-
Články
- Vzdělávání
- Časopisy
Top články
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The Characterization of Twenty Sequenced Human Genomes
We present the analysis of twenty human genomes to evaluate the prospects for identifying rare functional variants that contribute to a phenotype of interest. We sequenced at high coverage ten “case” genomes from individuals with severe hemophilia A and ten “control” genomes. We summarize the number of genetic variants emerging from a study of this magnitude, and provide a proof of concept for the identification of rare and highly-penetrant functional variants by confirming that the cause of hemophilia A is easily recognizable in this data set. We also show that the number of novel single nucleotide variants (SNVs) discovered per genome seems to stabilize at about 144,000 new variants per genome, after the first 15 individuals have been sequenced. Finally, we find that, on average, each genome carries 165 homozygous protein-truncating or stop loss variants in genes representing a diverse set of pathways.
Published in the journal: . PLoS Genet 6(9): e32767. doi:10.1371/journal.pgen.1001111
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001111Summary
We present the analysis of twenty human genomes to evaluate the prospects for identifying rare functional variants that contribute to a phenotype of interest. We sequenced at high coverage ten “case” genomes from individuals with severe hemophilia A and ten “control” genomes. We summarize the number of genetic variants emerging from a study of this magnitude, and provide a proof of concept for the identification of rare and highly-penetrant functional variants by confirming that the cause of hemophilia A is easily recognizable in this data set. We also show that the number of novel single nucleotide variants (SNVs) discovered per genome seems to stabilize at about 144,000 new variants per genome, after the first 15 individuals have been sequenced. Finally, we find that, on average, each genome carries 165 homozygous protein-truncating or stop loss variants in genes representing a diverse set of pathways.
Introduction
The technology to sequence entire human genomes has evolved rapidly in recent years. Massively-parallel sequencing techniques have been developed, and it is now possible to sequence an entire human genome in little more than a week. Programs to align these short reads and call the resulting variants are being developed and optimized [1], [2], and the cost to sequence a genome has plummeted. Single human genomes have been sequenced on a number of different next-generation sequencing platforms [3]–[6]. Whole genome sequencing has also been used to identify rare, disease-causing variants by sequencing the genome of one or a small number of affected individuals and then performing necessary follow-up work to confirm the variant [7]-[9], and it has also been used to study the patterns of variation that develop in cancerous cells [10], [11].
It will be essential going forward to be able to characterize the patterns of variation in larger sets of sequenced genomes. As a first step in that direction, we have characterized the patterns of variation observed in 20 human genomes that were sequenced at high coverage using the Illumina Genome Analyzer IIx platform.
Results
Study population
We sequenced individuals with hemophilia A who were thought to have been exposed to HIV-1, and who will contribute to a larger future study to identify genetic determinants of resistance to infection with HIV-1. We also considered ten genomes from various other non-HIV - related projects (Table S1), hereafter referred to collectively as “controls” for convenience. The ten hemophilia patients are all of European ancestry, as are seven of the controls. Two of the controls are Hispanic and one control is African American. This was confirmed using a principal component analysis [12].
Whole Genome Sequencing
The DNA for this study was extracted from blood samples or peripheral blood mononuclear cells (PBMCs). For each sequenced genome, we aimed to produce 70–80 billion bases that passed Illumina's quality filters. Some individuals were sequenced at much higher coverage (about 150–200 billion bases) to assess how coverage affects variant calling. To determine overall coverage, all gaps (stretches of N's) in the reference genome (NCBI human genome assembly build 36; Ensembl core database release 50_36l [13]) were excluded, resulting in the reference having 2,855,343,769 bases. After accounting for PCR duplicates and reads that did not align to the reference genome, genomic coverage of the autosomes ranged from 20× to 51× (Table 1). We further defined a “covered” base as a base with at least five reads where the Phred-like consensus score was greater than zero. On average across the autosomes, 97.45% of the reference genome was covered with at least five reads at each base, with a range of 92.49% to 99.65% coverage across the 20 genomes (Table 1).
Tab. 1. Summary of genomic and exonic coverage in the twenty sequenced genomes. 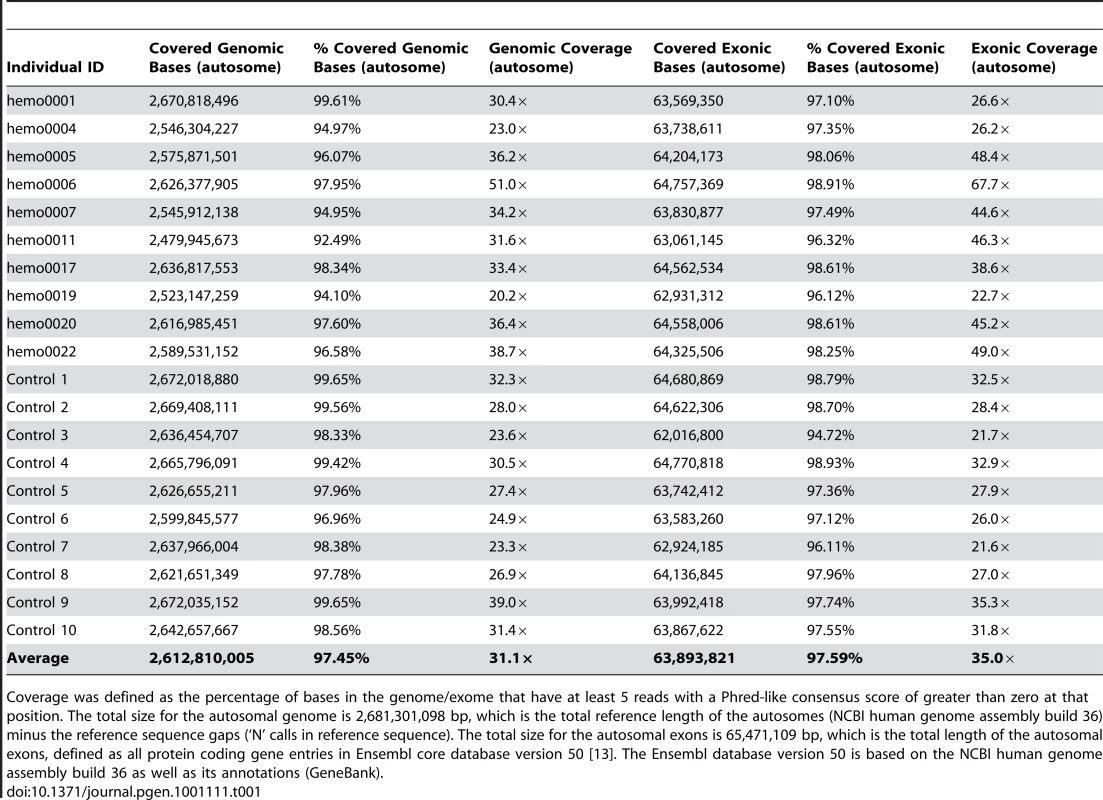
Coverage was defined as the percentage of bases in the genome/exome that have at least 5 reads with a Phred-like consensus score of greater than zero at that position. The total size for the autosomal genome is 2,681,301,098 bp, which is the total reference length of the autosomes (NCBI human genome assembly build 36) minus the reference sequence gaps (‘N’ calls in reference sequence). The total size for the autosomal exons is 65,471,109 bp, which is the total length of the autosomal exons, defined as all protein coding gene entries in Ensembl core database version 50 [13]. The Ensembl database version 50 is based on the NCBI human genome assembly build 36 as well as its annotations (GeneBank). Identifying single nucleotide variants (SNVs), small insertion/deletions (indels) and copy number variants (CNVs)
The short-reads were aligned with the Burrows-Wheeler Alignment tool (BWA) [1], and the genetic differences between our sequenced genomes and the reference were identified using modified settings in the SAMtools variant calling program [2]. On average, we identified approximately 3.5 million SNVs and 610,000 indels per genome (Table S2). Over 87% of the SNVs identified in each of the 20 genomes were found in the dbSNP database (Figure 1, Table S3), similar to what has been seen in other reports (Table S4), and 43.45% are in the HapMap project database (Table S3) [14]. The number of indels we observed is also similar to other reports (Table S5), although there were only a limited number of indels included in dbSNP (n = 13,727, version 129, validated) so overlap comparisons in this case are not informative. The average transition to transversion ratio is 2.08 and average homozygote to heterozygote ratio is 0.59 (Table S6), both consistent with what has been reported previously [3], [15], [16] (Table S7).
Fig. 1. Average per-genome overlap between SNVs in genomic databases and SNVs identified by whole-genome sequencing. 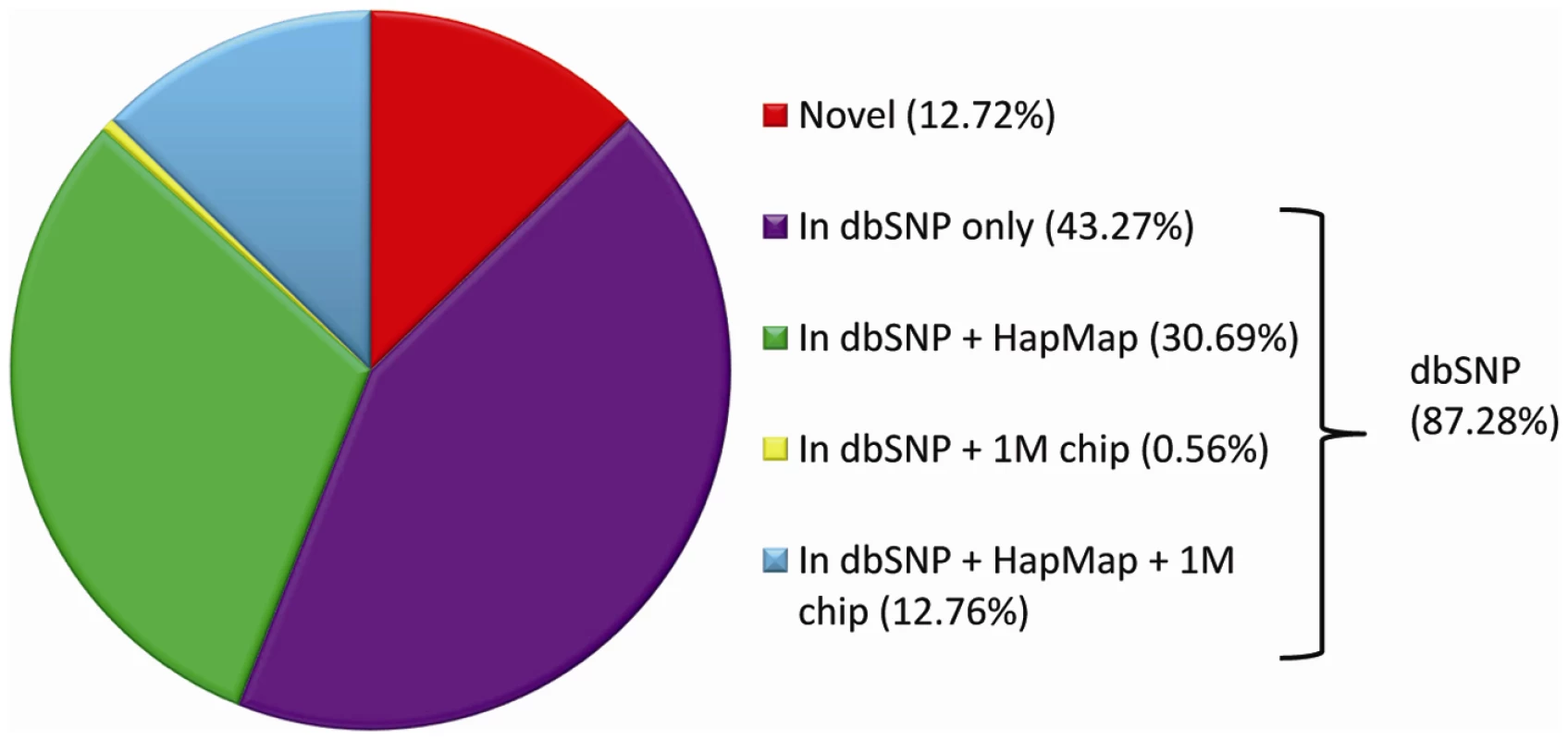
On average, 3,473,639 SNVs were observed in each genome (Table S2). A per-genome average of 87.28% of these SNVs were present in the dbSNP database (version 129, validated) (Table S3). We used the genomic distribution of the number of aligned reads (read depth) to infer copy number state in 2 kb windows using a hidden Markov model approach, incorporating SNV genotype status, implemented in a software package called “Estimation by Read Depth with SNVs” or ERDS [17]. We predicted 5,821 distinct copy number variants (CNVs) on the autosomes (6,204 across the whole genome) that were greater than 2 kb in length, and that had unique start and stop coordinates (we note, however, that even the same CNV will often be inferred to have slightly different start and stop points, meaning that it is in general not possible to determine which CNVs are identical across samples using only the location information). There was an average of 338 deletions (covering 0.72% of the autosomes) and 411 duplications (covering 0.79% of the autosomes) per genome. The median (mean) size of these CNVs was 34kb (58kb) [17]. We further note that this method is designed to detect test genomes that differ from the reference genome in genomic regions that are present in normal or nearly normal copy number state in the reference genome (corresponding to having a copy number count of two in typical genomic regions). ERDS is not designed to provide an absolute count for heavily amplified regions of the genome and in its current implementation will be insensitive to quantitative differences in highly amplified regions in comparison with the approach embodied, for example, in MrFAST [18].
Comparison of identified SNVs and CNVs to results from genotyping chip data
All samples were also run on either the Illumina Human1M-Duo version 3 or 610-Quad genotyping BeadChip, allowing comparison of SNV calls between the platforms, as well as comparison of structural variant calling. To assess concordance for SNVs, we considered all variants present on the relevant BeadChip: the concordance rate between sequencing and genotyping SNV calls ranged from 97.11% to 99.33% with an average rate of 98.58% (Figure 2, Table S8). To investigate the discordant SNVs in more detail, we split the discordant SNVs into two groups: category 1 - SNVs with homozygous calls by sequencing, but heterozygous call by genotyping BeadChip; and category 2 - any other mismatch. The majority (around 70%) of discordant calls were category 1 and preferentially observed at low-coverage sites. Category 2 discordance likely represented a mix of sequencing and genotyping errors.
Fig. 2. Concordance between sequencing and genotyping calls. 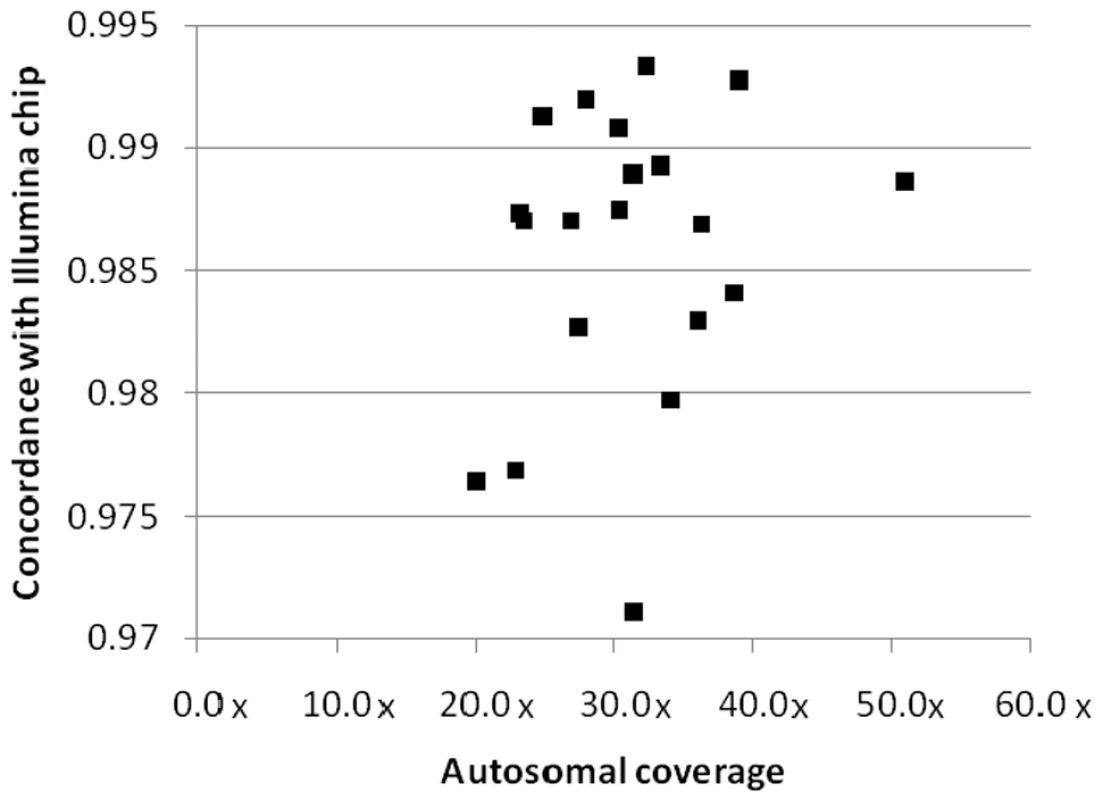
The sequenced samples were also run on either the Illumina Human 1M-Duo v3 BeadChip or the Illumina 610-Quad BeadChip. The concordance rate between the sequencing and the Illumina BeadChip genotype calls is plotted against sequencing coverage of the autosomes. A data point is plotted for each of the twenty genomes. We next compared structural variants called by ERDS with structural variants inferred from the BeadChip data using PennCNV [19]. Visual inspection of the BeadStudio data makes clear that the majority of discrepancies between ERDS and PennCNV calls for CNVs are due to the lack of sensitivity of PennCNV and the challenges in using chip data to call CNVs, especially small CNVs. Given these limitations, only 49.8% of the PennCNV-called CNVs are visually validated in BeadStudio, and only 11.7% of the deletions called by both ERDS and BreakDancer [20] can be detected by PennCNV. We also implemented several other key comparisons. First, we checked in our sample for common CNVs described by McCarroll et al [21] that have associated tagging SNVs. We checked for the presence of tagging SNVs, which indicated that the corresponding CNVs should be present. We then asked how many of the corresponding CNVs were called in our samples by PennCNV and ERDS, and we found that 9.8% (62/631) of such tagged CNVs were called by PennCNV, and 54.7% (345/631) were detected by ERDS. Furthermore, on average 43.1% of the ERDS-called CNVs had at least a 50% overlap with a common CNV entry in the Database of Genomic Variants (DGV) [22]. For those ERDS called CNVs that have been reported to be tagged by particular SNVs [21], 74.3% (371/499) of the corresponding SNVs are present in the genomes shown to carry the relevant CNV [17].
Evaluation of functional categories of identified variants
In order to classify variants in terms of functional potential, we developed a software environment called SequenceVariantAnalyzer or SVA [23] (http://www.svaproject.org/), which is a JAVA-based set of software tools that provides visualization and functional annotation of the called variants (Text S1). In particular, SVA is able to group the identified variants into functional classes (for example, non-synonymous, synonymous, stop gain), and to carry out simple frequency comparisons either internally (using our cases and controls) or externally, using, for example, data from HapMap [14] or the 1000 Genomes Project [24]. SVA can also filter for variants that meet certain quality control measures, or for genes in a certain pathway or gene ontology, or genes that are thought to have a certain function (for example, only protein-coding genes), or genes in a user-created list (for example, candidate genes based on user-defined criteria).
Using this tool, we evaluated the quantity of polymorphisms in different genomic regions, including intergenic, intronic, intron-exon boundary and exonic (Figure S1, Text S1). We also used SVA to characterize the numbers of variants in key functional categories including stop gain, stop loss, and nonsynonymous SNVs (Table S9) and frameshift coding indels (Table S10). Of particular note were variants that result in the truncation of a protein product. We define protein-truncating variants throughout this study to be any SNV that results in the gain of a stop codon, and any indel that results in a frameshift coding change. We also report some analyses that combined SNVs that result in the loss of a stop codon with those resulting in truncation to focus on a set of variants affecting the integrity of the protein product. We chose to evaluate these types of variants here and in subsequent analyses since they would be predicted to have the largest effect on protein activity. On average, each genome had 165 homozygous variants that were protein truncating or resulted in the loss of a stop codon (Tables S9 and S10). Across all 20 genomes we observed 563 different variants that were predicted to cause premature stops (n = 123), loss of a stop codon (n = 24), or a frameshift change (n = 416) in the coding regions of 484 unique genes, and which were predicted to be carried in their homozygous form by at least one of the twenty individuals. Out of these 563 variants located in 484 different genes, 21 variants, located in 20 genes, were observed in all 20 genomes. These may indicate that a less common allele is represented in the reference genome, or that the reference represents an error in the original sequencing of the human genome.
The number of protein-truncating or stop loss variants that we observed per genome was greater than what has been observed in studies that have sequenced whole exomes. Part of the reason is that we used the Ensembl transcript designations (Ensembl database versions 50_361) to screen for protein-truncating or stop loss variants. This database includes many putative protein-encoding genes that have not been confirmed to make a protein. We opted for this inclusiveness because of the possibility that poorly characterized transcripts may be of importance. For the purpose of comparison, however, we also evaluated the number of homozygous protein-truncating or stop loss variants that fell within the regions captured by the Agilent SureSelect Exome Targeted Enrichment system and that are in canonical transcripts. The number of coding indels and frameshift indels was similar to what has been observed previously [25] (Table S11).
We performed a pathway analysis using the Ingenuity Pathway Analysis (IPA) software to determine if the set of homozygous protein-truncating or stop loss variants were enriched for specific pathways. For this analysis we excluded the 21 variants present in all 20 genomes. The removal of these 21 variants removed 17 genes from our analysis. Of the remaining 467 genes, 330 were recognized by the HUGO Gene Nomenclature Committee (HGNC) database [26], and contained 364 unique homozygous protein truncating or stop loss variants. Of these, there were 228 genes with known functions (defined as a known gene ontology annotation; n = 50 for genes with premature stop SNV; n = 7 for genes with stop loss SNV; n = 177 for genes with frameshift indels). One gene has both a stop gain and a stop loss SNV, and five genes have both a stop gain SNV and a frameshift indel. The analysis did not result in a single significant canonical pathway. The most common genes with known functions were olfactory receptor genes (n = 32), followed by different protein-binding and DNA-binding genes. The enrichment of protein-truncating or stop loss variants in the olfactory receptor genes is not surprising because they make up a large gene family that is highly polymorphic for “pseudogenizing” polymorphisms [27]. Anything that is annotated as a pseudogene in Ensembl, including those in the olfactory receptor family, has not been included in this analysis.
Size and type of identified coding indels
We checked the size distribution of the coding indels that we observed in the study. The majority of the coding indels in our dataset were multiples of 3bp (51%), similar to what has been seen in other studies [28] (Figure 3).
Fig. 3. Coding indel length distribution. 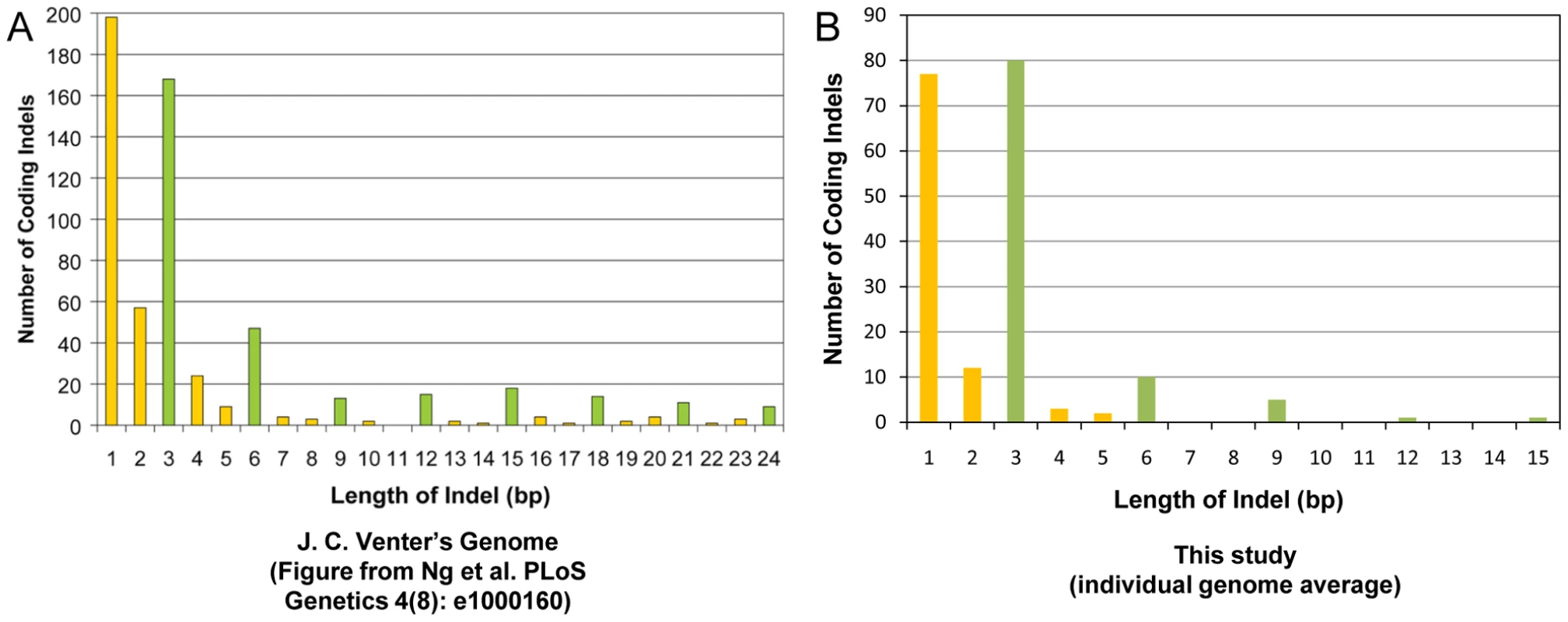
Shown is a side-by-side comparison of the length of the coding indels in this study as compared to a previous publication [26]. (A) Indel lengths observed in J.C. Venter's exome [26] versus (B) indel lengths observed in this study. The data from our study have been restricted to the canonical genes or transcripts that are captured by the Agilent SureSelect Targeted Enrichment system. Indels that are a multiple of 3bp in length are marked in green. We found 2,865 indels that cause a frameshift change and 3,737 coding indels that do not cause a frameshift change across our 20 genomes (Table S10). On average, we found 609,795 indels per genome, including 132 homozygous frameshift coding indels and 239 homozygous non-frameshift coding indels with at least 10× coverage at the variant positions (Tables S2 and S10). We also saw that the version of SAMtools that was used to make the variant calls had a large effect on the zygosity of the indels that were called (Table S1, Table S10). Inspection of discordant calls between the SAMtools versions showed that neither version was uniformly more accurate than the other.
Location of protein-truncating SNVs and indels
We investigated the locations within genes of the 554 identified stop gain SNVs and 2,865 frameshift indels. Consistent with previous reports [28], we found that these protein-truncating variants were not randomly located within genes, but rather enriched at both the N-termini and the C-termini, with a slightly higher frequency at the C-termini of the relevant proteins (Figure S2). We observed the same trend for both homozygotes and heterozygotes (data not shown).
Duplicated sequence can create apparent polymorphisms
On average, 11.2% of variants identified on the X-chromosome are assigned a heterozygote status in males (n = 17 males, Table S1). We investigated this phenomenon and found that 48.5% of them are in the pseudoautosomal regions (PARs), which are routinely masked for the Y portion [24]. Thus, the reads from the Y-chromosome PARs are free to align to the X-chromosome since there is no Y-chromosome PAR target. Because of the divergence between the chromosomes however, alignment of Y chromosome PAR sequence to the X chromosome can lead to variants being called. We found that 93.1% of this region is called as duplicated by ERDS in males. An additional 18.3% of the heterozygous SNVs on the X-chromosome in males fell in the parts of the centromeric regions that were not masked in the reference sequence, and which also appear as a duplicated region according to ERDS. The remainder of the heterozygous SNVs that were called on the X-chromosome in males may fall within the limits of a CNV that is too small to be picked up by ERDS, where the read depth signal is not strong enough to call as a duplication, or in amplified genomic regions that cannot be accurately identified as such using the ERDS approach.
While immediately recognizable on the X-chromosome in males, there is of course no reason that this phenomenon is restricted to the X-chromosome. To investigate the extent to which duplicated and diverged sequence contribute to called variants elsewhere in the genome, we note that when this happens, an excess of heterozygosity is likely and the genotype distributions will often be far out of Hardy-Weinberg equilibrium (HWE). For the autosomes, we therefore calculated HWE by Fisher's exact test for all SNVs for the whites in this study (n = 17, Table S1). We then focused on those polymorphisms with a p-value less than 0.01 in terms of where they fell across three distinct genomic regions: A) Genomic regions inferred to be duplicated in all samples relative to reference. B) Genomic regions inferred to be duplicated in one or more (but not all) samples. C) Genomic regions inferred to be in a non-duplicated region for all samples. We found that 0.72% of polymorphic loci (63,550/8,838,651) were not in Hardy-Weinberg equilibrium in the direction of excess heterozygote calls. Of those outliers, 72.0% fall in category A), which only encompassed 0.41% of genomic length of autosomes. This indicates that outliers of HWE were highly concentrated in duplication regions. An additional 11.2% of the variants fell into category B, which encompassed 1.16% of the autosomes. The remaining variants (16.8%) fell into category C, which encompassed the remaining 98.43% of the autosomes.
Experimental evaluation of a subset of the identified variants
We then used Sanger sequencing to evaluate a subset of identified protein-truncating variants. For this evaluation, we randomly selected 10 premature stop SNVs and 10 frameshift indels that appeared in homozygous form in multiple samples, and then evaluated the relevant genomic regions by Sanger sequencing in 16 samples (sufficient DNA for the follow-up work was available in only 16 of the 20 samples). Of the ten SNVs that we selected for evaluation, 8 were 100 percent concordant for all genotype calls across all samples between next-generation sequencing (NGS) and Sanger sequencing (Table S12). One variant occurred in a region of very high coverage (greater than 1000×), which is known to produce variant calling errors [29]. (We note that as the appropriate threshold for excluding variants due to high coverage is unclear, we carried all variants into SVA regardless of coverage and filtered at that point.) The final variant was concordant for all but two samples (where variants were called as heterozygotes by Sanger sequencing but were called as homozygotes by NGS, which is the most common SNV calling error (category 1, as described above). Excluding the high-coverage variant, our concordance rate for SNV genotype calls was 130/132 (98.5%). For the indels selected for evaluation, 7 variants were successfully sequenced, and 5 of these showed perfect concordance between NGS and Sanger sequencing for all genotype calls that pass our filters (Table S12). Two indels were incorrectly called, but closer inspection reveals the complexity of calling these two indels. For one of these, Sanger sequencing identified two indels within 12bp of one another. Both variants were called by SAMtools [2], but by default, when there is more than one indel within a 30bp window, SAMtools variant filtering will drop whichever indel has the lower quality score. The region containing the final indel also contains two SNVs, and the combined genotypes of these three variants sometimes made the region difficult to align and call properly. In total, we validated the genotype calls at 83/101 indels (82.2%), spread over these 7 variants in the 16 samples. Importantly, although indel genotype calls were inaccurate for two of the indels in a subset of the samples, all 7 indels identified by NGS were confirmed to be real indels in the predicted location by Sanger sequencing.
Additionally, we obtained an estimate of how well SNVs were called overall in this dataset using other experimental methods. To do this we identified a class of “vulnerable” SNVs as those that passed our variant calling criteria and were 1) observed in just one sample and 2) and were not listed in dbSNP. Of these “vulnerable” SNVs, we chose 20 at random. Since these SNVs were likely to have a higher than average false positive rate, this analysis should have given us a conservative estimate of how well SNVs are being called overall. We genotyped these 20 “vulnerable” SNVs by TaqMan in a total of 16 samples, including the sample putatively carrying each variant. We used Illumina Custom TaqMan SNP Genotyping Assays for genotyping. We then assessed whether the SNV showed evidence of variability in any of the samples, and if so, an individual blinded to the NGS data identified which sample contained the variant. We found that the genotypes we observed by TaqMan showed perfect concordance with the genotype observed by NGS sequencing for 18 of the 20 variants investigated. No variant was observed for the remaining two assays. Thus, at least 18 of these 20 variants are real, giving us a conservative estimate of 90% accuracy for calling “vulnerable” SNVs.
Comparison of cases to controls
To see whether we could easily identify the gene causing hemophilia in the cases, we next tested for enrichment of specific clearly functional variants in the set of 10 cases compared with the 10 controls. We identified first all protein-truncating or stop loss variants in homozygous form on the autosomes or present on the X chromosome, and we ranked all genes in the genome in terms of the number of cases affected. A variant was included in these counts as long as: 1) it was never observed in homozygous form in the controls, 2) no other protein-truncating or stop loss variant in the same gene was present in homozygous form in the controls, 3) it had a minor allele frequency of less than 25% in the controls (observed in heterozygous form in 5 or fewer of the 10 controls), 4) it had a minimum of 10× coverage on autosomes or 5× coverage on the sex chromosomes to be considered a homozygote, and 5) the affected gene was present in the HGNC database [26].
Not surprisingly, the gene with the most affected cases was Factor VIII (F8), mutations in which are known to cause hemophilia A. In our study, six of the hemophilia patients had identified protein truncating mutations (Table 2, Table S13, Figure S3). Table S14 lists all genes that fit the criteria and have protein-truncating or stop loss variants in at least 3 cases, regardless of HGNC classification. We did not identify disease causing mutations in the F8 gene for four of the hemophiliacs. However, it is well documented that a particular large inversion contributes to around 40% of all the severe type A hemophilia patients [30], [31]. Inversions are difficult to identify from short sequencing reads using current analysis tools, and in the case of F8, this is further complicated by regions of repetitive sequence that flank the known F8 inversion.
Tab. 2. Prioritization of protein-truncating or stop loss variants enriched in hemophilia samples. 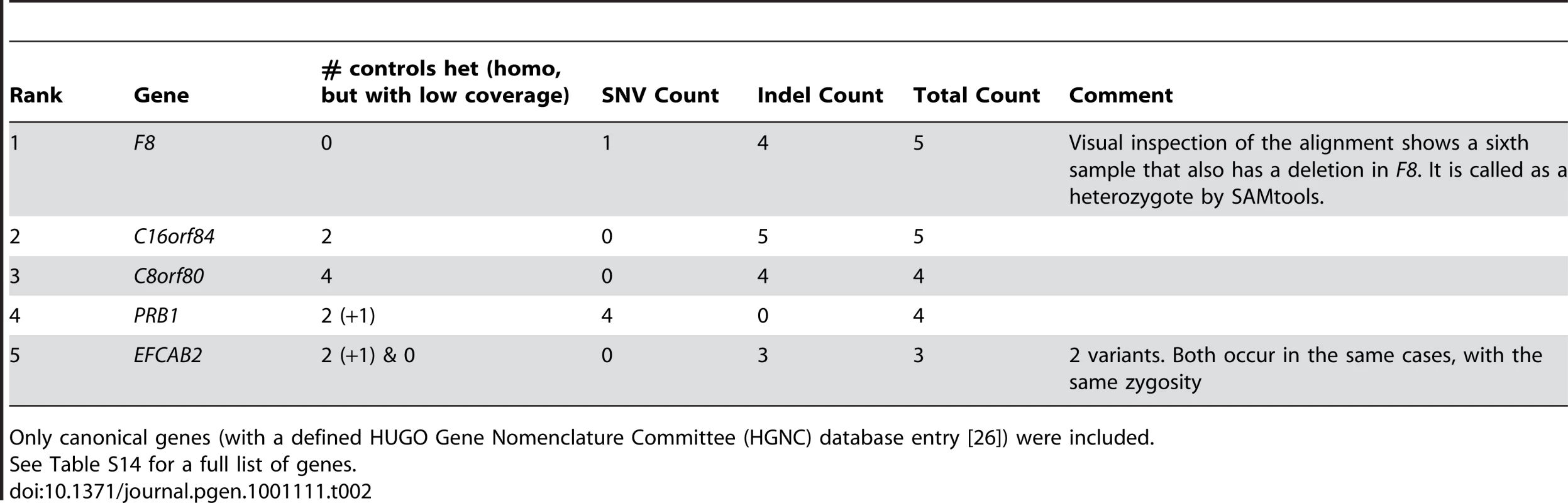
Only canonical genes (with a defined HUGO Gene Nomenclature Committee (HGNC) database entry [26]) were included. We also determined how many controls would be required to identify F8 in our 10 cases, by evaluating the rank of the F8 gene as defined above. We found that once we had five controls, F8 consistently ranked among the top five genes that showed an enrichment of variants in cases and not in the controls (Figure 4). Furthermore, the identification of the cluster of variants in F8 served as a proof-of-concept, by demonstrating that the technical and bioinformatic approaches that we used in this paper are sufficient to identify rare disease-causing variants.
Fig. 4. Rank of the F8 gene as the number of control genomes increases. 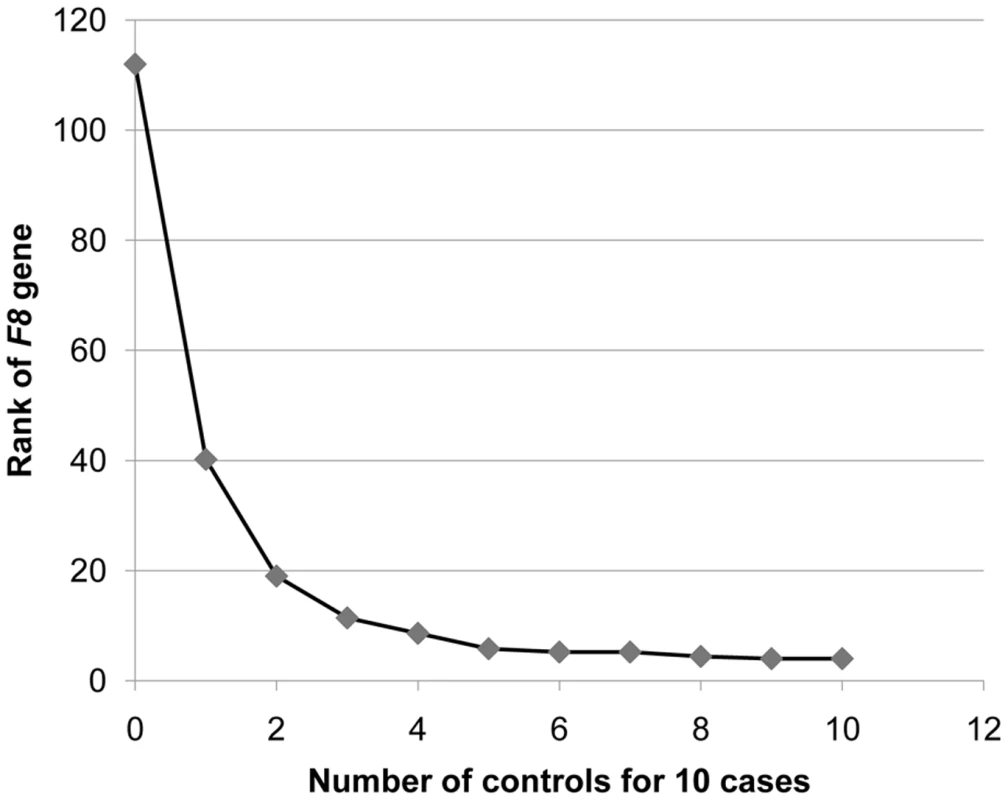
The gene ranking was ordered by the number of case genomes that carried protein-truncating or stop loss variants, in homozygous form or on the X-chromosome, that were not present in control genomes in homozygous form. Ranking was performed with a “gene prioritization” function implemented in the SVA software tool [21] (Text S1). Protein-truncating variants were defined as SNVs that cause a premature stop codon, and insertions or deletions that cause a frameshift coding change. The ranks represent an average taken from five permutations. When comparing 10 hemophilia cases to just one control, F8 ranks in the top 40 genes. Once 5 or more controls are available, it ranks in the top 5 genes. We next evaluated the scope for identifying specific coding variants that show a significant enrichment in our designated “cases.” In our dataset, there are 39,374 coding SNVs or indels that are present in at least two cases. Since variants in this class would be more likely to influence traits of interest than variants not annotated as functional, it seems reasonable to treat such groups of functional variants as a separate class in association studies [32]. The maximum imbalance that a variant could show in the current data set was to be present in the 10 cases and absent in the 10 controls, which would generate a p-value of 1×10−5. However, a Bonferroni correction for 39,374 tests requires a p-value of 1.3×10−6 to be significant at α = 0.05. Thus, it is not possible to reach significance in this size dataset, even for maximally imbalanced results.
Number of novel SNVs as a function of genomes considered
Finally, we evaluated how many novel SNVs were identified as the number of study subjects increased. Considering one of the genomes at random, we found on average 443,000 SNVs not in dbSNP. We then asked how many new SNVs were added per genome, considering both variants in dbSNP and variants “discovered” in the previously considered genomes. We permuted the order of genomes 1000 times and then took the mean of the number of SNVs added at each incremental step. The number of new SNVs per genome appeared to level off at around 144,000 novel SNVs by the 15th genome (Figure 5A, Text S1).
Fig. 5. Number of novel SNVs and novel knocked-out genes as the number of genomes increases. 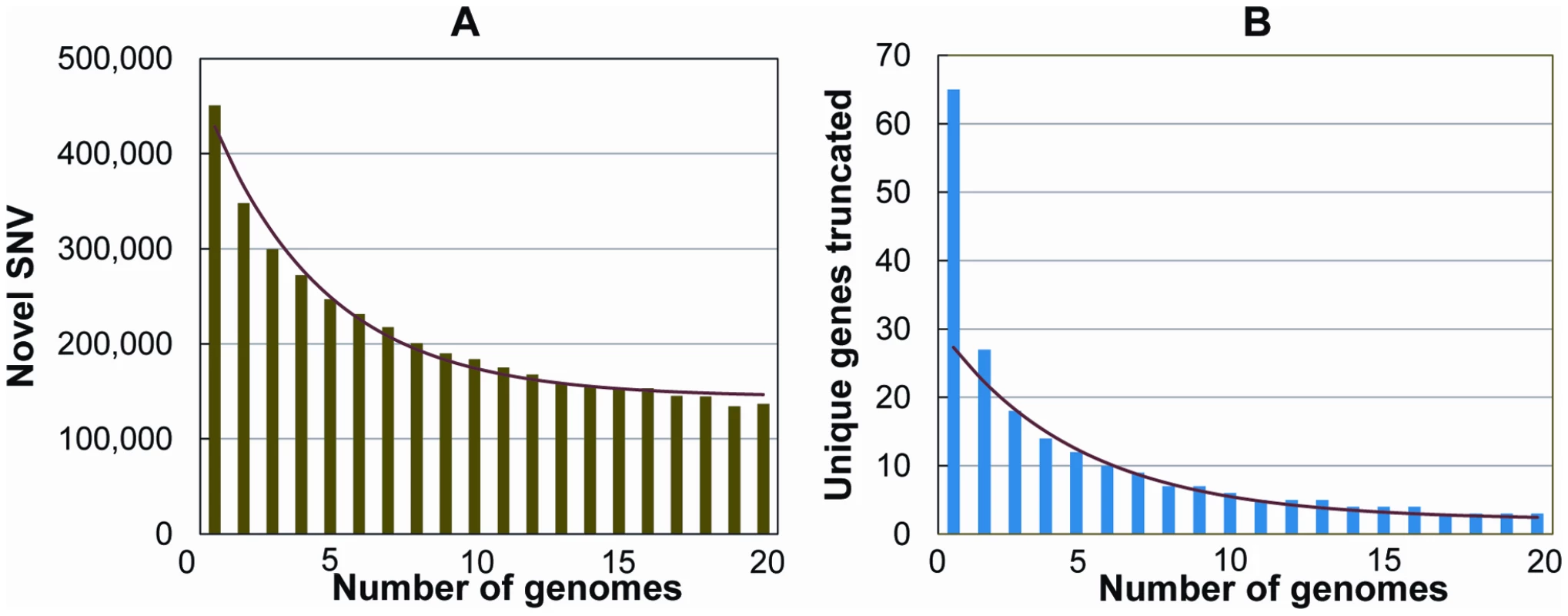
The total number of novel variants, and the total number of novel genes containing protein truncating or stop loss variants, continues to drop as additional genomes are added to the analysis. Shown are the number of unique SNVs (A) and unique genes carrying a homozygous protein-truncating or stop loss variant (B) per genome, as a function of the number of genomes already considered. The genomes were added in a random order to both analyses, and 1000 permutations were performed and averaged. We also considered the related question of how many new genes have been observed to be “knocked-out” by homozygous protein-truncating or stop loss variants per new genome that has been sequenced. Again, we permuted the order of genomes 1000 times and then took the mean of the number of new genes added with homozygous protein truncating or stop loss variants at each incremental step. The number of new genes appeared to level off around two new knocked-out genes per genome (Figure 5B). Assuming about two newly identified “knock out” genes per new genome sequenced, we may therefore estimate that sequencing around 10,000 genomes (20,000 genes/2 genes per genome) would be required to determine the number of genes that can be knocked out and still be compatible with life. At this point, it is not possible to more accurately estimate the true number of genomes that would be required. Here, we have simply propagated the trend observed in 20 genomes, with the caveats that we don't know how the rate of variant discovery will behave after 20 genomes, and given that we know that a portion of the protein-truncating indels included in the analyses will be either mis-genotyped (above) or artifacts. Nonetheless, it is clear that sequencing on the scale now possible will slowly identify individuals carrying homozygote knock-out mutations for a large catalogue of genes.
Discussion
Our evaluation of a broad range of overall coverage values has allowed us to identify an appropriate target coverage for discovery studies. Importantly, we found that once overall coverage exceeds about 30–35×, the concordance rate between sequencing and genotyping stabilizes at greater than 98.58% (Table 1, Figure 2). This suggests that between 30–35× is an appropriate target coverage for accurate variant calling.
The identification of the F8 gene as the top ranking gene, in terms of protein truncating genotypes, confirmed that the cause of a Mendelian disease can be identified by next-generation sequencing, as has been recently demonstrated by several groups [7]–[9], [25], [33], [34]. The majority of the causal mutations we observed in F8 were indels, and it is known that indel calling using next-generation sequence data is much less accurate than SNV calling (see above, and [35], [36]). Nevertheless, F8 is easily identified as responsible for hemophilia. This is consistent with our recent identification of a protein-truncating indel in the PTPN11 gene as responsible for metachondromatosis, a rare Mendelian disease affecting the skeleton [8]. Thus, even though indel calling remains a work in progress, it is nevertheless sensible to keep them in current variant calling pipelines.
Due to the limitations on obtaining whole-genome sequences to use as a control cohort, a handful of phenotypes were present in our controls (Table S1). However, they were still reasonable controls to use in our study design, since they represented a diversity of phenotypes and did not have hemophilia. Finally, the fact that we were only able to identify the causal variant for hemophilia in 6 out of the 10 patients also demonstrated the current limitation of using a next-generation sequencing study to identify certain types of genetic variants, including inversions.
The inclusion of 20 genomes in our analyses also allowed us to evaluate the discovery rate of new variants as additional genomes are added. Although the rate of variant discovery will continue to fall at an unknown rate, this nonetheless gives us a rough idea of how many genomes would be required to identify specific numbers of variations (or genotypes). In these analyses, the observation of greatest interest is that each genome carries many genes that are not expressed (knocked-out) and that a manageable sequencing effort of perhaps 10,000 individuals would allow the establishment of a cohort of individuals that are effective “knock outs” for many of the human genes which are not necessary for survival. It seems likely that such a cohort could be used much as mouse knock outs have been used, by inviting certain individuals to participate in intensive phenotyping to determine the effect of specific gene knockouts. Given the value of mouse knock outs in defining pathways and interactions, it seems likely that such a cohort will in time become a critical tool in human genetics. In addition, the catalogue of genes that can be knocked out with specific clinical consequences will also be of obvious immediate utility in the burgeoning effort to use whole-genome sequencing of defined cases to identify the genetic basis of common human disease, although considerable care would be required in the selection of the appropriate healthy controls for such a study.
Materials and Methods
Sample Preparation
Each sequenced sample was prepared according to the Illumina protocols. Briefly, one microgram of genomic DNA was fragmented by nebulization, the fragmented DNA was repaired, an ‘A’ was ligated to the 3′ end, Illumina adapters were then ligated to the fragments, and the sample was size selected aiming for a 350–400 base pair product. The size selected product was PCR amplified, and the final product was validated using the Agilent Bioanalyzer. Samples were then amplified on the flow cell and sequenced using the Genome Analyzer IIx, following the Illumina supplied protocols. The majority of sequence runs were paired end with 75 base reads. We aimed for 70–80 billion bases that passed the Illumina analysis filter per genome.
Analysis pipeline
All sequencing data was produced and curated by the Genomic Analysis Facility, part of the Center for Human Genome Variation, at Duke University. After the sequencing reactions were complete, the Illumina analysis pipeline was used to process the raw sequencing data (Firecrest, Bustard and Gerald). The resulting Eland alignment was used only to estimate an error rate for each read/cluster. If a sequencing run/lane had an error rate above 2%, the run was considered a failure and the data was not used. The quality of the sequencing runs were also assessed by evaluating the percentage of clusters passing filter and the percentage of reads that align to the reference genome. For a typical run, over 70% of clusters pass filter and over 85% align. Any major deviations from these values would trigger further evaluation (average intensity, error graphs, etc) and likely lead to these runs/lanes not being used. The FASTQ files were then ready for the next alignment step.
Once the raw sequence data was curated, the reads were aligned to a reference genome (NCBI human genome assembly build 36) using the BWA software [1]. Each alignment was assigned a mapping quality score by BWA, which is the Phred-scaled probability that the alignment is incorrect. The PCR amplification step will lead to the sequencing of identical DNA fragments. Not removing these PCR duplicates can lead to the miscalling of SNVs by overrepresentation of one allele. This is corrected by a quality control step to remove these potential PCR duplicates with SAMtools. Once all the reads have been aligned to the reference genome using BWA [1], we then used the SAMtools software [2] to produce a consensus genotype for each genomic position. The consensus genotype is the genotype which has the highest probability of occurring after consideration of a number of factors [37]. Each consensus genotype is then assigned a consensus quality, which is based on a Phred-scaled probability that the genotype call is incorrect. Single nucleotide variants (SNVs) and insertion/deletions (indels) were then identified based on differences between the consensus genotype and the reference allele at that position. SAMtools also assigns a Phred-scaled probability to each identified SNV/indel which indicates how likely it is that an inferred SNV/indel is identical to the reference. These SNVs and indels were then filtered by SAMtools' variation filter, changing only the maximum read depth parameter to call variants from its default value (100) to 10 million, to prevent the exclusion of SNVs and indels at a read depth greater than 100. The lists of SNVs/indels were then annotated in the SequenceVariantAnalyzer (SVA [23]). SVA was specifically designed to annotate the large number of identified SNVs/indels using a number of human genomic databases. In addition to looking at the SNVs/indels from the BWA alignment/SAMtools, we also predicted larger structural variation by developing an “Estimation by Read Depth with SNVs” or ERDS method, based on a Hidden Markov Model [17]. This was an extension to the methods described in Bentley et al. [3] and draft codes from Scally, A. [38]. Further details describing the analysis tools are presented in Text S1.
Data access
The raw reads for a portion of the genomes used in this study are available on the NCBI sequence read archive, under study ID SRP001691 (http://www.ncbi.nlm.nih.gov/sra/SRP001691). We do not have consent from the patients and/or permission from the Duke IRB to release the raw reads for several of the genomes used in this study.
Supporting Information
Zdroje
1. LiH
DurbinR
2009 Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25 1754 1760
2. LiH
HandsakerB
WysokerA
FennellT
RuanJ
2009 The Sequence Alignment/Map format and SAMtools. Bioinformatics 25 2078 2079
3. BentleyDR
BalasubramanianS
SwerdlowHP
SmithGP
MiltonJ
2008 Accurate whole human genome sequencing using reversible terminator chemistry. Nature 456 53 59
4. WangJ
WangW
LiR
LiY
TianG
2008 The diploid genome sequence of an Asian individual. Nature 456 60 65
5. WheelerDA
SrinivasanM
EgholmM
ShenY
ChenL
2008 The complete genome of an individual by massively parallel DNA sequencing. Nature 452 872 876
6. AhnSM
KimTH
LeeS
KimD
GhangH
2009 The first Korean genome sequence and analysis: full genome sequencing for a socio-ethnic group. Genome Res 19 1622 1629
7. LupskiJR
ReidJG
Gonzaga-JaureguiC
Rio DeirosD
ChenDC
2010 Whole-genome sequencing in a patient with Charcot-Marie-Tooth neuropathy. N Engl J Med 362 1181 1191
8. SobreiraNLM
CirulliET
AvramopoulosD
WohlerE
OswaldGL
2010 Whole genome sequencing of a single individual identifies a Mendelian disease gene. PLoS Genet 6 e1000991
9. RoachJC
GlusmanG
SmitAF
HuffCD
HubleyR
2010 Analysis of genetic inheritance in a family quartet by whole-genome sequencing. Science 328 636 639
10. LeyTJ
MardisER
DingL
FultonB
McLellanMD
2008 DNA sequencing of a cytogenetically normal acute myeloid leukaemia genome. Nature 456 66 72
11. LeeW
JiangZ
LiuJ
HavertyPM
GuanY
2010 The mutation spectrum revealed by paired genome sequences from a lung cancer patient. Nature 465 473 477
12. PriceAL
PattersonNJ
PlengeRM
WeinblattME
ShadickNA
2006 Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 38 904 909
13. HubbardTJ
AkenBL
AylingS
BallesterB
BealK
2009 Ensembl 2009. Nucleic Acids Res 37 D690 697
14. FrazerKA
BallingerDG
CoxDR
HindsDA
StuveLL
2007 A second generation human haplotype map of over 3.1 million SNPs. Nature 449 851 861
15. LevyS
SuttonG
NgPC
FeukL
HalpernAL
2007 The diploid genome sequence of an individual human. PLoS Biol 5 e254
16. HaleMC
McCormickCR
JacksonJR
DewoodyJA
2009 Next-generation pyrosequencing of gonad transcriptomes in the polyploid lake sturgeon (Acipenser fulvescens): the relative merits of normalization and rarefaction in gene discovery. BMC Genomics 10 203
17. ZhuM
NeedAC
GeD
SinghA
FengS
2010 Detection of copy number variation using whole genome sequence data from twenty human genomes. Manuscript in preparation
18. AlkanC
KiddJM
Marques-BonetT
AksayG
AntonacciF
2009 Personalized copy number and segmental duplication maps using next-generation sequencing. Nat Genet 41 1061 1067
19. WangK
LiM
HadleyD
LiuR
GlessnerJ
2007 PennCNV: an integrated hidden Markov model designed for high-resolution copy number variation detection in whole-genome SNP genotyping data. Genome Res 17 1665 1674
20. ChenK
WallisJW
McLellanMD
LarsonDE
KalickiJM
2009 BreakDancer: an algorithm for high-resolution mapping of genomic structural variation. Nat Methods 6 677 681
21. McCarrollSA
KuruvillaFG
KornJM
CawleyS
NemeshJ
2008 Integrated detection and population-genetic analysis of SNPs and copy number variation. Nat Genet 40 1166 1174
22. IafrateAJ
FeukL
RiveraMN
ListewnikML
DonahoePK
2004 Detection of large-scale variation in the human genome. Nat Genet 36 949 951
23. GeD
RuzzoEK
ShiannaKV
HeM
AllenA
2010 Annotation, visualization, and analysis of variants emerging from whole-genome and whole-exome sequencing using SVA. Manuscript in preparation
24. The 1000 Genomes Project 2009 http://www.1000genomes.org/page.php
25. NgSB
TurnerEH
RobertsonPD
FlygareSD
BighamAW
2009 Targeted capture and massively parallel sequencing of 12 human exomes. Nature 461 272 276
26. EyreTA
DucluzeauF
SneddonTP
PoveyS
BrufordEA
2006 The HUGO Gene Nomenclature Database, 2006 updates. Nucleic Acids Res 34 D319 321
27. GiladY
ManO
PaaboS
LancetD
2003 Human specific loss of olfactory receptor genes. Proc Natl Acad Sci U S A 100 3324 3327
28. NgPC
LevyS
HuangJ
StockwellTB
WalenzBP
2008 Genetic variation in an individual human exome. PLoS Genet 4 e1000160
29. MalhisN
JonesSJM
2010 High quality SNP calling using Illumina data at shallow coverage. Bioinformatics 26 1029 1035
30. NaylorJ
BrinkeA
HassockS
GreenPM
GiannelliF
1993 Characteristic mRNA abnormality found in half the patients with severe haemophilia A is due to large DNA inversions. Hum Mol Genet 2 1773 1778
31. AntonarakisSE
KazazianHH
TuddenhamEG
1995 Molecular etiology of factor VIII deficiency in hemophilia A. Hum Mutat 5 1 22
32. CirulliET
GoldsteinDB
2010 Uncovering the roles of rare variants in common disease through whole-genome sequencing. Nat Rev Genet 11 415 425
33. ChoiM
SchollUI
JiW
LiuT
TikhonovaIR
2009 Genetic diagnosis by whole exome capture and massively parallel DNA sequencing. Proc Natl Acad Sci U S A 106 19096 19101
34. NgSB
BuckinghamKJ
LeeC
BighamAW
TaborHK
2010 Exome sequencing identifies the cause of a mendelian disorder. Nat Genet 42 30 35
35. KrawitzP
RodelspergerC
JagerM
JostinsL
BauerS
2010 Microindel detection in short-read sequence data. Bioinformatics 26 722 729
36. KoboldtDC
2010 Challenges of sequencing human genomes. Brief Bioinform Advance publication 2 June 2010
37. LiH
RuanJ
DurbinR
2008 Mapping short DNA sequencing reads and calling variants using mapping quality scores. Genome Res 18 1851 1858
38. ScallyA
BentleyDR
2009 Personal Communication Hidden Markov Model for Copy-number Variation
Štítky
Genetika Reprodukční medicína
Článek Allelic Variation at the 8q23.3 Colorectal Cancer Risk Locus Functions as a Cis-Acting Regulator ofČlánek Allelic Selection of Amplicons in Glioblastoma Revealed by Combining Somatic and Germline AnalysisČlánek Lactic Acidosis Triggers Starvation Response with Paradoxical Induction of TXNIP through MondoAČlánek Rice a Cinnamoyl-CoA Reductase-Like Gene Family Member, Is Required for NH1-Mediated Immunity to pv.Článek Differentiation of Zebrafish Melanophores Depends on Transcription Factors AP2 Alpha and AP2 Epsilon
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 9- Akutní intermitentní porfyrie
- Souvislost haplotypu M2 genu pro annexin A5 s opakovanými reprodukčními ztrátami
- Růst a vývoj dětí narozených pomocí IVF
- Srdeční frekvence embrya může být faktorem užitečným v předpovídání výsledku IVF
- Vztah užívání alkoholu a mužské fertility
-
Všechny články tohoto čísla
- Optimal Strategy for Competence Differentiation in Bacteria
- Mutational Patterns Cannot Explain Genome Composition: Are There Any Neutral Sites in the Genomes of Bacteria?
- Frail Hypotheses in Evolutionary Biology
- Genetic Architecture of Complex Traits and Accuracy of Genomic Prediction: Coat Colour, Milk-Fat Percentage, and Type in Holstein Cattle as Contrasting Model Traits
- Allelic Variation at the 8q23.3 Colorectal Cancer Risk Locus Functions as a Cis-Acting Regulator of
- Allelic Selection of Amplicons in Glioblastoma Revealed by Combining Somatic and Germline Analysis
- Germline Variation Controls the Architecture of Somatic Alterations in Tumors
- Mice Doubly-Deficient in Lysosomal Hexosaminidase A and Neuraminidase 4 Show Epileptic Crises and Rapid Neuronal Loss
- Analysis of Population Structure: A Unifying Framework and Novel Methods Based on Sparse Factor Analysis
- FliO Regulation of FliP in the Formation of the Flagellum
- Cdc20 Is Critical for Meiosis I and Fertility of Female Mice
- dMyc Functions Downstream of Yorkie to Promote the Supercompetitive Behavior of Hippo Pathway Mutant Cells
- DCAF26, an Adaptor Protein of Cul4-Based E3, Is Essential for DNA Methylation in
- Genome-Wide Double-Stranded RNA Sequencing Reveals the Functional Significance of Base-Paired RNAs in
- An Immune Response Network Associated with Blood Lipid Levels
- Genetic Variants and Their Interactions in the Prediction of Increased Pre-Clinical Carotid Atherosclerosis: The Cardiovascular Risk in Young Finns Study
- The Histone H3K36 Methyltransferase MES-4 Acts Epigenetically to Transmit the Memory of Germline Gene Expression to Progeny
- Long- and Short-Term Selective Forces on Malaria Parasite Genomes
- Lactic Acidosis Triggers Starvation Response with Paradoxical Induction of TXNIP through MondoA
- Identification of Early Requirements for Preplacodal Ectoderm and Sensory Organ Development
- Orphan CpG Islands Identify Numerous Conserved Promoters in the Mammalian Genome
- Analysis of the Basidiomycete Reveals Conservation of the Core Meiotic Expression Program over Half a Billion Years of Evolution
- ETS-4 Is a Transcriptional Regulator of Life Span in
- The SR Protein B52/SRp55 Is Required for DNA Topoisomerase I Recruitment to Chromatin, mRNA Release and Transcription Shutdown
- The Baker's Yeast Diploid Genome Is Remarkably Stable in Vegetative Growth and Meiosis
- Chromatin Landscape Dictates HSF Binding to Target DNA Elements
- The APETALA-2-Like Transcription Factor OsAP2-39 Controls Key Interactions between Abscisic Acid and Gibberellin in Rice
- Accurately Assessing the Risk of Schizophrenia Conferred by Rare Copy-Number Variation Affecting Genes with Brain Function
- Widespread Over-Expression of the X Chromosome in Sterile F Hybrid Mice
- The Characterization of Twenty Sequenced Human Genomes
- The Genome of a Pathogenic : Cooptive Virulence Underpinned by Key Gene Acquisitions
- A Single Element Maintains Repression of the Key Developmental Regulator
- Identification of New Genetic Risk Variants for Type 2 Diabetes
- Effect of Correlated tRNA Abundances on Translation Errors and Evolution of Codon Usage Bias
- Evidence of Selection upon Genomic GC-Content in Bacteria
- Proteomic Changes Resulting from Gene Copy Number Variations in Cancer Cells
- Rice a Cinnamoyl-CoA Reductase-Like Gene Family Member, Is Required for NH1-Mediated Immunity to pv.
- Longitudinal Genome-Wide Association of Cardiovascular Disease Risk Factors in the Bogalusa Heart Study
- Response to Mechanical Stress Is Mediated by the TRPA Channel Painless in the Heart
- DNMT3L Modulates Significant and Distinct Flanking Sequence Preference for DNA Methylation by DNMT3A and DNMT3B
- Identifying Signatures of Natural Selection in Tibetan and Andean Populations Using Dense Genome Scan Data
- Incremental Genetic Perturbations to MCM2-7 Expression and Subcellular Distribution Reveal Exquisite Sensitivity of Mice to DNA Replication Stress
- Loss of Maternal ATRX Results in Centromere Instability and Aneuploidy in the Mammalian Oocyte and Pre-Implantation Embryo
- Comparative Genomic Hybridization (CGH) Reveals a Neo-X Chromosome and Biased Gene Movement in Stalk-Eyed Flies (Genus )
- Differentiation of Zebrafish Melanophores Depends on Transcription Factors AP2 Alpha and AP2 Epsilon
- Gene–Environment Interactions at Nucleotide Resolution
- Dementia Revealed: Novel Chromosome 6 Locus for Late-Onset Alzheimer Disease Provides Genetic Evidence for Folate-Pathway Abnormalities
- Critical Functions of Rpa3/Ssb3 in S-Phase DNA Damage Responses in Fission Yeast
- Preferential Re-Replication of Heterochromatin in the Absence of Geminin
- The Potential for Enhancing the Power of Genetic Association Studies in African Americans through the Reuse of Existing Genotype Data
- Evidence That Mutation Is Universally Biased towards AT in Bacteria
- Perturbation Analysis of Heterochromatin-Mediated Gene Silencing and Somatic Inheritance
- Diversity of Eukaryotic DNA Replication Origins Revealed by Genome-Wide Analysis of Chromatin Structure
- Genetic Deletion of the Desmosomal Component Promotes Tumor Microinvasion in a Mouse Model of Pancreatic Neuroendocrine Carcinogenesis
- The Metabolic Enzyme ManA Reveals a Link between Cell Wall Integrity and Chromosome Morphology
- SNPs Associated with Cerebrospinal Fluid Phospho-Tau Levels Influence Rate of Decline in Alzheimer's Disease
- Synthesizing and Salvaging NAD: Lessons Learned from
- A Central Regulatory System Largely Controls Transcriptional Activation and Repression Responses to Phosphate Starvation in Arabidopsis
- An Insect Herbivore Microbiome with High Plant Biomass-Degrading Capacity
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Synthesizing and Salvaging NAD: Lessons Learned from
- Optimal Strategy for Competence Differentiation in Bacteria
- Long- and Short-Term Selective Forces on Malaria Parasite Genomes
- Identifying Signatures of Natural Selection in Tibetan and Andean Populations Using Dense Genome Scan Data
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



