-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
JC Polyomavirus Infection Is Strongly Controlled by Human Leucocyte Antigen Class II Variants
JC virus infection can lead to progressive multifocal leukoencephalopathy in individuals with a compromised immune system, such as during HIV infections or when treated with immunosuppressive or immunomodulating therapies. Progressive multifocal leukoencephalopathy is a rare but potentially fatal disease characterized by progressive damage of the brain white matter at multiple locations. It is therefore of importance to understand the host genetic control of response to JC virus in order to identify patients that can be treated with immunomodulating therapies, common treatments for autoimmune diseases, without increased risk for progressive multifocal leukoencephalopathy. This may also lead to development of preventative or curative anti-JC virus therapies. We here identify genetic variants being associated with JC virus antibody development; a negative association with the human leucocyte antigen DRB1*15-DQA1*01 : 02-DQB1*06 : 02 haplotype and a positive association with the DRB1*13-DQA1*01 : 03-DQB1*06 : 03 haplotype among controls and patients with multiple sclerosis from Scandinavia. We confirmed the associations in patients with multiple sclerosis from Germany. These associations between JC virus antibody response and human leucocyte antigens imply that CD4+ T cells are crucial in the immune defence and lay the ground for development of therapy and prevention.
Published in the journal: . PLoS Pathog 10(4): e32767. doi:10.1371/journal.ppat.1004084
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004084Summary
JC virus infection can lead to progressive multifocal leukoencephalopathy in individuals with a compromised immune system, such as during HIV infections or when treated with immunosuppressive or immunomodulating therapies. Progressive multifocal leukoencephalopathy is a rare but potentially fatal disease characterized by progressive damage of the brain white matter at multiple locations. It is therefore of importance to understand the host genetic control of response to JC virus in order to identify patients that can be treated with immunomodulating therapies, common treatments for autoimmune diseases, without increased risk for progressive multifocal leukoencephalopathy. This may also lead to development of preventative or curative anti-JC virus therapies. We here identify genetic variants being associated with JC virus antibody development; a negative association with the human leucocyte antigen DRB1*15-DQA1*01 : 02-DQB1*06 : 02 haplotype and a positive association with the DRB1*13-DQA1*01 : 03-DQB1*06 : 03 haplotype among controls and patients with multiple sclerosis from Scandinavia. We confirmed the associations in patients with multiple sclerosis from Germany. These associations between JC virus antibody response and human leucocyte antigens imply that CD4+ T cells are crucial in the immune defence and lay the ground for development of therapy and prevention.
Introduction
Progressive multifocal leukoencephalopathy (PML) was first described neuropathologically during the fifties by Karl Erik Åström [1]. It took until 1971 when JC virus (JCV) was isolated from brain tissue of a patient with PML, since then JCV was accepted as the causative agent of PML [2]. PML used to be a rare demyelinating disease of the central nervous system, mainly seen in patients with lymphoproliferative disease or AIDS. Now several different drugs that interfere with immune functions, such as natalizumab, efalizumab, mycophenolate mofetil, fumaric acid, rituximab, tacrolimus, and possibly azathioprine, cyclosporine and cyclophosphamide have been associated with an increased risk of developing PML. For natalizumab and efalizumab the strongest associations were seen in patients without an underlying disease that predispose for PML itself [3]–[7]. Thus, it is of major importance to develop measures to prevent or treat the condition, including understanding of factors allowing persons to acquire the virus, as carriers, a requisite for later risk for PML.
In patients with multiple sclerosis (MS) treated with natalizumab previous immunosuppressive therapy, an increased duration of therapy, and the positive detection of anti-JCV IgG antibodies as surrogate for the infection with JCV have been established as risk factors for PML [8]–[12]. The anti-JCV antibody status in MS patients is determined by a commercial two step-ELISA. Around 40–50% of the adults are anti-JCV antibody negative [11], [13]–[15]. The cut-off of the commercial assay have been validated in large multicentre cohorts of MS patients with data on JC viruria available, and the false negative rate (sero-negative, but DNA excretion in urine) was estimated with around 2.5% [9]–[11]. In contrast, a recent study that also measured JCV excretion in urine in a comparably small study population (n = 67) indicated a much higher false negative rate of 37%, however, these cases displayed considerably lower JCV DNA copy numbers in the urine. Hypothetically, a vast majority of persons might be exposed to an ubiquitous virus such as JCV, proposed as contamination marker for human excretions, [16] but differ in replicative activity of a persistent asymptomatic infection, and potentially connected to this, the individual level of immune response to the virus. This view would fit with recent serological observations of a continuous anti-JCV reactivity in larger populations, [17] and might imply that actually not the true absence of the JCV infection, but rather the level of the replicative activity of the persistent JCV infection determines the individual risk of developing PML [18]. This risk might then critically depend on host genetic factors that determine the immune response to the virus, and protect from e.g. the spread of the virus from places of peripheral persistency or latency to the brain. Genes of particular interest in this respect are the HLA class I and class II genes where different variants with different peptide presenting abilities may affect the effectiveness of CD4+ and CD8+ T cell immune defence.
Our aim was therefore to test the host genetic regulation of HLA genes in the immune response to JCV. We used anti-JCV antibody status and anti-JCV antibody levels as surrogate for the identification of persons carrying a JCV infection in significant and clinically relevant levels and tested association to HLA class I and class II genes.
Results
Clinical characteristics and demographic data of the included patients and controls are displayed in table 1. Anti-JCV antibody status and levels were determined in the same laboratory for all individuals with an ELISA based method [9].
Tab. 1. Demographic information. 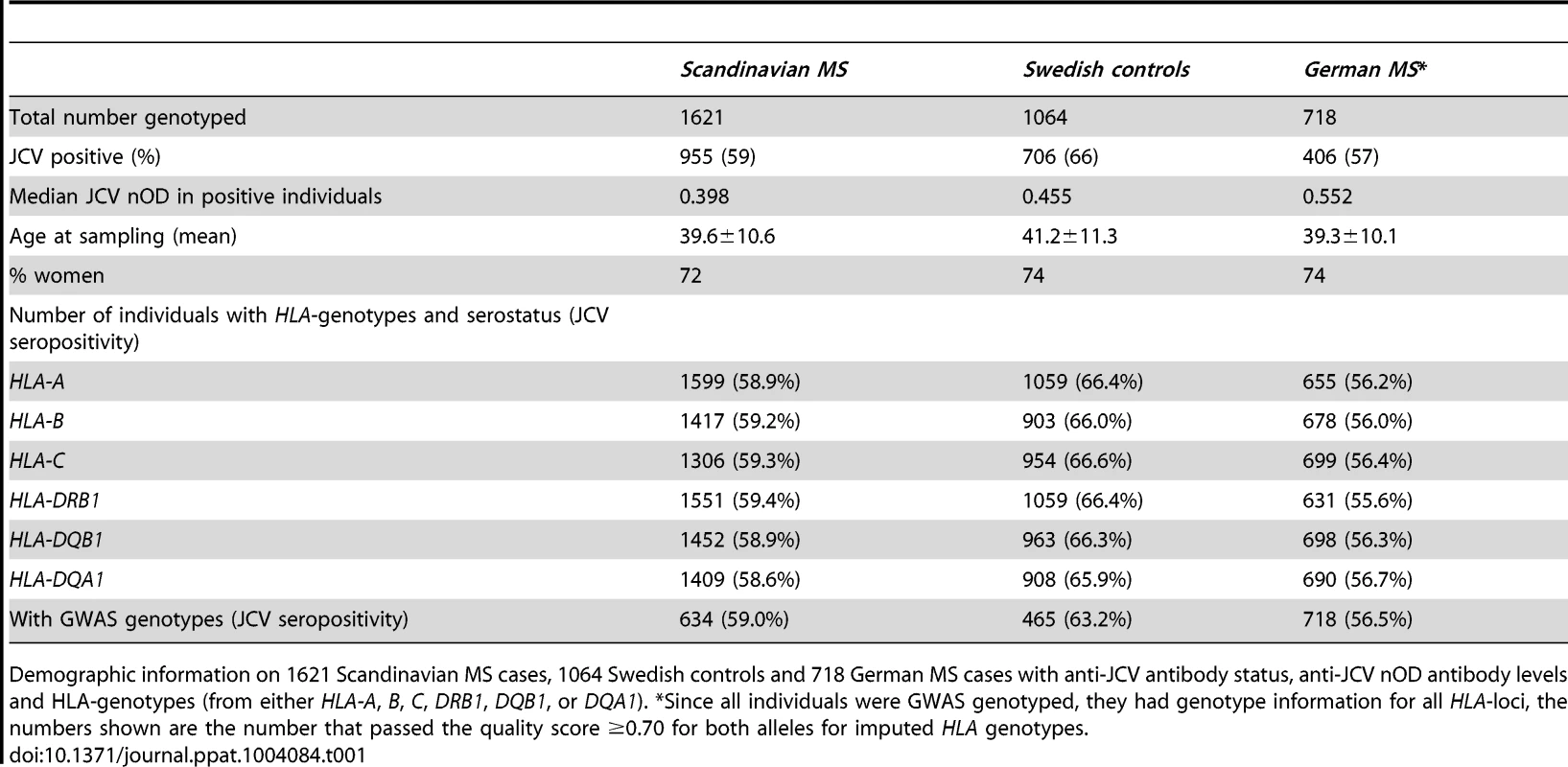
Demographic information on 1621 Scandinavian MS cases, 1064 Swedish controls and 718 German MS cases with anti-JCV antibody status, anti-JCV nOD antibody levels and HLA-genotypes (from either HLA-A, B, C, DRB1, DQB1, or DQA1). *Since all individuals were GWAS genotyped, they had genotype information for all HLA-loci, the numbers shown are the number that passed the quality score ≥0.70 for both alleles for imputed HLA genotypes. In this study we selected the HLA complex for scrutiny in view of its potent immune regulatory functions. We performed a meta-analysis of association of markers on chromosome 6 for both anti-JCV antibody status and normalized anti-JCV antibody levels (anti-JCV nOD) of results obtained in the three separate cohorts of individuals shown in table 1. This indicated a strong association signal in the HLA class II region for both anti-JCV antibody status and anti-JCV nOD values (figure 1). The most significant markers for the two analyses (rs34454257 for the anti-JCV antibody status and rs3129860 for anti-JCV nOD values) map 42.6 and 145.7 kb upstream of the HLA-DRB1 gene in the direction of the HLA class I genes.
Fig. 1. Association between JCA antibody response and markers in the Human Leuococyte region on chromosome 6. 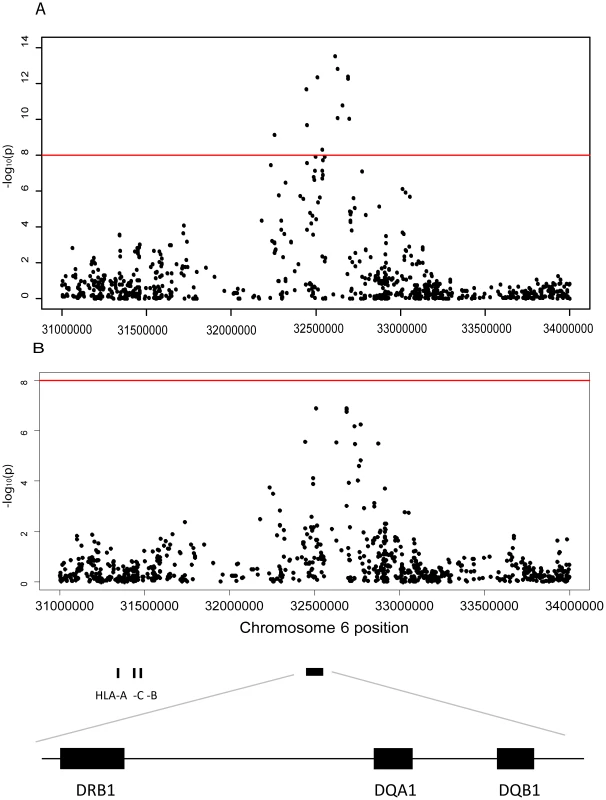
A Plot of the HLA region from the meta-analysis (random effects model) of the association between GWAS markers and JCV serostatus in the Scandinavian (n = 634) and German (n = 718) MS cases and the Swedish controls (n = 465) on chromosome 6. The horizontal line represent a p-values of and 1×10−8. All analyses were adjusted for gender, age at sampling, and principal components. The most significant SNP is rs34454237 (p<4×10−14) which maps 42.6 kb from the HLA-DRB1 gene towards the HLA class I genes. B Plot of the HLA region from the meta-analysis (random effects model) of the association between GWAS markers and transformed anti-JCV nOD levels in the anti-JCV antibody positive Scandinavian (n = 374) and German (n = 294) MS cases and the Swedish controls (n = 406). The horizontal lines represent a p-value of and 1×10−8. All analyses adjusted for gender, age at sampling, and principal components. The locations of the HLA-A, -C, -B, -DRB1, -DQA1 and -DRB1 loci are noted using genome build 36. The most significant SNP is rs3129860 (p<1×10−7) which maps 145.7 kb from the HLA-DRB1 gene in the direction of the HLA class I genes. With this association signal on chromosome 6p21, it was of interest to determine the particular class II gene variants which were associated. Several HLA-alleles showed association to anti-JCV antibody status in both Scandinavian MS cases and controls (Table 2). The table is organised based on common established extended haplotypes found in the Caucasian population [19]–[21]. It is noteworthy that the DRB1*15-DQA1*01 : 02-DQB1*06 : 02-haplotype, the most strongly associated MS genetic risk factor, was found to be negatively associated with the positive detection of anti-JCV antibodies. The OR for DRB1*15 was 0.42 in Scandinavian MS cases and 0.53 in controls. This association was replicated in German MS cases (OR for DRB1*15 0.54 Table 3). Other alleles in this haplotype, DQB1*06 : 02 and DQA1*01 : 02, also showed strong protective associations, as expected, since they are in LD with DRB1*15 : 01. In contrast, the DRB1*13-DQA1*01 : 03-DQB1*06 : 03-haplotype was positively associated with anti-JCV antibody status, with an OR = 1.62 in Scandinavian MS cases, OR = 1.55 in Swedish controls (Table 2) and OR = 1.58 in German MS cases (Table 3). In addition the DRB1*03-DQA1*05-DQB1*02 and DQA1*05-DQB1*03 : 01 haplotypes showed a positive association to anti-JCV antibody status, while the DQA1*05-DQB1*01 : 01-haplotype was negatively associated with anti-JCV antibody status among controls.
Tab. 2. HLA-associations to anti-JCV antibody status in Scandinavian cohort. 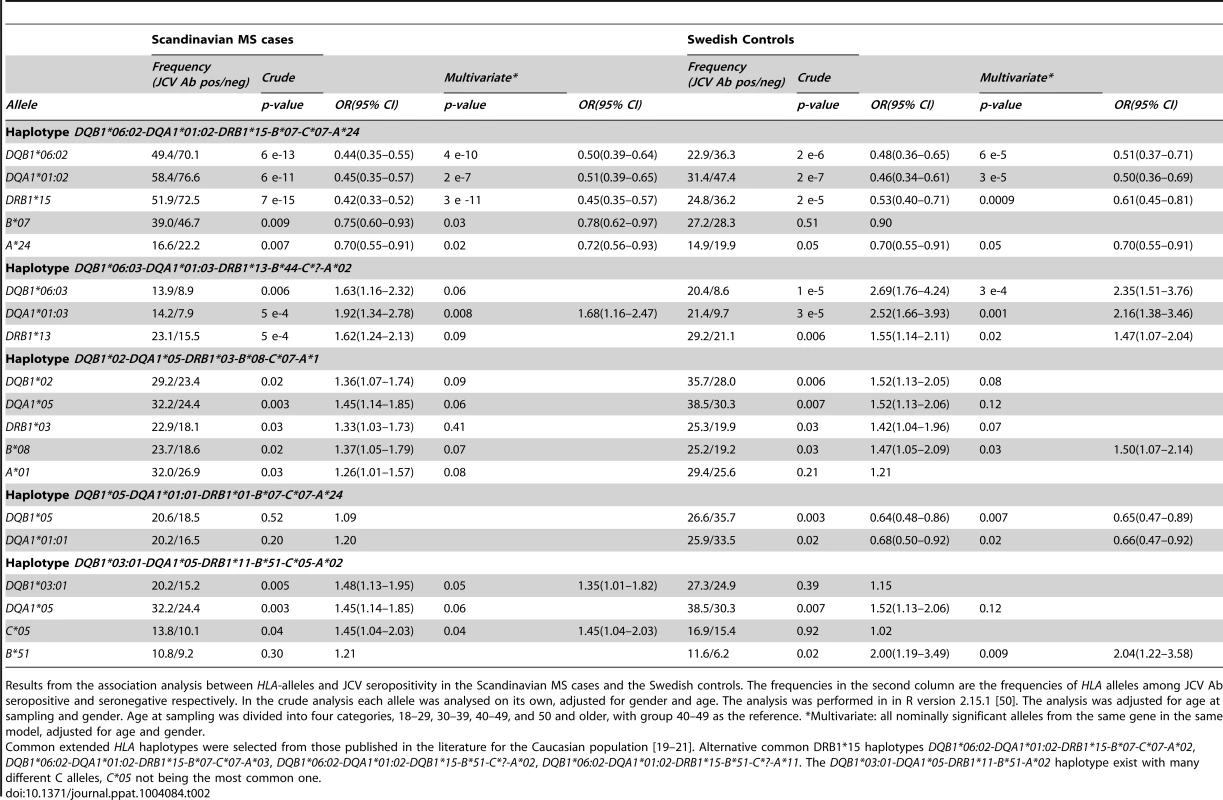
Results from the association analysis between HLA-alleles and JCV seropositivity in the Scandinavian MS cases and the Swedish controls. The frequencies in the second column are the frequencies of HLA alleles among JCV Ab seropositive and seronegative respectively. In the crude analysis each allele was analysed on its own, adjusted for gender and age. The analysis was performed in in R version 2.15.1 [50]. The analysis was adjusted for age at sampling and gender. Age at sampling was divided into four categories, 18–29, 30–39, 40–49, and 50 and older, with group 40–49 as the reference. *Multivariate: all nominally significant alleles from the same gene in the same model, adjusted for age and gender. Tab. 3. HLA-associations to anti-JCV antibody status among German MS patients. 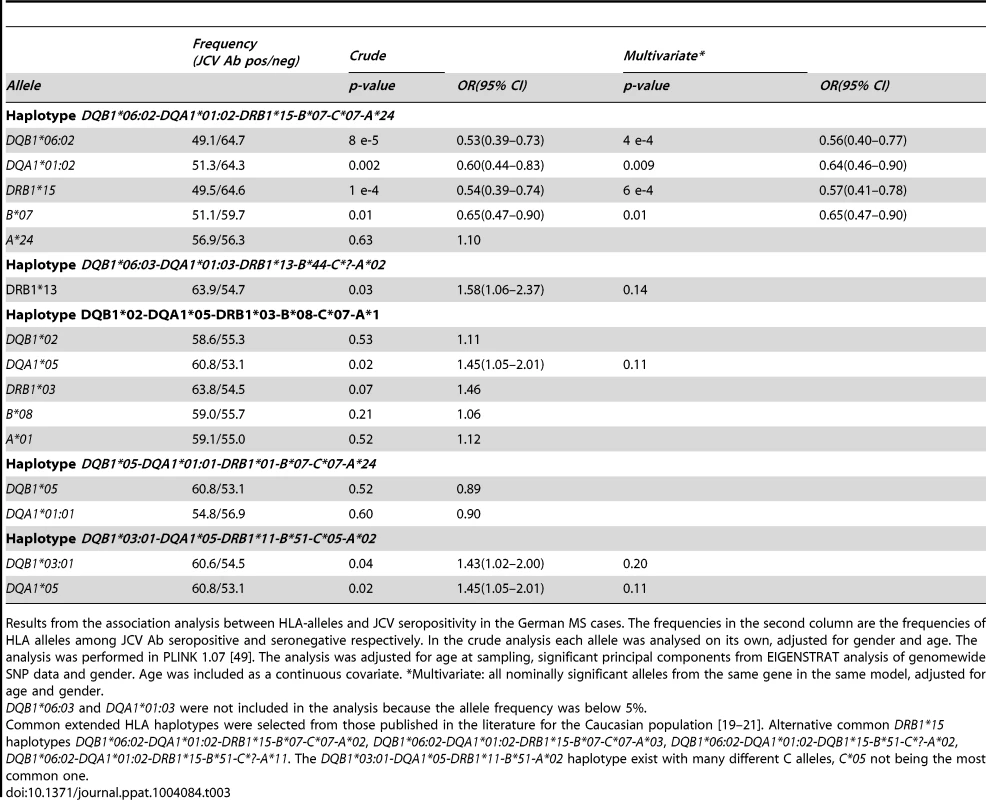
Results from the association analysis between HLA-alleles and JCV seropositivity in the German MS cases. The frequencies in the second column are the frequencies of HLA alleles among JCV Ab seropositive and seronegative respectively. In the crude analysis each allele was analysed on its own, adjusted for gender and age. The analysis was performed in PLINK 1.07 [49]. The analysis was adjusted for age at sampling, significant principal components from EIGENSTRAT analysis of genomewide SNP data and gender. Age was included as a continuous covariate. *Multivariate: all nominally significant alleles from the same gene in the same model, adjusted for age and gender. The DRB1*15-DQA1*01 : 02-DQB1*06 : 02-haplotype also showed an association to lower anti-JCV nOD levels in a linear regression analysis among JCV seropositive individuals, with a significance level of p≤0.001 in the Scandinavian cohorts (Table 4). For DQB1*06 : 02 beta was between −0.218 and −0.366 in the different cohorts (Table 4 and 5).
Tab. 4. HLA-association to transformed JCV nOD levels in Scandinavian cohort. 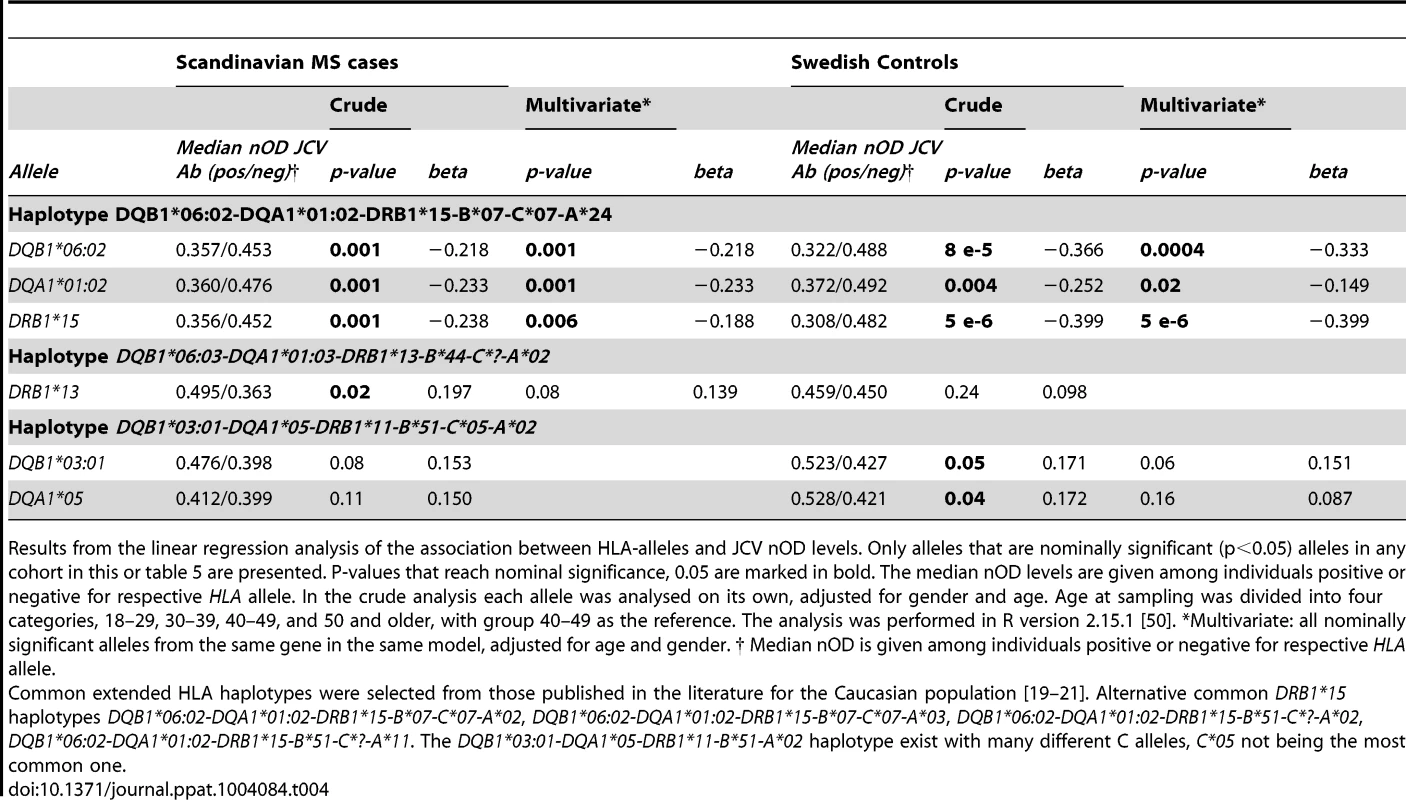
Results from the linear regression analysis of the association between HLA-alleles and JCV nOD levels. Only alleles that are nominally significant (p<0.05) alleles in any cohort in this or table 5 are presented. P-values that reach nominal significance, 0.05 are marked in bold. The median nOD levels are given among individuals positive or negative for respective HLA allele. In the crude analysis each allele was analysed on its own, adjusted for gender and age. Age at sampling was divided into four categories, 18–29, 30–39, 40–49, and 50 and older, with group 40–49 as the reference. The analysis was performed in R version 2.15.1 [50]. *Multivariate: all nominally significant alleles from the same gene in the same model, adjusted for age and gender. † Median nOD is given among individuals positive or negative for respective HLA allele. Tab. 5. HLA-association to transformed JCV nOD levels in German MS patients. 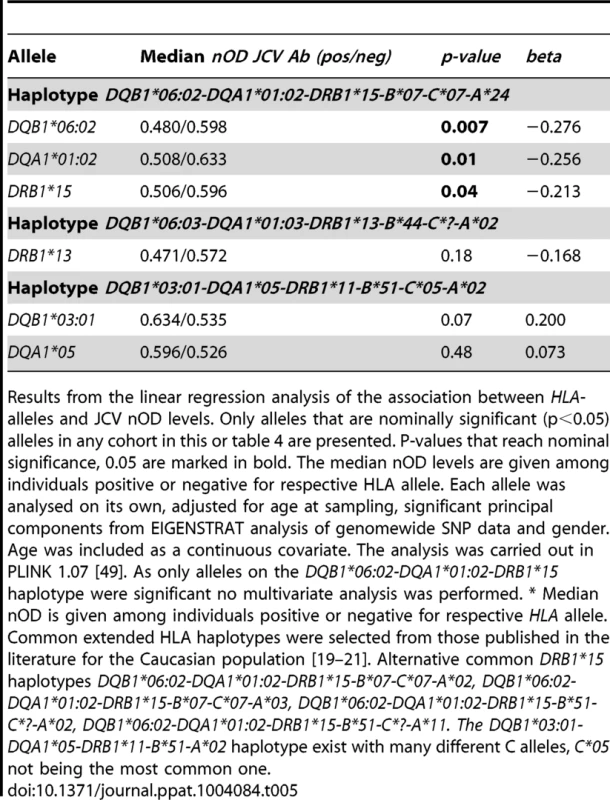
Results from the linear regression analysis of the association between HLA-alleles and JCV nOD levels. Only alleles that are nominally significant (p<0.05) alleles in any cohort in this or table 4 are presented. P-values that reach nominal significance, 0.05 are marked in bold. The median nOD levels are given among individuals positive or negative for respective HLA allele. Each allele was analysed on its own, adjusted for age at sampling, significant principal components from EIGENSTRAT analysis of genomewide SNP data and gender. Age was included as a continuous covariate. The analysis was carried out in PLINK 1.07 [49]. As only alleles on the DQB1*06:02-DQA1*01:02-DRB1*15 haplotype were significant no multivariate analysis was performed. * Median nOD is given among individuals positive or negative for respective HLA allele. DRB1*13 showed an association to higher anti JCV nOD levels among Scandinavian MS cases, p = 0.02, beta = 0.197, but not in German MS cases or Swedish controls (Table 4 and 5). In addition DQB1*03 : 01 and DQA1*05 showed an association to higher transformed anti-JCV nOD levels among Swedish controls (Table 4).
Most of the HLA associations to both anti-JCV antibody status and anti-JCV nOD levels remained similar when other nominally associated HLA alleles for the same gene are included in the regression analysis (Table 2 to 5).
The OR for the association of the presence of DRB1*15 and for DRB1*15 homozygotes for JCV antibody status did not differ, suggestive of a dominant DRB1*15 effect (Figure 2A). Conversely, the DRB1*13 homozygotes showed a slightly stronger association compared to presence of DRB1*13, although the 95%CI do overlap. DRB1*13/15 heterozygotes were not significantly associated with JCV seropositivity indicating that the effect of the two haplotypes counteract each other. Similar results were seen for the DQA1 locus (Figure 2B), but here the DQA1*01 : 03/05 heterozygotes are associated with an OR as high as 5.23.
Fig. 2. Analysis of association between HLA genotypes and anti-JCV antibody status in joint analysis of Swedish controls, Scandinavian and German MS patients. 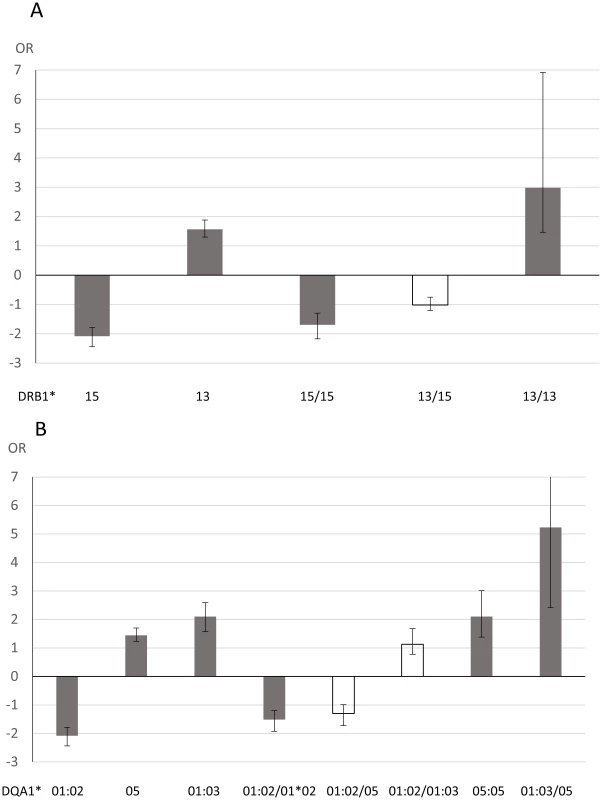
A Odds ratio (OR) for DRB1 alleles and genotypes from logistic regression analyses performed in R version 2.15.1 [50]. The analyses were adjusted for gender, cohort (Swedish controls, Scandinavian MS patients or German MS patients) and age at sampling. Age at sampling was divided into four categories, 18–29, 30–39, 40–49, and 50 and older, with group 40–49 as the reference. Error bars represents 95% confidence intervals. OR below 1 are plotted as −1/OR. Grey indicates associations with p<0.05, white p>0.05. B Odds ratio (OR) for DRB1 alleles and genotypes from logistic regression analyses performed in R version 2.15.1 [50]. The analyses were adjusted for gender, cohort (Swedish controls, Scandinavian MS patients or German MS patients) and age at sampling. Age at sampling was divided into four categories, 18–29, 30–39, 40–49, and 50 and older, with group 40–49 as the reference. Error bars represents 95% confidence intervals. OR below 1 are plotted as −1/OR. Grey indicates associations with p<0.05, white p>0.05. DQA1*01:03 homozygotes are not included as this combination was so rare (0.4%). Consistent with the effect on the qualitative anti-JCV status, the DRB1*15 haplotype appeared to act dominantly also on anti-JCV nOD levels as presence of DRB1*15 showed a similar association to DRB1*15 homozygotes, while the DRB1*11 haplotype had an additive effect (Table 6). The effect of these two haplotypes cancel each other out as DRB1*11/15 heterozygotes showed no association to anti-JCV nOD levels.
Tab. 6. Association of HLA genotypes to transformed JCV nOD levels in joint analysis of Swedish controls and Scandinavian and German MS patients. 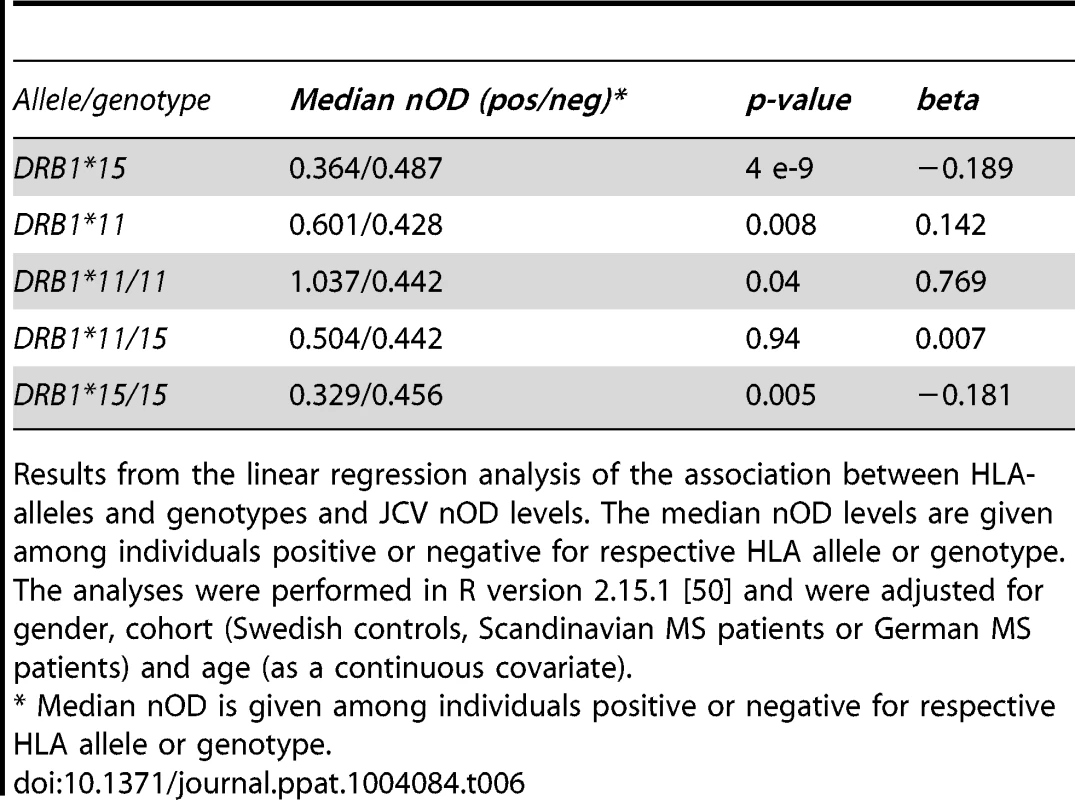
Results from the linear regression analysis of the association between HLA-alleles and genotypes and JCV nOD levels. The median nOD levels are given among individuals positive or negative for respective HLA allele or genotype. The analyses were performed in R version 2.15.1 [50] and were adjusted for gender, cohort (Swedish controls, Scandinavian MS patients or German MS patients) and age (as a continuous covariate). We reanalysed the association of SNPs on chromosome 6 to anti-JCV antibody status and anti-JCV nOD levels when including all HLA alleles that remained associated in the multivariate analysis as covariates. This lead to an almost complete abolishment of the association peak on chromosome 6, with the most significant remaining associations being p = 0.0001 for a handful of markers (data not shown). This indicates that the association we observed in the HLA region was almost completely explained by the HLA alleles listed in tables 2–4.
Discussion
We here demonstrate a host genetic HLA complex mediated influence on anti-JCV antibody status and anti-JCV antibody levels as surrogate for the susceptibility of the infection with JCV or the activity of the infection with JCV, respectively.
We report a strong negative association with anti-JCV antibody positivity, and to a lesser extent, to anti-JCV nOD levels, for the HLA-DRB1*15-DQA1*01 : 02-DQB1*06 : 02-haplotype in all three datasets. In contrast, the DRB1*13-DQA1*01 : 03-DQB1*06 : 03-haplotype is associated to increased signs of JCV carriage as assessed serologically. We further find that the DRB1*15-DQA1*01 : 02-DQB1*06 : 02 haplotype acts dominantly, one copy being sufficient to reduce the ability to form anti-JCV antibodies while the DRB1*13-DQA1*01 : 03-DQB1*06 : 03 haplotype acts in an additive fashion. Neither of the haplotypes dominates over the other. A recent study demonstrated considerable variation in which JCV peptides were recognized by T cells [22]. A most straight forward interpretation of the present findings is that the DRB1*15 : 01 haplotype displays class II molecules that are especially able to present JCV antigens/peptides that are instrumental in activating CD4+ T cells that support the elimination or control of the virus upon exposure to the host. Hence, the opposite would be valid for the haplotype associated with increased carriage of the JCV. Thus hypothetically a large proportion of those persons being sero-negative might have encountered the virus, but had an efficient immune response following primary infection, with low viral turnover or the lack of viral persistency, and low anti-JCV IgG as consequence.
Recent serological studies support such a concept: antibody reactivity as measured by ELISA resembles a continuum from non-reactive to highly reactive in particular in persons not excreting the virus in urine [9]. This led to the introduction of a second-step confirmation test when determining the anti-JCV sero-status. However, this pattern of continuous reactivity is also seen with alternative assay formats, which suggest that a vast majority of persons have been exposed to JCV, but have a level of the antibody response to JCV below the assay cut-off, possibly due to an efficient control of the virus with low viral turn-over [13],[17]. This would also explain the higher false negative rate of serological studies observed in recent publications [23], [24].
Although CD8+ cells, restricted by class I molecules are critical in eliminating virus infected cells, antigen specific CD4+ HLA class II restricted cells are crucial for providing T cell help through a variety of cytokines and activation of antigen presenting dendritic cells [25]. The findings may pave the way for finding epitopes in JCV critical for immune defence which could impact on vaccination strategies. Any direct clinical implications of the data, or use, for example in risk stratifications, remain to be determined.
The DRB1*13-DQA1*01 : 03-DQB1*06 : 03-haplotype shows a positive association to anti-JCV antibody serostatus, and was also to higher anti-JCV nOD levels. Hypothetically, a less effective viral immune control with higher viral turnover may be consistent with a chronically higher stimulation of the B cell arm of the immunity resulting in higher antibody levels. This might help us understand why patients that develop PLM during therapy with natalizumab had increased anti-JCV antibody levels already prior to development of PML, and why it might be rational to include the level of the anti-JCV response into PML risk stratification strategies [26].
Recent data suggests that PML-specific viral mutations are acquired intra-individually e.g. in the VP1-region and the regulatory region of the viral genome [27], [28]. It is tempting to speculate that viral PML-specific mutations, although being a random event, are more likely to occur in persons with an inefficient control of the infection with JCV. The host genetic data presented here might therefore be a first step helping to understand how the interplay of host - and viral genetic factors might lead to the development of PML in some, but not all persons exposed to certain immunosuppressive therapies. Our study is however lacking a sufficient number of cases of PML and is therefore not designed and empowered to test this directly.
There is one previous paper studying the HLA association to PLM [29]. In this paper 123 Caucasian PML cases, the majority whom were HIV positive, were compared with a large group of HIV positive individuals. The study was limited to the association of HLA class I antigens. While A3 was found to be nominally negatively associated with PML, B18 was found to be positively associated. We do not find any of these alleles associated with anti-JCV antibody formation in our study. However, the A3 association to PML possibly is explained by the same effect as the DRB1*15-DQA1*01 : 02-DQB1*06 : 02 association we see in our study, considering that A3 can be present on the same extended haplotype. Studies in larger cohorts of PML patients with appropriate controls testing the association of class II antigens are warranted. A recent investigation has studied the stimulation of CD4+ T cells by pools of JCV peptides among healthy donors with different HLA-DRB1 alleles [22]. For haplotypes where we see an increased OR for sero-status and positive correlation to JCV-Ab levels (DRB1*13 and DRB1*03) they observe reduced stimulation of CD4+ cells, while the opposite was true for DRB1*15. Hence, both antibody response and T-cell response to JCV infection are affected by HLA-class II antigens, which is consistent with our observations of potent HLA class II gene variant effects in large cohorts of persons.
HLA associations to some viral infections have been seen previously. The HLA class II genes were recently reported as host genetic factors influencing the IgG response to EBNA1, an Epstein Barr virus-related protein [30]. In addition, there are well documented class II allelic influences on Hepatitis C [31] and recently a highly associated SNP in the HLA region was demonstrated in relation to human papilloma virus infection [32]. HLA class II associations have also been seen in chronic hepatitis B infections as well as response to hepatitis B vaccination [33]–[35]. Association to the HLA class I related MICB gene have also been reported to hypovolemic shock caused by dengue viral infection and HIV viral load [36], [37]. Another MIC gene, MICA has been associated to hepatitis C virus induced hepato cellular carcinoma [38]. In our data, after adjusting the association for HLA class II associated alleles the most strongly associated marker in the class I region is rs3094014 (p<0.02) which is in LD with both the MICB (r2 0.76 for rs3132468 associated with dengue fever) and MICA (r2 0.90 for rs2596542 reported to be associated with hepatitis C virus induced hepato cellular carcinoma) using European 1000 genomes and HapMap data and may therefore represent the same signal.
Interestingly, the DRB1*15 haplotype is also the most strongly associated genetic risk factor for MS [39]. Consistent with this, the demographic data in our study suggests that anti-JCV antibody positivity is somewhat lower among MS cases (59%), compared to controls (66%, p = 0.02). A protection against the establishment of a persistent JCV infection with positive detection of anti-JCV antibodies provided by the DRB1*15 haplotype would then explain the lower sero-prevalence among cases. The presence of an association with the DRB1*15-haplotype in controls also indicates that the association is not likely due to an aberrant immune response to JCV infection in MS cases.
In conclusion, we here demonstrate strong associations of class II gene variants on JCV infection. Hence, CD4+ T cells, restricted by class II molecules are crucial in the host control of JCV infection. Our data is of importance for a better understanding of JCV infection and virus-host interactions, and might pave the way for new developments for an improved PML risk stratification, and preventive or curative future anti-JCV therapies.
Materials and Methods
Ethics statement
The study was approved by the regional ethical committees in each country involved; Stockholm regional Ethical Review Board (Sweden), the Ethical review boards at the Heinrich-Heine Universität Düsseldorf and the Technische Universität München (Germany) and the Danish Ethical Committee Review Board for Copenhagen and Frederiksberg (Denmark). All participants provided written informed consent.
Patients and controls
A Scandinavian dataset consisting of 2015 Swedish persons with MS from two separate studies EIMS [40] and IMSE [41] with 1259 population based controls, and 157 Danish MS patients treated with natalizumab in Copenhagen. HLA-genotypes and anti-JCV antibody status were available for 1621 MS cases and 1064 controls (table 1).
A German dataset of 745 MS patients, 718 with GWAS data, was used for replication. The cohort was recruited from multiple sites in Germany and included persons treated with interferon-beta for at least 6 months. GWAS genotyping for these MS cases had been performed in the same laboratory with the same chip as the Scandinavian datasets.
HLA and SNP-typing
HLA-genotypes came from three different sources. Low resolution Sequence Specific amplification (Olerup, Saltsjöbaden, Sweden) [42] were for genotyping of 2115 Swedish cases and controls for HLA-A, 2140 for HLA-DRB1, and 161 for HLA-C. For HLA-B a Luminex based reverse PCR-SSO (One Lambda, Inc., Canoga Park, CA, USA) was used for 173 persons [39]. And finally imputation either using HLA*IMP [43] with genotypes from the IMSGC WTCCC2 MS GWAS, [44] or with HLA*IMP:2 [45] using genotypes from the Immunochip [46] was used. The former was used for both the Danish and German cohort while both were used in the Swedish cohort. Imputed HLA data was available for 1105 Swedish persons from the IMSGC WTCCC2 and for 2220 Swedish persons using Immunochip genotypes. In cases where the genotypes for any individual were discordant between platforms, the following order of precedence was used: classical, Immunochip imputed, GWAS imputed. A quality value for allele probability of 0.7 was used as a threshold for imputed HLA genotypes.
In this study we used SNP genotypes from MS GWAS study to analyse association to the HLA region [44]. Genome-wide SNP markers were genotyped as part of the IMSGC WTCCC2 MS GWAS on the Human660-Quad chip, and genotype calling and markers were quality controlled as previously described [44].
Cases with previous intravenous IgG treatment were excluded. In the Scandinavian cohort all persons were of Scandinavian ancestry, and all MS cases fulfilled the McDonald or Poser criteria for MS. For the German MS cases, a total number of 749 cases were GWAs genotyped; 25 persons were removed as they were outliers in the principal component analysis (PCA), 4 were removed due to unsuccessful genotyping, and 2 because of natalizumab treatment at blood draw.
Anti-JCV antibody determination
JCV serology response was determined from plasma or serum using a two-step assay, [9] performed at Focus Diagnostics (Cypress, CA, USA) and sponsored by Biogen Idec (Cambridge, MA, USA). In the first step of the ELISA assay optical density (OD) were measured. Samples with OD>0.25 were considered positive while samples with OD<0.10 were considered negative. For samples in the intermediary interval (0.10–0.25) a second assay step was used to determine the percentage of inhibition during a pre-incubation with soluble JCV-like particles. Samples in this intermediary interval with an inhibition >40% were considered positive while those with an inhibition <40% were classified as negative.
The assay has been estimated to have a false negative rate for JCV carriage of 2–3%. For the quantitative analysis, normalised OD values (nOD) from the first step ELISA were transformed using rank based transformation in the GenABEL-package in R [47].
Statistical analysis
Association of GWAS-markers on chromosome 6 to anti-viral antibodies
To generate principal components and control for population stratification, an EIGENSTRAT analysis was performed in each cohort separately, using Eigensoft [48]. This analysis was performed after SNP-pruning with r2>0.2 were removed using PLINK 1.07 [49]. All principal components with a p-value below 0.05 were included as covariates in the regression models. All persons considered as outliers in the PCA were removed.
Logistic regression analysis (for anti-JCV antibody status) and linear regression analysis (for transformed anti–JCV nOD-levels among JCV seropositive individuals) were performed in PLINK 1.07, adjusting for age, gender, and principal components. The threshold for Hardy-Weinberg equilibrium test was p>0.001, and minor allele frequency >0.05. We also ran the same analysis, where we adjusted for all HLA-alleles associated with each outcome, with p<0.05 in the final model as cut-off for inclusion.
A meta-analysis of the results from the different cohorts was performed in PLINK 1.07, using both a fixed and a random effects model.
Analysis of HLA-association
MS cases and controls were analysed separately. Association to anti-JCV antibodies was tested separately for alleles with a frequency higher than 5% separately. As a second step, all nominally significant alleles from the same HLA-gene were analysed in a multivariate regression model. Association to anti-JCV serostatus was performed with logistic regression, and association to transformed anti-JCV nOD-values was performed with linear regression, in R [50] or PLINK 1.07 [49]. We adjusted for age at sampling and gender in all analyses.
For the German dataset, it was also possible to adjust for principal components, but for the Scandinavian dataset, this was not possible since the majority of samples were not genotyped with genome wide markers.
For the analysis of effect of genotypes a joint analysis was performed including all cohorts, in this analysis covariates for age at sampling, gender and cohort were included.
Analysis of the data was carried out by Emilie Sundqvist, Eva Albrecht and Ingrid Kockum.
Zdroje
1. AstromKE, MancallEL, RichardsonEPJr (1958) Progressive multifocal leuko-encephalopathy; a hitherto unrecognized complication of chronic lymphatic leukaemia and Hodgkin's disease. Brain 81 : 93–111.
2. PadgettBL, WalkerDL, ZuRheinGM, EckroadeRJ, DesselBH (1971) Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet 1 : 1257–1260.
3. CarsonKR, FocosiD, MajorEO, PetriniM, RicheyEA, et al. (2009) Monoclonal antibody-associated progressive multifocal leucoencephalopathy in patients treated with rituximab, natalizumab, and efalizumab: a Review from the Research on Adverse Drug Events and Reports (RADAR) Project. Lancet Oncol 10 : 816–824.
4. ErmisU, WeisJ, SchulzJB (2013) PML in a patient treated with fumaric acid. N Engl J Med 368 : 1657–1658.
5. PiccinniC, SacripantiC, PoluzziE, De PontiF (2013) Disproportionality signal of progressive multifocal leukoencephalopathy: monoclonal antibodies versus other immunosuppressants. Pharmacoepidemiol Drug Saf 22 : 443–445.
6. SchmedtN, AndersohnF, GarbeE (2012) Signals of progressive multifocal leukoencephalopathy for immunosuppressants: a disproportionality analysis of spontaneous reports within the US Adverse Event Reporting System (AERS). Pharmacoepidemiol Drug Saf 21 : 1216–1220.
7. van OostenBW, KillesteinJ, BarkhofF, PolmanCH, WattjesMP (2013) PML in a patient treated with dimethyl fumarate from a compounding pharmacy. N Engl J Med 368 : 1658–1659.
8. BloomgrenG, RichmanS, HotermansC, SubramanyamM, GoelzS, et al. (2012) Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med 366 : 1870–1880.
9. GorelikL, LernerM, BixlerS, CrossmanM, SchlainB, et al. (2010) Anti-JC virus antibodies: implications for PML risk stratification. Ann Neurol 68 : 295–303.
10. PlavinaT, BermanM, NjengaM, CrossmanM, LernerM, et al. (2012) Multi-site analytical validation of an assay to detect anti-JCV antibodies in human serum and plasma. J Clin Virol 53 : 65–71.
11. BozicC, RichmanS, PlavinaT, NatarajanA, ScanlonJV, et al. (2011) Anti-John Cunnigham virus antibody prevalence in multiple sclerosis patients: baseline results of STRATIFY-1. Ann Neurol 70 : 742–750.
12. SorensenPS, BertolottoA, EdanG, GiovannoniG, GoldR, et al. (2012) Risk stratification for progressive multifocal leukoencephalopathy in patients treated with natalizumab. Mult Scler 18 : 143–152.
13. WarnkeC, RamanujamR, PlavinaT, BergstromT, GoelzS, et al. (2013) Changes to anti-JCV antibody levels in a Swedish national MS cohort. J Neurol Neurosurg Psychiatry 84 : 1199–205.
14. TrampeAK, HemmelmannC, StroetA, HaghikiaA, HellwigK, et al. (2012) Anti-JC virus antibodies in a large German natalizumab-treated multiple sclerosis cohort. Neurology 78 : 1736–1742.
15. OutteryckO, OngagnaJC, DuhamelA, ZephirH, CollonguesN, et al. (2012) Anti-JCV antibody prevalence in a French cohort of MS patients under natalizumab therapy. J Neurol 259 : 2293–2298.
16. CalguaB, BarardiCR, Bofill-MasS, Rodriguez-ManzanoJ, GironesR (2011) Detection and quantitation of infectious human adenoviruses and JC polyomaviruses in water by immunofluorescence assay. J Virol Methods 171 : 1–7.
17. WarnkeC, PawlitaM, DehmelT, Posevitz-FejfarA, HartungHP, et al. (2013) An assay to quantify species-specific anti-JC virus antibody levels in MS patients. Mult Scler 19 : 1137–44.
18. BergerJR, HouffSA, GurwellJ, VegaN, MillerCS, et al. (2013) JC virus antibody status underestimates infection rates. Ann Neurol 74 : 84–90.
19. AskarM, DaghstaniJ, ThomasD, LeahyN, DunnP, et al. (2013) 16(th) IHIW: global distribution of extended HLA haplotypes. Int J Immunogenet 40 : 31–38.
20. BettensF, Nicoloso de FaveriG, TiercyJM (2009) HLA-B51 and haplotypic diversity of B-Cw associations: implications for matching in unrelated hematopoietic stem cell transplantation. Tissue Antigens 73 : 316–325.
21. AlperCA, LarsenCE, DubeyDP, AwdehZL, FiciDA, et al. (2006) The haplotype structure of the human major histocompatibility complex. Hum Immunol 67 : 73–84.
22. JelcicI, AlyL, BinderTM, Bofill-MasS, PlanasR, et al. (2013) T cell epitope mapping of JC polyoma virus-encoded proteome reveals reduced T cell responses in HLA-DRB1*04 : 01+ donors. J Virol 87 : 3393–3408.
23. BergerJR, HouffSA, GurwellJ, VegaN, MillerCS, et al. (2013) JC virus antibody status underestimates infection rates. Ann Neurol 74 : 84–90.
24. MajorEO, FrohmanE, DouekD (2013) JC viremia in natalizumab-treated patients with multiple sclerosis. N Engl J Med 368 : 2240–2241.
25. WhitmireJK (2011) Induction and function of virus-specific CD4+ T cell responses. Virology 411 : 216–228.
26. Plavina T, Subramanyam M, Bloomgren G, Richman S, Pace A, et al. (2013) JCV Antibody Index Stratifies PML Risk in Natalizumab-Treated MS Patients. In: The 27th Annual Meeting of the Consortium of Multiple Sclerosis Centers. Orlando, Florida, United States. pp. Paper1642.
27. GorelikL, ReidC, TestaM, BrickelmaierM, BossolascoS, et al. (2011) Progressive multifocal leukoencephalopathy (PML) development is associated with mutations in JC virus capsid protein VP1 that change its receptor specificity. J Infect Dis 204 : 103–114.
28. ReidCE, LiH, SurG, CarmilloP, BushnellS, et al. (2011) Sequencing and analysis of JC virus DNA from natalizumab-treated PML patients. J Infect Dis 204 : 237–244.
29. GheuensS, FellayJ, GoldsteinDB, KoralnikIJ (2010) Role of human leukocyte antigen class I alleles in progressive multifocal leukoencephalopathy. J Neurovirol 16 : 41–47.
30. RubiczR, YolkenR, DrigalenkoE, CarlessMA, DyerTD, et al. (2013) A Genome-Wide Integrative Genomic Study Localizes Genetic Factors Influencing Antibodies against Epstein-Barr Virus Nuclear Antigen 1 (EBNA-1). PLoS Genet 9: e1003147.
31. CangussuLO, TeixeiraR, CamposEF, RampimGF, MingotiSA, et al. (2011) HLA class II alleles and chronic hepatitis C virus infection. Scand J Immunol 74 : 282–287.
32. ChenD, McKayJD, CliffordG, GaborieauV, ChabrierA, et al. (2011) Genome-wide association study of HPV seropositivity. Hum Mol Genet 20 : 4714–4723.
33. KamataniY, WattanapokayakitS, OchiH, KawaguchiT, TakahashiA, et al. (2009) A genome-wide association study identifies variants in the HLA-DP locus associated with chronic hepatitis B in Asians. Nat Genet 41 : 591–595.
34. DavilaS, FroelingFE, TanA, BonnardC, BolandGJ, et al. (2010) New genetic associations detected in a host response study to hepatitis B vaccine. Genes Immun 11 : 232–238.
35. PngE, ThalamuthuA, OngRT, SnippeH, BolandGJ, et al. (2011) A genome-wide association study of hepatitis B vaccine response in an Indonesian population reveals multiple independent risk variants in the HLA region. Hum Mol Genet 20 : 3893–3898.
36. KhorCC, ChauTN, PangJ, DavilaS, LongHT, et al. (2011) Genome-wide association study identifies susceptibility loci for dengue shock syndrome at MICB and PLCE1. Nat Genet 43 : 1139–1141.
37. FellayJ, ShiannaKV, GeD, ColomboS, LedergerberB, et al. (2007) A whole-genome association study of major determinants for host control of HIV-1. Science 317 : 944–947.
38. KumarV, KatoN, UrabeY, TakahashiA, MuroyamaR, et al. (2011) Genome-wide association study identifies a susceptibility locus for HCV-induced hepatocellular carcinoma. Nat Genet 43 : 455–458.
39. LinkJ, KockumI, LorentzenAR, LieBA, CeliusEG, et al. (2012) Importance of human leukocyte antigen (HLA) class I and II alleles on the risk of multiple sclerosis. PLoS One 7: e36779.
40. HedstromAK, BaarnhielmM, OlssonT, AlfredssonL (2009) Tobacco smoking, but not Swedish snuff use, increases the risk of multiple sclerosis. Neurology 73 : 696–701.
41. HolmenC, PiehlF, HillertJ, Fogdell-HahnA, LundkvistM, et al. (2011) A Swedish national post-marketing surveillance study of natalizumab treatment in multiple sclerosis. Mult Scler 17 : 708–719.
42. OlerupO, ZetterquistH (1992) HLA-DR typing by PCR amplification with sequence-specific primers (PCR-SSP) in 2 hours: an alternative to serological DR typing in clinical practice including donor-recipient matching in cadaveric transplantation. Tissue Antigens 39 : 225–235.
43. DiltheyAT, MoutsianasL, LeslieS, McVeanG (2011) HLA*IMP–an integrated framework for imputing classical HLA alleles from SNP genotypes. Bioinformatics 27 : 968–972.
44. SawcerS, HellenthalG, PirinenM, SpencerCC, PatsopoulosNA, et al. (2011) Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 476 : 214–219.
45. DiltheyA, LeslieS, MoutsianasL, ShenJ, CoxC, et al. (2013) Multi-Population Classical HLA Type Imputation. PLoS Comput Biol 9: e1002877.
46. BeechamAH, PatsopoulosNA, XifaraDK, DavisMF, KemppinenA, et al. (2013) Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat Genet 45 : 1353–60.
47. AulchenkoYS, RipkeS, IsaacsA, van DuijnCM (2007) GenABEL: an R library for genome-wide association analysis. Bioinformatics 23 : 1294–1296.
48. PriceAL, PattersonNJ, PlengeRM, WeinblattME, ShadickNA, et al. (2006) Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 38 : 904–909.
49. PurcellS, NealeB, Todd-BrownK, ThomasL, FerreiraMA, et al. (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81 : 559–575.
50. R Development Core Team (2008) R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Affinity Proteomics Reveals Elevated Muscle Proteins in Plasma of Children with Cerebral MalariaČlánek The Transcriptional Activator LdtR from ‘ Liberibacter asiaticus’ Mediates Osmotic Stress ToleranceČlánek Complement-Related Proteins Control the Flavivirus Infection of by Inducing Antimicrobial PeptidesČlánek Fungal Chitin Dampens Inflammation through IL-10 Induction Mediated by NOD2 and TLR9 Activation
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 4- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostický algoritmus při podezření na syndrom periodické horečky
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- První vakcína proti klíšťové encefalitidě: vakcína FSME-IMMUN
-
Všechny články tohoto čísla
- , , , Genetic Variability: Cryptic Biological Species or Clonal Near-Clades?
- Early Mortality Syndrome Outbreaks: A Microbial Management Issue in Shrimp Farming?
- Wormholes in Host Defense: How Helminths Manipulate Host Tissues to Survive and Reproduce
- Plastic Proteins and Monkey Blocks: How Lentiviruses Evolved to Replicate in the Presence of Primate Restriction Factors
- The 2010 Cholera Outbreak in Haiti: How Science Solved a Controversy
- Affinity Proteomics Reveals Elevated Muscle Proteins in Plasma of Children with Cerebral Malaria
- Noncanonical Role for the Host Vps4 AAA+ ATPase ESCRT Protein in the Formation of Replicase
- Efficient Parvovirus Replication Requires CRL4-Targeted Depletion of p21 to Prevent Its Inhibitory Interaction with PCNA
- Host-to-Pathogen Gene Transfer Facilitated Infection of Insects by a Pathogenic Fungus
- The Transcriptional Activator LdtR from ‘ Liberibacter asiaticus’ Mediates Osmotic Stress Tolerance
- Coxsackievirus B Exits the Host Cell in Shed Microvesicles Displaying Autophagosomal Markers
- TCR Affinity Associated with Functional Differences between Dominant and Subdominant SIV Epitope-Specific CD8 T Cells in Rhesus Monkeys
- Coxsackievirus-Induced miR-21 Disrupts Cardiomyocyte Interactions via the Downregulation of Intercalated Disk Components
- Ligands of MDA5 and RIG-I in Measles Virus-Infected Cells
- Kind Discrimination and Competitive Exclusion Mediated by Contact-Dependent Growth Inhibition Systems Shape Biofilm Community Structure
- Structural Differences Explain Diverse Functions of Actins
- HSCARG Negatively Regulates the Cellular Antiviral RIG-I Like Receptor Signaling Pathway by Inhibiting TRAF3 Ubiquitination Recruiting OTUB1
- Vaginitis: When Opportunism Knocks, the Host Responds
- Complement-Related Proteins Control the Flavivirus Infection of by Inducing Antimicrobial Peptides
- Fungal Chitin Dampens Inflammation through IL-10 Induction Mediated by NOD2 and TLR9 Activation
- Microbial Pathogens Trigger Host DNA Double-Strand Breaks Whose Abundance Is Reduced by Plant Defense Responses
- Alveolar Macrophages Are Essential for Protection from Respiratory Failure and Associated Morbidity following Influenza Virus Infection
- An Interaction between Glutathione and the Capsid Is Required for the Morphogenesis of C-Cluster Enteroviruses
- Concerted Spatio-Temporal Dynamics of Imported DNA and ComE DNA Uptake Protein during Gonococcal Transformation
- Potent Dengue Virus Neutralization by a Therapeutic Antibody with Low Monovalent Affinity Requires Bivalent Engagement
- Regulation of Human T-Lymphotropic Virus Type I Latency and Reactivation by HBZ and Rex
- Functionally Redundant RXLR Effectors from Act at Different Steps to Suppress Early flg22-Triggered Immunity
- The Pathogenic Mechanism of the Virulence Factor, Mycolactone, Depends on Blockade of Protein Translocation into the ER
- Role of Calmodulin-Calmodulin Kinase II, cAMP/Protein Kinase A and ERK 1/2 on -Induced Apoptosis of Head Kidney Macrophages
- An Overview of Respiratory Syncytial Virus
- First Experimental Model of Enhanced Dengue Disease Severity through Maternally Acquired Heterotypic Dengue Antibodies
- Binding of Glutathione to Enterovirus Capsids Is Essential for Virion Morphogenesis
- IFITM3 Restricts Influenza A Virus Entry by Blocking the Formation of Fusion Pores following Virus-Endosome Hemifusion
- Parasite Fate and Involvement of Infected Cells in the Induction of CD4 and CD8 T Cell Responses to
- Deficient IFN Signaling by Myeloid Cells Leads to MAVS-Dependent Virus-Induced Sepsis
- Pernicious Pathogens or Expedient Elements of Inheritance: The Significance of Yeast Prions
- The HMW1C-Like Glycosyltransferases—An Enzyme Family with a Sweet Tooth for Simple Sugars
- The Expanding Functions of Cellular Helicases: The Tombusvirus RNA Replication Enhancer Co-opts the Plant eIF4AIII-Like AtRH2 and the DDX5-Like AtRH5 DEAD-Box RNA Helicases to Promote Viral Asymmetric RNA Replication
- Mining Herbaria for Plant Pathogen Genomes: Back to the Future
- Inferring Influenza Infection Attack Rate from Seroprevalence Data
- A Human Lung Xenograft Mouse Model of Nipah Virus Infection
- Mast Cells Expedite Control of Pulmonary Murine Cytomegalovirus Infection by Enhancing the Recruitment of Protective CD8 T Cells to the Lungs
- Cytosolic Peroxidases Protect the Lysosome of Bloodstream African Trypanosomes from Iron-Mediated Membrane Damage
- Abortive T Follicular Helper Development Is Associated with a Defective Humoral Response in -Infected Macaques
- JC Polyomavirus Infection Is Strongly Controlled by Human Leucocyte Antigen Class II Variants
- Cationic Antimicrobial Peptides Promote Microbial Mutagenesis and Pathoadaptation in Chronic Infections
- Estimating the Fitness Advantage Conferred by Permissive Neuraminidase Mutations in Recent Oseltamivir-Resistant A(H1N1)pdm09 Influenza Viruses
- Progressive Accumulation of Activated ERK2 within Highly Stable ORF45-Containing Nuclear Complexes Promotes Lytic Gammaherpesvirus Infection
- Caspase-1-Like Regulation of the proPO-System and Role of ppA and Caspase-1-Like Cleaved Peptides from proPO in Innate Immunity
- Is Required for High Efficiency Viral Replication
- Modified Vaccinia Virus Ankara Triggers Type I IFN Production in Murine Conventional Dendritic Cells via a cGAS/STING-Mediated Cytosolic DNA-Sensing Pathway
- Evidence That Bank Vole PrP Is a Universal Acceptor for Prions
- Rapid Response to Selection, Competitive Release and Increased Transmission Potential of Artesunate-Selected Malaria Parasites
- Inactivation of Genes for Antigenic Variation in the Relapsing Fever Spirochete Reduces Infectivity in Mice and Transmission by Ticks
- Exposure-Dependent Control of Malaria-Induced Inflammation in Children
- A Neutralizing Anti-gH/gL Monoclonal Antibody Is Protective in the Guinea Pig Model of Congenital CMV Infection
- The Apical Complex Provides a Regulated Gateway for Secretion of Invasion Factors in
- A Highly Conserved Haplotype Directs Resistance to Toxoplasmosis and Its Associated Caspase-1 Dependent Killing of Parasite and Host Macrophage
- A Quantitative High-Resolution Genetic Profile Rapidly Identifies Sequence Determinants of Hepatitis C Viral Fitness and Drug Sensitivity
- Histone Deacetylase Inhibitor Romidepsin Induces HIV Expression in CD4 T Cells from Patients on Suppressive Antiretroviral Therapy at Concentrations Achieved by Clinical Dosing
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The 2010 Cholera Outbreak in Haiti: How Science Solved a Controversy
- , , , Genetic Variability: Cryptic Biological Species or Clonal Near-Clades?
- Efficient Parvovirus Replication Requires CRL4-Targeted Depletion of p21 to Prevent Its Inhibitory Interaction with PCNA
- An Overview of Respiratory Syncytial Virus
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání


