-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaKu-Mediated Coupling of DNA Cleavage and Repair during Programmed Genome Rearrangements in the Ciliate
DNA double-strand breaks (DSBs) are potential threats for chromosome stability, but they are usually repaired by two major pathways, homologous recombination or non-homologous end joining (NHEJ). DSBs can also be essential during physiological processes, such as the programmed removal of germline sequences that takes place in various eukaryotes, including ciliates, during somatic differentiation. We use the ciliate Paramecium tetraurelia as a unicellular model to study how DNA breakage and DSB repair are coordinated during programmed genome rearrangements. In this organism, assembly of the somatic genome involves the elimination of ∼25% of germline DNA, including the precise excision of thousands of short Internal Eliminated Sequences (IES) scattered along germline chromosomes. A domesticated piggyBac transposase, PiggyMac, is required for double-strand DNA cleavage at IES ends and IES excision sites are very precisely repaired by the NHEJ pathway. Here, we report that a specialized Ku heterodimer, specifically expressed during programmed genome rearrangements, is an essential partner of PiggyMac and activates DNA cleavage. We propose that incorporation of DSB repair proteins in a pre-cleavage complex constitutes a safe and efficient way for Paramecium to direct thousands of programmed DSBs to the NHEJ pathway and make sure that somatic chromosomes are assembled correctly.
Published in the journal: . PLoS Genet 10(8): e32767. doi:10.1371/journal.pgen.1004552
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004552Summary
DNA double-strand breaks (DSBs) are potential threats for chromosome stability, but they are usually repaired by two major pathways, homologous recombination or non-homologous end joining (NHEJ). DSBs can also be essential during physiological processes, such as the programmed removal of germline sequences that takes place in various eukaryotes, including ciliates, during somatic differentiation. We use the ciliate Paramecium tetraurelia as a unicellular model to study how DNA breakage and DSB repair are coordinated during programmed genome rearrangements. In this organism, assembly of the somatic genome involves the elimination of ∼25% of germline DNA, including the precise excision of thousands of short Internal Eliminated Sequences (IES) scattered along germline chromosomes. A domesticated piggyBac transposase, PiggyMac, is required for double-strand DNA cleavage at IES ends and IES excision sites are very precisely repaired by the NHEJ pathway. Here, we report that a specialized Ku heterodimer, specifically expressed during programmed genome rearrangements, is an essential partner of PiggyMac and activates DNA cleavage. We propose that incorporation of DSB repair proteins in a pre-cleavage complex constitutes a safe and efficient way for Paramecium to direct thousands of programmed DSBs to the NHEJ pathway and make sure that somatic chromosomes are assembled correctly.
Introduction
DNA double strand breaks (DSBs) are among the most deleterious DNA lesions: if left unrepaired, a single DSB may trigger cell death, while incorrect repair can give rise to chromosome rearrangements [1]. Cells rely on two major pathways to repair DSBs. Homologous recombination (HR) uses a homologous template to restore the sequence of the broken chromosome, while non-homologous end joining (NHEJ) proceeds through the ligation of free DNA ends. Even though they can be very toxic, programmed DSBs are obligatory intermediates in essential biological processes, such as meiosis or acquired immune response. During meiosis, the Spo11 endonuclease cleaves DNA and DSB repair is carried out by HR [2]. In addition to favoring the exchange of parental alleles, HR ensures that homologous chromosomes are correctly paired before they are segregated during the first meiotic division. During lymphocyte differentiation, programmed genome rearrangements (PGR) mediated through V(D)J recombination generate the large diversity of immunoglobulin genes [3]. During V(D)J recombination, the domesticated transposase RAG1, associated with its partner RAG2, cleaves specific recombination sites. The resulting DSBs are repaired through classical NHEJ (C-NHEJ).
A critical step in C-NHEJ is the binding of the Ku70/Ku80 heterodimer to broken DNA ends [4]. Upon binding, Ku protects DNA ends from extensive resection [5] and, together with its facultative partner DNA-PKcs, facilitates the synapsis of two broken ends. Following recruitment of DNA processing enzymes, the Ligase IV-Xrcc4 complex mediates the joining of DNA ends. An alternative end joining pathway, referred to as alt-NHEJ (or MMEJ, for microhomology-dependent end joining), has been reported [6]. This poorly characterized pathway is independent of Ku and, to some extent, of Ligase IV. Because of the absence of Ku, alt-NHEJ involves limited 5′ to 3′ resection of broken DNA ends and generates deletions at DSB repair sites, which often involve microhomologies. When DSBs are repaired through HR, 5′ to 3′ resection also takes place, but two steps can be distinguished: initial short-range end resection relies on the same factors as alt-NHEJ [7], while subsequent long-range resection generates the long 3′ single strand that will invade a homologous DNA duplex [8].
Ciliates provide extraordinary models to study the interplay between DNA cleavage and DSB repair during PGR [9]. In these unicellular eukaryotes, two kinds of nuclei coexist in the same cytoplasm. The highly polyploid somatic macronucleus (MAC) is essential for gene expression but it is destroyed at each sexual cycle, while the diploid micronucleus (MIC) undergoes meiosis and transmits the germline genome to the new MIC and MAC of the next generation. In Paramecium, massive PGR take place in the new developing MAC, while the genome is amplified from 2n to 800n [10]. These PGR consist in the elimination of two types of germline-specific DNA. Regions of up to several kbp in length, often containing repeated sequences, are eliminated in a heterogeneous manner, leading to chromosome fragmentation or intra-chromosomal deletions. In addition, thousands of single-copy, short and non-coding Internal Eliminated Sequences (IES) are excised precisely. Because 47% of genes are interrupted by at least one IES in the germline genome [11], the precise excision of IESs is essential for the assembly of functional genes in the new MAC and the survival of the sexual progeny. Paramecium IESs are invariably flanked by one TA dinucleotide on each side and little additional information can be found in their nucleotide sequence, which raises the question of how these sequences are recognized and targeted for excision. In fact, the excision of an estimated one-third of Paramecium IESs is controlled maternally through a sequence homology-dependent mechanism [12]. For these so-called maternally-controlled IESs, a genome-wide comparison of the germline and rearranged versions of the genome involves non-coding RNAs [13], [14]. This epigenetic control drives the trans-generational inheritance of rearrangement patterns, from the old to the new MAC.
IES excision proceeds through a two-step “cut-and-close” mechanism. A domesticated piggyBac transposase, PiggyMac (Pgm), is essential to introduce the DNA cleavages that initiate the reaction, generating 4-bp staggered DSBs centered on the conserved TA at each IES boundary [15]. Following IES release, precise DSB repair is carried out through the C-NHEJ pathway and leaves a single TA at the IES excision site [16]. Providing their length allows enough DNA flexibility, the excised linear IESs are circularized, also through the C-NHEJ pathway, before they are actively degraded. During DSB repair, the flanking broken ends are thought to anneal through the pairing of the complementary TAs carried by their 4-base 5′ overhangs, and undergo limited 5′ and 3′ processing [17]. The final ligation step is mediated by the NHEJ-specific ligase complex, Ligase IV and its partner Xrcc4, both of which are essential for PGR. When LIG4 or XRCC4 genes are knocked down, Pgm-dependent DSBs are introduced normally, but they accumulate in the developing new MAC, which correlates with severely compromised DNA amplification and impairs the recovery of viable progeny [16].
Next-generation sequencing of the non-rearranged genome of Paramecium tetraurelia led to the identification of at least 45,000 IESs [11]. Therefore, a huge number of programmed DSBs have to be repaired precisely during MAC development. In the present study, we have addressed the question of how the C-NHEJ pathway is recruited to IES excision sites to carry out efficient and precise DSB repair. We have focused our analysis on the Ku heterodimer, which is the most upstream actor of the C-NHEJ pathway. Two KU70 and three KU80 genes were identified in the somatic genome [16]. We report here that development-specific KU genes are essential for PGR. Surprisingly, we demonstrate that Ku is required for the introduction of programmed DSBs at IES boundaries, and provide evidence that Ku interacts functionally and physically with Pgm. We propose that the Ku70a/Ku80c heterodimer forms a complex with Pgm and activates DNA cleavage during PGR in P. tetraurelia.
Results
Development-specific KU genes in P. tetraurelia
Two genes encoding Ku70 homologs, KU70a and KU70b, were identified in the macronuclear genome assembly of P. tetraurelia (Figure 1A and [16]). These two closely related copies arose from a recent whole genome duplication (WGD) that took place during the evolution of this species [18], and are referred to as ohnologs. We also identified three homologs of the human KU80 gene (Figure 1A). KU80a and KU80b are ohnologs from the recent WGD, while the more distant KU80c diverged after an earlier, “intermediate”, WGD.
Fig. 1. KU70 and KU80 genes in P. tetraurelia. 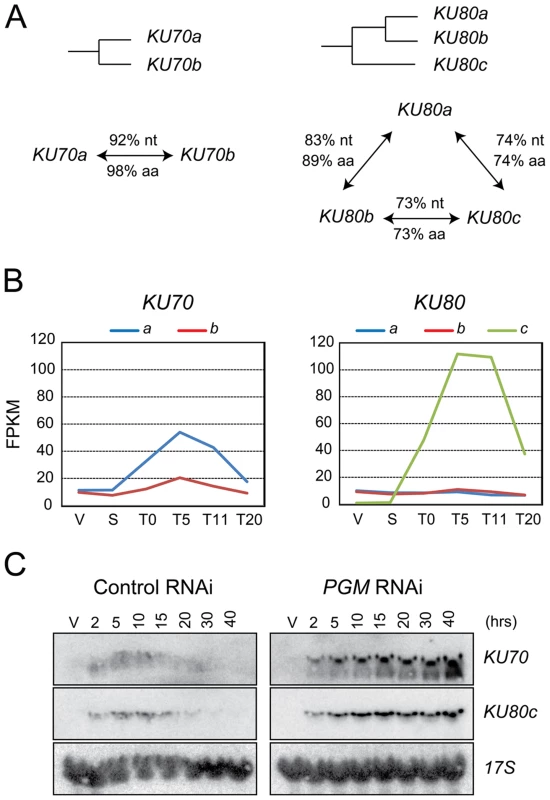
(A) Diagram of the WGD relationships between KU70 or KU80 genes in P. tetraurelia. The % of identity between genes (nt) and proteins (aa) are indicated along the arrows connecting two genes. (B) Transcription profiles of KU70 and KU80 genes during an autogamy time-course of strain 51, as determined by high-throughput RNA-seq. V: vegetative cells; S: starved or meiotic cells with intact parental MAC; T0: 50% of cells with fragmented MAC; the following time-points refer to hours after T0 [51]. On the vertical axis, FPKM represents the number of fragments per gene kb per million of fragments that were uniquely mapped on the genome. (C) Detection of KU70 and KU80c mRNA during autogamy, through northern blot hybridization. Control RNAi: RNAi against the nonessential ND7 gene [43], which encodes an exocytosis protein. V: vegetative cells. The times refer to hours after T0, the time at which 50% of cells have a fragmented MAC (Figure S1A). Sexual processes in Paramecium may occur in two different ways: during conjugation, following the mixing of two reactive partners with compatible mating types, or during a self-fertilization process called autogamy, in which MIC meiosis is induced upon starvation in cells of a single mating type [19]. A microarray analysis of the P. tetraurelia transcriptome during autogamy revealed that KU70a and KU80c are specifically induced during the development of the new MAC, when PGR take place [16], [20]. The microarray data were confirmed by high-throughput sequencing of polyadenylated RNAs extracted during an autogamy time-course (Figure 1B, Arnaiz et al. in prep.), and by northern blot hybridization with specific probes (Figures 1C and 3C, left panels; Malinsky et al. in prep.). After Pgm depletion, the transcription of KU70 and KU80c is switched on at the expected time-point during autogamy (Figure 1C, right panels), indicating that KU genes are not induced as a response to Pgm-induced DSBs, but more likely as part of a general transcription program during MAC development. Moreover, in contrast to control cells, the levels of KU70 and KU80c mRNAs do not decrease at later time-points after Pgm depletion, suggesting that the completion of PGR is a signal for transcriptional switch-off.
Ku70a and Ku80c localize in the developing new MAC
The expression of KU70a and KU80c is specifically induced during autogamy and reaches a peak when the developing new MACs start to be detected in the culture (T5 and T11 time-points in Figure 1B). Using N-terminal GFP fusions, we followed the cellular localization of Ku70a and Ku80c during autogamy. Transgenes expressing each fusion protein under the control of their respective endogenous promoters were microinjected into the MAC of vegetative cells. The resulting transformants were grown and starved to induce autogamy. Consistent with the transcriptome analysis, GFP fluorescence was stronger in autogamous cells with respect to vegetative cells for each fusion transgene (Figure 2). Both fusion proteins accumulated in the developing new MACs, suggesting a possible involvement of Ku70a and Ku80c in PGR during MAC development.
Fig. 2. Nuclear localization of GFP-Ku fusions during autogamy. 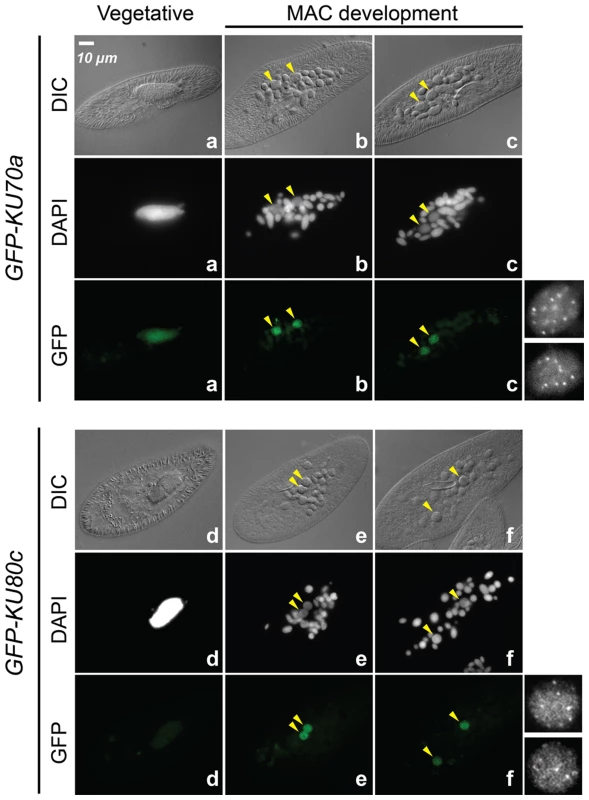
Cells were microinjected with fusion transgenes expressing GFP-Ku70a (panels a, b, c) and GFP-Ku80c (panels d, e, f) under the control of their respective transcription signals. In autogamous cells, developing MACs are indicated by yellow arrowheads, the other DAPI-stained nuclei are fragments from the old vegetative MAC. For each protein, the GFP fluorescence sometimes concentrated in nuclear foci of unclear biological significance (see cells in panels c and f, and enlarged inserts on the right). In this particular experiment, expression of the GFP fusions had no significant effect on the recovery of viable post-autogamous progeny (87% progeny with functional new MACs for GFP-Ku70a, 90% for GFP-Ku80c). Fig. 3. RNAi screen for essential KU genes during autogamy. 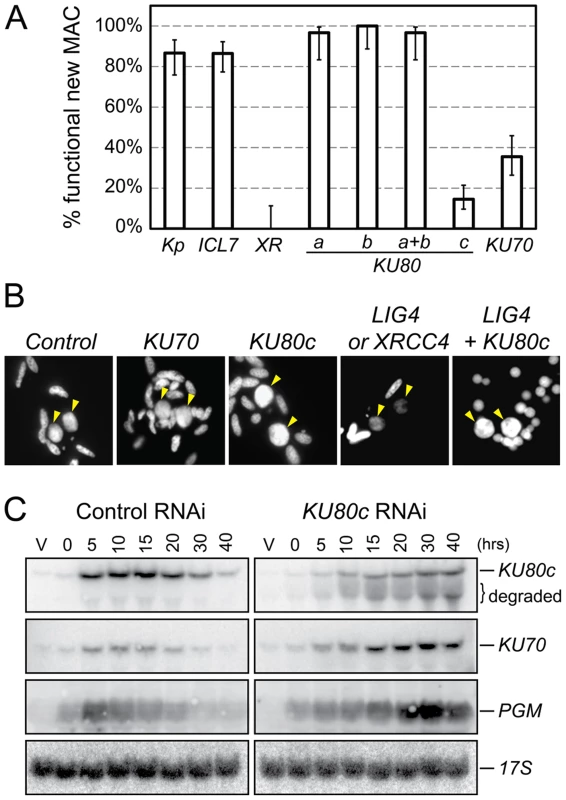
(A) Survival of the post-autogamous progeny of cells submitted to different combinations of RNAi. Kp: autogamy in standard K. pneumoniae medium; ICL7: RNAi against ICL7, a nonessential gene that encodes an infraciliary lattice centrin [47]; XR: RNAi against XRCC4. RNAi experiments against KU80 genes were performed using gene-specific inserts KU80-a2, KU80-b2 and KU80-c2 (see Figure S2). For each condition, 30 to ∼140 post-autogamous cells were analyzed. Each bar represents the percentage of viable post-autogamous cells carrying a functional new MAC, for each condition. Error bars represent the Wilson score intervals (95% confidence level), which are appropriate for a small number of trials or for values close to an extreme probability. (B) DAPI-staining of developing MACs during RNAi against ND7 (control), KU70, KU80c, XRCC4/LIG4 and LIG4 + KU80c. Developing MACs are indicated by yellow arrowheads. Upon LIG4 or XRCC4 RNAi, developing MACs exhibit faint DAPI staining, which correlates with a defect in DNA amplification [16]. (C) Northern blot hybridization of total RNA during a control time course experiment (ND7 RNAi) and in a KU80c RNAi. V: vegetative cells; T0: 60% of cells with fragmented MAC; other time-points refer to hours following T0 (Figure S1B). Ku70 and Ku80c are required for successful completion of autogamy
To test the implication of the different KU genes in MAC development, we knocked them down systematically by feeding wild-type P. tetraurelia cells on dsRNA-producing bacteria to induce RNA interference [21]. The very high percentage of identity between KU70a and KU70b made it impossible to design specific RNAi constructs for each individual gene. Therefore, we silenced them both together. In contrast, we designed gene-specific RNAi constructs for KU80a, KU80b and KU80c. Whenever possible, to make sure that the observed phenotypes would be attributable to the silencing of each targeted gene, we used RNAi constructs homologous to two different regions in each KU80 gene (Figure S2). After three days of starvation in each silencing medium, individual autogamous cells were transferred to standard growth medium and allowed to resume vegetative growth. The survivors were grouped in three categories: (i) those that were able to undergo a new round of autogamy following a few divisions, most likely because they had regenerated their old MAC, were not considered as bona fide post-autogamous progeny [16]; (ii) slow-growing survivors were counted as progeny with a defective new MAC; (iii) those survivors that remained in the vegetative state when starved after a few divisions were classified as fully viable post-autogamous progeny. Only the recovery of survivors from the third category indicated that the silenced cells had been able to form a functional new MAC (Figure 3A).
When KU80a or KU80b were knocked down, either individually or together, the progeny exhibited good survival rates when compared to a control RNAi against the nonessential ICL7 gene, or with respect to cells that underwent autogamy in standard medium (Figure 3A). In contrast, RNAi against KU80c or KU70 yielded only 10 to 30% viable sexual progeny, showing that the developmentally-induced KU80c gene and one or both KU70 genes are required for the completion of autogamy. Interestingly, we observed that new MACs develop normally at the cytological level in cells silenced for KU70 or KU80c (Figure 3B), while cells silenced for LIG4 or XRCC4 were previously shown to harbor small new developing MACs that remain faintly stained with DAPI, correlating with a block in DNA amplification [16]. Bright DAPI staining of the new MACs indicates that DNA amplification takes place normally in Ku-depleted cells.
Residual survival was observed reproducibly following Ku70 or Ku80c depletions, in contrast to the severe lethality phenotype of Xrcc4 depletion (Figure 3A). This phenotypic difference, which was also noted during mouse embryonic development [22], may be explained by the fact that Ku acts upstream of Ligase IV/Xrcc4 during C-NHEJ. Upon Ku depletion, alternative DSB repair pathways could restore chromosome integrity to some extent, while Ligase IV/Xrcc4 depletion would constitute a dead-end for DSBs, committed to C-NHEJ in the presence of Ku. We noted, indeed, that normal DAPI staining of the developing MAC is restored in Ligase IV-depleted cells by simultaneously depleting Ku80c (Figure 3B). At this stage of our study, this observation is consistent with the hypothesis that activation of alternative Ku-independent DSB repair pathways may rescue DNA amplification in the new MAC, therefore allowing the recovery of low amounts of post-autogamous survivors. An alternative explanation for the residual survival observed upon Ku80c depletion could also be that KU80c transcripts are only partially degraded by RNAi, as testified by the restoration of significant amounts of full-length mRNA at T30 and T40 (Figure 3C, top lane).
KU70 and KU80c are essential for the programmed elimination of germline DNA
We showed previously that the NHEJ-specific Ligase IV/Xrcc4 ligation complex joins broken DNA ends at IES excision sites [16]. Strikingly, after Ligase IV depletion, unrepaired broken ends remain very stable throughout autogamy, suggesting that Ku protects them against degradation. To gain further insight into the role of Ku during IES excision, we performed a molecular analysis of PGR in Ku-depleted cells. As predicted from the known role of Ku during C-NHEJ, we expected that DSBs would be introduced normally at IES boundaries, but that the broken DNA ends would not be directed to the C-NHEJ pathway.
We first focused our analysis on cells depleted in Ku80c. Using Southern blot hybridization, we monitored the excision of one particular IES, IES 51G4404 from the surface antigen G51 gene [23], during an autogamy time-course (Figure 4A). In a control RNAi (left panel), a major band corresponded to the IES− molecules from the old MAC and the newly rearranged molecules from the developing new MAC. A higher molecular weight species corresponding to the non-excised IES+ form was transiently and only barely detectable during MAC development (lanes 2′ to 4), and was completely absent at late time-points as a result of IES excision. In contrast, the IES+ form was clearly amplified during autogamy after Ku80c depletion (right panel). Using a related strategy, we tested chromosome fragmentation at one particular fragmentation site located downstream of the G51 gene (Figure 4B). We observed that non-rearranged molecules are amplified during MAC development in Ku80c-depleted cells, which indicates that Ku is also required for the elimination of the germline DNA that is associated with chromosome fragmentation.
Fig. 4. Molecular analysis of genome rearrangements after Ku80c depletion. 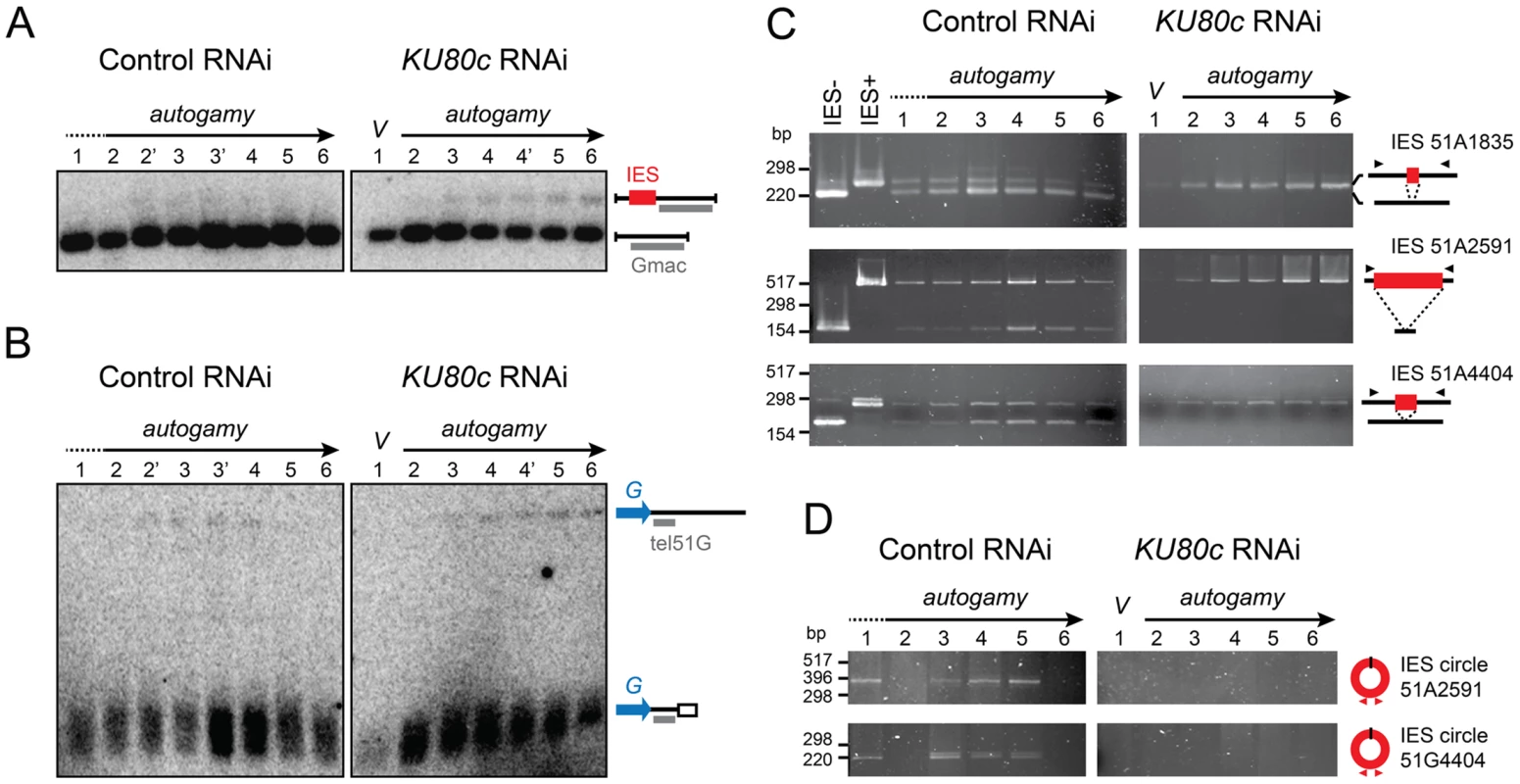
(A) Detection of DNA fragments with excised or non-excised IES 51G4404 from the surface antigen G51 gene. Total genomic DNA was extracted during autogamy of 51ΔA cells subjected to RNAi against ICL7 (control) or KU80c. To compare the two time-courses, similar autogamy stages were numbered from 1 to 6, based on the observation of DAPI-stained cells (Figure S1C). PstI-hydrolyzed total genomic DNA was run on 1% agarose gels. Southern blots were hybridized with the Gmac probe (in grey), which hybridizes to the flanking MAC DNA downstream of the IES. (B) Detection of fragmented or non-fragmented DNA downstream of the G51 gene by Southern blot hybridization of PstI-digested total genomic DNA (same samples as in A) run on 0.8% agarose gels. The subtelomeric tel51G probe is shown in grey. The white box in the bottom right diagram represents telomeric repeats. (C) PCR detection of de novo IES excision junctions during autogamy of 51ΔA cells, in an ICL7 (control) or a KU80c RNAi. Note that a few autogamous cells were present at time-point 1 in the control RNAi (see Figure S1C). (D) PCR detection of IES circle junctions during autogamy in an ICL7 (control) or a KU80c RNAi. Triangles in C and D represent PCR primers (see Table S1). The amplification of non-rearranged DNA indicates that PGR do not proceed normally after Ku80c depletion. However, because of the presence of old MAC DNA in our samples, the above experiment could not detect whether residual rearrangements had taken place in the new MAC. We could circumvent this problem, because the strain used in this experiment (51ΔA) harbors a wild-type germline genome, but carries a somatic deletion of the nonessential surface antigen gene A51. During autogamy, all IESs are excised normally from the A51 gene before the whole locus is deleted from the new somatic MAC [24]. Therefore, in the 51ΔA variant, all the IES− molecules originating from this locus may be attributed to de novo IES excision from the new MAC. Using PCR primers hybridizing in the flanking sequences, excised (IES−) and non-excised (IES+) molecules were readily detected in a control RNAi experiment, for IESs belonging to different classes (Figure 4C, left panel): short (51A1835 : 28 bp), intermediate (51A4404 : 77 bp) or long (51A2591 : 370 bp), maternally (51A2591) or non-maternally controlled (51A1835 and 51A4404). This stands in sharp contrast to the complete absence of de novo IES excision junctions after Ku depletion (right panel). Likewise, using IES-specific internal divergent primers, we followed the appearance of excised IES circular molecules during the autogamy of control and KU80c-silenced cells, but no circle junctions were detected following Ku80c depletion (Figure 4D).
To extend our analysis to the other KU genes, we submitted small-scale cultures of 51ΔA cells to RNAi against KU70 or individual KU80 genes, and tested their ability to complete IES excision during autogamy. When KU80a and KU80b were knocked down, together or separately, rearranged IES− molecules appeared at day 2 of starvation, with the same timing as in a control RNAi (Figure S3). In contrast, the appearance of de novo IES excision junctions was strongly impaired upon Ku70 depletion, similar to Ku80c depletion. Taken together, our molecular data indicate that Ku70 and the development-specific Ku80c are essential for the recovery of both precise chromosomal junctions and excised IES circles during PGR. Moreover, after Ku70 or Ku80c depletion, the non-rearranged version of the genome is amplified in the developing MAC, a strikingly different phenotype from that of Ligase IV or Xrcc4 depletions [16], but quite similar to Pgm depletion [15].
No DNA breaks can be detected by LMPCR in cells depleted for Ku80c
The absence of precise de novo IES excision junctions after Ku70 or Ku80c depletions could either reflect a problem in DSB repair, as established for Ligase IV depletion, or an inhibition of DNA cleavage, as demonstrated for Pgm depletion. Because Ku is essential for C-NHEJ in all organisms, a defect in end-joining was expected after Ku depletion in P. tetraurelia. However, the observation that non-excised IESs were amplified in the new MAC of cells depleted for Ku70/Ku80c raised the issue of whether DSBs were actually introduced at IES boundaries. We therefore used a sensitive ligation-mediated PCR assay (LMPCR) to search for DSBs at IES boundaries after Ku depletion. As previously published [17], for those IESs that were tested, free broken DNA ends at IES boundaries were detected at early autogamy time-points in cells subjected to a control RNAi (Figure 5A). DSBs disappeared later on during MAC development, indicative of efficient repair. After Ku80c depletion, no specific broken ends were detected on the MAC side or the IES side of the DSB (Figure 5A).
Fig. 5. Detection of programmed DSBs at IES boundaries during autogamy. 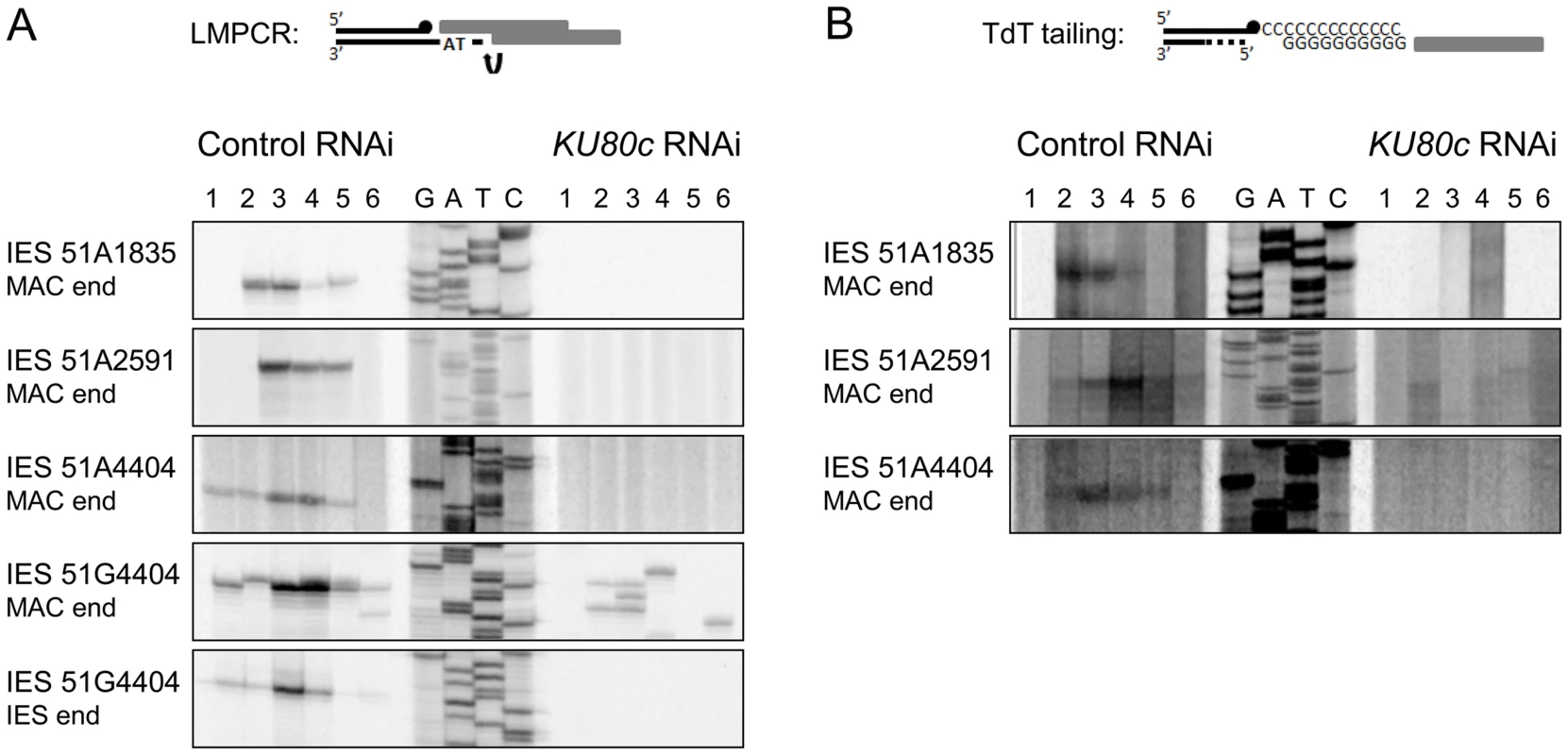
(A) LMPCR detection of DSBs at MAC or IES ends during autogamy of 51ΔA cells subjected to RNAi against ICL7 (control) or KU80c (same samples as in Figure 4). A Sanger DNA sequencing ladder provides size markers. On the diagram, the LMPCR linker is drawn as grey boxes and Paramecium DNA as black lines, with a black dot representing the 3′ end generated by Pgm-dependent cleavage. In the KU80c RNAi, the LMPCR signals at 51G4404 MAC ends are likely due to background DNA breaks generated at the MAC G51 locus during DNA extraction. This background is not detected for IESs of the A51 gene, because this locus is absent from the old MAC. (B) TdT tailing of free 3′OH ends during autogamy of 51ΔA cells subjected to RNAi against ICL7 (control) or KU80c (same samples as in (A)). On the diagram, the potentially resected 5′ end is represented by a dotted line. An important control was to verify whether Pgm is produced and imported normally into the developing MAC upon Ku depletion. Cells expressing a Pgm-GFP fusion under the control of the endogenous PGM transcription signals were subjected to a control RNAi or to RNAi against KU80c (Figure 6). In the control, the fusion protein appeared in the developing new MAC at day 2 of starvation, and formed small foci until day 3 (Figure 6A). Pgm-GFP foci disappeared at day 4, which corresponds to the time when most PGR are completed in control cells (see Figure S3). In a KU80c RNAi, the Pgm-GFP fusion also localized specifically to the developing new MAC (Figure 6B), indicating that Ku is not required for the nuclear import of Pgm. However, Ku80c depletion triggered a dramatic increase in the nuclear amount of the Pgm-GFP fusion (Figure 6B), which parallels the accumulation of the endogenous PGM mRNA observed upon KU80c RNAi in cells harboring no fusion transgene (Figure 3C, right panels). We also observed a striking difference in the subnuclear localization of Pgm, in a KU80c RNAi relative to the control. Indeed, at day 3, Pgm-GFP accumulated in large nuclear bodies, which were clearly detectable under differential interference contrast (DIC) and coincided with DAPI-free regions (Figure 6B, right panels). At day 4, GFP fluorescence was still high, but large nuclear bodies were no longer detectable. The apparently normal subnuclear organization of Pgm-GFP observed at day 4 correlated with the recovery of functional amounts of KU80c mRNA at very late time-points (Figure 3C, top right panel).
Fig. 6. Nuclear accumulation of a Pgm-GFP fusion in Ku80c-depleted cells. 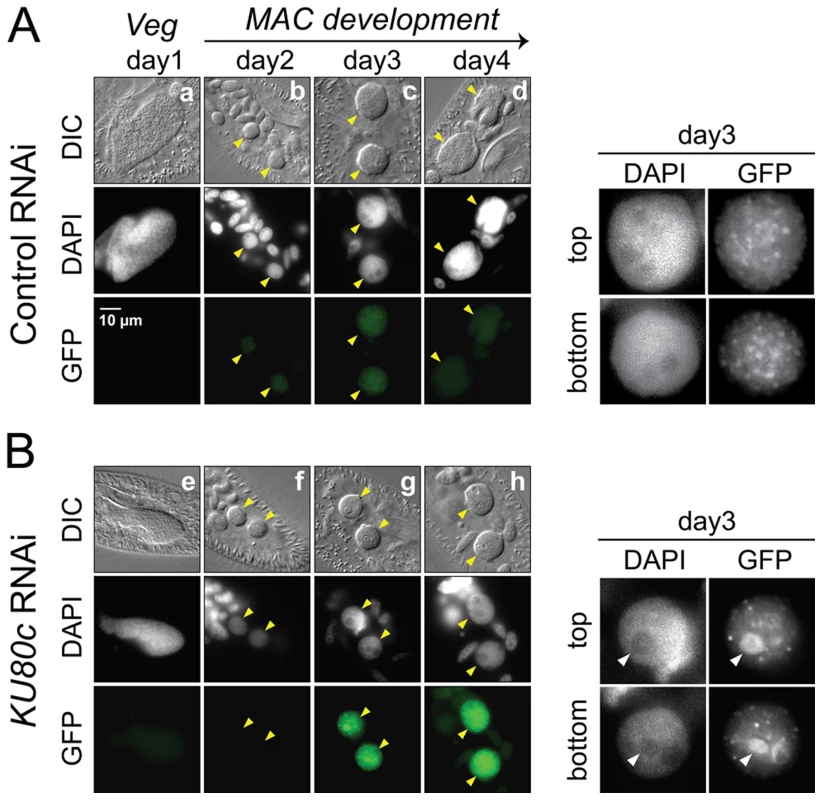
Cells were microinjected with a PGM-GFP fusion transgene and one transformant was submitted to RNAi against ICL7 (control: panel A) or KU80c (panel B). The progression of autogamy was monitored over a four-day starvation period (a and e: day 1; b and f: day 2; c and g: day 3; d and h: day 4). To compare the intensities of GFP fluorescence, signals were acquired with the same exposure time, and identical window settings were applied to the image display using the ImageJ software (National Institute of Health). Developing MACs are indicated by yellow arrowheads. In the enlarged inserts shown on the right of each panel, the display settings were modified to highlight the Pgm-GFP nuclear foci. The white arrowheads in panel B point to the DAPI-free regions, in which overproduced Pgm-GFP accumulates following KU80c RNAi. In the control RNAi, 93% of post-autogamous progeny had a functional new MAC, while the KU80c RNAi yielded no progeny with a functional new MAC. No detectable DSB repair intermediates in Ku-depleted cells
In other organisms, Ku is known to protect broken DNA ends against degradation [1], and Ku depletion may reveal alternative DSB repair pathways, such as alt-NHEJ or HR, by allowing 5′ to 3′ DNA end resection. Should resection occur upon Ku depletion in P. tetraurelia, the resected 5′ DNA ends would not be appropriate substrates for the LMPCR assay displayed in Figure 5, because the linkers that were used only allow detection of DSBs with a specific geometry (4 - or 3-base 5′ overhangs). We first investigated whether alt-NHEJ might rescue Ku depletions, by repeating the PCR assays shown in Figure 4C with more distant primers hybridizing 1 kb away from the IES excision site. This would have allowed us to detect alternative repair junctions with small deletions attributable to alt-NHEJ. Even under these conditions, however, no heterogeneous excision junctions could be detected (Figure S4). Second, because IES excision is concomitant with genome endoduplication, we reasoned that HR could use yet non-rearranged DNA molecules as templates to repair DSBs at IES excision sites: this would account for the amplification of IES+ molecules that we observed in the new MAC (Figure 4A). During HR-mediated DSB repair, the free 3′OH ends resulting from Pgm-dependent DNA cleavage would not be degraded during 5′ to 3′ resection, and should be detectable through polynucleotidyl terminal transferase (TdT) tailing [17]. In a control RNAi, indeed, free 3′OH ends were detected at IES boundaries (Figure 5B), with the same timing as the DSBs observed using LMPCR (Figure 5A). However, no free 3′OH ends were found at the expected position in a KU80c RNAi (Figure 5B). Taken together, our data do not support the hypothesis that, in cells depleted for Ku, broken IES excision sites are repaired through an alternative pathway involving 5′ to 3′ resection. Our results rather suggest that Ku is required for Pgm-dependent DNA cleavage itself.
Ku and Pgm form a complex in soluble cell extracts
A functional interaction between Ku and Pgm to activate DNA cleavage at IES boundaries may rely on the formation of a protein complex containing both proteins. To investigate this hypothesis, HA-tagged versions of Ku70a or Ku80c were produced in a heterologous insect cell system, together with Pgm fused to the maltose-binding protein (MBP) at its N-terminal end. For each condition, the MBP-Pgm protein was precipitated from soluble cell extracts using amylose magnetic beads, and co-precipitation of the Ku subunits was monitored on western blots (Figure 7A). We observed that Ku70a and Ku80c co-precipitated with MBP-Pgm, either individually or when the two subunits were co-expressed in the same cells. Control experiments confirmed that the enrichment in either Ku subunit is specific for the presence of MBP-Pgm in the extracts (Figure 7A). Reciprocally, we could co-immunoprecipitate Pgm with HA-tagged Ku from extracts of insect cells co-expressing both proteins, using magnetic beads coated with anti-HA antibodies (Figure 7B). The association of Pgm and Ku was resistant to DNase I (Figure 7C), suggesting that the formation of a Pgm/Ku complex does not depend upon the presence of DNA. Taken together, our data indicate that Pgm and Ku assemble in a higher-order complex in soluble cell extracts.
Fig. 7. Physical interaction between Pgm and Ku. 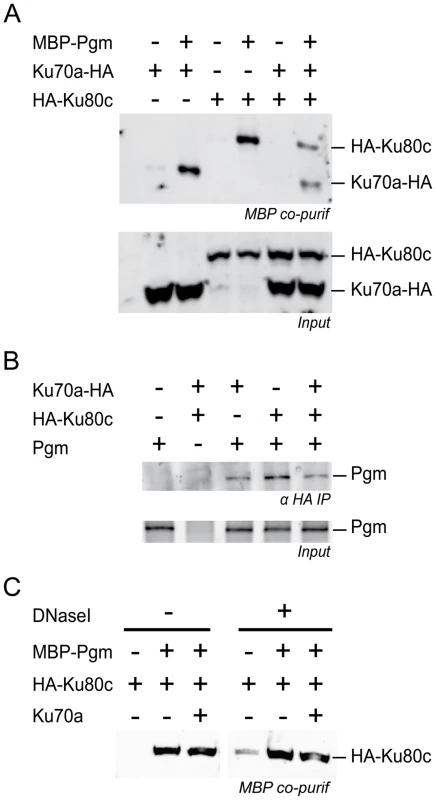
(A) Co-precipitation of HA-Ku80c and Ku70a-HA with MBP-Pgm from insect cell extracts (top panel), revealed on western blots using an anti-HA antibody. (B) Co-immunoprecipitation of Pgm with HA-Ku80c and/or Ku70a-HA from insect cell extracts, revealed on western blots using an anti-Pgm antibody. In A and B, the input proteins from each extract are displayed in the bottom panel. (C) The interaction between HA-Ku80c and Pgm is resistant to DNaseI treatment. The co-precipitation experiment was performed as described in A, using MBP-Pgm, HA-Ku80c and 6His-Ku70a recombinant proteins produced from baculovirus vectors. EDTA was removed from the lysis buffer and replaced by 10 mM MgCl2. Half of the sample was treated with 40 µg/mL of Dnase I during the 2-hr incubation with amylose-coupled magnetic beads. The presence of HA-Ku80c in the purified complexes was revealed on western blots using an anti-HA antibody. Discussion
A specific Ku heterodimer is required for MAC development as part of a developmental transcription program
Gene duplication has been proposed to be a driver of genome evolution, allowing the sub-functionalization of duplicated genes and sometimes leading to the emergence of novel cellular functions [25]. In P. tetraurelia, the presence of two KU70 and three KU80 genes has been the result of successive WGDs [18]. The KU80 family provides a nice example of sub-functionalization, with KU80a and KU80b being constitutively expressed throughout the life cycle, and KU80c exhibiting a characteristic induction pattern correlating with its specific function during MAC development. Alignment of the three Ku80 proteins reveals that Ku80c differs from Ku80a and Ku80b at several positions (Figure S5), some of which may lie on the exposed surface of the α/β domain [26] and might possibly interact with additional partners. Future studies should address the question of whether the specific function of KU80c has resulted only from its overexpression or also involves a specialization of the protein in assisting PiggyMac-dependent genome rearrangements. The function(s) of the KU70 genes could not be investigated separately through RNAi. The Ku70a and Ku70b proteins are 98% identical (see Figure S5), with only conservative amino acid changes in their α/β and β-barrel domains, which suggests that they perform similar functions. However, using RNA deep sequencing, we confirmed that the two recently duplicated KU70 genes exhibit distinct transcription patterns, with KU70a being overexpressed during MAC development. We propose that, similar to KU80c, KU70a might have been undergoing specialization to carry out an essential function in PGR.
According to previous microarray hybridization analyses, successive transcription induction peaks (early, intermediate and late) were identified at the genome-wide level during autogamy [20]. KU80c and PGM belong to the same “intermediate” cluster of genes that are induced by the time PGR take place during MAC development, and are repressed at later time-points during autogamy. Our study shows that the transcription of PGM is induced normally after Ku80c depletion. However, while PGM expression decreases at late time-points in a control, after PGR are essentially completed, it is continuously turned on in cells depleted in Ku80c, while PGR are strongly inhibited. Reciprocally, after Pgm depletion, which blocks PGR, the transcription of KU80c is induced normally but is not switched off. Quite interestingly, normal progression of autogamy is observed at the cytological level upon Ku80c or Pgm depletion, indicating that cytological progression of MAC development (DNA amplification, segregation of the new developing MACs into daughter cells) in Paramecium is uncoupled from the completion of PGR. Although alternative explanations might be proposed, the observation that similar transcriptional deregulation is observed at late autogamy time-points in KU80c and PGM knockdowns suggests that the cause of aberrant transcript accumulation for genes from the intermediate cluster is a failure to complete PGR. For instance, a transcriptional activator specific for the intermediate gene cluster may be expressed from an IES-containing gene in the developing MAC, and switched off as soon as IES excision has been completed. A regulatory mechanism relying on the retention of an IES overlapping a gene promoter has been shown to control the expression of a mating-type gene in P. tetraurelia [27]. In the ciliate Euplotes crassus, PGR also regulate the expression of a development-specific telomerase gene that is localized in the germline-restricted part of the genome and is, therefore, switched off naturally once it is eliminated [28]. The existence of feedback regulatory loops provides a nice illustration of how ciliates may take advantage of PGR to fine-tune gene expression during their sexual cycle.
The unexpected role of Ku in Paramecium: Coupling of DNA cleavage and DSB repair during PGR
Upon Ku70 or Ku80c depletions, we found that germline sequences are retained in the new MAC: both IES excision and chromosome fragmentation are inhibited, which confirms that the developmental-specific Ku70/Ku80c heterodimer plays an essential role in PGR. We confirmed that PGM expression, which is required for the two types of genome rearrangements [15], is still induced in Ku-depleted cells and that Pgm still localizes to the developing new MACs. As discussed above, the persisting overproduction of Pgm at late autogamy time-points appears to be a consequence, and not the cause, of defective genome rearrangements in Ku-depleted cells.
With regard to IES excision, no de novo precise excision junctions were detected upon Ku depletion, consistent with a defective C-NHEJ pathway. Neither did we detect any imprecise junction that may have resulted from alt-NHEJ. More surprisingly, we observed that the non-rearranged version of the genome is amplified in the new MAC, and that no DSBs with the expected geometry can be detected at IES boundaries using a sensitive LMPCR molecular approach. Because IES excision starts after 3 to 4 rounds of genome amplification in the new MAC [29], we considered the possibility that HR substitutes for end-joining during the repair of IES excision sites in Ku-depleted cells, which could restore IES+ chromosomes, supposing that a yet non-rearranged sister chromatid were used as a template. During HR-mediated repair, DSBs would be processed through 5′ to 3′ resection, which would make broken ends inappropriate substrates for LMPCR. However, using a sensitive TdT-tailing assay, we obtained no evidence that HR intermediates are formed, based on the absence of detectable free 3′ ends at IES boundaries. We therefore conclude that IESs are retained in the genome of Ku-depleted cells, as a consequence of defective programmed DNA cleavage at their boundaries. Our findings point to the participation of the development-specific Ku70-Ku80c heterodimer in Pgm-dependent DNA cleavage, upstream of its likely function in C-NHEJ-mediated DSB repair.
In another ciliate, Tetrahymena thermophila, IES excision is mediated by a Pgm homolog, Tpb2p, which is responsible for DNA cleavage at IES boundaries [30], [31]. T. thermophila harbors one KU80 and two KU70 genes: Tpb2p-dependent DNA cleavage does occur in a TKU80Δ strain, but the resulting DSBs are not repaired, leading to an arrest in MAC development and to DNA loss in the new MAC [32]. These phenotypes, which are quite similar to those described for Ligase IV or Xrcc4 depletions in P. tetraurelia [16], are fully consistent with a classical scenario, in which C-NHEJ factors are recruited to broken DNA ends after DNA cleavage at IES boundaries. Interestingly, the presence of Ku is not a prerequisite for DNA cleavage in T. thermophila, and Tku80p localization remains dispersed throughout the developing MAC when Tpb2p concentrates in large heterochromatin bodies, in which DNA elimination is thought to take place [32]. It is unclear, therefore, whether Ku interacts directly with Tpb2p during genome rearrangements in this ciliate. Together with the presence of a single KU80 gene in T. thermophila, the observed differences between the two ciliates suggest that specialization of KU80c in P. tetraurelia may have occurred after the divergence between Paramecium and Tetrahymena. Noteworthy, IES excision is rather imprecise in T. thermophila, and heterogeneity at IES excision junctions could be attributed to variability in the choice of Tpb2p cleavage sites [33] or to the participation of different mechanisms in the formation of IES excision junctions, such as C-NHEJ-mediated DSB repair [32] or trans-esterification if a single boundary is cleaved [34], [35]. Accordingly, T. thermophila appears to have avoided IES insertion into coding regions [36], where imprecise excision could be deleterious.
The situation is quite different in P. tetraurelia, in which 47% of genes are interrupted by at least one IES [11]. The pressure to assemble functional open reading frames in the somatic genome has driven the emergence of a highly efficient and precise IES excision mechanism. Several known features of the excision process contribute to this precision: (i) the establishment of a crosstalk between IES ends before DNA cleavage, within a transpososome-like complex [11], [24], and (ii) the precise positioning of Pgm-dependent cleavages on each flanking TA [17]. Here, we show that the development-specific Ku70/Ku80c heterodimer is required to activate Pgm-dependent DNA cleavage at IES boundaries in vivo. Although we excluded an inhibitory effect of Ku80c depletion on PGM transcription, we cannot formally rule out that a Ku-dependent upstream developmental event activates DNA cleavage. However, our observation that Ku70a and Ku80c interact with Pgm in pull-down experiments suggests that DNA cleavage and C-NHEJ-mediated DSB repair are tightly intertwined through the incorporation of the development-specific Ku70a/Ku80c heterodimer into the DNA cleavage complex itself (Figure 8): programmed DSBs, therefore, would be efficiently directed towards C-NHEJ-mediated precise repair. We propose that the Ku70/Ku80c heterodimer is an essential Pgm partner that activates the assembly of a transpososome-like nucleoprotein complex competent for DNA cleavage. In support to this model, we found that Ku70a and Ku80c interact with Pgm in cell extracts. During IES excision, Ku and Pgm may associate once Pgm is bound to IES boundaries (Figure 8A: activation of Pgm-dependent cleavage), or before Pgm interacts with DNA, as suggested by our observation that the Pgm/Ku complex is resistant to DNase I in vitro (Figure 8B: activation of the DNA binding activity of Pgm). Further biochemical work is needed to analyze the Ku/Pgm interaction and clarify the mechanisms involved in Ku-mediated activation of DNA cleavage. We cannot exclude at this stage that the DNA-PKcs is also a component of the DNA cleavage complex (Figure 8): indeed, a parallel study established that DNA-PKcs depletion impairs the excision of at least a subset of IESs, which exhibit a reduced efficiency of DNA cleavage at their boundaries (Malinsky et al., in prep.). The incorporation of early C-NHEJ proteins in the cleavage complex would allow the cell to face the challenge of repairing efficiently and precisely tens of thousands of DSBs during the short period of MAC development. In contrast, Ligase IV and Xrcc4 are clearly not required for DNA cleavage [16] and are probably not part of the cleavage complex.
Fig. 8. Models for the assembly of an active DNA cleavage complex. 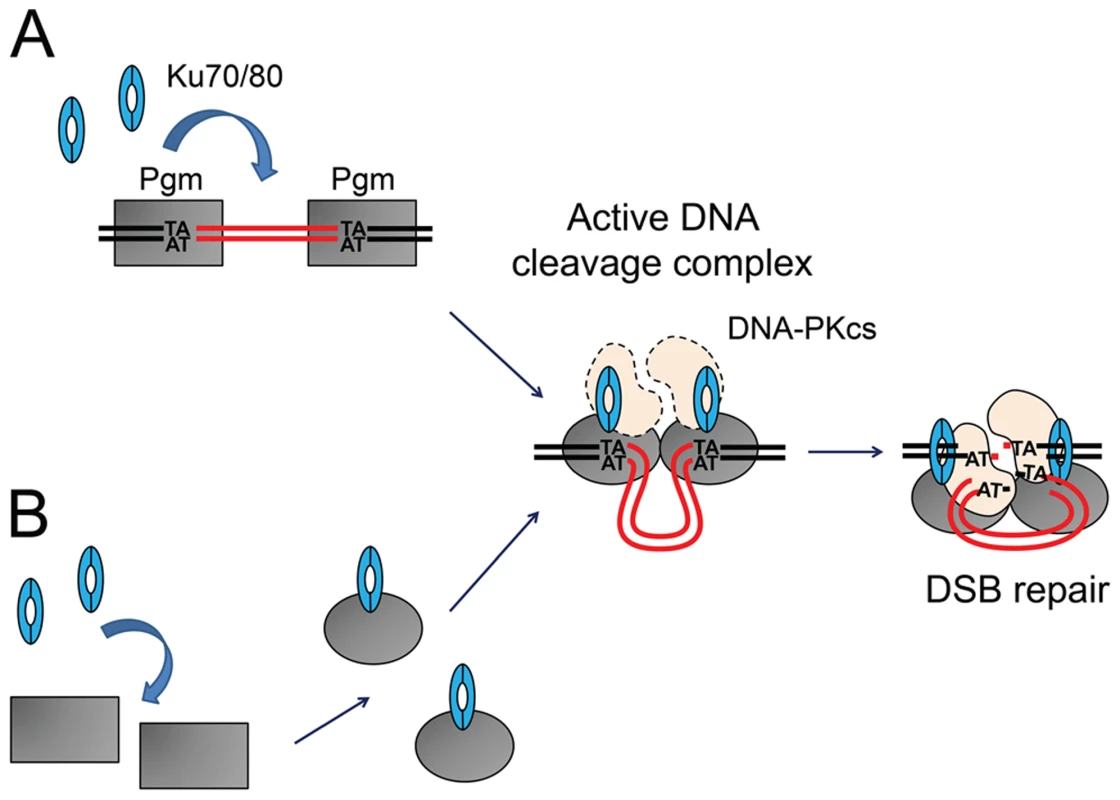
(A) Ku associates with DNA-bound Pgm and activates DNA cleavage. Pgm and its putative partners (in grey) would recognize and bind the boundaries of eliminated sequences. The binding of Ku (in blue) activates Pgm for DNA cleavage (symbolized by the switch from a rectangular box to an oval), perhaps by assisting the formation of a synapse between both IES ends, in a transpososome-like intermediate. The DNA-PKcs catalytic subunit (in peach) may also be part of the active DNA cleavage complex. (B) Ku forms a complex with Pgm in the absence of DNA, activating the DNA binding and/or cleavage activities of Pgm. Following DNA cleavage, conformational remodeling of the complex would position Ku on broken DNA ends and allow it to perform its classical role in C-NHEJ-mediated DSB repair. MAC DNA is represented in black, IES DNA in red. Integration of DNA cleavage and repair in recombination factories during PGR
Domesticated transposases from cut-and-paste DNA transposons might have been recruited to perform PGR in various systems, not only because of their DNA cleavage activities, but perhaps also because of the particular features of cut-and-paste transposition [37]. When transposons integrate into their target site, they duplicate a short sequence, the TSD (target site duplication), on each side of the integrated element. During the next round of transposition, the transposase cuts the DNA, excises the transposon and leaves a DSB at the donor site, which is repaired through cellular pathways. The study of cut-and-paste transposons has revealed that C-NHEJ, as opposed to alt-NHEJ, accounts for most end-joining events during DSB repair at transposon donor sites (reviewed in [5]). Moreover, several cut-and-paste transposons and/or their transposases interact with Ku. For instance, Ku70 binds the ends of the P element from Drosophila melanogaster and stimulates DSB repair at the donor sites [38]. In vitro, the transposase of Sleeping Beauty, a reconstructed Tc/mariner transposon, forms a complex with Ku70, and efficient transposition of Sleeping Beauty in a cellular system depends on the presence of DSB repair proteins [39]. However, it is not clear in vivo whether Ku/transposase interaction activates DNA cleavage at transposon ends or simply facilitates the recruitment of the C-NHEJ pathway once the donor site has been broken.
With regard to PGR, the formation of a complex involving a nuclease and DSB repair factors has been hypothesized during V(D)J recombination [40]. In vitro, Ku interacts with the domesticated transposase RAG1 [41], but no evidence has been provided that Ku is required in vivo for the introduction of RAG1-dependent programmed DSBs. As in Tetrahymena, the formation of nuclear recombination centers, or recombination factories, may facilitate V(D)J recombination by bringing together the recombination sites, the RAG1/RAG2 endonuclease and DSB repair factors. However, in this system, the presence of C-NHEJ proteins appears dispensable for DNA cleavage itself. The interplay between DNA cleavage and DSB repair has been pushed one step further in Paramecium. Indeed, the present study of IES excision in P. tetraurelia provides the first evidence that Ku is absolutely required in vivo to introduce programmed DSBs during PGR. The demonstration that DNA cleavage mediated by a transposase-related protein and C-NHEJ mediated repair are coupled during PGR in P. tetraurelia is reminiscent of the observation that, during meiosis in S. cerevisiae, the Mre11p HR protein is required for DNA cleavage by the topoisomerase-like Spo11 endonuclease [42]. These two systems support the notion that the presence of DSB repair factors in recombination factories may be a prerequisite for DNA cleavage during programmed genome rearrangements.
Materials and Methods
P. tetraurelia strains and growth conditions
For autogamy time-course experiments, we used P. tetraurelia strain 51 new (hereafter called 51) and its 51ΔA variant carrying a heritable deletion of the A gene in its MAC but harboring a wild-type MIC [24]. To facilitate the screening of transformants in microinjection experiments, we introduced the nd7-1 mutation [43] into strain 51 by conjugation, or used somatic variants carrying a MAC deletion of the ND7 gene [44]. Cells were grown at 27°C in a wheat grass infusion (WGP; Pines International Inc.) inoculated with Klebsiella pneumoniae. Autogamy was carried out through starvation as described [29]. Total RNA and genomic DNA were extracted from ∼400,000 cells for each time-point and quantified as described [15].
Molecular procedures
Oligonucleotides were purchased from Sigma-Aldrich or Eurofins MWG Operon (Table S1).
PCR amplifications were performed in a final volume of 25 µL, with 10 pmol of each primer, 5 nmol of each dNTP and 1 U of DyNAzyme II DNA polymerase (Finnzymes) or DreamTaq (Thermo Scientific) according to the enzyme suppliers' recommendations. PCR products were analyzed on 3% NuSieve GTG agarose gels (BioWhittaker Molecular Applications). LMPCR detection of double-strand breaks was performed as described [24]. Poly(C) tailing of free 3′ ends using terminal transferase was performed as described [17], using 500 ng of input total genomic DNA. Sanger DNA sequencing was performed at GATC Biotech, or using the fmol DNA Cycle Sequencing System (Promega). Northern and Southern blot hybridization with 32P-labeled probes was carried out as described [15]. The KU70 and KU80c probes are described in Figure S2 and Table S1. The sequence of the 17S rRNA oligonucleotide probe is shown in Table S1.
Protein depletion by RNAi
Description of RNAi plasmids
All RNAi plasmids are derivatives of vector L4440 [45] and carry a target gene fragment between two convergent T7 promoters. Each insert was chosen in order to minimize the risk of cross-silencing by using the “RNAi-off-target” tool of ParameciumDB [46]. Plasmids pXRCC4-R [16], pPGM-1 [15], p0ND7c [44] and pICL7a [47] carry RNAi inserts targeting the XRCC4, PGM, ND7 and ICL7a genes, respectively. RNAi plasmids targeting the KU genes were constructed as follows:
pL4440-KU70a-1: a 300-bp fragment from gene KU70a (bp 514–813 from ATG start codon) was amplified by PCR using primers ku70A-BamHI_1 and ku70A-KpnI_1, digested with BamHI and KpnI, and ligated between the BglII and KpnI sites of plasmid L4440.
pL4440-KU80a-1: a 233-bp fragment from gene KU80a (bp 40–272 from ATG start codon) was amplified by PCR using primers ku80A-BamHI_1 and ku80A-KpnI_1, digested with BamHI and KpnI, and ligated between the BglII and KpnI sites of plasmid L4440.
pL4440-KU80a-2: a 450-bp fragment from gene KU80a (bp 580–1029 bp from ATG start codon) was amplified by PCR using primers OMB223 and OMB224, digested with SpeI and ligated into the XbaI site of plasmid L4440.
pL4440-KU80b-1: a 233-bp fragment from gene KU80b (bp 40–272 from ATG start codon) was amplified by PCR using primers OMB219 and OMB220, digested with SpeI and ligated into the XbaI site of plasmid L4440.
pL4440-KU80b-2: a 450-bp fragment from gene KU80b (bp 580–1029 bp from ATG start codon) was amplified by PCR using primers OMB221 and OMB222, digested with SpeI and ligated into the XbaI site of plasmid L4440.
pL4440-KU80c-1: a 206-bp fragment from gene KU80c (bp 1021–1226 from ATG start codon) was amplified by PCR using primers ku80C-BamHI_1 and ku80C-KpnI_1bis, digested with BamHI and KpnI, and ligated between the BglII and KpnI sites of plasmid L4440.
pL4440-KU80c-2: a fragment from gene KU80C was amplified by PCR using primers OMB225 and ku80C-KpnI_1bis. Following restriction with SpeI, a 450-bp sub-fragment (bp 557–1006 from ATG start codon) was inserted into the XbaI site of plasmid L4440.
Outline of the “feeding” procedure
RNA interference was achieved as described [15], by feeding P. tetraurelia strains 51 or 51ΔA on Escherichia coli HT115 bacteria transformed with each plasmid and induced for the production of double-strand RNA corresponding to each RNAi insert. Survival of the progeny was tested at day 4 of starvation by transferring 30 individual autogamous cells to standard K. pneumoniae medium.
Determination of gene expression levels by RNA-seq
Strand-specific RNA-seq libraries were prepared from 50 ng polyA+ RNA following the directional mRNA-seq library preparation protocol provided by Illumina: RNAs were fragmented using fragmentation buffer, purified and treated with phosphatase and kinase prior to sequential ligation with different RNA adapters to the 3′ and 5′ ends. The ligated RNA fragments were reverse-transcribed, followed by PCR amplification. Each library was sequenced using an Illumina Genome Analyzer IIx to generate 75-nt paired-end reads. Reads were mapped with TopHat2 [48] (read-mismatches 1; min-intron-length 15; max-intron-length 100) on the P. tetraurelia strain 51 reference MAC genome [11]. Alignments were indexed using Samtools [49] and a custom perl script was used to count uniquely mapped fragments for each gene model. The counts were normalized to account for gene size and the total number of mapped fragments.
Construction of GFP fusions
For the construction of in-frame GFP-KU fusions, a GFP-coding fragment adapted to Paramecium codon usage [50] was added by PCR fusion to the 5′ end of the KU70a or KU80c genes. Each construct was inserted in a pUC18 plasmid between the SphI and SacI sites. As a result, the GFP is fused to the N-terminus of Ku70a and Ku80c and the fusion proteins are expressed under the control of the KU70a and KU80c transcription signals (promoters and 3′UTR), respectively. The PGM-GFP fusion will be described in detail elsewhere (Dubois et al., in prep.). Briefly, the GFP-coding fragment was added by PCR fusion to the 3′ end of the PGM gene carried by plasmid pPBL49g [15]: in the resulting construct, the PGM-GFP coding sequence is flanked by 96 bp upstream of the ATG (i.e. the putative endogenous PGM promoter) and 54 bp downstream of the TGA stop codon (including the endogenous 3′ UTR and the polyadenylation site).
Transformation of Paramecium cells and localization of fluorescent fusion proteins
Plasmids encoding GFP fusion proteins were linearized by appropriate restriction enzymes and microinjected into the MAC of vegetative 51 nd7-1 or 51ΔND7 cells, as described [15]. A complementing plasmid carrying a functional ND7 gene [43] was coinjected with the fusion transgenes to facilitate the selection of transformants. During autogamy, cells were permeabilized for 4 min in PHEM (60 mM Pipes, 25 mM Hepes, 10 mM EGTA, 2 mM MgCl2 pH 6.9) +1% Triton, then fixed for 10 min in PHEM +2% paraformaldehyde. All observations were performed using a Zeiss Axioplan 2 Imaging epifluorescence microscope. Developing MACs were identified using Nomarski differential interference contrast (DIC) combined with 4′,6-diamidino-2-phenylindole (DAPI) staining. No lethality was observed in the post-autogamous progeny of transformed cells.
Protein expression in insect cells and co-precipitation assays
Plasmids and vectors
DNA sequences coding for Pgm, Ku70a and Ku80c were obtained by gene synthesis (DNA 2.0 or Eurofins MWG/Operon). The synthetic PGM DNA sequence was first cloned into the pMAL-c2x vector (New England Biolabs). The MBP-PGM fusion, the MBP, the KU70a and the KU80c sequences were further introduced into the pVL-1392 vector (BD Biosciences). A HA tag was added to the C-terminus of Ku70a and the N-terminus of Ku80c. All plasmid sequences are provided in a supplementary information file (Text S1). Plasmids pVL1392-MBP-PGM, pVL1392-MBP, pVL1392-KU70a-HA and pVL1392-HA-KU80c were transfected individually into High Five cells together with the BD BaculoGold Linearized Baculovirus DNA (BD Biosciences).
Preparation of soluble protein extracts from baculovirus-infected High Five cells
High Five cells were grown in the EX-CELL 405 synthetic medium (Sigma Aldrich) supplemented with Penicillin and Streptomycin. For co-expression experiments, 106 cells are infected with MBP, MBP-PGM, KU70a-HA and/or HA-KU80c recombinant baculoviruses. In this system, each gene is expressed under the control of a late viral gene. At 48 hrs post-infection, cells were collected, washed with cold PBS buffer and re-suspended in 500 µl of lysis buffer containing 10 mM Tris-HCL pH 7.5, 150 mM NaCl, 1% Triton X-100(v/v), 1 mM EDTA, 1 mM DTT and 0.5% NaDesoxycholate (m/v) in the presence of protease inhibitors (Complete Protease Inhibitor Cocktail Tablets, Roche). Cells were lysed for 20 min on a rotating wheel, then centrifuged at 4°C for 15 min at 10,000 g.
Co-precipitation assays
For MBP-tagged proteins, soluble extracts were incubated for 2 hrs with 100 µg of amylose magnetic beads (New England Biolabs). For HA-tagged proteins, 1.5 µg of monoclonal anti-HA antibodies (HA-7 from Sigma Aldrich) were incubated overnight on a rotating wheel at 4°C with 10 µl of protein G magnetic beads (New England Biolabs), then cell extracts were incubated for 2 hrs with the coated beads. In both experiments, the beads were washed 3 times with 1 ml of lysis buffer, then re-suspended in Laemmli buffer before electrophoresis in SDS-polyacrylamide gels. Aliquots were saved for input control. HA-tagged Ku proteins were detected on western blots, using monoclonal anti-HA primary antibodies (same as above) and anti-mouse HRP-coupled secondary antibodies (Promega). MBP-tagged proteins were detected with anti-MBP HRP-coupled antibodies (New England Biolabs). Pgm was detected using polyclonal anti-Pgm rabbit primary antibodies (Proteogenics) raised against the DKNEKDAEDEFQDLNPSEHK peptide from the N-terminus of Pgm, and an anti-rabbit HRP-coupled secondary antibody (Promega).
Supporting Information
Zdroje
1. ChapmanJR, TaylorMR, BoultonSJ (2012) Playing the end game: DNA double-strand break repair pathway choice. Mol Cell 47 : 497–510.
2. LongheseMP, BonettiD, GueriniI, ManfriniN, ClericiM (2009) DNA double-strand breaks in meiosis: checking their formation, processing and repair. DNA Repair (Amst) 8 : 1127–1138.
3. SchatzDG, SwansonPC (2011) V(D)J recombination: mechanisms of initiation. Annu Rev Genet 45 : 167–202.
4. LieberMR (2010) The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu Rev Biochem 79 : 181–211.
5. BétermierM, BertrandP, LopezBS (2014) Is non-homologous end-joining really an inherently error-prone process? PLoS Genet 10: e1004086.
6. McVeyM, LeeSE (2008) MMEJ repair of double-strand breaks (director's cut): deleted sequences and alternative endings. Trends Genet 24 : 529–538.
7. TruongLN, LiY, ShiLZ, HwangPY, HeJ, et al. (2013) Microhomology-mediated End Joining and Homologous Recombination share the initial end resection step to repair DNA double-strand breaks in mammalian cells. Proc Natl Acad Sci U S A 110 : 7720–7725.
8. SymingtonLS, GautierJ (2011) Double-strand break end resection and repair pathway choice. Annu Rev Genet 45 : 247–271.
9. ChalkerDL, YaoMC (2011) DNA elimination in ciliates: transposon domestication and genome surveillance. Annu Rev Genet 45 : 227–246.
10. DuboisE, BischerourJ, MarmignonA, MathyN, RégnierV, et al. (2012) Transposon Invasion of the Paramecium Germline Genome Countered by a Domesticated PiggyBac Transposase and the NHEJ Pathway. Int J Evol Biol 2012 : 436196.
11. ArnaizO, MathyN, BaudryC, MalinskyS, AuryJM, et al. (2012) The Paramecium germline genome provides a niche for intragenic parasitic DNA: Evolutionary dynamics of internal eliminated sequences. PloS Genetics 8: e1002984.
12. CoyneRS, Lhuillier-AkakpoM, DuharcourtS (2012) RNA-guided DNA rearrangements in ciliates: is the best genome defence a good offence? Biol Cell 104 : 309–325.
13. LepèreG, BétermierM, MeyerE, DuharcourtS (2008) Maternal noncoding transcripts antagonize the targeting of DNA elimination by scanRNAs in Paramecium tetraurelia. Genes Dev 22 : 1501–1512.
14. LepèreG, NowackiM, SerranoV, GoutJF, GuglielmiG, et al. (2009) Silencing-associated and meiosis-specific small RNA pathways in Paramecium tetraurelia. Nucleic Acids Res 37 : 903–915.
15. BaudryC, MalinskyS, RestituitoM, KapustaA, RosaS, et al. (2009) PiggyMac, a domesticated piggyBac transposase involved in programmed genome rearrangements in the ciliate Paramecium tetraurelia. Genes Dev 23 : 2478–2483.
16. KapustaA, MatsudaA, MarmignonA, KuM, SilveA, et al. (2011) Highly precise and developmentally programmed genome assembly in Paramecium requires Ligase IV-dependent end joining. PloS Genetics 7: e1002049.
17. GratiasA, BétermierM (2003) Processing of double-strand breaks is involved in the precise excision of Paramecium IESs. Mol Cell Biol 23 : 7152–7162.
18. AuryJM, JaillonO, DuretL, NoelB, JubinC, et al. (2006) Global trends of whole-genome duplications revealed by the ciliate Paramecium tetraurelia. Nature 444 : 171–178.
19. BétermierM (2004) Large-scale genome remodelling by the developmentally programmed elimination of germ line sequences in the ciliate Paramecium. Res Microbiol 155 : 399–408.
20. ArnaizO, GoutJF, BétermierM, BouhoucheK, CohenJ, et al. (2010) Gene expression in a paleopolyploid: a transcriptome resource for the ciliate Paramecium tetraurelia. BMC Genomics 11 : 547.
21. GalvaniA, SperlingL (2002) RNA interference by feeding in Paramecium. Trends Genet 18 : 11–12.
22. FrankKM, SekiguchiJM, SeidlKJ, SwatW, RathbunGA, et al. (1998) Late embryonic lethality and impaired V(D)J recombination in mice lacking DNA ligase IV. Nature 396 : 173–177.
23. DuharcourtS, ButlerA, MeyerE (1995) Epigenetic self-regulation of developmental excision of an internal eliminated sequence in Paramecium tetraurelia. Genes Dev 9 : 2065–2077.
24. GratiasA, LepèreG, GarnierO, RosaS, DuharcourtS, et al. (2008) Developmentally programmed DNA splicing in Paramecium reveals short-distance crosstalk between DNA cleavage sites. Nucleic Acids Res 36 : 3244–3251.
25. TaylorJS, RaesJ (2004) Duplication and divergence: the evolution of new genes and old ideas. Annu Rev Genet 38 : 615–643.
26. WalkerJR, CorpinaRA, GoldbergJ (2001) Structure of the Ku heterodimer bound to DNA and its implications for double-strand break repair. Nature 412 : 607–614.
27. SinghDP, SaudemontB, GuglielmiG, ArnaizO, GoutJF, et al. (2014) Genome-defence small RNAs exapted for epigenetic mating-type inheritance. Nature 509 : 447–452.
28. KaramyshevaZ, WangL, ShrodeT, BednenkoJ, HurleyLA, et al. (2003) Developmentally programmed gene elimination in Euplotes crassus facilitates a switch in the telomerase catalytic subunit. Cell 113 : 565–576.
29. BétermierM, DuharcourtS, SeitzH, MeyerE (2000) Timing of developmentally programmed excision and circularization of Paramecium internal eliminated sequences. Mol Cell Biol 20 : 1553–1561.
30. ChengCY, VogtA, MochizukiK, YaoMC (2010) A domesticated piggyBac transposase plays key roles in heterochromatin dynamics and DNA cleavage during programmed DNA deletion in Tetrahymena thermophila. Mol Biol Cell 21 : 1753–1762.
31. VogtA, MochizukiK (2013) A Domesticated PiggyBac Transposase Interacts with Heterochromatin and Catalyzes Reproducible DNA Elimination in Tetrahymena. PLoS Genet 9: e1004032.
32. LinIT, ChaoJL, YaoMC (2012) An essential role for the DNA breakage-repair protein Ku80 in programmed DNA rearrangements in Tetrahymena thermophila. Mol Biol Cell 23 : 2213–2225.
33. SavelievSV, CoxMM (1995) Transient DNA breaks associated with programmed genomic deletion events in conjugating cells of Tetrahymena thermophila. Genes Dev 9 : 248–255.
34. SavelievSV, CoxMM (1996) Developmentally programmed DNA deletion in Tetrahymena thermophila by a transposition-like reaction pathway. EMBO J 15 : 2858–2869.
35. SavelievSV, CoxMM (2001) Product analysis illuminates the final steps of IES deletion in Tetrahymena thermophila. EMBO J 20 : 3251–3261.
36. FassJN, JoshiNA, CouvillionMT, BowenJ, GorovskyMA, et al. (2011) Genome-Scale Analysis of Programmed DNA Elimination Sites in Tetrahymena thermophila. G3 (Bethesda) 1 : 515–522.
37. FeschotteC, PrithamEJ (2007) DNA transposons and the evolution of eukaryotic genomes. Annu Rev Genet 41 : 331–368.
38. BeallEL, RioDC (1996) Drosophila IRBP/Ku p70 corresponds to the mutagen-sensitive mus309 gene and is involved in P-element excision in vivo. Genes Dev 10 : 921–933.
39. IzsvakZ, StuweEE, FiedlerD, KatzerA, JeggoPA, et al. (2004) Healing the wounds inflicted by sleeping beauty transposition by double-strand break repair in mammalian somatic cells. Mol Cell 13 : 279–290.
40. SchatzDG, JiY (2011) Recombination centres and the orchestration of V(D)J recombination. Nat Rev Immunol 11 : 251–263.
41. RavalP, KriatchkoAN, KumarS, SwansonPC (2008) Evidence for Ku70/Ku80 association with full-length RAG1. Nucleic Acids Res 36 : 2060–2072.
42. BordeV (2007) The multiple roles of the Mre11 complex for meiotic recombination. Chromosome Res 15 : 551–563.
43. SkouriF, CohenJ (1997) Genetic approach to regulated exocytosis using functional complementation in Paramecium: identification of the ND7 gene required for membrane fusion. Mol Biol Cell 8 : 1063–1071.
44. GarnierO, SerranoV, DuharcourtS, MeyerE (2004) RNA-mediated programming of developmental genome rearrangements in Paramecium tetraurelia. Mol Cell Biol 24 : 7370–7379.
45. KamathRS, Martinez-CamposM, ZipperlenP, FraserAG, AhringerJ (2001) Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol 2: RESEARCH0002.
46. ArnaizO, SperlingL (2011) ParameciumDB in 2011: new tools and new data for functional and comparative genomics of the model ciliate Paramecium tetraurelia. Nucleic Acids Res 39: D632–D636.
47. GogendeauD, KlotzC, ArnaizO, MalinowskaA, DadlezM, et al. (2008) Functional diversification of centrins and cell morphological complexity. J Cell Sci 121 : 65–74.
48. KimD, PerteaG, TrapnellC, PimentelH, KelleyR, et al. (2013) TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol 14: R36.
49. LiH, HandsakerB, WysokerA, FennellT, RuanJ, et al. (2009) The Sequence Alignment/Map format and SAMtools. Bioinformatics 25 : 2078–2079.
50. NowackiM, Zagorski-OstojaW, MeyerE (2005) Nowa1p and Nowa2p: novel putative RNA binding proteins involved in trans-nuclear crosstalk in Paramecium tetraurelia. Curr Biol 15 : 1616–1628.
51. NowakJK, GromadkaR, JuszczukM, Jerka-DziadoszM, MaliszewskaK, et al. (2011) Functional study of genes essential for autogamy and nuclear reorganization in Paramecium. Eukaryot Cell 10 : 363–372.
52. FinnRD, BatemanA, ClementsJ, CoggillP, EberhardtRY, et al. (2014) Pfam: the protein families database. Nucleic Acids Res 42: D222–230.
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 8- Akutní intermitentní porfyrie
- Souvislost haplotypu M2 genu pro annexin A5 s opakovanými reprodukčními ztrátami
- Příjem alkoholu a menstruační cyklus
- Doporučení pro diagnostiku a léčbu akutních jaterních porfyrií
- Hysteroskopická resekce děložního septa zlepšuje šanci na graviditu žen s jinak nevysvětlenou infertilitou
-
Všechny články tohoto čísla
- DNA Double Strand Break Repair in Mitosis Is Suppressed by Phosphorylation of XRCC4
- Inference of Transposable Element Ancestry
- The Population Genetics of Evolutionary Rescue
- Retinoic Acid Activates Two Pathways Required for Meiosis in Mice
- Pooled Segregant Sequencing Reveals Genetic Determinants of Yeast Pseudohyphal Growth
- Comprehensive Identification of Single Nucleotide Polymorphisms Associated with Beta-lactam Resistance within Pneumococcal Mosaic Genes
- SMA-Causing Missense Mutations in Display a Wide Range of Phenotypes When Modeled in
- Branch Migration Prevents DNA Loss during Double-Strand Break Repair
- Transcriptome Sequencing from Diverse Human Populations Reveals Differentiated Regulatory Architecture
- Genetic Deletion of SEPT7 Reveals a Cell Type-Specific Role of Septins in Microtubule Destabilization for the Completion of Cytokinesis
- Tethering Sister Centromeres to Each Other Suggests the Spindle Checkpoint Detects Stretch within the Kinetochore
- Global Genetic Variations Predict Brain Response to Faces
- Demography and the Age of Rare Variants
- The Response to High CO Levels Requires the Neuropeptide Secretion Component HID-1 to Promote Pumping Inhibition
- Sp6 and Sp8 Transcription Factors Control AER Formation and Dorsal-Ventral Patterning in Limb Development
- The Groucho Co-repressor Is Primarily Recruited to Local Target Sites in Active Chromatin to Attenuate Transcription
- A Transposable Element Insertion Confers Xenobiotic Resistance in Drosophila
- The Genomic Architecture of Population Divergence between Subspecies of the European Rabbit
- Human Social Genomics
- Gene Expansion Shapes Genome Architecture in the Human Pathogen : An Evolutionary Genomics Analysis in the Ancient Terrestrial Mucorales (Mucoromycotina)
- Canonical Non-Homologous End Joining in Mitosis Induces Genome Instability and Is Suppressed by M-phase-Specific Phosphorylation of XRCC4
- Roles of Type 1A Topoisomerases in Genome Maintenance in
- The TRIM-NHL Protein LIN-41 Controls the Onset of Developmental Plasticity in
- Wnt-Mediated Repression via Bipartite DNA Recognition by TCF in the Hematopoietic System
- KDM6 Demethylase Independent Loss of Histone H3 Lysine 27 Trimethylation during Early Embryonic Development
- Integration of UPR and Oxidative Stress Signaling in the Control of Intestinal Stem Cell Proliferation
- miR171-Targeted Scarecrow-Like Proteins Bind to GT -Elements and Mediate Gibberellin-Regulated Chlorophyll Biosynthesis under Light Conditions
- Syndecan-1 Is Required to Maintain Intradermal Fat and Prevent Cold Stress
- LIN-3/EGF Promotes the Programmed Cell Death of Specific Cells in by Transcriptional Activation of the Pro-apoptotic Gene
- A System for Genome-Wide Histone Variant Dynamics In ES Cells Reveals Dynamic MacroH2A2 Replacement at Promoters
- Multiple Regulation of Rad51-Mediated Homologous Recombination by Fission Yeast Fbh1
- A Genome-Wide Association Study of the Maize Hypersensitive Defense Response Identifies Genes That Cluster in Related Pathways
- The RNA Helicases AtMTR4 and HEN2 Target Specific Subsets of Nuclear Transcripts for Degradation by the Nuclear Exosome in
- Asymmetric Division and Differential Gene Expression during a Bacterial Developmental Program Requires DivIVA
- A Model-Based Approach for Identifying Signatures of Ancient Balancing Selection in Genetic Data
- Chromatin Insulator Factors Involved in Long-Range DNA Interactions and Their Role in the Folding of the Drosophila Genome
- Conditional Inactivation of Upstream Binding Factor Reveals Its Epigenetic Functions and the Existence of a Somatic Nucleolar Precursor Body
- Evidence for Divisome Localization Mechanisms Independent of the Min System and SlmA in
- Patterns of Admixture and Population Structure in Native Populations of Northwest North America
- Response Regulator Heterodimer Formation Controls a Key Stage in S Development
- A Genetic Strategy to Measure Circulating Insulin Reveals Genes Regulating Insulin Production and Secretion
- EVA-1 Functions as an UNC-40 Co-receptor to Enhance Attraction to the MADD-4 Guidance Cue in
- Dysfunction of the CNS-Heart Axis in Mouse Models of Huntington's Disease
- An Otx/Nodal Regulatory Signature for Posterior Neural Development in Ascidians
- Phosphorylation of a Central Clock Transcription Factor Is Required for Thermal but Not Photic Entrainment
- Genome-Wide Patterns of Genetic Variation within and among Alternative Selective Regimes
- EF-P Dependent Pauses Integrate Proximal and Distal Signals during Translation
- Ku-Mediated Coupling of DNA Cleavage and Repair during Programmed Genome Rearrangements in the Ciliate
- Functional Specialization Among Members Of Knickkopf Family Of Proteins In Insect Cuticle Organization
- Playing RNase P Evolution: Swapping the RNA Catalyst for a Protein Reveals Functional Uniformity of Highly Divergent Enzyme Forms
- The Translational Regulators GCN-1 and ABCF-3 Act Together to Promote Apoptosis in
- Meta-Analysis of Genome-Wide Association Studies in African Americans Provides Insights into the Genetic Architecture of Type 2 Diabetes
- A -Regulatory Mutation of Causes Silky-Feather in Chickens
- VIB1, a Link between Glucose Signaling and Carbon Catabolite Repression, Is Essential for Plant Cell Wall Degradation by
- A Population Genetic Signal of Polygenic Adaptation
- A Conserved Dopamine-Cholecystokinin Signaling Pathway Shapes Context–Dependent Behavior
- The MAP Kinase p38 Is Part of Circadian Clock
- The Cohesin Subunit Rad21 Is Required for Synaptonemal Complex Maintenance, but Not Sister Chromatid Cohesion, during Drosophila Female Meiosis
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Meta-Analysis of Genome-Wide Association Studies in African Americans Provides Insights into the Genetic Architecture of Type 2 Diabetes
- KDM6 Demethylase Independent Loss of Histone H3 Lysine 27 Trimethylation during Early Embryonic Development
- The RNA Helicases AtMTR4 and HEN2 Target Specific Subsets of Nuclear Transcripts for Degradation by the Nuclear Exosome in
- EF-P Dependent Pauses Integrate Proximal and Distal Signals during Translation
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



