-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Dietary Restriction Induced Longevity Is Mediated by Nuclear Receptor NHR-62 in
Dietary restriction (DR) extends lifespan in a wide variety of species, yet the underlying mechanisms are not well understood. Here we show that the Caenorhabditis elegans HNF4α-related nuclear hormone receptor NHR-62 is required for metabolic and physiologic responses associated with DR-induced longevity. nhr-62 mediates the longevity of eat-2 mutants, a genetic mimetic of dietary restriction, and blunts the longevity response of DR induced by bacterial food dilution at low nutrient levels. Metabolic changes associated with DR, including decreased Oil Red O staining, decreased triglyceride levels, and increased autophagy are partly reversed by mutation of nhr-62. Additionally, the DR fatty acid profile is altered in nhr-62 mutants. Expression profiles reveal that several hundred genes induced by DR depend on the activity of NHR-62, including a putative lipase required for the DR response. This study provides critical evidence of nuclear hormone receptor regulation of the DR longevity response, suggesting hormonal and metabolic control of life span.
Published in the journal: . PLoS Genet 9(7): e32767. doi:10.1371/journal.pgen.1003651
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003651Summary
Dietary restriction (DR) extends lifespan in a wide variety of species, yet the underlying mechanisms are not well understood. Here we show that the Caenorhabditis elegans HNF4α-related nuclear hormone receptor NHR-62 is required for metabolic and physiologic responses associated with DR-induced longevity. nhr-62 mediates the longevity of eat-2 mutants, a genetic mimetic of dietary restriction, and blunts the longevity response of DR induced by bacterial food dilution at low nutrient levels. Metabolic changes associated with DR, including decreased Oil Red O staining, decreased triglyceride levels, and increased autophagy are partly reversed by mutation of nhr-62. Additionally, the DR fatty acid profile is altered in nhr-62 mutants. Expression profiles reveal that several hundred genes induced by DR depend on the activity of NHR-62, including a putative lipase required for the DR response. This study provides critical evidence of nuclear hormone receptor regulation of the DR longevity response, suggesting hormonal and metabolic control of life span.
Introduction
Genetic and environmental factors can cause profound changes in organism lifespan. Genetic alterations that stimulate robust longevity across taxa include reduced insulin/IGF and TOR signaling, reduced mitochondrial function, and reduced signaling from germline stem cells [1]. One of the most pervasive environmental alterations that impacts longevity is dietary restriction (DR), a reduction in caloric uptake without malnutrition, which can increase health and life span in different organisms, including yeast, worms, flies, and rodents [2]. Whether DR induces longevity in non-human primates is still under debate, however there are clear health benefits observed [3], [4]. In humans, evidence indicates that DR lowers body temperature, insulin levels, and body fat [5], [6]. Moreover, improved serum cholesterol and lipid levels suggest a decreased risk for cardiovascular disease [7]. Conversely, overnutrition may be a risk factor for age-related disease including obesity, diabetes, heart disease, neurodegeneration, and cancer [8].
In Caenorhabditis elegans several different DR regimens can induce longevity. The two most widely used are dilution of bacterial food and the genetic DR mimetic eat-2. Dilution of bacterial food in liquid culture (BDR) was first demonstrated by Klass in 1977 to extend C. elegans life span, and variations of this method have been shown to enhance longevity by 20–100% [9], [10]. By this regimen, animals develop on bacterial plates ad libitum until adulthood, and then are shifted to liquid culture containing a dilution of bacterial food. The eat-2 mutation affects the function of a pharyngeal acetylcholine receptor subunit, which reduces pharyngeal pumping rate and subsequent food intake throughout the life of the animal, and extends life span by 15–40% [11]. Other ways of inducing DR in C. elegans adults include intermittent feeding (IF), in which worms are fed every two days, dietary deprivation (DD), in which adult worms are completely removed from food, and solid DR (sDR) where bacteria is diluted on solid agar plates [12]–[14]. Curiously, life extension by these regimens requires different sets of genes, indicating that DR is not a uniform process and could result from multiple responses [11]–[16].
From genetic studies in C. elegans, a few key transcriptional regulators of the DR response have started to emerge. PHA-4 is a FOXA homolog required for eat-2 and BDR induced longevity [10]. SKN-1 is an NF-E2 transcription factor required in a BDR model of DR longevity [17]. Additionally, heat shock factor and hypoxia inducible factor have been implicated in regimens resembling DD or IF [18], [19]. Reduced signaling through the nutrient sensor TOR kinase, and processes downstream of TOR that increase autophagy, reduce protein synthesis, and alter energy homeostasis may contribute to the DR response [12], [20], [21]. Nevertheless, the regulatory networks and the underlying mechanisms promoting longevity still remain unclear.
In an effort to identify regulators of DR-induced longevity, we specifically focused on nuclear hormone receptors (NHRs). Nuclear hormone receptors are transcription factors that respond to fat-soluble hormones, such as steroids and fatty acids, to directly regulate gene transcription. They are broadly implicated in the regulation of development, metabolism and homeostasis, and are well poised to coordinate events throughout the body in response to hormonal or nutritional signals [22], [23]. We hypothesized that NHRs may mediate metabolic states associated with DR, and thus could be important for DR-induced longevity. In this work we identify the HNF4α-like nuclear hormone receptor nhr-62 as required for DR-induced metabolic and longevity responses.
Results
NHR-62 is Necessary for eat-2 DR-induced Longevity
To test if NHRs mediate DR-induced longevity, we performed RNAi knockdown of NHRs in animals carrying an eat-2 mutation (a genetic DR mimetic), and screened for a loss of eat-2-induced longevity. We screened 246 of the 284 C. elegans NHRs in a genetic background more sensitive to RNAi (eat-2;nre-1;lin-15b). Knockdown of most NHR genes had no effect on eat-2 longevity (e.g., nhr-35 Figure 1A). As expected, knockdown of a few NHRs substantially shortened eat-2 and wild-type life span (e.g., nhr-49) yet largely maintained DR-induced life span extension (Figure S1). Interestingly, we found that only knockdown of nhr-62, an HNF4α related NHR, suppressed the longevity of eat-2;nre-1;lin-15b while having little effect on the longevity of nre-1;lin-15b control animals (Figure 1B).
Fig. 1. nhr-62 mediates the longevity response to dietary restriction. 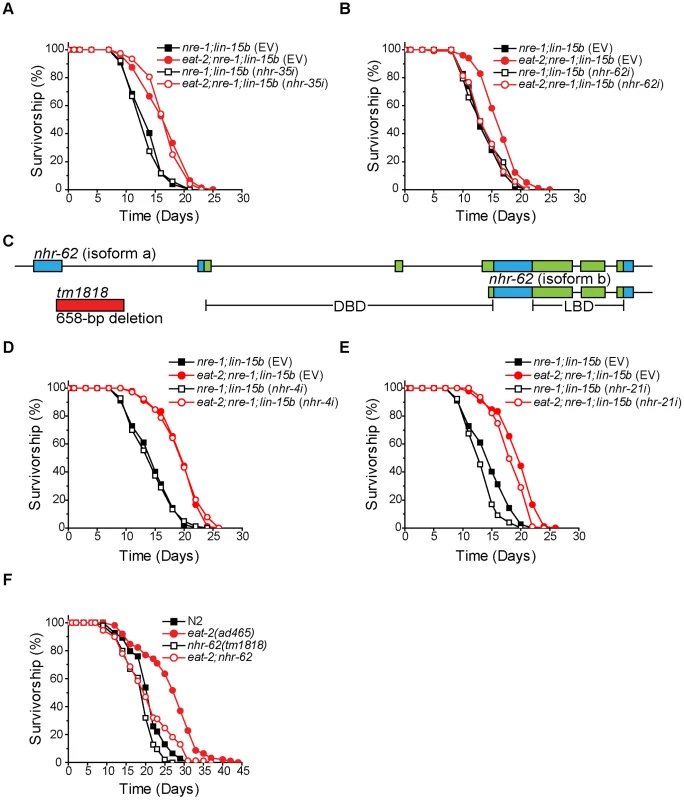
(A) eat-2;nre-1;lin-15b worms fed either empty vector or nhr-35 RNAi had a significant increase in lifespan compared to ad libitum controls (p<0.001). See Figure S1 for other NHRs. (B) eat-2;nre-1;lin-15b fed nhr-62 RNAi do not have a DR-induced lifespan extension (p = 0.907). (C) Gene model for nhr-62. Boxes indicate exons and lines indicate introns. The DNA binding domain (DBD) and the ligand binding domain (LBD) are indicated in green. (D,E) eat-2;nre-1;lin-15b worms fed either empty vector, nhr-4 or nhr-21 RNAi had a significant increase in lifespan compared to ad libitum controls (p<0.001). (F) eat-2(ad465) had a significant increase in lifespan compared to wild-type (N2) (p<0.001). Mutation of nhr-62 suppressed the lifespan extension of eat-2(ad465) worms (p<0.001). p-values calculated by the log-rank test. The nhr-62 locus encodes a predicted long isoform A (515 AA) consisting of DNA - and ligand-binding domains (DBD; LBD), and a short isoform B (353 AA) consisting of only the LBD (Figure 1C). nhr-62 shares 26–28% overall identity with vertebrate and insect HNF4α nuclear receptors across both domains. Mammalian HNF4α proteins function in many processes including lipid and glucose metabolism, and HNF4α mutations in humans are associated with maturity onset diabetes of the young type 1 [24], [25]. Similarly, the Drosophila melanogaster HNF4α homolog is important for lipid mobilization, fatty acid β-oxidation, and the starvation response [26]. However, 269 of the 284 NHRs in C. elegans represent a major expansion of the HNF4α family and are interrelated [27]. To determine specificity of the suppression of DR-induced longevity by nhr-62, we first compared the fraction of animals alive at day 15 between nre-1;lin-15b and eat-2;nre-1;lin-15b fed nhr-62's closest homologs, nhr-21, nhr-4, nhr-34, and nhr-100, and found that these genes were not required for longevity in eat-2;nre-1;lin-15b mutant animals (Figure S1). Secondly, we measured the lifespan of dietarily restricted worms subjected to either empty vector RNAi or RNAi specific to the NHRs with closest homology, nhr-21 and nhr-4, and again found that these genes were not required for DR-induced longevity (Figure 1D,E and Figure S1). These data suggest that nhr-62 uniquely regulates eat-2 DR-induced life span extension in C. elegans.
To validate our RNAi results, we obtained the deletion allele, nhr-62(tm1818), which removes a 658 bp region, including part of exon one, and results in an immediate stop codon, thus removing the DBD of the receptor. Although nhr-62(tm1818) is likely a strong loss-of-function allele, it may not be null because the predicted B isoform containing the LBD only is intact. We introduced this mutation into eat-2(ad465) animals and measured the lifespan of the double mutant. Similar to RNAi, nhr-62(tm1818) significantly suppressed the lifespan of eat-2(ad465) animals. Importantly, the nhr-62(tm1818) mutant had little or no effect on wild-type (N2), suggesting that nhr-62 does not suppress eat-2 longevity through general sickness (Figure 1F). Though nhr-62(tm1818) consistently suppressed eat-2(ad465) lifespan, suppression was not always complete, suggesting other activities still contribute to the response.
To confirm the role of nhr-62 in DR-induced longevity, we generated an extra chromosomal line, dhEx627, expressing wild-type nhr-62, and introduced it into eat-2;nhr-62 mutant animals to measure whether complementing nhr-62 function was sufficient to restore longevity. As expected, the dhEx627 array restored longevity to eat-2;nhr-62 double mutants (Figure 2A). Interestingly, when this array was crossed into the wild-type background, nhr-62 over-expressing worms displayed phenotypes similar to DR worms (e.g., small body size and reduced fat) without affecting pharyngeal pumping rate (Figure S2). Furthermore, the dhEx627 array significantly extended the life span of wild-type animals in four of eight independent experiments, suggesting that nhr-62 can be sufficient to promote longevity (Figure 2B and Table S1).
Fig. 2. nhr-62 specifically regulates DR-induced longevity. 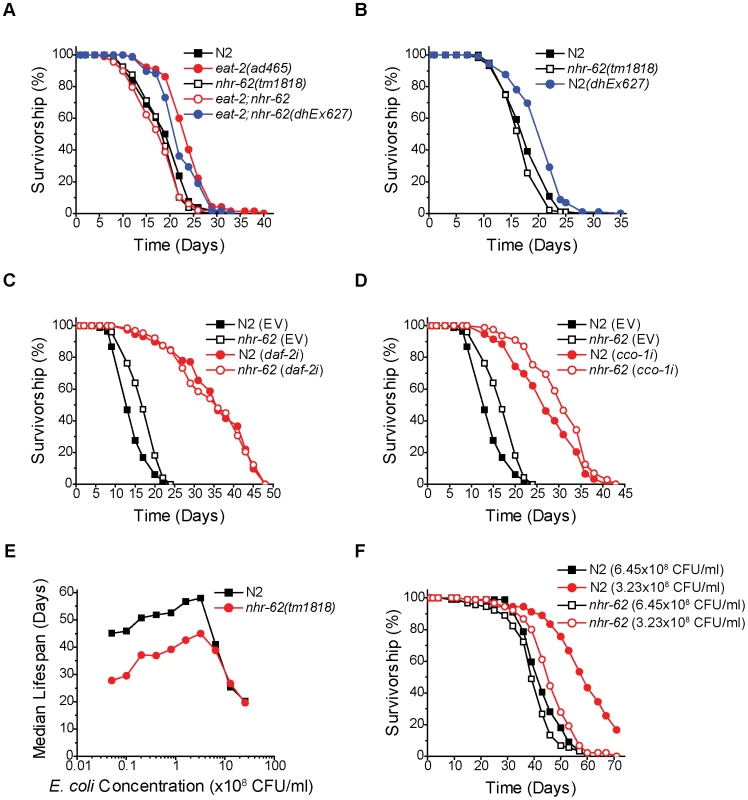
(A) Survivorship curves for wild-type (N2), eat-2(ad465), nhr-62(tm1818), eat-2;nhr-62, and eat-2;nhr-62(dhEx627). Extra chromosomal array dhEx627 (carrying a wild-type nhr-62) in eat-2;nhr-62 resulted in a significant increase in lifespan compared to both nhr-62(tm1818) and eat-2;nhr-62 mutants (p<0.001). (B) Wild-type (N2) worms with dhEx627 exhibited a significant increase in lifespan compared to wild-type (p<0.001). See Figure S2 for physiologic traits of overexpression. (C,D) Both wild-type (N2) and nhr-62(tm1818) worms fed daf-2 RNAi or cco-1 RNAi had a significant increase in lifespan when compared to controls (p<0.001). (E) BDR curve for wild-type (N2) and nhr-62(tm1818) worms. The lifespan of wild-type and nhr-62(tm1818) were not different at the three most concentrated food dilutions, but were significantly different at the remaining 7 dilutions (p<0.001). (F) Survivorship curves for wild-type (N2) and nhr-62(tm1818) fed either 6.45×108 CFU/ml or 3.23×108 CFU/ml. p-values calculated by the log-rank test. Reduced insulin/IGF receptor (IR) signaling or reduced mitochondrial function have both been shown to extend life span [28], [29]. To test if nhr-62 also modulates longevity of these pathways, we measured the lifespan of nhr-62(tm1818) animals fed daf-2 (IR) or cco-1 (cytochrome c oxidase) RNAi. As expected, daf-2 RNAi and cco-1 RNAi robustly increased the life span of wild-type animals. Moreover, RNAi knockdown similarly increased longevity in the nhr-62(tm1818) background (Figure 2C,D). These results reveal the nhr-62 mutation does not affect these pathways, but specifically modulates DR-induced longevity. Taken together, these data indicate that NHR-62 is a novel regulator of DR-induced longevity.
NHR-62 Mediates BDR Longevity Response at Lower Nutrient Levels
If NHR-62 is a robust mediator of DR-induced longevity, then it would be predicted to suppress longevity in a second DR regimen. To test whether nhr-62(tm1818) is also required for DR-induced longevity by BDR, we measured the lifespan of wild-type and nhr-62(tm1818) worms fed bacteria at ten different concentrations. We started with our ad libitum bacterial concentration of 2.5×109 CFU/ml followed by nine subsequent two-fold serial dilutions. At the level of DR associated with the longest lifespan (optimal; 3.23×108 CFU/ml), wild-type worms exhibited a 188% increase in median lifespan compared to ad libitum conditions (Figure 2E). When bacteria were diluted past this optimal DR condition, the median lifespan of wild-type worms decreased, resulting in a characteristic tent-shaped DR response curve. Unexpectedly, across the first two dilutions nhr-62(tm1818) worms exhibited a 40% increase in median lifespan compared to ad libitum conditions, identical to the wild-type DR response curve at these high nutrient conditions. However, at the optimal DR condition, the DR response curve for nhr-62(tm1818) mutants diverged from the wild-type curve; these animals were unable to respond to DR as effectively, with only a partial increase in the median lifespan at all subsequent concentrations (Figure 2E,F and Table S2). These results suggest that nhr-62 blunts the BDR response at lower nutrient concentrations.
NHR-62 Is Widely Expressed
We obtained a transgenic strain from the Hope laboratory containing 2 kb of the nhr-62 promoter fused to gfp (pnhr-62::gfp). As previously described, transgene expression was observed from embryo to adult in the pharynx and intestine. We also observed clear expression in various neurons including sensory neurons, motor neurons, hermaphrodite specific neuron, and pharyngeal neurons (data not shown). A similar pattern of expression was seen in the eat-2(ad465) background. A full-length integrated low copy nhr-62::gfp expressing strain from the TransgeneOme Project [30] was weakly expressed in many tissues including the nuclei of pharynx, sensory neurons, intestine, spermatheca, hypodermis, and excretory cell in both wild-type (Figure 3A–F) and eat-2 backgrounds. Again no obvious change in nhr-62::gfp intensity or localization was observed in the eat-2 background, nor were changes seen in nhr-62 mRNA levels as measured by qPCR. Notably several tissues that express nhr-62::gfp, such as the intestine, ASI sensory neurons, and hypodermis, are major endocrine tissues that coordinate metabolic states and have been implicated in aging [10], [17], [20], [31], [32].
Fig. 3. Expression pattern of nhr-62::gfp. 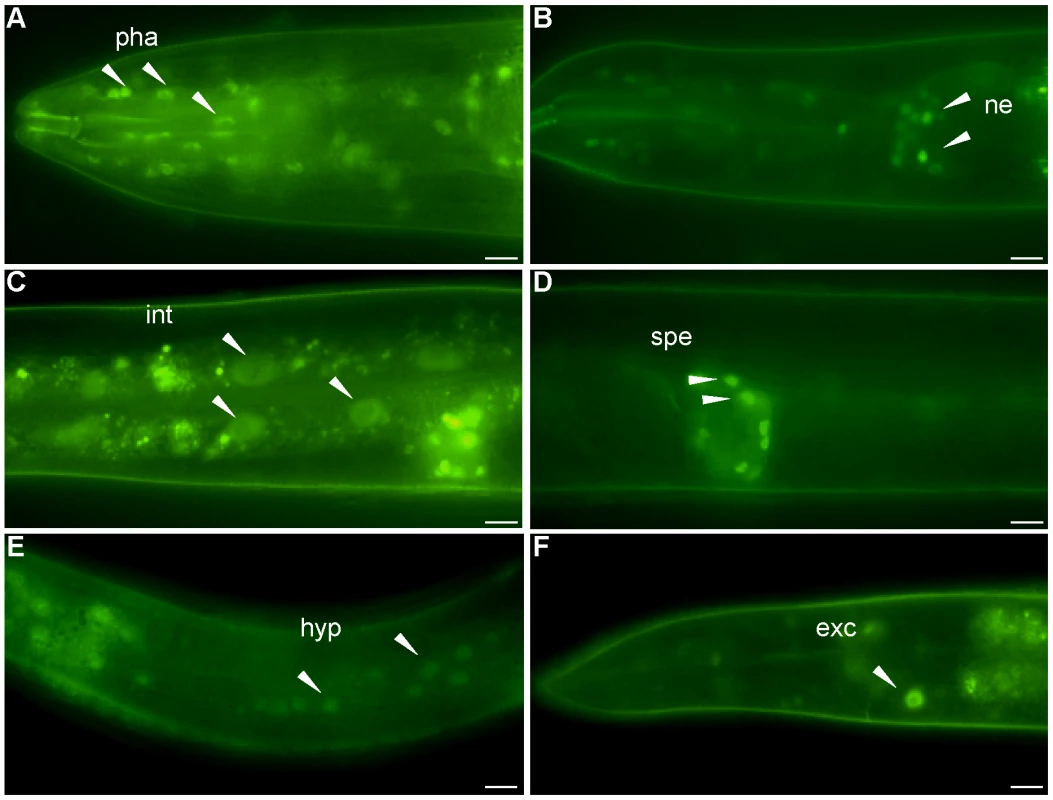
A full length fusion of nhr-62::gfp resides in the nuclei of (A) pharyngeal cells (pha), (B) sensory neurons (ne) (C) intestinal cells (int) (D) spermatheca (spe) (E) hypodermis (hyp) (F) excretory cell (exc). Scale bar is 10 µM. NHR-62 Modulates DR-Induced Fat Metabolism
Animals under DR generally display dramatic changes in stored fat compared to animals in ad libitum conditions. Since nhr-62 shares homology to HNF4α-like NHRs known to function in fat metabolism such as nhr-49 and nhr-80 [33], [34], we hypothesized that nhr-62 might be necessary for proper fat regulation under DR. To test this hypothesis, we treated wild-type, nhr-62(tm1818), eat-2(ad465), and eat-2;nhr-62 animals with the lysochrome dye Oil Red O, which stains neutral triglycerides and lipids (Figure 4A). The intensity of Oil Red O has been reported to correlate positively with triglyceride levels as measured by biochemical assays [35]. We found Oil Red O staining intensity in eat-2 mutant animals was noticeably less than wild-type. Notably, mutation of nhr-62 modestly increased staining intensity relative to wild-type or eat-2 controls. eat-2;nhr-62 mutants had a moderate but significant 14.1% increase in staining intensity compared to eat-2 animals (Figure 4B). This difference was not due to increased feeding as pumping rates of eat-2 and eat-2;nhr-62 were identical, as were body size and progeny production (Figure S3). We also measured the levels of triglycerides (TAGs) and similarly observed that eat-2 had a significant decrease in TAG/protein compared to both wild-type and nhr-62 mutants. Furthermore, nhr-62 partially reversed this effect, giving a significant 15.6% increase in total TAG/protein in eat-2;nhr-62 worms compared to eat-2 worms (Figure 4C). These data indicate a general role for nhr-62 in regulating lipid levels.
Fig. 4. nhr-62 modulates DR-induced fat metabolism. 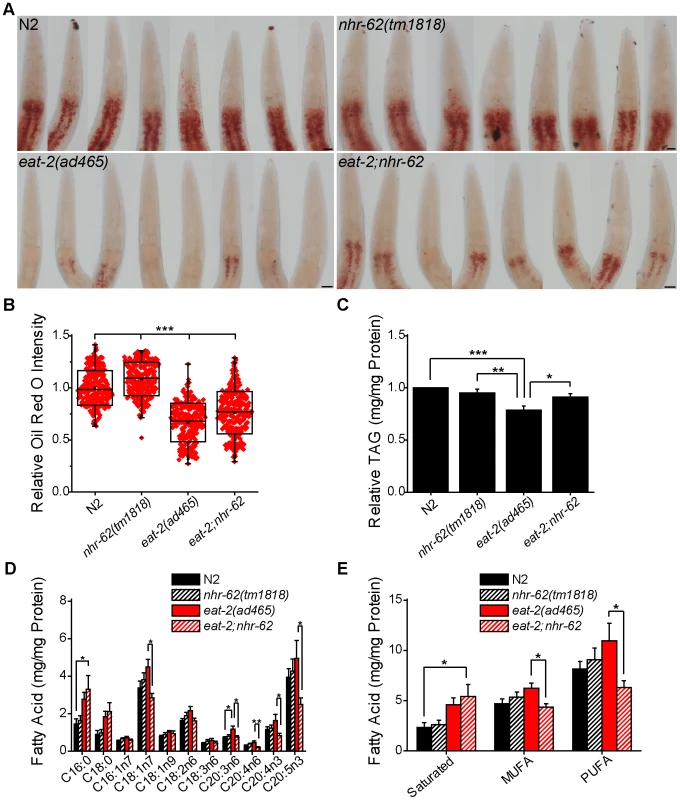
(A) Representative pictures of Oil Red O stained worms. Scale bar = 20 µM. (B) Oil Red O intensity is increased relative to wild-type (N2) or eat-2(ad465) controls by mutation of nhr-62 (combined data from 3 independent experiments). (C) Total triglycerides of day 1 adults relative to wild-type (N2) (combined data from 16 independent experiments). (D) Fatty acid profiles of day 1 adult animals (combined data from 11 independent experiments). (E) Total saturated, monounsaturated fatty acid (MUFA), and polyunsaturated fatty acid (PUFA) calculated from (D). eat-2;nhr-62 had increased saturated fat compared to wild-type (N2) and decreased MUFAs and PUFAs compared to eat-2(ad465). See Figure S3 for physiologic traits of nhr-62 mutation. *p<0.05, **p<0.01, ***p<0.001 by single factor ANOVA with Tukey test. Mean (Center Line) ± SD (Box) with bars representing an outlier coefficient of 1.5, or Mean±SEM. Longevity in C. elegans has been associated with altered fatty acid composition. For example, loss of germline stem cells, which increases life span, results in increased monounsaturated fatty acid content, and this longevity depends on production of oleic acid (C18 : 1n9) [36]. To determine if nhr-62 regulates fatty acid composition under DR, we quantified individual fats by gas chromatography. eat-2;nhr-62 double mutants exhibited increased levels of saturated fatty acids compared to wild-type. Moreover, eat-2;nhr-62 double mutants showed a significant reduction of various monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs) compared to eat-2 mutants alone (Figure 4D,E). These observations suggest that nhr-62 modulates changes in fatty acid composition under DR. Interestingly, we observed that the mRNA level of fat-2, a key enzyme in the production of PUFAs, was up-regulated 1.6-fold in eat-2 worms compared to wild-type as measured by qPCR. Mutation of nhr-62 dampened this up-regulation, although this effect did not reach statistical significance (Figure S4), suggesting that post-transcriptional regulation might also be at work.
Lipases are involved in the liberation of fatty acids from triglyceride stores. Given the changes in fatty acid composition and previous studies implicating lipases in modulating longevity [37], [38], we hypothesized that lipases might modulate DR-induced longevity. To test this hypothesis we screened through 34 predicted lipases in C. elegans for suppression of longevity in the eat-2;nre-1;lin-15b background (Table S3). Interestingly, we found that RNAi targeting one predicted lipase, C40H1.8, partially reduced longevity of dietary restricted animals, reaching significance in 4 out of 6 experiments (Figure 5A and Figure S5, Table S1). One explanation for a partial response is that C40H1.8 RNAi does not result in complete knockdown (Figure S6). Additionally, C40H1.8 has two closely related homologs, C40H1.7 and C40H1.9, which were unaffected by C40H1.8 RNAi knockdown and might compensate for its reduction of function (Figure S6). Consistent with a unified pathway, C40H1.8 knockdown did not further reduce the life span of eat-2;nhr-62 double mutants (Figure 5B). By qPCR analysis, we found that C40H1.8 was up-regulated five-fold under DR. In eat-2;nhr-62 animals, this up-regulation was attenuated by approximately 50%, suggesting some regulation of C40H1.8 expression by nhr-62 (Figure 5C). These data indicate that C40H1.8 may play at least a partial role in mediating eat-2-induced longevity. C40H1.8 contains a class 3 lipase domain, found in a variety of proteins predicted to have triacylglycerol lipase activity. In particular, C40H1.8 contains the catalytic triad comprised of serine, aspartate, and histidine (S198, D256, H316), a nucleophilic elbow, and other features typical of lipases (Figure S7) [39], [40]. Although C40H1.8 does not have a clear mammalian ortholog, class 3 lipases from other species include Atg15p, which is involved in autophagy in Saccharomyces cerevisiae and is required for DR-mediated longevity [38], and the Sn-1 specific diacylglycerol lipase implicated in endocannabinoid signaling in mice [41].
Fig. 5. nhr-62 regulates a DR-induced lipase and autophagy. 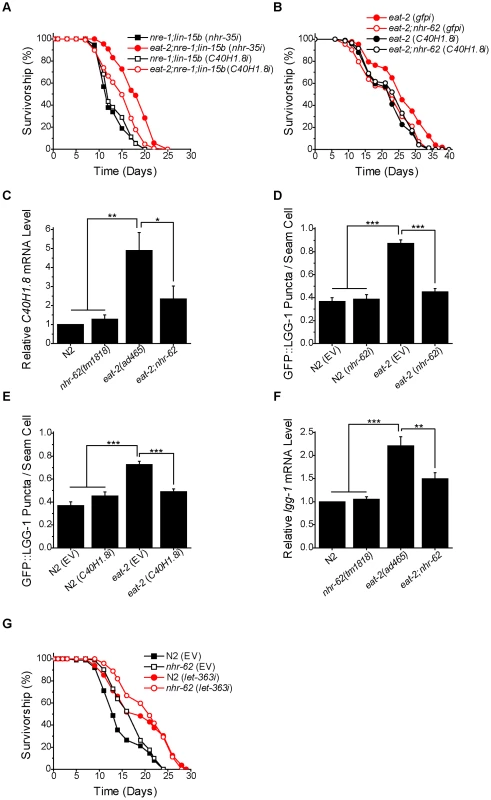
(A) eat-2;nre-1;lin-15b worms fed C40H1.8 RNAi had a significantly reduced lifespan compared to eat-2;nre-1;lin-15b worms fed nhr-35 control RNAi (log-rank test p<0.001). See Figure S5 for additional experiments. (B) C40H1.8 RNAi did not decrease the lifespan of eat-2;nhr-62 worms compared to eat-2;nhr-62 (gfpi). (log-rank test p = 0.4798). (C) The up-regulation of C40H1.8 expression under DR was significantly suppressed in eat-2;nhr-62. (D) The induction of GFP::LGG-1 puncta formation in eat-2(ad465) was significantly suppressed to ad libitum levels by nhr-62 RNAi (combined data from 3 independent experiments). (E) The induction of GFP::LGG-1 puncta formation in eat-2(ad465) was significantly suppressed to ad libitum levels on C40H1.8 RNAi (combined data from 3 independent experiments). (F) The up-regulation of lgg-1 expression under DR was significantly suppressed in eat-2;nhr-62. (G) Both wild-type (N2) and nhr-62(tm1818) worms fed let-363/TOR RNAi had a significant increase in lifespan when compared to controls (log-rank test p<0.001). EV = empty vector . *p<0.05, **p<0.01, ***p<0.001 by single factor ANOVA with Tukey test. Mean±SEM. NHR-62 Regulates DR-Induced Autophagy
Autophagy, the catabolic process involving the degradation of cellular components which are recycled to serve as a source of energy and precursors for biosynthesis, has also been linked to DR, as well as to fatty acid metabolism and lipolysis [20], [42], [43]. To determine if nhr-62 influenced DR induced autophagy, we utilized a GFP::LGG-1 reporter strain. LGG-1 is the worm ortholog of S. cerevisiae ATG8p, which is incorporated into pre-autophagosomal membranes, and is necessary for proper cellular degradation during autophagy [44]. Under normal conditions GFP::LGG-1 is cytoplasmic and diffuse in the worm's hypodermal seam cells, but under DR conditions, forms puncta which are associated with an increase in autophagy [20]. We observed a significant increase in the average number of GFP::LGG-1 puncta per seam cell in the eat-2 background, and found that nhr-62 RNAi suppressed puncta formation back to wild-type levels, suggesting that nhr-62 modulates autophagy up-regulation under DR (Figure 5D). Previous studies have shown that lipases are required for autophagy induction in the context of the gonadal longevity pathway [43]. We wondered whether the C40H1.8 lipase implicated here in eat-2 longevity played any role in autophagy, and found that C40H1.8 RNAi knockdown reduced the number of GFP::LGG1 puncta in the eat-2 background (Figure 5E). Additionally, levels of lgg-1 mRNA were likewise induced in eat-2 mutants in an nhr-62 dependent manner (Figure 5F). These results suggest that nhr-62 regulates DR-mediated longevity, possibly through lipolysis and autophagy.
A major regulator of the DR response is the target of rapamycin (TOR) kinase. Down-regulation of let-363/TOR induces autophagy and extends life span through DR pathways [21]. To see how nhr-62 interacts with TOR signaling, we reduced let-363/TOR by RNAi in the nhr-62(tm1818) mutants. We found that let-363 knockdown induced longevity in both wild-type and nhr-62 mutants (Figure 5G and Table S1). These results suggest that let-363/TOR acts downstream or parallel to nhr-62.
NHR-62 Regulates the DR-specific Transcriptome
Because nhr-62 promotes DR longevity and associated physiological processes, we speculated that it could modulate the overall transcriptional change upon DR. To test this hypothesis, we performed RNA-seq on wild-type, nhr-62(tm1818), eat-2(ad465), and eat-2;nhr-62 animals. This approach not only enabled us to identify genes regulated under DR, but also which genes showed nhr-62 dependence. Our criteria for differential gene regulation included a greater than 1.5-fold change in expression compared to wild-type, a false discovery rate less than 0.05, and greater than 10 reads when normalized to the base mean. For each sample we typically obtained 25–37 million unambiguous reads, and could assign these to approximately 15,000 genes. Heat maps of the mean Euclidean distance between samples showed that nhr-62 was most similar to wild-type, while eat-2 was most different, with eat-2;nhr-62 in between (Figure S8). qPCR on 12 of 13 regulated genes showed consistency with RNA-seq data, validating this approach for our analysis (Figure S8).
Global profiles comparing wild-type and eat-2 gave rise to over 3,000 genes with significant gene expression changes out of 17,788 examined genes, depicting an altered transcriptome for longevity induced by the eat-2 mutation (Figure 6A and Table S4). DAVID analysis [45], [46] revealed an enrichment of biological processes involved in oxidation/reduction, phosphorus metabolism, unsaturated fatty acid, eicosanoid and ceramide metabolism, lipid modification and transport, amino acid, amine and chitin metabolism, and neuropeptide signaling among others (Figure 6D and Table S5).
Fig. 6. Expression profile analysis of eat-2 and nhr-62 regulated genes. 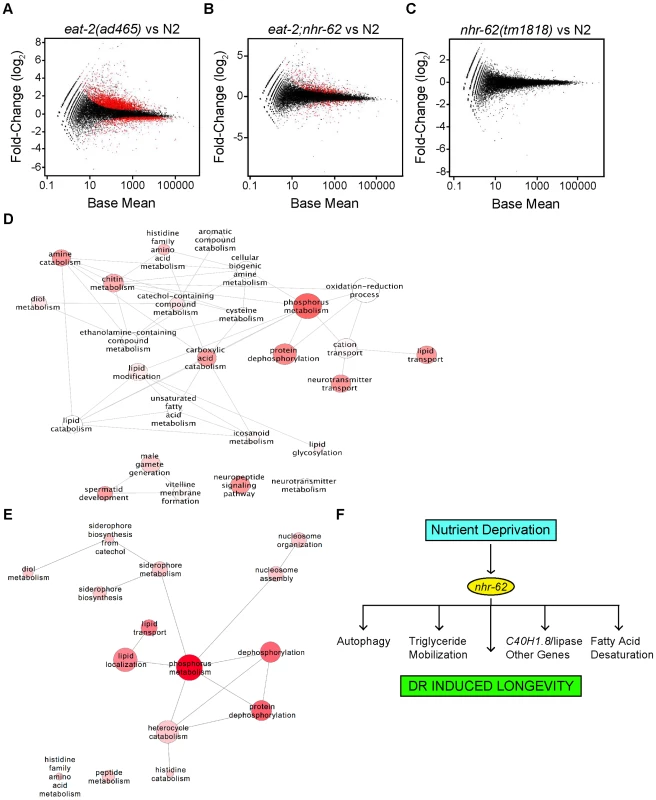
Scatter plots of log2 fold-changes versus mean expression level. Red dots highlight differentially expressed genes at FDR<0.05 for (A) eat-2(ad465) versus wild-type (N2) (B) eat-2;nhr-62 versus wild-type (N2) and (C) nhr-62(tm1818) versus wild-type (N2). (D) ReviGO analysis to summarize all GO-BP terms returned by DAVID with p value <0.1 when analyzing Gene-list “eat-2-vs-N2” and (E) list of DR regulated genes rescued by nhr-62(tm1818). Bubble size indicates the frequency of the GO term from the GOA database, bubble color is dictated by the p value with a darker color being a lower p value, and similar GO terms are linked by lines where the line thickness indicates degree of similarity. (F) Model for DR. See Figure S8 for Euclidean distances between samples, Venn diagram, and qPCR validation of RNA-seq data. Consistent with a physiological function in mediating DR, nhr-62 mutation restored expression of over 600 genes regulated by eat-2(ad465) back to wild-type levels (Figure 6B and Table S6). By contrast, little change was seen in the nhr-62 transcriptome compared to wild-type (Figure 6C), with only 17 genes differentially expressed. Enriched categories regulated by nhr-62 under DR included genes involved in fatty acid localization and transport (vit-1/2/3/4/5/6, lbp-8, ABCG transporters, wht-5 and wht-9), phosphorus metabolism (kinases gska-3 and T21G5.1, phosphatases Y57G11C.6 and B0280.11), nucleosome assembly and organization, (C50F4.6, ZC155.2) protein, amino acid, histidine, and heterocyclic amine metabolism (zmp-1, T25B9.6, B0019.1) and short chain dehydrogenase metabolism (dhs-25, dhs-14) (Figure 6E and Tables S6,S7). Other genes up-regulated by eat-2 but reversed by nhr-62 mutation include the glutathione S-transferase gst-21, collagen col-146, and the FMRFamide-related neuropeptide flp-21. Genes down-regulated under DR and reversed by nhr-62 include the acyl CoA dehydrogenase, acdh-2, lipid binding protein lbp-8, the conserved transmembrane protein C09B8.4, and the vitellogenins vit-1-6. The overall changes in transcription seen in nhr-62 mutants under DR, supports the notion that nhr-62 is an important mediator of the DR response.
Discussion
Nuclear hormone receptors are critical regulators of animal metabolism, development and homeostasis, and are particularly well suited to couple nutrient and lipid availability to transcriptional cascades. By screening through the set of NHR genes in C. elegans we identified the HNF4α homolog, nhr-62, as an important mediator of the DR response. Several lines of evidence are consistent with the notion that nhr-62 promotes aspects of DR-induced metabolism and longevity. RNAi knockdown or mutation of nhr-62 specifically suppressed eat-2 DR-induced longevity, but not that of reduced insulin/IGF receptor (daf-2) or mitochondrial function (cco-1) arguing for a DR specific function. Importantly, nhr-62 mutation prevented animals from fully responding to the longevity-inducing effects of bacterial food dilution (BDR), thereby making it one of only a handful of DR-regulating genes that clearly modulates multiple forms of DR. By comparing eat-2 to eat-2;nhr-62 mutants we found a reversal of Oil Red O staining intensity, triglyceride levels, and autophagy induction, without seeing a difference in body size or pumping rate. Additionally, we found that nhr-62 mutation decreased the mono - and polyunsaturated fatty acid content of dietary restricted worms. Finally, we found a reversal of the DR-induced transcriptional regulation of a predicted lipase required for DR, as well as a plethora of genes through RNA-seq analysis. These results point to a model in which a dietary restricted state promotes activation of nhr-62, the lipase C40H1.8, and numerous other genes to modulate DR-induced longevity, possibly through fat metabolism, autophagy, and other processes (Figure 6F). Although nhr-62 was the most visible candidate that emerged from our screens, it is possible that other NHRs may also play a role in the DR response, and were missed due to either incomplete knockdown or functional redundancy.
It was unexpected to see a partial BDR response by nhr-62(tm1818) mutants. One potential explanation for this observation is that by mutating nhr-62, the optimum DR threshold has been shifted to a new concentration. However, nhr-62(tm1818) mutants do not exhibit a globally shifted BDR response curve since the highest three food concentrations give median life spans that align precisely with wild-type. At further food dilution, nhr-62(tm1818) mutation significantly suppressed life span extension compared to wild-type, which results in a parallel response but with lower median life spans compared to wild-type. These results are consistent with a blunted response to DR in the lower nutrient range. In contrast to a “master regulator” of DR, which might completely abrogate life span extension at all food concentrations, this type of response curve could be indicative of a gene that plays a role in modulating or fine tuning the BDR longevity response at specific nutrient levels. It is also possible that nhr-62 mutation disrupts metabolism in a non-specific manner that is incompatible with a proper DR response. What argues against this is that nhr-62 mutation on its own had very little effect on gene expression or physiology under ad libitum conditions, suggesting nhr-62 is engaged in a regulatory role primarily under DR states.
nhr-62 exhibits several phenotypes suggesting it functions in mobilizing fat to partition energetic and biosynthetic demands under nutrient limitation. Notably nhr-62 mutation prevents some of the reduction of TAG and Oil Red O staining stores seen under DR longevity. Several dynamic changes in fatty acid metabolism have been observed upon DR shifts in other species. In flies there is a shift toward increasing fatty acid synthesis and breakdown, and inhibiting this suppresses DR longevity [47]. Mice initially increase endogenous fatty acid synthesis followed by prolonged fatty acid oxidation [48]. We observed that deletion of nhr-62 alters fatty acid composition in the eat-2 background, increasing the amount of the saturated fatty acid palmitate, and reducing the amount of various MUFAs and PUFAs, including C18 : 1n7, C20 : 3n6, C20 : 4n6, C20 : 4n3, and C20 : 5n3 fatty acids. It is possible that these changes could contribute towards the suppression of life span. Interestingly, a handful of these fatty acids have been recently implicated in longevity. The omega-6 fatty acid, DGLA (C20 : 3n6) stimulates autophagy and extends C. elegans life span [49]. Oleic acid (C18;1n7) has been shown to be important for life span extension upon germline removal [36]. It seems plausible that some of these lipids or their derivatives function in DR-mediated longevity. Additionally germline removal also results in up-regulation of the lipase, lipl-4, which stimulates autophagy [43]. Our results suggest a model in which lipolysis by C40H1.8 lipase could stimulate autophagy, since RNAi against this gene diminished DR longevity and GFP::LGG-1 punctual formation. Alternately, lipolysis could liberate fatty acids that serve a signaling role, binding to NHR-62 or related receptors. Future studies should further clarify the complex relationships between fatty acid composition, lipolysis, autophagy and longevity in the DR response.
By RNA-seq analysis we identified significant changes in the expression of approximately 3,000 genes comparing eat-2 to wild-type. Amongst enriched categories are genes involved in phosphorus metabolism, unsaturated fatty acid metabolism, eicosanoid and ceramide metabolism, lipid modification and transport, amino acid, amine and chitin metabolism, and neuropeptide signaling. Notably mutation of nhr-62 reversed the regulation of about 600 of these genes, suggesting a key role in mediating the DR response. These genes are candidates for nhr-62 targets, and possibly important effectors of the DR response (Table S6). Among them are genes whose molecular identity suggests a role in fat metabolism, including transport (apolipoproteins/vitellogenins, vit-1 through vit-6, ABCG transporters wht-5, wht-9), lipid binding (lbp-7, lbp-8), fatty acid remodeling (acyl coA thiolases F57F4.1), β-oxidation (acdh-2, ech-7, acyl transferase acl-13) and fatty acid elongation (elo-7, elo-8). Presumably some of these genes may mediate the physiologic changes relevant to DR. Indeed, vitellogenin knockdown has been previously shown to extend life [50].
Dietary restriction is associated with reduced TOR signaling, reduced protein synthesis, and increased autophagy [20], [21], [51]. Within the TOR pathway, the amino acid sensing G-proteins RAGA-1, RAGC-1 and TOR itself were down regulated about 20% but was significant for TOR only. Consistent with a possible downstream or parallel role, let-363/TOR RNAi induced longevity in the nhr-62 mutant background. We also found that the autophagy gene lgg-1 was up-regulated 1.5-fold, in a manner dependent on nhr-62. Consistently, we observed that autophagic vesicles visualized with GFP::LGG-1 were increased under DR in an nhr-62 dependent manner. Previous studies have shown that lgg-1 and other autophagy genes are required for DR and various longevity pathways [52]. However, other autophagy genes did not show significant regulation under DR, suggesting that post-transcriptional mechanisms contribute to this response.
Reduced protein translation extends life span and may be one of the important outputs of the DR response [53]. We observed significant down regulation of several translation initiation and release factors including inf-1, whose down-regulation has been shown to extend life span [54]. Additionally, a systematic, albeit small (approximately 20%) down-regulation of scores of ribosomal proteins was evident under DR; however none of these showed definitive nhr-62 dependence. It is possible that these minor transcriptional changes collectively recapitulate the physiologic effect of DR. Alternately, modest regulation of a critical enzyme or key regulator in the pathway might be sufficient. nhr-62 might also regulate unidentified post-transcriptional modifiers of these processes, especially given the many kinases and phosphatases affected (Tables S6,S7). Many of the genes regulated by nhr-62 also include nonconserved genes that fall into large families including F-box proteins, lectins, activated in blocked unfolded protein response (abu) and fungal induced proteins, which are speculated to be involved in innate immunity. In the future it will be important to understand how the diverse transcriptional output of nhr-62 relates to longevity and to identify direct targets by ChIP-seq.
It is currently unclear which mammalian homolog is most functionally analogous to nhr-62. The C. elegans genome has undergone a radical expansion of the HNF4 family of nuclear receptors, making an assignment of homologous function for nhr-62 challenging. One such HNF4-like nematode receptor, nhr-49, has been proposed as PPARα-like because it is involved in the starvation response, turns on genes implicated in β-oxidation, fatty acid desaturation, binding and transport. However, in our hands, nhr-49 knockdown did not abrogate the DR response. Conceivably, nhr-62 functions similar to the PPARs as well, since these nuclear receptors are known lipid sensors that regulate metabolism under different nutritional states. Corton et al originally observed a substantial overlap in the expression profile of DR and PPAR nuclear receptor regulation in mice [55]. Furthermore, in non-human primates, regulation of PPARs may be pivotal for the effects of DR [56]. Similar meta-analysis of expression profiles implicates PPARα in the DR response [57]. It is also possible that nhr-62 functions like HNF4, which regulates apolipoprotein levels and lipid and glucose homeostasis. Notably, both PPARs and HNF4 nuclear receptors can bind to fatty acids, which may be regulating their activity [58], [59]. The finding that nhr-62 is involved in fat metabolism raises the possibility it too is regulated by fatty acid like ligands.
Interestingly, the C. elegans transcription factor SKN-1/NF-E2 regulates DR-induced longevity from the pair of ASI sensory neurons [17]. This implies that cell non-autonomous signals downstream of SKN-1/NF-E2 mediate a systemic physiological response to DR. Conceivably, this DR response could be communicated through an endocrine mechanism, and suggest that specific hormones and hormone receptors such as NHR-62 are required for DR-induced longevity. If so, it would be exciting to identify the ligand for NHR-62 and determine if it could promote longevity under replete conditions. Alternately, NHR-62 may not be ligand regulated, but instead function as a competency factor instructed by other regulatory molecules that control its response to nutrient availability and facilitate metabolic remodeling. In the future it will be interesting to distinguish these possibilities. To our knowledge, this study is the first to find a nuclear hormone receptor specifically required for DR-induced longevity, potentially though regulation of fat metabolism and autophagy.
Materials and Methods
C. elegans Strains
All strains were grown and maintained on NGM agar seeded with E. coli (OP50) at 20°C unless otherwise noted. Standard procedures for culturing and maintaining strains were used [60]. Strains used: N2 (wild-type), eat-2(ad465), nhr-62(tm1818), nre-1(hd20);lin-15b(hd126), UL1385 mvEx5591(Y67A6A.2::gfp + unc-119), eat-2(ad465);mvEx5591(Y67A6A.2::gfp + unc-119), OP403 wgIs403(nhr-62::TY1 EGFP 3× FLAG (91H02);unc-119(+)); unc-119(tm4063), QU1 izEx1(plgg-1::gfp::lgg-1 + rol-6), eat-2(ad465); izEx1(plgg-1::gfp::lgg-1 + rol-6), eat-2(ad465);nhr-62(tm1818) dhEx627(pmyo-3::cfp + fosmid WRM065CF04). The dhEx627 extra-chromosomal rescue strain was generated by injecting DNA from worm fosmid WRM065CF04 (10 ng/µl), a co-injectable marker (pmyo-3::cfp at 20 ng/µl) and salmon sperm DNA at 70 ng/µl. Stable arrays were selected for and maintained based on expression of the co-injected marker. For imaging of nhr-62::gfp, day 1 adult worms were visualized using a Zeiss Axioskop2 Plus microscope and representative images were processed with ImageJ [61].
Lifespan Assays
Lifespans were recorded as previously described [62] unless otherwise noted. A log-rank p value of less than 0.001 was used for establishing significance. Experimental worms were grown at 20°C for three generations without starving and were staged as eggs on experimental plates using a timed egg lay. L4s were moved into the appropriate conditions for the start of the aging experiment at a density of 10–12 worms per 6 cm plate. At the L4 stage, or day 0 of aging experiment, worms were moved to fresh experimental plates at density of 10–12 worms per plate. Worms were scored every other day across their lifespan for movement. Worms that did not move on their own were gently touched with a sterilized platinum wire pick and monitored for movement. If there was no movement worms were scored as dead. During these experiments worms were transferred to fresh experimental plates every other day until the end of the reproductive period and then moved once a week. Animals that crawled off the plate, exploded due to a ruptured vulva, or became Egl (egg laying defective), in which unlaid eggs hatch inside the mothers, were censored from the aging experiment. The number of aging experiments performed, mean, median, and maximum lifespans, log-rank analysis, number of worms used in each experiment and worms censored were recorded (Table S1). For the high-throughput aging screen, worms were grown to gravid adult and bleached to collect staged eggs. To increase the efficacy of RNAi, RNAi sensitive strain nre-1(hd20);lin-15b(hd126) was used. Eggs were pipetted at a target density of 15 eggs per well into 12 well cell culture plates with RNAi NGM seeded with RNAi clone of interest. At L4, or day 0 of adult, 15 µl of FUdR (5-Fluoro-2′-deoxyuridine) (50 mM) was pipetted into each well to inhibit progeny production. On day 7 of adulthood, the number of worms alive was determined for each well. On day 15 of adulthood, the number of worms alive was again determined and the percent surviving was calculated. For RNAi experiments, worms were maintained on NGM plates with 20 µg/ml carbenicillin, 10 µg/ml tetracycline, and 1 mM IPTG. Worms were transferred to fresh RNAi plates every other day. For BDR longevity experiments, lifespans were recorded as previously described [10]. Worms were grown for three generations without starving on NGM plates seeded with OP50 and then staged using a timed egg lay. At L4, or day 0 of aging experiment, worms were transferred to NGM plates with FUdR (100 µg/mL). After 48 hours, worms were then washed for one hour in BDR liquid media (5.85 g NaCl, 1 g K2HPO4, 6 g KH2PO4, 1 ml cholesterol at 5 mg/mL in ethanol, and MilliQ H2O to 1 L) with carbenicillin (50 µg/ml), kanamycin (10 µg/ml), and tetracycline (1 µg/ml) added to inhibit bacteria replication. 90 worms were then moved into defined bacteria concentrations and scored every 3–4 days for survival at a density of 15 worms per well. On every score day worms were transferred to freshly made bacteria condition. FUdR was added to the BDR liquid media for the first 14 days of adulthood to inhibit progeny production. Bacteria concentration was determined through serial dilution, plating, and counting of colony forming units (CFUs). The number of aging experiments performed, mean, median, and maximum lifespans, log-rank analysis, number of worms used in each experiment and worms censored were recorded (Table S2).
Fat Analysis
Oil Red O protocol was adapted from [35]. 200–300 day 1 adult worms synchronized through a timed egg lay were washed with M9 and fixed with 50% isopropanol for 15 minutes. Oil Red O stock solution (0.5 g Oil Red O in 100 ml isopropanol) was diluted in water to 60% Oil Red O working solution and used to stain the worms overnight. Worms were then washed with PBS and 0.01% TritonX-100 (diluted in PBS) was added prior to mounting the worms. Images were captured using a DIC microscope and Zeiss AxioCam MRc5 camera. ImageJ was used to invert the color images and a 100 pixel diameter circle was drawn to quantify the intensity of the first intestinal cell and background was subtracted out. Statistics were done using GraphPad Prism software.
Biochemical triglyceride assays were performed using approximately 5,000 day 1 adult worms staged through timed egg lay. Worms were from NGM plates and washed 3 times in PBS then ground up using a pestle and homogenized using a Branson Sonifier Cell Disruptor. Triglyceride and protein levels were determined using a Triglyceride Colorimetric Assay Kit (Cayman Chemical Company) and the Pierce BCA Protein Assay Kit (Thermo Scientific) and analyzed on a Biotek Power Wave XS plate reader according to manufacturer's instructions. Three technical replicates were preformed for every biological replicate. Standards were also done in triplicate. Statistics were done using GraphPad Prism software.
GC Analysis of FAMEs
Day 1 adults animals were collected in 100 µl M9 buffer, washed 2-times, and run through 10-freeze/thaw cycles before being sonicated to lyse the cuticle and tissue. Protein concentration was determined using the Pierce BCA Protein Assay Kit (Thermo Scientific) according to manufacturer's instructions. 50 µl was removed to a 1.5 ml gas chromatography (GC) vial for derivatization. To each vial, 200 ng each of methyl C11 : 0, C13 : 0, and C23 : 0 (NuChek Prep, Inc.; 20 µg/ml stock in methanol) were added as internal reference standards. To convert fatty acids into their fatty acid methyl ester (FAME) derivatives, 0.5 ml of 2.5% H2SO4 (Sigma) in methanol (Biosolve) was added to each sample and incubated at 80°C for 20 minutes with shaking. 0.75 ml of water (Biosolve, ULC Grade) and 350 µl of hexane (Sigma) were next added and the sample was incubated with shaking for 10 min to extract FAMEs into the hexane layer. The hexane layer was removed, concentrated by evaporation, and used directly for analysis. FAME analysis was carried out using an Agilent 7890A GC equipped with a flame ionization detector (FID) (Agilent Technologies, Inc) and a DB-23 column (30 m×0.25 mm I.D., 0.25 µm, Agilent) using helium as a carrier gas at a flow rate of 3 ml/min. 1 µl per sample was injected in pulsed-splitless mode. The initial oven temperature was set to 80°C, held for 1 minute; increased to 170°C at 6.5°C/min; then increased to 215°C at 2.75°C/min. FAMEs were identified and compared against known reference standards for quantification.
qRT-PCR
Synchronized worms were collected into TRIzol (Invitrogen) when reaching adulthood but bearing no embryos. Total RNA and cDNA was prepared using RNeasy Mini kit (QIAGEN) and iScript cDNA Synthesis Kit (Bio-Rad) respectively. qRT-PCR was performed with Power SYBR Green master mix (Applied Biosystems) on a ViiA7 384 Real-Time PCR System (Applied Biosystems). A combination of ama-1 and cdc-42 was used as control. 4 biological replicates containing 300–400 worms each and four technical replicates were tested for each experiment. qPCR primers sequences are in Table S8.
Autophagy Marker GFP::LGG-1
Counting of puncta formation was done as previously described [20]. Briefly, young adults were transferred to RNAi plates targeting the gene of interest. L3 animals of the next generation were scored. The number of GFP puntca in each seam cell was recorded using a Zeiss Axioskop2 Plus microscope. For nhr-62 RNAi experiments, 3 biological replicates with 31 animals (345 seam cells) of GFP::LGG-1 (L4440), 26 animals (225 seams cells) of GFP::LGG-1 (nhr-62i), 62 animals (773 seams cells) of eat-2 GFP::LGG-1 (L4440), and 58 animals (598 seam cells) of eat-2 GFP::LGG-1 (nhr-62i) were scored. For C40H1.8 RNAi experiments, 3 biological replicates with 50 animals (323 seam cells) of GFP::LGG-1 (L4440), 43 animals (382 seams cells) of GFP::LGG-1 (C40H1.8i), 100 animals (933 seams cells) of eat-2 GFP::LGG-1 (L4440), and 99 animals (896 seam cells) of eat-2 GFP::LGG-1 (C40H1.8i) were scored.
RNA-seq and Bioinformatic Analysis
Worms of indicated genotypes were synchronized by two rounds of bleaching and approximately 300 worms for each genotype were handpicked into TRIzol (Invitrogen) when they just reached adulthood without carrying embryos. Total RNA was prepared with RNeasy Mini Kit (QIAGEN). cDNA library was subsequently constructed by TruSeq RNA Sample Preparation Kit (Illumina). Illumina sequencing (100 bp single end) was performed on 3 biological replicates for each of the 4 treatments eat-2, nhr-62;eat-2, nhr-62, N2, giving a total of 12 samples. Sequencing reads were clipped to remove adapter sequence using fastx_clipper (http://hannonlab.cshl.edu/fastx_toolkit/), clipped reads were aligned to the C. elegans reference genome (WBcel215.67) using tophat version 1.3 (Trapnell, Pachter & Salzberg 2009) with option −g1 to ensure unique mapping and option −G to pass the Caenorhabditis_elegans.WBcel215.67.gtf. The number of reads mapping to each Ensemble gene was counted with htseq-count (http://www-huber.embl.de/users/anders/HTSeq/doc/count.html#count). Statistical analysis and drawing of plots was performed in R (http://www.r-project.org/) using the bioconductor package Deseq (Anders, Huber 2010) and R function gplots (http://cran.r-project.org/web/packages/gplots/index.html). Scatterplots of the RNA-seq data were drawn to illustrate differential expression between samples. A heatmap showing Euclidean distances between samples (calculated from variance stabilized transformed data) was plotted in R. For each data comparison a list of “significant differentially expressed genes” was compiled from the Deseq-result as follows: FDR<0.05, mean normalized count >10 and absolute Foldchange (FC) >1.5. Furthermore a list of genes, which are up or down regulated by eat-2, compared to the control (eat-2 vs N2), but show that they are rescued in the nhr-62;eat-2 phenotype (nhr-62;eat-2 vs eat-2) was compiled and referred to as Rescued-Genes. Further analysis of “significant differentially expressed genes” and “Rescued-Genes” was performed as follows:
-
A Venn diagram was plotted in R for “significant differentially expressed genes” to show the overlap of the significant differentially expressed genes.
-
Annotation and Gene-enrichment analysis of “significant differentially expressed genes” and “Rescued-Genes” was performed with David [45], [46].
-
ReviGO analysis [63] was conducted on the DAVID GO-BP gene-enrichment result of “Rescued-Genes”. ReviGO summarizes GO-BP-IDs. The resultant network was uploaded into Cytoscape [64] for further editing and the image exported.
Supporting Information
Zdroje
1. KenyonCJ (2010) The genetics of ageing. Nature 464 : 504–512 doi:10.1038/nature08980.
2. RothLW, PolotskyAJ (2012) Can we live longer by eating less? A review of caloric restriction and longevity. Maturitas 71 : 315–319 doi:10.1016/j.maturitas.2011.12.017.
3. ColmanRJ, AndersonRM, JohnsonSC, KastmanEK, KosmatkaKJ, et al. (2009) Caloric restriction delays disease onset and mortality in Rhesus monkeys. Science 325 : 201–204 doi:10.1126/science.1173635.
4. MattisonJA, RothGS, BeasleyTM, TilmontEM, HandyAM, et al. (2012) Impact of caloric restriction on health and survival in Rhesus monkeys from the NIA study. Nature 489 : 318–321 doi:10.1038/nature11432.
5. RedmanLM, RavussinE (2011) Caloric restriction in humans: Impact on Physiological, Psychological and Behavioral Outcomes. Antioxidants & Redox Signaling 14 : 275–287.
6. WalfordRL, MockD, VerderyR, MaccallumT (2002) Calorie Restriction in Biosphere 2/: Alterations in Physiologic, Hematologic, Hormonal, and Biochemical Parameters in Humans Restricted for a 2-Year Period. Journal of Gerontology: Biological Sciences 57A: B211–B224.
7. OmodeiD, FontanaL (2011) Calorie restriction and prevention of age-associated chronic disease. FEBS Letters 585 : 1537–1542 doi:10.1016/j.febslet.2011.03.015.
8. StanfelMN, ShamiehLS, KaeberleinM, KennedyBK (2009) The TOR pathway comes of age. Biochimica et Biophysica Acta 1790 : 1067–1074 doi:10.1016/j.bbagen.2009.06.007.
9. KlassM (1977) Aging in the nematode Caenorhabditis elegans: major biological and environmental factors influencing life span. Mechanisms of Ageing and Development 6 : 413–429.
10. PanowskiSH, WolffS, AguilaniuH, DurieuxJ, DillinA (2007) PHA-4/Foxa mediates diet-restriction-induced longevity of C. elegans. Nature 447 : 550–555.
11. LakowskiB, HekimiS (1998) The genetics of caloric restriction in Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the United States of America 95 : 13091–13096.
12. GreerEL, DowlatshahiD, BankoMR, VillenJ, HoangK, et al. (2007) An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Current Biology 17 : 1646–1656 doi:10.1016/j.cub.2007.08.047.
13. KaeberleinTL, SmithED, TsuchiyaM, WeltonKL, ThomasJH, et al. (2006) Lifespan extension in Caenorhabditis elegans by complete removal of food. Aging Cell 5 : 487–494 doi:10.1111/j.1474-9726.2006.00238.x.
14. HonjohS, YamamotoT, UnoM, NishidaE (2009) Signalling through RHEB-1 mediates intermittent fasting-induced longevity in C. elegans. Nature 457 : 726–730 doi:10.1038/nature07583.
15. WangY, TissenbaumHA (2006) Overlapping and distinct functions for a Caenorhabditis elegans SIR2 and DAF-16/FOXO. Mechanisms of Ageing and Development 127 : 48–56 doi:10.1016/j.mad.2005.09.005.
16. MairW, PanowskiSH, ShawRJ, DillinA (2009) Optimizing dietary restriction for genetic epistasis analysis and gene discovery in C. elegans. PloS One 4: e4535 doi:10.1371/journal.pone.0004535.
17. BishopNA, GuarenteL (2007) Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature 447 : 545–549 doi:10.1038/nature05904.
18. SteinkrausKA, SmithED, DavisC, CarrD, PendergrassWR, et al. (2008) Dietary restriction suppresses proteotoxicity and enhances longevity by an hsf-1-dependent mechanism in Caenorhabditis elegans. Aging Cell 7 : 394–404 doi:10.1111/j.1474-9726.2008.00385.x.
19. ChenD, ThomasEL, KapahiP (2009) HIF-1 modulates dietary restriction-mediated lifespan extension via IRE-1 in Caenorhabditis elegans. PLoS Genetics 5: e1000486 doi:10.1371/journal.pgen.1000486.
20. HansenM, ChandraA, MiticLL, OnkenB, DriscollM, et al. (2008) A role for autophagy in the extension of lifespan by dietary restriction in C. elegans. PLoS Genetics 4: e24 doi:10.1371/journal.pgen.0040024.
21. HansenM, TaubertS, CrawfordD, LibinaN, LeeS-J, et al. (2007) Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell 6 : 95–110 doi:10.1111/j.1474-9726.2006.00267.x.
22. WollamJ, AntebiA (2011) Sterol regulation of metabolism, homeostasis, and development. Annual Review of Biochemistry 80 : 885–916 doi:10.1146/annurev-biochem-081308-165917.
23. MagnerDB, AntebiA (2008) Caenorhabditis elegans nuclear receptors: insights into life traits. Trends in Endocrinology and Metabolism 19 : 153–160 doi:10.1016/j.tem.2008.02.005.
24. JumpDB, BotolinD, WangY, XuJ, ChristianB, et al. (2005) Recent advances in nutritional sciences fatty acid regulation of hepatic gene transcription. The Journal of Nutrition 135 : 2503–2506.
25. SampathH, NtambiJM (2005) Polyunsaturated fatty acid regulation of genes of lipid metabolism. Annual Review of Nutrition 25 : 317–340 doi:10.1146/annurev.nutr.25.051804.101917.
26. PalankerL, TennessenJM, LamG, ThummelCS (2009) Drosophila HNF4 regulates lipid mobilization and beta-oxidation. Cell Metabolism 9 : 228–239 doi:10.1016/j.cmet.2009.01.009.
27. Robinson-RechaviM, Maina CV, GissendannerCR, LaudetV, SluderA (2005) Explosive lineage-specific expansion of the orphan nuclear receptor HNF4 in nematodes. Journal of Molecular Evolution 60 : 577–586 doi:10.1007/s00239-004-0175-8.
28. KenyonC, ChangJ, GenschE, RudnerA, TabtlangR (1993) A C. elegans Mutant That Lives Twice as Long as Wild Type. Nature 366 : 461–464.
29. DillinA, HsuA-L, Arantes-OliveiraN, Lehrer-GraiwerJ, HsinH, et al. (2002) Rates of Behavior and Aging Specified by Mitochondrial Function During Development. Science 298 : 2398–2401 doi:10.1126/science.1077780.
30. SarovM, MurrayJI, SchanzeK, PozniakovskiA, NiuW, et al. (2012) A Genome-Scale Resource for In Vivo Tag-Based Protein Function Exploration in C. elegans. Cell 150 : 855–866 doi:10.1016/j.cell.2012.08.001.
31. BermanJR, KenyonC (2006) Germ-cell loss extends C. elegans life span through regulation of DAF-16 by kri-1 and lipophilic-hormone signaling. Cell 124 : 1055–1068 doi:10.1016/j.cell.2006.01.039.
32. GerischB, RottiersV, LiD, MotolaDL, CumminsCL, et al. (2007) A bile acid-like steroid modulates Caenorhabditis elegans lifespan through nuclear receptor signaling. Proceedings of the National Academy of Sciences of the United States of America 104 : 5014–5019 doi:10.1073/pnas.0700847104.
33. Van GilstMR, HadjivassiliouH, JollyA, YamamotoKR (2005) Nuclear hormone receptor NHR-49 controls fat consumption and fatty acid composition in C. elegans. PLoS Biology 3: e53 doi:10.1371/journal.pbio.0030053.
34. BrockTJ, BrowseJ, WattsJL (2006) Genetic Regulation of Unsaturated Fatty Acid Composition in C. elegans. PLoS Genetics 2: e108 doi:10.1371/journal.pgen.0020108.
35. O'RourkeEJ, SoukasAA, CarrCE, RuvkunG (2009) C. elegans major fats are stored in vesicles distinct from lysosome-related organelles. Cell Metabolism 10 : 430–435 doi:10.1016/j.cmet.2009.10.002.
36. GoudeauJ, BelleminS, Toselli-MollereauE, ShamalnasabM, ChenY, et al. (2011) Fatty acid desaturation links germ cell loss to longevity through NHR-80/HNF4 in C. elegans. PLoS Biology 9: e1000599 doi:10.1371/journal.pbio.1000599.
37. WangM, O'RourkeE, RuvkunG (2008) Fat metabolism links germline stem cells and longevity in C. elegans. Science (New York, NY) 322 : 957–960.
38. TangF, WatkinsJW, BermudezM, GrayR, GabanA, et al. (2008) A life-span extending form of autophagy employs the vacuole-vacuole fusion machinery. Autophagy 4 : 874–886.
39. DerewendaZ, DerewendaU, DodsonG (1992) The crystal and molecular structure of the Rhizomucor miehei triacylglyceride lipase at 1.9 Å resolution. Journal of Molecular Biology 227 : 818–839.
40. WongH, SchotzMC (2002) The lipase gene family. The Journal of Lipid Research 43 : 993–999 doi:10.1194/jlr.R200007-JLR200.
41. BisognoT, HowellF, WilliamsG, MinassiA, CascioMG, et al. (2003) Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. The Journal of Cell Biology 163 : 463–468 doi:10.1083/jcb.200305129.
42. SinghR, KaushikS, WangY, XiangY, NovakI, et al. (2009) Autophagy regulates lipid metabolism. Nature 458 : 1131–1135 doi:10.1038/nature07976.
43. LapierreLR, GelinoS, MeléndezA, HansenM (2011) Autophagy and lipid metabolism coordinately modulate life span in germline-less C. elegans. Current Biology 21 : 1507–1514 doi:10.1016/j.cub.2011.07.042.
44. KirisakoT, BabaM, IshiharaN, MiyazawaK, OhsumiM, et al. (1999) Formation process of autophagosome is traced with Apg8/Aut7p in yeast. The Journal of Cell Biology 147 : 435–446.
45. HuangDW, ShermanBT, LempickiRA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Protocols 4 : 44–57 doi:10.1038/nprot.2008.211.
46. HuangDW, ShermanBT, LempickiRA (2009) Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Research 37 : 1–13 doi:10.1093/nar/gkn923.
47. KatewaSD, DemontisF, KolipinskiM, HubbardA, GillMS, et al. (2012) Intramyocellular fatty-acid metabolism plays a critical role in mediating responses to dietary restriction in Drosophila melanogaster. Cell Metabolism 16 : 97–103 doi:10.1016/j.cmet.2012.06.005.
48. BrussMD, KhambattaCF, RubyMA, AggarwalI, HellersteinMK (2010) Calorie restriction increases fatty acid synthesis and whole body fat oxidation rates. American Journal of Physiology Endocrinology and Metabolism 298: E108–16 doi:10.1152/ajpendo.00524.2009.
49. O'RourkeEJ, KuballaP, XavierR, RuvkunG (2013) ω-6 Polyunsaturated fatty acids extend life span through the activation of autophagy. Genes & Development 27 : 429–440 doi:10.1101/gad.205294.112.).
50. MurphyCT, McCarrollSA, BargmannCI, FraserA, KamathRS, et al. (2003) Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 424 : 277–283 doi:10.1038/nature01789.
51. KatewaSD, KapahiP (2010) Dietary restriction and aging, 2009. Aging Cell 9 : 105–112 doi:10.1111/j.1474-9726.2010.00552.x.
52. MeléndezA, HallDH, HansenM (2008) Monitoring the role of autophagy in C. elegans aging. Methods in Enzymology 451 : 493–520 doi:10.1016/S0076-6879(08)03229-1.
53. KapahiP, ChenD, RogersAN, KatewaSD, LiPW-L, et al. (2010) With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metabolism 11 : 453–465 doi:10.1016/j.cmet.2010.05.001.
54. CurranSP, RuvkunG (2007) Lifespan regulation by evolutionarily conserved genes essential for viability. PLoS Genetics 3: e56 doi:10.1371/journal.pgen.0030056.
55. CortonJC, ApteU, AndersonSP, LimayeP, YoonL, et al. (2004) Mimetics of caloric restriction include agonists of lipid-activated nuclear receptors. The Journal of Biological Chemistry 279 : 46204–46212 doi:10.1074/jbc.M406739200.
56. RezziS, MartinF-PJ, ShanmuganayagamD, ColmanRJ, NicholsonJK, et al. (2009) Metabolic shifts due to long-term caloric restriction revealed in nonhuman primates. Experimental Gerontology 44 : 356–362 doi:10.1016/j.exger.2009.02.008.
57. PlankM, WuttkeD, Van DamS, ClarkeSA, De MagalhãesJP (2012) A meta-analysis of caloric restriction gene expression profiles to infer common signatures and regulatory mechanisms. Molecular BioSystems 8 : 1339–1349.
58. HertzR, MagenheimJ, BermanI, Bar-TanaJ (1998) Fatty acyl-CoA thioesters are ligands of hepatic nuclear factor-4 alpha. Nature 392 : 512–516.
59. WahliW, MichalikL (2012) PPARs at the crossroads of lipid signaling and inflammation. Trends in Endocrinology and Metabolism 23 : 351–363 doi:10.1016/j.tem.2012.05.001.
60. BrennerS (1974) The genetics of Caenorhabditis elegans. Genetics 77 : 71–94.
61. AbràmoffMD, MagalhãesPJ, RamSJ (2004) Image processing with ImageJ. Biophotonics International 11 : 36–42.
62. GerischB, WeitzelC, Kober-EisermannC, RottiersV, AntebiA (2001) A hormonal signaling pathway influencing C. elegans metabolism, reproductive development, and life span. Developmental Cell 1 : 841–851.
63. SupekF, BošnjakM, ŠkuncaN, ŠmucT (2011) REVIGO summarizes and visualizes long lists of gene ontology terms. PloS One 6: e21800 doi:10.1371/journal.pone.0021800.
64. ShannonP, MarkielA, OzierO, BaligaNS, WangJT, et al. (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Research 13 : 2498–2504 doi:10.1101/gr.1239303.
Štítky
Genetika Reprodukční medicína
Článek Independent Evolution of Transcriptional Inactivation on Sex Chromosomes in Birds and MammalsČlánek The bHLH Subgroup IIId Factors Negatively Regulate Jasmonate-Mediated Plant Defense and DevelopmentČlánek Selective Pressures to Maintain Attachment Site Specificity of Integrative and Conjugative ElementsČlánek Reassembly of Nucleosomes at the Promoter Initiates Resilencing Following Decitabine ExposureČlánek Hepatocyte Growth Factor Signaling in Intrapancreatic Ductal Cells Drives Pancreatic Morphogenesis
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 7- Akutní intermitentní porfyrie
- Souvislost haplotypu M2 genu pro annexin A5 s opakovanými reprodukčními ztrátami
- Příjem alkoholu a menstruační cyklus
- Doporučení pro diagnostiku a léčbu akutních jaterních porfyrií
- Doc. Miloš Kubánek: Nemocní se srdeční amyloidózou jsou často skryti a sledováni pod jinými diagnózami
-
Všechny články tohoto čísla
- An Solution for Crossover Formation
- Genome-Wide Association Mapping in Dogs Enables Identification of the Homeobox Gene, , as a Genetic Component of Neural Tube Defects in Humans
- Independent Evolution of Transcriptional Inactivation on Sex Chromosomes in Birds and Mammals
- Stepwise Activation of the ATR Signaling Pathway upon Increasing Replication Stress Impacts Fragile Site Integrity
- Genomic Analysis of Natural Selection and Phenotypic Variation in High-Altitude Mongolians
- Modification of tRNA by Elongator Is Essential for Efficient Translation of Stress mRNAs
- Role of CTCF Protein in Regulating Locus Transcription
- Gene Set Signature of Reversal Reaction Type I in Leprosy Patients
- Mapping of PARK2 and PACRG Overlapping Regulatory Region Reveals LD Structure and Functional Variants in Association with Leprosy in Unrelated Indian Population Groups
- Is Required for Formation of the Genital Ridge in Mice
- Monopolin Subunit Csm1 Associates with MIND Complex to Establish Monopolar Attachment of Sister Kinetochores at Meiosis I
- Recombination Dynamics of a Human Y-Chromosomal Palindrome: Rapid GC-Biased Gene Conversion, Multi-kilobase Conversion Tracts, and Rare Inversions
- Mechanisms of Protein Sequence Divergence and Incompatibility
- Histone Methyltransferase DOT1L Drives Recovery of Gene Expression after a Genotoxic Attack
- Female Behaviour Drives Expression and Evolution of Gustatory Receptors in Butterflies
- Combinatorial Regulation of Meiotic Holliday Junction Resolution in by HIM-6 (BLM) Helicase, SLX-4, and the SLX-1, MUS-81 and XPF-1 Nucleases
- The bHLH Subgroup IIId Factors Negatively Regulate Jasmonate-Mediated Plant Defense and Development
- The Role of Interruptions in polyQ in the Pathology of SCA1
- Dietary Restriction Induced Longevity Is Mediated by Nuclear Receptor NHR-62 in
- Fine Time Course Expression Analysis Identifies Cascades of Activation and Repression and Maps a Putative Regulator of Mammalian Sex Determination
- Genome-scale Co-evolutionary Inference Identifies Functions and Clients of Bacterial Hsp90
- Oxidative Stress and Replication-Independent DNA Breakage Induced by Arsenic in
- A Moonlighting Enzyme Links Cell Size with Central Metabolism
- Budding Yeast Greatwall and Endosulfines Control Activity and Spatial Regulation of PP2A for Timely Mitotic Progression
- The Conserved Intronic Cleavage and Polyadenylation Site of CstF-77 Gene Imparts Control of 3′ End Processing Activity through Feedback Autoregulation and by U1 snRNP
- The BTB-zinc Finger Transcription Factor Abrupt Acts as an Epithelial Oncogene in through Maintaining a Progenitor-like Cell State
- The Cohesion Protein SOLO Associates with SMC1 and Is Required for Synapsis, Recombination, Homolog Bias and Cohesion and Pairing of Centromeres in Drosophila Meiosis
- The RNA-binding Proteins FMR1, Rasputin and Caprin Act Together with the UBA Protein Lingerer to Restrict Tissue Growth in
- Pattern Dynamics in Adaxial-Abaxial Specific Gene Expression Are Modulated by a Plastid Retrograde Signal during Leaf Development
- A Network of HMG-box Transcription Factors Regulates Sexual Cycle in the Fungus
- Bacterial Adaptation through Loss of Function
- ENU-induced Mutation in the DNA-binding Domain of KLF3 Reveals Important Roles for KLF3 in Cardiovascular Development and Function in Mice
- Interplay between Structure-Specific Endonucleases for Crossover Control during Meiosis
- FGF Signalling Regulates Chromatin Organisation during Neural Differentiation via Mechanisms that Can Be Uncoupled from Transcription
- The Arabidopsis RNA Binding Protein with K Homology Motifs, SHINY1, Interacts with the C-terminal Domain Phosphatase-like 1 (CPL1) to Repress Stress-Inducible Gene Expression
- Selective Pressures to Maintain Attachment Site Specificity of Integrative and Conjugative Elements
- The Conserved ADAMTS-like Protein Lonely heart Mediates Matrix Formation and Cardiac Tissue Integrity
- The cGMP-Dependent Protein Kinase EGL-4 Regulates Nociceptive Behavioral Sensitivity
- RBM5 Is a Male Germ Cell Splicing Factor and Is Required for Spermatid Differentiation and Male Fertility
- Disease-Related Growth Factor and Embryonic Signaling Pathways Modulate an Enhancer of Expression at the 6q23.2 Coronary Heart Disease Locus
- Yeast Pol4 Promotes Tel1-Regulated Chromosomal Translocations
- A Dual Role for SOX10 in the Maintenance of the Postnatal Melanocyte Lineage and the Differentiation of Melanocyte Stem Cell Progenitors
- SLC26A4 Targeted to the Endolymphatic Sac Rescues Hearing and Balance in Mutant Mice
- Odoriferous Defensive Stink Gland Transcriptome to Identify Novel Genes Necessary for Quinone Synthesis in the Red Flour Beetle,
- Prediction of Complex Human Traits Using the Genomic Best Linear Unbiased Predictor
- Gene × Physical Activity Interactions in Obesity: Combined Analysis of 111,421 Individuals of European Ancestry
- Reassembly of Nucleosomes at the Promoter Initiates Resilencing Following Decitabine Exposure
- Exquisite Light Sensitivity of Cryptochrome
- miR-133a Regulates Adipocyte Browning In Vivo
- Strabismus Promotes Recruitment and Degradation of Farnesylated Prickle in Planar Polarity Specification
- Hepatocyte Growth Factor Signaling in Intrapancreatic Ductal Cells Drives Pancreatic Morphogenesis
- Is a Potential Tumor Suppressor Gene Commonly Inactivated by Epigenetic Mechanisms in Colorectal Cancer
- Joint Molecule Resolution Requires the Redundant Activities of MUS-81 and XPF-1 during Meiosis
- The Mating Competence of Geographically Diverse Strains in Their Natural and Unnatural Sand Fly Vectors
- Defective Repair of Oxidative Base Lesions by the DNA Glycosylase Nth1 Associates with Multiple Telomere Defects
- Effective Blocking of the Enhancer Requires Cooperation between Two Main Mechanisms Suggested for the Insulator Function
- Trans-Ancestral Studies Fine Map the SLE-Susceptibility Locus
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- SLC26A4 Targeted to the Endolymphatic Sac Rescues Hearing and Balance in Mutant Mice
- Bacterial Adaptation through Loss of Function
- The Cohesion Protein SOLO Associates with SMC1 and Is Required for Synapsis, Recombination, Homolog Bias and Cohesion and Pairing of Centromeres in Drosophila Meiosis
- Gene × Physical Activity Interactions in Obesity: Combined Analysis of 111,421 Individuals of European Ancestry
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



