w1118), sulf1 and hs6st nulls, labeled with neural marker anti-Fasciclin II (FasII, red) and for phosphorylated Mothers against decapentaplegic (P-Mad; green) activated downstream of Gbb signaling. Arrows indicate representative P-Mad punctae in the indicated genotypes. (B) Representative ventral nerve cord (VNC) midlines from the same 3 genotypes, labeled with anti-FasII (red) and P-Mad (green). Labeled motor neuron nuclei are indicated by arrows. Quantification of the mean fluorescent intensity level of P-Mad labeling normalized to FasII co-label at the NMJ synapse (C) and in motor neuron nuclei (D), normalized to genetic control. Sample sizes are ≥14 animals per indicated genotypes. Statistically significant differences calculated using the Mann-Whitney test for non-parametric data, * p<0.05, ** p<0.01. Error bars indicate S.E.M. Trans-synaptic WNT/BMP signals genetically interact with sulf1 and hs6st nulls
In the sulf1 and hs6st nulls we identified a bi-directional change in synaptic functional differentiation, measured as evoked junction current amplitudes increased in sulf1 and decreased in hs6st null synapses (Figure 2). We therefore hypothesized that these functional changes are driven by the differential Wg and Gbb trans-synaptic signaling defects characterized above in sulf1 and hs6st mutants (Figure 3, Figure 4, Figure 5, Figure 6). We reasoned that correcting Wg and Gbb levels in sulf1 and hs6st nulls should restore neurotransmission to control levels. To test this hypothesis, we crossed heterozygous wg/+ and gbb/+ mutants into both sulf1 and hs6st homozygous null backgrounds, both singly and in combination, and compared them to both positive and negative controls. The resulting 9 genotypes were all assayed with TEVC electrophysiology to compare EJC transmission strength. A summary of these data is given in Figure 7.
Fig. 7. WNT and BMP signals genetically interact with sulf1 and hs6st nulls. 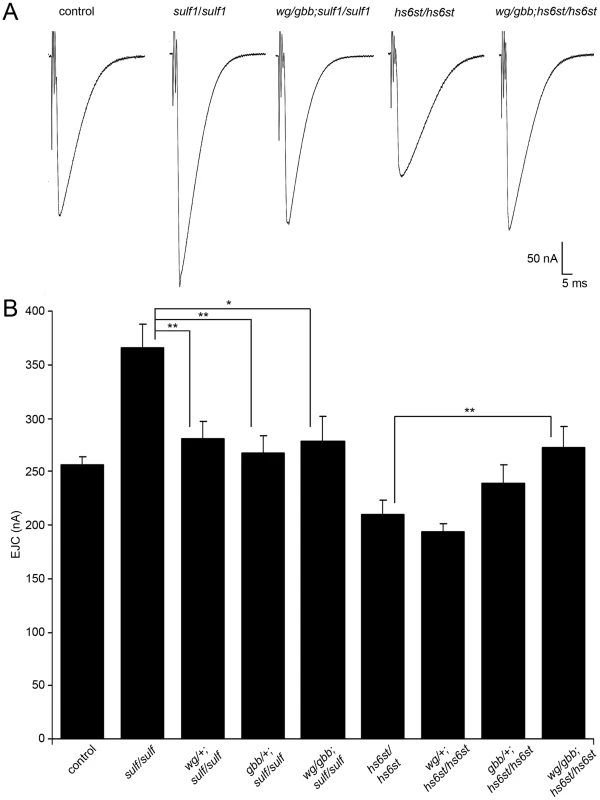
Genetic reduction of Wg and Gbb levels in sulf1 and hs6st homozygous conditions restore EJC amplitudes to control levels. (A) Representative excitatory junctional current (EJC) traces from control (w1118), homozygous sulf1Δ1 null, heterozygous wg/+ and gbb/+ in sulf1 null background (wgI-12/gbb2; sulf1Δ1/sulf1Δ1), homozygous hs6std770 null and heterozygous wg/+ and gbb/+ in hs6st null background (wgI-12/gbb2; hs6std770/hs6std770). The nerve was stimulated (arrows) in 1.0 mM external Ca2+, and TEVC records (−60 mV holding potential) made from muscle 6 in segment A3. Each trace was averaged from 10 consecutive evoked EJC recordings. (B) Quantified mean EJC amplitudes (nA) for the nine genotypes shown. Sample sizes are ≥7 animals per indicated genotype. Statistically significant differences calculated using student's t-test, * p<0.05, ** p<0.01. Error bars indicate S.E.M. Representative transmission records are shown as an average of 10 consecutive EJC responses (1.0 mM extracellular Ca2+) for the genotypes in Figure 7A, with quantification of mean peak amplitudes in all genotypes shown in Figure 7B. First testing sulf1 nulls, we examined the consequences of heterozygous genetic reduction of Wg and Gbb, alone and in combination. Compared to the elevated EJC amplitude of the sulf1 null condition (381.28±1 62.24 nA, p<0.01, n = 9; Figure 7B), genetic reduction of Wg (wg/+; sulf1/sulf1) caused very significantly reduced transmission, similar to genetic reduction of Gbb (gbb/+; sulf1/sulf1) with a comparable effect, restoring EJC amplitude to control levels (267.16±16.33, p<0.01, n = 9; Figure 7B). Combinatorial genetic reduction of both Wg and Gbb in the sulf1 null (wg/gbb;sulf1/sulf1) similarly returned EJC amplitudes to control levels (278.78±23.17, n = 7; Figure 7B). Secondly testing hs6st nulls, genetic reduction of either Wg or Gbb alone was not sufficient to significantly change the depressed synaptic function (Figure 7B). In this case, combinatorial genetic reduction of both Wg and Gbb in the hs6st null (wg/gbb;hs6st/hs6st) was required to raise the depressed EJC amplitude, a very significant increase back to control levels (272.98±18.58, p<0.01, n = 8; Figure 7B). Therefore, we conclude that combinatorial Wg and Gbb trans-synaptic signaling defects are causative for the observed bi-directional effects on synaptic functional differentiation in the sulf1 and hs6st null mutant conditions.
The sulf1 and hs6st mechanism regulates pre - and postsynaptic differentiation
The consequence of WNT (Wg) and BMP (Gbb) trans-synaptic signaling is nuclear import and transcriptional regulation in both synaptic partner cells [49], [51]. We therefore hypothesized that sulf1 and hs6st null mutants would show bidirectional changes in pre - and postsynaptic molecular components that would explain the bidirectional change in synaptic functional differentiation (Figure 2 and Figure 7). To test this hypothesis, we examined a key component of the presynaptic active zone (Bruchpilot; Brp) [63], and an essential subunit of the postsynaptic glutamate receptor (Bad Reception (Brec); GluRIID) [64]. In parallel, we also performed a miniature EJC (mEJC) analysis to compare functional presynaptic vesicle release probability and postsynaptic response amplitude. A summary of these data is shown in Figure 8.
Fig. 8. Bi-directional effects of sulf1 and hs6st nulls on synaptic assembly. 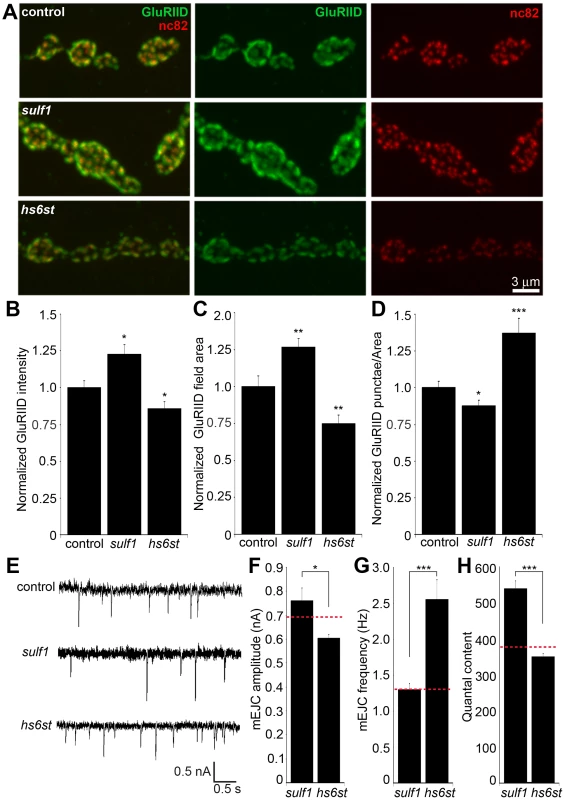
(A) Representative NMJ boutons from control (w1118), sulf1 and hs6st null genotypes, labeled for postsynaptic Bad Reception (Brec) glutamate receptor IID subunit (GluRIID, green) and presynaptic active zone Bruchpilot (anti-nc82, red). Quantification of GluRIID mean fluorescent intensity (≥18 animals per indicated genotype) (B), GluRIID field area (≥40 boutons from ≥9 animals per indicated genotype) (C), and GluRIID punctae number per synaptic bouton (≥40 boutons from ≥9 animals per indicated genotype) (D), all normalized to genetic control. (E) Representative mEJC traces from control (w1118), sulf1Δ1 and hs6std770 null alleles. Quantified mean mEJC amplitude (nA) (F), mean mEJC frequency (Hz) (G) and mean quantal content (H), with genetic control levels indicated as a dotted red line in each case. Sample sizes ≥15 recordings per indicated genotype. Statistically significant differences calculated using student's t-test or Mann-Whitney test for non-parametric data and indicated as, * p<0.05, ** p<0.01, *** p<0.001. Error bars indicate S.E.M. First, NMJ synapses were double-labeled for GluRIID recognized with anti-Brec (green) and Brp recognized with anti-nc82 (red) to compare genetic control (w1118) with sulf1 and hs6st nulls (Figure 8A). We found that GluRIID was very significantly elevated at sulf1 synapses compared to control (∼30% increased; p<0.01, n = 20; Figure 8B). In the opposing direction, hs6st null synapses showed a significant decrease in GluRIID abundance (∼15% reduced; p<0.05, n = 21; Figure 8B). The GluRIID field area per bouton and number of GluRIID punctae normalized to field area per synaptic bouton were also bidirectionally altered in the sulf1 and hs6st nulls (Figure 8C, 8D). GluRIID receptor field area was increased in sulf1 (∼30% greater; p<0.01, n = 47) but decreased in hs6st (∼25% reduced; p<0.01, n = 51). Conversely, measurements of GluRIID puncta normalized to field area per synaptic bouton were decreased in sulf1 (∼15% lower; p<0.05, n = 47), but increased in hs6st nulls (∼40% greater; p<0.01, n = 51, Figure 8D). The bi-directional differences between sulf1 and hs6st were very highly significant (p<0.001). The active zone protein Brp also showed opposite effects (Figure 8A). Although the difference between sulf1 null and control was not quite significant (p>0.05, n = 20), hs6st null synapses showed a very significant decrease in Brp compared to control (∼20% reduced; p<0.01, n = 21; Figure 8A).
Based on these results, we next tested pre - (Brp) and postsynaptic (Brec/GluRIID) changes in sulf1 and hs6st mutants with genetic reduction of Wg and Gbb (wg/gbb;sulf1/sulf1 and wg/gbb;hs6st/hs6st), as in Figure 7. Distribution changes of both pre - and postsynaptic components were assayed as measurements of glutamate receptor field and active zone areas (Figure S10A). To measure glutamate receptor distribution comparing wg/gbb;sulf1/sulf1 to matched control, we counted the number of GluRIID punctae per bouton (p = 0.73, n = 48; Figure S10B) and GluRIID area (p = 0.92, n = 48; Figure S10C), and found both corrected back to control levels. Likewise, for wg/gbb;hs6st/hs6st compared to control, GluRIID puncta number (p = 0.88, n = 48) and area (p = 0.41, n = 58) were both corrected to control levels. To measure Brp-positive presynaptic active zones comparing wg/gbb;sulf1/sulf1 to matched control, we counted the number of Brp punctae per bouton (p = 0.43, n = 48; Figure S10D) and Brp area (p = 0.39, n = 48; Figure S10D), and found both corrected back to control levels. Likewise, for wg/gbb;hs6st/hs6st compared to control, Brp number (p = 0.54, n = 58) and area (p = 0.19, n = 58) were also corrected back to control levels. These results provide strong genetic evidence that Wg and Gbb trans-synaptic signaling changes are causative for the pre - and postsynaptic molecular differentiation defects in the sulf1 and hs6st null mutants.
These bidirectional pre - and postsynaptic molecular changes parallel functional transmission changes in sulf1 and hs6st mutants (Figure 2). To assay function at the single synapse level, we finally assayed spontaneous synaptic vesicle fusion events. Representative mEJC traces for control compared to sulf1 and hs6st nulls are shown in Figure 8E. Consistent with observed bidirectional changes in evoked transmission, mEJC amplitudes in hs6st were ∼25% lower than in sulf1 nulls (hs6st, 0.60±0.02 nA vs. sulf1, 0.76±0.05 nA; p<0.5, n = 34; Figure 8F). Moreover, hs6st nulls had a ∼100% elevated mEJC frequency compared to sulf1 nulls (hs6st, 2.56±0.27 vs. sulf1, 1.30±0.09; p<0.001, n = 34; Figure 8G). Based on these mEJC measurements, there was a highly significant bidirectional change in quantal content between the two mutant conditions, with sulf1 quantal content ∼50% greater than hs6st (sulf1, 539.98±22.02 vs. hs6st, 350.69±8.92; p<0.001, n = 34; Figure 8H). Taken together, these results show a bi-directional change in presynaptic glutamate release machinery and vesicle fusion probability, as well as postsynaptic glutamate receptor levels and functional responsiveness. We conclude that these changes underlie the bi-directional switch in neurotransmission strength characterizing sulf1 and hs6st mutants.
Discussion
It is well known that synaptic interfaces harbor heavily-glycosylated membrane proteins, glycolipids and ECM molecules, but understanding of glycan-mediated mechanisms within this synaptomatrix is limited [9]. Our genomic screen aimed to systematically interrogate glycan roles in both structural and functional development in the genetically-tractable Drosophila NMJ synapse. 130 candidate genes were screened, classified into 8 functional families: N-glycan biosynthesis, O-glycan biosynthesis, GAG biosynthesis, glycoprotein/proteoglycan core proteins, glycan modifying/degrading enzymes, glycosyltransferases, sugar transporters and glycan-binding lectins. From this screen, 103 RNAi knockdown conditions were larval viable, whereas 27 others produced early developmental lethality. 35 genes had statistically significant effects on different measures of morphological development: 27 RNAi-mediated knockdowns increased synaptic bouton number, 9 affected synapse area (2 increased, 7 decreased) and 2 genes increased synaptic branch number. These data suggest that overall glycan mechanisms predominantly serve to limit synaptic morphogenesis. 13 genes had significant effects on the functional differentiation of the synapse, with 12 increasing transmission strength and only 1 decreasing function upon RNAi knockdown. Thus, glycan-mediated mechanisms also predominantly limit synaptic functional development. A very small fraction of tested genes (CG1597; pgant35A, CG7480; veg, CG6657; hs6st, CG4451; sulf1, CG6725 and CG11874) had effects on both morphology and function. A large percentage of genes (∼30%) showed morphological defects with no corresponding effect on function, while only 7% of genes showed functional alterations without morphological defects, and <5% of all genes affect both. These results suggest that glycans have clearly separable roles in modulating morphological and functional development of the NMJ synapse.
A growing list of neurological disorders linked to the synapse are attributed to dysfunctional glycan mechanisms, including muscular dystrophies, cognitive impairment and autism spectrum disorders [65], [66], [67]. Drosophila homologs of glycosylation genes implicated in neural disease states include ALG3 (CG4084), ALG6 (CG5091), DPM1 (CG10166), FUCT1 (CG9620), GCS1 (CG1597), MGAT2 (CG7921), MPDU1 (CG3792), PMI (CG33718) and PPM2 (CG12151) [65]. Two of these genes, Gfr (CG9620) and CG1597, showed synaptic morphology phenotypes in our RNAi screen. Given that connectivity defects are clearly implicated in cognitive impairment and autism spectrum disorders [68], [69], it would be of interest to explore the glycan mechanism affecting synapse morphology in Drosophila models of these disease states. Glycans are well known to modulate extracellular signaling, including ligands of integrin receptors, to regulate intercellular communication [70], [71]. In our genetic screen, several O-glycosyltransferases mediating this mechanism were identified to show morphological (GalNAc-T2, CG6394; pgant35A, CG7480, O-fut2, CG14789; rumi, CG31152) and functional (pgant5, CG31651; pgant35A, CG7480) synaptic defects upon RNAi knockdown. These findings suggest that known integrin-mediated signaling pathways controlling NMJ synaptic structural and functional development [16], [41], [72], [73] are modulated by glycan mechanisms. Our screen showed CG6657 RNAi knockdown affects functional differentiation, consistent with reports that this gene regulates peripheral nervous system development [74]. The corroboration of our screen results with published reports underscores the utility of RNAi-mediated screening to identify glycan mechanisms, and supports use of our screen results for bioinformatic/meta-analysis to link observed phenotypes to neurophysiological/pathological disease states and to direct future glycan mechanism studies at the synapse.
From our screen, the two functionally-paired genes sulf1 and hs6st were selected for further characterization. As in the RNAi screen, null alleles of these two genes had opposite effects on synaptic functional differentiation but similar effects on synapse morphogenesis, validating the corresponding screen results. The two gene products have functionally-paired roles; Hs6st is a heparan sulfate (HS) 6-O-sulfotransferase [43], and Sulf1 is a HS 6-O-endosulfatase [75]. These activities control sulfation of the same C6 on the repeated glucosamine moiety in HS GAG chains found on heparan sulfate proteoglycans (HSPGs). At the Drosophila NMJ, two HSPGs are known to regulate synapse assembly; the GPI-anchored glypican Dally-like protein (Dlp), and the transmembrane Syndecan (Sdc) [45]. In contrast, the secreted HSPG Perlecan (Trol) is not detectably enriched at the NMJ [76], and indeed appears to be selectively excluded from the perisynaptic domain. In other developmental contexts, the membrane HSPGs Dlp and Sdc are known to act as co-receptors for WNT and BMP ligands, regulating ligand abundance, presentation to cognate receptors and therefore signaling [20], [48]. Importantly, the regulation of HSPG co-receptor abundance has been shown to be dependent on sulfation state mediated by extracellular sulfatases [77]. Consistently, we observed upregulation of Dlp and Sdc in sulf1 null synapses, whereas Dlp was reduced in hs6st null synapses. In the developing Drosophila wing disc, HSPG co-receptors increase levels of the Wg ligand due to extracellular stabilization [78], and the primary function of Dlp in this developmental context is to retain Wg at the cell surface [21]. Likewise, in developing Drosophila embryos, a significant fraction of Wg ligand is retained on the cell surfaces in a HSPG-dependent manner [79], with the HSPG acting as an extracellular co-receptor. Syndecan also modulates ligand-dependent activation of cell-surface receptors by acting as a co-receptor [19], [20]. At the NMJ, regulation of both these HSPG co-receptors occurs in the closely juxtaposed region between presynaptic bouton and muscle subsynaptic reticulum, in the exact same extracellular space traversed by the secreted trans-synaptic Wg and Gbb signals [45]. We therefore proposed that altered Dlp and Sdc HSPG co-receptors in sulf1 and hs6st mutants differentially trap/stabilize Wg and Gbb trans-synaptic signals at the interface between motor neuron and muscle, to modulate the extent and efficacy of intercellular signaling driving synaptic development.
HS sulfation modification is linked to modulating the intercellular signaling driving neuronal differentiation [80]. In particular, WNT and BMP ligands are both regulated via HS sulfation of their extracellular co-receptors, and both signals have multiple functions directing neuronal differentiation, including synaptogenesis [49], [50], [51]. In the Drosophila wing disc, extracellular WNT (Wg) ligand abundance and distribution was recently shown to be strongly elevated in sulf1 null mutants [22]. Moreover, sulf1 has also recently been shown to modulate BMP signaling in other cellular contexts [81]. Consistently, we have shown here increased WNT Wg and the BMP Gbb abundance and distribution in sulf1 null NMJ synapses. The hs6st null also exhibits elevated Wg and Gbb at the synaptic interface, albeit the increase is lower and results in differential signaling consequences. In support of this contrasting effect, extracellular signaling ligands are known to bind HSPG HS chains differentially dependent on specific sulfation patterns [82], [83], [84]. It is important to note that the sulf1 and hs6st modulation of trans-synaptic signals is not universal, as Jelly Belly (Jeb) ligand abundance and distribution was not altered in the sulf1 and hs6st null conditions [17]. This indicates that discrete classes of secreted trans-synaptic molecules are modulated by distinct glycan mechanisms to control NMJ structure and function.
At the Drosophila NMJ, Wg is very well characterized as an anterograde trans-synaptic signal [23], [24], [85] and Gbb is very well characterized as a retrograde trans-synaptic signal [25], [26], [50], [86]. In Wg signaling, the dFz2 receptor is internalized upon Wg binding and then cleaved so that the dFz2-C fragment is imported into muscle nuclei [57], [59], [85]. In hs6st nulls, increased Wg ligand abundance at the synaptic terminal corresponds to an increase in dFz2C punctae in muscle nuclei as expected. In contrast, the increase in Wg at the sulf1 null synapse did not correspond to an increase in the dFz2C-terminus nuclear internalization, but rather a significant decrease. One explanation for this apparent discrepancy is the ‘exchange factor’ model based on the biphasic ability of the HSPG co-receptor Dlp to modulate Wg signaling [48]. In the Drosophila wing disc, this model suggests that the transition of Dlp co-receptor from an activator to repressor of signaling depends on Wg cognate receptor dFz2 levels, such that a low ratio of Dlp∶dFz2 potentiates Wg-dFz2 interaction, whereas a high ratio of Dlp∶dFz2 prevents dFz2 from capturing Wg [48]. In sulf1 null synapses, we observe a very great increase in Dlp abundance (∼40% elevated) with no significant change in the dFz2 receptor. In contrast, at hs6st null synapses there is a decrease in Dlp abundance (15% decreased) together with a significant increase in dFz2 receptor abundance (∼25% elevated). Thus, the higher Dlp∶dFz2 ratio in sulf1 nulls could explain the decrease in Wg signal activation, evidenced by decreased dFz2-C terminus import into the muscle nucleus. In contrast, the Dlp∶Fz2 ratio in hs6st is much lower, supporting activation of the dFz2-C terminus nuclear internalization pathway. This previously proposed competitive binding mechanism dependent on Dlp co-receptor and dFz2 receptor ratios predicts the observed synaptic Wg signaling pathway modulation in sulf1 and hs6st dependent manner [48].
At the Drosophila NMJ, Gbb is very well characterized as a retrograde trans-synaptic signal, with muscle-derived Gbb causing the receptor complex Wishful thinking (Wit), Thickveins (Tkv) and Saxaphone (Sax) to induce phosphorylation of the transcription factor mothers against Mothers against decapentaplegic (P-Mad) [25], [26], [87]. Mutation of Gbb ligand, receptors or regulators of this pathway have shown that Gbb-mediated retrograde signaling is required for proper synaptic differentiation and functional development [25], [52], [61], [86], [88]. Further, loss of Gbb signaling results in significantly decreased levels of P-Mad in the motor neurons [25]. We show here that accumulation of Gbb in sulf1 and hs6st null synapses causes elevated P-Mad signaling at the synapse and P-Mad accumulation in motor neuron nuclei. Importantly, sulf1 null synapses show a significantly higher level of P-Mad signaling compared to hs6st null synapses, and this same change is proportionally found in P-Mad accumulation within the motor neuron nuclei. These findings indicate differential activation of Gbb trans-synaptic signaling dependent on the HS sulfation state is controlled by the sulf1 and hs6st mechanism, similar to the differential effect observed on Wg trans-synaptic signaling. Our genetic interaction studies show that these differential effects on trans-synaptic signaling have functional consequences, and exert a causative action on the observed bi-directional functional differentiation phenotypes in sulf1 and hs6st nulls. Genetic correction of Wg and Gbb defects in the sulf1 null background restores elevated transmission back to control levels. Similarly, genetic correction of Wg and Gbb in hs6st nulls restores the decreased transmission strength back to control levels. These results demonstrate that the Wg and Gbb trans-synaptic signaling pathways are differentially regulated and, in combination, induce opposite effects on synaptic differentiation.
Both wg and gbb pathway mutants display disorganized and mislocalized presynaptic components at the active zone (e.g. Bruchpilot; Brp) and postsynaptic components including glutamate receptors (e.g. Bad reception; Brec/GluRIID) [23], [86], [89]. Consistently, the bi-directional effects on neurotransmission strength in sulf1 and hs6st mutants are paralleled by dysregulation of these same synaptic components. Changes in presynaptic Brp and postsynaptic GluR abundance/distribution causally explain the bi-directional effects on synaptic functional strength between sulf1 and hs6st null mutant states. Alterations in active zone Brp and postsynaptic GluRs also agree with assessment of spontaneous synaptic activity. Null sulf1 and hs6st synapses showed opposite effects on miniature evoked junctional current (mEJC) frequency (presynaptic component) and amplitude (postsynaptic component). Further, quantal content measurements also support the observation of bidirectional synaptic function in the two functionally paired nulls. Genetic correction of Wg and Gbb defects in both sulf1 and hs6st nulls restores the molecular composition of the pre - and postsynaptic compartments back to wildtype levels. When both trans-synaptic signaling pathways are considered together, these data suggest that HSPG sulfate modification under the control of functionally-paired sulf1 and hs6st jointly regulates both WNT and BMP trans-synaptic signaling pathways in a differential manner to modulate synaptic functional development on both sides of the cleft.
We present here the first systematic investigation of glycan roles in the modulation of synaptic structural and functional development. We have identified a host of glycan-related genes that are important for modulating neuromuscular synaptogenesis, and these genes are now available for future investigations, to determine mechanistic requirements at the synapse, and to explore links to neurological disorders. As proof for the utilization of these screen results, this study has identified extracellular heparan sulfate modification as a critical platform of the intersection for two secreted trans-synaptic signals, and differential control of their downstream signaling pathways that drive synaptic development. Other trans-synaptic signaling pathways are independent and unaffected by this mechanism, although it is of course possible that a larger assortment of signals could be modulated by this or similar mechanisms. This study supports the core hypothesis that the extracellular space of the synaptic interface, the heavily-glycosylated synaptomatrix, forms a domain where glycans coordinately mediate regulation of trans-synaptic pathways to modulate synaptogenesis and subsequent functional maturation.
Materials and Methods
Drosophila stocks and genetics
The glycan-related gene collection was generated using the KEGG glycan databases and Flybase annotation. The 163 UAS-RNAi lines tested were obtained from the Vienna Drosophila RNAi Center (VDRC) and Harvard TriP collection. Transgenic UAS-RNAi males were crossed to GAL4 driver females, with progeny raised at 25°C on standard food, controlling for density (3 ♀ crossed to 2 ♂). The UH1-GAL4 driver was used for ubiquitous knockdown of target gene expression [15]. Neural specific elav-GAL4 [90], muscle specific 24B-GAL4 [91] and glia specific repo-GAL4 lines [92] from Bloomington stock center were used to assay cell-targeted knockdown. The two sulf1 null alleles used were sulf1Δ1 [42] and sulf1ΔP1 [43]. The two hs6st null alleles used were hs6std770 and the deficiency Df(3R)ED6027 [93]. The wg allele wgI-12 [94] and gbb alleles gbb1 and gbb2 were used [25], [87]. Multiply mutant animals were made using standard genetic crosses. The trol-GFP line was obtained from Flytrap [76].
Antibody production
We generated a rabbit polyclonal anti-Gbb antibody using a 1∶1 combination of two Gbb-specific peptides (SHHRSKRSASHP, NDENVNLKKYRNMIVKSC) corresponding to amino acids 319–330 and 435–452 of Gbb (Young-In Frontier, Seoul, Korea). The antibody was purified by Protein A affinity chromatography, and antibody specificity demonstrated by examining immunoreactivity in the wandering third instar neuromusculature with gbb mutants and by expressing UAS-gbb9.1 under the control of the muscle driver BG57-GAL4 (Figure S11). Immunoreactivity in the wandering third instar neuromusculature was severely reduced in a strong hypomorphic gbb allele (gbb1/gbb2, UAS-gbb9.9), which has leaky expression of UAS-gbb9.9 in a null allelic combination [25], [87], [95]. In sharp contrast, the anti-Gbb signal was strongly elevated in BG57-GAL4/UAS-gbb9.1 relative to wildtype larvae.
Immunocytochemistry
Wandering third instars were dissected in Ca2+-free saline and then immediately fixed in either 4% paraformaldehyde for 10 minutes (all labels except anti-Dlp) or Bouin's fixative for 30 mins (anti-Dlp). Preparations were then washed in permeabilizing PBST (PBS+0.1% Triton-X) or detergent-free PBS for extracellular labeling only [16]. The following primary antibodies were used: rabbit or goat anti-HRP (1∶250; Jackson ImmunoResearch Laboratories); mouse anti-DLG (4F3; 1∶250; Developmental Studies Hybridoma Bank (DSHB)); mouse anti–Fasciclin II (1D4; 1∶5; DSHB); mouse anti-Dlp (13G8, 1∶5; DSHB) and rabbit anti-Syndecan (1∶200) [96]; mouse anti-Wg (4D4; 1∶2 DSHB) and rabbit anti-Gbb (1∶100); rabbit anti-PcanV (1∶1000) [97]; guinea pig anti-Jeb (1∶100) [17]; rabbit anti-dFz2-C (1∶500) and rabbit anti-dFz2-N (1∶100) [57]; rabbit anti-Htl (1∶100) [56]; rabbit anti-P-Mad (PS1; 1∶1000) [60]; rabbit anti-GluRIID (1∶500) [64] and mouse anti-BRP (1∶100; DSHB). Primary antibodies were incubated at 4°C overnight. Alexa-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories) were used at 1∶250 dilutions for 2 hours at room temperature. Staining with propidium iodide (Sigma Aldrich) to visualize cell nuclei was done at 1∶100 dilution of 1 mg/ml propidium iodide incubated for 30 minutes at room temperature.
Imaging quantification
Images were taken with on an upright Zeiss LSM 510 META laser-scanning confocal using a Plan Apo 63× oil objective. For structural quantification, including NMJ synapse branch number, bouton number and area, preparations were double-labeled with anti-HRP and anti-DLG, with counts made at muscle 4 in segment A3. For nuclear import studies, nuclei were identified by propidium iodide staining with fluorescent punctae counted and intensity quantified [59]. For synaptic functional protein quantitation, glutamate receptor and Brp punctae were quantified for muscle 4, segment 3. Glutamate receptor number and field area was quantified in consecutive boutons of >3 µm diameter. All preparations were fixed, stained and processed simultaneously to allow for intensity comparisons. All analyses were done with ImageJ software (National Institutes of Health) using the threshold function to outline areas and Z-stacks made using the maximum projection function. Statistics were done either with one-way ANOVA analysis followed by Dunnett's post-test, student's t-test or Mann-Whitney test for non-parametric data. All analyses were done blind to genotypes during all stages of experimentation and analysis. All figure images were projected in LSM Image Examiner (Zeiss) and exported to Adobe photoshop.
Heparin treatment
Stock solution of heparin (Sigma, H3393) in 1×PBS was prepared and serially diluted to obtain concentrations (e.g. 0.625, 0.315 and 0.156 mg/ml). Dissected wandering third instar larvae were incubated with these heparin concentrations for 5 minutes at RT, followed by a 1 minute wash with 1×PBS and then 10 minute fix with 4% paraformaldehyde in 1×PBS. After fixation, anti-Wg or anti-Gbb antibodies were used as above with appropriate secondary antibodies. Processed animals were analyzed for changes in intensity measurements as above in the image quantification section. All fluorescence intensity measurements were compared to preparations treated identically with only 1×PBS and no heparin, and the processed simultaneously for immunolabeling, microscopy and quantification.
Electrophysiology
Two-electrode voltage-clamp (TEVC) records were made from the wandering third instar NMJ as previously described [41]. In brief, staged control, mutant and transgenic RNAi animals were secured on sylgard-coated coverslips with surgical glue (liquid suture), dissected longitudinally along the dorsal midline, and glued flat. The segmental nerves were cut near the base of the ventral nerve cord. Recording was performed in 128 mM NaCl, 2 mM KCl, 4 mM MgCl2, 1.0 mM CaCl2, 70 mM sucrose, and 5 mM Hepes. Recording electrodes (1-mm outer diameter capillaries; World Precision Instruments) were filled with 3 M KCl and had resistances of >15 MΩ. Spontaneous mEJCs were collected using continuous (gap-free) recording and evoked EJC recordings were made from the voltage-clamped (Vhold = −60 mV) muscle 6 in segment A3 with a TEVC amplifier (Axoclamp 200B; MDS Analytical Technologies). The cut segmental nerve was stimulated with a glass suction electrode at a suprathreshold voltage level (50% above baseline threshold value) for a duration of 0.5 ms. Records were made with 0.2 Hz nerve stimulation in episodic acquisition setting and analyzed with Clampex software (version 7.0; Axon Instruments). Each n = 1 represents a recording from a different animal. Statistical comparisons were performed using student's t-test or the Mann-Whitney test for non-parametric data.
Supporting Information
Zdroje
1. IozzoRV (1998) Matrix proteoglycans: From molecular design to cellular function. Annual Review of Biochemistry 67 : 609–652.
2. VarkiA (2011) Evolutionary Forces Shaping the Golgi Glycosylation Machinery: Why Cell Surface Glycans Are Universal to Living Cells. Cold Spring Harbor Perspectives in Biology 3: a005462.
3. KleeneR, SchachnerM (2004) Glycans and neural cell interactions. Nature Reviews Neuroscience 5 : 195–208.
4. DityatevA, SchachnerM (2006) The extracellular matrix and synapses. Cell and Tissue Research 326 : 647–654.
5. Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, et al.. (2009) Essentials of glycobiology.
6. HagenKGT, ZhangLP, TianE, ZhangY (2009) Glycobiology on the fly: Developmental and mechanistic insights from Drosophila. Glycobiology 19 : 102–111.
7. BarrosCS, FrancoSJ, MuellerU (2011) Extracellular Matrix: Functions in the Nervous System. Cold Spring Harbor Perspectives in Biology 3.
8. VautrinJ (2010) The synaptomatrix: A solid though dynamic contact disconnecting transmissions from exocytotic events. Neurochemistry International 57 : 85–96.
9. DaniN, BroadieK (2012) Glycosylated synaptomatrix regulation of trans-synaptic signaling. Developmental Neurobiology 72 : 2–21.
10. RuppF, PayanDG, MagillsolcC, CowanDM, SchellerRH (1991) Structure and expression of rat agrin. Neuron 6 : 811–823.
11. TsimKWK, RueggMA, EscherG, KrogerS, McMahanUJ (1992) cDNA that encodes active agrin. Neuron 8 : 677–689.
12. TsenG, HalfterW, KrogerS, ColeGJ (1995) Agrin is a heparan-sulfate proteoglycan. Journal of Biological Chemistry 270 : 3392–3399.
13. McDonnellKMW, GrowWA (2004) Reduced glycosaminoglycan sulfation diminishes the agrin signal transduction pathway. Developmental Neuroscience 26 : 1–10.
14. ParkhomovskiyN, KammesheidtA, MartinPT (2000) N-acetyllactosamine and the CT carbohydrate antigen mediate agrin-dependent activation of MuSK and acetylcholine receptor clustering in skeletal muscle. Molecular and Cellular Neuroscience 15 : 380–397.
15. RohrboughJ, RushtonE, WoodruffE, FergestadT, VigneswaranK, et al. (2007) Presynaptic establishment of the synaptic cleft extracellular matrix is required for post-synaptic differentiation. Genes & Development 21 : 2607–2628.
16. RushtonE, RohrboughJ, BroadieK (2009) Presynaptic Secretion of Mind-the-Gap Organizes the Synaptic Extracellular Matrix-Integrin Interface and Postsynaptic Environments. Developmental Dynamics 238 : 554–571.
17. RohrboughJ, BroadieK (2010) Anterograde Jelly belly ligand to Alk receptor signaling at developing synapses is regulated by Mind the gap. Development 137 : 3523–3533.
18. RushtonE, RohrboughJ, DeutschK, BroadieK (2012) Structure-function analysis of endogenous lectin mind-the-gap in synaptogenesis. Developmental Neurobiology 72 : 1161–1179.
19. CareyDJ (1997) Syndecans: Multifunctional cell-surface co-receptors. Biochemical Journal 327 : 1–16.
20. DejimaK, KanaiMI, AkiyamaT, LevingsDC, NakatoH (2011) Novel Contact-dependent Bone Morphogenetic Protein (BMP) Signaling Mediated by Heparan Sulfate Proteoglycans. Journal of Biological Chemistry 286 : 17103–17111.
21. YanD, LinX (2009) Shaping Morphogen Gradients by Proteoglycans. Cold Spring Harbor Perspectives in Biology 1: a002493.
22. KleinschmitA, KoyamaT, DejimaK, HayashiY, KamimuraK, et al. (2010) Drosophila heparan sulfate 6-O endosulfatase regualtes Wingless morphogen gradient formation. Developmental biology 345 : 204–214.
23. PackardM, KooES, GorczycaM, SharpeJ, CumberledgeS, et al. (2002) The drosophila wnt, wingless, provides an essential signal for pre - and postsynaptic differentiation. Cell 111 : 319–330.
24. KorkutC, BudnikV (2009) WNTs tune up the neuromuscular junction. Nature Reviews Neuroscience 10 : 627–634.
25. McCabeBD, MarquesG, HaghighiAP, FetterRD, CrottyML, et al. (2003) The BMP homolog Gbb provides a retrograde signal that regulates synaptic growth at the Drosophila neuromuscular junction. Neuron 39 : 241–254.
26. KeshishianH, KimYS (2004) Orchestrating development and function: retrograde BMP signaling in the Drosophila nervous system. Trends in Neurosciences 27 : 143–147.
27. HoltCE, DicksonBJ (2005) Sugar codes for axons? Neuron 46 : 169–172.
28. MataniP, SharrowM, TiemeyerM (2007) Ligand, modulatory, and co-receptor functions of neural glycans. Frontiers in Bioscience 12 : 3852–3879.
29. YamaguchiY (2002) Glycobiology of the synapse: the role of glycans in the formation, maturation, and modulation of synapses. Biochimica Et Biophysica Acta-General Subjects 1573 : 369–376.
30. MartinPT (2002) Glycobiology of the synapse. Glycobiology 12 : 1R–7R.
31. MartinPT (2003) Glycobiology of the neuromuscular junction. Journal of Neurocytology 32 : 915.
32. KeshishianH, BroadieK, ChibaA, BateM (1996) The Drosophila neuromuscular junction: A model system for studying synaptic development and function. Annual Review of Neuroscience 19 : 545–575.
33. GramatesLS, BudnikV (1999) Assembly and maturation of the Drosophila larval neuromuscular junction. Neuromuscular Junctions in Drosophila 43 : 93–+.
34. Ruiz-CanadaC, BudnikV (2006) Introduction on the use of the Drosophila embryonic/larval neuromuscular junction as a model system to study synapse development and function, and a brief summary of pathfinding and target recognition. Fly Neuromuscular Junction: Structure and Function, Second Edition 75 : 1–31.
35. DietzlG, ChenD, SchnorrerF, SuKC, BarinovaY, et al. (2007) A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448 : 151–U151.
36. WodarzA, HinzU, EngelbertM, KnustE (1995) Expression of crumbs confers apical character on plasma-membrane domains of ectodermal epithelia of Drosophila. Cell 82 : 67–76.
37. KanehisaM, GotoS (2000) KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Research 28 : 27–30.
38. HoffmannR, ValenciaA (2004) A gene network for navigating the literature. Nature Genetics 36 : 664–664.
39. TweedieS, AshburnerM, FallsK, LeylandP, McQuiltonP, et al. (2009) FlyBase: enhancing Drosophila Gene Ontology annotations. Nucleic Acids Research 37: D555–D559.
40. GattoCL, BroadieK (2008) Temporal requirements of the fragile X mental retardation protein in the regulation of synaptic structure. Development 135 : 2637–2648.
41. BeumerKJ, RohrboughJ, ProkopA, BroadieK (1999) A role for PS integrins in morphological growth and synaptic function at the postembryonic neuromuscular junction of Drosophila. Development 126 : 5833–5846.
42. YouJ, BelenkayaT, LinX (2011) Sulfated Is a Negative Feedback Regulator of Wingless in Drosophila. Developmental Dynamics 240 : 640–648.
43. KamimuraK, FujiseM, VillaF, IzumiS, HabuchiH, et al. (2001) Drosophila heparan sulfate 6-O-sulfotransferase (dHS6ST) gene - Structure, expression, and function in the formation of the tracheal system. Journal of Biological Chemistry 276 : 17014–17021.
44. AiXB, DoAT, LozynskaO, Kusche-GullbergM, LindahlU, et al. (2003) QSulf1 remodels the 6-O sulfation states of cell surface heparan sulfate proteoglycans to promote Wnt signaling. Journal of Cell Biology 162 : 341–351.
45. JohnsonKG, TenneyAP, GhoseA, DuckworthAM, HigashiME, et al. (2006) The HSPGs syndecan and dallylike bind the receptor phosphatase LAR and exert distinct effects on synaptic development. Neuron 49 : 517–531.
46. LinXH, PerrimonN (2000) Role of heparan sulfate proteoglycans in cell-cell signaling in Drosophila. Matrix Biology 19 : 303–307.
47. HackerU, NybakkenK, PerrimonN (2005) Heparan sulphate proteoglycans: The sweet side of development. Nature Reviews Molecular Cell Biology 6 : 530–541.
48. YanD, WuY, FengY, LinS-C, LinX (2009) The Core Protein of Glypican Daily-Like Determines Its Biphasic Activity in Wingless Morphogen Signaling. Developmental Cell 17 : 470–481.
49. SalinasPC (2003) Synaptogenesis: Wnt and TGF-beta take centre stage. Current Biology 13: R60–R62.
50. MarquesG (2005) Morphogens and synaptogenesis in Drosophila. Journal of Neurobiology 64 : 417–434.
51. PackardM, MathewD, BudnikV (2003) Wnts and TGF beta in synaptogenesis: Old friends signalling at new places. Nature Reviews Neuroscience 4 : 113–120.
52. RawsonJM, LeeM, KennedyEL, SelleckSB (2003) Drosophila neuromuscular synapse assembly and function require the TGF-beta type I receptor saxophone and the transcription factor mad. Journal of Neurobiology 55 : 134–150.
53. Cox M, Nelson D (2004) Lehninger, Principles of Biochemistry. 1100.
54. ShimokawaK, Kimura-YoshidaC, NagaiN, MukaiK, MatsubaraK, et al. (2011) Cell Surface Heparan Sulfate Chains Regulate Local Reception of FGF Signaling in the Mouse Embryo. Developmental Cell 21 : 257–272.
55. SenA, YokokuraT, KankelMW, DimlichDN, ManentJ, et al. (2011) Modeling spinal muscular atrophy in Drosophila links Smn to FGF signaling. Journal of Cell Biology 192 : 481–495.
56. ShishidoE, OnoN, KojimaT, SaigoK (1997) Requirements of DFR1/heartless, a mesoderm-specific Drosophila FGF-receptor, for the formation of heart, visceral and somatic muscles, and ensheathing of longitudinal axon tracts in CNS. Development 124 : 2119–2128.
57. MathewD, AtamanB, ChenJY, ZhangYL, CumberledgeS, et al. (2005) Wingless signaling at synapses is through cleavage and nuclear import of receptor DFrizzled2. Science 310 : 1344–1347.
58. KimNMG (2010) Identification of downstream targets of the Bone Morphogenetic Protein pathway in the Drosophila nervous system. Developmental Dynamics 239 : 2413–2425.
59. MoscaTJ, SchwarzTL (2010) The nuclear import of Frizzled2-C by Importins-beta 11 and alpha 2 promotes postsynaptic development. Nature Neuroscience 13 : 935–U950.
60. PerssonU, IzumiH, SouchelnytskyiS, ItohS, GrimsbyS, et al. (1998) The L45 loop in type I receptors for TGF-beta family members is a critical determinant in specifying Smad isoform activation. Febs Letters 434 : 83–87.
61. NahmM, LongAA, PaikSK, KimS, BaeYC, et al. (2010) The Cdc42-selective GAP Rich regulates postsynaptic development and retrograde BMP transsynaptic signaling. Journal of Cell Biology 191 : 661–675.
62. Higashi-KovtunME, MoscaTJ, DickmanDK, MeinertzhagenIA, SchwarzTL (2010) Importin-beta 11 Regulates Synaptic Phosphorylated Mothers Against Decapentaplegic, and Thereby Influences Synaptic Development and Function at the Drosophila Neuromuscular Junction. Journal of Neuroscience 30 : 5253–5268.
63. WaghDA, RasseTM, AsanE, HofbauerA, SchwenkertI, et al. (2006) Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila (vol 49, pg 833, 2006). Neuron 51 : 275–275.
64. FeatherstoneDE, RushtonE, RohrboughJ, LieblF, KarrJ, et al. (2005) An essential Drosophila glutamate receptor subunit that functions in both central neuropil and neuromuscular junction. Journal of Neuroscience 25 : 3199–3208.
65. InlowJK, RestifoLL (2004) Molecular and comparative genetics of mental retardation. Genetics 166 : 835–881.
66. MuntoniF, TorelliS, BrockingtonM (2008) Muscular dystrophies due to glycosylation defects. Neurotherapeutics 5 : 627–632.
67. SchachterH, FreezeHH (2009) Glycosylation diseases: Quo vadis? Biochimica Et Biophysica Acta-Molecular Basis of Disease 1792 : 925–930.
68. BelmonteMK, AllenG, Beckel-MitchenerA, BoulangerLM, CarperRA, et al. (2004) Autism and abnormal development of brain connectivity. Journal of Neuroscience 24 : 9228–9231.
69. GattoCL, BroadieK (2011) Drosophila modeling of heritable neurodevelopmental disorders. Current Opinion in Neurobiology 21 (6) 834–41.
70. ZhangLP, ZhangY, Ten HagenKG (2008) A Mucin-type O-Glycosyltransferase Modulates Cell Adhesion during Drosophila Development. Journal of Biological Chemistry 283 : 34076–34086.
71. ZhangLP, TranDT, Ten HagenKG (2010) An O-Glycosyltransferase Promotes Cell Adhesion during Development by Influencing Secretion of an Extracellular Matrix Integrin Ligand. Journal of Biological Chemistry 285 : 19491–19501.
72. RohrboughJ, GrotewielMS, DavisRL, BroadieK (2000) Integrin-mediated regulation of synaptic morphology, transmission, and plasticity. Journal of Neuroscience 20 : 6868–6878.
73. BeumerK, MatthiesHJG, BradshawA, BroadieK (2002) Integrins regulate DLG/FAS2 via a CaM kinase II-dependent pathway to mediate synapse elaboration and stabilization during postembryonic development. Development 129 : 3381–3391.
74. ProkopenkoSN, HeYC, LuY, BellenHJ (2000) Mutations affecting the development of the peripheral nervous system in drosophila: A molecular screen for novel proteins. Genetics 156 : 1691–1715.
75. DhootGK, GustafssonMK, AiXB, SunWT, StandifordDM, et al. (2001) Regulation of Wnt signaling and embryo patterning by an extracellular sulfatase. Science 293 : 1663–1666.
76. MorinX, DanemanR, ZavortinkM, ChiaW (2001) A protein trap strategy to detect GFP-tagged proteins expressed from their endogenous loci in Drosophila. Proceedings of the National Academy of Sciences of the United States of America 98 : 15050–15055.
77. LaiJ-P, SandhuDS, YuC, HanT, MoserCD, et al. (2008) Sulfatase 2 up-regulates glypican 3, promotes fibroblast growth factor signaling, and decreases survival in hepatocellular carcinoma. Hepatology 47 : 1211–1222.
78. HanC, YanD, BelenkayaTY, LinXJ (2005) Drosophila glypicans Dally and Dally-like shape the extracellular Wingless morphogen gradient in the wing disc. Development 132 : 667–679.
79. PfeifferS, RicardoS, MannevilleJB, AlexandreC, VincentJP (2002) Producing cells retain and recycle wingless in Drosophila embryos. Current Biology 12 : 957–962.
80. GorsiB, StringerSE (2007) Tinkering with heparan sulfate sulfation to steer development. Trends in Cell Biology 17 : 173–177.
81. OtsukiS, HansonSR, MiyakiS, GroganSP, KinoshitaM, et al. (2010) Extracellular sulfatases support cartilage homeostasis by regulating BMP and FGF signaling pathways. Proceedings of the National Academy of Sciences of the United States of America 107 : 10202–10207.
82. BaegGH, PerrimonN (2000) Functional binding of secreted molecules to heparan sulfate proteoglycans in Drosophila. Current Opinion in Cell Biology 12 : 575–580.
83. BaegGH, LinXH, KhareN, BaumgartnerS, PerrimonN (2001) Heparan sulfate proteoglycans are critical for the organization of the extracellular distribution of Wingless. Development 128 : 87–94.
84. MohammadiM, OlsenSK, GoetzR (2005) A protein canyon in the FGF-FGF receptor dimer selects from an a la carte menu of heparan sulfate motifs. Current Opinion in Structural Biology 15 : 506–516.
85. AtamanB, AshleyJ, GorczycaD, GorczycaM, MathewD, et al. (2006) Nuclear trafficking of Drosophila Frizzled-2 during synapse development requires the PDZ protein dGRIP. Proceedings of the National Academy of Sciences of the United States of America 103 : 7841–7846.
86. MarquesG, BaoH, HaerryTE, ShimellMJ, DuchekP, et al. (2002) The Drosophila BMP type II receptor wishful thinking regulates neuromuscular synapse morphology and function. Neuron 33 : 529–543.
87. WhartonKA, CookJM, Torres-SchumannS, de CastroK, BorodE, et al. (1999) Genetic analysis of the bone morphogenetic protein-related gene, gbb, identifies multiple requirements during Drosophila development. Genetics 152 : 629–640.
88. McCabeBD, HomS, AberleH, FetterRD, MarquesG, et al. (2004) Highwire regulates presynaptic BMP signaling essential for synaptic growth. Neuron 41 : 891–905.
89. AberleH, HaghighiAP, FetterRD, McCabeBD, MagalhaesTR, et al. (2002) wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron 33 : 545–558.
90. LinDM, GoodmanCS (1994) Ectopic and increased expression of Fasciclin-II alters motoneuron growth cone guidance. Neuron 13 : 507–523.
91. BrandAH, PerrimonN (1993) Targeted gene-expression as a means of altering cell fates and generating dominant phenotypes. Development 118 : 401–415.
92. SeppKJ, SchulteJ, AuldVJ (2001) Peripheral glia direct axon guidance across the CNS/PNS transition zone. Developmental Biology 238 : 47–63.
93. KamimuraK, KoyamaT, HabuchiH, UedaR, MasuM, et al. (2006) Specific and flexible roles of heparan sulfate modifications in Drosophila FGF signaling. Journal of Cell Biology 174 : 773–778.
94. MarieB, PymE, BergquistS, DavisGW (2010) Synaptic Homeostasis Is Consolidated by the Cell Fate Gene gooseberry, a Drosophila pax3/7 Homolog. Journal of Neuroscience 30 : 8071–8082.
95. GooldCP, DavisGW (2007) The BMP ligand Gbb gates the expression of synaptic horneostasis independent of synaptic growth control. Neuron 56 : 109–123.
96. SpringJ, PainesaundersSE, HynesRO, BernfieldM (1994) Drosophila Syndecan - conservation of a cell-surface heparan-sulfate proteoglycan. Proceedings of the National Academy of Sciences of the United States of America 91 : 3334–3338.
97. FriedrichMVK, SchneiderM, TimplR, BaumgartnerS (2000) Perlecan domain V of Drosophila melanogaster - Sequence, recombinant analysis and tissue expression. European Journal of Biochemistry 267 : 3149–3159.
Štítky
Genetika Reprodukční medicína
Článek The Covariate's DilemmaČlánek Plant Vascular Cell Division Is Maintained by an Interaction between PXY and Ethylene SignallingČlánek Lessons from Model Organisms: Phenotypic Robustness and Missing Heritability in Complex Disease
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 11- Příjem alkoholu a menstruační cyklus
- Mateřský haplotyp KIR ovlivňuje porodnost živých dětí po transferu dvou embryí v rámci fertilizace in vitro u pacientek s opakujícími se samovolnými potraty nebo poruchami implantace
- Intrauterinní inseminace a její úspěšnost
- Růst a vývoj dětí narozených pomocí IVF
- Akutní intermitentní porfyrie
-
Všechny články tohoto čísla
- Inference of Population Splits and Mixtures from Genome-Wide Allele Frequency Data
- The Covariate's Dilemma
- Plant Vascular Cell Division Is Maintained by an Interaction between PXY and Ethylene Signalling
- Plan B for Stimulating Stem Cell Division
- Discovering Thiamine Transporters as Targets of Chloroquine Using a Novel Functional Genomics Strategy
- Is a Modifier of Mutations in Retinitis Pigmentosa with Incomplete Penetrance
- Evolutionarily Ancient Association of the FoxJ1 Transcription Factor with the Motile Ciliogenic Program
- Genome Instability Caused by a Germline Mutation in the Human DNA Repair Gene
- Transcription Factor Oct1 Is a Somatic and Cancer Stem Cell Determinant
- Controls of Nucleosome Positioning in the Human Genome
- Disruption of Causes Defective Meiotic Recombination in Male Mice
- A Novel Human-Infection-Derived Bacterium Provides Insights into the Evolutionary Origins of Mutualistic Insect–Bacterial Symbioses
- Trps1 and Its Target Gene Regulate Epithelial Proliferation in the Developing Hair Follicle and Are Associated with Hypertrichosis
- Zcchc11 Uridylates Mature miRNAs to Enhance Neonatal IGF-1 Expression, Growth, and Survival
- Population-Based Resequencing of in 10,330 Individuals: Spectrum of Genetic Variation, Phenotype, and Comparison with Extreme Phenotype Approach
- HP1a Recruitment to Promoters Is Independent of H3K9 Methylation in
- Transcription Elongation and Tissue-Specific Somatic CAG Instability
- A Germline Polymorphism of DNA Polymerase Beta Induces Genomic Instability and Cellular Transformation
- Interallelic and Intergenic Incompatibilities of the () Gene in Mouse Hybrid Sterility
- Comparison of Mitochondrial Mutation Spectra in Ageing Human Colonic Epithelium and Disease: Absence of Evidence for Purifying Selection in Somatic Mitochondrial DNA Point Mutations
- Mutations in the Transcription Elongation Factor SPT5 Disrupt a Reporter for Dosage Compensation in Drosophila
- Evolution of Minimal Specificity and Promiscuity in Steroid Hormone Receptors
- Blockade of Pachytene piRNA Biogenesis Reveals a Novel Requirement for Maintaining Post-Meiotic Germline Genome Integrity
- RHOA Is a Modulator of the Cholesterol-Lowering Effects of Statin
- MIG-10 Functions with ABI-1 to Mediate the UNC-6 and SLT-1 Axon Guidance Signaling Pathways
- Loss of the DNA Methyltransferase MET1 Induces H3K9 Hypermethylation at PcG Target Genes and Redistribution of H3K27 Trimethylation to Transposons in
- Genome-Wide Association Studies Reveal a Simple Genetic Basis of Resistance to Naturally Coevolving Viruses in
- The Principal Genetic Determinants for Nasopharyngeal Carcinoma in China Involve the Class I Antigen Recognition Groove
- Molecular, Physiological, and Motor Performance Defects in DMSXL Mice Carrying >1,000 CTG Repeats from the Human DM1 Locus
- Genomic Study of RNA Polymerase II and III SNAP-Bound Promoters Reveals a Gene Transcribed by Both Enzymes and a Broad Use of Common Activators
- Long Telomeres Produced by Telomerase-Resistant Recombination Are Established from a Single Source and Are Subject to Extreme Sequence Scrambling
- The Yeast SR-Like Protein Npl3 Links Chromatin Modification to mRNA Processing
- Deubiquitylation Machinery Is Required for Embryonic Polarity in
- dJun and Vri/dNFIL3 Are Major Regulators of Cardiac Aging in Drosophila
- CtIP Is Required to Initiate Replication-Dependent Interstrand Crosslink Repair
- Notch-Mediated Suppression of TSC2 Expression Regulates Cell Differentiation in the Intestinal Stem Cell Lineage
- A Combination of H2A.Z and H4 Acetylation Recruits Brd2 to Chromatin during Transcriptional Activation
- Network Analysis of a -Mouse Model of Autosomal Dominant Polycystic Kidney Disease Identifies HNF4α as a Disease Modifier
- Mitosis in Neurons: Roughex and APC/C Maintain Cell Cycle Exit to Prevent Cytokinetic and Axonal Defects in Photoreceptor Neurons
- CELF4 Regulates Translation and Local Abundance of a Vast Set of mRNAs, Including Genes Associated with Regulation of Synaptic Function
- Mechanisms Employed by to Prevent Ribonucleotide Incorporation into Genomic DNA by Pol V
- The Genomes of the Fungal Plant Pathogens and Reveal Adaptation to Different Hosts and Lifestyles But Also Signatures of Common Ancestry
- A Genome-Scale RNA–Interference Screen Identifies RRAS Signaling as a Pathologic Feature of Huntington's Disease
- Lessons from Model Organisms: Phenotypic Robustness and Missing Heritability in Complex Disease
- Population Genomic Scan for Candidate Signatures of Balancing Selection to Guide Antigen Characterization in Malaria Parasites
- Tissue-Specific Regulation of Chromatin Insulator Function
- Disruption of Mouse Cenpj, a Regulator of Centriole Biogenesis, Phenocopies Seckel Syndrome
- Genome, Functional Gene Annotation, and Nuclear Transformation of the Heterokont Oleaginous Alga CCMP1779
- Antagonistic Gene Activities Determine the Formation of Pattern Elements along the Mediolateral Axis of the Fruit
- Lung eQTLs to Help Reveal the Molecular Underpinnings of Asthma
- Identification of the First ATRIP–Deficient Patient and Novel Mutations in ATR Define a Clinical Spectrum for ATR–ATRIP Seckel Syndrome
- Cooperativity of , , and in Malignant Breast Cancer Evolution
- Loss of Prohibitin Membrane Scaffolds Impairs Mitochondrial Architecture and Leads to Tau Hyperphosphorylation and Neurodegeneration
- Microhomology Directs Diverse DNA Break Repair Pathways and Chromosomal Translocations
- MicroRNA–Mediated Repression of the Seed Maturation Program during Vegetative Development in
- Selective Pressure Causes an RNA Virus to Trade Reproductive Fitness for Increased Structural and Thermal Stability of a Viral Enzyme
- The Tumor Suppressor Gene Retinoblastoma-1 Is Required for Retinotectal Development and Visual Function in Zebrafish
- Regions of Homozygosity in the Porcine Genome: Consequence of Demography and the Recombination Landscape
- Histone Methyltransferases MES-4 and MET-1 Promote Meiotic Checkpoint Activation in
- Polyadenylation-Dependent Control of Long Noncoding RNA Expression by the Poly(A)-Binding Protein Nuclear 1
- A Unified Method for Detecting Secondary Trait Associations with Rare Variants: Application to Sequence Data
- Genetic and Biochemical Dissection of a HisKA Domain Identifies Residues Required Exclusively for Kinase and Phosphatase Activities
- Informed Conditioning on Clinical Covariates Increases Power in Case-Control Association Studies
- Biochemical Diversification through Foreign Gene Expression in Bdelloid Rotifers
- Genomic Variation and Its Impact on Gene Expression in
- Spastic Paraplegia Mutation N256S in the Neuronal Microtubule Motor KIF5A Disrupts Axonal Transport in a HSP Model
- Lamin B1 Polymorphism Influences Morphology of the Nuclear Envelope, Cell Cycle Progression, and Risk of Neural Tube Defects in Mice
- A Targeted Glycan-Related Gene Screen Reveals Heparan Sulfate Proteoglycan Sulfation Regulates WNT and BMP Trans-Synaptic Signaling
- Dopaminergic D2-Like Receptors Delimit Recurrent Cholinergic-Mediated Motor Programs during a Goal-Oriented Behavior
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Mechanisms Employed by to Prevent Ribonucleotide Incorporation into Genomic DNA by Pol V
- Inference of Population Splits and Mixtures from Genome-Wide Allele Frequency Data
- Zcchc11 Uridylates Mature miRNAs to Enhance Neonatal IGF-1 Expression, Growth, and Survival
- Histone Methyltransferases MES-4 and MET-1 Promote Meiotic Checkpoint Activation in
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Revma Focus: Spondyloartritidy
nový kurz
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



