-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Meta-analysis of Mutations in Autism Spectrum Disorders: A Gradient of Severity in Cognitive Impairments
Autism spectrum disorders (ASD) are a heterogeneous group of neurodevelopmental disorders. Mutations altering genes involved in the junction between brain cells have been repeatedly associated in ASD. For example, SHANK1, SHANK2 and SHANK3 emerged as one family of genes that are associated with ASD. However, little was known about the number of patients carrying these mutations and the clinical outcome. Here, we performed a new genetic screen of SHANK mutations and these results were analyzed in combination with those of the literature. In summary, SHANK mutations account for ∼1% of patients with ASD and were detected in the whole spectrum of autism with a gradient of severity in cognitive impairment: mutations in SHANK1 were rare (0.04%) and present in males with normal IQ and autism; mutations in SHANK2 were present in 0.17% of patients with ASD and mild intellectual disability; mutations in SHANK3 were present in 0.69% of patients with ASD and up to 2.12% of the cases with moderate to profound intellectual disability. Given the high frequency and impact of SHANK3 mutations in individuals with ASD and intellectual disability—more than 1 in 50—this gene should be screened for mutations in clinical practice.
Published in the journal: . PLoS Genet 10(9): e32767. doi:10.1371/journal.pgen.1004580
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004580Summary
Autism spectrum disorders (ASD) are a heterogeneous group of neurodevelopmental disorders. Mutations altering genes involved in the junction between brain cells have been repeatedly associated in ASD. For example, SHANK1, SHANK2 and SHANK3 emerged as one family of genes that are associated with ASD. However, little was known about the number of patients carrying these mutations and the clinical outcome. Here, we performed a new genetic screen of SHANK mutations and these results were analyzed in combination with those of the literature. In summary, SHANK mutations account for ∼1% of patients with ASD and were detected in the whole spectrum of autism with a gradient of severity in cognitive impairment: mutations in SHANK1 were rare (0.04%) and present in males with normal IQ and autism; mutations in SHANK2 were present in 0.17% of patients with ASD and mild intellectual disability; mutations in SHANK3 were present in 0.69% of patients with ASD and up to 2.12% of the cases with moderate to profound intellectual disability. Given the high frequency and impact of SHANK3 mutations in individuals with ASD and intellectual disability—more than 1 in 50—this gene should be screened for mutations in clinical practice.
Introduction
Autism spectrum disorders (ASD) are characterized by impairments in reciprocal social communication and stereotyped behaviors. There is strong evidence of the involvement of different forms of genetic variations in ASD [1], [2]. In particular, chromosomal rearrangements, rare de novo copy-number variants and de novo coding-sequence variants may account for more than 20% of the cases [1], [2]. These events have implicated more than 100 genes [3], but each gene or genomic alteration often accounts for less than 1% of the cases. Many of the genes associated with the disorder are involved in the development or functioning of neuronal circuits [4]. In particular, mutations in genes coding for synaptic cell adhesion molecules and scaffold proteins — such as neuroligins, neurexins and SHANK — have been repeatedly reported in individuals with ASD [5]–[10]. These proteins play a crucial role in the formation and stabilization of synapses [11], [12]. The synapse has therefore emerged as a common target for the different genetic mutations that affect chromatin remodeling, synaptic translation, formation and functioning [4].
Here, we focused on the three SHANK genes, which code for large synaptic scaffold proteins of the post-synaptic density [12]. Deletions, duplications and coding mutations in the SHANK genes have been recurrently reported in patients with ASD [6], [8]–[10], [13]–[20]. SHANK3 haploinsufficiency has been identified in more than 900 patients affected with chromosome 22q13 deletion syndrome, known as Phelan–McDermid syndrome [15]. The genomic rearrangements observed in these patients are diverse ranging from simple 22q13 deletions (72%), ring chromosomes (14%), unbalanced translocations (7%) to interstitial deletions (9%), all resulting in haploinsufficiency of the SHANK3 gene [21].
The majority of these patients have neonatal hypotonia, moderate to severe intellectual disability (ID), absent to severely delayed speech, and minor dysmorphic features [15]. In more than 80% of the cases, autism or autistic-like behavior is present [22]. De novo or truncating mutations in SHANK3 have also been observed in individuals with ASD [6], [13], [16], [17]. Few studies have explored SHANK1 and SHANK2 in ASD, but all have led to the conclusion that deleterious mutations in these genes contribute to the disorder [14], [18], [19].
Mice lacking any of the SHANK proteins display phenotypes relevant to ASD [23]. Shank1 knock-out mice show increased anxiety, decreased vocal communication, decreased locomotion and remarkably, enhanced working memory, but decreased long-term memory [24], [25]. Shank2 knock-out mice show hyperactivity, increased anxiety, repetitive grooming, and abnormalities in vocal and social behaviors [26], [27]. Shank3 knock-out mice show self-injurious repetitive grooming, and deficits in social interaction and communication [28]–[30].
While there is increasing evidence of an association between SHANK genes and ASD, SHANK mutations are considered to affect only a limited number of patients and as a consequence, these genes are not routinely sequenced in clinical practice. In addition, sequence gaps and annotation errors of SHANK2 and SHANK3 in the human genome assembly (hg19) have led to incorrect interpretations of sequencing results obtained in patients [15]. Finally, the clinical impact of the mutations in the SHANK genes is still largely unknown.
Our hypothesis was that mutations in SHANK genes might be more frequent in patients with ASD than previously suggested, and that each gene might be associated with specific clinical profiles. To conduct this study, we first corrected the reference sequence of SHANK2 and SHANK3 (Table S1, Figure S1 & S2 and Text S1: Supplementary Methods). We then analyzed a large number of individuals with ASD for SHANK copy-number variants and coding-sequence variants and combined these results with those reported in the literature. Finally, we performed an extended clinical investigation in all patients carrying de novo or truncating SHANK mutations.
Results
Cohorts used for the meta-analysis of SHANK mutations
We performed a meta-analysis of copy-number variants and coding sequence variants in all SHANK genes (Figure 1). This meta-analysis included the published data from 14 studies in addition to a new screening of SHANK copy-number variants and coding sequence variants. The number of individuals tested and the result of the meta-analysis are reported in the Table 1, Figures 2 and 3 and Table S2, S3, S4, S5, S6. In addition to the results from the literature, we performed a new copy-number variants analysis of 46 additional cases with ASD and 454 matched controls. We also performed a mutation screening of all SHANK1 exons in 743 independent individuals including 251 cases with ASD and 492 controls. Finally, we added 429 new independent cases with ASD and 80 new independent controls to our original screening of coding-sequence variants of SHANK3 [6]. When possible, the patients carrying truncating mutations altering SHANK genes underwent further clinical investigations (Table 2 & S7). The meta-analysis of the frequency of CNVs and coding-sequence variants altering SHANK genes in patients with ASD and in controls were also performed using IQ as a co-variable (Figure 4).
Fig. 1. SHANK variants in patients with ASD and controls. 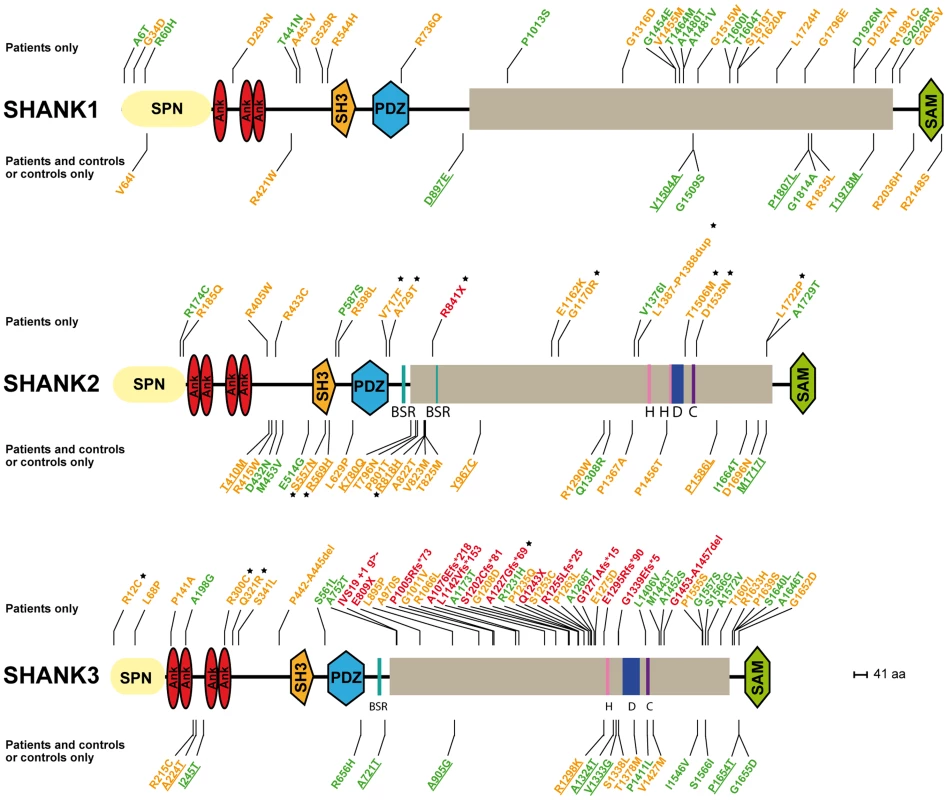
Coding-sequence variants identified only in patients with ASD (upper panel), shared by patients and controls (lower panel and underlined), and present only in controls (lower panel). Truncating variants are indicated in red. The variants predicted as deleterious or benign are indicated in orange and green, respectively. Coding-sequence variants with a proven in vitro functional impact are indicated with black stars. Conserved domains are represented in color: SPN (yellow), Ankyrin (red), SH3 (orange), PDZ (blue) and SAM (green). Fig. 2. Prevalence and meta-analysis of copy number variant studies in ASD. 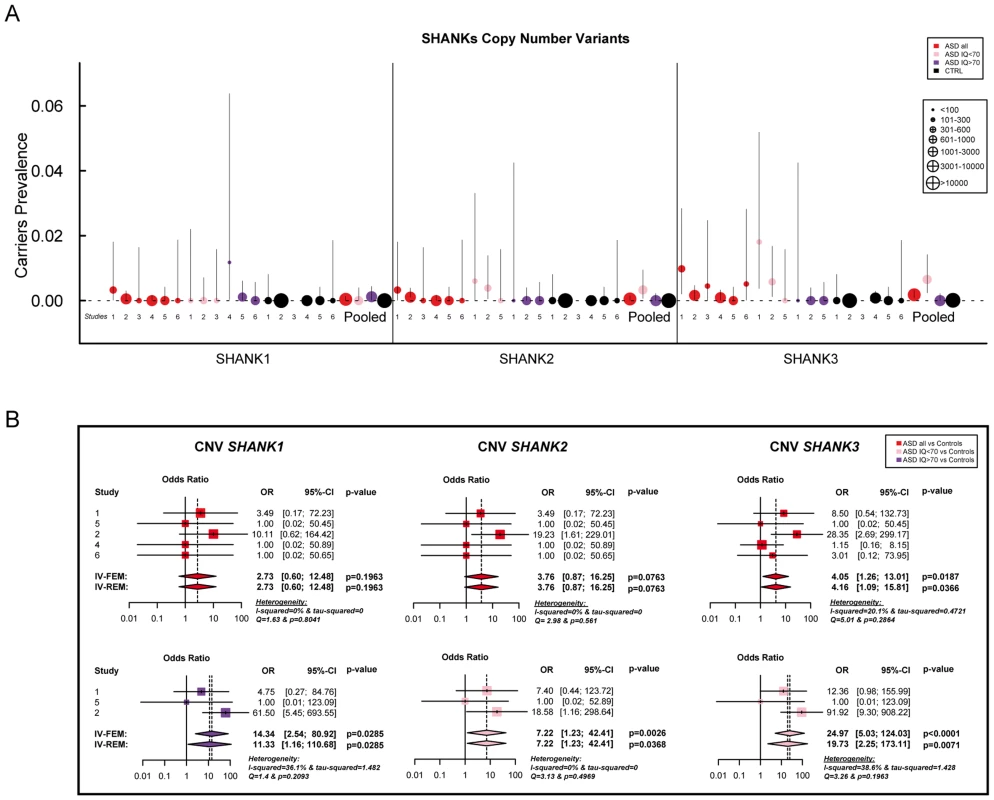
A. The prevalence and the confidence interval from a set of single copy number variant studies and the pooled prevalence and the confidence interval of the meta-analysis. The prevalence is indicated by circles in red, pink, purple and black for “ASD all” (all ASD patients), “ASD IQ<70” (patients with ID; IQ<70), “ASD IQ>70” (patients with IQ in the normal range), and “CTRL” (controls), respectively. The plotted circles are proportional to the corresponding sample size. B. Meta-analysis of the copy number variants altering SHANK genes. For each study, the Odds ratio and confidence interval are given. Each meta-analysis is calculated using inverse variance method for fixed (IV-FEM) and random effects (IV-REM). The statistics measuring heterogeneity (Q, I2 and Tau2) are indicated. The number under the scatter plot correspond to independent studies: 1 = “[The Paris cohort: this study+Durand et al. 2007 [6]; Sato et al. 2012 [19]; Leblond et al. 2012 [18]]”, 2 = “[Moessner et al. (2007) [13]; Marshall et al. (2008) [52]; Pinto et al. (2010) [8]; Berkel et al. (2010) [14]; Sato et al. (2012) [19]]”, 3 = “Bremer et al. (2010) [53]”, 4 = “Glessner et al. (2009) [34]”, 5 = “Sanders et al. (2011) [9]”, and 6 = “Sebat et al. (2007) [51]”. IV, Inverse Variance; FEM, Fixed Effect Method; REM, Random Effect Method; OR, Odds Ratio; CI, Confidence Interval; IQ, Intellectual Quotient; CNV, Copy Number Variant. Fig. 3. Prevalence and meta-analysis of coding-sequence variant studies in ASD. 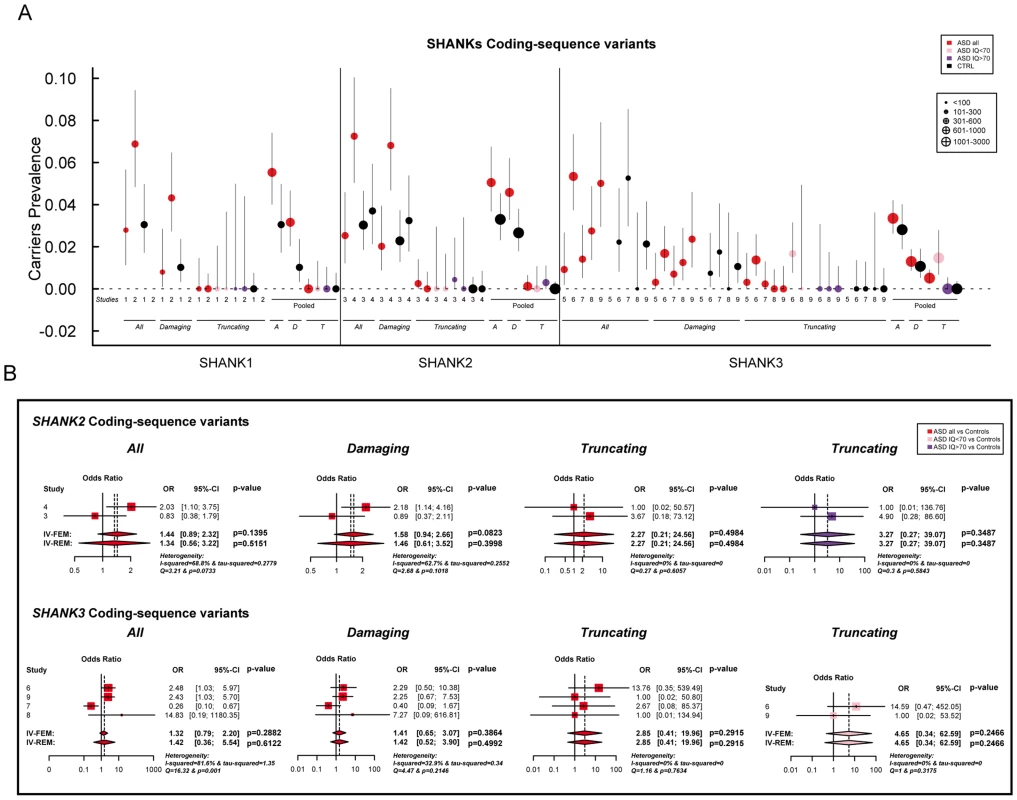
A. The prevalence and the confidence interval from a set of single coding-sequence variant studies, and the pooled prevalence and the confidence interval of the meta-analysis. The prevalence is indicated by circles in red, pink, purple and black for “ASD all” (all ASD patients), “ASD IQ<70” (patients with ID; IQ<70), “ASD IQ>70” (patients with normal IQ), and “CTRL” (controls), respectively. Three categories are used to study the prevalence of coding-sequence variants in ASD and controls: all or “A” (all mutation), Damaging or “D” (damaging missense mutation; score obtained from polyphen-2), and Truncating or “T” (mutation altering SHANK protein). The plotted circles are proportional to the corresponding sample size. B. Meta-analysis of coding-sequence variant studies altering SHANK genes. For each study, the Odds ratio and confidence interval is given. Each meta-analysis is calculated using inverse variance method for fixed (IV-FEM) and random effects (IV-REM). The statistics measuring heterogeneity (Q, I2 and Tau2) are indicated. The number under the scatter plot correspond to independent studies: 1 = “This study”, 2 = “ Sato et al. (2012) [19]”, 3 = “Berkel et al. (2010) [14]”, 4 = “Leblond et al. (2012) [18]”, 5 = “Boccuto et al. (2012) [17]”, and 6 = “[This Study and Durand et al. 2007 [6]]”, 7 = “[Gauthier et al. (2009–2010) [16], [47]]”, 8 = “Moessner et al. (2007) [13]”, 9 = “Schaff et al. (2011) [35]”. IV, Inverse Variance; FEM, Fixed Effect Method; REM, Random Effect Method; OR, Odds Ratio; CI, Confidence Interval; IQ, Intellectual Quotient; CNV, Copy Number Variant. Fig. 4. Scatter plots of the intellectual quotient and the Autism Diagnostic Interview-Revised (ADI-R) scores of the patients with ASD screened for SHANK1-3 mutations. 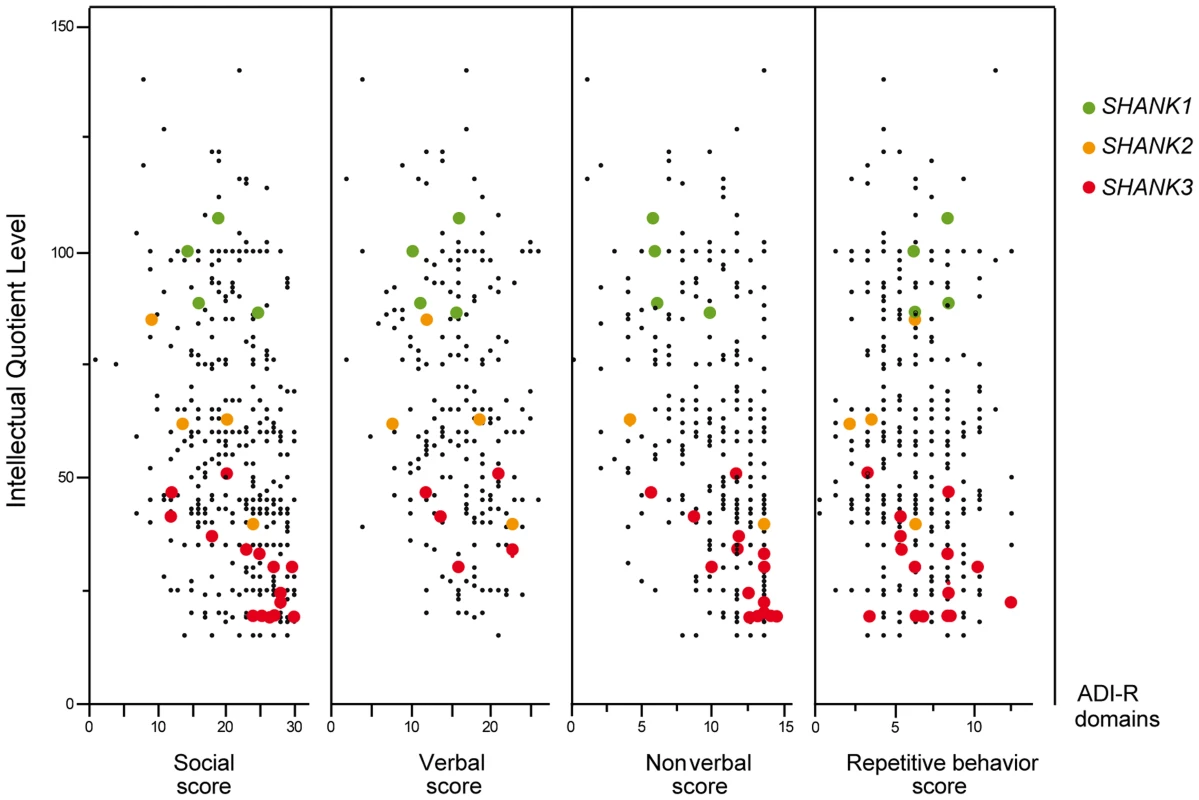
Mutations in SHANK1-3 are associated with a gradient of severity in cognitive impairment. SHANK1 mutations were reported in patients without ID (green dots). SHANK2 mutations were reported in patients with mild ID (orange dots). SHANK3 mutations were found in patients with moderate to severe deficit (red dots). Black dots correspond to the patients enrolled in the PARIS cohort screened for deleterious SHANK1-3 mutations (n = 498). In addition to the PARIS cohort [6], [8], [18], three patients with a SHANK1 deletion [19] and two patients with a SHANK2 deletion [14] were included in the scatter plot. A high score of the ADI-R is associated with a more severe profile. The threshold of the “Social”, “Verbal”, “Non-Verbal” and “Repetitive Behavior” Scores are 10, 8, 7 and 3, respectively. Tab. 1. Prevalence of SHANK rare coding-sequence and copy-number variants in patients with ASD and controls. 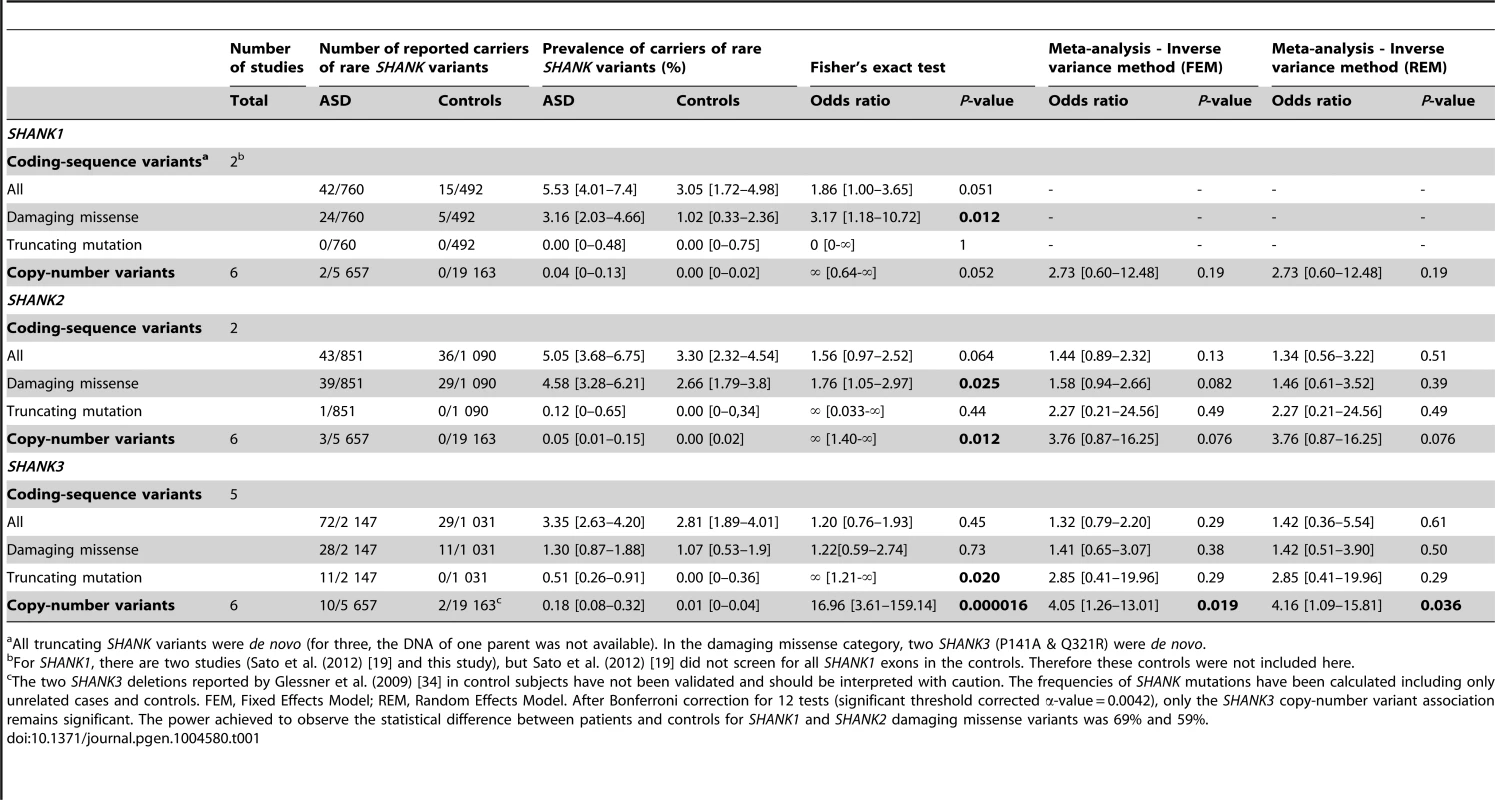
All truncating SHANK variants were de novo (for three, the DNA of one parent was not available). In the damaging missense category, two SHANK3 (P141A & Q321R) were de novo. Tab. 2. Clinical characteristics of the patients carrying de novo SHANK2 and SHANK3 mutations. 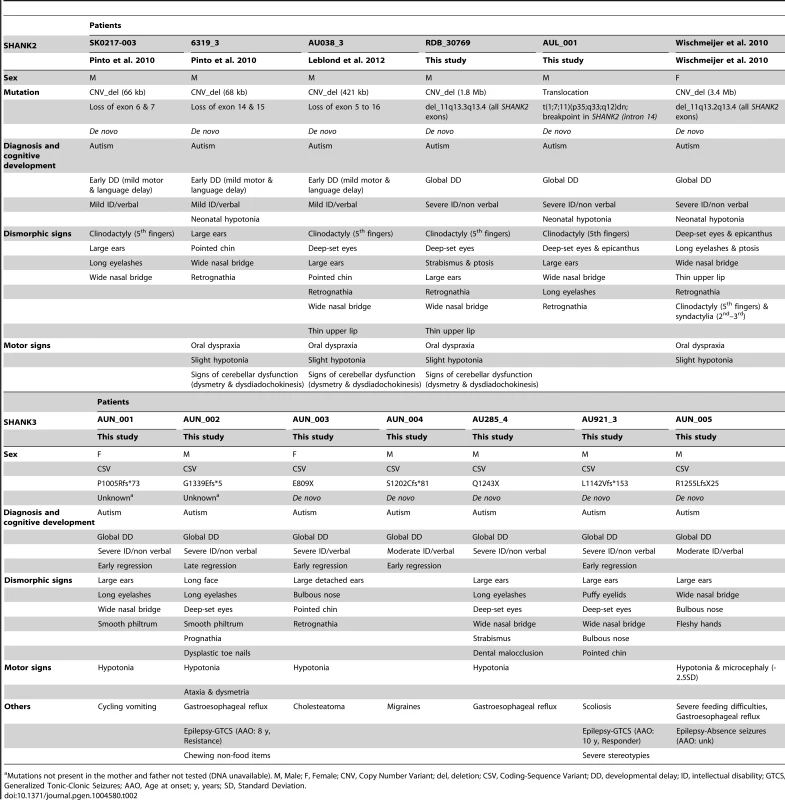
Mutations not present in the mother and father not tested (DNA unavailable). M, Male; F, Female; CNV, Copy Number Variant; del, deletion; CSV, Coding-Sequence Variant; DD, developmental delay; ID, intellectual disability; GTCS, Generalized Tonic-Clonic Seizures; AAO, Age at onset; y, years; SD, Standard Deviation. SHANK1 in ASD
Altogether, our new screening of 306 patients with ASD and 454 controls and the previously published copy-number variants studies (Table S3 & S5) showed that deletions disrupting SHANK1 were detected in 0.04% (n = 2/5 657) of patients with ASD and were never found in 19 163 controls (Meta-analysis – Inverse variance method - fixed effect model P = 0.19, OR = 2.73, 95% CI = 0.60–12.48) (Table 1 and Figure 2). The two independent families with SHANK1 deletions were reported by Sato et al. (2012) [19]. A de novo deletion of 63.4 kb altering both the synaptotagmin-3 gene (SYT3) and SHANK1 was detected in a Swedish male with normal IQ and ASD [19]. An inherited exonic deletion of 63.8 kb altering both SHANK1 and CLEC11A segregated in a four-generation Canadian family in which male carriers—but not female carriers—have ASD [19]. No SHANK1 duplications were found.
We screened a sample of 251 patients and 492 controls for SHANK1 coding exons. As for the SHANK1 mutation screening by Sato et al. (2012), no de novo truncating mutation sequences were identified. Based on the two cohorts of 760 patients with ASD and 492 controls (Table 1, Figure 1 & 3, Tables S4, S5, S6, S7 & S9), rare inherited coding-sequence variants predicted as damaging were, however, more frequent in patients with ASD than in controls (3.16% in ASD, 1.02% in controls, Fisher's exact test two-sided, P = 0.012, OR = 3.17, 95% CI = 1.18–10.72). The rare variants observed in patients with ASD were not observed in an additional sample of 500 control chromosomes.
The 4 males with SHANK1 deletions were diagnosed with ASD and an IQ in the normal range (mean IQ = 107) and good verbal ability without significant language delay [19] (Figure 4). Interestingly, sex differences might modulate the phenotype since females carrying an inherited SHANK1 deletion exhibited anxiety and shyness, but did not fulfill criteria for ASD [19].
SHANK2 in ASD
SHANK2 deletions were found in 0.05% (n = 3/5 657) of patients with ASD, and never in controls (n = 0/19 163; Meta-analysis – Inverse variance method - fixed effect model, P = 0.076, OR = 3.76, 95% CI = 0.87–16.25) (Table 1, S3 & S5, and Figure 2). All deletions were de novo and disrupted coding exons. No SHANK2 duplications were reported. We identified two patients with a SHANK2 de novo deletion (Table 2). For patient AUL_001, the breakpoints were previously sequenced using whole genome sequencing [31] (Table 2, Figure S4 and Text S1). The second patient (RDB_30769) carried a de novo deletion of 1.8 Mb encompassing SHANK2. These two patients were not included in the calculation of the prevalence since they were not part of our cohort screened for copy-number variants. They were identified during clinical screening and the exact number of patients with ASD investigated was not available.
Based on the mutation screening of Berkel et al. (2010) and Leblond et al. (2012), the prevalence of truncating SHANK2 coding-sequence variants was 0.12% in patients with ASD (n = 1/851), and such variants were not found in any of the 1 090 controls (Figure 1 and 3, Table 1, Table S4, S5, S6, S7 & S10). This prevalence is similar to the one reported in the four large-scale studies in ASD using exome sequencing [32] (de novo or truncating SHANK2 mutation 1/965, 0.10%). Finally, we observed rare coding-sequence variants predicted as damaging in 4.58% of the patients with ASD compared with 2.66% of the controls (Fisher's exact test two-sided, P = 0.025, OR = 1.76, 95% CI = 1.05–2.97).
The individuals carrying a SHANK2 de novo deletion were diagnosed with autistic disorder or pervasive developmental disorder not otherwise specified (PDD-NOS) in combination with mild to moderate ID (mean IQ = 62±17) (Figure 4). They displayed early signs of developmental delay, mild motor delay and significant language delay. They also displayed minor signs of dysmorphism (broad nasal bridge, thin upper lip, pointed chin, clinodactyly) and abnormal neurological examination (Table 2). Specifically, cases 6319-3 and AU038-3 had mild axial hypotonia, oral dyspraxia and minor signs or cerebellar dysfunction (including dysmetry and dysdiadochokinesis). These clinical signs are unspecific, but were also reported in patients with ASD with more complex chromosomal rearrangements encompassing SHANK2 [33]. The individual carrying the de novo truncating mutation R841X (SK 0441-003) had a normal IQ and diagnosed with ASD without any developmental delays or dysmorphic features.
SHANK3 in ASD
In our screening of 306 patients with ASD, we identified one patient (AU029) carrying a de novo SHANK3 deletion of 1.5 Mb (Figure S4). Altogether, SHANK3 deletions were detected in 0.18% of patients with ASD (n = 10/5 657) and in 0.01% of controls (n = 2/19 163) (Meta-analysis – Inverse variance method - fixed effect model, P = 0.019, OR = 4.05, 95% CI = 1.26–13.01) (Figure 2, Figure S4, Table 1 and Table S3, S4, S5, S6, S7). Deletions of SHANK3 have not been reported in controls before, so the two deletions reported by Glessner et al. (2009) [34], which have not been validated, should be interpreted with great caution. In three families from France and Canada, the SHANK3 deletions originated from a balanced translocation present in a healthy parent [6], [13]. Interestingly, in two families, a sibling carried the reciprocal SHANK3 duplication. In the French family, the elder brother carrying the SHANK3 duplication was diagnosed with Asperger syndrome [6]. In the Canadian family, the elder sister carrying the SHANK3 duplication was diagnosed with attention-deficit/hyperactivity disorder (ADHD) and developmental delay [13]. In a screen of 160 additional patients with ASD and ID using Multiplex Ligation-dependent Probe Amplification (MLPA) analysis, we observed two patients carrying a de novo deletion altering SHANK3 (Figure S4 and Text S1: Supplementary Methods). For patient AUN_006, the deletion breakpoint is located within intron 8 of SHANK3 and leads to the loss of SHANK3 (exons 9 to 22), ACR and RABL2B. For the second patient AUN_007, the deletion covers the exon 22 of SHANK3 and exons 1 to 3 of ACR.
We screened 429 patients with ASD for all coding exons of SHANK3 and found 8 patients carrying heterozygous truncating mutations (Figure 1 and 3, Figure S5, Table 1, Table S4, S5, S6, S7 and Table S11), including 6 that appeared de novo in the probands. For the remaining two, the mothers were not carriers, but the DNA of the fathers were not available. When all mutation screenings were included, truncating SHANK3 coding-sequence variants were found in 0.51% of the patients (n = 11/2 147) and were not found in 1031 controls (meta-analysis – inverse variance method - fixed effect model, P = 0.29, OR = 2.85, 95% CI = 0.41–19.96) (Table 1, S5 & S11, and Figure 1 & 3) [6], [13], [16], [17], [35]. We observed an enrichment of truncating mutations in exon 21a of SHANK3. We therefore screened an additional sample of 138 cases with ASD for exon 21a and identified a novel de novo stop mutation (Q1243X) in one boy with autism and moderate ID (Table 2 & Figure S5).
Individuals with SHANK3 truncating mutations displayed autism with moderate to severe/profound ID (mean IQ: 31±8) (Figure 4). The individuals carrying SHANK3 deletions had also manifestations of the Phelan-McDermid syndrome [15]. For example, the boy carrying the L1142Vfs*153 mutation was non-verbal, showed a global developmental delay with neonatal hypotonia and typical dysmorphic features of Phelan-McDermid syndrome, including wide nasal bridge, pointed chin, deep-set eyes, flat mid-face, large ears, long eyelashes, bulbous nose, and high-arched palate (Table 2). He also developed generalized epilepsy at the age of 10 years, which was characterized by intolerance and resistance to variety of anticonvulsant medications. By contrast, the boy carrying the de novo truncating mutation S1202Cfs*81 was verbal and had moderate ID. Although reduced in quality by the opposition of the patient, the clinical examination was considered in the normal range with no significant dysmorphic features. Thus, the phenotypic variability of the Phelan-McDermid syndrome, which was considered to result from the wide range of deletion sizes, was also observed for individuals carrying SHANK3 de novo or truncating mutations.
Discussion
Mutations of the SHANK genes were detected in the whole spectrum of ASD with a gradient of severity in cognitive impairment. SHANK1 mutations were detected in individuals with ASD and normal IQ, SHANK2 mutations were found in cases with ASD and mild ID, and SHANK3 mutations were mainly found in individuals with ASD combined with moderate to severe ID. In the whole spectrum of ASD, we estimated that 0.04%, 0.17% and 0.69% of cases with ASD had heterozygous truncating mutations in SHANK1, SHANK2 or SHANK3, respectively (Table 1). Recent exome sequencing studies only reported one de novo SHANK2 mutation out of 965 patients [32], [36], [37] and no truncating coding-sequence variation within SHANK1 and SHANK3. In contrast, we report 0.51% of cases with ASD carrying truncating coding-sequence variations in SHANK3. This difference could be explained by the very low sequencing coverage of SHANK3 using whole-exome sequencing technology leading to a low power of detection of SHANK3 mutations. This coverage issue of SHANK3 was indeed observed by other groups [38]. Interestingly, regions with low coverage of SHANK3 correlate with high percentage of GC; and the majority of the mutations detected in our study were located in the exonic region of SHANK3 showing a very low coverage (Figure S3).
The prevalence of each SHANK mutations appeared to be different when the severity of cognitive impairment was considered (Figure 4, Table 3 and Table S6). This was particularly relevant for SHANK3 in individuals with ASD and ID. The prevalence of de novo or truncating SHANK3 mutations in these patients was 2.12% (copy-number variants: 6/917 patients with IQ<70; prevalence = 0.65%; coding-sequence variants: 9/611 patients with IQ<70; prevalence = 1.47%), 0% in patients with ASD without ID and 0.01% in controls. Our prevalence of SHANK3 deletions in patients with ASD and ID is similar to that reported by Cooper et al. (2011) in a large sample of 1 379 patients with autism and developmental delay (0.87%) [10]. Altogether, in addition to the large deletions observed in Phelan-McDermid syndrome, mutations of SHANK3 account for more than 1 out of 50 cases diagnosed with the combination of ASD and ID. Detection of such mutations should therefore be considered in clinical practice. This clinical screening should: (i) improve the quality of genetic counseling of ASD and ID for patients and their family relatives; (ii) increase our understanding of the clinical features associated with SHANK mutations together with the developmental trajectories of the patients [39], (ii) enable the development of a large number of independent induced pluripotent stem cells (iPSC) carrying SHANK mutations [40], (iii) set the ground for future large scale clinical trials targeting these synaptic defects [41].
Tab. 3. Summary of the SHANK protein functions and of the main findings obtained for patients with ASD. 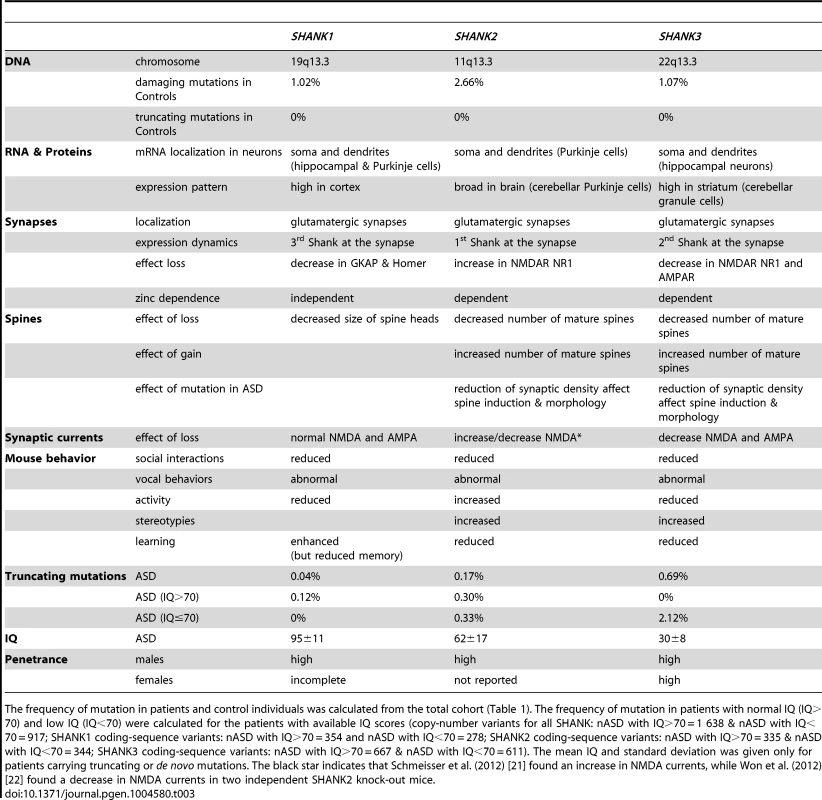
The frequency of mutation in patients and control individuals was calculated from the total cohort (Table 1). The frequency of mutation in patients with normal IQ (IQ>70) and low IQ (IQ<70) were calculated for the patients with available IQ scores (copy-number variants for all SHANK: nASD with IQ>70 = 1 638 & nASD with IQ<70 = 917; SHANK1 coding-sequence variants: nASD with IQ>70 = 354 and nASD with IQ<70 = 278; SHANK2 coding-sequence variants: nASD with IQ>70 = 335 & nASD with IQ<70 = 344; SHANK3 coding-sequence variants: nASD with IQ>70 = 667 & nASD with IQ<70 = 611). The mean IQ and standard deviation was given only for patients carrying truncating or de novo mutations. The black star indicates that Schmeisser et al. (2012) [21] found an increase in NMDA currents, while Won et al. (2012) [22] found a decrease in NMDA currents in two independent SHANK2 knock-out mice. Contrary to the de novo SHANK mutations, the role of the inherited sequence variants remains difficult to ascertain. Our study provides some insights regarding this issue. There is a trend for more SHANK1 (unadjusted P = 0.012) and SHANK2 (unadjusted P = 0.025) inherited deleterious mutations in patients with ASD than in controls (Table 1). However, these associations do not survive Bonferroni correction for multiple testing. Despite our meta-analysis that includes several mutations screening, we were underpowered to detect associations with low effect size (Table S12). The power to detect an odds ratio of 1.5 (two-sided) for SHANK1, SHANK2 and SHANK3 missense inherited damaging variants were 8%, 34% and 17%, respectively. Further studies with larger cohorts of patients stratified by IQ are therefore needed to achieve appropriate statistical power. Interestingly, in-frame deletions predicted to remove several amino acids in the SHANK2 and SHANK3 proteins were only detected in patients with ASD and their parents, never in controls. Previous functional studies have shown that inherited variants are associated with a statistically significant reduction in the density of synapses, although not as severe as the reduction caused by the de novo or truncating mutations [6], [18], [42]–[44]. Together, these genetic and functional data suggest that, although present in healthy parents, some inherited SHANK mutations might contribute to the development of ASD.
To date, only non-synonymous mutations in the known exons of the SHANK genes had been reported. However, all SHANK genes display several splicing isoforms and possibly some exons were not screened. In addition, other types of mutations such as synonymous mutations or variations in regulatory regions were rarely reported. In our cohort, we did not find synonymous mutations located at alternative splicing sites, but it is warranted that these variations should be reported in future screening.
It has been proposed that abnormal SHANK levels at the synapse might result in the mislocalization, de-clustering and/or functional impairment of several other crucial synaptic proteins such as cytoskeletal regulators and/or neurotransmitter receptors [12] (Table 3).
For SHANK1 mutations, it is expected that the number of dendrites and glutamatergic synapses will not be dramatically affected (if at all). SHANK1 mutations might rather lead to an immature neuronal network with a reduced number of large spine heads. Accordingly, male individuals carrying SHANK1 deletions do not present with language delay or ID and are diagnosed with normal IQ ASD or Asperger syndrome [19]. Interestingly, females who are carrier of a SHANK1 deletion seem to be protected against ASD suggesting that X-linked genes escaping the X-inactivation process and/or hormonal factors could buffer this type of synaptic alterations.
For SHANK2 and SHANK3 mutations, it is expected that affected neurons will have a reduced total number of dendritic spines and synapses. The reduction in mature glutamatergic synapses is expected to affect cognitive functions. Accordingly, most patients with SHANK2 and SHANK3 mutations have moderate to severe ID. Individuals with SHANK3 mutations are usually more severely affected than those carrying SHANK2 mutations. This difference in severity of cognitive impairment is in agreement with the observation that SHANK3 mutations are highly penetrant (to our knowledge only one validated SHANK3 deletion has been reported to be inherited from a mother with moderate ID [45]), while for SHANK2, additional genetic/epigenetic factors might be necessary to develop ASD [18], [19], [46].
In summary, our genetic and clinical findings provide additional support for considering SHANK mutations in a broad spectrum of patients with ASD. SHANK mutations are however not restricted to ASD. SHANK3 mutations have been identified in patients suffering from schizophrenia and bipolar disorder [45], [47]. More generally, other genes involved in the same synaptic pathway, including neurexin and neuroligin genes, appear to be associated with a variety of neuropsychiatric disorders [11]. Given the broad spectrum of disorders associated with this synaptic pathway, it is important to conduct fine-grained clinical investigations of patients in order to identify the factors that influence the clinical trajectory, clinical manifestations, and outcome of affected individuals [41].
Materials and Methods
Study samples for SHANK mutations in ASD
Mutation screening of the SHANK genes was performed in patients with ASD recruited by the PARIS (Paris Autism Research International Sibpair) study at specialized clinical neuropsychiatric centers located in France and Sweden (Table S2, S3, S4). Ethnicity of the patients and controls was ascertained using genetic data (Figure S6 and Text S1). The autism-spectrum diagnosis was based on the Autism Diagnostic Interview – Revised (ADI-R) [48] and for some of the patients, the Autism Diagnostic Observation Schedule (ADOS) [49]. In Sweden, in few cases, the Diagnostic Interview for Social and Communication Disorders (DISCO-10) [50] was used instead of the ADI-R. IQ was measured with an age-appropriate Weschler scale (WPPSI, Wechsler Preschool and Primary Scale of Intelligence; WISC, Wechsler Intelligence Scale for Children; or WASI, Wechsler Abbreviated Scale of Intelligence). For the most severe and/or non-verbal patients, the Raven's Standard Progressive Matrices were used to measure nonverbal IQ (NVIQ) and the Peabody Picture Vocabulary Test (PPVT-4th edition) to measured receptive vocabulary (RV). In addition, a physical exam was systematically performed to record specifically basic physical parameters (such as height, weight, cranial circumference), dysmorphic features and neurological symptoms. The patients used for the scatter plots of the intellectual quotient and the ADI-R scores, or for the clinical characteristics, or for the prevalence, are indicated in the Table S7.
Ethics statement
This study was approved by the local Institutional Review Board (IRB) and written informed consents were obtained from all participants of the study. For the patients who were unable to consent for themselves, a parent or legal guardian consented to the study on their behalf. The local IRB are the “Comité de Protection des Personnes” (Île-de-France Hôpital Pitié-Salpêtrière Paris, France); the “Comité de Protection des Personnes Sud Méditerrannée III” (centre hospitalier universitaire de Nîmes, France); the Sahlgrenska Academy Ethics committee (University of Gothenburg, Sweden); SickKids Research Ethics Board (Toronto, Ontario, Canada); Hamilton Integrated Research Ethic Board (HIREB) (Hamilton, Ontario, Canada) and Health Research Ethics Authority (HREA) (St. John's, Newfoundland, Canada).
SHANK copy-number and coding-sequence variants in ASD
SHANK copy-number variants were detected with the Illumina Human 1M-Duo BeadChip, and validated by quantitative PCR as previously described [8], [18]. For SHANK1 and SHANK2, the sequencing protocol was adapted from Leblond et al. (2012) [18] and Sato et al. (2012) [19] (Table S8). For SHANK3, because of its high GC content and the genomic sequence errors, several clinical and research centers could not screen exon 11 for mutations [13], [16]. We have now corrected the genomic sequence (Figure S2 and Text S1) and provided a new set of primers that successfully amplified and sequenced all SHANK3 exons. All SHANK primers and sequencing protocols are provided in Table S8.
To ascertain the frequency of SHANK mutations in ASD, we included all studies published before April 2012 reporting whole genome copy-number variant screening or SHANK mutation screening. We scanned the PubMed database (http://www.ncbi.nlm.nih.gov/pubmed) with combinations of the following keywords: “Autis*”, “mutation*” “Shank*”, Prosap*, “copy number variants”. For SHANK copy-number variants, a total of 5 657 cases and 19 163 controls were included, representing 11 studies including this one (Table S3) [8], [9], [13], [14], [18], [19], [34], [35], [51]–[53]. In order to avoid biasing the estimation of the frequency of the copy-number variants, we only included studies that analyzed large numbers of individuals (>200 individuals) and used similar mutation screening procedures (for details see Table S3 and Text S1). To ensure that the cohorts were constituted of independent and unrelated cases and controls, we contacted the corresponding authors of each study. For example, the cohorts used in the following studies: Pinto et al. (2010), Moessner et al. (2007), Marshall et al. (2008), Berkel et al. (2010), and Sato et al. (2012) contained overlapping samples. The number of non-overlapping samples from these five studies was 1 866 patients with ASD and 15 122 controls, and the total number of non-overlapping samples used in the meta-analysis was 5 657 patients with ASD and 19 163 controls. For patients with ASD, only copy-number variants validated by an independent method were included in the analysis. For controls, the two SHANK3 deletions reported by Glessner et al. (2009) among 2 519 individuals, were not validated and thus should be regarded with caution.
For SHANK coding-sequence variants, 10 studies including this one were used (Table S4) [13], [14], [16]–[19], [35], [47]. For all variants, the hg19 coordinates are given. Because of the very low coverage of whole exome sequencing (Figure S3 and Text S1), we excluded mutation screening performed using this approach. Taken together, a total of 760–2 147 patients and 492–1 090 controls were included in the analysis (SHANK1: 760 patients and 492 controls; SHANK2: 851 patients and 1 090 controls; SHANK3: 2 147 patients and 1 031 controls).
Statistics
The significance of observed differences in copy-number and coding-sequence variants was determined by a two-sided Fisher's exact test on a two-by-two contingency table. We used Bonferroni correction for the multiple testing correction (ntest = 12, significant threshold corrected α-value = 0.05/12 = 0.0042). We used G*Power (v3.1, http://www.psycho.uniduesseldorf.de/abteilungen/aap/gpower3) to compute for each test the achieved statistical power (for a two-sided Fisher's exact test) and the power to detect an odd ratio from 1.5 to 3 (two-sided) (Table S12). Prevalence and confidence intervals of single studies were evaluated using Clopper and Pearson method [54]. Heterogeneity between studies was assessed by the Q, I2 and Tau2 statistics. The Q statistic is a chi-square test for heterogeneity, and the I2 and Tau2 are the proportion of observed variance in effect sizes across studies for fixed effect model and random effect model, respectively [55]. Zero total event studies (no events in both ASD and controls) were included [56]. The meta-analysis was performed using the classical inverse variance for both fixed effects model and random effects model. To avoid population stratification bias in the calculation of the odds ratio (OR), studies without a control group were excluded (i.e. Boccuto et al. 2012 and Bremer et al. 2012) (Figure 2, 3, and Table 1). If any contingency tables contained zero values, a continuity correction was applied to the relevant tables [57]. For all the calculations and illustrations the R statistical software, and “metafor” and “meta” packages were used.
Supporting Information
Zdroje
1. GeschwindDH (2009) Advances in autism. Annu Rev Med 60 : 367–380.
2. HuguetG, EyE, BourgeronT (2013) The Genetic Landscapes of Autism Spectrum Disorders. Annu Rev Genomics Hum Genet 14 : 191–213.
3. BetancurC (2011) Etiological heterogeneity in autism spectrum disorders: More than 100 genetic and genomic disorders and still counting. Brain Res 1380 : 42–77.
4. ToroR, KonyukhM, DelormeR, LeblondC, ChasteP, et al. (2010) Key role for gene dosage and synaptic homeostasis in autism spectrum disorders. Trends Genet 26 : 363–372.
5. JamainS, QuachH, BetancurC, RastamM, ColineauxC, et al. (2003) Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet 34 : 27–29.
6. DurandCM, BetancurC, BoeckersTM, BockmannJ, ChasteP, et al. (2007) Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet 39 : 25–27.
7. SzatmariP, PatersonAD, ZwaigenbaumL, RobertsW, BrianJ, et al. (2007) Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet 39 : 319–328.
8. PintoD, PagnamentaAT, KleiL, AnneyR, MericoD, et al. (2010) Functional impact of global rare copy number variation in autism spectrum disorders. Nature 466 : 368–372.
9. SandersSJ, Ercan-SencicekAG, HusV, LuoR, MurthaMT, et al. (2011) Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron 70 : 863–885.
10. CooperGM, CoeBP, GirirajanS, RosenfeldJA, VuTH, et al. (2011) A copy number variation morbidity map of developmental delay. Nat Genet 43 : 838–846.
11. SudhofTC (2008) Neuroligins and neurexins link synaptic function to cognitive disease. Nature 455 : 903–911.
12. GrabruckerAM, SchmeisserMJ, SchoenM, BoeckersTM (2011) Postsynaptic ProSAP/Shank scaffolds in the cross-hair of synaptopathies. Trends Cell Biol 21 : 594–603.
13. MoessnerR, MarshallCR, SutcliffeJS, SkaugJ, PintoD, et al. (2007) Contribution of SHANK3 mutations to autism spectrum disorder. Am J Hum Genet 81 : 1289–1297.
14. BerkelS, MarshallCR, WeissB, HoweJ, RoethR, et al. (2010) Mutations in the SHANK2 synaptic scaffolding gene in autism spectrum disorder and mental retardation. Nat Genet 42 : 489–491.
15. PhelanK, McDermidHE (2012) The 22q13.3 Deletion Syndrome (Phelan-McDermid Syndrome). Mol Syndromol 2 : 186–201.
16. GauthierJ, SpiegelmanD, PitonA, LafreniereRG, LaurentS, et al. (2009) Novel de novo SHANK3 mutation in autistic patients. Am J Med Genet B Neuropsychiatr Genet 150B: 421–424.
17. BoccutoL, LauriM, SarasuaSM, SkinnerCD, BuccellaD, et al. (2012) Prevalence of SHANK3 variants in patients with different subtypes of autism spectrum disorders. Eur J Hum Genet 3 : 310–316.
18. LeblondCS, HeinrichJ, DelormeR, ProepperC, BetancurC, et al. (2012) Genetic and functional analyses of SHANK2 mutations suggest a multiple hit model of autism spectrum disorders. PLoS Genet 8: e1002521.
19. SatoD, LionelAC, LeblondCS, PrasadA, PintoD, et al. (2012) SHANK1 Deletions in Males with Autism Spectrum Disorder. Am J Hum Genet 90 : 879–887.
20. SooryaL, KolevzonA, ZweifachJ, LimT, DobryY, et al. (2013) Prospective investigation of autism and genotype-phenotype correlations in 22q13 deletion syndrome and SHANK3 deficiency. Mol Autism 4 : 18.
21. BonagliaMC, GiordaR, BeriS, De AgostiniC, NovaraF, et al. (2011) Molecular mechanisms generating and stabilizing terminal 22q13 deletions in 44 subjects with Phelan/McDermid syndrome. PLoS Genet 7: e1002173.
22. BetancurC, BuxbaumJD (2013) SHANK3 haploinsufficiency: a “common” but underdiagnosed highly penetrant monogenic cause of autism spectrum disorders. Mol Autism 4 : 17.
23. JiangYH, EhlersMD (2013) Modeling autism by SHANK gene mutations in mice. Neuron 78 : 8–27.
24. HungAY, FutaiK, SalaC, ValtschanoffJG, RyuJ, et al. (2008) Smaller dendritic spines, weaker synaptic transmission, but enhanced spatial learning in mice lacking Shank1. J Neurosci 28 : 1697–1708.
25. WohrM, RoulletFI, HungAY, ShengM, CrawleyJN (2011) Communication impairments in mice lacking Shank1: reduced levels of ultrasonic vocalizations and scent marking behavior. PLoS One 6: e20631.
26. SchmeisserMJ, EyE, WegenerS, BockmannJ, StempelAV, et al. (2012) Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature 486 : 256–260.
27. WonH, LeeH-R, GeeHY, MahW, KimJ-I, et al. (2012) Autistic-like social behaviour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature 486 : 261–265.
28. PecaJ, FelicianoC, TingJT, WangW, WellsMF, et al. (2011) Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature 472 : 437–442.
29. YangM, BozdagiO, ScattoniML, WoehrM, RoulletFI, et al. (2012) Reduced Excitatory Neurotransmission and Mild Autism-Relevant Phenotypes in Adolescent Shank3 Null Mutant Mice. Journal of Neuroscience 32 : 6525–6541.
30. WangX, McCoyPA, RodriguizRM, PanY, JeHS, et al. (2011) Synaptic dysfunction and abnormal behaviors in mice lacking major isoforms of Shank3. Hum Mol Genet 20 : 3093–3108.
31. Schluth-BolardC, LabalmeA, CordierMP, TillM, NadeauG, et al. (2013) Breakpoint mapping by next generation sequencing reveals causative gene disruption in patients carrying apparently balanced chromosome rearrangements with intellectual deficiency and/or congenital malformations. J Med Genet 50 : 144–150.
32. SandersSJ, MurthaMT, GuptaAR, MurdochJD, RaubesonMJ, et al. (2012) De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature 485 : 237–241.
33. WischmeijerA, MaginiP, GiordaR, GnoliM, CicconeR, et al. (2011) Olfactory Receptor-Related Duplicons Mediate a Microdeletion at 11q13.2q13.4 Associated with a Syndromic Phenotype. Mol Syndromol 1 : 176–184.
34. GlessnerJT, WangK, CaiG, KorvatskaO, KimCE, et al. (2009) Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature 459 : 569–573.
35. SchaafCP, SaboA, SakaiY, CrosbyJ, MuznyD, et al. (2011) Oligogenic heterozygosity in individuals with high-functioning autism spectrum disorders. Hum Mol Genet 20 : 3366–3375.
36. O'RoakBJ, VivesL, GirirajanS, KarakocE, KrummN, et al. (2012) Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature 485 : 246–250.
37. NealeBM, KouY, LiuL, Ma'ayanA, SamochaKE, et al. (2012) Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature 485 : 242–245.
38. KrummN, O'RoakBJ, ShendureJ, EichlerEE (2014) A de novo convergence of autism genetics and molecular neuroscience. Trends Neurosci 37 : 95–105.
39. GuilmatreA, HuguetG, DelormeR, BourgeronT (2013) The emerging role of SHANK genes in neuropsychiatric disorders. Dev Neurobiol 74 (2) 113–22.
40. ShcheglovitovA, ShcheglovitovaO, YazawaM, PortmannT, ShuR, et al. (2013) SHANK3 and IGF1 restore synaptic deficits in neurons from 22q13 deletion syndrome patients. Nature 503 : 267–271.
41. DelormeR, EyE, ToroR, LeboyerM, GillbergC, et al. (2013) Progress toward treatments for synaptic defects in autism. Nat Med 19 : 685–694.
42. DurandCM, PerroyJ, LollF, PerraisD, FagniL, et al. (2011) SHANK3 mutations identified in autism lead to modification of dendritic spine morphology via an actin-dependent mechanism. Mol Psychiatry 17 : 71–84.
43. AronsMH, ThynneCJ, GrabruckerAM, LiD, SchoenM, et al. (2012) Autism-Associated Mutations in ProSAP2/Shank3 Impair Synaptic Transmission and Neurexin-Neuroligin-Mediated Transsynaptic Signaling. J Neurosci 32 : 14966–14978.
44. BerkelS, TangW, TrevinoM, VogtM, ObenhausHA, et al. (2011) Inherited and de novo SHANK2 variants associated with autism spectrum disorder impair neuronal morphogenesis and physiology. Hum Mol Genet 21 : 344–357.
45. DenayerA, Van EschH, de RavelT, FrijnsJP, Van BuggenhoutG, et al. (2012) Neuropsychopathology in 7 Patients with the 22q13 Deletion Syndrome: Presence of Bipolar Disorder and Progressive Loss of Skills. Mol Syndromol 3 : 14–20.
46. ChilianB, AbdollahpourH, BierhalsT, HaltrichI, FeketeG, et al. (2013) Dysfunction of SHANK2 and CHRNA7 in a patient with intellectual disability and language impairment supports genetic epistasis of the two loci. Clin Genet 84 (6) 560–5 doi: 10.1111/cge.12105
47. GauthierJ, ChampagneN, LafreniereRG, XiongL, SpiegelmanD, et al. (2010) De novo mutations in the gene encoding the synaptic scaffolding protein SHANK3 in patients ascertained for schizophrenia. Proc Natl Acad Sci U S A 107 : 7863–7868.
48. LordC, RutterM, Le CouteurA (1994) Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 24 : 659–685.
49. LordC, RisiS, LambrechtL, CookEHJr, LeventhalBL, et al. (2000) The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 30 : 205–223.
50. WingL, LeekamSR, LibbySJ, GouldJ, LarcombeM (2002) The Diagnostic Interview for Social and Communication Disorders: background, inter-rater reliability and clinical use. J Child Psychol Psychiatry 43 : 307–325.
51. SebatJ, LakshmiB, MalhotraD, TrogeJ, Lese-MartinC, et al. (2007) Strong association of de novo copy number mutations with autism. Science 316 : 445–449.
52. MarshallCR, NoorA, VincentJB, LionelAC, FeukL, et al. (2008) Structural variation of chromosomes in autism spectrum disorder. Am J Hum Genet 82 : 477–488.
53. BremerA, GiacobiniM, NordenskjoldM, Brondum-NielsenK, MansouriM, et al. (2009) Screening for copy number alterations in loci associated with autism spectrum disorders by two-color multiplex ligation-dependent probe amplification. Am J Med Genet B Neuropsychiatr Genet 153B: 280–285.
54. ClopperCJ, PearsonES (1934) The Use of Confidence or Fiducial Limits Illustrated in the Case of the Binomial. Biometrika 26 : 404–413.
55. HigginsJP, ThompsonSG, DeeksJJ, AltmanDG (2003) Measuring inconsistency in meta-analyses. BMJ 327 : 557–560.
56. FriedrichJO, AdhikariNK, BeyeneJ (2007) Inclusion of zero total event trials in meta-analyses maintains analytic consistency and incorporates all available data. BMC Med Res Methodol 7 : 5.
57. LanePW (2013) Meta-analysis of incidence of rare events. Stat Methods Med Res 22 : 117–132.
Štítky
Genetika Reprodukční medicína
Článek An Evolutionarily Conserved Role for the Aryl Hydrocarbon Receptor in the Regulation of MovementČlánek Requirement for Drosophila SNMP1 for Rapid Activation and Termination of Pheromone-Induced ActivityČlánek Co-regulated Transcripts Associated to Cooperating eSNPs Define Bi-fan Motifs in Human Gene NetworksČlánek Identification of a Regulatory Variant That Binds FOXA1 and FOXA2 at the Type 2 Diabetes GWAS LocusČlánek tRNA Modifying Enzymes, NSUN2 and METTL1, Determine Sensitivity to 5-Fluorouracil in HeLa CellsČlánek Derlin-1 Regulates Mutant VCP-Linked Pathogenesis and Endoplasmic Reticulum Stress-Induced ApoptosisČlánek A Genetic Assay for Transcription Errors Reveals Multilayer Control of RNA Polymerase II FidelityČlánek The Proprotein Convertase KPC-1/Furin Controls Branching and Self-avoidance of Sensory Dendrites inČlánek Regulation of p53 and Rb Links the Alternative NF-κB Pathway to EZH2 Expression and Cell SenescenceČlánek BMPs Regulate Gene Expression in the Dorsal Neuroectoderm of and Vertebrates by Distinct MechanismsČlánek Unkempt Is Negatively Regulated by mTOR and Uncouples Neuronal Differentiation from Growth Control
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 9- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- Translational Regulation of the Post-Translational Circadian Mechanism
- An Evolutionarily Conserved Role for the Aryl Hydrocarbon Receptor in the Regulation of Movement
- Eliminating Both Canonical and Short-Patch Mismatch Repair in Suggests a New Meiotic Recombination Model
- Requirement for Drosophila SNMP1 for Rapid Activation and Termination of Pheromone-Induced Activity
- Co-regulated Transcripts Associated to Cooperating eSNPs Define Bi-fan Motifs in Human Gene Networks
- Targeted H3R26 Deimination Specifically Facilitates Estrogen Receptor Binding by Modifying Nucleosome Structure
- Role for Circadian Clock Genes in Seasonal Timing: Testing the Bünning Hypothesis
- The Tandem Repeats Enabling Reversible Switching between the Two Phases of β-Lactamase Substrate Spectrum
- The Association of the Vanin-1 N131S Variant with Blood Pressure Is Mediated by Endoplasmic Reticulum-Associated Degradation and Loss of Function
- Identification of a Regulatory Variant That Binds FOXA1 and FOXA2 at the Type 2 Diabetes GWAS Locus
- Regulation of Flowering by the Histone Mark Readers MRG1/2 via Interaction with CONSTANS to Modulate Expression
- The Actomyosin Machinery Is Required for Retinal Lumen Formation
- Plays a Conserved Role in Assembly of the Ciliary Motile Apparatus
- Hidden Diversity in Honey Bee Gut Symbionts Detected by Single-Cell Genomics
- Ribosome Rescue and Translation Termination at Non-Standard Stop Codons by ICT1 in Mammalian Mitochondria
- tRNA Modifying Enzymes, NSUN2 and METTL1, Determine Sensitivity to 5-Fluorouracil in HeLa Cells
- Causal Variation in Yeast Sporulation Tends to Reside in a Pathway Bottleneck
- Tissue-Specific RNA Expression Marks Distant-Acting Developmental Enhancers
- WC-1 Recruits SWI/SNF to Remodel and Initiate a Circadian Cycle
- Clonal Expansion of Early to Mid-Life Mitochondrial DNA Point Mutations Drives Mitochondrial Dysfunction during Human Ageing
- Methylation QTLs Are Associated with Coordinated Changes in Transcription Factor Binding, Histone Modifications, and Gene Expression Levels
- Differential Management of the Replication Terminus Regions of the Two Chromosomes during Cell Division
- Obesity-Linked Homologues and Establish Meal Frequency in
- Derlin-1 Regulates Mutant VCP-Linked Pathogenesis and Endoplasmic Reticulum Stress-Induced Apoptosis
- Stress-Induced Nuclear RNA Degradation Pathways Regulate Yeast Bromodomain Factor 2 to Promote Cell Survival
- The MAPK p38c Regulates Oxidative Stress and Lipid Homeostasis in the Intestine
- Widespread Genome Reorganization of an Obligate Virus Mutualist
- Trans-kingdom Cross-Talk: Small RNAs on the Move
- The Vip1 Inositol Polyphosphate Kinase Family Regulates Polarized Growth and Modulates the Microtubule Cytoskeleton in Fungi
- Myosin Vb Mediated Plasma Membrane Homeostasis Regulates Peridermal Cell Size and Maintains Tissue Homeostasis in the Zebrafish Epidermis
- GLD-4-Mediated Translational Activation Regulates the Size of the Proliferative Germ Cell Pool in the Adult Germ Line
- Genome Wide Association Studies Using a New Nonparametric Model Reveal the Genetic Architecture of 17 Agronomic Traits in an Enlarged Maize Association Panel
- Translational Regulation of the DOUBLETIME/CKIδ/ε Kinase by LARK Contributes to Circadian Period Modulation
- Positive Selection and Multiple Losses of the LINE-1-Derived Gene in Mammals Suggest a Dual Role in Genome Defense and Pluripotency
- Out of Balance: R-loops in Human Disease
- A Genetic Assay for Transcription Errors Reveals Multilayer Control of RNA Polymerase II Fidelity
- Altered Behavioral Performance and Live Imaging of Circuit-Specific Neural Deficiencies in a Zebrafish Model for Psychomotor Retardation
- Nipbl and Mediator Cooperatively Regulate Gene Expression to Control Limb Development
- Meta-analysis of Mutations in Autism Spectrum Disorders: A Gradient of Severity in Cognitive Impairments
- The Proprotein Convertase KPC-1/Furin Controls Branching and Self-avoidance of Sensory Dendrites in
- Hydroxymethylated Cytosines Are Associated with Elevated C to G Transversion Rates
- Memory and Fitness Optimization of Bacteria under Fluctuating Environments
- Regulation of p53 and Rb Links the Alternative NF-κB Pathway to EZH2 Expression and Cell Senescence
- Interspecific Tests of Allelism Reveal the Evolutionary Timing and Pattern of Accumulation of Reproductive Isolation Mutations
- PRO40 Is a Scaffold Protein of the Cell Wall Integrity Pathway, Linking the MAP Kinase Module to the Upstream Activator Protein Kinase C
- Low Levels of p53 Protein and Chromatin Silencing of p53 Target Genes Repress Apoptosis in Endocycling Cells
- SPDEF Inhibits Prostate Carcinogenesis by Disrupting a Positive Feedback Loop in Regulation of the Foxm1 Oncogene
- RRP6L1 and RRP6L2 Function in Silencing Regulation of Antisense RNA Synthesis
- BMPs Regulate Gene Expression in the Dorsal Neuroectoderm of and Vertebrates by Distinct Mechanisms
- Unkempt Is Negatively Regulated by mTOR and Uncouples Neuronal Differentiation from Growth Control
- Atkinesin-13A Modulates Cell-Wall Synthesis and Cell Expansion in via the THESEUS1 Pathway
- Dopamine Signaling Leads to Loss of Polycomb Repression and Aberrant Gene Activation in Experimental Parkinsonism
- Histone Methyltransferase MMSET/NSD2 Alters EZH2 Binding and Reprograms the Myeloma Epigenome through Global and Focal Changes in H3K36 and H3K27 Methylation
- Bipartite Recognition of DNA by TCF/Pangolin Is Remarkably Flexible and Contributes to Transcriptional Responsiveness and Tissue Specificity of Wingless Signaling
- The Olfactory Transcriptomes of Mice
- Muscular Dystrophy-Associated and Variants Disrupt Nuclear-Cytoskeletal Connections and Myonuclear Organization
- Interplay of dFOXO and Two ETS-Family Transcription Factors Determines Lifespan in
- Evidence for Widespread Positive and Negative Selection in Coding and Conserved Noncoding Regions of
- Genome-Wide Association Meta-analysis of Neuropathologic Features of Alzheimer's Disease and Related Dementias
- Rejuvenation of Meiotic Cohesion in Oocytes during Prophase I Is Required for Chiasma Maintenance and Accurate Chromosome Segregation
- Admixture in Latin America: Geographic Structure, Phenotypic Diversity and Self-Perception of Ancestry Based on 7,342 Individuals
- Local Effect of Enhancer of Zeste-Like Reveals Cooperation of Epigenetic and -Acting Determinants for Zygotic Genome Rearrangements
- Differential Responses to Wnt and PCP Disruption Predict Expression and Developmental Function of Conserved and Novel Genes in a Cnidarian
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Admixture in Latin America: Geographic Structure, Phenotypic Diversity and Self-Perception of Ancestry Based on 7,342 Individuals
- Nipbl and Mediator Cooperatively Regulate Gene Expression to Control Limb Development
- Genome Wide Association Studies Using a New Nonparametric Model Reveal the Genetic Architecture of 17 Agronomic Traits in an Enlarged Maize Association Panel
- Histone Methyltransferase MMSET/NSD2 Alters EZH2 Binding and Reprograms the Myeloma Epigenome through Global and Focal Changes in H3K36 and H3K27 Methylation
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



