-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaHIV prevalence and behavioral and psychosocial factors among transgender women and cisgender men who have sex with men in 8 African countries: A cross-sectional analysis
In a cross-sectional analysis of data from African countries, Tonia Poteat and colleagues report on risk factors for infection and HIV prevalence in transgender women and men who have sex with men.
Published in the journal: . PLoS Med 14(11): e32767. doi:10.1371/journal.pmed.1002422
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002422Summary
In a cross-sectional analysis of data from African countries, Tonia Poteat and colleagues report on risk factors for infection and HIV prevalence in transgender women and men who have sex with men.
Introduction
Sub-Saharan Africa bears more than 70% of the global burden of HIV [1]. Countries across the region have experienced broadly generalized HIV epidemics [2], and early studies focused on cisgender (non-transgender) heterosexual adults [3,4]. More recent epidemiological data have identified the uneven distribution of HIV risk, even within generalized epidemics [5,6]. As attention to the HIV-related needs of key affected populations such as men who have sex with men (MSM) and female sex workers grew [6,7], researchers began to explicitly assess gender diversity and notice higher HIV burdens among gender minorities [8,9]. However, very little research has addressed HIV among transgender women in sub-Saharan Africa [10–12], and HIV data from transgender women are often subsumed within MSM data [13].
Transgender women experience a disproportionate burden of HIV. The most recent global meta-analysis of HIV among transgender women examined laboratory-confirmed HIV data from 15 countries over the period of 2001–2011. The pooled HIV prevalence for transgender women was 19%, with this group having a 49-fold higher odds of infection compared with other reproductive age adults [14]. However, data were only available from countries with male-predominant epidemics, and no data were available from sub-Saharan Africa. A systematic review of the global HIV epidemiology among transgender populations was published in 2016, including research published between 2012 and 2015 [12]. Although data were available from more than 30 studies around the world, none were from sub-Saharan Africa. Of the 20 countries that reported HIV prevalence data on transgender people to the Joint United Nations Programme on HIV/AIDS (UNAIDS) in 2016, only 1, Democratic Republic of the Congo (DRC), was in sub-Saharan Africa [15]. DRC reported HIV prevalence of 7.9% among transgender people compared with 0.7% among adults 15–49 years old and 3.3% among MSM [16].
In 2016, Stahlman and colleagues published the first study of laboratory-confirmed HIV among transgender women in sub-Saharan Africa, with a focus on west Africa. They examined data from Togo, Burkina Faso, and Côte d’Ivoire [17] and found that 18% of 2,456 participants in studies targeting cisgender MSM (cis-MSM) identified as female or transgender. Compared with cis-MSM, transgender women in these studies reported greater levels of stigma and were more likely to be living with HIV. While sexual behavior stigma was not significantly associated with HIV prevalence, it was associated with condomless anal sex. This analysis was an important first step in filling the void of information on HIV in transgender women in sub-Saharan Africa. However, Togo, Burkina Faso, and Côte d’Ivoire have low-level epidemics with HIV prevalences of 2.4%, 0.8%, and 3.5%, respectively [2].
To date, no data have been published characterizing the HIV prevalence and associated risk factors among transgender women in eastern or southern Africa. To fill this gap, we analyzed data from HIV biobehavioral studies conducted between 2011 and 2016 across sub-Saharan Africa. The objectives of this analysis were to estimate HIV prevalence among transgender women, characterize psychosocial and behavioral risk factors, and distinguish HIV epidemiology among transgender women from that among cis-MSM.
Methods
This secondary analysis of pooled data from multiple cross-sectional, biobehavioral studies did not use a formal written protocol or prospective analysis plan. The analyses were guided by the a priori research objectives described above. Details of the analysis plan history are provided in S1 Text.
Study sample
Data for these analyses were collected in urban settings as part of larger cross-sectional studies initially tailored for MSM. Data collection took place from 2011 to 2016 at 14 sites across 8 countries: Bobo-Dioulasso and Ouagadougou in Burkina Faso (January–August 2013); Abidjan, Bouake, Gagnoa, and Yamoussoukro in Côte d’Ivoire (March 2015–February 2016); Banjul in The Gambia (July–December 2011); Maputsoe and Maseru in Lesotho (February–September 2014); Lilongwe in Malawi (July 2011–March 2012); Dakar in Senegal (February–November 2015); Mbabane in Swaziland (August–December 2011); and Kara and Lomé in Togo (January–June 2013).
Participants were recruited using respondent-driven sampling (RDS) [18], except in The Gambia where snowball sampling was used [19]. At sites that used RDS, recruitment was initiated using 3 to 6 seeds who were selected based on the recommendation of local community-based organizations. Seeds represented a range of characteristics in terms of age, education, socioeconomic status, and participation in lesbian, gay, bisexual, and transgender (LGBT) associations. In The Gambia, where there were no formal LGBT organizations, recruitment started with 10 highly motivated initial seeds who represented diversity in age and education levels and who were well-connected with members of the LGBT community.
Eligible participants were age 18 years and older in all countries except The Gambia, where enrollment included ages 16 years and older. Other eligibility criteria included being assigned male sex at birth and having had anal sex with a male partner in the prior 12 months. All participants provided verbal informed consent. Studies were approved by ethical review boards in each country as well as the Johns Hopkins Bloomberg School of Public Health Institutional Review Board.
Data collection
To facilitate the privacy and safety of participants, data collection took place in study-specific facilities. During the study visit, interviewers administered a structured questionnaire including modules on demographics, gender and sexual identity, mental health, alcohol and substance use, sexual risk practices, and experiences of stigma. Interviews were conducted in English, French, or another local language (per participant choice) by trained peer or LGBT-friendly interviewers who were fluent in the languages used. Rapid HIV tests were conducted by trained staff following survey administration. HIV testing and counseling were done according to national guidelines in each country, including pre-test counseling and optional, but encouraged, post-test counseling. A serial rapid HIV testing algorithm was implemented using Determine HIV-1/2 (Alere, Japan) for screening and Uni-Gold HIV (Trinity Biotech Ireland) for confirmation of positive screening test results [20]. All participants who tested HIV-positive were referred for HIV care.
Measures
Datasets from each country were merged, and all questions that were in at least 2 surveys were kept for analysis. Consistent with global standards [21], transgender women were defined as participants who were assigned male sex at birth and self-identified as transgender or female/woman. Three countries (Burkina Faso, Lesotho, and Togo) also included the option to identify as intersex; however, all intersex participants also identified as either male/men or female/woman/transgender. Therefore, gender was dichotomized as transgender women and cis-MSM. Demographic data included age in years and current employment status. Depression screening was conducted using an item that asked, “Have you ever felt sad or depressed in the last two weeks?” Suicidal ideation screening was conducted by “Have you ever felt like you wanted to end your life in the last two weeks?” Three questions assessed drug use: “Have you used needles to inject drugs in the last 12 months?” “If yes, have you used needles previously used by other people?” “Have you taken any drug that was not injected and not prescribed in the last 12 months?” Alcohol use was measured by “What is the average number of drinks you consume in one sitting?”
Sexual risk behaviors were assessed in multiple ways. In every country except Senegal and Côte d’Ivoire, participants were asked the number of male partners with whom they had anal sex in the prior 12 months. In Senegal and Côte d’Ivoire, respondents were asked about the number of anal sex acts or number of partners in the prior 30 days, but number of male anal sex partners could not be determined. Participants in all countries were asked whether a condom was used during the last time they had sex with a casual male partner and a regular male partner, and they were asked if they had condomless insertive and/or receptive anal sex with any of their reported male partners.
Scales used to measure access to condoms and lubricants varied by country. In 3 countries (Malawi, The Gambia, and Swaziland), participants were asked “What kind of access to condoms do you have when you need them?” with response options on a 4-point Likert scale where higher scores represent easier access. The same question was repeated replacing “condom” with “lubricant” in all countries except The Gambia, where lubricant access was not assessed. In 4 countries (Lesotho, Burkina Faso, Senegal, and Togo), participants were asked, “How difficult or easy is it for you to obtain condoms when you need them?” with response options on a 5-point Likert scale where higher scores represent easier access. The same question was repeated replacing “condom” with “lubricant.” Côte d’Ivoire data included the 5-point lubricant scale but no condom scale. Participants were also asked about history of sexually transmitted infection (STI) testing and diagnosis, and history of HIV testing and diagnosis.
Experiences of stigma based on sexual orientation/practice were measured using 13 questions representing 3 primary forms of stigma: enacted, anticipated, and perceived [22]. Questions asked about stigma from family, friends, and healthcare workers as well as in employment and education. Participants were asked about experiences of arrest, incarceration, torture, physical attacks, and rape. Finally, participants were asked about fear of walking in public spaces, fear of seeking health services, and denial of health services.
Data analysis
Statistical analyses were conducted using R, an open-source software environment (R Foundation for Statistical Computing, Vienna, Austria) [23]. Means and proportions were calculated for participant characteristics and variables of interest. The number of respondents for each question (rather than the full dataset) was used as the denominator for calculating item proportions. Odds ratios (ORs) were used to compare results between transgender women and cis-MSM using univariate logistic regression with a random intercept to account for clustering by site.
A stigma index was created for item reduction using exploratory factor analysis (EFA) [24]. Varimax rotation was used due to minimal overlap in variance, and tetrachoric correlations were calculated to account for the binary nature of the items. Item reduction took place using an iterative process by which individual items were deleted if they failed to load on any factor or loaded equally on multiple factors; then, the EFA was conducted again on the lower number of items. Scree plots were used to determine the number of factors to extract. This process was repeated until each of the remaining items loaded (>0.5) on at least 1 but no more than 1 factor.
A mixed effects logistic regression model was built to estimate the odds of HIV infection for transgender women compared with cis-MSM [25]. A random intercept was added by site to account for clustering. The adjusted model included age as a potential confounder as well as stigma factor score, positive depression screen, and condomless receptive anal sex as potential mediators. We hypothesized that gender would modify the relationship between condomless receptive anal sex and HIV; therefore, an interaction term for gender and condomless receptive anal sex was included in the final model.
Because we combined data from multiple studies, no adjustments were made for RDS. The proportion of missing data was calculated for each variable in the model. Missing data comprised less than 5% of all variables, except condomless anal intercourse, which was missing for approximately 16% of participants, with no difference in proportion of missing data by gender. No data were imputed.
Results
Participant characteristics
As presented in Table 1, all participants were assigned male at birth; however, 937 (20%) identified as transgender or female while 3,649 were cis-MSM. The largest proportion of transgender participants were from Côte d’Ivoire (33%), followed by Senegal (21%), Swaziland (13%), Burkina Faso (12%), Malawi (8%), Lesotho (8%), Togo (6%), and The Gambia (<1%). Approximately 3% of transgender women and 12% of cis-MSM also identified as intersex in the 3 countries where this was assessed. The mean age of study participants was approximately 24 years, with no difference between transgender participants and cis-MSM. There was no difference in employment status by gender. The most frequently reported status for both groups was student (34% of transgender women and 37% of cis-MSM), followed by unemployed (19%).
Tab. 1. Participant characteristics (N = 4,586). 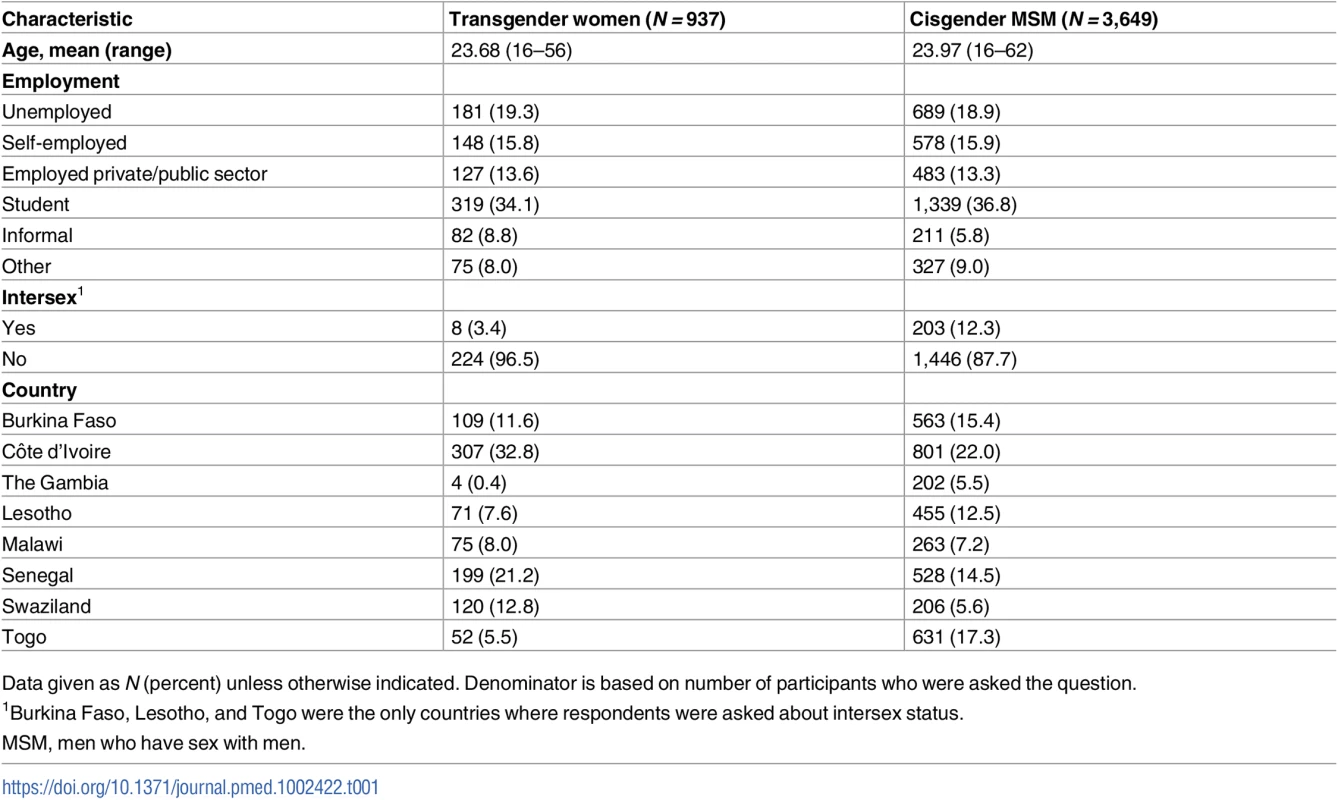
Data given as N (percent) unless otherwise indicated. Denominator is based on number of participants who were asked the question. Behavioral and psychosocial factors
Prevalence of injection drug use was less than 2% for all participants. However, among injectors, injecting with a previously used needle was common (40% among transgender women and 25% among cis-MSM) and did not differ significantly by gender. Approximately 18% of both transgender women and cis-MSM had taken a drug that was not prescribed or injected in the prior 12 months, and both groups drank an average of approximately 3 drinks per sitting. Overall, there were no significant differences by gender in alcohol and substance use. Depressive symptoms and suicidal ideation were common among both transgender women (57% and 19%) and cis-MSM (47% and 16%). However, transgender women were significantly more likely to report feeling sad or depressed (OR 1.30, 95% CI 1.12–1.52, p < 0.001).
Of the 13 stigma items assessed, transgender participants were significantly more likely than cis-MSM to report experiencing 6 of them (Table 2). There were no differences by gender in loss of employment, denial of educational opportunities, having been tortured, having been arrested, having been to jail or prison, being afraid to seek health services, and having been denied health services. However, transgender participants were significantly more likely to report exclusion from family gatherings (OR 1.75, 95% CI 1.42–2.16, p < 0.001), discriminatory remarks by family (OR 1.57, CI 1.33–1.85, p < 0.001), rejection by friends (OR 1.47, CI 1.23–1.75, p < 0.001), being beaten up (OR 1.70, CI 1.43–2.02, p < 0.001), rape (OR 1.95, 95% CI 1.63–2.36, p < 0.001), and fear of walking in public spaces (OR 1.58, 95% CI 1.31–1.91, p < 0.001). For transgender participants, discriminatory remarks from family members was most common (35%), followed by being beaten up (33%) and being rejected by friends (30%). The EFA supported a 3-factor index (Table 3) that included interpersonal stigma (3 items), law enforcement stigma (2 items), and violence (2 items).
Tab. 2. Psychosocial factors among transgender women and cisgender MSM. 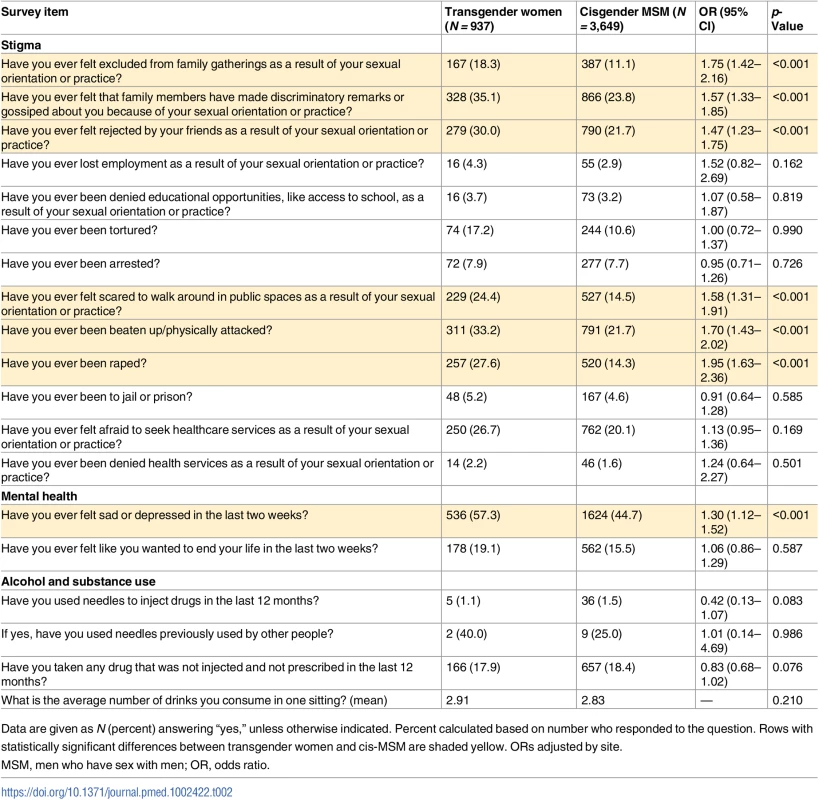
Data are given as N (percent) answering “yes,” unless otherwise indicated. Percent calculated based on number who responded to the question. Rows with statistically significant differences between transgender women and cis-MSM are shaded yellow. ORs adjusted by site. Tab. 3. Stigma index item factor loadings. 
Item loadings are bold in the column of their associated factor. Sexual risk behavior
Table 4 presents comparative data on sexual behavior, condom and lubricant access, and HIV and STI history. Transgender women had a higher median number of male anal sex partners than cis-MSM (3 versus 2, p = 0.004) and were less likely to have used a condom the last time they had sex with a regular partner (OR 0.8, CI 0.67–0.96, p = 0.16). However, there was no statistically significant difference by gender in condom use at last sex with a casual partner. Transgender women had more than twice the odds of reporting condomless receptive anal sex with male partners compared to cis-MSM (OR 2.44, 95% CI 2.05–2.90, p < 0.001). Both groups found it easier to access condoms than lubricants. Transgender women reported greater access to condoms when they needed them than cis-MSM (3.4 versus 3.1 on a 4-point Likert scale, p < 0.001), and they reported easier access to lubricant when they needed it (3.6 versus 3.0 on a 5-point Likert scale, p < 0.001). Transgender women were less likely to have been tested for STIs in the prior 12 months (OR 0.81, 95% CI 0.65–0.99, p = 0.046) but had no significant difference from cis-MSM in STI diagnosis (8% versus 7%). There was no difference by gender in history of ever having had an HIV test (78% versus 75%) or having been tested in the prior 12 months (51% versus 44%).
Tab. 4. Sexual risk and HIV/STIs among transgender women and cisgender MSM. 
Data are given as N (percent) answering “yes,” unless otherwise indicated. Percent calculated based on number who responded to the question. Rows with statistically significant differences between transgender women and cis-MSM are shaded yellow. ORs adjusted by site. HIV status
Compared with cis-MSM, transgender women were more likely to have been told they had HIV in the past (OR 2.78, 95% CI 2.03–3.81, p < 0.001) and to have tested positive on the rapid HIV test conducted during the study (25% versus 14%; OR 1.81, 95% CI 1.49–2.19, p < 0.001) (Table 4). As expected, HIV prevalence varied widely by country (Table 5). In 4 of the 8 countries (Côte d’Ivoire, Lesotho, Senegal, and Togo), transgender women had significantly higher HIV prevalence than cis-MSM, and, in the remainder, there was no statistically significant difference by gender. HIV prevalence was highest in Lesotho, at 59% among transgender women compared with 29% among cis-MSM (OR 3.63, 95% CI 2.17–6.17, p < 0.001).
Tab. 5. HIV prevalence by country. 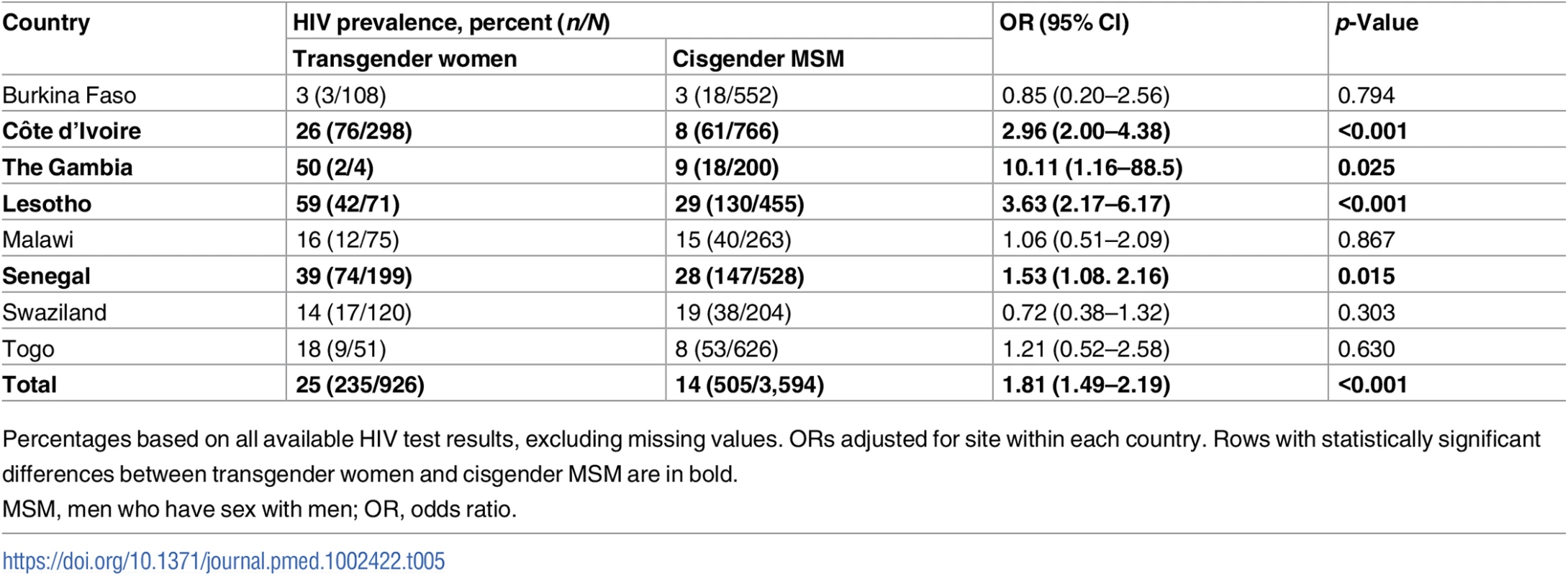
Percentages based on all available HIV test results, excluding missing values. ORs adjusted for site within each country. Rows with statistically significant differences between transgender women and cisgender MSM are in bold. In multivariable regression modeling including age, stigma experiences, condomless receptive anal sex, positive depression screening, and the interaction between gender and condomless receptive anal sex, transgender women had twice the odds of testing positive for HIV (OR 2.17, 95% CI 1.65–2.97) compared with cis-MSM (Table 6). The variables age, positive depression screen, condomless receptive anal sex, law enforcement stigma, violence, and gender by condomless receptive anal sex were significantly associated with HIV in the adjusted model, while interpersonal stigma was not statistically significant.
Tab. 6. Multivariable logistic regression of odds of HIV infection. 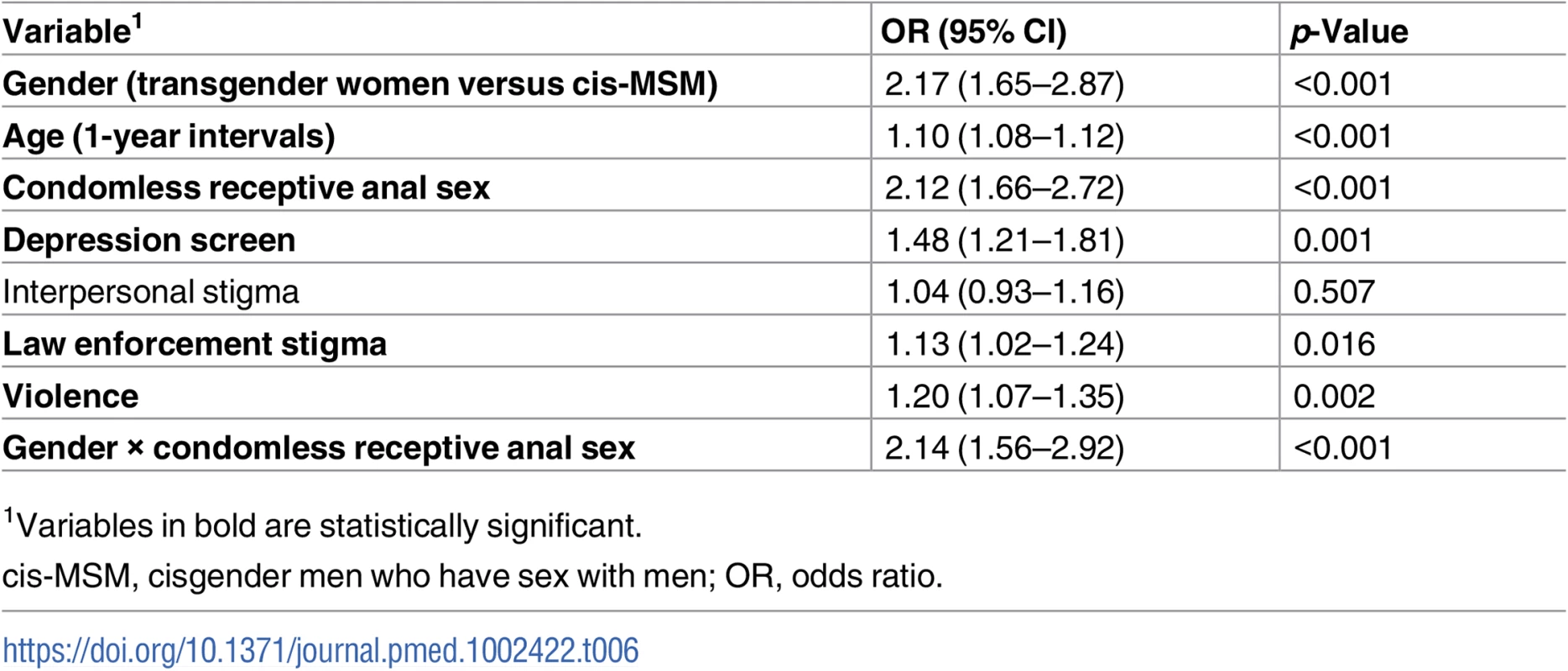
1Variables in bold are statistically significant. Discussion
In this analysis of data from MSM-tailored studies in 8 countries across sub-Saharan Africa, 1 out of every 5 participants (n = 937) identified as transgender or as a woman. Transgender women were more likely to report stigma, depressive symptoms, condomless receptive anal sex, and receipt of an HIV test within the prior 12 months compared with cis-MSM. HIV prevalence was 25% among transgender women and 14% among cis-MSM. In adjusted regression modeling, transgender women demonstrated a 2-fold higher odds of HIV infection than cis-MSM, with significant effect modification by the interaction between gender and condomless receptive anal intercourse.
These data contribute important nuance to understanding the HIV epidemic in sub-Saharan Africa, where essentialist notions of binary gender have been perpetuated in HIV research and surveillance [10]. Documentation of precolonial gender and sexual diversity in countries across the African continent has existed for more than 100 years [26]. In recent years, transgender Africans have demanded increased visibility—publishing narratives of their experience [27], establishing and leading advocacy organizations [28–30], and participating in feature stories [31] and documentaries about their lives [32]. By assessing gender identity separately from biological sex assigned at birth, HIV researchers and systems can make transgender participants visible within the data. These findings highlight the importance of collecting data on gender identity and disaggregating the data, even in countries with generalized HIV epidemics. Accurately characterizing HIV epidemiology by gender is essential to the development of appropriate strategies that reach key populations at highest risk for HIV infection [33].
Overall, 1 in 4 transgender women tested positive for HIV infection across these studies. While HIV prevalence varied greatly by country, wherever there was a statistically significant difference between transgender women and cis-MSM, transgender women had a higher HIV prevalence. Even in Lesotho, which has a broadly generalized epidemic and national HIV prevalence of 25% [34], the HIV prevalence among transgender women was 59%—representing a 3.6-fold increased odds of infection compared with cis-MSM. Such stark disparities are consistent with data from other regions of the world [12,14] and highlight the urgent need to address HIV prevention and care for transgender women across sub-Saharan Africa, where their needs have long been ignored [10]. The high HIV prevalence among such a young sample, with an average age of 24 years, reinforces the importance of intervening early to prevent HIV in this population.
Transgender women in this study were more likely than cis-MSM to report condom use with their last regular partner and easier access to condoms and lubricants. However, they also reported a greater number of sexual partners and were more likely to report condomless receptive anal sex than cis-MSM. Receptive condomless anal intercourse with a serodiscordant and viremic partner has a relatively high per-act HIV acquisition probability [35,36]; therefore, one would expect receptive condomless anal sex with a high number of partners to be associated with HIV infection. However, the significant effect modification by gender on the relationship between condomless receptive anal sex and HIV suggests a more complex relationship. Transgender women were more likely than cis-MSM to have been recently tested for HIV; therefore, one possibility is that those individuals who recently received an HIV diagnosis and post-test counseling were more likely to use condoms. Alternatively, these data may suggest the need to look beyond behavioral risks and examine psychosocial and structural drivers of HIV infection.
Fewer study participants reported substance use than in prior research among transgender women in places like Brazil [37], Thailand [38], and the United States [39] or among MSM in southern Africa [40–42]. However, depression, suicidal ideation, and experiences of stigma were common. Transgender participants were more likely to report experiences of stigma and poor mental health than cis-MSM. Our findings that law enforcement stigma, violence, and depression were significantly associated with HIV are consistent with syndemic theories that structural and psychosocial factors are important synergistic drivers of HIV in gender and sexual minority populations [12].
The UNAIDS 2016–2021 Strategy aims for 90% of people living with HIV to know their status, 90% of people with diagnosed HIV infection to receive antiretroviral treatment (ART), and 90% of people taking ART to be virally suppressed by 2020 [43]. The strategy also sets a target for 90% of key populations, including transgender people, to have access to HIV combination prevention services [44]. Combination prevention includes traditional interventions such as condoms and lubricants as well as newer strategies such as pre-exposure prophylaxis (PrEP) and suppressive ART [45]. HIV self-testing [46], mobile technologies [47], and long-acting antiretroviral agents [48] are being explored as strategies to increase access to effective HIV prevention and care. However, these strategies are unlikely to be effective for transgender women without an enabling environment that reduces stigma, addresses the associated mental health burden [49], and provides culturally and biomedically appropriate HIV services [50].
Emerging data suggest that provision of gender-affirming care may reduce HIV-related risk [51] and improve engagement in the HIV prevention and care continuum [52,53] for transgender women. Testing interventions among transgender women in sub-Saharan Africa and ensuring that the results are utilized will be critical to ensuring that transgender women in the region are not left behind as countries implement their national HIV plans. In particular, as many countries scale up access to PrEP, important questions of acceptability [54], adherence [55], and potential drug–drug interactions with feminizing hormone therapies [56] must be addressed to facilitate engagement of transgender women.
This study marks a significant step forward in raising the visibility of the HIV epidemic among transgender women in sub-Saharan Africa. However, the findings may not be generalizable to other transgender women in the region due to the use of sampling strategies tailored for cis-MSM. While studies in other regions that specifically recruited transgender women consistently demonstrated a high prevalence of HIV in this population [12], emerging transgender-specific data support associations between access to gender affirmation and HIV risk [51], and it remains a testable hypothesis that transgender women with early access to legal, medical, social, and psychological gender affirmation may be less likely to participate in cis-MSM social networks and also less likely to experience psychosocial drivers of HIV infection.
These data are limited by their cross-sectional nature, which precludes causal inference. The use of face-to-face interviews for data collection may have led to social desirability bias, such as underreporting of HIV risk behaviors. While merging datasets across multiple sites allowed for greater statistical power in the analyses, potentially relevant variables were not included if they only existed in 1 dataset. Finally, interpretation of experiences of stigma was limited because some sites asked participants to attribute their experiences specifically to sexual orientation and practices, while others did not.
Conclusions
Taken together, these data reinforce that gender identity is as complex in sub-Saharan Africa as in other regions, highlighting the need to collect and disaggregate data that distinguish assigned sex at birth from current gender identity. Most importantly, the data provide insights into where interventions are needed to mount an effective HIV response that considers the unique risks and vulnerabilities of transgender women in sub-Saharan Africa. Healthcare providers may use these data, particularly the high prevalence of stigma and violence, to motivate gender-affirming [51], trauma-informed care practices [57,58]. Globally relevant documents are available to guide healthcare providers in implementing HIV services for transgender individuals [59,60].
Appropriately powered studies specifically tailored for transgender women in sub-Saharan Africa would facilitate the robust analyses needed to fill gaps in knowledge of diverse transgender populations and to identify which transgender populations may be most vulnerable to HIV. Knowing that stigma drives both depression [61] and vulnerability to HIV infection, funders and national governments should invest in routine stigma surveillance [62] and the scale-up of effective transgender-inclusive stigma-reduction interventions [63,64] as key components of their HIV strategies. Transgender populations are emerging across sub-Saharan Africa: the data presented here suggest the need to understand the HIV epidemiology and specific HIV prevention and treatment needs of transgender populations in this region.
Supporting Information
Zdroje
1. Kharsany AB, Karim QA. HIV infection and AIDS in sub-Saharan Africa: current status, challenges and opportunities. Open AIDS J. 2016;10 : 34–48. doi: 10.2174/1874613601610010034 27347270
2. Joint United Nations Programme on HIV/AIDS. UNAIDS data 2017. Geneva: Joint United Nations Programme on HIV/AIDS; 2017 [cited 2017 Aug 24]. Available from: http://www.unaids.org/sites/default/files/media_asset/2017_data-book_en.pdf.
3. Plummer FA, Nagelkerke NJ, Moses S, Ndinya-Achola JO, Bwayo J, Ngugi E. The importance of core groups in the epidemiology and control of HIV-1 infection. AIDS. 1991;5(Suppl 1):S169–76.
4. Hunter DJ. AIDS in sub-Saharan Africa: the epidemiology of heterosexual transmission and the prospects for prevention. Epidemiology. 1993;4(1):63–72. 8420583
5. Delva W, Abdool Karim Q. The HIV epidemic in Southern Africa—is an AIDS-free generation possible? Curr HIV/AIDS Rep. 2014;11(2):99–108. doi: 10.1007/s11904-014-0205-0 24676559
6. Baral SD, Grosso A, Holland C, Papworth E. The epidemiology of HIV among men who have sex with men in countries with generalized HIV epidemics. Curr Opin HIV AIDS. 2014;9(2):156–67. doi: 10.1097/COH.0000000000000037 24445371
7. Djomand G, Quaye S, Sullivan PS. HIV epidemic among key populations in west Africa. Curr Opin HIV AIDS. 2014;9(5):506–13. doi: 10.1097/COH.0000000000000090 25010898
8. Baral SD, Ketende S, Mnisi Z, Mabuza X, Grosso A, Sithole B, et al. A cross-sectional assessment of the burden of HIV and associated individual - and structural-level characteristics among men who have sex with men in Swaziland. J Int AIDS Soc. 2013;16(Suppl 3):18768. doi: 10.7448/ias.16.4.18768 24321117
9. Wirtz AL, Trapence G, Kamba D, Gama V, Chalera R, Jumbe V, et al. Geographical disparities in HIV prevalence and care among men who have sex with men in Malawi: results from a multisite cross-sectional survey. Lancet HIV. 2017;4(6):e260–9. doi: 10.1016/S2352-3018(17)30042-5 28256422
10. Jobson GA, Theron LB, Kaggwa JK, Kim H-J. Transgender in Africa: invisible, inaccessible, or ignored? SAHARA J. 2012;9(3):160–3. doi: 10.1080/17290376.2012.743829 23237071
11. Evans MGB, Cloete A, Zungu N, Simbayi LC. HIV risk among men who have sex with men, women who have sex with women, lesbian, gay, bisexual and transgender populations in South Africa: a mini-review. Open AIDS J. 2016;10 : 49–64. doi: 10.2174/1874613601610010049 27347271
12. Poteat T, Scheim A, Xavier J, Reisner S, Baral S. Global epidemiology of HIV infection and related syndemics affecting transgender people. J Acquir Immune Defic Syndr. 2016;72(Suppl 3):S210–9. doi: 10.1097/qai.0000000000001087 27429185
13. Poteat T, German D, Flynn C. The conflation of gender and sex: gaps and opportunities in HIV data among transgender women and MSM. Glob Public Health. 2016;11(7–8):835–48. doi: 10.1080/17441692.2015.1134615 26785751
14. Baral SD, Poteat T, Strömdahl S, Wirtz AL, Guadamuz TE, Beyrer C. Worldwide burden of HIV in transgender women: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13(3):214–22. doi: 10.1016/S1473-3099(12)70315-8 23260128
15. Joint United Nations Programme on HIV/AIDS. Indicators: transgender people—HIV prevalence in transgender people. Geneva: Joint United Nations Programme on HIV/AIDS; 2016 [cited 2017 Aug 24]. Available from: http://aidsinfo.unaids.org/.
16. Joint United Nations Programme on HIV/AIDS. Factsheets: country fact sheets—Democratic Republic of the Congo. Geneva: Joint United Nations Programme on HIV/AIDS; 2016 [cited 2017 Aug 24]. Available from: http://aidsinfo.unaids.org/.
17. Stahlman S, Liestman B, Ketende S, Kouanda S, Ky-Zerbo O, Lougue M, et al. Characterizing the HIV risks and potential pathways to HIV infection among transgender women in Cote d’Ivoire, Togo and Burkina Faso. J Int AIDS Soc. 2016;19(3 Suppl 2):20774. doi: 10.7448/IAS.19.3.20774 27431465
18. Heckathorn DD. Snowball versus respondent-driven sampling. Sociol Methodol. 2011;41(1):355–66. doi: 10.1111/j.1467-9531.2011.01244.x 22228916
19. Mason K, Ketende S, Peitzmeier S, Ceesay N, Diouf D, Loum J, et al. A cross-sectional analysis of population demographics, HIV knowledge and risk behaviors, and prevalence and associations of HIV among men who have sex with men in the Gambia. AIDS Res Hum Retroviruses. 2013;29(12):1547–52. doi: 10.1089/AID.2013.0092 23875674
20. Mbachu II, Udigwe G, Joseph I, John O, Samuel UO, Joseph U, et al. The evaluation of accuracy of serial rapid HIV test algorithm in the diagnosis of HIV antibodies among pregnant women in south east Nigeria. BMC Res Notes. 2015;8 : 557. doi: 10.1186/s13104-015-1454-8 26459010
21. Winter S, Diamond M, Green J, Karasic D, Reed T, Whittle S, et al. Transgender people: health at the margins of society. Lancet. 2016;388(10042):390–400. doi: 10.1016/S0140-6736(16)00683-8 27323925
22. Stahlman S, Sanchez TH, Sullivan PS, Ketende S, Lyons C, Charurat ME, et al. The prevalence of sexual behavior stigma affecting gay men and other men who have sex with men across sub-Saharan Africa and in the United States. JMIR Public Health Surveill. 2016;2(2):e35. doi: 10.2196/publichealth.5824 27460627
23. R Core Team. R: A language and environment for statistical computing Vienna: R Foundation for Statistical Computing; 2016 [cited 2017 Oct 3]. Available from: http://www.R-project.org.
24. Flora DB, Flake JK. The purpose and practice of exploratory and confirmatory factor analysis in psychological research: decisions for scale development and validation. Can J Behav Sci. 2017;49(2):78–88. doi: 10.1037/cbs0000069
25. Wong GY, Mason WM. The hierarchical logistic regression model for multilevel analysis. J Am Stat Assoc. 1985;80(391):513–24. doi: 10.1080/01621459.1985.10478148
26. Murray SO, Roscoe W. Boy-wives and female-husbands: studies in African homosexualities. New York: St. Martin’s Press; 1998.
27. Morgan R, Marais C, Wellbeloved JR. Trans: transgender life stories from South Africa. Sister Namibia. 2009;21(4–5):32–3.
28. Transgender and Intersex Africa. 2017 [cited 2017 May 30]. Available from: http://transgenderintersexafrica.org.za/.
29. Astraea Lesbian Foundation for Justice. S.H.E, Social, Health and Empowerment Feminist Collective of Transgender Women of Africa. New York: Astraea Lesbian Foundation for Justice; 2017 [cited 2017 Oct 7]. Available from: https://www.astraeafoundation.org/stories/s-h-e-social-health-and-empowerment-feminist-collective-of-transgender-women-of-africa/.
30. The People’s Matrix Association. 2017 [cited 2017 May 30]. Available from: https://www.facebook.com/The-Peoples-Matrix-Association-294891844889/.
31. Migiro K. Meet one of Africa’s transgender pioneers. Los Angeles: TakePart; 2015 Apr 14 [cited 2017 May 30]. Available from: http://www.takepart.com/article/2015/04/14/meet-one-africas-transgender-warriors.
32. The Pearl of Africa. 2017 [cited 2017 May 30]. Available from: http://pearlofafrica.tv/.
33. Grubb IR, Beckham SW, Kazatchkine M, Thomas RM, Albers ER, Cabral M, et al. Maximizing the benefits of antiretroviral therapy for key affected populations. J Int AIDS Soc. 2014;17 : 19320. doi: 10.7448/IAS.17.1.19320 25043380
34. Joint United Nations Programme on HIV/AIDS. Fact sheets: country fact sheets—Lesotho. Geneva: Joint United Nations Programme on HIV/AIDS; 2016 [cited 2017 Aug 24]. Available from: http://aidsinfo.unaids.org/.
35. Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, Mermin J. Estimating per-act HIV transmission risk: a systematic review. AIDS. 2014;28(10):1509–19. doi: 10.1097/QAD.0000000000000298 24809629
36. Baggaley RF, White RG, Boily M - C. HIV transmission risk through anal intercourse: systematic review, meta-analysis and implications for HIV prevention. Int J Epidemiol. 2010;39(4):1048–63. doi: 10.1093/ije/dyq057 20406794
37. Martins TA, Kerr LR, Macena RH, Mota RS, Carneiro KL, Gondim RC, et al. Travestis, an unexplored population at risk of HIV in a large metropolis of northeast Brazil: a respondent-driven sampling survey. AIDS Care. 2013;25(5):606–12. doi: 10.1080/09540121.2012.726342 23082818
38. Guadamuz TE, Wimonsate W, Varangrat A, Phanuphak P, Jommaroeng R, McNicholl JM, et al. HIV prevalence, risk behavior, hormone use and surgical history among transgender persons in Thailand. AIDS Behav. 2011;15(3):650–8. doi: 10.1007/s10461-010-9850-5 21104008
39. Santos GM, Rapues J, Wilson EC, Macias O, Packer T, Colfax G, et al. Alcohol and substance use among transgender women in San Francisco: prevalence and association with human immunodeficiency virus infection. Drug Alcohol Rev. 2014;33(3):287–95. doi: 10.1111/dar.12116 24628655
40. Secor AM, Wahome E, Micheni M, Rao D, Simoni JM, Sanders EJ, et al. Depression, substance abuse and stigma among men who have sex with men in coastal Kenya. AIDS. 2015;29(Suppl 3):S251–9. doi: 10.1097/qad.0000000000000846 26562814
41. Lane T, Raymond HF, Dladla S, Rasethe J, Struthers H, McFarland W, et al. High HIV prevalence among men who have sex with men in Soweto, South Africa: results from the Soweto Men’s Study. AIDS Behav. 2011;15(3):626–34. doi: 10.1007/s10461-009-9598-y 19662523
42. Johnston LG, Holman A, Dahoma M, Miller LA, Kim E, Mussa M, et al. HIV risk and the overlap of injecting drug use and high-risk sexual behaviours among men who have sex with men in Zanzibar (Unguja), Tanzania. Int J Drug Policy. 2010;21(6):485–92. doi: 10.1016/j.drugpo.2010.06.001 20638262
43. Joint United Nations Programme on HIV/AIDS. 90-90-90: an ambitious treatment target to help end the AIDS epidemic. Geneva: Joint United Nations Programme on HIV/AIDS; 2014.
44. Joint United Nations Programme on HIV/AIDS. On the fast-track to end AIDS: UNAIDS 2016–2021 Strategy. Geneva: Joint United Nations Programme on HIV/AIDS; 2015.
45. World Health Organization. Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations—2016 update. Geneva: World Health Organization; 2016.
46. Stevens DR, Vrana CJ, Dlin RE, Korte JE. A global review of HIV self-testing: themes and implications. AIDS Behav. 2017 Feb 2. doi: 10.1007/s10461-017-1707-8 28155039
47. Ippoliti NB, L’Engle K. Meet us on the phone: mobile phone programs for adolescent sexual and reproductive health in low-to-middle income countries. Reprod Health. 2017;14(1):11. doi: 10.1186/s12978-016-0276-z 28095855
48. Nyaku AN, Kelly SG, Taiwo BO. Long-acting antiretrovirals: where are we now? Curr HIV/AIDS Rep. 2017;14(2):63–71. doi: 10.1007/s11904-017-0353-0 28303548
49. Chibanda D. Depression and HIV: integrated care towards 90-90-90. Int Health. 2017;9(2):77–9. doi: 10.1093/inthealth/ihw058 28115469
50. Mayer KH, Grinsztejn B, El-Sadr WM. Transgender people and HIV prevention: what we know and what we need to know, a call to action. J Acquir Immune Defic Syndr. 2016;72(Suppl 3):S207–9. doi: 10.1097/qai.0000000000001086 27429184
51. Sevelius JM. Gender affirmation: a framework for conceptualizing risk behavior among transgender women of color. Sex Roles. 2013;68(11–12):675–89. doi: 10.1007/s11199-012-0216-5 23729971
52. Grant RM, Sevelius JM, Guanira JV, Aguilar JV, Chariyalertsak S, Deutsch MB. Transgender women in clinical trials of pre-exposure prophylaxis. J Acquir Immune Defic Syndr. 2016;72(Suppl 3):S226–9. doi: 10.1097/qai.0000000000001090 27429187
53. Reisner SL, Radix A, Deutsch MB. Integrated and gender-affirming transgender clinical care and research. J Acquir Immune Defic Syndr. 2016;72(Suppl 3):S235–42. doi: 10.1097/qai.0000000000001088 27429189
54. Sevelius JM, Keatley J, Calma N, Arnold E. ‘I am not a man’: trans-specific barriers and facilitators to PrEP acceptability among transgender women. Glob Public Health. 2016;11(7–8):1060–75. doi: 10.1080/17441692.2016.1154085 26963756
55. Deutsch MB, Glidden DV, Sevelius J, Keatley J, McMahan V, Guanira J, et al. HIV pre-exposure prophylaxis in transgender women: a subgroup analysis of the iPrEx trial. Lancet HIV. 2015;2(12):e512–9. doi: 10.1016/S2352-3018(15)00206-4 26614965
56. Anderson PL, Reirden D, Castillo-Mancilla J. Pharmacologic considerations for preexposure prophylaxis in transgender women. J Acquir Immune Defic Syndr. 2016;72(Suppl 3):S230–4. doi: 10.1097/qai.0000000000001105 27429188
57. Eckstrand KL, Potter J. Trauma, resilience, and health promotion in LGBT patients: what every healthcare provider should know. New York: Springer; 2017.
58. Sales JM, Swartzendruber A, Phillips AL. Trauma-informed HIV prevention and treatment. Curr HIV/AIDS Rep. 2016;13(6):374–82. doi: 10.1007/s11904-016-0337-5 27704251
59. Wolf RC, Adams D, Dayton R, Verster A, Wong J, Romero M, et al. Putting the t in tools: a roadmap for implementation of new global and regional transgender guidance. J Int AIDS Soc. 2016;19(Suppl 2):20801.
60. Deutsch MB, Feldman JL. Updated recommendations from the World Professional Association for Transgender Health standards of care. Am Fam Phys2013;87(2):89–93.
61. Chakrapani V, Vijin PP, Logie CH, Newman PA, Shunmugam M, Sivasubramanian M, et al. Understanding how sexual and gender minority stigmas influence depression among trans women and men who have sex with men in India. LGBT Health. 2017;4(3):217–26. doi: 10.1089/lgbt.2016.0082 28422615
62. Stahlman S, Hargreaves JR, Sprague L, Stangl AL, Baral SD. Measuring sexual behavior stigma to inform effective HIV prevention and treatment programs for key populations. JMIR Public Health Surveill. 2017;3(2):e23. doi: 10.2196/publichealth.7334 28446420
63. Thapa S, Hannes K, Cargo M, Buve A, Aro AR, Mathei C. Building a conceptual framework to study the effect of HIV stigma-reduction intervention strategies on hiv test uptake: a scoping review. J Assoc Nurses AIDS Care. 2017;28(4):545–60. doi: 10.1016/j.jana.2017.04.004 28473183
64. Stangl AL, Lloyd JK, Brady LM, Holland CE, Baral S. A systematic review of interventions to reduce HIV-related stigma and discrimination from 2002 to 2013: how far have we come? J Int AIDS Soc. 2013;16(3 Suppl 2):18734. doi: 10.7448/IAS.16.3.18734 24242268
Štítky
Interní lékařství
Článek Contemporary disengagement from antiretroviral therapy in Khayelitsha, South Africa: A cohort studyČlánek Bioequivalence of twice-daily oral tacrolimus in transplant recipients: More evidence for consensus?
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2017 Číslo 11- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Ferinject: správně indikovat, správně podat, správně vykázat
- Optimální dávkování apixabanu v léčbě fibrilace síní
-
Všechny články tohoto čísla
- Labour trafficking: Challenges and opportunities from an occupational health perspective
- The end of HIV: Still a very long way to go, but progress continues
- Contemporary disengagement from antiretroviral therapy in Khayelitsha, South Africa: A cohort study
- Bioequivalence of twice-daily oral tacrolimus in transplant recipients: More evidence for consensus?
- Treatment guidelines and early loss from care for people living with HIV in Cape Town, South Africa: A retrospective cohort study
- Perinatal mortality associated with induction of labour versus expectant management in nulliparous women aged 35 years or over: An English national cohort study
- Core Outcome Set-STAndards for Development: The COS-STAD recommendations
- Closing the gaps in the HIV care continuum
- Association between the 2012 Health and Social Care Act and specialist visits and hospitalisations in England: A controlled interrupted time series analysis
- HIV pre-exposure prophylaxis and early antiretroviral treatment among female sex workers in South Africa: Results from a prospective observational demonstration project
- Sexual exploitation of unaccompanied migrant and refugee boys in Greece: Approaches to prevention
- Child sex trafficking in the United States: Challenges for the healthcare provider
- The expanding epidemic of HIV-1 in the Russian Federation
- Cardiovascular disease (CVD) and chronic kidney disease (CKD) event rates in HIV-positive persons at high predicted CVD and CKD risk: A prospective analysis of the D:A:D observational study
- Validity of a minimally invasive autopsy for cause of death determination in maternal deaths in Mozambique: An observational study
- malERA: An updated research agenda for malaria elimination and eradication
- malERA: An updated research agenda for health systems and policy research in malaria elimination and eradication
- A combination intervention strategy to improve linkage to and retention in HIV care following diagnosis in Mozambique: A cluster-randomized study
- Bioequivalence between innovator and generic tacrolimus in liver and kidney transplant recipients: A randomized, crossover clinical trial
- malERA: An updated research agenda for basic science and enabling technologies in malaria elimination and eradication
- Human trafficking and exploitation: A global health concern
- Virological response and resistance among HIV-infected children receiving long-term antiretroviral therapy without virological monitoring in Uganda and Zimbabwe: Observational analyses within the randomised ARROW trial
- Postmenopausal hormone therapy and risk of stroke: A pooled analysis of data from population-based cohort studies
- Lansoprazole use and tuberculosis incidence in the United Kingdom Clinical Practice Research Datalink: A population based cohort
- malERA: An updated research agenda for insecticide and drug resistance in malaria elimination and eradication
- Safety, pharmacokinetics, and immunological activities of multiple intravenous or subcutaneous doses of an anti-HIV monoclonal antibody, VRC01, administered to HIV-uninfected adults: Results of a phase 1 randomized trial
- HIV prevalence and behavioral and psychosocial factors among transgender women and cisgender men who have sex with men in 8 African countries: A cross-sectional analysis
- Treatment eligibility and retention in clinical HIV care: A regression discontinuity study in South Africa
- malERA: An updated research agenda for characterising the reservoir and measuring transmission in malaria elimination and eradication
- Effectiveness of a combination strategy for linkage and retention in adult HIV care in Swaziland: The Link4Health cluster randomized trial
- The value of confirmatory testing in early infant HIV diagnosis programmes in South Africa: A cost-effectiveness analysis
- HIV self-testing among female sex workers in Zambia: A cluster randomized controlled trial
- The US President's Malaria Initiative, transmission and mortality: A modelling study
- Comparison of two cash transfer strategies to prevent catastrophic costs for poor tuberculosis-affected households in low- and middle-income countries: An economic modelling study
- Direct provision versus facility collection of HIV self-tests among female sex workers in Uganda: A cluster-randomized controlled health systems trial
- malERA: An updated research agenda for diagnostics, drugs, vaccines, and vector control in malaria elimination and eradication
- malERA: An updated research agenda for combination interventions and modelling in malaria elimination and eradication
- HIV-1 persistence following extremely early initiation of antiretroviral therapy (ART) during acute HIV-1 infection: An observational study
- Respondent-driven sampling for identification of HIV- and HCV-infected people who inject drugs and men who have sex with men in India: A cross-sectional, community-based analysis
- Extensive virologic and immunologic characterization in an HIV-infected individual following allogeneic stem cell transplant and analytic cessation of antiretroviral therapy: A case study
- Measuring success: The challenge of social protection in helping eliminate tuberculosis
- Prospects for passive immunity to prevent HIV infection
- Reaching global HIV/AIDS goals: What got us here, won't get us there
- Evidence-based restructuring of health and social care
- Extreme exploitation in Southeast Asia waters: Challenges in progressing towards universal health coverage for migrant workers
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Postmenopausal hormone therapy and risk of stroke: A pooled analysis of data from population-based cohort studies
- Bioequivalence between innovator and generic tacrolimus in liver and kidney transplant recipients: A randomized, crossover clinical trial
- HIV pre-exposure prophylaxis and early antiretroviral treatment among female sex workers in South Africa: Results from a prospective observational demonstration project
- Bioequivalence of twice-daily oral tacrolimus in transplant recipients: More evidence for consensus?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Revma Focus: Spondyloartritidy
nový kurz
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



