-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Age-Dependent Cell Trafficking Defects in Draining Lymph Nodes Impair Adaptive Immunity and Control of West Nile Virus Infection
While West Nile virus (WNV) infection preferentially causes severe neuroinvasive disease in elderly humans, the basis for this epidemiological linkage has remained uncertain. Here, we studied the impact of aging on WNV pathogenesis and immune responses using a mouse model of infection. Old mice showed increased lethality after WNV infection compared to adult mice, and this phenotype was associated with delayed antibody responses, and higher levels of virus infection in the blood, spleen, and brain. Detailed immunological and microscopic analyses revealed defects in germinal center development in the draining lymph node and impaired migratory capacity of naïve CD4+ T cells within days of WNV infection. This deficit was worsened by a separate age-related deficiency in the production of several chemokines that recruit leukocytes to inflamed lymph nodes. Thus, age-dependent defects in naïve CD4+ T cell trafficking and in the draining lymph node environment result in delayed initiation of antiviral responses and a failure to control WNV, which ultimately contributes to higher rates of mortality after infection.
Published in the journal: . PLoS Pathog 11(7): e32767. doi:10.1371/journal.ppat.1005027
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1005027Summary
While West Nile virus (WNV) infection preferentially causes severe neuroinvasive disease in elderly humans, the basis for this epidemiological linkage has remained uncertain. Here, we studied the impact of aging on WNV pathogenesis and immune responses using a mouse model of infection. Old mice showed increased lethality after WNV infection compared to adult mice, and this phenotype was associated with delayed antibody responses, and higher levels of virus infection in the blood, spleen, and brain. Detailed immunological and microscopic analyses revealed defects in germinal center development in the draining lymph node and impaired migratory capacity of naïve CD4+ T cells within days of WNV infection. This deficit was worsened by a separate age-related deficiency in the production of several chemokines that recruit leukocytes to inflamed lymph nodes. Thus, age-dependent defects in naïve CD4+ T cell trafficking and in the draining lymph node environment result in delayed initiation of antiviral responses and a failure to control WNV, which ultimately contributes to higher rates of mortality after infection.
Introduction
Aging is linked to a decline in immunity that causes increased susceptibility to infectious diseases and reduced vaccine efficacy in the elderly population. This process, termed immunosenescence, is the consequence of age-dependent changes to multiple components of the innate and adaptive immune responses. For example, elderly individuals display elevated basal levels of pro-inflammatory cytokines and type I interferon (IFN) [1], and demonstrate reduced signaling following pathogen-associated molecular pattern (PAMP) recognition [2]; collectively this results in dysregulated cytokine responses [3]. Additionally, elderly individuals exhibit a shift from a naïve to a memory repertoire in CD4+ T cells, CD8+ T cells [4–6], and B cells [7], which decreases the number of cells capable of recognizing new antigens encountered during primary infections. Cell-intrinsic defects also have been identified in different arms of the adaptive response. Naïve CD4+ T cells from old individuals display reduced activation, immunological synapse formation, and proliferation upon T cell receptor (TCR) engagement [8,9]. This leads to a diminished ability of CD4+ T cells to prime B cells during germinal center reactions [10,11] and, along with intrinsic B cell defects [12], results in reduced affinity maturation, class switching, and memory responses [11].
West Nile Virus (WNV) is a globally important mosquito-transmitted RNA virus that causes severe disease preferentially in the elderly as a consequence of enhanced dissemination to the central nervous system (CNS) and infection and injury of neurons [13]. After inoculation in the skin, WNV infects dendritic cell subsets and traffics to the draining lymph node (DLN) where it replicates locally and spreads to other secondary lymphoid and visceral organs [14]. In most human cases, WNV is cleared after induction of a robust innate and adaptive immune response. However, in the elderly, the virus crosses the blood-brain barrier at increased frequency and infects neurons of the brain and spinal cord; this is associated with a 5 to 10% case-fatality rate [14]. Based on animal studies, rapid clearance of WNV infection requires the concerted actions of multiple aspects of the immune system, including antiviral cytokine responses by myeloid cells [15], complement activation [16], the rapid induction of virus-specific neutralizing antibodies [17,18], helper, effector, and regulatory functions of CD4+ T cells [19–22], and priming of cytotoxic CD8+ T cells [23,24].
Analogous to humans, old mice are more vulnerable to WNV infection. One study reported a decreased number and function of WNV-specific CD8+ T cells [21], although the mechanistic basis of this defect was not defined. We hypothesized that age-dependent defects in B cell responses also contributed to disease susceptibility in old mice. Following WNV infection of young adult mice, an early T-independent and T-dependent IgM response develops, which limits viremia and prevents spread to the brain [17,18]. Subsequently, T-dependent class-switching and affinity maturation leads to the development of plasma cells that secrete higher affinity IgG antibodies, which neutralize virus in peripheral tissues [22]. Some of these antibody-secreting cells migrate to the CNS where they likely help to control and clear WNV infection [25].
In this study, we detected age-dependent defects in the early antigen-specific IgM and IgG response that correlated with increased viral titers in the serum, spleen and brain, and higher rates of WNV mortality. Using a series of adoptive transfer experiments coupled with two-photon intravital microscopy, we identified cell-intrinsic defects in naïve CD4+ T cells as well as reduced cytokine and chemokine levels in the DLN environment, both of which contributed to reduced cellular accumulation, delayed germinal center development, and immune responses. The cell-intrinsic defect was observed specifically in naïve CD4+ T cells and not in CD19+ B cells and was associated with an inability to migrate efficiently into and within the inflamed DLN. Thus, age-dependent immune defects that occurred within the first few days of WNV infection resulted in delayed immune cell recruitment, antigen recognition, and priming. These very early defects contributed to the failure to control WNV infection and prevent death.
Results
Old mice are more vulnerable to WNV infection and have reduced antiviral antibody responses
We evaluated disease severity after WNV infection in adult and old mice. Compared to 4 month-old adult C57BL/6 mice, 18 month-old old syngeneic mice exhibited increased mortality (56% versus 15%, P < 0.01, Fig 1A) following subcutaneous infection with WNV (New York 2000 strain), corroborating results from a published report [21]. To understand the basis for the increased lethality, we measured viral burden in tissues. Older mice sustained increased levels of WNV in the serum at day 6 after infection (3.5-fold, P < 0.001 Fig 1B), in the spleen at days 4 (7-fold, P < 0.05) and 6 (17-fold, P < 0.01 Fig 1C) after infection, and in the brain at day 9 after infection (20-fold, P < 0.01 Fig 1D), which likely caused the higher mortality rate.
Fig. 1. Survival rate, viral burden, and B cell responses after WNV infection. 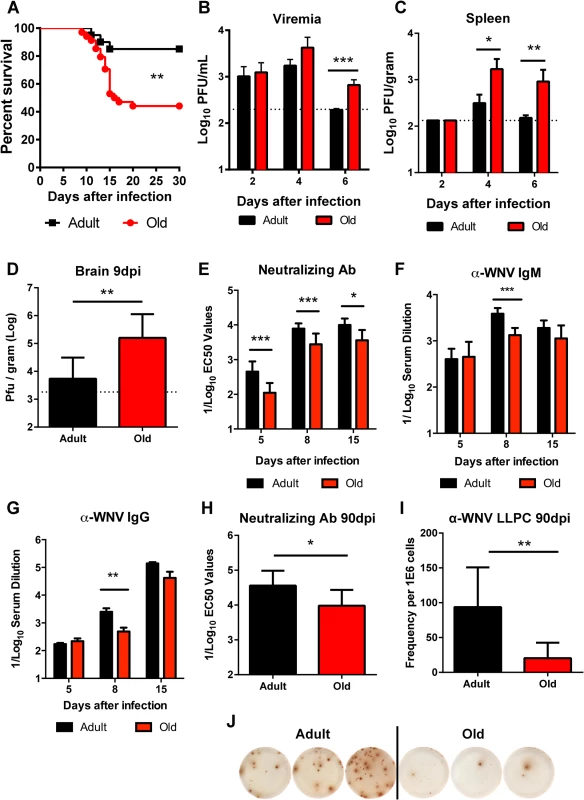
A. Adult or old mice were infected with 102 PFU of WNV (New York strain) via subcutaneous injection in the footpad. Survival was monitored for 30 days: n = 20 adult (16 weeks) and n = 34 old (18 months) mice from at least three independent experiments. The difference was statistically different (P < 0.01; log rank test). B-D. Viral burden after WNV infection of adult and old mice was measured by qRT-PCR from serum (B) and plaque assay from spleen and brain (C-D). The data is shown as the mean titer (log10 PFU/ml) ± standard deviation (SD) and reflects 8 to 9 mice per time point from at least two independent experiments. The dashed lines represent the limit of sensitivity of the assay. Asterisks indicate statistical significance (*, P < 0.05; **, P < 0.01; ***, P < 0.001; Mann-Whitney test). E-H. Serum was obtained from adult and old mice at days 5, 8, 15, and 90 after WNV infection. E and H. Neutralizing antibody titers were determined by a focus-reduction assay. Results are shown as the mean of the reciprocal log10 titer ± SD that yields 50% inhibition of infection and reflects data from 5 to 11 mice per group from three independent experiments. F-G. Anti-WNV IgM (F) and IgG (G) levels were measured by ELISA. Data is plotted as the reciprocal log10 titer, defined as three times greater than background. I-J. Ninety days after infection, WNV-specific antibody secreting long-lived plasma cells in the bone marrow (LLPC) were quantified. Shown is the LLPC frequency per 106 bone marrow cells ± SD (I), as well as representative ELISPOT images from three adult and three old mice (J). Data is pooled from 6 to 9 mice per group from three independent experiments. The early humoral immune response limits WNV infection and dissemination in adult mice [17,18]. We hypothesized that age-dependent defects in the WNV-specific humoral immunity might contribute to the more severe clinical phenotype in old mice. Accordingly, we assessed the WNV-specific antibody responses at days 5, 8, and 15 after infection in adult and old mice. Serum from old mice had less neutralizing activity than that from adult mice at all time points tested (2.7 to 4-fold, P < 0.05, Fig 1E). Also WNV-specific IgM and IgG antibody titers against the viral envelope (E) protein were lower at day 8 after infection in the aged mice (2.9 and 5.1-fold, P < 0.01, Fig 1F and 1G). By day 15, overall serum WNV E protein-specific IgM and IgG responses were equivalent in the surviving adult and old mice although a qualitative defect remained in the recognition of a dominant neutralizing epitope on domain III of the E protein [26,27] in old mice in both the IgM and IgG fractions (data not shown). Surviving old mice also had defects in durable humoral immunity, as their serum had reduced neutralization capacity at 90 days post infection (3.5-fold, P < 0.05, Fig 1H), which correlated with fewer WNV-specific long-lived plasma cells in the bone marrow (4.5-fold, P < 0.01 Fig 1I and 1J). Thus, old mice had delayed and decreased humoral immune responses during both the acute and memory phases after WNV infection.
Decreased germinal center development in old mice
To assess the basis for the defects in the early B cell response in WNV-infected old mice, we focused on the DLN. Following subcutaneous infection in the footpad, WNV traffics to the popliteal LN where an early B cell response is initiated [28]. Within the germinal centers of the DLN, T follicular helper (TFH) cells provide signals to germinal center B (GC B) cells to promote class switching and affinity maturation [29]. The accumulation of TFH (CD4+ PD1+ CXCR5+) and GC B (CD19+ Fas+ GL7+) cells was decreased in old mice relative to adult mice at days 4 and 6, although nearly equivalent levels of TFH and GC B cells were present at 8 days post infection (Fig 2A and 2C). These findings were corroborated by immunofluorescence microscopy analysis, which showed a reduced number of germinal centers at day 6 after infection (Fig 2E). The delayed germinal center development in the old mice matched the temporal pattern of antibody titers. At 6 days after infection, similar levels of CD28 were observed on CD4+ T cells and TFH cells from old and adult mice (S1A and S1B Fig), suggesting that decreased expression of co-stimulatory molecules was not responsible for the delayed germinal center development in old mice. Consistent with this pattern, old mice had a proportionately higher number of CD4+ T cells and TFH cells expressing the co-stimulatory molecule OX40 (CD134) (S1C and S1D Fig). TFH cells from old mice did exhibit a small yet statistically significant reduction (11%, P < 0.01) in CXCR5 expression at 6 days post infection (S1E Fig). By 8 days after infection, however, a higher percentage of the CD4+ T cells from old mice were phenotypically TFH, and adult and old mice had equivalent numbers of GC B cells (Fig 2A, 2B and 2D). Thus, in old mice, the blunted humoral response to WNV correlated with a delay in the kinetics of germinal center development.
Fig. 2. Delayed germinal center formation and decreased accumulation of CD4+ T cells and CD19+ B cells in DLN after WNV infection of old mice. 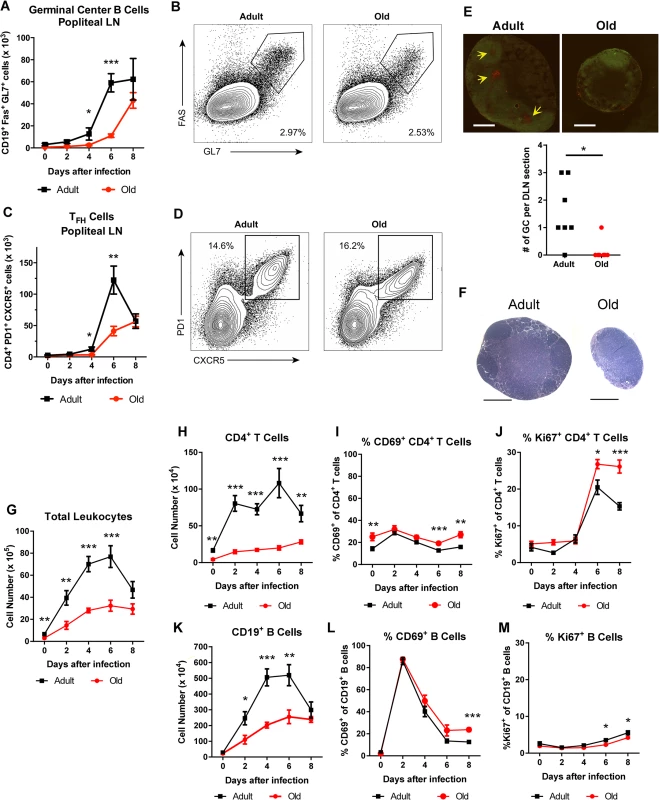
Mice were infected with 102 PFU of WNV (New York strain) in the footpad. A-D. Draining popliteal LN cells were analyzed by flow cytometry at the indicated days after infection. Numbers of GC B cells (CD19+ Fas+ GL7+ cells) (A) and TFH cells (CD4+ PD1+ CXCR5+ cells) were quantified (C). B and D. Representative flow cytometry plots of GC B cells (B, on gated CD19+ cells) and TFH cells (D, on gated CD4+ cells) from adult and old mice at 8 days post infection. E. Immunofluorescence staining of germinal centers in DLN from 6 days post WNV-infected adult and old mice. Slides were stained with GL7-PE, and IgD-FITC. Arrows point to sites of active GCs indicated by GL7 staining in the red channel. In the graph, the number of GCs per section is recorded with each point representing a section taken from the center of draining popliteal lymph nodes from adult or old mice from two independent experiments. F. Hematoxylin and eosin stained sections of the draining popliteal LN from adult and old mice 4 days post infection. G-M. At the indicated days post infection, the DLN was harvested, and total cells were counted (G). Antibody staining detected specific lymphocyte subsets including CD4+ T cells (H) and CD19+ B cells (K). CD4+ T cells (I, J) and CD19+ B cells (L, M) were co-stained with antibodies to CD69 (I, L) or Ki-67 (J, M) to establish their state of activation or proliferation. The results are pooled from at least three independent experiments with a total of 6 to 14 mice per group. Data is expressed as the mean ± the standard error of the mean (SEM). Asterisks indicate statistical significance (*, P < 0.05; **, P < 0.01; ***, P < 0.001; Mann-Whitney test). Reduced lymphocyte accumulation in the DLN of old mice
During isolation of the popliteal lymph nodes for GC analysis, we noticed DLN from adult mice were substantially larger than those from old mice after WNV infection (Fig 2E and 2F). Because of this observation, we assessed how aging affected the total number of immune cells in the DLN at different time points after infection. In naive mice, we found increased cell numbers in the adult DLN relative to those from the old mice (6.6 x 105 versus 3.0 x 105 cells, P < 0.01 Fig 2G). After subcutaneous WNV inoculation, cell numbers increased rapidly in the adult mice, peaking at day 6. Cell accumulation was delayed in the DLN of old mice with fewer cells observed at days 2, 4, and 6 after infection (Fig 2G).
Cell subset analysis revealed that old mice had fewer CD4+ T and B cells in the DLN after WNV infection (Fig 2H and 2K), which could reflect reduced in situ proliferation and/or defects in cell trafficking. We monitored cell activation, as judged by surface acquisition of the marker CD69, and proliferation through expression of the nuclear protein Ki-67. As reported previously [28], CD19+ B cells in the DLN of adult mice were activated shortly after WNV infection with greater than 85% of cells expressing CD69 by day 2 (Fig 2L). The kinetics of CD69 up-regulation were equivalent on B cells in adult and old mice, although in old mice CD69 expression remained higher at day 8 (Fig 2L and data not shown). An analogous pattern of CD69 expression was seen in adult and old CD4+ T cells, although a smaller subset of cells was activated (e.g., 31% at 2 days after infection) (Fig 2I). We observed a similar small fraction of B cells and CD4+ T cells staining for Ki-67 in the adult and old mice between days 0 and 4 after infection (Fig 2J and 2M). An increase in Ki-67 expression was noted at day 6 for CD4+ T cells in both age groups, with higher levels in old mice at both days 6 and 8 after infection (Fig 2J). We also detected a small decrease in the frequency of Ki-67-positive proliferating B cells in old mice at days 6 and 8 after infection (3.5% to 2.3% at day 6 and 5.5% to 4.2% at day 8, P < 0.05, Fig 2M), which could reflect delays in the germinal center reaction observed in the old DLN (Fig 2A and 2C). These findings suggest that impaired activation or proliferation of lymphocytes did not account for the reduced cellular accumulation in the DLN of the old mice.
Diminished cellularity of the DLN of old mice was not unique to viral infection, as similar data was obtained with an inflammatory stimulus. Old mice immunized with ovalbumin emulsified in complete Freund’s adjuvant (CFA) had fewer CD4+ T cells and B cells in the DLN at early time points compared to adult mice (Fig 3A–3C). Old mice also had fewer IL-2-secreting antigen-specific CD4+ T cells at day 7 after immunization, and fewer germinal center B cells and TFH cells at day 6 after immunization (Fig 3D–3F).
Fig. 3. Decreased accumulation of CD4+ T cells and CD19+ B cells in DLN of old mice after immunization with ovalbumin. 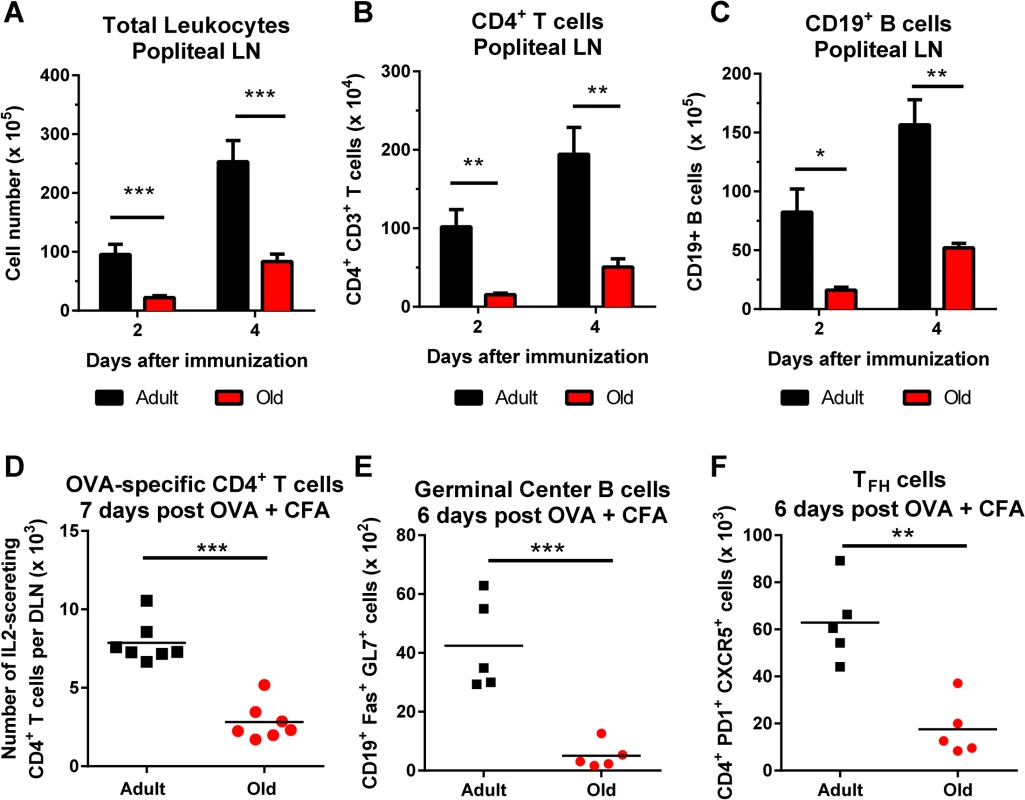
A-C. Adult and old mice were immunized with ovalbumin (OVA) complexed with complete Freund’s adjuvant in the footpad. At the indicated days post infection, the draining popliteal LN was harvested, and cells were counted (A). Antibody staining detected specific lymphocyte populations including CD4+ T cells (B) and CD19+ B cells (C). The results were pooled from two independent experiments with a total of 5 mice per group and data is expressed as the mean ± the standard error of the mean (SEM). D. Numbers of IL-2 secreting CD4+ T cells as judged by ELISPOT assay following OVA323–337 peptide stimulation in the DLN of adult and old mice at day 7 after immunization. E-F. Draining popliteal LN cells were analyzed by flow cytometry at 6 days after infection, and the numbers of GC B cells (CD19+ Fas+ GL7+) (E) and TFH cells (CD4+ PD1+ CXCR5+) (F) were quantified. Each data point represents and individual mice and the results are pooled from two independent experiments. Asterisks indicate statistical significance (*, P < 0.05; **, P < 0.01; ***, P < 0.001; unpaired t test). Cell-intrinsic and cell-extrinsic defects in T cell recruitment into the DLN
We hypothesized that the reduced lymphocyte accumulation in the DLN of old mice soon after WNV infection or ovalbumin immunization could be due to cell-intrinsic defects in the lymphocytes and/or environmental defects in the DLN. To evaluate these possibilities, we sorted naïve CD4+ CD44- CD62L+ T cells from lymphoid tissues of adult and old mice and labeled purified cells ex vivo with different fluorescent dyes (Fig 4A). As previously reported, old mice had fewer naïve CD4+ T cells compared to adult mice (S2 Fig and [5]). Equal numbers of differentially labeled adult and old naïve sorted CD4+ T cells were mixed at a 1 : 1 ratio and injected into recipient adult or old mice that had been infected two days earlier with the Kunjin strain of WNV (WNV-KUN), a less pathogenic variant that can be studied under A-BSL-2 conditions. Importantly, subcutaneous infection of old mice with WNV-KUN showed similar defects in cellular accumulation in the DLN compared to adult mice, with kinetics that mirrored that seen with the virulent WNV New York strain (S3 Fig and Fig 2). One hour after transfer of the labeled donor T cells into the WNV-KUN infected mice, the DLN of recipient mice were harvested. In both the adult and old recipients, greater numbers of adult donor T cells were detected within the DLN suggesting an intrinsic trafficking defect of the naïve CD4+ T cells from old mice (Fig 4B). We also observed reduced accumulation of adult and old donor cells in the old compared to adult recipients (Fig 4B), suggesting that the DLN environment separately contributed to the lower numbers of naïve CD4+ T cells recruited after infection. Similar defects were observed in the homing of cells to the spleen indicating that reduced trafficking could be a general property of naïve CD4+ T cells and the lymphoid environment of old mice (Fig 4C).
Fig. 4. Defects in migration of naïve CD4+ T cells from old mice into inflamed LN. 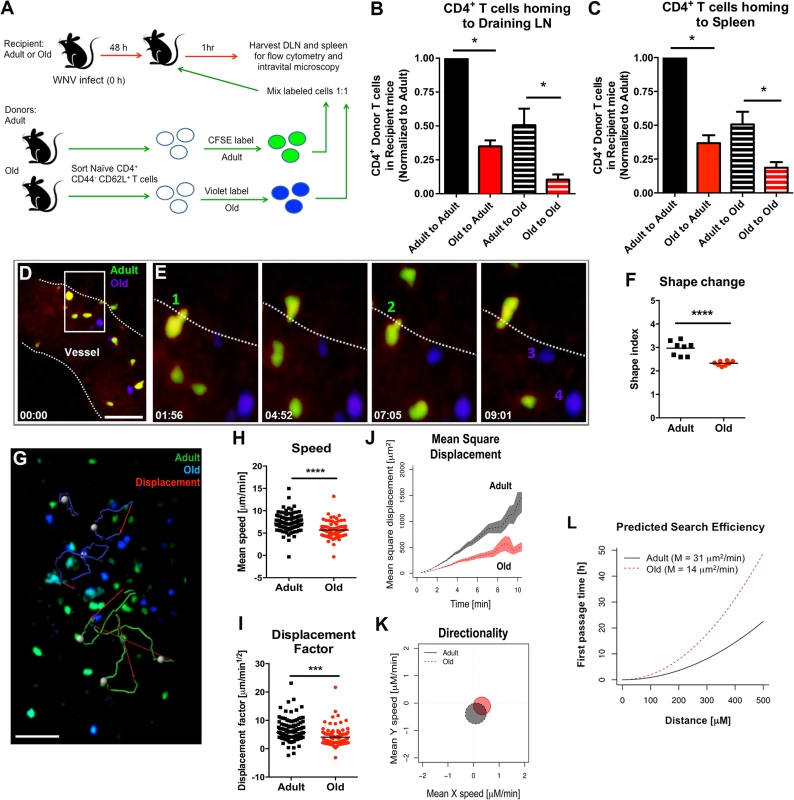
A. Scheme of adoptive transfer studies. 2 x 106 FACS-sorted naïve (CD44- CD62L+) CD4+ T cells from adult or old mice were differentially labeled with fluorescent dyes, mixed in a 1:1 ratio, and transferred to recipient adult or old mice that had been infected with WNV-KUN 48 hours earlier via a subcutaneous route. B-C. One hour later, draining popliteal LN (B) and spleen (C) were harvested. Cells were counted and the frequency of adult and old donor cells was determined by flow cytometry. In each tissue, the data was normalized to the levels of adult donor cells in the adult recipient organ and is a composite of four independent experiments. Asterisks indicate statistical significance (*, P < 0.05; Mann-Whitney test). D-E. Time-lapse two-photon intravital imaging of diapedesis of labeled donor naïve CD4+ T cells (adult = green; old = blue) in the HEV from the DLN of a recipient WNV-KUN-infected mouse. Images are individual frames from a continuous time-lapse movie (see S1 and S2 Movies). Relative time is displayed in min:sec. Panel D shows a picture of a Q-dot labeled vessel in a LN with multiple old (blue) and adult (green) CD4+ T cells adhering to the endothelium. The white dotted line is drawn to highlight the edge of the vessel lumen. The white box shows a zoomed in view of an extravasation "hot spot" that is then presented as a 4 time-lapse images in panel E. Two T cells (cells 1 and 2) from adult mice are shown undergoing diapedesis, whereas two representative T cells (cells 3 and 4) from old mice remain in the lumen over the same time frame. F. Cellular deformation of donor naïve CD4+ T cells in the HEV from the DLN of a recipient WNV-KUN-infected mouse as determined by analysis of two-photon microscopic images. G. Time-lapse two-photon imaging of movement of labeled donor naïve CD4+ T cells (adult = green; old = blue) in explanted DLN of a recipient WNV-KUN-infected mouse (see S3 Movie). The figure presents representative cell tracking for adult and old CD4+ T cells followed by evaluation of displacement. White opaque dots represent cells with tracked paths (green or blue). Red arrow indicates cell displacement. Scale bar (white): 40 μm. H-L. Analysis of movement parameters of adult and old donor naïve CD4+ T cells in explanted LN 6 to 8 hours post-transfer to recipient mice infected with WNV-KUN 48 hours earlier. Individual cells were tracked and (H) speed (μM/min), (I) cell displacement factor (μm/min1/2), (J) mean square cell displacement over time (μm2), (K) randomness of migration (Hotelling’s test of directionality) and (L) the predicted time of search efficiency for antigen. For panel K, the migration directionality was of a small magnitude, and no significant difference was observed between the cells from adult and old mice. For panel L, to judge the impact that the observed motility defects of old T cells would have on their ability to search antigen in DLN, we used a mathematical model [30] to predict the time needed by adult and old T cells to first reach increasingly distant locations (first passage time). The first passage time is proportional to the motility coefficient, which we estimated from the mean square displacement data. The predicted time to reach a location 500 μm away is ~20 hours for adult T cells, but ~50 hours for old T cells. The data in F, H and I are shown as a scatter plot and reflects three independent experiments. Asterisks indicate statistical significance (***, P < 0.001, ****, P < 0.0001; Mann-Whitney test). Defects in migration of naïve CD4+ T cells
We used intravital two-photon microscopy to evaluate the trafficking patterns of adult and old naïve CD4+ T cells in vivo within lymphoid tissues. Dye-labeled naïve CD4+ T cells from adult or old mice were mixed at a 1 : 1 ratio and adoptively transferred into adult recipient mice that had been infected with WNV-KUN two days prior. Donor cells from both adult and old mice efficiently rolled and arrested on the luminal side of the high endothelial venules (HEV) in the DLN of recipient infected mice (Fig 4D and 4E, S1 Movie and S2 Movie), although the appearance of donor T cells from old mice in the HEVs was delayed by ~15 minutes relative to donor cells from adult mice. Remarkably, naïve CD4+ T cells from old mice extravasated less efficiently compared to T cells from adult mice, with many cells unable to undergo the rapid cell shape deformation required for transendothelial migration across HEV (Fig 4F). This result suggested that although naïve CD4+ T cells from old mice could bind HEV, they were less efficient at entering the parenchyma of the DLN due to defective diapedesis. When the motility of extravasated T cells was analyzed in explanted lymph nodes from WNV-KUN infected adult recipient mice, adult donor naive CD4+ T cells moved with greater speed and displacement than the old donor naïve CD4+ T cells (Fig 4G–4I, S3 Movie and S4 Fig). However, neither adult nor old donor naive CD4+ T cells showed signs of chemotactically biased motion (Fig 4K). Computational analysis [30] predicted that the motility defects of naïve CD4+ T cells from old mice would delay encounter with antigen 500 μm away by ~30 hours (Fig 4L), which would contribute to the slowed kinetics of the humoral immune response in these animals. A trend towards reduced migratory capacity of old, compared to adult naïve CD4+ T cells also was observed in the LN of naive mice, although the magnitude of the difference was smaller (Fig 5A–5E).
Fig. 5. Migration of naïve CD4+ T cells from old mice into naive LN. 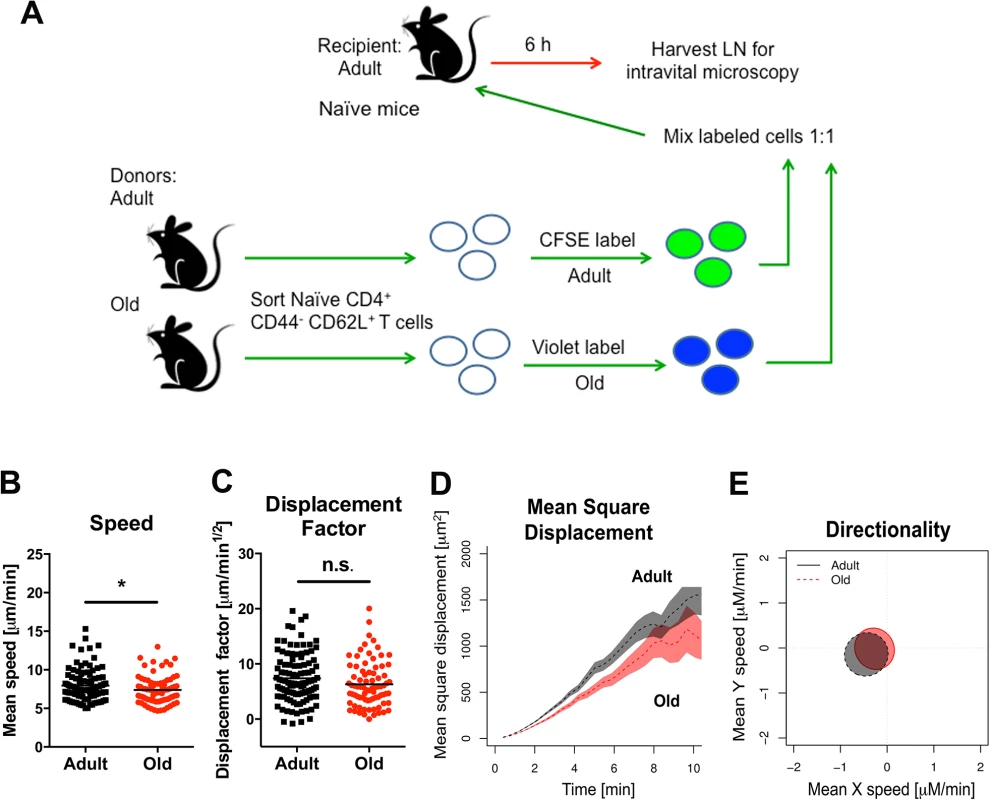
A. Scheme of adoptive transfer studies of naïve CD4+ T cells. 2 x 106 FACS-sorted naïve (CD44- CD62L+) CD4+ T cells from adult or old mice were differentially labeled with fluorescent dyes, mixed in a 1:1 ratio, and transferred to recipient naïve adult mice. B-E. Six hours later, LN was harvested for microscopy studies. Analysis of movement parameters of adult and old donor naïve CD4+ T cells in explanted LN from recipient mice. Individual cells were tracked and (B) speed (μM/min), (C) displacement factor (μm/min1/2), (D) mean square cell displacement over time (μm2), and (E) directionality (Hotelling’s test) were measured. The data in B-C are shown as a scatter plot and reflects two independent experiments. Asterisks indicate statistical significance (*, P < 0.05; Mann-Whitney test). Decreased expression of surface adhesion and chemoattractant receptors on naïve CD4+ T cells from old mice might explain the reduced transmigration and motility phenotype. However, when we assessed the expression of the surface adhesion molecules CD62L, PSGL1, LFA1, and VLA-4 as well as the chemokine receptors CCR7 and CXCR4 on naïve CD4+ T cells from adult and old mice, no significant differences in the geometric mean fluorescence intensity of expression of any of these molecules were observed (S5 Fig). We also did not find differences in the levels of filamentous actin in adult and old naïve CD4+ T cells following CCL19 (MIP-3β) and CCL21 (Exodus-2) stimulation (S6A Fig), suggesting that cytokine recognition, signaling through the Rac/Rho pathway, and actin polymerization/depolymerization were intact. Reduced T cell binding to ICAM-1 also could lead to diminished transmigration [31]. However, we observed no difference in the ability of naïve CD4+ T cells from adult or old mice to bind ICAM-1 coated substrates or migrate through ICAM-1 coated transwells towards CCL19 and CCL21 chemoattractants (S6B and S6C Fig).
Age-dependent cell-intrinsic defects in naïve CD4+ T cells lead to reduced GC responses
To link the reduced GC responses in old mice (Fig 2) with cell-intrinsic migratory defects of the naïve CD4+ T cells (Fig 4), we adoptively transferred sorted naïve CD4+ T cells from adult or old mice to recipient TCR β/δ -/-mice (lacking T cells) that had been infected 2 days earlier with WNV (Fig 6A). We quantified CD4+ T cell and GC responses 6 days later at 8 days after infection. In all recipient TCR β/δ -/-mice, very low GC responses were observed in the DLN, possibly because few antigen-specific CD4+ T cells reached this site (data not shown); however, GC responses were detected in the spleen. Animals receiving donor cells from old mice had a reduced percentage of CD4+ T cells in the spleen relative to those receiving adult donor cells (Fig 6B), and this corresponded with a lower percentage of TFH and GC B cells (Fig 6C and 6D). These data suggest that intrinsic CD4+ T cell defects alone, independent of other age-dependent immunological defects, contribute to the reduced GC response following WNV infection in old mice.
Fig. 6. Transfer of naïve CD4+ T cells from old mice results in reduced GC responses. 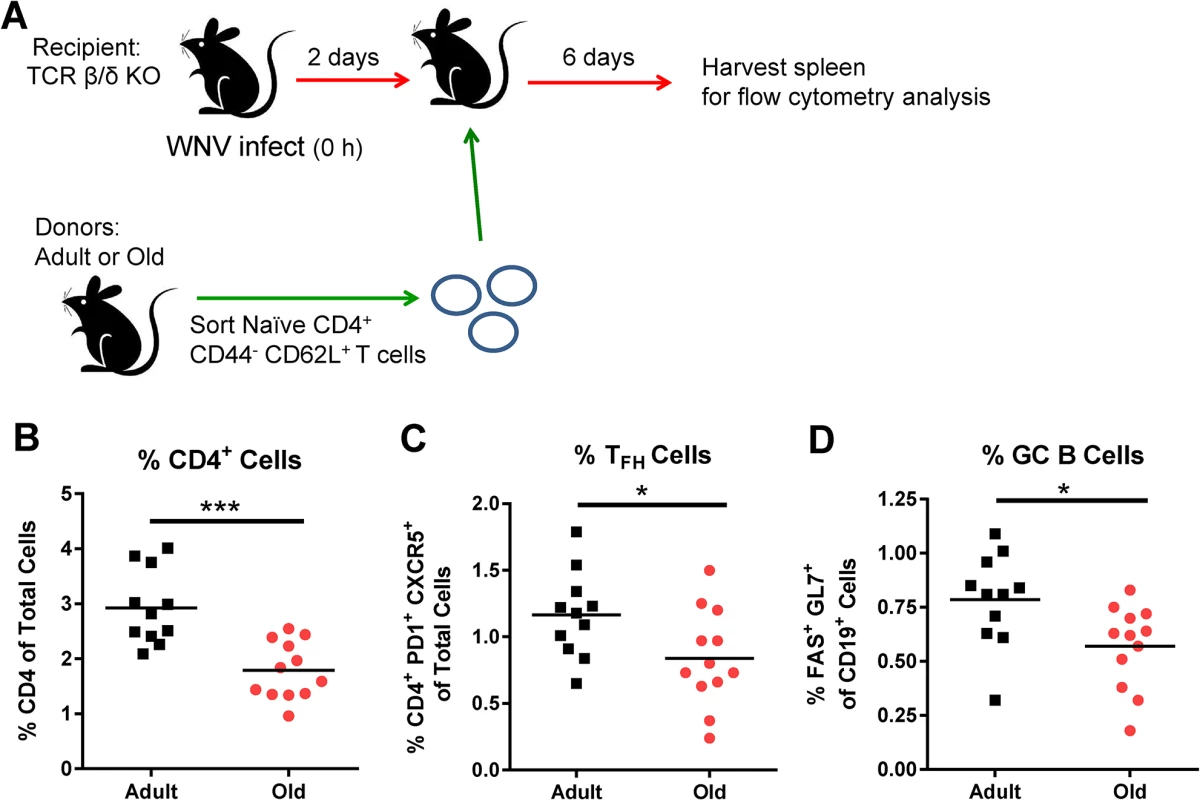
A. TCR β/δ-/- recipient mice were infected with WNV (New York strain). Two days after infection, 3 x 106 naïve sorted CD4+ T cells from adult or old mice were transferred adoptively into the recipient mice and 6 days post transfer (at 8 days post infection) spleens were analyzed for GC responses. Percentages of CD4+ T cells (B), TFH cells (CD4+ PD1+ CXCR5+ cells) (C), and GC B cells (Fas+ GL7+ of CD19+ cells) (D) were quantified. The results are pooled from three independent experiments with a total of 11 to 12 mice per group, and each data symbol represents a single mouse. Asterisks indicate statistical significance (*, P < 0.05; ***, P < 0.001; unpaired t-test). B cells from adult and old mice traffic to lymphoid tissues equivalently
We next tested whether B cells also had a trafficking defect by adoptively transferring a 1 : 1 ratio of differentially dye-labeled adult and old B cells into adult recipient WNV-KUN infected mice and quantifying cell recruitment. No major differences were observed in the numbers of adult and old donor B cells in lymphoid tissues at 6 hours after transfer (Fig 7A and 7B), suggesting that impaired cell accumulation was not a general phenomenon that affected all lymphocyte subsets from old mice. Similar results were obtained in the spleen at 1 hour after transfer (data not shown). CD19+ B cells also did not show defects in migration in explanted lymph nodes (Fig 7C–7F and 7K and 7L) or spleen (Fig 7G–7J and S4 Movie).
Fig. 7. Normal migration of CD19+ B cells from old mice. 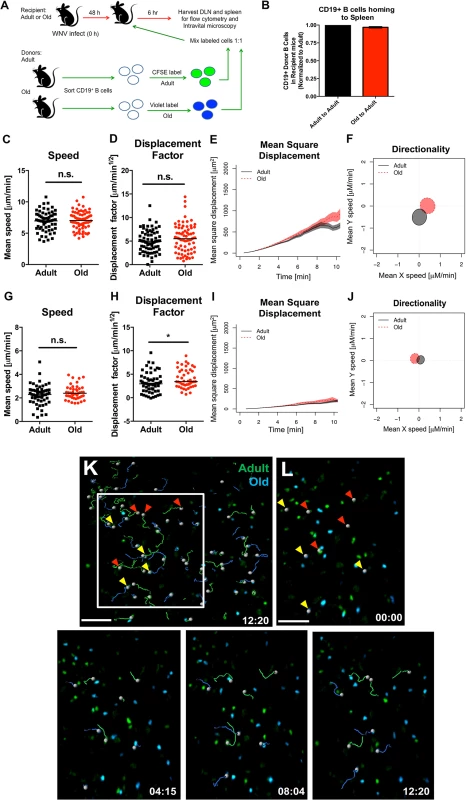
A. Scheme of adoptive transfer studies of CD19+ B cells. 5 x 106 isolated B cells from adult or old mice were differentially labeled with fluorescent dyes, mixed in a 1:1 ratio, and transferred to recipient adult mice infected with WNV-KUN 48 hours earlier via a subcutaneous route. B. Six hours later, spleens were harvested. Cells were counted and the frequency of adult and old donor cells was determined by flow cytometry. The data was normalized to the levels of adult donor cells in the recipient organ and is a composite of two independent experiments. C-J. Analysis of movement parameters of adult and old donor naïve CD19+ B cells in explanted LN (C-F) or spleen (G-J) 6 hours post-transfer to recipient mice that had been infected with WNV-KUN 48 hours earlier. Individual cells were tracked and (C, G) speed (μM/min), (D, H) displacement factor (μm/min1/2), (E, I) mean square displacement over time, and (F, J) directionality (Hotelling’s test) were determined. The data in C, D, G, and H are shown as a scatter plot and reflects two independent experiments. n.s. indicates the differences were not statistically significant; * indicates P < 0.05, Mann-Whitney test. K-L. Time-lapse image sequences of transferred adult and old CD19+ B cells in explanted LN in recipient mice 48 h after WNV-KUN infection. Differentially labeled (blue = old, green = adult) CD19+ B cells were adoptively transferred into WNV-KUN infected adult recipient mice (48 h after infection) and DLN were harvested 6 to 8 h later followed by ex vivo imaging. Panel K presents all tracked adult and old cells during the first 12 minutes within the cropped area with the white frame. Panel L and others presents time-lapse image sequences of adult and old cell tracking during first 12 minutes. Yellow and red arrows indicate which old and adult cells were analyzed and their starting points, respectively. Scale bar is indicated in white. Images are individual frames from a continuous time-lapse movie (S4 Movie). To further assess possible cell-intrinsic defects in B cells from old mice we adoptively transferred an equal number of bone marrow cells from adult or old CD45.2 mice into separately irradiated CD45.1 adult recipient mice. We also transferred bone marrow cells from adult B cell-deficient (μMT) to provide equivalent T cell help (Fig 8A). Twelve weeks later, the transfers were confirmed by analyzing circulating CD19+ B cells in blood (Fig 8B). Recipient mice were infected with WNV and serum was harvested for evaluation of WNV-specific antibody responses at days 5, 8, and 15 after infection. Notably, recipient mice reconstituted with B cells from adult or old mice had similar WNV-specific IgM and IgG responses (Fig 8C and 8D).
Fig. 8. Humoral immune response of donor bone marrow into irradiated recipient mice. 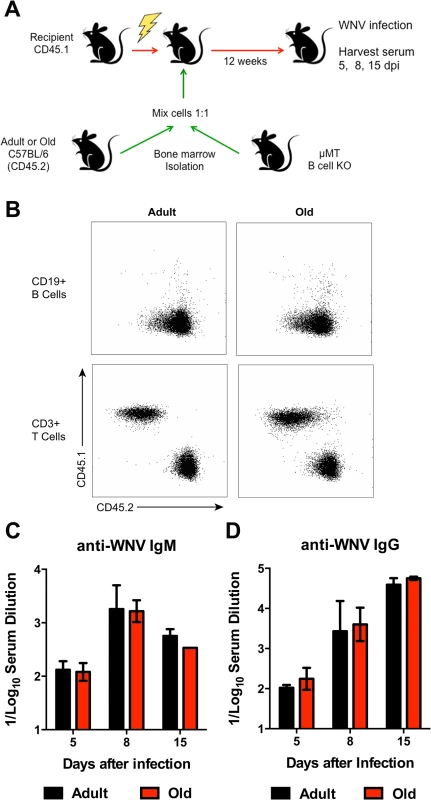
A. Scheme of reconstitution studies with bone marrow from adult or old mice. 107 bone marrow cells from adult or old mice were mixed with 107 bone marrow cells from μMT (B cell deficient) mice and transferred into irradiated recipient CD45.1 mice. After 12 weeks, mice were infected with WNV (New York strain) and serum was harvested 5, 8, and 15 dpi. B. After 12 weeks post transfer, blood was sampled and tested for reconstitution efficiency. The vast majority (>95%) of circulating CD19+ B cells was derived from the CD45.2 donor mice. C-D. IgM (C) and IgG (D) levels were measured by ELISA for reactivity with WNV E protein. Data is plotted as the reciprocal log10 titer and represents data from 5 mice per group. Reduced leukocyte migration and chemokines in the DLN
The reduced accumulation of adult or old donor cells in old recipient lymphoid tissue (Fig 4B and 4C) suggested a separate environmental defect independently contributed to the reduced migration of naïve CD4+ T cells. To address the basis for these findings, we measured the levels of pro-inflammatory cytokines and chemokines in the inflamed DLN (Fig 9). Within one to two days of WNV infection, lower levels of several chemokines were apparent in the homogenates of DLN from old mice. This included chemoattractants for monocytes (MCP-1 (CCL2)), granulocytes (MIP-1α (CCL3)), NK cells (MIP-1β (CCL4)), B cells (BLC (CXCL13)), and was associated with the subsequently decreased production of proinflammatory cytokines including IL-1α, IL-2, IL-6, and IFN-γ, and the anti-inflammatory cytokine IL-10. Relevant to our naïve CD4+ T cells findings, the DLNs from old mice had nearly a 10-fold decrease in the naïve T cell chemoattractant CCL21 at day 2 after infection (Fig 9N). Consistent with the blunted production of chemokines, we also observed diminished accumulation of NK cells, γδT cells, macrophages, and dendritic cells in the DLN of old mice after WNV infection (Fig 10). Thus, in addition to cell-intrinsic defects of naïve CD4+ T cells in old animals, the DLNs of old mice separately have attenuated inflammatory cytokine and chemokine responses, which reduced the recruitment of other cell types that orchestrate adaptive immunity against WNV infection.
Fig. 9. Decreased levels of cytokines and chemokines in the DLN of old mice. 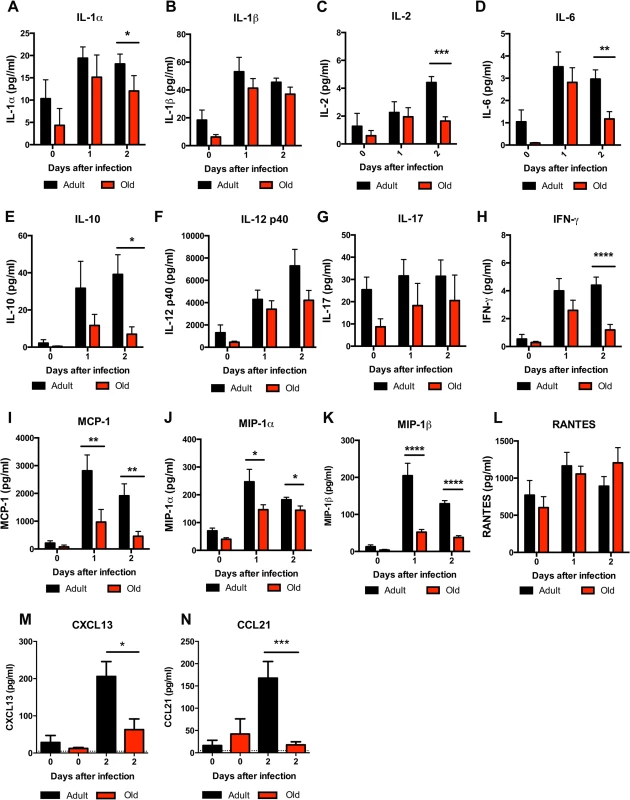
Adult and old mice were infected bilaterally with 102 PFU of WNV (New York strain) in the footpad and DLN were harvested at days 0, 1, and 2 after infection. A-L. Levels of cytokines (IL-1α (A), IL-1β (B), IL-2 (C), IL-6 (D), IL-10 (E), IL12 p40 (F), IL-17, (G), and IFN-γ (H)) and chemokines (MCP-1 (I), MIP-1α (J), MIP-1β (K), and RANTES (L)) in DLN were measured by Bio-Plex assay. M-N. Levels of chemokines (CXCL13 (M) and CCL21 (N)) in DLN were measured by ELISA. Data are presented as mean + SEM and reflects a total of 6 to 8 mice for each group from two independent experiments. Asterisks indicate statistical significance (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; Mann-Whitney test). Fig. 10. Leukocyte infiltration in the DLN of WNV-infected adult and old mice. 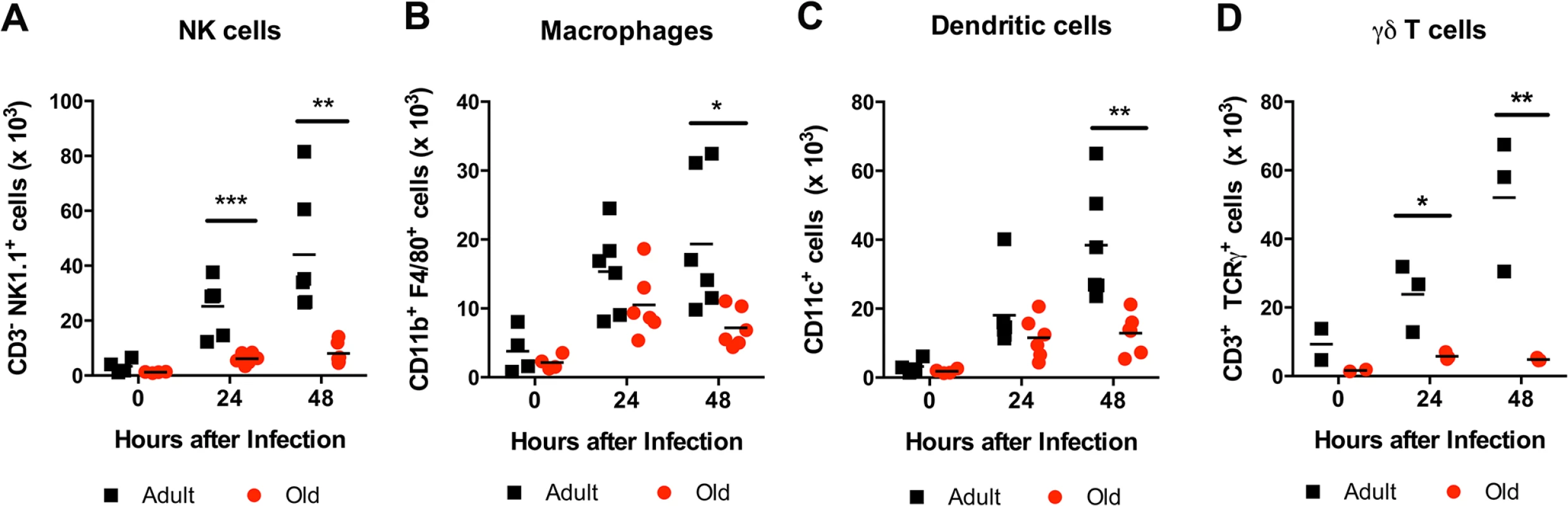
Adult and old mice were infected with 102 PFU of WNV (New York strain) in the footpad. One or two days later, the draining popliteal LN was harvested, cells were stained with antibodies to detect specific cell populations including (A) NK1.1+ NK cells, (B) CD11b+ F4/80+ macrophages, (C) CD11c+ dendritic cells, and (D) γδ T cells. The results are depicted as a scatter plot and are pooled from two independent experiments with a total of 6 mice per group (with the exception of γδ T cells, which reflects 3 mice per group). Asterisks indicate statistical significance (*, P < 0.05; **, P < 0.01; ***, P < 0.001; Mann-Whitney test). Discussion
The decline of the immune system with age correlates with increased susceptibility to many infectious diseases and a decreased response to vaccines. In this study, using a mouse model of aging in the context of WNV infection, we identified cell-intrinsic defects in naïve CD4+ T cells as well as environmental defects that resulted in delayed and reduced lymphocyte accumulation in the inflamed DLN of old mice at very early time points. These defects delayed the development of the early germinal center reaction, which resulted in blunted humoral immune responses that likely contributed to increased viral titers in the blood and peripheral organs and enhanced vulnerability to lethal infection. Even small differences in WNV-specific antibody levels at early times after infection affect dissemination to the brain and clinical outcome [17,18]. As mice lacking B cells uniformly succumb to WNV infection [17], the delayed adaptive immune response in old mice still provided a significant level (~50% survival) of protection. Old mice surviving the acute stage of infection eventually developed similar overall levels of WNV-specific antibody titers and germinal center B and TFH cells compared to adult mice, although qualitative defects in the humoral immunity remained. In addition, old mice had deficits in their long-lived plasma cell memory response relative to the adult mice. This suggests that despite GC B cells and TFH cells reaching equivalent levels in the surviving adult and old mice after WNV infection, functional defects persist in the germinal center reactions of old mice, which contribute to blunted memory responses. While further study is warranted, part of the age-dependent differences in memory phenotypes could be associated with differential expression of pro-survival (e.g., Bcl-2) or pro-apoptotic (e.g., TRAIL) proteins in B cells [32,33] or less likely the lower levels of CXCR5 observed on TFH cells.
Although the association between aging and susceptibility to viral infection has been described [34,35], our results support a model in which immune system deficits occurring shortly after infection determine clinical outcome. Within 48 hours of WNV infection, we detected a diminished immune response in the DLN of old mice that included reduced accumulation of NK cells, dendritic cells, macrophages, and B and T cells. Associated with this phenotype were decreased levels of several cytokines and chemokines, including the naïve T cell chemoattractant CCL21 and the B cell chemoattractant CXCL13. These results are consistent with reports showing disrupted cytokine production after pattern recognition receptor stimulation in innate immune cells from old mice and humans [3]. Defects in the cellular production of cytokines and chemokines in the LN of old mice likely contribute to the reduced accumulation of innate and adaptive immune cells after inflammatory or infectious stimuli. Consistent with this idea, reduced levels of CCL19 and CCL21 were detected in the spleen of old mice immunized with OVA absorbed on alum [36]. These chemokine and recruitment defects also may lead to defective priming of CD8+ T cells, as the DLN has an important role in priming these cells during a systemic viral infection [37]. Thus, our data provides an explanation why qualitative and quantitative defects in antigen-specific CD8+ T cells responses also exist after WNV infection of old mice [21].
Prior studies have reported reduced frequencies of naïve CD4+ T cells in old animals, similar to our observations, due to long-term antigen exposure, conversion to memory phenotypes, and repertoire contraction [5]. We also observed a separate, independent cell-intrinsic defect in the naïve CD4+ T cells from old mice. By adoptively transferring adult or old naïve CD4+ T cells into recipient mice lacking T cells, we found that intrinsic defects in the naïve CD4+ T cells of the old mice contributed to reduced GC responses independent of other factors, analogous to previous immunization models with transgenic donor cells [10]. Although others have identified naïve CD4+ T cell-intrinsic defects following antigen encounter [38], we identified an earlier, age-dependent deficit in the migratory capacity of the naïve CD4+ T cells. Soon after adoptive transfer of naïve CD4+ T cells from adult and old donors into adult recipient mice, cells from the old mice accumulated in DLNs to lower levels as compared to cells from adult mice. Intravital microscopy revealed that the trafficking of naïve CD4+ T cells from old mice was impaired at the diapedesis step specifically, as cell rolling and adhesion in HEVs was delayed only slightly relative to cells from adult mice. The reduced trafficking of naïve CD4+ T cells to DLN in old mice suggests that immune surveillance declines during aging. Once naïve CD4+ T cells from old mice entered the parenchyma of the inflamed LN, we also observed reduced motility. Although the T cell trafficking defects may seem modest, mathematical modeling [30] predicted that the reduced motility in the DLN could lead to many hours of delay in finding cognate antigen. Combined with the additional homing defects, this would hinder the induction of antigen-specific adaptive immune responses substantially. Due to the rapid nature of viral replication and dissemination, such a lag could and likely does alter disease outcome.
What is the cell-intrinsic defect of naïve CD4+ T cells from old animals that results in a reduced migratory capacity? A diminished capacity of naïve CD4+ T cells from old animals to activate and proliferate following TCR stimulation has been reported [38]; this has been ascribed to reduced cytoskeletal rearrangement [39,40], aberrant surface glycosylation [41], and altered phosphorylation of key signaling molecules [42]. These defects in naïve CD4+ T cells from old animals can be overcome to some extent by cytokine (e.g., IL-2) stimulation [43] or treatment with O-sialoglycoprotein endopeptidases [44]. We found no differences in the expression of cell-surface molecules important for chemokine binding or migration in adult or old mice. We also did not detect differences in the ability of naïve CD4+ T cells from adult and old mice to bind ICAM-1 or undergo cytoskeletal rearrangement after chemokine activation. Instead our studies suggest a key cell-intrinsic migratory defect in naïve CD4+ T cells from old mice that occurs in vivo prior to antigen stimulation at very early times relative to infection. Impaired function of non-muscle myosin IIA in CD4+ T cells from elderly humans and old mice has been linked to defective migration ex vivo [40] and an inability to deform nuclei [45] respectively, which are processes critical to successful diapedesis; however, we found no difference in the ability of naïve CD4+ T cells from adult and old mice to migrate across an ICAM-1-substrate ex vivo. Alternatively, mTOR inhibition rescues some of the age-related functional defects in naïve CD4+ T cells from old mice, suggesting that a reduced metabolic capacity could affect energy stores [44,46]. As migratory activities require extensive energy-dependent cytoskeletal rearrangements, generation of leading edges and uropodia, lower energy reserve levels could compromise this process.
In comparison to naïve CD4+ T cells, and even though humoral responses were blunted, we did not observe significant defects in the homing, motility, or function of CD19+ B cells from old mice. Intravital microscopy in explanted lymphoid tissues showed similar movement and displacement by B cells from old and adult mice. Moreover, in experiments in which bone marrow from old and adult mice were transferred into irradiated adult recipient mice and then infected with WNV, differences in the antiviral antibody response were not apparent; this confirms previous reports suggesting that newly derived B cells are functionally more intact than long-lived B cells in old animals [47]. These findings suggest that the defects in the naïve CD4+ T cells from old mice are not associated with a general functional decline of all lymphocyte subsets.
In summary, using a mouse model of WNV infection, we identified a specific age-dependent decline in the trafficking of naïve CD4+ T cells. Efficient T cell trafficking is required for optimal antigen recognition in the DLN and for timely initiation of T-helper dependent antibody induction. Because the rapid production of neutralizing and inhibitory antibodies is essential for limiting WNV replication and dissemination [17,18], even a modest delay in the kinetics of the humoral response in elderly persons could increase susceptibility to virus infection. As functional deficits in the cells that coordinate early adaptive immune response and the LN microenvironment exist, strategies to correct these defects in at-risk individuals could improve responses to vaccines and limit replication of microbes that promote rapid disease pathogenesis.
Materials and Methods
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocols were approved by the Institutional Animal Care and Use Committee at the Washington University School of Medicine (Assurance Number: A3381-01). Dissections and footpad injections were performed under anesthesia that was induced and maintained with ketamine hydrochloride and xylazine, and all efforts were made to minimize suffering.
Reagents and antibodies
Cell Trackers: Cell Tracker Orange CMTMR (5-(and-6)-(((4-Chloromethyl) Benzoyl) Amino) Tetramethylrhodamine), CellTrace CFSE (carboxyfluorescein diacetate, succinimidyl ester) and CellTrace Violet were purchased from Life Technologies. The following fluorochrome or biotin-conjugated antibodies were purchased from BD Biosciences, Biolegend, and eBioscience (clone name in parenthesis): CD3ε (145-2C11), CD4 (RM4-5), CD8α (53–6.7) CD11b (M1/70), CD11c (HL3), CD19 (1D3), CD28 (E18), CD44 (IM7), CD45R (RA3-6B2), CD62L (MEL-14), CD69 (H1.2F3), OX40/CD134 (OX-86), Ki67 (SolA15), PD1 (29F.1A12), FAS (Jo2), GL7, F4/80 (BM8), TCRγ (eBioGL3), NK1.1 (PK136), VLA4 (R1-2), LFA-1 (2D7), PSGL-1 (2PH1), CCR7 (4B12), CXCR4 (L276F12), and CXCR5 (2G8).
Viruses and cells
The WNV strain (3000.0259) was isolated in New York in 2000 and passaged once in C6/36 Aedes albopictus cells. The WNV-KUN strain (CH16532) was a gift of R. Tesh (World Reference Center of Emerging Viruses and Arboviruses, Galveston, TX) and amplified as described previously [48]. Viral titer was determined by plaque assay using Vero cells, as described previously [49].
Mouse experiments
C57BL/6 mice were purchased from The Jackson Laboratory (4 month-old) (Bar Harbor, ME) or acquired from the National Institute of Aging dedicated breeding colony at Charles River Laboratories (18 month-old) (Wilmington, MA). TCR β/δ-/- mice were purchased from The Jackson Laboratory and bred in the animal facilities at the Washington University School of Medicine. All mice were housed in a pathogen-free mouse facility at the Washington University. Mice were inoculated subcutaneously via footpad injection with 102 plaque-forming units (PFU) of WNV-NY or 104 PFU of WNV-KUN diluted in 50 μl of Hanks balanced salt solution (HBSS) supplemented with 0.1% heat-inactivated fetal bovine serum (FBS). For intravital imaging of the draining cervical lymph node, mice were infected subcutaneously in the nape of the neck.
Measurement of viral burden
At specified time points after WNV infection, serum was obtained by intracardiac heart puncture, followed by intracardiac perfusion (20 ml of PBS), and organ recovery. Organs were weighed, homogenized using a bead-beater apparatus, and titrated by plaque assay on BHK21-15 cells as described previously [49].
Bone marrow chimera and transfers
Bone marrow cells were collected from adult or old C57BL/6 (CD45.2) mice and adult B-cell deficient μMT mice. Adult or old bone marrow cells were mixed with an equal number of μMT bone marrow cells and 1 x 107 cells were transferred adoptively by retroorbital injection into 8 to 12-wk-old 800 cGy–irradiated B6.SJL (CD45.1) mice (National Cancer Institute). Twelve weeks later, reconstitution was validated by flow cytometry and mice were infected with WNV.
Ovalbumin immunizations
Mice were injected subcutaneously in the footpad with an emulsion containing CFA and 10 nmoles of OVA323–337 peptide, and DLN were analyzed at indicated time points. The ELISPOT assay has been described previously [50,51]. To evaluate MHC class II specific T cell responses in adult and old mice, 5 x 105 cells from the DLN were harvested at day 7 post-immunization and plated on 96-well plates with Immobilon-P membrane coated with anti-mouse IL-2 antibody. After overnight incubation, plates were washed, incubated with a secondary antibody and developed as previously described [50,51]. Spots were analyzed on dedicated equipment and software (Cellular Technology Ltd along with Immunospot software Version 5.0.9) to calculate the total number of antigen-specific CD4+ T cells in DLN.
Antibody responses
The levels of WNV-specific IgM and IgG were determined using an ELISA against purified WNV E protein [52]. Focus reduction neutralization assays were performed on Vero cells after mixing serial dilutions of serum with a fixed amount (100 FFU) of WNV [49].
ELISPOT assay
WNV antigen-specific antibody secreting cells were quantified via an enzyme-linked immunospot assay [28]. Briefly, mixed cellulose esters filter plates (Millipore) were coated with 20 μg/ml of purified WNV E protein. Bone marrow cells were harvested from infected mice 90 days post infection, and 200,000 cells were seeded per well with 8 wells per mouse. Antibody spots were developed with chromogen substrate (3-amino-9-ethyl-carbazole) and wells were imaged and enumerated with an ImmunoSpot plate reader from Cellular Technology Ltd.
Cell purification
To isolate naïve CD4+ T cells, splenocytes were isolated from mice and erythrocytes depleted with ACK lysis buffer (Invitrogen). Cells were then stained in buffer (PBS, 1% FBS, and 2 mM EDTA) with biotinylated antibodies against CD8, CD45R (B220), NK1.1, and MHC class II as well as fluorescently conjugated antibodies against CD4, CD44, and CD62L. Cells were washed with 10 mL of staining buffer and stained with anti-biotin magnetic beads (Miltenyi Biotec). Magnetically labeled cells were then depleted by passing through a LS magnetic column (Miltenyi Biotec) leaving roughly 90% pure CD4+ T cells. CD4+ CD44- CD62L+ were then isolated on an Aria-II (BD Biosciences) fluorescence-activated cell sorter (see S2 Fig). B cells were isolated using a B Cell Isolation Kit (Miltenyi Biotech). Briefly, cells were stained with a biotinylated-antibody cocktail followed by anti-biotin labeled magnetic beads. Cells were loaded onto a magnetic LS column and the flow-through was collected.
Adoptive cell transfers
Naïve CD4+ T cells or B cells from uninfected adult or old mice were sorted as detailed above. For cell tracking, isolated cells from adult and old donors were labeled differentially with vital dyes (CellTracker Orange CMTMR, CellTrace CFSE, or CellTrace Violet) according to the manufacturer recommendations. Importantly, dyes were switched between old and adult cells in some experiments and dye-dependent effects on cell trafficking were not observed under our staining conditions (data not shown). Isolated cells were incubated at 37°C and 5% CO2 in pre-warmed CO2-Independent Medium (Invitrogen) with 2 μM of CellTrace CFSE, 5 μM CellTracker Orange, or 10 μM of CellTrace Violet (Invitrogen) for 15 minutes. Subsequently, cells were centrifuged (300 x g, 5 minutes) and the pellet was resuspended in pre-warmed medium for another 30 minutes. The incubation was stopped after two washes with cold PBS. Finally, cells were counted and adjusted to equal number for homing or microscopy imaging experiments. Cells were transferred adoptively via an intravenous route into recipient mice that had been infected subcutaneously with WNV-KUNV 48 hours earlier. For transfer into TCR β/δ-/- mice, 3 x 106 sorted naïve CD4+ T cells were injected intravenously into each recipient mouse that had been infected subcutaneously with WNV-NY 48 hours earlier.
T cell homing experiments
For analysis of T cell homing to the LN and spleen, naïve CD4+ T cells or B cells were sorted from adult and old mice and labeled with different dyes. Cells were suspended in PBS at a ratio 1 : 1 (adult to old) and injected intravenously (2 x 106 from each donor). One hour post transfer, LN and spleens were harvested and processed into single cell suspensions. Cells were analyzed by flow cytometry on an LSRII (BD Biosciences).
Intravital two-photon microscopy
We analyzed the trafficking behavior of lymphocytes in lymphoid organs using published methods for ex vivo (LN explants) and with minor modifications for in vivo imaging of cervical LNs [53]. Recipient mice (adult or old) infected with WNV-KUN 48 hours earlier were given 5 x 106 cells CellTrace CFSE or Violet labeled naïve T cells (from adult and old donors). For ex vivo analysis, LN were harvested 6 to 8 hours later, glued to plastic cover slips with VetBond (3M), placed under the flow of warm (35°C to 37°C) oxygenated medium (DMEM, without phenol red) [54]. Intravital imaging of T cell extravasation was performed as described [55] with the following modifications. Mice were anesthetized with avertin (250 mg/kg) and connected to a MiniVet Mouse Ventilator (Harvard Apparatus) to reduce movement artifacts and improve mouse viability. The skin of the neck was opened and the cervical DLN exposed on a skin flap and the inner surface was attached to a plastic cover slip using VetBond with the LN protruding through a hole in the center. Imaging was performed on A custom-build two-photon microscope equipped with an Olympus 1.0 NA 20x water-immersion objective, a Vision II Coherent Ti:Sapphire laser and running ImageWarp (A&B Software) for hardware and acquisition control [56]. Dyes were excited at 820 nm and detected using 480 nm and 570 nm dichroic filters. T cell rolling in vessels was imaged at 25 f/sec and 3D images collected ~ 2 per minute with image dimensions of ~250 x 225 x 75 μm (X,Y,Z; 2.5 μm Z steps). Blood vessels in the LN were labeled with QTracker 655 (10 μl in 50 μl of PBS given intravenously, Life Technologies).
Image analysis
Multidimensional data rendering and cell tracking were performed using Imaris software (version 7.3, Bitplane). For quantitative and statistical analysis of T cell motility we used either, T Cell Analyzer Software (version 1.7.0, Dr. J. Dempster, University of Strathclyde, UK) and an open-source software suite developed by Dr. J Textor (University of Ultrecht, The Netherlands). For quantitative and statistical analysis of T and B cell motility, we used the X and Y dimensions of the cell tracks to avoid bias arising from the intrinsically poorer Z resolution [57]. Displacement factors were defined as the distance between the endpoints of a track divided by the square root of its duration. The unpaired Mann-Whitney U test was used to compare track motility parameters. To control for variation between experiments, data were normalized prior to comparison by equalizing the medians across independent experiments. Hotelling's test for directionality was performed as described previously [58]. Briefly, cell tracks were broken down into steps (each step being a pair of consecutive cell positions), and the mean speed vector was computed per population of interest. Under the null hypothesis, the cells perform a random walk and the mean speed vector is zero in each dimension. Ellipses in Figs 4K, 5E, 7F and 7J show 95% confidence regions for the mean speed vector. If an ellipse does not contain the origin (0,0), then the null hypothesis is rejected for that population, and there is some directionality in the cell motion. For all cell populations studied in this paper, directionality was of a small magnitude (<1 μm/min at mean speeds of 5–10 μm/min).
LN cell subset analysis
Adult or old draining popliteal LN were harvested from mice at the indicated times post infection. To generate a single cell suspension, LNs were placed in staining buffer and gently crushed with the back of a syringe plunger and cells passed through a 70 μm cell strainer. The total number of live cells was quantified by trypan blue exclusion in a hemocytometer. Cells were stained in buffer (PBS, 1% FBS, and 2 mM EDTA) with fluorescently conjugated antibodies for 30 minutes on ice. Cells were washed in buffer and then processed on an LSRII flow cytometer (BD Biosciences).
Histology
Draining lymph nodes were harvested at 6 days post infection with WNV-KUN. DLNs were frozen in O.C.T. compound, and 9-μm-thick sections were cut with a cryostat. Sections were mounted on microscope slides and stained with anti–GL7-PE (1 : 50), anti–IgD-FITC (1 : 50), and DAPI (1 μg/ml) in PBS for 1 hour at room temperature. Slides were washed in PBS and a coverslip mounted on top of the stained tissue sections.
Phenotyping of T cells
Splenocytes were isolated and erythrocytes were lysed with ACK buffer. Cells were stained with anti-CCR7 in staining buffer for 30 minutes at 37°C. Cells were washed and then incubated with anti-CD4, CD19, CD44, CD62L, LFA1, VLA4, PSGL1, and CXCR4 in staining buffer for 30 minutes on ice. Cells were processed on an LSRII flow cytometer. For filamentous actin staining, isolated CD4+ T cells were incubated with 100 ng/mL of CCL19 and CCL21 at 37°C in PBS + 0.1% BSA. Reactions were stopped by adding paraformaldehyde (3.6% final concentration), and cells were stained with anti-CD4, CD44, CD62L, and Alexa Fluor 647 phalloidin (Life Technologies). Cells were analyzed using an LSRII flow cytometer.
Cytokine analysis
Mice were infected subcutaneously with 102 PFU of WNV in the footpad. Draining popliteal LNs were collected at 24 or 48 hours post infection and placed in 200 μl of PBS containing 0.5% (w/v) BSA. DLN were homogenized and cytokines were quantified using a Bio-Plex Pro 23-plex group I cytokine kit (Bio-Rad) and Bio-Plex 200 (Bio-Rad). CXCL13 and CCL21 were measured using ELISA kits from R&D Systems.
ICAM-1 binding and transwell migration assays
ICAM-1 Fc (R&D Systems) was adsorbed to a polystyrene Petri dish at 4 or 40 μg/ml in PBS. After a one-hour incubation at 37°C, plates were rinsed with PBS and non-specific binding sites were blocked with 1% human serum albumin (Sigma-Aldrich) for one hour at room temperature. Freshly isolated naïve CD4+ T cells (5.5 x 105) were added to Petri dishes in 1.5 ml of L15 media containing 0.5% FBS and 10mM HEPES and supplemented with 200 ng/ml of CCL19 and CCL21. Dishes were incubated for one hour at 37°C and then fixed with 1% paraformaldehyde. Unbound cells were removed after extensive rinsing with PBS supplemented with 0.2% human serum albumin, 1 mM CaCl2, and 1 mM MgCl2. Dishes were imaged at 2.5X magnification and the number of adherent cells was counted. For the transwell migration assay, a 96-well modified Boyden chamber with 5 μm pore size was purchased (Neuro Probe). The top membrane was coated with 0, 4, or 40 μg/ml of ICAM-1 Fc in PBS for one hour at room temperature. CCL19 or CCL21 (0.1, 1, or 10 ng/ml) in migration buffer (RPMI 1640, 0.5% FBS, and 100U/ml penicillin-streptomycin) was plated in the bottom chamber. Freshly isolated naïve CD4+ T cells (5 x 104) were added to the top chamber and incubated for 4 hours at 37°C. Subsequently, the membrane was removed and cells in bottom chamber were counted with a hemocytometer.
Data analysis
All data was analyzed using Prism software (Graph Pad 6, San Diego, CA). Flow cytometry data was analyzed using FlowJo software (Tree Star Inc.). Kaplan-Meier survival curves were analyzed by the log rank test. All other statistical analyses were performed with Mann-Whitney or unpaired t-tests as indicated in the Figure legends.
Supporting Information
Zdroje
1. Cohen HJ, Harris T, Pieper CF (2003) Coagulation and activation of inflammatory pathways in the development of functional decline and mortality in the elderly. Am J Med 114 : 180–187. 12637131
2. Renshaw M, Rockwell J, Engleman C, Gewirtz A, Katz J, et al. (2002) Cutting edge: impaired Toll-like receptor expression and function in aging. J Immunol 169 : 4697–4701. 12391175
3. Shaw AC, Goldstein DR, Montgomery RR (2013) Age-dependent dysregulation of innate immunity. Nat Rev Immunol 13 : 875–887. doi: 10.1038/nri3547 24157572
4. Lerner A, Yamada T, Miller RA (1989) Pgp-1hi T lymphocytes accumulate with age in mice and respond poorly to concanavalin A. Eur J Immunol 19 : 977–982. 2666144
5. Haynes L, Swain SL (2012) Aged-related shifts in T cell homeostasis lead to intrinsic T cell defects. Semin Immunol 24 : 350–355. doi: 10.1016/j.smim.2012.04.001 22564707
6. Blackman MA, Woodland DL (2011) The narrowing of the CD8 T cell repertoire in old age. Curr Opin Immunol 23 : 537–542. doi: 10.1016/j.coi.2011.05.005 21652194
7. Frasca D, Blomberg BB (2011) Aging affects human B cell responses. J Clin Immunol 31 : 430–435. doi: 10.1007/s10875-010-9501-7 21318330
8. Linton PJ, Haynes L, Klinman NR, Swain SL (1996) Antigen-independent changes in naive CD4 T cells with aging. J Exp Med 184 : 1891–1900. 8920876
9. Garcia GG, Miller RA (2011) Age-related defects in the cytoskeleton signaling pathways of CD4 T cells. Ageing Res Rev 10 : 26–34. doi: 10.1016/j.arr.2009.11.003 19941976
10. Eaton SM, Burns EM, Kusser K, Randall TD, Haynes L (2004) Age-related defects in CD4 T cell cognate helper function lead to reductions in humoral responses. J Exp Med 200 : 1613–1622. 15611289
11. Linterman MA (2014) How T follicular helper cells and the germinal centre response change with age. Immunol Cell Biol 92 : 72–79. doi: 10.1038/icb.2013.77 24217812
12. Frasca D, Van der Put E, Riley RL, Blomberg BB (2004) Reduced Ig class switch in aged mice correlates with decreased E47 and activation-induced cytidine deaminase. J Immunol 172 : 2155–2162. 14764681
13. Chowers MY, Lang R, Nassar F, Ben-David D, Giladi M, et al. (2001) Clinical characteristics of the West Nile fever outbreak, Israel, 2000. Emerg Infect Dis 7 : 675–678. 11585531
14. Diamond MS (2013) Flaviviruses. In: Knipe DM, & Howley P.M., editor. Fields Virology. Philadelphia, PA: Lippincott Williams & Wilkins. pp. 747–792.
15. Samuel MA, Diamond MS (2005) Alpha/beta interferon protects against lethal West Nile virus infection by restricting cellular tropism and enhancing neuronal survival. J Virol 79 : 13350–13361. 16227257
16. Mehlhop E, Whitby K, Oliphant T, Marri A, Engle M, et al. (2005) Complement activation is required for induction of a protective antibody response against West Nile virus infection. J Virol 79 : 7466–7477. 15919902
17. Diamond MS, Shrestha B, Marri A, Mahan D, Engle M (2003) B cells and antibody play critical roles in the immediate defense of disseminated infection by West Nile encephalitis virus. J Virol 77 : 2578–2586. 12551996
18. Diamond MS, Sitati EM, Friend LD, Higgs S, Shrestha B, et al. (2003) A critical role for induced IgM in the protection against West Nile virus infection. J Exp Med 198 : 1853–1862. 14662909
19. Graham JB, Da Costa A, Lund JM (2014) Regulatory T cells shape the resident memory T cell response to virus infection in the tissues. J Immunol 192 : 683–690. doi: 10.4049/jimmunol.1202153 24337378
20. Lanteri MC, O'Brien KM, Purtha WE, Cameron MJ, Lund JM, et al. (2009) Tregs control the development of symptomatic West Nile virus infection in humans and mice. J Clin Invest 119 : 3266–3277. doi: 10.1172/JCI39387 19855131
21. Brien JD, Uhrlaub JL, Hirsch A, Wiley CA, Nikolich-Zugich J (2009) Key role of T cell defects in age-related vulnerability to West Nile virus. J Exp Med 206 : 2735–2745. doi: 10.1084/jem.20090222 19901080
22. Sitati EM, Diamond MS (2006) CD4+ T-cell responses are required for clearance of West Nile virus from the central nervous system. J Virol 80 : 12060–12069. 17035323
23. Brien JD, Uhrlaub JL, Nikolich-Zugich J (2007) Protective capacity and epitope specificity of CD8(+) T cells responding to lethal West Nile virus infection. Eur J Immunol 37 : 1855–1863. 17559175
24. Shrestha B, Diamond MS (2004) Role of CD8+ T cells in control of West Nile virus infection. J Virol 78 : 8312–8321. 15254203
25. Stewart BS, Demarest VL, Wong SJ, Green S, Bernard KA (2011) Persistence of virus-specific immune responses in the central nervous system of mice after West Nile virus infection. BMC Immunol 12 : 6. doi: 10.1186/1471-2172-12-6 21251256
26. Oliphant T, Engle M, Nybakken GE, Doane C, Johnson S, et al. (2005) Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nat Med 11 : 522–530. 15852016
27. Purtha WE, Tedder TF, Johnson S, Bhattacharya D, Diamond MS (2011) Memory B cells, but not long-lived plasma cells, possess antigen specificities for viral escape mutants. J Exp Med 208 : 2599–2606. doi: 10.1084/jem.20110740 22162833
28. Purtha WE, Chachu KA, Virgin HWt, Diamond MS (2008) Early B-cell activation after West Nile virus infection requires alpha/beta interferon but not antigen receptor signaling. J Virol 82 : 10964–10974. doi: 10.1128/JVI.01646-08 18786989
29. Crotty S (2011) Follicular helper CD4 T cells (TFH). Annu Rev Immunol 29 : 621–663. doi: 10.1146/annurev-immunol-031210-101400 21314428
30. Textor J, Henrickson SE, Mandl JN, von Andrian UH, Westermann J, et al. (2014) Random migration and signal integration promote rapid and robust T cell recruitment. PLoS Comput Biol 10: e1003752. doi: 10.1371/journal.pcbi.1003752 25102014
31. Muller WA (2011) Mechanisms of leukocyte transendothelial migration. Annu Rev Pathol 6 : 323–344. doi: 10.1146/annurev-pathol-011110-130224 21073340
32. van Grevenynghe J, Cubas RA, Noto A, DaFonseca S, He Z, et al. (2011) Loss of memory B cells during chronic HIV infection is driven by Foxo3a - and TRAIL-mediated apoptosis. J Clin Invest 121 : 3877–3888. doi: 10.1172/JCI59211 21926463
33. Sherwood EM, Xu W, Riley RL (2003) B cell precursors in senescent mice exhibit decreased recruitment into proliferative compartments and altered expression of Bcl-2 family members. Mech Ageing Dev 124 : 147–153. 12633934
34. Leng J, Goldstein DR (2010) Impact of aging on viral infections. Microbes Infect 12 : 1120–1124. doi: 10.1016/j.micinf.2010.08.009 20849976
35. Murasko DM, Jiang J (2005) Response of aged mice to primary virus infections. Immunol Rev 205 : 285–296. 15882361
36. Lefebvre JS, Maue AC, Eaton SM, Lanthier PA, Tighe M, et al. (2012) The aged microenvironment contributes to the age-related functional defects of CD4 T cells in mice. Aging Cell 11 : 732–740. doi: 10.1111/j.1474-9726.2012.00836.x 22607653
37. Olson MR, McDermott DS, Varga SM (2012) The initial draining lymph node primes the bulk of the CD8 T cell response and influences memory T cell trafficking after a systemic viral infection. PLoS Pathog 8: e1003054. doi: 10.1371/journal.ppat.1003054 23236277
38. Maue AC, Yager EJ, Swain SL, Woodland DL, Blackman MA, et al. (2009) T-cell immunosenescence: lessons learned from mouse models of aging. Trends Immunol 30 : 301–305. doi: 10.1016/j.it.2009.04.007 19541537
39. Garcia GG, Miller RA (2002) Age-dependent defects in TCR-triggered cytoskeletal rearrangement in CD4+ T cells. J Immunol 169 : 5021–5027. 12391217
40. Cane S, Ponnappan S, Ponnappan U (2012) Impairment of non-muscle myosin IIA in human CD4+ T cells contributes to functional deficits in the elderly. Cell Mol Immunol 9 : 86–96. doi: 10.1038/cmi.2011.41 21983869
41. Garcia GG, Miller RA (2003) Age-related defects in CD4+ T cell activation reversed by glycoprotein endopeptidase. Eur J Immunol 33 : 3464–3472. 14635057
42. Kirk CJ, Miller RA (1998) Analysis of Raf-1 activation in response to TCR activation and costimulation in murine T-lymphocytes: effect of age. Cell Immunol 190 : 33–42. 9826444
43. Haynes L, Linton PJ, Eaton SM, Tonkonogy SL, Swain SL (1999) Interleukin 2, but not other common gamma chain-binding cytokines, can reverse the defect in generation of CD4 effector T cells from naive T cells of aged mice. J Exp Med 190 : 1013–1024. 10510091
44. Perkey E, Miller RA, Garcia GG (2012) Ex vivo enzymatic treatment of aged CD4 T cells restores cognate T cell helper function and enhances antibody production in mice. J Immunol 189 : 5582–5589. doi: 10.4049/jimmunol.1200487 23136198
45. Jacobelli J, Estin Matthews M, Chen S, Krummel MF (2013) Activated T cell trans-endothelial migration relies on myosin-IIA contractility for squeezing the cell nucleus through endothelial cell barriers. PLoS One 8: e75151. doi: 10.1371/journal.pone.0075151 24069389
46. Mannick JB, Del Giudice G, Lattanzi M, Valiante NM, Praestgaard J, et al. (2014) mTOR inhibition improves immune function in the elderly. Sci Transl Med 6 : 268ra179. doi: 10.1126/scitranslmed.3009892 25540326
47. Keren Z, Naor S, Nussbaum S, Golan K, Itkin T, et al. (2011) B-cell depletion reactivates B lymphopoiesis in the BM and rejuvenates the B lineage in aging. Blood 117 : 3104–3112. doi: 10.1182/blood-2010-09-307983 21228330
48. Pinto AK, Richner JM, Poore EA, Patil PP, Amanna IJ, et al. (2013) A hydrogen peroxide-inactivated virus vaccine elicits humoral and cellular immunity and protects against lethal West Nile virus infection in aged mice. J Virol 87 : 1926–1936. doi: 10.1128/JVI.02903-12 23221549
49. Brien JD, Lazear HM, Diamond MS (2013) Propagation, quantification, detection, and storage of West Nile virus. Curr Protoc Microbiol 31 : 15D 13 11–15D 13 18.
50. Gmyrek GB, Akilesh HM, Graham DB, Fuchs A, Yang L, et al. (2013) Loss of DAP12 and FcRgamma drives exaggerated IL-12 production and CD8(+) T cell response by CCR2(+) Mo-DCs. PLoS One 8: e76145. doi: 10.1371/journal.pone.0076145 24155889
51. Gmyrek GB, Graham DB, Sandoval GJ, Blaufuss GS, Akilesh HM, et al. (2013) Polarity gene discs large homolog 1 regulates the generation of memory T cells. Eur J Immunol 43 : 1185–1194. doi: 10.1002/eji.201142362 23436244
52. Mehlhop E, Diamond MS (2006) Protective immune responses against West Nile virus are primed by distinct complement activation pathways. J Exp Med 203 : 1371–1381. 16651386
53. Cahalan MD, Parker I, Wei SH, Miller MJ (2002) Two-photon tissue imaging: seeing the immune system in a fresh light. Nat Rev Immunol 2 : 872–880. 12415310
54. Miller MJ, Wei SH, Parker I, Cahalan MD (2002) Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science 296 : 1869–1873. 12016203
55. Miller MJ, Wei SH, Cahalan MD, Parker I (2003) Autonomous T cell trafficking examined in vivo with intravital two-photon microscopy. Proc Natl Acad Sci U S A 100 : 2604–2609. 12601158
56. Zinselmeyer BH, Dempster J, Wokosin DL, Cannon JJ, Pless R, et al. (2009) Chapter 16. Two-photon microscopy and multidimensional analysis of cell dynamics. Methods Enzymol 461 : 349–378. doi: 10.1016/S0076-6879(09)05416-0 19480927
57. Beltman JB, Maree AF, de Boer RJ (2009) Analysing immune cell migration. Nat Rev Immunol 9 : 789–798. doi: 10.1038/nri2638 19834485
58. Textor J, Peixoto A, Henrickson SE, Sinn M, von Andrian UH, et al. (2011) Defining the quantitative limits of intravital two-photon lymphocyte tracking. Proc Natl Acad Sci U S A 108 : 12401–12406. doi: 10.1073/pnas.1102288108 21734152
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Transmission Properties of Human PrP 102L Prions Challenge the Relevance of Mouse Models of GSSČlánek Decline of FoxP3+ Regulatory CD4 T Cells in Peripheral Blood of Children Heavily Exposed to MalariaČlánek IFNγ and IL-12 Restrict Th2 Responses during Helminth/ Co-Infection and Promote IFNγ from Th2 CellsČlánek Exploiting Fungal Virulence-Regulating Transcription Factors As Novel Antifungal Drug Targets
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 7- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Basic Prion Science “Spreads” Insight
- Research Driven by Curiosity: The Journey from Basic Molecular Biology and Virology to Studies of Human Pathogenic Coronaviruses
- Cross Kingdom Activators of Five Classes of Bacterial Effectors
- Vaccination Drives Changes in Metabolic and Virulence Profiles of
- Expression of the Blood-Group-Related Gene Alters Susceptibility to Infection
- Transmission Properties of Human PrP 102L Prions Challenge the Relevance of Mouse Models of GSS
- Latent KSHV Infected Endothelial Cells Are Glutamine Addicted and Require Glutaminolysis for Survival
- The DSF Family of Cell–Cell Signals: An Expanding Class of Bacterial Virulence Regulators
- Intraperitoneal Infection of Wild-Type Mice with Synthetically Generated Mammalian Prion
- Vpr Promotes Macrophage-Dependent HIV-1 Infection of CD4 T Lymphocytes
- An In-Depth Comparison of Latency-Reversing Agent Combinations in Various and HIV-1 Latency Models Identified Bryostatin-1+JQ1 and Ingenol-B+JQ1 to Potently Reactivate Viral Gene Expression
- α-Macroglobulin Can Crosslink Multiple Erythrocyte Membrane Protein 1 (PfEMP1) Molecules and May Facilitate Adhesion of Parasitized Erythrocytes
- Should Symbionts Be Nice or Selfish? Antiviral Effects of Wolbachia Are Costly but Reproductive Parasitism Is Not
- A Unique Human Norovirus Lineage with a Distinct HBGA Binding Interface
- The Broad Neutralizing Antibody Responses after HIV-1 Superinfection Are Not Dominated by Antibodies Directed to Epitopes Common in Single Infection
- Rapidly Evolving Genes Are Key Players in Host Specialization and Virulence of the Fungal Wheat Pathogen ()
- MiR-21 in Extracellular Vesicles Leads to Neurotoxicity via TLR7 Signaling in SIV Neurological Disease
- Age-Dependent Cell Trafficking Defects in Draining Lymph Nodes Impair Adaptive Immunity and Control of West Nile Virus Infection
- Decline of FoxP3+ Regulatory CD4 T Cells in Peripheral Blood of Children Heavily Exposed to Malaria
- Dimerization-Induced Allosteric Changes of the Oxyanion-Hole Loop Activate the Pseudorabies Virus Assemblin pUL26N, a Herpesvirus Serine Protease
- Macrophages Subvert Adaptive Immunity to Urinary Tract Infection
- Mycolactone-Dependent Depletion of Endothelial Cell Thrombomodulin Is Strongly Associated with Fibrin Deposition in Buruli Ulcer Lesions
- Activation of TLR2 and TLR6 by Dengue NS1 Protein and Its Implications in the Immunopathogenesis of Dengue Virus Infection
- K-bZIP Mediated SUMO-2/3 Specific Modification on the KSHV Genome Negatively Regulates Lytic Gene Expression and Viral Reactivation
- Phosphoproteomic Analysis of KSHV-Infected Cells Reveals Roles of ORF45-Activated RSK during Lytic Replication
- CR3 and Dectin-1 Collaborate in Macrophage Cytokine Response through Association on Lipid Rafts and Activation of Syk-JNK-AP-1 Pathway
- IFNγ and IL-12 Restrict Th2 Responses during Helminth/ Co-Infection and Promote IFNγ from Th2 Cells
- THY-1 Cell Surface Antigen (CD90) Has an Important Role in the Initial Stage of Human Cytomegalovirus Infection
- Human Enterovirus Nonstructural Protein 2C Functions as Both an RNA Helicase and ATP-Independent RNA Chaperone
- IL-27 Signaling Is Crucial for Survival of Mice Infected with African Trypanosomes via Preventing Lethal Effects of CD4 T Cells and IFN-γ
- Synergistic Reactivation of Latent HIV Expression by Ingenol-3-Angelate, PEP005, Targeted NF-kB Signaling in Combination with JQ1 Induced p-TEFb Activation
- Vpu Exploits the Cross-Talk between BST2 and the ILT7 Receptor to Suppress Anti-HIV-1 Responses by Plasmacytoid Dendritic Cells
- Herpesvirus Genome Recognition Induced Acetylation of Nuclear IFI16 Is Essential for Its Cytoplasmic Translocation, Inflammasome and IFN-β Responses
- A Comprehensive Analysis of Replicating Merkel Cell Polyomavirus Genomes Delineates the Viral Transcription Program and Suggests a Role for mcv-miR-M1 in Episomal Persistence
- Analysis of the SUMO2 Proteome during HSV-1 Infection
- Capacity of Broadly Neutralizing Antibodies to Inhibit HIV-1 Cell-Cell Transmission Is Strain- and Epitope-Dependent
- A Novel Antiviral Target Structure Involved in the RNA Binding, Dimerization, and Nuclear Export Functions of the Influenza A Virus Nucleoprotein
- Deploying FLAREs to Visualize Functional Outcomes of Host—Pathogen Encounters
- Mosquitoes Reset Malaria Parasites
- The Lung Microbiome: New Principles for Respiratory Bacteriology in Health and Disease
- Extracellular Virions: The Advance Guard of Poxvirus Infections
- Risks of Antibiotic Exposures Early in Life on the Developing Microbiome
- RNA Virus Reassortment: An Evolutionary Mechanism for Host Jumps and Immune Evasion
- Exploiting Fungal Virulence-Regulating Transcription Factors As Novel Antifungal Drug Targets
- N-acetylglucosamine Regulates Virulence Properties in Microbial Pathogens
- Periodontal Diseases: Bug Induced, Host Promoted
- Mechanisms of Host Behavioral Change in Rodent Association
- The Endosymbiotic Bacterium Selectively Kills Male Hosts by Targeting the Masculinizing Gene
- HIV Reactivation from Latency after Treatment Interruption Occurs on Average Every 5-8 Days—Implications for HIV Remission
- Ubiquilin 1 Promotes IFN-γ-Induced Xenophagy of
- Transfer of Immunity from Mother to Offspring Is Mediated via Egg-Yolk Protein Vitellogenin
- Suppression of Long-Lived Humoral Immunity Following Infection
- The Role of VP1 Amino Acid Residue 145 of Enterovirus 71 in Viral Fitness and Pathogenesis in a Cynomolgus Monkey Model
- Utilizing Chemical Genomics to Identify Cytochrome as a Novel Drug Target for Chagas Disease
- The Emerging Role for RNA Polymerase II in Regulating Virulence Gene Expression in Malaria Parasites
- Turning Up the Heat: Inflammasome Activation by Fungal Pathogens
- On and Under the Skin: Emerging Basidiomycetous Yeast Infections Caused by Species
- EhVps32 Is a Vacuole-Associated Protein Involved in Pinocytosis and Phagocytosis of
- Characterization of a Prefusion-Specific Antibody That Recognizes a Quaternary, Cleavage-Dependent Epitope on the RSV Fusion Glycoprotein
- The Serine Protease EspC from Enteropathogenic Regulates Pore Formation and Cytotoxicity Mediated by the Type III Secretion System
- Existing Infection Facilitates Establishment and Density of Malaria Parasites in Their Mosquito Vector
- Evaluating Human T-Cell Therapy of Cytomegalovirus Organ Disease in HLA-Transgenic Mice
- Neuronal Interferon Signaling Is Required for Protection against Herpes Simplex Virus Replication and Pathogenesis
- Epstein-Barr Virus Proteins EBNA3A and EBNA3C Together Induce Expression of the Oncogenic MicroRNA Cluster miR-221/miR-222 and Ablate Expression of Its Target p57
- Colonization of the Mouse Gastrointestinal Tract Is Modulated by Wall Teichoic Acid, Capsule, and Surface Proteins
- Virulence of Group A Streptococci Is Enhanced by Human Complement Inhibitors
- Identification of Caspase Cleavage Sites in KSHV Latency-Associated Nuclear Antigen and Their Effects on Caspase-Related Host Defense Responses
- Calprotectin Increases the Activity of the SaeRS Two Component System and Murine Mortality during Infections
- Type VI Secretion System Transports Zn to Combat Multiple Stresses and Host Immunity
- Lv4 Is a Capsid-Specific Antiviral Activity in Human Blood Cells That Restricts Viruses of the SIV/SIV/HIV-2 Lineage Prior to Integration
- Phenylbutyrate Is Bacteriostatic against and Regulates the Macrophage Response to Infection, Synergistically with 25-Hydroxy-Vitamin D₃
- An Internally Translated MAVS Variant Exposes Its Amino-terminal TRAF-Binding Motifs to Deregulate Interferon Induction
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- RNA Virus Reassortment: An Evolutionary Mechanism for Host Jumps and Immune Evasion
- Activation of TLR2 and TLR6 by Dengue NS1 Protein and Its Implications in the Immunopathogenesis of Dengue Virus Infection
- N-acetylglucosamine Regulates Virulence Properties in Microbial Pathogens
- Characterization of a Prefusion-Specific Antibody That Recognizes a Quaternary, Cleavage-Dependent Epitope on the RSV Fusion Glycoprotein
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



