-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ENU-induced Mutation in the DNA-binding Domain of KLF3 Reveals Important Roles for KLF3 in Cardiovascular Development and Function in Mice
KLF3 is a Krüppel family zinc finger transcription factor with widespread tissue expression and no previously known role in heart development. In a screen for dominant mutations affecting cardiovascular function in N-ethyl-N-nitrosourea (ENU) mutagenized mice, we identified a missense mutation in the Klf3 gene that caused aortic valvular stenosis and partially penetrant perinatal lethality in heterozygotes. All homozygotes died as embryos. In the first of three zinc fingers, a point mutation changed a highly conserved histidine at amino acid 275 to arginine (Klf3H275R). This change impaired binding of the mutant protein to KLF3's canonical DNA binding sequence. Heterozygous Klf3H275R mutants that died as neonates had marked biventricular cardiac hypertrophy with diminished cardiac chambers. Adult survivors exhibited hypotension, cardiac hypertrophy with enlarged cardiac chambers, and aortic valvular stenosis. A dominant negative effect on protein function was inferred by the similarity in phenotype between heterozygous Klf3H275R mutants and homozygous Klf3 null mice. However, the existence of divergent traits suggested the involvement of additional interactions. We conclude that KLF3 plays diverse and important roles in cardiovascular development and function in mice, and that amino acid 275 is critical for normal KLF3 protein function. Future exploration of the KLF3 pathway provides a new avenue for investigating causative factors contributing to cardiovascular disorders in humans.
Published in the journal: . PLoS Genet 9(7): e32767. doi:10.1371/journal.pgen.1003612
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003612Summary
KLF3 is a Krüppel family zinc finger transcription factor with widespread tissue expression and no previously known role in heart development. In a screen for dominant mutations affecting cardiovascular function in N-ethyl-N-nitrosourea (ENU) mutagenized mice, we identified a missense mutation in the Klf3 gene that caused aortic valvular stenosis and partially penetrant perinatal lethality in heterozygotes. All homozygotes died as embryos. In the first of three zinc fingers, a point mutation changed a highly conserved histidine at amino acid 275 to arginine (Klf3H275R). This change impaired binding of the mutant protein to KLF3's canonical DNA binding sequence. Heterozygous Klf3H275R mutants that died as neonates had marked biventricular cardiac hypertrophy with diminished cardiac chambers. Adult survivors exhibited hypotension, cardiac hypertrophy with enlarged cardiac chambers, and aortic valvular stenosis. A dominant negative effect on protein function was inferred by the similarity in phenotype between heterozygous Klf3H275R mutants and homozygous Klf3 null mice. However, the existence of divergent traits suggested the involvement of additional interactions. We conclude that KLF3 plays diverse and important roles in cardiovascular development and function in mice, and that amino acid 275 is critical for normal KLF3 protein function. Future exploration of the KLF3 pathway provides a new avenue for investigating causative factors contributing to cardiovascular disorders in humans.
Introduction
Congenital heart defects are the most common congenital malformations in humans affecting 1–2% of live births [1] and 18% of stillbirths [2]. Causative mutations have been identified in families with inherited congenital heart defects [3] but in most cases remain unknown [2]. A strong genetic role is nevertheless likely given high heritability scores, for example >0.7 for left-sided congenital heart defects [4], [5], [6]. To discover new genes important in cardiovascular development, we measured aortic blood velocity in an ultrasound screen undertaken to assess left ventricular outflow function, in the offspring of N-ethyl-N-nitrosourea (ENU) mutagenized male mice [7]. One mutant had very high aortic blood velocities due to aortic valvular stenosis and this trait was heritable. Additional abnormalities in cardiovascular development and function were found in subsequent phenotyping of this mutant mouse line. A dominant point mutation in the region encoding the DNA binding domain of Klf3 was found by linkage analysis and gene sequencing. KLF3 is a zinc finger transcription factor that has discrete regions of expression that are widely distributed among embryonic and adult tissues in mice [8], [9]. KLF3 functions predominantly as a gene repressor [10] although it also has activator functions [11].
KLF3 had hitherto no described role in heart or vascular development or function. In prior work, homozygous deletion of the region encoding the Klf3 zinc finger DNA binding domain caused partially penetrant perinatal lethality in mice and significant abnormalities in adiposity [12], B cell development [13], and erythroid maturation [14] whereas cardiovascular defects were not reported. However, embryonic lethality [12] occurred at a stage of development consistent with death due to cardiovascular dysfunction [15]. Furthermore, several Klfs are expressed in cardiomyocytes and vascular smooth muscle cells [16] including Klf3 (current study and [17]). KLF3 is enriched at promoters of several muscle-specific genes including muscle creatine kinase (MCK) where it interacts with Serum Response Factor to act as a transcriptional activator [11]. Thus, despite known molecular mechanisms whereby KLF3 may alter cardiac or vascular development or function at a cellular level, a cardiovascular phenotype remained unidentified.
Herein we report the characterization of a new ENU-induced mouse mutant. Results reveal important and novel roles for KLF3 in cardiovascular development and function. Strong similarities in phenotype with homozygous Klf3 gene trap mice, where KLF3 is largely eliminated, suggest a predominantly dominant negative effect of the point mutant protein. However, intriguingly, the existence of divergent traits suggests the involvement of additional interactions. At the molecular level, the point mutation illuminates the critical importance of a highly conserved residue in the DNA binding domain of KLF3. The discoveries reported here provide impetus for exploring the KLF3 pathway to discover new causative factors contributing to cardiovascular disorders in humans.
Results
Breeding and mapping of the mutation
In a screen of 1770 adult heterozygous offspring from ENU mutagenized C57BL/6J male mice crossed with wild-type (WT) C3H/HeJ females, we identified a mutant mouse with an aortic blood velocity >7 standard deviations (SD) above the mean. Using a cut-off aortic blood velocity of 150 cm/s (i.e. >3 SD above the mean of all animals), we found that the trait (Figure 1A) was heritable when mutants were bred to BALB/cJ females although only 10% (17 of 165) had the trait. Nevertheless linkage analysis localized the mutation to chromosome 5 between 4.9 and 75.6 Mb (Figure S1A). The LOD score exceeded 4 in this interval (Figure S1B) whereas it was <2.5 elsewhere in the genome (not shown). The incidence of the trait was higher on a C57BL/6J (B6) background (136 of 584; 23%) so we performed fine mapping by crossing affected animals with B6–Chr 5 A/J consomic mice (incidence of trait was 40 of 183; 22%). We narrowed the interval to a 12.6 Mb region on chromosome 5 (Figure S1B), which contained 35 genes (Table S1). Genomic sequencing of 7 candidates (Table S1) revealed only one point mutation predicted to affect the protein product (Figure 1B). The mutation in exon 5 of Klf3 (Krüppel-like factor 3) (Figure 1C) changed a histidine residue (CAC) at amino acid 275 to arginine (CGC) (KLF3H275R) (Figure 1B). This histidine is conserved across species (www.ncbi.nlm.nih.gov/homologene) and across all but one of the 22 Sp/Klf family members [18]. It is the central of 3 amino acids predicted to make contact with DNA in the DNA binding region of the first of three zinc finger domains in KLF3 [18], [19]. We predicted that mutation at this site would be highly likely to affect the DNA binding function of KLF3 and thereby its function in transcriptional control. The Klf3H275R line was subsequently maintained by breeding with B6 mice.
Fig. 1. Mapping and identification of Klf3H275R mutation. 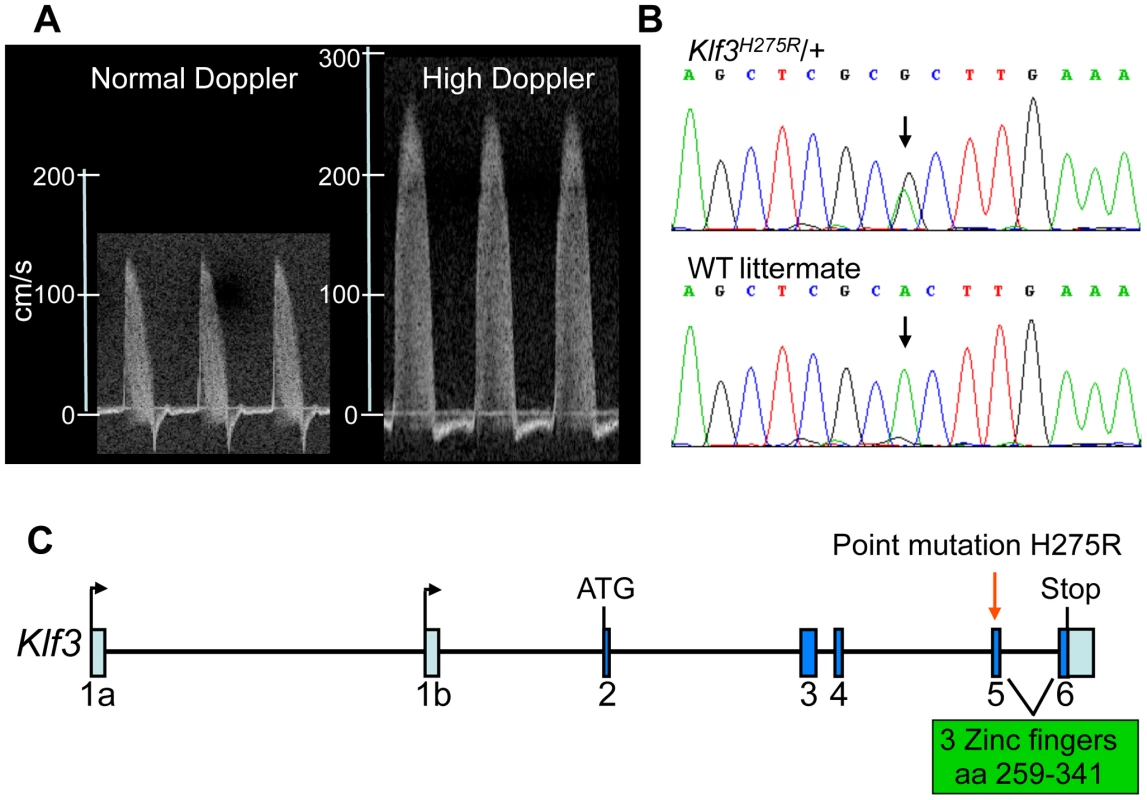
(A) Example of the ascending aortic blood velocity waveforms in an adult mouse with a normal waveform and one with the high Doppler trait that was used to identify mutants for mapping. (B) Sequencing of the candidate Klf3 gene revealed an A to G transition (at black arrows) in mice with mutant trait (top) but not in WT littermates (below) or in parental strains (not shown). This transition converts histidine to arginine at amino acid 275 in the KLF3 protein. (C) The location of the H275R point mutation (red arrow) is in exon 5 in the zinc finger region (amino acids 259–341) of the Klf3 gene. Aortic valvular stenosis in adult heterozygous Klf3 point mutants
High peak aortic blood velocity in adult heterozygous Klf3 point mutants (Klf3H275R/+) was caused by aortic valvular stenosis as shown by augmented valvular gradients in blood velocity (Figure 2A) and blood pressure in Klf3H275R/+ mice (Figure 2B,C), and by abnormal valve morphology detected by gross dissection (not shown), histopathology (Figure 3A), and scanning electron microscopy (Figure 3B). Aortic valves were tricuspid although bicuspid valves were occasionally observed. The leaflets were thickened, often partially fused, and sometimes exhibited blebs or small hematomas (Figure 3A). When genotype was used to identify mutants, most Klf3H275R/+ mice had peak velocities >150 cm/s (20 of 31 or 65%) in contrast with WT littermates (0 of 43 or 0%) (Figure 3D). Males and females were similarly affected. Significant aortic valve regurgitation was not observed. In humans, aortic valvular stenosis is often associated with post-stenotic aortic dilatation [20]. We therefore measured diastolic diameter of the ascending aorta in vivo and found a significant 27% post-stenotic enlargement in male and female Klf3H275R/+ mice (1.95±0.07 vs. 1.53±0.08 mm in males (n = 5) and 1.70±0.09 vs. 1.34±0.03 mm in females (n = 4); P<0.01) (Figure 3C).
Fig. 2. Hemodynamic evidence for aortic valvular stenosis in Klf3H275R/+ mice. 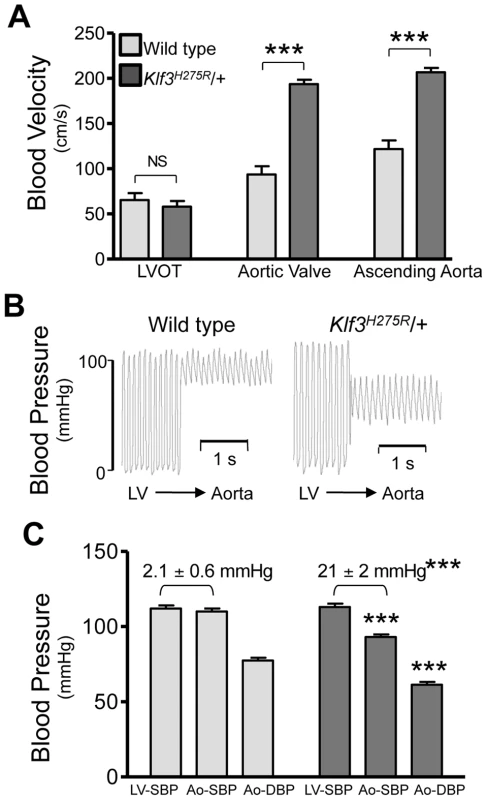
Adult Klf3H275R/+ mice with high aortic blood velocities and their cage-mate controls were studied. (A) Doppler ultrasound was used to measure transvalvular blood velocity in Klf3H275R/+ mutants (dark bars) relative to WT (light bars). Blood velocity in the left ventricular outflow tract (LVOT) was similar in mutants and WT but became elevated at the level of the aortic valve and remained elevated in the proximal ascending aorta (24–33 wk adult mice, n = 5 per group, mean ± SE). (B) The transvalvular pressure gradient was measured by pulling a catheter from the left ventricle (LV) to the aorta. The gradient in systolic blood pressure from the LV to the aorta was greater in Klf3H275R/+ mutant (right) than WT mice (left). (C) Left ventricular systolic blood pressures (LV-SBP) were similar but aortic systolic (Ao-SBP) and aortic diastolic (Ao-DBP) blood pressures were lower in Klf3H275R/+ mutants (dark bars on right) than WT mice (light bars on left). The LV-SBP to Ao-SBP gradient (mean ± SE above bars) was significantly greater in mutants suggesting aortic stenosis (18–25 wk adult mice, n = 10 WT, n = 9 mutants, mean ± SE). *** P<0.001 Klf3H275R/+ vs. WT. Fig. 3. Morphological evidence for aortic valvular stenosis in Klf3H275R/+ mice. 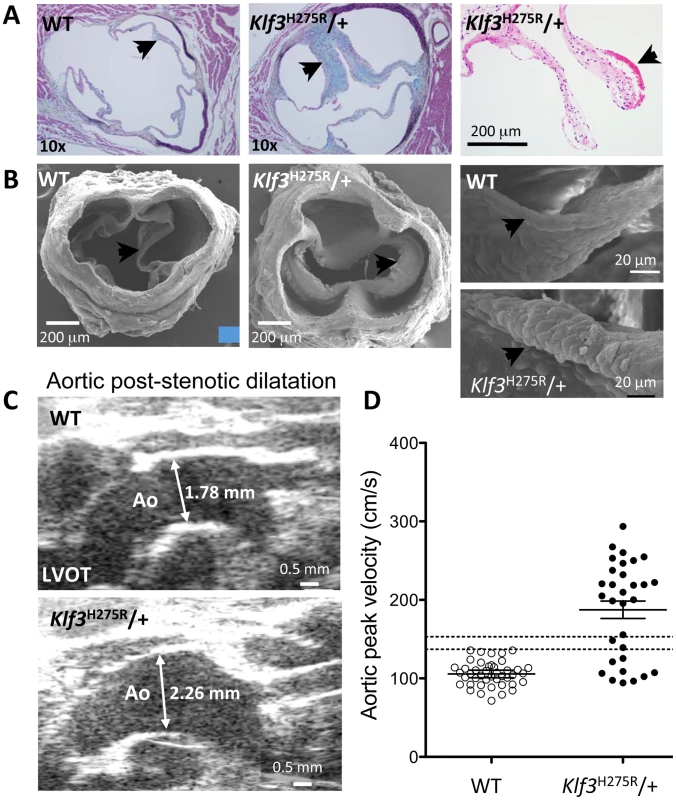
(A) Histological sections showing thickened aortic valve leaflets in adult Klf3H275R/+ (arrow in center and right panels) vs. WT (arrow in left panel). Blebs or hematomas were sometimes observed on aortic valve leaflets of Klf3H275R/+ mice (e.g. arrow in right panel). (B) Scanning electron microscopy images of adult aortic valves (arrows) from WT (left) and Klf3H275R/+ (center), with magnified images of valve leaflets (right), that show thickened leaflets in Klf3H275R/+ mice. (C) Micro-ultrasound images of aortic arch (Ao) in adult Klf3H275R/+ (below) vs. WT (above) showing aortic dilatation in the left ventricular outflow tract (LVOT) typical of Klf3H275R/+ mice. (D) Elevated peak blood velocity in the ascending aorta of Klf3H275R/+ (solid circles) relative to WT controls (open circles). Mean ± SE are shown for each group. The lower dashed line shows the WT mean + 2SD and the upper dashed line the WT mean + 3SD. 20 of 31 Klf3H275R/+ had peak velocities >3SD above WT. We next examined blood velocities through the other heart valves. Peak blood velocity was ∼30% higher in the main pulmonary artery, and at the atrioventricular valves during early ventricular filling (E-wave) in Klf3H275R/+ mice (Table 1). E-wave fusion with the atrial filling wave (A-wave) occurred significantly more often in the left or right filling waveforms in Klf3H275R/+ (9 of 20) than WT littermates (0 of 20). There were no abnormalities in peak inflow velocities during atrial contraction (i.e. A-wave) or in heart rate (Table 1), and no evidence of significant valve regurgitation in Doppler waveforms (not shown). No structural abnormalities in the pulmonary valves (Figure S2A,B) or the atrioventricular valves (not shown) were detected in adults by gross or histopathology examination. Thus, the mutation appeared to predominantly impact the aortic semilunar valve.
Tab. 1. Adult cardiac parameters measured by micro-ultrasound echocardiography. 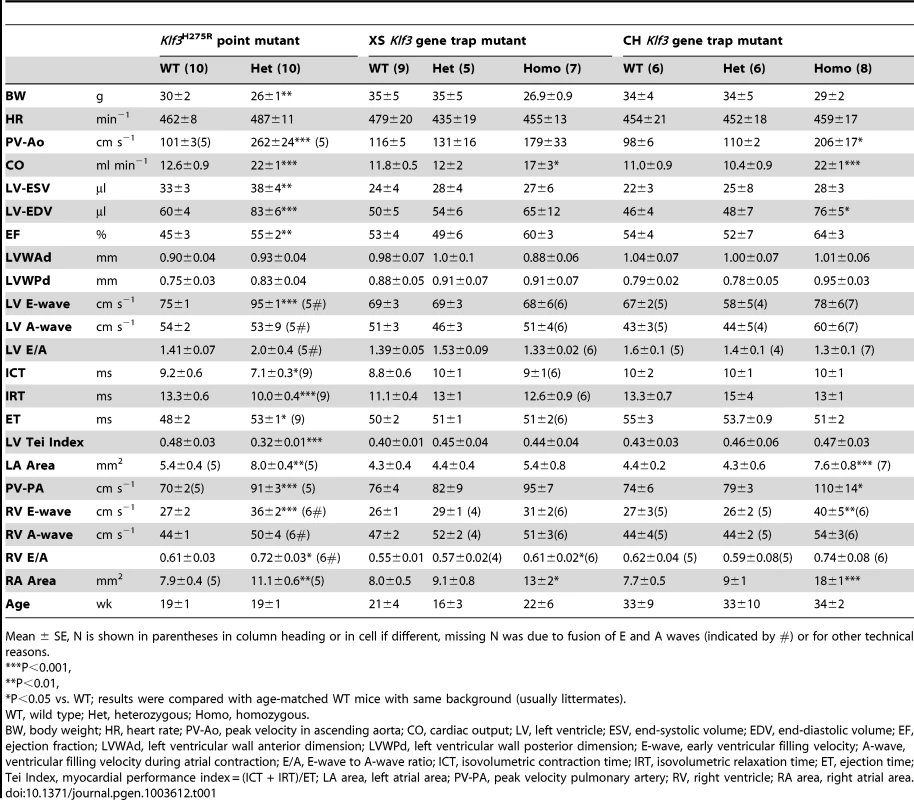
Mean ± SE, N is shown in parentheses in column heading or in cell if different, missing N was due to fusion of E and A waves (indicated by #) or for other technical reasons. Increased prenatal and postnatal mortality in Klf3H275R/+ and Klf3H275R/H275R mutants
At weaning, we observed 100% lethality of homozygous offspring and only ∼50% of the anticipated Klf3H275R/+ pups from Klf3H275R/+ intercross breeding (Table S2). At E14.5–16.5, significant lethality of homozygotes, but not heterozygotes, was observed (Table S2). We used histology to examine embryonic heart structure of Klf3H275R embryos at E12.5 (i.e. before the age of lethality) and at E14.5 in heterozygotes and in the few surviving homozygous embryos. At E12.5 (Figure 4A) and E14.5 (Figure 4B), homozygous embryos exhibited a thinned and disorganized ventricular myocardium and septum suggesting cardiac failure as the cause of their later demise. At E14.5, ventricular and atrial septation defects were also observed (Figure 4B). In contrast, heterozygotes at E12.5 had apparently normal cardiac anatomy (Figure 4A) and at E14.5 showed disorganization and thickening of the septal myocardium (Figure 4B,C). Some had atrial septation defects similar to homozygotes (Figure 4B) and enlarged atrioventricular cushion tissue that may have obstructed flow (Figure 4C). At birth, heterozygous neonates had abnormally thickened myocardial walls by magnetic resonance imaging (MRI) (Figure 5A) and aortic valve leaflets by histology (Figure 5B) and by optical projection tomography (Figure 5C). No abnormalities in placental weight or histology were detected at E12.5 and E14.5 (not shown) so placental dysfunction was unlikely to play a causative role (e.g. as in [21]).
Fig. 4. Abnormal cardiac histology in Klf3H275R embryos. 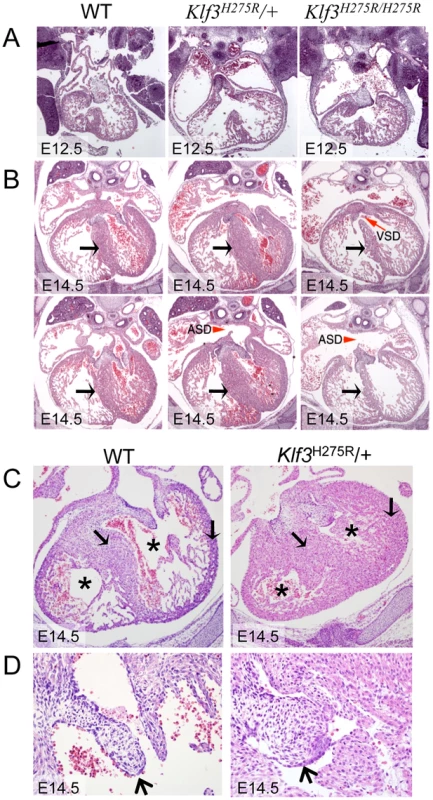
In homozygotes, histological images showed thinned and disorganized ventricular myocardium and septum (A) at E12.5 and (B) in a rare homozygous survivor at E14.5 (two section levels from same specimen are shown). At E14.5, ventricular (VSD) and atrial septation defects (ASD) were observed in some homozygote and heterozygote hearts. (B) The interventricular septum of heterozygotes at E14.5 appeared thickened relative to WT and homozygotes (black arrows). (C) Heterozygous Klf3H275R hearts at E14.5 exhibited thickened myocardial and septal wall thicknesses (arrows), diminished left and right ventricular lumens (*), and (D) abnormal hyperplasia of atrioventricular cushion tissue (arrows) (enlargement from (C)). All homozygotes died prenatally. About half of the heterozygotes died before weaning. Fig. 5. Abnormal hearts and aortic valves in Klf3H275R/+ neonates. 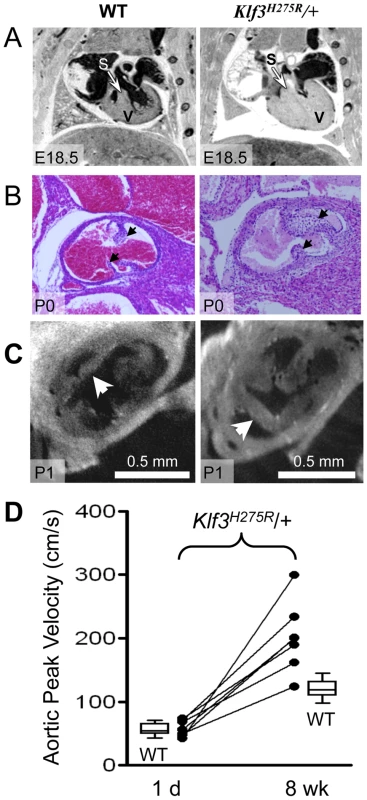
(A) Thoracic images of neonates delivered by cesarean-section at E18.5 (term). The heterozygous Klf3H275R neonate breathed occasionally for 30 min then stopped breathing and died (right). The wild type (WT) neonate (left) breathed and appeared normal before being euthanized for MRI. The ventricular myocardium (V) and septum (S) were markedly thickened in the heterozygous neonate that died. (B) Aortic valve leaflets (arrows) of a heterozygous Klf3H275R neonate found dead on postnatal day 0 (P0) were thickened (right) relative to a same age control (left). (C) Optical Projection Tomographic images of aortic valves from apparently healthy neonates on day 1 showing thickened aortic valve leaflets in Klf3H275R/+ (right) compared to WT (left). (D) Serial measurements of aortic peak blood velocity in Klf3H275R/+ as neonates on day 1 and at 8 wk as adults (n = 7; solid lines join points). Box plots show WT values for day 1 neonates (n = 14) and 8–14 wk adults (n = 21). At day 1, all 7 Klf3H275R/+ mice had aortic peak velocities within the normal range whereas 6 of 7 were elevated by 8 wk. To better define the age of lethality in heterozygotes, we delivered 3 litters of Klf3H275R/+ crossed with B6 mice by caesarean section at term (E18.5). Three Klf3H275R/+ embryos had recently died in utero and 2 Klf3H275R/+ died within 30 min with only occasional breathing (Table S2). Klf3H275R/+ pups that survived for up to 2 h were significantly smaller (1.02±0.02 g (n = 9)) than WT littermates (1.13±0.02 g (n = 10); P = 0.002). We next allowed 5 litters of Klf3H275R/+ crossed with B6 mice to deliver naturally at term. One day after birth, cardiac hypertrophy in Klf3H275R/+ pups was significant in surviving pups (7.5±0.2 mg/g body weight (n = 9) vs. WT 6.5±0.1 mg/g (n = 23); P <0.001) whereas it was striking in dead or dying pups on day 1 (15±2 mg/g (n = 3); P<0.001). Imaging showed that pups that died within 1 d of delivery had markedly diminished ventricular lumens, markedly thickened ventricular and septal myocardia, and aortic valve leaflets that were short and thick (Figure 5A,B,C). Thus, heterozygous Klf3H275R/+ pups that had the most pronounced ventricular hypertrophy apparently died in the perinatal period.
Cardiac hemodynamic dysfunction in postnatal Klf3H275R/+ mice
Aortic blood velocity was not higher in Klf3H275R/+ pups at day 1 of age (49±4 cm/s; n = 9) relative to WT littermates (48±4 cm/s; n = 21). In Klf3H275R/+ pups assessed on day 1 and again at 8 wk; 6 of 7 developed high velocities (>2 SD) by 8 wk (Figure 5D). Peak aortic blood velocity did not increase further between 9 wk and 1 y (n = 15; not shown). Thus, the Klf3 mutation may alter prenatal aortic valve development but the development of sufficient stenosis to elevate aortic blood velocity is a postnatal event occurring by 8 wk in Klf3H275R/+ mice.
Heterozygous mice that survived the perinatal period survived into adulthood. However the number of adults that died before 60 wk was significantly increased; 21% of Klf3H275R/+ mice with high aortic blood velocities at 8 wk died under 60 wk of age (41 of 195) vs. 4% of WT cage-mates (2 of 54) (P<0.0001). Premature death in adulthood was associated with a rapid deterioration in health and all 4 mice found moribund exhibited marked cardiac enlargement (e.g. Figure S3; heart weight 0.367±0.040 g (n = 3) vs. 0.159±0.008 g (n = 3) WT cage mates). In the Klf3H275R/+ group as a whole at ∼20 wk, cardiac enlargement was less pronounced and lung weight was not elevated (Table S3). Results are consistent with premature death caused by heart failure.
Cardiac structure and function in adult heterozygous Klf3 point mutants
We anticipated that intraventricular pressures would be elevated due to aortic valvular stenosis in surviving adult Klf3H275R/+ mutants and that this would lead to concentric ventricular hypertrophy (i.e. increased wall thickness) secondary to increased afterload. While the hearts were hypertrophic (i.e. the heart to body weight ratio was significantly elevated; Table S3), hypertrophy was not concentric because there was no increase in wall thickness in mutant adults (Table 1). Furthermore, the heart to body weight ratio did not correlate with aortic blood velocity (r2 = 0.17; P = 0.1; n = 13) or with the transvalvular pressure gradient (r2 = 0.06; P = 0.5; n = 7) in adult Klf3H275R/+ mice. Indeed, left ventricular systolic blood pressure was not significantly elevated when directly measured in isoflurane-anesthetized mice (Figure 2C). Instead, we found that surviving adult Klf3H275R/+ mice had eccentric hypertrophy (i.e. increased chamber dimensions). Thus, other prominent cardiovascular abnormalities were caused by the Klf3H275R allele; abnormalities not due to aortic valve defects.
Echocardiography on Klf3H275R/+ mice was therefore performed to discover other effects of this mutation on adult cardiac function. We found that the diastolic volume of the left ventricle was significantly increased, and cardiac output was nearly doubled despite their smaller body weight (Table 1). The left and right atrial areas measured by ultrasound were also significantly increased in Klf3H275R/+ hearts (Table 1), as was the heart to body weight ratio (Table S3). Left ventricular wall thicknesses and heart rate were unchanged (Table 1). Klf3H275R/+ mice had improved systolic function as suggested by a significant increase in ejection fraction (Table 1) and improved diastolic filling as suggested by a 30% increase in the early filling (E-wave) velocity for left and right atrioventricular filling (Table 1). The high E-wave may explain its more frequent fusion with the A-wave in Klf3H275R/+ mice (Table 1). Improved systolic and diastolic function was also supported by a change in the Tei Index (Table 1), a global indicator of cardiac function [22]. The change in the Tei Index occurred due to significantly shorter isovolumetric contraction and relaxation times, and a significantly longer ejection time (Table 1). Other than eccentric hypertrophy, there was no abnormality in histological structure of the heart, or atrial or ventricular myocardium detected by light microscopy (Figure S4A) or electron microscopy although myocardial contraction bands were more frequent in Klf3H275R/+ mice (Figure S4B).
The Klf3H275R/+ mutation caused a doubling of cardiac output (Table 1). Physiological increases in cardiac output can be evoked by increased metabolic rate or by reduced oxygen carrying capacity of the blood. Both would tend to increase tissue requirements for perfusion. We therefore measured oxygen consumption but found that it was only 15% higher in Klf3H275R/+ mice (3112±28 ml h−1 kg−1 vs. 2709±22 ml h−1 kg−1 in WT littermates, P = 0.03; n = 4 males per group averaged over 24 h) and thus was insufficient to explain the doubling in cardiac output in Klf3H275R/+ mice. Similarly, although Klf3H275R/+ mice were slightly anemic with a significant 10% reduction in RBC count (Figure S5) and haemoglobin concentration (not shown), reduced oxygen carrying capacity of the blood was insufficient to explain the large increase in cardiac output in these mutants.
Arterial hypotension in adult heterozygous Klf3 point mutants
High cardiac output can also be induced physiologically by low total peripheral vascular resistance. This mechanism was implicated by the significant reduction in arterial blood pressure in Klf3H275R/+ mice both when awake measured by tail cuff (98±2 mmHg vs. 108±1 mmHg in WT littermates at 9–12 wk; n = 10/genotype) and in the ascending aorta of anesthetised mice using a catheter-tip pressure transducer (Figure 2C). Heart rate did not significantly differ by genotype whether measured awake (677±13 min−1 Klf3H275R/+ vs. 636±21 min−1 in WT; n = 10/genotype) or under anesthesia (Table 1). We found that low blood pressure was not caused by low plasma volume. Indeed, plasma volume measured by Evan's Blue dilution was 34% greater in Klf3H275R/+ (44±3 ml/kg vs. 33±2 ml/kg in WT; P = 0.015; n = 6 per group). We were unable to find peripheral vascular malformations by color Doppler echocardiography, gross dissection, MRI or histology that could explain the low total peripheral vascular resistance.
These results suggest that the Klf3H275R/+ mutation caused low peripheral vascular resistance by increasing peripheral vascularity and/or vascular calibre. The resulting low arterial pressure resulted in intraventricular pressures that were not elevated despite aortic valvular stenosis and this likely explains the absence of left ventricular wall thickening in adult Klf3H275R/+ mutants. Thus the Klf3H275 mutation caused other prominent cardiovascular abnormalities in addition to defects in aortic valve development.
Body composition in heterozygous Klf3 point mutants
A defect in adipogenesis leading to reduced body weight and fat mass was the primary phenotype reported for homozygous mice with targeted deletion of the KLF3 zinc finger DNA binding domain [12]. An in vitro role for KLF3 in adipocyte differentiation was also found. In young adult Klf3H275R/+ mutants in our study, percent body fat was reduced by 14% (P<0.04; n = 13/genotype) and body weight by 8% (P<0.001; n = 13/genotype) in surviving Klf3H275R/+ mice at 10–11 wk. At 18–25 wk, the relative weight of the superficial abdominal fat pad was reduced by 43%, which contrasted with increased relative weights of the heart, spleen, kidney, and brain (Table S3). Relative lung and liver weights were not affected (Table S3). The similarity in body weight and fat mass phenotype between Klf3H275R/+ and Klf3 DNA binding domain deletion mutants [12] suggested that Klf3H275R is a loss of function allele. However, divergent traits (below) suggest that the interaction is more complex.
Effect of Klf3 point mutation on Klf3 mRNA and protein function
Klf3 mRNA (Figure 6A) and protein (Figure 6B) were expressed at wild-type levels in homozygous and heterozygous Klf3H275R embryos at E12.5. H275R protein exhibited reduced binding to KLF3's canonical CACCC binding region of the β-globin gene promoter both using recombinant bacterial GST-Klf3 zinc finger 1–3 protein (Figure 6C) or full length KLF3 protein expressed in COS cells (Figure 6D), but did not interfere with the ability of WT KLF3 to bind to DNA (Figure S6). In vivo, the H275R protein significantly opposed the repression of Lgals3, a gene that is normally repressed by KLF3 [14], in Klf3H275R homozygous embryos at E12.5 (Figure S7). However, expression of other known KLF3 targets including Klf8, Crip1, and Pqlc3 (Crossley M et al. unpublished) was unaffected (not shown).
Fig. 6. Klf3H275R mRNA, protein, and impairment in DNA binding. 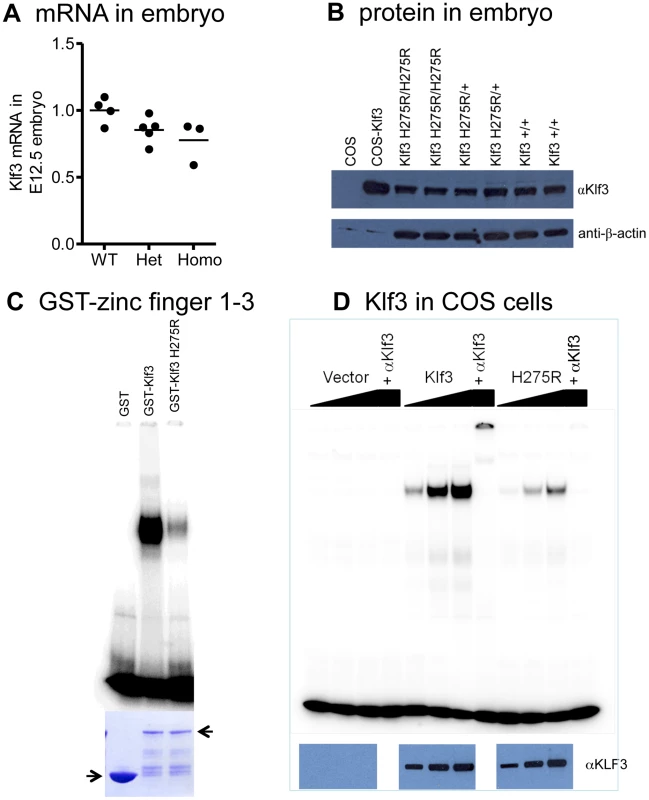
(A) Similar levels of Klf3 mRNA by qRT-PCR (P>0.05) and (B) KLF3 protein by Western blot (WB) in whole H275R homozygotes (Homo) and heterozygotes (Het) versus wild-type (WT) embryos at E12.5. (C) Bacterial expression of GST-zinc finger 1–3 of normal and mutant protein showing that H275R impaired DNA binding to CCACACCCT (canonical β-globin promoter) in electrophoretic mobility shift assay (coomassie blue-stained SDS PAGE gel (below)). (D) Dose-response showing H275R also impaired binding to CCACACCT in electrophoretic mobility shift assays when full length Klf3 was expressed in COS cells (western blot with αKlf3 antibody below). Protein identity was validated by eliminating binding with a KLF3 antibody (αKlf3). Klf3 deficiency causes abnormal cardiovascular development in zebrafish and mice
Because a role for KLF3 in cardiovascular development and function was hitherto unknown, we asked whether reduced KLF3 function could cause abnormal cardiovascular development in zebrafish embryos by using anti-klf3 morpholinos to inhibit translation of klf3 transcript. At 48 hour post-fertilization (hpf), embryos appeared to be developing normally suggesting that initial differentiation and morphogenesis of the heart proceeded normally. However by 72 hpf, 65% of klf3 knockdown embryos exhibited cardiac edema indicative of cardiovascular dysfunction (i.e. 49 of 59, and 27 of 58 in 2 replicate experiments) and some hearts were visibly dysmorphic and did not properly loop (Figure 7). Of those with edema, blood flow was visible in the embryonic vasculature in 60% of embryos at 72 hpf (i.e. 22 of 49, and 8 of 27 in the 2 replicates) with no occlusion of the outflow tract evident (data not shown). This supports cardiac dysfunction as the primary cause of cardiac edema and heart defects. Results were consistent over 6 replicate experiments (50–100 embryos per experiment). Injection of the mismatch control morpholino had no effect on the developing heart.
Fig. 7. Cardiac edema in zebrafish embryos injected with klf3 morpholinos. 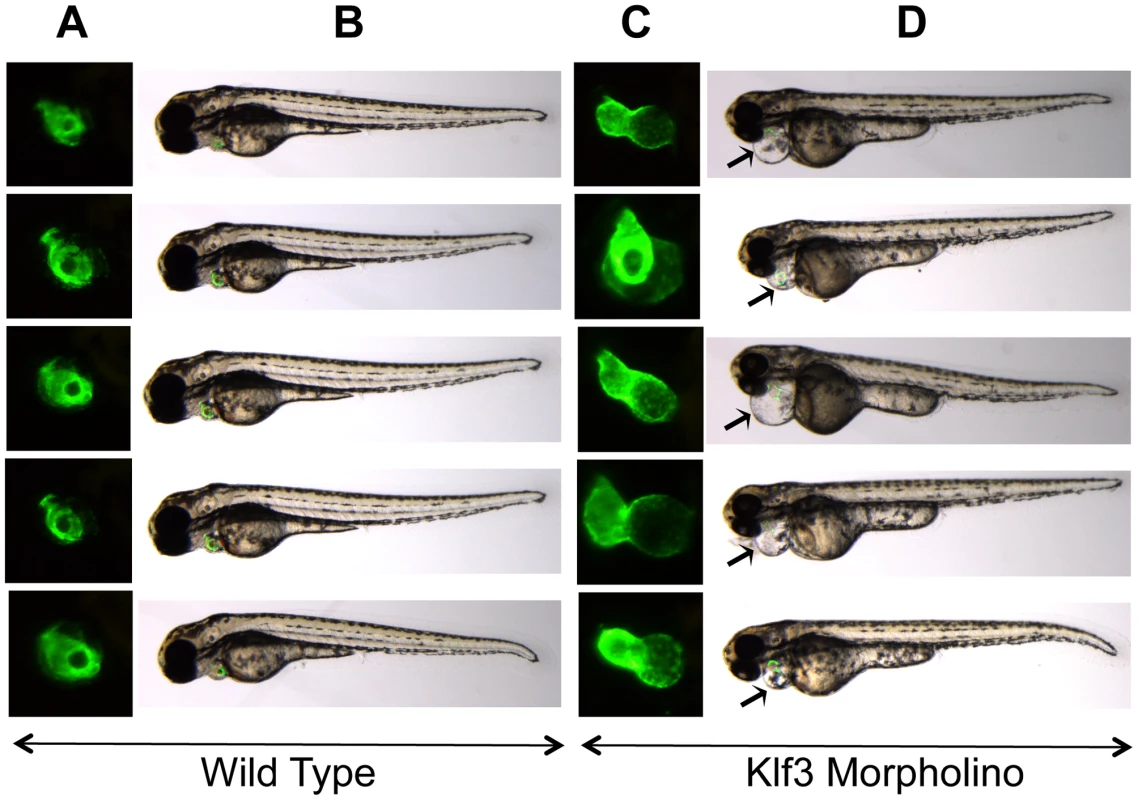
(A,C) Enlarged GFP images of embryonic hearts at 72 hpf. GFP expression was driven by the myl7 regulatory element (cardiac myosin light chain 2) and marks the myocardium of the ventricle (rostral) and atrium (caudal). (B,D) Light microscopy images of the corresponding zebrafish embryos with the GFP heart image shown in (A,C) superimposed. The GFP heart is in the same orientation as in (A) but has not yet been enlarged. Most embryos injected with klf3 morpholino exhibited cardiac abnormalities as shown in (C) and cardiac edema shown by arrows in (D) in comparison to wild type embryos shown in (A,B). Injection of the mismatch control morpholino had no effect on the developing zebrafish heart (not shown). Lethality and the cardiovascular phenotype of Klf3 gene trap mutants
To further validate that altered KLF3 function was the cause of the cardiovascular defects observed in Klf3H275R mutants, we generated mutant mice from two embryonic stem cell lines with gene trap vectors inserted near the start of the Klf3 gene (XS and CH; Figure 8A). These insertions largely eliminated Klf3 mRNA (Figure 8B) and KLF3 protein (Figure 8C) expression in homozygotes. If Klf3H275R was a simple loss of function allele, then these gene trap mutants would be anticipated to exhibit perinatal lethality and cardiovascular defects similar to Klf3H275R mutant mice.
Fig. 8. Gene trap insertion sites and effects on Klf3 mRNA and protein. 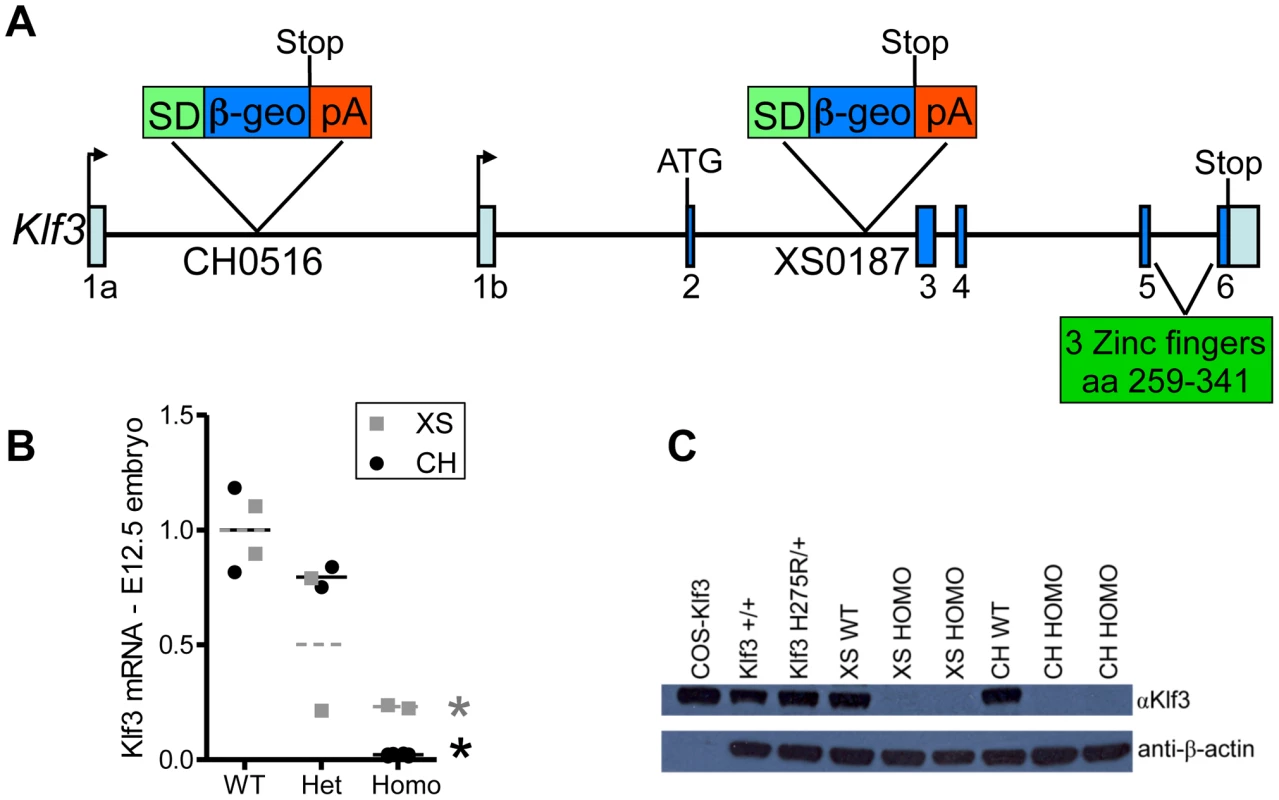
(A) Schematic of Klf3 gene showing the DNA insertion site of the gene trap marker in the XS line (XS0187) and in the CH line (CH0516). SD = splice donor site; β-geo = β-galactosidase and neomycin fusion protein; pA = polyadenylation. (B) Klf3 mRNA in E12.5 whole embryos from the XS (grey squares) and CH (black circles) gene trap lines measured by qRT-PCR was significantly decreased in homozygotes (homo) but not heterozygotes (het). (C) KLF3 protein in spleens of Klf3H275R/+ adults was similar to WT (i.e. Klf3+/+, XS WT, CH WT) whereas it was undetectable in XS and CH homozygote spleens by western blot. At weaning, heterozygosity did not affect survival in Klf3 gene trap mutants. This contrasted with ∼50% of Klf3H275R heterozygotes dying in the perinatal period. However, there was significant lethality prior to weaning age in homozygous gene trap mutants compared to WT littermates (Table S4). This was reported previously for homozygous mutants lacking Klf3's DNA binding region where ∼half those anticipated were found at weaning whereas the anticipated ratio was observed at E14.5 [12]). We found that some homozygous gene trap mutants survived to adulthood whereas homozygosity of the point mutation was always embryonic lethal.
To evaluate the effect of Klf3 gene trap mutations on the aortic valve, we measured ascending aortic blood velocity in homozygous adults. We found that aortic velocity was elevated in a significantly greater proportion of surviving homozygotes of both gene trap lines (Figure 9A). Heterozygotes were not significantly affected (not shown). Homozygote gene trap mutants often exhibited thickened aortic valve leaflets by gross morphology (5 of 7 mutants vs. 0 of 6 WT littermates) (e.g. Figure 9B). Thus, homozygous Klf3 gene trap mutations caused stenotic, malformed aortic semilunar valves that resembled those of the heterozygous point mutant mice (Klf3H275R/+).
Fig. 9. Cardiovascular changes in adult homozygous (homo) gene deletion mutants. 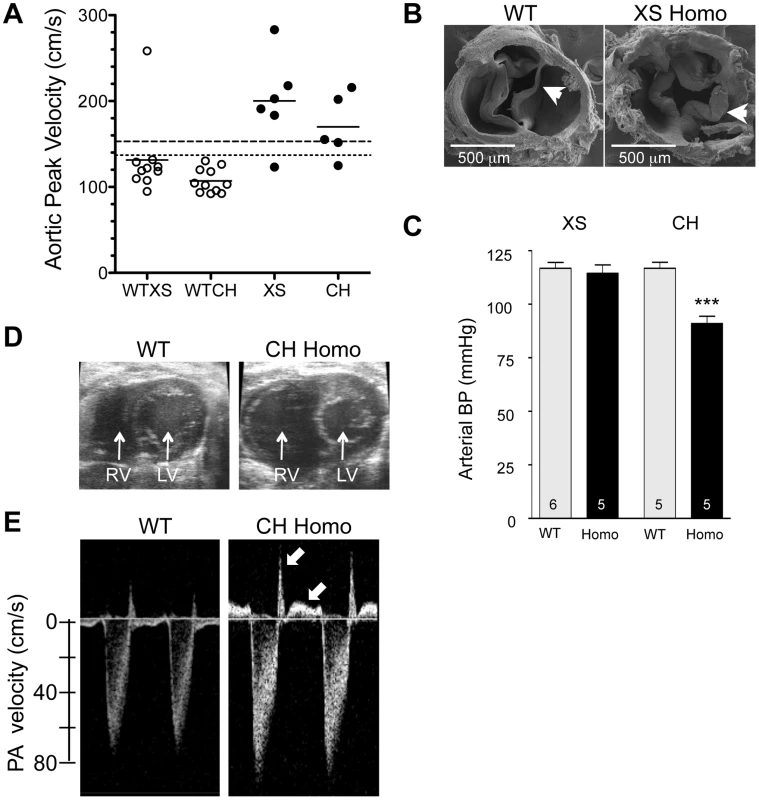
(A) Elevated peak blood velocity in the ascending aorta of XS and CH gene trap mutants (solid circles) relative to WT controls (open circles). Group means are indicated by horizontal lines. The lower dashed line shows the mean + 2SD and the upper dashed line the mean + 3SD for WT point mutant controls. 5 of 6 XS, and 4 of 5 CH mice had elevated peak velocities (>2SD relative to WT point mutants). (B) Scanning electron microscopy images of the aortic valve of a WT littermate (left) and a XS homozygote with aortic valvular stenosis (right). Note marked thickening of the valve leaflets of the mutant. (C) Arterial blood pressure by tail cuff plethysmography in XS and CH lines. *** P<0.001. N is shown in the bar. Mean ± SE. Note that significant hypotension was observed in the CH line only. (D) Cross-sectional view of the heart of a WT and CH homozygous mutant. Note the enlarged right ventricle (RV) in the mutant. Prominent RV enlargement was observed in 2 of 7 XS and 6 of 8 CH homozygotes but no WT controls. (E) Doppler velocity waveform in the proximal pulmonary artery in a WT and CH homozygous deletion mutant. Reversed diastolic blood velocities (arrows) indicate pulmonary valve regurgitation (observed in 5 of 7 XS and 8 of 8 CH homozygotes but no WT). Like adult Klf3H275R heterozygotes, homozygous gene trap mutants also had other diverse cardiac defects. They had significantly higher cardiac output (Table 1) with no change in left ventricular wall thickness or heart rate (Table 1) and, in homozygous CH mice, significantly higher left ventricular end diastolic volume (Table 1), consistent with a phenotype of left ventricular eccentric hypertrophy. Homozygous CH mice also had significantly reduced arterial pressure (−26 mmHg) (Figure 9C), which was similar to Klf3H275R heterozygotes (−10 mmHg) (Figure 2C). Also like adult Klf3H275R heterozygotes, homozygous gene trap mutants had significantly reduced body weights, enlarged hearts, and decreased abdominal fat pad weights (Table S5).
Homozygous Klf3 gene trap mutants exhibited a pronounced right ventricular trait not observed in Klf3H275R/+ mice. They often had marked pathological enlargement of the right ventricle (8 of 15; e.g. Figure 9D) and an abnormal leftward septal deviation in early diastole (Movie S1 and S2). Many also exhibited abnormally thickened pulmonary valve leaflets (Figure S2C,D), and pulmonary valve regurgitation (13 of 15) that was often associated with tricuspid valve regurgitation (7 of 15) (Figure 9E). Lung weight was significantly increased (Table S5) consistent with pulmonary congestion. No septal defects were detected. It is noteworthy that valve closure and septal deviation abnormalities could be secondary to a primary right ventricular enlargement defect.
There were also differences in the haematological phenotypes of the point mutant and gene trap mutants. All 3 mutant lines exhibited greater variation in red cell volume (%RDW; Figure S5) and a larger number of reticulocytes (immature RBC) in blood smears suggesting a defect in erythrocyte production, structure, and/or elimination. However, only Klf3H275R heterozygotes were anaemic and had increased RBC cell size, and only homozygous gene trap mutants had increased white blood cell counts (WBC) (Figure S5).
In Klf3H275R/+ mice, slight anaemia was deemed insufficient to explain the large increase in cardiac output in these mutants. This is supported by the finding that homozygous gene trap mutants had increased cardiac output (Table 1) but no significant change in RBC count (Figure S5) or haemoglobin concentration (not shown).
Organ distribution of Klf3 expression
Staining for LacZ generated by the XS Klf3 gene trap vector indicated strong Klf3 expression in the E10.5–12.5 embryonic aorta and cardiac outflow tract (where heart valve primordia form) (Figure 10A–D). LacZ staining in the embryonic myocardium was diffuse and punctate (not shown). LacZ staining was also observed in the adult atrial and ventricular myocardium, heart valves, and endothelial and vascular smooth muscle of the vasculature (Figure 10E–L, Figure S8). Thus local alterations in KLF3 transcriptional activity in cardiovascular cells may directly cause aortic valvular stenosis, hypotension, and abnormal myocardial growth. Strong LacZ staining was observed at other discrete albeit widespread sites within the embryo (e.g. Figure 10A,C) as observed previously by ISH in embryos [8], [23] and by qRT-PCR in adult tissues [9] so indirect hormonal or neural mechanisms may also play a role in cardiovascular abnormalities in Klf3 mutants.
Fig. 10. Klf3 gene expression detected by LacZ staining in the developing and adult heart and vasculature. 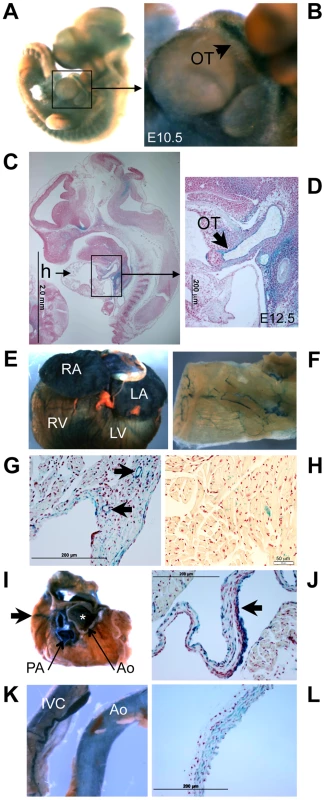
LacZ -staining (blue) in homozygous XS mice shows Klf3 gene expression is located at discrete but widespread sites in (A) the E10.5 embryo (whole mount) including (B) the embryonic outflow tract (OT), and (C) in histological sections of E12.5 embryos particularly in (D) the outflow tract. In adult XS mice, prominent Klf3 gene expression was observed in (E) the atria and ventricles and in (F) blood vessels including those of the skeletal muscle shown here in LacZ stained tissues. Histological images show Klf3 expression in the myocardium of the (G) atrium (where blood vessels are also heavily stained (arrows)) and in (H) the ventricle where staining is diffuse and punctate. (I) After removing the atria from the heart, prominent expression was seen in cardiac valves (asterisk indicates aortic valve location) and in the aorta (Ao) and pulmonary artery (PA), and coronary vessels (arrows) of the heart. Histological images show strong staining in (J) the aortic valve leaflets (arrow). Klf3 gene expression was prominent in (K) the inferior vena cava (left) and aorta (right) and in (L) histological images of vasculature where staining was found in both the smooth muscle and endothelium as shown here for the aorta. Ao, aorta; h, heart; IVC, inferior vena cava; LA, left atrium; LV, left ventricle; OT, outflow tract; PA, pulmonary artery; RA, right atrium; RV, right ventricle. Altered embryonic gene expression caused by Klf3H275R and CH Klf3 gene trap mutations
Gene expression was evaluated in Klf3H275R and CH homozygotes, Klf3H275R heterozygotes, and WT by microarray (Figure S9). To increase the likelihood of revealing immediate downstream targets of KLF3, we used mRNA isolated from whole embryos collected at E12.5 d of gestation. At this stage, Klf3H275R homozygosity was not yet lethal, and Klf3 LacZ expression was widespread (e.g. Figure 10A). When comparing Klf3H275R homozygotes, heterozygotes, or both groups combined versus WT, no genes were identified as significantly changed using a false discovery rate threshold of 0.1. For CH homozygotes vs. WT, 18 genes were changed significantly and 2 were changed >2-fold; Klf3 (0.07×) and Hsd3b6 (0.23×) (Table S6). Overall, CH homozygous embryos had 16 genes differentially expressed when compared to Klf3H275R homozygotes (including 4 genes changed by >2-fold; Klf3 (0.07×), Glmap4 (2.07×), Snora31 (2.20×), and Fah (2.31×)) and 185 genes were differentially expressed when compared to Klf3H275R heterozygotes (including 5 genes changed by >2-fold; Klf3 (0.07×), Hsd3b6 (0.38×), Mir1948 (0.48×), Snora31 (2.04×), and Slc6a16 (2.24×)) (Table S6). These differences in embryonic gene expression profiles may explain phenotype differences between heterozygous point mutant and homozygous gene trap lines that were observed later in development.
We then used qRT-PCR to validate the large down regulation of Hsd3b6 expression found in E12.5 embryos by microarray. Expression was significantly reduced in Klf3H275R heterozygotes (0.45×), and in Klf3H275R homozygotes (0.27×) vs. WT (Figure S10A) whereas a similar trend in CH homozygotes was not statistically significant possibly due to the smaller sample size (Figure S10B). We also evaluated Lilra6 by qRT-PCR; among genes with apparent up-regulation by visual inspection of the heat map (Figure S9), Lilra6 was the most consistently high in Klf3 mutant embryos (8 of 11) and low in WT (4 of 4). Lilra6 was significantly elevated in Klf3H275R heterozygotes (2.4×), and in Klf3H275R homozygotes (13.8×) (Figure S10C) whereas the much smaller increasing trend in CH homozygotes (2.2×) was not statistically significant (Figure S10D). These results show that the heterozygous and homozygous presence of the point mutant protein can down or up regulate normal gene expression in E12.5 embryos, and that effects may differ from that caused by reduced expression of the native protein (i.e. in CH homozygotes).
Discussion
Herein we report the discovery of novel and important roles for Klf3 in cardiovascular development and function. Although KLF3 was identified and cloned nearly 20 years ago [8] and Klf3 knock out mice have been studied [12], KLF3's role in cardiovascular biology had not been revealed until the current unbiased genome-wide ENU screen, in which prominent abnormalities were discovered in heterozygous Klf3H275R point mutants. We found abnormalities in embryonic gene expression during organogenesis and in heart morphology at E12.5, increased perinatal lethality associated with marked biventricular myocardial hypertrophy and aortic valve leaflet thickening, and adult survivors exhibited hypotension, aortic valvular stenosis, aortic dilatation, and myocardial hypertrophy with increased chamber size. KLF3 in vitro is enriched at promoters of muscle-specific genes [11], and in vivo is expressed by multiple cell types integral to the cardiovascular system including cardiomyocytes, heart valves, and vascular endothelial and smooth muscle cells based on LacZ expression patterns reported here. Cardiovascular abnormalities may therefore result directly from abnormal KLF3 function in cardiac and/or vascular cells. Abnormal renal or brain regulatory mechanisms may also contribute to hypotension given that Klf3 is expressed at these sites as well (e.g. figure 9 A,B; ref #10). Elucidating the likely multifactorial roles of KLF3 in cardiovascular development and function will require temporal - and cell-type specific control of Klf3 expression in future studies.
A role for KLF3 in cardiac valve development was detected in our ENU mutagenesis screen, in which high aortic blood velocity revealed aortic semilunar valve stenosis in heterozygous adults with Klf3H275R point mutations. In contrast, other cardiac valves were relatively unaffected although they similarly expressed Klf3 based on LacZ staining. Valve specificity may arise due to differences in developmental mechanisms in semilunar versus atrioventricular valves [24]. For example cells derived from the secondary heart field [25] and cardiac neural crest [26] selectively contribute only to semilunar valve formation. Homozygous gene trap mutants also exhibited a high incidence of aortic valvular stenosis in adults but, in contrast with point mutants, the pulmonary semilunar valve leaflets sometimes appeared thickened histologically and often failed to close sufficiently to prevent regurgitation. In gene trap mutants, it is possible that pulmonary valve defects were secondary to a primary chamber enlargement defect of the right ventricle, a trait that was also never observed in heterozygous Klf3H275R point mutants. Although aortic valve cusps were thickened in late gestation in Klf3H275R/+ embryos, aortic valvular stenosis sufficient to elevate aortic blood velocity developed only after birth when the normal separation, elongation, and thinning of the valve leaflets occurs [27], [28]. This suggests KLF3 plays a particularly critical role in these later events in aortic valve maturation. This finding is especially interesting given the paucity of knowledge about the genetic regulation of aortic semilunar valve development and the prevalence of aortic valve defects in humans [24].
Additional phenotypic characterization revealed broader roles for KLF3 in cardiovascular development and function, roles that were independent of its role in aortic valve development. In adults, high cardiac output and cardiac hypertrophy due to chamber enlargement (i.e. eccentric growth) with no change in left ventricular wall thickness was paradoxical given aortic valvular stenosis in adult Klf3 point mutant and gene trap mutants. This was explained by the surprising observation that stenosis did not elevate intraventricular systolic blood pressure in Klf3H275R/+ hearts relative to WT. Instead, normal intraventricular pressure, low arterial blood pressure, and augmented blood volume in adult Klf3H275R/+ were likely caused by low systemic vascular resistance, resulting in a hyperdynamic circulation and the high cardiac output that we observed. Intriguingly, hypotension in a wide variety of other mouse models does not elicit cardiac hypertrophy and/or an increase in cardiac output. Examples include transgenic mice with overexpression of eNOS (−18 mmHg and no change in heart weight to body weight ratio) [29], Rgs5-deficiency (>−20 mmHg and no increase in left ventricular inner diameter in diastole) [30], and overexpression of atrial natriuretic factor (−24 mmHg and no change in cardiac output) [31]. One exception is homozygous deletion of sarcomeric mitochondrial creatine kinase (Ckmt2) where hypotension, left ventricular hypertrophy and high cardiac output can occur depending on genetic background [32], [33]. Interestingly, KLF3 acts as a transcriptional activator of muscle creatine kinase [11] and interacts with promoters of several other muscle-specific genes but whether it influences Ckmt2 expression is not known. Thus, although a hyperdynamic circulation may cause eccentric cardiac growth, a direct myocardial hypertrophic mechanism may also be involved.
In the embryo at E12.5, prominent Klf3 LacZ expression was present in the ventricular outflow tract and vascular endothelium whereas LacZ expression in the myocardium was diffuse and punctate. It is therefore possible that the thin myocardium in Klf3H275R homozygote embryos was secondary to a vascular defect, which caused low peripheral vascular resistance and low intracardiac pressures. A thin myocardium also occurs by E12.5 in mouse embryos with endothelial-specific Klf2 gene deletion [34]. In that model, phenylephrine (a vasoconstrictor) reduced lethality at E14.5 in mutant mouse embryos, and in zebrafish embryos injected with anti-klf2 morpholino [34]. However, when we injected zebrafish with anti-klf3 morpholino, phenylephrine failed to significantly reduce lethality (not shown). Also we observed cardiac septal defects whereas none were reported in Klf2 endothelial-specific knockout mouse embryos [34]. Thus, at E12.5, cardiac defects were likely caused by the direct myocardial effects of Klf3H275R protein although contribution from vascular defects cannot be ruled out. Later in gestation, LacZ staining appeared to become more prominent in the heart and heart valves, ventricular wall thicknesses and heart weights were increased, ventricular chamber sizes were diminished, and aortic valves appeared abnormally thickened. Pressure loading of the heart due to outflow tract obstruction may have contributed to cardiac hypertrophy, which was dramatic in mutant embryos that died in the perinatal period. However, in surviving newborns, aortic blood velocities were not significantly elevated suggesting minimal aortic valvular stenosis. Nevertheless, left ventricles were significantly hypertrophied. These data suggest that at least a component of the perinatal hypertrophic cardiac phenotype was a direct effect of Klf3H275R on cardiac development and growth.
When we compared the phenotype of the H275R mutation with the loss of function gene trap mutations in Klf3, we found that there were considerable similarities, but also marked differences, between the heterozygous point mutants and the homozygous gene trap mutants. Similarities may be explained by the point mutation acting as a dominant negative so that it interfered with wild-type KLF3 function. However, the more severe embryonic lethality shown by the homozygous point mutants than the homozygous gene trap mutants, suggests that the point mutant form of KLF3 may also disrupt other proteins and pathways. It is not uncommon for point mutants to display phenotypes that are more robust and/or different than null mutants for the same gene [35] because point mutations can cause not only reduced gene function, but also enhanced or abnormal gene function, and/or generate proteins with dominant negative activity. Some phenotype differences between Klf3H275R mutants and Klf3 gene trap mutants may be due to differences in genetic background; phenotyping of Klf3H275R was largely on a B6 background whereas Klf3 gene trap mutants were on a mixed 129/B6 background. However, genetic background within the Klf3H275R strain cannot account for the more severe phenotype in homozygotes (lethal by ∼E14.5–16.5) than heterozygotes (∼50% survived to adulthood). Thus results are inconsistent with a simple dominant negative effect of the mutant protein. This is also supported by our finding that the mutant Klf3H275R protein exerted a dominant negative action on one of KLF3's gene targets (Lgals3) while sparing other normal targets. In this case, it is not clear how the point mutant protein would disable the native protein to exert a dominant negative effect because KLFs are not known to dimerize [36]. Furthermore, the point mutant protein did not interfere with the ability of WT KLF3 to bind to KLF3's canonical CACCC DNA binding region in vitro. Nevertheless, in vivo, the point mutant protein may have a greater affinity for rate-limiting co-factor binding proteins thereby competitively inhibiting the activity of native protein. In addition, alterations in the mutant protein's DNA binding specificity may cause the mutant protein to transcriptionally regulate additional genes not normally regulated by KLF3 (i.e. a gain of function effect).
A similarly complicated interaction caused by a single amino acid change in the second zinc finger of Klf1 (E339D) has also been found in mutant mice [36], [37], and the homologous mutation in humans causes human disease [38]. Similar to our case, the Klf1 mutation changed the central of 3 amino acids predicted to contact DNA in the zinc finger of a KLF protein. That mutation resulted in a mutant KLF1 protein that failed to bind and transactivate a subset of KLF1's downstream targets in a manner dependent on the target gene's DNA binding sequence [36]. While an association between the Klf3H275R mutation and human disease is not currently known, autosomal dominant point mutations, like H275R, are among the most common causes of human genetic disorders. With Klf1 as precedent, the current work provides strong impetus for searching for mutations in Klf3 in humans with cardiovascular dysfunction.
In conclusion, we have discovered important and hitherto unknown roles for Klf3 in cardiovascular development and function. We have also revealed the critical importance of amino acid 275 in the DNA binding region of the first of three zinc fingers, for normal DNA binding and transcriptional gene regulation, and its importance in cardiovascular development. This histidine residue is conserved in KLF3 across species from zebrafish to humans, and in all but one of 22 Sp/Klf family members [18], [28] (http://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Retrieve&db=homologene&dopt=MultipleAlignment&list_uids=7). Thus the identification of the critical importance of this histidine residue may have broad implications; it may apply across species, across the Sp/Klf family, and across other C2H2 zinc finger (C2H2-ZNF) proteins, one of the largest and most complex gene super-families with hundreds of members in the human and mouse genome [29]. Involvement of this histidine residue within the zinc finger domain in human disease warrants further investigation.
Materials and Methods
Ethics statement
All experimental procedures received approval from the Animal Care Committee of Mount Sinai Hospital and were conducted in accordance with the guidelines of the Canadian Council on Animal Care.
Generation of ENU mutant by Centre for Modeling Human Disease (www.cmhd.ca)
When male C57BL/6J mice (www.jax.org) regained fertility after ENU treatment (85 mg/kg i.p. 1/wk for 3 wk), they were bred to C3H/HeJ females (www.jax.org). Offspring were screened at 8–10 wk for high blood velocity in the ascending thoracic aorta. A male with an aortic blood velocity >7 SD above the mean of previous offspring was identified. It was bred to BALB/c females to test for heritability.
Genetic mapping, sequencing, and genotyping of point mutation
PCR amplification of individual microsatellite markers using fluorescently tagged primers (IDT, Coralville, IA) was performed on extracted tail DNA from offspring. Labeled products were multiplexed and analyzed on a BaseStation automated sequencer (MJ Research, Waltham, MA) to identify alleles from the mutagenized strain (C57BL/6J). Linkage analysis on BALB/c localized the causative mutation to chromosome 5 (Figure S1). Phenotype penetrance was greater on C57BL/6J so mice were then bred to the consomic chromosome substitution strain, C57BL/6J-Chr 5A/J/NaJ (www.jax.org, Stock Number 004383) and linkage analysis continued on chromosome 5 from the A/J strain. SNP markers unique to C57BL/6J and A/J strains were used to refine the linkage interval. Genomic sequencing of candidate genes revealed a point mutation in exon 5 of Klf3 (Krüppel-like factor 3) that changed a histidine residue (CAC) at amino acid 275 to arginine (CGC) (KLF3H275R). The mouse line has been named Klf3m1Jrt.
For genotyping the Klf3H275R point mutant mice, we distinguished the mutant Klf3 DNA from the endogenous wild type DNA using the base pair change from an A to a G, which introduced a novel BstUII restriction enzyme site (Table S7).
Phenotyping
Micro-ultrasound imaging and Doppler
The primary screen, high blood velocity in the ascending thoracic aorta, was assessed in isoflurane-anesthetized mice using pulsed Doppler ultrasound (20 MHz transducer; Indus Instruments, Houston, TX) while the rectal temperature was maintained at 37–38°C. Echocardiography was performed in isoflurane-anesthetized adults as a secondary screen (30–40 MHz; Vevo770 or Vevo2100, VisualSonics, Toronto, Canada) using published and/or standard methods [39], [40]. Peak ascending aortic blood velocity was assessed in isoflurane-anesthetized newborn mice one day after birth.
Arterial blood pressure
Arterial blood pressure and heart rate were measured in conscious mice using a computerized tail cuff system (MC-4000 Hatteras Instruments, NC; www.cmhd.ca) and/or in isoflurane-anesthetized mice with a catheter-tip pressure transducer (Ultra-Miniature Mikro-Tip; Millar Instruments, TX) inserted into the ascending aorta and left ventricle via the carotid artery.
Hematology and blood smears
Whole blood was collected from the saphenous vein into EDTA coated capillary tubes (Drummond Scientific Co., Broomall, PA) and analyzed on a Hemavet 950FS Hematology Analyzer (Drew Scientific, Waterbury, CT). Blood smears were stained with Wright – Giemsa SureStain (Fisher Scientific, Ottawa, Canada).
Metabolic rate
Oxygen consumption of males (n = 4 per group, 22–25 wk) was measured over 24 h by indirect calorimetry (Oxymax, Columbus Instruments, Columbus, OH) after 2 h acclimatization to the chamber.
Percent body fat
Body composition of anesthetized mice was assessed at 10 wk using Dual energy X-ray absorptiometry (DEXA) (PIXImus, Lunar Corp., Madison, WI) (www.cmhd.ca).
Magnetic resonance imaging (MRI), optical projection tomography (OPT), and micro-computed tomography (micro-CT)
Samples for MRI were prepared using methods adapted from [41] and imaged using standard methods [42]. Sample preparation for OPT was essentially as described [43] and images were acquired using standard methods [44], [45]. Micro-CT samples were immersed in iodine [46] to generate soft tissue contrast and imaging was performed as previously published [47], [48].
Histopathology
Animals were perfusion fixed with 10% neutral-buffered formalin, tissues were processed, embedded in paraffin, and 5 µm sections stained with hematoxylin and eosin, or Movat's. LacZ whole mount staining of embryos and slices of adult tissues were performed using standard methods [49] followed by routine histology.
Zebrafish morpholino injections
The zebrafish klf3 (Accession Number NM_131859) sequence was verified and targeted. A morpholino spanning the translation start site of the zebrafish klf3 gene (sequence: 5′ - agcatggctgcttccagtggaattt – 3′) was designed and ordered from Gene Tools (Philomath, Oregon). Following morpholino titration to determine the optimal concentration, 7 ng of morpholino was injected per embryo at the 1-cell stage using standard techniques. The myl7:EGFPtwu34 transgenic line was used to image the developing heart.
Embryonic stem cell lines
Embryonic stem cell lines XS0187 (XS) (MGI:4331780) and CH0516 (CH) (MGI:3872001) from the Sanger Institute Gene Trap Resource were obtained from the NIH/NCRR-sponsored Mutant Mouse Regional Resource Center at UC Davis. The location of the gene trap insertion was determined by long range PCR and confirmed by sequencing. 5′ RACE analysis (Sanger Institute Gene Trap Resource) placed the gene trap insertion cassette in Klf3 in intron 2 for XS and in intron 1 for CH. Primers for long-range PCR were designed at regular intervals spanning the preceding intronic sequence for the 5′ primer and a common sequence at the beginning of the B-geo fusion gene was used for the 3′ primer. PCR with each of the long-range primer sets was then undertaken systemically to narrow down the interval of the insertion site as determined by the production of a ∼1–2.5 kbp fragment. The resulting PCR product was purified and sent to The Centre for Applied Genomics (The Hospital for Sick Children, Toronto, Canada) for sequencing. To determine the exact site of insertion, sequencing results were compared to the intronic sequence of the Klf3 gene and the gene trap vector using the NCBI BLAST programme.
In XS, the gene trap cassette inserted 4105 bp downstream of the 5′ end of intron 2 (4,843 bp in total) (Figure 8A). In CH, the gene trap cassette inserted 3578 bp downstream from the start of intron 1 (12,943 bp in total) (Figure 8A). Primers were designed for genotyping mutants such that PCR and gel electrophoresis of genomic DNA yielded different sized products for the endogenous WT allele and for the allele with the gene trap insertion (Table S7). The mouse lines B6;129-Klf3Gt(XS0187)Wtsi/Cmhd (XS) and B6;129-Klf3Gt(CH0516)Wtsi/Cmhd (CH) were derived from embryonic stem cells by the Transgenic Core at the Toronto Centre for Phenogenomics.
Molecular methods
Vectors and cloning
The mammalian expression vector pMT3-Klf3 encoding full length murine Klf3 has been described previously [50]. The bacterial expression vector pGEX-6P-Klf3-F1-3, encoding residues 254–344 of Klf3 fused to glutathione S-transferase (GST), was kindly provided by Jacqui Matthews and Sandra Wissmueller (School of Molecular Bioscience, University of Sydney, NSW, Australia). The H275R mutation was incorporated into both of these vectors by overlap PCR to generate pMT3-Klf3H275R and pGEX-6P-Klf3-F1-3-H275R respectively.
COS transfections, preparation of nuclear extracts and analysis
COS cells were transfected with 1, 2 or 5 µg vector (pMT3, pMT3-Klf3 or pMT3-Klf3H275R) as described previously [9]. After 48 h, nuclear extracts were prepared and subjected to electrophoretic mobility shift assays using a radiolabelled DNA probe containing the mouse β-major globin promoter CACCC box as described previously [8]. Western blots were performed as described previously [50] using NuPAGE 10% Bis-Tris gels (Invitrogen, Carlsbad, CA) and antisera specific for KLF3 [8]. Detection was achieved using the Immobilon Western Chemiluminescent HRP Substrate kit (Millipore, Billerica, MA) and X-ray film (Eastman Kodak Company, Rochester, NY).
Bacterial expression, GST purification and analysis
Bacteria were transformed with pGEX-6P, pGEX-6P-Klf3-F1-3, and pGEX-6P-Klf3-F1-3-H275R respectively and expressed GST fusion proteins were purified on glutathione beads as described previously [51]. Proteins were analysed by electrophoretic mobility shift assay and by sodium dodecyl sulphate-polyacrylamide gel electrophoresis as above. Polyacrylamide gels were stained with Coomassie blue and subsequently destained by sequential 1 h immersions in solutions of 40% (v/v) methanol, 10% (v/v) acetic acid and 5% (v/v) methanol, 7% (v/v) acetic acid.
RNA extraction from embryos and real time PCR
Total RNA from E12.5 whole embryos and dissected livers were extracted using TRIZOL Reagent (Invitrogen), DNase-treated and cleaned up with RNeasy kits (QIAGEN) as described previously [9]. RNA (2.5 µg) was subsequently used as a template for cDNA synthesis using the SuperScript® VILO™cDNA Synthesis Kit (Invitrogen). Quantitative real time PCR (qRT-PCR) was performed as described [9] but using FastStart Universal SYBR Green Master (Rox) (Roche Diagnostics, Indianapolis, IN) or Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA), and the 7500 Fast Real-Time PCR System (Applied Biosystems). Primer sequences are shown in Table S8.
Nuclear protein extraction from embryos
Nuclear protein extracts were isolated from E12.5 whole embryos, analysed by Western blotting and visualized by chemiluminescence as described previously [50].
Statistical analysis
Significant differences (P<0.05) were tested using Student's t-test or, if normality failed, a Mann-Whitney Rank Sum Test. Proportions were tested using Chi Square or Fisher's Exact Test. Multiple groups were tested by 2-way or 3-way ANOVA as appropriate, and if significant, then a multiple comparison test was performed. If sex was not a significant factor, then combined data are shown (mean ± SE).
Microarrays
Total RNA was extracted from whole embryos (E12.5) for the following genotypes: wild type (n = 4), Klf3H275R/+ (n = 4), Klf3H275R/H275R (n = 3) and Klf3 CH homozygous (n = 4). RNA was prepared and hybridized to Affymetrix GeneChIP 2.0 ST arrays (Affymetrix, Santa Clara, CA) by the Ramaciotti Centre (University of New South Wales, Australia) as previously described [14]. Data were normalized using the robust multiarray average (RMA) algorithm and analyzed using Partek Genomic Suite version 6.6 (Partek Inc., St Louis, MO). Genes that showed greater than 1.5-fold deregulation of expression in Klf3H275R/H275R samples relative to wild type were used to construct the heat map (Figure S9). Microarray data were deposited in the Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/projects/geo) under accession number GSE43908.
Supporting Information
Zdroje
1. HoffmanJI, KaplanS (2002) The incidence of congenital heart disease. J Am Coll Cardiol 39 : 1890–1900.
2. TennstedtC, ChaouiR, KornerH, DietelM (1999) Spectrum of congenital heart defects and extracardiac malformations associated with chromosomal abnormalities: results of a seven year necropsy study. Heart 82 : 34–39.
3. BruneauBG (2008) The developmental genetics of congenital heart disease. Nature 451 : 943–948.
4. CripeL, AndelfingerG, MartinLJ, ShoonerK, BensonDW (2004) Bicuspid aortic valve is heritable. J Am Coll Cardiol 44 : 138–143.
5. McBrideKL, PignatelliR, LewinM, HoT, FernbachS, et al. (2005) Inheritance analysis of congenital left ventricular outflow tract obstruction malformations: Segregation, multiplex relative risk, and heritability. Am J Med Genet A 134A: 180–186.
6. HintonRBJr, MartinLJ, TabanginME, MazwiML, CripeLH, et al. (2007) Hypoplastic left heart syndrome is heritable. J Am Coll Cardiol 50 : 1590–1595.
7. JusticeMJ, NoveroskeJK, WeberJS, ZhengB, BradleyA (1999) Mouse ENU mutagenesis. Hum Mol Genet 8 : 1955–1963.
8. CrossleyM, WhitelawE, PerkinsA, WilliamsG, FujiwaraY, et al. (1996) Isolation and characterization of the cDNA encoding BKLF/TEF-2, a major CACCC-box-binding protein in erythroid cells and selected other cells. Mol Cell Biol 16 : 1695–1705.
9. FunnellAP, MaloneyCA, ThompsonLJ, KeysJ, TallackM, et al. (2007) Erythroid Krüppel Kruppel-like factor directly activates the basic Krüppel Kruppel-like factor gene in erythroid cells. Mol Cell Biol 27 : 2777–2790.
10. TurnerJ, CrossleyM (1998) Cloning and characterization of mCtBP2, a co-repressor that associates with basic Krüppel Kruppel-like factor and other mammalian transcriptional regulators. Embo J 17 : 5129–5140.
11. HimedaCL, RanishJA, PearsonRC, CrossleyM, HauschkaSD (2010) KLF3 regulates muscle-specific gene expression and synergizes with serum response factor on KLF binding sites. Mol Cell Biol 30 : 3430–3443.
12. SueN, JackBH, EatonSA, PearsonRC, FunnellAP, et al. (2008) Targeted disruption of the basic Krüppel Kruppel-like factor gene (Klf3) reveals a role in adipogenesis. Mol Cell Biol 28 : 3967–3978.
13. VuTT, GattoD, TurnerV, FunnellAP, MakKS, et al. (2011) Impaired B cell development in the absence of Krüppel Kruppel-like factor 3. J Immunol 187 : 5032–5042.
14. FunnellAP, NortonLJ, MakKS, BurdachJ, ArtuzCM, et al. (2012) The CACCC-binding protein KLF3/BKLF represses a subset of KLF1/EKLF target genes and is required for proper erythroid maturation in vivo. Mol Cell Biol 32 : 3281–3292.
15. ConwaySJ, Kruzynska-FrejtagA, KneerPL, MachnickiM, KoushikSV (2003) What cardiovascular defect does my prenatal mouse mutant have, and why? Genesis 35 : 1–21.
16. HaldarSM, IbrahimOA, JainMK (2007) Krüppel -like Factors (KLFs) in muscle biology. J Mol Cell Cardiol 43 : 1–10.
17. CullingfordTE, ButlerMJ, MarshallAK, Tham elL, SugdenPH, et al. (2008) Differential regulation of Krüppel-like factor family transcription factor expression in neonatal rat cardiac myocytes: effects of endothelin-1, oxidative stress and cytokines. Biochim Biophys Acta 1783 : 1229–1236.
18. KaczynskiJ, CookT, UrrutiaR (2003) Sp1 - and Krüppel-like transcription factors. Genome Biol 4 : 206.
19. KlevitRE (1991) Recognition of DNA by Cys2,His2 zinc fingers. Science 253 : 1367–1393.
20. Della CorteA, BanconeC, QuartoC, DialettoG, CovinoFE, et al. (2007) Predictors of ascending aortic dilatation with bicuspid aortic valve: a wide spectrum of disease expression. Eur J Cardiothorac Surg 31 : 397–404; discussion 404–395.
21. AdamsRH, PorrasA, AlonsoG, JonesM, VinterstenK, et al. (2000) Essential role of p38a MAP kinase in placental but not embryonic cardiovascular development. Mol Cell 6 : 109–116.
22. TeiC, NishimuraRA, SewardJB, TajikAJ (1997) Noninvasive Doppler-derived myocardial performance index: correlation with simultaneous measurements of cardiac catheterization measurements. J Am Soc Echocardiogr 10 : 169–178.
23. GrayPA, FuH, LuoP, ZhaoQ, YuJ, et al. (2004) Mouse brain organization revealed through direct genome-scale TF expression analysis. Science 306 : 2255–2257.
24. CombsMD, YutzeyKE (2009) Heart valve development: regulatory networks in development and disease. Circ Res 105 : 408–421.
25. LinCY, LinCJ, ChenCH, ChenRM, ZhouB, et al. (2012) The secondary heart field is a new site of calcineurin/Nfatc1 signaling for semilunar valve development. J Mol Cell Cardiol 52 : 1096–1102.
26. JainR, EnglekaKA, RentschlerSL, ManderfieldLJ, LiL, et al. (2011) Cardiac neural crest orchestrates remodeling and functional maturation of mouse semilunar valves. J Clin Invest 121 : 422–430.
27. HintonRBJr, LincolnJ, DeutschGH, OsinskaH, ManningPB, et al. (2006) Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ Res 98 : 1431–1438.
28. HintonRBJr, AlfieriCM, WittSA, GlascockBJ, KhouryPR, et al. (2008) Mouse heart valve structure and function: echocardiographic and morphometric analyses from the fetus through the aged adult. Am J Physiol Heart Circ Physiol 294: H2480–2488.
29. OhashiY, KawashimaS, HirataK, YamashitaT, IshidaT, et al. (1998) Hypotension and reduced nitric oxide-elicited vasorelaxation in transgenic mice overexpressing endothelial nitric oxide synthase. J Clin Invest 102 : 2061–2071.
30. ChoH, ParkC, HwangIY, HanSB, SchimelD, et al. (2008) Rgs5 targeting leads to chronic low blood pressure and a lean body habitus. Mol Cell Biol 28 : 2590–2597.
31. BarbeeRW, PerryBD, ReRN, MurgoJP, FieldLJ (1994) Hemodynamics in transgenic mice with overexpression of atrial natriuretic factor. Circ Res 74 : 747–751.
32. NahrendorfM, SpindlerM, HuK, BauerL, RitterO, et al. (2005) Creatine kinase knockout mice show left ventricular hypertrophy and dilatation, but unaltered remodeling post-myocardial infarction. Cardiovasc Res 65 : 419–427.
33. LygateCA, HunyorI, MedwayD, de BonoJP, DawsonD, et al. (2009) Cardiac phenotype of mitochondrial creatine kinase knockout mice is modified on a pure C57BL/6 genetic background. J Mol Cell Cardiol 46 : 93–99.
34. LeeJS, YuQ, ShinJT, SebzdaE, BertozziC, et al. (2006) Klf2 is an essential regulator of vascular hemodynamic forces in vivo. Dev Cell 11 : 845–857.
35. Acevedo-ArozenaA, WellsS, PotterP, KellyM, CoxRD, et al. (2008) ENU mutagenesis, a way forward to understand gene function. Annu Rev Genomics Hum Genet 9 : 49–69.
36. SiateckaM, SahrKE, AndersenSG, MezeiM, BiekerJJ, et al. (2010) Severe anemia in the Nan mutant mouse caused by sequence-selective disruption of erythroid Krüppel-like factor. Proc Natl Acad Sci U S A 107 : 15151–15156.
37. HeruthDP, HawkinsT, LogsdonDP, GibsonMI, SokolovskyIV, et al. (2010) Mutation in erythroid specific transcription factor KLF1 causes Hereditary Spherocytosis in the Nan hemolytic anemia mouse model. Genomics 96 : 303–307.
38. BorgJ, PatrinosGP, FeliceAE, PhilipsenS (2011) Erythroid phenotypes associated with KLF1 mutations. Haematologica 96 : 635–638.
39. ZhouYQ, DavidsonL, HenkelmanRM, NiemanBJ, FosterFS, et al. (2004) Ultrasound-guided left-ventricular catheterization: a novel method of whole mouse perfusion for microimaging. Lab Invest 84 : 385–389.
40. BrobergCS, PantelyGA, BarberBJ, MackGK, LeeK, et al. (2003) Validation of the myocardial performance index by echocardiography in mice: a noninvasive measure of left ventricular function. J Am Soc Echocardiogr 16 : 814–823.
41. PetietA, HedlundL, JohnsonGA (2007) Staining methods for magnetic resonance microscopy of the rat fetus. J Magn Reson Imaging 25 : 1192–1198.
42. SpringS, LerchJP, WetzelMK, EvansAC, HenkelmanRM (2010) Cerebral asymmetries in 12-week-old C57Bl/6J mice measured by magnetic resonance imaging. NeuroImage 50 : 409–415.
43. WallsJR, CoultasL, RossantJ, HenkelmanRM (2008) Three-dimensional analysis of vascular development in the mouse embryo. PLoS One 3: e2853.
44. SharpeJ, AhlgrenU, PerryP, HillB, RossA, et al. (2002) Optical projection tomography as a tool for 3D microscopy and gene expression studies. Science 296 : 541–545.
45. ZhouYQ, FosterFS, NiemanBJ, DavidsonL, ChenXJ, et al. (2004) Comprehensive transthoracic cardiac imaging in mice using ultrasound biomicroscopy with anatomical confirmation by magnetic resonance imaging. Physiol Genomics 18 : 232–244.
46. MetscherBD (2009) MicroCT for developmental biology: a versatile tool for high-contrast 3D imaging at histological resolutions. Dev Dyn 238 : 632–640.
47. MarxenM, ThorntonMM, ChiarotCB, KlementG, KoprivnikarJ, et al. (2004) MicroCT scanner performance and considerations for vascular specimen imaging. Med Phys 31 : 305–313.
48. KannoH, UtsugisawaT, AizawaS, KoizumiT, AisakiK, et al. (2007) Transgenic rescue of hemolytic anemia due to red blood cell pyruvate kinase deficiency. Haematologica 92 : 731–737.
49. CarmelietP, FerreiraV, BreierG, PollefeytS, KieckensL, et al. (1996) Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 380 : 435–439.
50. PerdomoJ, VergerA, TurnerJ, CrossleyM (2005) Role for SUMO modification in facilitating transcriptional repression by BKLF. Mol Cell Biol 25 : 1549–1559.
51. SmithDB, JohnsonKS (1988) Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene 67 : 31–40.
Štítky
Genetika Reprodukční medicína
Článek Independent Evolution of Transcriptional Inactivation on Sex Chromosomes in Birds and MammalsČlánek The bHLH Subgroup IIId Factors Negatively Regulate Jasmonate-Mediated Plant Defense and DevelopmentČlánek Selective Pressures to Maintain Attachment Site Specificity of Integrative and Conjugative ElementsČlánek Reassembly of Nucleosomes at the Promoter Initiates Resilencing Following Decitabine ExposureČlánek Hepatocyte Growth Factor Signaling in Intrapancreatic Ductal Cells Drives Pancreatic Morphogenesis
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 7- Akutní intermitentní porfyrie
- Souvislost haplotypu M2 genu pro annexin A5 s opakovanými reprodukčními ztrátami
- Příjem alkoholu a menstruační cyklus
- Doporučení pro diagnostiku a léčbu akutních jaterních porfyrií
- Doc. Miloš Kubánek: Nemocní se srdeční amyloidózou jsou často skryti a sledováni pod jinými diagnózami
-
Všechny články tohoto čísla
- An Solution for Crossover Formation
- Genome-Wide Association Mapping in Dogs Enables Identification of the Homeobox Gene, , as a Genetic Component of Neural Tube Defects in Humans
- Independent Evolution of Transcriptional Inactivation on Sex Chromosomes in Birds and Mammals
- Stepwise Activation of the ATR Signaling Pathway upon Increasing Replication Stress Impacts Fragile Site Integrity
- Genomic Analysis of Natural Selection and Phenotypic Variation in High-Altitude Mongolians
- Modification of tRNA by Elongator Is Essential for Efficient Translation of Stress mRNAs
- Role of CTCF Protein in Regulating Locus Transcription
- Gene Set Signature of Reversal Reaction Type I in Leprosy Patients
- Mapping of PARK2 and PACRG Overlapping Regulatory Region Reveals LD Structure and Functional Variants in Association with Leprosy in Unrelated Indian Population Groups
- Is Required for Formation of the Genital Ridge in Mice
- Monopolin Subunit Csm1 Associates with MIND Complex to Establish Monopolar Attachment of Sister Kinetochores at Meiosis I
- Recombination Dynamics of a Human Y-Chromosomal Palindrome: Rapid GC-Biased Gene Conversion, Multi-kilobase Conversion Tracts, and Rare Inversions
- Mechanisms of Protein Sequence Divergence and Incompatibility
- Histone Methyltransferase DOT1L Drives Recovery of Gene Expression after a Genotoxic Attack
- Female Behaviour Drives Expression and Evolution of Gustatory Receptors in Butterflies
- Combinatorial Regulation of Meiotic Holliday Junction Resolution in by HIM-6 (BLM) Helicase, SLX-4, and the SLX-1, MUS-81 and XPF-1 Nucleases
- The bHLH Subgroup IIId Factors Negatively Regulate Jasmonate-Mediated Plant Defense and Development
- The Role of Interruptions in polyQ in the Pathology of SCA1
- Dietary Restriction Induced Longevity Is Mediated by Nuclear Receptor NHR-62 in
- Fine Time Course Expression Analysis Identifies Cascades of Activation and Repression and Maps a Putative Regulator of Mammalian Sex Determination
- Genome-scale Co-evolutionary Inference Identifies Functions and Clients of Bacterial Hsp90
- Oxidative Stress and Replication-Independent DNA Breakage Induced by Arsenic in
- A Moonlighting Enzyme Links Cell Size with Central Metabolism
- Budding Yeast Greatwall and Endosulfines Control Activity and Spatial Regulation of PP2A for Timely Mitotic Progression
- The Conserved Intronic Cleavage and Polyadenylation Site of CstF-77 Gene Imparts Control of 3′ End Processing Activity through Feedback Autoregulation and by U1 snRNP
- The BTB-zinc Finger Transcription Factor Abrupt Acts as an Epithelial Oncogene in through Maintaining a Progenitor-like Cell State
- The Cohesion Protein SOLO Associates with SMC1 and Is Required for Synapsis, Recombination, Homolog Bias and Cohesion and Pairing of Centromeres in Drosophila Meiosis
- The RNA-binding Proteins FMR1, Rasputin and Caprin Act Together with the UBA Protein Lingerer to Restrict Tissue Growth in
- Pattern Dynamics in Adaxial-Abaxial Specific Gene Expression Are Modulated by a Plastid Retrograde Signal during Leaf Development
- A Network of HMG-box Transcription Factors Regulates Sexual Cycle in the Fungus
- Bacterial Adaptation through Loss of Function
- ENU-induced Mutation in the DNA-binding Domain of KLF3 Reveals Important Roles for KLF3 in Cardiovascular Development and Function in Mice
- Interplay between Structure-Specific Endonucleases for Crossover Control during Meiosis
- FGF Signalling Regulates Chromatin Organisation during Neural Differentiation via Mechanisms that Can Be Uncoupled from Transcription
- The Arabidopsis RNA Binding Protein with K Homology Motifs, SHINY1, Interacts with the C-terminal Domain Phosphatase-like 1 (CPL1) to Repress Stress-Inducible Gene Expression
- Selective Pressures to Maintain Attachment Site Specificity of Integrative and Conjugative Elements
- The Conserved ADAMTS-like Protein Lonely heart Mediates Matrix Formation and Cardiac Tissue Integrity
- The cGMP-Dependent Protein Kinase EGL-4 Regulates Nociceptive Behavioral Sensitivity
- RBM5 Is a Male Germ Cell Splicing Factor and Is Required for Spermatid Differentiation and Male Fertility
- Disease-Related Growth Factor and Embryonic Signaling Pathways Modulate an Enhancer of Expression at the 6q23.2 Coronary Heart Disease Locus
- Yeast Pol4 Promotes Tel1-Regulated Chromosomal Translocations
- A Dual Role for SOX10 in the Maintenance of the Postnatal Melanocyte Lineage and the Differentiation of Melanocyte Stem Cell Progenitors
- SLC26A4 Targeted to the Endolymphatic Sac Rescues Hearing and Balance in Mutant Mice
- Odoriferous Defensive Stink Gland Transcriptome to Identify Novel Genes Necessary for Quinone Synthesis in the Red Flour Beetle,
- Prediction of Complex Human Traits Using the Genomic Best Linear Unbiased Predictor
- Gene × Physical Activity Interactions in Obesity: Combined Analysis of 111,421 Individuals of European Ancestry
- Reassembly of Nucleosomes at the Promoter Initiates Resilencing Following Decitabine Exposure
- Exquisite Light Sensitivity of Cryptochrome
- miR-133a Regulates Adipocyte Browning In Vivo
- Strabismus Promotes Recruitment and Degradation of Farnesylated Prickle in Planar Polarity Specification
- Hepatocyte Growth Factor Signaling in Intrapancreatic Ductal Cells Drives Pancreatic Morphogenesis
- Is a Potential Tumor Suppressor Gene Commonly Inactivated by Epigenetic Mechanisms in Colorectal Cancer
- Joint Molecule Resolution Requires the Redundant Activities of MUS-81 and XPF-1 during Meiosis
- The Mating Competence of Geographically Diverse Strains in Their Natural and Unnatural Sand Fly Vectors
- Defective Repair of Oxidative Base Lesions by the DNA Glycosylase Nth1 Associates with Multiple Telomere Defects
- Effective Blocking of the Enhancer Requires Cooperation between Two Main Mechanisms Suggested for the Insulator Function
- Trans-Ancestral Studies Fine Map the SLE-Susceptibility Locus
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- SLC26A4 Targeted to the Endolymphatic Sac Rescues Hearing and Balance in Mutant Mice
- Bacterial Adaptation through Loss of Function
- The Cohesion Protein SOLO Associates with SMC1 and Is Required for Synapsis, Recombination, Homolog Bias and Cohesion and Pairing of Centromeres in Drosophila Meiosis
- Gene × Physical Activity Interactions in Obesity: Combined Analysis of 111,421 Individuals of European Ancestry
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



