-
Články
- Vzdělávání
- Časopisy
Top články
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
SUMO Pathway Dependent Recruitment of Cellular Repressors to Herpes Simplex Virus Type 1 Genomes
Components of promyelocytic leukaemia (PML) nuclear bodies (ND10) are recruited to sites associated with herpes simplex virus type 1 (HSV-1) genomes soon after they enter the nucleus. This cellular response is linked to intrinsic antiviral resistance and is counteracted by viral regulatory protein ICP0. We report that the SUMO interaction motifs of PML, Sp100 and hDaxx are required for recruitment of these repressive proteins to HSV-1 induced foci, which also contain SUMO conjugates and PIAS2β, a SUMO E3 ligase. SUMO modification of PML and elements of its tripartite motif (TRIM) are also required for recruitment in cells lacking endogenous PML. Mutants of PML isoform I and hDaxx that are not recruited to virus induced foci are unable to reproduce the repression of ICP0 null mutant HSV-1 infection mediated by their wild type counterparts. We conclude that recruitment of ND10 components to sites associated with HSV-1 genomes reflects a cellular defence against invading pathogen DNA that is regulated through the SUMO modification pathway.
Published in the journal: . PLoS Pathog 7(7): e32767. doi:10.1371/journal.ppat.1002123
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002123Summary
Components of promyelocytic leukaemia (PML) nuclear bodies (ND10) are recruited to sites associated with herpes simplex virus type 1 (HSV-1) genomes soon after they enter the nucleus. This cellular response is linked to intrinsic antiviral resistance and is counteracted by viral regulatory protein ICP0. We report that the SUMO interaction motifs of PML, Sp100 and hDaxx are required for recruitment of these repressive proteins to HSV-1 induced foci, which also contain SUMO conjugates and PIAS2β, a SUMO E3 ligase. SUMO modification of PML and elements of its tripartite motif (TRIM) are also required for recruitment in cells lacking endogenous PML. Mutants of PML isoform I and hDaxx that are not recruited to virus induced foci are unable to reproduce the repression of ICP0 null mutant HSV-1 infection mediated by their wild type counterparts. We conclude that recruitment of ND10 components to sites associated with HSV-1 genomes reflects a cellular defence against invading pathogen DNA that is regulated through the SUMO modification pathway.
Introduction
Herpesvirus infections are controlled by acquired and innate defences involving cellular, humoral and cytokine mediated responses (for reviews, see [1]). In recent years a concept has emerged of an additional antiviral defence mechanism that operates within individual cells. Unlike cytokine-mediated responses, intrinsic antiviral resistance involves the actions of pre-existing cellular proteins that, in the case of herpesviruses, act to repress viral transcription [2], [3], [4]. This defence is counteracted by viral regulatory proteins, for example the immediate-early (IE) proteins ICP0 of herpes simplex virus type 1 (HSV-1) [5], [6], [7], ie1 (IE72) of human cytomegalovirus (HCMV) [8], and HCMV virion component pp71 [9], [10], [11], [12], [13], [14]. One aspect of intrinsic resistance concerns cellular nuclear sub-structures known as ND10 or promyelocytic leukaemia (PML) nuclear bodies, and a number of their major components, namely PML itself, Sp100, hDaxx and ATRX. In HSV-1 infections, ICP0 overcomes the repressive properties of these proteins by inducing their degradation or dispersal [7], [15], [16], [17]. ICP0 null mutant HSV-1 exhibits a greatly reduced plaque forming efficiency, but this defect is partially reversed in cells depleted of PML, Sp100, hDaxx or ATRX [5], [6], [7].
A notable feature of PML and other ND10 components is their recruitment to novel ND10-like foci that are closely associated with parental HSV-1 genomes and early replication compartments during the initial stages of infection [18], [19]. The recruitment of PML to the virus-induced foci is not dependent on de novo viral protein expression and occurs extremely rapidly, indicating that the cell responds to the entry of viral genomes into the nucleus [18], [20]. Although the effect can be seen in wild type (wt) HSV-1 infections, it is short lived as the recruited proteins are rapidly degraded or dispersed through the effects of ICP0. During infection with ICP0 null mutant HSV-1, however, the ND10 proteins remain in these novel sites in a much longer-lived manner. The correlation between the biological activity of many ICP0 mutant proteins and their ability to counteract this recruitment process [21] suggests that this phenomenon reflects an aspect of intrinsic antiviral resistance. This model proposes that the recruited proteins generate a repressive environment that impedes viral transcription. This paper concerns the molecular mechanism by which ND10 components are recruited to the virus-induced foci and tests the hypothesis that the recruitment process contributes to intrinsic resistance to HSV-1 infection.
The formation of the virus-induced ND10-like structures is distinct from that of normal ND10 in uninfected cells because it is not dependent on PML or indeed any of the major ND10 components that have so far been studied [5], [6], [7], [20]. Here, we have used a depletion/reconstitution approach to analyze the molecular requirement for the recruitment of PML, Sp100 and hDaxx to HSV-1 genome-associated sites in newly infected cells. We found that in all cases the presence of a SUMO interaction motif (SIM) [22] is required for this property, and that the major SUMO modification sites of PML, but not Sp100, are also required. The virus-induced ND10-like foci also contain SUMO-2/3 conjugates and PIAS2β, a SUMO E3 ligase, even in the absence of PML. Unlike their wt counterparts, SIM deletion mutants of PML isoform I and hDaxx are unable to repress ICP0 null mutant HSV-1 infection. These data demonstrate that SUMO modification pathways play a key role in the recruitment of ND10 proteins to HSV-1 genome associated sites. We propose that this SUMO-dependent cellular response is an important component of intrinsic cellular defence against foreign DNA that has entered the nucleus.
Results
Recruitment of PML isoforms to sites associated with HSV-1 genomes
The recruitment of PML and other proteins to sites associated with parental HSV-1 genomes and early replication compartments can be detected by examination of cells at the periphery of developing viral plaques. The viral genomes enter the nucleus of newly infected cells in a directional and asymmetric manner, and remain close to the nuclear envelope. The viral IE transcriptional regulator ICP4 avidly binds to viral DNA, and therefore forms foci that, in cells at the early stages of infection, are commonly distributed along one interior edge of the nucleus. PML and other ND10 proteins then accumulate at novel sites that are closely associated with the viral genomes [18], [19]. This phenotype allows the unambiguous identification of proteins that have been recruited to and accumulate at the virus-induced sites. A typical example of endogenous PML recruitment in an ICP0-null mutant HSV-1 (ΔICP0) infected HepaRG cell expressing a control shRNA is shown in Figure 1A (left) in comparison with a similarly infected PML depleted cell (Figure 1A, right).
Fig. 1. The major nuclear isoforms of PML and their recruitment to sites associated with HSV-1 genomes. 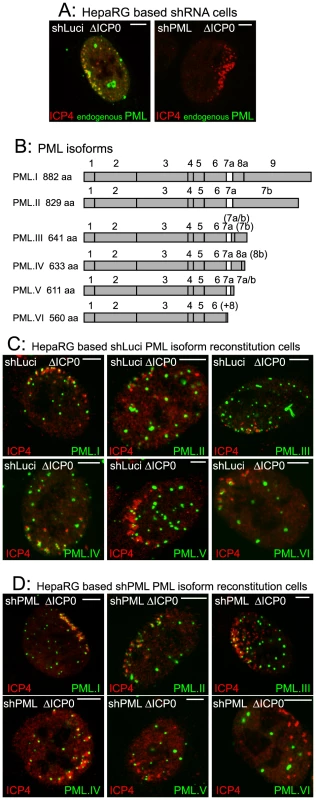
A. Typical examples of recruitment of endogenous PML (green) to sites associated with HSV-1 genomes (ICP4: red) in cells at the edges of ICP0 null mutant (ΔICP0) plaques in control and PML depleted cells. B. PML isoforms I to VI, noting the included exons and the translated product length. The bracketed exons indicate the use of alternative reading frames for these sequences. (+8) at the end of PML VI indicates an additional 8 residues following exon 6 as a result of alternative splicing that deletes exon 7a. Adapted from [23]. C and D. Recruitment of EYFP-PML isoforms in control (panel C) and PML depleted cells (panel D). Scale bars indicate 5 µm. Analysis of the localization of these proteins in uninfected cells and their expression levels is presented elsewhere [23]. We investigated the molecular characteristics of PML that are required for this recruitment by adopting a depletion/reconstitution approach [23]. All the PML isoforms studied (Figure 1B), expressed as EYFP fusions, were recruited to sites associated with ICP4 foci in cells containing endogenous PML (Figure 1C). In cells depleted of endogenous PML, however, while PML isoforms I to V were recruited, PML.VI remained entirely in large foci that were not associated with ICP4 (Figure 1D). The separated channels of the images in Figures 1C and 1D are shown in Figures S1 and S2. This assay is not amenable to precise quantification as the extent of recruitment varies between cells and is most obvious when the ICP4 foci are small. Nonetheless, when recruitment occurs it is evident in all cells with ICP4 foci near the nuclear periphery. A large number of cells were examined in each experiment, and we have scored a protein as defective in recruitment only when its behaviour was clearly different from the appropriate control. As an example, compare PML.VI in the control and PML depleted backgrounds in Figures 1C and D. Although recruitment of PML proteins that we have scored as recruitment positive occurred to some extent in all relevant cells, we noted some differences in PML isoform behaviour. For example, PML.I was recruited more extensively than PML.V (Figure 1D). We have not investigated the basis of these differences.
The defect in PML.VI recruitment to virus-induced foci also occurred in analogous human fibroblast (HF) derived cells. PML.I co-localized with Sp100 in both control and PML depleted HFs (Figure S3), both PML.I and PML.VI were recruited to the virus-induced in control HFs (Figure S4A), but PML.VI was not recruited in PML depleted HFs (Figure S4B).
PML isoform VI includes all the conserved exons present in the other isoforms, except exon 7a (Figure 1B). Therefore exon 7a includes sequences that are required for recruitment of PML to the virally induced foci. The defect in PML.VI recruitment is not exhibited when endogenous PML is present because PML interactions mediated through the coiled-coil motif [24], [25] enable the defective protein to be recruited in partnership with the endogenous isoforms.
PML exon 7a is required for recruitment to sites associated with HSV-1 genomes
PML exon 7a encodes an 18 amino acid segment containing a SIM that has been implicated in proper ND10 assembly [26]. Mutants of PML.I and PML.IV that lack exon 7a (Figure 2A). were expressed at levels similar to their wt versions, with similar patterns of SUMO modification, and co-localized with Sp100 in both control and PML depleted cells (Figure 2B and C). While both mutants were recruited to virus-induced foci in the presence of endogenous PML, both were defective in recruitment in PML depleted cells (Figures 2D and S5A). This defect of PML.I.Δ7a was confirmed in HF derived cells (Figure S5B and C; the localization of this protein in uninfected HFs is shown in Figure S3). EYFP-PML.I with substitution mutations in the SIM (PML.I.mSIM) exhibited properties very similar to those of PML.I.Δ7a in uninfected and ICP0-null mutant HSV-1 infected control and PML depleted cells (Figure S6). These data confirm that the lack of recruitment of PML.VI is due to the absence of the SIM in exon 7a.
Fig. 2. SIM deletion mutants of PML are defective in recruitment to sites associated with HSV-1 genomes. 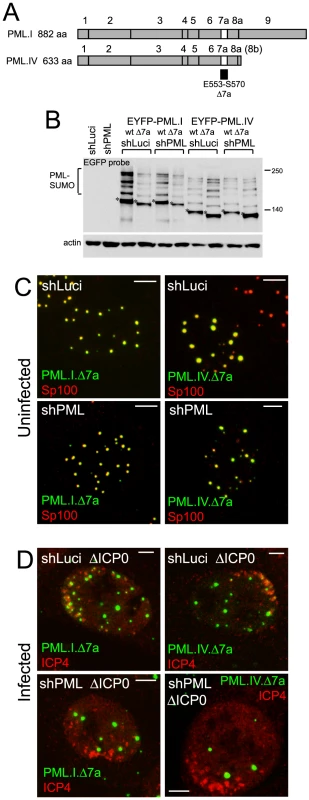
A. Maps of PML isoforms I and IV and derivatives lacking exon 7a. B. Western blot of the EYFP-linked proteins in control and PML depleted cells using an anti-EGFP antibody. The major unmodified bands of the EYFP-PML proteins are indicated by asterisks. C. Localization of EYFP-PML proteins (green) and endogenous Sp100 (red) in uninfected control and PML depleted cells. D. Typical assays of recruitment of EYFP-proteins (green) to sites associated with HSV-1 genomes (ICP4; red) in ICP0 null mutant (ΔICP0) infected control and PML depleted cells. Scale bars indicate 5 µm. The role of SUMO modification in the recruitment of PML to virus-induced foci
SUMO modification of PML is essential for ND10 assembly in uninfected cells [27]. Therefore we investigated whether this modification, in addition to the SIM, plays a role in PML recruitment to the virus-induced foci. Mutants of PML.I or PML.IV with lysine to arginine substitutions at residues 160 and 490 (PML.I.KK and PML.IV.KK; Figure 3A) exhibit substantial defects in SUMO modification, but some apparently modified species remain, especially in cells containing endogenous PML, and more so with PML.IV than PML.I. Modification of PML.I.KK in PML depleted cells was almost completely eliminated (Figure 3B).
Fig. 3. SUMO modification mutants of PML are defective in recruitment to sites associated with HSV-1 genomes. 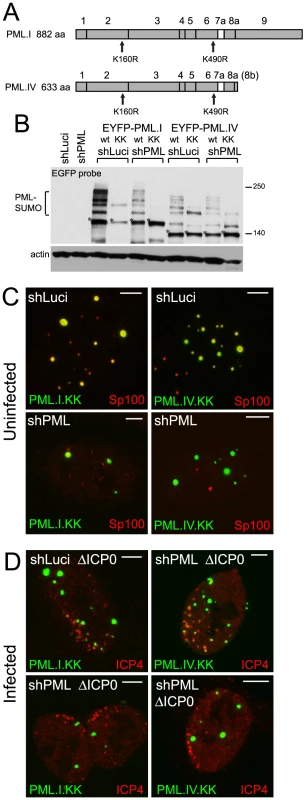
A. Maps of PML isoforms I and IV and derivatives with lysine to arginine substitutions at residues K160 and K490. B. Western blot of these proteins in control and PML depleted cells, using an anti-EGFP antibody. The major unmodified bands of the EYFP-PML proteins are indicated by asterisks. C. Localization of the EYFP-PML proteins (green) and endogenous Sp100 (red) in uninfected control and PML depleted cells. D. Typical assays of recruitment of these PML proteins (green) to sites associated with HSV-1 genomes (ICP4; red) in ICP0 null mutant (ΔICP0) infected control and PML depleted cells. Scale bars indicate 5 µm. PML.I.KK and PML.IV.KK localized to ND10 normally in cells expressing endogenous PML, probably through interactions with endogenous PML via their coiled-coil domains (Figure 3C, upper row). In contrast, and consistent with previous work [27], PML.I.KK exhibited a drastically altered localization in PML depleted cells, forming a reduced number of foci of increased size, some of which were in the cytoplasm. The nuclear foci contained greatly reduced amounts of Sp100. The aberrant PML.IV.KK foci were predominantly nuclear, again with little co-localization with Sp100 (Figure 3C, lower row). Residual co-localization with Sp100 of these PML mutants may be influenced by potential SUMO modification at other lysine residues. However, it is also affected by cell type since in the corresponding PML depleted HFs co-localization of PML.I.KK with Sp100 was more marked (Figure S9A).
To eliminate any residual SUMO modification of PML.I.KK we introduced additional mutations at either lysine 65 [28] or at lysine 616, which lies in a good match (LKID) to the consensus SUMO modification site (ΨKXE). Mutant PML.I.K123 (K65R, K160R, K490R) exhibited similar residual SUMO modification to that of PML.I.KK in cells expressing endogenous PML, whereas the K616R mutation virtually eliminated all modified bands in mutants PML.I.K234 (K160R, K490R, K616R) and PML.I.4KR (all indicated lysine residues substituted with arginine) (Figure S7). We conclude that PML.I is not detectably modified at lysine 65, but that lysine 616 is likely to be a SUMO modification site that is specific for PML.I and PML.IV (because it lies in exon 8a which is present in these two isoforms only). We note that lysine 65 is very close to zinc coordinating cysteine residues in the RING finger, so SUMO modification here would likely affect both the overall architecture of the RING and its interaction interfaces. PML.I.4KR co-localized with Sp100 in control but not PML depleted cells, in both HepaRG and HF backgrounds (Figures S8A and S9A). These data support the hypothesis that the sporadic co-localization of PML.I.KK with Sp100 in PML depleted cells (Figure 3C) is due to the remaining potential for SUMO modification.
As in the case of the SIM mutants, both PML.I.KK and PML.IV.KK could be recruited to virus-induced foci in cells expressing endogenous PML (Figure 3D, upper row), but recruitment was either absent or at greatly reduced levels in PML depleted cells (Figure 3D, lower row, and Figure S8B). Similar results were obtained in the equivalent HF derived cells (Figure S9B), and with the 4KR mutant in both HepaRG and HF backgrounds (Figures S8C and S9B). Therefore, in addition to the role of the SIM, mutation of the major SUMO modification sites of PML severely impedes the capacity of the protein to be recruited to sites associated with viral genomes, providing endogenous PML isoforms are absent.
Absence of the SIM does not affect the intrinsic mobility of PML
The defect of mutant PML proteins in recruitment to virus-induced foci could be explained by a decrease in intrinsic mobility. The dynamics of PML isoforms and SUMO modification deficient mutants in both the presence and absence of endogenous PML have been reported [18], [29], [30], [31], but the role of the SIM has not been investigated before. We analyzed the dynamics of all EYFP-linked PML isoforms, and the Δ7a and KK mutants of PML.I and PML.IV, in both control and PML depleted HepaRG cells. Typical FRAP recovery plots of PML.I and PML.I.KK in control cells are presented in Figure 4A, confirming previous conclusions that SUMO modification is required for the normal mobility of PML [30], [32]. The data for all PML isoforms and mutants in both cell backgrounds are presented as their degree of fluorescence recovery at the 112-second time point (Figure 4B). The results are broadly in agreement with previous work, with the exception that the relatively slow recovery of PML.V [30] was not reproduced here. The only major differences were between the SUMO modification deficient mutants and the wt forms. Importantly, the defect in recruitment to virus-induced foci of PML.VI and the PML.I and PML.IV mutants lacking exon 7a cannot be explained simply by reduced mobility, whereas in the case of the SUMO modification mutants this explanation cannot be excluded.
Fig. 4. FRAP analysis of wt PML isoforms and SUMO modification and SIM deletion mutants of PML.I and PML.IV in control and PML depleted cells. 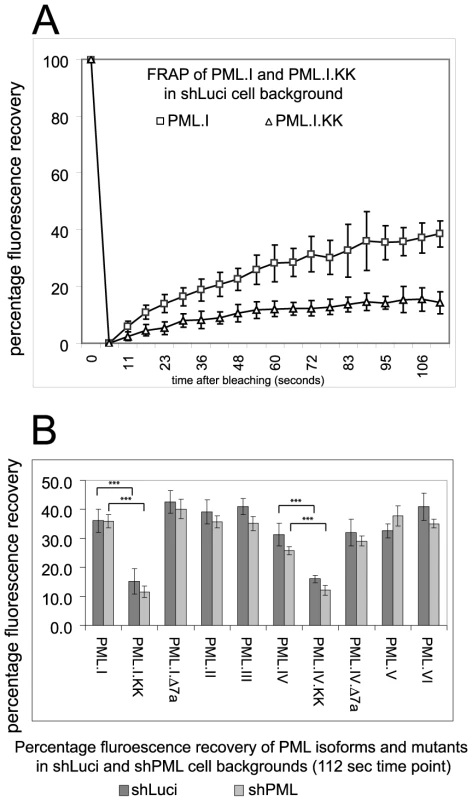
A. Examples of the original data of PML.I and PML.I.KK in control HepaRG shLuci cells.B. The means and standard deviations of the percentage recoveries at the final time point for each of the proteins in both cell backgrounds. Statistical significance between the indicated data sets was calculated by Student's two-tailed t-Test, p = <0.0001. The role of elements within the TRIM in recruitment of PML to virus-induced foci
Control and PML depleted cell lines that express EYFP-PML.I with a deletion of the coiled-coil or point mutations in the RING finger, B-Box 1 or B-Box 2 (Figure 5A) have been described previously [23]. Briefly, the RING finger mutant (ΔRING) co-localized with endogenous ND10 in control cells but was mostly nuclear diffuse in cells lacking endogenous PML, the coiled-coil deletion mutant (ΔCC) was nuclear diffuse in both cell backgrounds, the B-Box 2 mutant (ΔBB2) formed foci in both types of cell but in neither case were these co-localized with Sp100, and the B-Box 1 mutant (ΔBB1) co-localized with Sp100 in aberrant ND10-like structures in control cells and formed foci in a proportion of PML depleted cells, none of which co-localized with Sp100. SUMO modification of all tripartite motif (TRIM) mutants was highly compromised in both cell backgrounds [23].
Fig. 5. Most TRIM mutants of PML are defective in recruitment to sites associated with HSV-1 genomes. 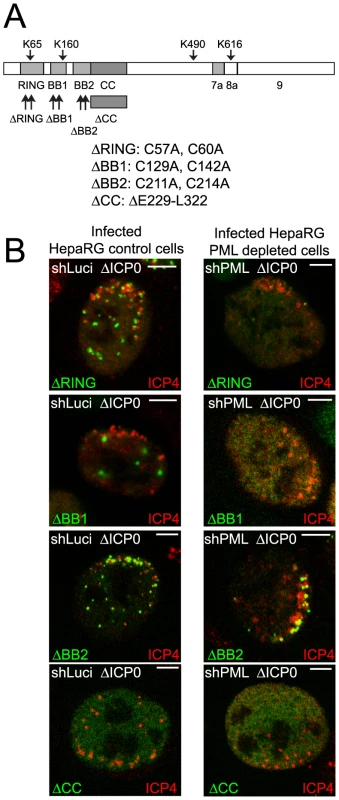
A. Map of the conserved PML.I showing the TRIM elements and the mutations introduced into EYFP-PML.I, adapted from [23]. Analysis of the localization of these proteins in uninfected cells and their expression levels is presented elsewhere [23]. B. Typical assays of recruitment of these PML proteins (green) to sites associated with HSV-1 genomes (ICP4; red) in cells at the edges of ICP0 null mutant (ΔICP0) plaques in control and PML depleted cells (left- and right-hand sets of images, respectively). Scale bars indicate 5 µm. The ΔRING and ΔBB2 mutants were recruited efficiently to the virus-induced foci in cells expressing endogenous PML, whereas the ΔBB1 and ΔCC mutants were not (Figures 5B and S10A). The ΔBB2 mutant was also efficiently recruited in PML depleted cells (Figure 5B), even though this mutant was not well SUMO-modified and did not efficiently co-localize with Sp100 in uninfected cells in either cell background [23]. Therefore the characteristics of PML required for nucleating an ND10-like structure in uninfected cells are distinguishable from those involved in recruitment to the novel virus-induced structures. As in cells expressing endogenous PML, the ΔBB1 and ΔCC mutants were not recruited into virus-induced foci in PML depleted cells (Figure 5B). The most variable results were obtained with the ΔRING mutant in PML depleted cells. In most cells infected with ICP0 null mutant HSV-1 this mutant remained nuclear diffuse, but in some cells it was recruited with variable efficiencies (Figure 5B). We conclude that whereas B-Box 1 and the coiled-coil are essential for PML recruitment to virus induced foci, B-Box 2 is dispensable, and inactivation of the RING greatly diminishes but does not always eliminate recruitment.
The SIM of Sp100 is required for recruitment to HSV-1 induced foci
Given that recruitment of PML to sites to HSV-1 induced foci is dependent on its SIM, we investigated whether recruitment of other ND10 proteins is also SIM dependent. Sp100A is the smallest and most abundantly expressed Sp100 isoform. It includes a SIM (residues 323–326), a major SUMO modification site at lysine 297, and a region near the N-terminus that is required for localization to ND10 the HSR domain (Figure 6A) [33], [34]. Control and Sp100-depleted HepaRG cells [6] were transduced with lentivirus vectors expressing EYFP-linked Sp100A and mutants lacking the HSR domain (ΔHSR), the SUMO modification site (K297R) or the SIM (mSIM), or a combination of both K297R and mSIM mutations (ΔSSIM). Consistent with previous studies [33], [34], none of the mutant proteins exhibited the SUMO modification pattern characteristic of wt Sp100A (Figure 6C). The mSIM mutant of Sp100A at least partially co-localized with PML in control cells, and also in Sp100 depleted cells expressing higher levels of the recombinant protein (Figure 7B, leftmost panels), although it was nuclear diffuse when weakly expressed in Sp100 depleted cells (not shown).
Fig. 6. Expression and analysis of mutants of Sp100A and their recruitment to sites associated with HSV-1 genomes. 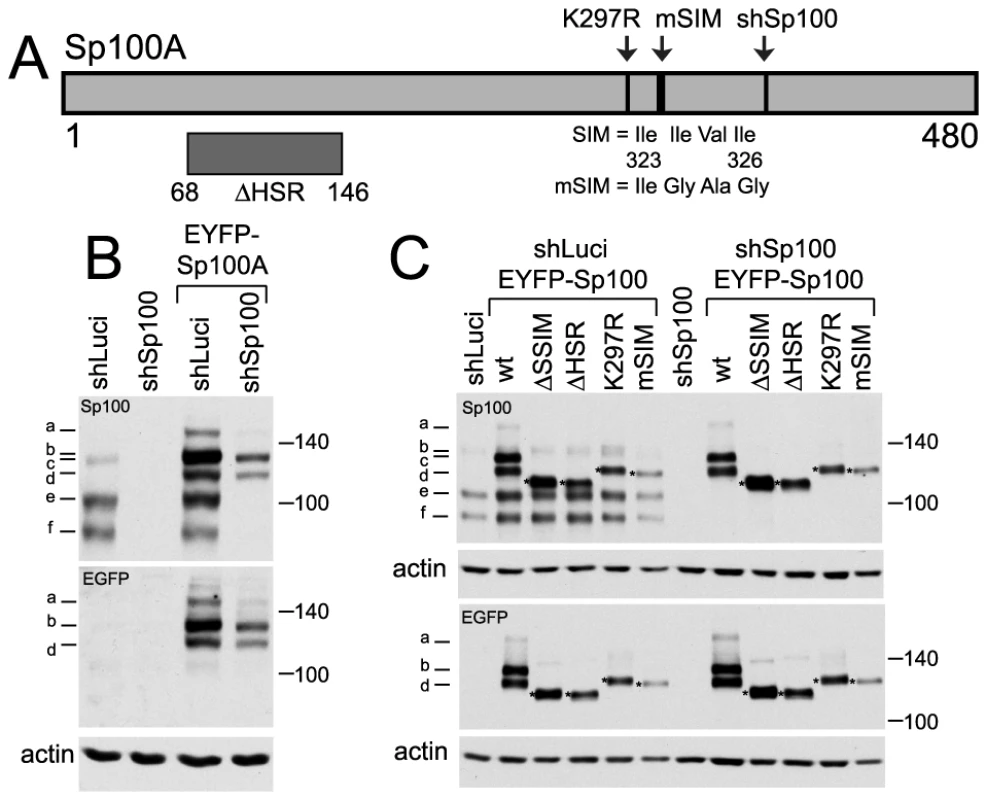
A. Map of Sp100A and mutations introduced into EYFP-Sp100A fusion proteins. The sequence targeted by the anti-Sp100 shRNA and the silent mutations that render the mRNA resistant is marked (shSp100). B. Western blot of wt EYFP-Sp100A in control and Sp100 depleted cells, with anti-Sp100 and anti-EGFP antibodies (upper and lower parts, respectively). The bands are as follows: a - double modification of EYFP-Sp100A; b - single SUMO modification of EYFP-Sp100A; c - double modification of endogenous Sp100; d - unmodified EYFP-Sp100A; e - single SUMO modification of endogenous Sp100A; f - unmodified endogenous Sp100A. C. Western blot of wt and mutant Sp100 proteins in control and Sp100 depleted cells. The mutant ΔSSIM deletes residues 289–327 encompassing both the K297 SUMO modification site and the SIM. Fig. 7. Immunofluorescence analysis of Sp100 reconstructed cells. 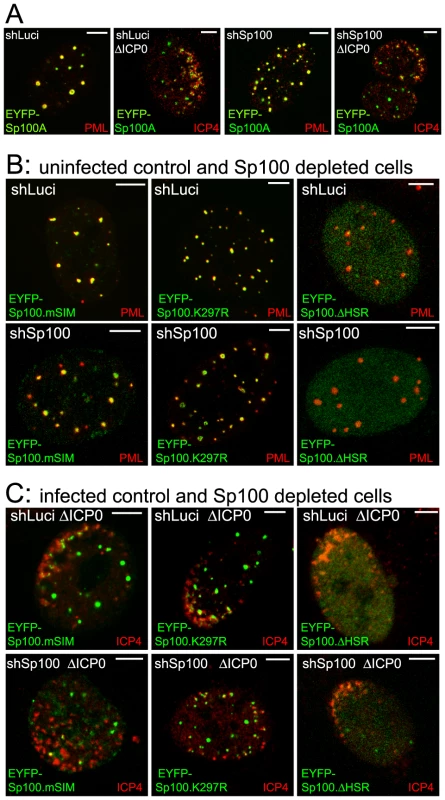
A. Typical images of wt EYFP-Sp100A (green) in uninfected control and Sp100 depleted cells co-stained for endogenous PML (red), and its recruitment to sites associated with HSV-1 genomes (ICP4; red) in cells at the edges of ICP0 null mutant (ΔICP0) plaques. B. Typical images of the mSIM, K297R and ΔHSR mutant proteins (green) in uninfected control and Sp100 depleted cells co-stained for endogenous PML (red). C. Assays of recruitment of these mutant proteins to sites associated with HSV-1 genomes (ICP4; red) in cells at the edges of ICP0 null mutant (ΔICP0) plaques. Scale bars indicate 5 µm. Both wt and K297R mutant EYFP-Sp100A were recruited to the virus-induced foci in both control and Sp100 depleted cells (Figure 7A and C), indicating that, unlike PML, the major SUMO modification site is not required for Sp100A recruitment. Although the HSR deletion mutant was not so recruited (Figure 7C, right-most panels, and Figure S10B), it is possible that this deletion causes major structural defects because this mutant does not localize to ND10 in uninfected cells (Figure 7B). The Sp100A.mSIM and ΔSSIM mutants were recruited to virus-induced foci in cells containing endogenous Sp100, but not in its absence (Figure 7C, leftmost panels, and Figure S10B; data not shown for ΔSSIM). These results indicate that the SIM of Sp100, like that of PML, is required for recruitment, but in its absence the mSIM mutant can be recruited through an interaction with endogenous Sp100, most likely through the HSR domain [34].
The SIM of hDaxx is required for its recruitment to HSV-1 induced foci
Like PML and Sp100, hDaxx includes a SIM (at its C-terminus; Figure 8A) and is also recruited to HSV-1 induced ND10-like foci [18], [35]. Both wt and mSIM mutant versions of hDaxx were expressed in control (data not shown) and hDaxx depleted cells (Figure 8B). The wt protein co-localized with PML in ND10 (Figure 8C, upper row, left), but the mSIM mutant was diffusely distributed in the nucleus (Figure 8C, upper row, right). As with PML and Sp100, wt but not SIM mutant EYFP-hDaxx was recruited to the virus-induced foci in hDaxx depleted cells (Figures 8C and S10C).
Fig. 8. Recruitment of hDaxx to sites associated with HSV-1 genomes is dependent on its SIM. 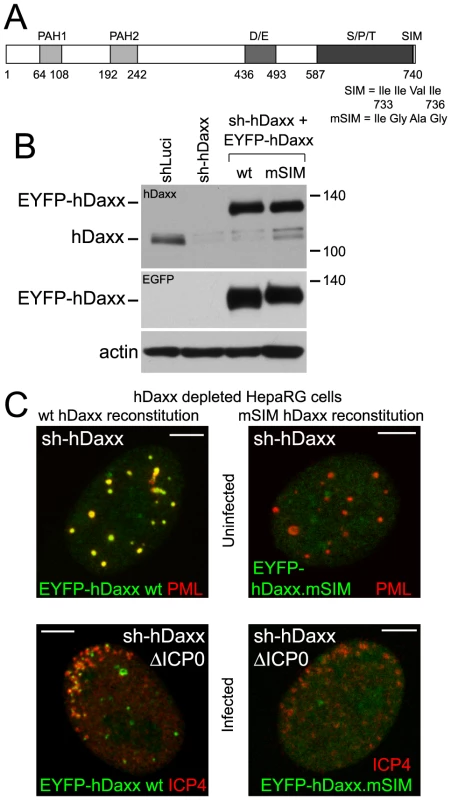
A. Map of hDaxx and its major features (PAH1 and PAH2 – paired amphipathic helices 1 and 2; D/E – aspartic and glutamic acid rich region; S/P/T – serine, proline and threonine rich region). The position and sequence of the SIM and the changes in the mSIM mutant are indicated. B. Western blot of control and hDaxx depleted cells, and depleted cells reconstituted with the wt and mSIM mutant EYFP-hDaxx, using anti-hDaxx (upper panel) and anti-EGFP (lower panel) antibodies. C. Typical images of wt and mSIM mutant EYFP-hDaxx (green) in uninfected hDaxx depleted cells co-stained for endogenous PML (red) (upper panels), and assays of their recruitment to sites associated with HSV-1 genomes (ICP4; red) in cells at the edges of ICP0 null mutant (ΔICP0) plaques (lower panels). Scale bars indicate 5 µm. PIAS2β and SUMO isoforms are present at HSV-1 induced foci
The requirement of the SIMs of PML, Sp100 and hDaxx for recruitment to the HSV-1 induced foci suggests that these proteins may be interacting with components of the SUMO conjugation pathway at these locations. Therefore we examined the behaviour of SUMO isoforms and the SUMO E3 ligase PIAS2β in this experimental system. We found that PIAS2β, as detected by an antibody that recognizes the endogenous protein, is an ND10 component (Figure 9C, upper left). This suggests that PIAS proteins could be involved in ND10 assembly, consistent with the observation that ectopically expressed tagged PIAS1 localizes to ND10 in Vero cells [36]. In ICP0-null mutant HSV-1 infected cells, SUMO-1, SUMO-2/3 and PIAS2β were clearly recruited to the virus-induced foci (Figure 9A–C, right-hand panels). It could be argued that the presence of SUMO isoforms would be expected because both PML and Sp100 are recruited to the foci, and both are heavily SUMO modified. However, recruitment of SUMO-2/3 was readily evident in PML depleted cells (in which SUMO modification of Sp100 is highly compromised) (Figure 9B), and although recruitment of SUMO-1 and PIAS2β was very weak at best in PML-depleted HepaRG cells (Figures 9A, 9C and S11D), recruitment of PIAS2β remained strong in PML-depleted HFs (Figure S11C). The presence of a SUMO E3 ligase in the virus-induced foci suggests that the recruited SUMO-2/3 signal could include newly generated SUMO conjugates, and it is possible that this activity drives the PML-independent but SIM-dependent recruitment of proteins such as hDaxx. Consistent with this hypothesis, we have found that the recruitment of PML and SUMO isoforms requires Ubc9 (the sole SUMO E2 conjugating enzyme), and that the overall level of high molecular weight SUMO conjugates increases in ICP0 null mutant HSV-1 infections (C. Boutell, D. Cuchet-Lourenço, E. Vanni, A. Orr, and R.D. Everett, unpublished data).
Fig. 9. Recruitment of SUMO family members and PIAS2β to HSV-1 induced foci. 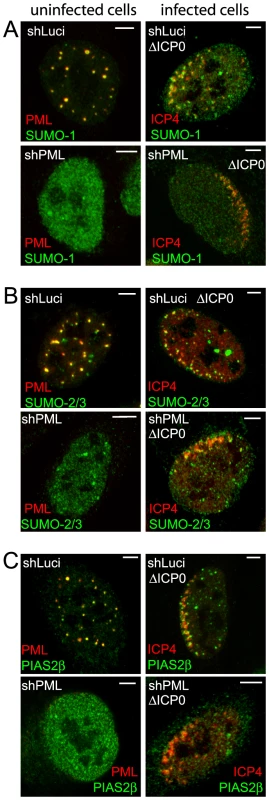
Left-hand images show uninfected cells and the co-localization of SUMO-1 (A), SUMO-2/3 (B) and PIAS2β (C) (green) with PML (red) in control (upper rows of each block of 4 images) and PML depleted (low rows of each block of 4 images) HepaRG cells. Right-hand images show typical examples of recruitment of the indicated proteins to sites associated with HSV-1 genomes (ICP4; red) in cells at the edges of ICP0 null mutant (ΔICP0) plaques in control and PML depleted HepaRG cells. Scale bars indicate 5 µm. Recruitment of hDaxx and PML.I to HSV-1 induced foci correlates with their antiviral properties
We investigated the biological significance of the recruitment of PML and hDaxx to sites associated with HSV-1 genomes by comparing the plaque formation efficiencies of wt and ICP0 null mutant HSV-1 in various control and reconstituted cells. Depletion of either PML or hDaxx increases the plaque formation efficiency of ICP0 mutant HSV-1 while not affecting that of the wt [5], [6], [7]. As expected, the plaque forming efficiency of the wt virus was not significantly different in any of the cell lines tested here (Figures 10A and 10B, upper histograms). Increased plaque formation of ICP0 null mutant HSV-1 was observed in cells depleted of either PML or hDaxx, and reintroduction of PML.I and hDaxx reversed these phenotypes partially and completely, respectively (Figures 10A and 10B, lower histograms), confirming previous work [7], [23]. However, reintroduction of the mSIM mutants of PML.I and hDaxx had no effect on ICP0 null mutant HSV-1 plaque formation (Figures 10A and 10B, lower histograms). It may be relevant that mutation of its SIM reduces the transcriptional repression activity of hDaxx [35]. We have reported previously that PML.I.KK, PML.I.Δ7a are unable to reproduce the repressive effect of PML.I on ICP0 null mutant HSV-1 plaque formation [23]. The analogous experiments with Sp100 were not informative because reintroduction of Sp100A into Sp100 depleted cells did not reverse the effect of Sp100 depletion (data not shown), possibly because it is the longer isoforms of Sp100 (Sp100B, -C and -HMG) that are thought to act as repressors, rather than Sp100A [37], [38], [39]. There is therefore a correlation between the recruitment of PML and hDaxx to foci associated with HSV-1 genomes and their involvement in intrinsic resistance to virus infection.
Fig. 10. The SIMs of PML and hDaxx are required for repression of ICP0 null mutant HSV-1 plaque formation. 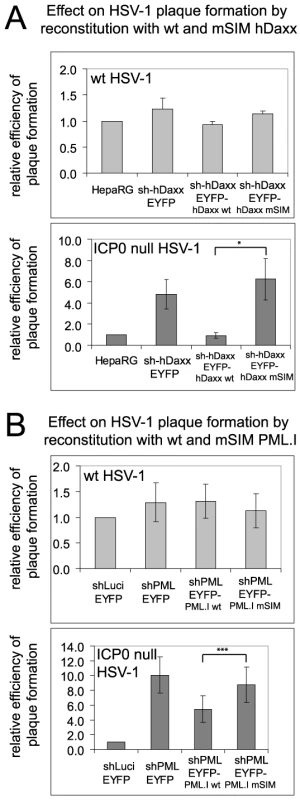
A. Relative plaque forming efficiencies of wt (upper histogram) and ICP0-null mutant (lower histogram) HSV-1 in control and hDaxx-depleted cells and in cells reconstituted with the wt and mSIM mutant proteins. The data show means and standard deviations from 3 independent experiments. The statistical significance between the ICP0 null mutant plaque forming efficiencies in wt and mSIM mutant reconstituted cells is marked (Student's two-tailed t-Test, p = <0.05). B. As in A, except using control, PML-depleted and the indicated PML reconstituted cell lines. The data show means and standard deviations from 5 independent experiments. Statistical significance shown as in A (Student's two-tailed t-Test, p = <0.001). Discussion
The recruitment of ND10 components to sites that are closely associated with parental HSV-1 genomes and early replication compartments is a dramatic cellular response to entry of the viral genome into the nucleus. It occurs very rapidly, being detectable as early as 30 minutes after addition of virus to a cell monolayer [20], and it occurs independently of de novo viral protein synthesis, implying that it is the viral DNA itself (perhaps in association with viral tegument proteins) that signals the response [18]. These observations raise several important questions, including the nature of the mechanism underlying the recruitment, the biological significance of the response, and the wider implications of these events.
We found that endogenous PML and Sp100 have dominant influences on the behaviour of introduced mutant forms of these proteins, obscuring the role of the mutated motifs (Figures 1–3, 5 and 7). Expression of the reintroduced proteins at close to endogenous levels is also important, as when expressed in excess a protein may be unable to interact efficiently with limiting binding partners in the cell. With these issues overcome, we found that the recruitment of PML to the virus-induced foci depends on its SIM, despite the fact that the mSIM mutant is indistinguishable from the wt in terms of localization and SUMO modification in uninfected cells (Figure S6). Recruitment of PML to the virus-induced sites is also compromised by mutation of the major SUMO modification sites (K160 and K490) and by alterations in the B-Box 1, coiled-coil and RING motifs (Figures 3 and 5). It is unsurprising that elements of the TRIM are important for PML behaviour because these mutations greatly reduce SUMO modification [23] and are likely to have major consequences on PML structure and interactions.
It has been reported that the SIM of PML is required for normal ND10 assembly [26]. Our finding that PML.I.mSIM and PML.I.Δ7a co-localize with Sp100 in PML depleted cells is not inconsistent with the data in this previous study, since the effect of removal of the SIM was observed only when the PML isoform used (PML.III) also lacked SUMO modification sites. Thus the SIM mutant of PML.III still co-localized with hDaxx and SUMO, even in PML (−/−) mouse fibroblasts [26]. That the SIM is not essential for ND10 localization is also consistent with previous studies utilizing PML.VI in PML depleted cells [8], [23], because this is a natural SIM deletion mutant.
The SIMs of both Sp100 and hDaxx are also important for their recruitment to the virus-induced foci (Figures 7 and 8). Given that SUMO modification of Sp100A is not required (Figures 6 and 7), and hDaxx appears not to be detectably SUMO modified in our system (Figure 8), we conclude that it is the SIM rather than SUMO modification that is the common essential feature for recruitment of these proteins to the virus-induced foci. The conclusion that SUMO modification of Sp100A is not required for this behaviour is supported by the observation that Sp100 is not SUMO modified in PML depleted cells, yet is still recruited to the viral foci [5], [6].
The importance of the SIM in recruitment to the virus-induced foci implies that PML, hDaxx and Sp100 are responding to an earlier SUMO-dependent event at these sites. This conclusion is supported by our recent data demonstrating that Ubc9 is required for efficient recruitment of PML to the virus induced foci (C. Boutell, D. Cuchet-Lourenco, E. Vanni, A. Orr, and R.D. Everett, unpublished data). This raises the question of which factors these ND10 components are interacting with, through their SIMs, to enable their recruitment to the virus-induced foci. Recruitment of PML is not dependent on de novo viral gene expression [18], implying that the initial event involves cellular proteins and their recognition of the viral genomes. Because the viral DNA is not chromatinized at the time of entry into the nucleus, its conformation is entirely different from cellular chromatin. It would be expected that many cellular proteins would become associated with the initially naked viral DNA, and one or more of these could initiate the SUMO-dependent recruitment process. The detection of PIAS2β in the novel foci implies that SUMO E3 ligases are involved in the assembly of the novel foci. This is the first report that PIAS2β is involved in ND10 biology, and it is intriguing that the protein has also been implicated in DNA damage and interferon pathways [40], [41].
The mechanisms that underlie formation of the virus-induced structures are distinct in a number of respects from those required for normal ND10 assembly. For example, PML is not required for recruitment of Sp100 and hDaxx to the virus-induced foci [5], [6], [20], and depletion of any one of these three proteins does not eliminate recruitment of the remaining two [6], [7]. Some of the PML mutants also illustrate the differences between normal and virus-induced ND10 related structures: PML.I.ΔBB2 does not colocalize with Sp100 in uninfected cells [23] but is recruited very efficiently to the virus-induced foci (Figure 5). On the other hand, recruitment of ATRX to both normal and virus-induced ND10 structures is dependent on hDaxx [7], illustrating that both specific protein-protein interactions and SIM dependent interactions are involved in building the virus-induced foci. PML-PML interactions through the coiled-coil and Sp100-Sp100 interactions through the HSR motif also influence assembly of the virus-induced structures (Figures 2, 3 and 7). A picture emerges that the building of these foci involves multiple interactions between distinct proteins, protein dimerization events, and SIM-SUMO interactions.
Our data imply a general importance for SUMO related pathways in the nucleation of the HSV-1 induced ND10-like foci. Because SUMO-2/3 and PIAS2β can also be recruited into these structures in a PML-independent manner (Figures 9 and S11), it is attractive to speculate that these events reflect ongoing SUMO conjugation events. We note that the genomes and replication compartments of many DNA viruses are closely associated with PML and ND10-like structures (reviewed in [42], [43]). It would be surprising if the principles revealed here concerning HSV-1 infection were not involved in ND10 association with other viral genomes, and there is evidence that this is the case in human cytomegalovirus infected cells [8], [44]. Therefore the events reported here are likely to reflect a more general cellular response to foreign DNA entering the nucleus. It is possible that the cell is responding to viral genomes in a manner related to that of the DNA damage response. Recent evidence demonstrates that SUMO modification is intimately involved in the assembly of DNA damage response foci, and SUMO conjugates are present at these locations [45], [46]. ICP0 inhibits the formation of these structures by inducing the degradation of RNF8 and RNF168 [47], but it is possible that ICP0 might also impede any recruitment of SUMO conjugated proteins to DNA damage foci, as it does in regard to the viral genome associated ND10-like foci (see below). These observations raise the intriguing possibility of commonalities between the DNA damage response, the assembly of HSV-1 induced ND10-like foci, and intrinsic resistance to HSV-1 infection.
The observations that the SIM mutants of PML.I and hDaxx are unable to reproduce the repression of ICP0 null mutant HSV-1 infection conferred by the wt proteins (Figure 10) imply that the recruitment of these proteins to the virus-induced foci is biologically significant and contributes to the repression of ICP0 null mutant HSV-1 infection. Recruitment of all ND10 component proteins so far studied is counteracted by ICP0 [7], [18], [20]. ICP0 inhibits PML recruitment by inducing its degradation [15], [17]. This simple mechanism does not, however, explain why recruitment of all ND10 proteins is inhibited by ICP0. Although ICP0 promotes the loss of SUMO modified Sp100 [16] this cannot explain why Sp100 recruitment is inhibited because the K297R mutant is still recruited (Figure 7). Furthermore, ICP0 does not promote the degradation of either hDaxx or ATRX [7]. Previous work has demonstrated that ICP0 induces the widespread degradation of SUMO conjugated proteins [15]. This activity provides an attractive explanation of how ICP0 inhibits recruitment of this group of proteins to virus-induced foci, since degradation of SUMO conjugates would eliminate SIM dependent interactions.
These arguments suggest a direct link between SUMO-dependent pathways and the mechanism of intrinsic cellular resistance to HSV-1 infection that is counteracted by ICP0. We propose that the cell responds to foreign DNA that enters the nucleus by stimulating SUMO conjugation events at sites associated with the introduced DNA, leading to recruitment of other proteins in a SIM dependent manner and resulting in a repressive environment. We note that there are several examples of factors involved in transcriptional repression that are regulated by SUMO modification [48], and that SUMO modification pathways have been linked to a general cellular response to pathogens [49]. This concept is strengthened by our related studies (C. Boutell, D. Cuchet-Lourenço, E. Vanni, A. Orr, and R.D. Everett, unpublished data) that demonstrate the involvement of Ubc9 in intrinsic antiviral resistance, and that ICP0 has SUMO-targeted ubiquitin ligase activities that play an important role in its ability to counteract this resistance.
Materials and Methods
Cells
U2OS, HEK-293T and human fibroblast cells were grown in Dulbecco's Modified Eagles' Medium with 10% fetal calf serum (FCS). Baby hamster kidney (BHK) cells were grown in Glasgow Modified Eagles' Medium with 10% new born calf serum and 10% tryptose phosphate broth. HepaRG cells [50] were grown in William's Medium E with 10% fetal bovine serum Gold (PAA Laboratories Ltd), 2 mM glutamine, 5 µg/ml insulin and 0.5 µM hydrocortisone. All cell growth media contained 100 units/ml penicillin and 100 µg/ml streptomycin.
Lentivirus expression vectors and cells
Lentivirus vector plasmids expressing shRNAs, EYFP-hDaxx, and EYFP-PML isoforms I to VI, mutants of PML.I with lesions in the RING finger (ΔRING), B-Box 1 (ΔBB1), B-Box 2 (ΔBB2), the coiled-coil motif (ΔCC) and at SUMO modification sites K160 and K490 were as described [6], [7], [23]. The following PML mutants were constructed using PCR splicing with mutagenic oligonucleotides: PML.I.Δ7a and PML.IV.Δ7a (precise deletions of exon 7a in PML.I and PML.IV cDNAs); PML.IV.KK (K160R, K490R mutations in the PML.IV background); PML.I.mSIM (residues 566–569 VVVI mutated to VGGG); PML.I.K123 (mutations K65R, K160R, K490R); PML.I.K234 (K160R, K490R, K616R); PML.I.K1-4 (lysine residues K65, K160, K490 and K160 mutated to arginine). Lentivirus transduced HepaRG cells expressing EYFP-linked proteins were sorted by FACS, as described [23]. HFs expressing control and anti-PML shRNAs [5] and EYFP-PML.I, PML.VI, PML.I.Δ7a, PML.I.KK and PML.I.K1-4 were isolated using the same methodology.
Sp100 and hDaxx expression vectors and cells
Lentivirus vectors expressing EYFP-Sp100 isoform A (using a cDNA resistant to the anti-Sp100 shRNA) and derivatives lacking the HSR region (Sp100.ΔHSR, deletion of residues 68–146), the major SUMO modification site and the SIM combined (Sp100.ΔSSIM, deletion of residues 289–327), the major SUMO modification site alone (Sp100.K297R) and with point mutations in the SIM alone (residues 323 to 326 IIVI changed to IGAG; Sp100.mSIM) were constructed using PCR splicing. A lentivirus vector expressing EYFP - hDaxx with lesions in the SIM (residues 733 to 736 IIVI changed to IGAG) was constructed by PCR directed mutagenesis. HepaRG cells expressing wt and mutant hDaxx were enriched by FACS.
Lentivirus transduction
Lentivirus vector plasmids were co-transfected into HEK-293T cells with helper plasmids pVSV-G and pCMV.DR.8.91, then the supernatants were used to transduce HF or HepaRG cells. All shRNA vectors express puromycin for selection (initially 1 µg/ml, then reduced to 0.5 µg/ml during subsequent passage), and all expressing a protein of interest confer G418 resistance (selection initially 1 mg/ml, then reduced to 0.5 mg/ml during subsequent passage).
HSV-1 strains and plaque assays
Wild type HSV-1 strain 17syn+ and its ICP0 null mutant derivative dl1403 [51] were grown in BHK cells and titrated in U2OS cells. Derivatives of wt and ICP0-null mutant HSV-1 that include a β-galactosidase gene linked to the human cytomegalovirus immediate-early promoter/enhancer (in1863 and dl1403/CMVlacZ) were used for plaque assays, as described [5].
Western blot analysis and antibodies
Cells in 24-well dishes at 1×105 cells per well were washed with phosphate buffered saline PBS) before harvesting in SDS-PAGE loading buffer. Proteins were resolved on 7.5% SDS-gels, then transferred to nitrocellulose membranes by western blotting. The following antibodies were used: anti-actin mAb AC-40 (Sigma-Aldrich); anti-PML mAb 5E10 [52]; anti-Sp100 rabbit serum SpGH [53]; anti-hDaxx rabbit polyclonal D7801 (Sigma-Aldrich); anti-EGFP rabbit polyclonal ab290 (Abcam).
Immunofluorescence and confocal microscopy
Cells on 13 mm glass coverslips were fixed using 1.5% (v/v) formaldehyde in PBS containing 2% sucrose then treated with 0.5% Nonidet P40 substitute (EuroClone S.p.A.) in PBS/10% sucrose. PML was detected with mAb 5E10 and ICP4 with mAb 58S. Rabbit polyclonal antibodies were used for Sp100 (SpGH), hDaxx (07-471, Upstate), SUMO-1 (ab32058, Abcam), SUMO-2/3 (ab3742-100, Abcam), PIAS2β (gifted by Mary Dasso). The secondary antibodies were FITC conjugated goat anti-mouse IgG (Sigma), Alexa 488 and Alexa 633 conjugated goat anti-rabbit and anti-mouse IgG, and Alexa 555 conjugated donkey anti-rabbit and anti-mouse IgG, (Invitrogen). A glycerol-based mounting medium was used (Citifluor AF1). The samples were examined using a Zeiss LSM 510 confocal microscope with 488 nm, 543 nm and 633 nm laser lines and a ×63 Plan-Apochromat oil immersion lens, NA 1.40. Exported images were processed using Adobe Photoshop with minimal adjustment, then assembled for presentation using Adobe Illustrator.
Fluorescence recovery after photobleaching
Cells were seeded into modified 35 mm dishes with the central area replaced by coverslip glass (MatTek Corporation). Fluorescence recovery after photobleaching (FRAP) was conducted using an LSM 510 META microscope with full environmental control. In each experiment, 3 PML foci in 10 different cells were subject to bleaching (100% power of the 514 nm laser, 10 reiterations), then 20 images were captured over a period of approximately two minutes during the recovery phase. Regions of interest were analyzed by subtracting the average pixel intensity in unbleached background areas and normalizing to any changes in overall intensity of a similar unbleached PML structure to control for any bleaching of the scanned areas during image acquisition. The data for each protein were assembled into an Excel file for graphical representation of the average plus standard deviation at each time point.
Supporting Information
Zdroje
1. KnipeDMHowleyPMGriffinDELambRAMartinMA 2006 Fields virology Philadelphia Lippincott Williams and Wilkins
2. SaffertRTKalejtaRF 2008 Promyelocytic leukemia-nuclear body proteins: herpesvirus enemies, accomplices, or both? Future Virology 3 265 277
3. TavalaiNStammingerT 2008 New insights into the role of the subnuclear structure ND10 for viral infection. Biochim Biophys Acta 1783 2207 2221
4. EverettRDChelbi-AlixMK 2007 PML and PML nuclear bodies: implications in antiviral defence. Biochimie 89 819 830
5. EverettRDRechterSPapiorPTavalaiNStammingerT 2006 PML contributes to a cellular mechanism of repression of herpes simplex virus type 1 infection that is inactivated by ICP0. J Virol 80 7995 8005
6. EverettRDParadaCGriponPSirmaHOrrA 2008 Replication of ICP0-null mutant herpes simplex virus type 1 is restricted by both PML and Sp100. J Virol 82 2661 2672
7. LukashchukVOrrAEverettRD 2010 Regulation of ICP0 null mutant HSV-1 infection by ND10 components ATRX and hDaxx. J Virol 84 4026 4040
8. TavalaiNPapiorPRechterSLeisMStammingerT 2006 Evidence for a role of the cellular ND10 protein PML in mediating intrinsic immunity against human cytomegalovirus infections. J Virol 80 8006 8018
9. CantrellSRBresnahanWA 2006 Human cytomegalovirus (HCMV) UL82 gene product (pp71) relieves hDaxx-mediated repression of HCMV replication. J Virol 80 6188 6191
10. LukashchukVMcFarlaneSEverettRDPrestonCM 2008 Human cytomegalovirus protein pp71 displaces the chromatin-associated factor ATRX from nuclear domain 10 at early stages of infection. J Virol 82 12543 12554
11. PrestonCMNichollMJ 2006 Role of the cellular protein hDaxx in human cytomegalovirus immediate-early gene expression. J Gen Virol 87 1113 1121
12. SaffertRTKalejtaRF 2007 Human cytomegalovirus gene expression is silenced by Daxx-mediated intrinsic immune defense in model latent infections established in vitro. J Virol 81 9109 9120
13. TavalaiNPapiorPRechterSStammingerT 2008 Nuclear domain 10 components promyelocytic leukemia protein and hDaxx independently contribute to an intrinsic antiviral defense against human cytomegalovirus infection. J Virol 82 126 137
14. WoodhallDLGrovesIJReevesMBWilkinsonGSinclairJH 2006 Human Daxx-mediated repression of human cytomegalovirus gene expression correlates with a repressive chromatin structure around the major immediate early promoter. J Biol Chem 281 37652 37660
15. EverettRDFreemontPSaitohHDassoMOrrA 1998 The disruption of ND10 during herpes simplex virus infection correlates with the Vmw110 - and proteasome-dependent loss of several PML isoforms. J Virol 72 6581 6591
16. ParkinsonJEverettRD 2000 Alphaherpesvirus proteins related to herpes simplex virus type 1 ICP0 affect cellular structures and proteins. J Virol 74 10006 10017
17. Chelbi-AlixMKde TheH 1999 Herpes virus induced proteasome-dependent degradation of the nuclear bodies-associated PML and Sp100 proteins. Oncogene 18 935 941
18. EverettRDMurrayJ 2005 ND10 components relocate to sites associated with herpes simplex virus type 1 nucleoprotein complexes during virus infection. J Virol 79 5078 5089
19. EverettRDSourvinosGLeiperCClementsJBOrrA 2004 Formation of nuclear foci of the herpes simplex virus type 1 regulatory protein ICP4 at early times of infection: localization, dynamics, recruitment of ICP27, and evidence for the de novo induction of ND10-like complexes. J Virol 78 1903 1917
20. EverettRDMurrayJOrrAPrestonCM 2007 Herpes simplex virus type 1 genomes are associated with ND10 nuclear substructures in quiescently infected human fibroblasts. J Virol 81 10991 11004
21. EverettRDParsyMLOrrA 2009 Analysis of the functions of herpes simplex virus type 1 regulatory protein ICP0 that are critical for lytic infection and derepression of quiescent viral genomes. J Virol 83 4963 4977
22. SongJDurrinLKWilkinsonTAKrontirisTGChenY 2004 Identification of a SUMO-binding motif that recognizes SUMO-modified proteins. Proc Natl Acad Sci U S A 101 14373 14378
23. CuchetDSykesAKNicolasAOrrAMurrayJ 2011 PML isoforms I and II participate in PML-dependent restriction of HSV-1 replication. J Cell Sci 124 280 291
24. JensenKShielsCFreemontPS 2001 PML protein isoforms and the RBCC/TRIM motif. Oncogene 20 7223 7233
25. KastnerPPerezALutzYRochette-EglyCGaubMP 1992 Structure, localization and transcriptional properties of two classes of retinoic acid receptor alpha fusion proteins in acute promyelocytic leukemia (APL): structural similarities with a new family of oncoproteins. Embo J 11 629 642
26. ShenTHLinHKScaglioniPPYungTMPandolfiPP 2006 The mechanisms of PML-nuclear body formation. Mol Cell 24 331 339
27. ZhongSMullerSRonchettiSFreemontPSDejeanA 2000 Role of SUMO-1-modified PML in nuclear body formation. Blood 95 2748 2752
28. KamitaniTKitoKNguyenHPWadaHFukuda-KamitaniT 1998 Identification of three major sentrinization sites in PML. J Biol Chem 273 26675 26682
29. BrandPLenserTHemmerichP 2010 Assembly dynamics of PML nuclear bodies in living cells. PMC Biophys 3 3
30. Weidtkamp-PetersSLenserTNegorevDGerstnerNHofmannTG 2008 Dynamics of component exchange at PML nuclear bodies. J Cell Sci 121 2731 2743
31. WiesmeijerKMolenaarCBekeerIMTankeHJDirksRW 2002 Mobile foci of Sp100 do not contain PML: PML bodies are immobile but PML and Sp100 proteins are not. J Struct Biol 140 180 188
32. DellaireGEskiwCHDehghaniHChingRWBazett-JonesDP 2006 Mitotic accumulations of PML protein contribute to the re-establishment of PML nuclear bodies in G1. J Cell Sci 119 1034 1042
33. KimETKimKKMatunisMJAhnJH 2009 Enhanced SUMOylation of proteins containing a SUMO-interacting motif by SUMO-Ubc9 fusion. Biochem Biophys Res Commun 388 41 45
34. SternsdorfTJensenKReichBWillH 1999 The nuclear dot protein sp100, characterization of domains necessary for dimerization, subcellular localization, and modification by small ubiquitin-like modifiers. J Biol Chem 274 12555 12566
35. LinDYHuangYSJengJCKuoHYChangCC 2006 Role of SUMO-interacting motif in Daxx SUMO modification, subnuclear localization, and repression of sumoylated transcription factors. Mol Cell 24 341 354
36. LeeJMKangHJLeeHRChoiCYJangWJ 2003 PIAS1 enhances SUMO-1 modification and the transactivation activity of the major immediate-early IE2 protein of human cytomegalovirus. FEBS Lett 555 322 328
37. NegorevDGVladimirovaOVIvanovARauscherF3rdMaulGG 2006 Differential role of Sp100 isoforms in interferon-mediated repression of herpes simplex virus type 1 immediate-early protein expression. J Virol 80 8019 8029
38. NegorevDGVladimirovaOVMaulGG 2009 Differential functions of interferon-upregulated Sp100 isoforms: herpes simplex virus type 1 promoter-based immediate-early gene suppression and PML protection from ICP0-mediated degradation. J Virol 83 5168 5180
39. WilcoxKWSheriffSIsaacATaylorJL 2005 SP100B is a repressor of gene expression. J Cell Biochem 95 352 365
40. FuJXiongYXuYChengGTangH 2011 MDA5 is SUMOylated by PIAS2beta in the upregulation of type I interferon signaling. Mol Immunol 48 415 422
41. WanzelMKleine-KohlbrecherDHeroldSHockABernsK 2005 Akt and 14-3-3eta regulate Miz1 to control cell-cycle arrest after DNA damage. Nat Cell Biol 7 30 41
42. EverettRD 2006 Interactions between DNA viruses, ND10 and the DNA damage response. Cell Microbiol 8 365 374
43. MaulGG 1998 Nuclear domain 10, the site of DNA virus transcription and replication. Bioessays 20 660 667
44. SourvinosGTavalaiNBerndtASpandidosDAStammingerT 2007 Recruitment of human cytomegalovirus immediate-early 2 protein onto parental viral genomes in association with ND10 in live-infected cells. J Virol 81 10123 10136
45. GalantyYBelotserkovskayaRCoatesJPoloSMillerKM 2009 Mammalian SUMO E3-ligases PIAS1 and PIAS4 promote responses to DNA double-strand breaks. Nature 462 935 939
46. MorrisJRBoutellCKepplerMDenshamRWeekesD 2009 The SUMO modification pathway is involved in the BRCA1 response to genotoxic stress. Nature 462 886 890
47. LilleyCEChaurushiyaMSBoutellCLandrySSuhJ 2010 A viral E3 ligase targets RNF8 and RNF168 to control histone ubiquitination and DNA damage responses. EMBO J 29 943 955
48. HayRT 2005 SUMO: a history of modification. Mol Cell 18 1 12
49. RibetDHamonMGouinENahoriMAImpensF 2010 Listeria monocytogenes impairs SUMOylation for efficient infection. Nature 464 1192 1195
50. GriponPRuminSUrbanSLe SeyecJGlaiseD 2002 Infection of a human hepatoma cell line by hepatitis B virus. Proc Natl Acad Sci U S A 99 15655 15660
51. StowNDStowEC 1986 Isolation and characterization of a herpes simplex virus type 1 mutant containing a deletion within the gene encoding the immediate early polypeptide Vmw110. J Gen Virol 67 2571 2585
52. StuurmanNde GraafAFlooreAJossoAHumbelB 1992 A monoclonal antibody recognizing nuclear matrix-associated nuclear bodies. J Cell Sci 101 773 784
53. SternsdorfTJensenKWillH 1997 Evidence for covalent modification of the nuclear dot-associated proteins PML and Sp100 by PIC1/SUMO-1. J Cell Biol 139 1621 1634
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek A Typhimurium-Typhi Genomic Chimera: A Model to Study Vi Polysaccharide Capsule Function In VivoČlánek A Structural Model for Binding of the Serine-Rich Repeat Adhesin GspB to Host Carbohydrate ReceptorsČlánek Dynamic Evolution of Pathogenicity Revealed by Sequencing and Comparative Genomics of 19 IsolatesČlánek Widespread Endogenization of Genome Sequences of Non-Retroviral RNA Viruses into Plant GenomesČlánek The Cost of Virulence: Retarded Growth of Typhimurium Cells Expressing Type III Secretion System 1Článek A Role for the Chemokine RANTES in Regulating CD8 T Cell Responses during Chronic Viral Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 7- Stillova choroba: vzácné a závažné systémové onemocnění
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Jak souvisí postcovidový syndrom s poškozením mozku?
-
Všechny články tohoto čísla
- What Do We Really Know about How CD4 T Cells Control ?
- “Persisters”: Survival at the Cellular Level
- E6 and E7 from Beta Hpv38 Cooperate with Ultraviolet Light in the Development of Actinic Keratosis-Like Lesions and Squamous Cell Carcinoma in Mice
- Selection of Resistant Bacteria at Very Low Antibiotic Concentrations
- The Extracytoplasmic Domain of the Ser/Thr Kinase PknB Binds Specific Muropeptides and Is Required for PknB Localization
- CD39/Adenosine Pathway Is Involved in AIDS Progression
- Hypoxia and a Fungal Alcohol Dehydrogenase Influence the Pathogenesis of Invasive Pulmonary Aspergillosis
- One Is Enough: Effective Population Size Is Dose-Dependent for a Plant RNA Virus
- Effects of Interferon-α/β on HBV Replication Determined by Viral Load
- A Typhimurium-Typhi Genomic Chimera: A Model to Study Vi Polysaccharide Capsule Function In Vivo
- Dual Chaperone Role of the C-Terminal Propeptide in Folding and Oligomerization of the Pore-Forming Toxin Aerolysin
- Rotavirus Stimulates Release of Serotonin (5-HT) from Human Enterochromaffin Cells and Activates Brain Structures Involved in Nausea and Vomiting
- Dissociation of Infectivity from Seeding Ability in Prions with Alternate Docking Mechanism
- The Impact of Recombination on dN/dS within Recently Emerged Bacterial Clones
- The Regulation of Sulfur Metabolism in
- Illumination of Parainfluenza Virus Infection and Transmission in Living Animals Reveals a Tissue-Specific Dichotomy
- A Permeable Cuticle Is Associated with the Release of Reactive Oxygen Species and Induction of Innate Immunity
- A Concerted Action of Hepatitis C Virus P7 and Nonstructural Protein 2 Regulates Core Localization at the Endoplasmic Reticulum and Virus Assembly
- SUMO Pathway Dependent Recruitment of Cellular Repressors to Herpes Simplex Virus Type 1 Genomes
- Re-localization of Cellular Protein SRp20 during Poliovirus Infection: Bridging a Viral IRES to the Host Cell Translation Apparatus
- Divergent Effects of Human Cytomegalovirus and Herpes Simplex Virus-1 on Cellular Metabolism
- A Structural Model for Binding of the Serine-Rich Repeat Adhesin GspB to Host Carbohydrate Receptors
- Transformation of Natural Genetic Variation into Genomes
- EBV Latency Types Adopt Alternative Chromatin Conformations
- Global mRNA Degradation during Lytic Gammaherpesvirus Infection Contributes to Establishment of Viral Latency
- Dynamic Evolution of Pathogenicity Revealed by Sequencing and Comparative Genomics of 19 Isolates
- Microbial Virulence as an Emergent Property: Consequences and Opportunities
- Widespread Endogenization of Genome Sequences of Non-Retroviral RNA Viruses into Plant Genomes
- Structural Basis of Chemokine Sequestration by CrmD, a Poxvirus-Encoded Tumor Necrosis Factor Receptor
- Cross-Species Transmission of a Novel Adenovirus Associated with a Fulminant Pneumonia Outbreak in a New World Monkey Colony
- An Interaction between KSHV ORF57 and UIF Provides mRNA-Adaptor Redundancy in Herpesvirus Intronless mRNA Export
- Elevated 17β-Estradiol Protects Females from Influenza A Virus Pathogenesis by Suppressing Inflammatory Responses
- The Role of IL-15 Deficiency in the Pathogenesis of Virus-Induced Asthma Exacerbations
- Fluorescence Lifetime Imaging Unravels Metabolism and Its Crosstalk with the Host Cell
- Programmed Death (PD)-1-Deficient Mice Are Extremely Sensitive to Murine Hepatitis Virus Strain-3 (MHV-3) Infection
- Hemoglobin Promotes Nasal Colonization
- Crystallography of a Lewis-Binding Norovirus, Elucidation of Strain-Specificity to the Polymorphic Human Histo-Blood Group Antigens
- The Cost of Virulence: Retarded Growth of Typhimurium Cells Expressing Type III Secretion System 1
- A Genome-Wide Approach to Discovery of Small RNAs Involved in Regulation of Virulence in
- Requires Glycerol for Maximum Fitness During The Tick Phase of the Enzootic Cycle
- C Metabolic Flux Analysis Identifies an Unusual Route for Pyruvate Dissimilation in Mycobacteria which Requires Isocitrate Lyase and Carbon Dioxide Fixation
- A Role for the Chemokine RANTES in Regulating CD8 T Cell Responses during Chronic Viral Infection
- Glycosaminoglycans and Sialylated Glycans Sequentially Facilitate Merkel Cell Polyomavirus Infectious Entry
- Regulation of Stomatal Tropism and Infection by Light in : Evidence for Coordinated Host/Pathogen Responses to Photoperiod?
- Multiple Translocation of the Effector Gene among Chromosomes of the Rice Blast Fungus and Related Species
- Comparative Genomics Yields Insights into Niche Adaptation of Plant Vascular Wilt Pathogens
- Unique Cell Adhesion and Invasion Properties of O:3, the Most Frequent Cause of Human Yersiniosis
- C-Terminal Region of EBNA-2 Determines the Superior Transforming Ability of Type 1 Epstein-Barr Virus by Enhanced Gene Regulation of LMP-1 and CXCR7
- Novel Chikungunya Vaccine Candidate with an IRES-Based Attenuation and Host Range Alteration Mechanism
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Requires Glycerol for Maximum Fitness During The Tick Phase of the Enzootic Cycle
- Comparative Genomics Yields Insights into Niche Adaptation of Plant Vascular Wilt Pathogens
- The Role of IL-15 Deficiency in the Pathogenesis of Virus-Induced Asthma Exacerbations
- “Persisters”: Survival at the Cellular Level
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



