-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaTrajectories of functional decline in older adults with neuropsychiatric and cardiovascular multimorbidity: A Swedish cohort study
Using data from a Swedish cohort study, Davide Vetrano and colleagues examine trajectories of functional decline in older adults with neuropsychiatric and cardiovascular multimorbidity.
Published in the journal: . PLoS Med 15(3): e32767. doi:10.1371/journal.pmed.1002503
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002503Summary
Using data from a Swedish cohort study, Davide Vetrano and colleagues examine trajectories of functional decline in older adults with neuropsychiatric and cardiovascular multimorbidity.
Introduction
Aging is accompanied by a number of cellular and molecular dysfunctions that are responsible for a progressive loss of physical and cognitive resilience [1]. When death does not occur, physical decline and disability become progressively more frequent and strongly impact people’s health [2]. However, even if the direction of this process is certain, its pace varies among individuals, and both individual and contextual factors play a role in this variation [2]. The co-occurrence of multiple age-related chronic diseases is one of these factors.
Approximately 55%–98% of people aged ≥60 years have at least two chronic diseases (multimorbidity) [3,4]. Chronic diseases result from the interplay between people’s genetic susceptibility and lifelong environmental exposure [5]. The accumulation of chronic diseases over time is associated with progressive dysfunction in several systems and organs. Moreover, the co-occurrence of chronic diseases has negative effects on quality of life, frequency of healthcare use, dependency, and survival, which are greater than the sum of the effects of the individual diseases [1,4–6]. A large collaborative study that included 1.2 million participants reported that co-occurring cardio-metabolic diseases had a multiplicative negative impact on survival [7]. Indeed, chronic diseases do not appear at random but follow specific patterns due to shared risk factors and common pathophysiological pathways [4,8]. Previous research has consistently found that clusters of cardiovascular (CV) and neuropsychiatric (NP) disease constitute the major patterns of chronic disease in older adults [8].
CV and NP diseases are among the main contributors to the global burden of disability-adjusted life years (DALYs) in older adults. CV diseases are responsible for 30% and NP diseases for 7% of the years older people live with disability. Because of the swift growth of the older population, the overall contribution of these diseases to DALYs is expected to double by 2030 [9–10].
The impact of multimorbidity on functional ability has been investigated in several previous studies. With few exceptions [11–13], the majority reported that functional disability increased along with the number of chronic conditions [14–17]. However, few studies have investigated the relationship between multimorbidity and physical performance, and fewer have attempted to identify the specific clinical patterns associated with worse prognosis [2,18]. We intend to help fill this gap by identifying specific health profiles that lead to a major risk of functional decline.
The current study thus aimed to detect the effect and to quantify the impact of different clinical patterns—characterised by the co-occurrence of chronic CV and NP diseases—on trajectories of functional decline in older adults. Specifically, we studied the co-occurrence of CV and NP diseases across three different domains of increasing complexity: (1) pure CV morbidity, (2) pure NP morbidity, and (3) combined CV/NP multimorbidity. Studying the joint impact and prognosis of major chronic diseases will provide valuable information to aid clinicians in their daily counselling and help healthcare systems better target at-risk populations [8].
Materials and methods
Study design and population
We used data from the 9-year follow-up of the population-based Swedish National study of Aging and Care in Kungsholmen (SNAC-K) [19]. This study consists of community-dwelling and institutionalised older adults ≥60 years. A random sample from 11 age cohorts born between 1892 and 1939 (the youngest and the oldest age cohorts were oversampled) living in the Kungsholmen district (Stockholm, Sweden) was invited to participate to the study. Those who accepted were evaluated for the first time between 2001 and 2004 and then followed up every 6 years (those aged <78 years) or every 3 years (those aged ≥78 years) [19]. At baseline, 3,363 people were examined (participation rate, 73%; S1 Text); 978 participants were excluded because they had been evaluated fewer than 2 times. Those excluded were older and more likely to be female, to live in an institution, and to have more chronic diseases than those included in the study (p < 0.001 for all). Fig 1 depicts the flow of participants through the study. The study was approved by the Regional Ethics Review Board in Stockholm. Participants in the study completed a written informed consent form as stipulated in the ethical approval. For participants with prevalent or incident cognitive impairment, consent was obtained from the next of kin. [19]. The results of the present study were reported in accordance with the STROBE guidelines (S1 Checklist).
Fig. 1. Flowchart of study participation over 9 years. 
Dropouts are due to either refusal of the participant/relative, loss of contact with the participant, or moving of the participant from the city where the study took place. FU1, follow-up 1; FU2, follow-up 2; FU3, follow-up 3; SNAC-K, Swedish National study of Aging and Care in Kungsholmen. Chronic disease assessment
During baseline and follow-up visits, SNAC-K participants underwent comprehensive clinical and functional assessments carried out by trained physicians, nurses, and neuropsychologists. Physicians collected information on diagnoses via physical examination, medical history, examination of medical charts, self-reported information, and/or proxy interviews [19]. Clinical parameters, lab tests, medications, and inpatient and outpatient care data from the Swedish National Patient Register were also used to identify specific conditions [20]. All diagnoses were coded in accordance with the International Classification of Diseases, 10th revision (ICD-10), and classified in accordance with a clinically driven methodology recently proposed by our group [20]. After this procedure, we identified 7 CV and 12 NP chronic diseases. Chronic conditions deemed to be risk factors were excluded (e.g., hypertension and dyslipidaemia; see S2 Text for details) [20].
We grouped participants into 7 mutually exclusive clinical patterns based on the presence of 0, 1, or 2 or more CV or NP diseases (Fig 2): (1) no CV/NP diseases (reference pattern), (2) 1 CV disease, (3) 2 or more CV diseases (CV multimorbidity), (4) 1 NP disease, (5) 2 or more NP diseases (NP multimorbidity), (6) 1 CV and one NP disease (mixed multimorbidity), (7) 2 or more CV or NP diseases, accompanied by at least 1 other NP or CV disease (complex multimorbidity). The reference group may also include participants with 1 or more chronic disorders other than CV and NP diseases (see S1 Table for more details). Our hypothesis is that CV and NP multimorbidity has a group effect on the outcomes that goes beyond the simple count of diseases and that exceeds the effect of generic multimorbidity (2 or more diseases, not including CV and NP diseases). This is the rationale for including participants with multimorbidity in the control group. Moreover, studying the impact of mixed and complex multimorbidity may also provide information on the effect of the number and type of diseases in participants who have ≥2 CV and/or NP diseases. For the present study, information on chronic diseases was available at baseline and at the 3 - and 6-year follow-ups.
Fig. 2. Model showing the grouping of chronic diseases. 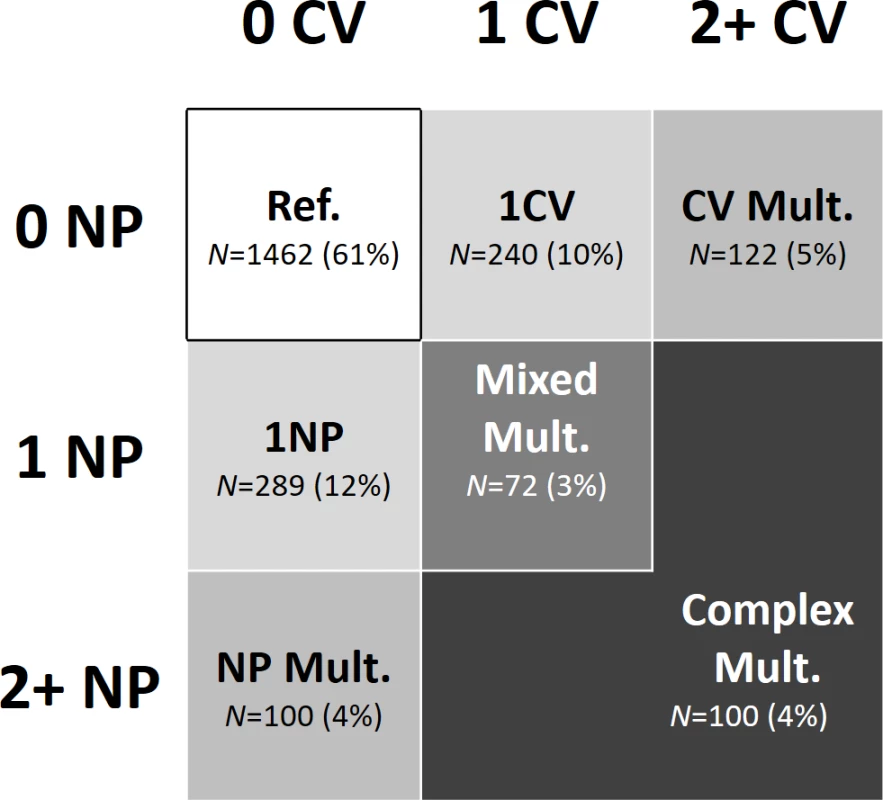
Participants were grouped by patterns of chronic diseases; that is, by number of CV and NP diseases. CV, cardiovascular; Mult., multimorbidity; NP, neuropsychiatric; Ref., reference group. Functional decline
Mobility was assessed with walking speed and independence as the ability to carry out activities of daily living (ADL). Changes in mobility and dependence were the outcomes of the present study. To assess walking speed, participants were asked to walk 6 meters at their usual speed, or alternatively, 2.4 meters. The shorter distance was used when participants reported that they walked slowly or when the assessment was carried out in restricted spaces, such as in homes or institutions. Walking speed was reported in meters/second. The ADL score was obtained by summing the number of intact domains from the following list: bathing, dressing, toileting, continence, transferring from bed, and eating. Both walking speed and ADL were assessed at baseline and at each follow-up for up to 9 years.
Covariates
Participants’ demographics (i.e., age, sex, and education) were collected through a questionnaire administered by the nurse [19]. Educational attainment was categorised as elementary, high school, and university or higher. Living arrangements (i.e., living in the community or in an institution) were registered. Body mass index (BMI) was obtained by dividing the participants’ weight by their squared height (kg/m2). A BMI <18.5 kg/m2 was considered indicative of malnutrition, in accordance with the recommendations of the European Society for Clinical Nutrition and Metabolism [21]. The total number of medications was obtained by counting the number of medications used at the moment of the assessment, coded in accordance with their Anatomical Therapeutic Chemical (ATC) classification. In the present study, institutionalization, malnutrition, and number of medications were used as global measures and proxies of disease severity. Data on BMI, living arrangements, and medications were available at baseline and at the 3 - and 6-year follow-ups. Vital status and date of death were obtained from the Swedish National Cause of Death Register and linked to SNAC-K data.
Statistical analyses
In a first analysis, trajectories of walking speed and ADL over 9 years were obtained with multilevel mixed-effect linear regression models. The clinical patterns at baseline were the exposures, and the analysis was adjusted for baseline age, sex, education, malnutrition, institutionalization, and number of medications. The reference group consisted of participants free from CV and NP diseases. Follow-up time was modelled through unrestricted cubic splines with 3 knots (at 0, 3, and 6 years). This analysis assessed how well the clinical patterns predicted functional decline at the individual level. In a second analysis, the association between clinical patterns and functional decline at the group level was estimated through mixed-effect regression models, in which clinical patterns and covariates that might change with time (i.e., number of medications, malnutrition, and institutionalization) were all included as time varying. This analysis assessed the cross-sectional association between clinical patterns and outcomes, considering the evolution of the exposure over time and taking intra - and interindividual variability into account. Interactions between clinical patterns and sex and age were tested, and stratified analyses were carried out. A p-value of <0.05 was considered statistically significant in all analyses. All analyses were carried out with Stata 14.0 (StataCorp LP) for Microsoft Windows. Compared with the original protocol (S1 Study Protocol), which included functional decline and mortality as outcomes, we later decided to focus our analyses on functional decline only, including walking speed (and not only disability) as outcome. Compared with the original plan, we also decided to model our outcomes through linear-mixed models instead of Cox regressions, in order to get the most from the repeated measures of both walking speed and ADL.
Sensitivity analyses
First, to investigate the role played by individual diseases in the robustness of our results, we tested the association between the number of CV and NP diseases and functional decline, excluding participants affected by the most common diseases, one disease at a time. Second, as death and dropout during follow-up may not have been at random, we repeated the analyses, excluding those who died or dropped out during follow-up. Third, incontinence occurs more frequently in females than in males. To check whether this difference had biased any estimation, we repeated the analyses, excluding incontinence from the ADL count.
Results
The mean age of the 2,385 participants was 73 years, and 65% were females (Table 1). As shown in Table 2, ischemic heart disease (13%), heart failure (7%), and atrial fibrillation (7%) were the 3 most frequent CV diseases at baseline. Depression and mood disorders (8%), dementia (6%), and cerebrovascular diseases (5%) were the 3 most frequent NP diseases. Chronic disease was more prevalent in participants ≥75 years than in younger participants. In the reference group (participants without CV and/or NP disease), 6% of participants were not affected by any disease, 17% had 1 disease, and 77% had 2 or more diseases. Chronic kidney disease (25%), osteoarthritis (13%), and thyroid diseases (9%) were the most common chronic diseases (S1 Table).
Tab. 1. Sample characteristics at baseline by patterns of CV and NP chronic diseases. 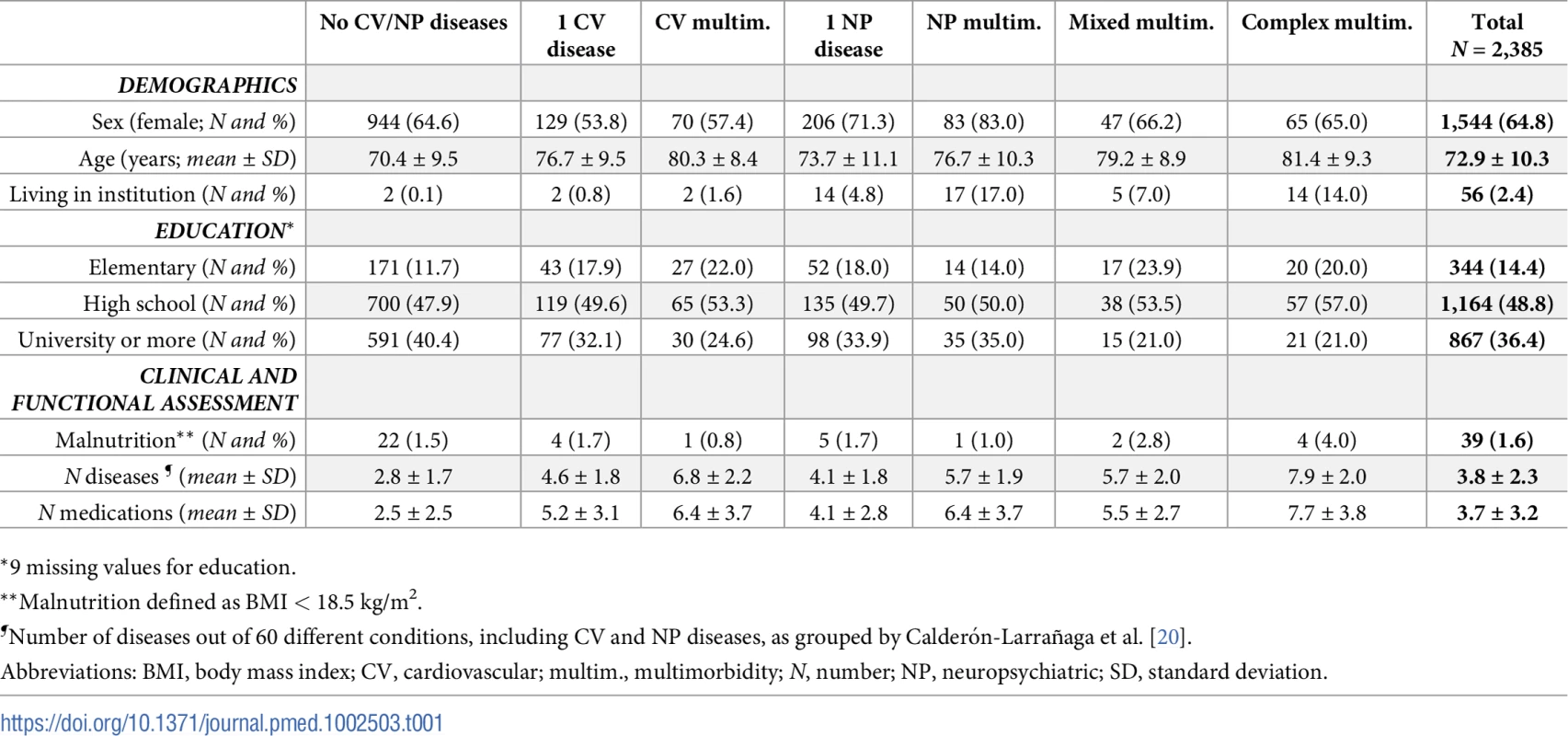
*9 missing values for education. Tab. 2. P/100 of CV and NP chronic diseases* and clinical patterns at baseline. 
* Details on the definition of listed chronic diseases are reported in S2 Text. Fig 3 depicts the trajectories of walking speed and ADL over time. Participants with multiple NP diseases, whether or not they combined with CV diseases, had the steepest decline in both walking speed and ADL. The rate of decline of participants with CV multimorbidity was significantly higher than that of the control group (participants free from CV and NP diseases), but only in walking speed. After 9 years, participants with multiple CV and NP diseases had the most meaningful deterioration in walking speed (up to 0.7 m/s, p < 0.001) and ADL (up to 3 ADL, p < 0.001), as compared with the reference group.
Fig. 3. Trajectories of walking speed and ADL impairment over 9 years by clinical patterns. 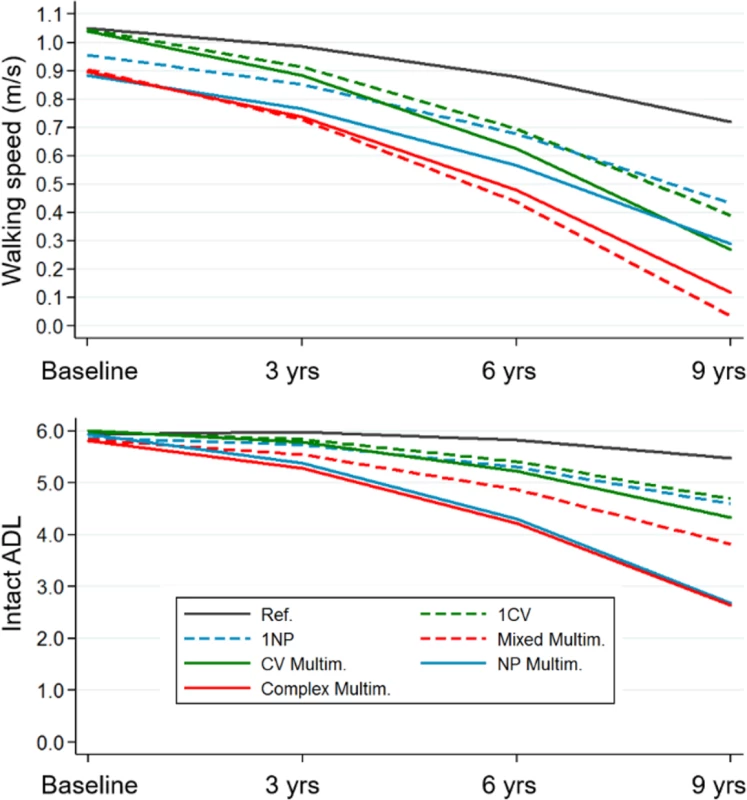
Trajectories derived from multilevel mixed-effect linear regression models adjusted for baseline age, sex, education, malnutrition, institutionalization, and number of medications. Reference group: participants free from cardiovascular and/or neuropsychiatric diseases. ADL, activities of daily living; CV, cardiovascular; Multim., multimorbidity; NP, neuropsychiatric; Ref., reference group. Fig 4 shows the association between the clinical patterns and functional decline at the group level, considering both the clinical patterns and the covariates as time varying. Having 1 CV disease was negatively associated with walking speed, but the association was of marginal significance (β = −0.03; 95% confidence interval [CI] −0.07–0.00). All other clinical patterns were significantly negatively associated with walking speed. The strongest associations were with NP multimorbidity (β = −0.20; 95% CI −0.24–−0.16) and complex multimorbidity (β = −0.21; 95% CI −0.25–−0.18). All clinical patterns that included at least 1 NP disease were negatively associated with ADL. Mixed multimorbidity (β = −0.20; 95% CI −0.30–−0.11) and complex multimorbidity (β = −0.27; 95% CI −0.34–−0.19) had the strongest associations. Notably, 1 of 4 participants had at least 1 NP disease. In general, participants with the most complex clinical patterns had 5%–20% lower functioning than those in the reference group.
Fig. 4. Association of chronic disease patterns with walking speed and number of intact ADL based on 6-year repeated measures of disease patterns, covariates, and outcomes. 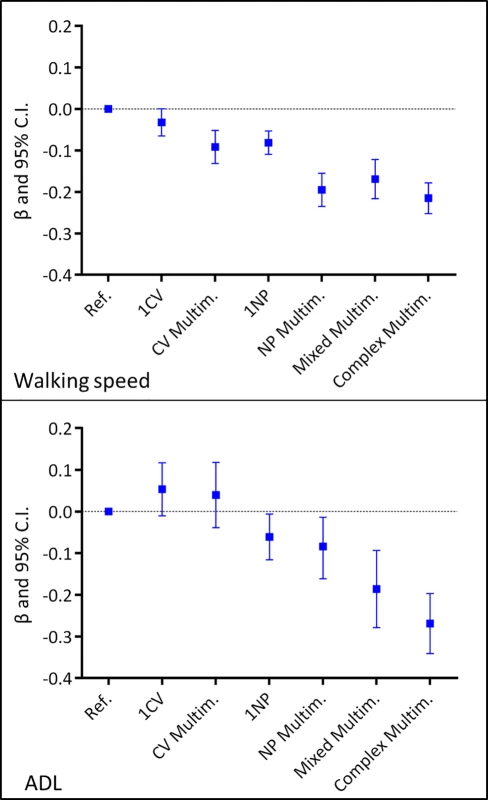
Multilevel mixed-effect linear regression models adjusted for age, sex, education, malnutrition, institutionalization, and number of medications. The exposure (the patterns of chronic diseases), the time-varying covariates (institutionalization, malnutrition, and number of medications), and the outcomes were introduced in the models as repeated measures. Reference group: participants free from CV/NP diseases. ADL, activities of daily living; CI, confidence interval; CV, cardiovascular; Multim., multimorbidity; NP, neuropsychiatric; Ref., reference group. The interaction and stratified analyses showed that older age and female sex generally tended to enhance the negative association between the clinical patterns and the outcomes (S2 and S3 Tables). In a sensitivity analysis, the exclusion of participants with the most prevalent CV and NP diseases (S1 Fig) did not change the strength of the association between the number of CV and NP diseases and functional decline. The results of further sensitivity analyses were consistent with the results of our main analyses.
Discussion
This study showed that patterns of CV and NP morbidity have different associations with functional decline in older adults over time. The pattern that included multiple CV and NP diseases was associated with the worst trajectory. NP diseases, alone or in association, are prevalent and major correlates of functional decline. Isolated CV multimorbidity was only associated with changes in mobility. In general, the combination of multiple CV and NP diseases (mixed and complex multimorbidity) was associated with 5%–20% reduced functioning than the reference group (people free from CV and NP diseases but with a high prevalence of generic multimorbidity).
A number of our results were consistent with those of previous studies. First, both CV and NP diseases were the most prevalent diseases. They also emerged as independent predictors of poor function in older adults, and NP diseases were stronger predictors than CV diseases [9]. Second, the coexistence of NP diseases boosted the association of each individual condition with functional decline, which suggests the existence of a biological interaction among NP diseases [22,23]. Third, when impairments in different systems coexist (as in mixed and complex multimorbidity), the association between the clinical patterns and the outcomes surpasses the sum of the individual associations with the outcomes and also exceeds the effect of generic multimorbidity [24,25].
Three studies have investigated the impact of multimorbidity patterns on disability, but none has looked at the impact on physical performance. Jackson et al. reported that CV and NP patterns—but not musculoskeletal pattern—were the main determinants of dependence in personal and instrumental ADL in a sample of 7,270 older women from the Australian Longitudinal Study on Women’s Health [22]. Quiñones et al. showed that a pattern of multimorbidity that included depressive symptoms, arthritis, and hypertension was associated with greater disability than other patterns in a sample of 8,782 older adults participating in the Health and Retirement Study [2]. The same group showed that in a sample of 4,017 older Medicare beneficiaries, combinations of somatic and mental diseases, in particular cognitive impairment and dementia, were associated with greater incident disability than combinations of somatic conditions only [25].
Only indirect comparisons can be made between our study and previous studies. In addition to differences in the sample selection, follow-up duration, and data collection, a number of aspects of our study require special attention: (1) unlike in previous studies, in which ad hoc lists of diseases were used, we relied on a comprehensive, clinically driven list of chronic diseases, for which the proof of concept was recently shown in the SNAC-K population [20]. (2) Our patterns of CV and NP diseases were not built with a data-driven procedure (e.g., factor analysis or prevalence), and we included all CV and NP diseases deemed chronic in the older population by Calderón-Larrañaga et al. [20]. (3) As opposed to previous studies, to test the association between disease patterns and functional status at the group level, we also took the within-individual, time-varying nature of multimorbidity into consideration and accounted for time variation in covariates (e.g., medication use and institutionalization). People age at different rates, and as a consequence, the development of multimorbidity follows different trajectories. Capturing the dynamic nature of multimorbidity in clinical and epidemiological studies is a scientific priority [6, 20].
Aging is a complex and multifaceted process, and underlying it are a large number of biological deficits that ultimately affect people’s resilience to external stressors [1,2]. The World Health Organization has defined healthy aging as the “process of developing and maintaining the functional ability that enables well-being in older age” [26]. Given the wide heterogeneity of clinical phenotypes in older people, it is preferable to use global measures of functioning to capture and assess their health status [27,28]. The National Institute for Health and Care Excellence (NICE) issued the first guidelines for the clinical assessment and management of multimorbidity in 2016. According to these guidelines, frailty and functional decline are both a component of multimorbidity and a condition that should be taken into account and assessed when managing the health and care of older adults with multimorbidity. [29]. In the present study, we chose walking speed and ADL as indicators of function in older adults. Slow walking speed is a strong and independent predictor of disability and a measure of physical frailty [28]. As shown by Santoni et al., slow walking speed and dependency in ADL optimally discriminate older adults’ health across different ages. Walking speed begins to decrease during the sixth decade of life, whereas ADL impairment tends to become evident only during the eighth decade [30]. In the present study, the effect of CV diseases and CV multimorbidity was more evident on mobility than on independence. Adequate walking speed and ADL both involve several systems and organs, and the integrity of mental, musculoskeletal, and CV functions are equally required [28,31]. However, unlike for ADL, walking—especially walking at an adequate speed—requires a certain level of CV fitness. The lack of such fitness due to the presence of 1 or more CV diseases might explain the selective association between CV diseases and walking speed [32]. NP conditions, on the other hand, were associated with both measures of functional decline. In particular, there was a clear dose-response association between walking speed and the higher number of coexisting NP conditions. Severe NP diseases, such as dementia and cerebrovascular disease (e.g., stroke), have a direct impact on quality of life and independence and are extremely burdensome, even in the presence of an intact CV system [9]. This finding has direct clinical implications. First, general practitioners and specialists should assess mobility in patients with multiple NP diseases more often than in others because of the steeper functional decline they are expected to experience. Similarly, physicians should consider the potential negative effect of medications for NP diseases on functional decline. Second, patients with multiple NP diseases and their caregivers are often told about the memory decline or NP symptoms they could develop but not about potential functional decline. Enhancing their awareness of this eventuality may help empower them to postpone or minimise the detrimental effects of diseases. For example, patients could ask for interventions to slow functional decline in the early stages. All these issues have clear public health implications. Finally, consistent with previous findings, the co-occurrence of diseases involving different systems (i.e., CV and NP) was associated with slower walking speed and greater ADL dependency. However, the strength of this association largely overlaps with that of NP multimorbidity.
Some limitations of this study need to be mentioned. First, studying the combined effect of different diseases belonging to the same body system (i.e., CV and NP) prevented us from looking at the differential impact of individual conditions. For example, we might expect a different prognosis for dementia and depression. However, in sensitivity analyses, excluding those with the most prevalent diseases, one disease at a time, did not change the association between the number of CV and NP diseases and the outcomes. This suggests that our hypothesis that homogenous patterns of multimorbidity convey an overall group-specific effect is plausible. Similar findings have been reported previously [7]. Second, we did not take the severity of diseases into account. However, we adjusted our analyses for a number of factors (i.e., malnutrition, institutionalization, and number of medications) that can be considered indirect proxies of disease severity and, at the same time, indicators of other comorbid conditions not explicitly accounted for. The same approach used to estimate the severity of single diseases might not work in the presence of multimorbidity. For this reason, using global and multiple proxies of complexity is warranted [33]. In addition, when we tested the overall association between disease patterns and functional status, these covariates were analyzed as time varying. Third, the study of the prognosis of heterogeneous and less prevalent disease patterns (i.e., mixed and complex multimorbidity) may have introduced some noise into our analyses. However, the strength and precision of the associations suggests that our hypothesis regarding the dose-response effect and interaction between multiple CV and NP diseases is plausible. Fourth, the SNAC-K sample selection (73% participation rate) might have affected the generalizability of the present study. In particular, nonparticipants had shorter survival (see S1 Text), which may mean that both multimorbidity and poor physical function were underestimated in the analyses, partially blunting the associations we observed between the clinical patterns and functional decline. Fifth, the exclusion of participants with less than 2 assessments may have led to an underestimation of the association between clinical patterns and walking speed and ADL, but only at baseline. However, as we were interested in long-term trajectories of functional decline, we believe that this exclusion criterion was reasonable. Notably, in the analyses that included all SNAC-K participants, the baseline association between clinical patterns and the outcomes was stronger than the baseline association in the main analysis, but rates of decline over the follow-up period were similar. Finally, as shown in previous studies, SNAC-K participants are relatively fit and wealthy. This might further limit the generalizability of our results to other groups of older adults.
Conclusion
In conclusion, our findings indicate that CV and NP multimorbidity had different associations with older adults’ health. NP, mixed, and complex multimorbidity were associated with the highest burden of functional decline, even with respect to participants with high prevalence of generic multimorbidity. CV diseases were less strongly associated with functional decline than were NP diseases; CV diseases were only associated with declines in mobility. Moreover, none of the diseases drove the association between the group to which it belonged and functional decline. This finding corroborates the idea that identifying patterns of chronic diseases can assist in managing the health and care of people with multimorbidity by providing useful prognostic information, highlighting clinical priorities, steering therapeutic interventions, and helping physicians counsel patients and their families.
Supporting Information
Zdroje
1. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K (2013) Frailty in elderly people. Lancet 381 : 752–762. doi: 10.1016/S0140-6736(12)62167-9 23395245
2. Newman AB, Sanders JL, Kizer JR, Boudreau RM, Odden MC, Zeki Al Hazzouri A, et al. (2016) Trajectories of function and biomarkers with age: the CHS All Stars Study. Int J Epidemiol 45 : 1135–1145. doi: 10.1093/ije/dyw092 27272182
3. Marengoni A, Angleman S, Meinow B, Santoni G, Mangialasche F, Rizzuto D, et al. (2016) Coexisting chronic conditions in the older population: Variation by health indicators. Eur J Intern Med 31 : 29–34. doi: 10.1016/j.ejim.2016.02.014 26944564
4. Marengoni A, Angleman S, Melis R, Mangialasche F, Karp A, Garmen A, et al. (2011) Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev 10 : 430–439. doi: 10.1016/j.arr.2011.03.003 21402176
5. Klimek P, Aichberger S, Thurner S (2016) Disentangling genetic and environmental risk factors for individual diseases from multiplex comorbidity networks. Sci Rep 6 : 39658. doi: 10.1038/srep39658 28008973
6. Vetrano DL, Calderón-Larrañaga A, Marengoni A, Onder G, Bauer JM, et al. (2017). (2017) An international perspective on chronic multimorbidity: approaching the elephant in the room. J Gerontol A Biol Sci Med Sci. Sep 16. doi: 10.1093/gerona/glx178
7. Di Angelantonio E, Kaptoge S, Wormser D, Willeit P, Butterworth AS, Bansal N, et al. (2015) Association of Cardiometabolic Multimorbidity With Mortality. Jama 314 : 52–60. doi: 10.1001/jama.2015.7008 26151266
8. Prados-Torres A, Calderon-Larranaga A, Hancco-Saavedra J, Poblador-Plou B, van den Akker M (2014) Multimorbidity patterns: a systematic review. J Clin Epidemiol 67 : 254–266. doi: 10.1016/j.jclinepi.2013.09.021 24472295
9. Prince MJ, Wu F, Guo Y, Gutierrez Robledo LM, O'Donnell M, Sullivan R, et al. (2015) The burden of disease in older people and implications for health policy and practice. Lancet 385 : 549–562. doi: 10.1016/S0140-6736(14)61347-7 25468153
10. European Union. (2015) The 2015 Ageing Report. [cited 2017 Aug 15]. Available from: http://ec.europa.eu/economy_finance/publications/european_economy/2015/pdf/ee3_en.pdf
11. Marengoni A, von Strauss E, Rizzuto D, Winblad B, Fratiglioni L (2009) The impact of chronic multimorbidity and disability on functional decline and survival in elderly persons. A community-based, longitudinal study. J Intern Med 265 : 288–295. doi: 10.1111/j.1365-2796.2008.02017.x 19192038
12. Hudon C, Soubhi H, Fortin M (2008) Relationship between multimorbidity and physical activity: secondary analysis from the Quebec health survey. BMC Public Health 8 : 304. doi: 10.1186/1471-2458-8-304 18775074
13. Lu FP, Chang WC, Wu SC (2016) Geriatric conditions, rather than multimorbidity, as predictors of disability and mortality among octogenarians: A population-based cohort study. Geriatr Gerontol Int 16 : 345–351. doi: 10.1111/ggi.12480 25907542
14. Koller D, Schon G, Schafer I, Glaeske G, van den Bussche H, Hansen H. (2014) Multimorbidity and long-term care dependency—a five-year follow-up. BMC Geriatr 14 : 70. doi: 10.1186/1471-2318-14-70 24884813
15. Garin N, Olaya B, Moneta MV, Miret M, Lobo A, Ayuso-Mateos JL, et al. (2014) Impact of multimorbidity on disability and quality of life in the Spanish older population. PLoS ONE 9: e111498. doi: 10.1371/journal.pone.0111498 25375890
16. Jindai K, Nielson CM, Vorderstrasse BA, Quinones AR (2016) Multimorbidity and Functional Limitations Among Adults 65 or Older, NHANES 2005–2012. Prev Chronic Dis 13: E151. doi: 10.5888/pcd13.160174 27809419
17. Tinetti ME, McAvay G, Chang SS, Ning Y, Newman AB, Fitzpatrick A, et al. (2011) Effect of chronic disease-related symptoms and impairments on universal health outcomes in older adults. Journal of the American Geriatrics Society 59 : 1618–1627. doi: 10.1111/j.1532-5415.2011.03576.x 21883120
18. Welmer AK, Kareholt I, Angleman S, Rydwik E, Fratiglioni L (2012) Can chronic multimorbidity explain the age-related differences in strength, speed and balance in older adults? Aging Clin Exp Res 24 : 480–489. doi: 10.3275/8584 22961066
19. Lagergren M, Fratiglioni L, Hallberg IR, Berglund J, Elmstahl S, Hagberg B, et al. (2004) A longitudinal study integrating population, care and social services data. The Swedish National study on Aging and Care (SNAC). Aging Clin Exp Res 16 : 158–168.
20. Calderon-Larranaga A, Vetrano DL, Onder G, Gimeno-Feliu LA, Coscollar-Santaliestra C, Carfi A, et al. (2016) Assessing and Measuring Chronic Multimorbidity in the Older Population: A Proposal for Its Operationalization. J Gerontol A Biol Sci Med Sci. 10 : 1417–1423
21. Cederholm T, Bosaeus I, Barazzoni R, Bauer J, Van Gossum A, Klek S, et al. (2015) Diagnostic criteria for malnutrition—An ESPEN Consensus Statement. Clin Nutr 34 : 335–340. doi: 10.1016/j.clnu.2015.03.001 25799486
22. Jackson CA, Jones M, Tooth L, Mishra GD, Byles J, Dobson A. (2015) Multimorbidity patterns are differentially associated with functional ability and decline in a longitudinal cohort of older women. Age Ageing 44 : 810–816. doi: 10.1093/ageing/afv095 26220988
23. Marventano S, Ayala A, Gonzalez N, Rodriguez-Blazquez C, Garcia-Gutierrez S, Forjaz MJ. (2014) Multimorbidity and functional status in community-dwelling older adults. Eur J Intern Med 25 : 610–616. doi: 10.1016/j.ejim.2014.06.018 24997486
24. Quinones AR, Markwardt S, Botoseneanu A (2016) Multimorbidity Combinations and Disability in Older Adults. J Gerontol A Biol Sci Med Sci 71 : 823–830. doi: 10.1093/gerona/glw035 26968451
25. Quiñones AR, Markwardt S, Thielke S, Rostant O, Vásquez E, Botoseneanu A. (2017) Prospective Disability in Different Combinations of Somatic and Mental Multimorbidity. J Gerontol A Biol Sci Med Sci. May 24. doi: 10.1093/gerona/glx100 28541396
26. World Health Organization. (2015) World report on ageing and health [cited 2017 Aug 15]. Available from: http://apps.who.int/iris/bitstream/10665/186463/1/9789240694811_eng.pdf
27. Christensen K, Doblhammer G, Rau R, Vaupel JW (2009) Ageing populations: the challenges ahead. Lancet 374 : 1196–1208. doi: 10.1016/S0140-6736(09)61460-4 19801098
28. Abellan van Kan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, et al. (2009) Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging 13 : 881–889. 19924348
29. National Institute for Health and Care Excellence (NICE). (2016) Multimorbidity: clinical assessment and management [cited 2017 Aug 15]. Available from: https://www.nice.org.uk/guidance/ng56/resources/multimorbidity-clinical-assessment-and-management-pdf-1837516654789
30. Santoni G, Angleman S, Welmer AK, Mangialasche F, Marengoni A, Fratiglioni L. (2015) Age-related variation in health status after age 60. PLoS ONE 10: e0120077. doi: 10.1371/journal.pone.0120077 25734828
31. Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. (2011) Gait speed and survival in older adults. Jama 305 : 50–58. doi: 10.1001/jama.2010.1923 21205966
32. Pulignano G, Del Sindaco D, Di Lenarda A, Alunni G, Senni M, Tarantini L, et al. (2016) Incremental Value of Gait Speed in Predicting Prognosis of Older Adults With Heart Failure: Insights From the IMAGE-HF Study. JACC Heart Fail 4 : 289–298. doi: 10.1016/j.jchf.2015.12.017 26970831
33. Boyd CM, Weiss CO, Halter J, Han KC, Ershler WB, Fried LP. (2007) Framework for evaluating disease severity measures in older adults with comorbidity. J Gerontol A Biol Sci Med Sci 62 : 286–295. 17389726
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2018 Číslo 3- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Ferinject: správně indikovat, správně podat, správně vykázat
- Optimální dávkování apixabanu v léčbě fibrilace síní
-
Všechny články tohoto čísla
- What is the value of multidisciplinary care for chronic kidney disease?
- 2017 Reviewer and Editorial Board Thank You
- Delays in completion and results reporting of clinical trials under the Paediatric Regulation in the European Union: A cohort study
- Surveillance of antimicrobial consumption in animal production sectors of low- and middle-income countries: Optimizing use and addressing antimicrobial resistance
- Causes of death and infant mortality rates among full-term births in the United States between 2010 and 2012: An observational study
- Time for high-burden countries to lead the tuberculosis research agenda
- The importance and challenges of shared decision making in older people with multimorbidity
- Trajectories of functional decline in older adults with neuropsychiatric and cardiovascular multimorbidity: A Swedish cohort study
- Mortality, ethnicity, and country of birth on a national scale, 2001–2013: A retrospective cohort (Scottish Health and Ethnicity Linkage Study)
- Validation of a genetic risk score for atrial fibrillation: A prospective multicenter cohort study
- Multimorbidity and survival for patients with acute myocardial infarction in England and Wales: Latent class analysis of a nationwide population-based cohort
- Integration of postpartum healthcare services for HIV-infected women and their infants in South Africa: A randomised controlled trial
- Cost-effectiveness of multidisciplinary care in mild to moderate chronic kidney disease in the United States: A modeling study
- Role of heme in lung bacterial infection after trauma hemorrhage and stored red blood cell transfusion: A preclinical experimental study
- Patterns and temporal trends of comorbidity among adult patients with incident cardiovascular disease in the UK between 2000 and 2014: A population-based cohort study
- Global child and adolescent mental health: The orphan of development assistance for health
- Cardiovascular disease and multimorbidity: A call for interdisciplinary research and personalized cardiovascular care
- Forced anal examinations to ascertain sexual orientation and sexual behavior: An abusive and medically unsound practice
- Primary prevention of cardiovascular disease: The past, present, and future of blood pressure- and cholesterol-lowering treatments
- Physical activity levels in adults and older adults 3–4 years after pedometer-based walking interventions: Long-term follow-up of participants from two randomised controlled trials in UK primary care
- Effect and cost-effectiveness of educating mothers about childhood DPT vaccination on immunisation uptake, knowledge, and perceptions in Uttar Pradesh, India: A randomised controlled trial
- Comorbidity health pathways in heart failure patients: A sequences-of-regressions analysis using cross-sectional data from 10,575 patients in the Swedish Heart Failure Registry
- Multimorbidity in patients with heart failure from 11 Asian regions: A prospective cohort study using the ASIAN-HF registry
- Antiviral efficacy of favipiravir against Ebola virus: A translational study in cynomolgus macaques
- Transmission of HIV-1 drug resistance mutations within partner-pairs: A cross-sectional study of a primary HIV infection cohort
- A clinical decision support tool for improving adherence to guidelines on anticoagulant therapy in patients with atrial fibrillation at risk of stroke: A cluster-randomized trial in a Swedish primary care setting (the CDS-AF study)
- Integrating HIV and hypertension management in low-resource settings: Lessons from Malawi
- The epidemiology of adolescents living with perinatally acquired HIV: A cross-region global cohort analysis
- Polycystic ovary syndrome, androgen excess, and the risk of nonalcoholic fatty liver disease in women: A longitudinal study based on a United Kingdom primary care database
- Blood pressure-lowering treatment strategies based on cardiovascular risk versus blood pressure: A meta-analysis of individual participant data
- Cerebral white matter disease and functional decline in older adults from the Northern Manhattan Study: A longitudinal cohort study
- HIV treatment eligibility expansion and timely antiretroviral treatment initiation following enrollment in HIV care: A metaregression analysis of programmatic data from 22 countries
- The current and potential health benefits of the National Health Service Health Check cardiovascular disease prevention programme in England: A microsimulation study
- Cardiovascular disease: The rise of the genetic risk score
- Comparative analysis of the association between 35 frailty scores and cardiovascular events, cancer, and total mortality in an elderly general population in England: An observational study
- Progression of diabetes, heart disease, and stroke multimorbidity in middle-aged women: A 20-year cohort study
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Forced anal examinations to ascertain sexual orientation and sexual behavior: An abusive and medically unsound practice
- Polycystic ovary syndrome, androgen excess, and the risk of nonalcoholic fatty liver disease in women: A longitudinal study based on a United Kingdom primary care database
- The current and potential health benefits of the National Health Service Health Check cardiovascular disease prevention programme in England: A microsimulation study
- Cardiovascular disease and multimorbidity: A call for interdisciplinary research and personalized cardiovascular care
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



