-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
DNA Ligase IV Supports Imprecise End Joining Independently of Its Catalytic Activity
DNA ligase IV (Dnl4 in budding yeast) is a specialized ligase used in non-homologous end joining (NHEJ) of DNA double-strand breaks (DSBs). Although point and truncation mutations arise in the human ligase IV syndrome, the roles of Dnl4 in DSB repair have mainly been examined using gene deletions. Here, Dnl4 catalytic point mutants were generated that were severely defective in auto-adenylation in vitro and NHEJ activity in vivo, despite being hyper-recruited to DSBs and supporting wild-type levels of Lif1 interaction and assembly of a Ku - and Lif1-containing complex at DSBs. Interestingly, residual levels of especially imprecise NHEJ were markedly higher in a deletion-based assay with Dnl4 catalytic mutants than with a gene deletion strain, suggesting a role of DSB-bound Dnl4 in supporting a mode of NHEJ catalyzed by a different ligase. Similarly, next generation sequencing of repair joints in a distinct single-DSB assay showed that dnl4-K466A mutation conferred a significantly different imprecise joining profile than wild-type Dnl4 and that such repair was rarely observed in the absence of Dnl4. Enrichment of DNA ligase I (Cdc9 in yeast) at DSBs was observed in wild-type as well as dnl4 point mutant strains, with both Dnl4 and Cdc9 disappearing from DSBs upon 5′ resection that was unimpeded by the presence of catalytically inactive Dnl4. These findings indicate that Dnl4 can promote mutagenic end joining independently of its catalytic activity, likely by a mechanism that involves Cdc9.
Published in the journal: . PLoS Genet 9(6): e32767. doi:10.1371/journal.pgen.1003599
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003599Summary
DNA ligase IV (Dnl4 in budding yeast) is a specialized ligase used in non-homologous end joining (NHEJ) of DNA double-strand breaks (DSBs). Although point and truncation mutations arise in the human ligase IV syndrome, the roles of Dnl4 in DSB repair have mainly been examined using gene deletions. Here, Dnl4 catalytic point mutants were generated that were severely defective in auto-adenylation in vitro and NHEJ activity in vivo, despite being hyper-recruited to DSBs and supporting wild-type levels of Lif1 interaction and assembly of a Ku - and Lif1-containing complex at DSBs. Interestingly, residual levels of especially imprecise NHEJ were markedly higher in a deletion-based assay with Dnl4 catalytic mutants than with a gene deletion strain, suggesting a role of DSB-bound Dnl4 in supporting a mode of NHEJ catalyzed by a different ligase. Similarly, next generation sequencing of repair joints in a distinct single-DSB assay showed that dnl4-K466A mutation conferred a significantly different imprecise joining profile than wild-type Dnl4 and that such repair was rarely observed in the absence of Dnl4. Enrichment of DNA ligase I (Cdc9 in yeast) at DSBs was observed in wild-type as well as dnl4 point mutant strains, with both Dnl4 and Cdc9 disappearing from DSBs upon 5′ resection that was unimpeded by the presence of catalytically inactive Dnl4. These findings indicate that Dnl4 can promote mutagenic end joining independently of its catalytic activity, likely by a mechanism that involves Cdc9.
Introduction
DNA double-strand breaks (DSBs) are potentially catastrophic chromosomal lesions. Accordingly, many proteins are recruited to DSBs for repair by non-homologous end joining (NHEJ) or homologous recombination (HR). NHEJ, a pathway conserved from yeast to humans, repairs DSBs by processing and directly ligating the DNA ends, often with little or no nucleotide loss [1], [2]. In Saccharomyces cerevisiae, the core components of the canonical NHEJ (c-NHEJ) machinery are the Ku (Yku70–Yku80), MRX (Mre11–Rad50–Xrs2) and DNA ligase IV (Dnl4–Lif1–Nej1) complexes [3]. The final step in c-NHEJ is DNA ligation, carried out by the DNA ligase IV catalytic subunit (Dnl4 in yeast, Lig4 in humans) in complex with its scaffolding protein XRCC4 (Lif1 in yeast) and supported by the XRCC4-like factor XLF (Nej1 in yeast) [1], [3], [4].
Dnl4/Lig4 is an ATP-dependent DNA ligase that functions exclusively in NHEJ [1], [3], [4]. The ATP-dependent DNA ligation mechanism has three distinct catalytic steps [4]. In step 1, the enzyme displaces pyrophosphate from ATP leading to covalent auto-adenylation of the ligase on its active site lysine (K282 in Dnl4), a reaction that can be reversed by incubating adenylated enzyme with excess pyrophosphate. This intermediate is long-lived such that most cellular ligase molecules are adenylated. In step 2, the activated AMP is transferred to a DNA 5′ phosphate via a 5′-5′ phosphoanhydride bond, which is finally cleaved in step 3 by the attack of an adjacent 3′ hydroxyl leading to DNA ligation and release of AMP. DNA ligases undergo profound conformational changes upon ATP and DNA binding [5], [6]. While the above properties are common to all DNA ligases, Dnl4/Lig4 has other unique but poorly understood properties presumably related to its role in DSB ligation, including an ability to ligate incompatible ends [7] and a slow rate of auto-adenylation [8].
There are many potential consequences of failed c-NHEJ ligation. Most importantly, 5′ resection of persistent DSB ends generates 3′-terminated single-stranded DNA tails that are essential for HR [9], [10]. Loss of c-NHEJ proteins in yeast, including Dnl4, has been reported to lead to increased rates of 5′ resection and HR [11], [12], suggesting that either the bound c-NHEJ protein complex and/or c-NHEJ ligation is in competition with HR. Additionally, because error-free HR is not always possible, DSB-derived deletions and other mutations catalyzed by alternative NHEJ (alt-NHEJ) pathways [13]–[16] or catastrophic chromosome loss and cell death can result from c-NHEJ ligation failure. The importance of these negative consequences is underscored by the DNA ligase IV deficiency syndrome, in which human patients with impaired Lig4 function display microcephaly, dysmorphology, developmental delay, bone marrow and immune deficiency, radiosensitivity and malignancy [17], [18].
The balance of the above outcomes might depend substantially on the reason for c-NHEJ ligation failure. However, most genetic studies of Dnl4/Lig4 function have been performed with gene deletion mutants, while most human mutations and polymorphisms are point changes [19]. For these reasons, we have performed mutational analysis of Dnl4 in S. cerevisiae, where an extensive set of outcomes can be assessed. We describe a distinct and mutagenic NHEJ repair behavior of a series of mutations in the ligase catalytic domain as compared to complete loss of the Dnl4 protein. Despite being severely catalytically defective, these mutants, along with the c-NHEJ complex including Ku and Lif1, hyper-accumulated at induced DSBs and supported imprecise NHEJ in some assays. Cdc9 likely represents the alternative ligase used in the presence of dysfunctional Dnl4, as evidenced by its appearance there in a time frame consistent with NHEJ catalysis. Finally, both Dnl4 mutants and Cdc9 were efficiently removed from DSBs and presented no impediment to 5′ resection, suggesting an active switch from NHEJ to HR.
Results
Dnl4 Catalytic Mutants Are Stable and Bind Lif1 Normally
We introduced mutations into the DNL4 chromosomal locus to explore the DSB repair behavior of catalytically defective Dnl4 (Figure 1A). Mutations in ligase Motif 1 (K282R, D284A) and Motif V (K466A) affect universally conserved residues predicted to be involved in ligation catalysis based on comparisons to structure-function studies of viral DNA ligases [20]–[22] and the crystal structure of human DNA ligase I [5] (Figure 1B,C). These residues were chosen on the hypothesis that they might differentially affect the three steps of ligation. K282R alters the lysine predicted to form the covalent AMP adduct and should be an obligatory step 1 mutant, a behavior validated by the complete inability of immunoprecipitated Dnl4-K282R to become adenylated in vitro (Figure 2A). By analogy to viral ligase mutants [20], [21] it was possible that D284A and K466A might be step 2 and step 3 mutants, respectively. However, each of these mutant proteins showed severely reduced in vitro adenylation following pre-incubation with excess pyrophosphate to remove pre-existent AMP adducts (Figure 2A). Because adenylation and pyrophosphorolysis are reverse reactions, these data indicate that Dnl4-D284A and Dnl4-K466A are also severely defective in ligation step 1.
Fig. 1. Dnl4 mutations under study. 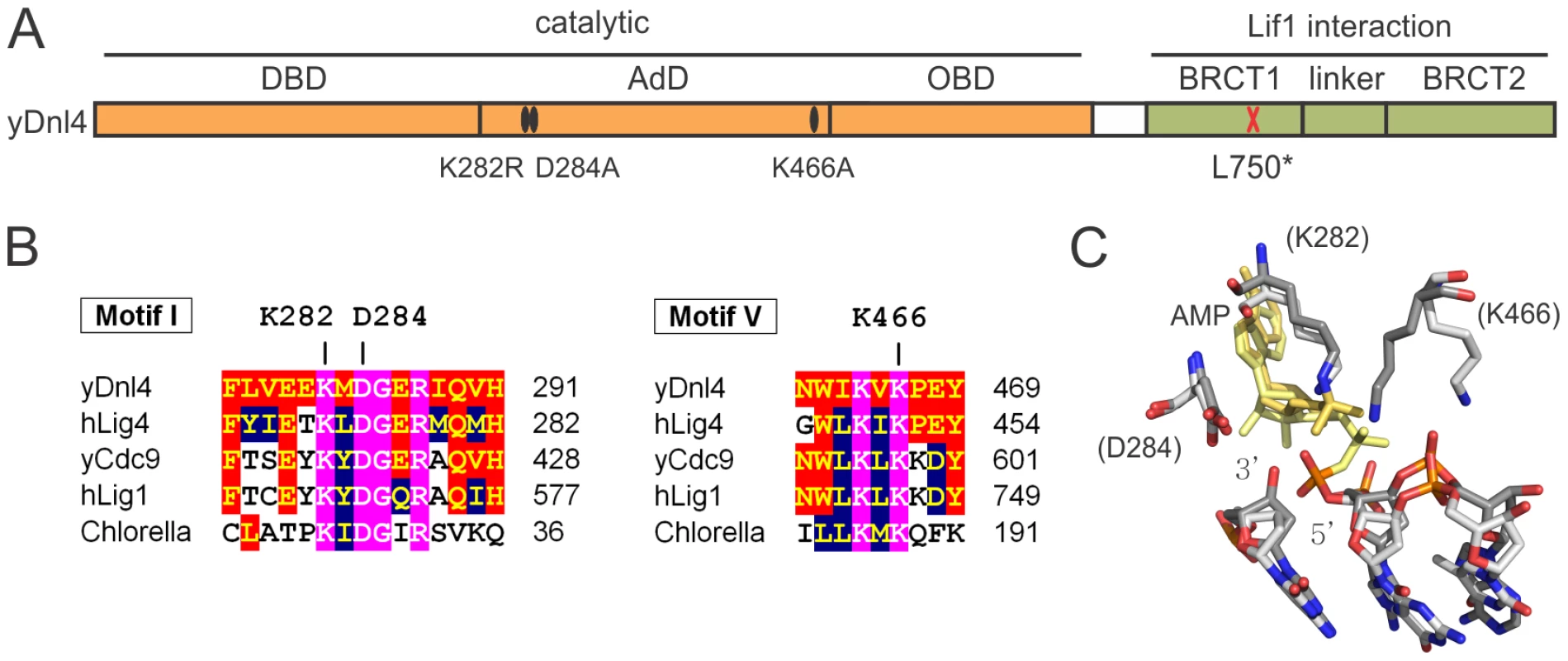
(A) Location of mutations made in this study relative to the functional domains of S. cerevisiae Dnl4 (yDnl4). DBD, DNA binding domain; AdD, adenylation domain; OBD, oligonucleotide binding domain; BRCT, BRCA1 C-terminal repeat; black oval, point mutation; red cross, stop codon. (B) Multiple sequence alignments surrounding conserved mutated yDnl4 positions. hLig4, human DNA ligase IV; yCdc9, S. cerevisiae DNA ligase I; hLig1, human DNA ligase I; Chlorella, chlorella virus DNA ligase. Magenta, identical among all proteins; red, identical to yDnl4; blue, conserved relative to yDnl4. (C) DNA ligase catalytic active site showing a structural alignment of hLig1 bound to a 5′-adenylated DNA nick (PDB 1X9N [5], shaded more lightly) and adenylated Chlorella virus ligase bound to a nick (PDB 2Q2T [22], shaded more darkly). Shown are the AMP (yellow), the substrate DNA strand with labeled 3′ and 5′ nick termini, and the universally conserved residues under study, labeled as the homologous positions in yDnl4. Protein and DNA are shaded by element. Fig. 2. Dnl4 catalytic mutations severely impair auto-adenylation and c-NHEJ efficiency. 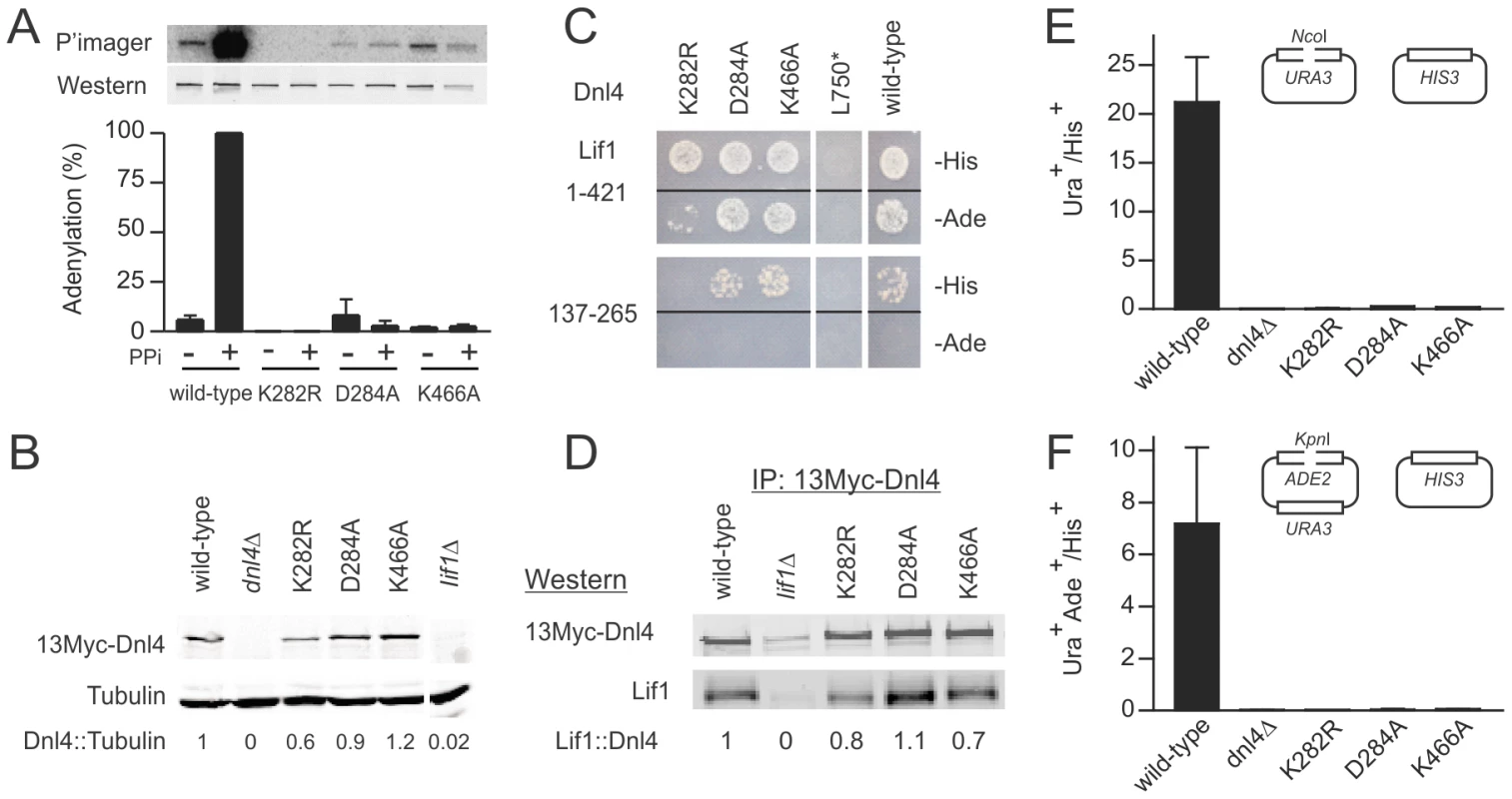
(A) Immunoprecipitated 13Myc-Dnl4 was incubated with α-32P ATP to form a radiolabeled enzyme-adenylate complex, with or without sodium pyrophosphate (PPi) pre-treatment to remove pre-existent covalently bound AMP. Phosphorimager (top panel) and immunoblot (bottom panel) exposures of an example blot are shown, as well as normalized results over three independent experiments (bottom panel). (B) Immunoblot of whole cell extracts from yeast expressing 13Myc-tagged Dnl4 using anti-Myc and anti-tubulin antibodies (Santa Cruz Biotechnology and Thermo Scientific, respectively). The normalized Dnl4 to tubulin signal ratio is shown. (C) Dnl4-Lif1 yeast two-hybrid analysis. Haploid strains expressing the full-length Dnl4 constructs (baits) were mated with strains expressing Lif1 1–421 or 137–265 (preys). The Dnl4-Lif1 interaction was scored by spotting to plates lacking either histidine (-His) or adenine (-Ade). (D) 13Myc-Dnl4 protein was immunoprecipitated from yeast in the same manner as (A) and immunoblotted for co-immunoprecipitated native Lif1 (antibody a kind gift Alan Tomkinson). Numbers are the Lif1/Dnl4 ratio normalized to wild-type Dnl4, after correcting for the background band co-migrating with Lif1. (E) and (F) Plasmid recircularization assay with 5′ and 3′overhangs, respectively. Results are the mean ± standard deviation of at least two independent experiments. We next examined the dependence of ligase stability and the Dnl4-Lif1 interaction on catalytic function. Western blots of whole cell extracts demonstrated that Dnl4-K282R was expressed at moderately reduced levels relative to wild-type Dnl4 (Figure 2B), suggesting a partial instability of this mutant form. In contrast, Dnl4-D284A and Dnl4-K466A were expressed normally (Figure 2B). These expression results mirrored the apparent strength of the Dnl4-Lif1 interaction as revealed by two-hybrid analysis (Figure 2C), but in fact all three catalytic mutants showed a good ability to co-immunoprecipitate Lif1 (Figure 2D). We interpret that the reduced Dnl4-K282R-Lif1 two-hybrid signal is most likely due to reduced Dnl4 expression rather than a defect in Lif1 binding, and thus that Dnl4 catalytic function is not required for Lif1 binding.
For comparison in repair studies below, we also created truncation mutant L750*, which lacks the core of the Lif1 interaction region [23] (Figure 1A). As expected, Dnl4-L750* was unable to bind Lif1 (Figure 2C).
Dnl4 Catalytic Mutants Are Strongly Defective in C-NHEJ
To determine the c-NHEJ phenotype of the catalytic Dnl4 mutants, corresponding strains were transformed by linearized plasmids bearing 5′ (NcoI) or 3′ (KpnI) overhanging DSBs, which is known to depend on Dnl4-mediated simple-religation c-NHEJ [24]–[26]. Consistent with their severe adenylation defect (Figure 2A), all three mutants were impaired in plasmid repair to nearly the same degree as a dnl4Δ mutant (Figure 2E,F), confirming the expected finding that catalysis by Dnl4 is required for c-NHEJ.
Catalytically Defective Dnl4 Binds To, and Is Removed From, Chromosomal DSBs
To determine whether catalytically defective Dnl4 mutants are recruited to DSBs in vivo, we employed a previously described system, GAL1-cs, in which a single DSB is introduced into the chromosomal GAL1 promoter by transient induction of HO endonuclease [27]. In this system, the accumulation of 13Myc-tagged Dnl4 at the DSB is measured by chromatin immunoprecipitation (ChIP) and any associated NHEJ repair is monitored by a parallel quantitative PCR assay. Unlike wild-type Dnl4, which showed an increase in intact HO cut sites from 24% at one hour to 72% at four hours after DSB induction, Dnl4-K282R, -D284A and -K466A were all unable to repair the cleaved allele (Figure 3A), consistent with results above. ChIP demonstrated that the NHEJ defect of the catalytic mutants was not a result of failed recruitment to the DSB. The three mutant proteins each accumulated at the DSB at one and two hours and in fact achieved even higher levels of binding than wild-type (Figure 3B), presumably because DSBs in these strains were not being repaired.
Fig. 3. Extensive recruitment of catalytically inactive Dnl4 and associated c-NHEJ factors to a chromosomal DSB. 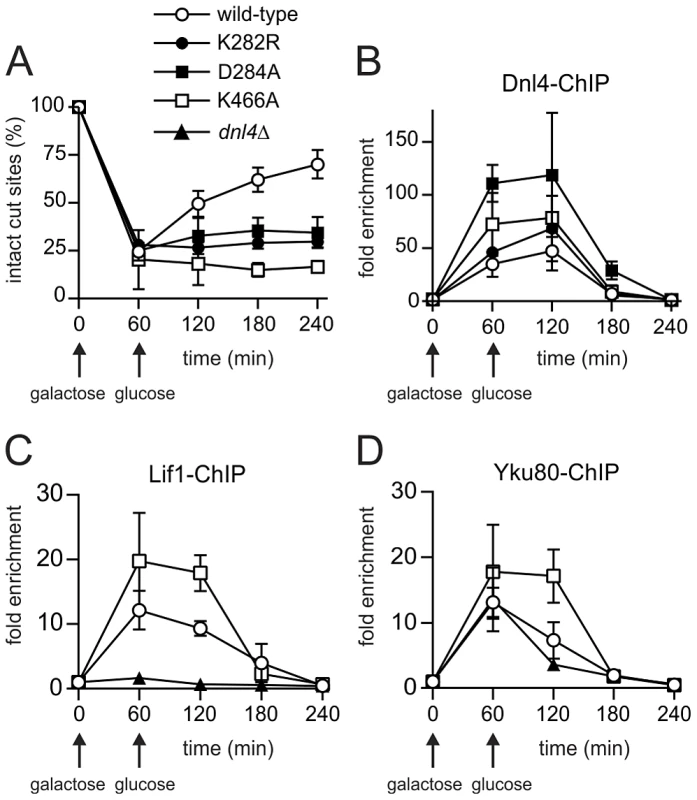
Yeast strains bearing the indicated Dnl4 mutations and the GAL1-cs allele were grown in galactose medium for 60 min to induce HO expression. Cells were then transferred to glucose to allow repair by NHEJ. (A) The fraction of intact GAL1-cs HO cut sites showing the extent of DSB formation and repair over time, determined by flanking PCR. (B) The corresponding enrichment of 13Myc-tagged Dnl4 at the GAL1-cs DSB relative to the ACT1 control locus as determined by ChIP from the same samples as (A). (C) Enrichment of 13Myc-tagged Lif1 and (D) 13Myc-tagged Yku80 at the GAL1-cs DSB. Results are the mean ± standard deviation of at least two independent experiments. Further ChIP analysis was used to explore whether an intact c-NHEJ complex, or just Dnl4, was accumulating at DSBs. Similar to Dnl4, Lif1 was hyper-recruited to DSBs at one and two hours in dnl4-K466A as compared to the wild-type strain, in marked contrast to the greatly impaired accumulation of Lif1 observed in our system and others in a dnl4Δ strain (Figure 3C) [11]. Yku80 showed a similarly enhanced ChIP signal in the dnl4-K466A mutant, while signal was slightly reduced at two hours in the dnl4Δstrain in a manner that likely reflects the reported stabilization of Ku by the ligase complex (Figure 3D) [11]. Thus, we conclude that typical, stable, but non-productive c-NHEJ complexes are assembled at DSBs in strains bearing catalytically defective Dnl4.
ChIP readouts also provided key information regarding the dissociation of Dnl4. We previously observed that Dnl4 and other c-NHEJ proteins begin rapidly disappearing from DSBs after two-hours in an MRX-dependent fashion [27], [28]. Figure 3B–D demonstrate that this disappearance is independent of successful c-NHEJ because non-productive c-NHEJ complexes were removed from DSBs with the same time course as when Dnl4 had successfully catalyzed DSB repair in many cells.
NHEJ, but Not Bound Dnl4, Restricts DSB Resection
We previously showed that disappearance of c-NHEJ proteins from DSBs correlates with the onset of 5′ resection in preparation for HR [27]. Others have suggested that the presence of DNA ligase IV and the c-NHEJ complex at a DSB is inhibitory to resection [11], [12]. However, the gene deletion mutations used in such studies both prevent productive c-NHEJ and disrupt c-NHEJ assembly. This distinction is important because experiments that rely on continued expression of HO allow for futile cycles in which DSBs are created, repaired and recreated. Observed effects of dnl4Δ mutants might result from interruption of this repair cycle rather than direct inhibition of resection by Dnl4. Dnl4 catalytic mutants provided a means of distinguishing these phenomena.
For monitoring resection we used a previously described system, ILV1-cs, in which a single HO DSB is introduced into the chromosomal ILV1 promoter [27]. Here a one-hour induction period was followed by the addition of glucose to turn off HO expression, so that either NHEJ or 5′ resection might ensue. These two downstream events were monitored and distinguished by Southern blotting. Surprisingly, glucose addition proved to be essential for DSB resection to occur (compare Figures 4A and 4B). We attribute this to the fact that our strains are gal1 due to fusion of HO to the native GAL1 promoter and therefore cannot utilize galactose as a carbon source. Consistently, reintroduction of the GAL1 gene on a plasmid restored resection without the addition of glucose (Figure S1), typical of an extensive yeast literature at various DSB sites. These results demonstrate that the metabolic state of a cell substantially influences resection efficiency.
Fig. 4. Catalytically inactive Dnl4 protein does not impede DSB resection. 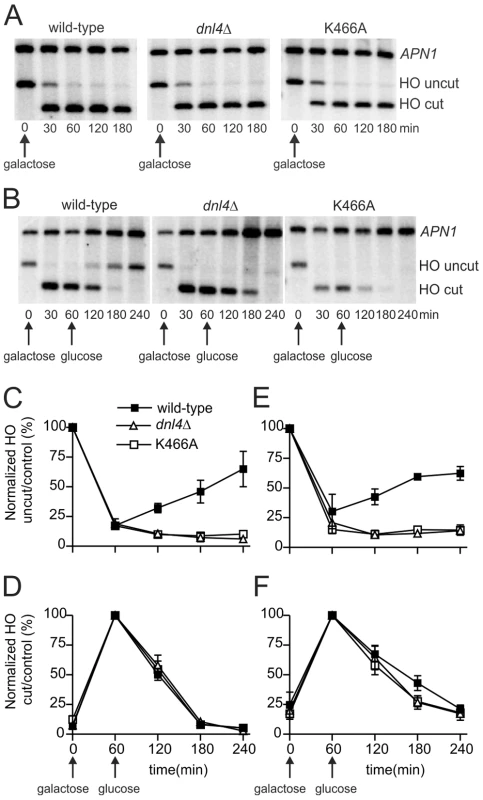
DSBs were induced in yeast strains bearing the ILV1-cs allele similarly to Figure 3 and monitored by Southern blotting. (A) Blot showing that DSB resection is not observed without the addition of glucose at 60 min. (B) Example blot showing formation of the HO-cut band and its subsequent disappearance by a combination of NHEJ and DSB resection. (C) and (E) The ratio of the HO-uncut band to the APN1 control was normalized to the ratio at time 0 to allow monitoring of DSB formation and repair by NHEJ. (D) and (F) The ratio of the HO-cut band to the APN1 control was normalized to the ratio at 60 min when DSB formation was maximal. Disappearance of the HO-cut band at subsequent times results from NHEJ (wild-type strain only) and/or DSB resection. (C) and (D) show results from asynchronous cells while (E) and (F) show results from cells arrested in G1 with α-factor. Results are the mean ± standard deviation of five independent experiments. Figure 4B–D shows ILV1-cs results with asynchronous cultures of yeast expressing wild-type Dnl4, no Dnl4 (dnl4Δ) or Dnl4-K466A. Once again, substantial NHEJ occurred upon glucose addition to the wild-type strain that was absent with both dnl4 mutants (Figure 4B,C). This repair contributed to the disappearance of the HO-cut alleles (Figure 4B,D). However, loss of the HO-cut band also reflects competing 5′ resection, and indeed it also disappeared from the dnl4 mutant strains. The rate of loss of the HO-cut band was equivalent regardless of whether productive c-NHEJ was occurring. This result indicates that resection did occur more robustly in the dnl4 mutant strains but that this difference was accounted for by the DSBs being repaired by c-NHEJ in wild-type. Moreover, the rate of HO-cut band loss, and thus resection, was identical when comparing dnl4Δ and K466A strains (Figure 4D). Similar to previous results [9], [11], [12], G1 arrest inhibited resection, although with ILV1-cs system this effect was incomplete (Figure 4E,F). In G1, we did observe a mildly accelerated loss of the HO-cut band in dnl4 mutant strains as compared to wild-type, similar to prior observations [11]. However, the resection rate was again no different between dnl4Δ and K466A (Figure 4F). Similarly, there was no difference in HR-mediated repair when a homologous donor allele was introduced into the various strains (Figure S2). We conclude that the increased rate of resection in dnl4 mutants can be attributed to the loss of competing NHEJ and is not correlated with the presence of Dnl4 at a DSB.
Dnl4 Promotes Imprecise NHEJ Independently of Its Catalytic Function
We further examined the NHEJ function of the catalytic mutants at two nearby chromosomal DSBs using the well-described suicide deletion assay [26], [29], [30]. Suicide deletion involves galactose-induced formation of two I-SceI DSBs flanking an internal cassette that are joined to recreate the ADE2 gene (Figure 5A). With this assay, dnl4-L750* was as deficient as dnl4Δ yeast (Table 1), confirming the known Dnl4 dependence of the assay [26], [30]. In marked contrast, we observed measureable rates of Ade+ colony formation with all of the Dnl4 catalytic mutants above that observed with dnl4Δ and L750* (Table 1 and Figure 5B). Critically, Ade+ colonies can only arise when the split halves of ADE2 are restored by NHEJ. To verify this, we re-expressed I-SceI in them and observed that, as expected, many repair joints could be re-cleaved by I-SceI and so occurred by simple religation (Table 1). These results indicate that residual end joining capacity exists that depends on Dnl4 protein but not its catalytic activity.
Fig. 5. Residual NHEJ in the chromosomal suicide deletion assay with catalytically defective Dnl4. 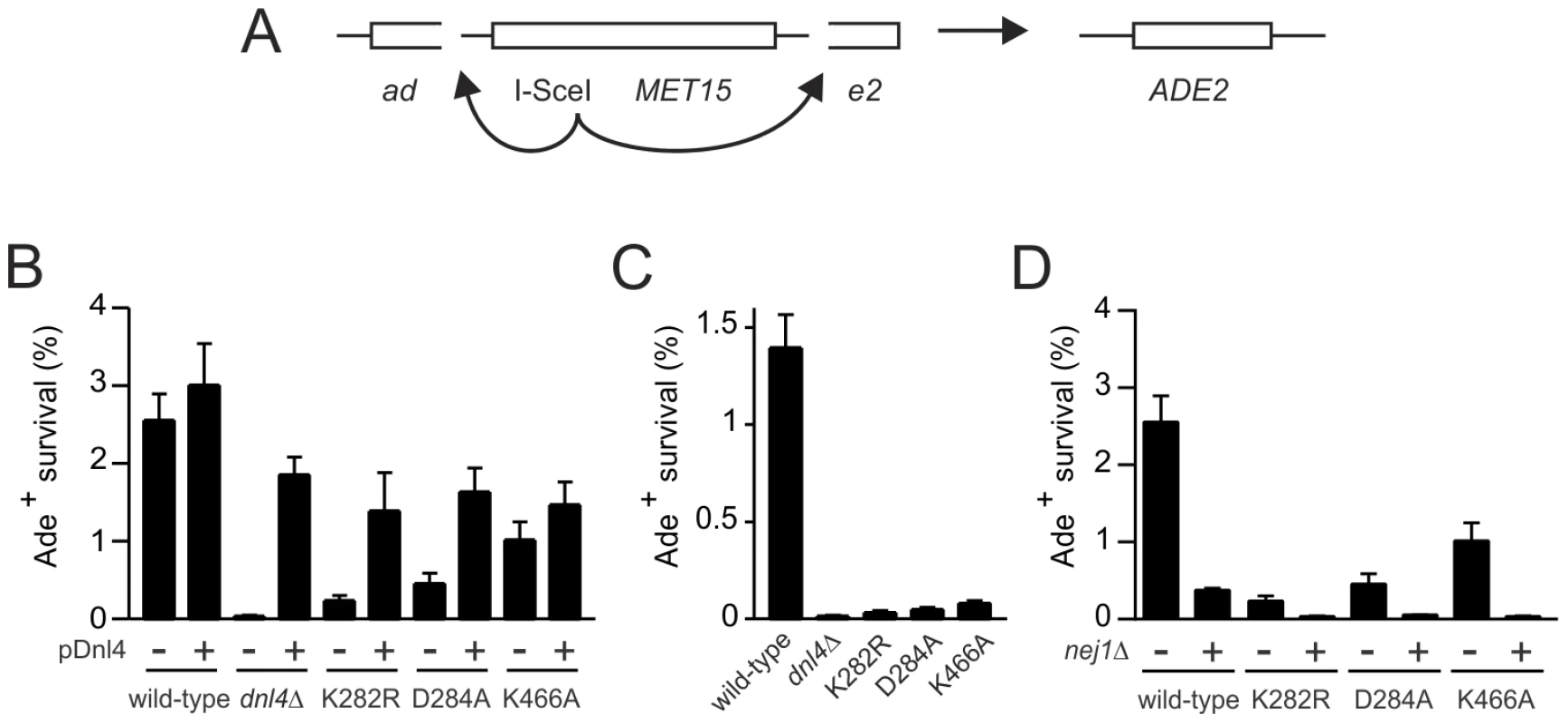
(A) Diagram of the suicide deletion chromosomal assay used to determine NHEJ efficiency in panels (B) to (D). (B) Cells were pre-grown to stationary phase in synthetic defined medium prior to plating to galactose. When indicated, the chromosomal dnl4 allele was complemented with a plasmid bearing wild-type DNL4 (pDNL4). (C) Cells were pre-grown to log phase in YPA-Glycerol. (D) Epistasis analysis of Dnl4 catalytic mutants with nej1Δ. Results are the mean ± standard deviation of at least three independent experiments. Tab. 1. NHEJ precision in Ade+ suicide deletion colonies. 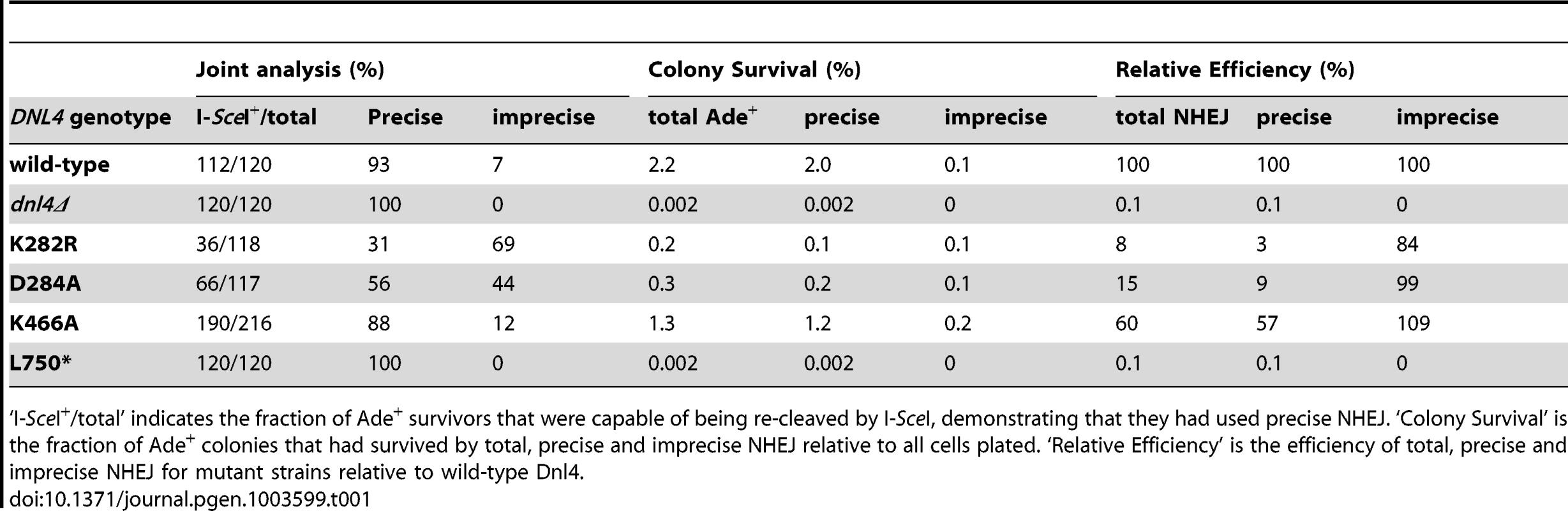
‘I-SceI+/total’ indicates the fraction of Ade+ survivors that were capable of being re-cleaved by I-SceI, demonstrating that they had used precise NHEJ. ‘Colony Survival’ is the fraction of Ade+ colonies that had survived by total, precise and imprecise NHEJ relative to all cells plated. ‘Relative Efficiency’ is the efficiency of total, precise and imprecise NHEJ for mutant strains relative to wild-type Dnl4. Sequencing the ADE2 alleles from a number of I-SceI-resistant Ade+ colonies revealed a similar and typical collection of in-frame but imprecise NHEJ junctions, including microhomologies, insertions and deletions, for both wild-type Dnl4 and the catalytic mutants (Figure S3A). Indeed, the absolute frequency of imprecise NHEJ was not substantially different for the catalytic point mutants as compared to wild-type Dnl4, in marked contrast to dnl4Δ and L750* yeast that showed a complete loss of imprecise junctions (Table 1). Thus, the joining pathway dependent on the Dnl4 protein, but not its activity, was substantially more mutagenic than that being catalyzed by Dnl4.
Ade− suicide deletion colonies are much rarer than Ade+ colonies and cannot be selected for when plating, preventing statistically meaningful frequency comparisons. However, we performed allelic analysis on the Ade−colonies we did recover (Table 2), first by scoring for status of the STE3-MET15 marker contained within the cassette that is lost upon suicide deletion (Figure 5A). We then attempted to amplify the DSB junctions from Ade− Met− colonies to determine whether they corresponded to imprecise NHEJ or large deletions of the ADE2 locus. Strikingly, dnl4Δ colonies never arose due to imprecise NHEJ but instead showed high frequencies of large deletions (Table 2). In contrast, the catalytic mutants showed imprecise NHEJ in a pattern that mirrored the Ade+ suicide deletion results (Table 2, Figures S3B). We conclude that imprecise NHEJ in the suicide deletion assay depends on Dnl4, but not its catalytic activity.
Tab. 2. Joint analysis of Ade− suicide deletion colonies. 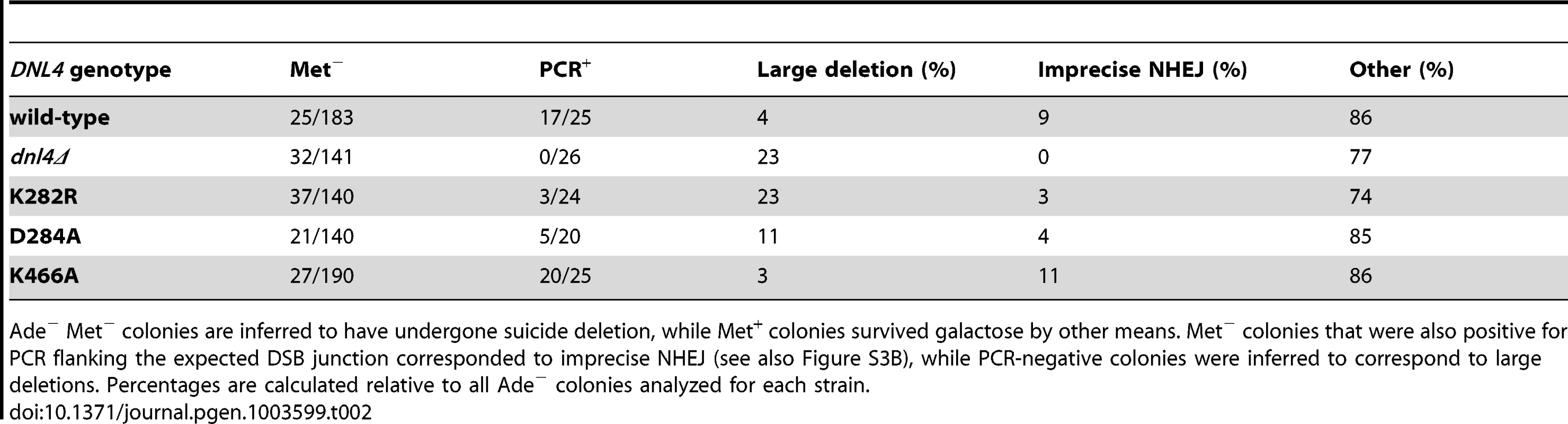
Ade− Met− colonies are inferred to have undergone suicide deletion, while Met+ colonies survived galactose by other means. Met− colonies that were also positive for PCR flanking the expected DSB junction corresponded to imprecise NHEJ (see also Figure S3B), while PCR-negative colonies were inferred to correspond to large deletions. Percentages are calculated relative to all Ade− colonies analyzed for each strain. The different result pattern when comparing the suicide deletion (Figure 5) and plasmid recircularization (Figure 2E,F) assays was striking, especially since the I-SceI and KpnI 3′ DSBs are in the same position in ADE2. Importantly, our standard suicide deletion protocol [26] entails pre-growth of cells to post-diauxic phase for two days, unlike the log-phase cells used in plasmid recircularization assays. Indeed, the residual suicide deletion repair with Dnl4 catalytic mutants was almost entirely suppressed when the experiment was repeated with log-phase cells (Figure 5C), demonstrating that, like resection, the mutagenic end joining pathway dependent on Dnl4 protein is strongly influenced by the metabolic state of the cell.
Residual NHEJ in the above suicide deletion experiments cannot have been catalyzed by Dnl4-K282R as it cannot be adenylated (see Figure 2A). We further assessed the in vivo adenylation state of Dnl4-K466A. Overexpressed Dnl4 proteins were excised from a gel (Figure S4A) and subjected to LC-MS/MS. Importantly, enhanced suicide-deletion NHEJ was still seen with overexpressed Dnl4-K466A (). Twelve adenylated peptides were recovered with wild-type Dnl4 out of 163 K282-containing peptides (Figure S4C). This fraction is smaller than expected and likely reveals instability of the adenylation intermediate during fragmentation. Nevertheless, no adenylated peptides were recovered out of 128 K282-containing peptides from Dnl4-K466A, consistent with the in vitro results that it is deficient in ligation step 1.
The above results with catalytically defective Dnl4 were reminiscent of prior observations that yeast lacking the Dnl4 accessory protein Nej1 display substantial residual suicide deletion NHEJ, unlike Ku, Lif1 and MRX mutants that phenocopy the near complete defect seen with dnl4Δ [30]. However, the residual NHEJ observed with nej1Δ and catalytically defective Dnl4 do not reflect the same phenomenon since nej1 dnl4 double mutants showed a synergistic decrease in suicide deletion survival (Figure 5D). Thus, Nej1 is required for the residual NHEJ observed with catalytically defective Dnl4. In turn, Dnl4 likely catalyzes the residual NHEJ observed with nej1Δ, demonstrating that c-NHEJ is differentially dependent on Nej1 and Lif1 in at least this assay.
A final possibility was that the Dnl4 catalytic mutants might act as dominant negatives if they could preclude the action of competent Dnl4 molecules. To test this, we transformed dnl4 mutant strains with a DNL4 plasmid. The same degree of complementation was observed with the three catalytic point mutants as with the dnl4Δ strain (Figure 5B), indicating that point mutant and wild-type Dnl4 had equal access to the DSB.
Dnl4-K466A Confers a Different Imprecise Joining Profile than Wild-Type
We next pursued high-throughput next-generation sequencing of NHEJ occurring at HO-induced DSBs in the ILV1-cs assay (i) to determine whether the findings regarding imprecise suicide deletion NHEJ could be confirmed using a single-DSB assay, and (ii) to dramatically improve the capacity with which imprecise joints could be scored to allow a more accurate comparison of the joining profiles of different strains. Here we did not add glucose following initial DSB induction in order to suppress 5′ resection (see Figure 4A) and to allow for continuous (re)cleavage of intact HO cut sites and thereby enrich for imprecise joining. Genomic DNA was collected from wild-type, dnl4-K466A, and dnl4Δ strains at 0-hour and 24-hour time points and PCR products flanking the HO DSB site were subjected to Illumina HiSeq sequencing.
As expected, all strains showed ≥98% intact cut sites from among all recovered reads before HO induction (File S1). At 24 hours, wild-type and K466A strains each showed an average across two independent replicates of 40% imprecise joints, consistent with the design of the experiment (Figure 6A). In marked contrast, the dnl4Δ strain yielded 97% precise joints and only 3% imprecise joints at 24 hours, a pattern more like the 0-hour time point than the other strains. The recovery of mainly intact cut sites in the most highly NHEJ deficient strain is explained by the presence of uncleaved alleles in dead cells combined with the near absence of bona fide repair events in live cells, given that results represent the frequency of sequences recovered from a sample, not their absolute efficiency. The inferred severe impairment of imprecise NHEJ only in the dnl4Δ strain closely parallels the findings from the suicide deletion assay.
Fig. 6. End joining profiles after extensive HO recleavage depend on Dnl4 status. 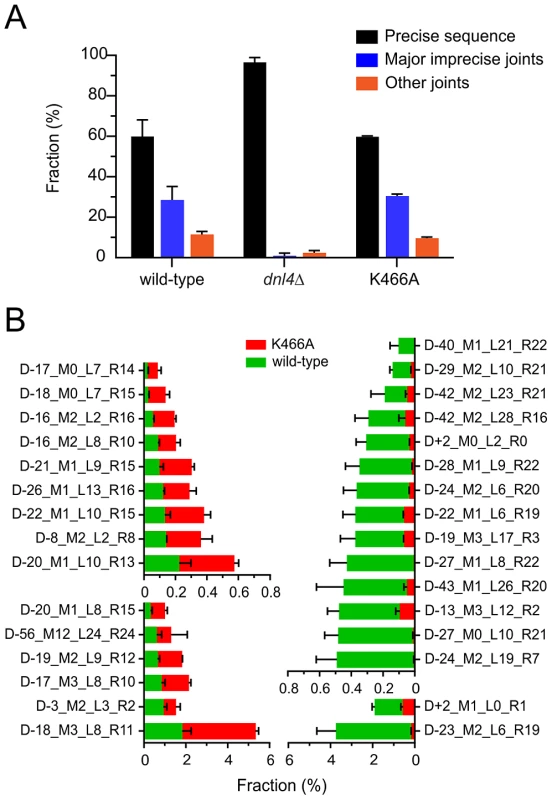
(A) Fractions of different ILV1-cs joint categories after 24 hours of HO induction, showing preserved imprecise joining with K466A. “Precise sequence” is the same as the input allele prior to DSB formation, “major imprecise joints” had a frequency ≥0.1% in any tested strain, and “other joints” are the remainder. (B) Individual major imprecise joints that showed significantly different frequencies between wild-type and K466A. The left half shows joints that had increased frequency in K466A as compared to wild-type. The right half shows joints that had a decreased frequency. Red bars, joint fraction in K466A; green bars, joint fraction in wild-type. See Figure S5 for a description of the joint identifiers and File S1 for their sequences. To compare the imprecise joining profiles of wild-type and Dnl4-K466A, joints were codified to names containing all information needed to uniquely define them (explained in Figure S5). The DESeq program [31] was then used to apply the negative binomial distribution to identify joints for which the frequency was significantly different between the two 24-hour replicates of K466A as compared to wild-type (Figure S6). To help ensure statistical validity, we further restricted our attention to only those joints that had a frequency >0.1% in any tested strain, for which robust counts had thus been obtained (File S1). Although wild-type and K466A strains showed a similar overall frequency for these major imprecise joints (Figure 6A), 15 (25%) and 16 (27%) individual joints showed significantly increased and decreased frequencies, respectively, when comparing K466A to wild-type (Figure 6B). These results establish that loss of Dnl4 catalytic activity confers differences in the quality of imprecise joints that are all nonetheless dependent on the Dnl4 protein.
Cdc9 Is Recruited to DSBs Coincident with Dnl4
A possible explanation for the above results is that Cdc9/DNA ligase I, the only other DNA ligase in S. cerevisiae, catalyzes the residual NHEJ when Dnl4 is present but inactive. It is difficult to perform NHEJ assays in cdc9 yeast as it is an essential gene, so to begin to assess its potential role in residual NHEJ we performed Cdc9 ChIP. A first experiment revealed the appearance of Cdc9 at the GAL1-cs DSB after one hour of galactose induction and its disappearance following glucose addition (Figure 7A,B). This pattern was very similar to Dnl4 (Figure 3), although the level of Cdc9 enrichment was lower. To make the signal more sustained we repeated the experiment by pre-growing the cells to post-diauxic phase and did not add glucose at one hour, which was expected to prevent 5′ resection and promote residual NHEJ according to results above. DSB induction was delayed in this protocol (Figure 7C), but Cdc9 enrichment was again observed that now persisted for four hours (Figure 7D). Given the lower DSB levels, this pattern suggests a more robust Cdc9 enrichment in post-diauxic than log phase cells. Controls lacking the anti-Myc ChIP antibody confirmed the specificity of this result, for which DSB induction in fact lowered the DSB locus signal relative to the ACT1 control locus, perhaps due to reduced non-specific binding of the DSB fragment resulting from local chromatin alterations upon DSB induction (Figure 7D) [32]. Finally, a pattern emerged in post-diauxic cells wherein Cdc9 signals were lowest in the complete absence of Dnl4 and highest in the presence of Dnl4-K466A (Figure 7D). This trend was again not predicted by the higher levels of DSB formation in the dnl4Δ strain, suggesting a promotion of Cdc9 retention by Dnl4 in post-diauxic cells.
Fig. 7. Cdc9 recruitment to a chromosomal DSB during the NHEJ repair phase. 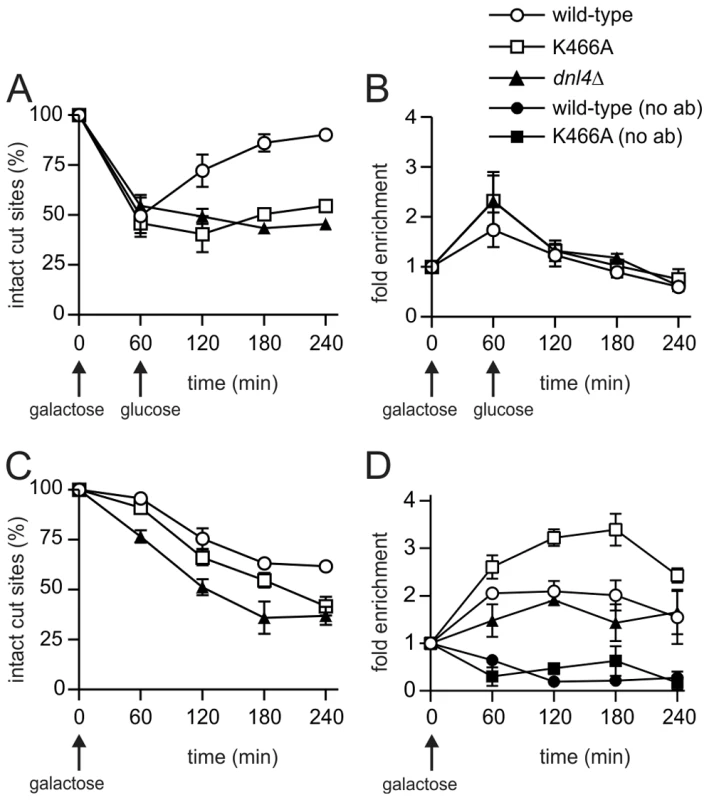
Yeast strains bearing 13Myc-tagged Cdc9 with the indicated Dnl4 mutations and the GAL1-cs allele were pre-grown to log phase in YPA-Glycerol (A, B) or post-diauxic phase (C, D) and treated with 2% galactose to induce HO expression and DSB formation. (A) and (C) show the fraction of intact GAL1-cs HO cut sites and thus the extent of DSB formation and repair over time as determined by flanking PCR. (B) and (D) show the corresponding enrichment of 13Myc-Cdc9 at the GAL1-cs DSB as determined by ChIP from the same samples as (A) and (C), respectively. ‘No ab’ indicates background signal obtained without antibody addition during immunoprecipitation. Results are the mean ± standard deviation of two independent experiments. Discussion
The central findings of this work are that (i) catalytically inactive yeast Dnl4/DNA ligase IV can promote imprecise NHEJ when it persists at DSBs, (ii) Cdc9/DNA ligase I is present at DSBs at times consistent with it catalyzing the observed residual NHEJ in place of Dnl4, (iii) these phenomenon and DSB 5′ resection are influenced by the metabolic state of the cells, and (iv) 5′ resection efficiency is reduced by c-NHEJ activity but not by the presence of Dnl4 protein at a DSB, suggesting an active process that removes NHEJ proteins and initiates 5′ resection.
Dnl4 Supports Mutagenic NHEJ Independently of Its Catalytic Activity
We made mutations in universally conserved Dnl4 residues that have been shown to create step-specific catalytic blocks in other ATP-dependent DNA ligases [20], [21]. Dnl4-K282R could not auto-adenylate, as expected, but we also observed severely impaired capacity of catalytic step 1 in vitro with Dnl4-D284A and Dnl4-K466A (Figure 2A) and commensurate undetectable auto-adenylation in vivo with Dnl4-K466A (Figure S4). This pattern of results emphasizes the integrated nature of the DNA ligase active site. Consistently, Dnl4-K282R, -D284A and -K466A mutants were all severely deficient in c-NHEJ in vivo in several assays (Figures 2–4). No mutant acted as a dominant negative (Figure 5B), despite the fact that all bound Lif1 (Figure 2C,D) and accumulated even more than wild-type at DSBs (Figure 3). Importantly, DSB recruitment must be distinguished from fully productive binding to DNA termini, which likely requires ligase auto-adenylation [5], [20].
Given the expected severe effect of Dnl4 catalytic mutation on ligase activity and c-NHEJ, it was striking that these same mutants supported residual NHEJ activity in the suicide deletion and ILV1-cs assays well above that observed in strains lacking Dnl4 (Figures 5 and 6). We considered a number of properties of the suicide deletion assay to account for this residual repair. The phenomenon did not depend on the use of two DSBs since a difference in residual NHEJ was also observed in the single-DSB ILV1-cs next-generation sequencing experiment (Figure 6). In contrast, increased survival due to residual suicide deletion NHEJ was observed only when cells were pre-grown to post-diauxic/early stationary phase (Figure 5). With the ILV1-cs assay, the detection of Dnl4 protein-dependent residual NHEJ entailed a growth protocol that promoted the recovery of imprecise joints by prolonged HO expression in the context of limited 5′ resection due to the inability of the cells to ferment galactose.
Despite the technical differences in execution, the suicide deletion and ILV1-cs assays led to the same conclusion that a substantial extent of imprecise NHEJ is preserved with catalytically defective Dnl4 but lost in the absence of Dnl4 protein. Thus, the form of NHEJ supported by Dnl4 protein was more mutagenic than the proper c-NHEJ catalyzed by wild-type Dnl4. This observation is reminiscent of findings that Dnl4 participates in some microhomology mediated end joining (MMEJ) through a poorly understood mechanism [33] and of mammalian studies documenting the mutagenic potential of DNA ligase IV-independent NHEJ [16], [34]. It also has implications for the human ligase IV syndrome in which mutations such as R278H, Q280R, and H282L (motif I) and G468E, G469E (motif V) are hypomorphic despite the fact that they severely alter the ATP binding properties of the enzyme [35], [36]. This pattern is very similar to the yeast mutants studied here and suggests the possibility for affected humans that inactive ligase might accumulate at a DSB and support mutagenic NHEJ.
Cdc9 as a Candidate Imprecise NHEJ Ligase
Joint analysis unequivocally established that residual repair observed with Dnl4 catalytic mutants occurred by NHEJ (Figure 6 and S3 and Table 1), demonstrating that NHEJ can be catalyzed by a ligase other than Dnl4. We cannot entirely rule out that residual repair was catalyzed by some Dnl4 mutants, but K282R is incapable of becoming adenylated (Figure 2A) and we found no evidence to support adenylation and c-NHEJ ligation by K466A (Figures 2 and S4). In contrast, Cdc9 was recruited to DSBs in vivo in a time frame consistent with its participation in NHEJ and in a manner that was enhanced by Dnl4 protein (Figure 7). The difference in the imprecise joint profile obtained with wild-type and Dnl4-K466A (Figures 6B and S6) provides further indirect support for the participation of a different ligase in the residual imprecise NHEJ. Dnl4-independent NHEJ is not especially surprising since even some precise NHEJ occurs in the complete absence of Dnl4 [25] and can become quite efficient as overhang lengths increase [37]. Moreover, DNA ligase III, which is missing from yeast, and to a lesser extent DNA ligase I have been shown to catalyze alt-NHEJ in higher eukaryotes [14], [15]. The key finding here is that use of an alternative ligase leading to especially imprecise NHEJ can be stimulated by the presence of Dnl4 protein at a DSB, to the point that one must consider whether many mutagenic NHEJ events are catalyzed by DNA ligase I/III even when DNA ligase IV is present, an idea supported by mammalian studies [14].
The exact mechanism by which Dnl4 protein might promote imprecise NHEJ by Cdc9 is unknown, but numerous observations suggest that it is the c-NHEJ complex that is responsible even if the events are not catalyzed by Dnl4. All tested c-NHEJ proteins accumulate at DSBs in strains bearing Dnl4 catalytic mutations in a manner that parallels Dnl4 itself (Figure 3), so all are likely present when residual NHEJ occurs. Further, it is well established for suicide deletion and other assays that loss of Ku, Lif1 and MRX alone leads to a severe loss of all NHEJ, including imprecise events, in the same fashion as dnl4Δ [30]. In the one case where we could specifically test a role of another c-NHEJ protein, we indeed found that the residual NHEJ observed in the absence of Dnl4 catalytic activity depends on Nej1 (Figure 5D). The mode of mutagenic NHEJ we describe here thus appears to be distinct from yeast MMEJ [33] or alt-NHEJ more generally, which are inhibited by the c-NHEJ assembly containing Ku [2]. Instead, Dnl4 protein likely promotes assembly of the c-NHEJ complex at the DSB, as has been proposed [11], for subsequent action not only by Dnl4 but also Cdc9 and likely many other enzymes. This concept is consistent with electron microscopy data that DNA ligase IV can execute end bridging in conjunction with Ku [38], as well as mammalian studies suggesting that DNA ligase IV acts non-catalytically at DSBs in the early stage of NHEJ [39], perhaps by supporting filament formation seen with XRCC4/XLF [40], [41].
Dnl4 Indirectly Inhibits DSB Resection
We monitored the flux of DSBs into the competing NHEJ and HR pathways to determine whether resection is inhibited by Dnl4 as has been suggested [11]. The results demonstrate that resection was inhibited by Dnl4 only because it catalyzed NHEJ that removed DSBs from the potential resection pool (Figure 4). DSBs that failed NHEJ were shunted to 5′ resection with equal efficiency whether the failure was created by loss of Dnl4 or by a Dnl4 point mutant that accumulated at the DSB. This behavior is explained by the observation that both wild-type and mutant Dnl4 (Figure 3), as well as Cdc9 (Figure 7), were efficiently removed from persistent DSBs. Although the mechanistic sequence remains to be established, these results support a model in which NHEJ protein removal is an active process initiated after an initial time period of end preservation to permit NHEJ [2], [27], [42]. The notion that it is an active process is supported by the observation that the utilizable carbon source, and by inference the energy status, had a large influence on the cell's ability to engage in 5′ resection (Figure 4) and ligase removal (Figure 7).
The combined observations on residual NHEJ activity and resection emphasize the impact that cell state, including both cell cycle stage and metabolic influences, has on DSB repair outcomes. Such ideas are relevant to human DNA repair since most cells in an adult mammal are in the G0 state and under careful metabolic control that is disrupted in cancer [43]. Indeed, recent studies demonstrate a strong influence of cell state on mutagenic NHEJ outcomes not unlike those observed here [44]. These insights also suggest possible unintended mutagenic consequences of DNA ligase IV inhibitors [45], agents which might lead to accumulation of catalytically ineffective c-NHEJ complexes at DSBs in a manner similar to the mutants studied here.
Materials and Methods
Protein Structural Analysis
Protein structural analysis was performed using The PyMOL Molecular Graphics System, Schrödinger, LLC.
Yeast Growth and Manipulation
Yeast strains used for NHEJ and chromatin immunoprecipitation (ChIP) assays (Table S1) were isogenic derivatives of BY4741 [46] as previously described [27], [28]. Point mutations were constructed in the native DNL4 gene using a pop-in/pop-out method [29]. L750* truncation was created as a stop codon after the indicated residue. All mutant alleles were confirmed by sequencing. Yeast were grown at 30°C in either rich medium containing 1% yeast extract, 2% peptone and 40 µg/ml adenine (YPA) or synthetic defined (SD) medium with either 2% glucose, 2% galactose or 3% glycerol as the carbon source [27], [28].
Yeast Two-Hybrid
The two hybrid indicator strain PJ69-4a/α, vectors pODB2 and pOAD, and full-length and truncated Dnl4 and Lif1 derivatives have been described [29], [47]. Dnl4 point mutant derivatives were made by transferring mutant chromosomal alleles into plasmids by gap repair [29]. Dnl4-Lif1 interaction was monitored by mating to strains carrying Lif1 constructs and spotting overnight cultures to plates lacking either histidine or adenine followed by 3 and 5 days growth, respectively.
Plasmid Recircularization Assay
pRS316 [46] was cut with NcoI to create a 5′ overhanging DSB within its URA3 marker. pK1827 [26] was cut with KpnI to create a 3′ overhanging DSB within its ADE2 marker, leaving its URA3 marker intact. Each cut plasmid (100 ng) was co-transformed into YW1228 derivatives along with 10 ng of supercoiled HIS3-marked pRS413 [46], as previously described [28]. c-NHEJ efficiency is reported as the ratio of Ura+ (pRS316) or Ura+/Ade+ colonies (pK1827) to His+ colonies.
Suicide Deletion Assay
The suicide deletion assay for monitoring NHEJ was previously described [26], [29], [30]. Here all strains (Table S1) bore the ade2::SD2-::STE3-MET15 allele for which HR is not possible. Cells were pre-grown either to stationary phase for 2 days in SD medium, our standard protocol [26], or to log phase overnight in YPA-Glycerol. NHEJ was scored as colony counts on galactose plates divided by counts on parallel glucose control plates. The nature of NHEJ events in Ade+ colonies was determined by mating to YW2083 carrying the I-SceI expression plasmid pTW334 [26]. If precise NHEJ had created an intact I-SceI site in the suicide deletion strain, it is recleaved and undergoes HR with the YW2083 ade2-M7 allele to give Ade−/red diploids. Ade− suicide deletion colonies were assessed by first ensuring an isolate was Met−, and thus had deleted STE3-MET15, and then attempting to amplify a 1.2 kb PCR product symmetrically flanking the expected DSB junction. No product indicated a large deletion. Inferred imprecise NHEJ alleles were finally sequenced.
Chromosomal DSB Induction
We previously described the GAL1-cs and ILV1-cs strains for creating single-site chromosomal DSBs [27]. In them, HO is expressed from the native chromosomal GAL1 promoter so that the strain is rendered Gal−. HO cut sites are present in either the same GAL1 promoter that expresses HO (GAL1-cs) or in the native ILV1 promoter (ILV1-cs). DSB induction and α-factor G1 arrest were as described [27].
Epitope Tagging and ChIP
GAL1-cs strains bore a 13Myc tag on the C-terminus of the native chromosomal DNL4, CDC9, LIF1 and YKU80 genes as previously described [27]. ChIP assays and parallel monitoring of DSB formation and repair were performed as described [27] except that quantitative PCR rather than competitive PCR was used to monitor Dnl4 binding to the DSB site, with fold enrichment determined from the Ct difference between GAL1 and the ACT1 control gene.
Immunoprecipitation and Adenylation Assays
GAL1-cs strains were grown overnight in YPA-Dextrose. Lysates were prepared from 1 g wet cell pellet in lysis buffer (10 mM Tris pH 7.5, 150 mM NaCl, 1 mM MgCl2, 2 mM CaCl2, 10% glycerol, and Roche complete mini tablet) using zirconia bead lysis followed by centrifugation at 15,000× g. Dnl4 was immunoprecipitated by overnight incubation with 80 µl anti-Myc conjugated agarose beads (Sigma). Samples were washed 3 times with lysis buffer, divided into two aliquots, and pre-incubated with 1 ml AMP buffer (60 mM Tris pH 8.0, 10 mM MgCl2, 2 mM DTT, and Roche complete mini tablet) with or without 0.5 mM sodium pyrophosphate for 15 min at room temperature. Beads were washed with 1 ml AMP buffer before addition of 15 µCi α-32P ATP to the residual volume for 30 min at room temperature. Beads were washed twice before boiling and electrophoresis on a 7.5% SDS-PAGE gel and transfer to nitrocellulose. The same membrane was used to obtain a phosphorimager screen exposure followed by Western blotting using anti-Myc antibody with detection by a LiCor Odyssey scanner. Results are expressed as the ratio of phosphorimager to Western blot signal, setting the wild-type Dnl4 sample with pyrophosphate treatment to 100%.
Resection Monitoring
Genomic DNA was extracted after galactose induction in the ILV1-cs system, digested with EcoRI and NcoI, and subjected to Southern blotting as previously described [27]. Results are expressed as a ratio of the intensity of the intact (2.6 kb) or HO-cut (1.1 kb) ILV1 fragment to that of the APN1 control fragment (3.5 kb), normalized to the ratio at either 0 or 60 minutes of induction, as indicated.
Recombination Assay
ILV1-cs yeast were transformed with pRS416 containing a 960 bp homologous ILV1 donor fragment. Cells were plated to glucose plates after varying times of DSB induction in galactose to determine cell survival relative to untreated cells.
Next Generation Sequencing
ILV1-cs yeast were pre-grown overnight in YPA-Glycerol. Genomic DNA was harvested before and 24 hours after galactose induction and subjected to PCR using primers flanking the ILV1 HO DSB site that additionally bore unique 6-base sequence indexes for each input sample and a 5′ extension matching Illumina paired-end sequencing adapters (5′-ACACTCTTTCCCTACACGACGCTCTTCCGATCTxxxxxxAGGGCAAAAAGAAAAAGCGCAG and 5′-CTCGGCATTCCTGCTGAACCGCTCTTCCGATCTxxxxxxGTTTTATCAAGGAAGGTGACA, where “xxxxxx” was replaced with specific index sequences). After 15 cycles of amplification, products were purified and subjected to an additional 16 PCR cycles using Illumina paired-end second-round primers. Products from all samples were pooled, 50% of a PhiX control library was added to facilitate cluster calling, and the mixture subjected to Illumina HiSeq 100 bp paired-end sequencing at the University of Michigan DNA Sequencing Core. Only forward reads crossed the ILV1 HO cut site and were used for further analysis. Reads matching PhiX were removed using Bowtie [48]. Custom Perl scripts (File S2) assigned the remaining reads to the input samples based on the index and counted the number that corresponded to the intact ILV1 HO cut site or any one of the possible blunt, microhomology, or insertion-mediated joints supported by the input DSB ends, allowing up to two inserted bases at the junction and one mismatched read base. Reads corresponding to more than one joint were called ambiguous. Joint counts were input into DESeq [31] as is, or expressed as a fraction of the total ILV1 read counts for graphical comparisons.
Dnl4 Mass Spectrometry
pTW644 (a kind gift Patrick Sung) expresses Dnl4 and His6-tagged Lif1 from divergent GAL1/10 promoters. Dnl4-K466A was introduced into this plasmid by PCR amplification and ligation into BamHI/SalI sites and confirmed by sequencing. Plasmids were transformed into YW1230 for suicide deletion analysis as described above and into protease-deficient YW2189 for protein purification. Yeast were grown to log phase in 1000 ml of YPA-Glycerol and expression induced by addition of 2% galactose for 4 hours. 5 g of cells were harvested, resuspended in buffer A [28], and lysed using zirconium beads (Biospec). The cleared lysate was incubated with 0.3 ml of nickel-NTA agarose beads (Qiagen) in the presence of 10 mM imidazole for 1 h at 4°C. The beads were washed three times with buffer A containing 20 mM imidazole and boiled in 1X SDS loading buffer. Following 10% SDS-PAGE and Novex colloidal blue staining (Invitrogen), Dnl4 bands were excised and subjected to trypsin digestion. Resulting peptides were resolved on a nano-capillary reverse phase column (Picofrit column, New Objective) using a 1% acetic acid/acetonitrile gradient, introduced into a linear ion-trap mass spectrometer (LTQ Orbitrap XL, ThermoFisher), and searched against the S. cerevisiae protein database using X!Tandem/TPP [49]. Oxidation of methionine (+15.9949 Da), carbamidomethylation of cysteines (+57.0214 Da), and AMP-lysine (+329.0525 Da) were allowed modifications. Precursor and fragment mass tolerance were 50 ppm and 0.8 Da, respectively. Matches to Dnl4 with a ProteinProphet probability of >0.9 (fdr <2%) were used to determine the adenylation state of K282-containing peptides.
Supporting Information
Zdroje
1. LieberMR (2010) The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu Rev Biochem 79 : 181–211.
2. ChiruvellaKK, LiangZ, WilsonTE (2013) Repair of double strand breaks by end-joining. Cold Spring Harb Perspect Biol 5(5): a012757.
3. DaleyJM, PalmbosPL, WuD, WilsonTE (2005) Nonhomologous end joining in yeast. Annu Rev Genet 39 : 431–451.
4. EllenbergerT, TomkinsonAE (2008) Eukaryotic DNA ligases: structural and functional insights. Annu Rev Biochem 77 : 313–338.
5. PascalJM, O'BrienPJ, TomkinsonAE, EllenbergerT (2004) Human DNA ligase I completely encircles and partially unwinds nicked DNA. Nature 432 : 473–478.
6. PascalJM, TsodikovOV, HuraGL, SongW, CotnerEA, et al. (2006) A flexible interface between DNA ligase and PCNA supports conformational switching and efficient ligation of DNA. Mol Cell 24 : 279–291.
7. MaY, LuH, TippinB, GoodmanMF, ShimazakiN, et al. (2004) A biochemically defined system for mammalian nonhomologous DNA end joining. Mol Cell 16 : 701–713.
8. RiballoE, WoodbineL, StiffT, WalkerSA, GoodarziAA, et al. (2009) XLF-Cernunnos promotes DNA ligase IV-XRCC4 re-adenylation following ligation. Nucleic Acids Res 37 : 482–492.
9. IraG, PellicioliA, BalijjaA, WangX, FioraniS, et al. (2004) DNA end resection, homologous recombination and DNA damage checkpoint activation require CDK1. Nature 431 : 1011–1017.
10. SymingtonLS, GautierJ (2011) Double-strand break end resection and repair pathway choice. Annu Rev Genet 45 : 247–71.
11. ZhangY, HefferinML, ChenL, ShimEY, TsengHM, et al. (2007) Role of Dnl4-Lif1 in nonhomologous end-joining repair complex assembly and suppression of homologous recombination. Nat Struct Mol Biol 14 : 639–646.
12. ClericiM, MantieroD, GueriniI, LucchiniG, LongheseMP (2008) The Yku70-Yku80 complex contributes to regulate double-strand break processing and checkpoint activation during the cell cycle. EMBO Rep 9 : 810–818.
13. MladenovE, IliakisG (2011) Induction and repair of DNA double strand breaks: the increasing spectrum of non-homologous end joining pathways. Mutat Res 711 : 61–72.
14. SimsekD, BrunetE, WongSY, KatyalS, GaoY, et al. (2011) DNA ligase III promotes alternative nonhomologous end-joining during chromosomal translocation formation. PLoS Genet 7: e1002080.
15. Della-MariaJ, ZhouY, TsaiMS, KuhnleinJ, CarneyJ, et al. (2011) hMre11/hRad50/Nbs1 and DNA ligase IIIα/XRCC1 act together in an alternative non-homologous end joining pathway. J Biol Chem 286 : 33845–33853.
16. BoboilaC, OksenychV, GostissaM, WangJH, ZhaS, et al. (2012) Robust chromosomal DNA repair via alternative end-joining in the absence of X-ray repair cross-complementing protein 1 (XRCC1). Proc Natl Acad Sci USA 109 : 2473–2478.
17. O'DriscollM, CerosalettiKM, GirardPM, DaiY, StummM, et al. (2001) DNA ligase IV mutations identified in patients exhibiting developmental delay and immunodeficiency. Mol Cell 8 : 1175–1185.
18. ChistiakovDA (2010) Ligase IV syndrome. Adv Exp Med Biol 685 : 175–185.
19. RucciF, NotarangeloLD, FazeliA, PatriziL, HickernellT, et al. (2010) Homozygous DNA ligase IV R278H mutation in mice leads to leaky SCID and represents a model for human LIG4 syndrome. Proc Natl Acad Sci USA 107 : 3024–3029.
20. SriskandaV, ShumanS (1998) Mutational analysis of Chlorella virus DNA ligase: catalytic roles of domain I and motif VI. Nucleic Acids Res 26 : 4618–4625.
21. SriskandaV, ShumanS (2002) Role of nucleotidyltransferase motifs I, III and IV in the catalysis of phosphodiester bond formation by Chlorella virus DNA ligase. Nucleic Acids Res 30 : 903–911.
22. NairPA, NandakumarJ, SmithP, OdellM, LimaCD, et al. (2007) Structural basis for nick recognition by a minimal pluripotent DNA ligase. Nat Struct Mol Biol 14 : 770–778.
23. DoreAS, FurnhamN, DaviesOR, SibandaBL, ChirgadzeDY, et al. (2006) Structure of an Xrcc4-DNA ligase IV yeast ortholog complex reveals a novel BRCT interaction mode. DNA Repair (Amst) 5 : 362–368.
24. DaleyJM, LaanRL, SureshA, WilsonTE (2005) DNA joint dependence of pol X family polymerase action in nonhomologous end joining. J Biol Chem 280 : 29030–29037.
25. WilsonTE, GrawunderU, LieberMR (1997) Yeast DNA ligase IV mediates non-homologous DNA end joining. Nature 388 : 495–498.
26. KarathanasisE, WilsonTE (2002) Enhancement of Saccharomyces cerevisiae end-joining efficiency by cell growth stage but not by impairment of recombination. Genetics 161 : 1015–1027.
27. WuD, TopperLM, WilsonTE (2008) Recruitment and dissociation of nonhomologous end joining proteins at a DNA double-strand break in Saccharomyces cerevisiae. Genetics 178 : 1237–1249.
28. PalmbosPL, WuD, DaleyJM, WilsonTE (2008) Recruitment of Saccharomyces cerevisiae Dnl4-Lif1 complex to a double-strand break requires interactions with Yku80 and the Xrs2 FHA domain. Genetics 180 : 1809–1819.
29. PalmbosPL, DaleyJM, WilsonTE (2005) Mutations of the Yku80 C terminus and Xrs2 FHA domain specifically block yeast nonhomologous end joining. Mol Cell Biol 25 : 10782–10790.
30. WilsonTE (2002) A genomics-based screen for yeast mutants with an altered recombination/end-joining repair ratio. Genetics 162 : 677–688.
31. AndersS, HuberW (2010) Differential expression analysis for sequence count data. Genome Biol 11: R106.
32. ShimEY, HongSJ, OumJH, YanezY, ZhangY, et al. (2007) RSC mobilizes nucleosomes to improve accessibility of repair machinery to the damaged chromatin. Mol and Cell Biol 27 : 1602–1613.
33. MaJL, KimEM, HaberJE, LeeSE (2003) Yeast Mre11 and Rad1 proteins define a Ku-independent mechanism to repair double-strand breaks lacking overlapping end sequences. Mol Cell Biol 23 : 8820–8828.
34. ChiruvellaKK, SebastianR, SharmaS, KarandeAA, ChoudharyB, et al. (2012) Time-dependent predominance of nonhomologous DNA end-joining pathways during embryonic development in mice. J Mol Biol 417 : 197–211.
35. RiballoE, DohertyAJ, DaiY, StiffT, OettingerMA, et al. (2001) Cellular and biochemical impact of a mutation in DNA ligase IV conferring clinical radiosensitivity. J Biol Chem 276 : 31124–31132.
36. GirardPM, KyselaB, HarerCJ, DohertyAJ, JeggoPA (2004) Analysis of DNA ligase IV mutations found in LIG4 syndrome patients: the impact of two linked polymorphisms. Hum Mol Genet 13 : 2369–2376.
37. DaleyJM, WilsonTE (2005) Rejoining of DNA double-strand breaks as a function of overhang length. Mol Cell Biol 25 : 896–906.
38. GrobP, ZhangTT, HannahR, YangH, HefferinML, et al. (2012) Electron microscopy visualization of DNA-protein complexes formed by Ku and DNA ligase IV. DNA repair 11 : 74–81.
39. CottarelJ, FritP, BombardeO, SallesB, NegrelA, et al. (2013) A noncatalytic function of the ligation complex during nonhomologous end joining. J Cell Biol 200 : 173–186.
40. MahaneyBL, HammelM, MeekK, TainerJA, Lees-MillerSP (2013) XRCC4 and XLF form long helical protein filaments suitable for DNA end protection and alignment to facilitate DNA double strand break repair. Biochem Cell Biol 91 : 31–41.
41. HammelM, YuY, FangS, Lees-MillerSP, TainerJA (2010) XLF regulates filament architecture of the XRCC4.ligase IV complex. Structure 18 : 1431–1442.
42. ShibataA, ConradS, BirrauxJ, GeutingV, BartonO, et al. (2011) Factors determining DNA double-strand break repair pathway choice in G2 phase. EMBO J 30 : 1079–1092.
43. OermannEK, WuJ, GuanKL, XiongY (2012) Alterations of metabolic genes and metabolites in cancer. Sem Cell Dev Biol 23 : 370–380.
44. BindraRS, GogliaAG, JasinM, PowellSN (2013) Development of an assay to measure mutagenic non-homologous end-joining repair activity in mammalian cells. Nucl Acids Res doi:10.1093. Epub ahead of print
45. SrivastavaM, NambiarM, SharmaS, KarkiSS, GoldsmithG, et al. (2012) An inhibitor of nonhomologous end-joining abrogates double-strand break repair and impedes cancer progression. Cell 151 : 1474–1487.
46. BrachmannCB, DaviesA, CostGJ, CaputoE, LiJ, et al. (1998) Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14 : 115–132.
47. UetzP, GiotL, CagneyG, MansfieldTA, JudsonRS, et al. (2000) A comprehensive analysis of protein-protein interactions in Saccharomyces cerevisiae. Nature 403 : 623–627.
48. LangmeadB, TrapnellC, PopM, SalzbergSL (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10: R25.
49. YatesJR, EngJK, McCormackAL, SchieltzD (1995) Method to correlate tandem mass spectra of modified peptides to amino acid sequences in the protein database. Anal Chem 67 : 1426–1436.
50. MooreJK, HaberJE (1996) Cell cycle and genetic requirements of two pathways of nonhomologous end-joining repair of double-strand breaks in Saccharomyces cerevisiae. Mol Cell Biol 16 : 2164–2173.
Štítky
Genetika Reprodukční medicína
Článek PARP-1 Regulates Metastatic Melanoma through Modulation of Vimentin-induced Malignant TransformationČlánek The Genome of : Evolution, Organization, and Expression of the Cyclosporin Biosynthetic Gene ClusterČlánek Distinctive Expansion of Potential Virulence Genes in the Genome of the Oomycete Fish PathogenČlánek USF1 and hSET1A Mediated Epigenetic Modifications Regulate Lineage Differentiation and TranscriptionČlánek Comprehensive High-Resolution Analysis of the Role of an Arabidopsis Gene Family in RNA EditingČlánek Extensive Intra-Kingdom Horizontal Gene Transfer Converging on a Fungal Fructose Transporter Gene
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 6- Akutní intermitentní porfyrie
- Souvislost haplotypu M2 genu pro annexin A5 s opakovanými reprodukčními ztrátami
- Vztah užívání alkoholu a mužské fertility
- Hysteroskopická resekce děložního septa zlepšuje šanci na graviditu žen s jinak nevysvětlenou infertilitou
- Srdeční frekvence embrya může být faktorem užitečným v předpovídání výsledku IVF
-
Všechny články tohoto čísla
- BMS1 Is Mutated in Aplasia Cutis Congenita
- High Trans-ethnic Replicability of GWAS Results Implies Common Causal Variants
- How Cool Is That: An Interview with Caroline Dean
- Genetic Architecture of Vitamin B and Folate Levels Uncovered Applying Deeply Sequenced Large Datasets
- Juvenile Hormone and Insulin Regulate Trehalose Homeostasis in the Red Flour Beetle,
- Meiosis-Specific Stable Binding of Augmin to Acentrosomal Spindle Poles Promotes Biased Microtubule Assembly in Oocytes
- Environmental Dependence of Genetic Constraint
- H3.3-H4 Tetramer Splitting Events Feature Cell-Type Specific Enhancers
- Network Topologies and Convergent Aetiologies Arising from Deletions and Duplications Observed in Individuals with Autism
- Effectively Identifying eQTLs from Multiple Tissues by Combining Mixed Model and Meta-analytic Approaches
- Altered Splicing of the BIN1 Muscle-Specific Exon in Humans and Dogs with Highly Progressive Centronuclear Myopathy
- The NADPH Metabolic Network Regulates Human Cardiomyopathy and Reductive Stress in
- Negative Regulation of Notch Signaling by Xylose
- A Genome-Wide, Fine-Scale Map of Natural Pigmentation Variation in
- Transcriptome-Wide Mapping of 5-methylcytidine RNA Modifications in Bacteria, Archaea, and Yeast Reveals mC within Archaeal mRNAs
- Multiplexin Promotes Heart but Not Aorta Morphogenesis by Polarized Enhancement of Slit/Robo Activity at the Heart Lumen
- Latent Effects of Hsp90 Mutants Revealed at Reduced Expression Levels
- Impact of Natural Genetic Variation on Gene Expression Dynamics
- DeepSAGE Reveals Genetic Variants Associated with Alternative Polyadenylation and Expression of Coding and Non-coding Transcripts
- The Identification of -acting Factors That Regulate the Expression of via the Osteoarthritis Susceptibility SNP rs143383
- Pervasive Transcription of the Human Genome Produces Thousands of Previously Unidentified Long Intergenic Noncoding RNAs
- The RNA Export Factor, Nxt1, Is Required for Tissue Specific Transcriptional Regulation
- Inferring Demographic History from a Spectrum of Shared Haplotype Lengths
- Histone Acetyl Transferase 1 Is Essential for Mammalian Development, Genome Stability, and the Processing of Newly Synthesized Histones H3 and H4
- PARP-1 Regulates Metastatic Melanoma through Modulation of Vimentin-induced Malignant Transformation
- DNA Methylation Restricts Lineage-specific Functions of Transcription Factor Gata4 during Embryonic Stem Cell Differentiation
- The Genome of : Evolution, Organization, and Expression of the Cyclosporin Biosynthetic Gene Cluster
- Distinctive Expansion of Potential Virulence Genes in the Genome of the Oomycete Fish Pathogen
- Deregulation of the Protocadherin Gene Alters Muscle Shapes: Implications for the Pathogenesis of Facioscapulohumeral Dystrophy
- Evidence for Two Different Regulatory Mechanisms Linking Replication and Segregation of Chromosome II
- USF1 and hSET1A Mediated Epigenetic Modifications Regulate Lineage Differentiation and Transcription
- Methylation of Histone H3 on Lysine 79 Associates with a Group of Replication Origins and Helps Limit DNA Replication Once per Cell Cycle
- A Six Months Exercise Intervention Influences the Genome-wide DNA Methylation Pattern in Human Adipose Tissue
- The Gene Desert Mammary Carcinoma Susceptibility Locus Regulates Modifying Mammary Epithelial Cell Differentiation and Proliferation
- Hooked and Cooked: A Fish Killer Genome Exposed
- Distinct Neuroblastoma-associated Alterations of Impair Sympathetic Neuronal Differentiation in Zebrafish Models
- Mutations in Cause Autosomal Recessive Congenital Ichthyosis in Humans
- Integrated Transcriptomic and Epigenomic Analysis of Primary Human Lung Epithelial Cell Differentiation
- RSR-2, the Ortholog of Human Spliceosomal Component SRm300/SRRM2, Regulates Development by Influencing the Transcriptional Machinery
- Comparative Polygenic Analysis of Maximal Ethanol Accumulation Capacity and Tolerance to High Ethanol Levels of Cell Proliferation in Yeast
- SPO11-Independent DNA Repair Foci and Their Role in Meiotic Silencing
- Budding Yeast ATM/ATR Control Meiotic Double-Strand Break (DSB) Levels by Down-Regulating Rec114, an Essential Component of the DSB-machinery
- Comprehensive High-Resolution Analysis of the Role of an Arabidopsis Gene Family in RNA Editing
- Functional Analysis of Neuronal MicroRNAs in Dauer Formation by Combinational Genetics and Neuronal miRISC Immunoprecipitation
- DNA Ligase IV Supports Imprecise End Joining Independently of Its Catalytic Activity
- Extensive Intra-Kingdom Horizontal Gene Transfer Converging on a Fungal Fructose Transporter Gene
- Heritable Change Caused by Transient Transcription Errors
- From Many, One: Genetic Control of Prolificacy during Maize Domestication
- Neuronal Target Identification Requires AHA-1-Mediated Fine-Tuning of Wnt Signaling in
- Loss of Catalytically Inactive Lipid Phosphatase Myotubularin-related Protein 12 Impairs Myotubularin Stability and Promotes Centronuclear Myopathy in Zebrafish
- H-NS Can Facilitate Specific DNA-binding by RNA Polymerase in AT-rich Gene Regulatory Regions
- Prophage Dynamics and Contributions to Pathogenic Traits
- Global DNA Hypermethylation in Down Syndrome Placenta
- Fragile DNA Motifs Trigger Mutagenesis at Distant Chromosomal Loci in
- Disturbed Local Auxin Homeostasis Enhances Cellular Anisotropy and Reveals Alternative Wiring of Auxin-ethylene Crosstalk in Seminal Roots
- Causes and Consequences of Chromatin Variation between Inbred Mice
- Genome-scale Analysis of FNR Reveals Complex Features of Transcription Factor Binding
- Distinct and Atypical Intrinsic and Extrinsic Cell Death Pathways between Photoreceptor Cell Types upon Specific Ablation of in Cone Photoreceptors
- Sex-stratified Genome-wide Association Studies Including 270,000 Individuals Show Sexual Dimorphism in Genetic Loci for Anthropometric Traits
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- BMS1 Is Mutated in Aplasia Cutis Congenita
- Sex-stratified Genome-wide Association Studies Including 270,000 Individuals Show Sexual Dimorphism in Genetic Loci for Anthropometric Traits
- Distinctive Expansion of Potential Virulence Genes in the Genome of the Oomycete Fish Pathogen
- Distinct Neuroblastoma-associated Alterations of Impair Sympathetic Neuronal Differentiation in Zebrafish Models
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



