-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Efficient Parvovirus Replication Requires CRL4-Targeted Depletion of p21 to Prevent Its Inhibitory Interaction with PCNA
Many DNA viruses induce and then exploit host cellular DNA damage responses to generate a suitable environment for their continued replication. Parvoviruses, important disease agents in both humans and animals, rely on host DNA polymerases to replicate their genomes in cell-cycle arrested cells. We show that efficient parvovirus replication requires the recruitment to viral replication compartments of a host cellular E3-ubiquitin ligase, CRL4Cdt2, to target the potent cell cycle regulator p21 for subsequent degradation. The DNA polymerase-δ cofactor PCNA provides a molecular platform for initial substrate recognition by this ligase, and subsequent p21 depletion prevents its continued interaction with PCNA which otherwise inhibits efficient viral replication. Virally-induced p21 degradation represents another way of promoting efficient replication of DNA polymerase-δ-dependent viruses.
Published in the journal: . PLoS Pathog 10(4): e32767. doi:10.1371/journal.ppat.1004055
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004055Summary
Many DNA viruses induce and then exploit host cellular DNA damage responses to generate a suitable environment for their continued replication. Parvoviruses, important disease agents in both humans and animals, rely on host DNA polymerases to replicate their genomes in cell-cycle arrested cells. We show that efficient parvovirus replication requires the recruitment to viral replication compartments of a host cellular E3-ubiquitin ligase, CRL4Cdt2, to target the potent cell cycle regulator p21 for subsequent degradation. The DNA polymerase-δ cofactor PCNA provides a molecular platform for initial substrate recognition by this ligase, and subsequent p21 depletion prevents its continued interaction with PCNA which otherwise inhibits efficient viral replication. Virally-induced p21 degradation represents another way of promoting efficient replication of DNA polymerase-δ-dependent viruses.
Introduction
Minute Virus of Mice (MVM) is an autonomously-replicating parvovirus which induces a DNA damage response resulting in substantial p53 activation which persists throughout the course of viral replication [1]. p53 is a well-established activator of p21WAF1/Cip1 (hereafter referred to as p21) expression. Transient expression of the MVM NS1 protein alone also leads to an increase in p21 levels [2], [3]. However, surprisingly, while these signals lead to an increase in p21 RNA accumulation, p21 protein levels remain low throughout the course of infection, including during the prolonged G2 phase in which the viral genome is replicated [2]. p21 can be a potent antiviral factor and possesses several potentially inhibitory activities including cyclin-dependent kinase (CDK) inhibition and repression of E2F1-mediated expression [4]. In addition, p21 has been shown to be an effective inhibitor of the DNA polymerase δ cofactor PCNA [5]–[7], and it has been shown to inhibit MVM replication in vitro [8]. p21 depletion during MVM infection was shown to be proteasomally mediated, suggesting that an E3 ubiquitin ligase was involved in targeting p21 for degradation [2].
Viruses often make use of the ubiquitin conjugation machinery to target for degradation cellular proteins that might otherwise negatively affect viral replication [9]. The Cullin-RING Ligase (CRL) CRL4Cdt2 consists of the scaffold protein Cullin 4 and the homo-trimeric protein DDB1 which serves as an adaptor for the putative substrate recognition protein Cdt2. This ligase has been shown to program the ubiquitination and subsequent degradation of p21 in response to DNA damaging agents such as UV treatment in order to ensure low p21 levels during S-phase [10]–[12]. Upon DNA damage or S-phase entry CRL4Cdt2 is recruited to chromatin via PCNA interaction where it targets substrate proteins for degradation [13].
We show here that efficient MVM replication in S/G2 arrested cells required the targeting for proteasomal degradation of p21 by the CRL4Cdt2 E3-ubiquitin ligase which was re-localized to viral chromatin within active MVM replication centers. PCNA provides a molecular platform that aids substrate recognition by the CRL4Cdt2 E3-ubiquitin ligase, and p21 targeting to this ligase during MVM infection required its interaction with PCNA. PCNA is also an important co-factor for DNA polymerase δ-dependent MVM replication which can be antagonized by p21 in vitro. Expression of a stable p21 mutant that retained interaction with PCNA inhibited MVM replication, while a stable p21 mutant which could no longer interact with PCNA did not. Introduction of a p21-derived peptide that bound to PCNA also substantially decreased viral replication. Our results suggest that interaction with PCNA was important for targeting p21 to the re-localized CRL4Cdt2 ubiquitin ligase, yet subsequent depletion of p21 was required to prevent its sustained interaction with PCNA which otherwise inhibited efficient viral replication.
Results
The CRL4Cdt2 ligase mediates p21 degradation during MVM infection
The CRL4Cdt2 E3 ubiquitin ligase has been implicated in targeting p21 for proteasomal degradation upon S-phase entry and after cellular DNA damage [10]–[12]. Was this ubiquitin ligase also enlisted to target p21 at late times during MVM infection when cells were blocked at the G2/M border? To test this possibility, DDB1 and Cdt2, components of this ligase which are not present in other E3 ubiquitin ligases known to modify p21 [13], [14], were targeted via RNAi in the protocol illustrated in Figure 1A.
Fig. 1. p21 degradation is mediated by the CRL4Cdt2 ligase complex. 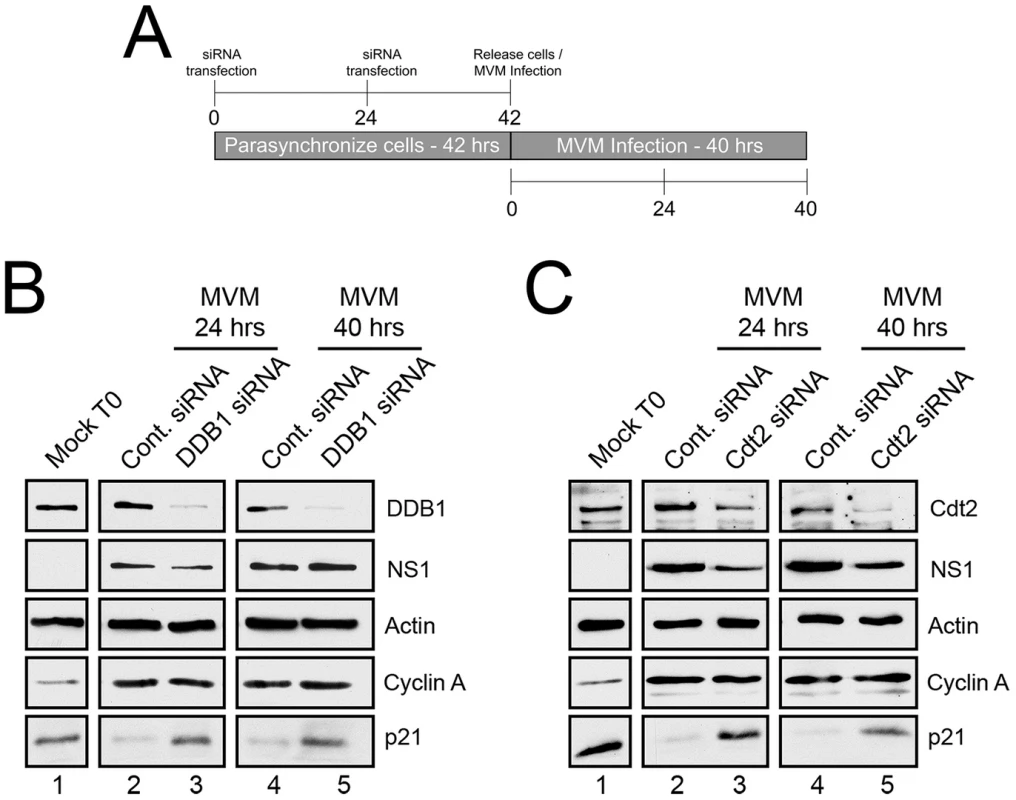
A) Schematic illustrating the experimental protocol for siRNA knockdown of ligase components in Figures 1B and 1C. B and C) p21 degradation requires DDB1 (B) and Cdt2 (C). Murine A9 cells were targeted with control siRNA or siRNA to DDB1 (B) or Cdt2 (C) as depicted in Figure 1A. Uninfected control cells were harvested at the time of release (Mock T0). Infections were done at the time of release at an MOI of 10 before harvest at 24 and 40 hr pi. Western blots were performed using antibodies against the indicated proteins. For these experiments cells were parasynchronized prior to infection to maximize the number of cells progressing uniformly through S-phase. This both synchronized infection and the characterization of p21 depletion. At the time of release from the synchronization procedure (as the cells progressed from G0 to G1) there were high levels of p21 expression (Mock T0, Figures 1B and 1C, lanes 1), which were reduced 24 hr post MVM infection (Figures 1B and 1C, lanes 2), and remained low up to 40 hr pi (Figures 1B and 1C, lanes 4). Targeting of endogenous DDB1 (panel 1B) or Cdt2 (panel 1C) by RNAi, which led to significant depletion of these proteins (Figure 1B and 1C, lanes 3 and 5), substantially prevented the loss of p21 both at 24 hr pi (Figures 1B and 1C, lanes 3), and also 40 hr pi (Figure 1B and 1C, lanes 5) – the latter being a point well past S-phase when infected cells are known to be arrested in G2 [1]. Expression of cyclin A indicated unperturbed entry into S-phase (Figures 1B and C, lanes 2–5), and expression of the viral NS1 protein indicated that the initiation of viral infection was unaffected by the RNAi protocol (Figures 1B and C, lanes 2–5). Similar results were also obtained following knockdown of Cullin 4A, a component of the CRL4Cdt2 ligase also present in several other ubiquitin ligases (data not shown). Taken together, these results indicated that the CRL4Cdt2 ligase was responsible for p21 degradation throughout virus infection.
CRL4Cdt2 also targets Set8 and Cdt1 for degradation during S-phase and in response to DNA damaging agents [15]–[17]. We also observed loss of Set8 in response to MVM infection (Figure S1). Cdt1 levels were not reduced for reasons not yet clear.
CRL4Cdt2 ligase function is required for efficient MVM replication
We next examined the functional consequence of CRL4Cdt2 E3 ligase depletion for viral replication using the protocol illustrated in Figure 2A. DDB1 knockdown resulted in an approximate 2-fold decrease in accumulated viral replicative forms at each time point compared to application of negative control siRNA (Figure 2B, compare lanes 1 to 2, 3 to 4, 5 to 6). Cdt2 knockdown during infection resulted in an approximate 2.5-fold decrease in accumulated viral replicative forms at each time point when compared to negative control siRNA (Figure 2C, compare lanes 1 to 2, 3 to 4, 5 to 6). Importantly, as mentioned above, expression of NS1 (Figure 1), and flow cytometric analysis (Figure S2), confirmed normal entry into S-phase following this synchronization and RNAi protocol which thus did not affect the S-phase dependent initiation of infection. These results demonstrated that the activity of the CRL4Cdt2 E3 ubiquitin ligase was necessary for efficient viral replication.
Fig. 2. The CRL4Cdt2 E3 ligase complex is important for MVM replication. 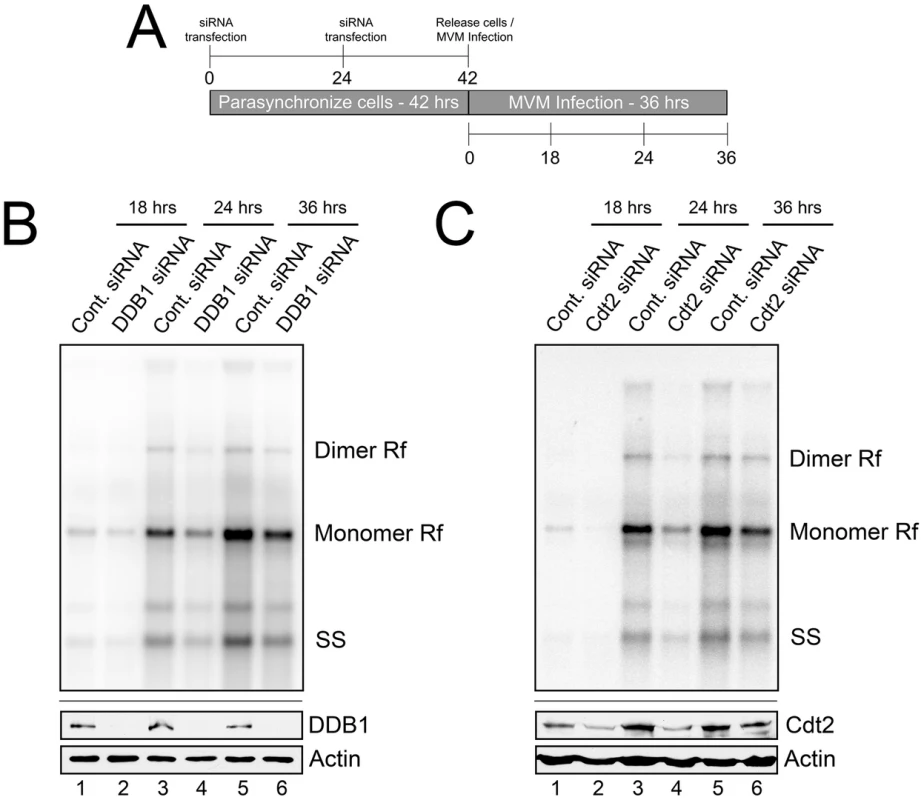
A) Schematic showing the experimental protocol for figures 2B and 2C. B and C) Upper panels: murine A9 cells treated with siRNA as shown in 2A were infected at an MOI of 0.5, harvested at the indicated time points and processed for Southern blotting using an MVM genomic probe. Rf - replicative forms. SS - single stranded genomic DNA. Representative Southern Blots are shown; quantifications in the text reflect two DDB1 and three Cdt2 separate knockdown experiments. Lower panels: western blots show knockdown of DDB1 and Cdt2 done in parallel experiments under identical conditions to replication assays. The CRL4Cdt2 ligase is recruited to viral replication compartments
MVM replicates in nuclear compartments termed autonomous parvovirus associated replication (APAR) bodies which have been shown to be enriched for cellular proteins such as DNA polymerase δ, RPA, cyclin A, and PCNA, which are also essential for parvovirus replication [18]. These nuclear bodies have been shown, via BrdU staining, to serve as sites of ongoing viral replication in infected cells and can be visualized by staining for the viral replicator protein NS1 [19]. Importantly, whereas punctate staining distributed throughout the nucleus was observed in mock treated cells, we detected recruitment of both DDB1and Cdt2 to NS1-containing viral replication compartments. Resistance to detergent pre-extraction prior to immunofluorescence (Figure 3A and 3B, note the merged images for each) also suggested that it may be bound to viral chromatin. These results indicated that MVM infection redirected the viral replication-enhancing CRL4Cdt2 ligase complex to APAR bodies. The recruitment was specific for the CRL4Cdt2 ligase because the APC/CCdc20 E3 ligase, which targets p21 for degradation after mitotic entry [20], was not similarly recruited (Figure S3). This is the first demonstration of the specific recruitment of a cellular E3 ubiquitin ligase to parvoviral replication compartments.
Fig. 3. The CRL4Cdt2 ligase is recruited to viral replication compartments. 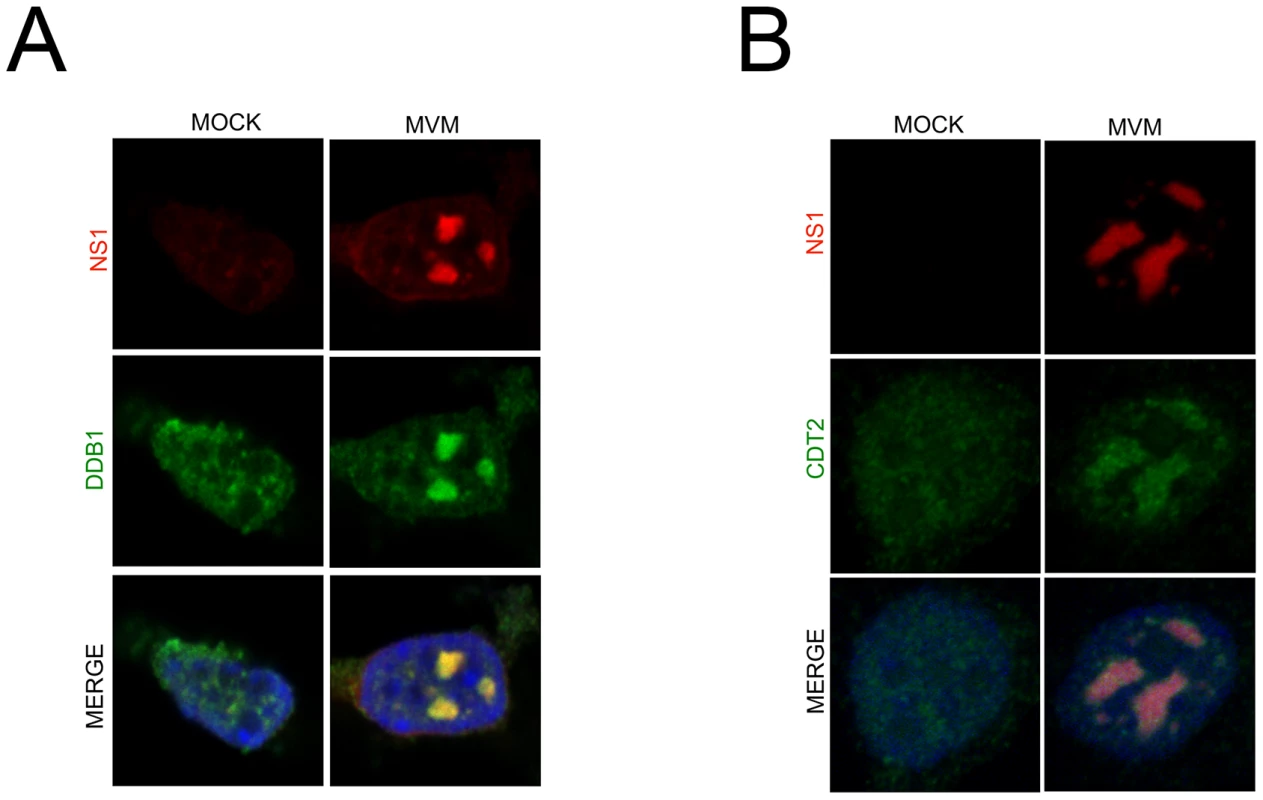
A and B) Murine A9 cells were mock infected or infected with MVM at an MOI of 10. At 24 hr pi cells were pre-extracted using cytoskeletal buffer and processed for IF using antibodies against NS1 and DDB1 (A) or Cdt2 (B). Depletion of p21 during MVM infection requires its interaction with the CRL4Cdt2 ligase and PCNA
The DNA polymerase δ cofactor PCNA is known to target the CRL4Cdt2 E3 ubiquitin ligase to cellular chromatin via its interaction with Cdt2 during normal cell division [13], [21]. Ubiquitin modification of p21 by the CRL4Cdt2 ligase requires Cdt2 interaction with the p21 degron motif, as well as interaction between p21 and PCNA via its PCNA-interaction (PIP) box [22]. p21 is recruited to UV-induced DNA lesions via interaction with PCNA, and a p21 mutant defective in binding to PCNA was resistant to degradation following UV treatment [10], [12].
To investigate the importance of these interactions for the MVM-dependent targeting of p21 by the CRL4Cdt2 ligase we generated stable murine cell lines via lentivirus transduction that conditionally expressed FLAG-tagged wild-type or mutant p21 (p21WT, p21ΔDegron, p21ΔPCNA, mutations shown in Figure 4A) in a doxycycline-responsive manner. As expected, MVM infection of a p21WT expressing cell line resulted in degradation of the tagged p21 (Figure 4C and 4E, compare lanes 2 to 4), which could be prevented by treating cells with the proteasome inhibitor MG132 (Figure S4, panel A), or via siRNA knockdown of CRL4Cdt2 components (Figure S4, panels B and C). These results suggested that the depletion of p21 in these cell lines occurred via a similar mechanism to that of the endogenous p21. The lysine at the p21 +4 position, 3′ to its PIP box, has been reported to be required for interaction of p21 with the CRL4Cdt2 ligase complex via Cdt2. Mutation of the +3 to +5 amino acids KRR to AAA (p21Δdegron) abolished p21 interaction with the CRL4Cdt2 complex following its transient transfection as reflected by loss of interaction with DDB1 (Figure 4B, compare lanes 2 to 3). Murine cell lines that expressed the p21Δdegron mutant were generated, and when infected with MVM, in contrast to cell lines expressing wild-type p21 (p21WT), the p21Δdegron protein was resistant to degradation (Figure 4C, compare lanes 6 and 8 to lanes 2 and 4). This suggested that MVM-induced p21 degradation required interaction of p21 with the CRL4Cdt2 ubiquitin ligase complex.
Fig. 4. p21 degradation during MVM requires interaction with PCNA and the CRL4Cdt2 ligase complex. 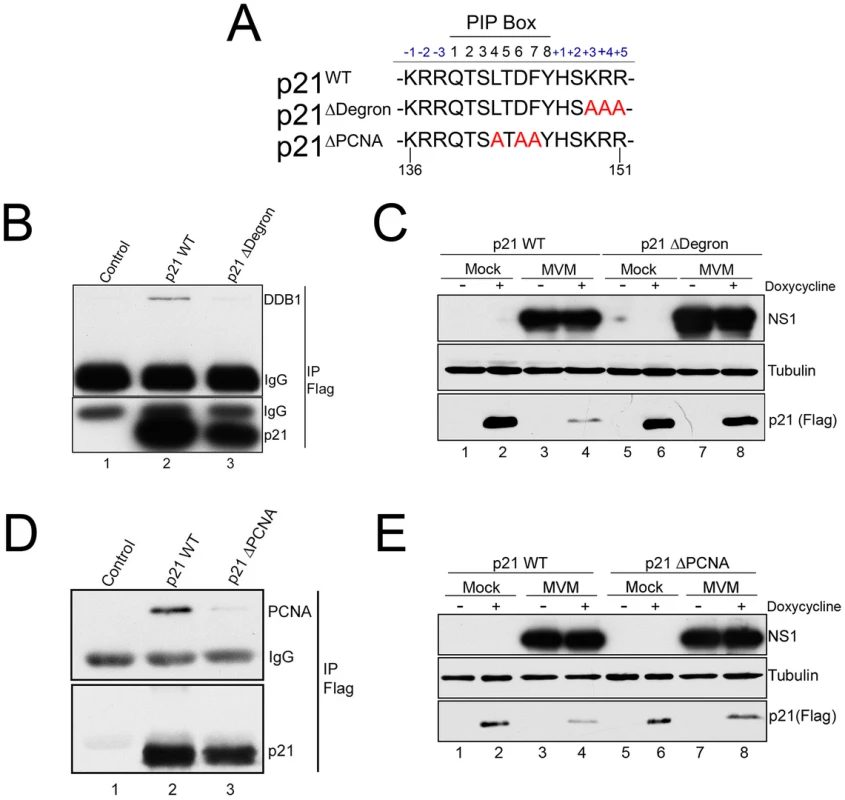
A) Illustration of the p21 PIP/degron region (amino acid 136 to 151) of wild-type murine p21 (p21WT), p21Δdegron, and p21ΔPIP. Mutations are shown in red. B) p21Δdegron does not interact with the CRL4Cdt2 complex. 293T were cells transfected with constructs expressing FLAG-tagged p21WT (lane 2), p21Δdegron (lane 3) or control plasmid (lane 1). Cells were harvested at 48 hr. Lysates were immunoprecipitated using with FLAG antibody and blotted against the indicated proteins. C) MVM degradation of p21 requires its interaction with the CRL4Cdt2 ligase complex. Murine A9 cell lines stably expressing p21WT and p21Δdegron were generated as described. Cells were parasynchronized, released into complete media and mock-infected or infected with MVM at an MOI of 10. At 20 hr pi cells were treated with doxycycline to induce p21 expression. Cells were harvested 6 hrs later and processed for western blotting using the indicated antibodies. D) p21ΔPCNA does not interact with PCNA. Experiment performed as for Figure 4B. E) MVM degradation of p21 requires its interaction with PCNA. Experiment performed as for 4C. The CRL4Cdt2 ligase also requires PCNA as a cofactor for targeting of its substrates [13]. Mutation of the p21 PIP box (p21ΔPCNA, Figure 4A) abrogated interaction with PCNA (Figure 4D, compare lane 3 to 2), and the p21ΔPCNA protein expressed in murine cells was not degraded as efficiently as the wild-type protein (p21WT) following infection (Figure 4E, compare lanes 8 and 6 to 4 and 2). These results suggested that PCNA-binding was also essential for effective CRL4Cdt2 targeting of p21 during MVM infection.
Stable p21 that retains interaction with PCNA inhibits MVM replication
p21 interaction with PCNA has been shown to interfere with DNA polymerase δ−mediated cellular DNA replication [5], [6]. PCNA is an important cofactor for MVM replication; it contributes to MVM replication in vitro [8], and is recruited to APAR bodies during infection. Thus, we investigated whether MVM-dependent depletion of p21 during infection, mediated by the CRL4Cdt2 E3 ubiquitin ligase, promoted efficient replication of the MVM genome by abrogating its inhibition of PCNA.
Unexpectedly, the p21ΔDegron mutant, although stable, interacted poorly with PCNA for reasons not yet clear (data not shown). As a result, we could not use cell lines expressing this mutant to determine whether stabilized, p21 affected MVM replication via PCNA binding. Thus we generated a murine cell line conditionally expressing a mutant p21 in which all seven lysines in p21 were changed to arginine [p21K7R, a similar mutation has been reported by others [23]]. Similar to the p21ΔDegron mutant, the p21K7R protein was resistant to degradation following MVM infection (Figure 5A, compare lanes 6 and 8 to 2 and 4), yet retained substantial interaction with PCNA in transient transfection assays (Figure 5B, compare lane 3 to lanes 2 and 4). Whereas induction of p21WT expression for 8 hrs after infection had little effect on MVM replication (Figure 5C, compare lanes 1 and 2), p21K7R expression reduced replication by up to 3 fold (Figure 5C, compare lanes 3 and 4). Importantly, in contrast, the p21ΔPCNA mutant-expressing cell line, which expressed a stable p21 which no longer could interact with PCNA (Figure 4D and 4E), failed to inhibit MVM replication upon induction (Figure 5D, compare lanes 3 and 4). This was also the case with the p21ΔDegron mutant-expressing cell lines (data not shown). Furthermore, cell lines expressing a mutant of p21K7R in the PIP box-mutated background (p21K7RΔPIP) (see Figure 5B, lane 4) also failed to inhibit MVM replication (Figure S5), demonstrating that absent PCNA binding, the K7R mutation itself had no deleterious effect on MVM replication. All the mutants tested were recruited to APAR bodies during MVM infection (Figure S6).
Fig. 5. A stabilized p21 that binds to PCNA inhibits MVM replication. 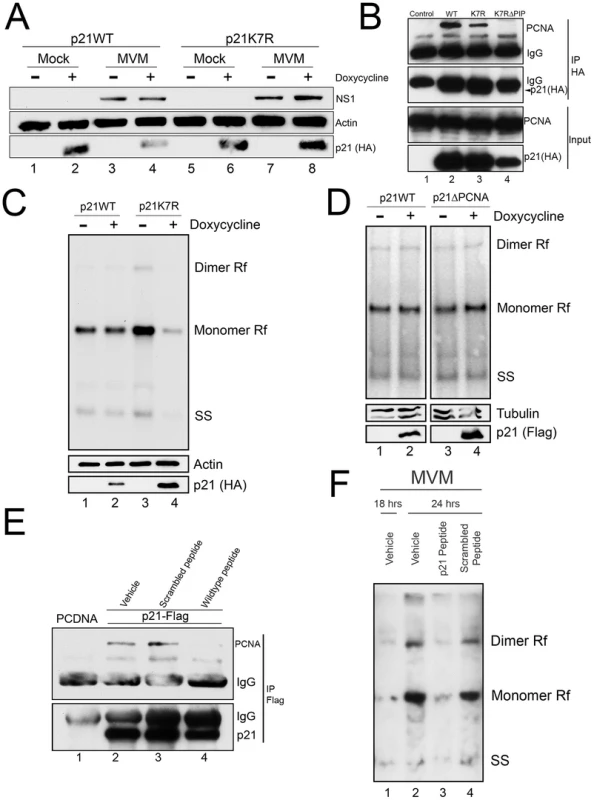
A) p21 degradation by MVM requires a ubiquitin-modifiable lysine. Murine A9 cell lines stably expressing HA-tagged wild-type p21 (p21WT) or a mutant in which all seven lysines have been mutated to arginines (p21K7R) were generated. p21 degradation assay was performed as described in 3C. B) p21K7R retains interaction with PCNA. Control plasmid (lane 1), p21WT (lane 2), p21K7R (lane 3), and p21K7R with additional mutations that disrupt PCNA interaction (p21K7RΔPIP, lane 4) were transfected into 293T cells. At 48 hr cells were lysed, immunoprecipitated with HA antibodies and blotted using the indicated antibodies. The p21K7RΔPIP was expressed at lower levels in this experiment however interaction with PCNA was not detected even upon longer exposure. C) p21K7R inhibits MVM replication. p21WT and p21K7R cell lines were parasynchronized, released and infected with MVM at an MOI of 0.5. At 16 hr pi cells were treated with doxycycline to induce p21 expression and harvested 8 hrs later. Cells were processed for Southern blotting (top panel), or for western blotting using the indicated antibodies (bottom panels). A representative experiment is shown; quantifications in the text reflect three independent experiments. D) p21ΔPCNA does not inhibit MVM replication. p21WT and p21ΔPCNA cells were treated, processed and standardized as in 4C. E) A p21 peptide competitively binds to PCNA. 293T cells were transfected with a construct expressing FLAG-tagged p21 (lanes 2 to 4) or control plasmid (PCDNA, lane 1) and processed for co-immunoprecipitation as in 4D. Treatment with wild-type but not scrambled peptide reduced interaction of p21 with PCNA. F) A p21-derived peptide inhibits MVM replication. Murine A9 cells were parasynchronized, released and infected with MVM at an MOI of 1. At 18 hr pi control cells were harvested (lane 1) and remainder were treated with vehicle (lane 2), wild-type peptide (lane 3) or scrambled peptide (lane 4) for 6 hrs. At 24 hr pi cells were harvested and processed for Southern blotting. A representative experiment is shown; the experiment was done three times. To confirm that the p21-PCNA interaction mediated the inhibitory role of p21 during infection, we made use of a previously described peptide containing 20 residues derived from sequences comprising the p21 PIP box [24] fused to a 16-mer penetratin motif to facilitate cellular entry [25]. A scrambled version of the p21 peptide linked to the penetratin peptide was used as control. Following application to murine cells, the wild-type (Figure 5E, lane 4), but not the scrambled version (Figure 5E, lane 3), disrupted the interaction between over-expressed FLAG-tagged p21 and endogenous PCNA, demonstrating that the peptide could competitively interact with PCNA. Subsequently, while treatment of cells with the scrambled peptide had no effect on MVM replication (Figure 5F, compare lanes 2 and 4), treatment of cells with the wild-type p21 PCNA-binding peptide significantly inhibited MVM replication (Figure 5F, compare lanes 2 and 3). These results indicated that a stabilized p21 interaction with PCNA was detrimental to viral replication. Thus, while interaction with PCNA was important for targeting p21 to the re-localized CRL4Cdt2 ligase, efficient viral replication required subsequent depletion of p21 and consequent abrogation of its inhibition of PCNA.
Discussion
Parvoviruses and other small DNA viruses rely on host polymerases to replicate their genomes. How the replication machinery is exploited to sustain parvovirus replication in G2-arrested cells, which normally contain potentially inhibitory cellular proteins such as p21, is not fully understood. p21 levels are high in G1, low in S-phase, restored in G2 phase, and are regulated by proteasomal degradation during cell cycle progression [4]. We and others have previously reported that expression of the parvoviral NS1 protein leads to increases in p21 levels [2], [3]. Additionally, p53, a transcriptional activator of p21, is significantly up-regulated and activated throughout MVM infection. Remarkably however, while these signals lead to increased p21 RNA accumulation, p21 protein levels remain low throughout infection [2]. Here we have identified the mechanism by which p21 was degraded upon parvovirus infection, and identified the consequence of this for virus replication.
Degradation of p21 during S-phase and in response to DNA damaging agents such as UV treatment is programmed by the CRL4Cdt2 ubiquitin ligase [13] and in this manuscript we have demonstrated that the same ligase targets p21 for degradation during MVM infection. The circumstances surrounding p21 degradation and the signals leading to it in the context of parvoviral infection, however, differ from how it occurs during S-phase. During MVM infection p21 loss persists for extended periods of time while virus is replicating in G2 arrested cells in the presence of high amounts of activated p53 and NS1 [2]. Additionally, whereas ATR activity is important for p21 degradation in response to various DDR-inducing agents [23], [26], the ATR substrate Chk1 is not activated during MVM replication (Adeyemi and Pintel, in preparation), suggesting that p21 degradation during infection may occur independently of ATR activity.
During MVM infection the CRL4Cdt2 ligase is recruited to viral replication centers. Recently, the CRL4Cdt2 ligase was shown to be recruited to cellular chromatin via direct PCNA interaction [21]. It is not yet clear whether similar mechanisms mediate CRL4Cdt2 recruitment to MVM APAR bodies; however, it appears that PCNA recruitment to MVM chromatin may represent a critical step leading to viral hijacking of the CRL4Cdt2 ligase. Neither interaction with PCNA nor the ligase was required for recruitment of p21 to replication centers, as all the mutants tested were relocalized to APAR bodies.
Stabilization of p21 via CRL4Cdt2 depletion led to reduced MVM replication subsequent to the initiation of genome replication following S-phase entry. This is the first published demonstration of the requirement and re-localization to replication centers of a specific cellular ubiquitin ligase during autonomous parvovirus replication. p21 is a potent inhibitor of CDKs and PCNA. Exactly how stabilized p21 inhibited MVM replication is not fully clear; however, interaction with PCNA mediated its inhibitory role. Using inducible cell lines expressing wild-type and mutant p21 proteins we demonstrated that p21 degradation during infection required motifs that mediate interaction with both Cdt2 and PCNA. PCNA is recruited to MVM chromatin [18] and is essential for parvovirus replication [27], [28]. Overexpression of a stable mutant p21 that retained interaction with PCNA, but could not be targeted for degradation by the CRL4Cdt2 ligase, led to reduced virus replication. Stable p21 mutants unable to bind PCNA did not affect MVM replication, indicating that other potential inhibitory functions of p21, such as CDK binding and promoter repression, were not detrimental to viral replication absent PCNA interaction. Additionally, introduction of a p21-derived peptide which maintained PCNA interaction abolished viral replication. Thus, p21 interaction with PCNA (and Cdt2) was necessary for targeting of p21 to the co-localized CRL4Cdt2 ligase, and its subsequent depletion also prevented its sustained interaction with PCNA that would otherwise be inhibitory to viral replication. Although our earlier work had suggested that Cdk2 activity was required for MVM replication and that p21 degradation might be necessary to prevent inhibition of CDKs [2], we have recently shown that Chk2 activation during MVM infection results in CDC25A degradation leading to partial CDK2 inhibition, independent of p21 [29]. Thus, while some level of CDK activity is required for MVM replication, prevention of CDK inhibition is not likely to be the critical reason for p21 degradation.
p21 has been shown to inhibit MVM replication in vitro, and this effect was shown to be overcome by the addition of increasing amounts of PCNA [8]. p21 binds to PCNA via its PIP box, a conserved motif shared by substrates of the CRL4Cdt2 ligase, as well as cellular proteins such as DNA polymerase δ, that are essential for replication but escape ubiquitin targeting due to the absence of a PIP degron [13]. The p68 subunit of DNA polymerase δ binds to PCNA via a similar hydrophobic pocket recognized by the p21 PIP box. Thus, while the mechanism of p21 inhibition of the DNA polymerase δ/PCNA complex has not been clearly resolved, a stabilized high-affinity interaction of p21 with the DNA polymerase δ binding pocket within PCNA could directly inhibit viral replication by competing with DNA polymerase δ for binding to its cofactor [30].
Due to its myriad effects on cell cycle progression and cancer, several viruses make use of different strategies to inactivate p21 during infection. For example, several oncogenic DNA viruses indirectly inhibit p21 by targeting p53 [31]. Additionally, papillomaviruses HPV E7 proteins sequester p21 thereby preventing its interaction with PCNA and CDKs [32], [33]. KSHV encodes a microRNA that down-regulates p21 in order to prevent cell cycle arrest [34]. p21 restricts HIV in myeloid cells of certain patients [35], [36], and a recent report demonstrated that this restriction may occur via ribonucleotide reductase-2 repression resulting in inhibition of dNTP biosynthesis [37]. Thus, cellular components required for viral genomic replication, such as dNTPs and polymerase cofactors, may be critically dependent on p21 depletion. Our findings present first a novel mechanism of p21 antagonism by a virus, namely, the use of a cellular E3 ligase recruited to viral replication factories. Further, we shown that efficient virus replication depends on the depletion of p21 to prevent its inhibition of PCNA. PCNA is an important cofactor for the replication of several DNA viruses. PCNA-mediated degradation of p21 in the context of viral infection may emerge as an important paradigm for allowing sustained viral replication of DNA polymerase-δ dependent viruses in infected cells.
Materials and Methods
Cell lines and drug treatments
Murine A9 and Human 293T cell lines were propagated as previously described [38], [39]. Stable doxycycline-inducible A9 cell lines were generated by infection of A9 cells with pseudotyped virus using the pINDUCER20 lentiviral system [40]. Cell lines were selected with 800 µg/ml of geneticin (Gibco) and maintained like regular A9s except for addition of geneticin. A9 cells were parasynchronized in G0 by isoleucine deprivation as previously described [1]. pINDUCER20 lentiviral transformed cell lines were induced with 500 ng/mL doxycycline hydrochloride (MP Biomedical). MG132 (Calbiochem) was added at a final concentration of 10 µM.
Viruses and infections
Wild-type MVMp was propagated and titered in murine A9 cell lines as described [1]. Pseudotyped viruses were generated by co-transfecting equal concentrations of HIV Gag/Pol, VSV-G and pINDUCER20 plasmids into 293T cells. Supernatants were collected and used to infect A9 cells.
Plasmids and DNA transfection
Murine wild-type p21 cDNA (Origene) was cloned into p3XFLAG-CMV 7.1 (Sigma). Additionally, p21 was tagged with a 3× HA tag by PCR mutagenesis. p21K7R was generated by PCR mutagenesis. p21ΔPCNA, p21Δdegron and p21K7RΔPIP (Q139A, L142A, F145A, Y146A) mutants were generated by site-directed mutagenesis (Agilent). FLAG and HA-tagged wild-type and mutant p21 were cloned into pDONR221 (Invitrogen) and pINDUCER20 using BP and LR clonase kits (Invitrogen) respectively. pINDUCER reagents were a gift from Guang Hu at NIH/NIEHS. DNA transfection was performed using LipoD293 (Signagen) or Lipofectamine (Invitrogen).
RNA interference
A9 cells were transfected twice with 40 nM Control siRNA (Qiagen #1022076), Cdt2 (DTL) siRNA (Dharmacon #L-045921-01-0005), Cul4A (Dharmacon #L-052208-00-0005) or DDB1 siRNA (Dharmacon #L-043146-01-0005). siRNA transfections were performed using HiPerfect (Qiagen).
Peptides
Wild-type p21 (KRRQTSMTDFYHSKRRLIFSRQIKIWFQNRRMKWKK) and scrambled p21 (KSTARHTKLSAQRYIRSFARRQIKIWFQNRRMKWKK) were purchased from Peptide 2.0 Inc. The peptides consist of p21-derived or scrambled sequences fused to penetratin – a 16 amino acid nuclear internalization sequence derived from the Antennapedia homeodomain [25]. Peptides were added to cells at 25 µM.
Antibodies
Commercially available antibodies used in this study were obtained from Bethyl (Cdc20, cat# A301-180A), Cell Signaling (Set8, cat# 2996S), Invitrogen (DDB1, cat# 399901; p21, cat# 556430), Millipore (Cdt1, cat# 06-1295; PCNA, cat# CBL407), Novus (Cullin 4A, cat# NB100-2267), Pierce (Actin, cat# MA515739), Sigma (FLAG, cat# F1804; HA, cat# H9658; Tubulin, cat# T4026), and Upstate (Cyclin A, cat# 06-138). Cdt2 antibody was a generous gift from Anindya Dutta (University of Virginia). All secondary antibodies were from Invitrogen. NS1 (CE10) and NS1/2 (M55) were previously described [1].
Immunoblot analysis
Immunoblots were performed as described previously [1]. Protein concentrations were quantified by Bradford assay and equal amounts of lysates were run.
Co-immunoprecipitation
FLAG and HA-tagged p21 was immunoprecipitated from 293T cells as previously described [12]. FLAG beads (Sigma) and HA antibodies were used for IP.
Immunofluorescence (IF)
For IF, A9 cells were grown on glass coverslips in 35 mm dishes and infected with MVMp at an MOI of 10. At 24 hr pi, cells were washed twice with cold phosphate-buffered saline (PBS) solution and then with cytoskeleton buffer [10 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES), pH 6.8, 100 mM NaCl, 300 mM sucrose, 1 mM MgCl2, 1 mM EGTA]. Afterwards cells were pre-extracted for in cytoskeletal buffer containing 0.5% Triton X-100, protease and phosphatase inhibitors for 3 minutes on ice, washed, fixed with 4% paraformaldehyde and stained for the indicated proteins. Nuclei were visualized by staining with To-Pro-3 (Invitrogen). The coverslips were mounted in Fluoromount-G (Southern Biotech) and images were acquired using a Zeiss LSM 510 META confocal microscope. All images were captured using an objective of 63×.
Analysis of viral DNA
Southern blots were carried out as previously described [38], using whole MVM genome probes. Loading of DNA samples was normalized using a nanodrop spectrophotometer. This procedure was verified using probes on Southern blots against mitochondrial DNA. Unless otherwise indicated, infections were carried out at a low MOI in order to maximize effects of siRNA knockdowns and overexpression analyses on viral replication. Representative Southern Blots are shown; quantifications in the text reflect multiple knockdown experiments.
Cell cycle analysis
A9 cells were fixed in 70% ethanol overnight at 4°C. Cells were then pelleted, washed in PBS and resuspended in PBS containing 0.2 mg/ml RNAse A for 1 hr at 37°C, then propidium iodide was added at 40 µg/ml. Flow cytometry was performed using FACScan (BD biosciences). Data were analyzed using Summit software (Beckman Coulter).
Supporting Information
Zdroje
1. AdeyemiRO, LandryS, DavisME, WeitzmanMD, PintelDJ (2010) Parvovirus minute virus of mice induces a DNA damage response that facilitates viral replication. PLoS Pathog 6: e1001141.
2. AdeyemiRO, PintelDJ (2012) Replication of minute virus of mice in murine cells is facilitated by virally induced depletion of p21. J Virol 86 : 8328–8332.
3. Op De BeeckA, Sobczak-ThepotJ, SirmaH, BourgainF, BrechotC, et al. (2001) NS1 - and minute virus of mice-induced cell cycle arrest: involvement of p53 and p21(cip1). J Virol 75 : 11071–11078.
4. AbbasT, DuttaA (2009) p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer 9 : 400–414.
5. ChenJ, JacksonPK, KirschnerMW, DuttaA (1995) Separate domains of p21 involved in the inhibition of Cdk kinase and PCNA. Nature 374 : 386–388.
6. ChenJ, PetersR, SahaP, LeeP, TheodorasA, et al. (1996) A 39 amino acid fragment of the cell cycle regulator p21 is sufficient to bind PCNA and partially inhibit DNA replication in vivo. Nucleic Acids Res 24 : 1727–1733.
7. WagaS, HannonGJ, BeachD, StillmanB (1994) The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature 369 : 574–578.
8. BashirT, HorleinR, RommelaereJ, WillwandK (2000) Cyclin A activates the DNA polymerase delta -dependent elongation machinery in vitro: A parvovirus DNA replication model. Proc Natl Acad Sci U S A 97 : 5522–5527.
9. RandowF, LehnerPJ (2009) Viral avoidance and exploitation of the ubiquitin system. Nat Cell Biol 11 : 527–534.
10. AbbasT, SivaprasadU, TeraiK, AmadorV, PaganoM, et al. (2008) PCNA-dependent regulation of p21 ubiquitylation and degradation via the CRL4Cdt2 ubiquitin ligase complex. Genes Dev 22 : 2496–2506.
11. KimY, StarostinaNG, KipreosET (2008) The CRL4Cdt2 ubiquitin ligase targets the degradation of p21Cip1 to control replication licensing. Genes Dev 22 : 2507–2519.
12. NishitaniH, ShiomiY, IidaH, MichishitaM, TakamiT, et al. (2008) CDK inhibitor p21 is degraded by a proliferating cell nuclear antigen-coupled Cul4-DDB1Cdt2 pathway during S phase and after UV irradiation. J Biol Chem 283 : 29045–29052.
13. HavensCG, WalterJC (2011) Mechanism of CRL4(Cdt2), a PCNA-dependent E3 ubiquitin ligase. Genes Dev 25 : 1568–1582.
14. SoriaG, GottifrediV (2010) PCNA-coupled p21 degradation after DNA damage: The exception that confirms the rule? DNA Repair (Amst) 9 : 358–364.
15. AbbasT, ShibataE, ParkJ, JhaS, KarnaniN, et al. (2010) CRL4(Cdt2) regulates cell proliferation and histone gene expression by targeting PR-Set7/Set8 for degradation. Mol Cell 40 : 9–21.
16. CentoreRC, HavensCG, ManningAL, LiJM, FlynnRL, et al. (2010) CRL4(Cdt2)-mediated destruction of the histone methyltransferase Set8 prevents premature chromatin compaction in S phase. Mol Cell 40 : 22–33.
17. JinJ, AriasEE, ChenJ, HarperJW, WalterJC (2006) A family of diverse Cul4-Ddb1-interacting proteins includes Cdt2, which is required for S phase destruction of the replication factor Cdt1. Mol Cell 23 : 709–721.
18. BashirT, RommelaereJ, CziepluchC (2001) In vivo accumulation of cyclin A and cellular replication factors in autonomous parvovirus minute virus of mice-associated replication bodies. J Virol 75 : 4394–4398.
19. CziepluchC, LampelS, GrewenigA, GrundC, LichterP, et al. (2000) H-1 parvovirus-associated replication bodies: a distinct virus-induced nuclear structure. J Virol 74 : 4807–4815.
20. AmadorV, GeS, SantamariaPG, GuardavaccaroD, PaganoM (2007) APC/C(Cdc20) controls the ubiquitin-mediated degradation of p21 in prometaphase. Mol Cell 27 : 462–473.
21. HavensCG, ShobnamN, GuarinoE, CentoreRC, ZouL, et al. (2012) Direct role for proliferating cell nuclear antigen in substrate recognition by the E3 ubiquitin ligase CRL4Cdt2. J Biol Chem 287 : 11410–11421.
22. HavensCG, WalterJC (2009) Docking of a specialized PIP Box onto chromatin-bound PCNA creates a degron for the ubiquitin ligase CRL4Cdt2. Mol Cell 35 : 93–104.
23. BendjennatM, BoulaireJ, JascurT, BricknerH, BarbierV, et al. (2003) UV irradiation triggers ubiquitin-dependent degradation of p21(WAF1) to promote DNA repair. Cell 114 : 599–610.
24. WarbrickE, LaneDP, GloverDM, CoxLS (1995) A small peptide inhibitor of DNA replication defines the site of interaction between the cyclin-dependent kinase inhibitor p21WAF1 and proliferating cell nuclear antigen. Curr Biol 5 : 275–282.
25. DerossiD, JoliotAH, ChassaingG, ProchiantzA (1994) The third helix of the Antennapedia homeodomain translocates through biological membranes. J Biol Chem 269 : 10444–10450.
26. LeeJY, YuSJ, ParkYG, KimJ, SohnJ (2007) Glycogen synthase kinase 3beta phosphorylates p21WAF1/CIP1 for proteasomal degradation after UV irradiation. Mol Cell Biol 27 : 3187–3198.
27. ChristensenJ, CotmoreSF, TattersallP (1997) A novel cellular site-specific DNA-binding protein cooperates with the viral NS1 polypeptide to initiate parvovirus DNA replication. J Virol 71 : 1405–1416.
28. ChristensenJ, TattersallP (2002) Parvovirus initiator protein NS1 and RPA coordinate replication fork progression in a reconstituted DNA replication system. J Virol 76 : 6518–6531.
29. AdeyemiRO, PintelDJ (2014) Parvovirus-induced depletion of cyclin b1 prevents mitotic entry of infected cells. PLoS Pathog 10: e1003891.
30. MoldovanGL, PfanderB, JentschS (2007) PCNA, the maestro of the replication fork. Cell 129 : 665–679.
31. GartelAL, RadhakrishnanSK (2005) Lost in transcription: p21 repression, mechanisms, and consequences. Cancer Res 65 : 3980–3985.
32. FunkJO, WagaS, HarryJB, EsplingE, StillmanB, et al. (1997) Inhibition of CDK activity and PCNA-dependent DNA replication by p21 is blocked by interaction with the HPV-16 E7 oncoprotein. Genes Dev 11 : 2090–2100.
33. JonesDL, AlaniRM, MungerK (1997) The human papillomavirus E7 oncoprotein can uncouple cellular differentiation and proliferation in human keratinocytes by abrogating p21Cip1-mediated inhibition of cdk2. Genes Dev 11 : 2101–2111.
34. GottweinE, CullenBR (2010) A human herpesvirus microRNA inhibits p21 expression and attenuates p21-mediated cell cycle arrest. J Virol 84 : 5229–5237.
35. ChenH, LiC, HuangJ, CungT, SeissK, et al. (2011) CD4+ T cells from elite controllers resist HIV-1 infection by selective upregulation of p21. J Clin Invest 121 : 1549–1560.
36. Saez-CirionA, HamimiC, BergamaschiA, DavidA, VersmisseP, et al. (2011) Restriction of HIV-1 replication in macrophages and CD4+ T cells from HIV controllers. Blood 118 : 955–964.
37. AllouchA, DavidA, AmieSM, LahouassaH, ChartierL, et al. (2013) p21-mediated RNR2 repression restricts HIV-1 replication in macrophages by inhibiting dNTP biosynthesis pathway. Proc Natl Acad Sci U S A 110: E3997–4006 doi: 10.1073/pnas.1306719110
38. ChoiEY, NewmanAE, BurgerL, PintelD (2005) Replication of minute virus of mice DNA is critically dependent on accumulated levels of NS2. J Virol 79 : 12375–12381.
39. NayakR, PintelDJ (2007) Positive and negative effects of adenovirus type 5 helper functions on adeno-associated virus type 5 (AAV5) protein accumulation govern AAV5 virus production. J Virol 81 : 2205–2212.
40. MeerbreyKL, HuG, KesslerJD, RoartyK, LiMZ, et al. (2011) The pINDUCER lentiviral toolkit for inducible RNA interference in vitro and in vivo. Proc Natl Acad Sci U S A 108 : 3665–3670.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Affinity Proteomics Reveals Elevated Muscle Proteins in Plasma of Children with Cerebral MalariaČlánek The Transcriptional Activator LdtR from ‘ Liberibacter asiaticus’ Mediates Osmotic Stress ToleranceČlánek Complement-Related Proteins Control the Flavivirus Infection of by Inducing Antimicrobial PeptidesČlánek Fungal Chitin Dampens Inflammation through IL-10 Induction Mediated by NOD2 and TLR9 ActivationČlánek Parasite Fate and Involvement of Infected Cells in the Induction of CD4 and CD8 T Cell Responses to
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 4- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- , , , Genetic Variability: Cryptic Biological Species or Clonal Near-Clades?
- Early Mortality Syndrome Outbreaks: A Microbial Management Issue in Shrimp Farming?
- Wormholes in Host Defense: How Helminths Manipulate Host Tissues to Survive and Reproduce
- Plastic Proteins and Monkey Blocks: How Lentiviruses Evolved to Replicate in the Presence of Primate Restriction Factors
- The 2010 Cholera Outbreak in Haiti: How Science Solved a Controversy
- Affinity Proteomics Reveals Elevated Muscle Proteins in Plasma of Children with Cerebral Malaria
- Noncanonical Role for the Host Vps4 AAA+ ATPase ESCRT Protein in the Formation of Replicase
- Efficient Parvovirus Replication Requires CRL4-Targeted Depletion of p21 to Prevent Its Inhibitory Interaction with PCNA
- Host-to-Pathogen Gene Transfer Facilitated Infection of Insects by a Pathogenic Fungus
- The Transcriptional Activator LdtR from ‘ Liberibacter asiaticus’ Mediates Osmotic Stress Tolerance
- Coxsackievirus B Exits the Host Cell in Shed Microvesicles Displaying Autophagosomal Markers
- TCR Affinity Associated with Functional Differences between Dominant and Subdominant SIV Epitope-Specific CD8 T Cells in Rhesus Monkeys
- Coxsackievirus-Induced miR-21 Disrupts Cardiomyocyte Interactions via the Downregulation of Intercalated Disk Components
- Ligands of MDA5 and RIG-I in Measles Virus-Infected Cells
- Kind Discrimination and Competitive Exclusion Mediated by Contact-Dependent Growth Inhibition Systems Shape Biofilm Community Structure
- Structural Differences Explain Diverse Functions of Actins
- HSCARG Negatively Regulates the Cellular Antiviral RIG-I Like Receptor Signaling Pathway by Inhibiting TRAF3 Ubiquitination Recruiting OTUB1
- Vaginitis: When Opportunism Knocks, the Host Responds
- Complement-Related Proteins Control the Flavivirus Infection of by Inducing Antimicrobial Peptides
- Fungal Chitin Dampens Inflammation through IL-10 Induction Mediated by NOD2 and TLR9 Activation
- Microbial Pathogens Trigger Host DNA Double-Strand Breaks Whose Abundance Is Reduced by Plant Defense Responses
- Alveolar Macrophages Are Essential for Protection from Respiratory Failure and Associated Morbidity following Influenza Virus Infection
- An Interaction between Glutathione and the Capsid Is Required for the Morphogenesis of C-Cluster Enteroviruses
- Concerted Spatio-Temporal Dynamics of Imported DNA and ComE DNA Uptake Protein during Gonococcal Transformation
- Potent Dengue Virus Neutralization by a Therapeutic Antibody with Low Monovalent Affinity Requires Bivalent Engagement
- Regulation of Human T-Lymphotropic Virus Type I Latency and Reactivation by HBZ and Rex
- Functionally Redundant RXLR Effectors from Act at Different Steps to Suppress Early flg22-Triggered Immunity
- The Pathogenic Mechanism of the Virulence Factor, Mycolactone, Depends on Blockade of Protein Translocation into the ER
- Role of Calmodulin-Calmodulin Kinase II, cAMP/Protein Kinase A and ERK 1/2 on -Induced Apoptosis of Head Kidney Macrophages
- An Overview of Respiratory Syncytial Virus
- First Experimental Model of Enhanced Dengue Disease Severity through Maternally Acquired Heterotypic Dengue Antibodies
- Binding of Glutathione to Enterovirus Capsids Is Essential for Virion Morphogenesis
- IFITM3 Restricts Influenza A Virus Entry by Blocking the Formation of Fusion Pores following Virus-Endosome Hemifusion
- Parasite Fate and Involvement of Infected Cells in the Induction of CD4 and CD8 T Cell Responses to
- Deficient IFN Signaling by Myeloid Cells Leads to MAVS-Dependent Virus-Induced Sepsis
- Pernicious Pathogens or Expedient Elements of Inheritance: The Significance of Yeast Prions
- The HMW1C-Like Glycosyltransferases—An Enzyme Family with a Sweet Tooth for Simple Sugars
- The Expanding Functions of Cellular Helicases: The Tombusvirus RNA Replication Enhancer Co-opts the Plant eIF4AIII-Like AtRH2 and the DDX5-Like AtRH5 DEAD-Box RNA Helicases to Promote Viral Asymmetric RNA Replication
- Mining Herbaria for Plant Pathogen Genomes: Back to the Future
- Inferring Influenza Infection Attack Rate from Seroprevalence Data
- A Human Lung Xenograft Mouse Model of Nipah Virus Infection
- Mast Cells Expedite Control of Pulmonary Murine Cytomegalovirus Infection by Enhancing the Recruitment of Protective CD8 T Cells to the Lungs
- Cytosolic Peroxidases Protect the Lysosome of Bloodstream African Trypanosomes from Iron-Mediated Membrane Damage
- Abortive T Follicular Helper Development Is Associated with a Defective Humoral Response in -Infected Macaques
- JC Polyomavirus Infection Is Strongly Controlled by Human Leucocyte Antigen Class II Variants
- Cationic Antimicrobial Peptides Promote Microbial Mutagenesis and Pathoadaptation in Chronic Infections
- Estimating the Fitness Advantage Conferred by Permissive Neuraminidase Mutations in Recent Oseltamivir-Resistant A(H1N1)pdm09 Influenza Viruses
- Progressive Accumulation of Activated ERK2 within Highly Stable ORF45-Containing Nuclear Complexes Promotes Lytic Gammaherpesvirus Infection
- Caspase-1-Like Regulation of the proPO-System and Role of ppA and Caspase-1-Like Cleaved Peptides from proPO in Innate Immunity
- Is Required for High Efficiency Viral Replication
- Modified Vaccinia Virus Ankara Triggers Type I IFN Production in Murine Conventional Dendritic Cells via a cGAS/STING-Mediated Cytosolic DNA-Sensing Pathway
- Evidence That Bank Vole PrP Is a Universal Acceptor for Prions
- Rapid Response to Selection, Competitive Release and Increased Transmission Potential of Artesunate-Selected Malaria Parasites
- Inactivation of Genes for Antigenic Variation in the Relapsing Fever Spirochete Reduces Infectivity in Mice and Transmission by Ticks
- Exposure-Dependent Control of Malaria-Induced Inflammation in Children
- A Neutralizing Anti-gH/gL Monoclonal Antibody Is Protective in the Guinea Pig Model of Congenital CMV Infection
- The Apical Complex Provides a Regulated Gateway for Secretion of Invasion Factors in
- A Highly Conserved Haplotype Directs Resistance to Toxoplasmosis and Its Associated Caspase-1 Dependent Killing of Parasite and Host Macrophage
- A Quantitative High-Resolution Genetic Profile Rapidly Identifies Sequence Determinants of Hepatitis C Viral Fitness and Drug Sensitivity
- Histone Deacetylase Inhibitor Romidepsin Induces HIV Expression in CD4 T Cells from Patients on Suppressive Antiretroviral Therapy at Concentrations Achieved by Clinical Dosing
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The 2010 Cholera Outbreak in Haiti: How Science Solved a Controversy
- , , , Genetic Variability: Cryptic Biological Species or Clonal Near-Clades?
- Efficient Parvovirus Replication Requires CRL4-Targeted Depletion of p21 to Prevent Its Inhibitory Interaction with PCNA
- An Overview of Respiratory Syncytial Virus
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



