-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Genomics of Cancer and a New Era for Cancer Prevention
A primary justification for dedicating substantial amounts of research funding to large-scale cancer genomics projects of both somatic and germline DNA is that the biological insights will lead to new treatment targets and strategies for cancer therapy. While it is too early to judge the success of these projects in terms of clinical breakthroughs, an alternative rationale is that new genomics techniques can be used to reduce the overall burden of cancer by prevention of new cases occurring and also by detecting them earlier. In particular, it is now becoming apparent that studying the genomic profile of tumors can help to identify new carcinogens and may subsequently result in implementing strategies that limit exposure. In parallel, it may be feasible to utilize genomic biomarkers to identify cancers at an earlier and more treatable stage using screening or other early detection approaches based on prediagnostic biospecimens. While the potential for these techniques is large, their successful outcome will depend on international collaboration and planning similar to that of recent sequencing initiatives.
Published in the journal: . PLoS Genet 11(11): e32767. doi:10.1371/journal.pgen.1005522
Category: Review
doi: https://doi.org/10.1371/journal.pgen.1005522Summary
A primary justification for dedicating substantial amounts of research funding to large-scale cancer genomics projects of both somatic and germline DNA is that the biological insights will lead to new treatment targets and strategies for cancer therapy. While it is too early to judge the success of these projects in terms of clinical breakthroughs, an alternative rationale is that new genomics techniques can be used to reduce the overall burden of cancer by prevention of new cases occurring and also by detecting them earlier. In particular, it is now becoming apparent that studying the genomic profile of tumors can help to identify new carcinogens and may subsequently result in implementing strategies that limit exposure. In parallel, it may be feasible to utilize genomic biomarkers to identify cancers at an earlier and more treatable stage using screening or other early detection approaches based on prediagnostic biospecimens. While the potential for these techniques is large, their successful outcome will depend on international collaboration and planning similar to that of recent sequencing initiatives.
Since the publication of the initial human genome sequence in 2002, at a cost of around US$3 thousand million, DNA sequencing has advanced to the extent where whole genomes can be sequenced in days for around one millionth of the cost [1]. This has led to a scientific tour de force in projects that aim to understand the genetics of cancer. Large-scale initiatives such as the International Cancer Genome Consortium (ICGC) and the Cancer Genome Atlas (TCGA) for somatic variation, as well as the OncoArray Network for genome-wide studies of germline variation, have harnessed international expertise in oncology, genomics, and bioinformatics with very high levels of funding and have resulted in the coordinated genotyping, sequencing, and cataloging of many thousands of cancer cases [2]. Comprehensive genomic data from all completed cases are being made available to the research community, along with basic clinical information on some, allowing for extensive additional analyses. This initiative has led to a new understanding of how to define specific cancer subtypes and has vastly increased the pace of progress in elucidating the underlying biology of cancer [3].
The most prominent visible outcome of the increased understanding of cancer biology is that targeted treatments have been developed or are being tested that aim to block specific molecules that spur the growth or spread of cancer. Although there are some exciting success stories such as the vastly improved survival with imatinib and chronic myelogenous leukemia (CML) or the increased efficacy of Herceptin treatment for women with Her2-positive breast cancer, most of this new generation of targeted treatments promise, at most, only a partial respite from the disease. The typical scenario is that the underlying cancer is not totally eradicated, remnants of the disease evolve and overcome any treatment, and the relapse is severe [4].
New targeted therapies are also expensive to develop and to prescribe, some costing over US$100,000 for each patient per year, while being applicable for a smaller number of patients with the relevant subtype of disease. Disease resistance may be overcome through new strategies that combine therapies for specific pathways, and combination therapy of two or more drugs that target independent pathways is likely to hold even greater promise for improving response [5]. Other approaches such as combined use of immune checkpoint inhibitors are also providing exciting results [6], although there remain concerns that the strategy of developing targeted therapies for late-stage disease may be fundamentally flawed, given the inherent complexity and heterogeneity of such tumors [7,8]. A complementary approach would be to focus also on early detection of localized cancer, including the use of screening, when survival is usually a lot more favorable [3], as well as primary prevention in identifying the causes and minimizing exposure. The role of genomics in primary and secondary prevention of cancer has received less attention than treatment, although it is perhaps here that genomics will have its most important contribution in the long term.
Primary Prevention of Cancer—Stopping the Disease Occurring
Some of the greatest public health successes in cancer prevention have arisen from identifying the causes of cancer and limiting or removing the exposure [9]. Obvious examples include identifying the role of smoking for lung cancer [10], and later for another 17 cancer types [11], implementation of Hepatitis-B vaccination programs against liver cancer [12], the role of Human Papilloma virus (HPV) in cervical cancer that led directly to the development of prophylactic vaccines [13], and the identification of specific occupations associated with very high cancer risk that has resulted in subsequent control of these exposures in many, but not all, parts of the world (e.g., workers exposed to asbestos and risk of mesothelioma). Overall, about 40% of cancer cases in high income countries appear to be attributable to known lifestyle factors, with tobacco explaining about half of this amount [14,15], indicating that much remains to be done in limiting the effects of this exposure. Such is the role of tobacco, that it can be useful to consider the proportion of cancers avoidable by known risk factors in smokers and nonsmokers separately. In an exercise for cancer deaths in the United States, about 70% of cancer deaths among smokers could be accounted for by known causes (60% due to tobacco and 10% due to other exposures), whereas only about 20% of cancer deaths among nonsmokers could be attributable to known causes [14].
There clearly remain important gaps in the litany of what causes cancers to occur, especially among the majority of the population who do not smoke. While some cancers are relatively rare in all populations, it is also the case that all cancers that are common in some populations are much rarer in others, usually by an order of magnitude or more [16]. It is clear from migrant studies and time trends that genetic susceptibility cannot explain these differences, implying underlying lifestyle and environmental factors. High quality cancer registries around the world are capable of accurately recording the numbers of new cases of each cancer type in a population, allowing for valid international comparisons [16]. The seven cancers listed in Table 1 make up over 25% of the global cancer burden, although only a small part of these international differences can currently be explained [16]. The incidence of some cancers has increased rapidly in recent years in different regions of the world, including testicular and renal cancers, as well as lymphomas. Despite multiple efforts, traditional epidemiology studies have not been able to explain these increases.
Tab. 1. Cancer registries with low and high incidence rates for selected cancers for which the etiology is not well understood. 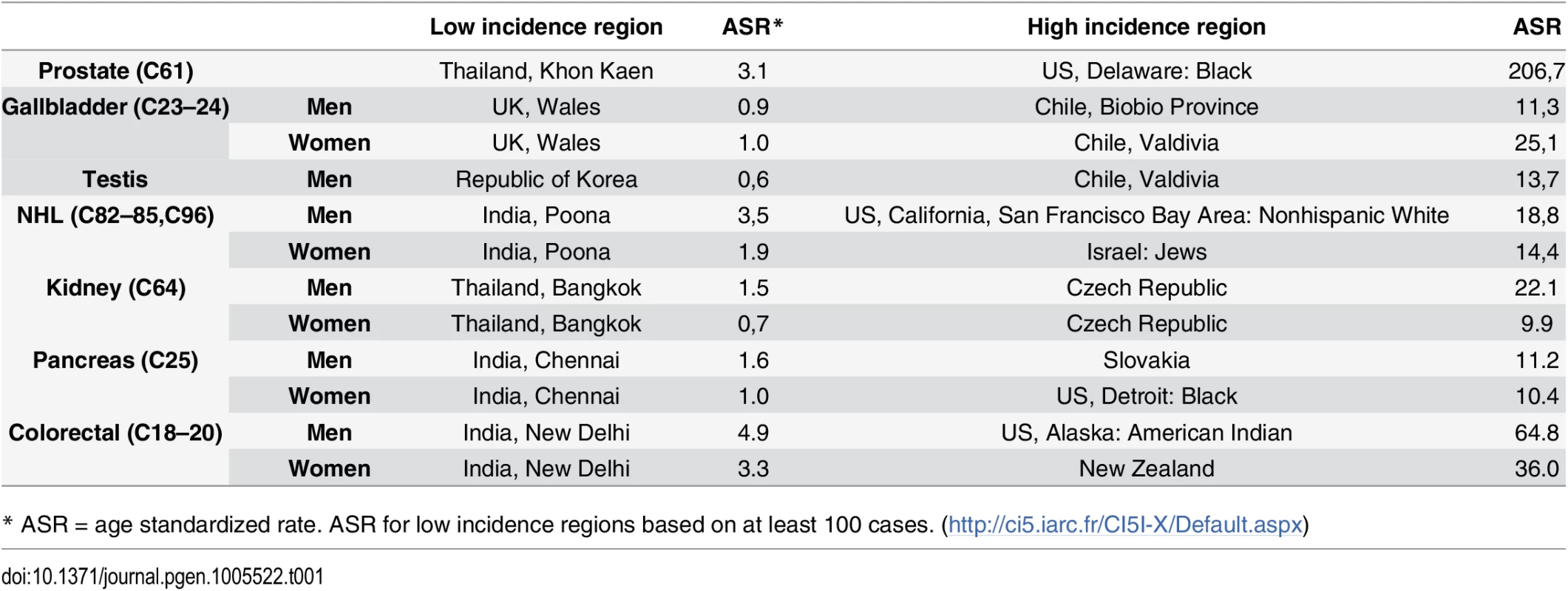
* ASR = age standardized rate. ASR for low incidence regions based on at least 100 cases. (http://ci5.iarc.fr/CI5I-X/Default.aspx) How Can Genomics Fill These Gaps?
DNA signatures of past exposures
Certain environmental exposures can leave a mutation “signature” in the tumor [17], providing evidence on the specific lifestyle and environmental exposures that caused the tumor to occur. For example, patterns of mutations in the TP53 gene, the most commonly mutated gene in cancer, differ strongly between smokers and nonsmokers who develop lung cancer, the former having a higher proportion of mutations that change a guanine base to a thymine (a G>T transversion). The changes are more likely to occur in a specific sequence context, with CpG dinucleotides being particularly enriched, and are more frequently found on the untranscribed DNA strand. The presence of such mutations can be explained by the direct mutagenic activity of specific compounds found in cigarette smoke, in particular polycyclic aromatic hydrocarbons (PAHs). The International Agency for Research on Cancer (IARC) P53 database has been documenting the pattern of p53 mutations within different cancers since 1989. Small cell lung cancers (SCLCs) occur almost exclusively among smokers, and among the 263 p53 mutations that have been recorded from 253 tumors, 32% are G>T transversions [18]. What is surprising is that the pattern of mutations across the whole genome of a single SCLC tumor is almost exactly the same as in the TP53 gene across 263 SCLC tumors. In 2009, a whole genome sequence of a SCLC identified 22,910 substitutions across the genome, with over one-third being G>T transversions [19] (Fig 1). While the vast majority of the mutations in any tumor have no functional impact, and are called “passenger mutations,” their pattern can be strongly indicative of the background exposures of the individual. Similar phenomenon can be observed for the pattern of mutations caused by sunlight and melanomas, or by aflatoxin B1 and liver cancer [20].
Fig. 1. Comparison of (i) the distribution of 22,910 mutations identified from sequencing on SCLC line [19], with (ii) 263 published mutations from 253 SCLCs. ![Comparison of (i) the distribution of 22,910 mutations identified from sequencing on SCLC line [<em class="ref">19</em>], with (ii) 263 published mutations from 253 SCLCs.](https://www.prolekare.cz/media/cache/resolve/media_object_image_small/media/image/e4ed73255e808e1ab12e44d910a4770d.png)
IARC p53 database [18], accessed March 2015. An example of how this type of investigation can expand the role of known carcinogens comes from the recently completed ICGC study of renal tumors [21]. Among 94 individuals with whole genome sequence data, recruited from four different countries, there was a sharp disparity among the pattern of A>T mutations among the 14 Romanian renal cancer cases when compared to the remaining 80 cases from the United Kingdom, Czech Republic, and Russia (Fig 2). A>T mutations are relatively rare in all tumor types, although many will occur as a result of exposure to aristolochic acid, a toxin that results from ingestion of the Aristolochia plant. Exposure is prevalent in parts of Asia, where it is common in traditional herbal remedies, and has been linked to rare cancers of the upper urinary tract that also exhibit a predominance of A>T mutations [20,22]. In the Balkan region of Southeastern Europe, the presence of Aristolochia clematitis (also known as European birthwort) is known to grow in wheat fields, contaminating the grain, and has been linked to Balkan endemic nephropathy, a renal disease that occurs in very specific regions along the Danube. The mutation profile in the 14 Romanian cases showed a predominance of A>T mutations on the untranscribed DNA strand and also occurred in a particular sequence context, two other facets of this signature that have been seen elsewhere. The results provide important clues that aristolochic acid exposure may be an important renal carcinogen in this part of Europe, going beyond the very specific region affected by Balkan endemic nephropathy.
Fig. 2. Mutation patterns from whole genome sequencing of 94 conventional renal carcinomas from four different countries showing a notable excess in the proportion of A>T mutations in cases from Romania. 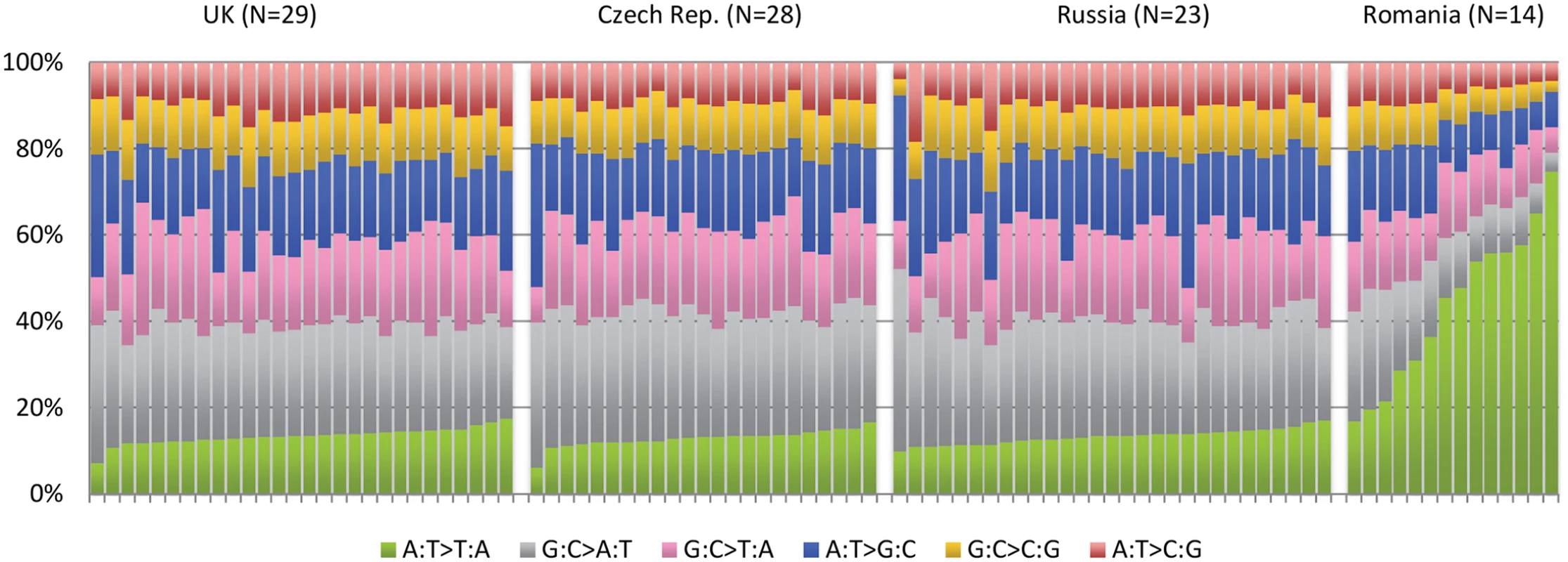
See Scelo et al. [21]. A catalogue of mutation signatures
In a comprehensive analysis of over 7,000 cancer cases with mutation data and close to 5 million mutations, Alexandrov and colleagues identified over 20 distinct mutation signatures, with most individual cancers showing evidence of more than one mutational signature [23]. Beyond those known to be caused by tobacco, UV light, and some specific alkylating agents, the cause of most of these signatures is not known or can only be hypothesized. While it cannot be expected that all of these mutation signatures will be linked to exogenous exposures, it is feasible that some will; and identifying the causes of specific mutation signatures and linking these to new cancer types will be an important step in expanding our knowledge on the causes of cancer. Evidence linking mutation signatures to specific exposures is likely to come about through two sources. One will be model systems whereby cell lines or other models are exposed to specific carcinogens and the resultant mutation profile is identified. For example, the human p53 knock-in (Hupki) mouse and derived immortalized mouse embryonic fibroblast models have been used to clarify mutation signatures for various exposures including UV light, benzo[a]pyrene, aristolochic acid, and aflatoxin B1 [24].
Another strategy to identify mutation signatures will be the comparison of sequence data from large numbers of cases included in studies such as ICGC and TCGA. An important characteristic of these studies up to now is that patients are recruited from single settings, with a strong focus on collecting high quality biological samples and accurate clinical data, but with only limited environmental or lifestyle data. An alternative approach that would maximize the possibility to find different mutation signatures would be to recruit an international series of cases that cover low and high incidence areas in a coordinated manner and also collect accurate information on lifestyle and environmental information. An additional strategy would be to select cases for sequencing based on the presence of a known or suspected carcinogen—the aim being to try to further define mutation signatures for an exposure. One could envisage a comparison of colorectal cancer tumors among individuals with high meat consumption compared to vegetarians, or a comparison of individuals with a history of heavy exposure to specific pesticides compared to no exposure for various cancers where this association has been hypothesized. While the large TCGA and ICGC sequencing initiatives were not established to identify lifestyle and environmental causes of cancer, there is an important opportunity to incorporate this aim in any future international initiative. This will require a commitment by those leading such studies to include key exposure data relevant to the cancer being studied.
Germline variation and Mendelian randomization
An important limitation of the branch of epidemiology research that seeks to identify causes is that it relies on observations, and lifestyle characteristics of individuals inevitably correlate, resulting in the potential for confounding. For example, heavy alcohol drinkers will have higher rates of lung cancer simply due to their increased propensity to smoke. Any causal role for alcohol is thought to be unlikely [25]. Less straightforward are epidemiological findings that highlight a strong association between a particular cancer and some nutrients or foods. For example, one of the reasons that a protective effect was hypothesized between lung cancer and beta carotene was because of the consistent results from observational studies showing a protective effect for specific food types rich in this compound [26]. Subsequent randomized trials to test this hypothesis proved negative and, if anything, found an association in the opposite direction [27]. While epidemiologists frequently try to untangle these disease–exposure relationships, it requires a complete knowledge of how exposures correlate and the ability to control for them or measure them accurately, something that is rarely the case. Unmeasured or poorly adjusted confounders are one of the primary reasons for why epidemiological studies are unable to investigate important exposure–disease relationships or even get the wrong answer [28].
An attractive option that can mitigate these shortcomings is Mendelian randomization [29]. This involves identifying a gene (or panel of genes) that is associated with the exposure and using this as an unconfounded “instrument” of the exposure instead of the exposure itself. It was Mendel who first recognized that genetic variation encapsulates information on physical attributes and that the information on different genes tends not to correlate (his Law of Independent Assortment). He also hypothesized that alleles are inherited in a random fashion from one generation to the next (his Law of Segregation). While these statements need certain clarifications, e.g., genes in close vicinity are likely to be inherited together, and Mendel did not use a terminology that included “genes” or “alleles”; in practical terms, this means that the selection of which individuals within a population who are more likely to have a genetic trait for smoking, drinking, or obesity is largely random, and these characteristics are inherited independent of other possible confounding factors. As an illustration, observational studies point to an association between alcohol consumption and increased blood pressure (arrow A), although there is much potential for confounding from lifestyle and socioeconomic risk factors that are also associated with both alcohol (arrow B) and hypertension, independently (arrow C) (Fig 3). The causality of the relationship (A) is unclear, as it may occur as a result of (B) and (C), and randomized studies are not feasible [30]. A Mendelian randomization analysis can utilize the ALDH2 gene as an unconfounded indicator of alcohol consumption (D). ALDH2 encodes for an enzyme that transforms acetaldehyde to acetic acid, and individuals who are homozygous for the null variant (2/2) drink considerably less than those who are homozygous for the active variant (1/1) with heterozygous (1/2) carriers in between. Further, ALDH2 genotype has been found to be not associated with other risk factors of blood pressure such as smoking, exercise, and obesity. ALDH2 is thus an indicator of drinking behavior that is inherited in a random fashion within a population and not associated with common confounders. Any effect of ALDH2 gene on blood pressure should be due simply to its effect on alcohol consumption patterns and be independent of potential confounders (i.e., [E], the dotted line in Fig 3). A combined analysis of multiple studies reported a clear association with the ALDH*1/1 genotype and both blood pressure levels and diagnosis of hypertension, providing persuasive evidence of a causal association [30].
Fig. 3. Causal pathway indicating how ALDH2 is an unconfounded marker (or instrument) of alcohol consumption in the association between alcohol and blood pressure. 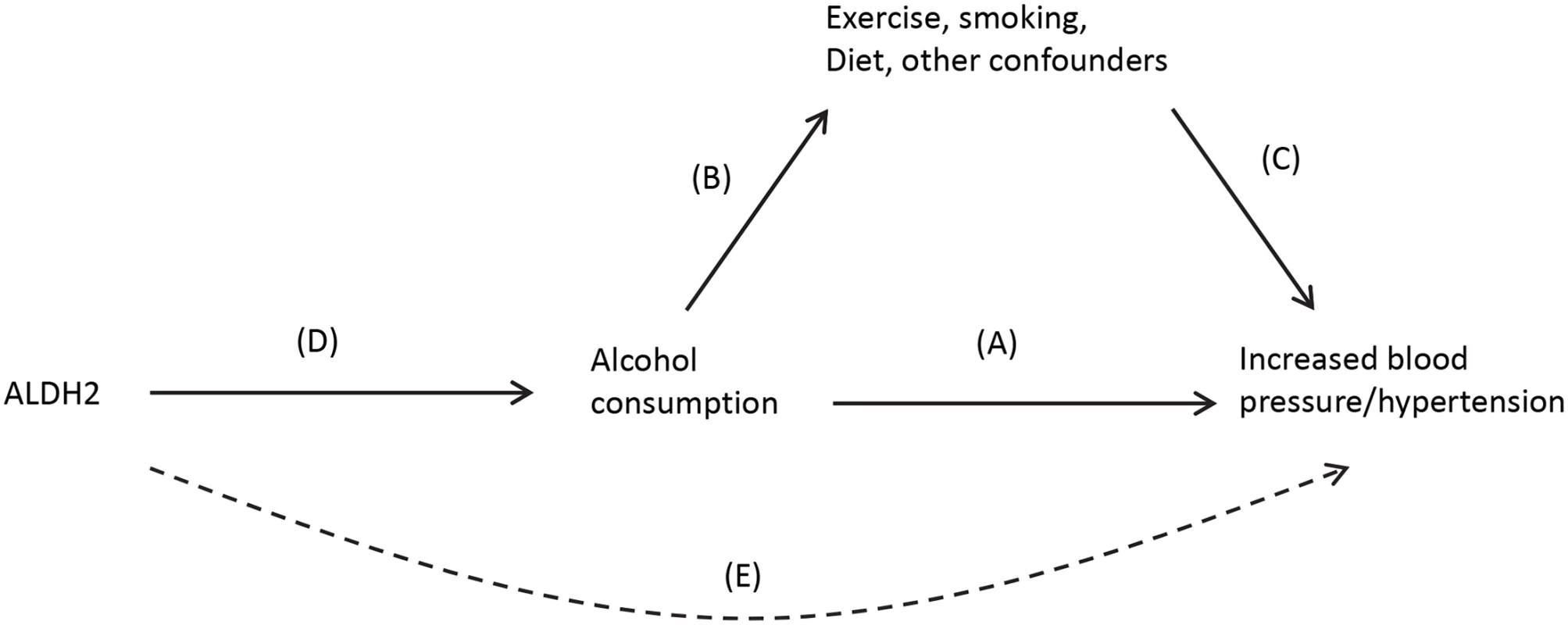
The potential for Mendelian randomization is apparent from recent publications on genes that influence obesity and adenocarcinoma of the esophagus [31], high fasting insulin levels and endometrial cancer risk [32], vitamin D genes and both all-cause and cancer-specific mortality [33], and also for cardiovascular disease and lipid levels [34–36]. The evidence linking obesity with esophageal adenocarcinoma is important as it is unlikely to be confounded by other potential risk factors including physical activity or specific dietary patterns. Similarly, the evidence linking endometrial cancer risk with insulin levels is helping to highlight the complex yet potentially causal relationship between risk factors for diabetes and cancer. Mendelian randomization studies have been compared to randomized control trials, and while Mendelian randomization studies have many attractions, they also have important limitations. In particular, one needs a genetic indicator (or instrument) for the exposure of interest. These have not yet been identified for many exposures including most lifestyle related factors, nor for many specific vitamins or nutrients. Given that extensive genome-wide analysis (GWA) data from very large population cohorts is becoming available, such as in UK Biobank, better instruments for Mendelian randomization studies are certain to be identified [37]. Additional issues include the pleiotropic nature of genes and that the genetic effect on the outcome trait is generally modest, meaning that very large studies are required to have a sufficient power to test the association between gene and outcome [38–41].
Secondary Prevention of Cancer—Catching It Early
There were about 8 million cancer deaths estimated to have occurred globally in 2012, compared to 14 million new cancer cases [42], providing a crude estimate that over half of cancer patients die from the disease worldwide. Even in countries classed as having a very high level of human development, the ratio of deaths to new cases is nearly one in two. Cancer survival in highly developed countries has shown some improvements over recent decades, although nothing like the improvements seen for cardiovascular disease [43]. While access to high quality coordinated treatment is a major driver in these improvements, detection of cancer at an early stage followed by access to high quality care are fundamental criteria in increasing the chances of surviving a cancer diagnosis. Cancers for which survival is particularly poor, for example lung, pancreatic, and liver, continue to be frequently identified at stage III or IV, even in highly developed countries. The overall cancer survival situation is far worse in less developed countries, where a majority of cancers is diagnosed at later stages, and access to effective cancer care is generally more limited.
Existing screening programs for cancer may be enhanced in the future by identification of individuals at increased genetic risk using combinations of rare high risk variants and multiple common yet low risk variants [44–46]. However, a major breakthrough for cancer prevention would be the identification of biomarkers for early cancer detection that are (i) easily measureable, (ii) sensitive for presymptomatic cancer (i.e., picking up a large proportion of cases), and (iii) specific (giving a negative response in the overwhelming majority of cases that do not have the disease). Some cancer biomarkers have been identified that are predictive of subsequent disease but lack the necessary accuracy for routine use in a population. One recent example of a highly specific, sensitive, and easily measurable biomarker linked to a preclinical cancer is antibodies to HPV16 E6 and cancer of the oropharynx [5]. This biomarker is detectable in the plasma of the majority of individuals who develop a HPV-associated oropharynx cancer up to 15 years before onset of clinical symptoms and is absent in over 99% of the comparable general population. The results using this biomarker are recent, and it is curious that it is not strongly predictive of other HPV-associated cancers such as cervix, although it does seem to predict a proportion of HPV positive anal cancers [47]. This biomarker is not currently ready as a screening tool primarily because even a specificity of 99% is too low for a very rare outcome such as oropharynx cancer, where the number of false positives would far outnumber true positives. The consequences of a positive HPV16 E6 result are also unclear, as precancerous lesions for oropharynx cancer have not been defined. While this may be seen as a proof of principle that sensitive and specific early detection cancer biomarkers can be developed, if a biomarker were to be identified for a common cancer with poor survival that had a similar sensitivity and specificity, the consequences for cancer prevention would be far reaching.
An emerging genomic technique that may prove key for the early detection of cancer is the analysis of circulating tumor DNA (ctDNA) in blood samples. The presence of cell-free DNA (cfDNA) in the blood has been recognized for many decades, with particularly high levels being observed in cancer patients [48]. A proportion of cfDNA in cancer patients is circulating tumor DNA (ctDNA) that is of much interest given its potential to act as a noninvasive biomarker for a malignancy. Indeed, ctDNA has been termed a “liquid biopsy” with potential applications including identifying response to treatment and relapse and even early-stage disease [49]. The proportion of ctDNA compared to the amount of cfDNA may be high (e.g., above 10%), especially for late-stage disease or for large tumors, while for early-stage disease this ratio is thought to be approximately 0.1%–1%. Extreme deep sequencing of 10,000 fold or more does appear capable of identifying such small concentrations, and a recent evaluation of 640 patients with various tumor types found that among early-stage cases that had not spread beyond the initial site, ctDNA could be detected in about 50% [49].
There are alternative techniques for detection of preclinical cancers that may prove to be more feasible than ctDNA in the long term and that are already providing exciting data. A good example is the analysis of panels of microRNAs (miRNAs) from plasma or serum samples. The number of human miRNAs is limited (around 1,000), and they have several attractive features as a biomarker, including their relative stability over time [50]. The most promising data is currently from within lung cancer screening studies, where several groups have independently reported panels of a modest number of miRNAs measured in blood samples taken prior to diagnosis that have good sensitivity and specificity for subsequent lung cancer risk [51,52]. Such analyses could be an important adjunct along with smoking history and other lung cancer risk markers when deciding who should undergo screening for lung cancer using low dose computed tomography. Early detection of cancer using ctDNA mutations or miRNA analysis represent just two possible strategies, with others including identification of circulating tumor cells, metabolomics or proteomics analysis, identification of aberrant immunology profiles, and also epigenetic analysis of circulating tumor DNA.
There are important challenges to identifying accurate biomarkers for cancer prior to clinical onset of symptoms. Difficulties include access to appropriate biological samples from population cohorts, lack of standard protocols for dealing with biological samples, sequencing analysis, and bioinformatics procedures. While impressive, these difficulties may not be insurmountable, and given the payback from the successful development of such techniques, it would seem important to consider how this line of research could be prioritized in a similar fashion to the coordinated international cancer sequencing programs that have proved so successful. This will involve establishing international partnerships of key laboratories, agreed goals for testing laboratory and bioinformatics protocols, and sharing of common sets of samples, and subsequently of data and results. The key ingredients to any successful program will be clear objectives, leadership, availability of large research infrastructures to test biomarkers, international willingness to collaborate, and financial backing.
Summary
It is known that the cancer burden will rapidly increase over the next 15 years, with an estimated annual number of new cases in excess of 20 million by 2030 [53]. Much of this increase will occur in parts of the world where the health systems are least capable of absorbing such an increase. New generations of cancer treatments often promise only an extended remission from disease and not cure and place an important financial burden on health services. There is no country in the world that will be able to treat its way out of this cancer problem [54].
Cancer epidemiology in the late 20th and early 21st centuries has made important contributions to reducing the numbers of cancers that would otherwise occur. By combining with the revolutionary new tools of genomics it can be expected to continue to produce similar findings that will lead to breakthroughs in identification of new causes of cancer and early detection. There is a danger, however, that, in the absence of a coordinated strategy on an international level, this progress will occur in fits and starts, leading to delays, waste of resources, and missed opportunities.
Zdroje
1. Wetterstrand K. DNA Sequencing Costs: Data from the NHGRI Genome Sequencing Program (GSP). www.genome.gov/sequencingcosts. Accessed 05-Mar-2015.
2. Hudson TJ, Anderson W, Artez A, Barker AD, Bell C, Bernabe RR, et al. International network of cancer genome projects. Nature. 2010;464(7291):993–8. doi: 10.1038/nature08987 20393554
3. Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA Jr., Kinzler KW. Cancer genome landscapes. Science (New York, NY). 2013;339(6127):1546–58.
4. Groenendijk FH, Bernards R. Drug resistance to targeted therapies: deja vu all over again. Molecular oncology. 2014;8(6):1067–83. doi: 10.1016/j.molonc.2014.05.004 24910388
5. Kreimer AR, Johansson M, Waterboer T, Kaaks R, Chang-Claude J, Drogen D, et al. Evaluation of human papillomavirus antibodies and risk of subsequent head and neck cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31(21):2708–15.
6. Riley JL. Combination checkpoint blockade—taking melanoma immunotherapy to the next level. N Engl J Med. 2013;369(2):187–9. doi: 10.1056/NEJMe1305484 23724866
7. Bozic I, Nowak MA. Cancer. Unwanted evolution. Science (New York, NY). 2013;342(6161):938–9.
8. Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481(7381):306–13. doi: 10.1038/nature10762 22258609
9. Colditz GA, Wolin KY, Gehlert S. Applying what we know to accelerate cancer prevention. Science translational medicine. 2012;4(127):127rv4. doi: 10.1126/scitranslmed.3003218 22461645
10. Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ. 2004;328(7455):1519. 15213107
11. IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol 100, A Review of Human Carcinogens. Lyon, France: International Agency for Research on Cancer; 2011. http://monographs.iarc.fr/ENG/Monographs/PDFs/index.php Accessed 05-Mar-2015.
12. Huang LM, Lu CY, Chen DS. Hepatitis B virus infection, its sequelae, and prevention by vaccination. Current opinion in immunology. 2011;23(2):237–43. doi: 10.1016/j.coi.2010.12.013 21257300
13. Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890–907. 17826171
14. Peto J. Cancer epidemiology in the last century and the next decade. Nature. 2001;411(6835):390–5. 11357148
15. Parkin DM, Boyd L, Walker LC. 16. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br J Cancer. 2011;105 Suppl 2:S77–81. doi: 10.1038/bjc.2011.489 22158327
16. Forman D BF, Brewster DH, Gombe Mbalawa C, Kohler B, Piñeros M, Steliarova-Foucher E, Swaminathan R and Ferlay J. eds (2013) Cancer Incidence in Five Continents, Vol. X (electronic version) Lyon, IARC. http://ci5.iarc.fr last accessed on 05-Mar-2015. (The printed version of this volume: IARC Scientific Publication No. 164, will be available in 2014)
17. Pfeifer GP. Environmental exposures and mutational patterns of cancer genomes. Genome medicine. 2010;2(8):54. doi: 10.1186/gm175 20707934
18. Petitjean A ME, Kato S, Ishioka C, Tavtigian SV, Hainaut P, Olivier M. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database. Hum Mutat. 2007;28(6):622–9 (Version R17, November 2013). 17311302
19. Pleasance ED, Stephens PJ, O'Meara S, McBride DJ, Meynert A, Jones D, et al. A small-cell lung cancer genome with complex signatures of tobacco exposure. Nature. 2010;463(7278):184–90. doi: 10.1038/nature08629 20016488
20. Pfeifer GP. How the environment shapes cancer genomes. Current opinion in oncology. 2015;27(1):71–7. doi: 10.1097/CCO.0000000000000152 25402978
21. Scelo G, Riazalhosseini Y, Greger L, Letourneau L, Gonzalez-Porta M, Wozniak MB, et al. Variation in genomic landscape of clear cell renal cell carcinoma across Europe. Nature communications. 2014;5 : 5135. doi: 10.1038/ncomms6135 25351205
22. Poon SL, Pang ST, McPherson JR, Yu W, Huang KK, Guan P, et al. Genome-wide mutational signatures of aristolochic acid and its application as a screening tool. Science translational medicine. 2013;5(197):197ra01.
23. Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–21. doi: 10.1038/nature12477 23945592
24. Olivier M, Weninger A, Ardin M, Huskova H, Castells X, Vallee MP, et al. Modelling mutational landscapes of human cancers in vitro. Sci Rep. 2014;4 : 4482. doi: 10.1038/srep04482 24670820
25. Korte JE, Brennan P, Henley SJ, Boffetta P. Dose-specific meta-analysis and sensitivity analysis of the relation between alcohol consumption and lung cancer risk. Am J Epidemiol. 2002;155(6):496–506. 11882523
26. Peto R, Doll R, Buckley JD, Sporn MB. Can dietary beta-carotene materially reduce human cancer rates? Nature. 1981;290(5803):201–8. 7010181
27. Goralczyk R. Beta-carotene and lung cancer in smokers: review of hypotheses and status of research. Nutr Cancer. 2009;61(6):767–74. doi: 10.1080/01635580903285155 20155614
28. Lawlor DA, Davey Smith G, Kundu D, Bruckdorfer KR, Ebrahim S. Those confounded vitamins: what can we learn from the differences between observational versus randomised trial evidence? Lancet. 2004;363(9422):1724–7. 15158637
29. Smith GD, Ebrahim S. 'Mendelian randomization': can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32(1):1–22. 12689998
30. Chen L, Smith GD, Harbord RM, Lewis SJ. Alcohol intake and blood pressure: a systematic review implementing a Mendelian randomization approach. PLoS Med. 2008;5(3):e52. doi: 10.1371/journal.pmed.0050052 18318597
31. Thrift AP, Shaheen NJ, Gammon MD, Bernstein L, Reid BJ, Onstad L, et al. Obesity and risk of esophageal adenocarcinoma and Barrett's esophagus: a mendelian randomization study. J Natl Cancer Inst. 2014;106(11).
32. Nead KT, Sharp SJ, Thompson DJ, Painter JN, Savage DB, Semple RK, et al. Evidence of a Causal Association Between Insulinemia and Endometrial Cancer: A Mendelian Randomization Analysis. J Natl Cancer Inst. 2015;107(9).
33. Trummer O, Pilz S, Hoffmann MM, Winkelmann BR, Boehm BO, Marz W, et al. Vitamin D and mortality: a Mendelian randomization study. Clinical chemistry. 2013;59(5):793–7. doi: 10.1373/clinchem.2012.193185 23319826
34. Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380(9841):572–80. doi: 10.1016/S0140-6736(12)60312-2 22607825
35. Ridker PM. LDL cholesterol: controversies and future therapeutic directions. Lancet. 2014;384(9943):607–17. doi: 10.1016/S0140-6736(14)61009-6 25131980
36. Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384(9943):626–35. doi: 10.1016/S0140-6736(14)61177-6 25131982
37. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age. PLoS Med. 2015;12(3):e1001779. doi: 10.1371/journal.pmed.1001779 25826379
38. Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23(R1):R89–98. doi: 10.1093/hmg/ddu328 25064373
39. Ebrahim S, Davey Smith G. Mendelian randomization: can genetic epidemiology help redress the failures of observational epidemiology? Hum Genet. 2008;123(1):15–33. 18038153
40. Burgess S, Thompson SG. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. 2011;40(3):755–64. doi: 10.1093/ije/dyr036 21414999
41. VanderWeele TJ, Tchetgen Tchetgen EJ, Cornelis M, Kraft P. Methodological challenges in mendelian randomization. Epidemiology. 2014;25(3):427–35. doi: 10.1097/EDE.0000000000000081 24681576
42. Ferlay J SI, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013 http://globocaniarcfr, accessed on 05-Mar-2015.
43. Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang XS, et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet. 2015;385(9972):977–1010. doi: 10.1016/S0140-6736(14)62038-9 25467588
44. Easton DF, Pharoah PD, Antoniou AC, Tischkowitz M, Tavtigian SV, Nathanson KL, et al. Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med. 2015;372(23):2243–57. doi: 10.1056/NEJMsr1501341 26014596
45. Jervis S, Song H, Lee A, Dicks E, Harrington P, Baynes C, et al. A risk prediction algorithm for ovarian cancer incorporating BRCA1, BRCA2, common alleles and other familial effects. J Med Genet. 2015;52(7):465–75. doi: 10.1136/jmedgenet-2015-103077 26025000
46. Szulkin R, Whitington T, Eklund M, Aly M, Eeles RA, Easton D, et al. Prediction of individual genetic risk to prostate cancer using a polygenic score. Prostate. 2015.
47. Kreimer AR, Brennan P, Lang Kuhs KA, Waterboer T, Clifford G, Franceschi S, et al. Human papillomavirus antibodies and future risk of anogenital cancer: a nested case-control study in the European prospective investigation into cancer and nutrition study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015;33(8):877–84.
48. Diehl F, Schmidt K, Choti MA, Romans K, Goodman S, Li M, et al. Circulating mutant DNA to assess tumor dynamics. Nature medicine. 2008;14(9):985–90. doi: 10.1038/nm.1789 18670422
49. Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, et al. Detection of circulating tumor DNA in early - and late-stage human malignancies. Science translational medicine. 2014;6(224):224ra24. doi: 10.1126/scitranslmed.3007094 24553385
50. Iorio MV, Croce CM. microRNA involvement in human cancer. Carcinogenesis. 2012;33(6):1126–33. doi: 10.1093/carcin/bgs140 22491715
51. Sozzi G, Boeri M, Rossi M, Verri C, Suatoni P, Bravi F, et al. Clinical utility of a plasma-based miRNA signature classifier within computed tomography lung cancer screening: a correlative MILD trial study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014;32(8):768–73.
52. Bianchi F, Nicassio F, Marzi M, Belloni E, Dall'olio V, Bernard L, et al. A serum circulating miRNA diagnostic test to identify asymptomatic high-risk individuals with early stage lung cancer. EMBO molecular medicine. 2011;3(8):495–503. doi: 10.1002/emmm.201100154 21744498
53. Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the Human Development Index (2008–2030): a population-based study. Lancet Oncol. 2012;13(8):790–801. doi: 10.1016/S1470-2045(12)70211-5 22658655
54. Wild CP, Bucher JR, de Jong BW, Dillner J, von Gertten C, Groopman JD, et al. Translational cancer research: balancing prevention and treatment to combat cancer globally. J Natl Cancer Inst. 2015;107(1):353. doi: 10.1093/jnci/dju353 25515230
Štítky
Genetika Reprodukční medicína
Článek A Hereditary Enteropathy Caused by Mutations in the Gene, Encoding a Prostaglandin TransporterČlánek Exocyst-Dependent Membrane Addition Is Required for Anaphase Cell Elongation and Cytokinesis inČlánek Spindle-F Is the Central Mediator of Ik2 Kinase-Dependent Dendrite Pruning in Sensory Neurons
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 11- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Délka menstruačního cyklu jako marker ženské plodnosti
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- Agricultural Genomics: Commercial Applications Bring Increased Basic Research Power
- Ernst Rüdin’s Unpublished 1922-1925 Study “Inheritance of Manic-Depressive Insanity”: Genetic Research Findings Subordinated to Eugenic Ideology
- Convergent Evolution During Local Adaptation to Patchy Landscapes
- The Locus Controls Age at Maturity in Wild and Domesticated Atlantic Salmon ( L.) Males
- A Hereditary Enteropathy Caused by Mutations in the Gene, Encoding a Prostaglandin Transporter
- Absence of Maternal Methylation in Biparental Hydatidiform Moles from Women with Maternal-Effect Mutations Reveals Widespread Placenta-Specific Imprinting
- Calibrating the Human Mutation Rate via Ancestral Recombination Density in Diploid Genomes
- Anaplastic Lymphoma Kinase Acts in the Mushroom Body to Negatively Regulate Sleep
- Connecting Replication and Repair: YoaA, a Helicase-Related Protein, Promotes Azidothymidine Tolerance through Association with Chi, an Accessory Clamp Loader Protein
- UFBP1, a Key Component of the Ufm1 Conjugation System, Is Essential for Ufmylation-Mediated Regulation of Erythroid Development
- Mosaic and Intronic Mutations in Explain the Majority of TSC Patients with No Mutation Identified by Conventional Testing
- Members of the Epistasis Group Contribute to Mitochondrial Homologous Recombination and Double-Strand Break Repair in
- QTL Mapping of Sex Determination Loci Supports an Ancient Pathway in Ants and Honey Bees
- Genetic Interactions Implicating Postreplicative Repair in Okazaki Fragment Processing
- Genomics of Cancer and a New Era for Cancer Prevention
- Adaptation to High Ethanol Reveals Complex Evolutionary Pathways
- Dynamics of Transcription Factor Binding Site Evolution
- Exocyst-Dependent Membrane Addition Is Required for Anaphase Cell Elongation and Cytokinesis in
- Enhancer Runaway and the Evolution of Diploid Gene Expression
- Cattle Sex-Specific Recombination and Genetic Control from a Large Pedigree Analysis
- Drosophila Mutants Model Cornelia de Lange Syndrome in Growth and Behavior
- Pleiotropic Effects of Immune Responses Explain Variation in the Prevalence of Fibroproliferative Diseases
- Leaderless Transcripts and Small Proteins Are Common Features of the Mycobacterial Translational Landscape
- Tissue-Specific Effects of Reduced β-catenin Expression on Mutation-Instigated Tumorigenesis in Mouse Colon and Ovarian Epithelium
- Genus-Wide Comparative Genomics of Delineates Its Phylogeny, Physiology, and Niche Adaptation on Human Skin
- Mapping of Craniofacial Traits in Outbred Mice Identifies Major Developmental Genes Involved in Shape Determination
- Conserved Genetic Interactions between Ciliopathy Complexes Cooperatively Support Ciliogenesis and Ciliary Signaling
- Metabolomic Quantitative Trait Loci (mQTL) Mapping Implicates the Ubiquitin Proteasome System in Cardiovascular Disease Pathogenesis
- DNA Repair Cofactors ATMIN and NBS1 Are Required to Suppress T Cell Activation
- Spindle-F Is the Central Mediator of Ik2 Kinase-Dependent Dendrite Pruning in Sensory Neurons
- Ernst Rüdin and the State of Science
- ABCs of Insect Resistance to Bt
- Epigenetic Control of O-Antigen Chain Length: A Tradeoff between Virulence and Bacteriophage Resistance
- Encodes Dual Oxidase, Which Acts with Heme Peroxidase Curly Su to Shape the Adult Wing
- The Fanconi Anemia Pathway Protects Genome Integrity from R-loops
- Controls Quantitative Variation in Maize Kernel Row Number
- Genome-Wide Association Study of Golden Retrievers Identifies Germ-Line Risk Factors Predisposing to Mast Cell Tumours
- Insect Resistance to Toxin Cry2Ab Is Conferred by Mutations in an ABC Transporter Subfamily A Protein
- A Cytosine Methytransferase Modulates the Cell Envelope Stress Response in the Cholera Pathogen
- Conserved piRNA Expression from a Distinct Set of piRNA Cluster Loci in Eutherian Mammals
- The Multi-allelic Genetic Architecture of a Variance-Heterogeneity Locus for Molybdenum Concentration in Leaves Acts as a Source of Unexplained Additive Genetic Variance
- The lncRNA Controls Cryptococcal Morphological Transition
- Sae2 Function at DNA Double-Strand Breaks Is Bypassed by Dampening Tel1 or Rad53 Activity
- A Tandem Duplicate of Anti-Müllerian Hormone with a Missense SNP on the Y Chromosome Is Essential for Male Sex Determination in Nile Tilapia,
- Ectodysplasin/NF-κB Promotes Mammary Cell Fate via Wnt/β-catenin Pathway
- The QTL within the Complex Involved in the Control of Tuberculosis Infection in Mice Is the Classical Class II Gene
- Identifying Loci Contributing to Natural Variation in Xenobiotic Resistance in
- Variation in Rural African Gut Microbiota Is Strongly Correlated with Colonization by and Subsistence
- A Flexible, Efficient Binomial Mixed Model for Identifying Differential DNA Methylation in Bisulfite Sequencing Data
- Competition between Heterochromatic Loci Allows the Abundance of the Silencing Protein, Sir4, to Regulate Assembly of Heterochromatin
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- UFBP1, a Key Component of the Ufm1 Conjugation System, Is Essential for Ufmylation-Mediated Regulation of Erythroid Development
- Metabolomic Quantitative Trait Loci (mQTL) Mapping Implicates the Ubiquitin Proteasome System in Cardiovascular Disease Pathogenesis
- Genus-Wide Comparative Genomics of Delineates Its Phylogeny, Physiology, and Niche Adaptation on Human Skin
- Encodes Dual Oxidase, Which Acts with Heme Peroxidase Curly Su to Shape the Adult Wing
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



