-
Články
- Vzdělávání
- Časopisy
Top články
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
A Role for in Higher Order Structure and Complement Binding of the Capsule
Polysaccharide capsules are important virulence factors in pathogenic microbes that provide a protective coat against host immunity. Cryptococcus neoformans is a pathogenic encapsulated yeast that is a major opportunistic infection, causing approximately 600,000 cases of meningitis per year in AIDS patients globally, and whose polysaccharide capsule is a major virulence factor. While extensive work has detailed the chemical components forming the cryptococcal capsule, the molecular events leading to the higher order assembly of the capsule, and its consequences on immune subterfuge remain unknown. In the present studies we used a proteomics method to identify a novel hydrolytic enzyme, lactonohydrolase (Lhc1) and used a variety of biophysical methods including dynamic and static light scattering as well as motility studies to show that extracted capsular polysaccharide undergoes remodeling in a LHC1-dependent fashion. This results in a more tightly compacted capsular structure that alters binding of anti-capsular antibodies and reduces binding by both human as well as mouse serum complement. Furthermore, LHC1-dependent capsular alterations serve to increase the virulence of the fungus in a mouse model, suggesting a novel role for this class of enzyme in capsular remodeling and immune evasion in microbial pathogenesis.
Published in the journal: . PLoS Pathog 10(5): e32767. doi:10.1371/journal.ppat.1004037
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004037Summary
Polysaccharide capsules are important virulence factors in pathogenic microbes that provide a protective coat against host immunity. Cryptococcus neoformans is a pathogenic encapsulated yeast that is a major opportunistic infection, causing approximately 600,000 cases of meningitis per year in AIDS patients globally, and whose polysaccharide capsule is a major virulence factor. While extensive work has detailed the chemical components forming the cryptococcal capsule, the molecular events leading to the higher order assembly of the capsule, and its consequences on immune subterfuge remain unknown. In the present studies we used a proteomics method to identify a novel hydrolytic enzyme, lactonohydrolase (Lhc1) and used a variety of biophysical methods including dynamic and static light scattering as well as motility studies to show that extracted capsular polysaccharide undergoes remodeling in a LHC1-dependent fashion. This results in a more tightly compacted capsular structure that alters binding of anti-capsular antibodies and reduces binding by both human as well as mouse serum complement. Furthermore, LHC1-dependent capsular alterations serve to increase the virulence of the fungus in a mouse model, suggesting a novel role for this class of enzyme in capsular remodeling and immune evasion in microbial pathogenesis.
Introduction
Polysaccharide capsules (PC) are highly diverse hydrated structures that provide microbes with a key defense against the host immune system [1]. For example, bacterial capsules confer resistance to complement-mediated opsonophagocytosis [2] and are an important property of highly virulent bacteria such as Neisseria meningitidis [3]. Among fungal pathogens, a prominent virulence factor of the opportunistic pathogen Cryptococcus neoformans is a large polysaccharide capsule with potent anti-phagocytic properties [4]. C. neoformans is a common cause of meningitis in parts of Africa [5], accounting for approximately 600,000 deaths annually [6].
The cryptococcal capsule is a hydrated polysaccharide gel, constituted by high-molecular weight polysaccharide polymers such as glucuronoxylomannan (GXM) which represents almost 90% of the total capsule with the remainder being glucuronoxylomannanogalactan (GXMGal) [7]. GXM is composed of a large backbone of 6-O-acetylated α-1,3-mannose residues with β-D-xylopyranosyl, β-D-glucuronosyl monosubstituted side chains [8]. Extensive work by numerous investigators has provided key insights into synthesis and virulence role of the capsular primary structure [7]. However, genes controlling or regulating higher order structures of the capsular polysaccharide have not been identified. This has been in part due to difficulties in assessing the tertiary structure of the cryptococcal polysaccharide.
Thus, to identify genes that may control the higher order organization of the capsular structure, we used a focused proteomic approach to identify capsular-associated proteins that may participate in remodeling of the cryptococcal capsule. Since current models suggest that the primary structure is synthesized within the cell cytoplasm [9], the hypothesis was that secreted proteins might be more likely to be involved in capsular tertiary structure. This approach identified a capsular lactonohydrolase of C. neoformans and a targeted mutant strain demonstrated a larger capsule size that was more permeable to dextran particles in a mutant strain defective in this hydrolytic activity. Recently applied biophysical methods [10] were then used to demonstrate that the mutant polysaccharide (PS) was larger, more hydrated and branched, evidenced by altered capsule nuclear magnetic spectra, zeta potential and polysaccharide hydrodynamic dimensions. The mutant also displayed an increase in antibody and serum-dependent phagocytosis by the macrophage cell line J774.16 cells, an increase in serum complement binding and reduced virulence in mice that could be reversed by depletion of complement using cobra-venom. These data thus identify LHC1 as a unique example of a gene locus involved in modification of higher order capsular structure of a microbial pathogen and its role in immune evasion.
Results
Isolation of capsular-associated proteins from C. neoformans by a focused proteomic approach
After extensive washing of cells, dimethyl sulfoxide (DMSO) was used to solubilize and remove the outer layers of the cryptococcal capsule without breakage of the cell wall as described previously [11]. Strain B-3501 was used because its smaller capsule produced relatively less capsular polysaccharide that could complicate protein purification. Interestingly, after recovery of crude protein from dialyzed DMSO-solubilized material by adsorption on diethylaminoethanol-agarose, only two prominent bands were identified on Coomassie-blue stained PAGE gels (Fig. 1A). Protein sequencing identified three cryptococcal proteins (see supplemental Table S1 in Text S1), each matching protein sequence within the serotype D (www.ncbi.nih.gov) as well as the H99 serotype A database (www.broad.mit.edu), indicating their presence in two strains representative of two important serotypes capable of causing human disease. The small number of protein bands was remarkable, considering the large number of secreted proteins of C. neoformans [12] and may be due to the presence of only a small population of capsular-associated proteins or to incomplete adsorption of proteins from solubilized capsule material by the DEAE-agarose matrix. Analysis of the CNAG_04753 amino acid sequence from the higher mobility band showed strong homology to a number of fungal lactonohydrolases including that from Fusarium oxysporum (E = e-119; Fig. 1B) and contained three conserved domains for this class of hydrolytic enzymes [13]. Interestingly, using the PROCARB carbohydrate binding prediction tool based on a database of known and modeled carbohydrate-binding protein structures [14], three putative amino acids were identified that could represent amino acids involved in such binding,W28, N454, and R456—all aromatic amino acids that have the capacity to form Pi(π) bond complexes with hexose sugars, a common mechanism of lectin binding to carbohydrates [15]. Sequence analysis of the lower mobility band (68 kDa) identified a mixture of a conserved hypothetical protein and a protein showing closest homology to Kex1 of yeast. Since these latter two proteins were less likely to be involved in capsular modifications, they were not analyzed further.
Fig. 1. Identification of a capsular-adherent putative lactonohydrolase from C. neoformans and role in virulence-related phenotypes. 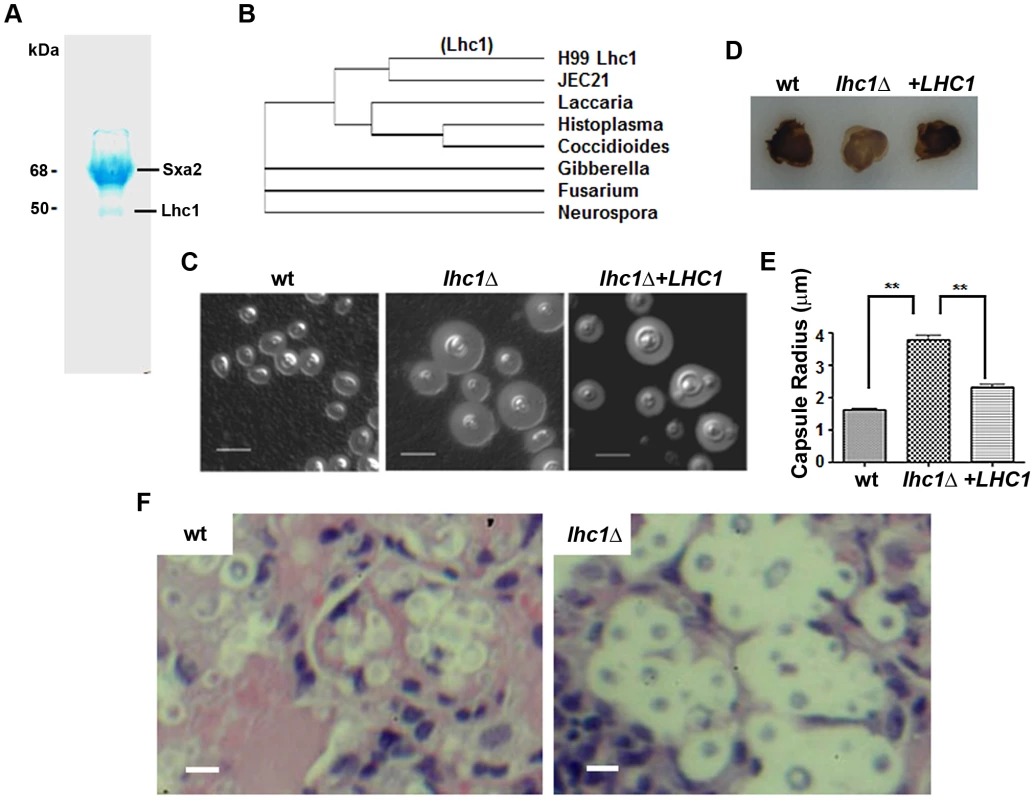
(A) SDS-PAGE of DMSO-solubilized capsular proteins adsorbed on DEAE-agarose. (B) Clustal-W comparison of proteins sequences of closest matches of the 50 kDa Lhc1 sequence. Indicated strains were assayed for (D) laccase by melanin formation and, (C) capsule by India Ink microscopy. (E) Capsule radius of India ink-stained cells was determined in 100 cells of the indicated strains. (F) Capsule of LHC1 strains during brain infection. Indicated strains (1×106) were inoculated intravenously and when moribund, mice were sacrificed and brains excised, sectioned and stained with H&E as described in methods. Bar = 5 microns. Analysis of the role of LHC1 in virulence-associated phenotypes of C. neoformans
A deletion strain was created in serotype A strain H99 to help identify a role for the putative lactonohydrolase from C. neoformans, Lhc1 using a strain of the serotype that is most predominant in human infections, serotype A [16]. As shown in Fig. 1C, a large increase in the size of the capsule was observed in the lhc1Δ mutant strain by India Ink microscopy grown in the presence of CO2, which was restored to approximately that of wild-type (wt) after complementation by a 3.6-kb fragment of the LHC1 gene. Larger capsule was also evident in YPD after a 1 day incubation that showed poor capsule induction in the wt strain or after capsule induction in ASN minimal media, 1∶10 Sabouraud or RPMI media (Fig. S1 in Text S1). In contrast, deletion of LHC1 had only a minor effect on other virulence factors such as laccase, measured by melanin formation (Fig. 1D) and no effect on urease activity or growth in YPD at 37°C (data not shown). Analysis of capsular radius of lhcΔ mutant cells using India ink microscopy induced by growth in the presence of 5% CO2 (Fig. 1E; p<0.01), ASN minimal media, 1∶10 SAB or RPMI demonstrated a significantly increased capsular radius compared to either the wt or complemented strains (Fig. S1 in Text S1; p<0.05). Interestingly, large capsules were also expressed by the lhc1Δ mutant in mouse brains (Fig. 1F, right panel) compared to that of wt (left panel) or the complemented strain (data not shown). These data establish a role for LHC1 in the wt capsular phenotype both in vitro and in vivo.
LHC1 expresses a capsular cryptococcal lactonohydrolase
To confirm the identity of LHC1, we assayed for hydrolysis of the aliphatic lactone, D-pantolactone (Fig. 2A) using a previously-described high performance liquid chromatographic method [17] after growth of wt and lhc1Δ mutant cells in minimal media for 3 days 30°C. As shown in Fig. 2B, wt fungal cells converted approximately 11.3+/−2.6% (SEM, N = 3) of the D-pantolactone to the corresponding acid in 30 min, whereas no significant hydrolysis was evident in the lhc1Δ mutant. A small shoulder on the substrate peak of the mutant reaction could represent an unknown breakdown product. However, we were not able to detect hydrolysis of the aromatic substrate 3,4-dihydrocoumarin by changes in UV absorption using a previously described method [18], suggesting a restriction to aliphatic lactones that might be expected within the polysaccharide matrix (data not shown). Recombinant Lhc1 was inactive in both assays which may be due to cryptococcal specific conformational modifications or a requirement for a specific carbohydrate binding cofactor for activity.
Fig. 2. C. neoformans possesses a LHC1-dependent lactonohydrolase activity that is localized to the capsule and expressed during human infection. 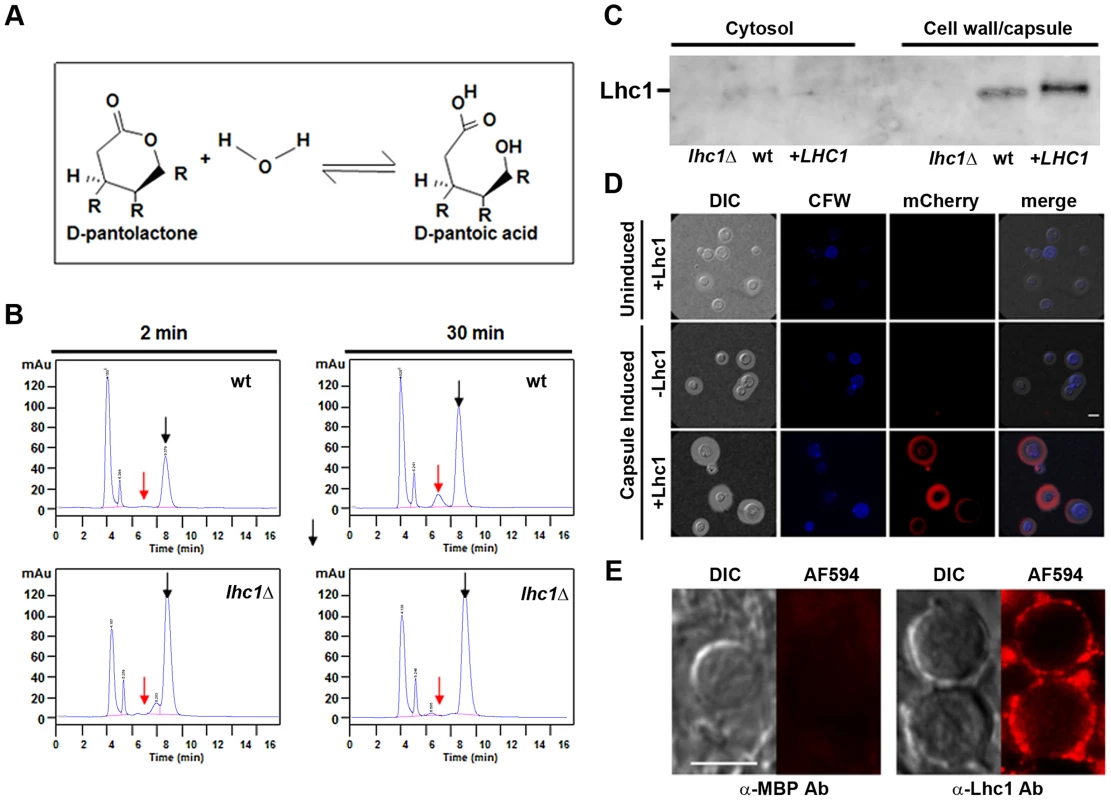
(A) Scheme of lactonohydrolase-dependent hydrolysis of D-pantolactone. (B) Indicated cells were grown in asparagine media (0.2% glucose) for 3 days, centrifuged and washed 3× in 10 mM Tris buffer, pH 7.0, incubated in the presence of 100 mM D-pantolactone at 37°C for the indicated times and the reaction terminated with methanol. Aliquots were then assayed for lactone (black arrow) or the hydrolyzed acid (red arrow) as determined using chemical standards. (C) Western blot analysis of polyclonal anti-Lhc1 reactivity against C. neoformans protein extracts. Protein supernatants of cell lysates (cytosol) or detergent-solubilized cell pellets (cell wall/capsule) from the indicated strains were prepared and western blot performed using polyclonal serum against the Lhc1 protein as described in Materials and Methods. (D) A C. neoformans lhc1Δ strain complemented with a vector expressing Lhc1-mCherry (+Lhc1) or empty vector alone (−Lhc1) was grown in YPD (uninduced) or induced on 1∶10 SAB (capsule induced) as described in Materials and Methods. (E) Sections of a brain autopsy specimen were obtained from a 30 y.o. female who died of severe and diffuse C. neoformans infection and stained with a mouse affinity-purified antibody against a maltose-binding protein-Lhc1 fusion protein (α-Lhc1 Ab) or anti-MBP polyclonal serum (α-MBP Ab) followed by incubation with a secondary Alexa Fluor 594 goat anti-rabbit IgG (AF494) according to Materials and Methods. Panels represent fluorescent antibody treated (AF594) or differential interference contrast microscopy (DIC) images. Cells were washed and mounted in anti-fade medium and imaged in an epifluorescence microscope using a 63× 1.4 NA objective. The exposure conditions were identical for each sample and are representative of 25 cells visualized. Bar = 5 microns. Further studies sought to confirm a capsular localization of Lhc1 suggested by DMSO-solubilization from intact cells. Western blots using mouse antiserum developed against a recombinant maltose-binding protein (MBP)-tagged Lhc1 fusion protein demonstrated an immunoreactive band from wt or LHC1 complemented, but not lhc1Δ mutant strains of the appropriate molecular mass from SDS extracts of pelleted fractions enriched in cell wall/capsule but not cell lysis supernatants enriched in cytosolic proteins after homogenization (Fig. 2C). Lhc1-immunoreactivity was also not detected from culture supernatants or 20× concentrated culture supernatants (data not shown), further suggesting that Lhc1 was a capsular-associated protein. Control antibody raised against MBP alone showed no cross-reactivity against C. neoformans cellular materials by western blot as described (Fig. S5 in Text S1) [19].
In addition, Lhc1 was expressed under its native promoter as a C. neoformans codon-optimized mCherry fusion protein (Fig. 2D), which suggested that 1) Lhc1 expression is repressed under nutrient-rich conditions where capsule is repressed and 2) is successfully expressed and localized to capsule under conditions where capsule is induced, including 1∶10 SAB (Fig. 2D), ASN minimal media or RPMI (Fig. S2 in Text S1). Regulation appeared to be at the transcriptional level as quantitative RT-PCR studies demonstrated induction under capsule inducing conditions in either ASN minimal media, 1∶10 SAB or RPMI media after 24 h incubation, which was present for both the serotype A H99 strain as well as the serotype D strain (B-3501) used to identify the Lhc1 protein (Fig. S3 in Text S1). Additional studies utilized fluorescence immune-microscopy of sectioned C. neoformans cells to demonstrate capsular reactivity in a human autopsy specimen of a 30 year old female who died of overwhelming C. neoformans meningoencephalitis (Fig. 2E). These data suggest a role for Lhc1 expression in human infections. In summary, lactonohydrolase expressed from C. neoformans was localized to the capsular matrix of the fungus, although removal with detergent and DMSO as well as migration within an SDS-PAGE gel matrix suggested a non-covalent interaction.
Role of LHC1 in the capsular structure determined by Nuclear Magnetic Resonance (NMR)
Because of the larger capsule size of the lhc1Δ mutant evident on India ink staining, additional studies were conducted to assess alterations in the chemical structure of the capsule. Neutral sugar analysis did not identify large changes in substituent sugars although some increases in xylose and glucuronic acid as well as reductions in glucose were noted (Table S2 in Text S1). NMR spectroscopy of isolated soluble GXM from wt and the lhc1Δ strain also showed subtle differences in the GXM spectra that suggested alterations in higher order structure of the polysaccharide (Fig. 3A). The acetylation ratio was almost identical to the wt; however, mannosyl residues substituted with glucuronic acid residues were more frequently also 6-O-acetylated in the mutant (M6G-ac in Fig. 3A) in contrast to the deacetylated residues in the wt (M6G-deac). The ratio of mannose: xylose: glucuronic acid residues was 3∶2.1∶1, similar to wt. However, the exact chemistry of the cross-linkage of GXM chains was difficult to determine for the lhc1Δ strain. Samples are usually prepared for NMR spectroscopy by sonication to break-up large polysaccharides into smaller repeating units to reduce relaxation times and hereby allow for 2D NMR correlation spectroscopy [20]. However, conventional preparation in this case did not result in sufficiently small polysaccharide fragments to yield usable 2D NMR data for the determination of the smallest repeating carbohydrate structure in the lhc1Δ strain. High-power sonication resulted in complete break-up into monosaccharide units, suggesting that the larger polysaccharide fragments of the mutant exhibited higher levels of structural complexity in the form of branching or intermolecular cross-links that required higher sonication energies.
Fig. 3. Deletion of LHC1 is associated with alterations in higher order polysaccharide structure of solubilized polysaccharide. 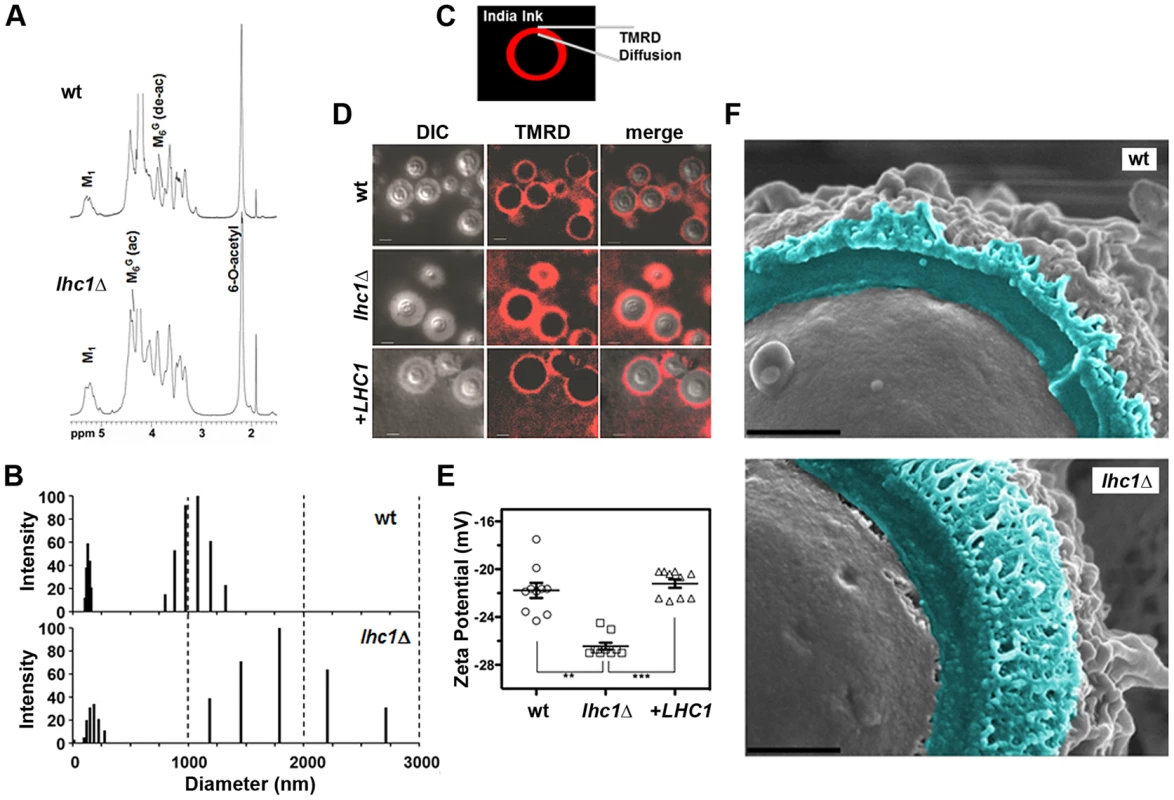
(A) Nuclear magnetic resonance (NMR) analysis of C. neoformans GXM. Isolated GXMs from indicated strains were dissolved in 0.5 ml of 99.96% D2O (Sigma, St. Louis, Mo.). NMR spectra were acquired with a Bruker Avance 600 NMR spectrometer, using a 5-mm (1H, 13C) inverse-detection dual-frequency probe, operating at 600.13 and 150.913 MHz, respectively. (B) TMRD diffusion was measured as the distance between the India Ink-capsule outer interface and the furthest region of diffusion of the fluorescent TMR-dextran. (C) TMRD diffusion: Fungal cells were induced for capsule, and treated with TMR-Dextran and India ink as described in Materials and Methods and observed for epifluorescence. Bar = 5 microns. (D) Capsule from the indicated strains was extracted with DMSO and polydispersity measured by Quasi elastic light scattering in a 90 Plus/Bi-MAS multi angle particle sizing analyzer as described in Materials and Methods. (E) Zeta potentials of capsular polysaccharide solutions from indicated strains at 1 mg/ml. Error bars represent SEM from 10 repeated measurements. (** p<0.01; *** p<0.001). (F) Fungal cells were subjected to cryo-scanning electron microscopy as described in Materials and Methods. Light blue coloring indicates outer region of cell wall and capsular structure exterior to the plasma membrane fracture plane. Bar = 500 nm. Alteration of capsule permeability to dextran after deletion of LHC1
Because NMR spectroscopy suggested alterations in the higher order structure of the capsule, permeability was assessed using a fluorescent-labeled dextran dye (2,000 kDa) used previously in this organism [21]. India ink was added to the dextran suspension to allow demarcation of the exterior surface of the capsule. Full capsule thickness was determined as the distance between the fungal cell wall and the outer edge of the India ink exclusion zone (Fig. 3C). Comparison of the three strains demonstrated increased permeability of the lhc1Δ mutant versus that of either the wt or the complemented strain, as determined by the width of the zones of red fluorescence (wt: 1.38±0.07; lhc1Δ: 2.41±0.06; lhc1Δ+LHC1: 1.53±0.12; p<0.0001 for lhc1Δ versus either wt or complemented strain-Fig. 3B, C). Ratios of dextran penetration versus full capsule thickness were also calculated, which showed increased fractional penetration of dextran in the lhc1Δ versus wt or complemented strains. (wt: 0.61±0.03; lhc1Δ: 0.72±0.03; lhc1Δ+LHC1: 0.46±0.03; p<0.05 for lhc1Δ versus either wt or complemented strain; Fig. S4 in Text S1). These data show that LHC1 plays a role in reducing capsule permeability of C. neoformans to large molecules.
Capsular PS of lhc1Δ mutant strains of C. neoformans exhibits higher dimensions and higher negative charge
Since altered cross-linking or branching of the capsular polymer may affect the dimensions of the C. neoformans capsular PS, hydrodynamic sizes of DMSO-extracted capsular PS were determined by dynamic light scattering as described [22]. These data (Fig. 3D) demonstrated two sets of particle distributions as described previously for the DMSO-extracted polysaccharide [23]. Interestingly, the particle distributions from the lhc1Δ mutant strain were much larger and more heterogeneous than those from the wt strain, suggesting a role for LHC1 in reducing capsular PS dimensions.
Zeta potential (ζ) of polysaccharide samples were also determined for the capsular material from the mutant strain. Zeta potential is a measurement of charge and is defined as the electric potential gradient between a boundary liquid in contact with a solid and the mobile diffuse layer in the body of the liquid. Colloidal suspensions having ζ that deviate from zero (>×30 mVolts) have greater solvent hydration and tend to remain in stable suspension, whereas those with values closer to zero tend to aggregate [24]. Using this approach ζ was found to be −28.28±0.30 mV for the wt strain and −34.46±0.61 mV for the lhc1Δ mutant (Fig. 3E; p<0.001). This suggests that the larger particles of the lhc1Δ mutant seen in the polydispersity profile (Fig. 3E) were also more highly hydrated either by increased cross-links/branching and/or by differences in glucuronic acid availability, the latter suggested by the neutral sugar analysis.
Deletion of LHC1 is associated with alterations in higher order polysaccharide structure
We next utilized light scattering to assess higher order capsular structure. These biophysical methods recently demonstrated evidence for branching/cross-linking within the polysaccharide matrix that is difficult to assess by chemical methods alone [10]. For these studies, DMSO-solubilized capsule was analyzed without size fractionation to reduce bias that could be introduced by excluding important capsular constituents. As shown in Table 1, deletion of LHC1 was associated with changes in a number of macromolecular parameters including average molecular mass (Mw), radius of gyration (Rg), hydrodynamic radius (Rh), mass density and the 2nd virial coefficient (A2). Interestingly, while shape factor and A2 were restored by complementation, the other parameters were not, suggesting a sensitivity to gene dosing of some of these parameters relative to wt cells by the heterologous insertion of the LHC1 gene as previously described [25] and examined more recently [26]. The significant increase in Mw and Rh are consistent with the capsular dimension results (Fig. 3E), demonstrating that the increase in capsule size observed in the mutants is due to the presence of larger polysaccharide molecules. Interestingly, the ratio of Rg/Rh, referred to as the shape factor, ρ, was much lower in the mutant than wt and the complemented strain. This was a key parameter demonstrating higher structural complexity of the cryptococcal polysaccharide with low values suggesting higher levels of branching [10]. In addition, the A2 coefficient was altered in the mutant. The A2 coefficient is a property which describes the interaction strength between the molecule and a solvent, giving insights into the tendency of polysaccharide-polysaccharide interactions in that solvent [27]. Solubilized material from the mutant strain manifested a negative A2 value (−2.6±1.1×10−4 cm3 mol/g2) compared to a positive value of both the wt (1.82±0.6×10−4 cm3 mol/g2) and the complemented strain (1.74±0.5×10−4 cm3 mol/g2), suggesting that the strength of molecular-solvent interactions in the mutant strain is lower than the molecular-molecular interactions, relative to the wt strain, and that intra-branch interactions in the mutant are stronger than in the wt strain. Thus, the lhc1Δ strain appeared from the biophysical data to produce a population of higher molecular weight capsular PS that showed more solvent hydration and exhibited a greater degree of branching/cross-linkage. To obtain additional structural data, wt and lhc1Δ cells were induced for capsule on 1∶10 SAB media and subjected to cryo EM. As shown in Fig. 3F, representative micrographs demonstrated a condensed PS structure in the wt with reduced radius and a larger, more highly branched PS structure in the mutant strain, consistent with the biophysical data. This again suggests a model whereby the capsular adherent lactonohydrolase either directly or indirectly results in the remodeling of secreted polysaccharide particles to reduce particle size and branching/cross-linkages.
Tab. 1. Molecular parameters of capsular PS samples performed by static and dynamic light scattering analysis. 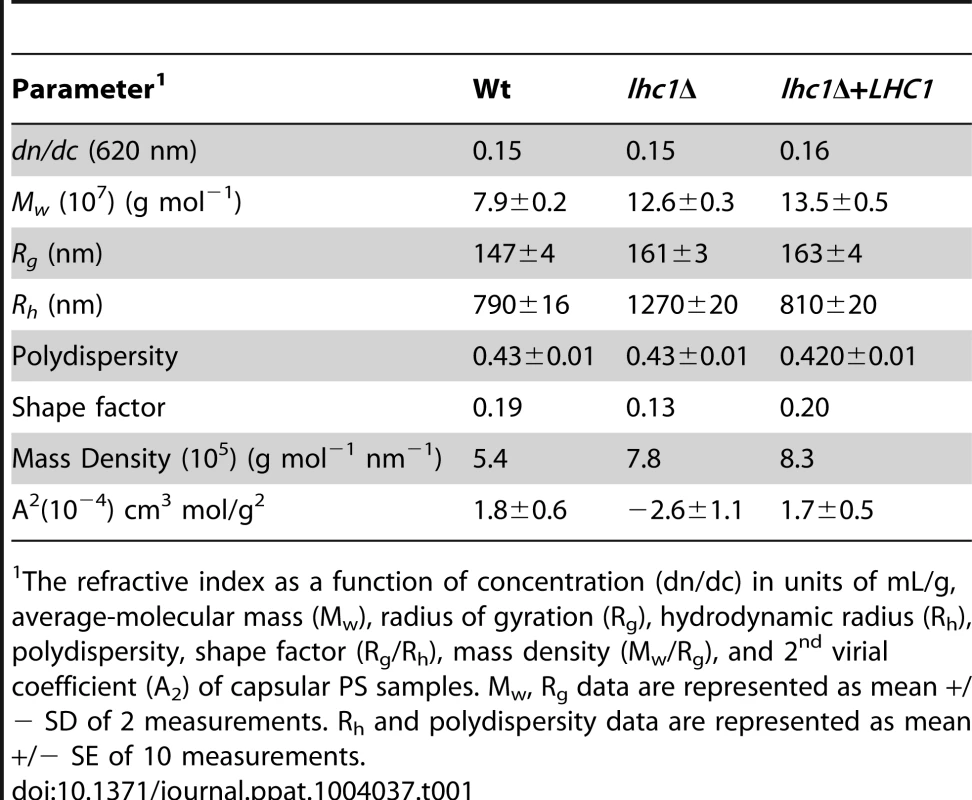
The refractive index as a function of concentration (dn/dc) in units of mL/g, average-molecular mass (Mw), radius of gyration (Rg), hydrodynamic radius (Rh), polydispersity, shape factor (Rg/Rh), mass density (Mw/Rg), and 2nd virial coefficient (A2) of capsular PS samples. Mw, Rg data are represented as mean +/− SD of 2 measurements. Rh and polydispersity data are represented as mean +/− SE of 10 measurements. LHC1-dependent changes in antibody binding and phagocytosis by the macrophage-like cell line, J774.16
To determine the functional significance of the LHC1-dependent altered higher order structure in the capsule, we compared binding of mAb to the capsule. Antibodies showed a punctate pattern of binding with subtle but significant differences in the number of puncta observed between the LHC1 strains (Fig. 4A, B). Antibody negative (Fig. 4A) or isotype controls (data not shown) showed no significant binding. Differences in puncta observed after antibody deposition on C. neoformans capsule have been associated with differences in antibody-mediated protection but the mechanism has not been elucidated [28]. Opsonization with the mAb 18B7 yielded increased phagocytosis of the lhc1Δ strain by the J774.16 macrophage-like cell line with an almost doubling of the phagocytic index defined as number of fungal cells/macrophage (wt: 1.4±0.3; lhc1Δ: 3.85±0.02; lhc1Δ+LHC1: 0.8±0.3; p<0.02 – lhc1Δ versus wt or complemented strain; Fig. 4C, left panel), with little phagocytosis evident, using a mouse IgG1 isotype control (Fig. 4C, right panel). Successful phagocytosis by macrophages plays a major role in killing of this facultative intracellular pathogen [29]. Opsonization was unsuccessful with mAbs 12A1 and 2D10 as IgM antibodies are not opsonizing, although these latter two IgM antibodies are capable of opsonizing serotype D C. neoformans strains through facilitation of a unusual conformational change in the capsule [30]. Increased antibody opsonization was also associated with decreased survival of the mutant strain after prolonged incubation in J774.16 cells (Fig. 4D). In summary, differences in capsular PS higher order structure as determined by biophysical methods translated into demonstrable changes in antibody-mediated rates of phagocytosis.
Fig. 4. wt and lhc1Δ mutant strains of C. neoformans differ in antibody binding and antibody mediated phagocytosis by a J774.16 macrophage-like cell line. 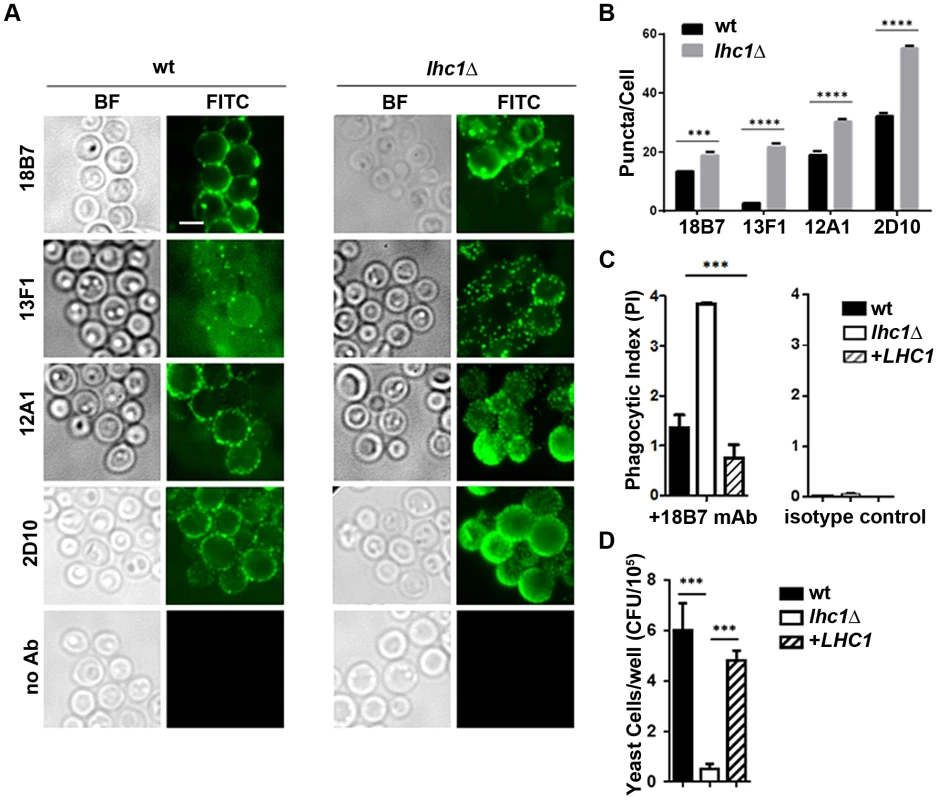
(A) Indicated strains were prepared and stained with indicated monoclonal antibodies as described in Material and Methods. (B) Puncta from cells labeled as in A were quantified from 50 cells. (C) Indicated strains were opsonized with the indicated antibody and then incubated with J774.16 cell monolayers and phagocytic index determined as in Materials and Methods (N = 4). (D) Fungal Killing Assay: Cells treated as in C, except that incubation was continued for 81 hours and fungal burden assayed by CFU after macrophage lysis (N = 4). *** p<0.001. Complement-mediated phagocytosis and virulence is altered in the lhc1Δ mutant strain
Similar to that found after antibody opsonization, the lhc1Δ mutant strain opsonized with human serum was more readily ingested than the wt strain (Fig. 5A-left panel), an effect that was abolished after heat inactivation (Fig. 5A-right panel), implying complement-dependent opsonization. The capsule serves as a site for deposition of C3 fragments of the alternative pathway of the complement cascade, which promotes C. neoformans phagocytosis [31], while the polysaccharide blocks activation of the classical pathway that can occur at the cell wall of avirulent non-encapsulated strains [32]. Incubation of fungal cells with human PBMC's after opsonization with fresh human serum resulted in increased fungal killing (Fig. 5B). Quantitation by flow cytometry demonstrated that C3 deposition was increased in the mutant strain, even after the addition of EGTA to inhibit the classical pathway, but was abolished (data not shown) after heat treatment (Fig. 5C). Complementation with the LHC1 locus led to a partial yet significant reduction in complement binding. Fluorescence microscopy using antibody to human C3 further demonstrated C3 binding within the enlarged capsule of the lhc1Δ strain (Fig. 5D) that was reduced after LHC1 complementation. Complement binding was heterogeneously deposited on the cells, most likely due to the presence of focal initiation sites, as previously described [32], [33]. Most of the C3 was identified in the form of iC3b (Fig. S6 in Text S1), as previously described [34]. To evaluate the consequences of the reduced LHC1-dependent complement binding in C. neoformans, we modeled the studies in mice, a species that would also allow testing for virulence [35]. As shown in Fig. 5E, opsonization with mouse serum reproduced the results using human serum and flow cytometry using antibody to C3 again demonstrated increased C3 binding. Inoculation of mice using an intravenous model showed reduced virulence of the lhc1Δ strain that was restored after complementation with the wt gene (Fig. 5G-left panel). Interestingly, after depletion of complement using cobra venom factor [33], the differences in virulence between wt and mutant strains disappeared (Fig. 5G-right panel), with a small increase in overall virulence of the wt strain, as previously described after complement depletion [36]. These data suggest a role for LHC1 in reducing mouse as well as human complement binding to C. neoformans and support a role for complement in mediating LHC1-dependent mammalian virulence.
Fig. 5. Deletion of LHC1 results in increased C3 binding from human or mouse serum and reduced virulence in a mouse model. 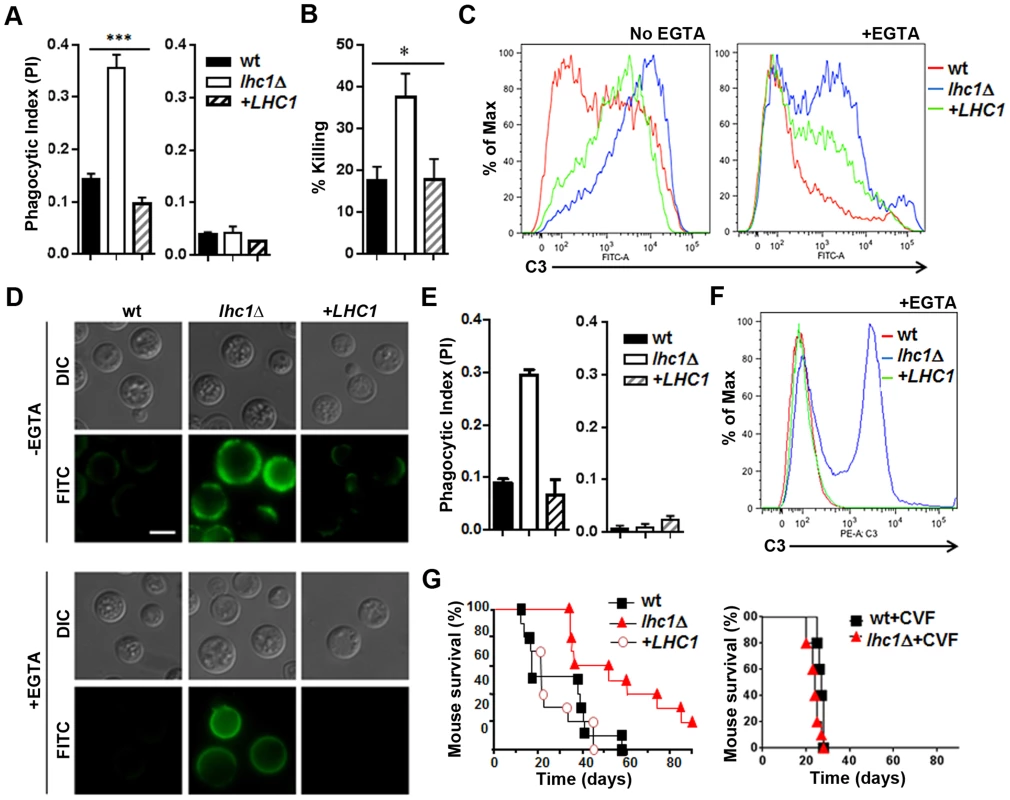
Human studies, A–D: (A) Indicated fungal strains were incubated with human serum (left panel) or heat killed serum (right panel) and subjected to phagocytosis using a J774.16 cell line according to Materials and Methods. (B) Strains indicated in B were incubated in the presence of serum and monocytes from a healthy volunteer and percent killing over 4 h measured according to Materials and Methods. (C, D) Indicated strains were incubated in the presence of human serum in the presence (+EGTA) or absence (−EGTA) of EGTA and analyzed by flow cytometry (C) or visualized by fluorescence microscopy (D) using an anti-human C3 antibody. For microscopic evaluation of C3 deposition, FITC-labeled goat anti-human C3 was added and samples resuspended in mounting medium, placed on glass slides and examined under oil immersion at 1000× for C3 deposition (FITC) or using differential interference contrast (DIC). Mouse studies, E–G: (E) Indicated strains were incubated with mouse serum (left panel) or heat killed serum (right panel) and subjected to phagocytosis using a J774.16 cell line according to Materials and Methods. (F) Indicated strains were incubated in the presence of mouse serum in the presence (+EGTA) or absence (−EGTA) of EGTA and analyzed by flow cytometry using an anti-mouse C3 antibody. (G) Cumulative mortality in CBA/J mice inoculated intravenously with 1×104 cells of the indicated strains (F-left panel). p<0.01 for the comparison of the lhc1Δ mutant versus either wt or the LHC1 complemented strain (F-right panel). Cumulative mortality of mice inoculated the same as in F-left panel, except mice were treated with cobra venom factor to deplete serum complement according to Materials and Methods (p = NS). Discussion
A number of formidable pathogenic microbes express PS capsules that are potent virulence factors. Despite their importance in pathogenesis, many aspects of capsular architecture remain poorly understood. The cryptococcal capsule is particularly large and complex, resulting in cells with diameters up to 50 µm in diameter that cannot be ingested by phagocytic cells [37]. The primary structure of the cryptococcal capsule has been well characterized [7]. Xylose and glucuronic acid substituents are attached to a mannose backbone that are readily detectable by NMR spectroscopy. However, given that GXM polymers have masses in excess of 1 mDa, current methods of analytical chemistry cannot reliably detect the rare sugar modifications that may be responsible for tertiary structural complexity [38]. Thus, to identify proteins possibly involved in the modification of the capsular structure, DMSO extraction of intact cells was followed by extensive dialysis and capture on a charged agarose matrix which identified a lactonohydrolase that co-localized with the capsule. Further studies utilizing an mCherry-tagged recombinant protein, biochemical as well immunolocalization confirmed this capsular localization. While the putative Lhc1 sequence does not contain a putative N-terminal leader sequence, several unconventional protein secretion mechanisms have been described including secretion of protein-filled exosomes [39]. Lactonohydrolases are hydrolytic enzymes that cleaves the lactone group within carbohydrates to produce the corresponding organic acid and are expressed by a wide variety of plants and plant-associated fungi but have not been implicated in capsule modifications [40].
Deletion of the LHC1 locus resulted in a larger capsule that blocked penetration by larger particles comprising India Ink, but allowed increased diffusion of a fluorescent dextran polymer. This phenotype was associated with a reduction in virulence in a mouse model and the increased capsule size persisted during infection in brain. Classical analytical approaches [8] yielded only subtle differences between the wt and mutant strain, including a slight increase in backbone sugars such as mannose and xylose [38] as well as branching glucuronic acids by neutral sugar analysis. NMR spectroscopy also suggested only subtle differences that reflected retention of much of the mannose backbone and primary branching, with a similar mannosyl∶xylosyl∶glucuronic acid ratio, but potentially stronger cross-linkage between GXM chains, given the resistance of the mutant PS to sonication. These results differed somewhat from the elemental analysis and may be due to the preparation of the material for NMR spectroscopy which used soluble GXM, rather than DMSO-extractable polysaccharide material used for the elemental analysis.
Such subtle changes in primary structure as well as increased diffusion of dextran dye in the mutant suggested a role for LHC1 in capsular tertiary structure that could result in a larger, more open structure in the mutant strain. Biophysical methods were thus used to study DMSO-extractable PS of the cryptococcal capsule that comprise the outer interface with phagocytes during infection [21] and also contained the adherent Lhc1 protein. Notable was the larger molecular mass and increased particle size of the PS from the lhc1Δ mutant by polydispersity measurements, which correlated with the overall larger capsule size visualized by India ink microscopy. Previous work had noted an association between extracted particle size and overall capsule size between cryptococcal strains [23]. In addition, the larger negative zeta potential of PS from the mutant strain suggested a more solvated surface for these larger molecules and a tendency towards less aggregation that could provide a more open structure for antibody and complement deposition. The mutant strain PS was also found to have a lower shape factor ρthan wt, determined by the ratio of Rg, the radius of gyration and Rh, the hydrodynamic radius, which provides an important measure of whether a molecule is loosely linear or branched [41], [42]. C. neoformans wt strains tend to produce PS which have low ρ, more similar to branched polysaccharides such as glycogen and amylopectin [10]. This suggests that the surface structure of the larger lhc1Δ mutant PS contains an even more highly-branched/cross-linked surface component that is also more efficiently solvated. Small changes in the number of glucuronic acid residues in the mutant strain, detected in the composition assay, could have contributed to this increased surface hydration, either alone or in combination with a more highly branched surface structure. A lower ρ value could also reflect aggregation of components of the PS capsule [43], but would be inconsistent with the more highly negative zeta potential value that suggests a lower tendency towards aggregation. In addition, examining the two values contributing to ρ, most of the change was due to that of the radius of hydration, which is a measure of the hydrated zone surrounding a given polysaccharide particle, with very little change in the radius of gyration, again suggesting a more branched, hydrated structure.
Combining the results of this study with prior contributions from several laboratories and more recent studies of the capsular architecture suggest a tentative model that may help to illustrate the modifications of C. neoformans capsular PS by Lhc1 and exclusion of anti-microbial products (Fig. 6). The location of a hydrolytic lactonohydrolase within the PS capsular structure and the smaller size of the PS particles in the wt strain suggest that the enzyme either directly or indirectly plays a role in remodeling secreted PS fibrils (Fig. 6A). Recent data has suggested that the capsule is assembled by non-covalent binding of PS fibrils that have a branched structure [10]. Hydrolysis of outer branching units within PS surface structure by Lhc1 in wt cells (Fig. 6B, right panel) could reduce the size of the capsular PS (compared to that of the lhc1Δ mutant; Fig. 6B, middle panel), and its overall branching if the outer hydrated segments were also highly branched. This would result in a reduced hydrated surface with a smaller radius of hydration and a less negative zeta potential in the wt cells, resulting in a smaller, more compact capsule. This structure was supported by cryo-scanning electron microscopy which demonstrated a compact structure in the wt cells composed of truncated fibrils, whereas the mutant strain demonstrated a much more open lattice composed of larger fibrils, likely representing the larger PS demonstrated by the polydispersity measurements. The poor fitting and better solvation of the unprocessed mutant PS within the capsular gel lattice in the lhc1Δ mutant thus resulted in increased diffusion of dextran particles and greater penetration by anti-microbial products or exposure of binding epitopes. PS processing by Lhc1 could be due to the presence of trace lactone units forming some of the PS crosslinks or other carbohydrate linkages specifically tailored to the hydrolytic activity of Lhc1. However, identifying trace lactone groups within a large polysaccharide background is particularly difficult. To put this problem in perspective, the molecular mass of GXM is >1 mDa [44]; thus, detection of rare lactone groups is probably beyond the current analytical horizon. If true, the occurrence of lactone-dependent crosslinking would imply that this step of capsular assembly occurs in the extracellular space and that random concentration gradients of reagents involved in such processes within the capsule could contribute to the remarkable antigenic variability reported within the capsule structure [45]. Localization of the protein to the capsule, suggested by the method of isolation, western blots of cell fractions and immune - and fluorescence-microscopy, also supports a remodeling role in capsular synthesis. Indeed, the mCherry-Lhc1 localization studies appear to suggest production of the enzyme during capsule induction at the outer region of the capsule which is the region that is thought to represent an inducible outer layer that forms on top of an older inner capsule layer [46]. Lhc1 protein production during capsular induction also suggests such an extracellular mechanism. However, an indirect or regulatory role cannot be ruled out. Interestingly, the gene has previously been found to be induced in response to hypoxia, which may facilitate virulence in the relatively hypoxic environment of infected tissue [47].
Fig. 6. Scheme of working model of capsule modification by Lhc1. 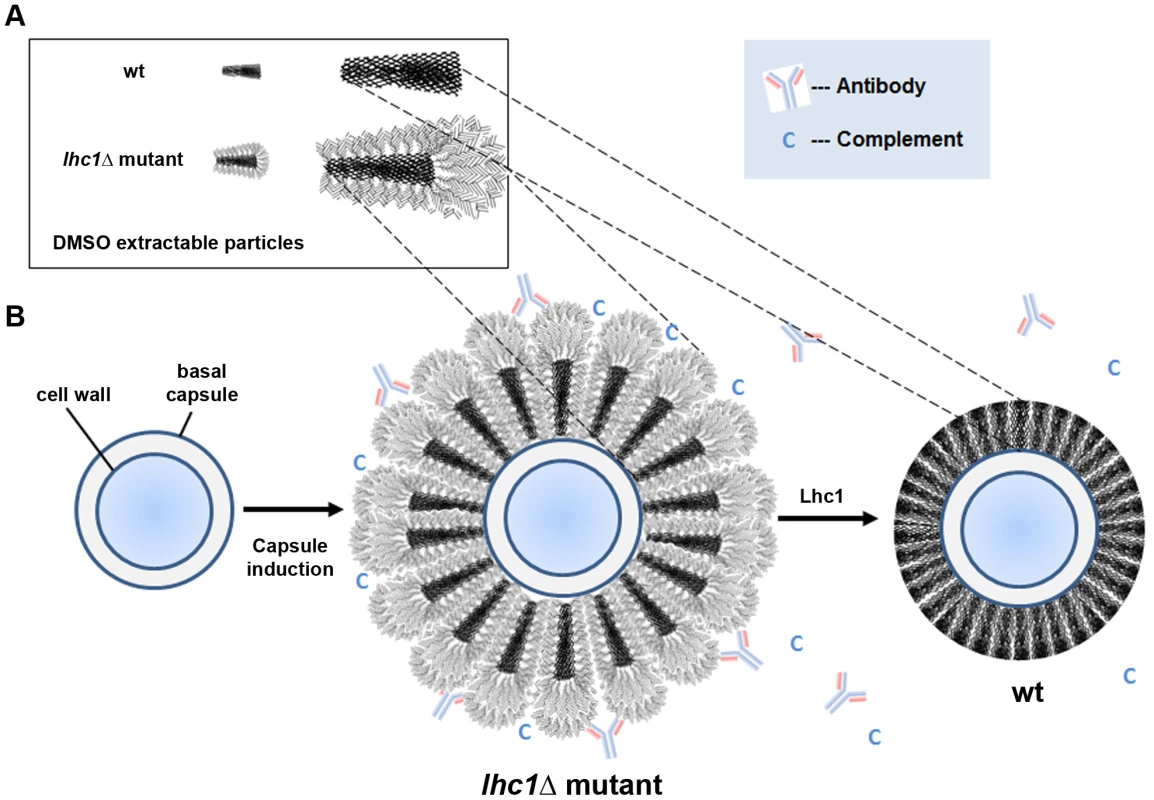
(A) Data from particle analysis of DMSO-solubilized PS and cryo-electron microscopy suggests hydrolytic, Lhc1-dependent processing of two populations of particles during capsule induction. Hydrolysis of outer branching units within the PS surface structure reduces both the size and overall branching as the wt particle adopts a surface having reduced hydration, radius of hydration and increased zeta potential. (B) Model of outer capsular synthesis. In the absence of Lhc1, unprocessed PS units with increased branching and increased surface hydration provide sites for antibody and complement binding, resulting in increased phagocytosis. Action of Lhc1 serves to provide a less hydrated, more compact capsule, resulting in better exclusion of innate immune products, reducing phagocytosis and increasing virulence potential. Events are not intended to be sequential; PS synthesis and LHC1-dependent remodeling likely are simultaneous. Representation does not intend to imply that PS particles are symmetrical or have regularly-spaced branches. Using either mAb 18B7 or serum as opsonin, the lhc1Δ mutant exhibited higher rates of phagocytosis than the wt or complemented strain. Patterns of antibody binding by the IgM mAb 12A1 and 2D10 were altered in the lhc1Δ mutant, whereas only subtle differences were noted using the IgG mAb, 18B7, which may have been due to the smaller size of the IgG antibody or to differences in epitopes to which they bind. As suggested by the serum-dependent phagocytosis data, more complement was deposited on the capsule of the lhc1Δ mutant than the wt strain both in the presence or absence of EGTA, which blocks the classical pathway. The increased deposition of C3 on the lhc1Δ capsule was accompanied by reduced virulence in a mouse model which was reversed by depletion of complement by cobra venom factor in the mice, suggesting a role for complement in the differential virulence between mutant and wt strains in complement-sufficient mice. There is strong evidence supporting a role for the alternative pathway in innate protection against Cryptococcus, since animals deficient in C3 but not C4 have reduced survival [48], [49]. Deposition of complement is important for efficient opsonization of the fungus, rather than direct killing by production of a membrane attack complex [50]. Complement fragments iC3b were also increased on the lhc1Δ mutant, which are important opsonic ligands and forms rapidly on the cryptococcal capsule after complement deposition [34]. Reducing alternative pathway activation by LHC1 thus facilitates a role for C. neoformans as an effective pathogen. In summary, the current studies demonstrate a role for LHC1 in the remodeling of the polysaccharide capsule of C. neoformans that represents a unique mechanism of virulence optimization among pathogenic microbes.
Materials and Methods
Ethics statement
Research involving human participants was approved by the NIAID intramural institutional review board and written, informed consent was obtained from all study participants before participation and was conducted according to the principals in the Declaration of Helsinki. All experimental procedures involving animals were conducted under guidelines of the National Institutes of Health and protocols approved by the Institutional Animal Care Committees (IACUC) of the Intramural NIH/NIAID and the University of Illinois at Chicago.
Fungal strains, plasmids and media
Cryptococcus neoformans ATCC 208821 (H99) was a generous gift of J. Perfect. Strain H99 ura5, [51] was employed as a recipient strain for deletion and expression studies and were maintained on media described in Supplemental Material and Methods. Plasmid pCIP containing the URA5 gene was a kind gift of K.J. Kwon-Chung.
Isolation of capsular proteins from DMSO-extracted capsule
C. neoformans strain B-3501 was grown at 30°C in RPMI supplemented with 2% glucose to stationary phase. Fifty grams of cells were harvested, washed extensively in 10 mM sodium phosphate, pH 7.0 and exchanged into dimethylsulfoxide as described [11] and incubated overnight with shaking at 37°C. Supernatant was harvested and dialyzed extensively against 10 mM sodium phosphate buffer, pH 7.0, followed by recovery of protein by passage of the dialysate on a 1 ml (DEAE)-Sepharose column. The column was extensively washed in 10 mM sodium phosphate, pH 7.0 and eluted with the same buffer containing 0.5 M NaCl. Eluate was again dialyzed and subjected to PAGE. Proteins were then transferred to nitrocellulose membranes and subjected to automated protein sequencing after protease Lys-C digestion as described [52].
Disruption and complementation of LHC1 in C. neoformans
Standard methods were used for disruption and complementation of the LHC1 gene in strain H99 as described previously using two PCR-amplified fragments and a 1.3-kb PCR fragment of the URA5 gene previously described to effect a 1.4-kb deletion within the LHC1 coding region (see Supplemental Materials and Methods in Text S1) and was complemented using a 3.6-kb genomic fragment of the LHC1 gene.
Analysis of lactonohydrolase activity
Fungal cells or recombinant enzyme were assayed for hydrolysis of the aliphatic lactone D-pantonylactone using a previously-described method [17] after induction in minimal media (0.1% glucose, 1 g/L asparagine, 20 mM sodium phosphate, 1 g/L YNB without amino acids and ammonium sulfate) for 3 days 30°C (see Supplemental Materials and Methods in Text S1) and assayed for hydrolysis of 100 mM D-pantonylactone by high performance liquid chromatography by reference to standard D-pantoic acid.
Preparation of a recombinant lactonohydrolase and generation of antibody to Lhc1
Full-length and an N-terminal fragment of lactonohydrolase was expressed in E. coli as a fusion protein with maltose-binding protein by using the pIH902 expression system (New England Biolabs, Beverly, Mass.) and the recombinant maltose-binding protein–lactonohydrolase fusion protein (MBP-Lhc1) purified on amylose-Sepharose according to the manufacturer's directions as described in Supplemental Materials and Methods. Mice were immunized by a standardized protocol with either MBP-Lhc1 or MBP alone as control and MBP antibodies removed as described in Supplemental Materials and Methods.
Immunolocalization studies
Histopathological material was prepared, embedded and fixed and incubated with either anti-Lhc1 or anti-MBP antibody and observed using fluorescence microscopy as described in Supplemental Materials and Methods.
Measurement of capsular size and permeability
To induce capsule, yeast cells were grown in 3 mL of RPMI in a 12-well plate incubated in a CO2 enriched environment (GasPak EZ CO2, Becton Dickinson) in a 37°C water jacketed incubator for 4 days. Alternatively, capsule was induced by growth on 1∶10 dilutions of Sabaraud's media at 30°C or RPMI agar for the indicated times and Lhc1 transcript was measured by quantitative RT-PCR and is described in Supplemental Material and Methods. TMR-Dextran 2,000 kDa (TMRD, Invitrogen) staining of C. neoformans capsule on cells grown under capsule-inducing conditions was performed as described previously [21]. The distance between the outside of the cell wall and the staining front was measured using Slidebook software. One-way ANOVA was used to assess statistical significance among the strains; Tukey's t test was used to perform pairwise analyses post-hoc.
Glycosyl composition of polysaccharide
Glycosyl composition analysis was performed on DMSO-extracted capsule by combined gas chromatography/mass spectrometry (GC/MS) of the per-O-trimethylsilyl (TMS) derivatives of the monosaccharide methyl glycosides produced from the sample by acidic methanolysis as described [53].
Analysis of capsule by nuclear magnetic resonance spectroscopy
Soluble glucoroxylomannan (GXM) was prepared and analyzed by NMR as previously reported [54]. Briefly, Isolated soluble GXMs were sonicated and lyophilized three times before being dissolved in 0.5 ml of 99.96% D2O (Sigma, St. Louis, Mo.) for nuclear magnetic resonance (NMR) analysis by standard methods [55]. NMR spectra were acquired with a Bruker Avance 600 NMR spectrometer, using a 5-mm (1H, 13C) inverse-detection dual-frequency probe, operating at 600.13 and 150.913 MHz, respectively as described.
Biophysical analysis and crypto-electron microscopy of DMSO-extracted capsular polysaccharide
Capsular PS samples from H99 wt, lhc1Δ and lhc1Δ LHC1 strains were isolated by DMSO extraction, prepared and subjected to light scattering analysis as described [10] and is described further in Supplemental Materials and Methods. Cryo-electron microscopy was performed on indicated cells and is described further in Supplemental Materials and Methods.
Virulence factor expression and virulence studies
Capsule was measured by microscopy after the fungal cells were suspended in India ink [56], urease production by incubation on Christensen's agar [57], and laccase by melanin production on nor-epinephrine agar [58]. Virulence studies were conducted according to a previously described intravenous mouse meningoencephalitis model [59] using 10 CBA/J mice for each C. neoformans strain. In a second experiment, animals were treated with cobra venom factor (CVF) according to Shapiro et al. [36], described in Supplemental Materials and Methods.
Phagocytosis and killing assays
Phagocytosis and fungal killing assays were conducted using J774.16 cells by the method of Shapiro et al [36] or the method of Miller and Mitchel [60] using human PBMCs and is described in Supplementary Materials and Methods. The phagocytosis index was determined by microscopic examination of the number of fungal cells ingested or adherent divided by the number of total macrophages.
Detection of C3 binding by flow cytometry and fluorescence microscopy
Fungal binding of C3 from mouse and human serum was determined by flow cytometry using goat anti-mouse C3-FITC (ICN), anti-human C3-FITC (Invitrogen) or anti-human iC3b (Quidel) in the presence or absence of EGTA by the method of [33] using a Becton Dickenson LSR Fortessa flow cytometer. C3 binding was determined by fluorescence microscopy using a FITC-labeled anti human C3 (Invitrogen) and visualized by fluorescence microscopy.
Statistics
The capsule radius was measured in India ink experiments using 10 cells for each strain and means compared using ANOVA with Tukey's test post hoc. Errors were expressed as standard error of the mean (SEM). Statistical significance of mouse survival times was assessed by Kruskall-Wallis analysis (ANOVA on Ranks). Statistical analyses for capsular biophysical measurements were carried out using Bi-ZPMwA Zimm Plot Software (Brookhaven Instruments).
90Plus/BI-MAS Software was used for effective diameter and polydispersity parameters (Brookhaven Instruments). Comparison of phagocytic index was performed by a non-parametric t-test with Welch's correction. Plots, curve fits, Pearson or Spearman correlations (r), and statistical analysis were performed using GraphPad Prism version 5.0a, GraphPad Software, San Diego, California, USA.
Supporting Information
Zdroje
1. RobertsIS, SaundersFK, BoulnoisGJ (1989) Bacterial capsules and interactions with complement and phagocytes. Biochem Soc Trans 17 : 462–464.
2. HorwitzMA, SilversteinSC (1980) Influence of the Escherichia coli capsule on complement fixation and on phagocytosis and killing by human phagocytes. J Clin Invest 65 : 82–94.
3. SchneiderMC, ExleyRM, RamS, SimRB, TangCM (2007) Interactions between Neisseria meningitidis and the complement system. Trends Microbiol 15 : 233–240.
4. VecchiarelliA (2000) Immunoregulation by capsular components of Cryptococcus neoformans. Med Mycol 38 : 407–417.
5. KisengePR, HawkinsAT, MaroVP, McHeleJP, SwaiNS, et al. (2007) Low CD4 count plus coma predicts cryptococcal meningitis in Tanzania. BMC Infect Dis 7 : 39.
6. ParkBJ, WannemuehlerKA, MarstonBJ, GovenderN, PappasPG, et al. (2009) Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. Aids 23 : 525–530.
7. KumarP, YangM, HaynesBC, SkowyraML, DoeringTL (2011) Emerging themes in cryptococcal capsule synthesis. Curr Opin Struct Biol 21 : 597–602.
8. CherniakR, SundstromJB (1994) Polysaccharide antigens of the capsule of Cryptococcus neoformans. Infect Immun 62 : 1507–1512.
9. DoeringTL (2009) How sweet it is! Cell wall biogenesis and polysaccharide capsule formation in Cryptococcus neoformans. Annu Rev Microbiol 63 : 223–247.
10. CorderoRJ, FrasesS, GuimaraesAJ, RiveraJ, CasadevallA (2011) Evidence for branching in cryptococcal capsular polysaccharides and consequences on its biological activity. Mol Microbiol 79 : 1101–1117.
11. GorenMB, MiddlebrookGM (1967) Protein conjugates of polysaccharide from Cryptococcus neoformans. J Immunol 98 : 901–913.
12. RodriguesML, NakayasuES, OliveiraDL, NimrichterL, NosanchukJD, et al. (2008) Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot Cell 7 : 58–67.
13. KataokaM, YamamotoK, ShimizuS, OhtaM, KitaA, et al. (1998) Crystallization and preliminary X-ray diffraction study of lactonohydrolase from Fusarium oxysporum. Acta Crystallogr D Biol Crystallogr 54 : 1432–1434.
14. MalikA, FirozA, JhaV, AhmadS (2010) PROCARB: A Database of Known and Modelled Carbohydrate-Binding Protein Structures with Sequence-Based Prediction Tools. Adv Bioinformatics 436036.
15. SvenssonC, TenebergS, NilssonCL, KjellbergA, SchwarzFP, et al. (2002) High-resolution crystal structures of Erythrina cristagalli lectin in complex with lactose and 2′-alpha-L-fucosyllactose and correlation with thermodynamic binding data. J Mol Biol 321 : 69–83.
16. BicanicT, HarrisonTS (2004) Cryptococcal meningitis. Br Med Bull 72 : 99–118.
17. ShimizuS, KataokaM, ShimizuK, HirakataM, SakamotoK, et al. (1992) Purification and characterization of a novel lactonohydrolase, catalyzing the hydrolysis of aldonate lactones and aromatic lactones, from Fusarium oxysporum. Eur J Biochem 209 : 383–390.
18. CasellasM, GrifollM, BayonaJM, SolanasAM (1997) New metabolites in the degradation of fluorene by Arthrobacter sp. strain F101. Appl Environ Microbiol 63 : 819–826.
19. ZhuX, GibbonsJ, Garcia-RiveraJ, CasadevallA, WilliamsonPR (2001) Laccase of Cryptococcus neoformans is a cell wall-associated virulence factor. Infect Immun 69 : 5589–5596.
20. JanbonG, HimmelreichU, MoyrandF, ImprovisiL, DromerF (2001) Cas1p is a membrane protein necessary for the O-acetylation of the Cryptococcus neoformans capsular polysaccharide. Mol Microbiol 42 : 453–467.
21. GatesMA, ThorkildsonP, KozelTR (2004) Molecular architecture of the Cryptococcus neoformans capsule. Mol Microbiol 52 : 13–24.
22. FonsecaFL, NimrichterL, CorderoRJ, FrasesS, RodriguesJ, et al. (2009) Role for chitin and chitooligomers in the capsular architecture of Cryptococcus neoformans. Eukaryot Cell 8 : 1543–1553.
23. FrasesS, PontesB, NimrichterL, VianaNB, RodriguesML, et al. (2009) Capsule of Cryptococcus neoformans grows by enlargement of polysaccharide molecules. Proc Natl Acad Sci U S A 106 : 1228–1233.
24. MullerRH, JacobsC, KayserO (2001) Nanosuspensions as particulate drug formulations in therapy. Rationale for development and what we can expect for the future. Adv Drug Deliv Rev 47 : 3–19.
25. ZhangS, HachamM, PanepintoJ, HuG, ShinS, et al. (2006) The Hsp70 member, Ssa1 acts as a DNA-binding transcriptional co-activator in Cryptococcus neoformans. Mol Microbol 62 : 1090–1101.
26. WangX, WangP, SunS, DarwicheS, IdnurmA, et al. (2012) Transgene induced co-suppression during vegetative growth in Cryptococcus neoformans. PLoS Genet 8: e1002885.
27. De JesusM, ChowSK, CorderoRJ, FrasesS, CasadevallA (2010) Galactoxylomannans from Cryptococcus neoformans varieties neoformans and grubii are structurally and antigenically variable. Eukaryot Cell 9 : 1018–1028.
28. MukherjeeJ, NussbaumG, ScharffMD, CasadevallA (1995) Protective and nonprotective monoclonal antibodies to Cryptococcus neoformans originating from one B cell. J Exp Med 181 : 405–409.
29. FeldmesserM, KressY, NovikoffP, CasadevallA (2000) Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect Immun 68 : 4225–4237.
30. TabordaCP, CasadevallA (2002) CR3 (CD11b/CD18) and CR4 (CD11c/CD18) are involved in complement-independent antibody-mediated phagocytosis of Cryptococcus neoformans. Immunity 16 : 791–802.
31. KozelTR (1993) Opsonization and phagocytosis of Cryptococcus neoformans. Arch Med Res 24 : 211–218.
32. KozelTR, WilsonMA, MurphyJW (1991) Early events in initiation of alternative complement pathway activation by the capsule of Cryptococcus neoformans. Infect Immun 59 : 3101–3110.
33. Mershon-ShierKL, VasuthasawatA, TakahashiK, MorrisonSL, BeenhouwerDO (2011) In vitro C3 deposition on Cryptococcus capsule occurs via multiple complement activation pathways. Mol Immunol 48 : 2009–2018.
34. KozelTR, PfrommerGS (1986) Activation of the complement system by Cryptococcus neoformans leads to binding of iC3b to the yeast. Infect Immun 52 : 1–5.
35. PanepintoJ, LiuL, RamosJ, ZhuX, Valyi-NagyT, et al. (2005) The DEAD-box RNA helicase Vad1 regulates multiple virulence-associated genes in Cryptococcus neoformans. J Clin Invest 115 : 632–641.
36. ShapiroS, BeenhouwerDO, FeldmesserM, TabordaC, CarrollMC, et al. (2002) Immunoglobulin G monoclonal antibodies to Cryptococcus neoformans protect mice deficient in complement component C3. Infect Immun 70 : 2598–2604.
37. Casadevall A, Perfect J (1998) Cryptococcus neoformans. Wash D.C.: ASM Press.
38. CherniakR, ValafarH, MorrisLC, ValafarF (1998) Cryptococcus neoformans chemotyping by quantitative analysis of 1H nuclear magnetic resonance spectra of glucuronoxylomannans with a computer-simulated artificial neural network. Clin Diagn Lab Immunol 5 : 146–159.
39. FevrierB, RaposoG (2004) Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol 16 : 415–421.
40. ShimizuS, KataokaM, HondaK, SakamotoK (2001) Lactone-ring-cleaving enzymes of microorganisms: their diversity and applications. J Biotechnol 92 : 187–194.
41. NilssonL, LeemanM, WahlundKG, BergenstahlB (2006) Mechanical degradation and changes in conformation of hydrophobically modified starch. Biomacromolecules 7 : 2671–2679.
42. MurakamiT, UchidaS, IshizuK (2008) Architecture of hyperbranched polymers consisting of a stearyl methacrylate sequence via a living radical copolymerization. J Colloid Interface Sci 323 : 242–246.
43. NimrichterL, FrasesS, CinelliLP, VianaNB, NakouziA, et al. (2007) Self-aggregation of Cryptococcus neoformans capsular glucuronoxylomannan is dependent on divalent cations. Eukaryot Cell 6 : 1400–1410.
44. McFaddenDC, De JesusM, CasadevallA (2006) The physical properties of the capsular polysaccharides from Cryptococcus neoformans suggest features for capsule construction. J Biol Chem 281 : 1868–1875.
45. McFaddenDC, FriesBC, WangF, CasadevallA (2007) Capsule structural heterogeneity and antigenic variation in Cryptococcus neoformans. Eukaryot Cell 6 : 1464–1473.
46. ZaragozaO, TelzakA, BryanRA, DadachovaE, CasadevallA (2006) The polysaccharide capsule of the pathogenic fungus Cryptococcus neoformans enlarges by distal growth and is rearranged during budding. Mol Microbiol 59 : 67–83.
47. ChunCD, LiuOW, MadhaniHD (2007) A link between virulence and homeostatic responses to hypoxia during infection by the human fungal pathogen Cryptococcus neoformans. PLoS Pathog 3: e22.
48. DiamondRD, MayJE, KaneM, FrankMM, BennettJE (1973) The role of late complement components and the alternate complement pathway in experimental cryptococcosis. Proc Soc Exp Biol Med 144 : 312–315.
49. MershonKL, VasuthasawatA, LawsonGW, MorrisonSL, BeenhouwerDO (2009) Role of complement in protection against Cryptococcus gattii infection. Infect Immun 77 : 1061–1070.
50. DiamondRD, MayJE, KaneMA, FrankMM, BennettJE (1974) The role of the classical and alternate complement pathways in host defenses against Cryptococcus neoformans infection. J Immunol 112 : 2260–2270.
51. ZhuX, WilliamsonPR (2004) Role of laccase in the biology and virulence of Cryptococcus neoformans. FEMS Yeast Res 5 : 1–10.
52. WilliamsonPR (1994) Biochemical and molecular characterization of the diphenol oxidase of Cryptococcus neoformans: identification as a laccase. J Bacteriol 176 : 656–664.
53. MerkelGJ, ScofieldBA (1994) Comparisons between in vitro glial cell adherence and internalization of non-encapsulated and encapsulated strains of Cryptococcus neoformans. J Med Vet Mycol 32 : 361–372.
54. MoyrandF, KlaprothB, HimmelreichU, DromerF, JanbonG (2002) Isolation and characterization of capsule structure mutant strains of Cryptococcus neoformans. Mol Microbiol 45 : 837–849.
55. DuusJ, GotfredsenCH, BockK (2000) Carbohydrate structural determination by NMR spectroscopy: modern methods and limitations. Chem Rev 100 : 4589–4614.
56. EricksonT, LiuL, GueyikianA, ZhuX, GibbonsJ, et al. (2001) Multiple virulence factors of Cryptococcus neoformans are dependent on VPH1. Mol Microbiol 42 : 1121–1131.
57. CoxG, MukherjeeJ, ColeG, CasadevallA, PerfectJ (2000) Urease as a virulence factor in experimental cryptococcosis. Infect Immun 68 : 443–448.
58. LiuL, WakamatsuK, ItoS, WilliamsonPR (1999) Catecholamine oxidative products, but not melanin, are produced by Cryptococcus neoformans during neuropathogenesis in mice. Infect Immun 67 : 108–112.
59. SalasSD, BennettJE, Kwon-ChungKJ, PerfectJR, WilliamsonPR (1996) Effect of the laccase gene CNLAC1, on virulence of Cryptococcus neoformans. J Exp Med 184 : 377–386.
60. MillerMF, MitchellTG (1991) Killing of Cryptococcus neoformans strains by human neutrophils and monocytes. Infect Immun 59 : 24–28.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Combined Systems Approaches Reveal Highly Plastic Responses to Antimicrobial Peptide Challenge inČlánek Two Novel Human Cytomegalovirus NK Cell Evasion Functions Target MICA for Lysosomal Degradation
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 5- Stillova choroba: vzácné a závažné systémové onemocnění
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
-
Všechny články tohoto čísla
- Surveillance for Emerging Biodiversity Diseases of Wildlife
- The Emerging Role of Urease as a General Microbial Virulence Factor
- PARV4: An Emerging Tetraparvovirus
- Epigenetic Changes Modulate Schistosome Egg Formation and Are a Novel Target for Reducing Transmission of Schistosomiasis
- The Human Adenovirus E4-ORF1 Protein Subverts Discs Large 1 to Mediate Membrane Recruitment and Dysregulation of Phosphatidylinositol 3-Kinase
- A Multifactorial Role for Malaria in Endemic Burkitt's Lymphoma Pathogenesis
- Structural Basis for the Ubiquitin-Linkage Specificity and deISGylating Activity of SARS-CoV Papain-Like Protease
- Cathepsin-L Can Resist Lysis by Human Serum in
- Epstein-Barr Virus Down-Regulates Tumor Suppressor Expression
- BCA2/Rabring7 Targets HIV-1 Gag for Lysosomal Degradation in a Tetherin-Independent Manner
- The Evolutionarily Conserved Mediator Subunit MDT-15/MED15 Links Protective Innate Immune Responses and Xenobiotic Detoxification
- Suppressor of Cytokine Signaling 4 (SOCS4) Protects against Severe Cytokine Storm and Enhances Viral Clearance during Influenza Infection
- T Cell Inactivation by Poxviral B22 Family Proteins Increases Viral Virulence
- Dynamics of HIV Latency and Reactivation in a Primary CD4+ T Cell Model
- HIV and HCV Activate the Inflammasome in Monocytes and Macrophages via Endosomal Toll-Like Receptors without Induction of Type 1 Interferon
- Virus and Autoantigen-Specific CD4+ T Cells Are Key Effectors in a SCID Mouse Model of EBV-Associated Post-Transplant Lymphoproliferative Disorders
- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Ion Channel Activity Promotes Virus Fitness and Pathogenesis
- Squalene Synthase As a Target for Chagas Disease Therapeutics
- The Contribution of Viral Genotype to Plasma Viral Set-Point in HIV Infection
- Combined Systems Approaches Reveal Highly Plastic Responses to Antimicrobial Peptide Challenge in
- Anthrax Lethal Factor as an Immune Target in Humans and Transgenic Mice and the Impact of HLA Polymorphism on CD4 T Cell Immunity
- Ly49C-Dependent Control of MCMV Infection by NK Cells Is -Regulated by MHC Class I Molecules
- Two Novel Human Cytomegalovirus NK Cell Evasion Functions Target MICA for Lysosomal Degradation
- A Large Family of Antivirulence Regulators Modulates the Effects of Transcriptional Activators in Gram-negative Pathogenic Bacteria
- Broad-Spectrum Anti-biofilm Peptide That Targets a Cellular Stress Response
- Malaria Parasite Infection Compromises Control of Concurrent Systemic Non-typhoidal Infection via IL-10-Mediated Alteration of Myeloid Cell Function
- A Role for in Higher Order Structure and Complement Binding of the Capsule
- Hip1 Modulates Macrophage Responses through Proteolysis of GroEL2
- CD8 T Cells from a Novel T Cell Receptor Transgenic Mouse Induce Liver-Stage Immunity That Can Be Boosted by Blood-Stage Infection in Rodent Malaria
- Phosphorylation of KasB Regulates Virulence and Acid-Fastness in
- HIV-Infected Individuals with Low CD4/CD8 Ratio despite Effective Antiretroviral Therapy Exhibit Altered T Cell Subsets, Heightened CD8+ T Cell Activation, and Increased Risk of Non-AIDS Morbidity and Mortality
- A Novel Mechanism Inducing Genome Instability in Kaposi's Sarcoma-Associated Herpesvirus Infected Cells
- Structural and Biochemical Characterization Reveals LysGH15 as an Unprecedented “EF-Hand-Like” Calcium-Binding Phage Lysin
- Hepatitis C Virus Cell-Cell Transmission and Resistance to Direct-Acting Antiviral Agents
- Different Modes of Retrovirus Restriction by Human APOBEC3A and APOBEC3G
- TNFα and IFNγ but Not Perforin Are Critical for CD8 T Cell-Mediated Protection against Pulmonary Infection
- Large Scale RNAi Reveals the Requirement of Nuclear Envelope Breakdown for Nuclear Import of Human Papillomaviruses
- The Cytoplasmic Domain of Varicella-Zoster Virus Glycoprotein H Regulates Syncytia Formation and Skin Pathogenesis
- A New Class of Multimerization Selective Inhibitors of HIV-1 Integrase
- Are We There Yet? The Smallpox Research Agenda Using Variola Virus
- High-Efficiency Targeted Editing of Large Viral Genomes by RNA-Guided Nucleases
- Dynamic Functional Modulation of CD4 T Cell Recall Responses Is Dependent on the Inflammatory Environment of the Secondary Stimulus
- Bacterial Superantigens Promote Acute Nasopharyngeal Infection by in a Human MHC Class II-Dependent Manner
- Follicular Helper T Cells Promote Liver Pathology in Mice during Infection
- A Nasal Epithelial Receptor for WTA Governs Adhesion to Epithelial Cells and Modulates Nasal Colonization
- Unexpected Role for IL-17 in Protective Immunity against Hypervirulent HN878 Infection
- Human Cytomegalovirus Fcγ Binding Proteins gp34 and gp68 Antagonize Fcγ Receptors I, II and III
- Expansion of Murine Gammaherpesvirus Latently Infected B Cells Requires T Follicular Help
- Venus Kinase Receptors Control Reproduction in the Platyhelminth Parasite
- Molecular Signatures of Hemagglutinin Stem-Directed Heterosubtypic Human Neutralizing Antibodies against Influenza A Viruses
- The Downregulation of GFI1 by the EZH2-NDY1/KDM2B-JARID2 Axis and by Human Cytomegalovirus (HCMV) Associated Factors Allows the Activation of the HCMV Major IE Promoter and the Transition to Productive Infection
- Inactivation of Fructose-1,6-Bisphosphate Aldolase Prevents Optimal Co-catabolism of Glycolytic and Gluconeogenic Carbon Substrates in
- New Insights into Rotavirus Entry Machinery: Stabilization of Rotavirus Spike Conformation Is Independent of Trypsin Cleavage
- Prophenoloxidase Activation Is Required for Survival to Microbial Infections in
- SslE Elicits Functional Antibodies That Impair Mucinase Activity and Colonization by Both Intestinal and Extraintestinal Strains
- Timed Action of IL-27 Protects from Immunopathology while Preserving Defense in Influenza
- HIV-1 Envelope gp41 Broadly Neutralizing Antibodies: Hurdles for Vaccine Development
- The PhoP-Dependent ncRNA Mcr7 Modulates the TAT Secretion System in
- Cellular Superspreaders: An Epidemiological Perspective on HIV Infection inside the Body
- The Inflammasome Pyrin Contributes to Pertussis Toxin-Induced IL-1β Synthesis, Neutrophil Intravascular Crawling and Autoimmune Encephalomyelitis
- Papillomavirus Genomes Associate with BRD4 to Replicate at Fragile Sites in the Host Genome
- Integrative Functional Genomics of Hepatitis C Virus Infection Identifies Host Dependencies in Complete Viral Replication Cycle
- Co-assembly of Viral Envelope Glycoproteins Regulates Their Polarized Sorting in Neurons
- Targeting Membrane-Bound Viral RNA Synthesis Reveals Potent Inhibition of Diverse Coronaviruses Including the Middle East Respiratory Syndrome Virus
- Dual-Site Phosphorylation of the Control of Virulence Regulator Impacts Group A Streptococcal Global Gene Expression and Pathogenesis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Venus Kinase Receptors Control Reproduction in the Platyhelminth Parasite
- Dual-Site Phosphorylation of the Control of Virulence Regulator Impacts Group A Streptococcal Global Gene Expression and Pathogenesis
- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Ion Channel Activity Promotes Virus Fitness and Pathogenesis
- High-Efficiency Targeted Editing of Large Viral Genomes by RNA-Guided Nucleases
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



