-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaMining Herbaria for Plant Pathogen Genomes: Back to the Future
article has not abstract
Published in the journal: . PLoS Pathog 10(4): e32767. doi:10.1371/journal.ppat.1004028
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1004028Summary
article has not abstract
Since the dawn of agriculture, plant pathogens and pests have been a scourge of humanity. Yet we have come a long way since the Romans attempted to mitigate the effects of plant disease by worshipping and honoring the god Robigus [1]. Books in the Middle Ages by Islamic and European scholars described various plant diseases and even proposed particular disease management strategies [1]. Surprisingly, the causes of plant diseases remained a matter of debate over a long period. It took Henri-Louis Duhamel du Monceau's elegant demonstration in his 1728 “Explication Physique” paper that a “contagious” fungus was responsible for a saffron crocus disease to usher in an era of documented scientific inquiry [2]. Confusion and debate about the exact nature of the causal agents of plant diseases continued until the 19th century, which not only saw the first detailed analyses of plant pathogens but also provided much-needed insight into the mechanisms of plant disease. An example of this is Anton de Bary's demonstration that a “fungus” is a cause, not a consequence, of plant disease [3]. This coming of age of plant pathology was timely. In the 19th century, severe plant disease epidemics hit Europe and caused economic and social upheaval. These epidemics were not only widely covered in the press but also recognized as serious political issues by governments [1], [4]–[6]. Many of the diseases, including late blight of potato, powdery and downy mildew of grapevine, as well as phylloxera, were due to exotic introductions from the Americas and elsewhere. These and subsequent epidemics motivated scientific investigations into crop breeding and plant disease management that developed into modern plant pathology science over the 20th century.
Nowadays, our understanding of plant pathogens and the diseases they cause greatly benefits from molecular genetics and genomics. All aspects of plant pathology, from population biology and epidemiology to mechanistic research, are impacted. The polymerase chain reaction (PCR) first enabled access to plant pathogen DNA sequences from historical specimens deposited in herbaria [7]–[9]. Historical records in combination with herbarium specimens have turned out to provide powerful tools for understanding the course of past plant epidemics. Recently, thanks to new developments in DNA sequencing technology, it has become possible to reconstruct the genomes of plant pathogens in herbaria [10], [11]. In this article, we first summarize how whole genome analysis of ancient DNA has been recently used to reconstruct the 19th-century potato-blight epidemic that rapidly spread throughout Europe and triggered the Irish potato famine. We then discuss the exciting prospects offered by the emergence of the discipline of ancient plant pathogen genomics.
What Is Ancient DNA?
DNA retrieved from historic and prehistoric sources such as museum specimens, archaeological finds, and fossil remains is collectively known as ancient DNA (aDNA) [12]. The aDNA field goes back to the 1980s. With the invention of PCR, aDNA research came into its own, and PCR-based methods were its mainstay for 20 years. Early on, mycologists recognized the value of herbaria in storage of pathogen aDNA and used PCR to decode fragments of aDNA from dried herbarium specimens [7]–[9], [13]. In contrast to DNA extracted from fresh tissue, aDNA comes, in general, in tiny amounts, is highly fragmented, and contains chemical modifications [12]. Many of the difficulties resulting from these characteristics have been recently overcome, thanks to the advent of high-throughput sequencing and the development of new library-based retrieval of aDNA fragments without relying on direct amplification by PCR [14], [15]. Nowadays, it is possible to sequence complete genomes from organisms that went extinct tens of thousands of years ago, providing unique insight into their history and evolution. For example, the sequencing of the complete genomes of two archaic hominins (Neanderthals and Denisovans) has opened a window to the past and profoundly changed our views on human origins [16]–[18].
Such a historic perspective is also starting to arise for infectious diseases. Past epidemics and disease emergence have been reconstructed by high-throughput sequencing of genomic fragments of ancient pathogens from human skeletal remains [19]. The complete genome sequences of medieval bacterial pathogens have started to answer questions about the origin and history of infectious human diseases such as bubonic plague and leprosy [20]–[22]. Such whole genome sequence analyses of historical pathogens have recently been extended to plant pathogens [10], [11], [23]. Using herbarium material, it was possible to describe not only sequence variation in 19th-century samples of the pathogen Phytophthora infestans, which triggered the Irish potato famine of 1845 to 1847, but also genomic variation in its potato host. The surprisingly high quality of aDNA in these samples suggests that the millions of dried plant samples that are stored in herbaria throughout the world hold great promise for the future study of past plant epidemics.
Using aDNA to Date Divergence Events
To understand disease dynamics, it is of particular importance to accurately date major events, such as epidemics' emergence and reemergence, and sudden changes in genetic diversity. These dated events can then be correlated with a timeline of historical and socioeconomic information. Fortunately, the genetic information obtained from aDNA sequences provides a unique opportunity to date divergence times in a phylogenetic tree. Genomes from historic samples will accumulate fewer nucleotide substitutions than modern samples, which have continued to accumulate substitutions for many more generations [18], [22]. This can be used to calculate substitution rates and subsequently divergence times using the sample dates as tip calibration points in a Bayesian phylogenetic framework [24], [25]. Herbaria samples usually contain collection date and geographic location, which makes them ideal calibration points in a phylogenetic tree [10]. Historic and modern samples that are more spread out in time yield more accurate calculations of divergence times [24].
Potato Late Blight: From the Irish Potato Famine to Modern Epidemics
The oomycete plant pathogen P. infestans causes late blight, the most destructive disease of potatoes and a major disease of tomatoes. Ever since the Irish potato famine in the 1840s, P. infestans has remained one of the more serious threats to food production, resulting in losses that add up to enough crop to feed hundreds of millions of people [26]. Similar to other oomycetes, P. infestans has a complex life cycle that includes both asexual and sexual phases. The ability of P. infestans to generate large amounts of asexual spores is a major determinant of its success as a destructive pathogen [27]. In agricultural systems, asexual reproduction is often dominant, with individual genotypes taking over large portions of the population and occasionally resulting in explosive epidemics (Figure 1) [28], [29]. Sexual reproduction is integral to the life cycle of P. infestans at its center of diversity in Mexico. In some agricultural systems, notably in the Netherlands and the Nordic countries, sexual reproduction is also common, generating an abundance of new pathogen genotypes [30], [31]. P. infestans genotypes with favorable phenotypes, such as increased virulence and spore production, can arise from pockets of sexual reproduction to become invasive when they spread to vulnerable potato production areas [28]. One example, illustrated in Figure 1, is clonal lineage 13_A2, an aggressive genotype that emerged in Europe in the early 21st century, rapidly displaced other genotypes [28], and more recently became pandemic with severe outbreaks in India [32] and China [33].
Fig. 1. The rise and fall of P. infestans lineages in the British Isles. 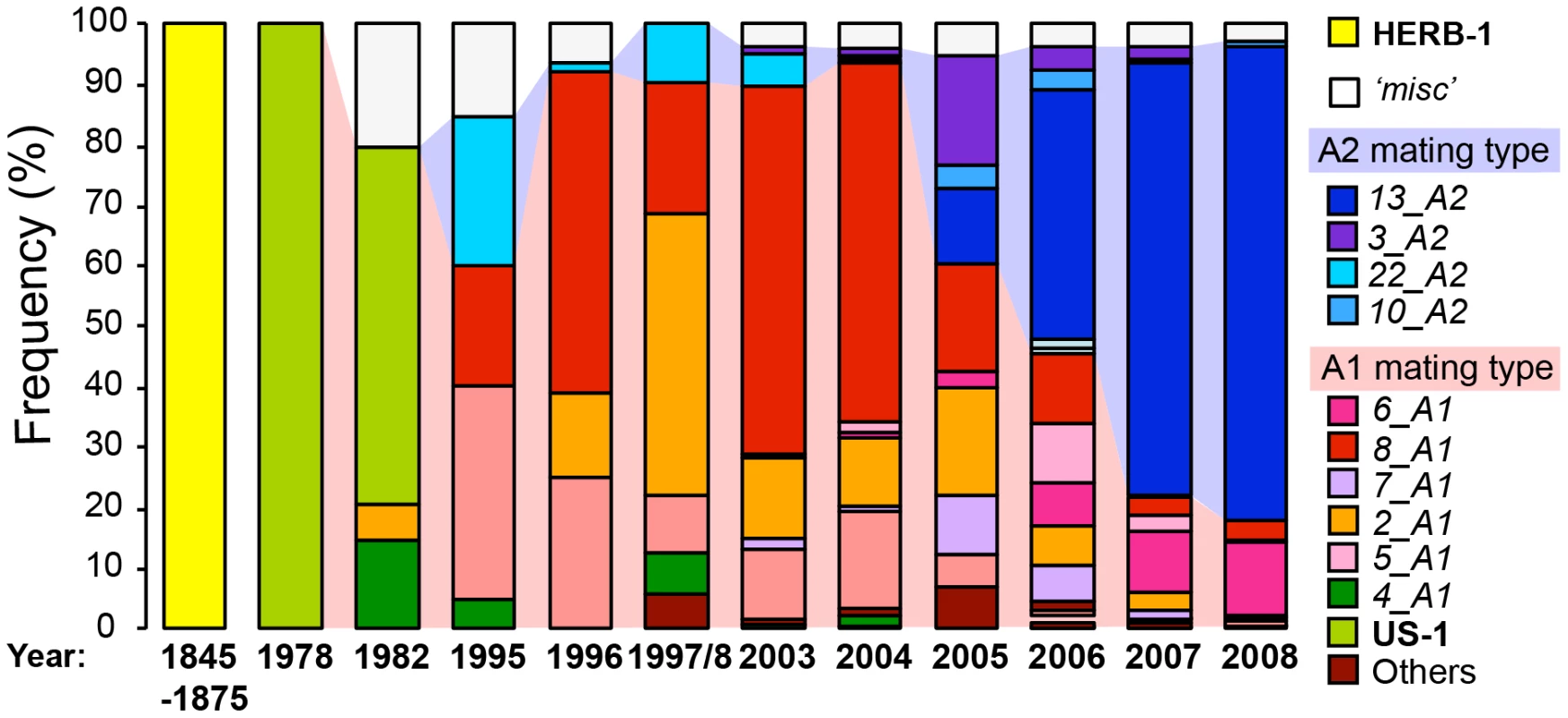
Frequency of genotypes over the years from the potato blight outbreaks in the British Isles (adapted from Cooke et al. (2012) [28]). Isolates of genotypes that occurred at a low frequency in a single year were grouped under the category termed ‘misc’. Two mating types of P. infestans, A1 and A2, are necessary for sexual reproduction. The shading between the bars indicates the proportion of A1- and A2-mating-type isolates, with pink referring to the A1 mating type and blue to A2. The data of 1845–1875 and 1978 were from Yoshida et al. (2013) [10] and Fry et al. (1992) [39], respectively. A template for the figure was kindly provided by Dr. David Cooke, James Hutton Institute, Dundee [28]. Reconstructing the 19th-Century Potato Blight Pandemic
A widely accepted hypothesis is that P. infestans has originated from Toluca Valley, Mexico, where it naturally infects and coevolves with wild Solanum relatives of the potato [31]. Although the Spanish introduced the potato to Europe in the 16th century, the crop remained free of late blight until the mid-19th century. In 1845, P. infestans finally reached Europe. Potato blight was first detected in Belgium at the end of June 1845. Over the summer, the disease rapidly spread over the European continent to reach the British Isles [4]. In Ireland, socioeconomic and political circumstances coincided to turn the disease into a tragic and deadly affair—the Great Famine triggered by potato late blight killed around one million people and forced even more to emigrate.
The identity of the P. infestans strain that caused the 19th-century epidemic and its relationship to modern isolates have long been controversial topics, even though historical records suggest that the strain(s) that first caused late blight in North America in 1843 moved to Europe in 1845 [4]. Based on mitochondrial markers in modern strains, Goodwin and colleagues [34] proposed in 1994 that a clonal lineage known as US-1, which dominated the world populations until the 1970s, was a direct descendant of the strain that caused the North American and European epidemics of the 1840s (Figure 2A). Ristaino and colleagues [8], [9] subsequently used PCR analyses to monitor polymorphic nucleotide positions in mitochondrial DNA from herbarium samples. Based on the results, the authors proposed a different scenario, that the 19th-century strain was different from the US-1 lineage, and proposed that the two genotypes caused unrelated pandemics that originated in South America (Figure 2B).
Fig. 2. Different models proposed for 19th-century P. infestans pandemics. 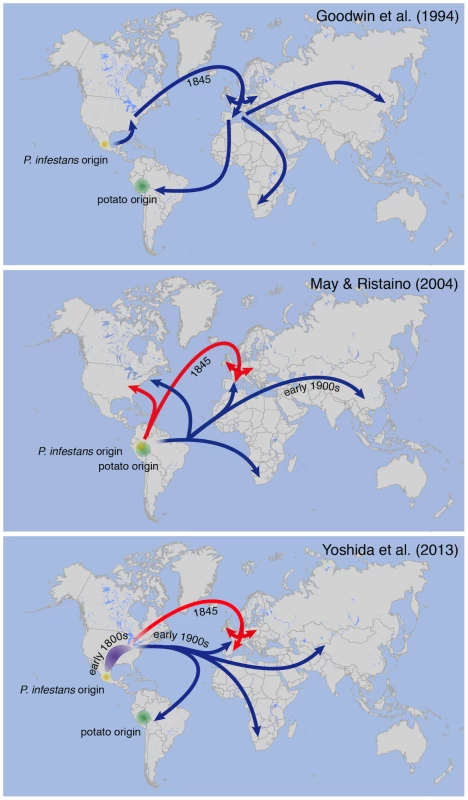
Migration paths of P. infestans lineages proposed by different authors [34], [8], [10]. The bottom panel of Figure 2 was adapted from Yoshida et al. (2013) [10] and reflects the most likely scenario based on the current data. The red arrows refer to the HERB-1 lineage. To resolve these contradictory findings, two recent studies performed genome analyses of P. infestans aDNA obtained from 19th-century herbarium material [10], [11]. Yoshida and colleagues (2013) [10] sequenced aDNA from 11 historical strains as well as 15 modern strains to various levels of genome coverage. Their analyses revealed that the 19th-century epidemic was caused by a single genotype, HERB-1, that was widespread in Europe from 1845 to 1877. Phylogenetic analyses supported HERB-1 being distinct from the 20th-century US-1 genotype but HERB-1 and US-1 being nevertheless closely related to each other (Figure 3). Yoshida et al. proposed that the 19th - and 20th-century pandemics were caused by distinct clonal lineages, HERB-1 and US-1, but that both genotypes most likely originated from a secondary metapopulation rather than from the Mexican center of origin (bottom panel of Figure 2). This interpretation was motivated by the high degree of P. infestans genetic diversity and preponderance of sexual reproduction in Mexico, making it less likely that closely related genotypes would have emerged independently and successfully spread from this region. The location of the metapopulation and origin of HERB-1 remain unclear. A search for the HERB-1 genotype in the past and modern American populations of P. infestans will help to answer these questions.
Fig. 3. The 19th-century P. infestans belong to a single clonal lineage, HERB-1. 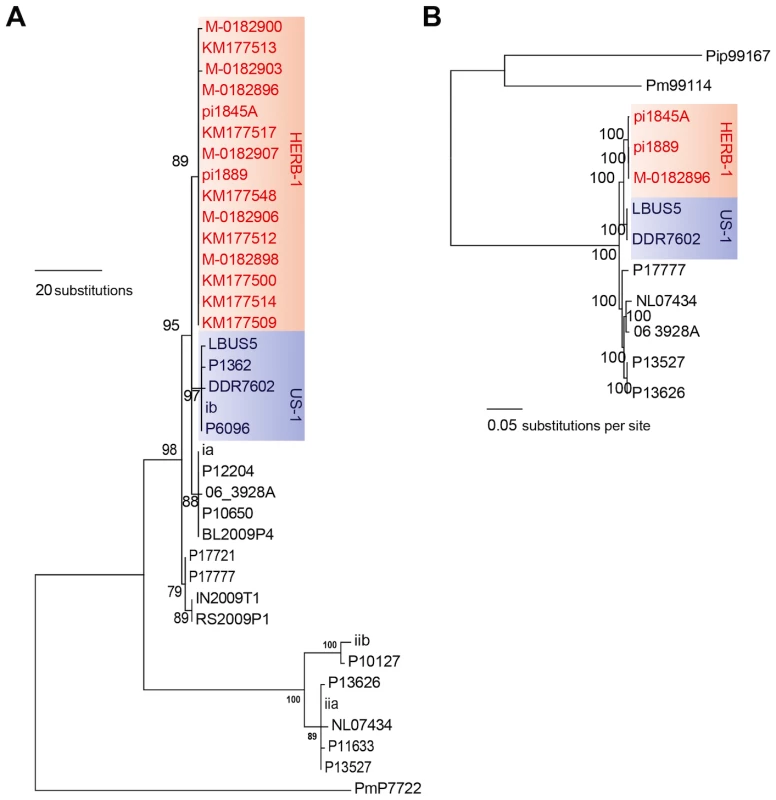
(A) Maximum-parsimony tree of complete mtDNA genomes. Sites with less than 90% information were not considered, leaving 24,526 sites in the final dataset. (B) Maximum-likelihood tree based on nuclear SNPs. Only sites with 100% information were considered, leaving 2,359,452 sites in the final dataset. Numbers at branches of both trees indicate bootstrap support (100 replicates), and scales indicate genetic distance. PmP7722 and Pm99114 are P. mirabilis. Pip99167 is P. ipomoeae. These three strains were used as outgroup species. The details of the tree construction were described in Yoshida et al. (2013) [10]. In an independent study, Martin and colleagues [11] reported on the genomes of P. infestans from another five 19th-century herbarium samples. Based on the observed number of single-nucleotide polymorphisms (SNPs) between two samples collected in 1845 and 1889, they proposed an alternative scenario, with multiple clonal lineages having been introduced to and spread over Europe. This study, however, did not address directly the relationship of the 19th-century P. infestans sequences to the US-1 genotype. To compare the two herbarium studies [10], [11], we have estimated genetic divergence for the high-coverage genome sequences of modern and historical samples from both studies and constructed phylogenetic trees. Genetic distances among the historical samples fell within the distance between sibling isolates of two modern clonal lineages: US-1 and the South American EC-1 (Table 1). Both mitochondria and nuclear trees show that the historic samples group into one single clade (Figure 3A, 3B). Based on these results, we conclude that the historic genome sequences reported in both recent studies [10], [11] are consistent with the hypothesis that a single clonal lineage, HERB-1, caused the 19th-century potato-blight pandemic (Figure 3A, 3B).
Tab. 1. Genetic distance between individual isolates of clonal lineages HERB-1, US-1, and EC-1 of P. infestans. 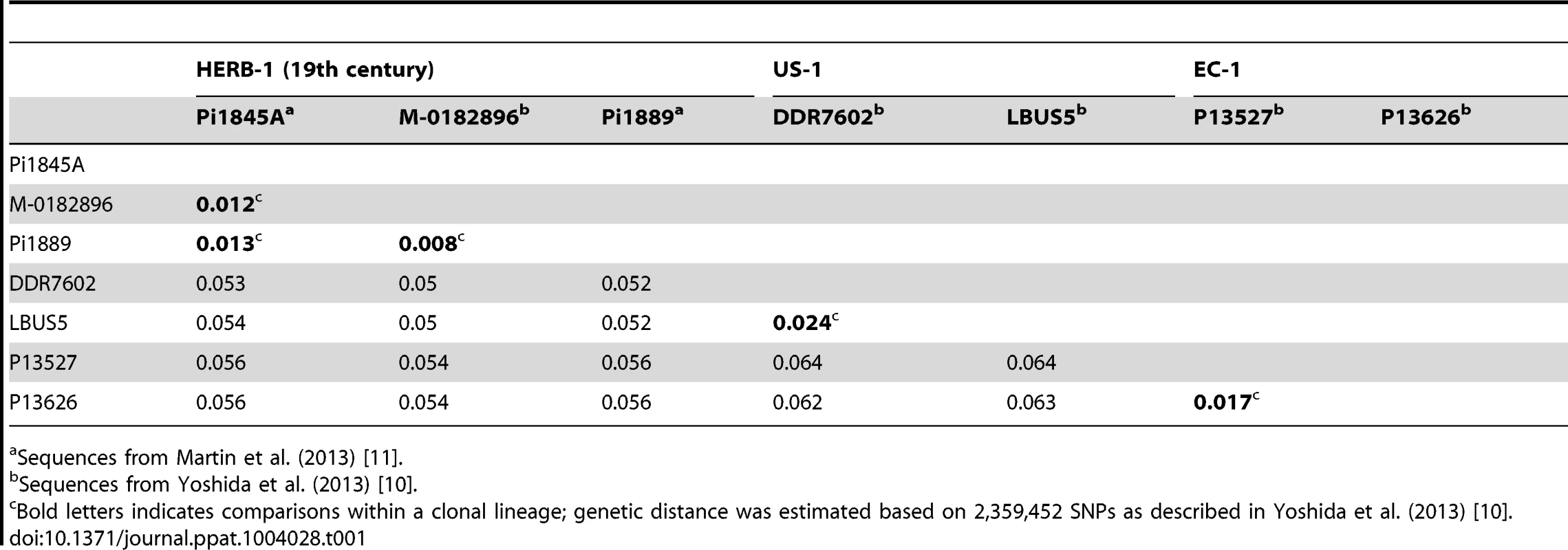
Sequences from Martin et al. (2013) [11]. Has Modern Potato Breeding Driven HERB-1 to Extinction?
An obvious question is whether P. infestans HERB-1 was so devastating because it was extraordinarily virulent. Several different lines of argument suggest that this was not the case. Rather, it was the extreme blight susceptibility of the potato cultivars that were grown in the 19th century, such as the Irish Lumper potato, that was responsible for the unusual severity of the disease [4], [35]. Later on, breeding for potato-blight resistance took off at the beginning of the 20th century based on introgression of disease resistance (R) genes from wild relatives of the potato. Among the first R genes to be bred, the R1, R2, R3a, and R4 genes were introduced into cultivated potatoes from the wild Mexican species Solanum demissum [35], [36]. These R genes encode immune receptors that detect specific avirulence effectors in P. infestans [37]. As expected from the historical records of plant breeding, HERB-1 carries isoforms of several pathogen effectors in their avirulence configuration [4], meaning that they would have triggered a resistance reaction by the R1, R2, R3a, and R4 genes as well as most of the other R genes that are present in modern potato cultivars. This indicates that the HERB-1 genotype of P. infestans would probably be incapable of infecting most modern potato varieties. Indeed, HERB-1 could not be identified among the modern isolates examined to date. However, an exhaustive survey of hundreds of P. infestans genotypes from all corners of the globe is needed before a definitive conclusion about HERB-1 extinction is reached [38]. Nonetheless, a plausible scenario is that the HERB-1 clonal lineage was displaced by US-1 with the emergence of modern potato breeding. Genome analyses of P. infestans population from the first half of the 20th century will help to shed light on this question.
An important insight from the herbarium analyses is that the displacement of HERB-1 by US-1 is reminiscent of modern population dynamics (Figure 1). For example, in the British Isles, different P. infestans genotypes have dominated the population since the 1970s (Figure 1) [28]. Waves of P. infestans clonal lineages rise and fall for a variety of ecological and agricultural reasons, including breeding of disease resistance genes, chemical treatments, and climate fluctuations [28], [31], [39].
Today, with both the A1 and A2 mating types of P. infestans coexisting in regional pockets outside of Mexico, the trend has accelerated with a notable increase in genetic diversity compared to the period before the 1980s when only the A1 mating type occurred outside of Mexico [31], [39].
Perspectives in Archaeogenomics of Plant Pathogens
Genome analyses of P. infestans aDNA have demonstrated that the potential of archaeogenomics to solve important questions in history and evolution does not apply to only humans and their pathogens but also to plant pathology. The budding field of archaeogenomics holds particular promise for plant pathogens because of the excellent preservation of genomic DNA in dried plant samples stored in herbaria. Thus, herbaria are hidden treasures, serving as unexploited archives of plant pathogen and plant genomes. Beyond the potato late blight, there are numerous interesting targets for investigation of plant pathogens. European herbaria hold over 800,000 specimens of rust fungi [40], a group of plant pathogens that are a recurring threat to world agriculture [41]. Another, well-sampled plant pathogen is the oomycete Plasmopara viticola, which caused epidemics of downy mildew on grapevine [5]. Mining these and other historic herbarium samples for plant pathogen genomes should inform past population and evolutionary dynamics of important plant pathogens, ultimately helping us to better prepare for future plant epidemics.
Zdroje
1. Large EC (1940) The Advance of Fungi. New York: Dover Publications Inc. 488 p.
2. DuhamelHL (1728) Explication physique d 'une maladie qui fait périr plusieurs plantes dans le Gatinois et particulièrement le safran. Mem Acad Roy Sci 100–112.
3. de BaryA (1863) Recherches sur le développement de quelques champignons parasites. Ann Sci Nat Bot Sér 4, 20 : 5–148.
4. BourkePMA (1964) Emergence of potato blight, 1843–46. Nature 203 : 805–808.
5. Viennot-Bourgin G (1981) History and importance of downy mildews. In: Spencer DM, editor. The Downy Mildews. London, United Kingdom: Academic Press. pp. 1–14.
6. PetersonPD (1992) James E. Teschemacher and the cause and management of potato blight in the United States. Plant Dis 76 : 754–756.
7. RistainoJB (1998) The importance of archival and herbarium materials in understanding the role of oospores in late blight epidemics of the past. Phytopathology 88 : 1120–1130.
8. RistainoJB, GrovesCT, ParraGR (2001) PCR amplification of the Irish potato famine pathogen from historic specimens. Nature 411 : 695–697.
9. MayKJ, RistainoJB (2004) Identity of the mtDNA haplotype(s) of Phytophthora infestans in historical specimens from the Irish potato famine. Mycol Res 108 : 471–479.
10. YoshidaK, SchuenemannV, CanoC, PaisP, MishraB, et al. (2013) The rise and fall of the Phytophthora infestans lineage that triggered the Irish potato famine. eLife 2: e00731.
11. MartinMD, CappelliniE, SamaniegoJA, ZepedaML, CamposPF, et al. (2013) Reconstructing genome evolution in historic samples of the Irish potato famine pathogen. Nat Commun 4 : 2172.
12. PääboS, PoinarH, SerreD, Jaenicke-DespresV, HeblerJ, et al. (2004) Genetic analyses from ancient DNA. Annu Rev Genetics 38 : 645–679.
13. BrunsTD, FogelR, TaylorJW (1990) Amplification and sequencing of DNA from fungal herbarium specimens. Mycologia 82 : 175–184.
14. GreenRE, KrauseJ, PtakSE, BriggsAW, RonanMT, et al. (2006) Analysis of one million base pairs of Neanderthal DNA. Nature 444 : 330–336.
15. PoinarHN, SchwarzC, QiJ, ShapiroB, MacpheeRD, et al. (2006) Metagenomics to paleogenomics: large-scale sequencing of mammoth DNA. Science 311 : 392–394.
16. GreenRE, KrauseJ, BriggsAW, MaricicT, StenzelU, et al. (2010) A draft sequence of the Neandertal genome. Science 328 : 710–722.
17. ReichD, GreenRE, KircherM, KrauseJ, PattersonN, et al. (2010) Genetic history of an archaic hominin group from Denisova Cave in Siberia. Nature 468 : 1053–1060.
18. MeyerM, KircherM, GansaugeMT, LiH, RacimoF, et al. (2012) A high-coverage genome sequence from an archaic Denisovan individual. Science 338 : 222–226.
19. SchuenemannVJ, BosK, DeWitteS, SchmedesS, JamiesonJ, et al. (2011) Targeted enrichment of ancient pathogens yielding the pPCP1 plasmid of Yersinia pestis from victims of the Black Death. Proc Natl Acad Sci USA 108: E746–E752.
20. BosKI, SchuenemannVJ, GoldingGB, BurbanoHA, WaglechnerN, et al. (2011) A draft genome of Yersinia pestis from victims of the Black Death. Nature 478 : 506–510.
21. BosKI, StevensP, NieseltK, PoinarHN, DewitteSN, et al. (2012) Yersinia pestis: new evidence for an old infection. PLoS One 7: e49803.
22. KrauseJ, FuQ, GoodJM, ViolaB, ShunkovMV, et al. (2010) The complete mitochondrial DNA genome of an unknown hominin from southern Siberia. Nature 464 : 894–897.
23. GibbonsA (2013) On the trail of ancient killers. Science 340 : 1278–1282.
24. DrummondAJ, NichollsGK, RodrigoAG, SolomonW (2002) Estimating mutation parameters, population history and genealogy simultaneously from temporally spaced sequence data. Genetics 161 : 1307–1320.
25. RambautA (2000) Estimating the rate of molecular evolution: incorporating non-contemporaneous sequences into maximum likelihood phylogenies. Bioinformatics 16 : 395–399.
26. FisherMC, HenkDA, BriggsCJ, BrownsteinJS, MadoffLC, et al. (2012) Emerging fungal threats to animal, plant and ecosystem health. Nature 484 : 186–194.
27. JudelsonHS, BlancoFA (2005) The spores of Phytophthora: weapons of the plant destroyer. Nat Rev Microbiol 3 : 47–58.
28. CookeDE, CanoLM, RaffaeleS, BainRA, CookeLR, et al. (2012) Genome analyses of an aggressive and invasive lineage of the Irish potato famine pathogen. PLoS Pathog 8: e1002940.
29. FryWE, McGrathMT, SeamanA, ZitterTA, McLeodA, et al. (2013) The 2009 late blight pandemic in Eastern USA. Plant Dis 97 : 296–306.
30. SjöholmL, AnderssonBr, HögbergN, WidmarkA-K, YuenJ (2013) Genotypic diversity and migration patterns of Phytophthora infestans in the Nordic countries. Fungal Biol 117 : 722–730.
31. Fry WE, Grünwald NJ, Cooke DEL, McLeod A, Forbes GA, et al.. (2008) Population genetics and population diversity of Phytophthora infestans. In: Lamour K, Kamoun S, editors. Oomycete Genetics and Genomics. Hoboken (New Jersey): John Wiley & Sons, Inc. pp. 139–164.
32. ChowdappaP, KumarNBJ, MadhuraS, KumarMSP, MyersKL, et al. (2013) Emergence of 13_A2 Blue Lineage of Phytophthora infestans was Responsible for Severe Outbreaks of Late Blight on Tomato in South-West India. J Phytopathol 161 : 49–58.
33. LiY, van der LeeT, ZhuJH, JinGH, LanCZ, et al. (2013) Population structure of Phytophthora infestans in China – geographic clusters and presence of the EU genotype Blue_13. Plant Pathol 62 : 932–942.
34. GoodwinSB, CohenBA, FryWE (1994) Panglobal distribution of a single clonal lineage of the Irish potato famine fungus. Proc Natl Acad Sci USA 91 : 11591–11595.
35. TurnerRS (2005) After the famine: Plant pathology, Phytophthora infestans, and the late blight of potatoes, 1845–1960. Hist Stud Phys Biol Sci 35 : 341–370.
36. Hawkes JG (1990). The Potato: Evolution, Biodiversity and Genetic Resources. Washington, D.C.: Smithsonian Institution Press. 259 p.
37. VleeshouwersVG, RaffaeleS, VossenJH, ChampouretN, OlivaR, et al. (2011) Understanding and exploiting late blight resistance in the age of effectors. Annu Rev Phytopathol 49 : 507–531.
38. BirchPRJ, CookeDE (2013) The early days of late blight. eLife 2: e00954.
39. FryWE, GoodwinSB, MatuszakJM, SpielmanLJ, MilgroomMG (1992) Population genetics and intercontinental migrations of Phytophthora infestans. Annu Rev Phytopathol 30 : 107–129.
40. HelferS, BerndtR, DenchevCM, MoriccaS, ScheuerC, et al. (2011) A call for renewed and pan-European strategic effort on the taxonomy of rust fungi (Uredinales). Mycol Balc 8 : 78–80.
41. SinghRP, HodsonDP, Huerta-EspinoJ, JinY, BhavaniS, et al. (2011) The emergence of Ug99 races of the stem rust fungus is a threat to world wheat production. Annu Rev Phytopathol 49 : 465–481.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Affinity Proteomics Reveals Elevated Muscle Proteins in Plasma of Children with Cerebral MalariaČlánek The Transcriptional Activator LdtR from ‘ Liberibacter asiaticus’ Mediates Osmotic Stress ToleranceČlánek Complement-Related Proteins Control the Flavivirus Infection of by Inducing Antimicrobial PeptidesČlánek Fungal Chitin Dampens Inflammation through IL-10 Induction Mediated by NOD2 and TLR9 ActivationČlánek Parasite Fate and Involvement of Infected Cells in the Induction of CD4 and CD8 T Cell Responses to
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 4- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
-
Všechny články tohoto čísla
- , , , Genetic Variability: Cryptic Biological Species or Clonal Near-Clades?
- Early Mortality Syndrome Outbreaks: A Microbial Management Issue in Shrimp Farming?
- Wormholes in Host Defense: How Helminths Manipulate Host Tissues to Survive and Reproduce
- Plastic Proteins and Monkey Blocks: How Lentiviruses Evolved to Replicate in the Presence of Primate Restriction Factors
- The 2010 Cholera Outbreak in Haiti: How Science Solved a Controversy
- Affinity Proteomics Reveals Elevated Muscle Proteins in Plasma of Children with Cerebral Malaria
- Noncanonical Role for the Host Vps4 AAA+ ATPase ESCRT Protein in the Formation of Replicase
- Efficient Parvovirus Replication Requires CRL4-Targeted Depletion of p21 to Prevent Its Inhibitory Interaction with PCNA
- Host-to-Pathogen Gene Transfer Facilitated Infection of Insects by a Pathogenic Fungus
- The Transcriptional Activator LdtR from ‘ Liberibacter asiaticus’ Mediates Osmotic Stress Tolerance
- Coxsackievirus B Exits the Host Cell in Shed Microvesicles Displaying Autophagosomal Markers
- TCR Affinity Associated with Functional Differences between Dominant and Subdominant SIV Epitope-Specific CD8 T Cells in Rhesus Monkeys
- Coxsackievirus-Induced miR-21 Disrupts Cardiomyocyte Interactions via the Downregulation of Intercalated Disk Components
- Ligands of MDA5 and RIG-I in Measles Virus-Infected Cells
- Kind Discrimination and Competitive Exclusion Mediated by Contact-Dependent Growth Inhibition Systems Shape Biofilm Community Structure
- Structural Differences Explain Diverse Functions of Actins
- HSCARG Negatively Regulates the Cellular Antiviral RIG-I Like Receptor Signaling Pathway by Inhibiting TRAF3 Ubiquitination Recruiting OTUB1
- Vaginitis: When Opportunism Knocks, the Host Responds
- Complement-Related Proteins Control the Flavivirus Infection of by Inducing Antimicrobial Peptides
- Fungal Chitin Dampens Inflammation through IL-10 Induction Mediated by NOD2 and TLR9 Activation
- Microbial Pathogens Trigger Host DNA Double-Strand Breaks Whose Abundance Is Reduced by Plant Defense Responses
- Alveolar Macrophages Are Essential for Protection from Respiratory Failure and Associated Morbidity following Influenza Virus Infection
- An Interaction between Glutathione and the Capsid Is Required for the Morphogenesis of C-Cluster Enteroviruses
- Concerted Spatio-Temporal Dynamics of Imported DNA and ComE DNA Uptake Protein during Gonococcal Transformation
- Potent Dengue Virus Neutralization by a Therapeutic Antibody with Low Monovalent Affinity Requires Bivalent Engagement
- Regulation of Human T-Lymphotropic Virus Type I Latency and Reactivation by HBZ and Rex
- Functionally Redundant RXLR Effectors from Act at Different Steps to Suppress Early flg22-Triggered Immunity
- The Pathogenic Mechanism of the Virulence Factor, Mycolactone, Depends on Blockade of Protein Translocation into the ER
- Role of Calmodulin-Calmodulin Kinase II, cAMP/Protein Kinase A and ERK 1/2 on -Induced Apoptosis of Head Kidney Macrophages
- An Overview of Respiratory Syncytial Virus
- First Experimental Model of Enhanced Dengue Disease Severity through Maternally Acquired Heterotypic Dengue Antibodies
- Binding of Glutathione to Enterovirus Capsids Is Essential for Virion Morphogenesis
- IFITM3 Restricts Influenza A Virus Entry by Blocking the Formation of Fusion Pores following Virus-Endosome Hemifusion
- Parasite Fate and Involvement of Infected Cells in the Induction of CD4 and CD8 T Cell Responses to
- Deficient IFN Signaling by Myeloid Cells Leads to MAVS-Dependent Virus-Induced Sepsis
- Pernicious Pathogens or Expedient Elements of Inheritance: The Significance of Yeast Prions
- The HMW1C-Like Glycosyltransferases—An Enzyme Family with a Sweet Tooth for Simple Sugars
- The Expanding Functions of Cellular Helicases: The Tombusvirus RNA Replication Enhancer Co-opts the Plant eIF4AIII-Like AtRH2 and the DDX5-Like AtRH5 DEAD-Box RNA Helicases to Promote Viral Asymmetric RNA Replication
- Mining Herbaria for Plant Pathogen Genomes: Back to the Future
- Inferring Influenza Infection Attack Rate from Seroprevalence Data
- A Human Lung Xenograft Mouse Model of Nipah Virus Infection
- Mast Cells Expedite Control of Pulmonary Murine Cytomegalovirus Infection by Enhancing the Recruitment of Protective CD8 T Cells to the Lungs
- Cytosolic Peroxidases Protect the Lysosome of Bloodstream African Trypanosomes from Iron-Mediated Membrane Damage
- Abortive T Follicular Helper Development Is Associated with a Defective Humoral Response in -Infected Macaques
- JC Polyomavirus Infection Is Strongly Controlled by Human Leucocyte Antigen Class II Variants
- Cationic Antimicrobial Peptides Promote Microbial Mutagenesis and Pathoadaptation in Chronic Infections
- Estimating the Fitness Advantage Conferred by Permissive Neuraminidase Mutations in Recent Oseltamivir-Resistant A(H1N1)pdm09 Influenza Viruses
- Progressive Accumulation of Activated ERK2 within Highly Stable ORF45-Containing Nuclear Complexes Promotes Lytic Gammaherpesvirus Infection
- Caspase-1-Like Regulation of the proPO-System and Role of ppA and Caspase-1-Like Cleaved Peptides from proPO in Innate Immunity
- Is Required for High Efficiency Viral Replication
- Modified Vaccinia Virus Ankara Triggers Type I IFN Production in Murine Conventional Dendritic Cells via a cGAS/STING-Mediated Cytosolic DNA-Sensing Pathway
- Evidence That Bank Vole PrP Is a Universal Acceptor for Prions
- Rapid Response to Selection, Competitive Release and Increased Transmission Potential of Artesunate-Selected Malaria Parasites
- Inactivation of Genes for Antigenic Variation in the Relapsing Fever Spirochete Reduces Infectivity in Mice and Transmission by Ticks
- Exposure-Dependent Control of Malaria-Induced Inflammation in Children
- A Neutralizing Anti-gH/gL Monoclonal Antibody Is Protective in the Guinea Pig Model of Congenital CMV Infection
- The Apical Complex Provides a Regulated Gateway for Secretion of Invasion Factors in
- A Highly Conserved Haplotype Directs Resistance to Toxoplasmosis and Its Associated Caspase-1 Dependent Killing of Parasite and Host Macrophage
- A Quantitative High-Resolution Genetic Profile Rapidly Identifies Sequence Determinants of Hepatitis C Viral Fitness and Drug Sensitivity
- Histone Deacetylase Inhibitor Romidepsin Induces HIV Expression in CD4 T Cells from Patients on Suppressive Antiretroviral Therapy at Concentrations Achieved by Clinical Dosing
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The 2010 Cholera Outbreak in Haiti: How Science Solved a Controversy
- , , , Genetic Variability: Cryptic Biological Species or Clonal Near-Clades?
- Efficient Parvovirus Replication Requires CRL4-Targeted Depletion of p21 to Prevent Its Inhibitory Interaction with PCNA
- An Overview of Respiratory Syncytial Virus
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



