-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaPlastic Proteins and Monkey Blocks: How Lentiviruses Evolved to Replicate in the Presence of Primate Restriction Factors
article has not abstract
Published in the journal: . PLoS Pathog 10(4): e32767. doi:10.1371/journal.ppat.1004017
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1004017Summary
article has not abstract
Introduction
Restriction factors are cellular factors that block virus infection, either by direct interaction with viral factors or by rendering the cellular environment incompatible with viral replication. Well-characterized restriction factors include SAMHD1 [1], [2], BST-2/tetherin [3], [4], APOBEC3G [5], and TRIM5α [6]. In general, viruses evolve resistance to the restriction factors of their natural hosts but may still be sensitive to homologs of the same restriction factors from other organisms. Thus, restriction factors are potentially major determinants of virus host range in nature. Much of the evidence favoring this hypothesis comes from comparative studies of the primate lentiviruses, including HIV-1, HIV-2, and the simian immunodeficiency viruses (SIV) of African primates. While cell-culture studies have deduced molecular details of restriction, comparative evolutionary analyses are helping to reveal the biological impact of restriction in nature.
How Have Primate Lentiviruses Helped Us Better Understand Restriction Factors?
Since the discoveries of HIV-1 and HIV-2 in humans and SIVmac in captive macaques, over 40 primate lentiviruses have been identified, all in African primates [7]. Phylogenetic comparisons revealed that HIV-1 groups M, N, O, and P arose by transmission of ape viruses (SIVcpz and SIVgor) to humans, while lentiviruses from sooty mangabeys (SIVsmm) also jumped to humans, giving rise to multiple HIV-2 groups [7]. Accidental transmission of SIVsmm occurred in colonies of captive macaques in the United States, emerging as SIVmac [7], [8]. In addition to these documented events, phylogenetic analyses suggest that the natural history of primate lentiviruses is rife with cross-species transmission events [7]. These complex retroviruses encode multiple accessory proteins that interfere with restriction, including Vif, Vpx, Vpr, Vpu, and Nef (Figure 1; Table 1). Thus, comparative studies of the primate lentiviruses and their hosts can shed light on the evolutionary and biological significance of restriction.
Fig. 1. Accessory proteins are the most diverse of the primate lentivirus proteins. 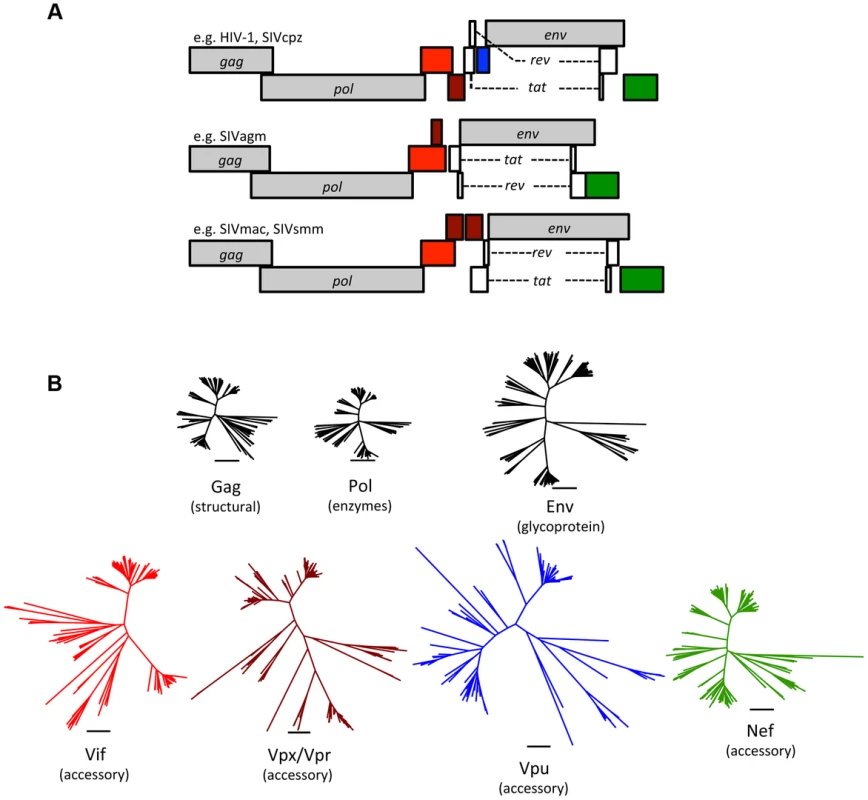
(A) Genomes of primate lentiviruses. Schematic representations of the three major types of genome organization found among primate lentiviruses. Genes encoding structural proteins (gag, pol, and env) are shown in gray. The regulatory genes, tat and rev, are in white. Accessory genes are color-coded to match the phylogenetic trees in the lower panel. (B) A comparison of genetic diversity among primate lentivirus proteins. Note that the accessory proteins are much more diverse than the structural proteins. Neighbor-Joining trees were generated using sequence alignments of primate lentiviruses available from Los Alamos National Labs (http://www.hiv.lanl.gov/). The Vpx and Vpr proteins are paralogs that arose by duplication during evolution of the primate lentiviruses and were therefore combined into a single tree. Tab. 1. Primate lentiviruses have an expanded repertoire of accessory genes. 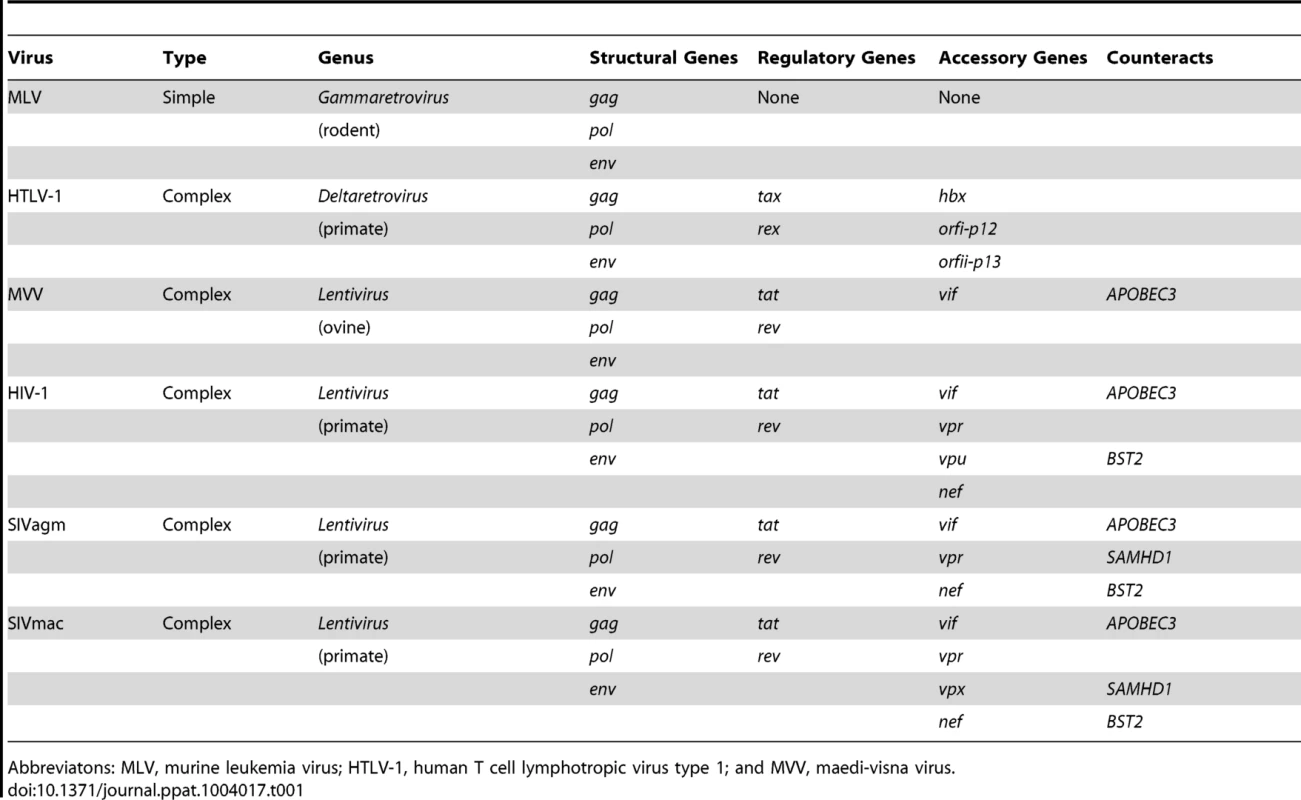
Abbreviatons: MLV, murine leukemia virus; HTLV-1, human T cell lymphotropic virus type 1; and MVV, maedi-visna virus. How Does a Virus Accessorize for (Evolutionary) Success?
All retroviruses share a core set of genes: gag, pro, pol, and env (Table 1). Gag encodes virion structural proteins; pol, the viral enzymes; and env, the viral glycoproteins. Complex retroviruses, including lentiviruses, encode a variable number of additional proteins that serve a variety of modulatory functions. Null mutations in these accessory genes often result in attenuation of replication in vivo, even when there is little or no effect on virus replication in cell culture [9]. The accessory genes are clustered along with env in the 3′ half of the viral genome, separate from gag, pro, and pol (Figure 1). This segregation is not unique to complex retroviruses, and analogous arrangements are found in many other viruses. It is tempting to speculate that physical separation of genes encoding conserved functions (structural proteins and enzymes) from variable functions (accessory proteins and surface glycoproteins) allows greater adaptive flexibility.
The accessory proteins are the most divergent lentivirus proteins (only the Env protein displays similar levels of diversity) (Figure 1). The complement of accessory genes is not identical for all primate lentiviruses; for example, several do not have a vpx gene (e.g., HIV-1 and SIVcpz), and many do not have vpu (e.g., SIVsmm and SIVmac) (Figure 1). If the comparison is expanded to include the “nonprimate” lentiviruses (e.g., lentiviruses found in cats, cattle, horses, and small ruminants), a distinct set of accessory genes is found, with only vif being common to both primate and nonprimate lentiviruses (Table 1). Thus, lentivirus accessory genes vary in primary sequence and overall composition. Such evolutionary plasticity is consistent with the notion that accessory genes help determine species tropism and may play a role in interspecies transmission and the emergence of lentiviruses.
Are No Two Restriction Factors Alike?
Comparative studies of HIV-1 and the other primate lentiviruses have revealed several host proteins that restrict viral replication. Four of these, SAMHD1, BST-2/tetherin, APOBEC3G, and TRIM5α, have been particularly well characterized and serve to demonstrate the diversity of restriction mechanisms.
TRIM5
The block imposed by TRIM5α occurs after viral entry but prior to provirus integration, whence it binds capsid cores in the cytoplasm and promotes premature uncoating and degradation [6], [10], [11]. Thus, the activity of TRIM5α is akin to cracking open the retroviral “egg” before the reverse transcriptase complex is ready to hatch. How TRIM5α recognizes divergent retroviruses is unknown but probably involves multimerization on the surface of incoming capsids through a series of high-avidity and low-affinity interactions [10]–[12]. Of the four examples, it is noteworthy that no viral antagonists of TRIM5α have been reported. Instead, resistance (or sensitivity) to TRIM5α is determined directly by variation in the viral capsid structure [6], [13], [14].
SAMHD1
SAMHD1, a deoxynucleoside triphosphate triphosphohydrolase, is targeted by lentiviral Vpx proteins [1], [2]. SAMHD1 depletes deoxyribonucleotide triphosphate (dNTP) pools within terminally differentiated target cells, such as the macrophage, thereby starving the reverse transcription complex of dNTPs [15]–[17]. Curiously, HIV-1 (and some other lentiviruses) do not encode Vpx or its functional equivalent, even though Vpx provided in trans dramatically enhances HIV-1 replication in the macrophage [15].
BST2
BST2 (also known as tetherin, CD31, or HM1.24) “traps” newly budded virions as they emerge from the surfaces of infected cells, even after complete scission of the virion and cellular membranes [3], [4], [11], [15]. These tethered virions are then removed from the cell surface by endocytosis and degraded inside the cell.
APOBEC3G
APOBEC3G is a cytosine deaminase that targets single-stranded DNA [5], [11], [15]. APOBEC3G expressed in the infected cell is incorporated into newly assembling retrovirus virions [5], [11], [15]. Reverse transcription in the next target cell produces a minus-strand DNA intermediate, which is attacked by APOBEC3G. Deamination of cytosines to uracils in the viral minus-strand DNA produces C-to-U mutations, resulting in a lethal dose of G-to-A substitutions in the coding strand [11], [15]
What Happens When a Virus Accessory Protein Meets a Host Restriction Factor?
SAMHD1, BST-2, and APOBEC3G are evolutionarily and mechanistically distinct from one another but share a common feature: the means by which they inhibit viruses are relatively nonspecific. For example, SAMHD1 inhibits viral replication indirectly by limiting the availability of precursors of DNA synthesis [1], [2], [15]–[17], potentially affecting any virus for which DNA synthesis is essential [18]. Likewise, BST-2 can, in theory, “capture” any membrane-enveloped structure (e.g., a virion) as it buds from the cell surface [11], [15]. APOBEC3G acts on single-stranded DNA produced during reverse transcription, but there is no evidence that the enzyme has a selective preference for viral DNA over other single-stranded DNAs. In other words, none of these factors targets a specific viral protein, and consequently, viral resistance does not result from escape mutations in a binding site or epitope. Instead, all three factors are targeted by viral accessory proteins, and interactions with these viral antagonists are primary determinants of viral sensitivity to restriction.
The Vif and Vpx proteins use similar mechanisms to overcome restriction by APOBEC3 and SAMHD1, respectively. In both cases, the viral protein couples its target to ubiquitin-ligase complexes, resulting in proteasome-mediated degradation of the restriction factor [11], [15]. Vpu also engages cellular ubiquitin ligase complexes, which may contribute, in part, to removal or sequestration of BST-2/tetherin from the cell surface. Vpr is a paralog of Vpx, and like Vpx, it interacts with the cellular ubitquitin-ligase machinery [1], [2], [19]. The cellular target of HIV-1 Vpr remains to be discovered, although in some SIV lineages Vpr has anti-SAMHD1 activity [20].
Lentiviral Nef proteins modulate cell-surface expression of many cellular proteins, and for some SIV strains, Nef is the primary antagonist of BST-2 [21], [22]. Interestingly, when SIVsmm jumped to humans and became HIV-2, the Nef protein could not interact with human BST-2 [7], [21], [22]. Consequently, the Env protein of HIV-2 evolved the capacity to counteract human BST-2 [23]. Similarly, SIVcpz Nef cannot engage human BST-2, and emergence of HIV-1 involved adaptation of Vpu to take on this function [7], [21], [22].
What Can Molecular Evolution Tell Us about the Significance of Restriction Factors?
SIVmac arose by unintentional transmission of SIVsmm from African mangabeys to Asian macaques in captivity. Because of similarities to HIV infection and AIDS, SIV infection of macaques is a major animal model for AIDS research. SIV strains with accessory genes inactivated (individually and in different combinations) are significantly attenuated in macaques, the first hint that the accessory functions are important in vivo [9]. One group retrospectively analyzed macaques that had been vaccinated with an SIV strain lacking nef [24]. The virus in these animals acquired adaptive changes in env, giving the viral glycoprotein antitetherin activity and making up for the loss of Nef—a case of neofunctionalization belying the in vivo significance of tetherin-mediated restriction. Historical emergence of SIVmac in macaques also required adaptations in capsid, rendering it resistant to macaque homologs of TRIM5α [13], and in Vif, conferring the ability to target macaque alleles of APOBEC3G for degradation [25]. Similar adaptations occur in macaques experimentally infected with strains of SIVsmm [13], [26]
If viruses have had a major impact on host evolution, it is reasonable to expect that host genes encoding restriction factors will bear signatures of selection by viral pathogens. Indeed, this is the case, and there is very strong evidence that ancient selective events occurred during primate evolution involving all four factors [19], [27], [28]. Most strikingly, individual residues that show the most variability between species are often those that interact physically with viral targets or viral antagonists. The assumption that similar interactions were responsible for selective events in the past, coupled with phylogenetic and molecular clock analyses, has pushed the estimated age of SIVs from less than 1 million years to at least 8–15 million years [14], [28], [29]
Summary
Taken together, the existence of viral accessory proteins dedicated to thwarting restriction, evidence that restriction factors evolve under positive selection, and the in vivo impact of restriction in SIV and AIDS models strengthen the hypothesis that restriction factors are major determinants of host range in nature, acting as selective barriers to cross-species transmission of viral pathogens. Novel mechanisms of restriction continue to be discovered. For example, Schlafen-11 exploits differences in human and viral codon usage to restrict HIV-1 [30], and MX2/MXB is a capsid-sensing component of the interferon-induced block to HIV-1 infection [31]–[33]. As more restriction factors are identified and their mechanisms deduced, it will be interesting to ask whether the interplay between restriction factors and viruses is a dominant theme in cross-species transmission, adaptation, and emergence of viruses.
Zdroje
1. LaguetteN, SobhianB, CasartelliN, RingeardM, Chable-BessiaC, et al. (2011) SAMHD1 is the dendritic - and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature 474 : 654–657.
2. HreckaK, HaoC, GierszewskaM, SwansonSK, Kesik-BrodackaM, et al. (2011) Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature 474 : 658–661.
3. NeilSJ, ZangT, BieniaszPD (2008) Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451 : 425–430.
4. Van DammeN, GoffD, KatsuraC, JorgensonRL, MitchellR, et al. (2008) The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe 3 : 245–252.
5. SheehyAM, GaddisNC, ChoiJD, MalimMH (2002) Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature 418 : 646–650.
6. StremlauM, OwensCM, PerronMJ, KiesslingM, AutissierP, et al. (2004) The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature 427 : 848–853.
7. SharpPM, HahnBH (2011) Origins of HIV and the AIDS pandemic. Cold Spring Harb Perspect Med 1: a006841.
8. ApetreiC, KaurA, LercheNW, MetzgerM, PandreaI, et al. (2005) Molecular epidemiology of simian immunodeficiency virus SIVsm in U.S. primate centers unravels the origin of SIVmac and SIVstm. J Virol 79 : 8991–9005.
9. DesrosiersRC, LifsonJD, GibbsJS, CzajakSC, HoweAY, et al. (1998) Identification of highly attenuated mutants of simian immunodeficiency virus. J Virol 72 : 1431–1437.
10. GrutterMG, LubanJ (2012) TRIM5 structure, HIV-1 capsid recognition, and innate immune signaling. Curr Opin Virol 2 : 142–150.
11. MalimMH, BieniaszPD (2012) HIV Restriction Factors and Mechanisms of Evasion. Cold Spring Harb Perspect Med 2: a006940.
12. Ganser-PornillosBK, ChandrasekaranV, PornillosO, SodroskiJG, SundquistWI, et al. (2011) Hexagonal assembly of a restricting TRIM5alpha protein. Proc Natl Acad Sci U S A 108 : 534–539.
13. KirmaierA, WuF, NewmanRM, HallLR, MorganJS, et al. (2010) TRIM5 suppresses cross-species transmission of a primate immunodeficiency virus and selects for emergence of resistant variants in the new species. PLoS Biol 8: e1000462.
14. McCarthyKR, SchmidtAG, KirmaierA, WyandAL, NewmanRM, et al. (2013) Gain-of-sensitivity mutations in a Trim5-resistant primary isolate of pathogenic SIV identify two independent conserved determinants of Trim5alpha specificity. PLoS Pathog 9: e1003352.
15. HarrisRS, HultquistJF, EvansDT (2012) The restriction factors of human immunodeficiency virus. J Biol Chem 287 : 40875–40883.
16. GoldstoneDC, Ennis-AdeniranV, HeddenJJ, GroomHC, RiceGI, et al. (2011) HIV-1 restriction factor SAMHD1 is a deoxynucleoside triphosphate triphosphohydrolase. Nature 480 : 379–382.
17. LahouassaH, DaddachaW, HofmannH, AyindeD, LogueEC, et al. (2012) SAMHD1 restricts the replication of human immunodeficiency virus type 1 by depleting the intracellular pool of deoxynucleoside triphosphates. Nat Immunol 13 : 223–228.
18. HollenbaughJA, GeeP, BakerJ, DalyMB, AmieSM, et al. (2013) Host factor SAMHD1 restricts DNA viruses in non-dividing myeloid cells. PLoS Pathog 9: e1003481.
19. JohnsonWE (2013) Rapid adversarial co-evolution of viruses and cellular restriction factors. Curr Top Microbiol Immunol 371 : 123–151.
20. LimES, FregosoOI, McCoyCO, MatsenFA, MalikHS, et al. (2012) The ability of primate lentiviruses to degrade the monocyte restriction factor SAMHD1 preceded the birth of the viral accessory protein Vpx. Cell Host Microbe 11 : 194–204.
21. JiaB, Serra-MorenoR, NeidermyerW, RahmbergA, MackeyJ, et al. (2009) Species-specific activity of SIV Nef and HIV-1 Vpu in overcoming restriction by tetherin/BST2. PLoS Pathog 5: e1000429.
22. ZhangF, WilsonSJ, LandfordWC, VirgenB, GregoryD, et al. (2009) Nef proteins from simian immunodeficiency viruses are tetherin antagonists. Cell Host Microbe 6 : 54–67.
23. Le TortorecA, NeilSJ (2009) Antagonism to and intracellular sequestration of human tetherin by the human immunodeficiency virus type 2 envelope glycoprotein. J Virol 83 : 11966–11978.
24. Serra-MorenoR, JiaB, BreedM, AlvarezX, EvansDT (2011) Compensatory changes in the cytoplasmic tail of gp41 confer resistance to tetherin/BST-2 in a pathogenic nef-deleted SIV. Cell Host Microbe 9 : 46–57.
25. KruppA, McCarthyKR, OomsM, LetkoM, MorganJS, et al. (2013) APOBEC3G polymorphism as a selective barrier to cross-species transmission and emergence of pathogenic SIV and AIDS in a primate host. PLoS Pathog 9: e1003641.
26. WuF, KirmaierA, GoekenR, OurmanovI, HallL, et al. (2013) TRIM5 alpha drives SIVsmm evolution in rhesus macaques. PLoS Pathog 9: e1003577.
27. MeyersonNR, SawyerSL (2011) Two-stepping through time: mammals and viruses. Trends Microbiol 19 : 286–294.
28. EmermanM, MalikHS (2010) Paleovirology—modern consequences of ancient viruses. PLoS Biol 8: e1000301.
29. ComptonAA, EmermanM (2013) Convergence and Divergence in the Evolution of the APOBEC3G-Vif Interaction Reveal Ancient Origins of Simian Immunodeficiency Viruses. PLoS Pathog 9: e1003135.
30. LiM, KaoE, GaoX, SandigH, LimmerK, et al. (2012) Codon-usage-based inhibition of HIV protein synthesis by human schlafen 11. Nature 491 : 125–128.
31. GoujonC, MoncorgeO, BaubyH, DoyleT, WardCC, et al. (2013) Human MX2 is an interferon-induced post-entry inhibitor of HIV-1 infection. Nature 502 : 559–562.
32. KaneM, YadavSS, BitzegeioJ, KutluaySB, ZangT, et al. (2013) MX2 is an interferon-induced inhibitor of HIV-1 infection. Nature 502 : 563–566.
33. LiuZ, PanQ, DingS, QianJ, XuF, et al. (2013) The interferon-inducible MxB protein inhibits HIV-1 infection. Cell Host Microbe 14 : 398–410.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Affinity Proteomics Reveals Elevated Muscle Proteins in Plasma of Children with Cerebral MalariaČlánek The Transcriptional Activator LdtR from ‘ Liberibacter asiaticus’ Mediates Osmotic Stress ToleranceČlánek Complement-Related Proteins Control the Flavivirus Infection of by Inducing Antimicrobial PeptidesČlánek Fungal Chitin Dampens Inflammation through IL-10 Induction Mediated by NOD2 and TLR9 ActivationČlánek Parasite Fate and Involvement of Infected Cells in the Induction of CD4 and CD8 T Cell Responses to
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 4- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- , , , Genetic Variability: Cryptic Biological Species or Clonal Near-Clades?
- Early Mortality Syndrome Outbreaks: A Microbial Management Issue in Shrimp Farming?
- Wormholes in Host Defense: How Helminths Manipulate Host Tissues to Survive and Reproduce
- Plastic Proteins and Monkey Blocks: How Lentiviruses Evolved to Replicate in the Presence of Primate Restriction Factors
- The 2010 Cholera Outbreak in Haiti: How Science Solved a Controversy
- Affinity Proteomics Reveals Elevated Muscle Proteins in Plasma of Children with Cerebral Malaria
- Noncanonical Role for the Host Vps4 AAA+ ATPase ESCRT Protein in the Formation of Replicase
- Efficient Parvovirus Replication Requires CRL4-Targeted Depletion of p21 to Prevent Its Inhibitory Interaction with PCNA
- Host-to-Pathogen Gene Transfer Facilitated Infection of Insects by a Pathogenic Fungus
- The Transcriptional Activator LdtR from ‘ Liberibacter asiaticus’ Mediates Osmotic Stress Tolerance
- Coxsackievirus B Exits the Host Cell in Shed Microvesicles Displaying Autophagosomal Markers
- TCR Affinity Associated with Functional Differences between Dominant and Subdominant SIV Epitope-Specific CD8 T Cells in Rhesus Monkeys
- Coxsackievirus-Induced miR-21 Disrupts Cardiomyocyte Interactions via the Downregulation of Intercalated Disk Components
- Ligands of MDA5 and RIG-I in Measles Virus-Infected Cells
- Kind Discrimination and Competitive Exclusion Mediated by Contact-Dependent Growth Inhibition Systems Shape Biofilm Community Structure
- Structural Differences Explain Diverse Functions of Actins
- HSCARG Negatively Regulates the Cellular Antiviral RIG-I Like Receptor Signaling Pathway by Inhibiting TRAF3 Ubiquitination Recruiting OTUB1
- Vaginitis: When Opportunism Knocks, the Host Responds
- Complement-Related Proteins Control the Flavivirus Infection of by Inducing Antimicrobial Peptides
- Fungal Chitin Dampens Inflammation through IL-10 Induction Mediated by NOD2 and TLR9 Activation
- Microbial Pathogens Trigger Host DNA Double-Strand Breaks Whose Abundance Is Reduced by Plant Defense Responses
- Alveolar Macrophages Are Essential for Protection from Respiratory Failure and Associated Morbidity following Influenza Virus Infection
- An Interaction between Glutathione and the Capsid Is Required for the Morphogenesis of C-Cluster Enteroviruses
- Concerted Spatio-Temporal Dynamics of Imported DNA and ComE DNA Uptake Protein during Gonococcal Transformation
- Potent Dengue Virus Neutralization by a Therapeutic Antibody with Low Monovalent Affinity Requires Bivalent Engagement
- Regulation of Human T-Lymphotropic Virus Type I Latency and Reactivation by HBZ and Rex
- Functionally Redundant RXLR Effectors from Act at Different Steps to Suppress Early flg22-Triggered Immunity
- The Pathogenic Mechanism of the Virulence Factor, Mycolactone, Depends on Blockade of Protein Translocation into the ER
- Role of Calmodulin-Calmodulin Kinase II, cAMP/Protein Kinase A and ERK 1/2 on -Induced Apoptosis of Head Kidney Macrophages
- An Overview of Respiratory Syncytial Virus
- First Experimental Model of Enhanced Dengue Disease Severity through Maternally Acquired Heterotypic Dengue Antibodies
- Binding of Glutathione to Enterovirus Capsids Is Essential for Virion Morphogenesis
- IFITM3 Restricts Influenza A Virus Entry by Blocking the Formation of Fusion Pores following Virus-Endosome Hemifusion
- Parasite Fate and Involvement of Infected Cells in the Induction of CD4 and CD8 T Cell Responses to
- Deficient IFN Signaling by Myeloid Cells Leads to MAVS-Dependent Virus-Induced Sepsis
- Pernicious Pathogens or Expedient Elements of Inheritance: The Significance of Yeast Prions
- The HMW1C-Like Glycosyltransferases—An Enzyme Family with a Sweet Tooth for Simple Sugars
- The Expanding Functions of Cellular Helicases: The Tombusvirus RNA Replication Enhancer Co-opts the Plant eIF4AIII-Like AtRH2 and the DDX5-Like AtRH5 DEAD-Box RNA Helicases to Promote Viral Asymmetric RNA Replication
- Mining Herbaria for Plant Pathogen Genomes: Back to the Future
- Inferring Influenza Infection Attack Rate from Seroprevalence Data
- A Human Lung Xenograft Mouse Model of Nipah Virus Infection
- Mast Cells Expedite Control of Pulmonary Murine Cytomegalovirus Infection by Enhancing the Recruitment of Protective CD8 T Cells to the Lungs
- Cytosolic Peroxidases Protect the Lysosome of Bloodstream African Trypanosomes from Iron-Mediated Membrane Damage
- Abortive T Follicular Helper Development Is Associated with a Defective Humoral Response in -Infected Macaques
- JC Polyomavirus Infection Is Strongly Controlled by Human Leucocyte Antigen Class II Variants
- Cationic Antimicrobial Peptides Promote Microbial Mutagenesis and Pathoadaptation in Chronic Infections
- Estimating the Fitness Advantage Conferred by Permissive Neuraminidase Mutations in Recent Oseltamivir-Resistant A(H1N1)pdm09 Influenza Viruses
- Progressive Accumulation of Activated ERK2 within Highly Stable ORF45-Containing Nuclear Complexes Promotes Lytic Gammaherpesvirus Infection
- Caspase-1-Like Regulation of the proPO-System and Role of ppA and Caspase-1-Like Cleaved Peptides from proPO in Innate Immunity
- Is Required for High Efficiency Viral Replication
- Modified Vaccinia Virus Ankara Triggers Type I IFN Production in Murine Conventional Dendritic Cells via a cGAS/STING-Mediated Cytosolic DNA-Sensing Pathway
- Evidence That Bank Vole PrP Is a Universal Acceptor for Prions
- Rapid Response to Selection, Competitive Release and Increased Transmission Potential of Artesunate-Selected Malaria Parasites
- Inactivation of Genes for Antigenic Variation in the Relapsing Fever Spirochete Reduces Infectivity in Mice and Transmission by Ticks
- Exposure-Dependent Control of Malaria-Induced Inflammation in Children
- A Neutralizing Anti-gH/gL Monoclonal Antibody Is Protective in the Guinea Pig Model of Congenital CMV Infection
- The Apical Complex Provides a Regulated Gateway for Secretion of Invasion Factors in
- A Highly Conserved Haplotype Directs Resistance to Toxoplasmosis and Its Associated Caspase-1 Dependent Killing of Parasite and Host Macrophage
- A Quantitative High-Resolution Genetic Profile Rapidly Identifies Sequence Determinants of Hepatitis C Viral Fitness and Drug Sensitivity
- Histone Deacetylase Inhibitor Romidepsin Induces HIV Expression in CD4 T Cells from Patients on Suppressive Antiretroviral Therapy at Concentrations Achieved by Clinical Dosing
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The 2010 Cholera Outbreak in Haiti: How Science Solved a Controversy
- , , , Genetic Variability: Cryptic Biological Species or Clonal Near-Clades?
- Efficient Parvovirus Replication Requires CRL4-Targeted Depletion of p21 to Prevent Its Inhibitory Interaction with PCNA
- An Overview of Respiratory Syncytial Virus
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



