-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaDirect provision versus facility collection of HIV self-tests among female sex workers in Uganda: A cluster-randomized controlled health systems trial
In a cluster-randomized trial, Katrina Ortblad and colleagues study the provision of HIV self-tests for female sex workers in Uganda.
Published in the journal: . PLoS Med 14(11): e32767. doi:10.1371/journal.pmed.1002458
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002458Summary
In a cluster-randomized trial, Katrina Ortblad and colleagues study the provision of HIV self-tests for female sex workers in Uganda.
Introduction
HIV testing is the important first step for both HIV treatment and HIV prevention interventions [1–5]. In sub-Saharan Africa, 20%–30% of people who are HIV positive still do not know their HIV status and can thus not utilize lifesaving HIV treatment [1,2,5–9]. Among people who are HIV-negative, frequent HIV testing is key to HIV prevention. In particular, frequent HIV testing is a necessary condition for treatment as prevention (TasP) [3,10], behavior change to prevent transmission [11,12], and HIV pre-exposure prophylaxis (PrEP) [4,13].
Frequent HIV testing is especially important for those key populations that face the highest HIV risks. In sub-Saharan Africa, the largest key population is female sex workers (FSWs) and their clients [1]. FSWs in sub-Saharan Africa have an approximately 5 times higher prevalence of HIV than the general population [14–17]. The World Health Organization recommends that FSWs test for HIV every 3 months [18,19], but many FSW communities in sub-Saharan Africa do not achieve this standard [20–22]. Frequently cited barriers to HIV testing for FSWs include healthcare provider stigma and discrimination [23–26], transport costs [16], and inconvenient location [16,24] and opening hours of testing centers [24]. HIV self-testing may allow FSWs to overcome several of these barriers: it does not require an interaction with a health worker, it does not require travel to a facility, and it can be carried out in any space and at any time.
Despite these advantages, few sub-Saharan African countries have introduced HIV self-testing because of concerns related to the specific features of self-testing: it decouples testing from standard pre - and post-test HIV counselling and it places testing outside healthcare facilities, in which other services are available [27,28]. As a result, HIV self-testing may lead to more frequent social or emotional harms following a test result and decrease linkage to HIV treatment and prevention services. Another important reason for governments’ reluctance to commit to HIV self-testing policies is that evidence on the impact of HIV self-testing on key populations is currently lacking [27,28].
The uptake of HIV self-testing will likely depend on the approach that is used to deliver it. The delivery model will determine the extent to which HIV self-testing can overcome the different types of barriers to HIV testing, such provider stigma, transport costs and inconvenient locations and opening hours of testing centers. In this study, we aimed to establish the effectiveness of HIV self-testing in increasing recent and repeat HIV testing among FSWs using 2 delivery models: (1) direct peer provision of an oral HIV self-test and (2) peer provision of a coupon for free collection of an oral HIV self-test in a healthcare facility.
We used FSWs who were trained as peer educators for this study because they have good access to other FSWs and are able to engage with FSWs who do not normally utilize the health system. FSWs tend to trust other FSWs, and trust is important in the context of introducing a new healthcare intervention and technology that may initially be perceived as threatening [29]. Additionally, FSW peer educators are a realistic platform for the future scale-up of HIV self-testing strategies [30]; they have previously been used to deliver many types of health interventions to FSWs in sub-Saharan African [30–33].
The direct provision of HIV self-tests to FSWs fully realizes the advantages of self-testing (i.e., testing that is independent of facility hours and location and does not require a health worker or any other person). A disadvantage of this model is, however, that it decouples HIV testing from the health system, in which counseling and HIV treatment and prevention services are provided. Without counselling following an HIV test, mental distress may be more common; without proximity to HIV treatment services, linkage to care may be delayed.
Facility-based collection of HIV self-tests is more like standard facility-based testing than the direct provision of HIV self-tests because it requires FSWs to go to a healthcare facility to be able to test for HIV. We included facility-based collection in our study because it does allow FSWs to freely choose the time and place of testing and because it closely resembles the standard HIV self-testing model that many countries will adopt—passive provision of HIV self-tests in facilities rather than active delivery of HIV self-tests to FSWs by peer educators.
A number of exploratory studies in sub-Saharan Africa have shown high acceptability and good performance of HIV self-testing [34–44]. Several HIV self-testing trials have been carried out in the general population [45] and in 2 key populations—the male partners of women attending antenatal care [46–49] and men who have sex with men [49–53]. Jointly with a concurrent study [54], this trial is to our knowledge the first to investigate the effects of HIV self-testing among FSWs in sub-Saharan Africa.
Methods
Ethics statement
Ethical approval for the study was granted by the Institutional Review Board at the Harvard T.H. Chan School of Public Health (IRB16-0885) and Mildmay Uganda Research Ethics Committee (REF 0105–2016).
Study setting
This study was conducted in Kampala, the capital city of Uganda, which has an estimated 13,000 FSWs [55,56]. About 1 in 3 FSWs in Uganda is HIV positive [55,56]. FSWs are a particular focus of the Ugandan Ministry of Health (MOH) for health and HIV interventions. In Kampala, FSWs have access to a range of HIV testing options through the country’s Most at Risk Populations Initiative (MARPI), including facility-, home-, and work-based HIV testing [57]. There are also 4 nongovernmental organizations (NGOs) operating within Kampala that use peer interventions to mobilize FSWs for HIV prevention and help provide health services and economic opportunities. To date, the Ugandan government has not issued a guideline or policy for HIV self-testing [58,59].
Study design
This 3-arm cluster-randomized controlled trial was designed to establish the effect of 2 different HIV self-testing delivery models for FSWs on HIV testing and linkage outcomes. All participating FSWs were organized into peer educator groups (1 peer educator and 8 FSWs). Peer educator groups were randomized into 1 of 3 study arms: (1) direct provision of an HIV self-test, (2) a coupon for free collection of an HIV self-test in a healthcare facility, and (3) standard of care HIV testing. Our study protocol and the prespecified primary and secondary outcomes of this trial can be found in the clinical trials registry and database run by the United States National Library of Medicine at the National Institutes of Health, ClinicalTrials.gov (NCT02846402). The trial’s Manual of Operations and Procedures is included in the Supporting Information (S1 Text).
FSW peer educators
All FSW peer educators in this study were affiliated with existing FSW NGOs or MARPI clinics throughout Kampala. There was no educational or age requirement for FSW peer educators; trust and respect within the local FSW community (determined by FSW NGO leaders and MARPI clinic coordinators) were the most important prerequisites. To ensure wide representation of FSWs in Kampala, an even number of peers (24) were recruited from each of the 5 Kampala divisions. Prior to participant enrollment, all peer educators completed a 2-day training during which they learned how to instruct participants to use the oral HIV self-test, interpret the results, and encourage linkage to HIV treatment and prevention services. The peer educators recruited potential study participants and referred them to research assistants, who first conducted a phone screening followed by an in-person eligibility assessment and enrollment. Peer educators were encouraged to recruit FSWs they already knew to ensure trust. We considered trust between the people delivering the trial interventions and the participants to be particularly important, because HIV self-testing is a new technology and might be feared to be harmful. The peer educators received 90,000 UGX per visit. At market exchange rates, this amount of UGX is about US$25; after adjusting for purchasing power parity (PPP), this amount of UGX equals about US$79 [60].
Study participants
Participants were eligible for trial enrollment if they (1) were 18 years or older; (2) reported the exchange of any vaginal, anal, or oral sex for money, goods, or other items of value; (3) said that they had never tested for HIV or self-reported negative HIV status without a recent HIV test (in the past 3 months); (4) had been working as FSWs in Kampala for at least 1 month prior to enrollment and planned on continuing to work as FSWs in Kampala for at least the next 4 months; and (5) had never used an oral HIV self-test. All eligible participants provided written informed consent; participants had to be of sound mind (i.e., not under the influence of drugs or alcohol) to be eligible for the informed consent process.
Peer educator groups
We used peer educators to recruit participants for our study. Peer educators are a common and tested approach for providing interventions to FSWs [27], including in public sector health systems in sub-Saharan Africa [30–33].
In Kampala, we had access to established FSW peer educators, who we could recruit and train to deliver our intervention. The use of peer educator groups in this trial guarded against intervention spillover effects. FSWs often cluster in particular locations (e.g., guest houses or brothels). Peers are likely to recruit from one such location and within pre-existing social networks. The peer educator-based trial assignment thus makes it more likely that any information (or HIV self-test) that is shared is shared among FSWs belonging to the same arm of the trial. The peer educator groups met once during the first peer educator visit; all subsequent peer educator visits were individual visits.
Randomization procedures
We randomized FSW peer educator groups 1 : 1:1 to 1 of 3 study arms. One of the authors, CEO, developed the randomization list using R Studio (Version 3.3.1, The R Foundation for Statistical Computing, Vienna, Austria) [61]. To ensure balance across study arms, we stratified the list by Kampala’s 5 divisions and evenly recruited peers from the divisions. To increase the probability that equal numbers of FSWs were allocated to all 3 study arms within each division, we used block randomization (using block sizes of 3, 6, and 9). Once the peer educator groups had been formed, the research assistants opened sealed randomization envelopes to assign the groups to one of the trial arms. Research assistants, peer educators, and participants were masked to study arm assignment prior to opening the envelopes.
Interventions
Fig 1 shows the study intervention activities in chronological order. Research assistants enrolled eligible participants. The research assistants gave all participants a referral card for facility-based HIV testing and a study card. The referral card could be used for HIV testing in 10 participating private healthcare facilities, which were evenly dispersed throughout Kampala’s 5 divisions. The study card included a toll-free hotline number, which participants were encouraged to call for referral to standard of care HIV testing and treatment services and to report potential adverse events. Participants in the HIV self-testing intervention arms were also encouraged to call the hotline number if they had any questions or concerns related to HIV self-testing. Individuals working at the hotline received training on HIV self-testing and the study procedures prior to participant enrollment.
Fig. 1. Time line of study interventions and assessments (conducted by research assistants, in blue). 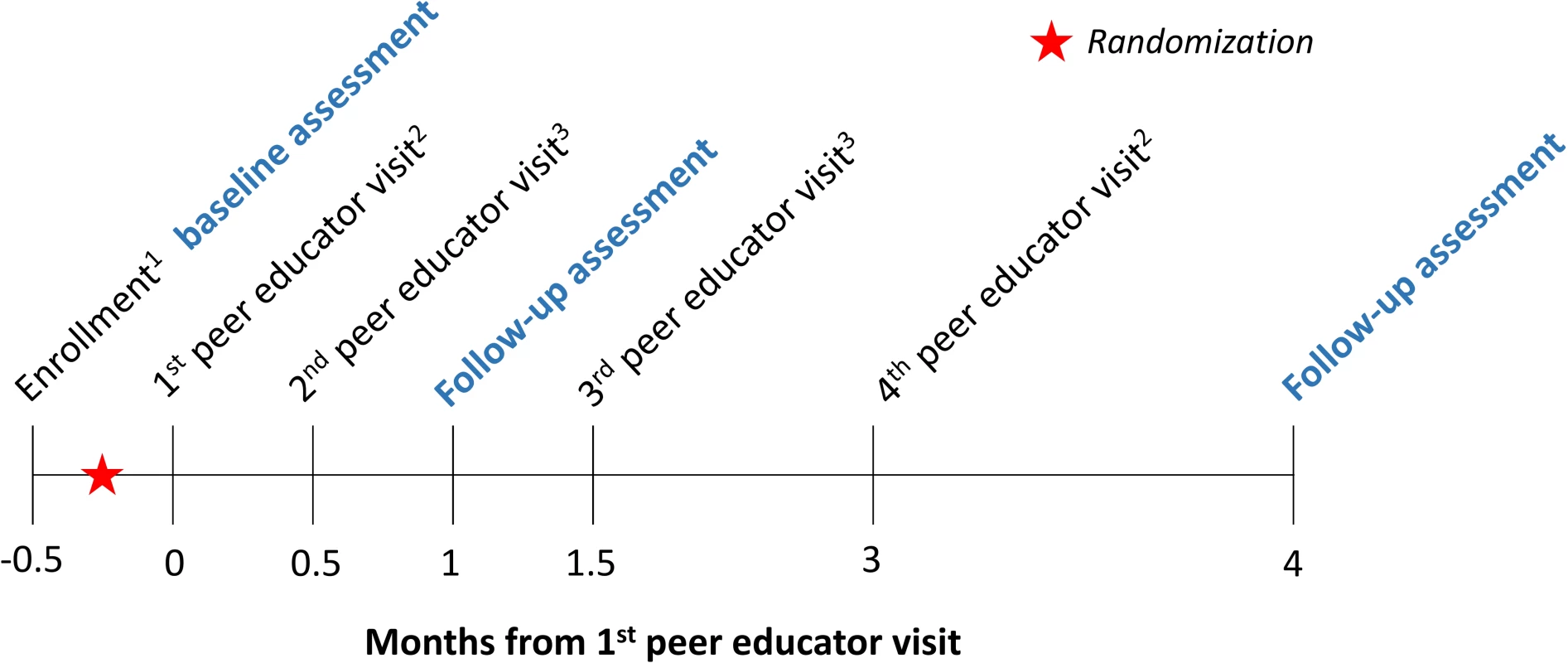
Participants were randomized in groups of 1 peer educator and 8 FSWs. The text following the subscripts below describe details about each peer educator visit: 1Research assistants gave all participants a referral card for free HIV testing and a study contact card. The referral card could be used at 10 private healthcare facilities participating in this study. The study contact card included a toll-free hotline number, which participants could call for information about linkage to care, to report potential adverse events, or to ask questions related to HIV self-testing (intervention arms only). 2The peer educators gave all participants condoms. In the direct provision arm, the peer educators additionally gave the participants oral HIV self-tests; in the facility collection arm, participants received coupons, which they could exchange for an HIV self-test at the participating healthcare facilities. 3The peer educators gave all participants condoms. The peer educators were scheduled to visit participants 4 times over the duration of the study. At all visits, the peer educators were to distribute condoms, refer participants to standard of care HIV testing services (including all public and private HIV testing facilities), and screen for potential adverse events. The first peer educator visit was a group visit so that the peer educators in the HIV self-testing intervention arms could provide participants with HIV self-test training, including self-test use, results interpretation, and linkage to care. The peer educators were trained to instruct participants that they should go to a healthcare facility for confirmatory testing if they said that they were HIV positive. The peer educators were also trained to instruct participants to test again 3 months after testing HIV negative. Research assistants observed the first peer educator visits to ensure participants received consistent information on oral HIV self-testing. The following 3 peer educator visits (at 2 weeks, at 1.5 months, and at 3 months) were individual visits to safeguard the confidentiality of participants who had learned that they were HIV-positive or wanted to report adverse events (Fig 1). Research assistants were instructed to call peer educators before and after each scheduled visit to ensure the visits happened and happened on time.
The peer educators in the intervention arms were trained to give participants in the intervention arms either 2 oral fluid rapid HIV self-tests (direct provision arm) or 2 coupons for free HIV self-tests (facility collection arm) over the duration of the study. Participants were to receive the first HIV self-test or coupon at the first peer educator visit and the second 3 months later, at the fourth peer educator visit. We used the OraQuick Rapid HIV-1/2 Antibody Test (OraSure Technologies, Bethlehem, PA); each test included pictorial and written step-by-step instructions (in the local language, Luganda, as well as in English) on test use, results interpretation, and follow-up care.
The participants in the facility collection arm of this study could exchange the coupon they received for HIV self-tests in the 10 private healthcare facilities described above. All 10 healthcare facilities were affiliated with our implementing partner, the Ugandan Health Marketing Group, which enabled us to closely monitor the distribution of the oral HIV self-test. Prior to enrollment, the research assistants trained the health workers in these 10 facilities in oral HIV self-test use and the study procedures. To minimize the risk of stigma and discrimination, the healthcare workers also received FSW sensitization training, which included skill building on eye contact, appropriate body language, and nonjudgmental spoken language. Two of the 10 private healthcare facilities provided free antiretroviral treatment for people who were HIV-positive.
Assessments
The research assistants conducted 3 quantitative outcome assessments with each study participant over the course of the study: a baseline assessment (post-enrollment and prior to randomization) and 2 follow-up assessments after the first peer educator visit (one at 1 month and the other at 4 months, Fig 1). The baseline assessment included questions on sociodemographic characteristics, sex work history, sexual behaviors, and past HIV testing (timing and location) as well as intimate partner violence (IPV). We measured both physical and sexual IPV. To measure physical IPV, we asked participants if a sexual partner had hit, slapped, punched, or shoved them or had done anything else to physically hurt them in the past month. To measure sexual IPV, we asked participants if they had sex against their will in the past month. The follow-up assessments included identical questions on sexual behavior, HIV testing, and IPV. Additionally, these follow-up assessments included questions about HIV self-test use and linkage to care. All data were collected electronically in face-to-face interviews using the CommCare electronic data platform (Dimagi Inc, Cambridge, MA). At the 4-month assessment, participants were offered free membership into a Kampala-based FSW organization (if they were not already a member). Upon completion of each assessment, participants received 16,500 Ugandan Shillings (UGX) (about 14 purchasing power parity [PPP]-adjusted US dollars [60]) as compensation for their time.
Outcomes
Our prespecified primary outcomes were any HIV testing following the first peer educator visit, measured at 1 month and at 4 months. To measure these outcomes, we asked participants when they last tested for HIV (last month, 2–3 months, >3–6 months, >6–12 months, >12 months, never tested) at each follow-up assessment. Our prespecified secondary outcomes were HIV self-test use, seeking HIV-related medical care, and ART initiation at 1 month and at 4 months. We also assessed adverse events related to HIV self-testing. In addition to the prespecified outcomes, we analyzed 2 additional secondary outcomes (at 1 month and at 4 months): to understand the intervention effects on frequent testing, we assessed testing twice for HIV; and to quantify substitution effects, we assessed facility-based HIV testing. Facility-based testing, i.e., testing in any public or private facility, was determined using a question about the location of HIV testing.
At both follow-up assessments, the research assistants asked participants to self-report the result of their last HIV test (HIV negative, HIV positive, inconclusive, unsure). If participants reported an HIV-positive test result, the research assistants asked them if they sought medical care for their HIV infection and if they were taking antiretroviral medicines. Throughout the study, the research assistants, hotline attendants, and peer educators screened for adverse events related to study participation or HIV self-testing, including physical, sexual, or verbal assault; unintentional HIV status disclosure; and self-harm.
Sample size
Each study arm received the same number of participants and peer educator groups. We used standard approaches for power calculations for cluster-randomized controlled trials [62]. Assuming 60% HIV testing 1 month following the first peer educator visit in the standard of care arm, 25% loss to follow-up for all participants, type I error probability of 0.05, and an intracluster correlation coefficient (ICC) of 0.02, we estimated that 960 participants in 120 peer educator groups would yield 90% power to detect a risk ratio (RR) of 1.25 in the pooled HIV self-testing arms compared to the standard of care arm. This sample size was also estimated to yield 90% power to detect an RR of 1.18 or larger in the direct provision arm compared to the facility collection arm.
Statistical analyses
All results are based on intention to treat (ITT) [63–65], complete-case analyses conducted at the unit of the individual with standard errors adjusted for clustering at the peer educator level. Our prespecified analyses were mixed-effect multilevel regression models. We calculated RRs for all binary outcomes using mixed-effects generalized linear models (with Poisson distribution, log link, and robust standard errors) [66], using a fixed effect for study arm and a random effect for peer educator (S2 Table). We chose to use modified Poisson models over log-binomial models because they generate similar outcomes and converge more easily when study outcomes are relatively common [66]. We also calculated risk differences for all binary outcomes using the mixed-effects generalized linear models with a fixed effect for study arm and a random effect for peer educator (S3 Table). All statistical tests were 2-sided with p < 0.05 considered statistically significant.
We conducted 4 sensitivity analyses. First, we pooled the data from the 2 intervention arms and calculated RRs for all outcomes that compared this pooled arm with the standard of care arm using the mixed-effects generalized linear models specified above (S4 Table). Second, we calculated the proportion of participants who presented each outcome in a peer educator group. We then used generalized linear models with study arm fixed effects to calculate risk differences for these outcome measures (S5 Table). Third, we redefined our outcomes at 4 months to cover shorter time periods than the entire observation period (i.e., the past 1 month, the past 3 months), where such data were available, and calculated RRs using the mixed-effects generalized linear models (described above) (S6 and S7 Tables). Fourth, for the linkage to care outcomes, we limited the sample to participants who reported testing HIV positive and calculated RRs (S8 and S9 Tables). We used Stata 13.1 (StataCorp, College Station, TX) for all analyses.
Results
Participants
From October to November 2016, 1,587 potential participants were assessed for eligibility via phone screening, of whom 997 were assessed in person for eligibility and 960 were enrolled in the trial (Fig 2). The most common reasons for exclusion from the study were HIV testing within 3 months (325/627, 52%) and self-reported positive HIV status (267/627, 43%). Of the enrolled 960 participants, 296 (31%) were randomized to the direct provision arm, 336 (35%) to the facility collection arm, and 328 (34%) to the standard of care arm. Participant baseline characteristics were balanced across the 3 study arms (Table 1). Participant retention at 1 and 4 months was 96.4% (925/960) and 89.7% (861/960), respectively. At 4 months, 18.7% (161/861) of all participants were already members of an FSW peer organization in Kampala. There were no statistically significant differences in loss to follow-up across study arms (S1 Table).
Fig. 2. Participant recruitment, eligibility, randomization, and follow-up. 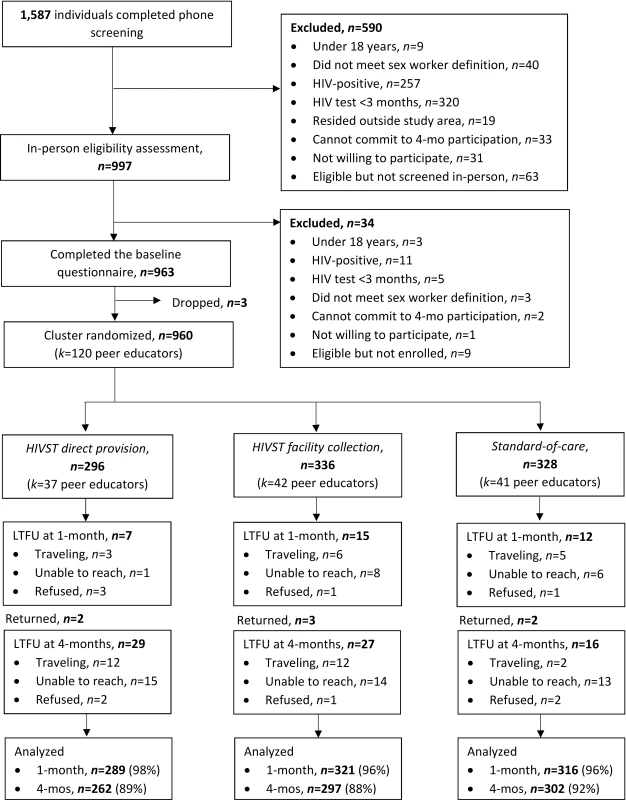
CONSORT flow diagram. HIVST, HIV self-test; n, number; k, clusters; LTFU, loss to follow-up; mo, month. Tab. 1. Participant baseline descriptive characteristics. 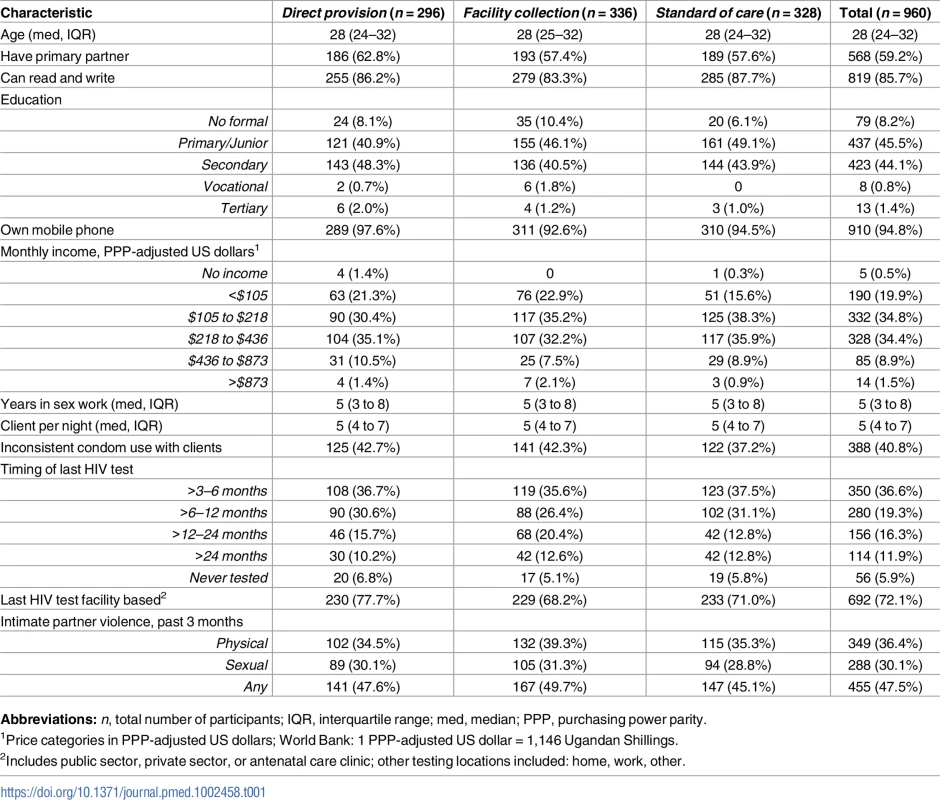
Abbreviations: n, total number of participants; IQR, interquartile range; med, median; PPP, purchasing power parity. Implementation activities
All peer educators reported completing 4 peer educator visits to research assistants over the duration of the study. At 4 months, 88.9% (233/262) of participants in the direct provision arm reported receiving 2 HIV self-tests and 89.9% (267/297) of participants in the facility collection arm reported receiving 2 coupons from their peer educator since the beginning of the study (Table 2). Only 4 participants, 2 in the direct provision arm and 2 in the facility collection arm, reported that they did not receive HIV self-tests or coupons from their peer educator. Among participants in the facility collection arm, 72.4% (215/297) reported exchanging 2 coupons for an HIV self-test at a participating healthcare facility and 4.7% (14/297) reported that they did not exchange any coupons for HIV self-tests (Table 2). The vast majority of all participants reported receiving condoms at every peer educator visit and there were no statistically significant differences across study arms (Table 2).
Tab. 2. Implementation activities reported by participants at 4 months. 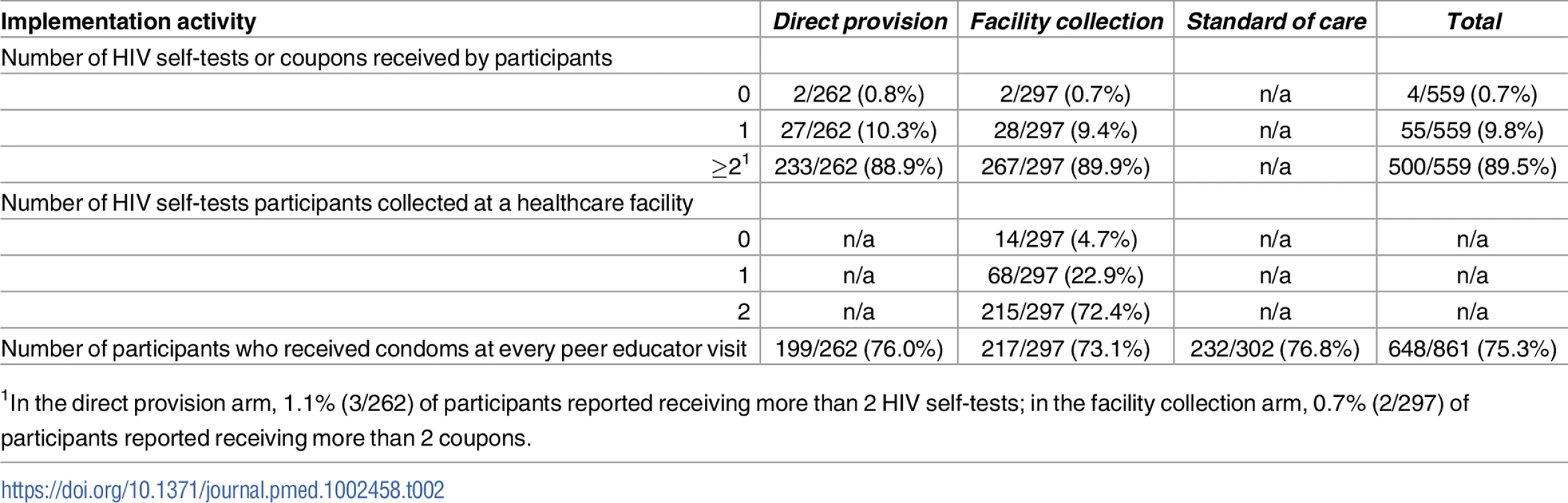
1In the direct provision arm, 1.1% (3/262) of participants reported receiving more than 2 HIV self-tests; in the facility collection arm, 0.7% (2/297) of participants reported receiving more than 2 coupons. Effects on HIV testing outcomes
The effects of HIV self-testing on our prespecified primary outcomes, any HIV testing measured at 1 month and 4 months, were greatest in the direct provision arm, followed by the HIV self-testing facility collection arm and the standard of care arm (“Tested for HIV” in Table 3). At 1 month, participants in the direct provision arm were significantly more likely to test for HIV compared to both those in the standard of care arm (RR 1.33, 95% confidence interval [CI] 1.17 to 1.51, p < 0.001; risk difference in, percentage points [PP] 24.2, 95% CI 13.9 to 34.5, p < 0.001) and those in the facility collection arm (RR 1.18, 95% CI 1.07 to 1.31, p = 0.001; PP 14.6, 95% CI 4.4 to 24.9; p = 0.005) (“Tested for HIV” in Fig 3, S2 and S3 Tables). There were no statistically significant differences in this outcome between participants in the facility collection arm and the standard of care arm (RR 1.12, 95% CI 0.96 to 1.32, p = 0.15; PP 9.6, 95% CI −0.4 to 19.6, p = 0.06). These effect sizes imply that, compared to the standard of care arm at 1 month, 1 additional participant tested for HIV for every 4 (95% CI 3–7) HIV self-tests (in the direct delivery arm); and, had there been a significant increase in testing at 1 month in the facility collection arm, 1 additional participant could have tested for HIV for every 10 (95% CI 5–250) coupons (in the facility collection arm). The observed ICC for any HIV testing at 1 month was 0.45 (95% CI 0.33–0.58).
Fig. 3. Effect size estimates for impact of HIV self-testing on any HIV testing. 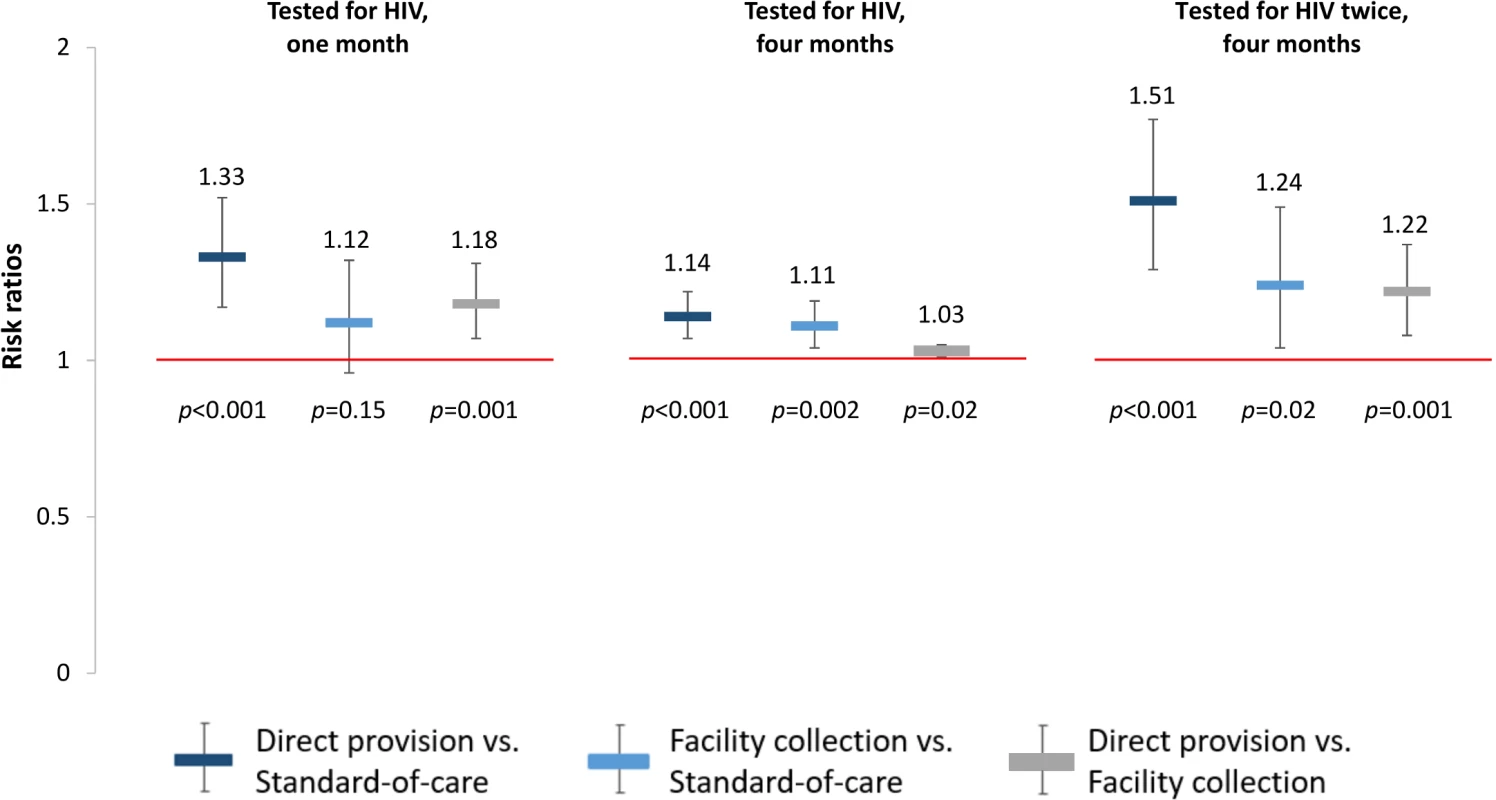
All outcomes since study start. Prespecified primary outcomes were any testing at 1 month and at 4 months. Comparisons between study arms: direct provision versus standard of care (dark blue), facility collection versus standard of care (light blue), direct provision versus facility collection (gray). Tab. 3. Primary and secondary study outcomes at 1 month and 4 months. 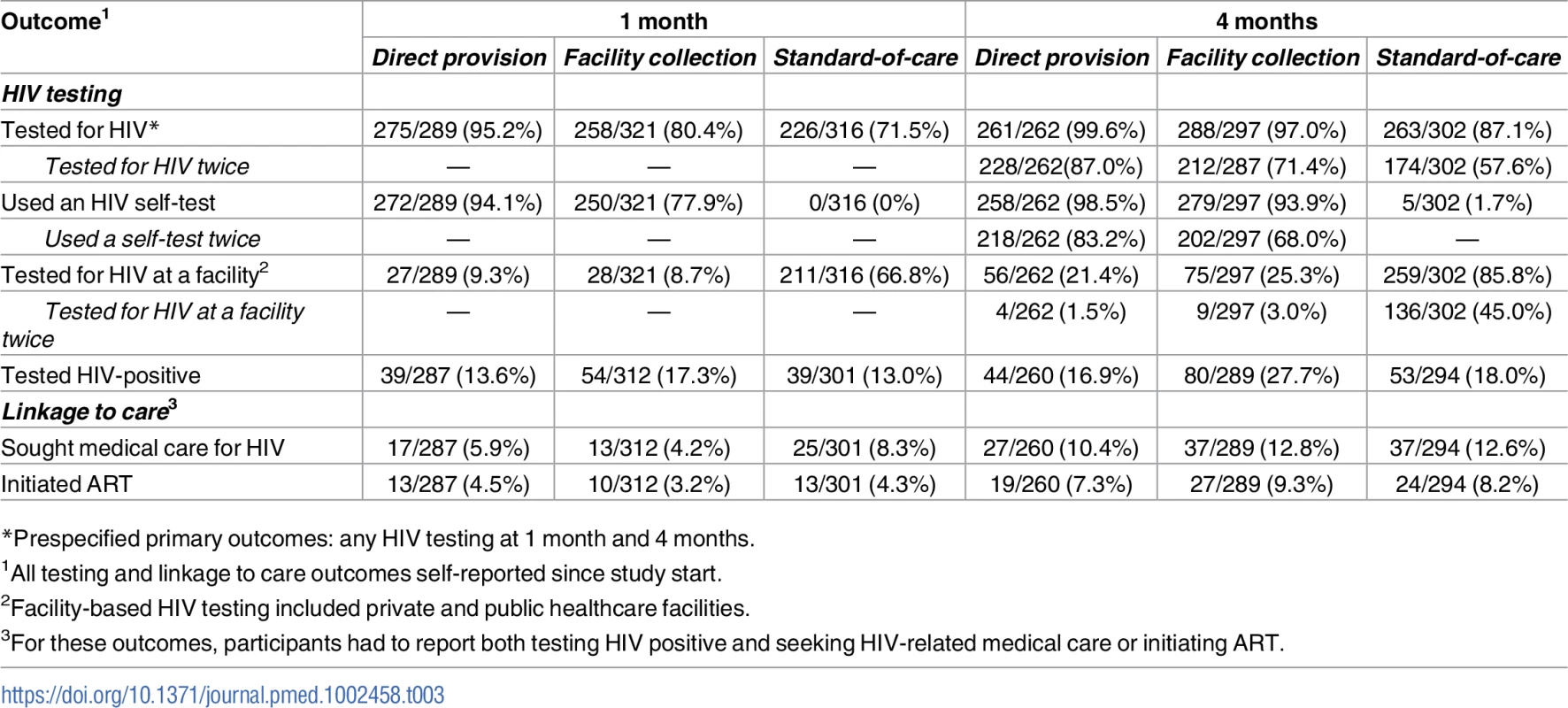
*Prespecified primary outcomes: any HIV testing at 1 month and 4 months. At 4 months, participants in the direct provision arm were more likely to test for HIV compared to both those in the standard of care arm (RR 1.14, 95% CI 1.07–1.22, p < 0.001; PP 12.9, 95% CI 7.6–18.2, p < 0.001) and those in the facility collection arm (RR 1.03, 95% CI 1.01–1.05, p = 0.02; PP 2.6, 95% CI −2.7–7.8, p = 0.34). Participants in the facility collection arm were significantly more likely to test for HIV at 4 months compared to those in the standard of care arm (RR 1.11, 95% CI 1.04–1.19, p = 0.002; PP 10.3, 95% CI 5.2–15.4, p < 0.001) (“Tested for HIV” in Fig 3, S2 and S3 Tables). These effect sizes imply that, compared to standard of care at 4 months, 1 additional participant tested for HIV for every 8 (95% CI 6–13) HIV self-tests (in the direct delivery arm) and for every 10 (95% CI 7–20) coupons (in the facility collection arm).
To better understand the effects of the HIV self-testing interventions, we also determined the effect of the interventions on repeated HIV testing. At 4 months, participants in both self-testing intervention arms were significantly more likely to have tested for HIV twice compared to those in the standard of care arm (direct provision RR 1.51, 95% CI 1.29–1.77, p < 0.001; facility collection RR 1.24, 95% CI 1.04–1.49, p = 0.02), and participants in the direct provision arm were significantly more likely to have tested for HIV twice compared to those in the facility collection arm (RR 1.22, 95% CI 1.08–1.37, p = 0.001) (“Tested for HIV twice” in Fig 3, S2 and S3 Tables). Finally, to better understand the relationship between the availability of the HIV self-testing technology and HIV testing outcomes, we additionally assessed the effect of the HIV self-testing interventions on facility-based testing. Facility-based testing was significantly lower among participants in the HIV self-testing intervention arms compared to the standard of care arm, both at 1 month (direct provision RR 0.14, 95% CI 0.09–0.22, p < 0.001; facility collection RR 0.13, 95% CI 0.08–0.21, p < 0.001) and at 4 months (direct provision RR 0.25, 95% CI 0.18–0.34, p < 0.001; facility collection RR 0.29, 95% CI: 0.23–0.37, p < 0.001), (“Tested for HIV at a facility” in Fig 4, S2 and S3 Tables). In the standard of care arm, private facility-based testing was more common than public facility-based testing at both 1 month (private 40.5%, 128/316; public 26.6%, 83/316) and 4 months (private 56.6%, 171/302; public 31.8%, 96/302) (S10 and S11 Tables).
Fig. 4. Effect size estimates for impact of HIV self-testing on facility-based HIV testing. 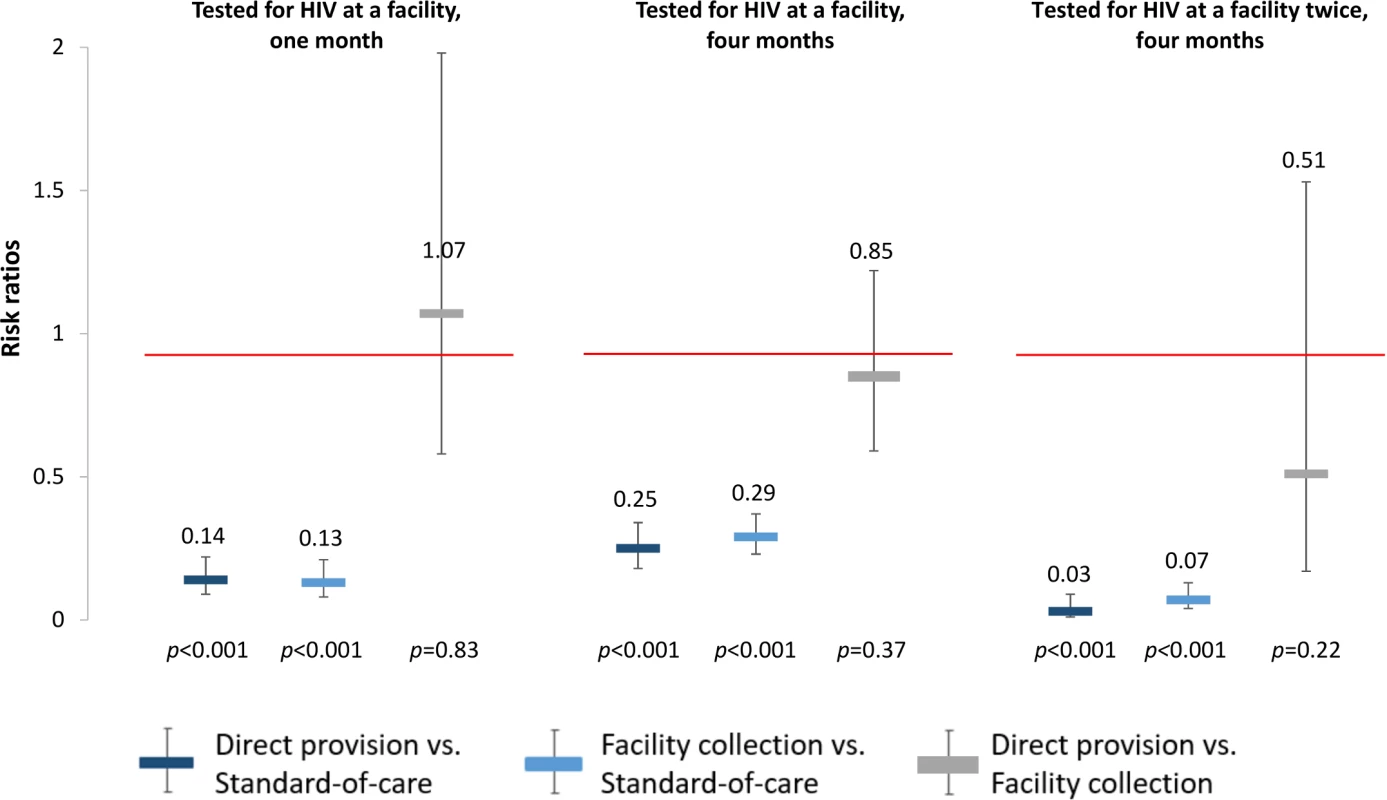
All outcomes since study start. Facility-based testing included private and public healthcare facilities. Comparisons between study arms: direct provision versus standard-of-care (dark blue), facility collection versus standard-of-care (light blue), direct provision versus facility collection (gray). Effects on linkage to care outcomes
Linkage to care outcomes were low at both 1 month and 4 months (“Sought medical care for HIV” and “Initiated ART” in Table 3). Overall, few people tested HIV positive and were eligible for linkage to care (because FSWs with known positive HIV status were excluded from our study). Seeking HIV-related medical care appeared to be lower in both the HIV self-testing intervention arms compared to the standard of care arm at 1 month (direct provision RR 0.65, 95% CI 0.30–1.41, p = 0.28; facility collection RR 0.50, 95% CI 0.24–1.04, p = 0.06), but these differences were not statistically significant and were reduced in magnitude at 4 months (“Sought medical care for HIV” in Table 3 and in S2 and S3 Tables). There were no statistically significant differences in ART initiation across study arms (“Initiated ART” in Table 3 and in S2 and S3 Tables). However, the CIs around the linkage to care effect point estimates are very wide, indicating a high degree of uncertainty regarding this finding.
Sensitivity analyses
All of our sensitivity analyses confirmed our main analyses. In the analysis pooling the data from the 2 HIV self-testing intervention arms, any HIV testing was significantly higher in the pooled arm compared to the standard of care arm at both 1 month (RR 1.22, 95% CI 1.07–1.40, p = 0.004) and 4 months (RR 1.13, 95% CI 1.05–1.21, p < 0.001) (“Tested for HIV” in S4 Table). In the analysis using the proportion of participants in a peer educator group, the outcome any HIV testing was significantly higher in the direct provision arm than in the standard of care arm at both 1 month (PP 24.4, 95% CI 14.1–34.7, p < 0.001) and 4 months (PP 13.1, 95% CI 7.9–18.4, p < 0.01) (“Tested for HIV” in S5 Table). In the sensitivity analyses using outcomes at 4 months that covered shorter time periods than the entire observation period, any HIV testing in the past month was significantly higher in the direct provision arm (RR 1.35, 95% CI 1.08–1.69, p = 0.009) and the facility collection arm (RR 1.29, 95% CI 1.00–1.66, p = 0.05) compared to the standard of care arm (“Tested for HIV (past month)” in S6 and S7 Tables). All of the other ITT sensitivity analyses also demonstrated robustness of the results described above (S4–S7 Tables).
In the linkage to care sensitivity analysis, which was conditioned on participants who reported testing HIV positive, seeking HIV-related medical care was again lower in the HIV self-testing intervention arms compared to the standard of care arm, but now this difference among participants in the facility collection arm was statistically significant at both 1 month (RR 0.38, 95% CI 0.21–0.67, p = 0.001) and 4 months (RR 0.66, 95% CI 0.47–0.94, p = 0.02) (“Sought medical care for HIV” in S8 Table). There were no statistically significant differences in ART initiation across study arms in this analysis, confirming the results from the ITT analysis (“Initiated ART” in S8 Table). Limiting the sample size to participants who reported testing HIV positive post-randomization could have introduced selection biases. In as far as we can test such biases through balance tests of observed participants’ baseline characteristics across study arms, any selection biases seem unlikely to be severe. We only found a statistically significant lack of balance in one of the characteristics we tested (mobile phone ownership, S9 Table). Additionally, the significance of linkage findings remained robust in models that adjusted for participant’s age, highest level of education, and monthly income (S8 Table).
Adverse events
Adverse events were rare; 4 were reported over the duration of the study. Two were related to HIV self-testing: (1) interpersonal violence (verbal abuse from boyfriend, in the facility collection arm) and (2) mental distress following a positive HIV self-test result (the participant later tested HIV negative at a healthcare facility, in the direct provision arm). The additional 2 adverse events were interpersonal violence related to FSW status disclosure. Use of the study hotline was uncommon; only 7% (59/861) of participants called the hotline by 4 months and only 8 (14%) of these calls were for reasons related to HIV self-testing (questions regarding HIV self-test use or results interpretation).
Discussion
We find that oral HIV self-testing is safe and effective at increasing HIV testing among FSWs. In our study done in Kampala, Uganda, the provision of HIV self-tests significantly increased the likelihood that FSWs participated in HIV testing at 1 month and additionally resulted in almost universal HIV testing at 4 months. Within a 4-month period, FSWs in the HIV self-testing arms were also significantly more likely to test twice for HIV compared to those in the standard of care arm. Universal and repeated HIV testing is particularly important for FSWs because of the high risk of HIV acquisition they face in their daily lives [14,15].
For their own health, frequent HIV testing will allow FSWs to detect HIV infection early and initiate treatment with minimal delay. Frequently repeated HIV testing is also a prerequisite for PrEP, which is becoming increasingly available to FSWs in sub-Saharan Africa. PrEP requires frequent HIV testing to detect breakthrough infections [13]. Our results suggest that HIV self-testing could be an important approach to ensure that FSWs who are taking PrEP regularly check their HIV status [67]. Of course, the viability of combining PrEP and HIV self-testing will depend on further investigation of the performance of the HIV self-tests in detecting HIV among PrEP users [68].
For the health of others, frequent HIV testing is necessary for successful TasP and positive prevention strategies [11,12]. FSWs have larger numbers of sex partners than most other population groups [15] and are thus at increased risk of spreading the virus following infection [14–16]. Frequent HIV testing will ensure early detection of infection, which is needed for early treatment initiation and behavior change to prevent onward HIV transmission. Our findings regarding the effect of HIV self-testing on frequent testing thus suggests that self-testing could play an important role in reducing transmission risk among FSWs.
While our findings indicate that HIV self-testing is effective overall in increasing recent and repeat HIV testing, the effectiveness of one of the two delivery models that we tested—direct provision of HIV self-tests—was better than that of the other model—facility collection of HIV self-tests. Direct provision of HIV self-testing eliminates more potential barriers to HIV self-testing than facility collection, requiring neither an interaction with a health worker nor money or time for travel to test for HIV. In contrast, facility collection of HIV self-tests requires some interaction with health workers, who might stigmatize FSWs. Moreover, to collect an HIV self-test in a healthcare facility, an FSW needs to spend amounts of time and money similar to the amounts necessary for facility-based testing.
We included the facility collection arm in our study because it closely resembles the likely default strategy to HIV self-testing that governments in sub-Saharan Africa will choose. In fact, in South Africa [69] and Kenya [70], HIV self-tests have already become available for over-the-counter purchase in pharmacies [71]. Our results show that for FSWs, such passive provision of HIV self-tests in facilities is inferior to the active delivery of HIV self-tests through peer educators. In adopting HIV self-testing policies, governments in sub-Saharan Africa should consider peer-supported strategies of direct HIV self-test delivery for FSWs as well as for other key populations that are likely to face healthcare provider stigma and lack the time and money for frequent travel to healthcare facilities.
Another important secondary finding of our study is that the HIV self-testing interventions not only increase overall HIV testing but also lead to a very high degree of substitution of facility-based testing with self-testing. At 1 month, less than 10% of all testing was facility based in the self-testing intervention arms, while more than 60% of testing was facility based in the standard of care arm; at 4 months, about one-fourth of all testing was facility based in the self-testing arms, while more than 80% was facility-based in the standard of care arm. This substitution has several important implications. First, it signals a high degree of acceptance of HIV self-testing among FSWs in Uganda, which bodes well for future routine government rollout of HIV self-testing strategies in the country. Second, in the direct provision arm, the large substitution effect implies substantial money and time savings for FSWs compared to facility-based HIV testing. These savings are an additional benefit of direct provision of HIV self-tests and are particularly important because FSWs are typically very poor [15]. Third, HIV self-testing moves HIV testing outside the regulated environment of the health system. Here, correct HIV status knowledge depends on the tester's correct interpretation of the self-test results. Previous studies have found high sensitivity and specificity of self-interpreted HIV self-test results [43,44,72,73], but none of these studies were conducted among FSWs. The overall value of HIV self-testing as an approach to increase recent and frequent HIV testing among FSWs will critically depend on ensuring that FSWs have the skills to correctly interpret the self-test results.
The large substitution effects also raise the worry of potential negative consequences for linkage to care. Self-testing will typically take place outside a healthcare facility and often far from the closest facility where HIV treatment and other services are available. Moreover, self-testing will generate an HIV test result without accompanying pre - and post-test counselling by a specifically trained health worker, as is the standard in facility-based testing. Both of these characteristics of self-testing could decrease linkage to care. Our linkage to care analyses suggest that this may be an important concern. In both the ITT analyses and the sensitivity analyses estimating linkage effects only among those FSWs who reported that they tested HIV positive, the percentage of participants who reported seeking HIV-related medical care at 1 month was lower in the HIV self-testing intervention arms compared to the standard of care arms. This difference was not statistically significant and disappeared by 4 months in the ITT analyses, but it was statistically significant and remained so at 4 months in the conditional sensitivity analyses.
It is important to note in this regard that the sensitivity analyses estimating linkage effects of HIV self-testing only among those FSWs who reported that they tested HIV positive will likely be biased. Randomization only ensures that we are comparing “like” and “like” in ITT analysis; in contrast, analyses that are conditional on events that occur after randomization can suffer from selection biases. One such type of event is the process that leads to HIV testing and reporting of a positive HIV test result. The intervention itself could influence this selection process, leading to biased effect size estimation when outcomes are conditional on participants reporting positive HIV status, as is the case for the linkage analysis conditional on positive status. For instance, the option to test oneself for HIV may induce some FSWs who would never test in a facility to test for HIV for the first time. These FSWs will be in the denominator of persons who are HIV-positive in the intervention arms, but they will not be in the same denominator in the standard of care arm. If the characteristics that prevented these FSWs from testing in healthcare facilities—e.g., fear of provider stigma—also prevents them from linking to HIV care, the effect size estimation in the conditional analysis will be biased.
Future studies are needed to provide stronger evidence of the impact of HIV self-testing on linkage. These studies will require substantial investments. Compared to previous studies [46–49], in our study a large number of people (a total of 177 across the 3 arms) tested HIV positive and were thus eligible for linkage. Nevertheless, eligibility for linkage was still comparatively rare among study participants and our study lacked sufficient power for strong conclusions on the effects of HIV self-testing on linkage to care. Until better evidence becomes available, HIV self-testing policies for FSWs should ideally include strong linkage interventions. Such interventions are available. Successful examples of linkage-enhancing interventions include counseling by peer educators [74,75], home - and community-based ART [76,77], and financial incentives [78,79].
Our results indicate that FSWs preferred the direct provision of HIV self-tests by peer educators over access to HIV self-testing via a coupon that could be exchanged for an HIV self-test in healthcare facilities. It is possible that this preference is due to the time and monetary costs incurred when traveling to a facility to collect an HIV self-test. In future research, it would be interesting to compare peer-provided HIV rapid testing with peer-provided HIV self-tests. Both of these HIV testing modalities eliminate the costs of traveling to a healthcare facility. However, peer-provided HIV self-tests differ from peer-provided HIV rapid testing in several ways: the former strongly protects the privacy of test results, while the latter implies disclosure of results to a peer; the former allows FSWs to mentally prepare for an HIV test and to test at the preferred time and location, while the latter requires HIV testing when and where the peer educator is available. Our 3-arm comparison allows some inferences regarding the testing preferences relevant to judge these 2 alternative testing options. At the time of our study, there were several HIV testing initiatives targeted to FSWs in Kampala, including home - and work-based HIV testing. The fact that FSWs in both intervention arms almost exclusively used HIV self-tests instead of other HIV testing options suggests that FSWs highly valued the distinguishing characteristics of HIV self-tests (strong privacy protection, unconstrained choice of testing time and location), independent of the delivery model used to provide the self-test. It thus seems likely that many FSWs will prefer peer-provided HIV self-testing over peer-provided HIV rapid testing. At the same time, the difference in the effects of the direct provision and the facility collection approaches to HIV self-testing was likely caused by the different costs associated with these 2 delivery models. It therefore seems likely that many FSWs will prefer peer-provided HIV rapid testing over facility collection of HIV self-tests.
Our study has several important strengths, including the testing of 2 different HIV self-testing delivery models, the large sample size, the low loss to follow-up, and the exclusive focus on FSWs. Our study also has important limitations. For one, we rely on self-reported outcomes, which could be biased by social desirability and other reporting distortions. For instance, participants in the direct provision arm might have felt more shame for not using an HIV self-test than those in the facility collection arm, because they received an HIV self-test directly from their peer educators and did not have to invest time and money to obtain a self-test in a facility. The participants in the direct provision arm might thus have felt more pressure to over-report HIV testing and self-test use.
In addition, the external validity of our results may be limited. First, all participants in our study received the peer educator intervention, which included condom distribution, information about HIV testing options, and encouragements to test for HIV. Thus, we were unable to measure the effect of HIV self-testing in the absence of these peer educator activities. Second, the peer educators delivering the interventions in our trial reached out to fellow sex workers within their social networks. This recruitment process might further threaten the external validity of our findings. For instance, peer educators may have selected those FSWs who they knew would be interested in exploring new HIV testing options. Third, FSWs in urban Kampala are also highly organized and benefit from ongoing policy initiatives by the Ugandan government aimed at providing HIV services to FSWs. The high background levels of HIV testing in our study population are likely a result of these services. Our findings may thus not generalize to FSWs in settings with fewer HIV testing interventions targeting FSWs. Fourth, FSWs enrolled in this study were recognized as such by their peers. Sex work, however, manifests in a variety of different forms, and peer-based HIV self-testing delivery models might have different effects in less formal FSW populations, such as girls who date sugar daddies and barmaids who only occasionally sell sex.
In sum, oral HIV self-testing could be an important component of HIV policies to achieve near-universal and frequent HIV testing among FSWs. In particular, HIV self-testing could support HIV interventions that require frequent HIV testing, such as TasP, behavior change for transmission reduction, and PrEP. In designing HIV self-testing policies for FSWs, governments should consider direct provision of HIV self-tests rather than merely making HIV self-tests available in healthcare facilities. HIV self-testing policies for FSWs should be accompanied by strong interventions to support linkage to care.
Supporting Information
Zdroje
1. The Joint United Nations Programme on HIV/AIDS. Ending AIDS: Progress Towards the 90-90-90 Targets. Geneva: Joint United Nations Programme on HIV/AIDS; 2017 [cited 2017 Aug 1]. Available from: http://www.unaids.org/sites/default/files/media_asset/Global_AIDS_update_2017_en.pdf
2. The Joint United Nations Programme on HIV/AIDS. 90–90–90—An ambitious treatment target to help end the AIDS epidemic. Geneva: Joint United Nations Programme on HIV/AIDS; 2017 [cited 2017 Nov 14]. Available from: http://www.unaids.org/en/resources/documents/2014/90-90-90
3. Donnell D, Baeten JM, Kiarie J, Thomas KK, Stevens W, Cohen CR, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010 Jun;375(9731):2092–8. doi: 10.1016/S0140-6736(10)60705-2 20537376
4. Centers for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States—2014; a clinical practice guideline. Atlanta, GA: Centers for Disease Control and Prevention; 2014 [cited 2017 Jul 7]. Available from: https://www.cdc.gov/hiv/pdf/prepguidelines2014.pdf
5. Fox MP, Rosen S. A new cascade of HIV care for the era of “treat all.” PLoS Med. 2017 Apr 11;14(4):e1002268. doi: 10.1371/journal.pmed.1002268 28399160
6. Iwuji C, Orne-Gliemann J, Balestre E. The impact of universal test and treat on HIV incidence in a rural South African population: ANRS 12249 TasP trial, 2012–2016. 21st International AIDS Conference; July 18-22, 2016; Durban, South Africa.
7. Haber N, Tanser F, Bor J, Naidu K, Mutevedzi T, Herbst K, et al. From HIV infection to therapeutic response: a population-based longitudinal HIV cascade-of-care study in KwaZulu-Natal, South Africa. Lancet HIV. 2017 May;4(5):e223–e230. doi: 10.1016/S2352-3018(16)30224-7
8. Nsanzimana S, Kanters S, Remera E, Forrest JI, Binagwaho A, Condo J, et al. HIV care continuum in Rwanda: a cross-sectional analysis of the national programme. Lancet HIV. 2015 May;2(5):e208–215. doi: 10.1016/S2352-3018(15)00024-7 26423003
9. Kranzer K, Govindasamy D, Ford N, Johnston V, Lawn SD. Quantifying and addressing losses along the continuum of care for people living with HIV infection in sub-Saharan Africa: a systematic review. J Int AIDS Soc. 2012 Nov 19;15(2):17383. doi: 10.7448/IAS.15.2.17383
10. Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 Infection with Early Antiretroviral Therapy. N Engl J Med. 2011 Aug 11;365(6):493–505. doi: 10.1056/NEJMoa1105243 21767103
11. Bunnell R, Mermin J, Cock KMD. HIV Prevention for a Threatened Continent: Implementing Positive Prevention in Africa. JAMA. 2006 Aug 16;296(7):855–8. doi: 10.1001/jama.296.7.855 16905790
12. Kennedy CE, Medley AM, Sweat MD, O’Reilly KR. Behavioural interventions for HIV positive prevention in developing countries: a systematic review and meta-analysis. Bull World Health Organ. 2010 Aug;88(8):615–23. doi: 10.2471/BLT.09.068213 20680127
13. The World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Geneva: World Health Organization; 2015 [cited 2017 Nov 14]. Available from: http://www.who.int/hiv/pub/guidelines/earlyrelease-arv/en/
14. Baral S, Beyrer C, Muessig K, Poteat T, Wirtz AL, Decker MR, et al. Burden of HIV among female sex workers in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis. 2012 Jul 1;12(7):538–49. doi: 10.1016/S1473-3099(12)70066-X 22424777
15. Shannon K, Strathdee SA, Goldenberg SM, Duff P, Mwangi P, Rusakova M, et al. Global epidemiology of HIV among female sex workers: influence of structural determinants. Lancet. 2015 Jan 3;385(9962):55–71. doi: 10.1016/S0140-6736(14)60931-4 25059947
16. The Joint United Nations Programme on HIV/AIDS. The Gap Report. Geneva: Joint United Nations Programme on HIV/AIDS; 2014 [cited 2017 Nov 14]. Available from: http://www.unaids.org/sites/default/files/media_asset/UNAIDS_Gap_report_en.pdf
17. The World Health Organization. Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations. Geneva, Switzerland: World Health Organization; 2016 Update [cited 2017 Nov 14]. Available from: http://apps.who.int/iris/bitstream/10665/246200/1/9789241511124-eng.pdf
18. The World Health Organization. Consolidated Guidelines on HIV Testing Services; 5Cs: Consent, Confidentiatlity, Counseling, Correct Results and Connection. Geneva, Switzerland: World Health Organization; 2015 Jul.
19. The World Health Organization. Prevention and Treatment of HIV and Other Sexually Transmitted Infections for Sex Workers in Low - and Middle-Income Countries. Geneva, Switzerland: World Health Organization; 2012 Dec [cited 2017 Aug 19]. Available from: http://apps.who.int/iris/bitstream/10665/77745/1/9789241504744_eng.pdf
20. Schwartz S, Lambert A, Phaswana-Mafuya N, Kose Z, Mcingana M, Holland C, et al. Engagement in the HIV care cascade and barriers to antiretroviral therapy uptake among female sex workers in Port Elizabeth, South Africa: Findings from a respondent-driven sampling study. Sex Transm Infect. 2017 Jun;93(4):290–296. doi: 10.1136/sextrans-2016-052773
21. Cowan FM, Davey CB, Fearon E, Mushati P, Dirawo J, Cambiano V, et al. The HIV Care Cascade Among Female Sex Workers in Zimbabwe: Results of a Population-Based Survey From the Sisters Antiretroviral Therapy Programme for Prevention of HIV, an Integrated Response (SAPPH-IRe) Trial. J Acquir Immune Defic Syndr. 2017 Apr 1;74(4):375–82. doi: 10.1097/QAI.0000000000001255 27930599
22. Gupta S, Granich R. National HIV Care Continua for Key Populations: 2010 to 2016. J Int Assoc Provid AIDS Care JIAPAC. 2017 Mar 1;16(2):125–32. doi: 10.1177/2325957416686195 28090799
23. Chanda M, Perez-Brumer A, Ortblad K, Mwale M, Chongo S, Kamungoma N, et al. Barriers and facilitators to HIV testing among Zambia female sex workers in three transit hubs. AIDS Patient Care STDs. 2017 Jul;31(7):290–296. doi: 10.1089/apc.2017.0016 28581820
24. Wanyenze RK, Musinguzi G, Kiguli J, Nuwaha F, Mujisha G, Musinguzi J, et al. “When they know that you are a sex worker, you will be the last person to be treated”: Perceptions and experiences of female sex workers in accessing HIV services in Uganda. BMC Int Health Hum Rights. 2017 May 5;17(1):11. doi: 10.1186/s12914-017-0119-1 28476153
25. Lancaster KE, Cernigliaro D, Zulliger R, Fleming PF. HIV care and treatment experiences among female sex workers living with HIV in sub-Saharan Africa: A systematic review. Afr J AIDS Res AJAR. 2016 Dec;15(4):377–86. doi: 10.2989/16085906.2016.1255652 27974017
26. Lafort Y, Lessitala F, Candrinho B, Greener L, Greener R, Beksinska M, et al. Barriers to HIV and sexual and reproductive health care for female sex workers in Tete, Mozambique: results from a cross-sectional survey and focus group discussions. BMC Public Health. 2016;16 : 608. doi: 10.1186/s12889-016-3305-5 27440108
27. Napierala Mavedzenge S, Baggaley R, Corbett EL. A Review of Self-Testing for HIV: Research and Policy Priorities in a New Era of HIV Prevention. Clin Infect Dis. 2013 Jul 1;57(1):126–38. doi: 10.1093/cid/cit156 23487385
28. Brown AN, Djimeu EW, Cameron DB. A Review of the Evidence of Harm from Self-Tests. AIDS Behav. 2014;18(Suppl 4):445–9.
29. Medley A, Kennedy C, O’Reilly K, Sweat M. Effectiveness of Peer Education Interventions for HIV Prevention in Developing Countries: A Systematic Review and Meta-Analysis. AIDS Educ Prev. 2009 Jun 1;21(3):181–206. doi: 10.1521/aeap.2009.21.3.181 19519235
30. George A, Blankenship KM. Peer Outreach Work as Economic Activity: Implications for HIV Prevention Interventions among Female Sex Workers. PLoS ONE. 2015 Mar 16;10(3):e0119729. doi: 10.1371/journal.pone.0119729 25775122
31. Luchters S, Chersich MF, Rinyiru A, Barasa M - S, King’ola N, Mandaliya K, et al. Impact of five years of peer-mediated interventions on sexual behavior and sexually transmitted infections among female sex workers in Mombasa, Kenya. BMC Public Health. 2008;8 : 143. doi: 10.1186/1471-2458-8-143 18445258
32. Ngugi EN, Wilson D, Sebstad J, Plummer FA, Moses S. Focused Peer-Mediated Educational Programs Among Female Sex Workers To Reduce Sexually Transmitted Disease And Human Immunodeficiency Virus Transmission In Kenya And Zimbabwe. J Infect Dis. 1996 Oct 1;174(Supplement_2):S240–7.
33. Shahmanesh M, Patel V, Mabey D, Cowan F. Effectiveness of interventions for the prevention of HIV and other sexually transmitted infections in female sex workers in resource poor setting: a systematic review. Trop Med Int Health. 2008 May 1;13(5):659–79. doi: 10.1111/j.1365-3156.2008.02040.x 18266784
34. Pai NP, Sharma J, Shivkumar S, Pillay S, Vadnais C, Joseph L, et al. Supervised and Unsupervised Self-Testing for HIV in High - and Low-Risk Populations: A Systematic Review. PLoS Med. 2013 Apr 2;10(4):e1001414. doi: 10.1371/journal.pmed.1001414 23565066
35. Krause J, Subklew-Sehume F, Kenyon C, Colebunders R. Acceptability of HIV self-testing: a systematic literature review. BMC Public Health. 2013 Aug 8;13(1):735.
36. Mokgatle MM, Madiba S. High Acceptability of HIV Self-Testing among Technical Vocational Education and Training College Students in Gauteng and North West Province: What Are the Implications for the Scale Up in South Africa? PLoS ONE. 2017 Jan 31;12(1):e0169765. doi: 10.1371/journal.pone.0169765 28141858
37. Zerbe A, DiCarlo A, Mantell J, Remien R, Morris D, Frederix K, et al. Acceptability and uptake of home-baed HIV self-testing in Lesotho. Conference on Retroviruses and Opportunistic Infections; February 23-26, 2015; Seattle, USA.
38. Figueroa C, Johnson C, Verster A, Baggaley R. Attitudes and Acceptability on HIV Self-testing Among Key Populations: A Literature Review. AIDS Behav. 2015 Jun 9;19(11): 1–17, 1949–65.
39. Pérez GM, Cox V, Ellman T, Moore A, Patten G, Shroufi A, et al. “I Know that I Do Have HIV but Nobody Saw Me”: Oral HIV Self-Testing in an Informal Settlement in South Africa. PLoS ONE. 2016 Apr 4;11(4):e0152653. doi: 10.1371/journal.pone.0152653 27044006
40. Kumwenda M, Munthali A, Phiri M, Mwale D, Gutteberg T, MacPherson E, et al. Factors shaping initial decision-making to self-test amongst cohabiting couples in urban Blantyre, Malawi. AIDS Behav. 2014 Jul;18 Suppl 4:S396–404.
41. Brown W, Carballo-Diéguez A, John RM, Schnall R. Information, Motivation, and Behavioral Skills of High-Risk Young Adults to Use the HIV Self-Test. AIDS Behav. 2016 Sep 1;20(9):2000–9. doi: 10.1007/s10461-016-1309-x 26885813
42. Mugo PM, Micheni M, Shangala J, Hussein MH, Graham SM, Wit TFR de, et al. Uptake and Acceptability of Oral HIV Self-Testing among Community Pharmacy Clients in Kenya: A Feasibility Study. PLoS ONE. 2017 Jan 26;12(1):e0170868. doi: 10.1371/journal.pone.0170868 28125699
43. Choko AT, MacPherson P, Webb EL, Willey BA, Feasy H, Sambakunsi R, et al. Uptake, Accuracy, Safety, and Linkage into Care over Two Years of Promoting Annual Self-Testing for HIV in Blantyre, Malawi: A Community-Based Prospective Study. PLoS Med. 2015 Sep;12(9):e1001873. doi: 10.1371/journal.pmed.1001873 26348035
44. Choko AT, Desmond N, Webb EL, Chavula K, Napierala-Mavedzenge S, Gaydos CA, et al. The Uptake and Accuracy of Oral Kits for HIV Self-Testing in High HIV Prevalence Setting: A Cross-Sectional Feasibility Study in Blantyre, Malawi. PLoS Med. 2011 Oct 4;8(10):e1001102. doi: 10.1371/journal.pmed.1001102 21990966
45. Ayles H, Floyd S, Mulubwa C, Hensen B, Schaap A, Phiri B, et al. Increasing knowledge of HIV status among men: a cluster-randomised trial of community-based distribution of oral HIV self-test kits nested in four HPTN 071 communities in Zambia. 9th IAS Conference on HIV Science; July 23-26, 2017; Paris, France; 2017 [cited 2017 Nov 14]. Available from: http://programme.ias2017.org/Abstract/Abstract/5600
46. Gichangi A, Wambua J, Gohole A, Mutwiwa S, Njogu R, Bazant E. Provision of oral HIV self-test kits triples uptake of HIV testing among male partners of antenatal care clients: results of a randomized trial in Kenya. 21st International AIDS Conference; July 18-22, 2016; Durban, South Africa.
47. Masters SH, Agot K, Obonyo B, Mavedzenge SN, Maman S, Thirumurthy H. Promoting Partner Testing and Couples Testing through Secondary Distribution of HIV Self-Tests: A Randomized Clinical Trial. PLoS Med. 2016 Nov 8;13(11):e1002166. doi: 10.1371/journal.pmed.1002166 27824882
48. Thirumurthy H, Masters SH, Mavedzenge SN, Maman S, Omanga E, Agot K. Promoting male partner HIV testing and safer sexual decision making through secondary distribution of self-tests by HIV-negative female sex workers and women receiving antenatal and post-partum care in Kenya: a cohort study. Lancet HIV. 2016 Jun;3(6):e266–274. doi: 10.1016/S2352-3018(16)00041-2 27240789
49. Johnson CC, Kennedy C, Fonner V, Siegfried N, Figueroa C, Dalal S, et al. Examining the effects of HIV self-testing compared to standard HIV testing services: a systematic review and meta-analysis. J Int AIDS Soc. 2017 May 15;20(1):21594. doi: 10.7448/IAS.20.1.21594
50. Wang Z, Lau J, Ip M, Ho S, Mo P, Latkin C, et al. A randomized controlled trial evaluating efficacy of promoting a home-based HIV self-testing with online counseling on increasing HIV testing among men who have sex with men. AIDS Behav. 2017 Aug. doi: 10.1007/s10461-017-1887-2. [Epub ahead of print] 28831616
51. Wang Z, Lau J, Ip M, Ho S. A randomized controlled trial evaluating the efficacy of promoting HIV self-testing and online real-time counseling on increasing HIV testing among men who have sex with men in Hong Kong. International Congress of Behavioral Medicine. December 7–10, 2016. Melbourne, Australia.
52. Katz D, Golden M, Hughes J, Farquhar C, Stekler J. HIV self-testing increases HIV testing frequency among high-risk men who have sex with men: a randomized controlled trial. IAS 2015. 8th Conference on HIV Pathogenesis, Treatment and Prevention. July 19–22, 2015. Vancouver, Canada. Abstract MOPDC0103.
53. Jamil MS, Prestage G, Fairley CK, Grulich AE, Smith KS, Chen M, et al. Effect of availability of HIV self-testing on HIV testing frequency in gay and bisexual men at high risk of infection (FORTH): a waiting-list randomised controlled trial. Lancet HIV. 2017 Jun;4(6):e241–50. doi: 10.1016/S2352-3018(17)30023-1 28219619
54. Chanda MM, Ortblad KF, Mwale M, Chongo S, Kanchele C, Kamungoma N, et al. (2017) HIV self-testing among female sex workers in Zambia: A cluster randomized controlled trial. PLoS Med 14(11):e1002442. doi: 10.1371/journal.pmed.1002442
55. Centers for Disease Control and Prevention. The Crane Survey: Female sex workers (FSW) in Kampala. Entebbe: Centers for Disease Control and Prevention; 2009 Dec [cited 2017 Mar 28]. Available from: https://docs.google.com/viewer?a=v&pid=sites&srcid=ZGVmYXVsdGRvbWFpbnxjcmFuZXN1cnZleXxneDo3NzE4MjNjM2YwYzRkY2E4
56. The Joint United Nations Programme on HIV/AIDS. Global AIDS Update 2016. Geneva: Joint United Nations Programme on HIV/AIDS; 2016 [cited 2017 Nov 14]. Available from: http://www.unaids.org/sites/default/files/media_asset/global-AIDS-update-2016_en.pdf
57. The Ugandan Ministry of Health. MARPI—Most at Risk Populations Initiatives. [cited 2017 Apr 27]. Available from: http://www.marpi.org/marpi/about_us
58. HIV self-testing research and policy hub: Uganda. 2017 [cited 2017 Jun 7]. Available from: http://hivst.org/policy/uganda
59. The Ugandan Ministry of Health. Consolidated Guidelines for Prevention and Treatment of HIV in Uganda. Kampala, Uganda: Ugandan Ministry of Health; 2016 Dec.
60. The World Bank Group. PPP conversion factor, GDP (LCU per international $). Washington, DC: World Bank; 2017 [cited 2017 Sep 28]. Available from: https://data.worldbank.org/indicator/PA.NUS.PPP
61. Kang M, Ragan BG, Park J-H. Issues in Outcomes Research: An Overview of Randomization Techniques for Clinical Trials. J Athl Train. 2008 Mar 1;43(2):215–21. doi: 10.4085/1062-6050-43.2.215 18345348
62. Hemming K, Marsh J. A menu-driven facility for sample-size calculations in cluster randomized controlled trials. Stata J. 2013;13(1):114–35.
63. Montori VM, Guyatt GH. Intention-to-treat principle. CMAJ Can Med Assoc J. 2001 Nov 13;165(10):1339–41.
64. Gupta SK. Intention-to-treat concept: A review. Perspect Clin Res. 2011;2(3):109–12. doi: 10.4103/2229-3485.83221 21897887
65. Ranganathan P, Pramesh CS, Aggarwal R. Common pitfalls in statistical analysis: Intention-to-treat versus per-protocol analysis. Perspect Clin Res. 2016;7(3):144–6. doi: 10.4103/2229-3485.184823 27453832
66. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004 Apr 1;159(7):702–6. 15033648
67. Ngure K, Heffron R, Mugo N, Thomson KA, Irungu E, Njuguna N, et al. Feasibility and acceptability of HIV self-testing among pre-exposure prophylaxis users in Kenya. J Int AIDS Soc. 2017 10;20(1):21234. doi: 10.7448/IAS.20.1.21234 28362073
68. Suntharasamai P, Martin M, Choopanya K, Vanichseni S, Sangkum U, Tararut P, et al. Assessment of Oral Fluid HIV Test Performance in an HIV Pre-Exposure Prophylaxis Trial in Bangkok, Thailand. PLoS ONE. 2015 Dec 30;10(12):e0145859. doi: 10.1371/journal.pone.0145859 26717405
69. Southern African HIV Clinicians Society. South African HIV Self-Testing Policy and Guidance Considerations: A supplement to the National HIV Testing Services Policy 2016. Johannesburg, South Africa: Southern African HIV Clinicians Society, 2017.
70. The Kenyan Ministry of Health. The Kenya HIV Testing Services Guidelines. Nairobi, Kenya: Kenyan Ministry of Health; 2015 Oct.
71. HIVST.org. Policy: HIV self-testing research and policy hub. HIVST.org. 2017 [cited 2017 Aug 20]. Available from: http://www.hivst.org/policy
72. Asiimwe S, Oloya J, Song X, Whalen CC. Accuracy of Un-supervised Versus Provider-Supervised Self-administered HIV Testing in Uganda: A Randomized Implementation Trial. AIDS Behav. 2014 Apr 2;18(12):2477–84. doi: 10.1007/s10461-014-0765-4 24691923
73. Kurth AE, Cleland CM, Chhun N, Sidle JE, Were E, Naanyu V, et al. Accuracy and Acceptability of Oral Fluid HIV Self-Testing in a General Adult Population in Kenya. AIDS Behav. 2016;20 : 870–9. doi: 10.1007/s10461-015-1213-9 26438487
74. Arem H, Nakyanjo N, Kagaayi J, Mulamba J, Nakigozi G, Serwadda D, et al. Peer health workers and AIDS care in Rakai, Uganda: a mixed methods operations research evaluation of a cluster-randomized trial. AIDS Patient Care STDs. 2011 Dec;25(12):719–24. doi: 10.1089/apc.2010.0349 21391828
75. Chang LW, Kagaayi J, Nakigozi G, Ssempijja V, Packer AH, Serwadda D, et al. Effect of peer health workers on AIDS care in Rakai, Uganda: a cluster-randomized trial. PLoS ONE. 2010 Jun 2;5(6):e10923. doi: 10.1371/journal.pone.0010923 20532194
76. Kipp W, Konde-Lule J, Saunders LD, Alibhai A, Houston S, Rubaale T, et al. Results of a community-based antiretroviral treatment program for HIV-1 infection in Western Uganda. Curr HIV Res. 2010 Mar;8(2):179–85. 20163349
77. Iwuji CC, Orne-Gliemann J, Larmarange J, Okesola N, Tanser F, Thiebaut R, et al. Uptake of Home-Based HIV Testing, Linkage to Care, and Community Attitudes about ART in Rural KwaZulu-Natal, South Africa: Descriptive Results from the First Phase of the ANRS 12249 TasP Cluster-Randomised Trial. PLoS Med. 2016 Aug;13(8):e1002107. doi: 10.1371/journal.pmed.1002107 27504637
78. Bassett IV, Wilson D, Taaffe J, Freedberg KA. Financial incentives to improve progression through the HIV treatment cascade. Curr Opin HIV AIDS. 2015 Nov;10(6):451–63. doi: 10.1097/COH.0000000000000196 26371461
79. Govindasamy D, Meghij J, Negussi EK, Baggaley RC, Ford N, Kranzer K. Interventions to improve or facilitate linkage to or retention in pre-ART (HIV) care and initiation of ART in low - and middle-income settings–a systematic review. J Int AIDS Soc. 2014 Aug 1;17(1). Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4122816/
Štítky
Interní lékařství
Článek Contemporary disengagement from antiretroviral therapy in Khayelitsha, South Africa: A cohort studyČlánek Bioequivalence of twice-daily oral tacrolimus in transplant recipients: More evidence for consensus?
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2017 Číslo 11- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Ferinject: správně indikovat, správně podat, správně vykázat
- Optimální dávkování apixabanu v léčbě fibrilace síní
-
Všechny články tohoto čísla
- Labour trafficking: Challenges and opportunities from an occupational health perspective
- The end of HIV: Still a very long way to go, but progress continues
- Contemporary disengagement from antiretroviral therapy in Khayelitsha, South Africa: A cohort study
- Bioequivalence of twice-daily oral tacrolimus in transplant recipients: More evidence for consensus?
- Treatment guidelines and early loss from care for people living with HIV in Cape Town, South Africa: A retrospective cohort study
- Perinatal mortality associated with induction of labour versus expectant management in nulliparous women aged 35 years or over: An English national cohort study
- Core Outcome Set-STAndards for Development: The COS-STAD recommendations
- Closing the gaps in the HIV care continuum
- Association between the 2012 Health and Social Care Act and specialist visits and hospitalisations in England: A controlled interrupted time series analysis
- HIV pre-exposure prophylaxis and early antiretroviral treatment among female sex workers in South Africa: Results from a prospective observational demonstration project
- Sexual exploitation of unaccompanied migrant and refugee boys in Greece: Approaches to prevention
- Child sex trafficking in the United States: Challenges for the healthcare provider
- The expanding epidemic of HIV-1 in the Russian Federation
- Cardiovascular disease (CVD) and chronic kidney disease (CKD) event rates in HIV-positive persons at high predicted CVD and CKD risk: A prospective analysis of the D:A:D observational study
- Validity of a minimally invasive autopsy for cause of death determination in maternal deaths in Mozambique: An observational study
- malERA: An updated research agenda for malaria elimination and eradication
- malERA: An updated research agenda for health systems and policy research in malaria elimination and eradication
- A combination intervention strategy to improve linkage to and retention in HIV care following diagnosis in Mozambique: A cluster-randomized study
- Bioequivalence between innovator and generic tacrolimus in liver and kidney transplant recipients: A randomized, crossover clinical trial
- malERA: An updated research agenda for basic science and enabling technologies in malaria elimination and eradication
- Human trafficking and exploitation: A global health concern
- Virological response and resistance among HIV-infected children receiving long-term antiretroviral therapy without virological monitoring in Uganda and Zimbabwe: Observational analyses within the randomised ARROW trial
- Postmenopausal hormone therapy and risk of stroke: A pooled analysis of data from population-based cohort studies
- Lansoprazole use and tuberculosis incidence in the United Kingdom Clinical Practice Research Datalink: A population based cohort
- malERA: An updated research agenda for insecticide and drug resistance in malaria elimination and eradication
- Safety, pharmacokinetics, and immunological activities of multiple intravenous or subcutaneous doses of an anti-HIV monoclonal antibody, VRC01, administered to HIV-uninfected adults: Results of a phase 1 randomized trial
- HIV prevalence and behavioral and psychosocial factors among transgender women and cisgender men who have sex with men in 8 African countries: A cross-sectional analysis
- Treatment eligibility and retention in clinical HIV care: A regression discontinuity study in South Africa
- malERA: An updated research agenda for characterising the reservoir and measuring transmission in malaria elimination and eradication
- Effectiveness of a combination strategy for linkage and retention in adult HIV care in Swaziland: The Link4Health cluster randomized trial
- The value of confirmatory testing in early infant HIV diagnosis programmes in South Africa: A cost-effectiveness analysis
- HIV self-testing among female sex workers in Zambia: A cluster randomized controlled trial
- The US President's Malaria Initiative, transmission and mortality: A modelling study
- Comparison of two cash transfer strategies to prevent catastrophic costs for poor tuberculosis-affected households in low- and middle-income countries: An economic modelling study
- Direct provision versus facility collection of HIV self-tests among female sex workers in Uganda: A cluster-randomized controlled health systems trial
- malERA: An updated research agenda for diagnostics, drugs, vaccines, and vector control in malaria elimination and eradication
- malERA: An updated research agenda for combination interventions and modelling in malaria elimination and eradication
- HIV-1 persistence following extremely early initiation of antiretroviral therapy (ART) during acute HIV-1 infection: An observational study
- Respondent-driven sampling for identification of HIV- and HCV-infected people who inject drugs and men who have sex with men in India: A cross-sectional, community-based analysis
- Extensive virologic and immunologic characterization in an HIV-infected individual following allogeneic stem cell transplant and analytic cessation of antiretroviral therapy: A case study
- Measuring success: The challenge of social protection in helping eliminate tuberculosis
- Prospects for passive immunity to prevent HIV infection
- Reaching global HIV/AIDS goals: What got us here, won't get us there
- Evidence-based restructuring of health and social care
- Extreme exploitation in Southeast Asia waters: Challenges in progressing towards universal health coverage for migrant workers
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Postmenopausal hormone therapy and risk of stroke: A pooled analysis of data from population-based cohort studies
- Bioequivalence between innovator and generic tacrolimus in liver and kidney transplant recipients: A randomized, crossover clinical trial
- HIV pre-exposure prophylaxis and early antiretroviral treatment among female sex workers in South Africa: Results from a prospective observational demonstration project
- Bioequivalence of twice-daily oral tacrolimus in transplant recipients: More evidence for consensus?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Revma Focus: Spondyloartritidy
nový kurz
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



