-
Články
- Vzdělávání
- Časopisy
Top články
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The Cost of Virulence: Retarded Growth of Typhimurium Cells Expressing Type III Secretion System 1
Virulence factors generally enhance a pathogen's fitness and thereby foster transmission. However, most studies of pathogen fitness have been performed by averaging the phenotypes over large populations. Here, we have analyzed the fitness costs of virulence factor expression by Salmonella enterica subspecies I serovar Typhimurium in simple culture experiments. The type III secretion system ttss-1, a cardinal virulence factor for eliciting Salmonella diarrhea, is expressed by just a fraction of the S. Typhimurium population, yielding a mixture of cells that either express ttss-1 (TTSS-1+ phenotype) or not (TTSS-1− phenotype). Here, we studied in vitro the TTSS-1+ phenotype at the single cell level using fluorescent protein reporters. The regulator hilA controlled the fraction of TTSS-1+ individuals and their ttss-1 expression level. Strikingly, cells of the TTSS-1+ phenotype grew slower than cells of the TTSS-1− phenotype. The growth retardation was at least partially attributable to the expression of TTSS-1 effector and/or translocon proteins. In spite of this growth penalty, the TTSS-1+ subpopulation increased from <10% to approx. 60% during the late logarithmic growth phase of an LB batch culture. This was attributable to an increasing initiation rate of ttss-1 expression, in response to environmental cues accumulating during this growth phase, as shown by experimental data and mathematical modeling. Finally, hilA and hilD mutants, which form only fast-growing TTSS-1− cells, outcompeted wild type S. Typhimurium in mixed cultures. Our data demonstrated that virulence factor expression imposes a growth penalty in a non-host environment. This raises important questions about compensating mechanisms during host infection which ensure successful propagation of the genotype.
Published in the journal: . PLoS Pathog 7(7): e32767. doi:10.1371/journal.ppat.1002143
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002143Summary
Virulence factors generally enhance a pathogen's fitness and thereby foster transmission. However, most studies of pathogen fitness have been performed by averaging the phenotypes over large populations. Here, we have analyzed the fitness costs of virulence factor expression by Salmonella enterica subspecies I serovar Typhimurium in simple culture experiments. The type III secretion system ttss-1, a cardinal virulence factor for eliciting Salmonella diarrhea, is expressed by just a fraction of the S. Typhimurium population, yielding a mixture of cells that either express ttss-1 (TTSS-1+ phenotype) or not (TTSS-1− phenotype). Here, we studied in vitro the TTSS-1+ phenotype at the single cell level using fluorescent protein reporters. The regulator hilA controlled the fraction of TTSS-1+ individuals and their ttss-1 expression level. Strikingly, cells of the TTSS-1+ phenotype grew slower than cells of the TTSS-1− phenotype. The growth retardation was at least partially attributable to the expression of TTSS-1 effector and/or translocon proteins. In spite of this growth penalty, the TTSS-1+ subpopulation increased from <10% to approx. 60% during the late logarithmic growth phase of an LB batch culture. This was attributable to an increasing initiation rate of ttss-1 expression, in response to environmental cues accumulating during this growth phase, as shown by experimental data and mathematical modeling. Finally, hilA and hilD mutants, which form only fast-growing TTSS-1− cells, outcompeted wild type S. Typhimurium in mixed cultures. Our data demonstrated that virulence factor expression imposes a growth penalty in a non-host environment. This raises important questions about compensating mechanisms during host infection which ensure successful propagation of the genotype.
Introduction
The ability to infect a host and elicit disease is dictated by the virulence factors expressed by a given pathogen. This may include, but is not limited to, protective factors neutralizing antibacterial defenses, enzymes involved in nutrient acquisition within the host, regulators of virulence factor expression and toxins or secretion systems for subverting host cell signal transduction. The coordinated expression of such virulence factors enhances colonization, growth/survival within the host and transmission. However, most studies of virulence factor function and pathogen fitness have been performed in bulk assays, averaging the phenotypes over large pathogen populations of genetically identical cells. In contrast, little is known about the potential advantages, costs or burdens arising from virulence factor expression by an individual cell of the pathogen population. Therefore, single cell analyses might be of significant interest, in particular if virulence factors, which are expressed in a bistable fashion by some but not all members of a pathogen population, e.g. the ttss-1 system of S. Typhimurium [1], [2], [3], [4], [5], as described in this paper.
Bistable gene expression is genetically encoded. In most cases, one particular genotype expresses one predictable phenotype in a given environment. However, in some cases, two different phenotypes are expressed by isogenic organisms living in the same environment. This is termed phenotypic variation, bimodal gene expression or bistability and represents a special case of gene expression [6]. The importance of bistability for pathogenic bacterial fitness and evolution is just beginning to be understood.
Like other cases of gene expression, bistability is generally observed in response to particular environmental cues. The response is driven by a dedicated (set of) regulator(s), which responds to environmental signals (operon model of Jacob [7]). This response is subject to stochastic fluctuations. In particular in the case of regulators expressed in a few copies per cell, this can significantly affect the active regulator concentration thus randomizing the corresponding phenotype in a population [8], [9]. In combination with non-linear responses (e.g. regulator multimerization, feedback loops), this can lead to formation of phenotypically distinct and stable subpopulations of isogenic bacteria [6], [8], [9], [10], [11]. In terms of evolution, two models may explain the advantage of bistability: i. in “bet hedging”, the optimally adapted phenotype will prevail and ensure the survival of the shared genotype in a changing environment [12]. ii. in “division of labor”, both phenotypes cooperate to ensure survival of the shared genotype [4]. In either way, the bistable expression of certain genes is thought to promote the survival of the genotype. However, it has remained poorly understood whether/how bistability may affect the lifestyle of pathogenic bacteria.
Salmonella enterica subspecies 1 serovar Typhimurium (S. Tm) is a pathogenic Gram-negative bacterium causing numerous cases of diarrhea, worldwide. Its' type III secretion system 1 (TTSS-1) was recently identified as an example for bistable gene expression [1], [3], [5], [13]. TTSS-1 is a well-known virulence determinant of S. Tm required for eliciting diarrheal disease [14], [15], [16]. The needle like TTSS-1 apparatus injects effector proteins into host epithelial cells, thus triggering host cell invasion and pro-inflammatory responses [17], [18], [19]. TTSS-1 is encoded on a genomic island (Salmonella pathogenicity island 1 (SPI-1)), which also harbors genes for effector proteins and for several regulators of ttss-1 expression, e.g. hilA, hilC and hilD [20], [21].
The bistable ttss-1 expression is controlled by a complex regulatory network, which includes coupled positive feedback loops, controls the threshold for ttss-1 induction and amplifies ttss-1 expression [5], [22]. Bistable ttss-1 expression is observed in “ttss-1 inducing” environments, i.e. the gut lumen of infected mice or in non-host environments, e.g. when S. Tm is grown to late logarithmic phase in LB [1], [2], [4], [5]. This yields mixed populations of isogenic S. Tm cells that express ttss-1 (TTSS-1+ phenotype), or do not (TTSS-1− phenotype), in a bimodal fashion. In the mouse gut, only the TTSS-1+ cells can actively invade the mucosal tissue and efficiently trigger inflammation [4], [18]. This inflammatory response may help to overcome the commensal microflora, thus enhancing Salmonella growth and transmission [23], [24], [25], [26], [27], [28], [29]. Experimental data indicate that bistable ttss-1 expression might represent an example of “division of labor” [4], but further data is required to settle this point. At any rate, ttss-1 expression seems to be instrumental for eliciting diarrheal disease and enhancing pathogen transmission. But the functional properties of the TTSS-1+ phenotype are not well understood.
The complex setting of the infected animal gut has hampered the analysis of the TTSS-1+ phenotype. In vitro experiments are essential for gaining detailed mechanistic insights. Here, we have analyzed the induction of ttss-1 expression and its effects on the growth rate of the TTSS-1+ phenotype by single cell reporter assays, competitive growth experiments and mathematical modeling. In such non-host environments, expression of the ttss-1 virulence system expression imposed a growth penalty on the TTSS-1+ cells. This may have important implications with respect to compensatory mechanisms during the infection of animal hosts.
Results
Single cell reporters for studying the TTSS-1+ phenotype
We started our analysis of the TTSS-1+ phenotype by probing ttss-1 expression at the single cell level. For this purpose, we chose the sicA promoter (PsicA), which controls expression of the chromosomal sicAsipBCDA operon (Fig. S1C). This operon encodes key parts of the TTSS-1 virulence system. On the one hand, we employed a transcriptional sipA-tsrvenus reporter gene cassette placing the reporter downstream of the sicAsipBCDA operon (Fig. S1; [2], [3]). Due to its localization at the bacterial poles, the tsrvenus reporter allows detecting <10 proteins per cell [30]. Thus, sipA-tsrvenus provides a highly sensitive reporter for the TTSS-1+ phenotype.
Next, we verified the performance of the sipA-tsrvenus reporter. sipA-tsrvenus expression was bistable and TTSS-1− and TTSS-1+ individuals were distinguishable by the presence/absence of Tsrvenus spots at the bacterial poles ([30]; Fig. 1A; Fig. S1D). TTSS-1 expression and virulence were not compromised (Fig. 1B). The accurate response of sipA-tsrvenus to Salmonella signaling cascades was established by disturbing known elements of the TTSS-1 gene regulation network and FACS analysis of sipA-tsrvenus expression (Fig. 1C, D). In line with the published work on ttss-1 regulation (Fig. 1D): i. Over-expression of positive TTSS-1 regulators increased the abundance of tsrvenus-expressing individuals (Fig. 1C; Fig. S1D). In particular, hilA, hilC and hilD over-expression increased the fraction of sipA-tsrvenus expressing individuals from ∼20% to 80–100%. ii. The median signal intensity per sipA-tsrvenus expressing cell increased when positive regulators were over-expressed (philA: 3.8±0.3-fold; philC: 4.0±0.1-fold; philD: 4±0.1-fold; median ± s.d.). iii. Control experiments in a ΔhilA mutant verified that expression of the TTSS-1+ phenotype depended on the ttss-1 master-regulator, HilA (Fig. 1C; open bars) and iv. The average HilA protein levels of the analyzed strains correlated positively with the fraction of tsrvenus-expressing individuals (r2 = 0.78; quantitative Western blot; Fig. 1E). These data verified the accurate performance of the sipA-tsrvenus reporter and demonstrated that hilA-dependent regulation affects both, the fraction of TTSS-1+ individuals and the level of ttss-1 expression per cell.
Fig. 1. sipA-tsrvenus as a single cell reporter for ttss-1 expression. 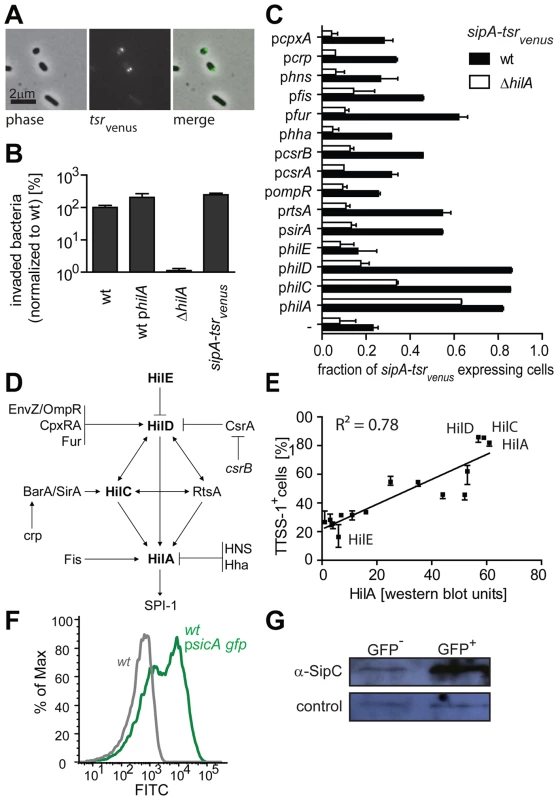
A) Bistable expression of sipA-tsrvenus in wt S. Tm (M2001). Living bacteria (4 h in LB) were imaged by fluorescence- and phase contrast microscopy. Bar, 2 µm; B) Invasion into MDCK cells (3 indep. experiments; ±s.d.; Materials and Methods). C) Response of the sipA-tsrvenus reporter to over-expression of known ttss-1 regulators. Wt S. Tm (sipA-tsrvenus; M2001; black bars) or ΔhilA (sipA-tsrvenus; M2018; open bars) harboring the indicated regulator-expression plasmids (Table S2) were cultured for 4 h in LB and FACS-analyzed (triplicates ±s.d.). D) ttss-1 regulation cascade depicting the regulators analyzed in C) and E; adapted from [35], [36], [59], [60], [61], [62]). E) Correlation between HilA protein levels and the fraction of ttss-1 expressing individuals. The fraction of cells with the TTSS-1+ phenotype (from C) was plotted against the average HilA expression (average of ≥3 independent quantitative Western blots per regulator and strain). F) Bistable expression of psicA-gfp in S. Tm SL1344 determined and separated by FACS; G) Western blot analysis of TTSS-1- and TTSS-1+ subpopulations from F) using a polyclonal rabbit α-SipC antibody. In addition, we employed psicA-gfp, a reporter plasmid expressing gfp under control of the sicA promoter. This construct yielded brighter fluorescence than the chromosomal sipA-tsrvenus and was better suited for FACS analysis. Again, this reporter yielded a bistable expression pattern (Fig. 1F). Using wt S. Tm psicA-gfp we separated TTSS-1+ and TTSS-1 - subpopulations by FACS. Western blot analysis of the FACS-sorted subpopulations verified coincident expression of psicA-gfp and the TTSS-1 protein SipC (Fig. 1F, G). This indicated that our fluorescent reporter constructs are faithful reporters of the bistable expression of the TTSS-1+ phenotype.
Time-lapse microscopy reveals retarded growth of TTSS-1+ individuals
During our experiments, we observed that hilA, hilC and hilD over-expression led to reduced culture densities (e.g. OD600 for wt sipA-tsrvenus: 3.4±0.3 vs. wt sipA-tsrvenus philA: 2.0±0.3; mean ± s.d.). This was a first hint suggesting that retarded growth might be a general feature of the TTSS-1+ phenotype. However, it remained to be shown whether growth retardation occurs in wild type cells expressing normal levels of hilA, hilC and hilD.
The growth rate of the TTSS-1+ individuals was analyzed by time-lapse microscopy. Wild type S. Tm harboring gfp - or tsrvenus-reporters for ttss-1 expression were placed on an agar pad (LB, 1.5% agarose), the TTSS-1+ individuals were identified by fluorescence microscopy and growth was analyzed by time-lapse phase contrast microscopy (1 frame/30 min; Fig. 2A). Imaging did not impose detectable photo damage to the bacteria, as indicated by the unaltered growth rate (Fig. S2). Strikingly, TTSS-1+ individuals grew slower than TTSS-1− individuals (wt S. Tm sipA-tsrvenus (M2001); µT1+ = 0.90 h−1 vs. µT1− = 1.30 h−1; p = 0.027 for the factor ‘phenotype’ in a two-way ANOVA; Fig. 2B). The negative control strain ΔhilA sipA-tsrvenus yielded only TTSS-1− individuals, which grew at the “fast” rate (µT1− = 1.16 h−1; Fig. 2B). Thus, TTSS-1+ individuals seemed to grow at a reduced rate.
Fig. 2. Time-lapse microscopy reveals retarded growth of TTSS-1+ individuals. 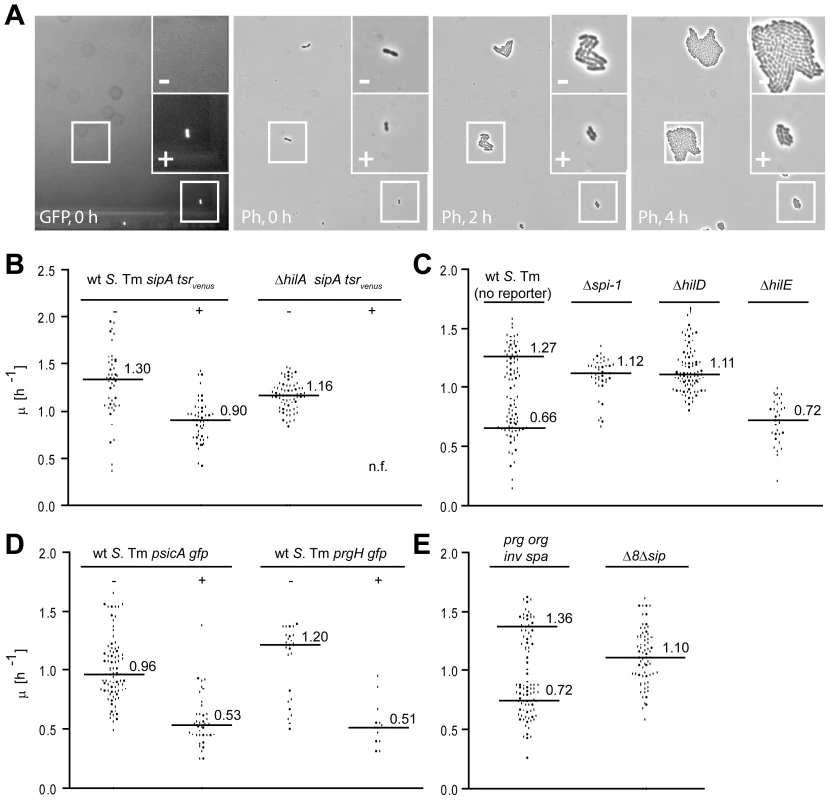
Bacteria (4 h LB subculture, OD600 = 1), were placed on an agar pad (37°C) and imaged to detect ttss-1 expression (fluorescence) and growth (phase contrast; 1 frame/30 min). A) Sample images from a typical time-lapse microscopy experiment with wt S. Tm (SL1344, psicA-gfp). B)-D): Time-lapse microscopy experiments with wt S. Tm (M2001; sipA-tsrvenus) and an isogenic hilA mutant (M2018; sipA-tsrvenus; B); wt S. Tm (SL1344; no reporter) and mutants lacking ttss-1, hilD or hilE (no reporter); C); wt S. Tm (SL1344; psicA-gfp and an isogenic wt reporter strain (SL1344 prgH-gfp; D); mutants lacking most genes encoding the TTS apparatus (prg-org, inv-spa) or most effector proteins and the translocon (Δ8Δsip); E). Each data point represents the growth rate of an individual micro colony. Data were from ≥3 independent experiments. Black line, median; Numbers, median growth rates. To exclude potential artifacts attributable to the sipA-tsrvenus reporter, we analyzed unmodified wild type S. Tm not harboring any reporter (Fig. 2C; Fig. S2). Using a maximum likelihood approach, we identified two populations with distinct growth rates (likelihood ratio test for two populations versus one population, p<0.001, µslow = 0.66 h−1 vs. µfast = 1.27 h−1; Fig. 2C), very similar to the ones described above (Fig. 2B). Furthermore, unmarked mutants lacking the entire SPI-1 region (Δspi-1) or the positive ttss-1 regulator hilD yielded exclusively fast growing cells, while deletion of the negative ttss-1 regulator hilE yielded only slow growing cells (Fig. 2C). Finally, wild type S. Tm harboring psicA-gfp or a chromosomal gfp-reporter for the TTSS-1 gene prgH [1] yielded slow growing TTSS-1+ and fast growing TTSS-1− cells (µT1+ = 0.51 h−1 vs. µT1− = 1.2 h−1; p = 0.006 for the factor ‘phenotype’ in a two-way ANOVA; Fig. 2D). Bacteria expressing the psicA-gfp or prgH-gfp reporters grew even slower than the TTSS-1− sipA-tsrvenus bacteria or the slow-growing wt S. Tm subpopulation (Fig. 2BC). Presumably, this was attributable to the additional “burden” conferred by the GFP expression, as described, before [31].
Thus, the time-lapse microscopy experiments verified bistable ttss-1 expression and revealed that the TTSS-1− phenotype has a reduced growth rate, even at wild type HilA and TTSS-1 levels (µT1+ in the range of 0.7 h−1 vs. µT1− in the range of 1.3 h−1). This was confirmed in a dye dilution assay (Fig. S3).
Our data suggested that ttss-1 expression represents a “cost” to the bacterial cell. However the mechanism explaining this growth retardation had remained unclear. We speculated that expression of the TTS apparatus itself or the sheer load of the proteins transported by the TTSS-1 (effectors, translocon proteins) might play a role. To test these hypotheses, we analyzed two additional S. Tm mutants. In the first mutant, termed Δprg-orgΔinv-spa, we deleted most apparatus-encoding genes (Table S1). This mutant formed two populations with distinct growth rates (likelihood ratio test for two populations versus one population, p<0.001, µslow = 0.72 h−1 vs. µfast = 1.36 h−1; Fig. 2E), very similar to those described for wild type S. Tm (Fig. 2C). The second mutant, termed Δ8Δsip, was lacking the genes for most TTSS-1 effector proteins and the secreted translocon components including sipB, sipC, sipD, sipA, sptP, sopE, sopE2, sopB and sopA (Tab. S1). In contrast to wild type S. Tm, we could not distinguish two subpopulations in this mutant (likelihood ratio test for two populations versus one population, p = 0.73; Fig. 2E). Instead, this mutant displayed a median growth rate of µ = 1.10 h−1, similar to the fast growing subpopulation of S. Tm wt and the mutants Δspi-1 and hilD (Fig. 2C). This data suggests, that expression of the effector proteins and translocon components is “costly” and provides at least in part a mechanistic explanation for the growth retardation of wild type S. Tm cells of the TTSS-1+ phenotype.
Retarded growth and ttss-1 induction determine the fraction of TTSS-1+ individuals: a mathematical analysis
When monitoring growth and bistable ttss-1 expression in a wt S. Tm (psicA-gfp) culture, the fraction of TTSS-1+ individuals began to rise after 2.5 h as soon as the culture entered the late logarithmic phase, increased in a linear fashion, and reached approx. 60% after 7 h once the culture entered the stationary phase (Fig. 3A).
Fig. 3. Time course experiment analyzing the initiation of ttss-1 expression. 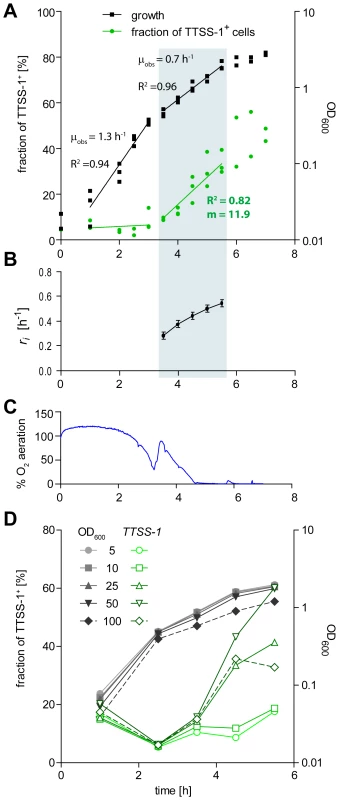
A) Wt S. Tm (SL1344, psicA-gfp) was sub-cultured under mild aeration in LB. Growth (OD600, black) and ttss-1 expression (FACS, green) was analyzed and fitted separately for early and late log phase. Gray: late logarithmic phase. m: apparent initiation rate of ttss-1 expression, as determined from the slope of the fitted line. B) Calculation of the mean value of ri(t) during the late log phase using eq. 4, data from A) and 86 individual µT1− values for S. Tm psicA gfp (from Fig. 2D); error bars depict the SEM. C) pO2 during the experiment. D) Growth (OD600, black) and ttss-1 expression (FACS, green) in 250 ml flasks (shaken 160 rpm, 37°C) harboring the indicated volume of LB (inoculation: 1/100 from a 12 h S. Tm psicA-gfp culture). Our results implied that two different parameters affect the fraction of TTSS-1+ individuals and the overall growth progression in the late logarithmic phase: i. Competitive growth. TTSS-1+ individuals are steadily outgrown by the fast-growing TTSS-1− individuals (µT1+<µT1−; Fig. 2); this constantly reduces the size of the TTSS-1+ subpopulation. ii. ttss-1 induction. Presumably, initiation of ttss-1 expression in TTSS-1− individuals compensates the “TTSS-1+ losses” attributable to competitive growth and explains the increasing fractions of TTSS-1+ individuals during the late logarithmic phase.
To infer the dynamic initiation rate ri of ttss-1 expression in the late logarithmic phase from our experimental data, we devised a mathematical model describing the growth of the TTSS-1+ (NT1+; growth rate µT1+) and the TTSS-1− population (NT1−; growth rate µT1−) as a function of time (t): (1)(2)
It should be noted that the model does not include a term for “switching off” ttss-1 expression. This was justified by our failure to observe “off switching” in the experiments shown in Fig. 2 and further supported by other data (Fig. S2 and data shown below). During the late logarithmic phase, the relative abundance of the TTSS-1+ individuals increased, and the fraction α of TTSS-1− individuals (NT1-) decreased in a linear fashion (Fig. 3A):(3)
Equation (2) can be rearranged to calculate ri(t) (see Text S1 for details):(4)
With the data from Fig. 3A and by using equation (3) we could determine NT1− (t) and, after fitting an empirical function to NT1− (t), also dNT1−/dt. Using equation (4), this allowed calculating ri(t) during the late logarithmic phase (see Text S1 for details). We found that the mean initiation rate (ri) of ttss-1 expression increased continuously during the late logarithmic phase, e.g. from 0.28 h−1 at 3.5 h to 0.54 h−1 at 5.5 h (SEM = 0.03 h−1; Fig. 3B).
Environmental signals affecting ttss-1 expression in the late logarithmic phase
The initiation rate of ttss-1 expression seemed to increase upon entry into the late logarithmic growth phase (Fig. 3A). Therefore, it might be induced by growth-related environmental signals (e.g. oxygen depletion, quorum signals, nutrient depletion, metabolite accumulation). To address this, we analyzed the partial oxygen pressure (pO2) during growth. As expected, pO2 declined to <30% relative aeration during the first three hours (Fig. 3C). After approximately 3.5 h, we detected a transient rebound of the oxygen pressure followed by a steady decline to <3% relative aeration during the next hour. This undulation of oxygen pressure is indicative of a change in the growth physiology at 3.5 h and was in line with the reduced growth rate (Fig. 3A, shaded area).
The data suggested that altered metabolism, nutrient availability, waste product accumulation, the reduced growth rate or the low oxygen pressure might represent cues inducing ttss-1 expression. As a first approach to test the role of pO2, we performed batch culture growth experiments in identical 250 ml culture flasks filled with the indicated volumes of media (wt S. Tm psicA gfp grown in 5, 10, 25, 50 or 100 ml LB; Fig. 3D). This setup allowed analyzing the effect of reduced pO2 (i.e. in larger, poorly aerated culture volumes) at equivalent growth rates. We observed that the fraction of ttss-1 expressing cells increased in larger culture volumes. Therefore, low oxygen tension might represent one environmental cue directly or indirectly inducing bistable ttss-1 expression. However, the evidence is merely circumstantial at this moment and other cues might well be involved. Identification of these cues will benefit from the strategies for determining ri as described above.
Time lapse microscopy detects the emergence and the reduced growth rate of TTSS-1+ cells
In liquid culture, the initiation of ttss-1 expression occurred in the late logarithmic phase. However, our initial time lapse microscopy data for bacteria sampled from this growth phase did not show initiation of ttss-1 expression (Fig. 2). We reasoned that this might be attributable to the lack of inducing environmental signals, as these experiments had been performed on agar pads soaked with fresh LB medium. To test this hypothesis, we modified the time lapse microscopy experiment and imaged bacteria (S. Tm psicA-gfp) placed on agar pads soaked with filter-sterilized spent medium taken from a culture at the same growth phase (OD600 = 0.9, see Materials and Methods). We analyzed growth of 191 micro colonies. At the beginning, 135 did not express ttss-1. But remarkably, we observed 15 of 135 initially TTSS-1− micro colonies, in which individual bacteria induced ttss-1 expression during the course of our imaging experiment (e.g. Fig. 4A, Fig. S4; Video S1). After induction, the TTSS-1+ cells grew at a slower rate than their TTSS-1− siblings. In addition, we observed numerous TTSS-1+ bacteria (56 micro colonies) and TTSS-1− bacteria (120 micro colonies) which did not “switch” their ttss-1 expression status. In line with the results above, ttss-1 expression and the interval between two cell divisions was negatively correlated (Fig. 4A,B,C, Spearman's rho = −0.747, p<0.0001, N = 29).
Fig. 4. Time-lapse microscopy shows onset of ttss-1 expression and concomitant growth retardation. 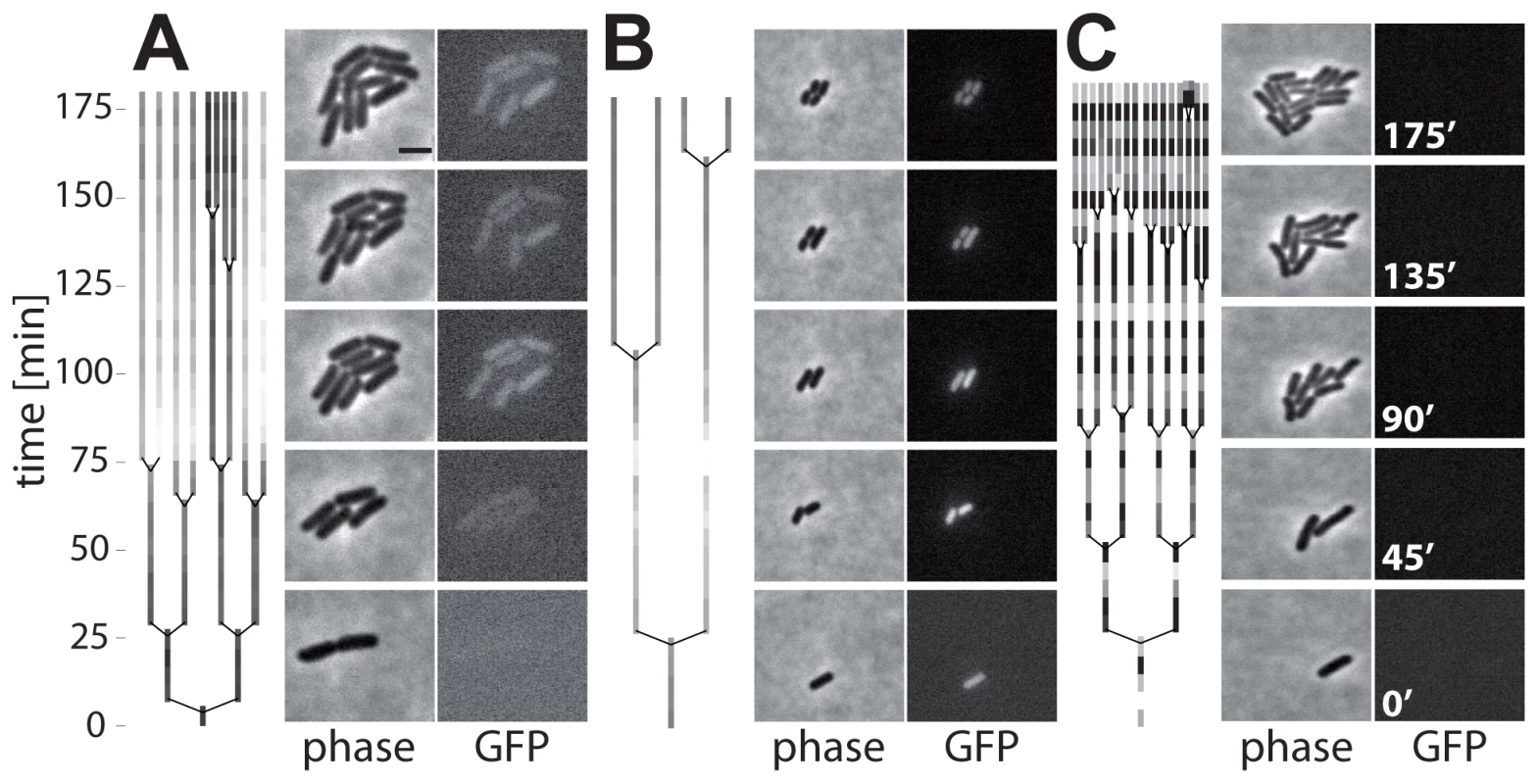
Lineage trees with corresponding phase contrast and GFP images of S. Tm (M556; psicA-gfp) grown on agar pads with spent LB. Coloring of the lineage trees reflects the relative mean GFP intensity of individual cells (dark = low; light = high; scaled to the highest fluorescence in tree). A) On-switching of ttss-1 expression in a fraction of the micro colony. B) Micro colony uniformly expressing ttss-1 throughout the assay. C) Micro colony not expressing ttss-1 throughout the assay. Scale bar, 2 µm; see also Fig. S4. These experiments support the stochastic initiation of ttss-1 expression. But the initiation rate of ttss-1 expression (<0.04 h−1) was lower than that predicted from the batch culture experiment shown in Fig. 3 (ri = 0.18−0.45 h−1). This might be attributable to the lack of some environmental cue, e.g. low oxygen pressure, as time lapse microscopy was performed at ambient atmosphere. Only two micro colonies showed a decrease in fluorescence as expected for “off-switching”. Hence, the rate of off-switching is not substantial. This indicated that our mathematical model, which assumed that “switching off” the ttss-1 expression would be negligible, was justified (equation (1) did not include ri(t)NT1-(t)). These experiments verified that ttss-1 expression is initiated in a stochastic fashion under “inducing” environmental conditions and that the TTSS-1+ phenotype exhibits a growth defect.
Handicap of wt S. Tm in a competitive growth experiment
Finally, we confirmed the growth penalty attributable to ttss-1 expression in the late logarithmic phase in competition experiments. Wt S. Tm expresses ttss-1 in a bistable fashion and forms a significant fraction of slow-growing TTSS-1+ cells during the late logarithmic phase (Fig. 3). This slows down the apparent growth of the total wild type population (see above). In contrast, hilA or hilD mutants, which do not express ttss-1, yield a pure population of fast-growing TTSS-1− cells (Figs. 1 and 2). Thus, in a mixed culture, hilA or hilD mutants should outgrow wt S. Tm. Indeed, both mutants out-competed the wt strain during the late logarithmic phase of the mixed culture (ΔhilA, ΔhilD; Fig. 5A,B). In contrast, a hilE mutant, which forms a larger fraction of TTSS-1+ cells than wt S. Tm (Fig. 2), was outcompeted by wt S. Tm in this type of assay (ΔhilE, Fig. 5C). This verified the growth penalty of TTSS-1+ cells in LB batch cultures.
Fig. 5. Competitive growth experiment confirming that ttss-1 expression retards growth. 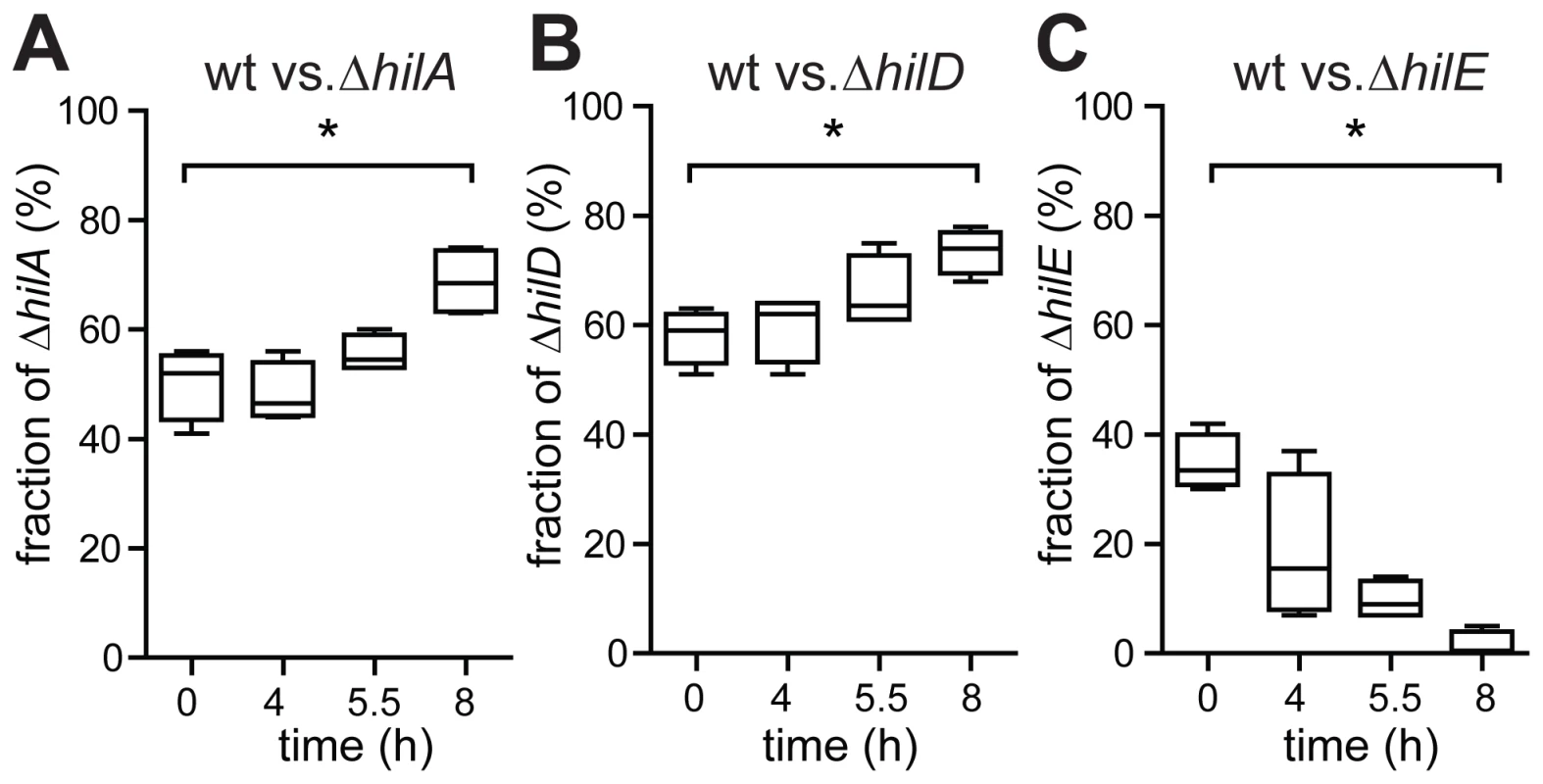
A) Wt S. Tm (ATCC14028, kmS) and an isogenic hilA mutant (M2005, kmR), were used to inoculate a sub-culture at a ratio of approx. 1∶1. Growth of the mixed culture was monitored via OD600. B) Competitive growth between wt S. Tm and an isogenic hilD mutant (M2007, kmR), resp. an isogenic hilE mutant (M2008, cmR), C). The fraction of wt S. Tm was determined by differential plating on LB agar (50 µg/ml kanamycin, resp. 30 µg/ml chloramphenicol) at the indicated time points. Data were derived from four experiments (±s.d., p = 0.014). Discussion
The effect of virulence factor expression on the fitness of an individual pathogen cell has remained unclear. We have analyzed the fitness costs associated with the expression of ttss-1, which encodes a key virulence function of S. Tm. An in vitro system was chosen for a detailed analysis of the growth phenotype of TTSS-1+ cells. We found that these cells have a reduced growth rate. This established that ttss-1 expression represents a burden (and not an advantage) at the level of the individual cell, at least in the non-host environment of our assay system. The growth penalty affects the fraction of TTSS-1+ individuals and the overall growth progression in a S. Tm culture. Mathematical modeling and experimental data demonstrated that this growth penalty and an increasing initiation rate of ttss-1 expression during the late logarithmic growth phase were sufficient to explain the dynamic abundance of TTSS-1+ and TTSS-1− individuals in a clonal S. Tm batch culture.
Evidence for bistability of ttss-1 expression has only recently been accumulated. Under inducing conditions, single cell reporters for expression of ttss-1 or effector proteins yielded cells in the “on” and cells in the “off” state [1], [2], [3], [5], [32]. The regulatory network controlling ttss-1 expression includes at least three positive feedback loops and this architecture is thought to set the threshold for initiating ttss-1 expression and to amplify the level of expression [5], [32], [33]. The TTSS-1+ phenotype can persist for several hours, even if the bacteria are shifted into environments normally not inducing ttss-1 expression (histeresis; shift to fresh LB, Fig. 2; Fig. S2). However, it should also be noted that it has not been possible to define unequivocally where stochasticity is introduced. In fact, stochastic initiation of ttss-1 expression might hinge on different regulators in different environments.
TTSS-1+ cells have at least two important characteristics. First, they express the virulence factors enabling host manipulation and elicitation of disease [13], [17], [18]. Second, as we have found here, they grow at a reduced rate. ttss-1 expression may represent a “burden” in itself. The mechanism explaining the growth defect of TTSS-1+ cells is of significant interest. A partial disruption of the proton gradient by “leaky” TTSS assembly-intermediates and/or the metabolic energy required for biosynthesis of the TTSS may offer plausible explanations. Typical TTSS-1+ cells are estimated to express 20–200 TTS apparatuses and approx. 3−10×104 effector proteins, amounting to a significant fraction of the total cellular protein [2], [3]. Indeed, deleting the translocon and most effector proteins significantly increased the growth rate of the TTSS-1+ cells (Δ8Δsip; Fig. 2E), indicating that these proteins account at least in part for the cost of ttss-1 expression. However, the growth rate of Δ8Δsip (µ = 1.10 h−1) was still lower than that of the TTSS-1− subpopulation of wt S. Tm (µfast = 1.27 h−1), suggesting that other factors do also contribute to growth retardation.
An alternative explanation for the reduced growth rate of TTSS-1+ cells might reside in coordinated expression of a complex regulon. This might be reminiscent of the prf virulence regulon of Listeria monocytogenes, which coordinates metabolism and virulence gene expression thus controlling environment-specific fitness phenotypes in vitro and in vivo [34]. Several global regulators (e.g. crp, mlc, fur; [7], [35], [36]) and silencing proteins (hns, hha; [37], [38]) can control ttss-1 expression. Moreover, HilA may control multiple loci apart from ttss-1 (25). And we have observed co-expression of ttss-1 and of fliC, which encodes a key structural component of the flagella, in the late logarithmic phase (Fig. S5). Accordingly, ttss-1 expression might be one feature of a “differentiated” state which also includes adaptations reducing the growth rate. It is tempting to speculate that this state might be particularly adapted for mucosal tissue invasion. This would be an important topic for future research.
Interestingly, similar phenomena have been observed in other ttss-expressing pathogens. In Pseudomonas aeruginosa, growth in suboptimal media was shown to result in bistable ttss expression [39]. But it remained unclear whether growth might be affected. In contrast, the plasmid-encoded TTSS of Yersinia spp. is well known to cause growth retardation in response to host cell contact or low calcium environments [40], [41]. However, in this case, ttss induction seems to be uniform even in suboptimal media [42]. Thus, bistability and growth retardation do occur in other ttss expressing bacteria, but specific adaptations may exist for each pathogen.
Which environmental cues induce ttss-1 expression in S. Tm? ttss-1 is expressed in the lumen of the host's intestine and in the late logarithmic phase in LB-batch culture. Low oxygen pressure is common to both environments and may represent an inducing signal (see Fig. 3C). In line with this hypothesis, Shigella flexneri, a closely related gut pathogen, can modulate the activity of its TTSS in response to low oxygen pressures typically observed at the gut wall [43]. Similarly, HilA-mediated ttss-1 expression is known to respond to oxygen pressure [21], [44]. In addition, numerous other internal and external cues are known to affect ttss-1 expression, including osmolarity, pH, growth rate, or the presence of short chain fatty acids like acetate [45], [46], [47], [48], [49], [50], [51]. The sum of these environmental cues seems to determine the level of ttss-1 induction. This might explain our observation of a low, but detectable initiation rate of ttss-1 expression on agar pads soaked with spent medium (Fig. 4). This environment should harbor most cues present in the late log culture medium, but lacks low oxygen pressure, which could not be established in the real time microscopy setup.
In summary, our findings indicate that the TTSS-1+ phenotype is more complex than previously anticipated. Currently, we can only speculate how this affects the real infection and transmission in vivo. Our results suggest that the TTSS-1+ subpopulation is constantly drained by the burdens inflicted by immune defenses within the infected gut mucosa [4] and by the reduced growth rate (this work). The latter should represent a competitive disadvantage against all other bacteria (commensals and TTSS-1− S. Tm cells) present in the gut lumen. Moreover, this burden should materialize even before invading the gut tissue and may explain why ttss-1 defective mutants are sometimes (though rarely) found in infected animal flocks and isolated in one case of a human outbreak [52], [53]. In order to explain the evolution and mainentance of bistable ttss-1 expression and the successful propagation of the ttss-1 genotype, one has to predict that the TTSS-1+ phenotype must confer some type of advantage. According to the “division of labor” model, the advantage might emanate from a “public good”, i.e. the TTSS-1 induced gut inflammation fostering Salmonella growth in the gut lumen and enhancing transmission. Alternatively, the TTSS-1+ phenotype might include (unidentified) features enhancing the survival and growth of the ttss-1 expressing bacteria themselves, e.g. in permissive niches of the host's intestine or by enhancing the chances of chronic infection and long-term shedding. Identifying these mechanisms will represent an important step for understanding the evolution of bistable ttss-1 expression.
Materials and Methods
Bacteria
All strains were derivatives of Salmonella Typhimurium SL1344 or ATCC14028 (see Tab. S1 and Text S2 for references). All plasmids and primers are shown in Tab. S2 and S3. Bacteria were inoculated (1∶100 in LB) from 12 h overnight cultures (LB, supplemented with the appropriate antibiotics) and grown under mild aeration for 4 h at 37°C, if not stated otherwise. In Fig. 1C,E, the medium included 0.01% arabinose.
The mutants were constructed using the lambda red recombination system [54]. The chloramphenicol or kanamycin resistance cassette of pKD3 (cat) resp. pKD4 (aphT) were amplified by PCR using the primer pairs ÄhilA::kan-fw and ÄhilA::kan-rev, ÄhilD::kan-fw and ÄhilD::kan-rev, ÄhilE::cat-fw and ÄhilE::cat-rev and electroporated into SL1344 harboring pKD46 to generate the regulator mutants M2005 (ÄhilA::aphT), M2007 (ÄhilD::aphT) and M2008 (ÄhilE::cat). Mutants were selected by plating on LB-Agar (50 µg/ml kanamycin or 30 µg/ml chloramphenicol). M2072 (termed Δprg-orgΔinv-spa in this paper) was also generated using the lambda red system using the primers invG-fw and spaS-rev as well as prgH-fw and orgC–rev and the plasmids pKD3 and pKD4 to generate prgHIJKorgABC::aphT, invGEABCIJspaOPQRS::cat, a mutant lacking most genes of the TTS apparatus. For construction of strain M2532 (termed Δ8Δsip in this paper), we transduced the ÄsipBCDA-sptP::aphT allele from SB245 (SL1344, ÄsipBCDA-sptP::aphT fliGHI::Tn10; K. Kaniga and J. E. Galan, unpublished data) via P22 into M2400 (SL1344, ÄsopE, ÄsopE2, ÄsopB, ÄsipA, ÄsptP, ÄsopA, ÄspvB, ÄspvC), which has been previously described [55]. M2532 fails to express most TTSS-1 effector proteins and the translocon components.
To create the suicide plasmid pM2002, pVS152Tsr [30] was digested with the restriction endonucleases Eco47III and XmaI. The tsrvenus encoding fragment was ligated into pM1300 (digested with MslI and XmaI, [56]) downstream of a truncated sipA fragment (nt 1156–2058 of the orf), to finally create pM2002 and introduced by homologous recombination into the genome of ATCC14028 to generate the reporter strain M2001. To obtain the tsrvenus reporter for hilA (M2076), the c-terminal region of hilA (nt 114 to 1661 of the orf) was amplified using the primer pair hilA-fw-XmaI-NcoI and hilA-rev-NheI-XbaI and cloned into pBluescriptII (Invitrogen) using the restriction endonucleases XmaI and XbaI, yielding pM2090. This plasmid was digested with NheI and NotI to introduce the tsrvenus encoding PCR fragment (template pM2002, primers: venus-NheI-fw and venus-NotI-rev, digested with NheI and NotI) to obtain pM2095. The entire region ranging from hilA to tsrvenus was cloned into pSB377 using the restriction enzymes NotI and XmaI yielding the suicide plasmid pM2080. This plasmid was used to generate the hilA reporter strain M2076 by homologous recombination into the genome of ATCC14028. To obtain the tsrvenus reporter for fliC, tsrvenus was amplified by PCR (primers: tsr-XmaI-fw and venus-XbaI-rev) and cloned into pBluescriptII using XmaI and XbaI thus yielding pM2533. After amplification of fliC by PCR using SL1344 chromosomal DNA as template and primers fliC-XhoI-fw and fliC-HindIII-rev, the fliC encoding fragment was cloned via XhoI and HindIII upstream of the tsrvenus gene into pM2533, thus yielding pM2539. Subsequently, the construct was moved via XhoI and XbaI into the suicide plasmid pGP704, thus yielding pM2819. This plasmid was used to create the fliC-tsrvenus reporter strain M2821 by homologous recombination into the genome of SL1344.
All over-expression plasmids from pM2010 to pM2042 were obtained by digesting the indicated PCR fragments (Table S2 and S3 for plasmids and primers) with EcoRI and XbaI into pBAD24.
All mutations were verified by PCR or DNA sequencing.
HilA expression was analyzed by quantitative Western blot using an affinity-purified rabbit α-HilA antiserum (Fig. 1E). Recombinant HilA was used for normalization. SipC was detected using an α-SipC serum (Fig. 1G).
For invasion, MDCK cells were grown in MEM (Invitrogen), infected for 30 min (MOI = 5; [57], washed and incubated in MEM (400 µg/ml gentamicin; 1 h). Intracellular bacteria were enumerated by plating.
FACS
Prior to analysis, fluorophore formation was ensured (2 h, RT, 30 µg/ml chloramphenicol). Tsrvenus and Gfp emission was analyzed at 530 nm (supplement; FACSCalibur 4-color, Becton Dickinson). Bacteria were identified by side scatter (SSC). Data were analyzed with FlowJo software (Tree Star, Inc.). For Tsrvenus (Fig. 1), ln-transformed fluorescence values for 40000 events were median-normalized (subtraction) and compared to the similarly normalized data from the reporterless control strain, thus yielding the fraction of TTSS-1+ individuals. For sorting bacterial cells, S. Tm (psicA-gfp) cells were sorted by FACS (Aria Becton Dickinson, FACSDiva Software).
Time-lapse microscopy
Bacteria were placed on a 1.5% agarose pad equilibrated with LB, sealed under a glass coverslip and mounted (37°C temp. control; Axioplan2; Plan-APOCHROMAT 63x/1.4 oil; Zeiss or IX81, UPlanFLN 100x/1.3 Oil, Olympus). Reporter fluorescence (Exc. 470/20 nm; BP 495 nm; Em. 505–530 nm) and micro colony growth (phase contrast) were monitored and evaluated using Axiovision software (Zeiss). The slope of the ln-tranformed bacterial numbers (t), as determined from the logarithmic growth phase, yielded the growth rate µ. For sipA-tsrvenus and prgH-gfp, the micro colonies were scored visually as TTSS-1+ or TTSS-1−. To analyze differences in growth rates between TTSS-1+ and TTSS-1− micro colonies, we performed a full-factorial analysis of variance with the two factors phenotype (fixed) and experiment (random). Variance was analyzed in SPSS 17.0 (SPSS Inc. - Chicago, IL).
Growth rates w/o reporter were analyzed via a maximum likelihood approach to test for two subpopulations with different growth rates. The growth rate measurements from five independent experiments (87 micro colonies) were combined. Using maximum likelihood, we fitted a bi-modal distribution (the sum of two normal probability density functions) and a unimodal (normal) distribution, and compared the two fits with a likelihood ratio test using R software [58].
In Fig. 4, cell growth and ttss-1 expression were analyzed using a modified version of the cell tracking software described in [9]. The first cell in each micro colony that could be observed over a whole division was used to analyze the statistical association between ttss-1 expression and the interval between two divisions (by non-parametric correlation analysis using PASW Statistics 18.0.0). 157 micro colonies were analyzed to estimate the fraction of micro colonies in which all cells, none of the cells, and a fraction of the cells expressed ttss-1. These groupings were based on visual inspection of each micro colony.
Supporting Information
Zdroje
1. HautefortIProencaMJHintonJC 2003 Single-copy green fluorescent protein gene fusions allow accurate measurement of Salmonella gene expression in vitro and during infection of mammalian cells. Appl Environ Microbiol 69 7480 7491
2. SchlumbergerMCMullerAJEhrbarKWinnenBDussI 2005 Real-time imaging of type III secretion: Salmonella SipA injection into host cells. Proc Natl Acad Sci U S A 102 12548 12553
3. WinnenBSchlumbergerMCSturmASchupbachKSiebenmannS 2008 Hierarchical effector protein transport by the Salmonella Typhimurium SPI-1 type III secretion system. PLoS ONE 3 e2178
4. AckermannMStecherBFreedNESonghetPHardtWD 2008 Self-destructive cooperation mediated by phenotypic noise. Nature 454 987 990
5. SainiSEllermeierJRSlauchJMRaoCV 2010 The role of coupled positive feedback in the expression of the SPI1 type three secretion system in Salmonella. PLoS Pathog 6 e1001025
6. SmitsWKKuipersOPVeeningJW 2006 Phenotypic variation in bacteria: the role of feedback regulation. Nat Rev Microbiol 4 259 271
7. LimSYunJYoonHParkCKimB 2007 Mlc regulation of Salmonella pathogenicity island I gene expression via hilE repression. Nucleic Acids Res 35 1822 1832
8. ElowitzMBLevineAJSiggiaEDSwainPS 2002 Stochastic gene expression in a single cell. Science 297 1183 1186
9. RosenfeldNYoungJWAlonUSwainPSElowitzMB 2005 Gene regulation at the single-cell level. Science 307 1962 1965
10. RajAvan OudenaardenA 2008 Nature, nurture, or chance: stochastic gene expression and its consequences. Cell 135 216 226
11. RaserJMO'SheaEK 2005 Noise in gene expression: origins, consequences, and control. Science 309 2010 2013
12. KussellEKishonyRBalabanNQLeiblerS 2005 Bacterial persistence: a model of survival in changing environments. Genetics 169 1807 1814
13. SchlumbergerMCHardtWD 2006 Salmonella type III secretion effectors: pulling the host cell's strings. Curr Opin Microbiol 9 46 54
14. WatsonPRGalyovEEPaulinSMJonesPWWallisTS 1998 Mutation of invH, but not stn, reduces Salmonella-induced enteritis in cattle. Infect Immun 66 1432 1438
15. TsolisRMAdamsLGFichtTABaumlerAJ 1999 Contribution of Salmonella typhimurium virulence factors to diarrheal disease in calves. Infect Immun 67 4879 4885
16. BarthelMHapfelmeierSQuintanilla-MartinezLKremerMRohdeM 2003 Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect Immun 71 2839 2858
17. WallisTSGalyovEE 2000 Molecular basis of Salmonella-induced enteritis. Mol Microbiol 36 997 1005
18. HapfelmeierSHardtWD 2005 A mouse model for S. typhimurium-induced enterocolitis. Trends Microbiol 13 497 503
19. SantosRLRaffatelluMBevinsCLAdamsLGTukelC 2009 Life in the inflamed intestine, Salmonella style. Trends Microbiol 17 498 506
20. SchechterLMDamrauerSMLeeCA 1999 Two AraC/XylS family members can independently counteract the effect of repressing sequences upstream of the hilA promoter. Mol Microbiol 32 629 642
21. BajajVHwangCLeeCA 1995 hilA is a novel ompR/toxR family member that activates the expression of Salmonella typhimurium invasion genes. Mol Microbiol 18 715 727
22. EllermeierJRSlauchJM 2007 Adaptation to the host environment: regulation of the SPI1 type III secretion system in Salmonella enterica serovar Typhimurium. Curr Opin Microbiol 10 24 29
23. StecherBRobbianiRWalkerAWWestendorfAMBarthelM 2007 Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol 5 2177 2189
24. RaffatelluMGeorgeMDAkiyamaYHornsbyMJNuccioSP 2009 Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine. Cell Host Microbe 5 476 486
25. LuppCRobertsonMLWickhamMESekirovIChampionOL 2007 Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe 2 204
26. StecherBBarthelMSchlumbergerMCHaberliLRabschW 2008 Motility allows S. Typhimurium to benefit from the mucosal defence. Cell Microbiol 10 1166 1180
27. StecherBHardtWD 2008 The role of microbiota in infectious disease. Trends Microbiol 16 107 114
28. LawleyTDBouleyDMHoyYEGerkeCRelmanDA 2008 Host transmission of Salmonella enterica serovar Typhimurium is controlled by virulence factors and indigenous intestinal microbiota. Infect Immun 76 403 416
29. WinterSEKeestraAMTsolisRMBaumlerAJ 2010 The blessings and curses of intestinal inflammation. Cell Host Microbe 8 36 43
30. YuJXiaoJRenXLaoKXieXS 2006 Probing gene expression in live cells, one protein molecule at a time. Science 311 1600 1603
31. WendlandMBumannD 2002 Optimization of GFP levels for analyzing Salmonella gene expression during an infection. FEBS Lett 521 105 108
32. TemmeKSalisHTullman-ErcekDLevskayaAHongSH 2008 Induction and relaxation dynamics of the regulatory network controlling the type III secretion system encoded within Salmonella pathogenicity island 1. J Mol Biol 377 47 61
33. Bailly-BechetMBeneckeAHardtWDLanzaVSturmA 2010 An externally modulated, noise-driven switch for the regulation of SPI1 in Salmonella enterica serovar Typhimurium. J Math Biol [Epub ahead of print]
34. BrunoJCJrFreitagNE 2010 Constitutive activation of PrfA tilts the balance of Listeria monocytogenes fitness towards life within the host versus environmental survival. PLoS One 5 e15138
35. TeplitskiMGoodierRIAhmerBM 2006 Catabolite repression of the SirA regulatory cascade in Salmonella enterica. Int J Med Microbiol 296 449 466
36. EllermeierJRSlauchJM 2008 Fur regulates expression of the Salmonella pathogenicity island 1 type III secretion system through HilD. J Bacteriol 190 476 486
37. NavarreWWPorwollikSWangYMcClellandMRosenH 2006 Selective silencing of foreign DNA with low GC content by the H-NS protein in Salmonella. Science 313 236 238
38. OlekhnovichINKadnerRJ 2007 Role of nucleoid-associated proteins Hha and H-NS in expression of Salmonella enterica activators HilD, HilC, and RtsA required for cell invasion. J Bacteriol 189 6882 6890
39. RietschAMekalanosJJ 2006 Metabolic regulation of type III secretion gene expression in Pseudomonas aeruginosa. Mol Microbiol 59 807 820
40. BrubakerRRSurgallaMJ 1964 The Effect of Ca++ and Mg++ on Lysis, Growth, and Production of Virulence Antigens by Pasteurella Pestis. J Infect Dis 114 13 25
41. HiguchiKKupferbergLLSmithJL 1959 Studies on the nutrition and physiology of Pasteurella pestis. III. Effects of calcium ions on the growth of virulent and avirulent strains of Pasteurella pestis. J Bacteriol 77 317 321
42. WileyDJRosqvistRSchesserK 2007 Induction of the Yersinia type 3 secretion system as an all-or-none phenomenon. J Mol Biol 373 27 37
43. MarteynBWestNPBrowningDFColeJAShawJG 2010 Modulation of Shigella virulence in response to available oxygen in vivo. Nature 465 355 358
44. SchiemannDAShopeSR 1991 Anaerobic growth of Salmonella typhimurium results in increased uptake by Henle 407 epithelial and mouse peritoneal cells in vitro and repression of a major outer membrane protein. Infect Immun 59 437 440
45. LostrohCPLeeCA 2001 The Salmonella pathogenicity island-1 type III secretion system. Microbes Infect 3 1281 1291
46. HuangYSuyemotoMGarnerCDCicconiKMAltierC 2008 Formate acts as a diffusible signal to induce Salmonella invasion. J Bacteriol 190 4233 4241
47. LeeCAFalkowS 1990 The ability of Salmonella to enter mammalian cells is affected by bacterial growth state. Proc Natl Acad Sci U S A 87 4304 4308
48. ErnstRKDombroskiDMMerrickJM 1990 Anaerobiosis, type 1 fimbriae, and growth phase are factors that affect invasion of HEp-2 cells by Salmonella typhimurium. Infect Immun 58 2014 2016
49. LeeCAJonesBDFalkowS 1992 Identification of a Salmonella typhimurium invasion locus by selection for hyperinvasive mutants. Proc Natl Acad Sci U S A 89 1847 1851
50. LawhonSDMaurerRSuyemotoMAltierC 2002 Intestinal short-chain fatty acids alter Salmonella typhimurium invasion gene expression and virulence through BarA/SirA. Mol Microbiol 46 1451 1464
51. GalanJECurtissR3rd 1990 Expression of Salmonella typhimurium genes required for invasion is regulated by changes in DNA supercoiling. Infect Immun 58 1879 1885
52. HuQCoburnBDengWLiYShiX 2008 Salmonella enterica serovar Senftenberg human clinical isolates lacking SPI-1. J Clin Microbiol 46 1330 1336
53. RahnKDe GrandisSAClarkeRCMcEwenSAGalanJE 1992 Amplification of an invA gene sequence of Salmonella typhimurium by polymerase chain reaction as a specific method of detection of Salmonella. Mol Cell Probes 6 271 279
54. DatsenkoKAWannerBL 2000 One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97 6640 6645
55. HoffmannCGalleMDillingSKappeliRMullerAJ 2010 In macrophages, caspase-1 activation by SopE and the type III secretion system-1 of S. typhimurium can proceed in the absence of flagellin. PLoS One 5 e12477
56. EhrbarKHapfelmeierSStecherBHardtWD 2004 InvB is required for type III-dependent secretion of SopA in Salmonella enterica serovar Typhimurium. J Bacteriol 186 1215 1219
57. EhrbarKFriebelAMillerSIHardtWD 2003 Role of the Salmonella pathogenicity island 1 (SPI-1) protein InvB in type III secretion of SopE and SopE2, two Salmonella effector proteins encoded outside of SPI-1. J Bacteriol 185 6950 6967
58. Team RDC 2009 R: A language and environment for statistical computing. R Foundation for Statistical Computing
59. EllermeierCDEllermeierJRSlauchJM 2005 HilD, HilC and RtsA constitute a feed forward loop that controls expression of the SPI1 type three secretion system regulator hilA in Salmonella enterica serovar Typhimurium. Mol Microbiol 57 691 705
60. SchechterLMJainSAkbarSLeeCA 2003 The small nucleoid-binding proteins H-NS, HU, and Fis affect hilA expression in Salmonella enterica serovar Typhimurium. Infect Immun 71 5432 5435
61. HumphreysSRowleyGStevensonAAnjumMFWoodwardMJ 2004 Role of the two-component regulator CpxAR in the virulence of Salmonella enterica serotype Typhimurium. Infect Immun 72 4654 4661
62. AltierCSuyemotoMLawhonSD 2000 Regulation of Salmonella enterica serovar typhimurium invasion genes by csrA. Infect Immun 68 6790 6797
63. IlgKEndtKMisselwitzBStecherBAebiM 2009 O-antigen-negative Salmonella enterica serovar Typhimurium is attenuated in intestinal colonization but elicits colitis in streptomycin-treated mice. Infect Immun 77 2568 2575
64. BonifieldHRHughesKT 2003 Flagellar phase variation in Salmonella enterica is mediated by a posttranscriptional control mechanism. J Bacteriol 185 3567 3574
65. FreedNESilanderOKStecherBBohmAHardtWD 2008 A simple screen to identify promoters conferring high levels of phenotypic noise. PLoS Genet 4 e1000307
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek A Typhimurium-Typhi Genomic Chimera: A Model to Study Vi Polysaccharide Capsule Function In VivoČlánek SUMO Pathway Dependent Recruitment of Cellular Repressors to Herpes Simplex Virus Type 1 GenomesČlánek A Structural Model for Binding of the Serine-Rich Repeat Adhesin GspB to Host Carbohydrate ReceptorsČlánek Dynamic Evolution of Pathogenicity Revealed by Sequencing and Comparative Genomics of 19 IsolatesČlánek Widespread Endogenization of Genome Sequences of Non-Retroviral RNA Viruses into Plant GenomesČlánek A Role for the Chemokine RANTES in Regulating CD8 T Cell Responses during Chronic Viral Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 7- Stillova choroba: vzácné a závažné systémové onemocnění
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Jak souvisí postcovidový syndrom s poškozením mozku?
-
Všechny články tohoto čísla
- What Do We Really Know about How CD4 T Cells Control ?
- “Persisters”: Survival at the Cellular Level
- E6 and E7 from Beta Hpv38 Cooperate with Ultraviolet Light in the Development of Actinic Keratosis-Like Lesions and Squamous Cell Carcinoma in Mice
- Selection of Resistant Bacteria at Very Low Antibiotic Concentrations
- The Extracytoplasmic Domain of the Ser/Thr Kinase PknB Binds Specific Muropeptides and Is Required for PknB Localization
- CD39/Adenosine Pathway Is Involved in AIDS Progression
- Hypoxia and a Fungal Alcohol Dehydrogenase Influence the Pathogenesis of Invasive Pulmonary Aspergillosis
- One Is Enough: Effective Population Size Is Dose-Dependent for a Plant RNA Virus
- Effects of Interferon-α/β on HBV Replication Determined by Viral Load
- A Typhimurium-Typhi Genomic Chimera: A Model to Study Vi Polysaccharide Capsule Function In Vivo
- Dual Chaperone Role of the C-Terminal Propeptide in Folding and Oligomerization of the Pore-Forming Toxin Aerolysin
- Rotavirus Stimulates Release of Serotonin (5-HT) from Human Enterochromaffin Cells and Activates Brain Structures Involved in Nausea and Vomiting
- Dissociation of Infectivity from Seeding Ability in Prions with Alternate Docking Mechanism
- The Impact of Recombination on dN/dS within Recently Emerged Bacterial Clones
- The Regulation of Sulfur Metabolism in
- Illumination of Parainfluenza Virus Infection and Transmission in Living Animals Reveals a Tissue-Specific Dichotomy
- A Permeable Cuticle Is Associated with the Release of Reactive Oxygen Species and Induction of Innate Immunity
- A Concerted Action of Hepatitis C Virus P7 and Nonstructural Protein 2 Regulates Core Localization at the Endoplasmic Reticulum and Virus Assembly
- SUMO Pathway Dependent Recruitment of Cellular Repressors to Herpes Simplex Virus Type 1 Genomes
- Re-localization of Cellular Protein SRp20 during Poliovirus Infection: Bridging a Viral IRES to the Host Cell Translation Apparatus
- Divergent Effects of Human Cytomegalovirus and Herpes Simplex Virus-1 on Cellular Metabolism
- A Structural Model for Binding of the Serine-Rich Repeat Adhesin GspB to Host Carbohydrate Receptors
- Transformation of Natural Genetic Variation into Genomes
- EBV Latency Types Adopt Alternative Chromatin Conformations
- Global mRNA Degradation during Lytic Gammaherpesvirus Infection Contributes to Establishment of Viral Latency
- Dynamic Evolution of Pathogenicity Revealed by Sequencing and Comparative Genomics of 19 Isolates
- Microbial Virulence as an Emergent Property: Consequences and Opportunities
- Widespread Endogenization of Genome Sequences of Non-Retroviral RNA Viruses into Plant Genomes
- Structural Basis of Chemokine Sequestration by CrmD, a Poxvirus-Encoded Tumor Necrosis Factor Receptor
- Cross-Species Transmission of a Novel Adenovirus Associated with a Fulminant Pneumonia Outbreak in a New World Monkey Colony
- An Interaction between KSHV ORF57 and UIF Provides mRNA-Adaptor Redundancy in Herpesvirus Intronless mRNA Export
- Elevated 17β-Estradiol Protects Females from Influenza A Virus Pathogenesis by Suppressing Inflammatory Responses
- The Role of IL-15 Deficiency in the Pathogenesis of Virus-Induced Asthma Exacerbations
- Fluorescence Lifetime Imaging Unravels Metabolism and Its Crosstalk with the Host Cell
- Programmed Death (PD)-1-Deficient Mice Are Extremely Sensitive to Murine Hepatitis Virus Strain-3 (MHV-3) Infection
- Hemoglobin Promotes Nasal Colonization
- Crystallography of a Lewis-Binding Norovirus, Elucidation of Strain-Specificity to the Polymorphic Human Histo-Blood Group Antigens
- The Cost of Virulence: Retarded Growth of Typhimurium Cells Expressing Type III Secretion System 1
- A Genome-Wide Approach to Discovery of Small RNAs Involved in Regulation of Virulence in
- Requires Glycerol for Maximum Fitness During The Tick Phase of the Enzootic Cycle
- C Metabolic Flux Analysis Identifies an Unusual Route for Pyruvate Dissimilation in Mycobacteria which Requires Isocitrate Lyase and Carbon Dioxide Fixation
- A Role for the Chemokine RANTES in Regulating CD8 T Cell Responses during Chronic Viral Infection
- Glycosaminoglycans and Sialylated Glycans Sequentially Facilitate Merkel Cell Polyomavirus Infectious Entry
- Regulation of Stomatal Tropism and Infection by Light in : Evidence for Coordinated Host/Pathogen Responses to Photoperiod?
- Multiple Translocation of the Effector Gene among Chromosomes of the Rice Blast Fungus and Related Species
- Comparative Genomics Yields Insights into Niche Adaptation of Plant Vascular Wilt Pathogens
- Unique Cell Adhesion and Invasion Properties of O:3, the Most Frequent Cause of Human Yersiniosis
- C-Terminal Region of EBNA-2 Determines the Superior Transforming Ability of Type 1 Epstein-Barr Virus by Enhanced Gene Regulation of LMP-1 and CXCR7
- Novel Chikungunya Vaccine Candidate with an IRES-Based Attenuation and Host Range Alteration Mechanism
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Requires Glycerol for Maximum Fitness During The Tick Phase of the Enzootic Cycle
- Comparative Genomics Yields Insights into Niche Adaptation of Plant Vascular Wilt Pathogens
- The Role of IL-15 Deficiency in the Pathogenesis of Virus-Induced Asthma Exacerbations
- “Persisters”: Survival at the Cellular Level
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



