-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Decline of FoxP3+ Regulatory CD4 T Cells in Peripheral Blood of Children Heavily Exposed to Malaria
In malaria endemic regions, immunity is slow to develop and does not provide substantial protection against reinfection. Rather, following repeated exposure, older children and adults eventually develop protection from most symptomatic manifestations of the infection. This may be due in part to the induction of immunoregulatory mechanisms by the P. falciparum parasite, such as FoxP3+ regulatory T cells (Tregs). Prior human studies have shown that Tregs are induced by malaria parasites both in vivo and in vitro, but the role of these cells in immunity in children who are chronically exposed to malaria remains unclear. In this study, we assessed the frequency and features of Tregs among children from areas of high malaria transmission in Uganda. We found that this regulatory T cell population declined markedly with increasing malaria episodes. This loss was associated with decreased expression of TNFR2, which is a protein implicated in stability of Tregs. Additionally, T cells from highly malaria exposed children demonstrated a reduced propensity to differentiate into Tregs following parasite stimulation. Together our data suggest that repeated episodes of malaria alter Treg homeostasis, which may influence the development of immunity to malaria in children.
Published in the journal: . PLoS Pathog 11(7): e32767. doi:10.1371/journal.ppat.1005041
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1005041Summary
In malaria endemic regions, immunity is slow to develop and does not provide substantial protection against reinfection. Rather, following repeated exposure, older children and adults eventually develop protection from most symptomatic manifestations of the infection. This may be due in part to the induction of immunoregulatory mechanisms by the P. falciparum parasite, such as FoxP3+ regulatory T cells (Tregs). Prior human studies have shown that Tregs are induced by malaria parasites both in vivo and in vitro, but the role of these cells in immunity in children who are chronically exposed to malaria remains unclear. In this study, we assessed the frequency and features of Tregs among children from areas of high malaria transmission in Uganda. We found that this regulatory T cell population declined markedly with increasing malaria episodes. This loss was associated with decreased expression of TNFR2, which is a protein implicated in stability of Tregs. Additionally, T cells from highly malaria exposed children demonstrated a reduced propensity to differentiate into Tregs following parasite stimulation. Together our data suggest that repeated episodes of malaria alter Treg homeostasis, which may influence the development of immunity to malaria in children.
Introduction
FoxP3+ regulatory CD4 T cells (Tregs) play a central role in preventing autoimmunity and maintaining self-tolerance. In the setting of infection, Tregs help to maintain the delicate balance between pathogen-specific immunity and immune-mediated pathology. Preserving this equilibrium requires a complicated balance between regulatory and effector T cell activity. For instance, in the murine leishmania model, Treg-mediated suppression of effector immune responses interferes with complete parasite clearance—but paradoxically, the resulting pathogen persistence fosters the long-term maintenance of effector immune responses that are required for protection from reinfection [1,2]. Given their central role in immunoregulation, the timing, magnitude, and duration of Treg activity must be fine-tuned for promote resolution of the effector immune response only after control of the pathogen has been achieved. Malaria, like many other parasite infections, has been reported to induce an expansion of the Treg population [3]. However, the factors governing Treg homeostasis in the setting of P. falciparum infection, which in high transmission regions is characterized by both recurrent symptomatic episodes in young children and persistent asymptomatic infection in older individuals, remain unclear, as does the role of Tregs in the immunopathogenesis of malaria.
P. falciparum infection in humans induces multiple immunoregulatory pathways that likely evolved to protect the host from severe malaria by down-modulating the acute inflammatory response, perhaps at the cost of interfering with clearance of parasitemia and development of immunologic memory. Several lines of evidence suggest that Tregs are induced during human P. falciparum infection and play a role in modulating the host response. Following experimental P. falciparum sporozoite infection of naïve human subjects, FOXP3 mRNA is upregulated and peripheral blood CD25+CD4+ T cells expand [4]. In rural Gambia, the percentage and absolute count of FoxP3+CD127low CD4 T cells were shown to increase following the malaria transmission season, and are significantly higher among malaria-exposed rural Gambians than among ethnically matched urban Gambians with no malaria exposure [5]. Moreover, a number of studies have shown that peripheral Treg frequencies correlate with parasite burden in infected individuals [6–8]. Together these data suggest that Tregs are induced by P. falciparum infection in vivo. This conclusion is further supported by in vitro studies demonstrating that FoxP3+ Tregs are induced by co-culture of PBMC with P. falciparum-infected red blood cells or parasite schizont extracts [9–13].
Induction of Tregs by parasite antigens may have implications for the development of a host-protective immune response. FOXP3 mRNA levels in children with acute malaria have been shown to correlate inversely with the magnitude of the subsequent Th1 memory response to P. falciparum measured 28 days after infection [6]. Similarly, FOXP3 expression among malaria-naive adults following experimental sporozoite vaccination correlates inversely with the subsequent Th1 memory response [14]. It is possible that P. falciparum induction of Tregs may contribute to the failure of the adaptive immune response to mediate parasite clearance, as has been demonstrated in other parasitic infections such as leishmania and filariasis [1,2,15]. However, the role of Tregs in protection or risk from symptomatic malaria remains unclear. High frequencies of CD25high T cells (putatively regulatory T cells) were associated with increased risk of malaria in one prospective cohort study [16]. Consistent with this, among previously naïve adults experimentally infected with malaria, Treg induction was associated with increased parasite replication rates [4]. Further, a recent study in children and adults in Indonesian Papua found a trend towards lower proportions of activated Tregs in individuals who had asymptomatic infection compared to symptomatic malaria or healthy controls, suggesting dampened activation of Tregs may be associated with decreased risk of disease [17]. However, it has also been suggested that Tregs may serve a protective role in preventing immunopathology during infection [18,19]. Murine studies have failed to provide clear resolution of this issue, as different models have yielded conflicting data. Early reports described enhanced control of parasitemia and improved survival in mice experimentally depleted of Tregs [20], but subsequent studies that used more precise definitions of Tregs, different depletion regimens, or different parasite strains have failed to demonstrate a consistent host-protective role (summarized in [19]).
To better understand the role of Tregs in the immunopathogenesis of malaria in the setting of chronic exposure, we assessed the frequencies and phenotypic features of Tregs in Ugandan children of varying ages and malaria exposure histories. Our results indicate that while Treg frequencies are expanded in a high compared to low transmission settings, in high transmission settings children with repeated malaria infection experience a marked and progressive decline in peripheral blood Tregs, accompanied by reduced in vitro induction of Tregs by parasite antigen and decreased expression of TNFR2. This loss of circulating Tregs may have implications for the development of protective immunity to malaria, and suggests that chronic antigen stimulation, such as that observed in areas of chronic Plasmodium infection, may result in pathogen-driven alteration of Treg homeostasis.
Results
The frequency of FoxP3+ regulatory CD4 T cells in peripheral blood declines with increasing prior malaria exposure
To investigate the relationship between Treg frequencies and prior malaria exposure, we measured peripheral blood Treg frequencies in 2 separate cohorts of children in the high malaria transmission region of Tororo District, Uganda (annual entomological inoculation rate (aEIR) 310 bites ppy [21]). In both cohorts, participants were followed prospectively from enrollment at approximately 6 months of age, with comprehensive documentation of all malaria episodes at a dedicated study clinic, and at the time of analysis were either 2 years old (PROMOTE cohort, no chemoprevention control arm, n = 82) or 4 years old (TCC cohort, n = 75) (S1 Table). Treg frequencies were measured as the percentage of CD4 T cells that were FoxP3+CD25+ (for gating strategy see S1 Fig). Within both the 2-year-old and 4-year-old cohorts, there was a strong inverse relationship between Treg frequencies and prior malaria incidence (Spearman’s r = -0.27, p = 0.01, and r = -0.28, p = 0.01 respectively; Fig 1A and 1B). This inverse relationship was strengthened by further gating on the CD127dim subset, which more stringently defines suppressive Tregs (Spearman’s r = -0.36, p = 0.001; assessed in 4-year-old cohort only, for gating strategy see S1B Fig). The frequency of Tregs among children who had asymptomatic P. falciparum infection at the time of assessment (determined by blood smear) did not differ from that of uninfected children (Wilcoxon ranksum p = 0.951). Furthermore, there was no relationship between the frequency of Tregs and the duration of time since the last malaria episode, which might be expected if Tregs transiently increase in response to acute malaria (Spearman’s r = 0.094, p = 0.422), similar to what has been shown in malaria-naïve adults [4]. We measured CD4 T cell responses to P. falciparum-infected red blood cells from blood samples obtained concurrently (TCC cohort, n = 56), but we observed no statistical relationship between the frequency of Tregs and other effector or regulatory T cell populations, including cells producing IFNγ (p = 0.65), TNFα (p = 0.17), or the recently described IL10-producing “self-regulatory” CD4 T cells (p = 0.99) [22–25].
Fig. 1. Regulatory T cells decline with increasing prior malaria incidence and mosquito exposure among children in a high transmission setting. 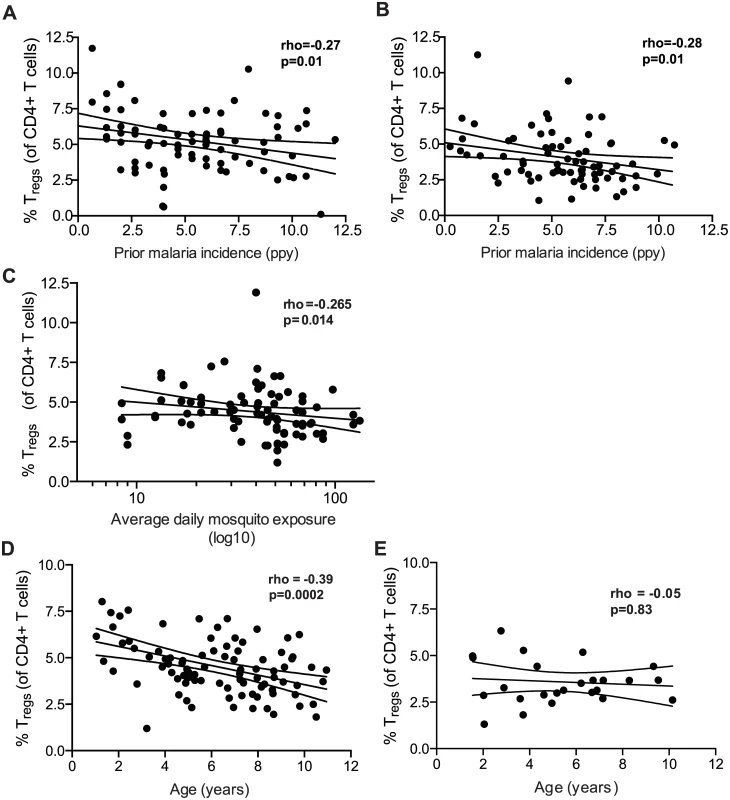
Regulatory T cell frequencies were analyzed as the percentage of FoxP3+CD25+ of CD4+ T cells from (A) fresh whole blood in 2-year old (PROMOTE-cohort, no chemoprevention control arm) and (B) and frozen PBMCs from 4-year olds (TCC) and the association with prior malaria incidence analyzed. In both 2 and 4 year olds, Treg frequencies declined with increasing prior malaria incidence. (C/D) Regulatory T cell frequencies, analyzed as the percentage of FoxP3+CD25+CD127dim of CD4+ T cells from 1 to 11 year old children (PRISM cohort, high transmission Nagongera, Tororo District), declined with increasing mean daily household mosquito exposure (from monthly CDC light traps) (C) and age (D). (E) The relationship between Treg frequencies and age was analyzed in children from the low transmission Jinja District; there was no decline in Treg frequencies with age in children from the low malaria transmission settings. For all analyses, Spearman’s rho and p are indicated. The cross-sectional data above are consistent with either a malaria-driven decline in peripheral Treg frequencies or an increased susceptibility to symptomatic malaria among children whose Treg frequencies are inherently low. To distinguish between these possibilities, we measured Treg frequencies in a third cohort of children residing in the same high transmission Nagongera, Tororo District (PRISM cohort, age 1 to 11 years, n = 91 [21]), in whom mosquito exposure was directly measured using CDC light traps within the homes of individual cohort participants [26]. In this cohort, we observed an inverse relationship between Treg frequencies and mean daily household mosquito exposure, consistent with a parasite-driven decline in Tregs (Spearman’s rho = -0.265, p = 0.014, Fig 1C). In contrast to the younger cohorts of children described above, we did not observe an inverse correlation between Tregs and the incidence of prior clinical malaria in this cohort (Spearman’s rho = 0.043, p = 0.685), likely because older children do not develop symptomatic clinical malaria with each P. falciparum infection, and thus malaria incidence is not a good measure of total P. falciparum exposure beyond early childhood. There was, however, a strong inverse relationship between Tregs and age (Spearman’s rho = -0.385, p = 0.0002; Fig 1D), suggesting that Tregs progressively decline with age in this high endemnicity setting. This decline was not attributable to age-related changes in total lymphocyte counts, as a similar relationship was observed when absolute numbers of Tregs (per μl of blood) were calculated by normalization to absolute CD4 cell counts in a subset of children (r = -0.424, p = 0.025; n = 28, S2 Fig). To investigate whether the age-related decline in Treg frequencies was unique to this high malaria transmission setting, we compared Treg frequencies among children age 1.5 to 11 years who were enrolled in the observational malaria cohort (PRISM), but at the low transmission Jinja District (aEIR 2.8 bites ppy [21]). Among children at the lower transmission site, Treg frequencies did not decline with age (r = -0.05, p = 0.83; n = 34; Fig 1E). Together these data suggest that exposure to malaria parasites may contribute to a loss of peripheral blood Tregs in this high transmission setting.
Longitudinal decline in Treg frequencies within children correlates with higher intercurrent malaria exposure
To investigate whether changes in Treg frequencies within individual subjects over time correlates with their malaria incidence, we measured Treg frequencies longitudinally in 41 subjects at 16, 24 and 36 months of age (PROMOTE cohort, SP chemoprevention arm). Subjects were stratified into tertiles based on their number of malaria infections between 16 and 36 months. Among children in the highest tertile of malaria incidence (n = 14), there was a consistent decline in Treg frequencies between 16 and 36 months of age (Wilcoxon signed rank test, p = 0.009, Fig 2A). In contrast, among children in the lowest tertile of incidence (n = 13), there was no change in Treg frequencies between 16 and 36 months (Wilcoxon signed rank test p = 0.54). Repeated-measures analysis using generalized estimating equations confirmed that changes in Treg frequencies over time differed between the exposure groups (p = 0.0011, Fig 2B). Together, these data suggest that very high malaria exposure during childhood results in the loss of peripherally circulating Tregs within individuals over time.
Fig. 2. Regulatory T cells decrease over time in individuals with high but not low malaria incidence. 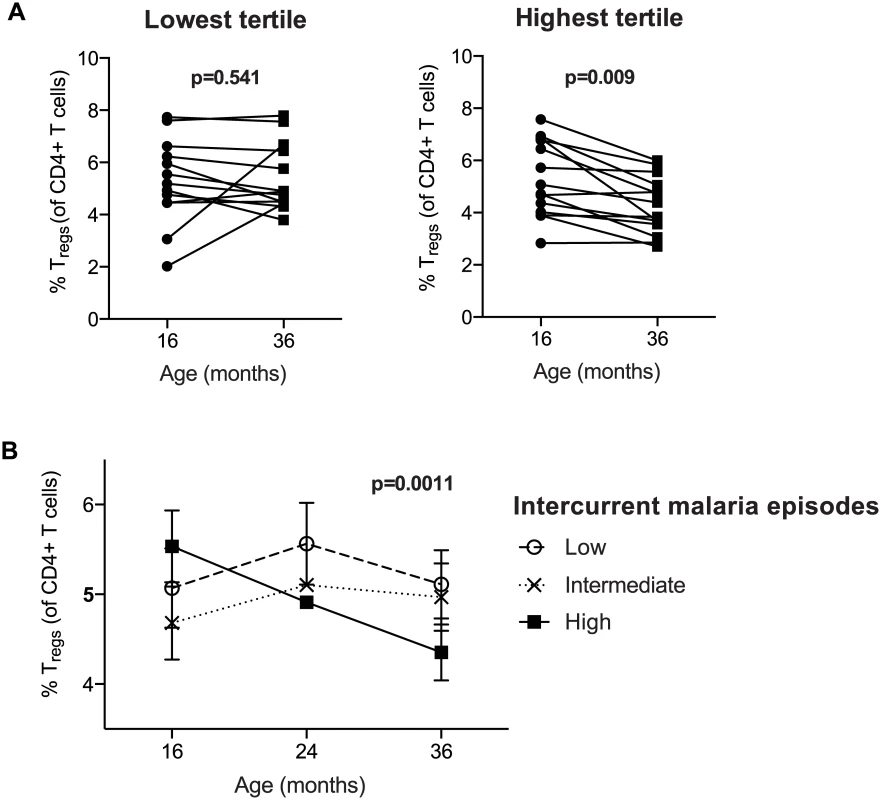
Treg frequencies of CD4+ T cells (FoxP3+CD25+CD127dim) were measured at 16, 24 and 36 months of age (PROMOTE- SP arm). (A) Children were divided into tertiles based on incidence of malaria between 16 and 36 months; lowest tertile median incidence 4.0 episodes ppy (IQR 3.0–5.5); intermediate tertile median incidence 8.2 episodes ppy (IQR 7.3–9.2); highest tertile median incidence 13.7 episodes ppy (IQR 10.3–14.6). The median duration since last malaria infection in these three tertiles was 8.5, 20, and 100 days, respectively. Wilcoxon matched pairs signed rank test p values indicated. Between 16 and 36 months, Treg frequencies declined in individuals in the highest but not lowest tertile of malaria incidence. (B) Changes in Treg frequencies between 16, 24 and 36 months were compared between children in the lowest, intermediate, and highest tertiles of malaria exposure by generalized estimate equations, accounting for repeated measures, age, duration since last malaria episode and parasite status at time of sampling. Treg dynamics during childhood differ between high and low malaria transmission settings
Prior studies have shown that experimental and natural P. falciparum infection induces the expansion of regulatory T cells in vivo [4,5]. To investigate whether and how Treg dynamics differ between settings of high and low exposure, we compared Treg frequencies between children over a range of ages in the high transmission district of Tororo to children from the low transmission district of Jinja (PRISM cohort age 1 to 11 years). Children in the high transmission Tororo District experienced a much higher malaria incidence (median 3.6 vs. 0 ppy) and a much shorter duration since last infection (median 62 vs. 230 days) than children in the low transmission Jinja District (full details in S1 Table). Overall, Treg frequencies (FoxP3+CD25+CD127dim) were higher in children from the high transmission district compared to the lower transmission district across all age groups (Wilcoxon ranksum p<0.0001, Fig 3A), and this difference was most marked in the youngest age group (Fig 3B), possibly reflecting an earlier expansion of Tregs in response to initial infections during early childhood or even in utero [27–30]. The difference in Tregs frequencies between the low and high transmission study sites decreased with increasing age, and this trend extended to adulthood (Fig 3B). Thus, our data suggest that in areas of high malaria transmission malaria infections early in life induce Tregs, as has been previously described among naïve or comparatively low-exposure individuals [4,5,7,8]. However, in areas of intense and continual malaria exposure, parasite-driven induction of Tregs is diminished, and instead there is a progressive decline of Tregs with repeated malaria episodes. This decline does not appear to be transient, as Treg frequencies do not correlate with the duration of time since last malaria episode or asymptomatic parasite infection. Instead, there appears to be sustained and progressive loss of Tregs with age among children heavily exposed to malaria.
Fig. 3. FoxP3+ regulatory T cells are increased in high compared to low transmission settings, but decrease with age only in highly exposed children. 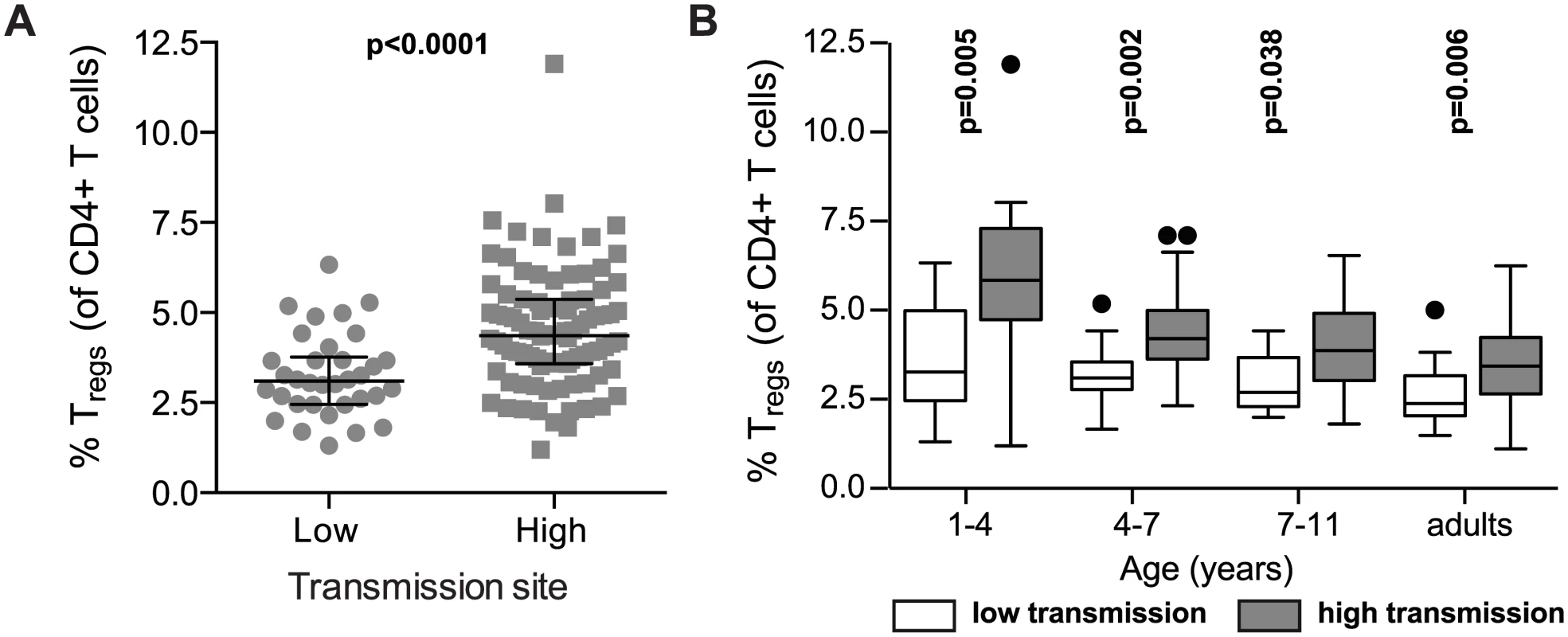
(A) FoxP3+CD25+CD127dim regulatory T cell frequencies in children age 1 to 11 years (PRISM cohort) residing in the low transmission Jinja District (n = 34) were compared to children in the high transmission Tororo District (n = 91). Wilcoxon ranksum p value indicated. (B) FoxP3+CD25+CD127dim regulatory T cell frequencies were compared between children from low and high transmission areas at age 1–4 (n = 11 and n = 18), 4–7 (n = 14 and n = 35) and 7–11 (n = 9 and n = 38) years of age and adults (n = 9 and n = 37). Wilcoxon ranksum for age group comparisons, p values indicated. Reduced TNFR2 expression on Tregs following chronic malaria exposure
We next assessed expression of TNFR2 on Treg cells, as this receptor has been shown to be critical for both proliferative expansion of Tregs and maintenance of FOXP3 expression in inflammatory environments [31,32]. Furthermore, Tregs expressing TNFR2 have been shown to have enhanced suppressive capacity [8,33,34], and are increased during malaria infection [8]. We found that the percentage of Tregs expressing TNFR2 was significantly lower among PRISM cohort children from the high transmission Tororo District than among children of similar age from the low transmission Jinja district (p<0.0001, Fig 4A, see S3 Fig for gating strategy). Among Tororo children, expression of TNFR2 was inversely correlated with number of recent malaria episodes (Coef = -0.31, p = 0.032, Fig 4B), although expression was slightly higher on Tregs from children currently PCR-positive for P. falciparum infection (p = 0.043). These data suggest that TNFR2 expression is transiently up-regulated during parasitemia but declines over time following repeated malaria episodes. This decrease in TNFR2 expression could contribute to the loss of FoxP3+ Tregs from peripheral blood by decreasing the stability of FOXP3 expression [31,32].
Fig. 4. TNFR2 expression on FoxP3+ regulatory T cells declines with increasing prior malaria incidence. 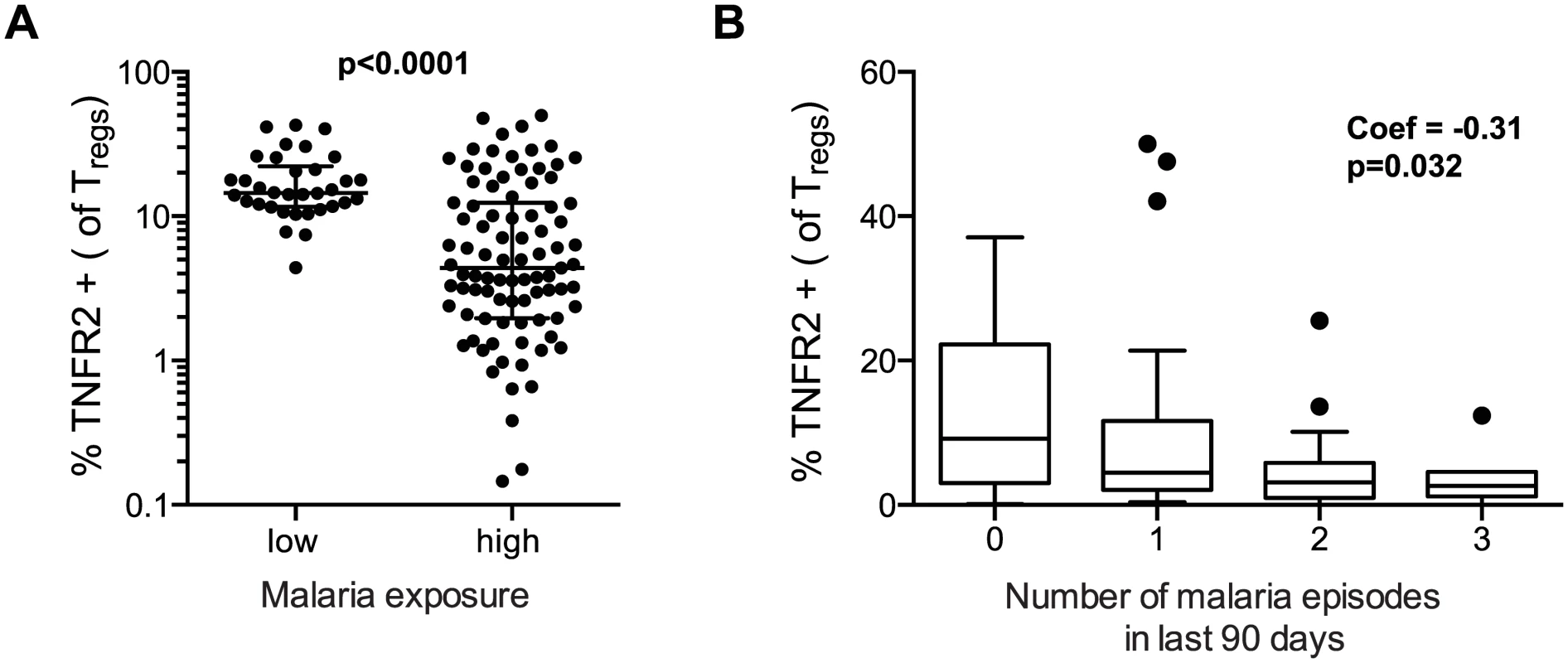
A. Frequencies of TNFR2 expressing Tregs (FoxP3+CD25+CD127dim) were compared between children in the high malaria transmission area (Tororo District) and in the low transmission area (Jinja district). TNFR2 expression on Tregs was higher in low compared to high transmission settings. Wilcoxon signed rank test indicated. B. The association between frequencies of TNFR2 expressing Tregs and number of recent malaria episodes was analyzed among children from high malaria exposure area (Tororo District). TNFR2 expression declined with increasing number of malaria episodes in last 90 days. Regression coefficient and p value are indicated. Altered homeostasis of Tregs following intense malaria exposure
The progressive decline in circulating Tregs in children heavily exposed to malaria could be explained by changes in Treg homeostasis such as decreased induction, increased loss (due to apoptosis or downregulation of FOXP3), or both. Therefore, we next examined whether heavy prior malaria infection altered the propensity for Treg induction or apoptosis. It has previously been shown that in vitro stimulation of adult PBMCs with P. falciparum antigen induces regulatory T cells [9,10,12,35]. To investigate whether the propensity of CD4 T cells to differentiate into Tregs in response to parasite antigen is influenced by age and/or prior malaria exposure, we measured induction of Tregs following in vitro stimulation with P. falciparum schizont extracts (PfSE) in malaria-naïve adults, malaria-exposed children (28 months of age), and malaria-exposed adults from the high incidence district of Tororo (gating strategy and ex vivo Treg frequencies shown in S4 Fig). As previously reported, co-culture of PBMCs from naïve adults with PfSE resulted in consistent induction of Tregs (Fig 5A and S4C Fig). However, using PBMC from malaria-exposed children and adults, induction of Tregs was reduced compared to naïve adults (Fig 5A). Further, whereas all children with low prior malaria exposure (<2 episodes ppy) exhibited Treg induction (fold change >1), only 55% of children with high prior malaria exposure (>6 episodes ppy) induced Tregs following PfSE stimulation (p = 0.03; Fig 5B). This suggests that heavy prior exposure to malaria may limit the propensity of CD4 cells to differentiate into Tregs upon re-encounter with parasite antigens.
Fig. 5. Evidence for changed homeostasis of Tregs in children with high malaria exposure. 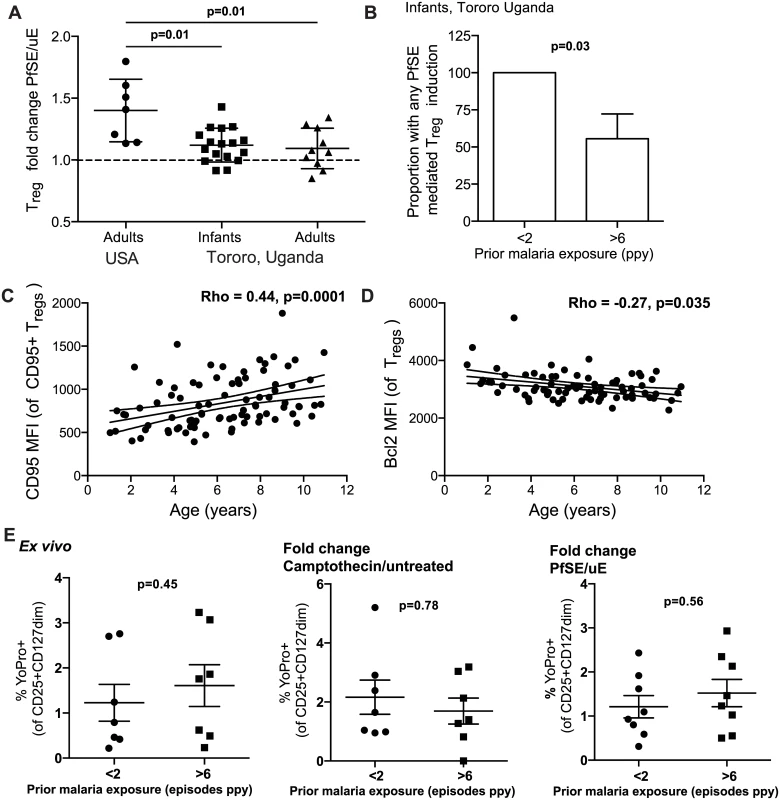
A. PBMCs from 28 month old children (PROMOTE no chemoprevention control arm), malaria-exposed adults (PRISM cohort, Nagongera, Tororo District) and naïve adults were incubated for 7 days with protein extract from mature stage P. falciparum infected RBCs (PfSE) or uninfected RBCs (uE). Treg frequencies were enumerated (FoxP3+CD25+CD127dim) and induction factor was calculated based on frequency fold change between PfSE and uE stimulated PBMCs. Parasite induction of Tregs was reduced in exposed compared to naïve individuals. Wilcoxon signed rank test indicated for comparison between naïve-adults and exposed infant and adult samples. B. The proportion of children with any Treg induction by PfSE was compared between those with low (<2 episodes ppy, n = 10) and high (>6 episodes ppy, n = 10) prior malaria incidence. There was a reduced proportion of infants with any Treg induction in those who had high compared to low prior malaria incidence. Chi-square test indicated. C. Pro-apoptotic marker CD95 was measured on Tregs in children (PRISM cohort) from the high transmission area (Tororo District). Association between the level of CD95 expression on Tregs, as measured by MFI of CD95+ Tregs, and age was analyzed. The level of CD95 expression increased with age. D. Pro-survival Bcl2 was measured on Tregs in children (PRISM cohort, Nagongera, Tororo) from the high transmission area. Association between the expression of Bcl2 on the Treg population and age was analyzed. The level of Bcl2 expression decreased with age. Spearman’s rho and p indicated. E. YoPro staining of Tregs from 28 month old children (PROMOTE, no chemoprevention control arm, with low (<2 episodes ppy) and high (>6 episodes ppy) prior malaria incidence was measured ex vivo and following stimulation with camptothecin (an activator of apoptosis) or P. falciparum antigen. Data from YoPro staining is representative of all measures of apoptosis (see S5 Fig additional measures of apoptosis including activated Caspase 3 and AnnexinV). There was no difference in sensitivity to apoptosis between infants with low and high prior malaria incidence. Wilcoxon signed rank test indicated. We next investigated whether heavy prior malaria exposure increased the susceptibility of Tregs to apoptosis, as has been shown in chronic HIV-1 infection [36]. The percentage of Tregs expressing the pro-apoptotic marker CD95 increased with age among Tororo children (Rho = 0.175, p = 0.079), as did the level of CD95 expression (as calculated by MFI of CD95 on CD95+ Tregs, Spearman’s Rho = 0.44, p = 0.0001) (Fig 5C). Conversely, expression of the anti-apoptotic marker Bcl2 on Tregs declined with age (Rho = -0.266 p = 0.035) (Fig 5D). However there was no independent relationship between expression of these markers and prior malaria incidence, current parasite infection, nor time since last malaria episode, suggesting that age may independently affect the sensitivity of Tregs to apoptosis. To further investigate this, three distinct measures of apoptosis (YoPro, Annexin V and activated Caspase 3) were measured on Tregs both ex vivo and following stimulation with camptothecin (an activator of apoptosis) or PfSE in 28-month infants with low or high prior malaria incidence (PROMOTE no-chemoprevention control arm). There was no difference in sensitivity to apoptosis as measured by any of the markers either ex vivo or following stimulation with camptothecin or parasite antigen; the frequencies of positively stained cells ex vivo, and the fold change of apoptosis staining, were the same regardless of prior malaria exposure (Fig 5E and S5 Fig). Together these data suggest that Treg homeostasis may be altered in the setting of heavy malaria exposure, in part due to reduced induction of peripheral Treg cells, with little evidence for increased susceptibility to antigen-driven apoptosis.
Lower Treg frequencies may be associated with a decreased risk of symptoms following parasite infection
We finally asked whether the frequency of circulating Tregs influences susceptibility to malaria. We assessed the influence of Treg frequencies on protection from malaria in both the 2-year-old PROMOTE no chemoprevention control arm and 4-year-old TCC cohorts using two methods; a time-to-event analysis (time to next malaria episode), and negative binomial regression of the relationship of Treg frequencies to malaria incidence in the year following assessment. Among 2-year-olds, we found that higher Treg frequencies were associated with an increased time to next malaria episode and a lower future malaria incidence in univariate analysis (Table 1). However, after adjusting for prior malaria incidence in a multivariate model to account for heterogeneity in environmental exposure to infected mosquitoes [22,37,38], this relationship was no longer significant, suggesting that differences in environmental exposure intensity may underlie this association [37]. Among 4-year-olds, Treg frequencies were not associated with time to next malaria infection or malaria incidence during follow-up. Similarly, no relationships between Treg frequencies and malaria incidence in follow-up or time to next malaria episode were observed in the PRISM 1–11 year old cohorts, in either the low or the high transmission study sites, even after adjustment for household mosquito exposure. Thus we did not find clear evidence that Tregs are associated with the risk of clinical malaria.
Tab. 1. Relationship between Treg frequencies and prospective risk of malaria. 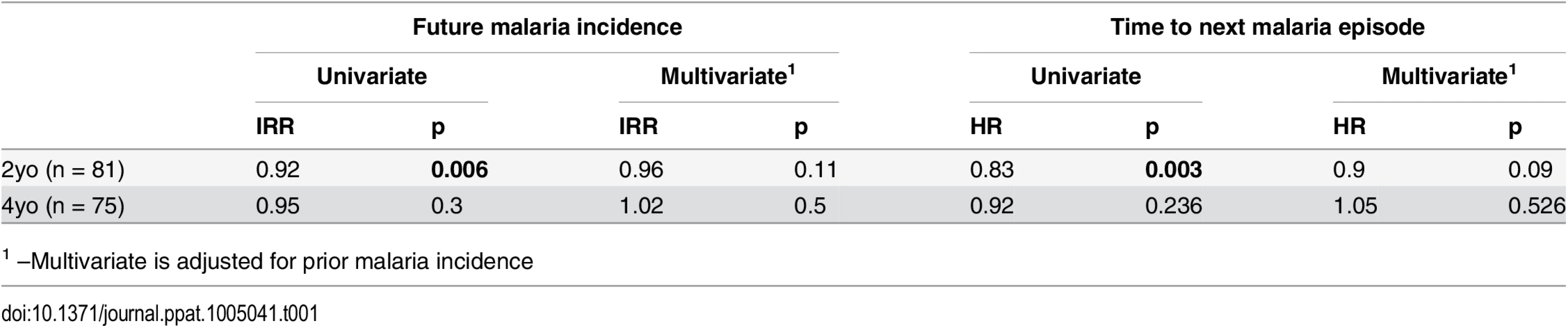
1 –Multivariate is adjusted for prior malaria incidence Given their immunoregulatory role, is also possible that Tregs play a role in protecting the host from the symptomatic manifestations of malaria once P. falciparum infection is established [1,2,15,19]. To assess whether Tregs may influence the risk of clinical disease once infected, we analyzed the relationship between Treg frequencies and the probability of symptoms once parasitemic using generalized estimate equations with robust standard errors, accounting for repeated measures [16,39]. Comparing children with the lowest compared to highest tertiles of Tregs, lower Treg frequencies were associated with an increased monthly probability of infection, consistent with the exposure induced decline in Tregs described. However, lower Treg frequencies were also associated with an overall decreased probability of becoming symptomatic once infected (2 year old PROMOTE cohort; OR = 0.4, p = 0.039, 4 year old TCC cohort; OR = OR = 0.37, p = 0.06), suggesting that the decline in circulating Tregs may be associated with the acquisition of clinical immunity.
Discussion
Here, we have shown through both cross-sectional and longitudinal studies that the percentage and absolute number of FoxP3+ Tregs in peripheral blood are influenced by repeated exposure to malaria. While children in settings of intense exposure have higher Treg frequencies during early childhood, frequencies decline throughout childhood in settings of high (but not low) exposure, and the extent of Treg loss correlates with the intensity of P. falciparum exposure. We provide both in vivo and in vitro evidence that among children in high exposure settings, there is a reduction of parasite induced Treg expansion during infection. Further, we show a down-regulation of TNFR2, which is required for stabilization of the FoxP3+ regulatory phenotype in inflammatory environments [31]. These data demonstrate that chronic exposure to malaria results in altered Treg homeostasis in vivo, which may have a downstream impact on the acquisition of immunity and control of infection.
Our data indicate that the dynamics of Treg induction and homeostasis differ markedly between high and low malaria transmission settings. Although children residing in high transmission areas had higher Treg frequencies overall, perhaps in response to a parasite-driven Treg expansion in early childhood or in utero [27–30], we observed a marked decline in Treg frequencies beginning at 1–2 years of age, which appeared to be driven by persistent parasite exposure. Further, among highly exposed children, we saw no association between Treg frequencies and current or recent infection, suggesting that Tregs have reduced in vivo induction during infection of these chronically exposed children. Consistent with this, we demonstrated that induction of Tregs following parasite stimulation of PBMC was diminished in heavily exposed adults and children, providing in vitro evidence that chronic antigen exposure may blunt the proliferative expansion of Tregs in response to malaria. This is in contrast to published studies suggesting that Tregs expand in response to malaria in vivo and in vitro [4,7–13,18,19]. The most likely explanation for the difference in our findings is that these earlier studies were performed largely on malaria-naïve volunteers or relatively low-exposed populations. Overall our data suggest that while Tregs may be induced in initial encounters with parasites, induction capacity is diminished after repeated parasite exposure and instead Tregs undergo a steady decline in the periphery. The induction of Tregs by Plasmodium is believe to occur through activation of latent membrane-bound TGFβ [11,40], which can be blocked by antibodies to the P. falciparum thrombospondin-related adhesive protein (PfTRAP) [11]. This Treg induction mechanism is shared by related protozoal pathogens Toxoplasma and Leishmania [35] and may represent an immune evasion strategy. The reduced capacity of parasite antigen to induce Tregs in heavily malaria exposed children suggests that the host may be able to circumvent parasite induction of Tregs, potentially enabling enhanced control of infection.
Another potential mechanism for the observed decline in Tregs among children chronically exposed to malaria is via loss of FOXP3 expression by “unstable” Tregs, which has been reported to occur in highly inflammatory immune environments [41–45]. Sustained expression of the canonical transcription factor FOXP3 by Tregs is critical for maintenance of regulatory function [46]. Several recent studies suggest that Tregs can become “unstable” and lose FOXP3 expression in response to cues in the microenvironment, although the significance, extent, and triggers of this phenomenon remain subject to considerable debate [44,45,47–52]. Lineage tracking experiments have elegantly shown that antigen-driven activation and inflammation can drive a subset of FoxP3hi Tregs to lose both FOXP3 expression and suppressor function [44], and even acquire an effector phenotype [53]. Further, repeated TCR stimulation leads to the loss of FOXP3 expression and the conversion to pro-inflammatory cytokine producing cells in natural Tregs in vitro [54]. Our data suggest a potential mechanism for such Treg destabilization in malaria infection, as recurrent exposure resulted in down-regulated Treg expression of TNFR2, which has been shown to be critical for both the proliferative expansion of Tregs and stabilization of their FOXP3 expression in inflammatory environments [31,32,55]. In the setting of malaria, TNFR2+ Tregs have previously been shown to have higher FOXP3 expression and enhanced suppressive function [8]. Thus our data are consistent with mounting evidence suggesting that peripherally induced Tregs have significant plasticity in response to inflammatory environments such as that observed in malaria infection, which may culminate in loss of FOXP3 expression and suppressive function.
Several additional processes might contribute to the observed loss of Tregs in peripheral blood. Because Tregs track to sites of inflammation, it is possible that Tregs induced by P. falciparum traffic to the liver, spleen, or secondary lymphoid organs during infection. Invasive sampling was not possible in our study cohorts; therefore we were unable to exclude a preferential sequestration of Tregs in tissues or lymphoid organs. However, we did not observe any statistical relationship of Treg frequencies with the presence of parasitemia, nor with the amount of time elapsed since the last P. falciparum infection, as might be expected if Tregs migrate to sites of local inflammation during active infection. Alternatively, loss of Tregs through apoptosis might contribute to their decline following repeated malaria infections. However, we observed no relationship between prior malaria incidence and the expression of the pro-apoptotic molecule CD95 or the anti-apoptotic marker Bcl2 on Tregs (after controlling for age), nor did we observe a differential susceptibility towards apoptosis ex vivo, or following in vitro re-stimulation with parasite antigens.
The observed decline in Treg frequencies with increasing prior malaria incidence contrasts with that of another regulatory T lymphocyte population, IL10-producing Th1 cells, which we have recently shown to dominate the P. falciparum-specific CD4 T cell response among heavily malaria-exposed children, including among children from both the TCC and the PRISM Nagongera, Tororo cohorts tested here [22,56]. This “autoregulatory” population consists predominantly of IL10/IFNγ co-producing cells that express the canonical Th1 transcription factor Tbet and appear to be short-lived in the periphery, exhibiting a strong association with recent infection [22]. We observed no statistical relationship between frequencies of IL10-producing Th1 cells and conventional FoxP3+ Tregs, in contrast to an earlier small cohort study that reported a positive correlation between these two regulatory cell populations [57]. This suggests that in highly exposed children, the loss of peripherally circulating Tregs is not directly compensated by increased frequencies of IL10 producing CD4 responses. In addition, we did not observe any statistical relationships between Treg frequencies and P. falciparum-specific CD4 effector responses. In prior studies, FOXP3 mRNA levels measured during acute malaria were shown to correlate inversely with the magnitude of the subsequent Th1 memory response to P. falciparum measured 28 days after infection [6]. Similarly, FOXP3 expression among malaria-naive adults following experimental sporozoite vaccination was shown to correlate inversely with the Th1 memory response measured >100 days later [14]. Thus, while Tregs are likely to influence the development of parasite-specific T cell memory responses, no relationship between these populations could be demonstrated through our concurrent measurements in peripheral blood, which maybe due in part to the chronicity of malaria exposure in these children and/or the substantial heterogeneity within the cohort with regard to time elapsed since the last infection. Furthermore, additional parameters of Treg function that cannot readily be measured in peripheral blood in large cohorts, such as suppression of T cell proliferation and modulation of APC function, are likely to influence the cellular immune response to malaria, but could not be assessed in the present study.
While our results clearly suggest that repeated malaria impacts peripherally circulating Tregs in children, the role of these cells in protection from malaria and the development of immunity remains unclear. We observed no association between Treg frequencies and future malaria incidence or time to next malaria episodes in any of our cohorts. However, our data suggest that although children with the lowest Treg frequencies had a higher monthly probability of infection, they were less likely to become symptomatic once infected compared to children with the highest Treg frequencies over the entire study period. While these data suggest that clinical immunity is acquired as Tregs decline, the role of Tregs in mediating clinical immunity remains unclear, and may not be causal—rather, declining Treg frequencies may coincide with other immune changes that mediate protection. Because all children in our study cohorts have easy access to dedicated study clinics and prompt antimalarial drug treatment, the incidence of severe malaria was extremely low, preventing assessment of the potential role of Tregs in protection from severe disease. We were similarly unable to assess the impact of Treg activity on pathogen persistence following infection, because all cases of symptomatic malaria were promptly treated with potent artemisinin-based drugs, thus altering the natural course of infection. In other protozoal infections, such as leishmaniasis and toxoplasmosis, pathogen-induced Tregs have been reported to curb the inflammatory response, allowing long-term pathogen persistence [1,2,15]. Indeed, in murine models of leishmania, pathogen persistence resulting from Treg-mediated immune suppression has been shown to be a requirement for immunity to re-infection [1]. The long-term asymptomatic maintenance of low-burden P. falciparum infection that is commonly observed among adults in high-transmission areas [58] may represent a similar phenomenon, but the role of Tregs in mediating this process is not known. Although we did not observe higher frequencies of peripheral blood Tregs among children with asymptomatic P. falciparum infection, which is not routinely treated in Uganda, this does not exclude a role for Tregs in maintaining this state of host-parasite equilibrium.
In conclusion, we observed a progressive loss of Tregs from the peripheral blood of children following chronic repeated malaria infections, accompanied by downregulation of TNFR2 and diminished in vitro induction of Tregs by parasite antigen. Together these data demonstrate that the impact of chronic malaria antigen exposure on the FoxP3+ regulatory T cell population is quite different from that of acute infection of malaria-naïve individuals. Our findings also add to mounting data suggesting that the stability and homeostasis of FoxP3+ Tregs are perturbed under highly inflammatory conditions. The implications of this pathogen-driven Treg loss for pathogen clearance, host-parasite equilibrium, and the development of clinical immunity in regions of intense malaria transmission require further investigation.
Materials and Methods
Ethics approval
Written informed consent was obtained from the adult individual or parent/guardian of all study participants. Study protocols were approved by the Uganda National Council of Science and Technology and the institutional review boards of the University of California, San Francisco, Makerere University and the Centers of Disease Control and Prevention.
Study participants
Samples for this study were obtained from children enrolled in 3 longitudinal childhood malaria cohort studies conducted in Tororo District and Jinja District of eastern Uganda. Cohort characteristics are described in S1 Table. For all cohorts, samples were selected on the bases of availability of PBMCs.
The PROMOTE-Chemoprevention Study was conducted from 2010–2013 and enrolled 400 children who were randomized to receive chemoprevention with monthly sulfadoxine-pyrimethamine (SP), daily trimethoprim-sulfamethoxazole (TS), monthly dihydroartemisinin-piperaquine (DP) or no chemoprevention (control arm) from 6–24 months of age, then followed for an additional year after the intervention ended. Results of this trial have been published [59]. In this report we only include data from children who were randomized to receive “no chemoprevention (control arm)” or SP, which was found to have no efficacy for prevention of malaria [59]. Samples from this cohort were taken at 16, 24, 28 and 36 months of age as indicated.
The Tororo Child Cohort Study (TCC) was conducted from 2007–2012 in Tororo district, and enrolled children at approximately 6 months of age and followed until age 5. Results of this study have previously been published [60]. Samples used here are from patients who were HIV-negative children born to HIV-negative mothers, taken at age 4 years.
The PRISM cohort was initiated in 2011 and is ongoing. This longitudinal observational cohort consists of 200 households across two study sites, the Nagongera sub-county in Tororo district and the Walukuba sub-county in Jinja district. Description of the study and results have been published in [21] In all households, one adult caregiver and all eligible children aged 6 months to 11 years were enrolled into the study. Samples used here were taken from cross-sectional bleeds of study participants taken between August 2013 and March 2014. Household-level mosquito exposure was calculated based on mosquito counts obtained from CDC light traps placed monthly within the household of each individual trial participant in the 2012 [26].
For the PROMOTE-Chemoprevention, TCC and PRISM Nagongera high transmission area, the estimated entomological inoculation rate (aEIR) is approximately 310 bites ppy. In contrast, at the PRISM Walukuba low transmissions site the aEIR is estimated at 2.8 [21].
Clinical management and measurement of malaria incidence
On enrollment all study participants were given an insecticide treated bed net and followed for all medical care at dedicated study clinics. Children who presented with a fever (tympanic temperature ≥38.0°C) or history of fever in the previous 24 hours had blood obtained by finger prick for a thick smear. If the thick smear was positive for malaria parasites, the patient was diagnosed with malaria regardless of parasite density and treatment with artemether-lumefantrine or dihydroartemisinin-piperaquine for all episodes of malaria. Incident episodes of malaria were defined as all febrile episodes accompanied by any parasitemia requiring treatment, but not preceded by another treatment in the prior 14 days. The incidence of malaria was calculated as the number of episodes per person years (ppy) from the time of enrolment into the cohort. In a subset of PRISM cohort children used to assess TNFR2 expression on Tregs parasite infection was assessed via PCR from dried blood spots as previously described [61].
FoxP3+ Regulatory T cells measurements and P. falciparum specific CD4 responses
Treg frequencies were enumerated from whole blood and fresh and cryopreserved PBMCs as indicated below. For enumeration of Tregs from whole blood (PROMOTE-Chemoprevention, control arm 2-year-old samples), 100 μl of fresh whole blood was stained with BD Pharmingen anti-CD3-FITC (UCHT1), anti-CD4-PE-CY7 (SK3), and CD25-APC (M-A251) for 20 minutes and then lysed and permeabilized with eBioscience RBC lysis buffer. Cells were washed and then incubated with eBioscience anti-FoxP3-PE (PCH101). Samples were acquired on Accuri C6 Cytometer.
For analysis of Tregs from fresh PBMCs (PRISM Nagongera cohort), PBMCs were isolated by Ficoll density gradient centrifugation and rested over night in 10% fetal bovine serum. PBMCs were stained with BD Pharmingen anti-CD3 PerCP (SK7), anti-TNFR2-Alexa646 (hTNFR-M1), anti-CD95-PECy7 (DX2) and Biolegend anti-CD4-APC-Cy7 (OKT4), anti-CD25-BrillantViolet510 (M-A251), anti-CD127-PacificBlue (A019D5). Following surface staining, cells were fixed and permeabilized with eBioscience FoxP3 staining set and intracellular stained with FoxP3-PE (PCH101) and BD Pharmingen anti-Bcl2-FITC (Bcl-2/100) as per manufacturers protocol. Samples were acquired on three laser BD FACsCantoII with FACSDiva software.
For analysis of Tregs from frozen PBMCs (Tororo Child Cohort 4-year-olds, PROMOTE-Chemoprevention SP arm longitudinal samples at 16, 24, and 28 months of age, PRISM Walukuba cohort), cryopreserved PBMCs were thawed using standard methods, and immediately stained with the following panels of antibodies; BD Pharmingen anti-CD3-FITC (UCHT1), anti-CD4-PE-CY7 (SK3), CD25-APC (M-A251) and Biolegend anti-CD127 Pacific Blue (A019D5); or Biolegend anti-CD3-BrilliantViolet650 (OKT3), anti-CD4-PerCP (OKT4), anti-CD127-FITC (A019D5); or Biolegend anti-CD3-PerCP (OKT3), anti-CD4-APC-Cy7 (OKT4), anti-CD25-BrillantViolet510 (M-A251), anti-CD127-PacificBlue (A019D5), anti-TNFR2-APC (3G7A02). Live/dead aqua amine (Invitrogen) was included in all panels. Following surface staining, cells were fixed and permeabilised with eBioscience FoxP3 staining set and intracellular stained with FoxP3-PE (PCH101) as per manufacturers protocol. Samples were acquired on LSR2 three laser flow cytometer (Becton Dickinson) with FACSDiva software.
For calculation of absolute Tregs counts (i.e. cells per μl, PRISM Nagongera cohort), peripheral blood CD4 T cell concentrations were measured from whole blood using counting beads, and Treg frequencies were calculated by normalization to total CD4 T cell numbers.
CD4 T cell responses to P. falciparum infected RBCs
Analysis of CD4+ T cell responses to P. falciparum infected RBCs via intracellular cytokine staining was performed as previously described [22,56]. PBMCs were stimulated with P. falciparum infected RBCs or uninfected RBCs and CD4 T cell production of IFNγ, IL10, and TNFα were measured via intracellular staining.
Induction of Regulatory T cells by P. falciparum in vitro
PBMCs from PROMOTE subjects (28 months of age; no chemoprevention control arm) and adults from the high malaria transmission region of Tororo were thawed and washed in 10% Human serum (AB) media (Gemini), and 3–6X106 PBMC were labeled with 1 ml of 1.25 mM 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes) for seven minutes. CFSE-labeled PBMC were incubated in 96-well, deep-well culture plates (Nunc, Roskilde, Denmark) at 106 PBMC/ well in 1 ml for 7 days with P. falciparum schizont extract (PfSE) (W2 strain) or protein extract from uninfected RBCs (uE) at a effector to target ratio equivalent to 1 : 1 PBMC:infected RBC. PHA (1μg/ml) was used as a positive control. PfSE extracts were made from the W2 stain grown in standard culture conditions and confirmed to be free of mycoplasma contamination using MycoAlert (Lonza). Mature stage parasites were magnet purified from culture using MACs purification columns. Purified parasites or uninfected RBCs were freeze thawed 3+ times (via snap freezing on liquid nitrogen and then transfer to 37°C water bath) to produce PfSE and uE and stored at -20°C. Following culture of PBMCs with protein extracts, cells were treated with 100 units of DNase I (Invitrogen) in culture media for 5 minutes and then surface stained with Biolegend anti-CD3-BrilliantViolet650 (OKT3), anti-CD4-PerCP (OKT4), anti-CD25-PE-Cy7 (BC96), anti-CD127 Pacific Blue (A019D5), BD Pharmingen anti-CD8-ABC-H7 (SK1) and Live/dead aqua amine (Invitrogen cells). Following surface staining, cells were fixed and permeabilized with eBioscience FoxP3 staining set and stained with FoxP3-PE (PCH101). Proliferation with PHA was used to ensure cell viability, and cells incubated with uE were used as a background control. Of the infants tested, the prior median malaria incidence was 1.2 episodes ppy in the low exposed group and 8.5 episodes ppy in the high exposed group.
Susceptibility of Tregs to apoptosis
PBMCs from PROMOTE subjects (28 months of age; no chemoprevention control arm) were thawed and rested overnight either in standard media (untreated), 5uM camptothecin (Sigma) or P. falciparum schizont extract (PfSE) or protein extract from uninfected RBCs (uE) at an effector:target ratio of 3 : 1. To test for induction of apoptosis, stains for AnnexinV (Biolegend) or YoPro (Invitrogen), or activated Caspase 3 FITC (BD) were used according to the manufacturer’s instructions in combination with the following antibodies: AnnexinV and YoPro staining—CD3 (OKT3) Brilliant Violet 650, CD4 (RPA-T4) APC-Cy7, CD127 (A019D5) APC, CD25 (BC96) PE-Cy7 from Biolegend; for Caspase3—CD3 (OKT3) Brilliant Violet 650, CD4 (RPA-T4) PerCP, CD127 (A019D5) Pacific Blue, CD25 (BC96) PE-Cy7. Tregs were gated as CD3+CD4+CD25+CD127dim. Sensitivity to apoptosis was measured ex vivo (untreated control), after induction with camptothecin (fold change compared to untreated), and after stimulation with P. falciparum schizont extract (fold change comparing PfSE to uE).
Flow cytometry data analysis
Unless otherwise indicated, samples were acquired on an LSR2 flow cytometer (Becton Dickinson) with FACSDiva software. Flow cytometry data were analysed using FlowJo software (Tree Star, San Carlos, CA). Color compensation was performed using single color cell controls or beads stained for each fluorochrome. Gating strategies are outlined in Supplementary Figures. Fluorescence minus one controls were used for gating of CD25, CD95, HLA-DR and Bcl2. For FoxP3 staining, an anti-Rabbit-Isotype control was used.
Statistical analysis
Data analysis was performed using Stata version 12 (Stata Corp, College Station, Tx) and PRISM version 6 (Graph Pad). Associations between Treg frequencies and other continuous variables (prior malaria incidence, age, time since last malaria episode) were assessed using Spearman’s correlation. Changes in Treg frequencies within an individual over time were assessed using the Wilcoxon signed rank test. All other two-group comparisons of continuous variables were performed using the Wilcoxon rank sum test. Repeated measures analysis of longitudinal changes in Treg frequencies was performed using generalized estimating equations, with adjustment for concurrent parasitemia, age and duration since last malaria episode. Categorical variables were compared using Chi sq test. Associations between Treg frequencies and time to next malaria episode were evaluated using the Kaplan-Meier product limit formula, and a multivariate cox proportional hazards model was used to adjust for surrogates of malaria exposure (cumulative episodes since enrollment in study for TCC and PROMOTE cohorts, or age for PRISM cohorts). Negative binomial regression was used to estimate associations between Treg frequencies and the prospective incidence of malaria in the following year (incidence rate ratios, IRR) and prevalence of asymptomatic parasitemia in the following year (prevalence rate ratios, PRR), adjusting for malaria exposure as above. Two-sided p-values were calculated for all test statistics and p<0.05 was considered significant. In the PRMOTE and TCC cohorts, associations between the highest and lowest tertiles of Treg frequencies and the monthly risk of parasitemia, probability of symptoms if parasitemic, and incidence of malaria, stratified by year of age, were evaluated using generalized estimating equations with robust SEs accounting for repeated measures in the same patient, for the period of the inter study (6 months to 3 or 5 years of age) [62].
Supporting Information
Zdroje
1. Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL (2002) CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420 : 502–507. doi: 10.1038/nature01152 12466842
2. Belkaid Y, Blank RB, Suffia I (2006) Natural regulatory T cells and parasites: a common quest for host homeostasis. Immunol Rev 212 : 287–300. doi: 10.1111/j.0105-2896.2006.00409.x 16903921
3. Velavan TP, Ojurongbe O (2011) Regulatory T cells and parasites. J Biomed Biotechnol 2011 : 520940. doi: 10.1155/2011/520940 22262943
4. Walther M, Tongren JE, Andrews L, Korbel D, King E, et al. (2005) Upregulation of TGF-beta, FOXP3, and CD4+CD25+ regulatory T cells correlates with more rapid parasite growth in human malaria infection. Immunity 23 : 287–296. doi: 10.1016/j.immuni.2005.08.006 16169501
5. Finney OC, Nwakanma D, Conway DJ, Walther M, Riley EM (2009) Homeostatic regulation of T effector to Treg ratios in an area of seasonal malaria transmission. Eur J Immunol 39 : 1288–1300. doi: 10.1002/eji.200839112 19338000
6. Walther M, Jeffries D, Finney OC, Njie M, Ebonyi A, et al. (2009) Distinct roles for FOXP3 and FOXP3 CD4 T cells in regulating cellular immunity to uncomplicated and severe Plasmodium falciparum malaria. PLoS Pathog 5: e1000364. doi: 10.1371/journal.ppat.1000364 19343213
7. Bueno LL, Morais CG, Araújo FF, Gomes JAS, Corrêa-Oliveira R, et al. (2010) Plasmodium vivax: induction of CD4+CD25+FoxP3+ regulatory T cells during infection are directly associated with level of circulating parasites. PLoS ONE 5: e9623. doi: 10.1371/journal.pone.0009623 20224778
8. Minigo G, Woodberry T, Piera KA, Salwati E, Tjitra E, et al. (2009) Parasite-dependent expansion of TNF receptor II-positive regulatory T cells with enhanced suppressive activity in adults with severe malaria. PLoS Pathog 5: e1000402. doi: 10.1371/journal.ppat.1000402 19390618
9. Scholzen A, Cooke BM, Plebanski M (2014) Plasmodium falciparum induces Foxp3hi CD4 T cells independent of surface PfEMP1 expression via small soluble parasite components. Front Microbiol 5 : 200. doi: 10.3389/fmicb.2014.00200 24822053
10. Scholzen A, Mittag D, Rogerson SJ, Cooke BM, Plebanski M (2009) Plasmodium falciparum-mediated induction of human CD25Foxp3 CD4 T cells is independent of direct TCR stimulation and requires IL-2, IL-10 and TGFbeta. PLoS Pathog 5: e1000543. doi: 10.1371/journal.ppat.1000543 19680449
11. Clemente A, Caporale R, Sannella AR, Majori G, Severini C, et al. (2011) Plasmodium falciparum soluble extracts potentiate the suppressive function of polyclonal T regulatory cells through activation of TGFβ-mediated signals. Cell Microbiol 13 : 1328–1338. doi: 10.1111/j.1462-5822.2011.01622.x 21699642
12. Finney OC, Lawrence E, Gray AP, Njie M, Riley EM, et al. (2012) Freeze-thaw lysates of Plasmodium falciparum-infected red blood cells induce differentiation of functionally competent regulatory T cells from memory T cells. Eur J Immunol 42 : 1767–1777. doi: 10.1002/eji.201142164 22585585
13. Finney OC, Riley EM, Walther M (2010) Phenotypic analysis of human peripheral blood regulatory T cells (CD4+FOXP3+CD127lo/-) ex vivo and after in vitro restimulation with malaria antigens. Eur J Immunol 40 : 47–60. doi: 10.1002/eji.200939708 19877016
14. Todryk SM, Walther M, Bejon P, Hutchings C, Thompson FM, et al. (2009) Multiple functions of human T cells generated by experimental malaria challenge. Eur J Immunol 39 : 3042–3051. doi: 10.1002/eji.200939434 19658096
15. Taylor MD, van der Werf N, Maizels RM (2012) T cells in helminth infection: the regulators and the regulated. Trends Immunol 33 : 181–189. doi: 10.1016/j.it.2012.01.001 22398370
16. Todryk SM, Bejon P, Mwangi T, Plebanski M, Urban B, et al. (2008) Correlation of memory T cell responses against TRAP with protection from clinical malaria, and CD4 CD25 high T cells with susceptibility in Kenyans. PLoS ONE 3: e2027. doi: 10.1371/journal.pone.0002027 18446217
17. Kho S, Marfurt J, Noviyanti R, Kusuma A (2015) Preserved dendritic cell HLA-DR expression and reduced regulatory T cell activation in asymptomatic Plasmodium falciparum and P. vivax infection. Infection and …. doi: 10.1128/IAI.00226-15
18. Scholzen A, Minigo G, Plebanski M (2010) Heroes or villains? T regulatory cells in malaria infection. Trends Parasitol 26 : 16–25. doi: 10.1016/j.pt.2009.10.004 19914134
19. Finney OC, Riley EM, Walther M (2010) Regulatory T cells in malaria—friend or foe? Trends Immunol 31 : 63–70. doi: 10.1016/j.it.2009.12.002 20056484
20. Hisaeda H, Maekawa Y, Iwakawa D, Okada H, Himeno K, et al. (2004) Escape of malaria parasites from host immunity requires CD4+ CD25+ regulatory T cells. Nat Med 10 : 29–30. doi: 10.1038/nm975 14702631
21. Kamya MR, Arinaitwe E, Wanzira H, Katureebe A, Barusya C, et al. (2015) Malaria transmission, infection, and disease at three sites with varied transmission intensity in Uganda: implications for malaria control. Am J Trop Med Hyg 92 : 903–912. doi: 10.4269/ajtmh.14-0312 25778501
22. Jagannathan P, Eccles-James I, Bowen K, Nankya F, Auma A, et al. (2014) IFNγ/IL-10 co-producing cells dominate the CD4 response to malaria in highly exposed children. PLoS Pathog 10: e1003864. doi: 10.1371/journal.ppat.1003864 24415936
23. Portugal S, Moebius J, Skinner J, Doumbo S, Doumtabe D, et al. (2014) Exposure-dependent control of malaria-induced inflammation in children. PLoS Pathog 10: e1004079. doi: 10.1371/journal.ppat.1004079 24743880
24. Gitau EN, Tuju J, Karanja H, Stevenson L, Requena P, et al. (2014) CD4+ T cell responses to the Plasmodium falciparum erythrocyte membrane protein 1 in children with mild malaria. J Immunol 192 : 1753–1761. doi: 10.4049/jimmunol.1200547 24453249
25. Gitau EN, Tuju J, Stevenson L, Kimani E, Karanja H, et al. (2012) T-cell responses to the DBLα-tag, a short semi-conserved region of the Plasmodium falciparum membrane erythrocyte protein 1. PLoS ONE 7: e30095. doi: 10.1371/journal.pone.0030095 22272280
26. Wanzirah H, Tusting LS, Arinaitwe E, Katureebe A, Maxwell K, et al. (2015) Mind the gap: house structure and the risk of malaria in Uganda. PLoS ONE 10: e0117396. doi: 10.1371/journal.pone.0117396 25635688
27. Bisseye C, van der Sande M, Morgan WD, Holder AA, Pinder M, et al. (2009) Plasmodium falciparum infection of the placenta impacts on the T helper type 1 (Th1)/Th2 balance of neonatal T cells through CD4(+)CD25(+) forkhead box P3(+) regulatory T cells and interleukin-10. Clin Exp Immunol 158 : 287–293. doi: 10.1111/j.1365-2249.2009.04014.x 19758375
28. Brustoski K, Moller U, Kramer M, Hartgers FC, Kremsner PG, et al. (2006) Reduced cord blood immune effector-cell responsiveness mediated by CD4+ cells induced in utero as a consequence of placental Plasmodium falciparum infection. J Infect Dis 193 : 146–154. doi: 10.1086/498578 16323143
29. Mackroth MS, Malhotra I, Mungai P, Koech D, Muchiri E, et al. (2011) Human cord blood CD4+CD25hi regulatory T cells suppress prenatally acquired T cell responses to Plasmodium falciparum antigens. J Immunol 186 : 2780–2791. doi: 10.4049/jimmunol.1001188 21278348
30. Flanagan KL, Halliday A, Burl S, Landgraf K, Jagne YJ, et al. (2010) The effect of placental malaria infection on cord blood and maternal immunoregulatory responses at birth. Eur J Immunol 40 : 1062–1072. doi: 10.1002/eji.200939638 20039298
31. Chen X, Wu X, Zhou Q, Howard OMZ, Netea MG, et al. (2013) TNFR2 is critical for the stabilization of the CD4+Foxp3+ regulatory T. cell phenotype in the inflammatory environment. J Immunol 190 : 1076–1084. doi: 10.4049/jimmunol.1202659 23277487
32. Okubo Y, Mera T, Wang L, Faustman DL (2013) Homogeneous expansion of human T-regulatory cells via tumor necrosis factor receptor 2. Sci Rep 3 : 3153. doi: 10.1038/srep03153 24193319
33. Chen X, Subleski JJ, Hamano R, Howard OMZ, Wiltrout RH, et al. (2010) Co-expression of TNFR2 and CD25 identifies more of the functional CD4+FOXP3+ regulatory T cells in human peripheral blood. Eur J Immunol 40 : 1099–1106. doi: 10.1002/eji.200940022 20127680
34. Chen X, Subleski JJ, Kopf H, Howard OMZ, Männel DN, et al. (2008) Cutting edge: expression of TNFR2 defines a maximally suppressive subset of mouse CD4+CD25+FoxP3+ T regulatory cells: applicability to tumor-infiltrating T regulatory cells. J Immunol 180 : 6467–6471. doi: 10.4049/jimmunol.180.10.6467 18453563
35. Clemente AM, Severini C, Castronovo G, Tanturli M, Perissi E, et al. (2014) Effects of soluble extracts from Leishmania infantum promastigotes, Toxoplasma gondii tachyzoites on TGF-β mediated pathways in activated CD4+ T lymphocytes. Microbes Infect 16 : 778–787. doi: 10.1016/j.micinf.2014.08.002 25130316
36. Xing S, Fu J, Zhang Z, Gao Y, Jiao Y, et al. (2010) Increased turnover of FoxP3high regulatory T cells is associated with hyperactivation and disease progression of chronic HIV-1 infection. J Acquir Immune Defic Syndr 54 : 455–462. doi: 10.1097/QAI.0b013e3181e453b9 20585263
37. Greenhouse B, Ho B, Hubbard A, Njama-Meya D, Narum DL, et al. (2011) Antibodies to Plasmodium falciparum antigens predict a higher risk of malaria but protection from symptoms once parasitemic. Journal of Infectious Diseases 204 : 19–26. doi: 10.1093/infdis/jir223 21628654
38. Jagannathan P, Nankya F, Stoyanov C, Eccles-James I, Sikyomu E, et al. (2015) IFNγ Responses to Pre-erythrocytic and Blood-stage Malaria Antigens Exhibit Differential Associations With Past Exposure and Subsequent Protection. Journal of Infectious Diseases 211 : 1987–1996. doi: 10.1093/infdis/jiu814 25520427
39. Jagannathan P, Kim CC, Greenhouse B, Nankya F, Bowen K, et al. (2014) Loss and dysfunction of Vδ2⁺ γδ T cells are associated with clinical tolerance to malaria. Science Translational Medicine 6 : 251ra117. doi: 10.1126/scitranslmed.3009793 25163477
40. Omer FM, de Souza JB, Corran PH, Sultan AA, Riley EM (2003) Activation of transforming growth factor beta by malaria parasite-derived metalloproteinases and a thrombospondin-like molecule. J Exp Med 198 : 1817–1827. doi: 10.1084/jem.20030713 14676296
41. Wan YY, Flavell RA (2007) Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature 445 : 766–770. doi: 10.1038/nature05479 17220876
42. Zheng S-G, Wang J, Horwitz DA (2008) Cutting edge: Foxp3+CD4+CD25+ regulatory T cells induced by IL-2 and TGF-beta are resistant to Th17 conversion by IL-6. J Immunol 180 : 7112–7116. 18490709
43. Koenen HJPM, Smeets RL, Vink PM, van Rijssen E, Boots AMH, et al. (2008) Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood 112 : 2340–2352. doi: 10.1182/blood-2008-01-133967 18617638
44. Bailey-Bucktrout SL, Martínez-Llordella M, Zhou X, Anthony B, Rosenthal W, et al. (2013) Self-antigen-driven activation induces instability of regulatory T cells during an inflammatory autoimmune response. Immunity 39 : 949–962. doi: 10.1016/j.immuni.2013.10.016 24238343
45. Li Z, Li D, Tsun A, Li B (2015) FOXP3(+) regulatory T cells and their functional regulation. Cell Mol Immunol. doi: 10.1038/cmi.2015.10
46. Williams LM, Rudensky AY (2007) Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat Immunol 8 : 277–284. doi: 10.1038/ni1437 17220892
47. Sakaguchi S, Vignali DAA, Rudensky AY, Niec RE, Waldmann H (2013) The plasticity and stability of regulatory T cells. Nat Rev Immunol 13 : 461–467. doi: 10.1038/nri3464 23681097
48. Miyao T, Floess S, Setoguchi R, Luche H, Fehling HJ, et al. (2012) Plasticity of Foxp3(+) T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity 36 : 262–275. doi: 10.1016/j.immuni.2011.12.012 22326580
49. Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, et al. (2010) Stability of the regulatory T cell lineage in vivo. Science 329 : 1667–1671. doi: 10.1126/science.1191996 20929851
50. Bailey-Bucktrout SL, Bluestone JA (2011) Regulatory T cells: stability revisited. Trends Immunol 32 : 301–306. doi: 10.1016/j.it.2011.04.002 21620768
51. Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martínez-Llordella M, et al. (2009) Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol 10 : 1000–1007. doi: 10.1038/ni.1774 19633673
52. Komatsu N, Mariotti-Ferrandiz ME, Wang Y, Malissen B, Waldmann H, et al. (2009) Heterogeneity of natural Foxp3+ T cells: a committed regulatory T-cell lineage and an uncommitted minor population retaining plasticity. Proc Natl Acad Sci USA 106 : 1903–1908. doi: 10.1073/pnas.0811556106 19174509
53. Oldenhove G, Bouladoux N, Wohlfert EA, Hall JA, Chou D, et al. (2009) Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity 31 : 772–786. doi: 10.1016/j.immuni.2009.10.001 19896394
54. Hoffmann P, Boeld TJ, Eder R, Huehn J, Floess S, et al. (2009) Loss of FOXP3 expression in natural human CD4+CD25+ regulatory T cells upon repetitive in vitro stimulation. Eur J Immunol 39 : 1088–1097. doi: 10.1002/eji.200838904 19283780
55. Kleijwegt FS, Laban S, Duinkerken G, Joosten AM, Zaldumbide A, et al. (2010) Critical role for TNF in the induction of human antigen-specific regulatory T cells by tolerogenic dendritic cells. J Immunol 185 : 1412–1418. doi: 10.4049/jimmunol.1000560 20574005
56. Boyle MJ, Jagannathan P, Bowen K, McIntyre TI, Vance HM, et al. (2015) Effector Phenotype of Plasmodium falciparum-Specific CD4+ T Cells Is Influenced by Both Age and Transmission Intensity in Naturally Exposed Populations. Journal of Infectious Diseases. doi: 10.1093/infdis/jiv054
57. Jangpatarapongsa K, Chootong P, Sattabongkot J, Chotivanich K, Sirichaisinthop J, et al. (2008) Plasmodium vivax parasites alter the balance of myeloid and plasmacytoid dendritic cells and the induction of regulatory T cells. Eur J Immunol 38 : 2697–2705. doi: 10.1002/eji.200838186 18825754
58. Doolan DL, Dobaño C, Baird JK (2009) Acquired immunity to malaria. Clinical Microbiology Reviews 22 : 13–36–TableofContents. doi: 10.1128/CMR.00025-08 19136431
59. Bigira V, Kapisi J, Clark TD, Kinara S, Mwangwa F, et al. (2014) Protective efficacy and safety of three antimalarial regimens for the prevention of malaria in young ugandan children: a randomized controlled trial. PLoS Med 11: e1001689. doi: 10.1371/journal.pmed.1001689 25093754
60. Wanzira H, Kakuru A, Arinaitwe E, Bigira V, Muhindo MK, et al. (2014) Longitudinal outcomes in a cohort of Ugandan children randomized to artemether-lumefantrine versus dihydroartemisinin-piperaquine for the treatment of malaria. Clin Infect Dis 59 : 509–516. doi: 10.1093/cid/ciu353 24825870
61. Schwartz A, Baidjoe A, Rosenthal PJ, Dorsey G, Bousema T, et al. (2015) The Effect of Storage and Extraction Methods on Amplification of Plasmodium falciparum DNA from Dried Blood Spots. Am J Trop Med Hyg 92 : 922–925. doi: 10.4269/ajtmh.14-0602 25758652
62. Zeger SL, Liang KY (1986) Longitudinal data analysis for discrete and continuous outcomes. Biometrics 42 : 121–130. 3719049
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Transmission Properties of Human PrP 102L Prions Challenge the Relevance of Mouse Models of GSSČlánek IFNγ and IL-12 Restrict Th2 Responses during Helminth/ Co-Infection and Promote IFNγ from Th2 CellsČlánek Exploiting Fungal Virulence-Regulating Transcription Factors As Novel Antifungal Drug Targets
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 7- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Basic Prion Science “Spreads” Insight
- Research Driven by Curiosity: The Journey from Basic Molecular Biology and Virology to Studies of Human Pathogenic Coronaviruses
- Cross Kingdom Activators of Five Classes of Bacterial Effectors
- Vaccination Drives Changes in Metabolic and Virulence Profiles of
- Expression of the Blood-Group-Related Gene Alters Susceptibility to Infection
- Transmission Properties of Human PrP 102L Prions Challenge the Relevance of Mouse Models of GSS
- Latent KSHV Infected Endothelial Cells Are Glutamine Addicted and Require Glutaminolysis for Survival
- The DSF Family of Cell–Cell Signals: An Expanding Class of Bacterial Virulence Regulators
- Intraperitoneal Infection of Wild-Type Mice with Synthetically Generated Mammalian Prion
- Vpr Promotes Macrophage-Dependent HIV-1 Infection of CD4 T Lymphocytes
- An In-Depth Comparison of Latency-Reversing Agent Combinations in Various and HIV-1 Latency Models Identified Bryostatin-1+JQ1 and Ingenol-B+JQ1 to Potently Reactivate Viral Gene Expression
- α-Macroglobulin Can Crosslink Multiple Erythrocyte Membrane Protein 1 (PfEMP1) Molecules and May Facilitate Adhesion of Parasitized Erythrocytes
- Should Symbionts Be Nice or Selfish? Antiviral Effects of Wolbachia Are Costly but Reproductive Parasitism Is Not
- A Unique Human Norovirus Lineage with a Distinct HBGA Binding Interface
- The Broad Neutralizing Antibody Responses after HIV-1 Superinfection Are Not Dominated by Antibodies Directed to Epitopes Common in Single Infection
- Rapidly Evolving Genes Are Key Players in Host Specialization and Virulence of the Fungal Wheat Pathogen ()
- MiR-21 in Extracellular Vesicles Leads to Neurotoxicity via TLR7 Signaling in SIV Neurological Disease
- Age-Dependent Cell Trafficking Defects in Draining Lymph Nodes Impair Adaptive Immunity and Control of West Nile Virus Infection
- Decline of FoxP3+ Regulatory CD4 T Cells in Peripheral Blood of Children Heavily Exposed to Malaria
- Dimerization-Induced Allosteric Changes of the Oxyanion-Hole Loop Activate the Pseudorabies Virus Assemblin pUL26N, a Herpesvirus Serine Protease
- Macrophages Subvert Adaptive Immunity to Urinary Tract Infection
- Mycolactone-Dependent Depletion of Endothelial Cell Thrombomodulin Is Strongly Associated with Fibrin Deposition in Buruli Ulcer Lesions
- Activation of TLR2 and TLR6 by Dengue NS1 Protein and Its Implications in the Immunopathogenesis of Dengue Virus Infection
- K-bZIP Mediated SUMO-2/3 Specific Modification on the KSHV Genome Negatively Regulates Lytic Gene Expression and Viral Reactivation
- Phosphoproteomic Analysis of KSHV-Infected Cells Reveals Roles of ORF45-Activated RSK during Lytic Replication
- CR3 and Dectin-1 Collaborate in Macrophage Cytokine Response through Association on Lipid Rafts and Activation of Syk-JNK-AP-1 Pathway
- IFNγ and IL-12 Restrict Th2 Responses during Helminth/ Co-Infection and Promote IFNγ from Th2 Cells
- THY-1 Cell Surface Antigen (CD90) Has an Important Role in the Initial Stage of Human Cytomegalovirus Infection
- Human Enterovirus Nonstructural Protein 2C Functions as Both an RNA Helicase and ATP-Independent RNA Chaperone
- IL-27 Signaling Is Crucial for Survival of Mice Infected with African Trypanosomes via Preventing Lethal Effects of CD4 T Cells and IFN-γ
- Synergistic Reactivation of Latent HIV Expression by Ingenol-3-Angelate, PEP005, Targeted NF-kB Signaling in Combination with JQ1 Induced p-TEFb Activation
- Vpu Exploits the Cross-Talk between BST2 and the ILT7 Receptor to Suppress Anti-HIV-1 Responses by Plasmacytoid Dendritic Cells
- Herpesvirus Genome Recognition Induced Acetylation of Nuclear IFI16 Is Essential for Its Cytoplasmic Translocation, Inflammasome and IFN-β Responses
- A Comprehensive Analysis of Replicating Merkel Cell Polyomavirus Genomes Delineates the Viral Transcription Program and Suggests a Role for mcv-miR-M1 in Episomal Persistence
- Analysis of the SUMO2 Proteome during HSV-1 Infection
- Capacity of Broadly Neutralizing Antibodies to Inhibit HIV-1 Cell-Cell Transmission Is Strain- and Epitope-Dependent
- A Novel Antiviral Target Structure Involved in the RNA Binding, Dimerization, and Nuclear Export Functions of the Influenza A Virus Nucleoprotein
- Deploying FLAREs to Visualize Functional Outcomes of Host—Pathogen Encounters
- Mosquitoes Reset Malaria Parasites
- The Lung Microbiome: New Principles for Respiratory Bacteriology in Health and Disease
- Extracellular Virions: The Advance Guard of Poxvirus Infections
- Risks of Antibiotic Exposures Early in Life on the Developing Microbiome
- RNA Virus Reassortment: An Evolutionary Mechanism for Host Jumps and Immune Evasion
- Exploiting Fungal Virulence-Regulating Transcription Factors As Novel Antifungal Drug Targets
- N-acetylglucosamine Regulates Virulence Properties in Microbial Pathogens
- Periodontal Diseases: Bug Induced, Host Promoted
- Mechanisms of Host Behavioral Change in Rodent Association
- The Endosymbiotic Bacterium Selectively Kills Male Hosts by Targeting the Masculinizing Gene
- HIV Reactivation from Latency after Treatment Interruption Occurs on Average Every 5-8 Days—Implications for HIV Remission
- Ubiquilin 1 Promotes IFN-γ-Induced Xenophagy of
- Transfer of Immunity from Mother to Offspring Is Mediated via Egg-Yolk Protein Vitellogenin
- Suppression of Long-Lived Humoral Immunity Following Infection
- The Role of VP1 Amino Acid Residue 145 of Enterovirus 71 in Viral Fitness and Pathogenesis in a Cynomolgus Monkey Model
- Utilizing Chemical Genomics to Identify Cytochrome as a Novel Drug Target for Chagas Disease
- The Emerging Role for RNA Polymerase II in Regulating Virulence Gene Expression in Malaria Parasites
- Turning Up the Heat: Inflammasome Activation by Fungal Pathogens
- On and Under the Skin: Emerging Basidiomycetous Yeast Infections Caused by Species
- EhVps32 Is a Vacuole-Associated Protein Involved in Pinocytosis and Phagocytosis of
- Characterization of a Prefusion-Specific Antibody That Recognizes a Quaternary, Cleavage-Dependent Epitope on the RSV Fusion Glycoprotein
- The Serine Protease EspC from Enteropathogenic Regulates Pore Formation and Cytotoxicity Mediated by the Type III Secretion System
- Existing Infection Facilitates Establishment and Density of Malaria Parasites in Their Mosquito Vector
- Evaluating Human T-Cell Therapy of Cytomegalovirus Organ Disease in HLA-Transgenic Mice
- Neuronal Interferon Signaling Is Required for Protection against Herpes Simplex Virus Replication and Pathogenesis
- Epstein-Barr Virus Proteins EBNA3A and EBNA3C Together Induce Expression of the Oncogenic MicroRNA Cluster miR-221/miR-222 and Ablate Expression of Its Target p57
- Colonization of the Mouse Gastrointestinal Tract Is Modulated by Wall Teichoic Acid, Capsule, and Surface Proteins
- Virulence of Group A Streptococci Is Enhanced by Human Complement Inhibitors
- Identification of Caspase Cleavage Sites in KSHV Latency-Associated Nuclear Antigen and Their Effects on Caspase-Related Host Defense Responses
- Calprotectin Increases the Activity of the SaeRS Two Component System and Murine Mortality during Infections
- Type VI Secretion System Transports Zn to Combat Multiple Stresses and Host Immunity
- Lv4 Is a Capsid-Specific Antiviral Activity in Human Blood Cells That Restricts Viruses of the SIV/SIV/HIV-2 Lineage Prior to Integration
- Phenylbutyrate Is Bacteriostatic against and Regulates the Macrophage Response to Infection, Synergistically with 25-Hydroxy-Vitamin D₃
- An Internally Translated MAVS Variant Exposes Its Amino-terminal TRAF-Binding Motifs to Deregulate Interferon Induction
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- RNA Virus Reassortment: An Evolutionary Mechanism for Host Jumps and Immune Evasion
- Activation of TLR2 and TLR6 by Dengue NS1 Protein and Its Implications in the Immunopathogenesis of Dengue Virus Infection
- N-acetylglucosamine Regulates Virulence Properties in Microbial Pathogens
- Characterization of a Prefusion-Specific Antibody That Recognizes a Quaternary, Cleavage-Dependent Epitope on the RSV Fusion Glycoprotein
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



