-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Treatment guidelines and early loss from care for people living with HIV in Cape Town, South Africa: A retrospective cohort study
In a retrospective cohort study, Ingrid Katz and colleagues report on the continuity of care for people with HIV in Gugulethu Township, South Africa
Published in the journal: . PLoS Med 14(11): e32767. doi:10.1371/journal.pmed.1002434
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002434Summary
In a retrospective cohort study, Ingrid Katz and colleagues report on the continuity of care for people with HIV in Gugulethu Township, South Africa
Introduction
South Africa has the world’s largest HIV epidemic, which has been met with an ever expanding and increasingly robust response since 2004, enabling the development of the single biggest antiretroviral therapy (ART) program globally. There are now over 3 million people on treatment in South Africa, which represents roughly half of the people living with HIV (PLWH) in the country [1,2]. The expansion in treatment availability, first ushered in by the US President’s Emergency Plan for AIDS Relief (PEPFAR) and the Global Fund and its partners [3,4] and now predominantly run through a governmental response, has increased the availability of ART for healthier PLWH [5,6].
South Africa has undergone multiple expansions in ART eligibility, with an increasing immunological threshold for ART initiation, from a CD4+ threshold of ≤200 cells/μl at the start of the treatment program to providing ART for all PLWH as of September 2016. The expansion of ART eligibility has resulted in a larger number of individuals being screened for treatment. Simultaneously, funding has shifted from programs primarily funded through external donors, such as PEPFAR and the Global Fund, to clinics run by the South African Department of Health (DOH). This has resulted in transitions from centralized, physician-managed programs to more decentralized, nurse-managed clinics [7–10].
While guidelines have shifted to expand earlier access to ART, it is unknown whether the expansion of treatment eligibility and availability has had an impact on patients’ engagement in care and early retention on treatment. This is particularly critical in the context of a new era of “test and treat” that has shifted the care cascade to be more focused on earlier ART initiation and durable retention [11]. Prior research in the earliest phases of treatment availability in South Africa showed that risk of loss to follow-up and virological failure both increased over successive calendar periods as the number of patients per clinic provider increased markedly over time [5]. An individual site in Durban undergoing a rapid transfer of care from a robust PEPFAR-funded program to community-based clinics reported loss of up to 20% of patients, who may have experienced a treatment interruption [12]. Similarly, a study of over 5,000 patients transitioning care from a physician-managed clinic to a nurse-managed program showed a significantly higher rate of loss to follow-up among patients who were down-referred [13].
We performed a retrospective cohort analysis of data from a large, urban community HIV treatment site in Cape Town to assess the association of South Africa’s HIV treatment eligibility guidelines with pre-ART attrition and early loss from care (<16 weeks). We hypothesized that increasing the CD4+ threshold to access ART would increase pre-treatment and early losses. The rationale for this hypothesis was that there could be a healthy cohort effect and/or that the programmatic shift to expanded treatment eligibility could result in a higher patient to nurse ratio, resulting in a crowding effect.
Methods
Ethics statement
Data collection on this cohort was approved by the research ethics committee of the University of Cape Town and the Partners HealthCare institutional review board, and patients gave written informed consent to have data collected anonymously for research purposes.
Treatment cohort
This cohort of PLWH accessing care in a DOH community health center in Gugulethu Township, a poor peri-urban area within Cape Town, South Africa, has been previously well characterized and is affiliated with the Desmond Tutu HIV Centre [14,15]. Treatment was made available at this site starting in September 2002, and as of 2013, over 5,257 patients were in care. While patients were not transitioned from this site during expansions in ART eligibility, there was a shift in funding starting in 2009 that resulted in an overall decrease in the number of physicians on site, while increasing the number of nurses providing care. Treatment is provided to patients free of charge. Patients have routine clinical assessments every 2 weeks prior to ART (the standard of care during this period required 8 clinic visits for patients initiating treatment) and again after 4, 8, and 16 weeks of treatment, and 16-weekly thereafter. CD4+ cell count testing is performed at baseline, and HIV-1 viral load is checked at 16 weeks after initiating ART.
Provision of patient care is supported by peer counselors, most of whom are living with HIV and are in care themselves [16]. Each new patient enrolling into the clinic is allocated to a peer counselor living in the same area. Through group sessions and individual home visits, patients are educated about the need for medication adherence and provided with counseling support.
Study design
While no prospective protocol was published or registered for this observational study, we adhered to an analysis plan that was developed in advance of our study (S2 Text). Specifically, data were abstracted retrospectively on all participants on ART at the Gugulethu clinic from electronic health data collected during routine care, from first clinic access through 16 weeks after ART initiation. These data included clinical variables, treatment outcomes (including death, loss to follow-up, and transfer out), ART regimens, and laboratory data derived from patient notes and pharmacy and laboratory records. ART-naïve patients aged ≥18 years who were eligible for treatment and enrolled in this cohort between 1 January 2009 and 31 December 2013 were eligible for this analysis. Women who were pregnant were excluded from this dataset. All data that were available for analysis that met the inclusion and exclusion criteria were used. The inclusion/exclusion criteria and statistical analyses for the study were established at the outset and were not changed.
Definitions of outcomes
Our outcomes were defined at the outset and were based on prior studies that we and others have published. Specifically, we defined “early mortality” as death from all causes prior to starting ART or death within the first 16 weeks on treatment. Pre-ART loss from care was defined as attrition between the time of learning ART eligibility and starting treatment. Early loss from care was defined as early discontinuation of treatment (within the first 16 weeks on ART). We used World Health Organization (WHO) clinical staging and immunological classification of HIV infection to assess disease status. The scale was developed in 1990 and is used only once an HIV infection has been established through a blood test [17].
Data collection and analysis
Data were abstracted from electronic records and paper charts and included baseline CD4+, age at referral, WHO stage, decision-making regarding ART initiation, and early treatment outcome (up to 16 weeks on ART). WHO stage was used as a proxy measure of baseline disease severity, where those with stage 1 are predominantly asymptomatic and those with stage 4 demonstrate more pronounced symptoms. Analyses were retrospective, and treatment discontinuation was confirmed through patient tracking involving up to 3 home visits if a patient had failed to attend the clinic for ≥12 weeks and had not been traced to another regional treatment center. Participants were examined in the context of an early cohort (patients enrolled into care at the clinic between 1 January 2009 and 31 August 2011), during which time the threshold for ART initiation was CD4+ ≤ 200 cells/μl, and a later cohort (patients enrolled into care between 1 September 2011 and 31 December 2013), when the treatment threshold had been expanded to CD4+ ≤ 350 cells/μl.
Demographic and clinical factors were compared in a bivariate analysis of before and after the policy change using chi-squared tests to identify potentially confounding covariates. Baseline age was calculated from date of birth, if available, and entry into the clinical cohort. In the bivariate analysis, CD4+ cell count among the earlier cohort was compared to that of those entering in the later cohort, using a Wilcoxon rank sum test. In the logistic regression models, CD4+ cell count was dichotomized to >200 and ≤200 based on the clinical definition of an AIDS diagnosis. We used p < 0.20 to identify any potential confounders, and logistic regression models were then used to estimate the adjusted risk of early loss (<16 weeks) from care controlling for age, baseline CD4+ cell count, and WHO stage. Multiple logistic regression was used to estimate the risk of early loss from care, pre - and post-ART initiation, adjusting for relevant baseline covariates, including calendar period of enrollment, which was included as a key variable of interest in this model. Post hoc relative goodness of fit of the logistic model was verified using a log-likelihood ratio to estimate a chi-squared value. Final models were checked using standard regression diagnostics for logistic regression. Wald confidence limits were calculated for all multivariate models. All statistical tests were 2-sided at alpha of 0.05. SAS statistical software, version 9.4, was used for all analyses (SAS Institute, Cary, North Carolina).
Results
In all, 4,025 ART-eligible PLWH who were referred to the treatment clinic between 1 January 2009 and 31 December 2013 were included in our sample. The median age in our population was 34 years (IQR 28–41 years) (see Table 1). Nearly 62% were female, and the median CD4+ count was 173 cells/μl (IQR 92–254 cells/μl). Overall, individuals in the earlier cohort had significantly more advanced disease than those in the later cohort, with lower CD4+ counts at the time of ART initiation (146 cells/μl versus 214 cells/μl, respectively, p < 0.001), and a larger percentage were classified as having a higher WHO stage (61.1% with stage 3/4 versus 42.8%, respectively, p < 0.001).
Tab. 1. Baseline characteristics of all participants by qualification period. 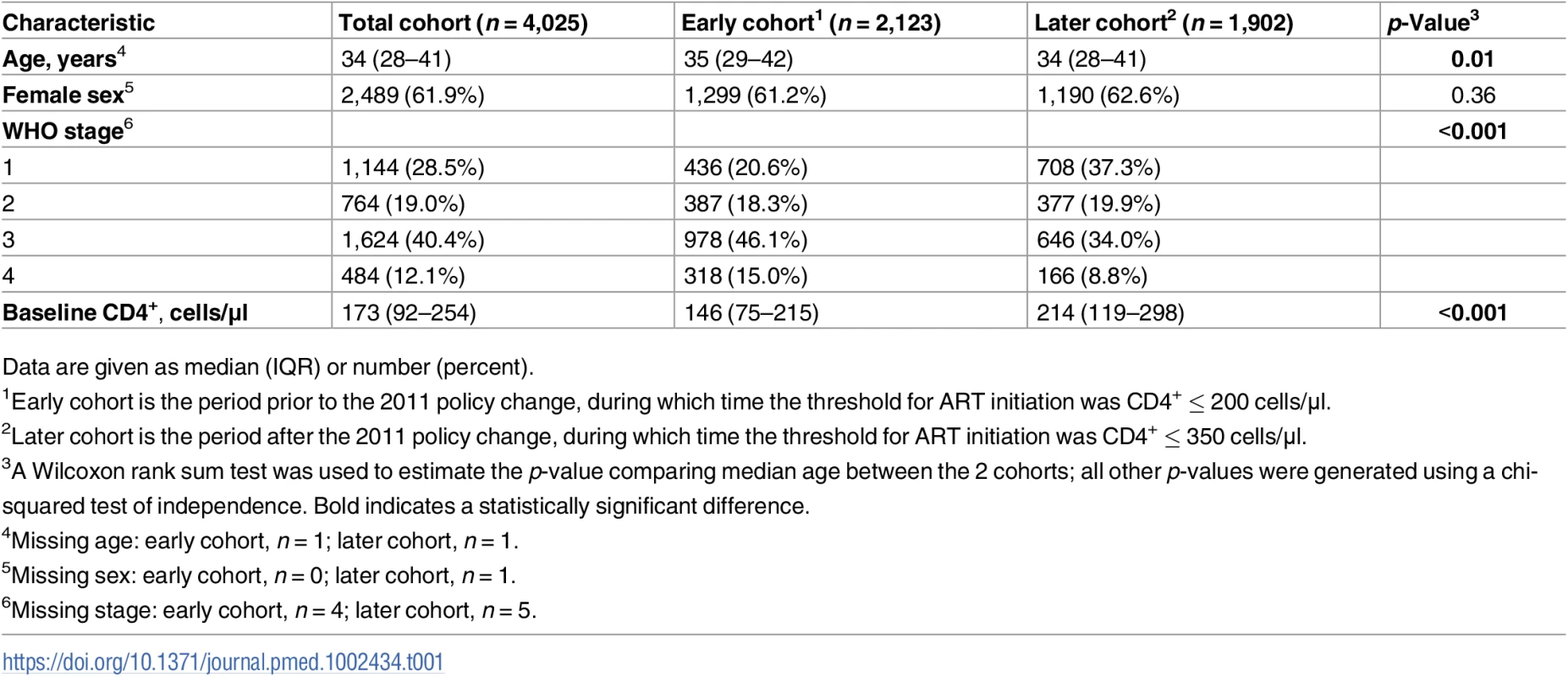
Data are given as median (IQR) or number (percent). Ninety percent (n = 3,640) of the population initiated ART within 16 weeks of first entering the clinic, per pharmacy records (see Table 2). There was no significant difference in the percentage of the population who initiated ART within 16 weeks between the earlier and later cohorts (90.6% versus 90.2%, p = 0.7). Of the 9.6% (n = 385) who did not start ART, 25.7% died prior to starting treatment. The rate of pre-ART death in the earlier cohort was twice that of the later cohort (34.2% versus 16.7%, p < 0.001).
Tab. 2. Early loss from care and mortality in Cape Town, South Africa. 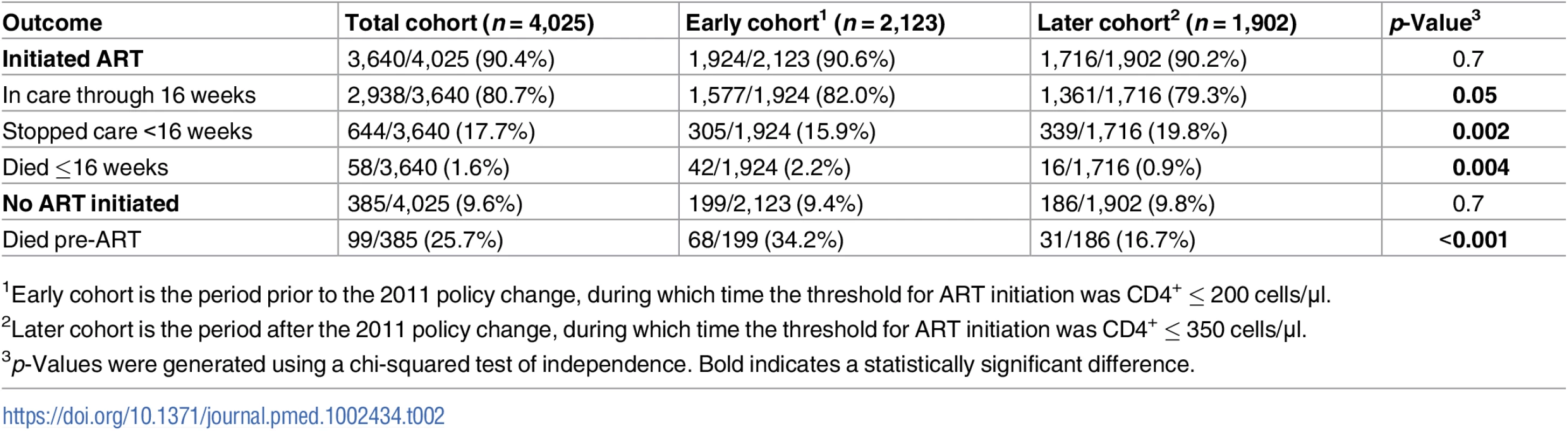
1Early cohort is the period prior to the 2011 policy change, during which time the threshold for ART initiation was CD4+ ≤ 200 cells/μl. Among the cohort who initiated treatment, 17.7% stopped accessing treatment within 16 weeks of ART initiation, and 1.6% died within the first 16 weeks. ART-eligible individuals in the later cohort were significantly more likely to discontinue care <16 weeks into treatment compared to those initiating treatment prior to 31 August 2011 (19.8% versus 15.8%, odds ratio [OR] = 1.32, p = 0.002). After controlling for baseline CD4+, WHO stage, and age, this effect remained significant (adjusted OR [aOR] = 1.30, 95% CI 1.09–1.55). When the analysis was restricted to only the individuals who would have qualified for ART in either cohort (CD4+ ≤ 200 cells/μl), the difference in early ART discontinuation remained significant (aOR = 1.34, 95% CI 1.06–1.67).
Fig 1 shows attrition across the cascade from the pre-ART period through to 16 weeks on ART in both the early and later cohorts. Across the full cohort, 157 (3.9%) ART-eligible PLWH died, and 930 (23.1%) were lost from care prior to ART initiation or within the first 16 weeks of starting treatment. This resulted in a total combined early loss of 1,087 (27.0%) ART-eligible PLWH. Over the 5 years of the study period, the total number of people entering care increased over 2-fold, from 776 entering care in 2009 to 1,506 entering care in 2013. During this time, the Gugulethu clinic transitioned from 5 doctors and 5 nurses in 2009 to 3 doctors and 7 nurses in 2013. The standard of care during this period required 8 clinic visits for patients initiating treatment.
Fig. 1. Attrition in the care cascade. 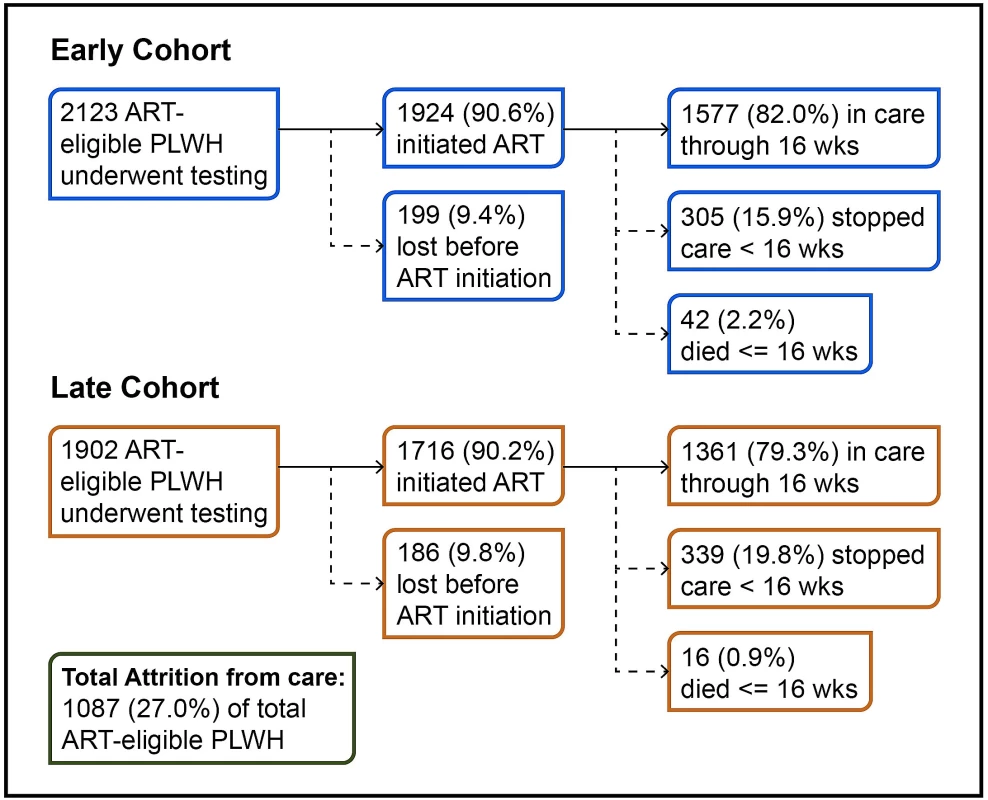
PLWH, people living with HIV. Discussion
In this cohort study, we assessed early losses from care over a 5-year period as South Africa was expanding its ART eligibility, and shifting from a centralized HIV treatment program with a high number of medical doctors, funded through PEPFAR, the Global Fund, and other partners, to a more decentralized, nurse-led system, supported largely through the South African government. Overall, we found that over one-quarter of this well-established ART-eligible cohort never achieved the long-term benefits of treatment and viral load suppression due to early mortality, failure to start ART, or ART discontinuation <16 weeks from the time of initiation. Patients who entered care in the later cohort were significantly more likely to discontinue treatment early.
Estimates of pre-ART attrition from care and early loss from care on ART among ART-eligible PLWH have consistently been estimated at 20%–30% of patients in South Africa [18–21]. What has remained less clear is the impact of guideline changes and shifting delivery systems on attrition and early loss over time. In 2013, WHO published consolidated guidelines on the use of antiretroviral drugs for HIV treatment and prevention across all age groups and populations, that were then revised based on new scientific evidence in 2015 [22]. WHO set forth these guidelines to increase treatment CD4+ thresholds, with the explicit goal of reducing transmission between sexual partners [23] and providing health benefits for patients taking treatment [24–26].
As the South African government has moved to incorporate these recommendations, expanding treatment to all PLWH, more people have become eligible to start ART. However, challenges remain in both ART initiation and retention in care, resulting in the failure of these strategies to achieve the expected benefits for the individual, let alone at a population level [27]. Recent research by Haber et al. showed that in a large population of PLWH in South Africa, the early stages of the cascade were the most vulnerable to losses [28]. South Africa has faced the added challenge of transitioning from a well-resourced HIV care program supported by donors to a decentralized system of care though a vast network of public health clinics, while expanding enrollment into ART programs throughout the country.
The impact of policy-level changes on treatment outcomes remains uncertain. In this study, the rate of early loss continued to rise throughout the study period. While the later cohort was significantly healthier than the earlier cohort, the increase in loss from care persisted in adjusted analyses and thus did not appear to reflect differences in CD4 count and WHO stage. We believe this trend may reflect large programmatic shifts in care and clinic crowding, as efforts have continued to expand to accommodate millions more PLWH into treatment programs despite constrained resources and a limited number of healthcare providers [3,29]. In addition, our prior qualitative research [30], along with prior research conducted by Fox et al. [31] and Duff et al. [32], suggests that perceptions of health and illness may be a strong driver of ART decision-making, and that these perceptions may not always be correlated with actual CD4+ counts [33]. As such, the goal of getting patients onto treatment earlier may remain a challenge if PLWH perceive themselves to be healthy, especially in settings where clinic crowding remains significant.
Our data have several limitations and a number of strengths. First, we are limited by the fact that these data were accumulated at a single high-volume site. Therefore, it is unclear if these findings are generalizable. Despite this, our findings are consistent with data from other multi-site, large cohorts [27,28,34]. Second, despite our access to staffing numbers, we are unable to formally investigate whether a crowding effect was the true cause of higher rates of loss from care in the later cohort. Third, our sample cannot account for patients who have potentially left the area and accessed care in other provinces. While this is a challenge consistently noted in previous research [35,36], this clinic utilized a robust tracking system when patients did not return for treatment, including active tracing of patients who missed clinic visits through home visits by community care workers. Finally, these data were collected from a site where treatment and testing were available. As such, it remains unclear if the linkage rates observed would be as high if the site was a stand-alone testing site where patients then had to link to a new site for care.
In conclusion, over one-quarter of this well-established ART-eligible cohort did not achieve the long-term benefits of treatment due to early mortality, failure to start ART, or ART discontinuation <16 weeks in care. Early ART discontinuation, which appeared to be independent of CD4+ count or WHO stage, likely reflected larger programmatic trends towards higher volume treatment centers that result in clinic crowding. Future interventions should focus on those most at risk for pre-ART attrition and early loss from care as programs continue to expand in an era of treatment for all in South Africa.
Supporting Information
Zdroje
1. United Nations Joint Programme on HIV/AIDS. The gap report. Geneva: United Nations Joint Programme on HIV/AIDS; 2014 [cited 2017 May 16]. http://www.refworld.org/docid/53f1e1604.html.
2. United Nations Joint Programme on HIV/AIDS. UNAIDS announces that the goal of 15 million people on life-saving HIV treatment by 2015 has been met nine months ahead of schedule. Geneva: United Nations Joint Programme on HIV/AIDS; 2015 [cited 2017 Jul 31]. http://www.unaids.org/en/resources/presscentre/pressreleaseandstatementarchive/2015/july/20150714_PR_MDG6report.
3. Katz IT, Bassett IV, Wright AA. PEPFAR in transition—implications for HIV care in South Africa. N Engl J Med. 2013;369(15):1385–7. doi: 10.1056/NEJMp1310982 24106930
4. U.S. President’s Emergency Plan for AIDS Relief. PEPFAR latest global results—2016. Washington (DC): U.S. President’s Emergency Plan for AIDS Relief; 2016 [cited 2017 May 15]. https://www.pepfar.gov/documents/organization/264882.pdf.
5. Nglazi MD, Lawn SD, Kaplan R, Kranzer K, Orrell C, Wood R, et al. Changes in programmatic outcomes during 7 years of scale-up at a community-based antiretroviral treatment service in South Africa. J Acquir Immune Defic Syndr. 2011;56(1):e1–8. doi: 10.1097/QAI.0b013e3181ff0bdc 21084996
6. South African Department of Health. The South African antiretroviral treatment guidelines—2010. Pretoria: South African Department of Health; 2010 [cited 2017 May 15]. http://apps.who.int/medicinedocs/documents/s19153en/s19153en.pdf.
7. Bekker LG, Venter F, Cohen K, Goemare E, Van Cutsem G, Boulle A, et al. Provision of antiretroviral therapy in South Africa: the nuts and bolts. Antivir Ther. 2014;19(Suppl 3):105–16. doi: 10.3851/IMP2905 25310359
8. Collins C, Beyrer C. Country ownership and the turning point for HIV/AIDS. Lancet Glob Health. 2013;1(6):e319–20. doi: 10.1016/S2214-109X(13)70092-5 25104588
9. South African Department of Health. National consolidated guidelines for the prevention of mother-to-child transmission of HIV (PMTCT) and the management of HIV in children, adolescents and adults Pretoria, South Africa. Pretoria: South African Department of Health; 2014 [cited 2017 May 15]. http://www.kznhealth.gov.za/family/HIV-Guidelines-Jan2015.pdf.
10. United Nations Joint Programme on HIV/AIDS. Country ownership for a sustainable AIDS response: from principles to practice. Geneva: United Nations Joint Programme on HIV/AIDS; 2012 [cited 2017 May 15]. http://www.unaids.org/sites/default/files/sub_landing/files/20120717_JC2134_UNAIDS_Country_Ownership_Discussion_Paper.pdf.
11. Fox MP, Rosen S. A new cascade of HIV care for the era of “treat all”. PLoS Med. 2017;14(4):e1002268. doi: 10.1371/journal.pmed.1002268 28399160
12. Cloete C, Regan S, Giddy J, Govender T, Erlwanger A, Gaynes MR, et al. The linkage outcomes of a large-scale, rapid transfer of HIV-infected patients from hospital-based to community-based clinics in South Africa. Open Forum Infect Dis. 2014;1(2):ofu058. doi: 10.1093/ofid/ofu058 25734128
13. Grimsrud A, Kaplan R, Bekker LG, Myer L. Outcomes of a nurse-managed service for stable HIV-positive patients in a large South African public sector antiretroviral therapy programme. Trop Med Int Health. 2014;19(9):1029–39. doi: 10.1111/tmi.12346 25041716
14. Lawn SD, Little F, Bekker LG, Kaplan R, Campbel E, Orrell C, et al. Changing mortality risk associated with CD4 cell response to antiretroviral therapy in South Africa. AIDS. 2009;23(3):335–42. doi: 10.1097/QAD.0b013e328321823f 19114870
15. Njuguna C, Orrell C, Kaplan R, Bekker LG, Wood R, Lawn SD. Rates of switching antiretroviral drugs in a primary care service in South Africa before and after introduction of tenofovir. PLoS ONE. 2013;8(5):e63596. doi: 10.1371/journal.pone.0063596 23717451
16. Lawn SD, Kaplan R, Wood R, Bekker LG. Promoting retention in care: an effective model in an antiretroviral treatment service in South Africa. Clin Infect Dis. 2007;45(6):803. doi: 10.1086/521173 17712771
17. World Health Organization. WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. Geneva: World Health Organization; 2007 [cited 2017 Aug 3]. http://www.who.int/hiv/pub/guidelines/HIVstaging150307.pdf.
18. Plazy M, Dray-Spira R, Orne-Gliemann J, Dabis F, Newell ML. Continuum in HIV care from entry to ART initiation in rural KwaZulu-Natal, South Africa. Trop Med Int Health. 2014;19(6):680–9. doi: 10.1111/tmi.12301 24654990
19. Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS Med. 2011;8(7):e1001056. doi: 10.1371/journal.pmed.1001056 21811403
20. Clouse K, Pettifor AE, Maskew M, Bassett J, Van Rie A, Behets F, et al. Patient retention from HIV diagnosis through one year on antiretroviral therapy at a primary health care clinic in Johannesburg, South Africa. J Acquir Immune Defic Syndr. 2013;62(2):e39–46. doi: 10.1097/QAI.0b013e318273ac48 23011400
21. Katz IT, Essien T, Marinda ET, Gray GE, Bangsberg DR, Martinson NA, et al. Antiretroviral therapy refusal among newly diagnosed HIV-infected adults. AIDS. 2011;25(17):2177–81. doi: 10.1097/QAD.0b013e32834b6464 21832935
22. World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Geneva: World Health Organization; 2015 [cited 2017 May 16]. http://www.who.int/hiv/pub/guidelines/earlyrelease-arv/en/.
23. Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med. 2016;375(9):830–9. doi: 10.1056/NEJMoa1600693 27424812
24. TEMPRANO ANRS 12136 Study Group, Danel C, Moh R, Gabillard D, Badje A, Le Carrou J, et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373(9):808–22. doi: 10.1056/NEJMoa1507198 26193126
25. Bor J, Herbst AJ, Newell ML, Barnighausen T. Increases in adult life expectancy in rural South Africa: valuing the scale-up of HIV treatment. Science. 2013;339(6122):961–5. doi: 10.1126/science.1230413 23430655
26. Grinsztejn B, Hosseinipour MC, Ribpaudo HJ, Swindells S, Eron J, Chen YQ, et al. Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: results from the phase 3 HPTN 052 randomised controlled trial. Lancet Infect Dis. 2014;14(4):281–90. doi: 10.1016/S1473-3099(13)70692-3 24602844
27. Plazy M, Dabis F, Naidu K, Orne-Gliemann J, Barnighausen T, Dray-Spira R. Change of treatment guidelines and evolution of ART initiation in rural South Africa: data of a large HIV care and treatment programme. BMC Infect Dis. 2015;15 : 452. doi: 10.1186/s12879-015-1207-2 26497054
28. Haber N, Tanser F, Bor J, Naidu K, Mutevedzi T, Herbst K, et al. From HIV infection to therapeutic response: a population-based longitudinal HIV cascade-of-care study in KwaZulu-Natal, South Africa. Lancet HIV. 2017;4(5):e223–30. doi: 10.1016/S2352-3018(16)30224-7 28153470
29. Katz IT, Bogart LM, Cloete C, Crankshaw TL, Giddy J, Govender T, et al. Understanding HIV-infected patients’ experiences with PEPFAR-associated transitions at a Centre of Excellence in KwaZulu Natal, South Africa: a qualitative study. AIDS Care. 2015;27(10):1298–303. doi: 10.1080/09540121.2015.1051502 26300297
30. Katz IT, Dietrich J, Tshabalala G, Essien T, Rough K, Wright AA, et al. Understanding treatment refusal among adults presenting for HIV-testing in Soweto, South Africa: a qualitative study. AIDS Behav. 2015;19(4):704–14. doi: 10.1007/s10461-014-0920-y 25304330
31. Fox MP, Mazimba A, Seidenberg P, Crooks D, Sikateyo B, Rosen S. Barriers to initiation of antiretroviral treatment in rural and urban areas of Zambia: a cross-sectional study of cost, stigma, and perceptions about ART. J Int AIDS Soc. 2010;13 : 8. doi: 10.1186/1758-2652-13-8 20205930
32. Duff P, Kipp W, Wild TC, Rubaale T, Okech-Ojony J. Barriers to accessing highly active antiretroviral therapy by HIV-positive women attending an antenatal clinic in a regional hospital in western Uganda. J Int AIDS Soc. 2010;13 : 37. doi: 10.1186/1758-2652-13-37 20863399
33. Katz IT, Dietrich JJ, Bogart LM, Leone D, Courtney I, Tshabalala G, et al. A prospective multi-site cohort study of pre-ART losses and ART refusal in South Africa. Abstract number 1014. Conference on Retroviruses and Opportunistic Infections; 2016 Feb 22–25; Boston, MA, US. 2016 [cited 2017 Oct 13]. http://www.croiconference.org/sessions/prospective-multisite-cohort-study-pre-art-losses-and-art-refusal-south-africa.
34. Mugglin C, Estill J, Wandeler G, Bender N, Egger M, Gsponer T, et al. Loss to programme between HIV diagnosis and initiation of antiretroviral therapy in sub-Saharan Africa: systematic review and meta-analysis. Trop Med Int Health. 2012;17 : 1509–20. doi: 10.1111/j.1365-3156.2012.03089.x 22994151
35. Lippman SA, Shade SB, El Ayadi AM, Gilvydis JM, Grignon JS, Liegler T, et al. Attrition and opportunities along the HIV care continuum: findings from a population-based sample, North West Province, South Africa. J Acquir Immune Defic Syndr. 2016;1;73(1):91–9. doi: 10.1097/QAI.0000000000001026 27082505
36. Fox MP, Shearer K, Maskew M, Meyer-Rath G, Clouse K, Sanne I. Attrition through multiple stages of pre-treatment and ART HIV care in South Africa. PLoS ONE. 2014;9(10): e110252. doi: 10.1371/journal.pone.0110252 25330087
Štítky
Interní lékařství
Článek Contemporary disengagement from antiretroviral therapy in Khayelitsha, South Africa: A cohort studyČlánek Bioequivalence of twice-daily oral tacrolimus in transplant recipients: More evidence for consensus?
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2017 Číslo 11- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Antikoagulační léčba u pacientů před operačními výkony
-
Všechny články tohoto čísla
- Labour trafficking: Challenges and opportunities from an occupational health perspective
- The end of HIV: Still a very long way to go, but progress continues
- Contemporary disengagement from antiretroviral therapy in Khayelitsha, South Africa: A cohort study
- Bioequivalence of twice-daily oral tacrolimus in transplant recipients: More evidence for consensus?
- Treatment guidelines and early loss from care for people living with HIV in Cape Town, South Africa: A retrospective cohort study
- Perinatal mortality associated with induction of labour versus expectant management in nulliparous women aged 35 years or over: An English national cohort study
- Core Outcome Set-STAndards for Development: The COS-STAD recommendations
- Closing the gaps in the HIV care continuum
- Association between the 2012 Health and Social Care Act and specialist visits and hospitalisations in England: A controlled interrupted time series analysis
- HIV pre-exposure prophylaxis and early antiretroviral treatment among female sex workers in South Africa: Results from a prospective observational demonstration project
- Sexual exploitation of unaccompanied migrant and refugee boys in Greece: Approaches to prevention
- Child sex trafficking in the United States: Challenges for the healthcare provider
- The expanding epidemic of HIV-1 in the Russian Federation
- Cardiovascular disease (CVD) and chronic kidney disease (CKD) event rates in HIV-positive persons at high predicted CVD and CKD risk: A prospective analysis of the D:A:D observational study
- Validity of a minimally invasive autopsy for cause of death determination in maternal deaths in Mozambique: An observational study
- malERA: An updated research agenda for malaria elimination and eradication
- malERA: An updated research agenda for health systems and policy research in malaria elimination and eradication
- A combination intervention strategy to improve linkage to and retention in HIV care following diagnosis in Mozambique: A cluster-randomized study
- Bioequivalence between innovator and generic tacrolimus in liver and kidney transplant recipients: A randomized, crossover clinical trial
- malERA: An updated research agenda for basic science and enabling technologies in malaria elimination and eradication
- Human trafficking and exploitation: A global health concern
- Virological response and resistance among HIV-infected children receiving long-term antiretroviral therapy without virological monitoring in Uganda and Zimbabwe: Observational analyses within the randomised ARROW trial
- Postmenopausal hormone therapy and risk of stroke: A pooled analysis of data from population-based cohort studies
- Lansoprazole use and tuberculosis incidence in the United Kingdom Clinical Practice Research Datalink: A population based cohort
- malERA: An updated research agenda for insecticide and drug resistance in malaria elimination and eradication
- Safety, pharmacokinetics, and immunological activities of multiple intravenous or subcutaneous doses of an anti-HIV monoclonal antibody, VRC01, administered to HIV-uninfected adults: Results of a phase 1 randomized trial
- HIV prevalence and behavioral and psychosocial factors among transgender women and cisgender men who have sex with men in 8 African countries: A cross-sectional analysis
- Treatment eligibility and retention in clinical HIV care: A regression discontinuity study in South Africa
- malERA: An updated research agenda for characterising the reservoir and measuring transmission in malaria elimination and eradication
- Effectiveness of a combination strategy for linkage and retention in adult HIV care in Swaziland: The Link4Health cluster randomized trial
- The value of confirmatory testing in early infant HIV diagnosis programmes in South Africa: A cost-effectiveness analysis
- HIV self-testing among female sex workers in Zambia: A cluster randomized controlled trial
- The US President's Malaria Initiative, transmission and mortality: A modelling study
- Comparison of two cash transfer strategies to prevent catastrophic costs for poor tuberculosis-affected households in low- and middle-income countries: An economic modelling study
- Direct provision versus facility collection of HIV self-tests among female sex workers in Uganda: A cluster-randomized controlled health systems trial
- malERA: An updated research agenda for diagnostics, drugs, vaccines, and vector control in malaria elimination and eradication
- malERA: An updated research agenda for combination interventions and modelling in malaria elimination and eradication
- HIV-1 persistence following extremely early initiation of antiretroviral therapy (ART) during acute HIV-1 infection: An observational study
- Respondent-driven sampling for identification of HIV- and HCV-infected people who inject drugs and men who have sex with men in India: A cross-sectional, community-based analysis
- Extensive virologic and immunologic characterization in an HIV-infected individual following allogeneic stem cell transplant and analytic cessation of antiretroviral therapy: A case study
- Measuring success: The challenge of social protection in helping eliminate tuberculosis
- Prospects for passive immunity to prevent HIV infection
- Reaching global HIV/AIDS goals: What got us here, won't get us there
- Evidence-based restructuring of health and social care
- Extreme exploitation in Southeast Asia waters: Challenges in progressing towards universal health coverage for migrant workers
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Postmenopausal hormone therapy and risk of stroke: A pooled analysis of data from population-based cohort studies
- Bioequivalence between innovator and generic tacrolimus in liver and kidney transplant recipients: A randomized, crossover clinical trial
- HIV pre-exposure prophylaxis and early antiretroviral treatment among female sex workers in South Africa: Results from a prospective observational demonstration project
- Bioequivalence of twice-daily oral tacrolimus in transplant recipients: More evidence for consensus?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



