-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaMultimorbidity in patients with heart failure from 11 Asian regions: A prospective cohort study using the ASIAN-HF registry
Using data from the ASIAN-HF Registry, Carolyn Lam and colleagues examine multimorbidity patterns in patients with heart failure from 11 Asian countries
Published in the journal: . PLoS Med 15(3): e32767. doi:10.1371/journal.pmed.1002541
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002541Summary
Using data from the ASIAN-HF Registry, Carolyn Lam and colleagues examine multimorbidity patterns in patients with heart failure from 11 Asian countries
Introduction
Multimorbidity, the presence of 2 or more chronic medical conditions in an individual, is highly prevalent in patients with heart failure (HF) [1–3]. Indeed, with aging populations worldwide, patients with age-related multimorbidity are becoming the norm rather than the exception. This is especially so in Asia, with the most rapidly aging populations in the world, where almost two-thirds of patients with HF were found to have multimorbidity [4]. Comorbidities and their treatments may complicate the diagnosis, treatment, and outcomes of patients with HF, affect patient preferences for care, and negatively impact patient outcomes.
Within the HF syndrome, we currently distinguish HF with reduced ejection fraction (HFrEF) from HF with preserved ejection fraction (HFpEF). Early HF trials defined HF using a reduced left ventricular ejection fraction (LVEF) as an entry criterion, leading to the distinction of HFrEF from HFpEF since large trials of medications (e.g., renin-angiotensin-aldosterone system blockers) that showed improved survival in HFrEF later failed to improve outcomes in similar trials for HFpEF [5]. Cardiac structure and function are distinct between the HF groups: patients with HFrEF mostly display left ventricular (LV) eccentric remodeling with systolic dysfunction, whereas patients with HFpEF more often have concentric remodeling with preserved LV pump function but prominent diastolic dysfunction and increased filling pressures [6]. The underlying basis for these differences remains poorly understood and has been postulated to be related to the different comorbidity burdens in these patients [7].
Most prior clinical research has focused on individual comorbidities in isolation and has not studied the burden and patterns of multimorbidity in HF. Understanding how comorbidities cluster in individuals, and the impact of clustering of comorbidities on patient outcomes, is an important step towards personalizing HF treatment approaches for better outcomes [5,8–13].
Thus, we sought to identify the patterns and burden of multimorbidity in Asian patients with HF, as well as the association of specific multimorbidity patterns with patients’ quality of life (QoL), cardiac remodeling, and health outcomes. We hypothesized that comorbidities would cluster in specific multimorbidity groups, regardless of ejection fraction, and that these groups would differentially influence patients’ QoL, cardiac remodeling and health outcomes. Furthermore, we hypothesized that regional variation would exist across Asia, providing important insights for healthcare resource allocation and a tailored approach to patients from different Asian regions.
Methods
Study design, study population, and setting
This study is reported as per the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (S1 Checklist). Ethics approvals were obtained from the relevant human ethics committees at all sites. All patients included provided written informed consent, and this study adheres to the principles of medical research as laid down in the Declaration of Helsinki. We studied comorbidities in 6,480 HF patients enrolled (1 October 2012 and 6 October 2016) in the Asian Sudden Cardiac Death in Heart Failure (ASIAN-HF) registry. The prospective study design of the ASIAN-HF registry has been published previously [4,14]. The primary analysis described in the prospective study design related to sudden cardiac death and utilization of implantable cardiac defibrillators in HFrEF, and the primary outcomes have been published [15]. Subsequent publications from the ASIAN-HF registry are guided by a publication charter and overseen by a publications committee.
In brief, the ASIAN-HF registry is a multinational registry of Asian patients with HF from 46 medical centers across 11 regions (Taiwan, Hong Kong, China, India, Malaysia, Thailand, Singapore, Indonesia, Philippines, Japan, and Korea; S1 Table). Patients included in the ASIAN-HF registry were all eligible patients at enrollment sites who met predetermined inclusion and exclusion criteria and provided informed written consent for participation. Recruitment sites were selected to include a broad spectrum of medical, cardiology, and HF specialty units that regularly manage and follow patients with chronic HF. Patients included in the ASIAN-HF registry were >18 years of age with symptomatic HF (at least 1 previous episode of decompensated HF in the previous 6 months resulting in a hospital admission or treatment in outpatient clinic). Patients with severe valvular heart disease as a cause of HF, with a life-threatening comorbidity with a life expectancy <1 year, or unable or unwilling to give consent were excluded. The ASIAN-HF registry was originally designed to include only patients with HFrEF (LVEF < 40%) [4,14], but in 2013 the study underwent a protocol amendment to also include patients with HFpEF (LVEF ≥ 50%). Recruitment of patients with HFpEF started later than the recruitment of patients with HFrEF, for funding reasons. However, the delay was only 1 year (1 October 2012 versus 9 September 2013), and for the majority of the recruitment period (until 6 October 2016) there was overlap in recruitment of both types of HF. We do not anticipate that there were substantial shifts in epidemiology or treatment of patients with HFrEF or HFpEF during this short period of 1 year that may have biased the regional patterns of multimorbidity groups, although the potential for bias cannot be excluded. Data on demographics, previous medical history, clinical symptoms, and functional status were collected. According to the protocol, patients underwent standard 12-lead electrocardiography (ECG) and transthoracic echocardiography at inclusion.
Study definitions
The definitions of comorbidities in the ASIAN-HF registry have previously been described [4,14]. Obesity was defined according to the standard body mass index (BMI) cutoff defined by the World Health Organization (WHO) (≥30 kg/m2). Coronary artery disease (CAD) was defined as angiographically documented presence of significant coronary obstruction, history of myocardial infarction, or prior revascularization. Hypertension was defined as any past or current history of hypertension and treatment for hypertension. Diabetes was defined as having a (prior) diagnosis of diabetes. Estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease Study equation, and chronic kidney disease (CKD) was defined as eGFR < 60 ml/min/1.73 m2. Anemia was defined according to WHO criteria: hemoglobin <13 g/dl for men and <12 g/dl for women. Atrial fibrillation (AF) was defined as having a medical history or AF on ECG. Peripheral arterial and venous disease (PAVD), previous stroke, chronic obstructive pulmonary disease (COPD), peptic ulcer, renal artery stenosis, dementia, liver disease, cancer, and depression were identified by medical history.
QoL was measured using the Kansas City Cardiomyopathy Questionnaire (KCCQ), a 23-item self-administered HF-specific questionnaire validated in multiple HF-related disease states [16–21]. KCCQ domain scores range from 0 to 100; higher scores represent better QoL. Ethnicity was self-reported. Region income level was defined according to World Bank criteria: low—Indonesia, Philippines, and India; middle—China, Thailand, and Malaysia; high—Singapore, Hong Kong, Taiwan, South Korea, and Japan.
Medications by therapeutic class were identified, including angiotensin-converting enzyme inhibitors (ACEis) or angiotensin receptor blockers (ARBs), beta-blockers, mineralocorticoid receptor antagonists (MRAs), and diuretics. Medication use was captured at baseline.
Outcomes
The primary outcome of this study was all-cause death or HF hospitalization within 1 year. In all, 5,875 (90.7%) patients had outcome data available, whereas 605 (9.3%) patients were lost to follow-up. Patients with less than 1 year of follow-up available were censored at their last known visit date. Outcomes were adjudicated by an independent committee. Secondary outcomes were all-cause mortality alone and hospitalization for HF alone. All data were captured prospectively in an electronic database, with registry operations and data management handled by Quintiles Outcomes as the contract research organization appointed by the academic executive committee.
Echocardiography
The collection and processing of echocardiographic data has previously been described [14]. Echocardiography was performed at each center according to internationally accepted guidelines [22]. LVEF, LV dimensions, left atrial dimensions, LV diastolic function, stroke volume, and cardiac output were measured. The Cardiovascular Imaging Core Laboratory of the National University Health System, Singapore, provided oversight and imaging protocol guidelines as well as quality assurance of echocardiograms. Accuracy and reproducibility of interpreted results were ensured through consistent training and systematic analytical processes provided by the core laboratory according to international guidelines [22]. For further calculations, LV mass (LVM) was calculated from linear dimensions and indexed to height2.7 as well as to body surface area (BSA) [22]. Relative wall thickness (RWT) was calculated by the formula (2 × diastolic posterior wall thickness)/diastolic LV internal diameter. LV hypertrophy (LVH) was determined as LVM indexed to BSA >115 g/m2 in men and >95 g/m2 in women [22]. Normal cardiac geometry was defined as having no LVH and a RWT ≤ 0.42. Abnormal cardiac geometry (cardiac remodeling) was classified as concentric remodeling (no LVH and RWT > 0.42), concentric hypertrophy (LVH and RWT > 0.42), or eccentric hypertrophy (LVH and RWT ≤ 0.42). Left atrial size was indexed to BSA [22].
Statistical analysis
Latent class analysis (LCA) was performed using the poLCA package in the R statistical package [23] to identify groups of patients with patterns of comorbidities. Briefly, a comprehensive list of comorbidities—which included AF, CAD, stroke, CKD, obesity, hypertension, COPD, peptic ulcer, renal artery stenosis, cancer, liver disease, dementia, anemia, depression, diabetes, and PAVD—was analyzed to identify group membership of individual patients. Maximum likelihood estimations were used to identify patient groups based on multimorbidity type for a range of 2–10 groups. The optimal number of groups was identified using the first minimum of the Bayesian information criterion (BIC). The BIC is suggested to provide for the most parsimonious model selection and is recommended in LCA [23–25]. poLCA uses random starts; therefore, each model was estimated with 10 replications. Cases with missing covariates were removed in this process. In this study, the optimal number of classes was 5 (S2 Table). Patients’ individual class membership was then derived using a Bayesian approach [23]. After determining the optimal number of clusters, the partial probabilities were averaged over the 10 replications. These partial probabilities were then used to calculate each group membership in a Bayesian fashion using the probabilities listed in S3 Table. By multiplying each probability corresponding to each variable, a patient’s probability of belonging to a group was determined. Final group selection was based on the patient’s highest probability of a group. Baseline echocardiographic characteristics and KCCQ domain scores were stratified according to group membership and are presented as means and standard deviations, medians and IQRs, or numbers and percentages, as appropriate. Differences between multimorbidity groups in the entire HF cohort were tested with 1-way analysis of variance (ANOVA), Kruskal–Wallis test, or the χ2 test, where appropriate. We corrected for multiple testing in the tables using the Benjamini–Hochberg correction, using a false discovery rate of 0.05. In addition, we tested for interaction between group membership and HF type (HFrEF or HFpEF) and stratified our analyses by HF type in the presence of significant interaction. For logistic regressions, the young group was used as the referent. In logistic regression, we further corrected for age, sex, inpatient versus outpatient enrollment, ethnicity, and New York Heart Association (NYHA) class. Kaplan–Meier curves stratified by group membership are shown, with differences between groups tested using the log-rank test for survival. Multivariable Cox regression analysis was used to test for differences between multimorbidity groups in all-cause mortality and HF-related hospitalization within 1 year, with the young group used as a referent. We corrected for confounders selected based on clinical considerations in a stepwise manner. In model 1 we corrected for age and sex. In model 2 we corrected for variables included in model 1 and geographic zone, previous hospitalization for HF (yes/no), NYHA class, and HFrEF versus HFpEF. In model 3 we corrected for all variables in model 2 and usage of ACEis/ARBs, beta-blockers, MRAs, and diuretics at baseline. When analyzing HF hospitalizations alone, all-cause mortality was used as a competing risk.
Prior to performing this study, we planned LCA and analyses regarding the differences between possible multimorbidity groups for the primary combined outcome as well as differences in clinical characteristics and echocardiographic parameters and regional distribution of multimorbidity groups. Based on recommendations during the peer-review process, we conducted additional sensitivity analyses investigating the differences between multimorbidity groups within a single ethnicity (Chinese) between 2 zones: Northeast Asia (South Korea, Japan, Taiwan, Hong Kong, and China) and Southeast Asia (Thailand, Malaysia, Philippines, Indonesia, and Singapore). Additionally, we included analyses of all-cause mortality alone and hospitalizations for HF alone (with all-cause mortality as a competing risk) based on recommendations from the peer-review process.
All tests were performed 2-sided, and p-values of <0.05 were considered statistically significant. Statistical analyses were performed using STATA 13.0 (StataCorp, College Station, TX, US) and R version 3.4.
Results
Multimorbidity groups identified by LCA
Overall, patients were on average 62 years old, and 27% were female (Table 1). Patients were primarily of Chinese (33%) and Indian (30%) ethnicity, and the majority of patients were in NYHA class II or III. The median number of comorbidities was 3, and 81% of patients had ≥2 comorbidities in addition to HF. Among all comorbidities, hypertension (55%) was the most common, followed by CAD (46%) and CKD (45%).
Tab. 1. Baseline characteristics according to multimorbidity group. 
Data are given as mean (SD), median (IQR), or number (percent). In the entire cohort, 5 multimorbidity groups of relatively equal size (N = 1,048–1,759) were identified, each characterized by a different combination of comorbidities: elderly/AF, metabolic, young, ischemic, and lean diabetic.
Patients in the elderly/AF group, were the oldest (mean age 68.2 years), had the highest prevalence of AF (67.6%) and stroke (19.8%), and had a comparatively high prevalence of CKD (63.6%). They were also more likely to be of Chinese, Japanese, or Korean ethnicity and from high-income regions.
Patients in the metabolic group had the highest mean BMI (29 kg/m2) and prevalence of obesity (45.1%), combined with a high prevalence of hypertension (87.8%) and diabetes (63.5%). These patients were relatively young (mean age 59.1 years), often of Malay ethnicity, and most often on ACEis/ARBs.
Patients in the young group were the youngest (mean age 55.6 years) and had an exceptionally low proportion of all comorbidities, with high prevalence on non-ischemic etiology of HF (77.8%). These patients were primarily of Indian or Chinese ethnicity and from low-income regions and were most the likely to be treated with MRAs. These patients had the lowest absolute number of comorbidities (0, IQR 0,1).
Patients in the ischemic group were of intermediate age (mean age 62.4 years); this group had the highest proportion of men (83%) compared to the other groups. Overall, these patients were most often Indian and had ischemic etiology of HF (71%), with lower prevalence of diabetes (43%) compared to the young group but the highest prevalence of CAD (88%; p for all comparisons < 0.001) and high prevalence of anemia (69%).
The lean diabetic group consisted of patients of intermediate age (mean age 66.1 years) with a strikingly high prevalence of diabetes (97%) despite a low prevalence of obesity (22%). They also had high prevalence of hypertension (95%), CKD (89%), anemia (78.5%), and CAD (76%). These patients were commonly of Malay ethnicity and from high-income regions (60%). These also appeared to be the sickest patients, with the worst signs and symptoms of HF and frequent history of hospitalization for HF (69%). These patients had the highest absolute number of comorbidities (5, IQR 5, 6).
Patients from the lean diabetic group had the worst overall QoL, while patients from the young group had the best QoL, comparing overall summary scores (Table 2). Similarly, the lean diabetic group had poorer QoL as compared to the young group in the domains of total symptoms and social limitations.
Tab. 2. Kansas City Cardiomyopathy Questionnaire domain scores according to multimorbidity group. 
*Significant after Benjamini–Hochberg correction using a false discovery rate of 0.05. Distribution of multimorbidity groups by region
The distribution of multimorbidity groups by region is summarized in Fig 1. In China and Thailand, the young group was most prevalent (Fig 2A). In Hong Kong, the majority of patients belonged to the lean diabetic and elderly/AF groups. Indian and Indonesian patients most often belonged to the young and ischemic groups. Japanese and Korean patients most often belonged to the elderly/AF and young groups. Overall, patients from Singapore and Malaysia were in either the lean diabetic or metabolic group. Patients from the Philippines and Taiwan were most often in the metabolic group.
Fig. 1. Concept figure summarizing the most important findings of this study. 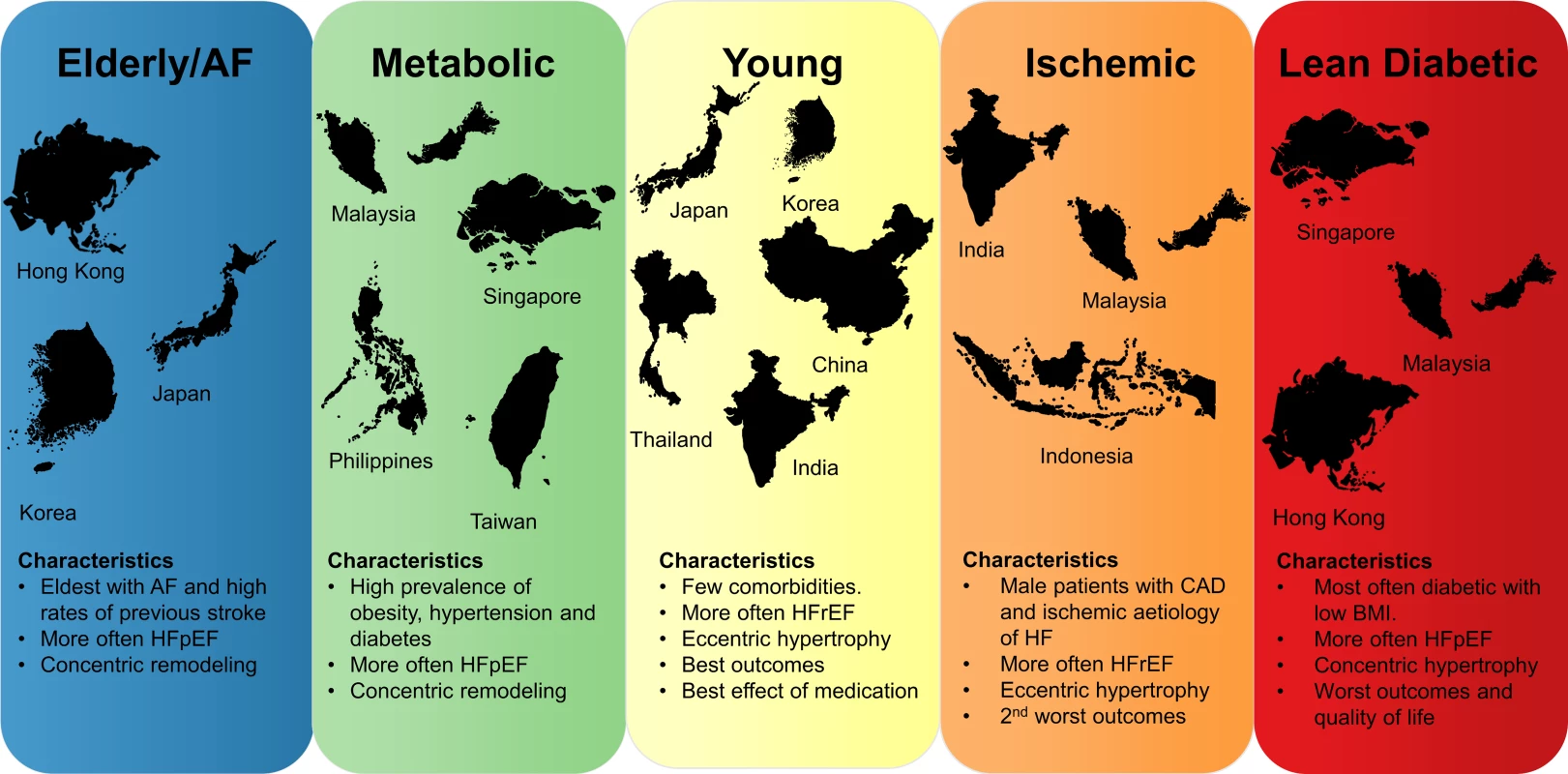
Region sizes in the figure are not to scale. AF, atrial fibrillation; BMI, body mass index; CAD, coronary artery disease; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction. Fig. 2. Bar graphs showing the distribution of multimorbidity groups across regions and HFrEF/HFpEF. 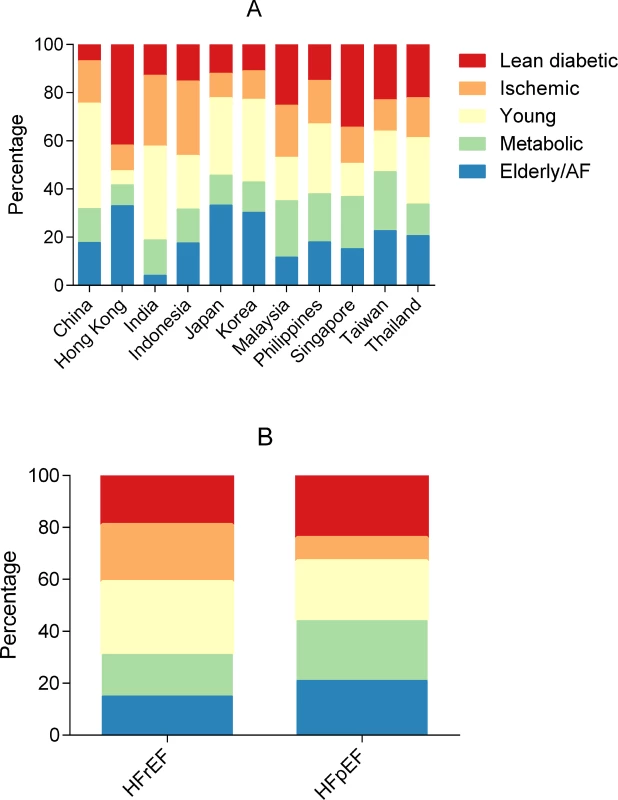
By region (A) and HFrEF versus HFpEF (B). AF, atrial fibrillation; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction. We performed sensitivity testing restricted to a single ethnicity (Chinese) in 2 different zones (Southeast Asia and Northeast Asia) and found that the same phenotypic groups emerged in each zone, with similar characteristics within each group. This suggests that the phenotypic groups were not simply due to ethnic or regional differences in inclusion criteria, but may represent underlying biological differences.
Distribution of multimorbidity groups by type of HF
The relative prevalence of the ischemic and young groups was higher in HFrEF, while the elderly/AF, metabolic, and lean diabetic groups had a higher relative prevalence in HFpEF (Fig 2B). When adjusted for age, sex, inpatient versus outpatient enrollment, ethnicity, and NYHA class, patients in the metabolic group were more likely to have HFpEF, while patients in the ischemic group were more likely to have HFrEF.
Differences in cardiac structure and function by multimorbidity group
Overall, the metabolic and lean diabetic groups had the highest proportions of concentric hypertrophy, and the young group had the highest proportion of eccentric hypertrophy (Fig 3A). When correcting for age, sex, inpatient versus outpatient enrollment, ethnicity, NYHA class, and HFrEF versus HFpEF, the ischemic group (odds ratio [OR] 0.73, 95% CI 0.61–0.87) and lean diabetic group (OR 0.71, 95% CI 0.59–0.85) had less LVH compared to the young group. In contrast to the young group, the elderly/AF (OR 1.91, 95% CI 1.52–2.40), metabolic (OR 2.46, 95% CI 1.98–3.06), and lean diabetic (OR 2.59, 95% CI 2.09–3.21) groups had more concentric remodeling after multivariable correction.
Fig. 3. Bar graphs showing cardiac geometry across multimorbidity groups. 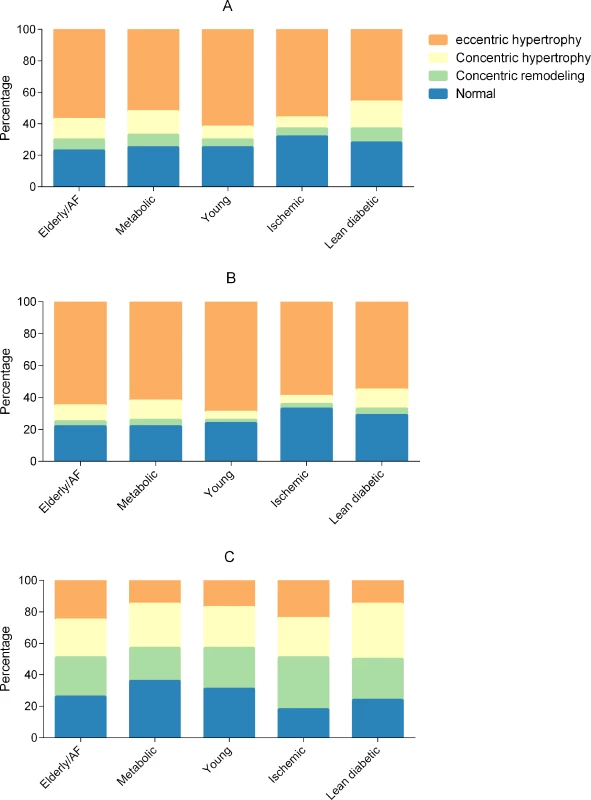
Total cohort (A), heart failure with reduced ejection fraction (B), and heart failure with preserved ejection fraction (C). AF, atrial fibrillation. Echocardiographic data stratified by multimorbidity group and HF type (HFrEF or HFpEF) are presented in Tables 3 and 4. To study whether multimorbidity group affected cardiac geometry differently in patients with HFrEF and HFpEF, we studied interactions between multimorbidity group and HF type (HFrEF or HFpEF). We observed a significant interaction between multimorbidity group and HF type for both concentric remodeling (P = 0.001) and LVH (P = 0.011). In HFrEF, the metabolic (OR 2.53, 95% CI 1.84–3.47) and lean diabetic (OR 2.39, 95% CI 1.72–3.33) groups were more likely to have concentric remodeling as compared to the young group, after adjusting for age, sex, inpatient versus outpatient enrollment, ethnicity, and NYHA class. The ischemic group was less likely to have LVH than the young group (OR 0.65, 95% CI 0.53–0.78). In HFrEF, the young group had the highest prevalence of eccentric hypertrophy, followed by the elderly/AF group. In HFpEF, the lean diabetic group had the highest proportion of concentric remodeling (Fig 3B and 3C).
Tab. 3. Echocardiographic characteristics. 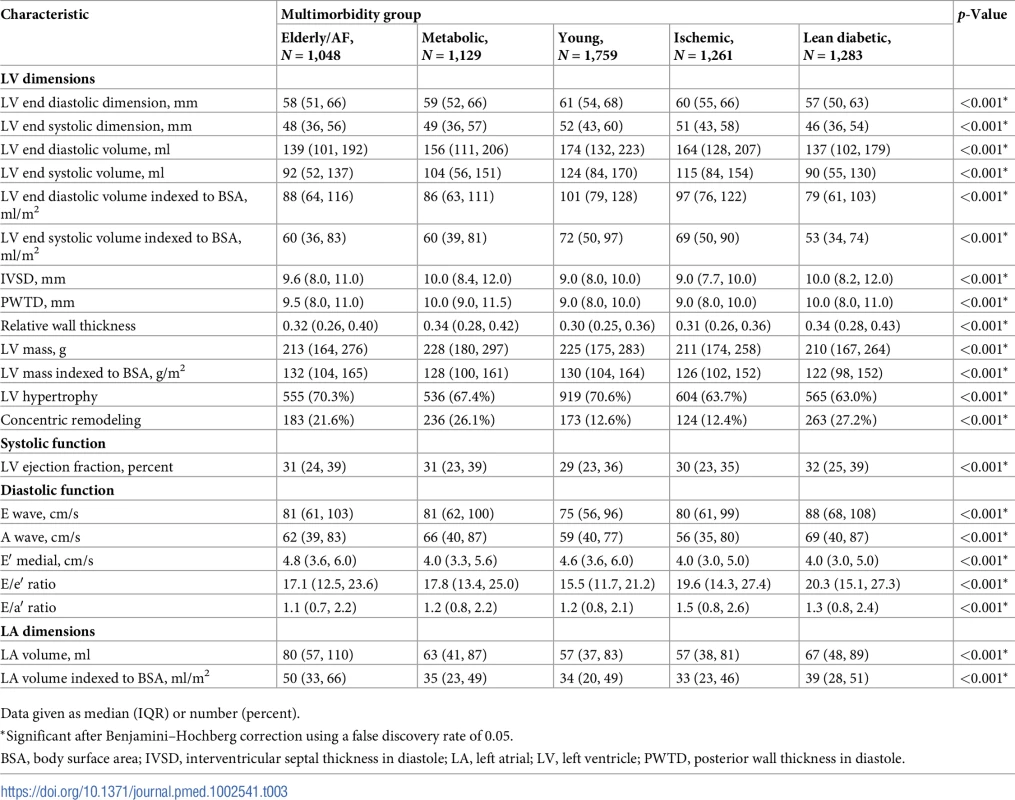
Data given as median (IQR) or number (percent). Tab. 4. Echocardiographic characteristics, stratified by multimorbidity groups and heart failure type. 
Data given as median (IQR). Outcomes by multimorbidity group
In the overall cohort, 1,125 (19.2%) patients experienced the primary combined outcome of all-cause mortality or hospitalization for HF within 1 year. Regarding secondary outcomes, 564 (9.6%) patients died, and 679 (11.6%) patients were hospitalized within 1 year.
There were clear differences in the primary combined outcome between multimorbidity groups (P < 0.001; Fig 4). Particularly, the lean diabetic group had the highest proportion of events of the combined outcome (HF hospitalization or mortality) within 1 year (29%), while the young group had the lowest (11%). In model 3, the lean diabetic group remained associated with the highest proportion of events of the combined outcome (hazard ratio [HR] 1.79, 95% CI 1.46–2.22) compared to the young group (Table 5). Similarly, the elderly/AF (HR 1.57, 95% CI 1.26–1.96), metabolic (HR 1.28, 95% CI 1.02–1.60), and ischemic groups (HR 1.52, 95% CI 1.22–1.88) had higher rates of the combined outcome than the young group. Differences in survival remained after adjusting for systolic function (LVEF), diastolic function (E/e′), and cardiac geometry across groups. After correcting for number of comorbidities, the predictive power of multimorbidity group remained; here particularly the ischemic group was associated with a higher proportion of the combined outcome (HR 1.47, 95% CI 1.08–1.99). When investigating mortality alone, the elderly/AF group had the highest hazards for dying within 1 year (HR 1.71, 95% CI 1.26–2.32). For hospitalizations for HF, the lean diabetic group had the highest hazards (HR 1.99, 95% CI 1.52–2.60).
Fig. 4. Kaplan–Meier curve showing differences for the primary combined outcome of all-cause mortality and HF-related hospitalization within 1 year across multimorbidity groups. 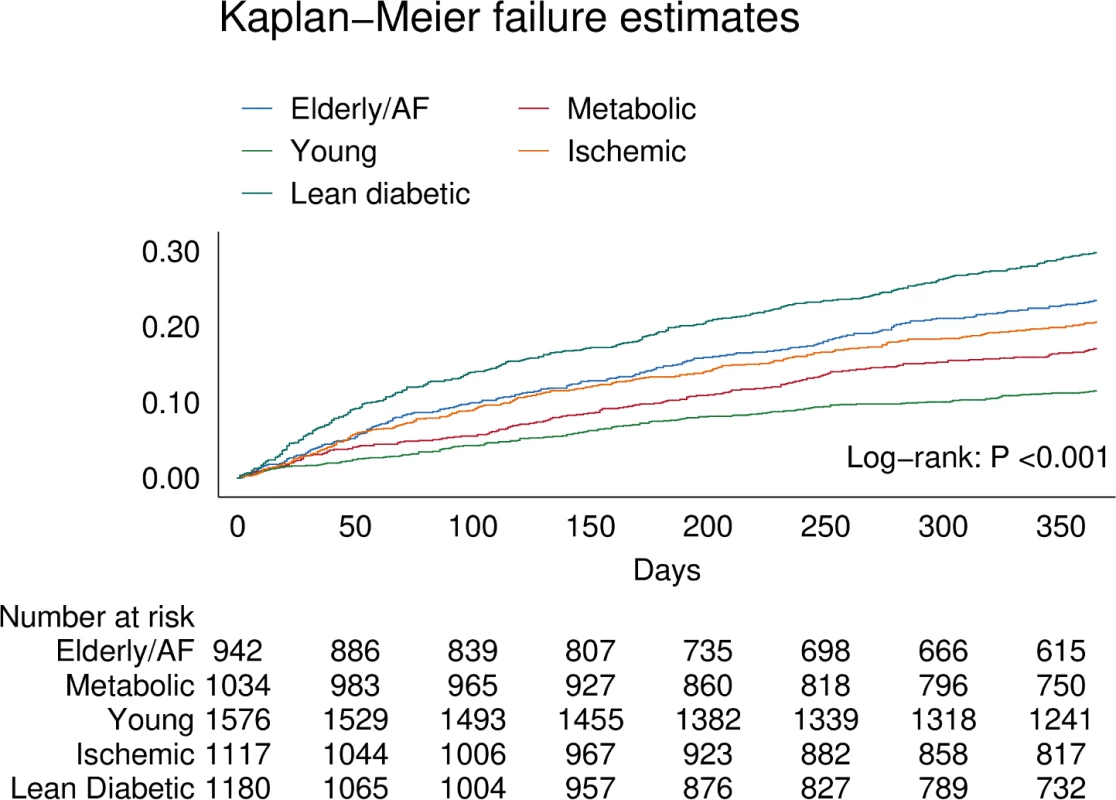
AF, atrial fibrillation. Tab. 5. Results of Cox regression analysis across multimorbidity groups for the combined outcome of all-cause mortality and hospitalization for heart failure, all-cause mortality alone, and hospitalization for heart failure alone. 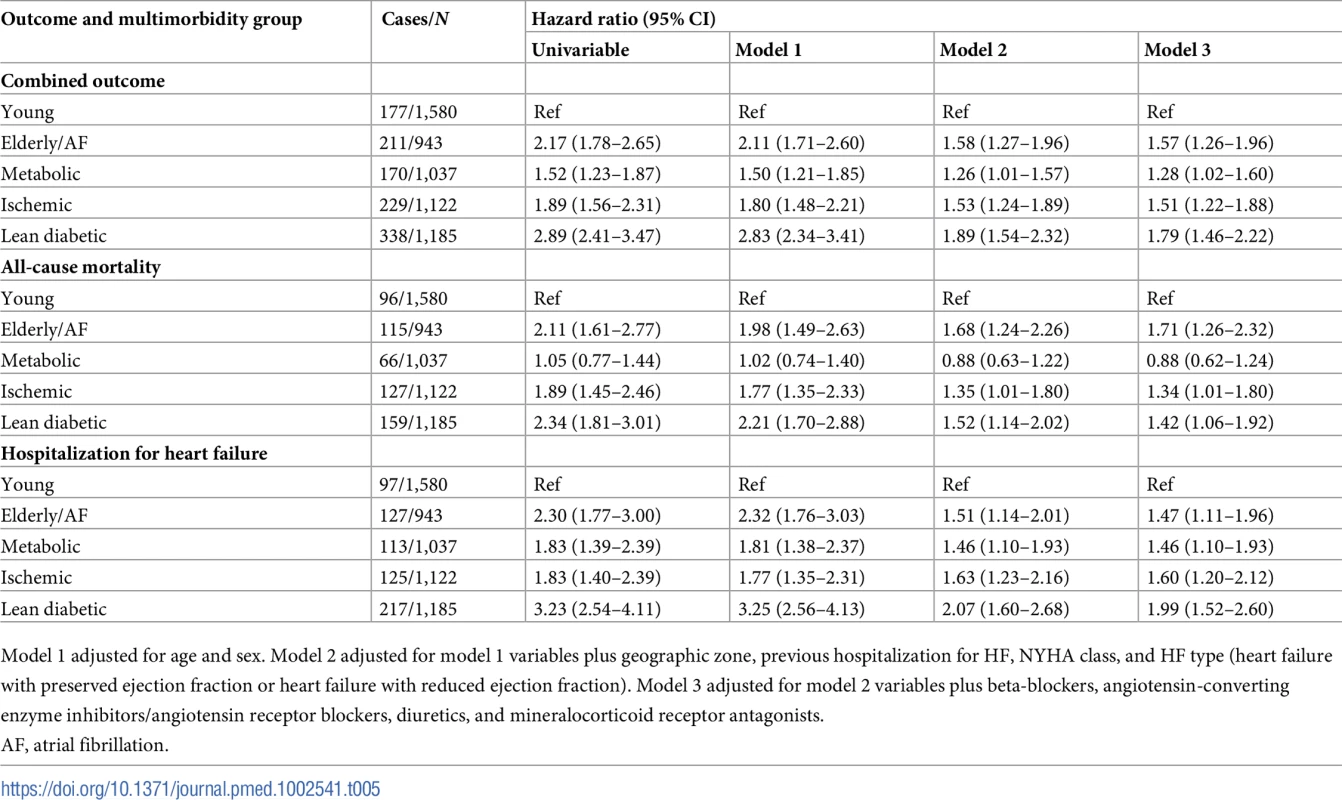
Model 1 adjusted for age and sex. Model 2 adjusted for model 1 variables plus geographic zone, previous hospitalization for HF, NYHA class, and HF type (heart failure with preserved ejection fraction or heart failure with reduced ejection fraction). Model 3 adjusted for model 2 variables plus beta-blockers, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, diuretics, and mineralocorticoid receptor antagonists. The type of HF (HFrEF or HFpEF) modified the associations of multimorbidity groups with the primary combined outcome (Pinteraction = 0.008), such that in HFpEF, only the lean diabetic group was associated with a higher proportion of the primary combined outcome compared to the young group (HR 2.57, 95% CI 1.19–5.59) when adjusted for age, sex, geographic zone, previous hospitalization for HF, and NYHA class.
Discussion
To the best of our knowledge this study provides the first prospective multinational data on multimorbidity patterns among Asian patients with HF. We found several interesting results. First, rather than occurring in isolation, comorbidities naturally clustered among Asian patients with HF and could be categorized into 5 distinct patterns: elderly/AF, metabolic, young, ischemic, and lean diabetic. Second, different patterns of multimorbidity were associated with different underlying patterns of cardiac remodeling. Third, striking geographic differences were observed in the distribution of multimorbidity groups across Asia. Fourth, and most importantly, multimorbidity groups were differentially associated with the prespecified primary combined outcome of all-cause mortality and HF-related hospitalization. These data highlight the importance of multimorbidity in patients with HF, improve our understanding of the role of multimorbidity in the pathophysiology of HF, and pave the way for a tailored approach to patients with HF.
Previous studies have identified subgroups in HF using cluster analyses [10,26–28]. Ahmad et al. reported one of the first applications of a cluster analysis to identify clinical phenotypes of patients with HFrEF in the HF-ACTION study [10]. Based on clinical characteristics including ECG data, biomarkers (NT-proBNP), and signs and symptoms of HF, the authors identified 4 groups: a young group with high BMI, an elderly group with high rates of comorbidities, an ischemic cardiomyopathy group, and a non-ischemic cardiomyopathy group [10]. However, this study included only patients with HFrEF, and only patients from a single clinical trial, which predominantly included white men with ischemic cardiomyopathy. Similar studies have been performed in HFpEF patients alone [28,29], with similar subgroup findings. Based on the selected variables, these prior studies have been postulated to classify patients predominantly based on HF severity, with differences in survival driven mainly by differences in age and NT-proBNP [30]. Another study, by Lee et al., investigated comorbidity profiles in hospitalized HF patients using ICD codes in a US nationwide database [31]. The authors found a lifestyle profile, with high rates of diabetes and obesity, a renal profile, with high rates of renal disease and hypertension, a neurovascular profile (hypertension plus cerebrovascular disease), and a common group (high rate of hypertension). The hypertensive (common group) patients comprised the highest proportion (47%) among patients hospitalized for HF in the US, while the renal patients comprised the second highest proportion (30%), followed by the lifestyle (20%) and neurovascular (4%) patients with HF. This study relied on data from the Nationwide Inpatient Sample (NIS) database, and the depth of investigation was limited by the quality and detail of data collected [31].
Our study extends the prior literature by providing data on multimorbidity patterns and their echocardiographic correlates and association with QoL, mortality, and hospitalization for HF, in a large, well-characterized multinational Asian cohort of patients with HFrEF and HFpEF. We found novel multimorbidity patterns unique to Asia such as the lean diabetic group. Most noteworthy in our study was the prominence of the lean diabetic group in Southeast Asia (particularly Malaysia and Singapore). This was surprising given the rise in obesity in this zone [32]. Southeast Asia is home to a rapidly growing population of >600 million people, and is notable for its rapid epidemiological transition from “the age of receding pandemics” to “the age of degenerative and man-made disease” and now “the age of declining cerebrovascular mortality, ageing, lifestyle modifications, and resurgent diseases” [33,34] within the generation of adults now presenting with HF. The thrifty gene hypothesis [35] may explain the extraordinarily high rates of diabetes as a risk factor for HF in spite of the absence of overt obesity. Indeed, previous studies have shown that the prevalence of diabetes among Asian individuals is far greater than among white individuals and that diabetes occurs on average at a far lower BMI [36]. Furthermore, diabetes is associated with higher rates of mortality and hospitalization for HF in Asian patients with HF than in white patients with HF. Here we showed that among Asian patients with HF, the lean diabetic phenotype was associated with the highest rates of the primary combined outcome, with more than twice as many deaths or hospitalizations for HF compared to the young group. This is potentially driven by the high proportion of CKD in these patients, which is a strong determinant of mortality and hospitalizations [37]. Of note, the lean diabetic patients experienced higher rates of the primary combined outcome compared to obese diabetic patients in the metabolic group.
In Asia, the healthcare topography in terms of government health expenditure, availability of universal health insurance coverage, and reliance on private payment varies greatly, and this may contribute to disparities in care across the region. For instance, we have previously shown that there was enormous variation in utilization of implantable cardiac defibrillators in eligible patients in our cohort, which was associated with geographic variations in out-of-pocket health expenditure and total government health expenditure [15]. The extent to which these factors may have contributed to the regional differences in multimorbidity phenotypes and differences in all-cause mortality and hospitalization for HF warrants further study. Given that genetic background may be determined by ethnicity [38], future studies are warranted to determine possible genetic factors underlying the predominance of particular multimorbidity groups in different ethnicities.
Comorbidities are associated with certain pattern of cardiac structural and functional changes in HF [39]. Previous studies have shown that single comorbidities such as CKD, diabetes, and obesity affect cardiac structure and function both in patients with HF and in the general population [39–42]. Furthermore, a greater burden (number) of comorbidities is associated with indices of cardiac mechanics [43]. However, prior studies did not examine the cumulative effect of specific combinations of comorbidities. The prospective design of our study, with standardized echocardiography by protocol, enabled our detailed interrogation into cardiac structural and functional changes that potentially underlie the different clinical behaviors of patient groups. We found an expected association between the metabolic group and HFpEF, as well as between the ischemic group and HFrEF. More surprising was the association of the lean diabetic group with the greatest extent of concentric remodeling, LVH, and diastolic dysfunction, even more so than in the obese diabetic metabolic group in HFpEF, thus offering a potential explanation for the higher rates of the primary combined outcome seen in the lean diabetic group. Importantly, this provides clinical evidence of cardiometabolic disturbance as a key driver of cardiac dysfunction, apart from the confounding influence of weight gain per se, and supports the recent development of drugs targeting cardiometabolic pathways in HF [44]. In fact, our data suggest that these cardiometabolic agents may have unique application in specific Asian populations, as opposed to weight loss as a therapeutic strategy in Western populations [45]. Surprising was the association of the young group with the greatest prevalence of eccentric hypertrophy, even more so than the ischemic group in HFrEF, and despite the relative youth and strikingly low prevalence of comorbidities of individuals in the young group.
Our findings carry implications for clinical surveillance and management of patients with HF in different regions of Asia, as well as for design of global clinical trials in HF. This study shows that comorbidities in patients with HF cluster into distinct multimorbidity groups that affect mortality and hospitalization for HF beyond the sum of their parts. Future studies should take the combinations of comorbidities into account, which could drive decisions in personalized patient care based on survival as well as time to hospitalization for HF. Furthermore, patients from Southeast Asia with diabetes, even in the absence of obesity, warrant surveillance for HFpEF, and trials targeting HFpEF may enrich their populations by including lean diabetic patients from the region.
Strengths and limitations
We acknowledge potential bias in site selection and willingness of patients to participate in a prospective registry, particularly across a huge geography of 11 regions, with disparate healthcare systems at different stages of evolution [15]. Site selection in the ASIAN-HF registry was based on the size of the region, geographic location of the site within the region, patient population served, HF patient volume, and availability of expertise in echocardiography. Screening logs were encouraged but not available from all sites. Nevertheless, every effort was made to ensure protocol adherence and standardization, including language translations specific to each region, on-site investigator training, regular monitoring (both in person and remote), and centralized database management. The representativeness of the ASIAN-HF registry has been discussed previously [4]. There is a paucity of multinational data on patients with HF in Asia. Therefore, we can only rely on comparisons to single-center studies or studies reporting on only a few countries in Asia. Previous results have shown that data on patients in the ASIAN-HF registry are consistent with prior reports from single Asian nations [46–50]. This suggests that patients included in ASIAN-HF registry are representative of patients with HF in the region. Although our cohort was prospectively enrolled and followed up, we included prevalent HF cases and their risk factors at baseline, with the potential for survival bias and reverse causality. For instance, the fact that the highest risk of the primary combined outcome was in the lean diabetic group may have been because these patients were frailer or had lost weight in the months leading up to inclusion. Of note, baseline severity of HF as measured by NYHA class was similar between the ischemic and metabolic groups. Nonetheless, while every effort has been made to correct for potential confounders in survival analyses, some unmeasured factors might have influenced differences in survival between groups. Particular strengths of this study include the prospective design, uniform comprehensive data collection, detailed echocardiographic characterization, and close follow-up with independent adjudication of outcomes. We also used state-of-the-art statistical methods: LCAs are hypothesis generating and provide us with potential new insights into multimorbidity profiles of patients with HF.
Conclusion
These first prospective multinational data on multimorbidity patterns among Asian patients with HF showed that comorbidities naturally clustered in 5 distinct groups: elderly/AF, metabolic, young, ischemic, and lean diabetic. Different multimorbidity groups were associated with different underlying patterns of cardiac remodeling, and were differentially related to the primary combined outcome of all-cause mortality and hospitalization for HF, as well as to the secondary outcomes of all-cause mortality alone and hospitalization for HF alone. Striking geographic differences were observed in the distribution of multimorbidity groups across Asia. These data underscore the importance of multimorbidity in patients with HF and the need for more comprehensive approaches in phenotyping patients with HF and multimorbidity.
Supporting Information
Zdroje
1. Mentz RJ, Kelly JP, von Lueder TG, Voors AA, Lam CSP, Cowie MR, et al. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol. 2014;64 : 2281–93. doi: 10.1016/j.jacc.2014.08.036 25456761
2. Ather S, Chan W, Bozkurt B, Aguilar D, Ramasubbu K, Zachariah AA, et al. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol. 2012;59 : 998–1005. doi: 10.1016/j.jacc.2011.11.040 22402071
3. van Deursen VM, Urso R, Laroche C, Damman K, Dahlström U, Tavazzi L, et al. Co-morbidities in patients with heart failure: an analysis of the European Heart Failure Pilot Survey. Eur J Heart Fail. 2014;16 : 103–11. doi: 10.1002/ejhf.30 24453099
4. Lam CSP, Teng T-HK, Tay WT, Anand I, Zhang S, Shimizu W, et al. Regional and ethnic differences among patients with heart failure in Asia: the Asian sudden cardiac death in heart failure registry. Eur Heart J. 2016; 3141–53. doi: 10.1093/eurheartj/ehw331 27502121
5. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18 : 891–975. doi: 10.1002/ejhf.592 27207191
6. Shah AM. Ventricular remodeling in heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2013;10 : 341–9. doi: 10.1007/s11897-013-0166-4 24097113
7. Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62 : 263–71. doi: 10.1016/j.jacc.2013.02.092 23684677
8. Bayes-Genis A, Voors AA, Zannad F, Januzzi JL, Richards AM, Díez J. Transitioning from usual care to biomarker-based personalized and precision medicine in heart failure: call for action. Eur Heart J. 2017;133 : 226–31. doi: 10.1093/eurheartj/ehx027 28204449
9. Kirchhof P, Sipido KR, Cowie MR, Eschenhagen T, Fox KAA, Katus H, et al. The continuum of personalized cardiovascular medicine: a position paper of the European Society of Cardiology. Eur Heart J. 2014;35 : 3250–7. doi: 10.1093/eurheartj/ehu312 25148837
10. Ahmad T, Pencina MJ, Schulte PJ, O’Brien E, Whellan DJ, Piña IL, et al. Clinical implications of chronic heart failure phenotypes defined by cluster analysis. J Am Coll Cardiol. 2014;64 : 1765–74. doi: 10.1016/j.jacc.2014.07.979 25443696
11. Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, et al. Phenotype-specific treatment of heart failure with preserved ejection fraction. Circulation. 2016;134 : 73–90. doi: 10.1161/CIRCULATIONAHA.116.021884 27358439
12. Tromp J, Meyer S, Mentz RJ, O’Connor CM, Metra M, Dittrich HC, et al. Acute heart failure in the young: clinical characteristics and biomarker profiles. Int J Cardiol. 2016;221 : 1067–72. doi: 10.1016/j.ijcard.2016.06.339 27448534
13. Tromp J, Khan MAF, Klip IT, Meyer S, de Boer RA, Jaarsma T, et al. Biomarker profiles in heart failure patients with preserved and reduced ejection fraction. J Am Heart Assoc. 2017;6:e003989. doi: 10.1161/JAHA.116.003989 28360225
14. Lam CSP, Anand I, Zhang S, Shimizu W, Narasimhan C, Park SW, et al. Asian Sudden Cardiac Death in Heart Failure (ASIAN-HF) registry. Eur J Heart Fail. 2013;15 : 928–36. doi: 10.1093/eurjhf/hft045 23568645
15. Chia YMF, Teng T-HK, Tan ESJ, Tay WT, Richards AM, Chin CWL, et al. Disparity between indications for and utilization of implantable cardioverter defibrillators in Asian patients with heart failure. Circ Cardiovasc Qual Outcomes. 2017;10:e003651. doi: 10.1161/CIRCOUTCOMES.116.003651 29150533
16. Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35 : 1245–55. 10758967
17. Joseph SM, Novak E, Arnold SV, Jones PG, Khattak H, Platts AE, et al. Comparable performance of the Kansas City Cardiomyopathy Questionnaire in patients with heart failure with preserved and reduced ejection fraction. Circ Heart Fail. 2013;6 : 1139–46. doi: 10.1161/CIRCHEARTFAILURE.113.000359 24130003
18. Comín-Colet J, Garin O, Lupón J, Manito N, Crespo-Leiro MG, Gómez-Bueno M, et al. Validation of the Spanish version of the Kansas City Cardiomyopathy Questionnaire. Rev Esp Cardiol. 2011;64 : 51–8. doi: 10.1016/j.recesp.2010.10.003 21194819
19. Patidar AB, Andrews GR, Seth S. Prevalence of obstructive sleep apnea, associated risk factors, and quality of life among Indian congestive heart failure patients: a cross-sectional survey. J Cardiovasc Nurs. 2011;26 : 452–9. doi: 10.1097/JCN.0b013e31820a048e 21372733
20. Chen H-M, Clark AP, Tsai L-M, Lin C-C. Self-reported health-related quality of life and sleep disturbances in Taiwanese people with heart failure. J Cardiovasc Nurs. 2010;25 : 503–13. doi: 10.1097/JCN.0b013e3181e15c37 20938252
21. Luo N, Teng T-HK, Tay WT, Anand IS, Kraus WE, Liew HB, et al. Multi-national and multi-ethnic variations in health-related quality of life in patients with chronic heart failure. Am Heart J. 2017;191 : 75–81. doi: 10.1016/j.ahj.2017.06.016 28888273
22. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28 : 1–39.e14. doi: 10.1016/j.echo.2014.10.003 25559473
23. Linzer DA, Lewis JB. poLCA: an R package for polytomous variable latent class analysis. J Stat Softw. 2011;42 : 1–29. doi: 10.18637/jss.v042.i10
24. Forster MMR. Key concepts in model selection: performance and generalizability. J Math Psychol. 2000;44 : 205–31. doi: 10.1006/jmps.1999.1284 10733865
25. Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6 : 461–4. doi: 10.1214/aos/1176344136
26. Shah AM, Solomon SD. Phenotypic and pathophysiological heterogeneity in heart failure with preserved ejection fraction. Eur Heart J. 2012;33 : 1716–7. doi: 10.1093/eurheartj/ehs124 22730487
27. Kao DP, Wagner BD, Robertson AD, Bristow MR, Lowes BD. A personalized BEST: characterization of latent clinical classes of nonischemic heart failure that predict outcomes and response to bucindolol. PLoS ONE. 2012; 7(11): e48184. doi: 10.1371/journal.pone.0048184 23144856
28. Kao DP, Lewsey JD, Anand IS, Massie BM, Zile MR, Carson PE, et al. Characterization of subgroups of heart failure patients with preserved ejection fraction with possible implications for prognosis and treatment response. Eur J Heart Fail. 2015;17 : 925–35. doi: 10.1002/ejhf.327 26250359
29. Shah SJ, Katz DH, Selvaraj S, Burke MA, Yancy CW, Gheorghiade M, et al. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation. 2015;131 : 269–79. doi: 10.1161/CIRCULATIONAHA.114.010637 25398313
30. Francis GS, Cogswell R, Thenappan T. The heterogeneity of heart failure: will enhanced phenotyping be necessary for future clinical trial success? J Am Coll Cardiol. 2014;64 : 1775–6. doi: 10.1016/j.jacc.2014.07.978 25443697
31. Lee CS, Chien C V, Bidwell JT, Gelow JM, Denfeld QE, Masterson Creber R, et al. Comorbidity profiles and inpatient outcomes during hospitalization for heart failure: an analysis of the U.S. Nationwide inpatient sample. BMC Cardiovasc Disord. 2014;14 : 73. doi: 10.1186/1471-2261-14-73 24898986
32. World Health Organization. Global Health Observatory (GHO) data: overweight and obesity. Geneva: World Health Organization; 2017 [cited 2018 Jan 31]. Available from: http://www.who.int/gho/ncd/risk_factors/overweight/en/.
33. Omran AR. The epidemiologic transition theory revisited thirty years later. World Health Stat Q. 1998;51 : 99–119.
34. Omran AR. The epidemiologic transition theory. A preliminary update. J Trop Pediatr. 1983;29 : 305–16. 6672237
35. Sellayah D, Cagampang FR, Cox RD. On the evolutionary origins of obesity: a new hypothesis. Endocrinology. 2014;155 : 1573–88. doi: 10.1210/en.2013-2103 24605831
36. Bank IEM, Gijsberts CM, Teng T-HK, Benson L, Sim D, Yeo PSD, et al. Prevalence and clinical significance of diabetes in Asian versus white patients with heart failure. JACC Heart Fail. 2017;5 : 14–24. doi: 10.1016/j.jchf.2016.09.015 28447583
37. Damman K, Valente MAE, Voors AA, O’Connor CM, van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J. 2014;35 : 455–69. doi: 10.1093/eurheartj/eht386 24164864
38. Jorde LB, Wooding SP. Genetic variation, classification and “race.” Nat Genet. 2004;36:S28–33. doi: 10.1038/ng1435 15508000
39. Mohammed SF, Borlaug BA, Roger VL, Mirzoyev SA, Rodeheffer RJ, Chirinos JA, et al. Comorbidity and ventricular and vascular structure and function in heart failure with preserved ejection fraction: a community-based study. Circ Heart Fail. 2012;5 : 710–9. doi: 10.1161/CIRCHEARTFAILURE.112.968594 23076838
40. Gori M, Senni M, Gupta DK, Charytan DM, Kraigher-Krainer E, Pieske B, et al. Association between renal function and cardiovascular structure and function in heart failure with preserved ejection fraction. Eur Heart J. 2014;35 : 3442–51. doi: 10.1093/eurheartj/ehu254 24980489
41. Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. 2017;136 : 6–19. doi: 10.1161/CIRCULATIONAHA.116.026807 28381470
42. Lam CSP, Roger VL, Rodeheffer RJ, Bursi F, Borlaug BA, Ommen SR, et al. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from Olmsted County, Minnesota. Circulation. 2007;115 : 1982–90. doi: 10.1161/CIRCULATIONAHA.106.659763 17404159
43. Selvaraj S, Aguilar FG, Martinez EE, Beussink L, Kim K-YA, Peng J, et al. Association of comorbidity burden with abnormal cardiac mechanics: findings from the HyperGEN Study. J Am Heart Assoc. 2014;3:e000631. doi: 10.1161/JAHA.113.000631 24780206
44. Noordali H, Loudon BL, Frenneaux MP, Madhani M. Cardiac metabolism—a promising therapeutic target for heart failure. Pharmacol Ther. 2017;182 : 95–114. doi: 10.1016/j.pharmthera.2017.08.001 28821397
45. Kitzman DW, Brubaker P, Morgan T, Haykowsky M, Hundley G, Kraus WE, et al. Effect of Caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction. JAMA. 2016;315 : 36. doi: 10.1001/jama.2015.17346 26746456
46. Choi D-J, Han S, Jeon E-S, Cho M-C, Kim J-J, Yoo B-S, et al. Characteristics, outcomes and predictors of long-term mortality for patients hospitalized for acute heart failure: a report from the Korean Heart Failure Registry. Korean Circ J. 2011;41 : 363–71. doi: 10.4070/kcj.2011.41.7.363 21860637
47. Harikrishnan S, Sanjay G, Anees T, Viswanathan S, Vijayaraghavan G, Bahuleyan CG, et al. Clinical presentation, management, in-hospital and 90-day outcomes of heart failure patients in Trivandrum, Kerala, India: the Trivandrum Heart Failure Registry. Eur J Heart Fail. 2015;17 : 794–800. doi: 10.1002/ejhf.283 26011246
48. Shiba N, Nochioka K, Miura M, Kohno H, Shimokawa H, CHART-2 Investigators. Trend of westernization of etiology and clinical characteristics of heart failure patients in Japan—first report from the CHART-2 study. Circ J. 2011;75 : 823–33. 21436596
49. Lee R, Chan S-P, Chan Y-H, Wong J, Lau D, Ng K. Impact of race on morbidity and mortality in patients with congestive heart failure: a study of the multiracial population in Singapore. Int J Cardiol. 2009;134 : 422–5. doi: 10.1016/j.ijcard.2007.12.107 18372060
50. Leong KTG, Goh PP, Chang BC, Lingamanaicker J. Heart failure cohort in Singapore with defined criteria: clinical characteristics and prognosis in a multi-ethnic hospital-based cohort in Singapore. Singapore Med J. 2007;48 : 408–14. 17453098
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2018 Číslo 3- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Ferinject: správně indikovat, správně podat, správně vykázat
- Optimální dávkování apixabanu v léčbě fibrilace síní
-
Všechny články tohoto čísla
- What is the value of multidisciplinary care for chronic kidney disease?
- 2017 Reviewer and Editorial Board Thank You
- Delays in completion and results reporting of clinical trials under the Paediatric Regulation in the European Union: A cohort study
- Surveillance of antimicrobial consumption in animal production sectors of low- and middle-income countries: Optimizing use and addressing antimicrobial resistance
- Causes of death and infant mortality rates among full-term births in the United States between 2010 and 2012: An observational study
- Time for high-burden countries to lead the tuberculosis research agenda
- The importance and challenges of shared decision making in older people with multimorbidity
- Trajectories of functional decline in older adults with neuropsychiatric and cardiovascular multimorbidity: A Swedish cohort study
- Mortality, ethnicity, and country of birth on a national scale, 2001–2013: A retrospective cohort (Scottish Health and Ethnicity Linkage Study)
- Validation of a genetic risk score for atrial fibrillation: A prospective multicenter cohort study
- Multimorbidity and survival for patients with acute myocardial infarction in England and Wales: Latent class analysis of a nationwide population-based cohort
- Integration of postpartum healthcare services for HIV-infected women and their infants in South Africa: A randomised controlled trial
- Cost-effectiveness of multidisciplinary care in mild to moderate chronic kidney disease in the United States: A modeling study
- Role of heme in lung bacterial infection after trauma hemorrhage and stored red blood cell transfusion: A preclinical experimental study
- Patterns and temporal trends of comorbidity among adult patients with incident cardiovascular disease in the UK between 2000 and 2014: A population-based cohort study
- Global child and adolescent mental health: The orphan of development assistance for health
- Cardiovascular disease and multimorbidity: A call for interdisciplinary research and personalized cardiovascular care
- Forced anal examinations to ascertain sexual orientation and sexual behavior: An abusive and medically unsound practice
- Primary prevention of cardiovascular disease: The past, present, and future of blood pressure- and cholesterol-lowering treatments
- Physical activity levels in adults and older adults 3–4 years after pedometer-based walking interventions: Long-term follow-up of participants from two randomised controlled trials in UK primary care
- Effect and cost-effectiveness of educating mothers about childhood DPT vaccination on immunisation uptake, knowledge, and perceptions in Uttar Pradesh, India: A randomised controlled trial
- Comorbidity health pathways in heart failure patients: A sequences-of-regressions analysis using cross-sectional data from 10,575 patients in the Swedish Heart Failure Registry
- Multimorbidity in patients with heart failure from 11 Asian regions: A prospective cohort study using the ASIAN-HF registry
- Antiviral efficacy of favipiravir against Ebola virus: A translational study in cynomolgus macaques
- Transmission of HIV-1 drug resistance mutations within partner-pairs: A cross-sectional study of a primary HIV infection cohort
- A clinical decision support tool for improving adherence to guidelines on anticoagulant therapy in patients with atrial fibrillation at risk of stroke: A cluster-randomized trial in a Swedish primary care setting (the CDS-AF study)
- Integrating HIV and hypertension management in low-resource settings: Lessons from Malawi
- The epidemiology of adolescents living with perinatally acquired HIV: A cross-region global cohort analysis
- Polycystic ovary syndrome, androgen excess, and the risk of nonalcoholic fatty liver disease in women: A longitudinal study based on a United Kingdom primary care database
- Blood pressure-lowering treatment strategies based on cardiovascular risk versus blood pressure: A meta-analysis of individual participant data
- Cerebral white matter disease and functional decline in older adults from the Northern Manhattan Study: A longitudinal cohort study
- HIV treatment eligibility expansion and timely antiretroviral treatment initiation following enrollment in HIV care: A metaregression analysis of programmatic data from 22 countries
- The current and potential health benefits of the National Health Service Health Check cardiovascular disease prevention programme in England: A microsimulation study
- Cardiovascular disease: The rise of the genetic risk score
- Comparative analysis of the association between 35 frailty scores and cardiovascular events, cancer, and total mortality in an elderly general population in England: An observational study
- Progression of diabetes, heart disease, and stroke multimorbidity in middle-aged women: A 20-year cohort study
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Forced anal examinations to ascertain sexual orientation and sexual behavior: An abusive and medically unsound practice
- Polycystic ovary syndrome, androgen excess, and the risk of nonalcoholic fatty liver disease in women: A longitudinal study based on a United Kingdom primary care database
- The current and potential health benefits of the National Health Service Health Check cardiovascular disease prevention programme in England: A microsimulation study
- Cardiovascular disease and multimorbidity: A call for interdisciplinary research and personalized cardiovascular care
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



