-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Regulation of Metamorphosis by Xenobiotic Response Regulators
Mammalian Nrf2-Keap1 and the homologous Drosophila CncC-dKeap1 protein complexes regulate both transcriptional responses to xenobiotic compounds as well as native cellular and developmental processes. The relationships between the functions of these proteins in xenobiotic responses and in development were unknown. We investigated the genes regulated by CncC and dKeap1 during development and the signal transduction pathways that modulate their functions. CncC and dKeap1 were enriched within the nuclei in many tissues, in contrast to the reported cytoplasmic localization of Keap1 and Nrf2 in cultured mammalian cells. CncC and dKeap1 occupied ecdysone-regulated early puffs on polytene chromosomes. Depletion of either CncC or dKeap1 in salivary glands selectively reduced early puff gene transcription. CncC and dKeap1 depletion in the prothoracic gland as well as cncCK6/K6 and dKeap1EY5/EY5 loss of function mutations in embryos reduced ecdysone-biosynthetic gene transcription. In contrast, dKeap1 depletion and the dKeap1EY5/EY5 loss of function mutation enhanced xenobiotic response gene transcription in larvae and embryos, respectively. Depletion of CncC or dKeap1 in the prothoracic gland delayed pupation by decreasing larval ecdysteroid levels. CncC depletion suppressed the premature pupation and developmental arrest caused by constitutive Ras signaling in the prothoracic gland; conversely, constitutive Ras signaling altered the loci occupied by CncC on polytene chromosomes and activated transcription of genes at these loci. The effects of CncC and dKeap1 on both ecdysone-biosynthetic and ecdysone-regulated gene transcription, and the roles of CncC in Ras signaling in the prothoracic gland, establish the functions of these proteins in the neuroendocrine axis that coordinates insect metamorphosis.
Published in the journal: . PLoS Genet 9(2): e32767. doi:10.1371/journal.pgen.1003263
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003263Summary
Mammalian Nrf2-Keap1 and the homologous Drosophila CncC-dKeap1 protein complexes regulate both transcriptional responses to xenobiotic compounds as well as native cellular and developmental processes. The relationships between the functions of these proteins in xenobiotic responses and in development were unknown. We investigated the genes regulated by CncC and dKeap1 during development and the signal transduction pathways that modulate their functions. CncC and dKeap1 were enriched within the nuclei in many tissues, in contrast to the reported cytoplasmic localization of Keap1 and Nrf2 in cultured mammalian cells. CncC and dKeap1 occupied ecdysone-regulated early puffs on polytene chromosomes. Depletion of either CncC or dKeap1 in salivary glands selectively reduced early puff gene transcription. CncC and dKeap1 depletion in the prothoracic gland as well as cncCK6/K6 and dKeap1EY5/EY5 loss of function mutations in embryos reduced ecdysone-biosynthetic gene transcription. In contrast, dKeap1 depletion and the dKeap1EY5/EY5 loss of function mutation enhanced xenobiotic response gene transcription in larvae and embryos, respectively. Depletion of CncC or dKeap1 in the prothoracic gland delayed pupation by decreasing larval ecdysteroid levels. CncC depletion suppressed the premature pupation and developmental arrest caused by constitutive Ras signaling in the prothoracic gland; conversely, constitutive Ras signaling altered the loci occupied by CncC on polytene chromosomes and activated transcription of genes at these loci. The effects of CncC and dKeap1 on both ecdysone-biosynthetic and ecdysone-regulated gene transcription, and the roles of CncC in Ras signaling in the prothoracic gland, establish the functions of these proteins in the neuroendocrine axis that coordinates insect metamorphosis.
Introduction
Cellular responses to many xenobiotic compounds, including various toxins and pharmacological agents, are controlled by mammalian Nrf2 and Keap1, and by the homologous Drosophila CncC and dKeap1 proteins [1], [2], [3]. The Nrf2-Keap1 complex has multiple effects on carcinogenesis. Nrf2-deficient mice have increased susceptibility to chemical carcinogens, potentially because of defective activation of cytoprotective genes in response to carcinogen exposure [4]. Mutations in Nrf2 and Keap1 that are predicted to disrupt their interactions are found in many human cancers, suggesting that Nrf2 interactions with Keap1 counteract cancer progression [1], [5]. Conversely, the deletion of Nrf2 suppresses pancreatic and lung tumorigenesis in a mouse model with constitutively active K-RasG12D expression [6]. The mechanisms whereby Nrf2 promotes tumorigenesis in conjunction with K-RasG12D are not known. Nrf2 and Keap1 are investigated as potential targets for therapeutic interventions in cancer, neurodegenerative diseases and developmental disorders [1], [7].
Nrf2 (NF-E2-Related Factor 2) is a bZIP family transcription factor that can bind to genes whose transcription is induced by xenobiotic compounds [1]. Keap1 (Kelch-like ECH-Associated Protein 1) is a Kelch family protein that can interact with the N-terminal region of Nrf2, and inhibits the activation of many genes activated by Nrf2 [8]. Studies in cultured mammalian cells indicate that Keap1 is predominantly localized to the cytoplasm [9], where it promotes Nrf2 degradation and inhibits its accumulation in the nucleus [8], [10], [11], [12].
Studies of the Drosophila homologues of Nrf2 and Keap1 have provided insights into the functions of these protein families in adult flies. The Drosophila cap‘n’collar locus encodes CncC, which contains a bZIP domain homologous to that of Nrf2 and N-terminal DLG and ETGE motifs homologous to those that mediate Nrf2 interaction with Keap1 [13] (Figure 1A). Drosophila dKeap1 contains Kelch repeats homologous to those that mediate Keap1 interaction with Nrf2 as well as a sequence motif that is required for mammalian Keap1 export from the nucleus [3], [10]. Overexpression of CncC and depletion of dKeap1 in adult flies activates the transcription of many genes that protect cells from xenobiotic compounds, whereas dKeap1 overexpression represses their transcription, indicating that the functions of these protein families in the xenobiotic response are conserved between mammals and Drosophila [2], [3].
Fig. 1. CncC and dKeap1 subcellular localization and polytene chromosome occupancy. 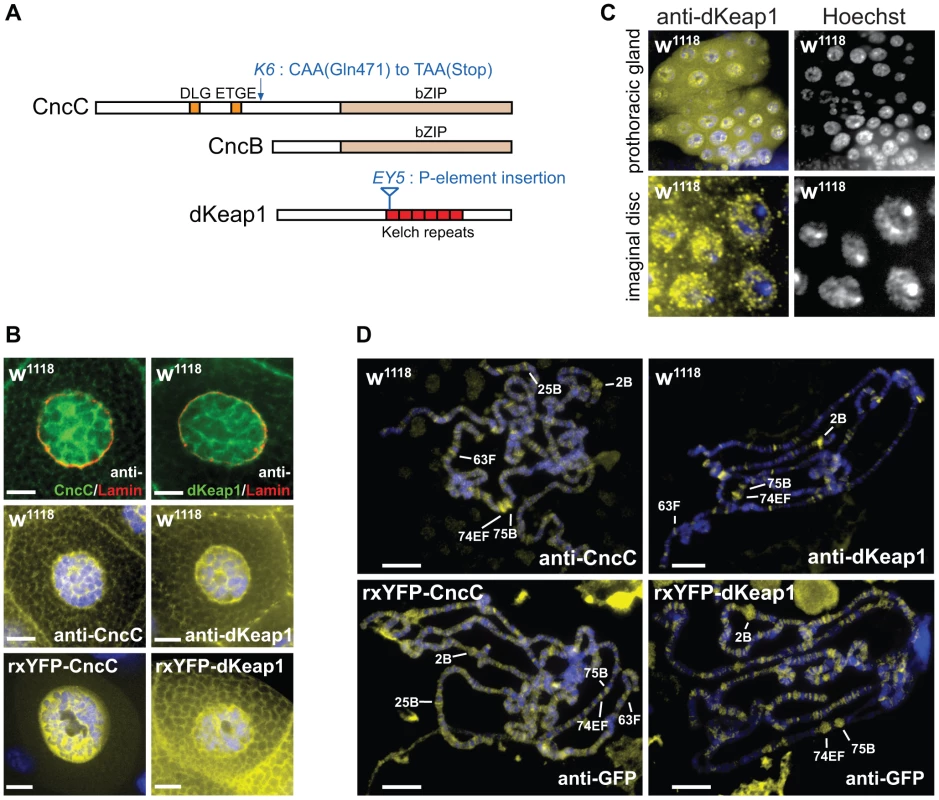
(A). Conserved sequences and mutations in CncC, CncB and dKeap1. Regions that are conserved between Drosophila and mammalian proteins are shown in color. The locations of the cncK6 and dKeap1EY5 mutations are indicated [3], [15]. (B). Visualization of the subcellular distributions of CncC and dKeap1 in salivary glands. [Upper panels] Endogenous CncC and dKeap1 distributions in wild type (w1118) salivary glands stained using anti-CncC or anti-dKeap1 (green) and anti-Lamin Dm0 (red) antibodies. [Middle panels] Wild type (w1118) salivary glands were stained with anti-CncC or anti-dKeap1 antibodies (yellow) and Hoechst (blue). [Lower panels] Ectopic CncC and dKeap1 distributions in live salivary glands that expressed rxYFP-CncC and rxYFP-dKeap1, respectively. The intrinsic fluorescence (yellow) was superimposed on Hoechst fluorescence (blue). The salivary glands were isolated from early wandering 3rd instar larvae. The scale bars are 10 µm. (C). Visualization of the localization of endogenous dKeap1 in the prothoracic gland and in imaginal disc cells. Endogenous dKeap1 was visualized by immunostaining using anti-dKeap1 antibodies (yellow) superimposed on Hoechst staining (blue). The separate images are shown to the right of each color image. (D). Visualization of the loci occupied by CncC and dKeap1 on polytene chromosomes. [Upper panels] The loci occupied by endogenous CncC and dKeap1 were visualized on polytene chromosome from wild type larvae (w1118) by staining with anti-CncC and anti-dKeap1 antibodies, respectively. [Lower panels] The loci occupied by ectopic CncC and dKeap1 were visualized on polytene chromosomes from larvae that expressed rxYFP-CncC and rxYFP-dKeap1, respectively, by staining with anti-GFP antibodies. The immunofluorescence (yellow) was superimposed on Hoechst fluorescence (blue). The major ecdysone-regulated early puffs are indicated. The polytene chromosomes were prepared from the salivary glands of early wandering 3rd instar larvae. Several lines of evidence suggest that CncC and dKeap1 also affect cell proliferation and development. CncC overexpression and dKeap1 depletion inhibit intestinal stem cell proliferation, and counteract the proliferative effects of environmental stress in these cells [14]. Loss of function mutations in cncC and dKeap1 cause larval lethality [3], [15]. The genes regulated by CncC and dKeap1 during larval development had not been established. Elucidation of the relationship between CncC and dKeap1 functions in xenobiotic responses and in development is important to define how the transcription regulatory functions of CncC and dKeap1 are regulated in response to intrinsic and extrinsic stimuli.
In Drosophila and in other holometabolous insects, the onset of metamorphosis is triggered by an increase in the level of the endocrine hormone ecdysone [16], [17]. Ecdysone is synthesized in the prothoracic gland (PG) by a series of cytochrome P450 enzymes [18]. The expression of these ecdysone-biosynthetic genes and the timing of pupation are regulated by Ras signaling in response to prothoracicotropic hormone (PTTH) binding to the Torso receptor [19], [20]. Ecdysone facilitates the onset of metamorphosis by regulating transcription in many tissues, including the salivary glands where ecdysone-regulated transcription is manifest by puffs at specific polytene chromosome loci [21]. The transcription factors that bind to the ecdysone biosynthetic gene promoters and activate their transcription have remained unknown.
In the work presented here, we found that CncC and dKeap1 occupied the classical ecdysone-regulated puffs on polytene chromosomes. Depletion of CncC or of dKeap1 in salivary glands reduced ecdysone-regulated gene transcription. Depletion of CncC or of dKeap1 in the PG as well as cncC and dKeap1 loss of function mutations reduced ecdysone biosynthetic gene transcription in larvae and in embryos, respectively. The reduced ecdysteroid levels caused by CncC and by dKeap1 depletion in the PG delayed pupation and suppressed the premature pupation caused by constitutive Ras signaling. These observations establish roles for CncC and dKeap1 in transcriptional programs in different tissues that coordinate metamorphosis.
Results
Nuclear localization of CncC and dKeap1
To investigate if the subcellular localization of CncC was regulated by dKeap1 in the manner that has been reported for mammalian Nrf2 and Keap1, we determined the distributions of CncC and dKeap1. Both CncC and dKeap1 immunoreactivity were predominantly nuclear in Drosophila salivary gland cells (Figure 1B, Figure S1A). Likewise, ectopic CncC and dKeap1 fused to fluorescent proteins were enriched within the nuclei of live salivary gland cells (Figure 1B, Figure S1A). CncC and dKeap1 were also present in the nuclei of prothoracic gland, imaginal disc and gut cells, though the proportions that were localized to the nucleus varied in different tissues (Figure 1C, Figure S1B). The intensity of anti-dKeap1 immunoreactivity was markedly reduced in dKeap1EY5/EY5 mutant larvae, and the bands corresponding to endogenous dKeap1 and CncC were not detected by immunoblotting of extracts from dKeap1EY5/EY5 and cncK6/K6 mutant larvae, demonstrating the specificity of these antibodies (Figure S1C, S1D). These observations establish that both endogenous as well as ectopically expressed CncC and dKeap1 were localized to the nuclei in many different tissues, in contrast to the predominantly cytoplasmic localization observed for Keap1 and Nrf2 in many cultured mammalian cell lines.
CncC and dKeap1 occupancy at ecdysone-regulated puffs on polytene chromatin
To establish if CncC and dKeap1 bound to specific chromatin loci, we visualized their occupancy on polytene chromosomes by immunostaining. Anti-CncC and anti-dKeap1 antibodies recognized overlapping sets of loci, including a majority of the classical ecdysone-regulated early puffs on polytene chromosomes (e.g. 2B, 74EF, 75B, 63F, and 25B) (Figure 1D). Anti-CncC antibodies also recognized several loci that were not detected by anti-dKeap1 antibodies (e.g. 22B and 97B) and vice versa (e.g. 50C and 94C). CncC and dKeap1 occupied many non-puff loci, and did not occupy all puffs, indicating that their occupancy was not controlled solely by chromatin decondensation. Ectopically expressed CncC and dKeap1 fusion proteins occupied loci that overlapped those occupied by endogenous CncC and dKeap1, though they also occupied additional loci (Figure 1D). Few other sequence-specific DNA binding proteins have been identified that bind to ecdysone-regulated puffs [22], [23], [24]. The overlapping sets of loci occupied by endogenous and ectopic CncC and dKeap1, as detected by several different antibodies, corroborate the specificity of CncC and dKeap1 binding at these loci.
Regulation of ecdysone response genes by CncC and dKeap1 in salivary glands
To test if CncC and dKeap1 regulated transcription of the early puff genes that they occupied on polytene chromosomes, we investigated the effects of CncC as well as dKeap1 depletion in salivary glands on transcription of ecdysone-regulated genes. Expression of an shRNA that targets CncC [3] under the control of either the 71B-GAL4 or the Sgs3-GAL4 driver reduced the levels of almost all of the ecdysone-regulated early puff and glue gene transcripts examined (Figure 2A). In contrast, transcription of most of the late puff genes that were not prominently occupied by CncC or dKeap1 was not affected by CncC depletion (Figure 2A). 71B-GAL4 directs expression throughout salivary gland development and in imaginal discs [25]; Transcription directed by Sgs3-GAL4 is detected only in late 3rd instar salivary glands [22], establishing that the change in transcription of ecdysone-regulated genes was due to CncC depletion in salivary glands. Expression of a different shRNA that targets all Cnc isoforms also reduced the levels of all of the early puff and glue gene transcripts examined (Figure 2A). The cncC-RNAi transgene had no detectable effects on transcription in larvae that lacked a GAL4 driver (Figure S2A).
Fig. 2. Regulation of early puff gene transcription by CncC and dKeap1. 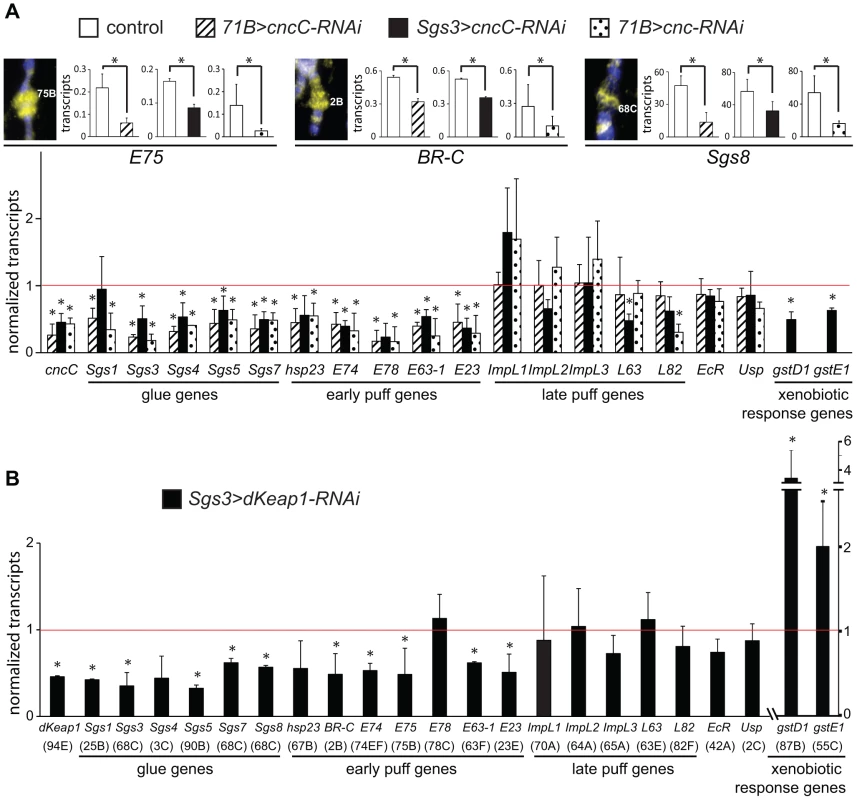
(A). Effects of CncC depletion in the salivary glands on the transcription of ecdysone-regulated genes and xenobiotic response genes. The images show wild type polytene chromosomes stained using anti-CncC antibodies. The levels of the transcripts indicated below the bar graphs were measured in the salivary glands of early wandering 3rd instar larvae. Transcript levels were compared in control larvae [71B-GAL4 (71B>), Sgs3-GAL4 (Sgs3>); open bars] and in larvae that expressed the shRNA targeting CncC under the control of the 71B-GAL4 (71B>cncC-RNAi; solid bars) or the Sgs3-GAL4 (Sgs3>cncC-RNAi; striped bars) driver, as well as in larvae that expressed a different shRNA targeting all Cnc isoforms under the control of the 71B-GAL4 driver (71B>cnc-RNAi; dotted bars). Larvae with different drivers and shRNAs were analyzed in separate experiments under different conditions (see methods). To facilitate comparison, the transcript levels in the lower graph were normalized by the levels of the transcripts in the control larvae. (B). Effects of dKeap1 depletion in the salivary glands on the transcription of ecdysone-regulated genes and xenobiotic response genes. The levels of the transcripts indicated below the bar graphs were measured in the salivary glands of early wandering 3rd instar larvae that expressed the shRNA targeting dKeap1 under the control of the Sgs3-GAL4 driver as described for the lower graph in panel A. The data in panels A and B represent the means and standard deviations from three or four separate experiments each (*, p<0.05). The corresponding loci are indicated in parentheses below the lower graph. Expression of an shRNA that targets dKeap1 [3] under the control of the Sgs3-GAL4 driver also reduced the levels of almost all of the ecdysone-regulated early puff and glue gene transcripts examined, but had no effect on most of the late puff gene transcripts (Figure 2B). In contrast to the concordant effects of CncC and dKeap1 depletion on ecdysone-regulated early puff gene transcription, CncC versus dKeap1 depletion had opposite effects on transcription of the gstD1 and gstE1 xenobiotic response genes (Figure 2A, 2B) [3], [26].
To examine if CncC and dKeap1 depletion affected early puff gene transcription through indirect mechanisms, we measured the levels of ecdysone receptor subunit transcripts and ecdysteroids. CncC and dKeap1 depletion in the salivary glands had no effect on the levels of the ecdysone receptor (EcR) or ultraspiracle (usp) transcripts in the salivary glands (Figure 2A, 2B). CncC depletion in the salivary glands also had no effect on the level of 20-hydroxyecdysone (20E) in the larvae (Figure S2B). There was no detectable effect on the size or the morphology of the salivary glands, or on the time of pupation. CncC and dKeap1 therefore likely regulated transcription of the ecdysone-regulated genes directly by binding to these loci.
Regulation of ecdysone biosynthetic genes by CncC and dKeap1
The effects of CncC and dKeap1 on ecdysone-regulated gene transcription in salivary glands, the arrested development of cncK6/K6 and dKeap1EY5/EY5 mutant larvae, and the presence of both CncC and dKeap1 in prothoracic gland nuclei prompted us to investigate their roles in ecdysone biosynthetic gene transcription. We investigated the effects of CncC and dKeap1 depletion in the prothoracic gland (PG) on ecdysone biosynthetic gene transcription. We measured the levels of the neverland (nvd), spookie (spok), phantom (phm), disembodied (dib), shadow (sad), and shade (shd) transcripts in the brain complexes of larvae that expressed the shRNA targeting CncC or dKeap1 in the PG. Expression of the shRNA targeting CncC under the control of either the 5015-GAL4 or the phm-GAL4 driver reduced the levels of all ecdysone biosynthetic gene transcripts that are expressed exclusively in the PG (Figure 3A, Figure S3A). 5015-GAL4 directs expression in the PG, the salivary glands and the lymph gland [27]; phm-GAL4 directs expression in the PG and at low levels in the wing and leg discs of 3rd instar larvae [28]. Expression of the shRNA targeting CncC also reduced Sad immunoreactivity in the PG (Figure 3C, Figure S3B). Expression of the shRNA targeting dKeap1 under the control of the phm-GAL4 driver reduced the levels of nvd, spok, phm, but not the levels of dib and sad in the brain complex (Figure 3A). CncC depletion in the PG therefore reduced transcription of all known ecdysone biosynthetic genes that are selectively expressed in the PG, and dKeap1 depletion reduced transcription of a subset of these genes.
Fig. 3. Regulation of ecdysone biosynthetic gene transcription by CncC and dKeap1. 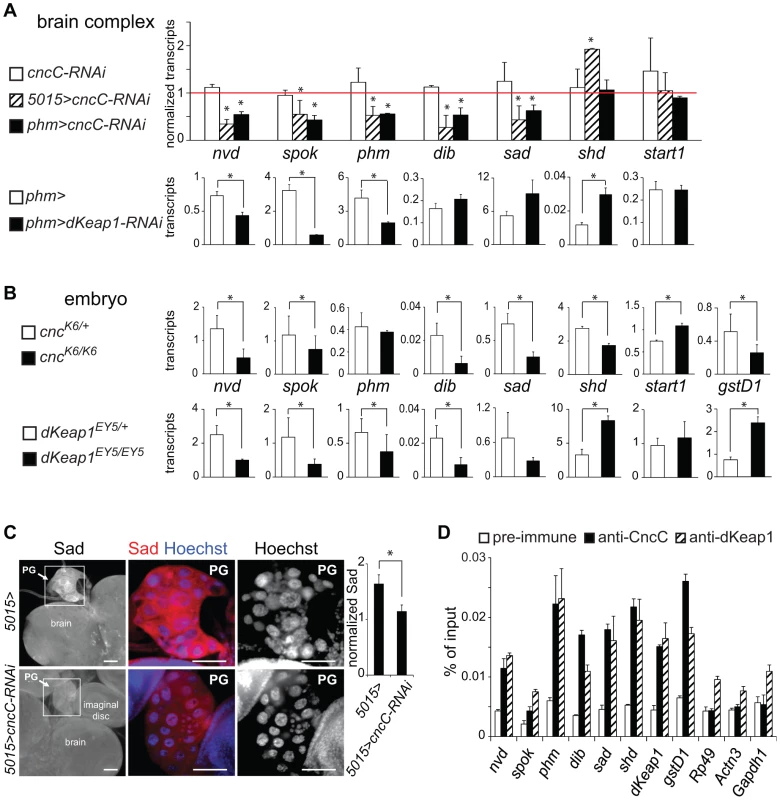
(A). Effects of CncC and dKeap1 depletion in the prothoracic gland on ecdysone biosynthetic gene transcription. [Upper graph] The transcripts indicated below the bars were measured in the brain complexes from early wandering 3rd instar larvae that expressed the shRNA targeting CncC under the control of the 5015-GAL4 (5015>cncC-RNAi, striped bar) or the phm-GAL4 (phm>cncC-RNAi, solid bar) driver, and from larvae that lacked a GAL4 driver (cncC-RNAi, open bar). To facilitate comparison, the transcript levels were normalized by the levels of the transcripts in control larvae (5015>, phm> and w1118). The data represent the means and standard deviations of the ratios of transcript levels between larvae that expressed the cncC-RNAi shRNA constructs and the corresponding control larvae from two independent experiments (*, p<0.05). [Lower graphs] The transcripts indicated above the graphs were measured in the brain complexes from early wandering 3rd instar larvae that expressed the shRNA targeting dKeap1 under the control of the phm-GAL4 (phm>cncC-RNAi, solid bar) driver, and from control larvae (phm>, open bar). The transcript levels were normalized by the levels of Rp49 transcripts divided by 100 and represent the means and standard deviations from two separate experiments (*, p<0.05). (B). Effects of CncC and dKeap1 loss of function mutations on ecdysone biosynthetic gene and xenobiotic response gene transcription in late embryos. The transcripts indicated between the graphs were measured in heterozygous (cncK6/+ or dKeap1EY5/+, open bar) and homozygous (cncK6/K6 or dKeap1EY5/EY5, solid bar) stage 14–16 embryos. The transcript levels were normalized by the levels of Rp49 transcripts divided by 1000 and represent the means and standard deviations from two separate experiments (*, p<0.05). (C). Effects of CncC depletion on PG morphology and Sad protein expression. The brain complexes of control larvae (5015>) and larvae that expressed the shRNA targeting CncC in the PG (5015>cncC-RNAi) were stained using anti-Sad (red) and Hoechst (blue). The ratio of Sad immunostaining in the PG relative to the brain is plotted in the graph to the right of the images (*, p<0.05). (D). Analysis of CncC and dKeap1 occupancy at the promoter regions of ecdysone biosynthetic genes in late embryos. Chromatin isolated from stage 14–16 embryos was precipitated using anti-CncC (solid bars), anti-dKeap1 (striped bars), and pre-immune (open bars) sera. The promoter regions of the genes indicated below the bars were quantified using qPCR. gstD1 was used as a positive control and Rp49, Actn3 and Gapdh1 were used as negative controls. The data represent the mean values and standard deviations of replicate qPCR reactions, and are representative of two experiments using independently prepared embryos. To determine the specificity of the reduction in ecdysone biosynthetic gene transcription upon CncC or dKeap1 depletion in the PG, we examined transcription of shd, which is expressed throughout the brain, and start1, which is expressed predominantly in the PG [29]. The levels of shd and start1 transcripts in the brain complex were not reduced by CncC or dKeap1 depletion in the PG (Figure 3A, Figure S3A). Expression of the shRNAs targeting CncC or dKeap1 also did not alter the size, morphology or the number of nuclei in the PG (Figure 3C, Figure S3B and S3C). It is therefore unlikely that the effects of CncC or dKeap1 depletion on ecdysone biosynthetic gene transcription were caused by a disruption of PG development.
To examine if CncC or dKeap1 affected ecdysone biosynthetic gene transcription at a different stage of development, we examined the effects of the cncK6 and dKeap1EY5 loss of function mutations on transcription of these genes in late embryos. The levels of nvd, spok, dib, sad, and shd transcripts were lower in cncK6/K6 homozygous than in cncK6/+ heterozygous embryos (Figure 3B). Likewise, the levels of nvd, spok, phm, dib, and sad transcripts were lower in dKeap1EY5/EY5 homozygous than in dKeap1EY5/+ heterozygous embryos, whereas the level of shd transcripts was higher in the homozygous than in heterozygous embryos (Figure 3B). The moderate effects of the cncK6 and dKeap1EY5 loss of function mutations on ecdysone biosynthetic gene transcription and the consequent lack of complete developmental arrest during embryogenesis could be due to maternal deposition of CncC and dKeap1 mRNA or proteins in the egg. The effects of these mutations on the levels of ecdysone biosynthetic gene transcripts in embryos corroborate the effects of CncC and dKeap1 depletion on transcription of these genes in the PG. In contrast to the concordant effects of the cncK6 and dKeap1EY5 loss of function mutations on ecdysone biosynthetic gene transcription, these mutations had opposite effects on transcription of the gstD1 xenobiotic response gene (Figure 3B). We were not able to determine the effects of CncC or dKeap1 depletion on the level of gstD1 in the PG since gstD1 is expressed throughout the brain.
The cncK6 and dKeap1EY5 mutations could affect transcription of the ecdysone biosynthetic genes through several mechanisms, including direct binding to the promoters and indirect effects on other transcription factors. To test if CncC and dKeap1 bound to the ecdysone biosynthetic genes, we measured CncC and dKeap1 occupancy at their promoter regions in late embryos using ChIP analysis. CncC and dKeap1 occupancy were observed at the phm, shd, dib and sad genes at levels that were comparable to their occupancy at the dKeap1 and gstD1 genes (Figure 3D). Their occupancy was higher near the sad promoter compared to flanking regions (Figure S3D). No CncC occupancy above background and only low dKeap1 occupancy was observed at the Rp49, Actn3, and Gapdh1 housekeeping genes. CncC and dKeap1 are therefore likely to regulate ecdysone biosynthetic gene expression directly by binding to their promoter regions.
Effects of CncC and dKeap1 on ecdysteroid production and pupation
Defects in ecdysteroid biosynthesis in the PG can delay pupation and increase the size of the pupae [19]. We investigated if the reduction in ecdysone biosynthetic gene transcription caused by CncC or dKeap1 depletion affected the timing of pupation by altering larval ecdysteroid levels. Expression of the shRNA targeting CncC under the control of the phm-GAL4 or the 5015-GAL4 driver extended the average time between third instar molting and pupation by 40–125% (Figure 4A). Expression of a different shRNA targeting all Cnc isoforms under the control of the phm-GAL4 driver also delayed the time of pupation (Figure 4A). The cncC-RNAi transgene alone had no detectable effect. The mean size of the pupae formed by larvae that expressed the shRNA targeting CncC in the PG was larger than the mean size of the pupae formed by control larvae (Figure 4B), indicating that the delayed pupation was not a secondary consequence of a reduced rate of larval growth. Some larvae continued to grow and formed giant semi-pupae (Figure S4A).
Fig. 4. Effects of CncC and dKeap1 depletion on the time of pupation, pupal size, and ecdysteroid levels. 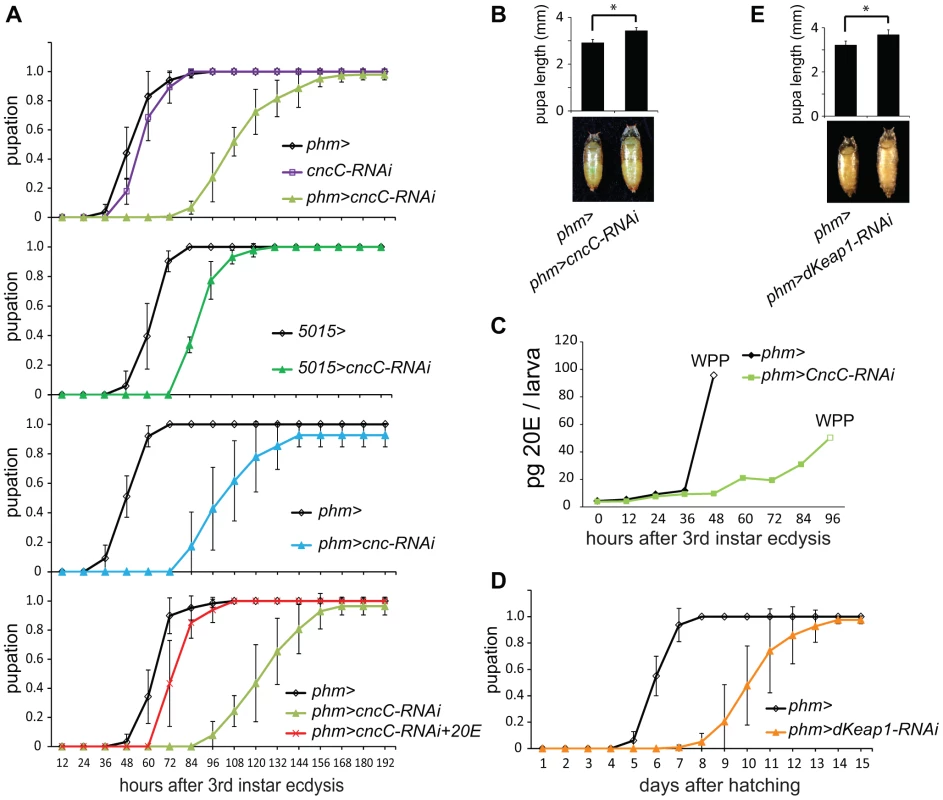
(A). Effects of expression of the shRNA targeting CncC in the PG on the time of pupation. The proportion of larvae that had formed pupae was plotted as a function of the time after 3rd instar ecdysis for control larvae (phm> or 5015>, black; cncC-RNAi, purple), for larvae that expressed the shRNA targeting CncC (phm>cncC-RNAi, green; 5015>cncC-RNAi, dark green), as well as for larvae that expressed a different shRNA targeting all Cnc isoforms (phm>cnc-RNAi, cyan) in the PG. Pupation by the larvae the expressed the shRNA targeting CncC was also examined when the food was supplemented with 0.5 mg/ml 20E (phm>cncC-RNAi+20E, red). The data in the different graphs were obtained from separate experiments and represent the means and standard deviations from two to four repeats using 20–30 larvae in each. (B). Effects of expression of the shRNA targeting CncC in PG on the sizes of pupae. The lengths of the pupae formed by control larvae (phm>) and larvae that expressed the shRNA targeting CncC in the PG (phm>cncC-RNAi) were measured. The data represent the mean and standard deviation of 30 pupae of each genotype (*, p<0.001). (C). Effects of expression of the shRNA targeting CncC in the PG on 20-hydroxyecdysone (20E) levels. The levels of 20E in larvae and white pre-pupae (WPP, open symbols) were measured in control (phm>, black) and transgenic Drosophila that expressed the shRNA targeting CncC in the PG (phm>cncC-RNAi, green). The 20E level at each time point was measured in 10 larvae or pre-pupae. (D). Effects of expression of the shRNA targeting dKeap1 in the PG on the time of pupation. The proportion of larvae that had formed pupae was plotted as a function of the time after hatching for control larvae (phm>, black) and for larvae that expressed the shRNA targeting dKeap1 (phm>dKeap1-RNAi, orange) in the PG. The data represent the means and standard deviations from four separate experiments using 20–30 larvae in each. The time of pupation was measured after hatching since it was more difficult to obtain a sufficient number of larvae synchronized at 3rd instar ecdysis. Based on observation of a small number of larvae (>10), the time between 3rd instar ecdysis and pupation was delayed by about 3 days upon dKeap1 depletion in the PG. The delay in pupation was therefore primarily due to extension of the 3rd instar larval stage. (E). Effects of expression of the shRNA targeting dKeap1 in the PG on the sizes of pupae. The lengths of the pupae formed by control larvae (phm>) and larvae that expressed the shRNA targeting dKeap1 in the PG (phm>dKeap1-RNAi) were measured. The data represent the mean and standard deviation of 20 pupae of each genotype (*, p<0.001). To evaluate the role of ecdysteroid levels in the delayed pupation, we measured the level of 20E in the larvae. Expression of the shRNA targeting CncC in the PG delayed the rise in 20E after third instar molting (Figure 4C, Figure S4B). To establish if the reduced level of 20E was the cause of the delay in pupation, we added 20E to the food for the larvae that expressed the shRNA targeting CncC in the PG. Supplementation with 20E shortened the time between third instar molting and pupation in these larvae by almost 50%, restoring their time of pupation nearly to that of wild-type larvae (Figure 4A).
Expression of the shRNA targeting dKeap1 under the control of the phm-GAL4 driver extended the average length of the larval stage by 4 days (Figure 4D). The mean size of the pupae formed by larvae that expressed the shRNA targeting dKeap1 in the PG was larger than the mean size of the pupae formed by control larvae (Figure 4E). Taken together, these results establish that CncC and dKeap1 affected the time of pupation through their effects on ecdysone biosynthetic gene transcription and on the level of 20E.
Functions of CncC in response to Ras signaling
We examined the functions of CncC in relation to the Ras signaling pathway, which controls the timing of pupation in response to prothoracicotropic hormone (PTTH) binding to the Torso receptor [19]. Constitutively active RasV12 expression in the PG causes early pupation and a smaller pupal size [20] (Figure 5A). Moreover, deletion of Nrf2 in mice suppresses the lung and pancreatic tumorigenesis caused by constitutively active K-RasG12D expression [6]. We determined the effect of CncC depletion in combination with RasV12 expression in the PG on the time of pupation and on pupal size. When the shRNA targeting CncC was co-expressed with RasV12 in the PG, the premature pupation was suppressed and the pupae were restored to nearly normal size (Figure 5A, 5B). CncC depletion in the PG not only suppressed premature pupation caused by RasV12 expression, but delayed pupation relative to wild type larvae, suggesting that CncC was required for both ectopic and endogenous Ras signaling.
Fig. 5. Interrelationships between Ras signaling and CncC functions. 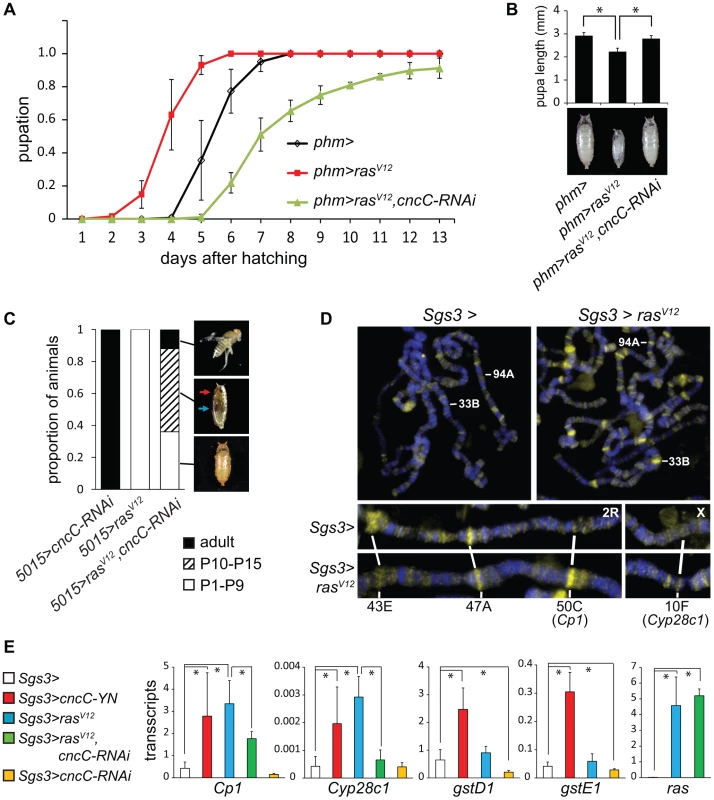
(A). Effects of CncC depletion on the time of pupation by larvae that expressed RasV12 in the PG. The proportion of larvae that had formed pupae was plotted as a function of the time after hatching for control larvae (phm>, black), larvae that expressed either RasV12 alone (phm>rasV12, red) or RasV12 together with the shRNA targeting CncC (phm>rasV12, cncC-RNAi, green) in the PG. The data represent the means and standard deviations from three repeats using 20–30 larvae in each. (B). Effects of CncC depletion on the sizes of pupae formed by larvae that expressed RasV12 in the PG. The lengths of pupae formed by the larvae described in part A were measured. The data represent the means and standard deviations of 30 pupae of each genotype (*, p<0.001). (C). Effects of CncC depletion and RasV12 expression in the PG on pupal development. The terminal stage of development was recorded for pupae formed by larvae that expressed either the shRNA targeting CncC alone (5015>cncC-RNAi), RasV12 alone (5015>rasV12), or RasV12 in combination with the shRNA targeting CncC (5015>rasV12, cncC-RNAi) in the PG. The proportion of pupae that arrested at early (P1–P9, open bar) and late (P10–P15, striped bar) pupal stages and that eclosed (adult, solid bar) are indicated (images shown on the right). Early and late stage pupae were distinguished by the absence or presence of red eye pigment (red arrow) and dark wings (blue arrow). Approximately 100 animals of each genotype were scored. (D). Effects of RasV12 expression in salivary glands on CncC occupancy on polytene chromosomes. Polytene chromosomes from control larvae (Sgs3>) and larvae that expressed RasV12 (Sgs3>rasV12) were stained using anti-CncC antibody. Selected loci whose occupancy by CncC changed upon RasV12 expression are labeled. (E). Effects of RasV12 and CncC fusion or CncC-RNAi expression in salivary glands on transcription of genes whose occupancy was affected by RasV12 expression and of control xenobiotic response genes. The levels of the transcripts indicated above the graphs were measured in salivary glands that expressed the proteins or the shRNA indicated. All transcript levels were normalized by the level of Rp49 transcripts. The data represent the means and standard deviations from two separate experiments (*, p<0.05). We further examined if CncC depletion affected the consequences of constitutive Ras signaling for pupal development. Most of the animals that expressed RasV12 alone arrested at early pupal stages with no detectable eye pigmentation or wings (Figure 5C, Figure S5A). In contrast, co-expression of the shRNA targeting CncC with RasV12 enabled a majority of the pupae to develop to late stages, and some to eclose and produce adult flies (Figure 5C, Figure S5A). It is unlikely that CncC depletion affected RasV12 expression in the PG since CncC depletion did not alter the level of rasV12 transcripts in salivary glands (Figure 5E). The genetic interactions between CncC depletion and RasV12 expression suggest that CncC mediated the regulation of pupation by the Ras signaling pathway.
To determine if Ras signaling affected CncC binding to chromatin, we investigated if RasV12 expression in salivary glands affected endogenous CncC occupancy on polytene chromosomes. RasV12 expression increased both the number of loci occupied by CncC and the level of CncC occupancy at most loci, but did not affect the level of cncC transcripts in salivary glands (Figure 5D, Figure S5B). RasV12 expression reduced CncC binding at some loci (Figure 5D). Ras signaling therefore regulated both the efficiency and the specificity of CncC binding to chromatin.
To establish if Ras signaling and CncC affected gene transcription in concert, we examined the effects of ectopic RasV12 and CncC expression on transcription of genes at two of the loci where RasV12 expression affected CncC occupancy in salivary glands (Figure 5D, lower panels). Both RasV12 as well as CncC fusion protein expression activated transcription of these genes (Figure 5E). Conversely, CncC depletion by shRNA expression counteracted the activation of these genes by RasV12 expression. RasV12 expression had selective effects on the transcription of genes at these loci since the transcription of other CncC target genes, including gstD1 and gstE1, was not detectably affected by RasV12 expression (Figure 5E). These results suggest that Ras signaling regulated CncC transcriptional activity by altering its occupancy at selected target genes.
Discussion
Visualization of the subcellular distributions of CncC and dKeap1 and their occupancy on polytene chromosomes revealed that both CncC and dKeap1 were predominantly nuclear and occupied specific chromatin loci. The nuclear localization of dKeap1 and its occupancy of specific chromatin loci indicated that it has functions distinct from those that have been previously attributed to mammalian Keap1. Analysis of the transcriptional and developmental consequences of tissue-specific depletion of CncC and dKeap1 as well as of mutations in cncC and dKeap1 established that these proteins control transcriptional regulons in different organs that coordinate the onset of metamorphosis. The direct roles of CncC and dKeap1 both in ecdysone biosynthetic gene transcription in the PG as well as in ecdysone-regulated gene transcription in salivary glands establish mechanistic links between these central processes in Drosophila metamorphosis.
Concordant versus opposing effects of CncC and dKeap1 on transcription of different classes of genes
Both CncC and dKeap1 depletion reduced transcription of ecdysone-regulated early puff genes. These loci were occupied by both CncC and dKeap1, suggesting that CncC and dKeap1 activated transcription of these genes in concert. Similarly, transcription of most ecdysone biosynthetic genes was reduced by both CncC and dKeap1 depletion as well as by the cncK6/K6 and dKeap1EY5/EY5 loss of function mutations in larvae and embryos, respectively. The ecdysone biosynthetic genes were also occupied by both CncC and dKeap1 in embryos, suggesting that CncC and dKeap1 activated their transcription in concert. In contrast, CncC and dKeap1 depletion as well as the cncK6/K6 and dKeap1EY5/EY5 mutations had opposite effects on transcription of the gstD1 and gstE1 xenobiotic response genes in salivary glands and in embryos, respectively. Similarly, opposite effects of CncC and dKeap1 on transcription of other xenobiotic response genes have been previously reported in adult Drosophila [2]. CncC and dKeap1 therefore regulated transcription of different classes of genes through distinct mechanisms. Whereas xenobiotic response genes are regulated by antagonistic effects of dKeap1 on transcription activation by CncC, ecdysone biosynthetic and response genes were activated by concerted chromatin binding by CncC and dKeap1. Chromatin binding by dKeap1 as well as its cooccupancy and cooperation with CncC have potential implications for Keap1 function and its effects on Nrf2 activity in mammalian cells. Keap1 can shuttle into the nucleus in some cells [10], [12], and could bind chromatin in association with Nrf2 or other interaction partners.
CncC and dKeap1 regulate the onset of metamorphosis by controlling transcriptional regulons in different organs
The effects of CncC and dKeap1 depletion on ecdysone biosynthetic gene transcription and on the timing of pupation indicate that CncC and dKeap1 are important components of the transcription regulatory circuit that controls ecdysone biosynthesis (Figure 6). Many parts of the neuro-endocrine signaling axis that induces ecdysone biosynthesis have been characterized [19], [20], [30], [31]. Previous studies had not identified the transcription factors that bind and regulate ecdysone biosynthetic genes. dSmad2 depletion in the PG reduces ecdysone biosynthetic gene transcription and inhibits pupation. dSmad2 depletion also reduces torso and InR transcription, and RasV12 or InR co-expression in combination with dSmad2 depletion restores both ecdysone-biosynthetic gene transcription as well as pupation [30]. It is therefore likely that dSmad2 affects ecdysone production indirectly by altering Torso or Insulin signaling. In contrast, CncC depletion suppressed the premature pupation caused by RasV12 expression in the PG, and RasV12 expression in salivary glands altered the loci occupied by CncC on polytene chromosomes. These results, together with CncC occupancy and regulation of ecdysone biosynthetic genes in embryos, suggest that CncC mediated the effects of Ras signaling in the PG on pupation by regulating ecdysone biosynthetic gene transcription.
Fig. 6. Model for the regulation of the onset of metamorphosis by CncC and dKeap1. 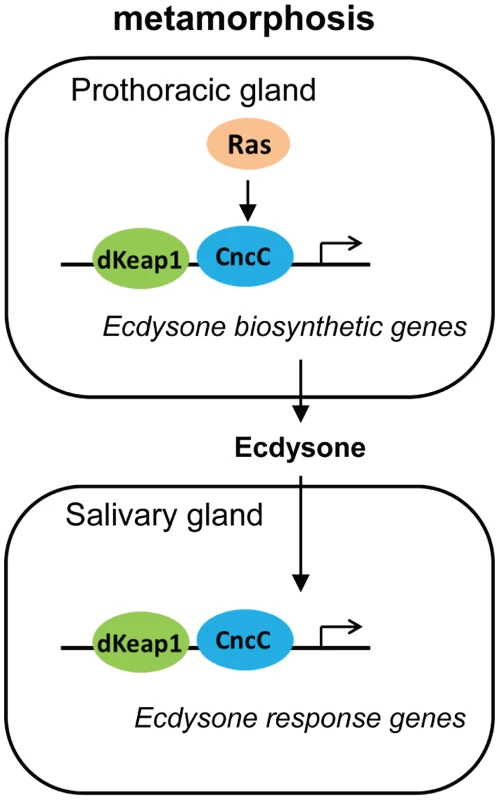
The roles of Ras signaling and ecdysone biosynthesis in the control of metamorphosis were previously established [17], [18], [19]. Our results show that CncC and dKeap1 regulate ecdysone biosynthetic genes and that CncC links Ras signaling to ecdysone biosynthetic gene transcription in the PG. The consequent modulation of ecdysone biosynthesis can regulate the onset of metamorphosis. CncC and dKeap1 also regulate the transcription of ecdysone-inducible genes in the salivary gland. We propose that CncC and dKeap1 coordinate the developmental programs that regulate the onset of metamorphosis by controlling both ecdysone biosynthetic and response genes in different tissues. CncC and dKeap1 also regulated transcription of the early ecdysone-inducible genes in the salivary gland. CncC and dKeap1 binding at ecdysone-inducible early puffs, and the absence of effects of CncC depletion on ecdysone receptor subunit or on late puff gene transcription indicate that CncC and dKeap1 regulated early puff gene transcription directly. The functions of CncC and dKeap1 in regulation of genes that control both ecdysteroid synthesis as well as the transcriptional responses to this hormone place CncC-dKeap1 complex at the nexus of a regulatory network that that coordinates the onset of insect metamorphosis (Figure 6).
Relationships between xenobiotic responses and Drosophila development
The discovery that CncC and dKeap1 coordinate Drosophila metamorphosis has identified novel functions of Nrf2-Keap1 family proteins in normal cellular processes and development. The regulation of both metamorphosis and xenobiotic responses by CncC and dKeap1 suggests that these processes either share a common evolutionary ancestry, or that they are mechanistically or functionally interrelated. Most of the ecdysone biosynthetic genes encode cytochrome P450 class oxidoreductases [18]. P450 class oxidoreductases are also key mediators of the metabolic detoxification of many xenobiotic compounds [32].
The genes that were regulated by CncC and dKeap1 in the salivary and prothoracic glands during larval development and those that are regulated by CncC and dKeap1 in adult flies [2], [3] were mostly non-overlapping. Among the genes identified in this study, only nvd among the ecdysone biosynthetic genes and Sgs5 among the ecdysone-regulated genes were detected by microarray analysis of transcripts induced by CncC expression in adult flies [2]. Thus, the effects of CncC and dKeap1 on the transcription of most of the genes that controlled the onset of metamorphosis were restricted to specific tissues and stages of development.
The functions of CncC and dKeap1 in both hormonal regulation of development and in responses to toxic compounds and environmental stress could represent a mechanism that controls development in response to environmental conditions. Imaginal disc damage inhibits PTTH synthesis, resulting in reduced ecdysone synthesis and a delayed pupation [33]. Modulation of TOR signaling in the PG regulates ecdysone biosynthetic and ecdysone-regulated gene transcription and the timing of pupation [31]. Activation of TOR signaling in the PG suppressed the pupation delay caused by larval starvation, indicating that TOR signaling affected developmental timing in response to nutrient stress. Nutrient restriction and heat stress alter 20E and juvenile hormone levels in the ovaries, arresting oogenesis [34]. The interaction between CncC and dKeap1 could mediate responses to both external as well as endogenous signals that modulate developmental progression. Future studies of the effects of environmental stresses on the developmental functions of CncC and dKeap1 will test this hypothesis.
Regulation of Nrf/CncC family protein functions by Ras signaling
The premature pupation and developmental arrest caused by constitutively active RasV12 expression were suppressed by CncC depletion in the PG. Similarly, the lung and pancreatic tumorigenesis caused by constitutive K-RasG12D expression are suppressed by Nrf2 deletion in mice [6]. K-RasG12D expression can cause a two-fold increase in Nrf2 transcription, but the significance of this change in Nrf2 transcription for tumorigenesis has not been established. RasV12 expression in Drosophila did not alter the level of CncC transcription, but increased the overall level of CncC binding to chromatin, shifted the loci occupied by CncC on polytene chromosomes, and activated genes at those loci in concert with CncC. These results suggest that Ras signaling can regulate the functions of CncC/Nrf family proteins by altering their target gene specificities or transcriptional activities. The mechanisms whereby Ras regulated CncC occupancy remain to be determined, but are likely to include phosphorylation as the MAPK pathway has been proposed to regulate both Nrf2 and the C. elegans homologue of CncC [35], [36], [37].
Relationships between the functions of CncC and mammalian Nrf family proteins
The relationships between the roles of CncC and dKeap1 in Drosophila metamorphosis and the functions of their mammalian homologues in development remain to be elucidated. Two of the mammalian homologues of CncC, Nrf1 and Nrf2, appear to have partially overlapping functions during mouse development [38], [39], [40], [41]. Genome-wide analyses have identified many genes occupied by Nrf2 that have no known functions in the xenobiotic response [42], [43]. Although ecdysteroids are unique to invertebrates, steroid hormones have central roles in many aspects of mammalian physiology. Nrf2 can mediate the 1α,25-dihydroxyvitamin D3-induced differentiation of acute myeloid leukemia cells through multiple mechanisms, including VDR/RXRα transcription [44]. Further studies of the mechanisms of action of CncC/Nrf and dKeap1/Keap1 family proteins in different phyla are required to establish the evolutionary relationships among these proteins and their functions in development and disease.
Materials and Methods
Drosophila stocks
Plasmids encoding CncC, CncB, and dKeap1 fused to intact fluorescent proteins and fluorescent protein fragments were constructed as described in supplemental materials and methods. Transgenic Drosophila lines carrying these expression constructs were generated by microinjection in the w1118 background. The transgenic lines carrying UAS-cncC-RNAi, UAS-dKeap1-RNAi and UAS-rasV12 transgenes were as described [3], [20]. The transgenic line carrying UAS-cnc-RNAi expressed an shRNA that targets all of the Cnc. Double transgenic lines were produced by crosses with Sgs3-GAL4, 71B-GAL4, 5015-GAL4, phm-GAL4 and tub-GAL4 driver lines [19], [22], [25], [27]. To minimize external sources of stress, all studies were conducted with larvae and embryos maintained at 25°C with the exception for larvae carrying the UAS-cnc-RNAi and UAS-dKeap1-RNAi transgenes, which were maintained at 29°C to improve the efficiencies of CncC and dKeap1 depletion. Homozygous and heterozygous embryos carrying the cncK6 and dKeap1EY5 alleles were identified by using the Dfd-YFP marker.
Antisera, polytene chromosome squash, immunostaining, and imaging
Anti-CncC and anti-dKeap1 antisera were raised against proteins encompassing residues 88–344 of CncC and residues 620–776 of dKeap1 fused to GST. The antigens were immobilized and used for affinity purification of the antibodies. Polytene chromosome spreads isolated from the salivary glands of early wandering 3rd instar larvae were prepared and immunolabeled as described in supplemental information. Salivary glands, brain complexes (including brain and prothoracic gland), and imaginal discs were isolated from early wandering 3rd instar larvae and were immunolabeled as described in supplemental information.
Transcript quantitation and chromatin immunoprecipitation
mRNA was isolated from the salivary glands and brain complexes of early wandering 3rd instar larvae as well as embryos, and was quantified by RT-qPCR. The relative transcript levels were calculated by assuming that they were proportional to 2−Cp, and were normalized by the levels of Rp49 transcripts. For ChIP analysis, chromatin was isolated from dechorionated embryos, sheared by sonication, and precipitated using the antisera indicated. The precipitated DNA was quantified by qPCR.
Analysis of the time of pupation and 20E levels
Newly molted 3rd instar larvae or newly hatched 1st instar larvae were collected and transferred into vials. The number of white prepupae (WPP) was scored every 12 hours or 24 hours. To determine the effect of 20E feeding on pupation, the larvae were grown on feeding plates topped with yeast paste containing 0.5 mg/ml 20E. 20E was extracted from larvae and white pre-pupae and was quantified using an enzyme immunoassay kit (Cayman Chemical). Detailed experimental procedures and references are included in Text S1, Table S1, Table S2.
Supporting Information
Zdroje
1. TaguchiK, MotohashiH, YamamotoM (2011) Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes Cells 16 : 123–140.
2. MisraJR, HornerMA, LamG, ThummelCS (2011) Transcriptional regulation of xenobiotic detoxification in Drosophila. Genes Dev 25 : 1796–1806.
3. SykiotisGP, BohmannD (2008) Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev Cell 14 : 76–85.
4. SlocumSL, KenslerTW (2011) Nrf2: control of sensitivity to carcinogens. Arch Toxicol 85 : 273–284.
5. PadmanabhanB, TongKI, OhtaT, NakamuraY, ScharlockM, et al. (2006) Structural basis for defects of Keap1 activity provoked by its point mutations in lung cancer. Mol Cell 21 : 689–700.
6. DeNicolaGM, KarrethFA, HumptonTJ, GopinathanA, WeiC, et al. (2011) Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 475 : 106–109.
7. CalabreseV, CorneliusC, Dinkova-KostovaAT, CalabreseEJ, MattsonMP (2010) Cellular stress responses, the hormesis paradigm, and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid Redox Signal 13 : 1763–1811.
8. ItohK, WakabayashiN, KatohY, IshiiT, IgarashiK, et al. (1999) Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev 13 : 76–86.
9. WataiY, KobayashiA, NagaseH, MizukamiM, McEvoyJ, et al. (2007) Subcellular localization and cytoplasmic complex status of endogenous Keap1. Genes Cells 12 : 1163–1178.
10. SunZ, ZhangS, ChanJY, ZhangDD (2007) Keap1 controls postinduction repression of the Nrf2-mediated antioxidant response by escorting nuclear export of Nrf2. Mol Cell Biol 27 : 6334–6349.
11. KobayashiA, KangMI, OkawaH, OhtsujiM, ZenkeY, et al. (2004) Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol 24 : 7130–7139.
12. NguyenT, SherrattPJ, NioiP, YangCS, PickettCB (2005) Nrf2 controls constitutive and inducible expression of ARE-driven genes through a dynamic pathway involving nucleocytoplasmic shuttling by Keap1. J Biol Chem 280 : 32485–32492.
13. McMahonM, ThomasN, ItohK, YamamotoM, HayesJD (2006) Dimerization of substrate adaptors can facilitate cullin-mediated ubiquitylation of proteins by a “tethering” mechanism: a two-site interaction model for the Nrf2-Keap1 complex. J Biol Chem 281 : 24756–24768.
14. HochmuthCE, BiteauB, BohmannD, JasperH (2011) Redox regulation by Keap1 and Nrf2 controls intestinal stem cell proliferation in Drosophila. Cell Stem Cell 8 : 188–199.
15. VeraksaA, McGinnisN, LiX, MohlerJ, McGinnisW (2000) Cap ‘n’ collar B cooperates with a small Maf subunit to specify pharyngeal development and suppress deformed homeotic function in the Drosophila head. Development 127 : 4023–4037.
16. ThummelCS (2001) Molecular mechanisms of developmental timing in C. elegans and Drosophila. Dev Cell 1 : 453–465.
17. DubrovskyEB (2005) Hormonal cross talk in insect development. Trends Endocrinol Metab 16 : 6–11.
18. RewitzKF, RybczynskiR, WarrenJT, GilbertLI (2006) The Halloween genes code for cytochrome P450 enzymes mediating synthesis of the insect moulting hormone. Biochem Soc Trans 34 : 1256–1260.
19. RewitzKF, YamanakaN, GilbertLI, O'ConnorMB (2009) The insect neuropeptide PTTH activates receptor tyrosine kinase torso to initiate metamorphosis. Science 326 : 1403–1405.
20. CaldwellPE, WalkiewiczM, SternM (2005) Ras activity in the Drosophila prothoracic gland regulates body size and developmental rate via ecdysone release. Curr Biol 15 : 1785–1795.
21. AshburnerM (1972) Patterns of puffing activity in the salivary gland chromosomes of Drosophila. VI. Induction by ecdysone in salivary glands of D. melanogaster cultured in vitro. Chromosoma 38 : 255–281.
22. CherbasL, HuX, ZhimulevI, BelyaevaE, CherbasP (2003) EcR isoforms in Drosophila: testing tissue-specific requirements by targeted blockade and rescue. Development 130 : 271–284.
23. HillRJ, SegravesWA, ChoiD, UnderwoodPA, MacavoyE (1993) The reaction with polytene chromosomes of antibodies raised against Drosophila E75A protein. Insect Biochem Mol Biol 23 : 99–104.
24. FletcherJC, ThummelCS (1995) The ecdysone-inducible Broad-complex and E74 early genes interact to regulate target gene transcription and Drosophila metamorphosis. Genetics 141 : 1025–1035.
25. BussonD, PretAM (2007) GAL4/UAS targeted gene expression for studying Drosophila Hedgehog signaling. Methods Mol Biol 397 : 161–201.
26. LiHM, BuczkowskiG, MittapalliO, XieJ, WuJ, et al. (2008) Transcriptomic profiles of Drosophila melanogaster third instar larval midgut and responses to oxidative stress. Insect Mol Biol 17 : 325–339.
27. YoshiyamaT, NamikiT, MitaK, KataokaH, NiwaR (2006) Neverland is an evolutionally conserved Rieske-domain protein that is essential for ecdysone synthesis and insect growth. Development 133 : 2565–2574.
28. MirthC, TrumanJW, RiddifordLM (2005) The role of the prothoracic gland in determining critical weight for metamorphosis in Drosophila melanogaster. Curr Biol 15 : 1796–1807.
29. RothGE, GierlMS, VollbornL, MeiseM, LintermannR, et al. (2004) The Drosophila gene Start1: a putative cholesterol transporter and key regulator of ecdysteroid synthesis. Proc Natl Acad Sci U S A 101 : 1601–1606.
30. GibbensYY, WarrenJT, GilbertLI, O'ConnorMB (2011) Neuroendocrine regulation of Drosophila metamorphosis requires TGFbeta/Activin signaling. Development 138 : 2693–2703.
31. LayalleS, ArquierN, LeopoldP (2008) The TOR pathway couples nutrition and developmental timing in Drosophila. Dev Cell 15 : 568–577.
32. GuengerichFP (2006) Cytochrome P450s and other enzymes in drug metabolism and toxicity. AAPS J 8: E101–111.
33. HalmeA, ChengM, HariharanIK (2010) Retinoids regulate a developmental checkpoint for tissue regeneration in Drosophila. Curr Biol 20 : 458–463.
34. GruntenkoNE, RauschenbachIY (2008) Interplay of JH, 20E and biogenic amines under normal and stress conditions and its effect on reproduction. J Insect Physiol 54 : 902–908.
35. ZipperLM, MulcahyRT (2003) Erk activation is required for Nrf2 nuclear localization during pyrrolidine dithiocarbamate induction of glutamate cysteine ligase modulatory gene expression in HepG2 cells. Toxicol Sci 73 : 124–134.
36. SunZ, HuangZ, ZhangDD (2009) Phosphorylation of Nrf2 at multiple sites by MAP kinases has a limited contribution in modulating the Nrf2-dependent antioxidant response. PLoS ONE 4: e6588 doi:10.1371/journal.pone.0006588.
37. InoueH, HisamotoN, AnJH, OliveiraRP, NishidaE, et al. (2005) The C. elegans p38 MAPK pathway regulates nuclear localization of the transcription factor SKN-1 in oxidative stress response. Genes Dev 19 : 2278–2283.
38. ChanJY, KwongM, LuR, ChangJ, WangB, et al. (1998) Targeted disruption of the ubiquitous CNC-bZIP transcription factor, Nrf-1, results in anemia and embryonic lethality in mice. EMBO J 17 : 1779–1787.
39. ChanK, LuR, ChangJC, KanYW (1996) NRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proc Natl Acad Sci U S A 93 : 13943–13948.
40. ChenL, KwongM, LuR, GinzingerD, LeeC, et al. (2003) Nrf1 is critical for redox balance and survival of liver cells during development. Mol Cell Biol 23 : 4673–4686.
41. LeungL, KwongM, HouS, LeeC, ChanJY (2003) Deficiency of the Nrf1 and Nrf2 transcription factors results in early embryonic lethality and severe oxidative stress. J Biol Chem 278 : 48021–48029.
42. MalhotraD, Portales-CasamarE, SinghA, SrivastavaS, ArenillasD, et al. (2010) Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucleic Acids Res 38 : 5718–5734.
43. ChorleyBN, CampbellMR, WangX, KaracaM, SambandanD, et al. (2012) Identification of novel NRF2-regulated genes by ChIP-Seq: influence on retinoid X receptor alpha. Nucleic Acids Res 40 : 7416–7429.
44. BobilevI, NovikV, LeviI, ShpilbergO, LevyJ, et al. (2011) The Nrf2 transcription factor is a positive regulator of myeloid differentiation of acute myeloid leukemia cells. Cancer Biol Ther 11 : 317–329.
Štítky
Genetika Reprodukční medicína
Článek MSH3 Polymorphisms and Protein Levels Affect CAG Repeat Instability in Huntington's Disease MiceČlánek Yemanuclein and HIRA Cooperate for Assembly of H3.3-Containing Nucleosomes in the Male Pronucleus
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 2- Akutní intermitentní porfyrie
- Souvislost haplotypu M2 genu pro annexin A5 s opakovanými reprodukčními ztrátami
- Vztah užívání alkoholu a mužské fertility
- Doporučení pro diagnostiku a léčbu akutních jaterních porfyrií
- Doc. Miloš Kubánek: Nemocní se srdeční amyloidózou jsou často skryti a sledováni pod jinými diagnózami
-
Všechny články tohoto čísla
- Complex Inheritance of Melanoma and Pigmentation of Coat and Skin in Grey Horses
- A Meta-Analysis of Thyroid-Related Traits Reveals Novel Loci and Gender-Specific Differences in the Regulation of Thyroid Function
- Genetic Landscape of Open Chromatin in Yeast
- Deleterious Alleles in the Human Genome Are on Average Younger Than Neutral Alleles of the Same Frequency
- Age-Dependent Transition from Cell-Level to Population-Level Control in Murine Intestinal Homeostasis Revealed by Coalescence Analysis
- Next-Generation Sequencing Identifies the Danforth's Short Tail Mouse Mutation as a Retrotransposon Insertion Affecting Expression
- ImmunoChip Study Implicates Antigen Presentation to T Cells in Narcolepsy
- Massive Mitochondrial Gene Transfer in a Parasitic Flowering Plant Clade
- Comment on “Genomic Hypomethylation in the Human Germline Associates with Selective Structural Mutability in the Human Genome”
- The Prefoldin Bud27 Mediates the Assembly of the Eukaryotic RNA Polymerases in an Rpb5-Dependent Manner
- Genetic Determinants of Trabecular and Cortical Volumetric Bone Mineral Densities and Bone Microstructure
- Encodes a Novel and -Genus-Specific Regulator of Photoperiodic Flowering in Rice
- Only One Isoform of CTP Synthase Forms the Cytoophidium
- Mechanisms Involved in the Functional Divergence of Duplicated GroEL Chaperonins in DK1622
- A Genome-Wide RNAi Screen in Identifies the Nicotinic Acetylcholine Receptor Subunit ACR-7 as an Antipsychotic Drug Target
- Autophagy Induction Is a Tor- and Tp53-Independent Cell Survival Response in a Zebrafish Model of Disrupted Ribosome Biogenesis
- Ancient DNA Reveals Prehistoric Gene-Flow from Siberia in the Complex Human Population History of North East Europe
- Inflammation-Mediated Genetic and Epigenetic Alterations Drive Cancer Development in the Neighboring Epithelium upon Stromal Abrogation of TGF-β Signaling
- MicroRNA-3148 Modulates Allelic Expression of Toll-Like Receptor 7 Variant Associated with Systemic Lupus Erythematosus
- RNAi–Based Functional Profiling of Loci from Blood Lipid Genome-Wide Association Studies Identifies Genes with Cholesterol-Regulatory Function
- CELF Family RNA–Binding Protein UNC-75 Regulates Two Sets of Mutually Exclusive Exons of the Gene in Neuron-Specific Manners in
- Coordination of Chromatid Separation and Spindle Elongation by Antagonistic Activities of Mitotic and S-Phase CDKs
- The Ubiquitin Ligase Subunit Acts in Target Tissue to Restrict Tracheal Terminal Cell Branching and Hypoxic-Induced Gene Expression
- Mitotic Evolution of Shows a Stable Core Genome but Recombination in Antigen Families
- Tysnd1 Deficiency in Mice Interferes with the Peroxisomal Localization of PTS2 Enzymes, Causing Lipid Metabolic Abnormalities and Male Infertility
- A Regulatory Pathway, Ecdysone-Transcription Factor Relish-Cathepsin L, Is Involved in Insect Fat Body Dissociation
- PcG-Mediated Higher-Order Chromatin Structures Modulate Replication Programs at the BX-C
- MSH3 Polymorphisms and Protein Levels Affect CAG Repeat Instability in Huntington's Disease Mice
- JNK-Interacting Protein 3 Mediates the Retrograde Transport of Activated c-Jun N-Terminal Kinase and Lysosomes
- Discovery of a Splicing Regulator Required for Cell Cycle Progression
- Rearrangements of 2.5 Kilobases of Noncoding DNA from the Locus Define Predictive Rules of Genomic -Regulatory Logic
- Admixture Mapping in Lupus Identifies Multiple Functional Variants within IFIH1 Associated with Apoptosis, Inflammation, and Autoantibody Production
- Roles of the Developmental Regulator Homothorax in Limiting Longevity in
- miR-199a-5p Is Upregulated during Fibrogenic Response to Tissue Injury and Mediates TGFbeta-Induced Lung Fibroblast Activation by Targeting Caveolin-1
- A Kinome-Wide RNAi Screen in Glia Reveals That the RIO Kinases Mediate Cell Proliferation and Survival through TORC2-Akt Signaling in Glioblastoma
- Assembly of the Auditory Circuitry by a Genetic Network in the Mouse Brainstem
- SOX2 Co-Occupies Distal Enhancer Elements with Distinct POU Factors in ESCs and NPCs to Specify Cell State
- Retrotransposon Activates Ectopic Expression: A Short Tail
- Confounding by Repetitive Elements and CpG Islands Does Not Explain the Association between Hypomethylation and Genomic Instability
- Cell Reprogramming Requires Silencing of a Core Subset of Polycomb Targets
- Properties and Modeling of GWAS when Complex Disease Risk Is Due to Non-Complementing, Deleterious Mutations in Genes of Large Effect
- Essential Developmental, Genomic Stability, and Tumour Suppressor Functions of the Mouse Orthologue of
- Conditional Inactivation of the DNA Damage Response Gene in Mouse Testis Reveals Separable Roles for Components of the RAD9-RAD1-HUS1 Complex in Meiotic Chromosome Maintenance
- Genome-Wide Analysis Points to Roles for Extracellular Matrix Remodeling, the Visual Cycle, and Neuronal Development in Myopia
- Patterning of Leaf Vein Networks by Convergent Auxin Transport Pathways
- An Evolutionary Perspective on Epistasis and the Missing Heritability
- A Retrotransposon Insertion in the 5′ Regulatory Domain of Ptf1a Results in Ectopic Gene Expression and Multiple Congenital Defects in Danforth's Short Tail Mouse
- The Mub1/Ubr2 Ubiquitin Ligase Complex Regulates the Conserved Dsn1 Kinetochore Protein
- Mutations Can Cause Enamel-Renal Syndrome (ERS)
- Yemanuclein and HIRA Cooperate for Assembly of H3.3-Containing Nucleosomes in the Male Pronucleus
- Hepatocyte Growth Factor, a Determinant of Airspace Homeostasis in the Murine Lung
- ISWI and CHD Chromatin Remodelers Bind Promoters but Act in Gene Bodies
- COM-1 Promotes Homologous Recombination during Meiosis by Antagonizing Ku-Mediated Non-Homologous End Joining
- Control of Multicellular Development by the Physically Interacting Deneddylases DEN1/DenA and COP9 Signalosome
- Antagonism Versus Cooperativity with TALE Cofactors at the Base of the Functional Diversification of Hox Protein Function
- Dynamic Association of NUP98 with the Human Genome
- Ectopic Expression of Induces Spinal Defects, Urogenital Defects, and Anorectal Malformations in Mice
- Regulation of Contributes to the Lineage Potential of Neurogenin3+ Endocrine Precursor Cells in the Pancreas
- Gene-Based Testing of Interactions in Association Studies of Quantitative Traits
- The Amidation Step of Diphthamide Biosynthesis in Yeast Requires , a Gene Identified through Mining the - Interaction Network
- Plant-Symbiotic Fungi as Chemical Engineers: Multi-Genome Analysis of the Clavicipitaceae Reveals Dynamics of Alkaloid Loci
- Genome-Wide Diversity in the Levant Reveals Recent Structuring by Culture
- DNA Methylation Mediated Control of Gene Expression Is Critical for Development of Crown Gall Tumors
- Identification of the SlmA Active Site Responsible for Blocking Bacterial Cytokinetic Ring Assembly over the Chromosome
- Expression of a Novel P22 ORFan Gene Reveals the Phage Carrier State in Typhimurium
- Altered Cohesin Gene Dosage Affects Mammalian Meiotic Chromosome Structure and Behavior
- Quantitative Analysis of Histone Modifications: Formaldehyde Is a Source of Pathological N-Formyllysine That Is Refractory to Histone Deacetylases
- Duplicate Abalone Egg Coat Proteins Bind Sperm Lysin Similarly, but Evolve Oppositely, Consistent with Molecular Mimicry at Fertilization
- Lessons from on the Strengths and Weaknesses of Structured Association Mapping
- DNA–Methylome Analysis of Mouse Intestinal Adenoma Identifies a Tumour-Specific Signature That Is Partly Conserved in Human Colon Cancer
- Transposon Variants and Their Effects on Gene Expression in
- Polygenic Modeling with Bayesian Sparse Linear Mixed Models
- Single Transmembrane Peptide DinQ Modulates Membrane-Dependent Activities
- The JNK Signaling Pathway Activates Expression of Stress Response Genes by Derepressing the Fos/HDAC Repressor Complex
- The Interaction of CtIP and Nbs1 Connects CDK and ATM to Regulate HR–Mediated Double-Strand Break Repair
- Regulation of Metamorphosis by Xenobiotic Response Regulators
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Complex Inheritance of Melanoma and Pigmentation of Coat and Skin in Grey Horses
- Coordination of Chromatid Separation and Spindle Elongation by Antagonistic Activities of Mitotic and S-Phase CDKs
- Autophagy Induction Is a Tor- and Tp53-Independent Cell Survival Response in a Zebrafish Model of Disrupted Ribosome Biogenesis
- Assembly of the Auditory Circuitry by a Genetic Network in the Mouse Brainstem
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



