-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaSurveillance of antimicrobial consumption in animal production sectors of low- and middle-income countries: Optimizing use and addressing antimicrobial resistance
In a policy forum, Daniel Schar and colleagues discuss the need for surveillance of antimicrobial consumption in animals in low - and middle-income countries and propose the establishment of antimicrobial consumption monitoring systems.
Published in the journal: . PLoS Med 15(3): e32767. doi:10.1371/journal.pmed.1002521
Category: Policy Forum
doi: https://doi.org/10.1371/journal.pmed.1002521Summary
In a policy forum, Daniel Schar and colleagues discuss the need for surveillance of antimicrobial consumption in animals in low - and middle-income countries and propose the establishment of antimicrobial consumption monitoring systems.
Summary points
Antimicrobial use in low - and middle-income country (LMIC) food animal production sectors is accelerating, commensurate with expanded intensive production to meet rapidly increasing demand for animal-source nutrition.
However, antimicrobial consumption in animal production contexts of LMICs remains largely undocumented, limiting the ability to establish and monitor progress toward achieving consumption targets.
We propose the establishment of antimicrobial consumption monitoring systems in a phased manner that will be responsive and adaptive to LMIC contexts, while directing a path toward incremental enhancement of monitoring structures.
This phased approach enables implementation of systems yielding standardized, globally comparable antimicrobial consumption data, which could inform policies to optimize antimicrobial usage in food animal production.
The approach should be complemented by efforts to strengthen animal production systems, eliminate medically important antimicrobial growth promoters, and reduce reliance upon prophylactic antimicrobial use.
Global antimicrobial consumption in terrestrial and aquatic food animal production is accelerating, associated with expanded production to meet increasing demand for animal-source nutrition [1]. In South Asia, for example, demand for poultry between 2000 and 2030 is expected to increase by 725% [2]. Overall, growth in demand for animal-source nutrition through 2030 is anticipated to be higher in low - and middle-income countries (LMICs) as compared with high-income countries [2].
Efforts to meet rising demand for animal-source nutrition in LMICs are driving a shift in animal production from small holder, mixed crop, and livestock operations to increasingly intensive, large-scale, and specialized commercialization [1,3]. Intensive production systems have historically employed nontherapeutic antimicrobial use, particularly during transitionary stages [1]. Nontherapeutic antimicrobial use includes both mass administration for prevention and control of disease and antimicrobial growth promoter (AGP) use. AGPs are antimicrobials administered at subtherapeutic doses, are intended to enhance growth and production efficiencies, and are generally delivered in feed or water for extended duration. Although AGPs are not authorized in many countries, LMIC regulatory structures are often insufficient to monitor and enforce AGP bans.
Quantitative volumes of AGP usage from LMICs are not available. However, studies from LMICs indicate substantial nontherapeutic use. In the Mekong Delta of Vietnam, 84% of poultry farms surveyed indicated that antimicrobials were used for prophylactic rather than therapeutic purposes, and nearly a third of overall usage involved antimicrobial classes on the WHO list of highest priority, critically important antimicrobials for human medicine [4]. As a consequence of this changing landscape of animal production, without appropriate policy to optimize use, global antimicrobial consumption in food-producing animals, estimated at 131,109 tons in 2013, is projected to increase over 50% by 2030 to 200,235 tons [5]. In the BRICS countries—Brazil, Russia, India, China, and South Africa—antimicrobial consumption in food animals is projected to double [1].
The discovery and commercialization of antimicrobials stands as a defining achievement of 20th century medicine. As usage patterns drive rising rates of antimicrobial resistance (AMR)-linked morbidity and mortality [6]—compounded by a formidable cross-sectoral economic burden [7]—the global community faces an unprecedented challenge of preserving—and maintaining in perpetuity—antimicrobial efficacy.
Antimicrobial usage (AMU) in humans and animals exerts selection pressure potentiating AMR [1,5–8]; policy goals aim to reduce antimicrobial use where effective alternatives are available. A standardized framework guiding collection of incrementally detailed, internationally comparable antimicrobial consumption data from animal production could accelerate progress toward optimizing global antimicrobial use (Box 1). Yet, no such standardized framework relevant to LMIC animal production contexts exists [9,10], challenging the characterization of usage and limiting government policies aimed at optimizing and monitoring AMU in animal production sectors.
Box 1. Benefits expected from establishing a standardized framework for antimicrobial consumption in animal production
Monitor consumption trends and benchmarking for optimization of antimicrobial consumption.
Facilitates identification of trends and usage profiles,
Permits establishment of time-bound consumption targets,
Monitors progress toward achieving targets,
Guides policy and targeted interventions optimizing antimicrobial use.
Comparison of antimicrobial consumption data across countries, species, farms, and with human consumption.
Comparison of consumption data ultimately disaggregated by species and comparable across human and animal sectors,
Antimicrobial consumption data become globally comparable,
Contributes to international benchmarking,
Creates a globally standardized monitoring architecture, upon which a future international agreement capping consumption could be developed.
Builds a platform for studying the association between antimicrobial consumption and AMR surveillance data.
The challenge of establishing surveillance of antimicrobial consumption in animal production
The measurement of AMU in human health and animal health and production settings is a central goal of the Global Action Plan on Antimicrobial Resistance [11] and the complementary plans and strategies developed by the Food and Agriculture Organization of the United Nations (FAO) and World Organization for Animal Health (OIE) [12,13].
The ecosystem in which veterinary antimicrobials are produced, distributed, and utilized in LMICs is complex and variable. A majority of LMICs currently lack structures capable of quantifying consumption at sufficient resolution to provide usage data by antimicrobial class, animal species, production context, purpose of usage, and route of administration, which are necessary to facilitate effective interventions to optimize use [14]. Of the 74 LMIC OIE member states, 54 (73%) reported AMU to the OIE in 2015, while 20 (27%) provided no reporting at all. Of the 54 member states reporting, 30 (55.6%) were able to provide some quantitative usage data but were largely unable to report that data disaggregated by the aforementioned parameters [14].
Patterns of antimicrobial use differ widely across animal production value chains, as evidenced by macrolide, fluoroquinolone, and tetracycline residue detection in animal products marketed for human consumption [15], but species-level consumption data from LMICs are largely unavailable [16]. Reports are inconsistent, challenging international comparison due to lack of measurement standardization. A more complete picture of consumption variability is imperative in identifying targeted action toward optimizing usage.
Despite calls for establishing usage thresholds and targets [5,7,17], in the absence of an implementable, globally standardized methodology for quantifying consumption, progress toward setting, measuring, and achieving targeted usage benchmarks will remain limited.
Other challenges frequently shared by LMICs (Box 2) are equally important in realizing progress toward optimizing usage, and addressing them will require a foundational evidence base on consumption.
Box 2. Challenges experienced by LMICs in optimizing antimicrobial consumption in animal production sectors
Low rates of AMR awareness and risk perception among farmers and veterinarians [15],
Inconsistent policies governing antimicrobial use in animal production,
Absent AMU regulation and enforcement structures enabling wide accessibility to critically important antimicrobials without prescription,
Where a legal veterinarian–client–patient relationship exists governing prescription use of antimicrobials in animals, the link between prescription and sales presents a sales volume profit incentive, and
Lack of systematic post-market quality surveillance that would enable recall of substandard and counterfeit veterinary antimicrobial products impacting therapeutic efficacy.
Standardizing measurement methodologies in the context of LMICs
Multiple methodologies for quantifying usage have been variously employed, hindering data comparability across countries and production sectors. The OIE terrestrial animal health code, the guiding framework for its 180 member nations, outlines the minimum standard as measuring gross usage by weight of active ingredient per year [18].
Through the European Surveillance of Veterinary Antimicrobial Consumption (ESVAC) activity, European Union member states voluntarily report antimicrobial use by food animal–producing species [19]. The ESVAC guidance aims to harmonize antimicrobial consumption data and enable comparison with AMR surveillance data.
Antimicrobial consumption in food animals can be crudely estimated by quantifying the total weight of the active ingredient adjusted for biomass eligible for treatment, usually expressed as mg/kg population correction unit (PCU), where PCU is defined as total live and slaughtered animal weight per year in a prescribed geographic area. While this measurement of total volume of active ingredient per biomass considers neither the concentration of administered product nor its pharmacokinetics in individual species, it is a readily calculated estimate of usage. ESVAC guidelines use the mg/PCU methodology and introduce both defined daily dose in animals (DDDvet) and defined course dose in animals (DCDvet) [20], serving two functions: comparability across a range of species, usage, pharmaceutical formulations, and dosing regimens and aligning AMU methodologies in animals to those in humans, namely the defined daily dose (DDD) per 1,000 population per day endorsed as a global standard by WHO [21]. However, DDDvet and DCDvet—proprietary ESVAC reporting nomenclature similar to animal defined daily dose (ADDD) and animal defined course dose (ADCD), respectively—are not suited to contexts in which measurement must also capture continuous-use, subtherapeutic dosing, as in LMICs where policies governing AGP use vary significantly.
A phased capacity framework: Toward a global architecture for quantifying AMU in animal production
Accurate accounting of antimicrobial consumption in food-producing animals of LMICs must capture both therapeutic and nontherapeutic use, including AGPs and mass administration of antimicrobials delivered in medicated, premixed feed. Antimicrobial consumption monitoring is particularly important in aquaculture, in which mass administration of antimicrobials in medicated feed for disease prevention and control is a standard practice [22].
We propose a standardized, internationally-endorsed, phased approach that accounts for the context and currently available structures for collecting AMU data from food-producing animals—both terrestrial and aquatic species—in LMICs. The foundation is built upon the annual measurement of total sales of antimicrobials by class in mg/PCU, which can be derived from the total sales by class for member states reporting to the OIE divided by animal biomass data. Consistency in deriving the numerator—active pharmaceutical ingredient (API)—across multiple formulations of veterinary antimicrobials necessitates that products be managed through a standardized classification scheme, such as the Anatomical Therapeutic Chemical (ATCvet) system established for veterinary medical products [23]. Similarly, the animal biomass PCU denominator must employ a clearly and consistently applied calculation, using standardized production cycle and weight parameters [19] and accounting for animals locally produced, minus import and plus export of animals across borders. Leveraging existing, aggregate antimicrobial importation data; API domestic manufacturing and export data; antimicrobial sales data; and all-species animal biomass estimates publicly available [24] permit the rapid adoption of this foundational measurement scheme and bypasses substantial challenges in some LMICs of bottom-up quantification at the farm level, where legal requirements for identifying antimicrobials premixed into animal feed do not presently exist.
As countries establish refined data collection structures (Box 3), measurements could correspondingly evolve in subsequent phases to encompass more detailed parameters, including disaggregation by species and production type, and enhanced methodologies, such as number of animal defined daily doses (Table 1). Advanced phases could also involve collection sources more proximal to the site of usage—ultimately, at the individual animal or herd/flock/pond level—providing increasingly rich data sources directing optimal usage profiles and enabling usage audits and gap analysis for continued professional education. Achieving this detail requires both verifiable on-farm record keeping and longitudinal or serial, cross-sectional surveys capturing usage. Linkages with legislation and enforcement of appropriate incentive–penalty structures and establishment of an independent audit system will enhance compliance.
Box 3. Application of the phased antimicrobial consumption monitoring approach in Thailand
In 2015, Thailand’s International Health Policy Program and One Health partners established a human and animal national Surveillance for Antimicrobial Consumption (Thai SAC) system [25,26]. Thailand’s 1967 Drug Act mandates pharmaceutical importers and producers to submit an annual report on total volume of importation and manufacture of all medicines, including antimicrobials, to the Thai Food and Drug Administration. This total national antimicrobial sales data forms the foundation of Thai SAC, although the current reporting format does not require disaggregation by animal species. A 4-year (2014–2017) retrospective consumption study, with expected completion by mid-2018, will report mg active ingredient per PCU totaled across all animal species. Illustrating the application of an incremental, phased approach, in 2017, electronic information systems are being developed to facilitate e-reporting total sales of antimicrobial classes by animal species [27].
Tab. 1. Phased capacity approach to establishing surveillance for antimicrobial consumption in food animal production context of LMICs. 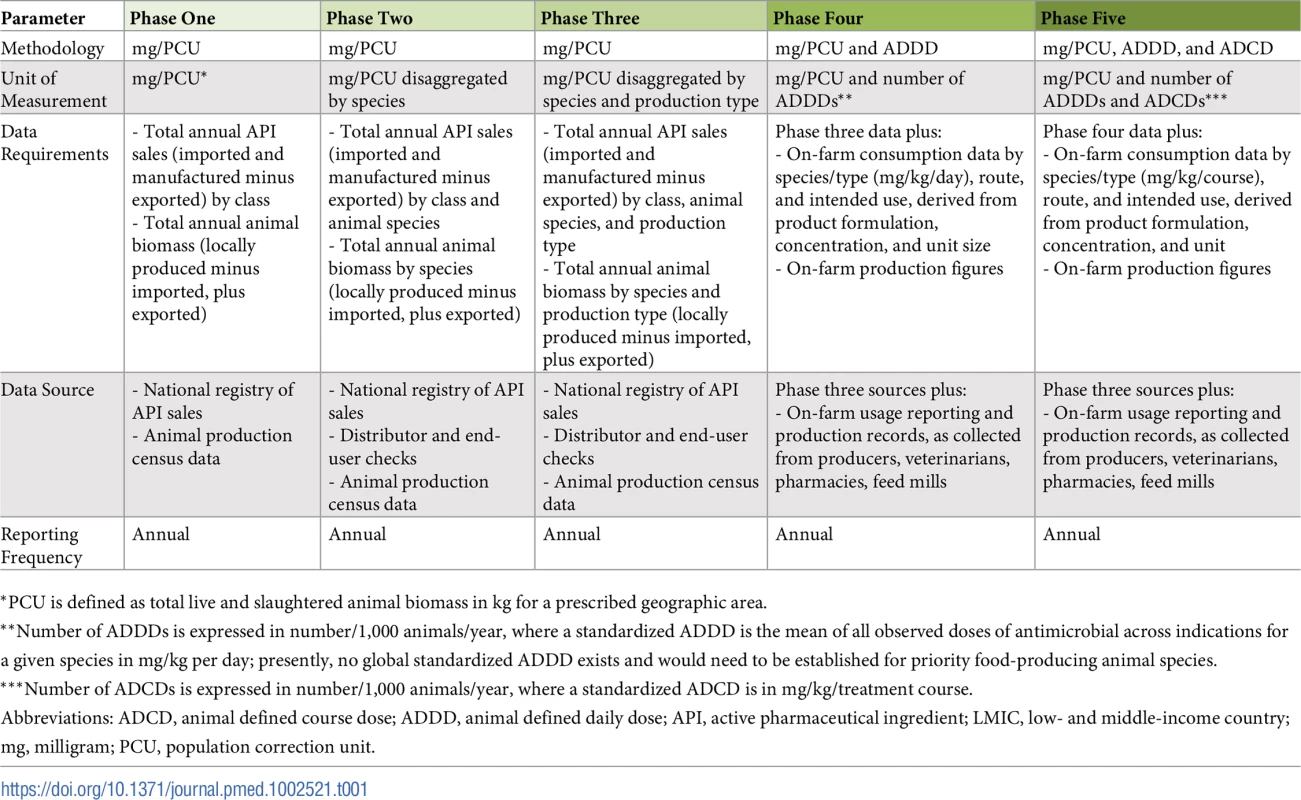
*PCU is defined as total live and slaughtered animal biomass in kg for a prescribed geographic area. Technical assistance could be housed within existing or newly established coordinating bodies and should prioritize intraregional LMIC exchanges and solutions relevant to unique regional capacities and contexts. An intergovernmental panel on AMR has been suggested [28], similar to structures designed to catalyze global action on climate change. The United Nations ad hoc Interagency Coordination Group on Antimicrobial Resistance (IACG), formed at the 71st UN General Assembly in 2016, comprises agencies including the WHO-FAO-OIE tripartite, which can jointly coordinate technical and operational assistance between member countries [29].
The path forward in optimizing AMU in food animal production sectors
Documenting usage—particularly for WHO-classified highest priority critically important antimicrobials—permits trends monitoring and identification of animal production sectors in which targeted interventions hold promise of arresting drivers of resistance. Conceivably, a reversion to susceptibility is achievable, as noted in Salmonella enterica Serovar Heidelberg following elimination of third-generation cephalosporins from poultry hatcheries in Quebec, Canada [30].
Establishing antimicrobial consumption monitoring systems in animal production sectors should be a central, near-term goal of multisectoral national AMR action plans aligned with the WHO Global Action Plan and the focus of advocacy and support from the UN FAO, OIE, and WHO tripartite. Embedding this approach within national AMR action plans promotes monitoring capacities endorsed across ministries, including those vested with budget authority, and costed, resourced, and prioritized for implementation.
In parallel, the WHO Joint External Evaluation (JEE) tool, which explicitly considers AMR and is increasingly being utilized by countries as an independent assessment of International Health Regulations (2005) capacity requirements [31], could develop an indicator evaluating AMU monitoring capacities. The JEE’s 1 (no capacity) to 5 (sustainable capacity) scoring structure is well matched to the phased, incrementally enhanced monitoring pathway presented here (Table 1), and incorporating a distinct indicator would ensure dedicated consideration of usage monitoring in human and animal sectors.
Antimicrobial consumption monitoring systems alone, however, will be insufficient to realize progress in directing prudent AMU in food-producing animals. Concurrently, support for strengthened farm and market chain biosecurity, enhanced livestock vaccination coverage, and improved uptake of good animal husbandry and nutrition practices will be necessary in achieving optimized usage, particularly in transitioning food animal production contexts of LMICs. New production facilities in LMICs should be designed to achieve high standards of husbandry and biosecurity, enabling more rapid phaseout of AGPs. Eliminating AGPs can be achieved at negligible cost to productivity, particularly in the context of such strengthened production systems [32].
The framework presented here should provide LMIC policy makers with high-quality antimicrobial consumption data that can be used to establish usage targets, while building incrementally enhanced monitoring capacities. The analyses derived from this approach could steer targeted policies optimizing antimicrobial consumption and scale back selective pressures currently driving AMR in animal production, with benefits expected to extend broadly across animal and human health.
Disclaimer
The authors’ views expressed in this publication do not necessarily reflect the views of the United States Agency for International Development or the United States Government.
Zdroje
1. Van Boeckel TP, Brower C, Gilbert M, Grenfell BT, Levin SA, Robinson TP, et al. Global trends in antimicrobial use in food animals. Proc Natl Acad Sci USA. May 5, 2015. vol. 112 (18). 5649–5654. doi: 10.1073/pnas.1503141112 25792457
2. Robinson TP, Pozzi F. Mapping supply and demand for animal-source foods to 2030. Animal Production and Health Working Paper No. 2. Rome: Food and Agriculture Organisation (FAO) of the United Nations. 2011. Available from: http://www.fao.org/docrep/014/i2425e/i2425e00.pdf Cited 13 February 2018.
3. UN FAO. The State of Food and Agriculture 2009. Available from: http://www.fao.org/docrep/012/i0680e/i0680e.pdf Cited 13 February 2018.
4. Carrique-Mas JJ, Trung NV, Hoa NT, Mai HH, Thanh TH, Campbell JI, et al. Antimicrobial Usage in Chicken Production in the Mekong Delta of Vietnam. Zoonoses Public Health. 2015; 62 : 70–78. doi: 10.1111/zph.12165 25430661
5. Van Boeckel TP, Glennon EE, Chen D, Gilbert M, Robinson TP, Grenfell BT, et al. Reducing antimicrobial use in food animals. Science. 29 Sep 2017: Vol. 357, Issue 6358, pp. 1350–1352. doi: 10.1126/science.aao1495 28963240
6. Laxminarayan R, Duse A, Wattal C, Zaidi AKM, Wertheim HFL, Sumpradit N, et al. Antibiotic resistance—the need for global solutions. Lancet Infect Dis. Volume 13, Issue 12, December 2013, Pages 1057–1098. https://doi.org/10.1016/S1473-3099(13)70318-9 24252483
7. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. London: Review on Anti-Microbial Resistance. 2014. Available from: https://amr-review.org/Publications.html Cited 13 February 2018.
8. Smith DL, Dushoff J, Morris JG Jr. Agricultural Antibiotics and Human Health. PLoS Med. 2005; 2(8): e232. https://doi.org/10.1371/journal.pmed.0020232 15984910
9. Parathon H, Kuntaman K, Widiastoety TH, Muliawan BT, Karuniawati A, Qibtiyah M, et al. Progress towards antimicrobial resistance containment and control in Indonesia. BMJ. 2017; 358:j3808. http://dx.doi.org/10.1136/bmj.j3808 28874346
10. Laxminarayan R, Chaudhury RR. Antibiotic Resistance in India: Drivers and Opportunities for Action. PLoS Med. 2016; 13(3): e1001974. https://doi.org/10.1371/journal.pmed.1001974 26934098
11. WHO. Global Action Plan on Antimicrobial Resistance. 2015. Available from: http://apps.who.int/iris/bitstream/10665/193736/1/9789241509763_eng.pdf Cited 13 February 2018.
12. UN FAO. The FAO Action Plan on Antimicrobial Resistance2016-2020. Available from: http://www.fao.org/3/a-i5996e.pdf Cited 13 February 2018.
13. OIE. The OIE Strategy on Antimicrobial Resistance and the Prudent Use of Antimicrobials. November 2016. Available from: http://www.oie.int/fileadmin/Home/eng/Media_Center/docs/pdf/PortailAMR/EN_OIE-AMRstrategy.pdf Cited 13 February 2018.
14. Moulin G, Góchez D, Diaz F, Szabo M, Lasley J, Erlacher-Vindel E. Outcomes from the OIE’s questionnaire on antimicrobial use in animals in 2015. Available from: http://www.oie.int/fileadmin/Home/eng/Our_scientific_expertise/docs/pdf/AMR/A_Bull_2016-3_Moulin.pdf Cited 13 February 2018.
15. Pham DK, Chu J, Do NT, Brose F, Degand G, Delahaut P, et al. Monitoring Antibiotic Use and Residue in Freshwater Aquaculture for Domestic Use in Vietnam. Ecohealth. 2015; 12 : 480. https://doi.org/10.1007/s10393-014-1006-z 25561382
16. Gandra S, Joshi J, Trett A, Sankhil Lamkang A, Laxminarayan R. Scoping Report on Antimicrobial Resistance in India. Washington, DC: Center for Disease Dynamics, Economics & Policy. November 2017. Available from: http://www.dbtindia.nic.in/wp-content/uploads/ScopingreportonAntimicrobialresistanceinIndia.pdf Cited 13 February 2018.
17. Laxminarayan R, Sridhar D, Blaser M, Wang M, Woolhouse M. Achieving global targets for antimicrobial resistance. Science. 18 Aug 2016; 353 (6302), 874–875. doi: 10.1126/science.aaf9286 27540009
18. OIE Terrestrial Animal Health Code, Chapter 6.8. Available from: http://www.oie.int/fileadmin/Home/eng/Health_standards/tahc/current/chapitre_antibio_monitoring.pdf Cited 13 February 2018.
19. European Medicines Agency. Guidance on provision of data on antimicrobial use by animal species from national data collection systems. 24 March 2017. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2017/03/WC500224492.pdf Cited 13 February 2018.
20. European Medicines Agency. Principles on assignment of defined daily dose for animals (DDDvet) and defined course dose for animals (DCDvet). 23 June 2015. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/06/WC500188890.pdf Cited 13 February 2018.
21. WHO. WHO methodology for a global programme on surveillance of antimicrobial consumption (version 1.0). 2017. Available from: www.who.int/medicines/areas/rational_use/WHO_AMCsurveillance_1.0.pdf?ua=1 Cited 13 February 2018.
22. Cabello FC. Heavy use of prophylactic antibiotics in aquaculture: A growing problem for human and animal health and for the environment. Environ Microbiol. 2006; 8(7):1137–1144. doi: 10.1111/j.1462-2920.2006.01054.x 16817922
23. WHO Collaborating Center for Drug Statistics Methodology. Available from: https://www.whocc.no/atcvet/atcvet_index/ Cited 13 February 2018.
24. FAOSTAT. Available from: http://www.fao.org/faostat/en/#home Cited 13 February 2018.
25. Tangcharoensathien V, Sattayawutthipong W, Kanjanapimai S, Kanpravidth W, Brown R, Sommanustweechaia A. Antimicrobial resistance: from global agenda to national strategic plan, Thailand. Bull World Health Organ. 2017; 95 : 599–603. http://dx.doi.org/10.2471/BLT.16.179648 28804172
26. Food and Drug Administration and International Health Policy Program, Ministry of Public Health, Thailand. Development of Thailand Surveillance of Antimicrobial Consumption: the 2017 national health and welfare survey in Thailand the role of processes and technical capacities [Internet]. Bangkok: Ministry of Public Health, Thailand; 2018 [cited 2018 Feb 13]. Available from: http://www.ihppthaigov.net/wpdm-package/development-of-thailand/
27. Tangcharoensathien V, Sommanustweechai A, Chanthong B, Sumpradit N, Sakulbumrungsil R, Jaroenpoj S, et al. Surveillance of antimicrobial consumption: methodological review for systems development in Thailand. J Glob Health. 2017: Vol. 7; 010307. doi: 10.7189/jogh.07.010307 28702173
28. Woolhouse M, Farrar J. Policy: An intergovernmental panel on antimicrobial resistance. Nature. 29 May 2014. 509, 555–557. doi: 10.1038/509555a 24877180
29. Ad Hoc Interagency Coordination Group On Antimicrobial Resistance (IACG). 29 May 2017. Available from: http://www.who.int/antimicrobial-resistance/interagency-coordination-group/IACG-AMR-ToR.pdf Cited 13 February 2018.
30. Dutil L, Irwin R, Finley R, Ng LK, Avery BP, Boerlin P, et al. Ceftiofur Resistance in Salmonella enterica Serovar Heidelberg from Chicken Meat and Humans, Canada. Emerg Infect Dis. 2010 Jan; 16(1): 48–54. doi: 10.3201/eid1601.090729 20031042
31. WHO. Joint External Evaluation Tool: International Health Regulations (2005). 2016. Available from: http://apps.who.int/iris/bitstream/10665/204368/1/9789241510172_eng.pdf Cited 13 February 2018.
32. Laxminarayan R, Van Boeckel TP, Teillant A. The economic costs of withdrawing antimicrobial growth promoters from the livestock sector. Paris: Organization for Economic Cooperation and Development. 2015. Available from: http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=TAD/CA/APM/WP(2014)34/FINAL&docLanguage=En Cited 13 February 2018.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2018 Číslo 3- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Ferinject: správně indikovat, správně podat, správně vykázat
- Optimální dávkování apixabanu v léčbě fibrilace síní
-
Všechny články tohoto čísla
- What is the value of multidisciplinary care for chronic kidney disease?
- 2017 Reviewer and Editorial Board Thank You
- Delays in completion and results reporting of clinical trials under the Paediatric Regulation in the European Union: A cohort study
- Surveillance of antimicrobial consumption in animal production sectors of low- and middle-income countries: Optimizing use and addressing antimicrobial resistance
- Causes of death and infant mortality rates among full-term births in the United States between 2010 and 2012: An observational study
- Time for high-burden countries to lead the tuberculosis research agenda
- The importance and challenges of shared decision making in older people with multimorbidity
- Trajectories of functional decline in older adults with neuropsychiatric and cardiovascular multimorbidity: A Swedish cohort study
- Mortality, ethnicity, and country of birth on a national scale, 2001–2013: A retrospective cohort (Scottish Health and Ethnicity Linkage Study)
- Validation of a genetic risk score for atrial fibrillation: A prospective multicenter cohort study
- Multimorbidity and survival for patients with acute myocardial infarction in England and Wales: Latent class analysis of a nationwide population-based cohort
- Integration of postpartum healthcare services for HIV-infected women and their infants in South Africa: A randomised controlled trial
- Cost-effectiveness of multidisciplinary care in mild to moderate chronic kidney disease in the United States: A modeling study
- Role of heme in lung bacterial infection after trauma hemorrhage and stored red blood cell transfusion: A preclinical experimental study
- Patterns and temporal trends of comorbidity among adult patients with incident cardiovascular disease in the UK between 2000 and 2014: A population-based cohort study
- Global child and adolescent mental health: The orphan of development assistance for health
- Cardiovascular disease and multimorbidity: A call for interdisciplinary research and personalized cardiovascular care
- Forced anal examinations to ascertain sexual orientation and sexual behavior: An abusive and medically unsound practice
- Primary prevention of cardiovascular disease: The past, present, and future of blood pressure- and cholesterol-lowering treatments
- Physical activity levels in adults and older adults 3–4 years after pedometer-based walking interventions: Long-term follow-up of participants from two randomised controlled trials in UK primary care
- Effect and cost-effectiveness of educating mothers about childhood DPT vaccination on immunisation uptake, knowledge, and perceptions in Uttar Pradesh, India: A randomised controlled trial
- Comorbidity health pathways in heart failure patients: A sequences-of-regressions analysis using cross-sectional data from 10,575 patients in the Swedish Heart Failure Registry
- Multimorbidity in patients with heart failure from 11 Asian regions: A prospective cohort study using the ASIAN-HF registry
- Antiviral efficacy of favipiravir against Ebola virus: A translational study in cynomolgus macaques
- Transmission of HIV-1 drug resistance mutations within partner-pairs: A cross-sectional study of a primary HIV infection cohort
- A clinical decision support tool for improving adherence to guidelines on anticoagulant therapy in patients with atrial fibrillation at risk of stroke: A cluster-randomized trial in a Swedish primary care setting (the CDS-AF study)
- Integrating HIV and hypertension management in low-resource settings: Lessons from Malawi
- The epidemiology of adolescents living with perinatally acquired HIV: A cross-region global cohort analysis
- Polycystic ovary syndrome, androgen excess, and the risk of nonalcoholic fatty liver disease in women: A longitudinal study based on a United Kingdom primary care database
- Blood pressure-lowering treatment strategies based on cardiovascular risk versus blood pressure: A meta-analysis of individual participant data
- Cerebral white matter disease and functional decline in older adults from the Northern Manhattan Study: A longitudinal cohort study
- HIV treatment eligibility expansion and timely antiretroviral treatment initiation following enrollment in HIV care: A metaregression analysis of programmatic data from 22 countries
- The current and potential health benefits of the National Health Service Health Check cardiovascular disease prevention programme in England: A microsimulation study
- Cardiovascular disease: The rise of the genetic risk score
- Comparative analysis of the association between 35 frailty scores and cardiovascular events, cancer, and total mortality in an elderly general population in England: An observational study
- Progression of diabetes, heart disease, and stroke multimorbidity in middle-aged women: A 20-year cohort study
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Forced anal examinations to ascertain sexual orientation and sexual behavior: An abusive and medically unsound practice
- Polycystic ovary syndrome, androgen excess, and the risk of nonalcoholic fatty liver disease in women: A longitudinal study based on a United Kingdom primary care database
- The current and potential health benefits of the National Health Service Health Check cardiovascular disease prevention programme in England: A microsimulation study
- Cardiovascular disease and multimorbidity: A call for interdisciplinary research and personalized cardiovascular care
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



